Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
157 results about "Benzisoxazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C₇H₅NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
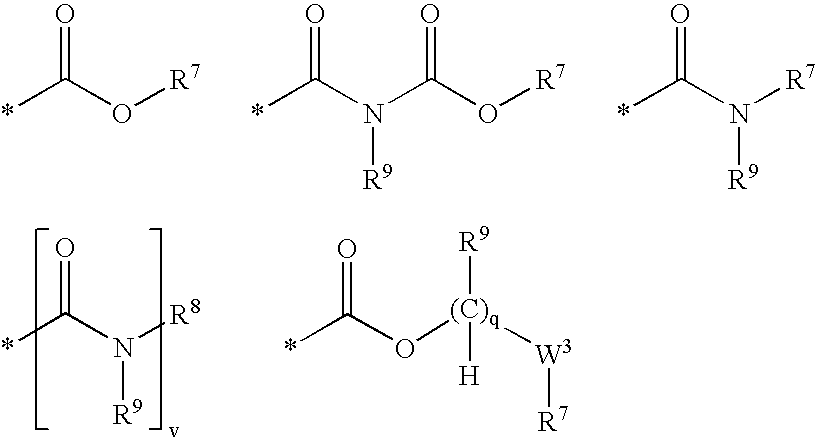
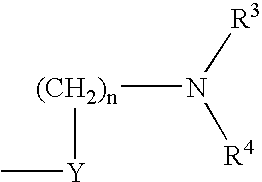
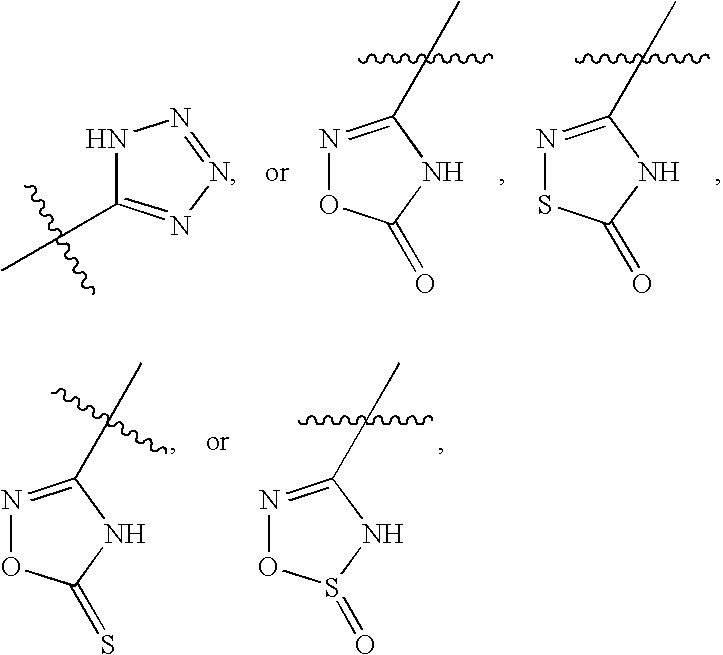
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Methods and dosage forms for reducing side effects of benzisozazole derivatives
InactiveUS20050208132A1Stable conditionReduce high blood pressureBiocideNervous disorderSide effectDosage form
Disclosed are dosage forms and methods comprising benzisoxazole derivatives. More particularly, disclosed are dosage forms, methods, and new uses of benzisoxazole derivatives that substantially reduce or substantially eliminate certain side effects of the benzisoxazole derivatives when dosed to a patient.
Owner:ALZA CORP
Aryl- and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
ActiveUS20060052378A1Good curative effectQuick effectBiocideNervous disorderBenzoxazoleChemical structure
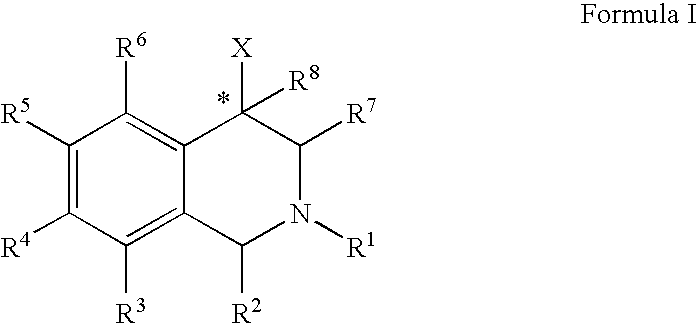
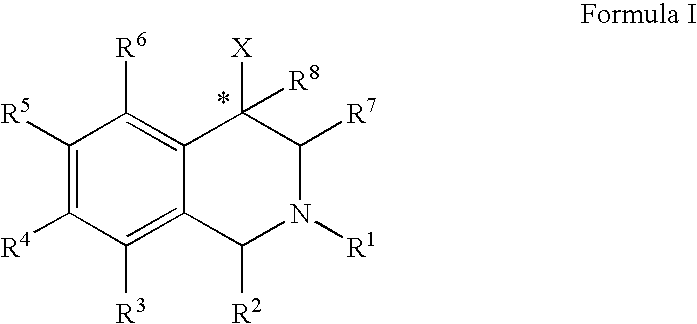
