Method for preparing high-purity nalmefene hydrochloride
A technology of nalmefene hydrochloride and nalmefene, which is applied in the field of medicine, can solve problems such as toxicity, achieve low cost, simplify the quality inspection process, and ensure safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
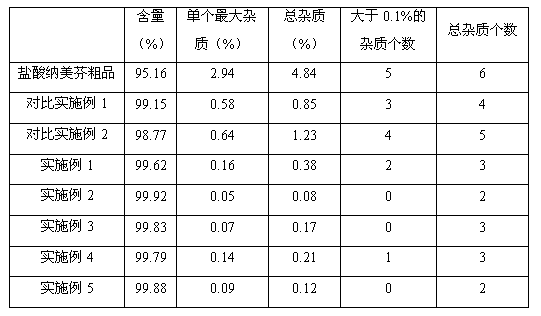
[0024] Add 100.0g of crude nalmefene hydrochloride (HPLC content 95.16%) and 400.0ml of distilled water into the three-necked flask, raise the temperature to 85-95°C, and stir to completely dissolve nalmefene hydrochloride. After dissolving, add activated carbon 0.8g, decolorize for 20 minutes, then filter while hot, wash the filter cake with a small amount of hot water, and collect the filtrate. The filtrate was naturally cooled to room temperature, and white crystals were precipitated during the cooling process. The crystallized material was placed in a freezer at 5°C to allow the crystals to fully separate out. After suction filtration, the filter cake was washed with a small amount of cold water and dried at 60°C to obtain 86.3 g of high-purity nalmefene hydrochloride. The HPLC analysis results are shown in Table 1.
Embodiment 2
[0026] Add 100.0g of crude nalmefene hydrochloride (HPLC content 95.16%) and 200.0ml of distilled water into the three-necked flask, raise the temperature to 85-95°C, and stir to completely dissolve nalmefene hydrochloride. After dissolving, add 0.04g of activated carbon, decolorize for 20 minutes, then filter while hot, wash the filter cake with a small amount of hot water, and collect the filtrate. The filtrate was naturally cooled to room temperature, and white crystals were precipitated during the cooling process. The crystallized material was placed in a freezer at 5°C to allow the crystals to fully separate out. After suction filtration, the filter cake was washed with a small amount of cold water and dried at 60°C to obtain 90.5 g of high-purity nalmefene hydrochloride. The HPLC analysis results are shown in Table 1.
Embodiment 3
[0028] Add 100.0g of crude nalmefene hydrochloride (HPLC content 95.16%) and 100.0ml of distilled water into the three-necked flask, raise the temperature to 90-100°C, and stir to completely dissolve nalmefene hydrochloride. After dissolving, add 0.05 g of activated carbon, decolorize for 10 minutes, then filter while hot, wash the filter cake with a small amount of hot water, and collect the filtrate. The filtrate was naturally cooled to room temperature, and white crystals were precipitated during the cooling process. The crystallized material was placed in a freezer at 0°C to allow the crystals to fully separate out. After suction filtration, the filter cake was washed with a small amount of cold water and dried in vacuum at 40°C to obtain 91.8 g of high-purity nalmefene hydrochloride. The HPLC analysis results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com