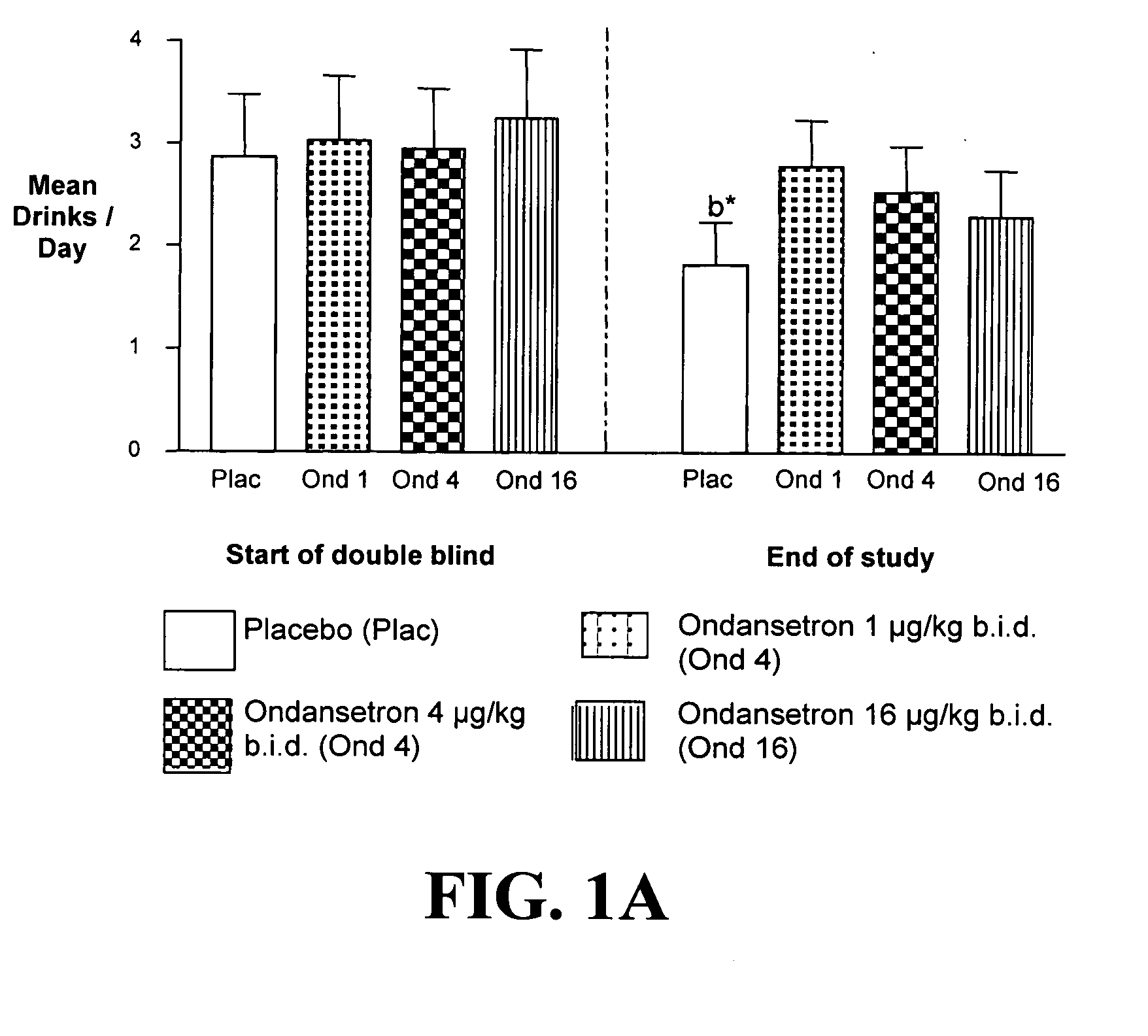
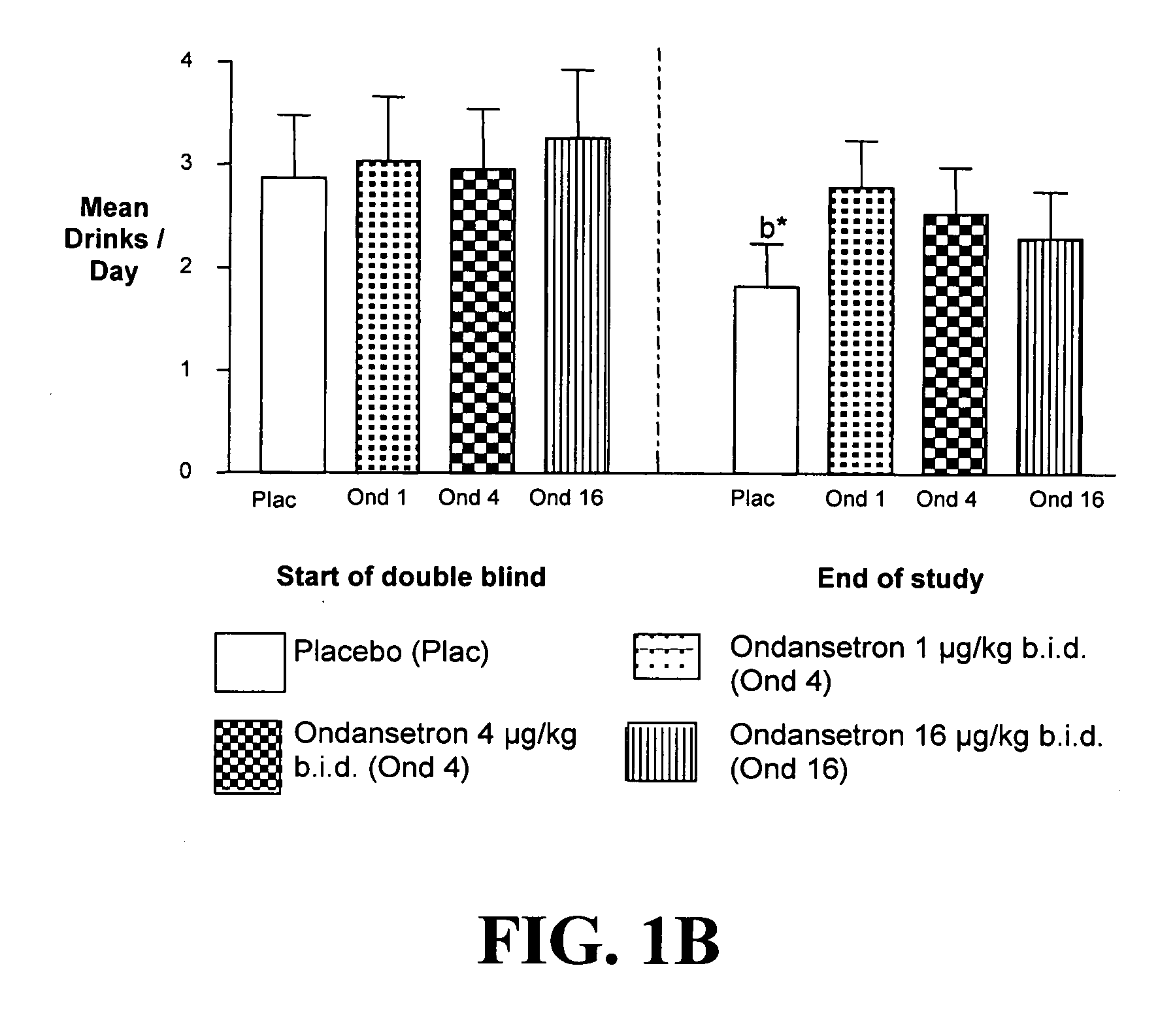
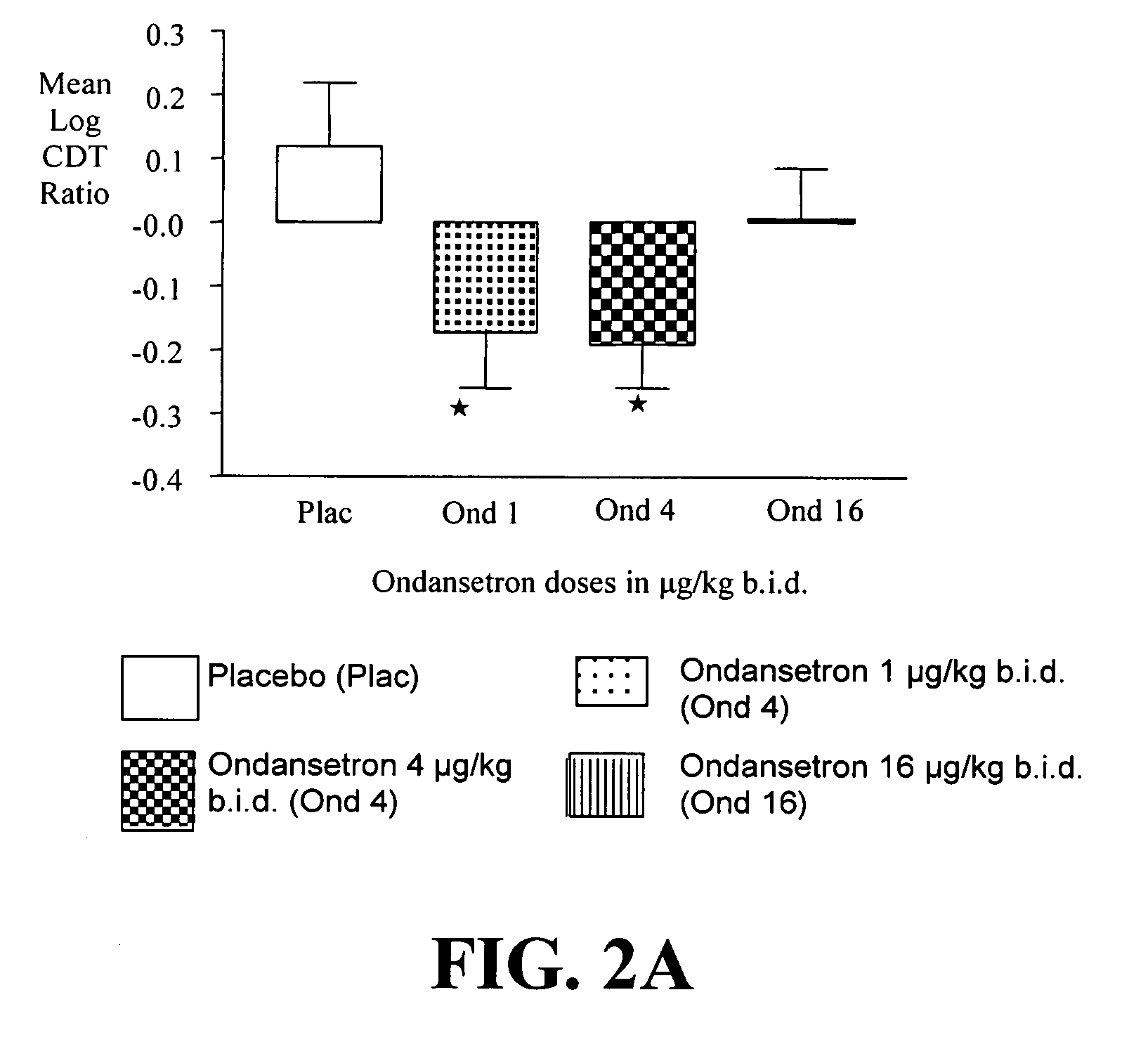
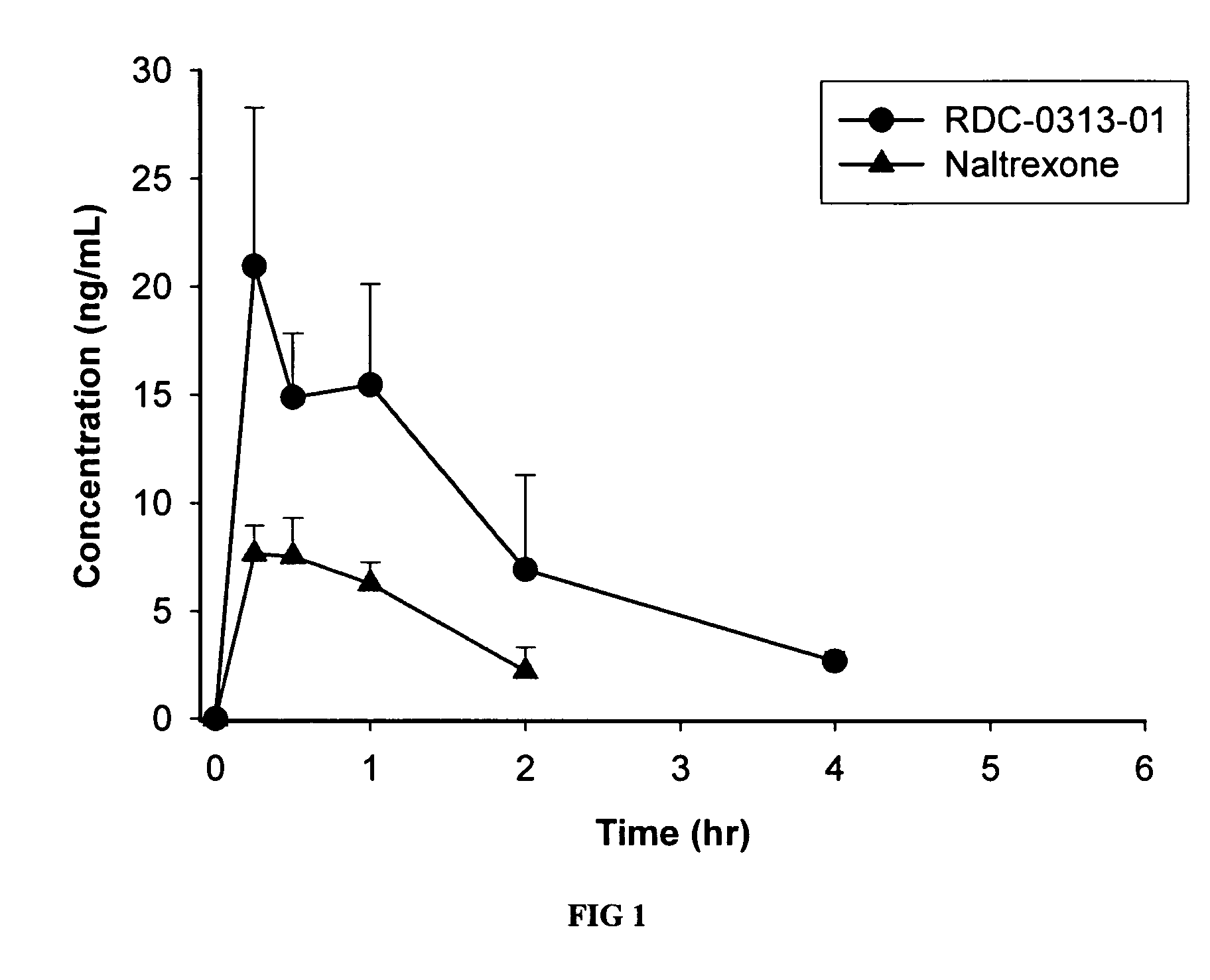
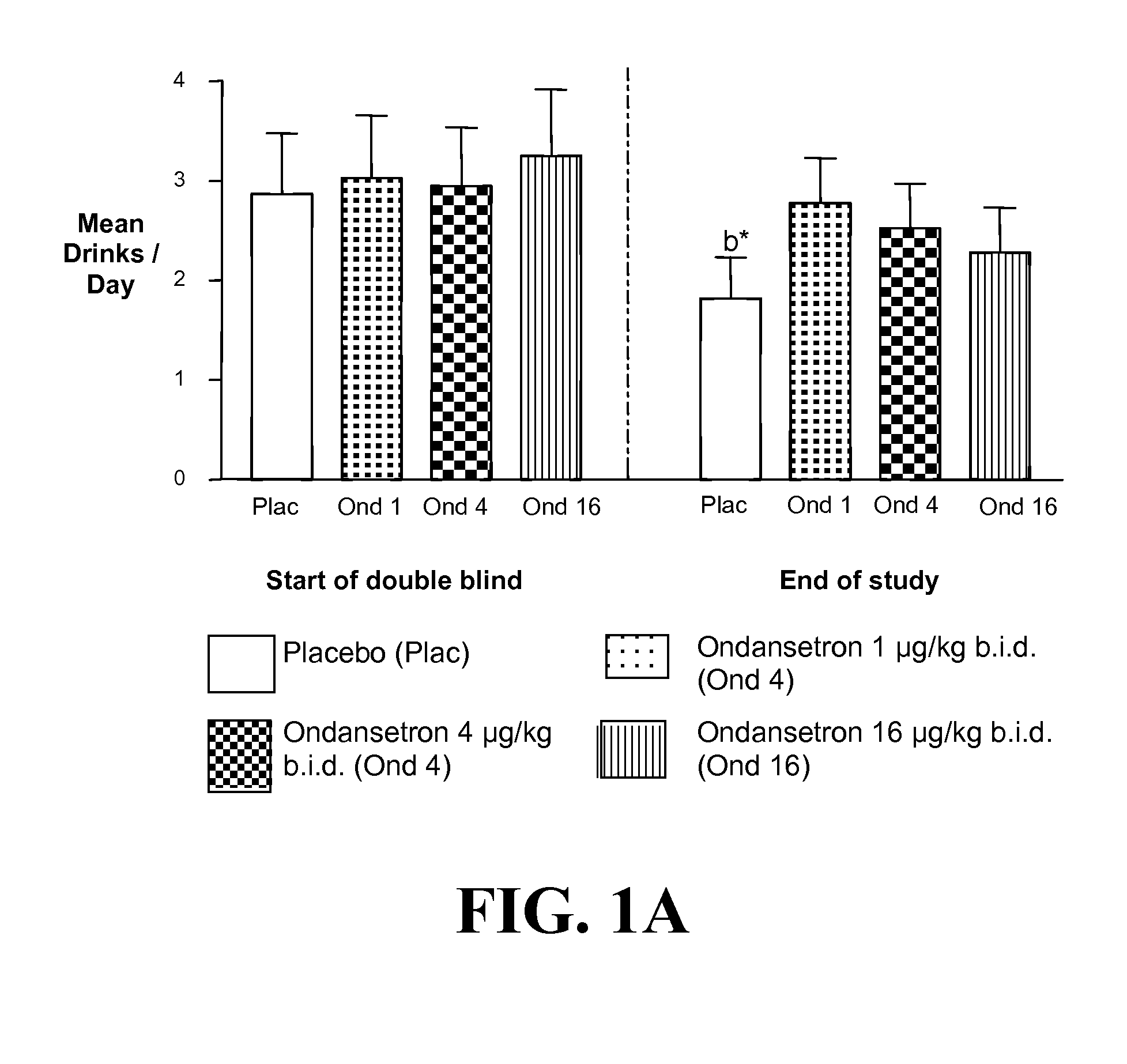
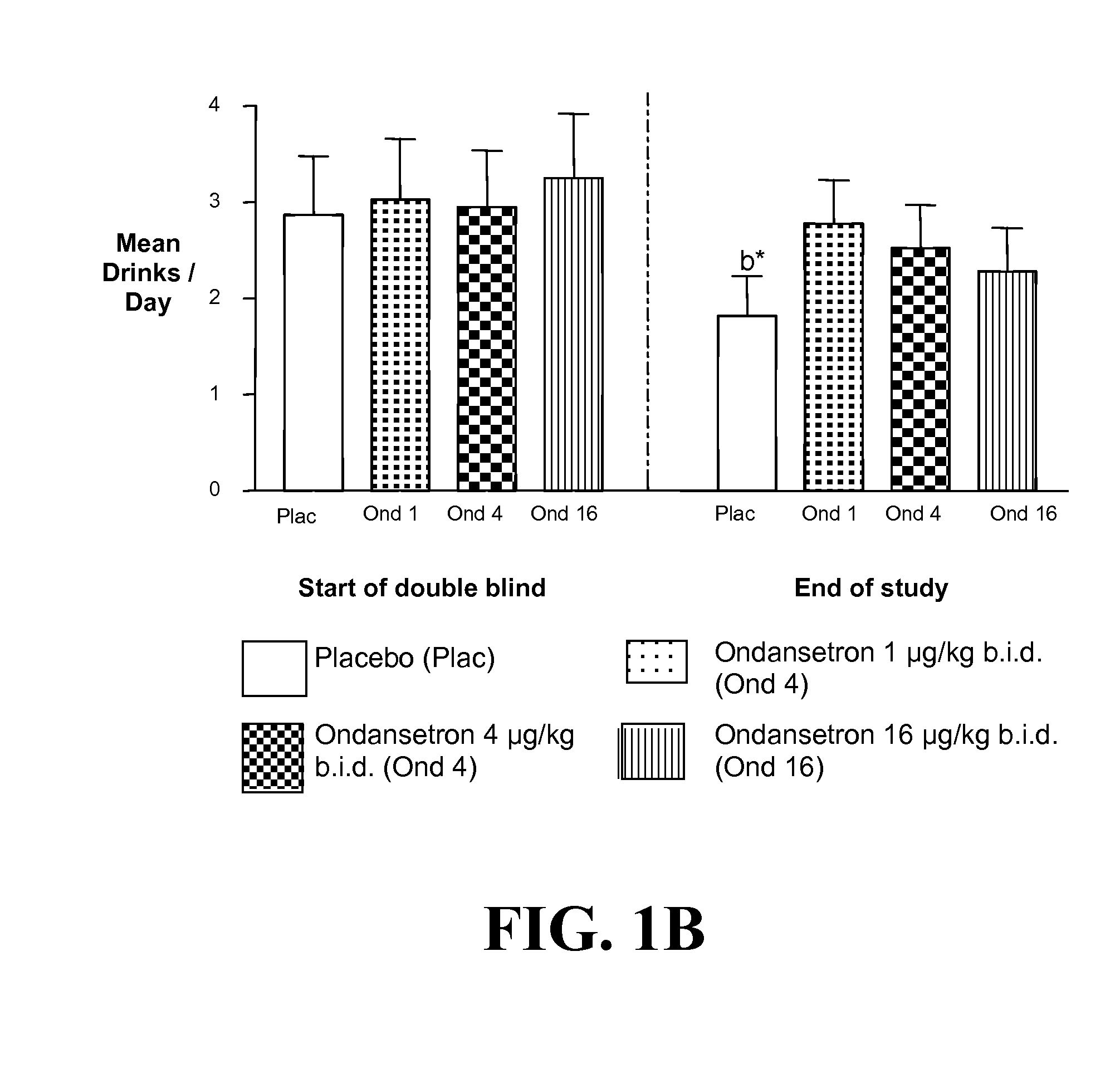
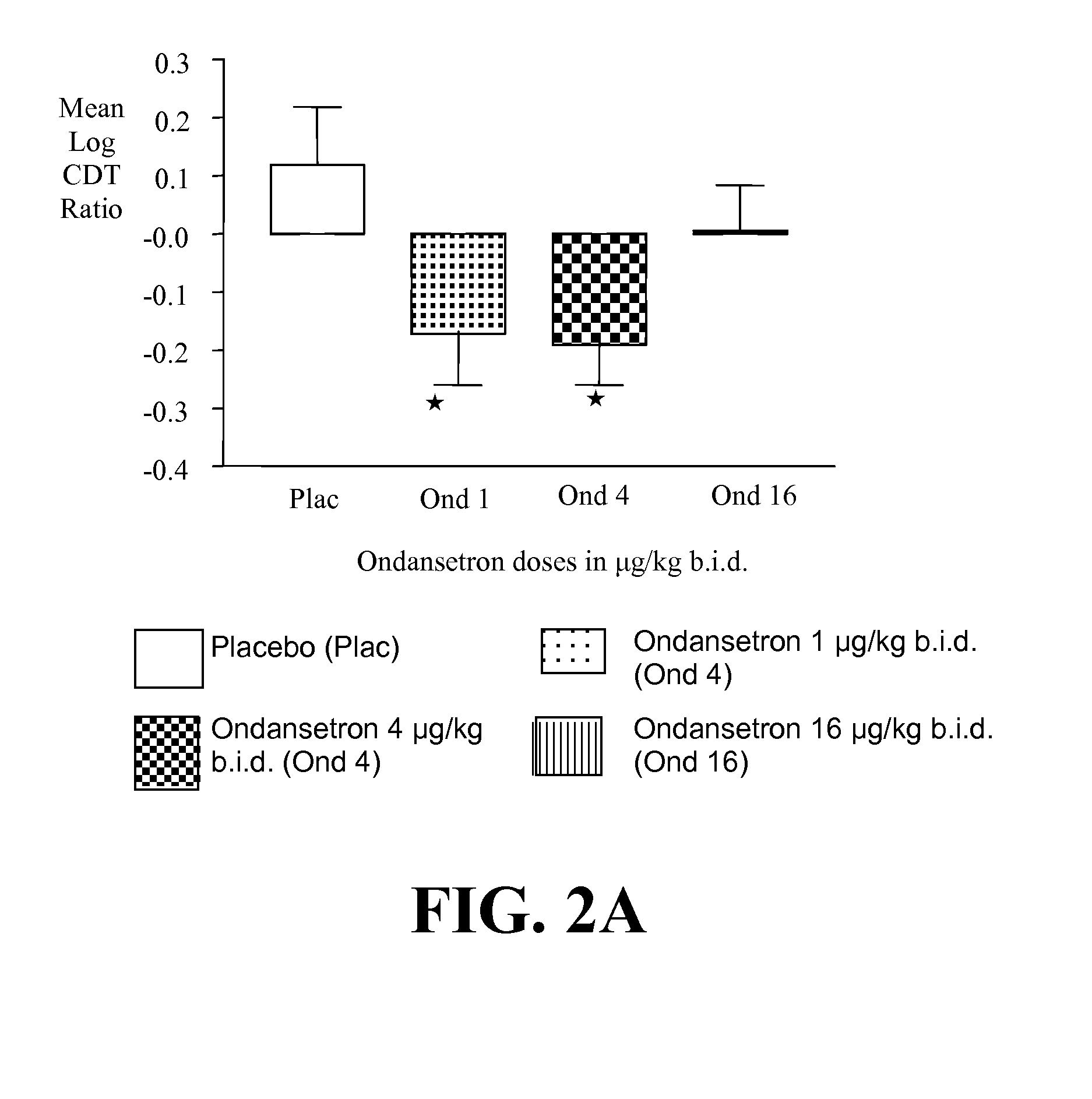
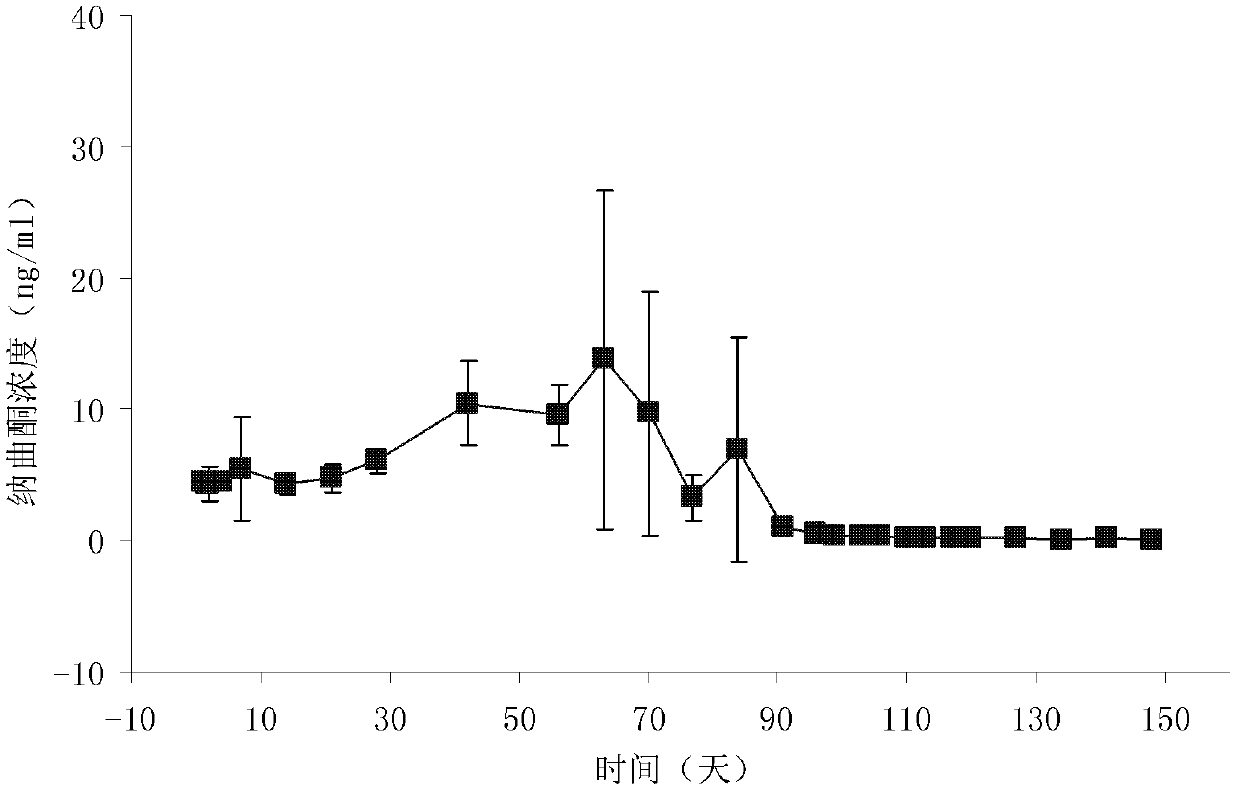
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Naltrexone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to prevent people who have been addicted to certain drugs (opiates) from taking them again. It is used as part of a complete treatment program for drug abuse (e.g., compliance monitoring, counseling, behavioral contract, lifestyle changes). This medication must not be used in people currently taking opiates, including methadone.
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
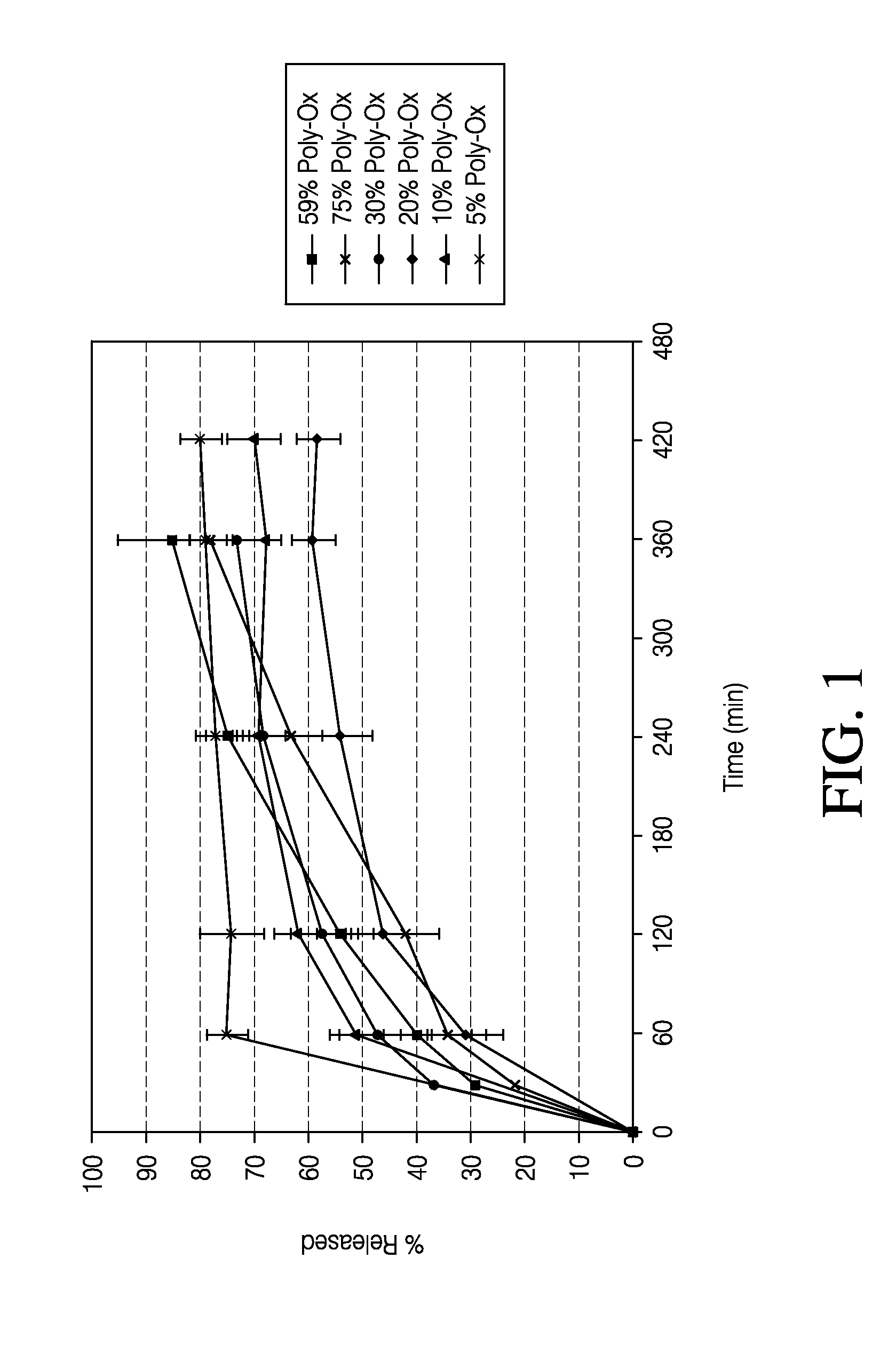
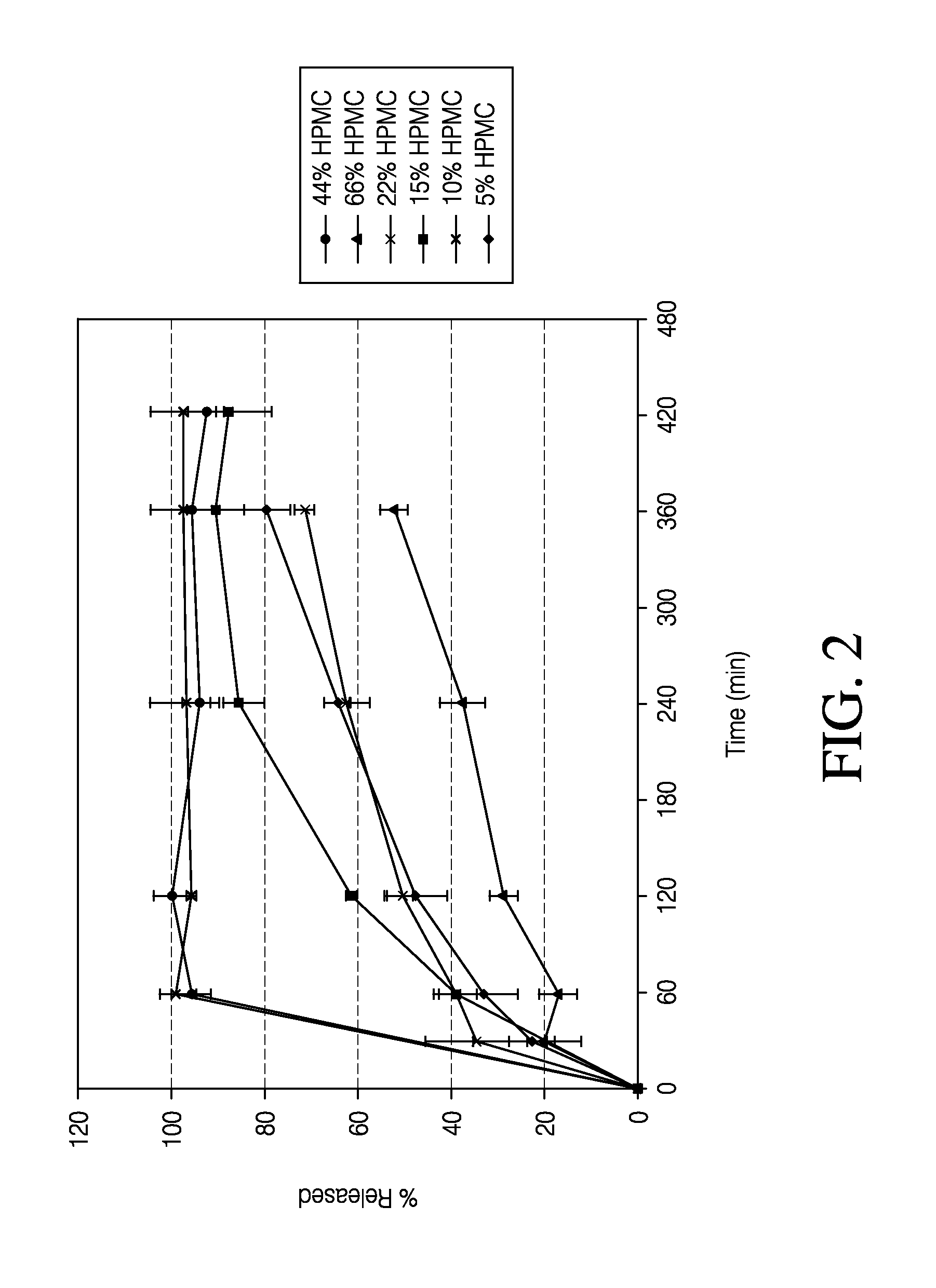
