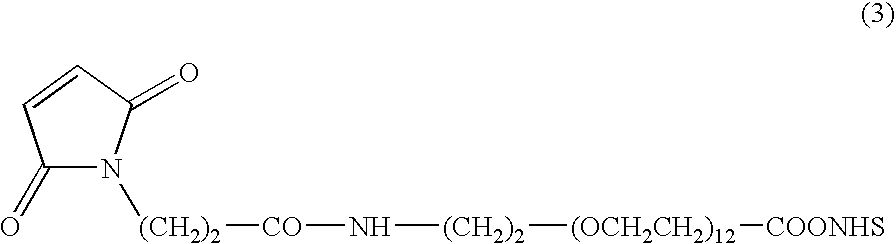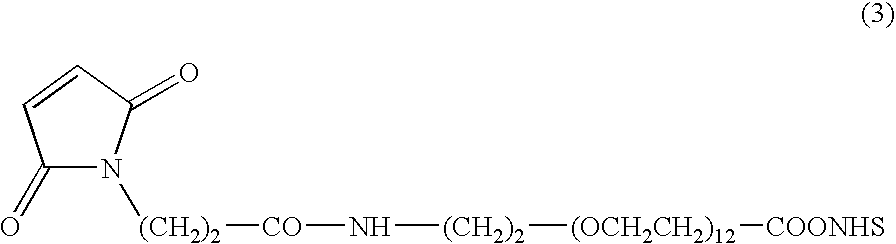Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
255results about How to "Receive treatment well" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
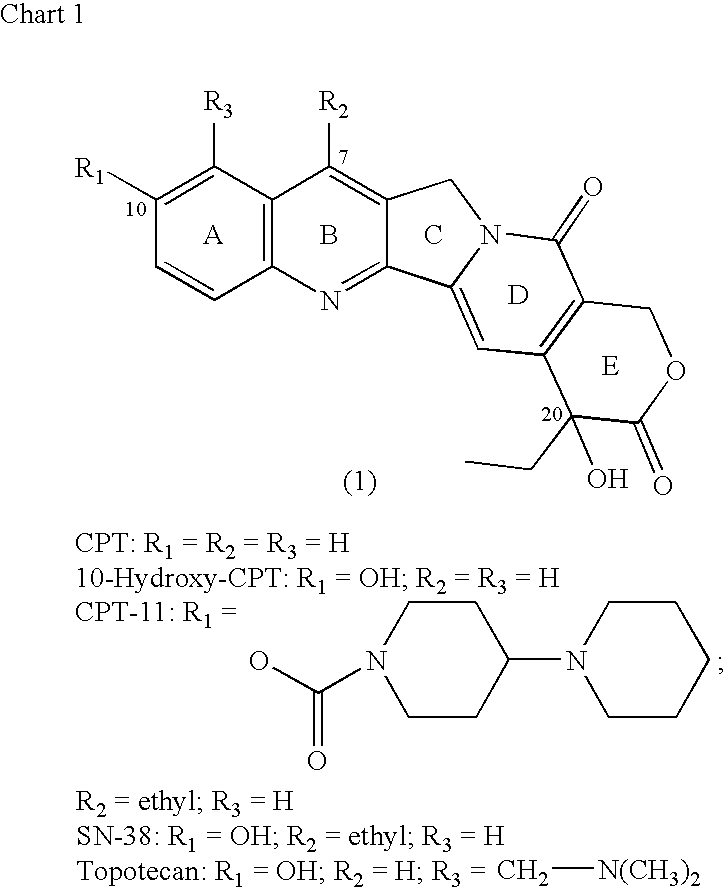
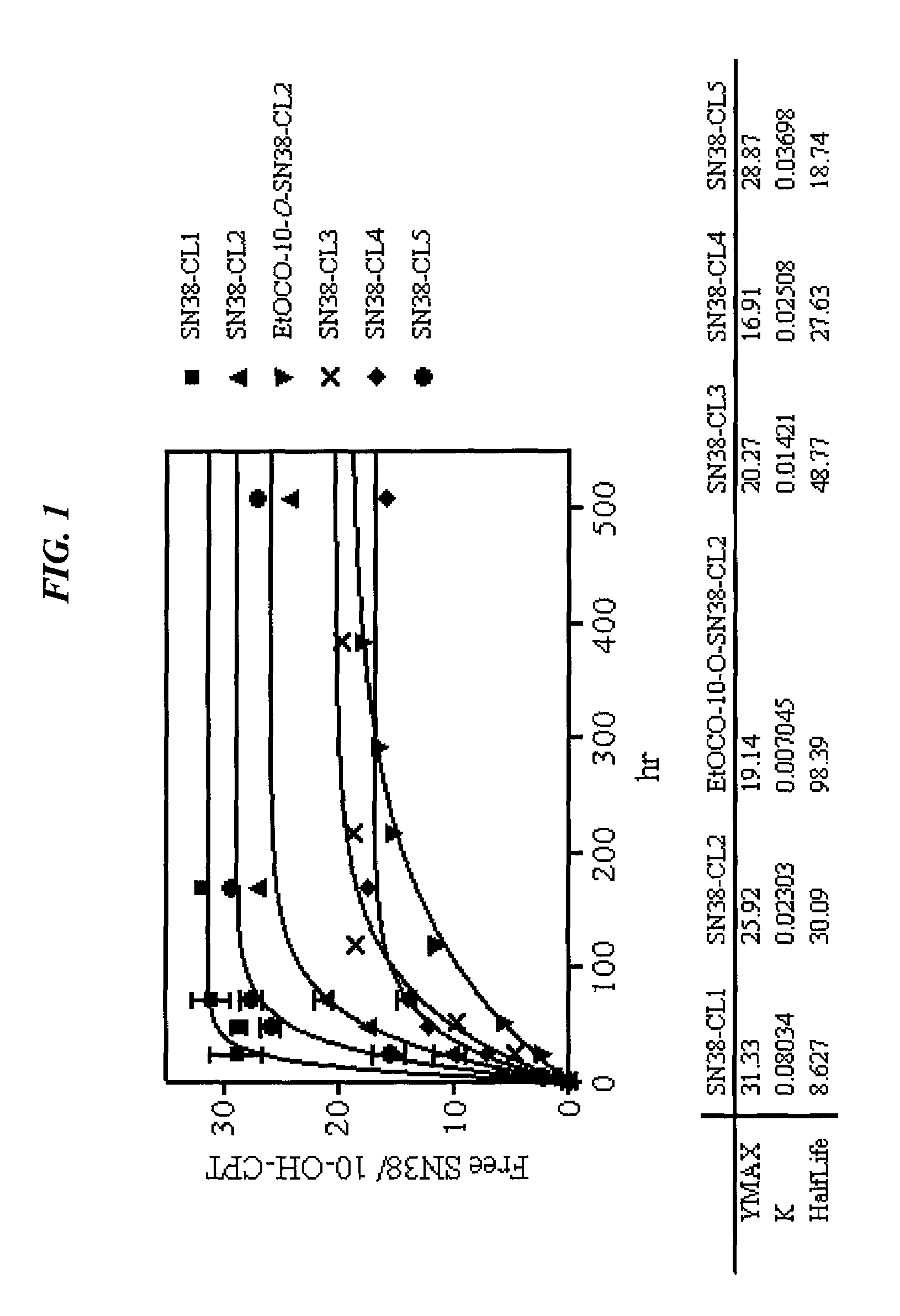
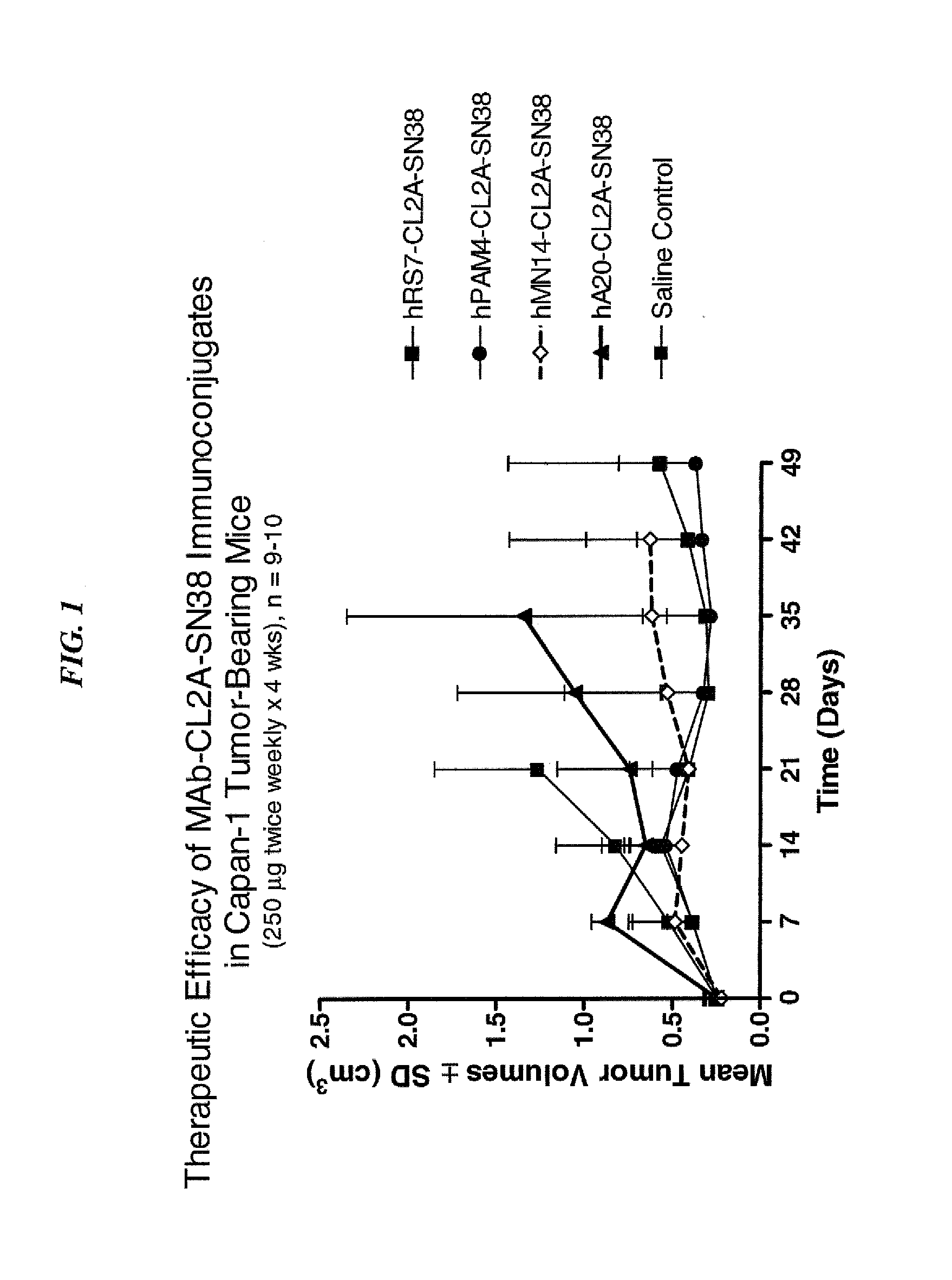
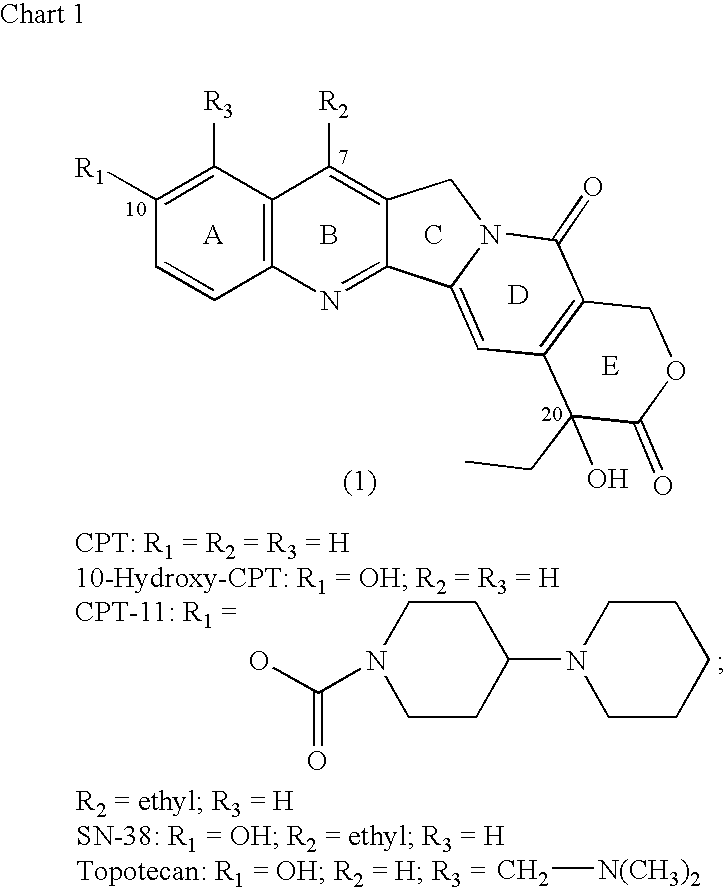
Camptothecin-binding moiety conjugates
ActiveUS20060193865A1High affinityReducing certain severe side effectsAntibacterial agentsNervous disorderAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
Immunoconjugates with an intracellularly-cleavable linkage
InactiveUS7999083B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsOrganic active ingredientsIntracellularAntibody fragments
Owner:IMMUNOMEDICS INC
Camptothecin-Binding Moiety Conjugates
ActiveUS20080166363A1Reducing certain severe side effectsReceive treatment wellAntibacterial agentsAntimycoticsAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
Immunoconjugates with an Intracellularly-Cleavable Linkage
ActiveUS20100104589A1Reducing certain severe side effectsReceive treatment wellAntibacterial agentsOrganic active ingredientsAntibody fragmentsChemistry
Owner:IMMUNOMEDICS INC
Camptothecin-binding moiety conjugates
ActiveUS7591994B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsAntimycoticsAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
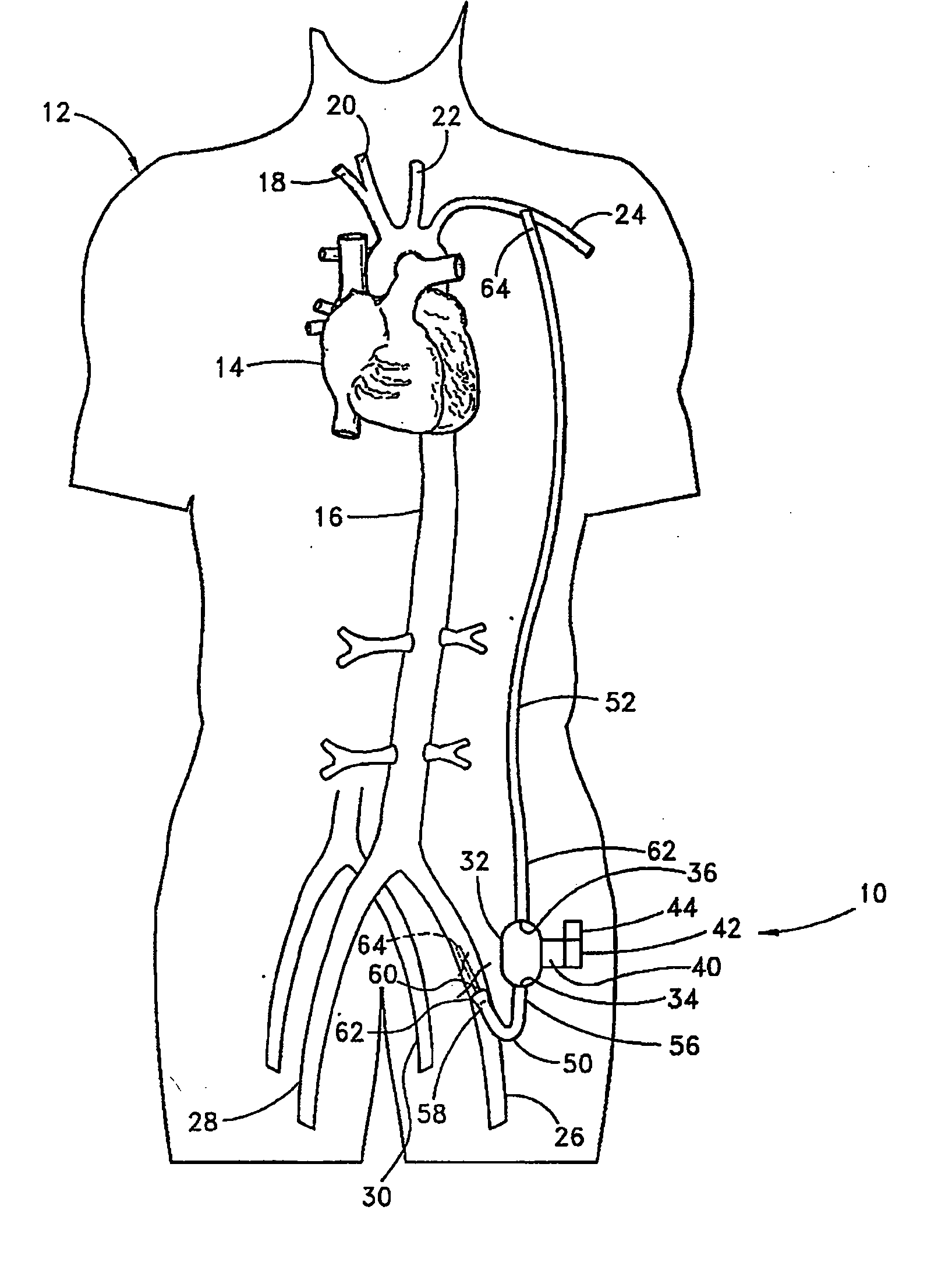
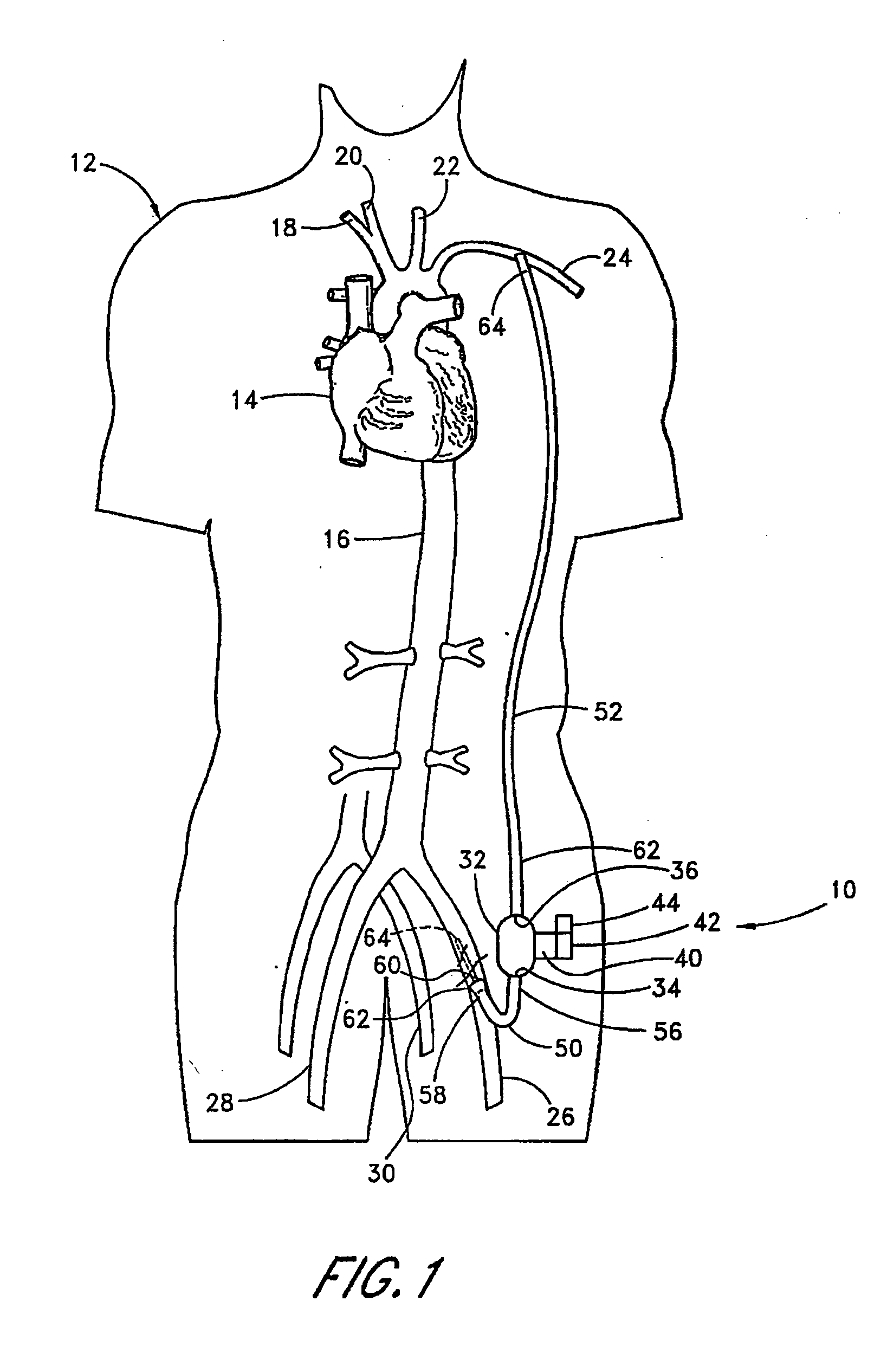
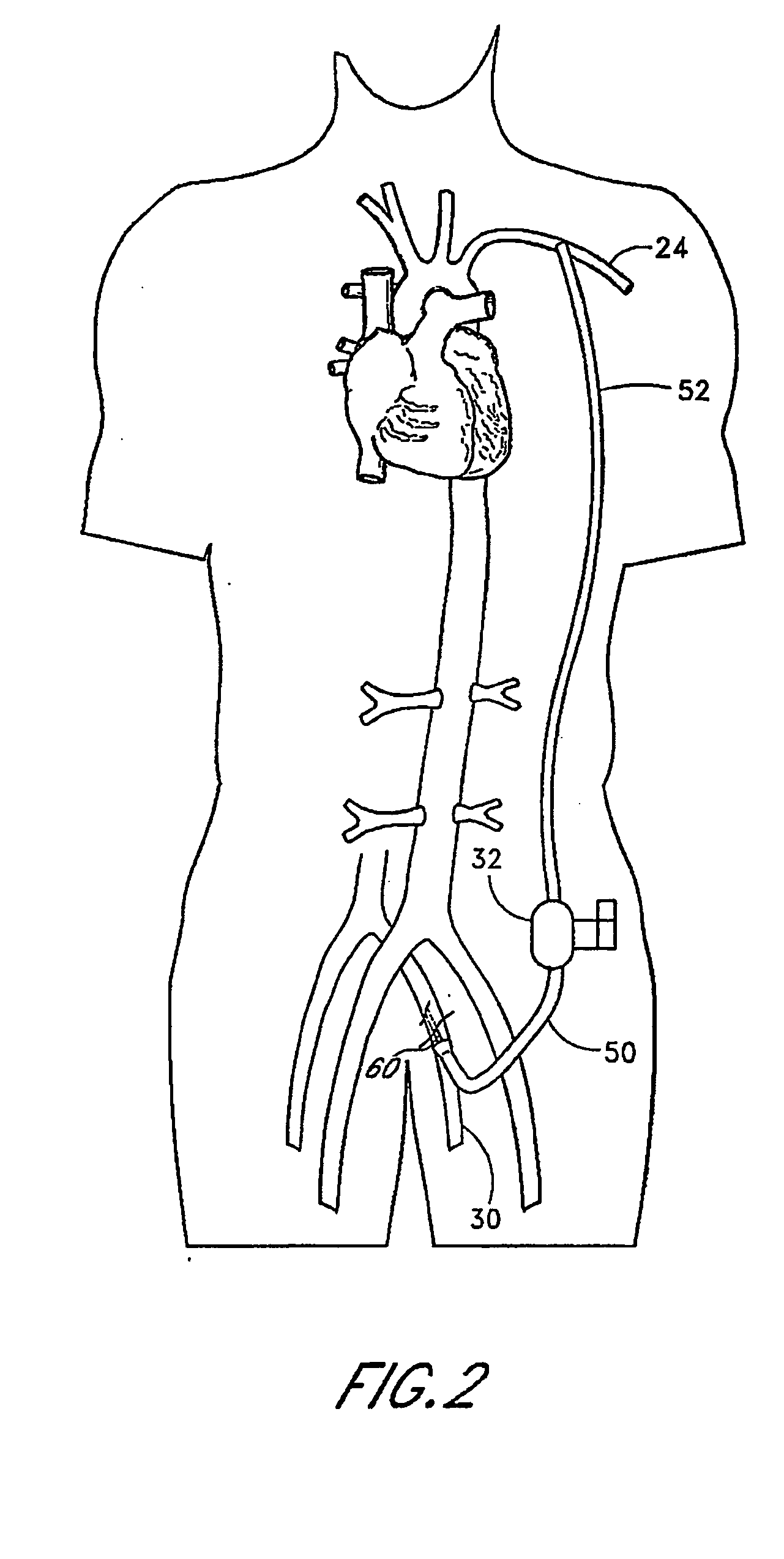
Implantable heart assist system and method of applying same
InactiveUS20050085683A1Reduce usageReceive treatment wellControl devicesBlood pumpsVia renal arteryMinimally invasive procedures
An intravascular extracardiac pumping system for increasing perfusion through a renal artery to tissues of a patient without any component thereof being connected to the patient's heart is provided. The system includes means for pumping blood and a portion that houses the pumping means. The portion that houses the pumping means is configured to direct blood from a location upstream of the pumping means to a location within a renal artery. The pumping means and the portion that houses the pumping means are configured to be insertable into a non-primary vessel subcutaneously in an minimally-invasive procedure for positioning within the patient's vasculature.
Owner:TC1 LLC
Delivery device for localized delivery of a therapeutic agent
InactiveUS20110137155A1Receive treatment wellImprove usabilityStentsBalloon catheterLesionBlood vessel
Therapeutic agent delivery devices and methods for delivering a therapeutic agent to a target location as well as methods for determining the location of a lesion on a vessel wall are disclosed. Various embodiments disclose an expandable member comprising a drug delivery matrix for selectively delivering a therapeutic agent to a lesion on a vessel wall. The drug delivery matrix may comprise one or more sensors and an electroactive polymer for releasing the therapeutic agent. Other embodiments disclose an expandable member comprising a plurality of radially-expanding flexible walls forming a plurality of channels for selectively delivering therapeutic agent to a target area adjacent one or more of the channels. Detecting a lesion may comprise using a plurality of Hall effect sensors disposed on a distal end of a catheter.
