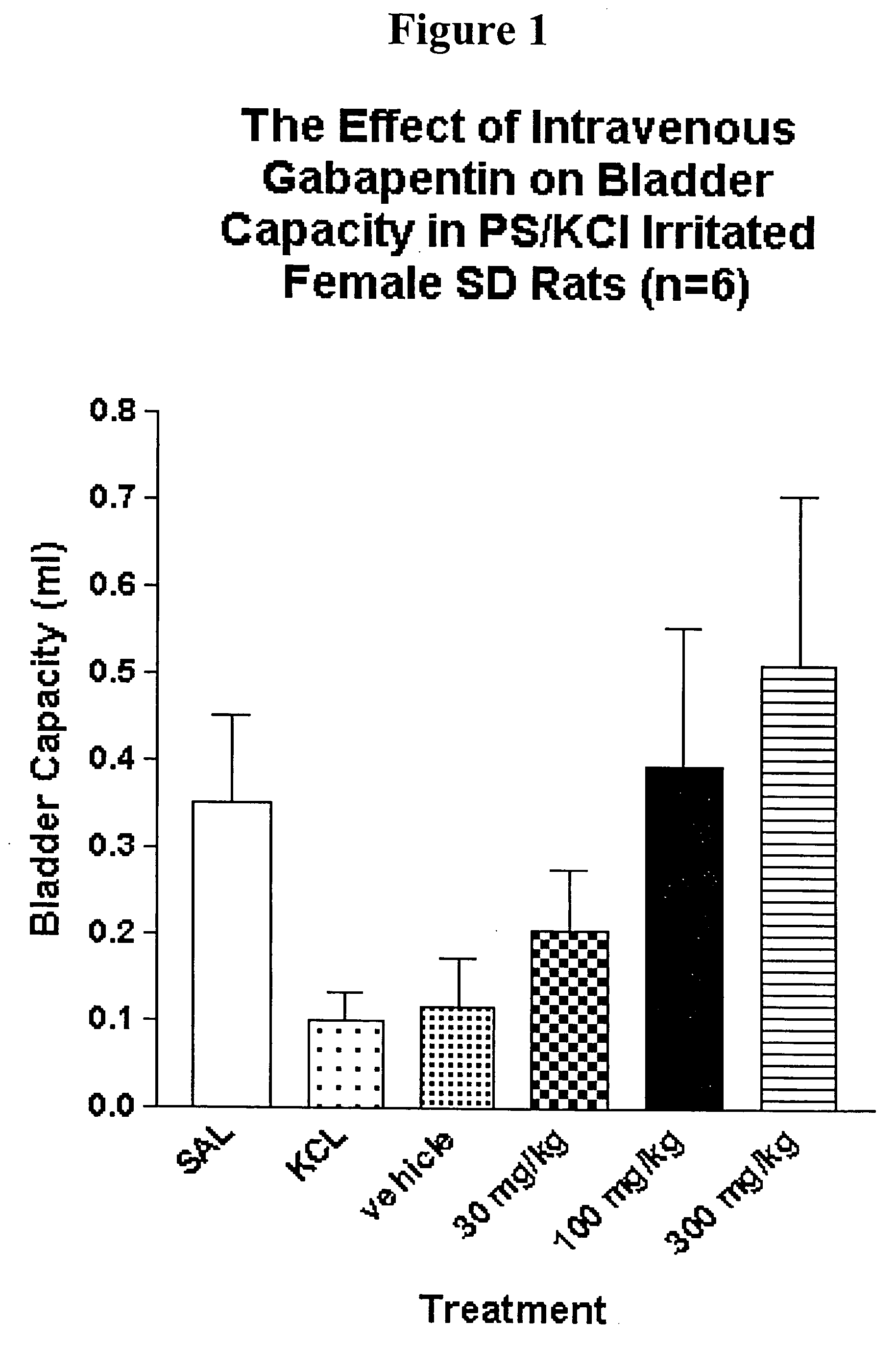
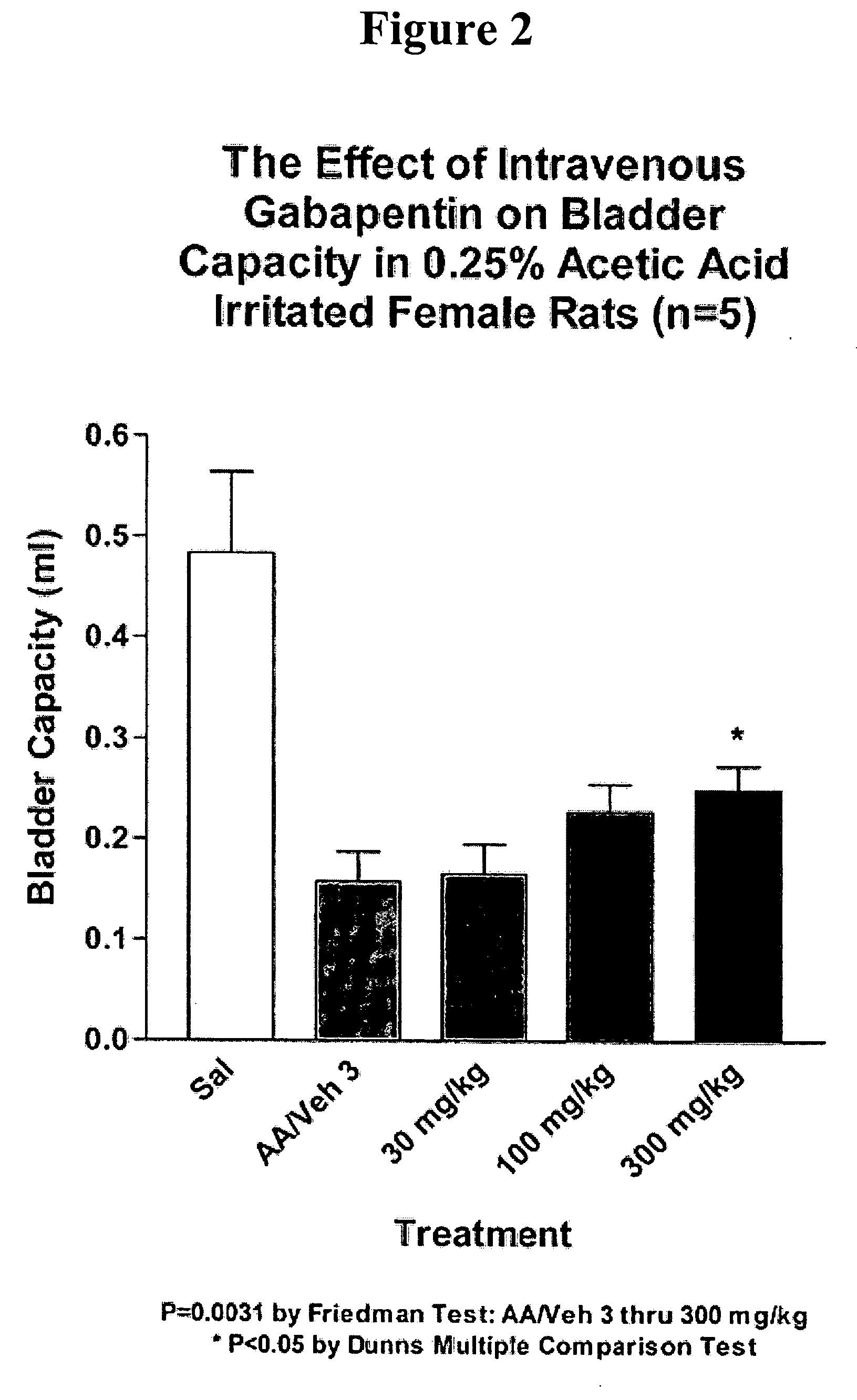
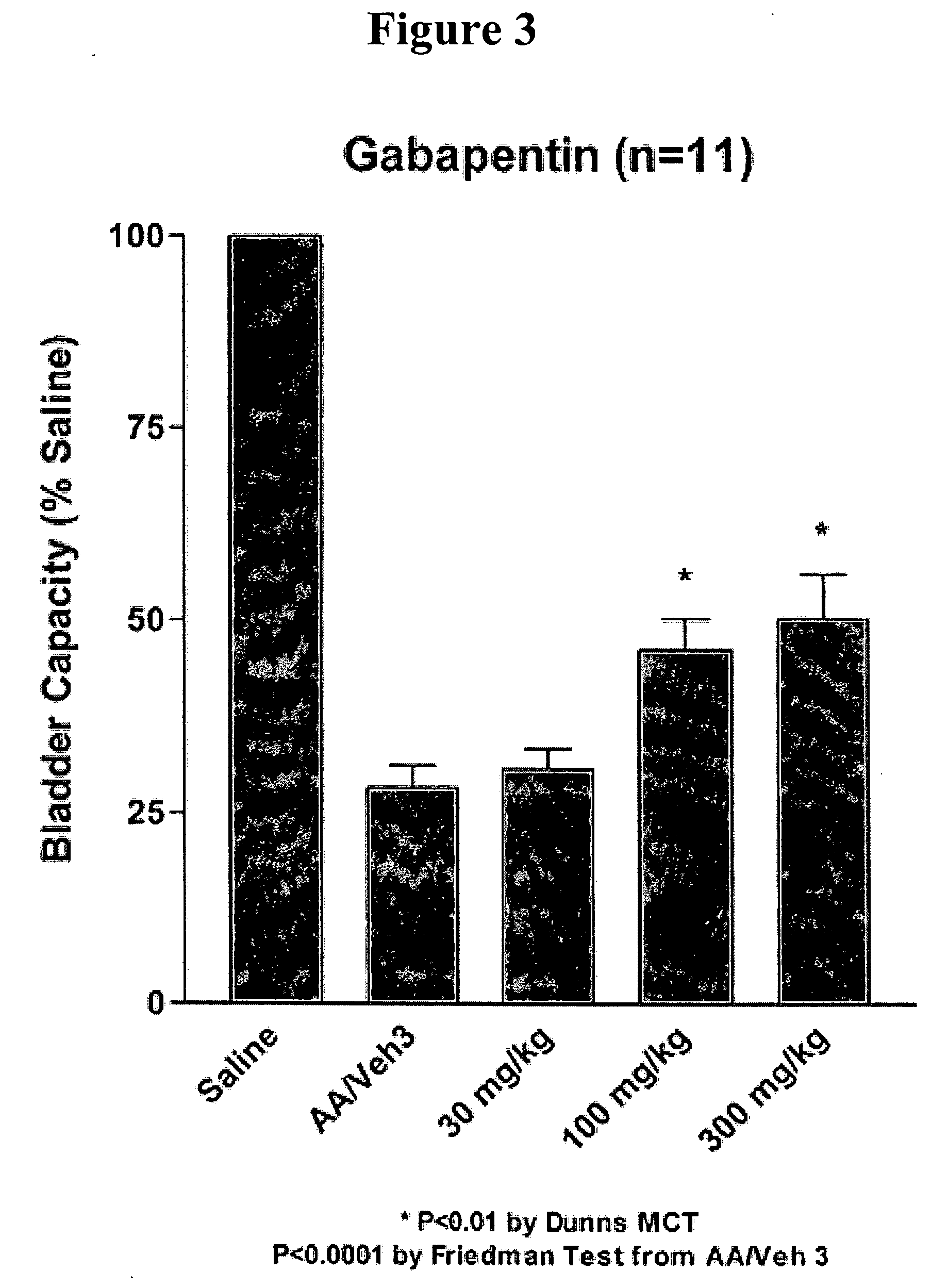
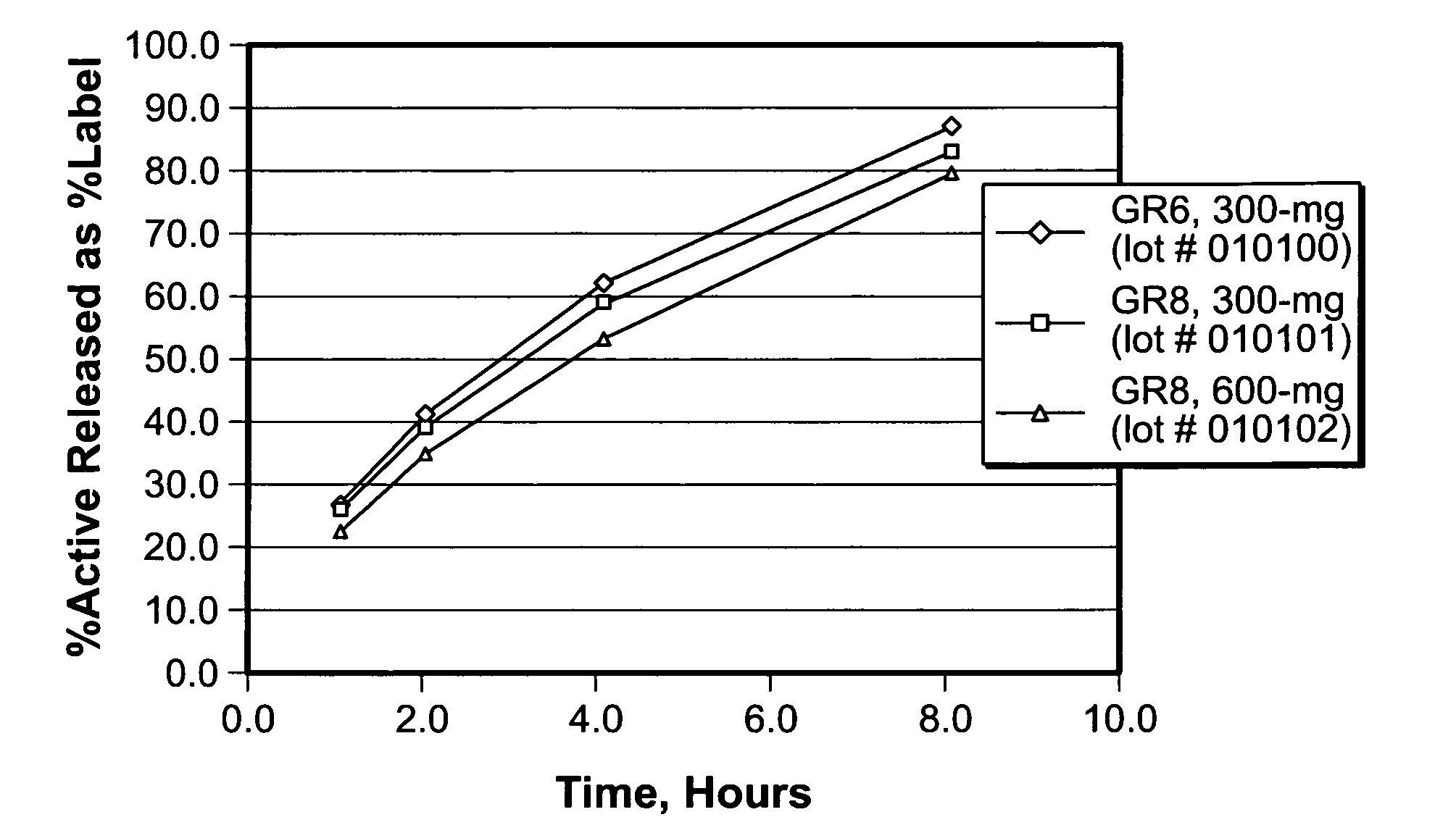
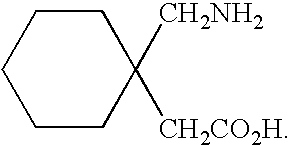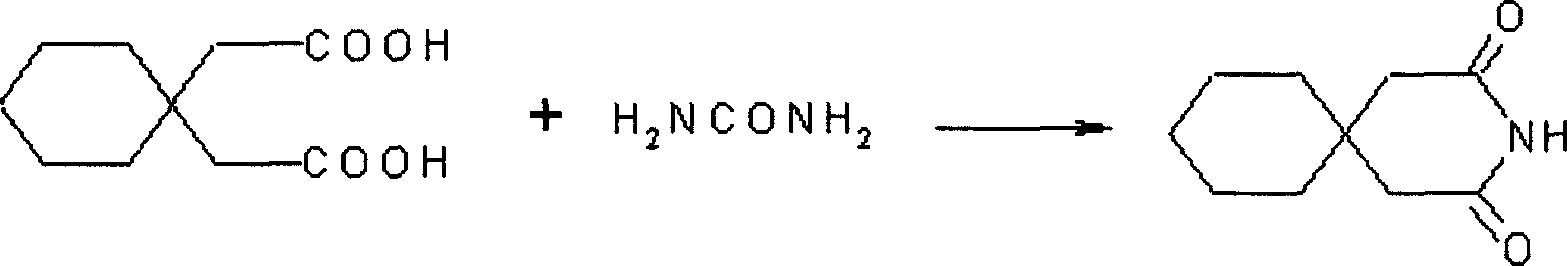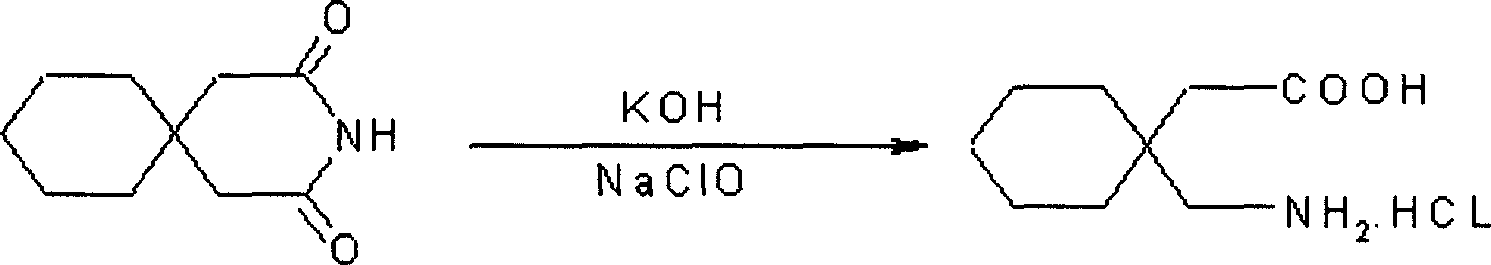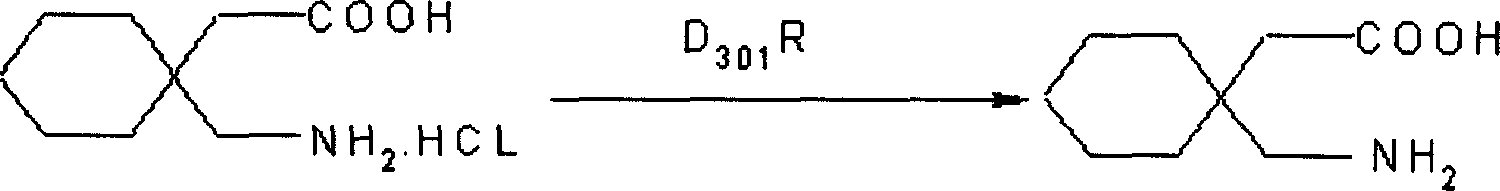Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
163 results about "Gabapentin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults.