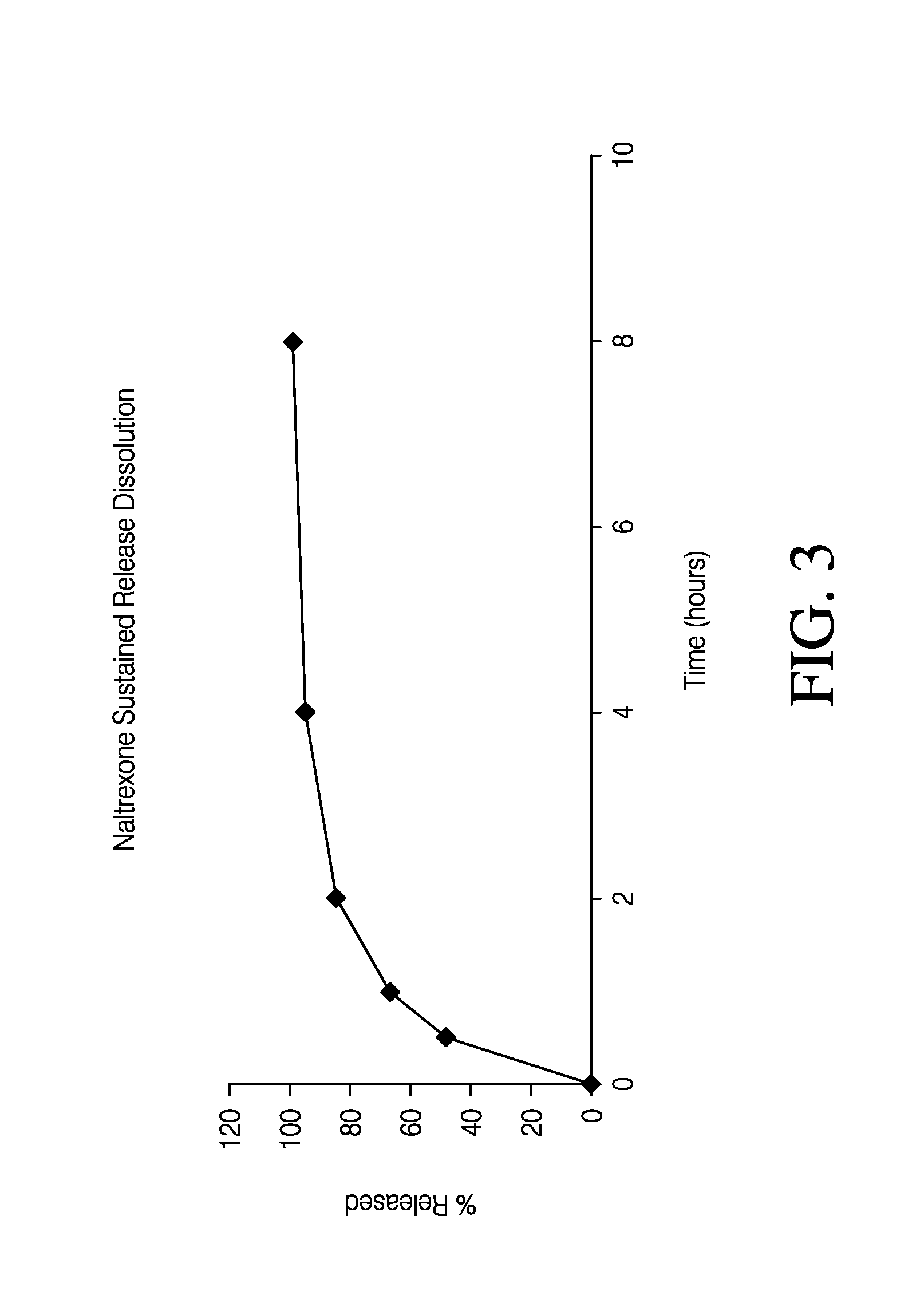
Sustained release formulation of naltrexone
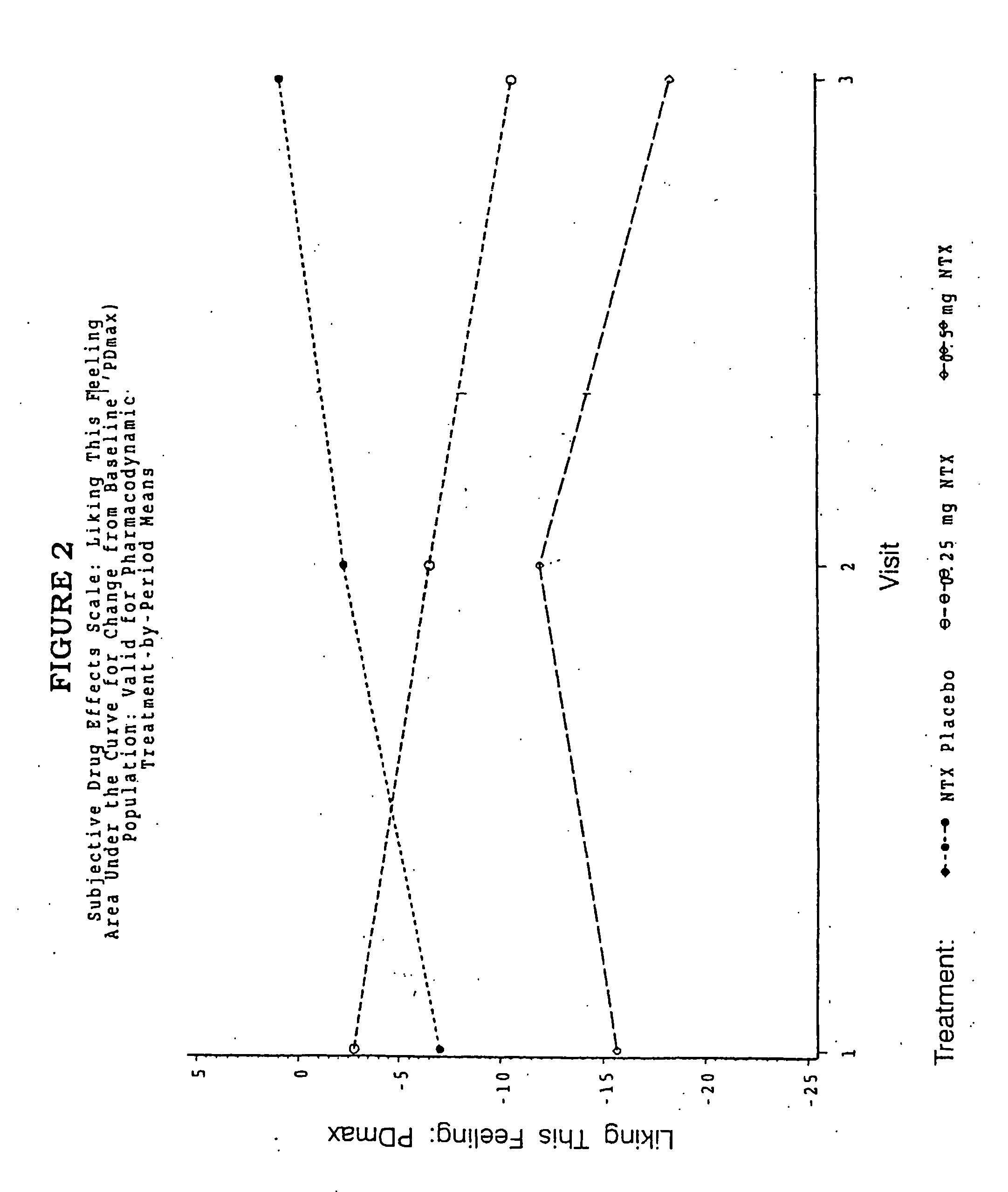
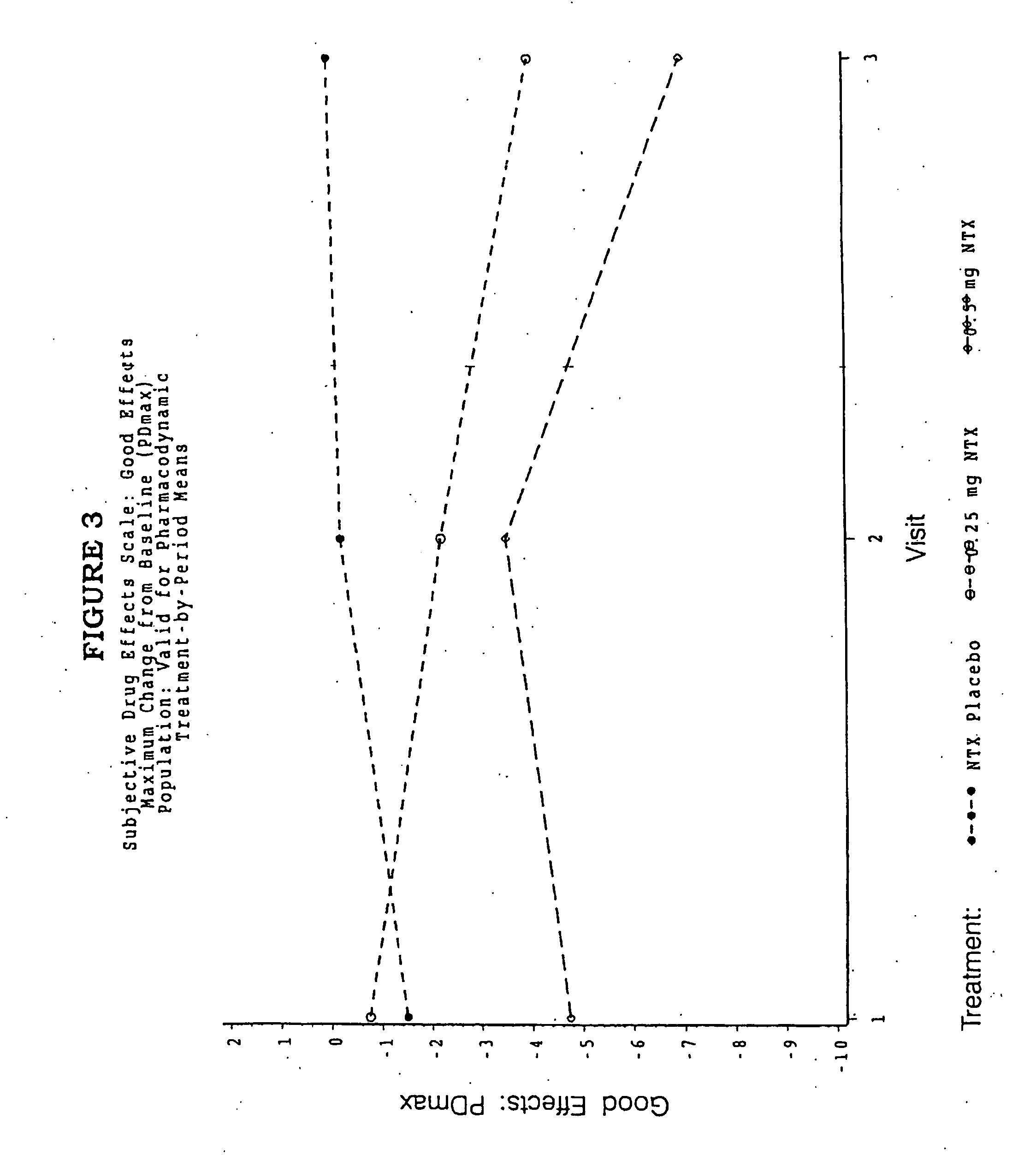
ActiveUS20070281021A1Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectCompound (substance)
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
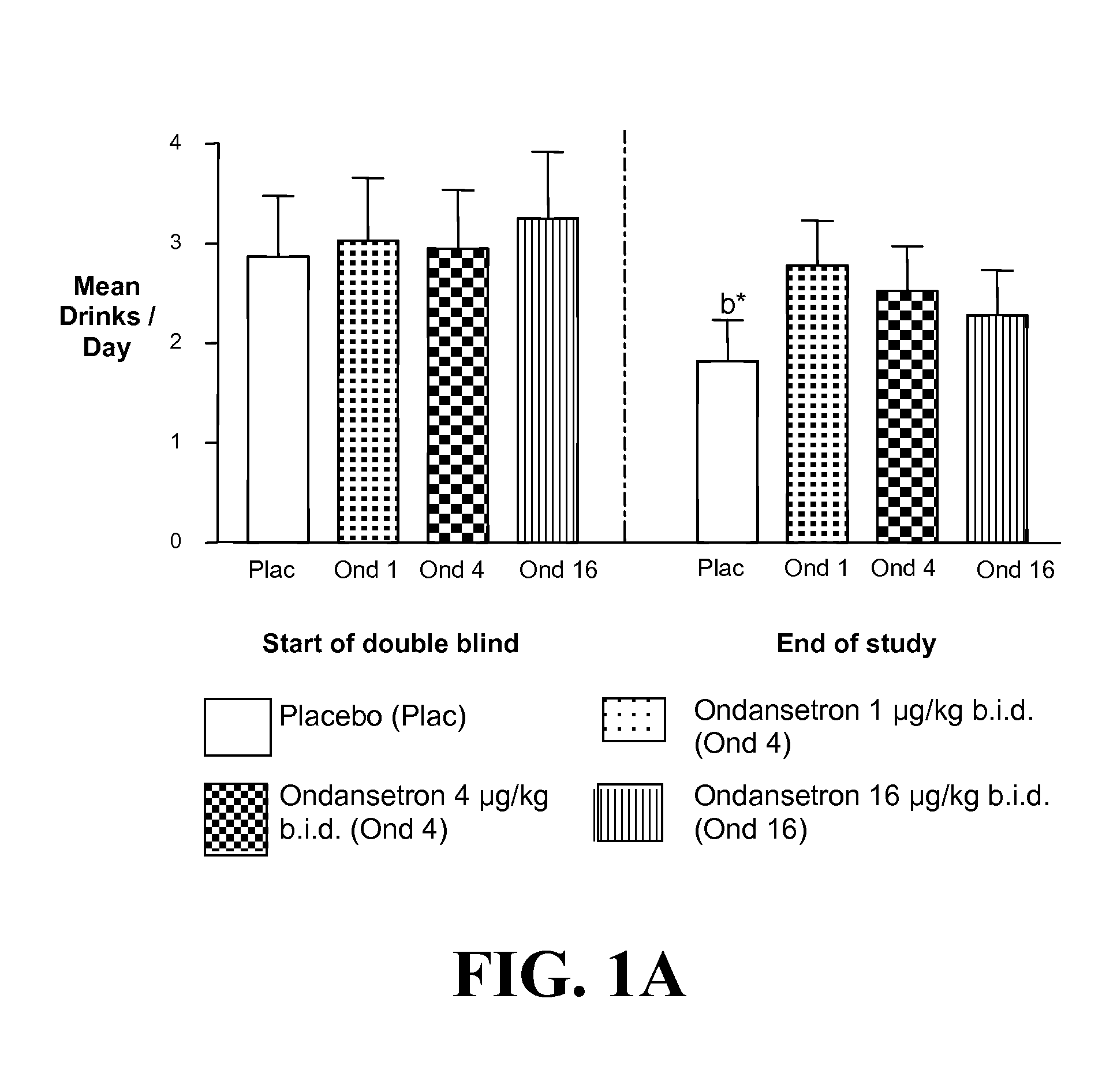
Medication Combinations for the Treatment of Alcoholism and Drug Addiction
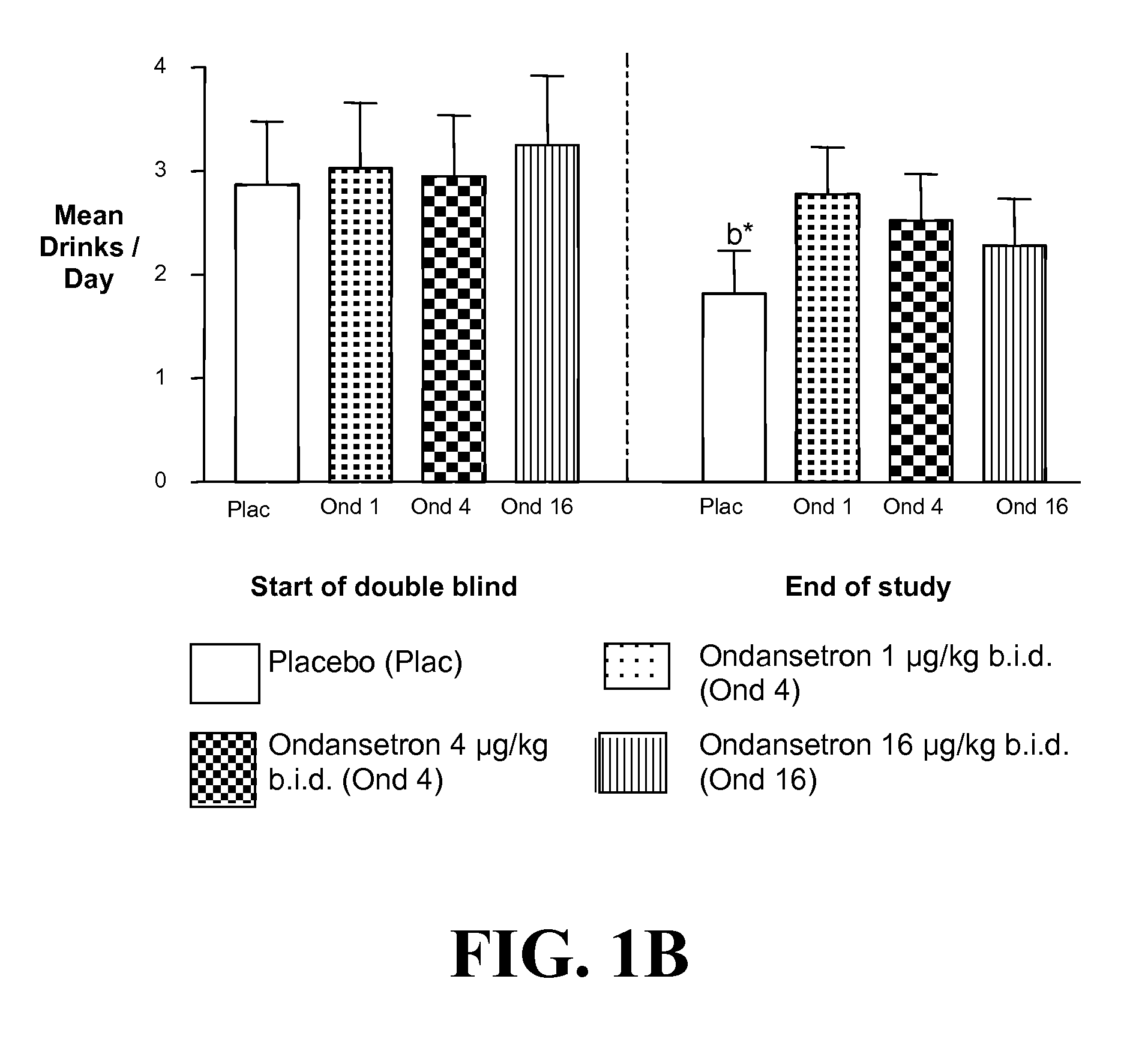
InactiveUS20110065628A1Decrease and cessationReverses effectBiocideNervous disorderAlcoholismsOndansetron
The present invention provides for the use of combinations of drugs to treat addictive disorders. More specifically, the present invention provides compositions and methods for treating disorders using combinations of drugs such as topiramate, ondansetron, and naltrexone.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Methods and compositions for the treatment of brain reward system disorders by combination therapy
The present invention is directed to a combination treatment of an opioid antagonist e.g., naltrexone and a second compound selected from the group consisting of a GABA B agonist, an NMDA antagonist, a serotonin antagonist, and a cannabinoid antagonist is the key to the successful treatment of a brain reward system disorder. A brain reward system, include but are not limited, to pathological gambling, compulsive alcohol consumption, compulsive over-eating and obesity, compulsive smoking, and drug addiction. The compounds and methods of the present invention effectively reduce the cravings, withdrawal symptoms and negative drug side effects associated with a monotherapy. As such, patient compliance is greatly increased, thereby decreasing relapse of a brain reward system disorder.
