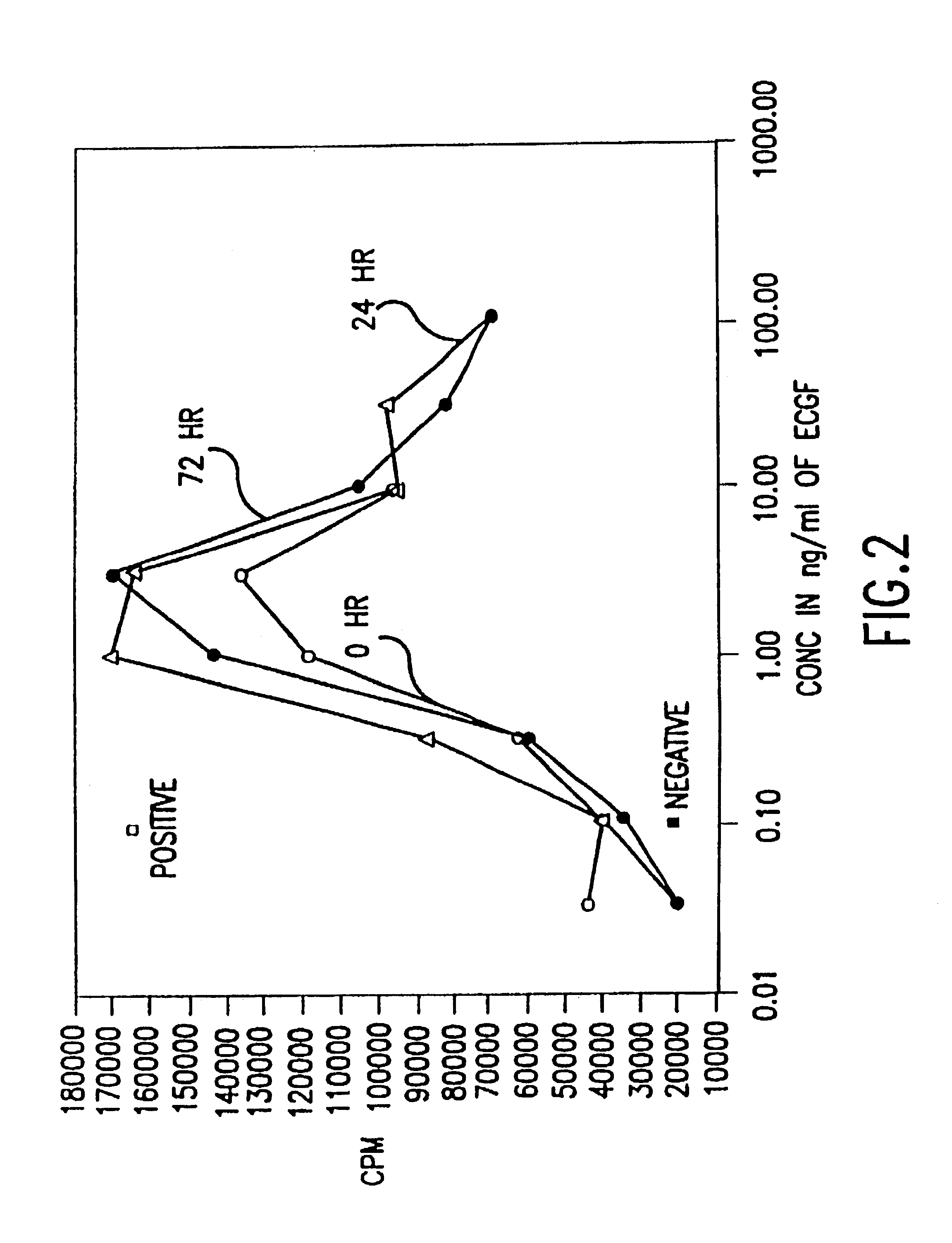
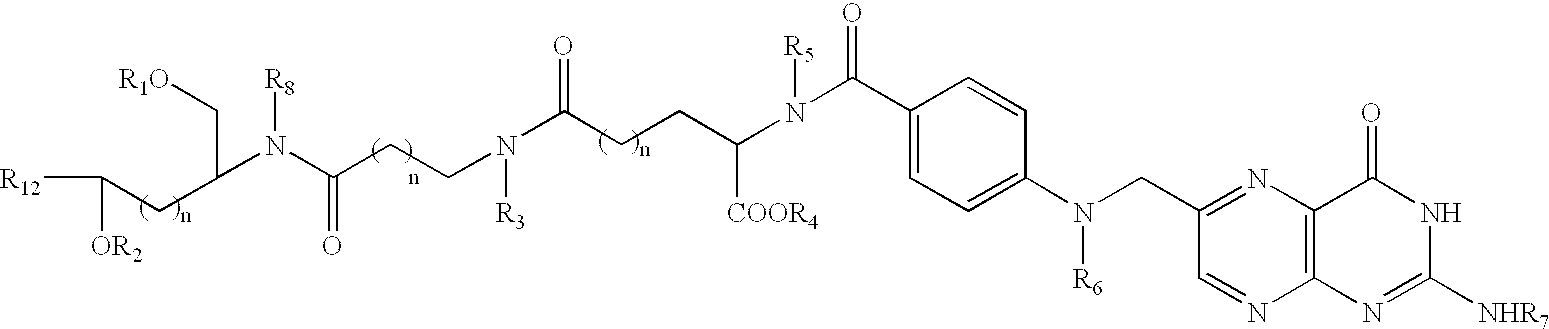
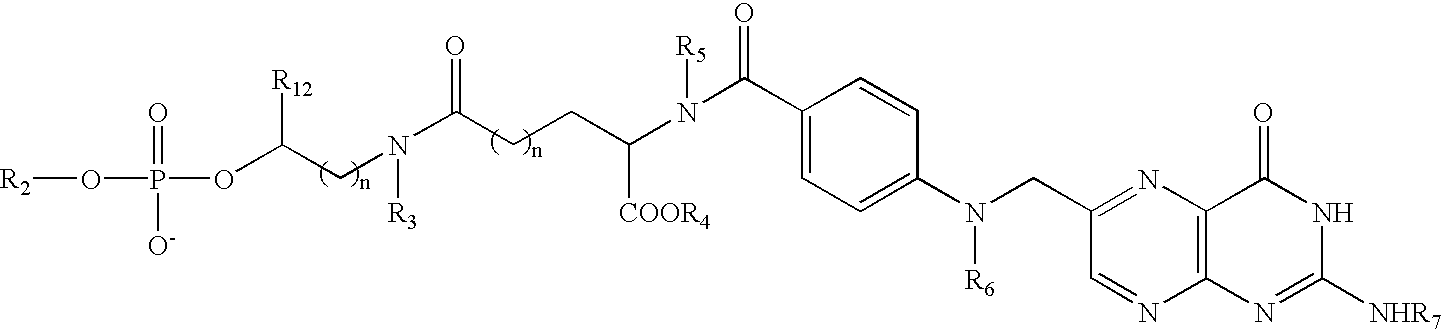
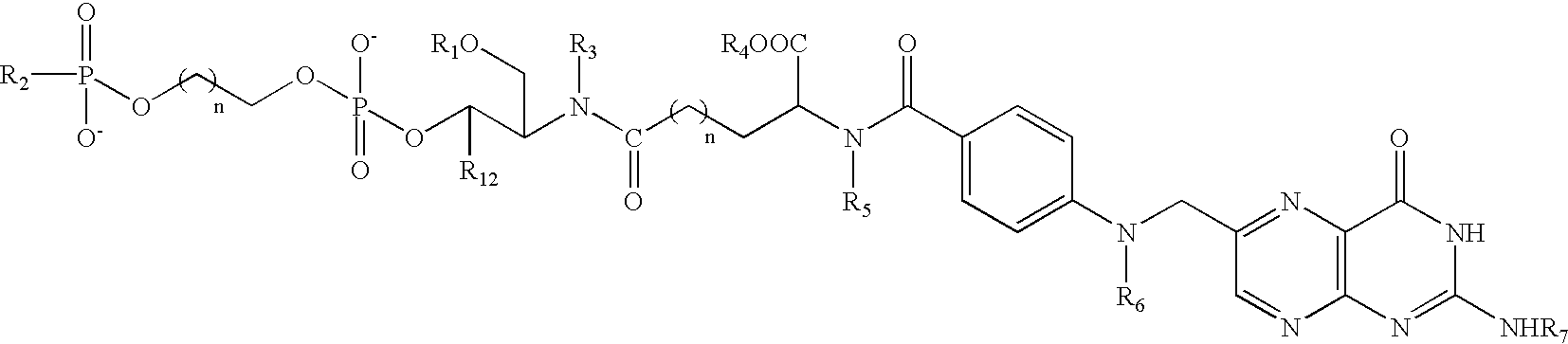
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
177results about How to "Improve drug delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
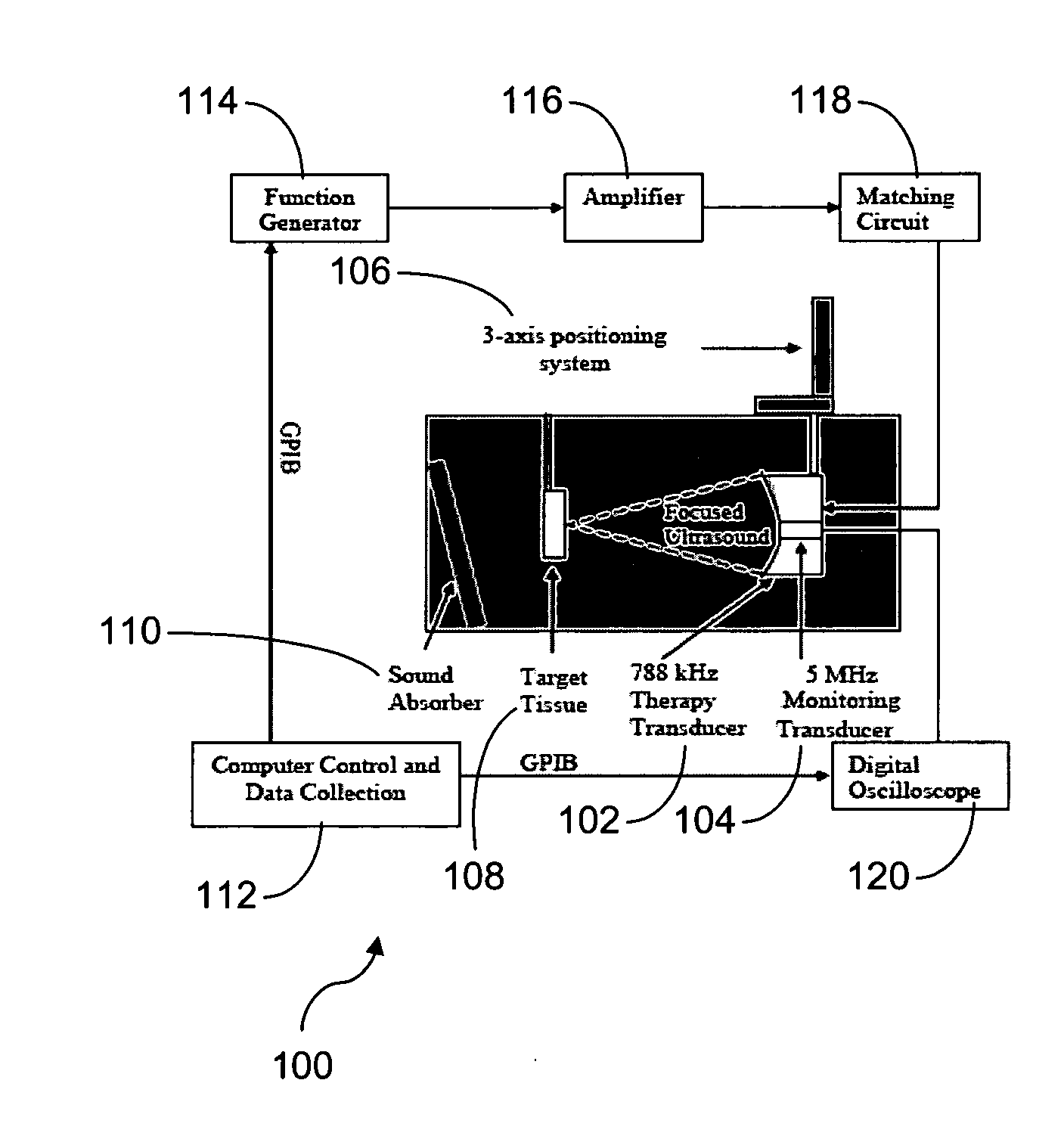
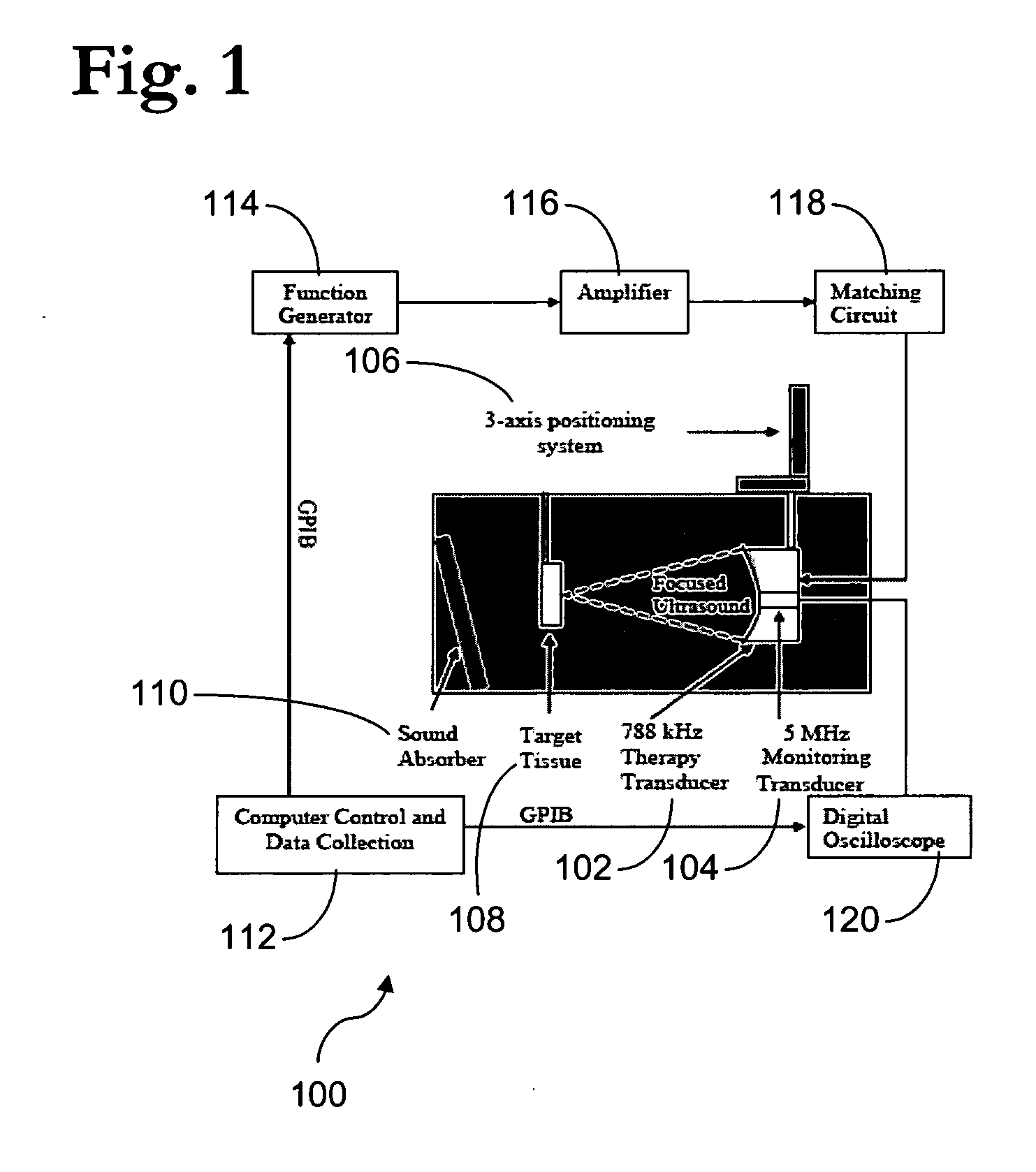
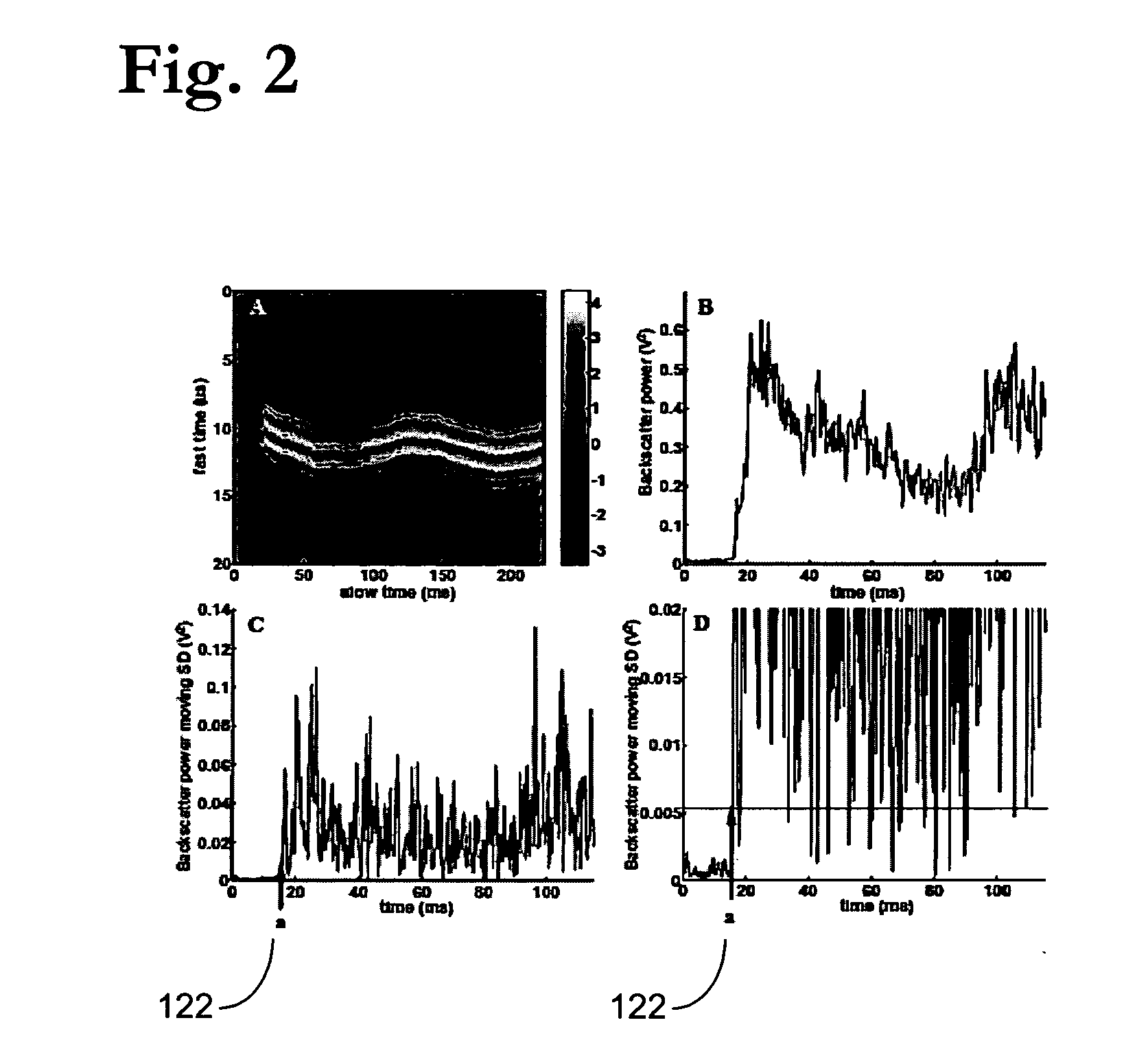
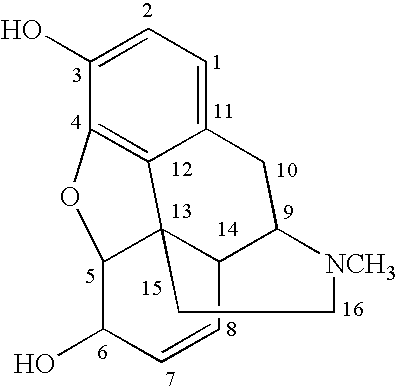
Pulsed cavitational ultrasound therapy
InactiveUS20070083120A1Enhance deliveryEnhance transportUltrasonic/sonic/infrasonic diagnosticsSurgeryBubble cloudMicrobubbles
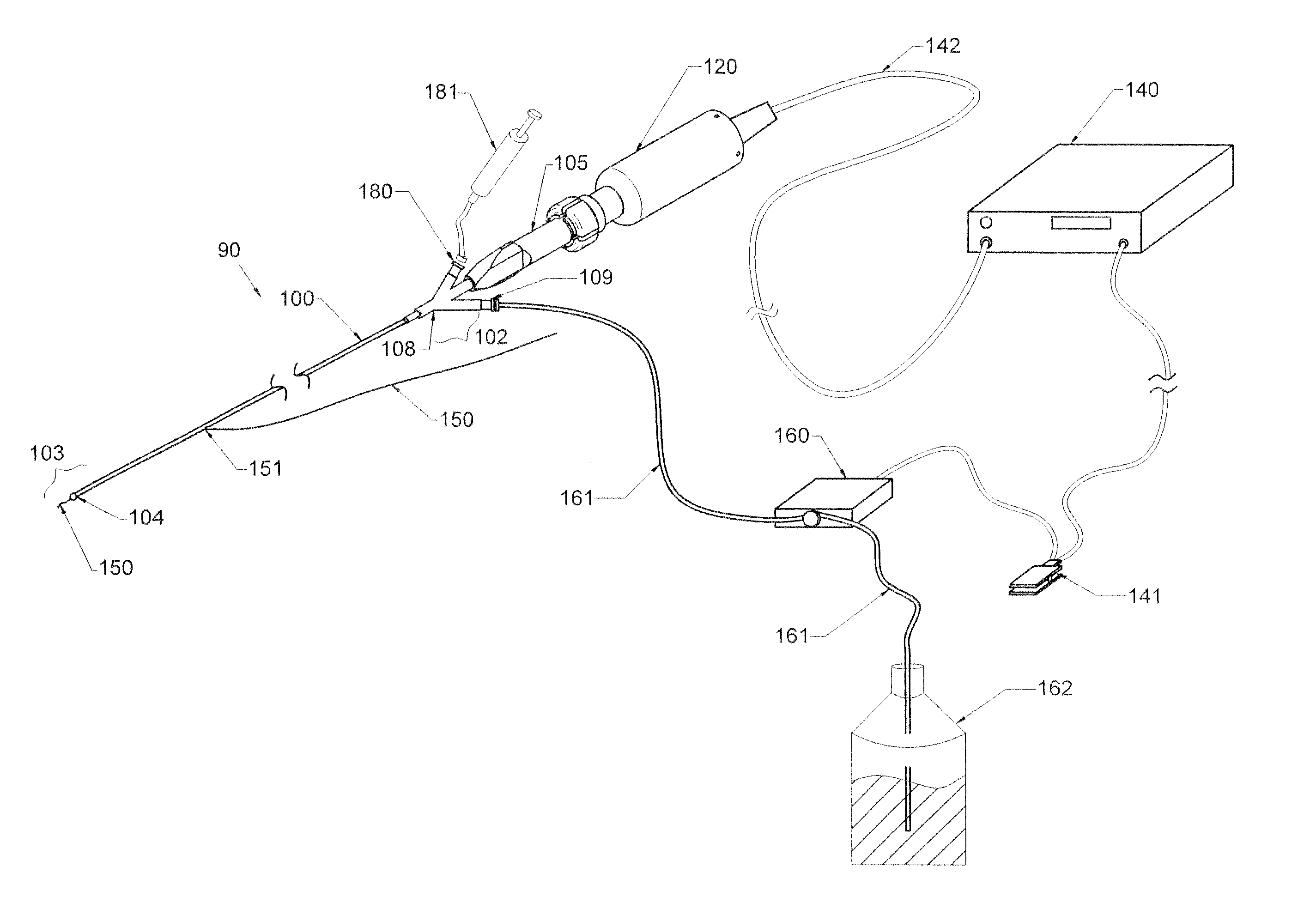
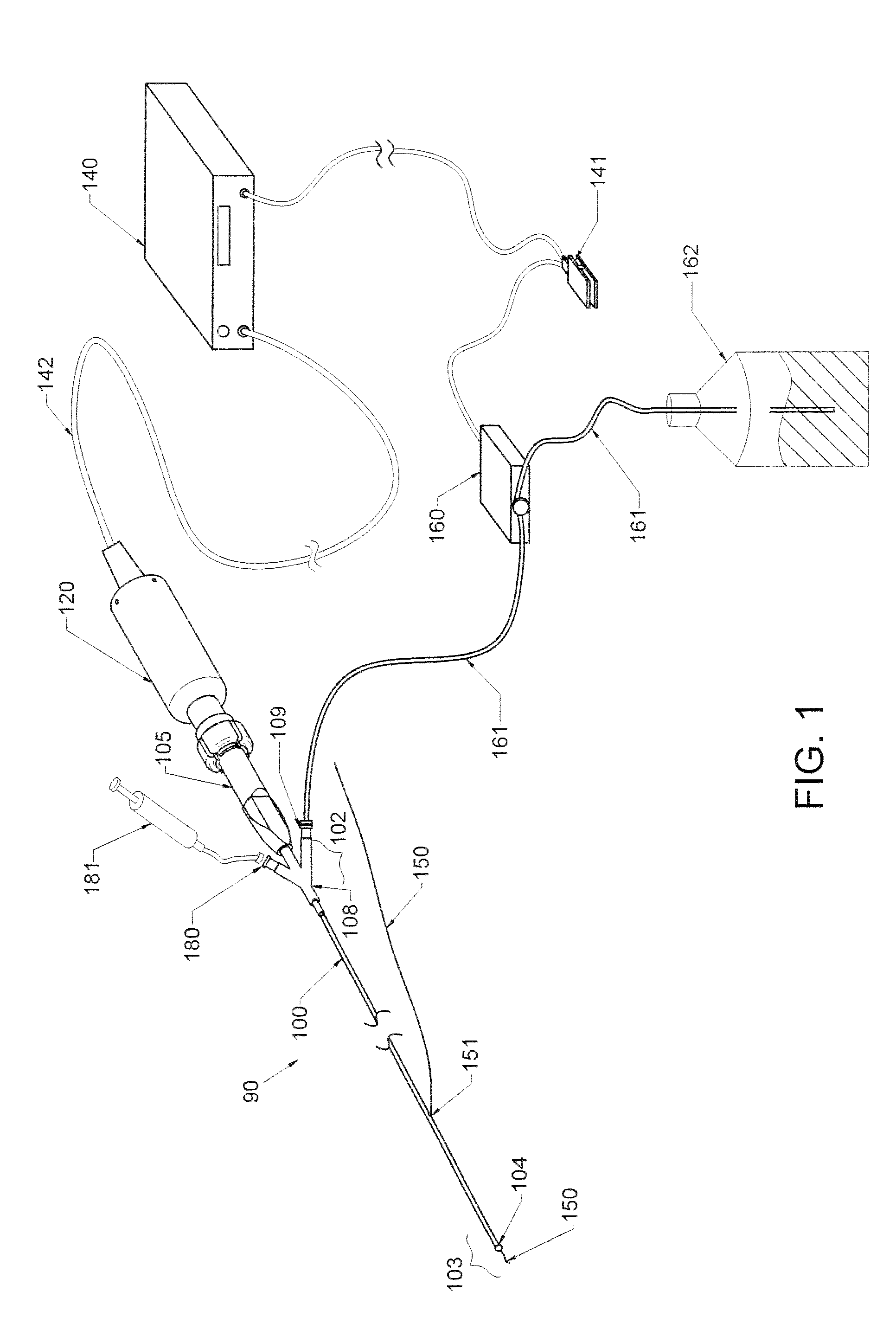
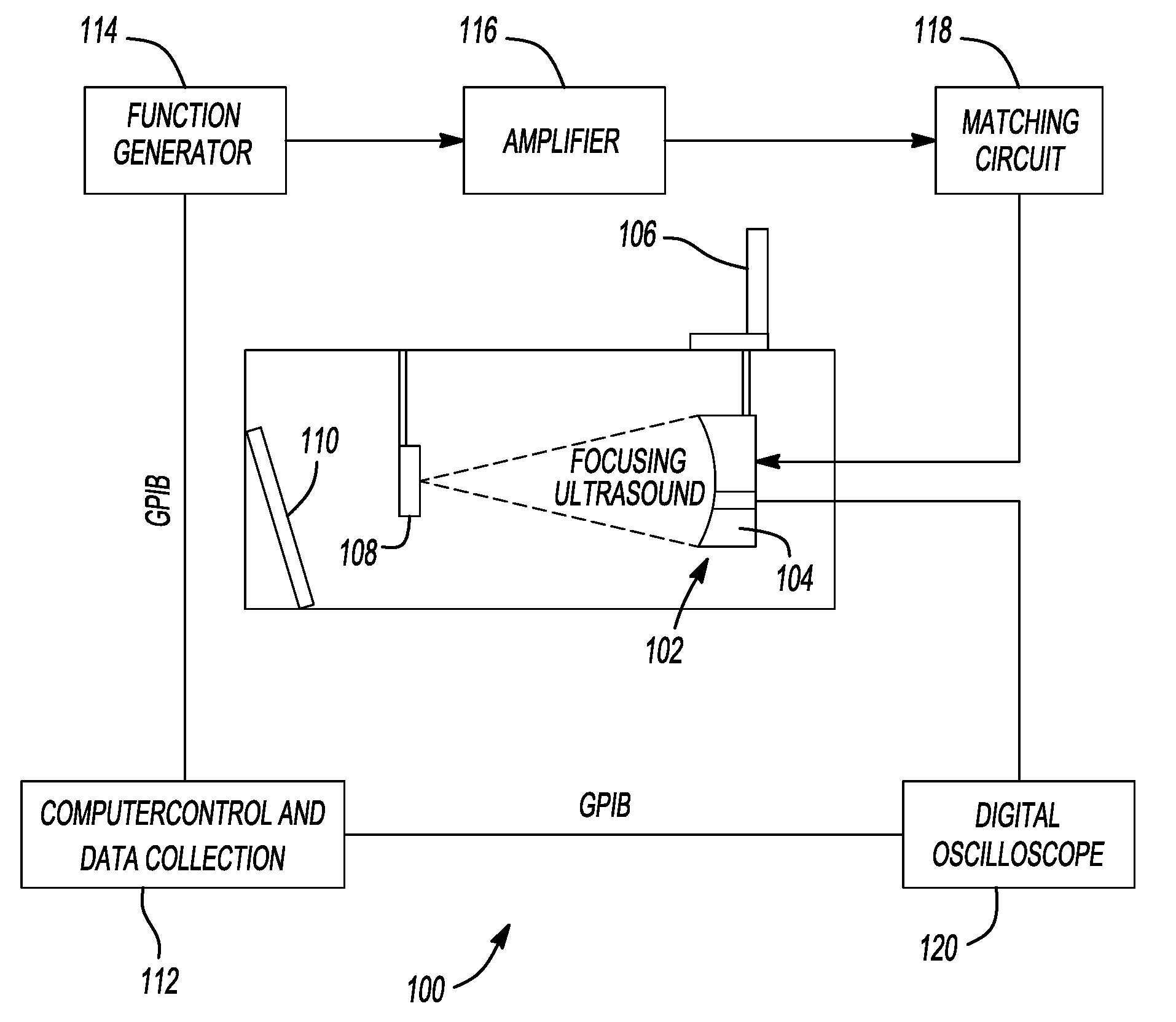
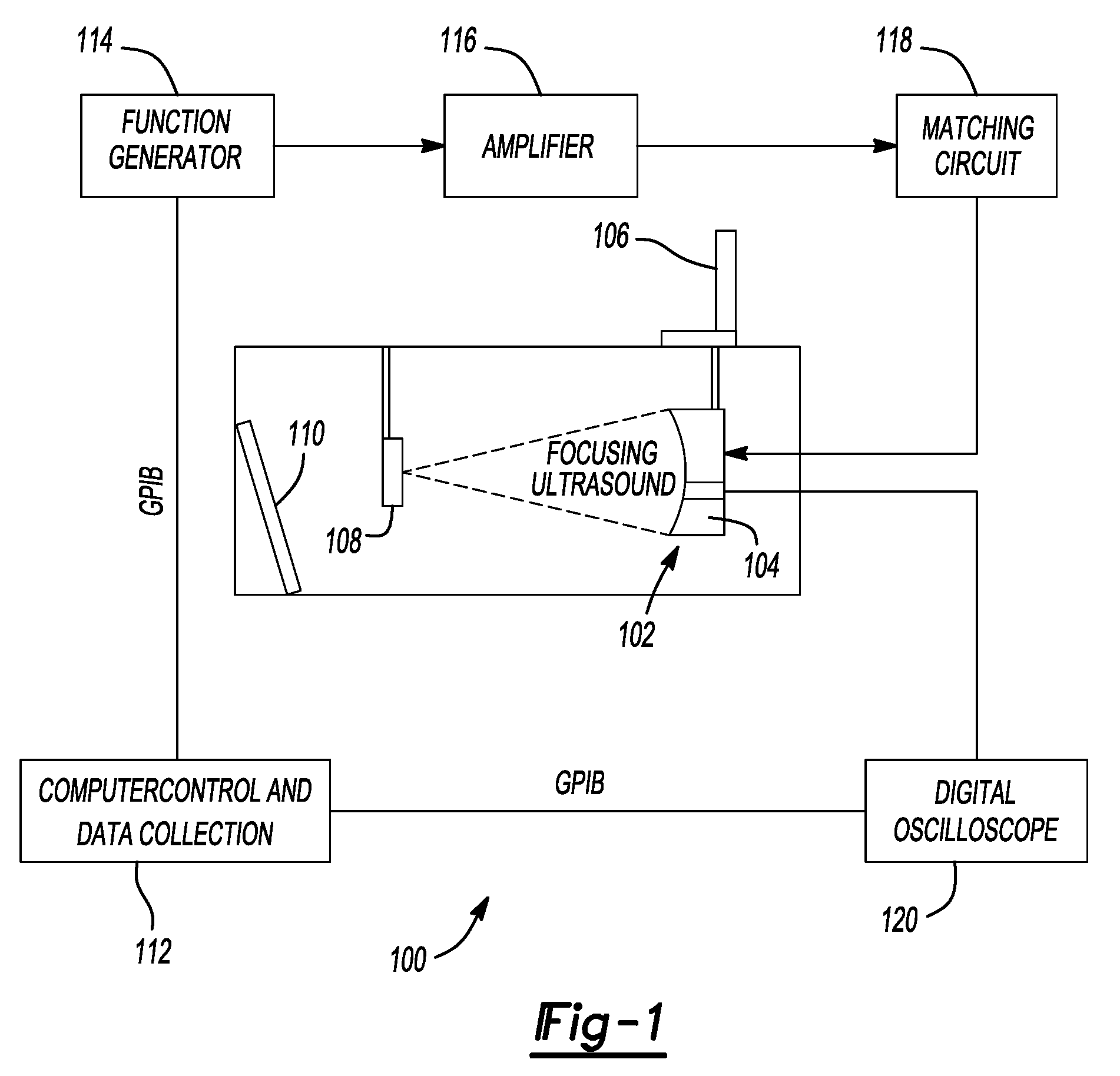
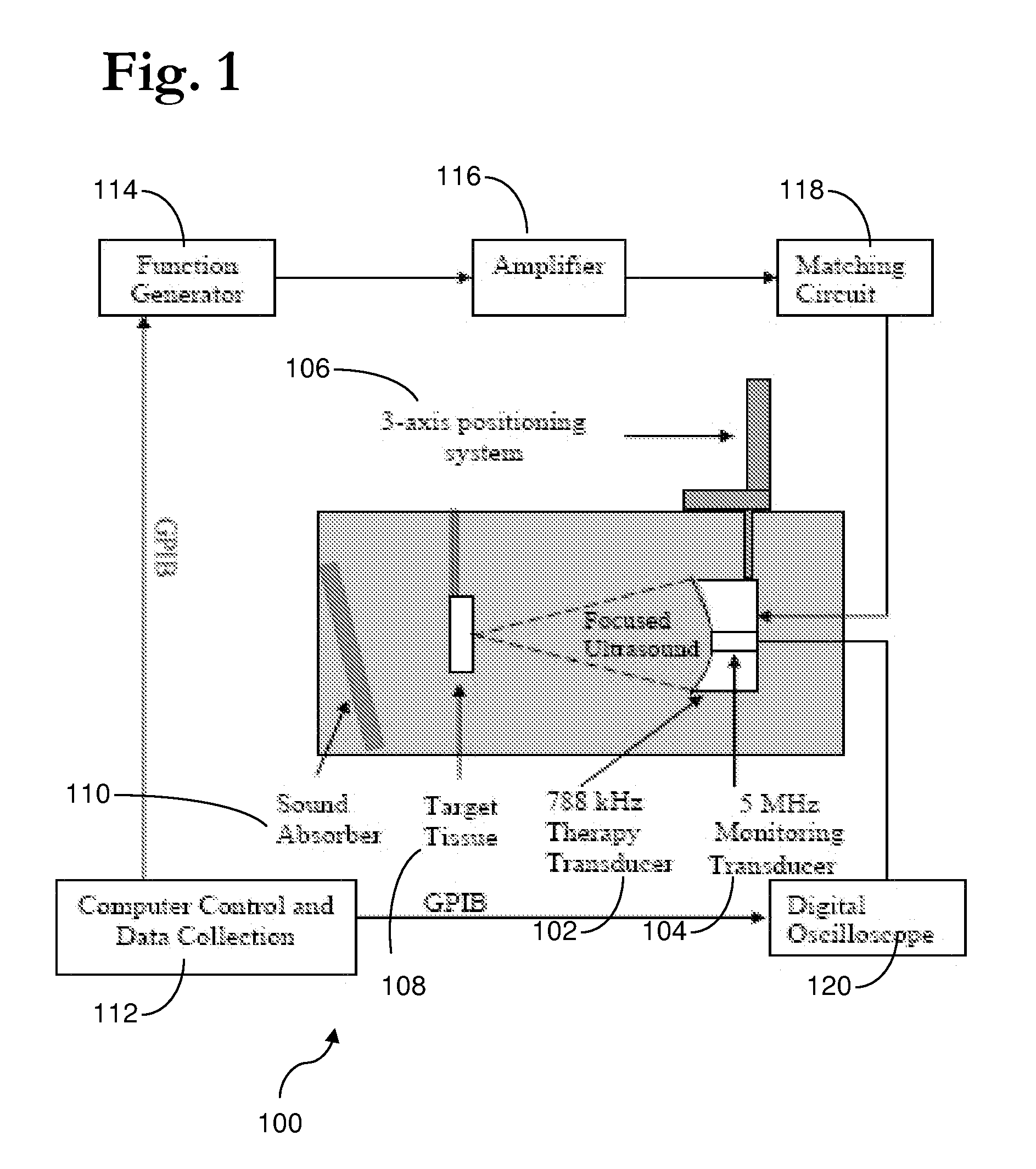
Therapy methods using pulsed cavitational ultrasound therapy can include the subprocesses of initiation, maintenance, therapy, and feedback of the histotripsy process, which involves the creation and maintenance of ensembles of microbubbles and the use of feedback in order to optimize the process based on observed spatial-temporal bubble cloud dynamics. The methods provide for the subdivision or erosion of tissue, liquification of tissue, and the enhanced delivery of therapeutic