Owner:BOSTON SCI SCIMED INC
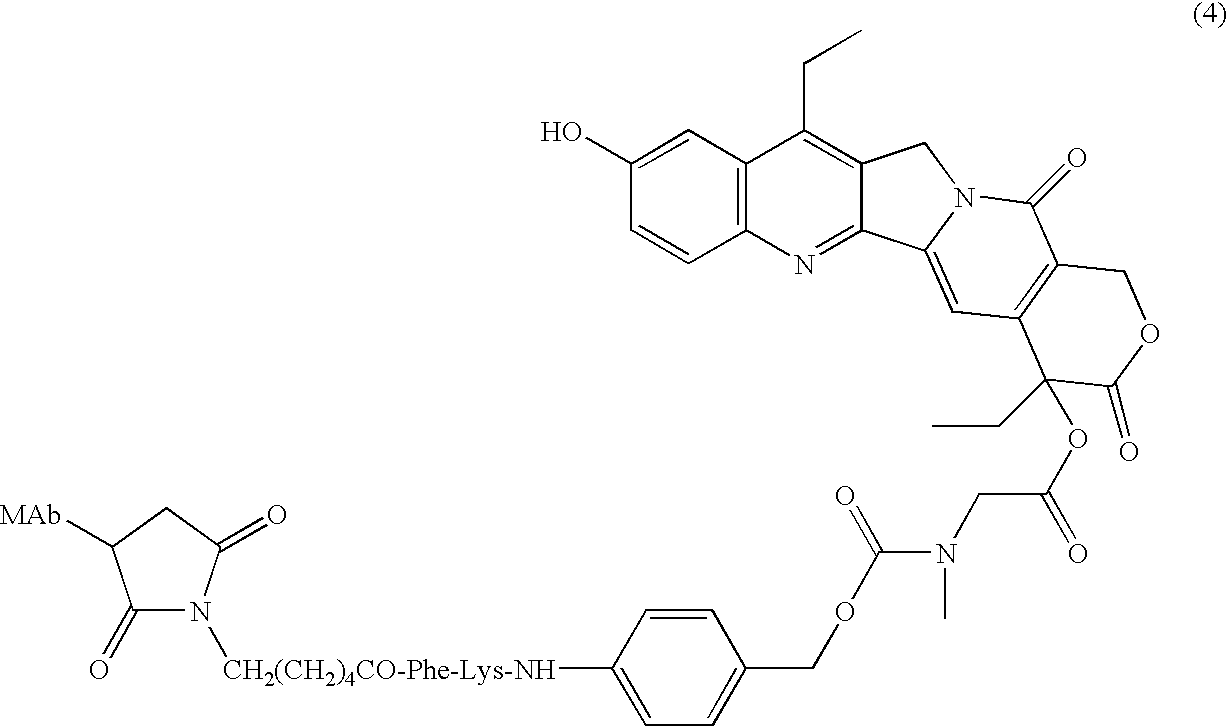
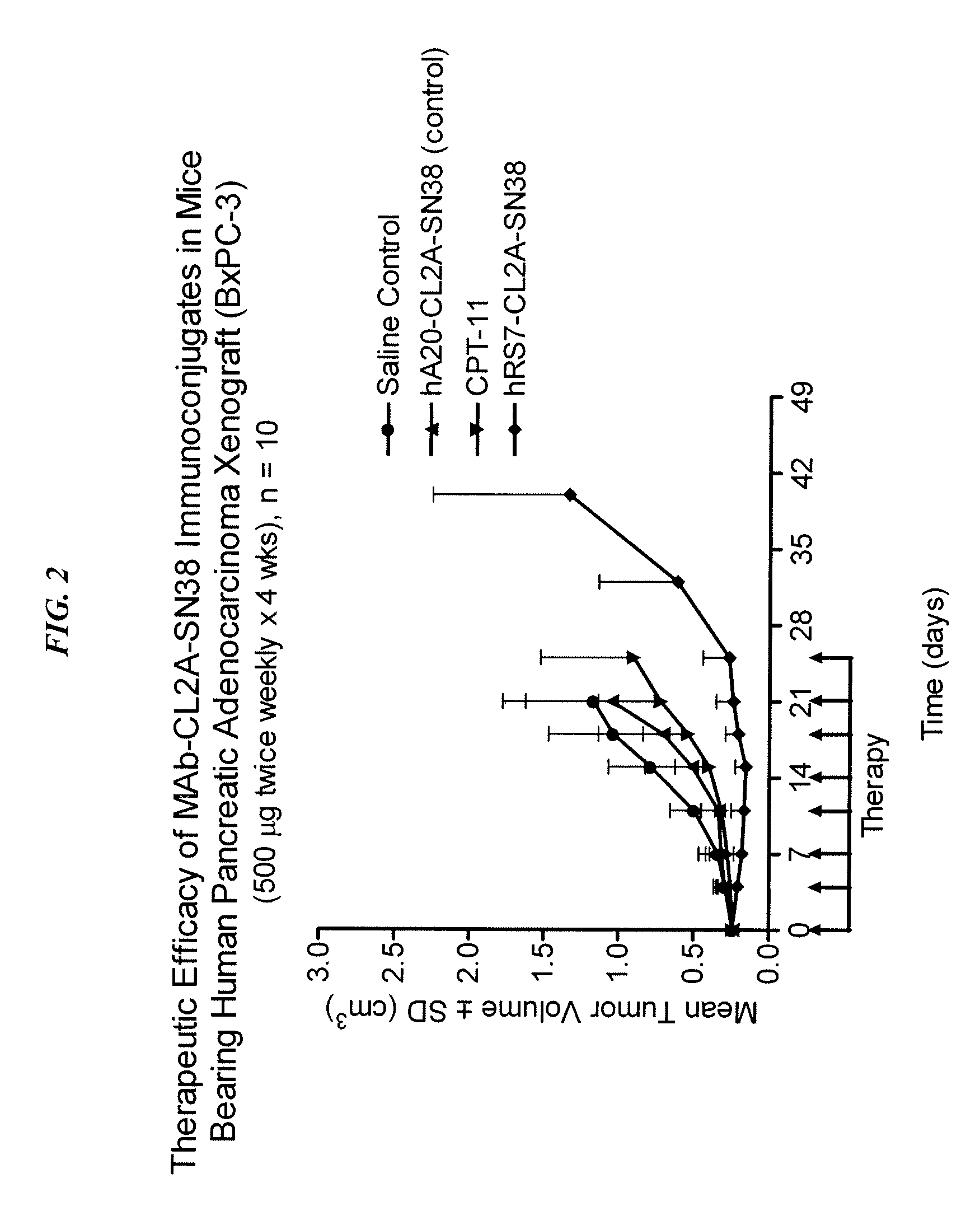
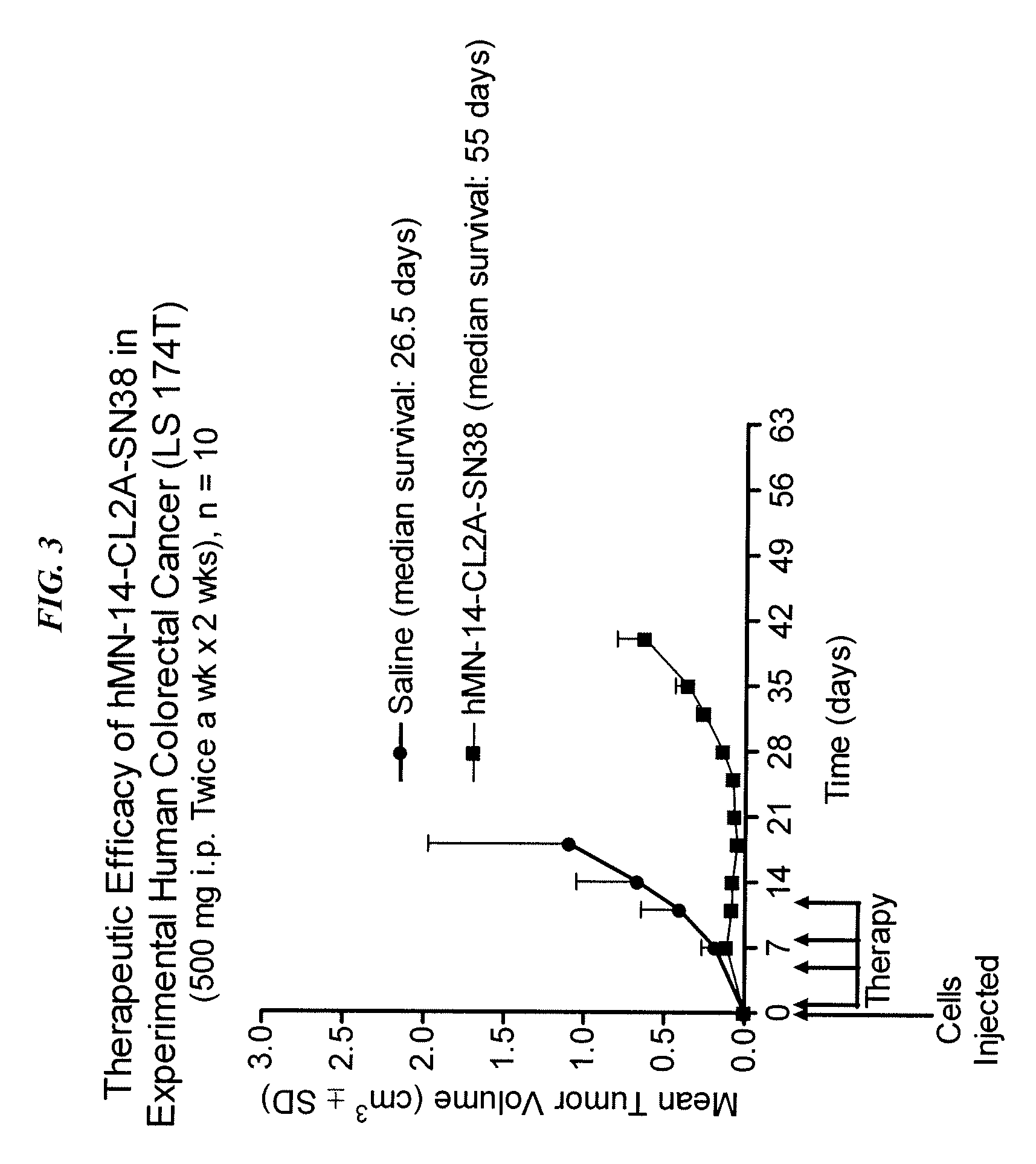
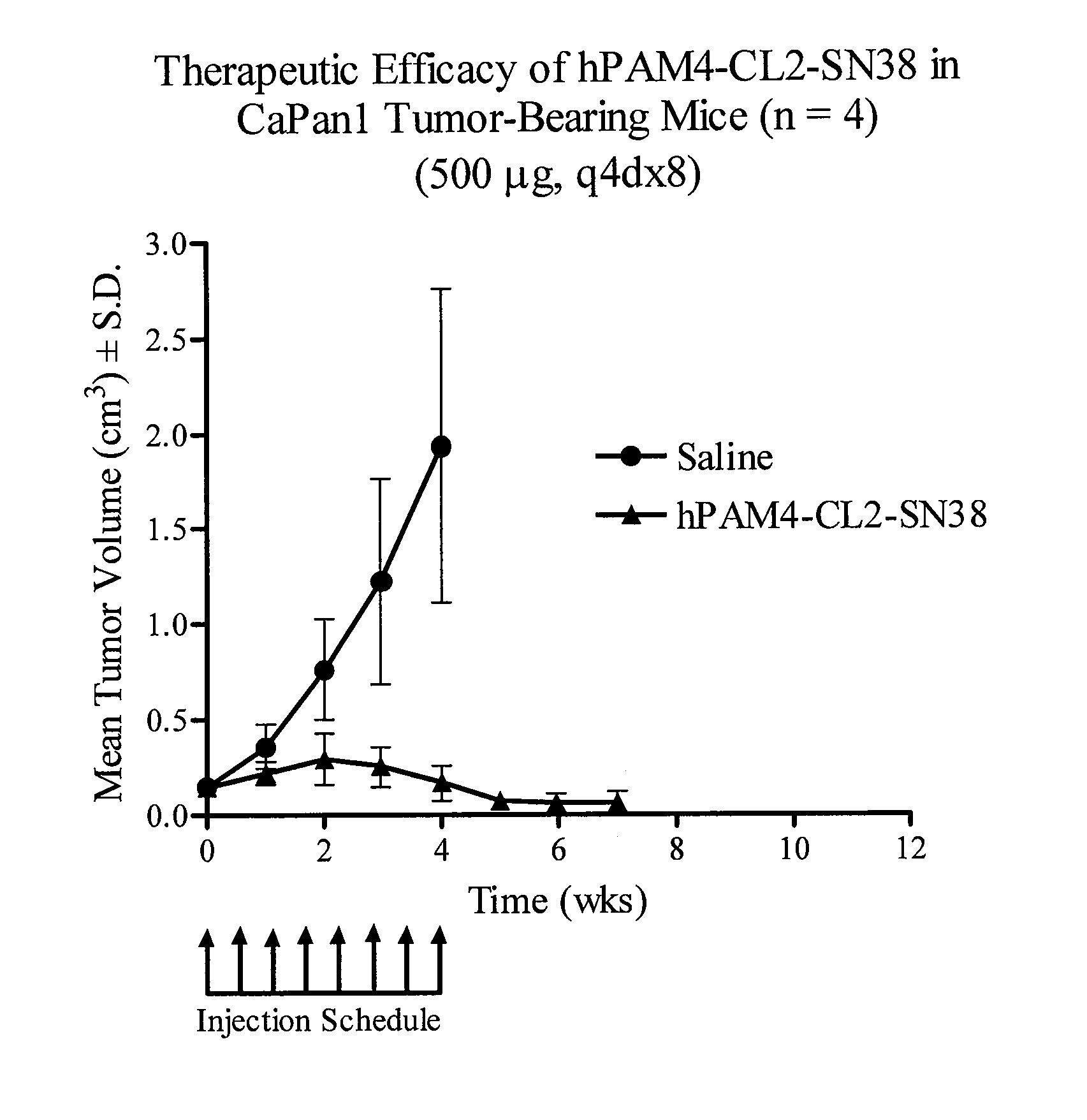
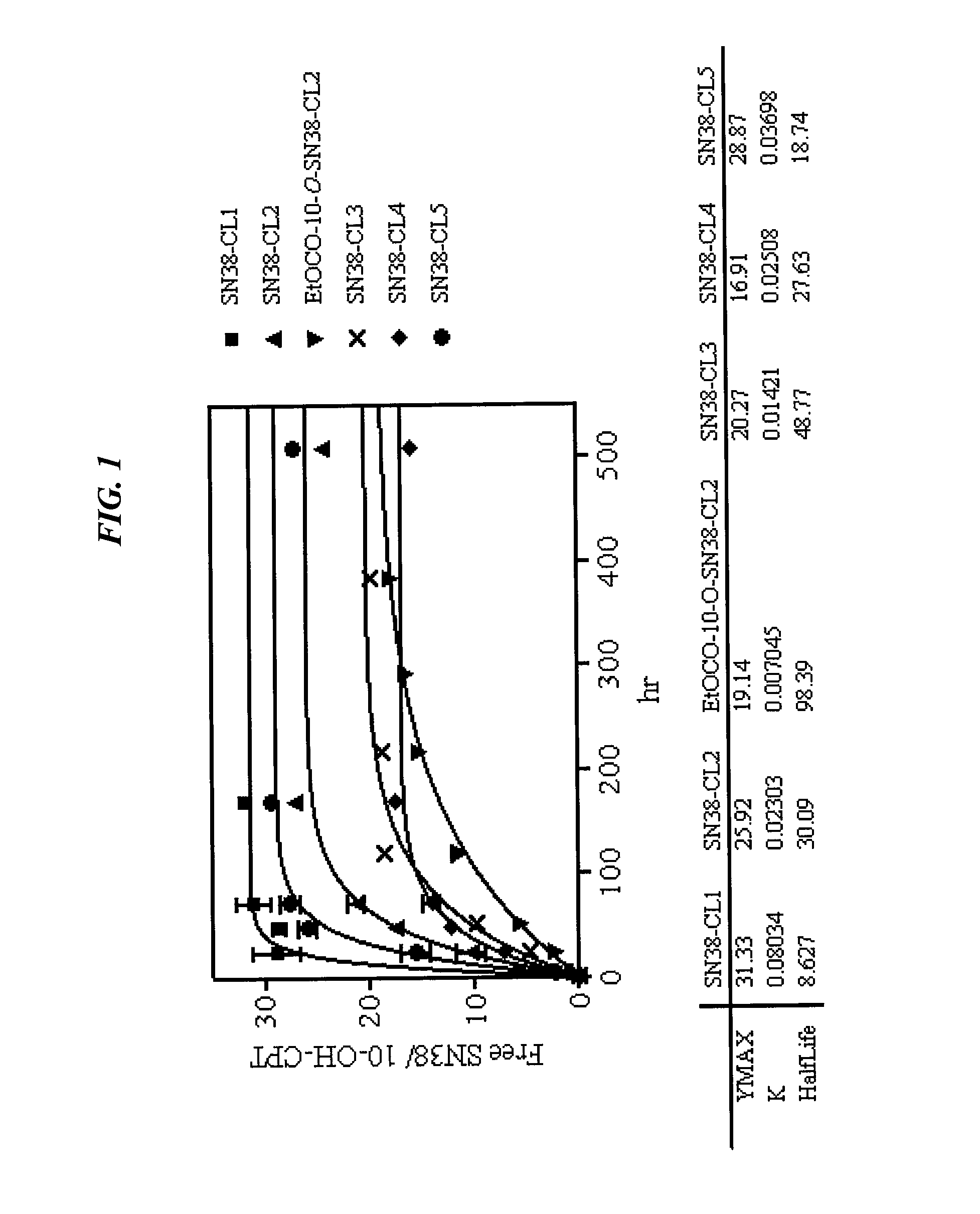
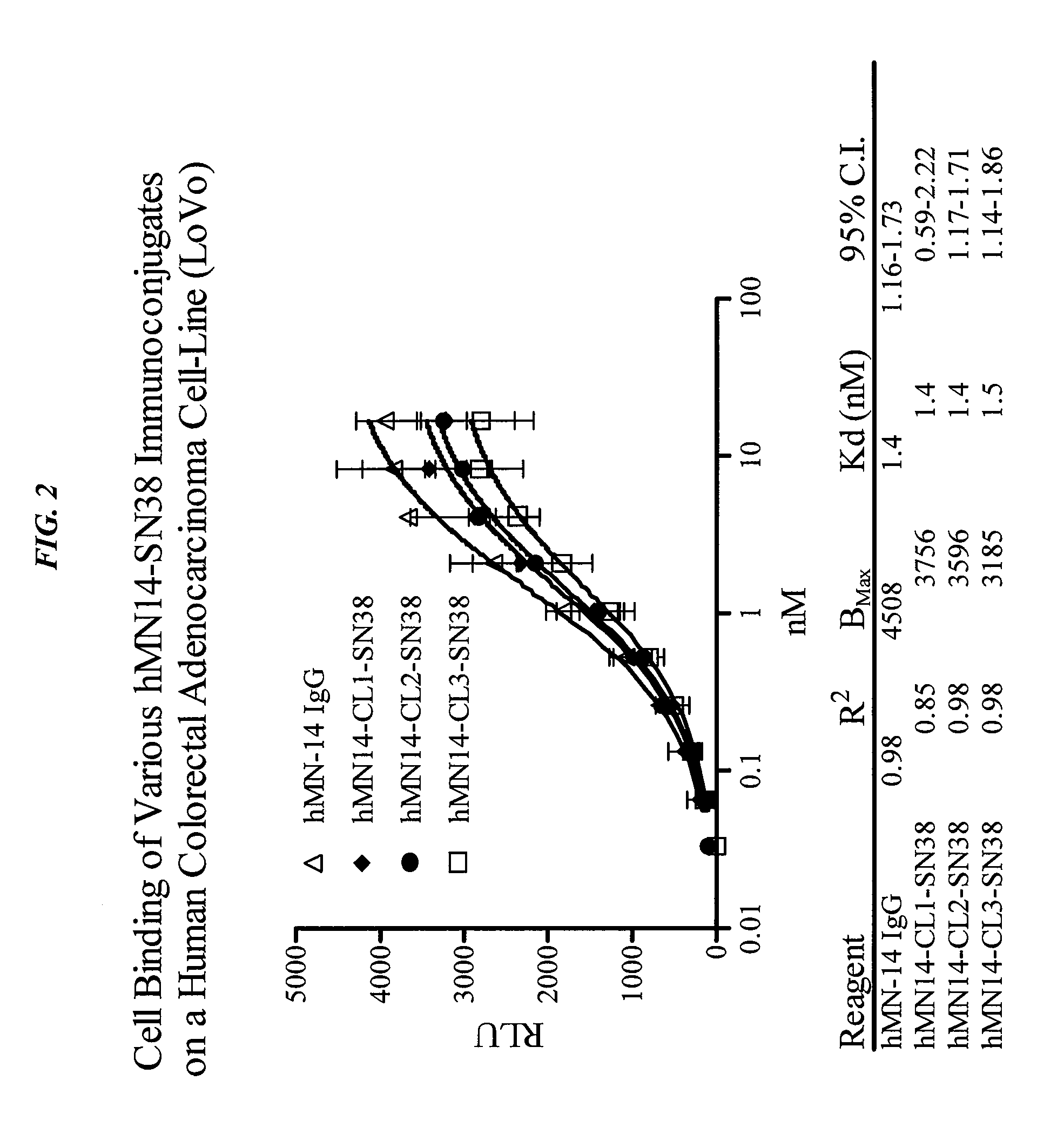
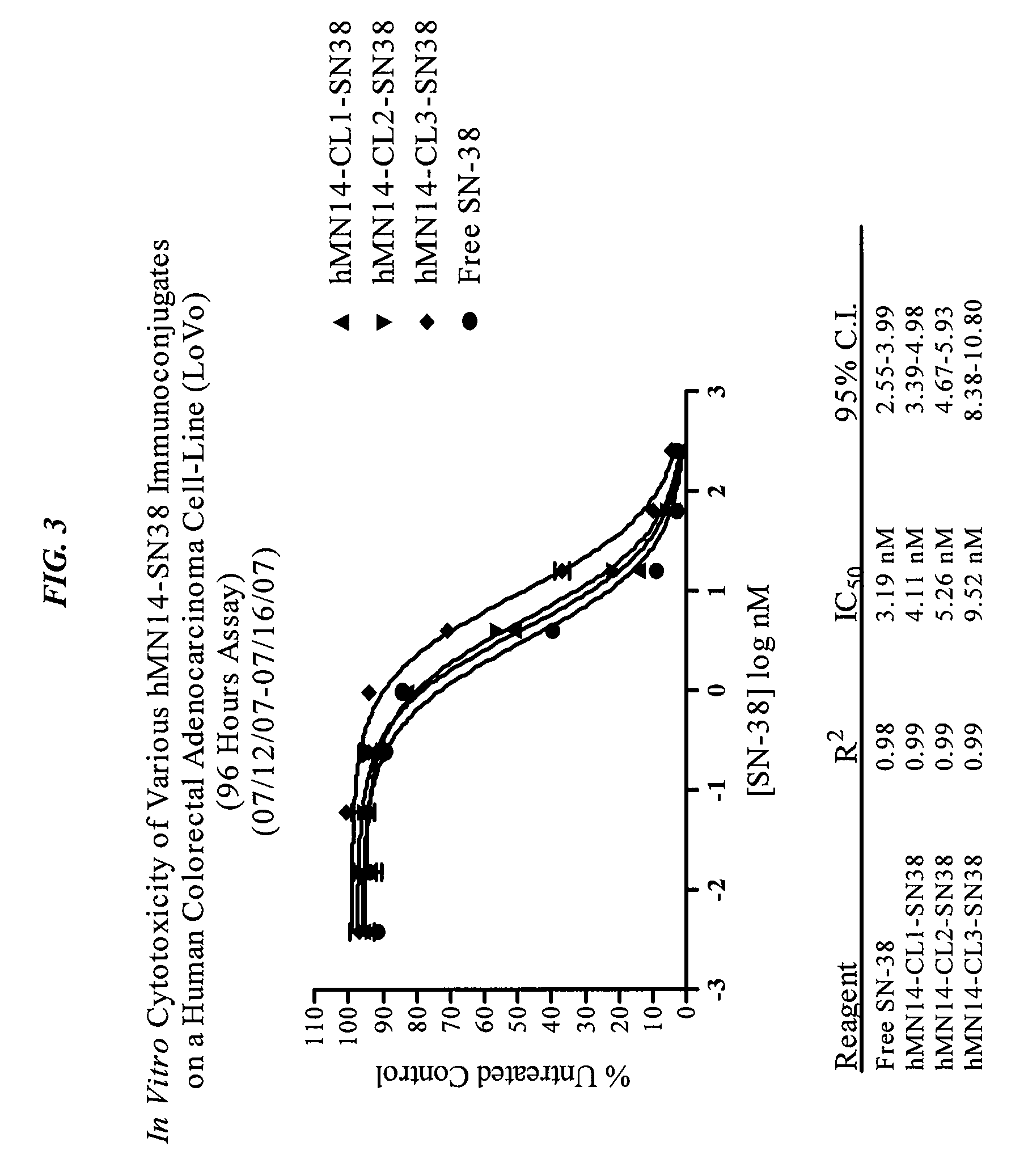
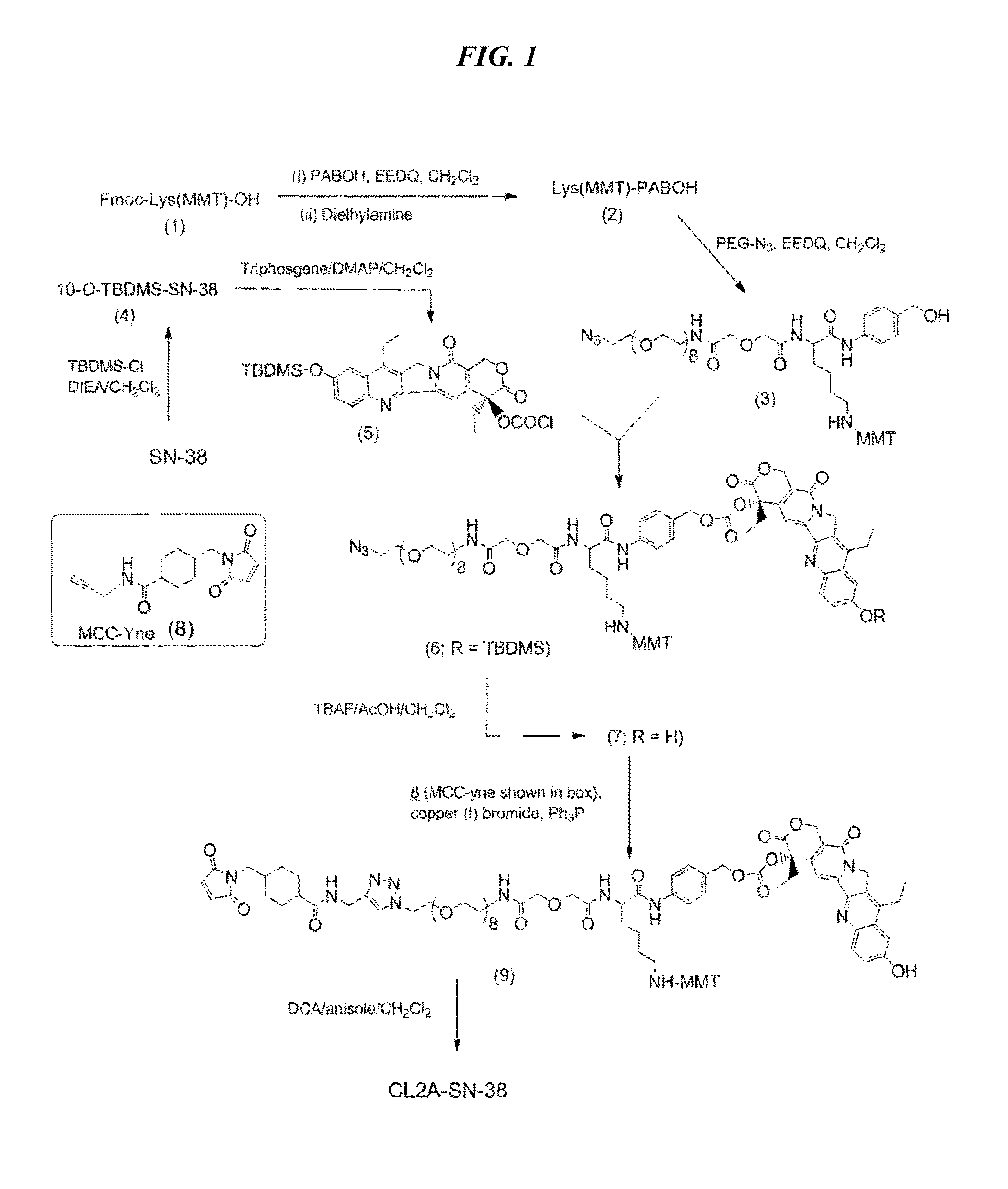
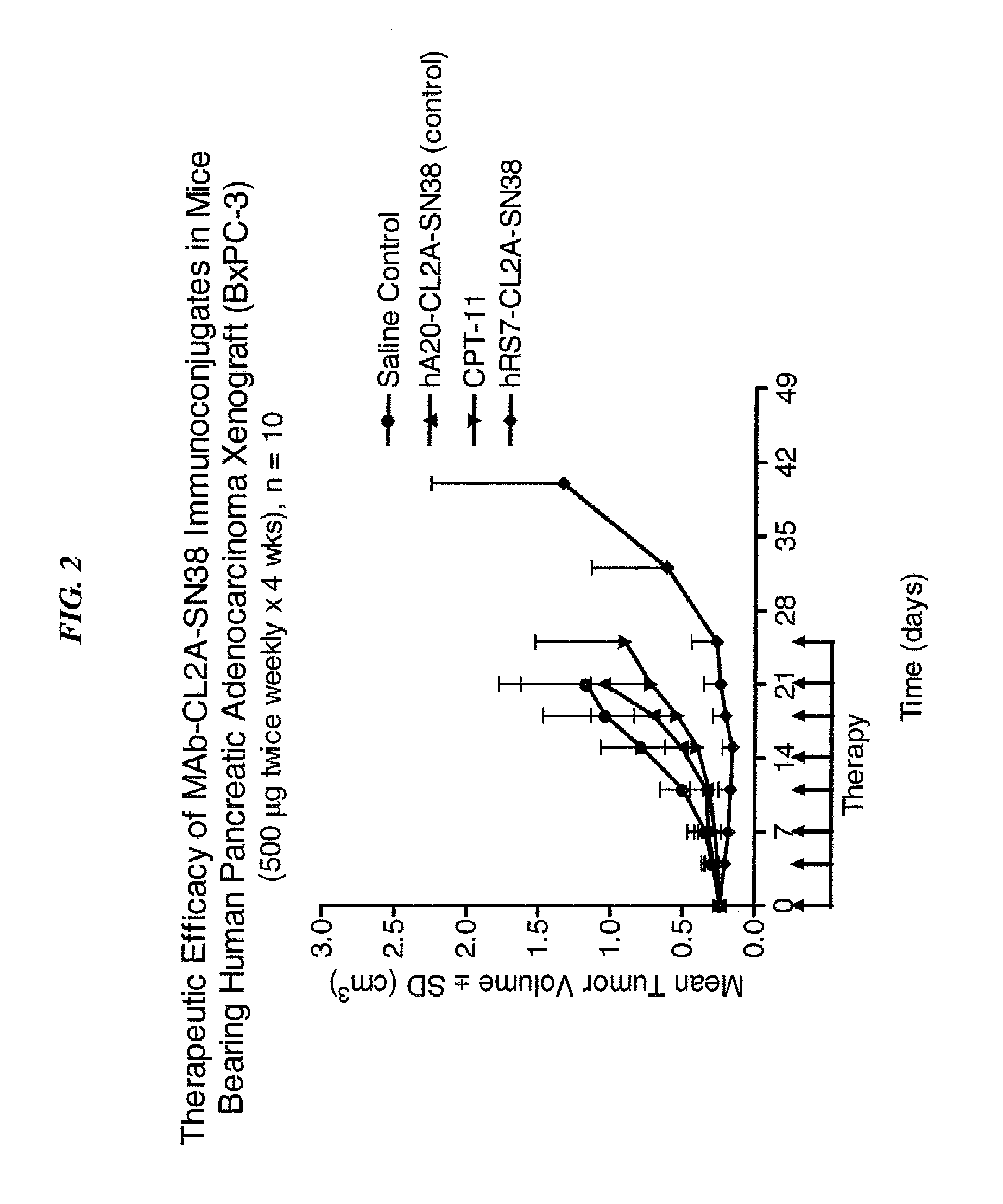
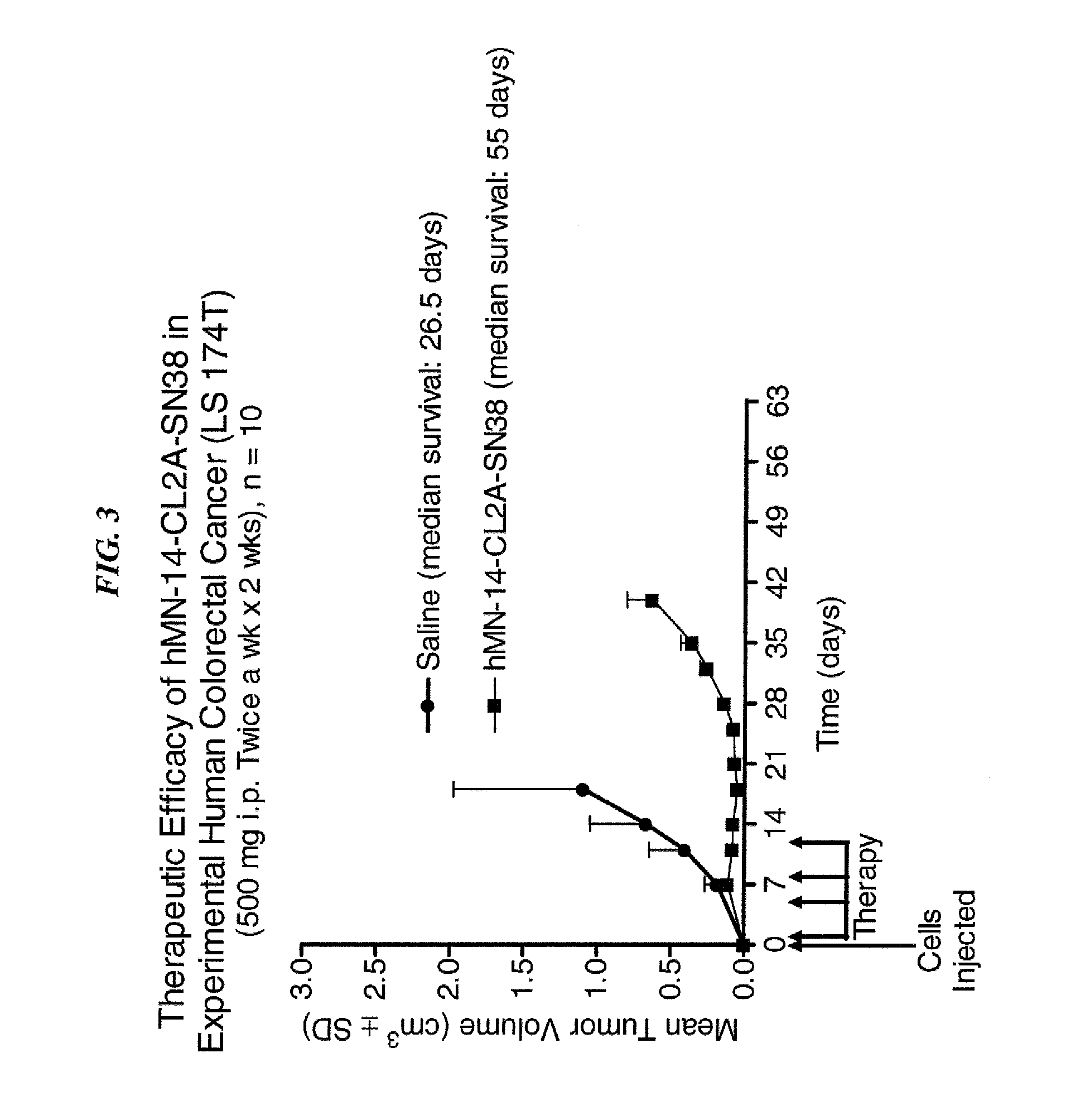
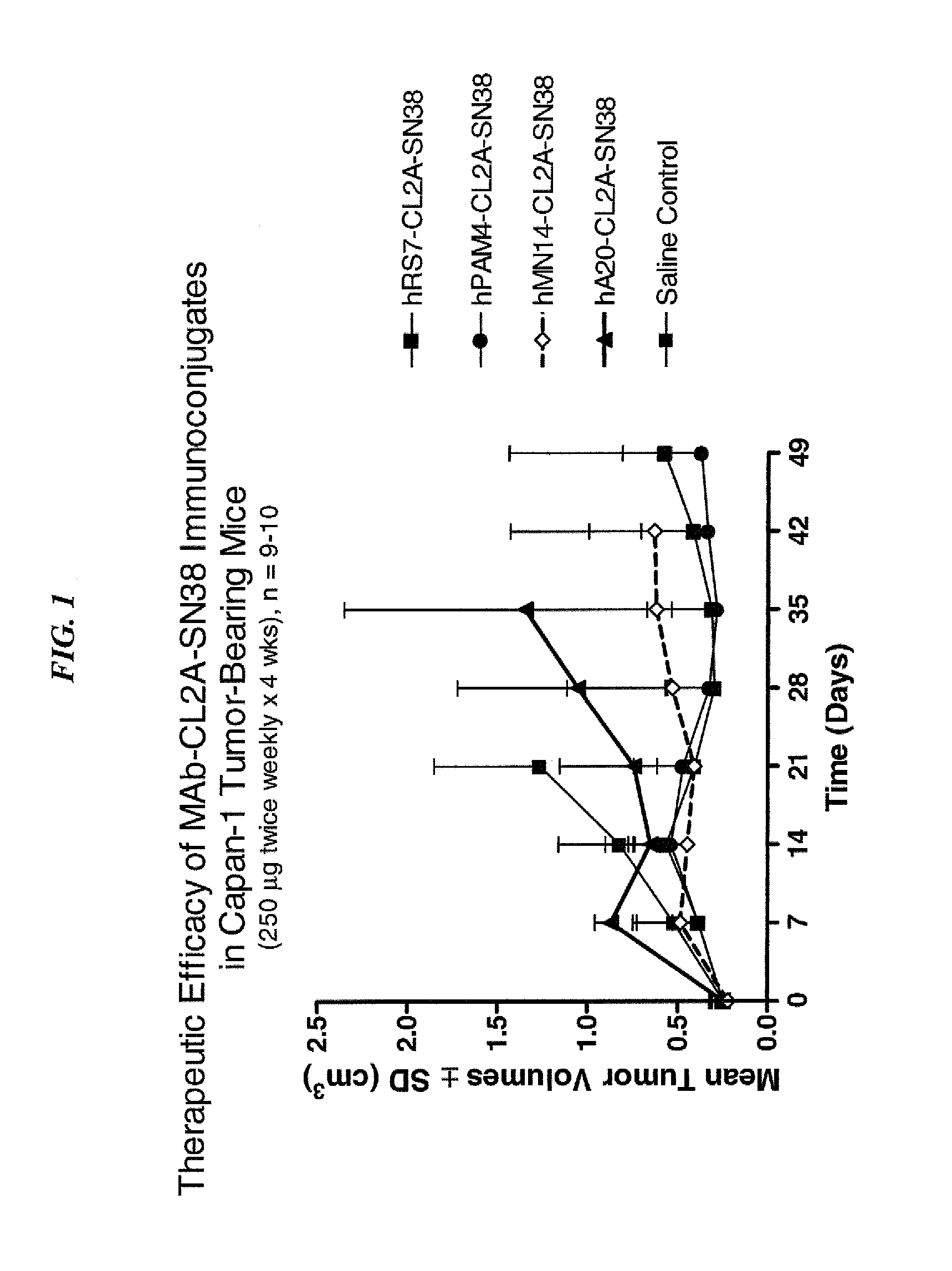
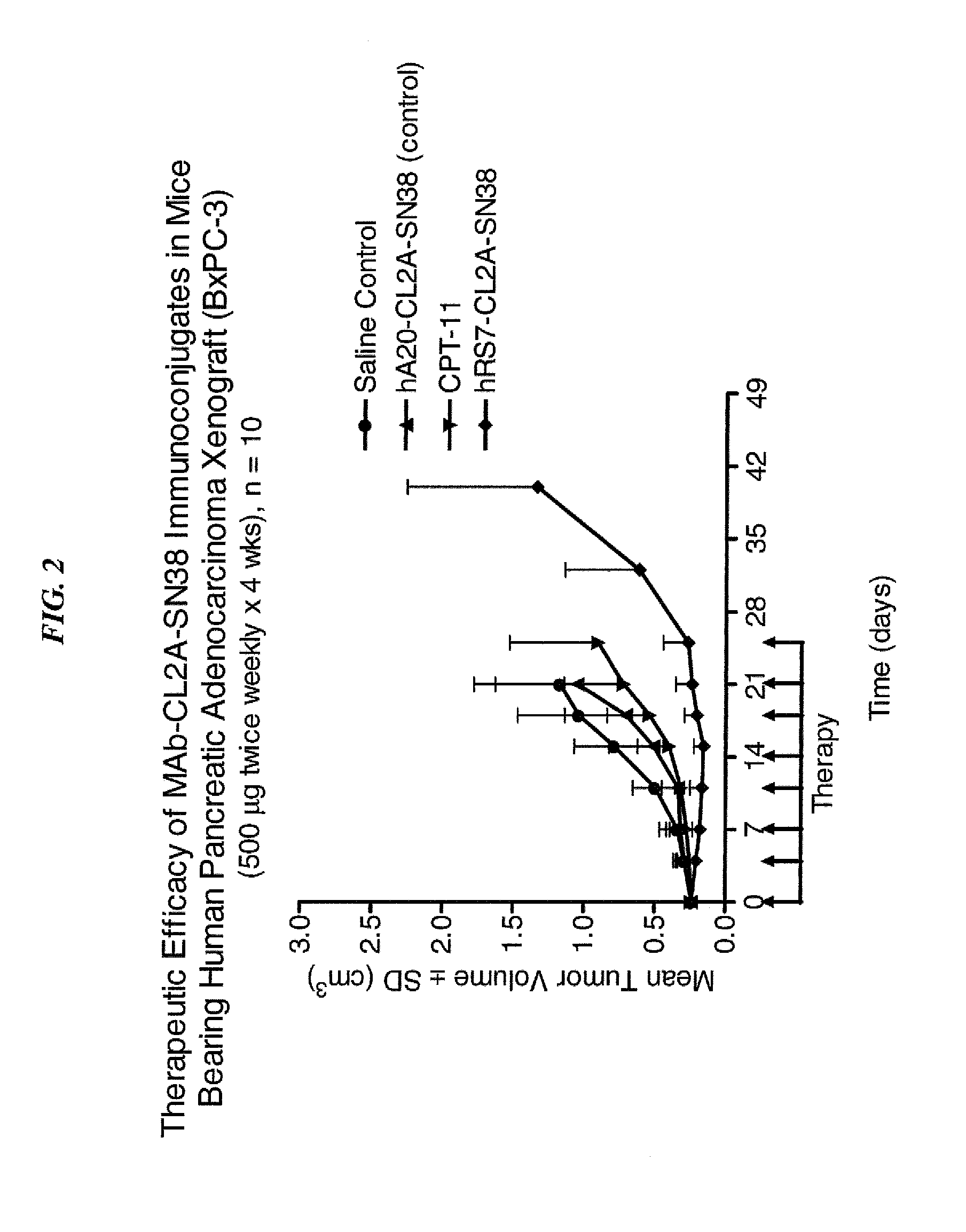