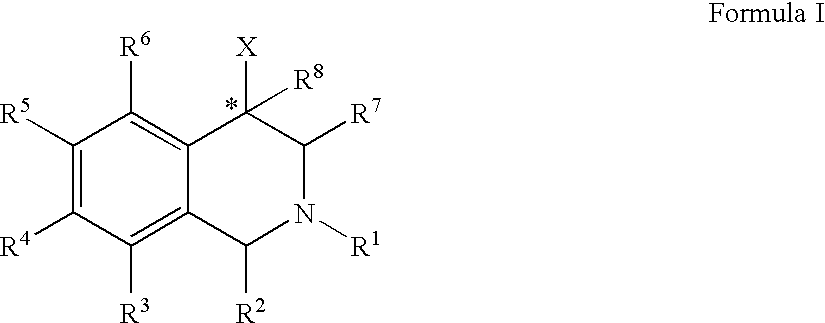
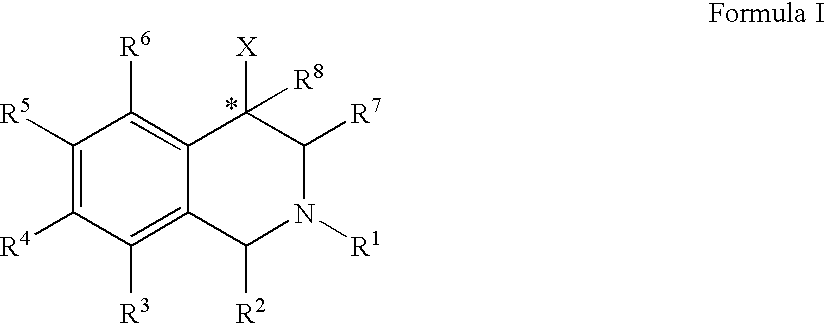
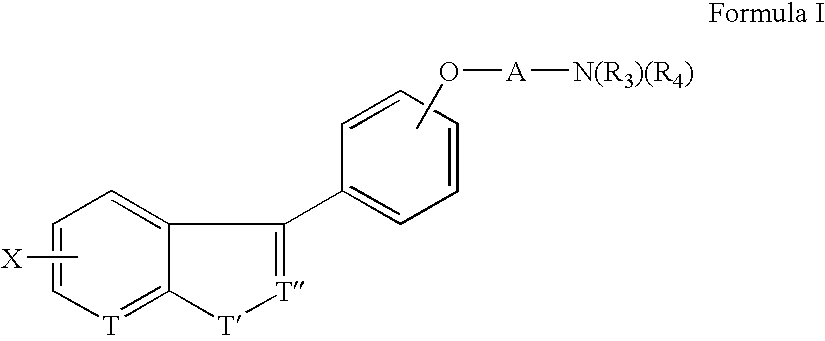
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Use of aryl- and heteroaryl-substituted tetrahydroisoquinolines to block reuptake of norepinephrine, dopamine, and serotonin
InactiveUS20060063766A1Little and no activityMinimal potential for substance abuseBiocideNervous disorderBenzoxazoleBenzene
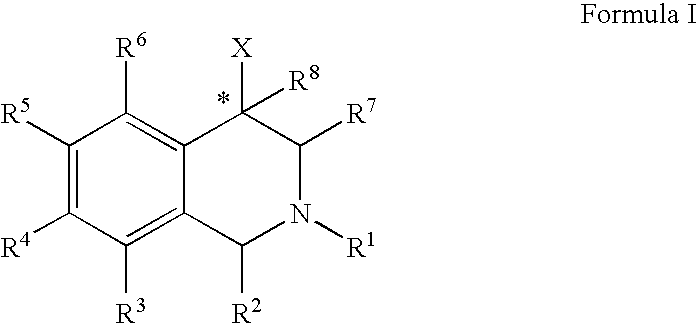
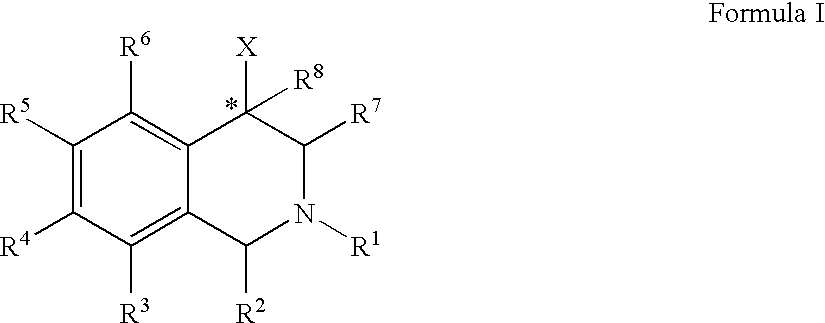
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
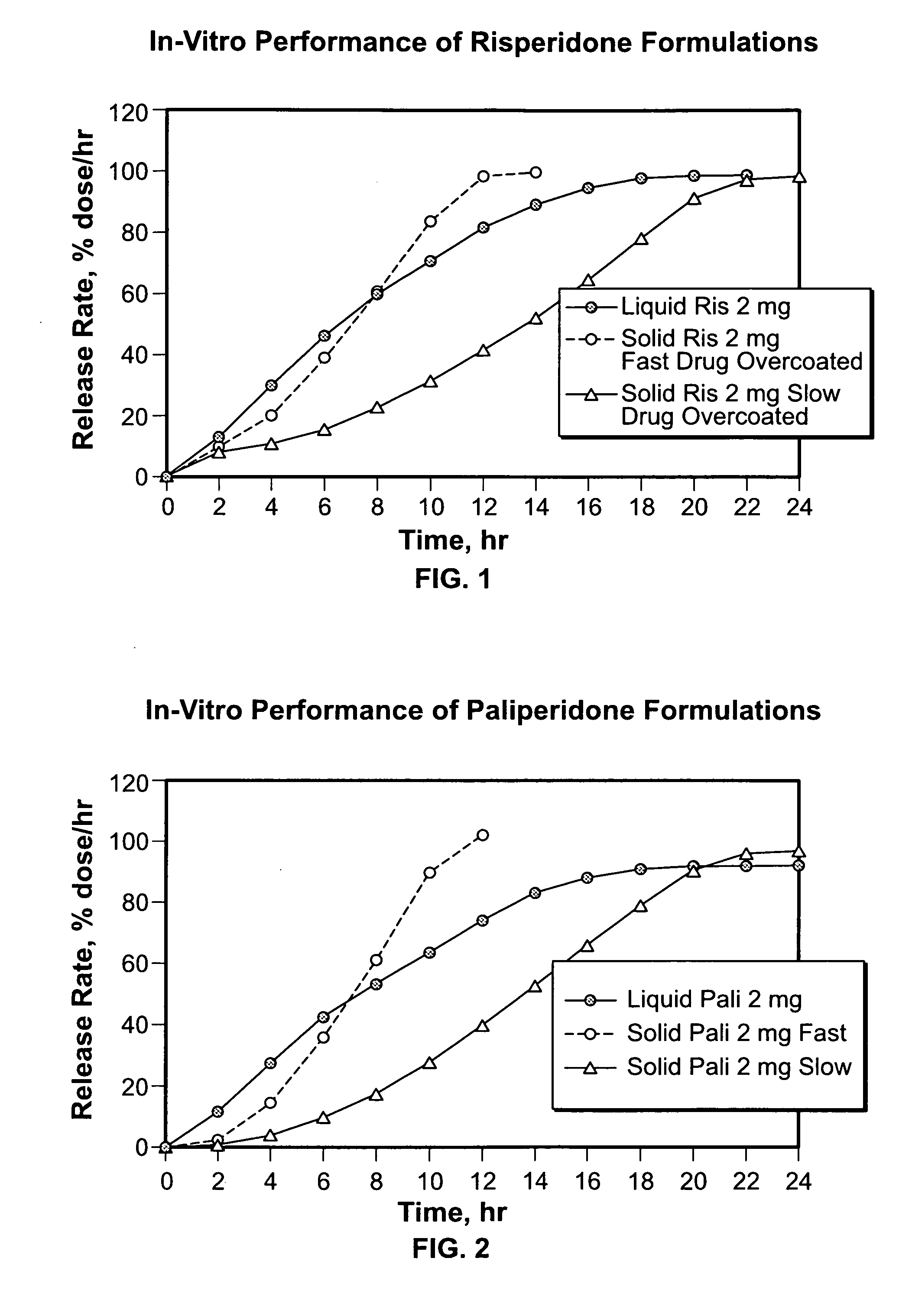
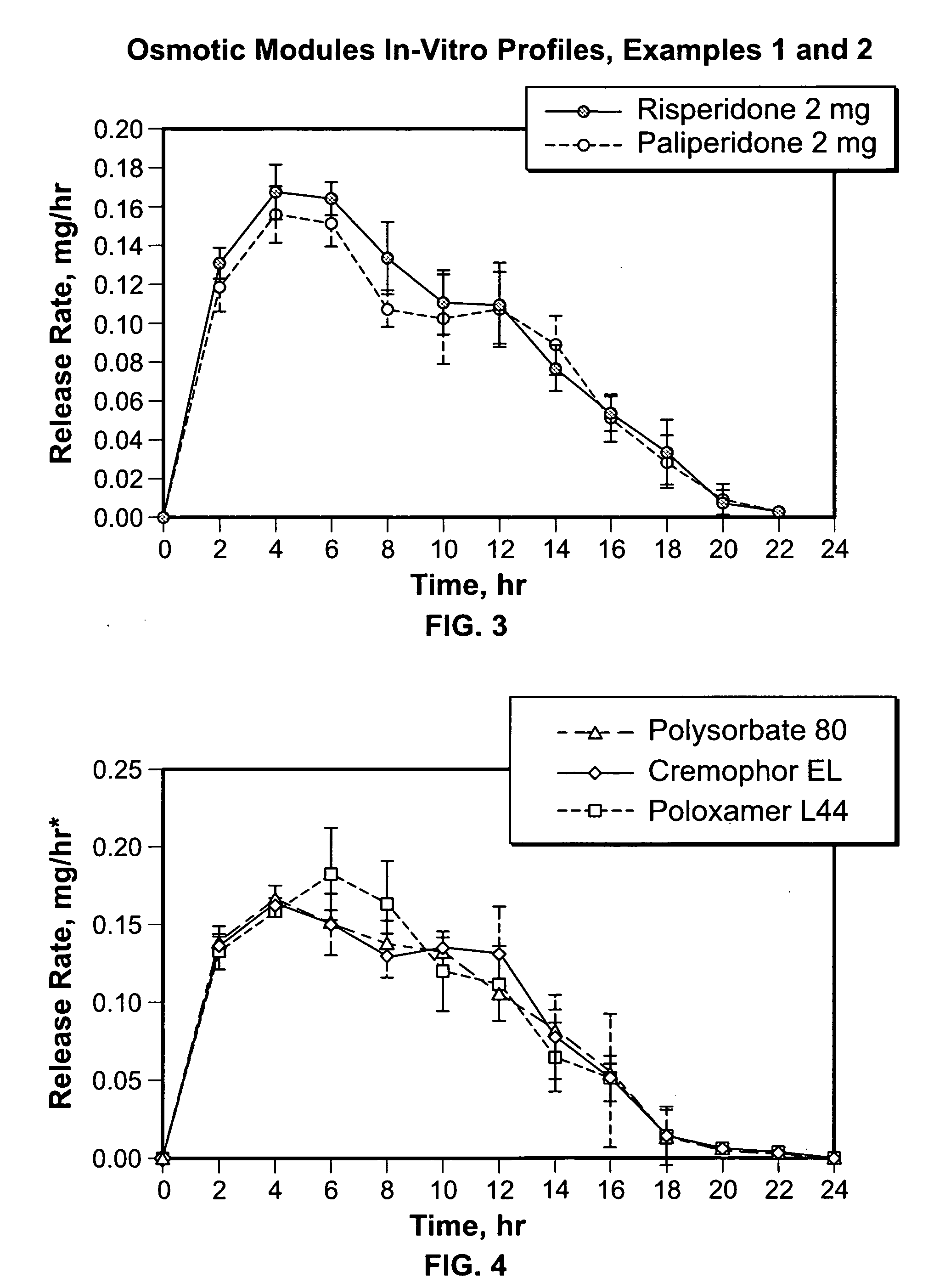
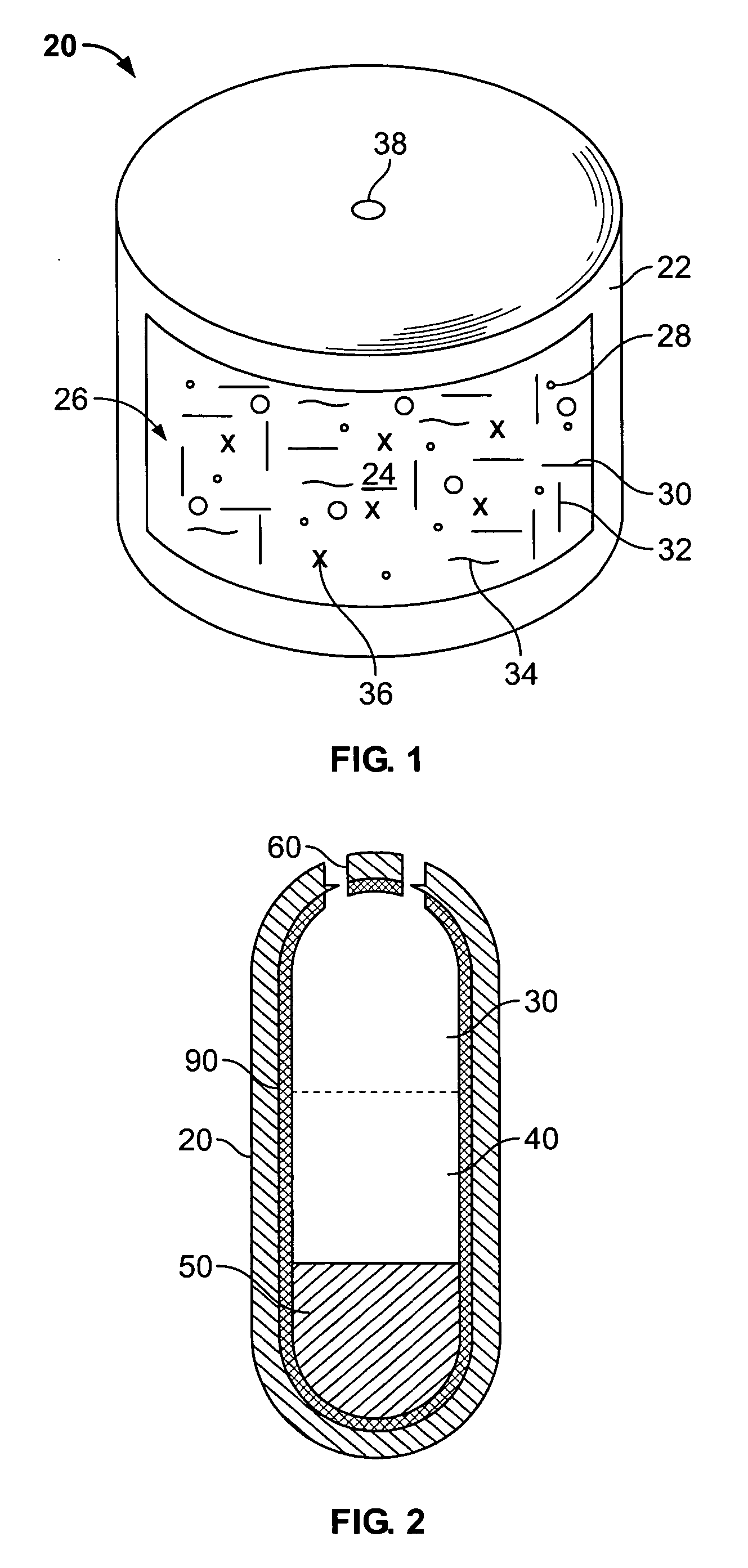
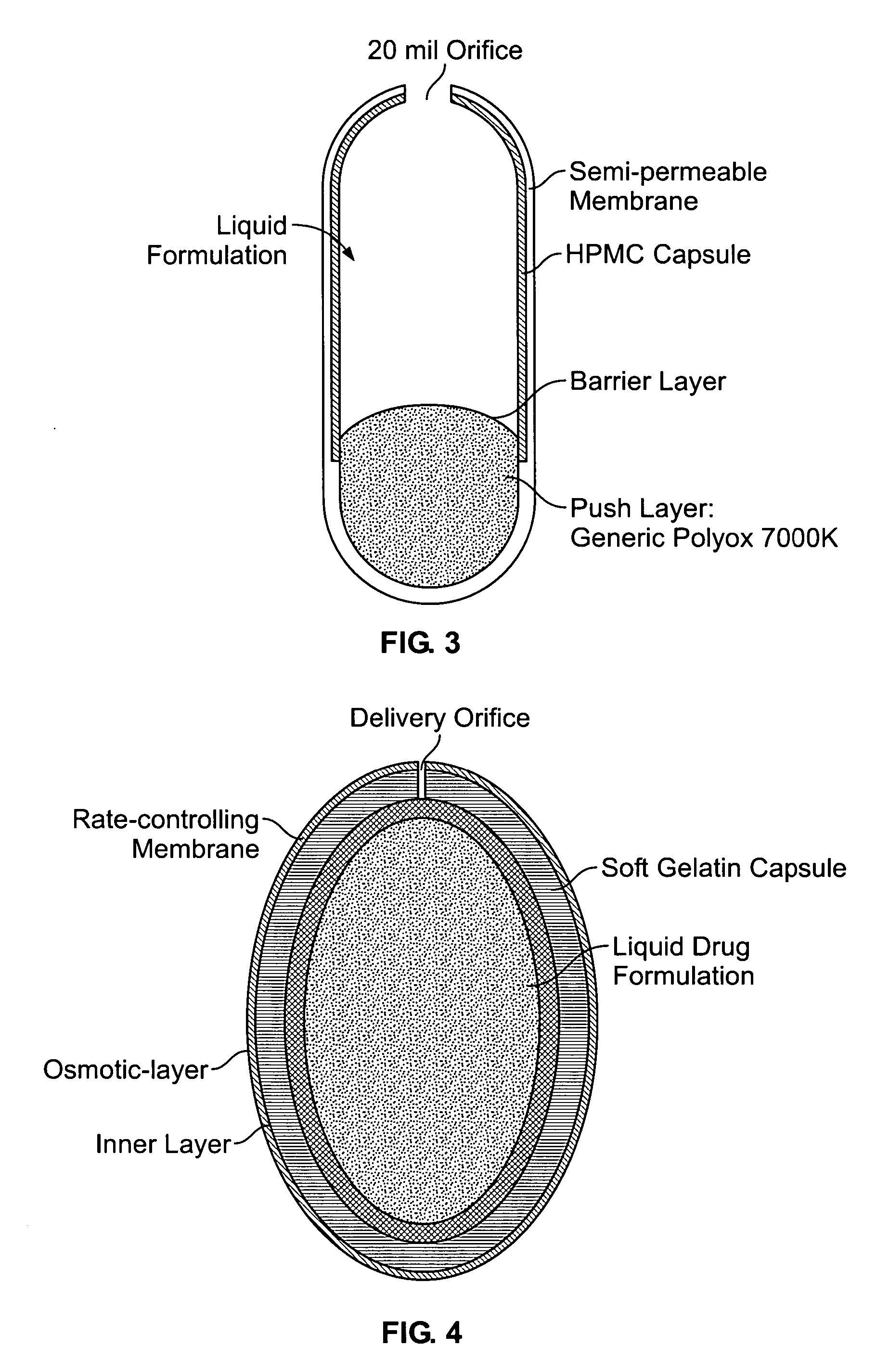
Liquid formulations for controlled delivery of benzisoxazole derivatives
Disclosed are dosage forms including a controlled release dosing structure; and a liquid formulation contained within the controlled release dosing structure; wherein the liquid formulation comprises a benzisoxazole derivative and a liquid carrier. Also disclosed are methods of making and using such dosage forms.
