Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Tissue augmentation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
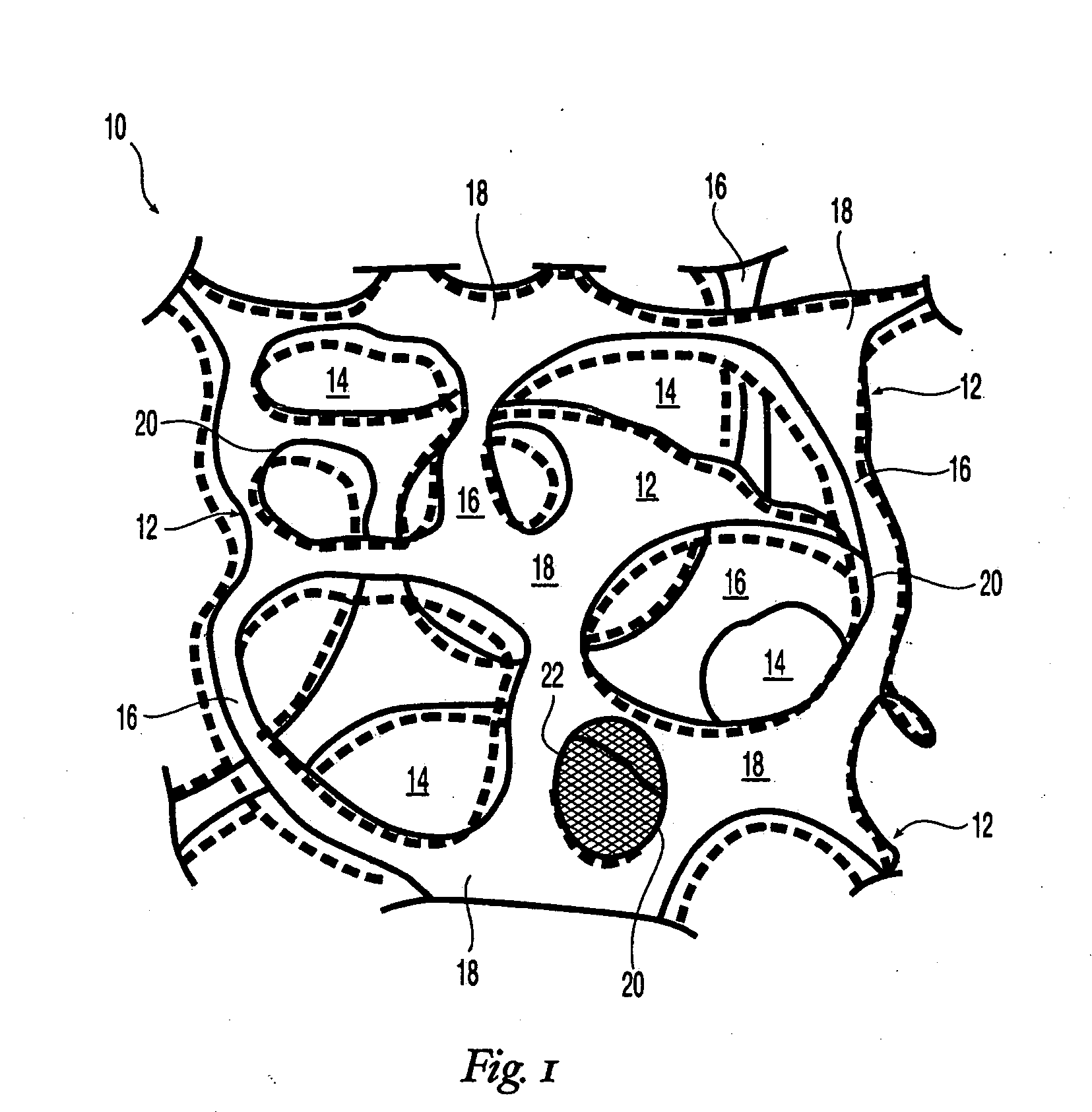
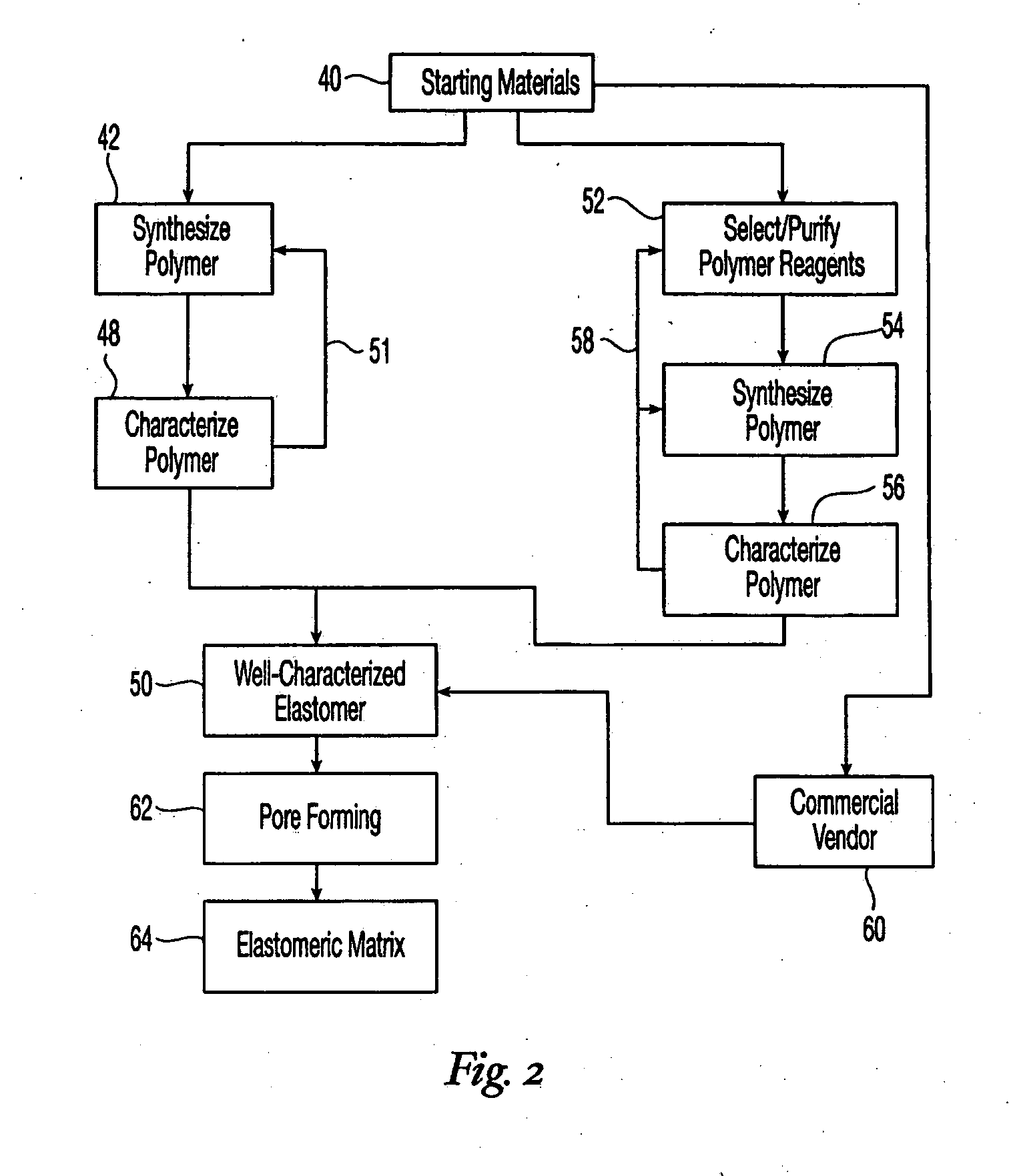
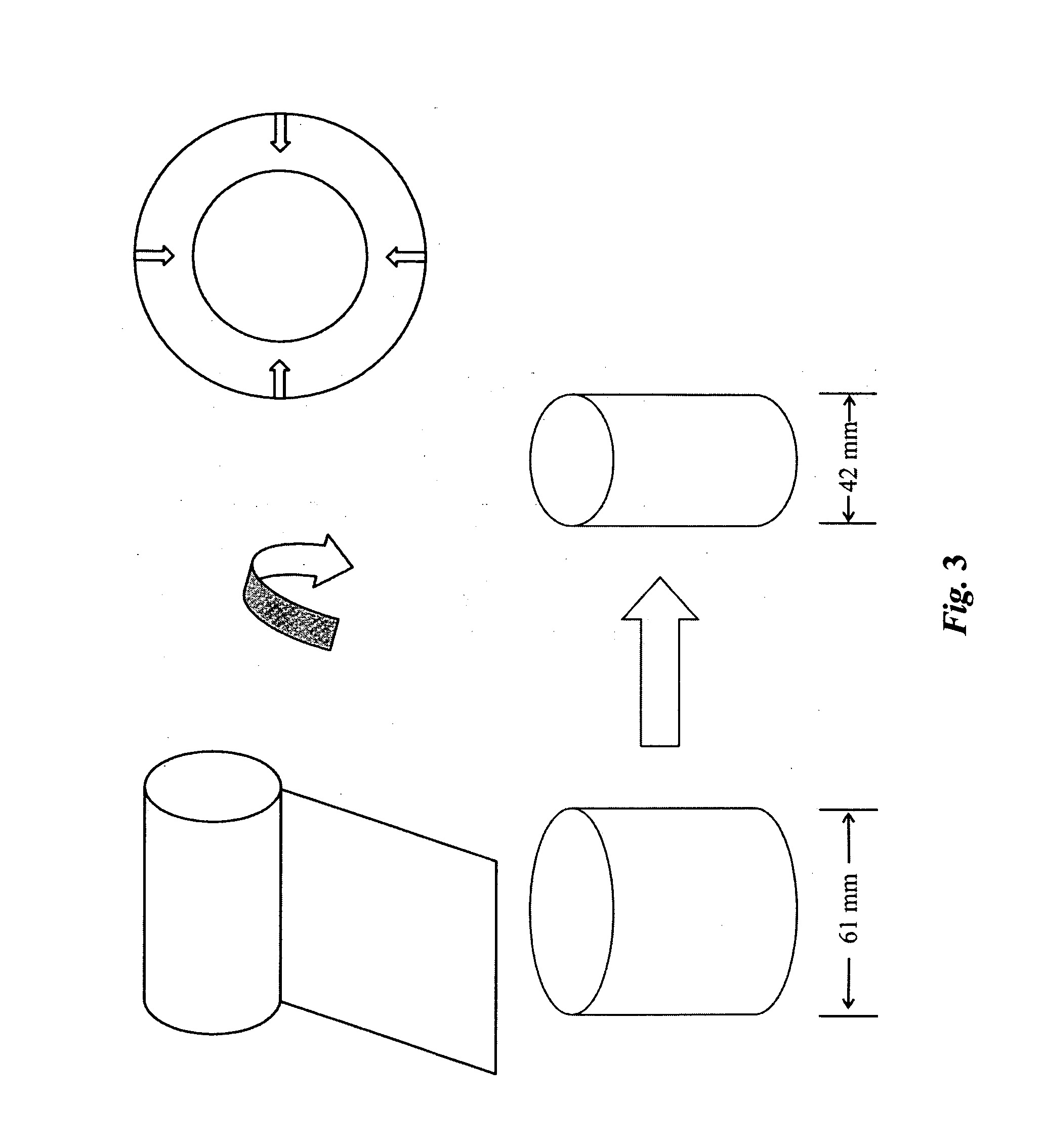
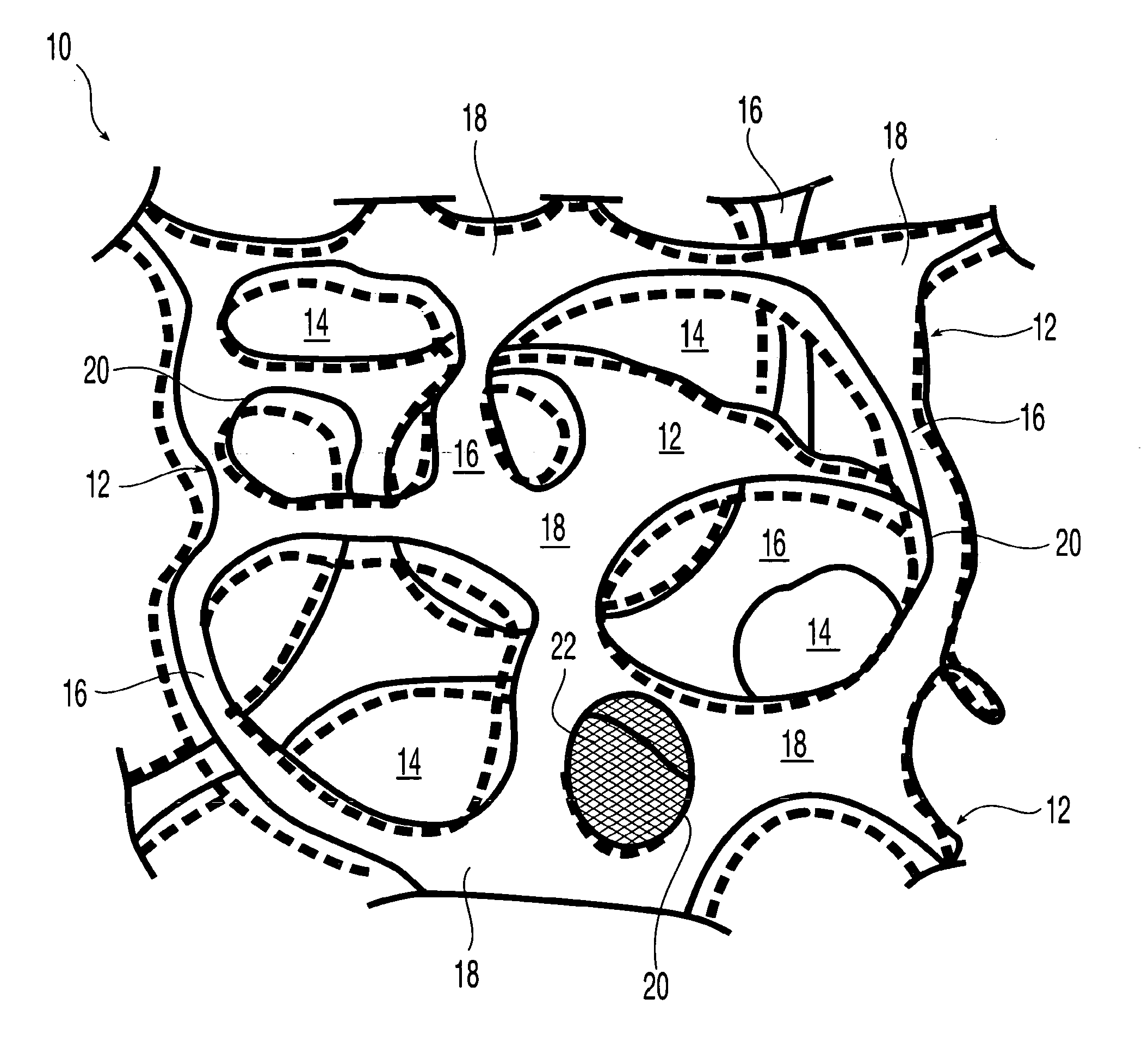
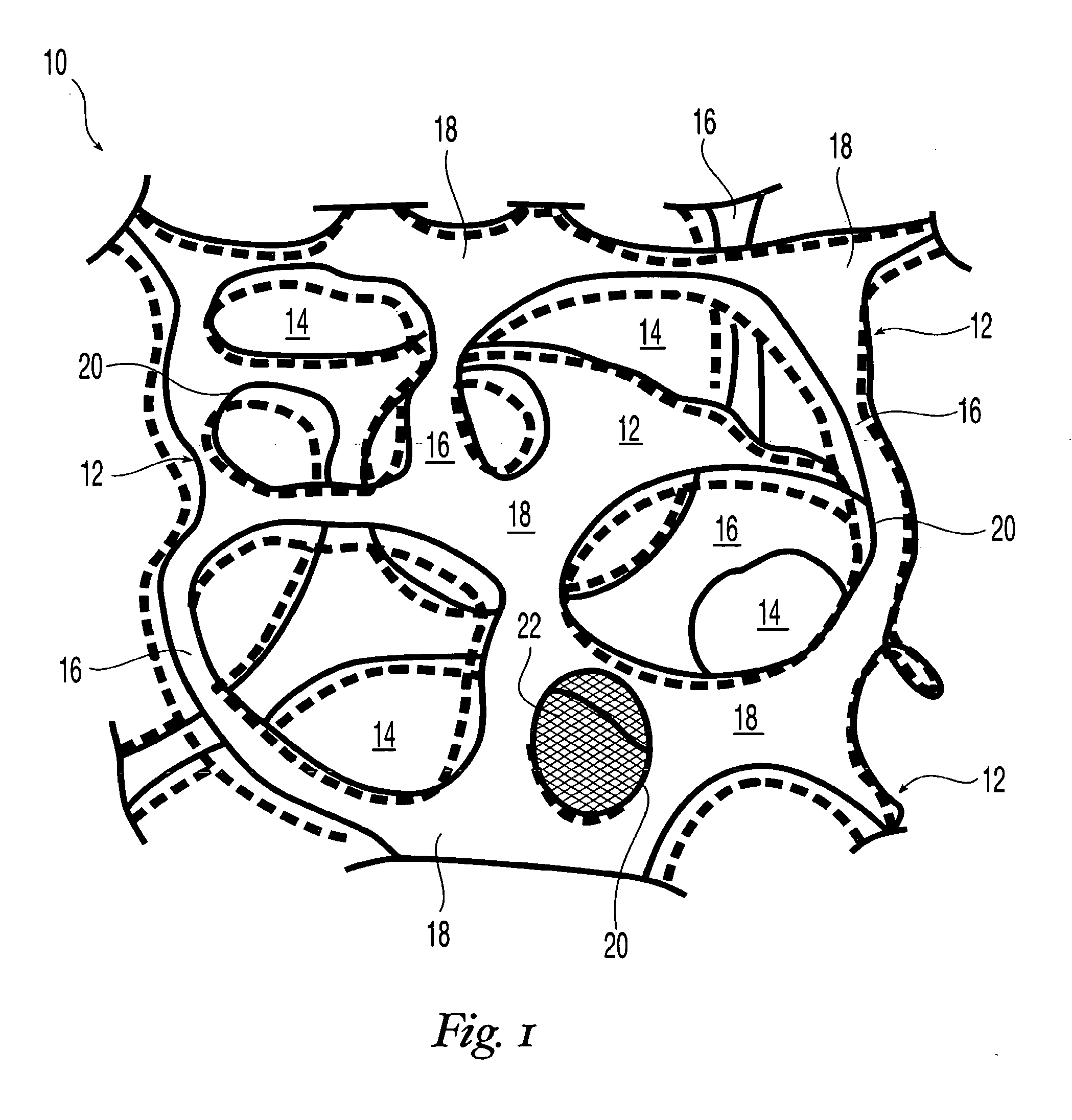
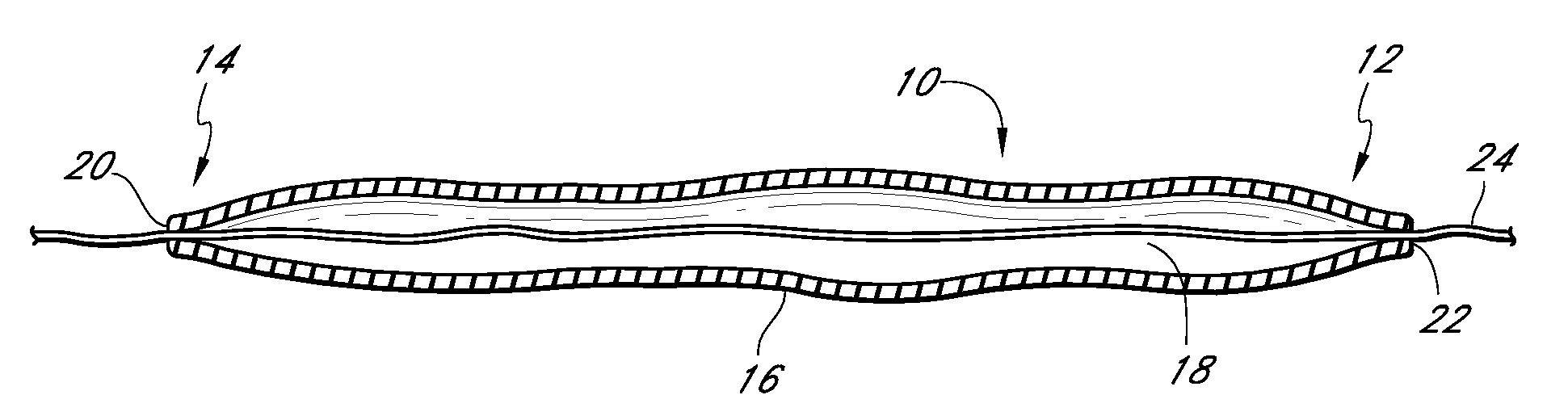
High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair
InactiveUS20070190108A1Different and simple configurationEfficiently employedProsthesisElastomerSurgical operation
This invention relates to reticulated elastomeric matrices, their manufacture, their post-processing, such as their reinforcement, compressive molding or annealing, and uses including uses for implantable devices into or for topical treatment of patients, such as humans and other animals, for surgical devices, tissue augmentation, tissue repair, therapeutic, nutritional, or other useful purposes.
