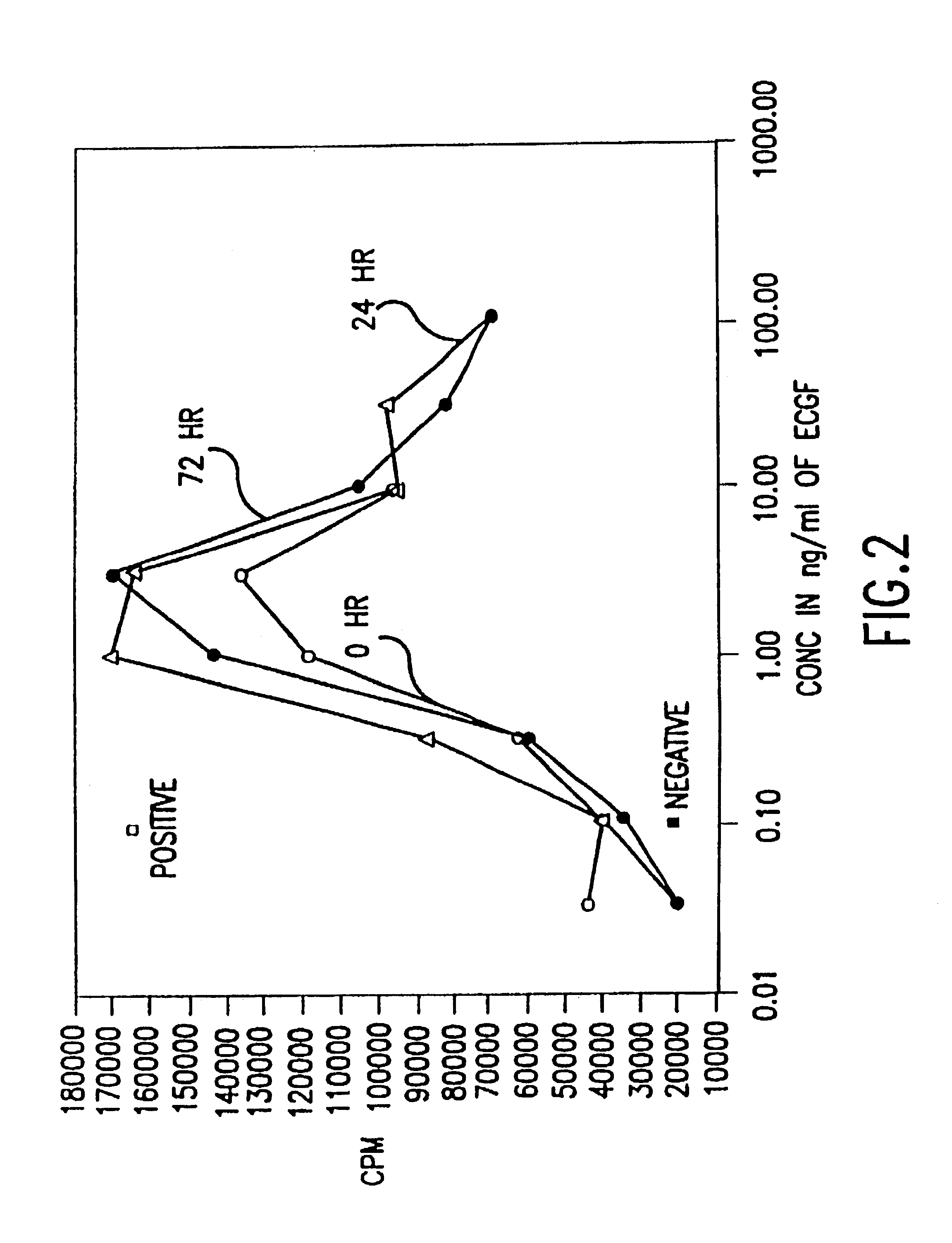
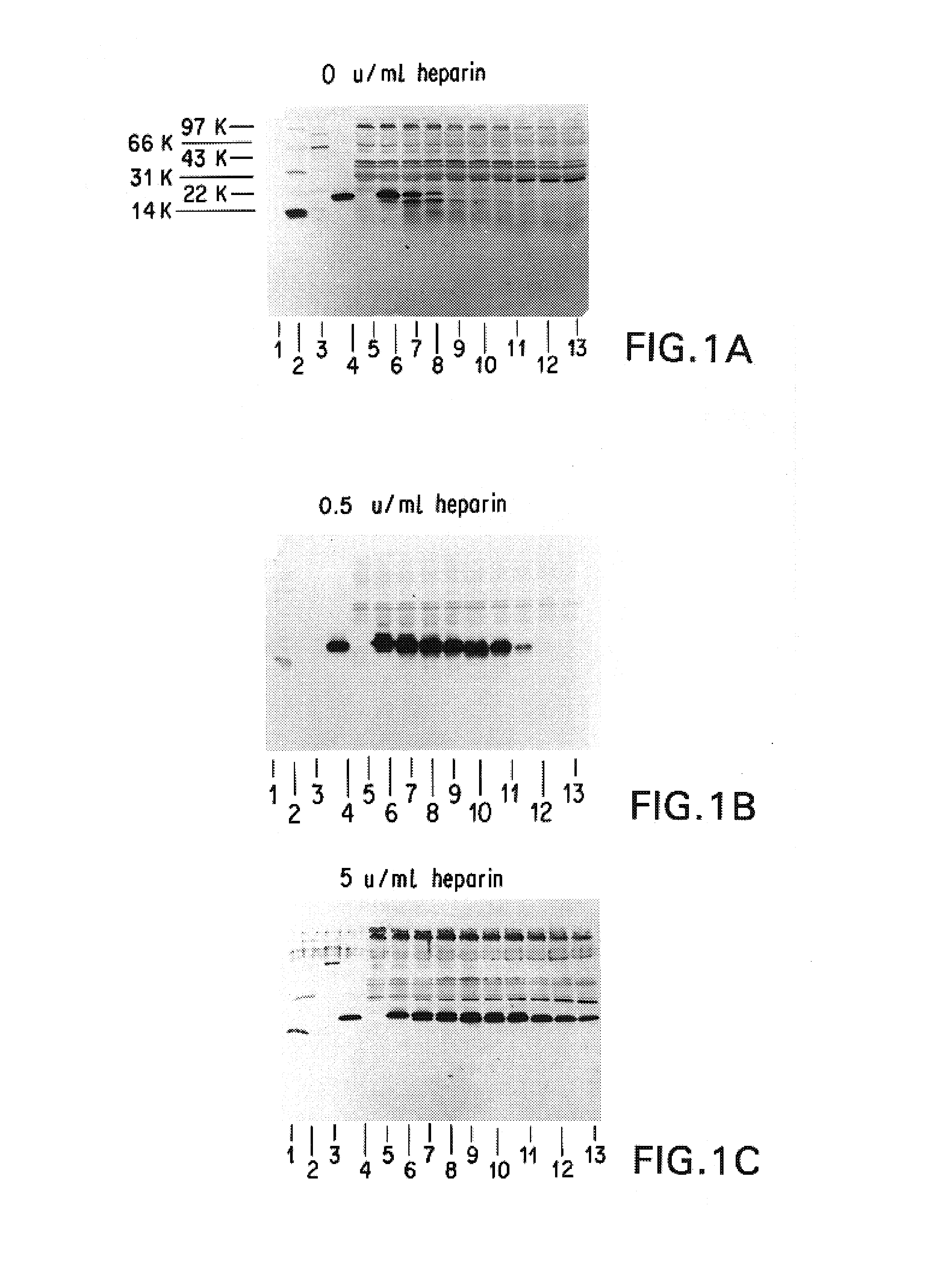
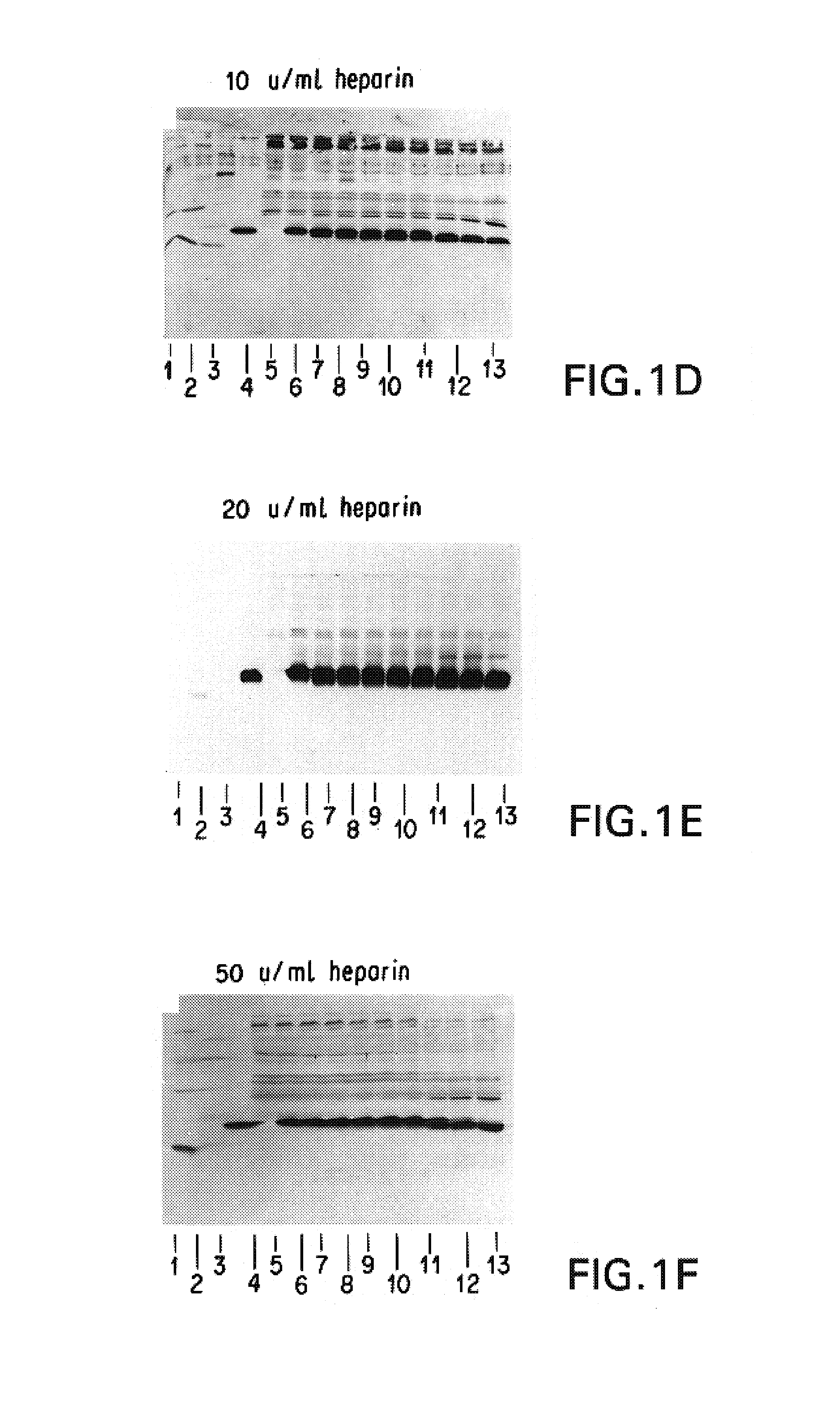
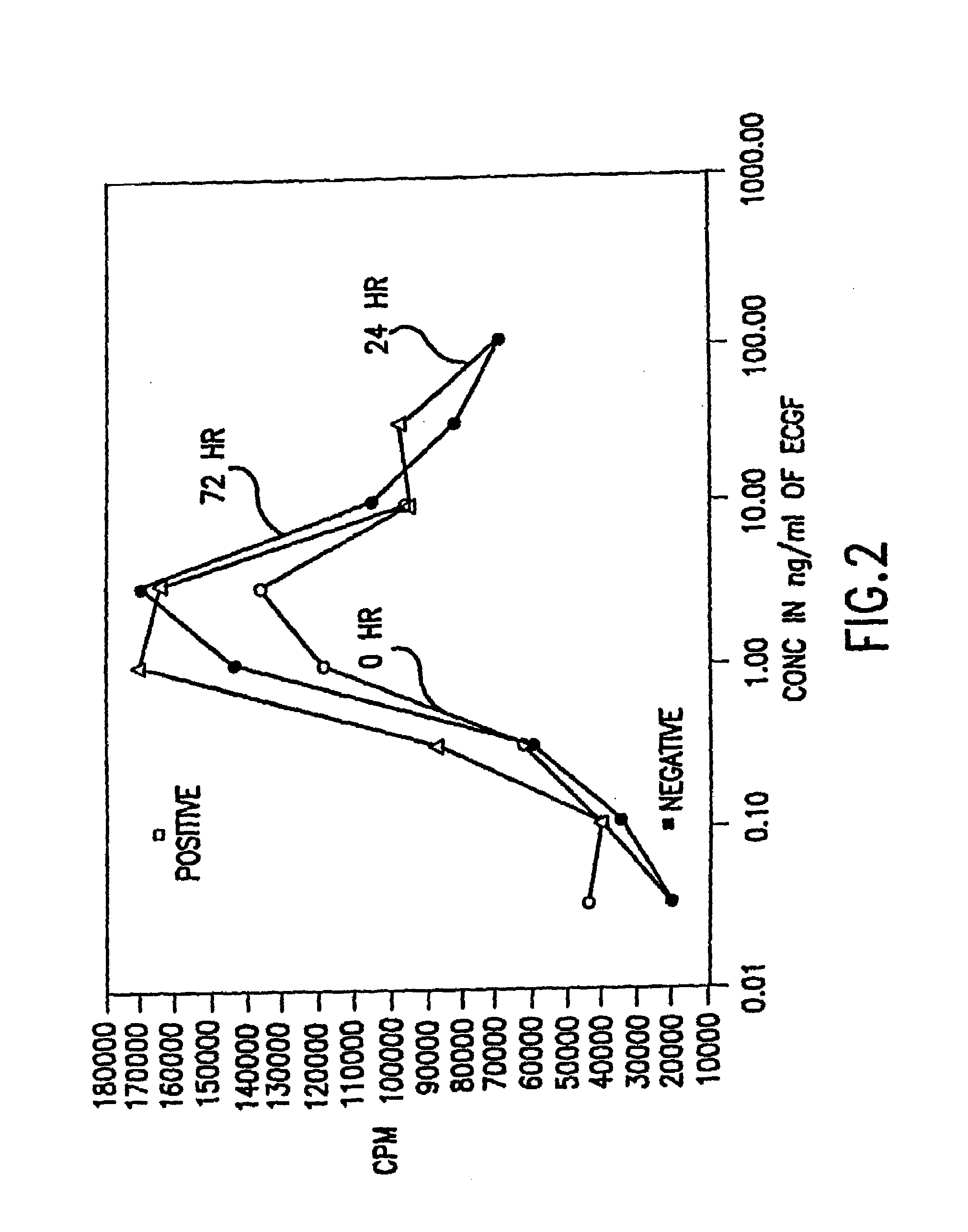
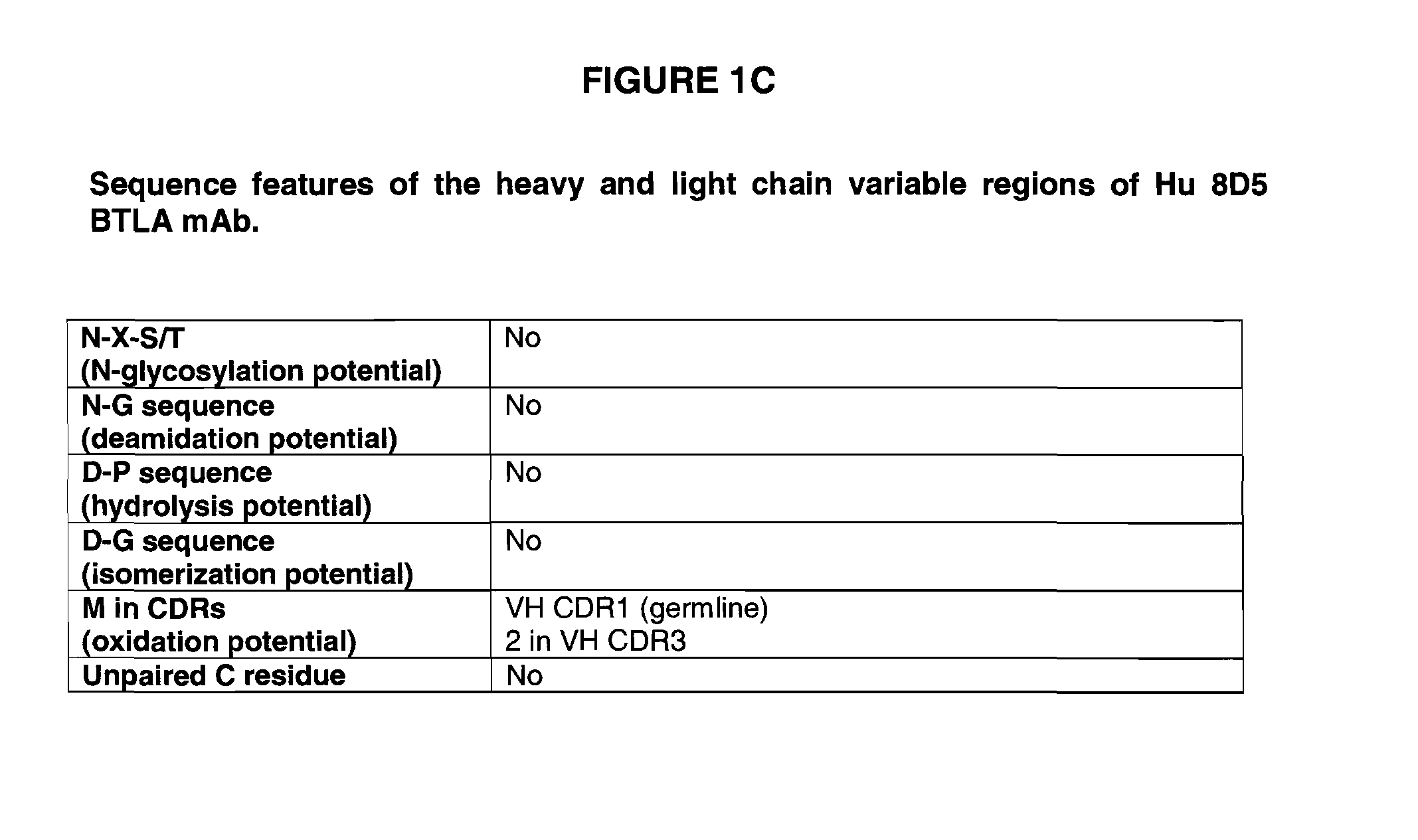
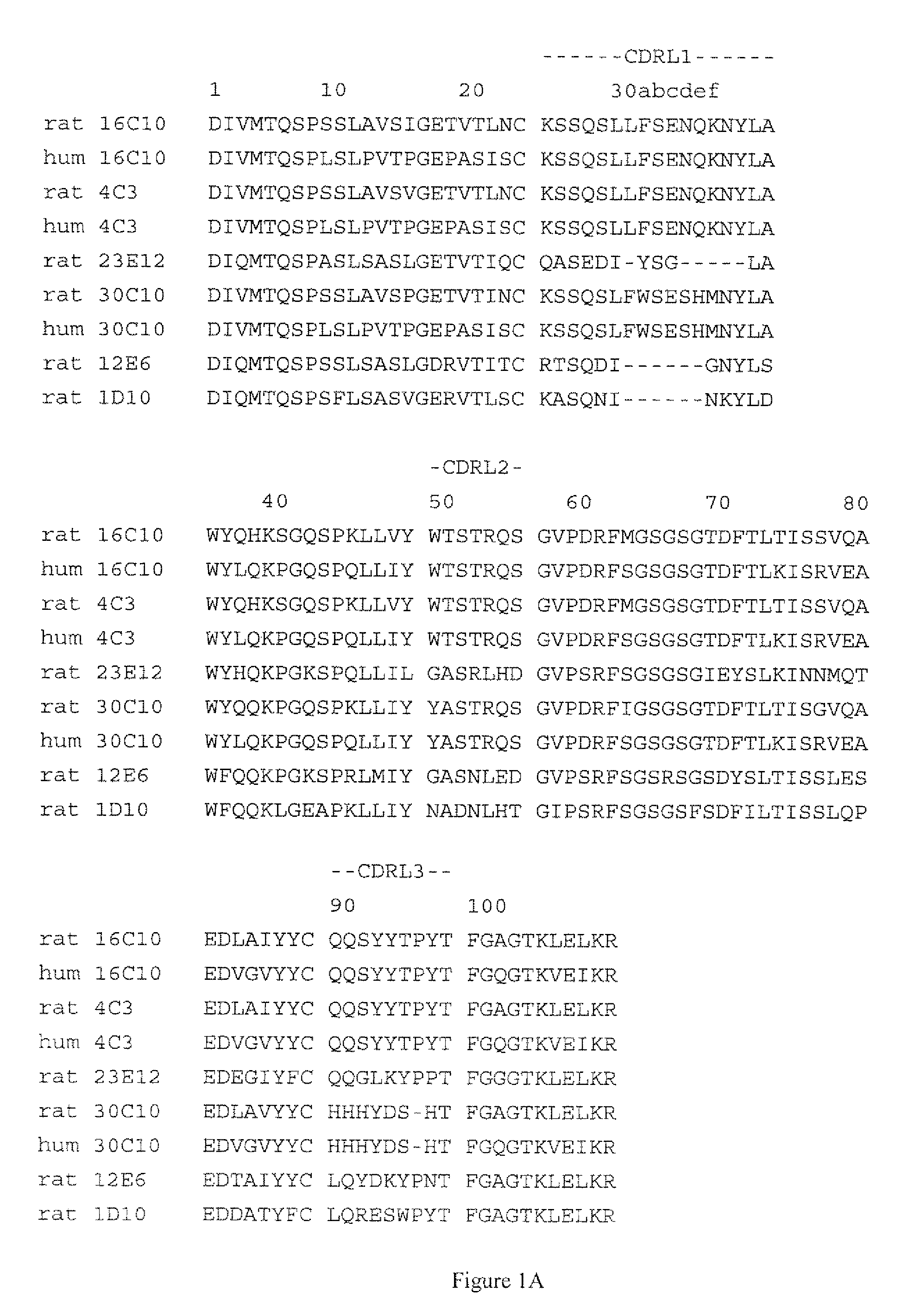
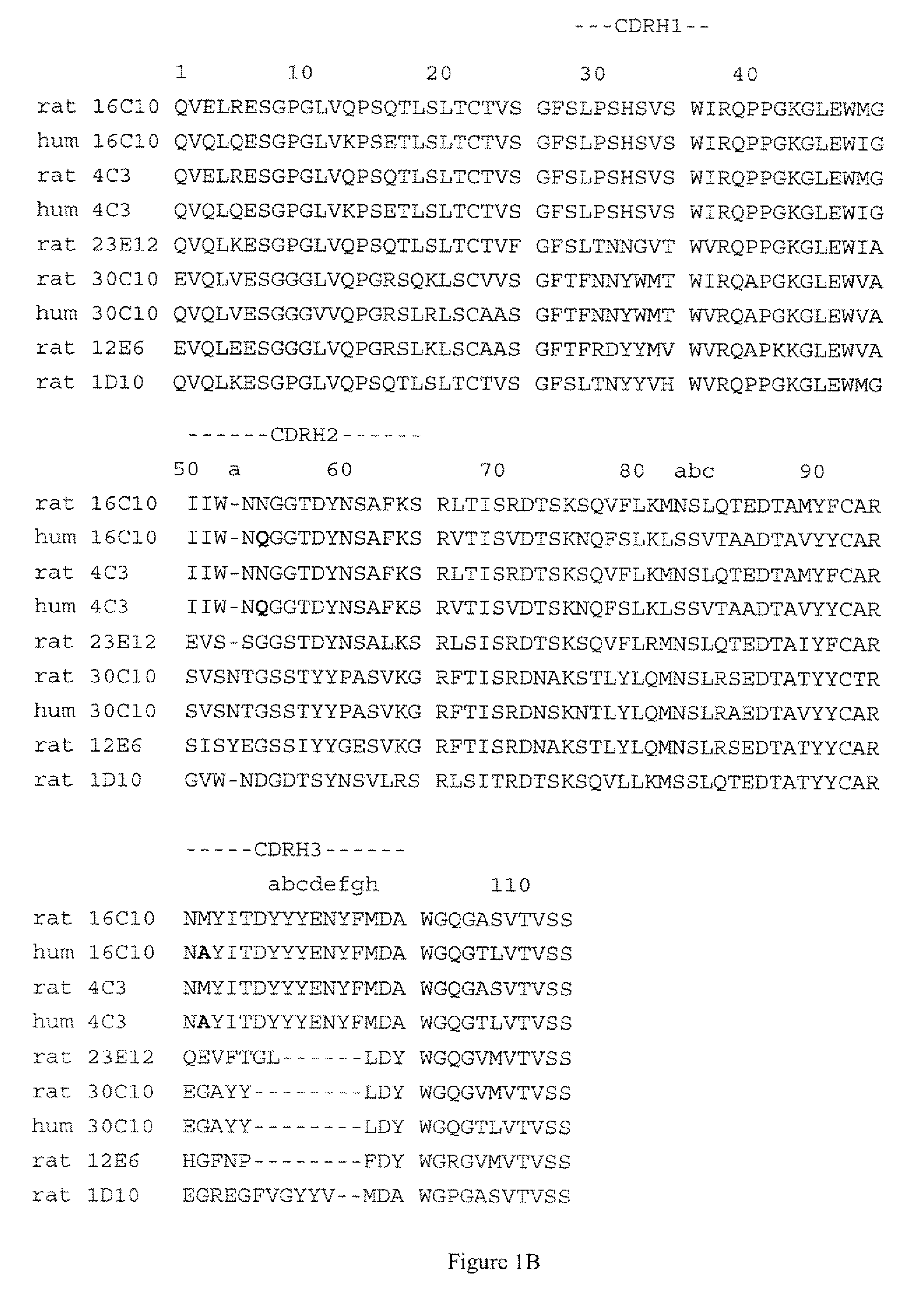
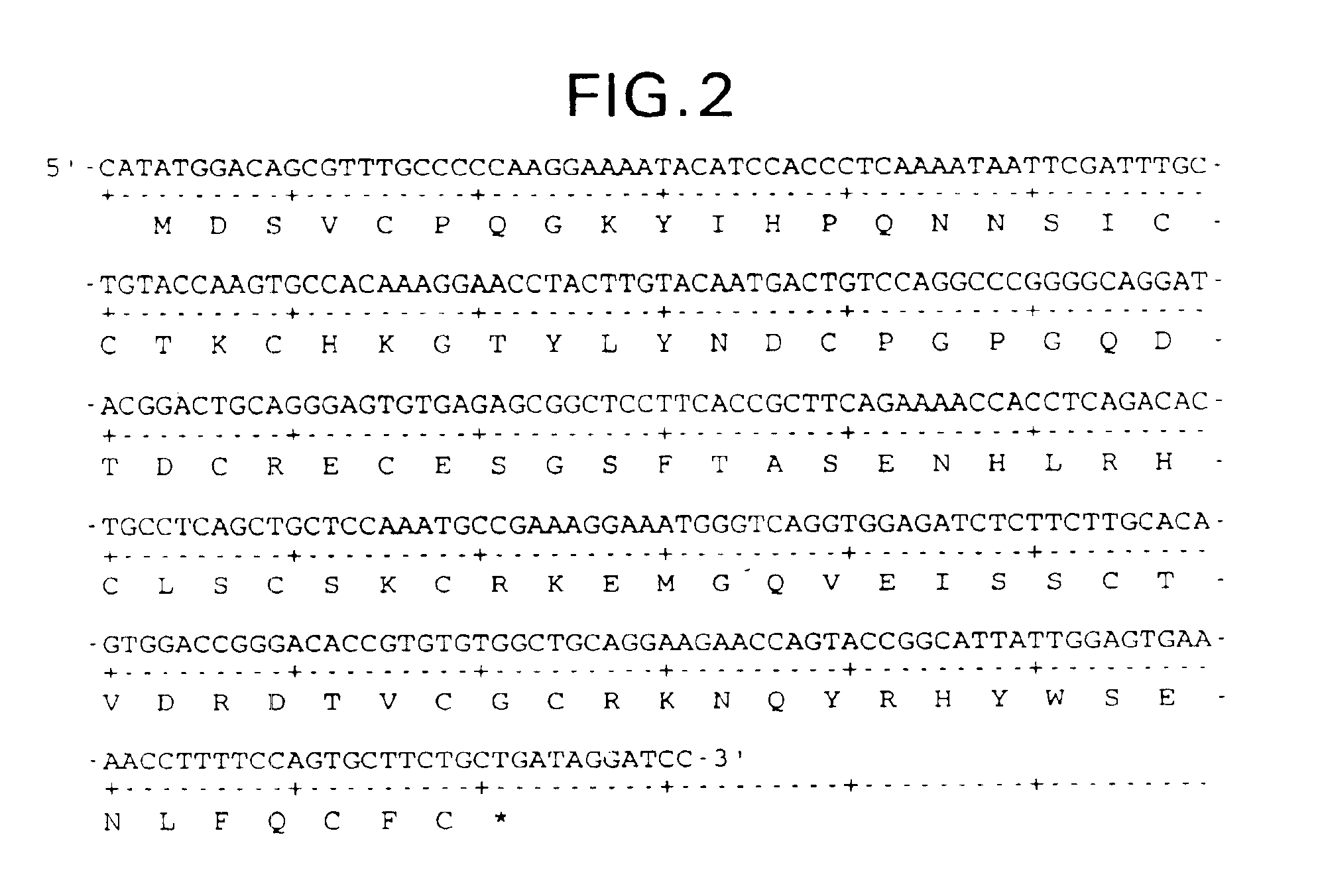
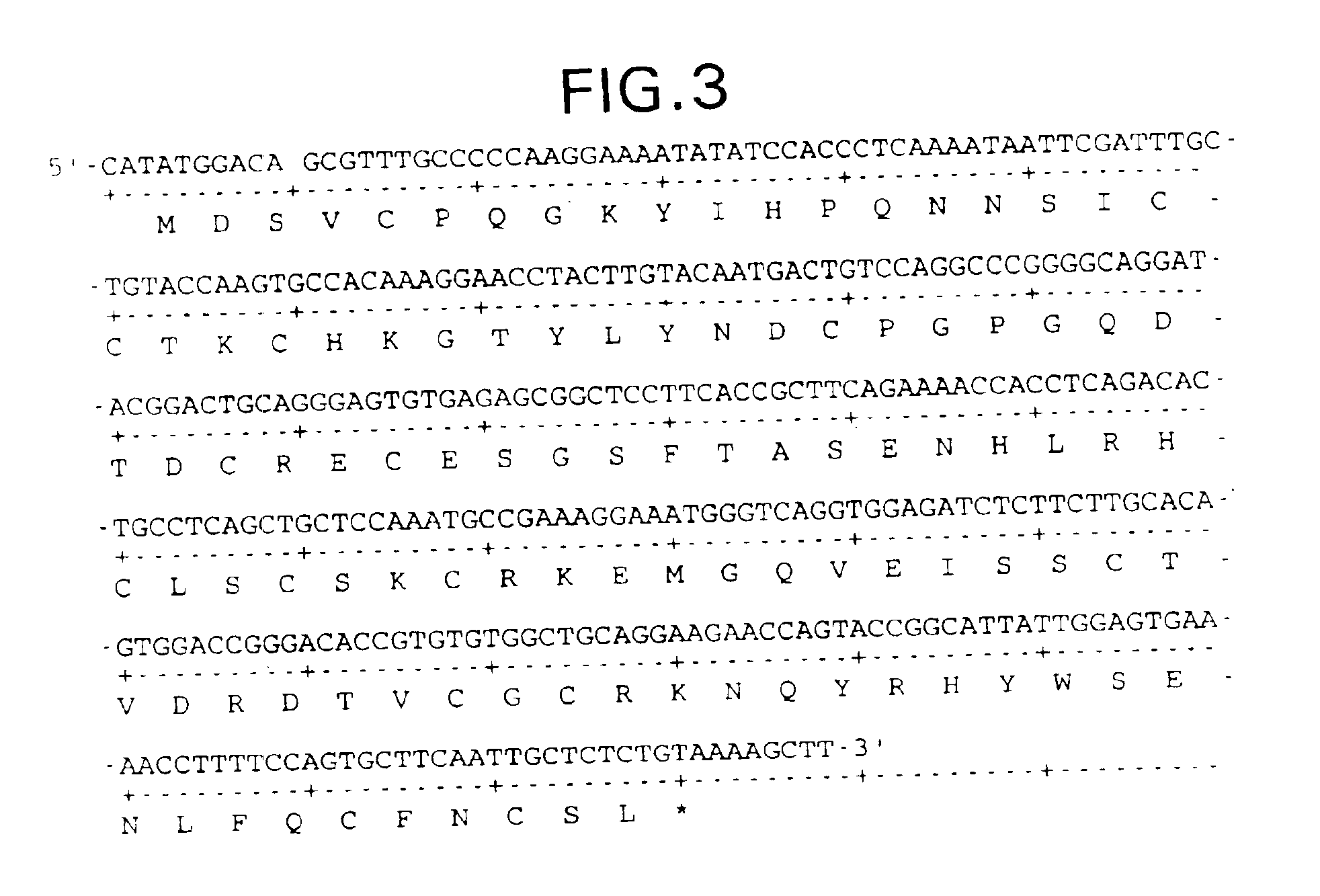