Owner:ALZA CORP
Enhanced efficacy benzisoxazole derivative dosage forms and methods
Disclosed are dosage forms and methods comprising benzisoxazole derivatives. More particularly, disclosed are dosage forms, methods, and new uses of benzisoxazole derivatives that provide enhanced efficacy when used in the treatment of schizophrenia and / or bipolar mania.
Owner:KRAMER MICHELLE +3
Compositions for treating CNS disorders
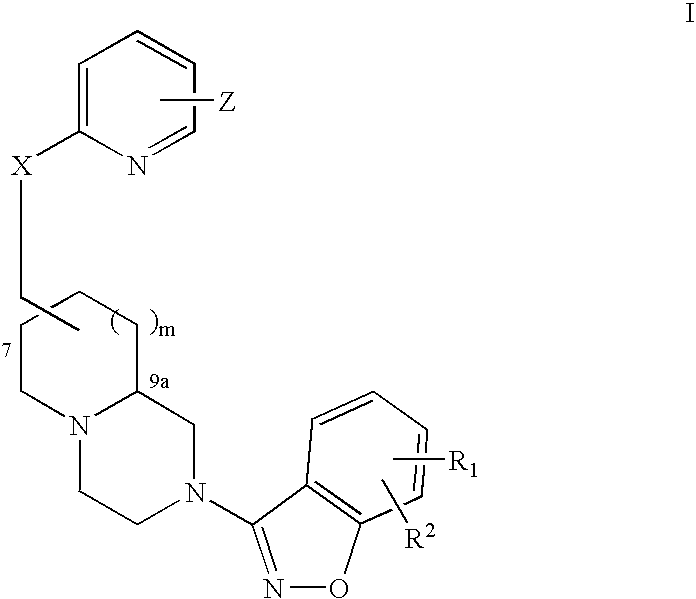
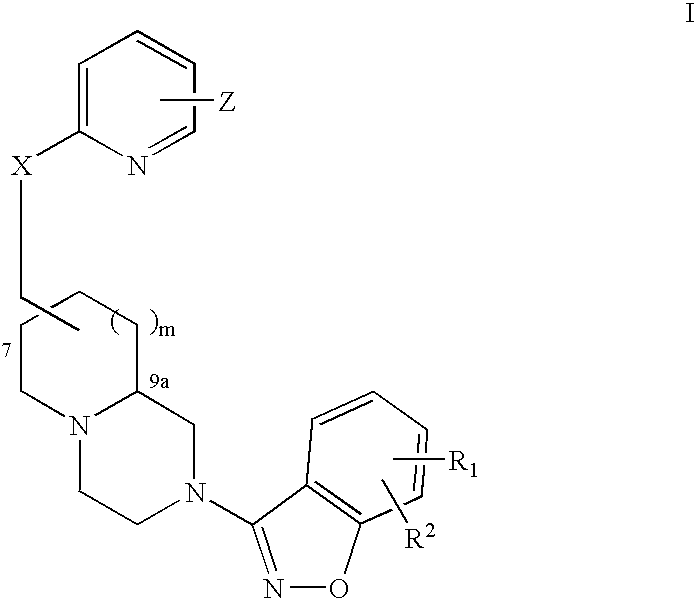
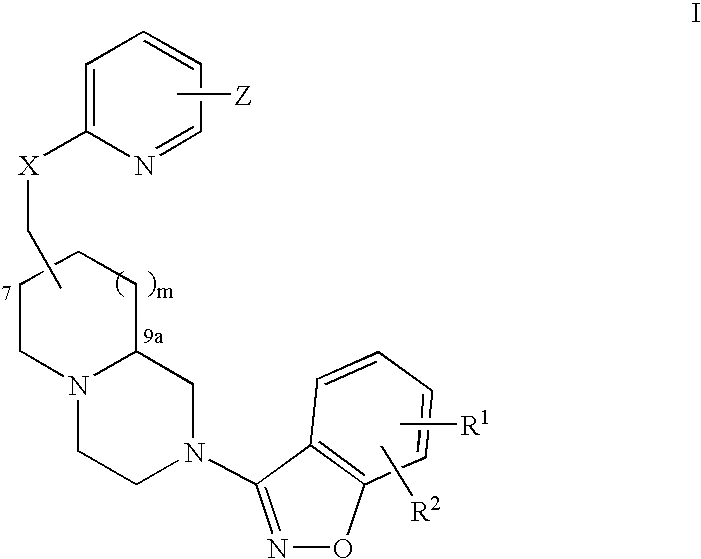
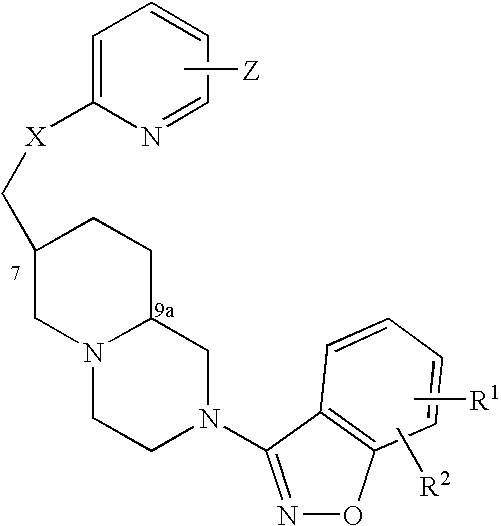
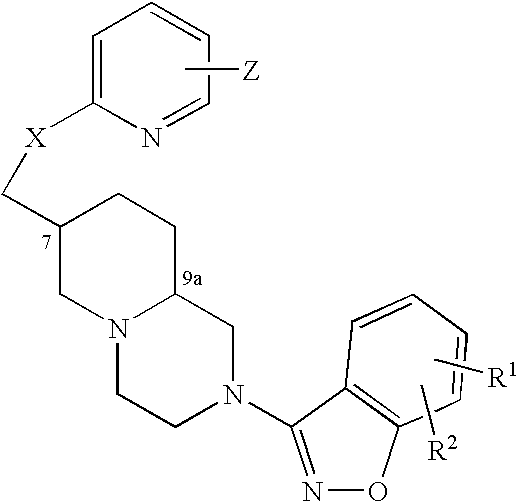
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating one or more CNS or other disorders, including concurrent treatment of disorders such as schizophrenia and depression.
Owner:PFIZER INC
3-amido-1,2-benzoisoxazole derivatives, process for preparation, and use thereof
InactiveUS7291638B2Improve bioavailabilityImprove their bioavailabilitiesBiocideOrganic chemistryLeukotriene BBioavailability
