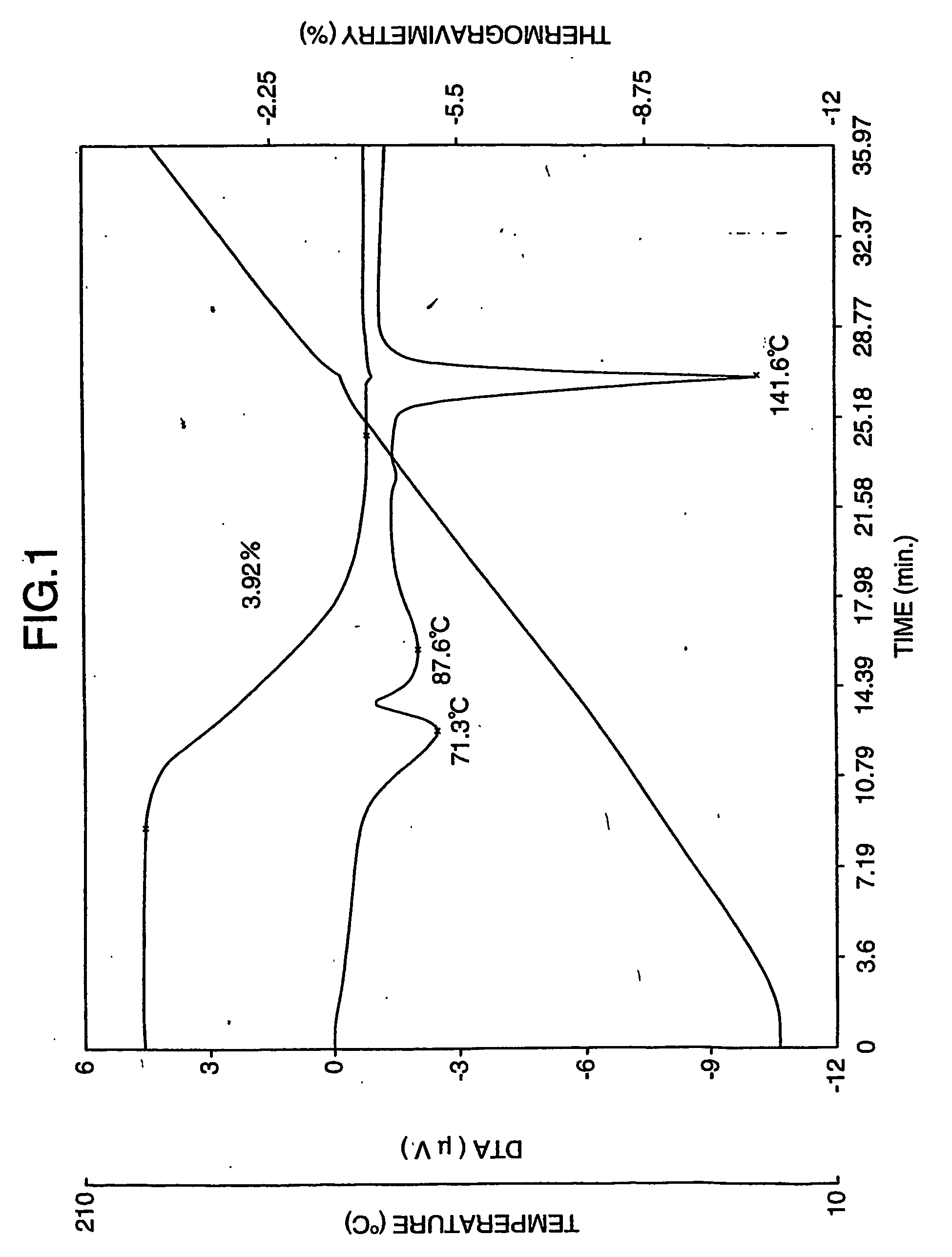
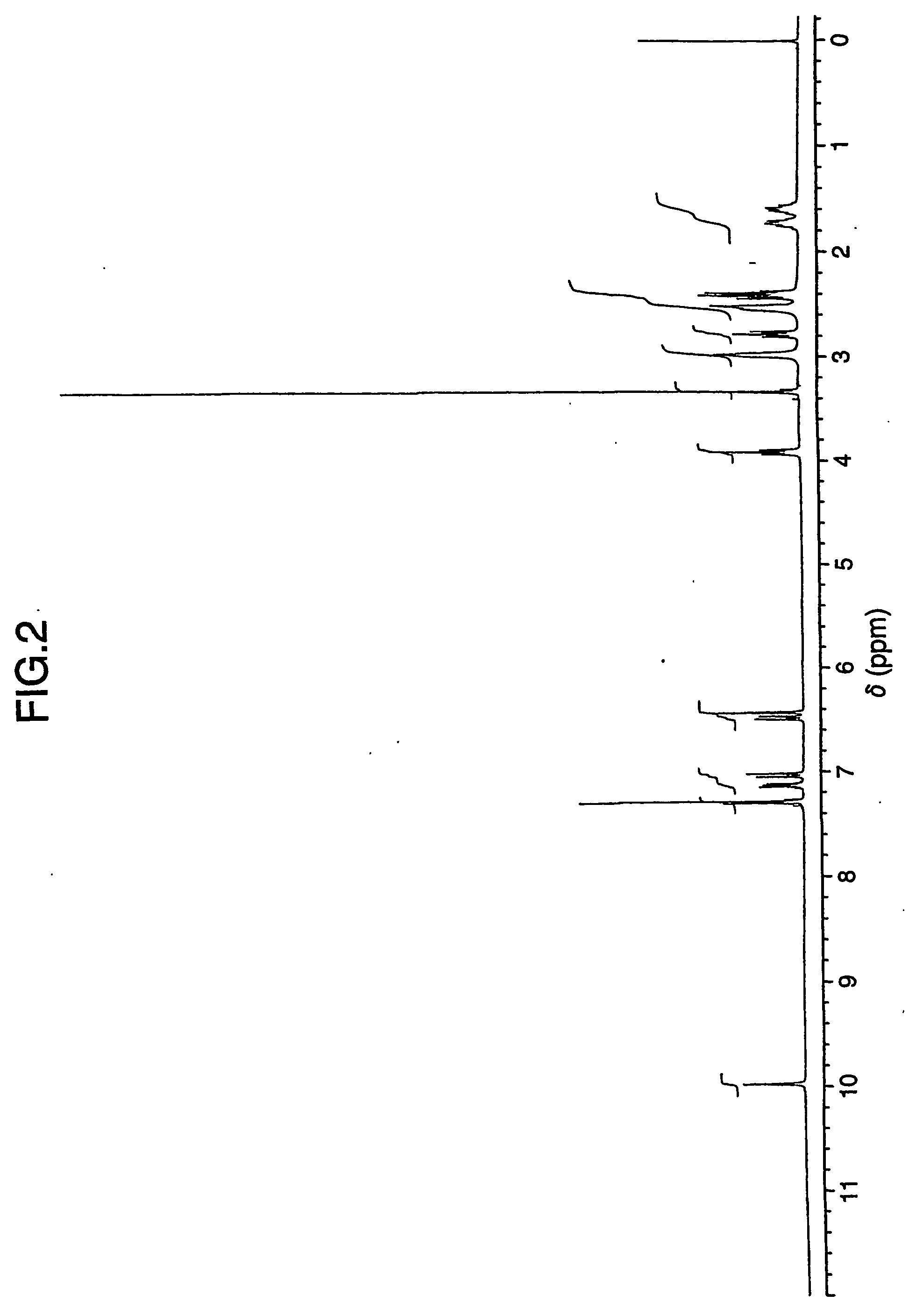
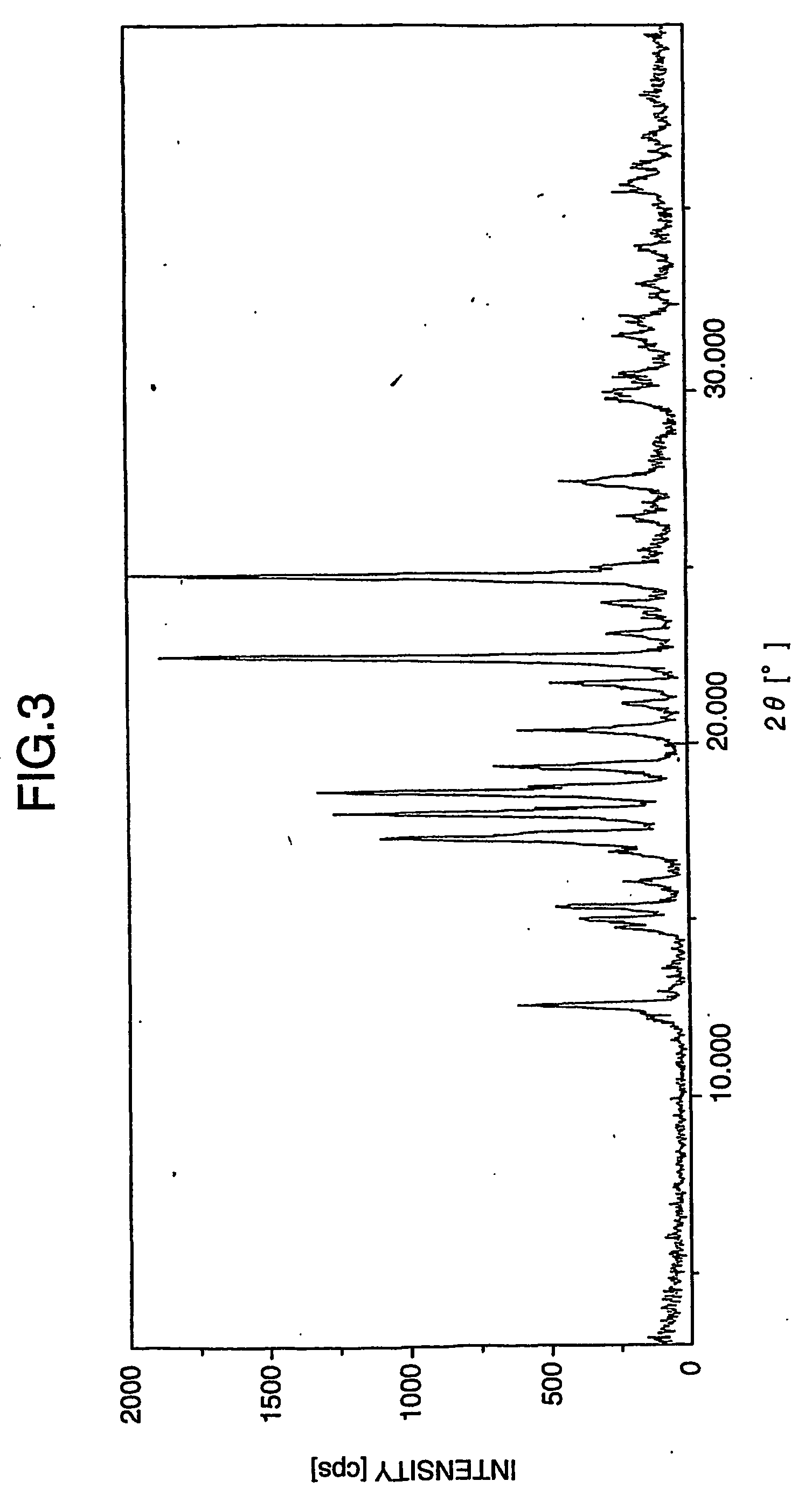
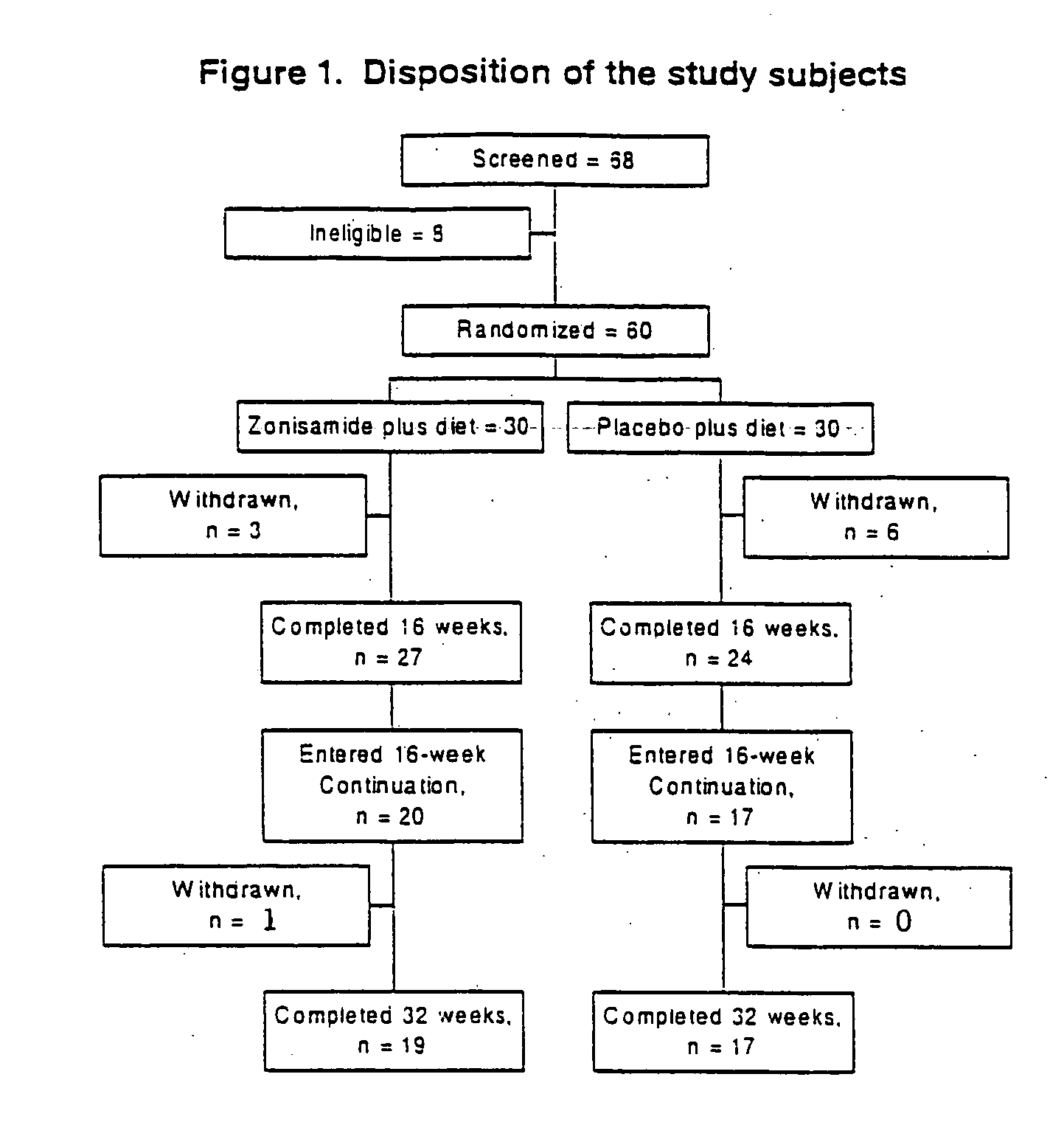
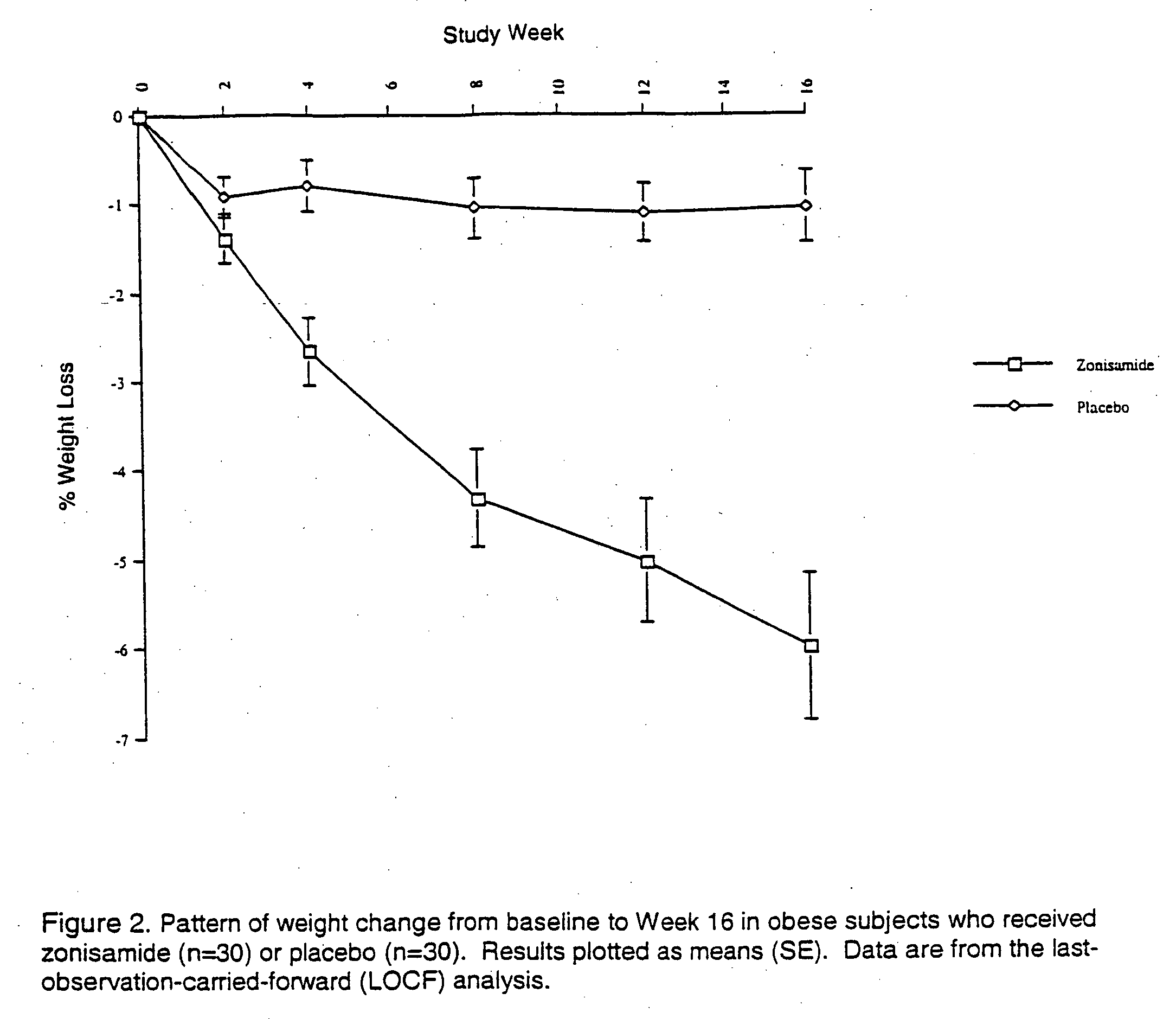
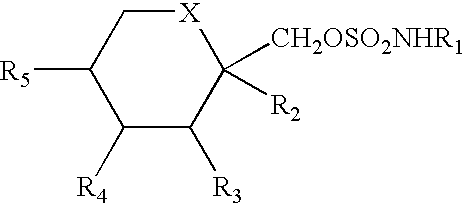
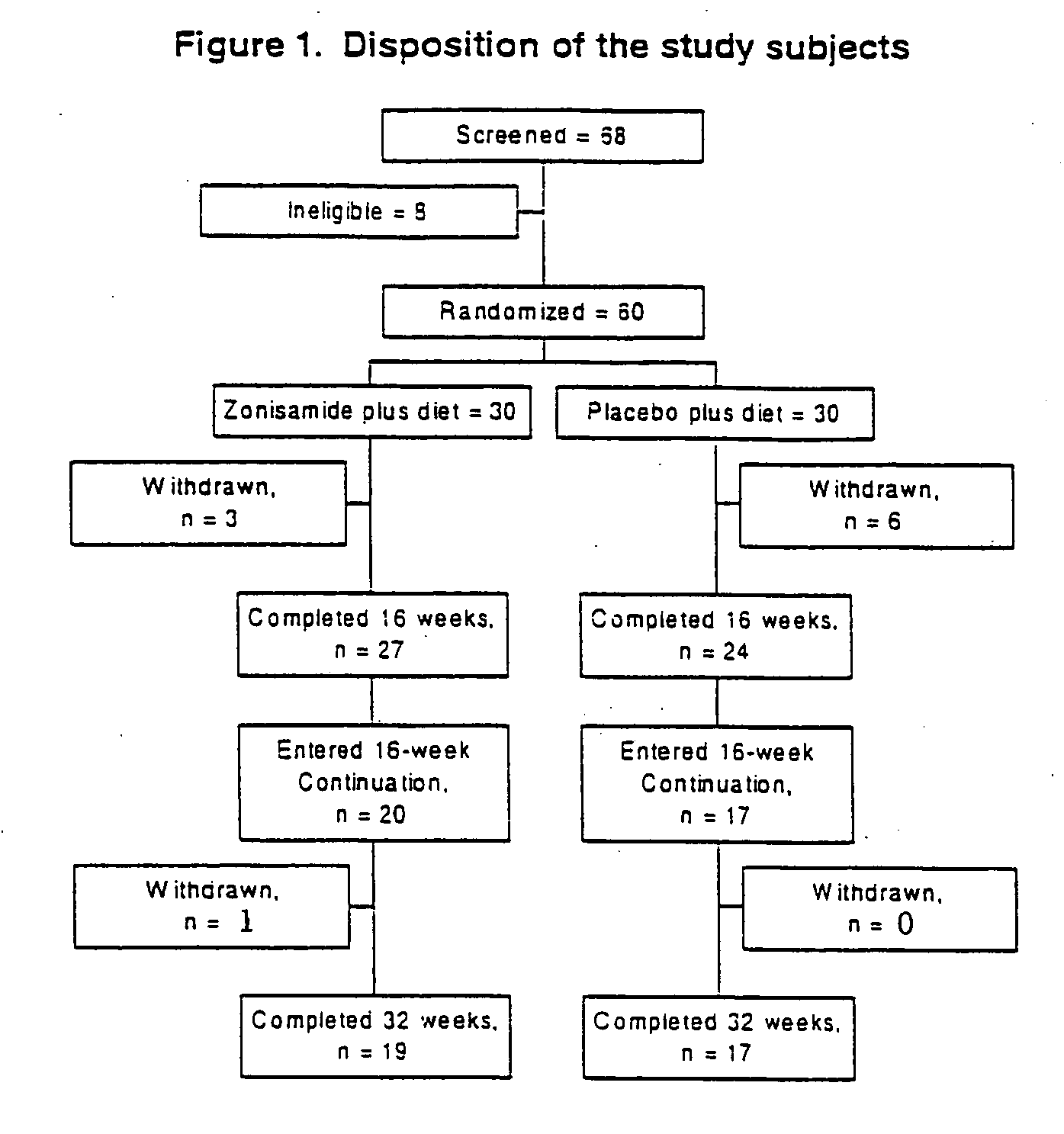
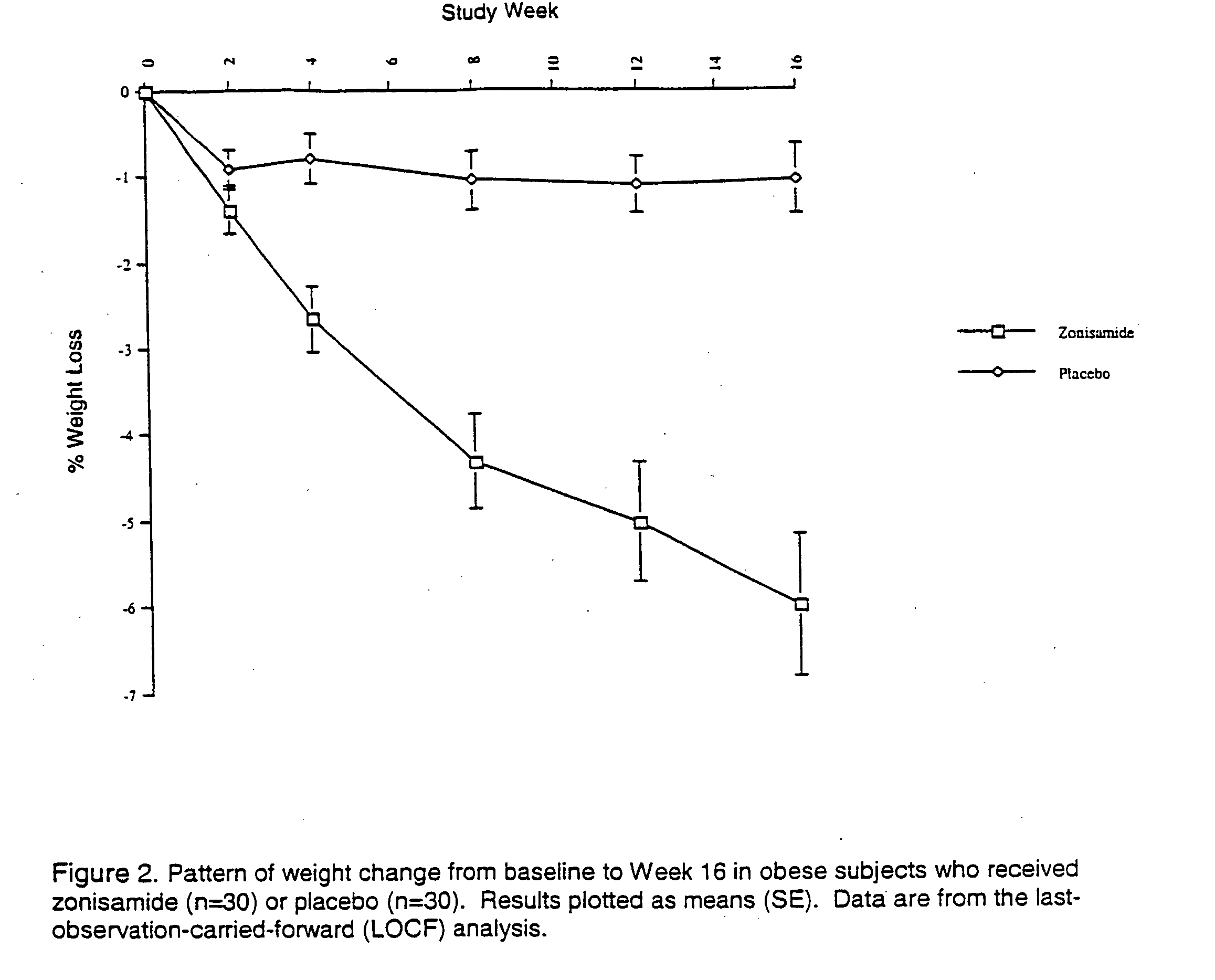
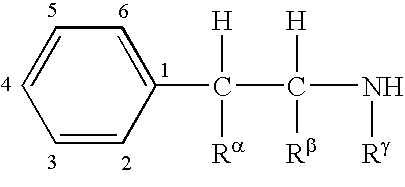
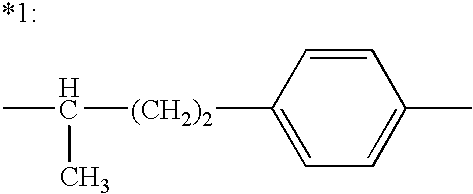
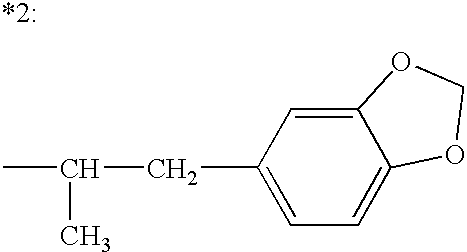
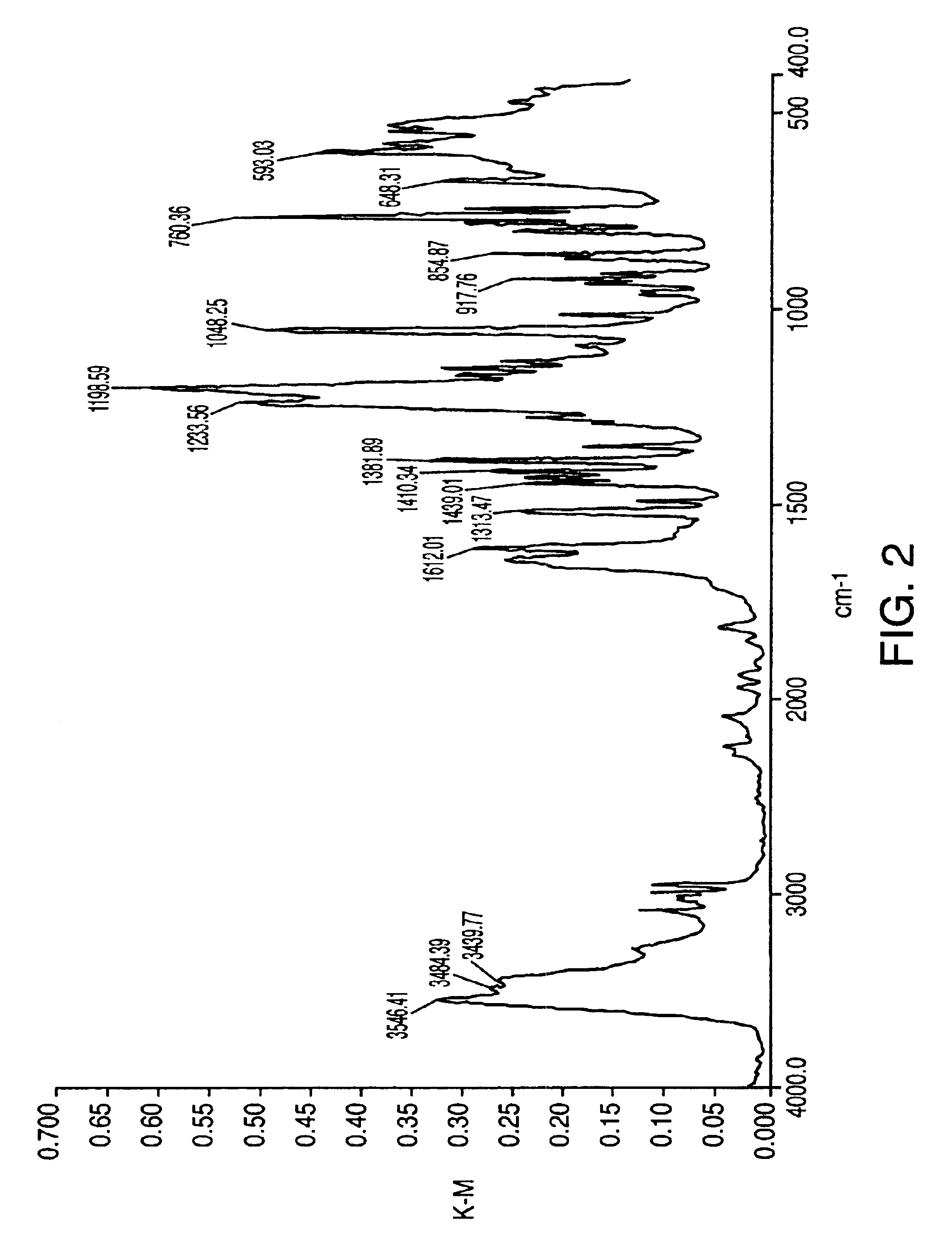
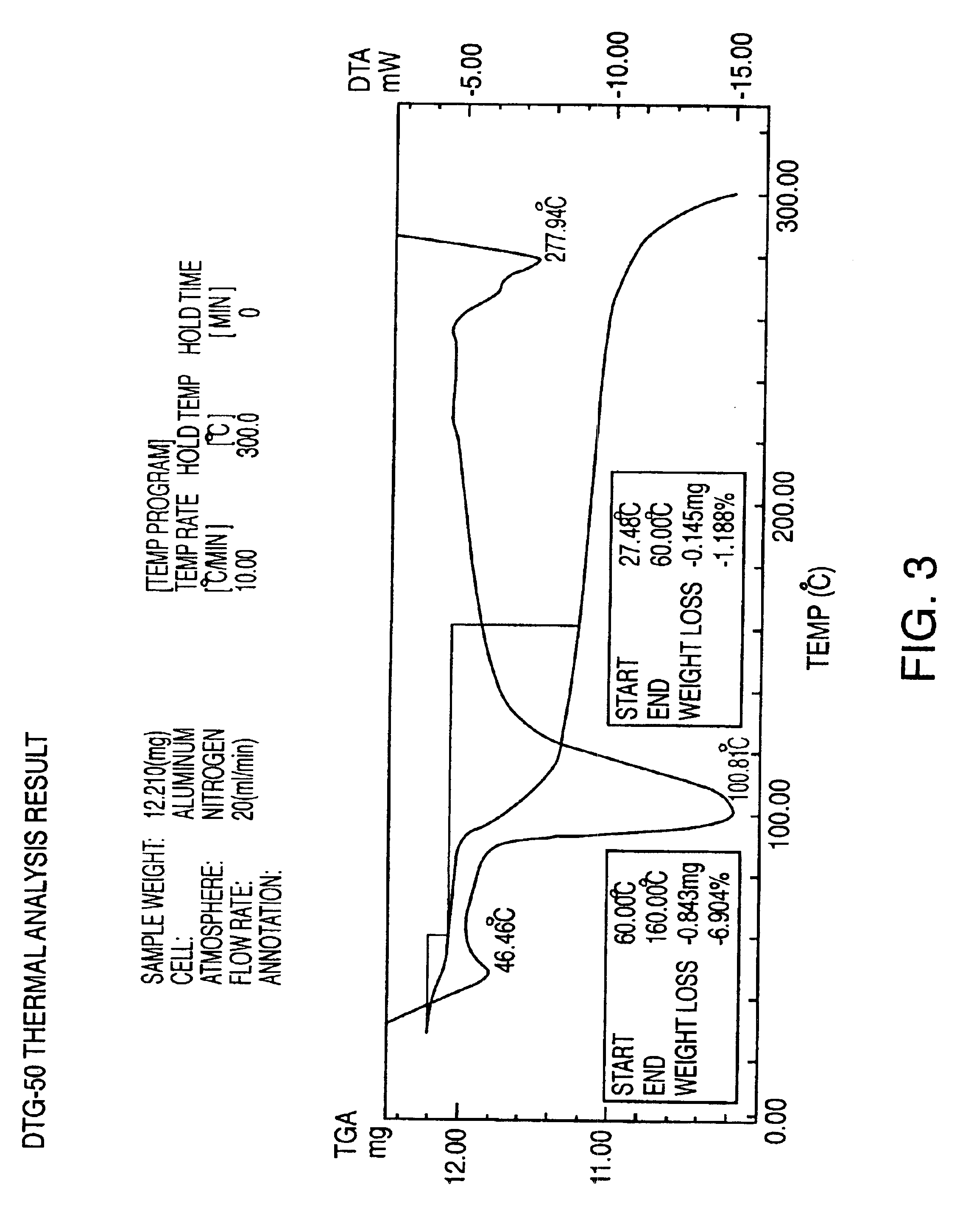
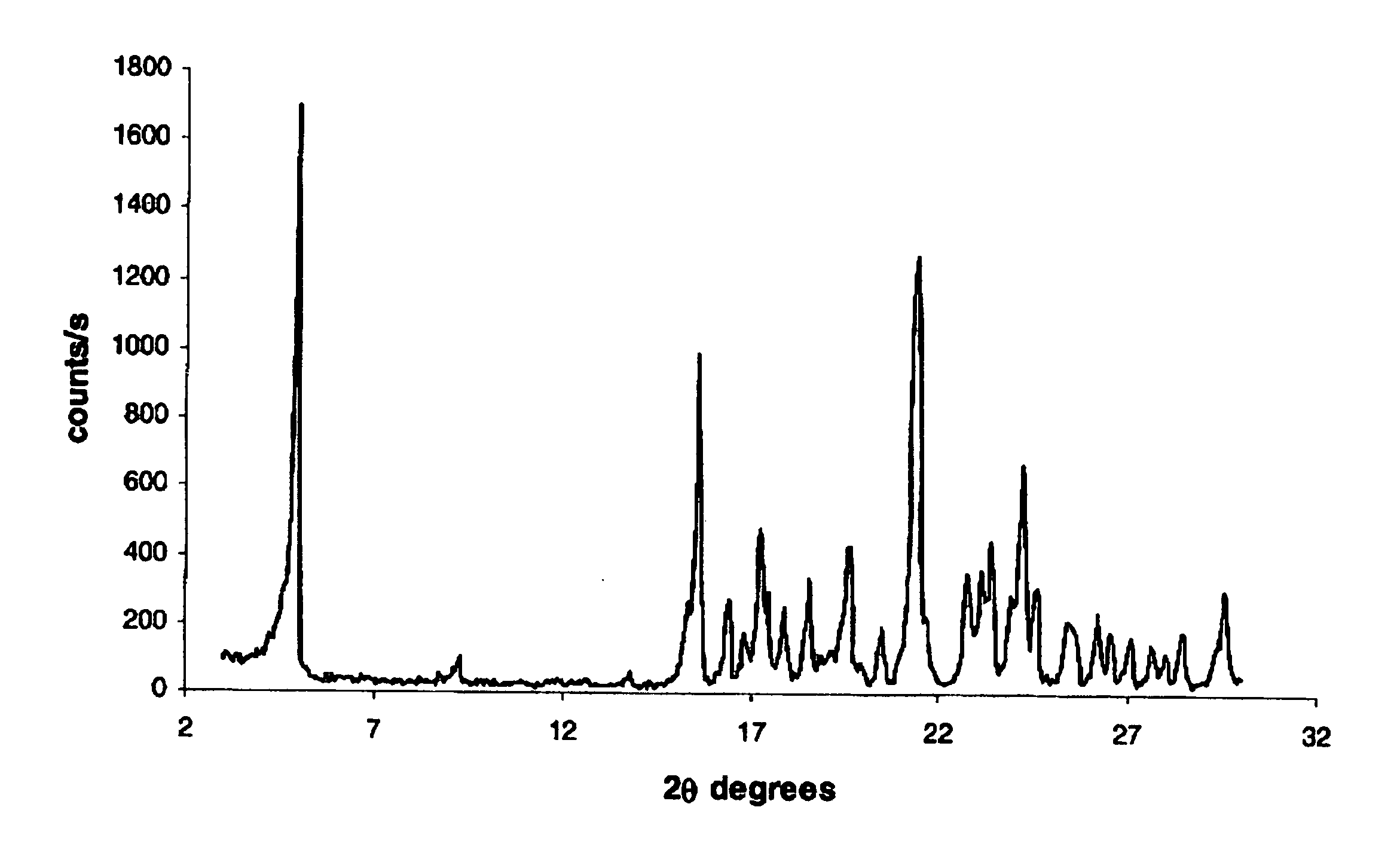
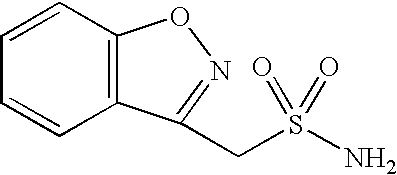
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
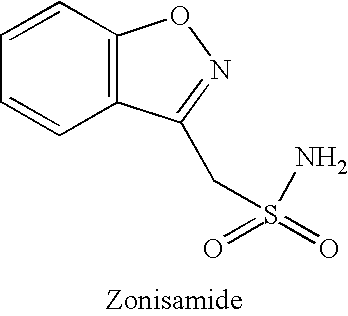
64 results about "Zonisamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
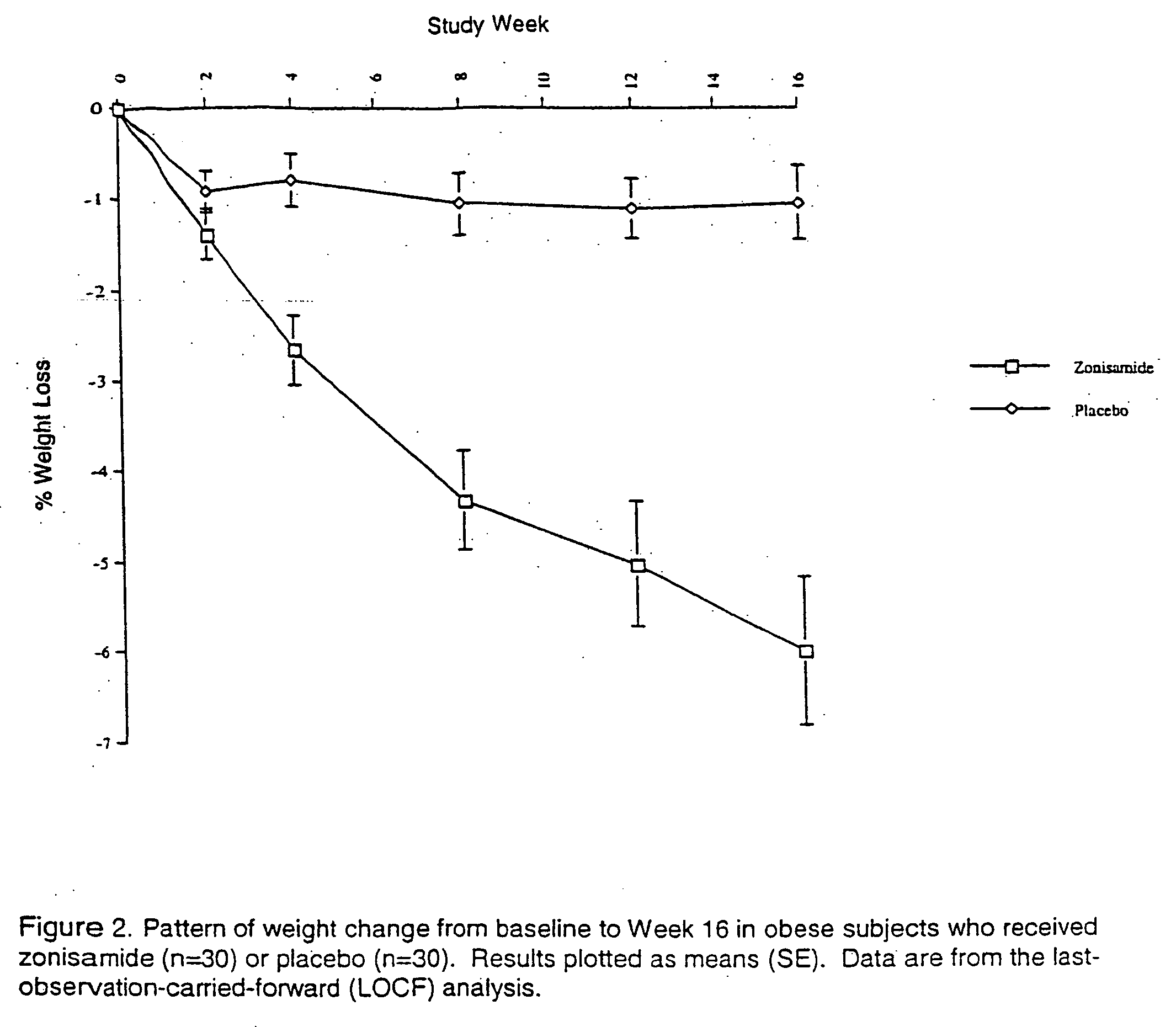
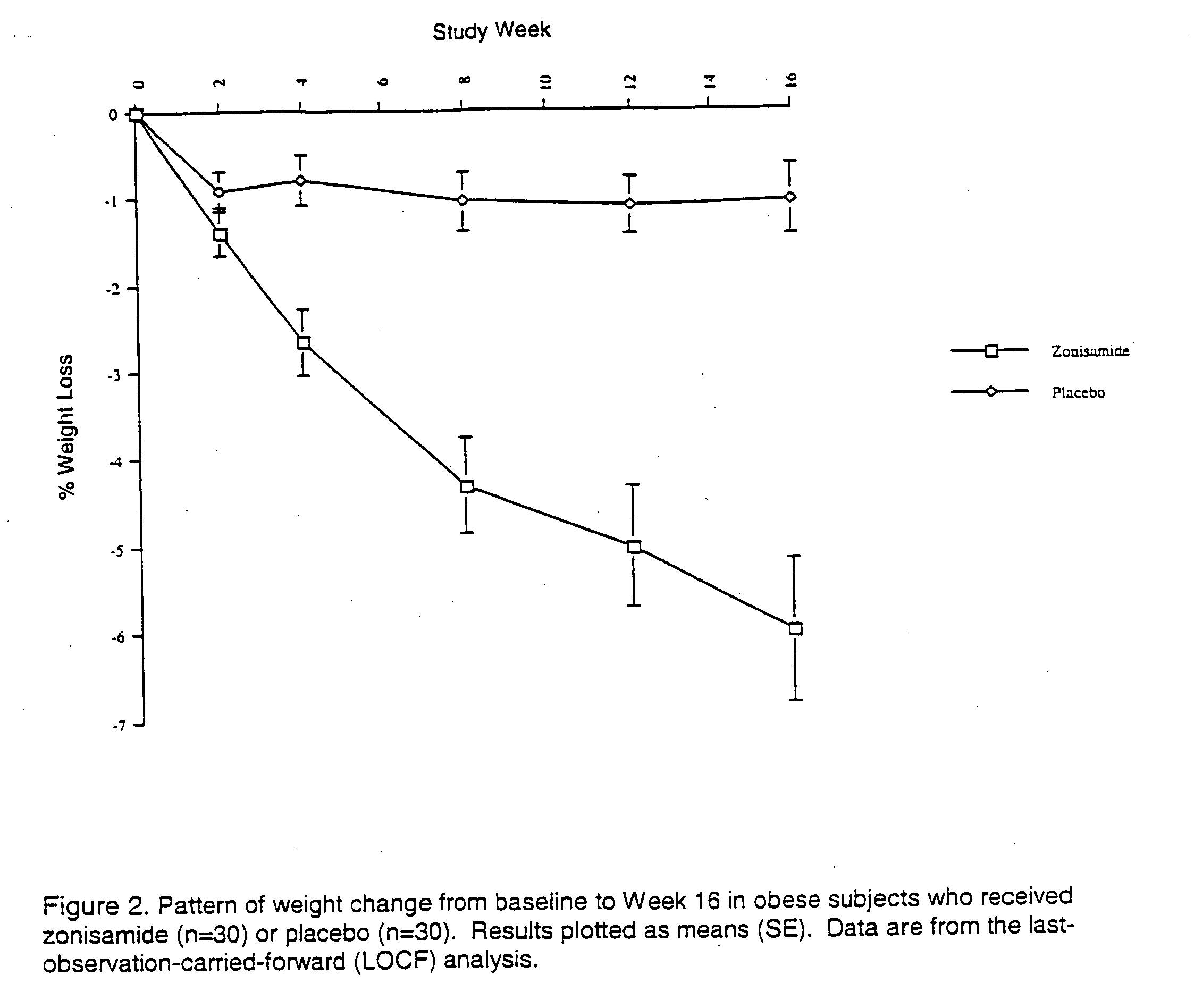
Zonisamide is used with other medications to prevent and control seizures (epilepsy).
