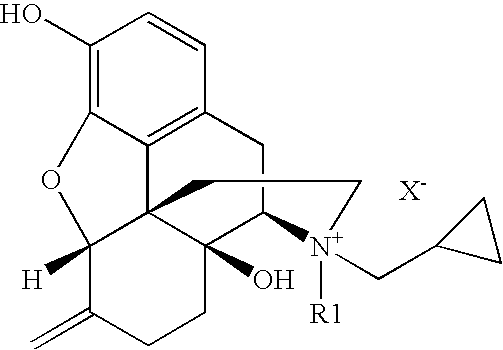
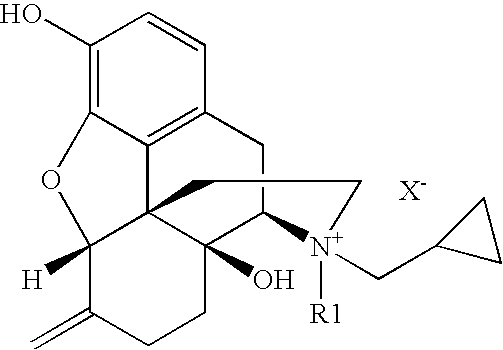
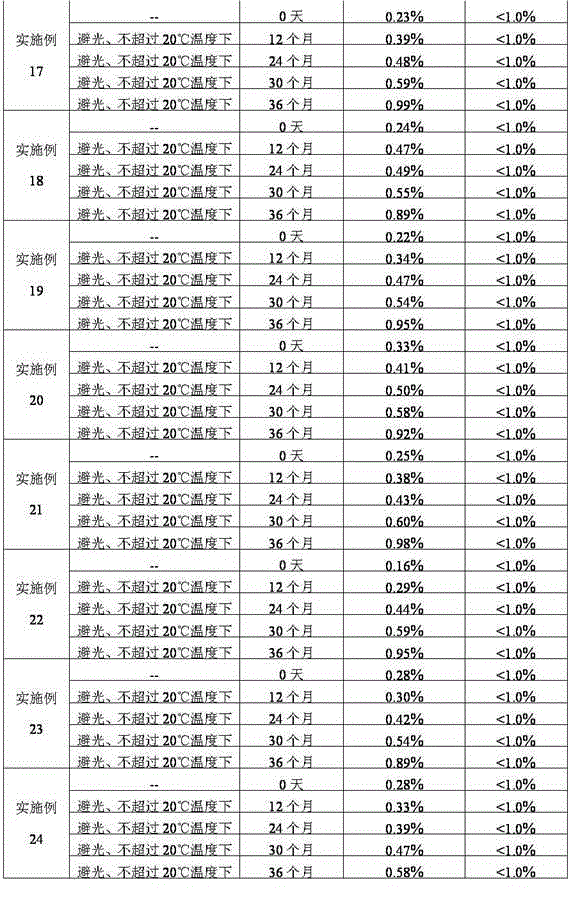
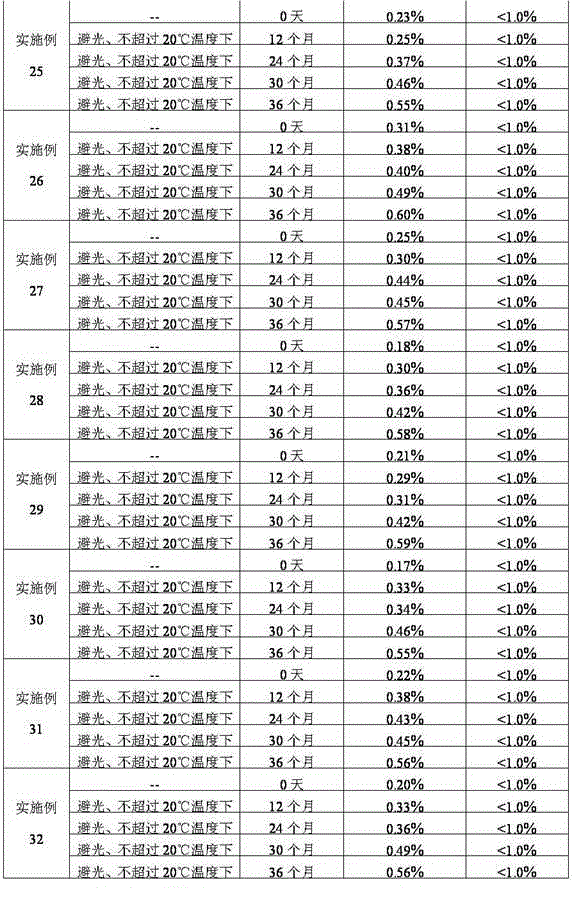
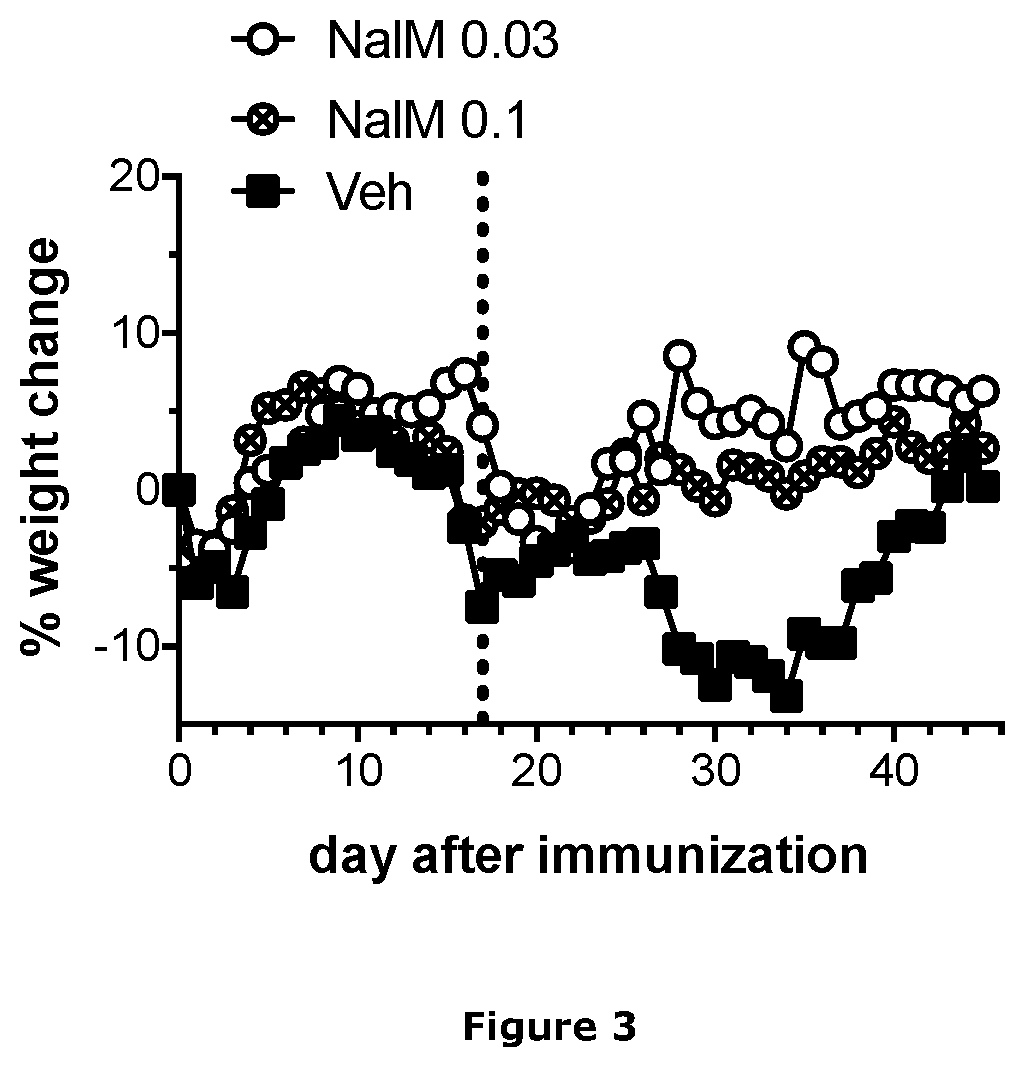
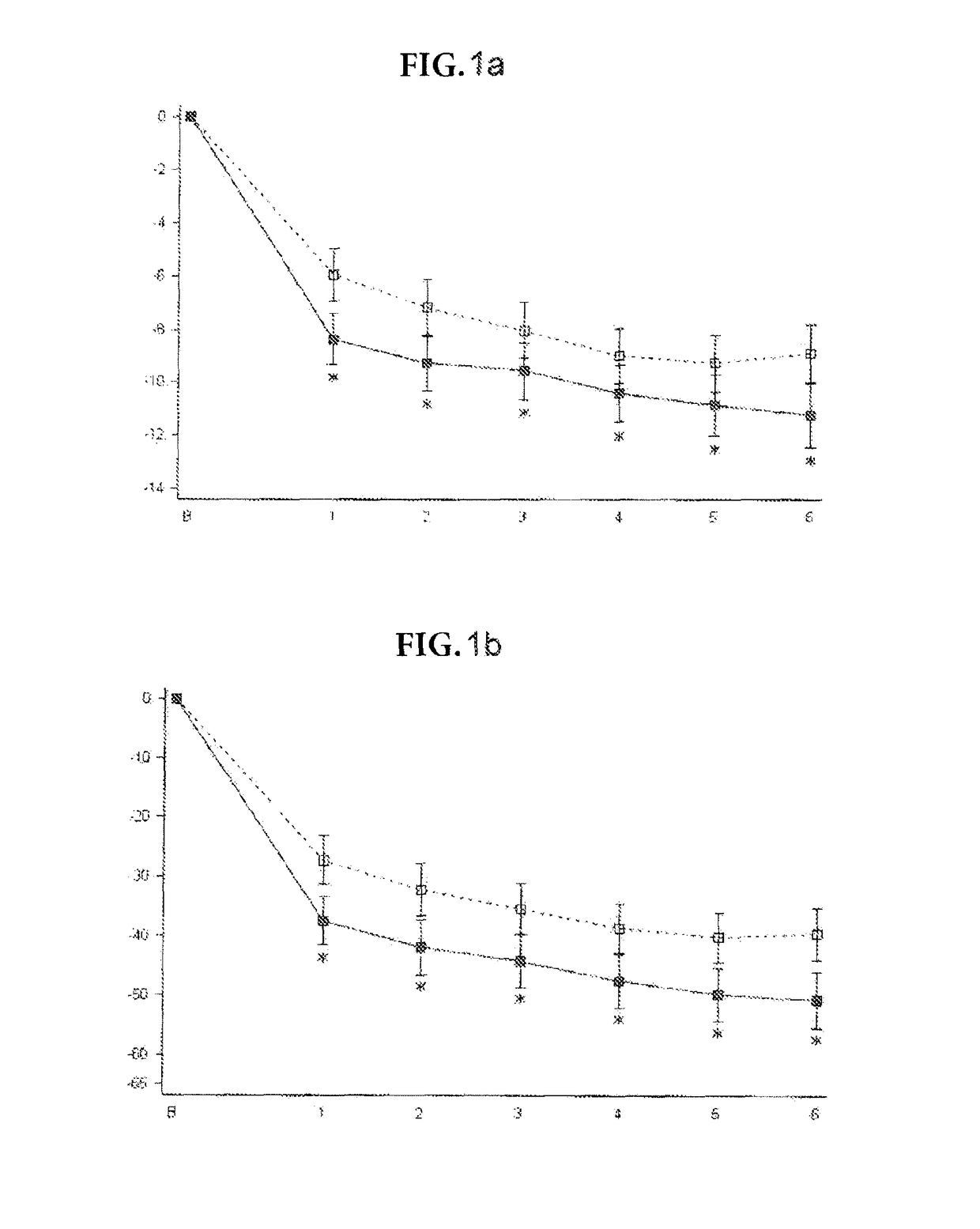
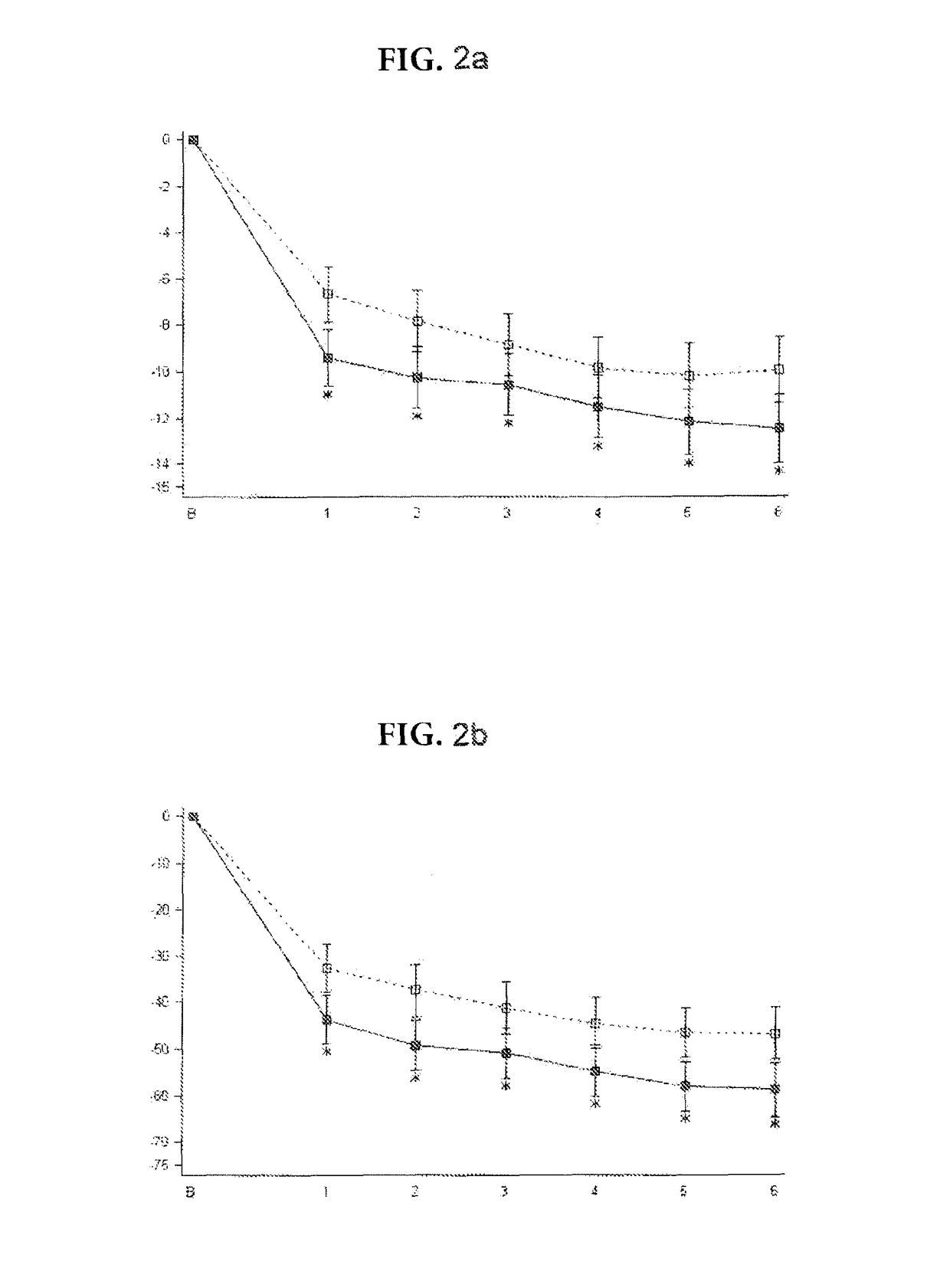
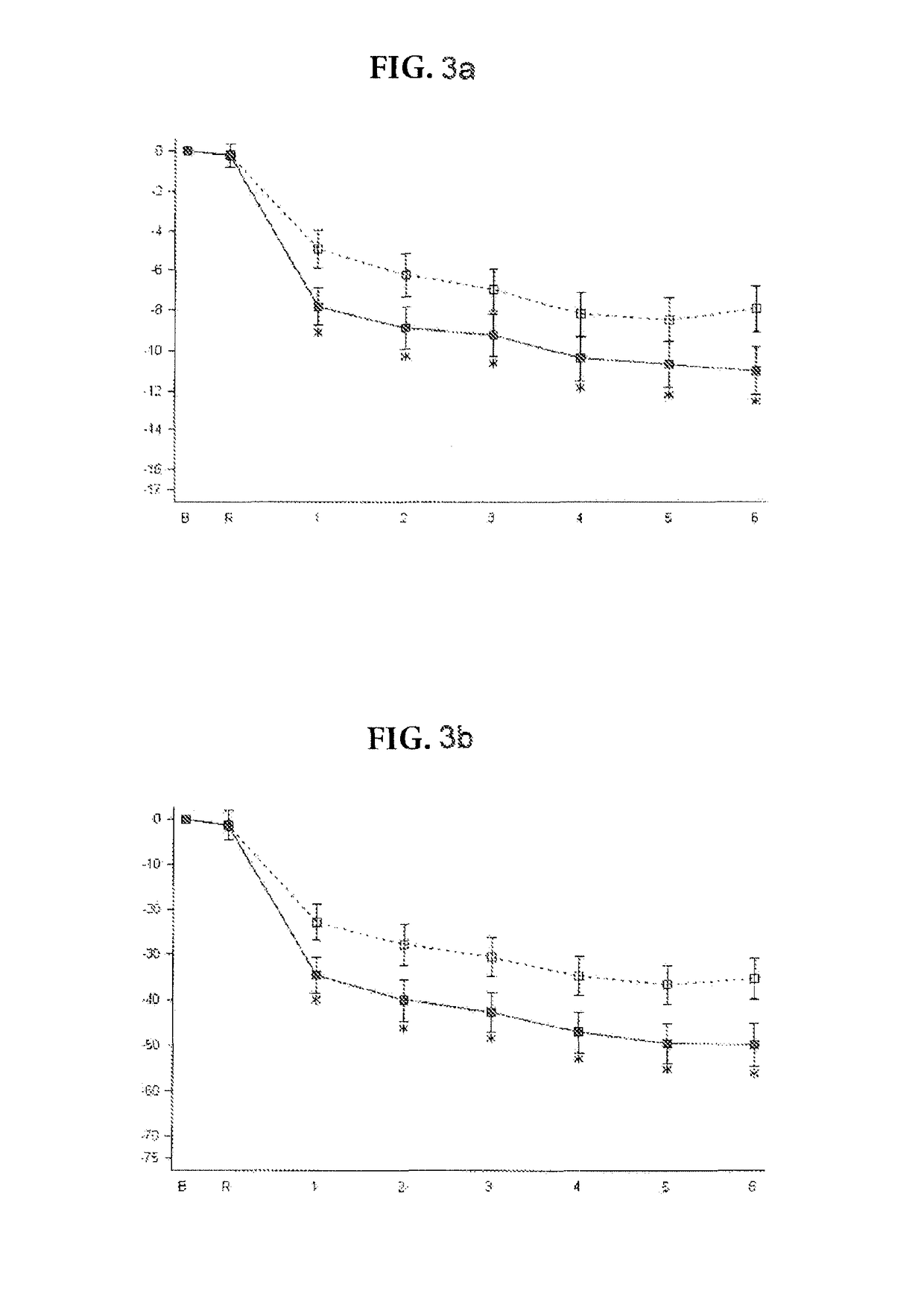
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Nalmefene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
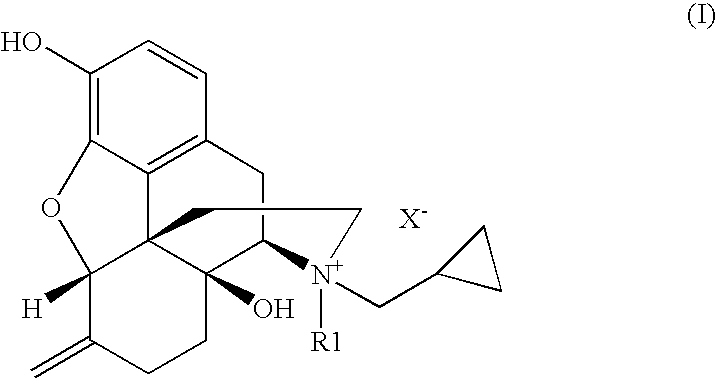
Nalmefene (originally known as nalmetrene; trade name Selincro) is an opioid antagonist used primarily in the management of alcohol dependence. It has also been investigated for the treatment of other addictions such as pathological gambling.
