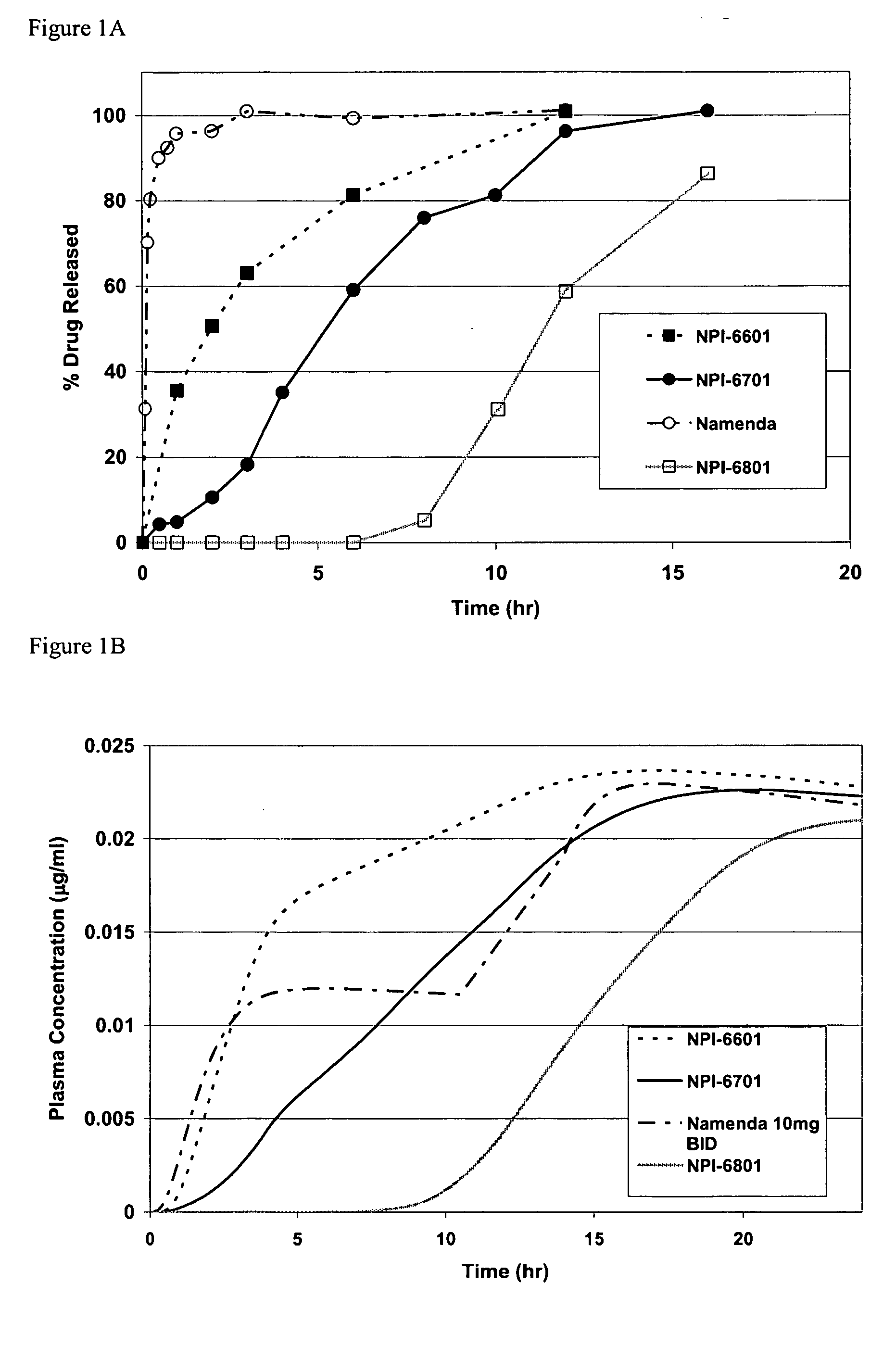
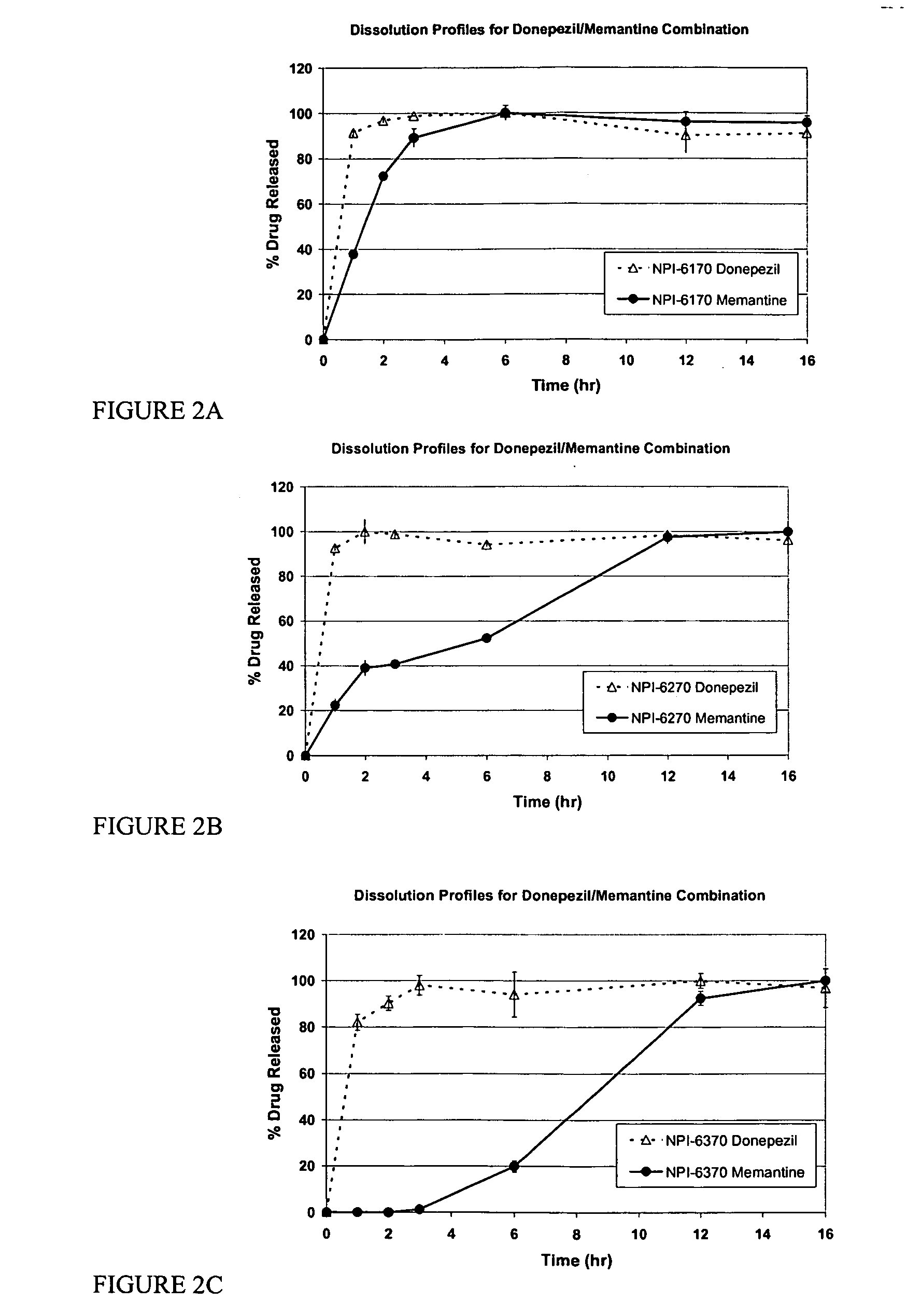
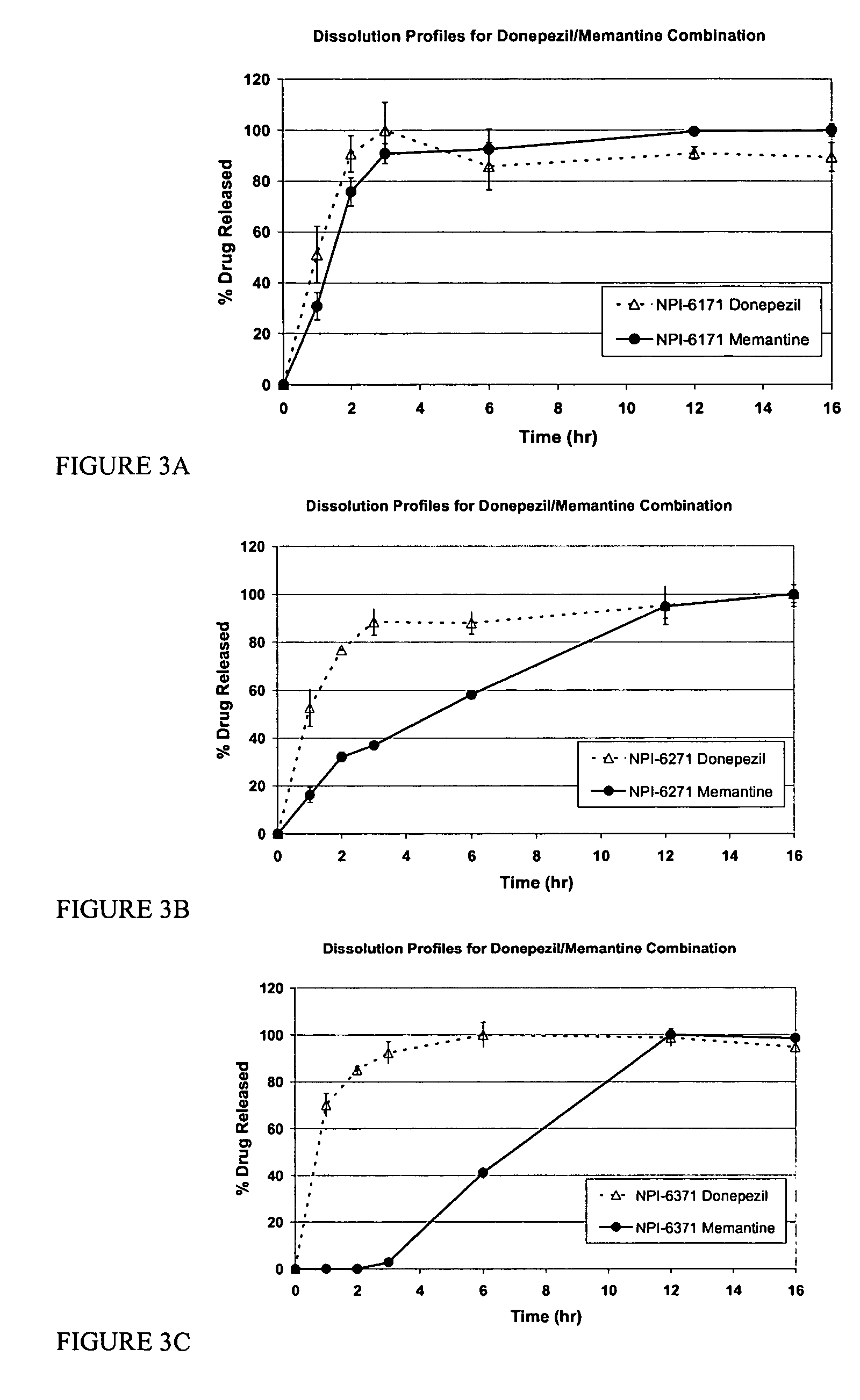
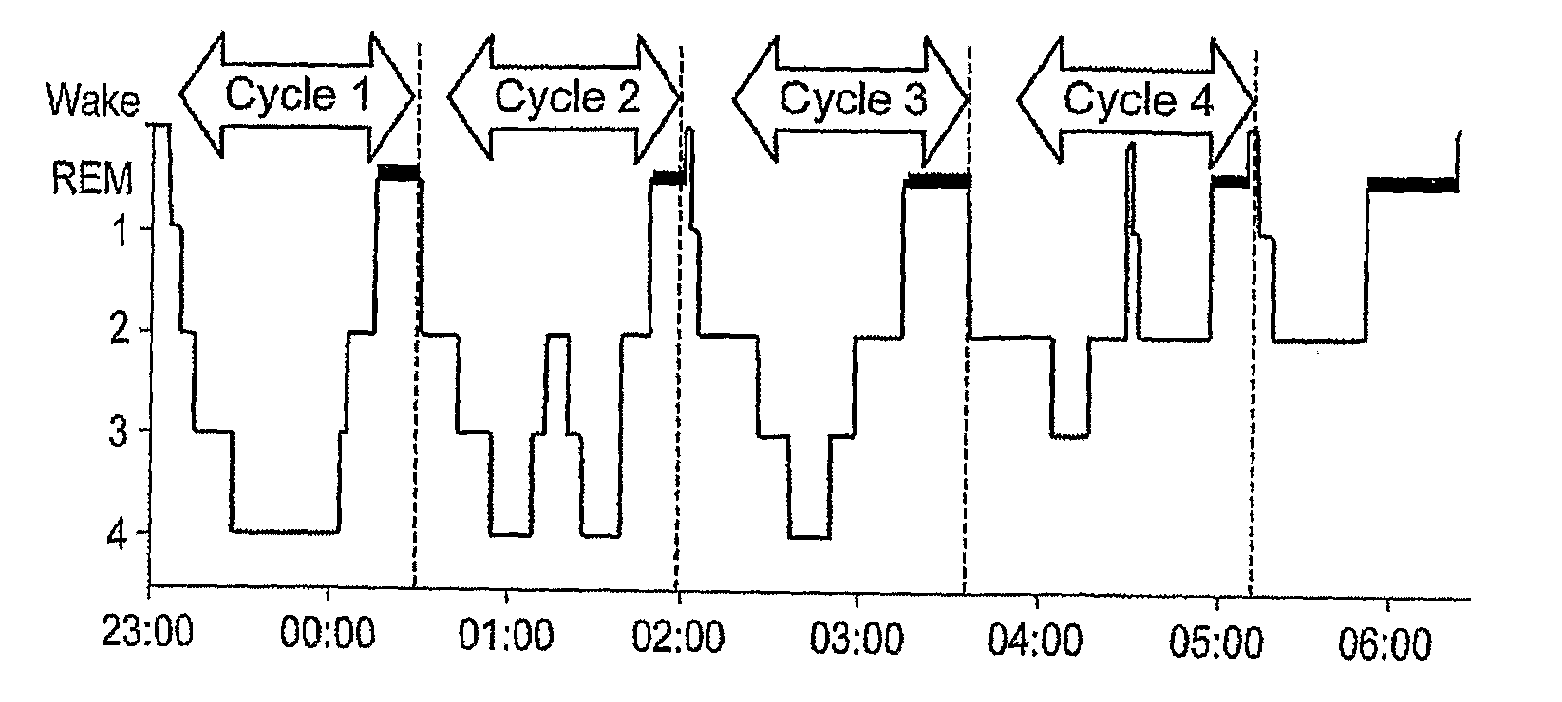
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2170 results about "Psychiatry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Psychiatry is the medical specialty devoted to the diagnosis, prevention, and treatment of mental disorders. These include various maladaptations related to mood, behaviour, cognition, and perceptions. See glossary of psychiatry.
