Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Dermal Fillers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
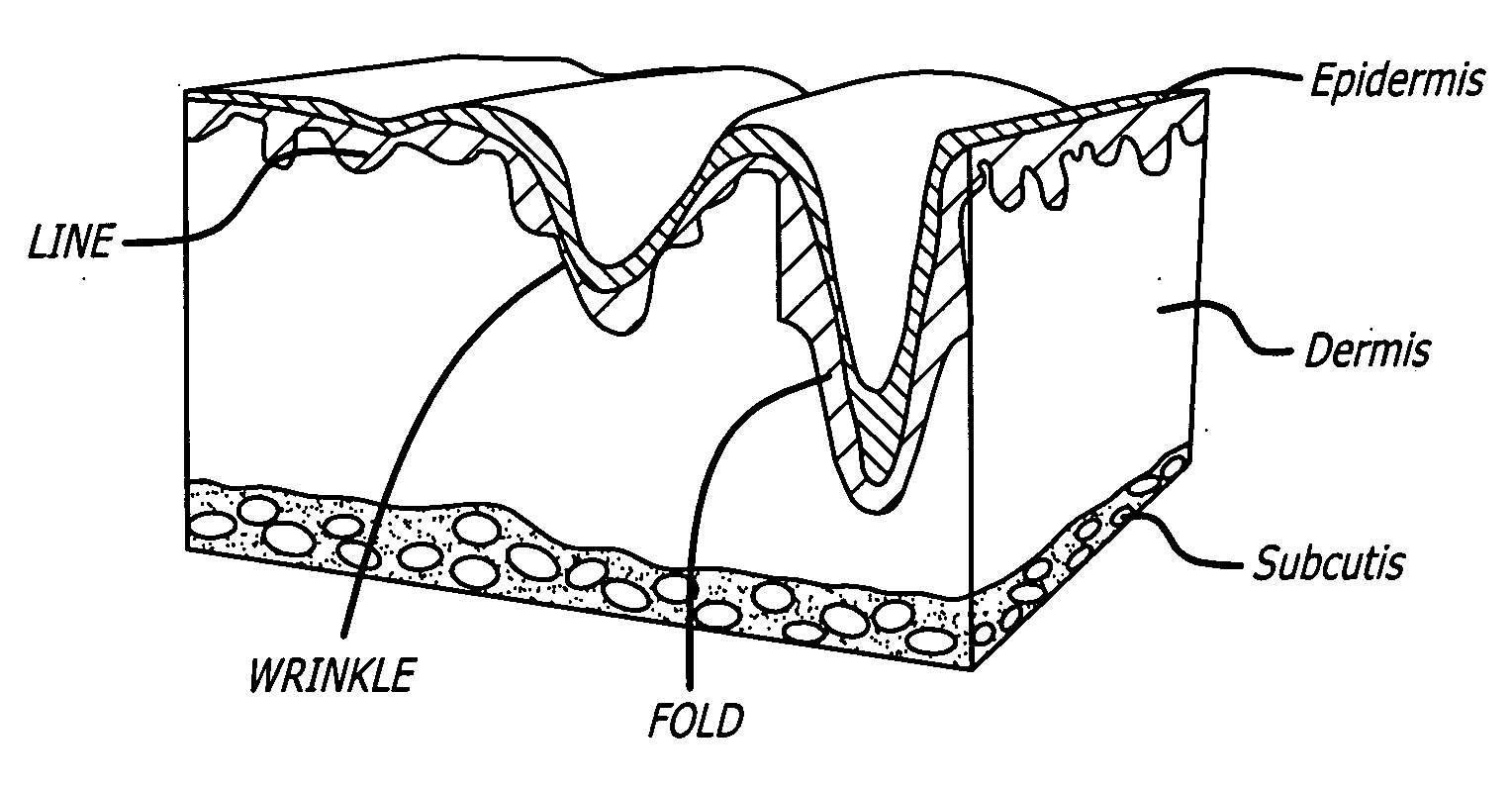
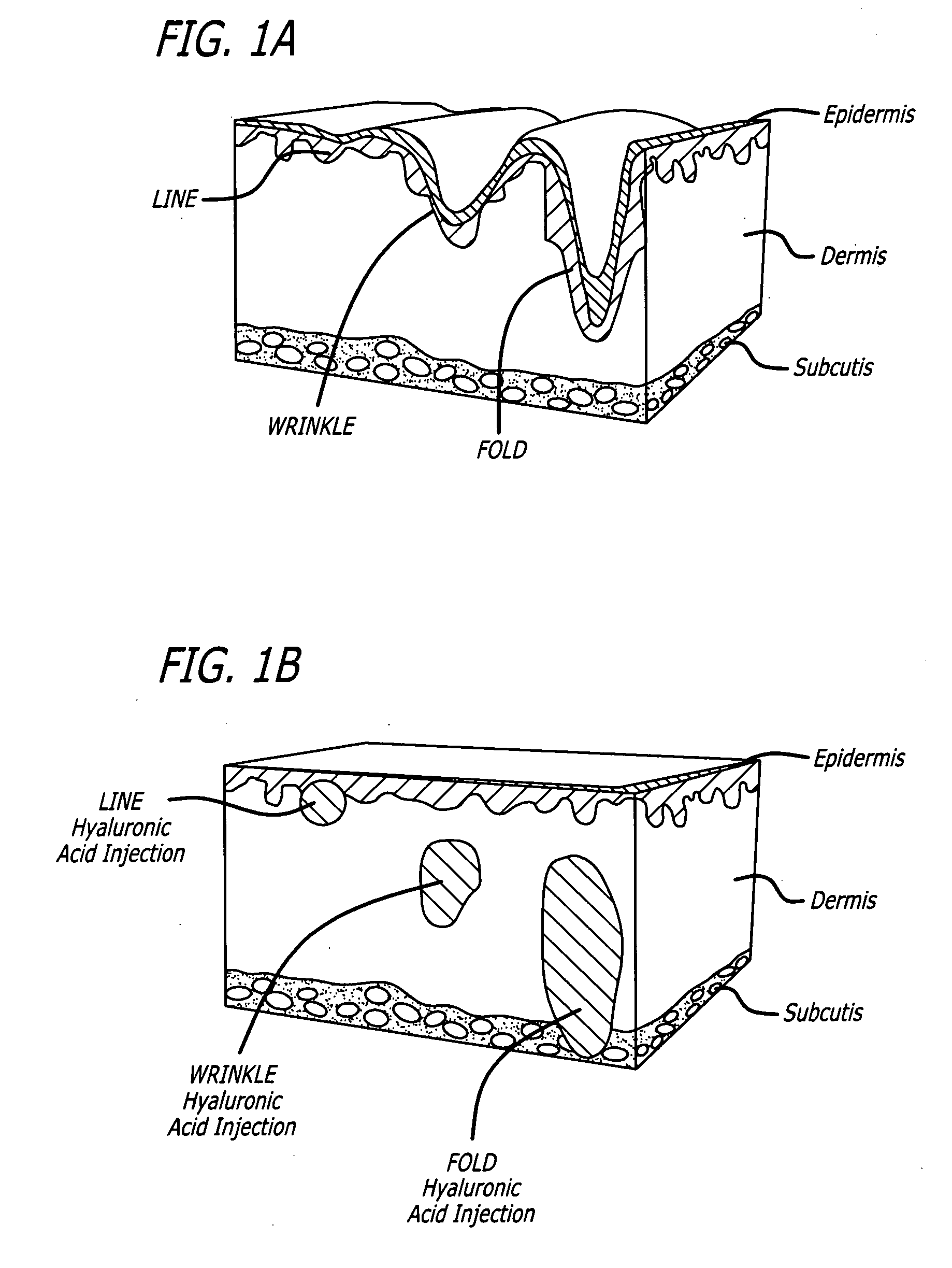
Materials such as COLLAGEN or HYALURONIC ACID that are injected or deposited into the DERMIS for the purpose of skin augmentation.
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20070212385A1Quality improvementExtending and improving qualityBiocideCosmetic preparationsBiologyTissue augmentation
Owner:KYTHERA BIOPHARMLS INC
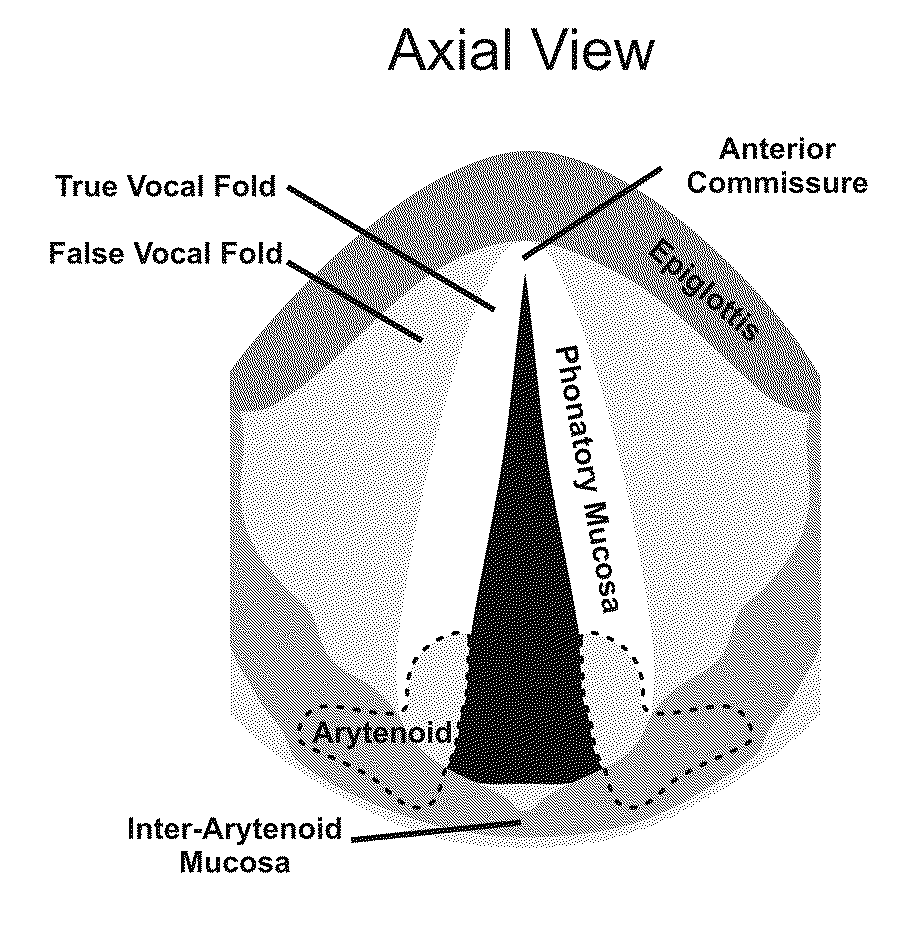
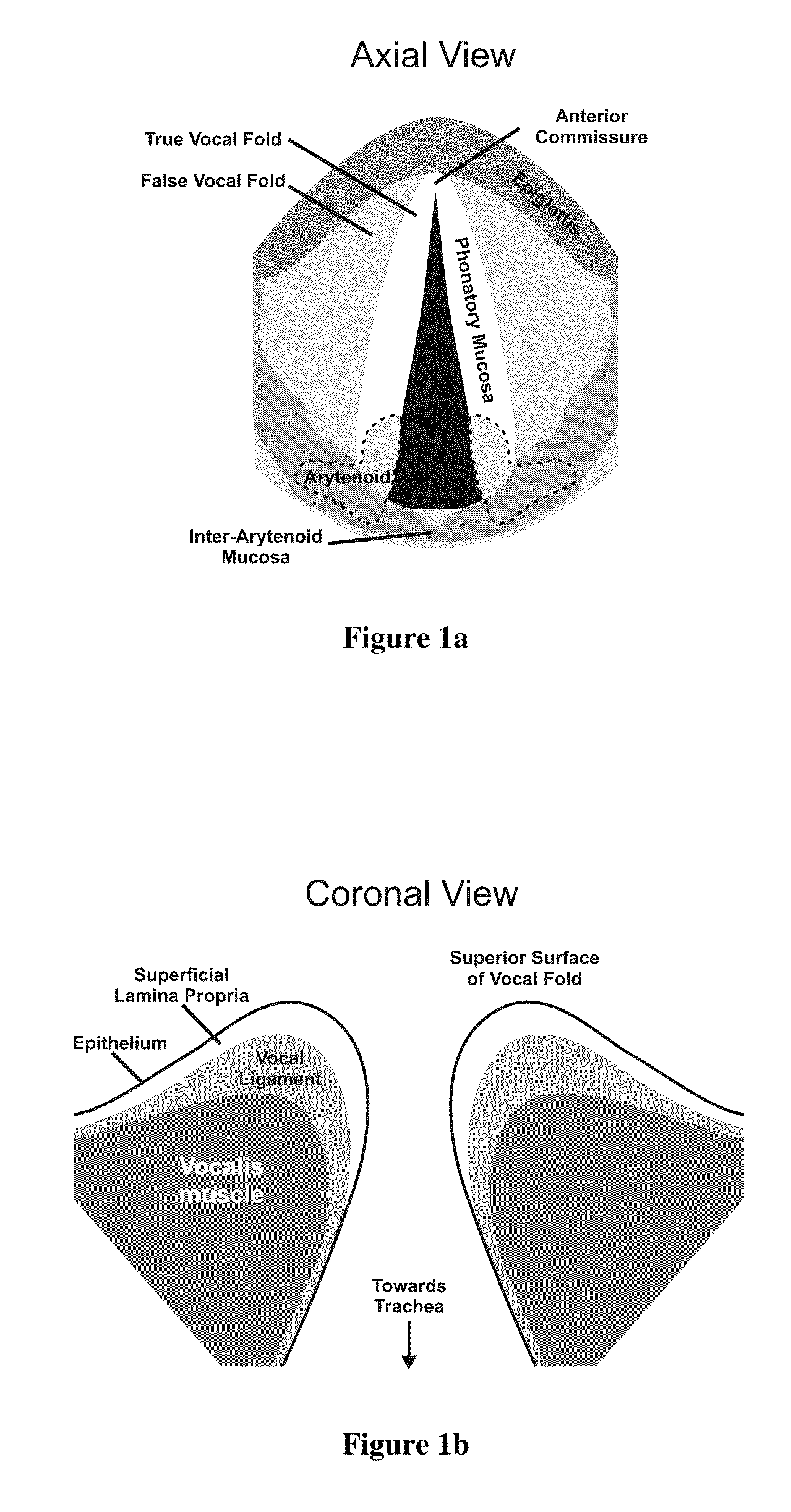
Hydrogels for vocal cord and soft tissue augmentation and repair
ActiveUS20100055184A1Repairing pliabilityDiminished functional vibratory capacityBiocideOrganic active ingredientsEpitheliumBreast implant
The present invention provides hydrogels and compositions thereof for vocal cord repair or augmentation, as well as other soft tissue repair or augmentation (e.g., bladder neck augmentation, dermal fillers, breast implants, intervertebral disks, muscle-mass). The hydrogels or compositions thereof are injected into the superficial lamina propria or phonatory epithelium to restore the phonatory mucosa of the vocal cords, thereby restoring a patient's voice. In particular, it has been discovered that hydrogels with an elastic shear modulus of approximately 25 Pa are useful in restoring the pliability of the phonatory mucosa. The invention also provides methods of preparing and using the inventive hydrogels.