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Method for treating obesity
InactiveUS20050143322A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicCompound (substance)
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:DUKE UNIV
Method for treating obesity
InactiveUS20050137144A1Minimizing metabolic risk factorHigh activityAntibacterial agentsBiocideZonisamideAdrenergic
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:GADDE KISHORE M +1
Compositions and methods for treating obesity and related disorders
InactiveUS20080255093A1Reduces unwanted side effectGood for weight lossBiocideNervous disorderSYMPATHOMIMETIC AGENTSSerotonin receptor agonist
The present invention is drawn to combinations of pharmaceutical agents having similar chemical and / or pharmacological properties, wherein the combinations maximize the therapeutic effect of the drug while minimizing their adverse effects. The methods and compositions of the invention are particularly useful in the treatment of obesity and related conditions which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) or bupropion in combination with an anti-epileptic agent (e.g., topiramate, zonisamide), CB1 antagonists (e.g., rimonabant), or a 5HT2C-selective serotonin receptor agonist, (e.g., lorcaserin) for the treatment of obesity and related conditions. The invention also features kits for use in the practice of these novel therapies.
Owner:VIVUS
Method for treating obesity
InactiveUS20060009514A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicMetabolic risk
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:GADDE KISHORE M +1
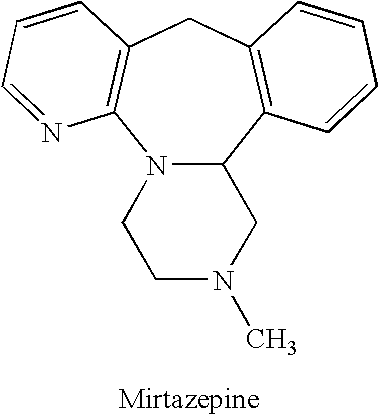
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
Method for treating obesity
InactiveUS20050215552A1Minimizing metabolic risk factorHigh activityBiocideMetabolism disorderMetaboliteAdrenergic