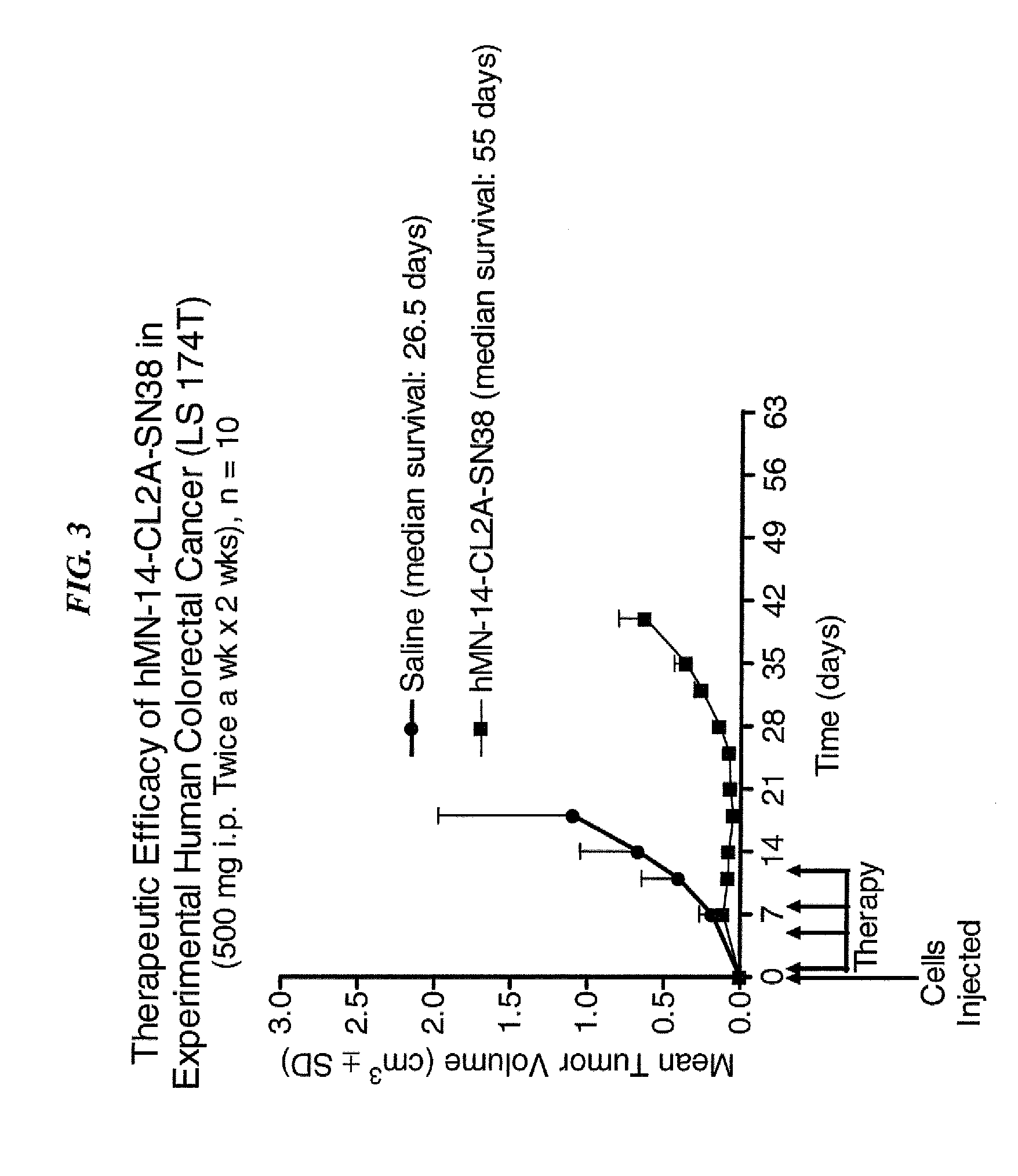
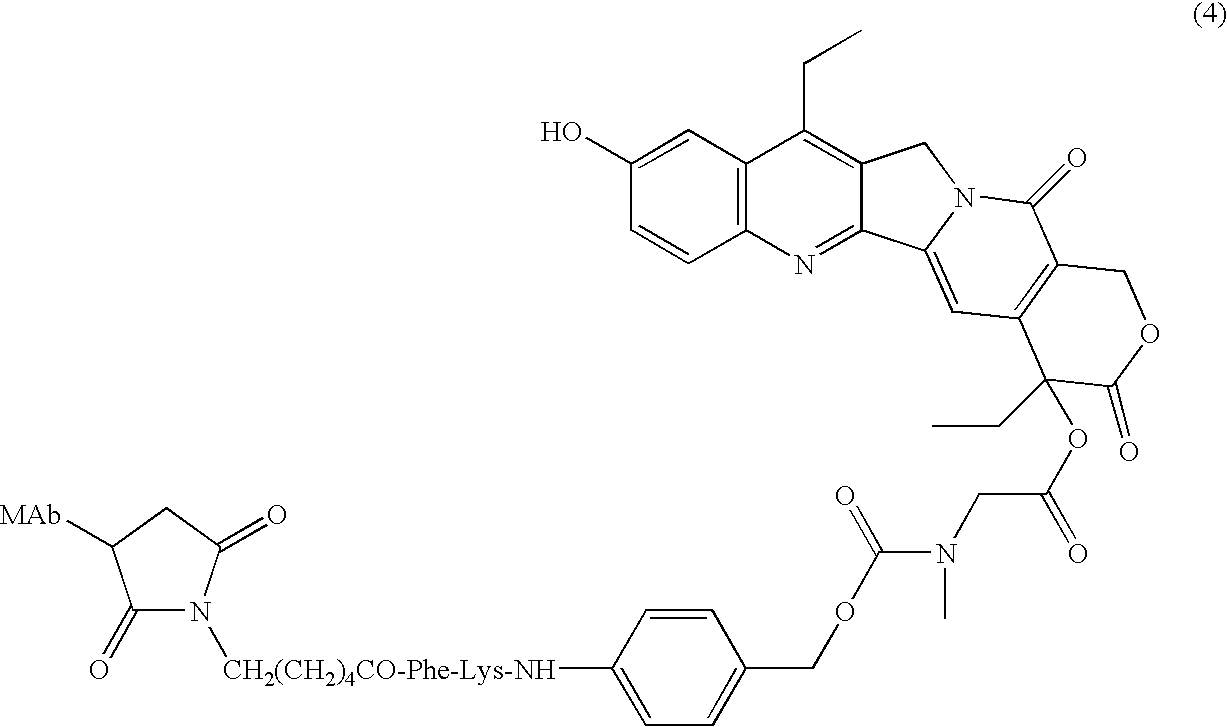
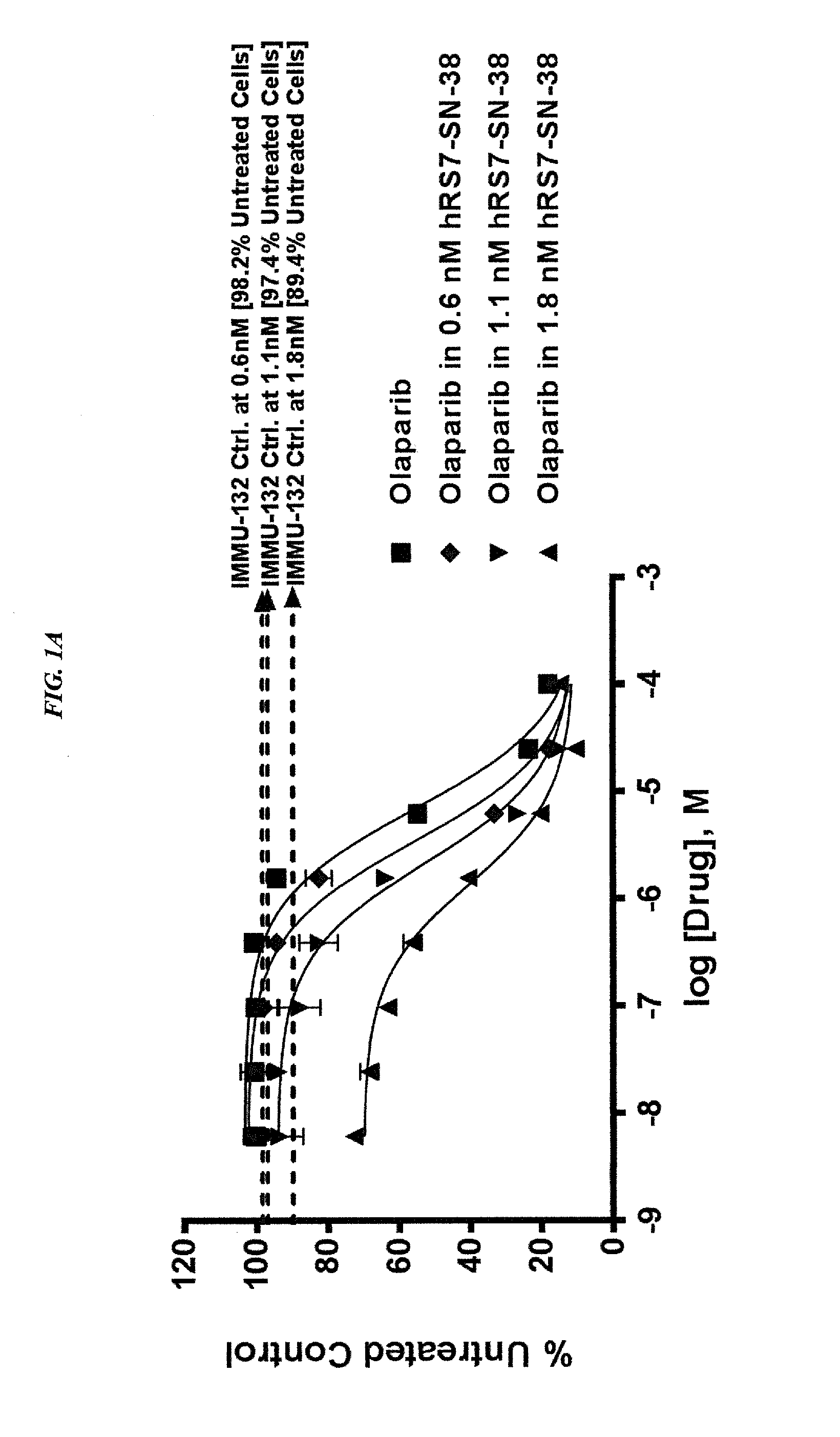
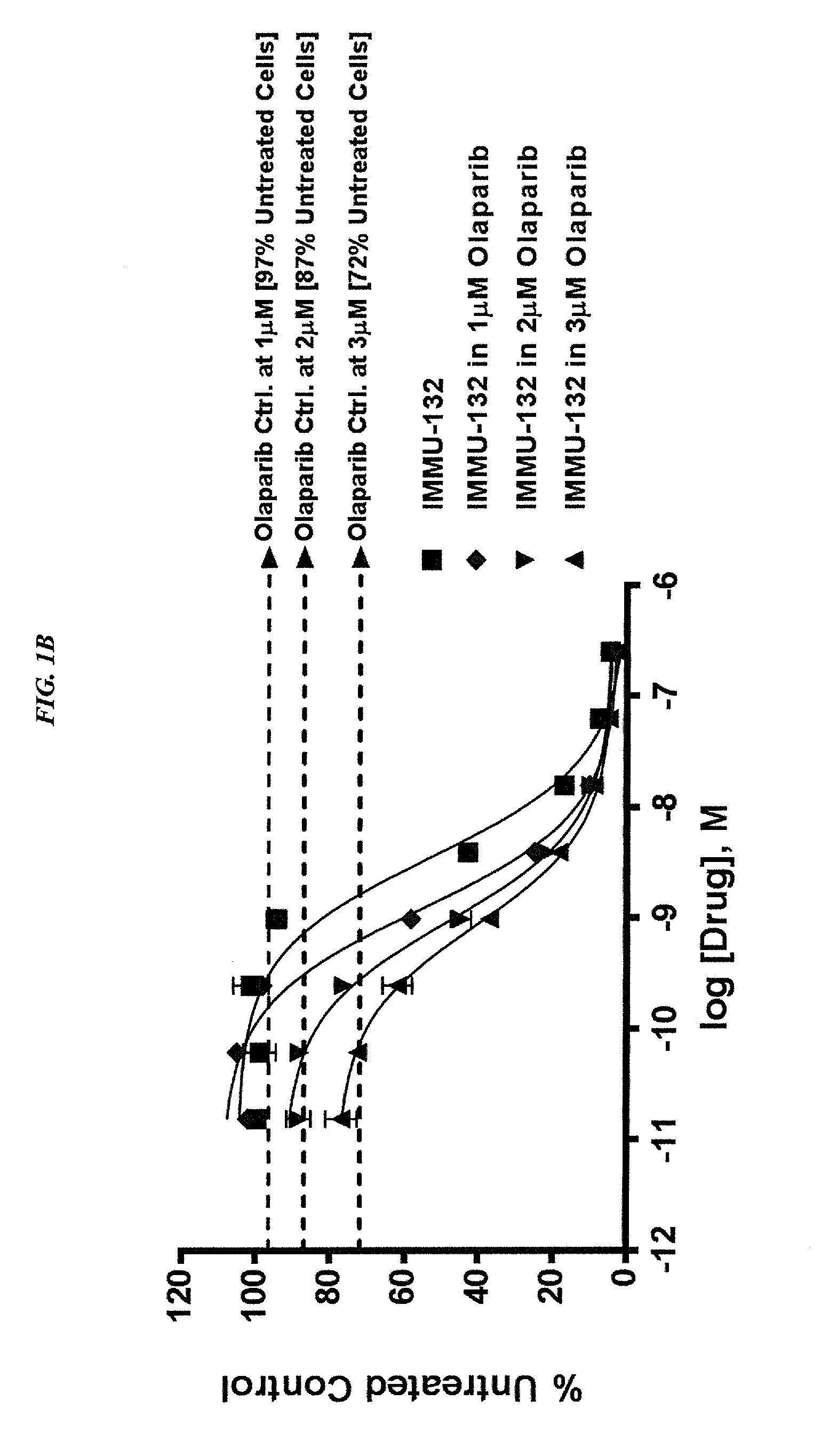
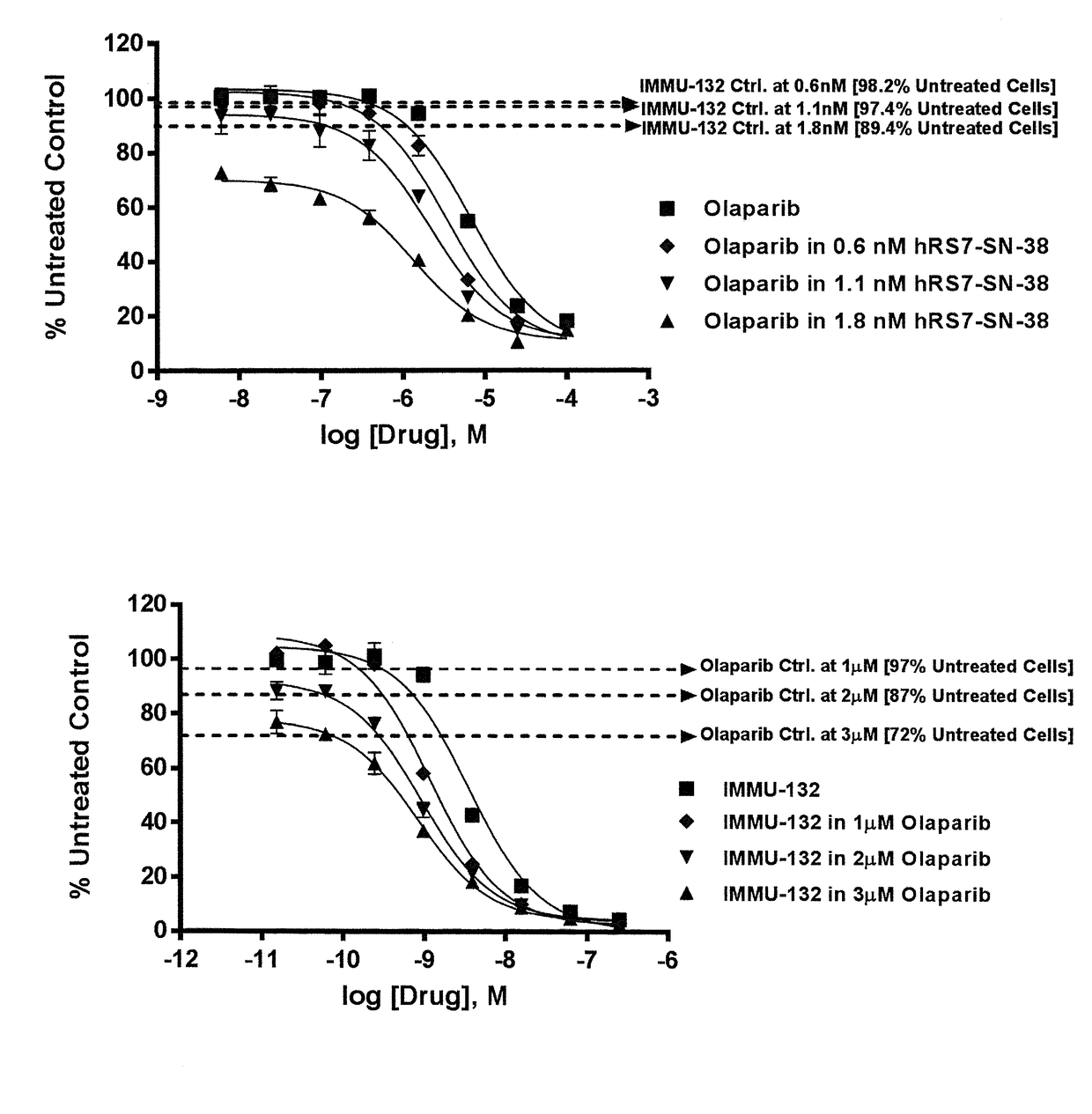
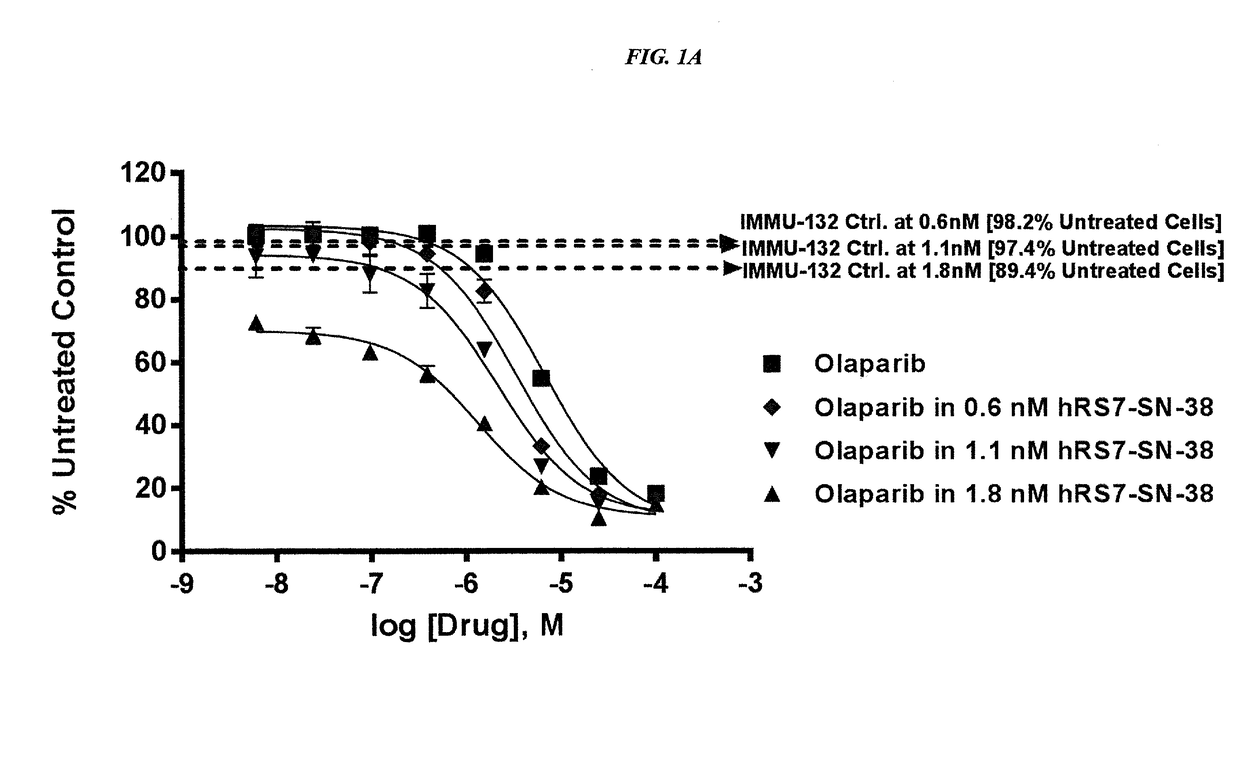
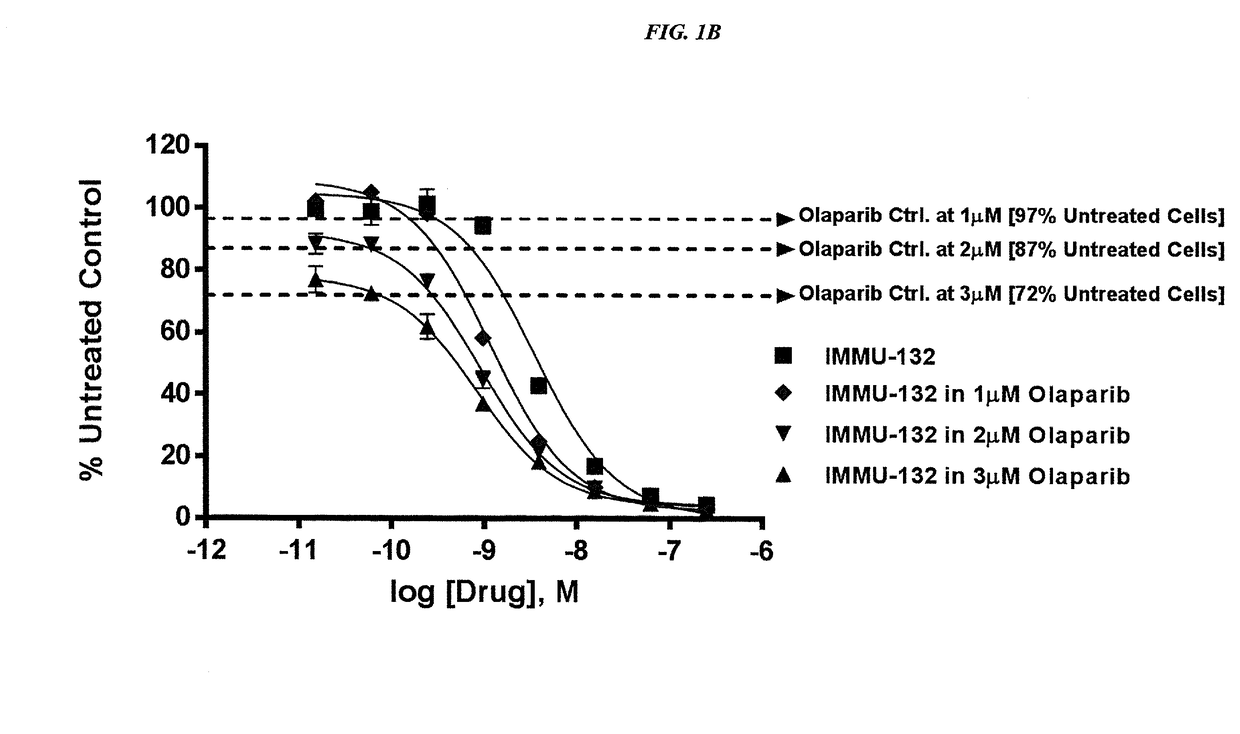
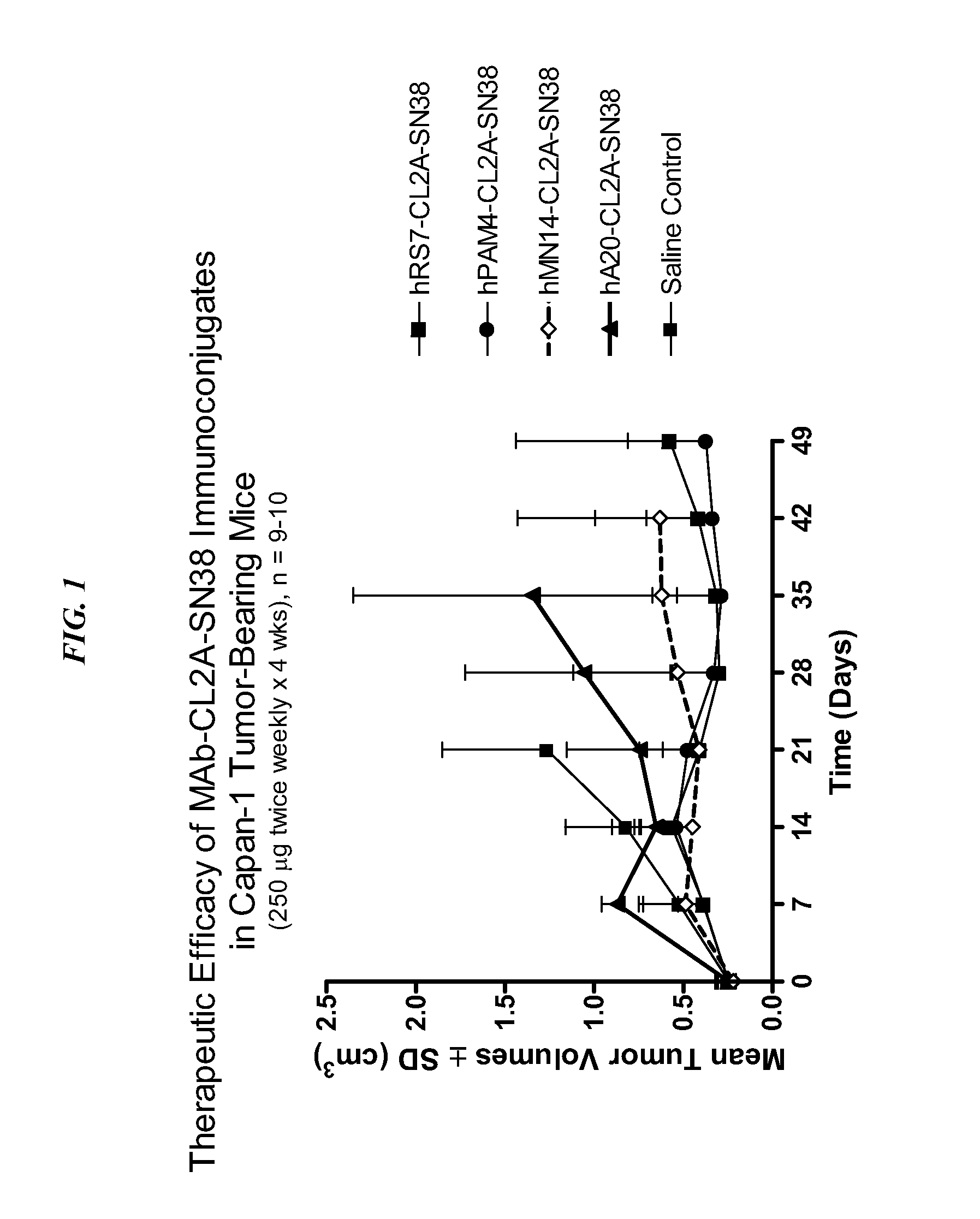
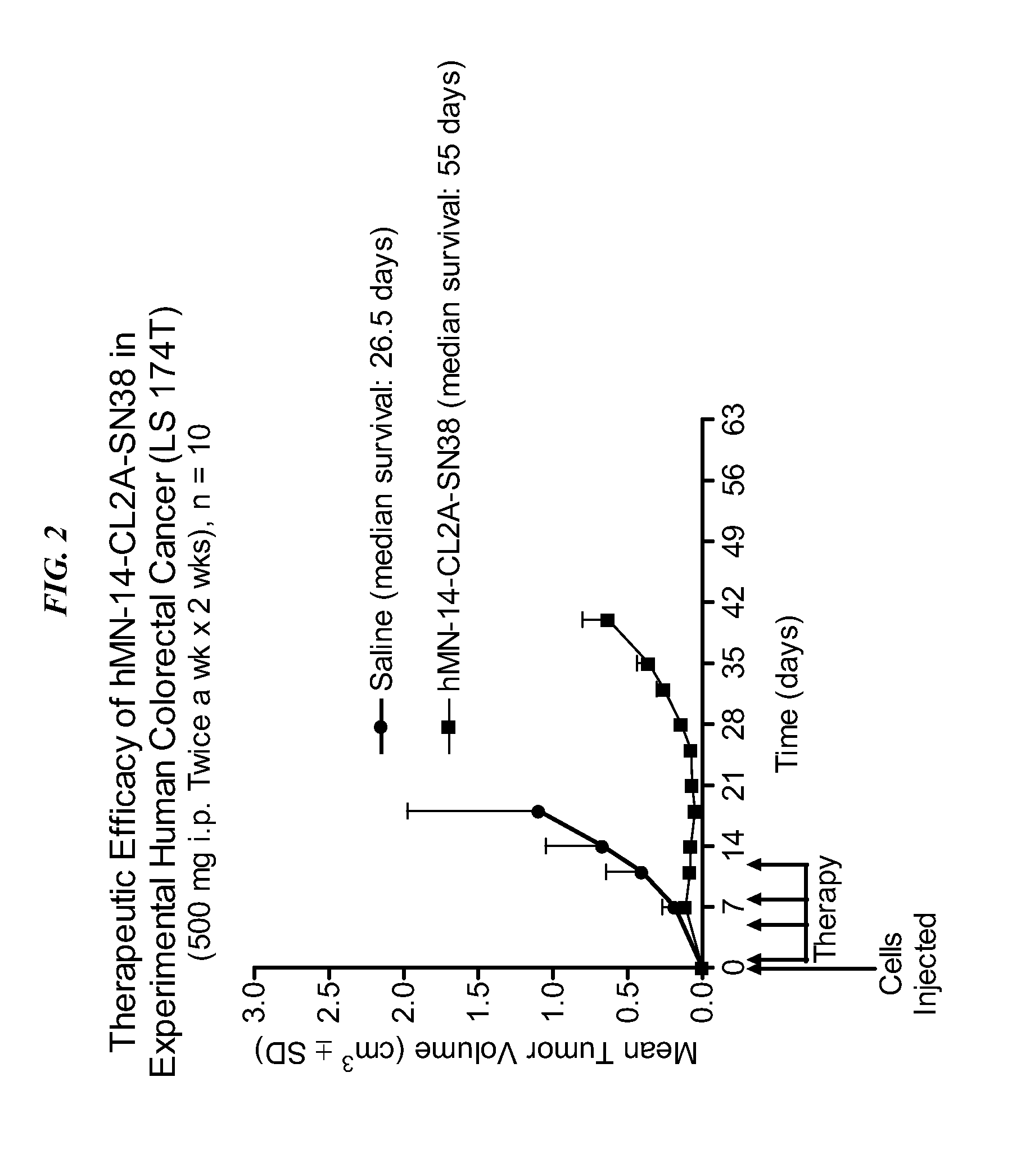
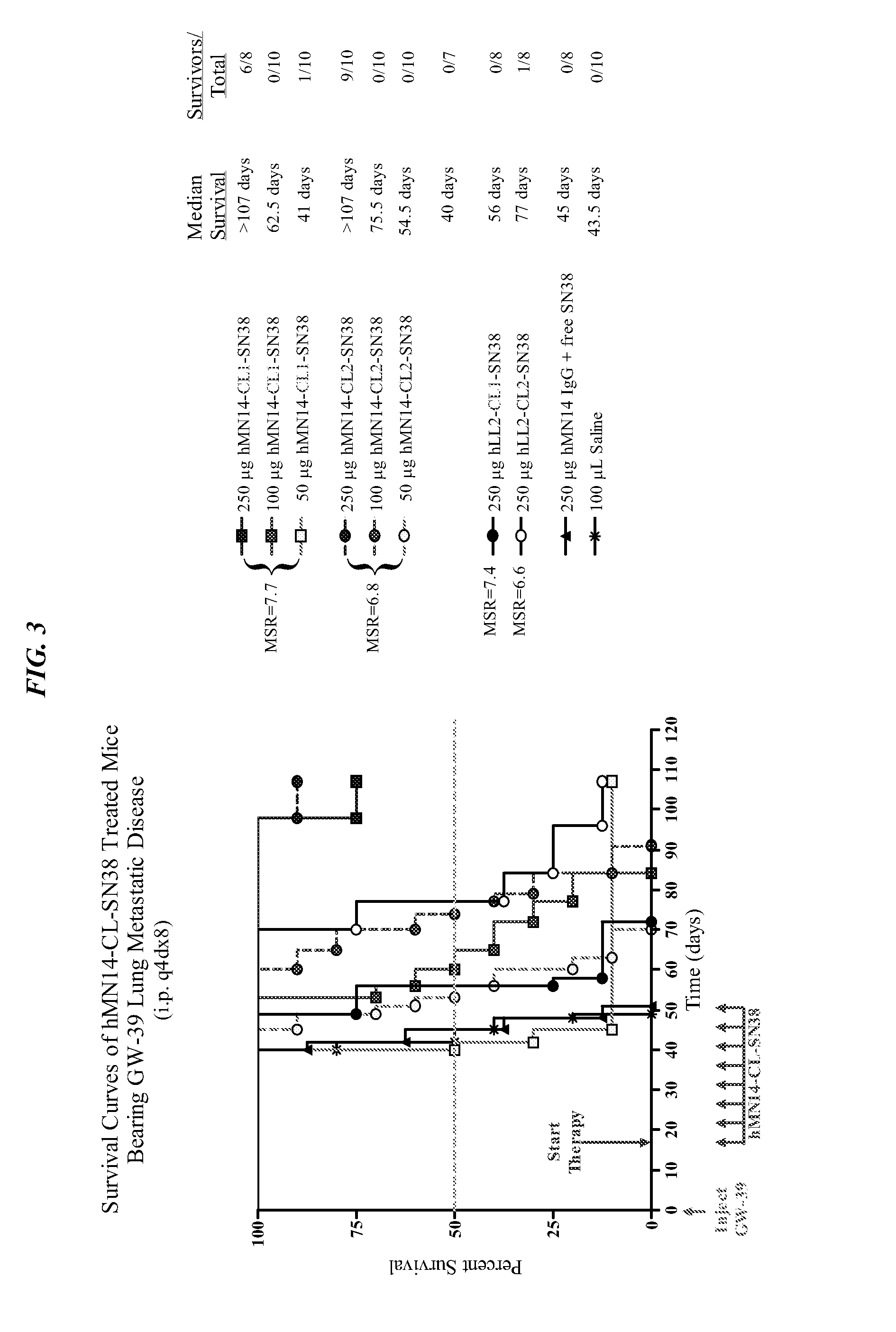
Antibody-sn-38 immunoconjugates with a cl2a linker
ActiveUS20140227180A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Delivery device and related methods
ActiveUS20090013994A1Receive treatment wellImprove drug deliveryRespiratorsLiquid surface applicatorsBiomedical engineeringDrug
Owner:MANTA DEVICES
Delivery device and related methods
InactiveUS20100180894A1Receive treatment wellImprove drug deliveryRespiratorsMedical devicesMedicine
A delivery device that includes a cutter for opening a barrier layer to provide fluid access to a dose chamber, and a diverting structure for direct air flow toward the dose chamber.