Owner:MASSACHUSETTS INST OF TECH
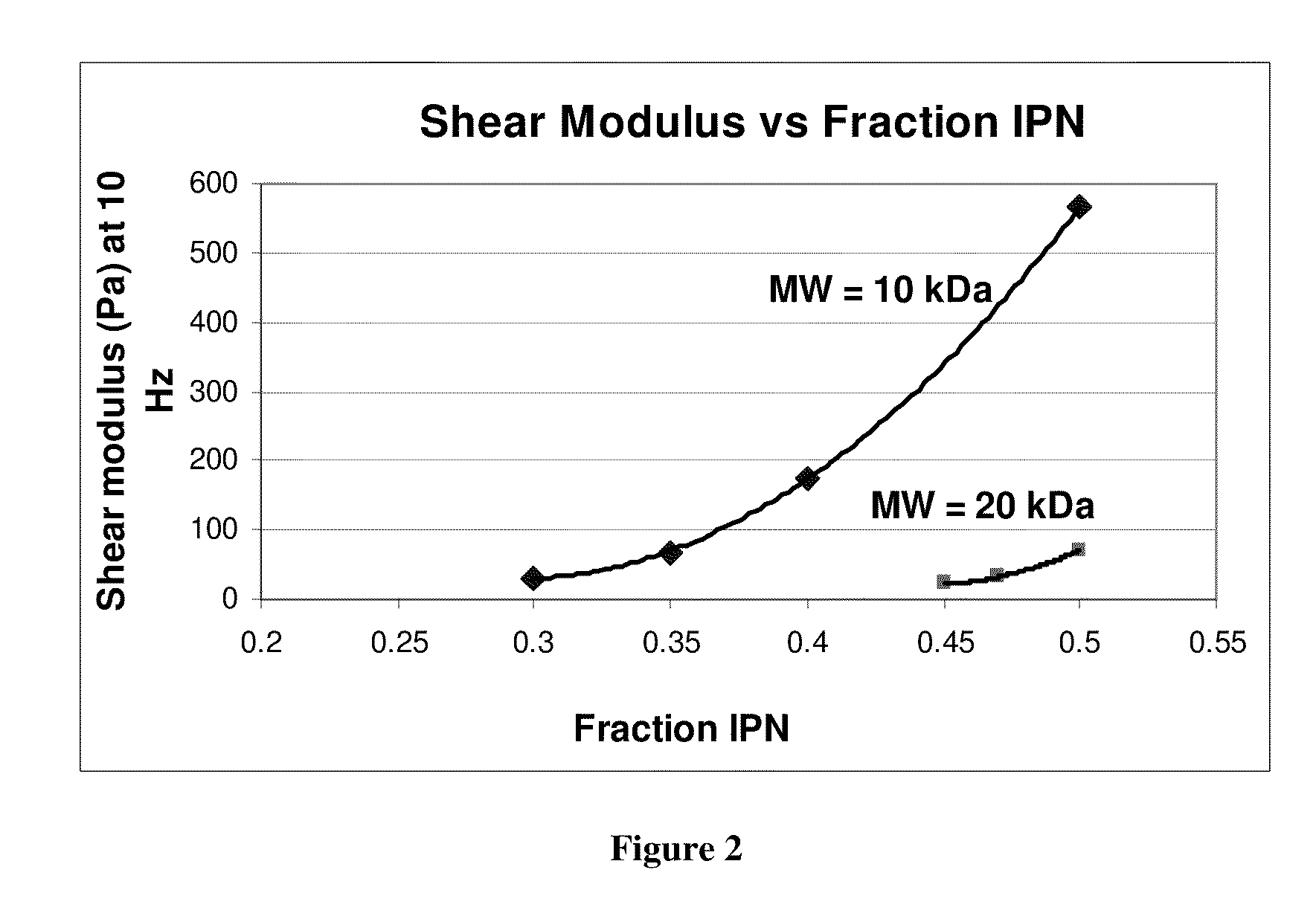
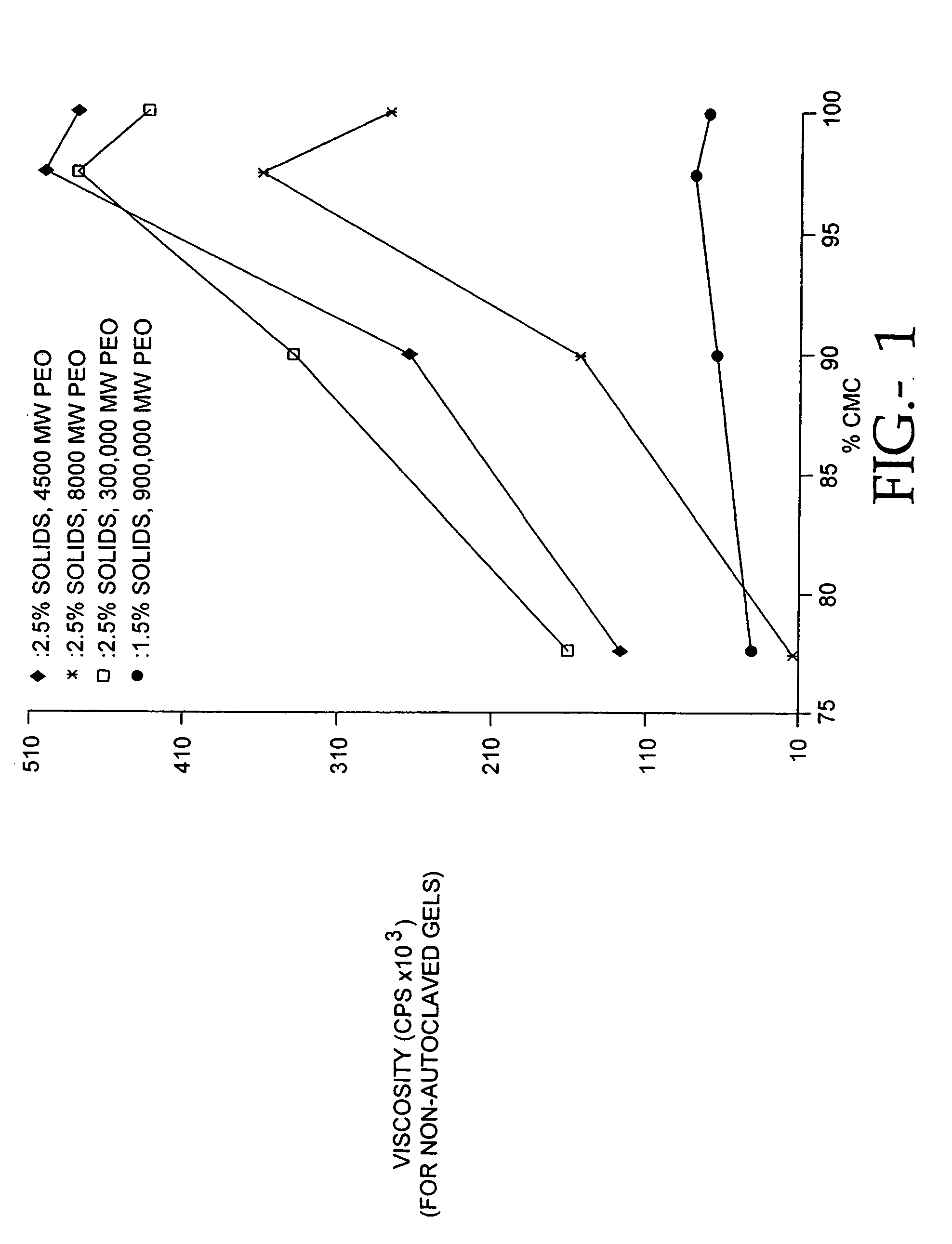
Compositions of polyacids and polyethers and methods for their use as dermal fillers
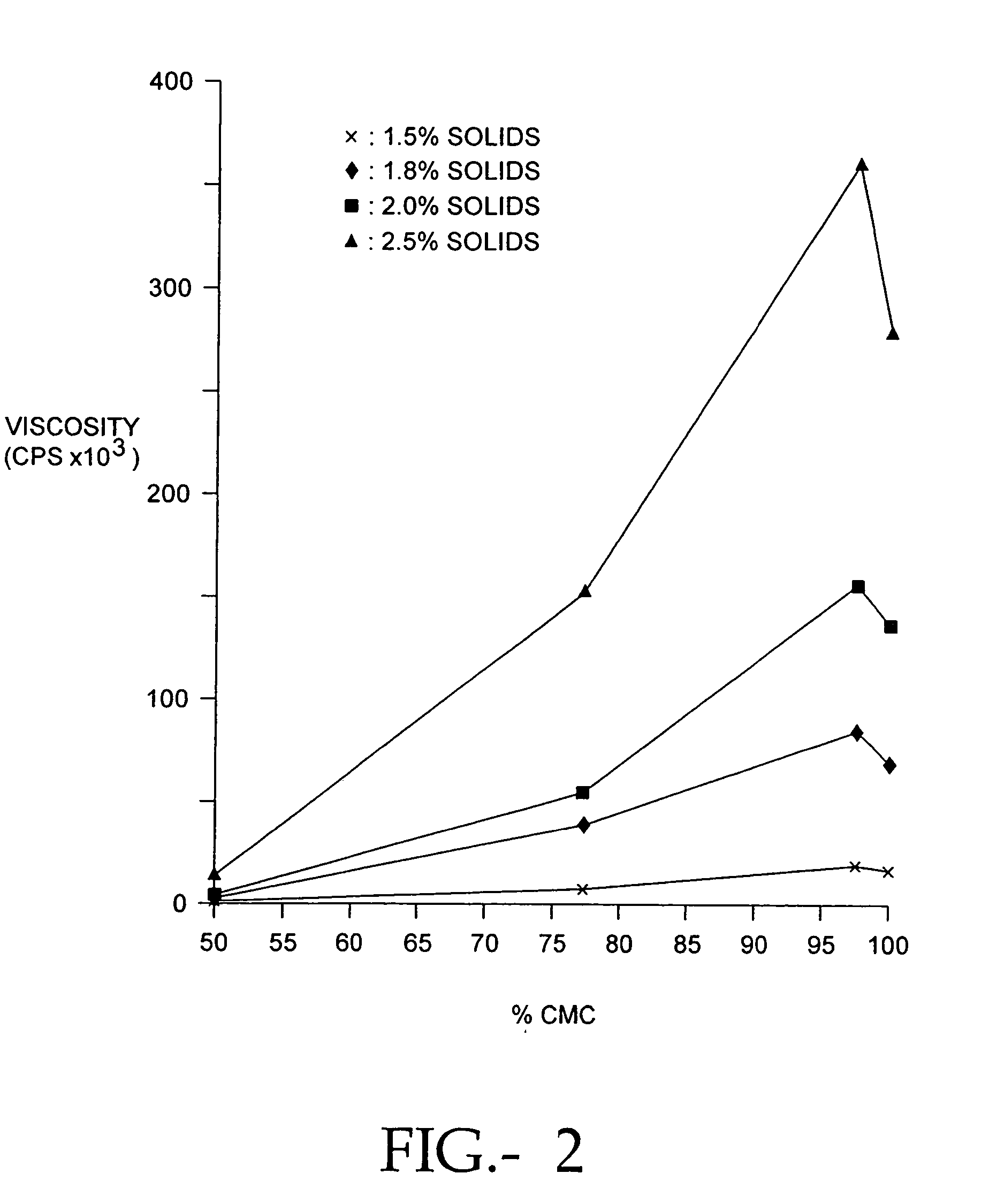
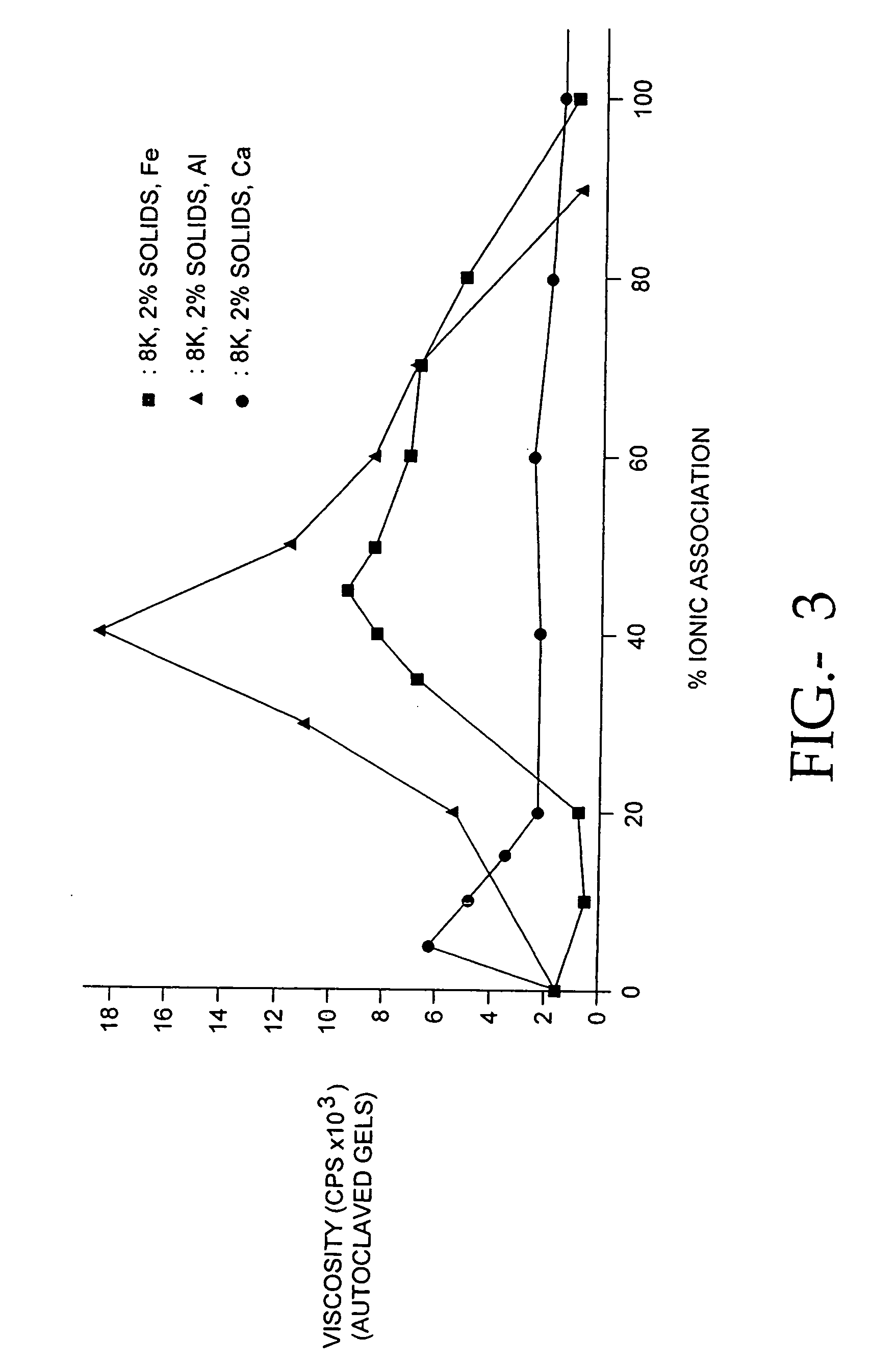
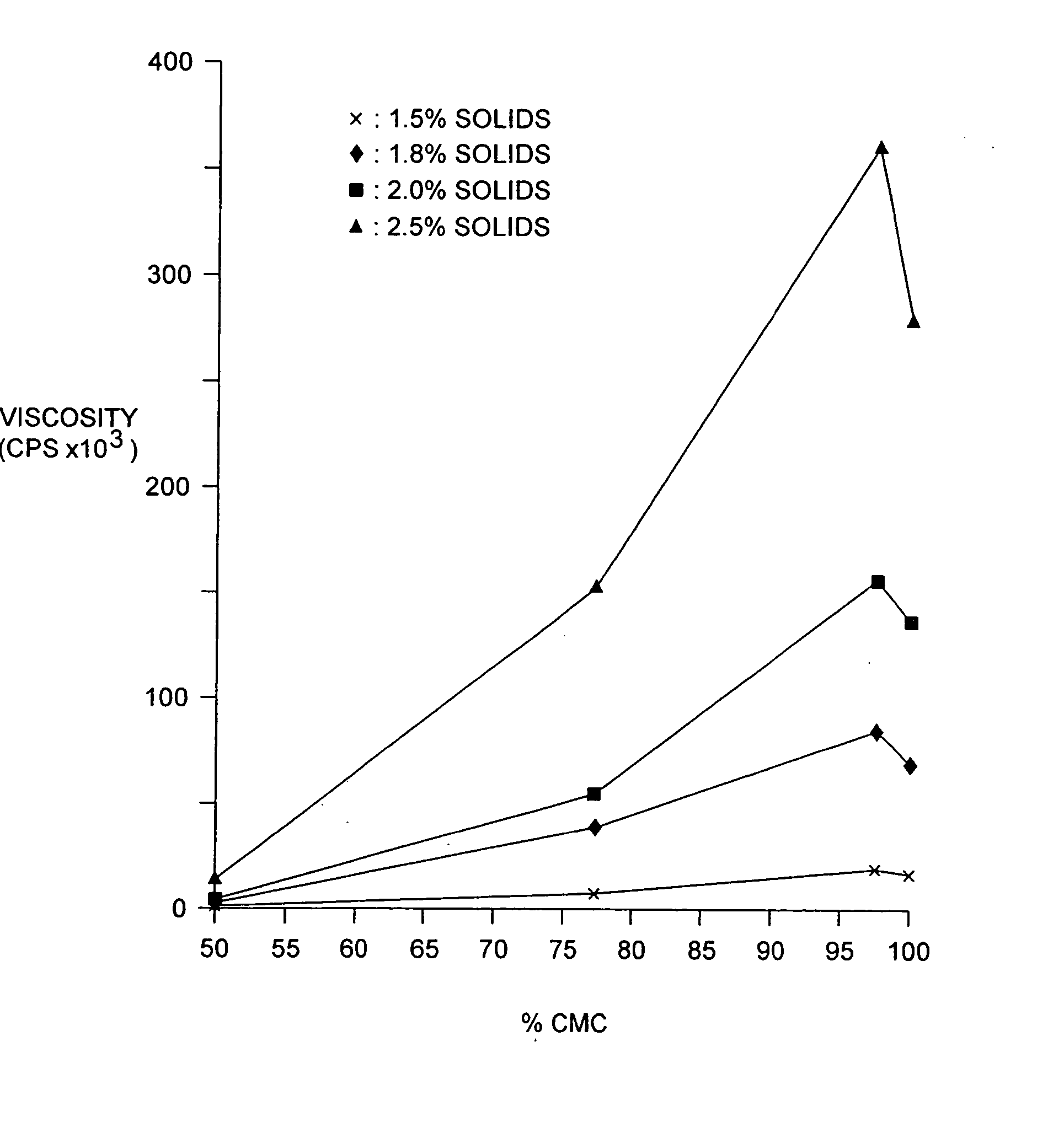
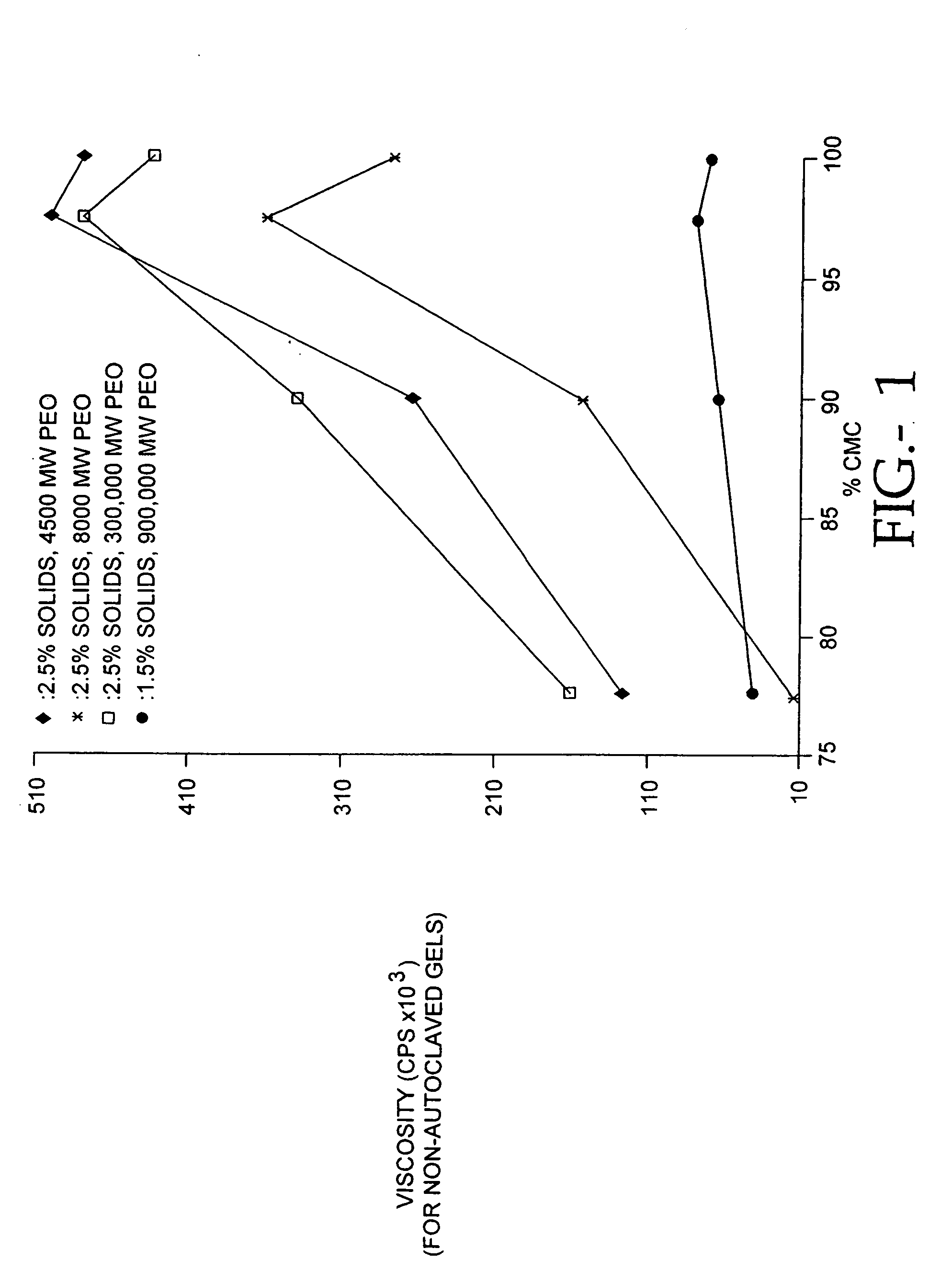
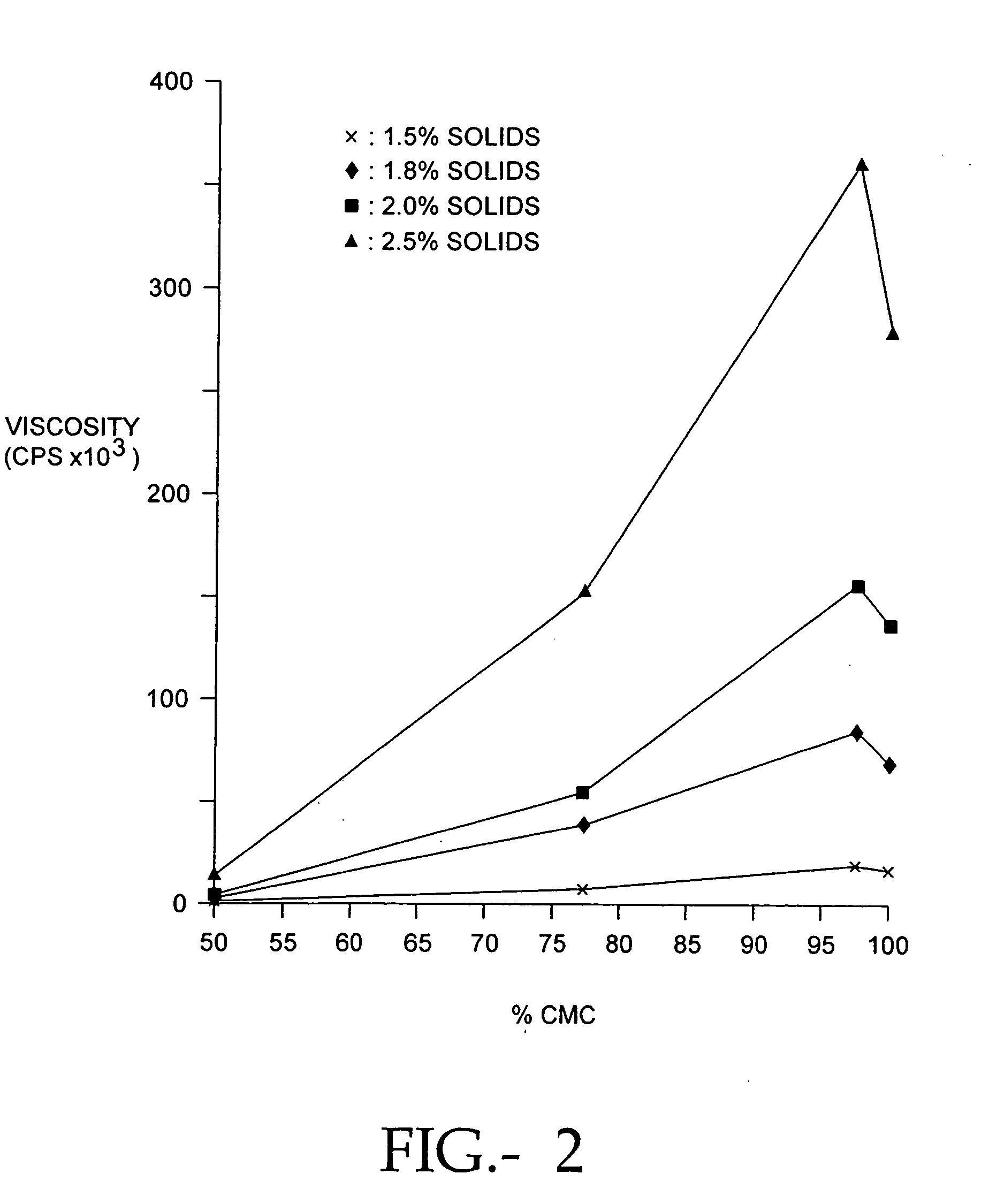
The present invention relates to improved methods for filling the skin for cosmetic or medical purposes. Compositions comprising carboxymethyl cellulose (CMC), polyethylene oxide (PEO) and calcium ions can be made and have physical properties that depend on the amounts and types of CMC, PEO, and calcium ions to form ioniclaly cross-linked gels. Compositions can be formed into microspheres, coascervates, gels, or membranes. Gels, microspheres and coascervates can be injected directly into a site for dermal filling. Membranes can be surgically introduced, where they swell to form hydrated gels. After introduction, the dermal filler persists for a period of time and then can disintegrate and be removed from the body.