The present invention relates generally to methods of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight loss-promoting anti-convulsant either alone or in combination with bupropion or metabolites thereof or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:OREXIGEN THERAPEUTICS INC
Method for treating sleep-related breathing disorders
InactiveUS20060039866A1Excellent toneSuppressing REM sleepBiocideAerosol deliveryHypopneaPositive airway pressure
Compositions and methods for the treatment of sleep related breathing disorders are provided. Compositions include mirtazapine in combination with other active pharmaceutical ingredients, such as zonisamide, topiramate or modafinil. The treated sleep related breathing disorders include sleep apnea and sleep hypopnea. In some embodiments, the pharmaceutical compounds are used as adjuvant therapy with positive airway pressure (PAP) therapy, thereby lowering the pressure required to maintain airway patency during PAP therapy.
Owner:CYPRESS BIOSCI
Zonisamide and nsaid nanoparticulate formulations
The present invention is directed to compositions comprising zonisamide, or a salt or derivative thereof, and at least one nanoparticulate NSAID. The zonisamide and NSAID combination preferably includes nanoparticulate NSAID particles of the composition with an effective average particle size of less than about 2000 nm. The zonisamide and NSAID combination is useful in the treatment of migraine and acute migraine pain and related conditions.
Owner:ELAN PHRMA INT LTD
Method for treating sleep-related breathing disorders with setiptiline
InactiveUS20060039867A1Excellent toneSuppressing REM sleepBiocideAerosol deliveryHypopneaPositive airway pressure
Compositions and methods for the treatment of sleep related breathing disorders are provided. Compositions include setiptiline in combination with other active pharmaceutical ingredients, such as zonisamide, topiramate or modafinil. The treated sleep related breathing disorders include sleep apnea and sleep hypopnea. In some embodiments, the pharmaceutical compounds are used as adjuvant therapy with positive airway pressure (PAP) therapy, thereby lowering the pressure required to maintain airway patency during PAP therapy.
Owner:CYPRESS BIOSCI
Methods of improving the safety of zonisamide therapy
InactiveUS20050043773A1Removal or tapering off of zonisamideImprove securityOrganic active ingredientsElectrotherapySide effectPancreatitis
Methods to increase the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of pancreatitis as a possible side effect wherein both the patient and treating physicians and other medical care providers may monitor effectively for pancreatitis and methods for increasing the probability of a positive outcome in the few patients who experience pancreatitis associated with zonisamide therapy.
