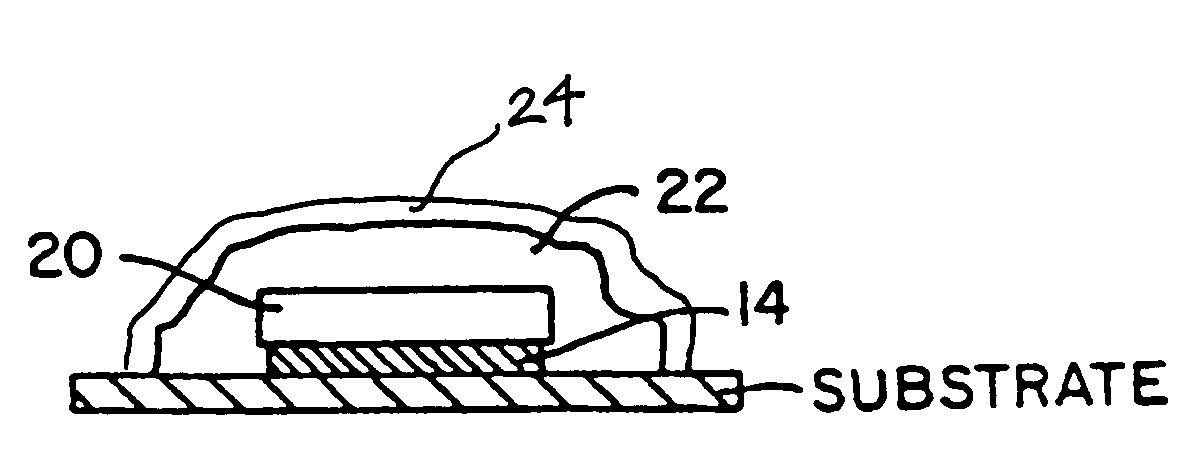
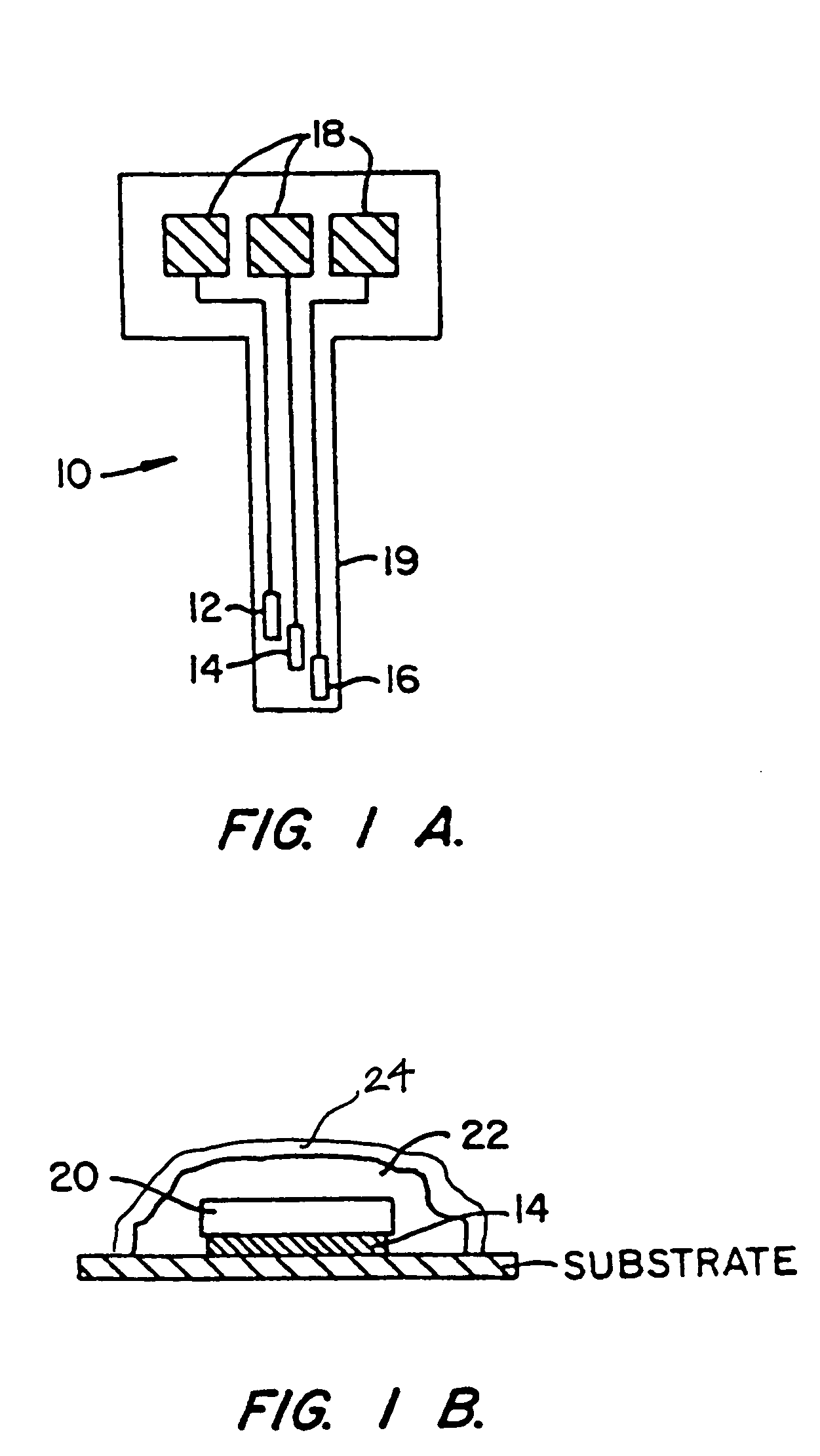
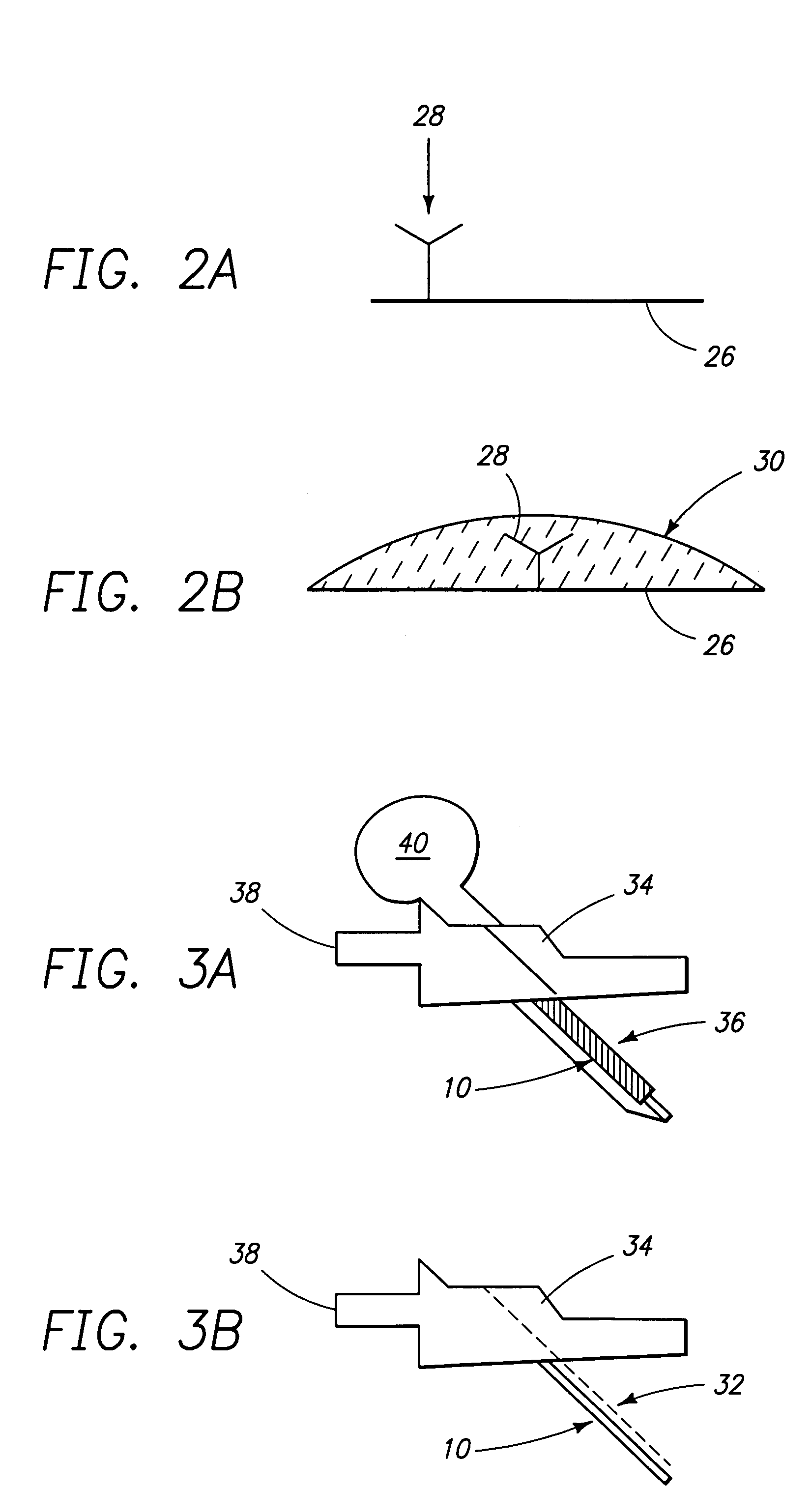
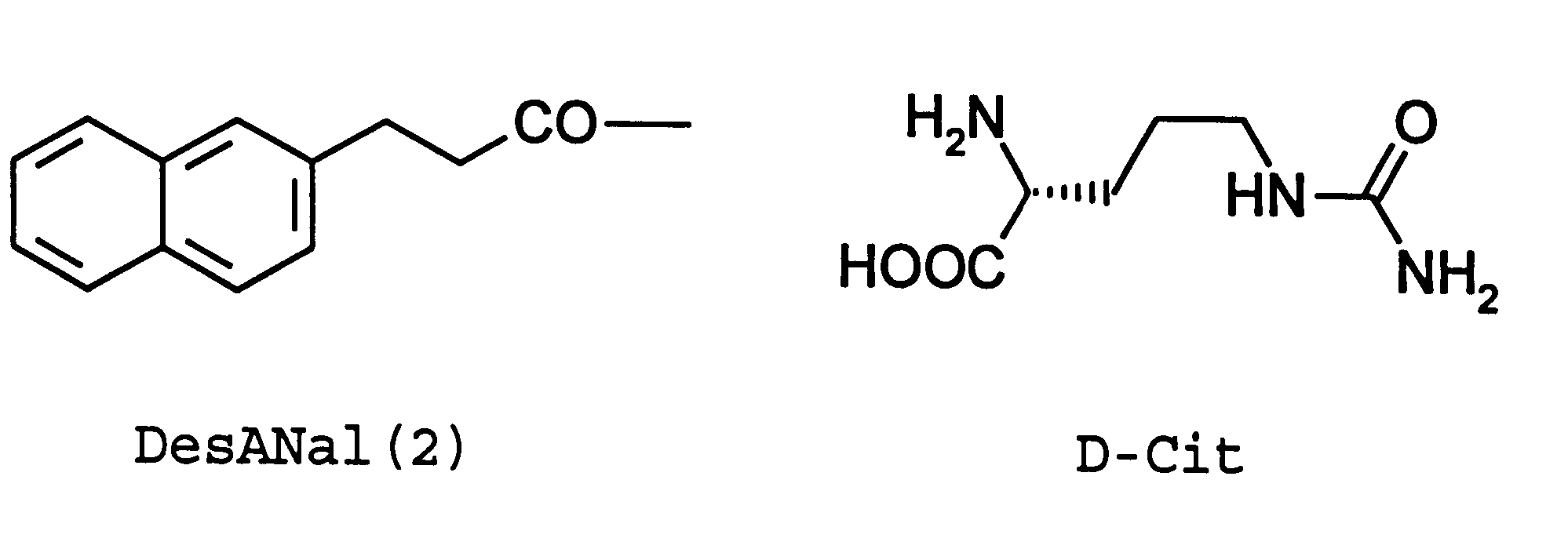
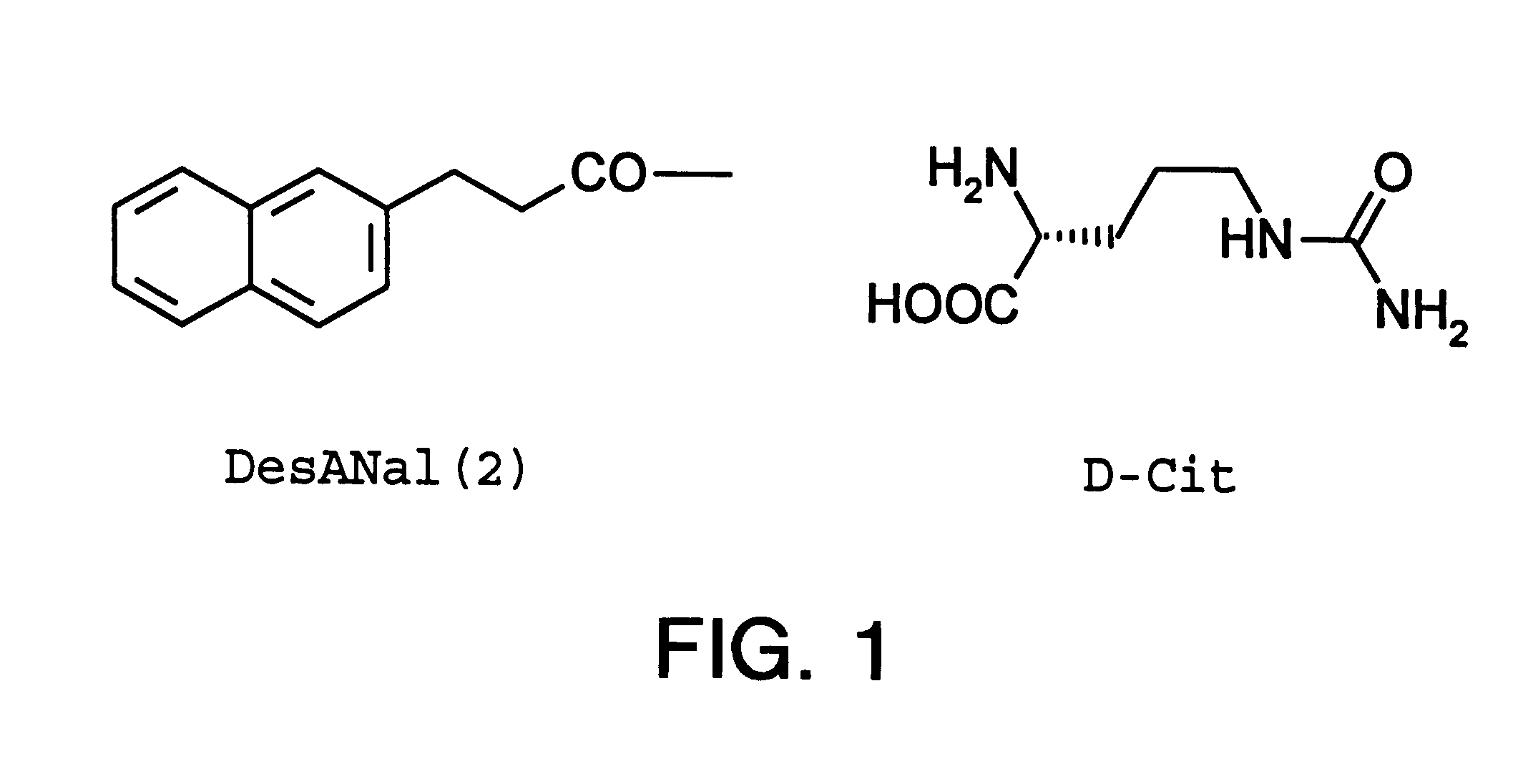
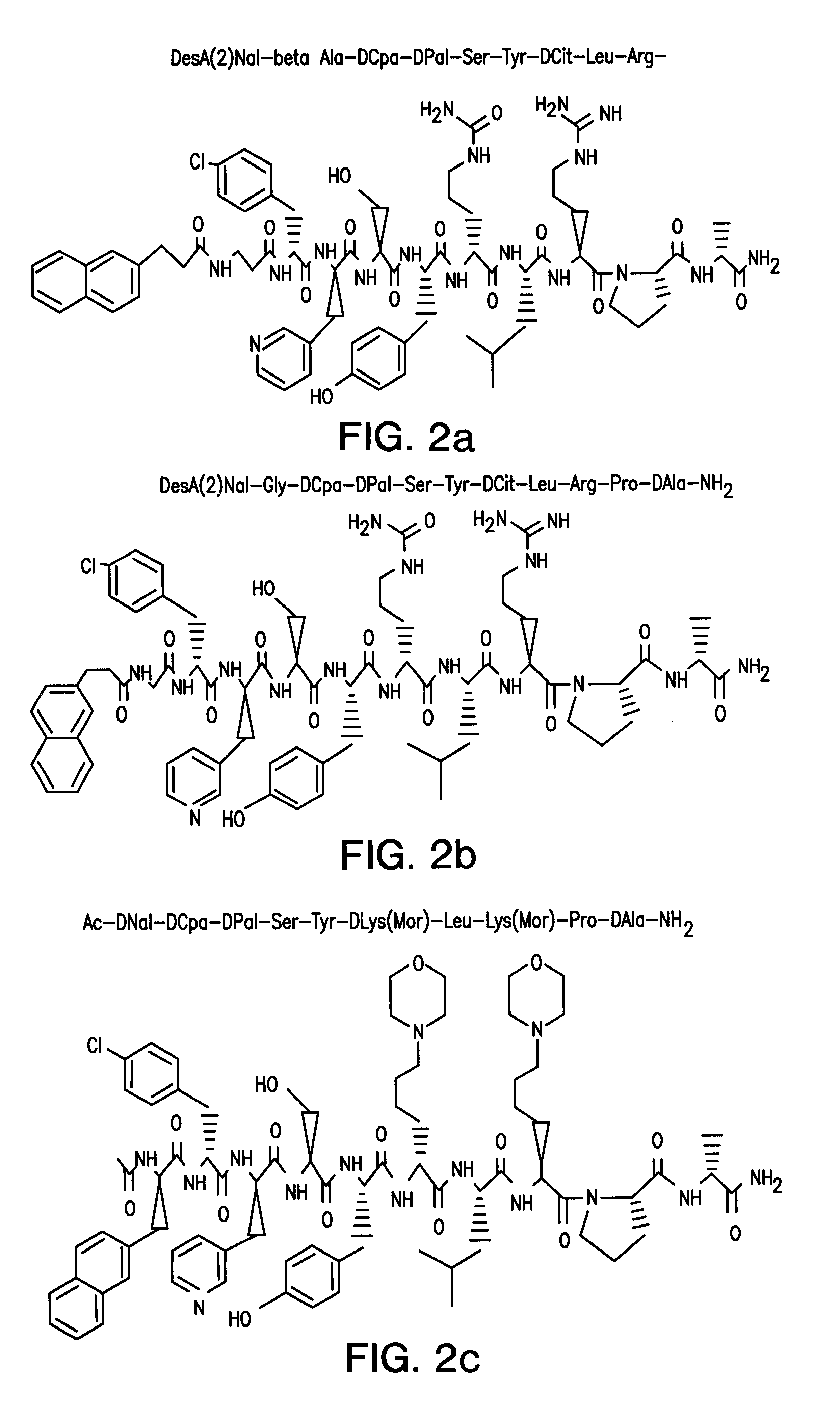
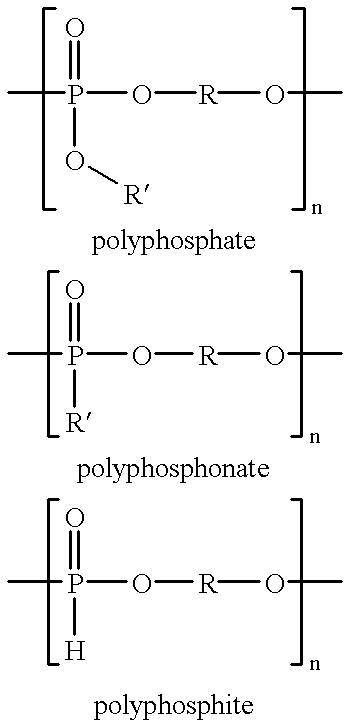
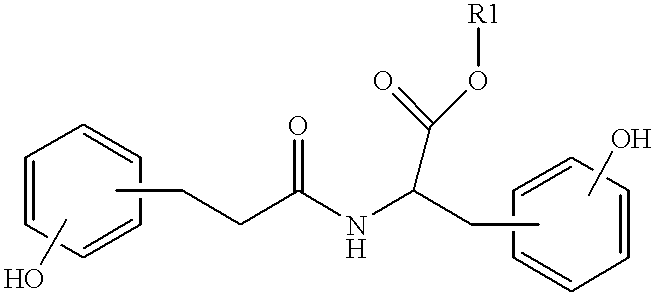
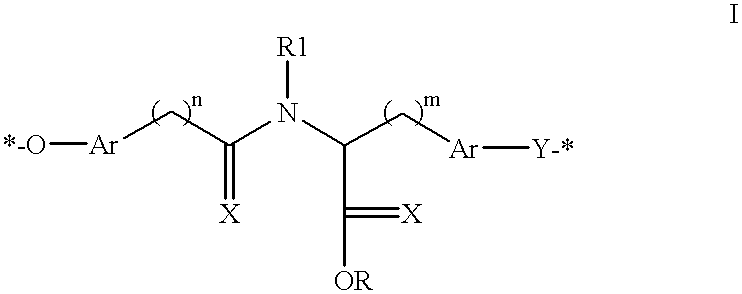
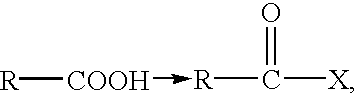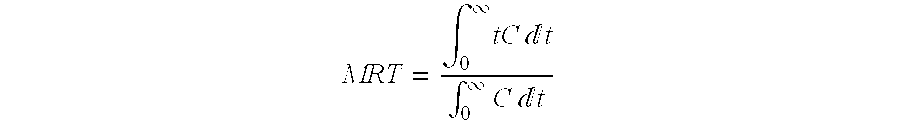Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
943 results about "Biologically active substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The conjugate as a biologically active substance carrier is characterised in that the biologically active substance is selected from a group comprising proinsulin, tyrosyl-2-alanyl-glyceryl-phenylalanyl-leucyl-arginine diacetate, alcalase, hyaluronidase, somatotropin and in that the carrier is in the form of a pharmacologically acceptable...