Owner:BIOMERIX CORP
Devices and methods for pericardial access
InactiveUS20060247672A1Facilitate pericardial accessReliable holdCannulasDiagnosticsPericardiumTissue augmentation
Devices and methods for establishing pericardial access to facilitate therapeutic and / or diagnostic applications. Pericardial access is facilitated, in part, by a tissue grasping device that reliably holds pericardial tissue, even in the presence of fatty deposits. The tissue grasping portion may include a tissue penetrating tip, a tissue dilating distal section, a tissue retention neck, and a tissue stop. When advanced into the pericardium, the tip may serve to create an opening (e.g., pierce, cut, etc.) in the pericardium, the distal section may serve to dilate the opening, the neck may serve to hold the tissue upon recoil of the dilated opening, and the stop may serve to limit further penetration once tissue is retained in the neck.
Owner:EDWARDS LIFESCIENCES LLC
System for delivery of biologically active substances with actuating three dimensional surface
InactiveUS20080140002A1Reduce the overall diameterDelivery be eliminatedStentsBalloon catheterTissue augmentationThree dimensional surface
Tissue expanding and drug delivery systems with actuating three-dimensional surfaces are described for controlling the delivery and release of therapeutic agents against or upon tissue regions of interest. Such treatments devices and methods may include systems utilizing pores having various pore architectures to control the release of one or more drugs from an outer layer of an expandable delivery instrument, such as a balloon.
Owner:DIETCH LAURA N +2
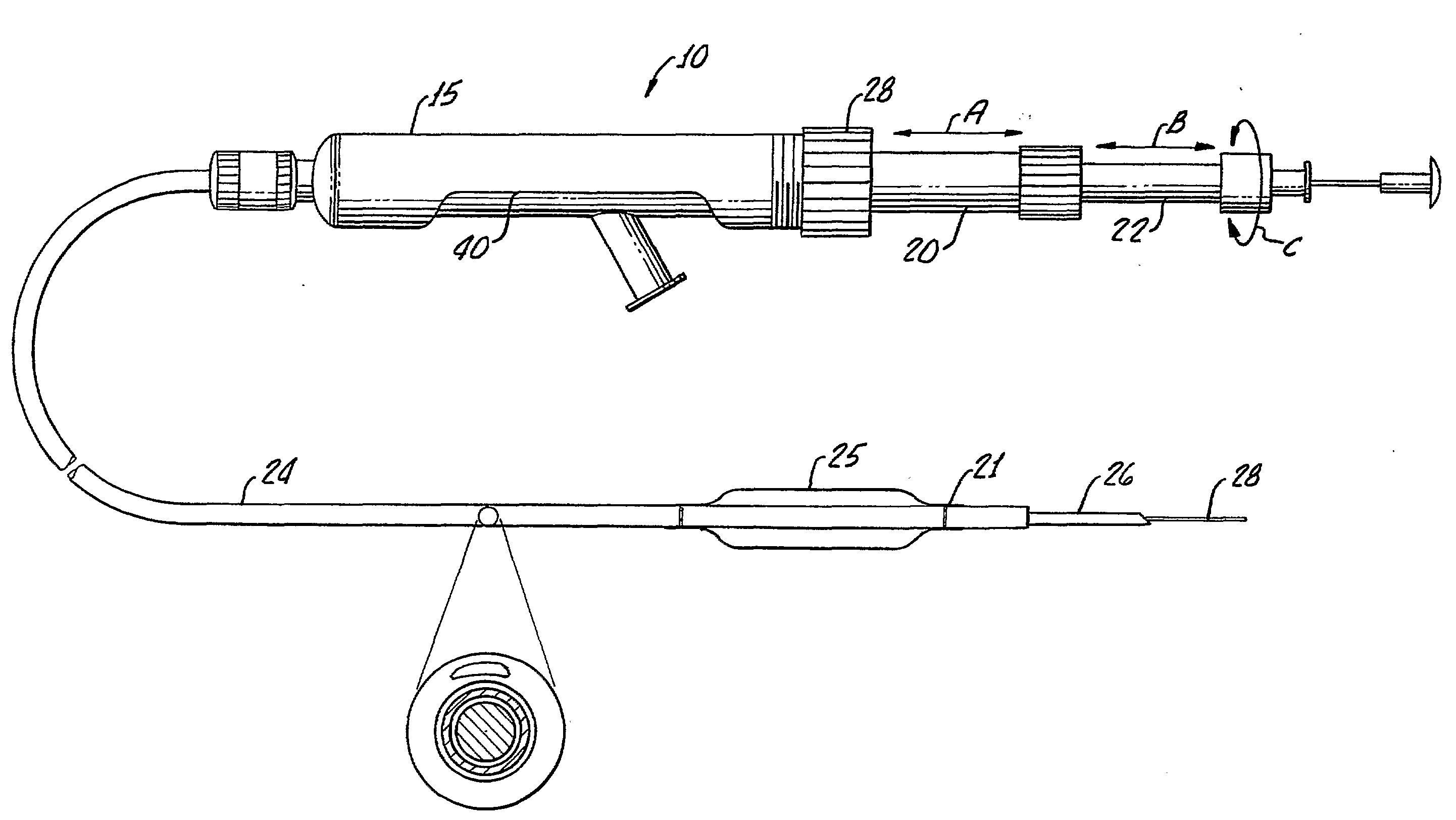
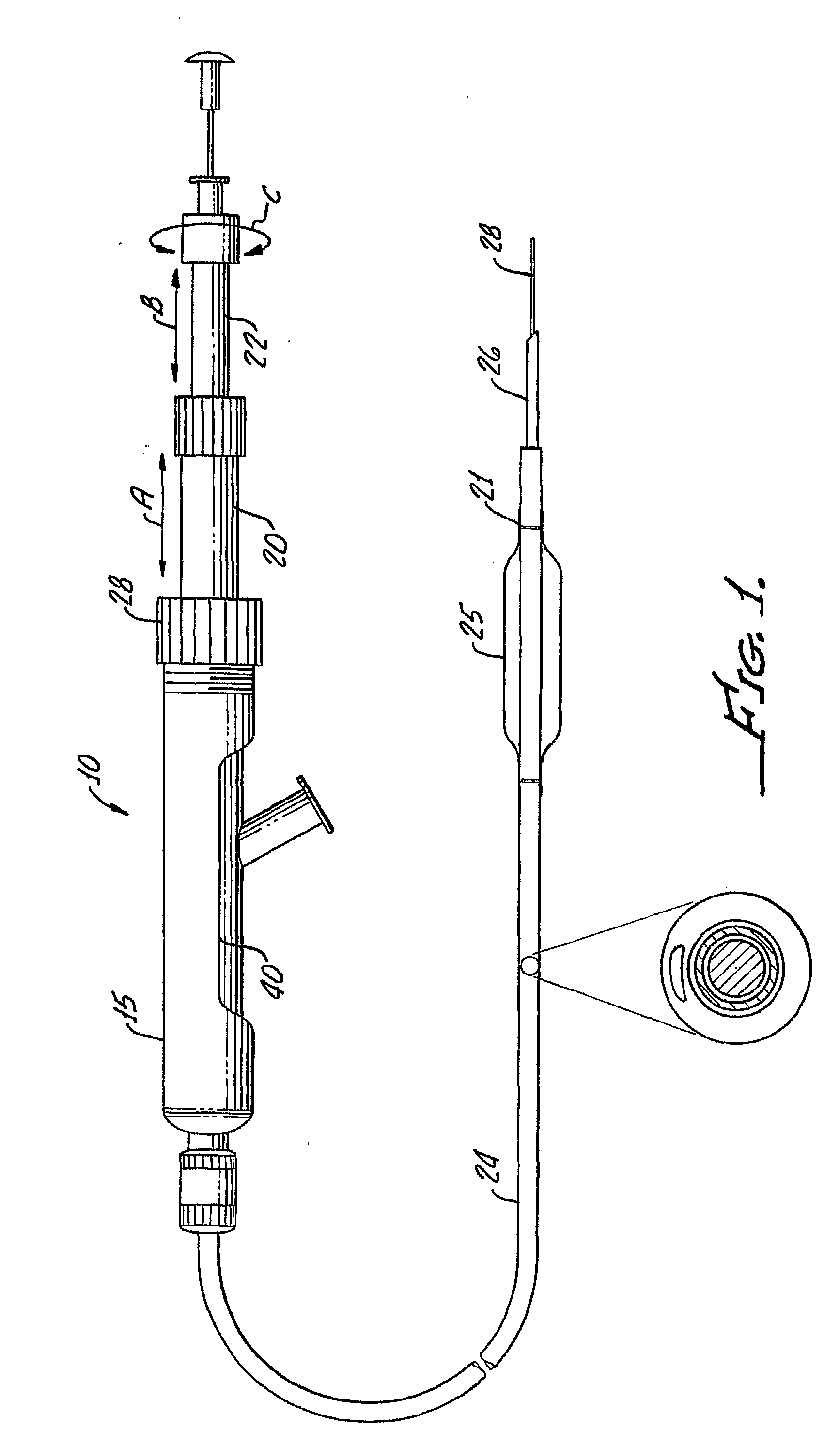
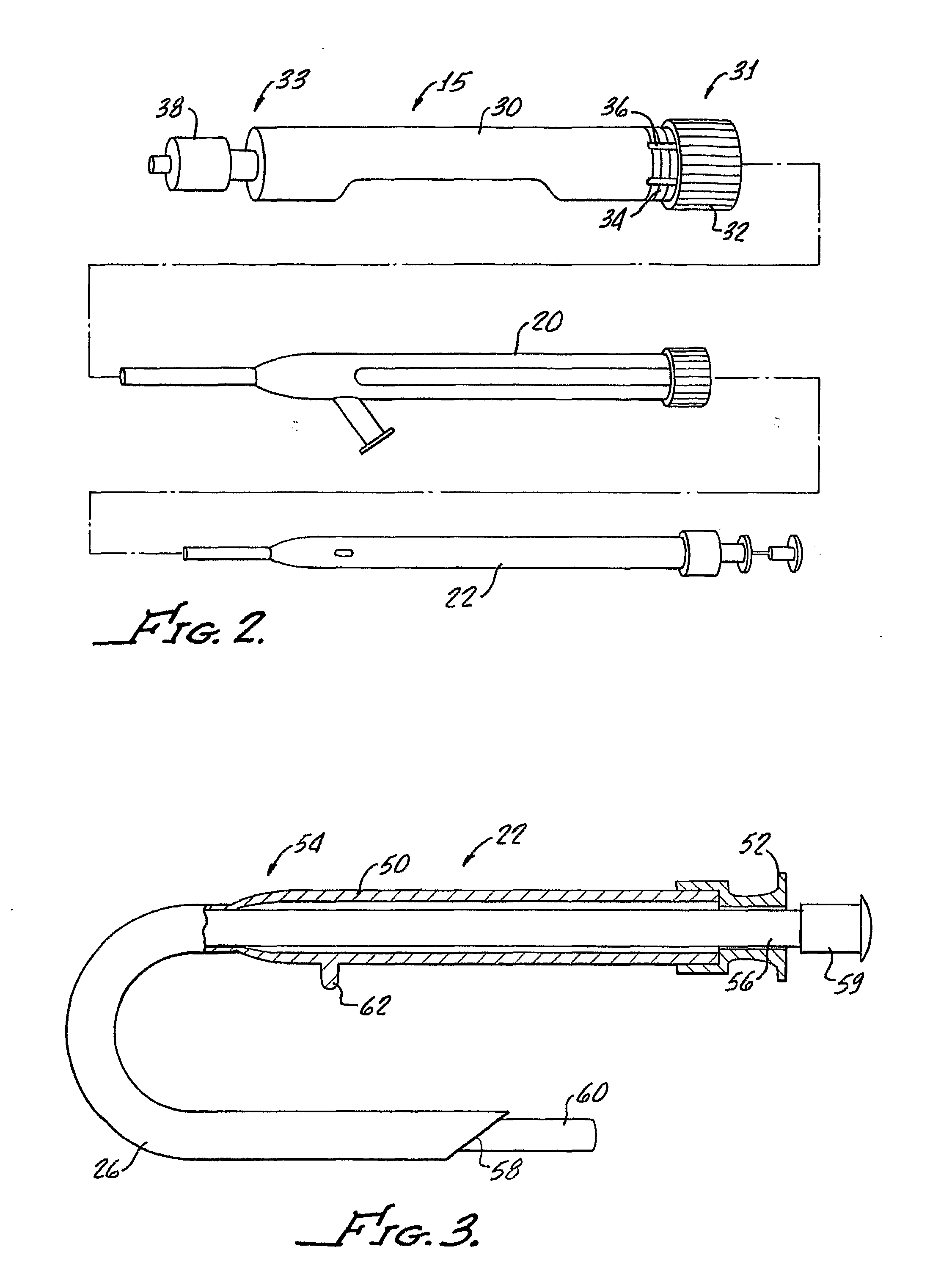
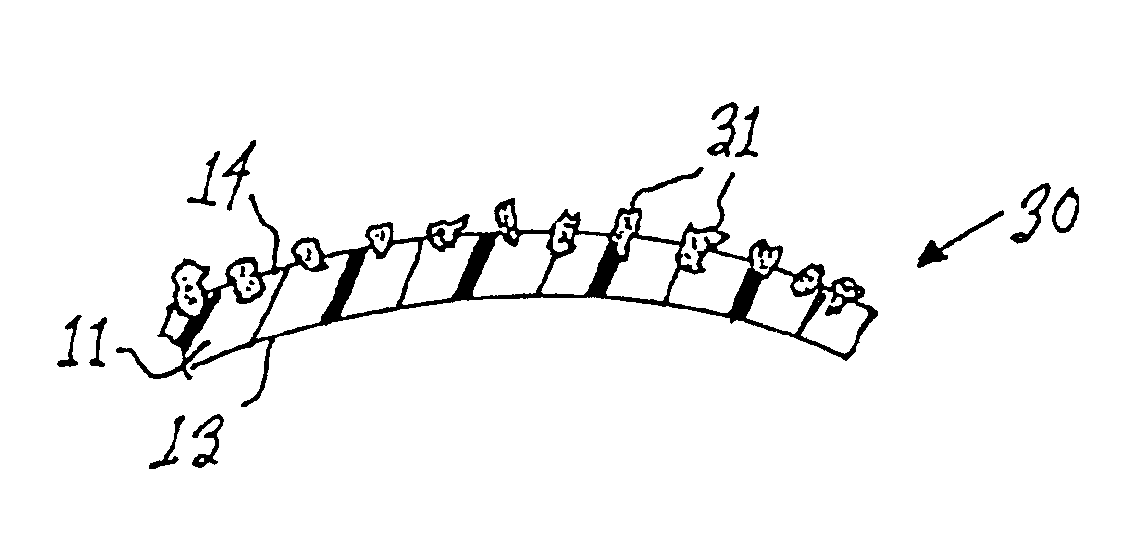
Tissue augmentation methods using a medical injection apparatus
InactiveUS20070078435A1Simple processReduce effortInfusion syringesMedical devicesHand heldInjection device
An injection apparatus that includes components that facilitate injection of relatively viscous materials into a subject is provided as well as methods of use. An injection apparatus may include a transition-bore needle apparatus, which has a proximal end, a distal end, and a lumen extending from the proximal end to the distal end, in which the diameter of the proximal end is greater than the diameter of the distal end. An injection apparatus may include a hand-held injection facilitation apparatus, which may be coupled to a syringe. The hand-held injection facilitation apparatus can include a pivot arm and a body with a rod disposed within the body and coupled to the pivot arm. Movement of the pivot arm results in a proximal or distal movement of the rod within the body to effectively cause material to be expelled from the syringe. An injection apparatus may include a transition-bore needle apparatus and a hand-held injection facilitation apparatus in combination.