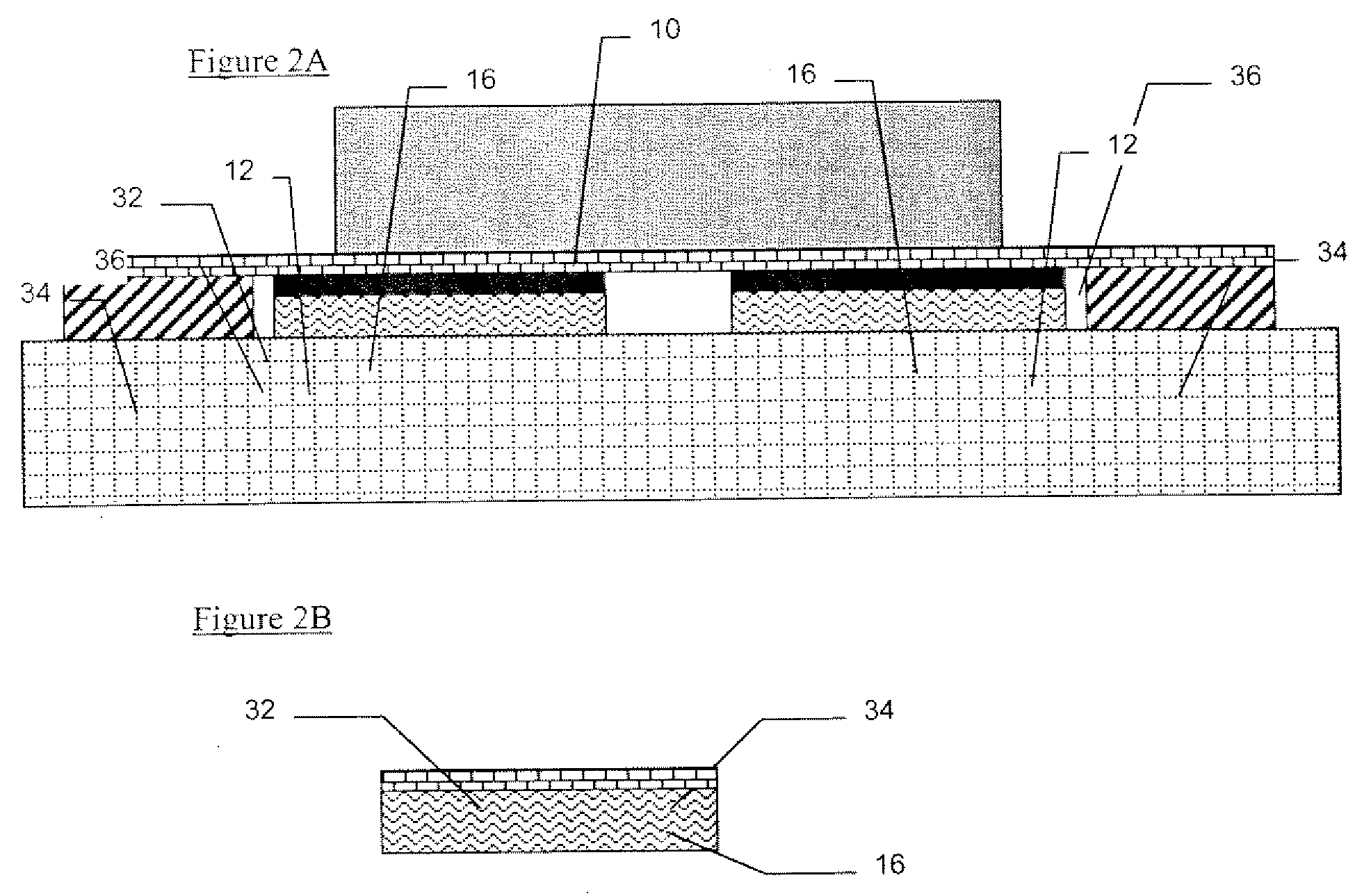
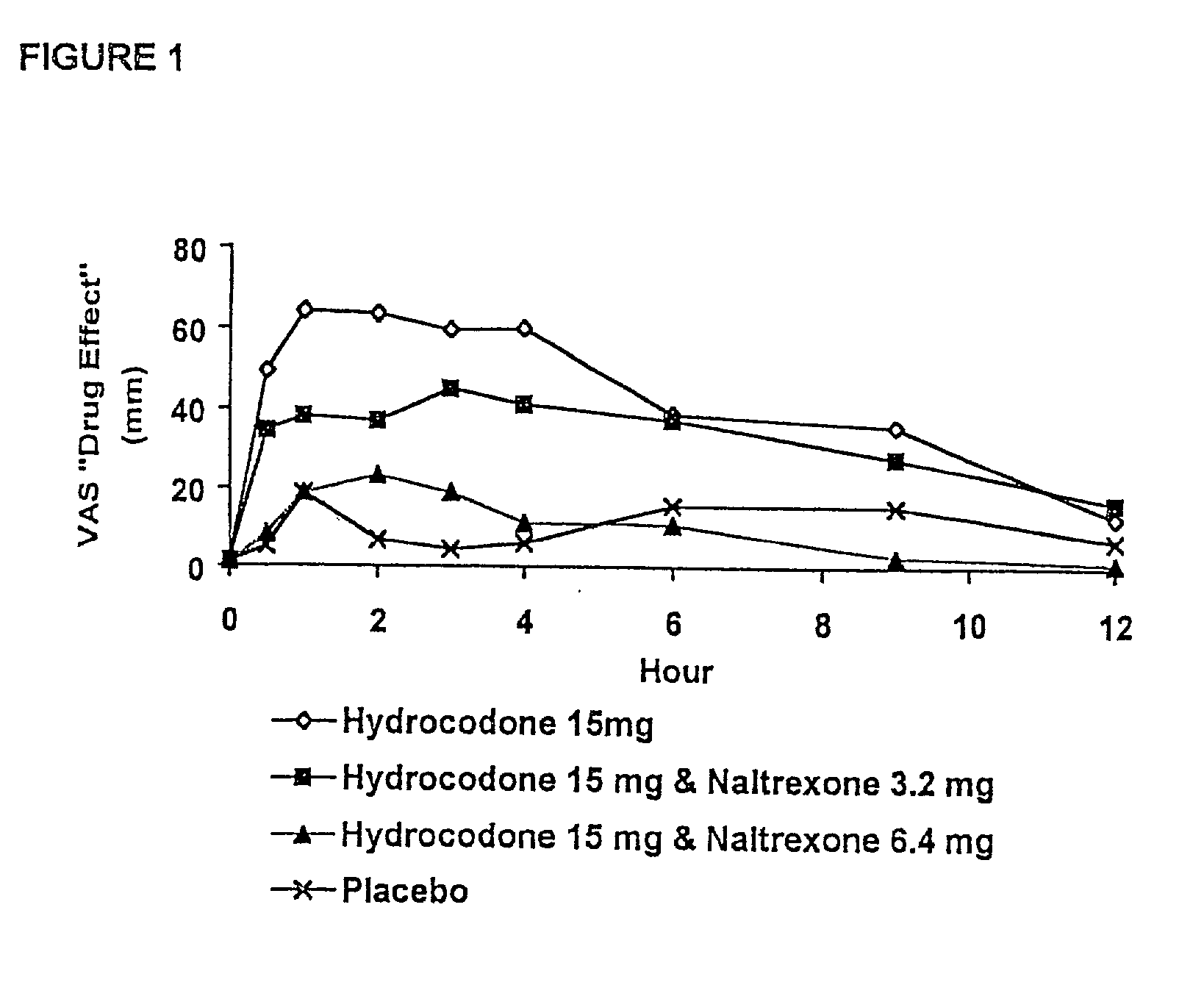
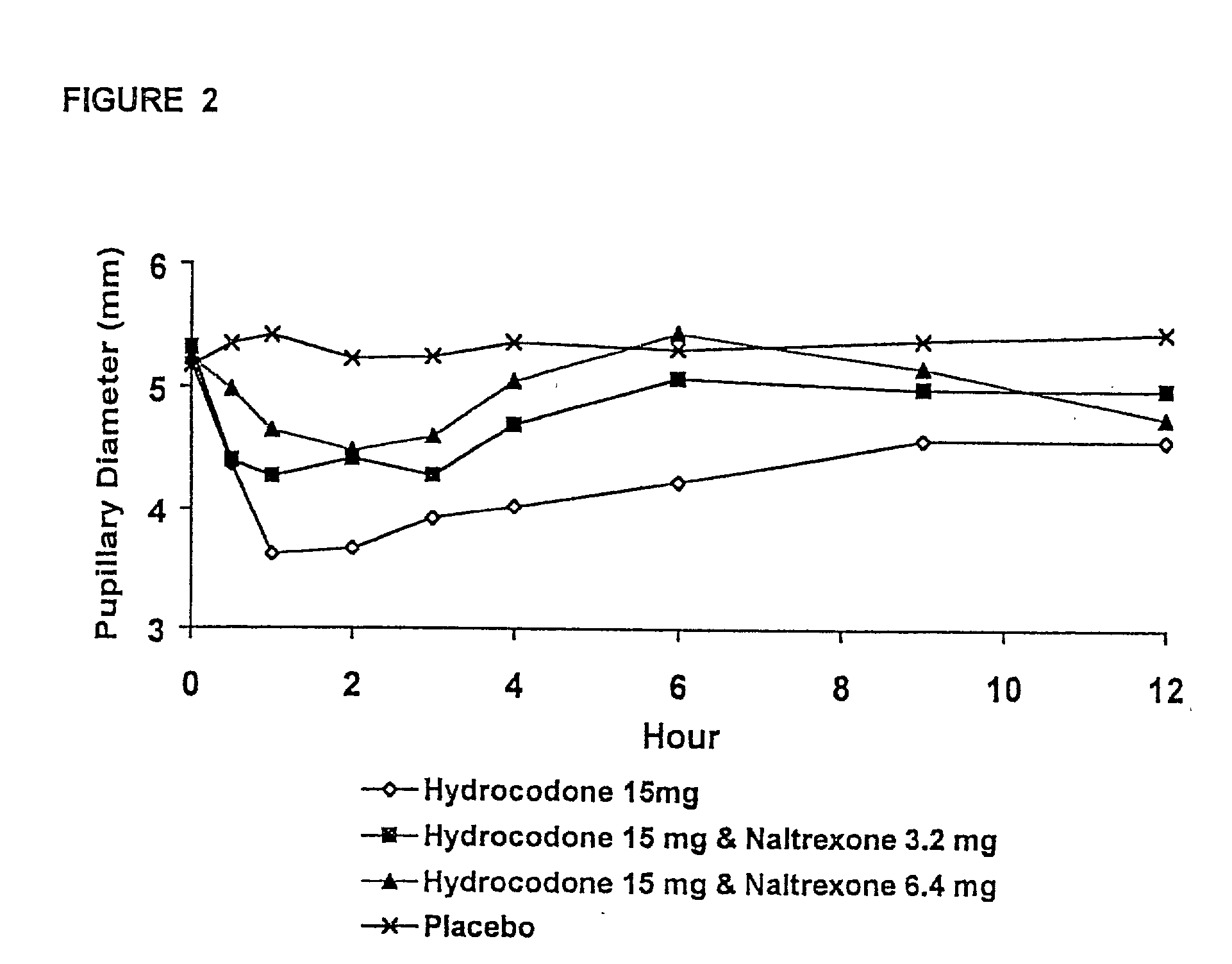
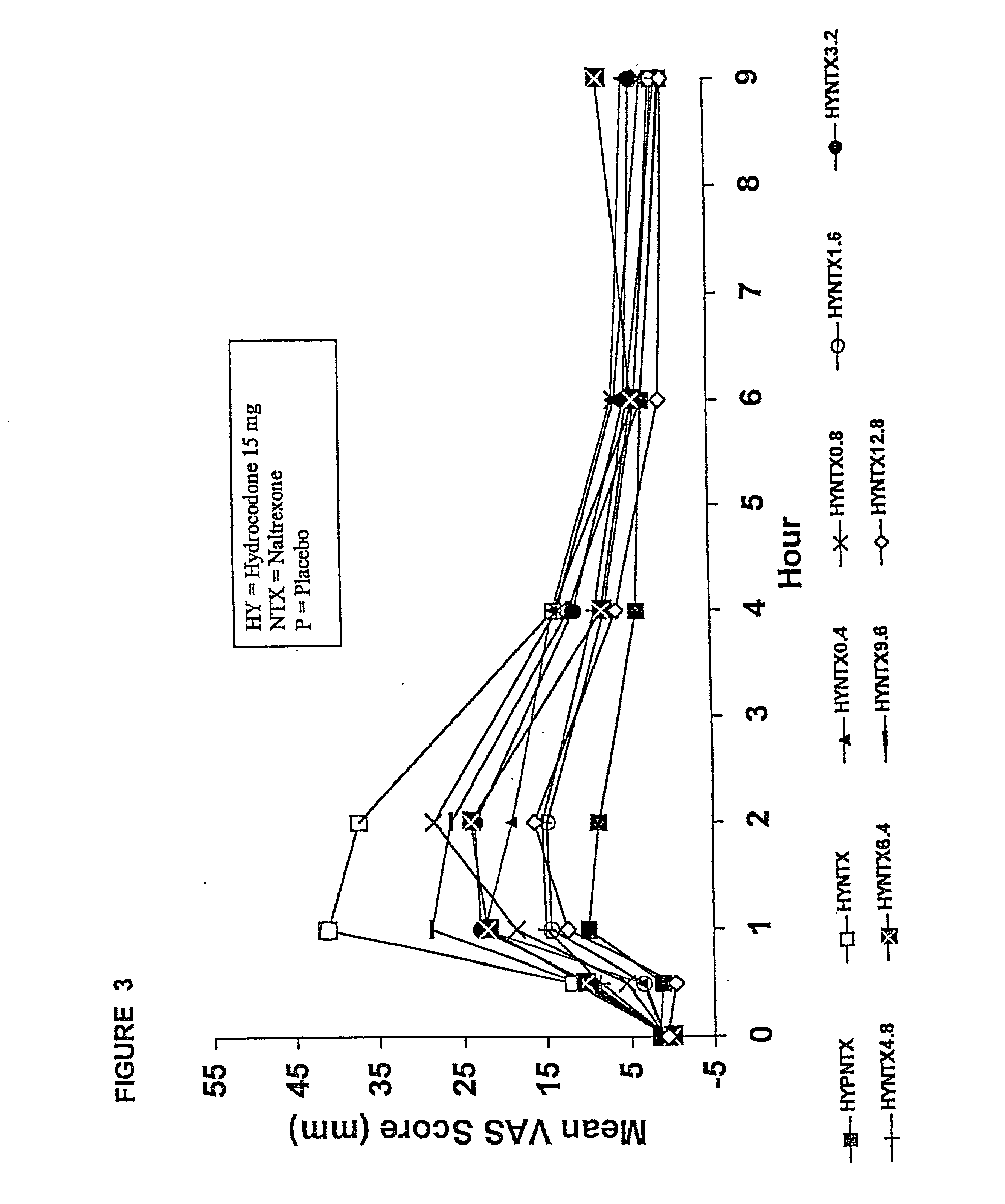
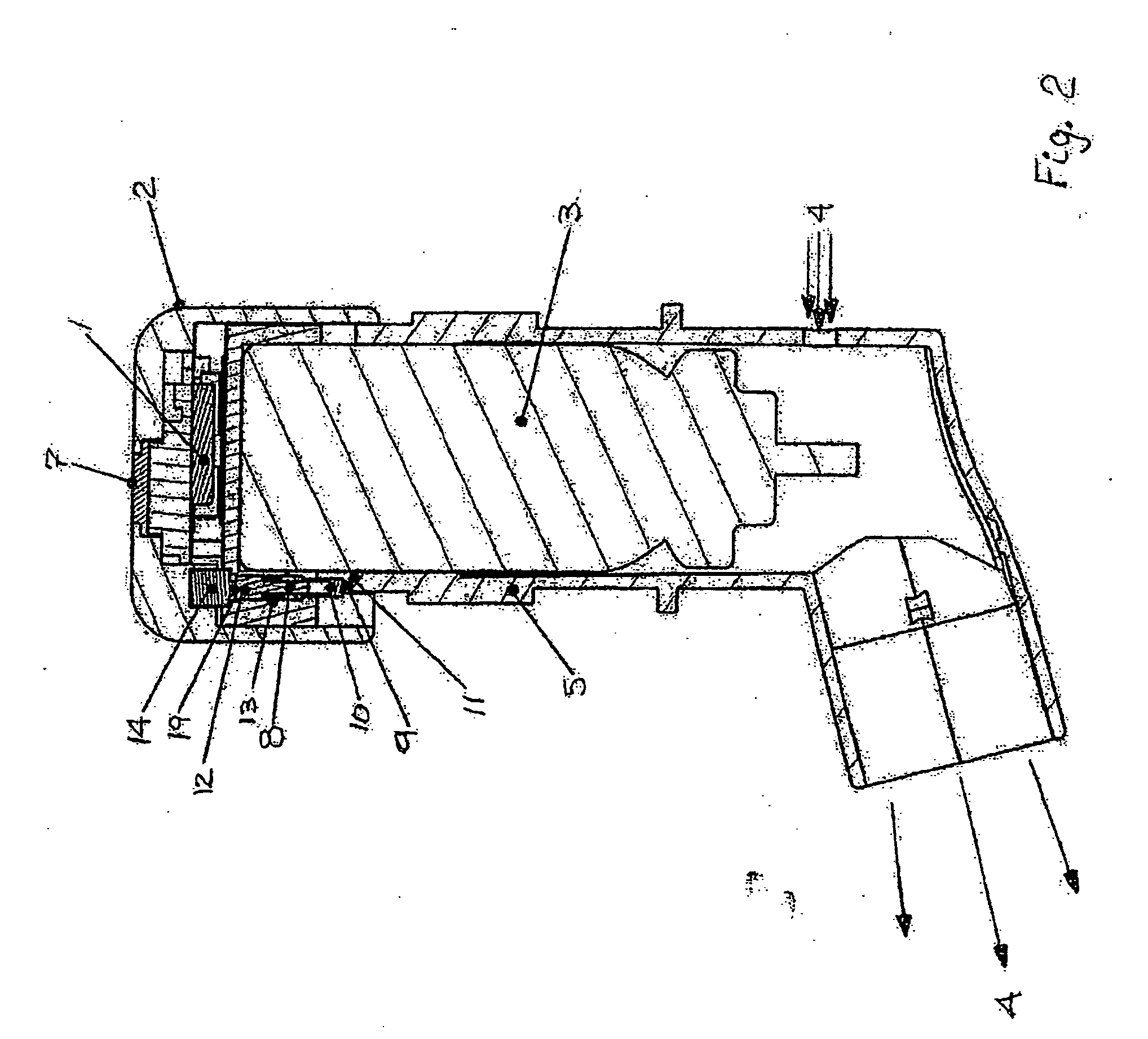
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
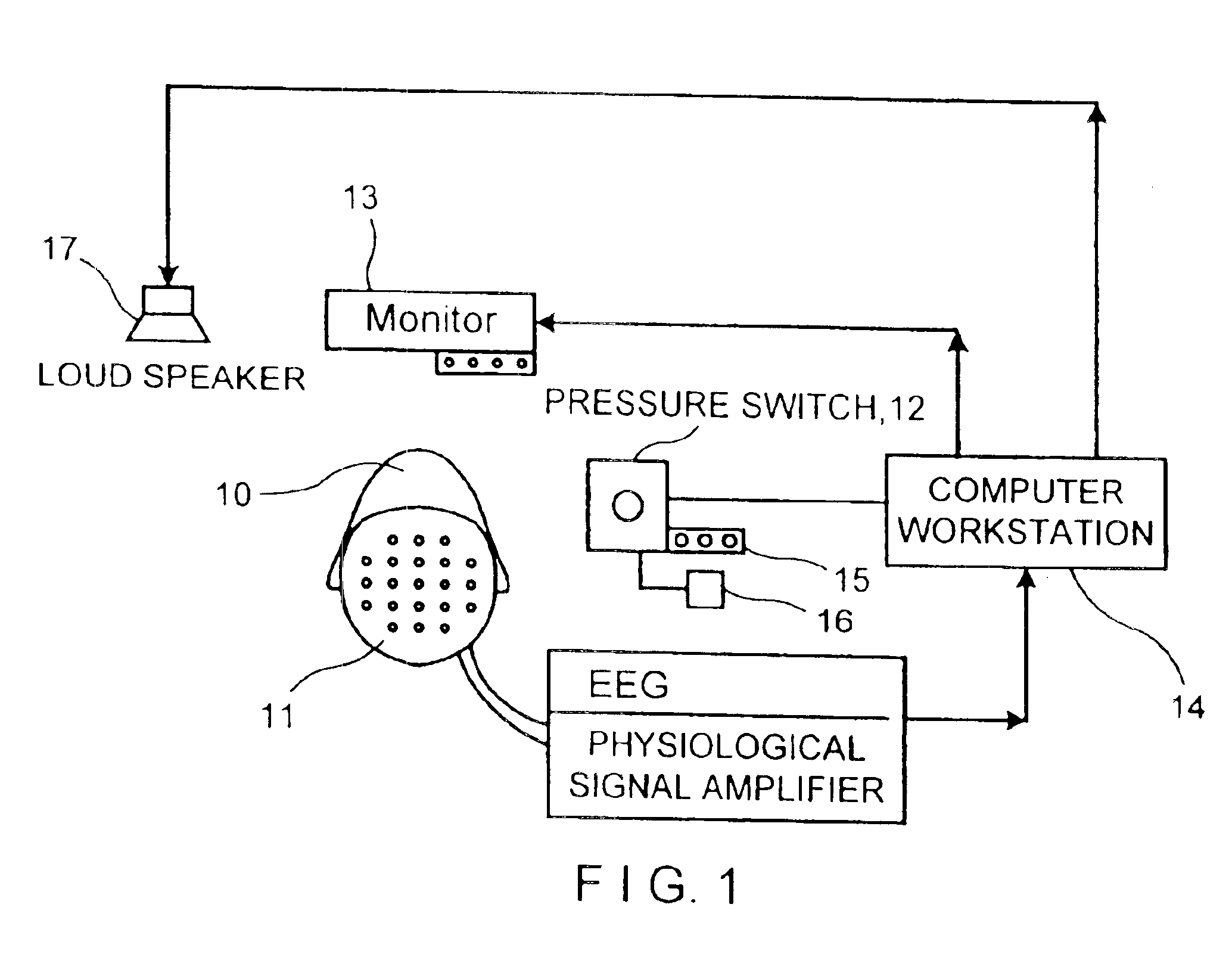
Collective brain measurement system and method
InactiveUS20050273017A1ElectroencephalographyMedical automated diagnosisPsychiatryDiagnosis treatment
A method of providing diagnosis capability, diagnosis of the effects of treatment or diagnosis of distinctive capabilities of a test subject, the method can comprise the steps of: (a) carrying out a series of tests on a group of subjects of at least two modal measures, the modal measures comprising brain-body function, brain structure, neuropsychological, personality, genetics, personal history, performance and behaviour; and (b) examining the inter-relationships between the modal measures to output an analysis of the inter-relationships of two or more measures of the tests results of the group of subjects.
Owner:THE BRAIN RESOURCE
Compositions and methods for treating contracture
InactiveUS20050186261A1Prevent and minimize contracture formationPrevent relapseBiocideMuscular disorderPsychiatryContracture
A method for treating contracture is provided that includes administering to a patient in need thereof a composition that includes a therapeutic agent effective in treating contracture. Compositions, devices, and kits for use in treating contracture are also described.
Owner:ANGIOTECH INT AG (CH)
Method of treating depression using a TNF-alpha antibody
InactiveUS20070041905A1Inhibiting TNFα activityImprove depressionSalicyclic acid active ingredientsBiocideAntiendomysial antibodiesAntigen binding
The invention describes methods of treating depression comprising administering a TNFα antibody, such as a human TNFα antibody. The invention also provides a method for treating depression comprising inhibiting TNFα activity in a subject suffering from depression by systemically administering to the subject a human anti-TNFα antibody, or an antigen-binding portion thereof, such that depression is treated. Also described is a method for the treatment or alleviation of depression or other affective disorders comprising administering an amount of an anti-inflammatory agent effective to treat or alleviate depression or other affective disorder to a subject in need thereof, wherein said anti-inflammatory agent down-regulates peripheral cytokine levels to thereby treat or alleviate depression or other affective disorder.