Owner:MANTA DEVICES
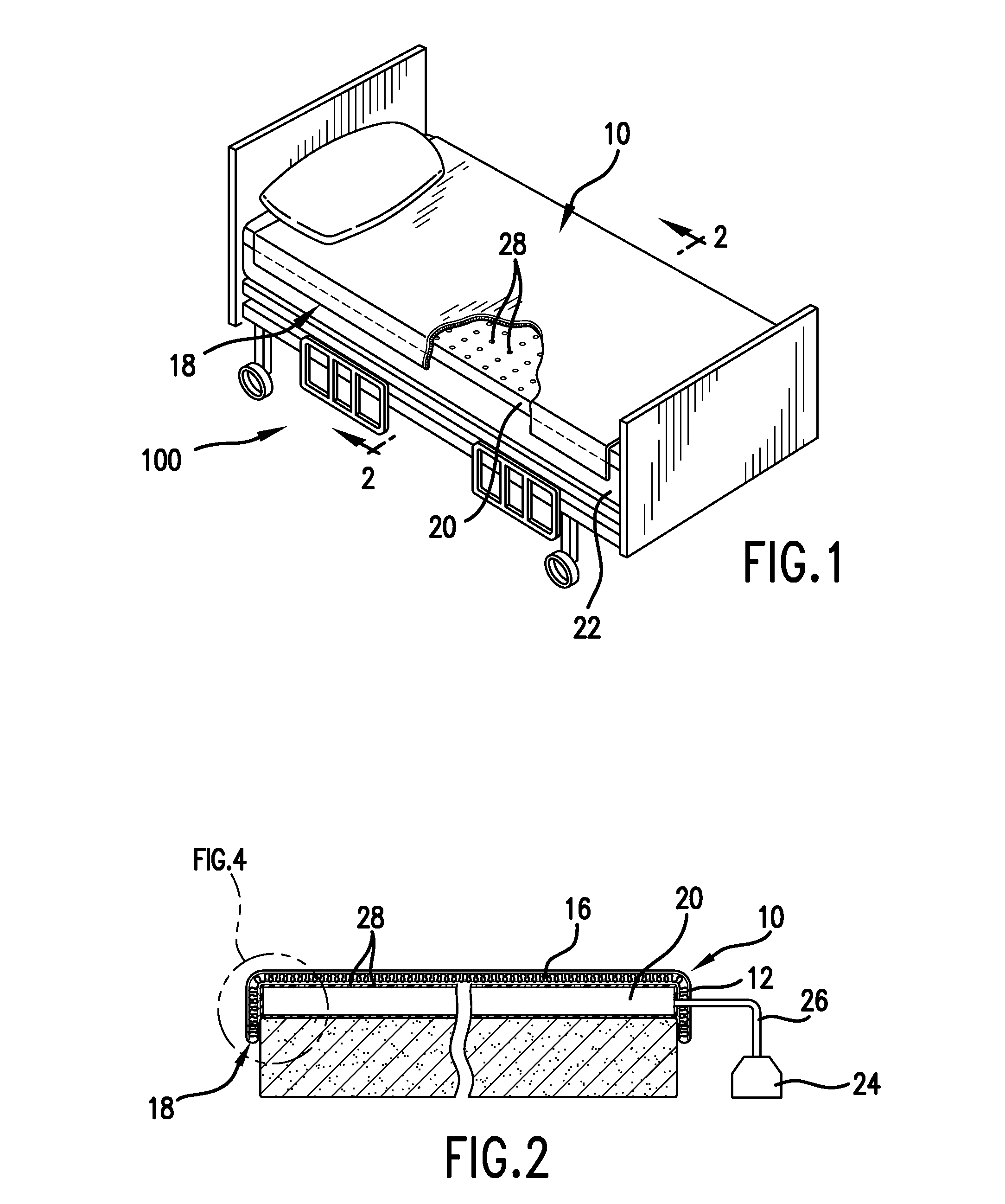
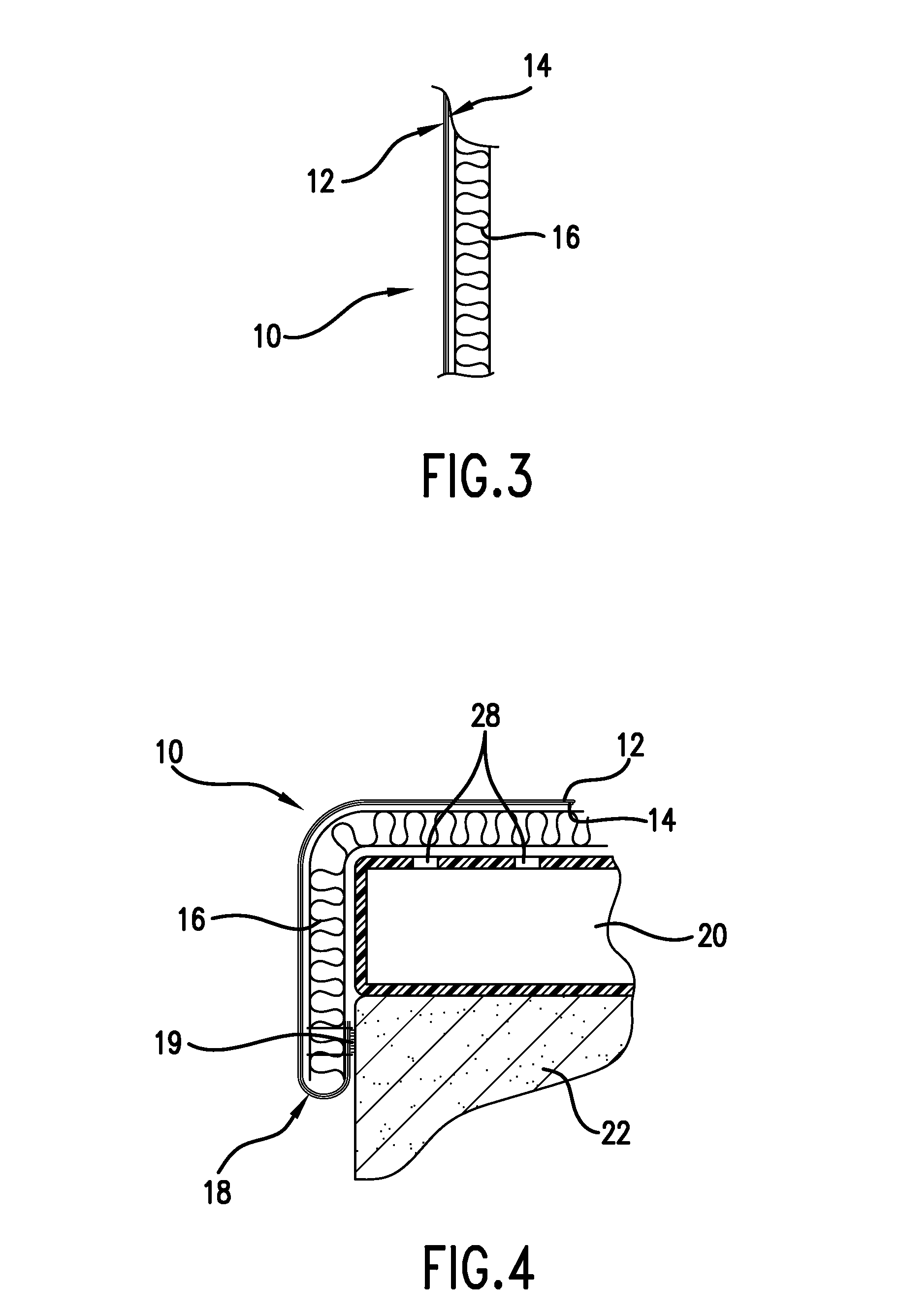
Mattress cover for convalescing patient
InactiveUS20090056030A1Receive treatment wellReduce and treat decubitus ulcersStuffed mattressesOrnamental textile articlesEngineeringWeight-bearing
There is provided an inflatable mattress and coverlet used to reduce and treat decubitus ulcers. The mattress is an air inflatable mattress and is particularly adapted for use on a bed in a hospital, nursing home, health care facility or the like. The air inflatable mattress has a series of apertures on the top allowing small amounts of air to pass through to the underside of the coverlet. According to one aspect of the invention the coverlet is placed over the top of air inflatable mattress in the central portion thereof between the head of the bed and the foot of the bed. The coverlet is of sufficient length to extend under the weight bearing portions of the patient. The coverlet has as a first layer a moisture wicking fabric, such as a polyester, and on one side of the moisture wicking fabric there is attached a microporous polymeric member which allows air from the mattress to pass through the coverlet to the patient. Adjacent the polymeric member is another layer comprising a spacer fabric. The side edges of the coverlet form a drip edge and may be attached to the mattress by suitable means.
Owner:IPM
Methods for application of reduced pressure therapy
ActiveUS8007491B2Reduce pressureReceive treatment wellNon-adhesive dressingsSurgical needlesDensity of airSealant
The tissue therapy device includes a sealant layer and a suction apparatus. The sealant layer creates a sealed enclosure between it and the surface of a patient by forming an airtight seal around an area of tissue. The suction apparatus is in fluid communication with the sealant layer and together, create a closed, reduced pressure therapy system. The suction apparatus self-creates reduced pressure by decreasing the density of air molecules underneath the sealant layer by expanding the volume of the air molecules.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
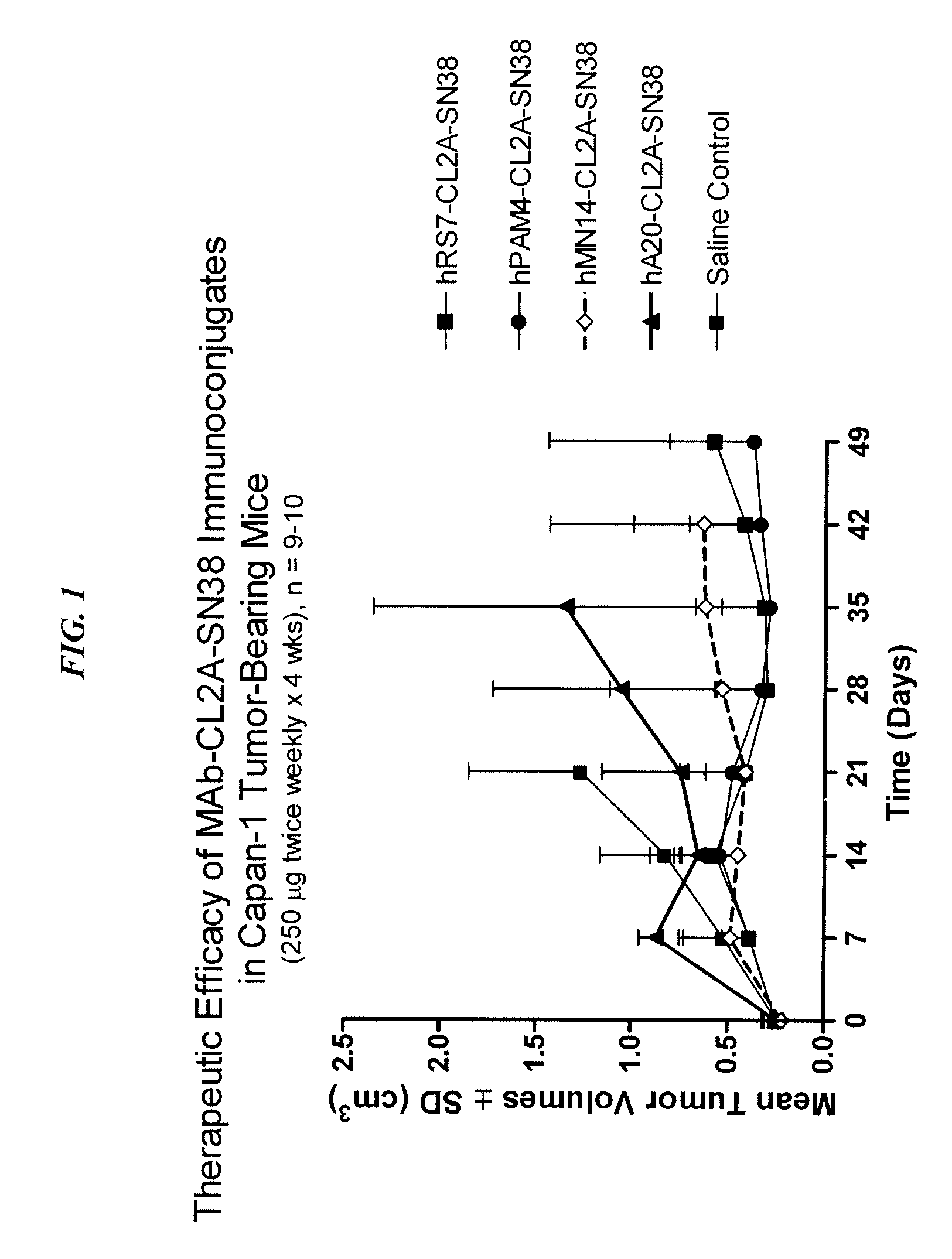
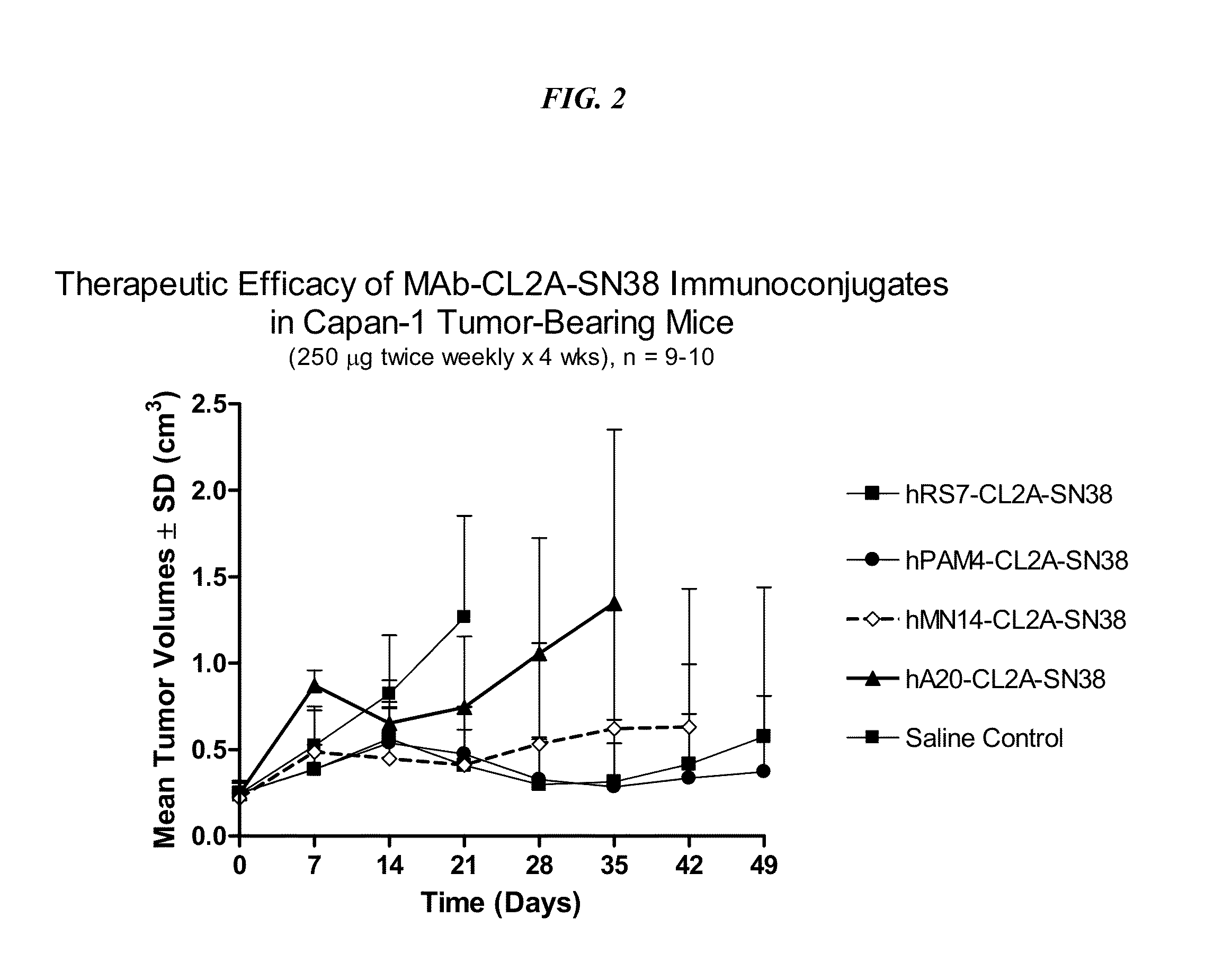
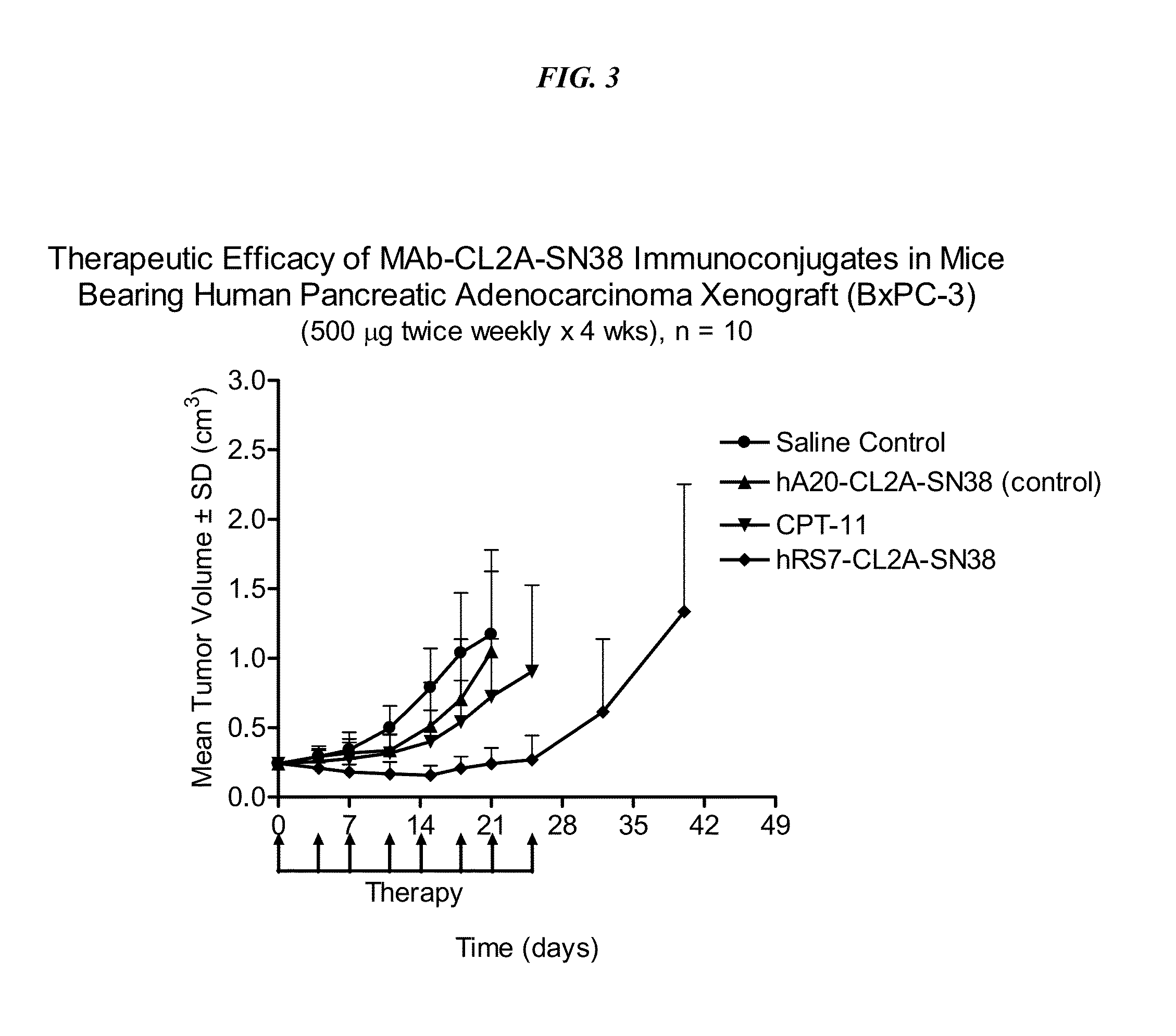
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
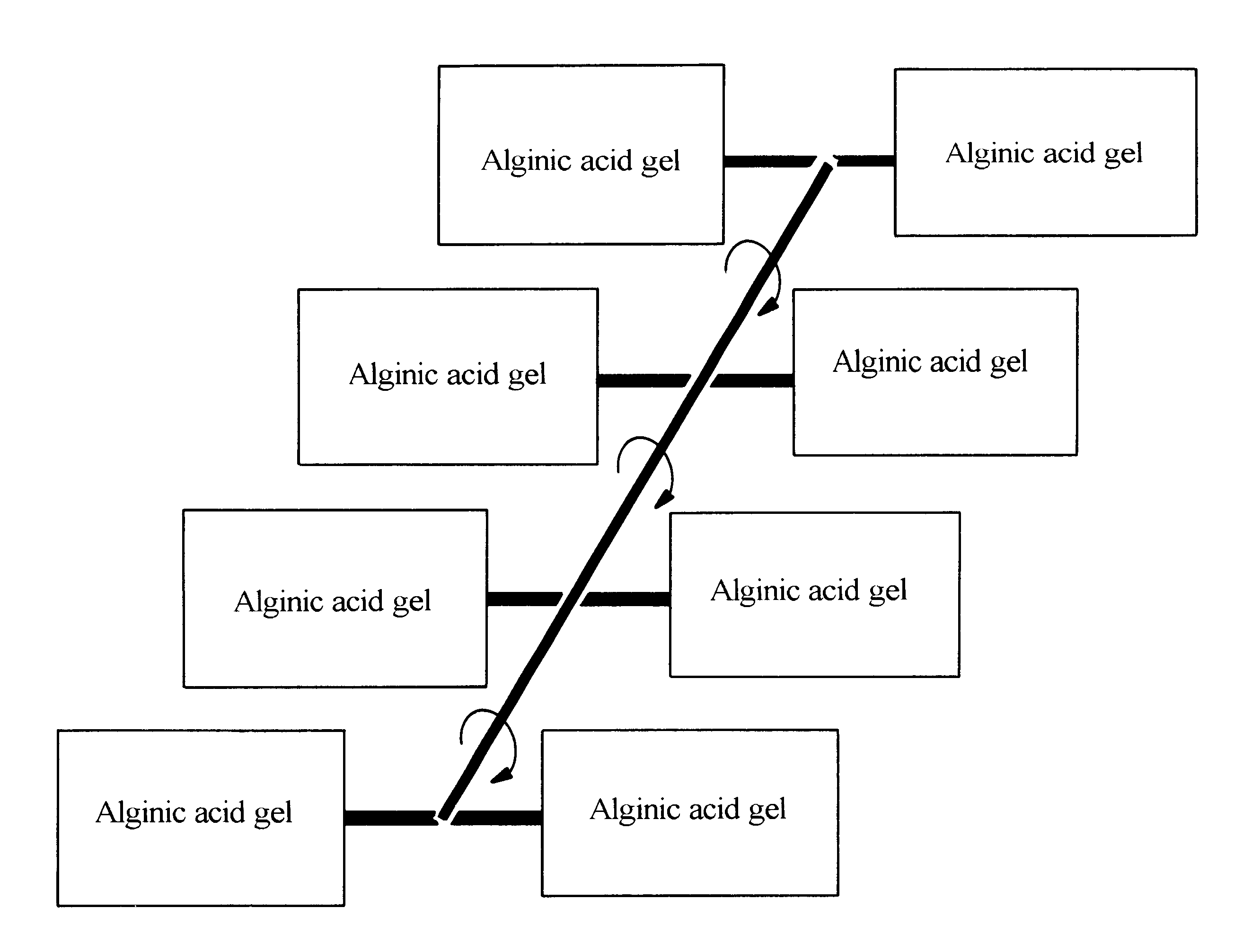
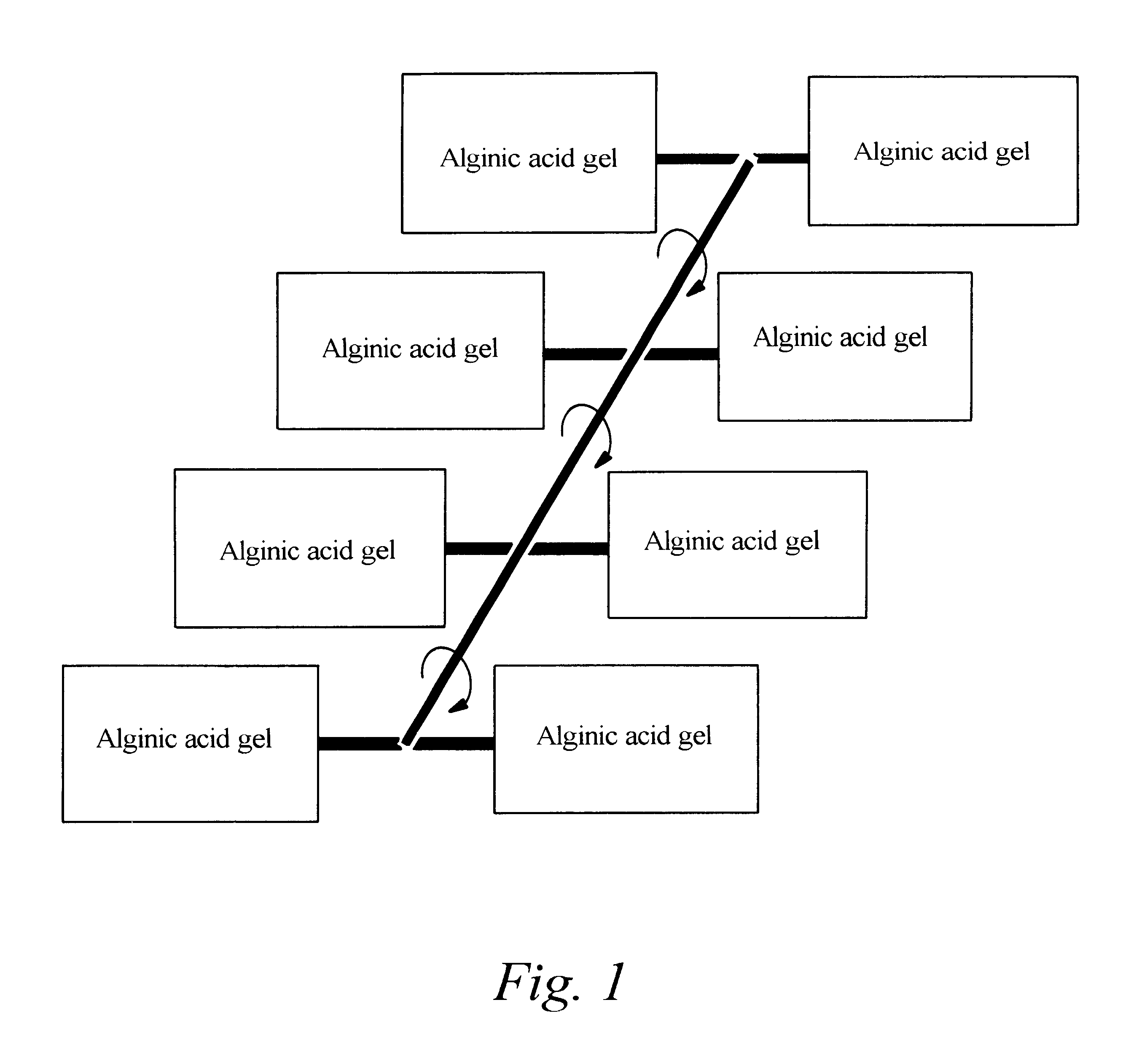
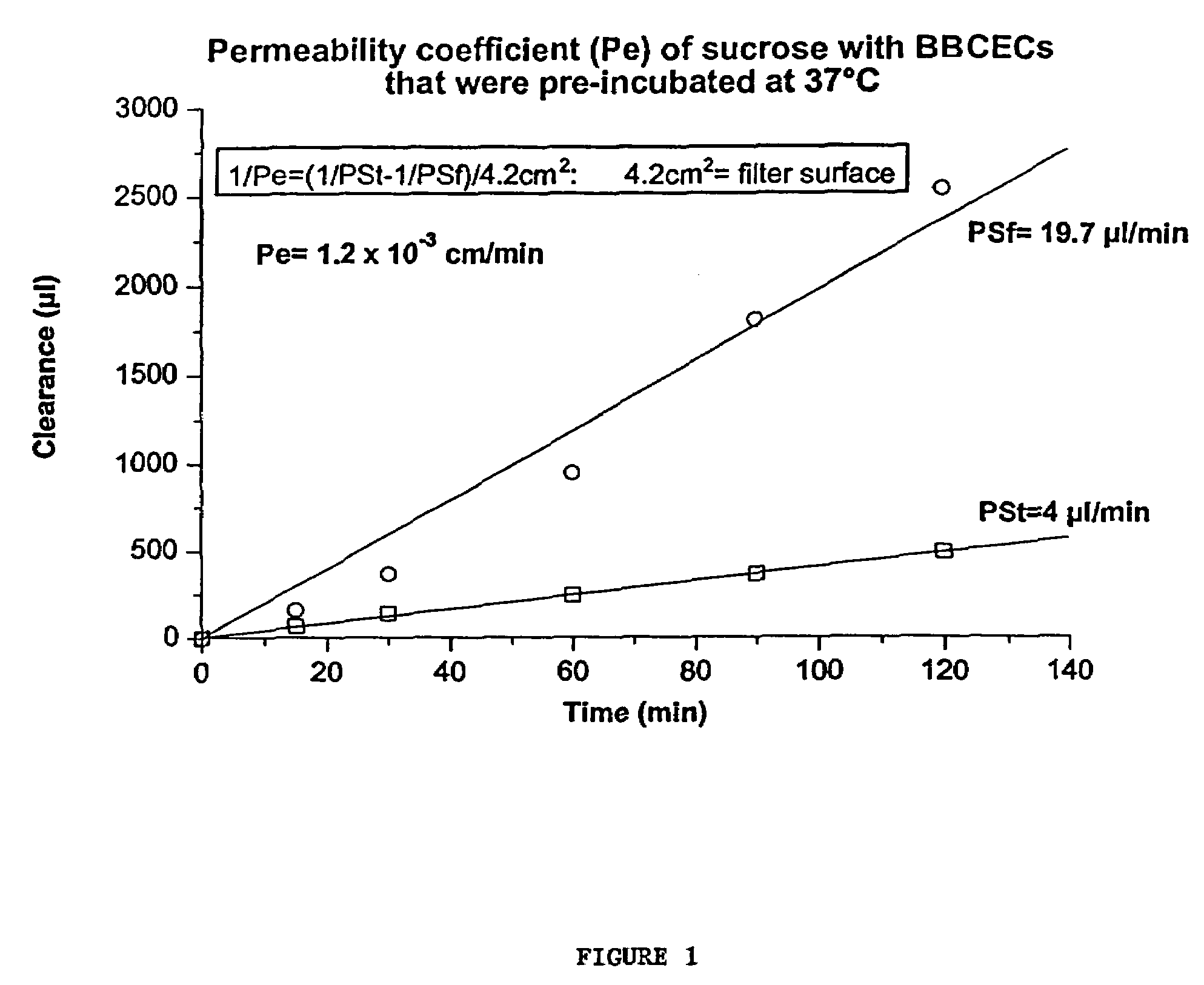
Alginate gel based adsorbents for heavy metal removal
InactiveUS6989102B1Receive treatment wellEasy to useOther chemical processesWater contaminantsIonActivated carbon
An alginate gel adsorbent to remove heavy metal ions according to the present invention is prepared by adding dropwisely 0.1-5 wt % alginate solution to a polyvalent cationic solution thereby cross-linking alginic acid with polyvalent cations. An alginate gel adsorbent containing activated carbon capable of simultaneously removing heavy metal ions and organotoxic materials, that is, activated carbon / alginate gel adsorbent, is prepared by adding dropwisely a mixed solution of 0.17-10 wt % of alginate and 0.1-10 wt % of activated carbon powder to a polyvalent cationic solution thereby cross-linking alginic acid with polyvalent cations in order to immobilize polyvalent cation to alginic acid containing activated carbon. The polyvalent cationic solution is selected from the group consisting of calcium chloride (CaCl2), strontium chloride (SrCl2), barium chloride (BaCl2) and aluminium chloride (AlCl3). A flat board or a thin membrane coated alginate gel adsorbent can be prepared by coating alginate gels onto a supporter such as paper, wood plate and textile fabrics. The alginate gel adsorbent for water purification can be applied to a water purifier as well as a process of wastewater treatment. The alginate gel coated onto a supporter is prepared by immersing a supporter in 0.05-5 wt % alginate solution so that the supporter may uniformly adsorb the alginate solution, immersing the supporter in a polyvalent cationic solution thereby forming alginate gel on the surface of the supporter, and drying the supporter formed with alginate gel thereon.