agents. Various feedback mechanisms allow variation of ultrasound parameters and provide control over the pulsed cavitational process, permitting the process to be tuned for a number of applications. Such applications can include specific tissue erosion, bulk tissue homogenization, and delivery of therapeutic agents across barriers.
Owner:THE RGT OF THE UNIV OF MICHIGAN
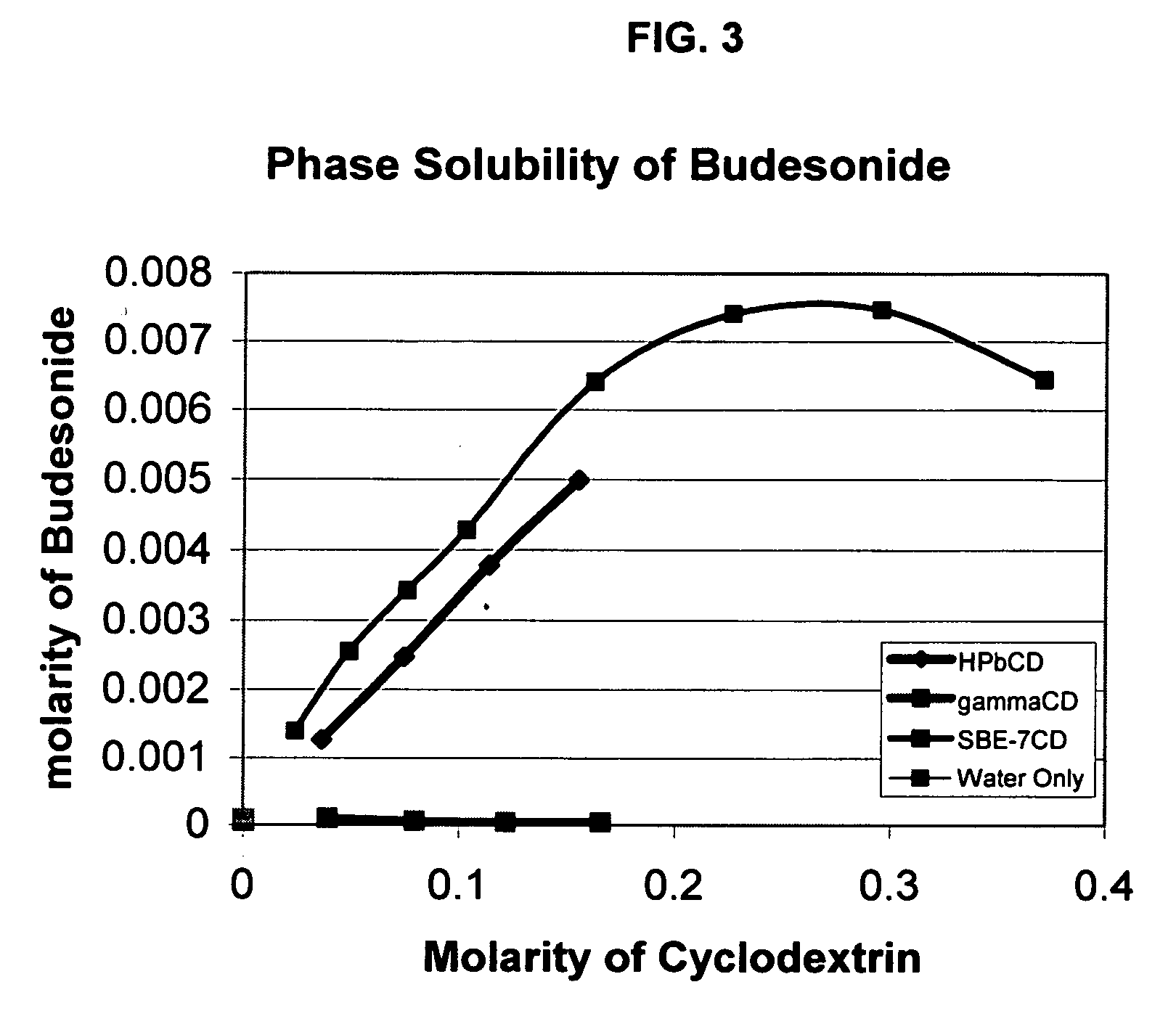
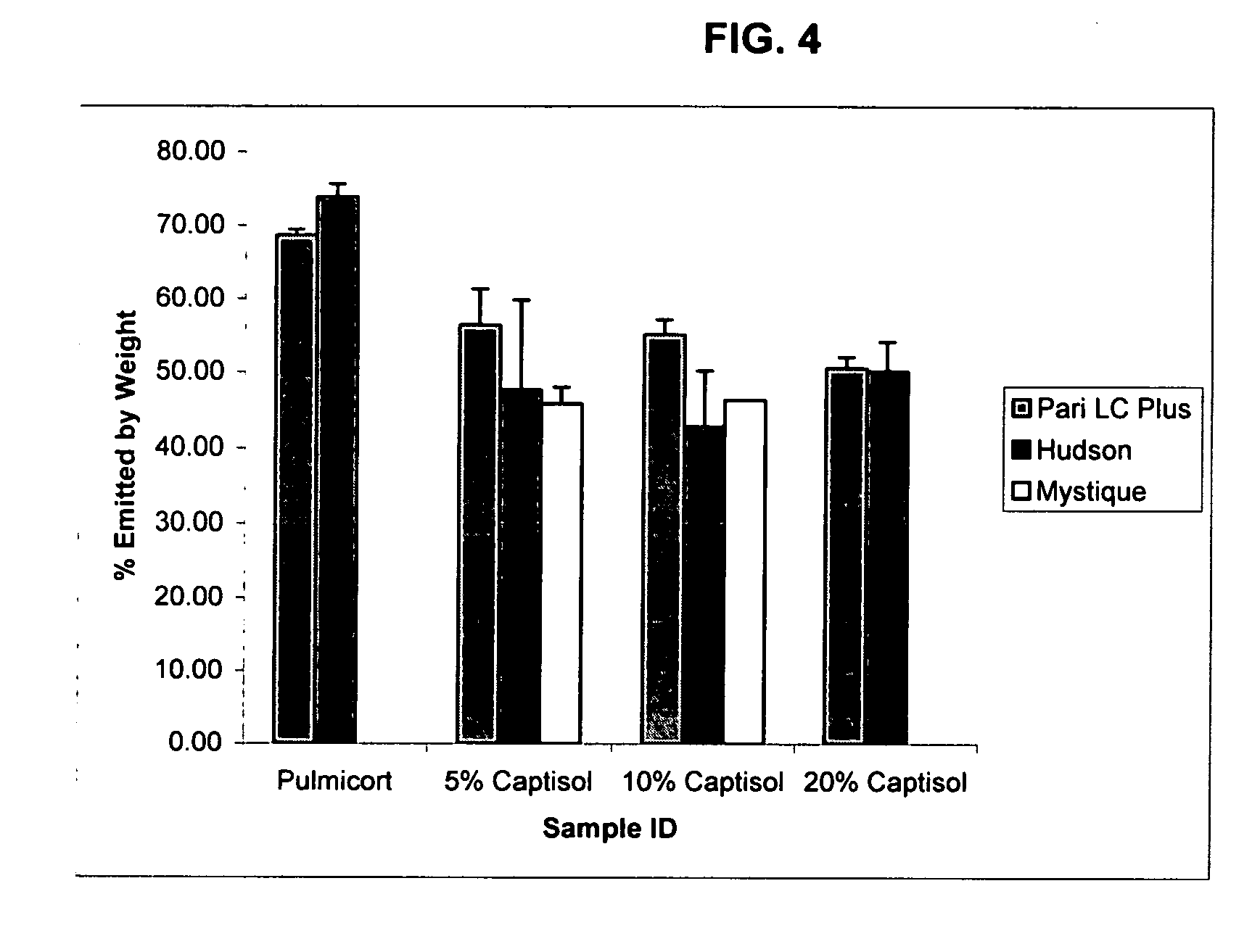
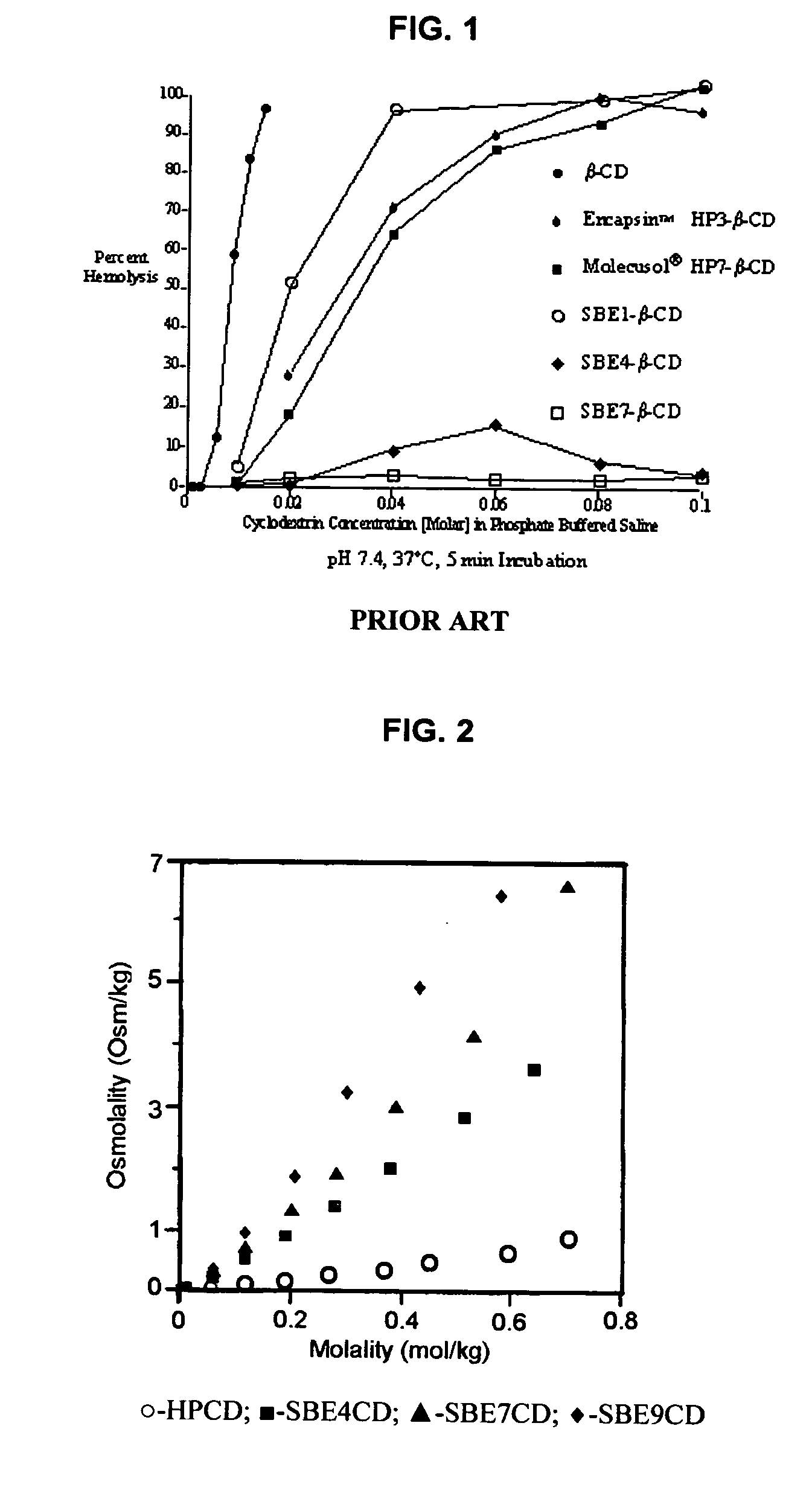
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
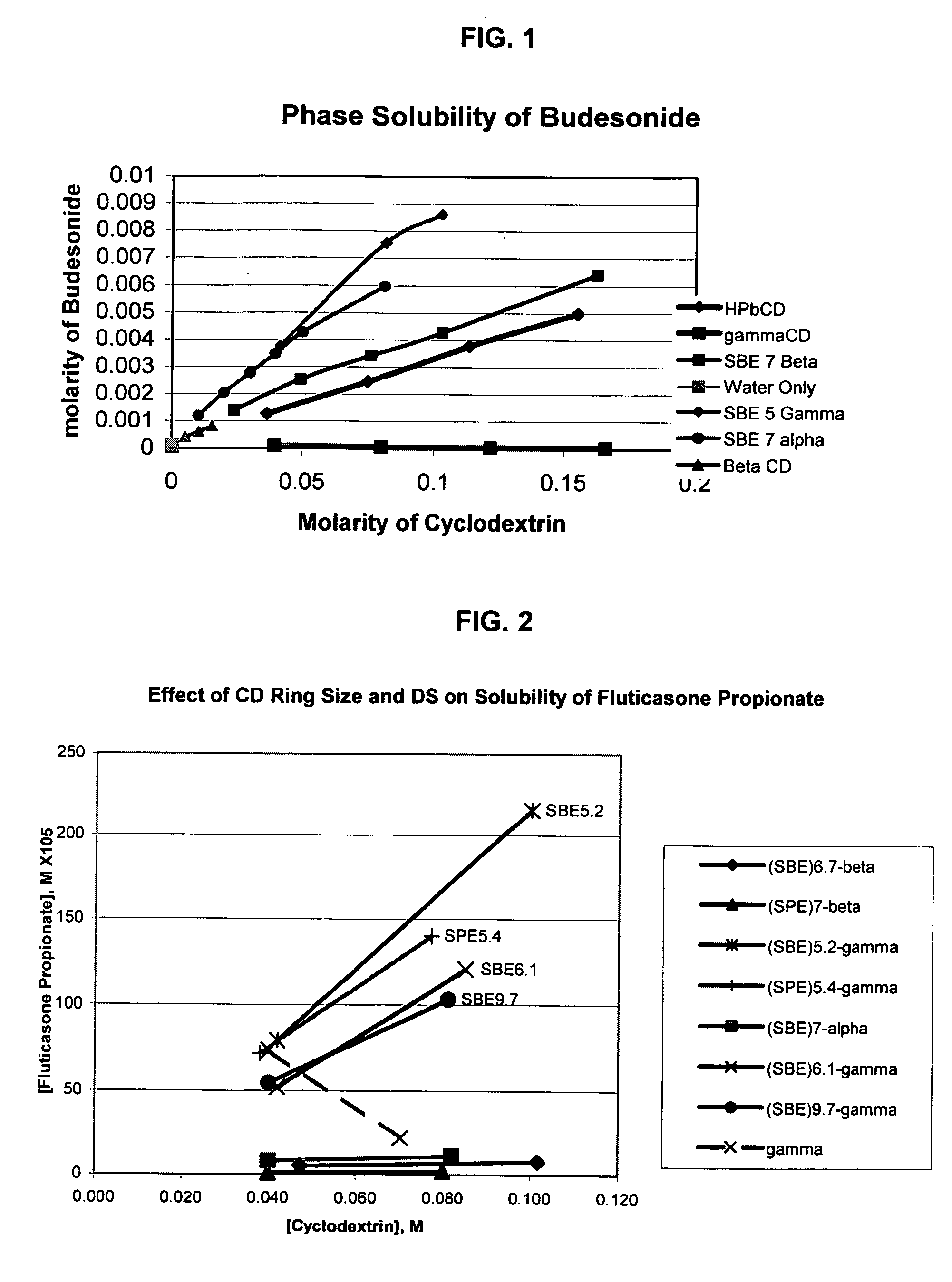
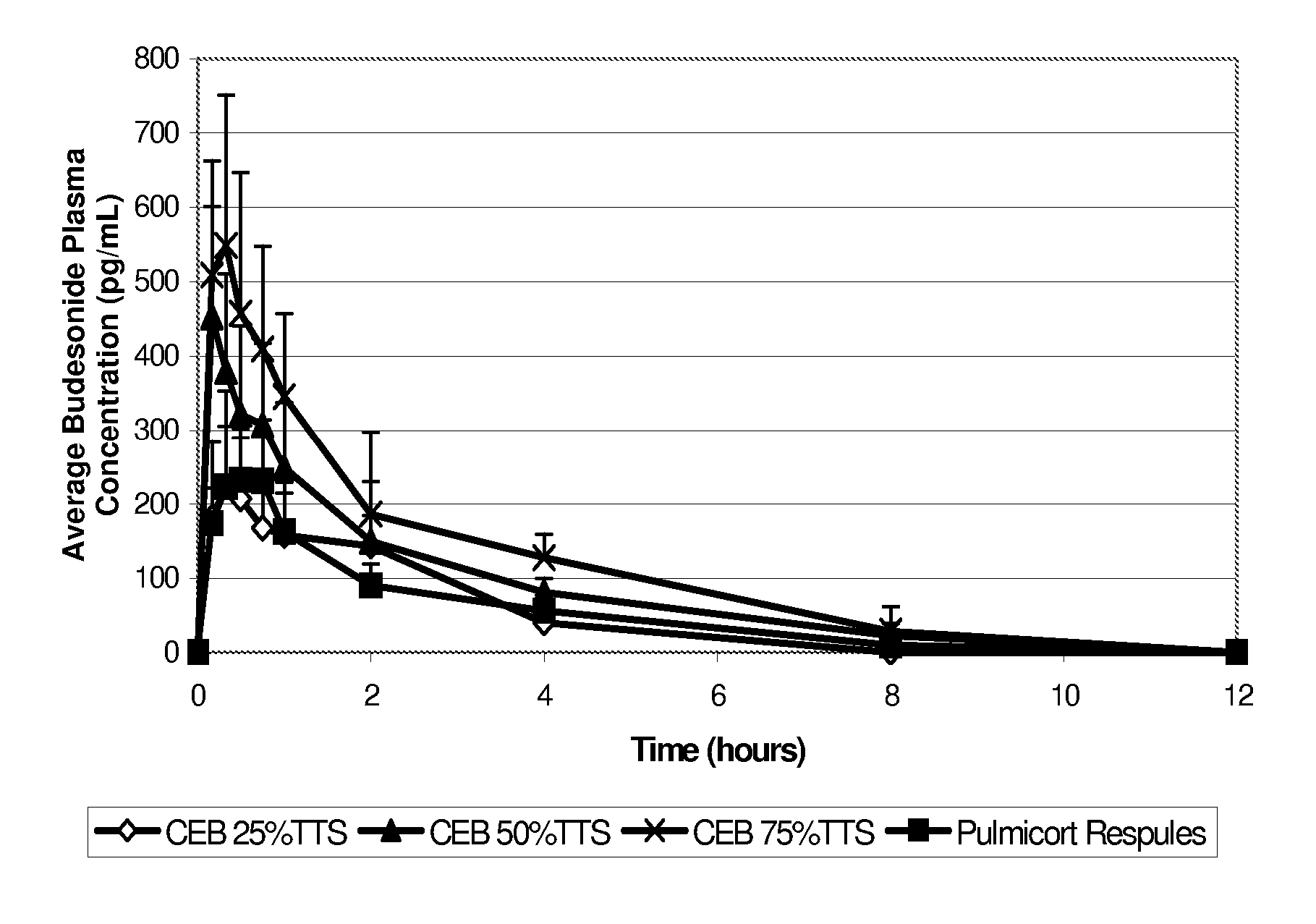
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
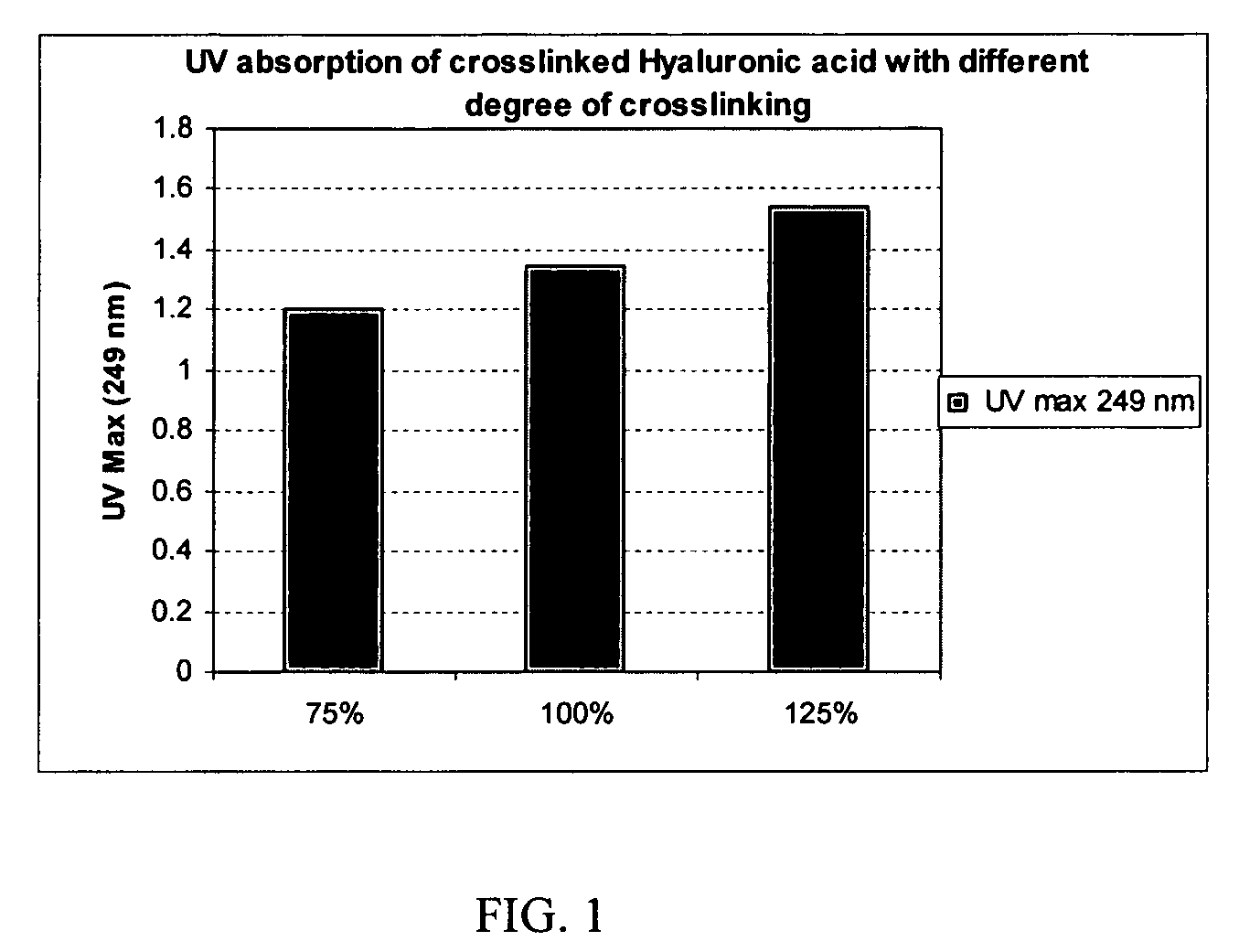
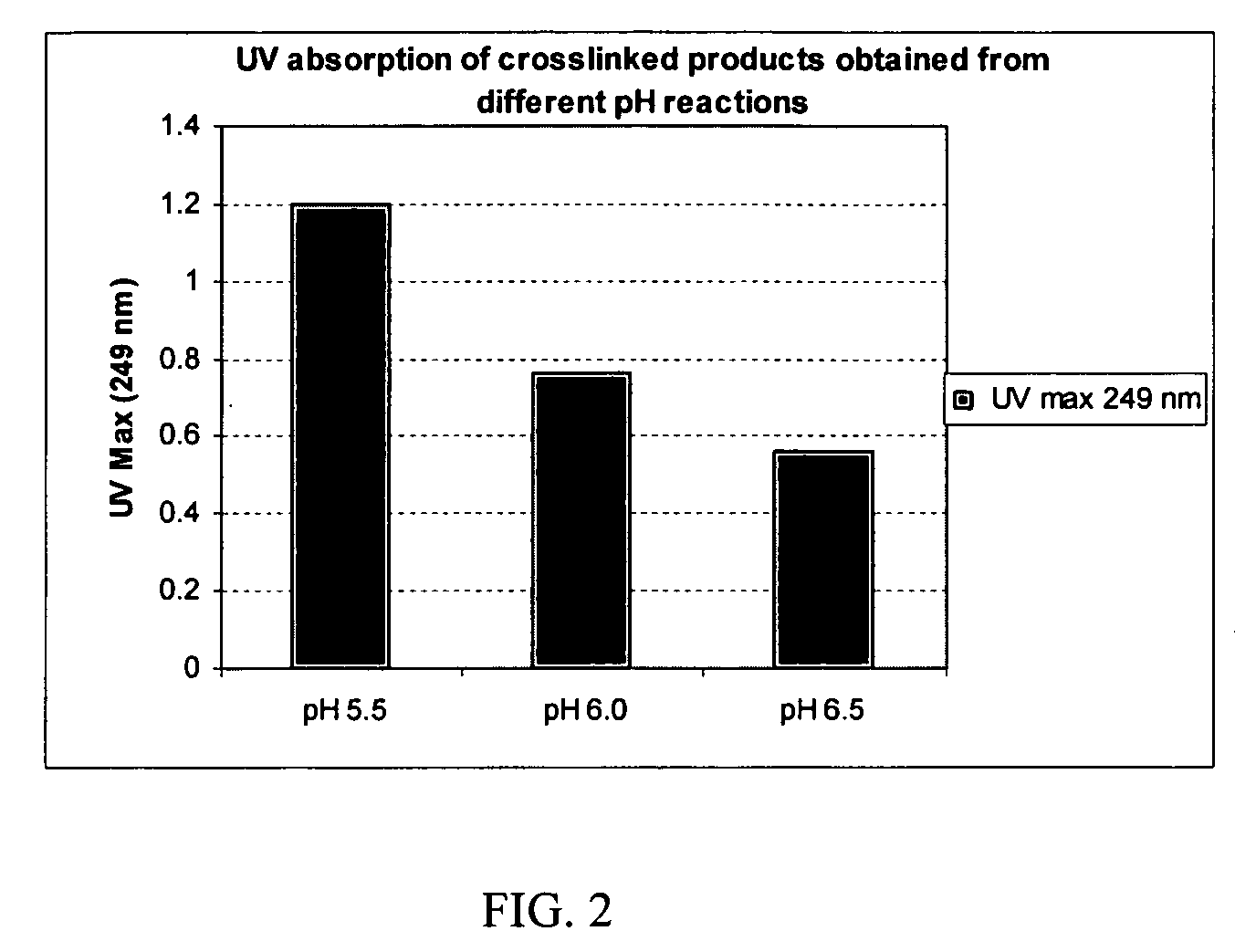
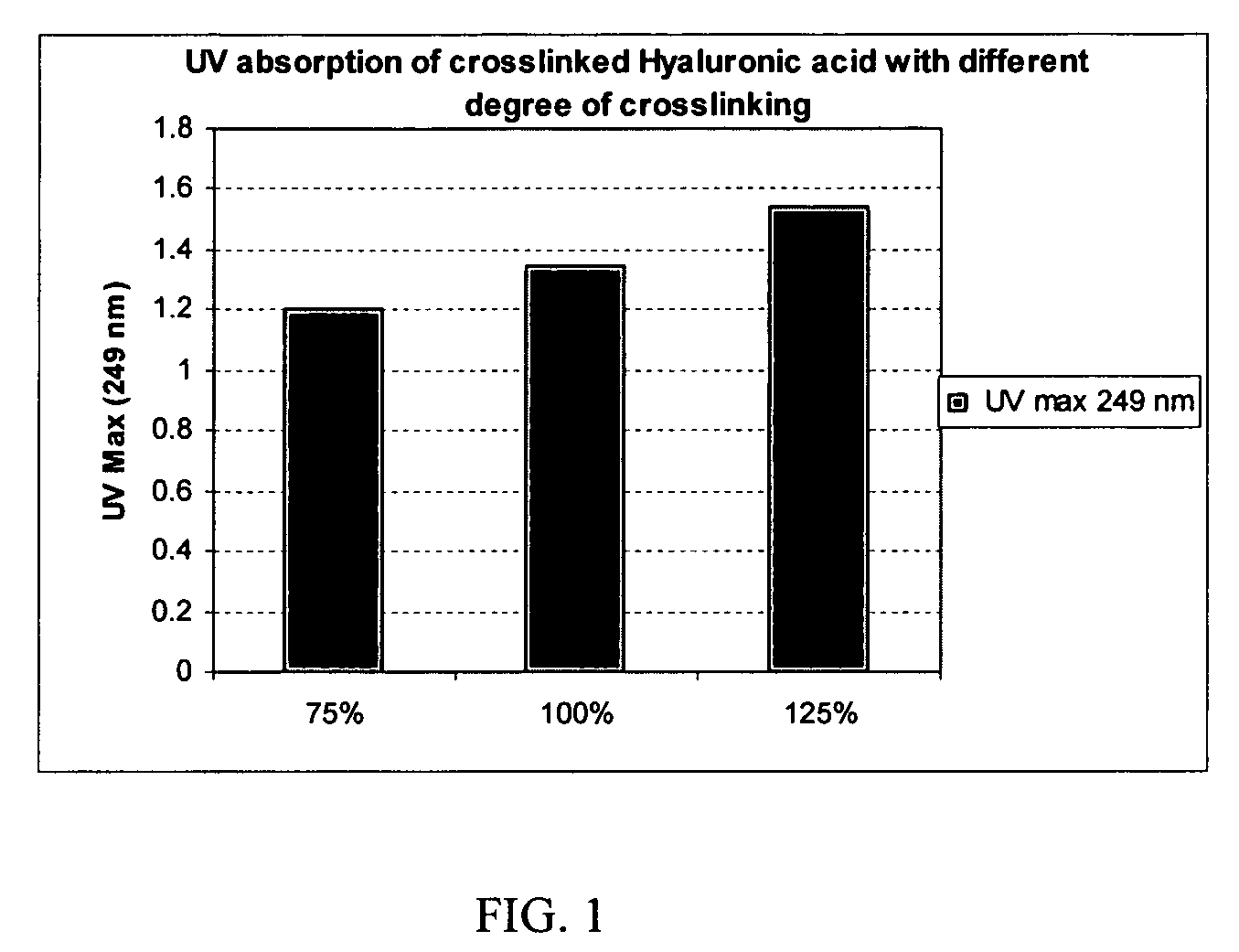
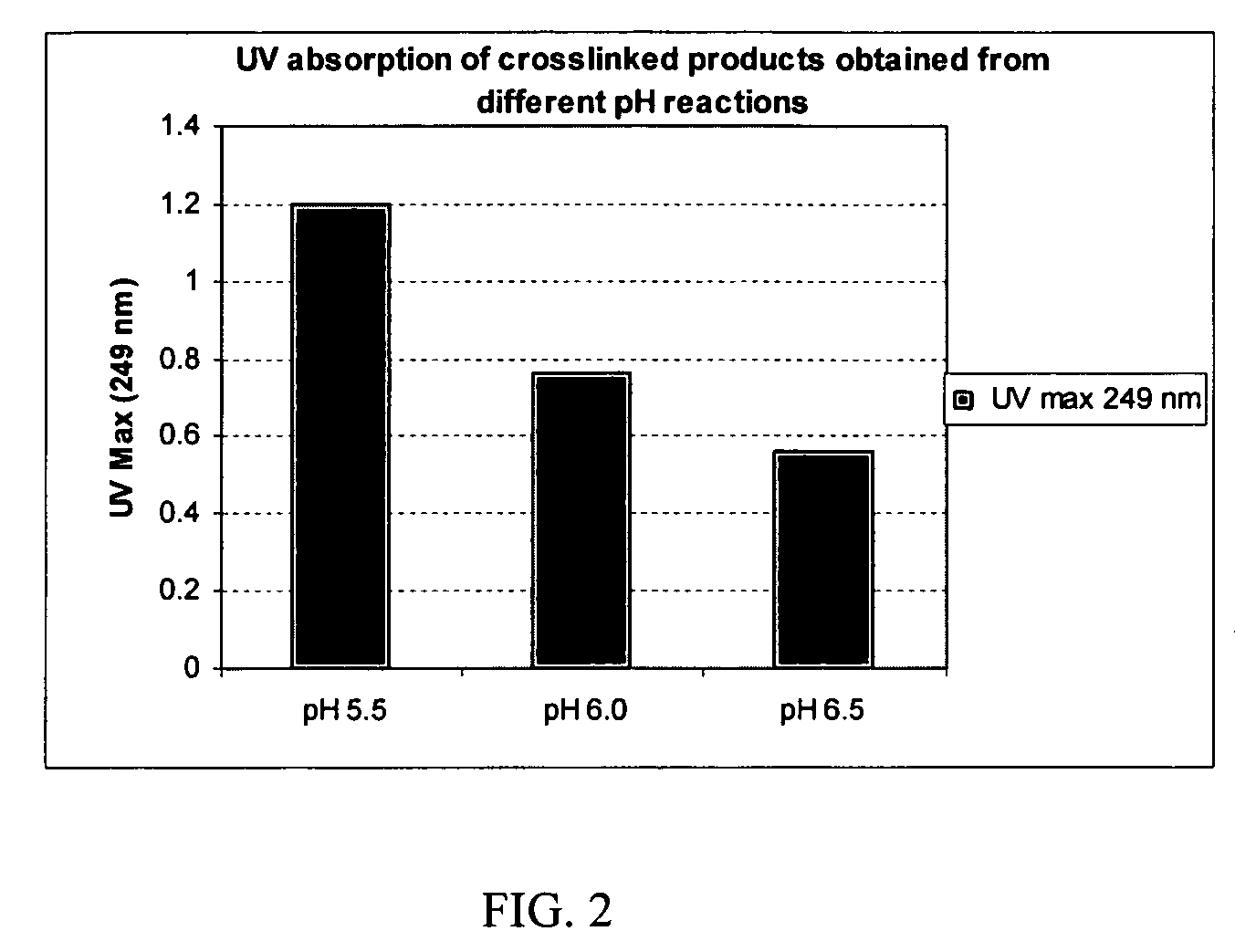
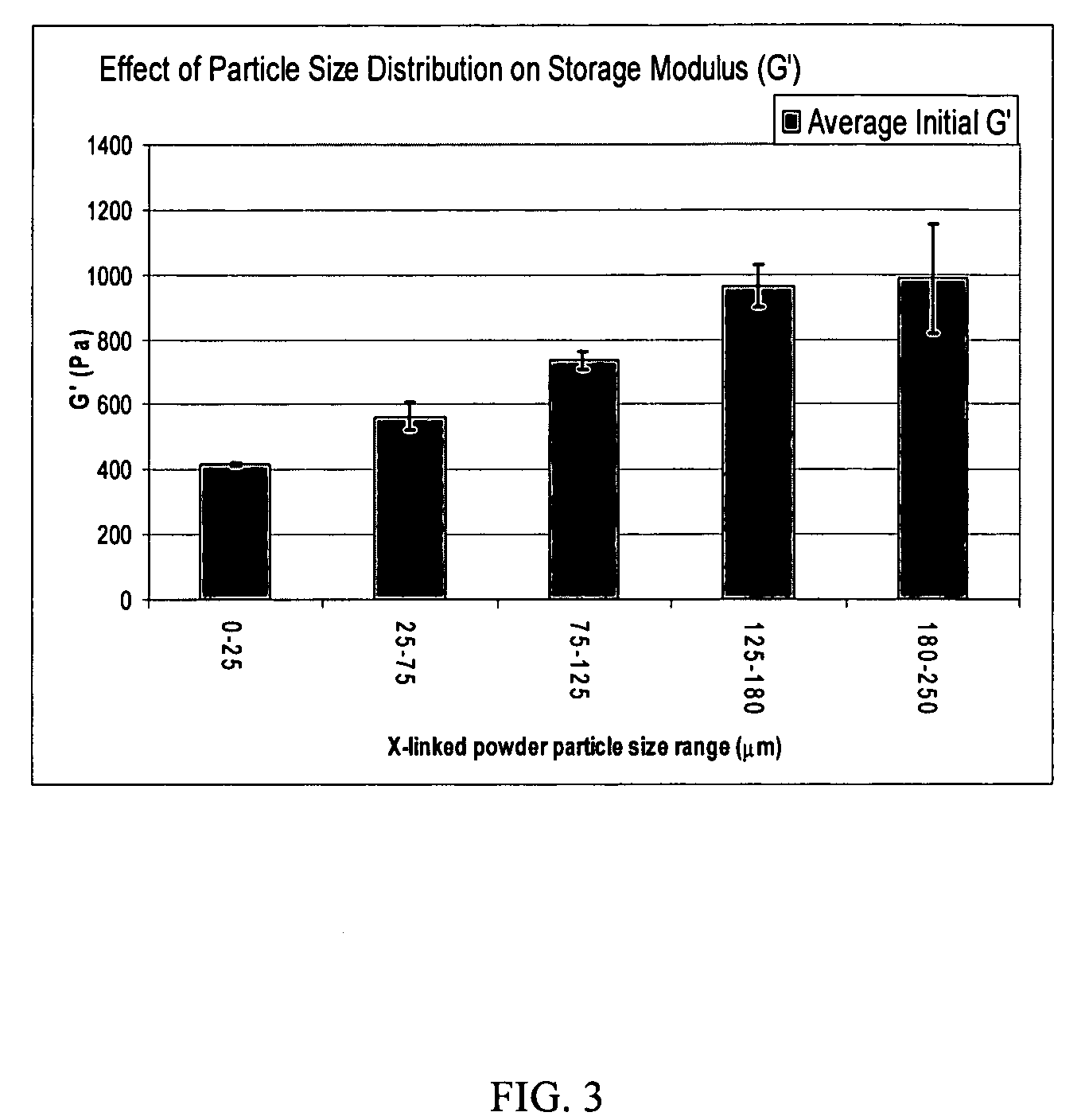
Crosslinked hyaluronic acid compositions for tissue augmentation
ActiveUS20050136122A1Improve drug deliveryReduce frequencyAntibacterial agentsBiocideAqueous solutionAverage diameter
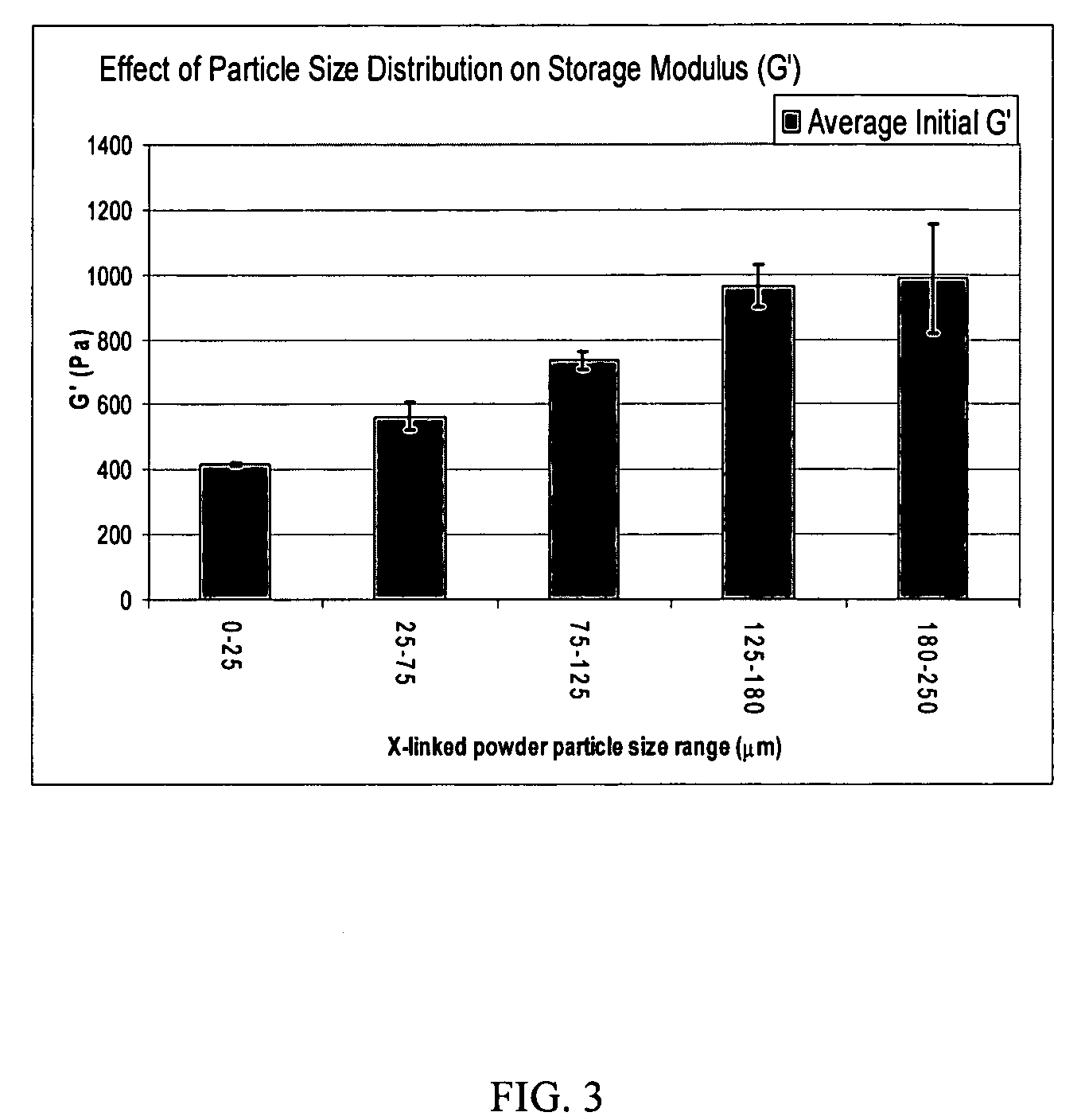
A hyaluronic acid (HA) composition includes crosslinked, water-insoluble, hydrated HA gel particles. The HA includes crosslinks represented by the following structural formula: HA—U—R2—U—HA The variables are defined herein. A method of augmenting tissue in a subject includes inserting a needle into a subject at a location in the subject that is in need of tissue augmentation, wherein the needle is coupled to a syringe loaded with the HA composition, and applying force to the syringe, to deliver the HA composition into the subject. A method of preparing the HA composition, includes forming water-insoluble, dehydrated crosslinked HA particles, separating the water-insoluble, dehydrated particles by average diameter, selecting a subset of particles by average diameter, and hydrating the subset of dehydrated particles with a physiologically compatible aqueous solution. Another method of preparing the crosslinked HA composition includes crosslinking a precursor of the crosslinked HA with a biscarbodiimide in the presence of a pH buffer and dehydrating the crosslinked HA. Also included is a method of augmenting tissue in a subject that is in need of tissue augmentation. A method of stabilizing crosslinked HA includes hydrating water-insoluble, dehydrated crosslinked HA with a physiologically compatible aqueous solution that includes a local anesthetic, wherein the value of storage modulus G′ for the stabilized composition is at least about 110% of the value of G′ for a non-stabilized composition,. Also included is the stabilized HA composition.