Owner:HOFFMAN REBECCA S +3
Formulations of nonopioid and confined opioid analgesics
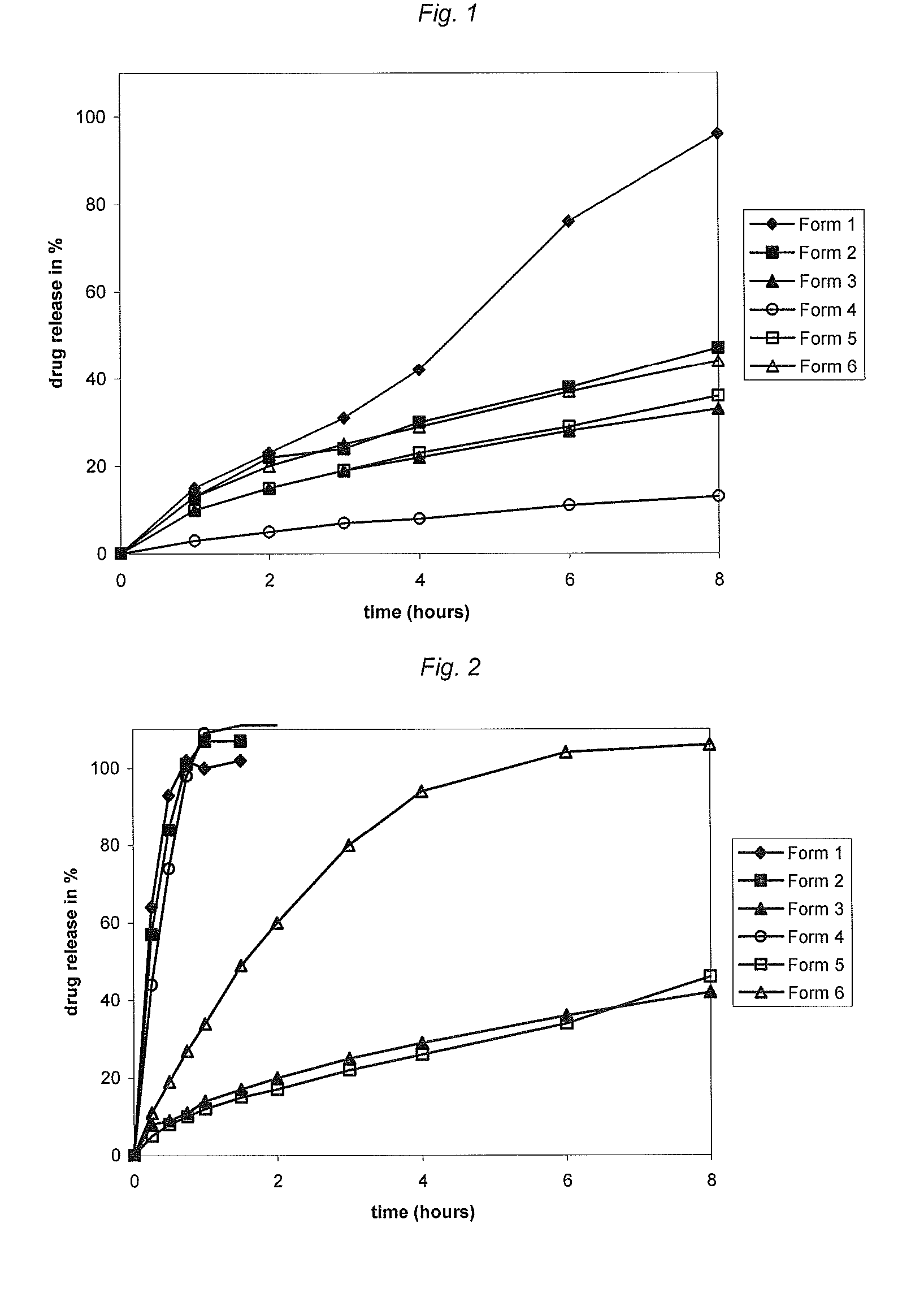
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Dosage forms for the delivery of drugs of abuse and related methods
InactiveUS20070190142A1Release drugOrganic active ingredientsNervous disorderDrugs of abuseInitial burst
A dosage form and method for the delivery of drugs, particularly drugs of abuse, characterized by resistance to solvent extraction, tampering, crushing, or grinding, and providing an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Systems and methods for detecting deception by measuring brain activity
Owner:MUSC FOUND FOR RES DEV
Method and apparatus for diagnosis of a mood disorder or predisposition therefor
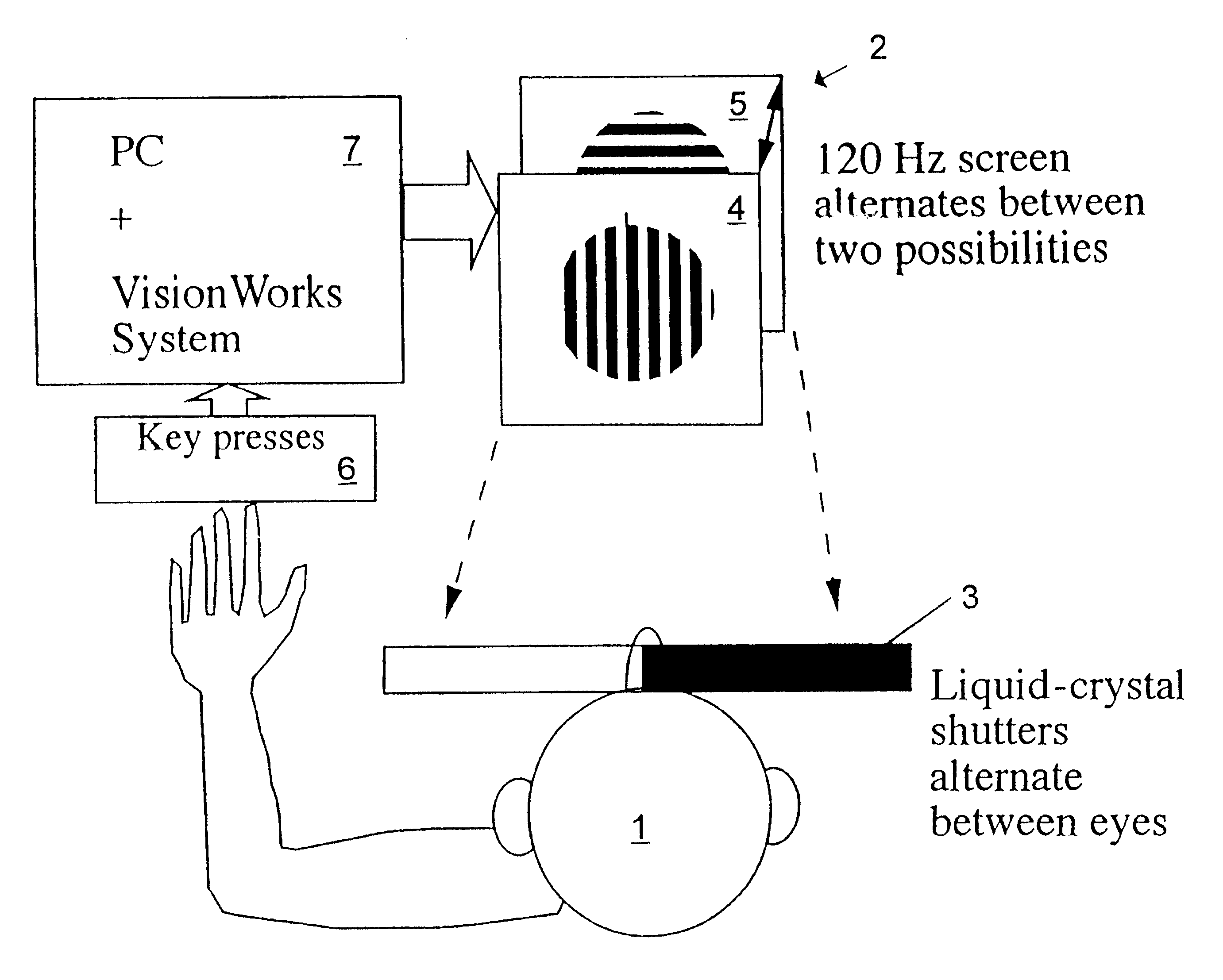
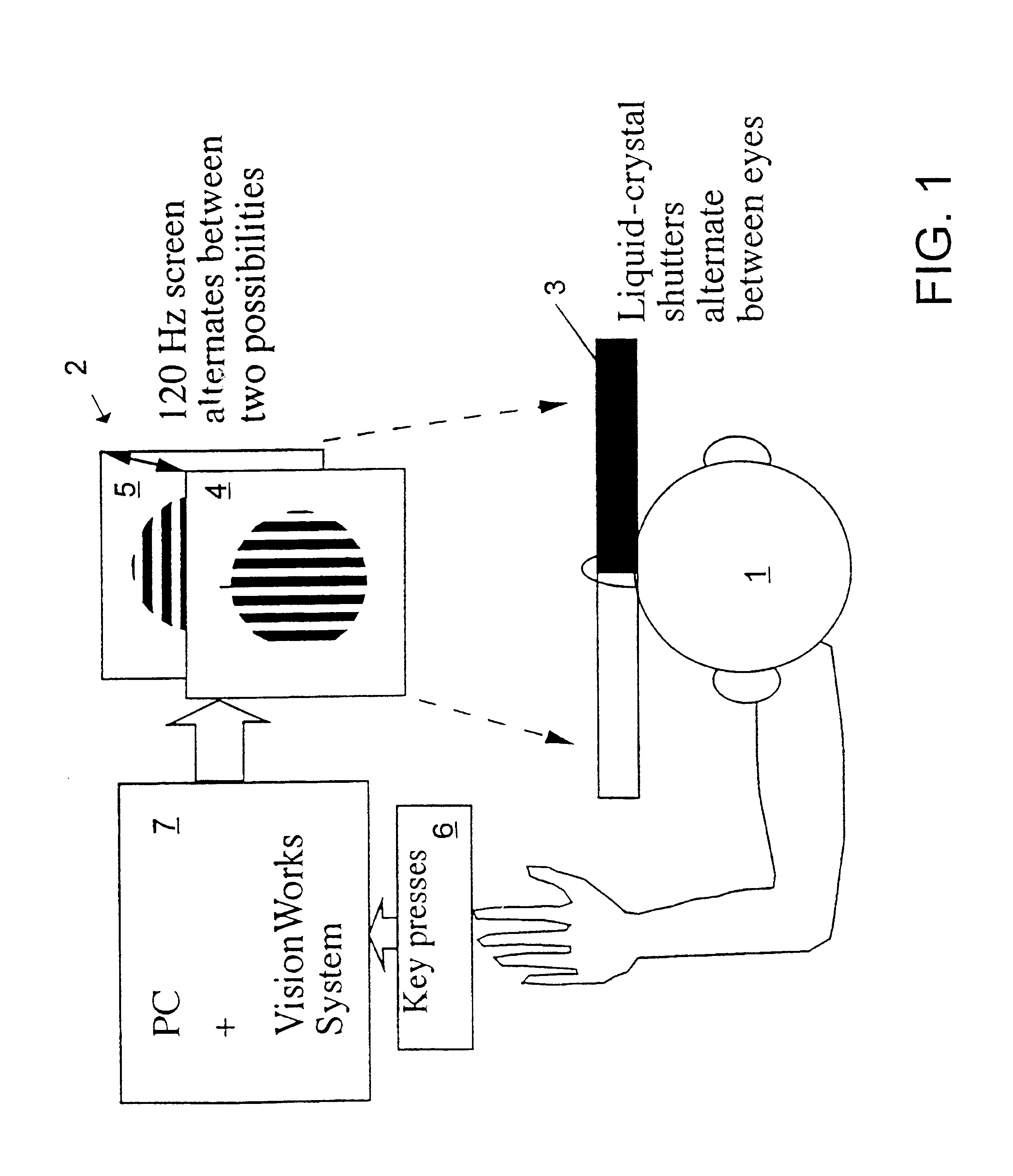
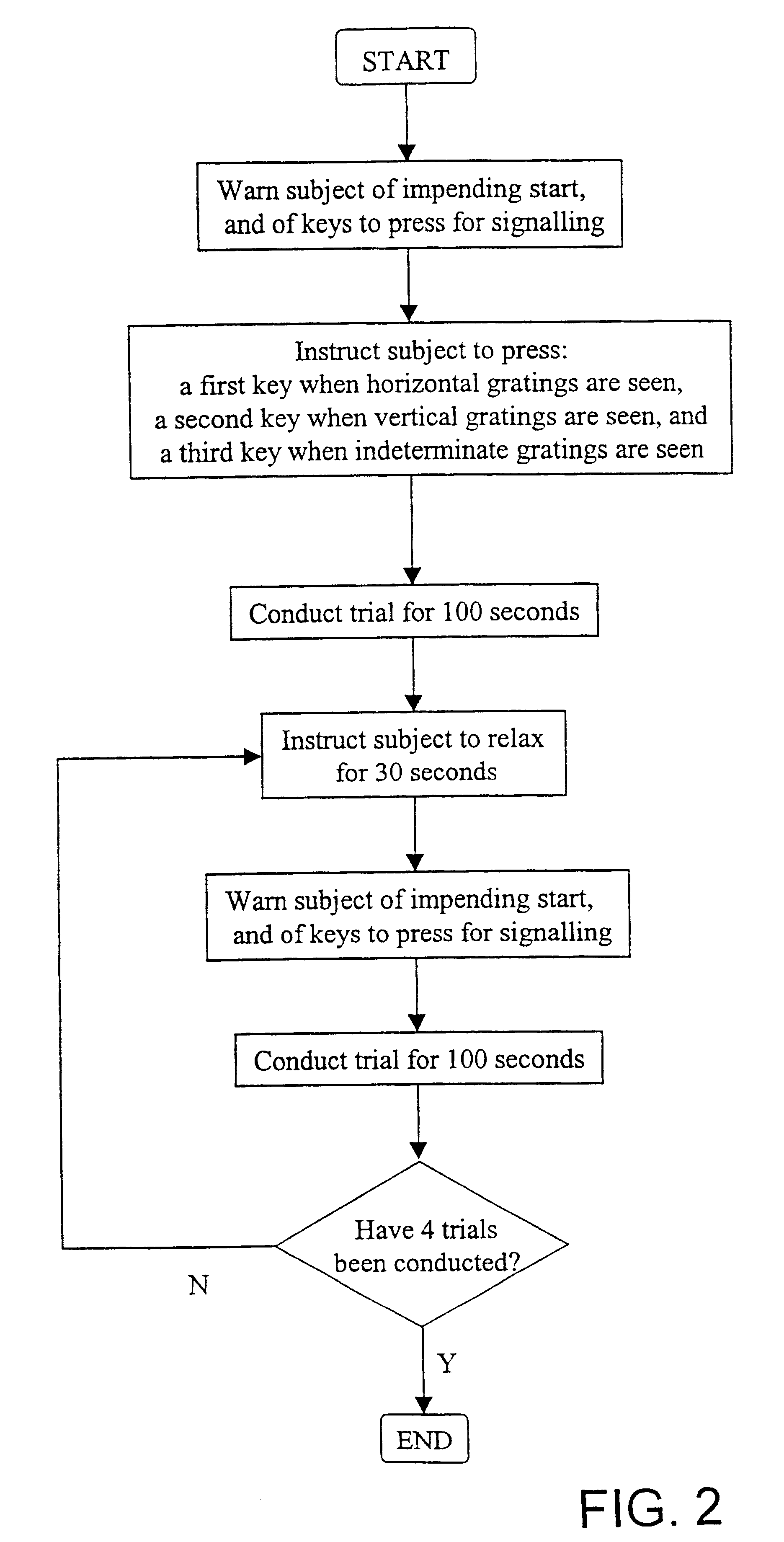
InactiveUS6629935B1Great absolute magnitude of changeEasy to changeIndividual molecule manipulationSensorsDiseaseBinocular rivalry
A method for diagnosis of a mood disorder or predisposition therefor in a test subject is disclosed. The method includes the steps of determining an interhemispheric switch rate of the test subject, and comparing the switch rate with a corresponding reference switch rate to diagnose presence or absence of the mood disorder or predisposition therefor. In a preferred embodiment, the interhemispheric switch rate is determined by measuring the rate of binocular rivalry in the test subject. Also disclosed is an apparatus for diagnosis of a mood disorder or predisposition therefor, use of the diagnostic method in genetic linkage studies for the identification of the molecular defect(s) underlying these disorders, and for the identification of compounds which may alleviate such disorders.