Smoking cessation treatments using naltrexone and related compounds
InactiveUS6541478B1Reduce weight gainPrevent relapseBiocideNervous disorderOpioid antagonistAntianxiety Agent
Nicotine dependency is treated by administration of an opioid antagonist. In some embodiments, rapid or ultra rapid detoxification techniques include using a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone or naltrexone or a mixture of any one of these, and either clonidine or related compounds either while awake, or while under sedation or anesthesia, followed by continued administration of an effective amount of an opioid antagonist with or without agents that enhance nicotine dependency treatment. Persons are also treated for nicotine dependency with more gradual detoxification methods using administration of a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone, naltrexone, or a mixture of any of these, and an effective amount of agents used to treat nicotine withdrawal including nicotine, such as that delivered by a nicotine patch, nicotine chewing gum, nicotine inhaler or other methods for delivering nicotine, antidepressants and antianxiety agents, and / or clonidine and related compounds. Administration of an effective amount of an opioid antagonist to prevent relapse, attenuate craving, and reduce weight gain during and after treatment for nicotine dependency is continued in some embodiments.
Owner:YALE UNIV
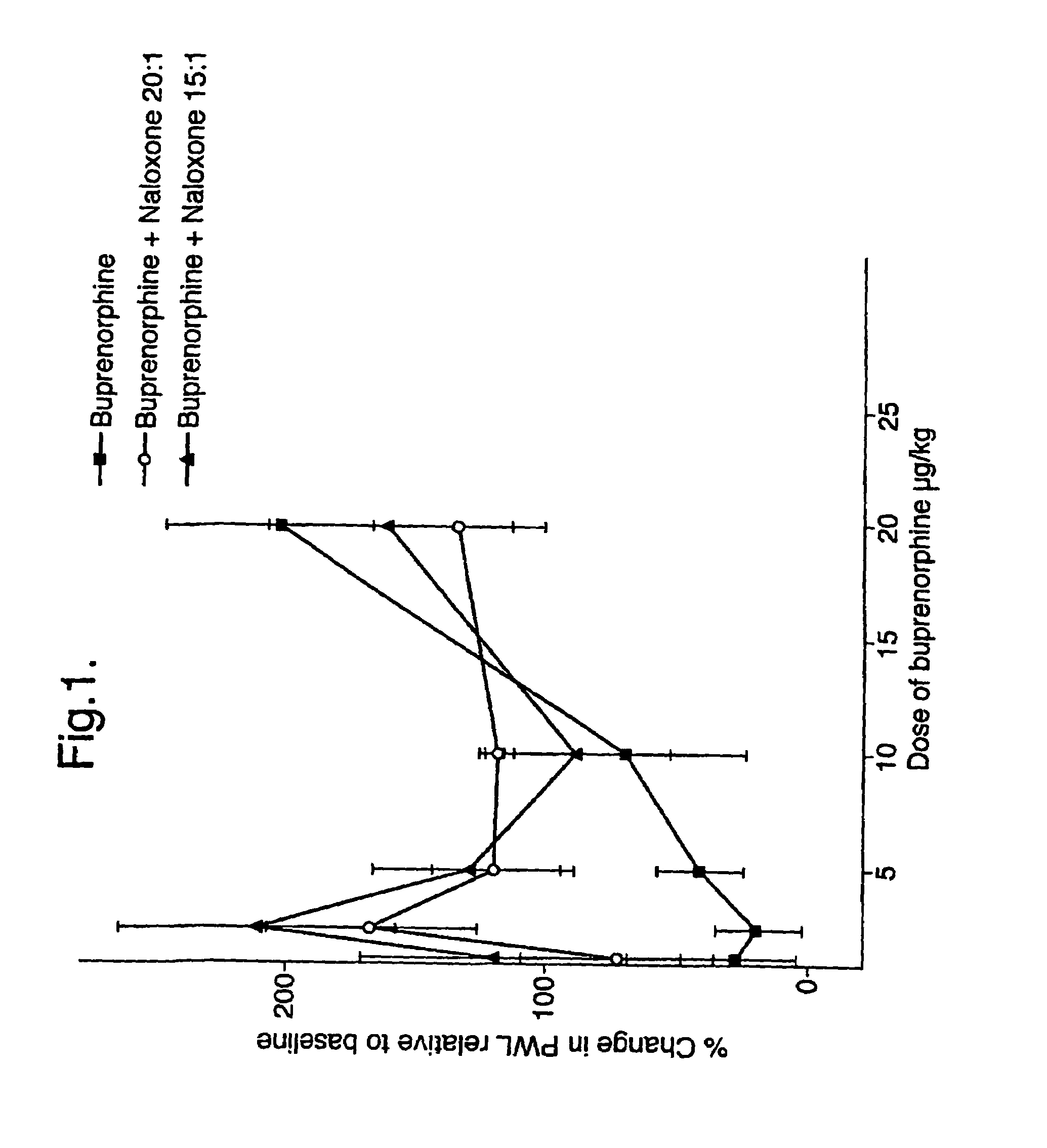
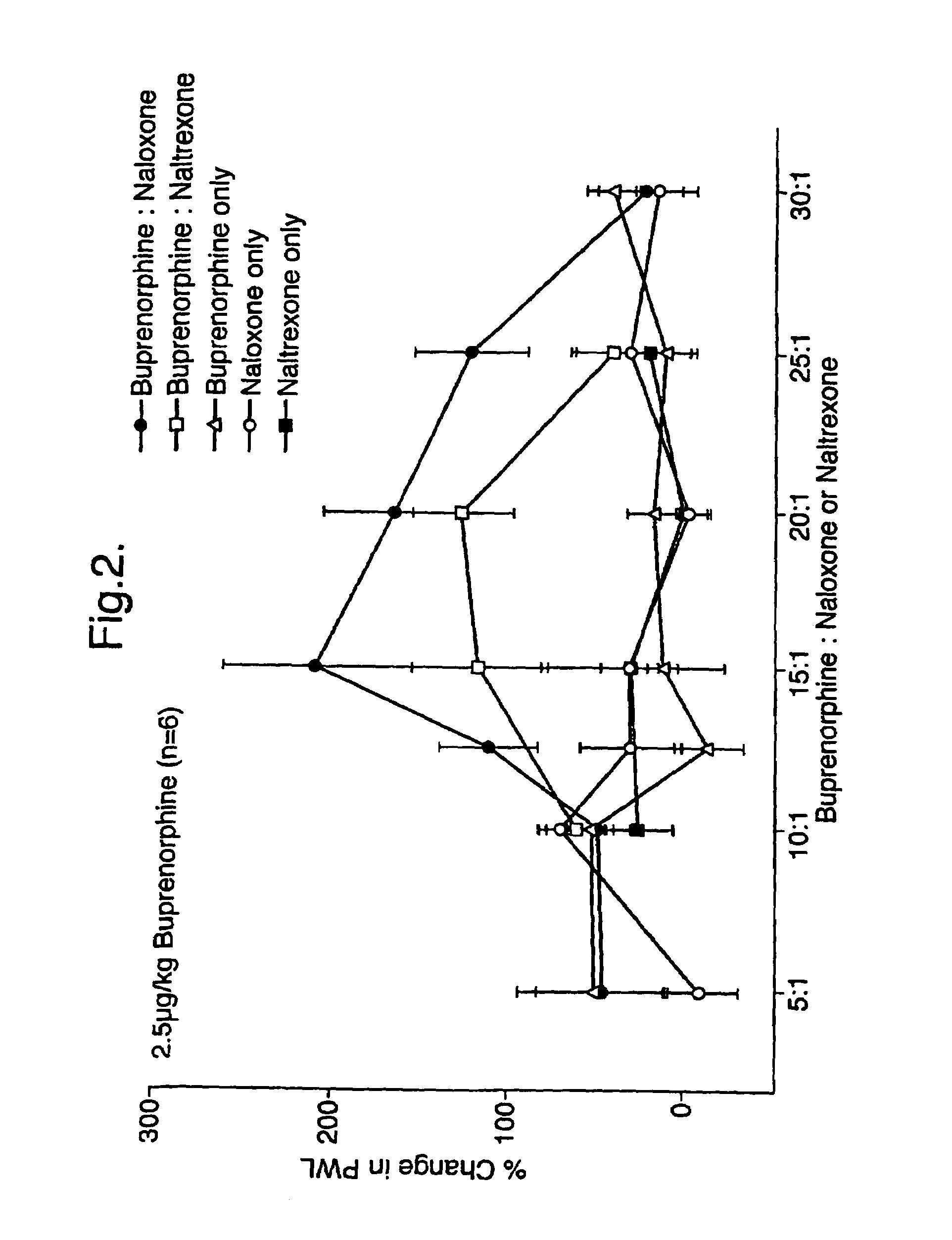
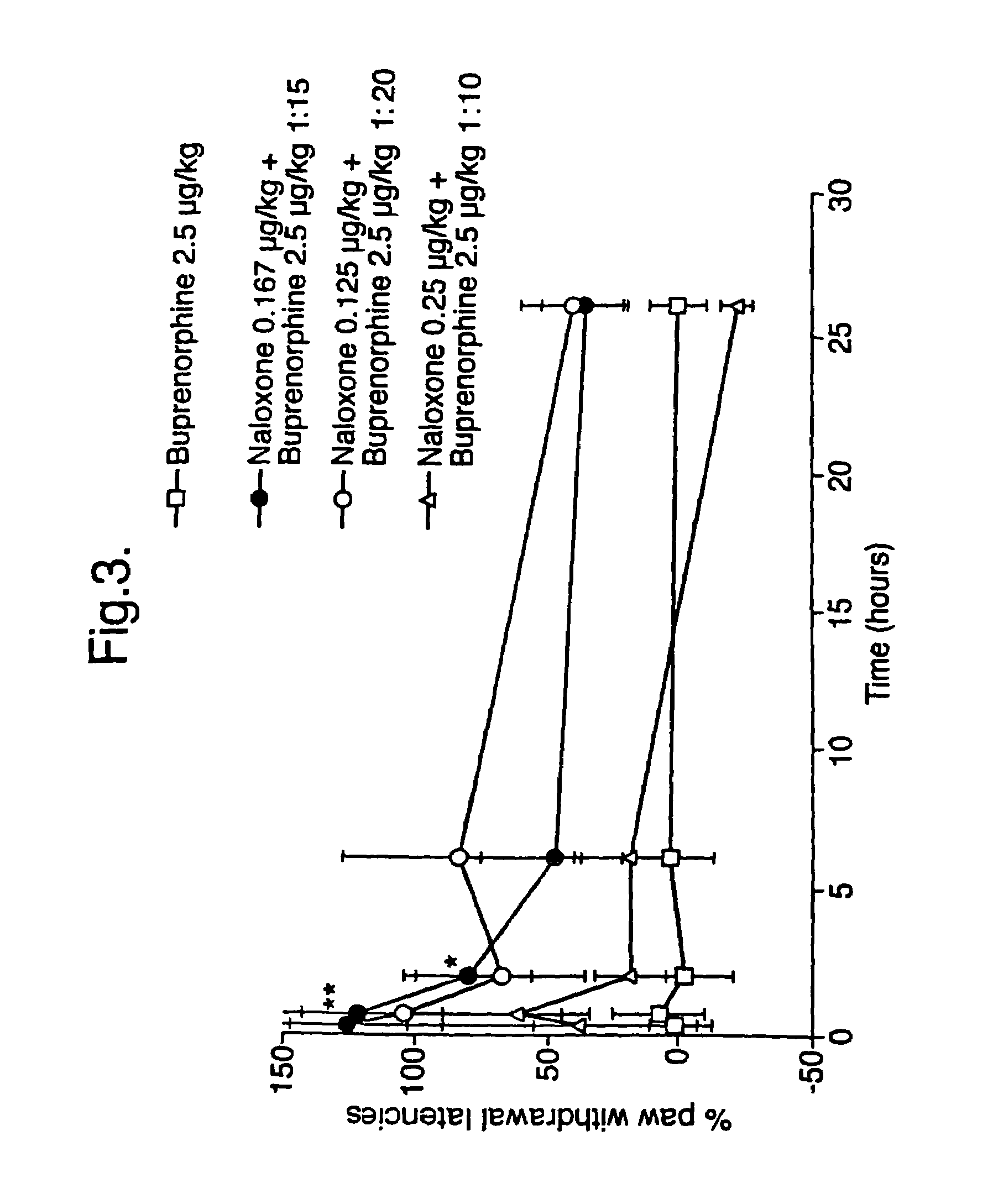
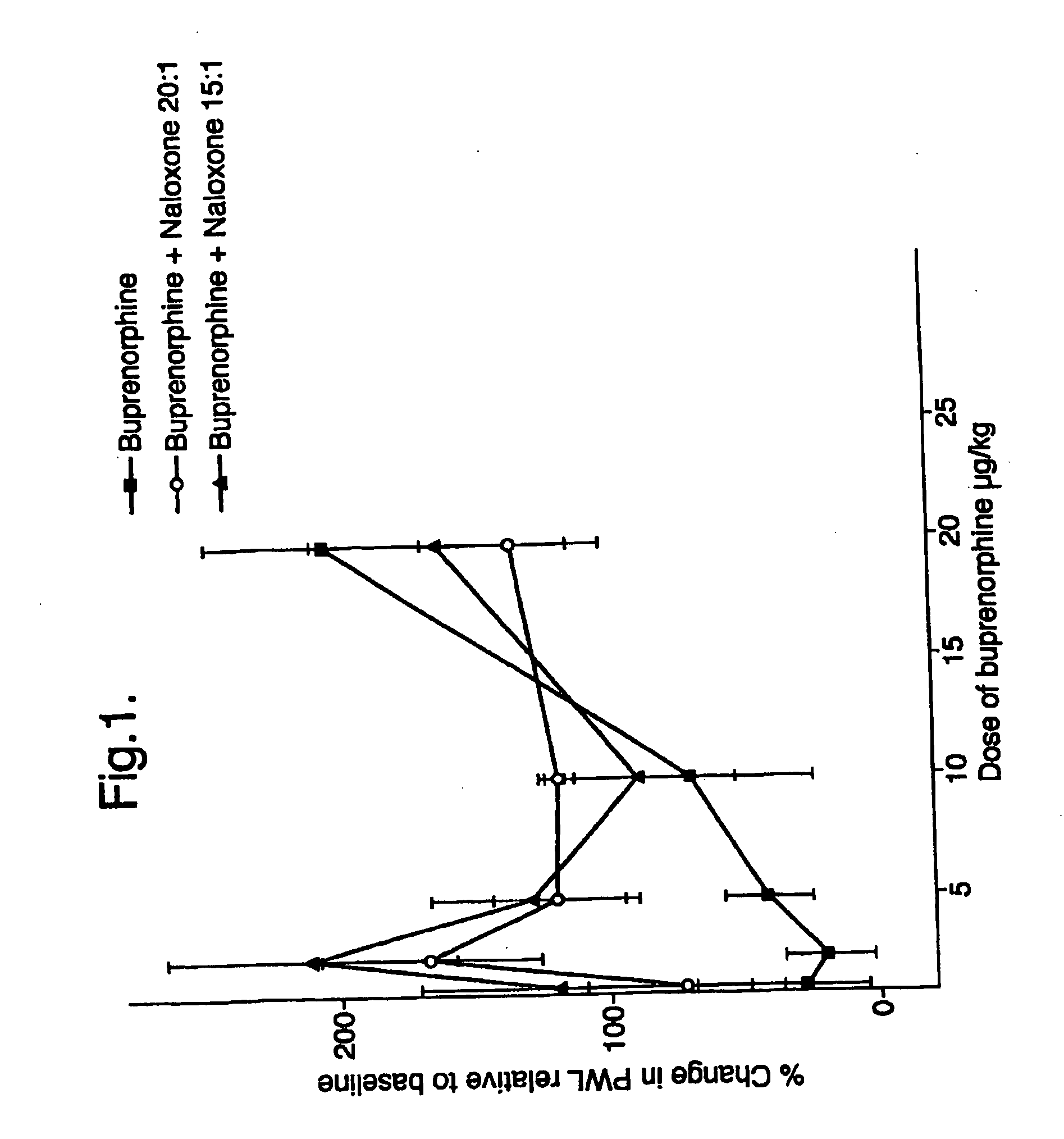
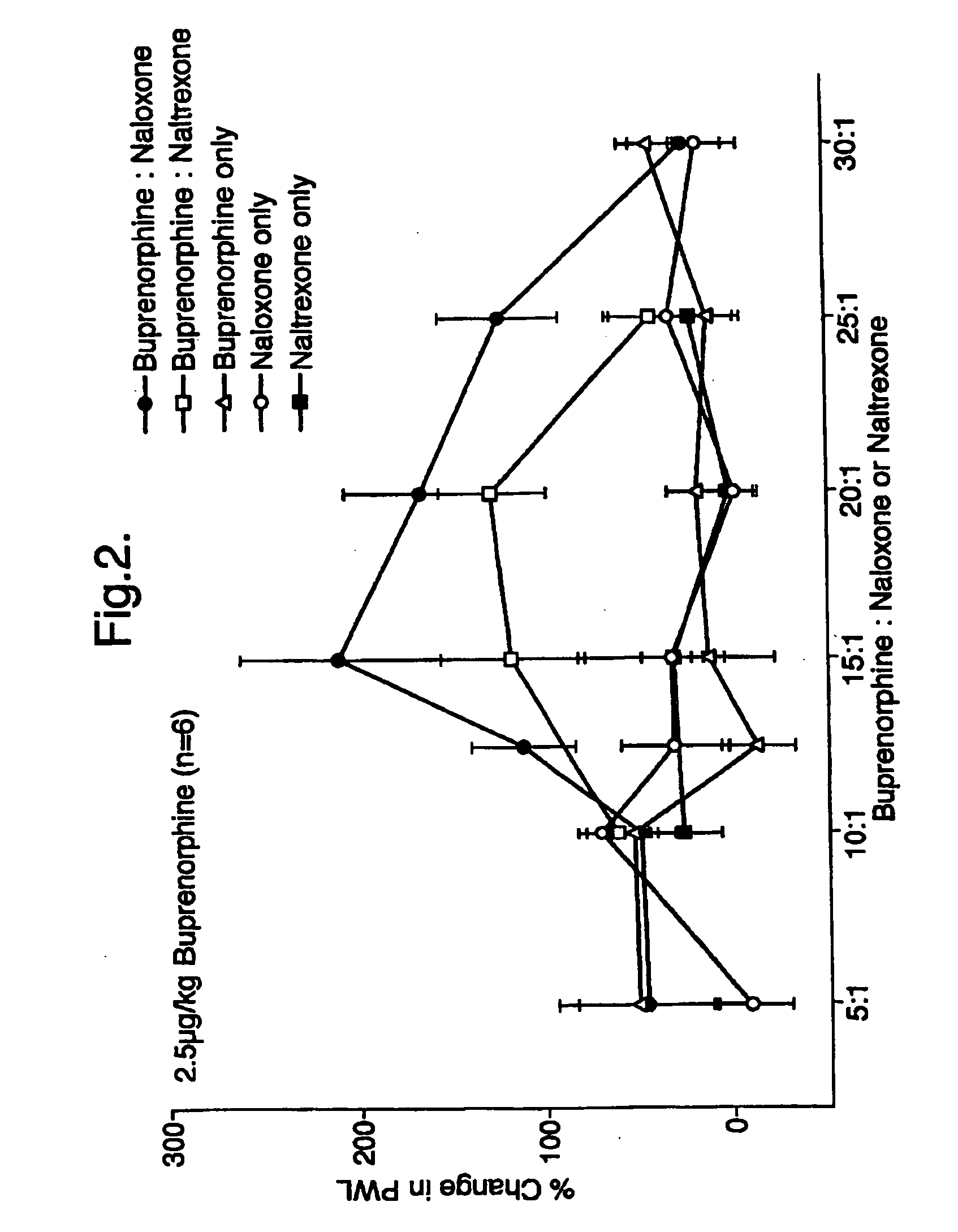
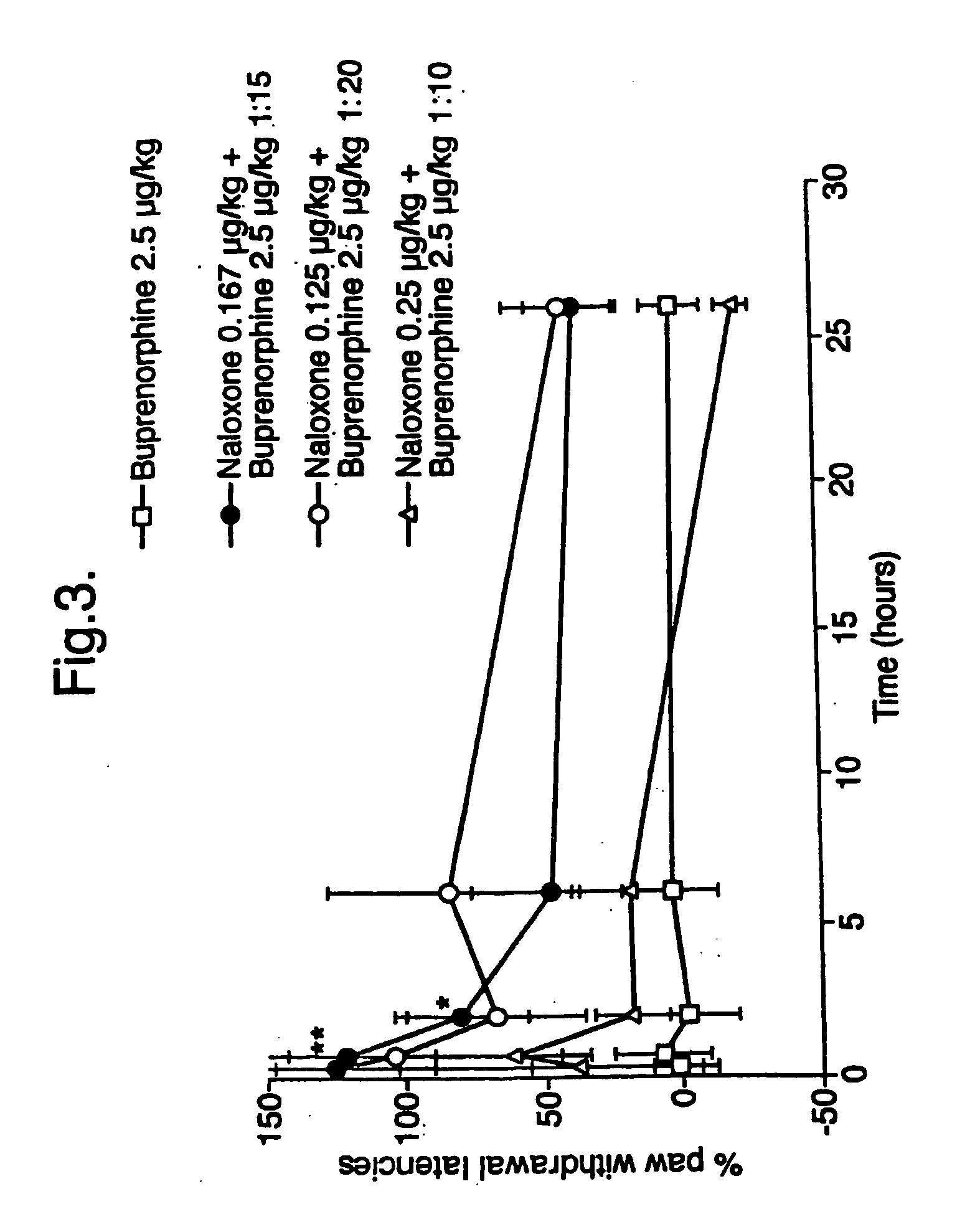