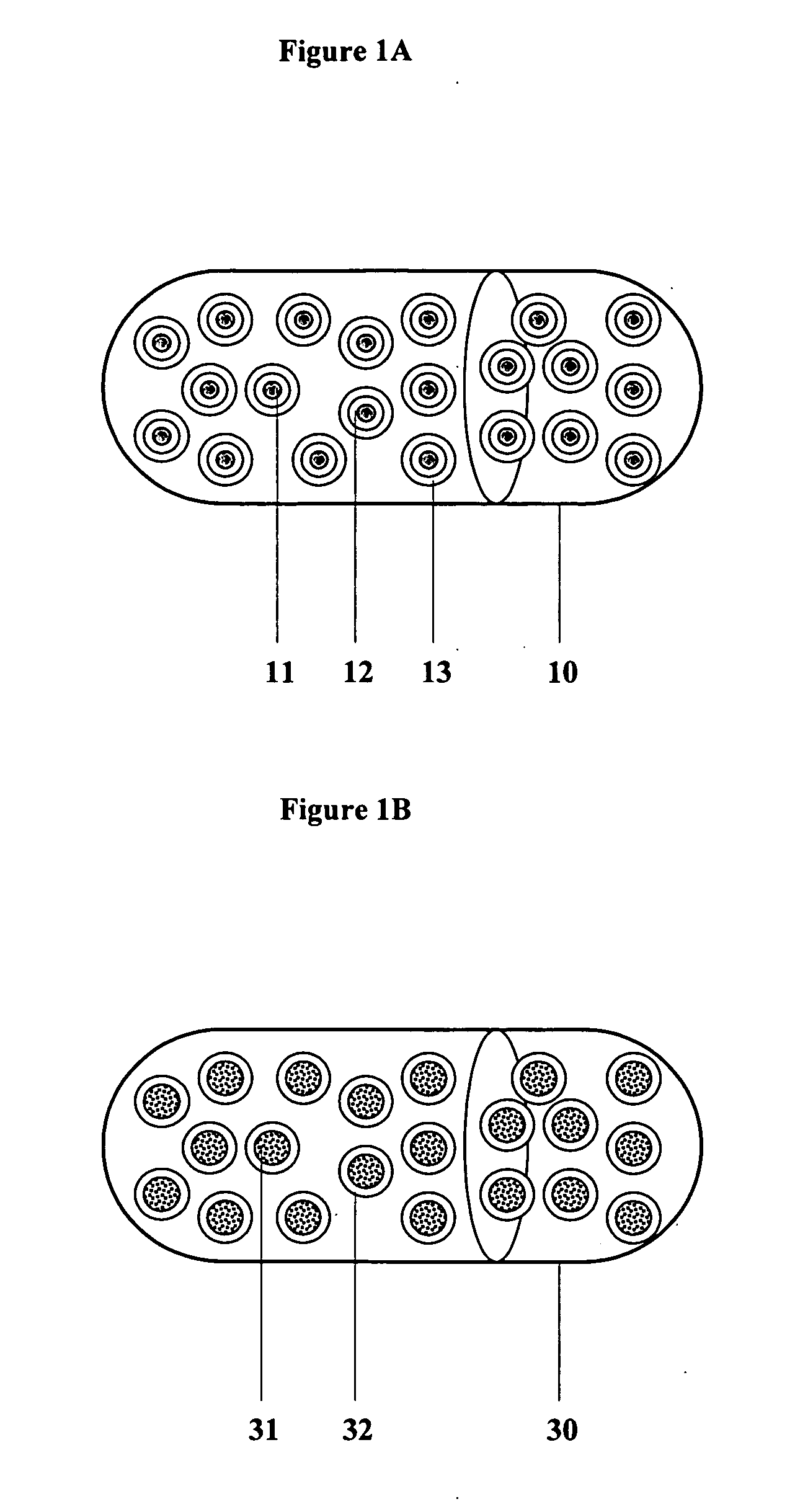
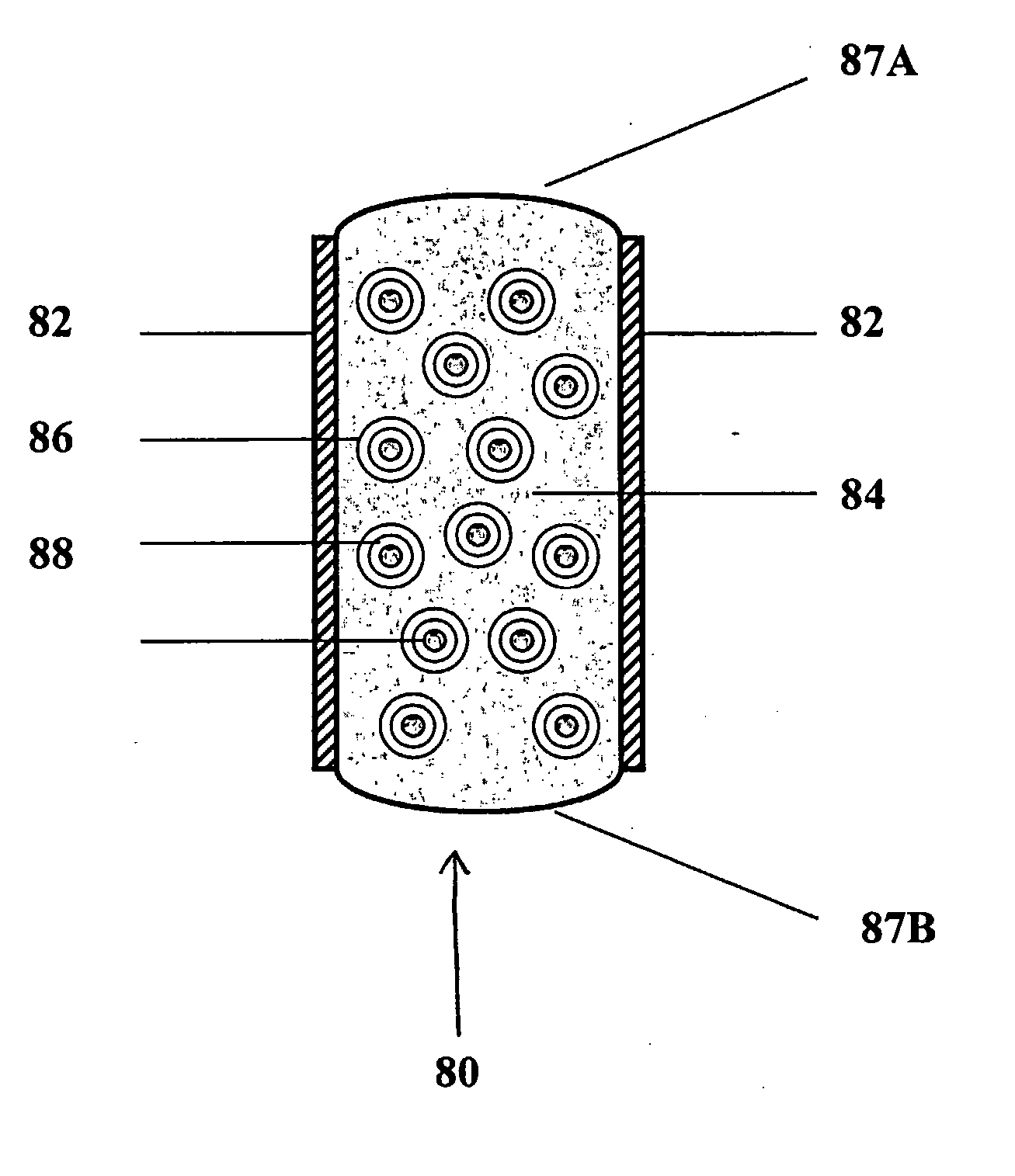
Controlled regional oral delivery
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
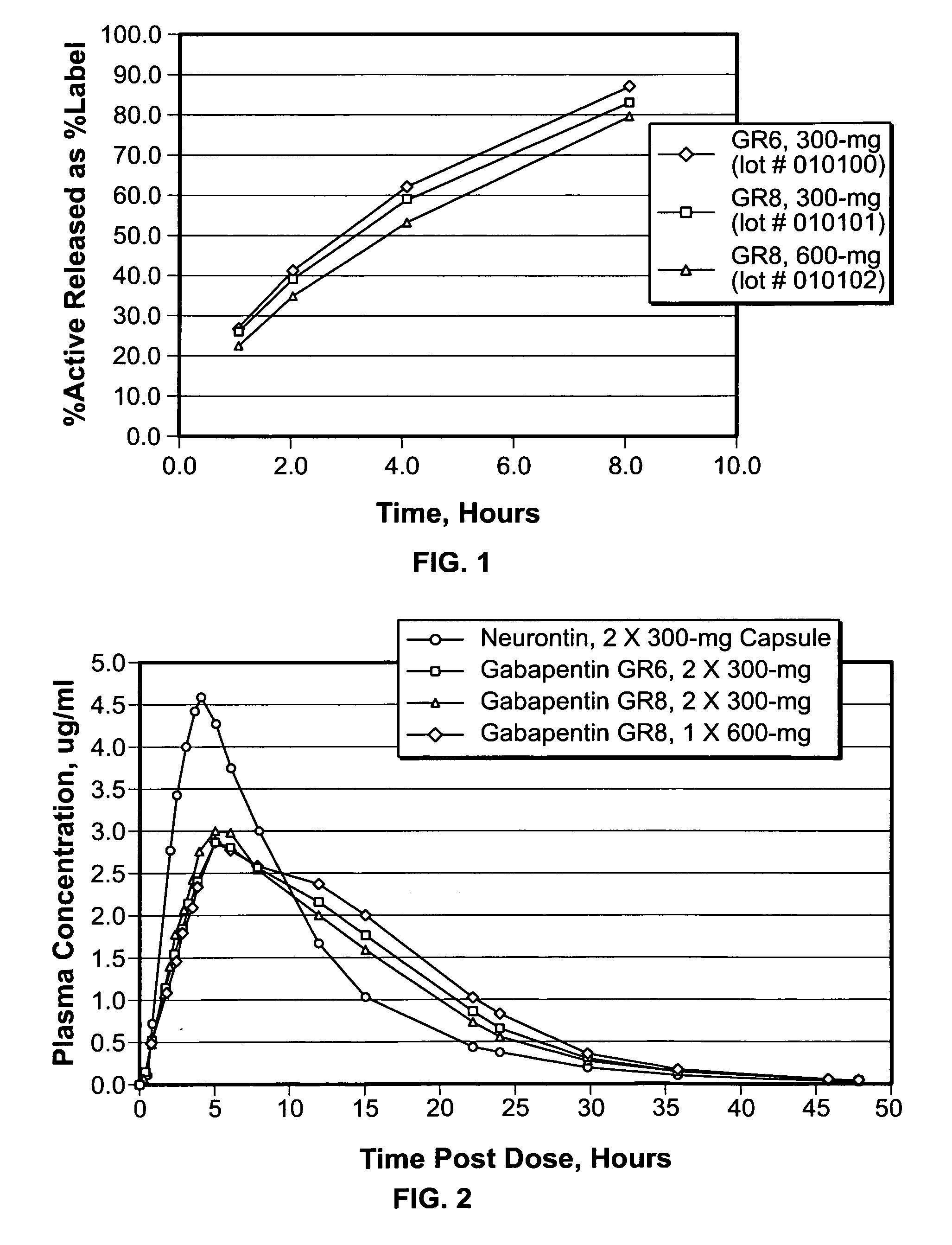
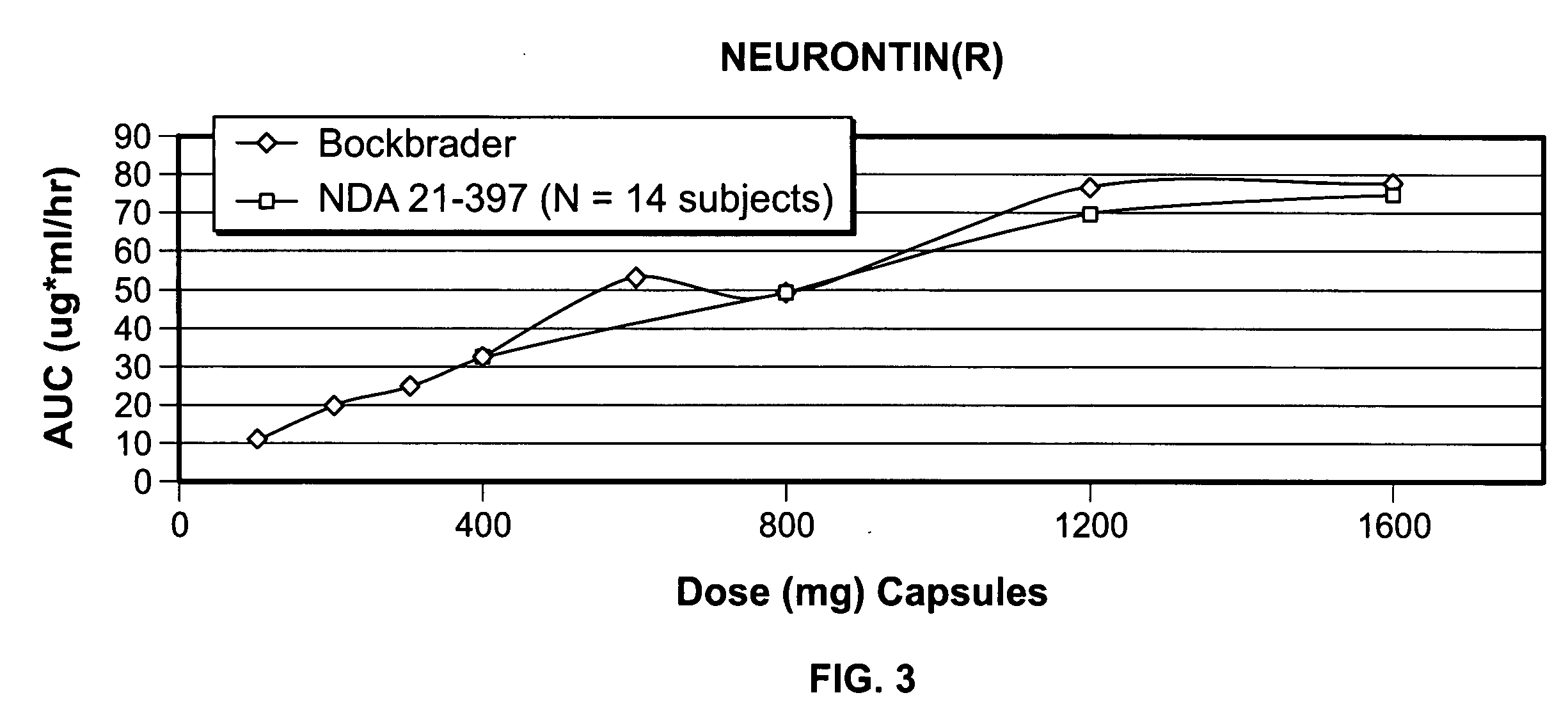
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Pain relief lollipop compositions and methods
InactiveUS20090191257A1Without pain and invasivenessFast pain reliefOrganic active ingredientsNervous disorderGabapentinOpioid Agonist
A pain relief lollipop comprises a candy matrix comprising (a) an opioid agonist, (b) an N-methyl-D-aspartate receptor antagonist different from the opioid agonist, (c) gabapentin, or a pharmaceutically acceptable salt thereof, and, optionally, a muscle relaxant, sedative, anxiolytic, and / or antidepressant. A patient can self-administer small amounts of the pain relief drug as needed by simply licking or sucking on the lollipop in response to his subjective experience of pain.
Owner:INNOVATIVE PHARMA
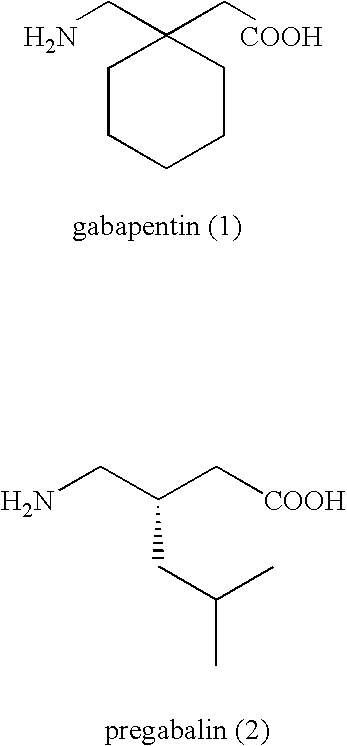
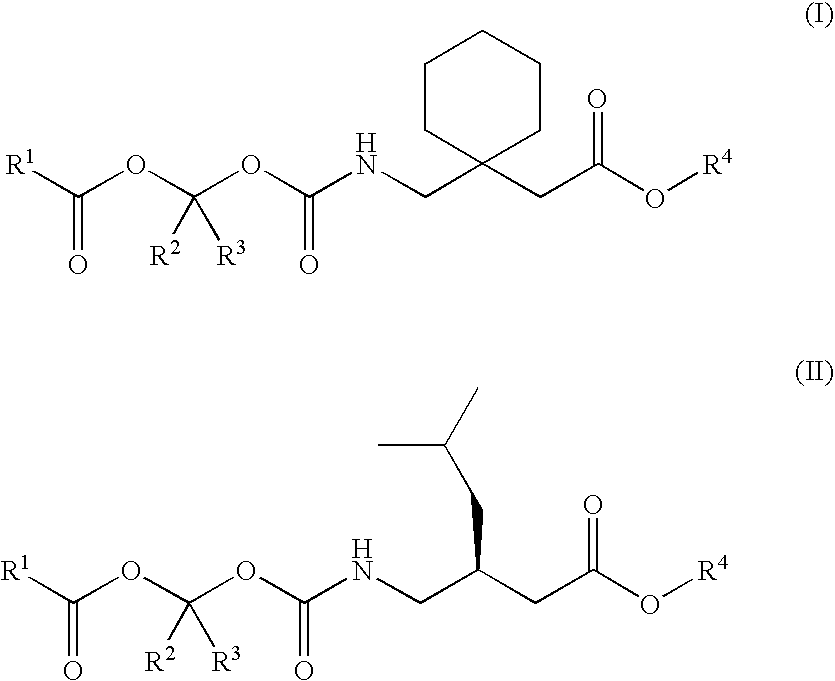
Methods for synthesis of prodrugs from 1-acyl-alkyl derivatives and compositions thereof
Disclosed is a method for synthesizing 1-(acyloxy)-alkyl derivatives of primary or secondary amine drugs from 1-acyl-alkyl derivatives of primary or secondary amine drugs, which typically proceeds stereospecifically, in high yield, does not require the use of activated intermediates and / or toxic compounds and is readily amenable to scale-up. For example, 1-acyl-alkyl derivatives of gabapentin and pregabalin are oxidized to yield 1-(acyloxy)-alkyl derivatives of gabapentin and pregabalin, respectively.
Owner:XENOPORT
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Sodium channel blocker compositions and the use thereof
Methods of treating or preventing chronic pain or convulsion are disclosed by administering to an animal a sodium channel blocker and at least one of gabapentin and pregabalin. Also disclosed are pharmaceutical compositions and kits for the treatment or prevention of chronic pain or convulsion.
