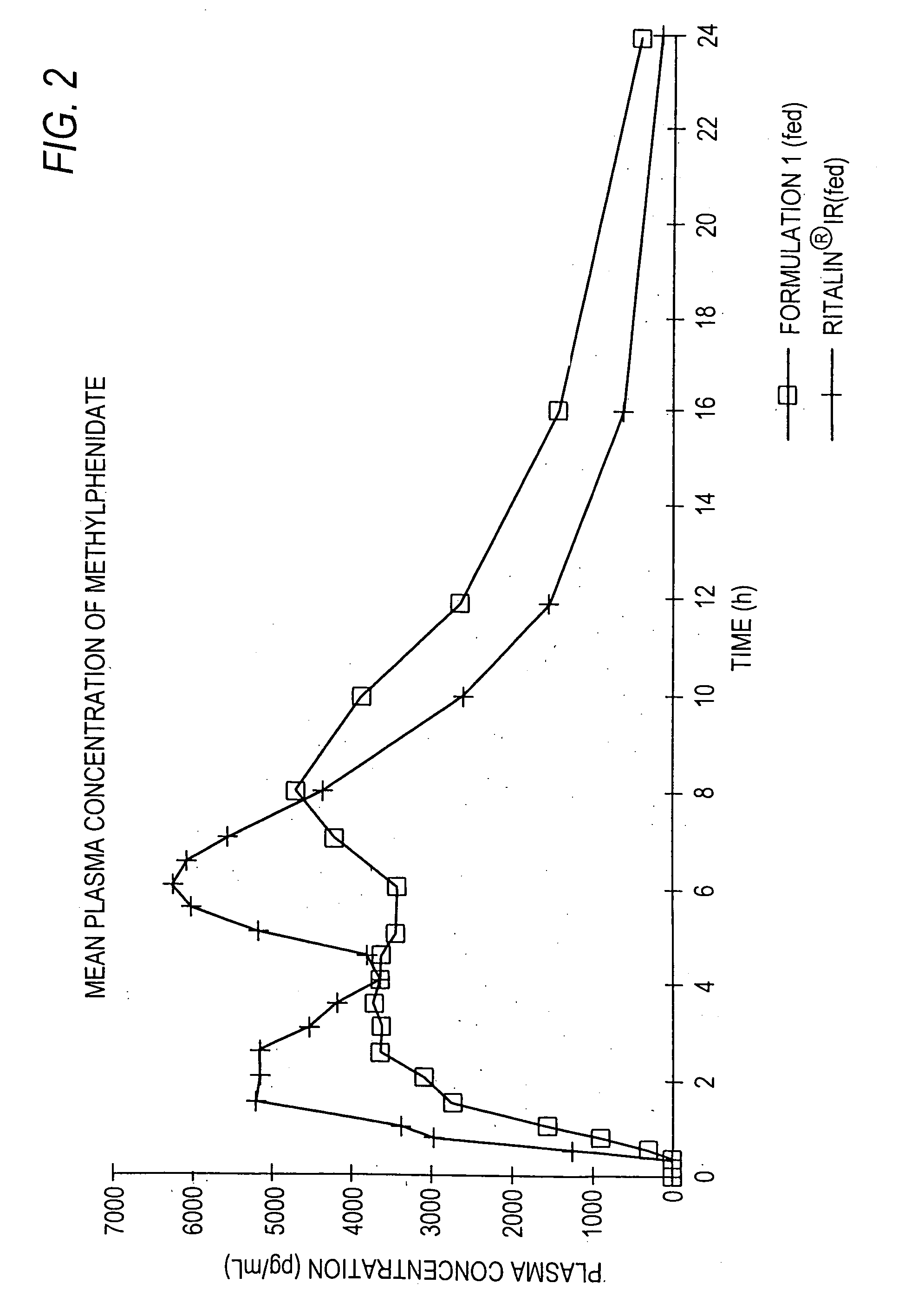
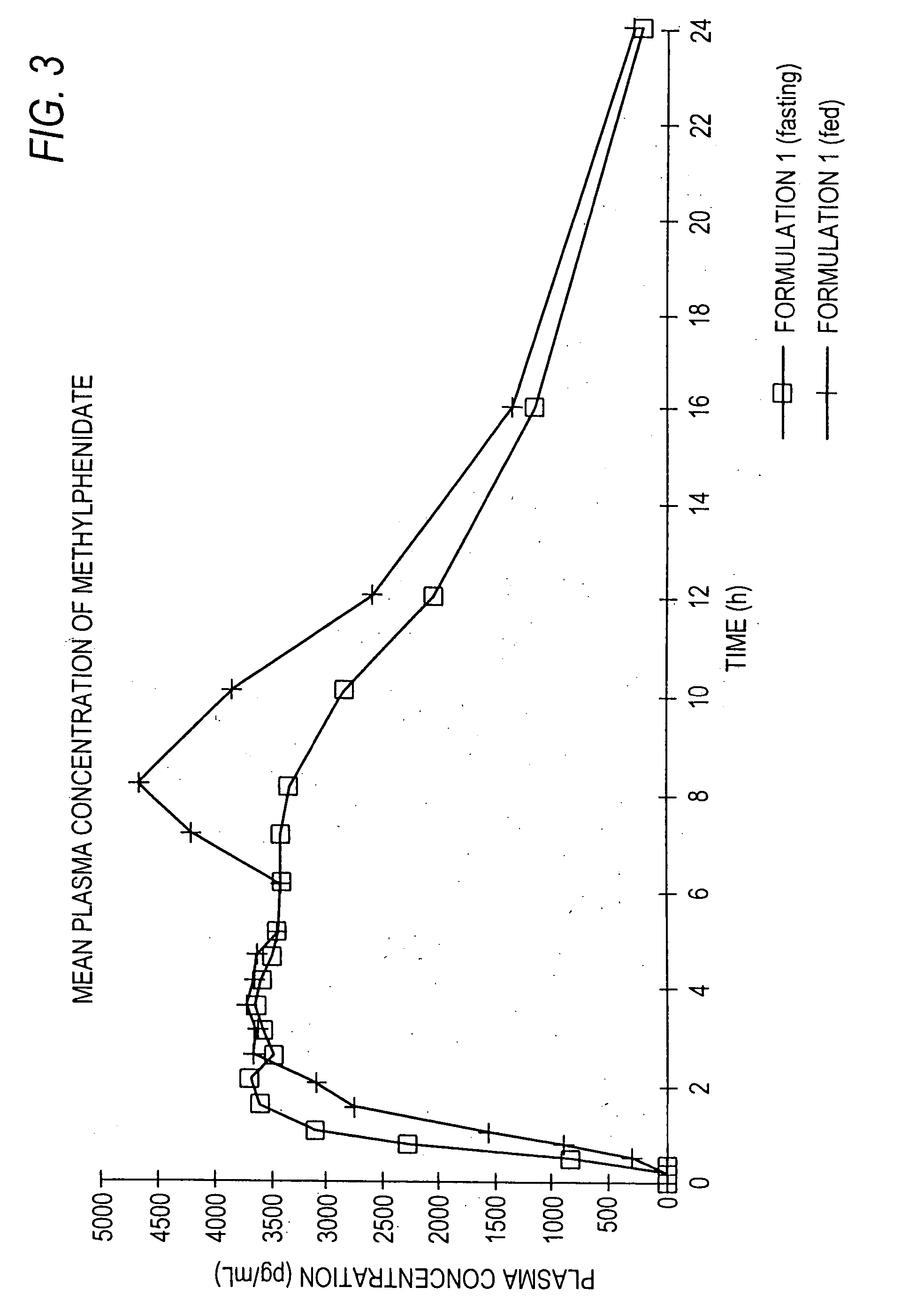
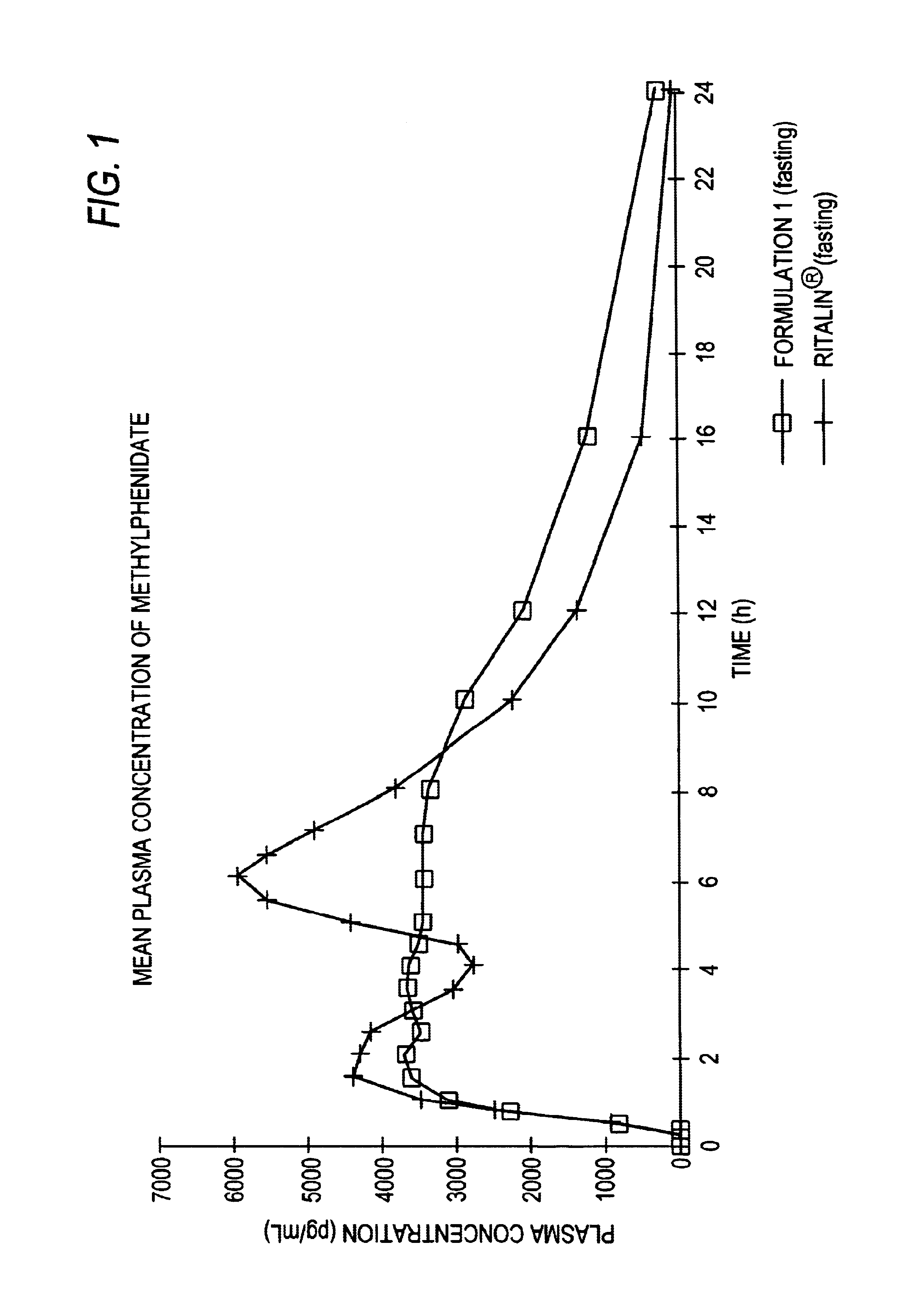
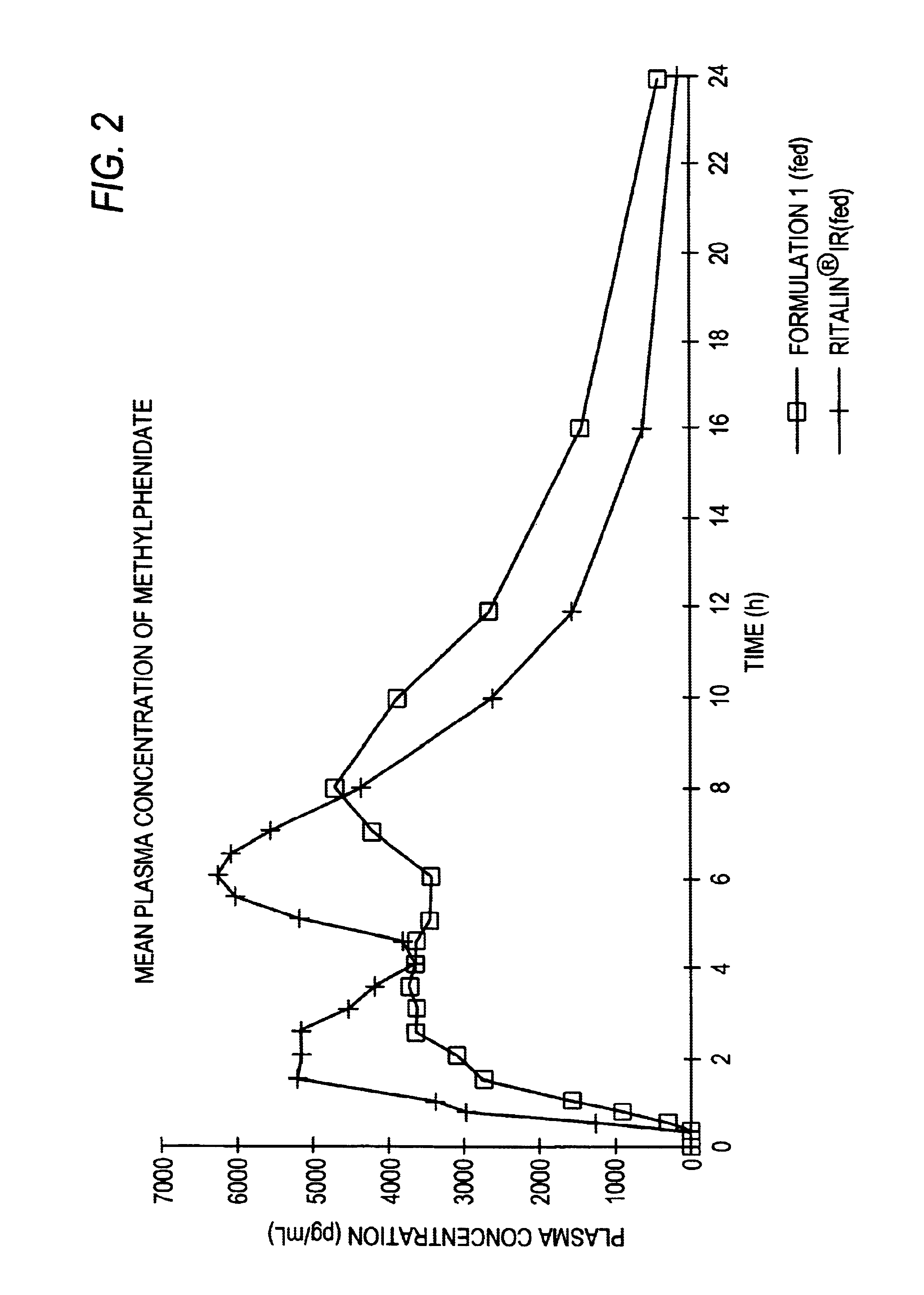
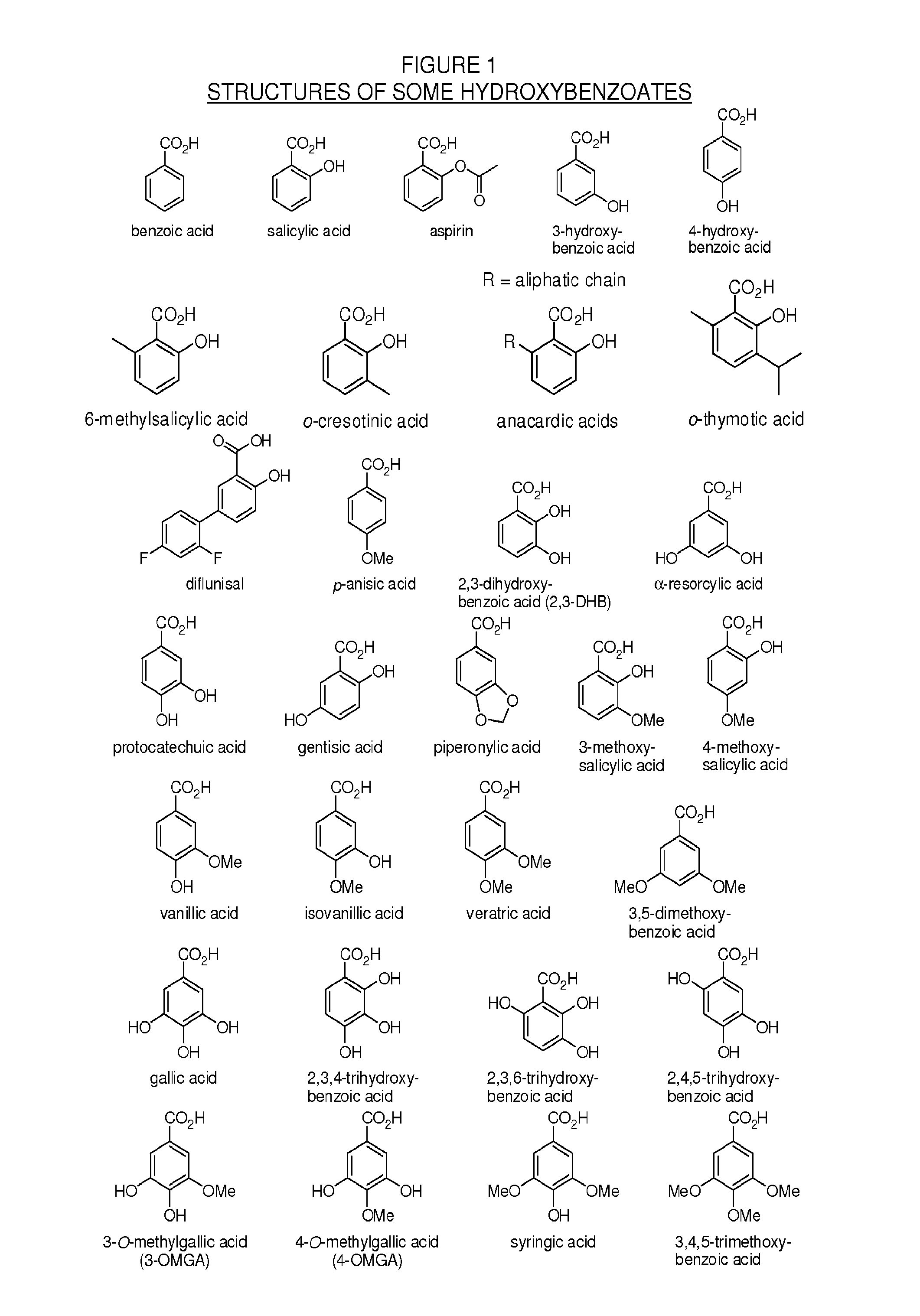
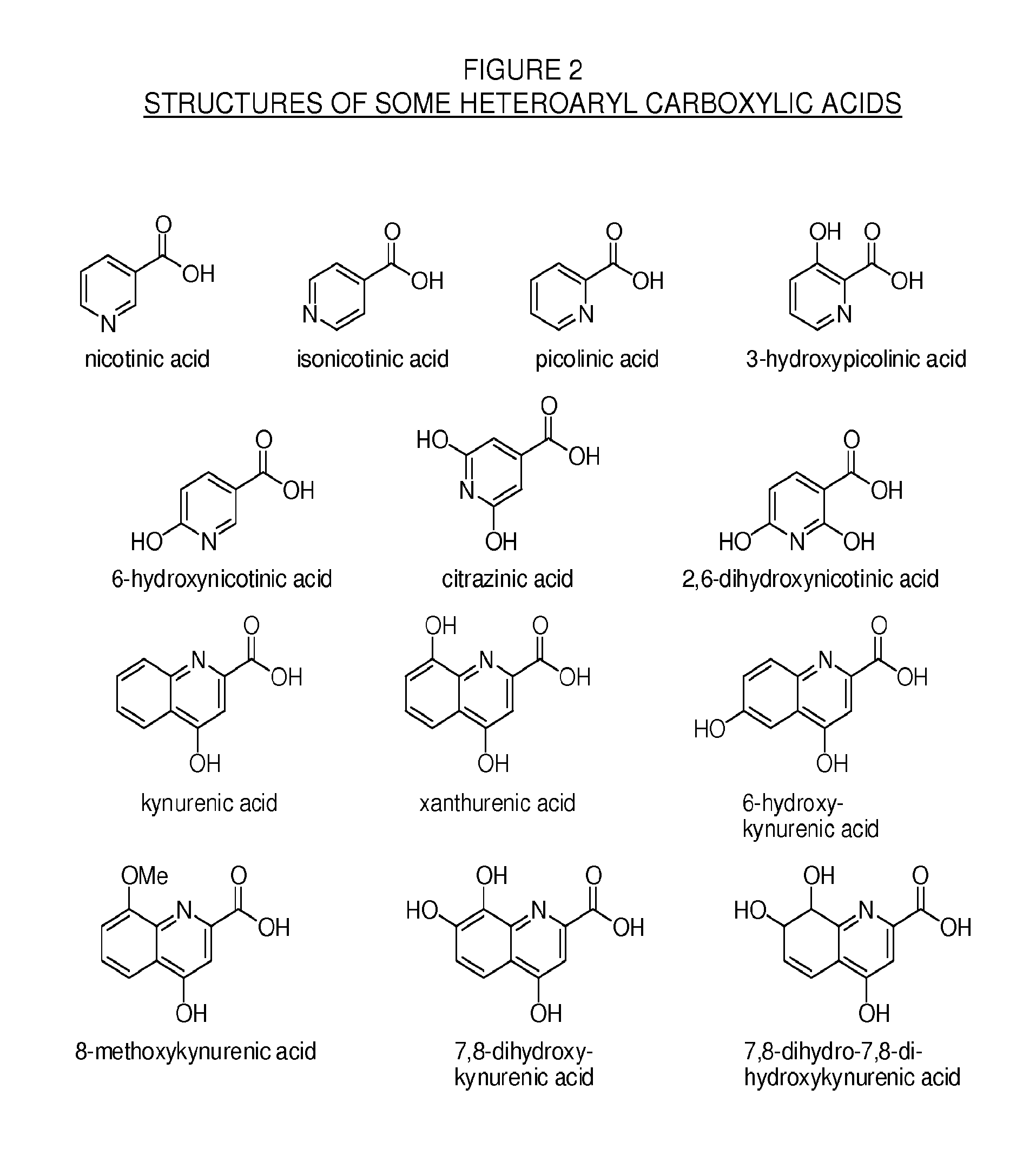
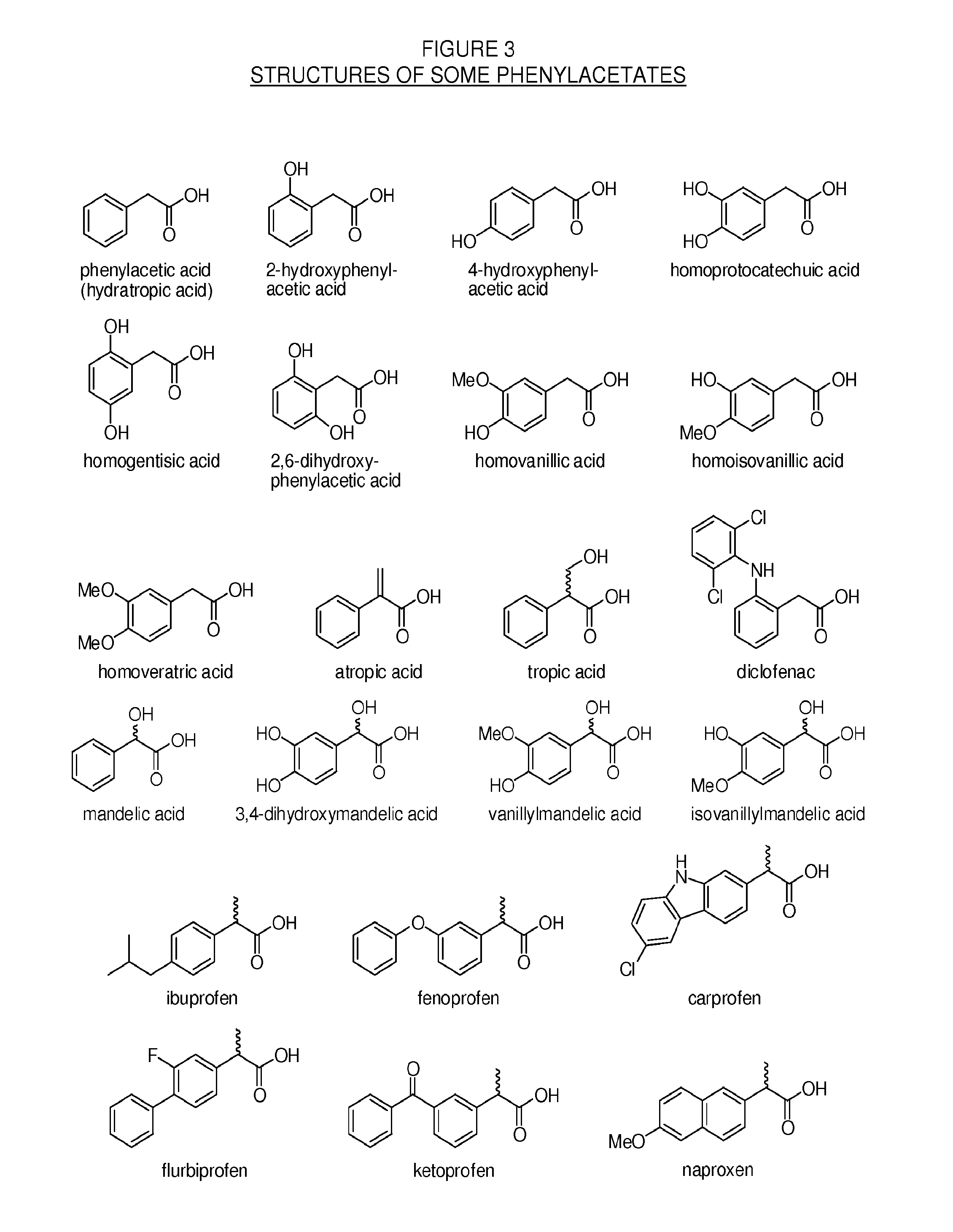
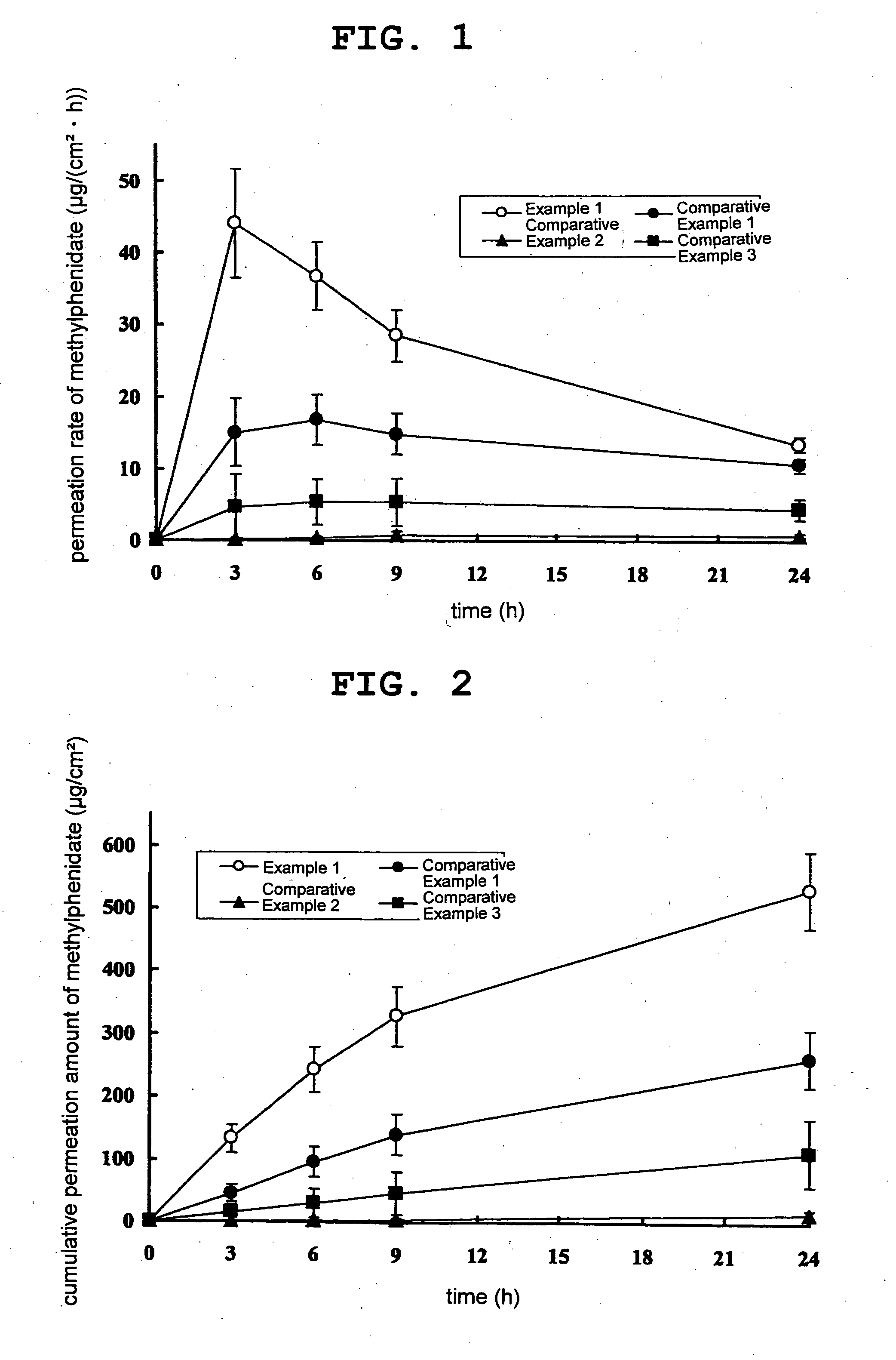
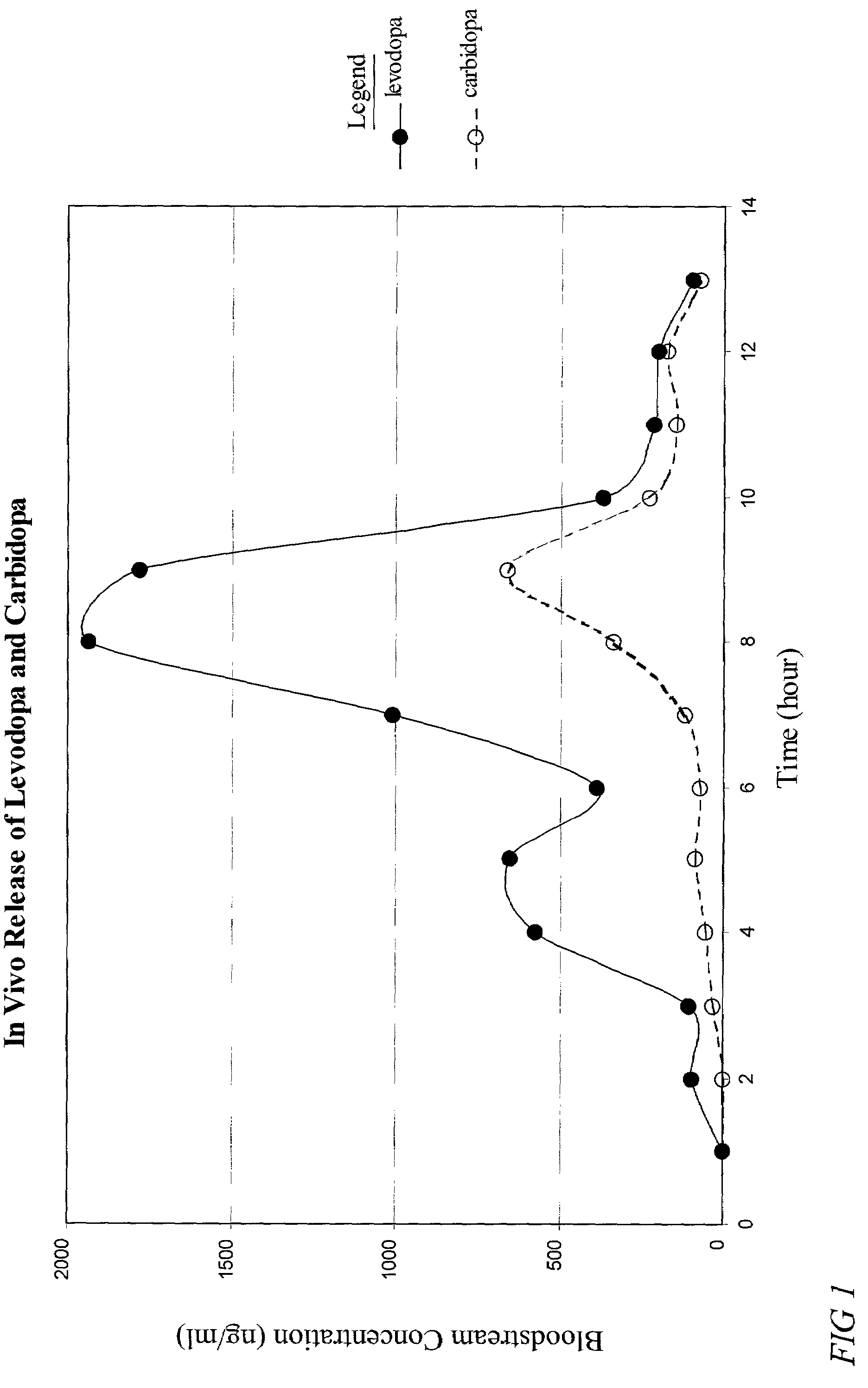
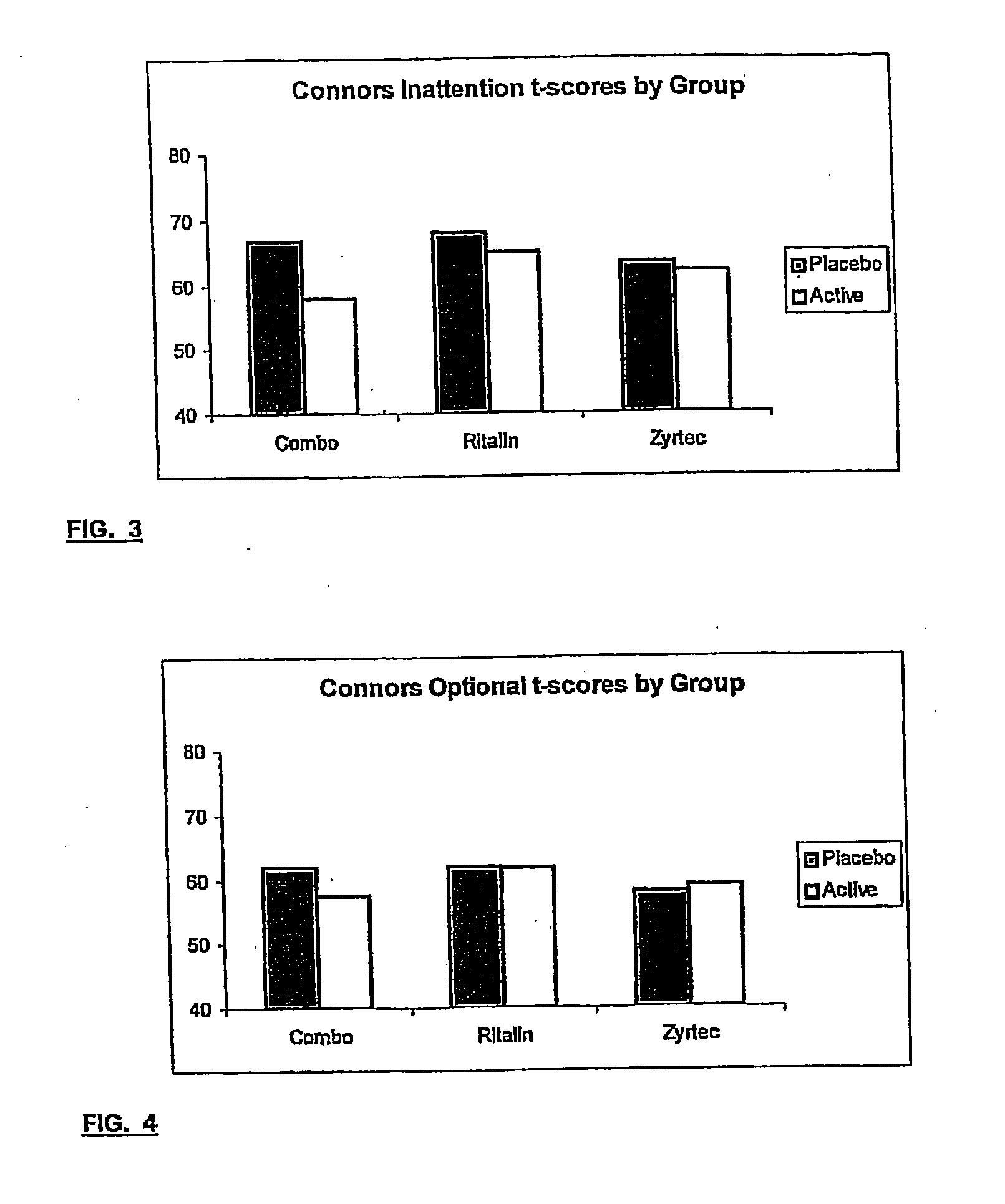
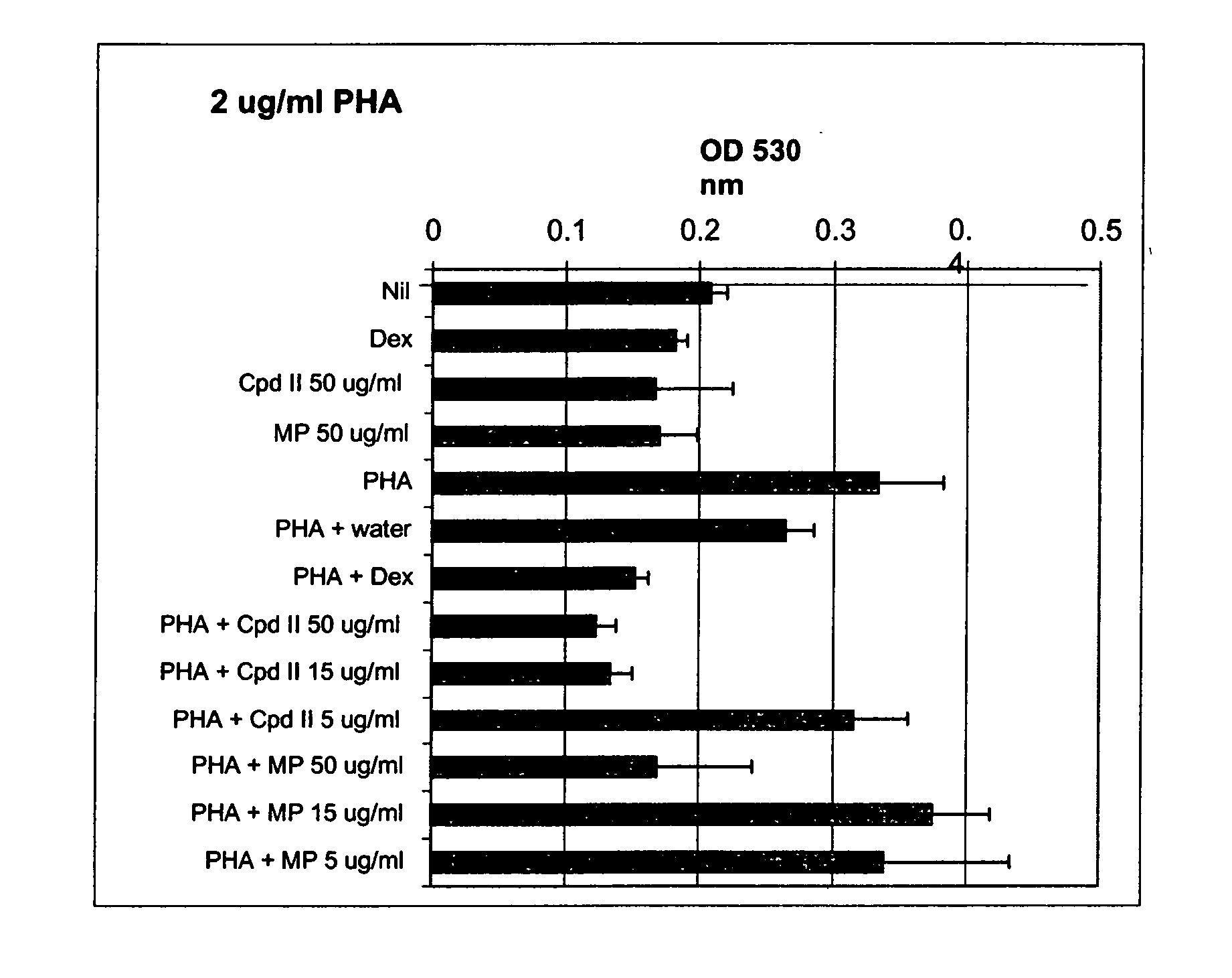
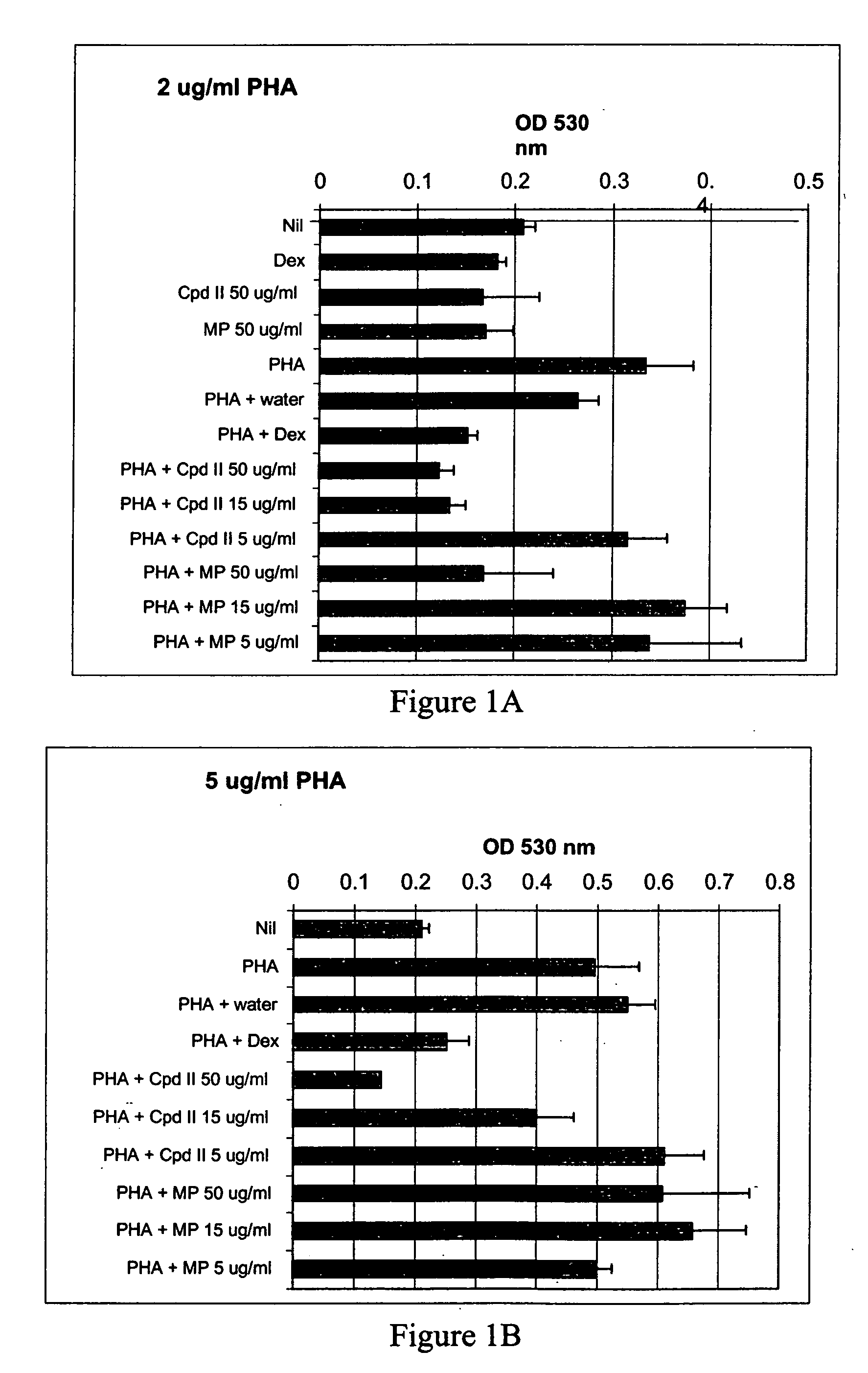