Owner:SAMSUNG ATOFINA CO LTD
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Camptothecin-binding moiety conjugates
InactiveUS8877901B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsNervous disorderAntibody fragmentsCamptothecin
Owner:IMMUNOMEDICS INC
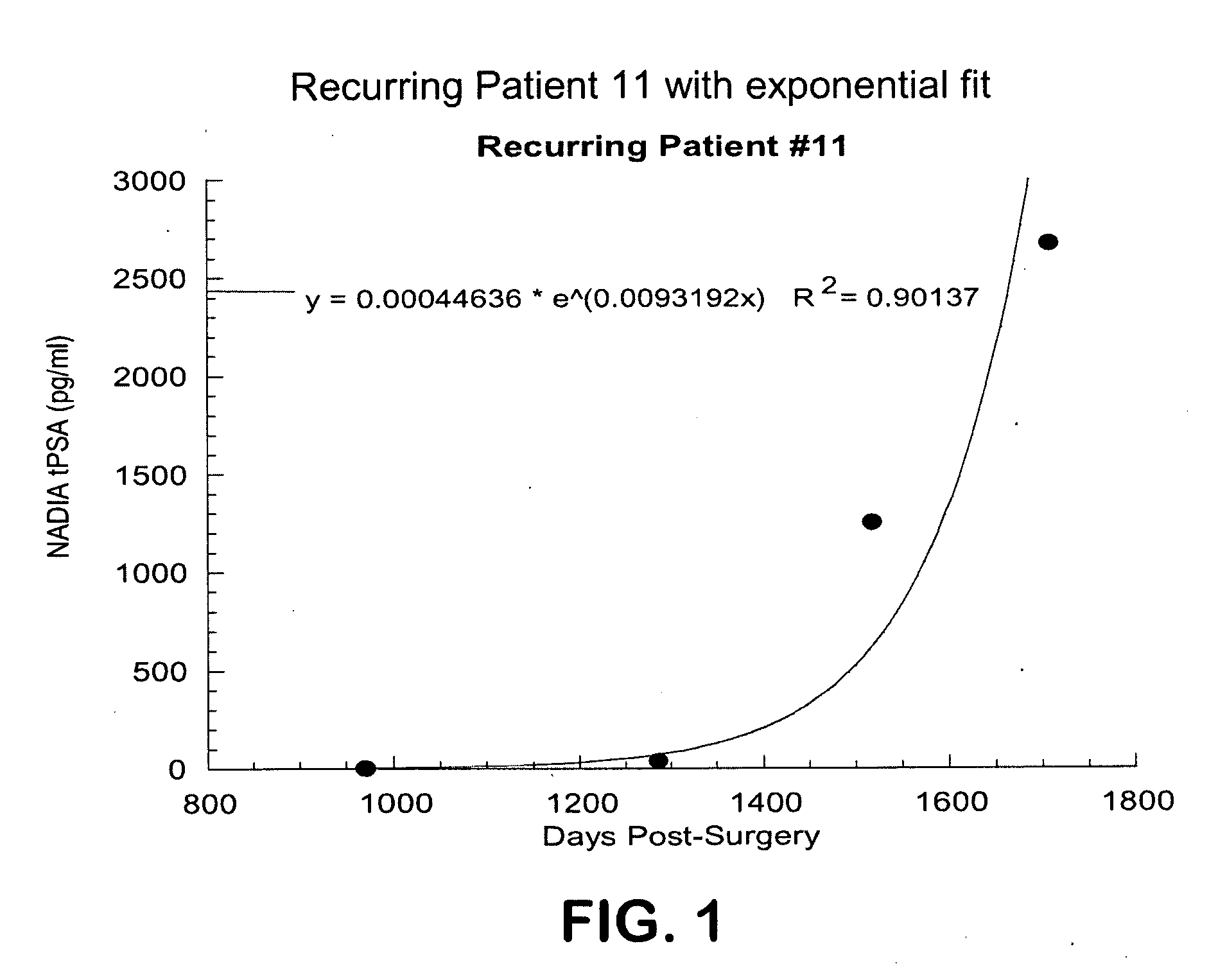
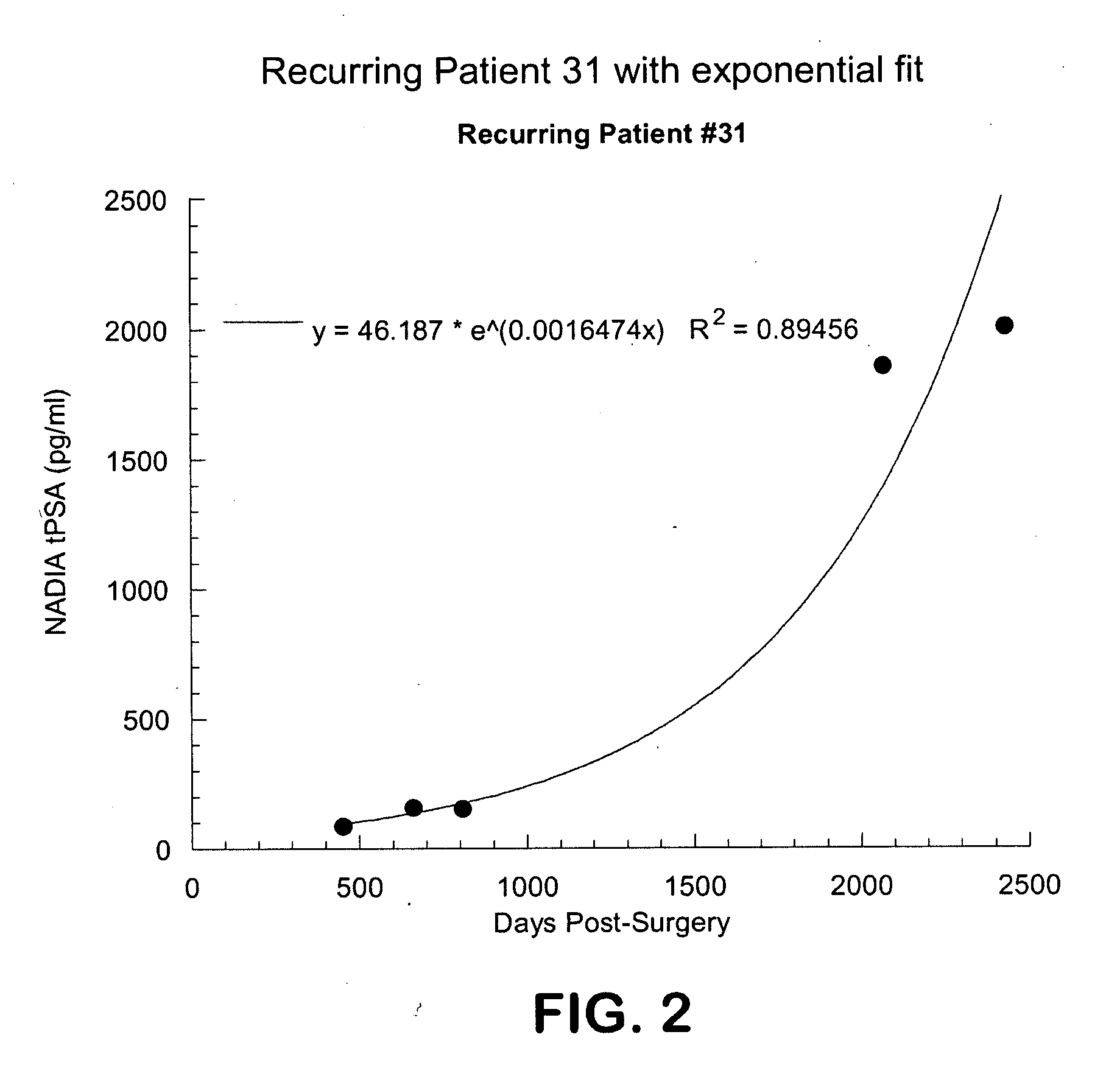
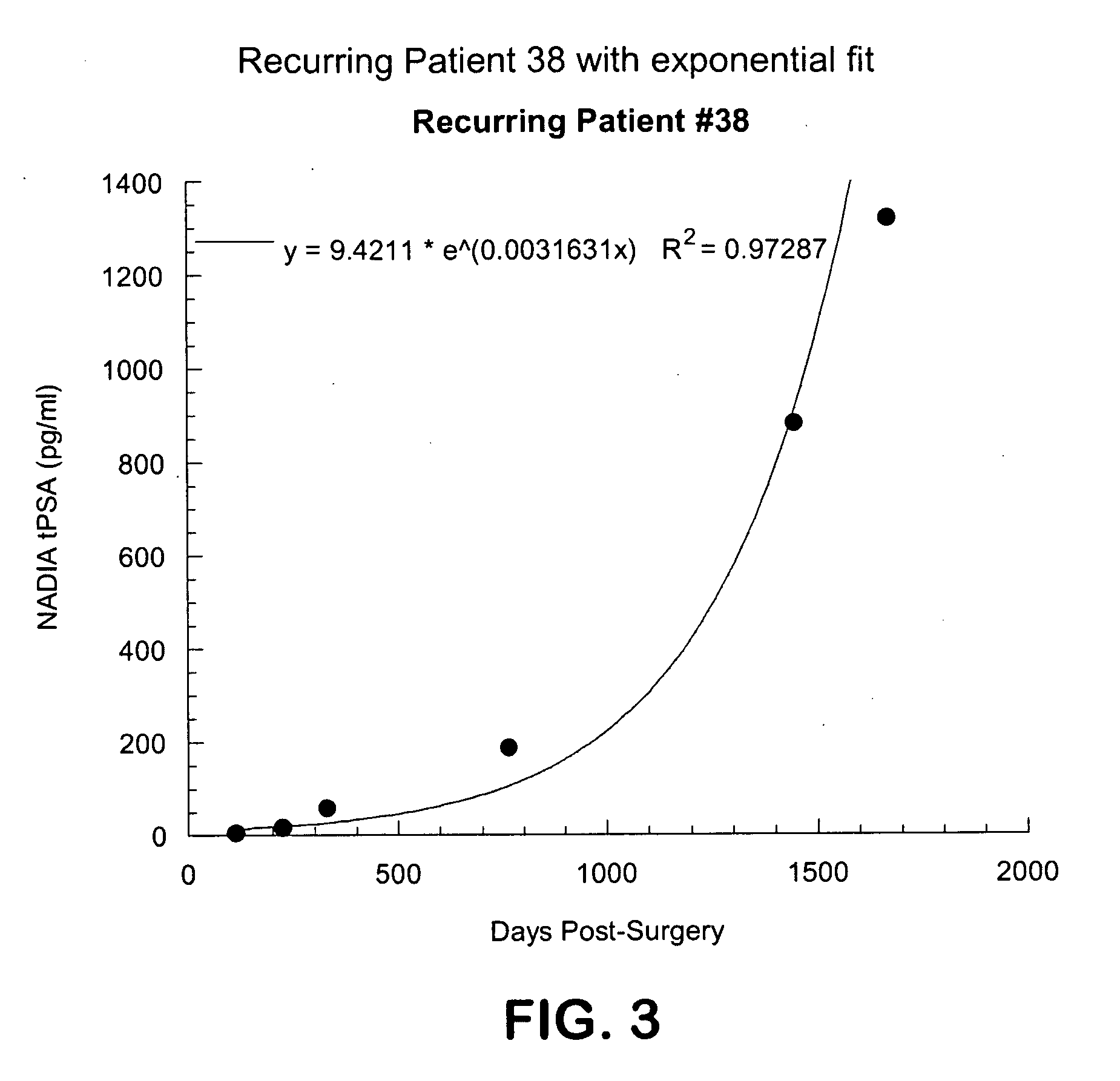
Method for early determination of recurrence after therapy for prostate cancer
InactiveUS20090246781A1Lower levelIncrease probabilityMicrobiological testing/measurementDisease diagnosisProstate diseaseStable Disease
This invention describes compositions and methods for use in PSA assays having low functional sensitivity which are useful, for example, in the detection of early stage recurrence of prostate disease following treatment and in the determination of whether patients have early stage biochemical reoccurrence (ES-BCR) or stable disease.
Owner:IRIS INT
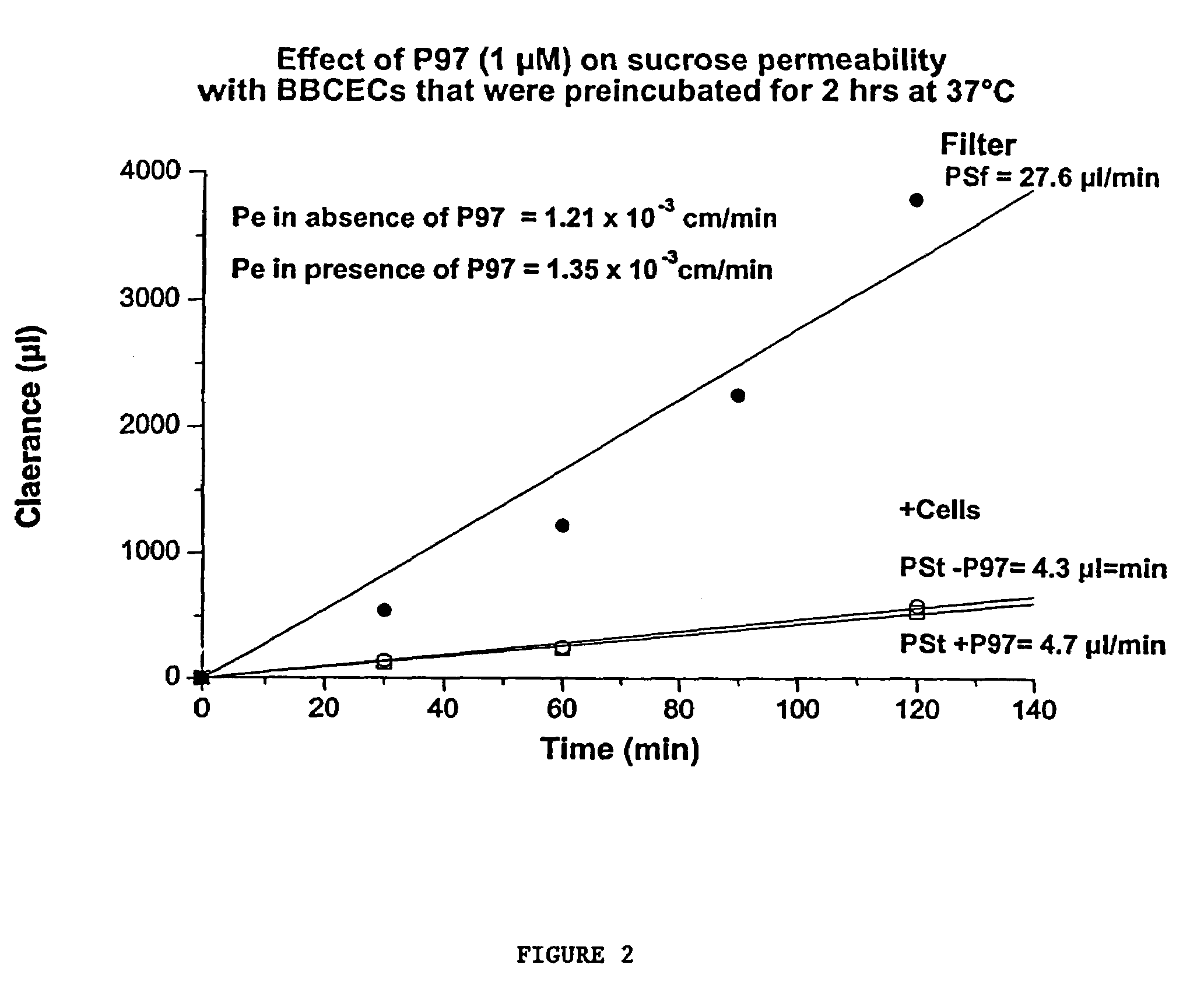
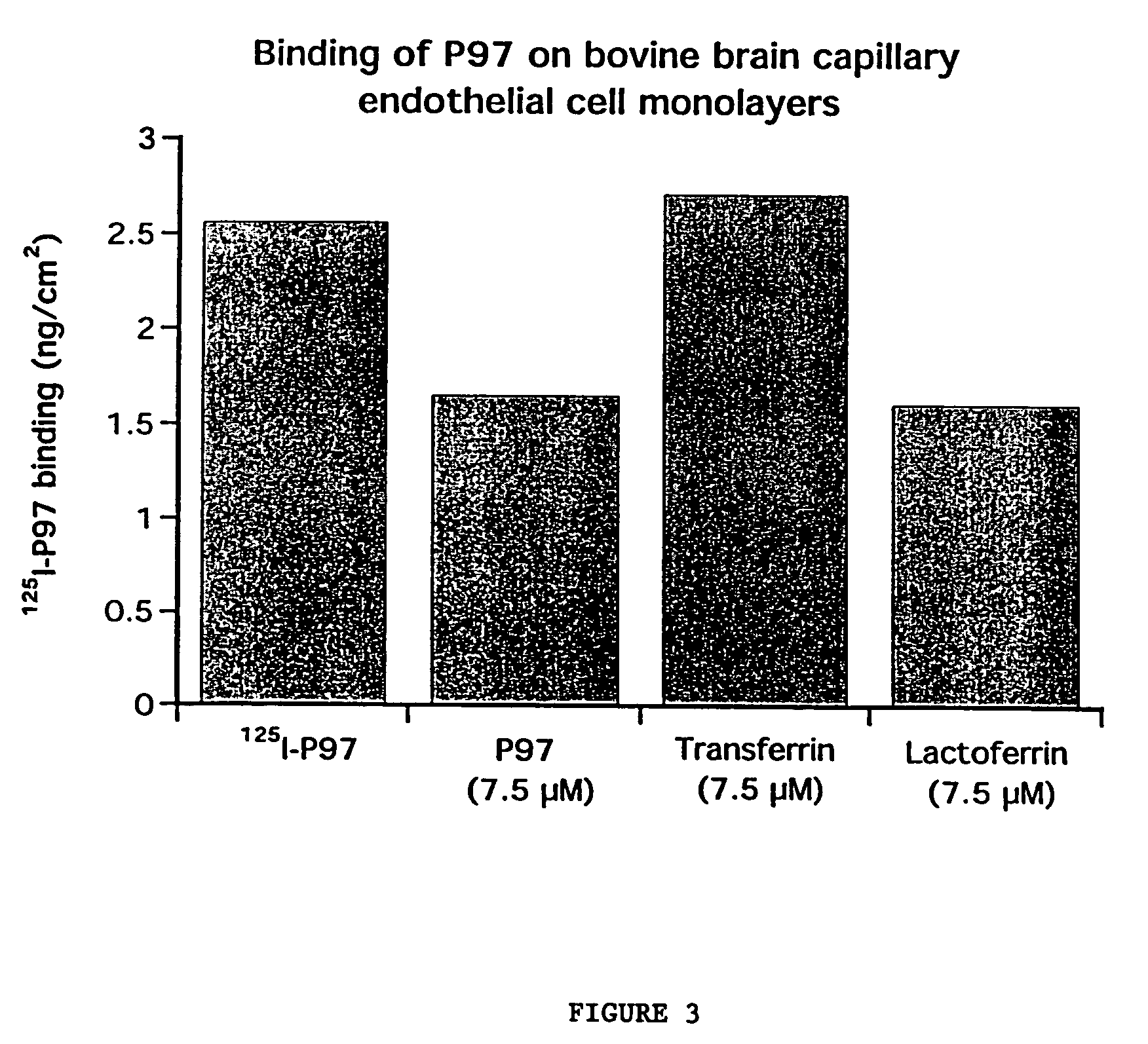
Compositions for modulating blood-brain barrier transport
InactiveUS7700554B2Reducing a neurological side-effectReceive treatment wellOrganic active ingredientsNervous disorderActive agentLysosome
This invention provides conjugates of therapeutic or active agents with melanotransferrin or with other ligands of a melanotransferrin receptor, melanotransferrin receptor modulators, and related compositions and methods for modulating blood-brain barrier transport by providing methods of screening and selecting such conjugates, ligands, and modulators in vitro and in vivo, and methods of use of such conjugates, modulators and ligands in diagnosis and the treatment of diseases, including particularly diseases of the central nervous system or lysosomal storage diseases.