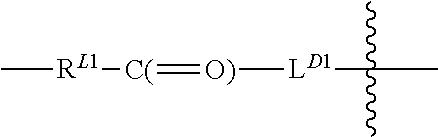Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
436results about How to "Low antigenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
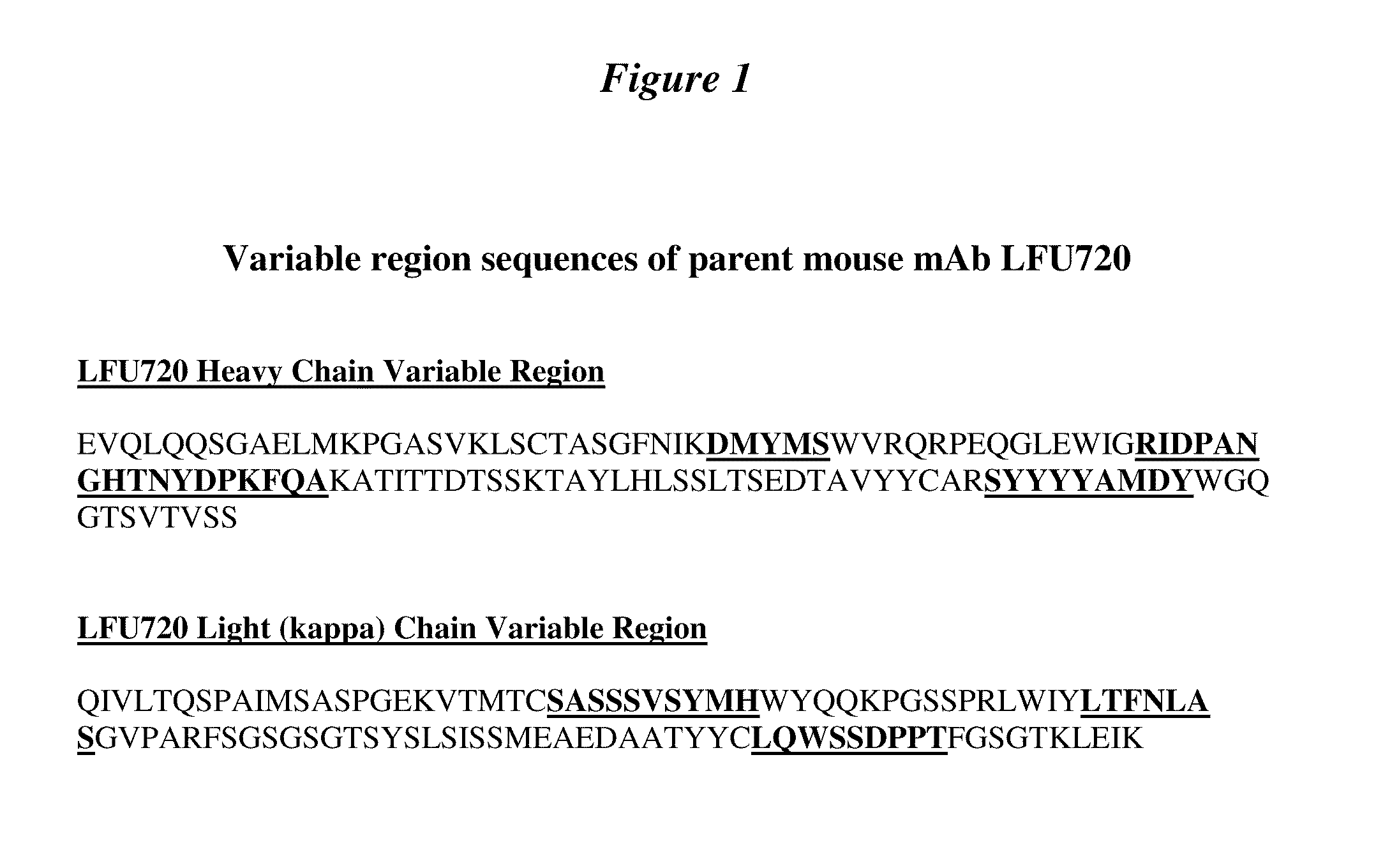
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
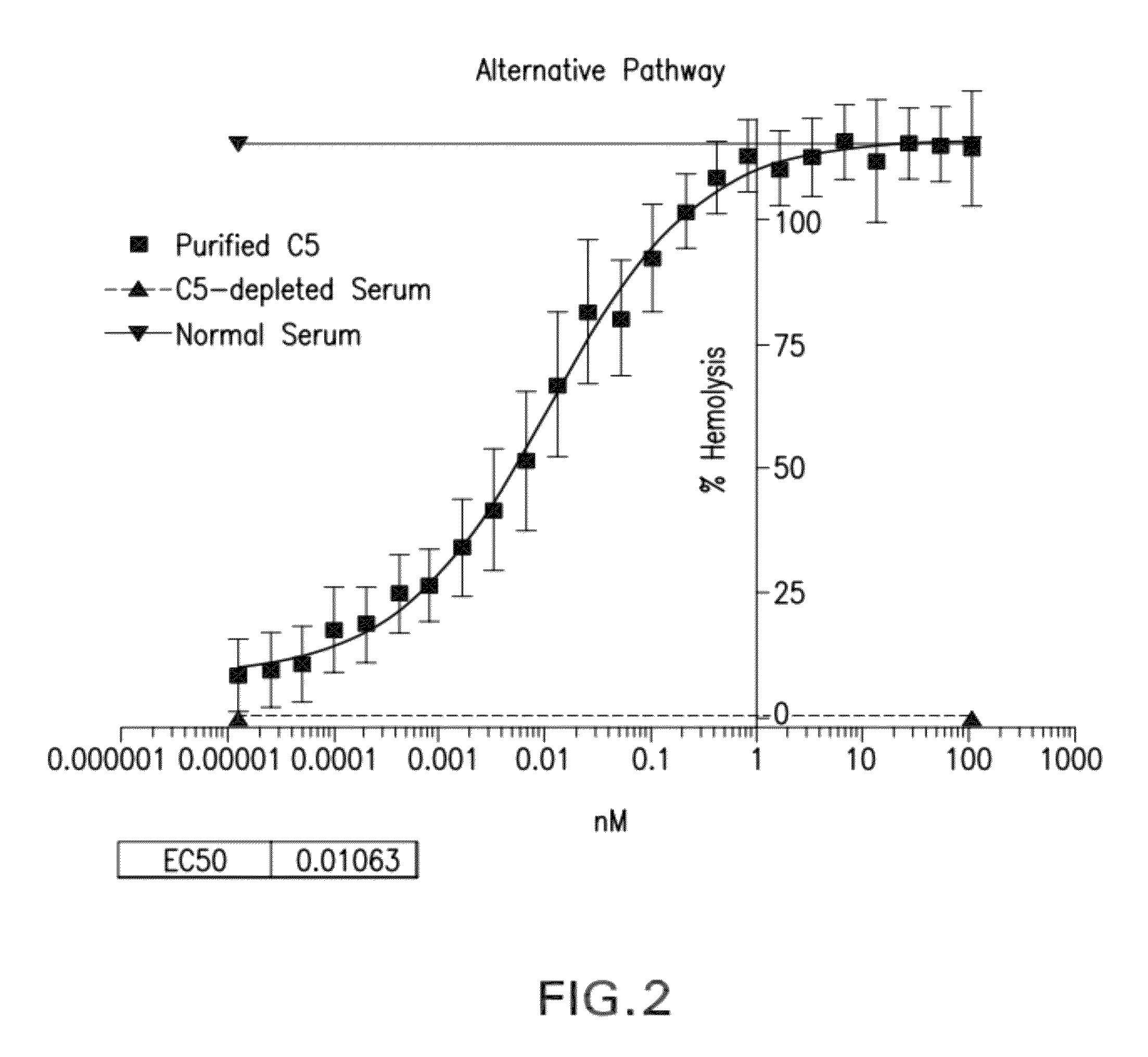