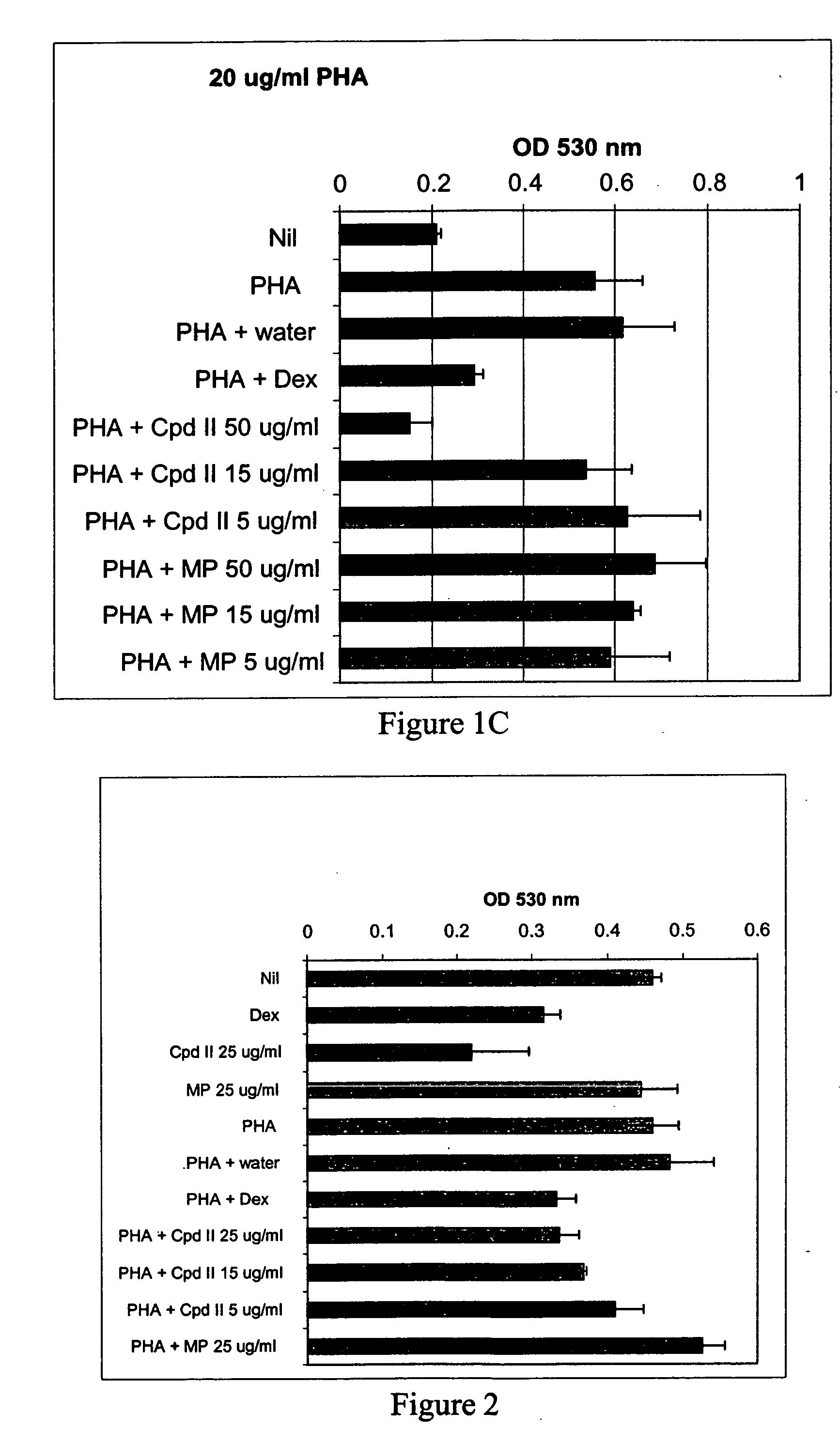
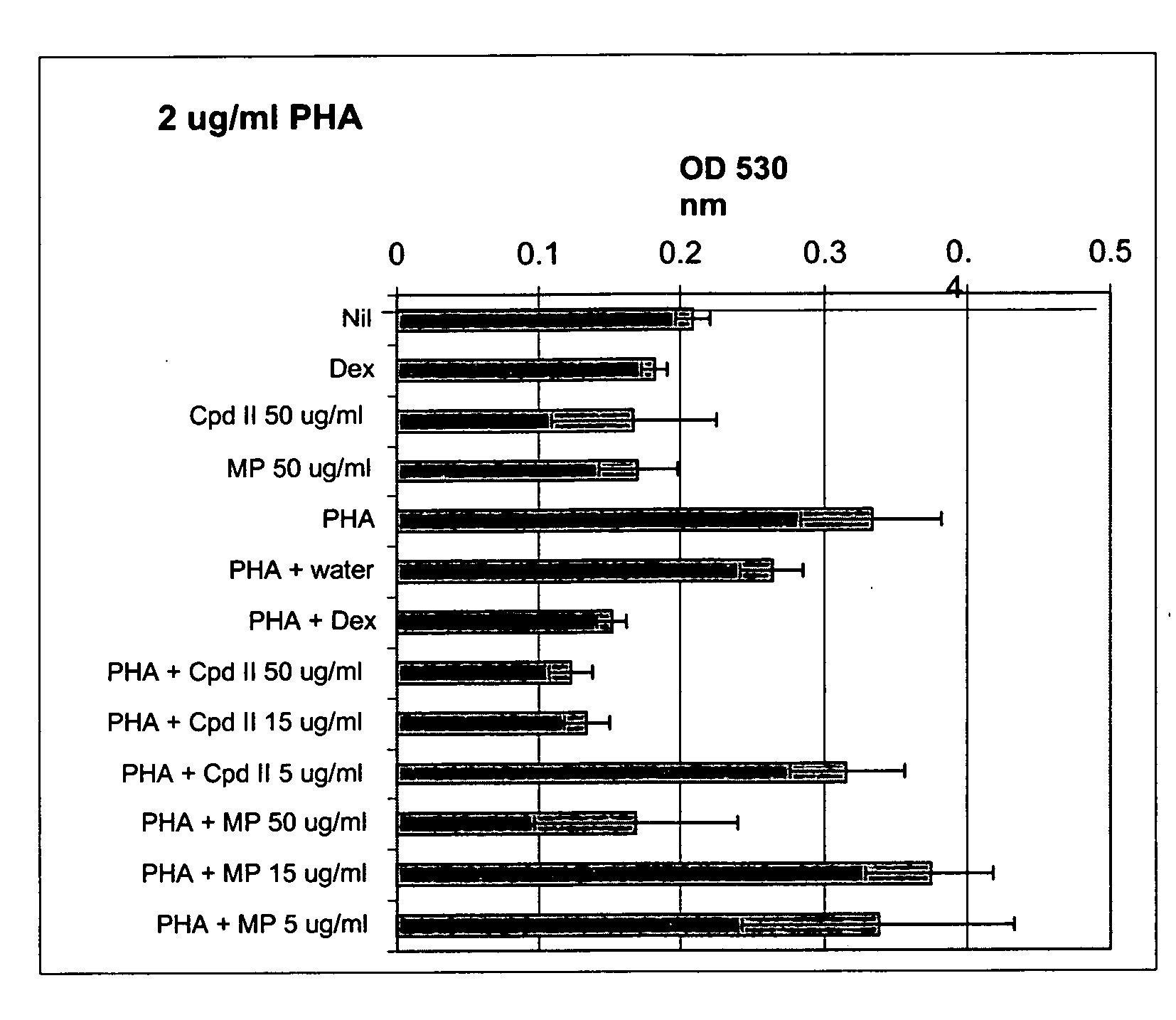
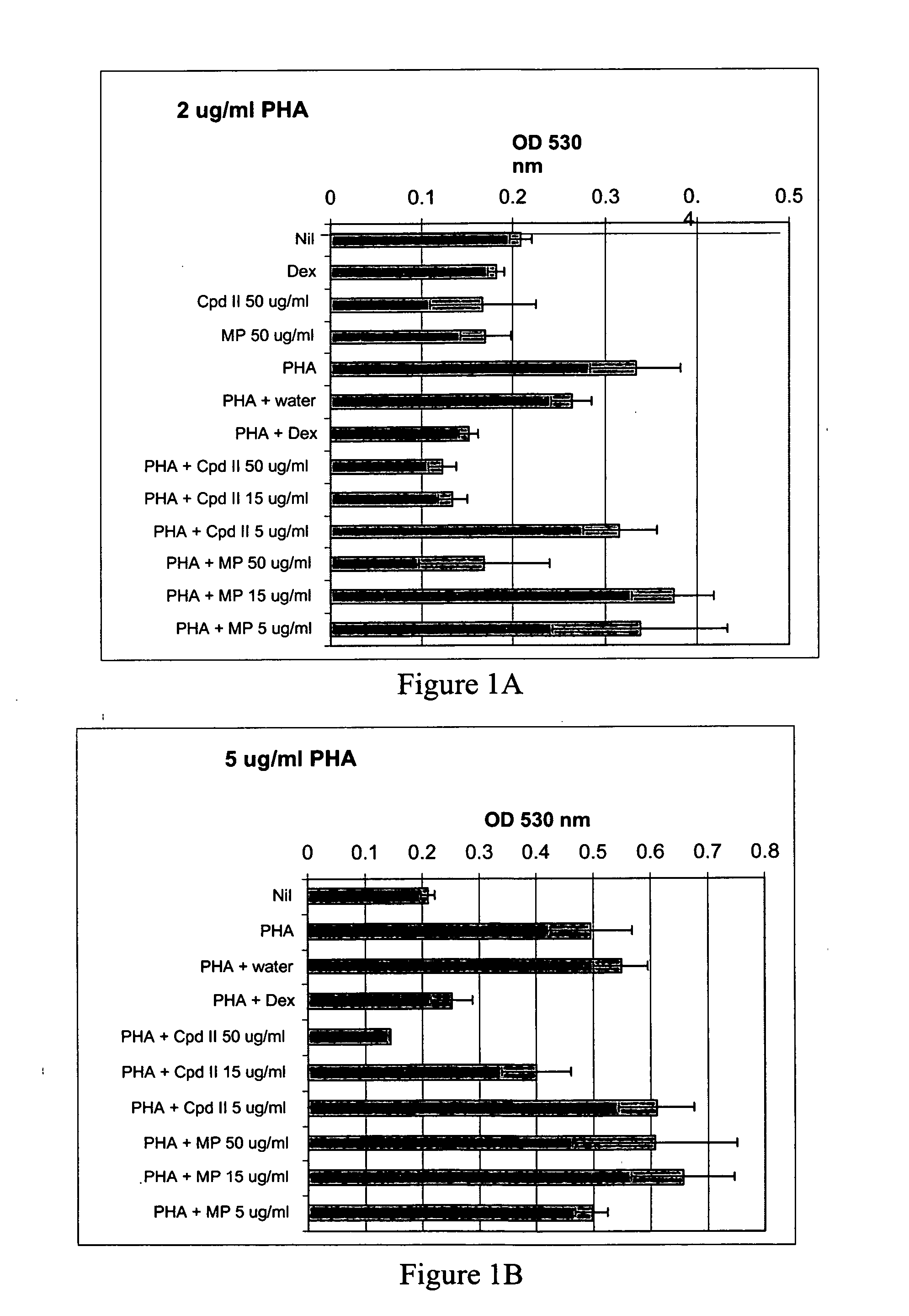
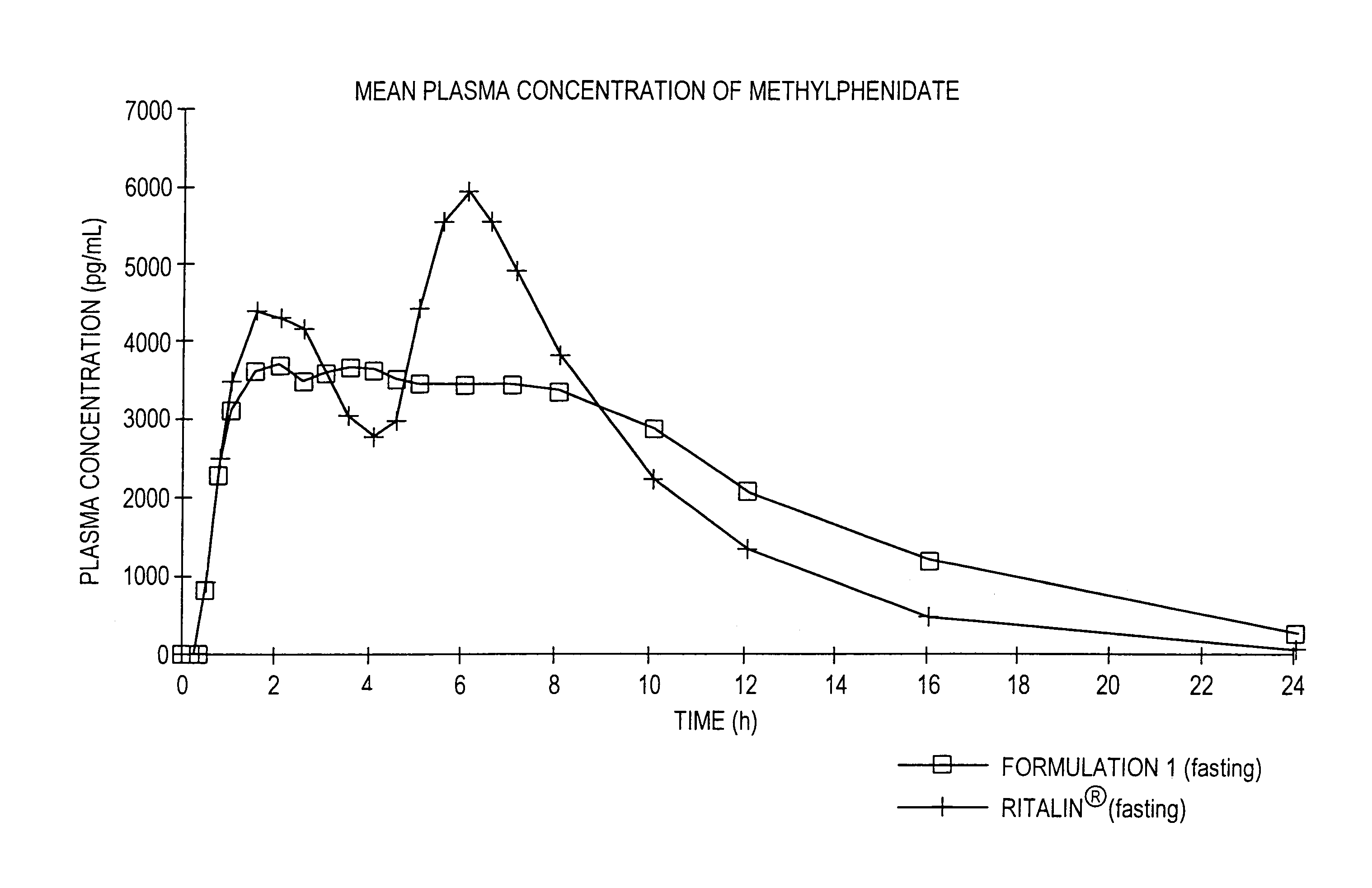Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
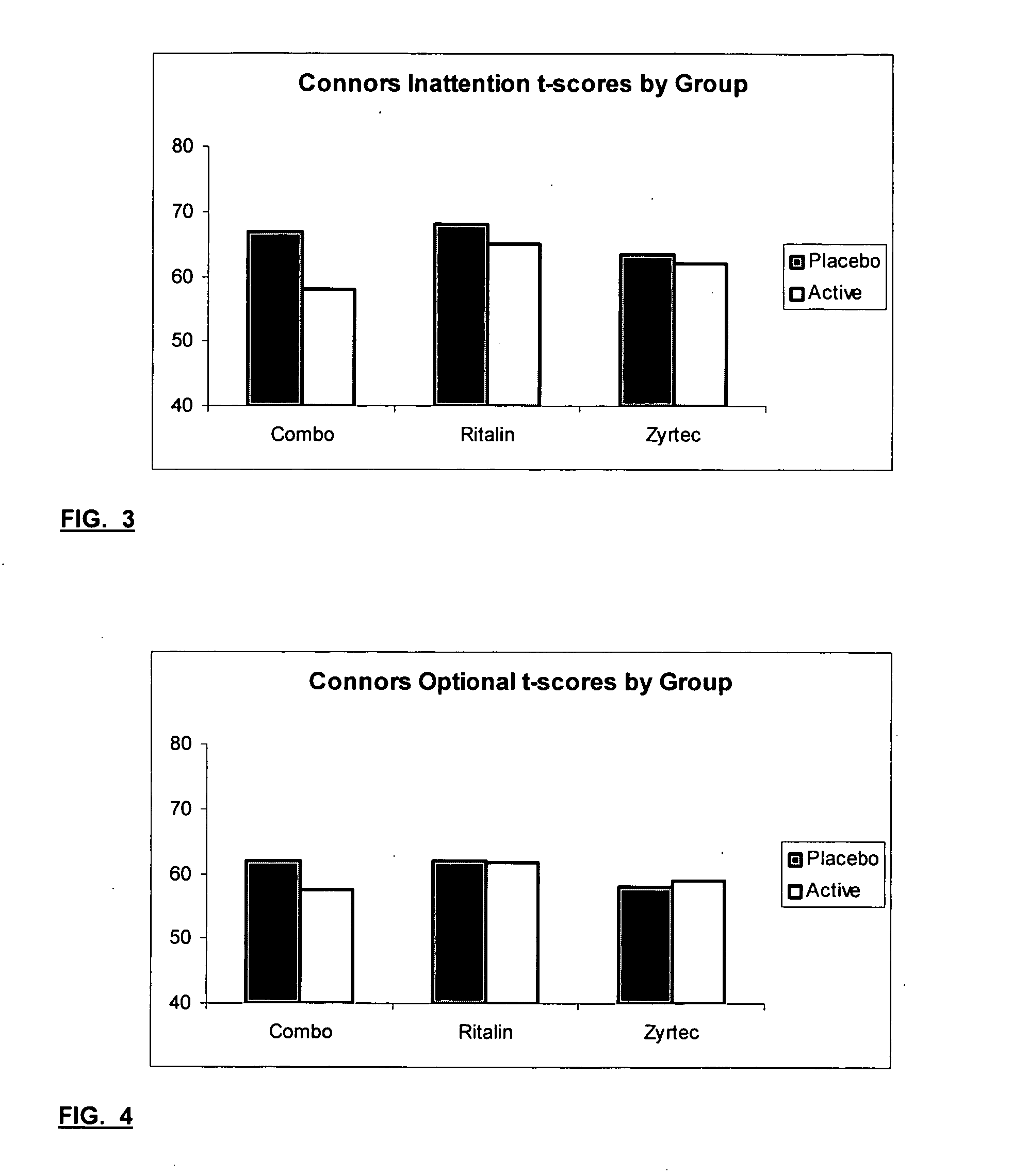
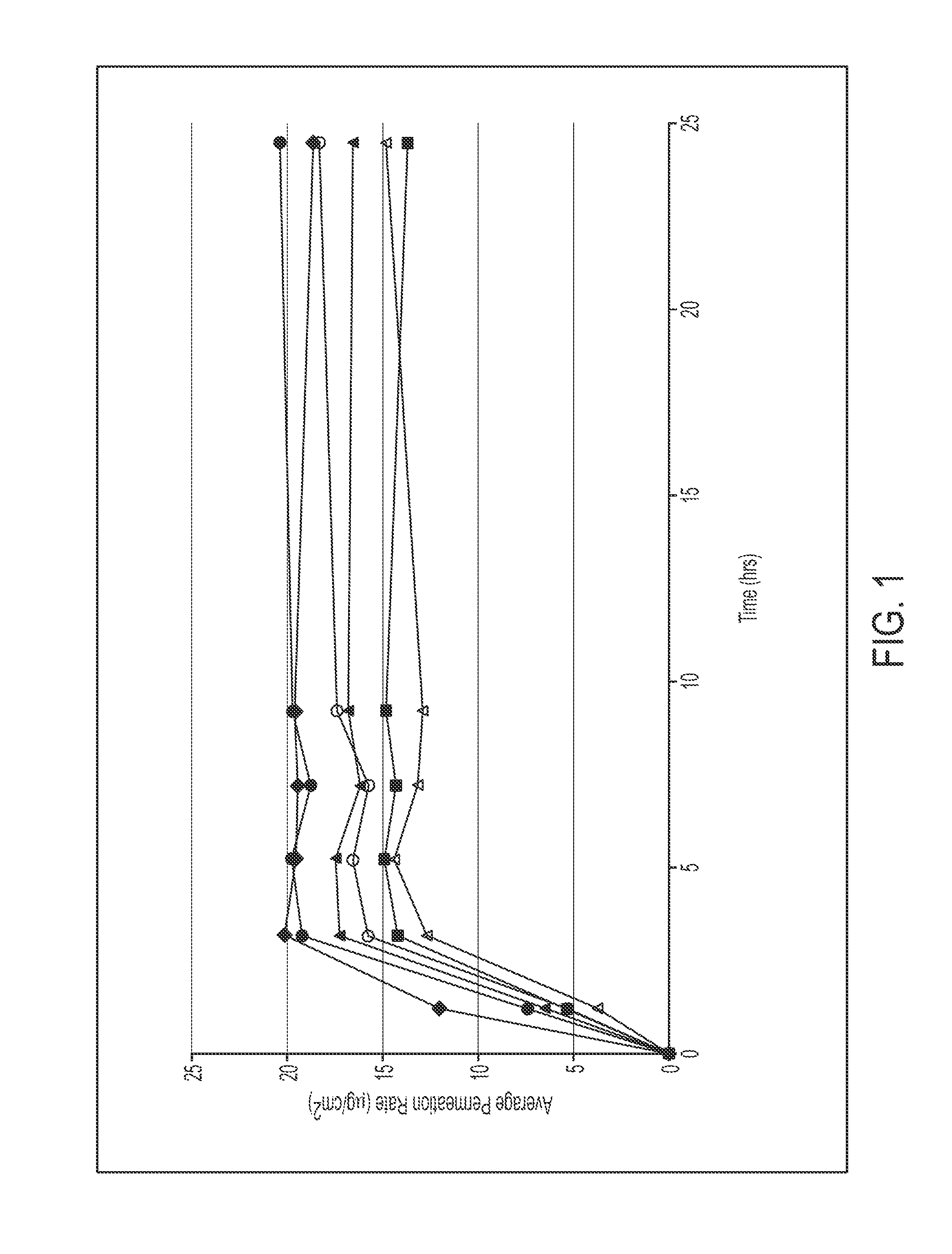
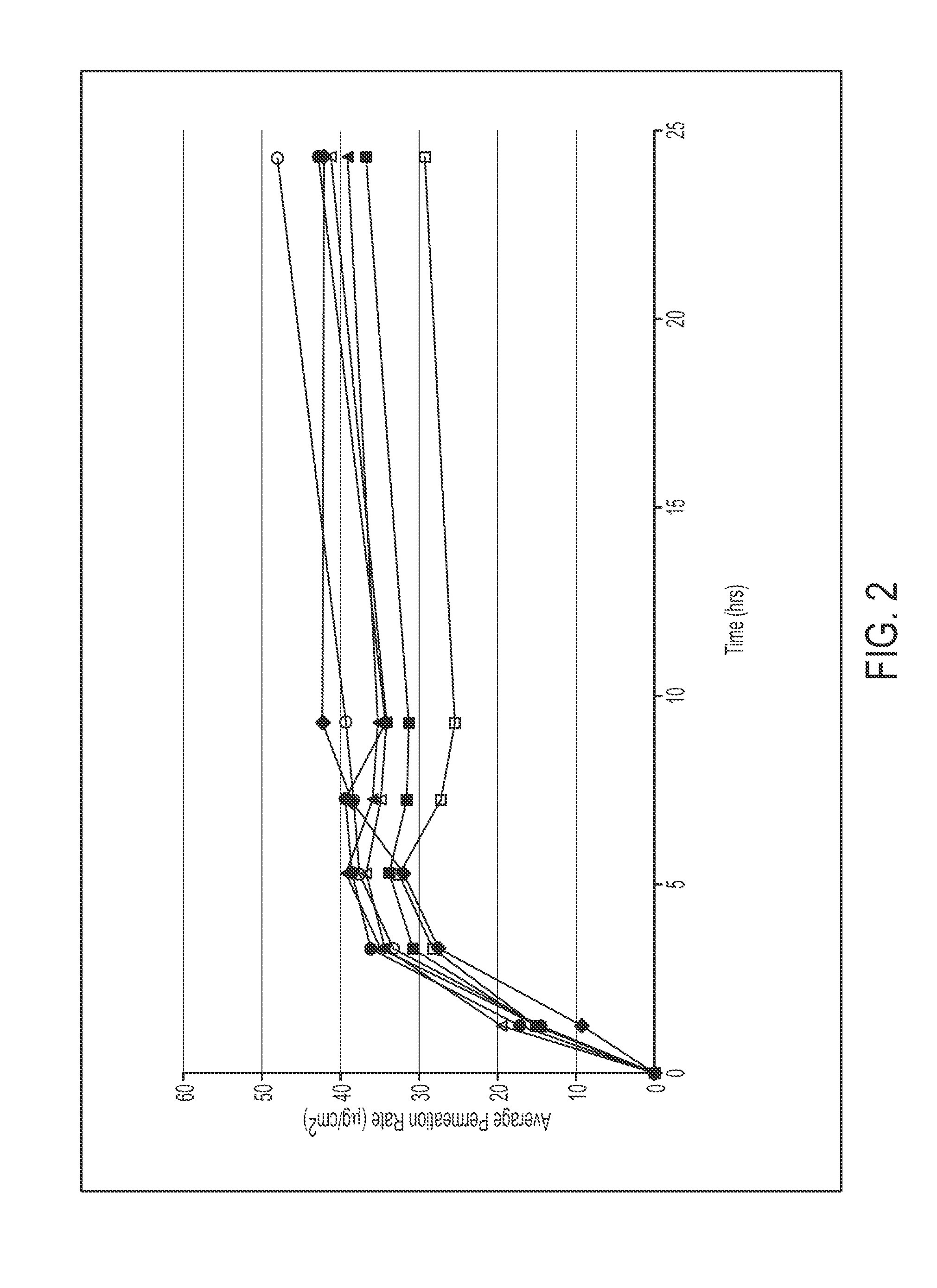
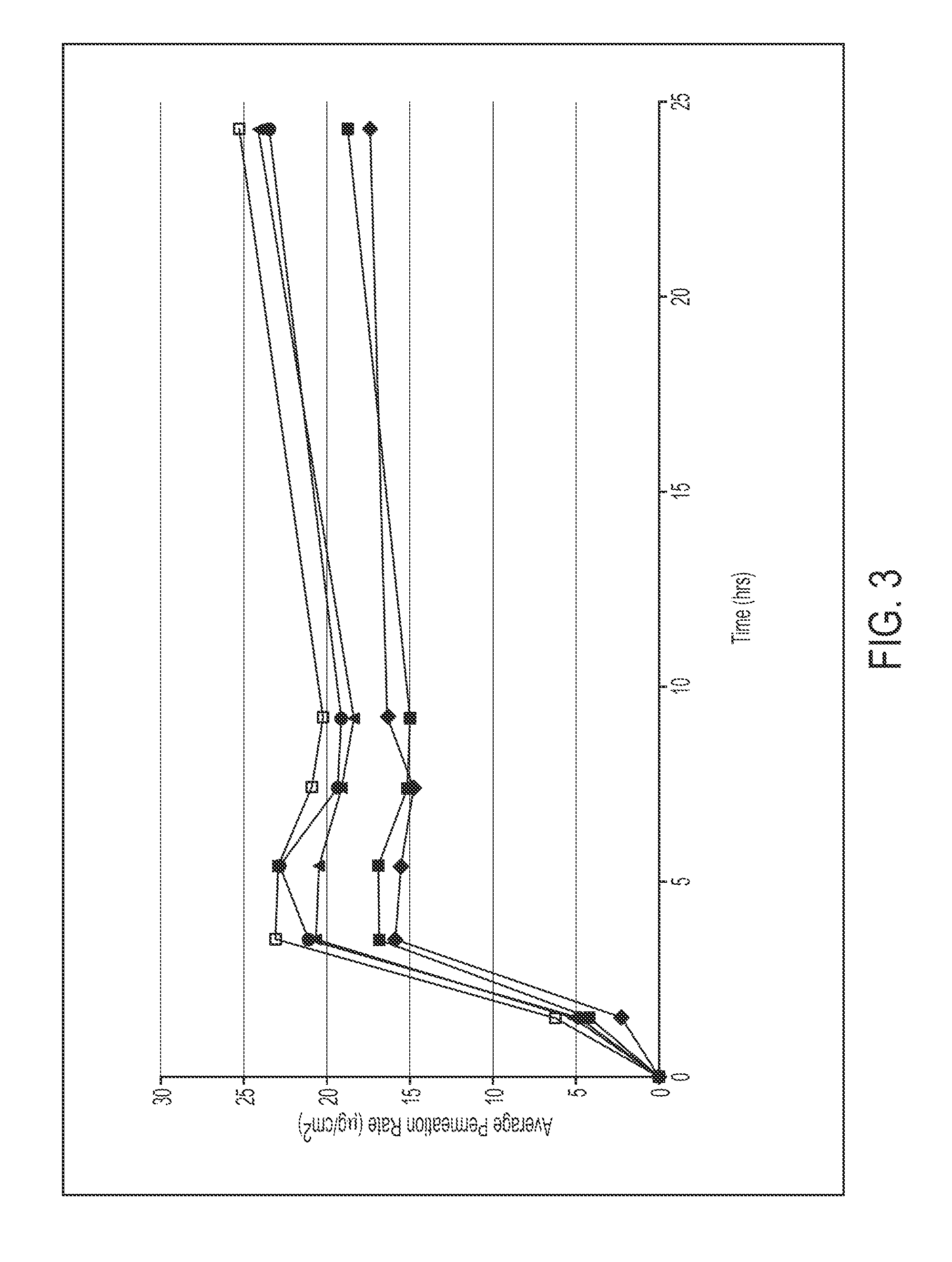
79 results about "Methylphenidate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