Owner:ANIKA THERAPEUTICS INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
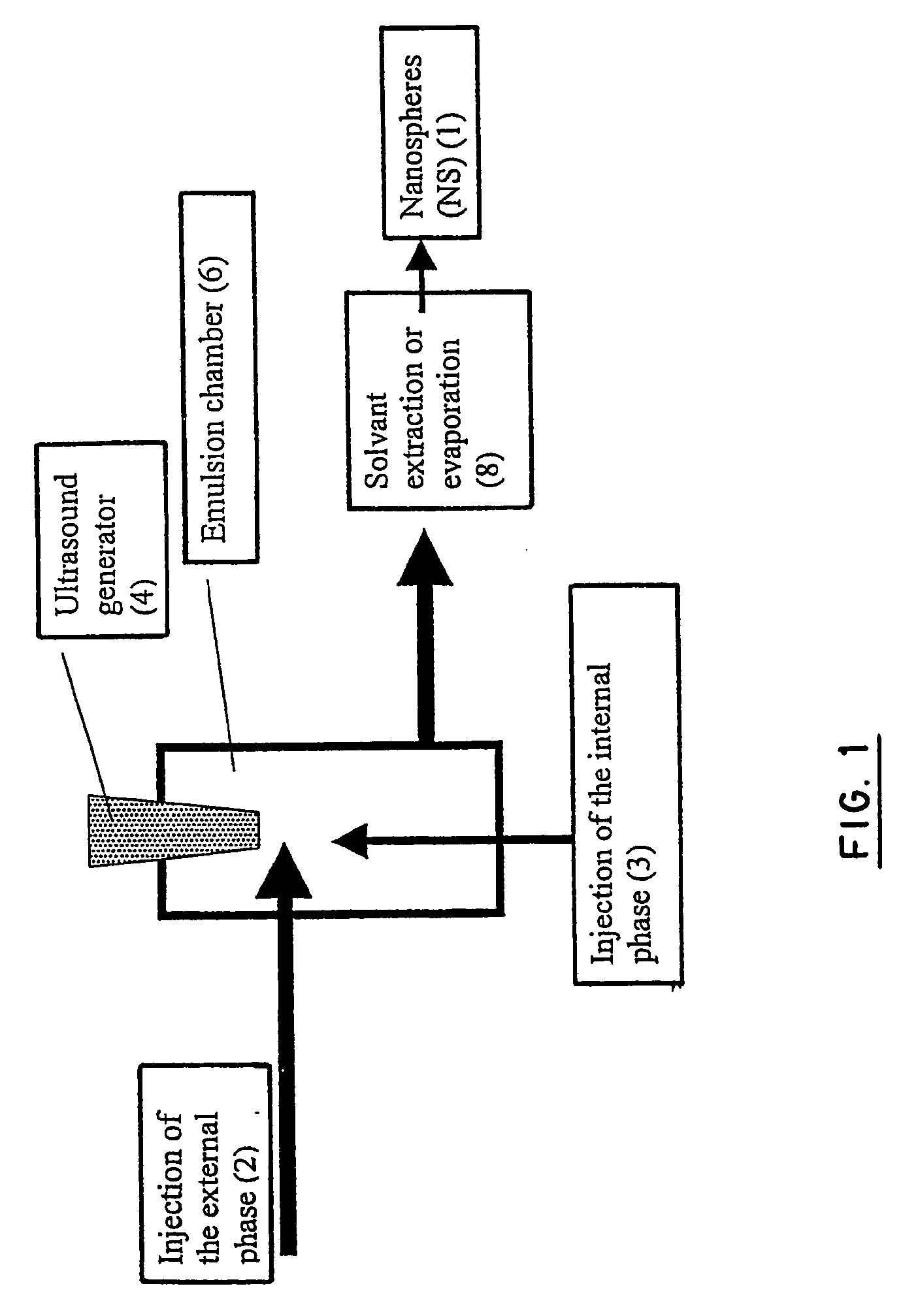
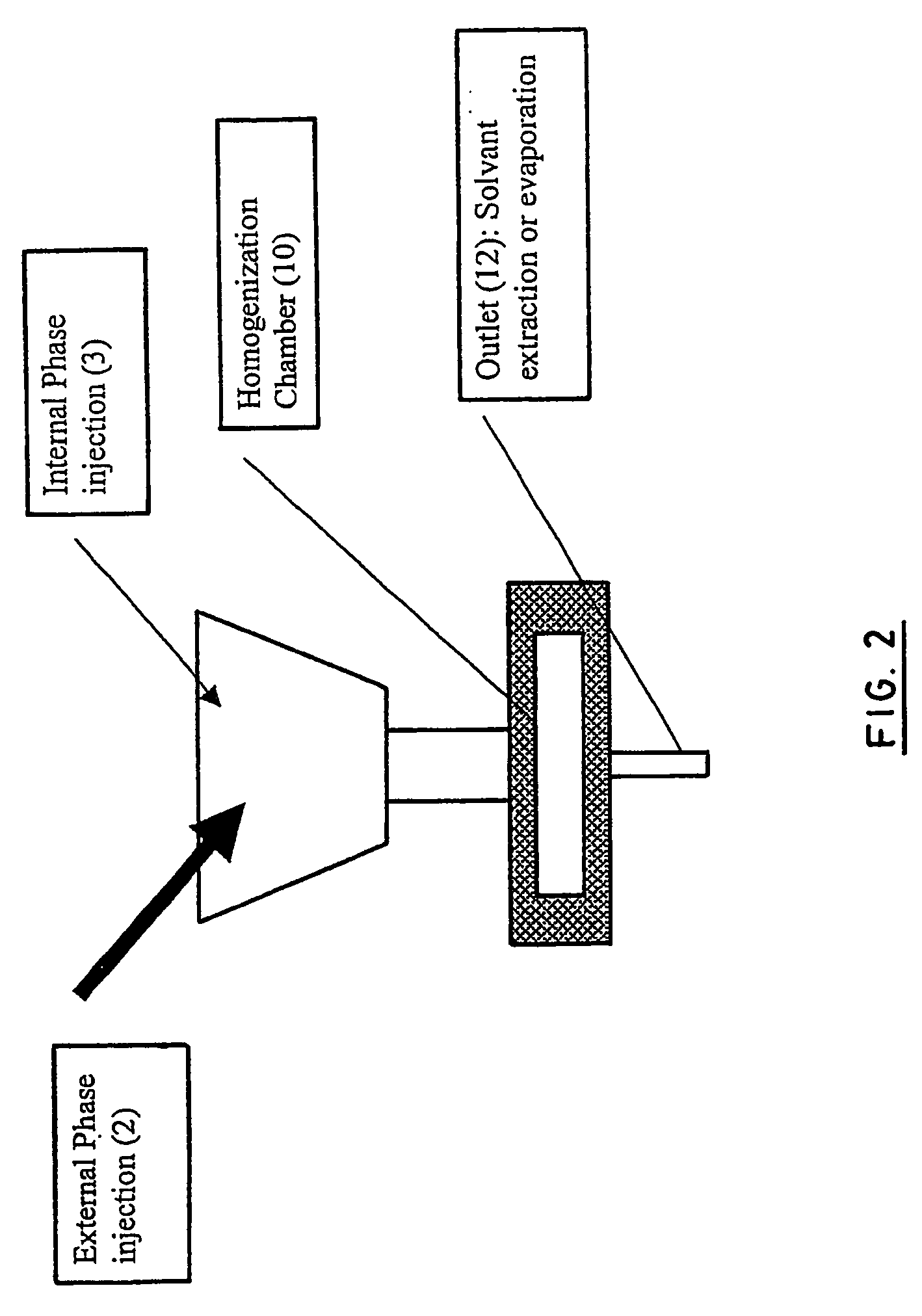
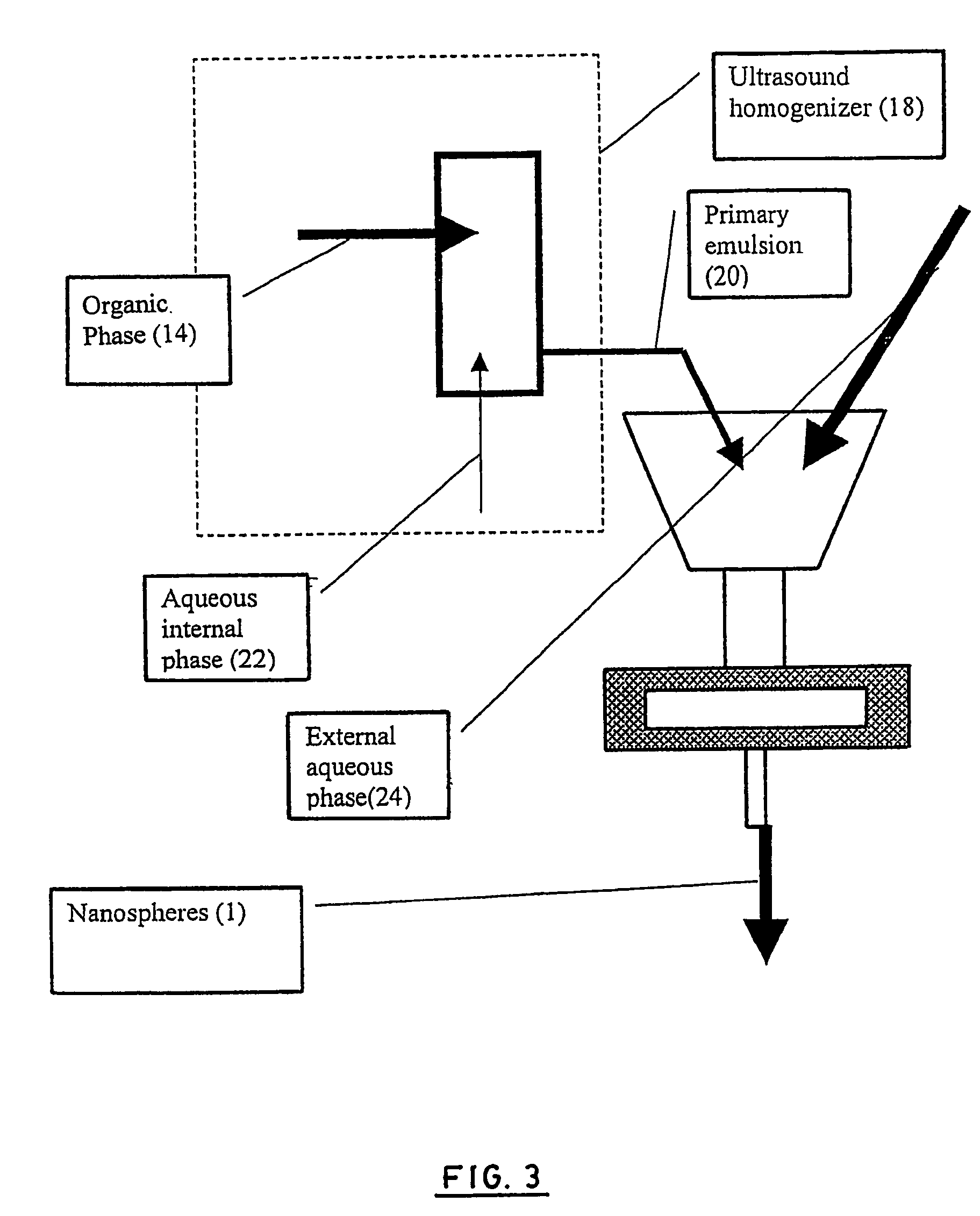
Stealthy polymeric biodegradable nanospheres and uses thereof
InactiveUS20060165987A1Maintain stable propertiesImprove drug deliverySynthetic resin layered productsCellulosic plastic layered productsPolyesterDrug compound
Disclosed herein are stealthy polymeric biodegradable nanospheres each comprising: (i) a polyester-polyethylene multiblock copolymer; (ii) optionally a polyester entangled with the multiblock copolymer to give rigidity to the nanospheres; and (iii) optionally a pharmaceutical compound incorporated therein. Also disclosed is the use of such nanospheres for the preparation of a medicament having a long-term and non-toxic release of a pharmaceutical compound into a mammal, and the method for preparing a stealthy polymeric biodegradable nanospheres.
Owner:VALORISATION RECH SOC & COMMANDITE
Crosslinked hyaluronic acid compositions for tissue augmentation
ActiveUS8124120B2Improve drug deliveryReduce frequencyAntibacterial agentsOrganic active ingredientsMedicineWater insoluble
Disclosed are hyaluronic acid (HA) compositions including crosslinked, water-insoluble, hydrated HA gel particles. Also disclosed are methods of making the HA compositions, and methods of using the HA composition to augment tissue in a subject.
Owner:ANIKA THERAPEUTICS INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Conjugates and compositions for cellular delivery
ActiveUS7109165B2Reduce deliveryPromotes associationOrganic active ingredientsBiocideAntisense nucleic acidNucleotide
This invention features conjugates, compositions, methods of synthesis, and applications thereof, including folate derived conjugates of nucleosides, nucleotides, non-nucleosides, and nucleic acids including enzymatic nucleic acids and antisense nucleic acid molecules.
Owner:SIRNA THERAPEUTICS INC
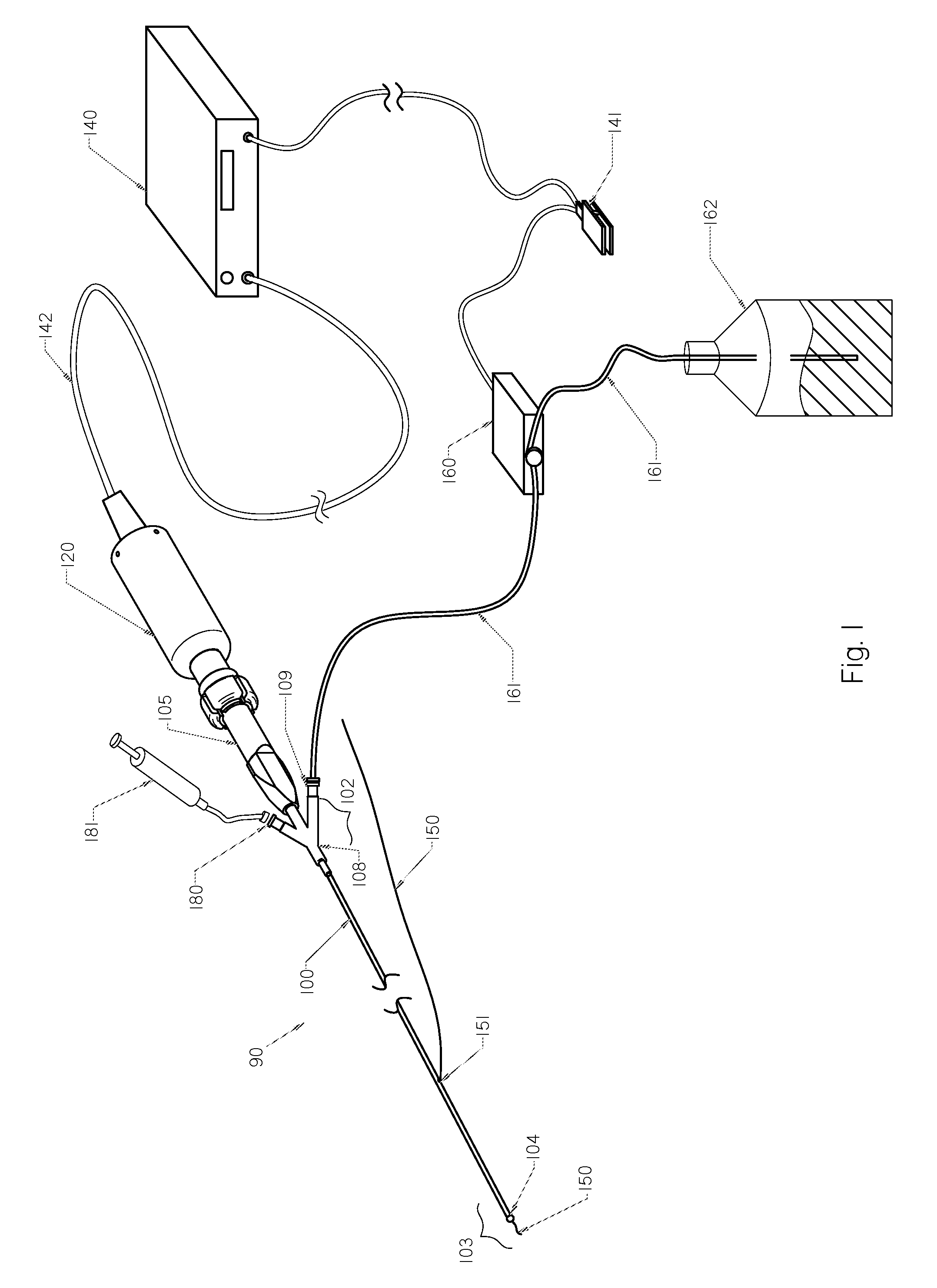
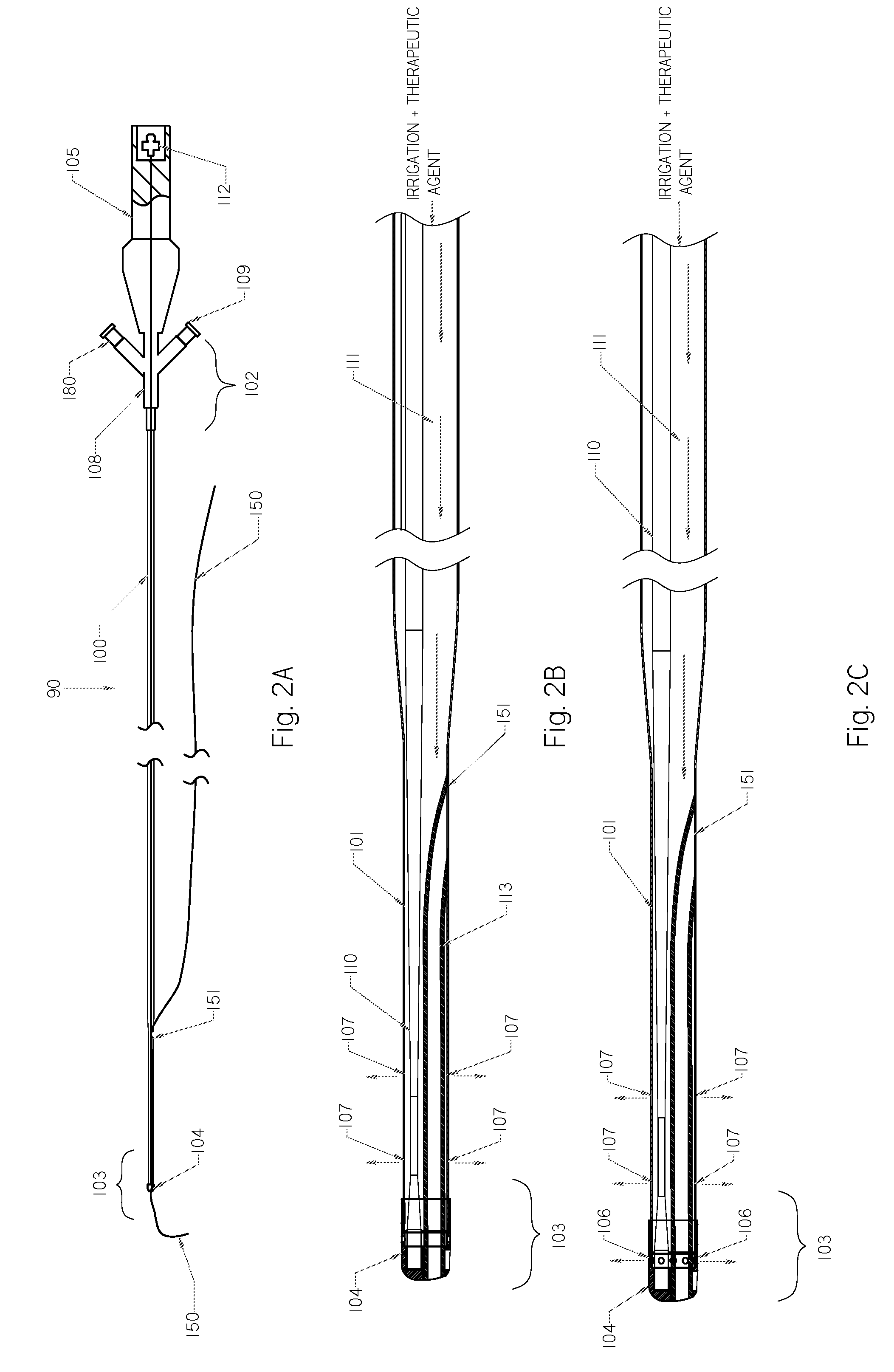
Methods and devices for endovascular therapy
ActiveUS20160270806A1Increase vascular permeabilityReduce stenosisMedical devicesCatheterDiseaseEndovascular therapy
The present invention provides methods and devices for treating endovascular disease. Vibrational energy is delivered to change compliance and increase permeability at the treatment area. To improve clinical outcomes, one or more therapeutic drugs may be delivered to the treatment area.