Owner:DONG WHA PHARM CO LTD
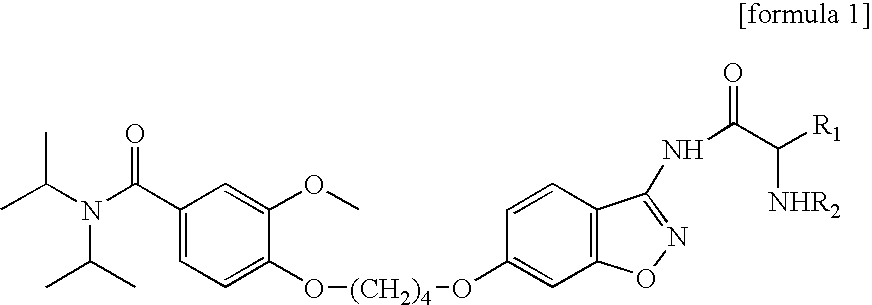
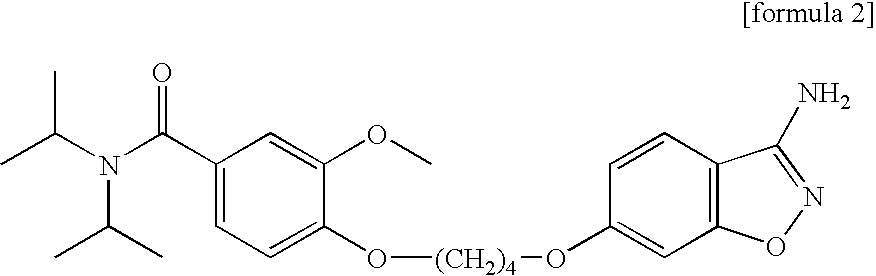
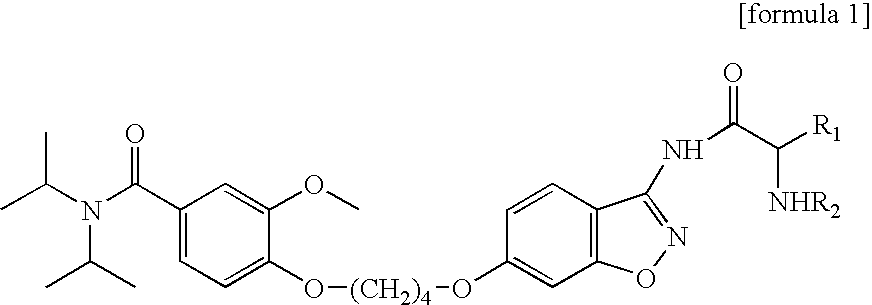
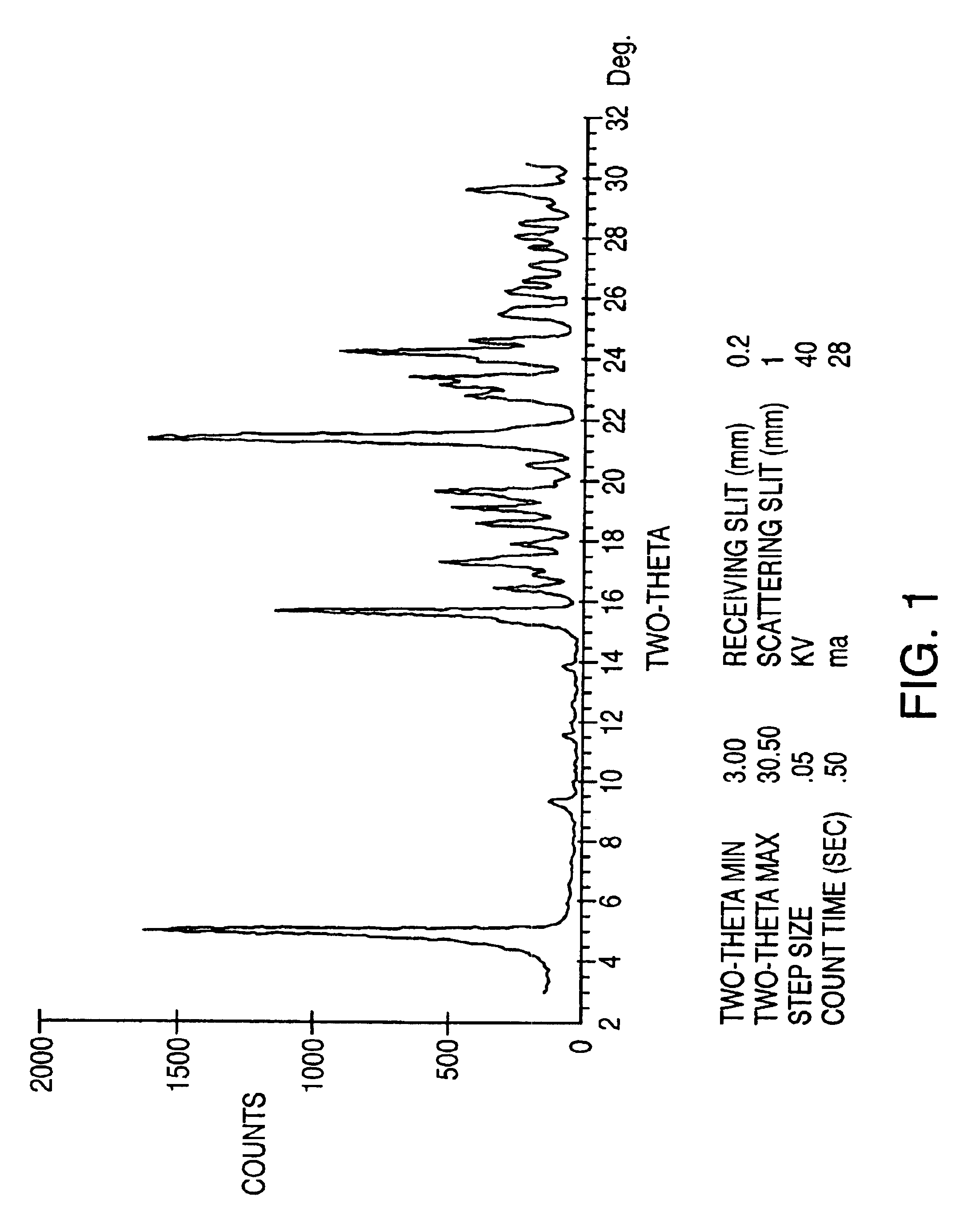
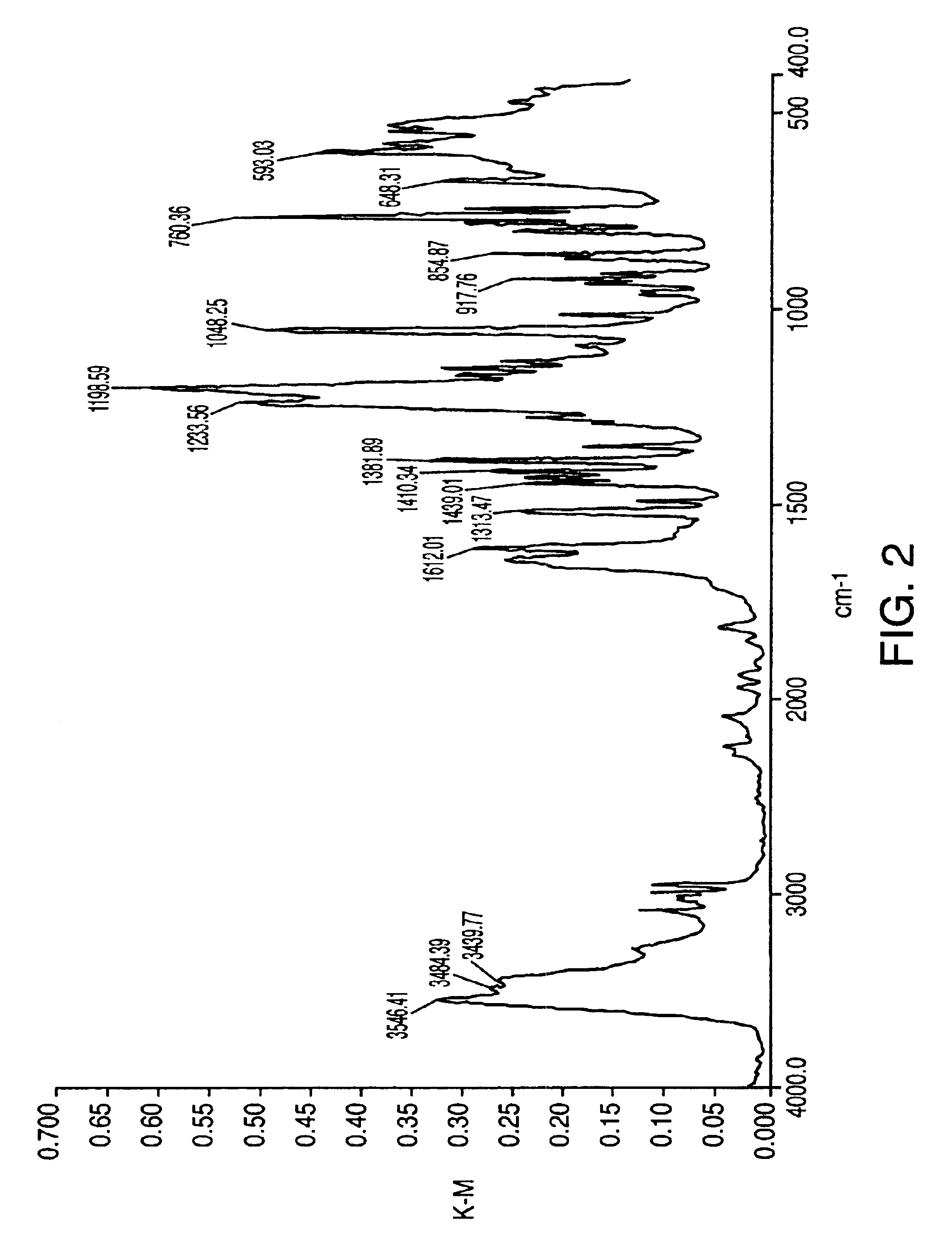
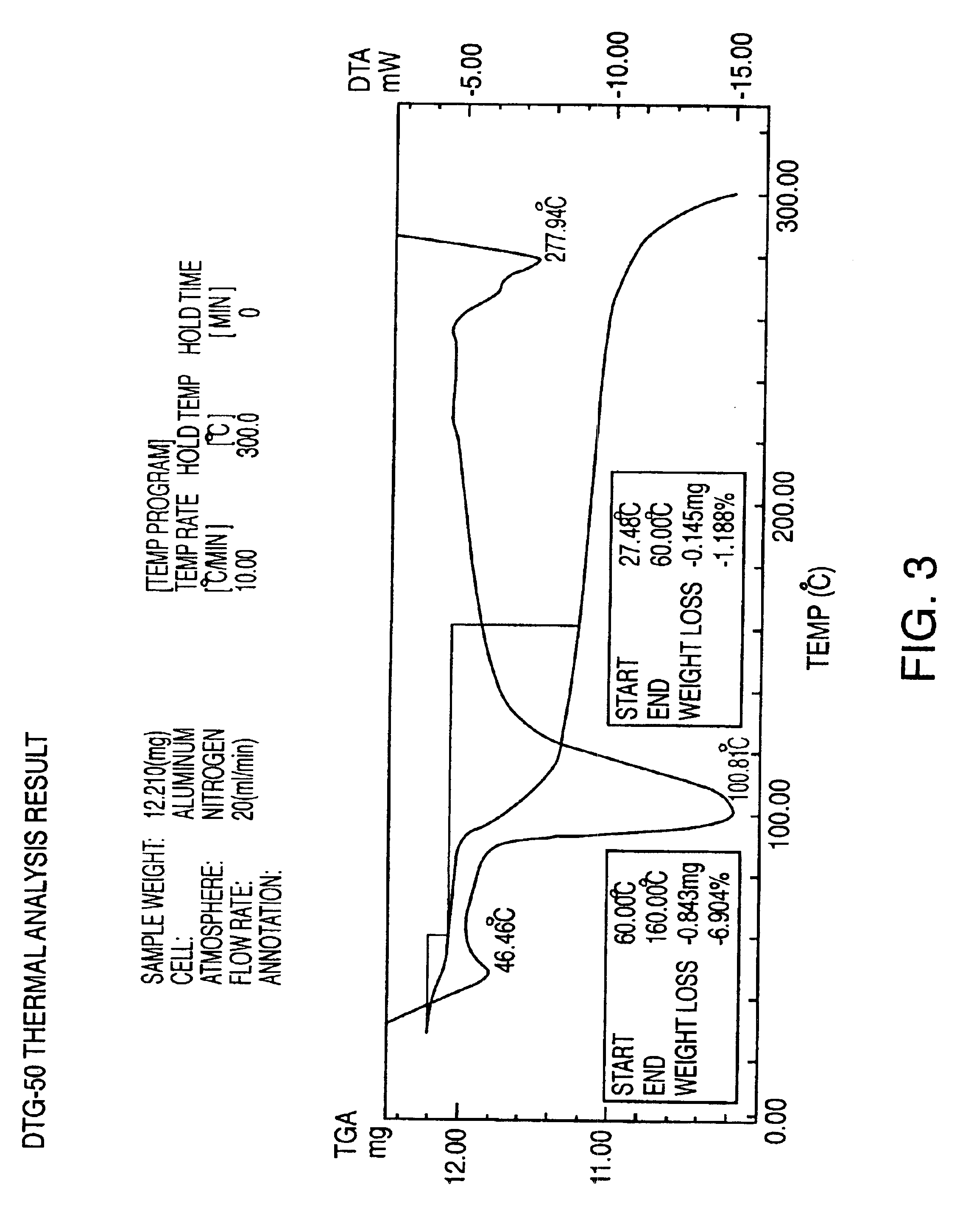
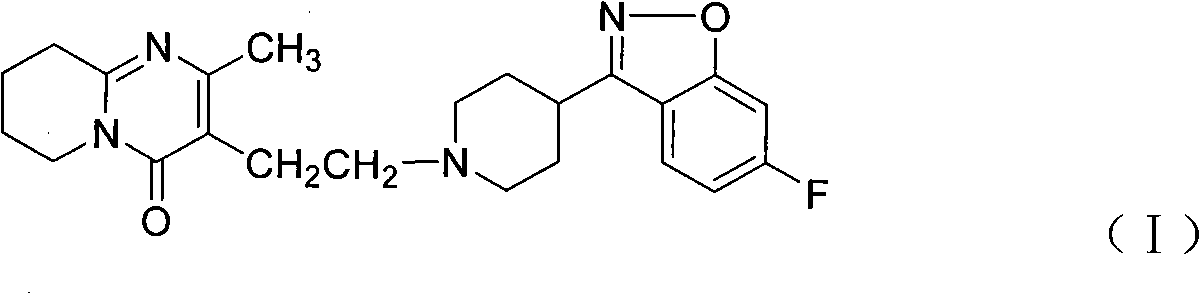
Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester
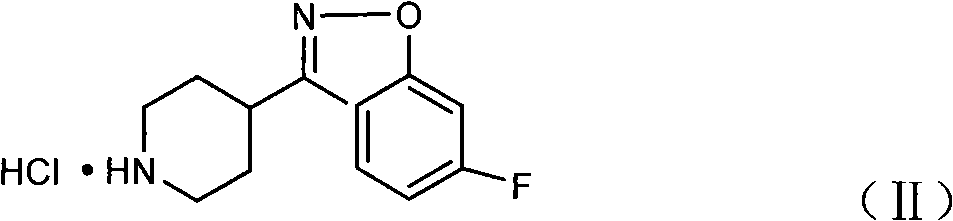
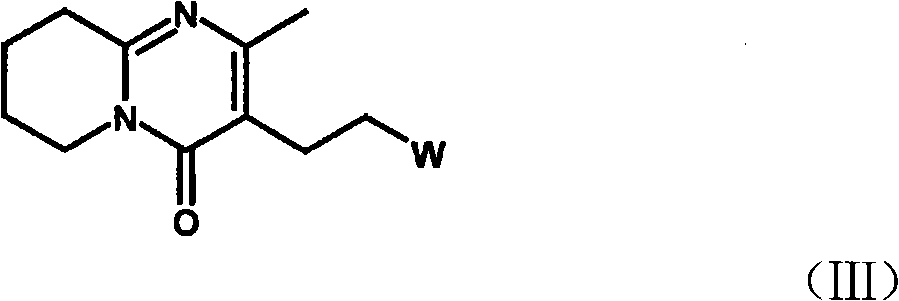
The present invention concerns a process for preparing aseptic crystalline 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one palmitate ester (I) substantially free of 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one (II-a), 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3 -yl)-1-piperidinyl]ethyl]-6,7-dihydro-2-methyl-4H-pyrido[1,2-a]-pyrimidin-4-one (II-b), and 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-ethyl]-6,7,8,9-tetrahydro-2-methyl-9-pentadecyl-4H-pyrido[1,2-a]pyrimidin-4-one (III), and having an average particle size ranging from 20 to 150 μm.