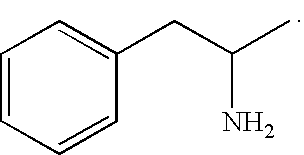
This medication is used to treat attention deficit hyperactivity disorder - ADHD.
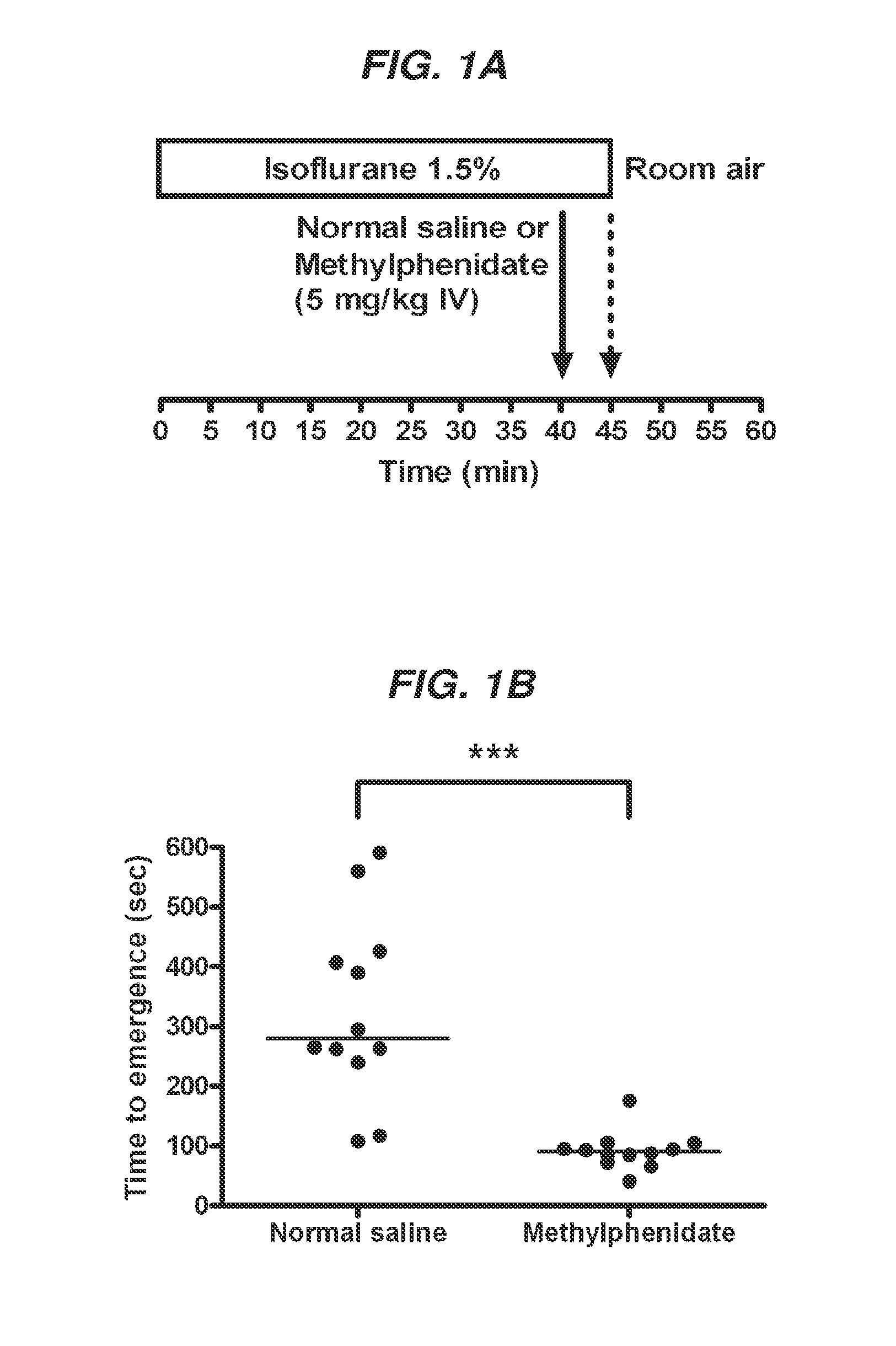
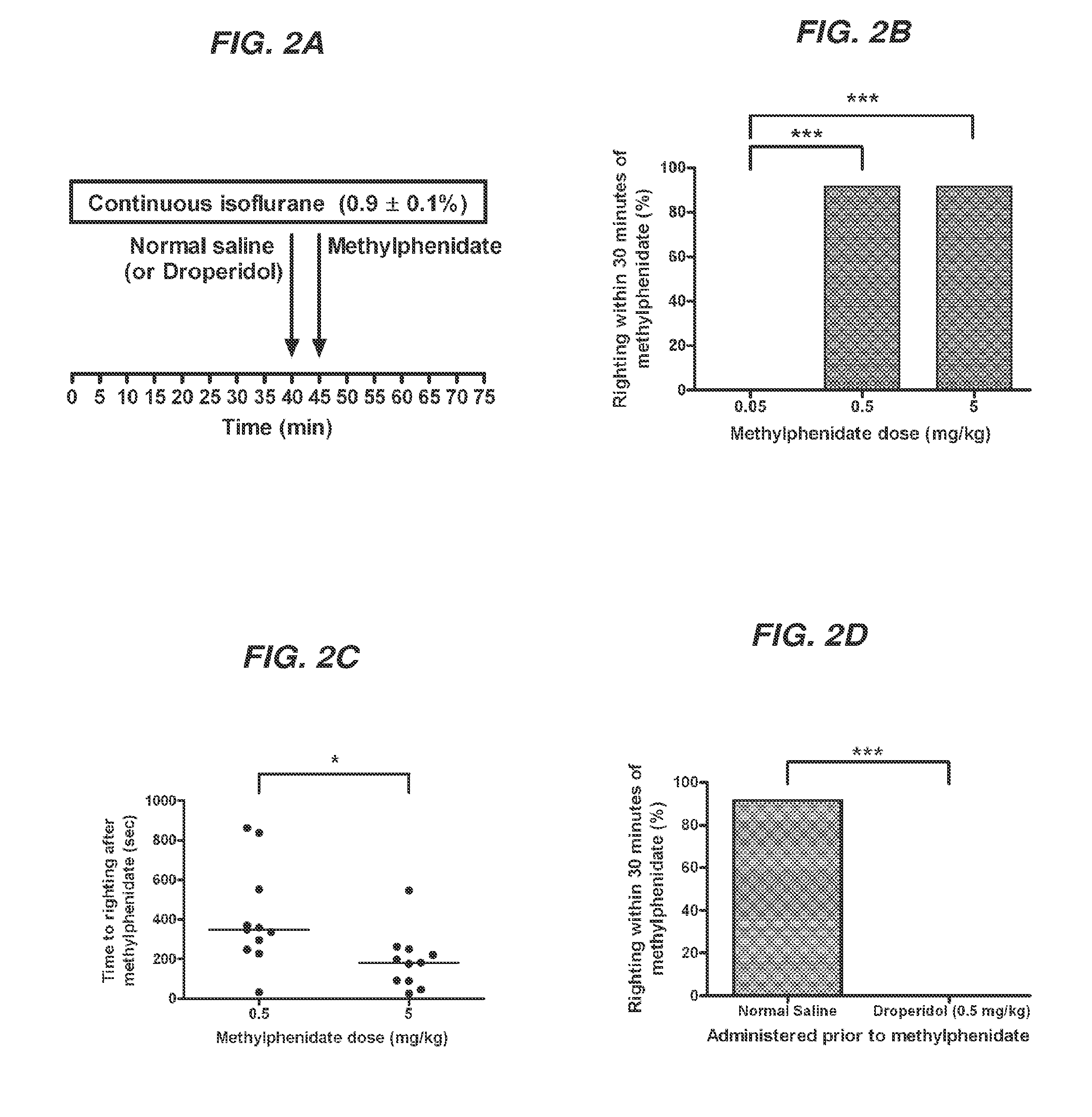
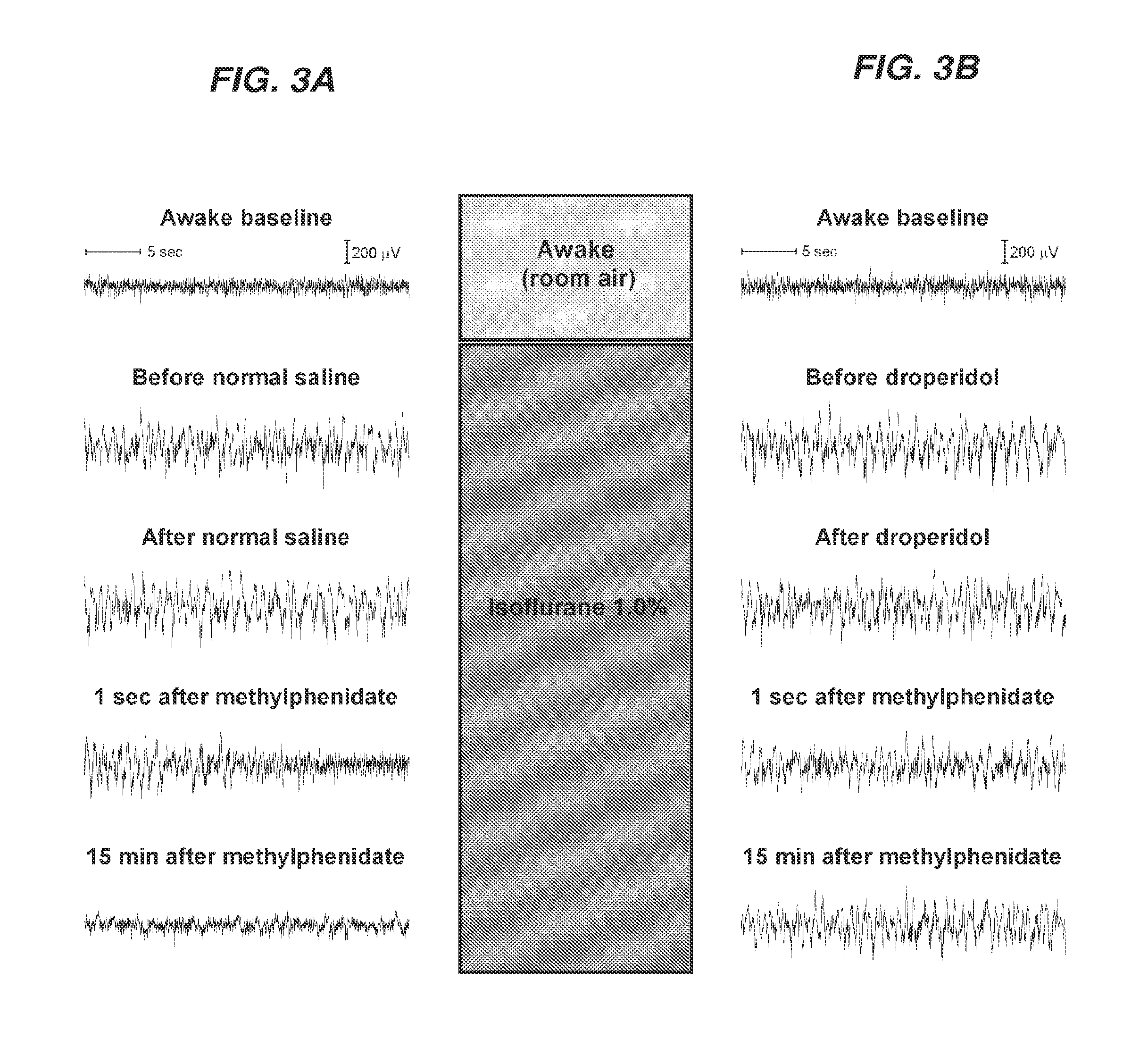
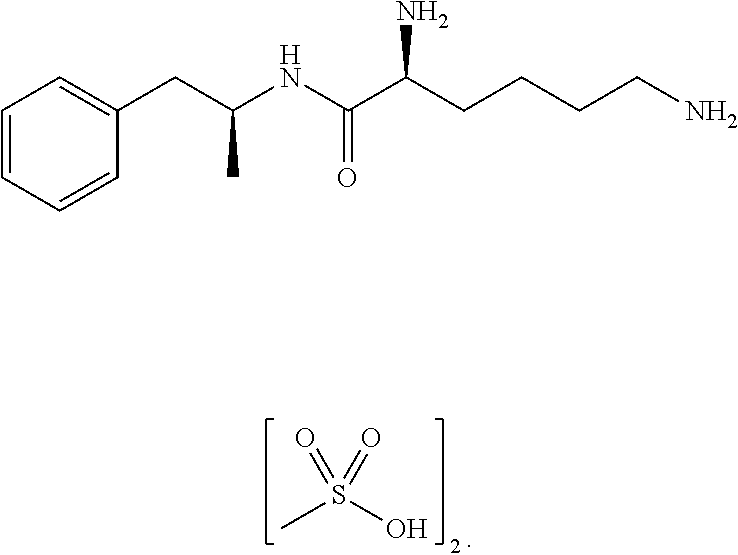
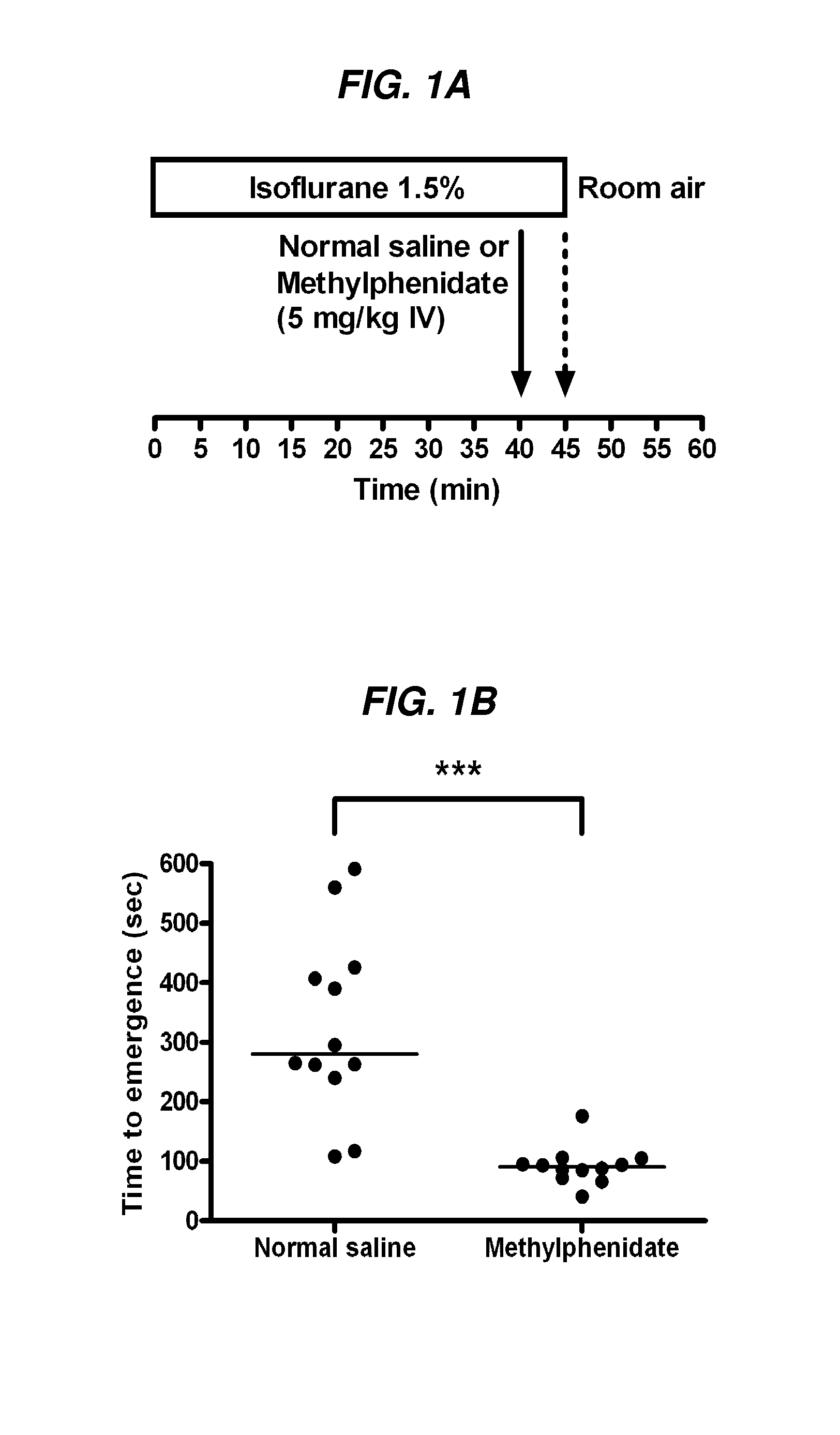
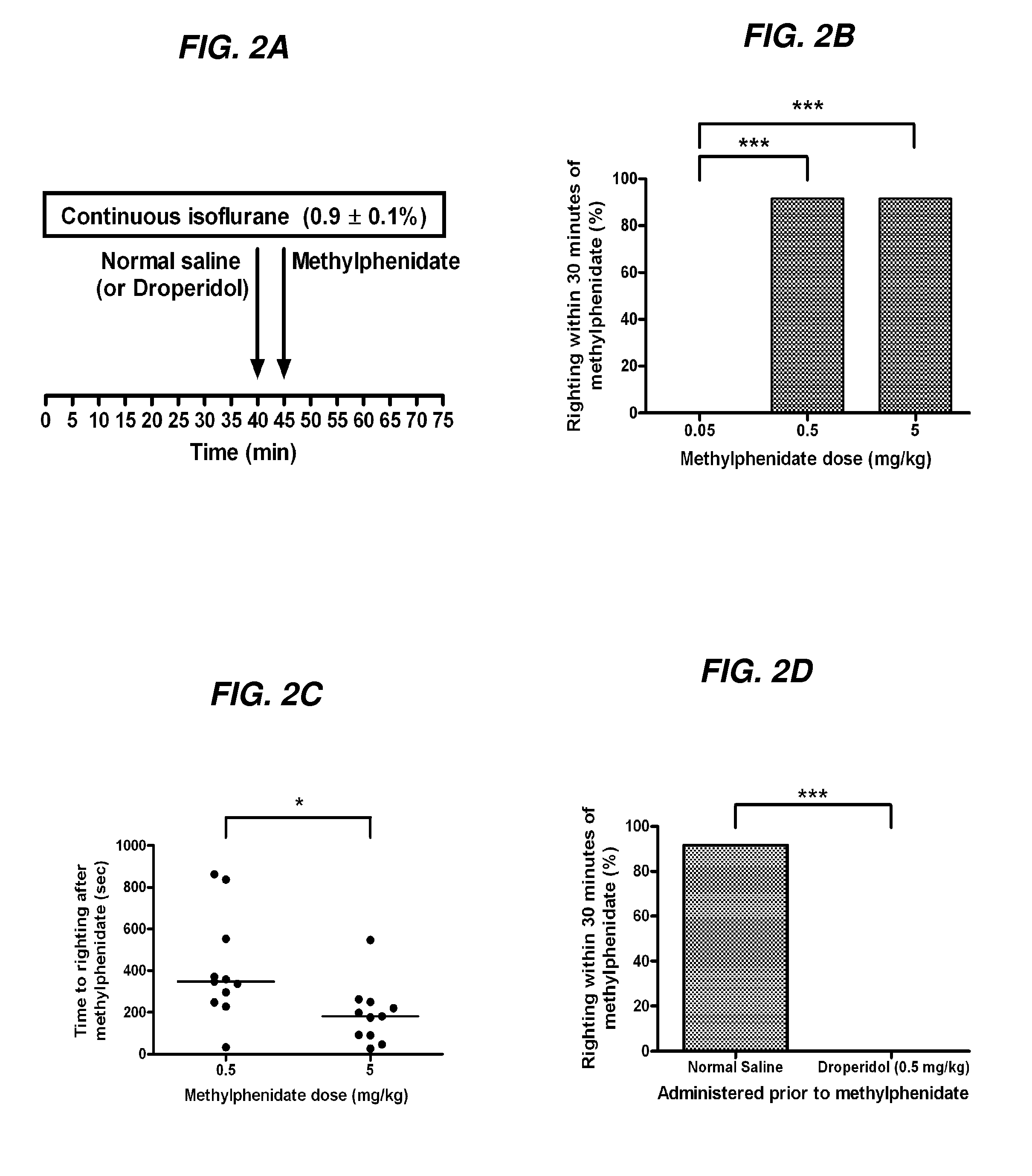
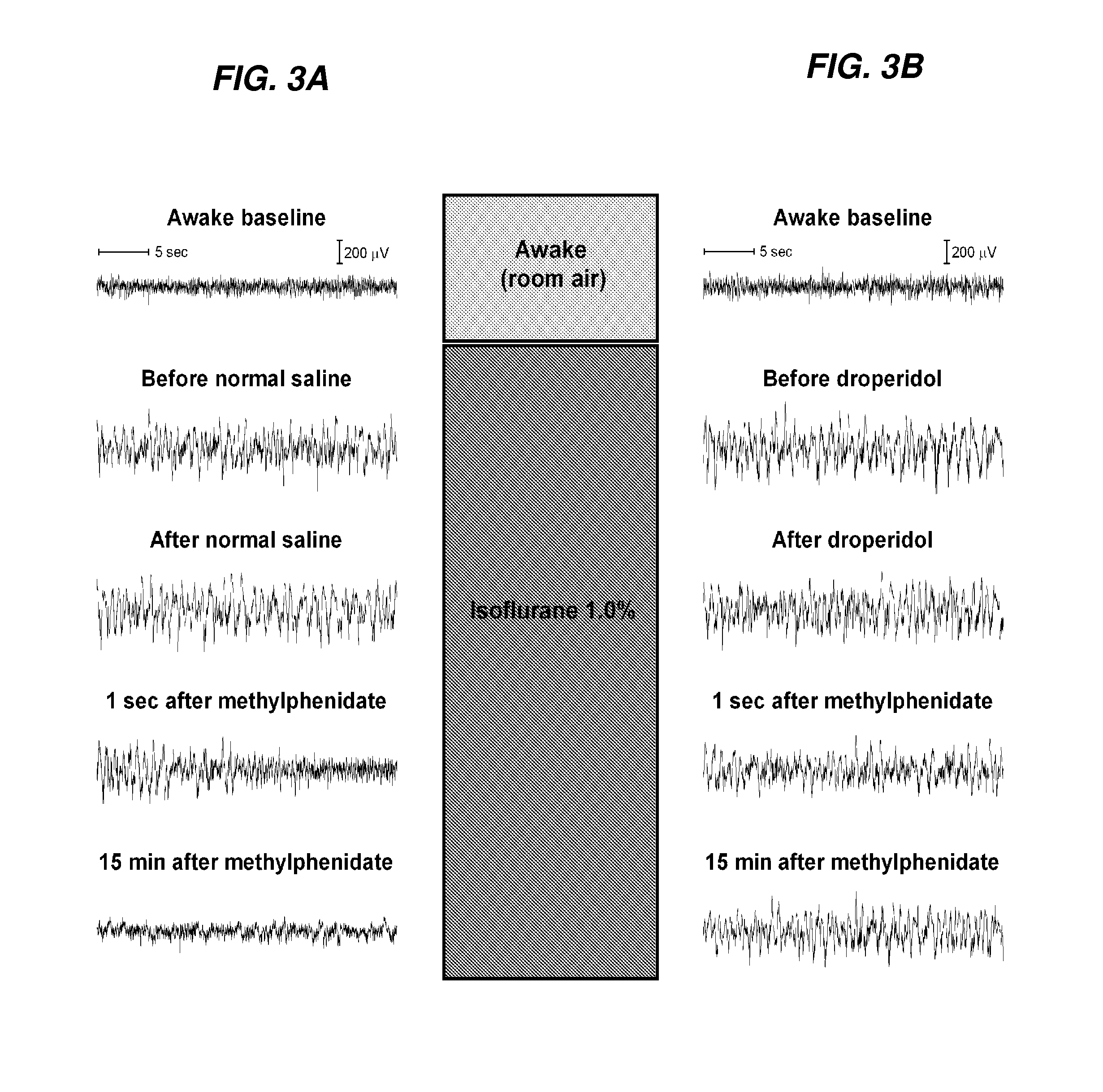
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
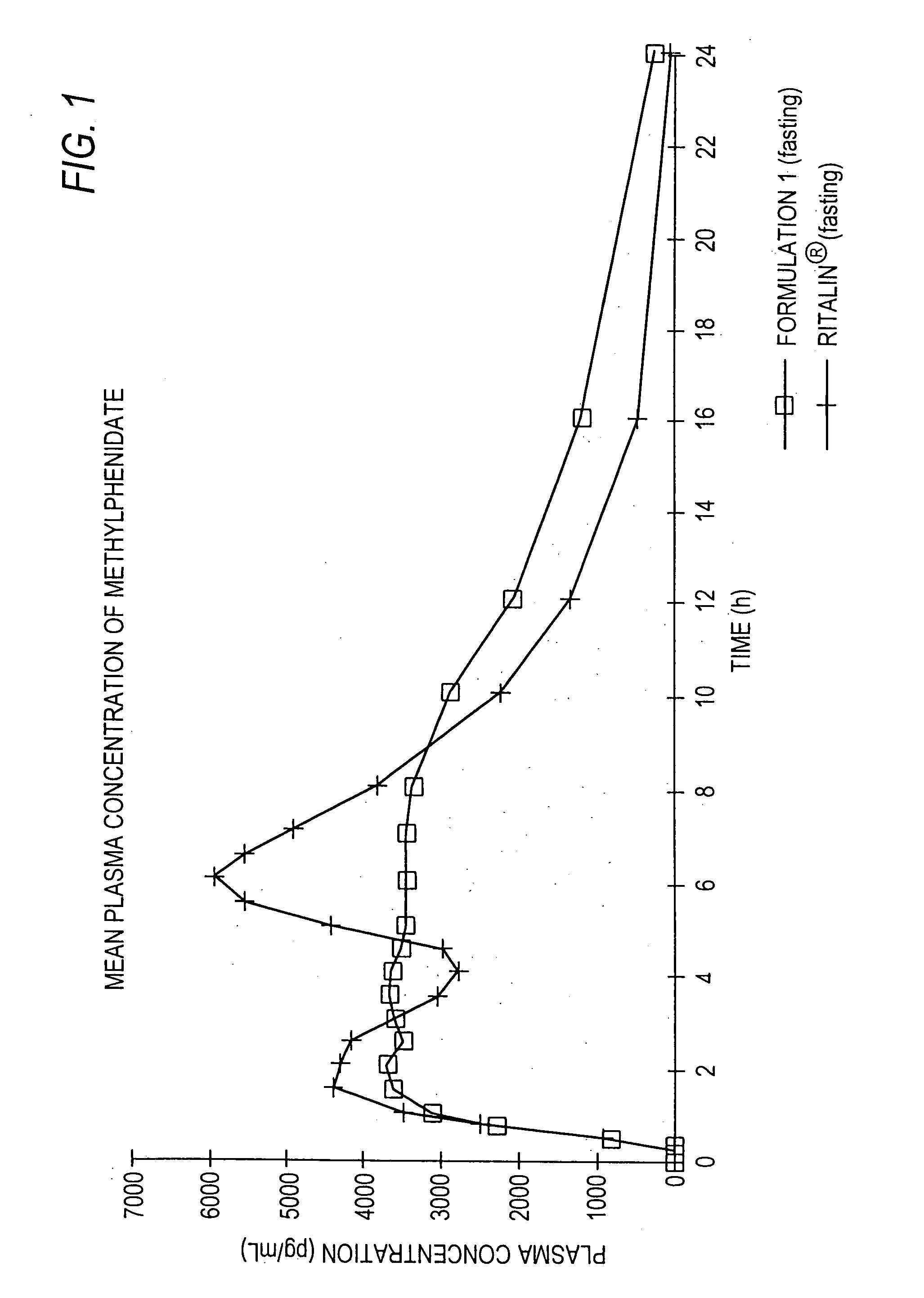