Owner:CARDIOPROLIFIC INC
Pulsed cavitational ultrasound therapy
ActiveUS8057408B2Improve efficacyPrevent and reduce actionOrgan movement/changes detectionSurgerySonificationMicrobubbles
Owner:RGT UNIV OF MICHIGAN
Methods and devices for endovascular therapy
ActiveUS9375223B2Improve drug deliveryReduce breakageMedical devicesCatheterDiseaseEndovascular therapy
The present invention provides methods and devices for treating endovascular disease. Vibrational energy is delivered to change compliance and increase permeability at the treatment area. To improve clinical outcomes, one or more therapeutic drugs may be delivered to the treatment area.
Owner:CARDIOPROLIFIC INC
Methods and devices for endovascular therapy
ActiveUS20160367274A1Improve drug deliveryReduce breakageMedical devicesCatheterDiseaseEndovascular therapy
Owner:CARDIOPROLIFIC INC
Methods and devices for endovascular therapy
ActiveUS20160367275A1Improve drug deliveryReduce breakageMedical devicesSurgical instrument detailsDiseaseEndovascular therapy
The present invention provides methods and devices for treating endovascular disease. Vibrational energy is delivered to change compliance and increase permeability at the treatment area. To improve clinical outcomes, one or more therapeutic drugs may be delivered to the treatment area.
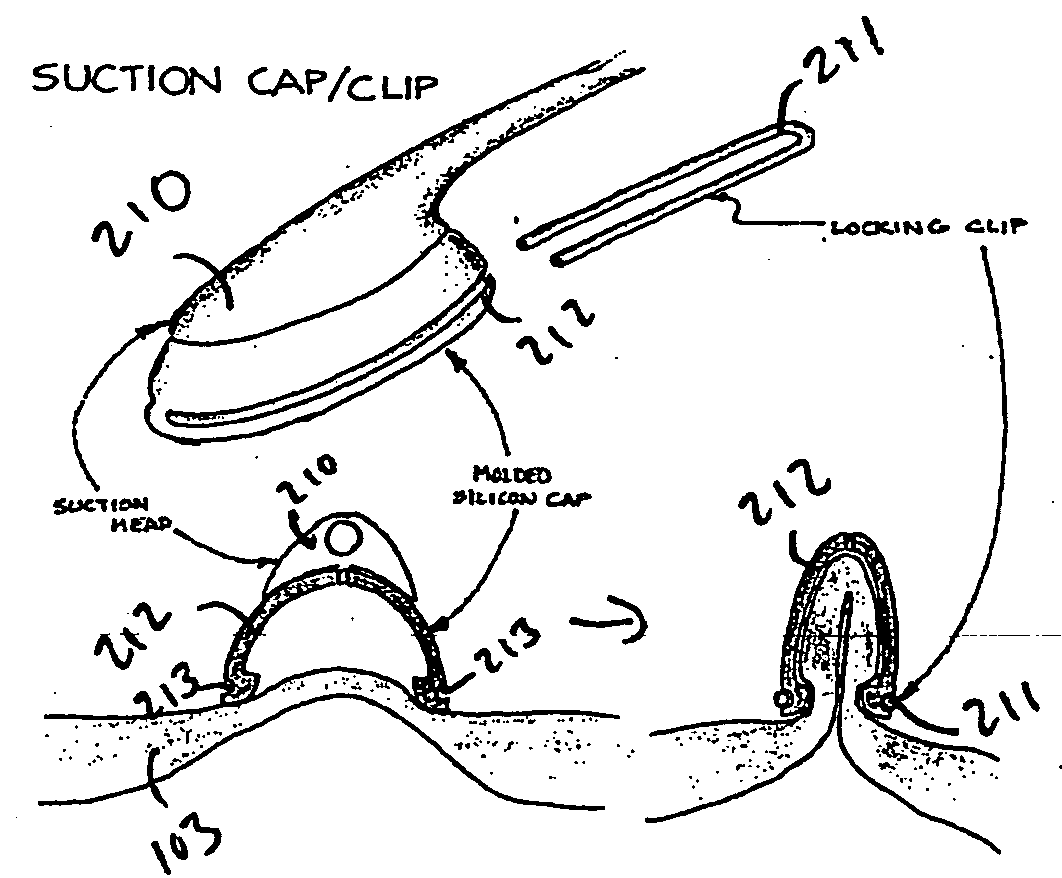
Owner:CARDIOPROLIFIC INC
Method and device for treating dysfunctional cardiac tissue
ActiveUS20070073274A1Avoid problemsSmooth delivery rateSuture equipmentsDiagnosticsFunctional disturbanceVentricular volume
Various methods and devices are provided for reducing the volume of the ventricles of the heart. In one embodiment, a method for reducing the ventricular volume of a heart chamber is provided including the steps of inserting an anchoring mechanism onto dysfunctional cardiac tissue, deploying one or more anchors into the dysfunctional cardiac tissue, raising the dysfunctional cardiac tissue using the anchors, and securing the anchors to hold the dysfunctional cardiac tissue in place. Further, a device for reducing the volume of the ventricles of a heart chamber is provided where the device has one or more clips for placement on dysfunctional cardiac tissue of a heart, one or more anchors for deployment and securement into the dysfunctional cardiac tissue, and a lifting mechanism for raising the one or more anchors and the dysfunctional cardiac tissue.
Owner:BIOVENTRIX A CHF TECH
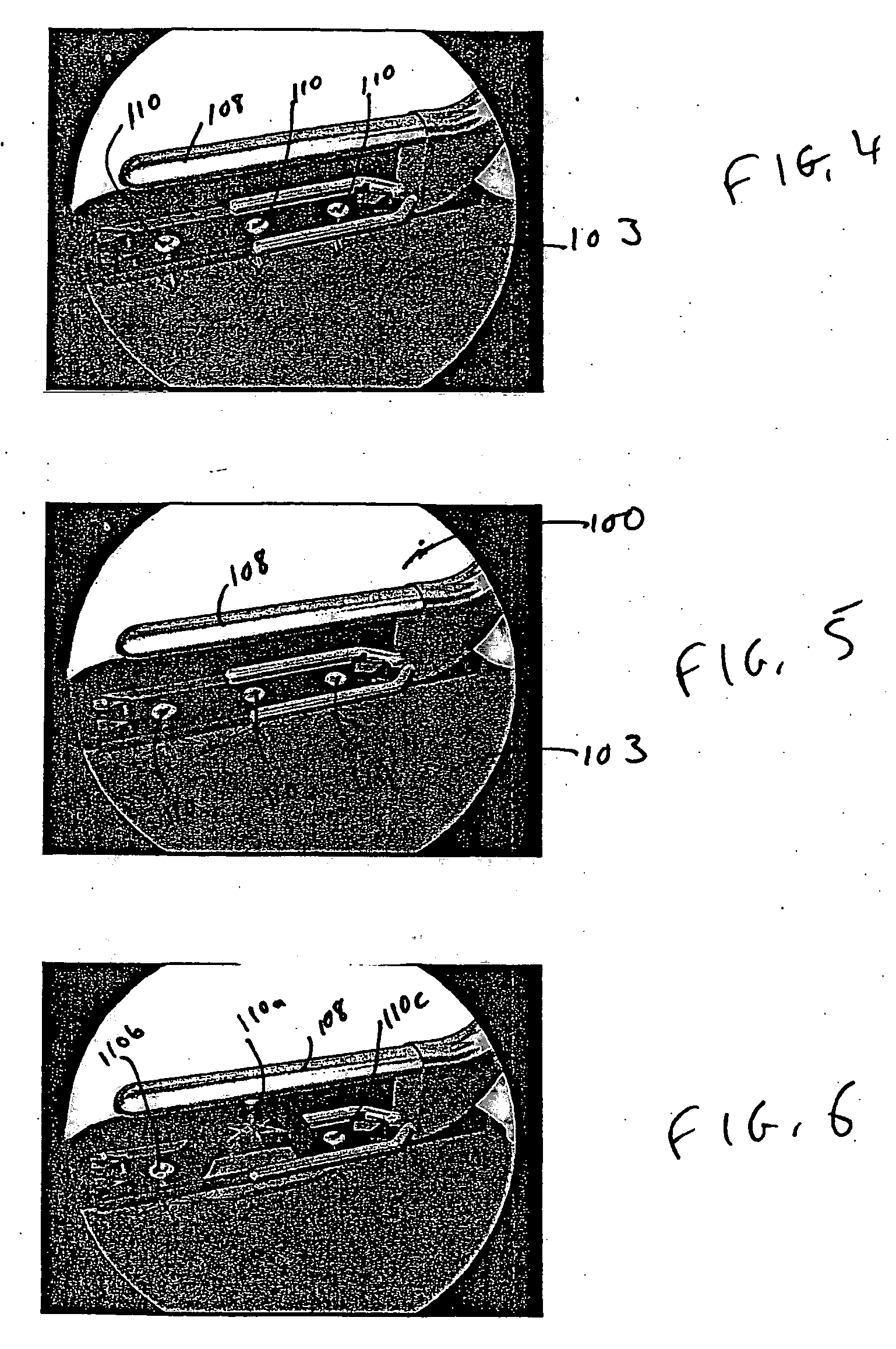
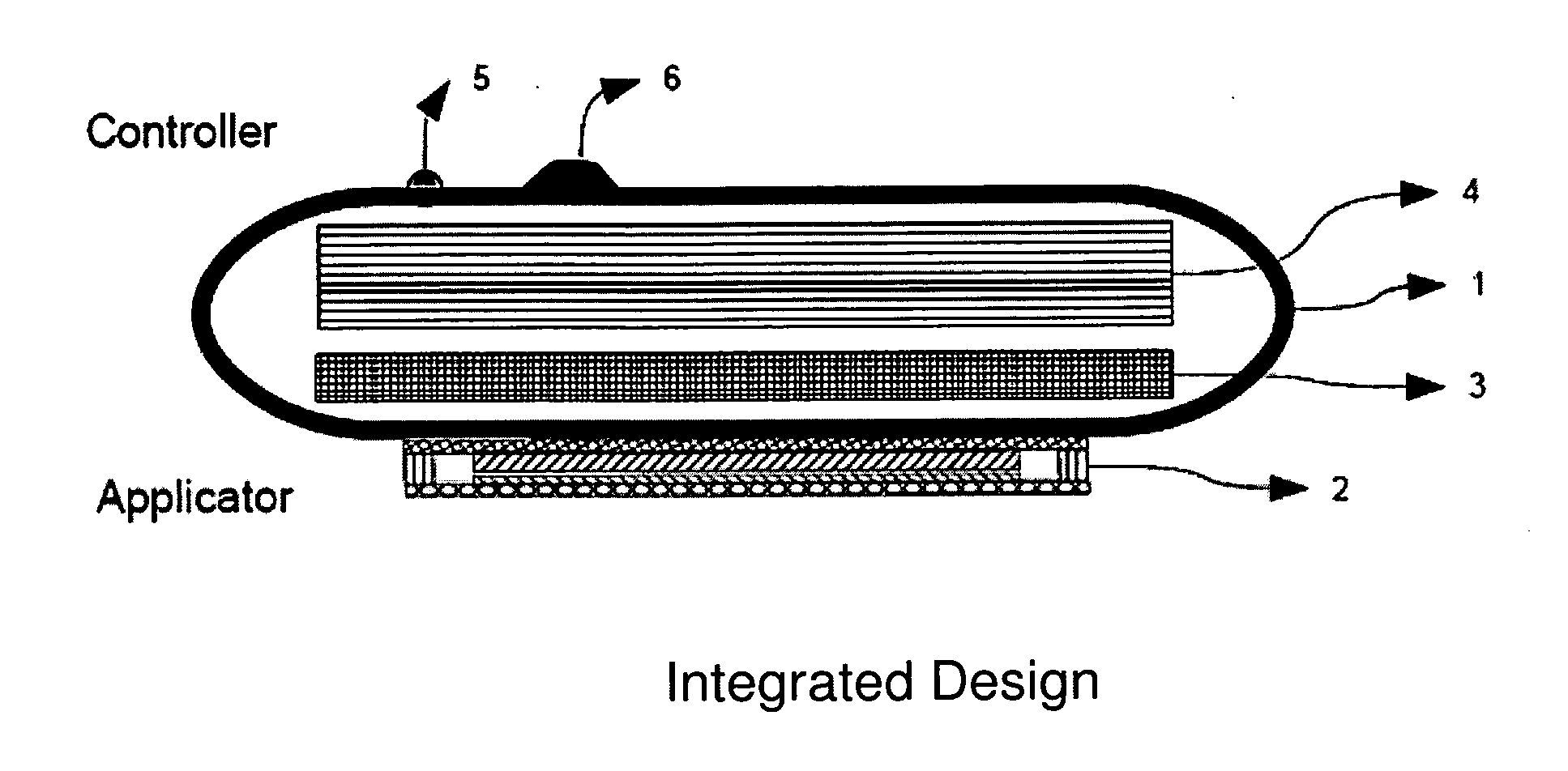
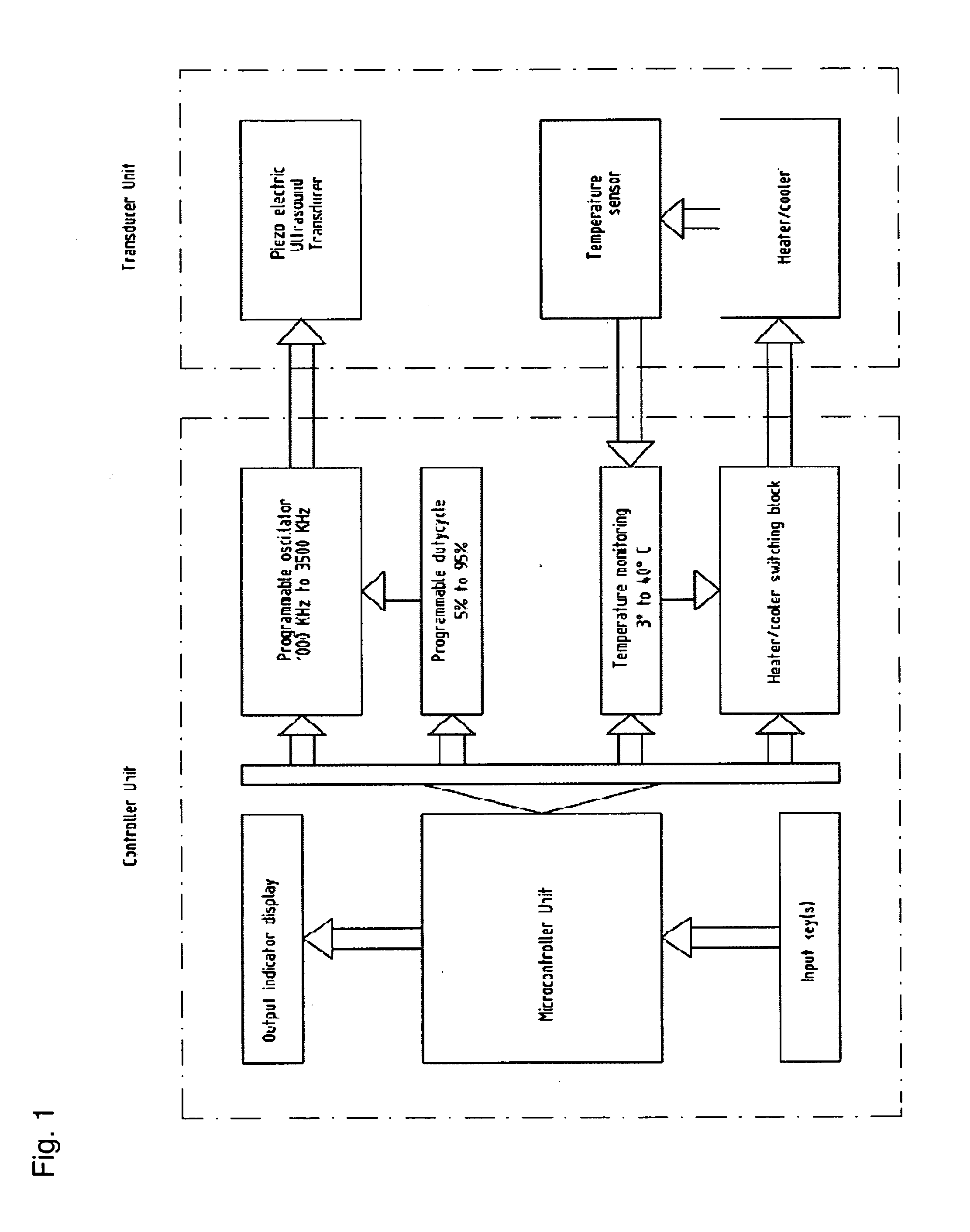
Portable electronic therapy device and the method thereof
InactiveUS20140200487A1Physiological effect is goodProduce heatUltrasound therapyElectrotherapyAutomatic controlPain management
The present disclosure relates to an electronic therapy device including automatic controlled application of energies along with feedback control using sensors for improved synergistic effects and further the device is configured to be used for longer periods of time for improved and optimal therapeutic results without causing any adverse effects, the device can be used for pain management, healing, fitness, cosmetic and topical delivery related applications and a method for performing electronic therapy using the said portable electronic device.