Anti-inflammatory biosensor for reduced biofouling and enhanced sensor performance
A biosensor including an external surface, and an accessory material in close proximity to the external surface. The accessory material includes a coating containing a hydrophilic material and / or a fiber modified to deliver a therapeutic agent. The biosensor modifies a biological response to the biosensor upon contact with a tissue, such as upon implantation into the skin of a subject, thereby reducing biofouling, inflammation and other undesirable tissue responses that interfere with biosensor performance. The biosensor can be any biocompatible sensor, suitable for short- or long-term use. Preferably, the biosensor is an enzymatic or electrochemical sensor, such as a glucose sensor. Also provided are a method of producing a biosensor and a method of delivering a biologically active substance to a subject.
Owner:MEDTRONIC MIMIMED INC
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
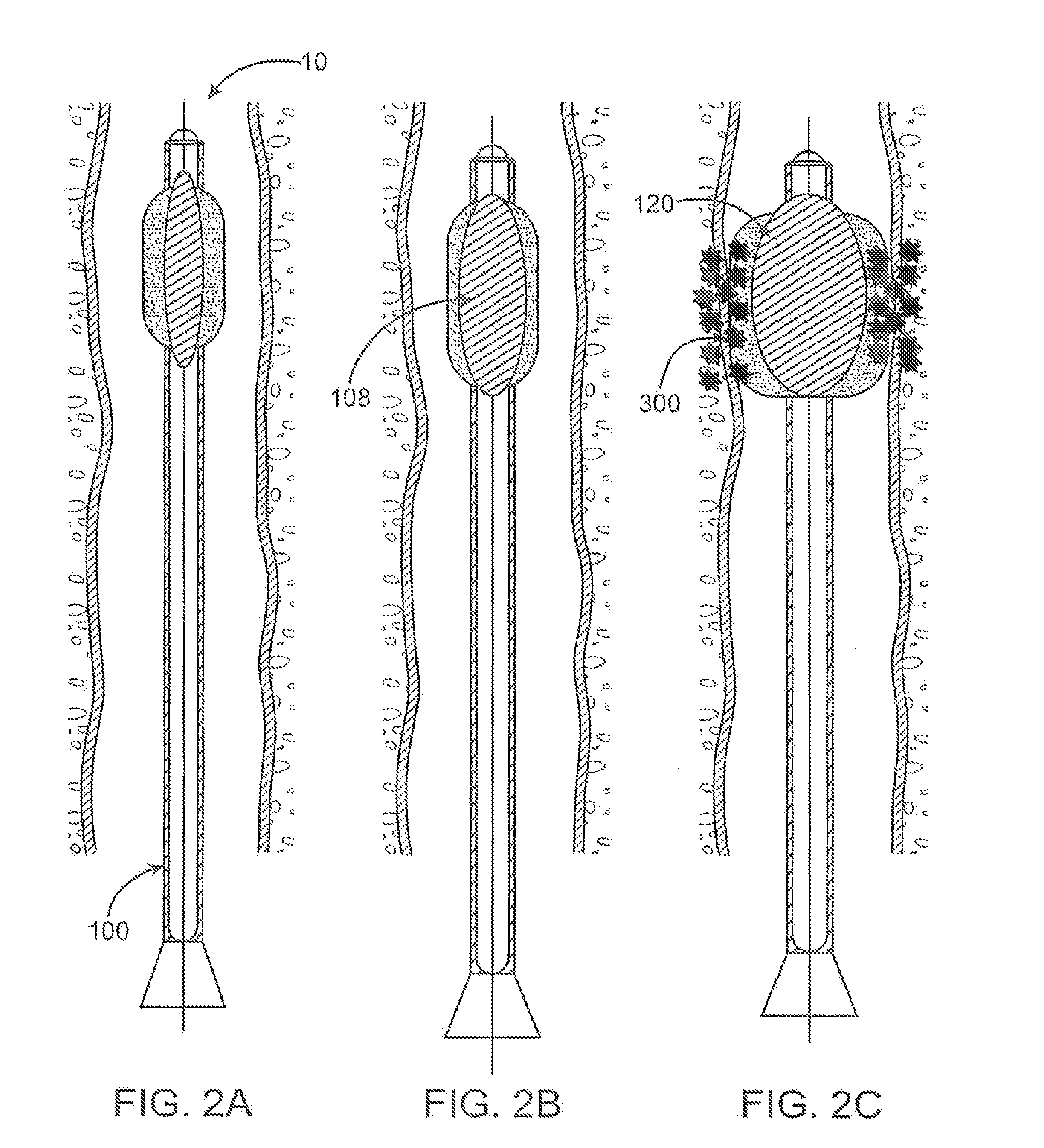
System for delivery of biologically active substances with actuating three dimensional surface
InactiveUS20080140002A1Reduce the overall diameterDelivery be eliminatedStentsBalloon catheterTissue augmentationThree dimensional surface
Tissue expanding and drug delivery systems with actuating three-dimensional surfaces are described for controlling the delivery and release of therapeutic agents against or upon tissue regions of interest. Such treatments devices and methods may include systems utilizing pores having various pore architectures to control the release of one or more drugs from an outer layer of an expandable delivery instrument, such as a balloon.