Owner:EURO-CELTIQUE SA
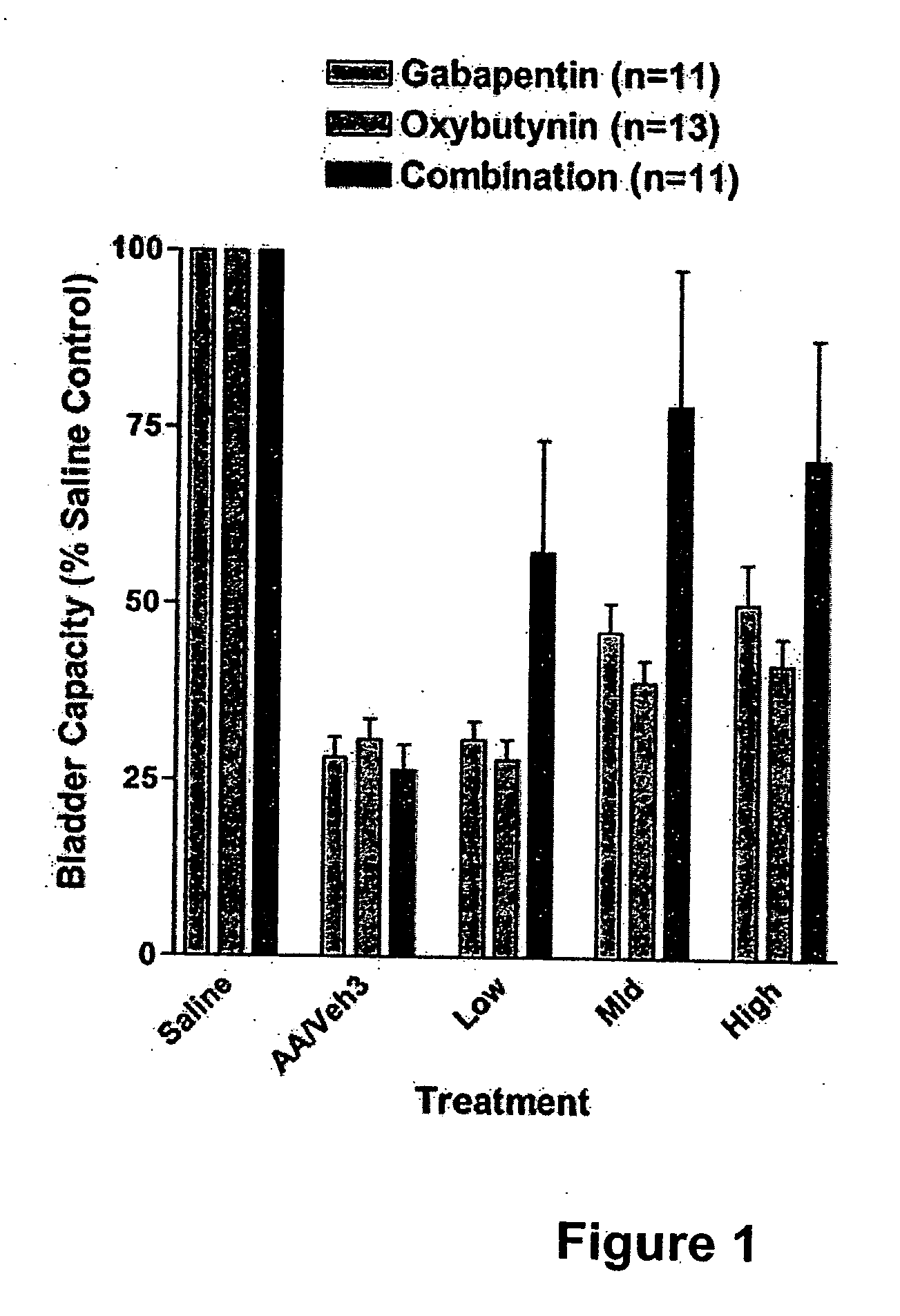
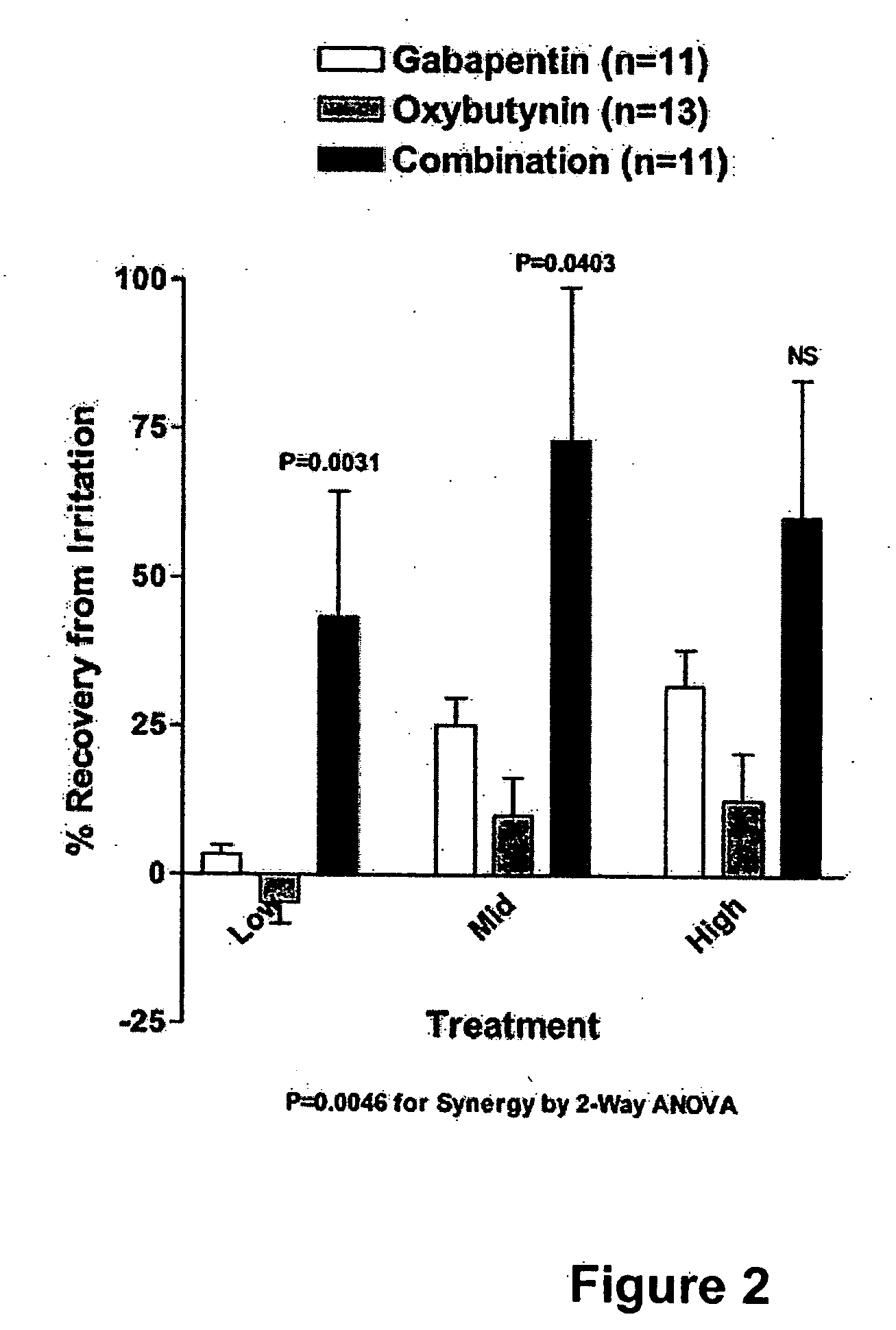
Methods for treating pain using smooth muscle modulators and a2 subunit calcium channel modulators
InactiveUS20060264509A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsGabapentinAdrenergic receptor agonists
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat pain. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g., gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Sodium Channel Blocker Compositions and the Use Thereof
Methods of treating or preventing chronic pain or convulsion are disclosed by administering to an animal a sodium channel blocker and at least one of gabapentin and pregabalin. Also disclosed are pharmaceutical compositions and kits for the treatment or prevention of chronic pain or convulsion.
Owner:PURDUE PHARMA LP
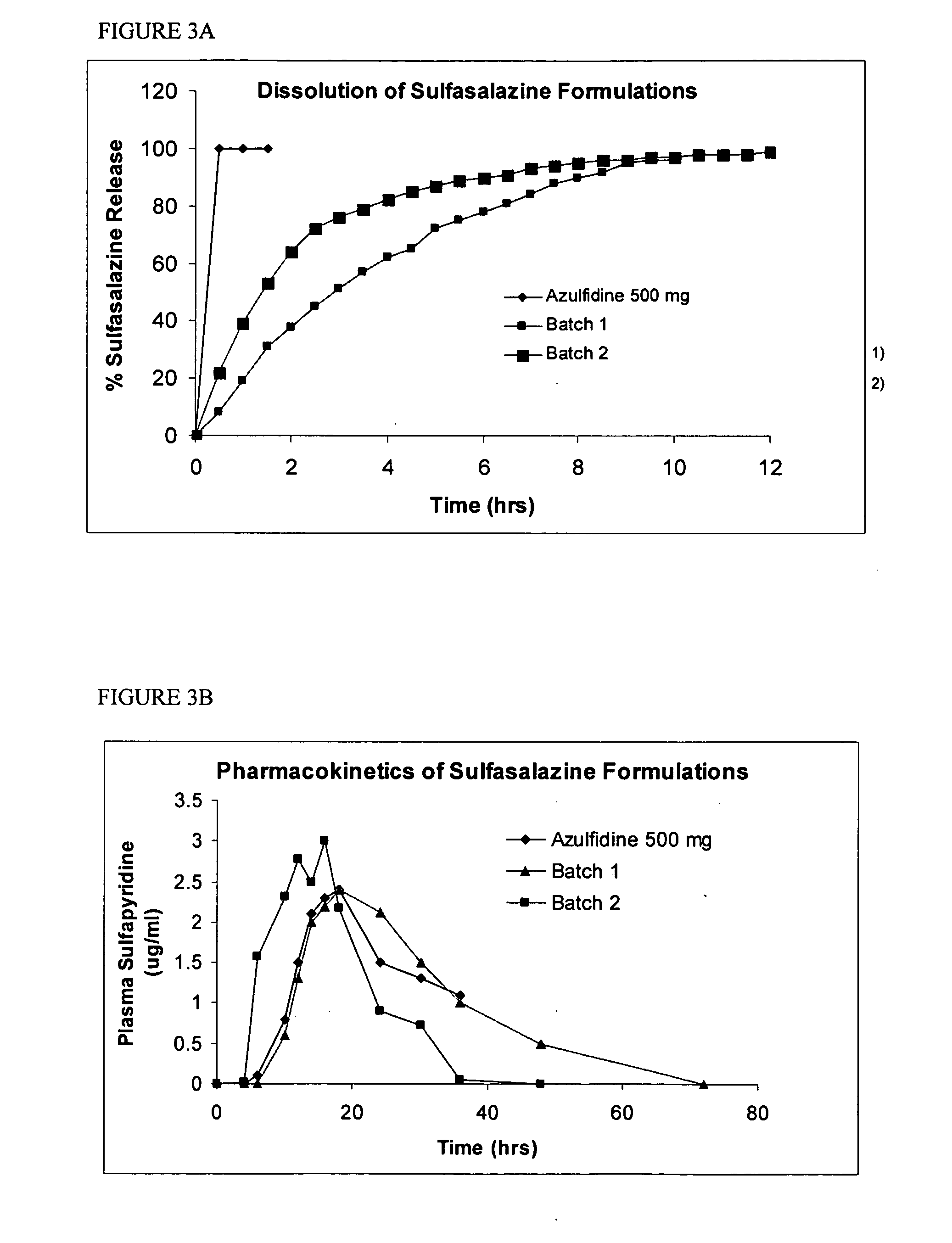
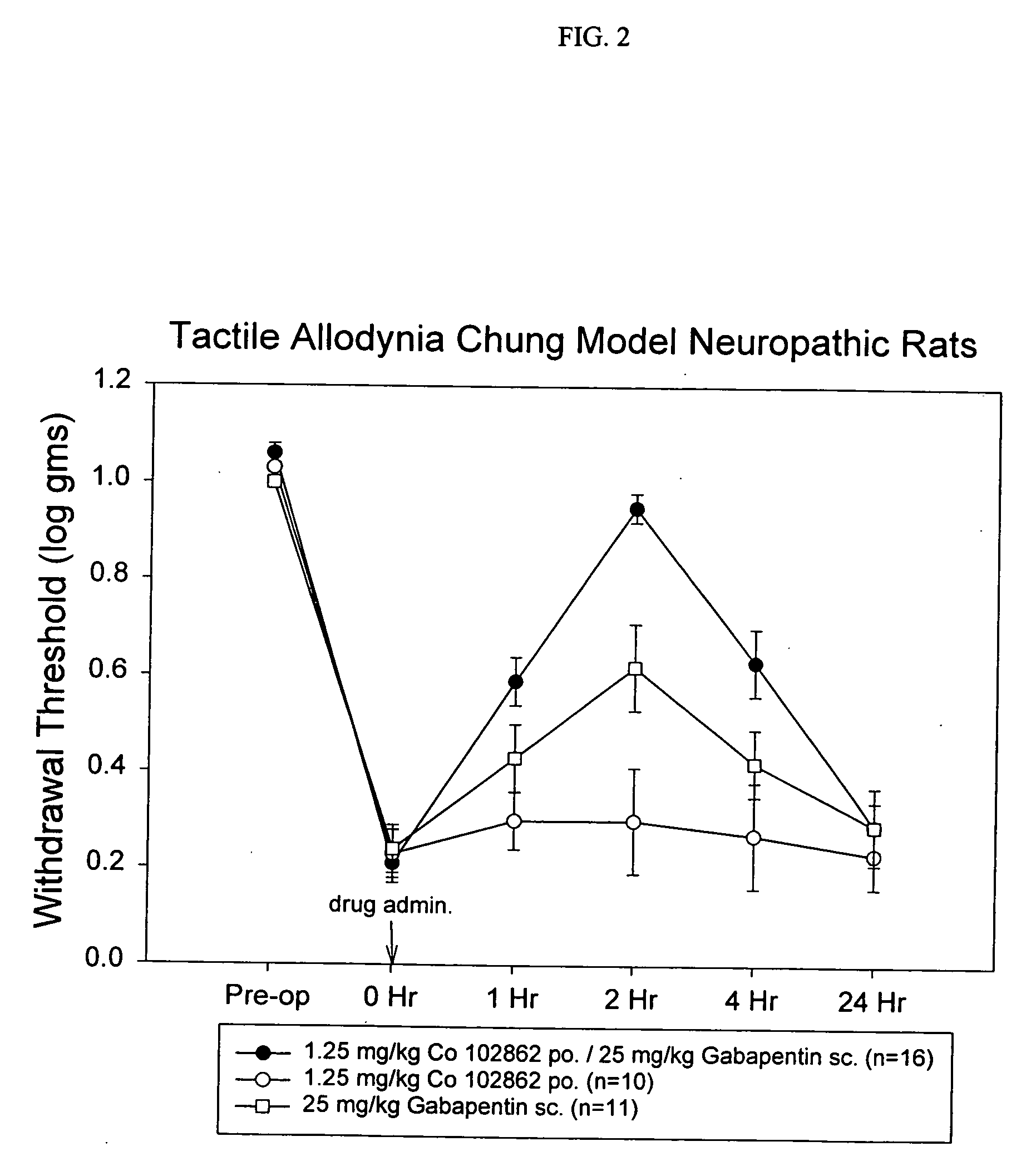
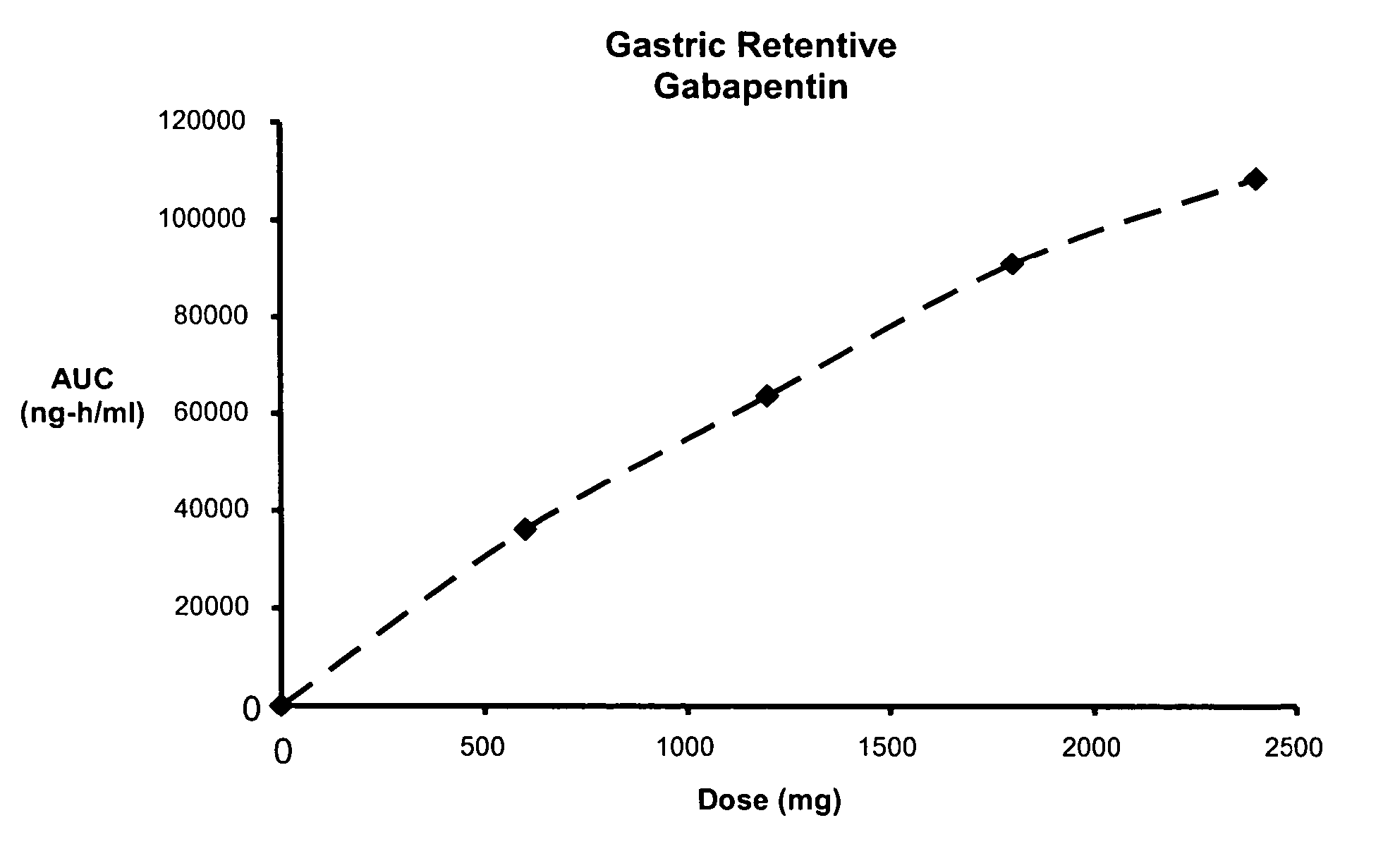
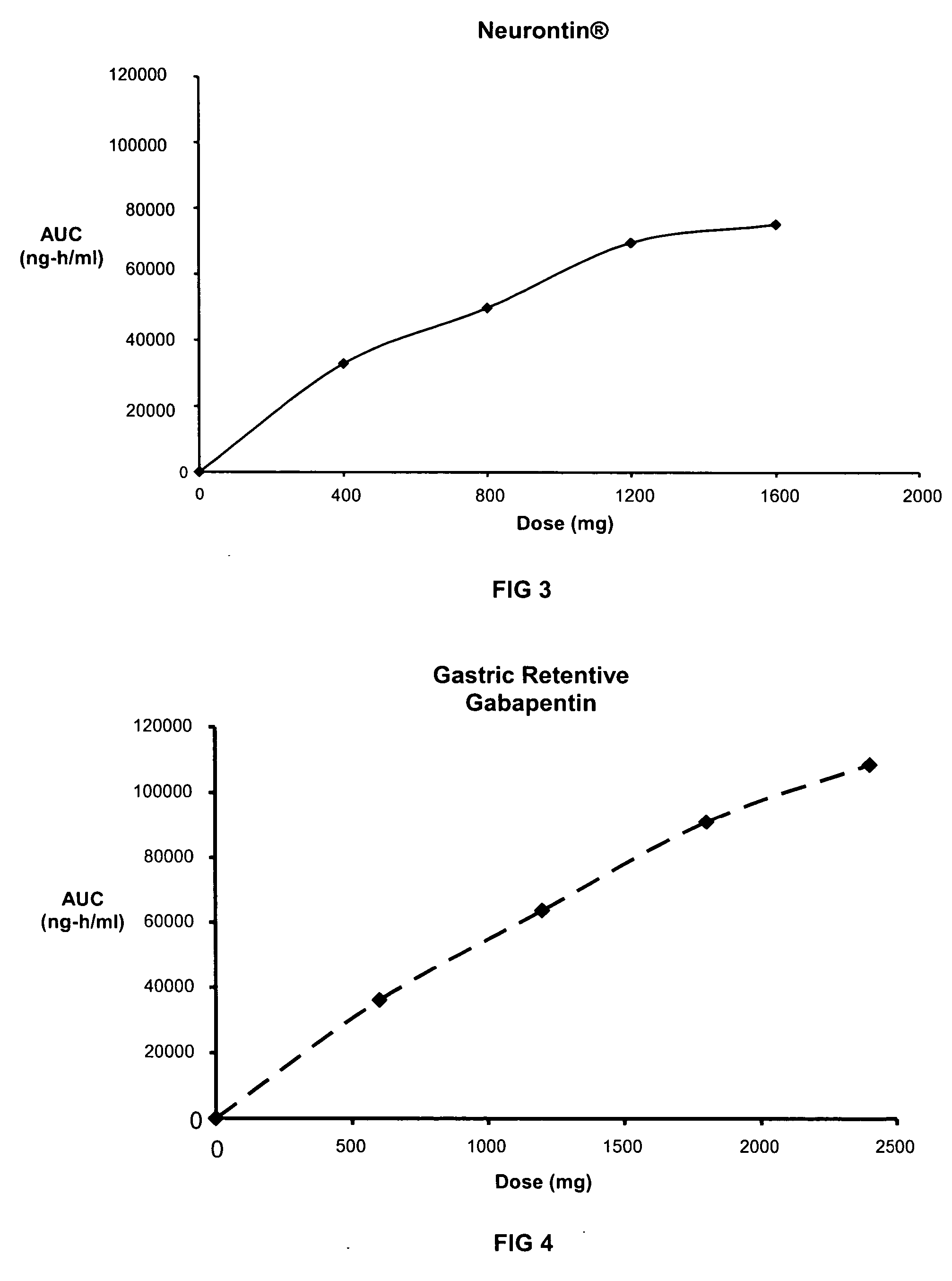
Methods of treating non-nociceptive pain states with gastric retentive gabapentin
InactiveUS20060159743A1Bioavailability (AUC)Improve complianceBiocideOrganic active ingredientsGabapentinDosing regimen
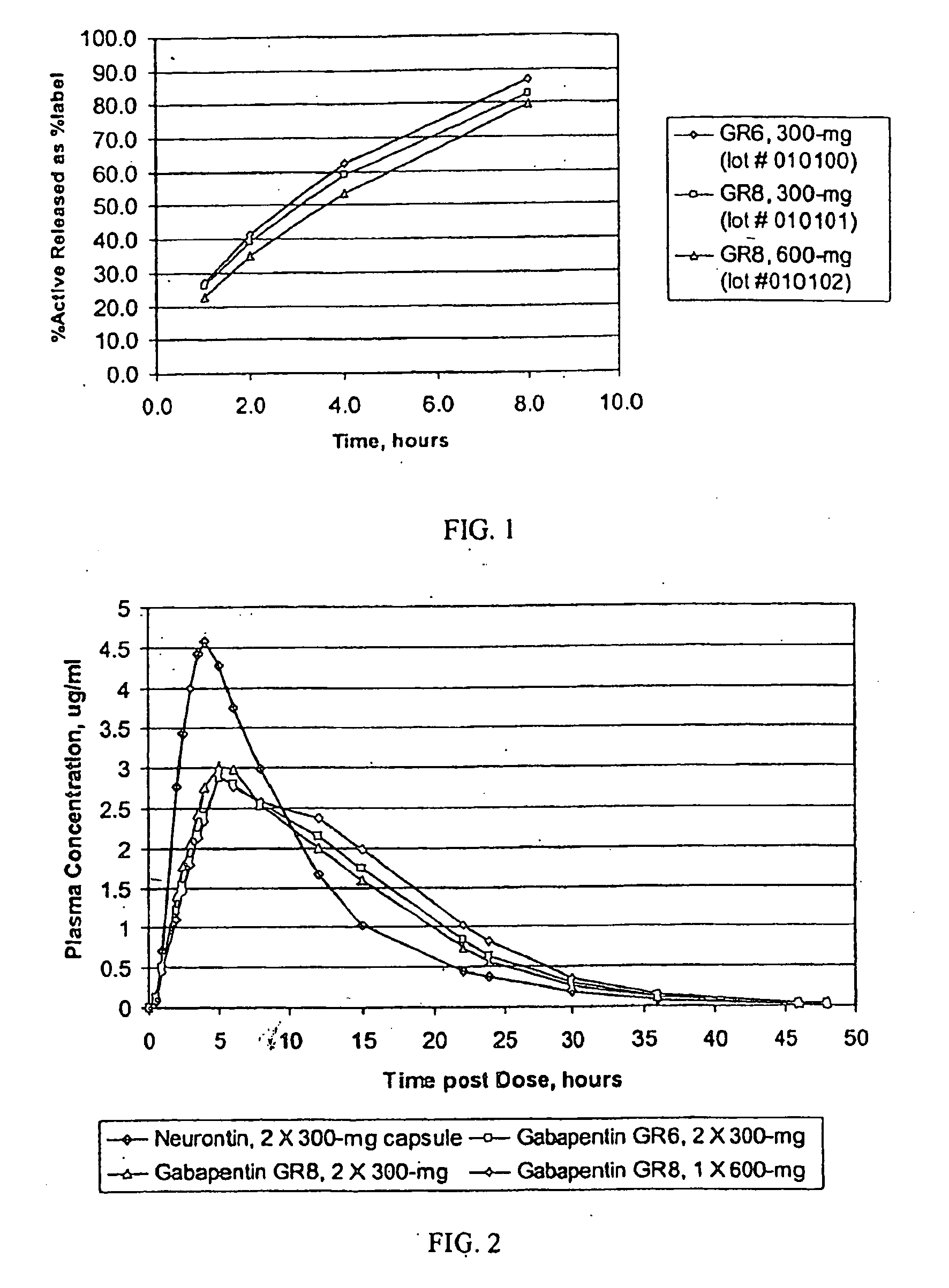
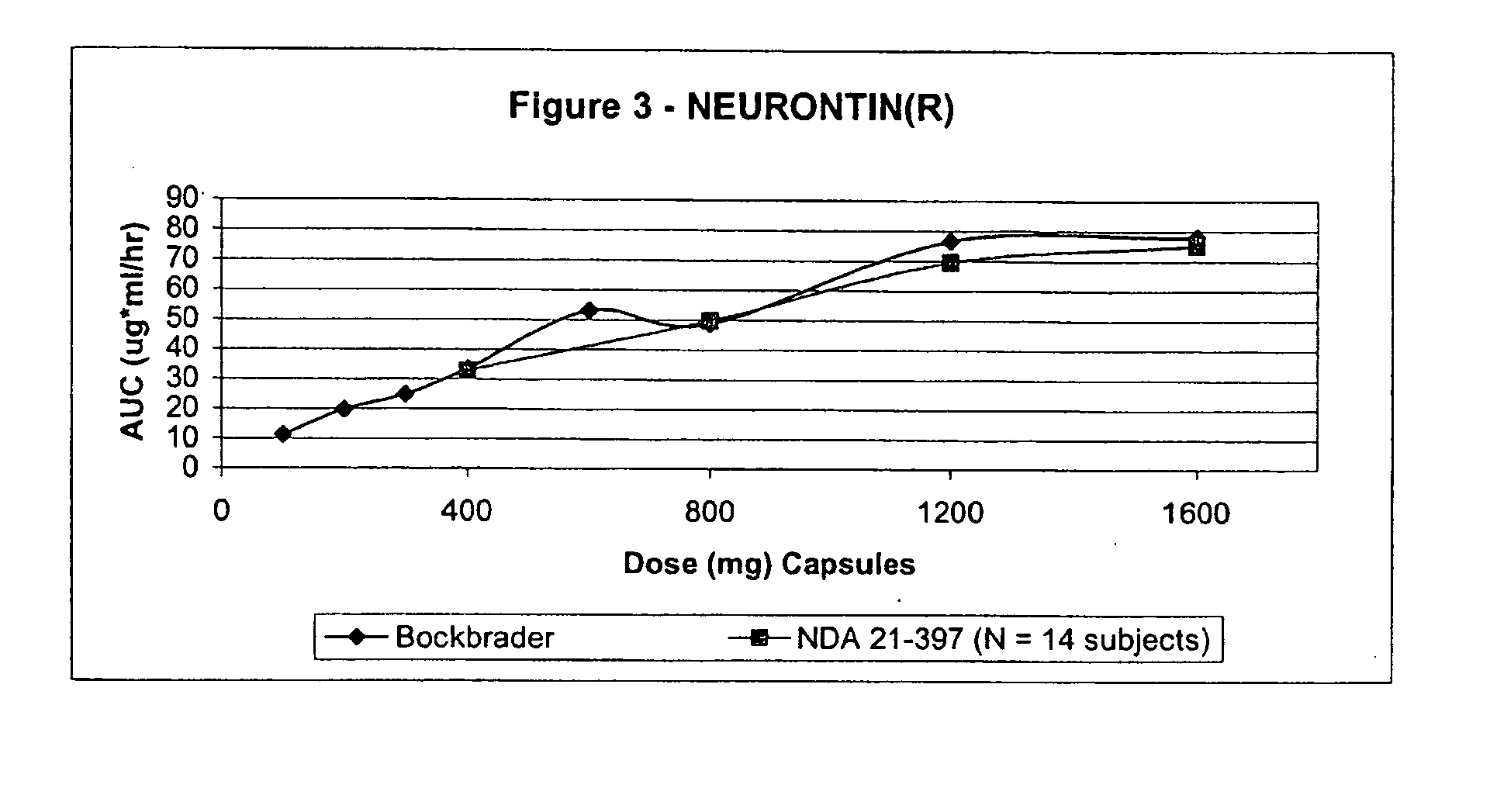
Provided is a method of treating a patient suffering from a pain state by administering to the patient a gastric retentive dosage form of gabapentin that is capable of administration in once-daily or twice daily dosing regimens. By reducing the need to administer gabapentin from the thrice-daily