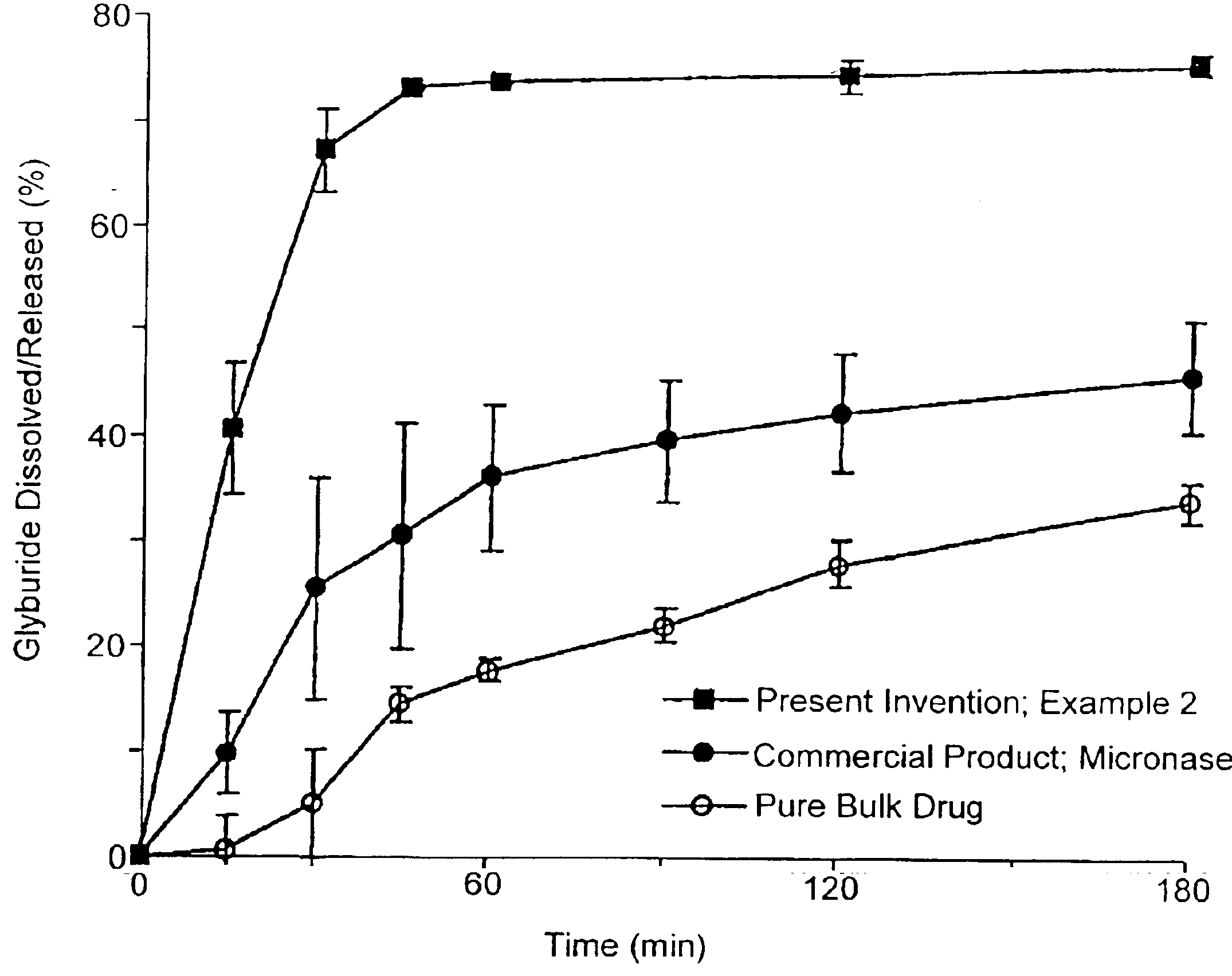
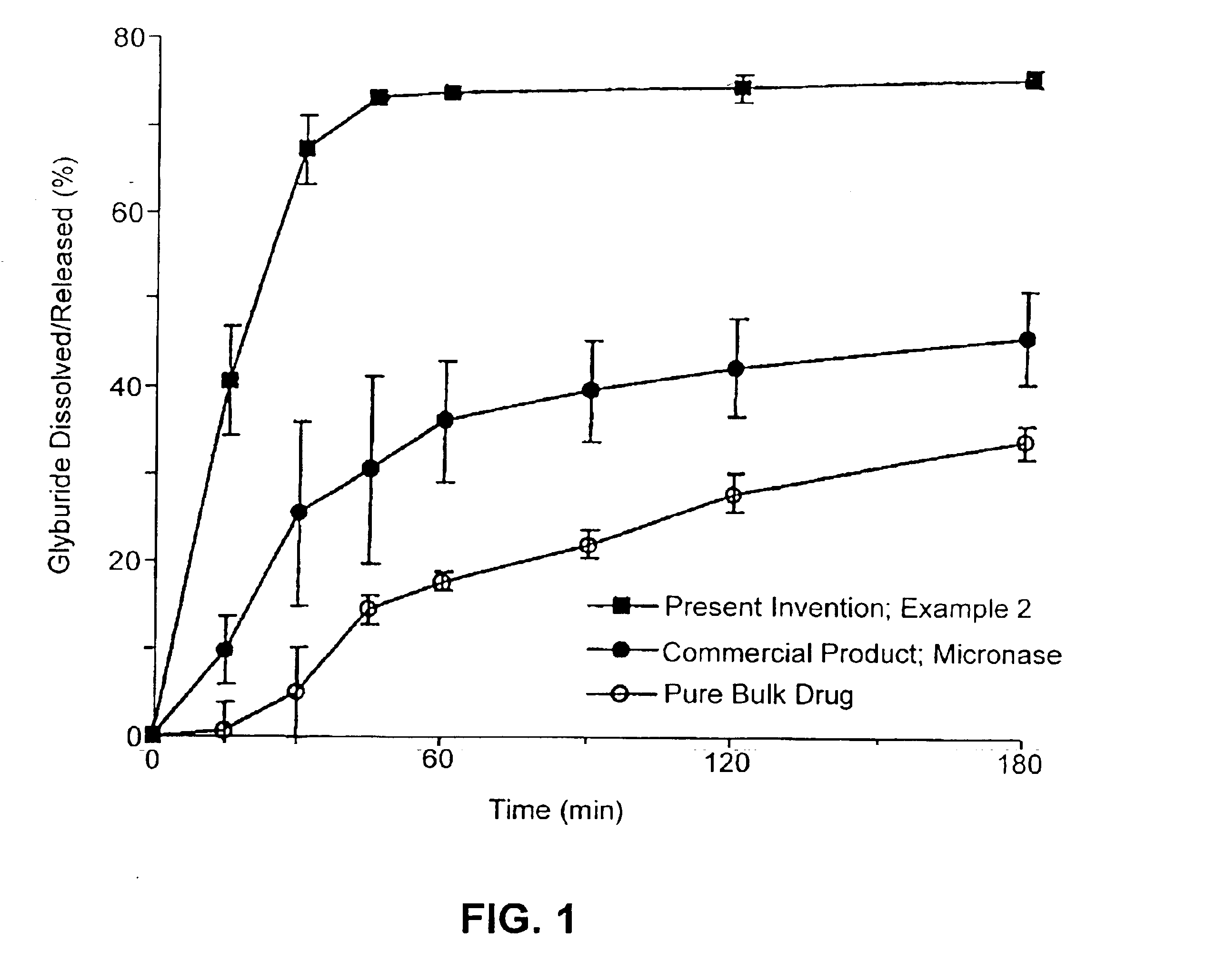
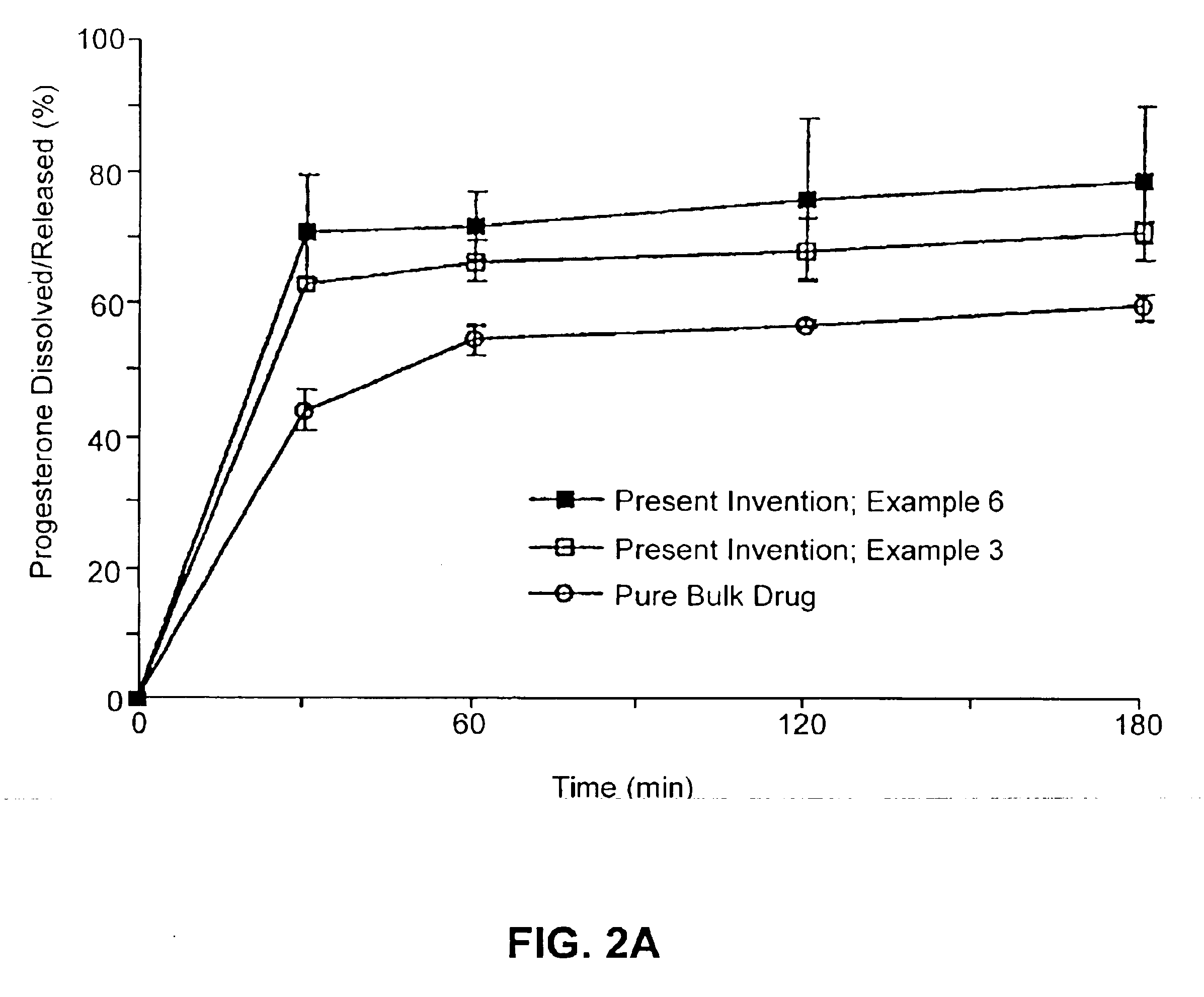
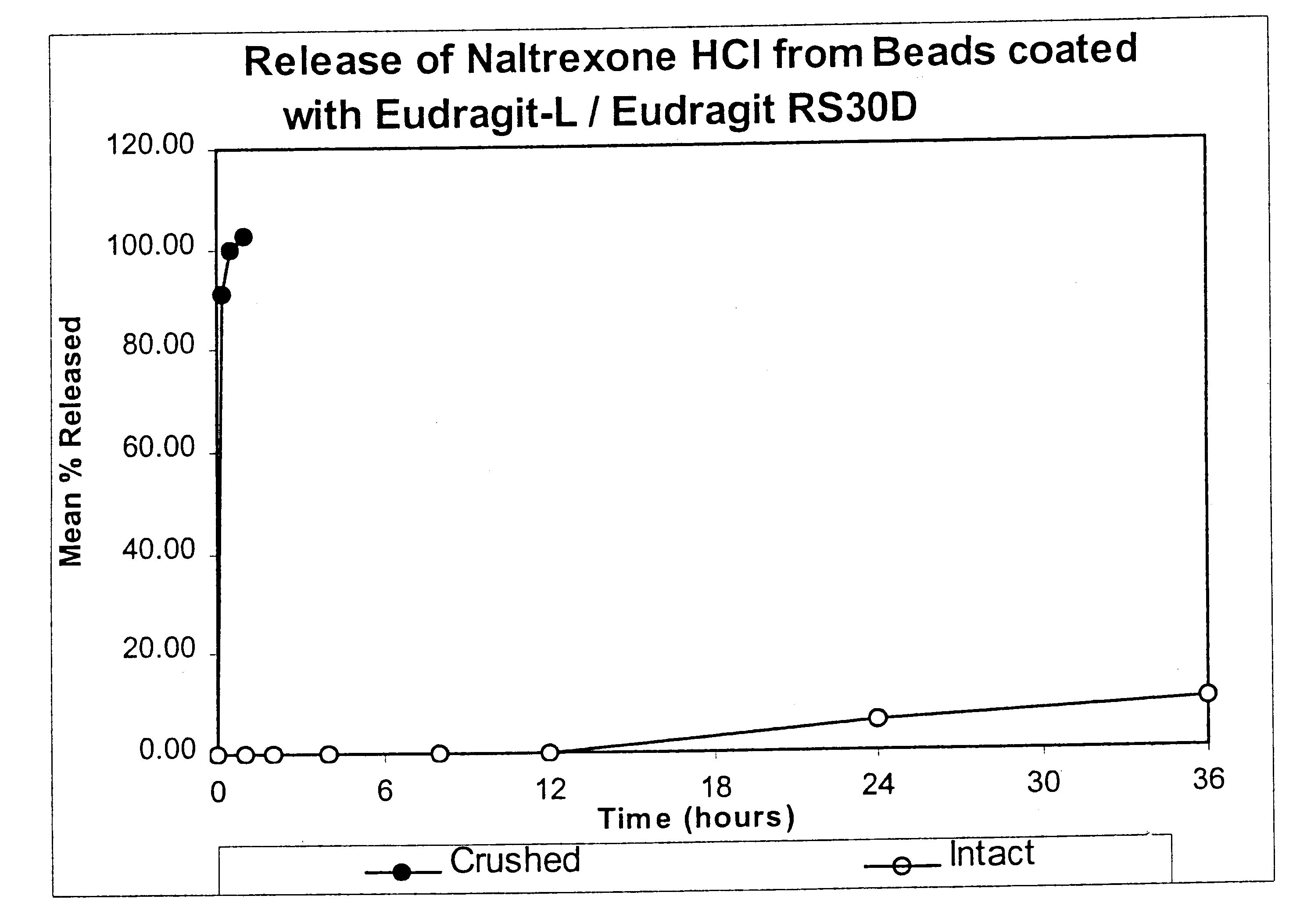
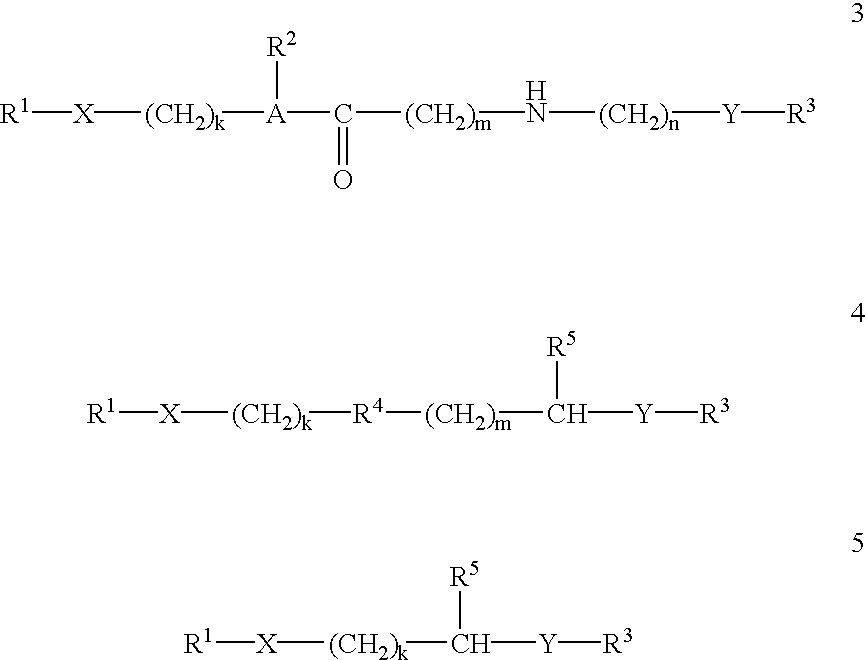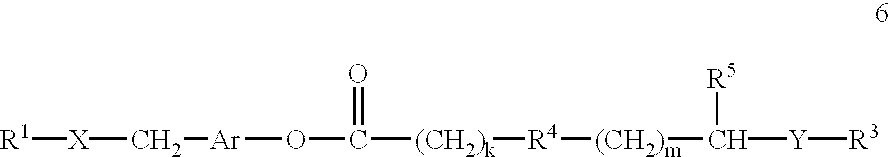Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2111results about "Suppositories delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
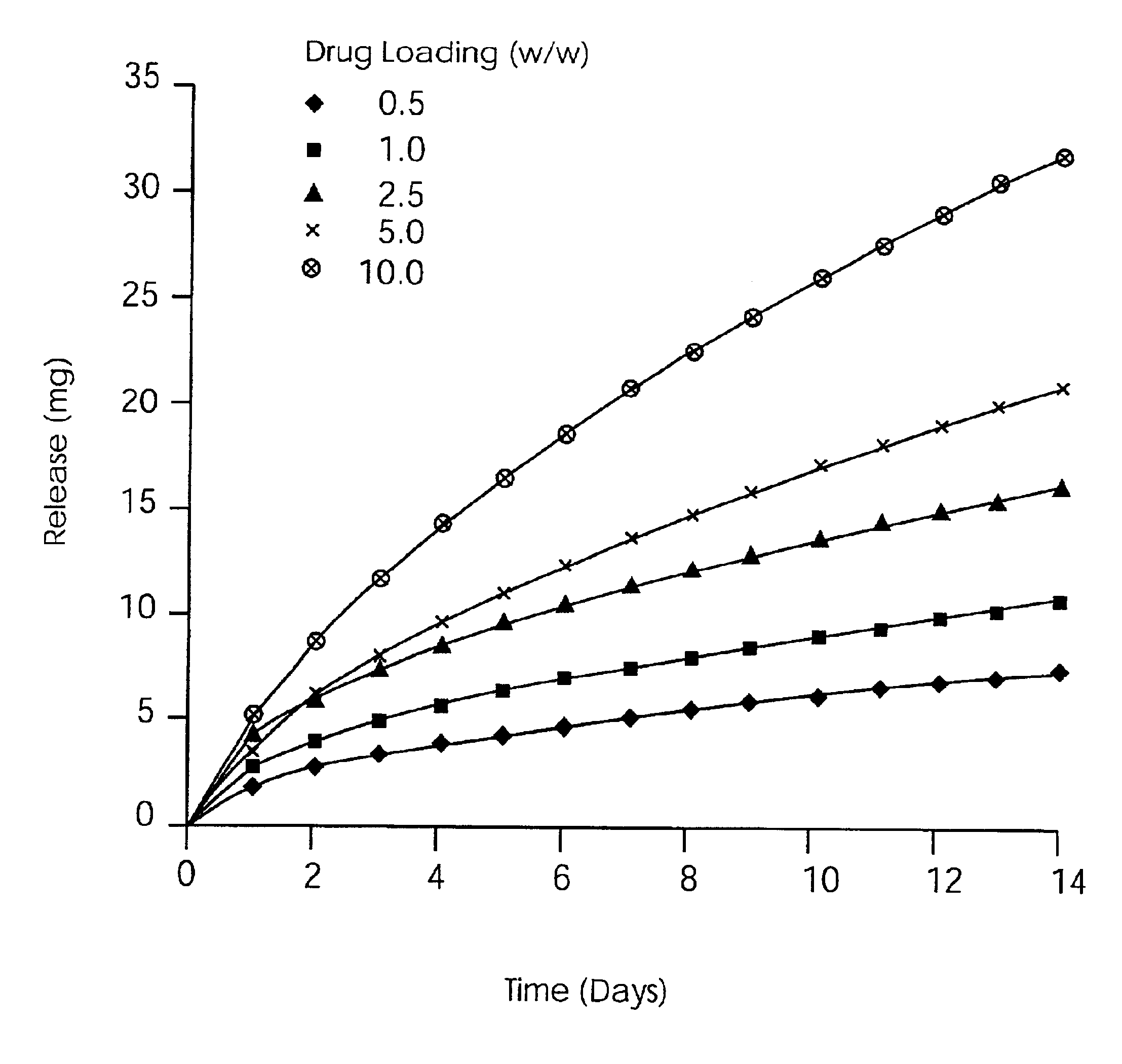
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
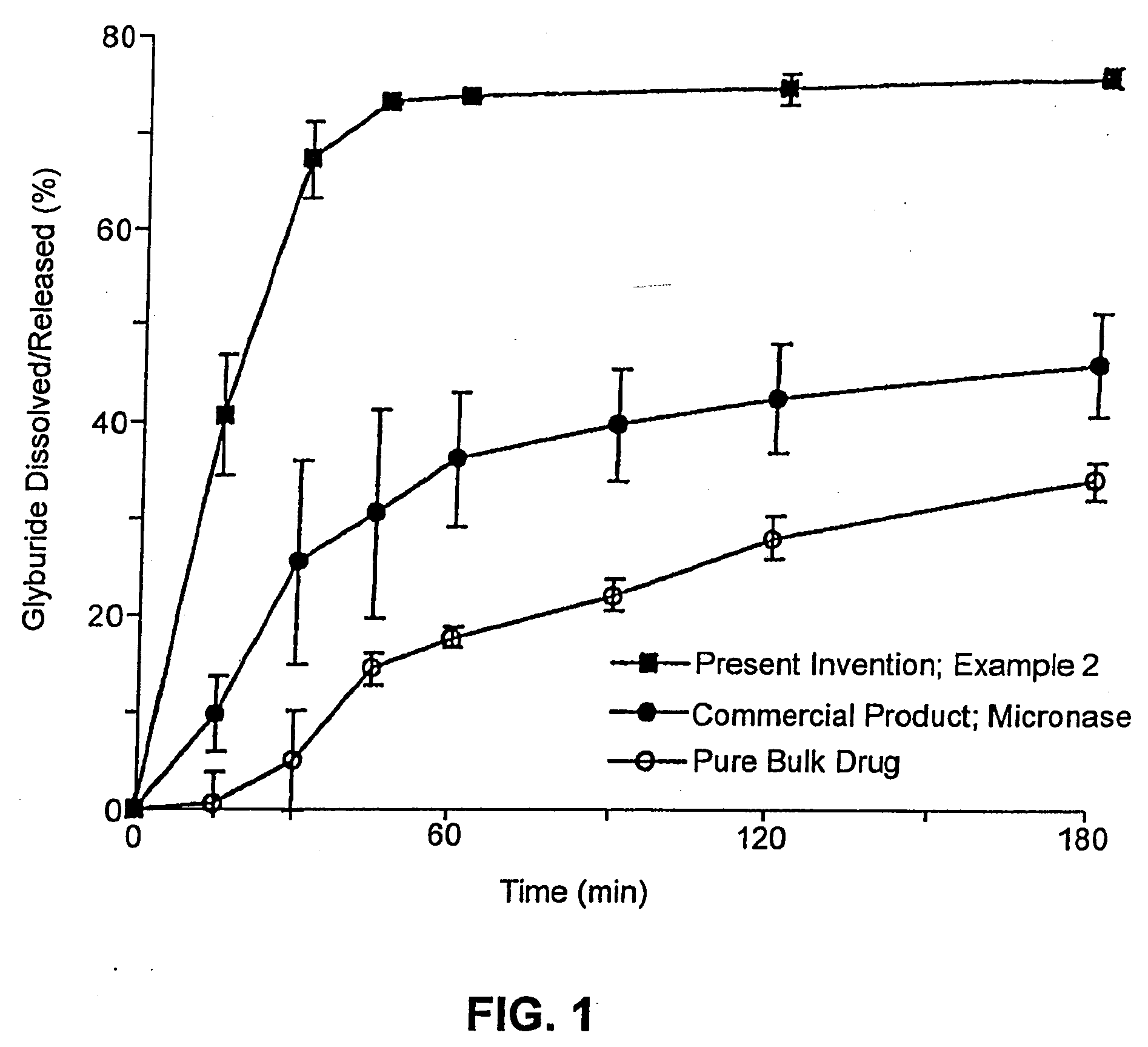
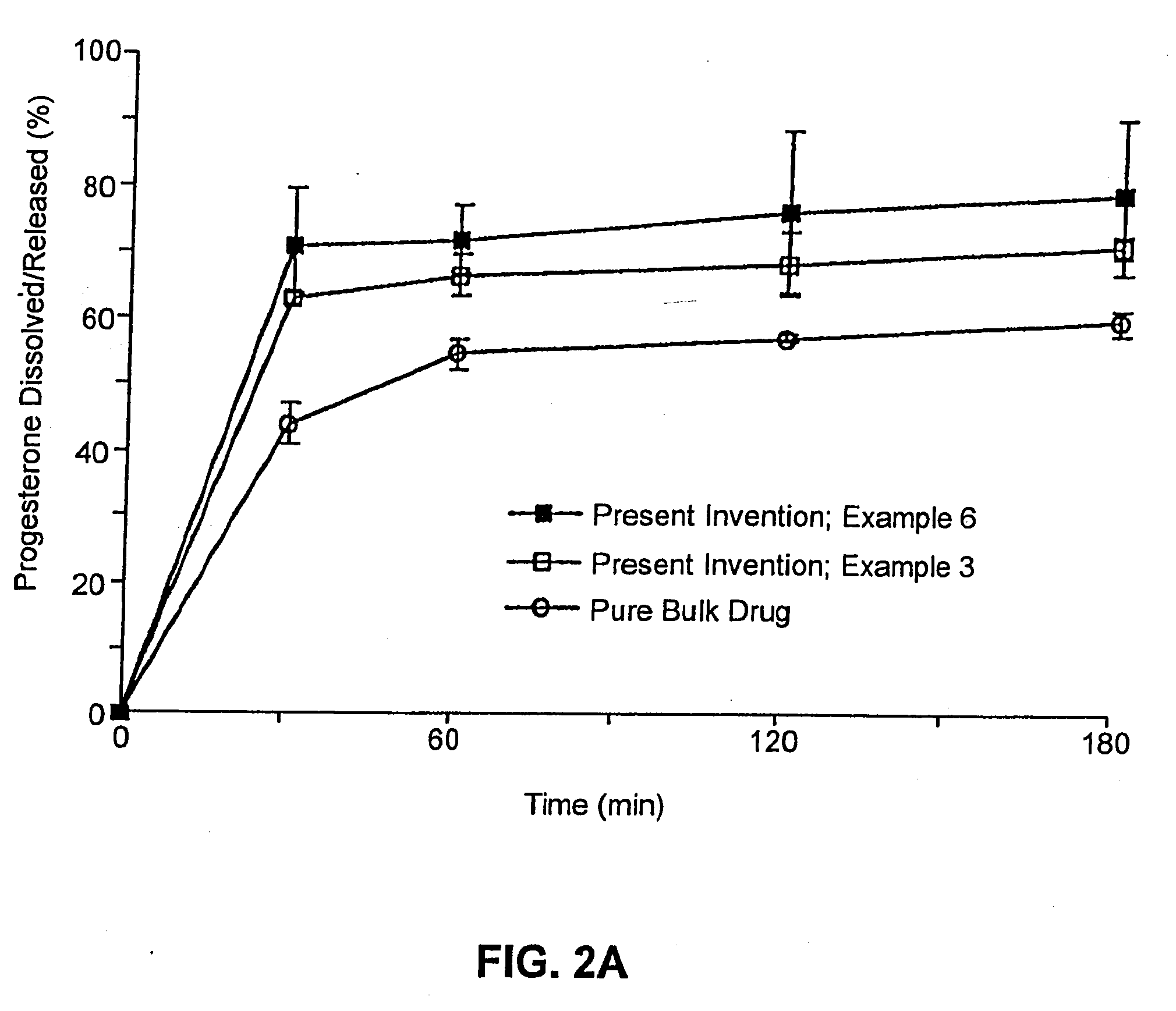
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
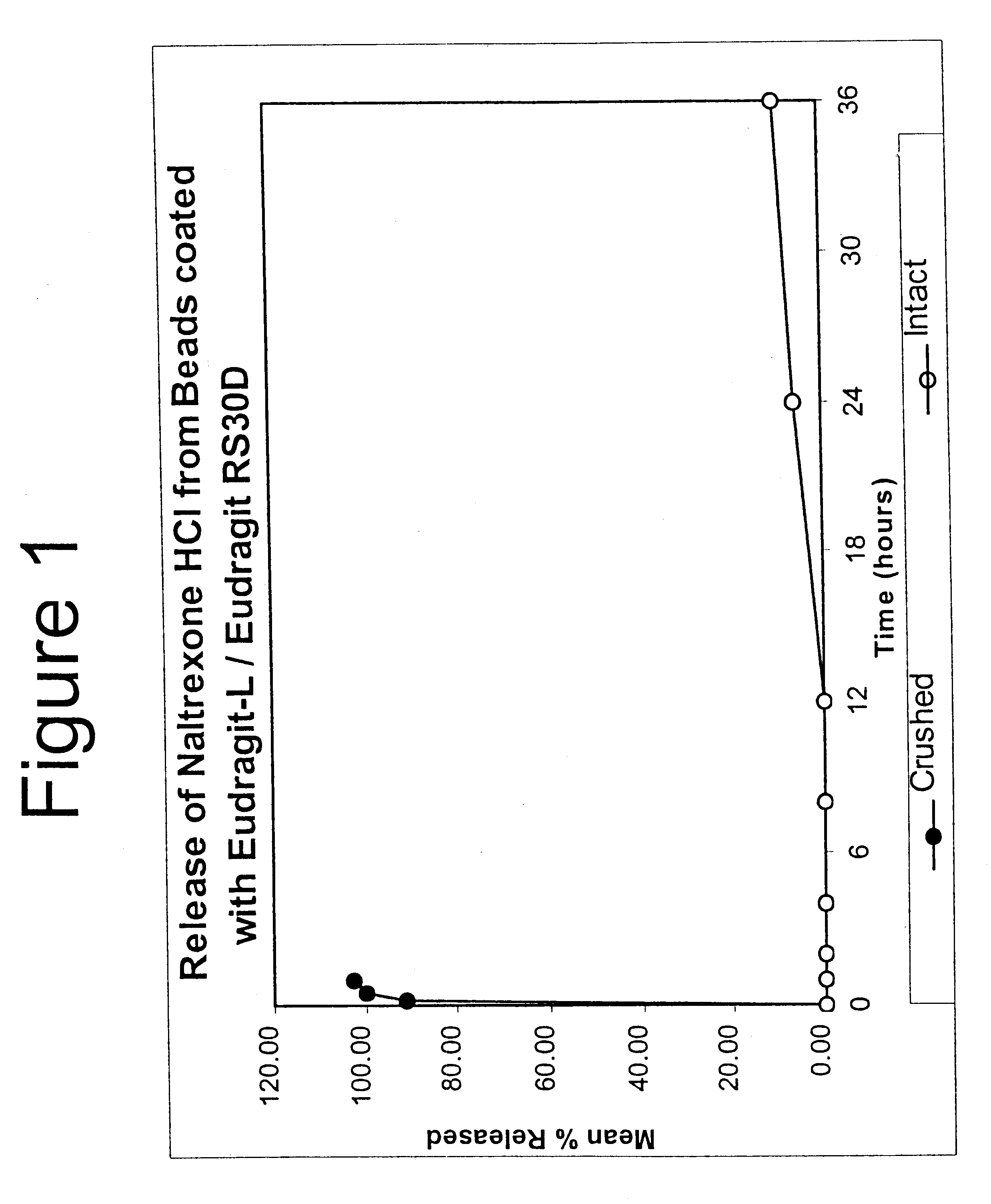
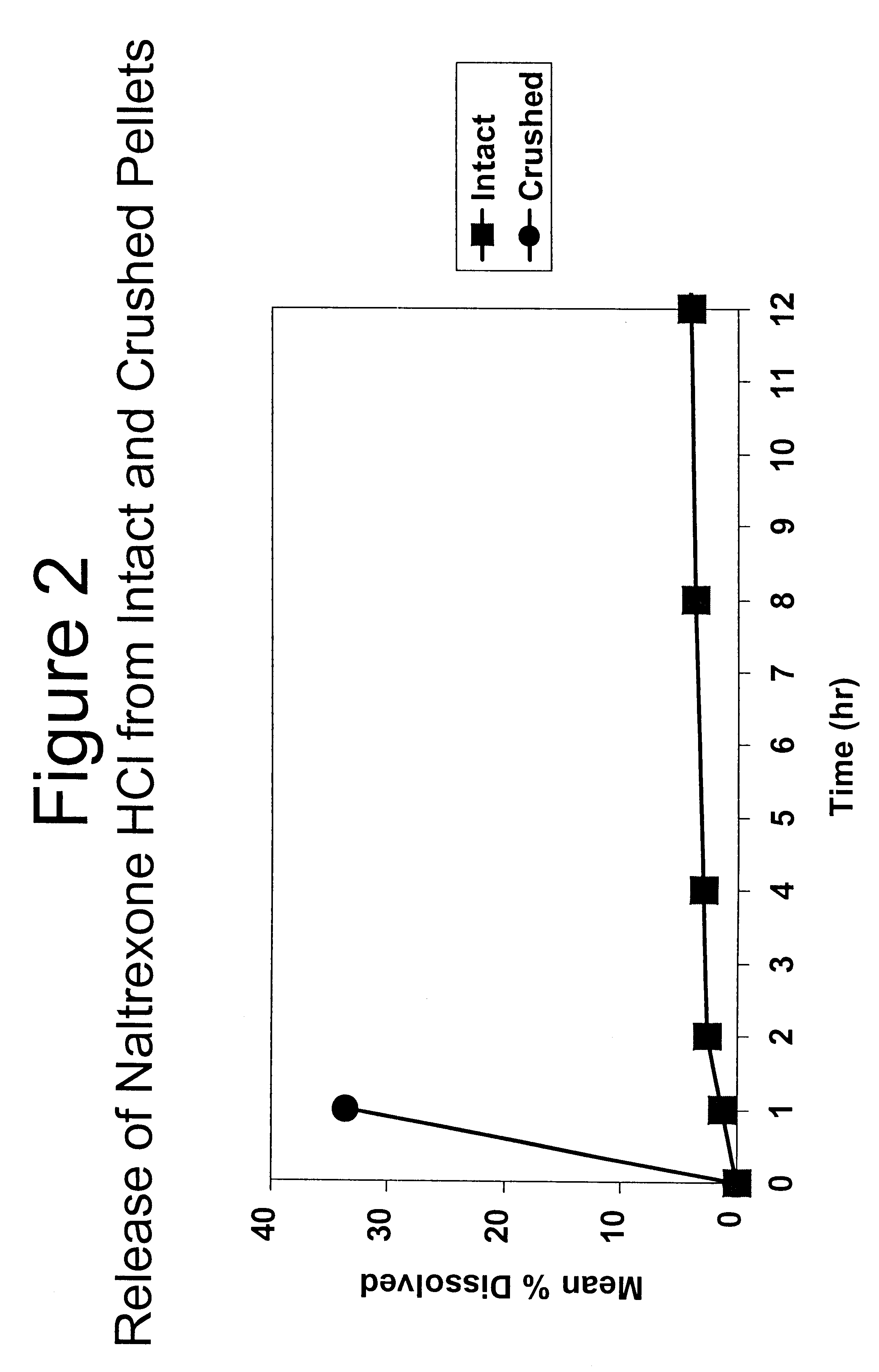
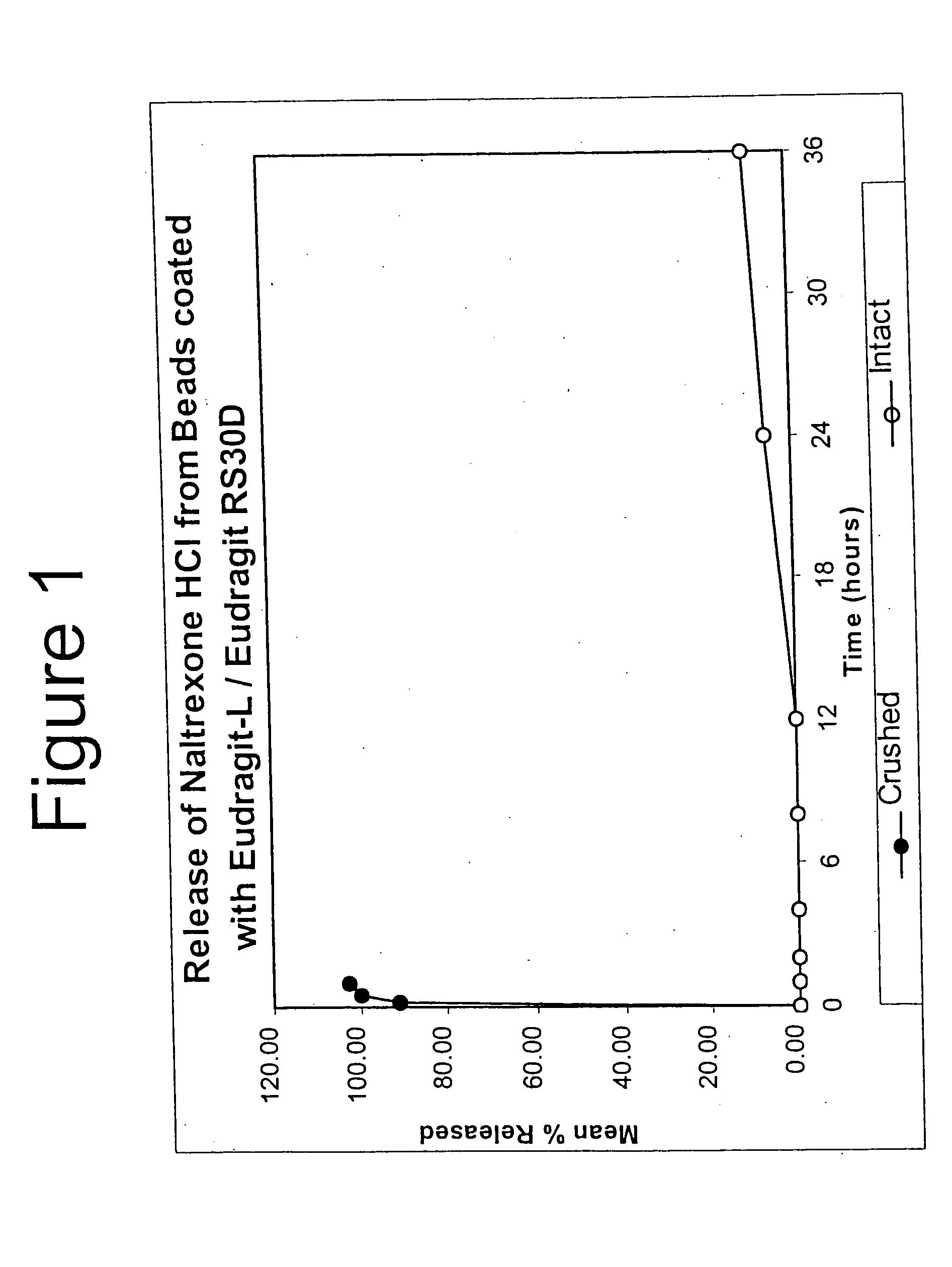
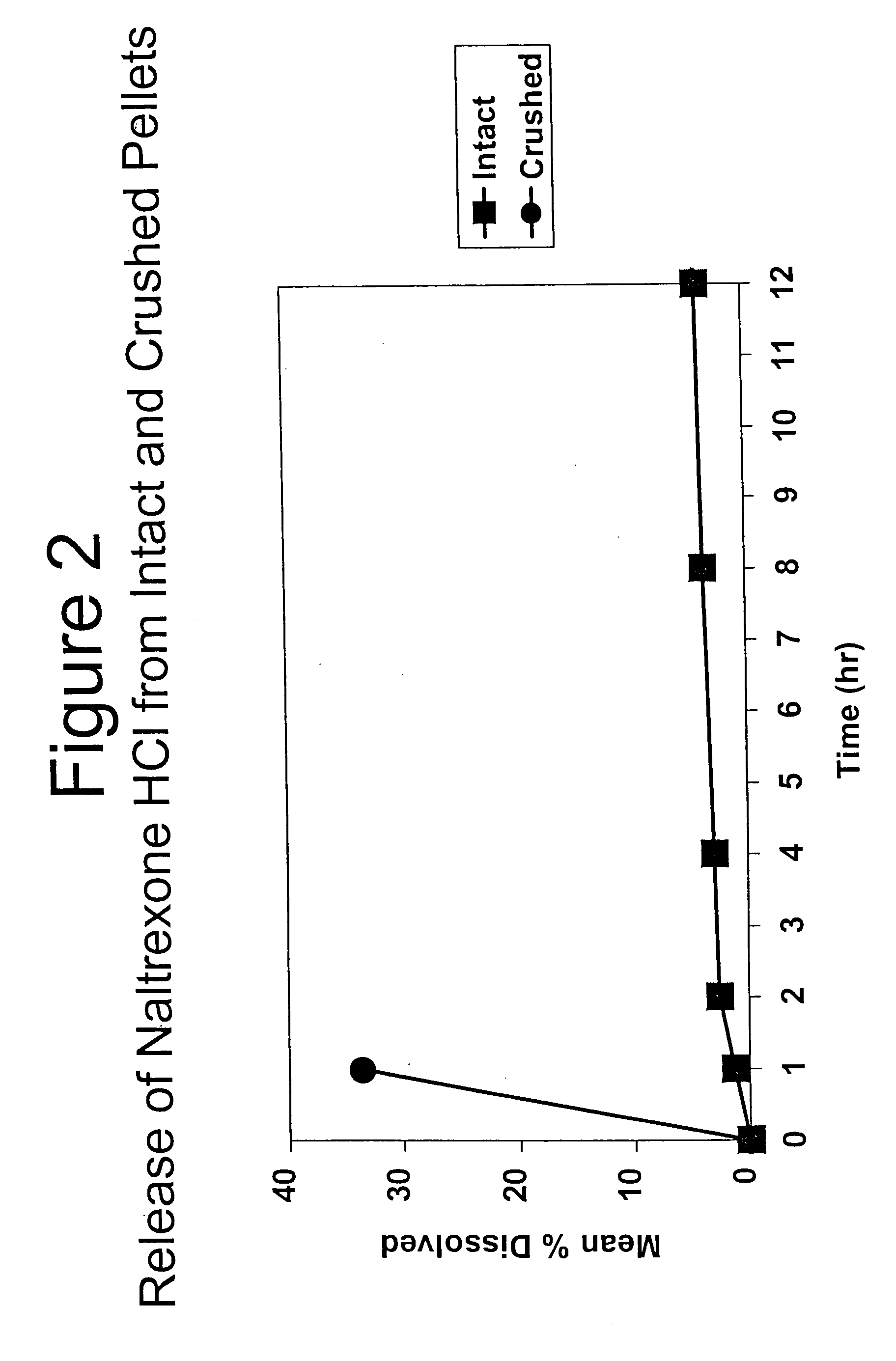