Owner:QUEENSLAND UNIV OF THE
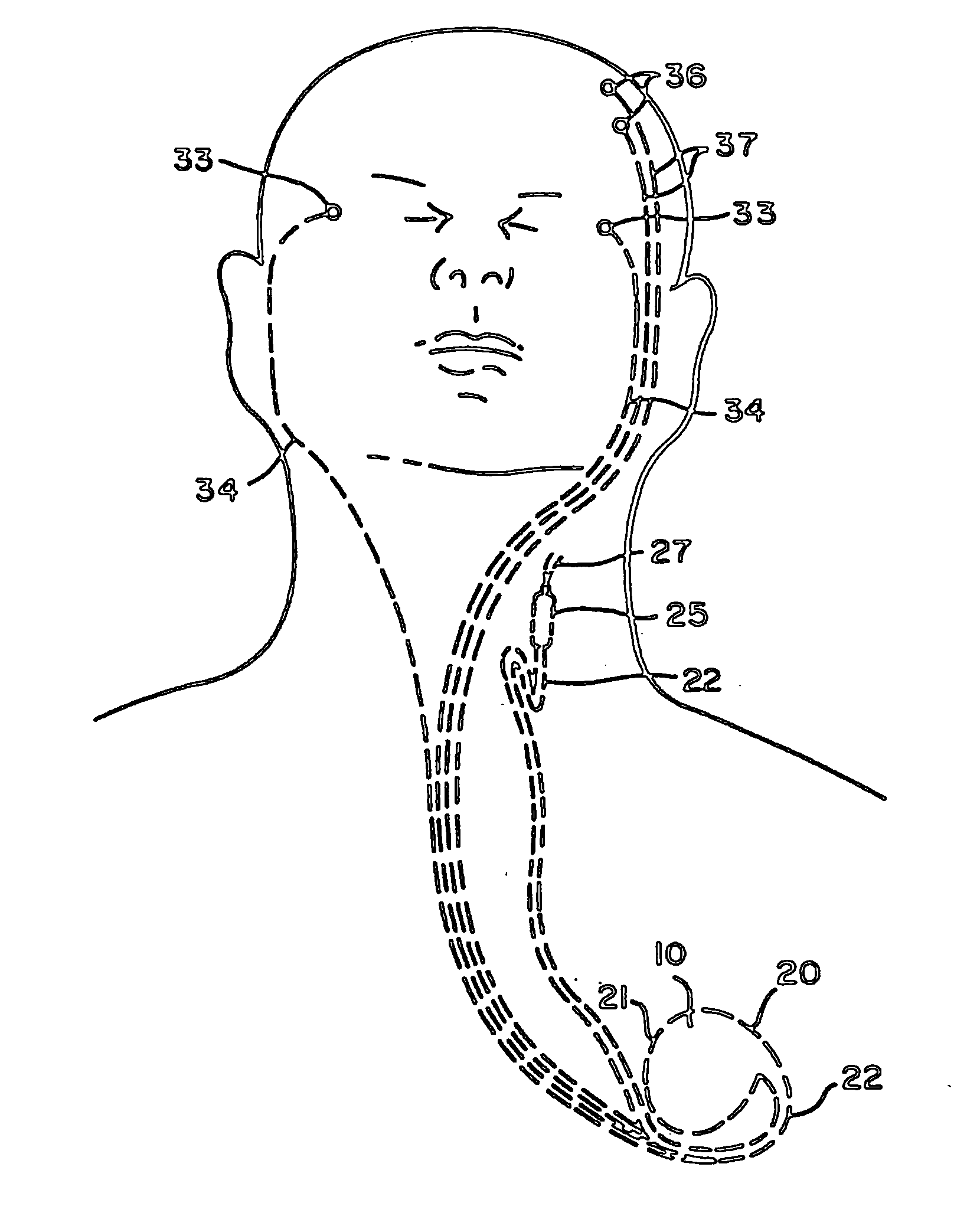
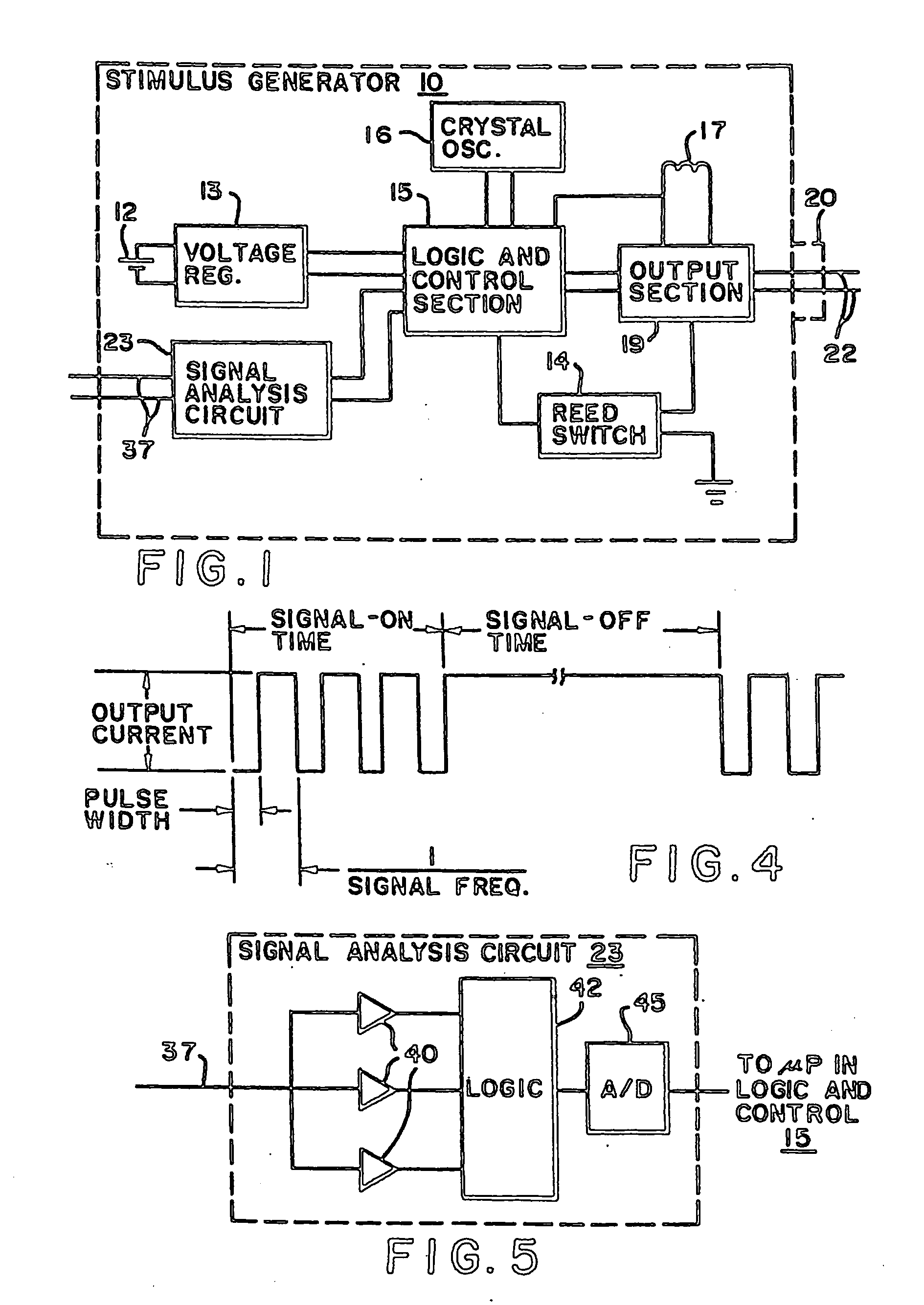
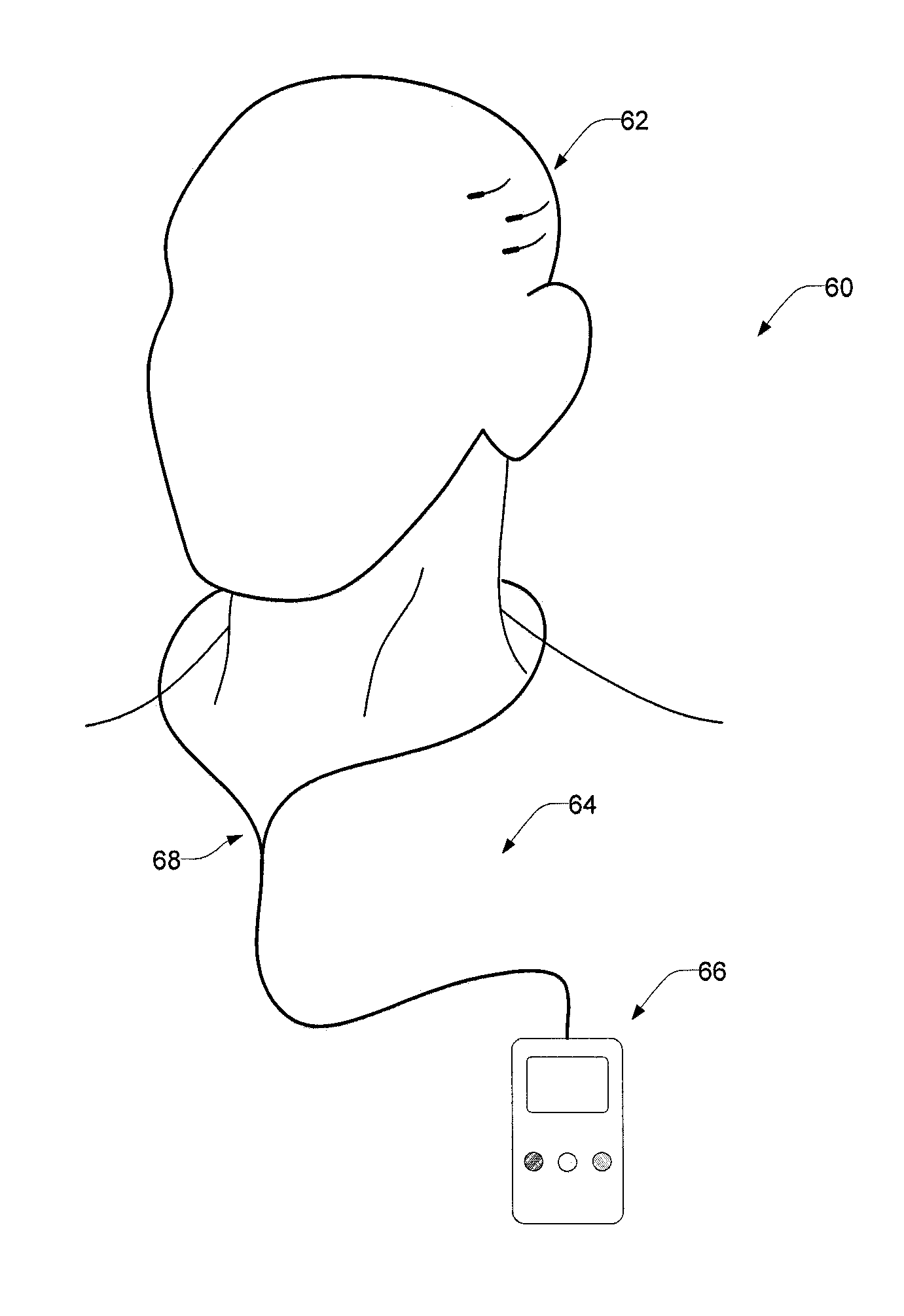
Vagus nerve stimulation for treatment of depression with therapeutically beneficial parameter settings
Method and apparatus for treating a patient with depression comprising continuously providing a therapy to treat the patient's depression. The therapy, in one embodiment, comprises stimulating a patient's vagus nerve for about 30 seconds at a current of about 0.75 mA followed by a cessation of vagus nerve stimulation of about 5 minutes. Further still, the therapy comprises a pulse width of about 500 μs and a frequency of about 20 Hz. In another embodiment, the therapy comprises stimulating a patient's vagus nerve for about 25.07 seconds at a current of about 0.85 mA followed by a cessation of vagus nerve stimulation of about 4.07 minutes. This latter therapy also comprises a pulse width of about 415.20 μs and a frequency of about 20.07 Hz.
Owner:LIVANOVA USA INC
Microcurrent device with a sensory cue
The present invention is directed to an apparatus that includes a microcurrent delivery device portion and at least one independent sensory cue delivery means. The independent sensory cue delivery means can provide an independent sensory cue selected from the group consisting of vibration, heat, cool, skin irritation, tingling, fragrance or auditory.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Sleep management method and system for improving sleep behaviour of a human or animal in the care of a carer
InactiveUS8398538B2Local control/monitoringAuscultation instrumentsSleep managementInteraction device
A sleep management method and system for use by a carer to improve the sleep behavior of a child in their care by monitoring one or more objective parameters relevant to sleep quality and providing one or more recommendations for behavioral programs and / or actions to the carer via a portable user interaction device such as a mobile phone. Such a system can also be adapted for use in looking after adults and animals such as domestic pets.
Owner:SHARP KK
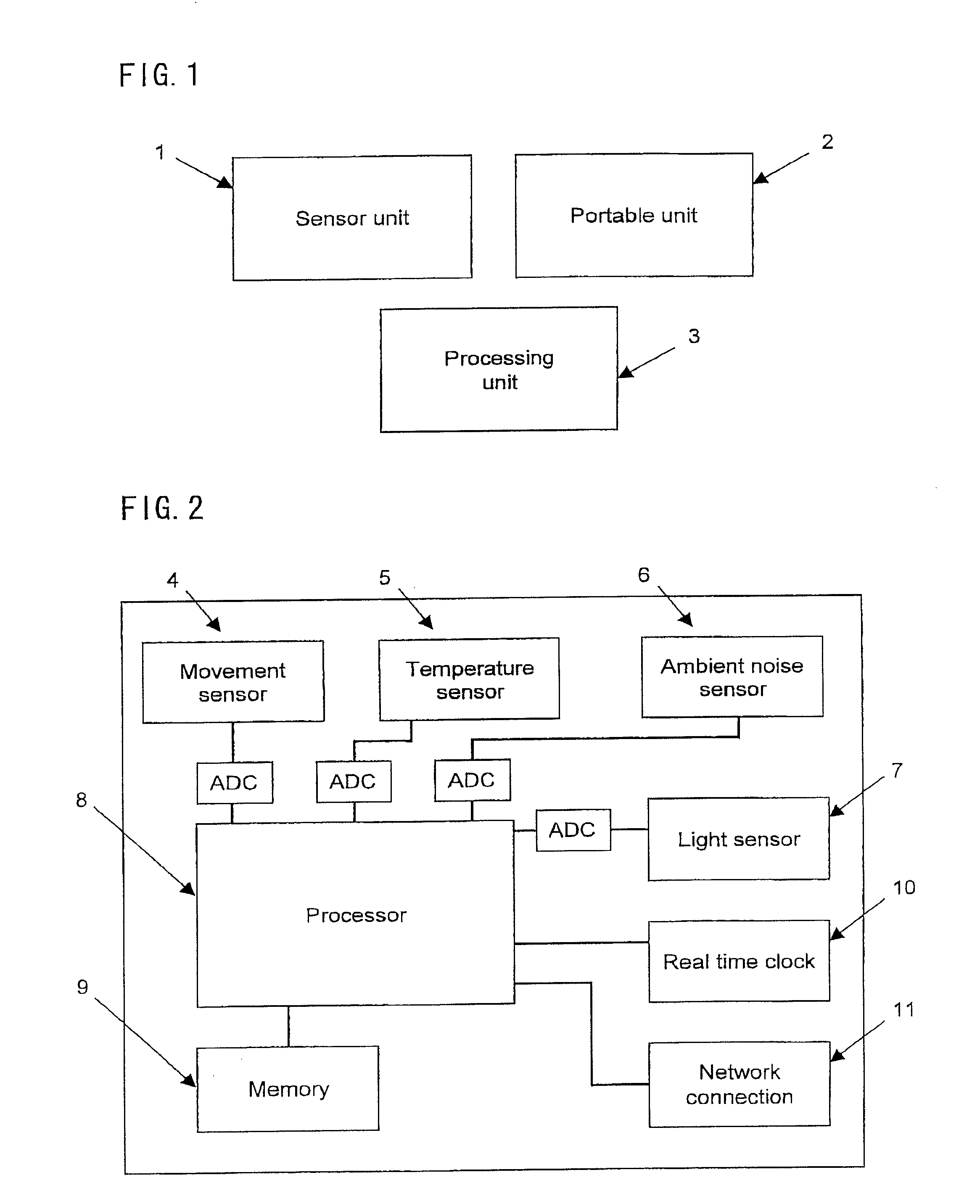
Methods and Systems for Facilitating Clinical Trials
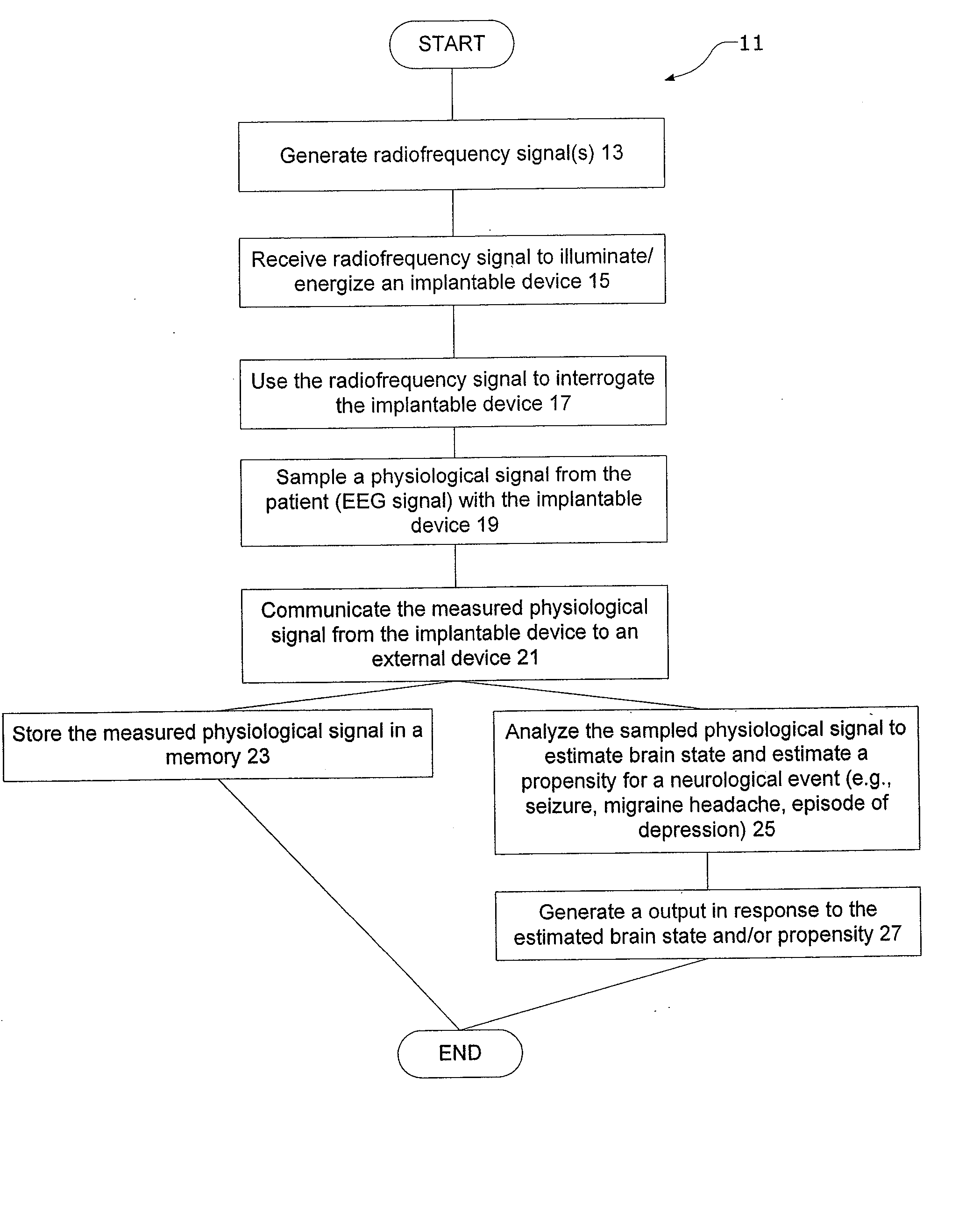
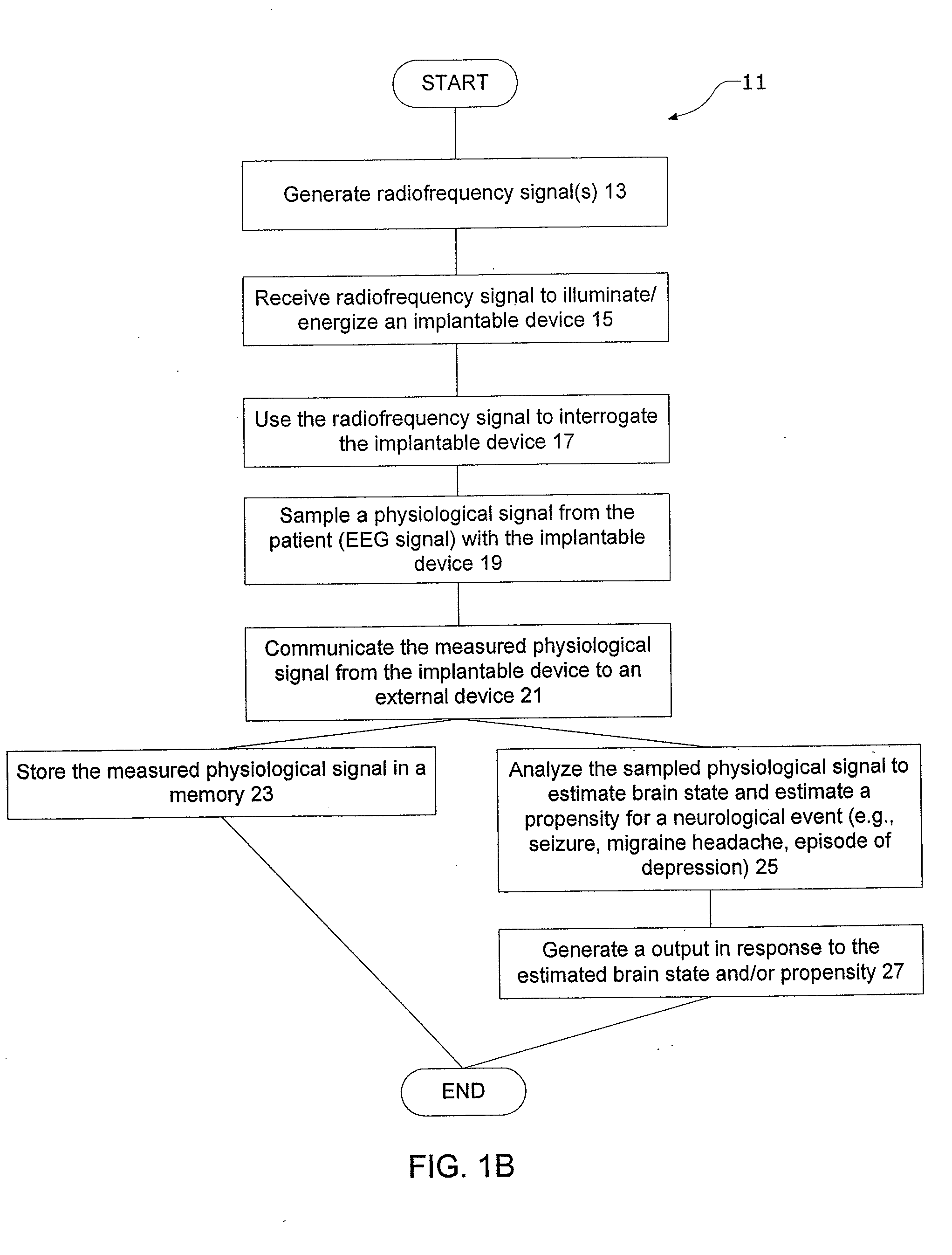
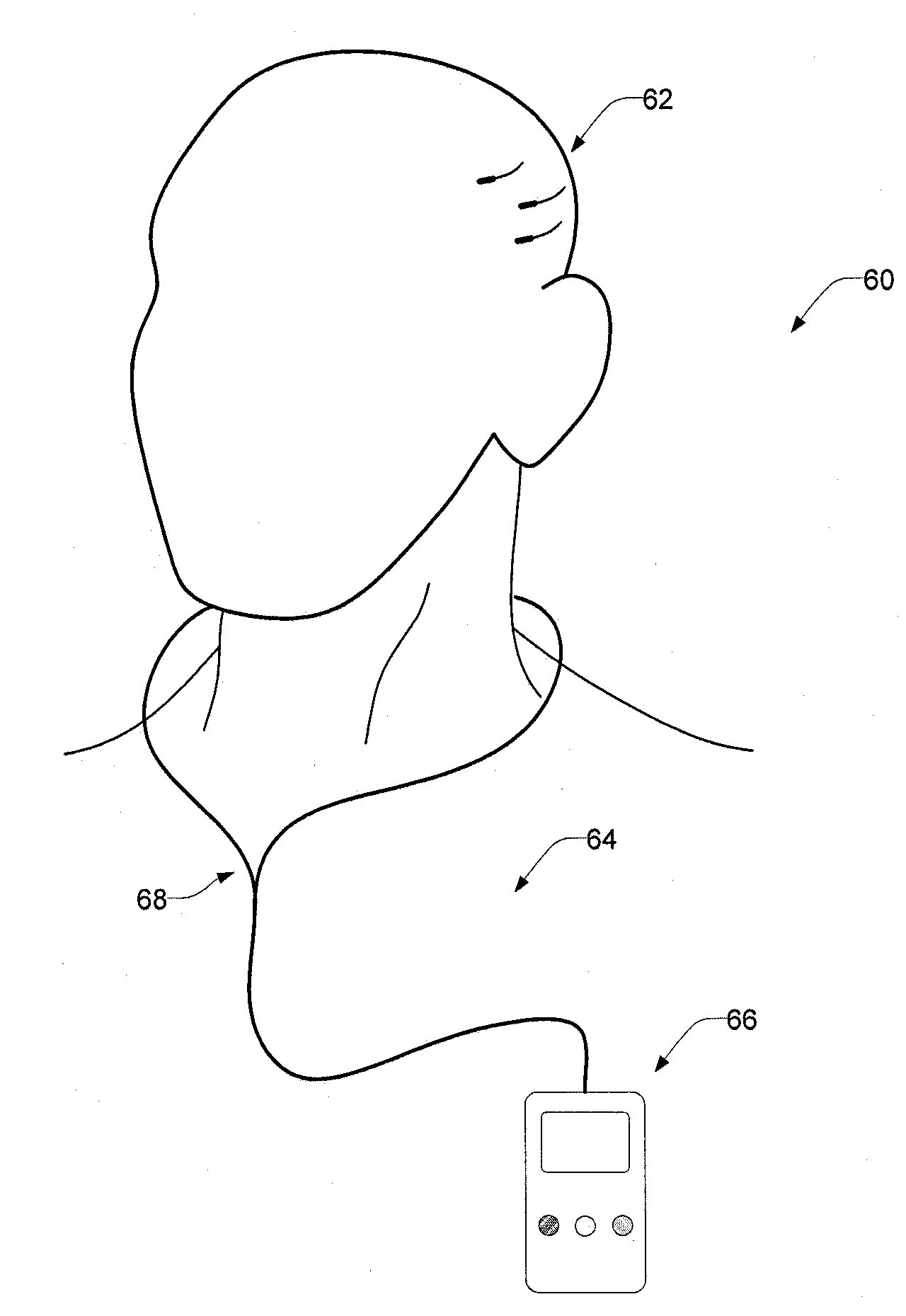
InactiveUS20080021341A1Easy data transferElectroencephalographyElectrotherapyClinical trialImplanted device
The present invention provides methods and systems for improving clinical trials of experimental therapies. The present invention uses minimally invasive, leadless implantable devices that facilitate long term monitoring of a physiological signal (e.g., neural signals) from a patient population which may provide an indication of the effect of the experimental therapy on the patient population. In preferred embodiments, neural signals are sampled from the patients in the patient population with an externally powered, leadless implanted device and are transmitted to an external device for further processing.