Owner:SPITTAELS THOMAS FREDERIK ERNESTINE +3
Pyridyloxymethyl and benzisoxazole azabicyclic derivatives
Owner:PFIZER INC
Benzisoxazolyl-, pyridoisoxazolyl-and benzthienyl-phenoxy derivatives useful as D4 antagonists
The compounds are of the class of benzisoxazolyl-, pyridoisoxazolyl- and benzthienyl-phenoxy derivatives, useful as D4 antagonists. Said compounds are useful for the treatment of medical conditions mediated by inhibition of D4 receptor. These conditions comprise, for example, Attention Deficit Hyperactivity Disorder, Obsessive-Compulsive Disorder, Psychoses, Substance Abuse, Substance Dependence, Parkinson's Disease, Parkinsonism, Tardive Diskinesia, Gilles de la Tourette Syndrome, Conduct Disorder, and Oppositional Defiant Disorder. A further aspect of the invention is to provide a pharmaceutical composition, intermediates, and a method of making said class of compounds.
Owner:AVENTIS PHARMA INC
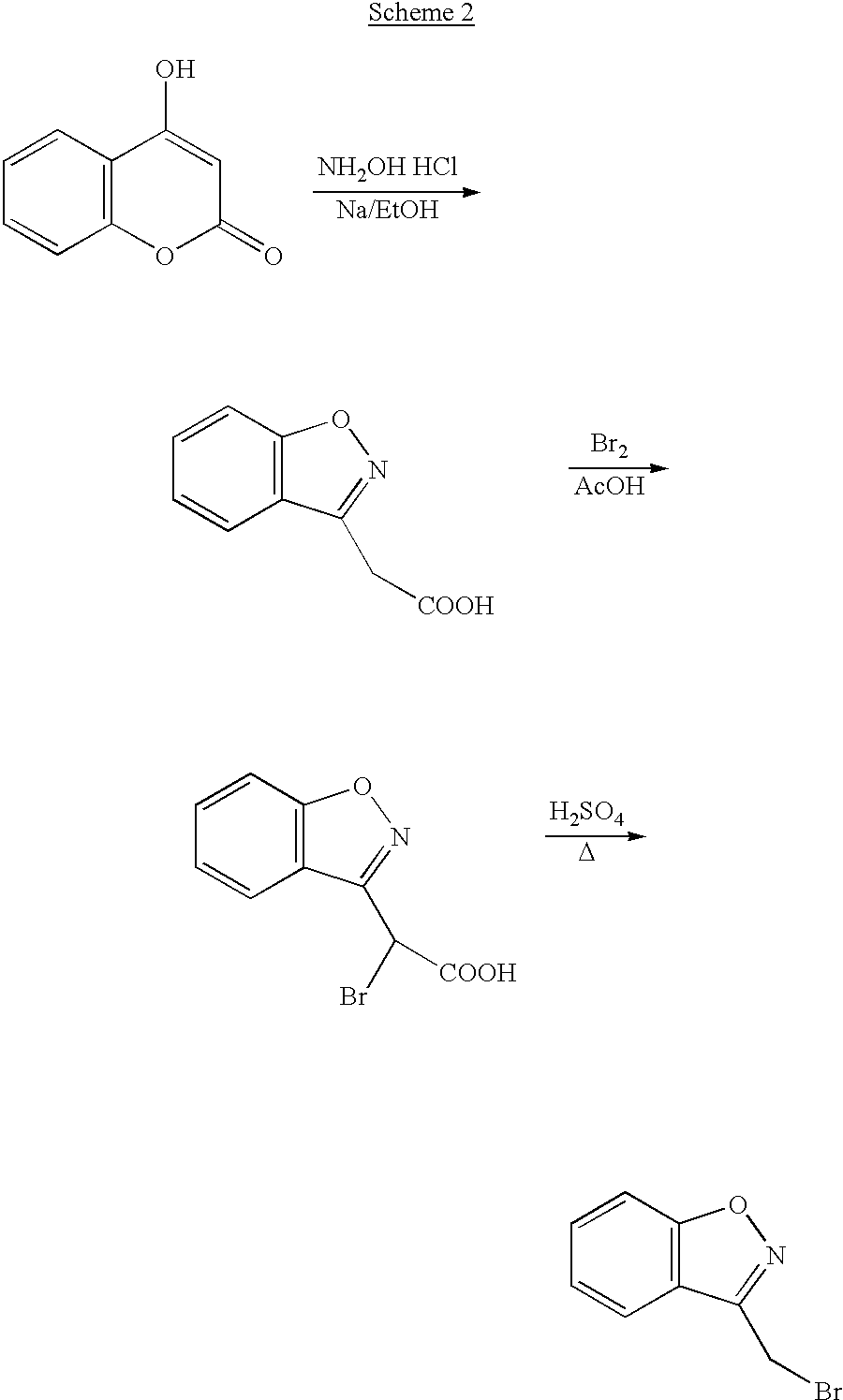
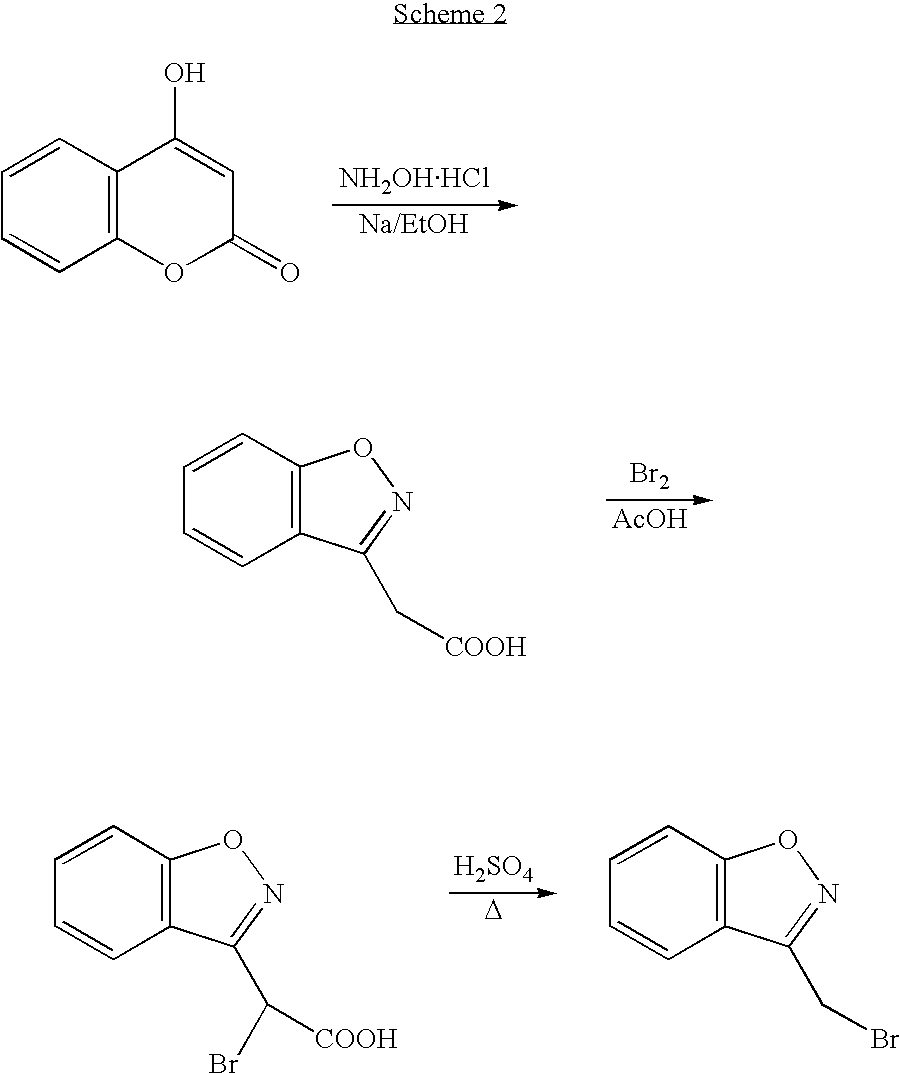
Process for the preparation of 1,2-benzisoxazole-3-acetic acid
InactiveUS20020183525A1High puritySpeed up the processOrganic chemistryAcetic acidMethane sulfonic acid
The present invention provides a process for preparing 1,2-benzisoxazole-3-acetic acid, comprising the step of reacting 4-hydroxy-coumarin with hydroxyl-amine in the presence of a base. The present invention further provides a process for preparing a salt of benzisoxazole methane sulfonic acid, comprising the steps of 1) sulfonating 1,2-benzisoxazole-3-acetic acid using chlorosulfonic acid in a solvent mixture comprising methylene chloride and sodium hydroxide; and 2) isolating the salt of benzisoxazole methane sulfonic acid.
Owner:TEVA PHARM USA INC
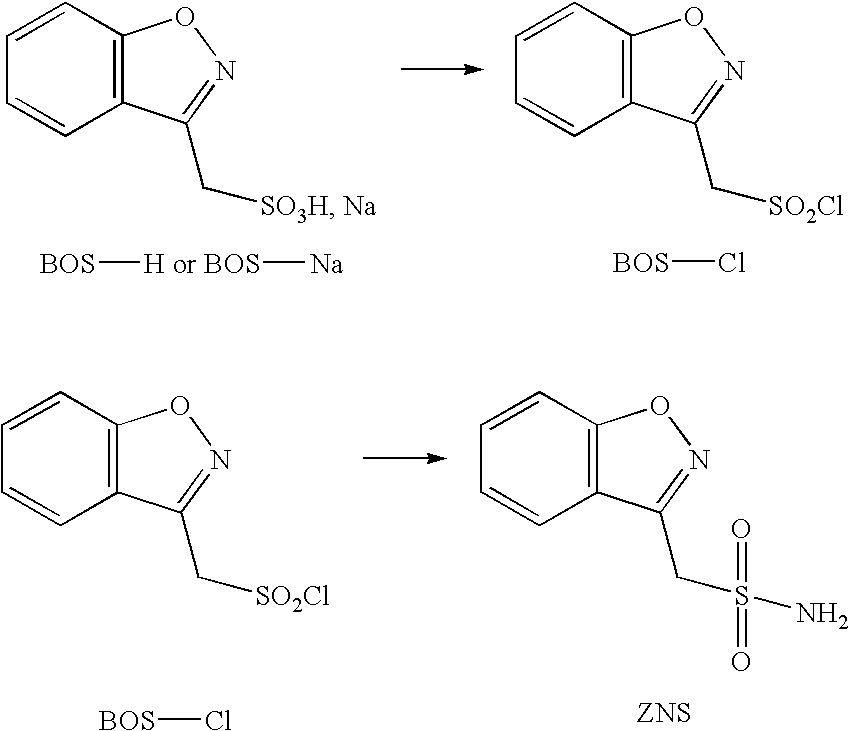
Sulfonation method for zonisamide intermediate in zonisamide synthesis and their novel crystal forms
The present invention relates to a novel sulfonation of an intermediate of zonisamide. The sulfonation processes using chlorosulfonic acid as well as acetic anhydride and sulfuric acid in an organic solvent are disclosed. Crystalline forms of benzisoxazole methane sulfonic acid (BOS-H) and its salts (BOS-Na, BOS-Ca, and BOS-Ba) and their novel preparation processes are disclosed.
Owner:TEVA PHARM USA INC
Method for preparing 6-fluoro-3-(4- piperidyl)-1,2-benzo isoxazole hydrochlorate
ActiveCN101328173APrevent participation in responseSolve the problems of many types, complicated operation and low yieldNervous disorderOrganic chemistryCyclopropanationPetroleum ether
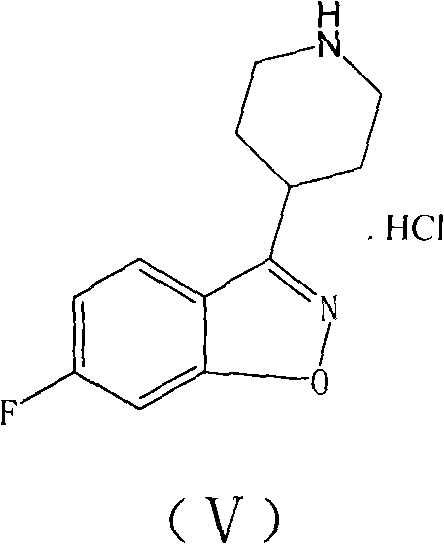
The invention relates to a method for preparing a hydrochloride of 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole. The method is to subject 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to cyclopropanation and salifying in an aprotic solvent containing an alkali metal hydroxdide, wherein the molar ratio of the 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to the alkali metal hydroxide is 1 to between 1 and 3. The method can effectively prevent F atoms on para positions from participating in the reaction and avoid the production of a dipolymer (V), thereby avoiding the production of a dipolymer (VI) during the following preparation of risperidone. The method crystallizes crystals of a target substance directly by salifying, thereby solving the problems of use of a plurality of kinds of organic solvents, complex operation and low yield due to the extraction by toluene, condensation, and crystallization of petroleum ether. The yield rate of the target substance is more than 80 percent, and the purity of target substance is more than 99 percent.