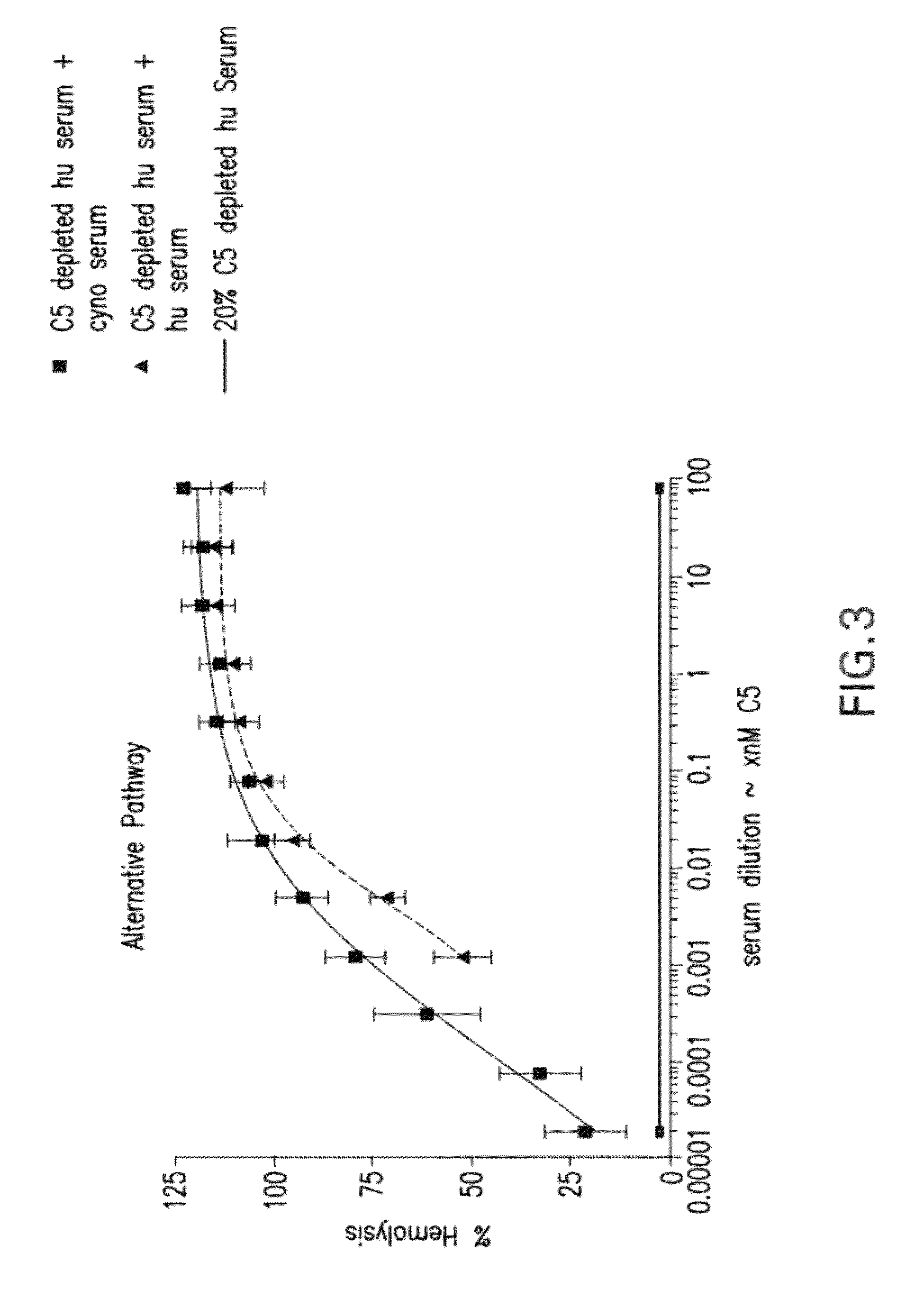
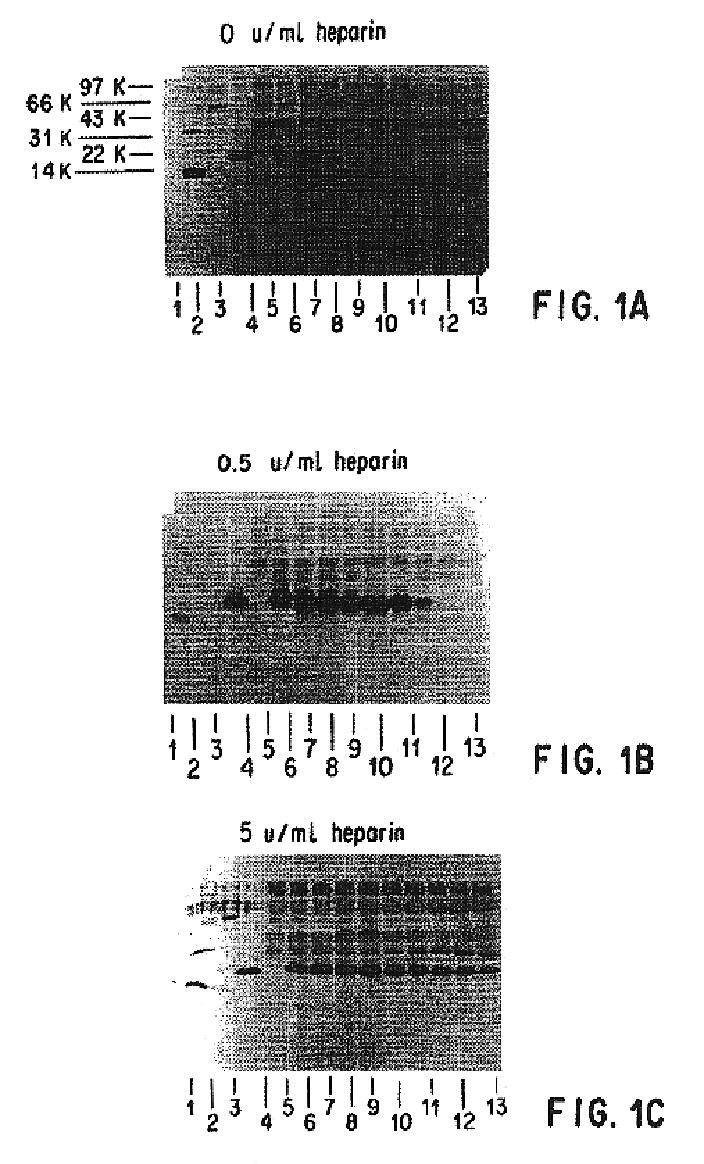
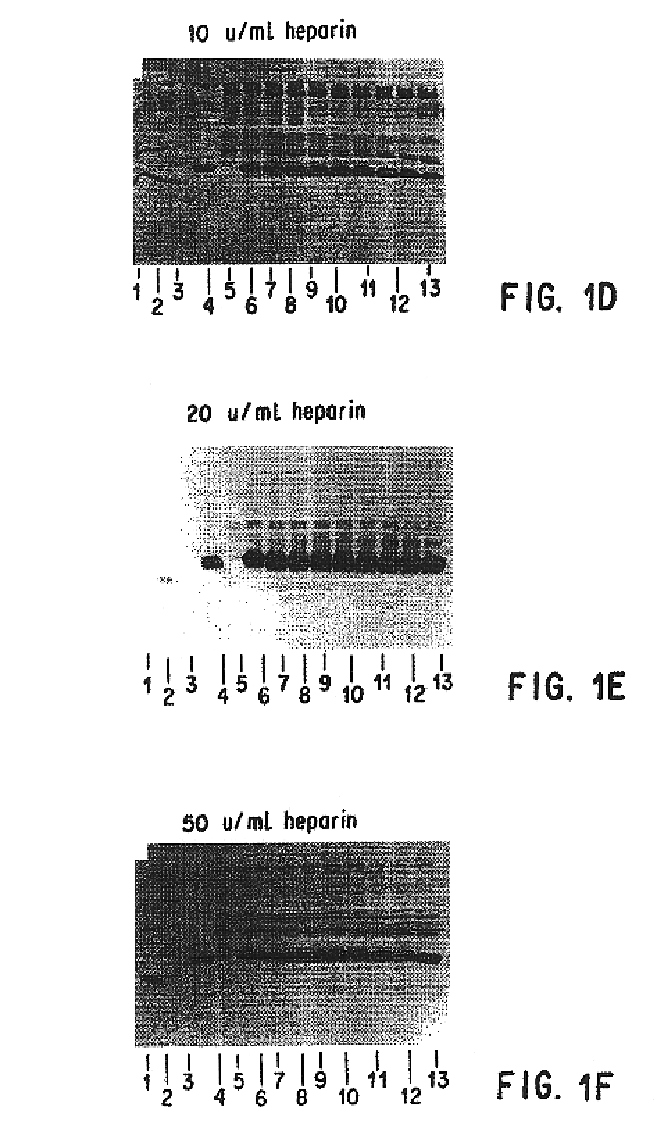
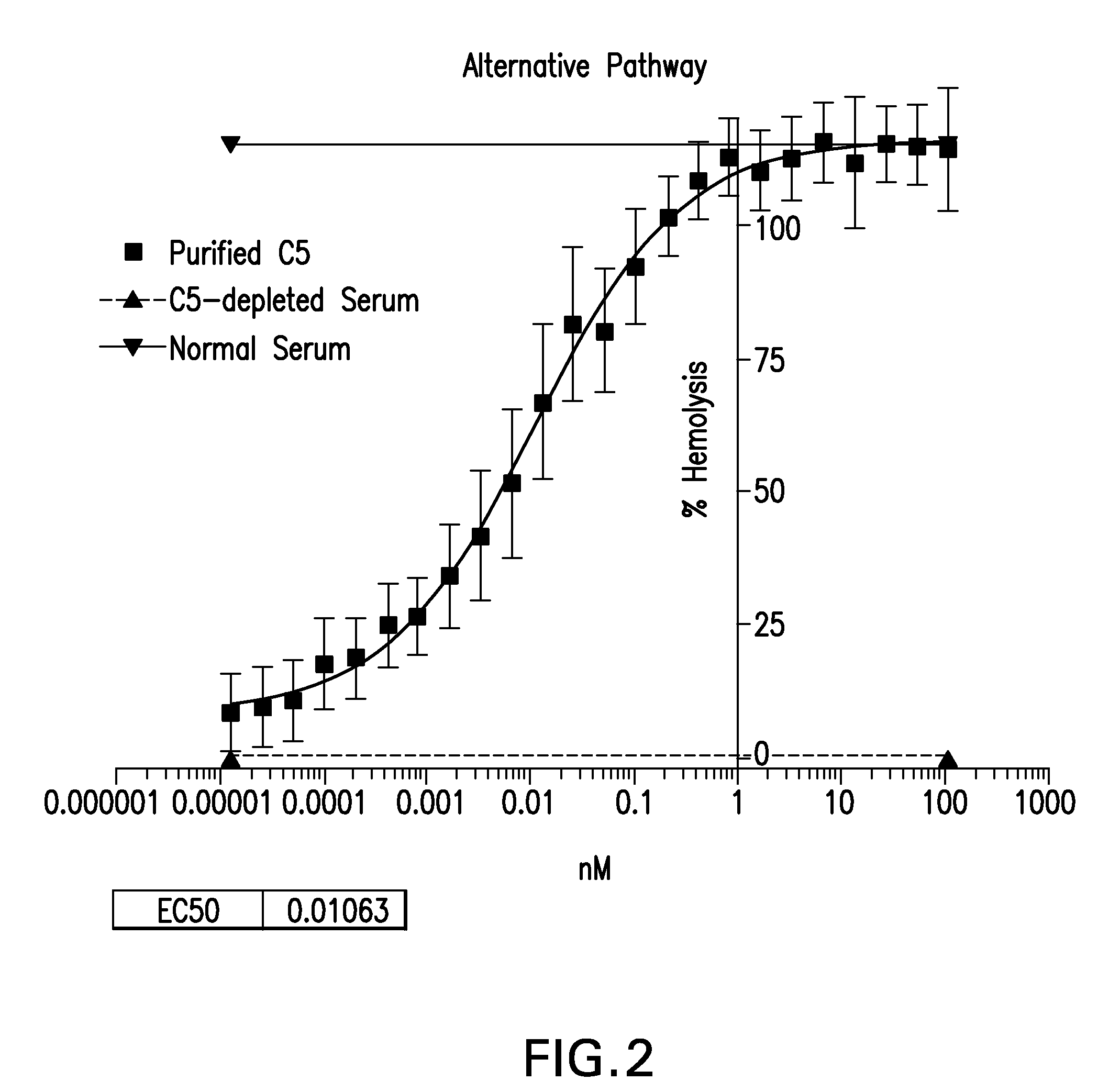
Compositions and methods for antibodies targeting complement protein C5
The present invention relates to antibodies targeting complement protein C5 and compositions and methods of use thereof.