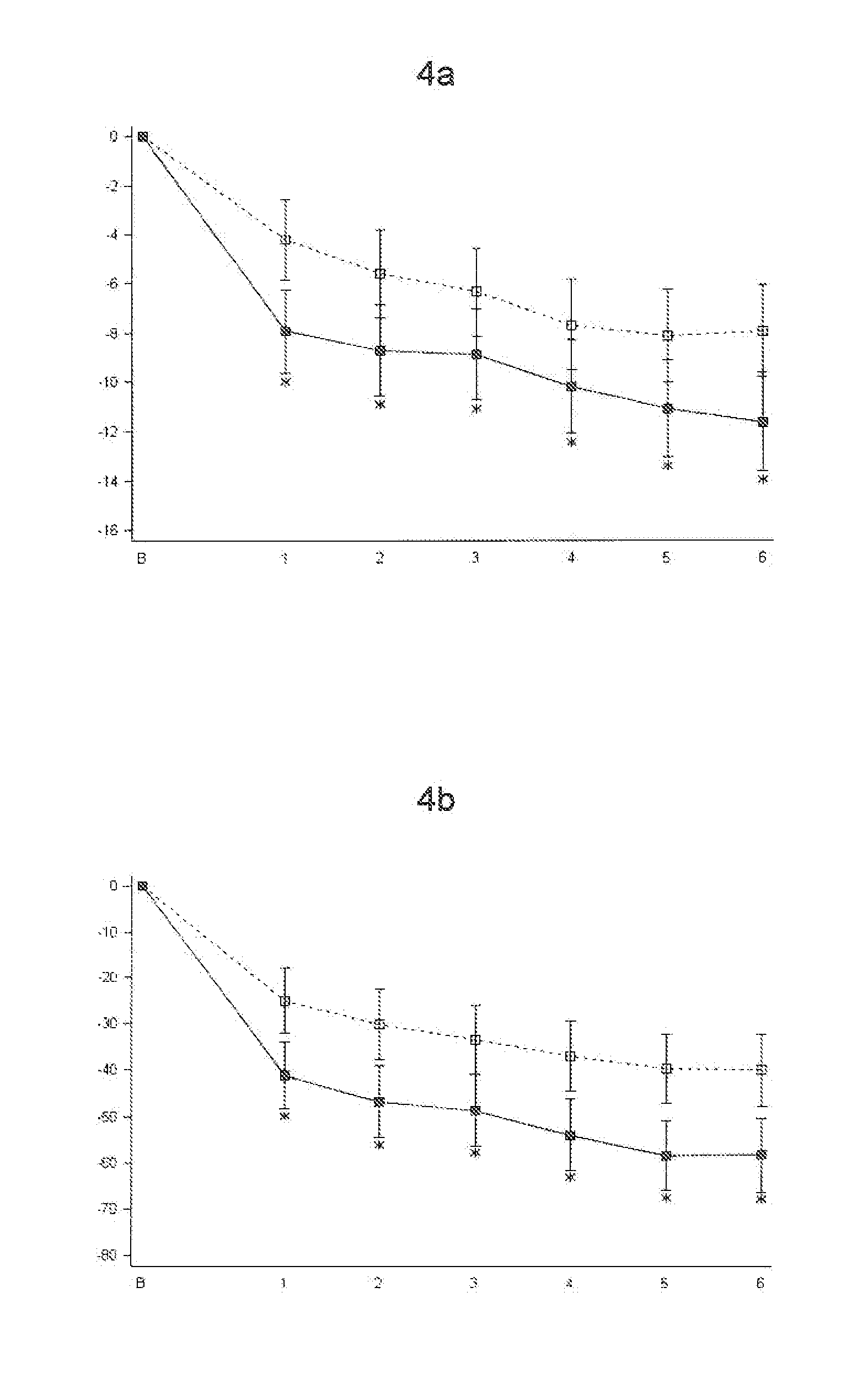
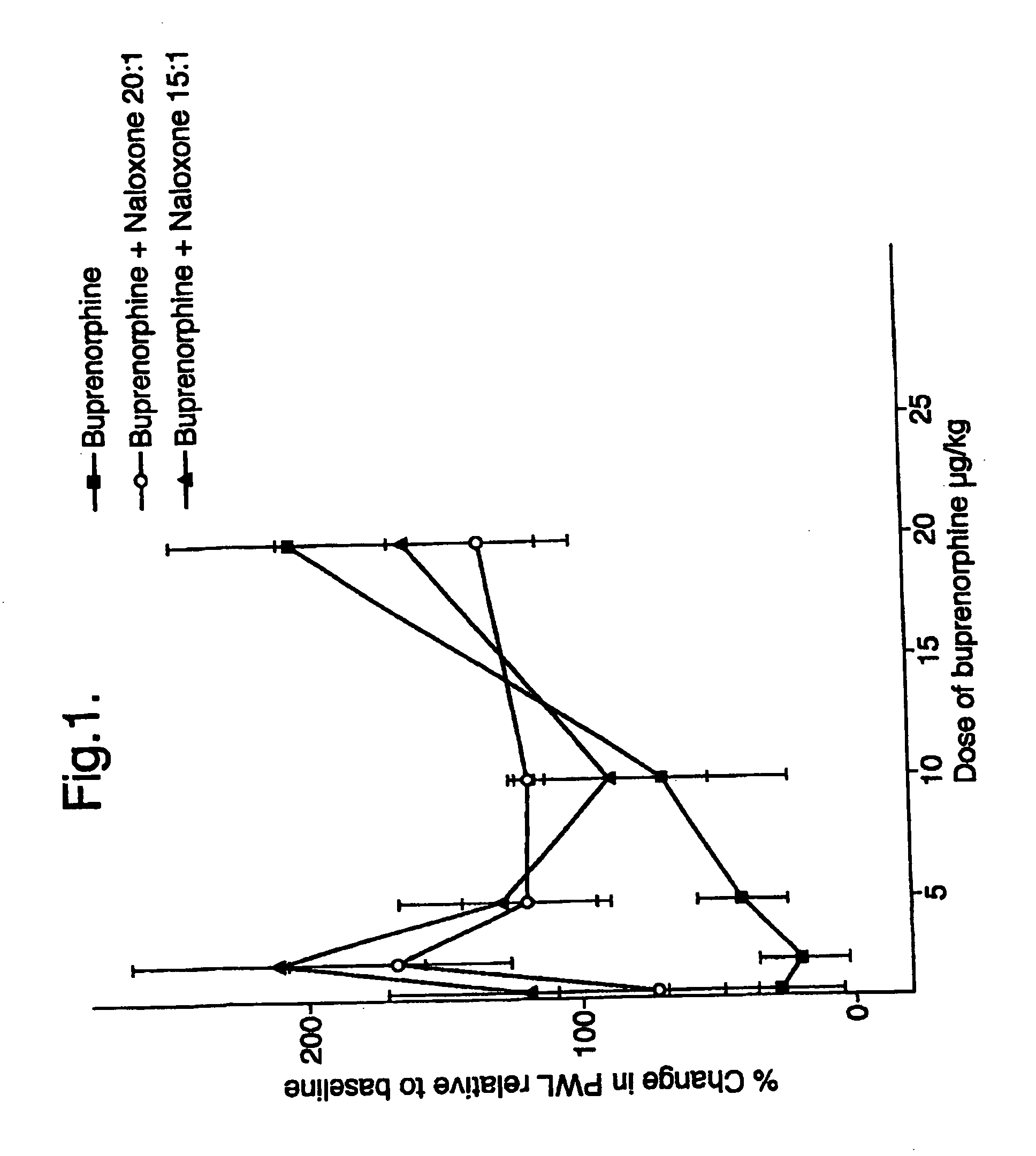
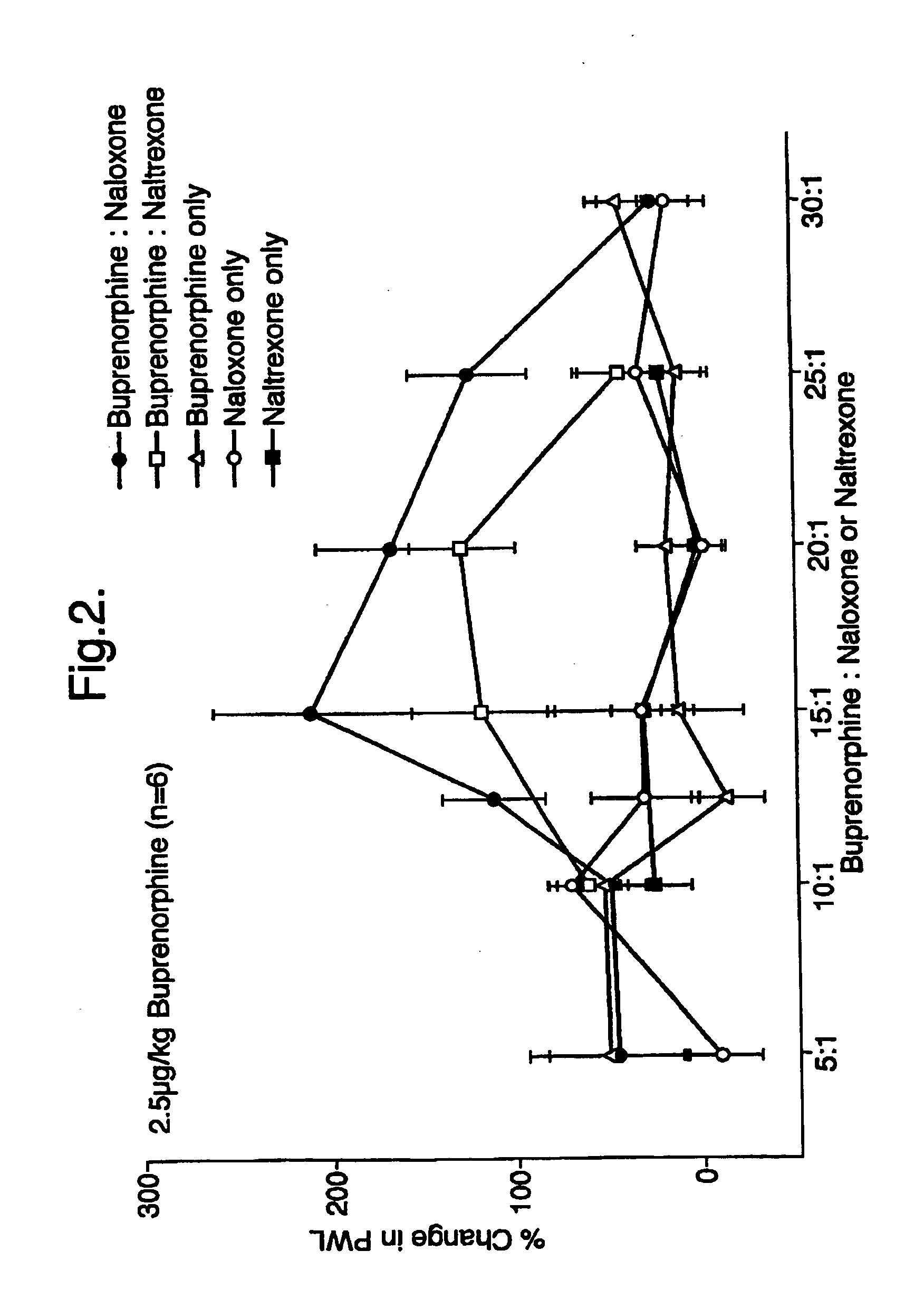
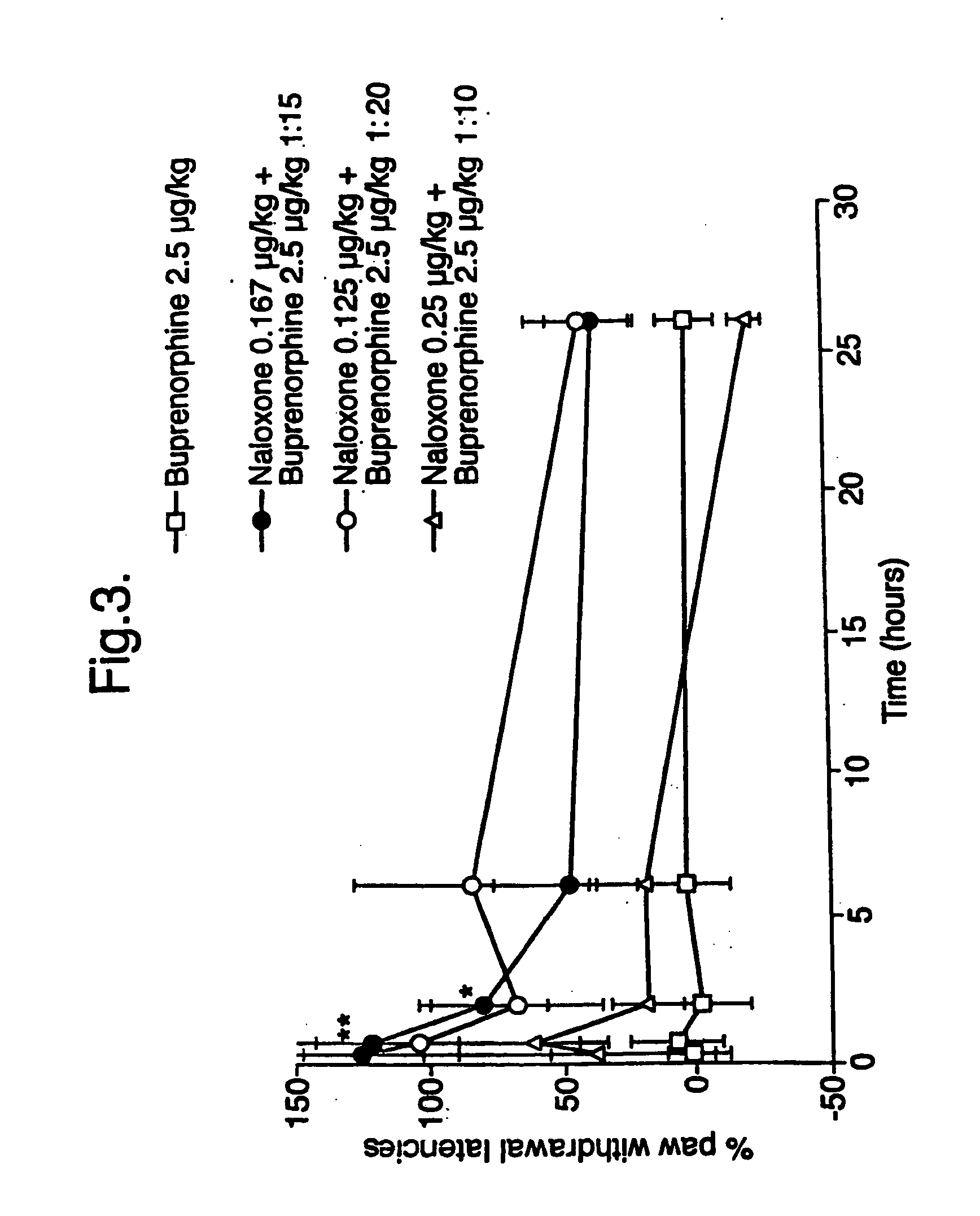
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
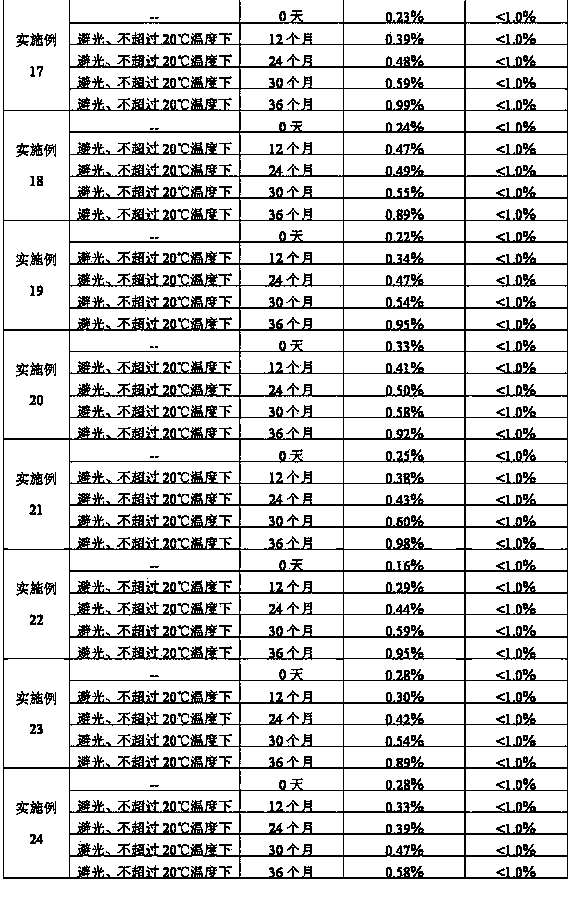
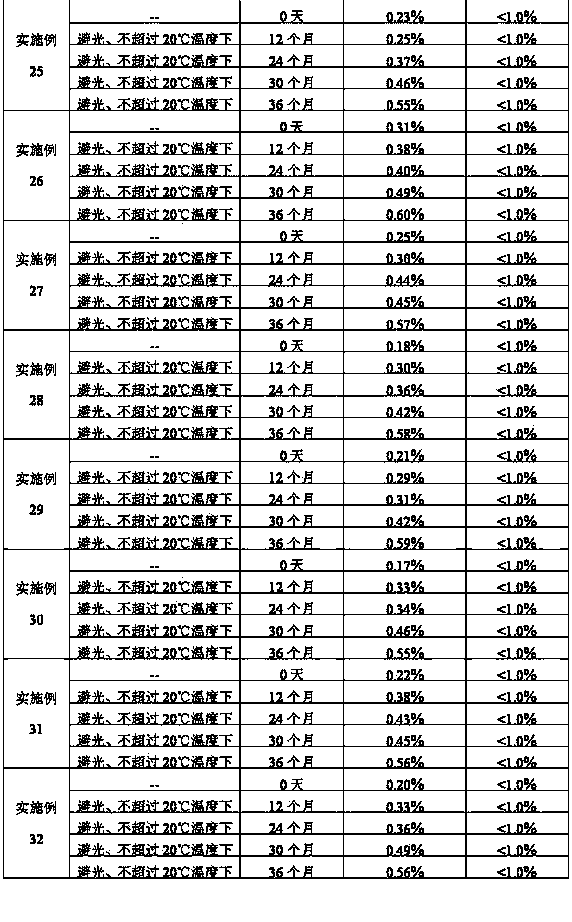
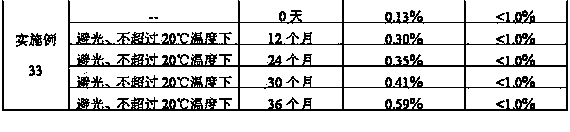
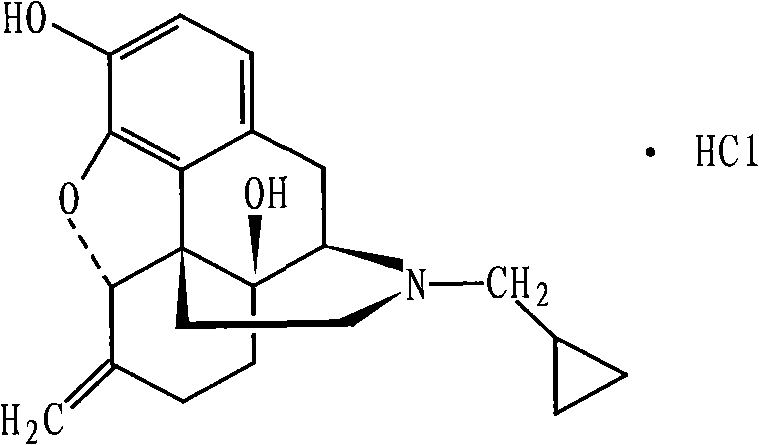
Stabilized nalmefene hydrochloride injection and its preparation
ActiveCN1895251AReasonable compositionSimple processOrganic active ingredientsNervous disorderGlucose polymersD-Glucose
A high-stability nalmefene hydrochloride injection is prepared from nalmefene hydrochloride (0.005-0.2 W / v %) and the medicinal carrier chosen from sodium chloride, glucose, beta-cyclodextrin, dextran, pectose, sorbitol, etc. Its preparing process is also disclosed.