Owner:ARTES MEDICAL USA
Method and Apparatus for Performing Needle Guided Interventions
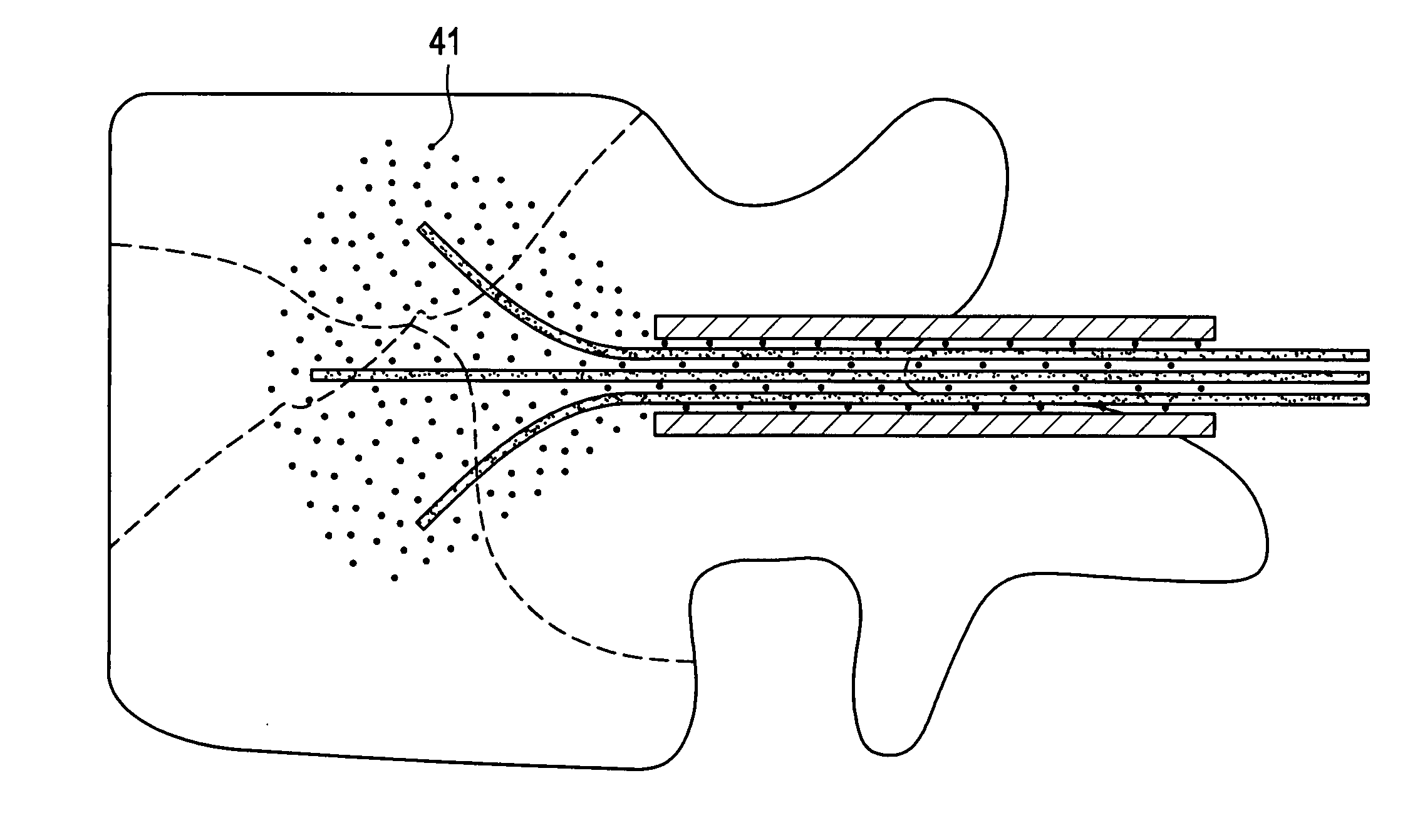
An apparatus and method for performing needle guided interventions and especially needle guided dilations of tissue to create a therapeutic conduit between two luminal organs or structures. The device is particularly useful for creation of an artificial lumen between two hollow body organs using the working lumen of an endoscope.
Owner:BOSTON SCI SCIMED INC
Medical implant having bioabsorbable textured surface
A hybrid medical implant having a biocompatible, nonabsorbable core portion and a bioabsorbable textured outer surface portion overlying the core portion. The hybrid implant is useful as a prosthesis for tissue augmentation and / or reconstruction. The core portion of the implant includes a body formed from a nonabsorbable, biocompatible implantable material such as silicone or urethane elastomer. The core portion may be either a solid body, a viscous gel body or a fluid-filled shell. The textured outer surface portion envelops the core portion and presents an irregular, bioabsorbable textured surface to the exterior environment. As a capsule forms around the implant following implantation, the irregular contour of the outer surface of the implant disorients structural proteins in the capsule to impede spherical contraction thereof. Either during the formation of the capsule and / or after the capsule is formed, the outer bioabsorbable surface portion of the implant is absorbed by the body of the host. After bioabsorbtion of the bioabsorbable outer surface portion, the remaining core portion of the implant remains enveloped by the capsule but unattached to capsular tissue. The outer bioabsorbable portion of the hybrid implant may include more than one biocompatible, bioabsorbable material.