Owner:HIREVUE +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
Pcsk9 antagonists
ActiveUS20110142849A1Reduce severitySufficient amountPeptide/protein ingredientsGenetic material ingredientsKexinSubtilisins
The present invention provides antibody antagonists against proprotein convertase subtilisin / kexin type 9a (“PCSK9”) and methods of using such antibodies.
Owner:NOVARTIS AG
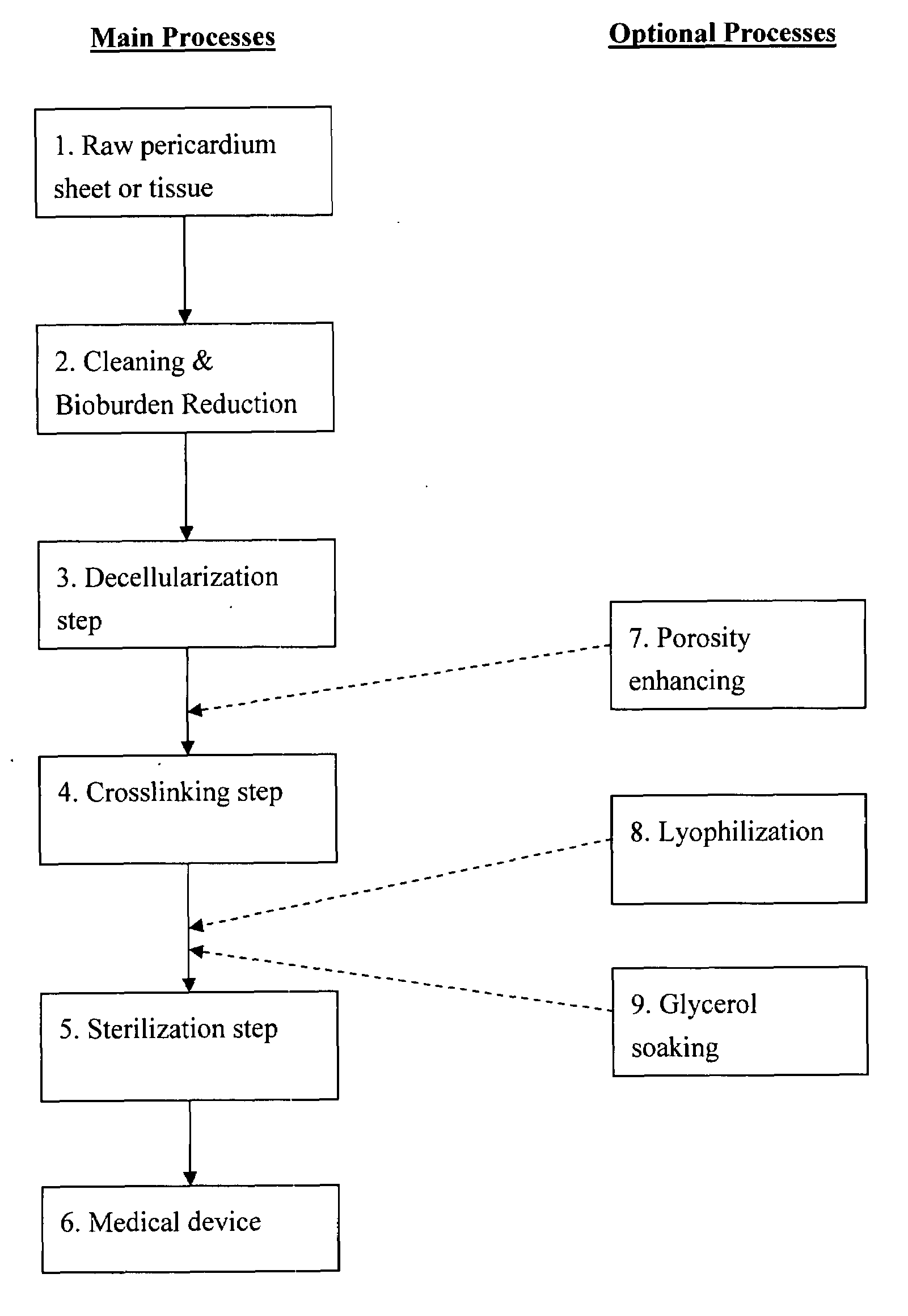
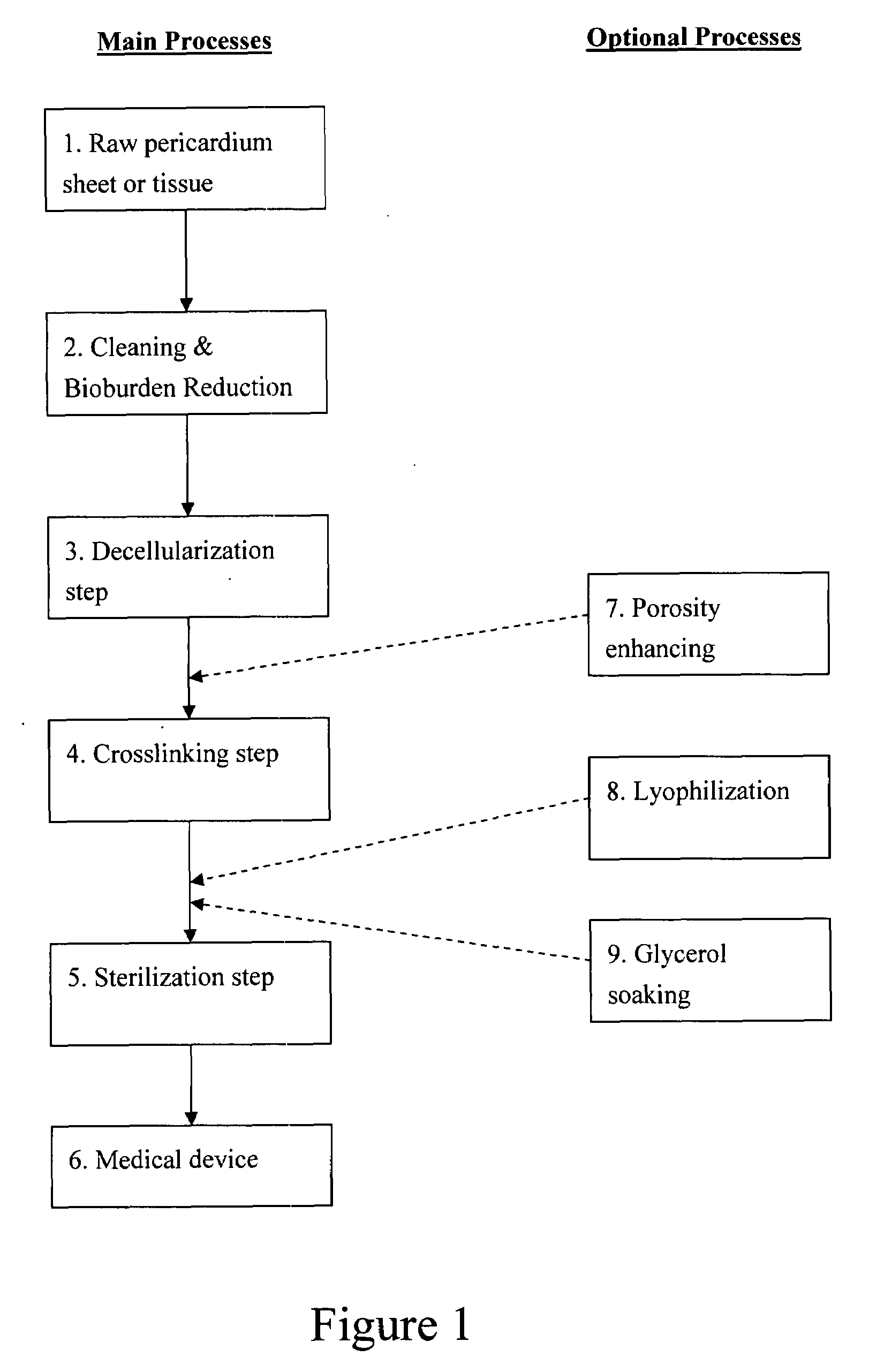
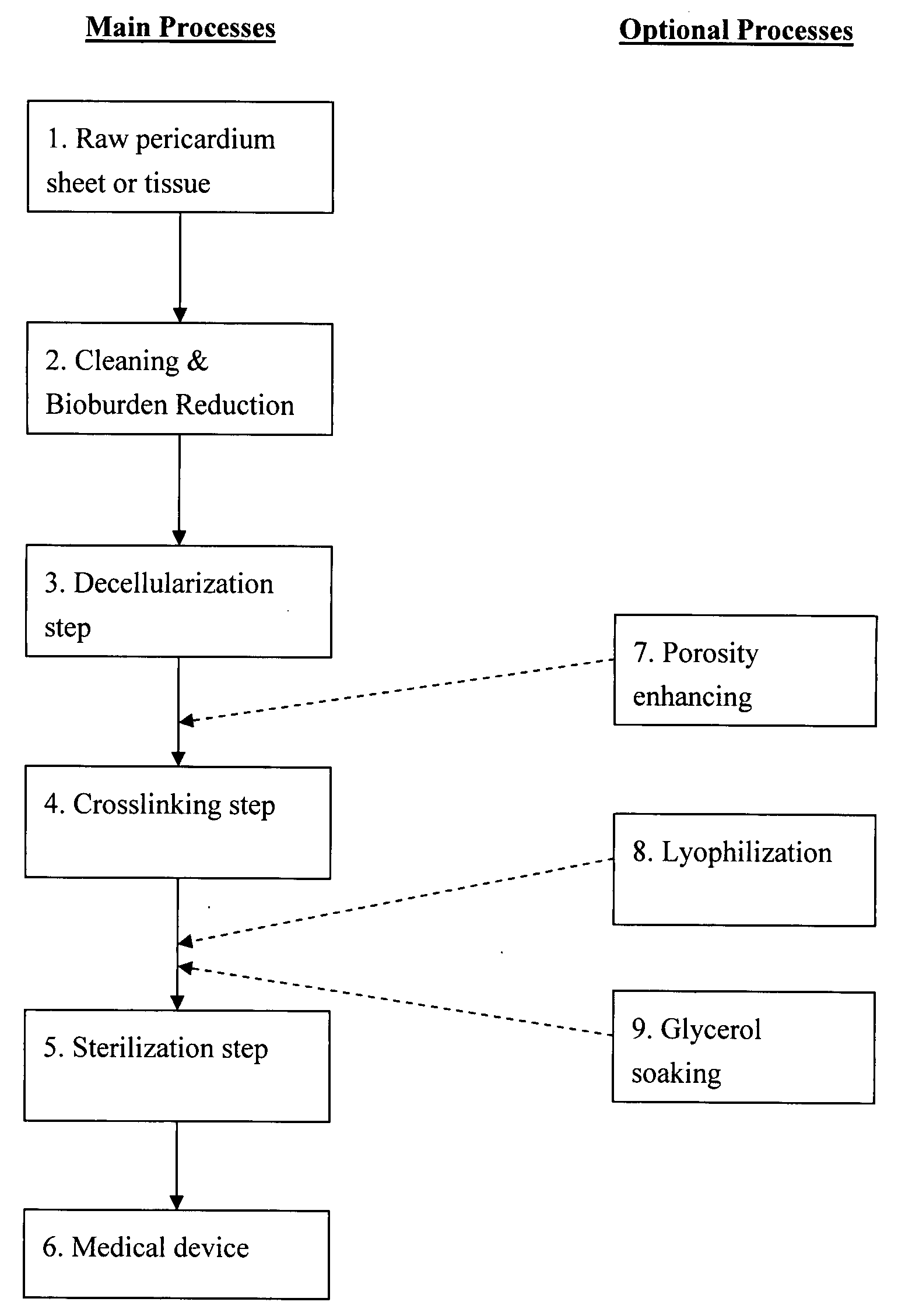
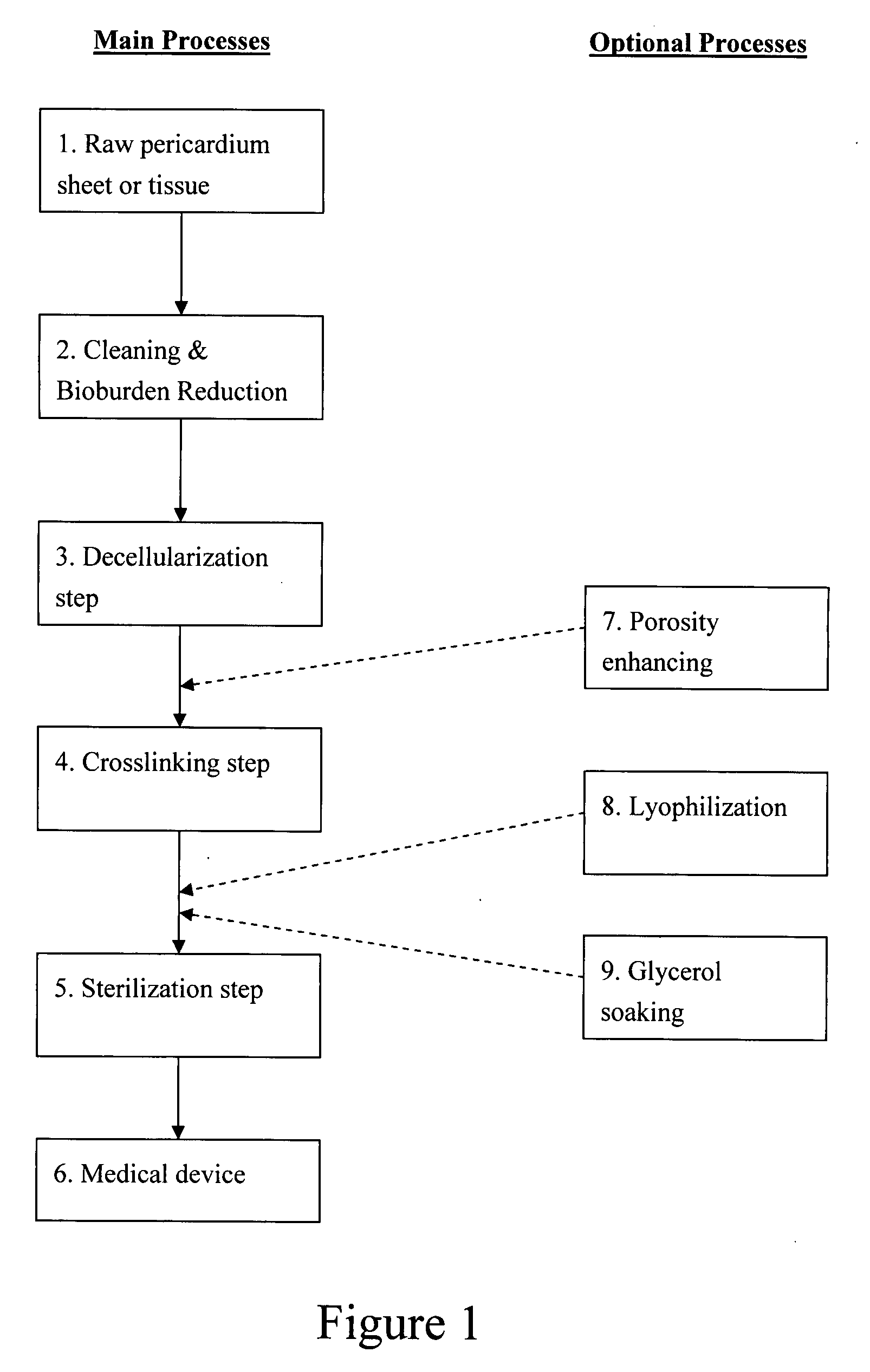
Pericardial tissue sheet
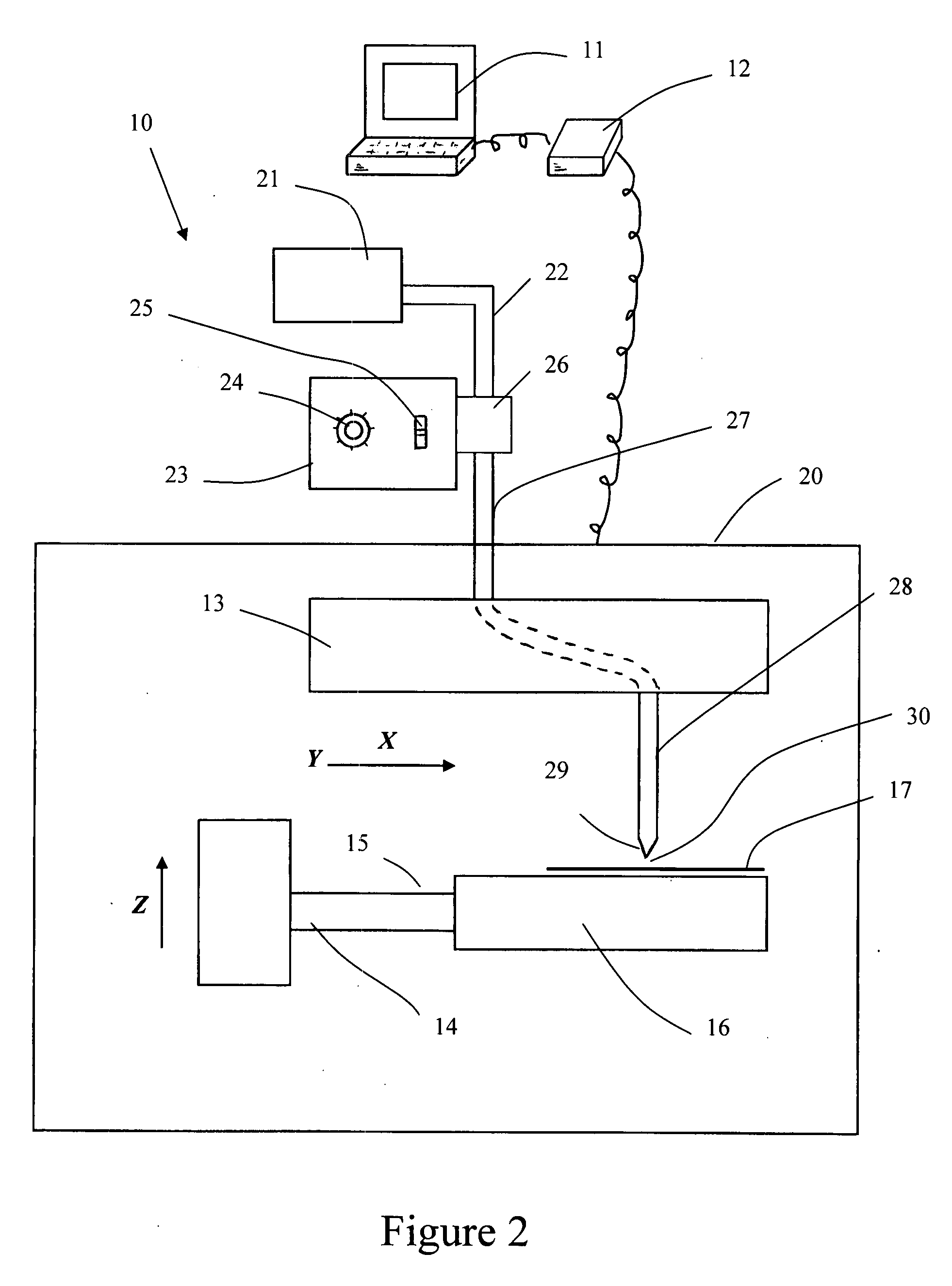
InactiveUS20080195230A1Low antigenicityLow immunogenicityFluid jet surgical cuttersSurgical instruments for heatingThermal energyTissue material
A method of cutting tissue material of biology origin employs a plotted water-jet or RF cutting system. The cutting system is computer controlled and includes a water-jet or RF cutting means combined with a motion system. The cutting energy is selected so that communication of thermal energy into the segment beyond the edge is minimized to avoid damaging the segment adjacent the edge.