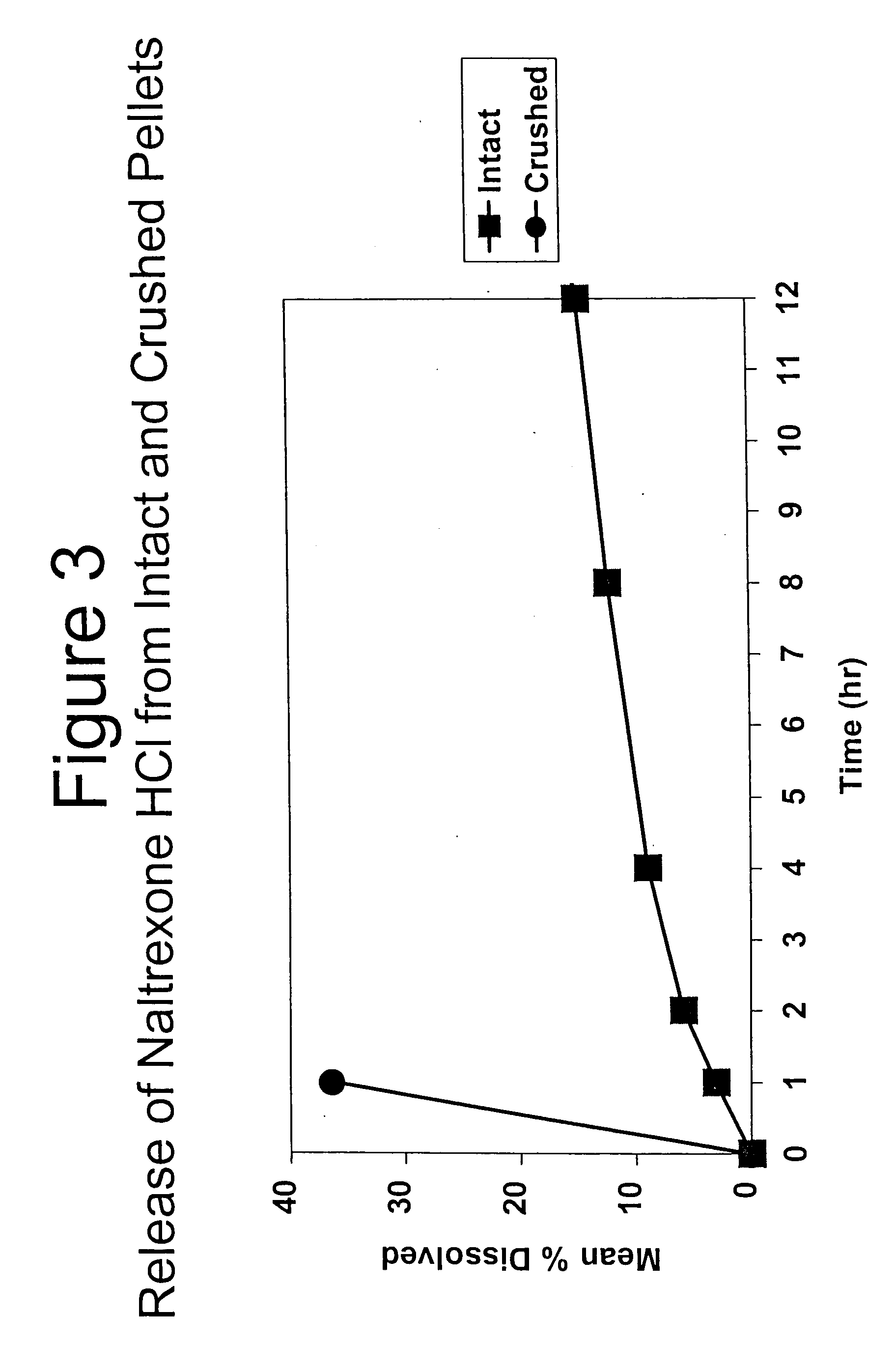
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
Use of mechanical dilator devices to enlarge ostia of paranasal sinuses and other passages in the ear, nose, throat and paranasal sinuses
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Coated vaginal devices for vaginal delivery of therapeutically effective and/or health-promoting agents
A vaginal device for delivering therapeutical and / or health-promoting agents. The vaginal device partly or completely coated by, covered by or combined with a coating or covering comprising a film, foam, strip, cap, cup or particles. The coating of the device comprises a mucoadhesive composition comprising a therapeutical and / or health-promoting agent.