Owner:西藏易明西雅医药科技股份有限公司
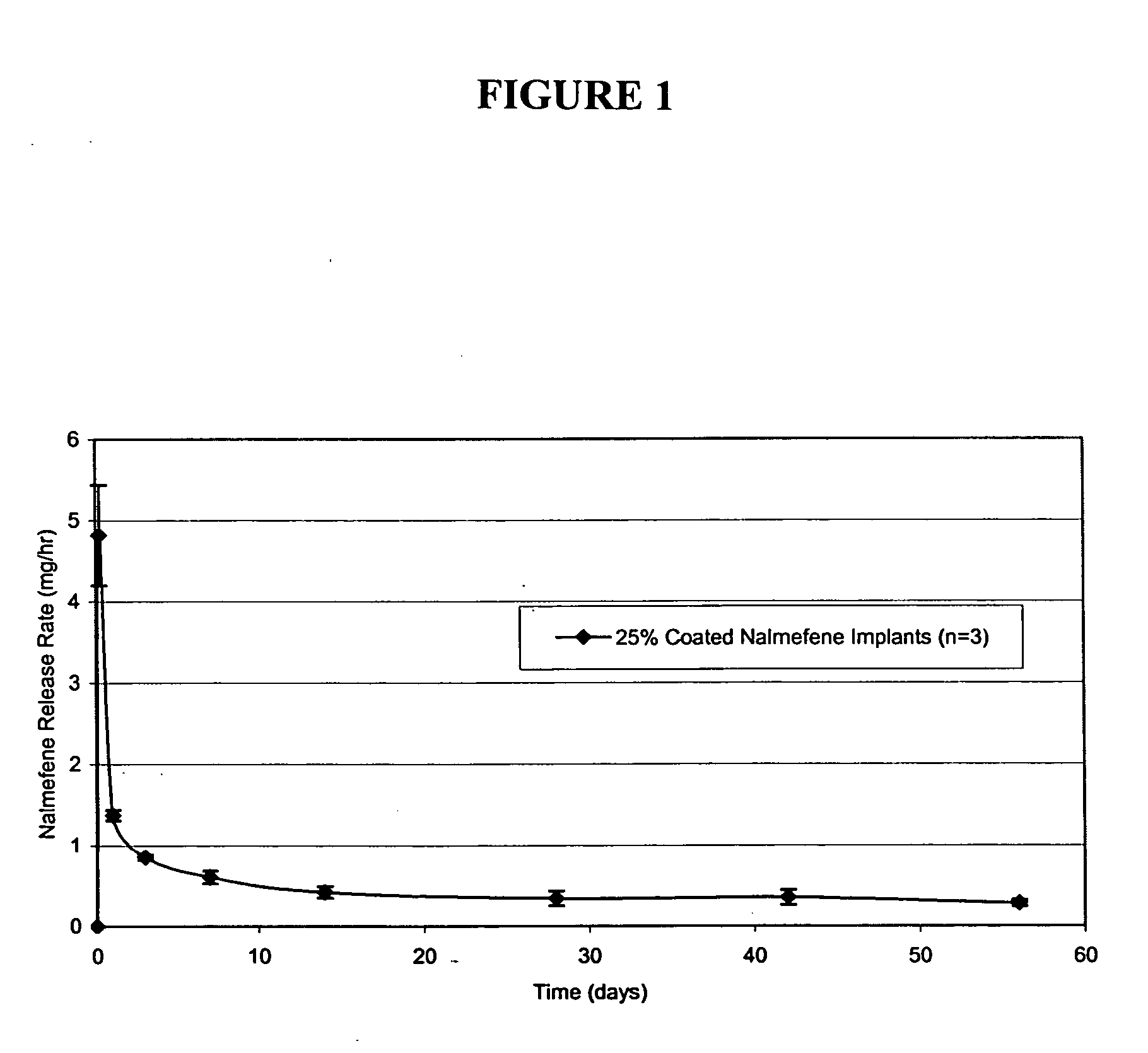
Implantable polymeric device for sustained release of nalmefene
The present invention provides compositions, methods, and kits for administration of nalmefene for treatment of alcoholism, nicotine dependence, or another condition for which treatment with nalmefene is therapeutically beneficial. The invention provides a biocompatible nonerodible polymeric device which releases nalmefene continuously with generally linear release kinetics for extended periods of time. Nalmefene is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with nalmefene.
Owner:TITAN PHARMA
Compositions and methods for enhancing analgesic potency of covalently bound-compounds, attenuating its adverse side effects, and preventing their abuse
InactiveUS20100144645A1Lower potentialAmenable to synthesizing conjugatesBiocideNervous disorderChemical MoietyOpioid antagonist
The invention generally relates to compositions and methods with covalently bound compounds, such as controlled substances covalently attached to a chemical moiety, and opioid antagonists or covalently bound opioid antagonists to enhance analgesic potency and / or attenuate one or more adverse effects of covalently bound compounds, including adverse side effect(s) in humans such as nausea, vomiting, dizziness, headache, sedation (somnolence), physical dependence or pruritis. This invention relates to compositions and methods for selectively enhancing the analgesic potency of a covalently bound compound and simultaneously attenuating anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects associated with the administration of a covalently bound compound. The methods of the invention comprise administering to a subject an analgesic or sub-analgesic amount of a covalently bound compound and an amount of excitatory opioid receptor antagonist such as naltrexone or nalmefene effective to enhance the analgesic potency of a covalently bound compound and attenuate the anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects of covalently bound compound. The invention also relates to the addition of covalently-bound opioid antagonists to the compositions containing covalently bound compounds such that if the compositions are subjected to manipulation by illicit chemists, the opioid antagonist is released effectively reducing or eliminating the euphoric effect of the covalently bound compounds.
Owner:SHIRE PLC
Method of treating alcoholism or alcohol abuse
The present invention relates to a method of treating alcoholism or alcohol abuse by administering to a subject a pharmaceutically effective amount of an opioid antagonist before imminent drinking. Particularly, the present invention relates to a method of treating alcoholism or alcohol abuse by administering transmucosally to a subject a pharmaceutically effective amount of an opioid antagonist before imminent drinking. Preferably, the opioid antagonist used in the method is nalmefene or a pharmaceutically acceptable salt thereof. The invention also relates to a method of treating alcoholism or alcohol abuse by administering to a subject before imminent drinking a transmucosal preparation comprising a pharmaceutically effective amount of an opioid antagonist, wherein the transmucosal preparation has rapid onset of action. Advantageously, a FAH+ subject is treated. Further, the invention relates to a method of treating alcoholism or alcohol abuse of a FAH+ subject, comprising extinguishing an alcohol-drinking response by administering to the FAH+ subject a pharmaceutically effective amount of nalmefene or a pharmaceutically acceptable salt thereof.
Owner:CONTRAL PHARMA
Opiates painkiller and opiate receptor antagonist-containing medicinal composition
ActiveCN102068697AGood analgesic effectPrevent and/or mitigate adverse effectsOrganic active ingredientsNervous disorderSide effectNK1 receptor antagonist
The invention provides an opiates painkiller and opiate receptor antagonist-containing medicinal composition. In the medicinal composition, an opiates painkiller is fentanyl, remifentanil, sufentanil, alfentanil and pharmaceutically acceptable salts thereof; and an opiate receptor antagonist is naloxone, naltrexone, nalmefene and pharmaceutically acceptable salts thereof. The medicinal composition has a pharmacological effect on analgesia. Compared with using the opiates painkiller singly, the composition can prevent and / or lighten side effects in pain treatment, reduce abuse and improve adaptability, and has a reinforcing effect on an analgesic effect of the opiates painkiller.
Owner:YICHANG HUMANWELL PHARMA
Nalmefene hydro chloride lyophilized powder formulation for injection
InactiveCN1813739AImprove solubilitySimple manufacturing processOrganic active ingredientsPowder deliveryFreeze-dryingDextran
The present invention relates to a nalmefene hydrochloride freeze-dried powder injection preparation for injection. Said preparation is formed from effective component malmefene hydrochloride and proper medicinal carrier, in which the content range of nalmefene hydrochloride in the preparation is 0.1-4.5 mg generally, the medicinal carrier can be one or several kinds selected from mannitol, glucose, sodium chloride, beta-cyclodextrin, glucosan, fructose and sorbitol. The content range of said medicinal carrier can be 10-100 mg. the pH value of said preparation is 5.0-7.0.
Owner:王颖
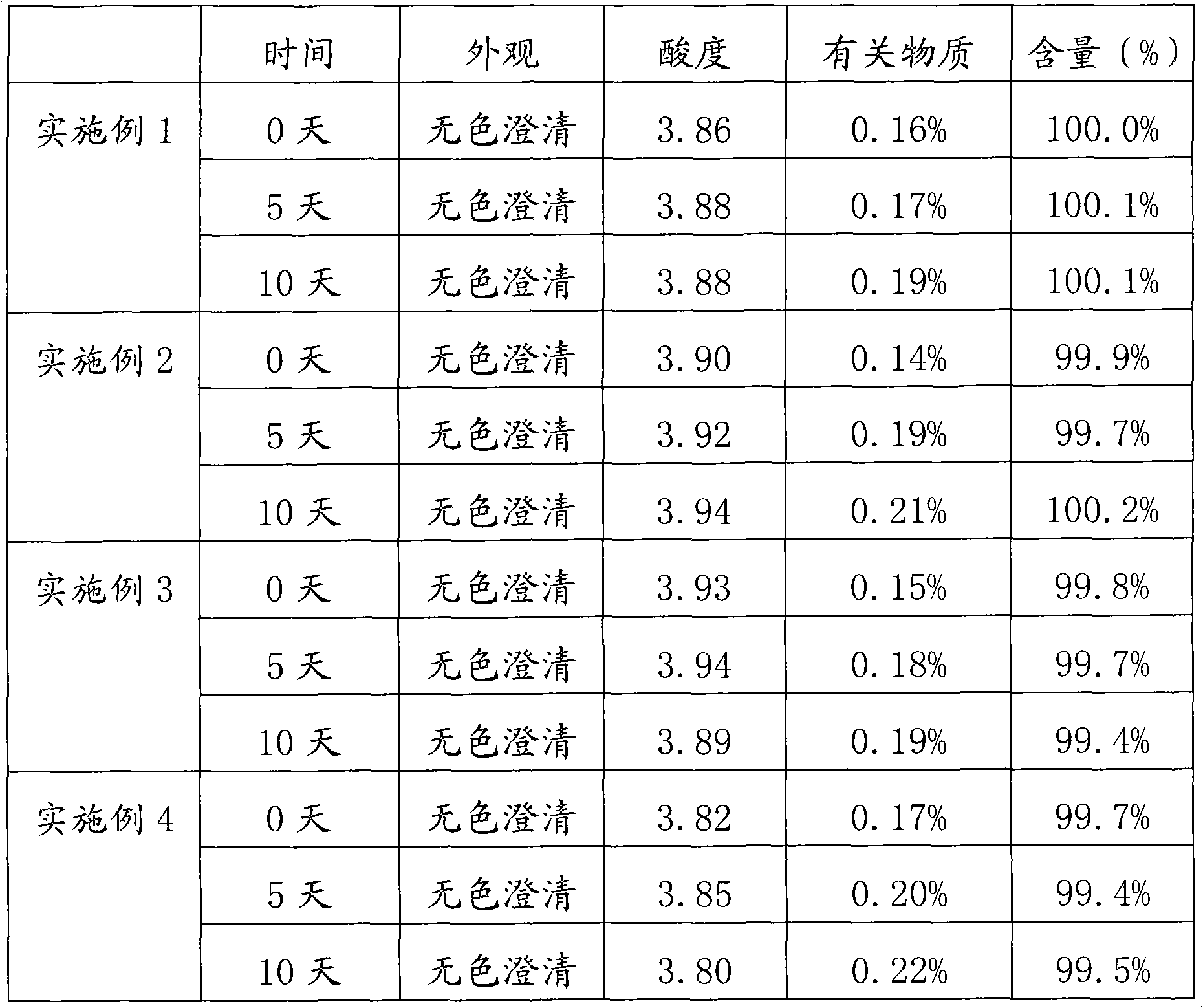
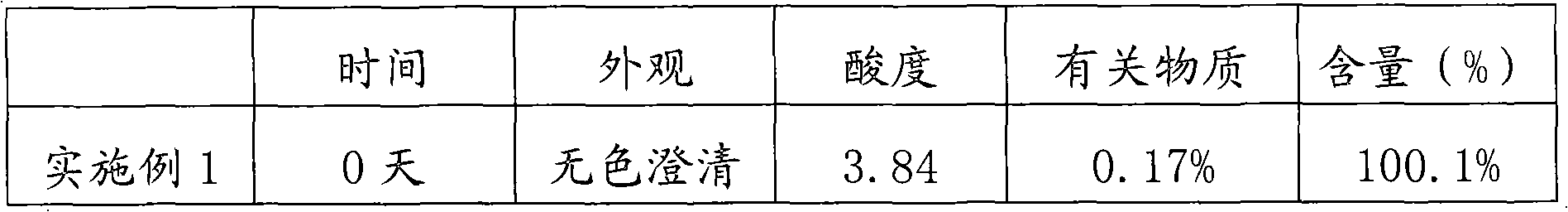
Method for preparing nalmefene hydrochloride injection and prepared nalmefene hydrochloride injection
InactiveCN103202806AExcellent pHImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSample appearanceAdditive ingredient
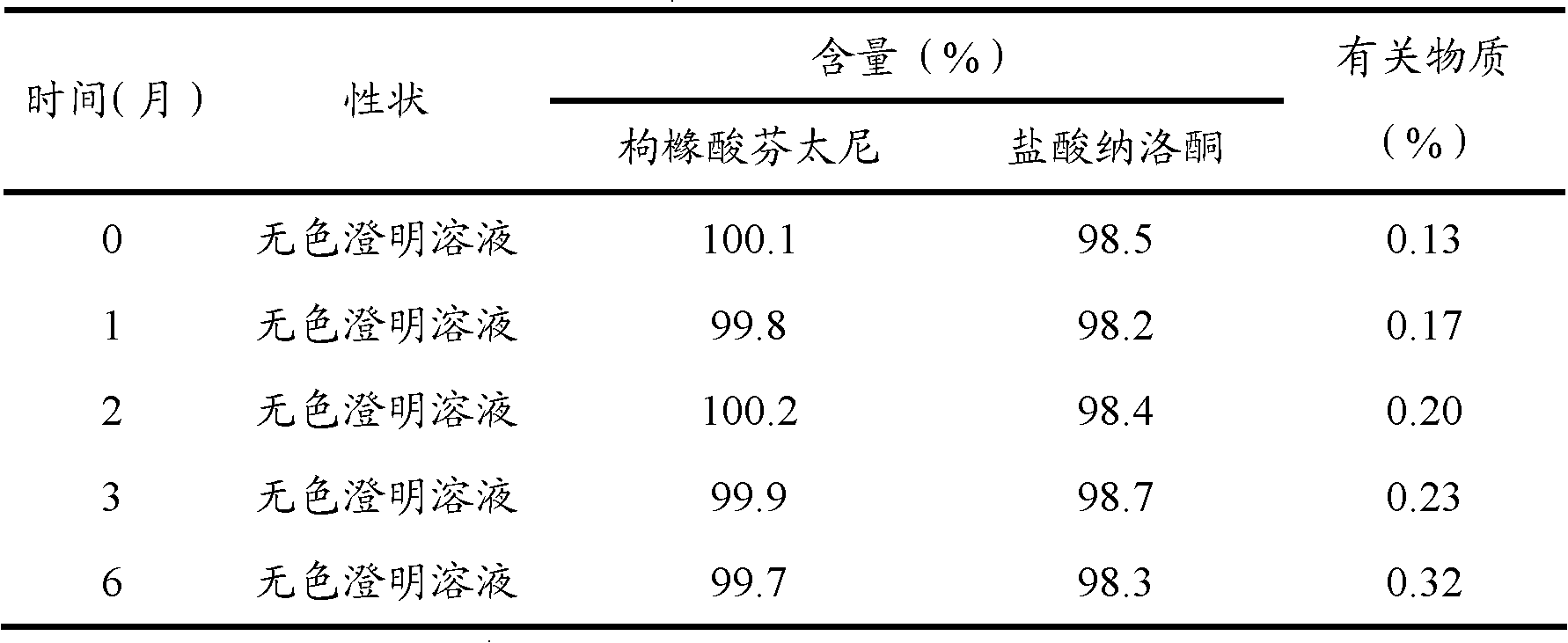
The invention discloses a method for preparing nalmefene hydrochloride injection. The method comprises the steps of adding a certain amount of citric acid or citrate auxiliary material to the preparation when the nalmefene hydrochloride injection is prepared according to a common method, wherein the adding amount is 5-15g of citric acid or citrate added to each liter of injection; firstly, taking the prescribed auxiliary material in preparation, adding injection water to dissolve, adding active carbon to agitate and adsorb, filtering and removing impurities; adding the prescribed nalmefene hydrochloride under the protection of nitrogen, fully dissolving and sizing to an appointed constant volume by water, adjusting the pH value to 3.5-4.5; and finally conducting split charging, charging nitrogen, sealing and sterilizing after filtering by a filter. By adopting the method disclosed by the invention, the validity period of the nalmefene hydrochloride injection can be obviously prolonged; a specific citric acid pH value stabilizer is adopted; and the nalmefene hydrochloride injection has good stability, and stable sample appearance, pH value, effective ingredient content and related substances, and the like. Therefore, the safety of medication is ensured.