Owner:QUIJANO RODOLFO C +1
Acellular biological material chemically treated with genipin
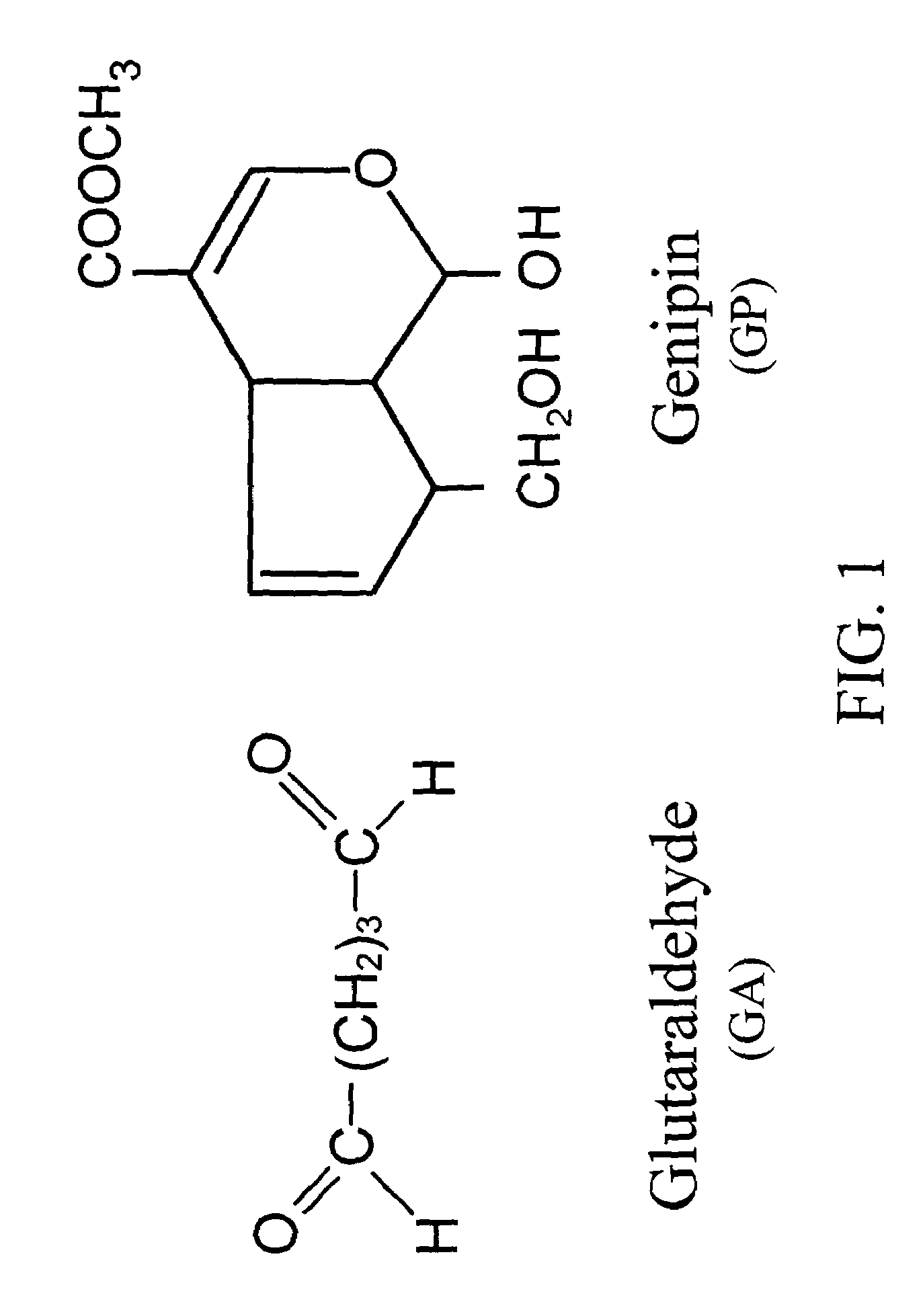
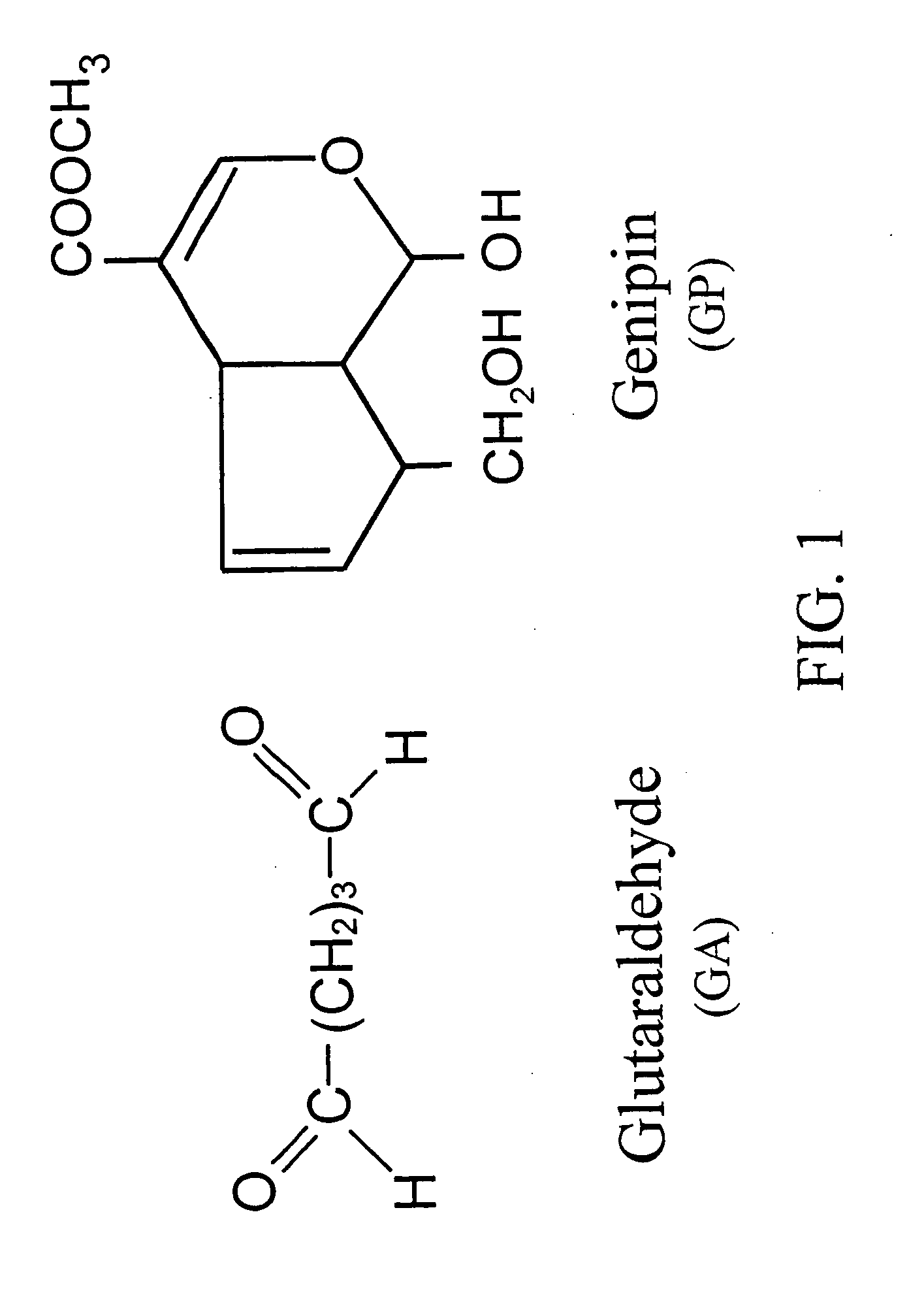
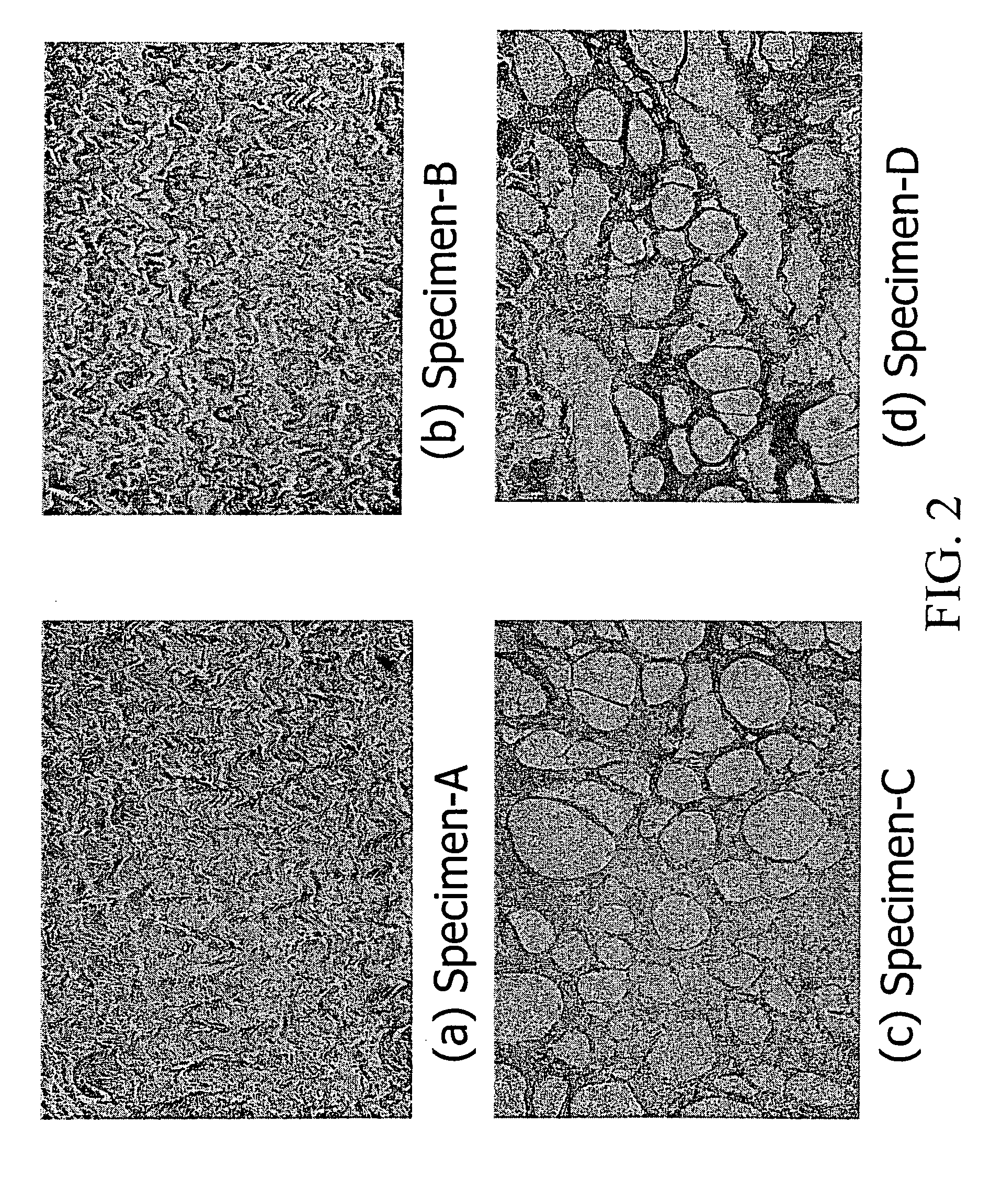
InactiveUS6998418B1Low antigenicityLow immunogenicityBiocidePeptide/protein ingredientsGenipinCytotoxicity
A method for promoting autogenous ingrowth of damaged or diseased tissue selected from a group consisting of ligaments, tendons, muscle and cartilage, the method comprising a step of surgically repairing the damaged or diseased tissue by attachment of a tissue graft, wherein the tissue graft is formed from a segment of connective tissue protein after an acellularization process, the segment being crosslinked with genipin, its analog or derivatives. The genipin-fixed acellular tissue provides a microenvironment for tissue regeneration adapted for use as a biological implant device due to its low cytotoxicity.
Owner:GP MEDICAL
Crosslinkable biological material and medical uses
InactiveUS7101857B2Low antigenicityLow immunogenicityStentsBiocideMedicineAngiogenesis growth factor
A method for promoting angiogenesis in a patient comprising providing crosslinkable biological solution to the target tissue, wherein the crosslinkable biological solution is loaded with at least one angiogenic agent. In one embodiment, the at least one angiogenic agent is a non-protein factor selected from a group consisting of ginsenoside Rg1, ginsenoside Re, combination thereof and the like. In another embodiment, the crosslinkable biological solution of the present invention is broadly defined in a form or phase of solution, paste, gel, suspension, colloid or plasma that may be solidifiable thereafter.