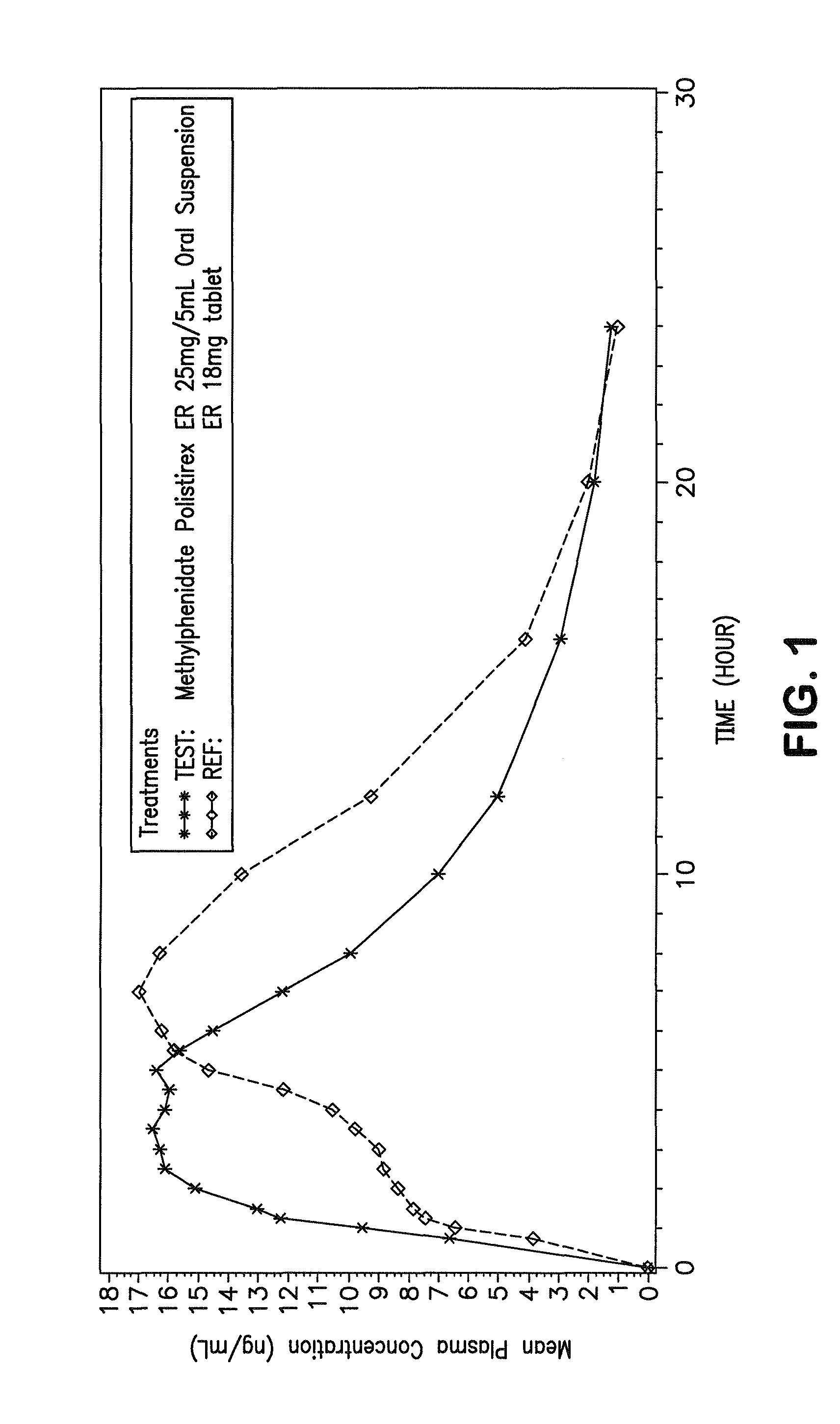
Controlled/modified release oral methylphenidate formulations
InactiveUS20040131680A1Patient compliance is goodPowder deliveryOrganic active ingredientsDuration of effectMethylphenidate
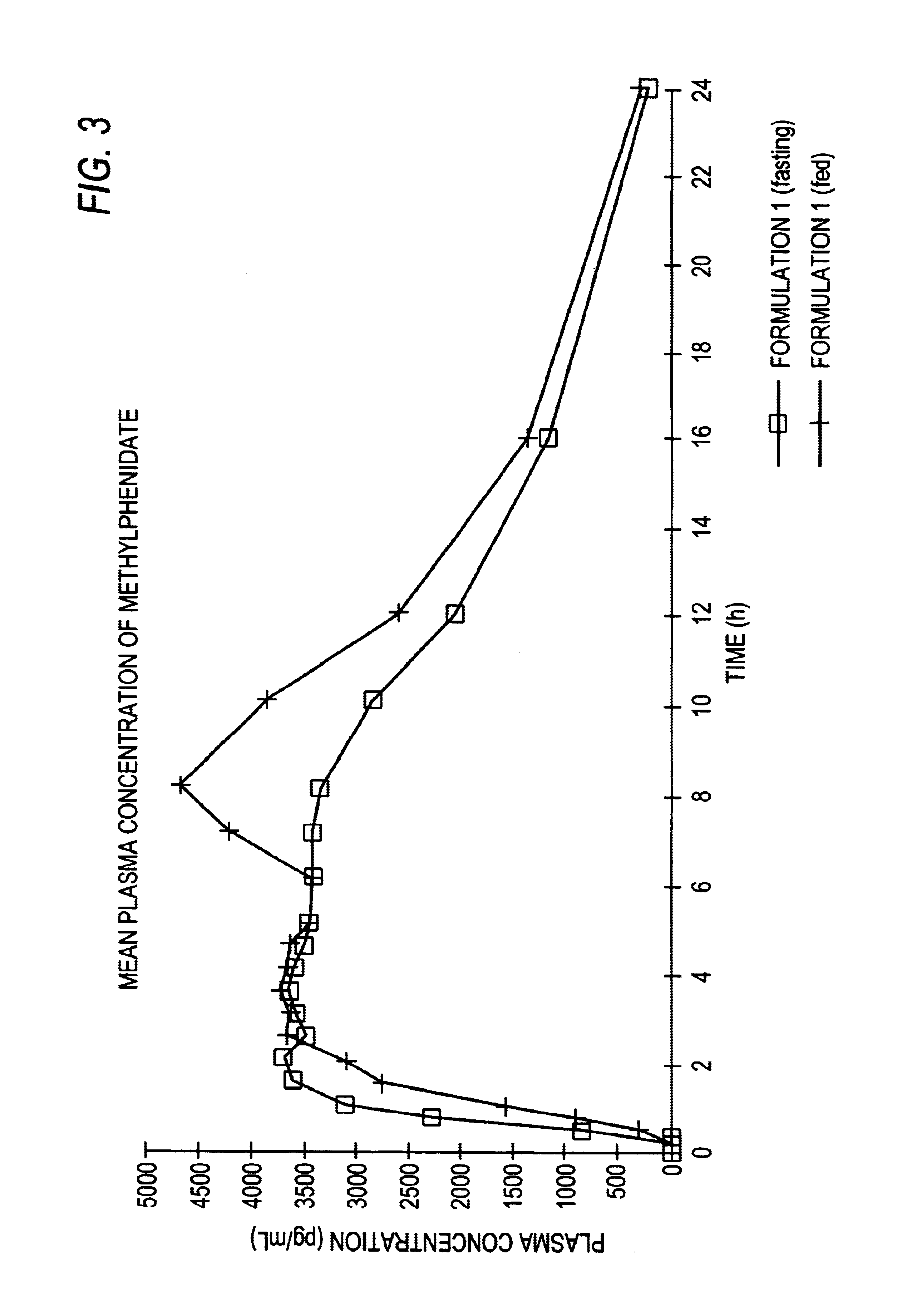
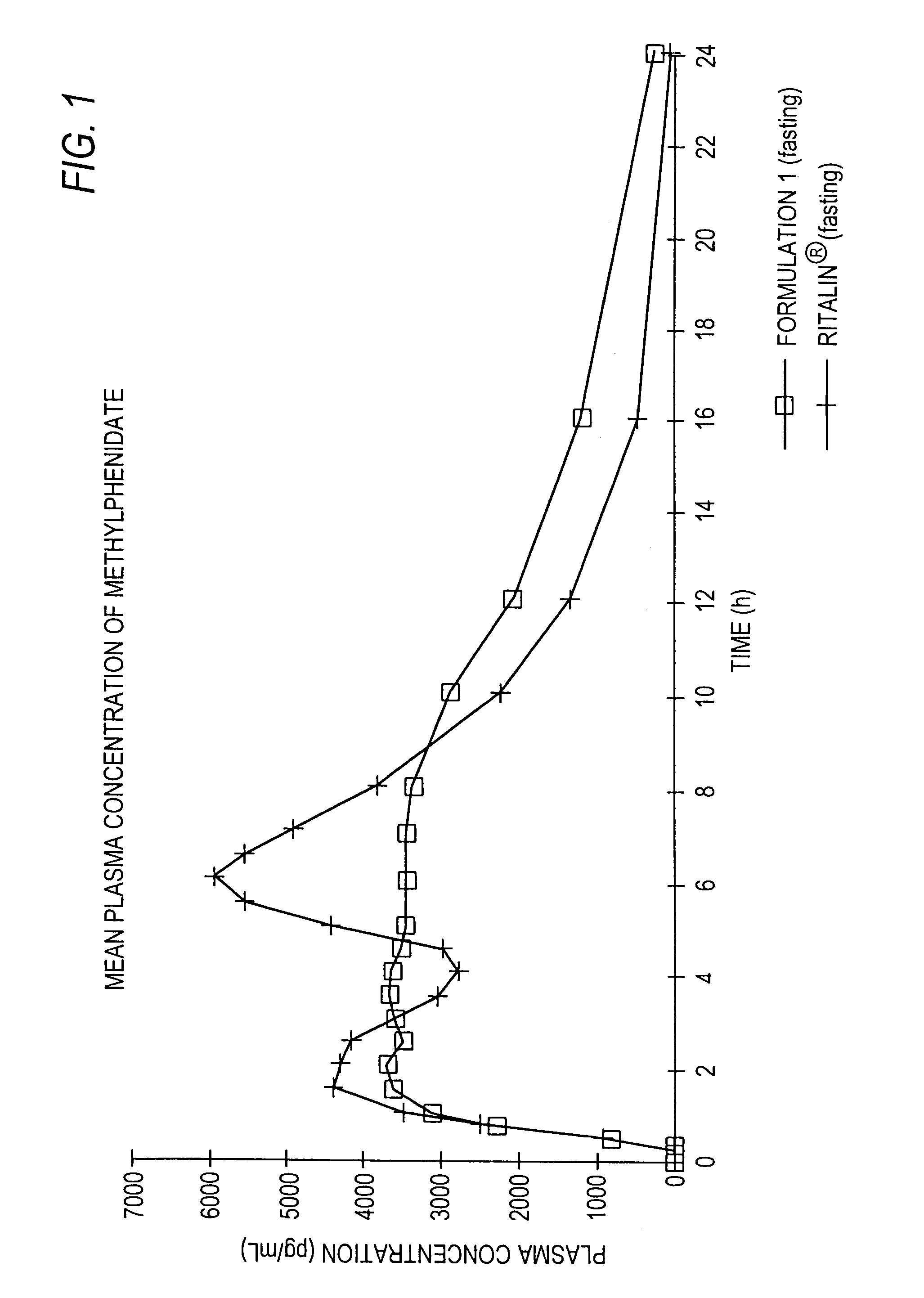
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Controlled/modified release oral methylphenidate formulations
InactiveUS6673367B1Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Oral pharmaceutical dosage forms
InactiveUS20100260844A1Enhanced delivery kineticsEasy constructionBiocidePowder deliveryControlled releaseMethylphenidate
Controlled release oral dosage forms suitable for administration of methylphenidate are provided. Abuse-resistant controlled release oral dosage forms suitable for administration of methylphenidate are also provided. Methods of treating ADD and ADHD using the oral dosage forms are also provided.