Owner:ANHUI HEALSTAR PHARM CO LTD
Pharmaceutical composition containing nalmefene hydrochloride and preparation method of same
ActiveCN102415993ASimple ingredientsLess impuritiesOrganic active ingredientsNervous disorderEthylene diamine tetra aceticEthylene diamine
The invention relates to a pharmaceutical composition containing nalmefene hydrochloride. Nalmefene hydrochloride injection is a product of the pharmaceutical composition. 1ml injection consists of 0.1mg or 1mg nalmefene hydrochloride, 9.0 mg sodium chloride, 0.3mg ethylene diamine tetraacetic acid, hydrochloric acid and the remaining of water for injection, wherein the hydrochloric acid is used for regulating pH of the injection to 3.75-3.85. A preparation method of the pharmaceutical composition comprises the following steps of: mixing the nalmefene hydrochloride, the sodium chloride and the ethylene diamine tetraacetic acid, dissolving the mixture in the water for injection, uniformly mixing the mixture, and adding the water for injection to full; adding 0.1mol / L hydrochloric acid in to the mixture to regulate the pH value of medicine liquid to 3.75-3.85; filtering the medicine liquid by using a microporous filtering film with the thickness of 0.22 microns; and sterilizing the medicine liquid in a water bath with the temperature of 121 DEG C for 15 minutes to obtain the nalmefene hydrochloride injection. The pharmaceutical composition containing the nalmefene hydrochloride is simple components; and the product has the advantages of less foreign matters as well as high stability and safety based on tests on influencing factors, long-term stability and safety.
Owner:ZHUHAI TONGYUAN PHARMA CO LTD
Method for preparing high-purity nalmefene hydrochloride
ActiveCN103012416AReduce typesReduce contentOrganic active ingredientsNervous disorderOrganic solventImpurity
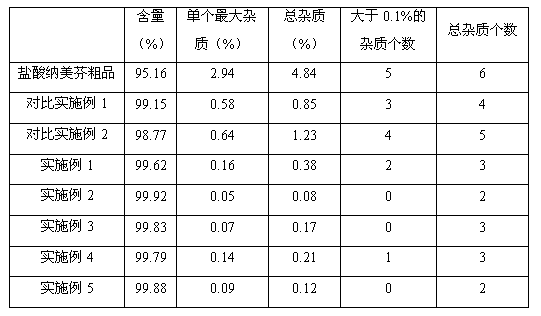
The invention relates to a method for preparing high-purity nalmefene hydrochloride, and in particular relates to a method for refining the nalmefene hydrochloride. The method comprises the steps of dissolving a crude product of the nalmefene hydrochloride in right amount of hot water, cooling and separating out the high-purity nalmefene hydrochloride. The method has the following advantages: (1) through refining, the content of the nalmefene hydrochloride is up to more than 99.5%, the content of the maximum single impurity is less than 0.2%, the content of total impurities is less than 0.5% and the prepared nalmefene hydrochloride is excellent in storage stability and suitable for preparing the preparations of parenteral administration; (2) the method is simple in process, simple and convenient for operation and low in cost and suitable for industrial production; and (3) as no organic solvent is used, the damage to operating personnel and environment is avoided, the finished product has no residual organic solvent problem, the quality inspection step is simplified and the medication safety is guaranteed.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Medicinal use of nalmefene hydrochloride
ActiveCN102048733AReduce aggregation rateImproved prognosisOrganic active ingredientsDigestive systemFatty liverLiver function
The invention relates to medicinal use of nalmefene hydrochloride. The nalmefene hydrochloride can improve lipid metabolism and improve prognosis of a fatty liver patient by reducing the platelet aggregation rate, improving the liver function state and resisting oxidation. The nalmefene hydrochloride can serve as a medicine for resisting human / animal fatty liver.
Owner:ZHUHAI TONGYUAN PHARMA CO LTD
Novel therapeutic uses for nalmefene
InactiveUS20060235038A1Easy to superviseAvoids gastrointestinal discomfortBiocideAnimal repellantsOpioid antagonistAlcoholisms
The present invention relates to novel compositions, methods and therapeutic uses for nalmefene, a unique opioid antagonist drug. The invention teaches administering nalmefene by means that function to produce optimal steady-state plasma or serum concentrations. These means plus functions are claimed for treating alcoholism and pathological gambling.
Owner:SIMON DAVID LEW
Pharmaceutical composition containing nalmefene hydrochloride for injection
ActiveCN103705448AExcellent long-term storage stabilityMaintain binalmefene contentOrganic active ingredientsNervous disorderActivated carbonNalmefene
The invention discloses a formula of a pharmaceutical composition containing nalmefene hydrochloride for injection. The pharmaceutical composition is characterized in that an injection does not contain a chelating agent, and comprises effective dose of nalmefene hydrochloride and a defined amount of pharmaceutical carrier, wherein the concentration of the nalmefene hydrochloride in the injection can be 0.01-0.2 percent (w / v), the content of the nalmefene hydrochloride in each unit of injection is 0.1-2.0mg; an osmotic pressure regulator can be sodium chloride or glucose, preferably, sodium chloride, the content of the osmotic pressure regulator in each unit of injection is 4.5-22.5mg; the pH value of the injection is regulated to be 3.2-4.5 by using hydrochloric acid. The invention further discloses a preparation method of the formula of the injection. The preparation method comprises the steps of in a blending process, regulating the pH value of a solution to be 6.0-9.0 by using alkaline in first time, and after adsorbing by using active carbon, regulating the pH value of the solution to be 3.2-4.5 by using acid in second time. The formula of the injection containing the nalmefene hydrochloride is simple, and is stable in quality; the preparation method is simple in process, simple and convenient to operate, and more suitable for industrialized production.
Owner:CHENGDU GUOHONG PHARMA
Methods and Compositions for the Treatment of Pruritus
InactiveUS20090093509A1Reductions of inflammatory cell infiltrateRelieve itchingBiocideAnimal repellantsDiseasePrurigo
The present invention is based on the discovery that a nalmefene salt or a peripherally acting analogue of nalmefene is capable of providing long-acting activity against pruritus, when applied topically. In addition, treatment with a nalmefene salt or peripherally acting analogue of nalmefene, can provide disease-modifying effects against the chronic symptoms of atopic dermatitis, including reductions of inflammatory cell infiltrate into the skin, epidermal hyperplasia and trans-epidermal water loss. Compositions of the invention have an inhibitory effect on the itch-scratch-cycle, leading to both relief of pruritus and disease-modifying activity.
Owner:SERENTIS LTD
Pharmaceutical composition containing nalmefene hydrochloride for injection
ActiveCN103705448BExcellent long-term storage stabilityMaintain binalmefene contentOrganic active ingredientsNervous disorderActivated carbonNalmefene
Owner:CHENGDU GUOHONG PHARMA
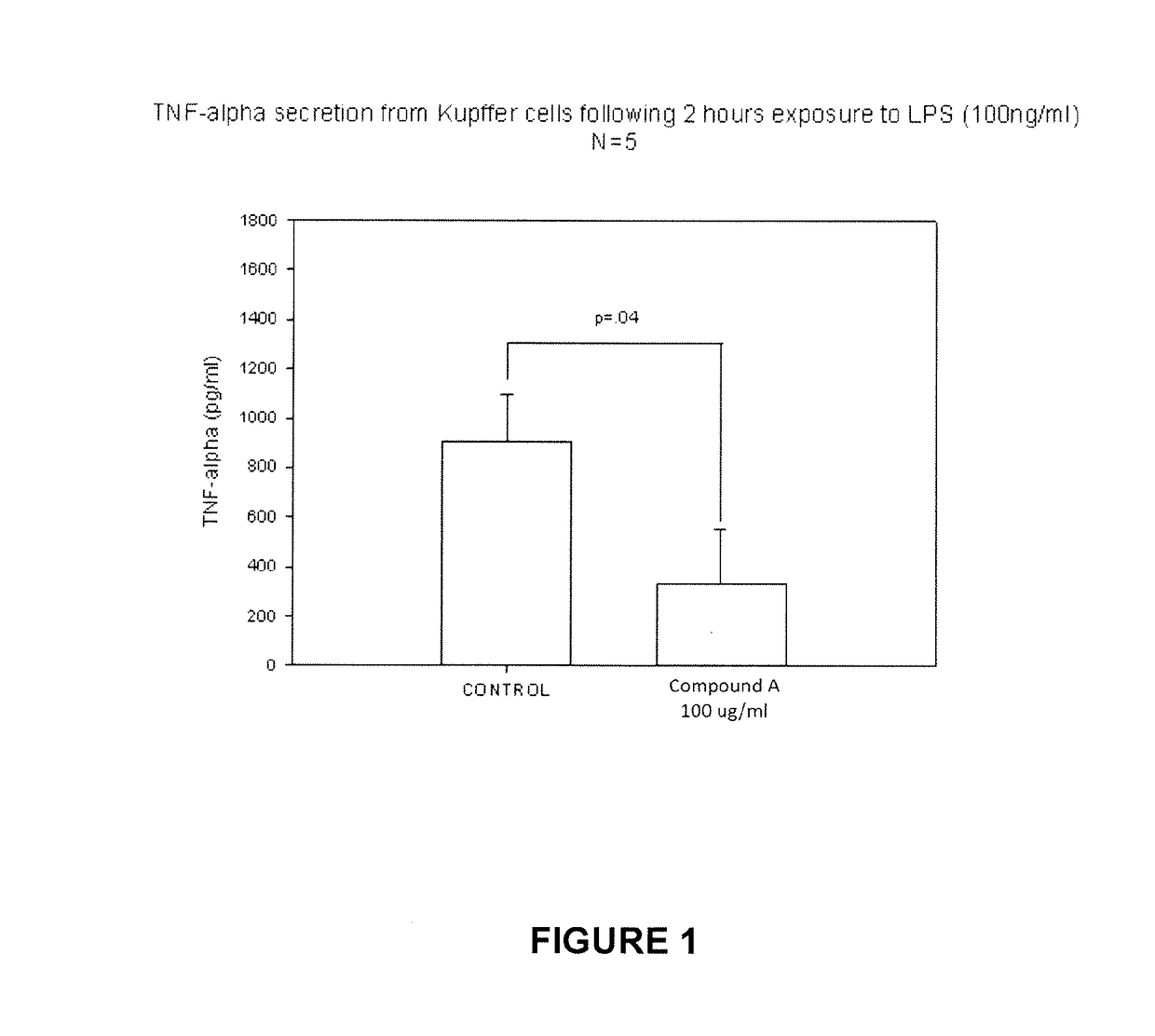
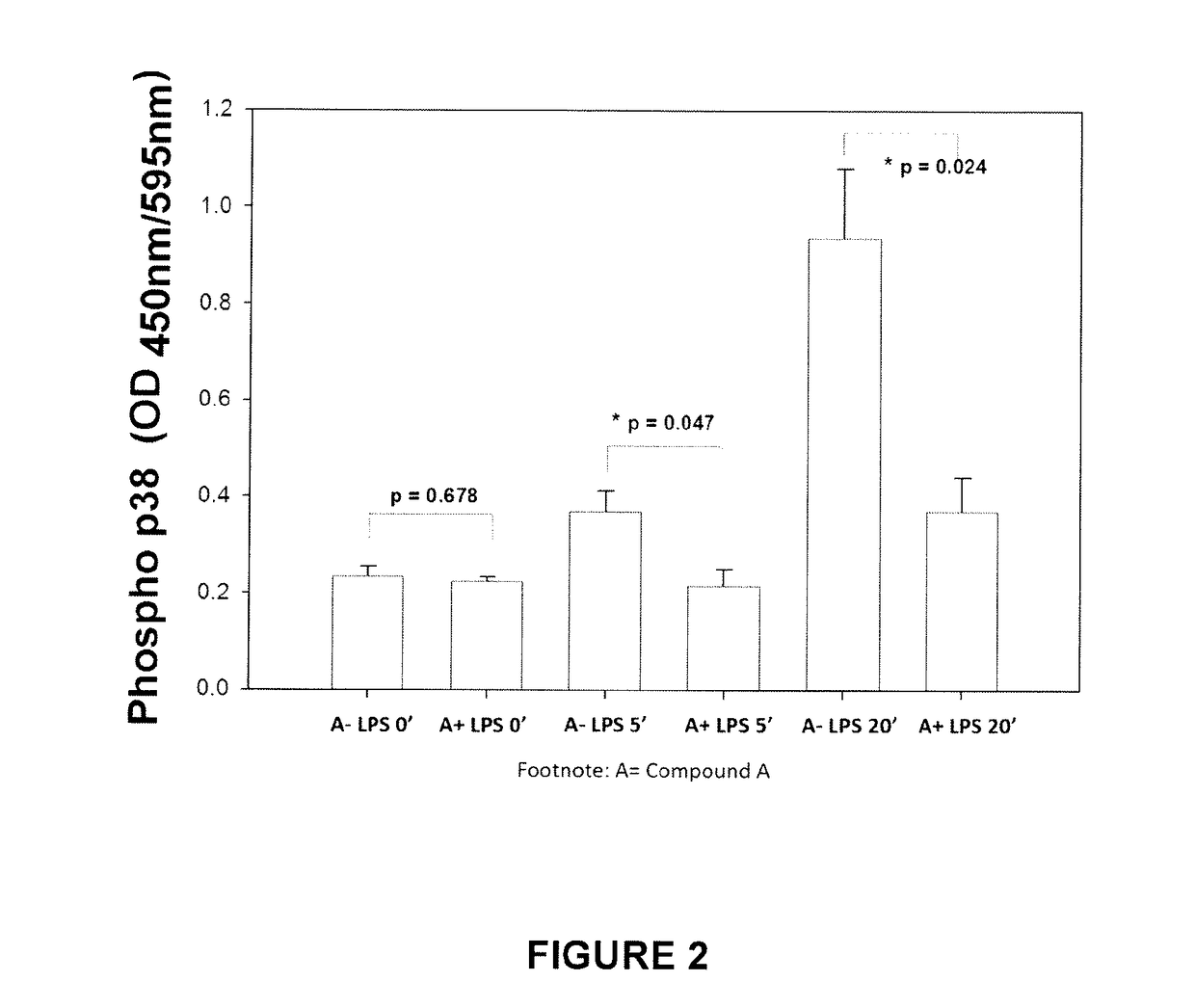
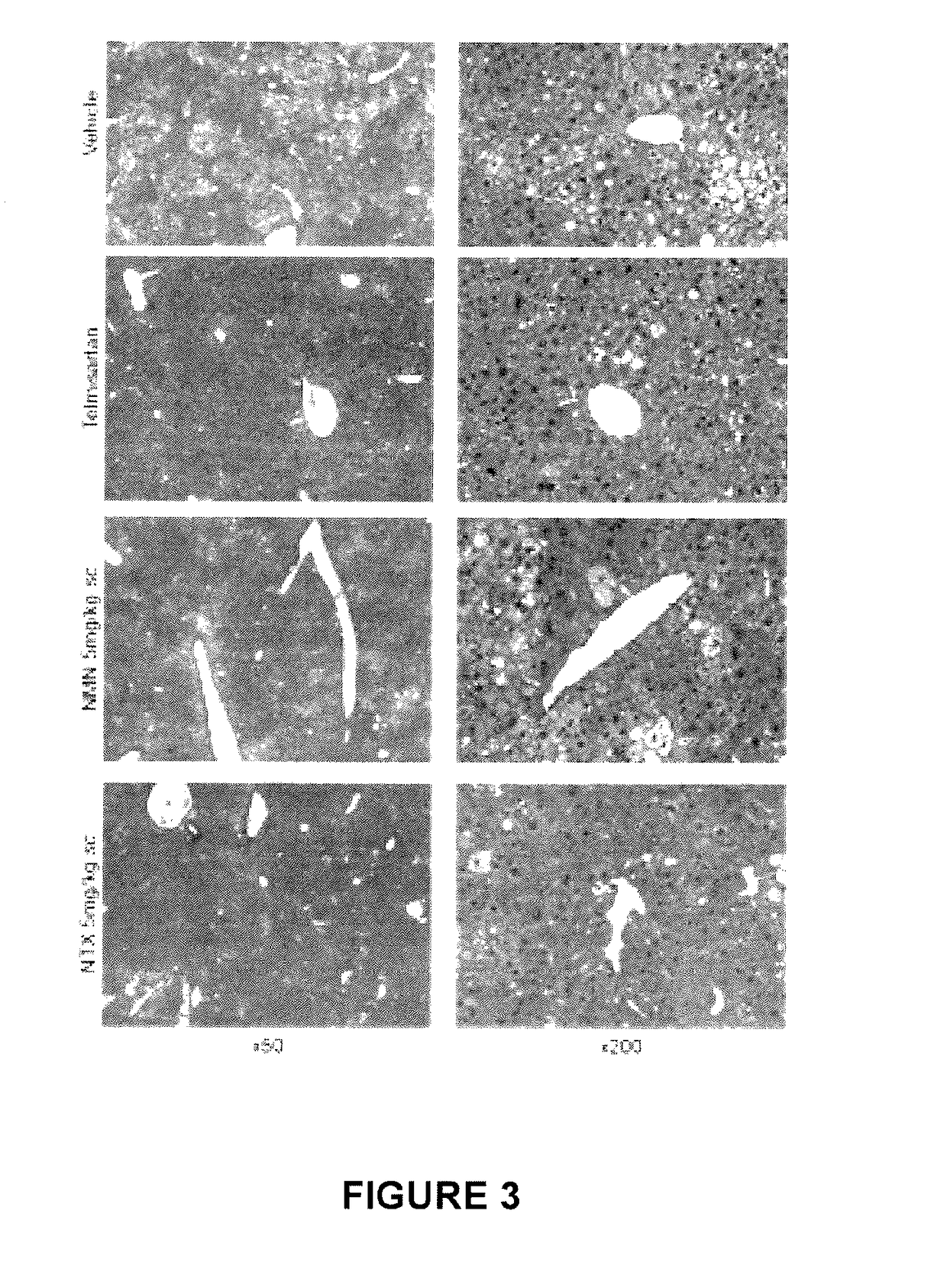
Use of nalmefene in non-alcoholic steatohepatitis
The present invention relates to new medical uses of morphinans such as nalmefene and naltrexone and their related derivatives, pharmaceutical formulations thereof, and use thereof for prevention and treatment of NASH, NAFLD, and / or ASH.