Owner:UNIVERSITY OF MINNESOTA DULUTH
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
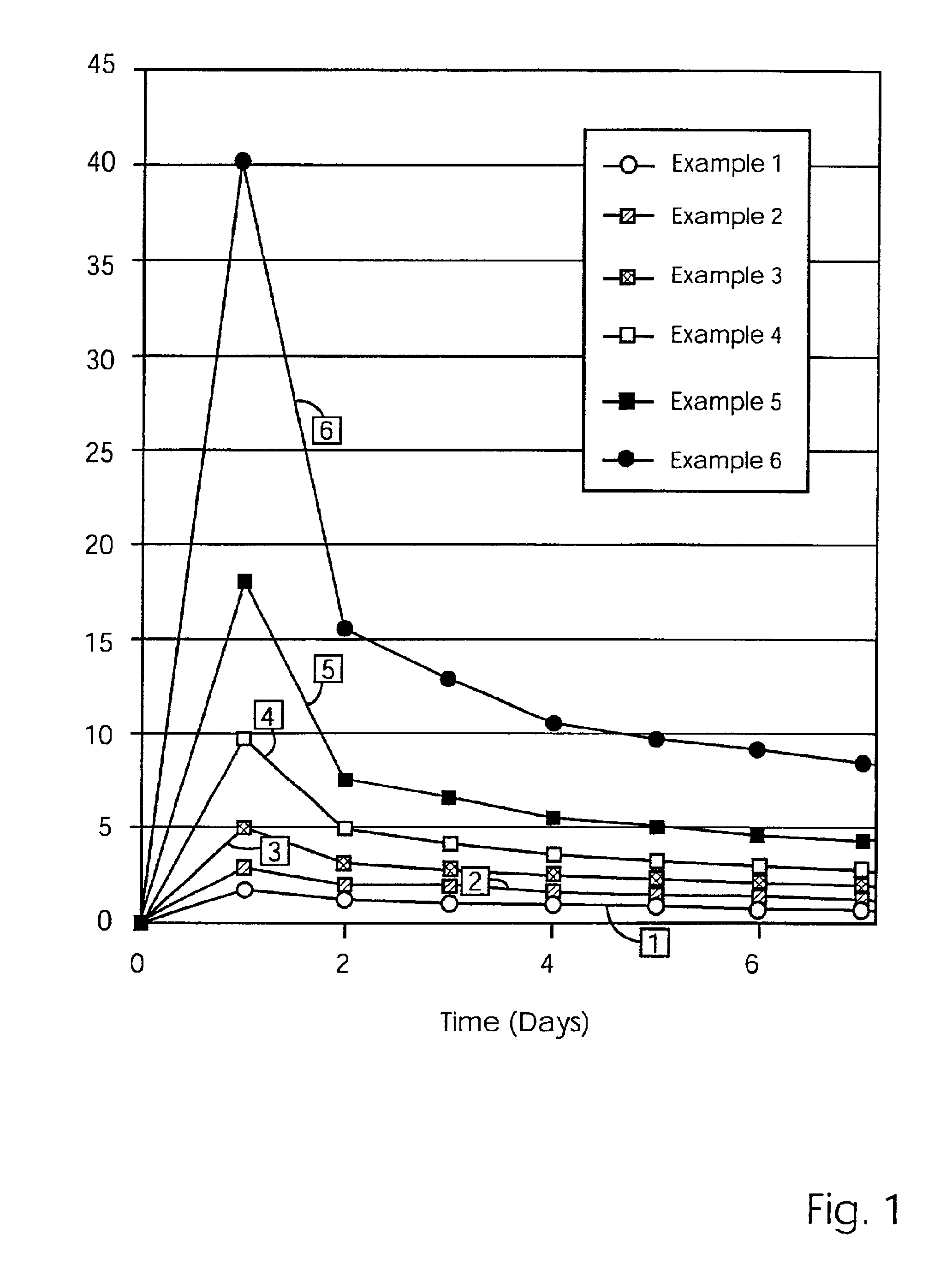
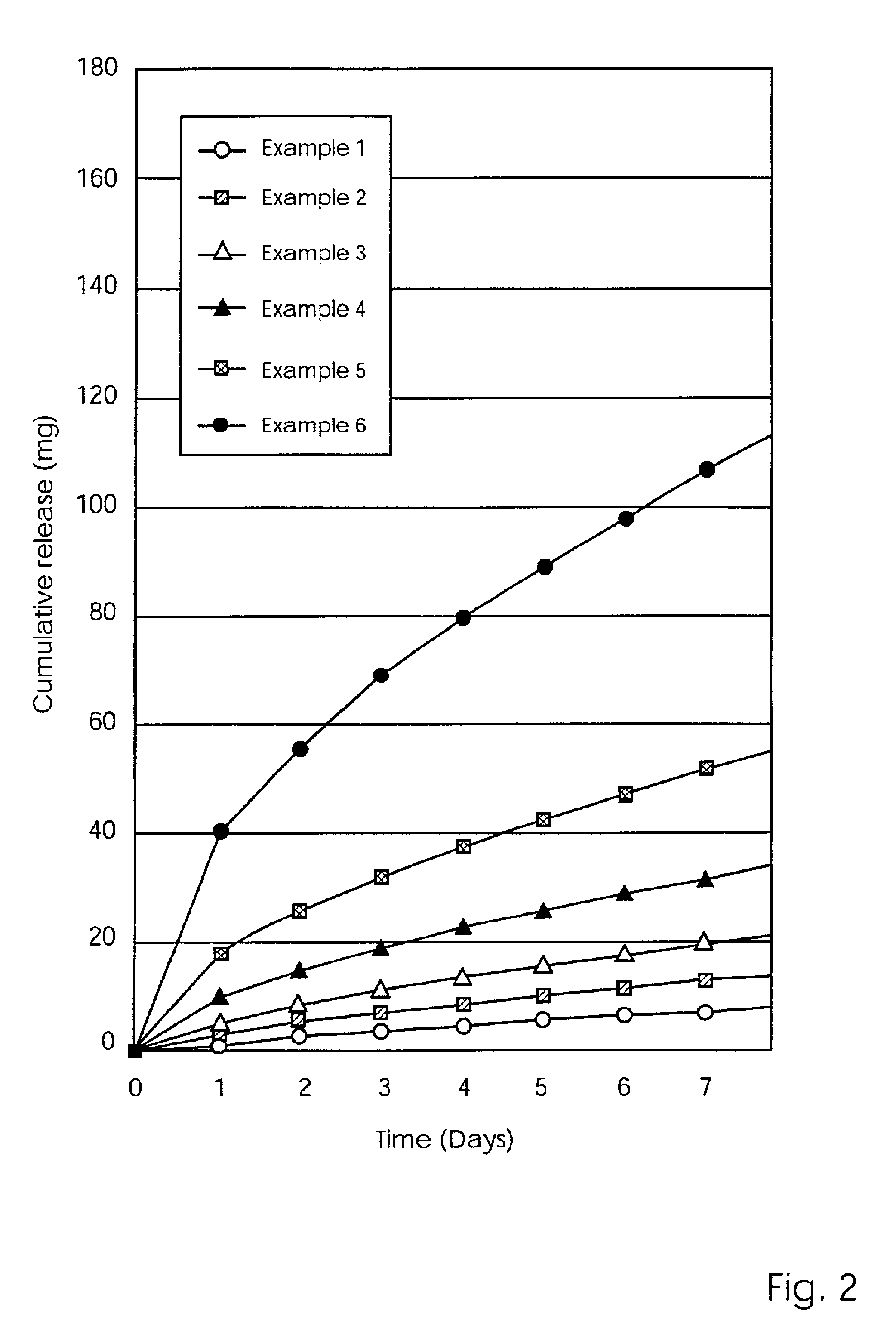
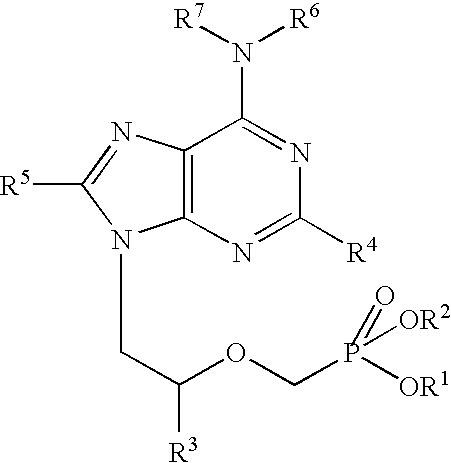
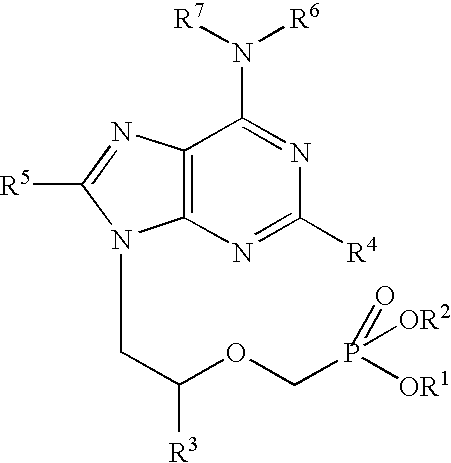
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Intravaginal drug delivery devices for the administration of an antimicrobial agent
InactiveUS6951654B2Convenient and high complianceReduce in quantityAntibacterial agentsTetracycline active ingredientsAntimicrobial drugAnti-Microbial Agents
An intravaginal antimicrobial drug delivery device is disclosed having an antimicrobial agent dispersed throughout a biocompatible elastomeric system. Also disclosed is a method of making the antimicrobial drug delivery device.