Owner:FZIOMED
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20090110736A1Reduce wrinklesReduce scarsPowder deliveryCosmetic preparationsFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Coated Hyaluronic Acid Particles
The present invention generally relates to particles comprising hyaluronic acid, wherein the particles are coated or encapsulated with a coating. The coating preferably comprises a polymer, protein, polysaccharide, or combination thereof that decreases the rate of degradation of the hyaluronic acid once the particles are placed in an aqueous environment, such as inside mammalian skin. The compositions of the present invention comprising such coated hyaluronic acid are useful for soft tissue augmentation, and are particularly useful as dermal fillers.
Owner:ALLERGAN INC
Tunable crosslinked polysaccharide compositions
InactiveUS20120071437A1Eliminate symptomsImprove the situationBiocideOrganic active ingredientsPolyethylene glycolPolysaccharide
The present specification generally relates to injectable dermal fillers including multifunctional polyethylene glycol-based crosslinking agents, hydrogel compositions comprising a matrix polymer crosslinked with such crosslinking agents, and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN INC
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20080038306A1Extending and improving qualityImprove sturdinessCosmetic preparationsBiocideTissue augmentationDermal Fillers
Owner:KYTHERA BIOPHARMLS INC +1
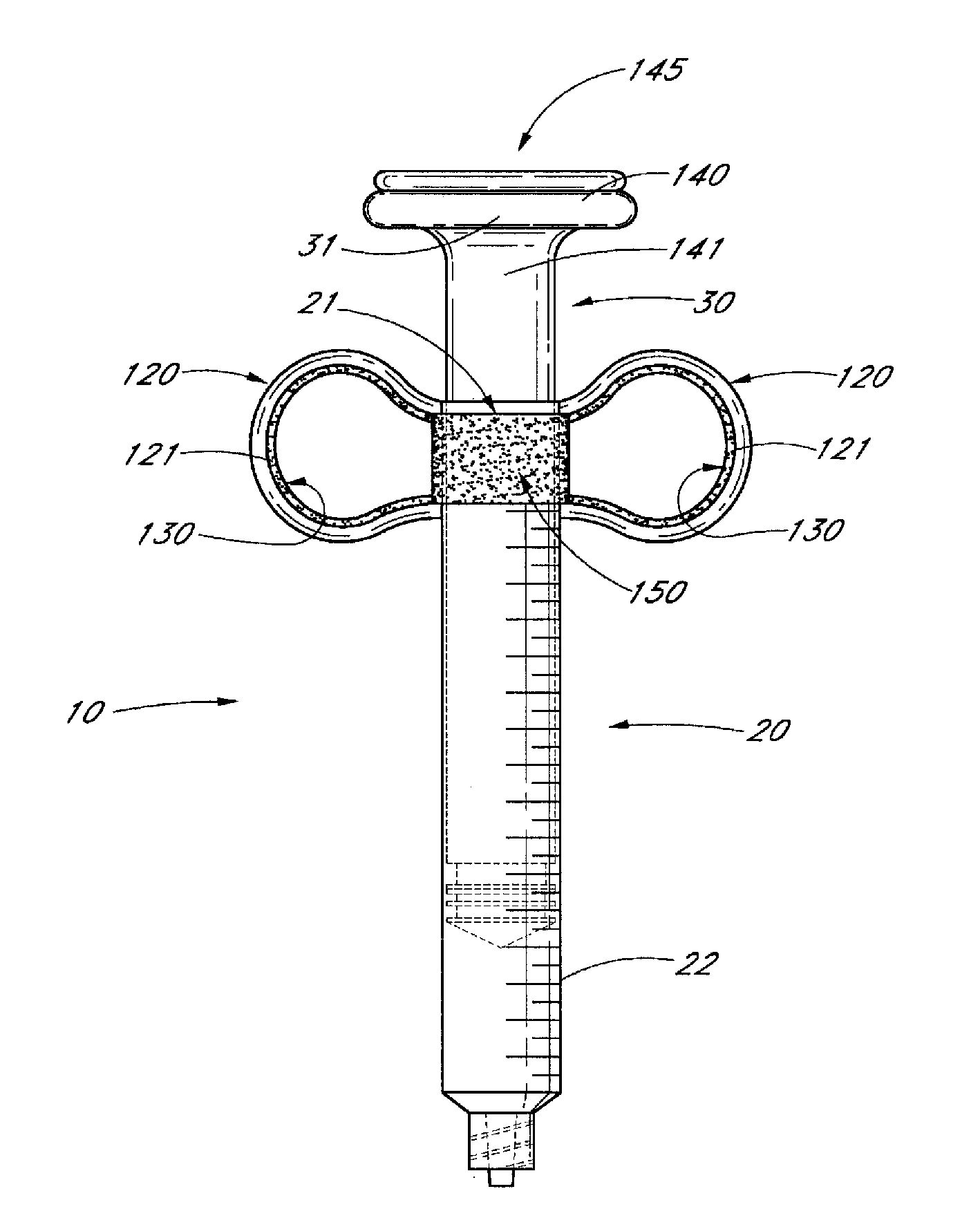
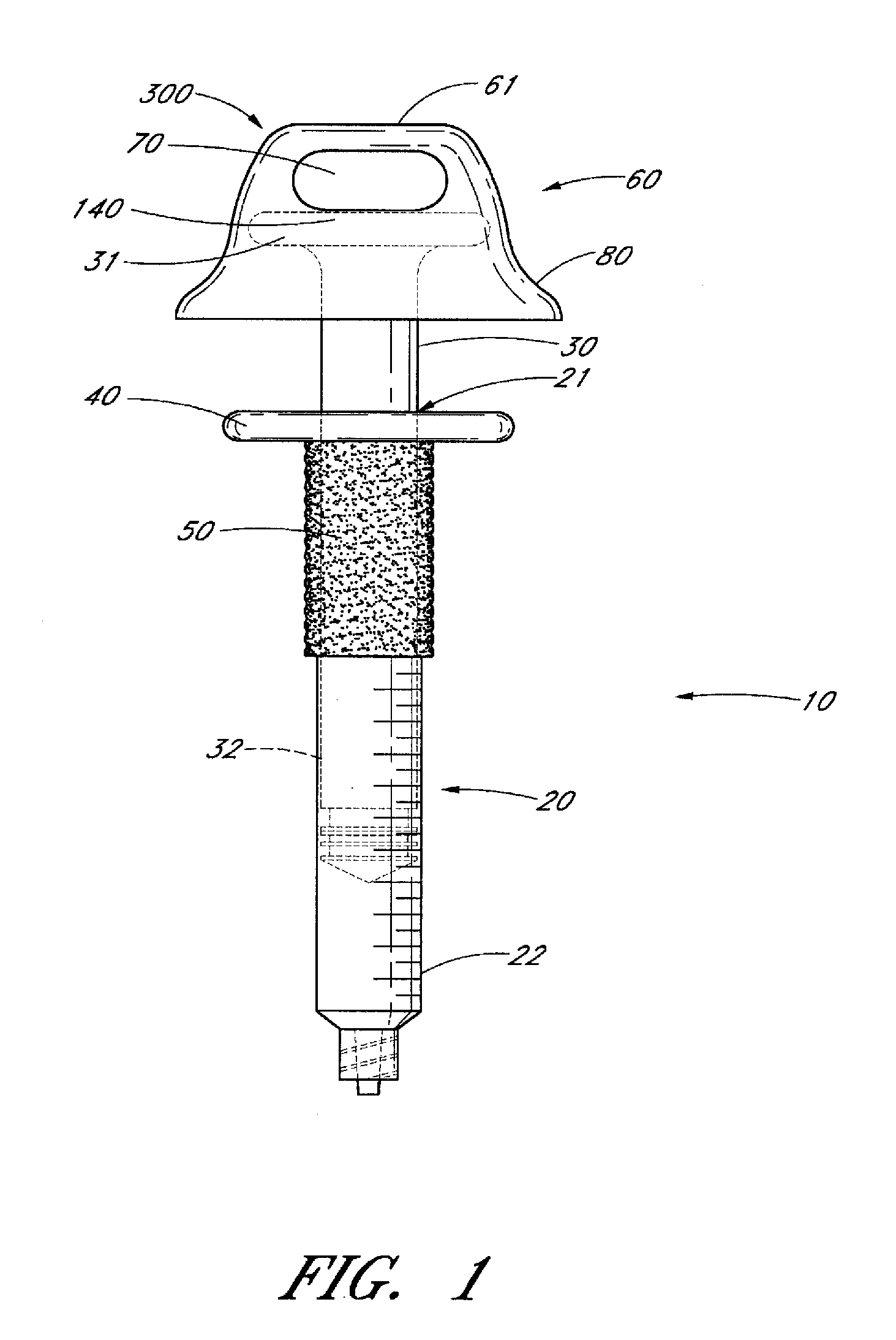
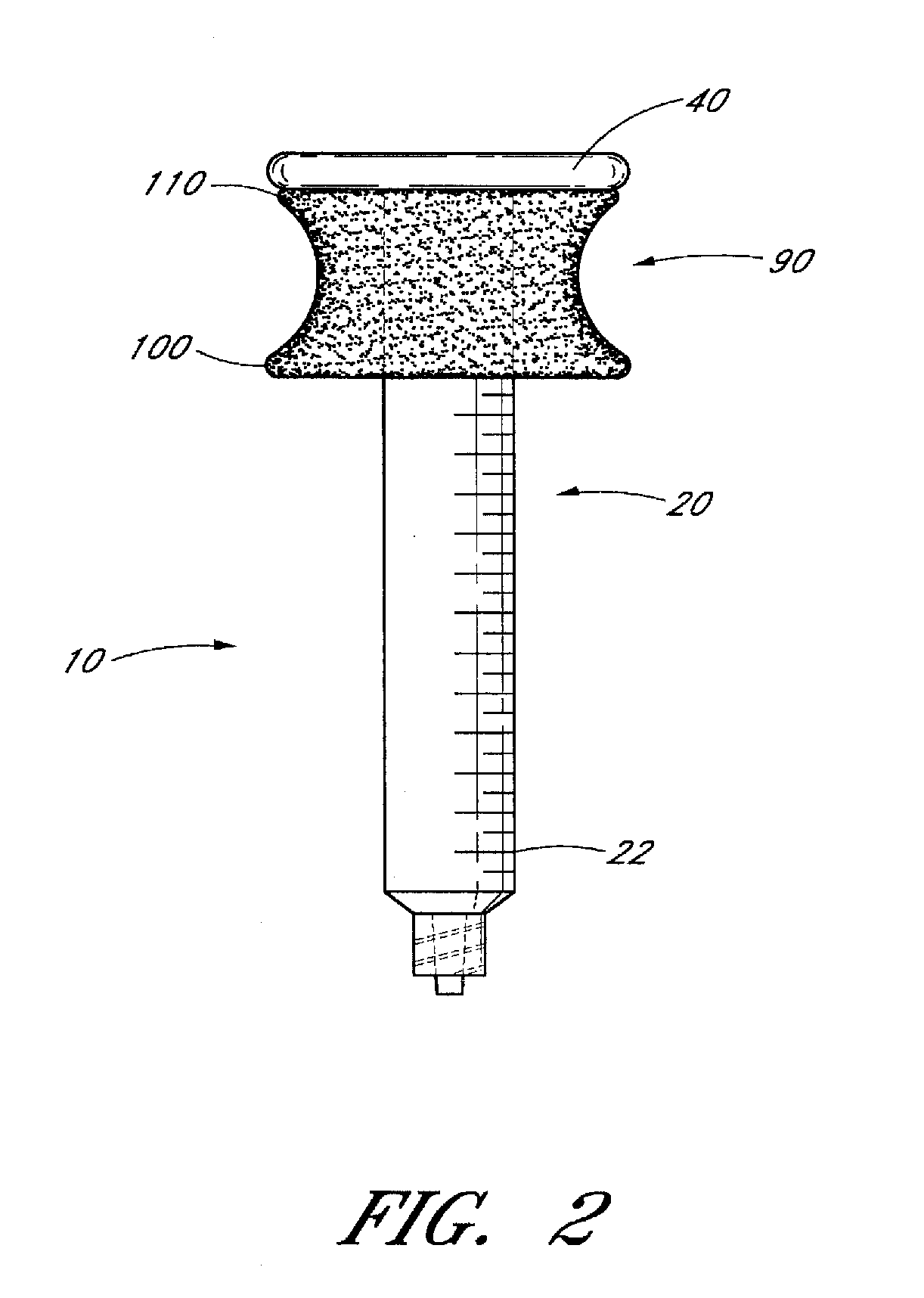
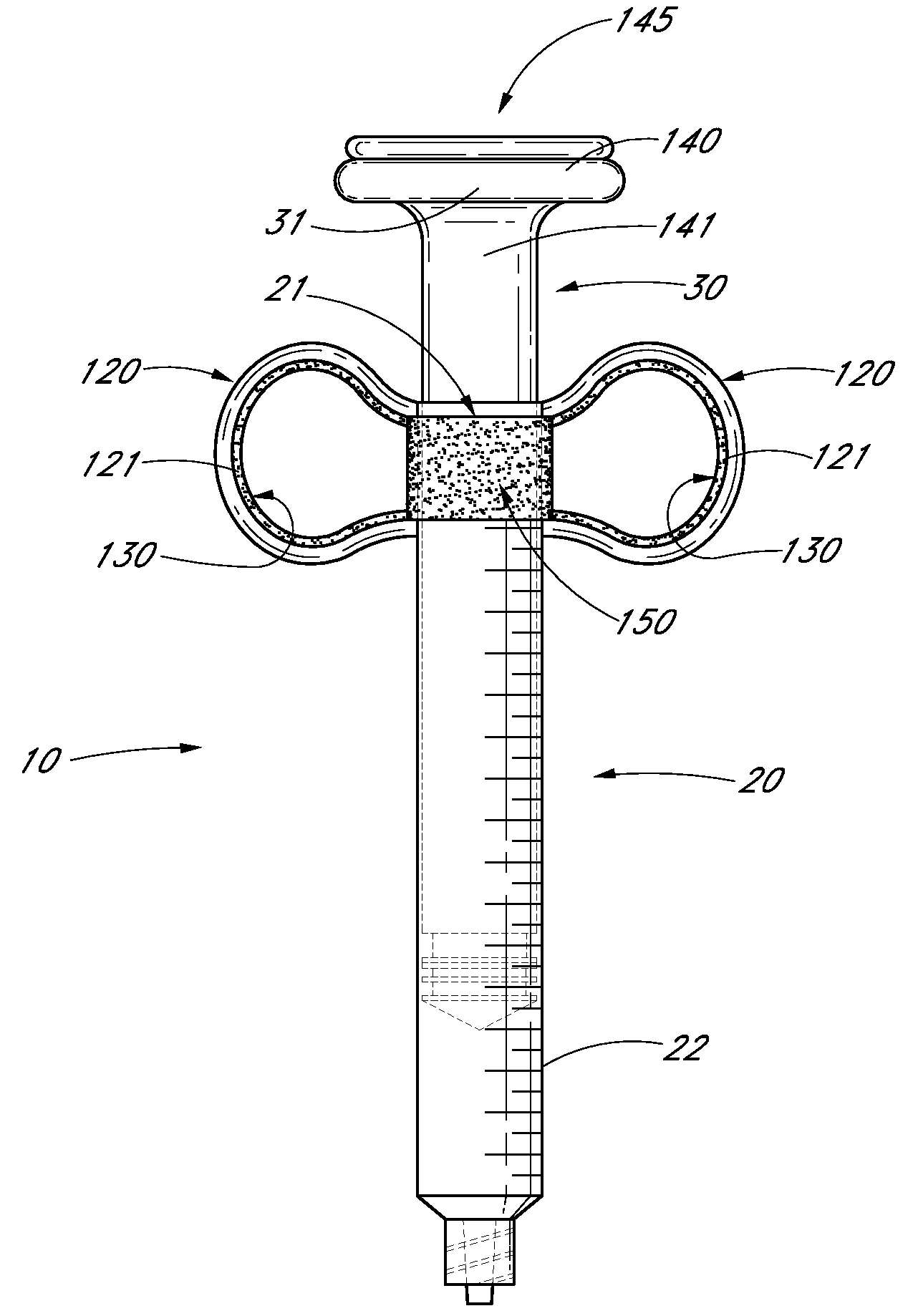
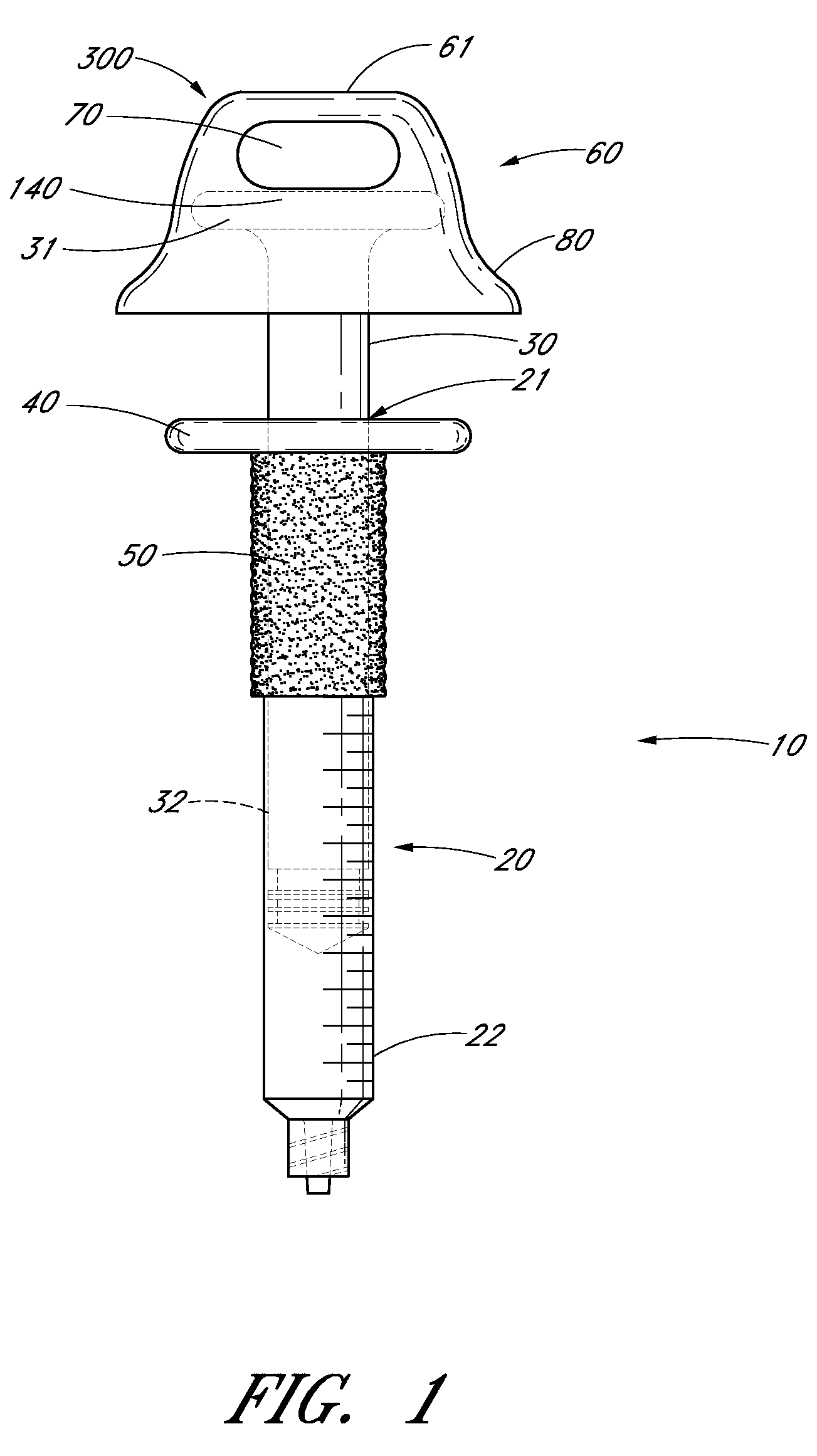
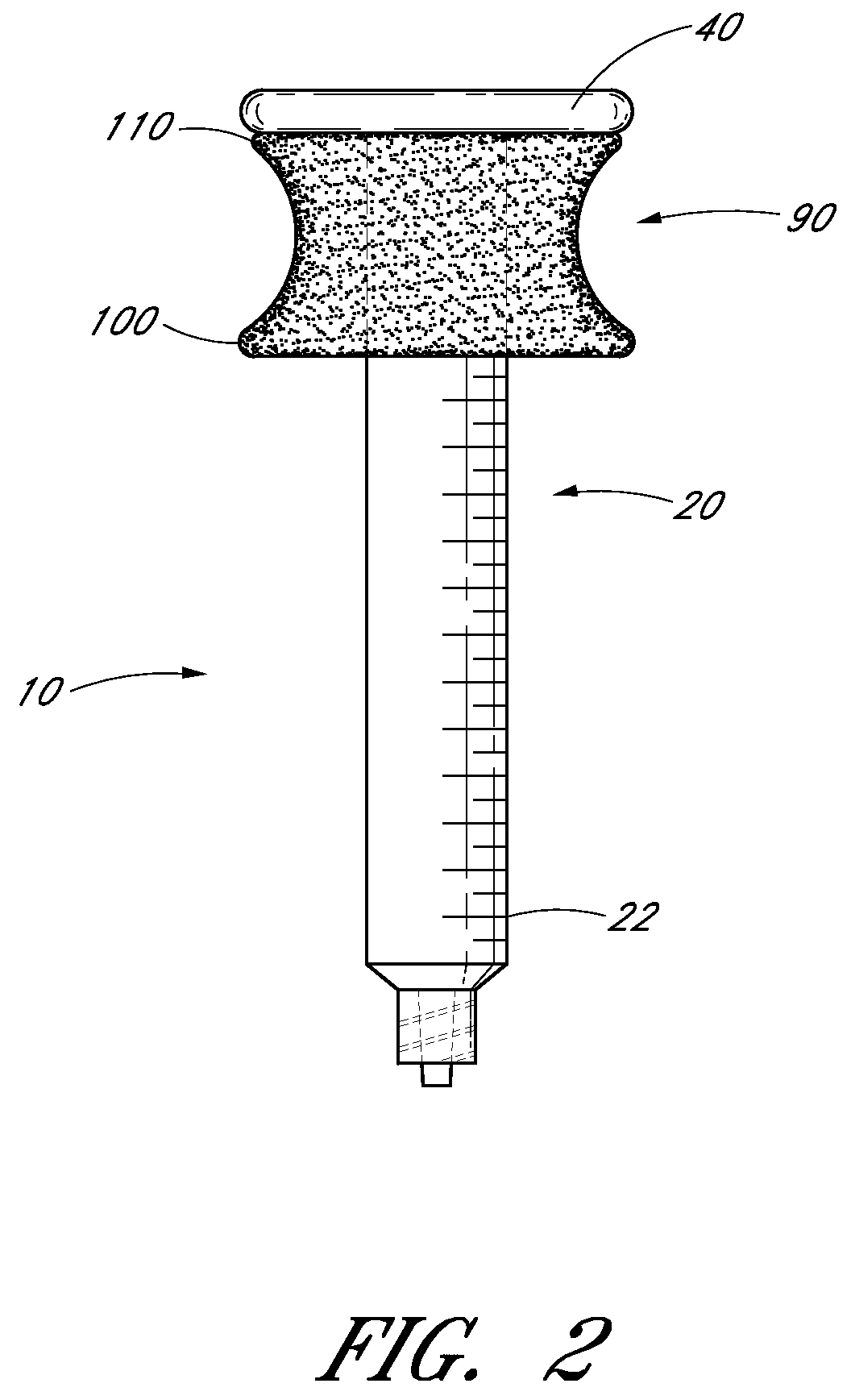
Ergonomic syringe
InactiveUS20120220948A1Reduce riskConvenient and accurate and comfortableInfusion syringesMedical devicesBiomedical engineeringLow volume
The present embodiments relate to devices and methods for injection. More specifically, the devices and methods provide an ergonomic aspect. In some embodiments, the devices and methods are especially useful in the administration of low volumes of dermal fillers to a subject.
Owner:IPSYRNG CAPITAL DEV
Ergonomic syringe
InactiveUS20090093787A1Reduce riskConvenient and accurate and comfortableInfusion syringesMedical devicesBiomedical engineeringLow volume
The present embodiments relate to devices and methods for injection. More specifically, the devices and methods provide an ergonomic aspect. In some embodiments, the devices and methods are especially useful in the administration of low volumes of dermal fillers to a subject.
Owner:BARBOUR JENNIFER
Preparations Derived From Placental Materials and Methods of Making and Using Same
Preparations derived from placental materials and methods of making and using same, the preparations including a first preparation composed of placental membranes digested in collagenase, a second preparation composed of multipotent cells derived from the collagenase digested placental membranes and that are grown in adherent culture beyond confluence and a third preparation composed of ground placental membranes re-suspended in a fluid containing hyaluronic acid. The preparations can be used for regenerating damaged or defective tissue including connective tissue, nerve tissue, muscle tissue, skin tissue, cartilage tissue and bone tissue. The preparations can also be used as dermal fillers in cosmetic and plastic surgery applications.
Owner:NUTECH MEDICAL
Alloplastic injectable dermal filler and methods of use thereof