Owner:GP MEDICAL
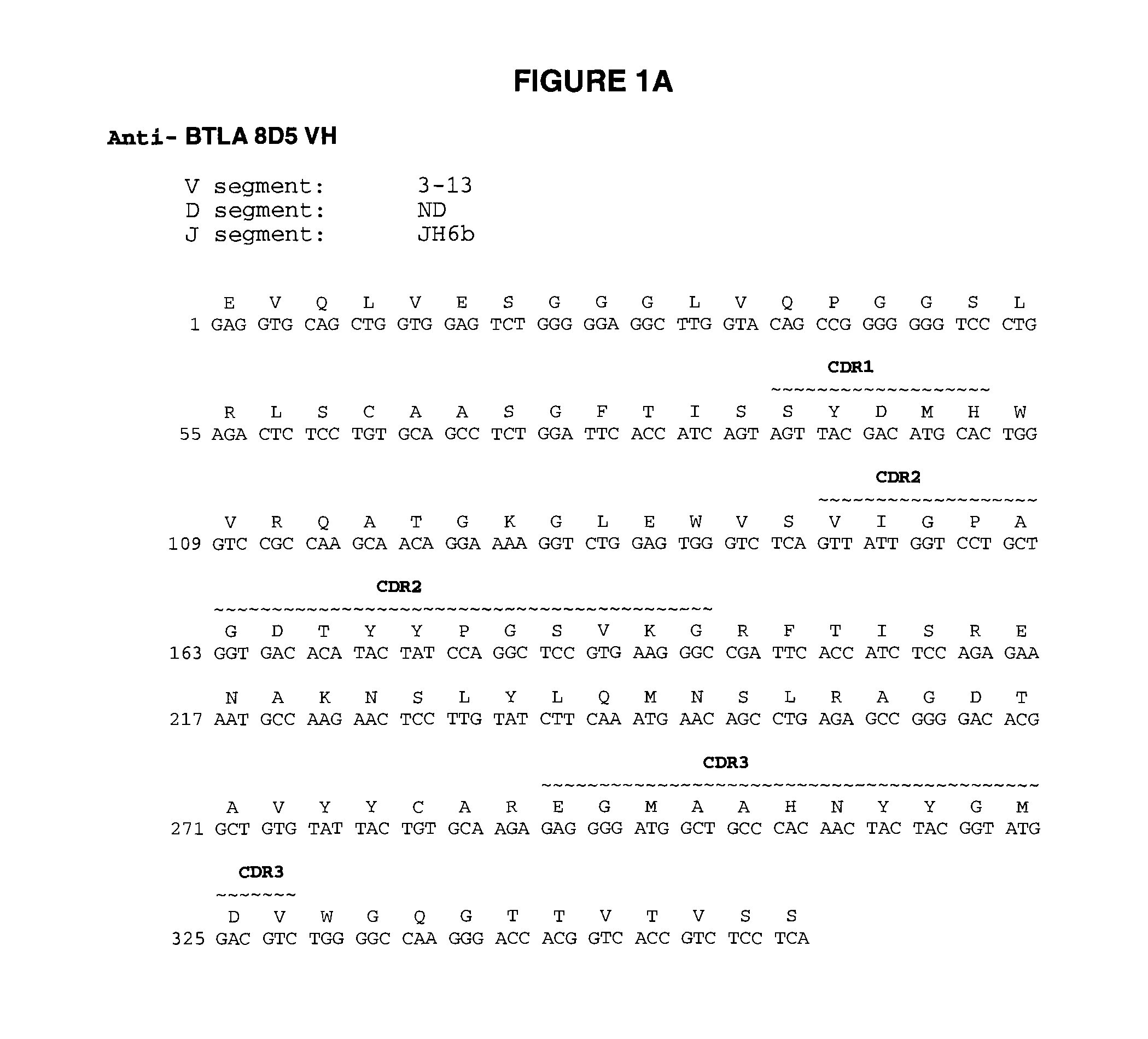
Fully human antibodies to BTLA
ActiveUS8563694B2High binding affinityImprove pharmacokineticsSenses disorderNervous disorderAntibodyLuetic disease
Owner:ER SQUIBB & SONS INC
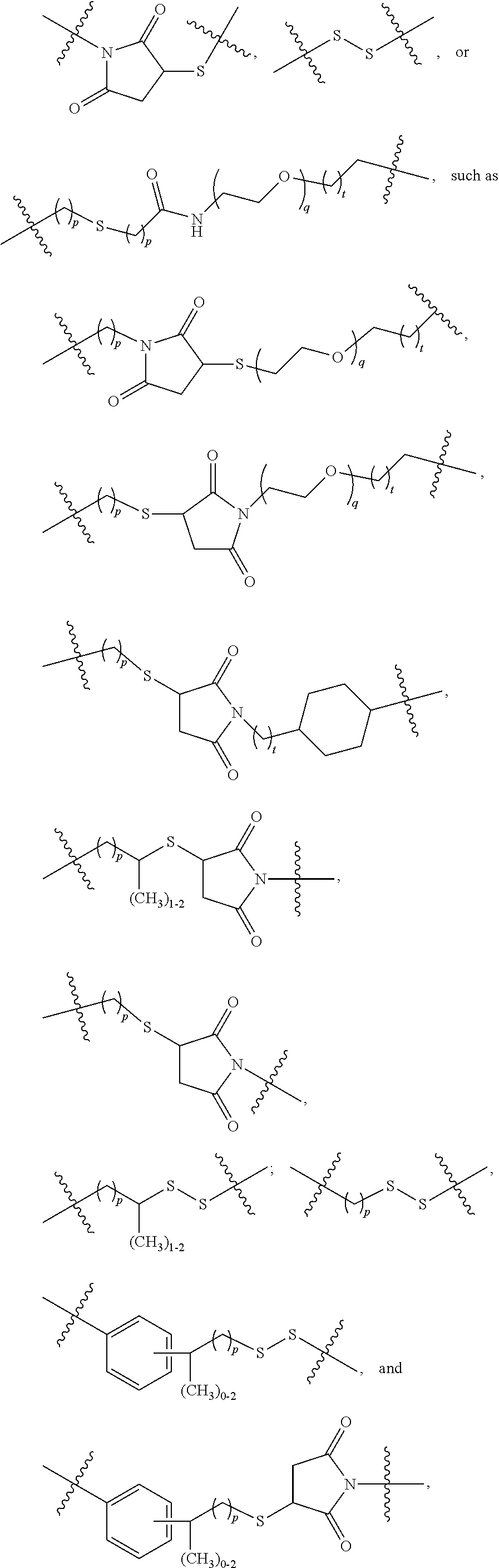
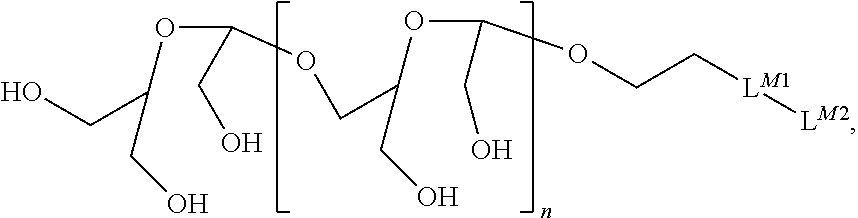
Terminally Modified Polymers and Conjugates Thereof
InactiveUS20140017265A1Reduce degradationProlong lifeTetrapeptide ingredientsImmunoglobulinsPolymer sciencePolymer
A terminally modified polymer is provided herein. At least one terminus of the polymer is —O—(CH2)2-LM or —O—CH2—CH(OH)—CH2—CR1═CR2R3. LM, R1, R2, and R3 are defined herein Also disclosed are terminal conjugates comprising the polymer and a pharmaceutically useful modifier, as well as compositions comprising the conjugates, methods of their preparation, and methods of treating various disorders with the conjugates or their compositions.
Owner:MERSANA THERAPEUTICS INC
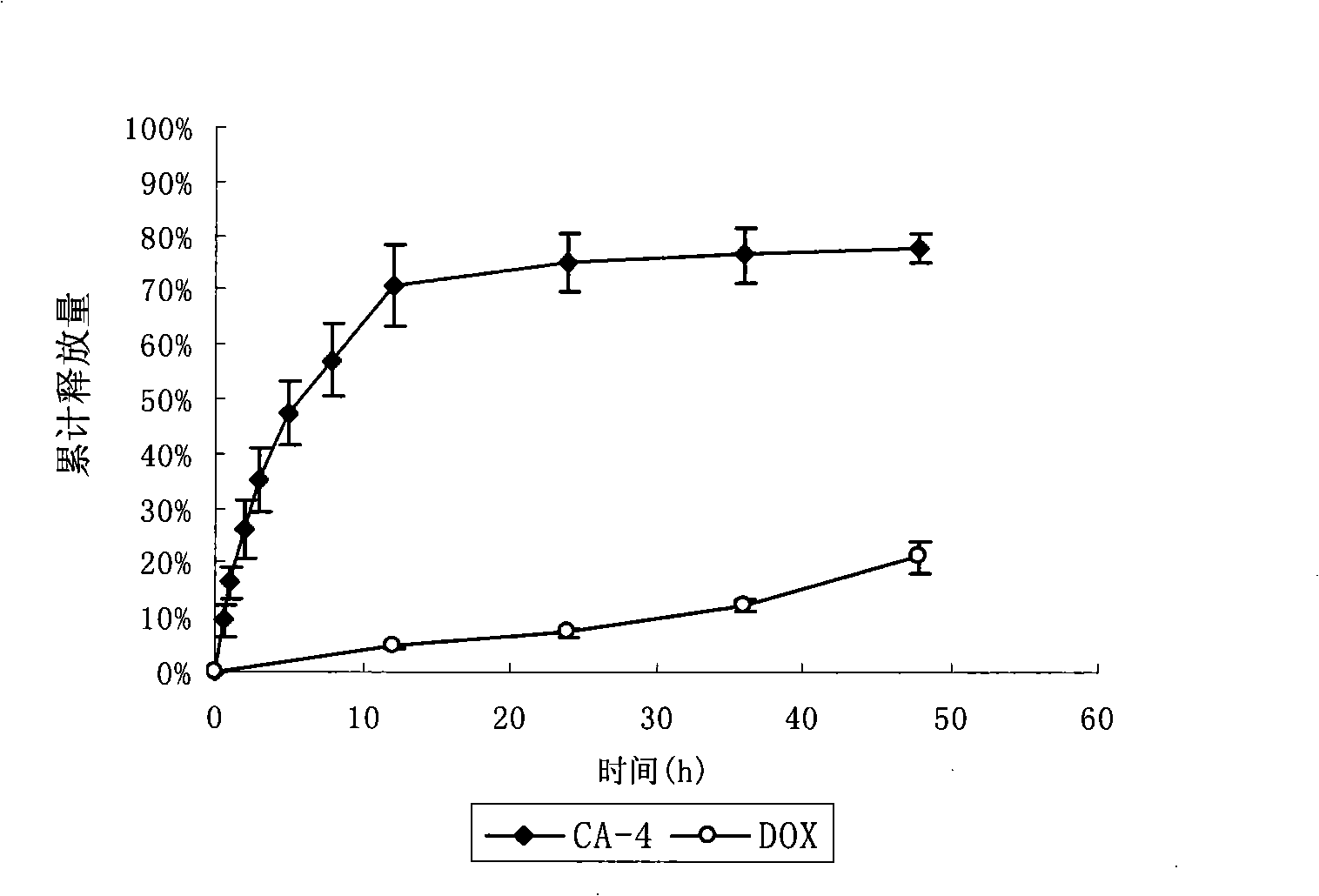
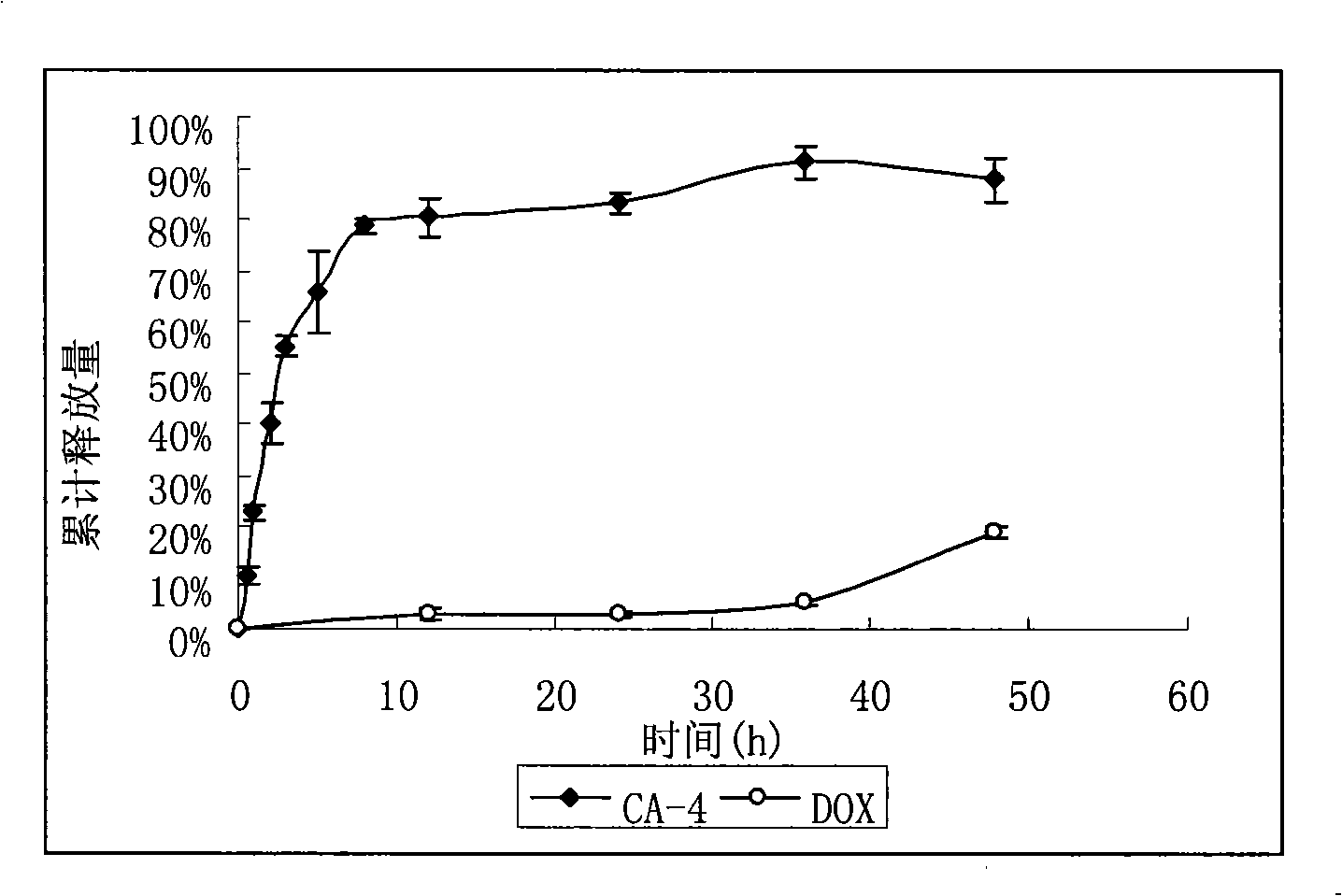
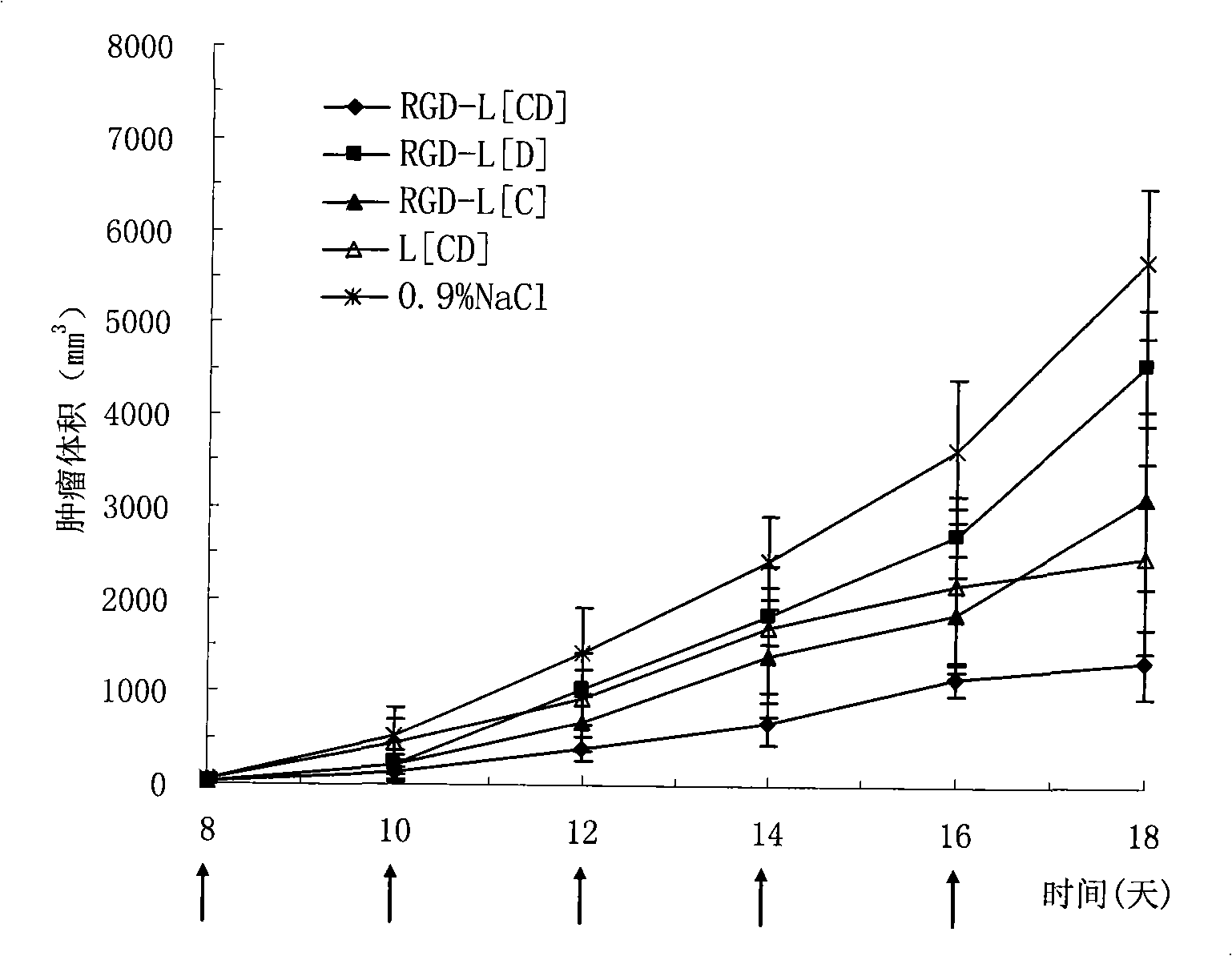
Anti-tumor long-circulating target liposomes for injections
InactiveCN101327190AGood treatment effectImprove specific binding abilityMacromolecular non-active ingredientsAntineoplastic agentsAnti-angiogenic drugsTumor targeting
The invention relates to an injection anticancer long circulating targeting liposome which is characterized in that anti-angiogenic drug is combined with anticancer drug, the liposome modified by polypeptide with tumor targeting function and hydrophilic polyethyleneglycol is adopted for loading and transporting the two drugs to tumor positions, and the tumor curative effect is enhanced through the different releasing rates and action mechanism of the two drugs.
Owner:PEKING UNIV
Decellularized pericardial tissue
InactiveUS20080195229A1Low antigenicityLow immunogenicityTissue regenerationProsthesisChemical treatmentPericardium
The invention discloses a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts as a medical device and process of manufacture.
Owner:QUIJANO RODOLFO C +1
Artificial blood vessel
InactiveUS6136024AEasy to getLittle rejectionTissue regenerationBlood vesselsUltra fineBiomedical engineering
PCT No. PCT / JP97 / 04387 Sec. 371 Date Jun. 4, 1999 Sec. 102(e) Date Jun. 4, 1999 PCT Filed Dec. 2, 1997 PCT Pub. No. WO98 / 24385 PCT Pub. Date Jun. 11, 1998The present invention provides an artificial blood vessel provided with collagen layer composed of ultra-fine fibers on at least the outside of a tube composed of a supporting framework material, and a method for producing the same. The present invention offers advantages such that its materials are easily acquired, it causes very little rejection after being transplanted into the body, it allows sutures to be maintained for a long time after transplant, and all or a portion is absorbed and degraded in the body after a predetermined period of time.
Owner:TAPIC INT
Self-expanding valve for the venous system
InactiveUS20090062907A1Minimize turbulenceAcceptable cytotoxicityVenous valvesBlood vesselsVenous ValvesVenous vessel
A venous valve device and method of formation are described to provide antegrade blood flow in the deep venous vessels of the leg or in other venous vessels of the body having incompetent or irreversibly dysfunctional valves. The venous valve device is made of a sheet of biocompatible material, comprising a longitudinal wire-frame structure that is a continuous seamless wire loop and plural anchoring mesh-lattice wing members spaced apart and connected to the base of the wire-frame structure.