Owner:DIETCH LAURA N +2
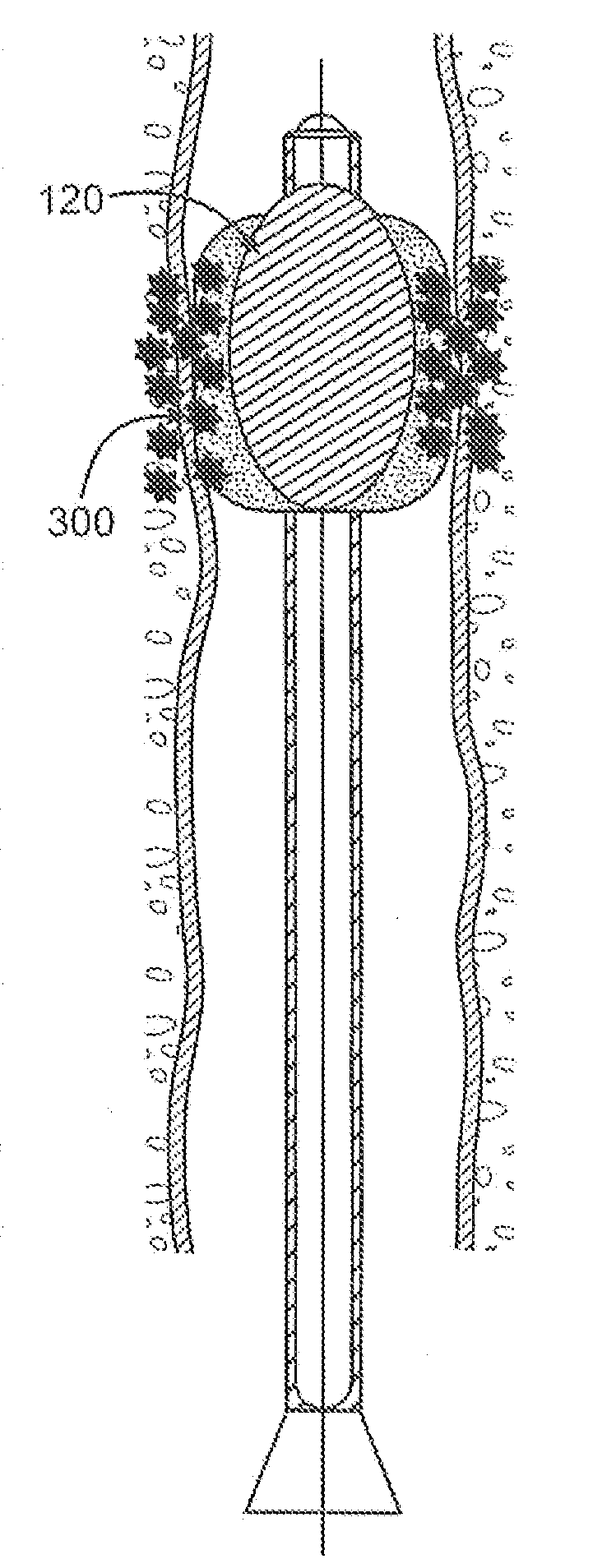
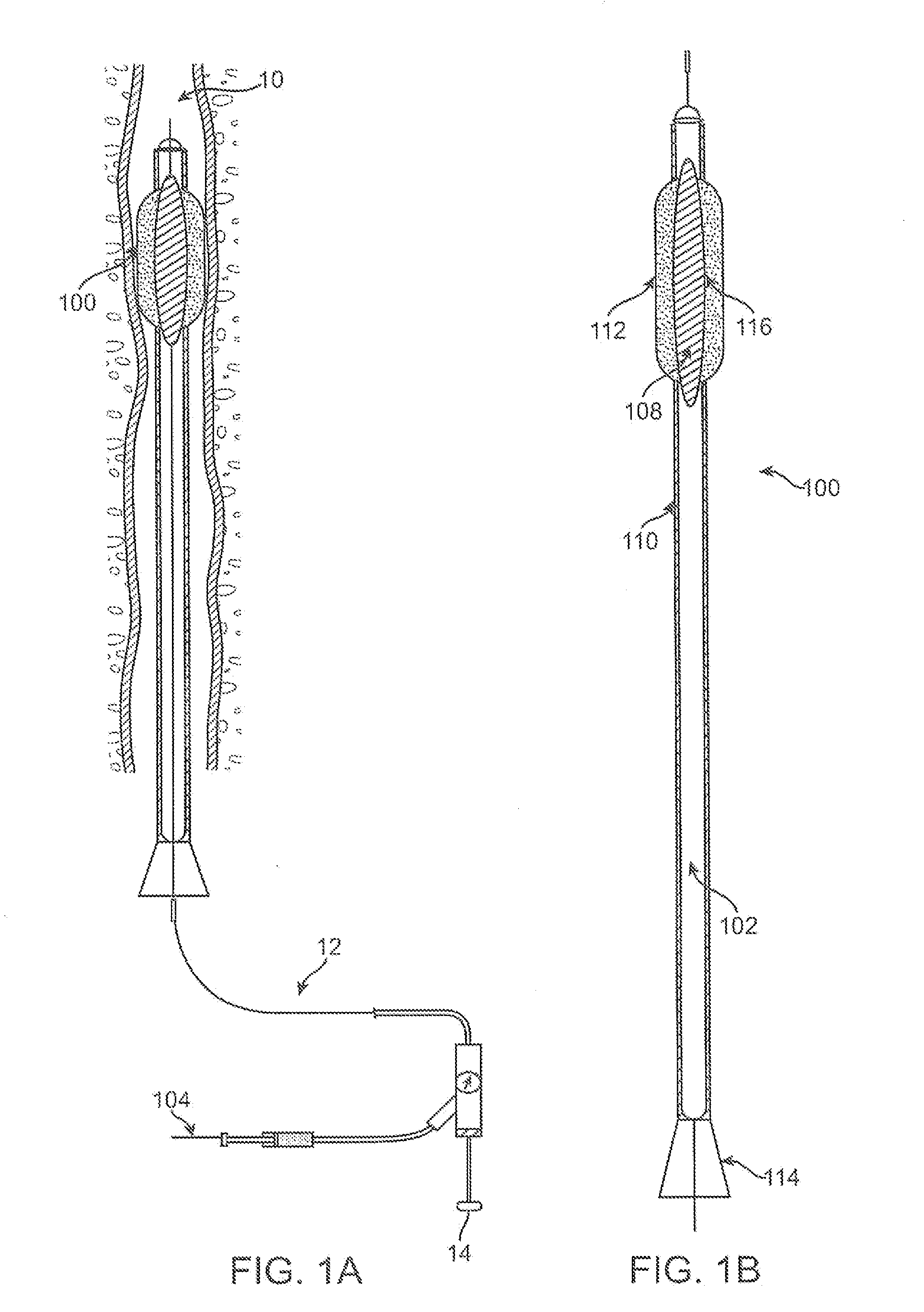
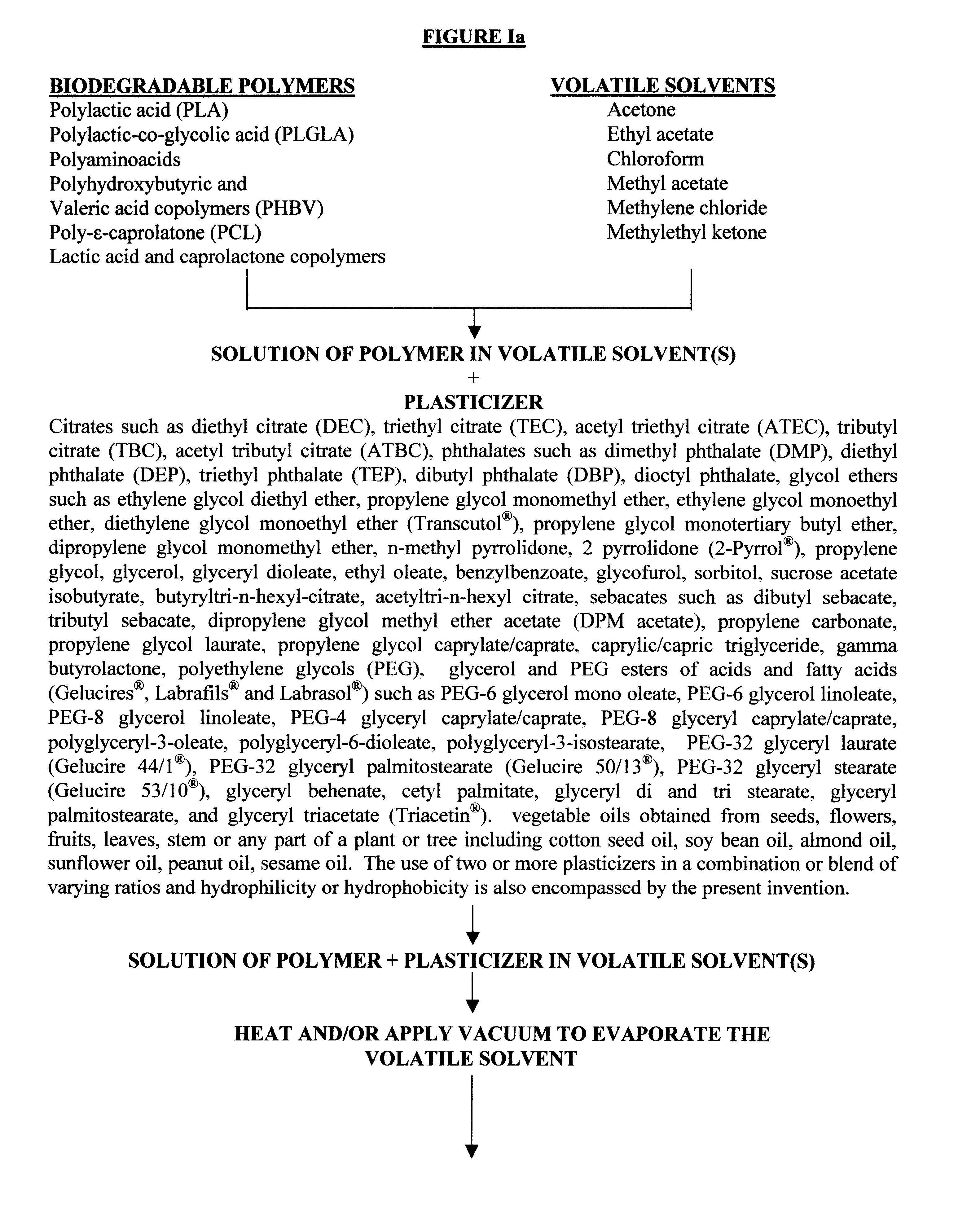
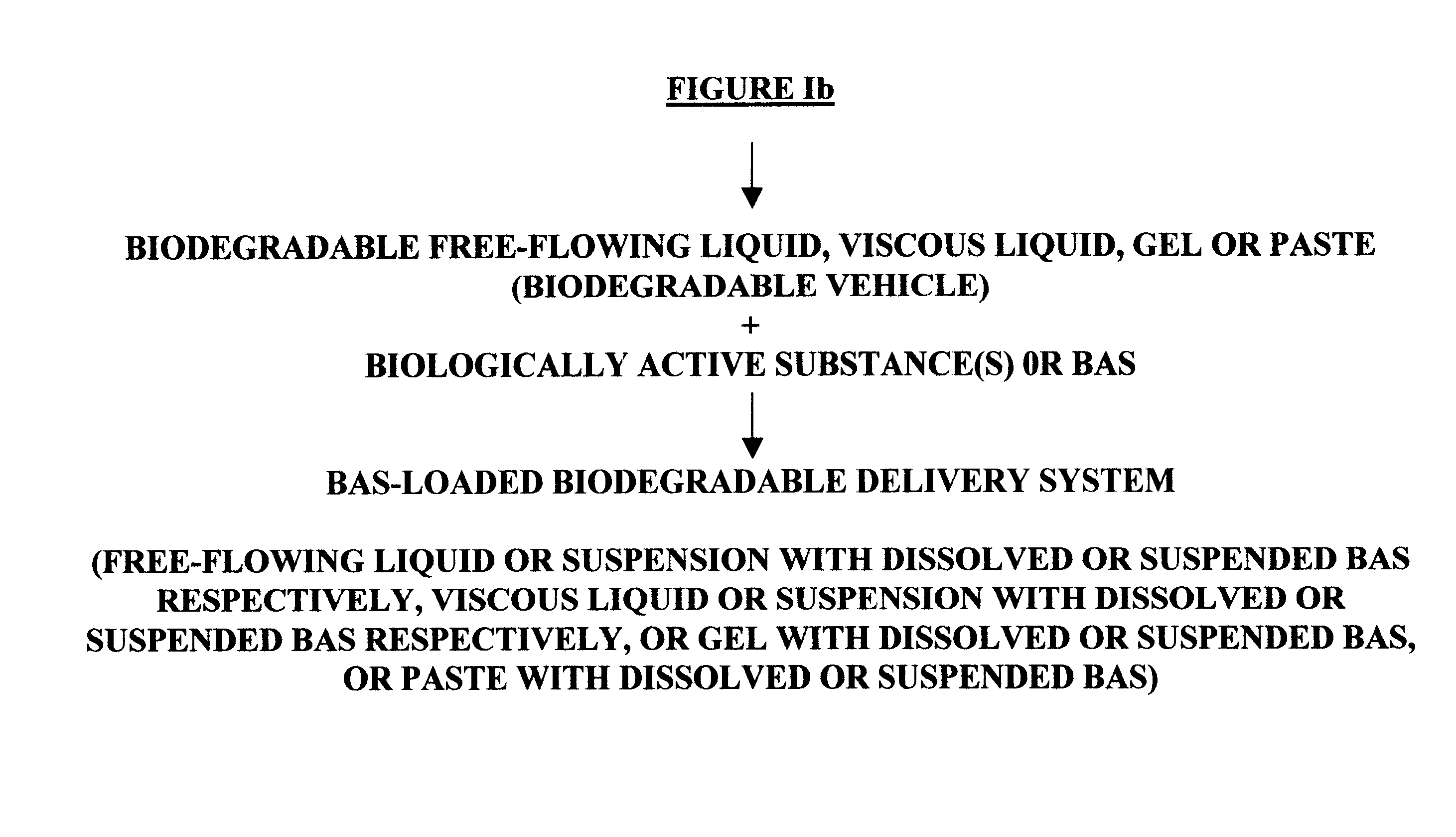
Biodegradable vehicle and filler
InactiveUS6432438B1Improve stabilityWell mixedOrganic active ingredientsPharmaceutical delivery mechanismPolymer sciencePlasticizer
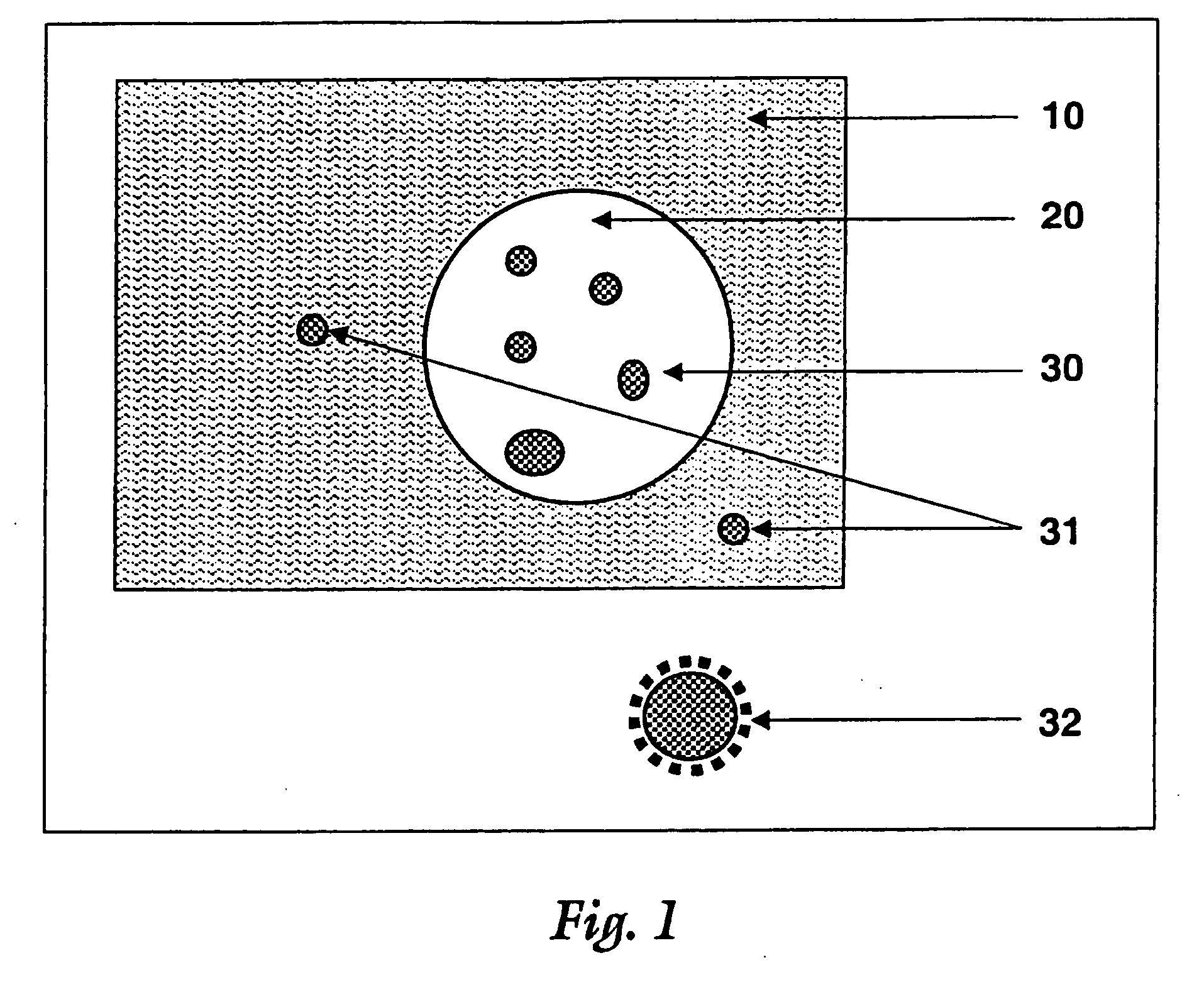
A biodegradable vehicle and filler (referred to in this invention as biodegradable vehicle), which can be mixed with one or more biologically active substances (BAS), or can be used as a biodegradable filler to fill in cavities or body tissues in animals, birds and humans. The consistency and rheology, hydrophilicity and hydrophobicity, and in vivo degradation rates of the biodegradable vehicle is controlled by modulating the molecular weight of polymers and copolymers, concentration of plasticizers, ratios of two or more plasticizer in the blends, types of polymers and copolymers, copolymer ratios, and ratios of blends of polymers with different molecular weights or different copolymers. The biodegradable vehicle is mixed with one or more BAS (which is separately stored away from the biodegradable vehicle in an appropriate container) just prior to use. Mixing of the BAS with the biodegradable vehicle can be accomplished by simply stirring the mixture with a stirring device, or by triturating the mixture or employing an ointment mill or a suitable device or apparatus or equipment that can be used for blending / mixing. Alternatively, a device, which resembles two syringes, attached together with a removable partition or a valve assembly can also be used to uniformly mix the BAS with the biodegradable vehicle. The mixing is performed in order to dissolve or uniformly suspend the BAS particles in the biodegradable vehicle. Modulating the polymer to plasticizer ratio, polymer molecular weight, copolymer ratio, and hydrophobicity and hydrophilicity of the plasticizer controls the release of the BAS from the biodegradable vehicle.
Owner:SHUKLA ATUL J
Apparatus and formulations for suprachoroidal drug delivery
InactiveUS20070202186A1Avoid traumaMinimally-invasive deliveryBiocidePowder deliveryPosterior regionPharmaceutical formulation
Drug formulations, devices and methods are provided to deliver biologically active substances to the eye. The formulations are delivered into scleral tissues adjacent to or into the suprachoroidal space without damage to the underlying choroid. One class of formulations is provided wherein the formulation is localized in the suprachoroidal space near the region into which it is administered. Another class of formulations is provided wherein the formulation can migrate to another region of the suprachoroidal space, thus allowing an injection in the anterior region of the eye in order to treat the posterior region.
Owner:CLEARSIDE BIOMEDICAL
Apparatus and method for flow electroporation of biological samples