Owner:ALKERMES INC
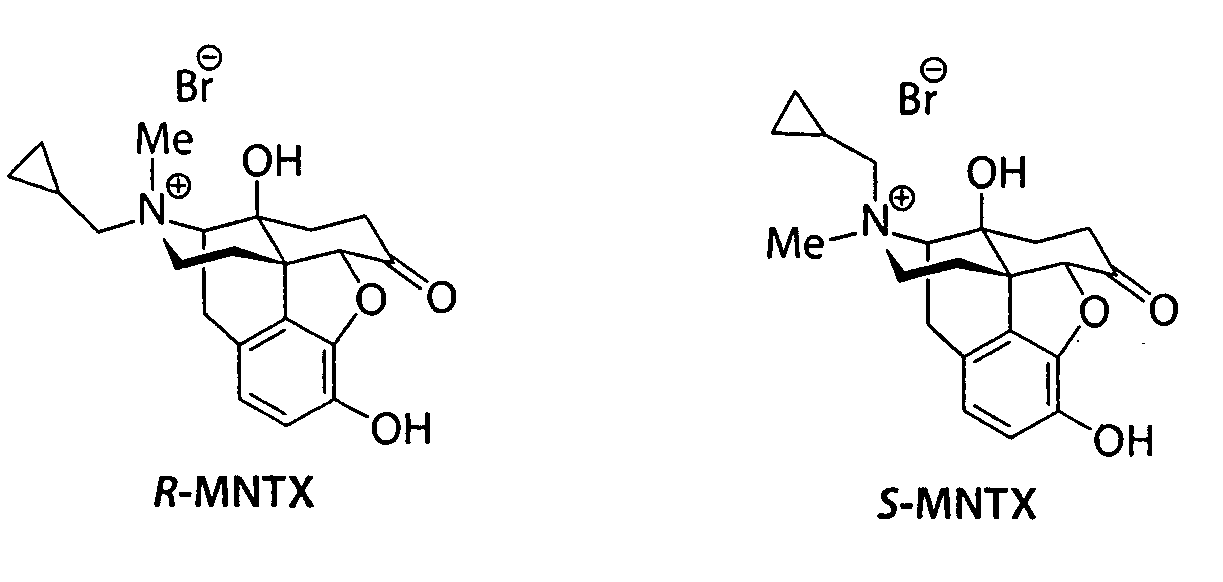
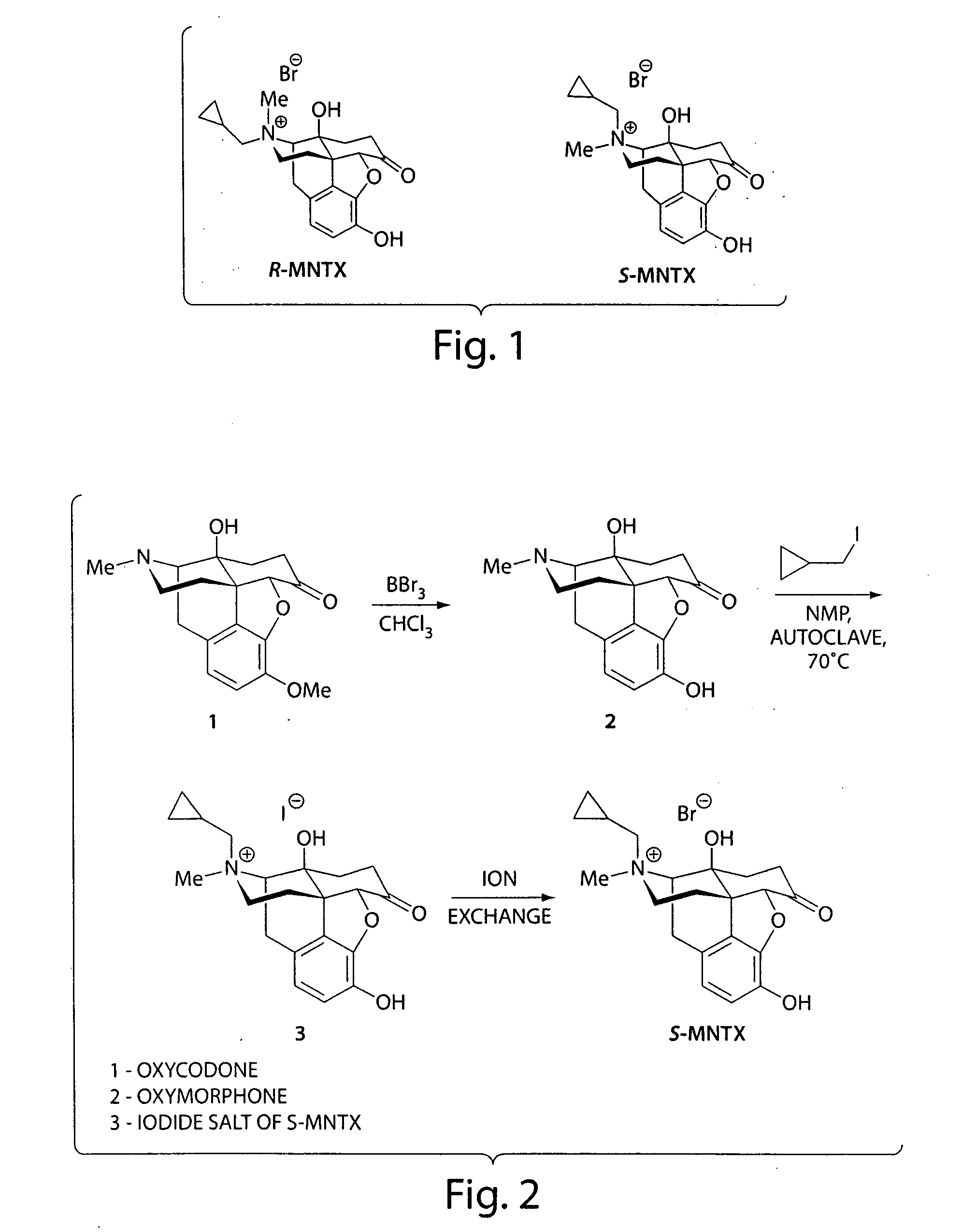
(S)-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
Process and compounds for the production of (+)opiates
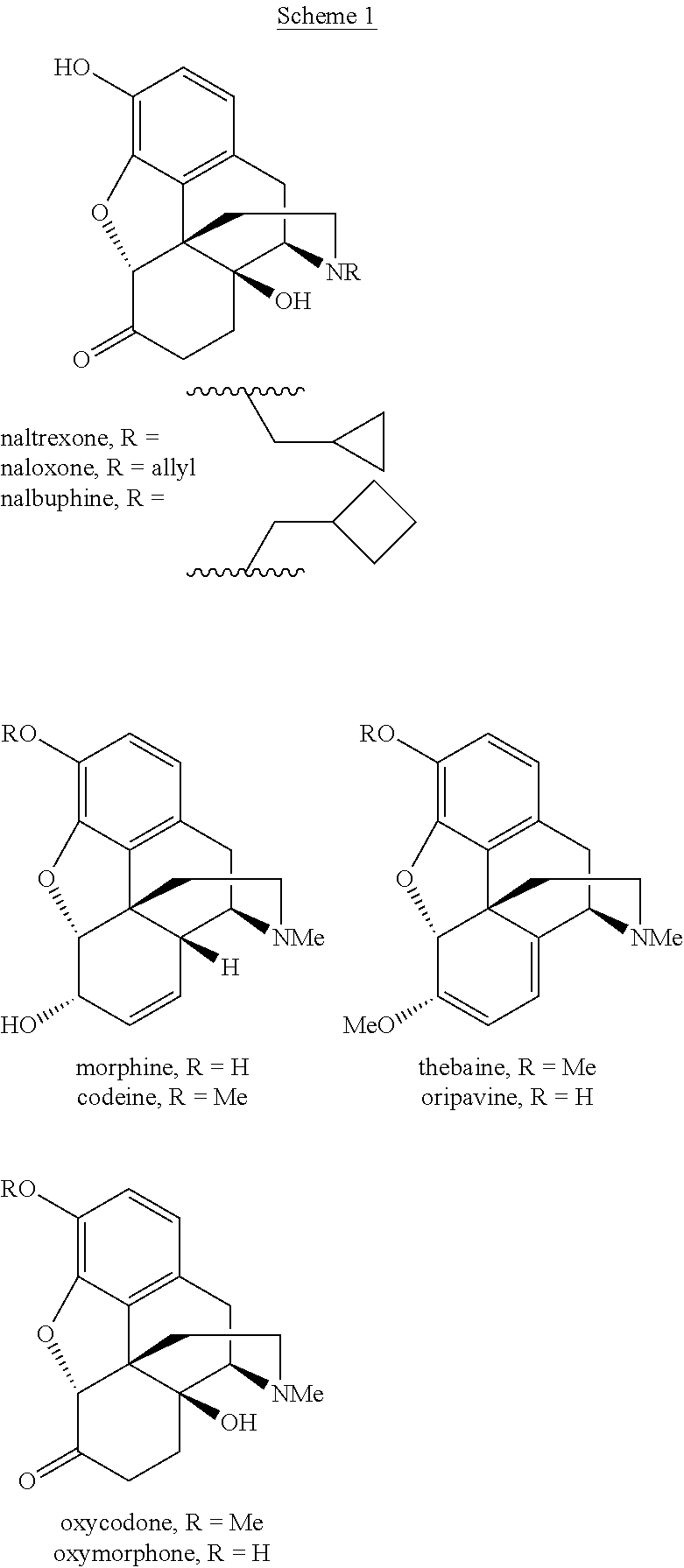
The invention generally provides processes and intermediate compounds useful for the production of (+)-opiates. Non-limiting examples of (+) opiates that may be derived from one or more compounds of the invention include (+)-noroxymorphone, (+)-naltrexone, (+)-naloxone, (+)-N-cyclopropylmethylnorhydrocodone, (+)-N-cycloproylmethylnorhydromorphone, (+)-N-allylnorhydrocodone, (+)-N-allylnorhydromorphone, (+)-noroxycodone, (+)-naltrexol, (+)-naloxol, and (+)-3-O-methyl-naltrexone.
Owner:SPECGX LLC
Pharmaceutical combinations of hydrocodone and naltrexone
InactiveUS20060194826A1Reducing abuse potential of dosage formLess abuse potentialBiocideNervous disorderHydrocodoneNaltrexone
Disclosed is a pharmaceutical composition comprising from about 5 to about 20 mg of hydrocodone or a pharmaceutically acceptable salt thereof and from 0.055 to about 0.56 mg naltrexone or pharmaceutically acceptable salt thereof.
Owner:PURDUE PHARMA LP
Pharmaceutical Compositions Comprising an Opioid Receptor Antagonist and Methods of Using Same
The present invention features compositions for intranasal administration comprising an opioid receptor antagonist. The invention also features methods of using such compositions in the treatment of various diseases and disorders, such as the treatment of alcoholism. In certain embodiments, the opioid receptor antagonist is naltrexone or a pharmaceutically acceptable salt thereof.
Owner:INTRANASAL THERAPEUTICS +1
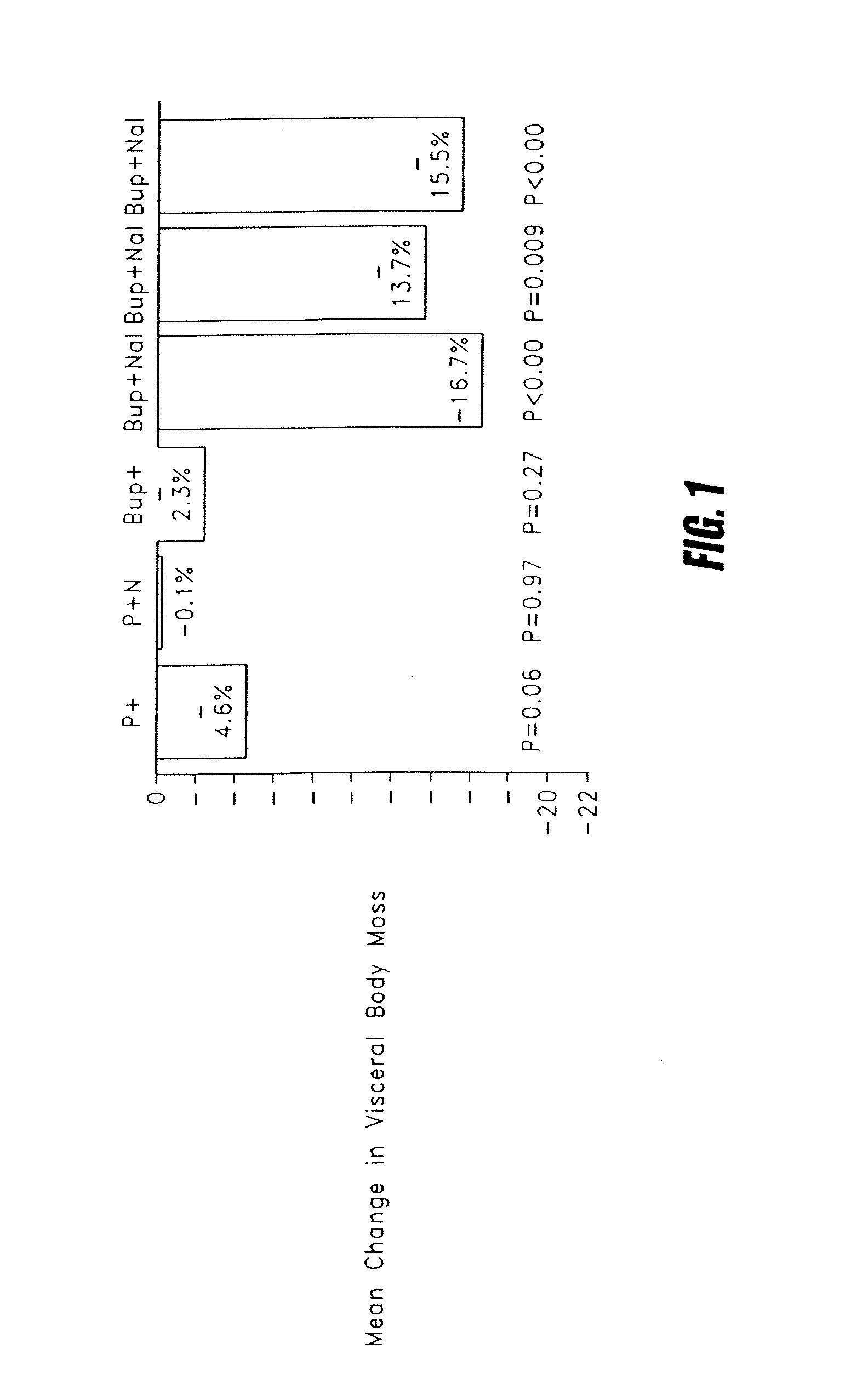
Methods for treating visceral fat conditions
Disclosed are methods and compositions for treating visceral fat conditions and / or metabolic syndrome using combinations of naltrexone and bupropion.