Owner:CSPC OUYI PHARM CO LTD
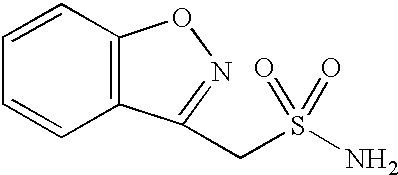
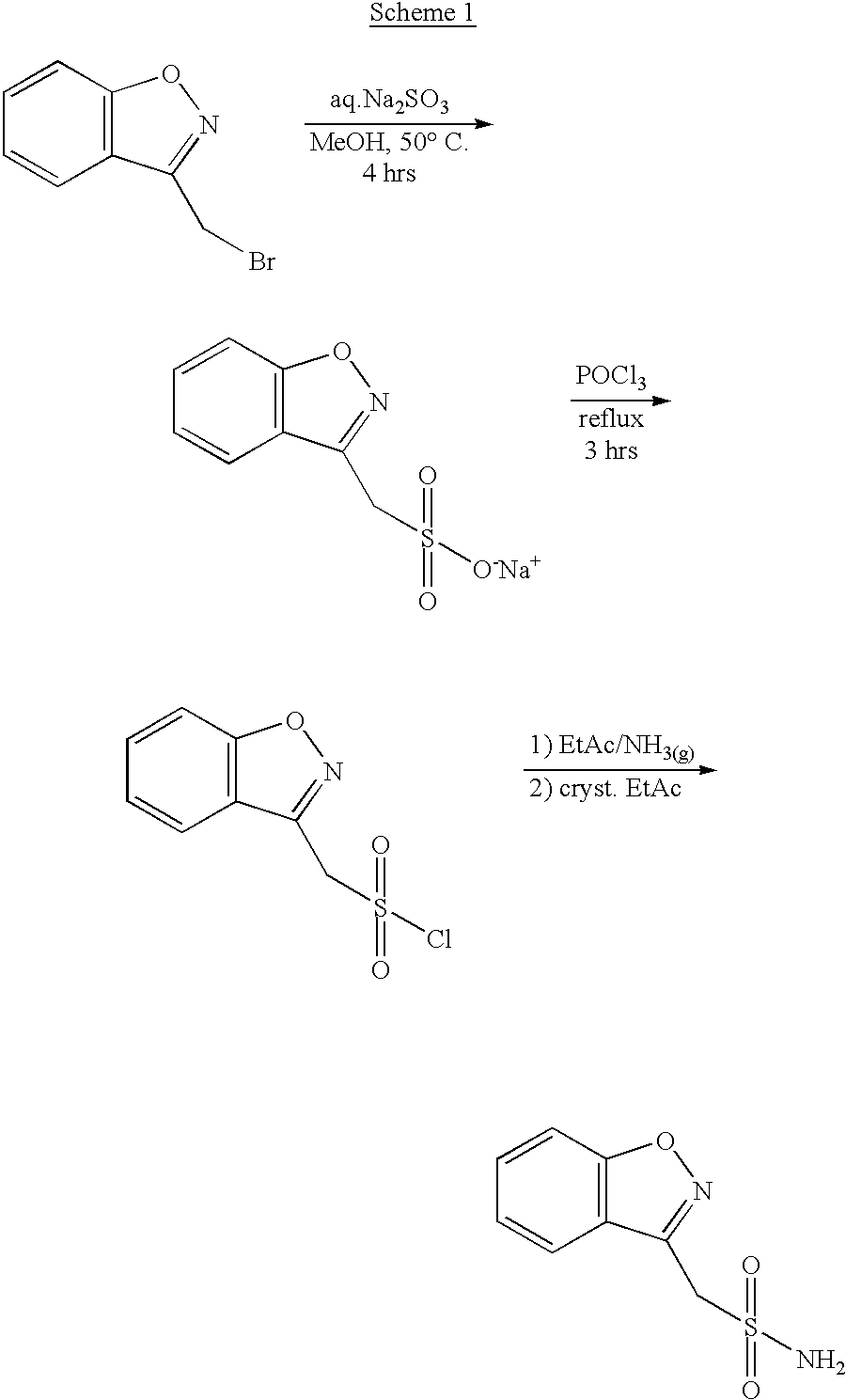
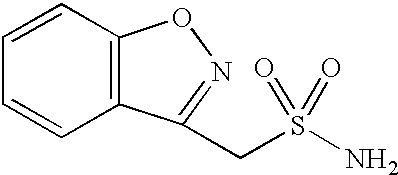
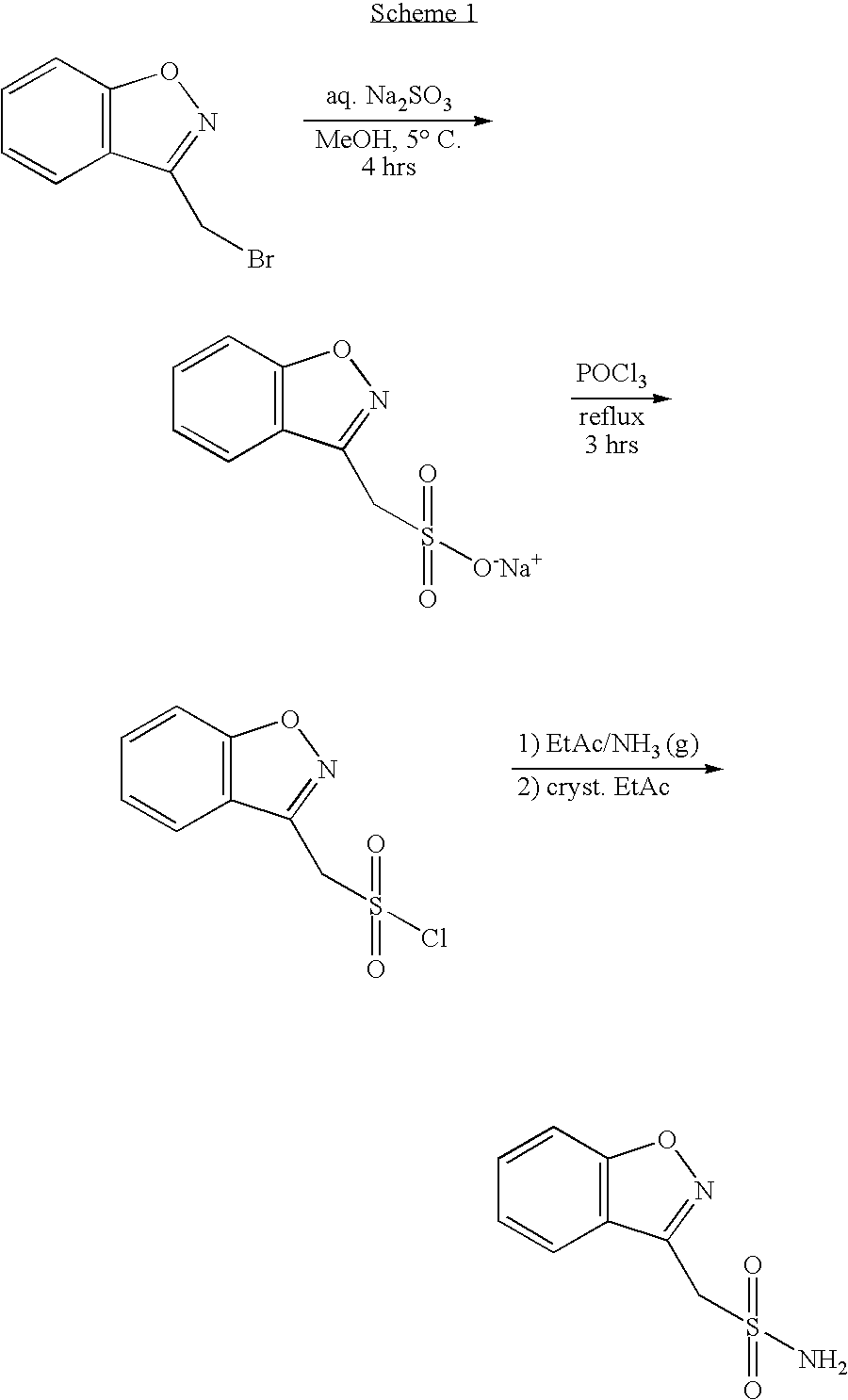
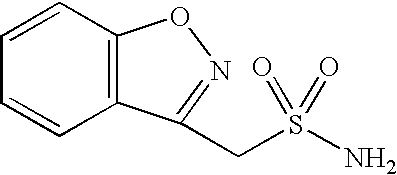
One-pot process for the preparation of 1,2-benzisoxazole-3-methanesulfonamide
A process for the preparation of 1,2-benzisoxazole-3-methane-sulfonamide without isolation of intermediates in solid form, by using 4-hydroxycoumarin as a starting compound, and water and 1,2-dichloro-ethane as solvents; and an industrially useful process for the preparation of 1,2-benzisoxazole-3-acetic acid, by reacting 4-hydroxycoumarin and hydroxylamine in water.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
New preparation method of iloperidone
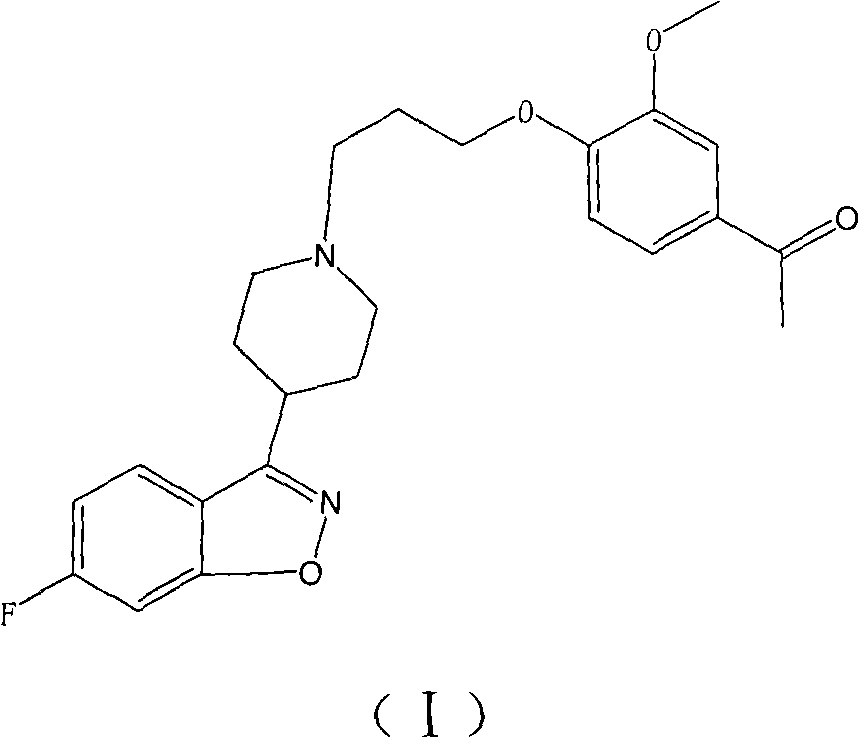
The invention provides a new preparation method of iloperidone, comprising leading 6 - F -3 - (4 - piperidinyl) -1,2 - benzisoxazole hydrochloride and 1 - [4 - (3 - chloropropoxy) -3 - methoxyphenyl] ethyl ketone to react in the inorganic alkaline water solution to obtain the iloperidone. The advantages of the invention are as follows: the method has high yield, low cost, no adverse effects for operators and environment, and is very suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Benzisoxazole piperazine compounds and methods of use thereof
InactiveUS20070004752A1Shorten time to sleepIncrease the lengthOrganic active ingredientsNervous disorderPiperazineChemistry
The invention includes benzisoxazole piperazine compositions and methods of using them for modulating sleep.