Owner:ITRACE BIOMEDICAL
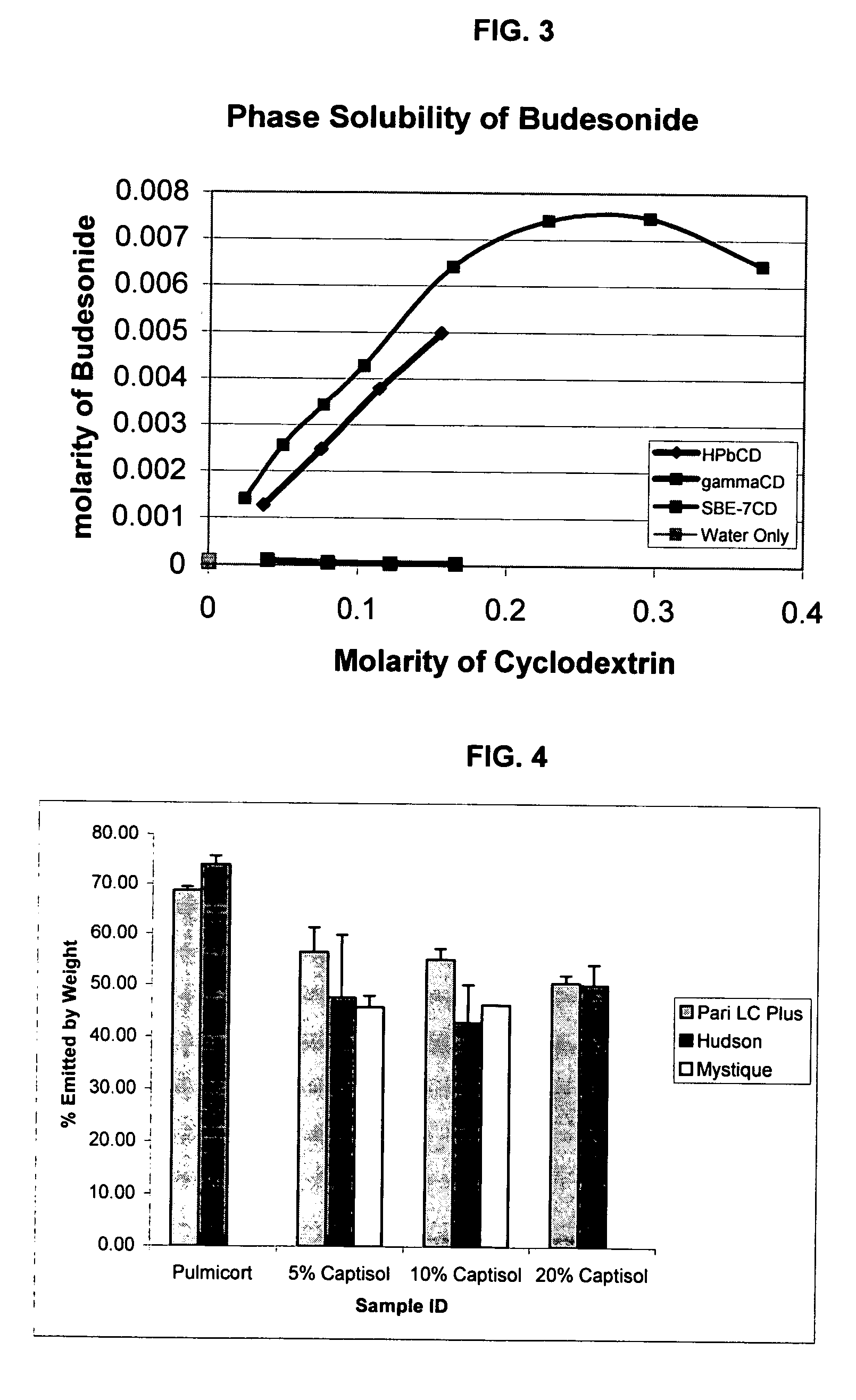
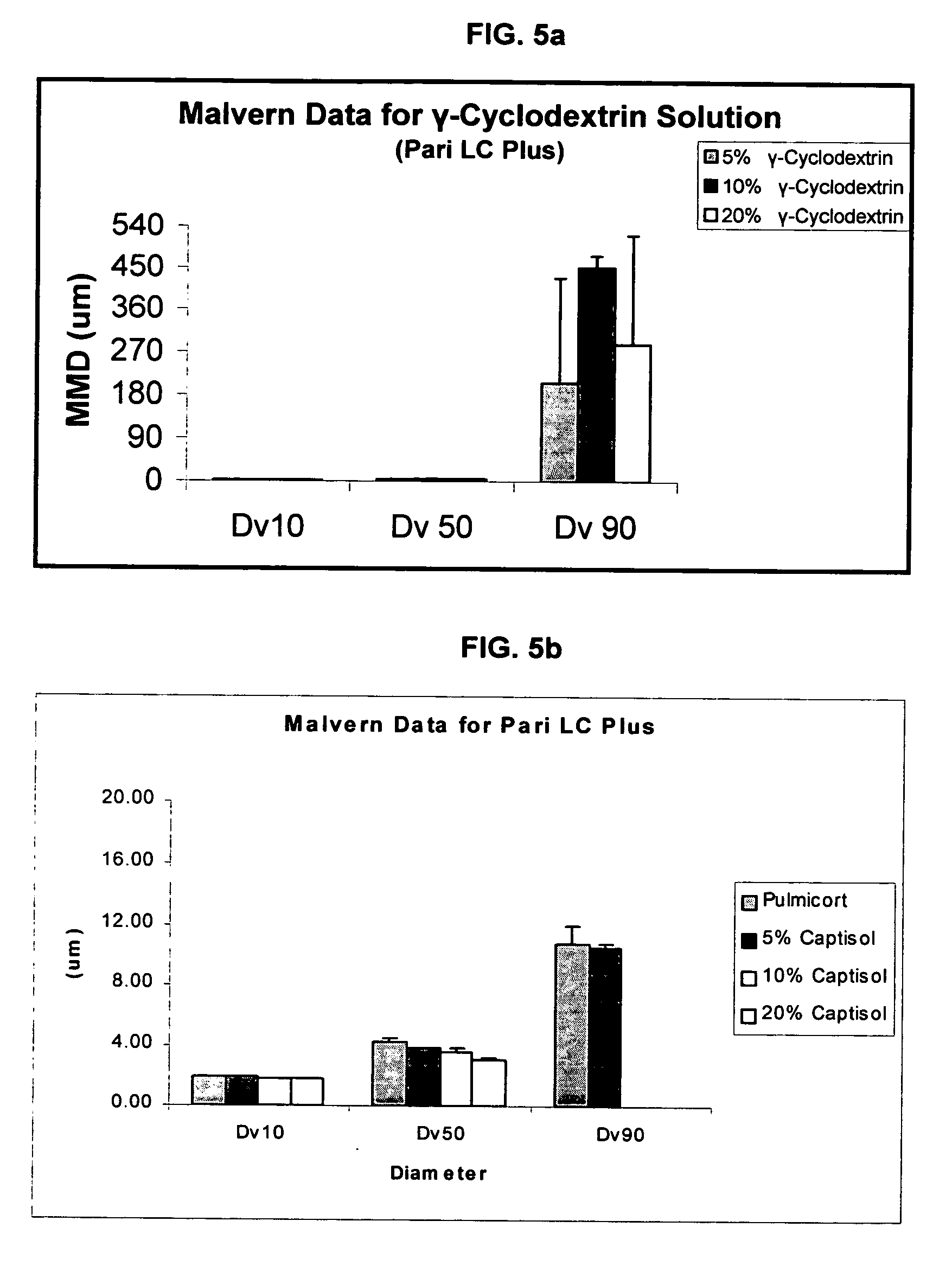
Inhalant formulation containing sulfoalkyl ether gamma-cyclodextrin and corticosteroid
InactiveUS20070020298A1Reduce the degradation rateIncrease productivityOrganic active ingredientsBiocideNasal cavityNebulizer
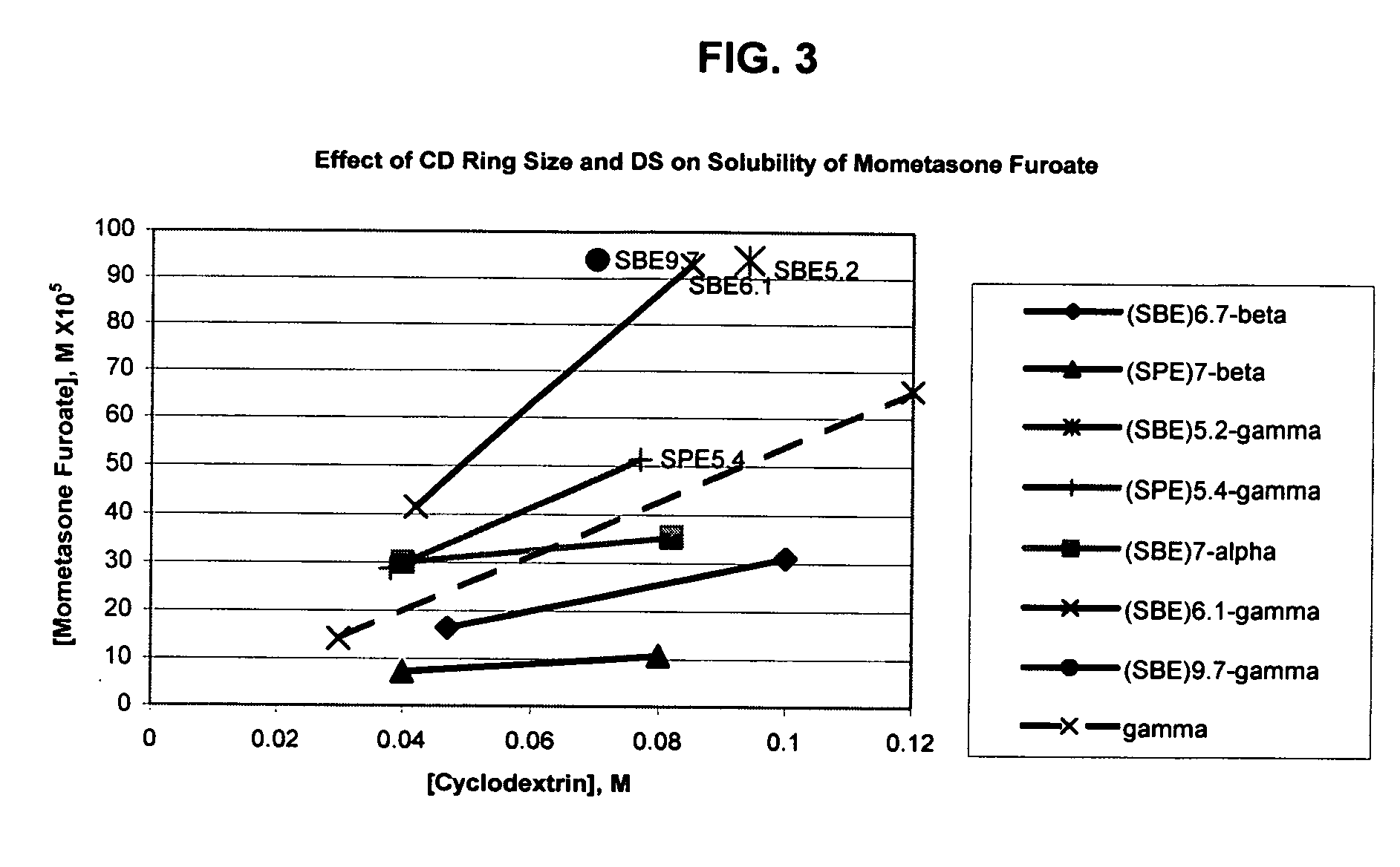
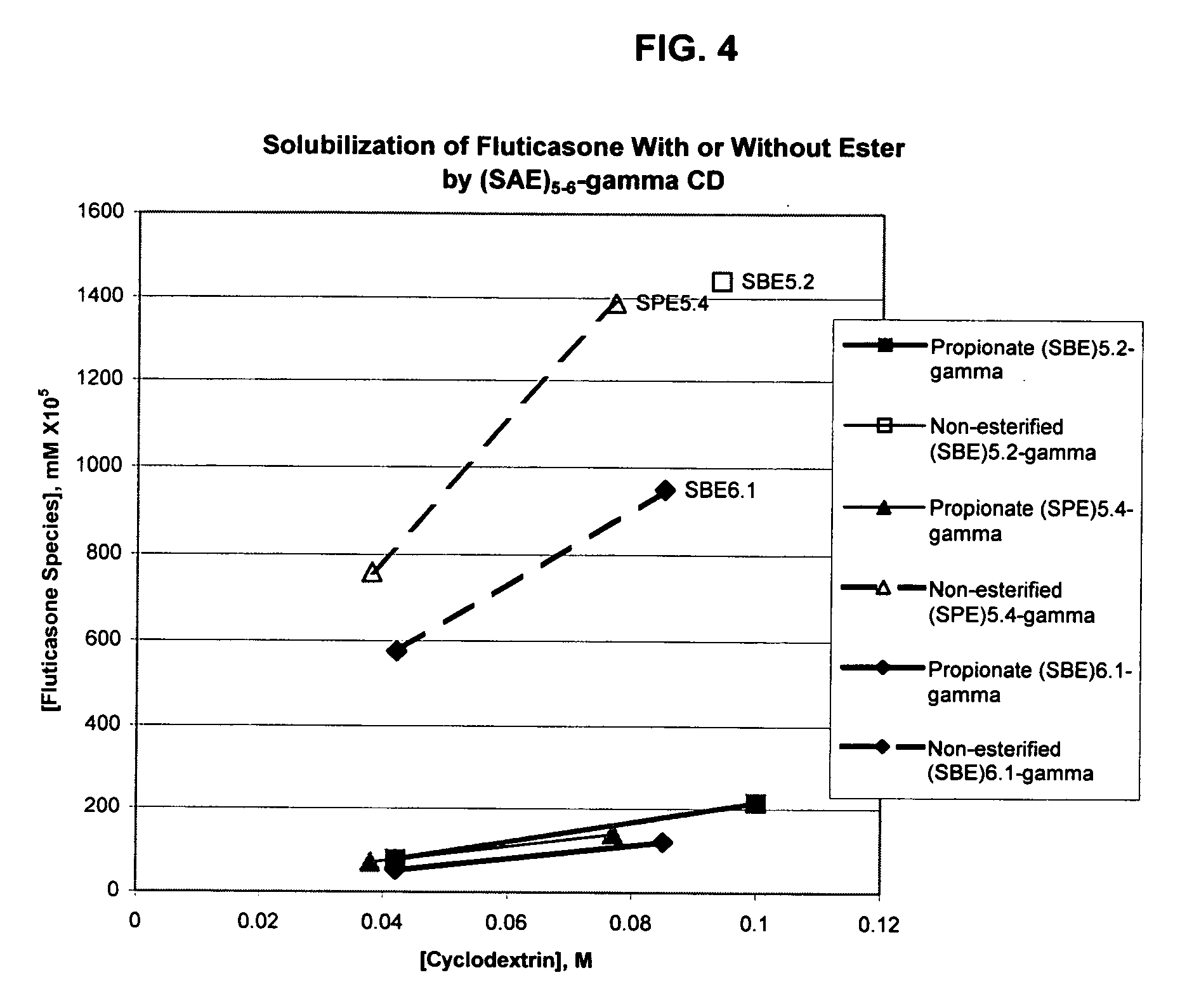
An inhalable formulation containing SAE-γ-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-γ-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation can include one or more additional therapeutic agents for use in combination with the corticosteroid. SAE-γ-CD is especially useful for solubilizing esterified corticosteroids.
Owner:CYDEX PHARMACEUTICALS INC
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
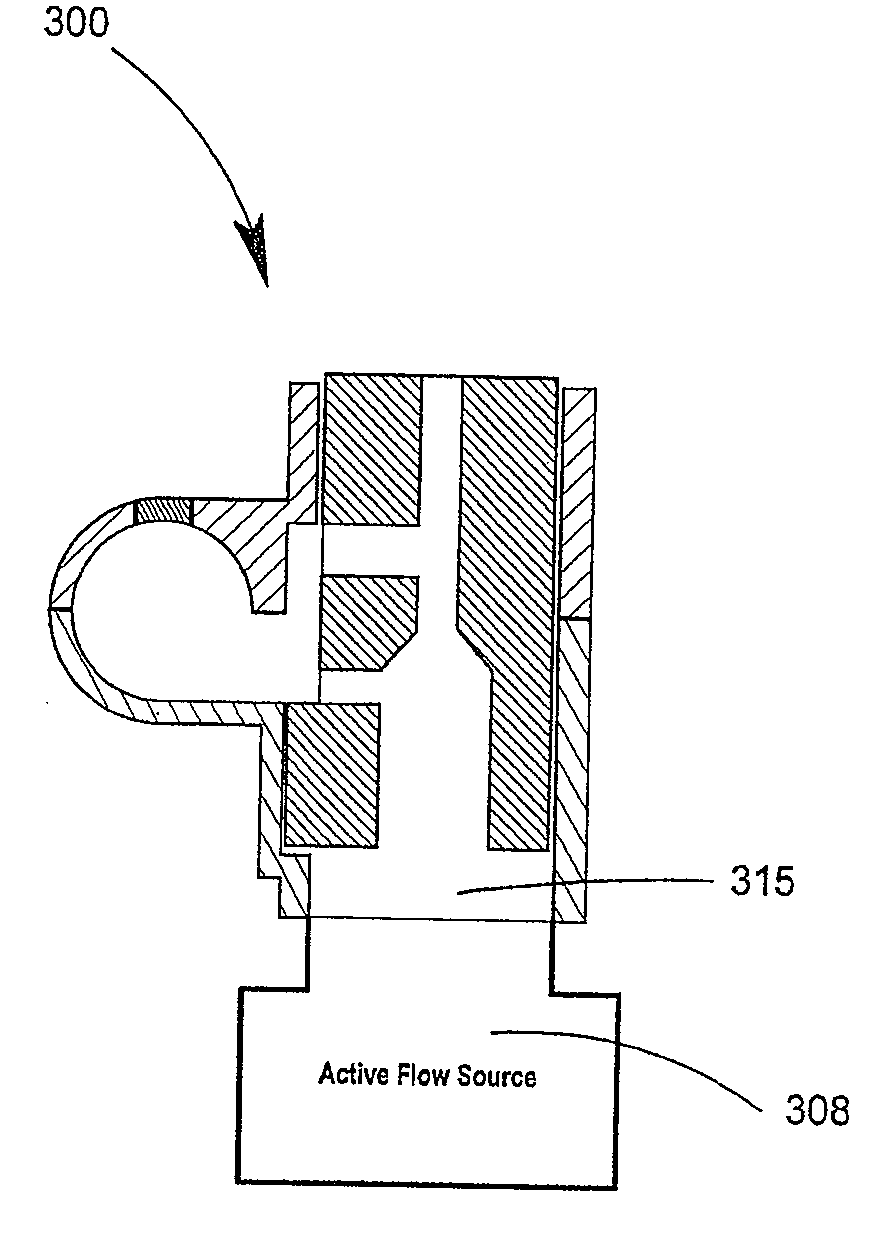
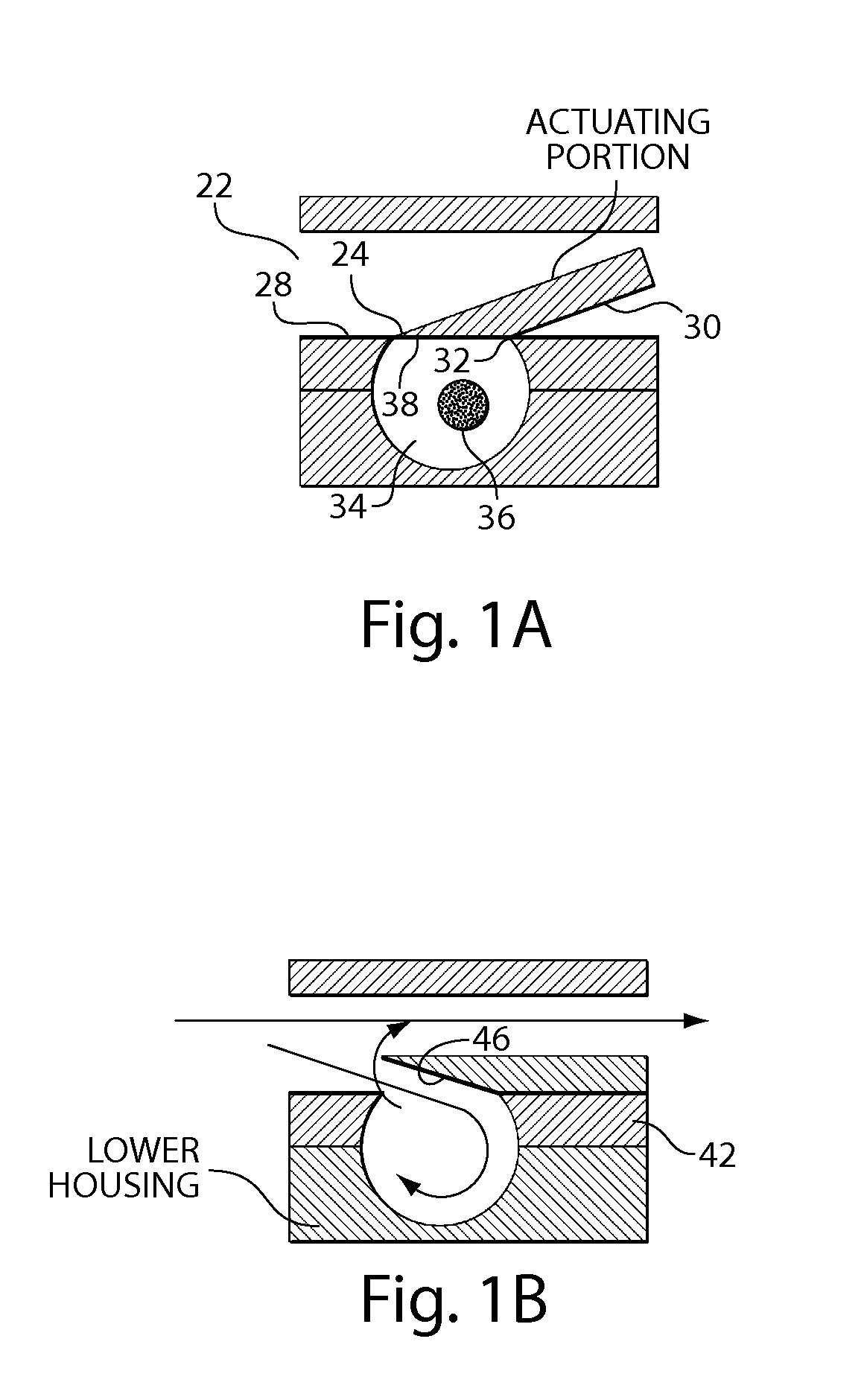
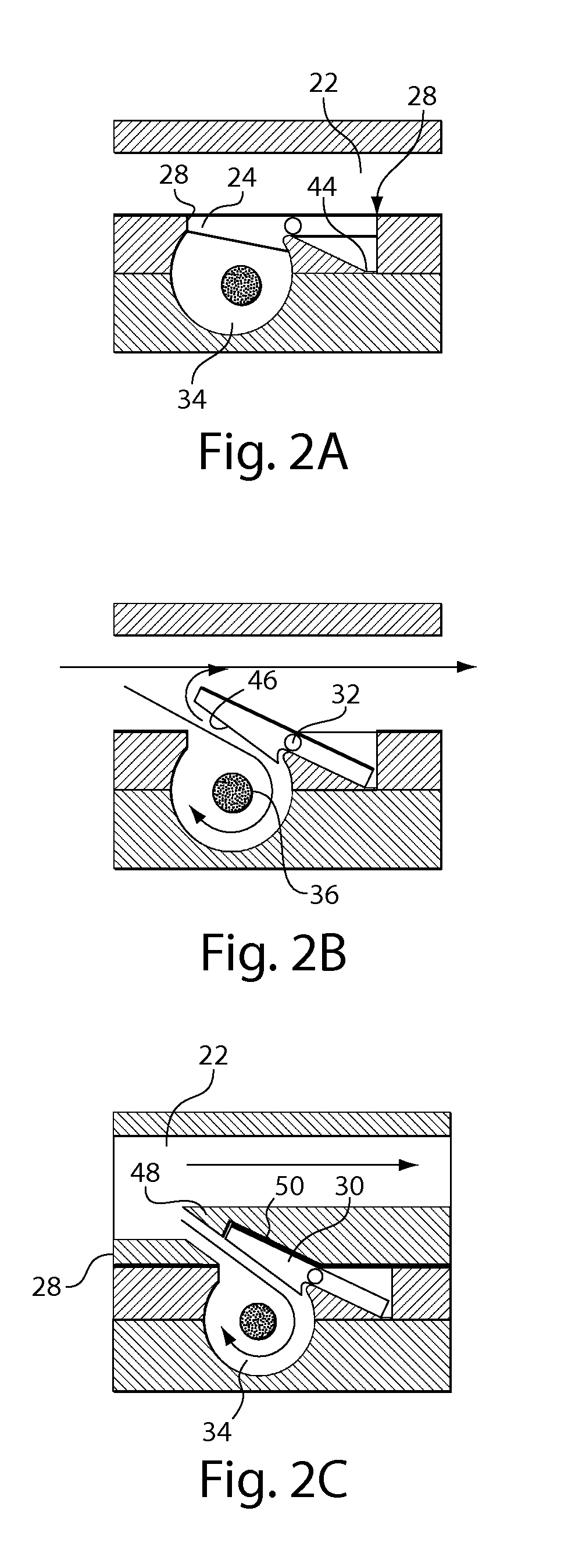
Delivery device and related methods
ActiveUS20090013994A1Receive treatment wellImprove drug deliveryRespiratorsLiquid surface applicatorsBiomedical engineeringDrug
Owner:MANTA DEVICES
Delivery device and related methods
InactiveUS20100180894A1Receive treatment wellImprove drug deliveryRespiratorsMedical devicesMedicine
A delivery device that includes a cutter for opening a barrier layer to provide fluid access to a dose chamber, and a diverting structure for direct air flow toward the dose chamber.
Owner:MANTA DEVICES
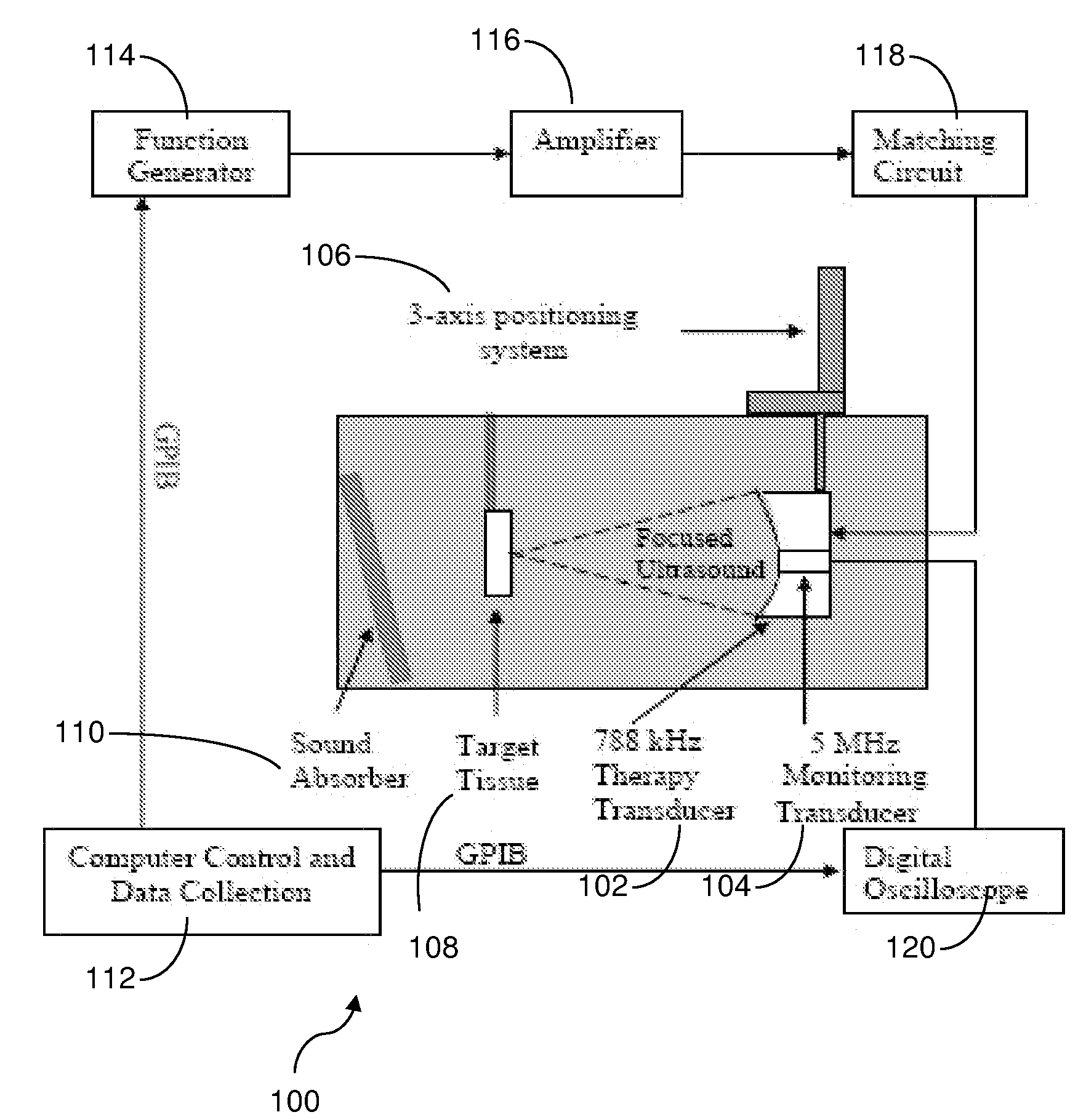
Low intensity directed ultrasound (LODUS) mediated blood brain barrier disruption
InactiveUS7896821B1Facilitate therapeutic drug deliveryReduce frequencyElectrotherapyChiropractic devicesCavitationRadiology
A method and device selectively and reversibly disrupts the blood-brain barrier (BBB) in a selected volume of the brain without the need for exogenous agents. The method and device employ low intensity directed ultrasound (LODUS) that is safe, reduces the danger of cavitation and thermal tissue damage, and is able to expose small or large regions of the brain to achieve a desired therapeutic or prophylactic effect.
Owner:KYLE ALBERT S
Method and device for treating dysfunctional cardiac tissue
ActiveUS8506474B2Avoid problemsSmooth delivery rateSuture equipmentsDiagnosticsFunctional disturbanceVentricular volume
Various methods and devices are provided for reducing the volume of the ventricles of the heart. In one embodiment, a method for reducing the ventricular volume of a heart chamber is provided including the steps of inserting an anchoring mechanism onto dysfunctional cardiac tissue, deploying one or more anchors into the dysfunctional cardiac tissue, raising the dysfunctional cardiac tissue using the anchors, and securing the anchors to hold the dysfunctional cardiac tissue in place. Further, a device for reducing the volume of the ventricles of a heart chamber is provided where the device has one or more clips for placement on dysfunctional cardiac tissue of a heart, one or more anchors for deployment and securement into the dysfunctional cardiac tissue, and a lifting mechanism for raising the one or more anchors and the dysfunctional cardiac tissue.