Owner:OREXIGEN THERAPEUTICS INC
Topiramate plus naltrexone for the treatment of addictive disorders
InactiveUS20120302592A1Treatment safetyLess susceptible to neuroadaptationBiocideNervous disorderDiseaseTopiramate
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
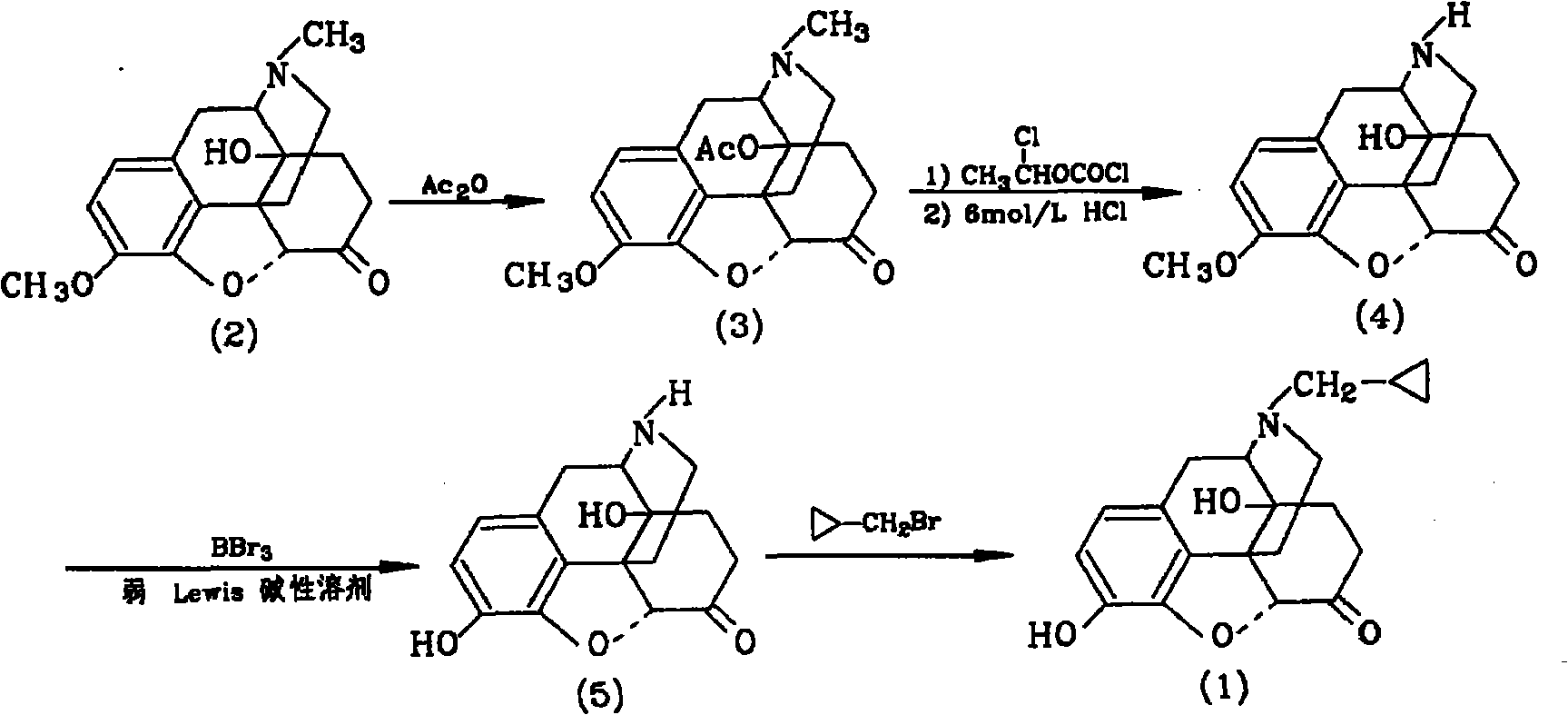
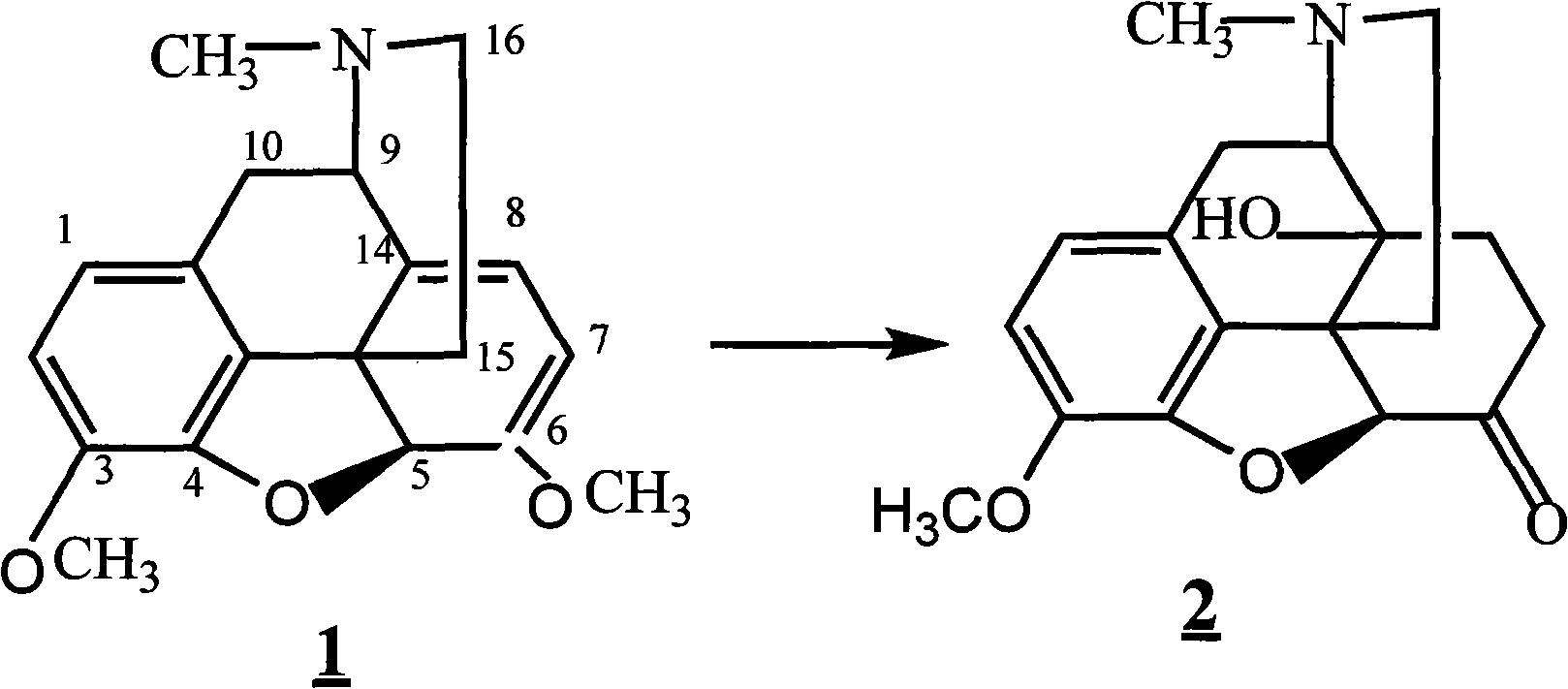
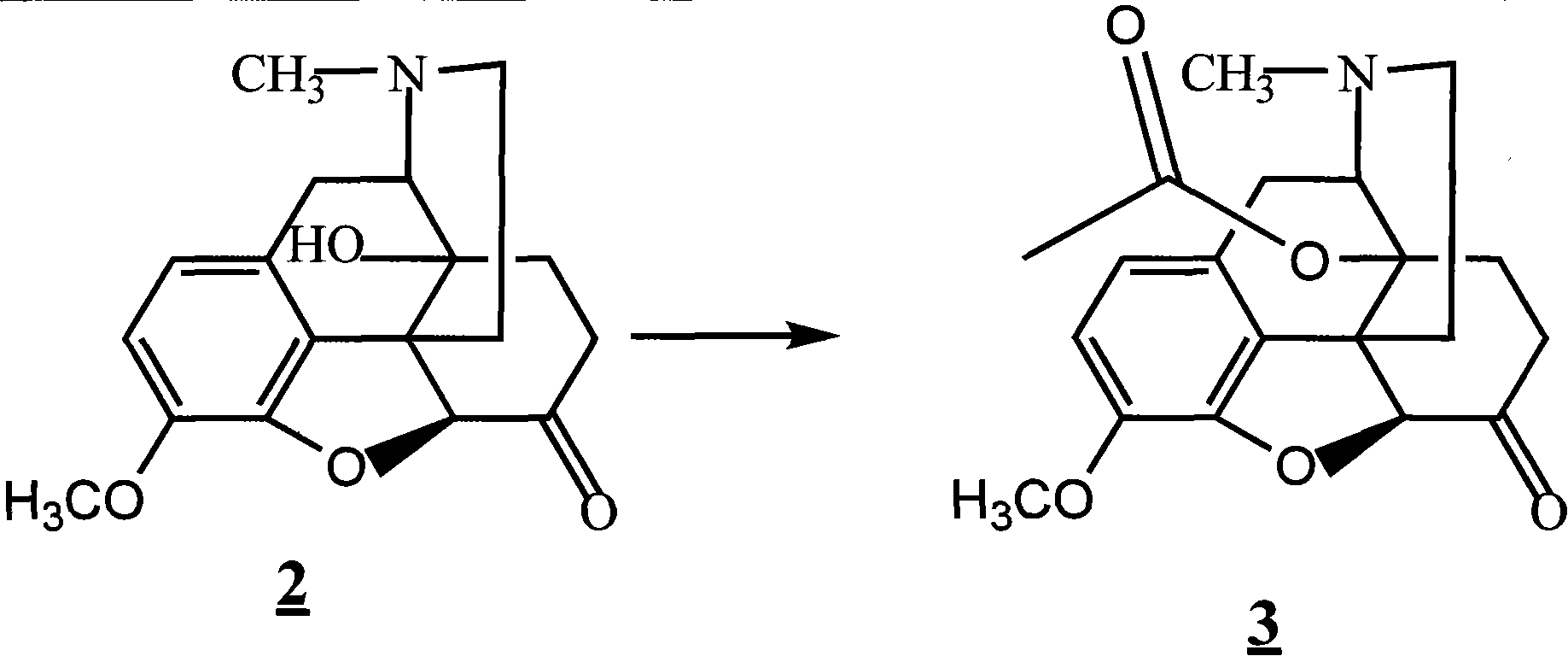
Method for synthesizing naloxone or naltrexone
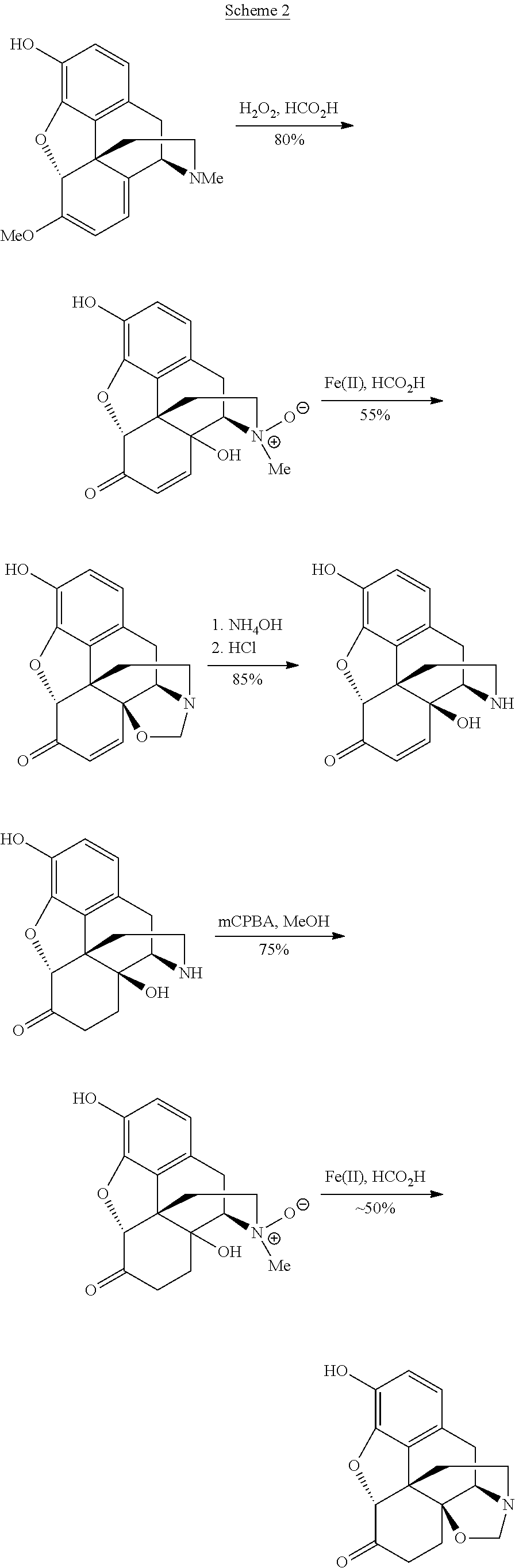
The invention provides a method for synthesizing naloxone or naltrexone, which comprises the following steps of: dissolving thebaine in formic acid, uniformly stirring, dripping an oxidant, keeping the temperature of between 20 and 40 DEG C for 3 to 7 hours, displacing gas in a reaction vessel by inert gas serving as protective gas for 3 to 5 times, adding a metallic framework catalyst, displacing the gas by hydrogen for 3 to 5 times, keeping the temperature of between 25 and 45 DEG C and stabilizing a system for 7 to 13 hours to obtain a compound 2; reacting the compound 2 with acetic anhydride at the temperature of between 60 and 100 DEG C for 1 to 2 hours to obtain a compound 3; taking the inert gas as the protective gas, adding toluene, chloroformic acid-1-chloroethyl ester and potassium bicarbonate into the compound 3, heating to the temperature of between 75 and 100 DEG C and reacting for 20 to 40 hours, concentrating under reduced pressure until the system is fully dry, adding 10 percent hydrochloric acid, and heating and refluxing for 2 to 6 hours to obtain a compound 4; dissolving the compound 4 and at least one alkylation reagent in an organic solvent 1 and reacting with alkali at the temperature of between 50 and 100 DEG C to obtain a compound 5; and reacting the compound 5 with boron tribromide in an organic solvent 2 at the temperature of between -10 and 40 DEG C for 2 to 4 hours to obtain a compound 6, namely the naloxone or naltrexone.
Owner:甘肃普安制药股份有限公司
Topiramate Plus Naltrexone for the Treatment of Addictive Disorders
InactiveUS20100076006A1Treatment safetyLess susceptible to neuroadaptationBiocideNervous disorderDiseaseTopiramate
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
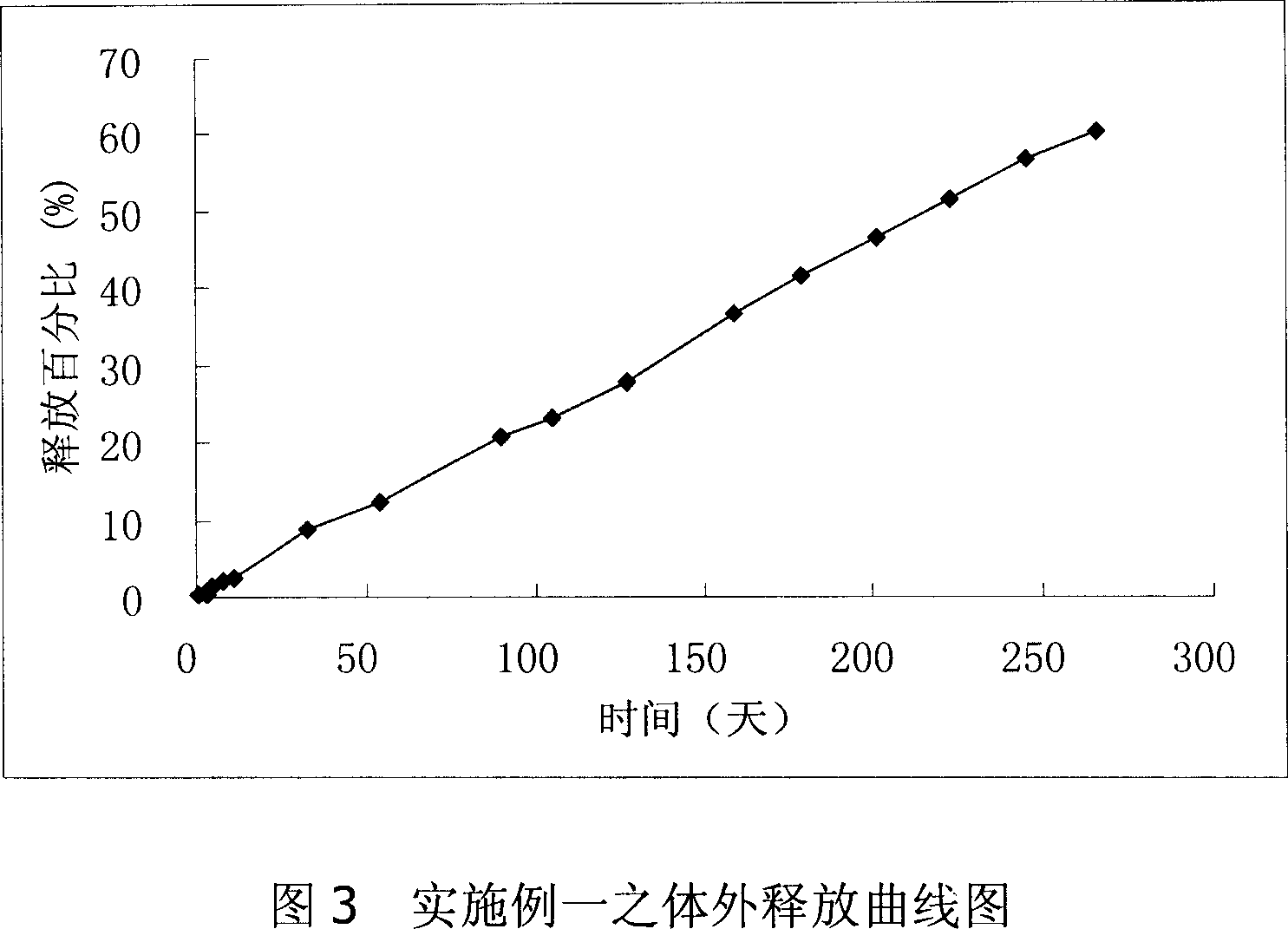
Long-acting naltrexone implant and preparation method thereof
ActiveCN102512399AFacilitated releaseRelease stabilityOrganic active ingredientsNervous disorderSide effectCurative effect
The invention relates to a long-acting naltrexone implant and a preparation method thereof, which belong to the field of pharmaceutical preparations. The long-acting naltrexone implant comprises a polylactic acid and naltrexone, and is characterized in that the viscosity coefficient of the polylactic acid is 0.5-2.5dl / g (chloroform, 30 DEG C); the ratio of the polylactic acid to the naltrexone is1:(0.5-1.8) in parts by weight; the composition structure of the long-acting naltrexone implant is a particle tabletted and coated structure; coated particles are slow release pellets which can be sieved by a 20-60 mesh screen in particle size; the slow release pellets comprise the polylactic acid and the naltrexone; the ratio of the polylactic acid to the naltrexone in the slow release pellets is 1:(1-2) in part by weight; and a coating layer is a DL-polylactic acid. The long-acting naltrexone implant has a good curative effect, better drug-loading rate and better drug release completeness, and has small side effect on human bodies.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Pharmaceutical Composition Comprising Opioid Agonist And Sequestered Antagonist