Owner:EISAI INC
Novel methods using zonisamide
The present invention relates to usefulness of zonisamide as a pharmaceutical composition for treating dementia or cognitive impairment.
Owner:EISIA R&D MANAGEMENT CO LTD
Combination Therapy
The present invention features a novel therapy for treating diabetes, hypertension, migraine, epilepsy, sleep apnea, depression, impulse control disorders or alcohol addiction which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate compound (e.g., topiramate) or an anticonvulsive sulfonylurea compound (e.g. zonisamide).
Owner:VIVUS
Methods of using zonisamide as an adjunctive therapy for partial seizures
InactiveUS20050043705A1Reduce removalImprove securityOrganic active ingredientsElectrotherapySide effectPancreatitis
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of pancreatitis as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for pancreatitis and employ methods that will improve the therapeutic outcome in the few patients who experience pancreatitis associated with zonisamide therapy.
Owner:EISAI INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
InactiveUS20050043704A1Reduce removalImprove securityOrganic active ingredientsElectrotherapySide effectPancreatitis
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of pancreatitis as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for pancreatitis and employ methods that will improve the therapeutic outcome in the few patients who experience pancreatitis associated with zonisamide therapy.
Owner:EISAI INC
Prevention and treatment of hearing disorders
Compositions, and methods of use thereof, are provided for the prevention, treatment or alleviation of symptoms of hearing are provided. Embodiments of the methods employ zonisamide as the sole active pharmaceutical agent or a combination of zonisamide and another pharmaceutical agent, such as an antioxidant, a NMDA antagonist, an SSRI or a combined SSRI / NMDA antagonist agent. Other embodiments of the method involve the use of zonisamide alone or in combination with another API to prevent, treat or ameliorate one or more symptoms of hearing loss. Hearing disorders treatable with the invention include noise-induced hearing loss, drug-induced hearing loss, central auditory hearing disorder (CAPD), tinnitus and presbyacusis.
Owner:CYPRESS BIOSCI
Method for treating obesity
InactiveUS20090076108A1Minimize risk factorHigh activityBiocideMetabolism disorderAdrenergicMetabolite
Owner:OREXIGEN THERAPEUTICS INC
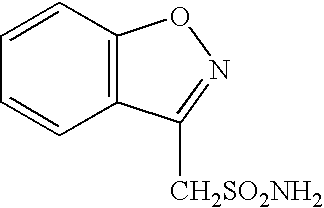
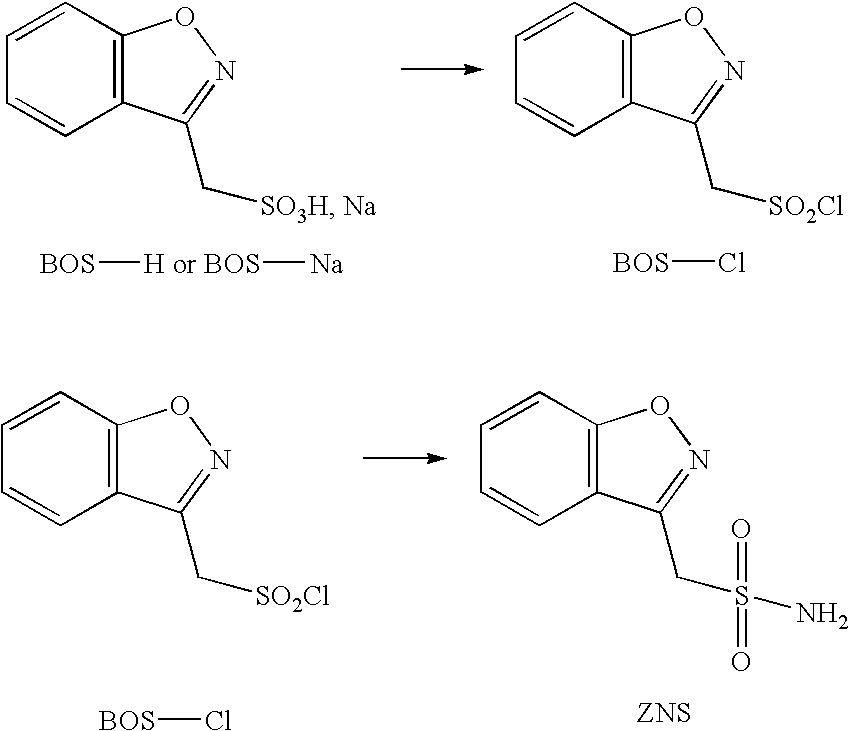
Sulfonation method for zonisamide intermediate in zonisamide synthesis and their novel crystal forms
The present invention relates to a novel sulfonation of an intermediate of zonisamide. The sulfonation processes using chlorosulfonic acid as well as acetic anhydride and sulfuric acid in an organic solvent are disclosed. Crystalline forms of benzisoxazole methane sulfonic acid (BOS-H) and its salts (BOS-Na, BOS-Ca, and BOS-Ba) and their novel preparation processes are disclosed.
Owner:TEVA PHARM USA INC
Methods for prophylatic appetite suppression
Pharmaceutical formulations comprise sustained-release zonisamide. Methods of preparing such pharmaceutical formulations involve intermixing zonisamide with a suitable excipient configured to control the dissolution profile of the zonisamide. Methods of treatment involve administering the pharmaceutical formulations to patients in need of such treatment.
Owner:OREXIGEN THERAPEUTICS INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of rhabdomyolysis and / or elevated CPK as possible side effects; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for such conditions and employ methods that will improve the therapeutic outcome in the few patients who experience rhabdomyolysis and / or elevated CPK associated with zonisamide therapy.
Owner:EISAI INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of hyperammonemia as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for hyperammonemia and employ methods that will improve the therapeutic outcome in the few patients who experience hyperammonemia associated with zonisamide therapy.
Owner:EISAI INC
Novel crystalline forms of sodium 1,2-benzisoxazole-3-methanesulfonate, processes of preparing same and use thereof in the synthesis of zonisamide
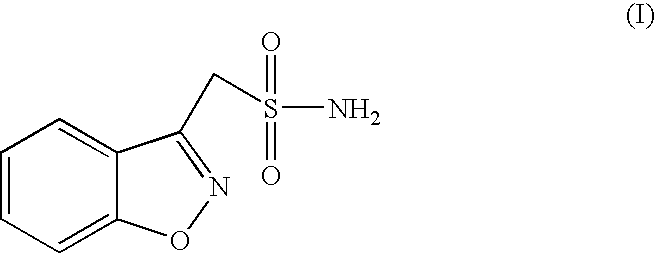
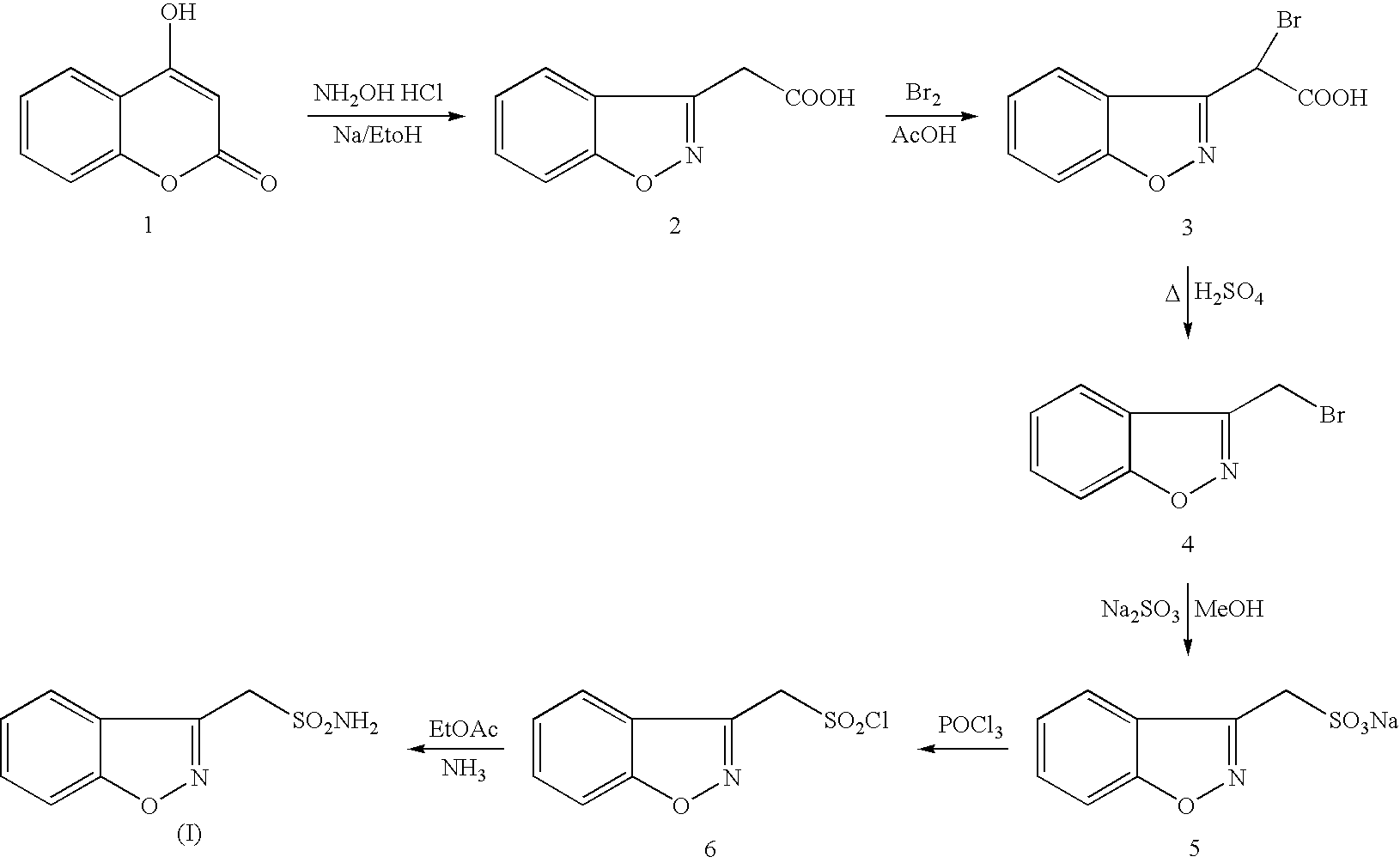