Owner:TAIWANJ PHARMA
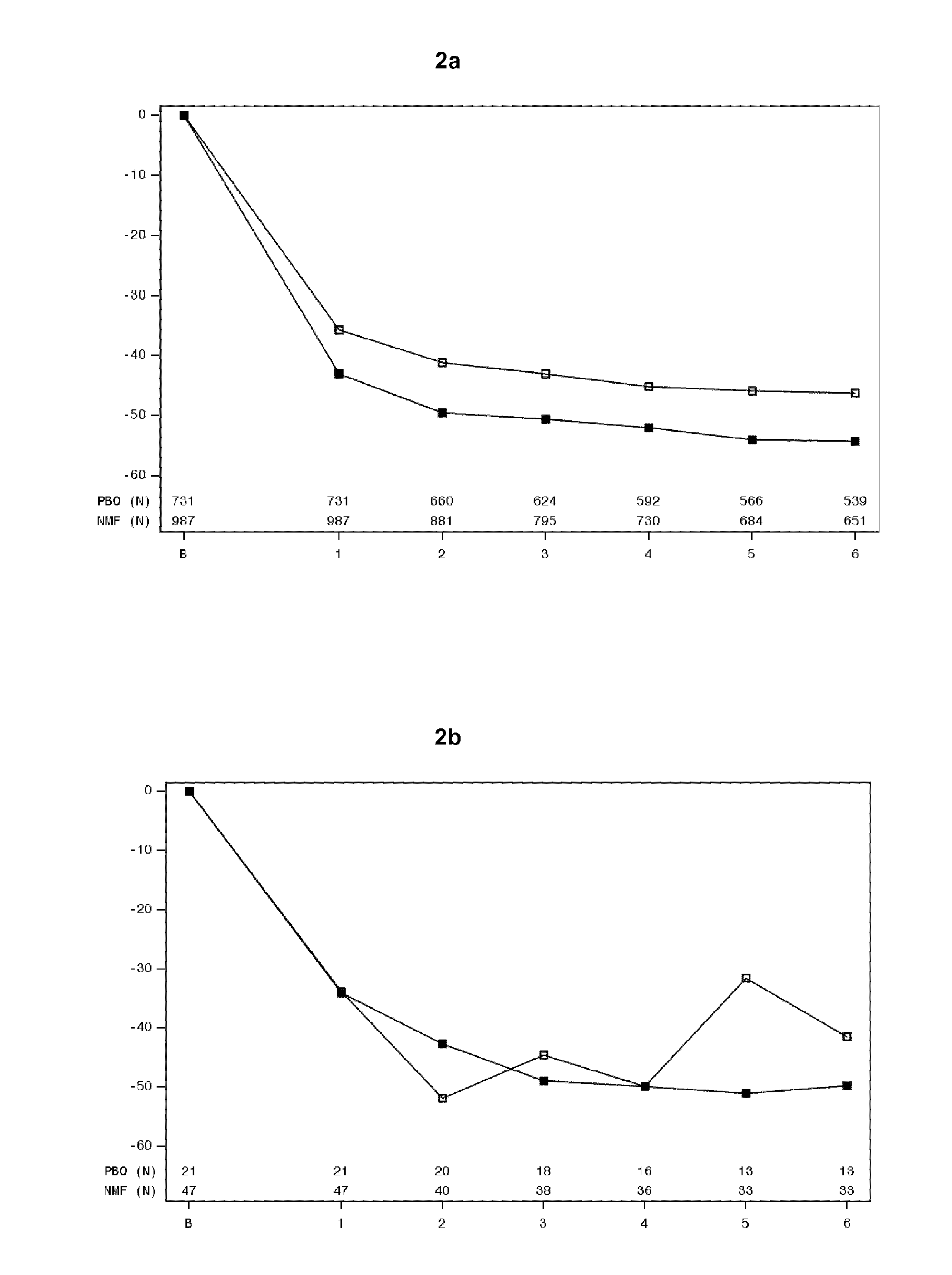
Nalmefene for Treatment of Patients with Anxiety Disorder
The present invention relates to nalmefene for use in the treatment of anxiety disorders. The present invention further relates to nalmefene for use in the treatment of patients with alcohol dependence who have a co-morbid anxiety disorder. The invention further relates to nalmefene for use in the treatment of an anxiety disorder in said patients.
Owner:H LUNDBECK AS
Nalmefene hydrochloride injection and preparation method thereof
ActiveCN101658489AImprove stabilityEnsure safetyOrganic active ingredientsNervous disorderAntioxidantNalmefene
The invention discloses a nalmefene hydrochloride injection, which is solution prepared from nalmefene hydrochloride and usable pharmaceutical excipients dissolved in injection water. The pharmaceutical excipients comprise an antioxidant and an osmotic pressure modifier. The nalmefene hydrochloride injection is characterized in that: the weight ratio of nalmefene to the antioxidant in the nalmefene hydrochloride is 1:0.1-1.0; and the antioxidant is mixed by one or more of tertiary butyl hydroxy anisole, 2,6-di-tert-butyl-4-methyl-phenol and rosemary. The injection is stable in property and ensures medication safety.
Owner:BEIJING SIHUAN PHARMA +1
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:RECKITT BENCKISER HEALTHCARE (UK) LTD
Nose cavity administering formulation of nalmefene
The invention provides a nalmefene preparation to be administered through nasal cavity, which comprises nalmefene, namefene free alkali or other pharmaceutically acceptable medicinal salt of nalmefene and absorption promoting agent, a large number of tests have shown that, the nasal cavity administered medicinal preparation by the invention can make the medicament absorbed through the path of nasal mucosa, and enter into blood circulation for actions. The advantages of the preparation include stabilized performance, controlled quality and non-stimulation to nasal cavity.
Owner:广东同德药业有限公司
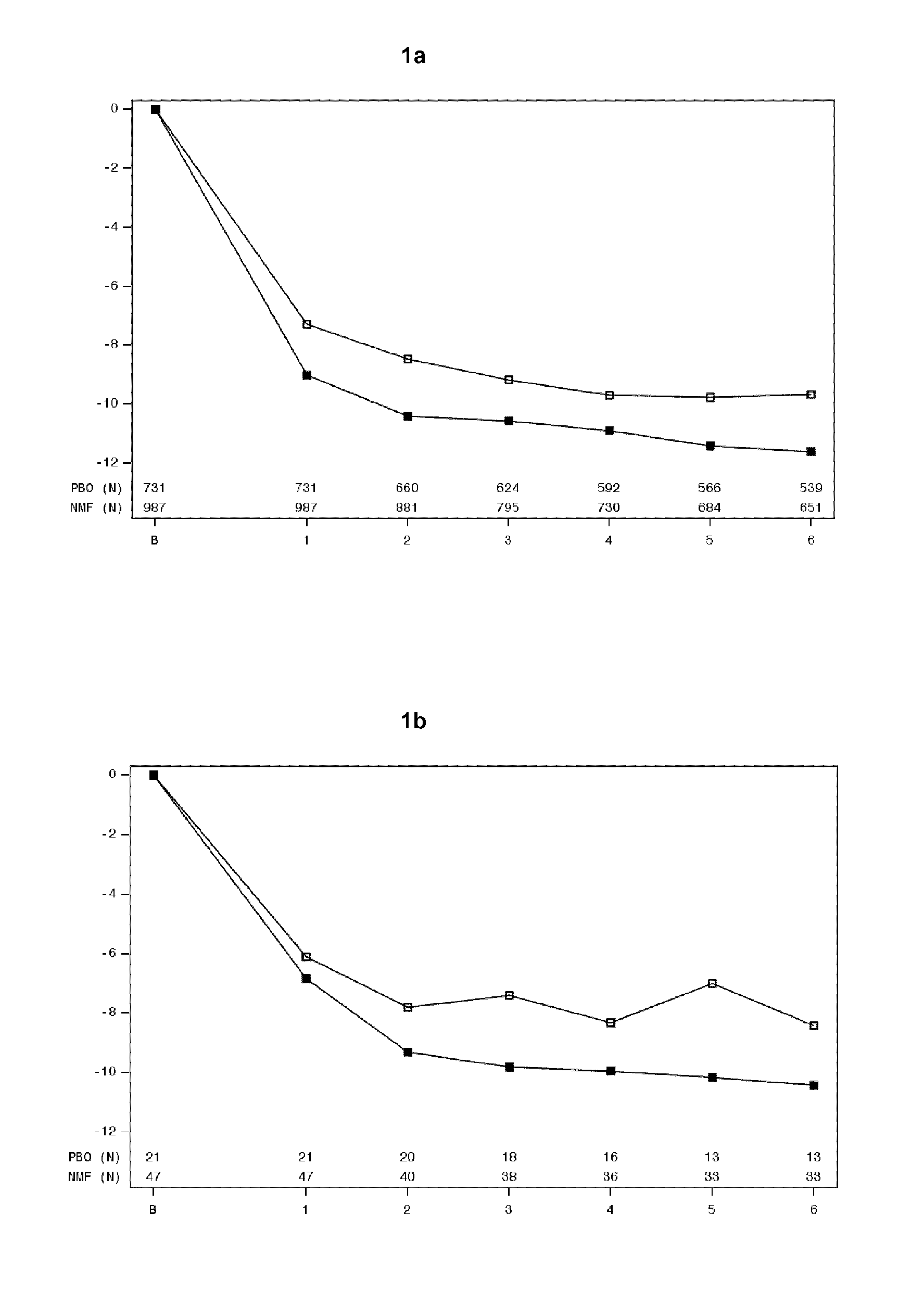
Nalmefene for reduction of alcohol consumption in specific target populations
The present invention relates to nalmefene for use in the reduction of alcohol consumption in a patient with alcohol dependence who has a high drinking risk level. The present invention also relates to nalmefene for use in the reduction of alcohol consumption in a patient with alcohol dependence who maintains a high DRL after an observation period following initial assessment.
Owner:H LUNDBECK AS
Use of Opioid Antagonists for the Preparation of a Medicament in the Treatment of Retinal Degenerative Diseases
Retinal degenerative diseases affect the delicate layer of retina tissue that lines the inside back of the eye leading to gradual vision loss. The use of opioid anatgonists for the preparation of a medicament for the selective blocking of the body's opioid receptors sites is a method of treating this human suffering by daily administration to the patient of from about 0.5 to about 10 mg of drugs such as naltrexone, naloxone or nalmefene. They act primarily by normalizing derangements in the human complement system that may occur in the disease etiology. Therapeutical approach for macular degeneration and retinitis pigmentosa is primarily intended. Oral, parenteral uses as well as topical applications may all be considered. They may be administered in one or divided doses daily for the best receptor blocking activity, preferably in the evening hours. Low Dose Naltrexone suitable for oral administration is most preferred regimen at about 4.5 mg / day.
Owner:IMUNEKS FARMA ILAC SANAYI VE TICARET
Nalmefene hydrochloride purification method
ActiveCN106167492AThe production method is economical and practicalProduction method safetyOrganic chemistryAcetic acidPurification methods
The invention belongs to the technical field of medicines, and particularly relates to a nalmefene hydrochloride purification method. Nalmefene is used as a raw material, and nalmefene hydrochloride with purity of 99.95% or more is prepared under the action of solvents such as ethyl acetate, acetone and water.
Owner:西藏易明西雅医药科技股份有限公司
Nose cavity administering formulation of nalmefene
The invention provides a nalmefene preparation to be administered through nasal cavity, which comprises nalmefene, namefene free alkali or other pharmaceutically acceptable medicinal salt of nalmefene and absorption promoting agent, a large number of tests have shown that, the nasal cavity administered medicinal preparation by the invention can make the medicament absorbed through the path of nasal mucosa, and enter into blood circulation for actions. The advantages of the preparation include stabilized performance, controlled quality and non-stimulation to nasal cavity.