Owner:DURECT CORP
Controlled/modified release oral methylphenidate formulations
InactiveUS7083808B2Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Method of treating depressive disorders
The invention provides methods of treating depressive disorders, in particular major depression but other depressive orders also, with prodrug stimulants or analogs including amphetamine prodrugs, methylphenidate prodrugs, and methylphenidate analogs, Such methods of treatment may utilize the prodrug stimulant or analog as monotherapy or, more commonly, as an adjunct to antidepressant medication treatment to augment their effect. The invention includes combination methods of treatment in which an amphetamine prodrug, methylphenidate prodrug, or methylphenidate analog is administered to an individual in need with one or more other active agents, either in separate forms or as a single pharmaceutical formulation. Packaged pharmaceutical compositions containing an amphetamine or methylphenidate prodrug, instructions for using the prodrug to treat certain disorders, and optionally one or more other active agents are provided by the invention.
Owner:LUCERNE BIOSCI
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS20040234608A1Improved gastric retentionHigh retention ratePowder deliveryOrganic active ingredientsGastric fluidAttention deficits
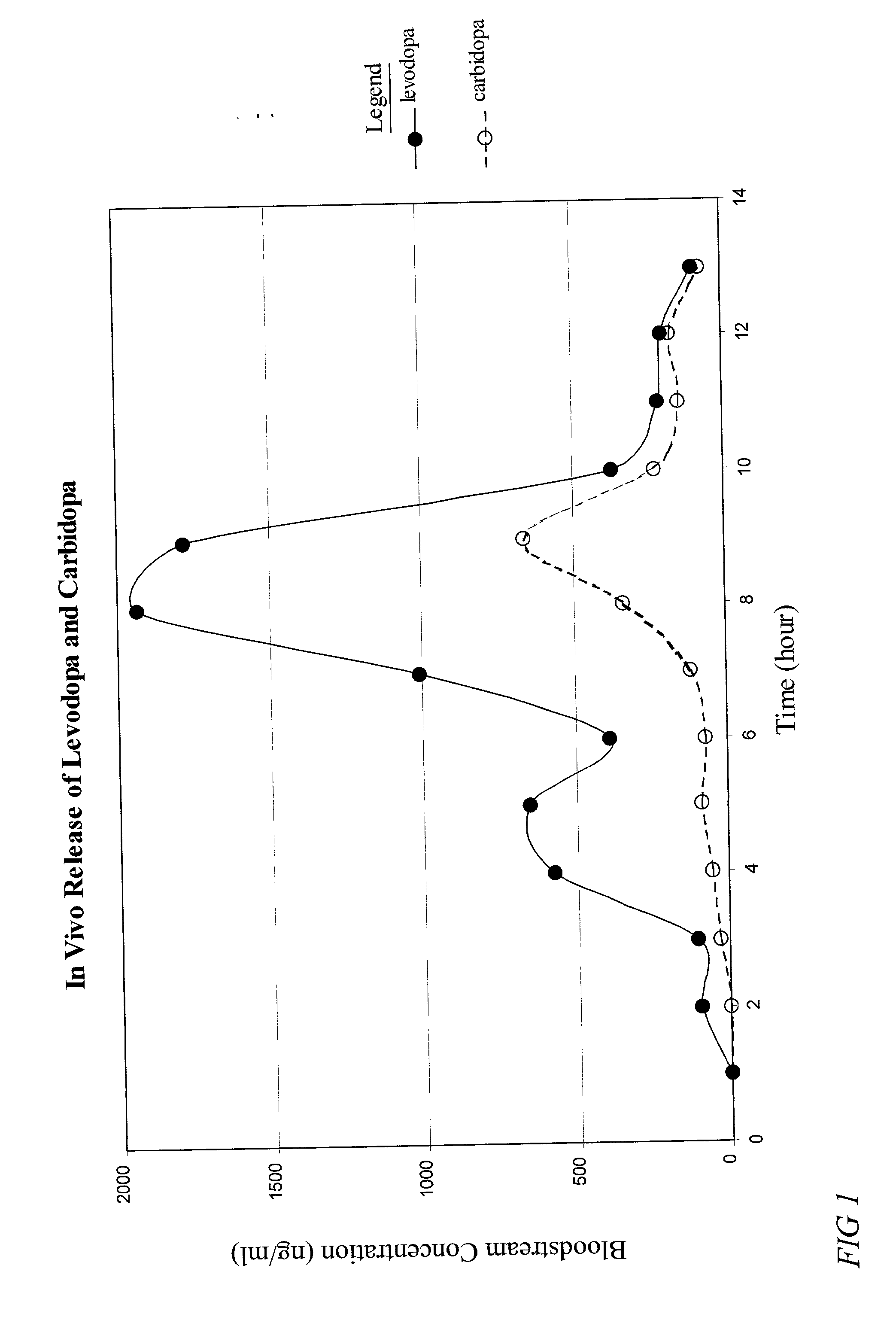
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
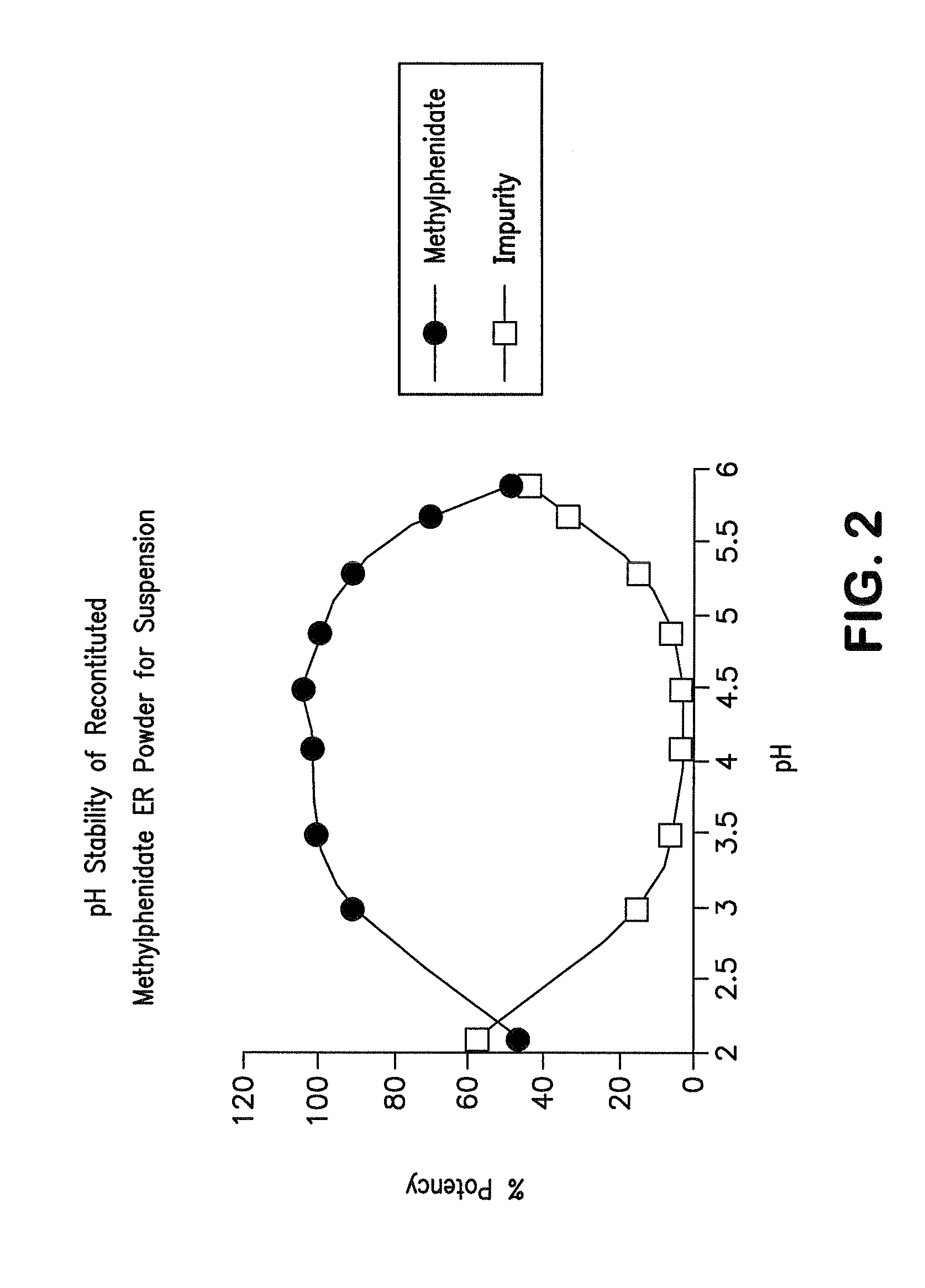
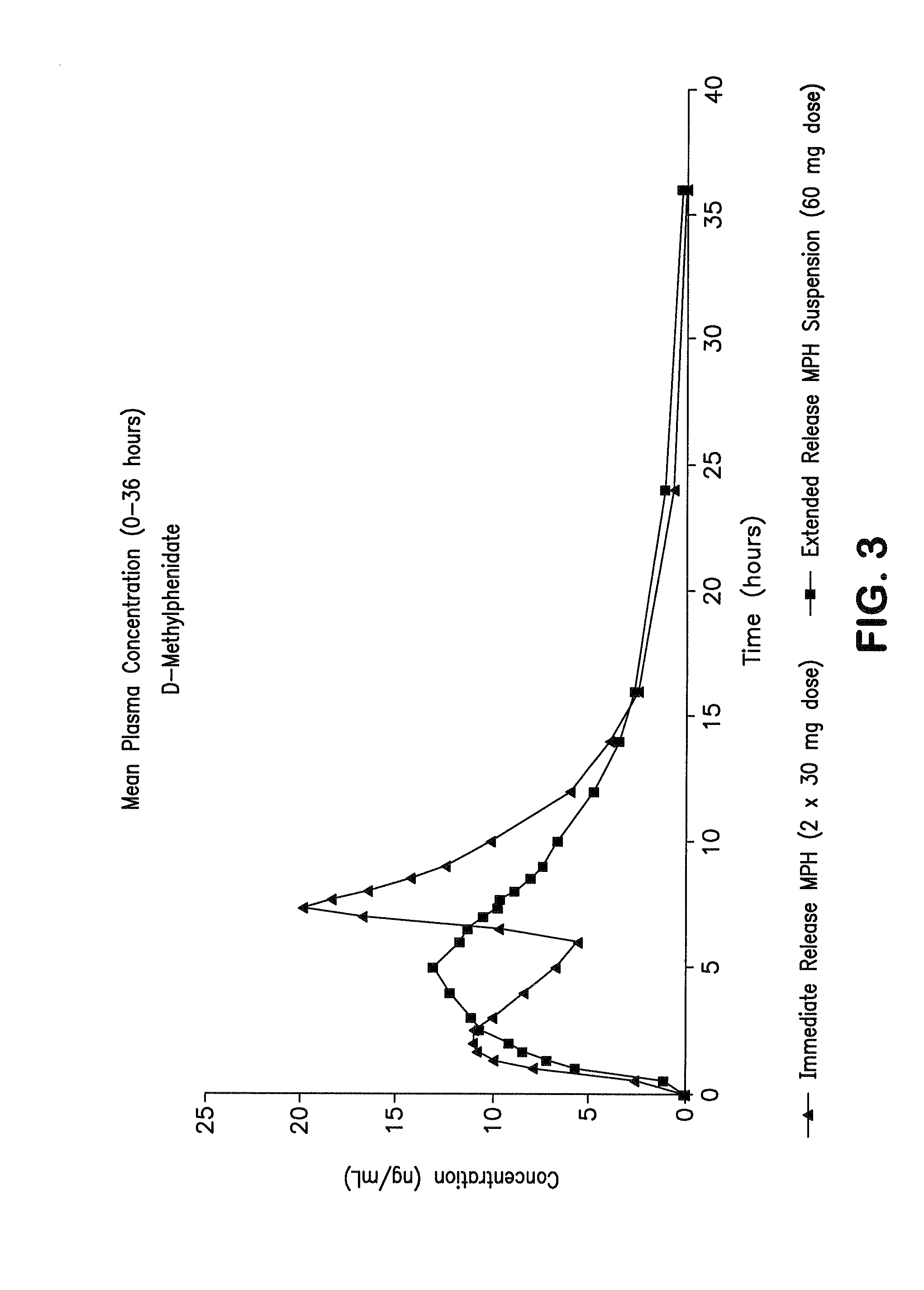
Orally effective methylphenidate extended release powder and aqueous suspension product
An oral methylphenidate powder which is reconstitutable into a final oral aqueous sustained release formulation containing at least about 50%, or at least about 80% by weight water based on the total weight of the suspension, is provided. The powder is a blend containing a combination of an uncoated methylphenidate-ion exchange resin complex, a barrier coated methylphenidate-ion exchange resin complex-matrix, and a water soluble buffering agent such that upon formed into an aqueous liquid formulation, the formulation has a pH in the range of about 3.5 to about 5, or about 4 to about 4.5. Following administration of a single dose of the oral aqueous methylphenidate suspension, a therapeutically effective amount of methylphenidate is reached in less than one hour and the composition provides a twelve-hour extended release profile.
Owner:TRIS PHARMA
Methods of treating pervasive development disorders
InactiveUS20080219966A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsPervasive developmental disorderMethylphenidate
A therapeutic method for treating an individual diagnosed with PDD pervasive developmental disorder comprises determining the efficacy of digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level. A method for reducing the amount of methylphenidate (Ritalin) being taken by an individual with attention deficit disorder (ADD) or attention deficit hyperactivity disorder (ADHD) by administering a therapeutic amount of digestive enzymes is also provided.
Owner:CUREMARK
Silicone-containing acrylic polymers for transdermal drug delivery compositions
Described herein are silicone-containing acrylic polymers useful, for example, in transdermal drug delivery compositions, to methods of making and using them, to transdermal drug delivery compositions comprising them, and to methods of making and using such transdermal drug delivery compositions. The polymers are particular suitable for formulating amine drugs, such as amphetamine, methylphenidate, rivastigmine, paroxetine and clonidine.
Owner:NOVEN PHARMA
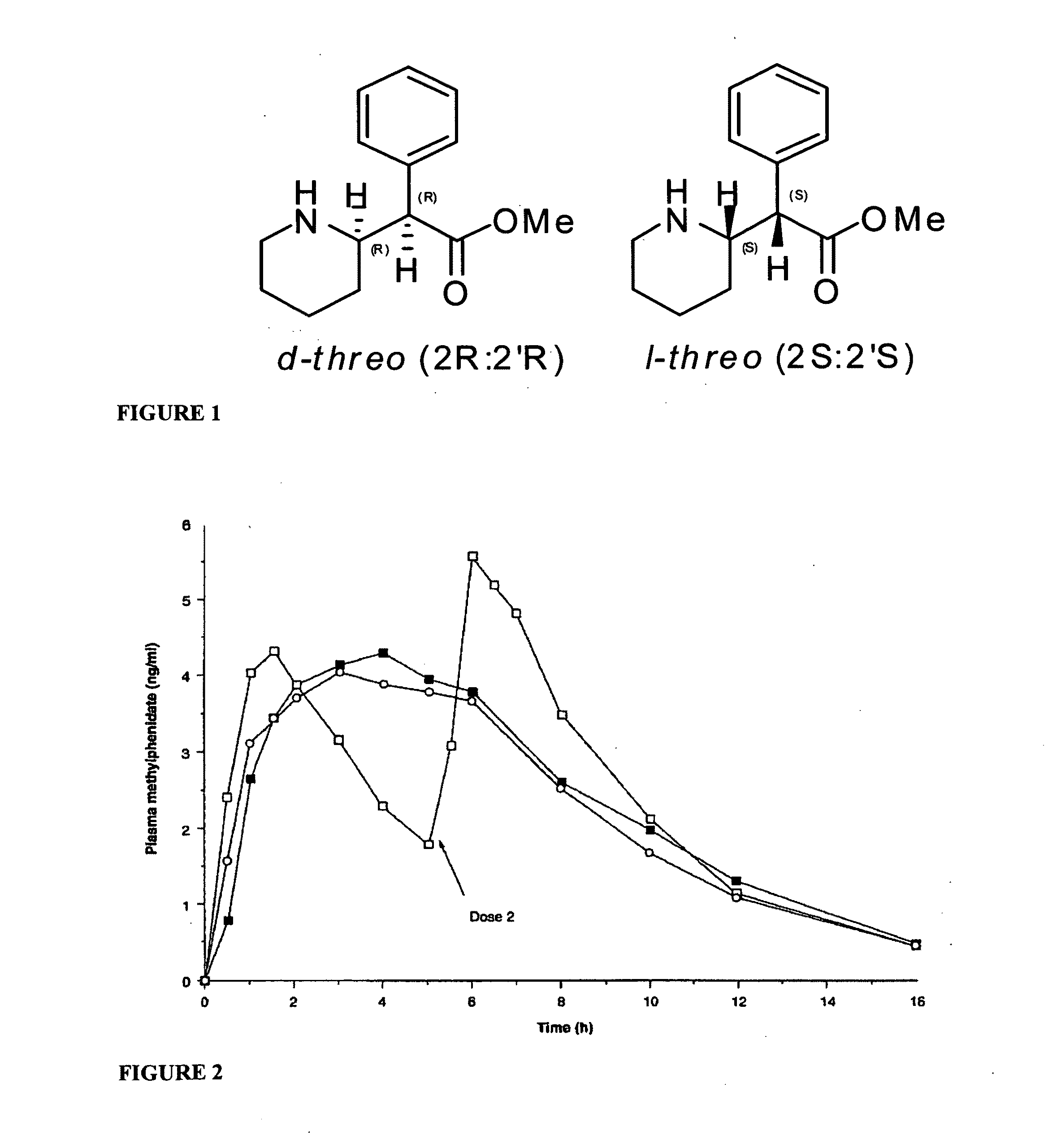
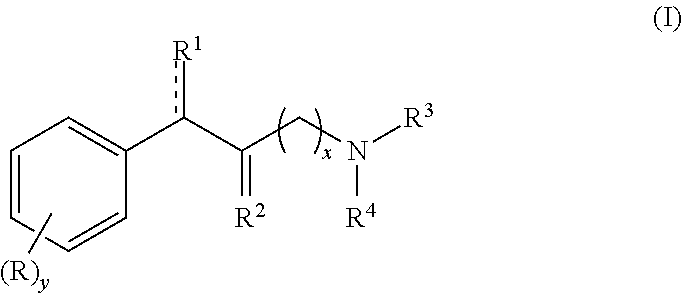
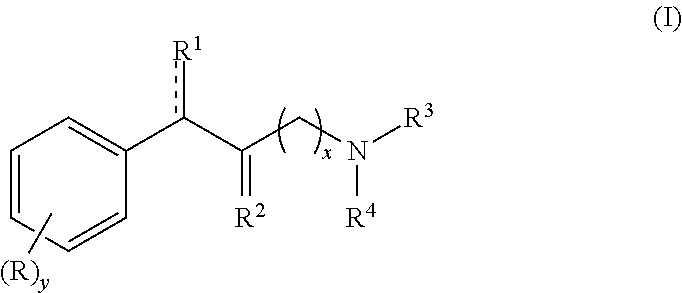
Methylphenidate analogs and methods of use thereof
Provided are analogs of methylphenidate (“MPH”) that are useful for the treatment of drug addiction, attention deficit disorder, attention deficit hyperactivity disorder, and depression. The MPH analogs are extended duration compounds that bind to the dopamine transporter and the reuptake of dopamine in the afflicted individual's brain. Because of the extended duration of the MPH analogs, administration of the compounds is only required on a once or twice daily schedule.
Owner:FROIMOWITZ MARK +1
Methods for treating fibromyalgia
The invention provides methods for treating or ameliorating cognitive dysfunction, fatigue, energy, concentration, mood, and pain associated with fibromyalgia using compositions containing methylphenidate or pharmaceutically equivalents thereof.
Owner:KATZ ROBERT S +1
Method of treating binge eating disorder and obesity resulting from binge eating behavior
Owner:LUCERNE BIOSCI
Reversal of general anesthesia by administration of methylphenidate, amphetamine, modafinil, amantadine, and/or caffeine
InactiveUS20130310422A1High speedReduces and eliminates effectBiocidePharmaceutical delivery mechanismUnconsciousnessWhole body
The present invention generally relates to compositions comprising anesthesia-reversing agents which facilitate or increase the time of awakening or reverse the effects of general anesthesia-induced unconsciousness. In some embodiments, the anesthesia reversing agent can be selected from any or a combination of methylphenidate (MPH), amphetamine, modafinil, amantadine, caffeine, or analogues or derivatives thereof. In some embodiments, compositions comprising at least one or more anesthesia-reversing agents can be used to facilitate awakening from anesthesia without or decreasing occurrence of delirium, and can be used in methods to treat or prevent the symptoms associated with emergence delirium, as well as treat a subject oversedated with general an esthesia. The invention also relates to methods for administering these compositions comprising anesthesia-reversing agents to subjects and for use.