Owner:HYPNION INC
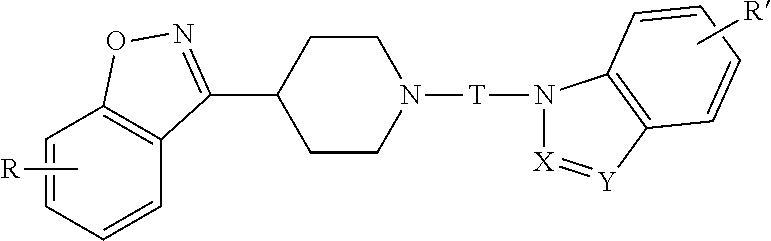
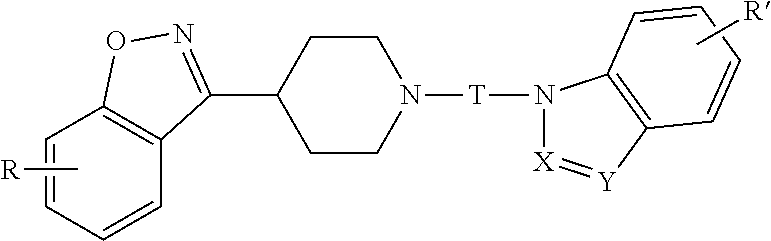
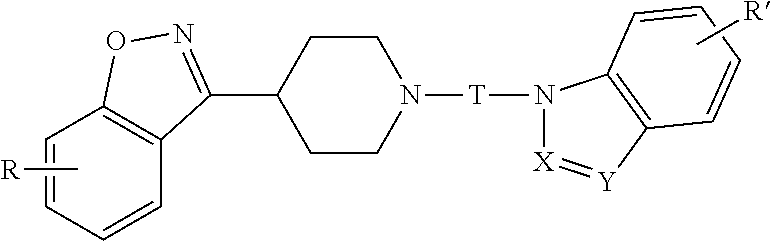
Benzisoxazole piperidinyl derivatives, pharmaceutical compositions comprising the derivatives and their use
InactiveUS20110306638A1Useful propertyImprove toleranceBiocideNervous disorderSide effect5 ht reuptake
The invention relates to a benzisoxazolyl piperidine derivative having the following general formula, a salt or a hydrate thereof,wherein R, X, Y, R′ and T are defined as in the specification. Such compounds have serotonin system modulating effects such as antagonizing effect on 5-HT2A and inhibitory effect on 5-HT reuptake. The compounds have good analgesic and sedative activities with mild toxic and side effects. The invention also relates to a composition comprising said derivative and the use thereof.
Owner:NHWA PHARMA CORPORATION
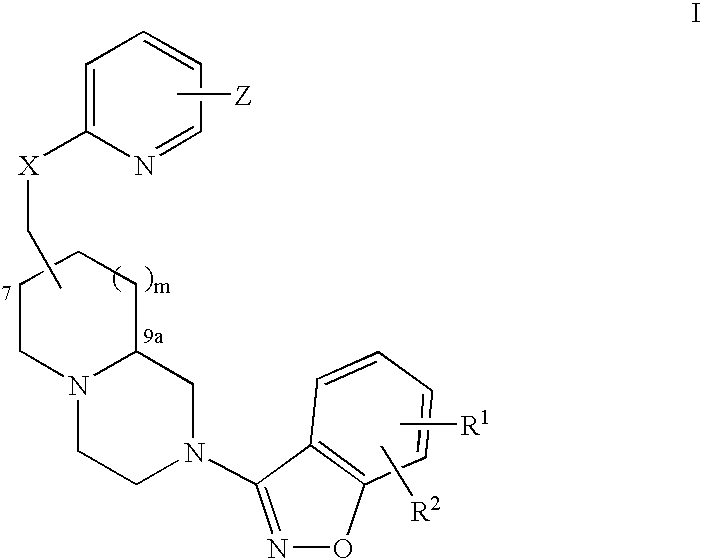
Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
InactiveUS6897308B1Inexpensive and economicalHigh purityNervous disorderOrganic chemistryHydrogenMetal catalyst
A process for the preparation of 3-substituted ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2,-a]pyrimidin-4-one of the formula IIB: where X may be halo, acyloxy, or sulfonyloxy such as tosyloxy or mesyloxy, an intermediate in the synthesis of the anti-psychotic risperidone. The process comprises hydrogenation of 3-substituted ethyl-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one in aqueous inorganic acid medium at atmospheric to 60 psi at 0-100° C. in the presence of a metal catalyst and the product is isolated. A process for the preparation of risperidone of the formula I: comprising condensation of 3-substituted ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2,-a]pyrimidin-4-one with 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole in water in the presence of an inorganic base at 25-100° C. and the product is isolated.
Owner:RPG LIFE SCIENCES
Methods of Treating Mood Disorders Using Pyridyloxymethyl and Benzisoxazole Azabicyclic Derivatives
InactiveUS20080318926A1Effective inhibitory activityIncrease in horizontal locomotor activityBiocideNervous disorderAzabicyclo CompoundsAntisocial personality disorder
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating a mood disorder selected from the group consisting of Somatization Disorder, Borderline Personality Disorder, Narcissistic Personality Disorder, Suicidal Ideation, and Antisocial Personality Disorder.
Owner:PFIZER INC
Process for the preparation of 1,2-benzisoxazole-3-acetic acid
InactiveUS6677458B2High puritySpeed up the processOrganic chemistryMethane sulfonic acidAcetic acid
The present invention provides a process for preparing 1,2-benzisoxazole-3-acetic acid, comprising the step of reacting 4-hydroxy-coumarin with hydroxyl-amine in the presence of a base. The present invention further provides a process for preparing a salt of benzisoxazole methane sulfonic acid, comprising the steps of 1) sulfonating 1,2-benzisoxazole-3-acetic acid using chlorosulfonic acid in a solvent mixture comprising methylene chloride and sodium hydroxide; and 2) isolating the salt of benzisoxazole methane sulfonic acid.
Owner:TEVA PHARM USA INC
Hybrid Composite Yarn
This invention is a hybrid composite yarn comprising: a first polyolefin yarn having >about 80% crystallinity according to WAXS measuring techniques; a second yarn taken from the group consisting of: glass; quartz; carbon; poly(p-phenylene terephthalamide), poly(m-phenylene terephthalamide); poly(vinyl alcohol); poly(1,4-phenylene-2,14-benzibisoxazole) (PBO); poly(1,4-phenylene-2,14-benzobisthiazole) (PBT); poly(benzimidizole) (PBI); poly(ethylene-2,14-naphthalate) (PEN); lyotropic liquid crystalline polymers formed by polycondensation of aromatic organic monomers to form aromatic polyesters, polyamides, aluminia-silicates, basalt, regenerated cellulosic materials and ultra-high molecular weight polyethylene (UHMWPE); and, wherein the first polyolefin yarn and the second yarn are physically combined to form a composite yarn having at least one of the following properties: tenacity greater than about 100% of the expected tenacity based on the volume fraction of the second; an initial modulus >about 100% of the expected modulus based on the volume fraction of the second; elongation at break <about 320% of the elongation at break of the second.
Owner:INNEGRITY C +1
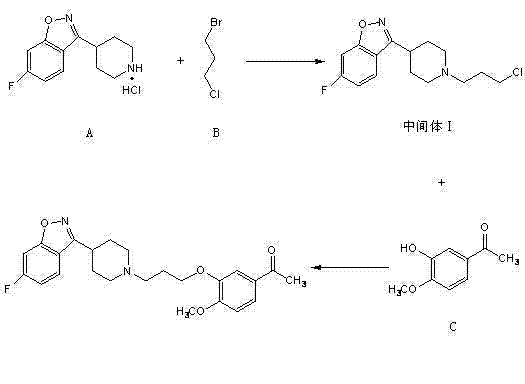
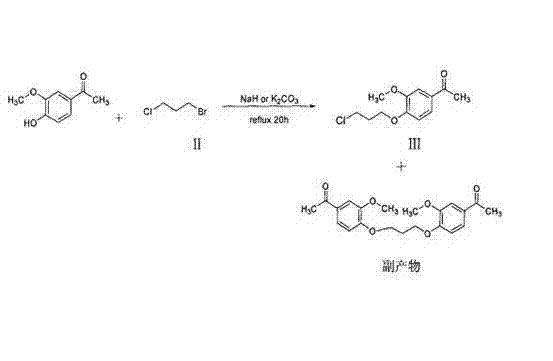
Method for preparing iloperidone
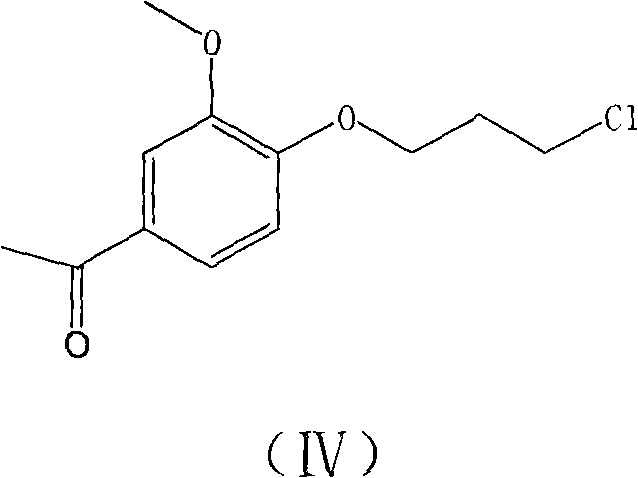
The invention belongs to the technical field of medicines. The invention discloses a method for preparing iloperidone. The method comprises the following steps of: reacting 6-fluoro-3-(4-piperidinyl)-1,2 benzisoxazole monohydrochloride and 1-bromine-3-chloropropane, which are both used as raw materials, to obtain 3-[1-(3-chloropropyl)piperidine-4-radical]-6-fluoro-1,2 benzoisoxazole, and reacting the obtained product and 4-hydroxyl-3-methoxyacetophenone serving as a raw material to obtain the iloperidone. Research shows that the byproducts generated in the method can be easily removed in the purifying process, so that the method has the advantages of high yield, high product purity and the like.
Owner:北京美迪康信医药科技有限公司
Novel crystalline forms of sodium 1,2-benzisoxazole-3-methanesulfonate, processes of preparing same and use thereof in the synthesis of zonisamide
Disclosed is a process of preparing 1,2-benzisoxazole-3-methanesulfonamide (zonisamide). Also disclosed is a method of dehydrating sodium 1,2-benzisoxazole-3-methanesulfonate, a compound useful in the preparation of 1,2-bisoxazole-3-methanesulfonamide (zonisamide) as well as new crystalline forms of sodium 1,2-benzisoxazole-3-methanesulfonate.
Owner:CHEMAGIS
Method for preparing benzisoxazole methane sulfonyl chloride and its amidation to form zonisamide
The present invention relates to a process of preparing benzisoxazole methane sulfonic acid-chloride (BOS—Cl) as an zonisamide intermediate via chlorination of benzisoxazole methane sulfonate. The present invention also discloses a process of preparing zonisamide via amidation of BOS—Cl. More particularly, the present invention provides a process of preparing zonisamide, comprising the steps of: a) chlorinating BOS, salts or esters thereof, with SOCl2 in an organic solvent and / or in the presence of a catalyst to form BOS—Cl; and b) amidating BOS—Cl in the presence of ammonia selected from the group consisting of aqueous ammonia in a biphasic system, masked ammonia and dry ammonia to form zonisamide.
Owner:TEVA PHARM USA INC
Stable Aqueous Solution
InactiveUS20150366966A1Improve stabilityImproving stability of aqueous solutionBiocideOrganic active ingredientsAqueous solutionChemistry
The present invention provides an aqueous liquid preparation having high stability to light and heat, which contains (3-{2-[4-isopropyl-2-(4-trifluoromethyl)phenyl-5-thiazolyl]ethyl}-5-methyl-1,2-benzisoxazol-6-yl)oxyacetic acid or a pharmaceutically acceptable salt thereof, and tyloxapol or octoxynol.