Owner:广东同德药业有限公司
Compositions and methods for opioid antagonist delivery
PendingUS20200138805A1Reduce riskOrganic active ingredientsNervous disorderOpioid antagonistAdjuvant
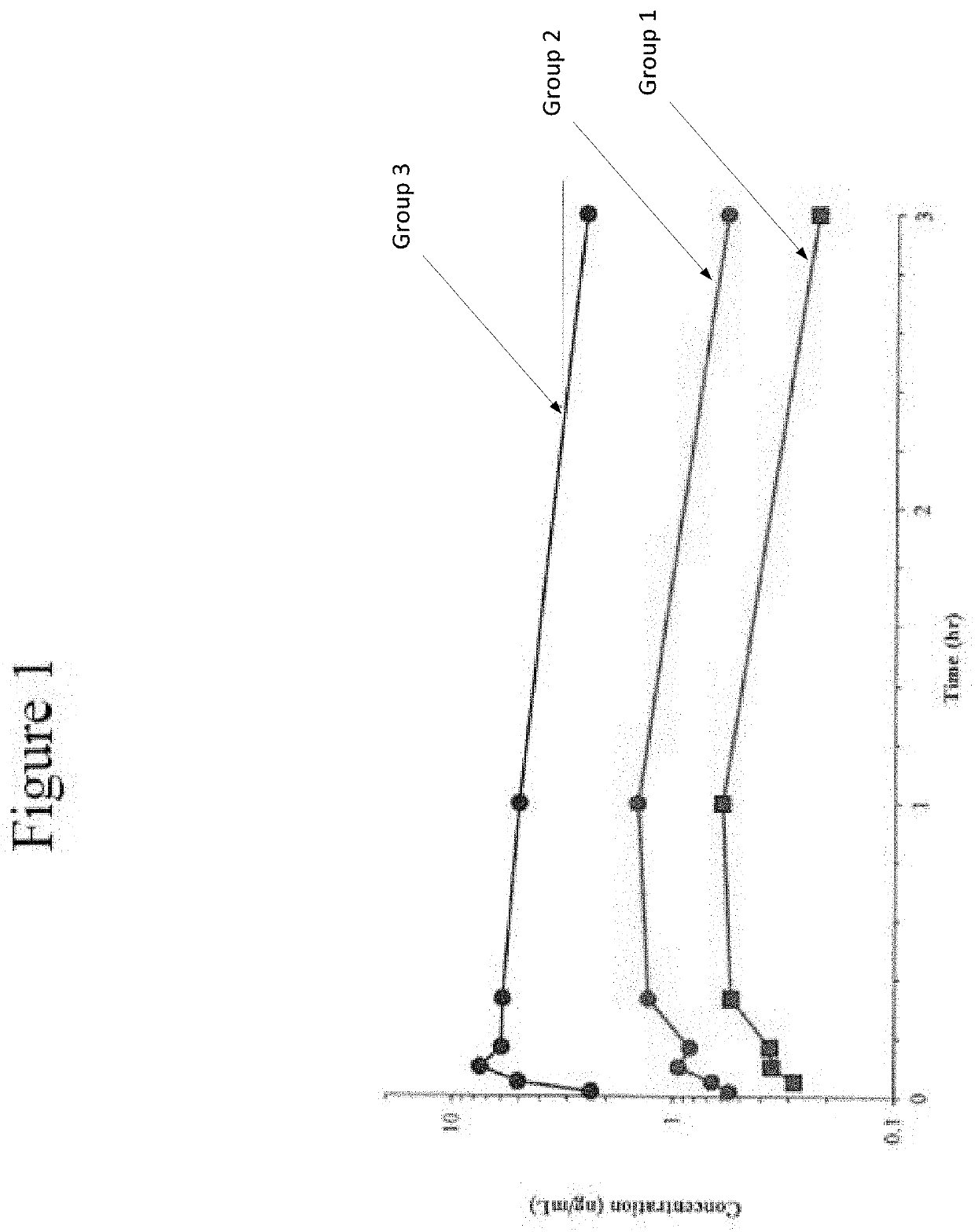
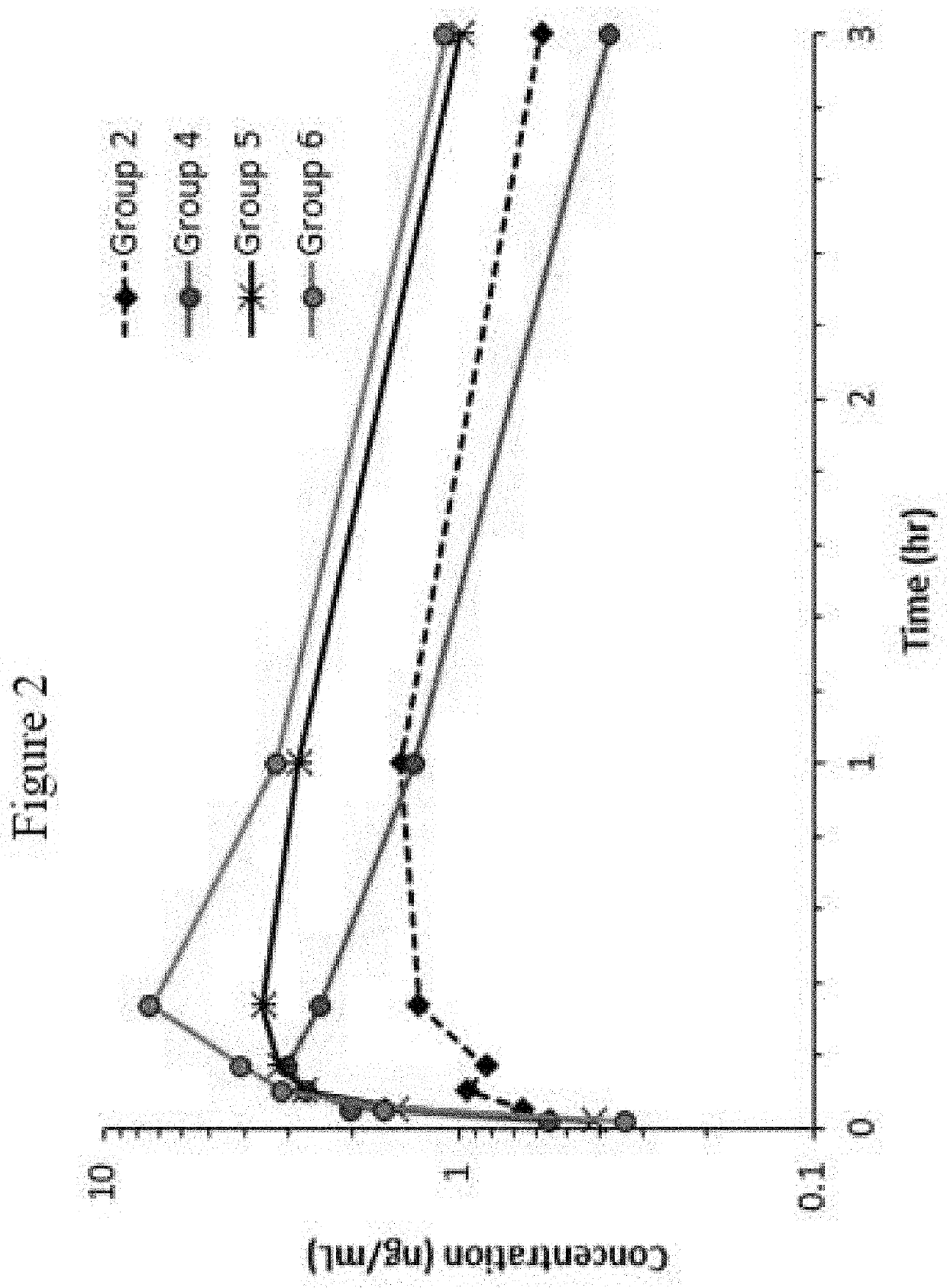
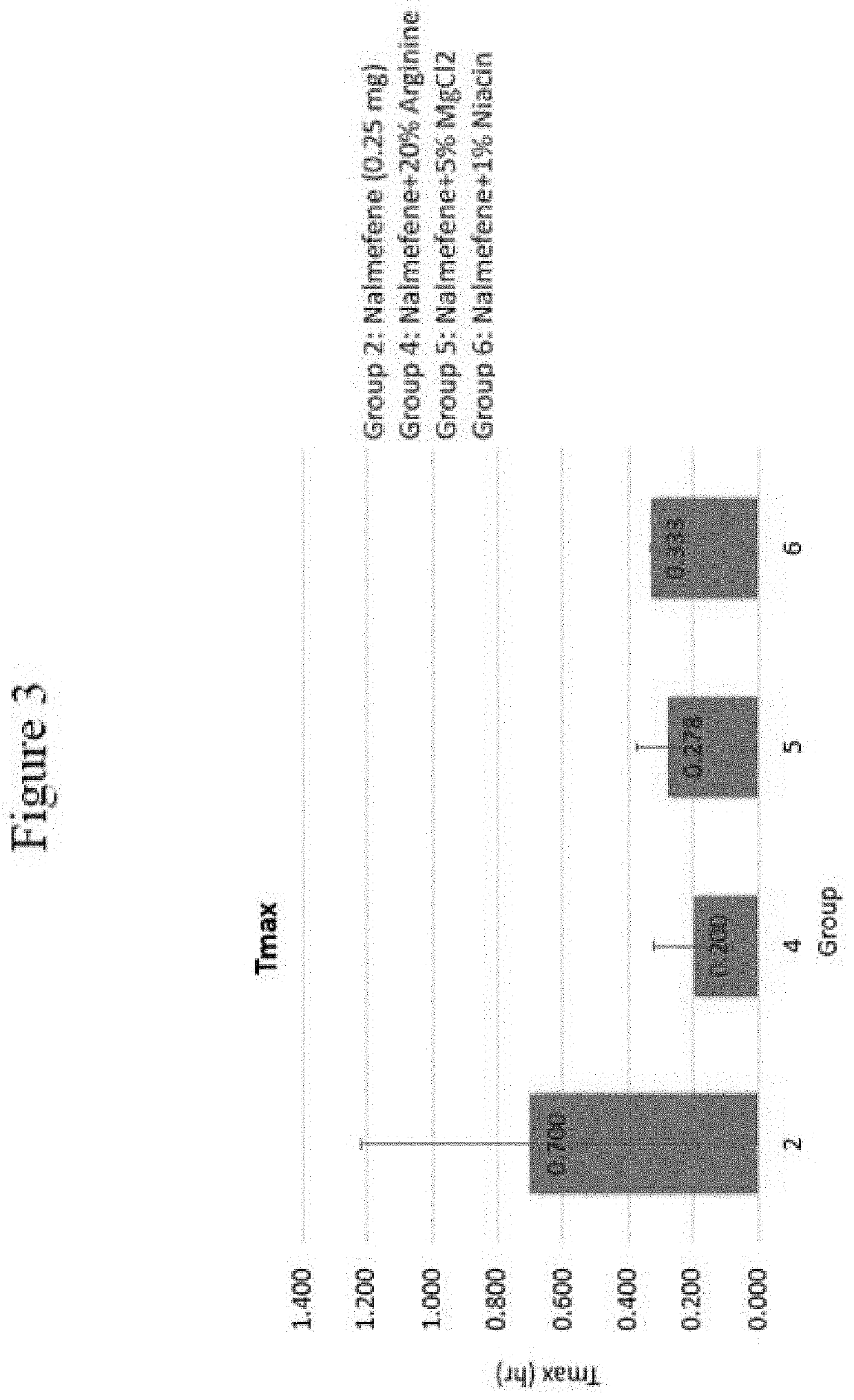
Disclosed in certain embodiments is a pharmaceutical formulation (e.g., parenteral formulation) comprising a therapeutically effective amount of nalmefene or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable adjuvant (e.g., parenterally acceptable adjuvant) that promotes the rate at which the nalmefene or salt thereof is more rapidly absorbed into the systemic circulation of a subject identified as in need thereof.
Owner:PURDUE PHARMA LP
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
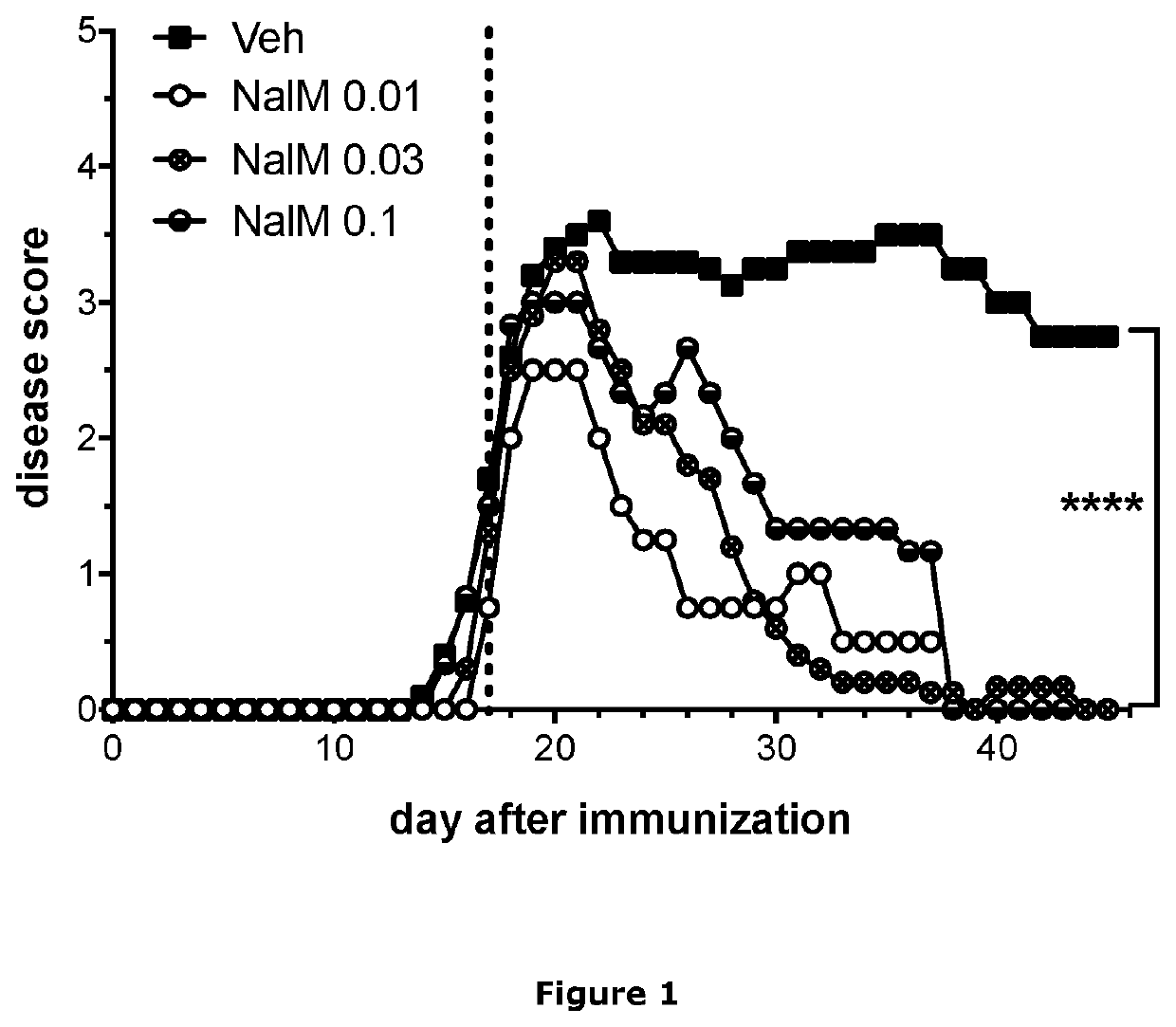
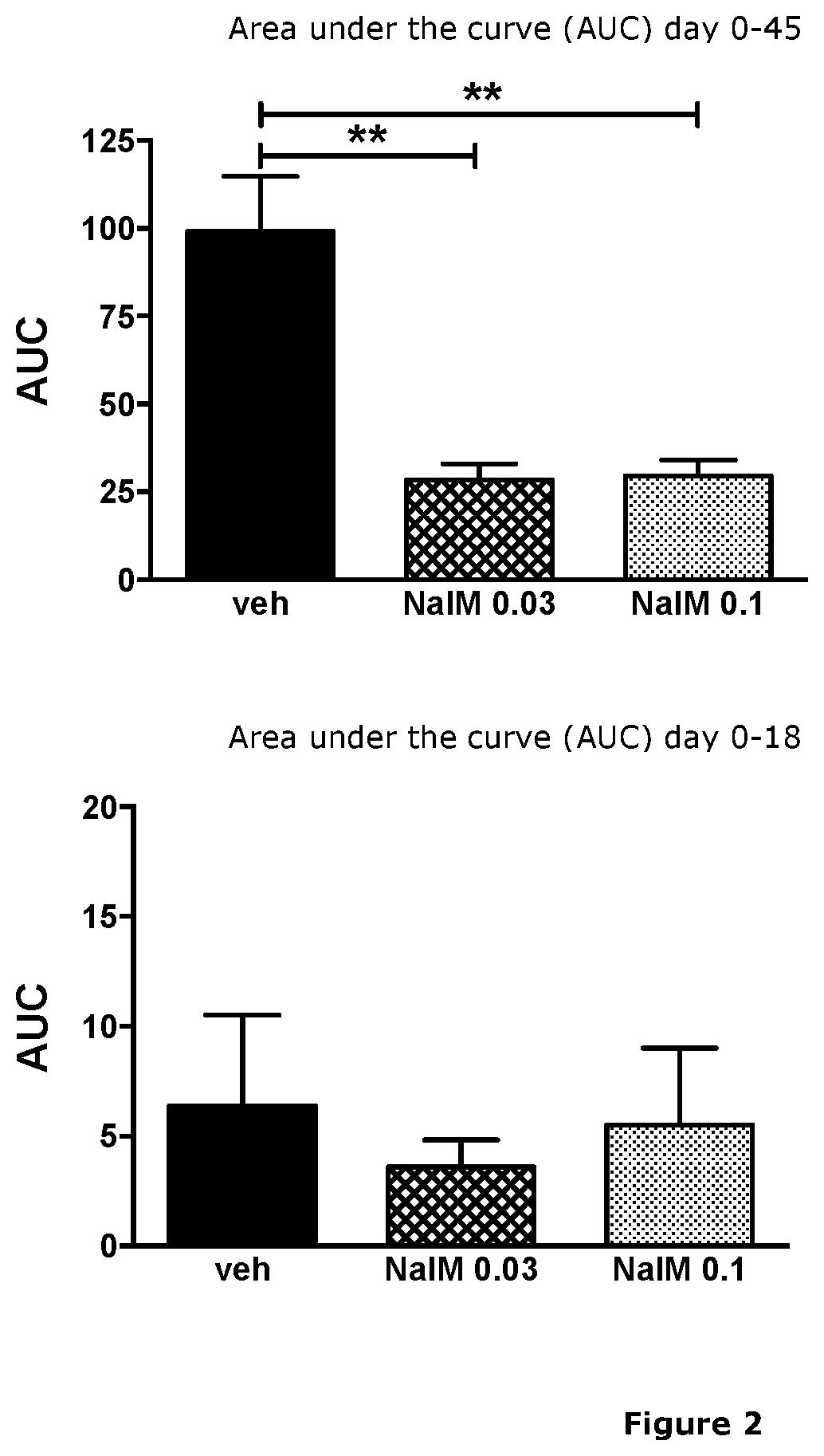
Treatment of demyelinating diseases
InactiveUS20210023074A1High remission rateOrganic active ingredientsNervous disorderDiseaseDemyelinated diseases
The present invention relates generally to methods of using nalmefene for treating and / or preventing demyelinating disease in a subject, and in particular for treating and / or preventing multiple sclerosis (MS). Also disclosed is nalmefene for use in treating and / or preventing MS as well as pharmaceutical compositions and unit dosage forms comprising nalmefene for use for treating and / or preventing demyelinating disease in a subject, and in particular for treating and / or preventing MS.
Owner:UNIVERSITY OF KANSAS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com