Owner:APTALIS PHARMA
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Chinese-medicinal composition for treating neck, shoulder, waist and legs diseases and its making method
A Chinese medicine for treating cervical spondylopathy, periomethritis, lumbar intervertebral disc protrusion and hyperosteogeny is prepared from 79 Chinese-medicinal materials including centipede, ground beetle, Chuan-xiong rhizome, myrrh, etc. Its preparing process is also disclosed.
Owner:侯保君
Multi-site drug delivery platform
Owner:PEDIAMED PHARMA
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20090074859A1Dissolve fastReduce deliveryAntibacterial agentsPowder deliveryDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
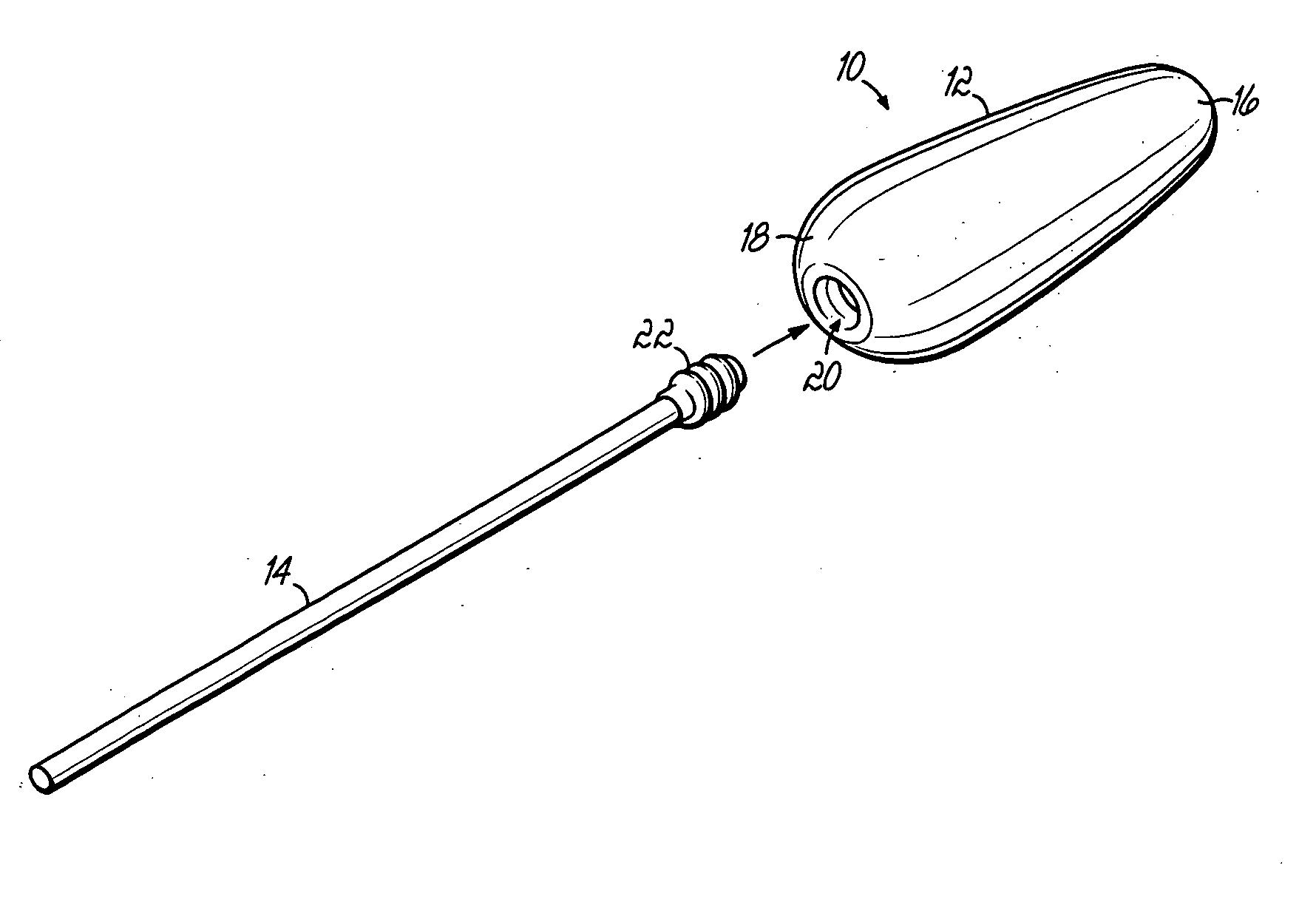
Applicator for semi-solid medications
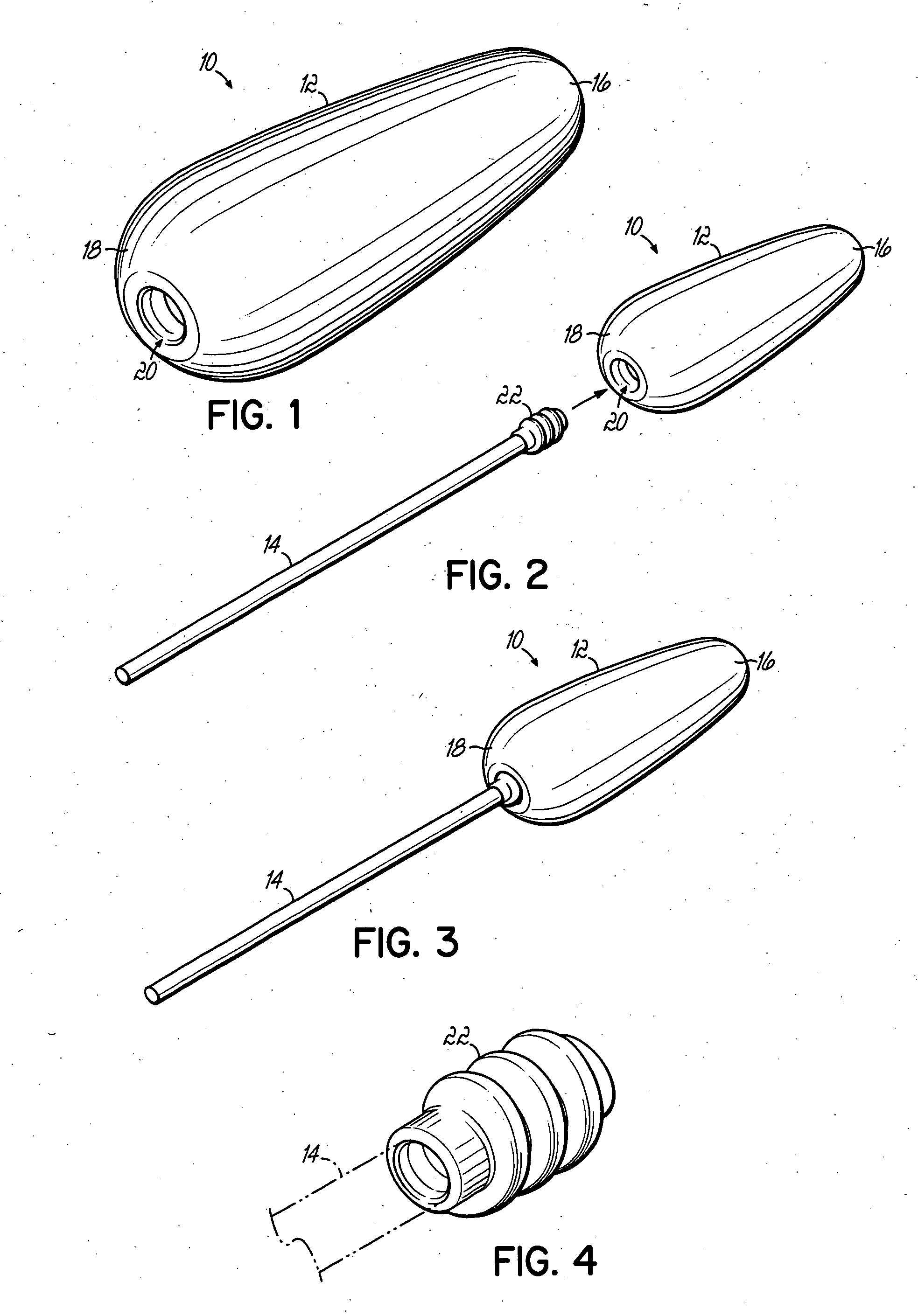
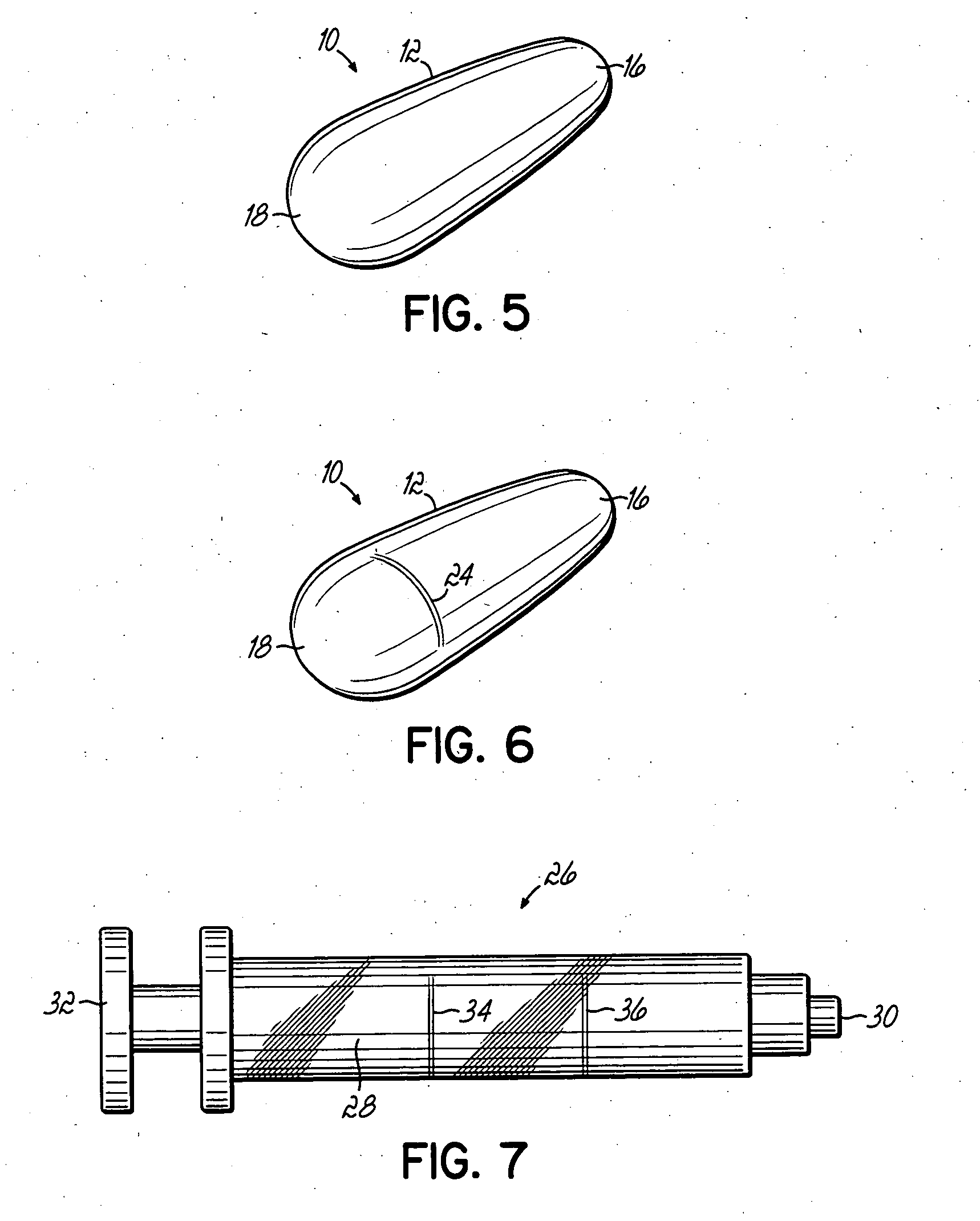
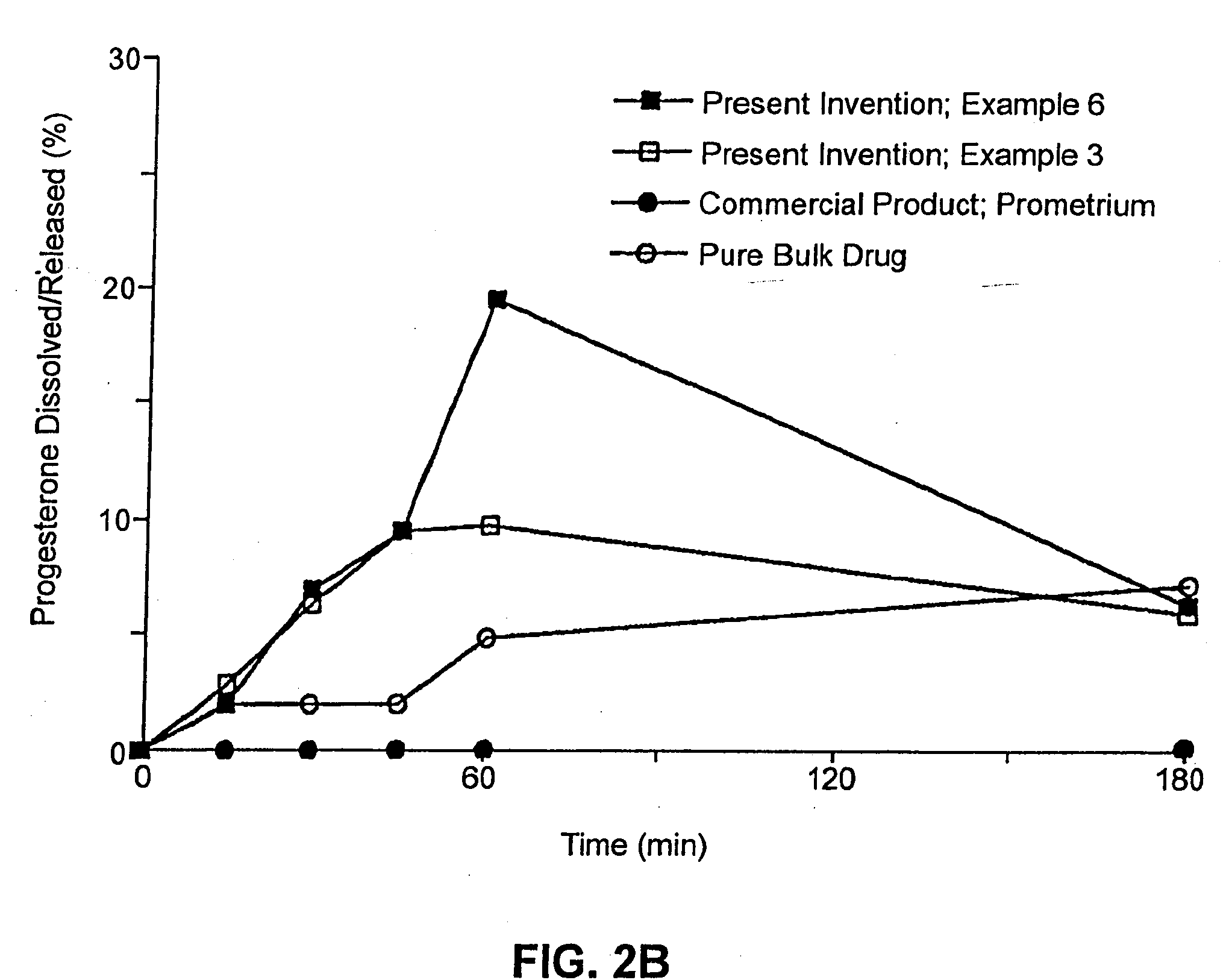
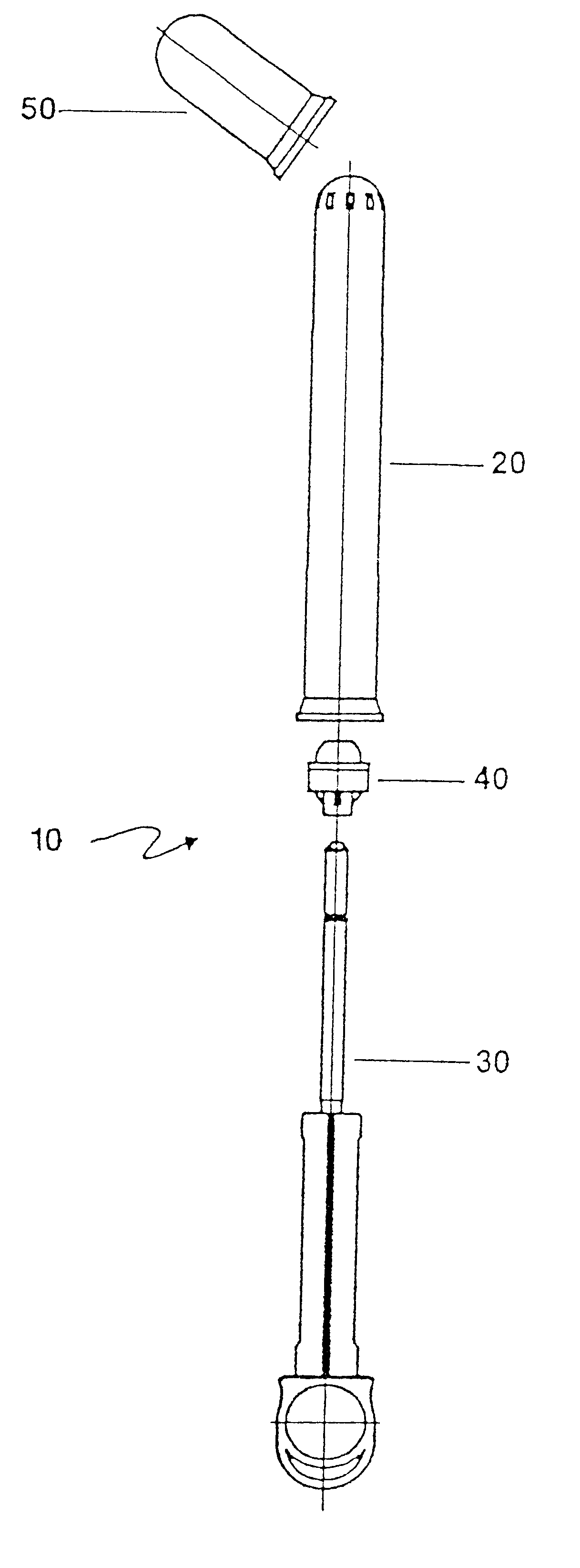
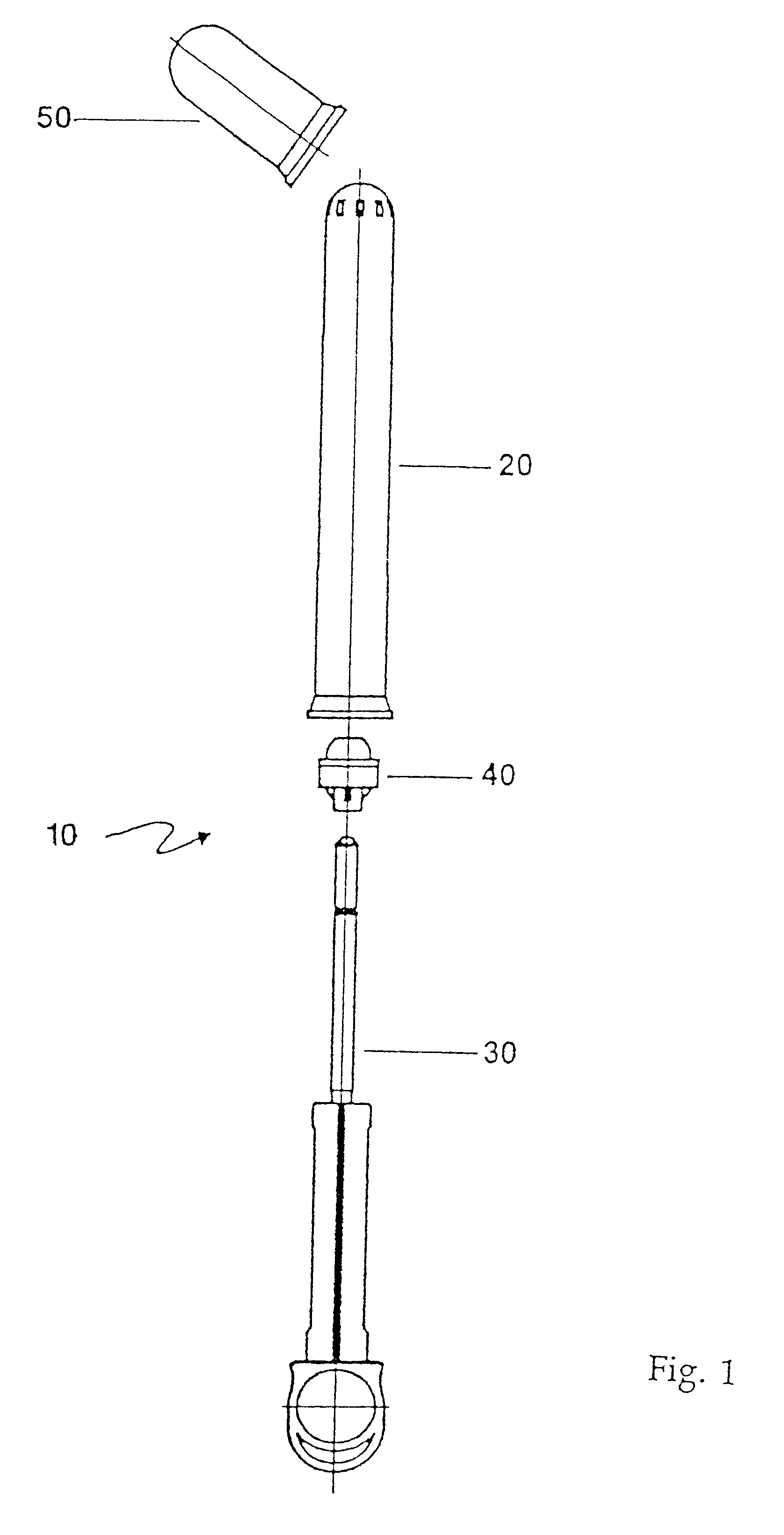
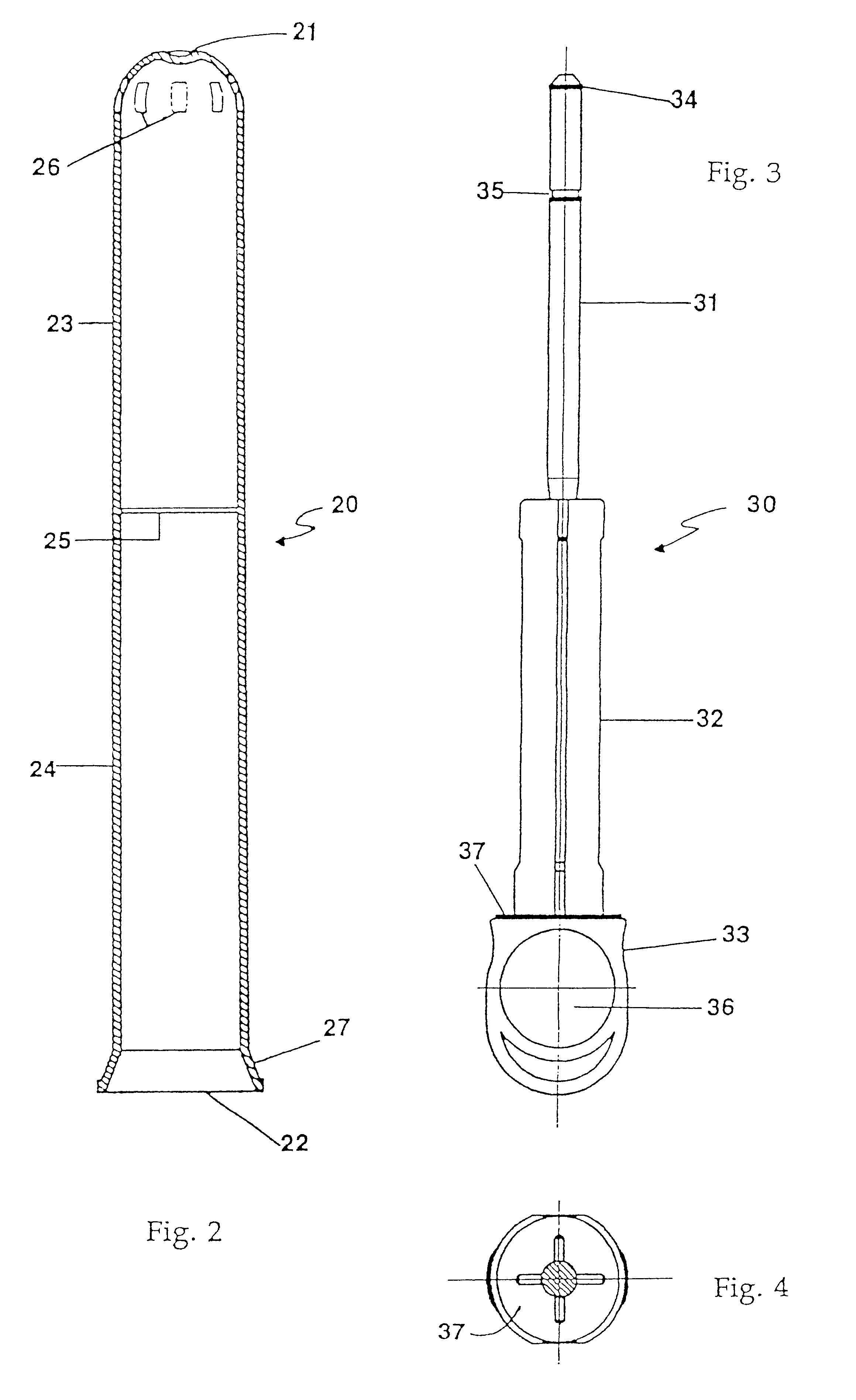
An applicator for semi-solid medications comprises a tubular body (20) having a rounded dispensing end (21) provided with at least one opening (26) and a grasping end (22), the inner surface of a proximal portion of said tubular body defining a reservoir for the medication, a plunger (30), initially housed inside the tubular body, which plunger is provided with a rod (31) and grasping means (33), a piston (40), positioned in sealing contact with the inner surface of said tubular body providing a closure for the medication reservoir, wherein said piston has a longitudinal hole (45) through which rod (31) of the plunger is disposed, and wherein said piston initially abuts on a stop means (25) when the applicator is received by the user, a coupling means (35, 46) between rod (31) and piston (40), a closure means for sealingly closing off said opening (26) on said dispensing end, wherein the plunger (30) is extractable from the tubular body until the rod (31) becomes engaged with the piston (40) by the coupling means, whereupon the plunger together with the piston is displaceable along the tubular body towards the dispensing end (21) for expelling the medication through opening (26), and wherein the stops means (25) is provided in the form of a projection on the inner surface of the tubular body.