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent comprising particles of the unsubstituted acrylate / substituted acrylate copolymer with a diameter of less than about 100μ. Methods for making a composition comprising an alloplastic injectable suspension for use as a dermal filler are provided. Methods of augmenting soft tissue to provide long-term reduction of a skin defect and the stimulation of collagen production are also provided.
Owner:BOUTROS AYMAN
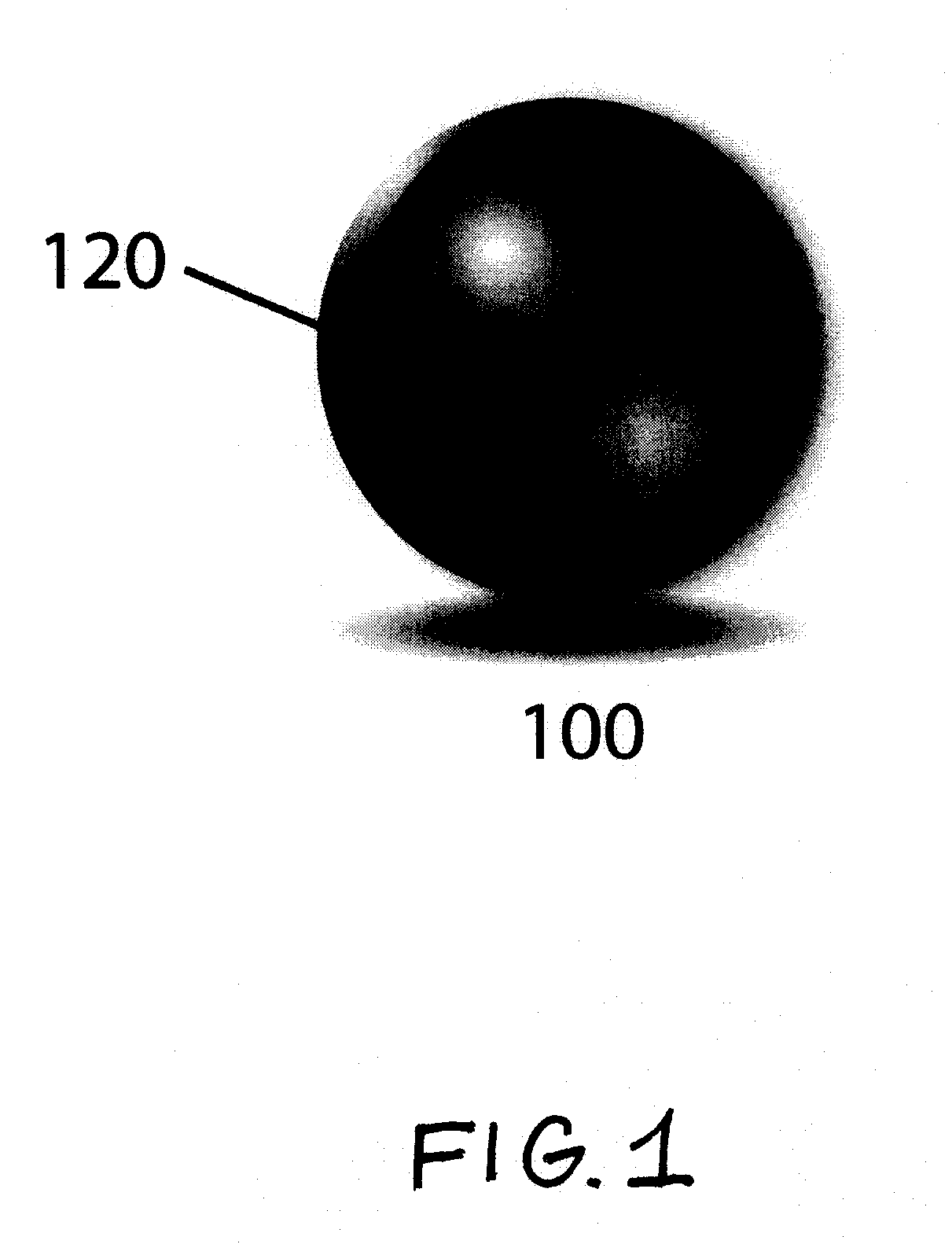
Color-Coded Polymeric Particles of Predetermined Size for Therapeutic and/or Diagnostic Applications and Related Methods
InactiveUS20100028260A1Minimizes probabilityPowder deliveryGenetic material ingredientsPolyphosphazeneMedicine
Various embodiments are directed to color-coded and size-calibrated polymeric particles comprising an acrylate-based hydrogel core incorporating one or more chromophores of interest, and an outer shell comprising polyphosphazenes of formula I, useful for various therapeutic and / or diagnostic procedures. In various embodiments, the color-coded and size-calibrated polymeric particles can be employed in any particle-mediated procedure, including as embolic agents, dermal fillers, and various implantable devices for a broad range of clinical and cosmetic applications. The incorporation of a particular chromophore formulation that correlates with a pre-determined size specificity for implantable and loadable polymeric particles (“color-coded and size-calibrated”) enables the visual detection and identification of particles exhibiting a particular size of interest, and minimizes the probability of user-introduced or procedural errors.
Owner:VARIAN MEDICAL SYSTEMS
Terminal sterilization of injectable collagen products
ActiveUS20060280769A1Lower Level RequirementsAvoid radiationBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineVolumetric Mass Density
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5kGy and 12kGy and more preferably between 6kGy and 8kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
Filler composition comprising beta-glucans
InactiveUS20130196944A1Significant positive effectPositive influenceOrganic active ingredientsCosmetic preparationsCross-linkMedicine
The present invention pertains to a filler composition comprising β-glucan moieties and optionally a cosmetically and / or pharmaceutically acceptable carrier. It further relates to a filler composition, wherein the β-glucan moieties are cross-linked. in one embodiment of the instant invention the filler composition is a dermal filler. In one further embodiment of the present invention the filler composition is for the treatment of wrinkles and / or folds. In another embodiment of the instant invention the filler composition is for use in the treatment of a medical condition. The filler composition provided in the present invention may further comprise one or more active pharmaceutical ingredients. Further, the present invention pertains to a process for preparing the filler composition as claimed herein.
Owner:MERZ PHARMA GMBH & CO KGAA
Polymer-flavonoid conjugates and hydrogels for biomedical applications
InactiveCN105658633AImprove bioavailabilityImprove biological activityOrganic active ingredientsCosmetic preparationsThiolViscosupplements
There is provided polymer-flavonoid conjugates. Flavonoid-grafted and flavonoid-terminated polymer conjugates are disclosed according to the invention. The linkage of flavonoids to the polymers has been achieved via thiol linkages. The inventive processes allow for making of the conjugates in high yield avoiding complex purification steps. The conjugates can be easily autoxidized to hydrogels with uses in many biomedical applications where a higher stability of the flavonoid is necessary. The hydrogels can be potentially used as viscosupplement, anti-adhesion film or dermal filler.