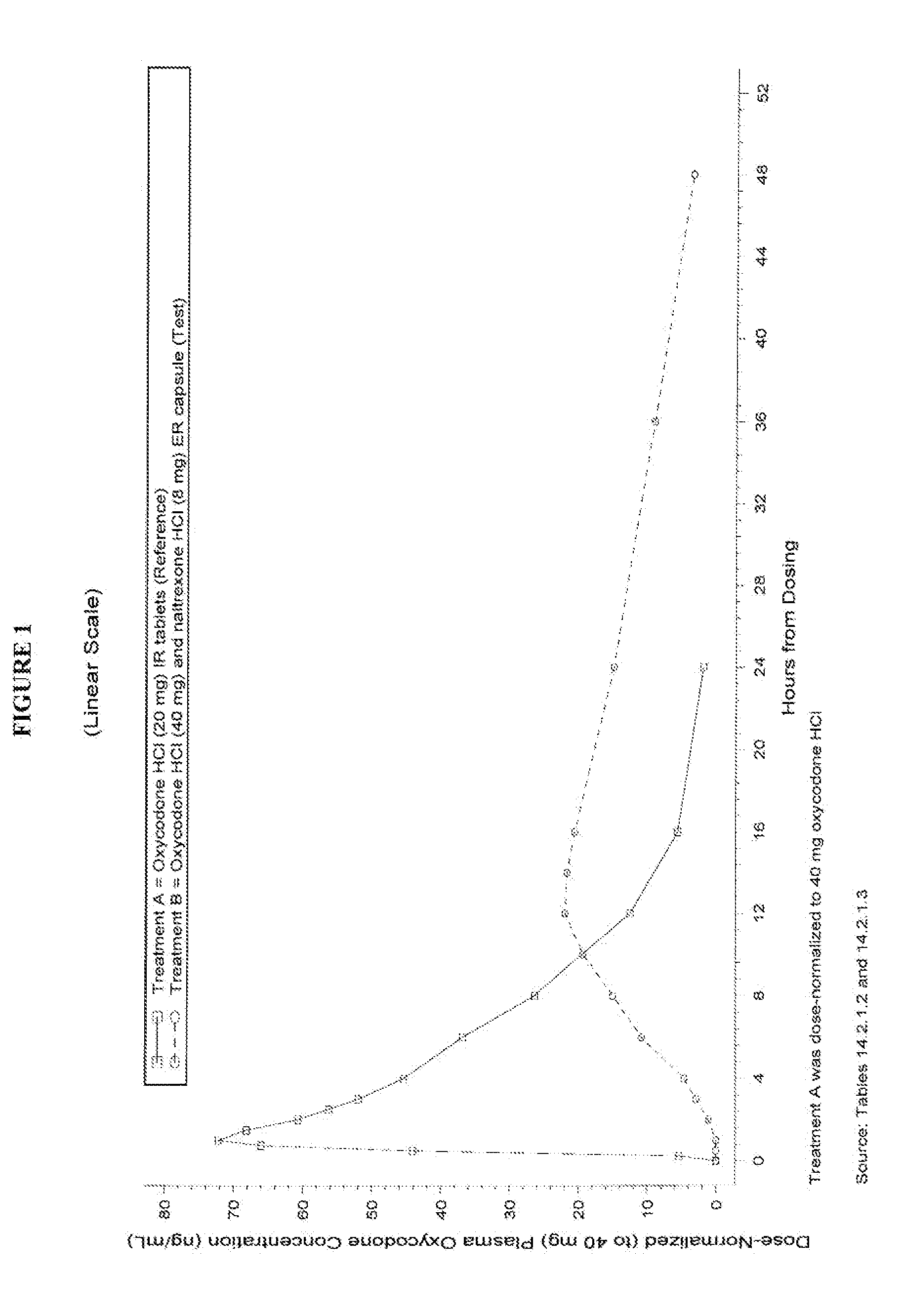
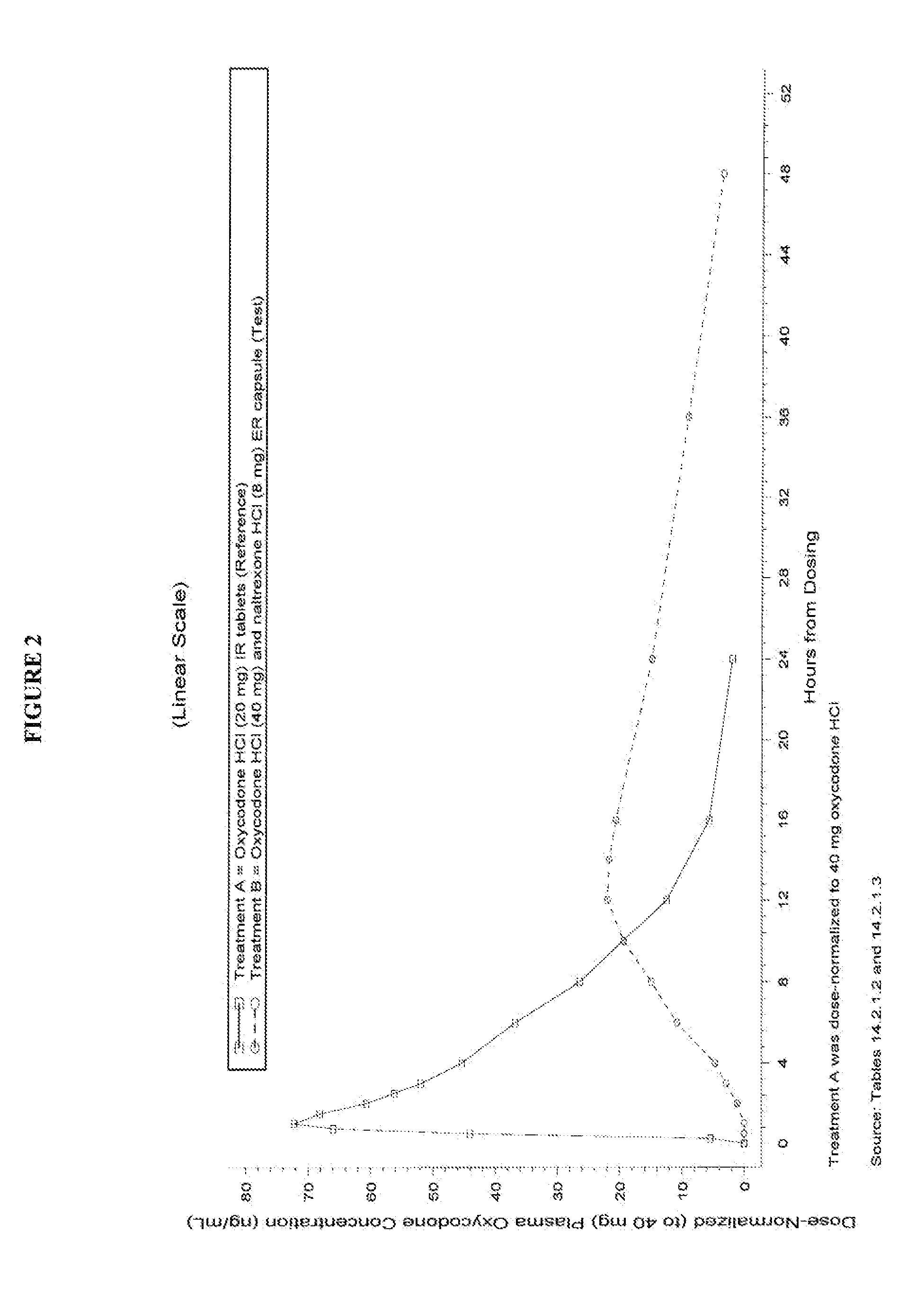
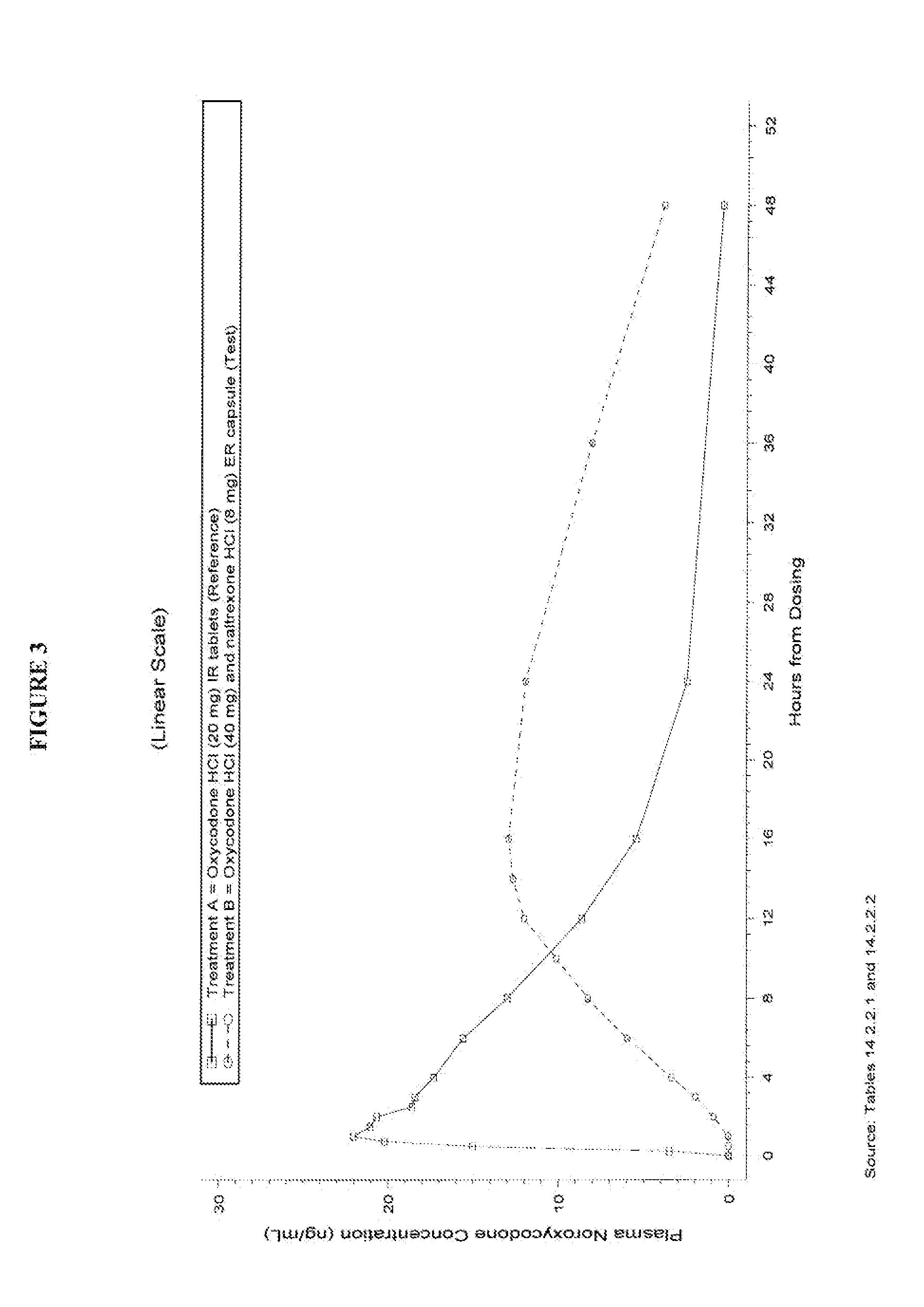
This invention pertains to pharmaceutical composition comprising a plurality of multi-layered beads having an oxycodone layer and a sequestering subunit comprising a naltrexone and a blocking agent, in particular pharmaceutical compositions comprising a higher level of naltrexone, and related compositions and methods of use, such as in the prevention of abuse of a therapeutic agent. The compositions of the present invention also have a long Tmax for oxycodone release and a flatter release profile of oxycodone over time.
Owner:WILSON EDWARD S
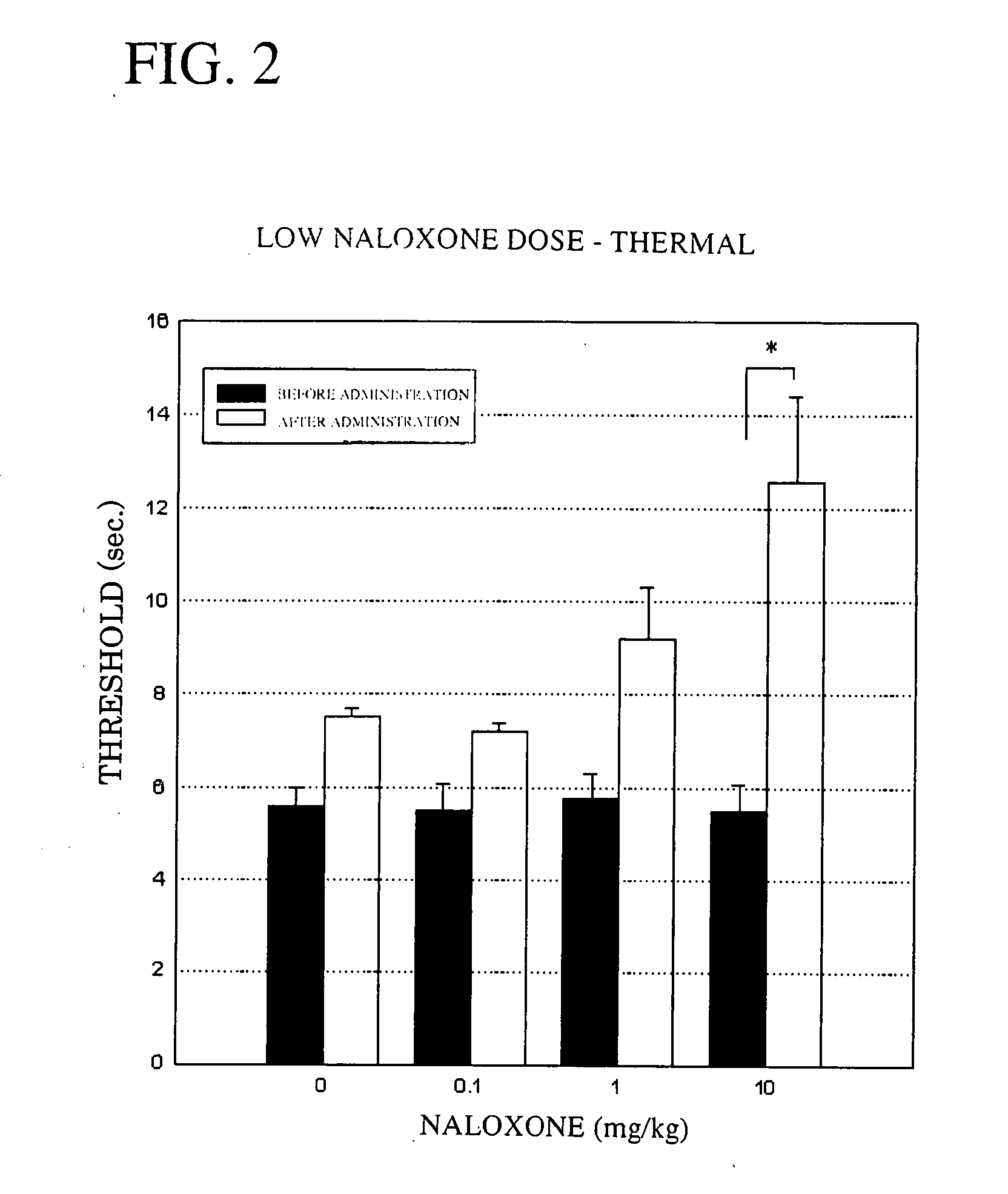
Therapeutic Agent for Neuropathic Pain
InactiveUS20070299098A1Easy to useEffective treatmentBiocideNervous disorderTreatment effectNaltrindole
The present invention provides therapeutic agents for neuropathic pain, the agents having excellent therapeutic effects on neuropathic pain, which is an intractable disorder. More specifically, the invention provides therapeutic agents for neuropathic pain comprising, as the active ingredient, an opioid receptor antagonist (particularly naloxone, naltrexone, naloxonazine, naltrindole, etc.), pharmaceutical compositions for treating neuropathic pain comprising an opioid receptor antagonist as the active ingredient, and a method for treating neuropathic pain using opioid receptor antagonists.
Owner:JAPAN SCI & TECH CORP +1
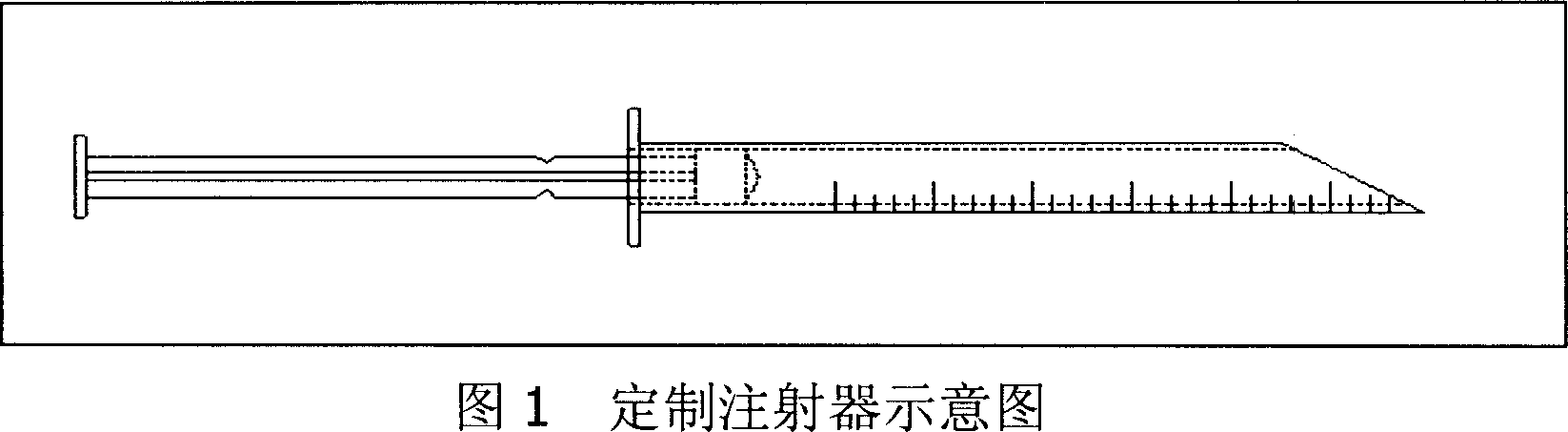
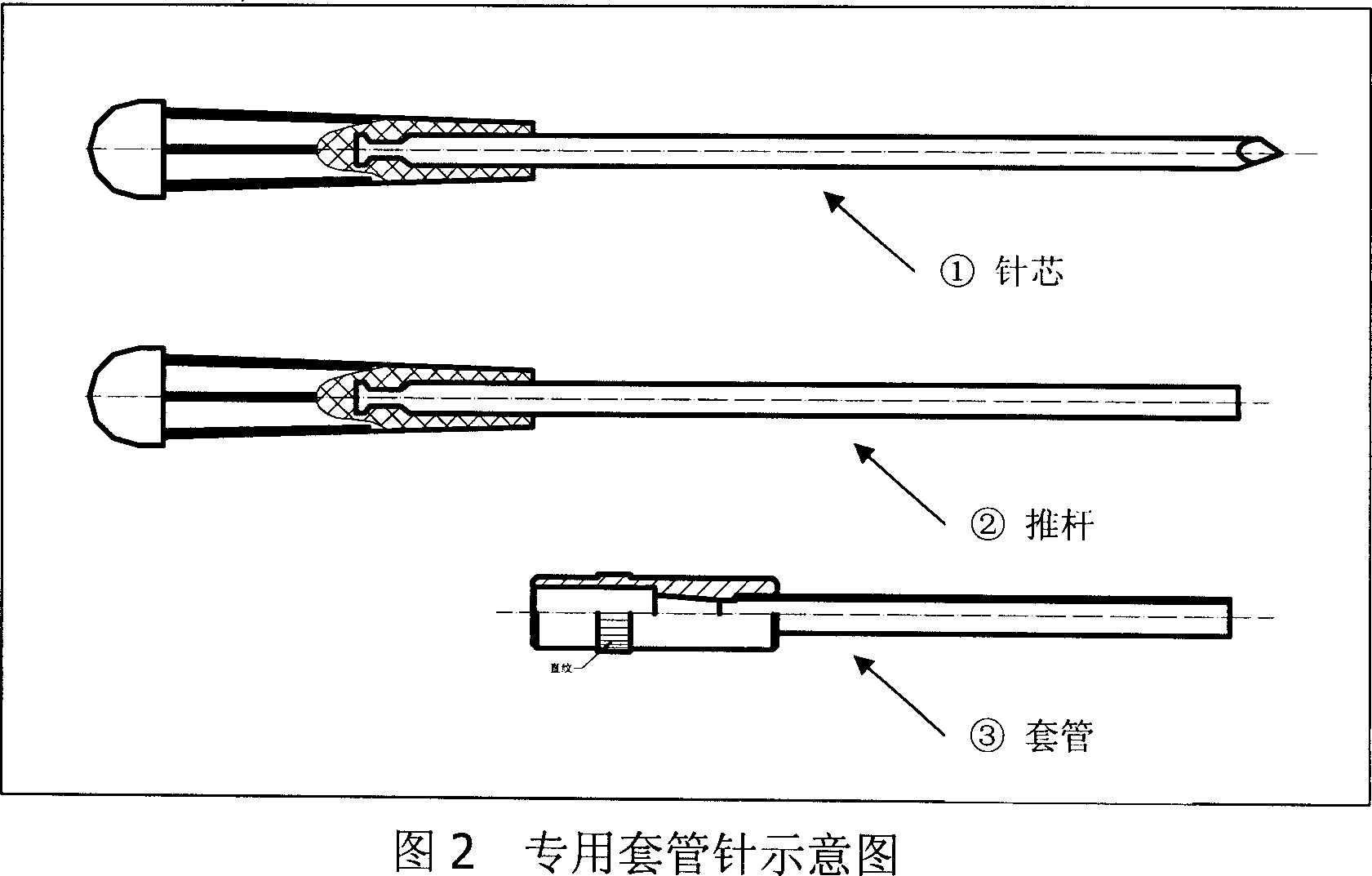
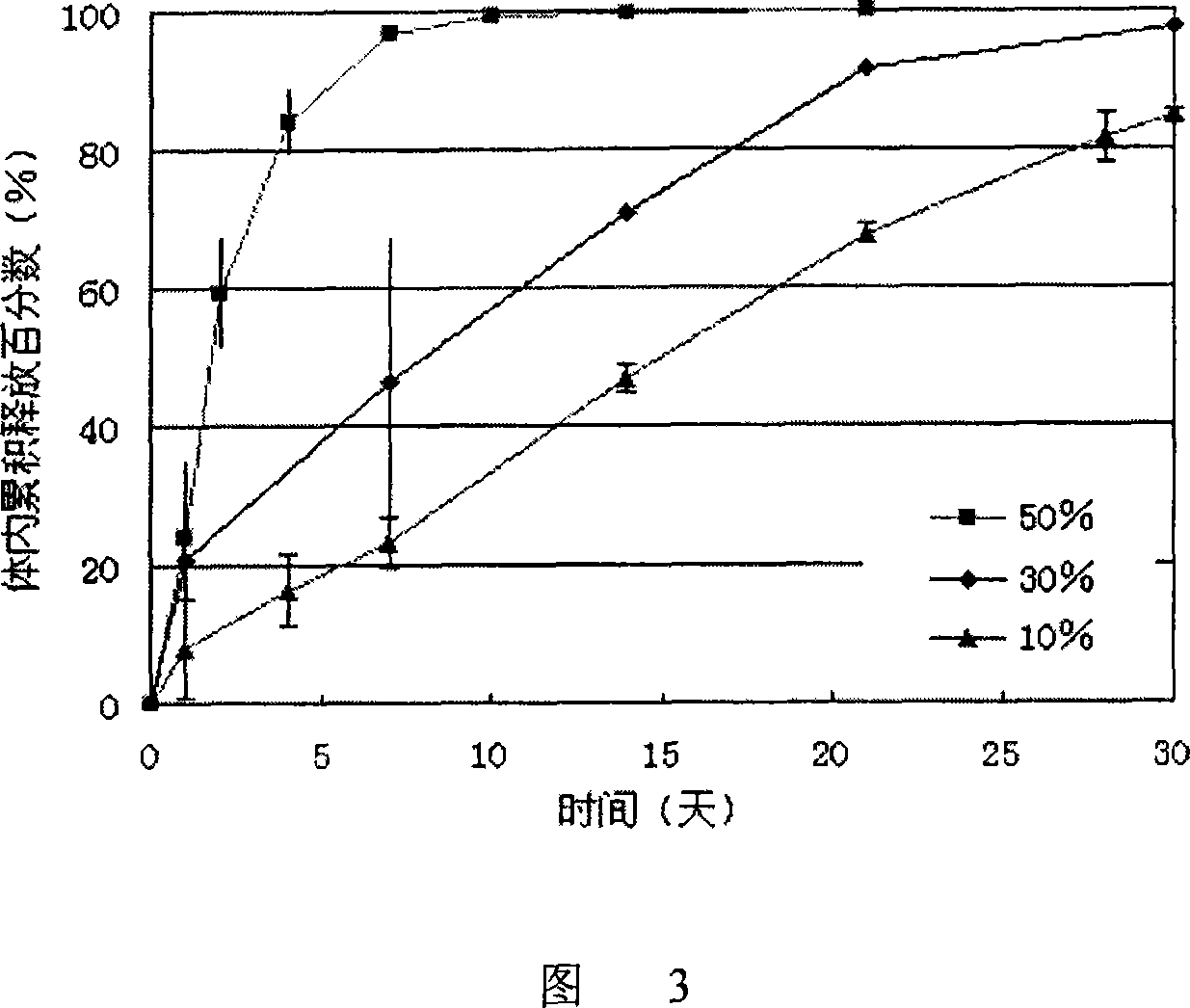
Long acting slow releasing drug addiction eliminating prepn and its prepn process and use
ActiveCN1973840ASolve the sudden release problemRelease stabilityOrganic active ingredientsNervous disorderMicrosphereSolvent
The present invention provides the preparation process and usage of slow released naltrexone (NTX) preparation, which is used in the rehabilitation after eliminating opium addiction. The NTX preparation includes NTX as the opium receptor antagonist and matrix of biodegradable polymer material polylactic acid. The preparation process includes emulsification and solvent volatilization to prepare microsphere, pressing into tablet, coating with polylactic acid and other steps. The NTX preparation has great medicine carrying amount and high encapsulating rate, and may reach blood medicine concentration for over 360 days. When it is used, the NTX preparation is injected with special injector into subcutaneous fat for NTX to release slowly and persistently to maintain the effective blood medicine concentration.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Injectable opioid partial agonist or opioid antagonist microparticle compositions and their use in reducing consumption of abused substances
InactiveUS20030152638A1Avoid prolonged useReadily injected intramuscularlyBiocidePowder deliveryOpioid antagonistControl manner
An injectable slow-release partial opioid agonist or opioid antagonist formulation is provided comprising a partial opioid agonist or opioid antagonist in a poly(D,L-lactide) excipient with a small amount of residual ethyl acetate. Upon intramuscular injection of the composition, a partial opioid agonist or opioid antagonist is released in a controlled manner over an extended period of time. The composition finds use in the treatment of heroin addicts and alcoholics to reduce consumption of the abused substances. Of particular interest are the drugs buprenorphine, methadone and naltrexone.
