Method of treating depressive disorders
a depressive disorder and treatment method technology, applied in the field of treating depressive disorders, can solve the problems difficult treatment of binge eating disorder, and significant medical and psychiatric risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
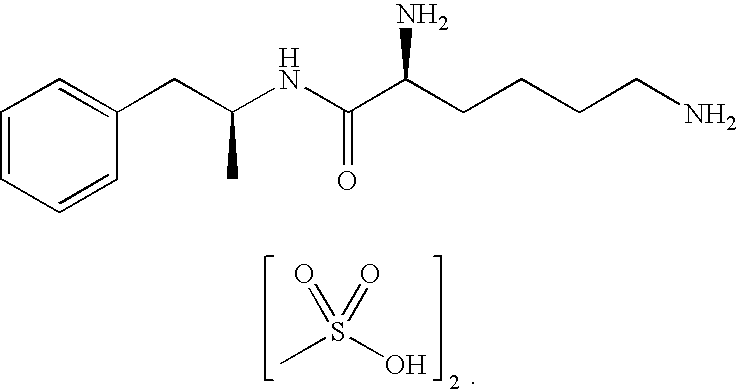
Treatment of a Patient with Major Depressive Disorders and Binge Eating Disorder Using the Amphetamine Prodrug Lisdexamfetamine Dimesylate
[0117]Patient 1 is an adult, non-geriatric patient with a history of a major depressive disorder, attention deficit hyperactivity disorder, and binge eating disorder. Throughout Patient 1's entire adult life, there were reportedly periodic depressive episodes and symptoms. Patient 1 also indicated a history of binge eating disorder for approximately one year, characterized by eating unusually large amounts of junk food, often until feeling nauseated, and then feeling very guilty about the binging behavior. Such symptoms, though intermittently present for the past two decades, had escalated to an average of nearly every other day for about 12 months. During this time Patient 1 was being treated with PAXIL (paroxetine) and then ZOLOFT (sertraline) daily. Patient 1 participated in group therapy to address mood and eating symptoms. However, group ther...
example 2
Treatment of a Patient with Binge Eating Disorder and Major Depressive Disorders Using Lisdexamfetamine Dimesylate
[0119]Patient 2 is a non-geriatric adult with a history of attention-deficit hyperactivity disorder, polysubstance dependence, major depressive disorder, generalized anxiety disorder, and binge eating disorder. The patient has been treated in the past with multiple medication trials, either alone or in combination, including: WELLBUTRIN (bupropion HCl), EFFEXOR (venlafaxine HCl), CELEXA (citalopram), LAMICTAL (lamotrigine), RISPERDAL (risperidone), NEURONTIN (gabapentin), KLONOPIN (clonazepam), STRATTERA (atomoxetine) CONCERTA (methylphenidate), RITALIN SR (methylphenidate), ADDERAL XR (dextroamphetamine+amphetamine), and PROVIGIL (modafinil). The patient also was previously treated with intensive psychotherapy and received various forms of substance abuse counseling in the past. Lisdexamfetamine dimesylate was initiated for treatment of the patient's ADHD to address ‘we...
example 3
Treatment of a Patient with ADHD, Generalized Anxiety Disorder, and Obsessive-Compulsive Behavior Using Lisdexamfetamine Dimesylate
[0122]The patient is a non-geriatric adult diagnosed with a history of ADHD, inattentive type, and comorbid Generalized Anxiety Disorder, though had no prior treatment. Presenting ADHD symptoms included difficulty sustaining attention and attending to details, difficulty organizing tasks with tendencies toward avoidance, distractibility, and problems finishing tasks that have been initiated. Symptoms of Generalized Anxiety Disorder included frequent and intense ruminative worrying, feeling overly fatigued, muscle tension and intermittent problems with sleep. History suggests that a perseverative pattern of thinking and compulsive worrying may have evolved from deficits in attention and information processing. The patient was started on VYVANSE 30 mg in the morning, along with TRAZODONE 50 mg at bedtime. VYVANSE was maintained at 30 mg once daily in the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com