Owner:AGENCY FOR SCI TECH & RES
Compositions of polyacids and polyethers and methods for their use as dermal fillers
The present invention relates to improved methods for filling the skin for cosmetic or medical purposes. Compositions comprising carboxymethyl cellulose (CMC), polyethylene oxide (PEO) and calcium ions can be made and have physical properties that depend on the amounts and types of CMC, PEO, and calcium ions to form ioniclaly cross-linked gels. Compositions can be formed into microspheres, coascervates, gels, or membranes. Gels, microspheres and coascervates can be injected directly into a site for dermal filling. Membranes can be surgically introduced, where they swell to form hydrated gels. After introduction, the dermal filler persists for a period of time and then can disintegrate and be removed from the body.
Owner:FZIOMED
Cationic steroidal antimicrobial compositions for the treatment of dermal tissue
ActiveUS20170258963A1Reduce dirtReduce the risk of infectionAntibacterial agentsOrganic active ingredientsSkin treatmentsMedicine
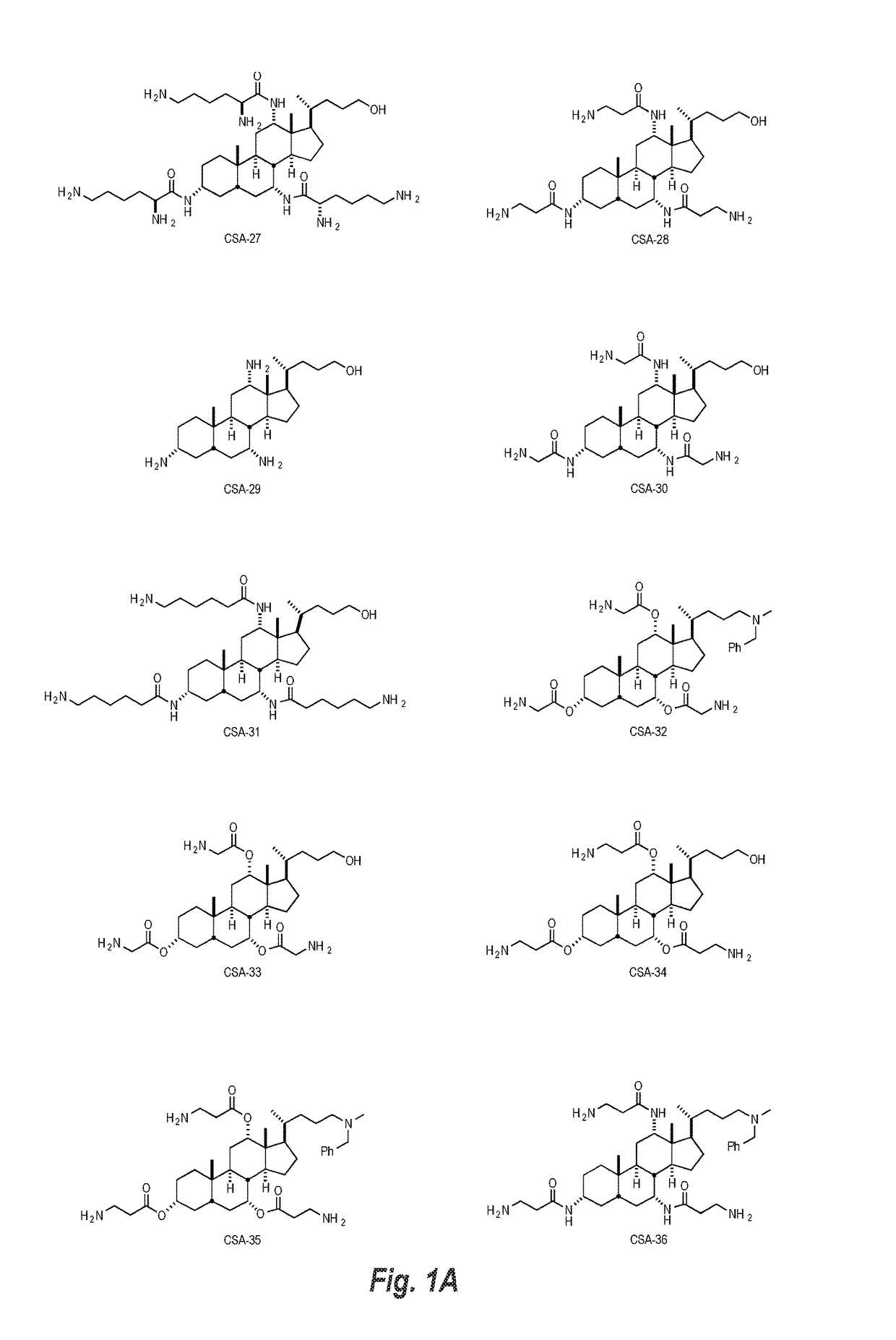
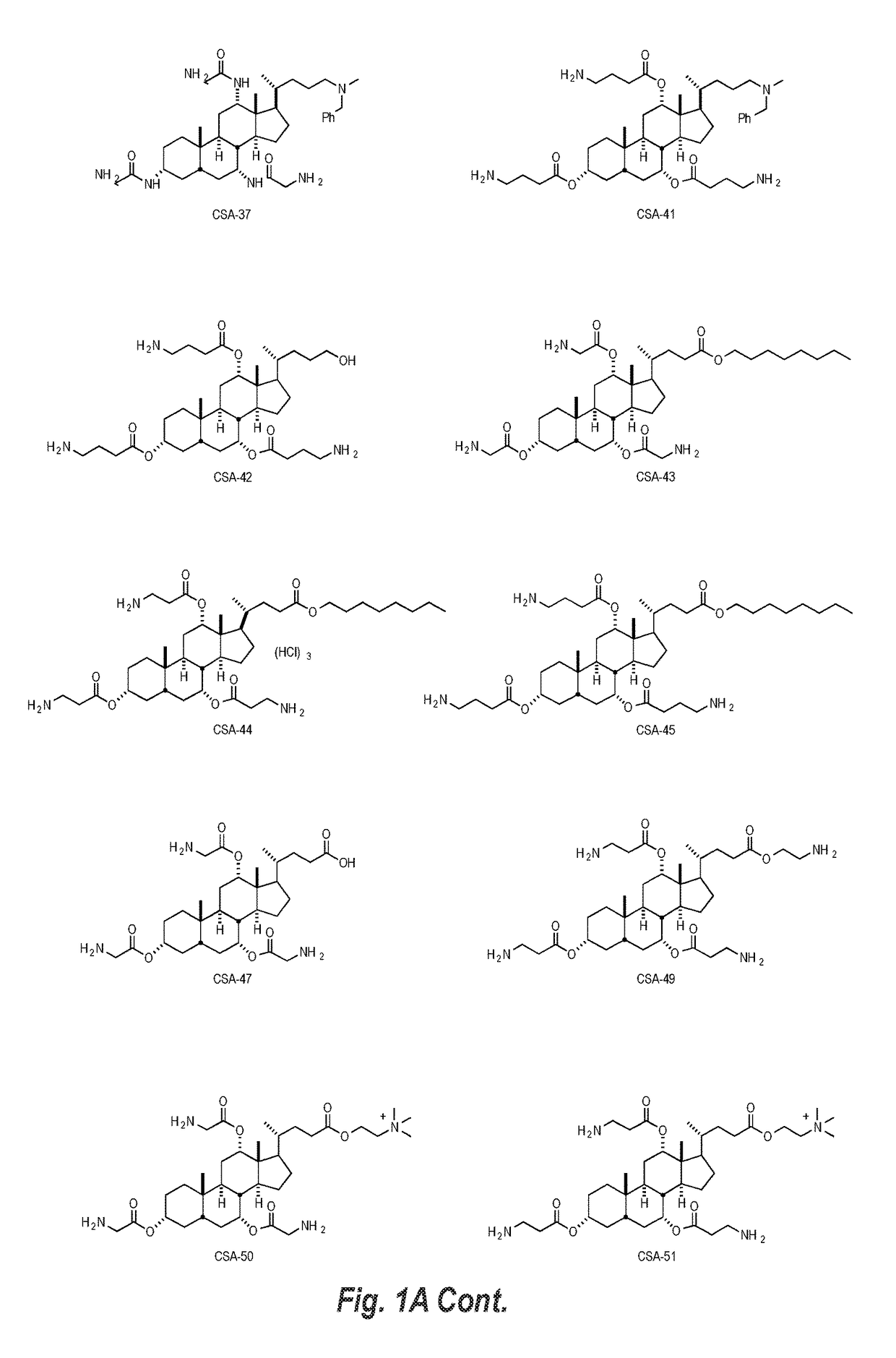
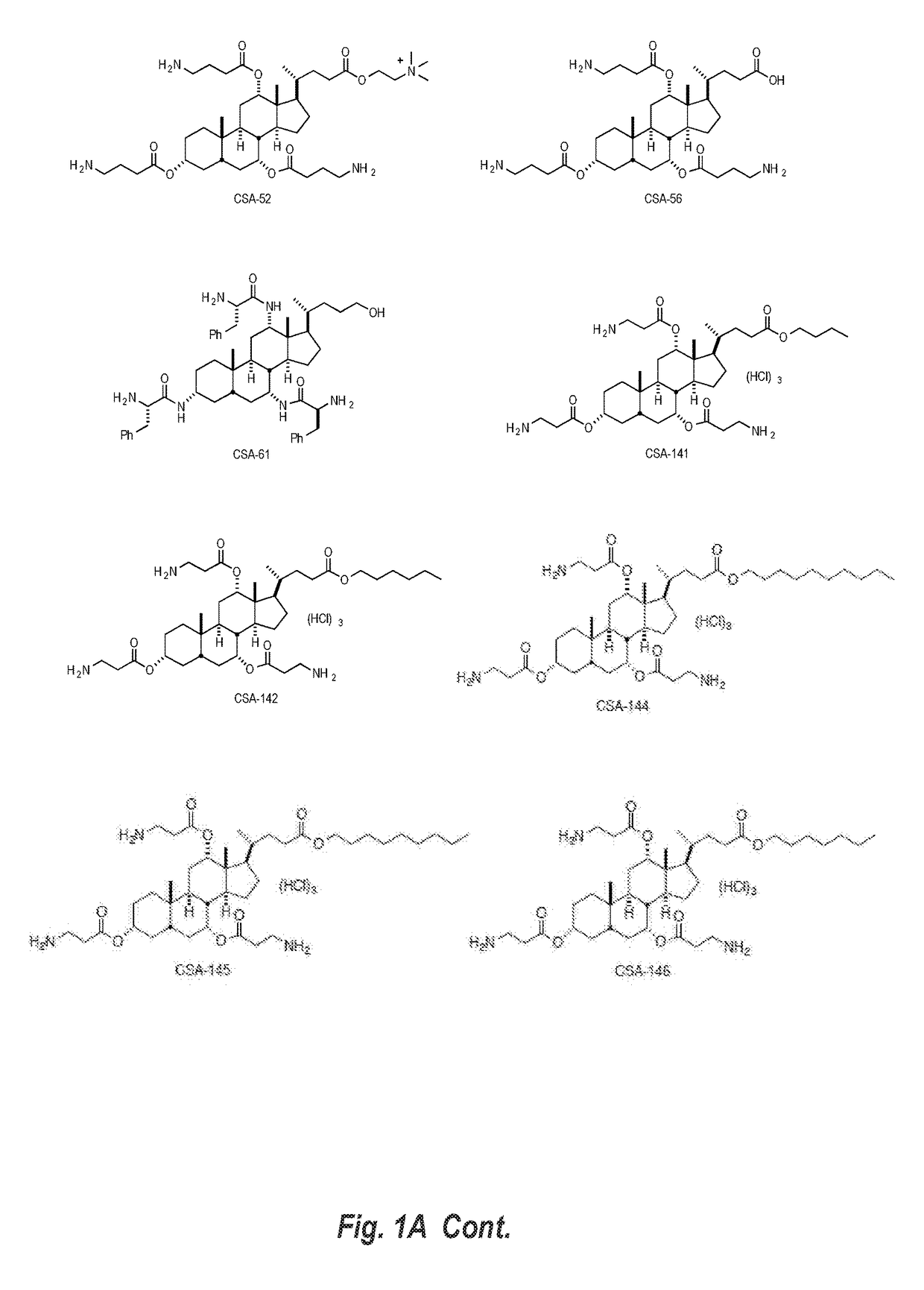
This disclosure relates to dermal treatment compositions, such as dermal fillers and tissue glues, and injectable compositions that incorporate one or more cationic steroidal antimicrobials (CSAs). The CSAs are incorporated into the dermal treatment compositions to provide effective antimicrobial, anti-inflammatory, analgesic, anti-swelling and / or tissue-healing properties. A treatment composition includes a component formed from a biologically compatible material suitable for injection into and / or application onto tissue at a treatment site. One or more CSA compounds are mixed with the biologically compatible material so that the one or more CSA compounds are incorporated within the composition, forming a reservoir of CSA compounds within the resulting bolus of the treatment composition after injection and / or application.
Owner:BRIGHAM YOUNG UNIV
Chitosan beads and filler comprising such beads
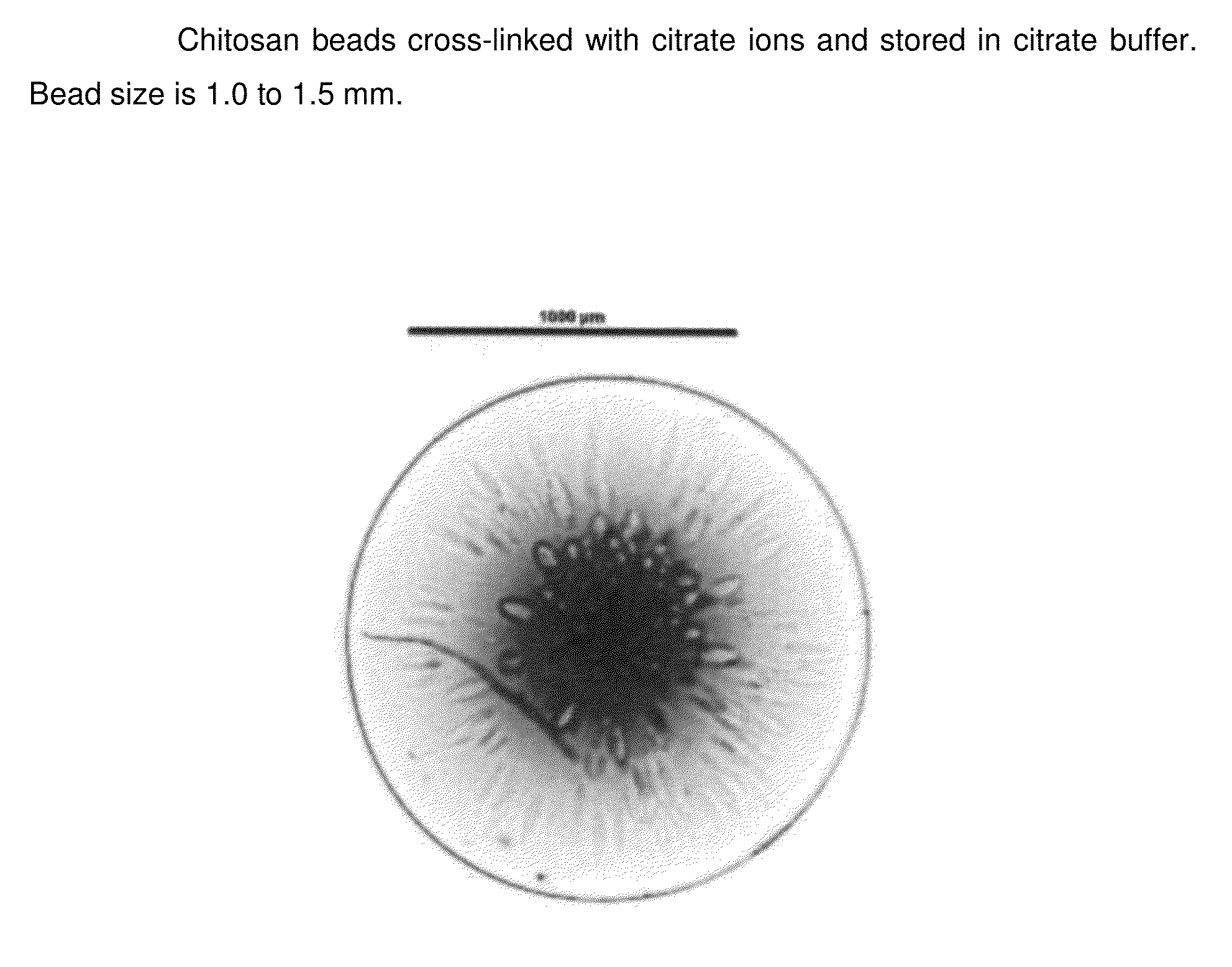
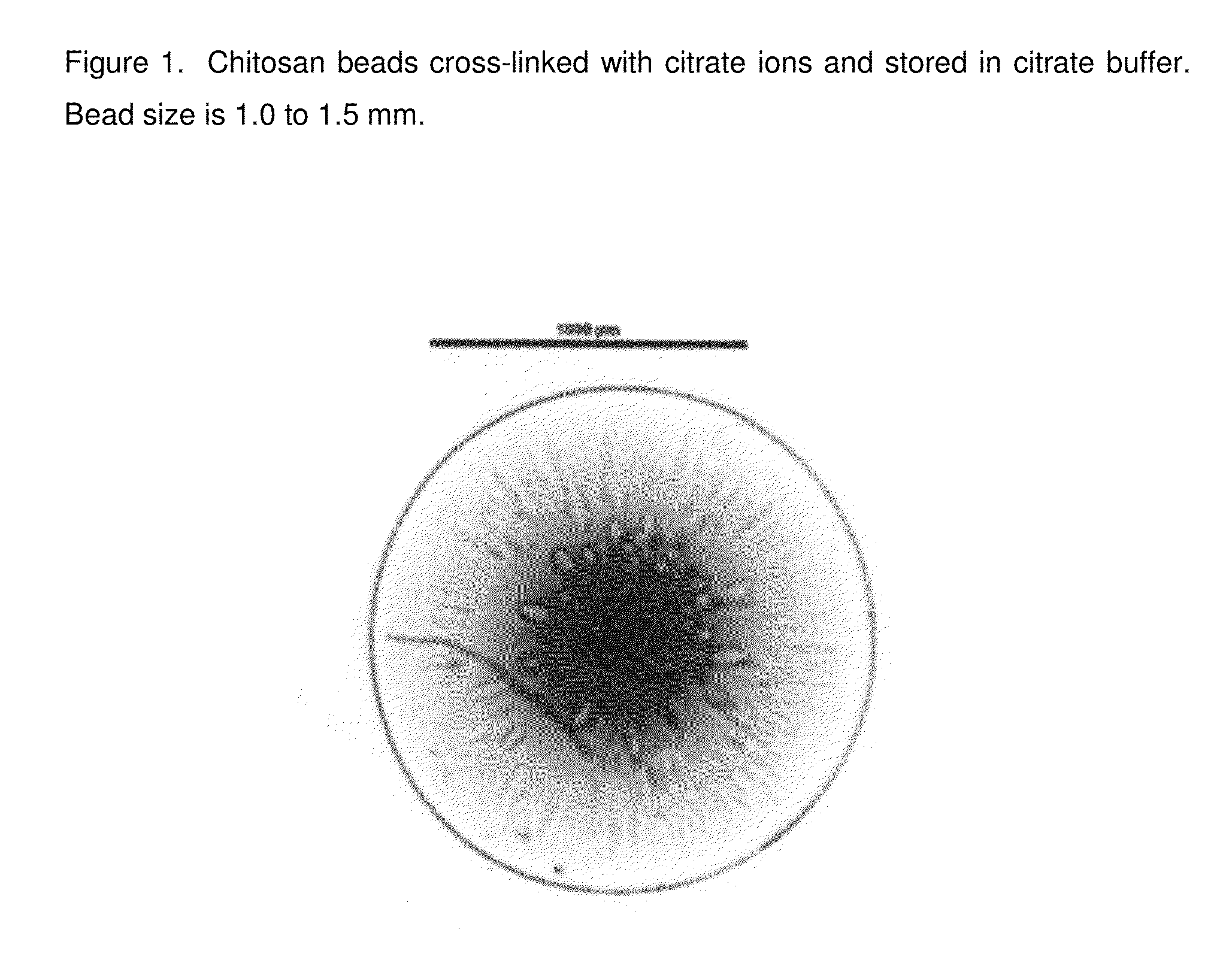
InactiveUS20140377368A1Lasting effectRapidly volumeBiocideCosmetic preparationsCross-linkCITRATE ESTER
The present invention pertains to chitosan beads consisting of chitosan cross-linked with citrate ions. The present invention furthermore pertains to a filler comprising such chitosan-citrate beads. In one embodiment of the instant invention the filler is a dermal filler. In one further embodiment of the present invention the dermal filler is for the treatment of wrinkles and / or folds. In another embodiment of the instant invention the filler is for use in the treatment of a medical condition. The filler provided in the present invention may further comprise one or more active pharmaceutical ingredients. Further, the present invention pertains to a process for preparing the filler as claimed herein.