Owner:WHD ELEKTRONISCHE PRUEFTECHN
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20070212385A1Quality improvementExtending and improving qualityBiocideCosmetic preparationsBiologyTissue augmentation
Owner:KYTHERA BIOPHARMLS INC
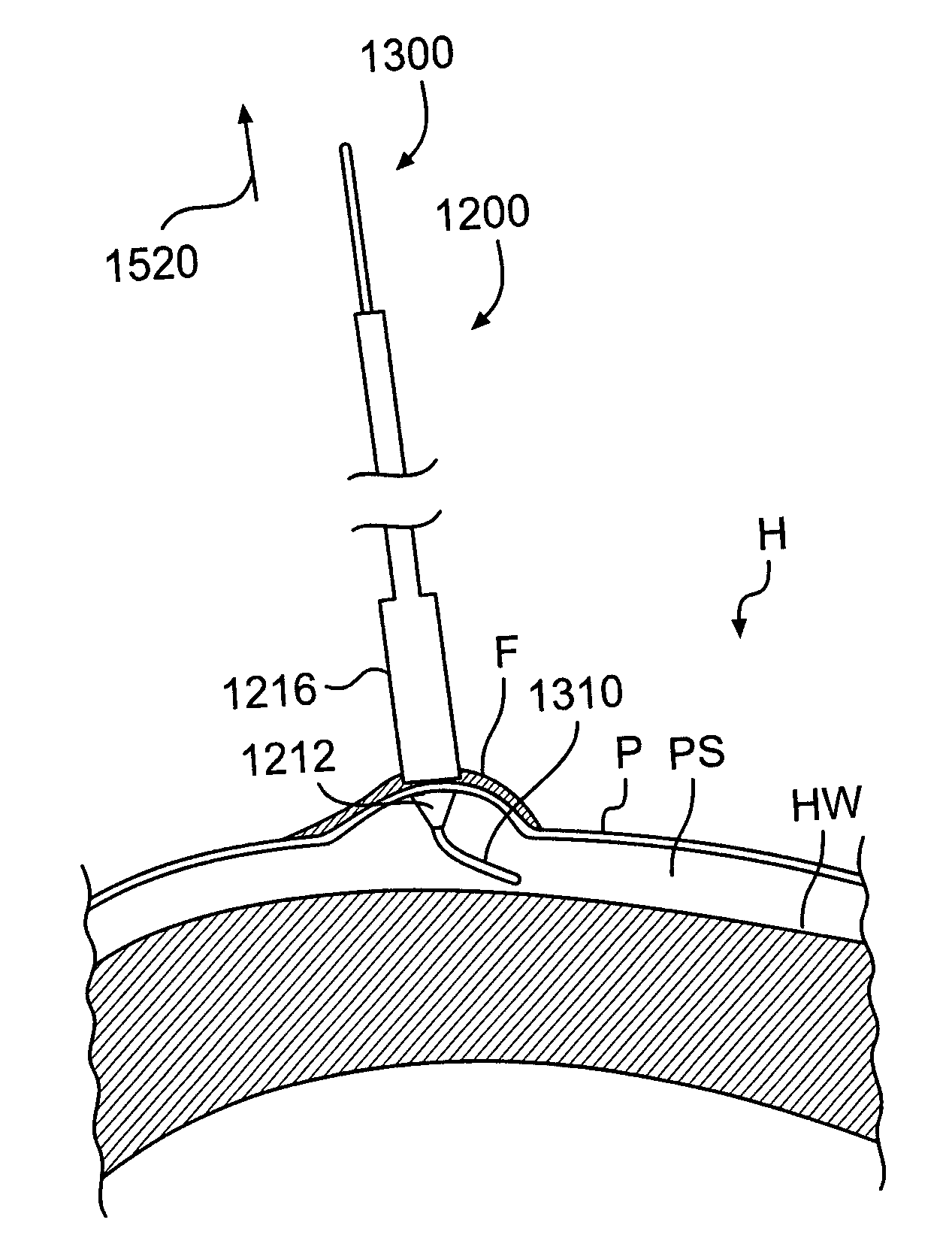
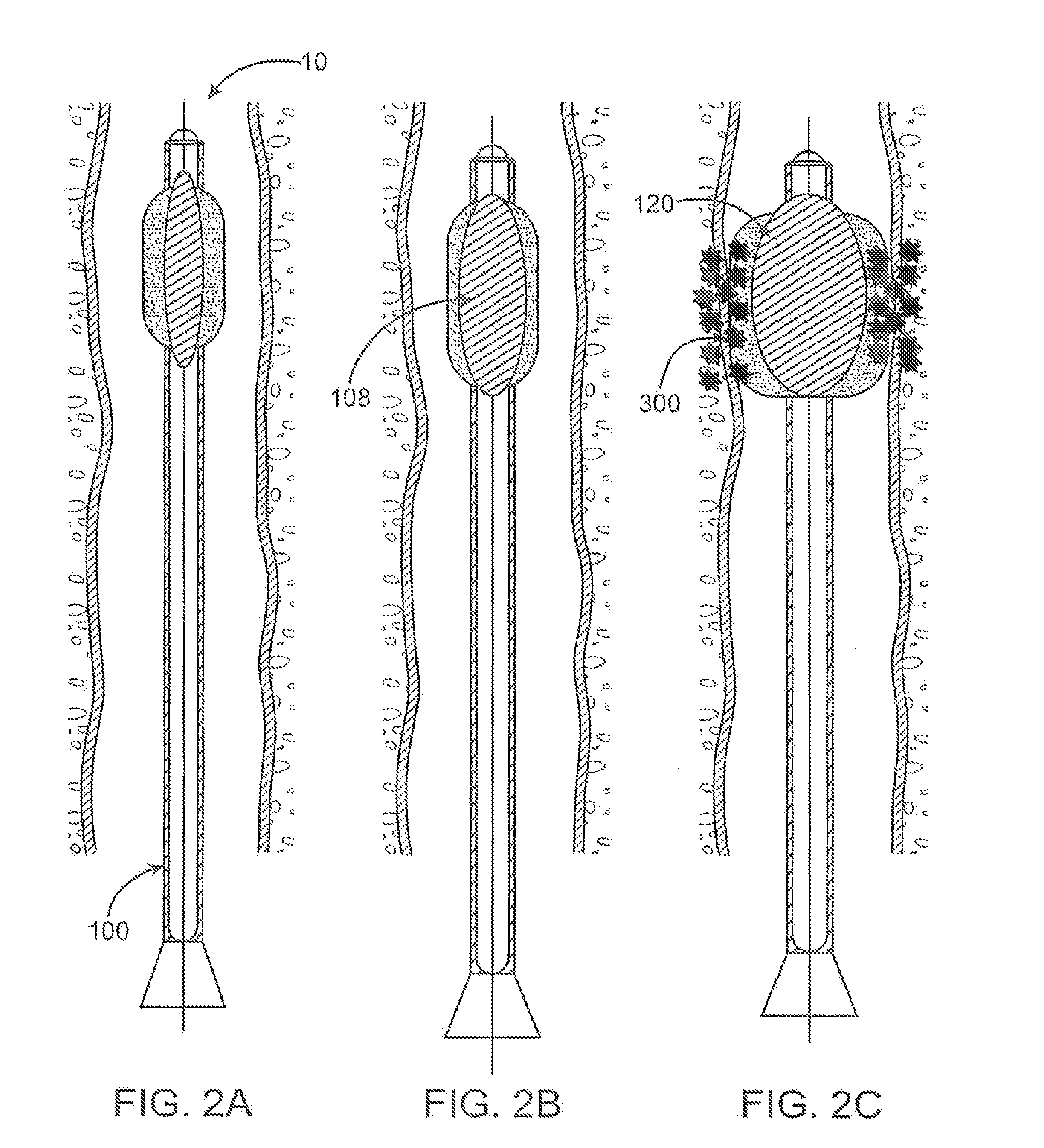
Tissue augmentation device
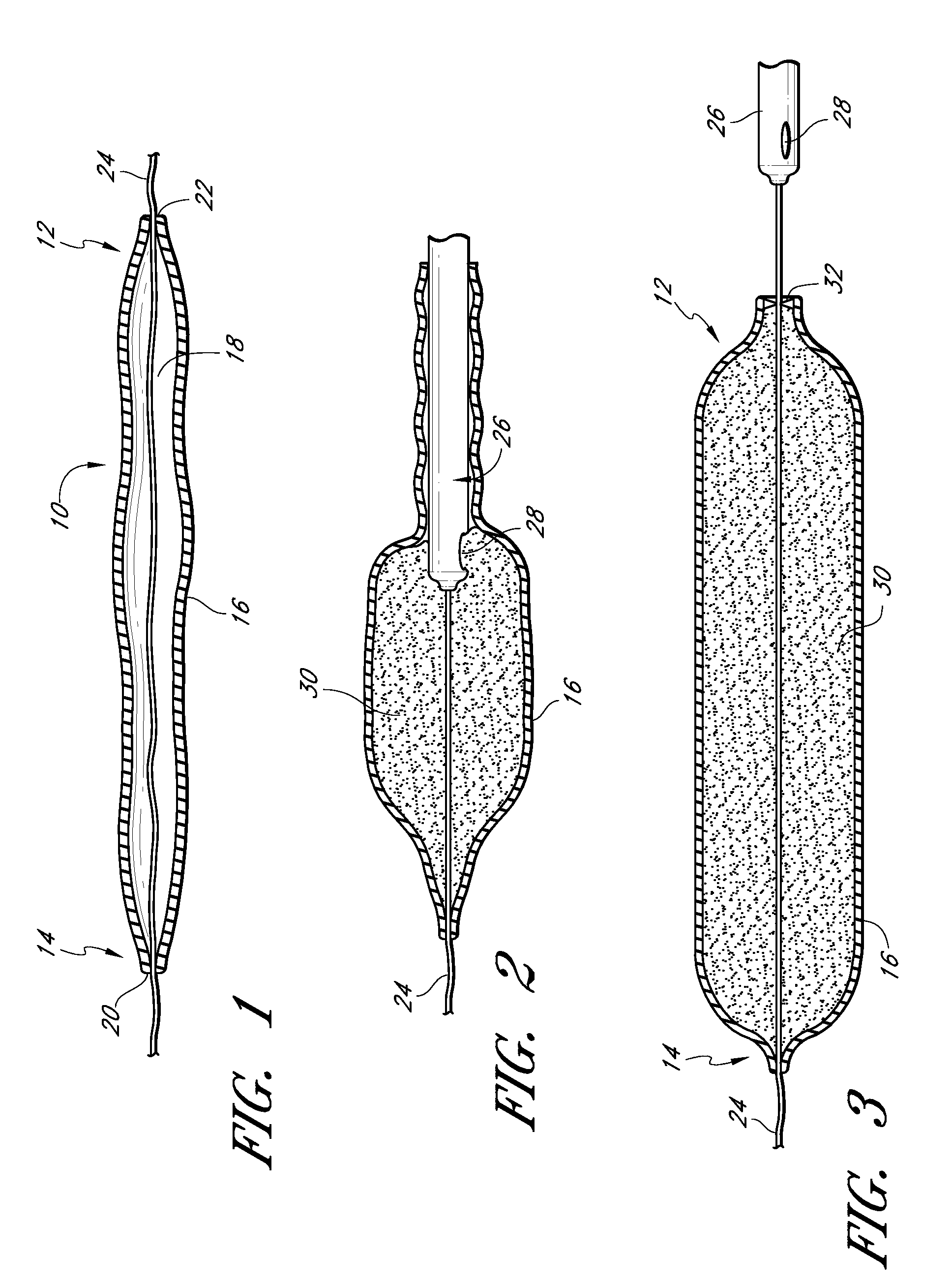
InactiveUS20060161253A1Flatten nasolabial foldEnhance lipMammary implantsCosmetic implantsTissue augmentationVALVE PORT
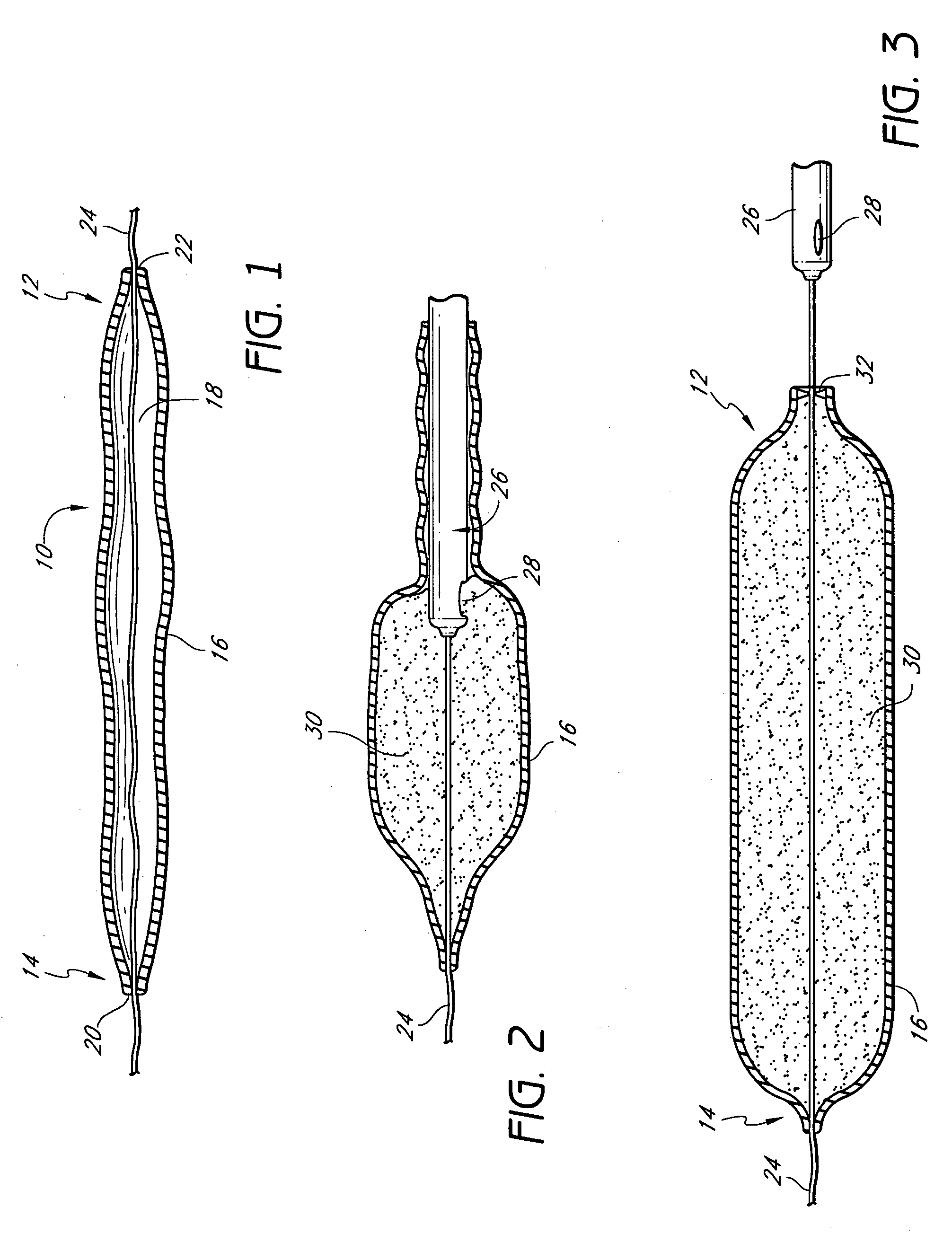
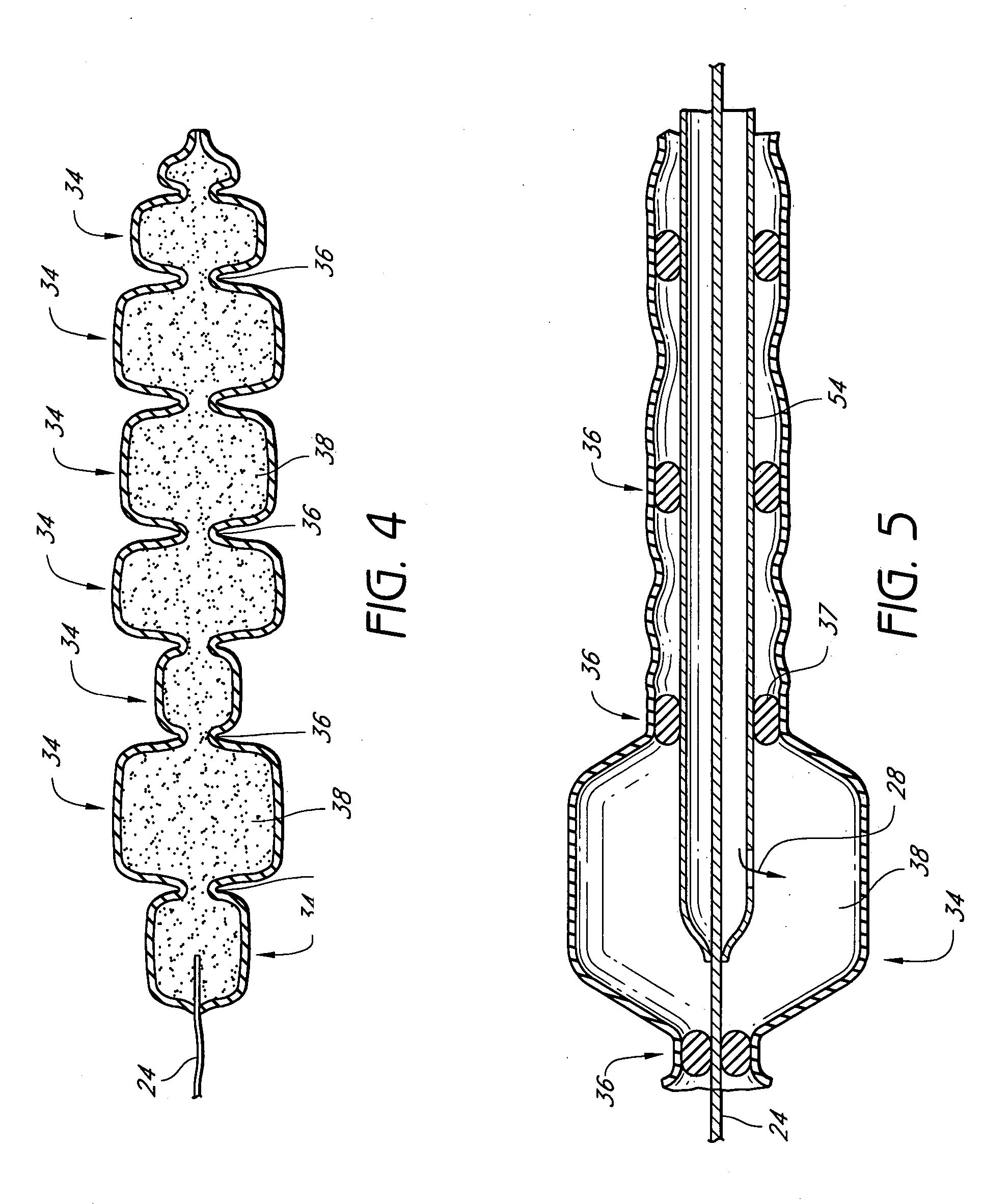
Disclosed are implantable tissue augmentation devices, methods, and associated tools. The devices include an inflatable body, having an inner layer and an outer layer. A valve is provided for permitting the introduction of and retaining inflation media. At least one pull tab is provided on an end of the implant, to assist in positioning the implant. Kits and systems are also disclosed.
Owner:EVERA MEDICAL
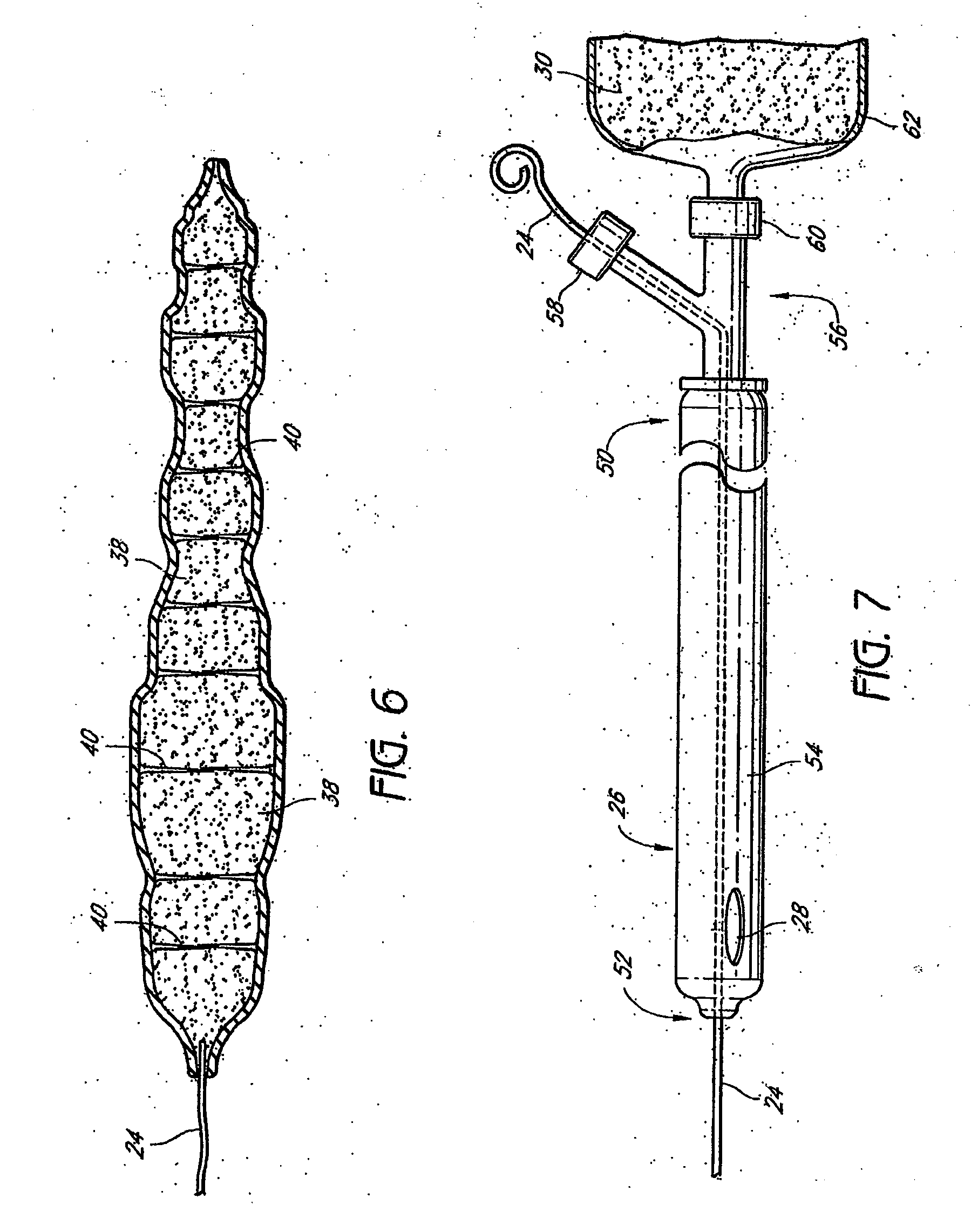
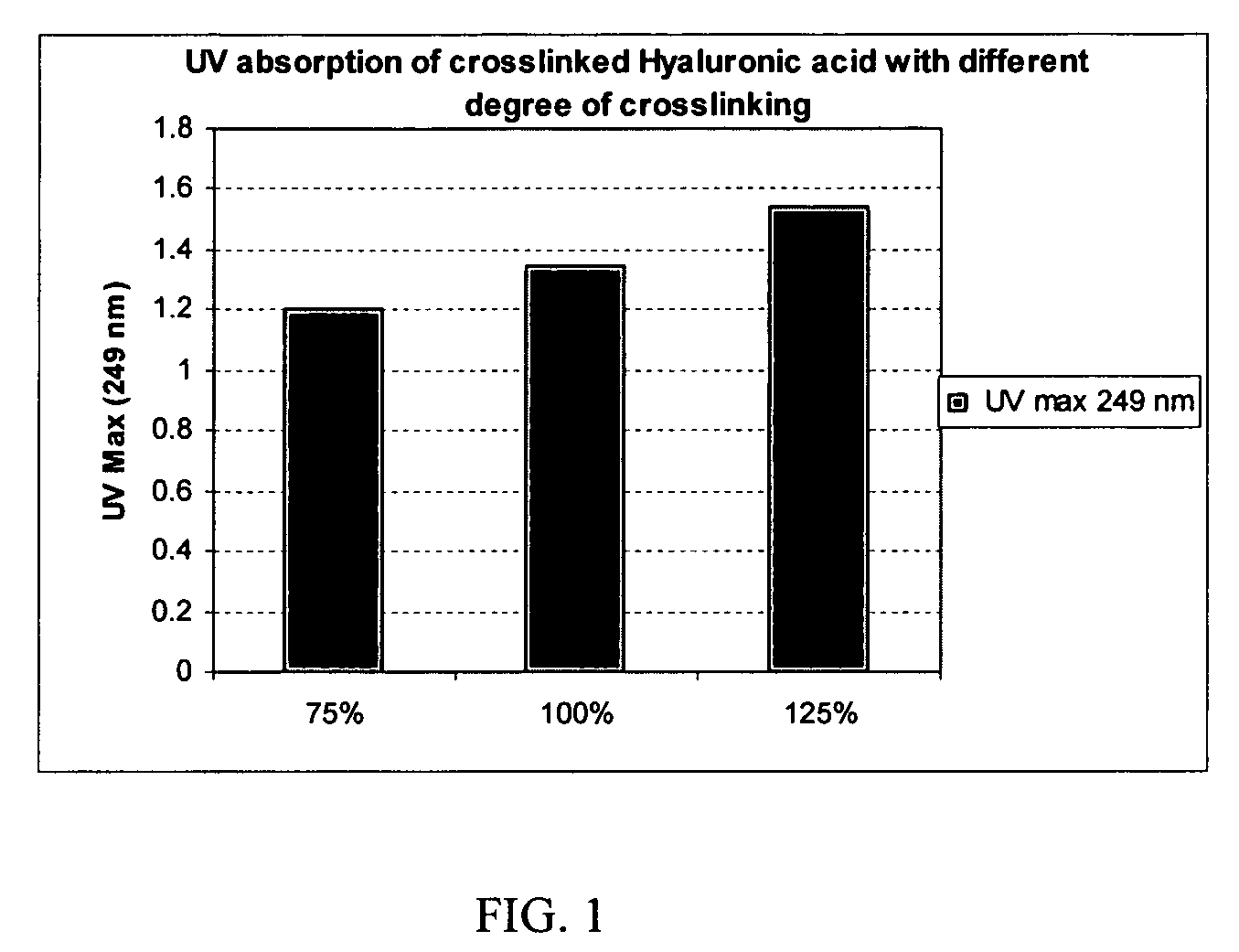
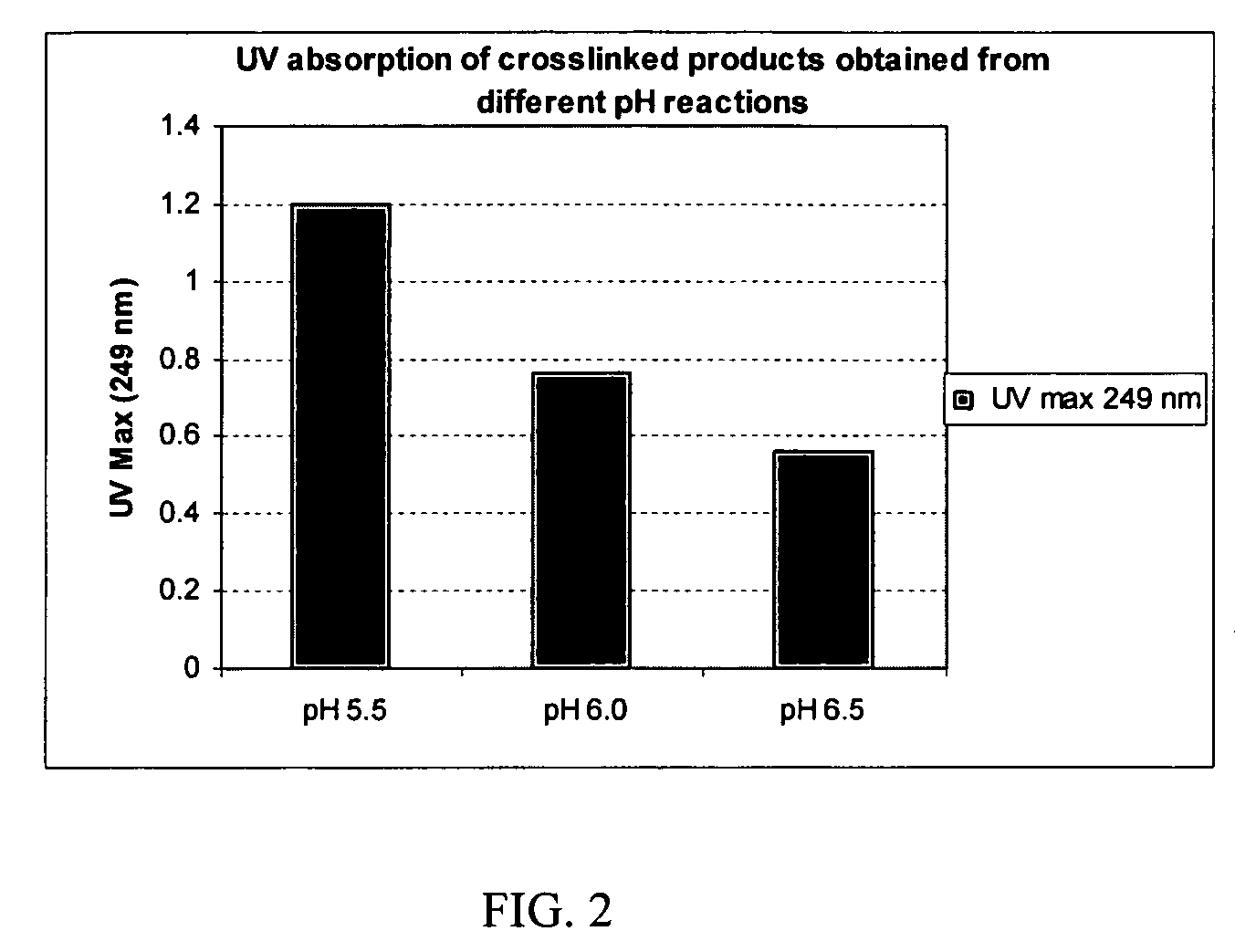
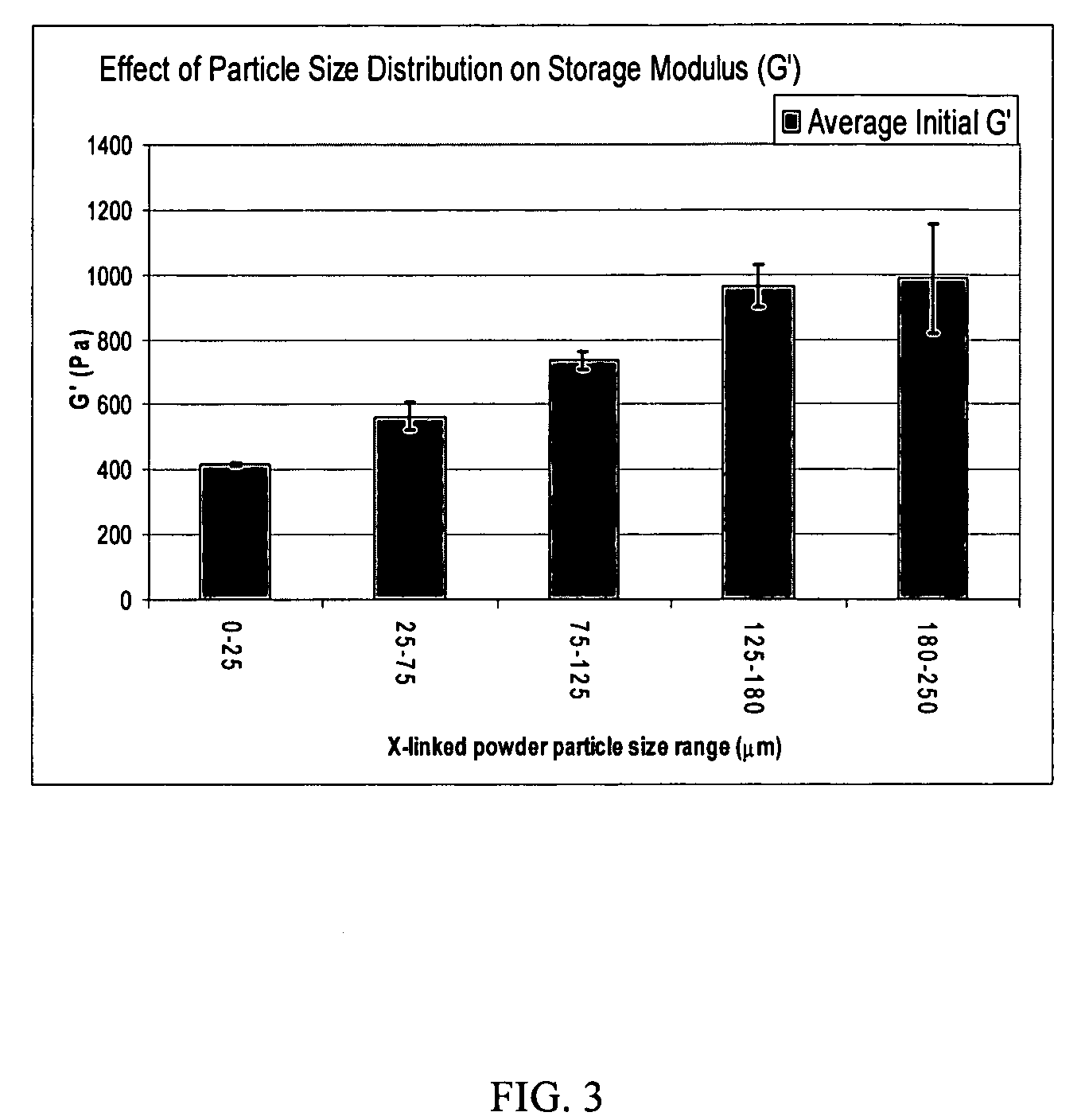
Crosslinked hyaluronic acid compositions for tissue augmentation
ActiveUS8124120B2Improve drug deliveryReduce frequencyAntibacterial agentsOrganic active ingredientsMedicineWater insoluble
Disclosed are hyaluronic acid (HA) compositions including crosslinked, water-insoluble, hydrated HA gel particles. Also disclosed are methods of making the HA compositions, and methods of using the HA composition to augment tissue in a subject.