Owner:HORIZON ORPHAN LLC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS20160296633A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadTreatment effect
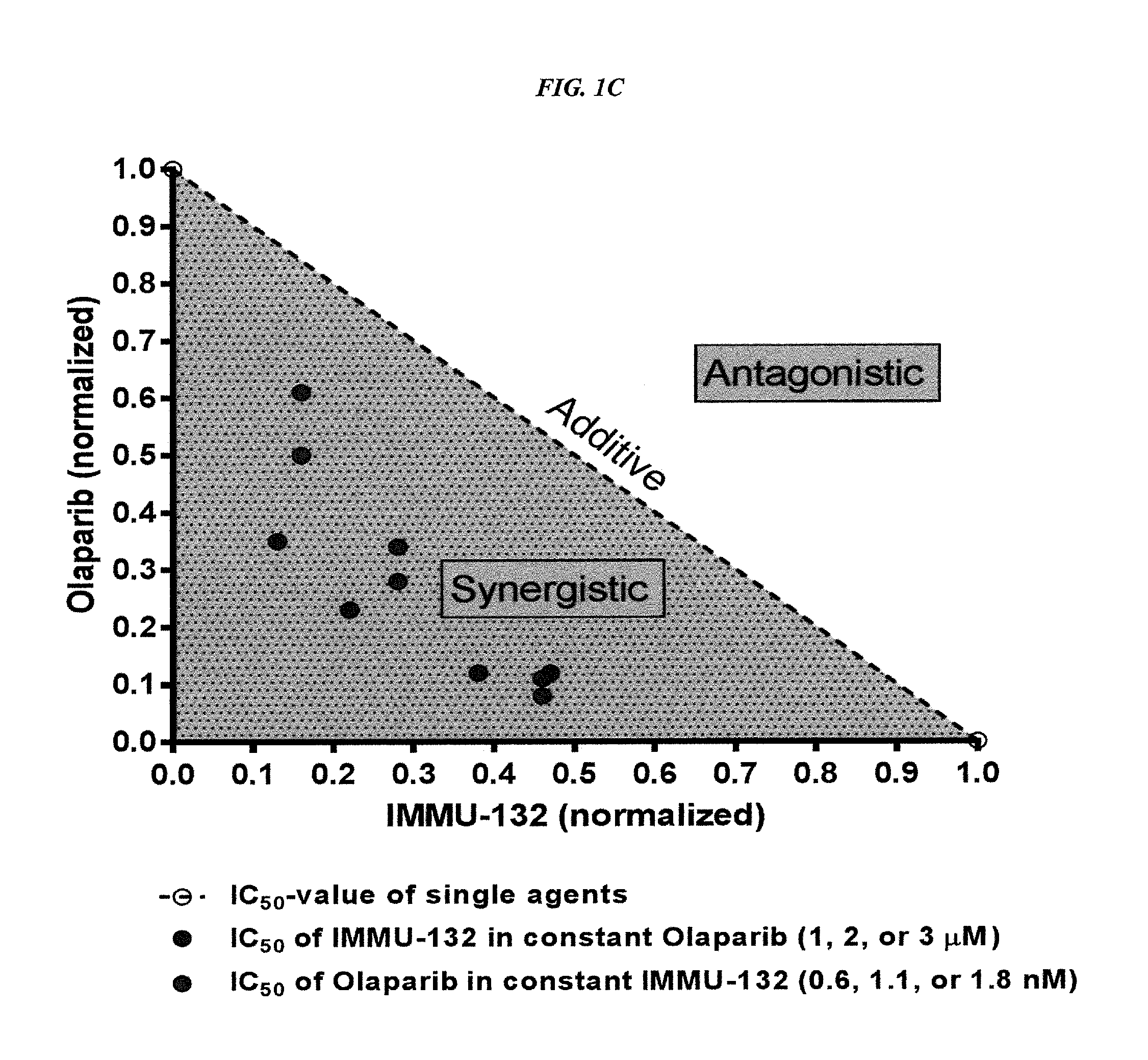
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Plasma processing apparatus and plasma processing method
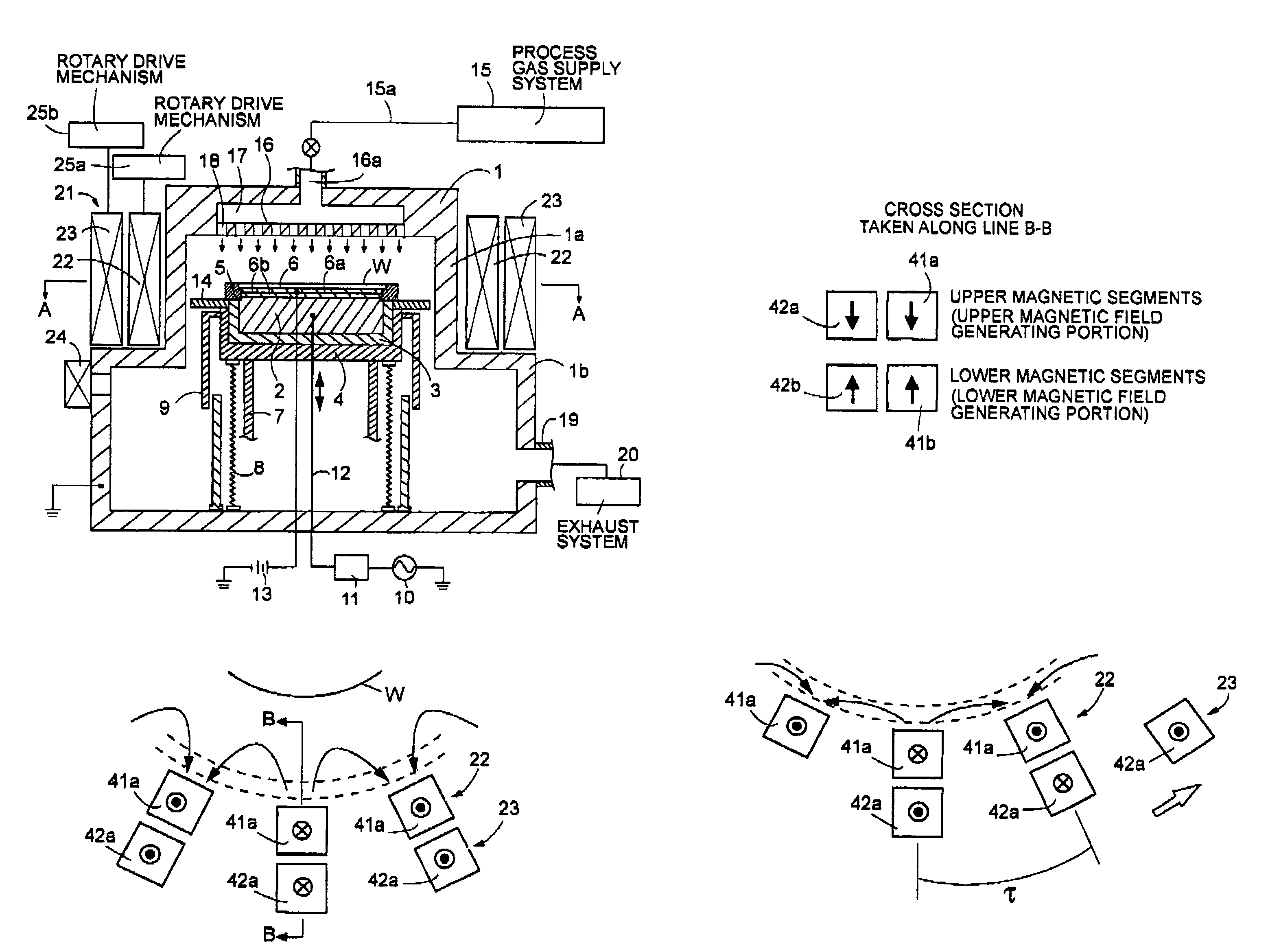
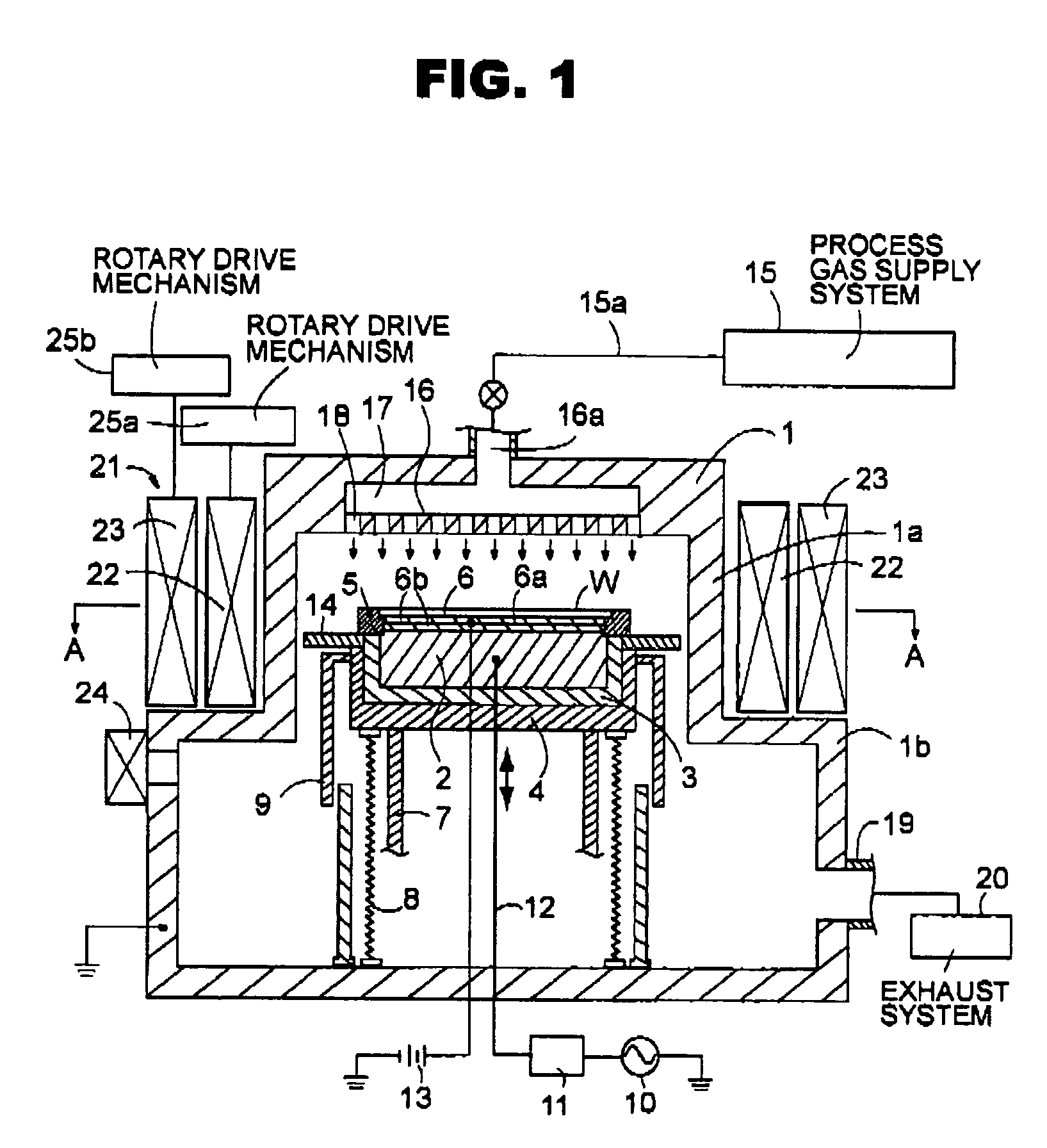
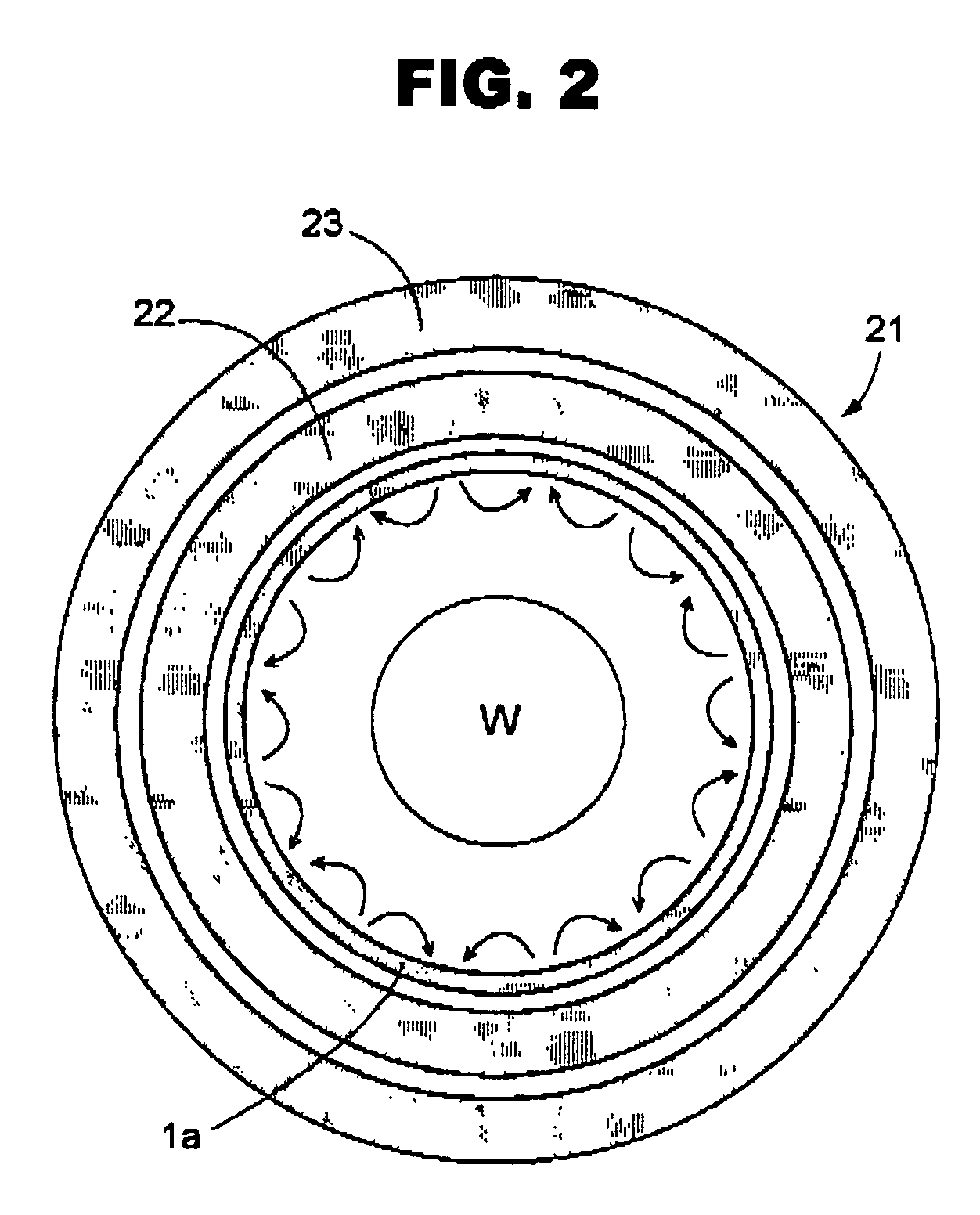
ActiveUS7438783B2Receive treatment wellOptimization mechanismCellsElectric discharge tubesPlasma generatorVacuum chamber
Owner:SHIN ETSU CHEM CO LTD +1
Pharmaceutical compositions comprising tyrphostins
InactiveUS20030013748A1Good treatment effectConvenient treatmentBiocideOrganic chemistryProtein Tyrosine Kinase InhibitorsTyrphostin Compound
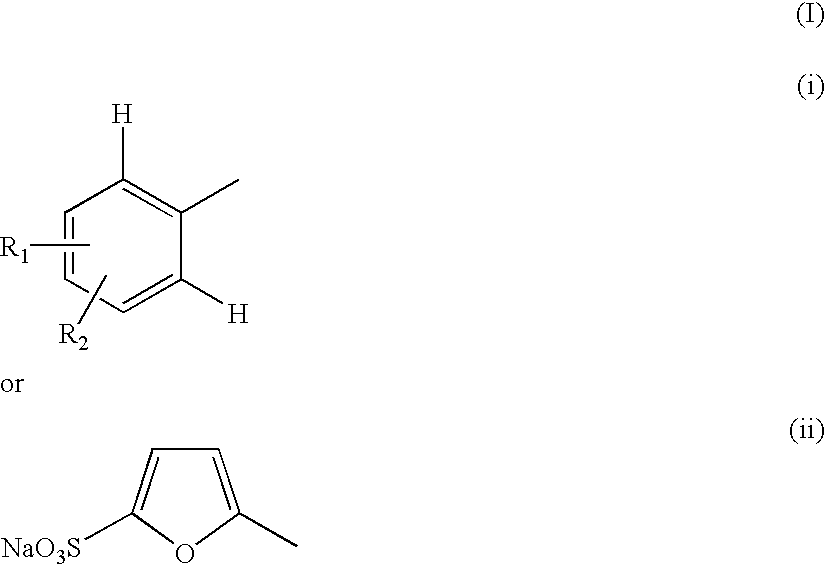
Compounds useful for countering undesired toxic effects to cells, tissues or organs having formula (I) wherein: Ar is a group of formulae (i) or (ii), n is O or, when Ar has formula (i) above, then n may also be 1, R is CN, -GC(S)NH2, -C(O)NHR3 or, when R1 is 4-NO2 and R2 is H or 3-OH, then R may also be a group of formulae (iii), (iv), (v), (vi) where R3 is H, phenyl, phenyl(lower alkyl) or pyridylmethyl; R1 and R2 are each independently H, OH, NO2 or, when R is CN, also CH3, F, or CF3, provided that both R1 and R2 are simultaneously H.
Owner:NOTOX
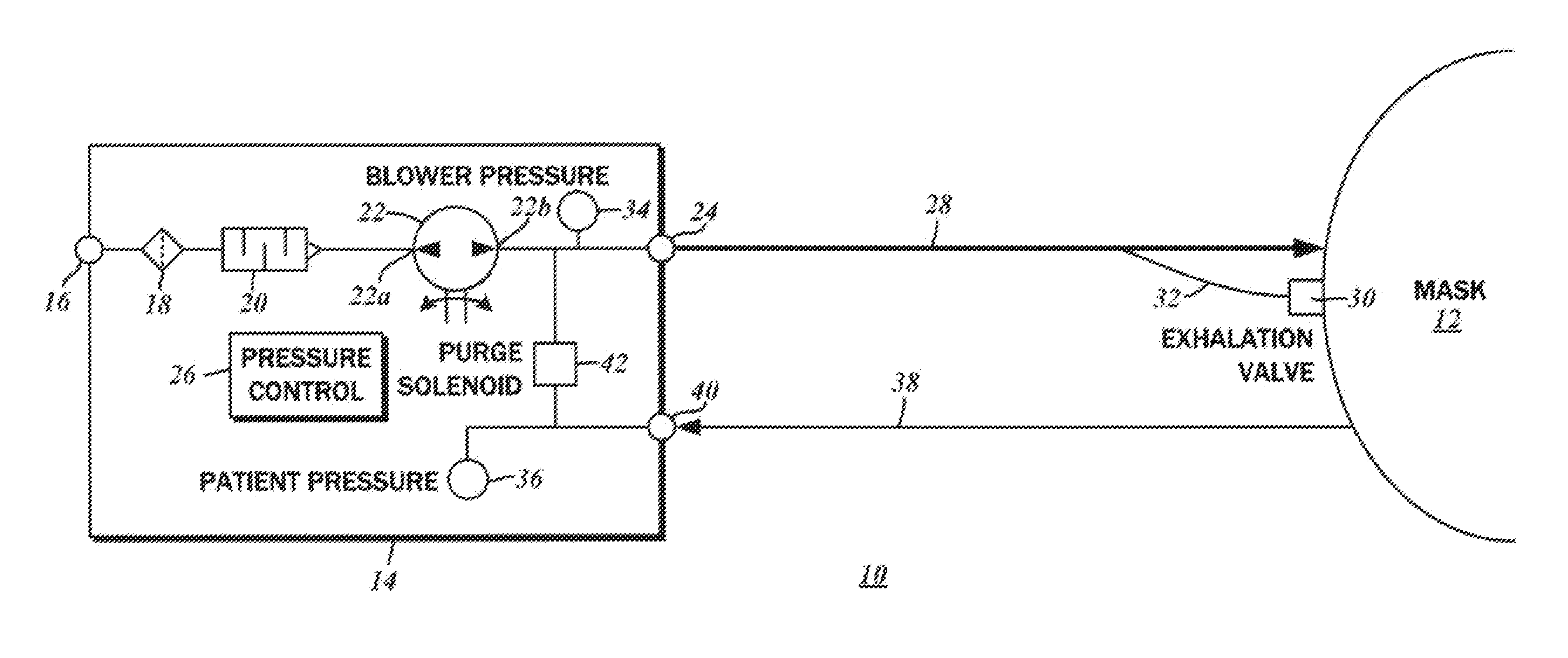
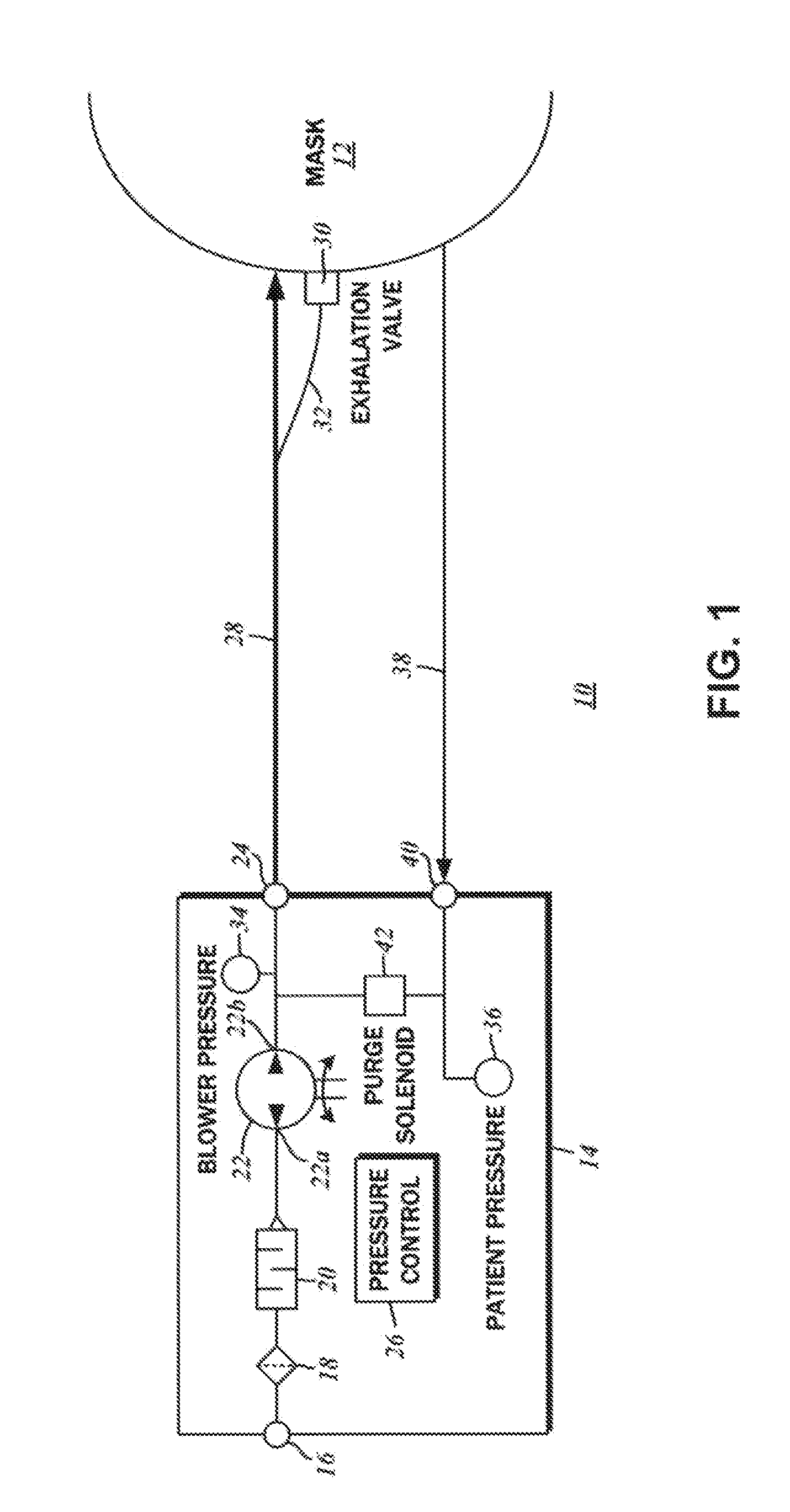
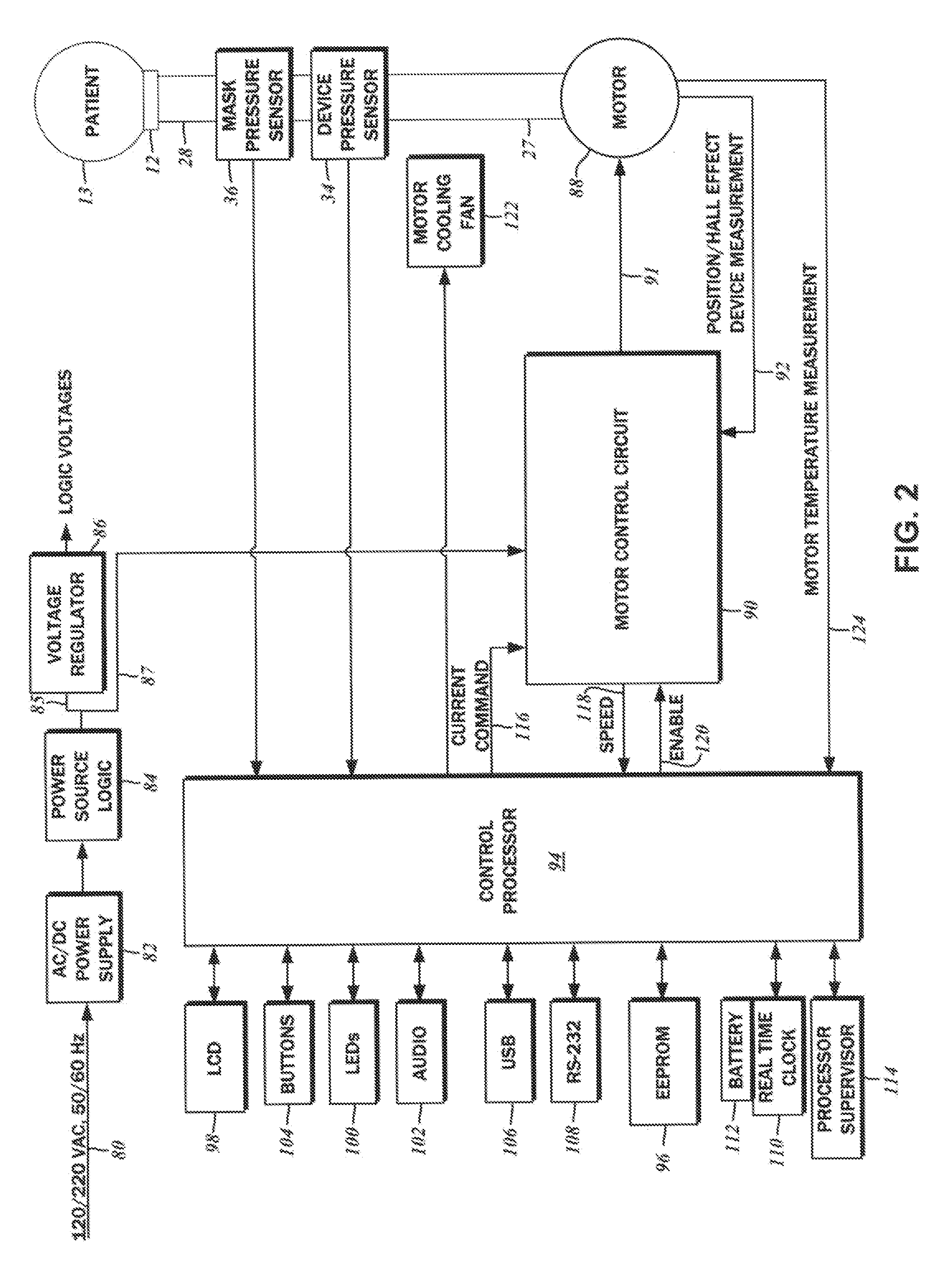
Dual pressure sensor continuous positive airway pressure (CPAP) therapy
ActiveUS20130228180A1Relieve pressureReceive treatment wellOperating means/releasing devices for valvesRespiratory masksPositive pressurePressure difference
A continuous positive airway pressure (CPAP) apparatus for respiratory assistance of a pattern is disclosed. There is a blower having an output connectible to a ventilation mask wearable by the patient. A first pressure sensor measures blower pressure at the output of the blower, and a second pressure sensor that is connectible to the ventilation mask measures mask pressure therein. A pressure controller is connected to the first pressure sensor and the second pressure sensor, and a patient inspiratory phase and a patient expiratory phase is be detectable by the pressure controller to regulate therapeutic pressure at the patient mask, based upon pressure differentials between the mask pressure and the blower pressure.
Owner:BREATHE TECHNOLOGIES INC
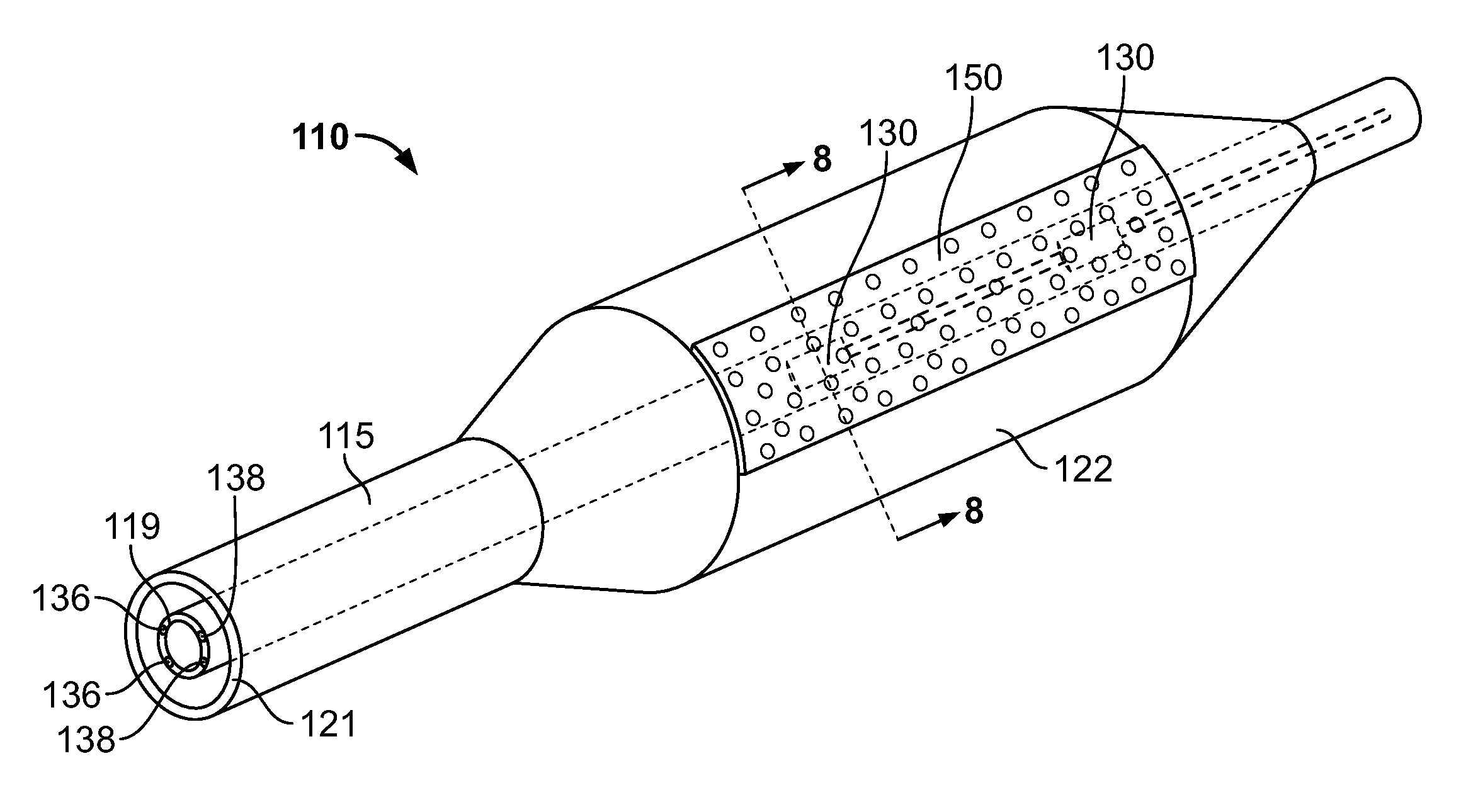
Stent Design Allowing Extended Release of Drug and/or Enhanced Adhesion of Polymer to OD Surface
The invention is directed to mechanisms and methods that reduce the delamination of a therapeutic agent from a stent. The mechanisms include holes (channels, wells, and other hole configurations), protrusions, sintered metal cores, clamps / staples, pins, and stainless steel shields.
Owner:BOSTON SCI SCIMED INC
Medicine for curing rheumatoid arthritis
ActiveCN102920984ASignificant effectHigh cure rateHeavy metal active ingredientsAnthropod material medical ingredientsDrugMedical product
The invention discloses medicine for curing rheumatoid arthritis and belongs to the technical field of medical products. The medicine is a medicine composition prepared by all natural plants, animals, mineral and other ingredients which serve as raw materials according to a certain process. The medicine has unique effects on the rheumatoid arthritis and scapulohumeral periarthritis and remarkable effects on rheumatoid arthritis, osteonecrosis of the femoral head and lumbar disc herniation. The medicine is mainly characterized by expelling wind and removing cold, clearing and activating channels and collaterals, activating blood and dissolving stasis, and relieving swelling and pain; and diseases do not recur after heal. The medicine is wide in the raw materials, low in cost, simple in preparation method and free of emission of three wastes in production.