Owner:THE GENERAL HOSPITAL CORP
Methylphenidate-oxoacid conjugates, processes of making and using the same
ActiveUS20140243291A1Improve bioavailabilityGood water solubilityBiocideNervous disorderThiolAlcohol
The present technology is directed to prodrugs and compositions for the treatment of various diseases and / or disorders comprising methylphenidate, or methylphenidate derivatives, conjugated to at least one alcohol, amine, oxoacid, thiol, or derivatives thereof. In some embodiments, the conjugates further include at least one linker. The present technology also relates to the synthesis of methylphenidate, or methylphenidate derivatives, conjugated to at least one alcohol, amine, oxoacid, thiol, or derivatives thereof or combinations thereof.
Owner:KEMPHARM INC
Methods and compositions to prevent addiction
InactiveUS20120302590A1Decrease and lessening of aversionDecrease and lessening of and addictionBiocideNervous disorderNervous systemStimulant
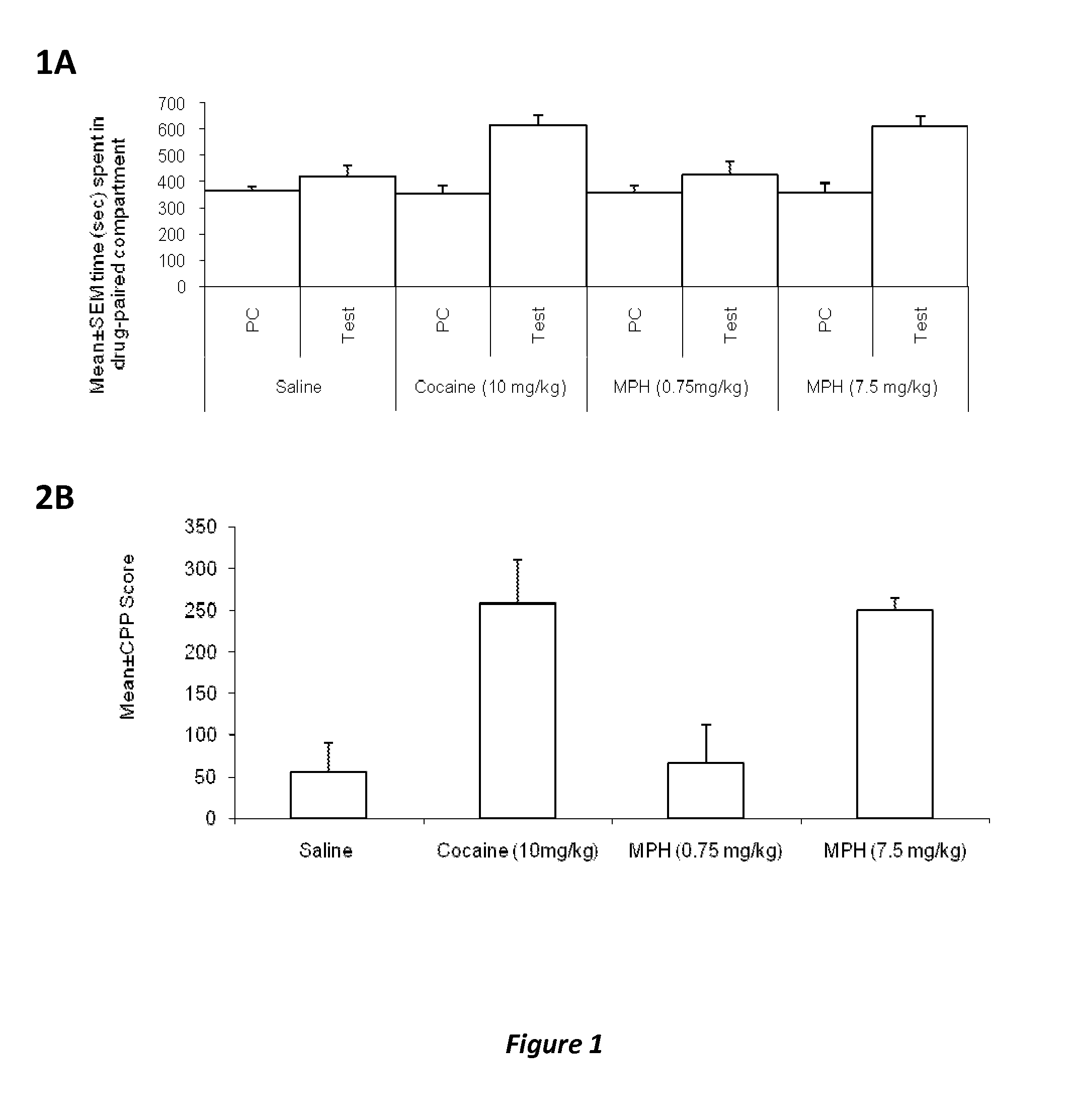
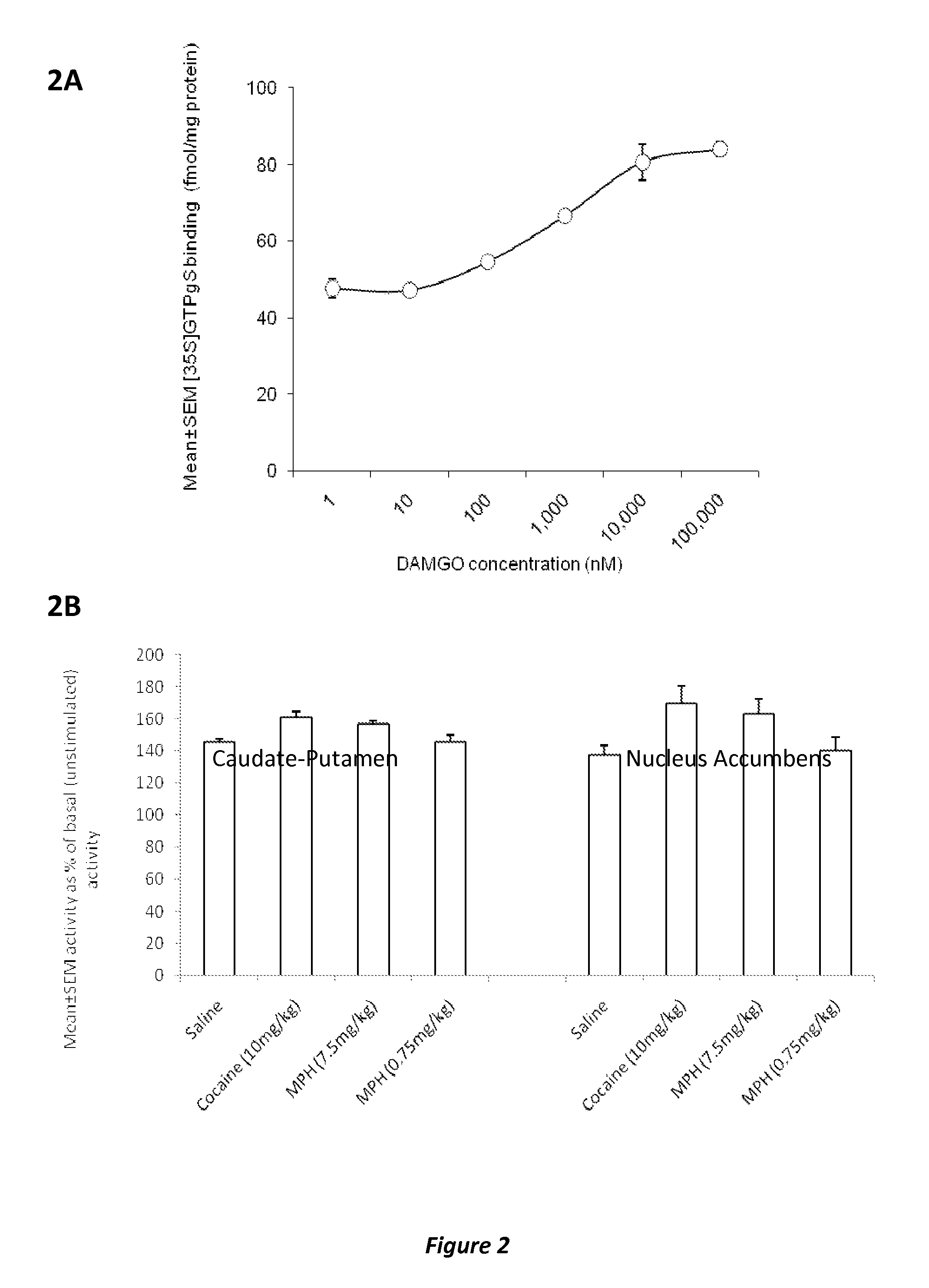
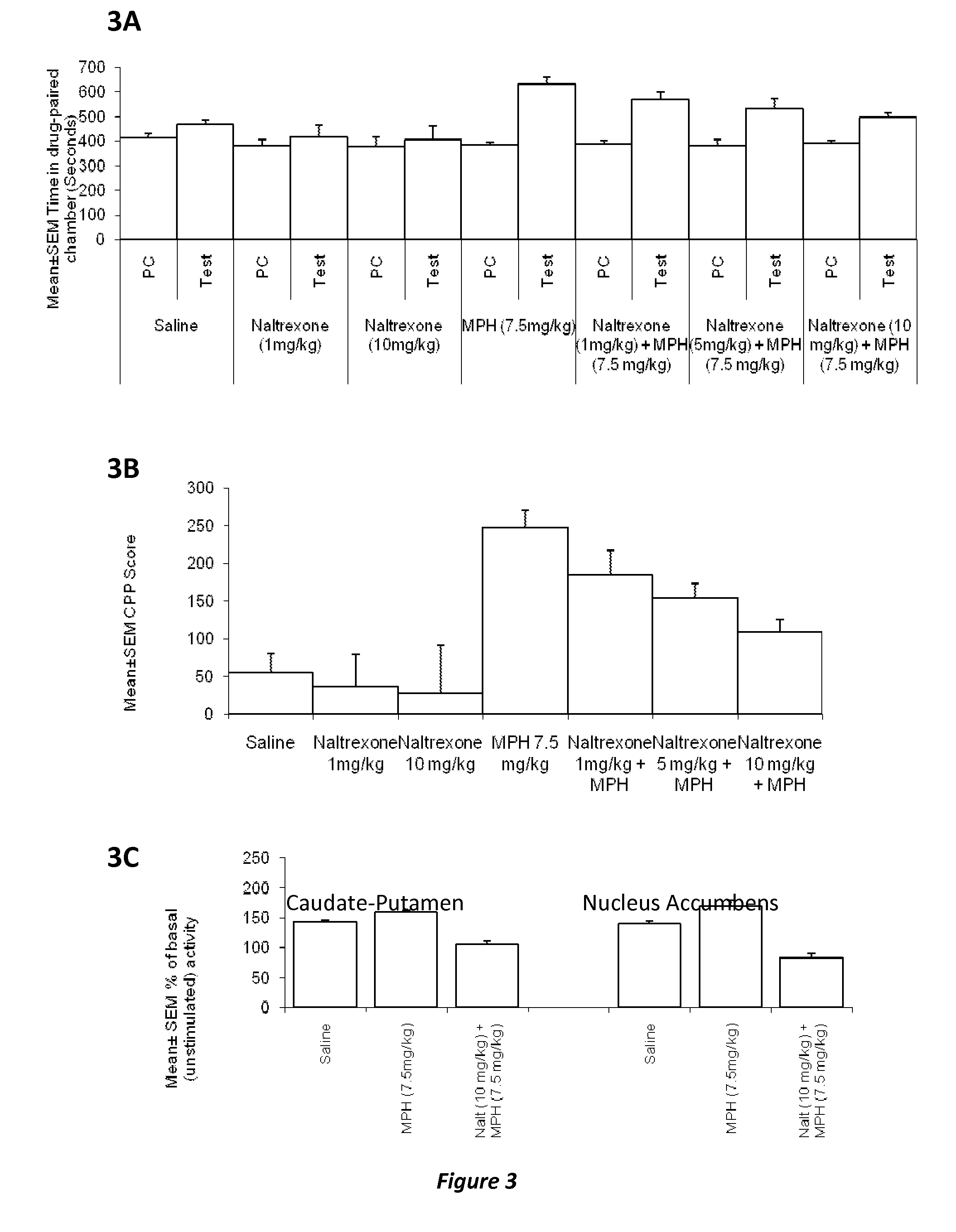
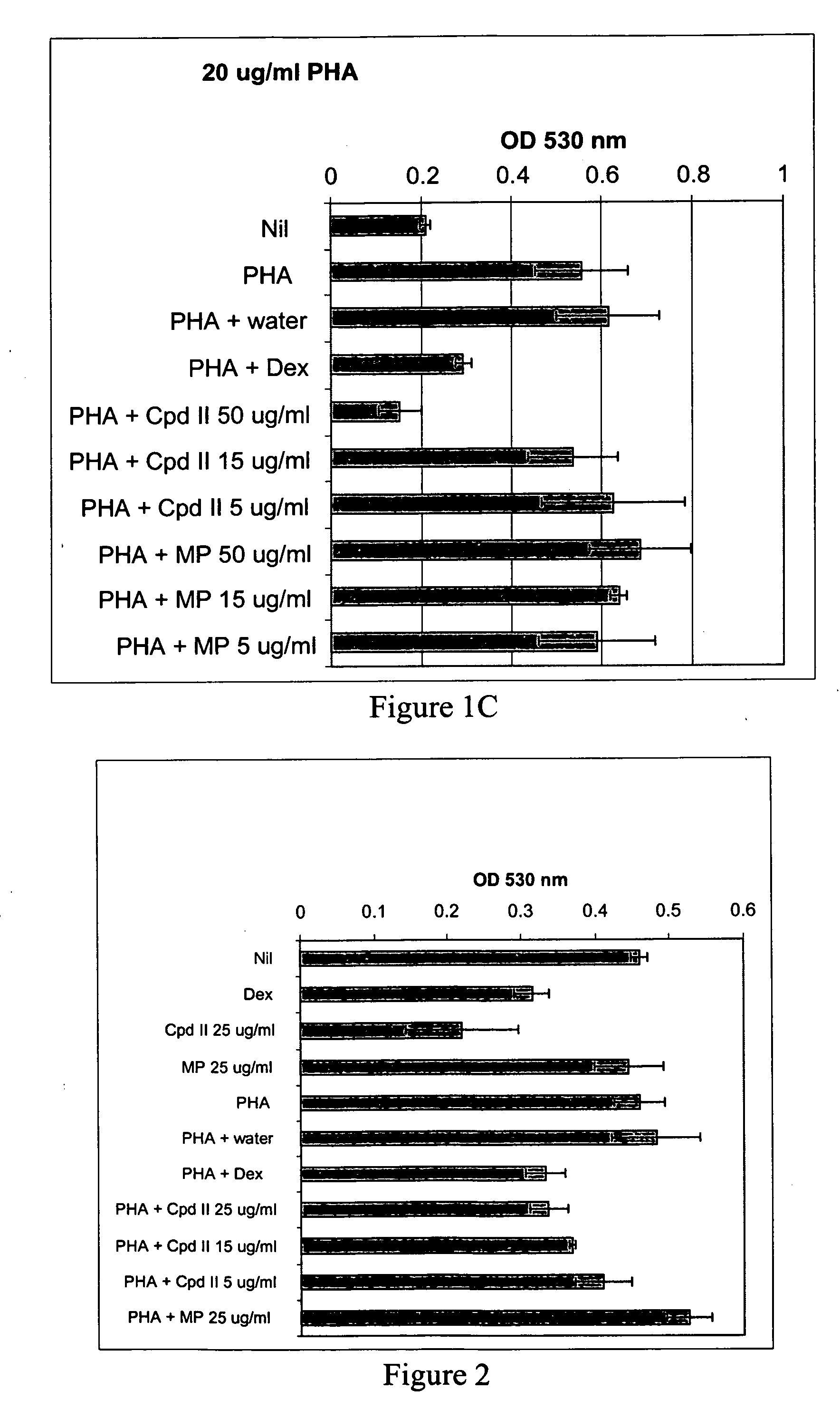
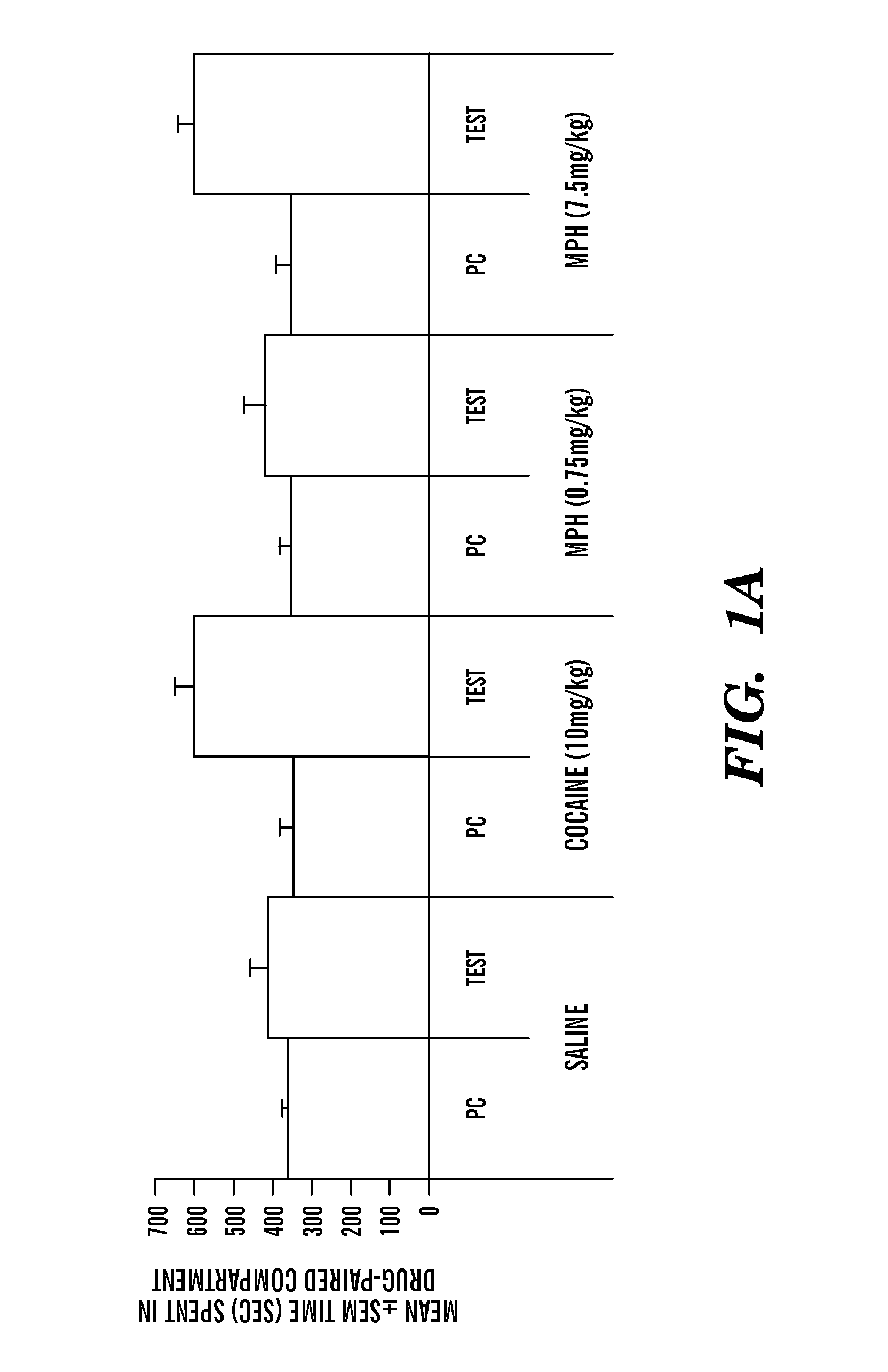
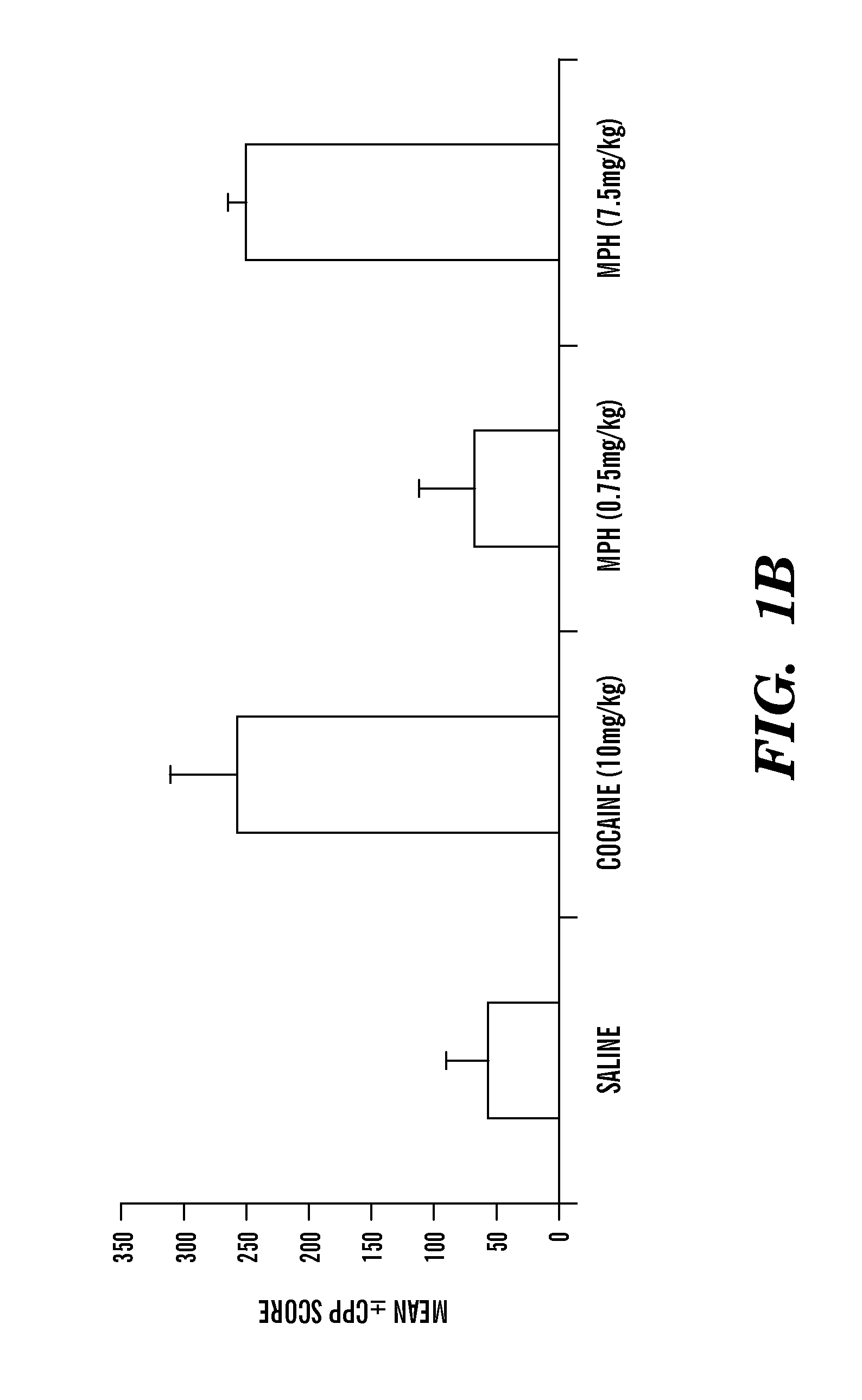
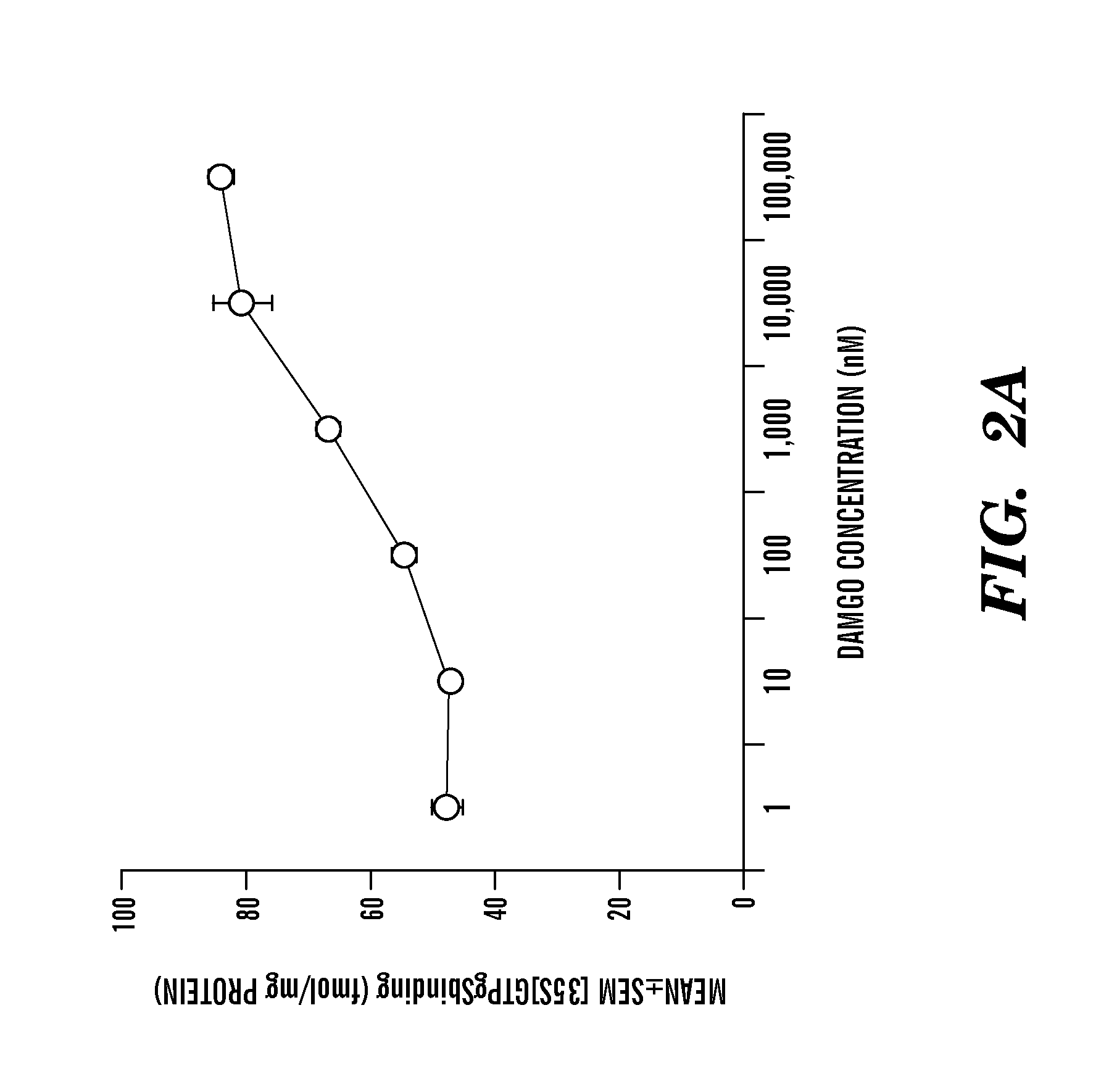
Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject comprising, administering a therapeutic amount of the neurological stimulant and administering an antagonist of the kappa opioid receptor, to thereby reduce or prevent the development of aversion to the CNS stimulant in the subject. Also disclosed is a method of reducing or preventing the development of addiction to a CNS stimulant in a subject, comprising, administering the CNS stimulant and administering a mu opioid receptor antagonist to thereby reduce or prevent the development of addiction to the CNS stimulant in the subject. Also disclosed are pharmaceutical compositions comprising a central nervous system stimulant and an opioid receptor antagonist. Examples of central nervous system stimulants (such as methylphenidate) and opioid receptor antagonists (such as naltrexone) are provided.