Owner:CYBERONICS INC
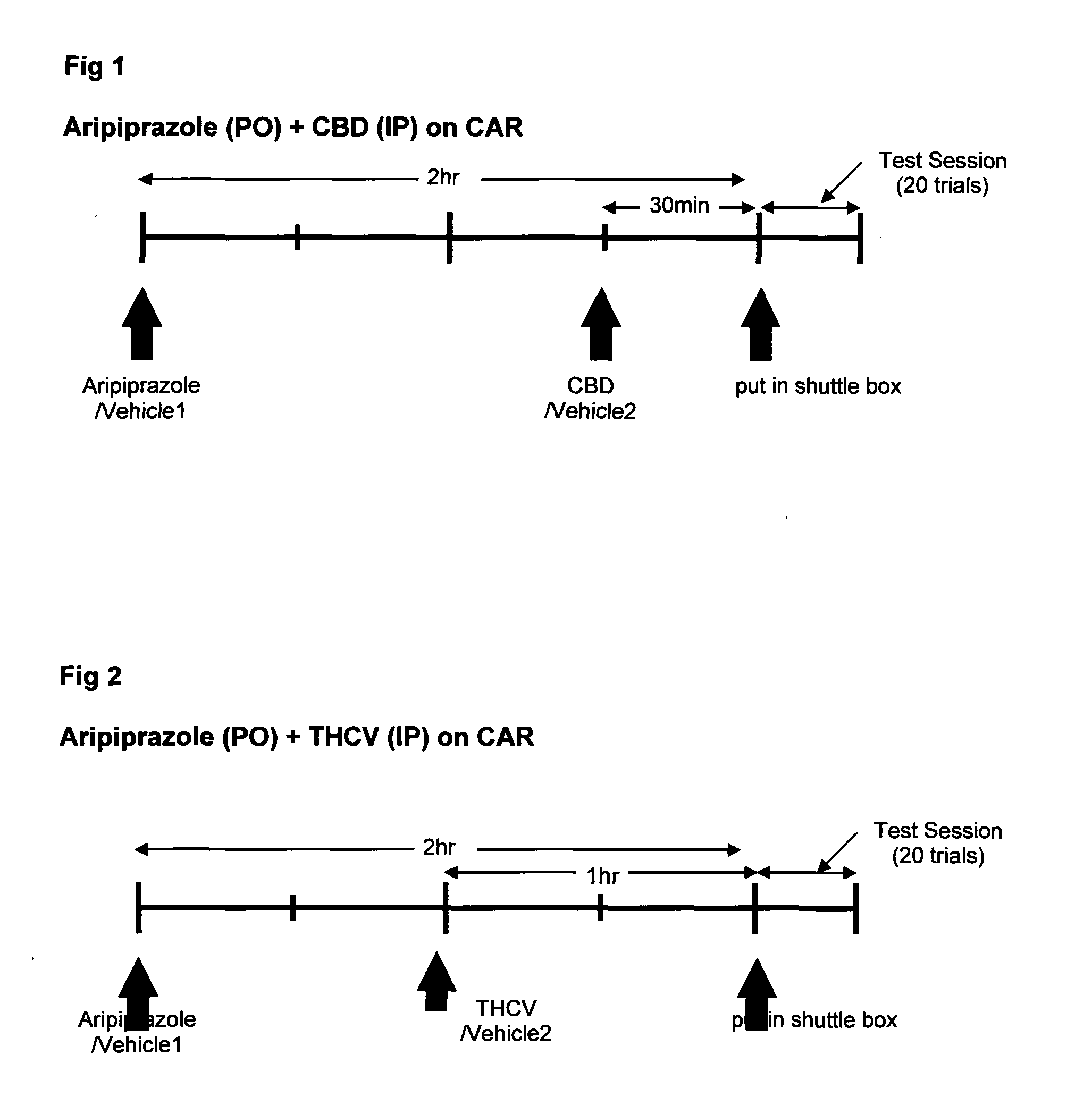
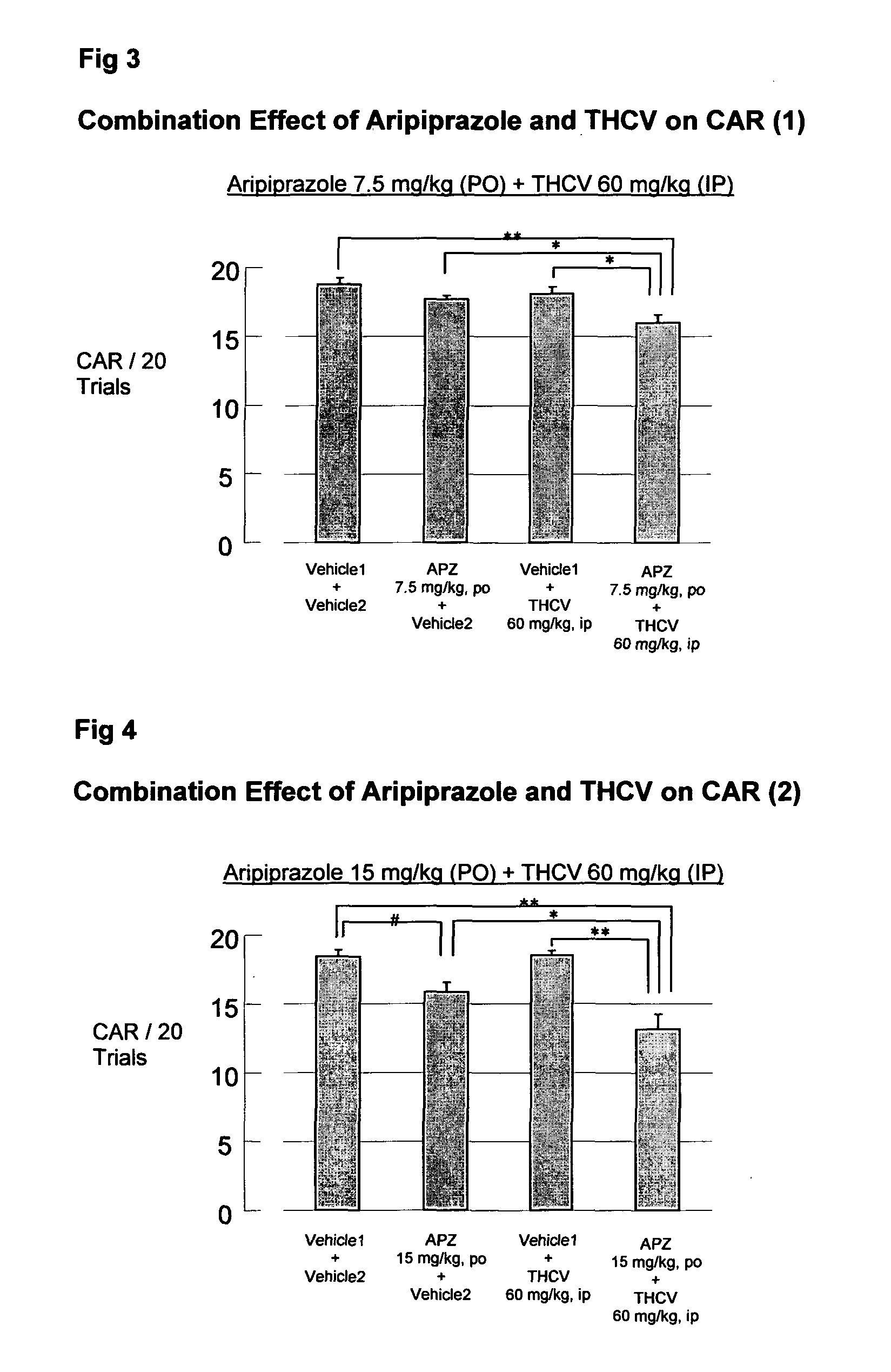
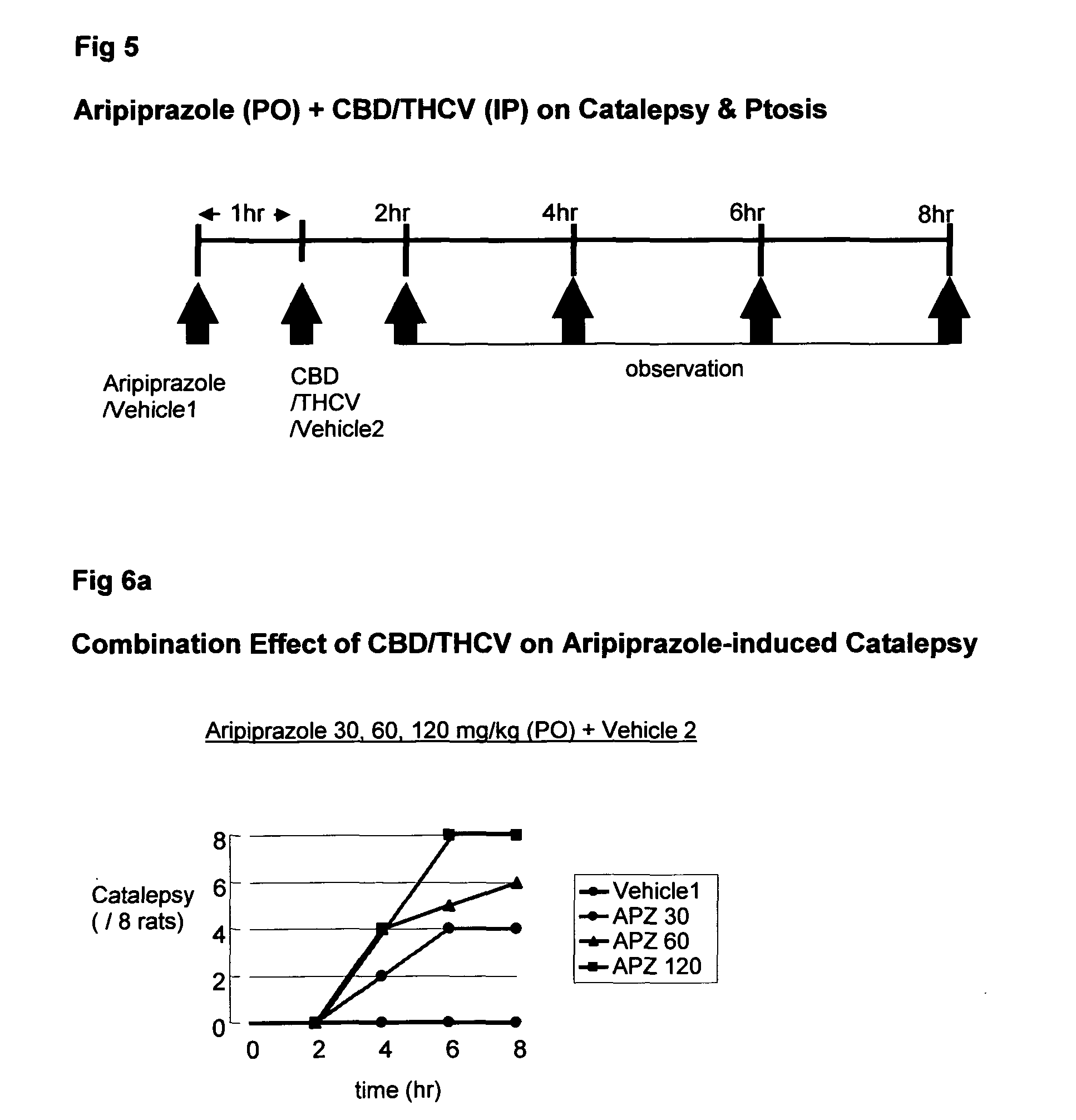
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
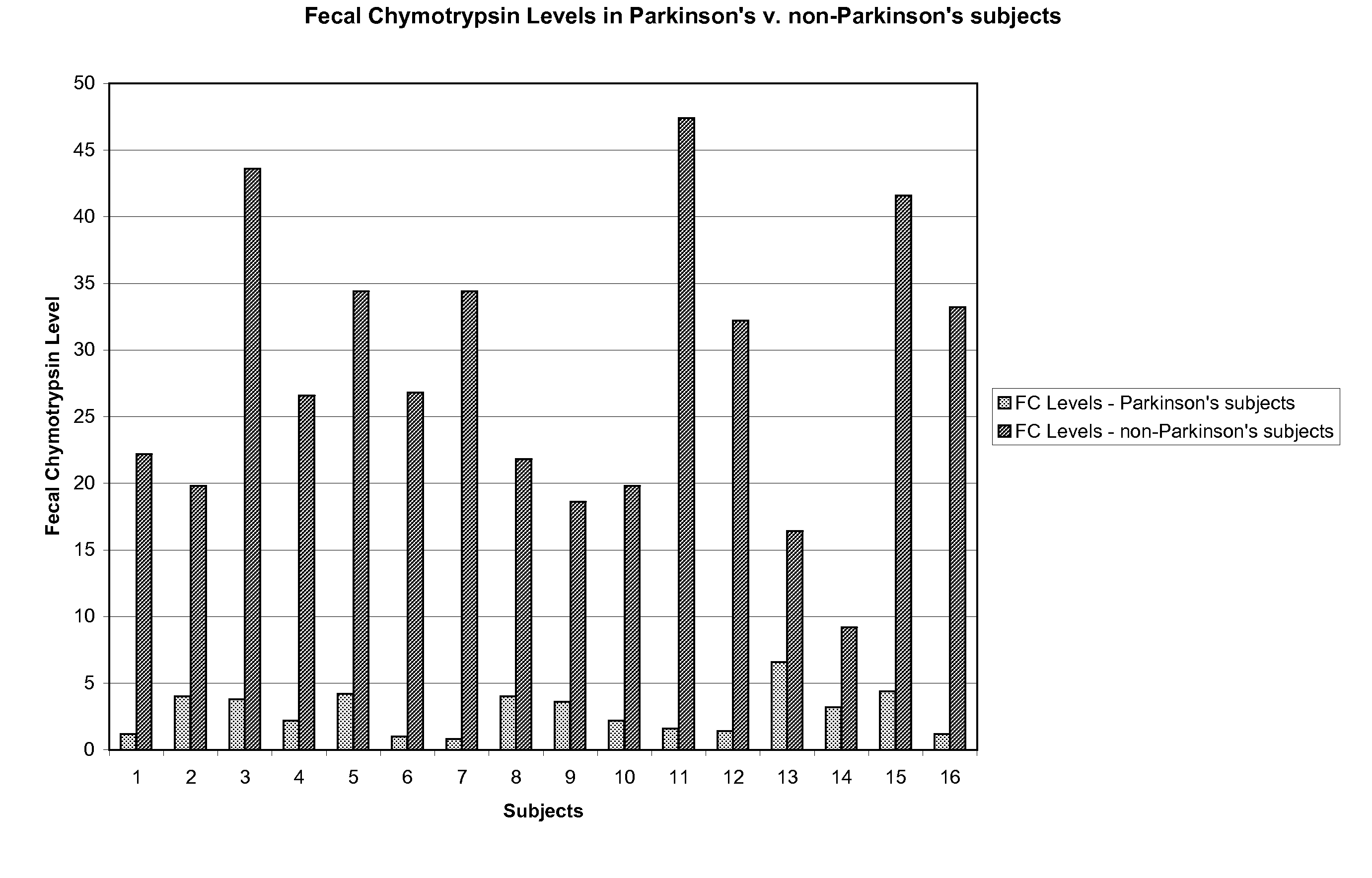
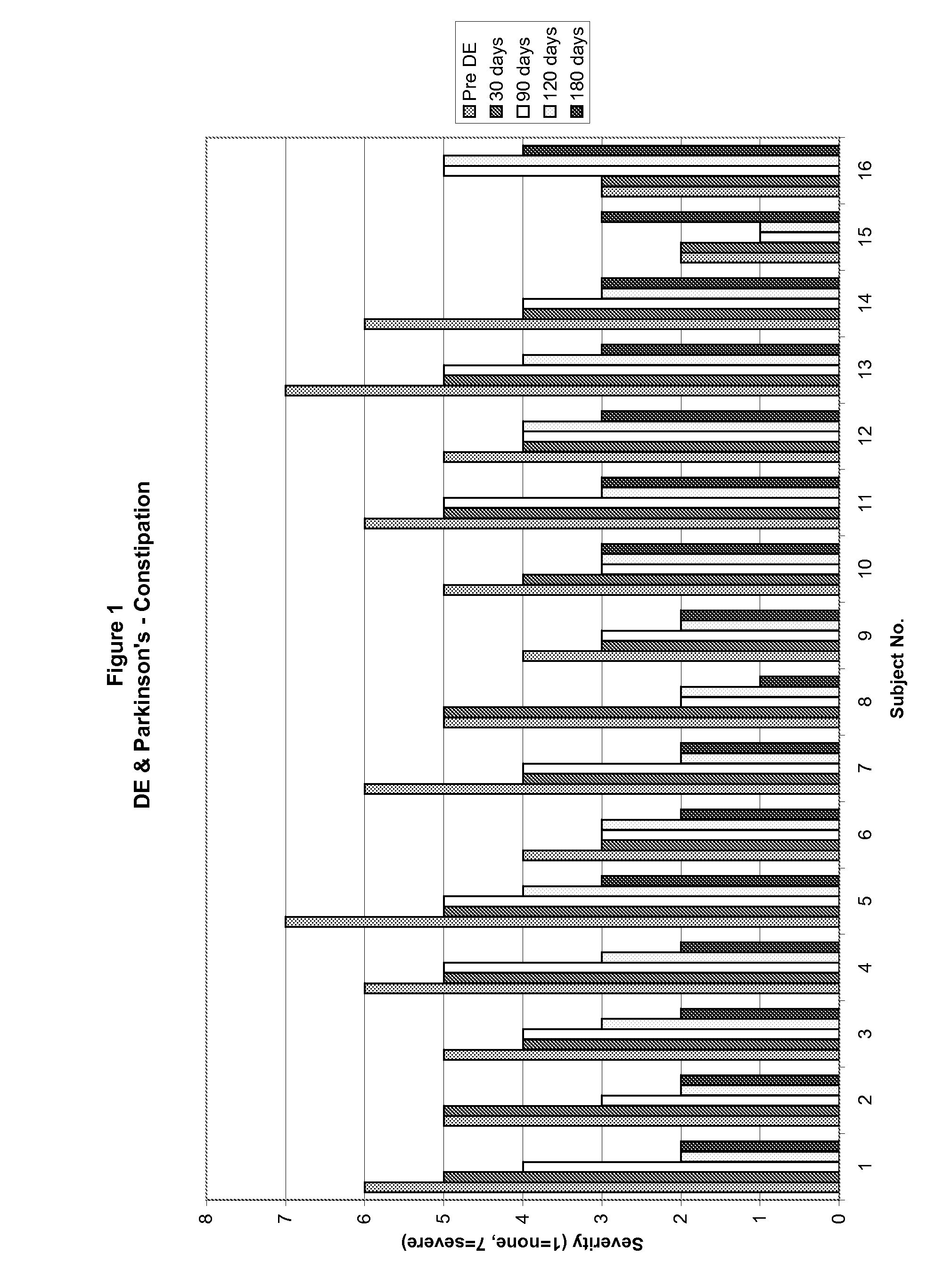
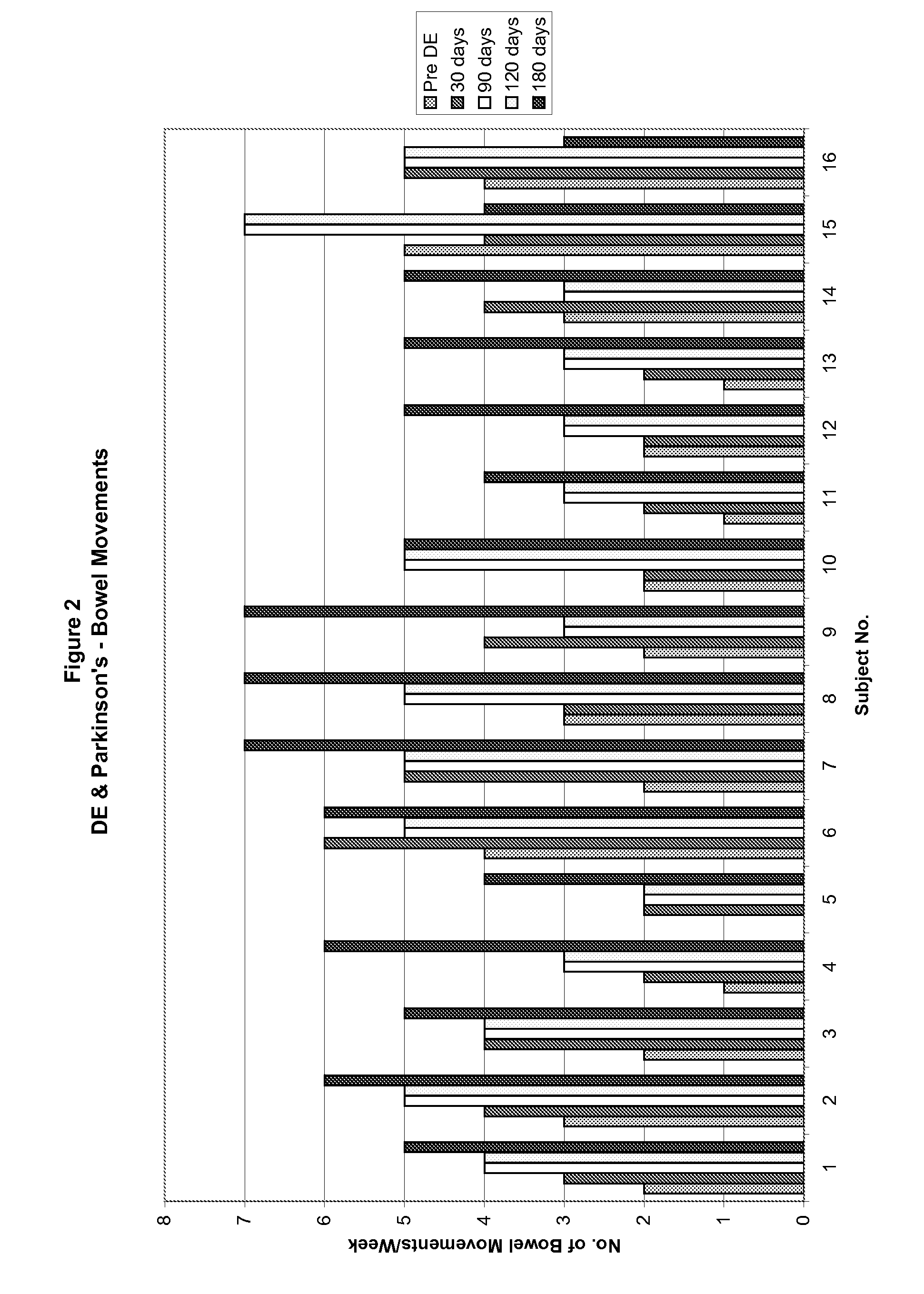
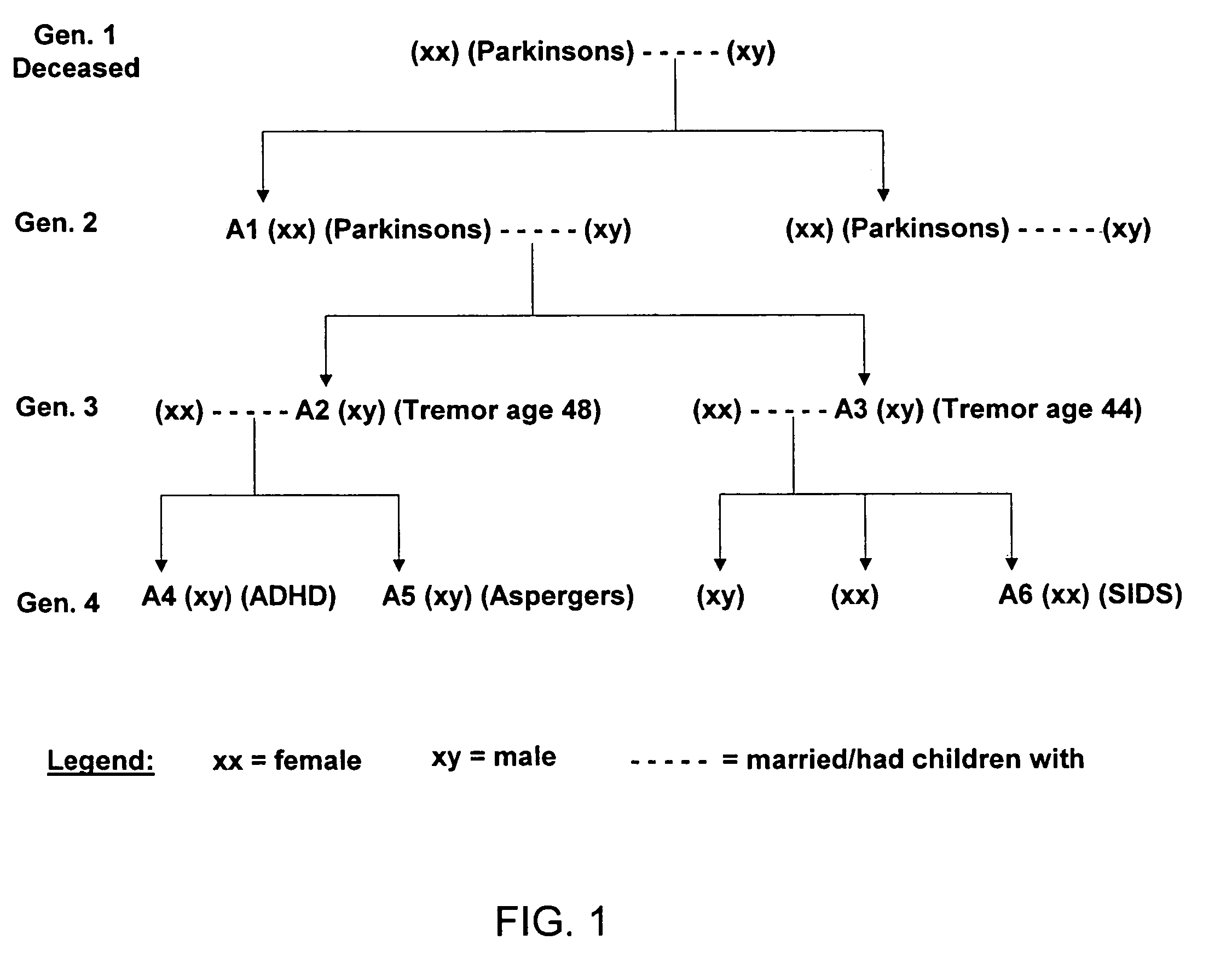
Method of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20070053895A1Symptoms improvedNervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
Immunotherapy Regimes Dependent On APOE Status
InactiveUS20090155256A1Improve securityReducing brain volume declineNervous disorderData processing applicationsDiseaseGenotype
The invention provides methods of immunotherapy of Alzheimer's and similar diseases in which the regime administered to a patient depends on the ApoE genotype of the patient.
Owner:WYETH LLC +1
Methods and compositions for the treatment of CNS-related conditions
ActiveUS20060252788A1Reduce and even minimize variationMinimizing CratioBiocideNervous disorderDiseasePsychiatry