administrations characteristic of immediate release gabapentin, the gastric retentive gabapentin dosage forms provided herein have the advantages of improving patient compliance for gabapentin treatment. In addition to the foregoing, the gastric retentive gabapentin dosages forms also exhibit decreased blood plasma concentrations and increased bioavailability throughout the dosing regimen.
Owner:DEPOMED SYST INC
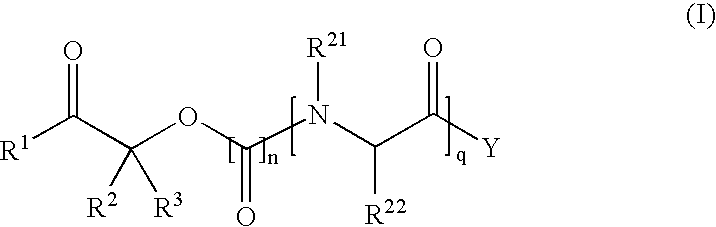
Gabapentin tablets and method for their preparation
InactiveUS20060039968A1Treat painOrganic active ingredientsPeptide/protein ingredientsGabapentinDry mixing
The present invention is generally directed to methods for preparing stable gabapentin tablets by wet granulation. A wet granulation method for preparing gabapentin tablets includes forming a mixture by dry mixing of a first portion of a binder with the gabapentin, one or more excipients, or a combination of the gabapentin and the one or more excipients; and adding a second portion of the binder to the mixture, wherein the second portion of the binder is in the form of a solution or dispersion.
Owner:RANBAXY LAB LTD
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
Gastric retentive gabapentin dosage forms and methods for using same
InactiveUS20070184104A1Good curative effectReduce morbidityBiocidePeptide/protein ingredientsDosing regimenGabapentin