Owner:苏瑞芳
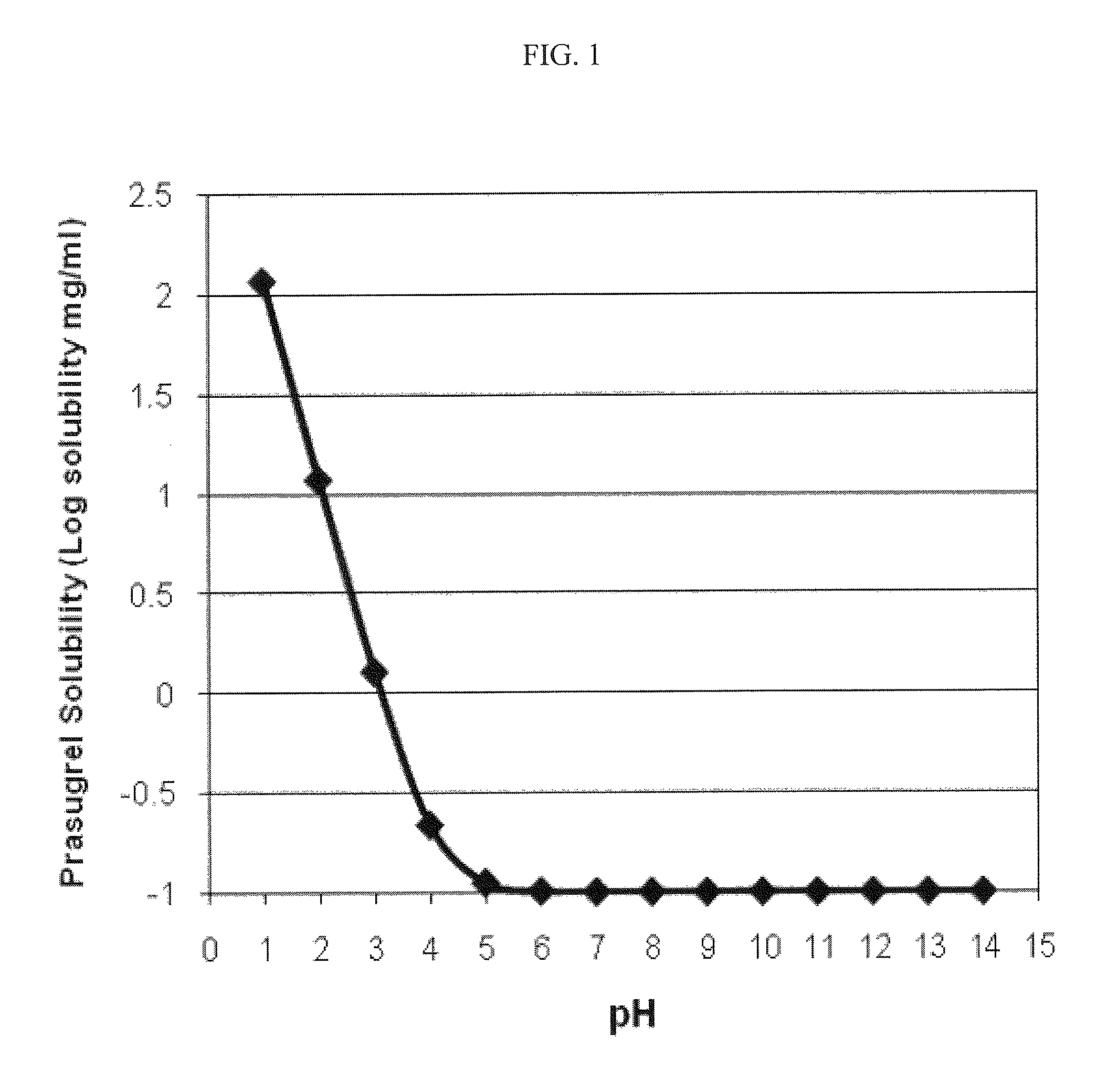
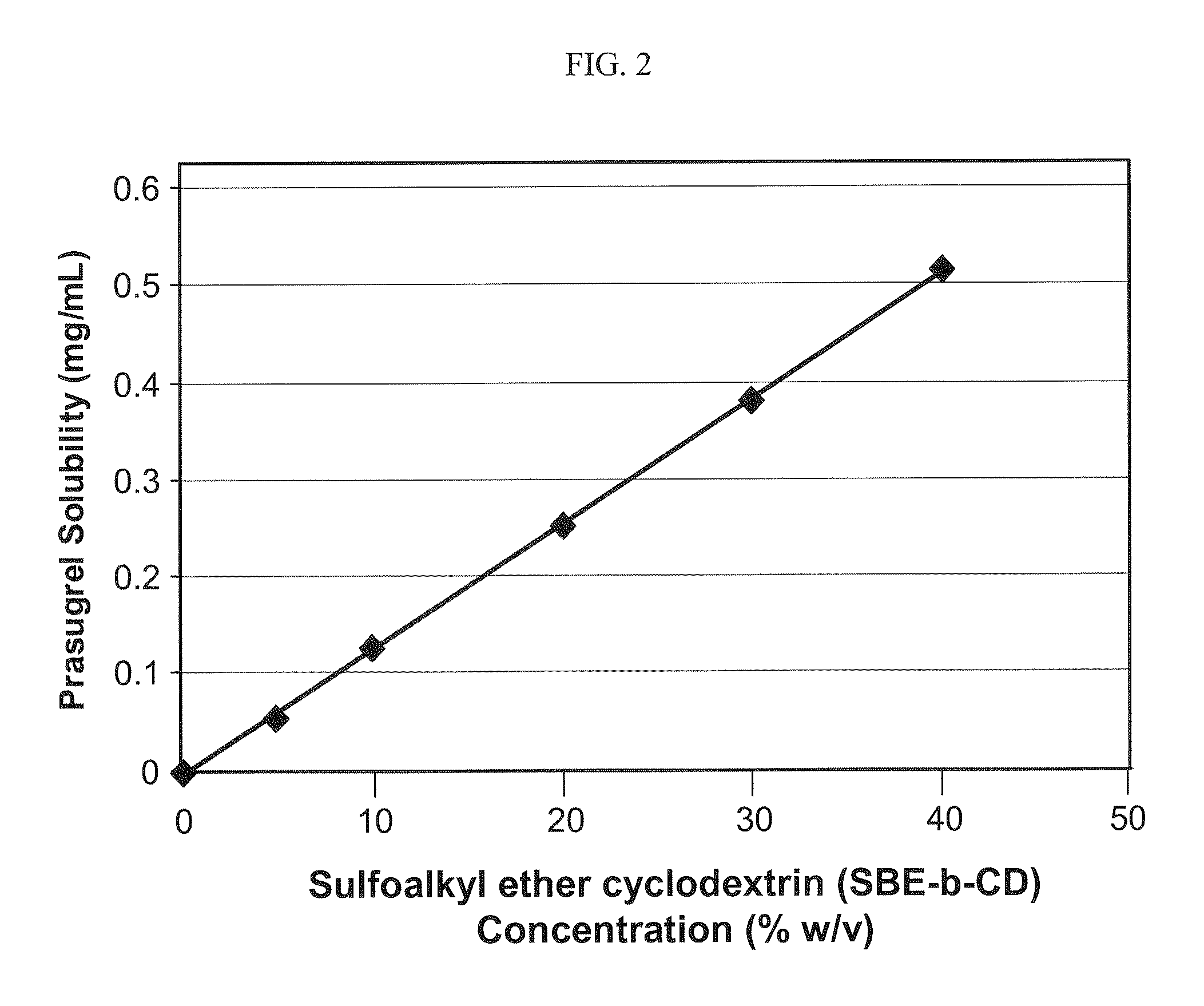
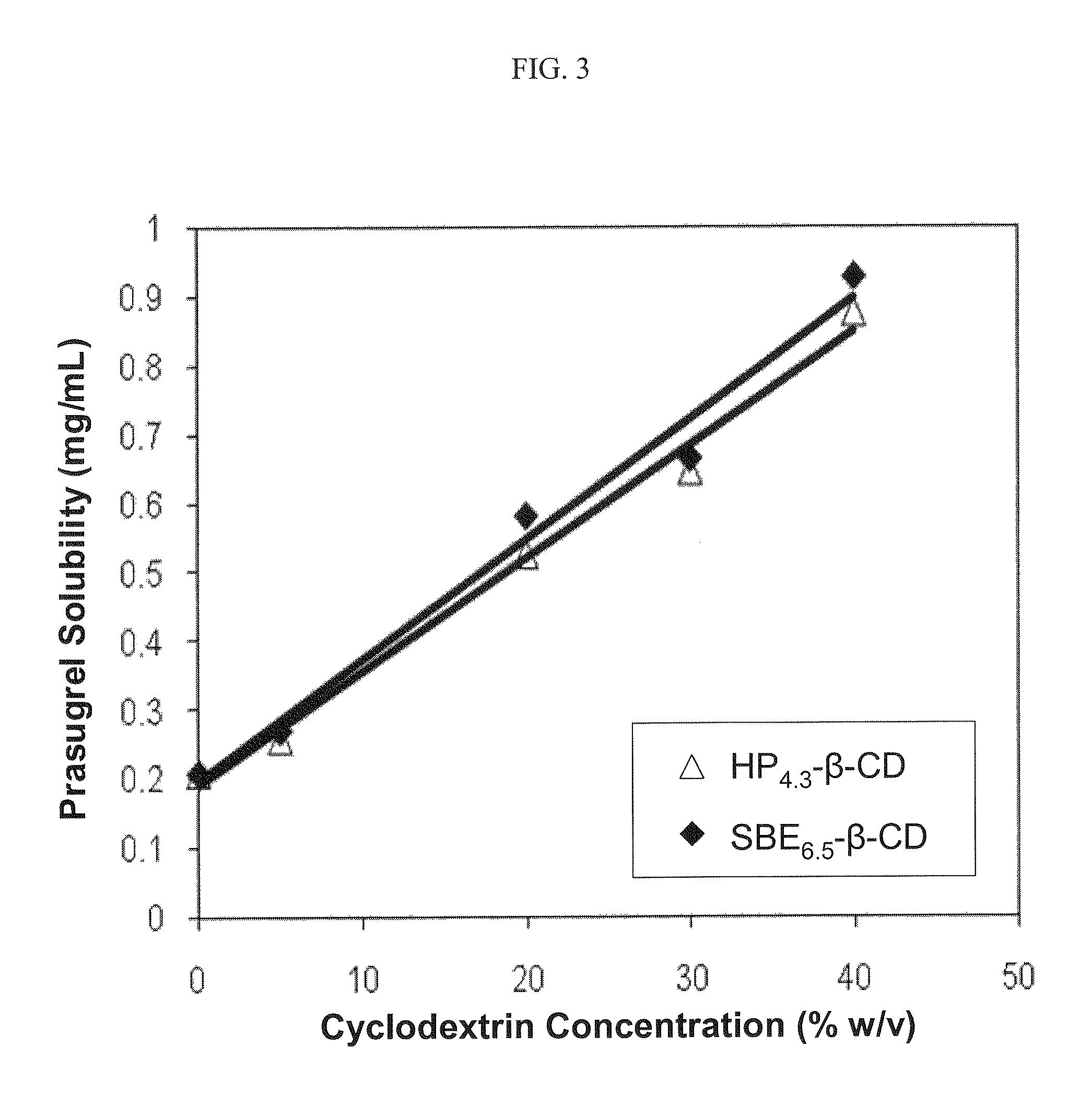
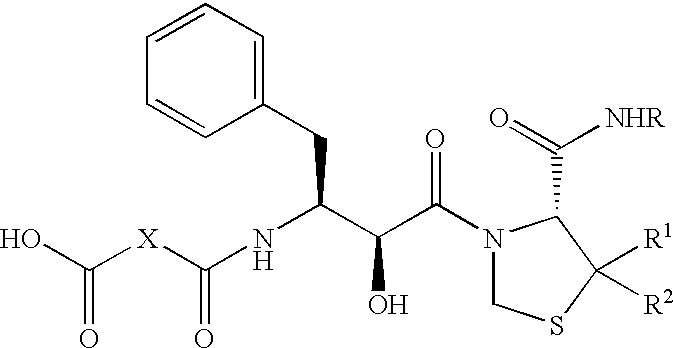
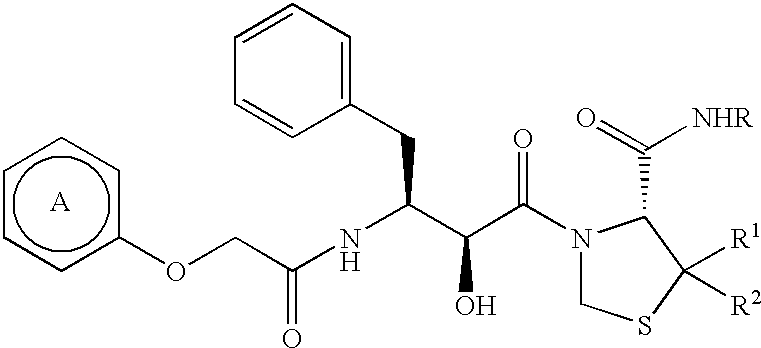
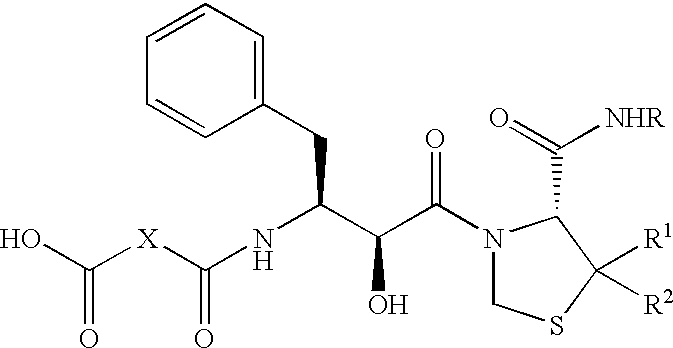
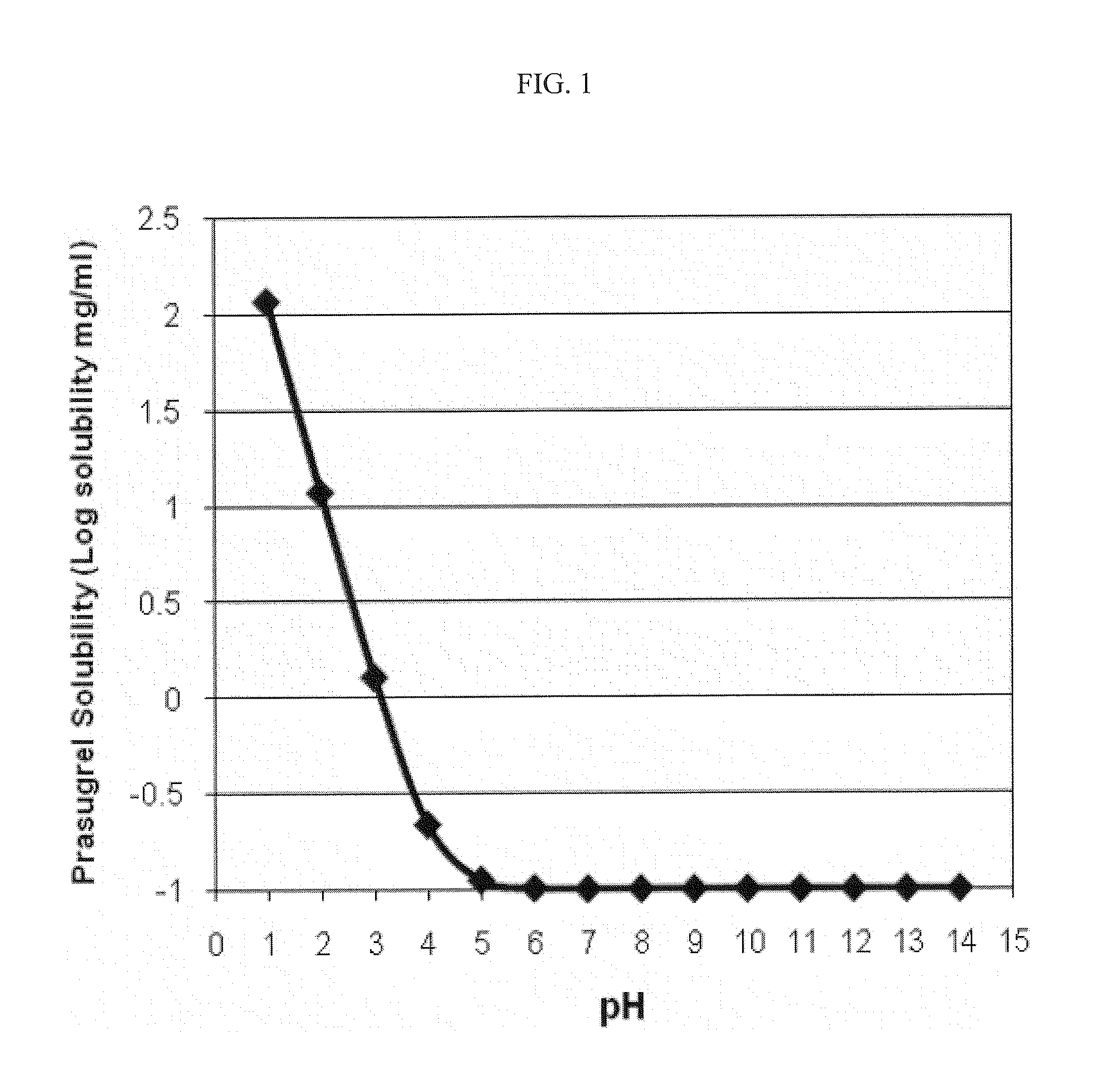
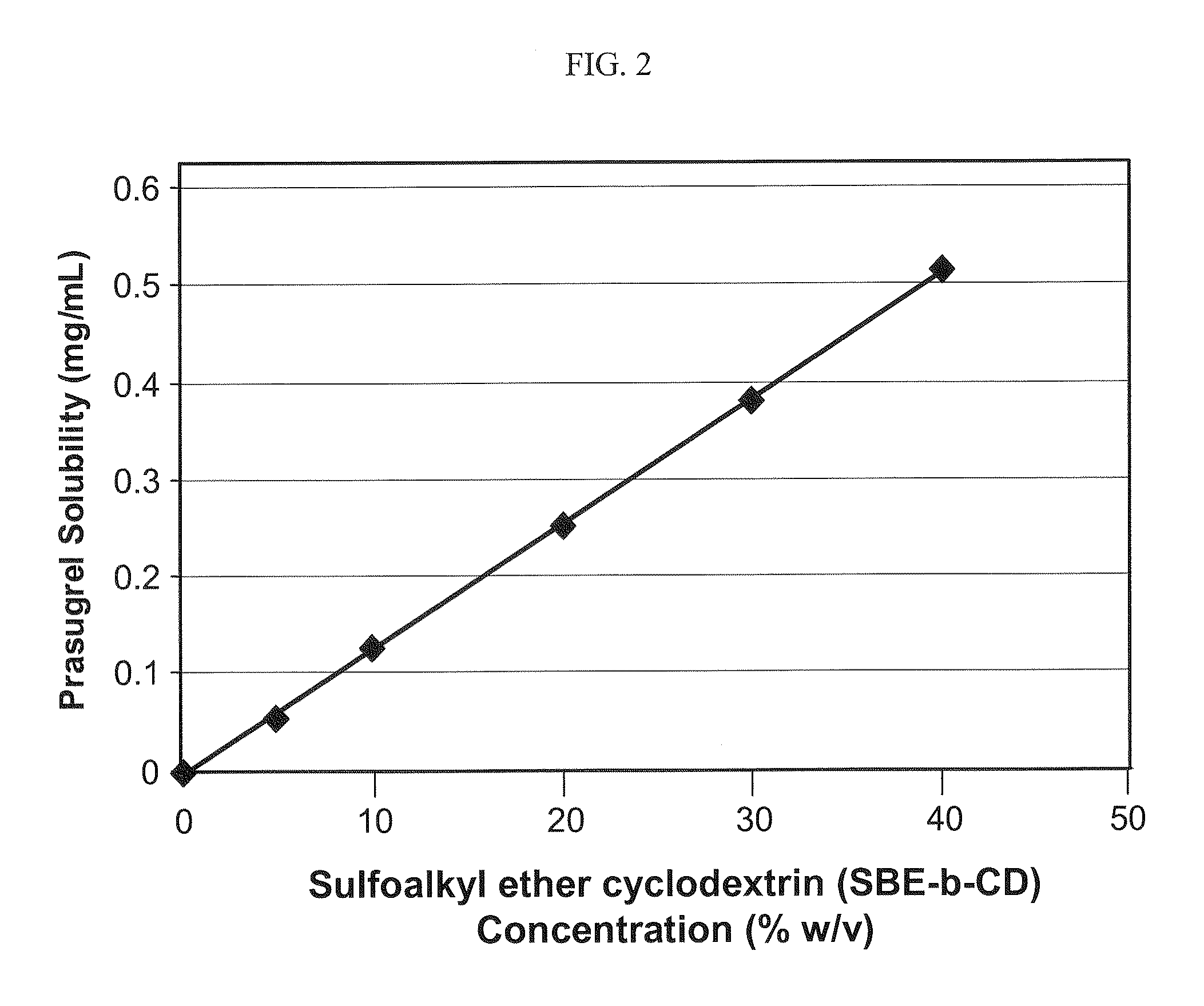
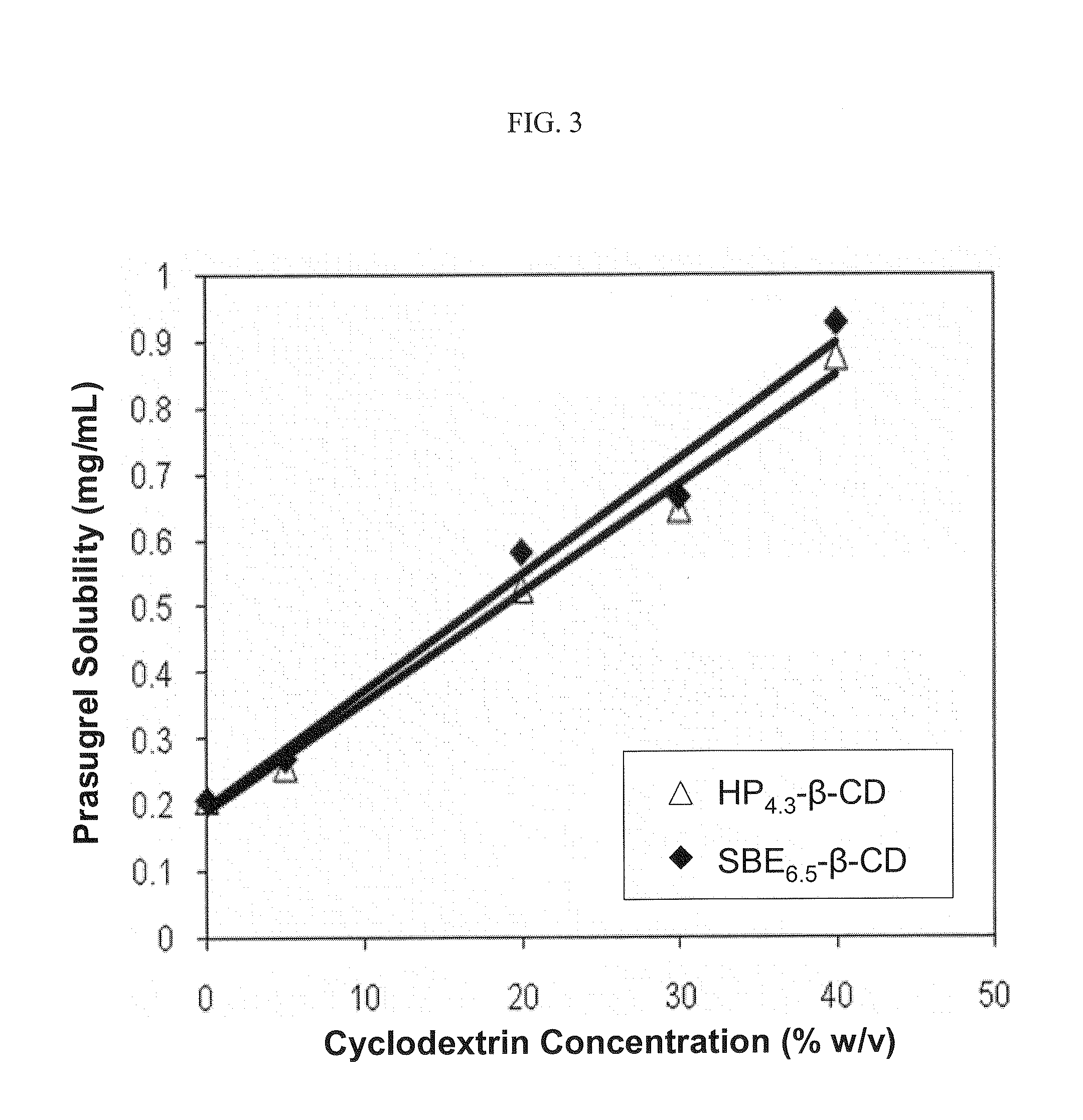
Pharmaceutical compositions comprising prasugrel and cyclodextrin derivatives and methods of making and using the same
ActiveUS8236782B2Minimize toxicology and side-effect profileTitrated more safely and/or easilyBiocidePeptide/protein ingredientsCyclodextrin DerivativesPrasugrel
The present invention is directed to pharmaceutical compositions comprising prasugrel and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMACEUTICALS INC
Multiple-agents-binding compound, production and use thereof
InactiveUS6440946B1Prevent goodConvenient treatmentBiocideAntiviralsChemical compoundCell Surface Proteins
The present invention is to provide a multiple-agents-binding compound comprising a compound having anti-HIV activity and having no affinity for cell surface protein bound together with a same or different kind of at least one compound having anti-HIV activity and having no affinity for cell surface protein, or a salt thereof, and a pharmaceutical composition for the prevention or treatment of infectious diseases of HIV or AIDS comprising said multiple-agents-binding compound.
Owner:YOSHIAKI KISO
Pharmaceutical Compositions Comprising Prasugrel and Cyclodextrin Derivatives and Methods of Making and Using the Same
ActiveUS20100291056A1Minimize toxicologyMinimize side-effect profileBiocidePeptide/protein ingredientsCyclodextrin derivativePrasugrel
The present invention is directed to pharmaceutical compositions comprising prasugrel and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMACEUTICALS INC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
InactiveUS20170274093A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Humanized Anti-ceacam5 antibody and uses thereof
InactiveUS20150125386A1Overcome tumorImprove targetingImmunoglobulins against animals/humansRadioactive preparation carriersDrug conjugationWhole body
The present invention concerns compositions and methods of use of a humanized Class III anti-CEA antibody, comprising the heavy and light amino acid sequences SEQ ID NO:1 and SEQ ID NO:2. The antibody is effective to treat CEACAM5-expressing tumors, either alone or in combination with one or more therapeutic agents. Drug conjugated Class III anti-CEA antibodies, such as SN-38 or P2PDox immunoconjugates, are particularly efficacious. Surprisingly, the antibody-drug conjugates (ADCs) exhibit high anti-cancer efficacy, while exhibiting low levels of systemic toxicity that are readily treated with standard amelioration techniques. Antibodies and / or immunoconjugates comprising the amino acid sequences SEQ ID NO:1 and SEQ ID NO:2 are surprisingly efficacious for therapy of solid tumors, even when the tumor has proven resistant to standard anti-cancer therapies.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com