Owner:J URIACH Y CIA SA
Compositions and methods for enhancing drug delivery across and into epithelial tissues
InactiveUS20020127198A1Reduce deliveryAntibacterial agentsOrganic active ingredientsSide chainGastrointestinal tract
This invention provides compositions and methods for enhancing delivery of drugs and other agents across epithelial tissues, including the skin, gastrointestinal tract, pulmonary epithelium, ocular tissues and the like. The compositions and methods are also useful for delivery across endothelial tissues, including the blood brain barrier. The compositions and methods employ a delivery enhancing transporter that has sufficient guanidino or amidino sidechain moieties to enhance delivery of a compound conjugated to the reagent across one or more layers of the tissue, compared to the non-conjugated compound. The delivery-enhancing polymers include, for example, poly-arginine molecules that are preferably between about 6 and 25 residues in length.
Owner:KAI PHARMA
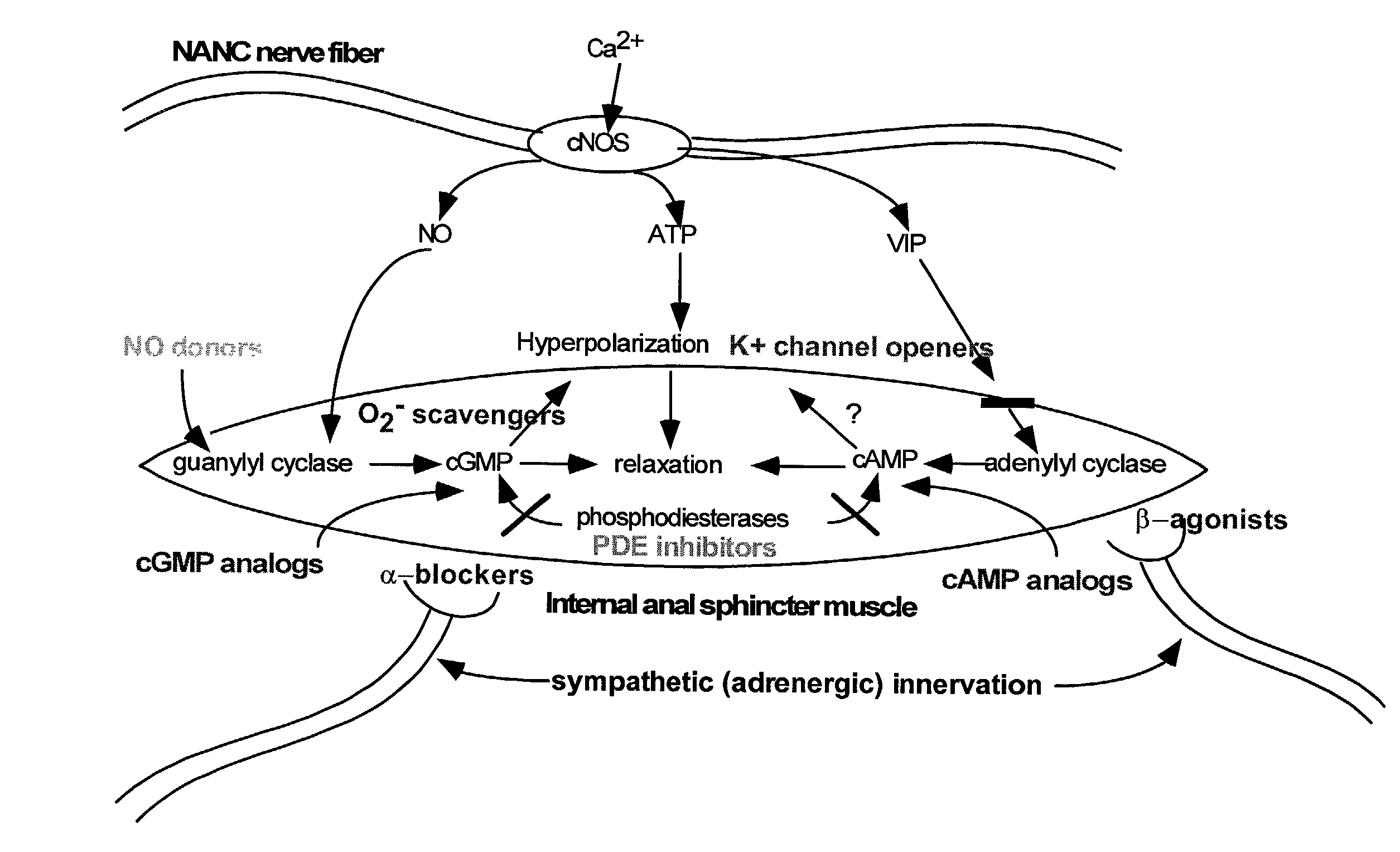
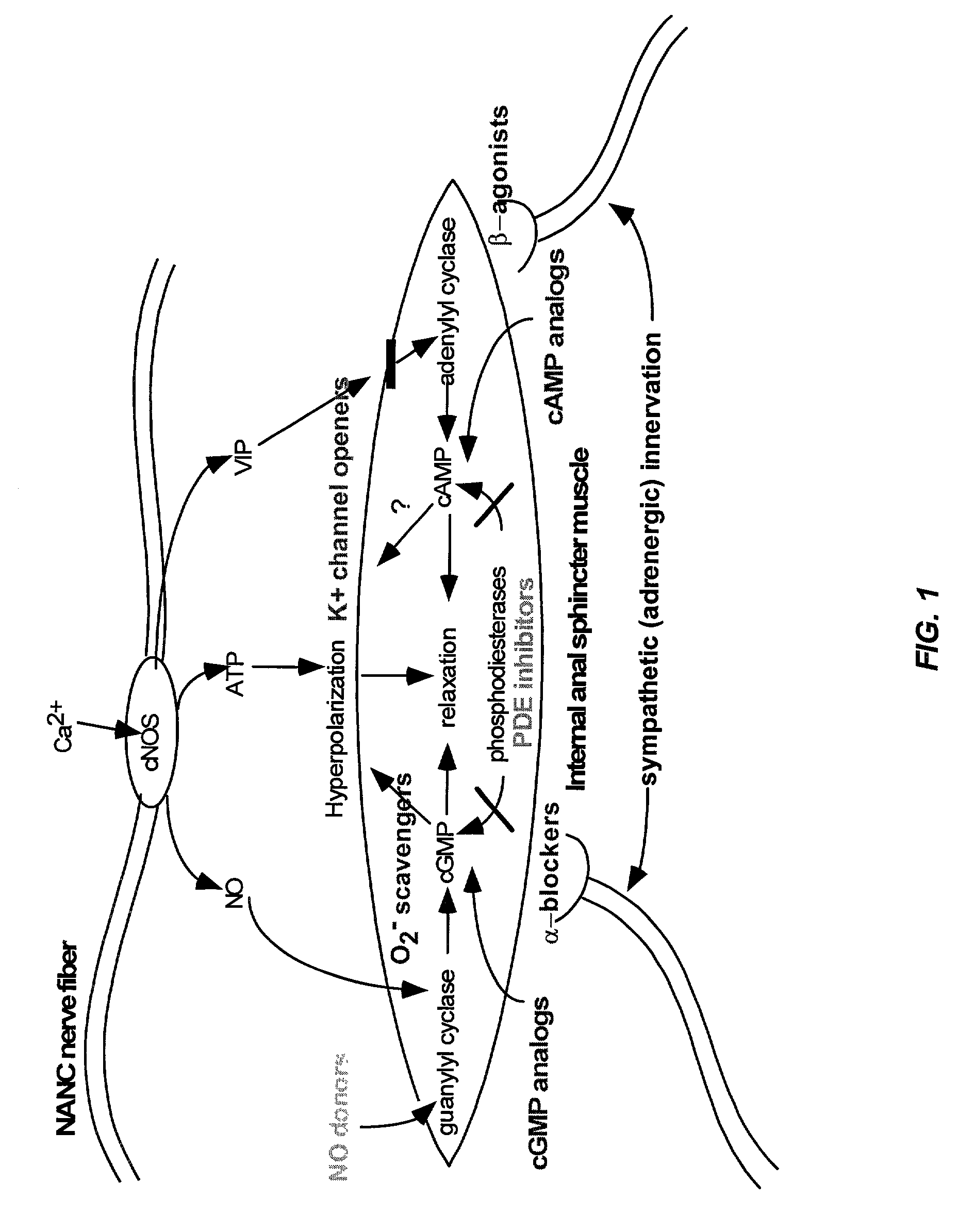
Nitric oxide donor composition and method for treatment of anal disorders
A pharmaceutical composition contains a nitric oxide donor and advantageously an optional corticosteroid and / or topical anesthetic. The composition is useful in a method for treating anal disorders such as anal fissure, anal ulcer, hemorrhoidal disease, levator spasm, and so forth, by topical application to or proximate the affected area.
Owner:STRAKAN INT S A R L
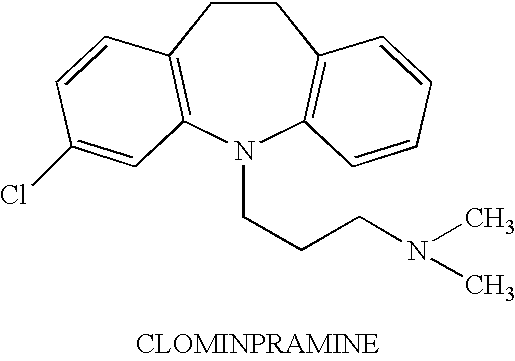
On demand administration of clomipramine and salts thereof to treat premature ejaculation
InactiveUS6495154B1Delaying the onset of ejaculationDelay the onset of ejaculationPowder deliveryAerosol deliveryActive agentWhole body
A method is provided for delaying the onset of ejaculation in an individual. The method involves systemic and on demand administration to an individual of a pharmaceutical formulation containing an amount of an active agent selected from the group consisting of clomipramine and pharmacologically acceptable acid addition salts thereof. Drug delivery may be accomplished via any route designed to provide systemic levels of the active agent effective to delay the onset of ejaculation. Pharmaceutical formulations and dosage forms are provided as well.