Provided is a method of treating a patient suffering from a pain state by administering to the patient a gastric retentive dosage form of gabapentin that is capable of administration in once-daily or twice daily dosing regimens. By reducing the need to administer gabapentin from the thrice-daily administrations characteristic of immediate release gabapentin, the gastric retentive gabapentin dosage forms provided herein have the advantages of improving patient compliance for gabapentin treatment. In addition to the foregoing, the gastric retentive gabapentin dosages forms also exhibit decreased blood plasma concentrations and increased bioavailability throughout the dosing regimen.
Owner:DEPOMED SYST INC
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
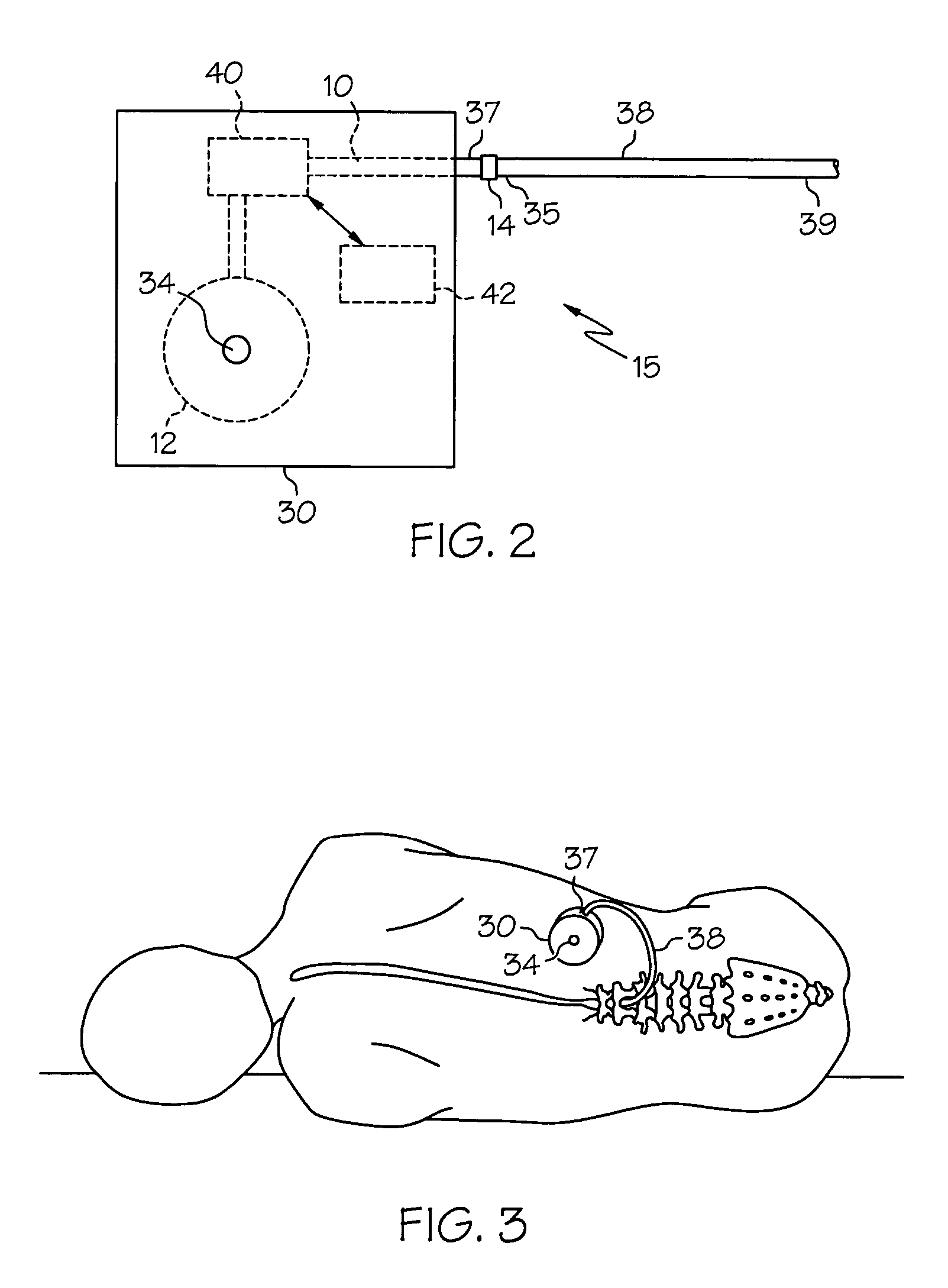
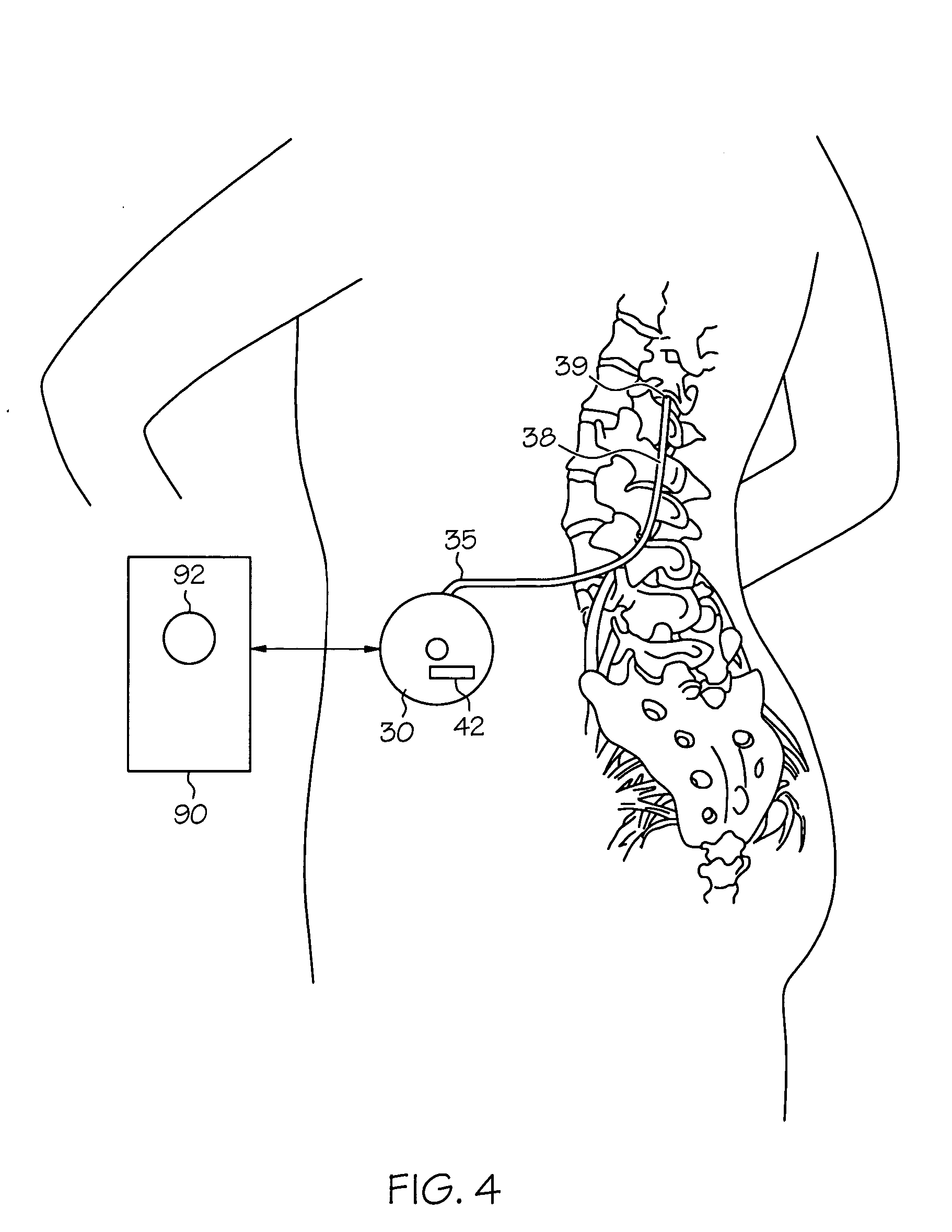
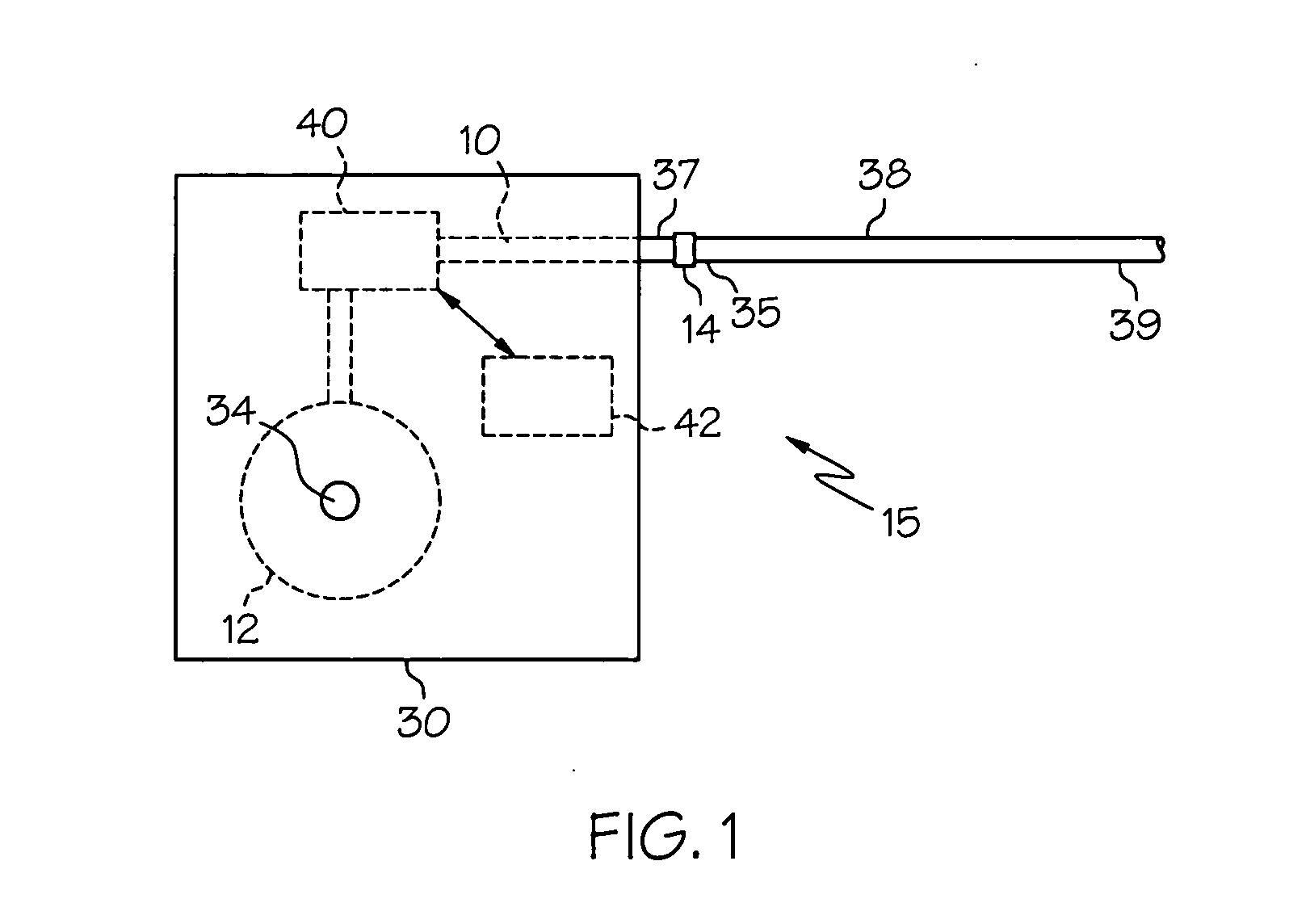
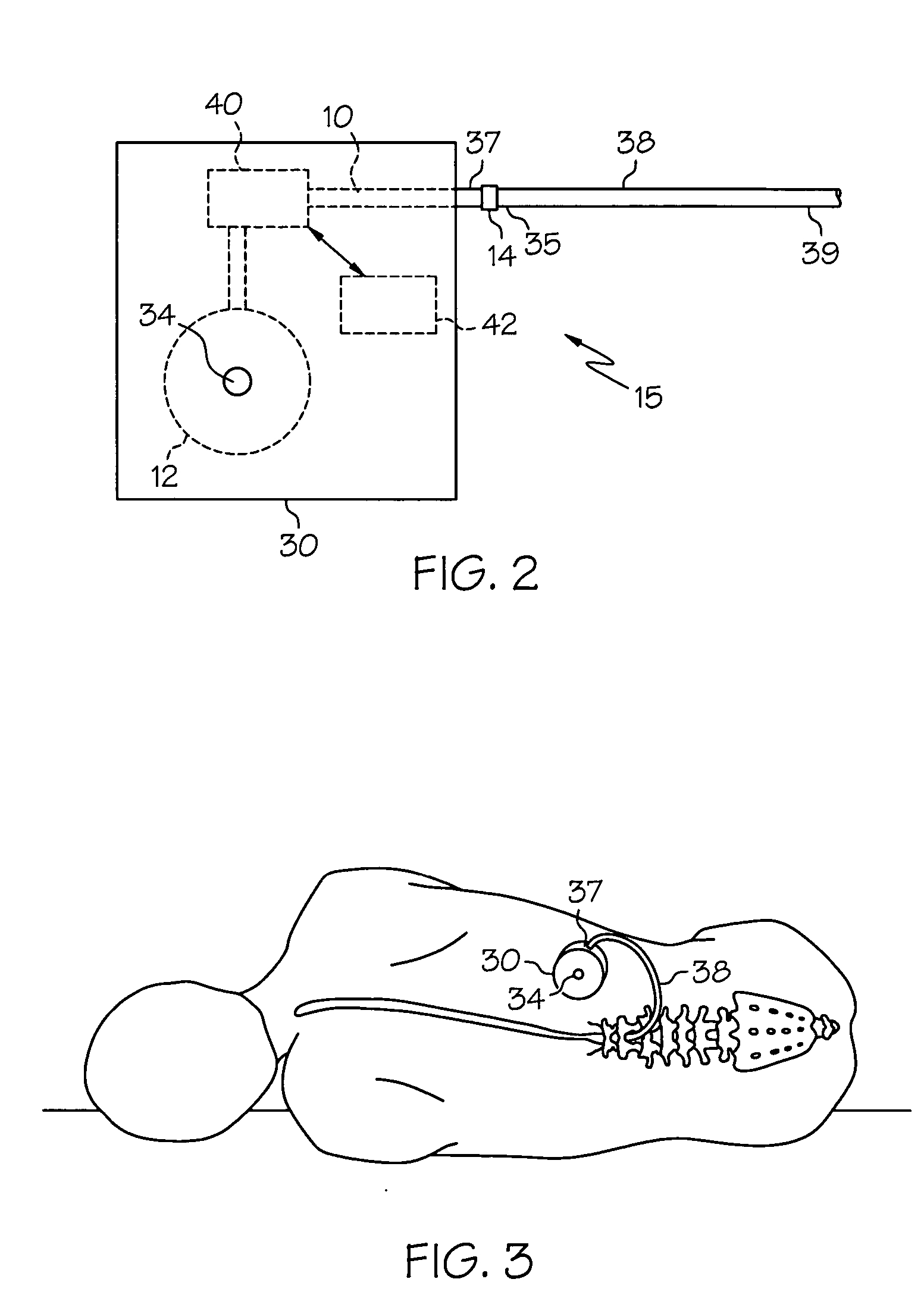
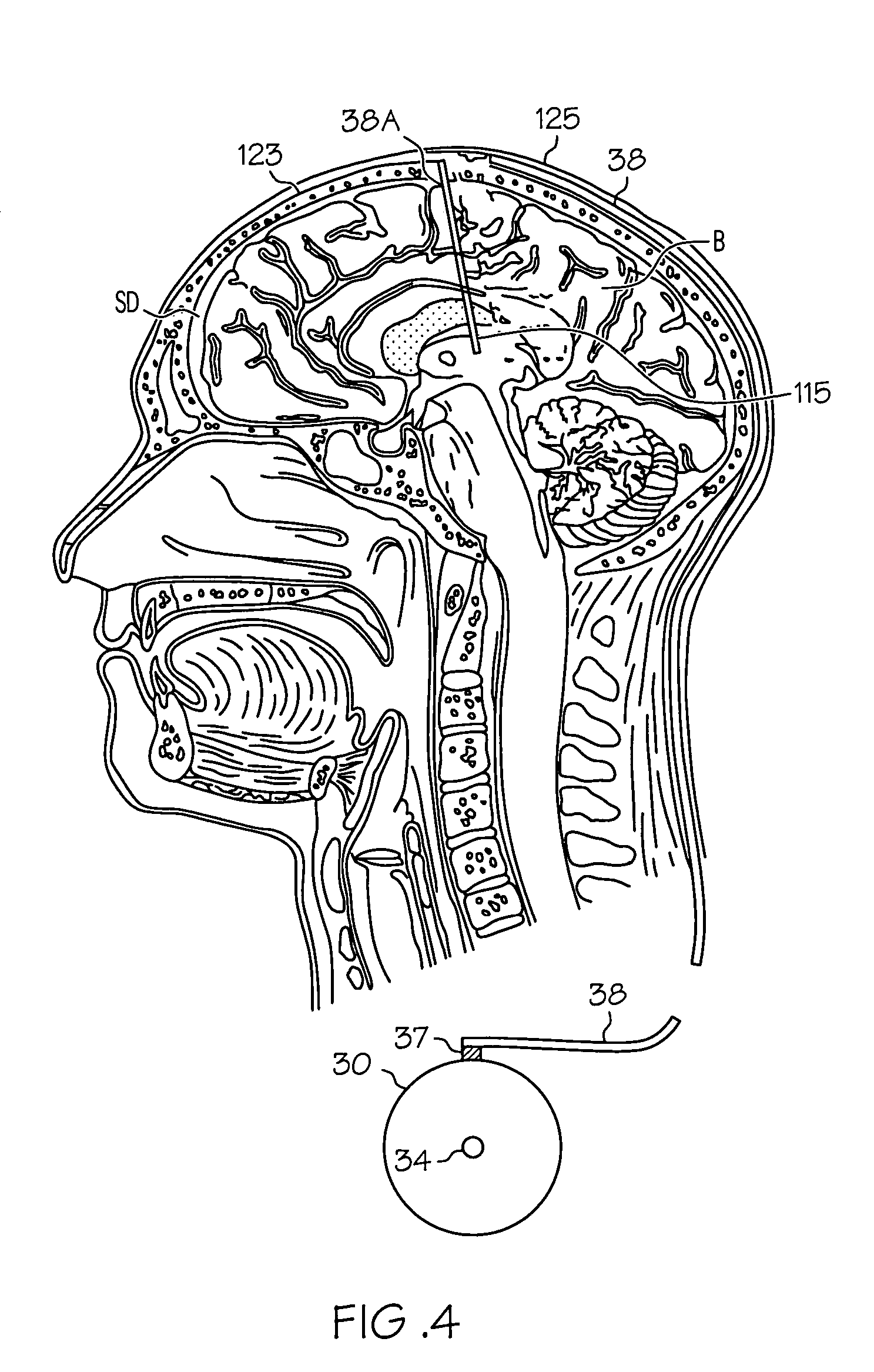
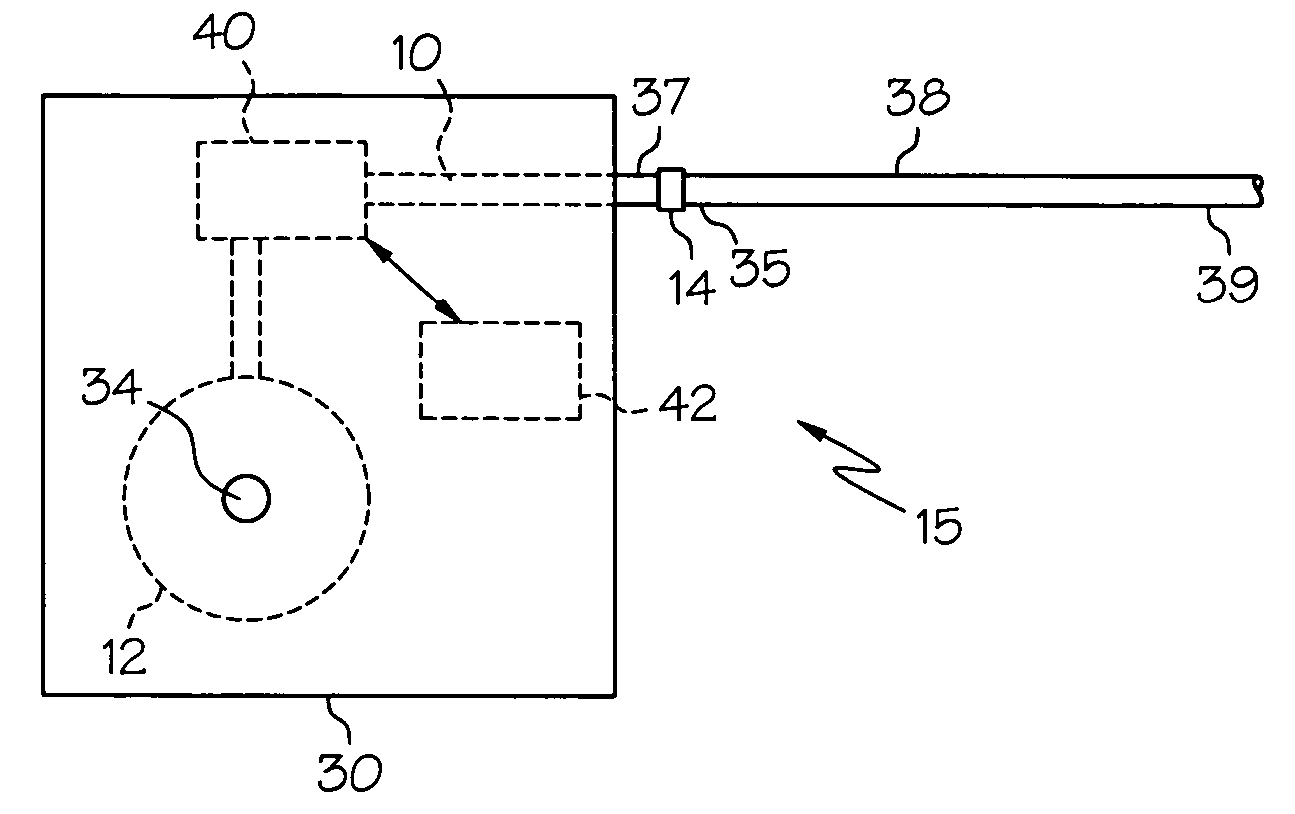
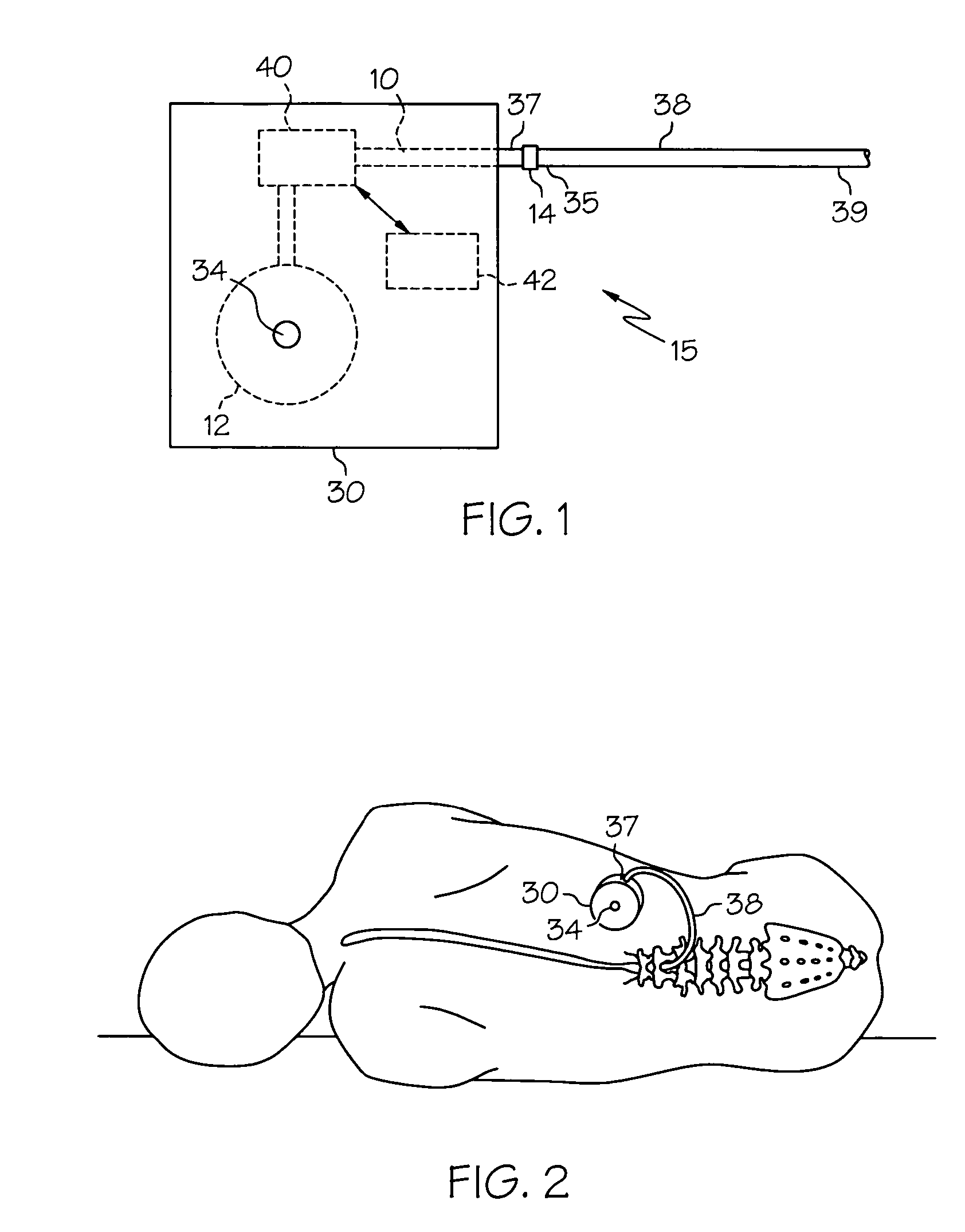
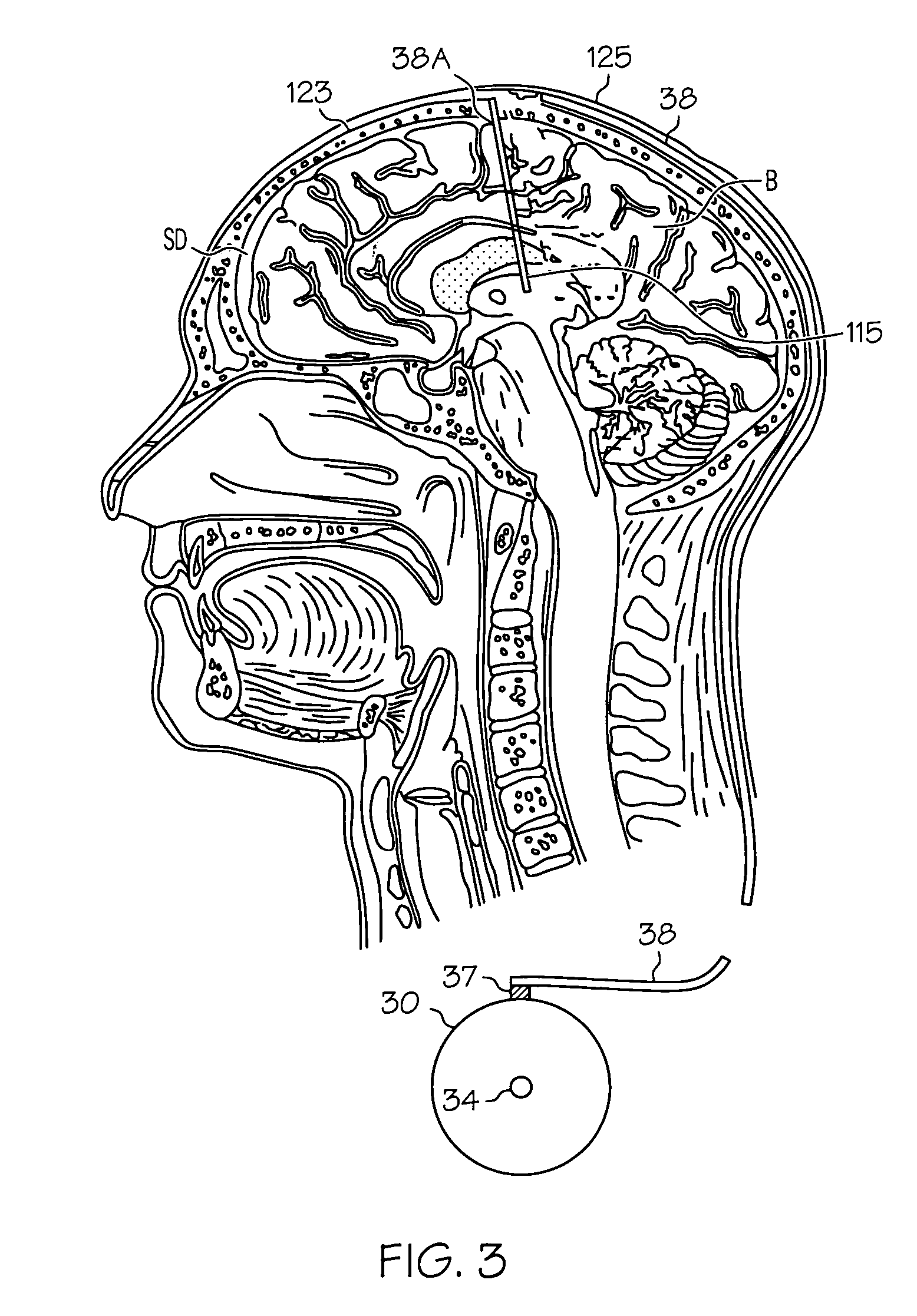
Intrathecal gabapentin for treatment of pain
InactiveUS20050090549A1Easy to controlGood curative effectBiocideOrganic active ingredientsGabapentinDistal portion
Methods for treating pain by administering gabapentin to cerebrospinal fluid of a patient are discussed. Compositions, particularly injectable compositions, containing gabapentin are also discussed. In addition, systems including an implantable pump having a reservoir for housing a composition, a catheter having a proximal portion coupled to the pump and having a distal portion adapted for administrating a composition to a cerebrospinal fluid of a patient, and a composition containing gabapentin, which composition is housed in the reservoir of the pump, is also discussed.