Owner:THE GENERAL HOSPITAL CORP
Methylphenidate-oxoacid conjugates, processes of making and using the same
ActiveUS9079928B2Good water solubilityIncrease capacityOrganic active ingredientsNervous disorderThiolAlcohol
Owner:KEMPHARM INC
Multi-Layered Controlled-Release Methylphenidate Pellet
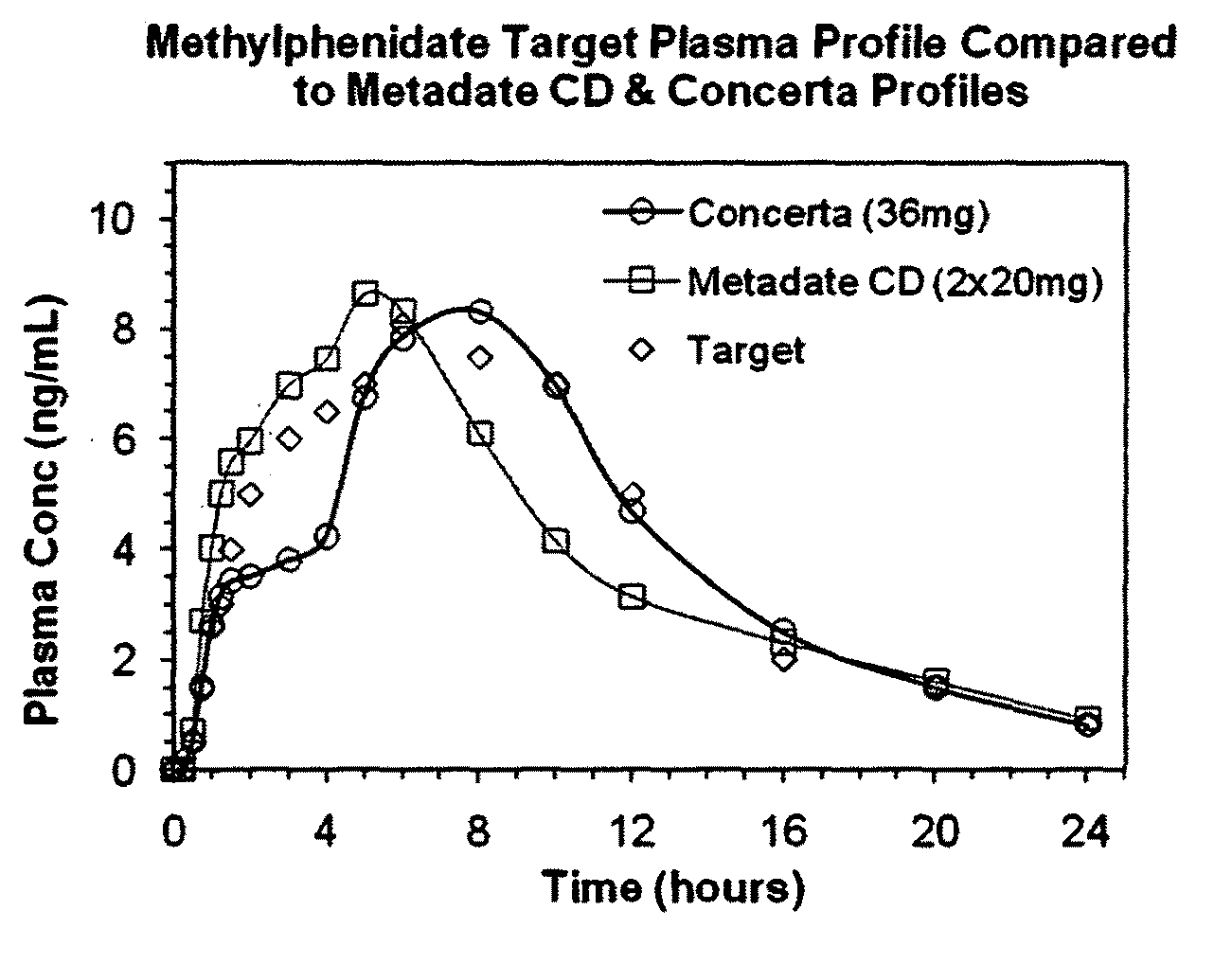
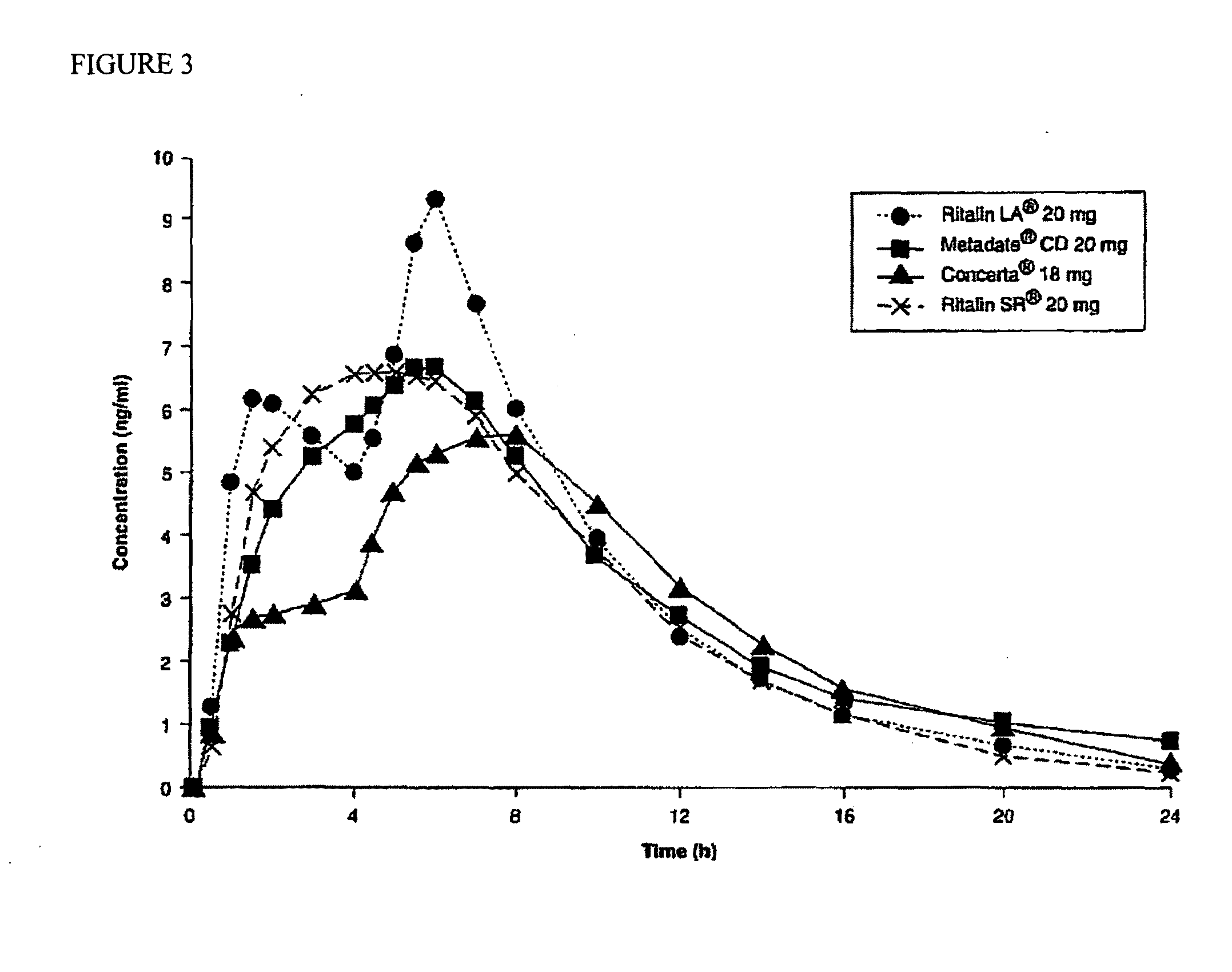
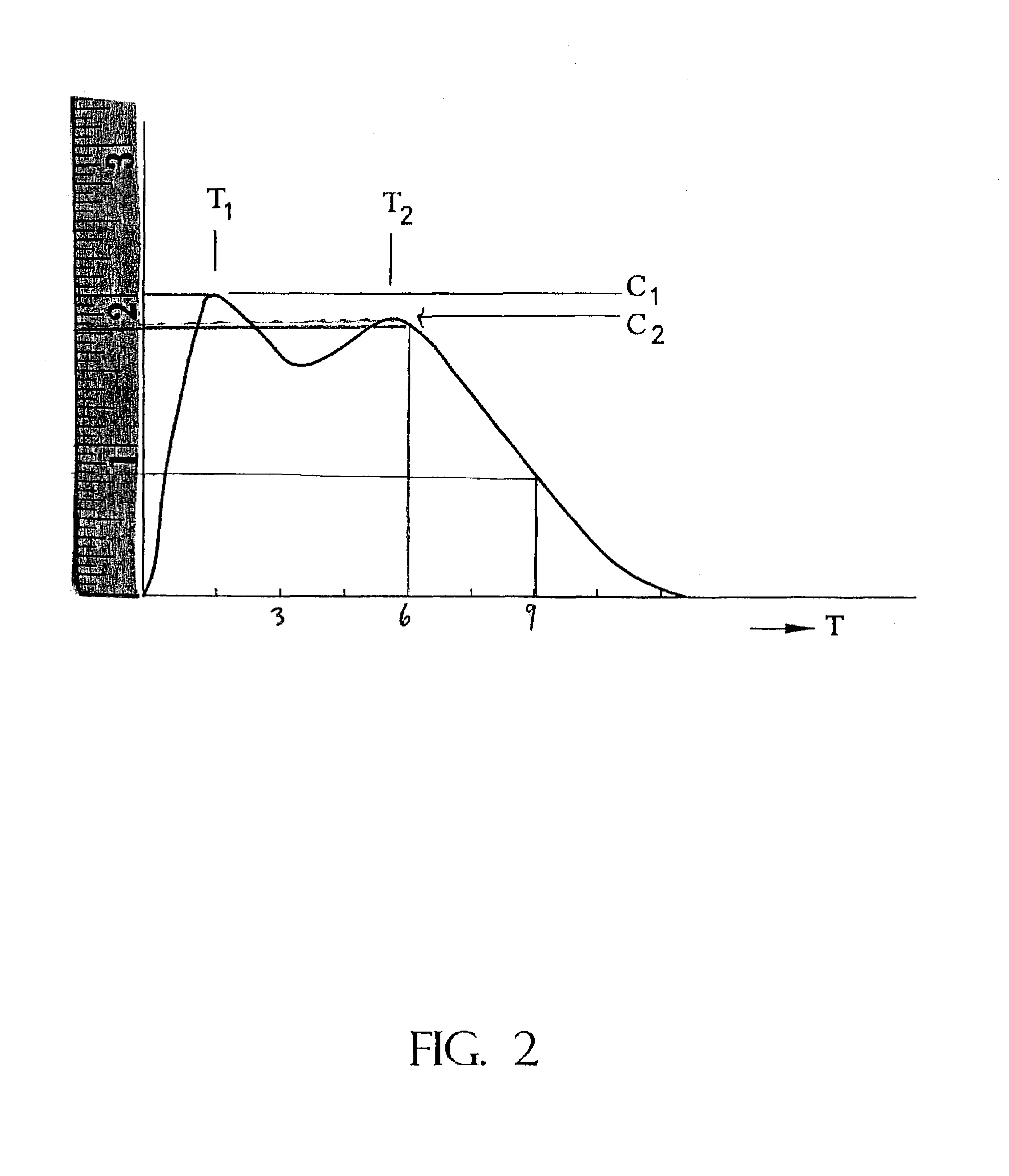
The present invention relates to a type of controlled-release multilayered methylphenidate pellet that does not need to be combined with another type of pellet. It allows the maintenance of therapeutic levels in the plasma during 12 hours with a single daily dose, avoiding repeated administrations throughout the day. The controlled-release multi-layered pellet is comprised of an inert core, a first layer that contains methylphenidate and an acid buffering system, a protective layer, a layer of ethylcellulose, that performs the function of controlling the extended release of most of the methylphenidate, and a second layer of methylphenidate, that is responsible for the immediate release of the aforesaid within one hour of administration. The weight ratio between the methylphenidate present in the first active layer and the ethylcellulose is between 1.40:1 and 1.90:1. The multi-layered pellet may possibly have an external coating to protect it from erosion during processing.
Owner:LAB RUBII12
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Transdermal methylphenidate compositions with acrylic block copolymers
Described herein are transdermal compositions comprising methylphenidate in a polymer matrix comprising a non-reactive random acrylic polymer and an acrylic block copolymer. Method of making such compositions, and therapeutic methods using them also are disclosed.
Owner:NOVEN PHARMA
Methylphenidate patch preparation
InactiveUS20110200663A1Decrease can be minimizedImprove skinBiocideAnimal repellantsSkin permeabilityPlasticizer
Provided is a methylphenidate patch preparation superior in the stability of a drug (methylphenidate and / or a salt thereof) in the patch preparation, skin permeability of a drug during use of the patch preparation, and methylphenidate availability. A patch preparation having a support and an adhesive layer formed on at least one surface of the support, wherein the adhesive layer contains methylphenidate and / or a salt thereof, polyisobutylene and a liquid plasticizer. The liquid plasticizer preferably has an HLB value of 1.0-3.3.
Owner:AVEVA DRUG DELIVERY SYST
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS7674480B2Less timeHigh speedPowder deliveryOrganic active ingredientsAdditive ingredientAttention deficits
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
Treatment of Behavioral Disorders
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as cetirizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxiety, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Uses of methylphenidate derivatives
InactiveUS20060183773A1Prevent proliferationAntibacterial agentsBiocideMethylphenidateCompound (substance)
Owner:AMPIO PHARMA
Pharmaceutical composition which reduces or eliminates drug abuse potential
InactiveUS20070148247A1Reduces and eliminates drug abuse potentialPrevents nasal absorption and injectabilityOrganic active ingredientsBiocideStimulantMethylphenidate
A pharmaceutical composition which reduces or eliminates the drug abuse potential of central nervous system stimulant comprising: (a) a drug selected from the group consisting of methylphenidate, amphetamine, methamphetamine, and combinations thereof; and (b) a gel forming polymer wherein the gel forming polymer is a polymer that forms a gel when contacted with moisture or placed in an aqueous solution. The present invention is based on the discovery that a central nervous system stimulant such as methylphenidate in combination with a gel forming polymer reduces or eliminates drug abuse potential by swelling in the presence of moisture, and thus, preventing nasal absorption and injectability of the drug.
Owner:JOSHI YATINDRA +1
Methylphenidate derivatives and uses of them
InactiveUS20060189655A1Inhibit angiogenesisBiocideSenses disorderCompound (substance)Methylphenidate
Owner:AMPIO PHARMA
Pharmaceutical compositions for release control of methylphenidate
Disclosed is a pharmaceutical composition for release control comprising a plurality of particles for release control. The plurality of particles for release control comprise a core material containing methylphenidate and a polymer coating layer for release control formed on the core material. The plurality of particles for release control are divided into two or more groups based on the average thickness of the polymer coating layer for release control. The particle groups are identical in terms of the composition of the polymer in the polymer coating layer, but are different in terms of the average thickness of the coated layer. The pharmaceutical composition for release control according to the present invention may control the release pattern of methylphenidate contained in the core material as desired, and can be used as an oral formulation in a variety of forms such as orally disintegrating tablets, etc.
Owner:SAMYANG HLDG CORP
Carboxylesterase-1 Polymorphisms and Methods of Use Therefor
InactiveUS20110020801A1Improve throughputEfficient CatalysisMicrobiological testing/measurementBiological material analysisCaucasian populationNucleotide
Methods and kits are provided for detecting polymorphisms in carboxylesterase-1 (CES1). Several single nucleotide polymorphisms (SNPs) in CES1 in humans, and methods for detecting the same, are provided (e.g., Gly143Glu, 12754T>del). Results indicate that the Gly143Glu (9486G>A) polymorphism has an allelic frequency of 1.5% in the Caucasian population. Polymorphisms of the present invention may alter the function of the carboxylesterase-1 enzyme (hCES1). Thus, the methods and kits of the present invention may be used to personalize a therapy and / or avoid adverse consequences of altered metabolism of a therapeutic or compound (e.g., enalapril, methylphenidate, etc.) which may result due to a CES1 polymorphism. In addition, recombinant cells lines overexpressing wild-type CES1 or expressing CES1 mutants are provided. Such cell lines may be used to assess the effects of candidate compounds on CES1, and the action of CES1 on these candidate compounds.
Owner:MARKOWITZ JOHN S +1
Methods and compositions to prevent addiction
Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject comprising, administering a therapeutic amount of the neurological stimulant and administering an antagonist of the kappa opioid receptor, to thereby reduce or prevent the development of aversion to the CNS stimulant in the subject. Also disclosed is a method of reducing or preventing the development of addiction to a CNS stimulant in a subject, comprising, administering the CNS stimulant and administering a mu opioid receptor antagonist to thereby reduce or prevent the development of addiction to the CNS stimulant in the subject. Also disclosed are pharmaceutical compositions comprising a central nervous system stimulant and an opioid receptor antagonist. Examples of central nervous system stimulants (such as methylphenidate) and opioid receptor antagonists (such as naltrexone) are provided.
Owner:FLORIDA STATE UNIV RES FOUND INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com