Owner:MERZ PHARMA GMBH & CO KGAA
Terminal sterilization of injectable collagen products
ActiveUS7902145B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluenceVolumetric Mass Density
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5 kGy and 12 kGy and more preferably between 6 kGy and 8 kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
Soft tissue augmentation by needle-free injection
ActiveUS20090030367A1Accurate placementJet injection syringesAutomatic syringesWrinkle skinNeedle free
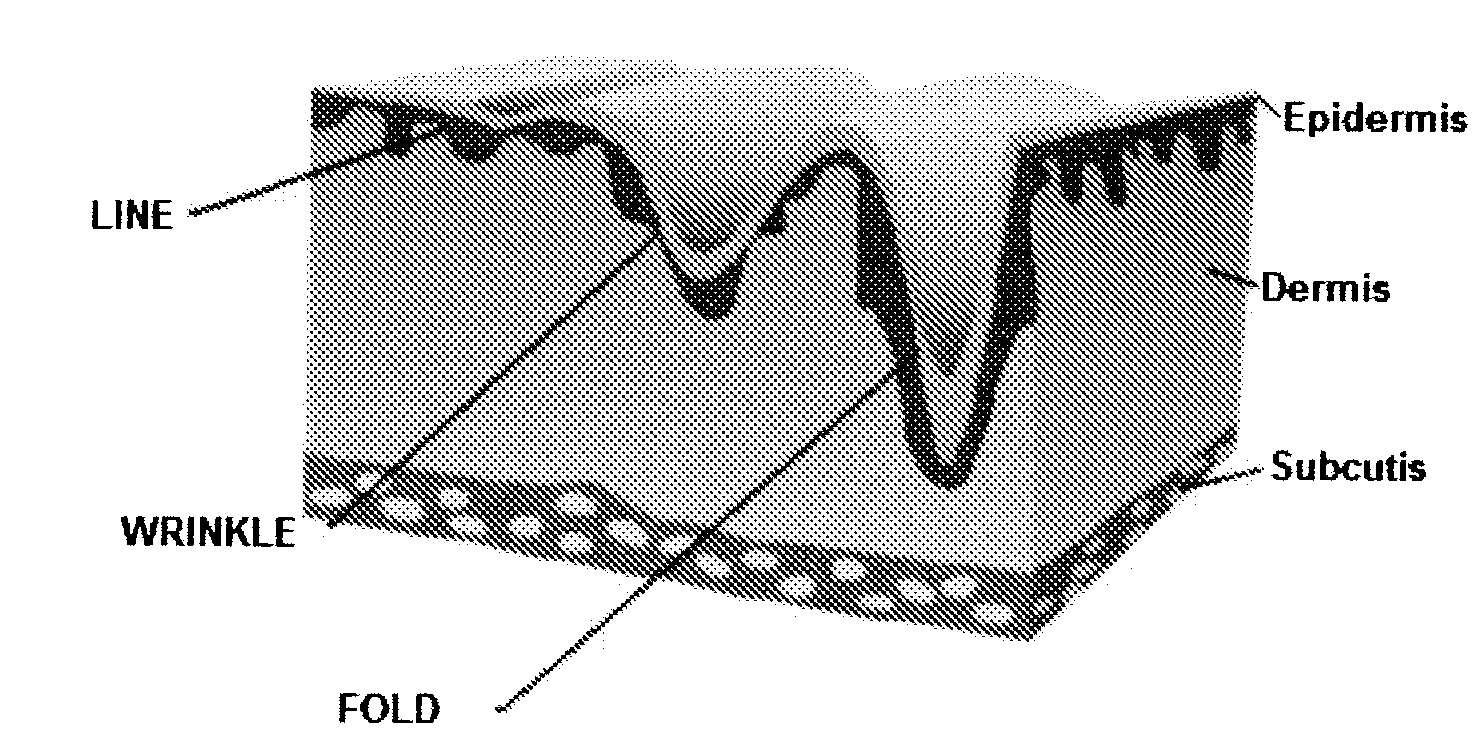
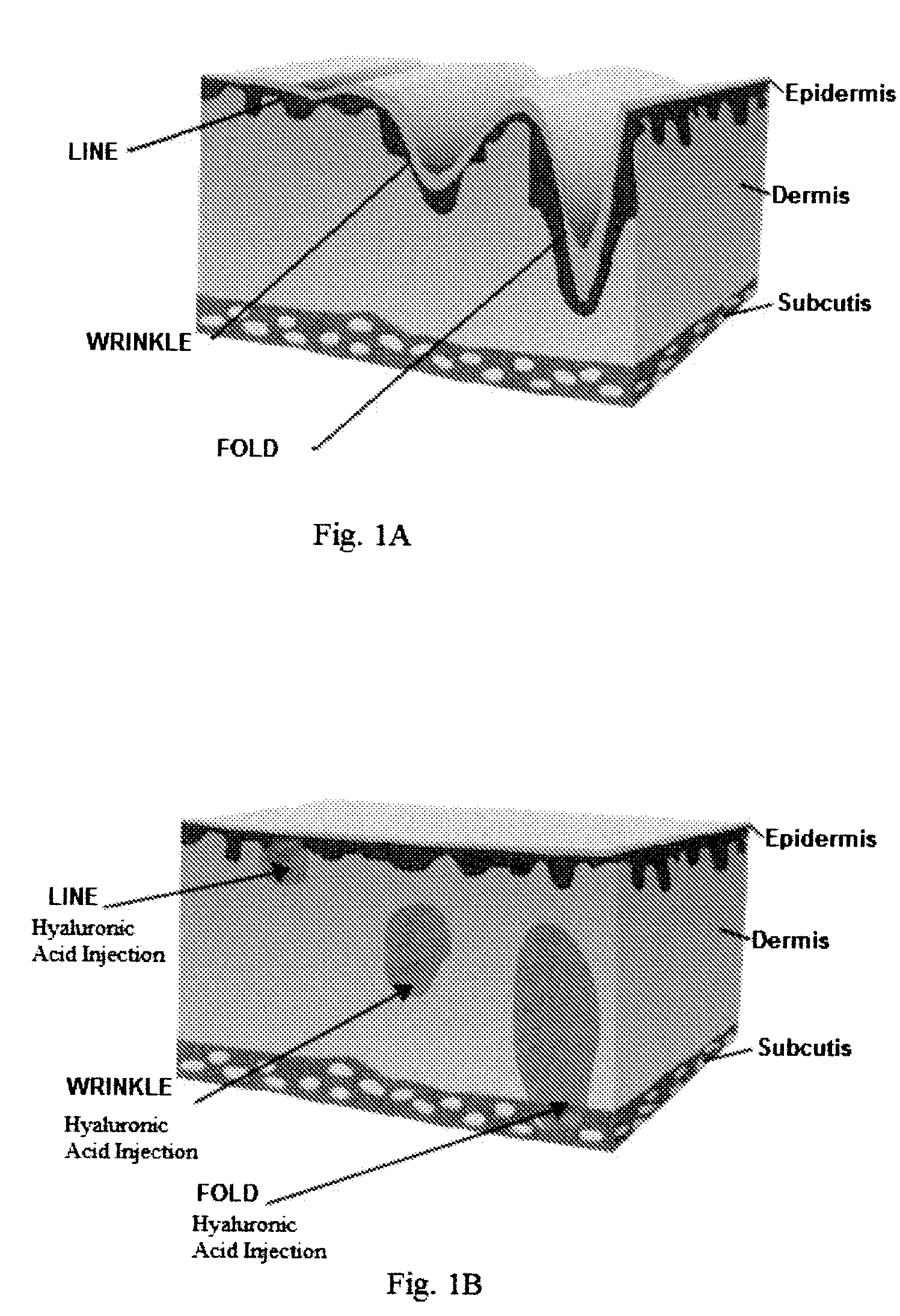
The invention relates to needle-free apparatus that can be used to augment soft tissue. More specifically, the needle-free injectors of the present invention allow injection of more viscous materials such as collagen, hyaluronic acid, and other polymers that are useful as dermal fillers. The needle-free injectors of the present invention allow injection of such materials to fill the undesired lines, wrinkles, and folds of a patient. The present invention also relates to kits comprising such needle-free injectors and a quantity of dermal filling material. In addition, the present invention relates to methods of augmenting soft tissue using needle-free apparatus.
Owner:ALLERGAN INC
Alloplastic injectable dermal filler and methods of use thereof
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Dermal filler and method of using same
Polyglutamic acid (PGA) formulations are provided for use as a dermal filler. Also provided are methods of use of such formulations for treatment of cosmetic defects.
Owner:PRESCOTT ALBERT G
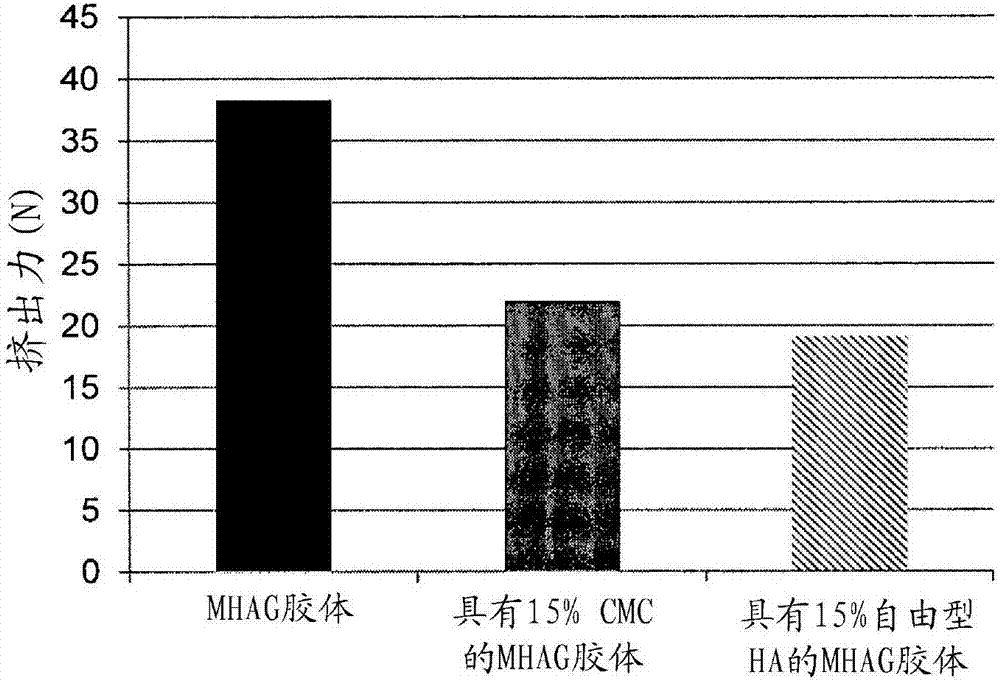
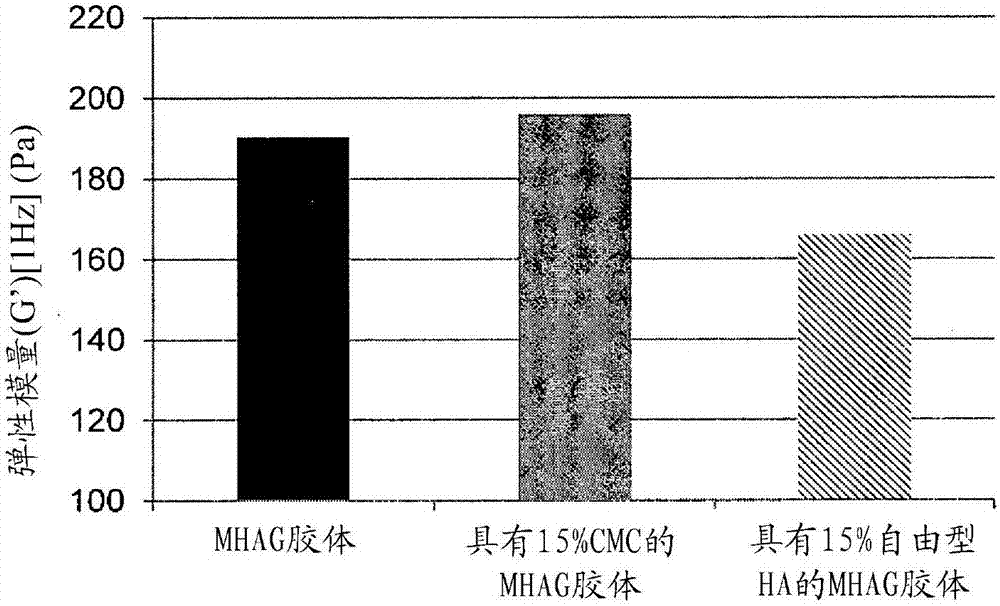
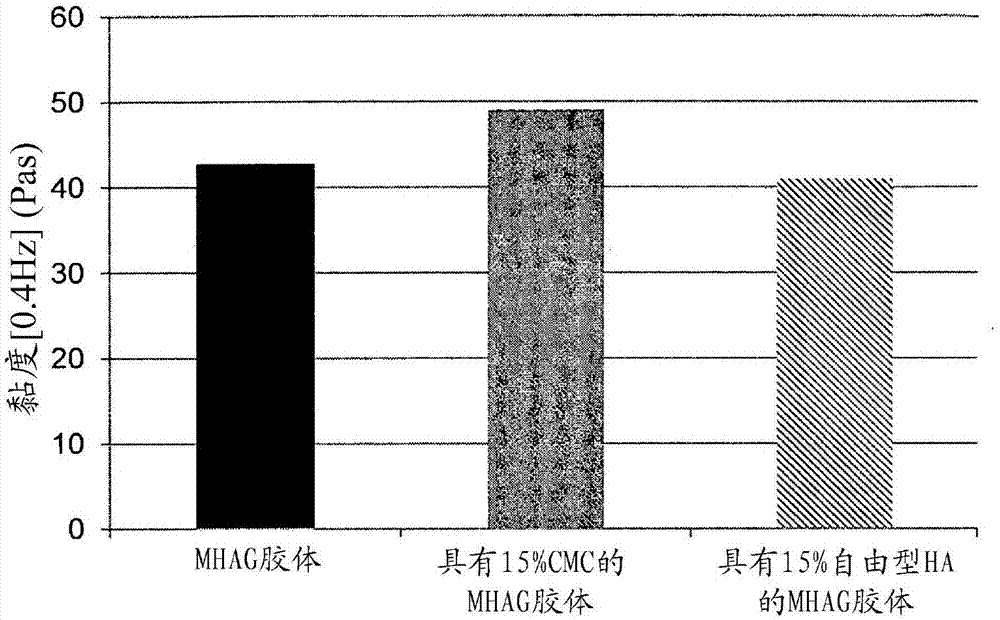
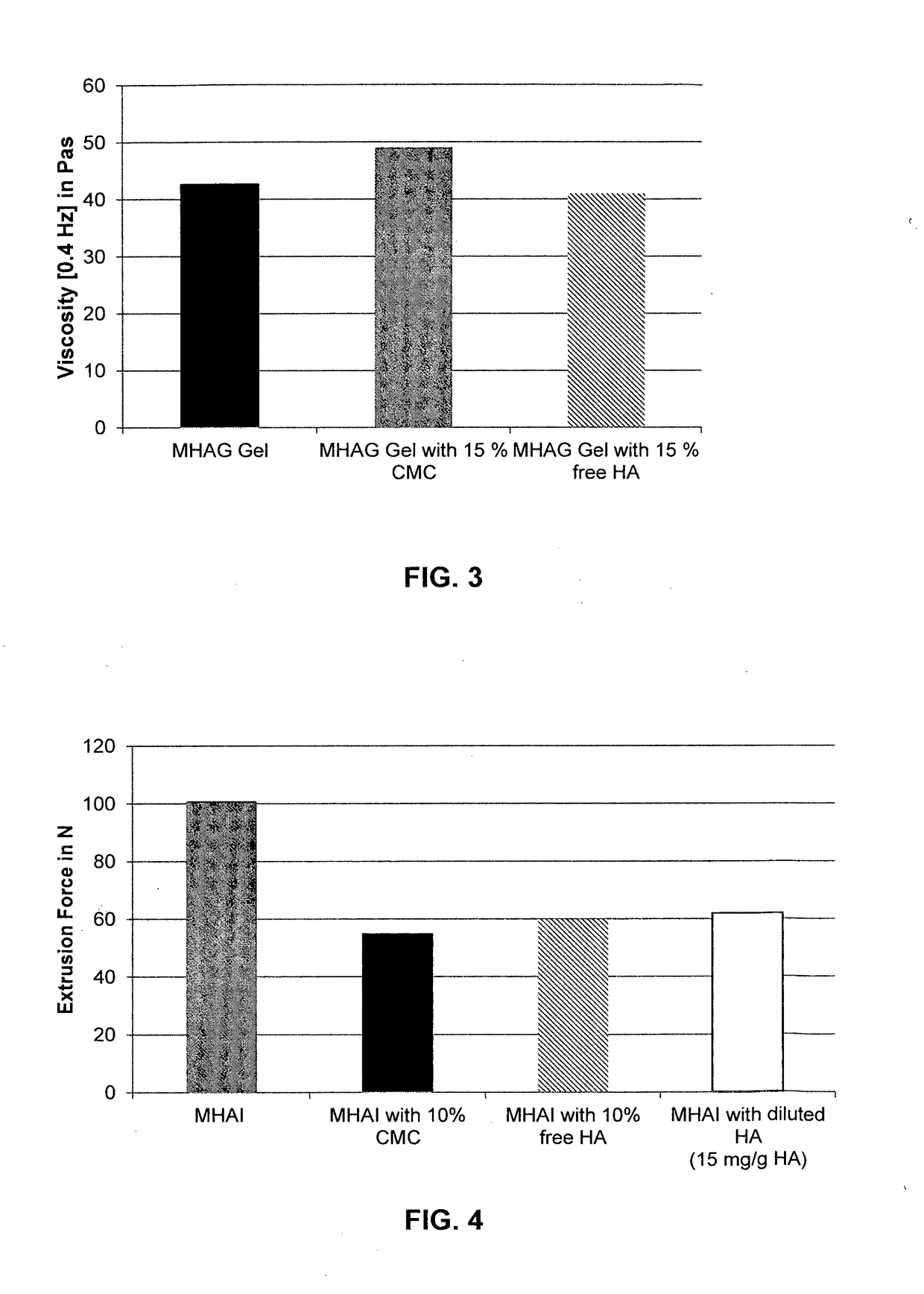
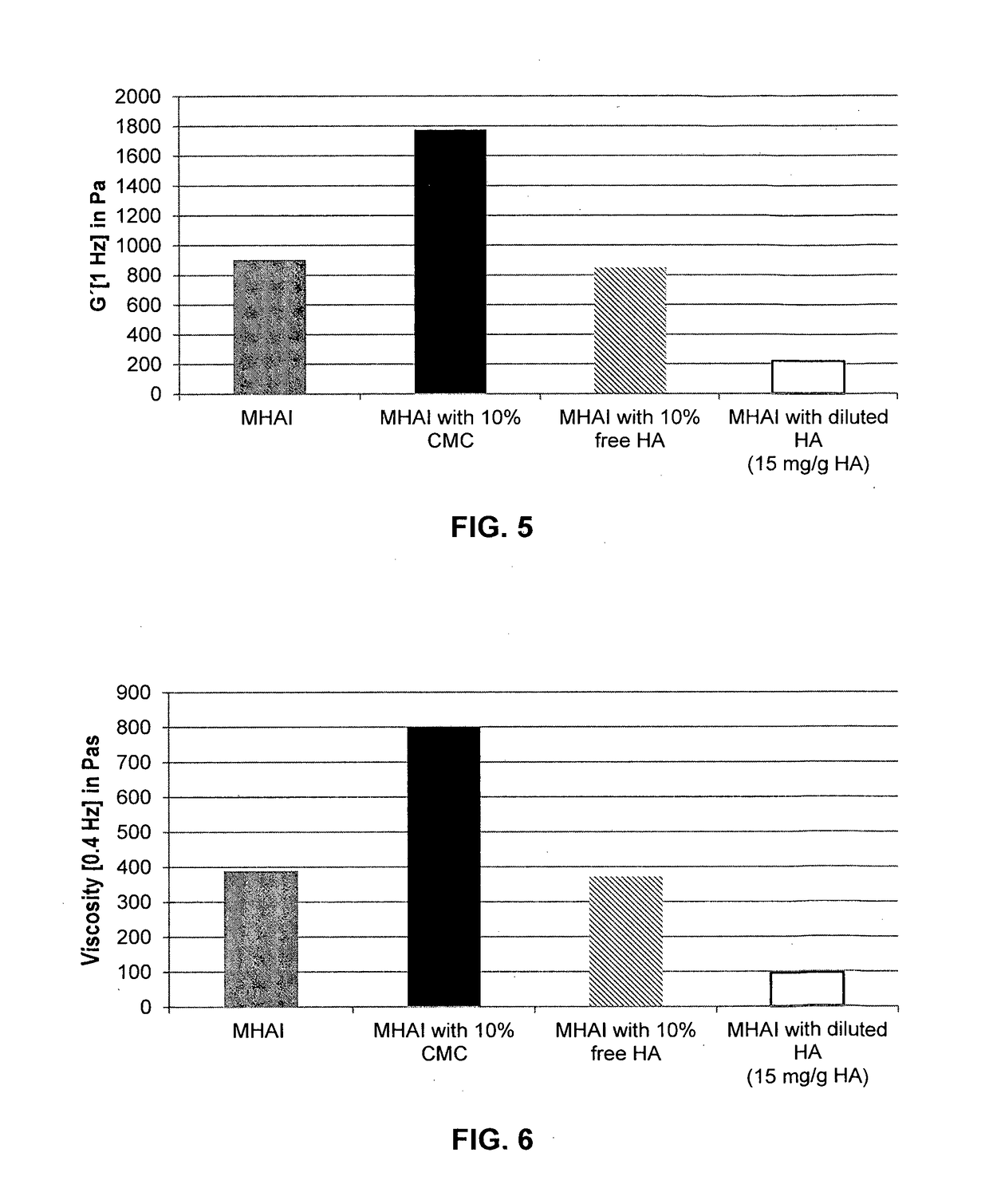
Dermal filler based on crosslinked hyaluronic acid and carboxymethyl cellulose lubricant
ActiveCN106999625APharmaceutical delivery mechanismAnaestheticsCarboxymethyl celluloseFiller Excipient
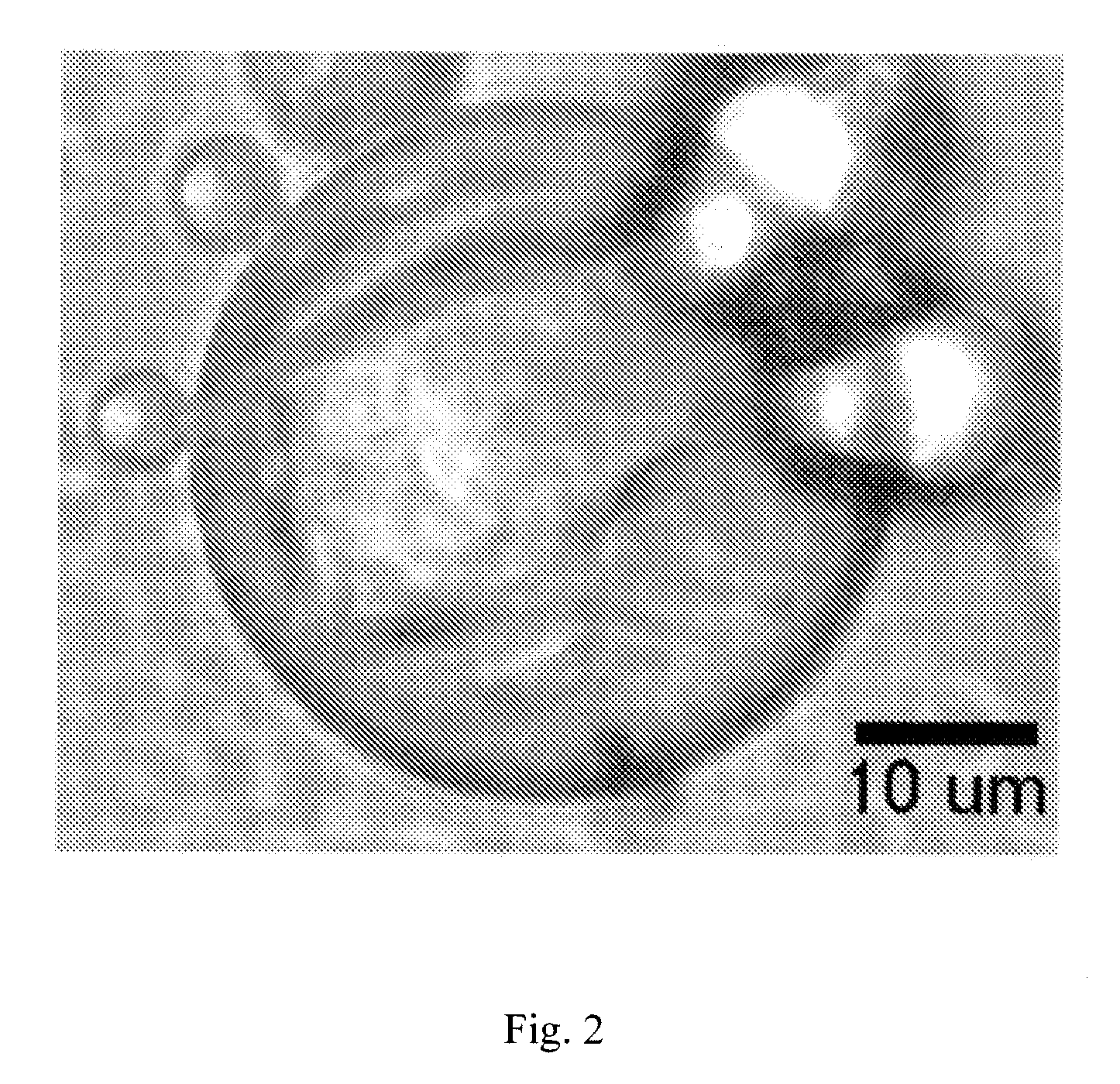
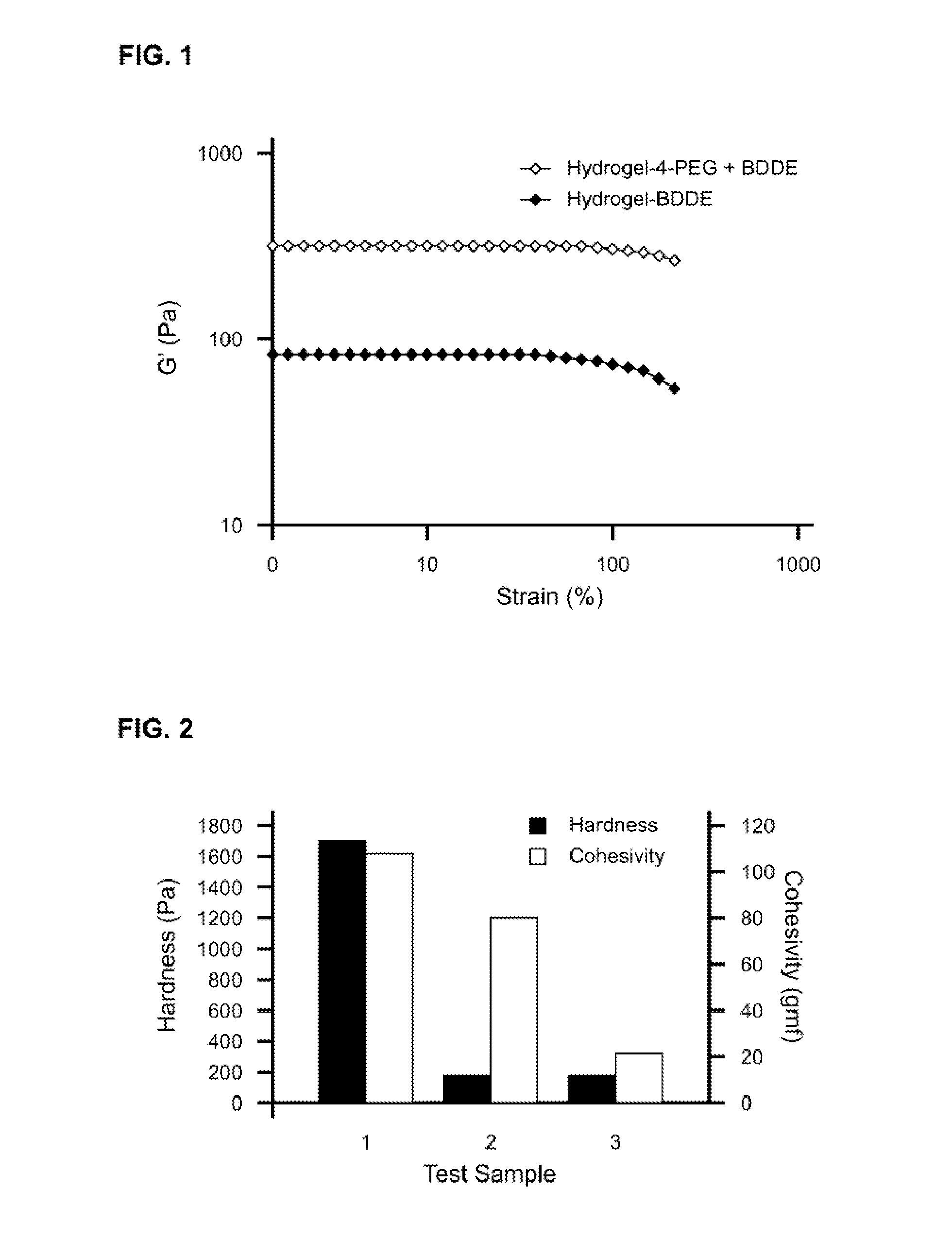
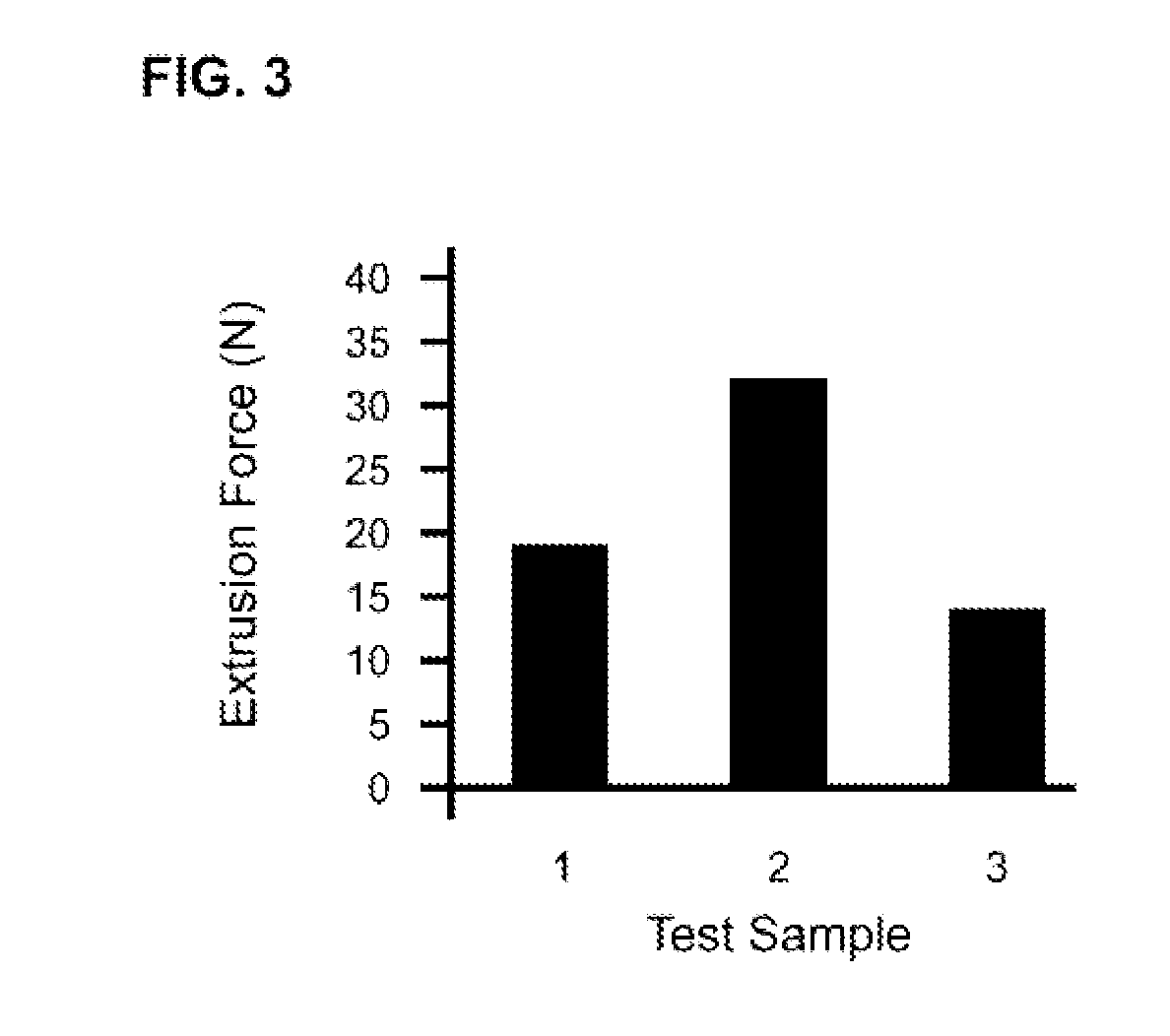
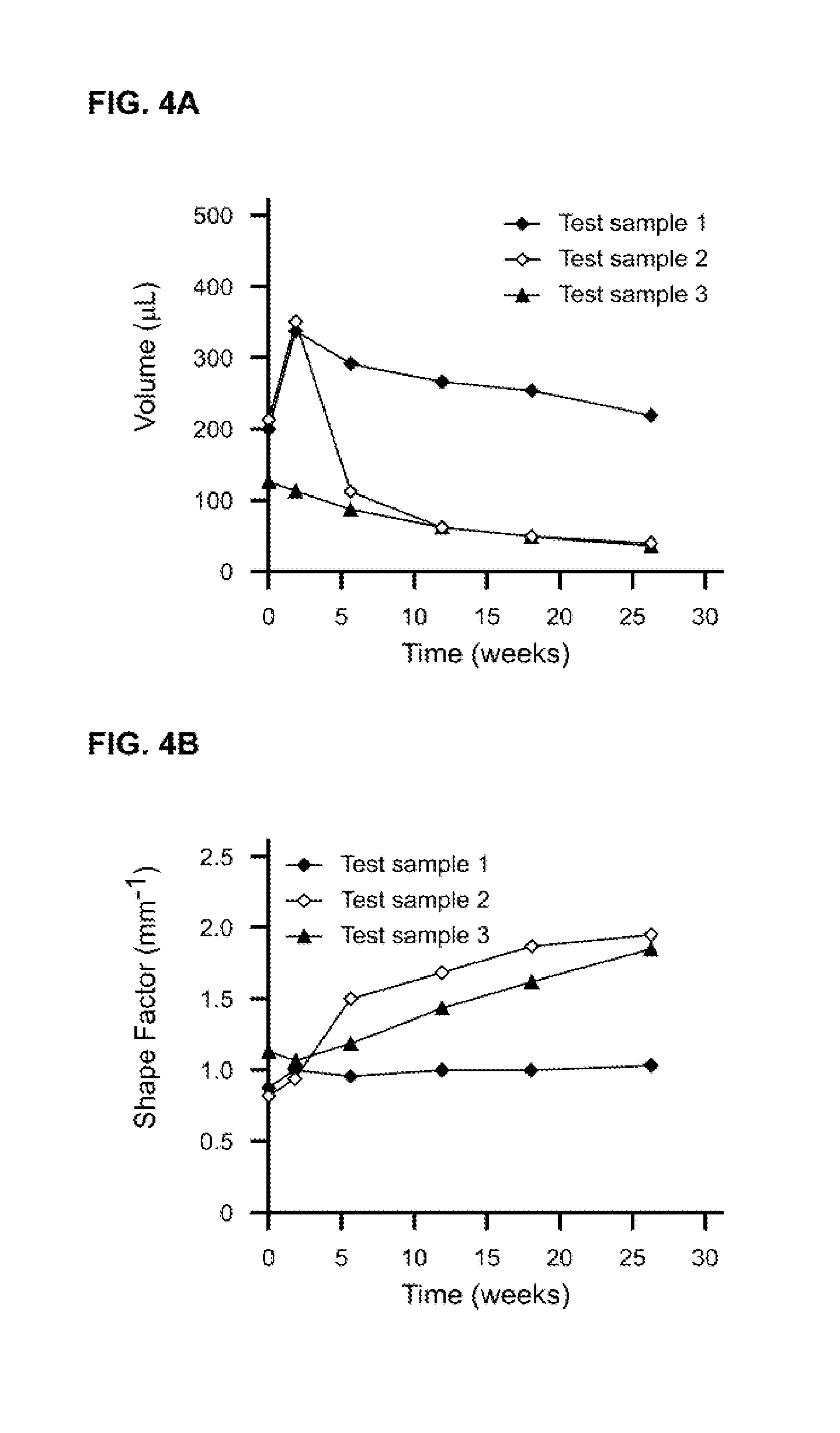
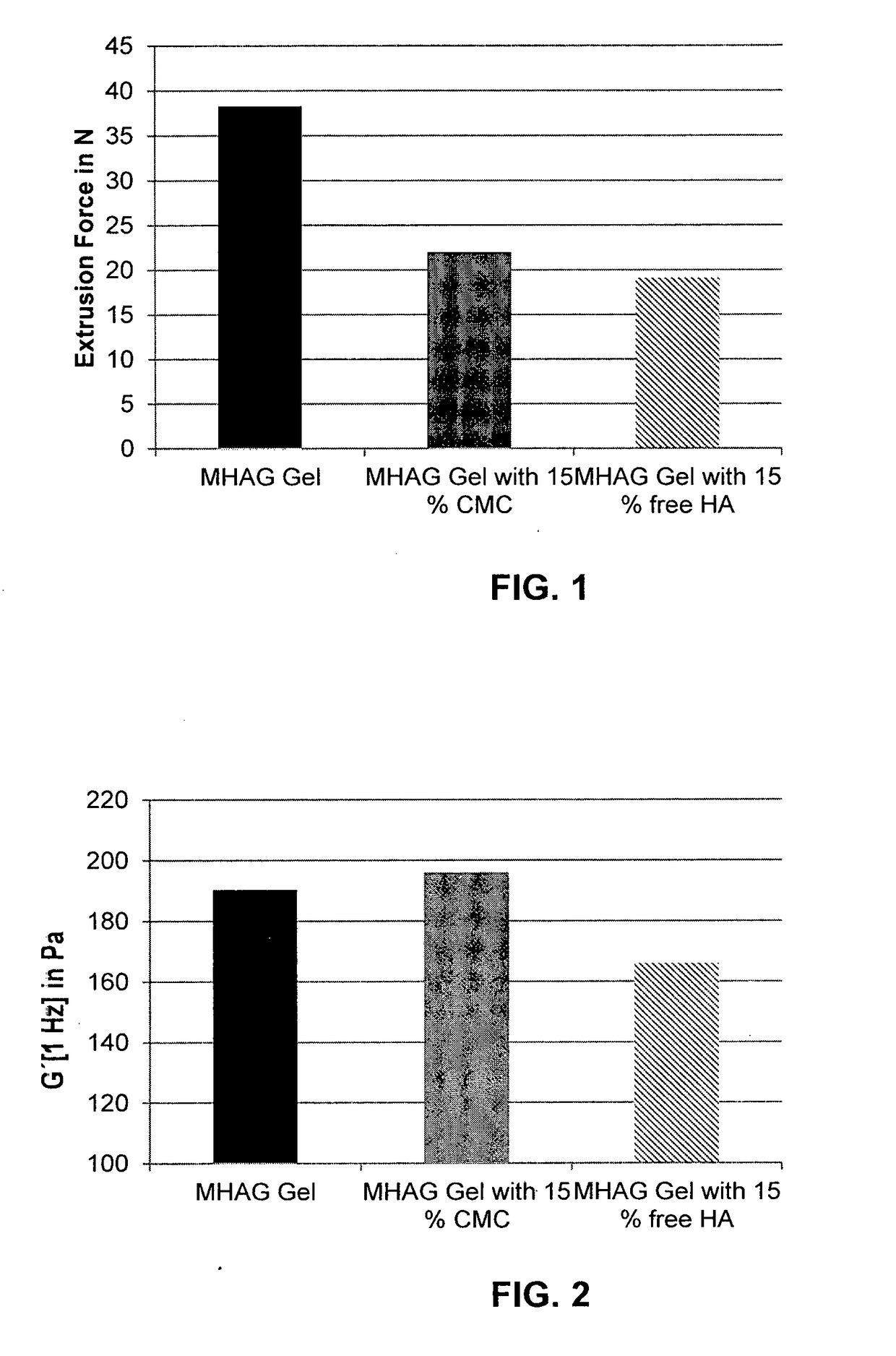
The present invention relates to injectable dermal filler compositions in the form of a gel, comprising hyaluronic acid (HA), carboxymethyl cellulose (CMC) and, optionally, microparticles such as calcium hydroxyapatite (CaHAP) microparticles. The injectable dermal filler compositions have improved rheological properties while at the same time have low extrusion forces. The present invention further relates to a method for preparing such injectable dermal filler compositions and their use for cosmetic and therapeutic purposes.