Owner:ANIKA THERAPEUTICS INC
Tissue augmentation material and method
InactiveUS7060287B1Reduce deliveryImpression capsAnti-incontinence devicesUnilateral vocal cord paralysisSphincter
A permanent, biocompatible material for soft tissue augmentation. The biocompatible material comprises a matrix of smooth, round, finely divided, substantially spherical particles of a biocompatible ceramic material, close to or in contact with each other, which provide a scaffold or lattice for autogenous, three dimensional, randomly oriented, non-scar soft tissue growth at the augmentation site. The augmentation material can be homogeneously suspended in a biocompatible, resorbable lubricious gel carrier comprising a polysaccharide. This serves to improve the delivery of the augmentation material by injection to the tissue site where augmentation is desired. The augmentation material is especially suitable for urethral sphincter augmentation, for treatment of incontinence, for filling soft tissue voids, for creating soft tissue blebs, for the treatment of unilateral vocal cord paralysis, and for mammary implants. It can be injected intradermally, subcutaneously or can be implanted.
Owner:BIOFORM
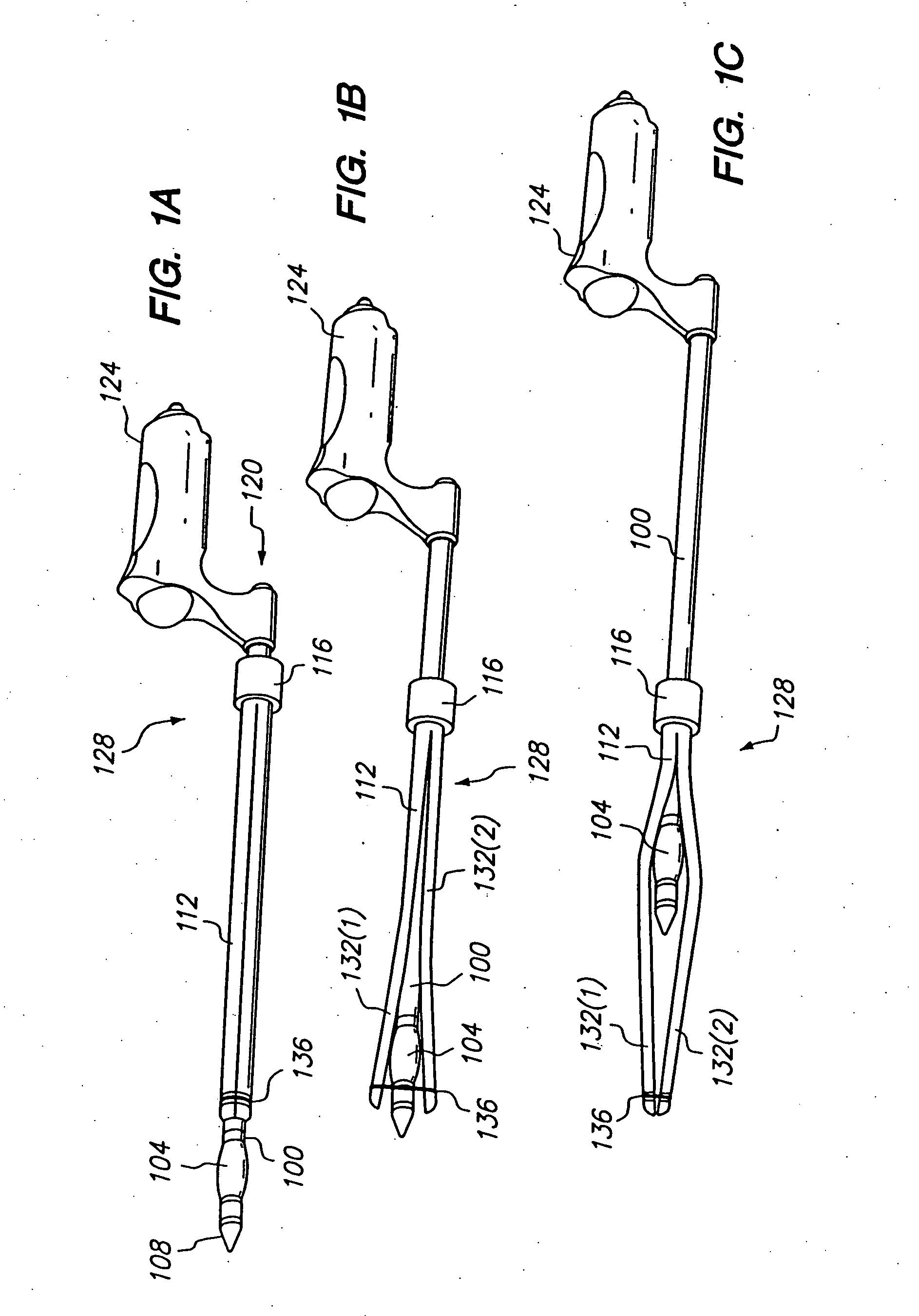
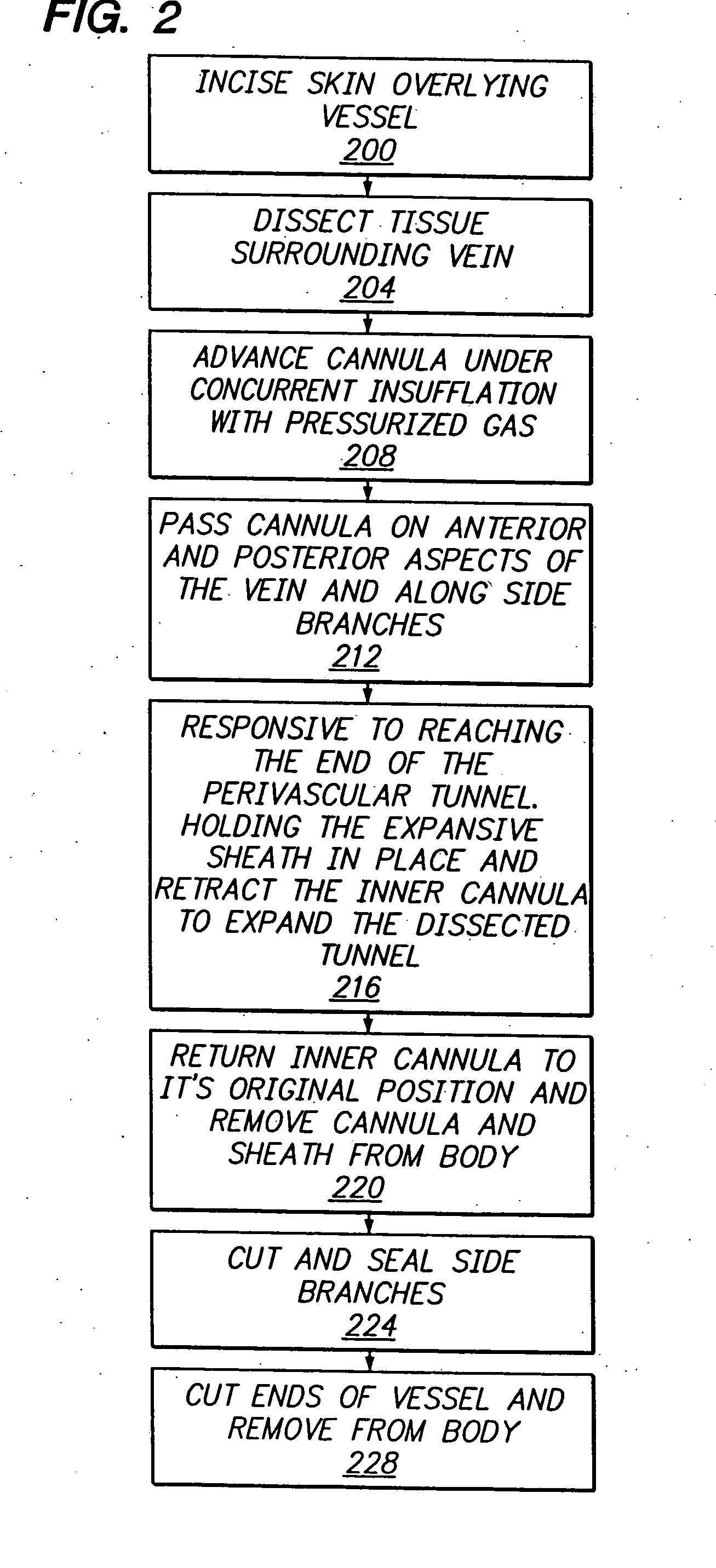
Longitudinal dilator
Apparatus and method for dilation of tissue utilize a tissue expansion device positioned on an inner cannula with an outer overlying expansive sheath that expands upon translation of the tissue expansion device therethrough. The tissue expansion device may be an olive or wedge formed near the tip of the cannula, and the expansible sheath includes two elongated shells that are fixably attached near proximal ends, and that are resiliently connected near distal ends. Translating the tissue expansion device through the expansible sheath expands the dimension of the shells to provide even dilation of surrounding tissue. Additionally, tissue dilation is performed in one continuous motion of retracting the inner cannula through the expansible sheath or pushing the tissue expansion device through the expansible sheath. The outer expansible sheath may be removed from the inner cannula to provide a dissection instrument having minimal outer diameter. The tissue expansion device may provide two stage expansion from a minimal outer dimension in one configuration to a second larger outer dimension in response to an applied axial force to provide enhanced tissue dilation.
Owner:MAQUET CARDIOVASCULAR LLC
Implantation Compositions for Use in Tissue Augmentation
InactiveUS20100041788A1Good compatibilityMinimized immuno-histo tissue responseOrganic active ingredientsSurgical adhesivesPolymer solutionPolysaccharide
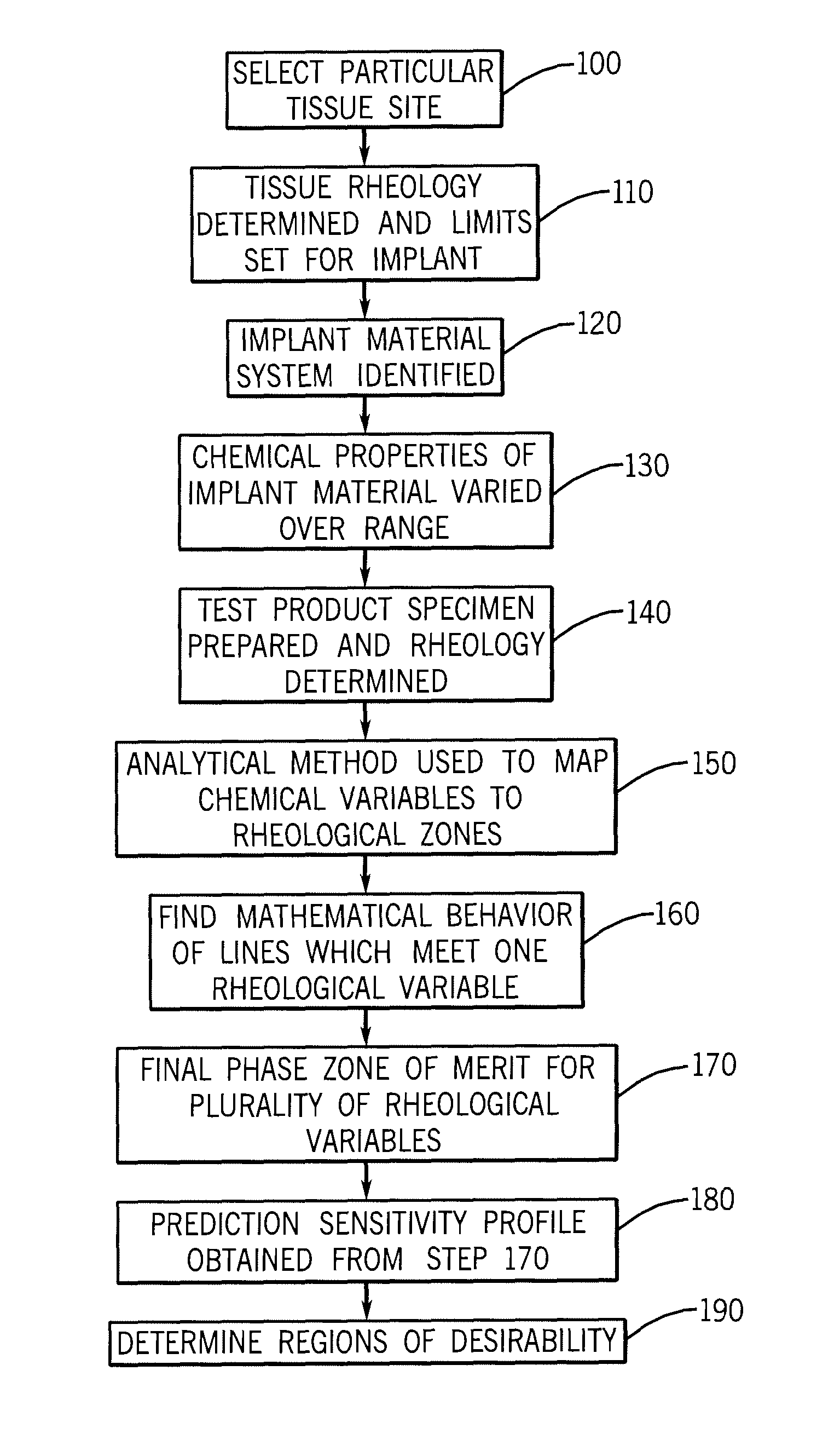
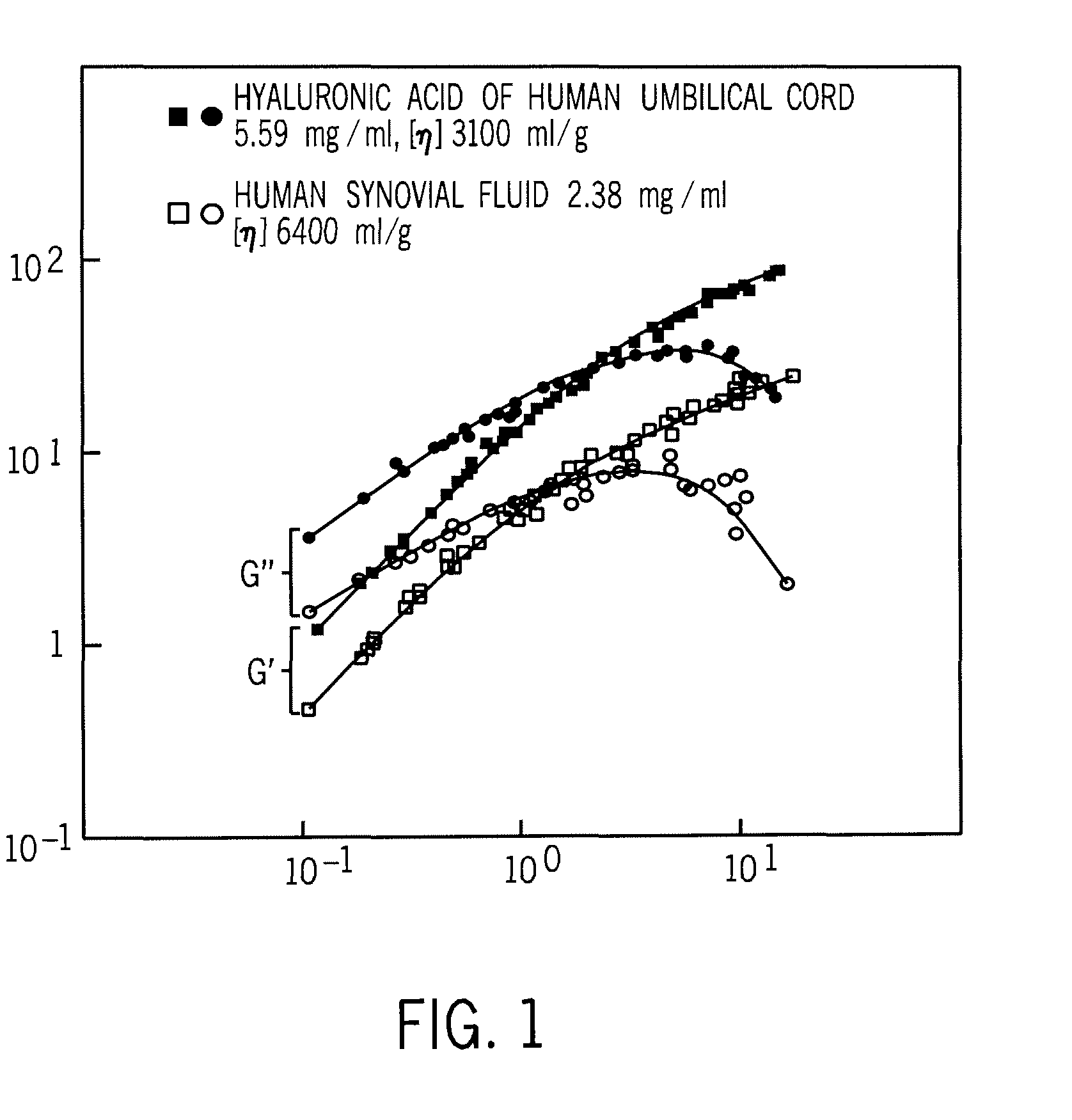
A composition of matter and method for preparation of a tissue augmentation material. A polysaccharide gel composition is prepared with rheological properties selected for a particular selected application. The method includes preparing a polymeric polysaccharide in a buffer to create a polymer solution or gel suspending properties in the gel and selecting a rheology profile for the desired tissue region.