ActiveUS20030059945A1Large populationOvercome limitationsBioreactor/fermenter combinationsBiological substance pretreatmentsBiologically active substancesPopulation
The present invention relates to methods and apparatus for the encapsulation of biologically-active substances in various cell populations. More particularly, the present invention relates to a method and apparatus for the encapsulation of biologically-active substances in various cell populations in blood by electroporation to achieve therapeutically desirable changes in the physical characteristics of the various cell populations in blood.
Owner:MAXCYTE
Deployable multifunctional hemostatic agent
InactiveUS20050123588A1Good hemostasisRapid and effective hemostasisSuture equipmentsPharmaceutical non-active ingredientsVeinMicrosphere
This invention relates to deployable hemostatic materials comprising chitosan fibers upon which hemostatic microporous polysaccharide microspheres and a medicament or biologically active substance are deposited. The hemostatic materials are suitable for use in controlling active bleeding from artery and vein lacerations, sealing femoral artery punctures, and controlling oozing from tissue.
Owner:LOMA LINDA UNIV MEDICAL CENT
Method of producing morphologically uniform microcapsules and microcapsules produced by this method
InactiveUS6294204B1Readily water-miscibleSpeed up concentrationPowder deliveryBiocidePolymer dissolutionEmulsion
The invention relates to a process for the production of morphologically uniform microcapsules that contain peptides, proteins or other water-soluble biologically active substances as active ingredients as well as microcapsules that are produced according to this process with a degree of concentration of between 3 to 30% by weight and a diameter<=8 mum.According to the invention, biodegradable polymers are dissolved in a halogen-free solvent or solvent mixture, and the buffered active ingredient solution, which has a pH of between 6.0 to 8.0, is dispersed into this solution. Then, an aqueous solution that contains a surface-active substance (W / O / W-emulsion) is added to this W / O-emulsion, and the solvent is removed.The microcapsules that are produced with this process do not show any tendency toward agglomeration. The encapsulation efficiency of the process is approximately 90 to 95%.
Owner:ALRISE BIOSYST
Biodegradable polymers, compositions, articles and methods for making and using the same
Biodegradable polymer compositions that degrade in vivo into non-toxic residues are described. In part, the present invention is directed to such polymers containing phosphorus and desaminotyrosyl L-tyrosine linkages in the polymer backbone. Processes for preparing such polymers, compositions containing such polymers and biologically active substances, articles useful for implantation or injection into the body fabricated from the compositions, and methods for controllably releasing biologically active substances using the polymers, are also described.