Owner:SENJU PHARMA CO LTD
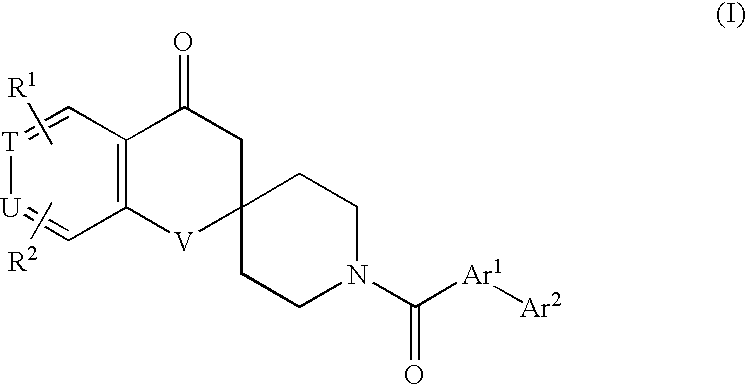
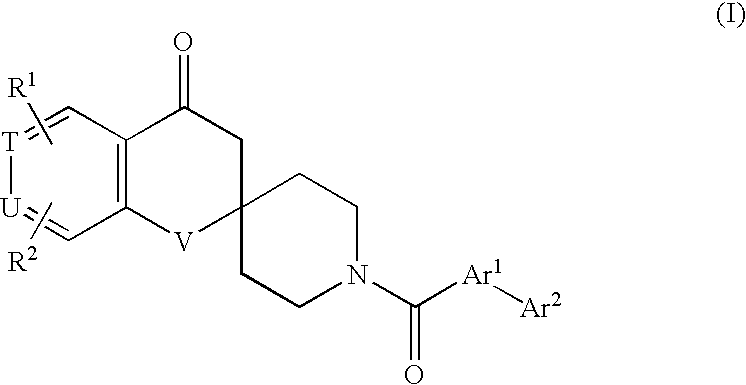
Spirochromanon derivatives
The invention relates to a compound of a general formula (I): wherein Ar1 represents a group formed from an aromatic ring selected from a group consisting of benzene, pyrazole, isoxazole, pyridine, indole, 1H-indazole, 1H-furo[2,3-c]pyrazole, 1H-thieno[2,3-c]pyrazole, benzimidazole, 1,2-benzisoxazole, imidazo[1,2-a]pyridine, imidazo[1,5-a]pyridine and 1H-pyrazolo[3,4-b]pyridine, having Ar2, and optionally having one or two or more substituents selected from R3: R1 and R2 each independently represent a hydrogen atom, a halogen atom, a cyano group, a C2-C6 alkenyl group, a C1-C6 alkoxy group, a C2-C7 alkanoyl group, a C2-C7 alkoxycarbonyl group, an aralkyloxycarbonyl group, a carbamoyl-C1-C6 alkoxy group, a carboxy-C2-C6 alkenyl group, or a group of -Q1-N(Ra)-Q2-Rb; or a C1-C6 alkyl group optionally having a substituent; or an aryl or heterocyclic group optionally having a substituent; or a C1-C6 alkyl group or a C2-C6 alkenyl group having the aryl or heterocyclic group; T and U each independently represent a nitrogen atom or a machine group; and V represents an oxygen atom or a sulfur atom. The compound of the invention is useful as therapeutical agents for various ACC-related diseases.
Owner:MSD KK +1
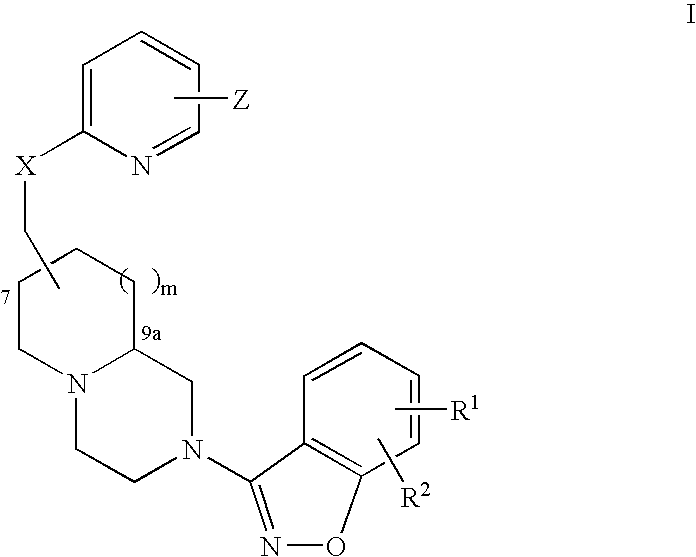
Methods of Treating Cognitive Disorders Using Pyridyloxymethyl and Benzisoxazole Azabicyclic Derivatives
InactiveUS20070270430A1Effective inhibitory activityIncrease in horizontal locomotor activityBiocideNervous disorderAzabicyclo CompoundsCognitive diseases
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating a cognitive disorder selected from the group consisting of Asperger's disorder, Autistic Disorder, Oppositional Defiant Disorder, and Conduct Disorder.
Owner:PFIZER INC
Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process
InactiveUS20090099369A1Easy to prepareReduce environmental impactPreparation by ester-hydroxy reactionOrganic compound preparationPropionatePropanoic acid
A preparation method using as an intermediate 6-(halomethyl)-1,2-benzisoxazol-3(2H)-one derivative represented by general formulawherein R5 is a methyl group that is substituted with one or more optionally substituted phenyl groups, or an optionally substituted oxygen-containing heterocyclic group; X represents a halogen atom, can be used as a method for safely and easily preparing 3-{5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-hydroxy-1,2-benzisoxazol-6-yl)methoxy]phenyl}propionic acid, which is useful as an antirheumatic agent, with a high yield.
Owner:TOYAMA CHEM CO LTD
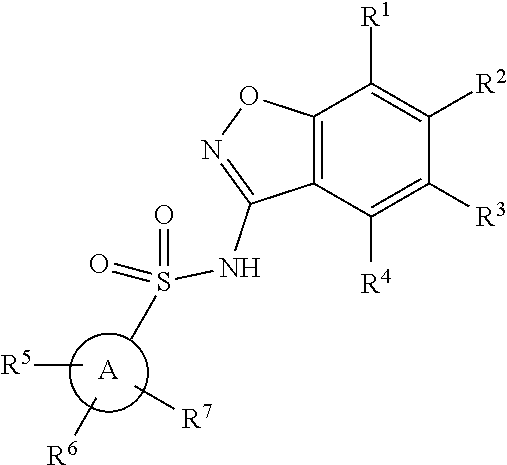
Benzisoxazole Sulfonamide Derivatives
ActiveUS20200399258A1Organic active ingredientsOrganic chemistry methodsPerylene derivativesPharmaceutical Substances
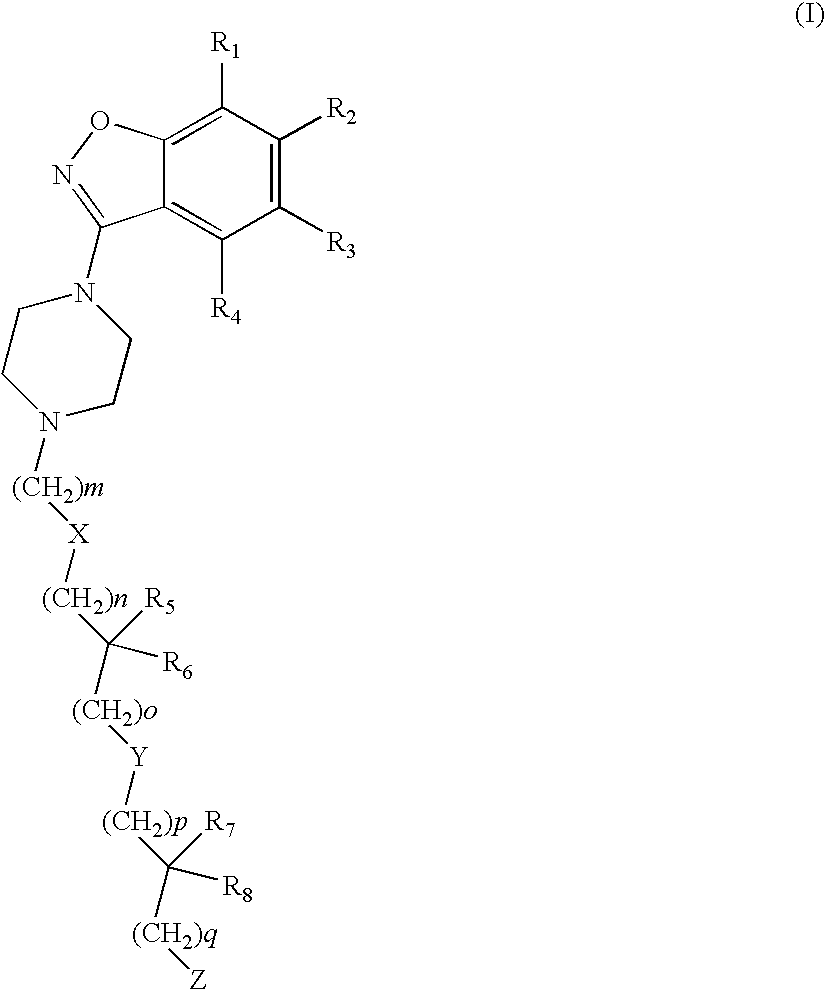
The present invention relates to compounds of formula (1)or pharmaceutically acceptable salts thereof, wherein Ring A, R1-R8, and n are defined herein. The novel benzisoxazole sulfonamide derivatives are useful in the treatment of abnormal cell growth, such as cancer, in patients. Additional embodiments relate to pharmaceutical compositions containing the compounds and to methods of using the compounds and compositions in the treatment of abnormal cell growth in patients.
Owner:PFIZER INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ecfc6078-703f-4745-a8bd-cbce93460d6e/US20080214808A1-20080904-C00001.png)
![Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ecfc6078-703f-4745-a8bd-cbce93460d6e/US20080214808A1-20080904-C00002.png)
![Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester Preparation of Aseptic 3-[2-[4-((6-Fluoro-1,2-Benzisoxazol-3-Yl)-1-Piperidinyl]-6,7,8,9-Tetrahydro-9-Hydroxy-2-Methyl-4H-Pyrido[1,2-a]Pyrimidin-4-One Palmitate Ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ecfc6078-703f-4745-a8bd-cbce93460d6e/US20080214808A1-20080904-C00003.png)
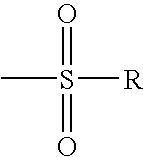
![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00001.png)
![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00002.png)
![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00003.png)
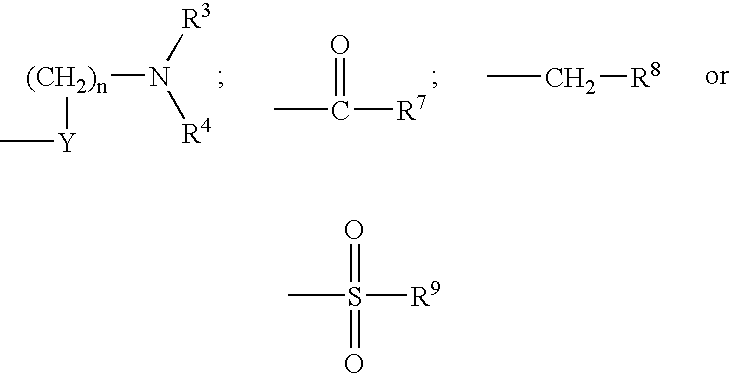





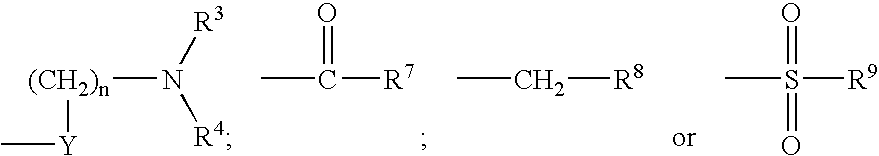
![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00001.png)
![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00002.png)
![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00003.png)