Owner:MERZ AESTHETICS
Compositions and systems for forming crosslinked biomaterials and methods of preparation and use
InactiveUS20060210602A1Organic active ingredientsProtein waste adhesivesActive agentBiological materials
Crosslinkable compositions are provided that readily crosslink in situ to provide biocompatible, nonimmunogenic crosslinked materials that may be used as adhesive compositions. The compositions comprise collagen and a plurality of crosslinkable components having reactive functional groups thereon, with the functional groups selected so as to enable inter-reaction between the components, i.e., crosslinking. Methods for preparing and using the compositions are also provided. Exemplary uses include tissue augmentation, biologically active agent delivery, bioadhesion, prevention of adhesions following surgery or injury, and coating of surgically acceptable patches and solid implants, the latter including sutures.
Owner:ANGIODEVICE INT GMBH
Thermopolymer composition and related methods
A thermopolymer composition is disclosed suitable for use in filling voids within a human body, including but not limited to orthopedic joints (i.e. the discs of the spine and joints of the extremities), spaces between bone fractures or separations, and / or voids created within muscle and / or viscera for the purpose of tissue augmentation. The thermopolymer composition of the present invention may be heated and injected into the body in flowable form and thereafter cooled to body temperature to become a flexible, yet relatively solid material.
Owner:NUVASIVE
Compositions and systems for forming crosslinked biomaterials and methods of preparation of use
Crosslinkable compositions are provided that readily crosslink in situ to provide biocompatible, nonimmunogenic crosslinked materials that may be used as adhesive compositions. The compositions comprise collagen and a plurality of crosslinkable components having reactive functional groups thereon, with the functional groups selected so as to enable inter-reaction between the components, i.e., crosslinking. Methods for preparing and using the compositions are also provided. Exemplary uses include tissue augmentation, biologically active agent delivery, bioadhesion, prevention of adhesions following surgery or injury, and coating of surgically acceptable patches and solid implants, the latter including sutures.
Owner:ANGIODEVICE INT GMBH
Polysaccharide compositions for use in tissue augmentation
A composition of matter and method for preparation of a tissue augmentation material. A polysaccharide gel composition is prepared with a programmable rheology for a particular selected application. The method includes preparing a polymeric polysaccharide in a buffer to create a polymer solution or gel suspending particles in the gel and selecting a rheology profile for the desired tissue region.
Owner:BIOFORM MEDICAL
Implantable collagen compositions
InactiveUS20060100138A1Improve durabilityImproved ease of handlingConnective tissue peptidesSurgical adhesivesTissue augmentation
The present invention relates to collagen compositions suitable for use in various tissue augmentation procedures.
Owner:FIBROGEN INC
Methods of forming a multilayer tissue implant to create an accordion effect on the outer layer
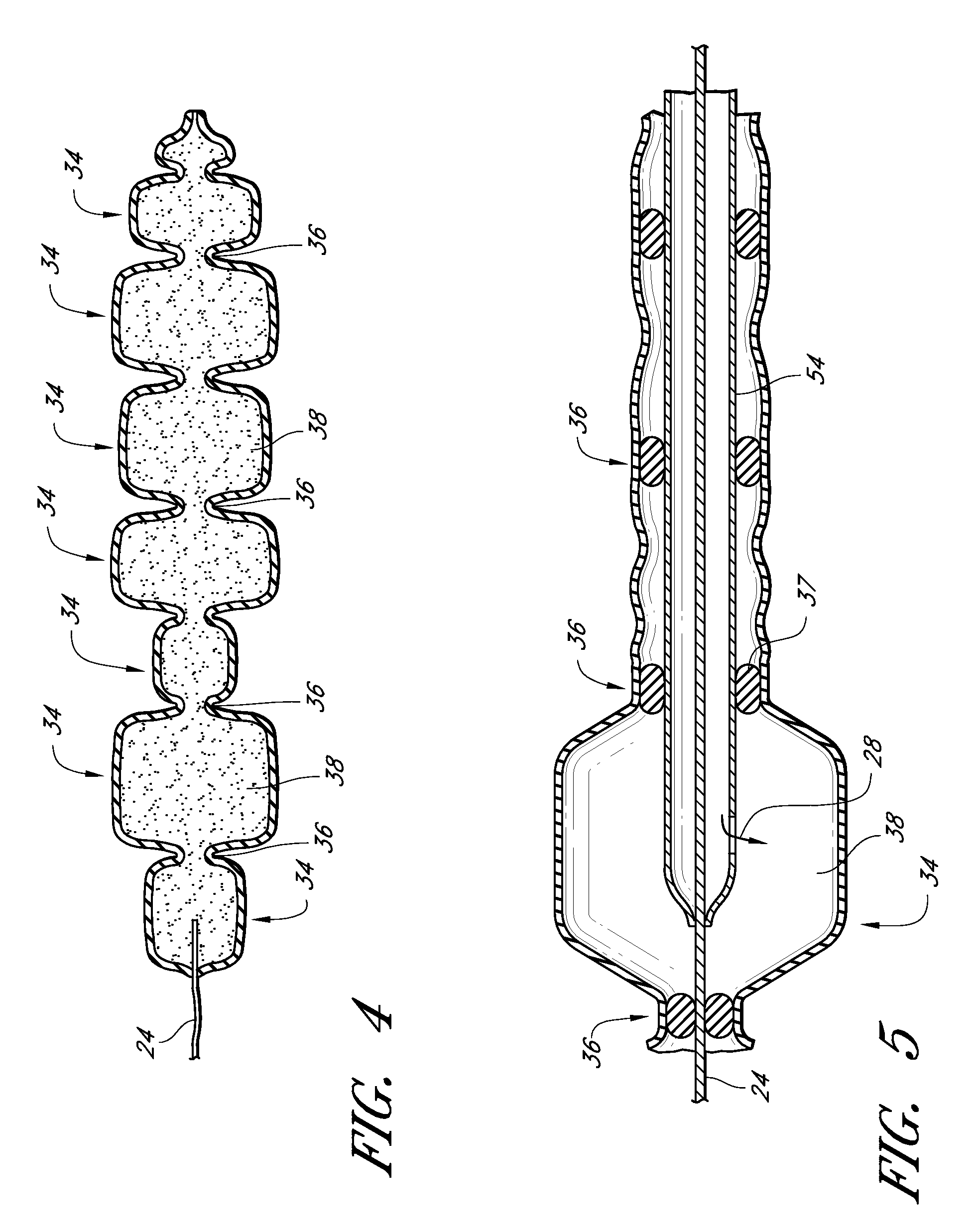
InactiveUS20090024227A1Increase flexibilityMammary implantsCosmetic implantsAccordion effectTissue augmentation
Disclosed are implantable tissue augmentation devices, methods, and associated tools. The devices include an inflatable body, having an inner layer and an outer layer. A valve is provided for permitting the introduction of and retaining inflation media. At least one pull tab is provided on an end of the implant, to assist in positioning the implant. Kits and systems are also disclosed.
Owner:EVERA MEDICAL
Prolonged delivery of heparin-binding growth factors from heparin-derivatized collagen
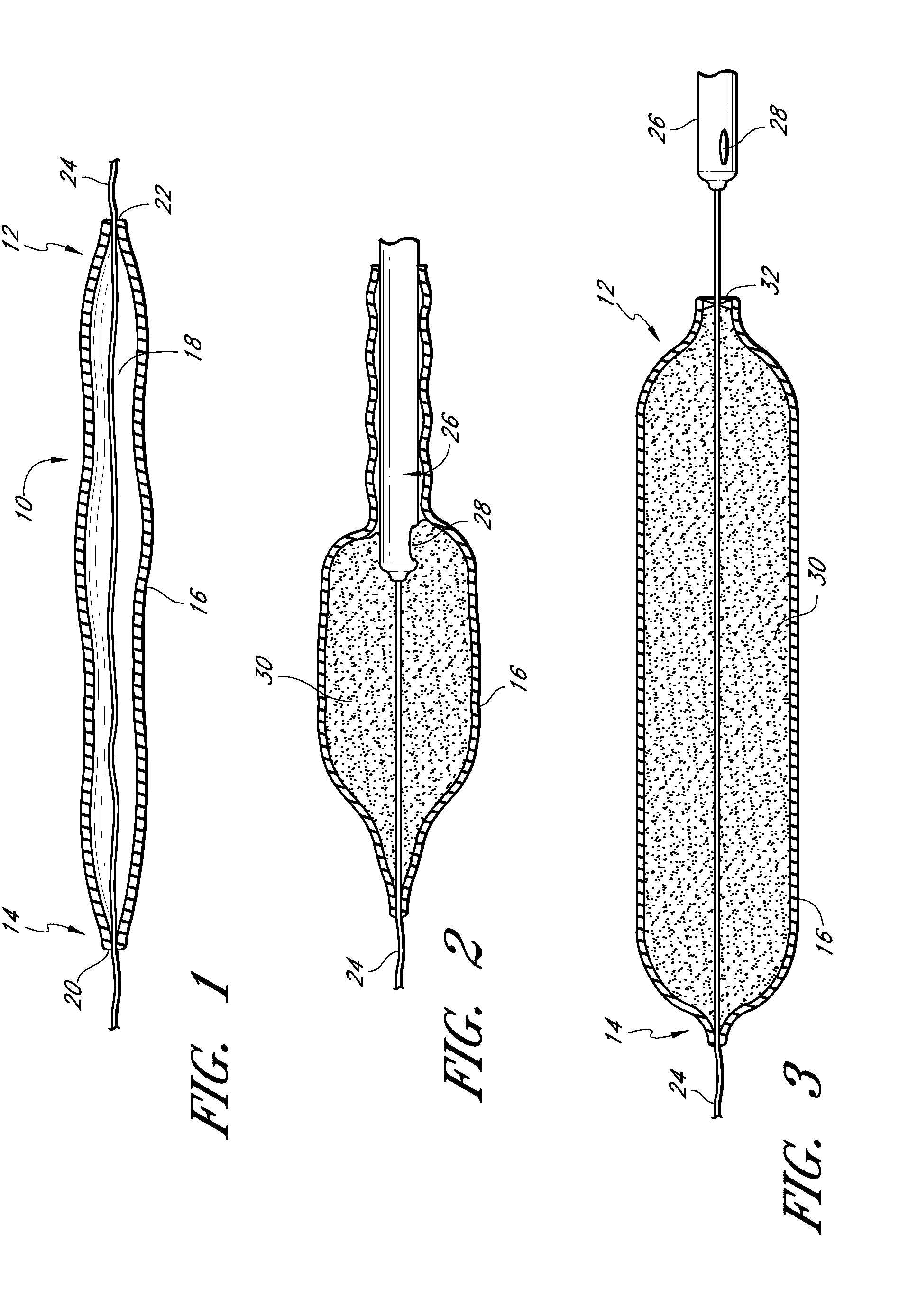
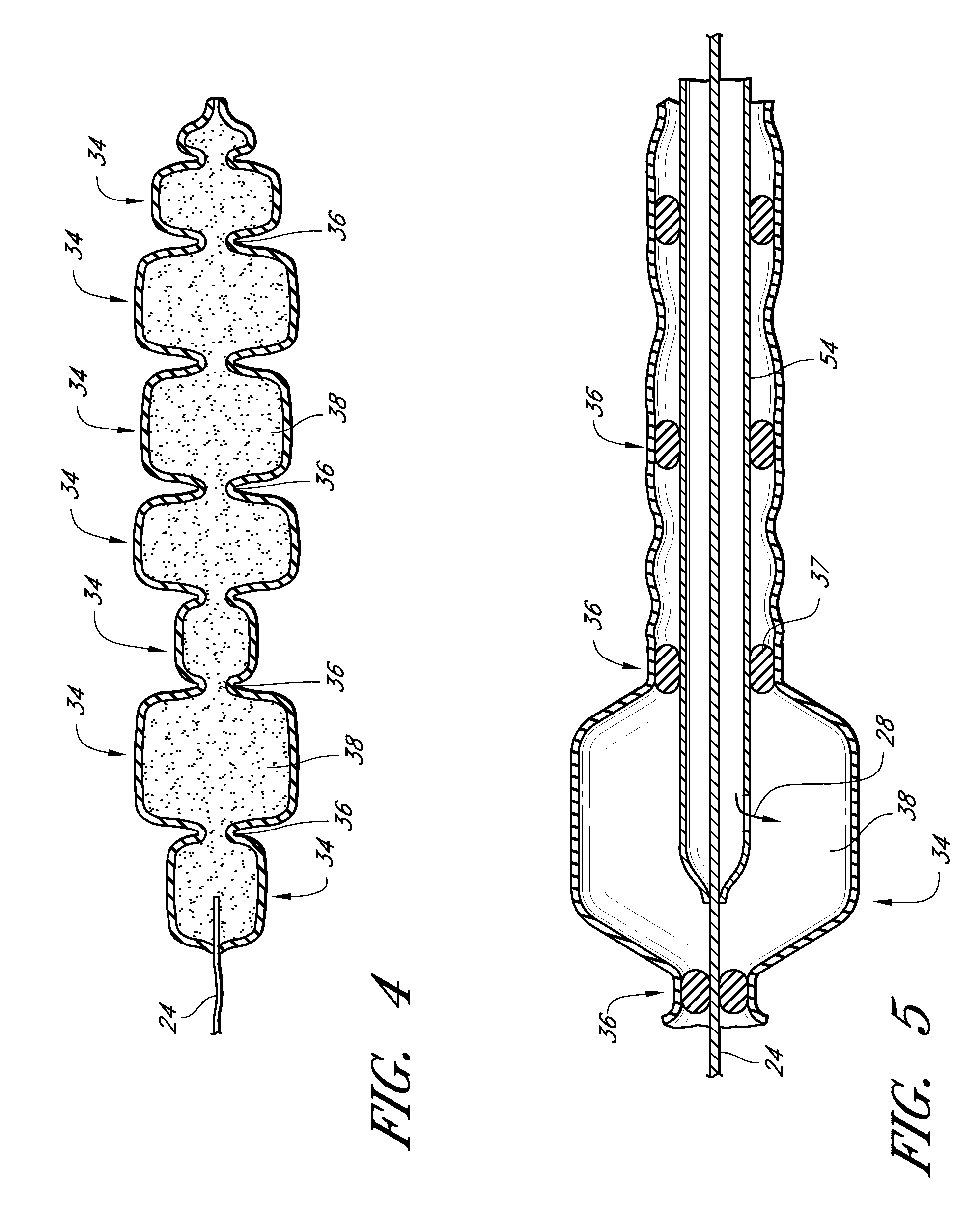
InactiveUS20090192079A1Improve biological activityPeptide/protein ingredientsGenetic therapy composition manufactureMuscle tissueCollagen scaffold
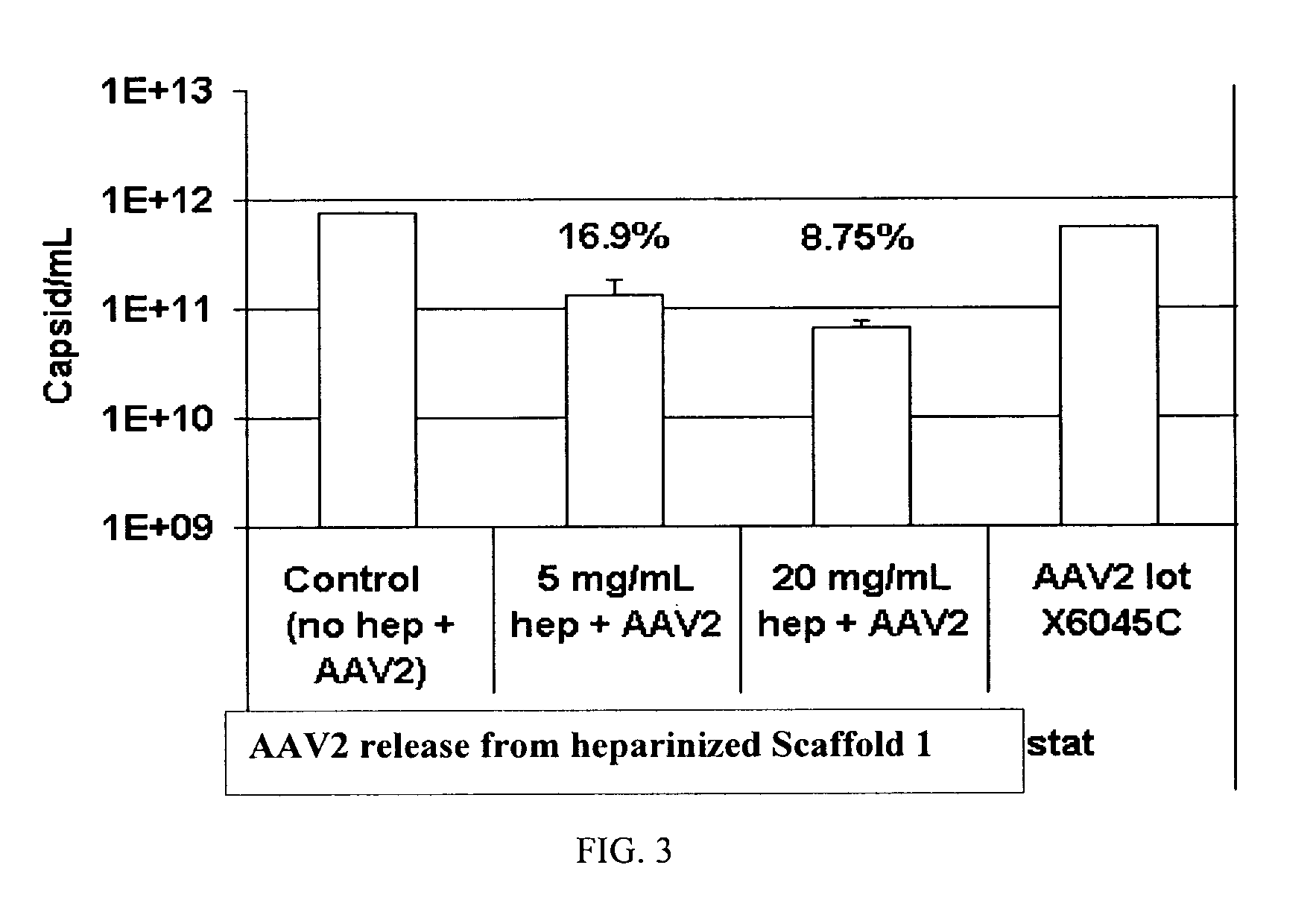
The present invention relates to a heparin-derivatized collagen matrix comprising a fragment of heparin covalently linked to a collagen scaffold, wherein the fragment of heparin has molecular weight of less than about 15 kDa, and at least one heparin-binding growth factor (HBGF) or heparin-binding adeno-associated virus (HB-AAV) or a combination thereof and methods for promoting bone growth, bone repair, cartilage repair, bone development, neo-angiogensis, wound healing, tissue engraftment and muscle tissue regeneration and / or tissue augmentation comprising administering a heparin-derivatized collagen matrix that includes at least one heparin-binding growth factor or heparin-binding adeno-associated virus or a combination thereof.