The present invention provides novel methods and compositions for the treatment and prevention of CNS-related conditions. One of the CNS-related conditions treated by the methods and compositions of the invention is Alzheimer's disease.
Owner:ADAMAS PHARMA LLC
Method and apparatus for assessing sleep quality
Systems and / or methods for assessing the sleep quality of a patient in a sleep session are provided. Data is collected from the patient and / or physician including, for example, sleep session data in the form of one or more physiological parameters of the patient indicative of the patient's sleep quality during the sleep session, a subjective evaluation of sleep quality, etc.; patient profile data; etc. A sleep quality index algorithm, which optionally may be an adaptive algorithm, is applied, taking into account some or all of the collected data. Sleep quality data may be presented to at least the patient, and it may be displayed in any suitable format (e.g., a format useful for the patient to be appraised on the progress of the treatment, a format useful for a sleep clinician to monitor progress and / or assess the effectiveness of differing treatment regimens, etc).
Owner:RESMED LTD
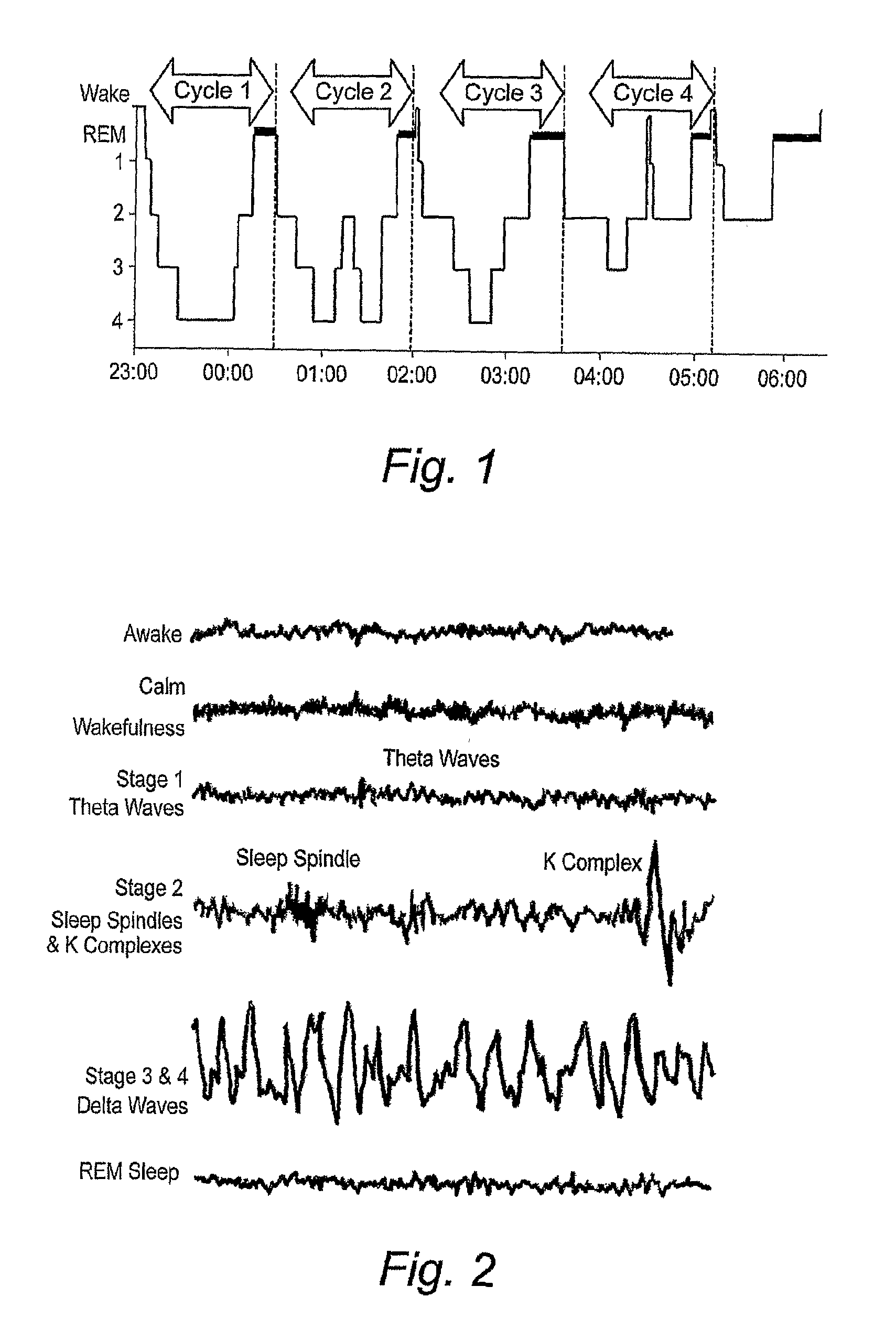