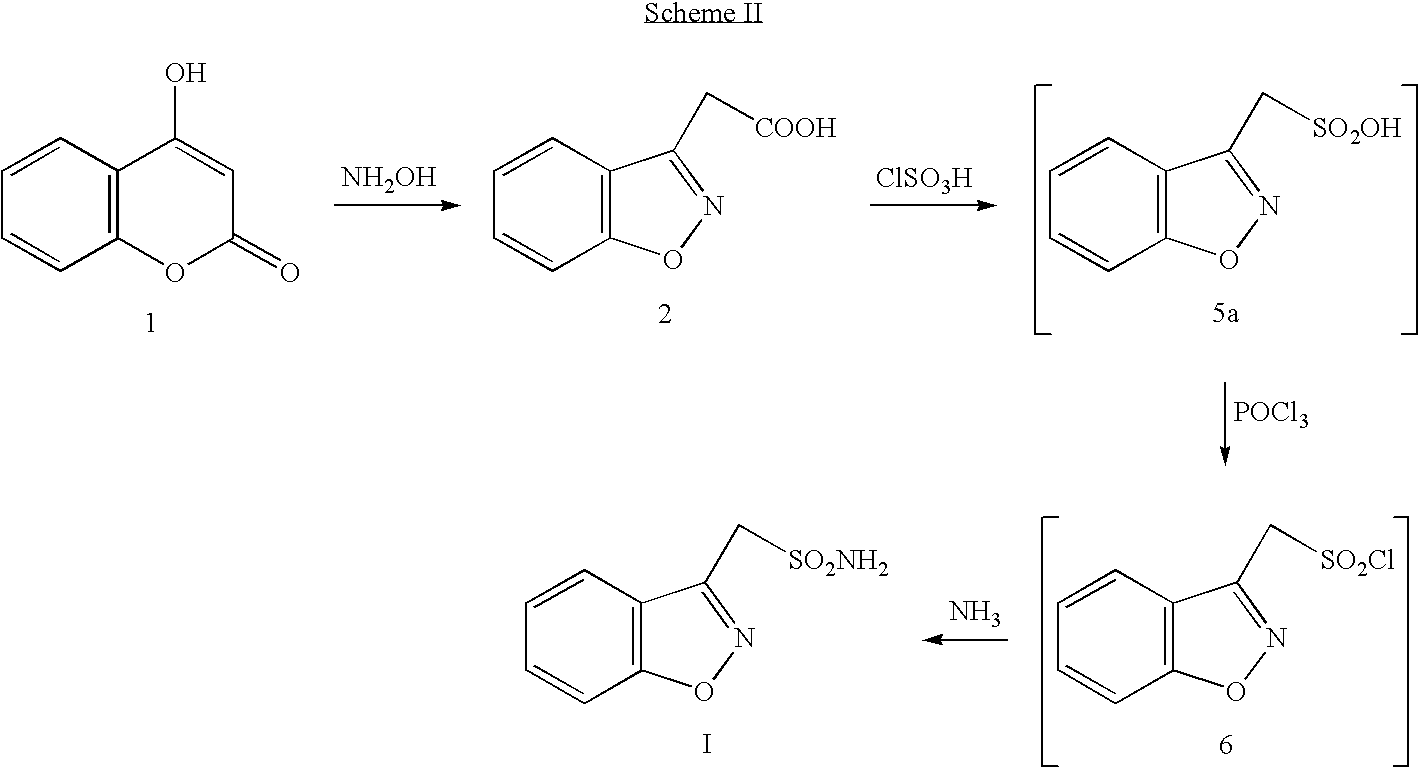
Disclosed is a process of preparing 1,2-benzisoxazole-3-methanesulfonamide (zonisamide). Also disclosed is a method of dehydrating sodium 1,2-benzisoxazole-3-methanesulfonate, a compound useful in the preparation of 1,2-bisoxazole-3-methanesulfonamide (zonisamide) as well as new crystalline forms of sodium 1,2-benzisoxazole-3-methanesulfonate.
Owner:CHEMAGIS
Method for preparing benzisoxazole methane sulfonyl chloride and its amidation to form zonisamide
The present invention relates to a process of preparing benzisoxazole methane sulfonic acid-chloride (BOS—Cl) as an zonisamide intermediate via chlorination of benzisoxazole methane sulfonate. The present invention also discloses a process of preparing zonisamide via amidation of BOS—Cl. More particularly, the present invention provides a process of preparing zonisamide, comprising the steps of: a) chlorinating BOS, salts or esters thereof, with SOCl2 in an organic solvent and / or in the presence of a catalyst to form BOS—Cl; and b) amidating BOS—Cl in the presence of ammonia selected from the group consisting of aqueous ammonia in a biphasic system, masked ammonia and dry ammonia to form zonisamide.
Owner:TEVA PHARM USA INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of neuroleptic malignant syndrome (NMS) as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for NMS and employ methods that will improve the therapeutic outcome in the few patients who experience NMS associated with zonisamide therapy.
Owner:EISAI INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of hyperammonemia as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for hyperammonemia and employ methods that will improve the therapeutic outcome in the few patients who experience hyperammonemia associated with zonisamide therapy.
Owner:EISAI INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of rhabdomyolysis and / or elevated CPK as possible side effects; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for such conditions and employ methods that will improve the therapeutic outcome in the few patients who experience rhabdomyolysis and / or elevated CPK associated with zonisamide therapy.
Owner:EISAI INC
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of neuroleptic malignant syndrome (NMS) as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for NMS and employ methods that will improve the therapeutic outcome in the few patients who experience NMS associated with zonisamide therapy.
Owner:EISAI INC
Process for the preparation of 1,2-dichloroethane free crystals of zonisamide
InactiveUS20040138474A1Improve securityNervous disorderOrganic chemistryAlcoholAzeotropic distillation
A process for the preparation of crystals of zonisamide containing residual 1,2-dichloroethane of not more than 5 ppm, which comprises adding an aqueous C2-4 alcohol to crystals of zonisamide containing residual 1,2-dichloroethane of more than 5 ppm, removing said 1,2-dichloroethane by azeotropic distillation, followed by collecting the precipitated crystals from the residual mixture.
Owner:UENO YOSHIKAZU +1
Process for the preparation of zonisamide
InactiveUS20060084814A1Good lookingAvoid isolationOrganic chemistryMethane sulfonic acidOrganic solvent
The present invention provides an improved process for the preparation of zonisamide or a derivative thereof comprising (a) reacting 1,2-benzisoxazole-3-methane-sulfonic acid with a halogenating agent in a first organic solvent to provide benzisoxazole methane sulfonyl halide; and, (b) reacting benzisoxazole methane sulfonyl halide with an amine in a second organic solvent to form zonisamide or a derivative thereof.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Methods of using zonisamide as an adjunctive therapy for partial seizures
InactiveUS20050059718A1Reduce removalQuickly recognizing and minimizing a serious side effectBiocideAnimal repellantsSide effectMonoclonal gammopathy of undetermined significance
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), and / or multiple myeloma (MM) as possible side effects; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for such conditions and employ methods that will improve the therapeutic outcome in the few patients who experience MGUS, SMM, and / or MM associated with zonisamide therapy.
Owner:EISAI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com