Owner:GENZYME CORP
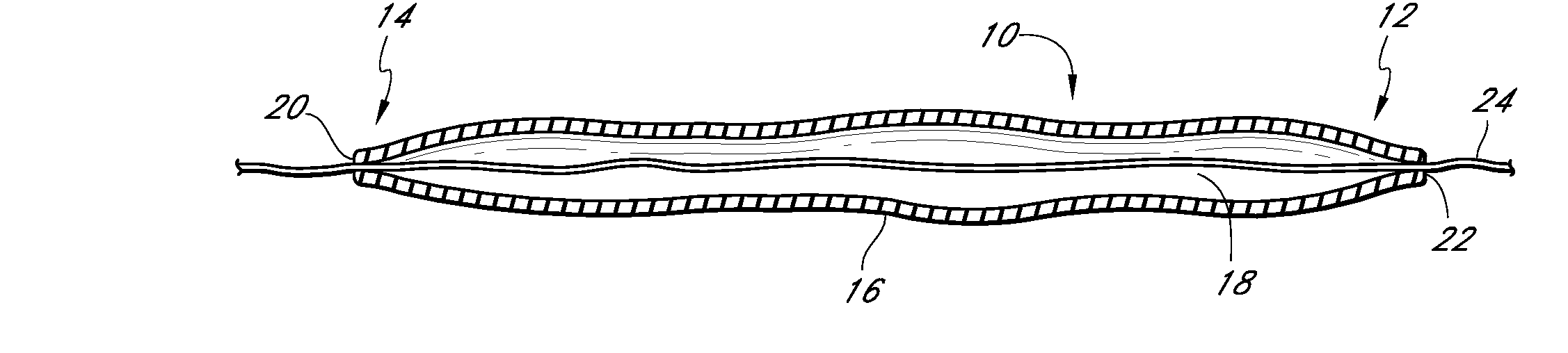
Valved tissue augmentation implant
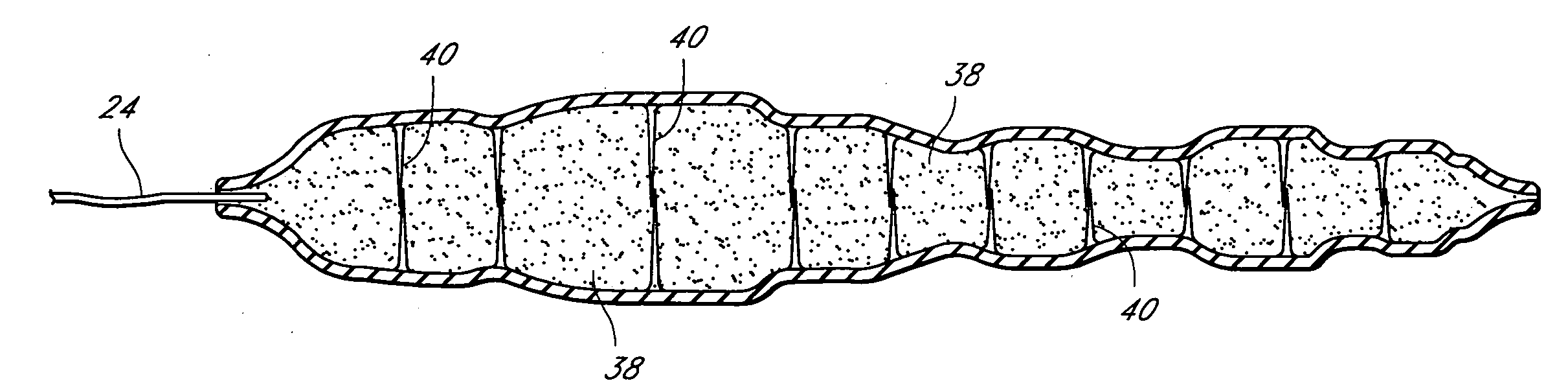
A medical device and methods for filling tissue are disclosed. The device has a first configuration wherein the device can pass through a small catheter or needle placed in the tissue to be filled and a second configuration in which the device is expanded within the tissue to be filled. The exterior profile of the expanded device may be customized along its length, to achieve a desired cosmetic or therapeutic result. In one embodiment, the device comprises an outer sleeve for containing filler and a valve on the sleeve for retaining the filler therein. The device is adapted to be placed through a needle in the skin to reduce facial wrinkles or augment facial features such as the lips.
Owner:EVERA MEDICAL
High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair
InactiveUS20110184530A1Different and simple configurationEfficiently employedProsthesisElastomerTissue repair
This invention relates to reticulated elastomeric matrices, their manufacture, their post-processing, such as their reinforcement, compressive molding or annealing, and uses including uses for implantable devices into or for topical treatment of patients, such as humans and other animals, for surgical devices, tissue augmentation, tissue repair, therapeutic, nutritional, or other useful purposes.
Owner:BIOMERIX CORP
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20080038306A1Extending and improving qualityImprove sturdinessCosmetic preparationsBiocideTissue augmentationDermal Fillers
Owner:KYTHERA BIOPHARMLS INC +1
Tissue Augmentation With Active Agent For Wound Healing
The invention relates to a bioactive settable hydrogel matrix having a pore creating material, which may also carry a therapeutic agent, and methods of using the same, for example, use in promoting internal wound healing, tissue repair, tissue regeneration.
Owner:WARSAW ORTHOPEDIC INC
Tissue implant having a biased layer and compliance that simulates tissue
InactiveUS20090024228A1Increase flexibilityMammary implantsCosmetic implantsTissue augmentationVALVE PORT
Disclosed are implantable tissue augmentation devices, methods, and associated tools. The devices include an inflatable body, having an inner layer and an outer layer. A valve is provided for permitting the introduction of and retaining inflation media. At least one pull tab is provided on an end of the implant, to assist in positioning the implant. Kits and systems are also disclosed.
Owner:EVERA MEDICAL
Tissue augmentation material and method
InactiveUS7968110B2Reduce deliveryAnti-incontinence devicesPharmaceutical delivery mechanismCelluloseCarrageenan
Owner:MERZ NORTH AMERICA
High swell, long-lived hydrogel sealant
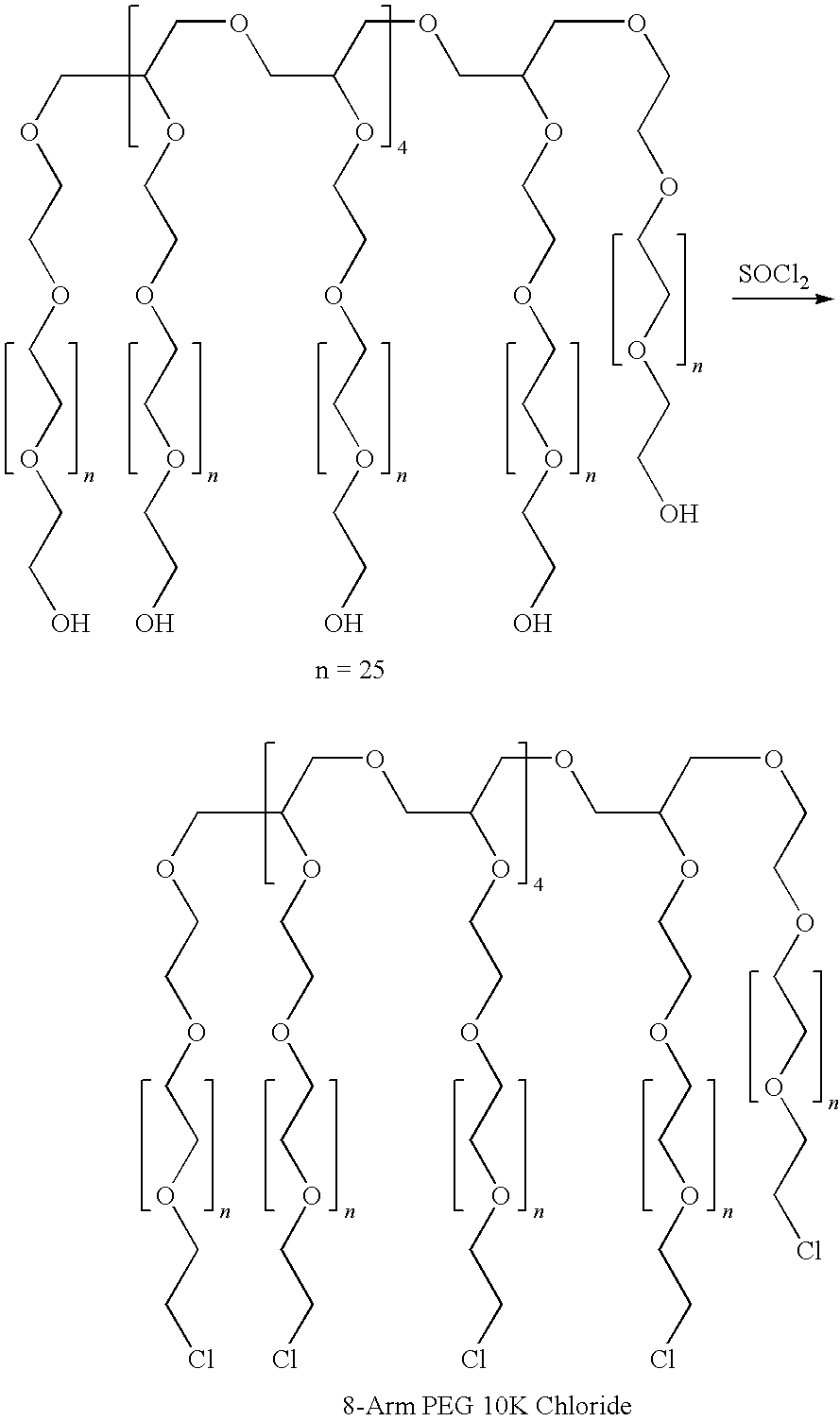
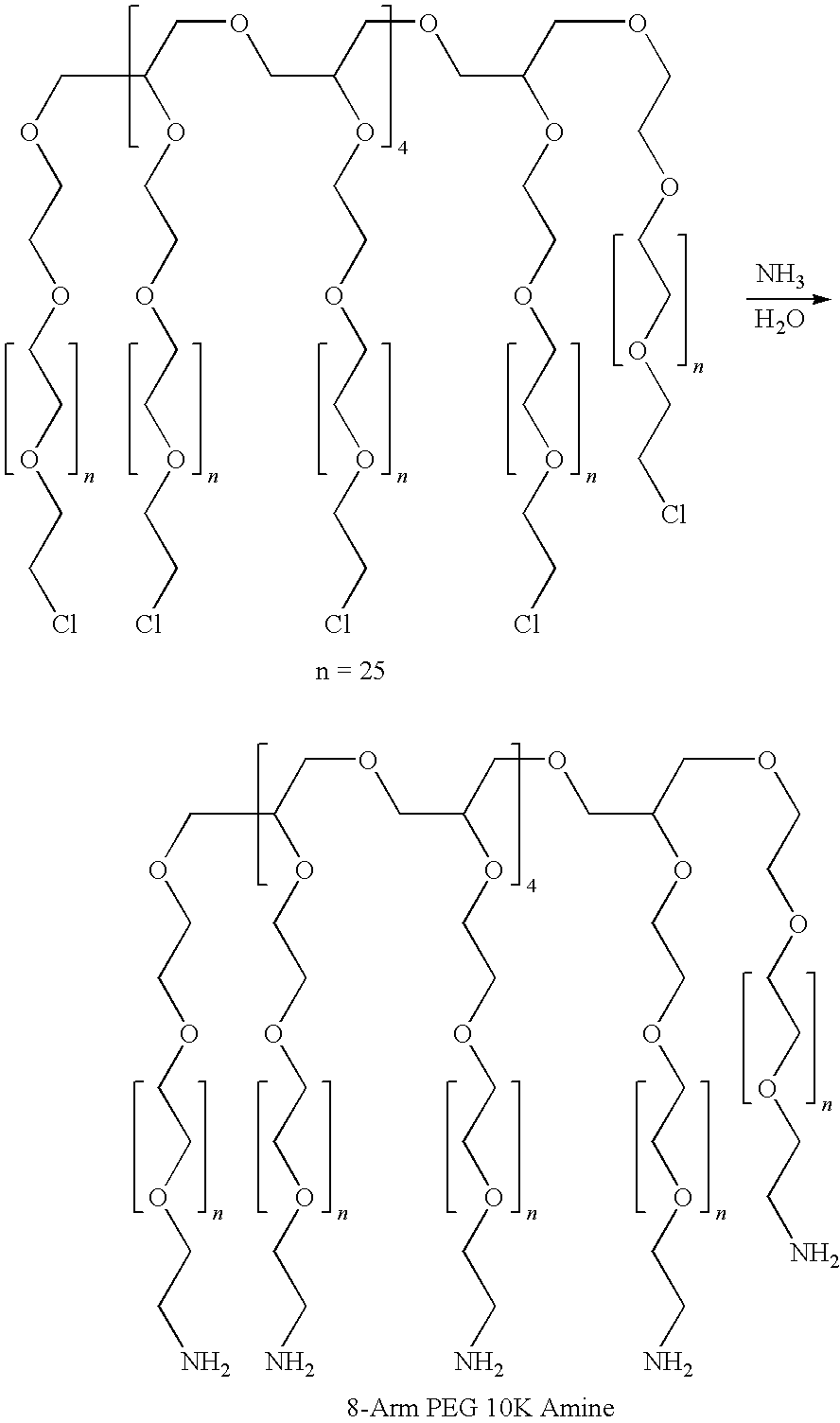
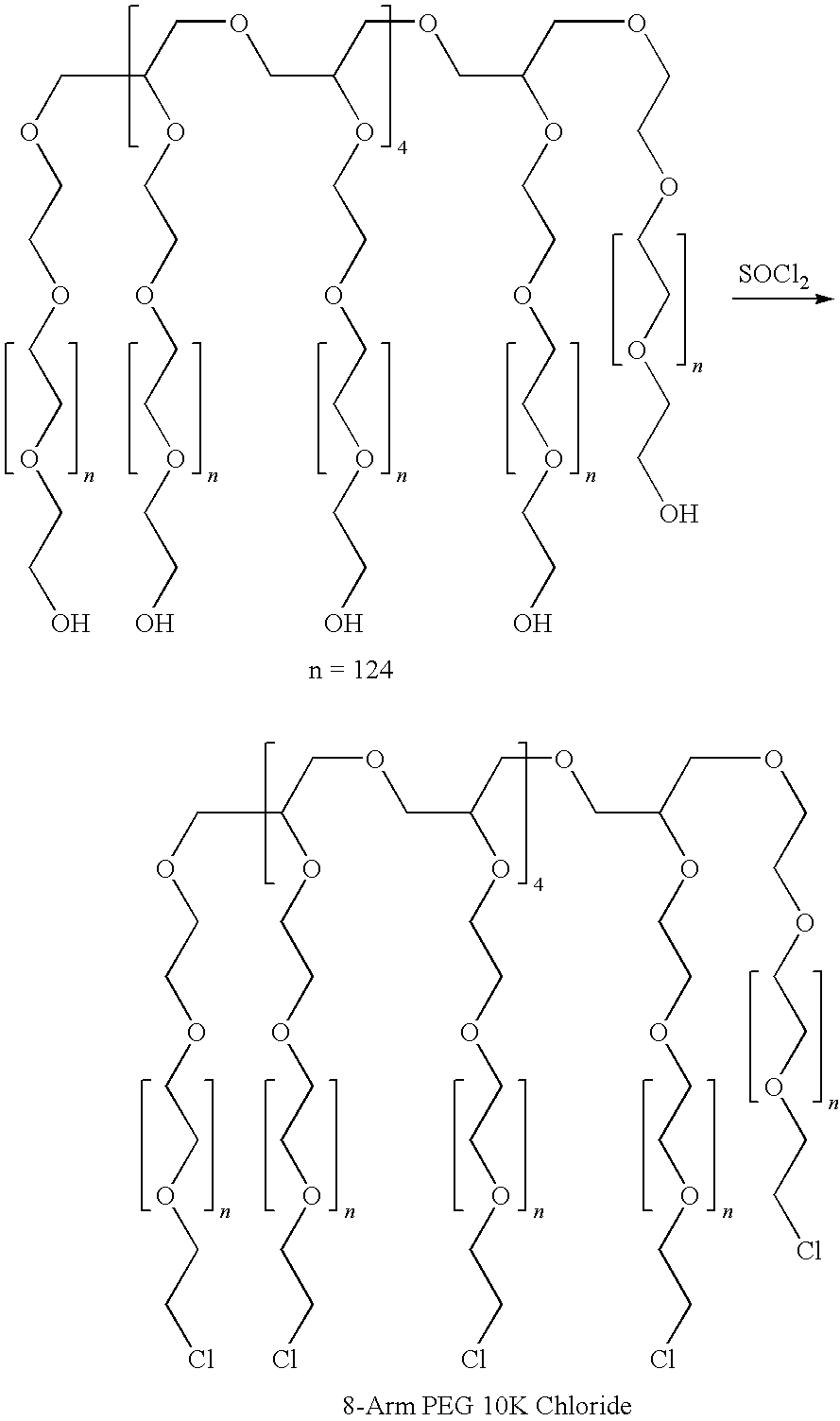
A high swell, long-lived hydrogel sealant formed by reacting a highly oxidized polysaccharide containing aldehyde groups with a multi-arm amine is described. The hydrogel sealant may be particularly suitable for applications requiring high swell and slow degradation, for example, tissue augmentation, both cosmetic and reconstructive; void filling; tissue bulking, for example treatment of urinary incontinence and acid reflux; and embolization. The high swell, long-lived hydrogel sealant may also be useful as a tissue sealant and adhesive, and as an anti-adhesion barrier.