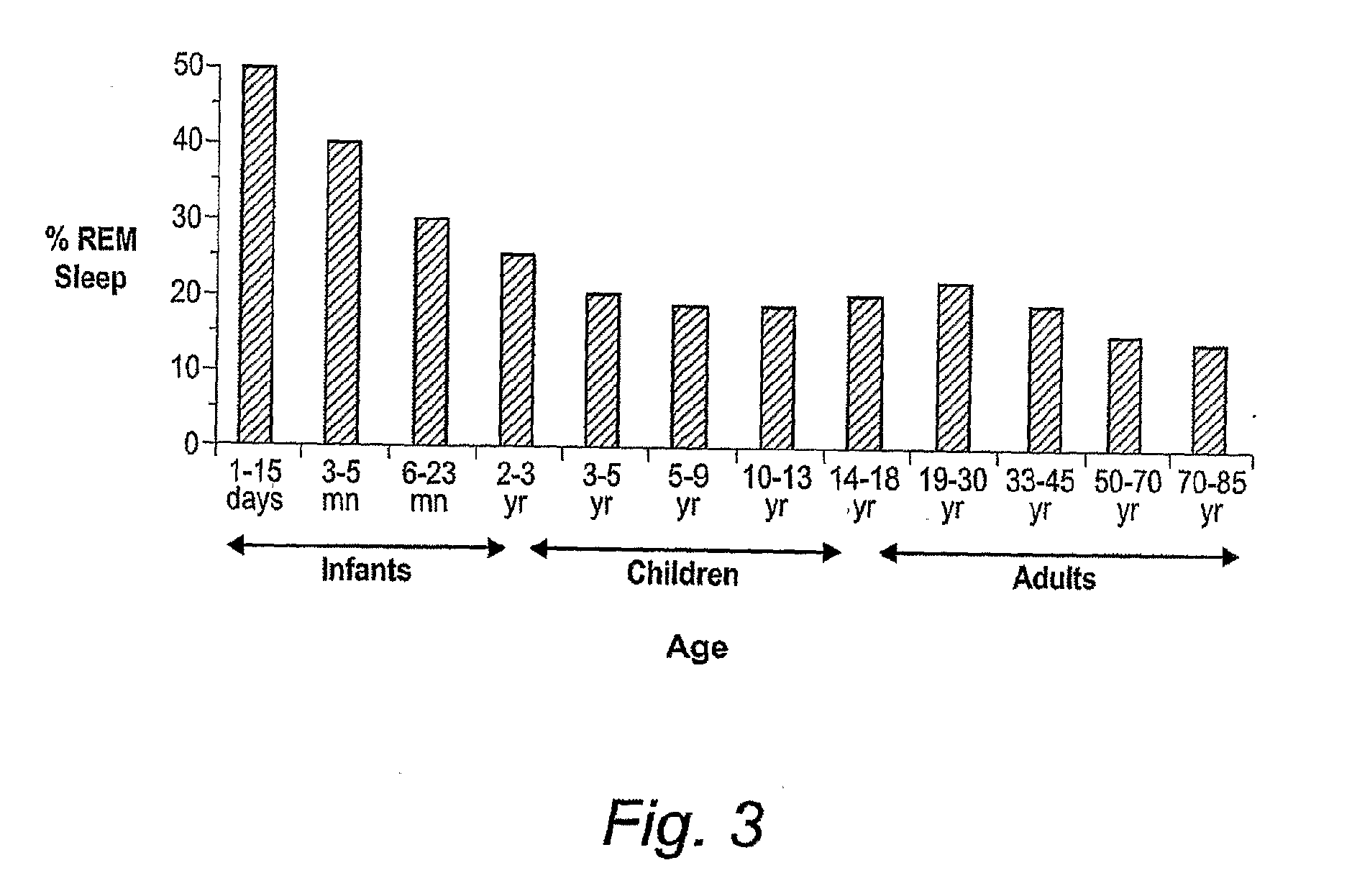
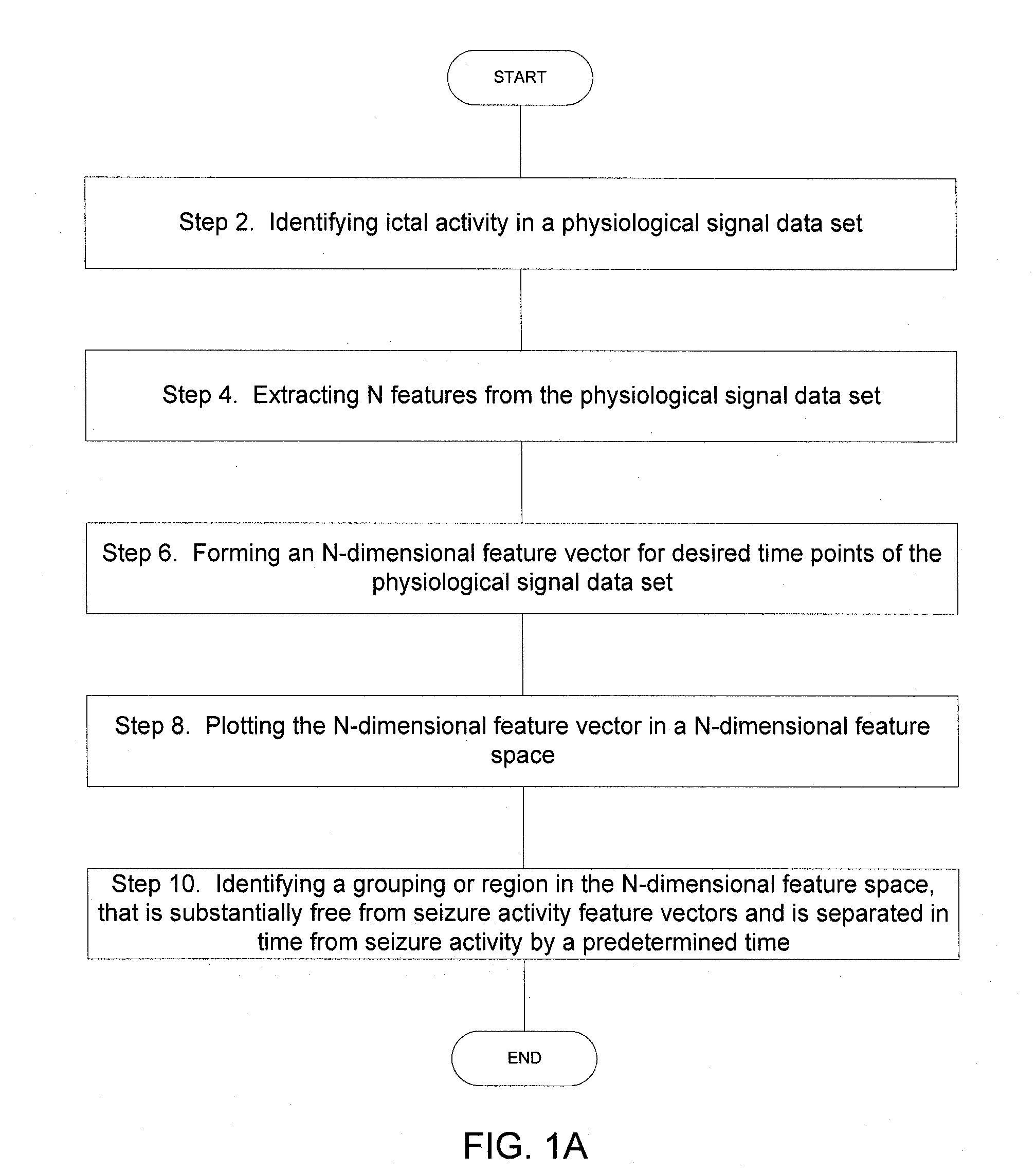
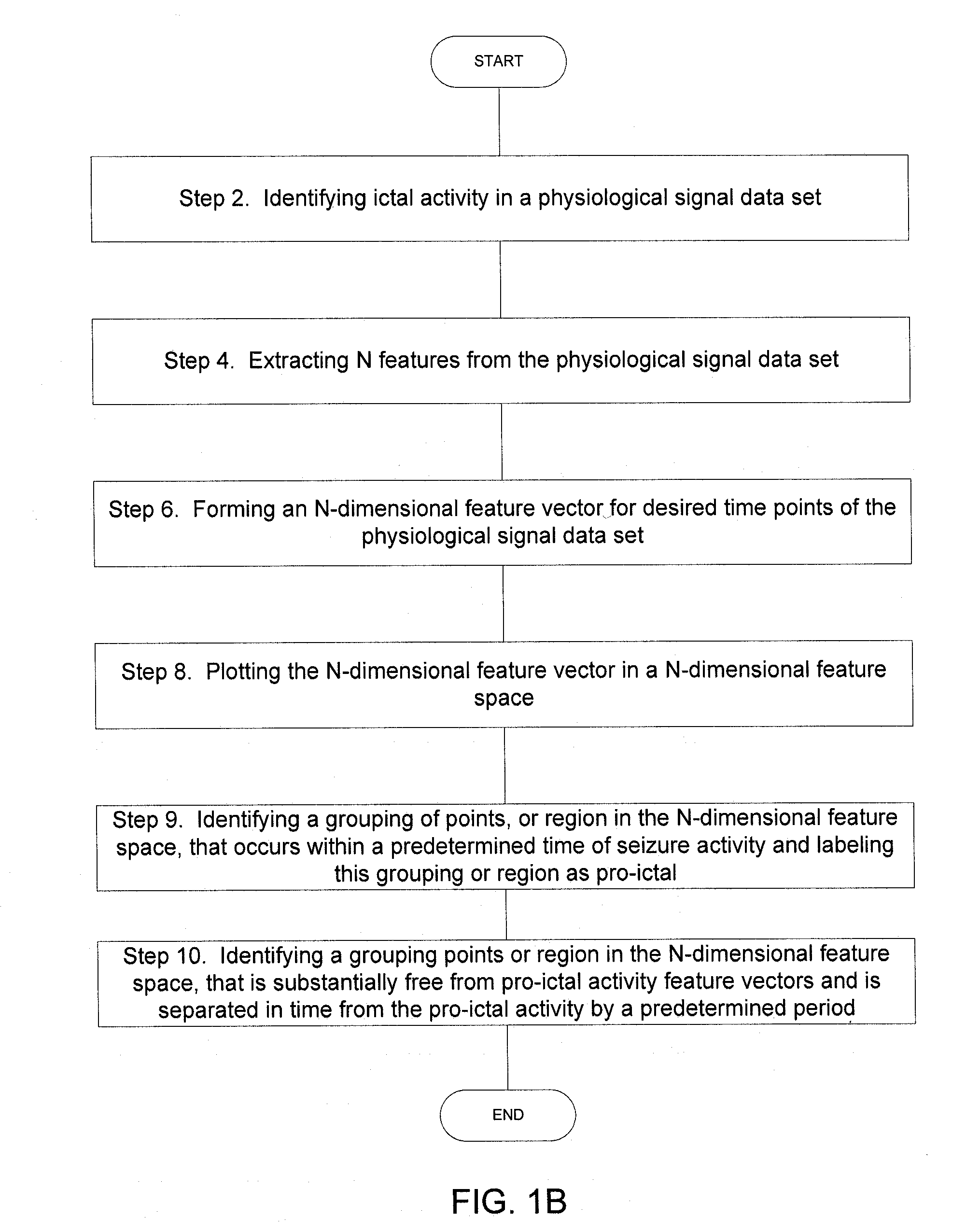
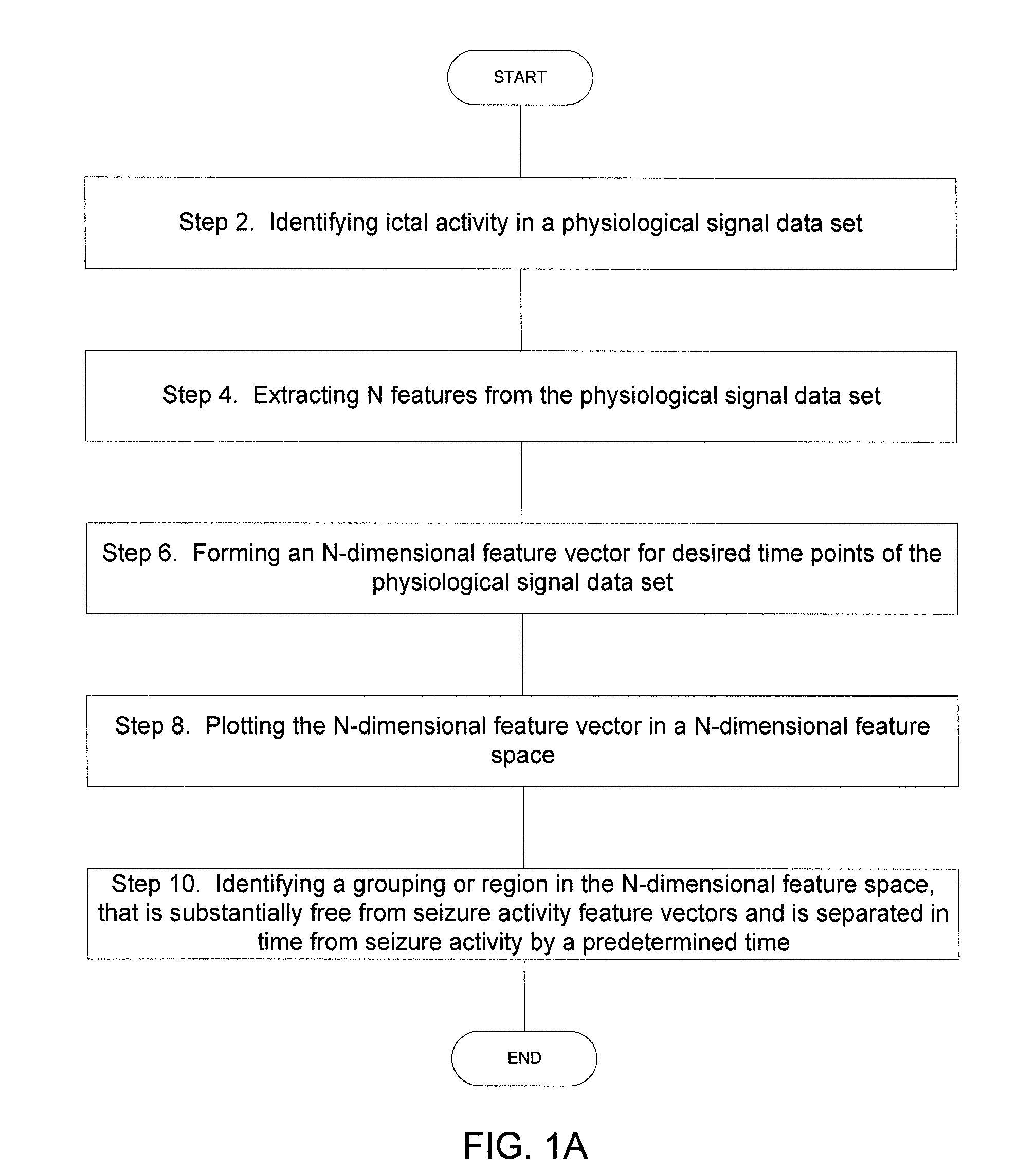
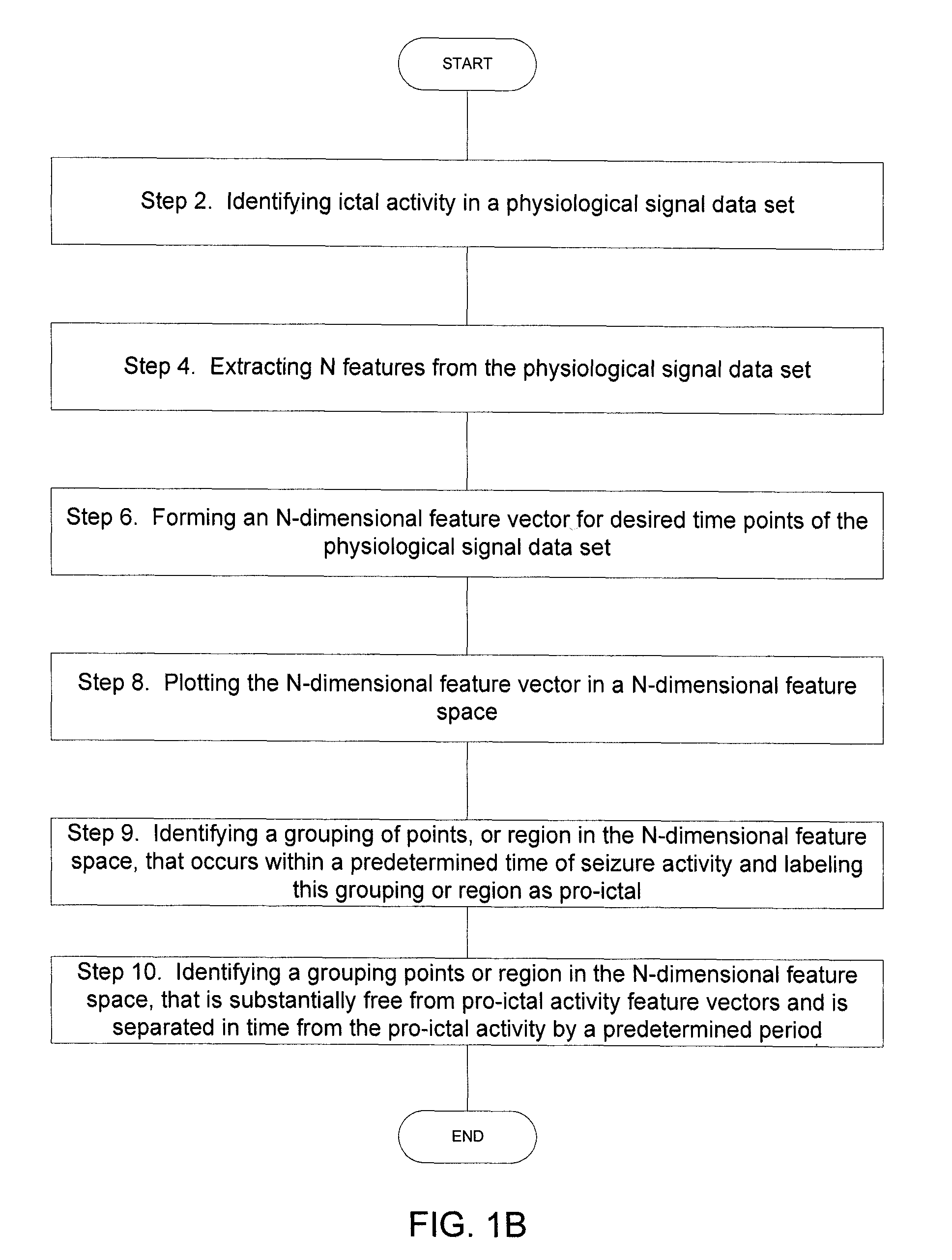
Implantable Systems and Methods for Identifying a Contra-ictal Condition in a Subject
ActiveUS20080234598A1Increased sensitivityReduce sensitivityElectroencephalographyElectrocardiographyPsychiatryRed light
Systems and methods of monitoring a subject's neurological condition are provided. In some embodiments, the method includes the steps of analyzing a physiological signal (such as an EEG) from a subject to determine if the subject is in a contra-ictal condition; and if the subject is in a contra-ictal condition, providing an indication (e.g., to the subject and / or to a caregiver) that the subject is in the contra-ictal condition. The systems and methods may utilize a minimally invasive, leadless device to monitor the subject's condition. In some embodiments, if the subject is in a pro-ictal condition, the method includes the step of providing an indication (such as a red light) that the subject is in the pro-ictal condition.