Owner:BIOVENTRIX A CHF TECH
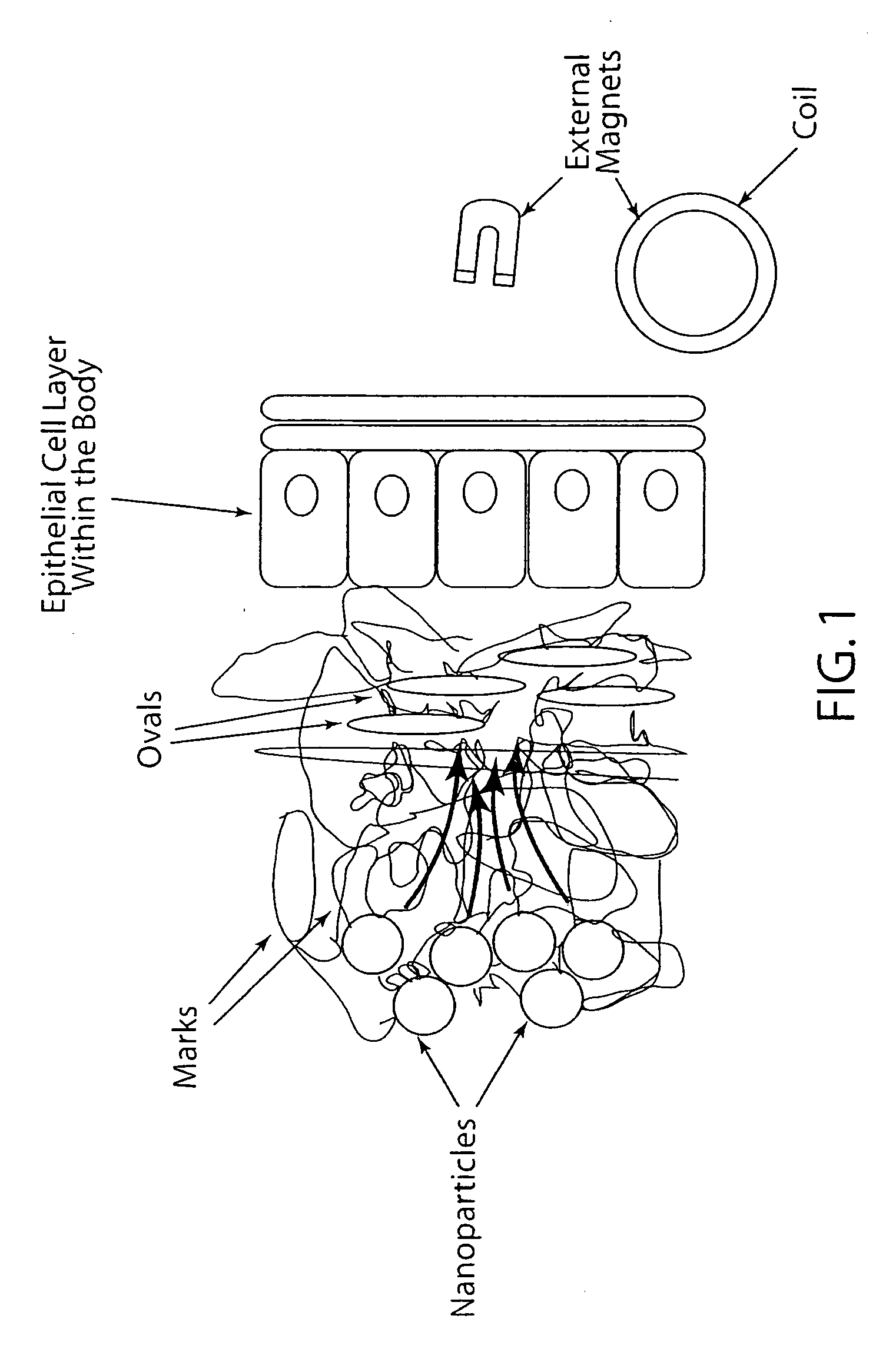
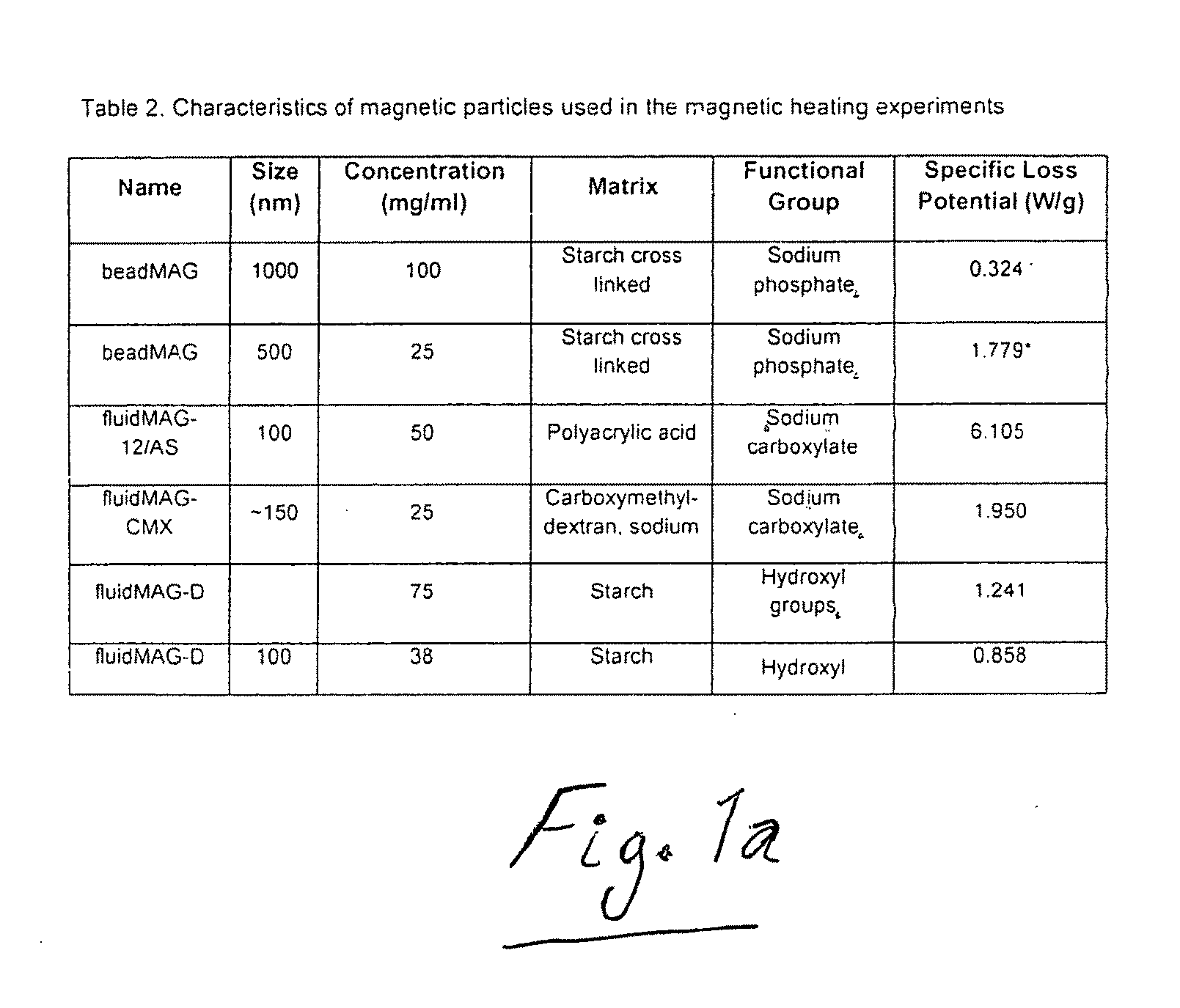
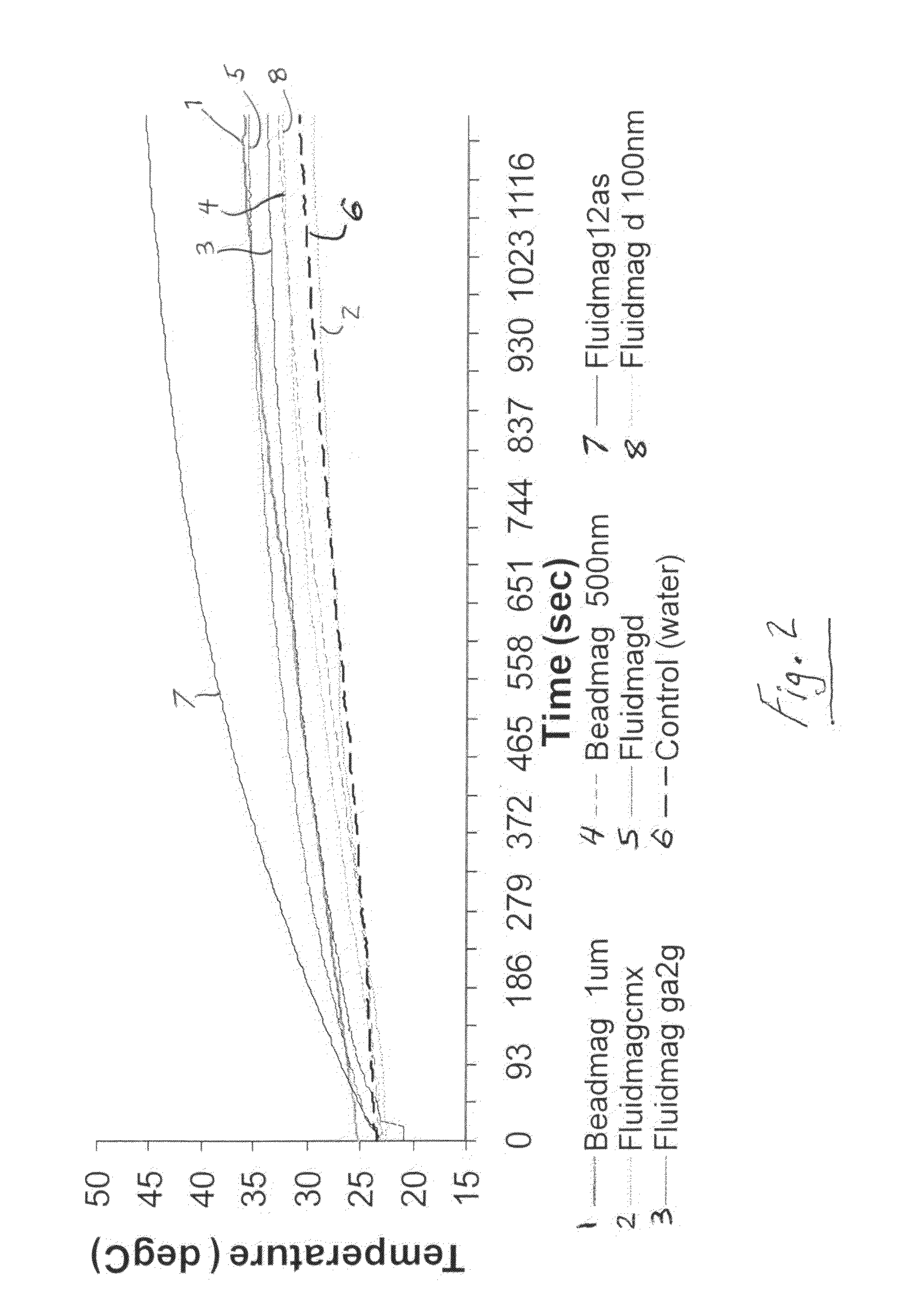
Active nanoparticles and method of using
ActiveUS20090226521A1Improve efficacyImprove distributionPowder deliveryOrganic active ingredientsMultifunctional nanoparticlesGene
Active multifunctional nanoparticles provide significant enhancement of the efficacy of model therapeutic and gene agents due to increased diffusion and penetration through mucus and biological barriers under the influence of a magnetic field.
Owner:STC UNM
Pulsed cavitational ultrasound therapy
InactiveUS20100069797A1Improve efficacyPrevent and reduce actionUltrasonic/sonic/infrasonic diagnosticsUltrasound therapySonificationMicrobubbles
Therapy methods using pulsed cavitational ultrasound therapy can include the subprocesses of initiation, maintenance, therapy, and feedback of the histotripsy process, which involves the creation and maintenance of ensembles of microbubbles and the use of feedback in order to optimize the process based on observed spatial-temporal bubble cloud dynamics. The methods provide for the subdivision or erosion of tissue, liquification of tissue, and the enhanced delivery of therapeutic agents. Various feedback mechanisms allow variation of ultrasound parameters and provide control over the pulsed cavitational process, permitting the process to be tuned for a number of applications. Such applications can include specific tissue erosion, bulk tissue homogenization, and delivery of therapeutic agents across barriers.
Owner:RGT UNIV OF MICHIGAN
Glucosamine and glucosamine/Anti-inflammatory mutual prodrugs, compositions, and methods
ActiveUS20120021046A1Reduce deliveryEasy to degradeBiocideCosmetic preparationsAnti-inflammatoryChemistry
Mutual prodrugs of glucosamine, and derivatives and analogs of glucosamine and an anti-inflammatory agent, compositions thereof, and methods for, e.g., treating disorders and conditions by administration of the compositions are provided. Topical compositions of glucosamine, and derivatives and analogs of glucosamine are also provided.
Owner:UNIV OF GEORGIA RES FOUND INC
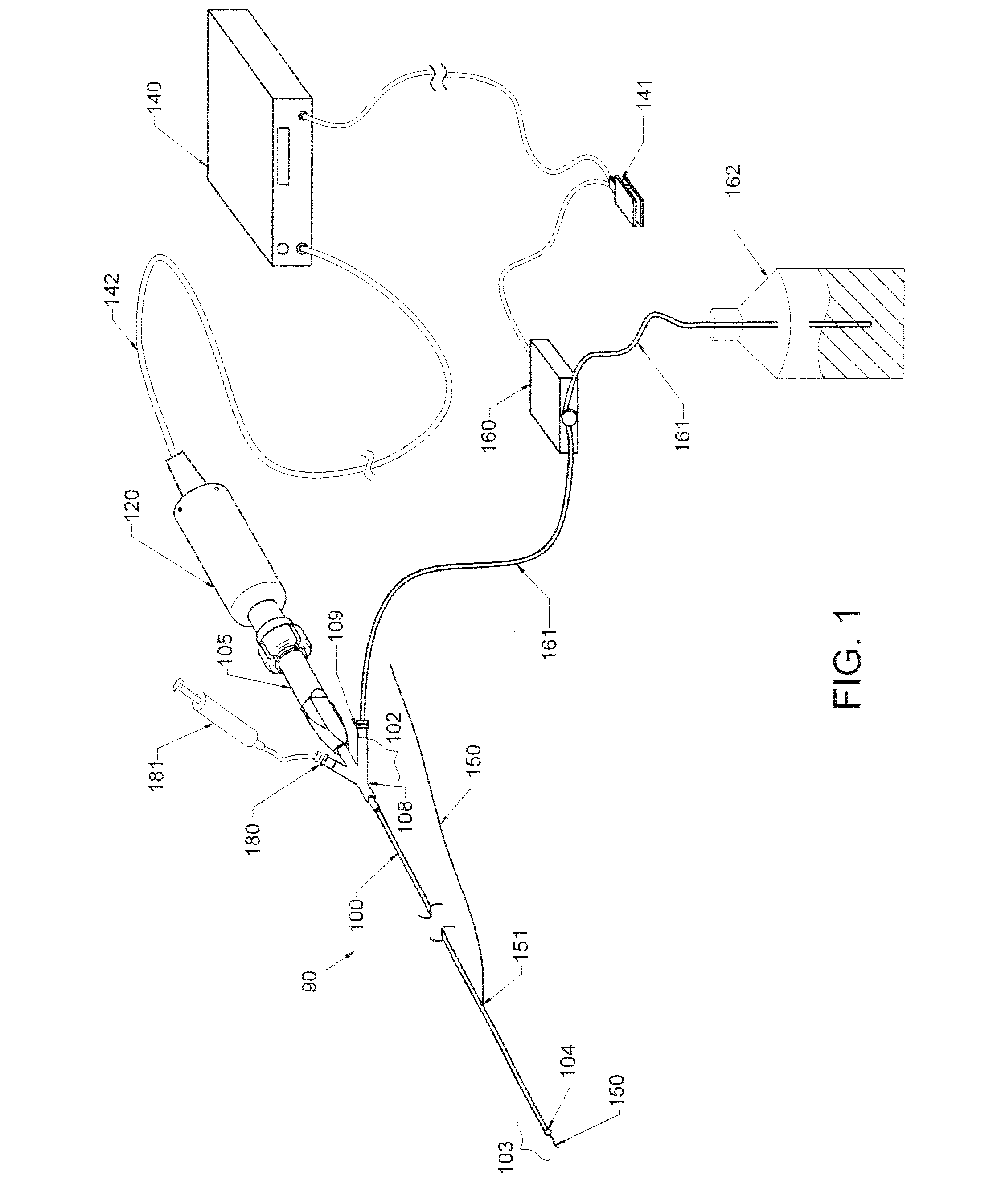
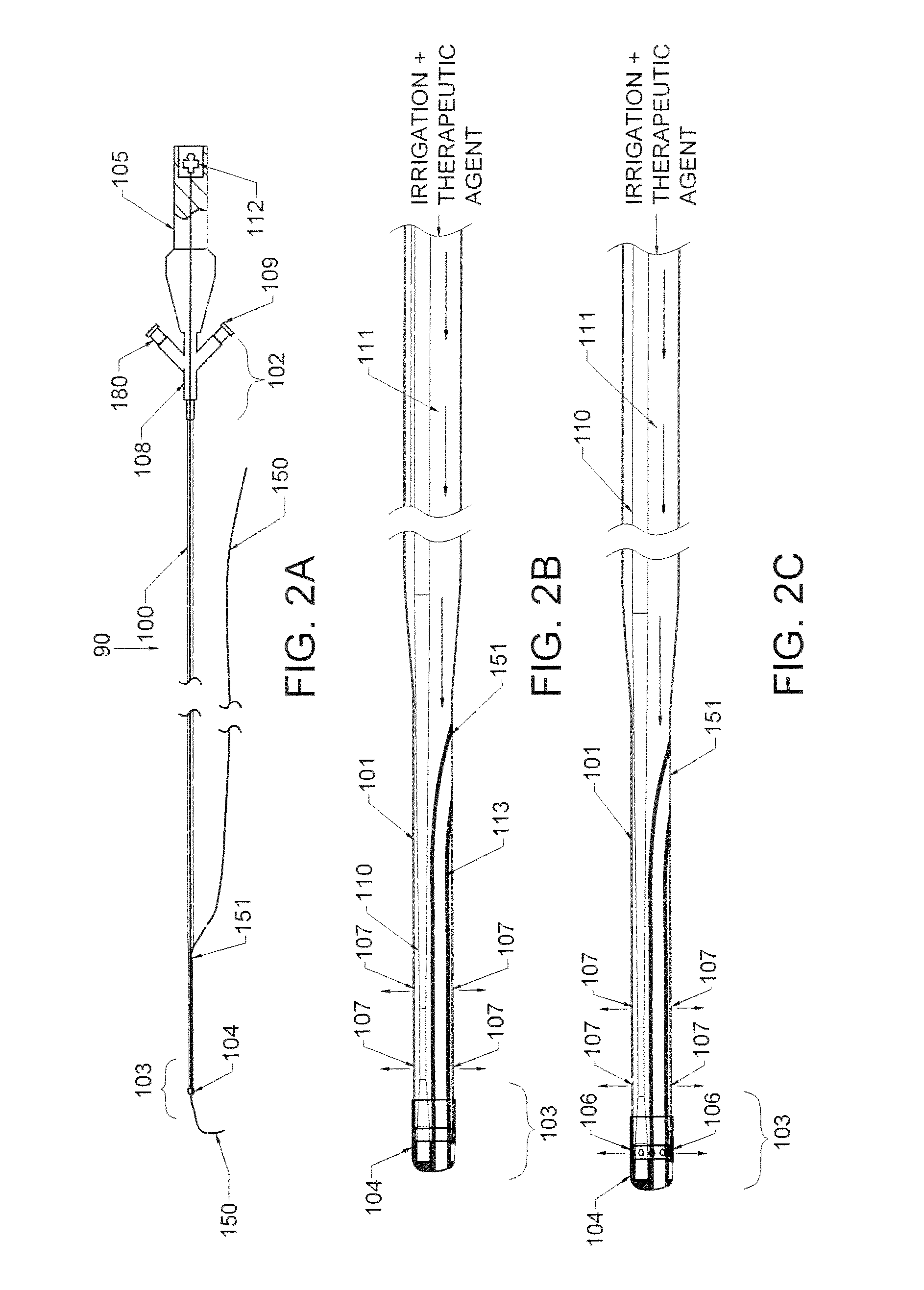
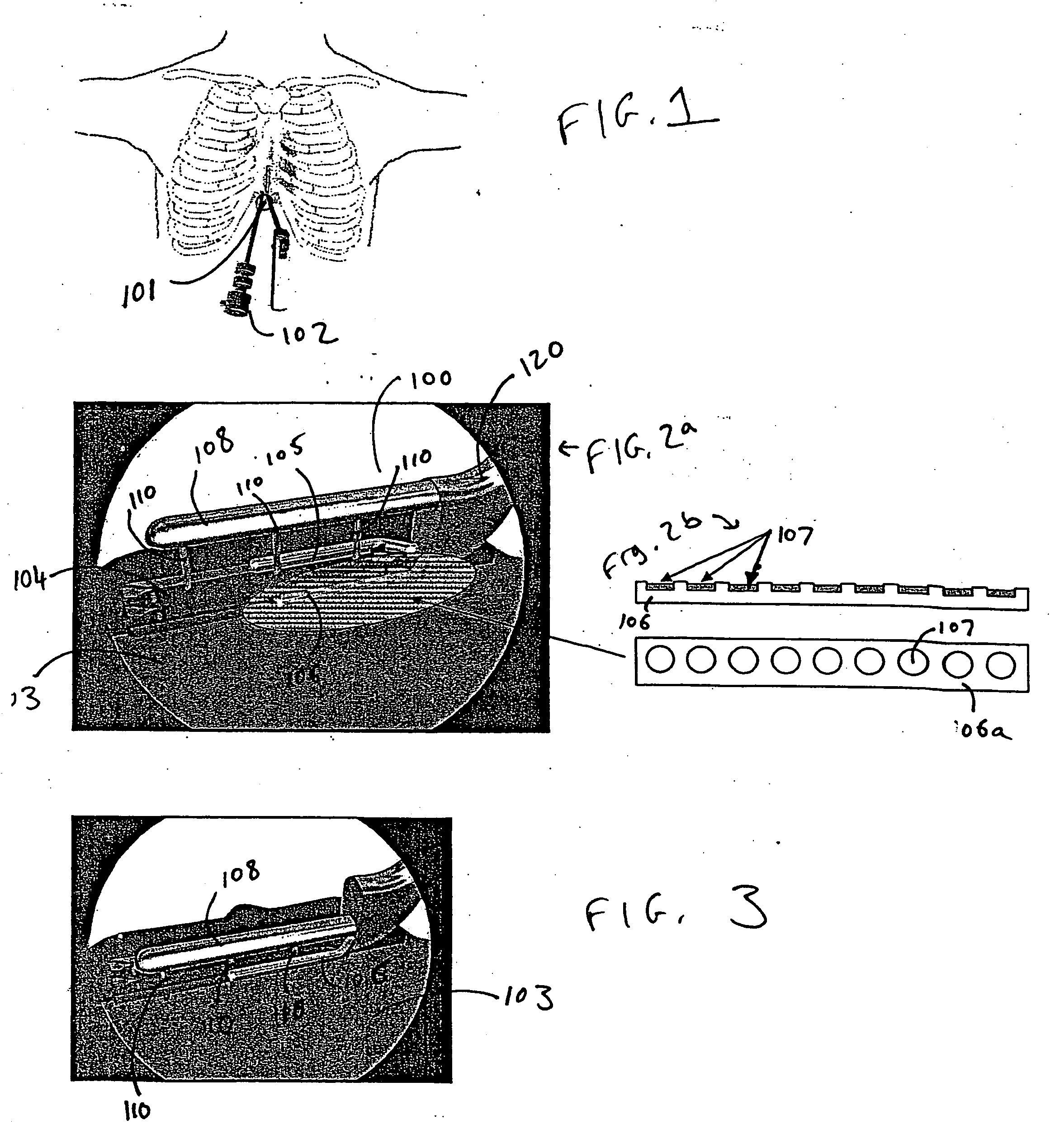
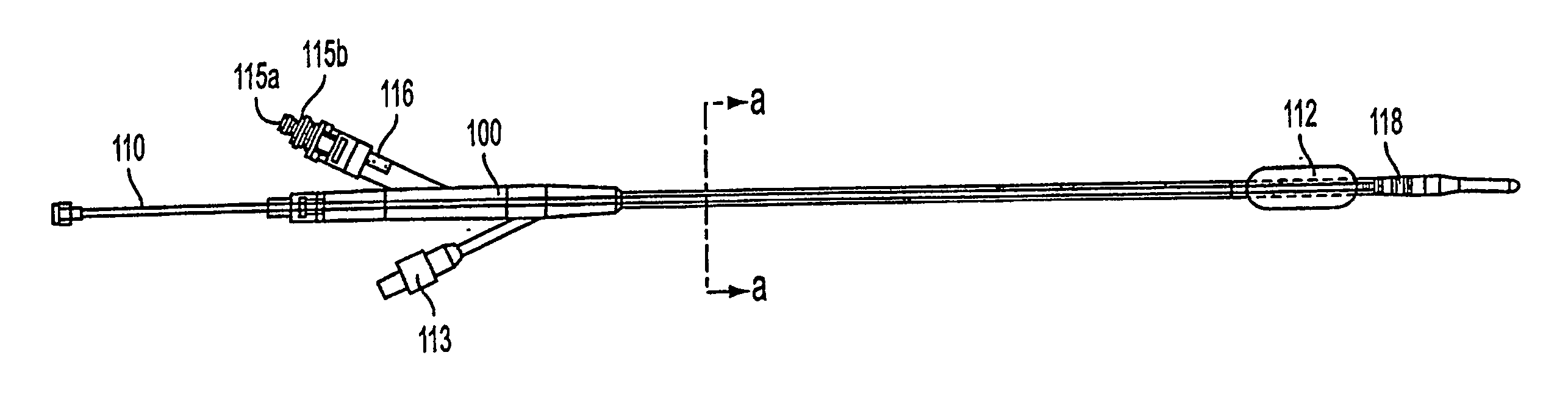
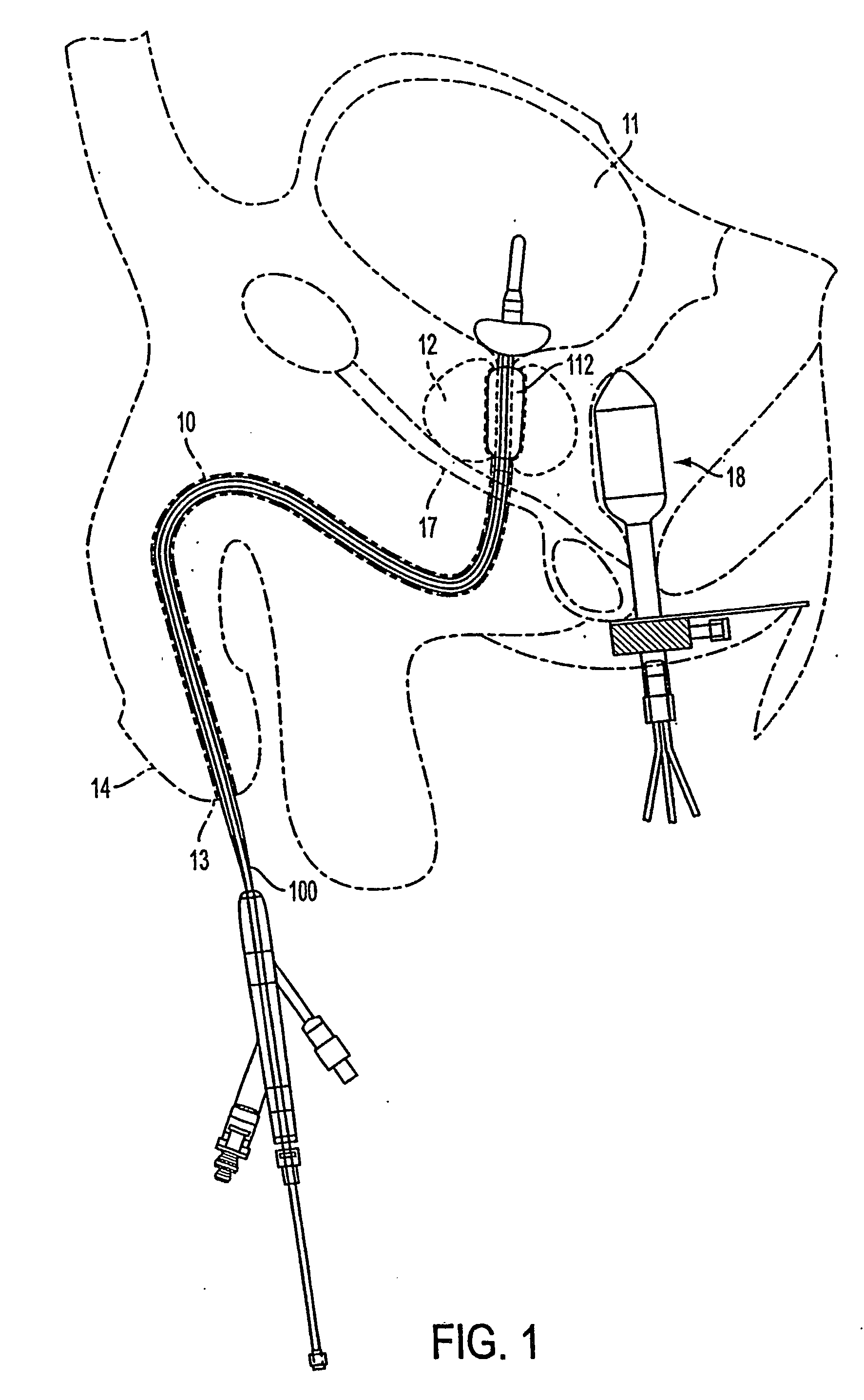
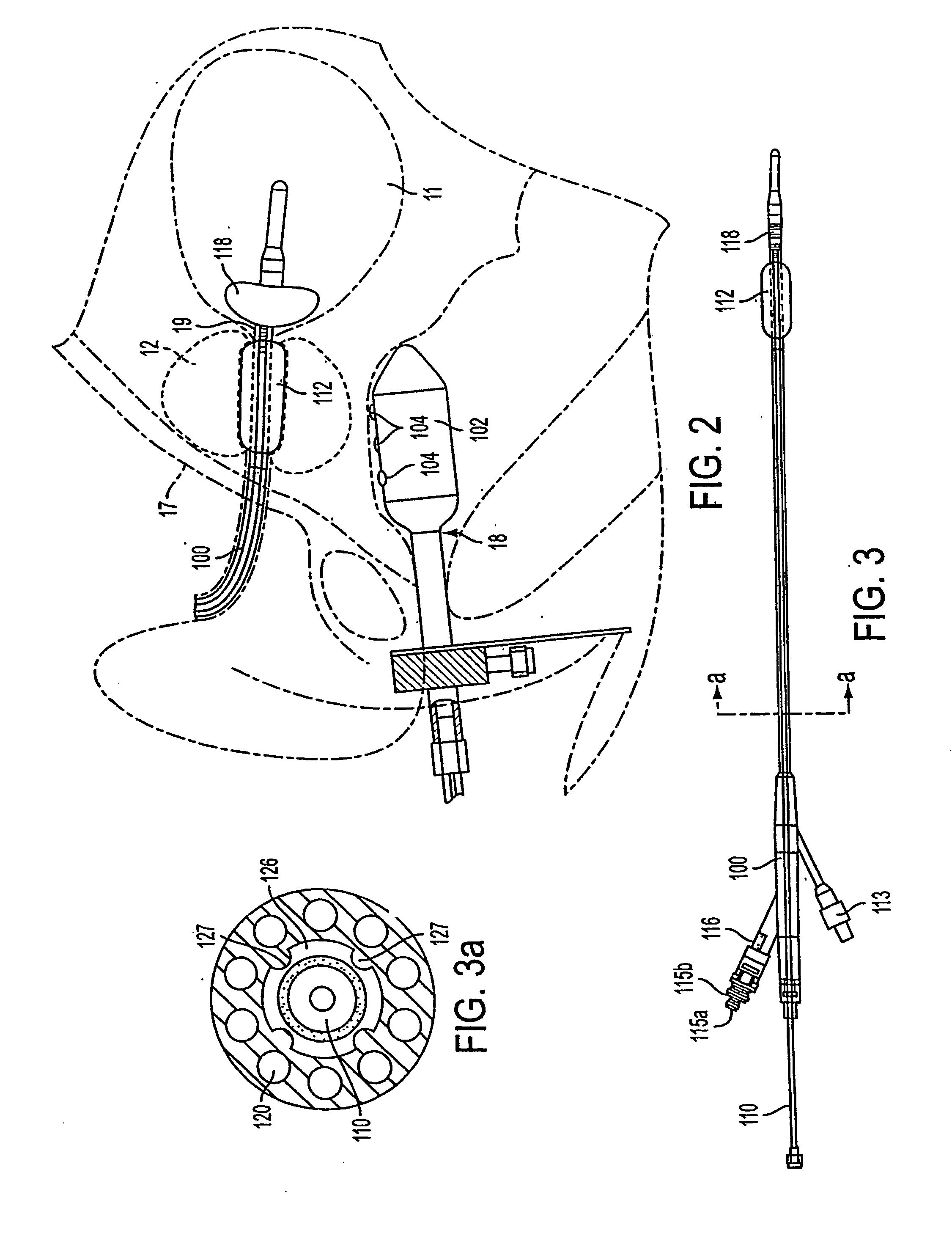
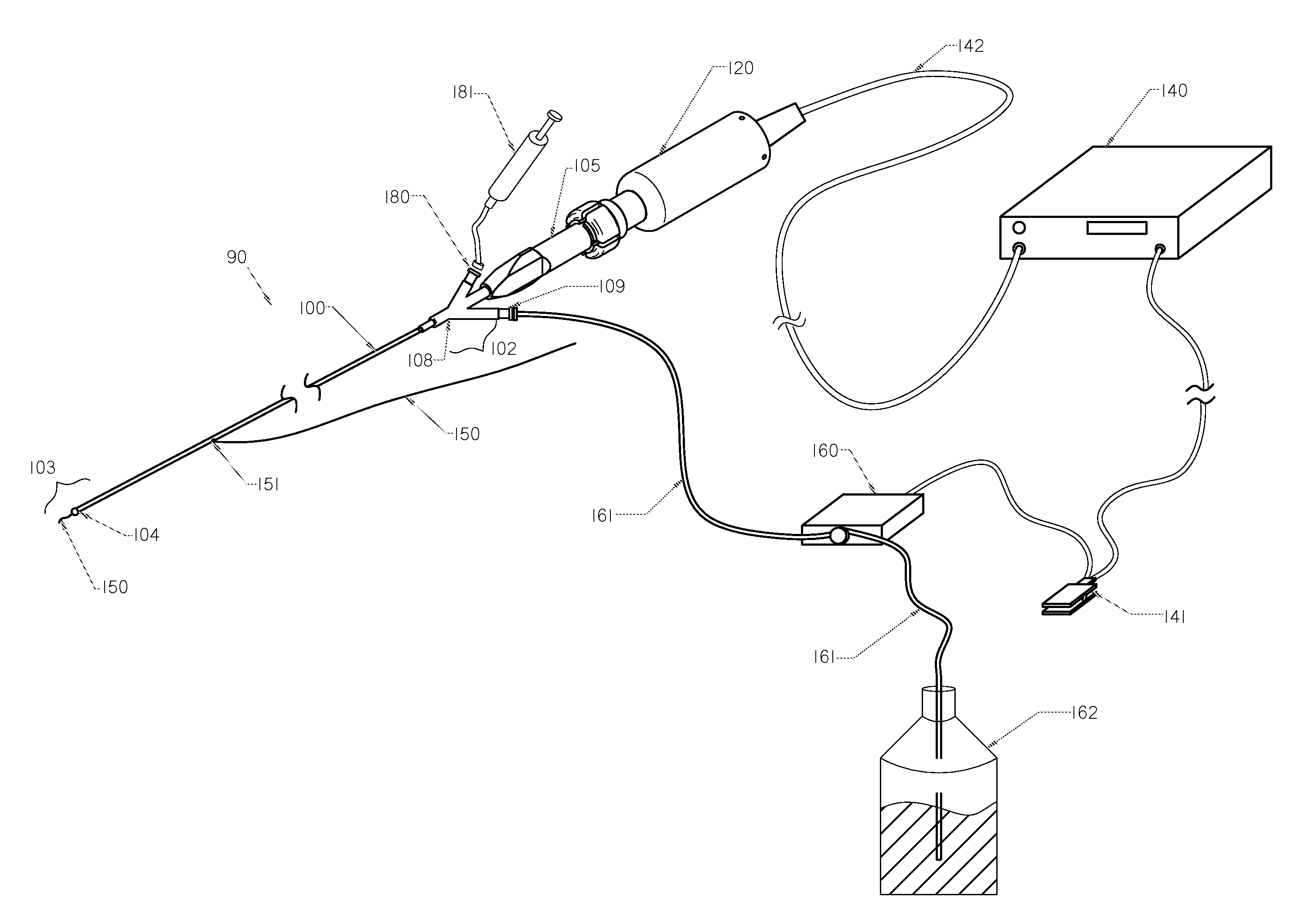
Method and apparatus treating tissue adjacent a bodily conduit with thermocompression and drugs
InactiveUS20050203498A1Efficient transferImprove drug deliveryElectrotherapyDrug compositionsInsertion stentHeat sensitive
A method and apparatus (100) of treating tissue adjacent a bodily conduit using thermotherapy, while preventing obstructions of the bodily conduit due to edema, includes injection of a drug-encapsulated within a heat-sensitive carrier, such as a liposome, within a region of tissue to be treated. The heat produced by the energy-emitting source (110) heats a portion of the tissue surrounding the bodily conduit to a temperature of approximately 43° C. for a time sufficient to destroy the heated portion of the tissue. In addition, the heat produced by the energy-emitting source (110) activates the heat-sensitive carrier to activate the release of the encapsulated drug and the drug targets the tissue to be heated. The focused energy of the energy-emitting source together with the compression acting on the target area can assist in delivering drugs to the target area so that a natural stent has a long term efficacy.
Owner:MEDIFOCUS
Devices and Methods for Endovascular Therapies
InactiveUS20130023897A1Improve clinical outcomeFacilitate distribution , delivery , absorption and/or efficacySurgical instrument detailsEndovascular therapyPercent Diameter Stenosis