Owner:MEDTRONIC INC
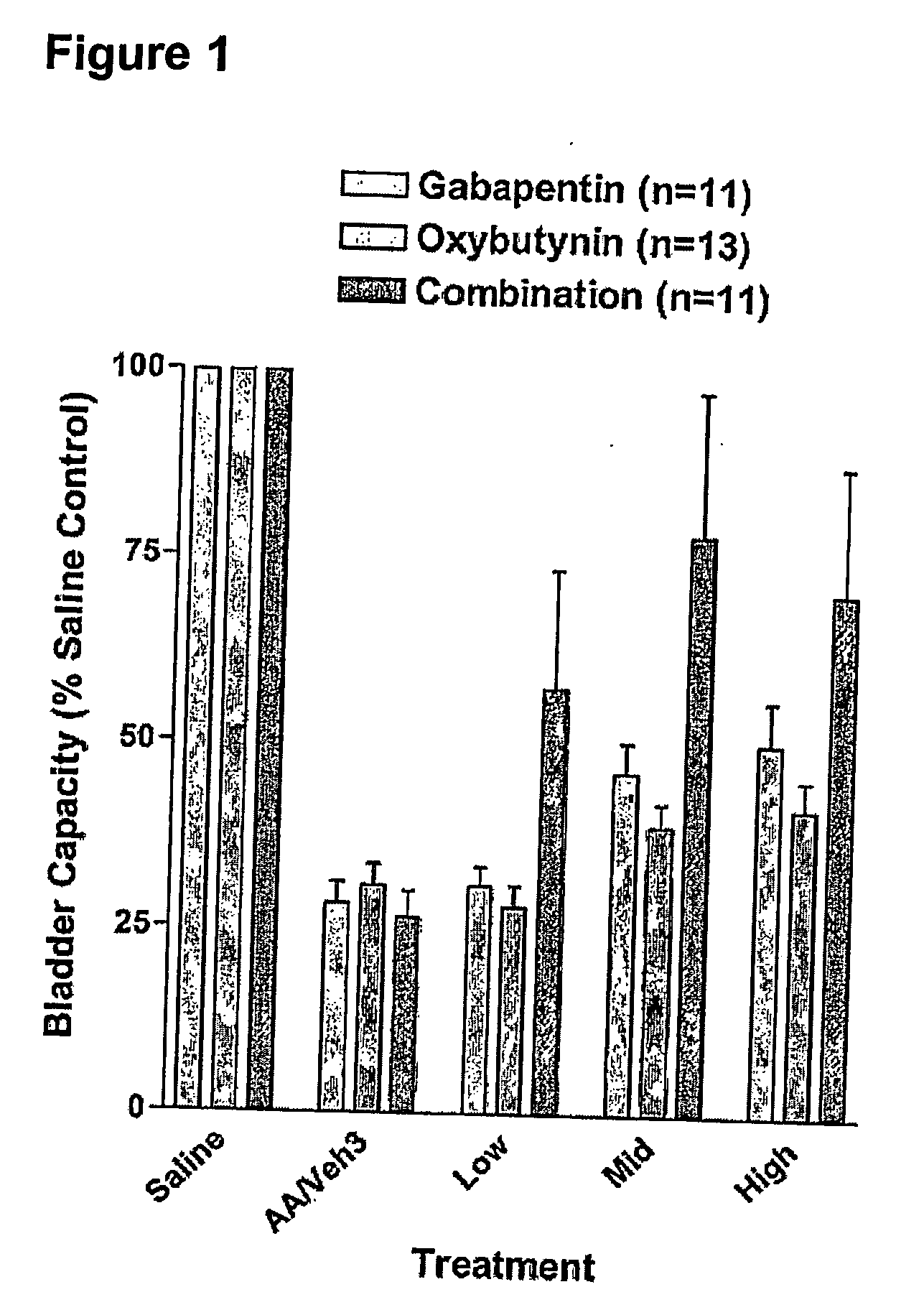
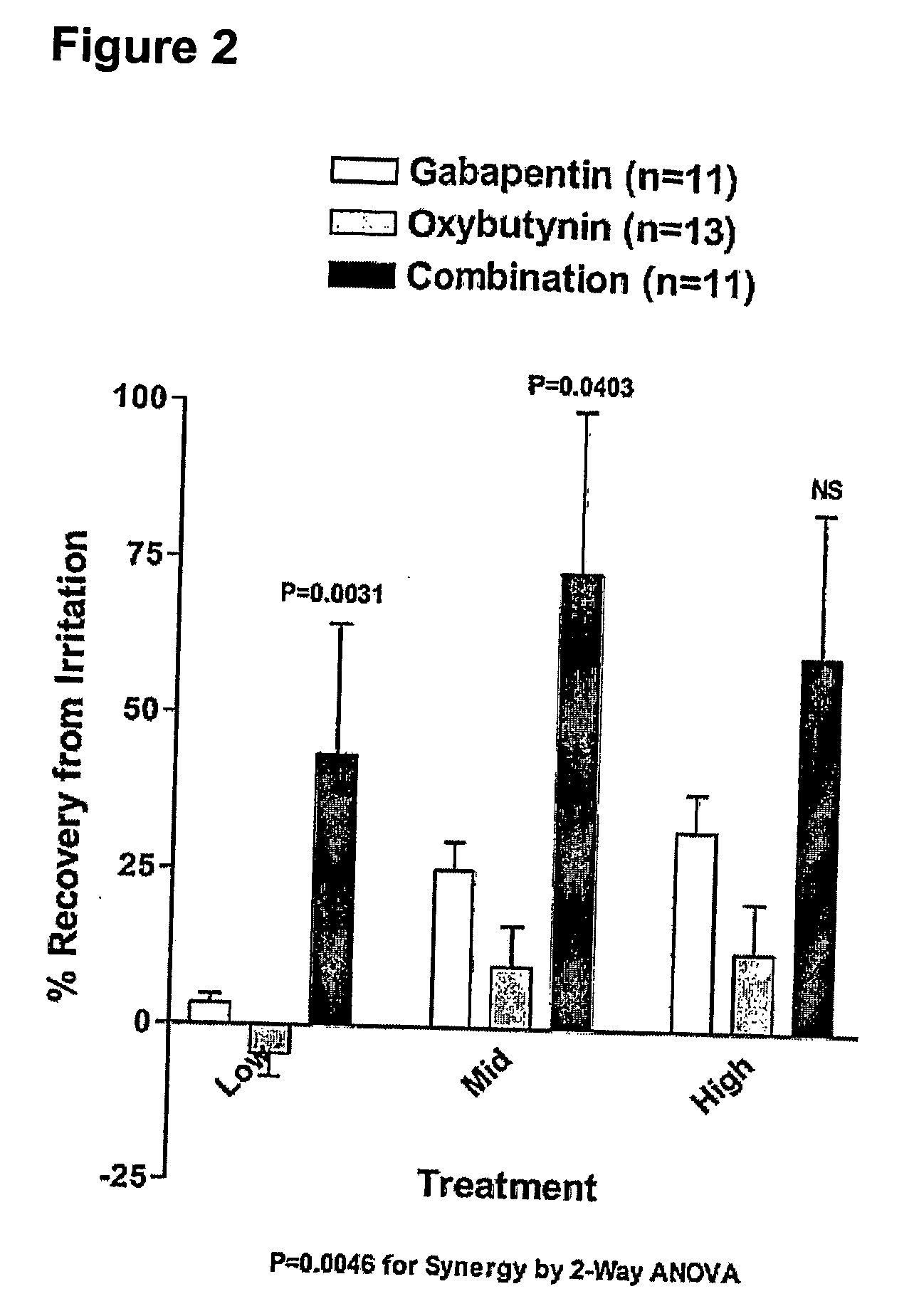
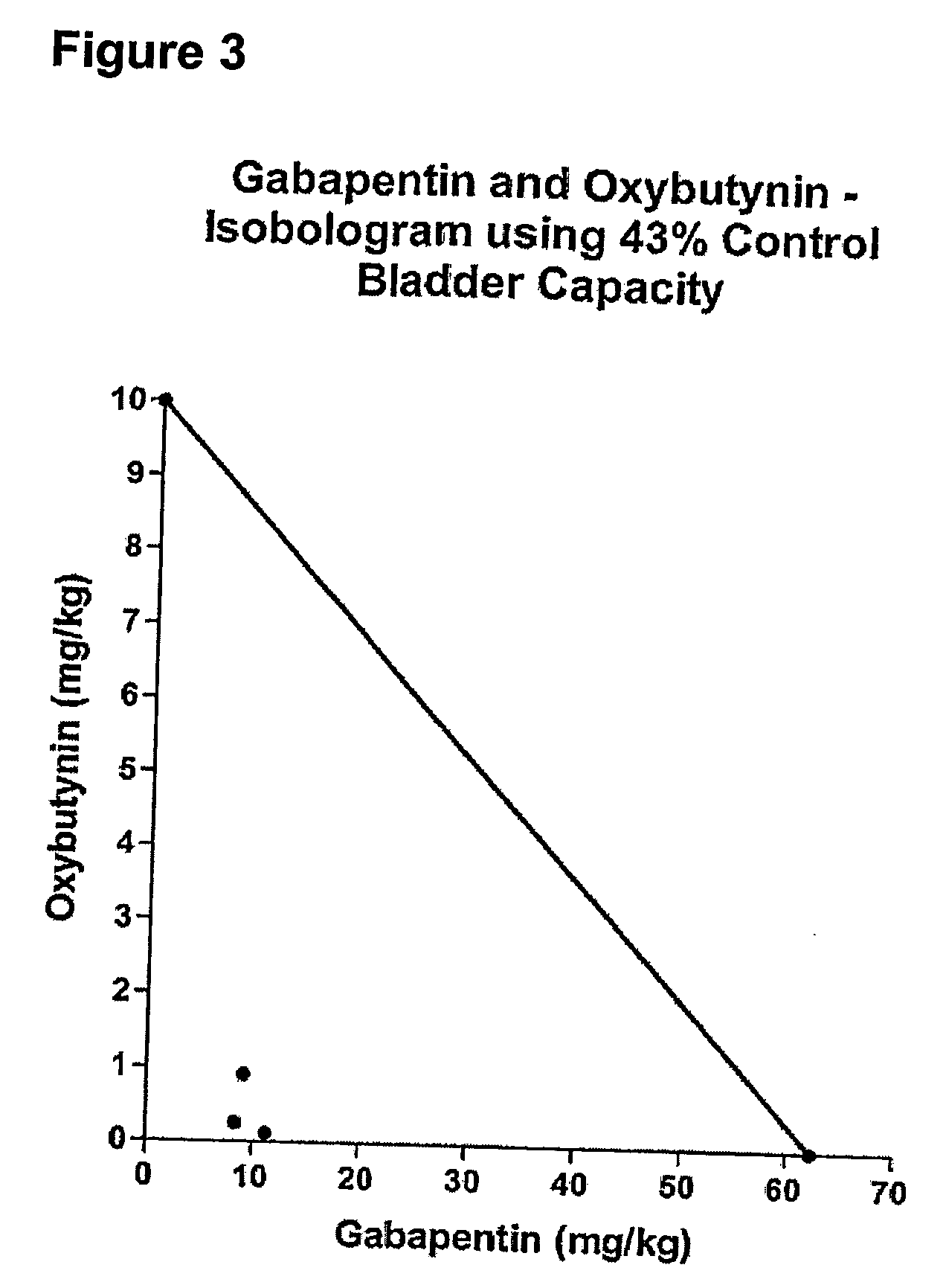
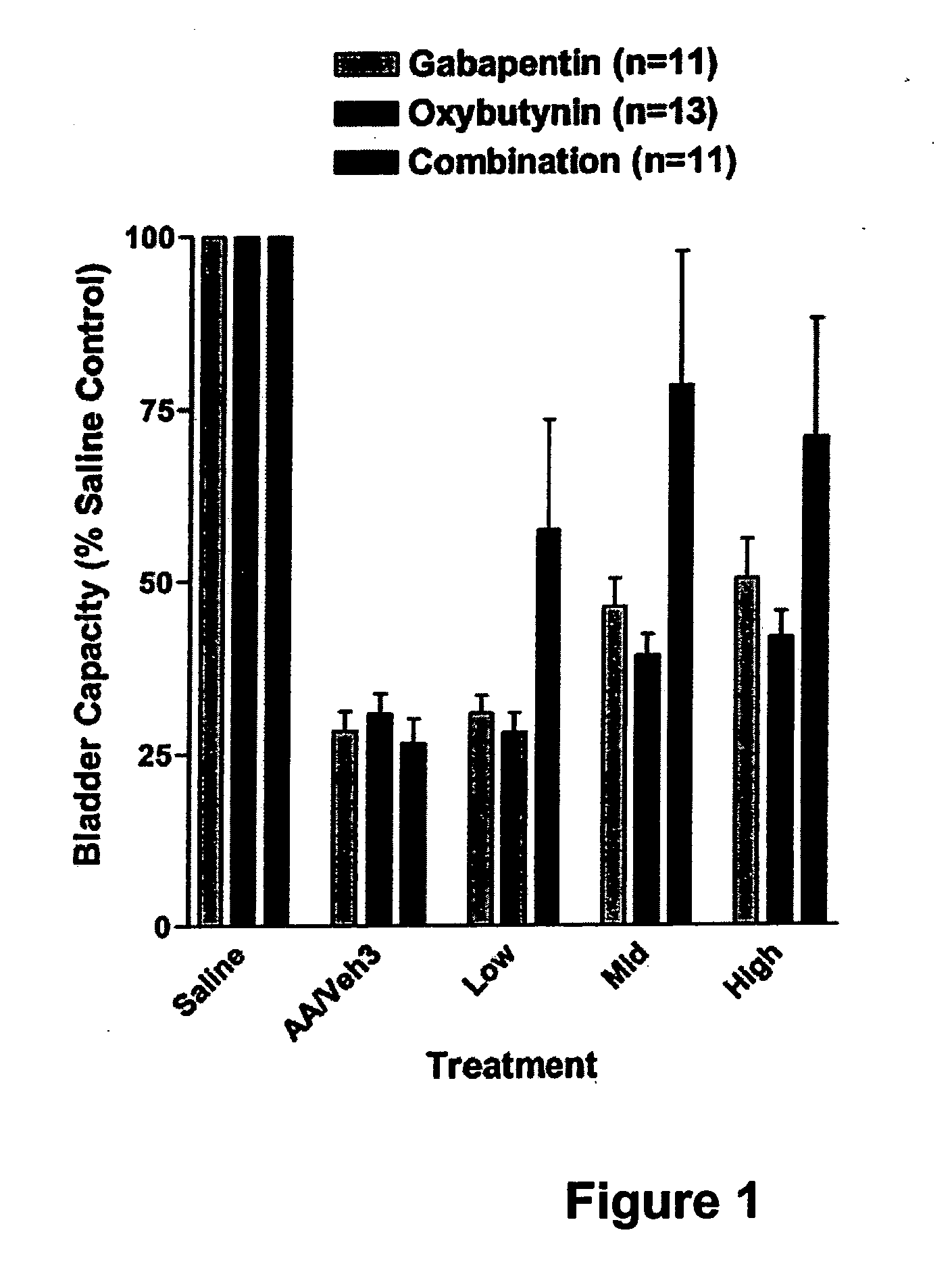
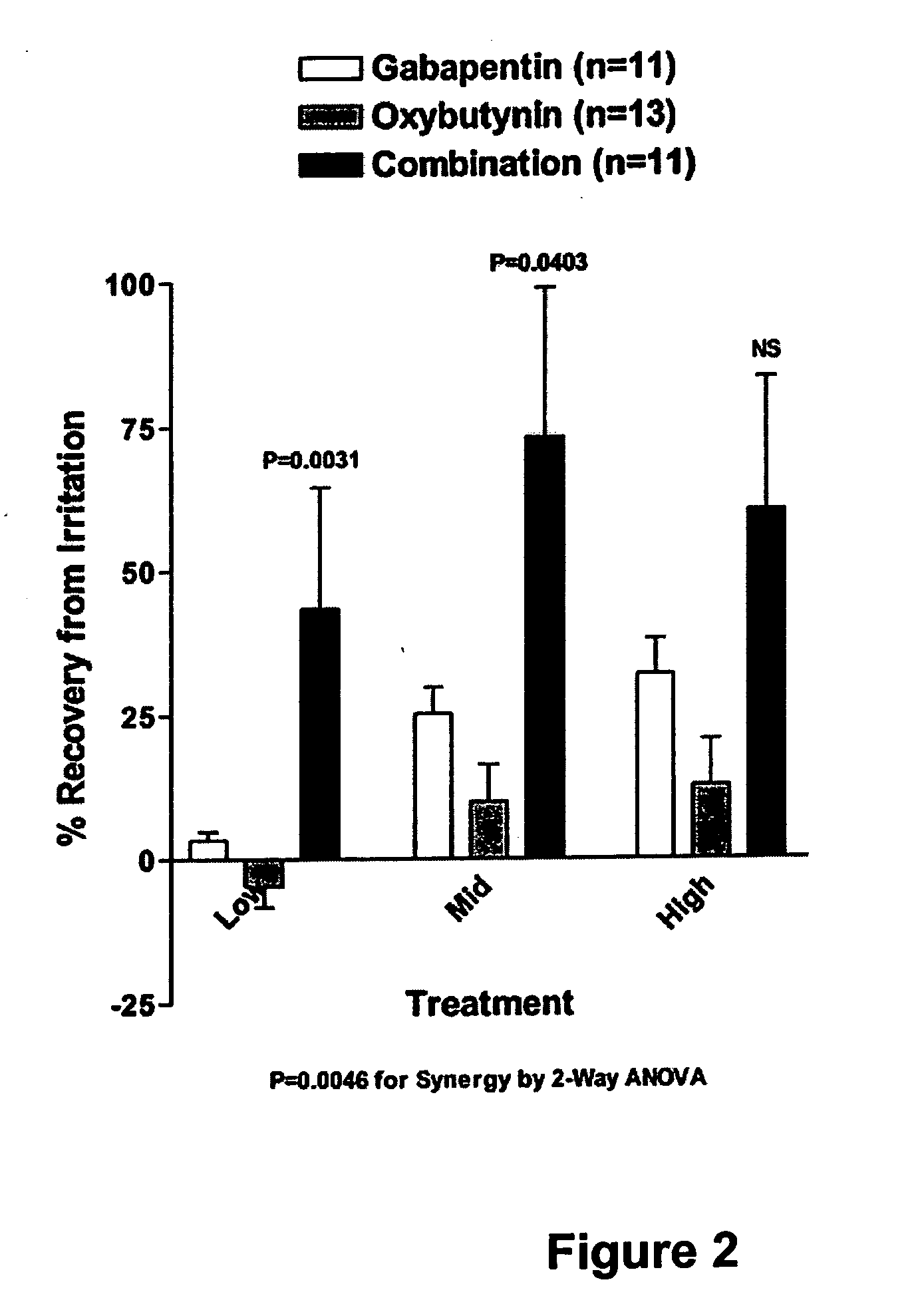
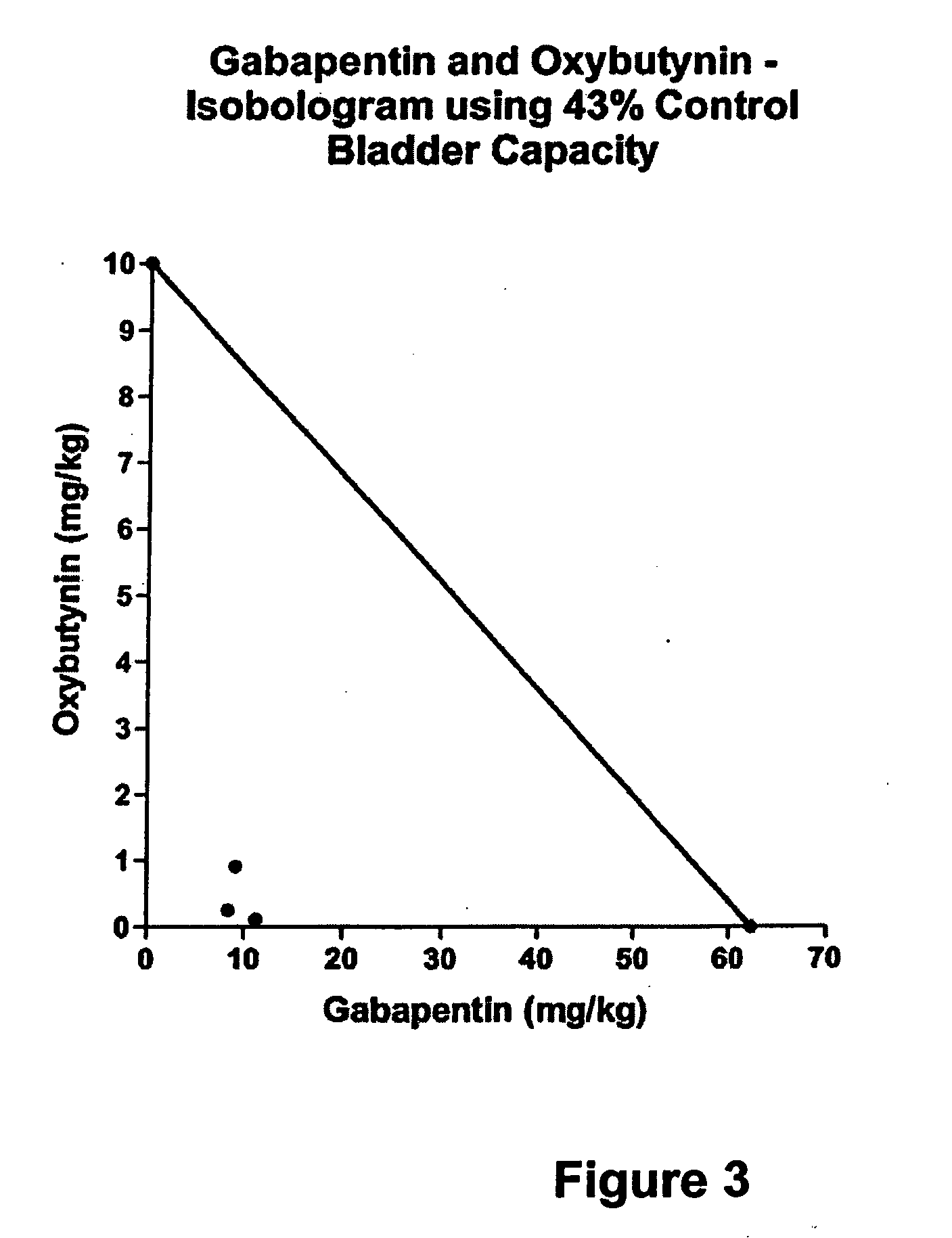
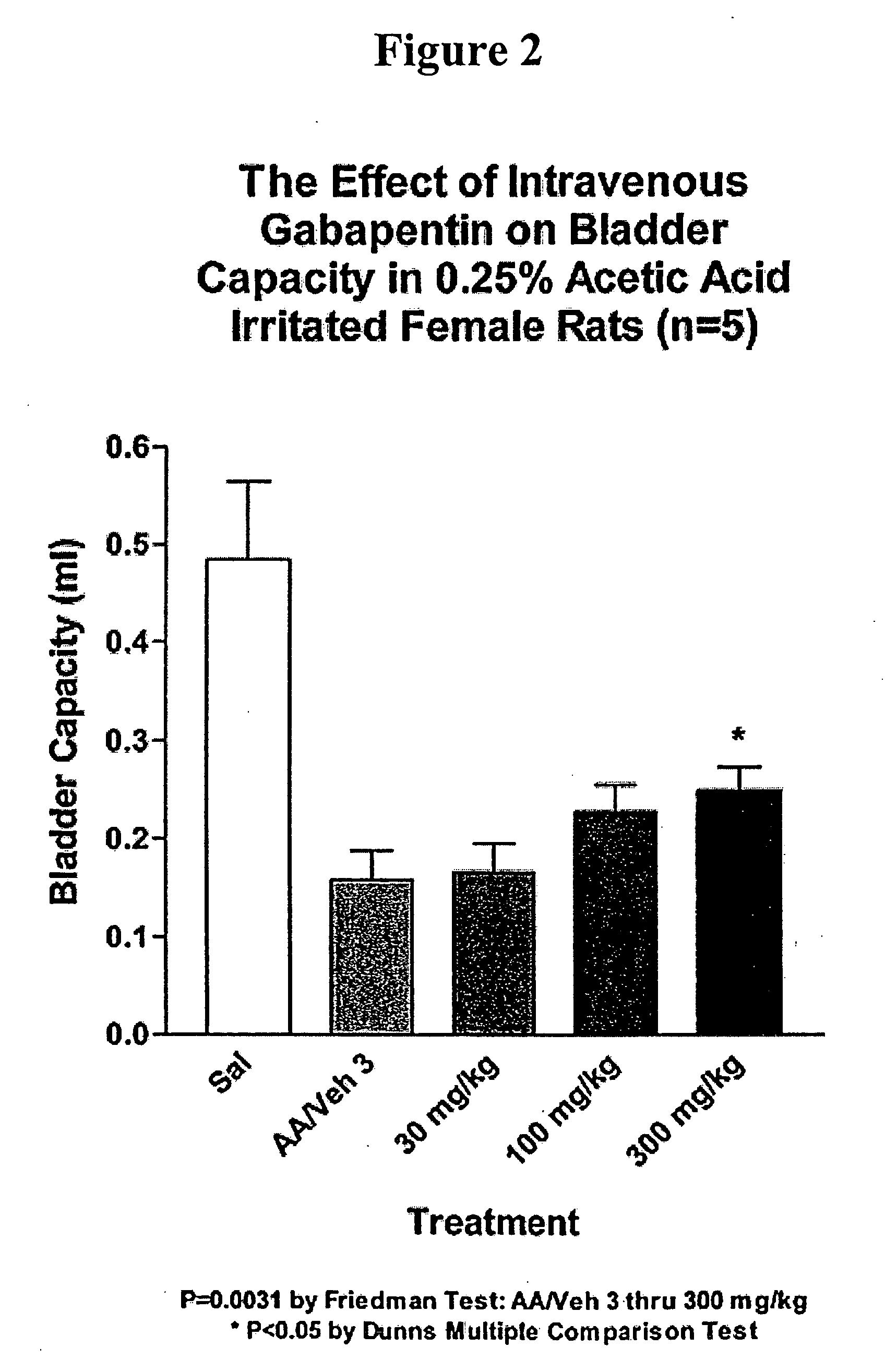
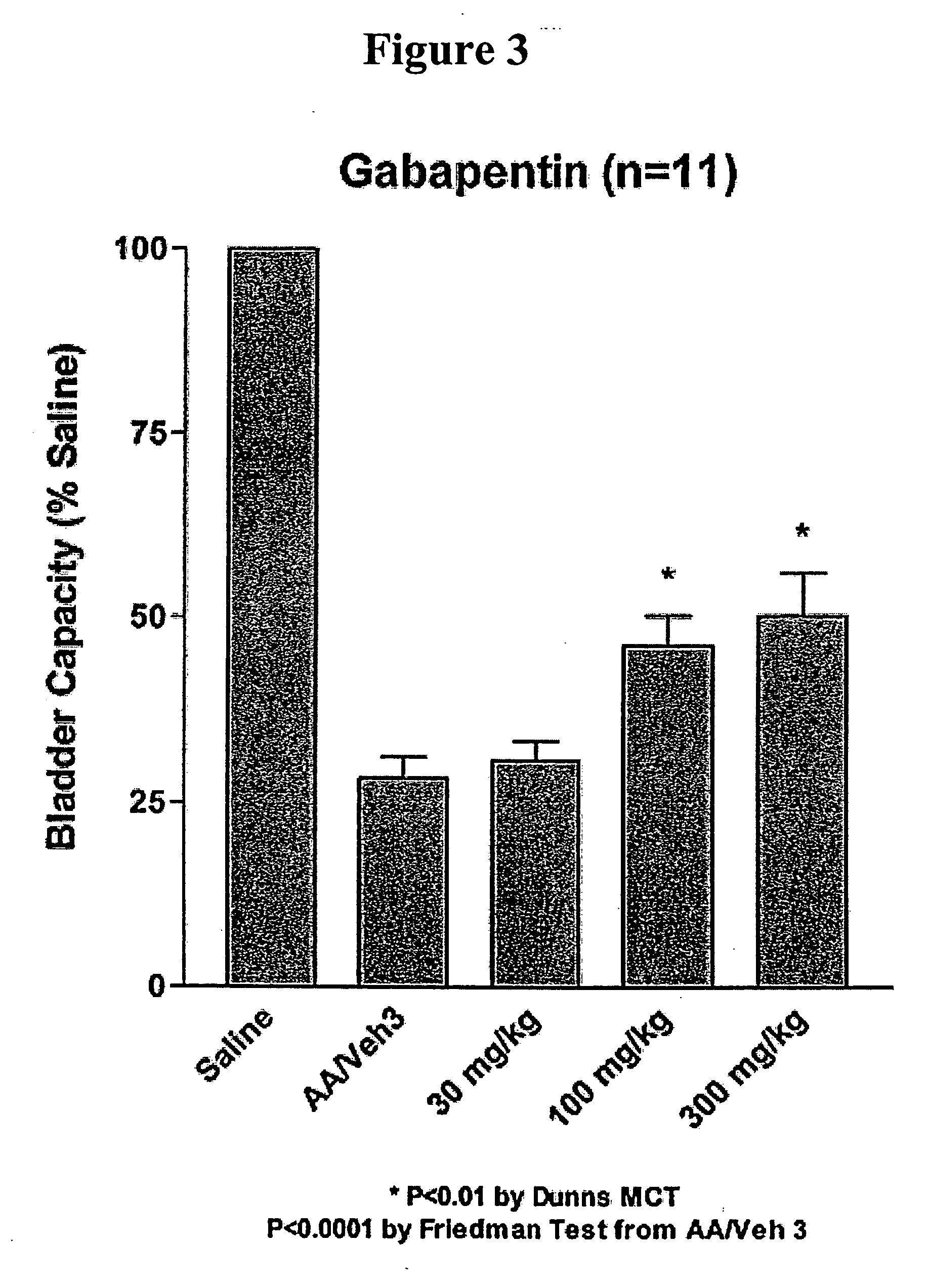
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:EDUSA PHARMA
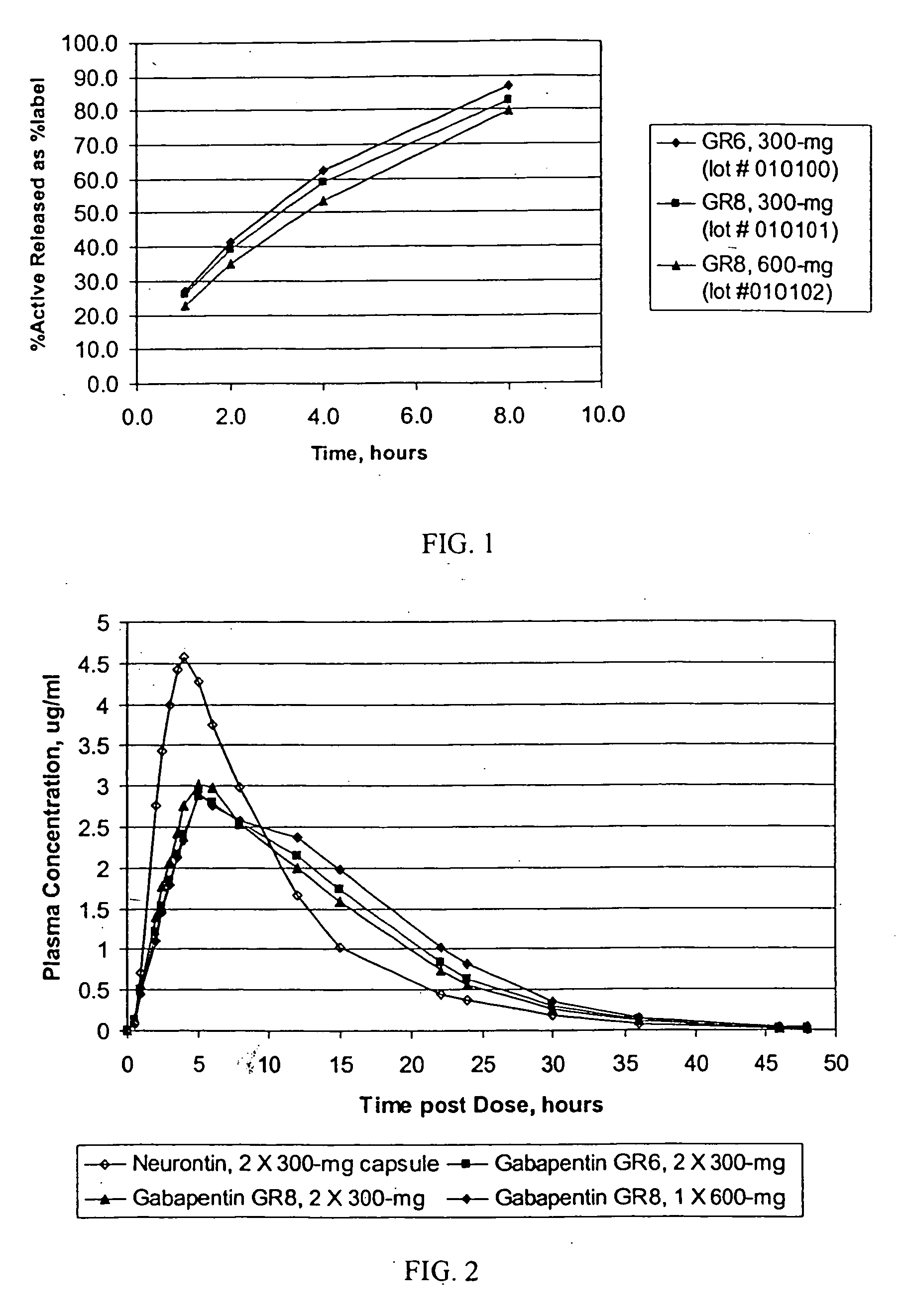
Sustained release oral dosage forms of gabapentin
InactiveUS20050158380A1Extended gastric residence timeGood sustained releaseOrganic active ingredientsBiocideGabapentinSustained Release Tablet
The present invention relates to sustained release oral dosage forms of gabapentin and at least one rate controlling polymer, and a process for the preparation of the sustained release oral dosage forms, and a process for the preparation thereof. The sustained release tablet includes gabapentin or a pharmaceutically acceptable salt or hydrates thereof and at least one rate-controlling polymer such that the tablet provides therapeutically effective plasma levels of gabapentin for a period of up to about 12 hours.
Owner:RANBAXY LAB LTD
Novel Pharmaceutical Compositions for Treating Chronic Pain and Pain Associated with Neuropathy
InactiveUS20130189354A1Reduced plasma concentrationEfficient managementOrganic active ingredientsBiocideGabapentinChronic pain
The present invention relates to compositions and methods for treating pain wherein the compositions comprise a combination of tramadol or a pharmaceutically acceptable salt thereof, magnesium or a pharmaceutically acceptable salt thereof; and gabapentin or pregabalin. The therapeutic combination can further contain capsaicin or an ester of capsaicin.