Owner:QUIJANO RODOLFO C +1
Fibronectin-based binding molecules and their use
InactiveUS20100322930A1Improve stabilityExtended half-lifeSenses disorderPeptide/protein ingredientsHalf-lifeMammalian cell
The invention provides fibronectin-based binding molecules and methods for introducing donor CDRs into a fibronectin-based binding scaffold, in particular, Fn3. The fibronectin-based binding molecules of the invention may be further conjugated to another moiety, for example, Fc, anti-FcRn, HSA, anti-HSA, and PEG, for improved half life and stability, particularly in mammalian cells. The invention also provides methods for screening such molecules for binding to a target antigen as well as the manufacture and purification of a candidate binder.
Owner:NOVARTIS AG
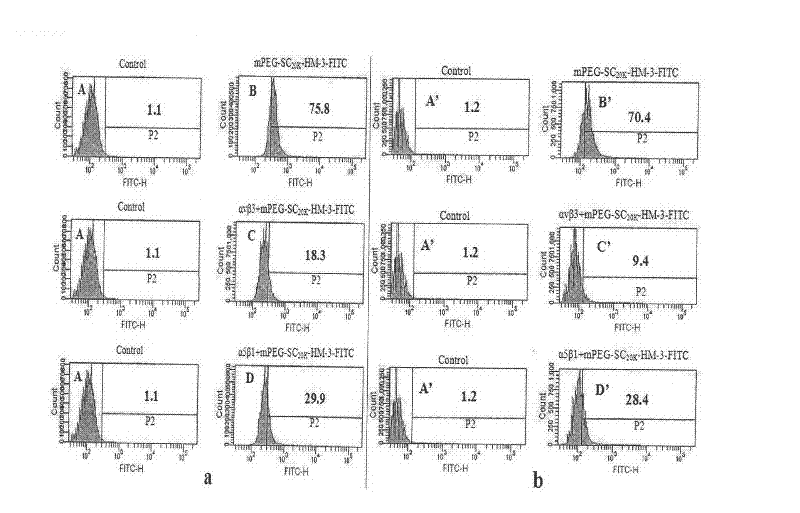
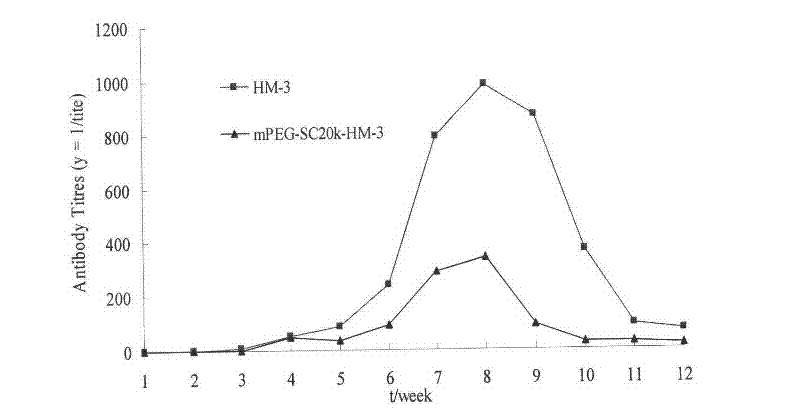
Polyethylene glycol-modified integrin blocking agent HM-3 and application thereof
InactiveCN102417540AExtended half-lifeDoes not affect the activity in vivo and in vitroConnective tissue peptidesPeptide/protein ingredientsTumor angiogenesisPolyethylene glycol
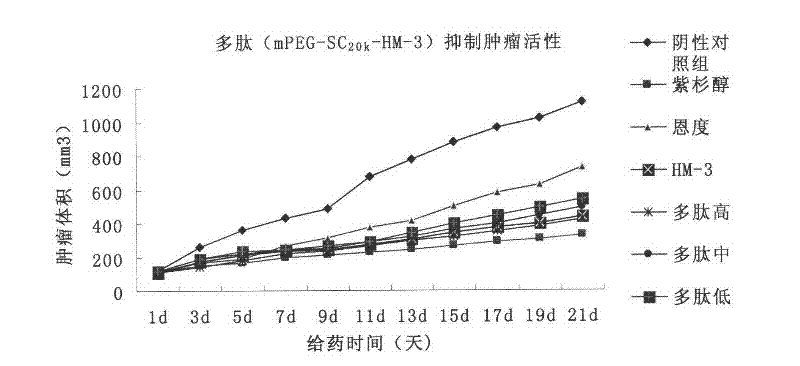
The invention relates to the field of medicaments, in particular to an integrin blocking agent HM-3 which has the function of inhibiting tumor angiogenesis, integrin affinity and a bonding capacity and application thereof. The blocking agent is a polypeptide modified with polyethylene glycol, and the modified integrin blocking agent polypeptide can be applied to treatment of solid tumors. During application of the integrin blocking agent to preparation of a tumor treatment medicament, the sequence and structure of the integrin blocking agent is mPEG-SC20k-Ile-Val-Arg-Arg-Ala-Asp-Arg-Ala-Ala-Val-Pro-Gly-Gly-Gly-Gly-Arg-Gly-Asp. The integrin blocking agent polypeptide designed in the invention is scientific, reasonable, practical and effective, can be used for preparing a treatment medicament for treating human solid tumors, and has remarkable social value and market value; and the treatment spectrum of the integrin blocking agent is expanded greatly, and novel thought and prospect are provided for future development of medicaments.
Owner:CHINA PHARM UNIV
Bracket material for bone tissue engineer and preparation method thereof
InactiveCN101417145AImprove mechanical propertiesGood biocompatibilityProsthesisDrug biological activityProtein C
The invention relates to a bracket material used for an osseous tissue project, and a preparation method thereof. Firstly, the bracket of a type I collagen is extracted from a small fresh pigskin by a mature extracting technique. Then, the bracket is further expanded in a Tris cushion liquid, the pH of which is equal to 8.8 to obtain a natural porous collagen bracket by the treatments of freezing and drying. The bracket is respectively and repeatedly mineralized in a CaCl2 liquid and in (NH4)2HPO4 liquid or mineralized in simulated body fluid for a long period to lead the weakly crystallized HA to be uniformly settled into the collagen bracket; and then a pigskin collagen-hydroxyapatite ossein is obtained by the treatments of freezing and drying to replace the natural bracket material. The invention not only maintains the natural bracket structure of the collagen in an organism, but also has the advantages of low material cost, simple devices, short period and easy operation. The obtained compound bracket material used for the pigskin collagen-hydroxyapatite osseous tissue project has the characteristics of high intensity, large toughness, non-antigenicity, higher biological activity as well as degradation and releasing control.
Owner:SHANDONG UNIV
Guided tissue regeneration membrane and its preparation method
The invention relates to a guided tissue regeneration membrane, which comprises a compact coating and a loose coating, wherein the compact coating is composed of collagen-I or collagen-I composite hyaluronic acid; and the loose coating is composed of collagen-I or collagen-I composite calcium salt. A preparation method of the guided tissue regeneration membrane comprises the following steps of: preparing a collagen solution or a collagen and hyaluronic acid mixed solution by the use of acetic acid; injecting the solution into a self-made die, and drying it in the air to prepare the compact coating; injecting the collagen solution or a mixed liquor of the collagen solution and calcium salt onto the compact coating, and uniformly spreading; precooling the die at ultralow temperature, followed by freeze-drying to prepare the guided membrane with the compact coating and loose coating structure; carrying out vacuum high-temperature crosslinking and chemical crosslinking; and finally cleaning. The regeneration membrane has good histocompatibility and mechanical strength, low antigenicity and strong guided tissue regeneration capability. Its degradation rate in vivo is 3-8 months. The method is easy to operate, is suitable for automatic and continuous large-scale production, and solves the problems of large size of collagen membrane and material uniformity during industrial production.
Owner:SHENZHEN LANDO BIOMATERIALS
Compositions and Methods for Antibodies Targeting Complement Protein C5
The present invention relates to antibodies targeting complement protein C5 and compositions and methods of use thereof.
Owner:HIREVUE +1
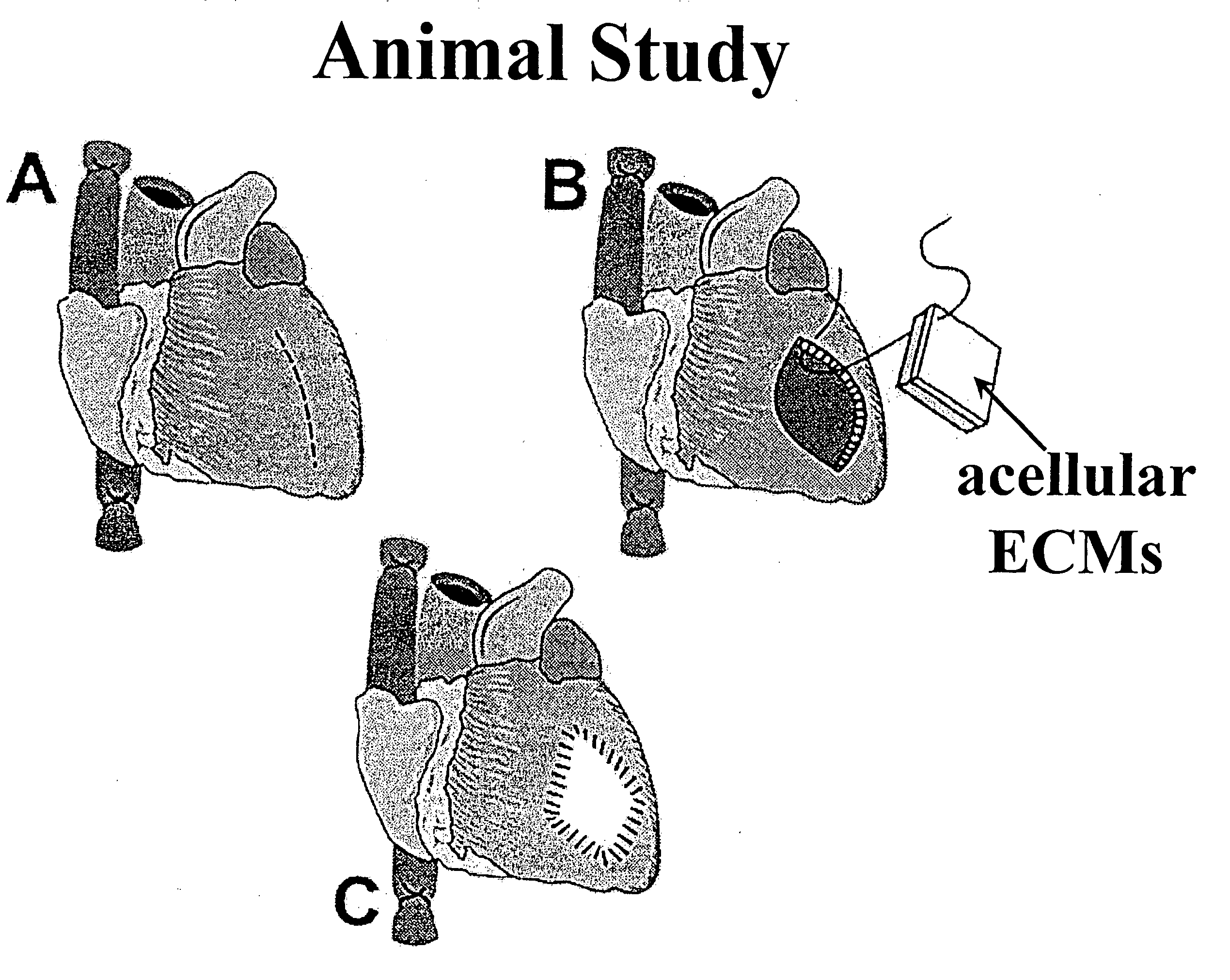
Peritoneal regeneration with acellular pericardial patch
InactiveUS20050171616A1Low antigenicityLow immunogenicityHeart valvesTubular organ implantsGenipinPericardial patch
The invention discloses a method of using acellular bovine pericardia fixed with genipin as a surgical-repair material to fix an abdominal wall defect.
Owner:GP MEDICAL
Root canal filling point
InactiveUS6024569AEasy-to-use and physicochemically stableLow antigenicityTeeth fillingTeeth cappingCopolymerEthylene
A new root canal filling point which is physicochemically stable, is not toxic to periapical tissue, can be sterilized and x-rayed; and has high elasticity and fracture resistance which enables the root canal filling point to be pressure-inserted into an even narrow or curved root canal. The root canal filling point is made of a copolymer consisting of propylene and ethylene, and a contrast medium.