Owner:VIVUS
Methods of delivering stable topical drug compositions
InactiveUS20060127469A1Easy and administration routeNot to wasteOrganic active ingredientsPeptide/protein ingredientsRoom temperatureTopical drug
A method of delivering a drug composition comprises providing a carrier having a phosphatidylcholine component and a drug entrapped therein, and applying the composition to the skin for transdermal delivery of the drug, wherein the composition is stable at room temperature.
Owner:TRANSDERMAL BIOTECHNOLOGY INC
Topical Antiviral Formulations
InactiveUS20080035155A1Reduce riskPrevent and reduce riskBiocideOrganic active ingredientsTopical antiviralReverse transcriptase
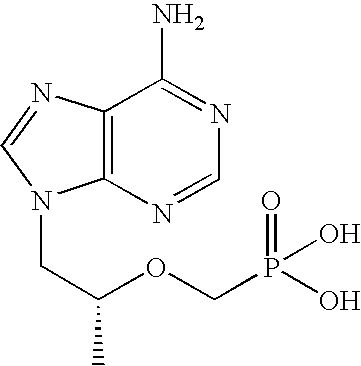
The present invention relates to formulations of nucleotide reverse transcriptase inhibitors (N(t)RTIs), preferably [2-(6-Amino-purin-9-yl)-1-methyl-ethoxymethyl]-phosphonic acid (tenofovir, PMPA), or a physiologically functional derivative thereof, suitable for topical application and their use in the prevention of HIV infections.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
Tamper-resistant oral opioid agonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Topical drug delivery using phosphatidylcholine
InactiveUS20060105955A1Topical deliveryEasier and pleasanterOrganic active ingredientsPeptide/protein ingredientsTopical drugTransdermal medication
The present invention relates to compositions and methods for transdermal drug delivery comprising formulating a phosphatidylcholine carrier composition containing the drug and applying the composition to the skin.
Owner:TRANSDERMAL BIOTECHNOLOGY INC
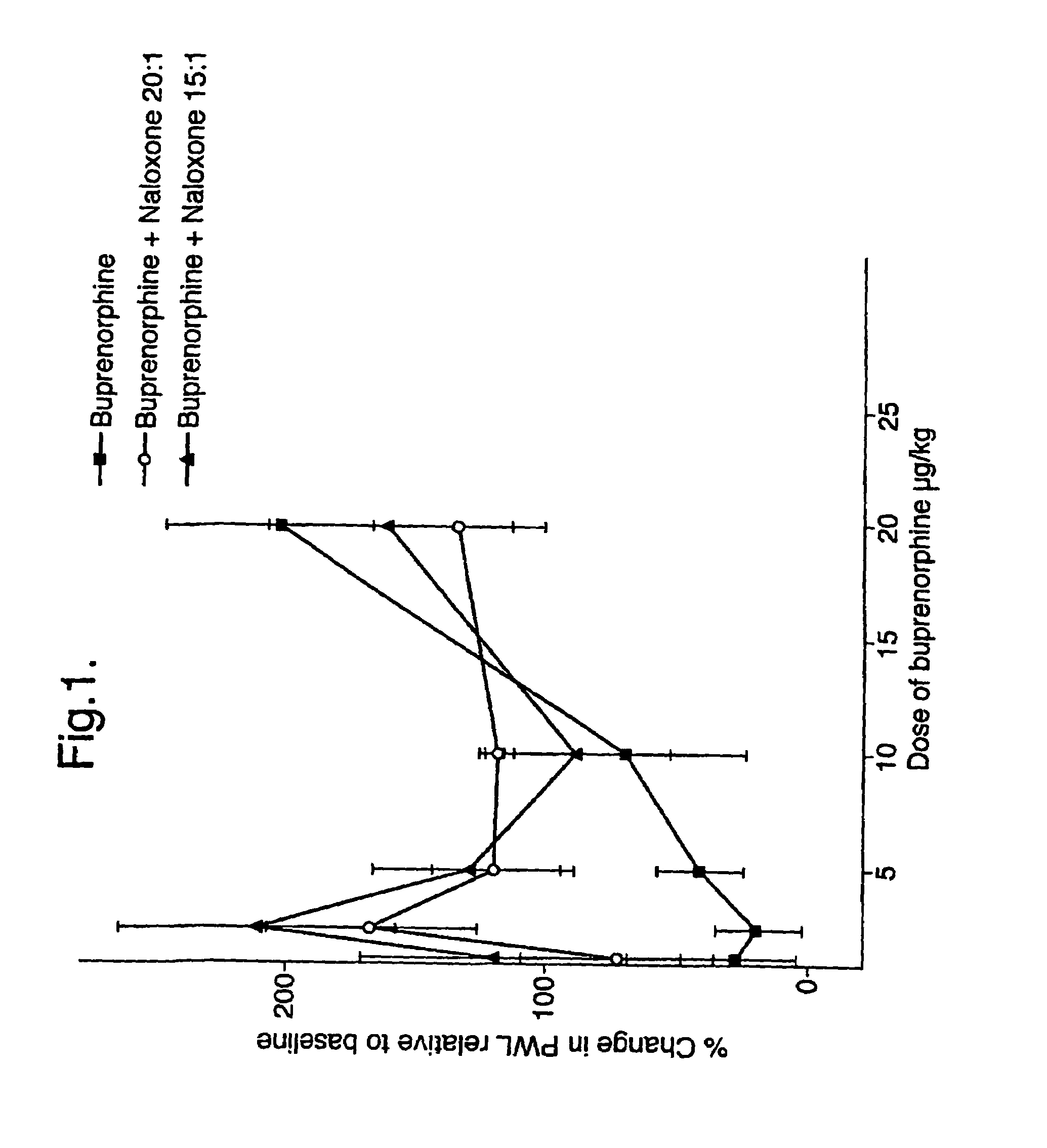
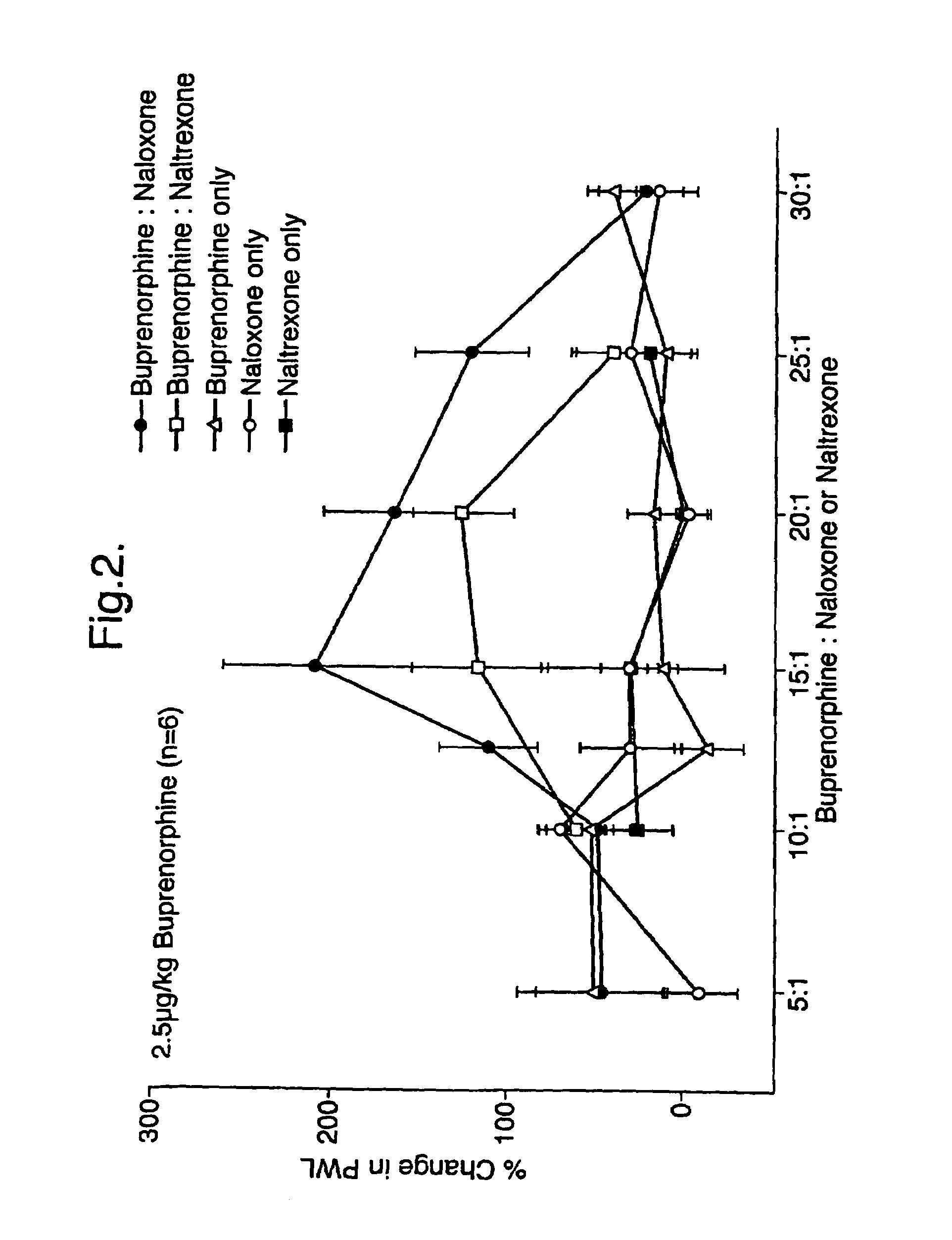
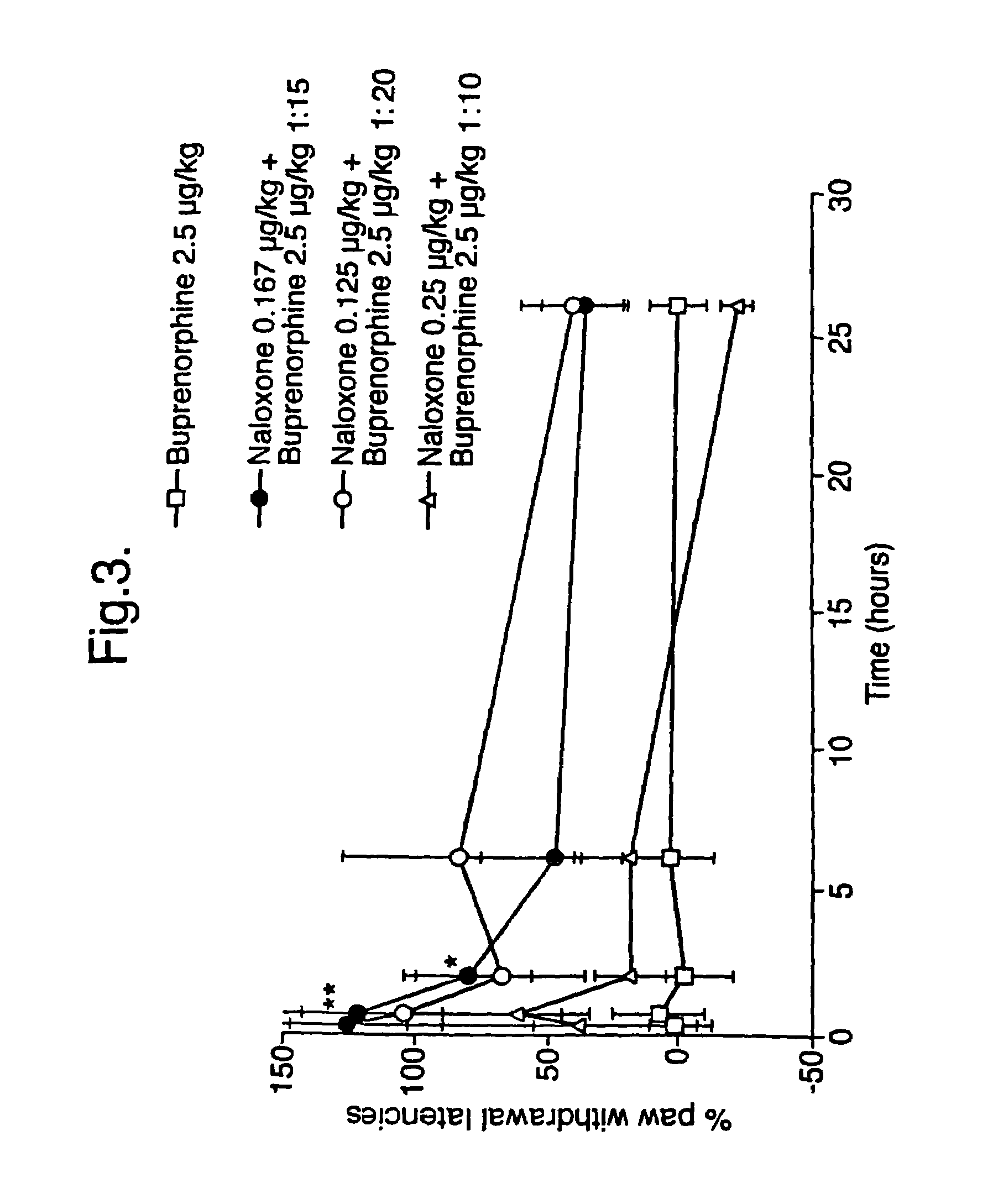
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
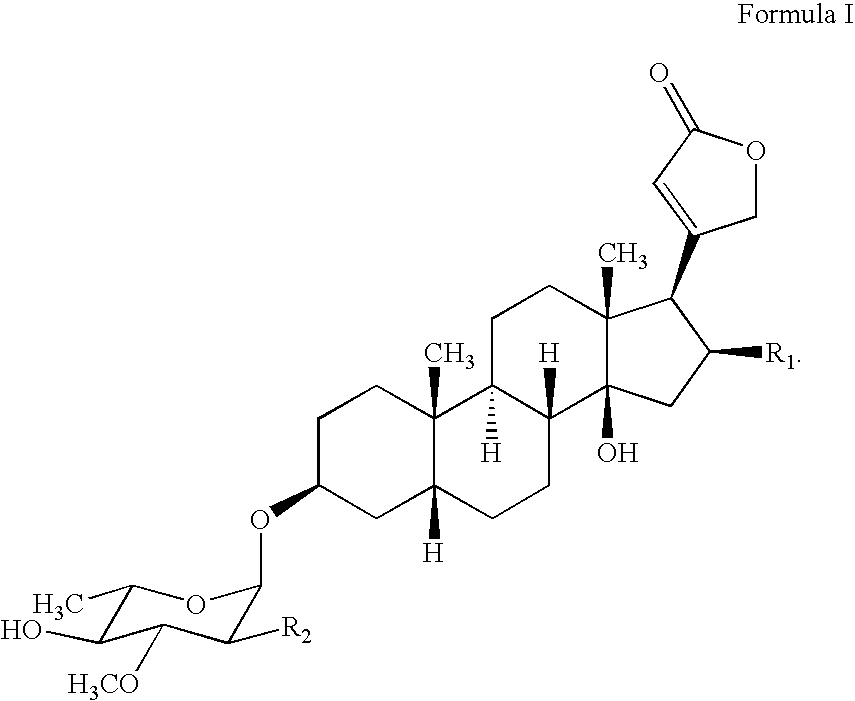
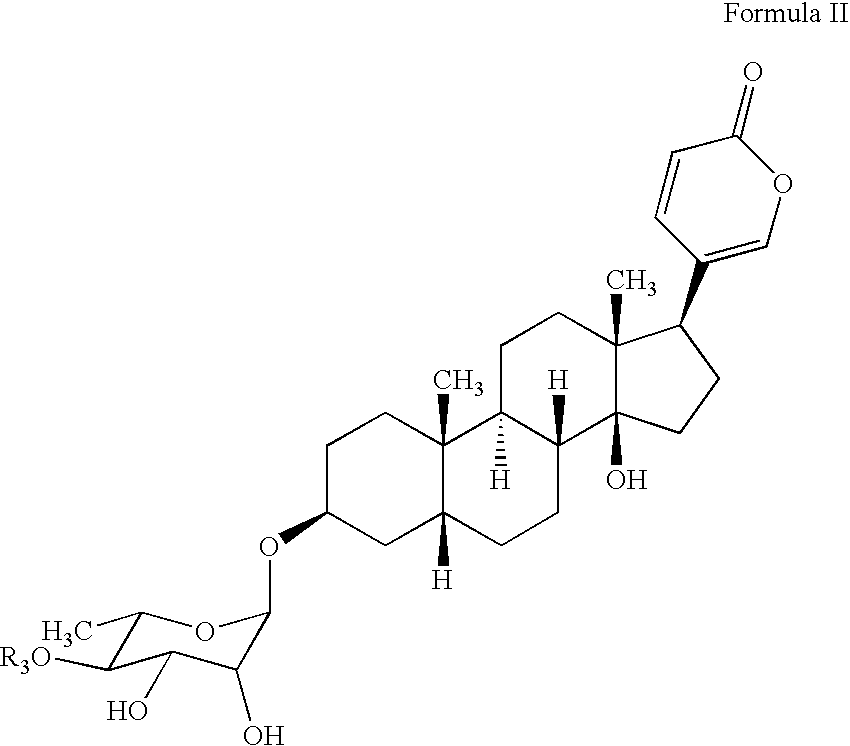
Water soluble formulations of digitalis glycosides for treating cell-proliferative and other diseases
The present invention provides method, preparation and use of a variety of pharmaceutical composition containing at least one digitalis glycosides such as oleandrin, odoroside-A, neriifolin, proscillaridin-A, methyl-proscillaridin-A, digitoxin, digoxin and amorphous cyclodextrins. In another aspect, the present invention provides an effective method to reduce the growth of cancers or reducing the incidence of metastases. In yet another aspect, the present invention provides an effective method for treating diseases in a warm-blooded animal.