Owner:MERZ PHARMA GMBH & CO KGAA
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20100322982A1Reduce wrinklesReduce scarsCosmetic preparationsPowder deliveryFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
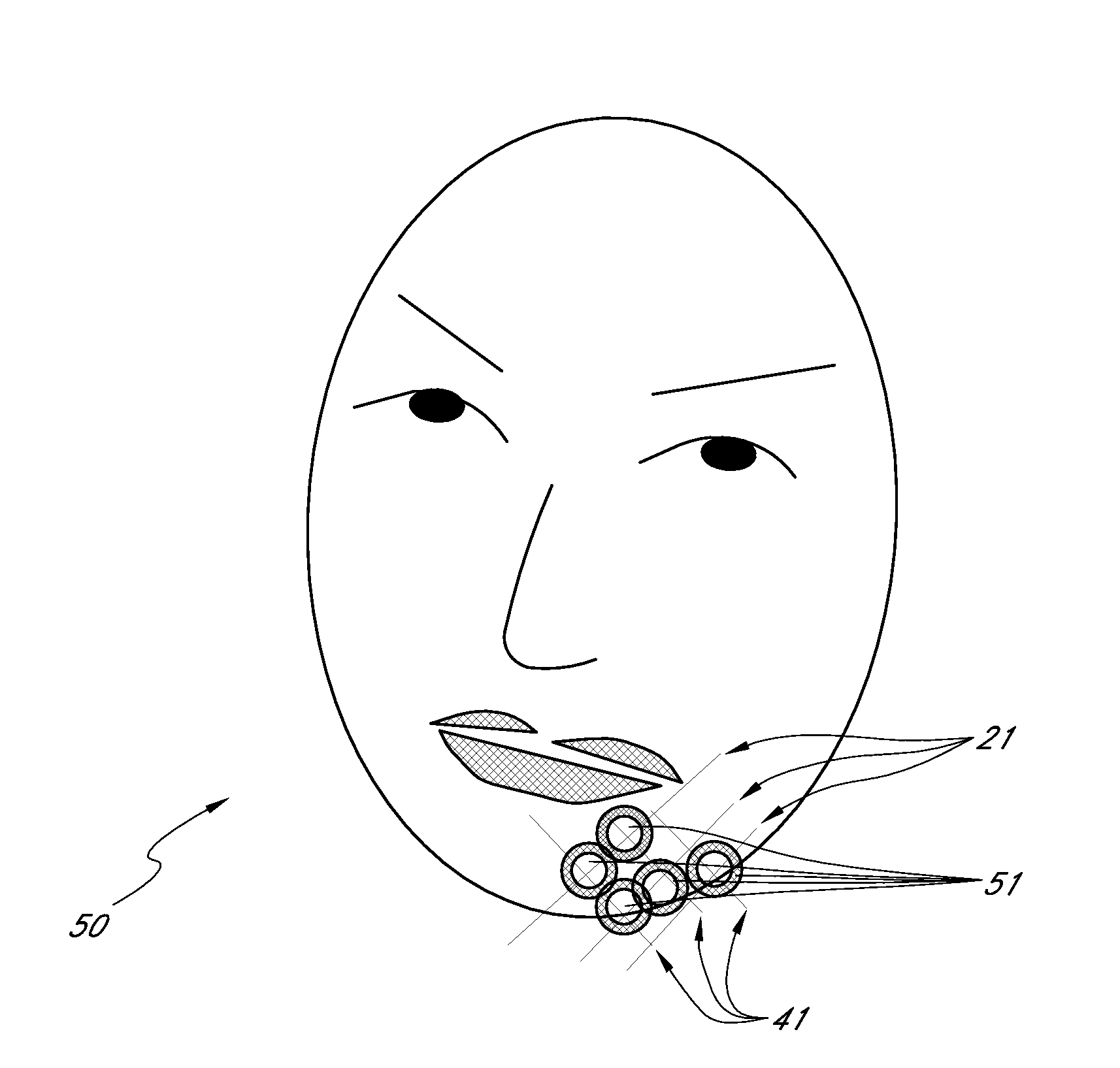
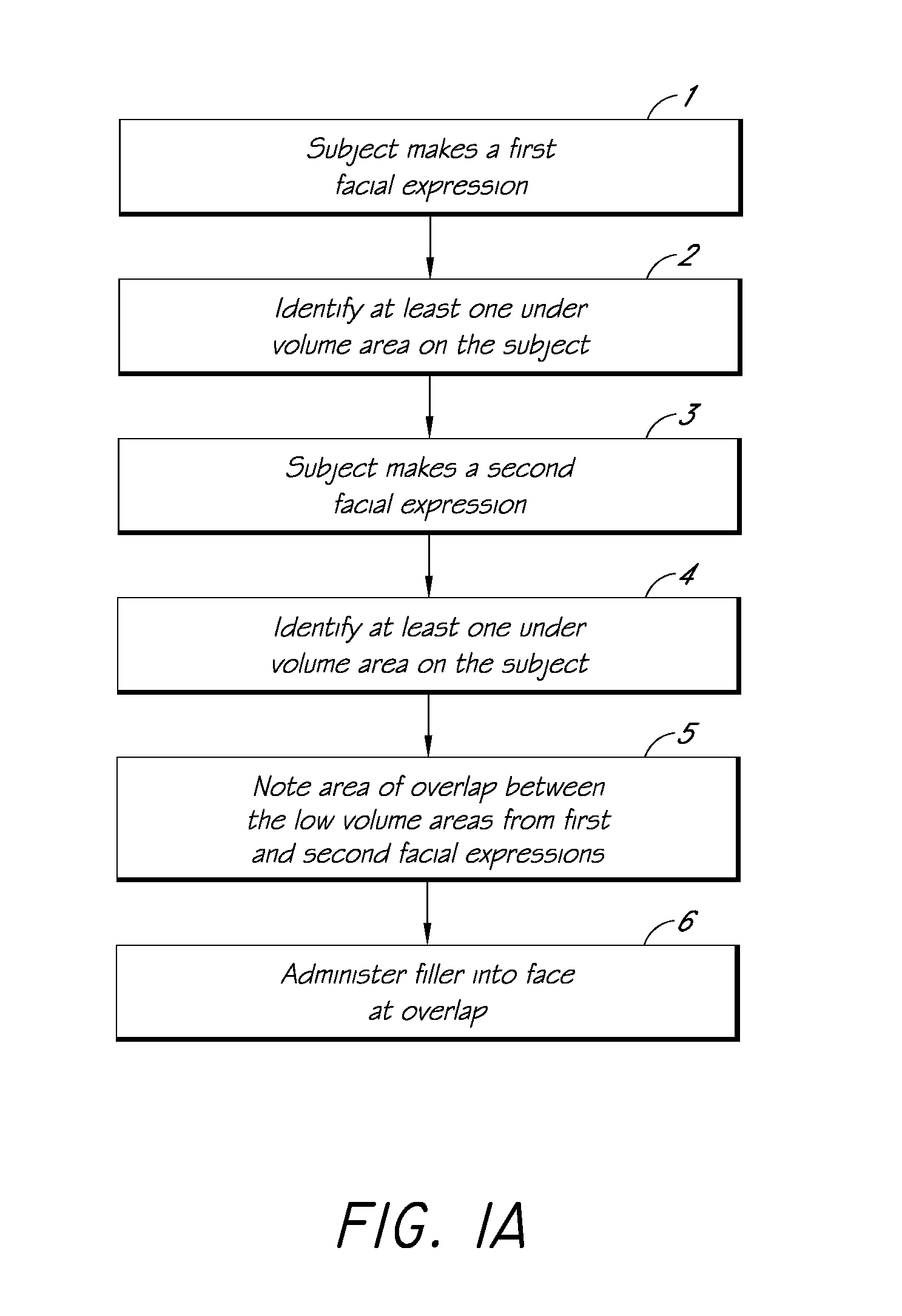
Methods for Identifying Areas of a Subject's Skin that Appear to Lack Volume
InactiveUS20100160849A1Increase aesthetic benefitImprove adverse effectsElectrotherapyPeptide/protein ingredientsMedicineSKIN REGIONS
The present embodiments relate to methods and systems for identifying areas of a subject's skin that lack sufficient volume. In some embodiments, this can be used to direct the administration of filler compositions or the application of techniques that increase the volume or firmness of the area. In some embodiments, the methods and systems provide for improved aesthetic benefit as well as suitability for instruction and training. In some embodiments, the methods are especially useful in the administration of dermal fillers to a subject.
Owner:IPSYRNG CAPITAL DEV
Swellable hyaluronic acid particles
InactiveUS20100217403A1Extend your lifeOrganic active ingredientsTissue regenerationMedicinePolysaccharide
Owner:ALLERGAN INC
Dermal filler composition
ActiveCN101426451ALong-term stable volume expansion effectPromote amplificationSkin implantsPenis implantsCross-linkFiller Excipient
Disclosed herein is a dermal filler composition. The composition includes polymethylmethacrylate (PMMA), cross-linked dextran, hydroxypropyl methylcellulose (HPMC), and physiological saline or distilled water. The composition rapidly restores volume at application sites by injection, does not require pre-testing, such as allergic skin testing, because it does not cause severe allergic reactions, is cheap, and is not easily degraded or absorbed in the body, thus ensuring a long-lasting volume augmentation effect. Due to the characteristics described above, the composition facilitates volume correction requiring a large amount (20 cc or greater) of dermal filler such as in augmentation phalloplasty.
Owner:曹康善 +1
Dermal filler composition
ActiveCN102247618ASimple production processReduce manufacturing costPharmaceutical delivery mechanismProsthesisPenisCross-link