Owner:AYTEC JAPAN CORP
Bispecific molecules and uses thereof
InactiveUS20040180046A1Reduce clearanceRapidly and efficiently clearedFungiBacteriaCross-linkPrimate
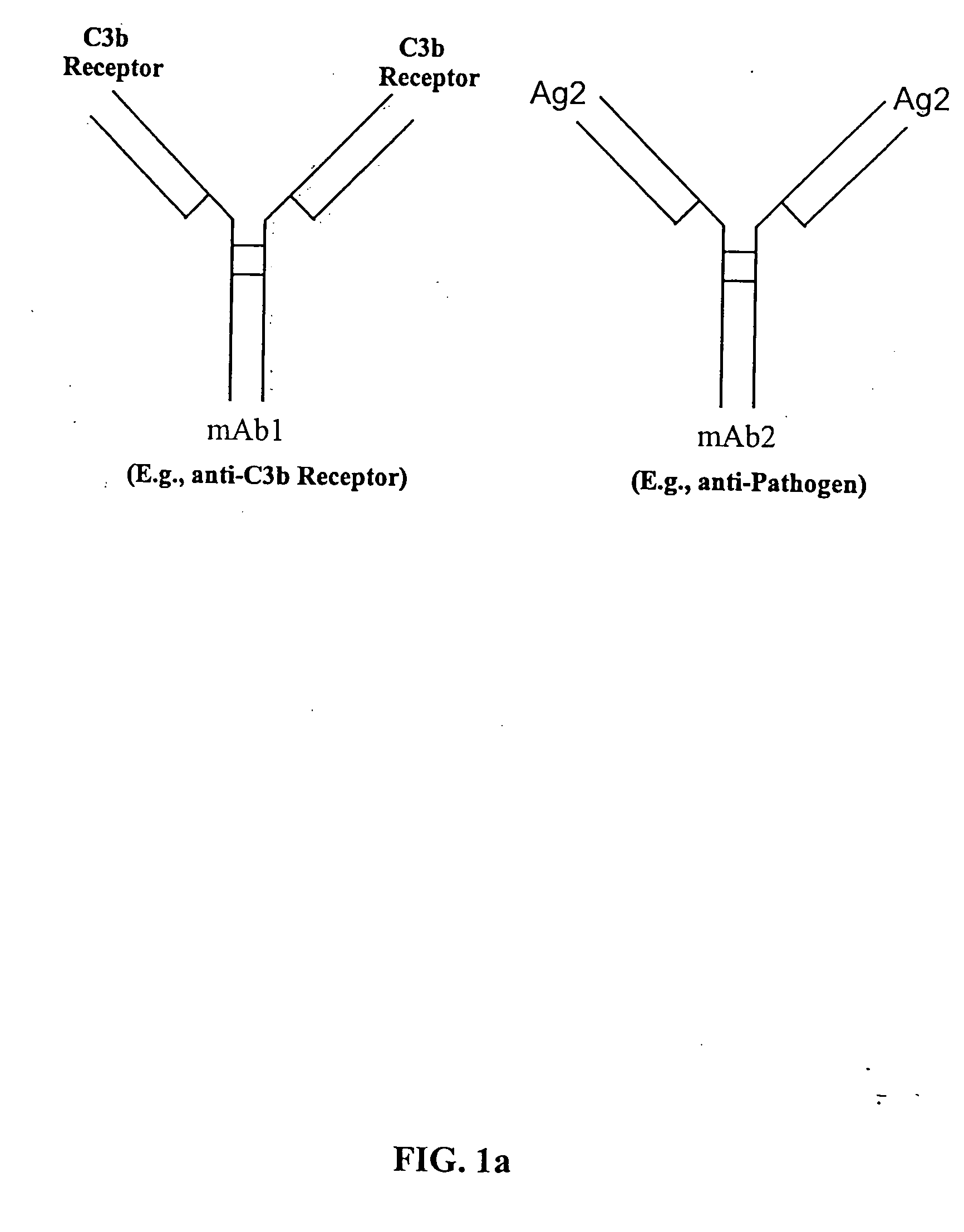
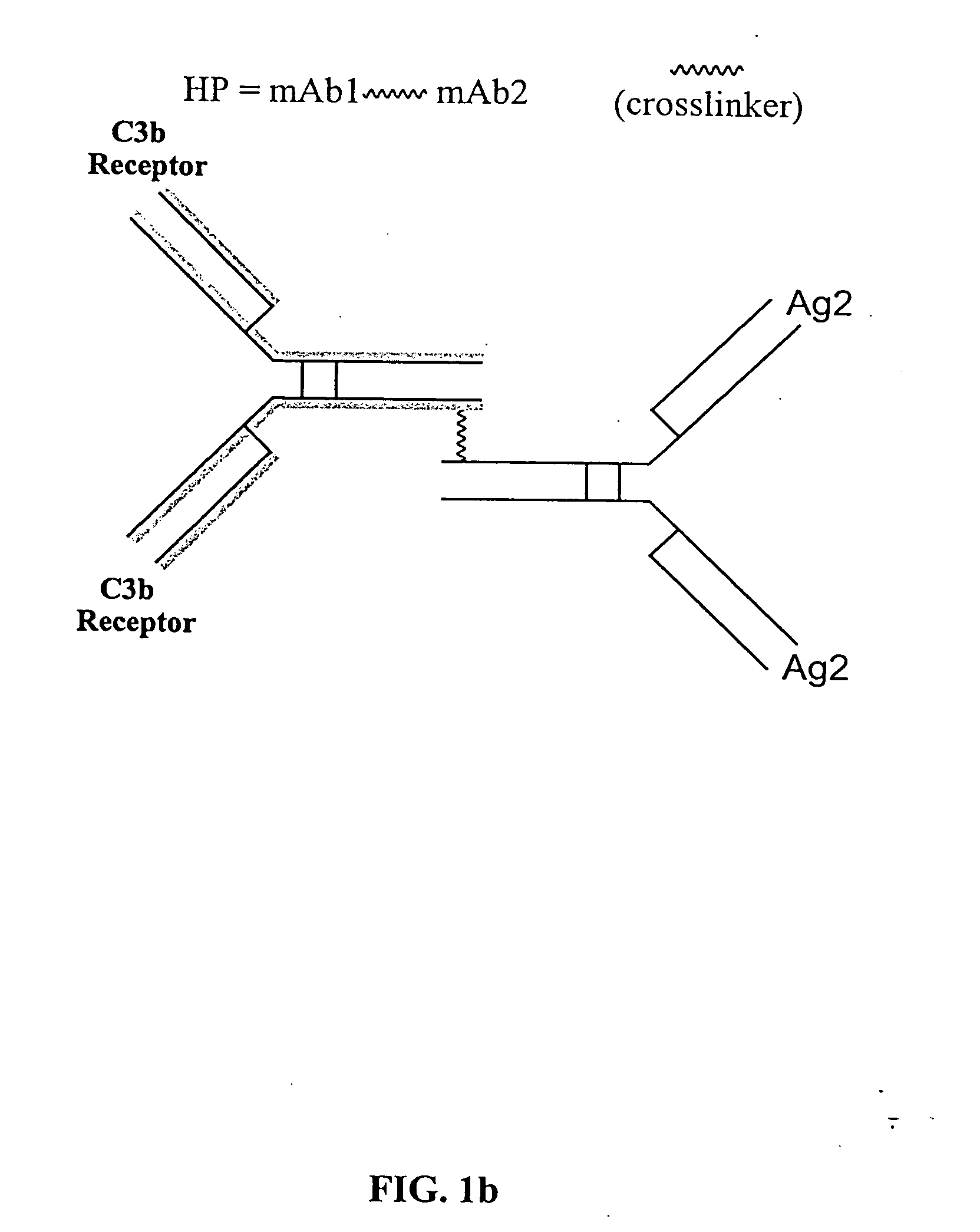
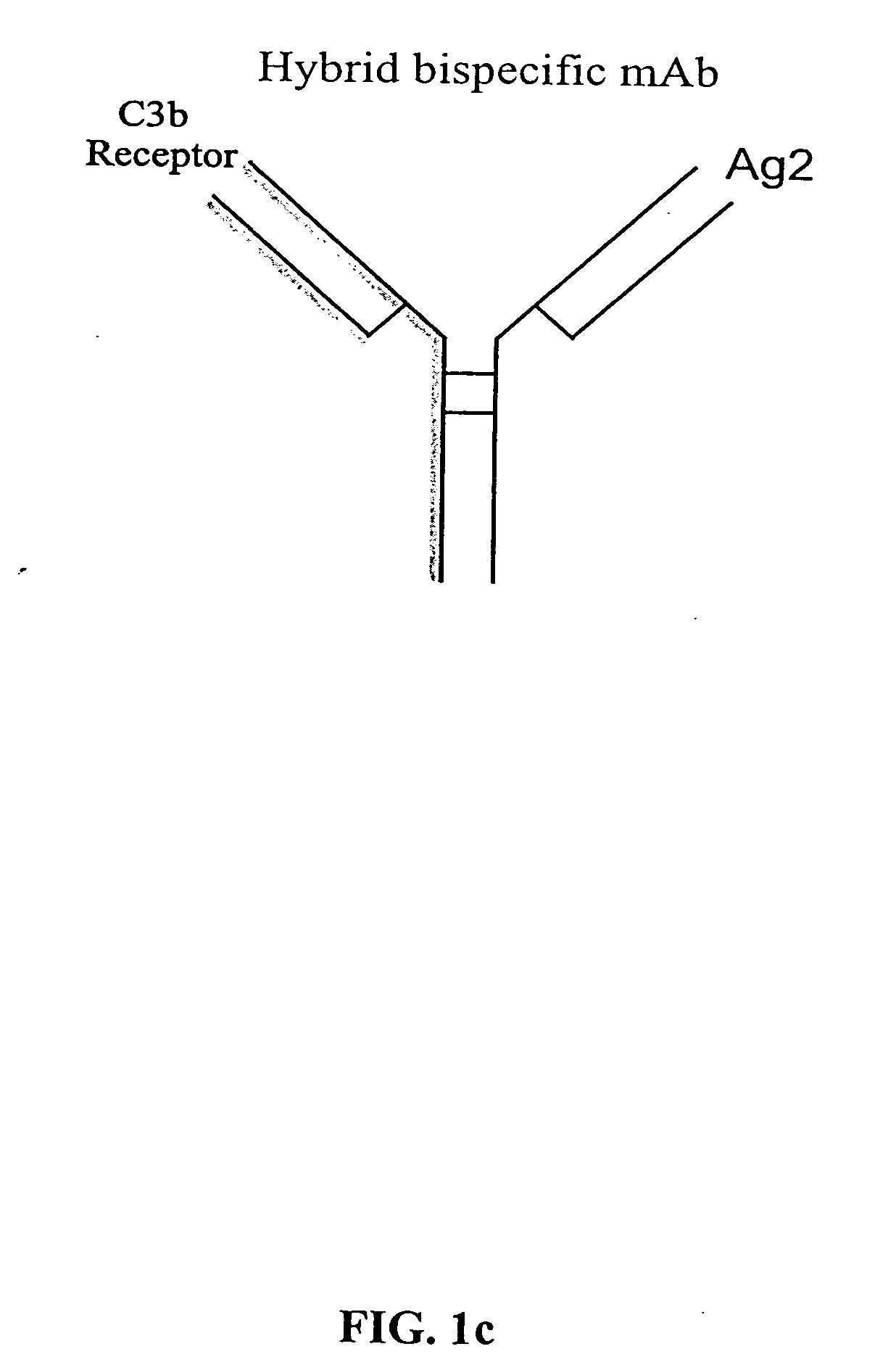
The present invention relates to bispecific molecules that are characterized by having a first binding domain which binds an antigen present in the circulation of a mammal and a second binding domain which binds the C3b-like receptor (known as complement receptor 1 (CR1) or CD35 in primates). The bispecific molecules do not consist of a first monoclonal antibody to CR1 that has been chemically cross-linked to a second monoclonal antibody. The invention also relates to methods of making the bispecific molecules and therapeutic uses thereof, as well as to kits containing the bispecific molecules. The invention further provides polyclonal populations of bispecific molecules, which comprise populations of bispecific molecules with different antigen recognition specificities. Such polyclonal populations of bispecific molecules can be used for targeting multiple epitopes of a pathogenic antigenic molecule and / or multiple variants of a pathogenic antigenic molecule.
Owner:ELUSYS THERAPEUTICS
Electrostatic spinning nanofiber-extracellular matrix composite material as well as preparation method and application thereof
InactiveCN103877622AEasy to stick value-addedAdhesion promotionSkeletal/connective tissue cellsNon-woven fabricsCell-Extracellular MatrixCell adhesion
The invention discloses an electrostatic spinning nanofiber-extracellular matrix composite material as well as a preparation method and application thereof. The preparation method is simple and feasible in steps, and large-sized equipment is not needed. The prepared composite material has the advantages of electrostatic spinning nanofibers and an extracellular matrix. Compared with a pure electrostatic spinning nanofiber material or a pure extracellular matrix, the composite material has very high biocompatibility and very high mechanical property, is easier for cell adhesion and proliferation, has very good application prospect and high practical value in mechanical tissue engineering repair, and can be used for effectively promoting the adhesive growth, proliferation, migration and differentiation and amplifying and culturing stem cells on a large scale. Moreover, the composition material is low in antigenicity, and the risk of disease transmission is eliminated by means of strict screening.
Owner:SUN YAT SEN UNIV
Biological ink material for 3D printing and preparation method and application thereof
ActiveCN105885436AEasy to sprayWon't clogAdditive manufacturing apparatusProsthesisBiological macromoleculeAntigenicity
The invention discloses biological ink material for 3D printing and a preparation method and application thereof. The biological ink material is prepared by, by mass concentration percentage, 5-18% of biological macromolecules, 76-93.5% of water or 76-93.5% of pre-crosslinking agent, and 1.5-6% of coagulant, wherein the sum of the mass concentration percentage of the above raw materials is 100%. The biological ink material is characterized in that water washing and crosslinking are performing for shaping after printing. The biological ink material has the advantages that the biological ink material can be easily squeezed out during printing, does not block printing needles and can be easily shaped after being squeezed out; the biological ink material is low in antigenicity in the bodies of organisms, low in caused rejection reaction, biodegradable, nontoxic to the organisms and high in safety.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39192E1Increased longevityImprove stabilityAntibacterial agentsPowder deliveryTissue sealantVascular dilatation
This invention provides supplemented tissue sealants, methods for their production and use thereof. Disclosed are tissue sealants supplemented with at least one cytotoxin or cell proliferation inhibiting composition. The composition may be further supplemented with, for example, one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, cytokines, drugs, growth factors, interferons, hormones, lipids, deminearlized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like.
Owner:AMERICAN NAT RED CROSS
Methods of treatment and compositions therefor
InactiveUS20050070474A1Maximize efficacyHigh activityOrganic active ingredientsPeptide/protein ingredientsEndocrinology
Owner:AUCKLAND UNISERVICES LTD
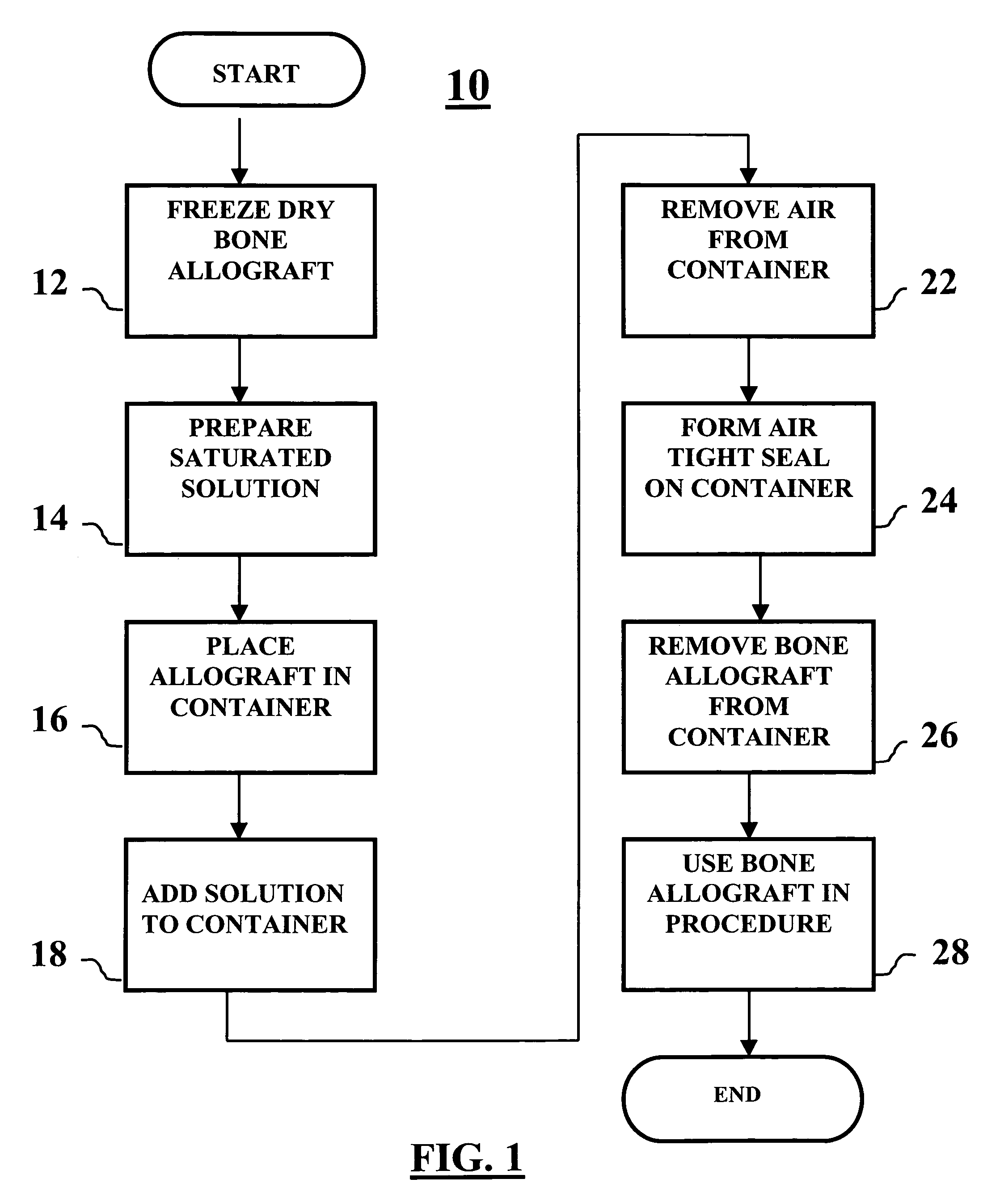
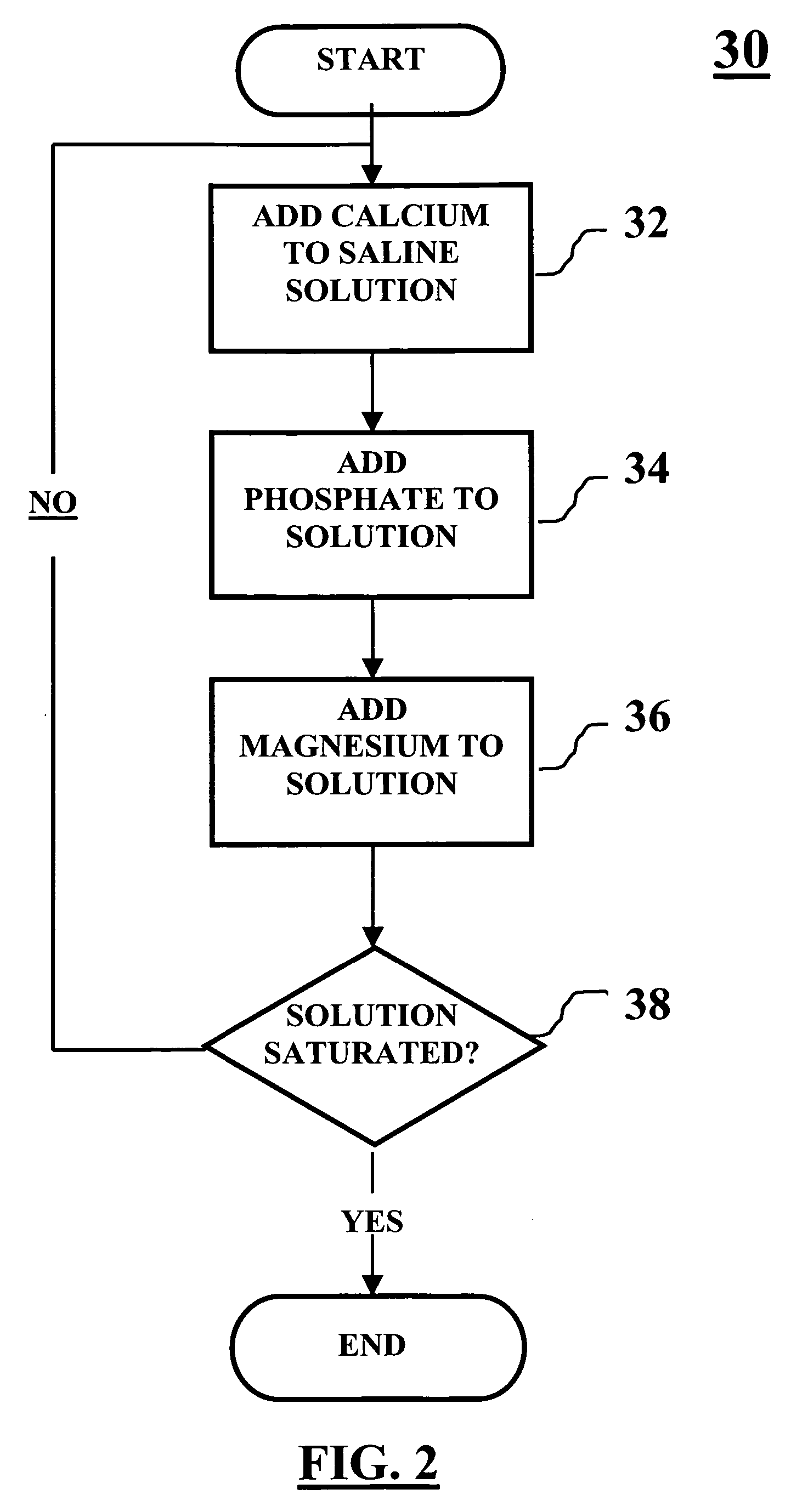
Method of packaging a bone allograft intended for a spinal fusion procedure
A system and method of packaging a bone allograft for use in a future medical procedure in which the bone allograft is stored in a saturated saline solution in an airtight container. The saline solution keeps the allograft hydrated and may be saturated with a combination of calcium, phosphate, or magnesium to inhibit mineral leaching out of the allograft during storage. The container may be deformable to conform to the shape of the allograft material as the container is “shrink-fitted” to the allograft. Optionally, the bone allograft may also be freeze-dried prior to placement in the container.
Owner:NUVASIVE
C5 antibody and method for preventing and treating complement-related diseases
ActiveUS20160068592A1Low antigenicityEffective diagnosisSenses disorderBacteriaAntibodyDisease cause
Owner:IMMUNABS INC
Antibodies to IL-17A
Owner:MERCK SHARP & DOHME LLC
Truncated soluble tumor necrosis factor type-I and type-II receptors
InactiveUS6989147B2Improve performanceHigh molecular weightAntibacterial agentsOrganic active ingredientsADAMTS ProteinsFactor ii
Disclosed are novel proteins, referred to as tumor necrosis factor binding proteins, that modulate the activity of tumor necrosis factor. Also disclosed are processes for obtaining the tumor necrosis binding proteins by recombinant genetic engineering techniques.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com