Owner:EVONIK CORP
Sustained release formulation of naltrexone
ActiveUS8916195B2Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectSustained-Release Preparations
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
Methods and compositions to prevent addiction
InactiveUS20120302590A1Decrease and lessening of aversionDecrease and lessening of and addictionBiocideNervous disorderNervous systemStimulant
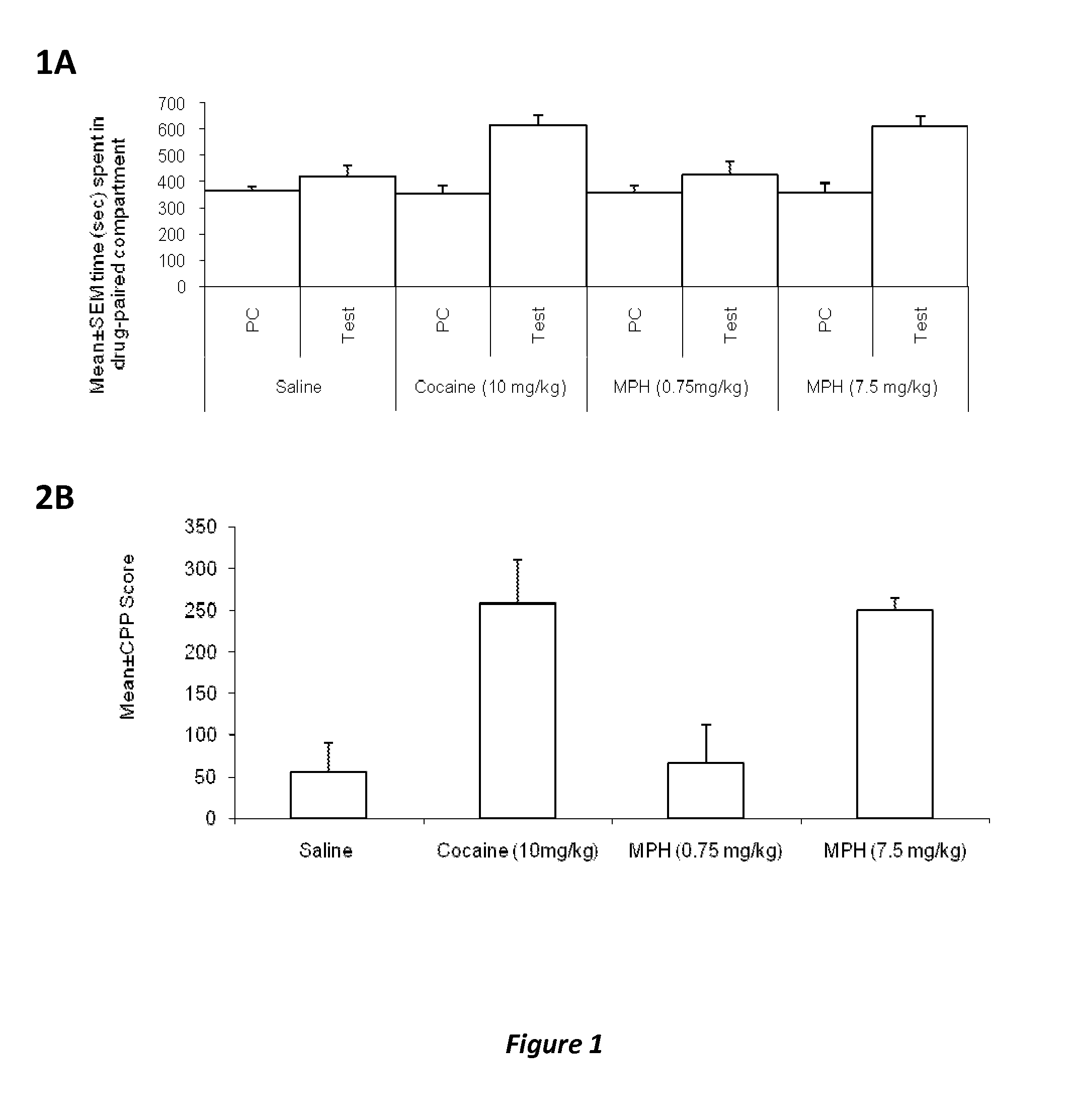
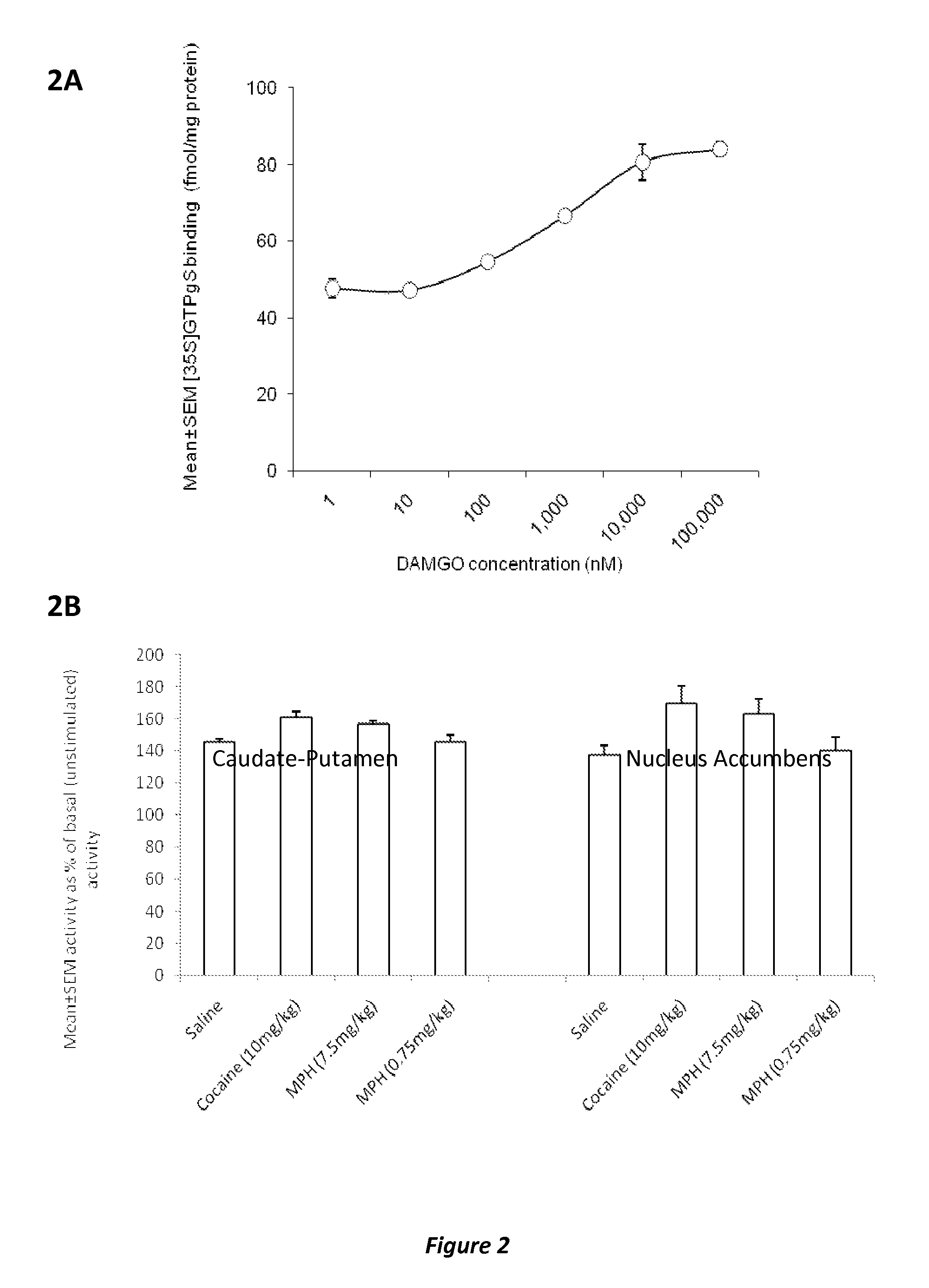
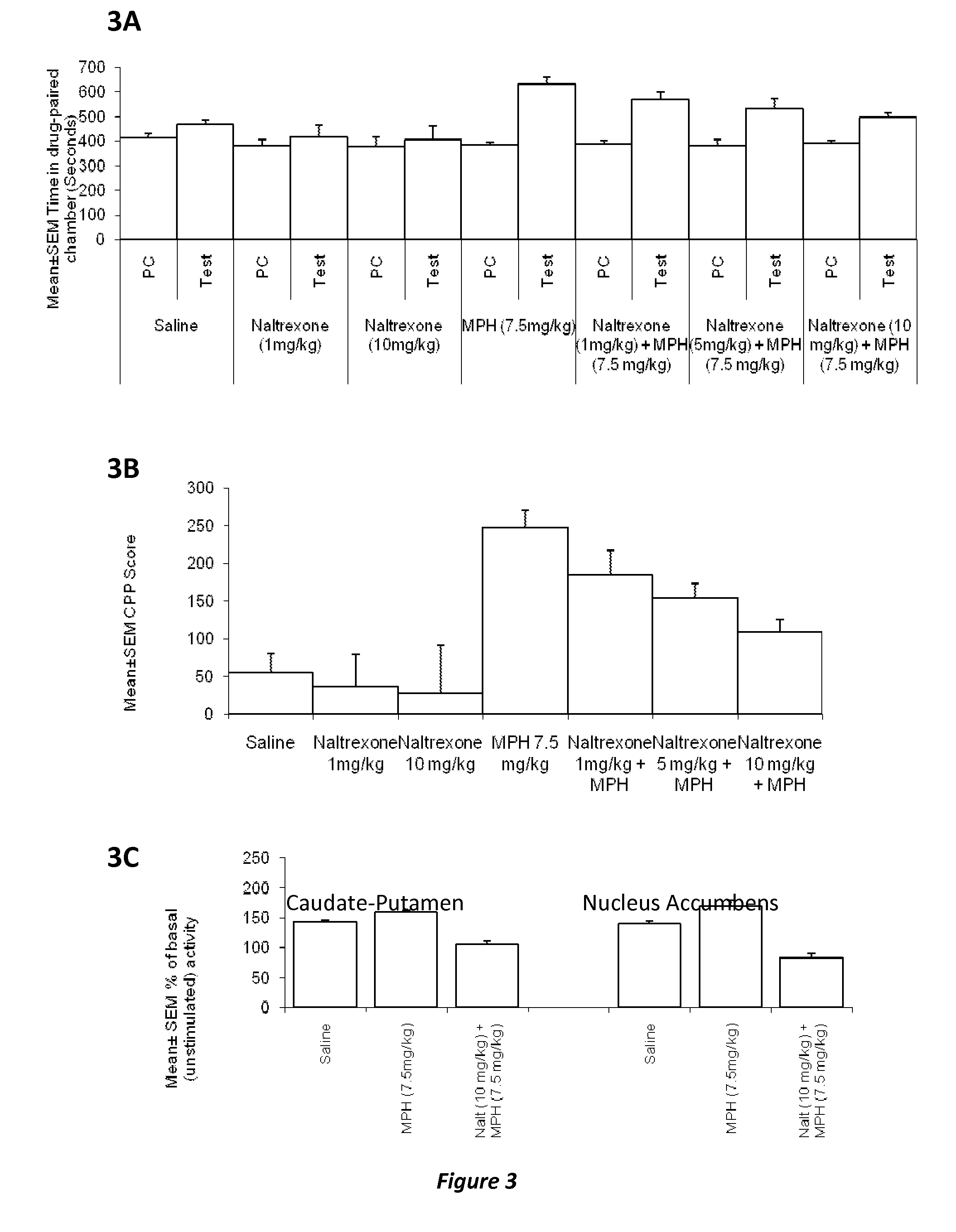
Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject comprising, administering a therapeutic amount of the neurological stimulant and administering an antagonist of the kappa opioid receptor, to thereby reduce or prevent the development of aversion to the CNS stimulant in the subject. Also disclosed is a method of reducing or preventing the development of addiction to a CNS stimulant in a subject, comprising, administering the CNS stimulant and administering a mu opioid receptor antagonist to thereby reduce or prevent the development of addiction to the CNS stimulant in the subject. Also disclosed are pharmaceutical compositions comprising a central nervous system stimulant and an opioid receptor antagonist. Examples of central nervous system stimulants (such as methylphenidate) and opioid receptor antagonists (such as naltrexone) are provided.
Owner:THE GENERAL HOSPITAL CORP
Pharmaceutical formulation
InactiveUS20100261745A1Eliminate side effectsReduce secretionBiocideNervous disorderPharmaceutical formulationMethylnaltrexone
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Pharmaceutical formulation
ActiveUS20100261744A1Eliminate side effectsReduce secretionBiocideNervous disorderPharmaceutical formulationMethylnaltrexone
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
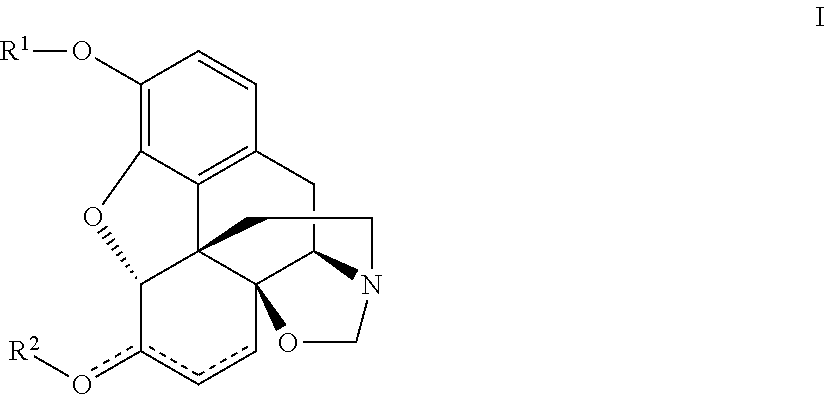
Processes and Intermediates in the Preparation of Morphine Analogs via N-Demethylation of N-Oxides Using Cyclodehydration Reagents
InactiveUS20120283443A1Efficient conversionOrganic chemistryBulk chemical productionMorphinansOxymorphone
A high-yielding method for the N-demethylation of oxycodone- and oxymorphone-N-oxides by the reaction of these compounds with cyclodehydration reagents has been performed. This method has been utilized to improve the synthesis of various morphine analogs, such as naltrexone, nalbuphone and naloxone.
Owner:BROCK UNIVERSITY
Methods for treating visceral fat conditions
Disclosed are methods and compositions for treating visceral fat conditions and / or metabolic syndrome using combinations of naltrexone and bupropion.