Owner:JOHNS HOPKINS UNIV SCHOOL OF MEDICINE
Injectable hyaluronic acid derivative with pharmaceuticals/cells
InactiveUS6699471B2Improve bioavailabilityPrevent further deteriorationBiocideOrganic active ingredientsCross-linkMicrosphere
Owner:ANIKA THERAPEUTICS SRL
Isolatable, water soluble, and hydrolytically stable active sulfones of poly(ethylene glycol) and related polymers for modification of surfaces and molecules
A poly(ethylene glycol) derivative is disclosed that is activated with a sulfone moiety for selective attachment to thiol moieties on molecules and surfaces. The activated PEG is water soluble, hydrolytically stable for extended periods, and forms hydrolytically stable linkages with thiol moieties. The linkages generally are not reversible in reducing environments. The PEG derivative is useful for modifying the characteristics of substances including modifying biologically active molecules and surfaces for biocompatibility. Methods for synthesizing the active PEG and for preparing conjugates of the active PEG and other substances, including biologically active substances, are also disclosed.
Owner:NEKTAR THERAPEUTICS INC
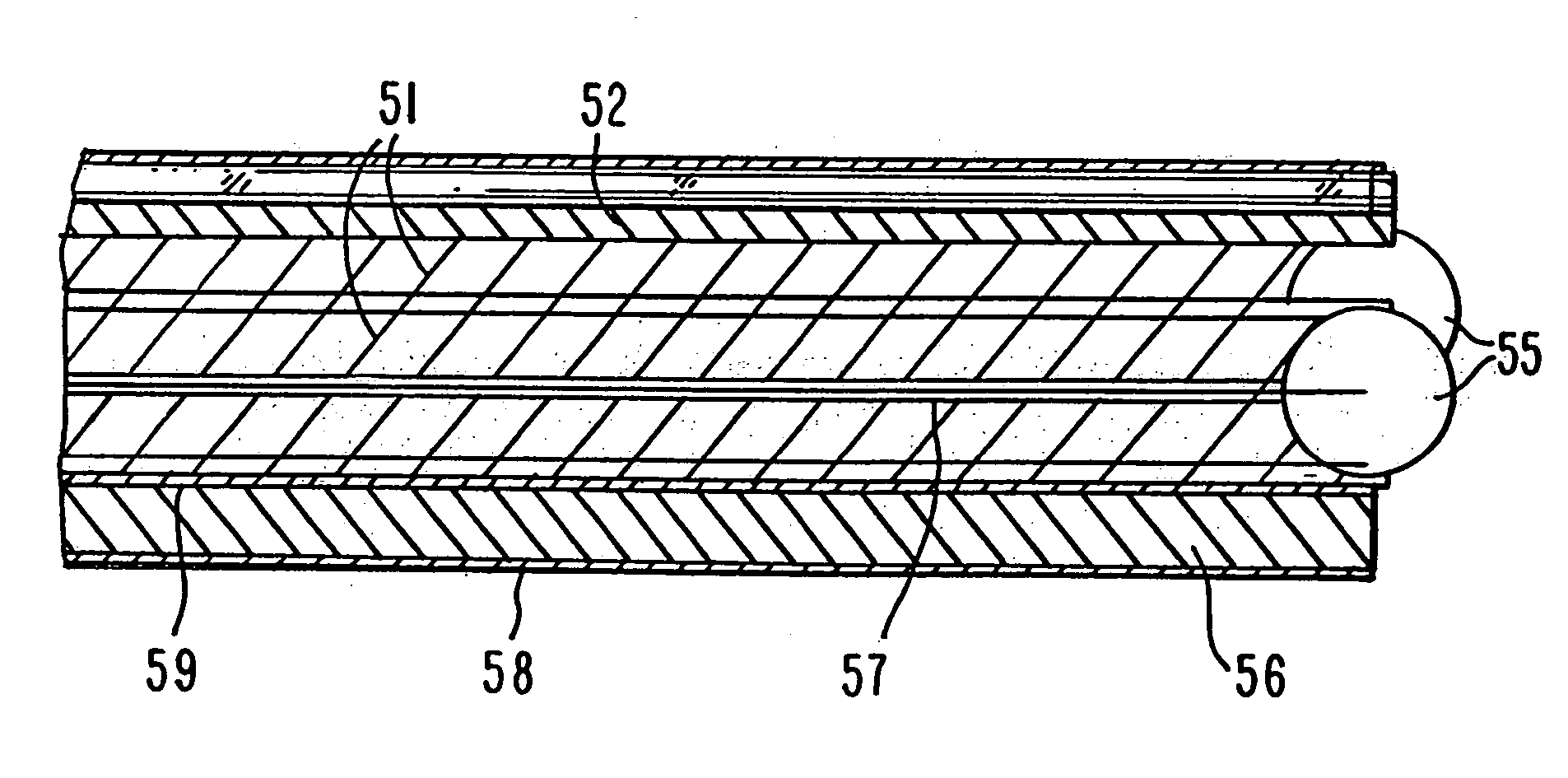
Minimally invasive surgery device
Provided is a surgical instrument comprising a node rotatably mounted within a restraining structure at the distal end of a shaft, The node can be rotated allowing manipulation and orientation of a surgical tool extending from the node at the distal end of the shaft through control remote from the distal end of the shaft. Cameras may also be located at the distal end of the shaft allowing stereoscopic imaging to be conveyed to an operator. The surgical instrument is well suited for administering biologically active substances to a desired location. The method of administration comprises insertion of a flexible shaft comprising a channel, controlling the location of the distal end of the shaft through control cables within the shaft, and projecting the biologically active substance from the end of the channel at the desired location.
Owner:STELZER PAUL +1
Medical implant
PCT No. PCT / EP96 / 05506 Sec. 371 Date Aug. 12, 1998 Sec. 102(e) Date Aug. 12, 1998 PCT Filed Dec. 10, 1996 PCT Pub. No. WO97 / 22308 PCT Pub. Date Jun. 26, 1997The invention relates to a medical implant, in particular a dental implant, intended for implantation in available cavities. Dental implants are implanted in extraction sockets. The implant is provided with reservoirs for a biologically active substance. An advantage of the implant is that, for dental procedures, it can be manufactured and implanted as part of a single therapeutic treatment. However, the implant can also be used as a release system for biologically active substances.
Owner:DR JOUKO SUHONEN +1
Apparatus and method for electroporation of biological samples
InactiveUS7141425B2Simple and efficientBioreactor/fermenter combinationsElectrotherapyElectroporationBiochemistry
The present invention relates to methods and apparatus for the encapsulation of biologically-active substances in various cell populations. More particularly, the present invention relates to a method and apparatus for the encapsulation of biologically-active substances in various cell populations in blood by electroporation to achieve therapeutically desirable changes in the physical characteristics of the various cell populations in blood.
Owner:MAXCYTE
Multi-functional polymeric materials and their uses
InactiveUS20050169882A1Easy to controlReduce deliveryPowder deliveryMaterial nanotechnologyBiomedical engineeringPolymer
Multifunctional polymers are disclosed having a smart segment and a biodegradable segment. Advantageously, the biodegradable segment includes a hydrophilic segment and a hydrophobic segment. Embodiments include combining the multifunctional polymeric material with a biologically active substance in an aqueous loading environment and administering the composition as a drug delivery vehicle to a human subject.
Owner:HUANG XIAO +2
Apparatus and method for flow electroporation of biological samples
InactiveUS7029916B2Overcome limitationsBioreactor/fermenter combinationsBiological substance pretreatmentsElectroporationBiochemistry
The present invention relates to methods and apparatus for the encapsulation of biologically-active substances in various cell populations. More particularly, the present invention relates to a method and apparatus for the encapsulation of biologically-active substances in various cell populations in blood by electroporation to achieve therapeutically desirable changes in the physical characteristics of the various cell populations in blood.
Owner:MAXCYTE
Biodegradable and Thermosensitive Poly(Organophosphazene) Hydrogel, Preparation Method Thereof and Use Thereof
The present invention relates to a biodegradable and thermosensitive poly(organophosphazene) with a functional group, a preparation method thereof, and a use thereof for delivery of bioactive substances. According to the present invention, poly(organophosphazene) is a phosphagen-based polymer showing biodegradability, thermosensitivity, and sol-gel phase transition depending on temperature change, whereby when administered into a living body with bioactive substances such as drugs, the poly(organophosphazene) forms a gel-phase at body temperature to be capable of controlled release of the bioactive substances. Further, the poly(organophosphazene) has functional groups to chemically bind with bioactive substances through an ionic bond, covalent bond, or coordinate covalent bond to be capable of a sustained release of the bioactive substances due to its good binding property. Therefore, the poly(organophosphazene) is useful as a delivery material for bioactive substances.
Owner:KOREA INST OF SCI & TECH
Pharmaceutical and cosmetic carrier or composition for topical application
InactiveUS20040253275A1Antibacterial agentsOrganic active ingredientsPharmaceutical SubstancesHydrophobe
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Surface modified particulate compositions of biologically active substances
InactiveUS20060210622A1Easy to useEfficient deliveryPowder deliveryOrganic active ingredientsParticulatesWater insoluble
This invention disclosure relates to compositions for the delivery of stable surface modified sub-micron and micron sized particles of water-insoluble biologically active substances from a non-aqueous medium that self-disperses on exposure to an aqueous environment.