Owner:TRINITY LAB INC
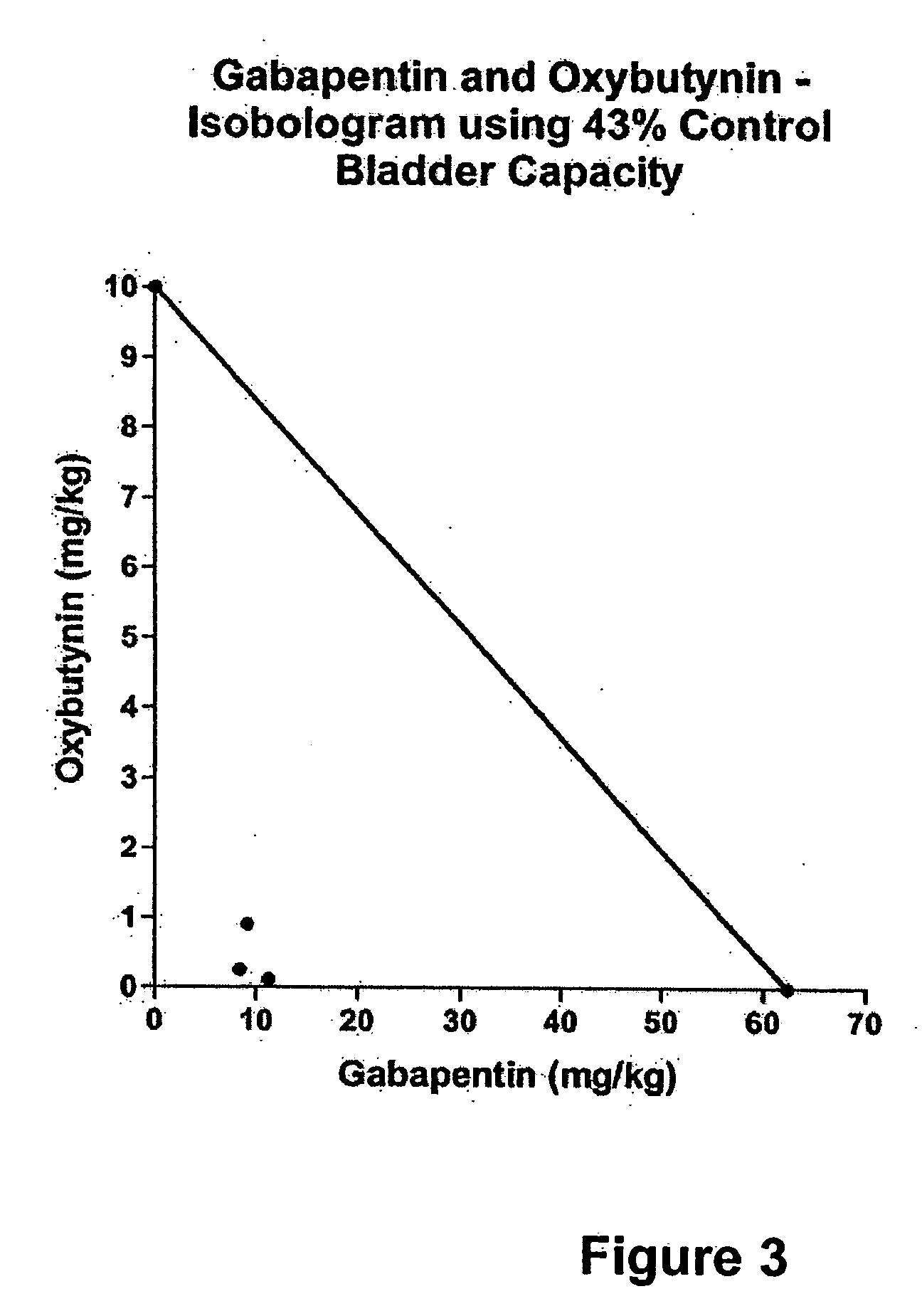
Methods for decreasing detrusor muscle overactivity
InactiveUS20050239890A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsDiseaseGabapentin
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs, e.g., gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Compounded transdermal pain management
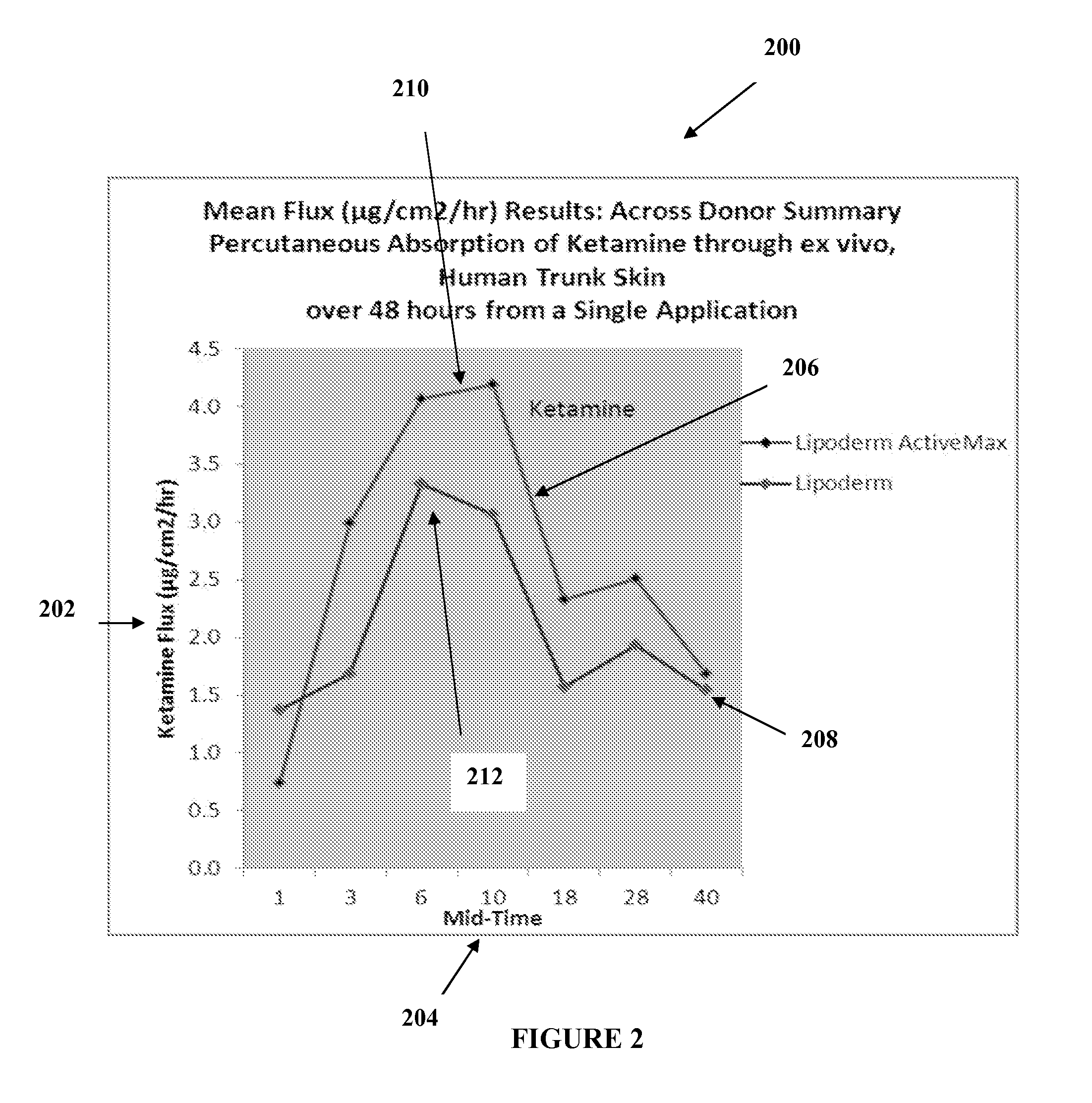
The present embodiments relate to topically delivered medication (compounded) for treatment of pain, inflammation, muscle fatigue, spasms, and / or other ailments. A transdermal cream may provide the effective topical administration of multiple medications simultaneously. The transdermal cream may include a salt load of approximately 30% or greater. The transdermal cream may include a unique base composition such that the transdermal cream may be able to remain stable and avoid degradation for six months or more and capable of effective delivery of active ingredient concentrations exceeding approximately 40% or more of the total formulation weight. The active ingredients may include a nerve depressant, NSAID, muscle relaxant, opiate agonist, local anesthetic, NMDA receptor antagonist, and a tricyclic antidepressant. In one embodiment, the transdermal cream may comprise ketamine HCL, gabapentin, clonidine HCL and baclofen. The transdermal cream may deliver an enhanced topical delivery flux of ketamine via a single transdermal application.