Owner:OREXIGEN THERAPEUTICS INC
Opiates painkiller and opiate receptor antagonist-containing medicinal composition
ActiveCN102068697AGood analgesic effectPrevent and/or mitigate adverse effectsOrganic active ingredientsNervous disorderSide effectNK1 receptor antagonist
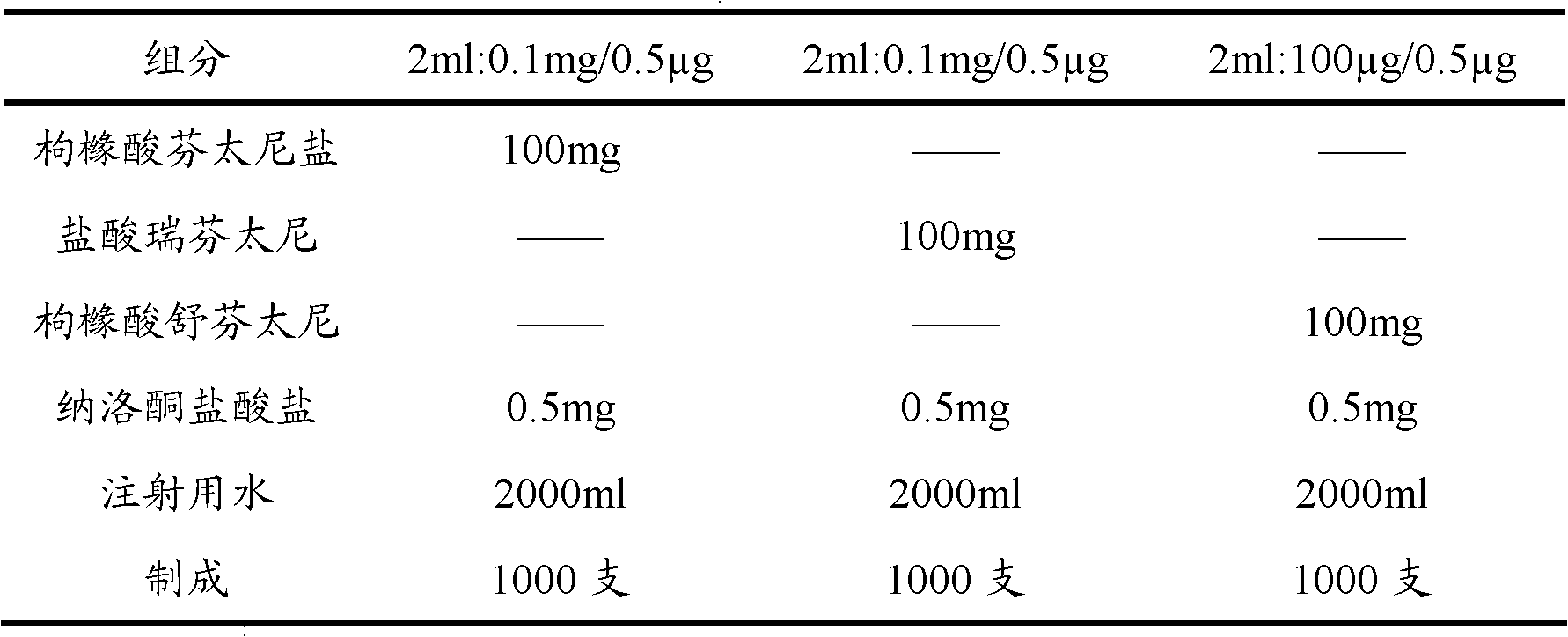
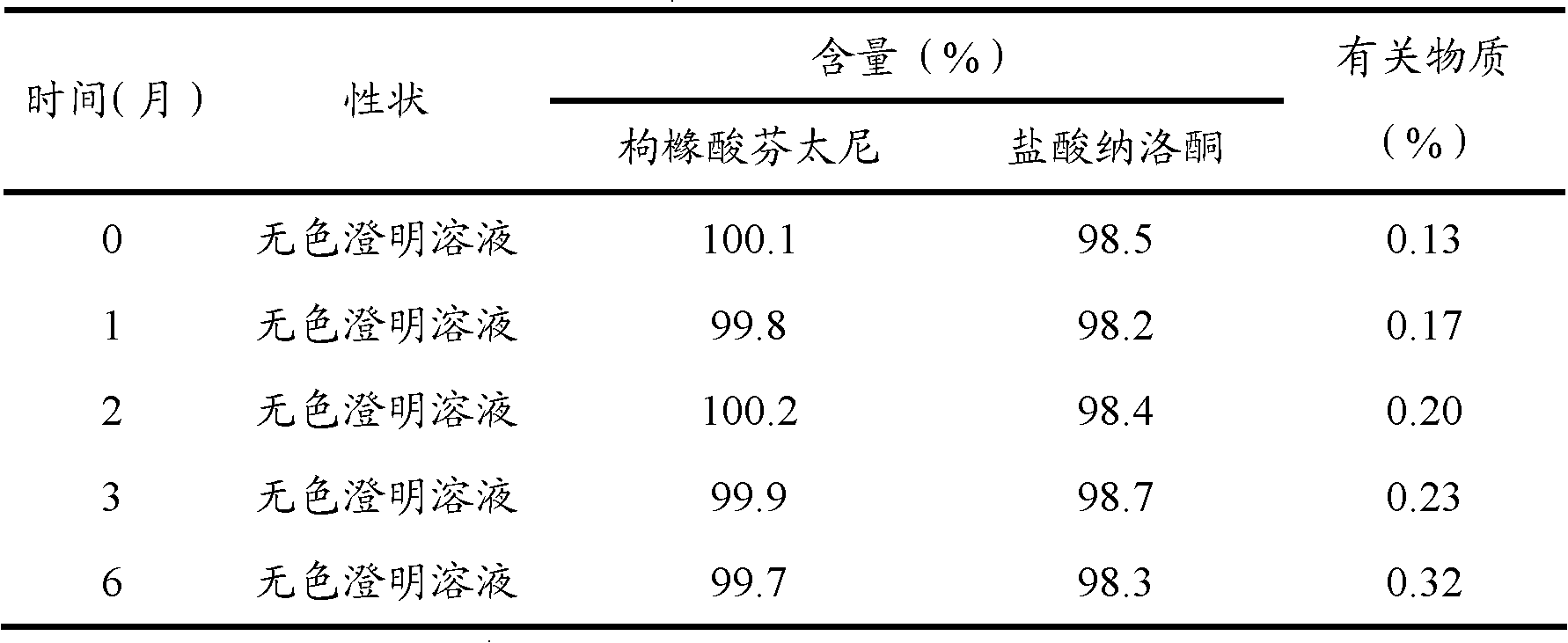
The invention provides an opiates painkiller and opiate receptor antagonist-containing medicinal composition. In the medicinal composition, an opiates painkiller is fentanyl, remifentanil, sufentanil, alfentanil and pharmaceutically acceptable salts thereof; and an opiate receptor antagonist is naloxone, naltrexone, nalmefene and pharmaceutically acceptable salts thereof. The medicinal composition has a pharmacological effect on analgesia. Compared with using the opiates painkiller singly, the composition can prevent and / or lighten side effects in pain treatment, reduce abuse and improve adaptability, and has a reinforcing effect on an analgesic effect of the opiates painkiller.
Owner:YICHANG HUMANWELL PHARMA
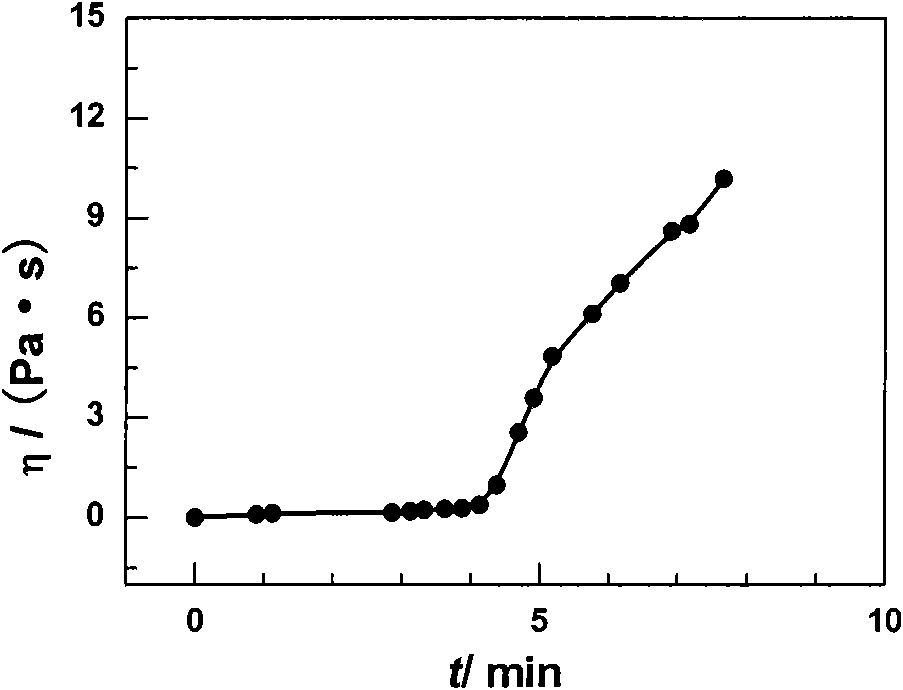
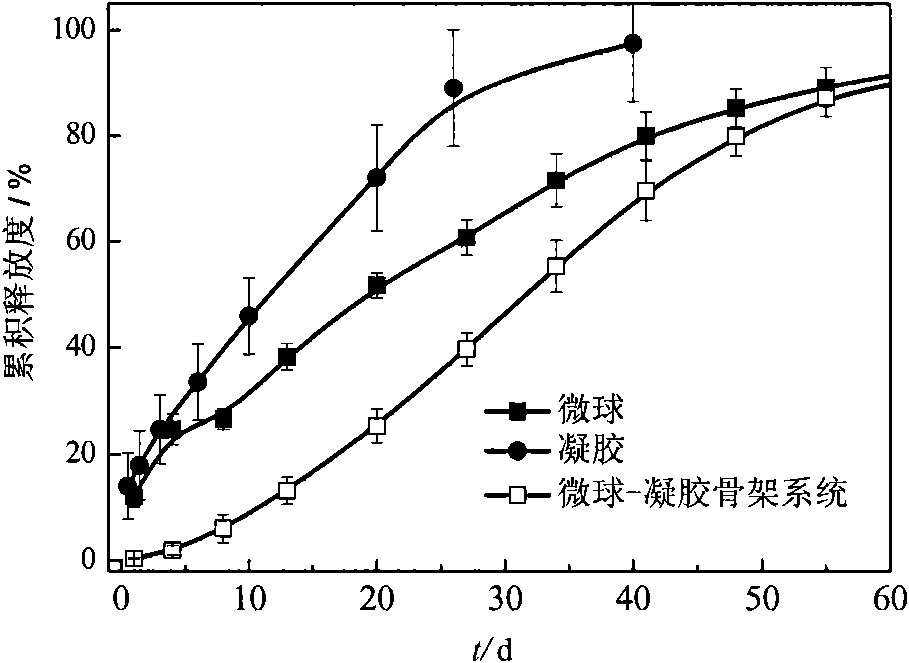
In-situ implantation drug delivery system of naltrexone microsphere-hydrogel matrix
InactiveCN101612437AStable release rateReduce high speed releaseSurgeryPharmaceutical delivery mechanismMicrosphereDetoxification Process
The invention discloses an in-situ implantation drug delivery system of microsphere-hydrogel matrix of detoxification drug naltrexone, belonging to the field of medicinal preparation. The drug naltrexone is embedded in microspheres of biodegradable materials polylactic acid / glycolic acid polymer; the microspheres disperse in reverse temperature sensitive hydrosol of methylcellulose; the hydrosol is solidified into a hydrogel matrix at body temperature during hypodermic injection to form a composite system of microsphere-hydrogel matrix and to control the release of naltrexone. The invention integrates the advantages of medicine carrying controlled release particles and temperature sensitive gel matrix in-situ implantation system, realizes injected implantation, realizes release at a constant speed for 60 days after implantation, and can adjust drug release speed by adjusting the content of naltrexone microspheres in hydrogel, thus having great significance in improving clinical application effect of naltrexone and in solving the problem of relapse in the detoxification process.
Owner:TSINGHUA UNIV
New preparation method for raising rate of packaging microspheres of naltrexone
InactiveCN101049288AHigh encapsulation efficiencyOrganic active ingredientsNervous disorderOrganic solventEmulsion
A process for increasing the encapsulating rate of naltrexone microball by O / W- emulsifying-solidifying method includes such steps as adding the dispersed phase cotaining maltrexone and glycollide-lactide copolymer to the first continuous phase, emulsifying to become O / W emulsion, adding the second continuous phase for removing solvent and solidifying.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Medication and treatment for disease
Owner:ALTMAN ENTERPRISES
Pharmaceutical compositions for treatment of addiction
The invention provides transdermal patches comprising an adhesive and a composition comprising naltrexone and either methyl oleate or isopropyl myristate, a kit comprising a plurality of such transdermal patches, a composition comprising naltrexone and methyl oleate, such a composition for use as a medicament, and compositions comprising naltrexone and methyl oleate or isopropyl myristate for use in the treatment of alcoholism or opiate addiction.
Owner:LIBERO PHARMA LTD
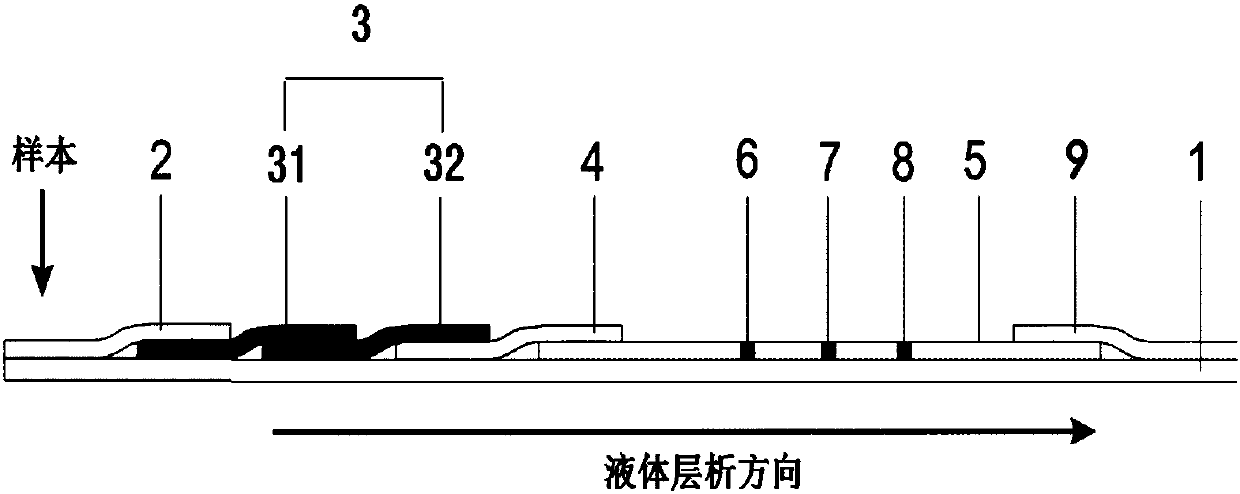
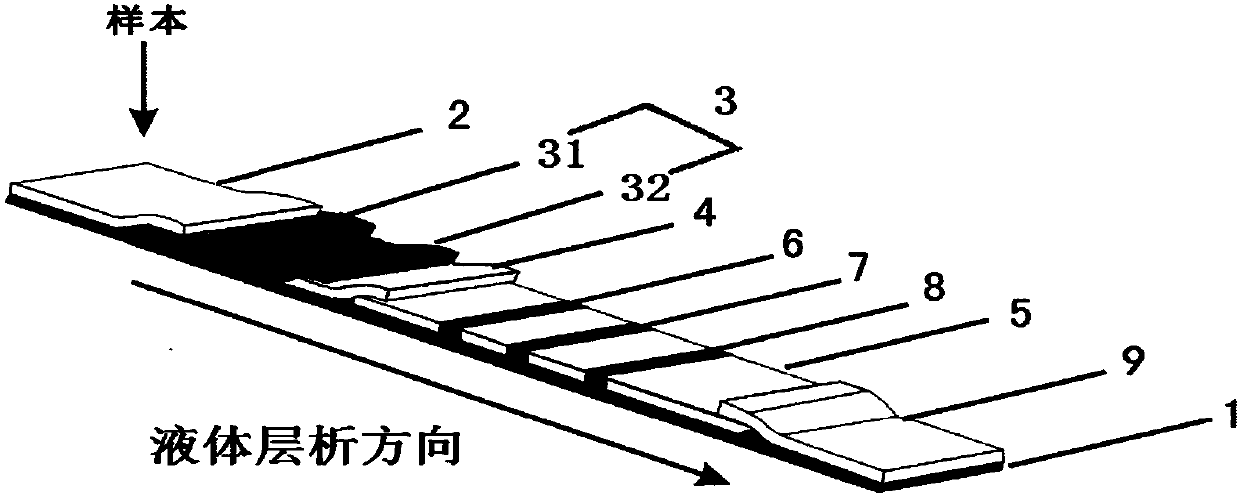
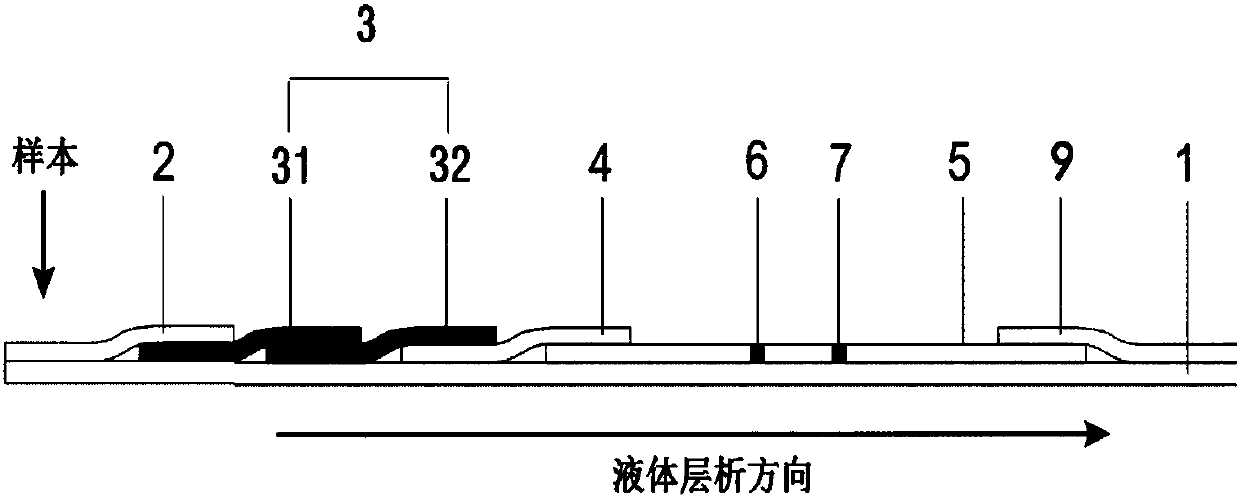
NTx (Naltrexone) detection test paper, kit and preparation method thereof
InactiveCN107688090AImprove detection stabilityEnables direct detectionMaterial analysisOsteoporosisTest strips
The invention discloses an NTx (Naltrexone) detection test strip. The NTx detection test strip comprises a chromatography membrane and a conjugate pad; an immunolabelled NTx antibody and an immunolabelled first antibody are loaded on the conjugate pad; the chromatography membrane is provided with an NTx protein covered detection line and a second antibody covered colorimetric line along a liquid chromatography direction. When a sample is detected, the colorimetric line correspondingly displays the chroma of specific protein concentration after the second antibody is specifically combined withthe first antibody; NTx protein on the detection line and the NTx protein in the sample are competitively combined with the immunolabelled NTx antibody and the color developing depth of the detectionline and the colorimetric line is directly compared so that semi-quantitative detection of the NTx protein is realized. The invention discloses a preparation method of the NTx detection test strip; the preparation method is used for preparing an NTx protein semi-quantitative detection test strip which is rapid and sensitive in detection and does not depend on an instrument. The invention disclosesan NTx detection kit; the NTx detection kit comprises the NTx detection test strip and can provide effective information for early diagnosis and treatment of osteoporosis.
Owner:RUNBIO BIOTECH CO LTD
Pharmaceutical compositions comprising an opioid receptor antagonist and methods of using same
The present invention features compositions for intranasal administration comprising an opioid receptor antagonist. The invention also features methods of using such compositions in the treatment of various diseases and disorders, such as the treatment of alcoholism. In certain embodiments, the opioid receptor antagonist is naltrexone or a pharmaceutically acceptable salt thereof.
Owner:ALCOMED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com