Owner:TRINITY LAB INC
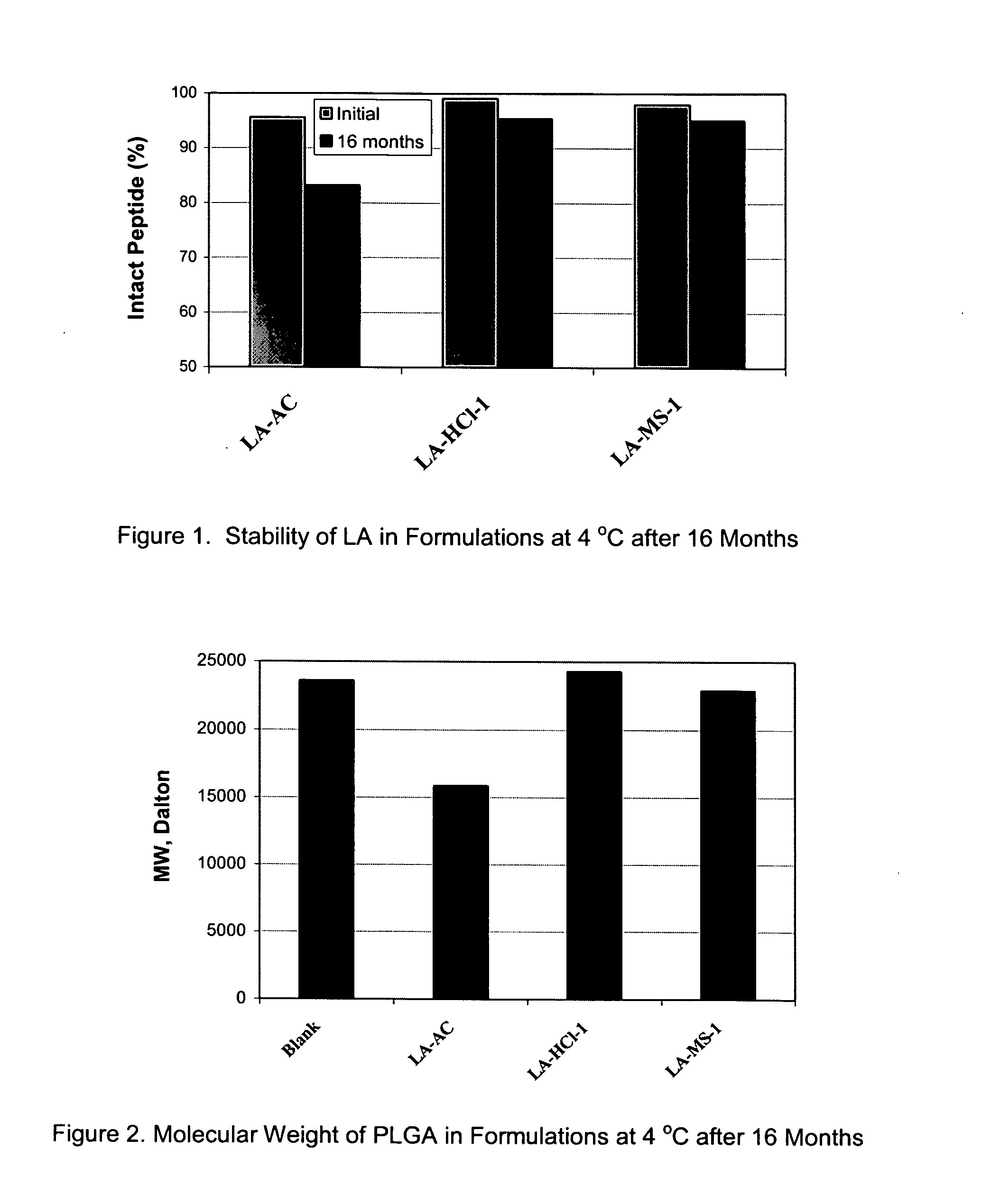
Pharmaceutical compositions with enhanced stability
InactiveUS20070196416A1Improve stabilitySatisfied with stabilityPowder deliveryPeptide/protein ingredientsOrganic solventControl release
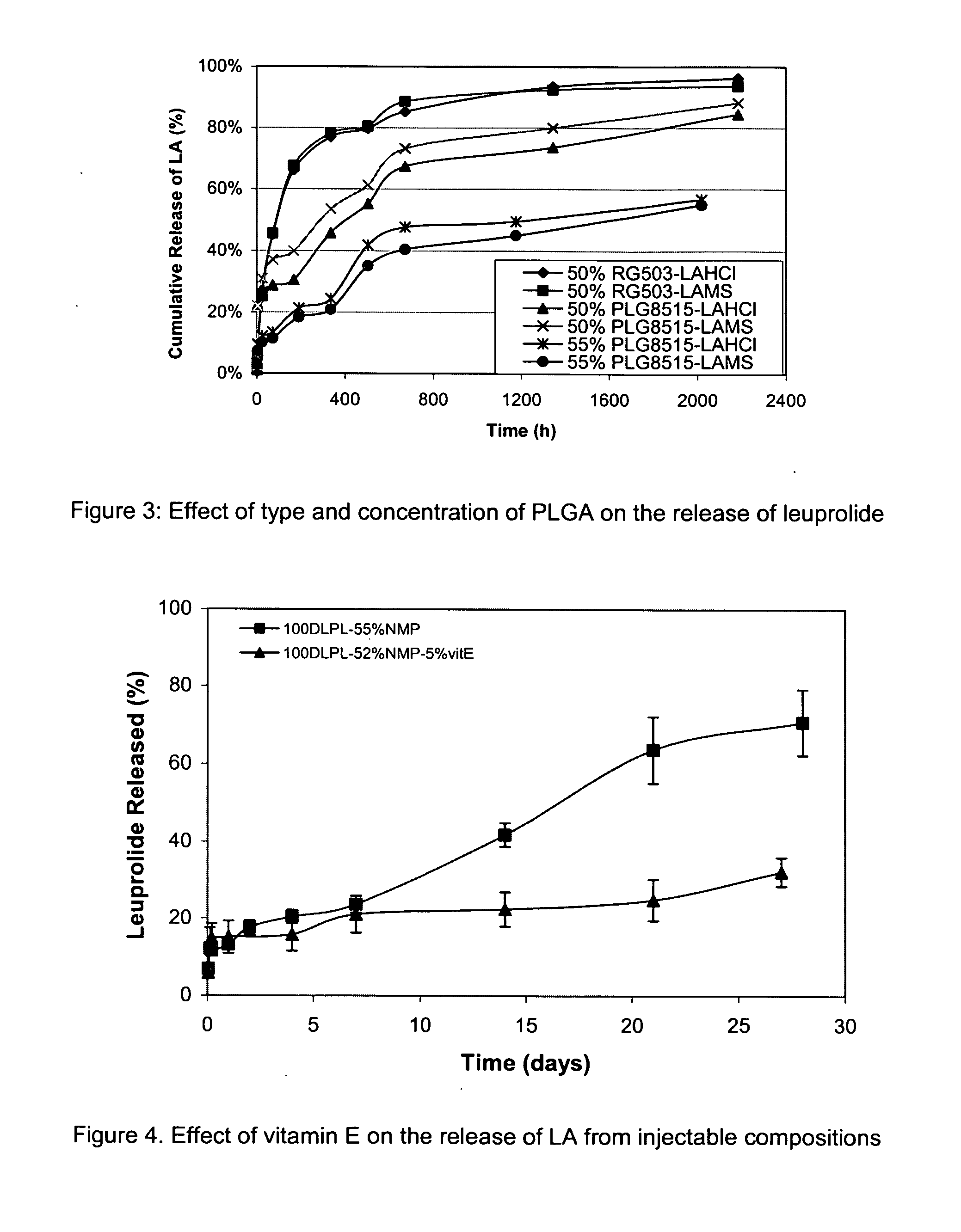
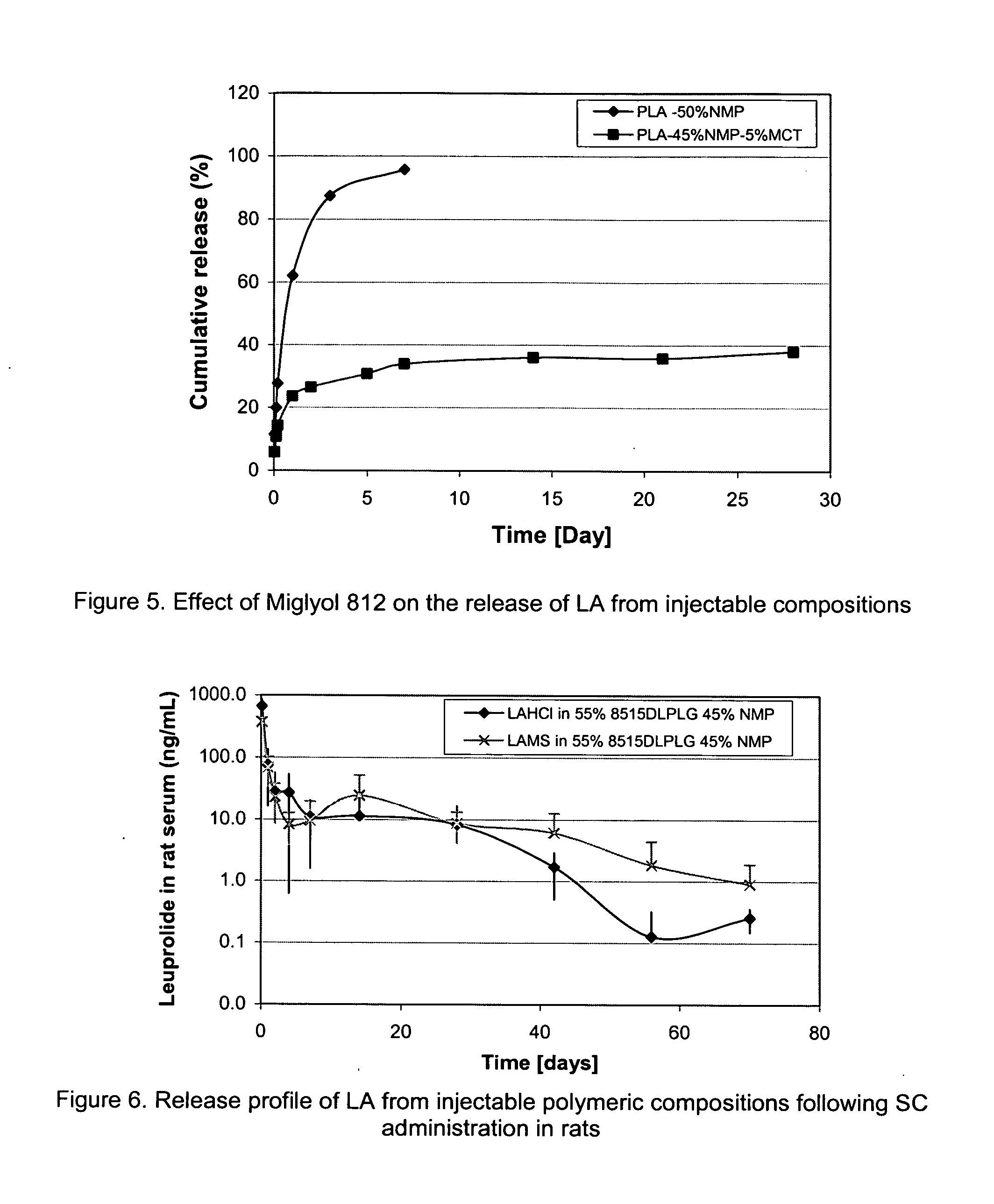
The present invention provides for a stabilized biodegradable polymeric composition useful as a controlled release delivery system for peptide agents. The compositions of the present invention comprise a) a beneficial salt of a peptide agent formed with a strong acid that minimizes or prevents the interaction / reaction between the peptide agent and the polymer in an organic solution; b) a biodegradable polymer; c) a pharmaceutically acceptable organic solvent; and d) optionally one or more excipients. The present invention also relates to a method of manufacturing and a method of use thereof.
Owner:FORESEE PHARMA CO LTD
Medical usage of 2beta-hydroxyilicicacid in inhibiting hepatitis B
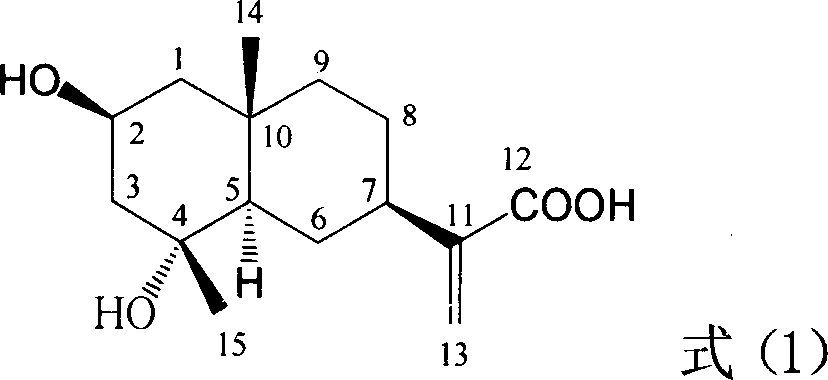
InactiveCN1951378APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsAerosol deliveryDiseaseHepatitis b surface antigen
The invention relates to a hemiterpene derivant 2-Hydroxyilicic acid, as formula (1) 2beta-hydroxy-5alphaH-eudesmane-11(13)-allyl-12-acid and relative compounds which can be used to prepare the drug treating hepatitis B disease. The inventive compound can restrain the copy of hepatitis B surface antigen (HBsAg) and the hepatitis B deoxyribonucleic acid (HBV-DNA), while its HBsAg restrain ability is higher than positive contrast difuradin; in the density as 100mug / ml, 20mug / ml, and 4mug / ml, it can restrain the copy of hepatitis B virus HBV-DNA.
Owner:WENZHOU MEDICAL UNIV +1
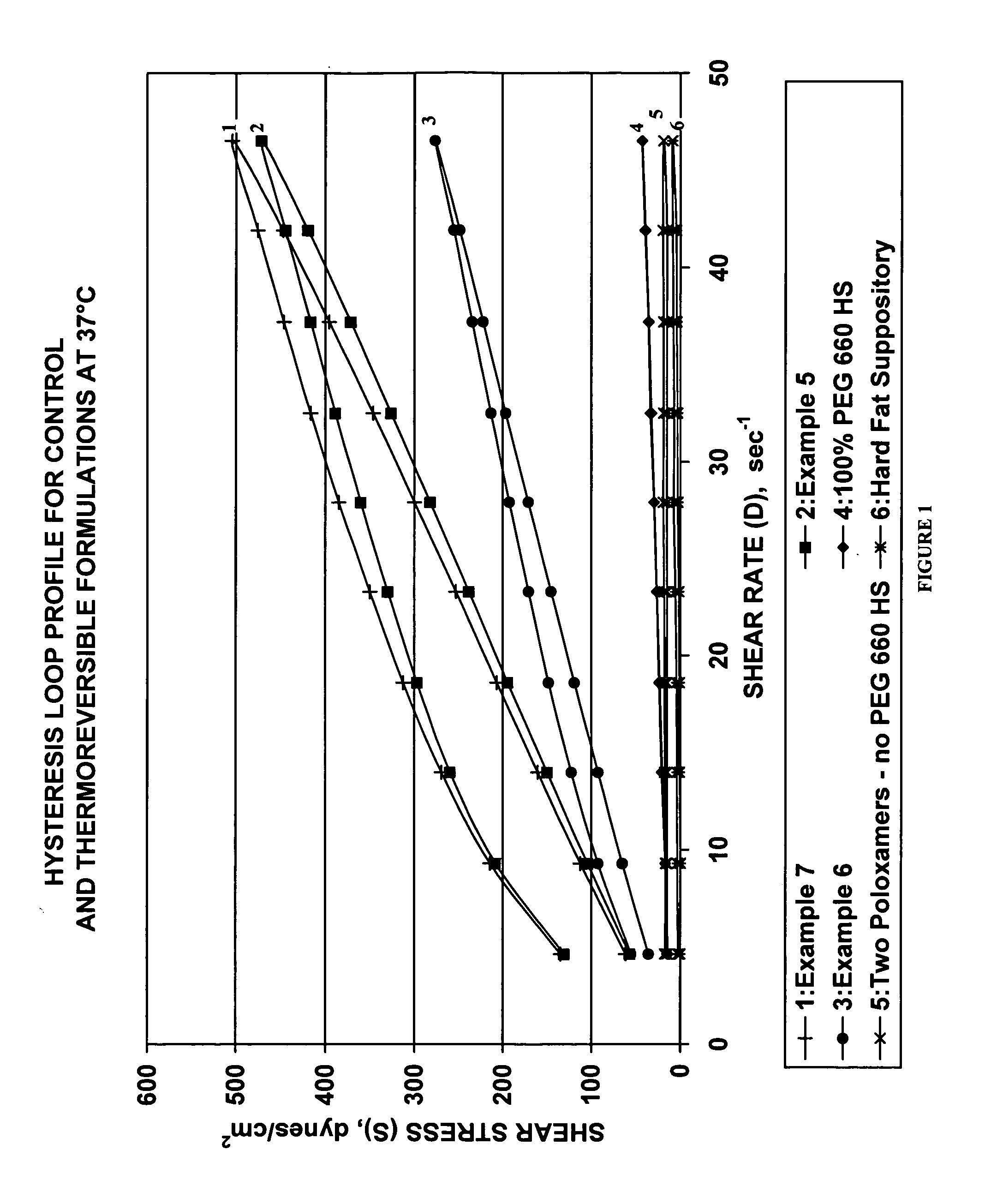
Thermoreversible pharmaceutical formulation for anti-microbial agents comprising poloxamer polymers and hydroxy fatty acid ester of polyethylene glycol
The present invention provides a pharmaceutical formulation having thermoreversible properties, comprising: a) an anti-microbial agent; b) a poloxamer mixture containing at least two poloxamer polymers; and c) a hydroxy fatty acid ester of polyethylene glycol, wherein the formulation is a solid at room temperature and is a liquid-gel at body temperature. The thermoreversible pharmaceutical formulation has a viscosity of about 8,500 cP to about 400,000 cP at room temperature, and a viscosity of about 1,000 cP to about 8,000 cP at body temperature and exhibits a hysteresis loop behavior. The present invention further provides a process of preparing as well as a method of treating a microbial infection in a mammal using the same.