The present invention relates to a novel dermal filler composition and to a method for preparing same. The composition of the present invention comprises, as a main component, cross-linked dextran, the molecular weight of which is 30,000 to 100,000. The composition can rapidly augment a defective area of the skin and maintain softness to the touch even when used alone. The composition eliminates the necessity of a pretreatment, such as an allergy test, which might otherwise be required prior to injection, is inexpensive, and is not easily decomposed or absorbed in vivo, thereby maintaining the tissue-volume augmentation effects thereof over a long period of time after injection. Therefore, the composition is suitable for use in a procedure such as penile augmentation or the like which requires the injection of a large amount of dermal filler, i.e. more than 20 cc. The composition of the present invention can be prepared through a simplified process to make the composition easily usable. The composition is soft to the touch when injected under the skin, and can thus be applicable not only to the skin of the penis but also to the skin of other parts of the human body, Including the face.
Owner:曹康善 +3
Dermal filler based on crosslinked hyaluronic acid and carboxymethyl cellulose lubricant
ActiveUS20170333596A1Improve rheologyEasy injectionPharmaceutical delivery mechanismAnaestheticsCarboxymethyl celluloseFiller Excipient
The present invention relates to injectable dermal filler compositions in the form of a gel, comprising hyaluronic acid (HA), carboxymethyl cellulose (CMC) and, optionally, microparticles such as calcium hydroxyapatite (CaHAP) microparticles. The injectable dermal filler compositions have improved rheological properties while at the same time have low extrusion forces. The present invention further relates to a method for preparing such injectable dermal filler compositions and their use for cosmetic and therapeutic purposes.
Owner:MERZ PHARMA GMBH & CO KGAA
Composition, in an aqueous medium, including at least one hyaluronic acid and at least one sucrose octasulphate water-soluble salt
ActiveCN104394934AMaintain rheological propertiesImprove durabilityOrganic active ingredientsCosmetic preparationsSucroseWater soluble
A composition including at least one crosslinked or non-crosslinked hyaluronic acid, or one of its salts, and at least one water-soluble salt of sucrose octasulphate, to processes for the manufacture of said composition and to the use of said composition for the formulation of a viscosupplementation composition or for the formulation of a composition as a dermal filler or for the formulation of a cosmetic composition.
Owner:LABES VIVACY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com