Owner:CMPD LICENSING
Analgesic composition for topical use
An analgesic composition, is disclosed which comprises a mixture of piroxicam, dexamethasone, ketamine, lidocaine injection, dimethyl sulfoxide, gabapentin and Vanicream™, preferably in the form of a cream or ointment. The composition is applied topically for the relief of pain of arthritis, neuropathy, post-herpetic (shingles) conditions, sore muscles, tendons and ligaments, and local reactions to insect bites or stings.
Owner:CATHCART CELEVATORON H
Intrathecal gabapentin compositions
InactiveUS20050004221A1Reduced hypertonicityAvoid damageBiocidePeptide/protein ingredientsGabapentinTonicity
Injectable compositions containing gabapentin and having reduced tonicity are discussed. One such injectable gabapentin composition contains greater than about 30 mg / ml gabapentin and has a tonicity of less than about 900 mOsm. Another such injecable gabapentin composition contains less that 0.9% sodium chloride. A process for preparing injectable gabapentin compositions are also discussed. The process includes determining and optionally adjusting tonicity of a gabapentin composition.
Owner:MEDTRONIC INC +1
Topical formulations for treatment of neuropathy
Topical treatments for neuropathy are described. The treatments include topical formulations of NMDA antagonists and one additional active ingredient. In one example, the formulation includes ketamine and gabapentin for the treatment of a subject's neuropathy. These transdermal or topical compositions provide a surprising degree of effective relief from the symptoms of peripheral neuropathy and can be administered to subjects to treat various neuropathies.
Owner:TARAXOS
Intrathecal gabapentin for treatment of epilepsy
InactiveUS20050090548A1Easy to controlImprove the level ofBiocideOrganic active ingredientsGabapentinImplanted device
Methods for treating epilepsy by administering gabapentin to cerebrospinal fluid and brain tissue of a patient are discussed. Compositions, particularly injectable compositions, containing gabapentin are also discussed. In addition, systems including an implantable device having a pump coupled to a reservoir for housing a composition, a catheter having a proximal end coupled to the pump and having a distal end adapted for administrating a composition to a cerebrospinal fluid of a patient, and a composition containing gabapentin, which composition is housed in the reservoir of the pump, are also discussed.
Owner:MEDTRONIC INC
Pump systems including injectable gabapentin compositions
InactiveUS20050004219A1Reduced hypertonicityReducing solvent tonicityOrganic active ingredientsBiocideGabapentinTonicity
A system including a reservoir, a pump coupled to the reservoir, a catheter coupled to the pump and adapted for delivering a therapeutic agent to a cerebrospinal fluid of a patient; and an injectable gabapentin composition housed in the reservoir and deliverable through the catheter, is discussed. The injectable gabapentin composition may have reduced tonicity. One such injectable gabapentin composition contains greater than about 30 mg / ml gabapentin and has a tonicity of less than about 900 mOsm. Another such injecable gabapentin composition contains less that 0.9% sodium chloride.
Owner:MEDTRONIC INC
Methods for decreasing detrusor
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
Gastric retentive gabapentin dosage forms and methods for using same
InactiveUS20090176882A1Good curative effectReduce incidenceOrganic active ingredientsBiocideDosing regimenGabapentin
Provided is a method of treating a patient suffering from a pain state by administering to the patient a gastric retentive dosage form of gabapentin that is capable of administration in once-daily or twice daily dosing regimens. By reducing the need to administer gabapentin from the thrice-daily administrations characteristic of immediate release gabapentin, the gastric retentive gabapentin dosage forms provided herein have the advantages of improving patient compliance for gabapentin treatment. In addition to the foregoing, the gastric retentive gabapentin dosages forms also exhibit decreased blood plasma concentrations and increased bioavailability throughout the dosing regimen.
Owner:DEPOMED SYST INC
Pharmaceutical composition containing gabapentin or pregabalin and N-type calcium channel antagonist
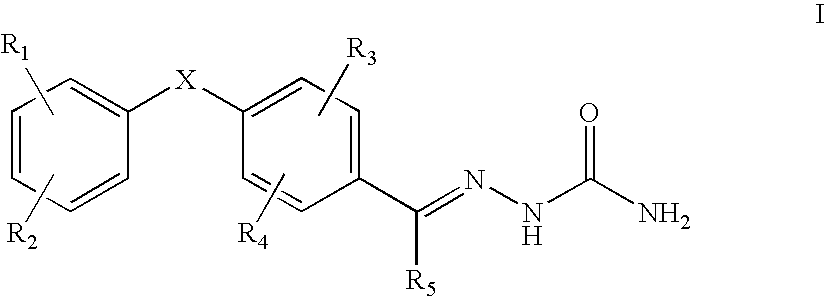
The present invention relates to a pharmaceutical composition useful for preventing / treating pain, which comprises combination of gabapentin or pregabalin, or pharmaceutically acceptable salts thereof and N-type calcium channel antagonists or pharmaceutically acceptable salts thereof such as a compound having the following structure.
Owner:AJINOMOTO CO INC
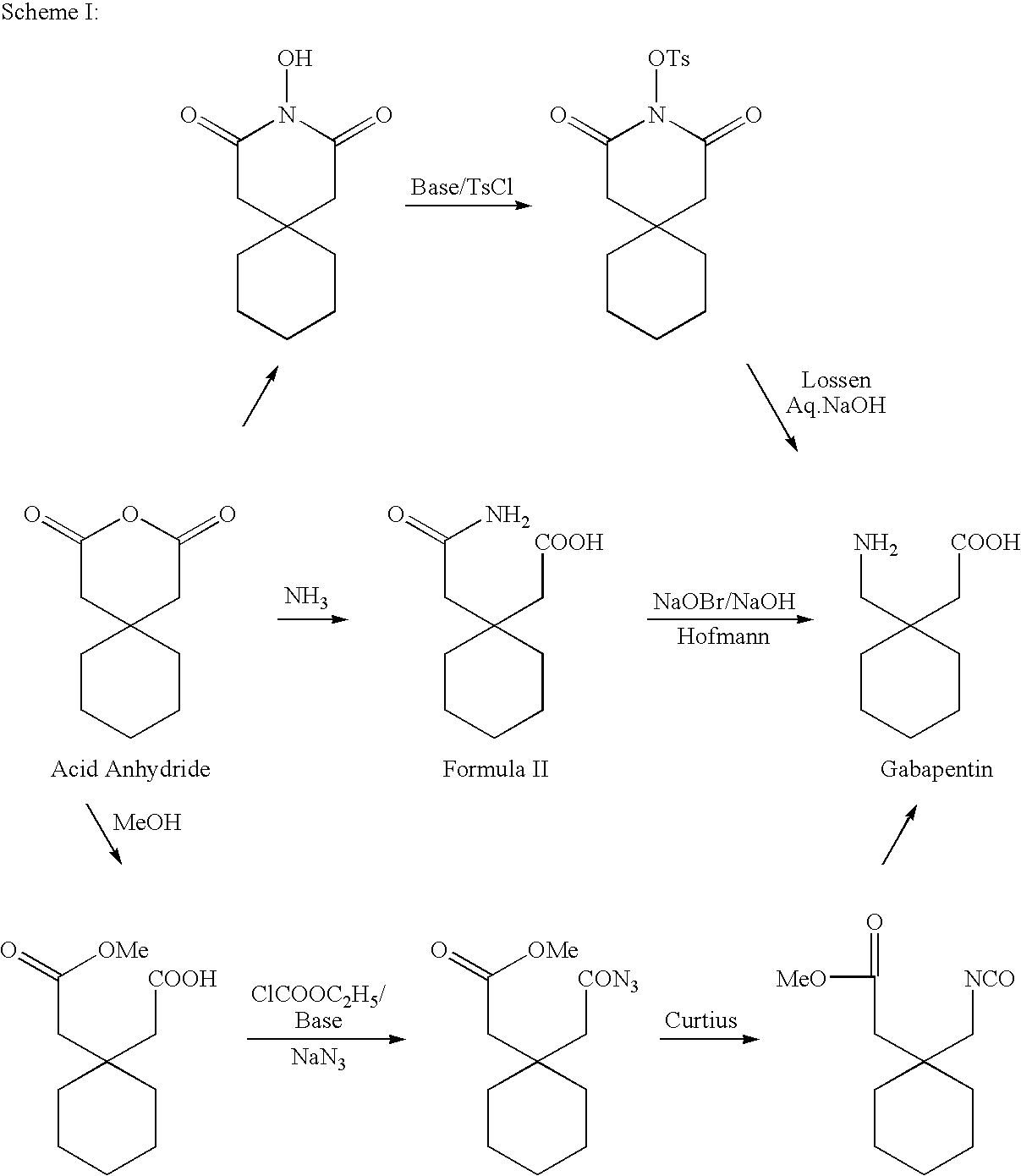
Process For Synthesis Of Gabapentin
InactiveUS20080103334A1Increase productionMinimizes by-products/impuritiesOrganic compound preparationOrganic chemistry methodsGabapentinAmmonia
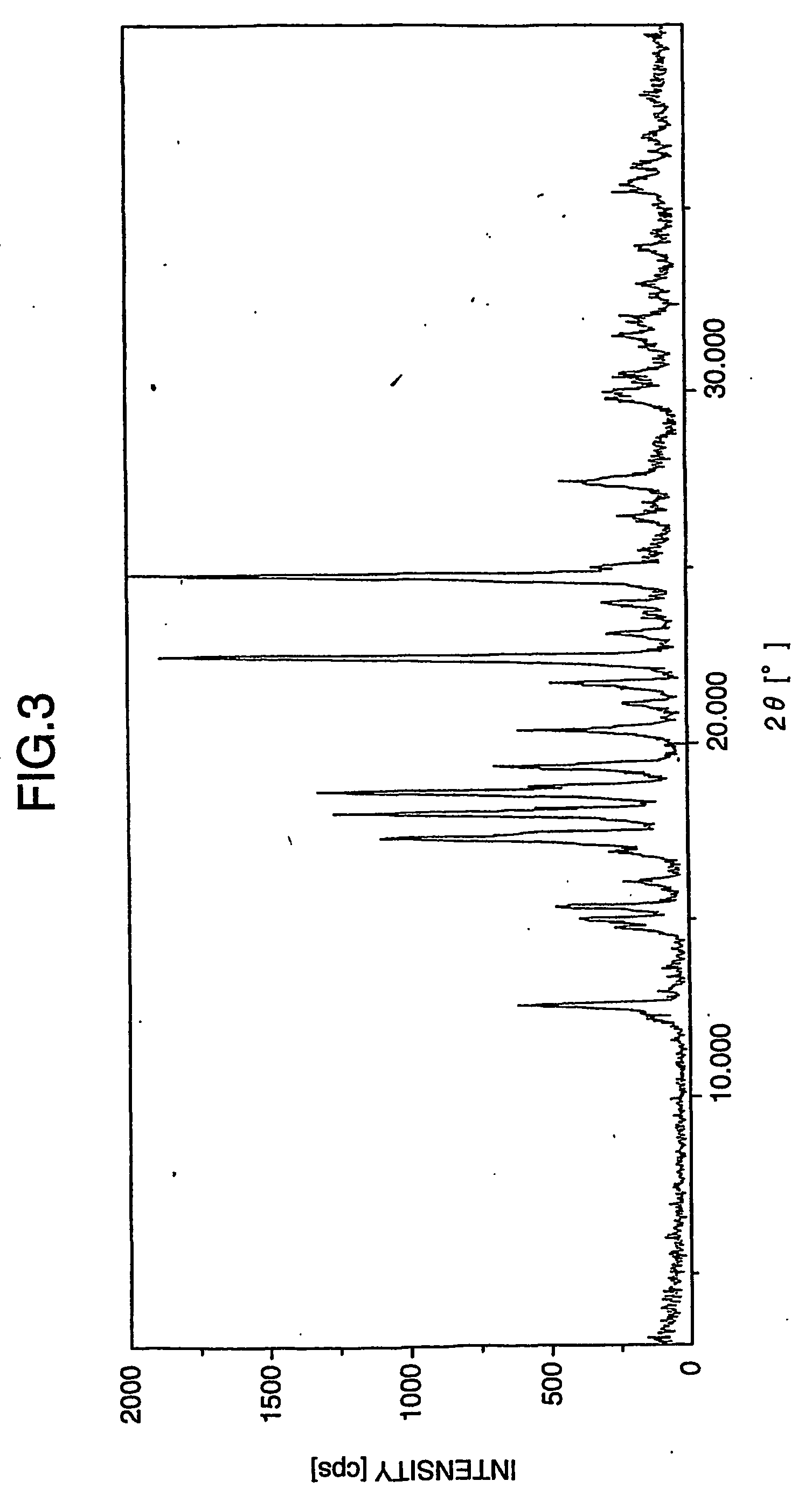
A process for preparation of gabapentin comprising a step of obtaining 1,1-cyclohexane diacetic acid monoamide from 1,1-cyclohexane diacetic acid anhydride, wherein said reaction is characterized by the use of ammonia precursor or pre-generated ammonia-isopropanol solution. The invention further discloses preparation of gabapentin and isolation of gabapentin in polymorphic Form II with high yield and purity.
Owner:IPCA LAB LTD
Gabapentin hydrochloride and its intermediate preparation method
ActiveCN1880299AReduce generationReduced post-treatment processOrganic compound preparationAmino-carboxyl compound preparationGabapentinHydrochloride
This invention relates to a method for producing gabapentin and intermediate thereof, comprising: 1,1-cyclohexanediacctic acid reacts with urea to produce the gabapentin intermediate 3,3-pentamethyleneglutarimide. This invention is characterized of easy-obtained raw material, low cost, simple operation, and easy-controlled procedures, which makes it much qualified for mass industrial production.
Owner:NHWA PHARMA CORPORATION
Methods of treating non-painful bladder disorders using alpha2delta subunit calcium channel modulators
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
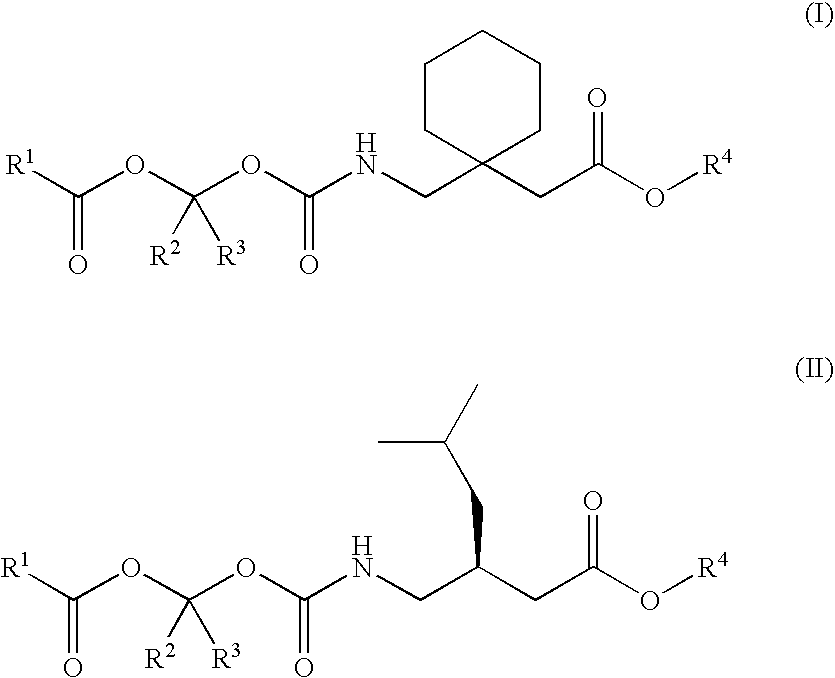
Treating premature ejaculation using gabapentin and pregabalin prodrugs
InactiveUS20070049626A1Improve side effectsRapid clearance/shortBiocideAnimal repellantsGabapentinDrug
Disclosed herein are methods of using prodrugs of gabapentin and pregabalin, and pharmaceutical compositions thereof, to treat premature ejaculation in male humans, and pharmaceutical compositions of prodrugs of gabapentin and pregabalin useful in treating premature ejaculation.
Owner:XENOPORT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com