Owner:TARO PHARMA INDS
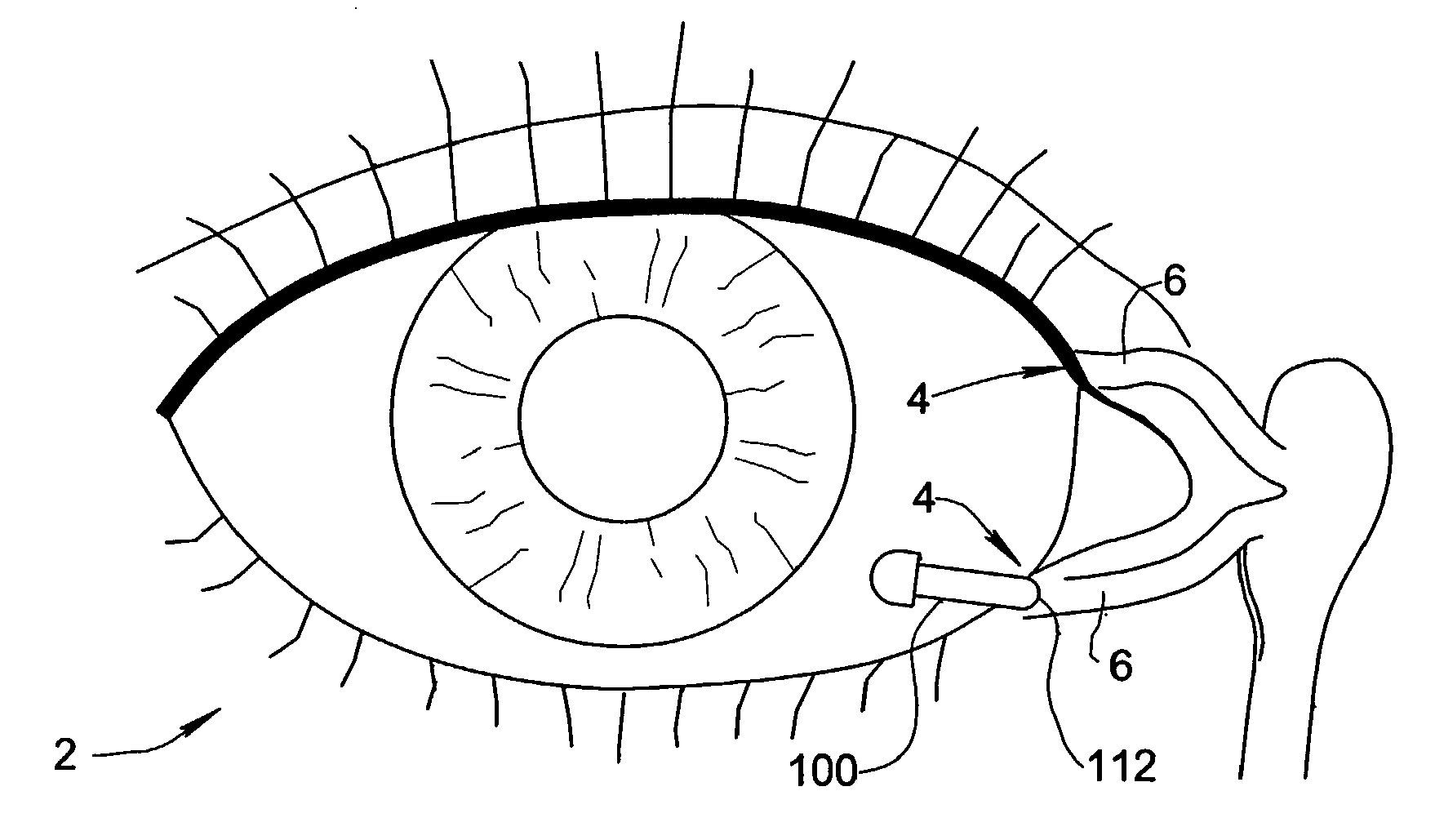
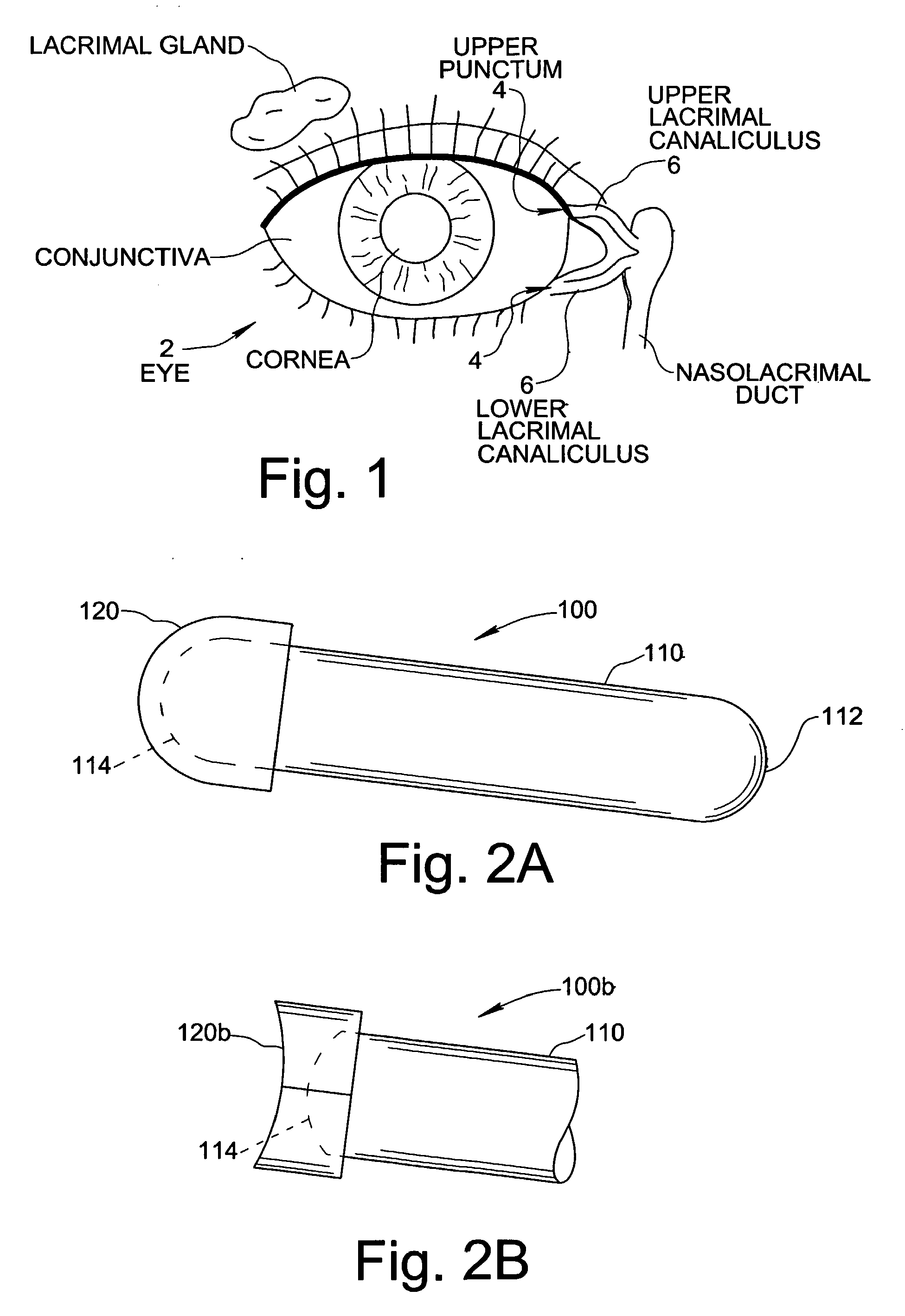
Treatment Medium Delivery Device and Methods for Delivery of Such Treatment Mediums to the Eye Using such a Delivery Device
ActiveUS20090118702A1Extended retention timePrevent drainageOrganic active ingredientsIn-vivo radioactive preparationsOphthalmology
Owner:MATI THERAPEUTICS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Expandable vaginal suppository
ActiveCN103181889AImprove bioavailabilityDifficult to cleanSuppositories deliveryTamponsVaginal SuppositoryPhysiology
The invention relates to an expandable vaginal suppository which comprises a first expansion carrier; a stype layer comprising active constituents and a matrix is used for at least covering an end part of the first expansion carrier; the vaginal suppository is expanded in a water solution with the pH value of 3.0-8.0 according to the predetermined expansion value; and the expansion value of the first expansion carrier in the saturated water absorption process is 1-30. The vaginal suppository can be fully and selectively contacted with vaginal epithelium and mucosal wall, the bioavailability of active drugs is averagely improved by 20-40%, and different positions of a suppository body can be expanded to different degree, so that the problem of low possibility of cleaning a sliver vaginal suppository is thoroughly solved and the problems of poor user compliance, strong discomfort and the like are avoided.
Owner:哈尔滨田美药业股份有限公司
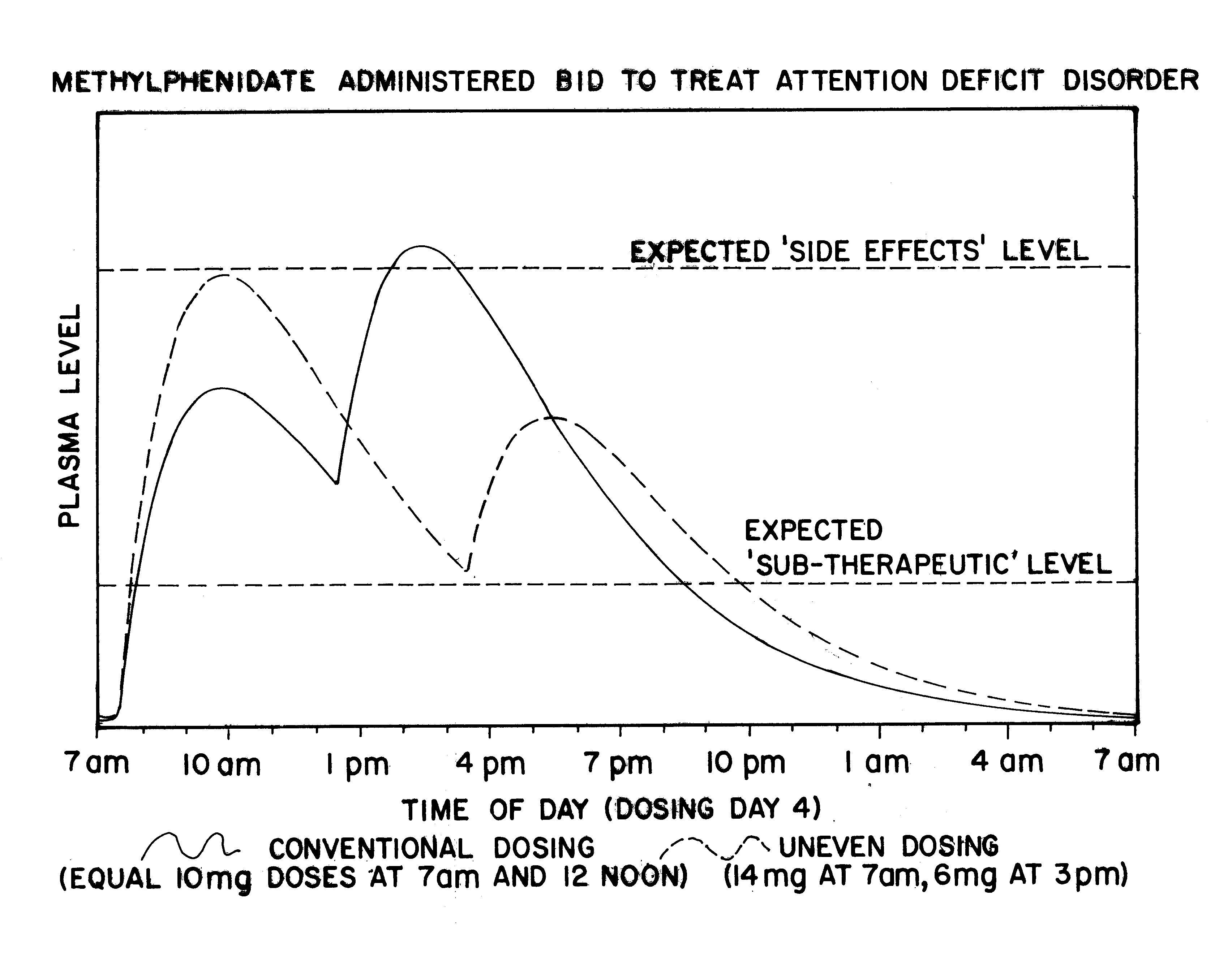
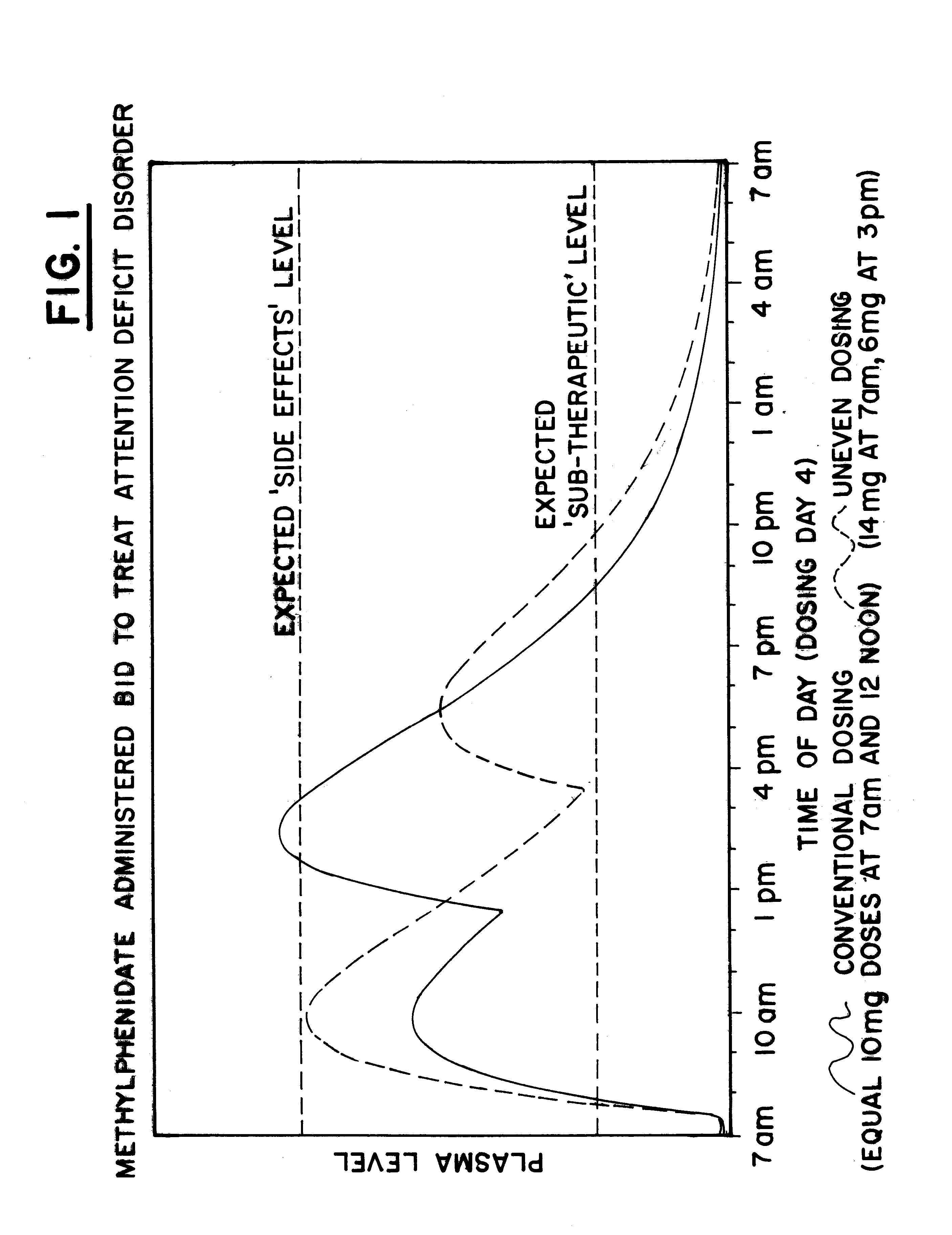
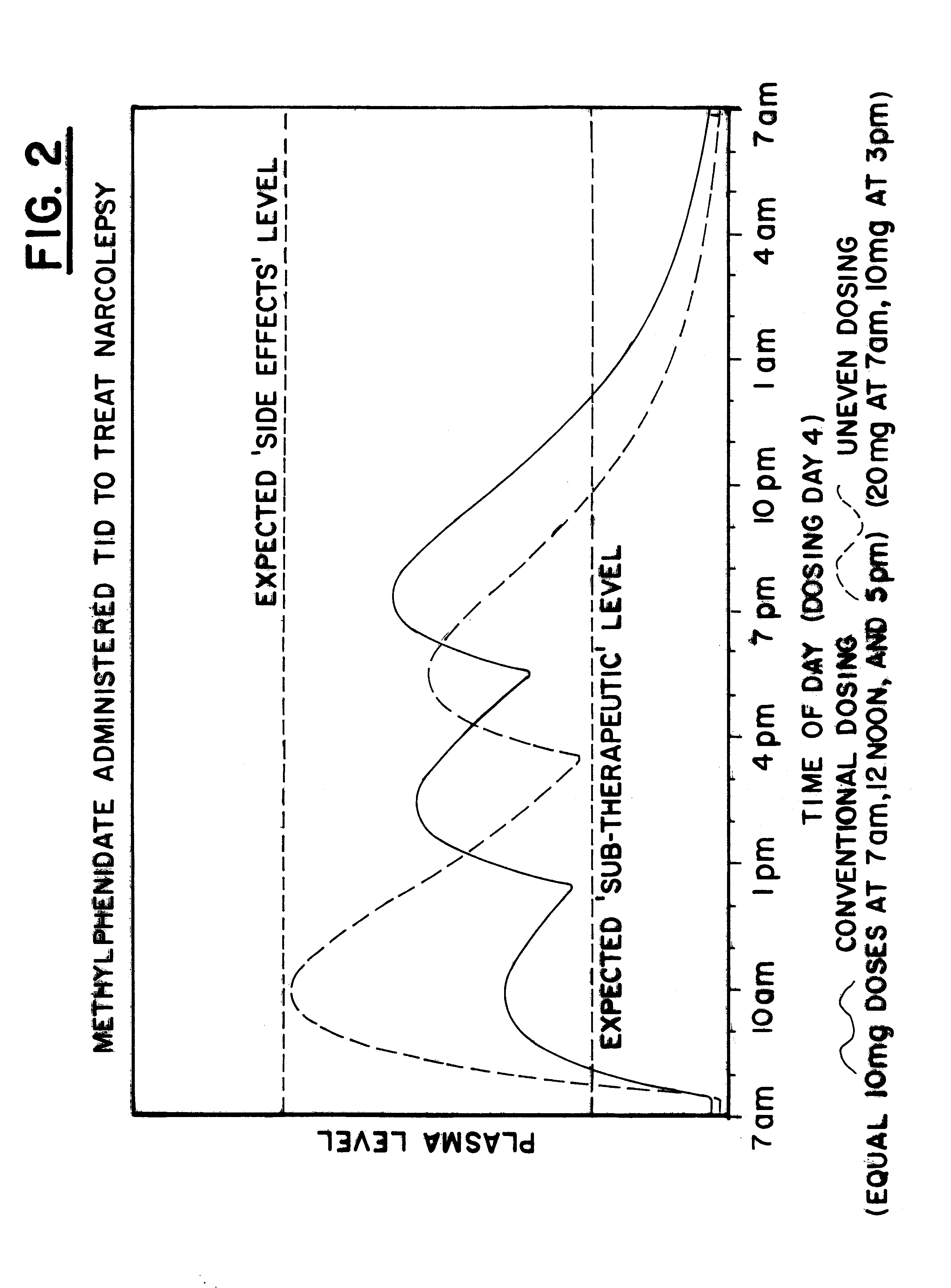
Maximizing effectiveness of substances used to improve health and well being
The present disclosure relates to novel dosage forms, drug delivery regimens, methods and pharmaceutical compositions which optimize the therapeutic effects of active therapeutic substances through the application of the concept of uneven dosing.
Owner:LUMARA HEALTH IP
Compounds and methods for the treatment of urogenital disorders
InactiveUS6987129B2Reduce painLess discomfortBiocidePeptide/protein ingredientsDiseaseFemale Sexual Arousal Disorder
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com