Medical devices to treat stenosis, inhibiting restenosis, plaque removal, crossing totally occluded arteries or veins, treatment of vulnerable plaque, as well as removal of blood clots from the patient body arc disclosed. Such devices maybe used alone or in combination with therapeutic drugs. In some embodiments, flow protection devices are used for homogeneous drug delivery and removal from the patient to minimize the systemic effect. In some other embodiments, ablated tissue or blood clots are removed from the body after the procedure.
Owner:CARDIOPROLIFIC INC
Portable electronic therapy device and the method thereof
InactiveUS9710607B2Increase permeationImprove drug deliveryUltrasound therapyElectrotherapyPhysical medicine and rehabilitationAutomatic control
The present disclosure relates to an electronic therapy device including automatic controlled application of energies along with feedback control using sensors for improved synergistic effects and further the device is configured to be used for longer periods of time for improved and optimal therapeutic results without causing any adverse effects, the device can be used for pain management, healing, fitness, cosmetic and topical delivery related applications and a method for performing electronic therapy using the said portable electronic device.
Owner:ITRACE BIOMEDICAL
Compositions and methods for oral drug delivery
InactiveUS20110142889A1Promote absorptionImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsPermeationExcipient
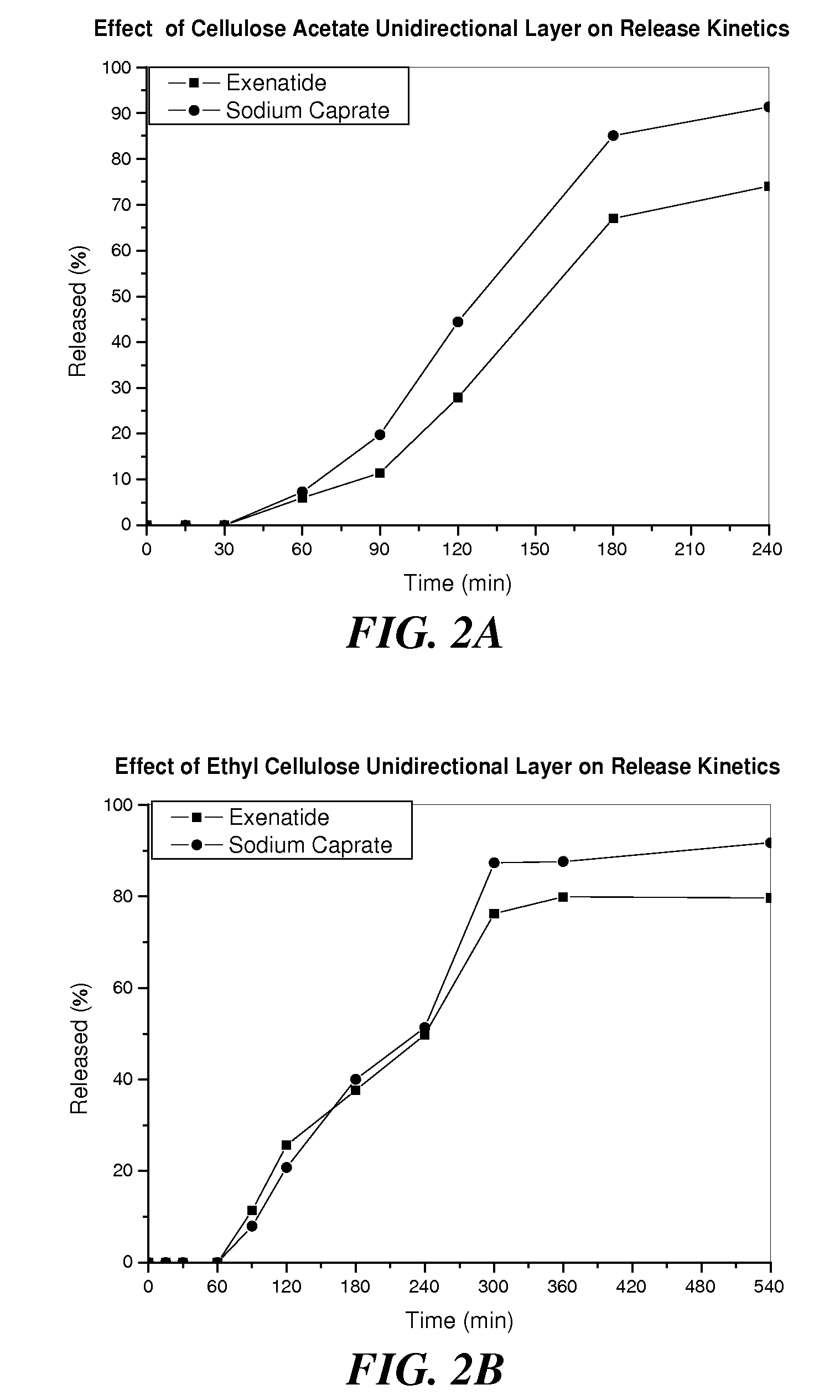
The invention provides a pharmaceutical composition for oral drug delivery comprising a solid dosage form containing an effective amount of a therapeutic agent, a permeation enhancer and a pharmaceutically acceptable excipient and a bioadhesive layer containing a bioadhesive polymer, and optionally comprising an impermeable or semi-permeable layer having an opening capable of directing a unidirectional release of the therapeutic agent and the permeation enhancer from the solid dosage form. Methods of making and using the present pharmaceutical composition are also provided.
Owner:NOD PHARMA
Phosphate derivatives of pharmaceutical products
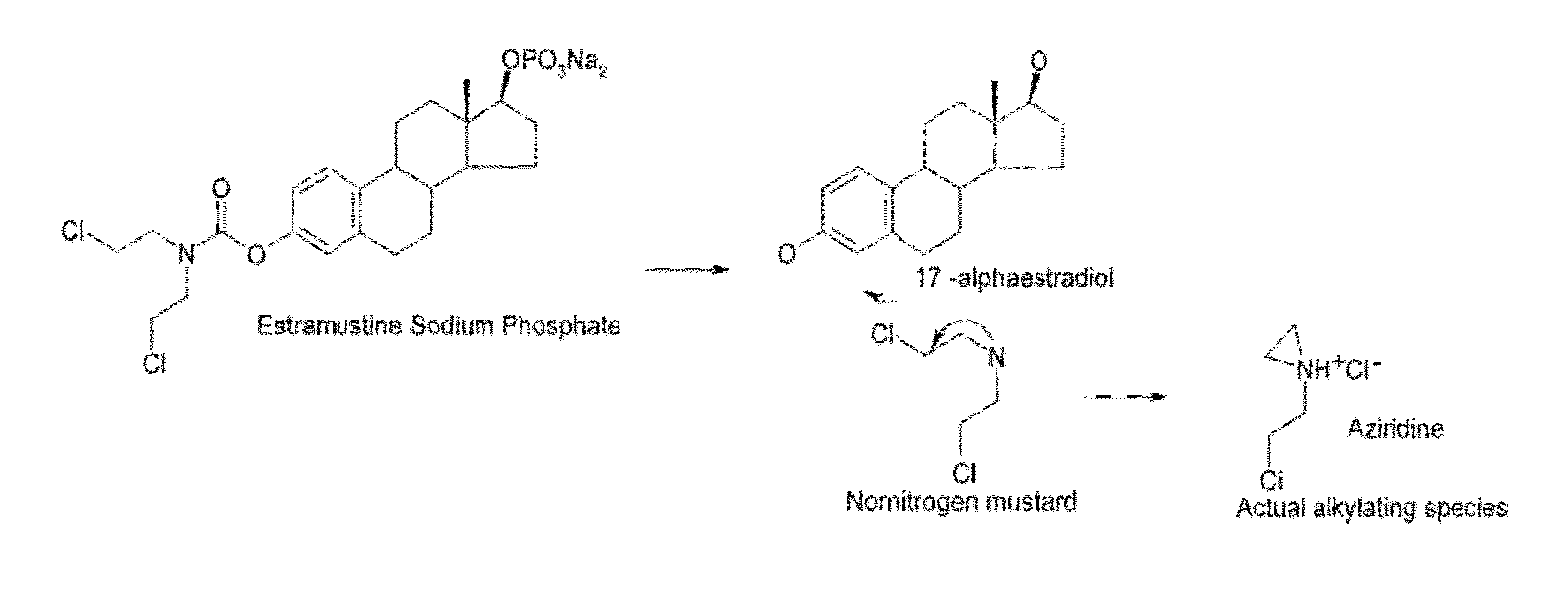
InactiveUS20070042999A1Quick conversionReduce solubilityBiocideNervous disorderAnesthetic AgentPhosphate
According to the invention, there is provided a complex of a pharmaceutical compound selected from the group consisting of opioids, hormones, anaethetics and chemotherapeutic agents comprising the reaction product of: (a) one or more phosphate derivatives of one or more opioids, steroid hormones, thyroid hormones, anaesthetics or chemotherapeutic agents having a phenolic, primary alcohol, secondary alcohol or tertiary hydroxyl group; and (b) a complexing agent selected from the group comprising amphoteric surfactants, cationic surfactants, amino acids having nitrogen functional groups and proteins rich in these amino acids.
Owner:VITAL HEALTH SCIENCES PTY LTD
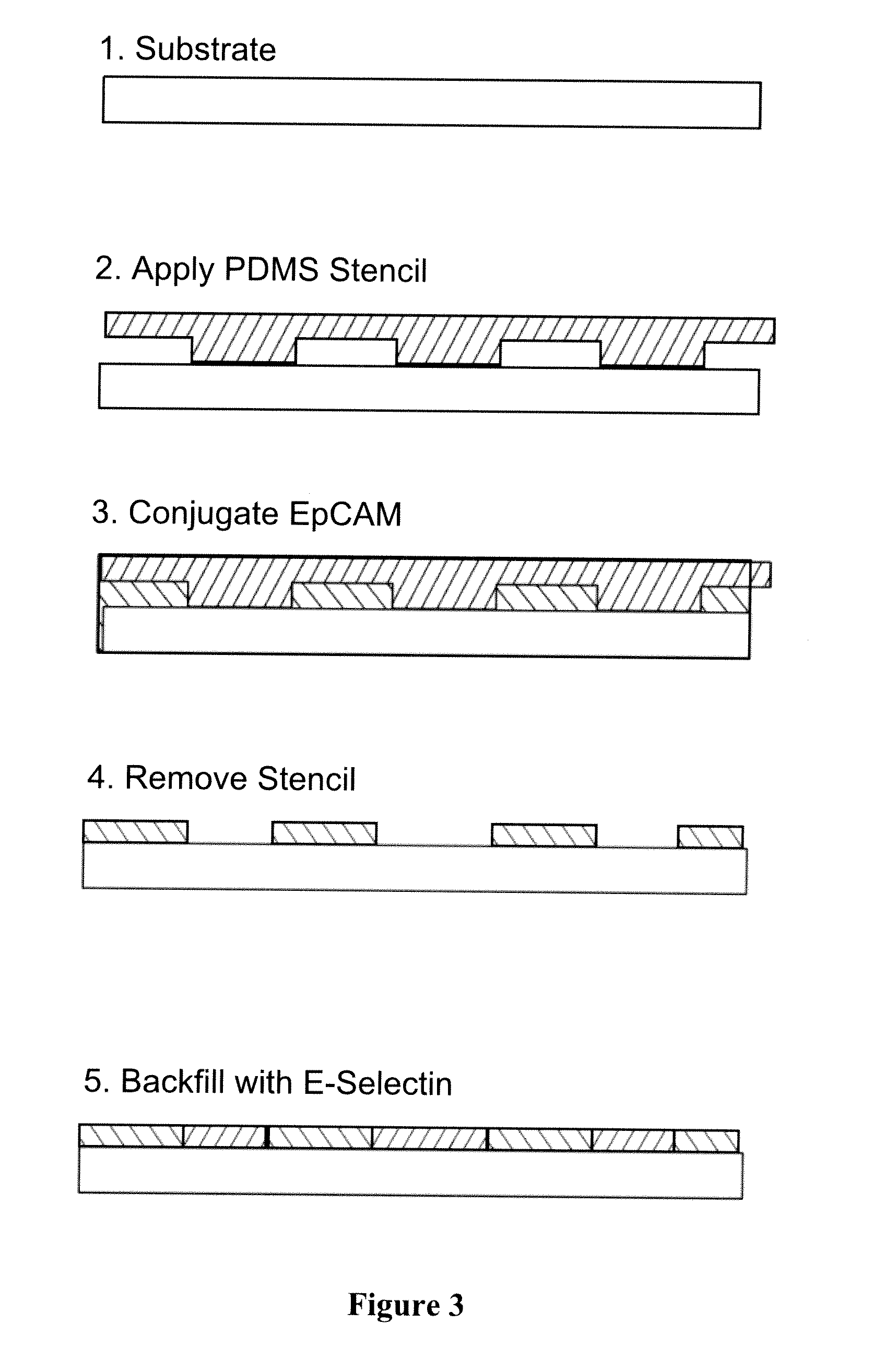
Methods and Devices for Capturing Circulating Tumor Cells
InactiveUS20120077246A1Enhance active targeting efficacyIncrease interacting surface areaLaboratory glasswaresOn/in biological cellCirculating cancer cellBiology
A method of capturing a Circulating Tumor Cell (CTC) from a sample includes introducing a sample into a microfluidic device having a cell capture surface and a flow modification surface under conditions that allow a CTC to bind to a cell rolling-inducing agent and a capturing agent disposed on the cell capture surface. The flow modification surface induces a rotational flow within the sample as it flows through the microfluidic device.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com