Owner:SKYEPHARMA CANADA INC
Microparticle preparation
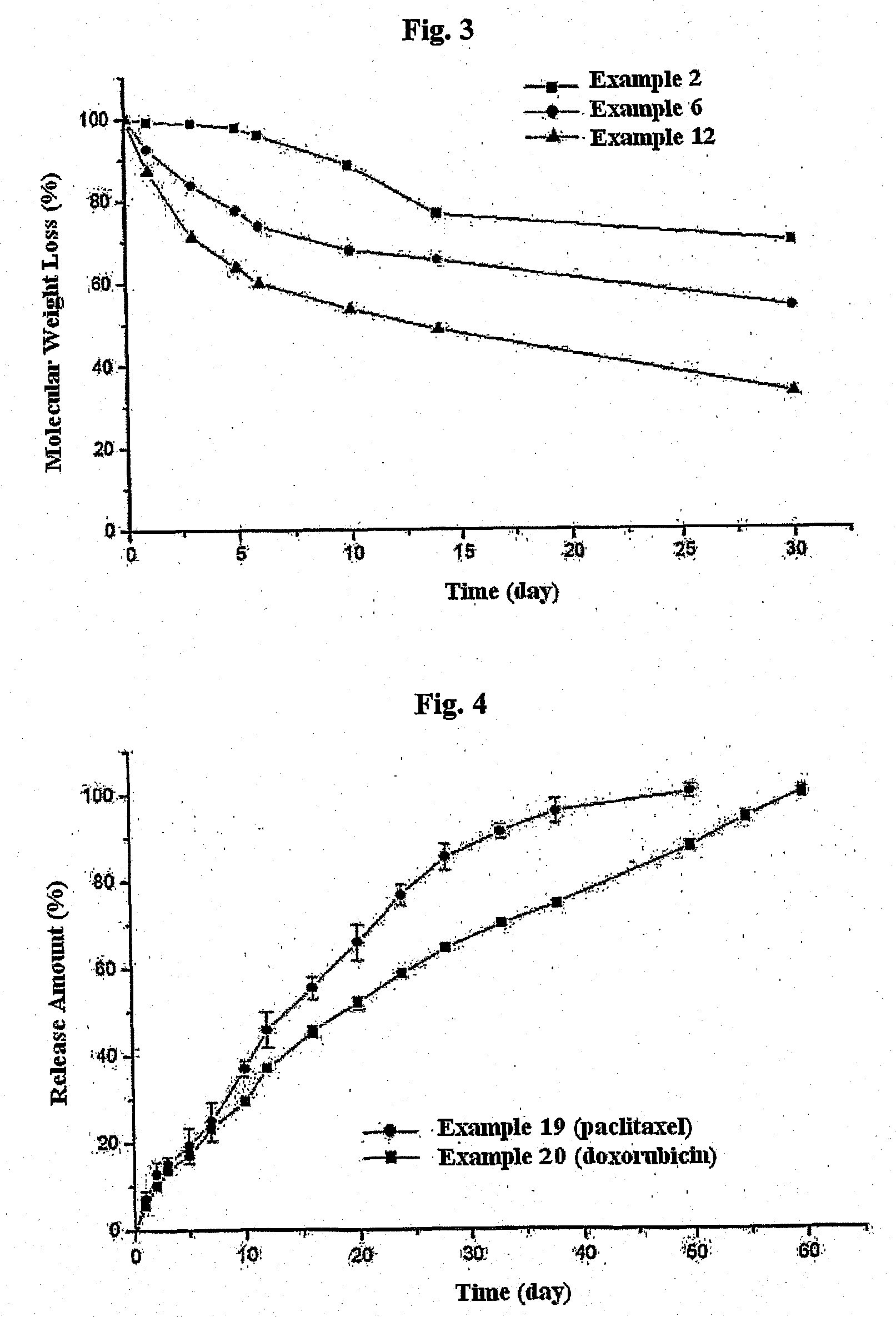
A parenterally administrable, biodegradable microparticle preparation containing a biologically active substance which, during the first 24 hours after injection, exhibits a release of the active substance that is less than 25% of the total release, determined from a concentration-time curve in the form of the ratio between the area under the curve during the said first 24 hours and the total area under the curve in question
Owner:PACIRA PHARMA INC
Dermatological application with solidified fat compositions
InactiveUS20030157138A1Antibacterial agentsEdible oils/fats ingredientsFirming agentCosmetic vehicle
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Energetically controlled delivery of biologically active material from an implanted medical device
An implantable medical assembly that contains a substrate, and nanomagnetic material and a therapeutic agent located over the substrate. A barrier is located between the therapeutic agent and biological material. When the assembly is exposed to electromagnetic radiation, the barrier between the biological material and the therapeutic agent is removed.
Owner:BIOPHAN TECH
Biologically active molecules having thiol moiety conjugated to polymers containing ethyl sulfone moiety
InactiveUS6894025B2Stable waterSpecific reactivityBiocidePeptide/protein ingredientsThiolBiocompatibility Testing
A poly(ethylene glycol) derivative is disclosed that is activated with a sulfone moiety for selective attachment to thiol moieties on molecules and surfaces. The activated PEG is water soluble, hydrolytically stable for extended periods, and forms hydrolytically stable linkages with thiol moieties. The linkages generally are not reversible in reducing environments. The PEG derivative is useful for modifying the characteristics of substances including modifying biologically active molecules and surfaces for biocompatibility. Methods for synthesizing the active PEG and for preparing conjugates of the active PEG and other substances, including biologically active substances, are also disclosed.
Owner:NEKTAR THERAPEUTICS INC
Pharmaceutical compositions based on a microemulsion
InactiveUS20100034880A1Efficient deliveryIncrease concentrationOrganic active ingredientsPowder deliveryPropylene carbonateBiological membrane
The invention provides a transdermal, transmucosal pharmaceutical composition suitable for substantially extra-vascular application of at least one biologically active substance to biological membranes of a mammal, comprising a pharmaceutical or cosmetic composition comprising propylene carbonate at least one oil or source of fatty acid or surfactant; and water; in combination with the at least one biologically active substance wherein the propylene carbonate is adapted to enhance the bioavailability of the at least one biologically active substance.
Owner:NANODERMA
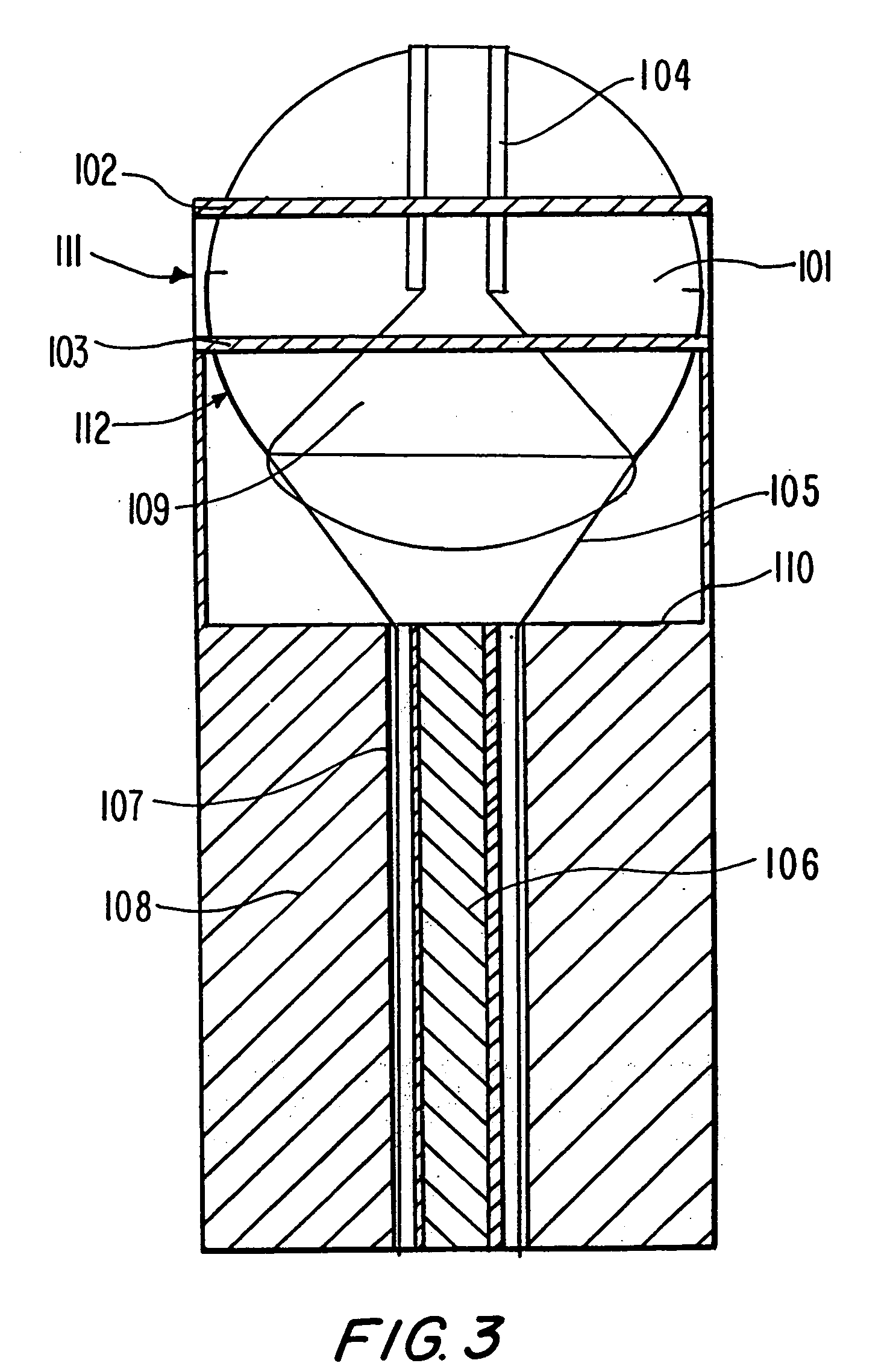
Photokinetic ocular drug delivery methods and apparatus
The present invention relates generally to transscleral, transcorneal, and transocular delivery of biologically active substances through the tissues, blood vessels and cellular membranes of the eyes of patients without causing damage to the cellular surface, tissue or membrane. The invention provides compositions and methods for enhanced transscleral, transcorneal and transocular delivery of biologically active substances using pulsed incoherent light, and particularly the transcleral, transcorneal or transocular delivery of high molecular weight biologically active substances to a patient using pulsed incoherent light. The invention further provides a device for the application of the pulsed incoherent light to cellular surfaces and membranes of the eye of a subject using those compositions and methods.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Apparatus and formulations for suprachoridal drug delivery
InactiveUS20130245600A1Minimally-invasive deliveryAvoid traumaPowder deliveryOrganic active ingredientsPosterior regionPharmaceutical formulation
Drug formulations, devices and methods are provided to deliver biologically active substances to the eye. The formulations are delivered into scleral tissues adjacent to or into the suprachoroidal space without damage to the underlying choroid. One class of formulations is provided wherein the formulation is localized in the suprachoroidal space near the region into which it is administered. Another class of formulations is provided wherein the formulation can migrate to another region of the suprachoroidal space, thus allowing an injection in the anterior region of the eye in order to treat the posterior region.
Owner:CLEARSIDE BIOMEDICAL
Oral liposomal delivery system
A liposome-capsule dosage unit system for the delivery of a biologically active material is formed by encapsulating a biologically active materials in liposomes and then placing the liposome encapsulated material into a capsule. The capsule is typically a soft gel capsule or a two piece capsule capable of tolerating a certain amount of water. A less water tolerant capsule can be employed if the liposomes are dehydrated prior to placement within the capsule. Biologically active material include drugs, nutritional supplements, vitamins, minerals, enzymes, hormones, proteins and polypeptides. The system is especially suited for the delivery of materials with poor oral solubility, materials that are not absorbed or are poorly absorbed from the gastrointestinal tract, and materials that have conventionally been given by an invasive route. The system can be administered orally, intra-occularly, intranasally, rectally, or vaginally.