Owner:ACTAMAX SURGICAL MATERIALS
Compositions and methods of using a transient colorant
This invention relates generally to compositions and systems for forming biomaterials containing a transient colorant for visualizing tissue or surgical materials coated with such biomaterials, to methods of using such compositions as bioadhesives, for tissue augmentation, in the prevention of surgical adhesions, for coating surfaces of synthetic implants, as drug delivery matrices, for ophthalmic applications, and in other applications.
Owner:SURGICAL SPECIALTIES CORP LTD
Active tissue augmentation materials and method
InactiveUS7326172B2Avoid absorptionPrevent circulatory insufficiencyAnti-incontinence devicesSurgical needlesMedicineInjected material
Active tissue augmenting agents, compositions and methods for use are disclosed. In a typical embodiment, the active augmenting agents of the invention can be used to form an artificial sphincter around a lumen of a human or animal body. In one embodiment, the active augmenting agent comprises magnitizable particles which can provide occlusion of a lumen, such as the urethral lumen, by circumferential attraction of the injected material toward the center of the lumen by the inherent magnetic flux field created from the magnetic dipoles of the magnetic particles.
Owner:TORAX MEDICAL
Tissue augmentation material and method
InactiveUS20060173551A1Reduce deliveryAnti-incontinence devicesPharmaceutical delivery mechanismUnilateral vocal cord paralysisPolysaccharide
A permanent, biocompatible material for soft tissue augmentation. The biocompatible material comprises a matrix of smooth, round, finely divided, substantially spherical particles of a biocompatible ceramic material, close to or in contact with each other, which provide a scaffold or lattice for autogenous, three dimensional, randomly oriented, non-scar soft tissue growth at the augmentation site. The augmentation material can be homogeneously suspended in a biocompatible, resorbable lubricious gel carrier comprising a polysaccharide. This serves to improve the delivery of the augmentation material by injection to the tissue site where augmentation is desired. The augmentation material is especially suitable for urethral sphincter augmentation, for treatment of incontinence, for filling soft tissue voids, for creating soft tissue blebs, for the treatment of unilateral vocal cord paralysis, and for mammary implants. It can be injected intradermally, subcutaneously or can be implanted.
Owner:MERZ AESTHETICS
Tissue augmentation, stabilization and regeneration Technique
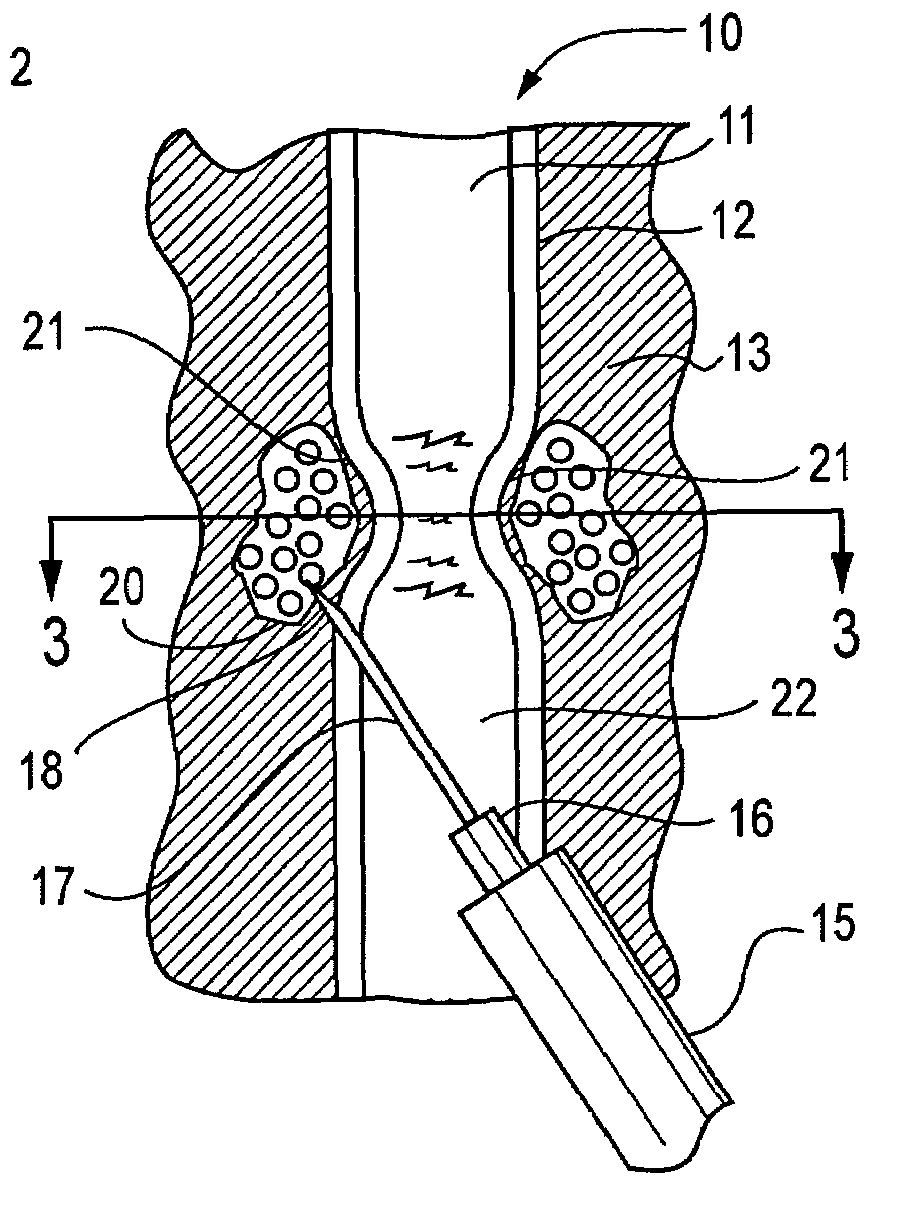
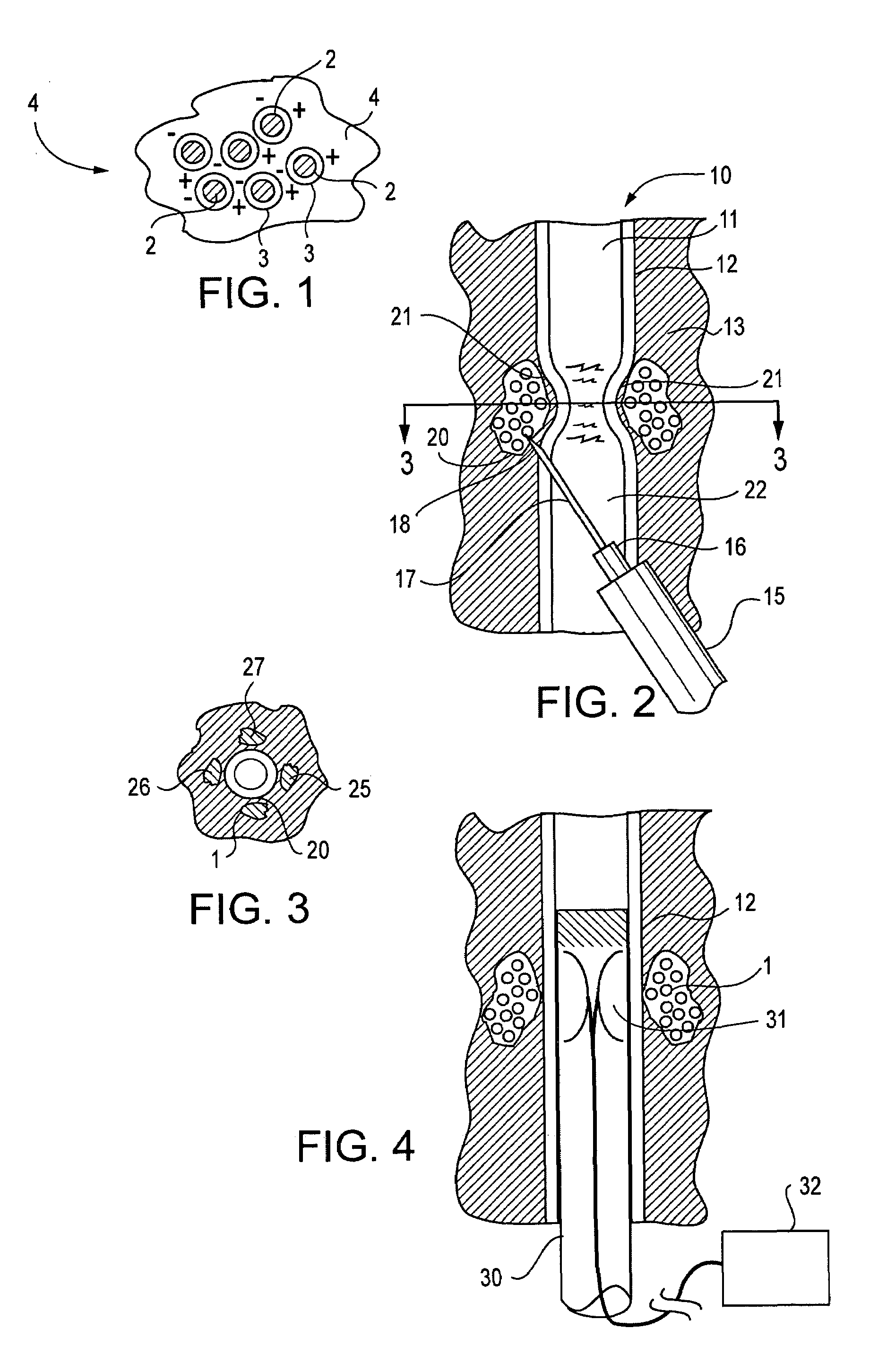
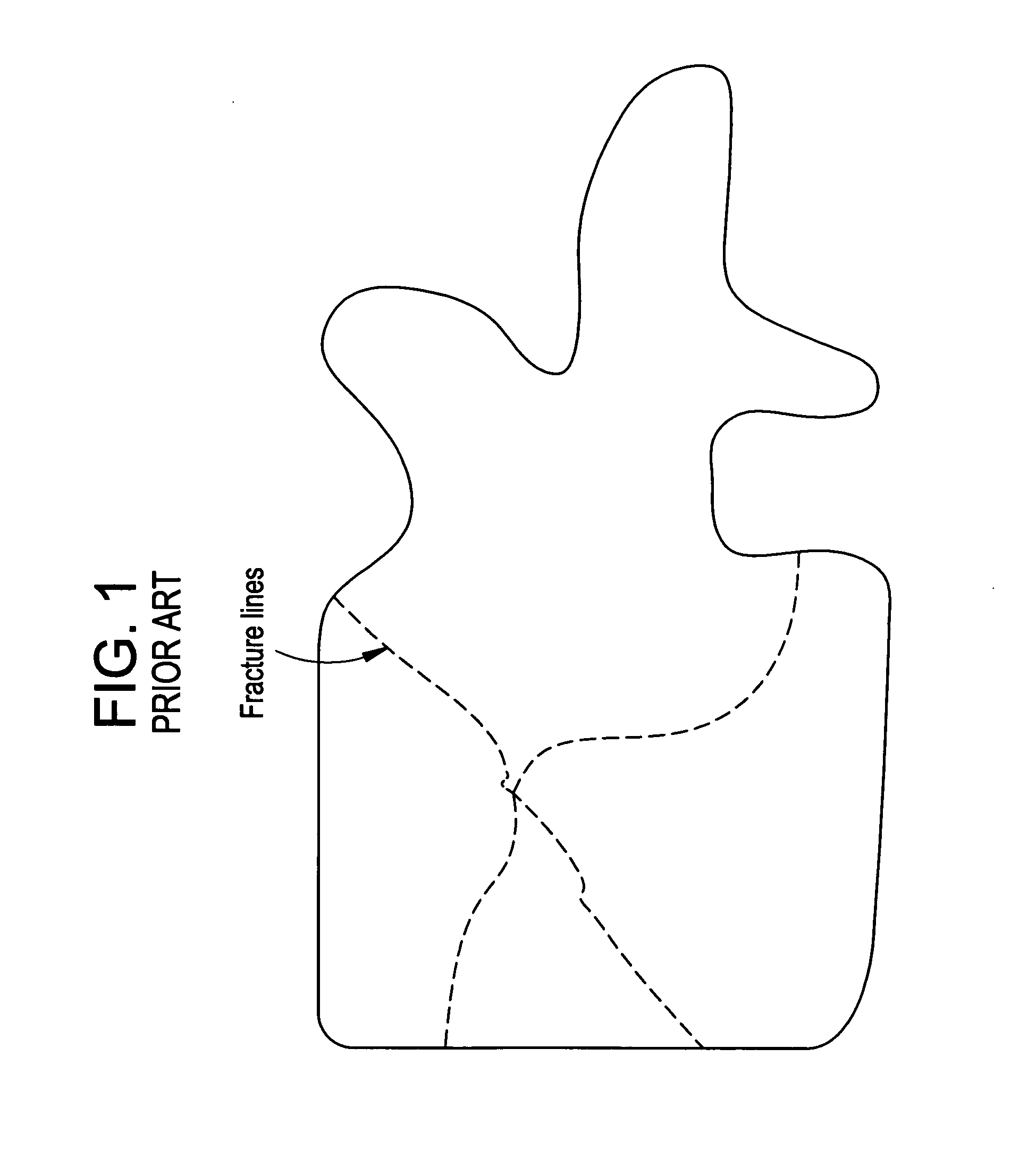
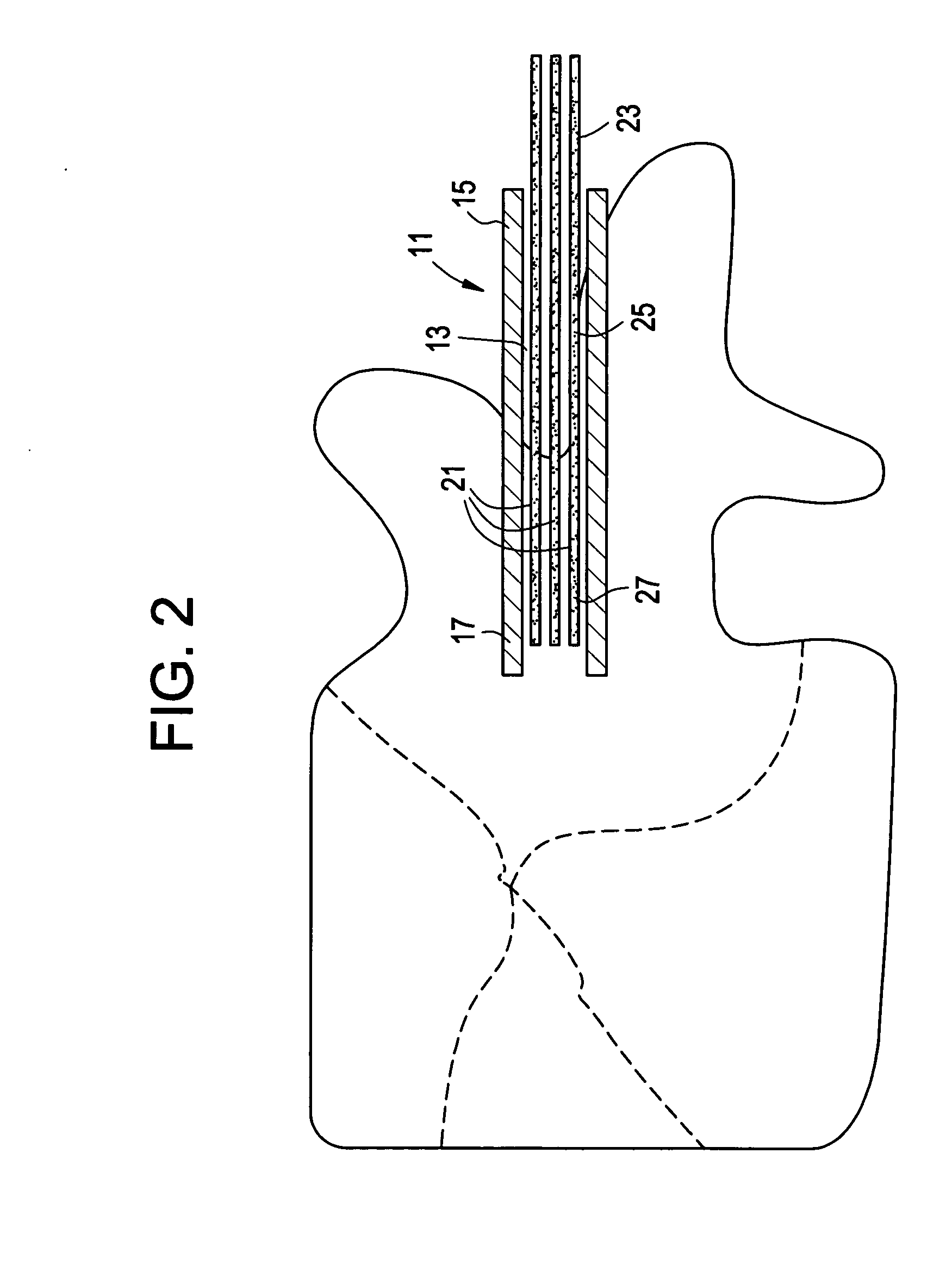
InactiveUS20070083205A1Promote new bone growthStabilizing the fracture siteInternal osteosythesisBone implantStable fractureFracture plane
A method of treating a fractured vertebral body by using a) a plurality of reinforcement rods combined with the b) a bone growth agent, wherein the reinforcement rods act to mechanically join disparate bone fragments across the fracture planes, thereby stabilizing the fracture site, and the bone growth agent promotes the growth of new bone across the fracture planes, thereby permanently replacing the fracture site with new bone.
Owner:DEPUY SPINE INC (US)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com