Owner:BIOZONE LAB
Isolated population of plant single cells and method of preparing the same
ActiveUS8053238B2Stable productionAvoid excessive changesFermentationPlant cellsPlant SourcesCell growth
This invention is a method of minimizing the variation of cell growth and production through homogeneous cell line development. To be more specific, it is the method of isolating and proliferating single cell clone from the procambium to promote the stability of the plant-derived biologically active substances production by solving the problems of decrease in cell growth and the productivity during the long term culture.
Owner:WELLKEY HLDG LTD
Delivery of large molecular weight biologically active substances
The invention relates generally to intradermal, transdermal, and / or transmembrane delivery of biologically active substances in the epidermis and / or through the skin, sub-dermal tissues, blood vessels and cellular membranes without causing damage to the cellular surface, tissue or membrane. The biologically active substances may have a molecular weight no less than about 5.8 kDa to about 2,500 kDa, such as Hyaluronic Acid (HA). The biologically active substances may be deposited in a dermal patch containing a red algae polysaccharide-based matrix, wherein the red algae polysaccharide is an extract of Chondrus crispus at 2% by weight of the dermal patch. The invention provides systems and methods for enhanced intradermal, transdermal, and / or transmembrane delivery of such biologically active substances using pulsed incoherent light. The invention further provides a device for the application of the pulsed incoherent light to cellular surfaces and membranes using those compositions and methods.
Owner:PHOTOKINETIX HLDG
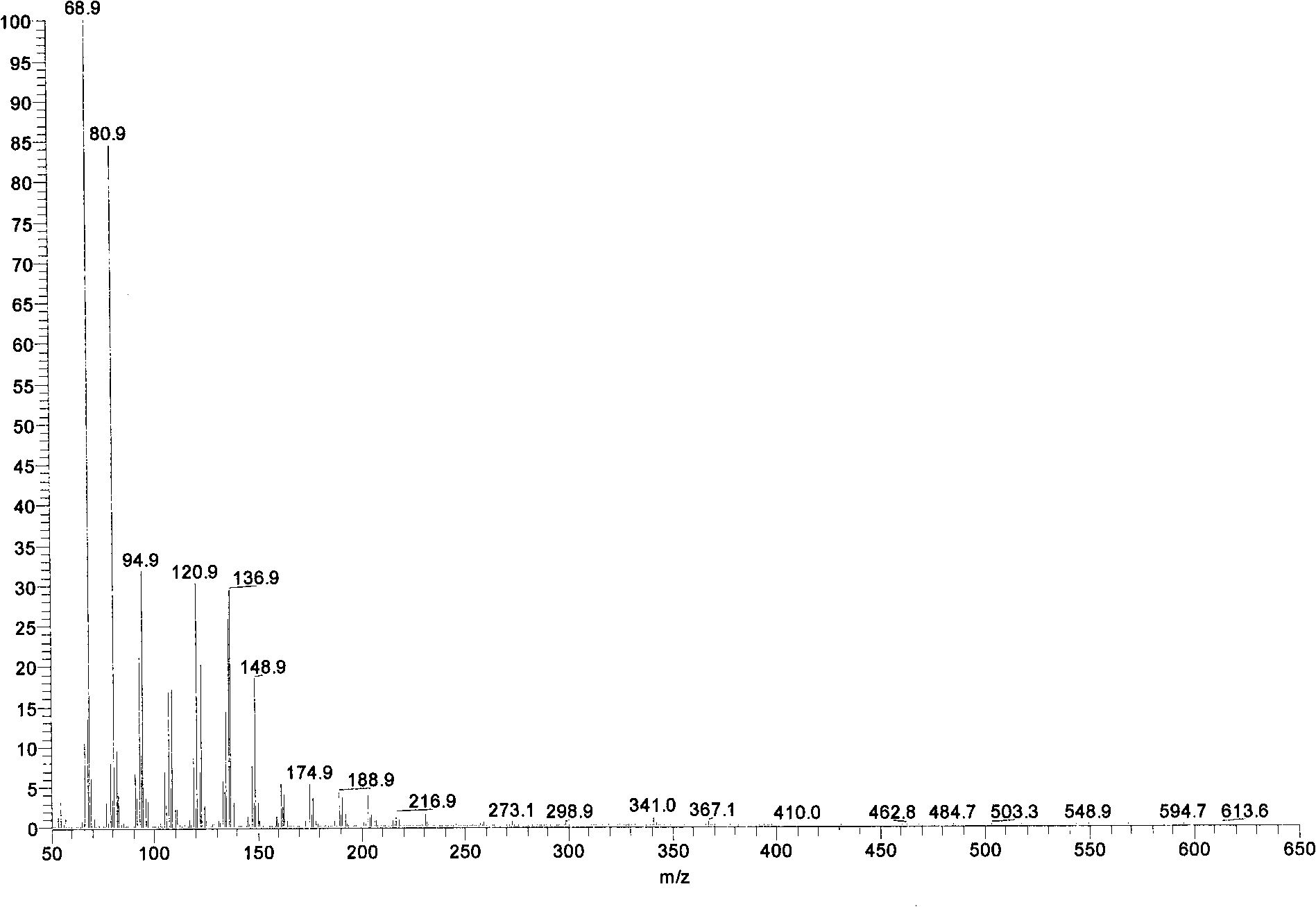
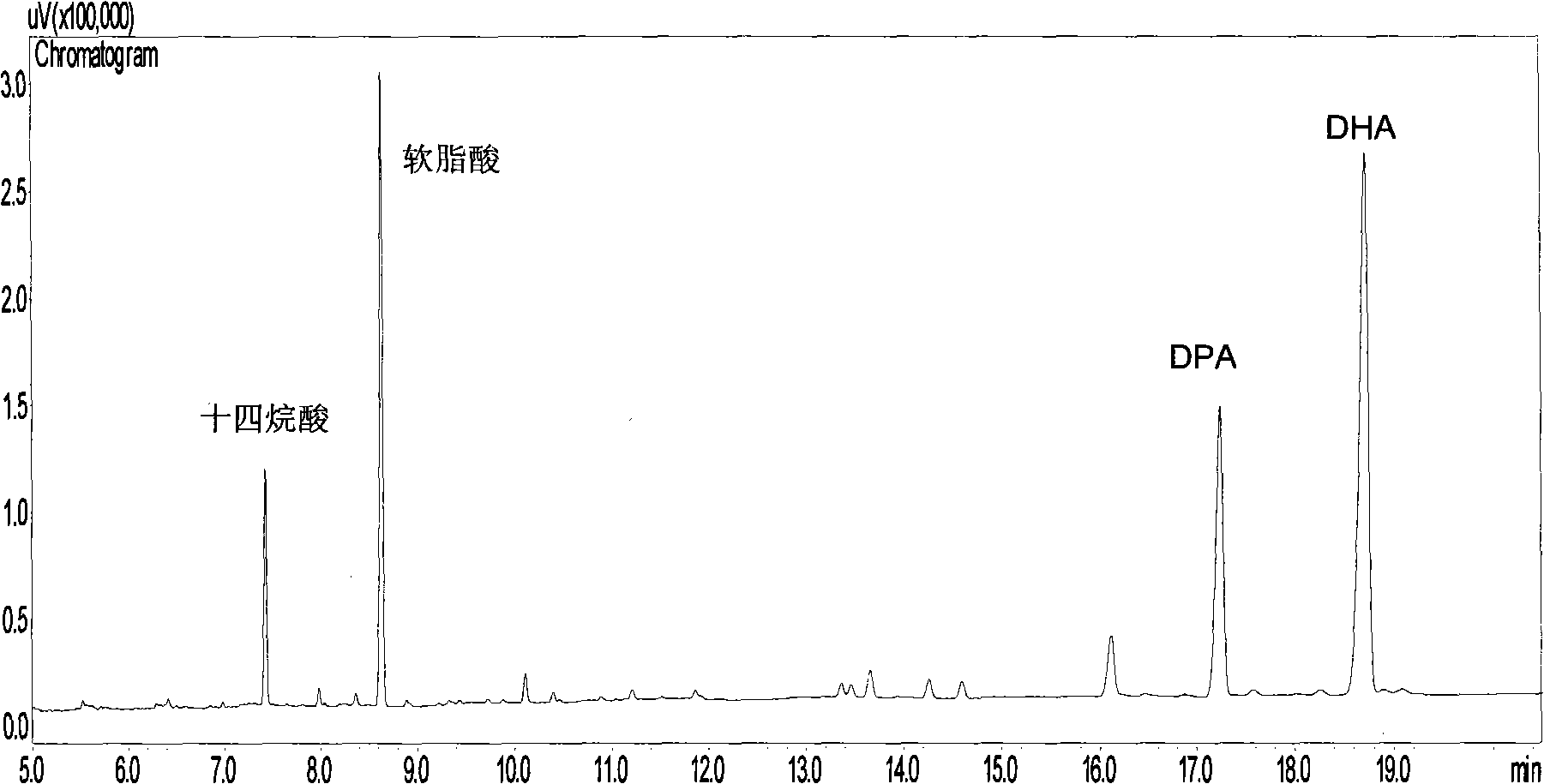
Schizochytrium sp. and method for producing DHA lipa by using same
ActiveCN101575584AIncrease biomassHigh in DHABacteriaMicroorganism based processesFood additiveSchizochytrium sp.
The invention discloses a Schizochytrium sp., which has a category name of Schizochytrium sp., is preserved in Common Microorganisms Center of China Committee for Culture Collection of Microorganisms and has a preservation number of CCTCC No.209059. The invention also discloses a method for producing DHA lipa by using the Schizochytrium sp. The method optimizes a strain fermentation medium from the perspectives of osmotic pressure and element supply and combines a fed-batch strategy to achieve high-density fermentation of microalgae, thus the final dry cell weight reaches 70 grams per liter, the lipa content reaches 31.5 grams per liter, and the DHA accounts for more than 35 percent of the total fatty acid content. All indexes of the DHA essential oil obtained by the method accord with the standard of food additives, and the DHA essential oil contains a bioactive substance of squalene. The method has the advantages of simple entire process, convenient operation, and high biomass and DHA content, reduces the fermentation cost, and is suitable for industrial production.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com