Owner:CYBERONICS INC
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Phospholipase c beta1 (plcbeta1) knockout mice as a model system for testing schizophrenia drugs
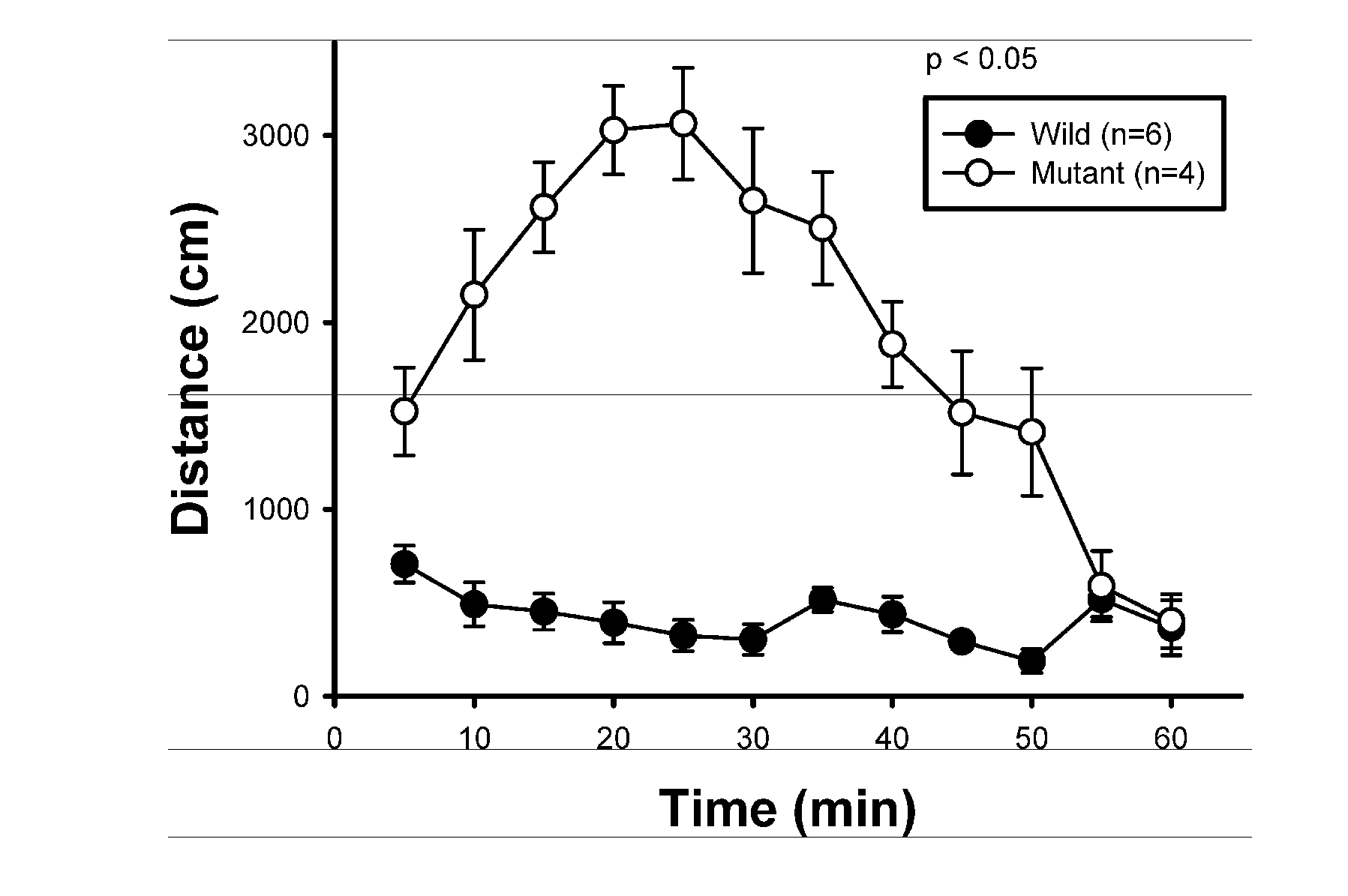
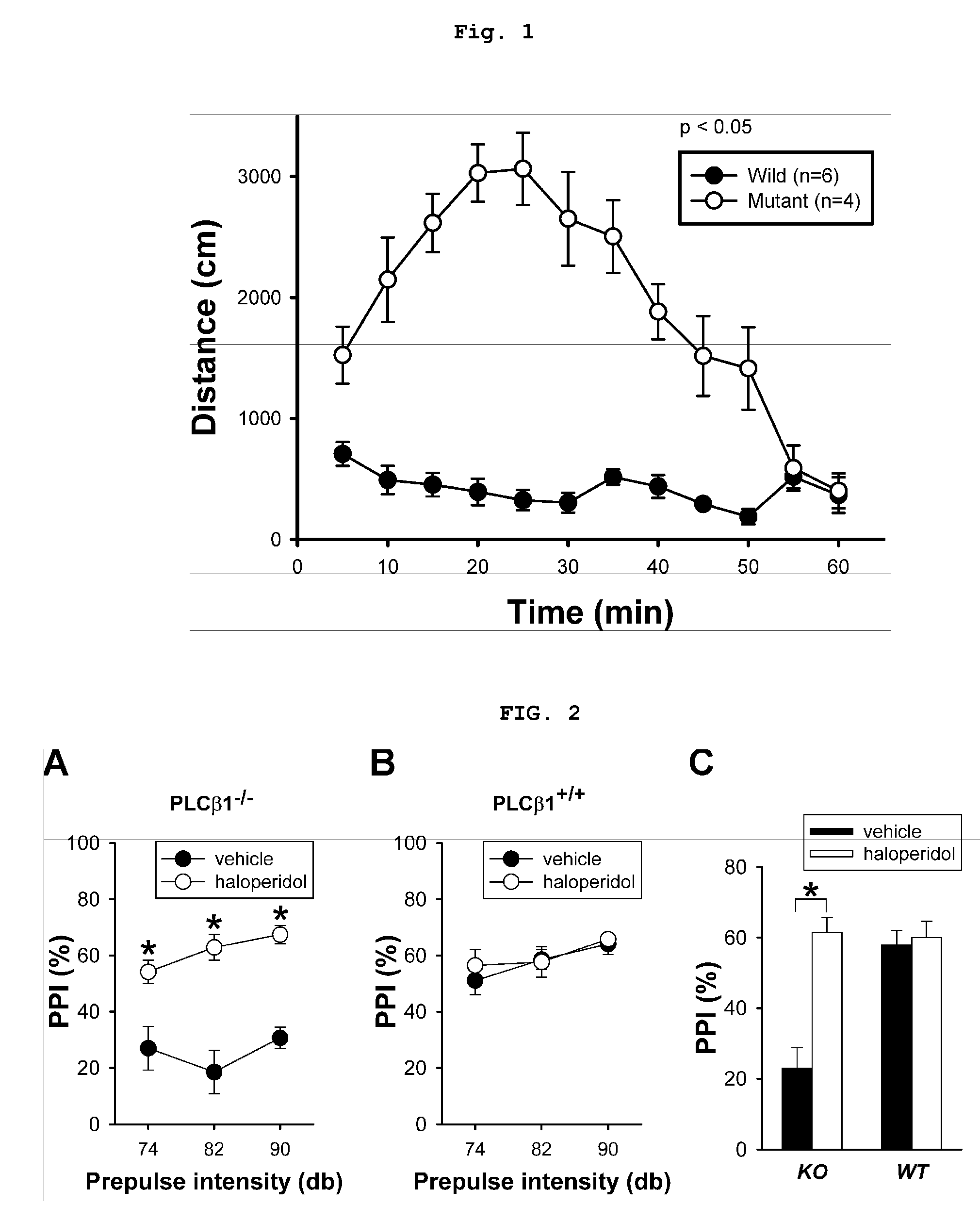
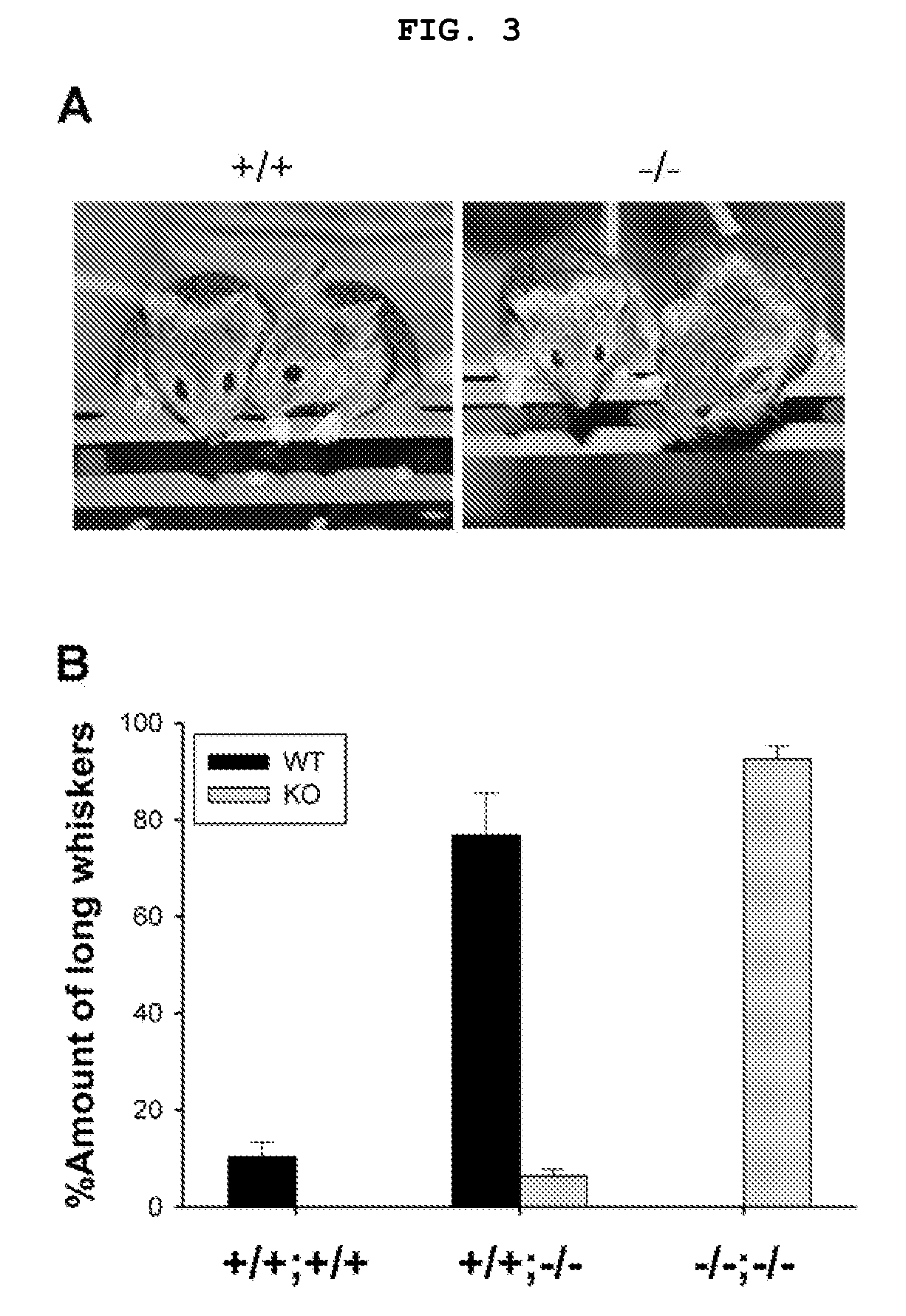
The present invention relates to a method for screening therapeutic drugs of schizophrenia using an animal model of the disease. More specifically, this invention relates to a screening method based on the phospholipase C β1 (PLCβ1) knockout mouse as an animal model of schizophrenia with all the major symptoms of the human disease. This knockout mouse exhibits symptoms similar to human schizophrenia such as locomotor hyperactivity, impaired prepulse inhibition of the startle response, lack of barbering and nesting behaviors, socially subordinate status, impaired learning, and lack of type II theta rhythm which has been implicated in working memory. Thus, the knockout mouse of the present invention can be useful as an animal for screening therapeutic drugs against schizophrenia.
Owner:KOREA INST OF SCI & TECH
Brain, spinal, and nerve injury treament
InactiveUS20030109417A1Significant morbidityHigh incidenceBiocideNervous disorderSubstance P Receptor AntagonistsSpinal cord
A treatment for brain, spinal and nerve injury comprising use of a substance P receptor antagonist optionally in combination with a magnesium compound. There is also provided a formulation for use in this treatment comprising a substance P receptor antagonist and a magnesium compound.
Owner:EUSTRALIS PHARMA LTD
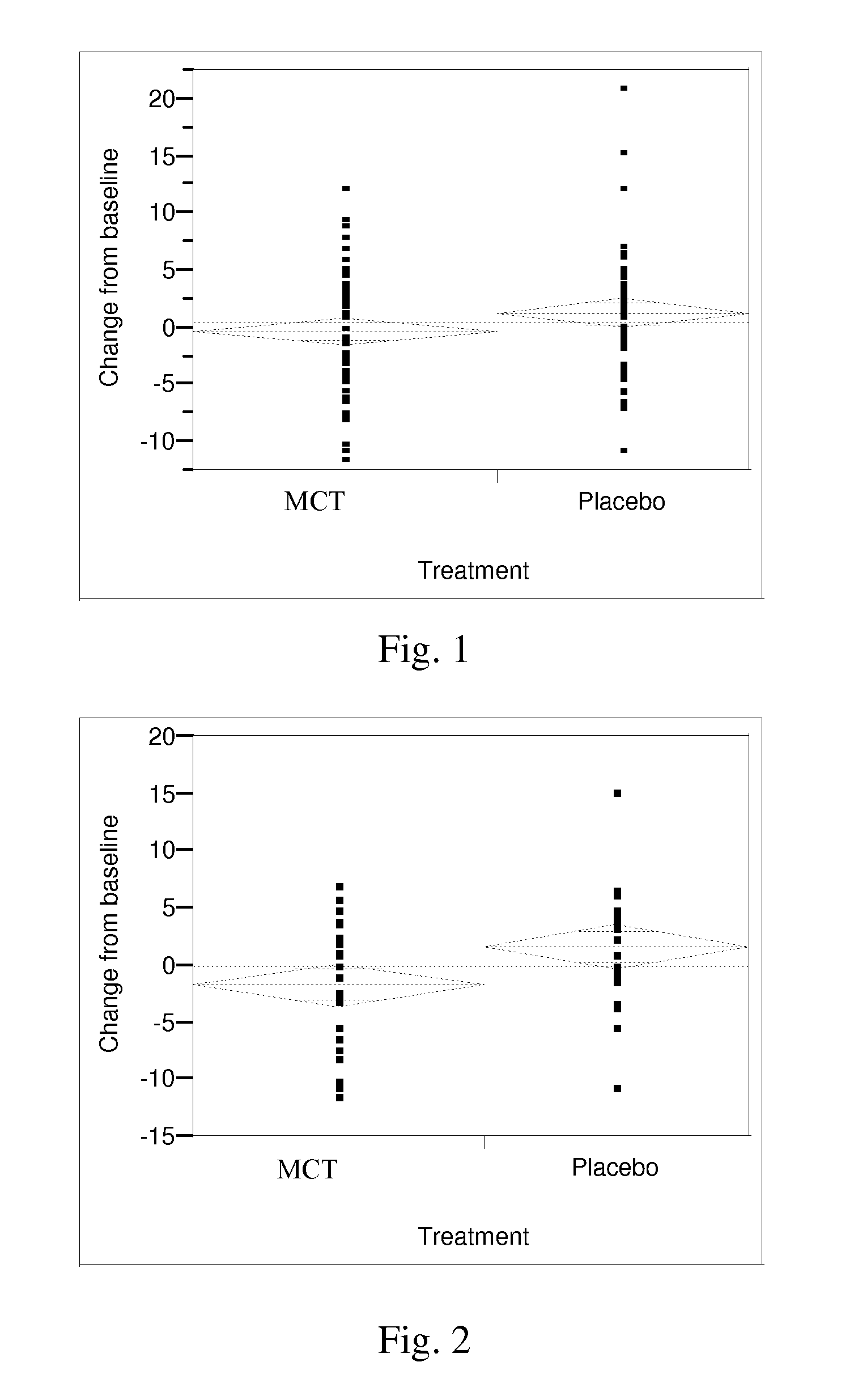
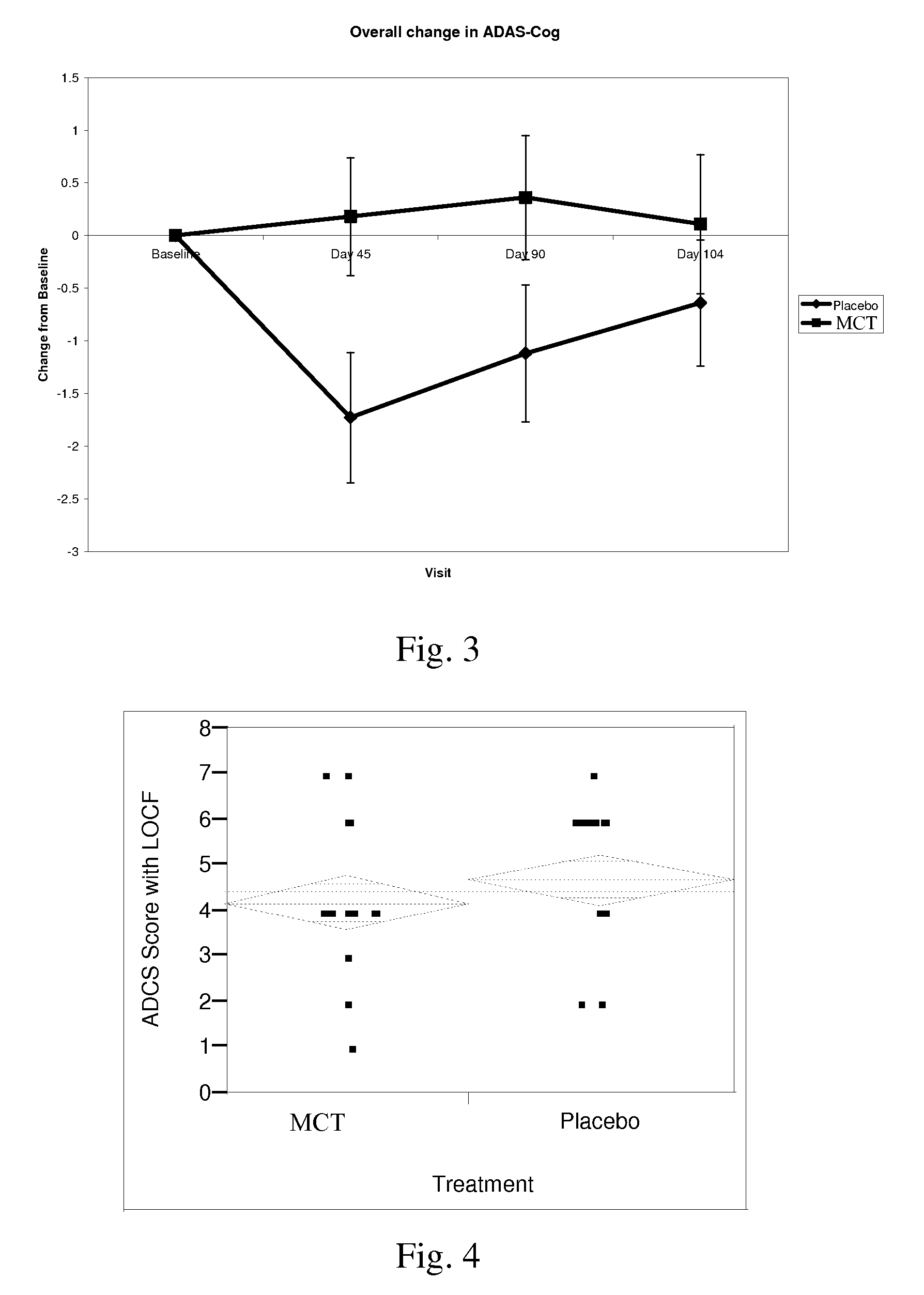
Combinations of medium chain triglycerides and therapeutic agents for the treatment and prevention of alzheimers disease and other diseases resulting from reduced neuronal metabolism
InactiveUS20080009467A1Increase utilityBiocideNervous disorderAnti atheroscleroticAnti-diabetic Agent
Methods and compositions for treating or preventing, the occurrence of senile dementia of the Alzheimer's type, mild cognitive impairment, or other conditions arising from reduced neuronal metabolism and leading to lessened cognitive function are described. In a preferred embodiment the administration of triglycerides or fatty acids with chain lengths between 5 and 12, to said patient at a level to produce an improvement in cognitive ability, and a therapeutic agent selected from the group consisting of anti-Alzheimer's agents, anti-diabetic agents, agents capable of increasing utilization of lipids, anti-atherosclerotic agents, anti-hypertensive agents, anti-inflammatory agents, anti-obesity agents, and combinations thereof. Preferred therapeutic agents include donepezil, rivastigmine, galantamine, and memantine.
Owner:ACCERA INC
Implantable systems and methods for identifying a contra-ictal condition in a subject
Systems and methods of monitoring a subject's neurological condition are provided. In some embodiments, the method includes the steps of analyzing a physiological signal (such as an EEG) from a subject to determine if the subject is in a contra-ictal condition; and if the subject is in a contra-ictal condition, providing an indication (e.g., to the subject and / or to a caregiver) that the subject is in the contra-ictal condition. The systems and methods may utilize a minimally invasive, leadless device to monitor the subject's condition. In some embodiments, if the subject is in a pro-ictal condition, the method includes the step of providing an indication (such as a red light) that the subject is in the pro-ictal condition.
Owner:CYBERONICS INC
Dose counting in metered dose inhaler
A counter for indicating the number of doses left in a canister that is suitable for use in a metered dose inhaler. The counter is affixed to the canister and includes a module for providing an indication of the number of doses left in the canister and a triggering mechanism for updating the indication in response to activation of the inhaler.
Owner:GENOVA PERRY +2
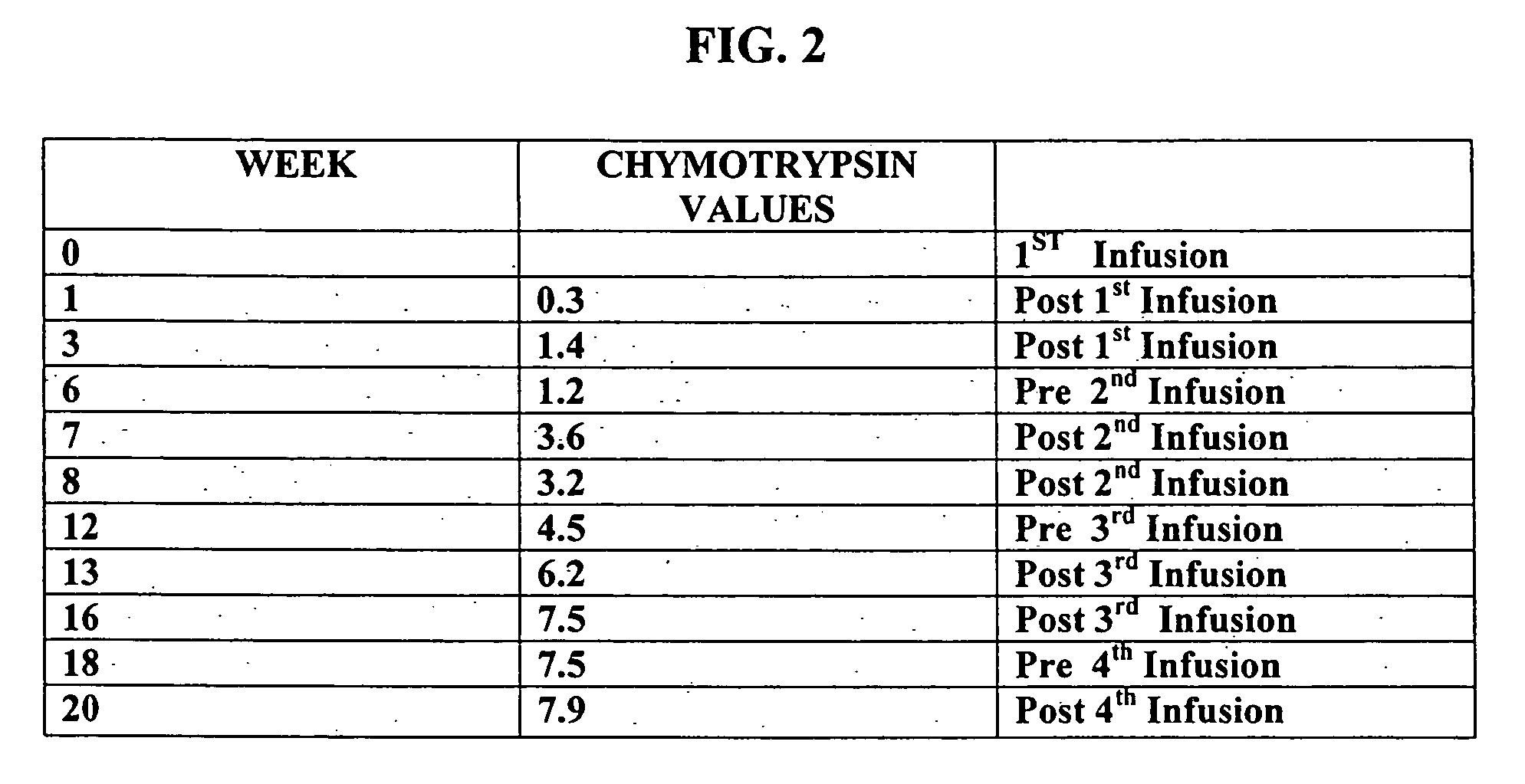
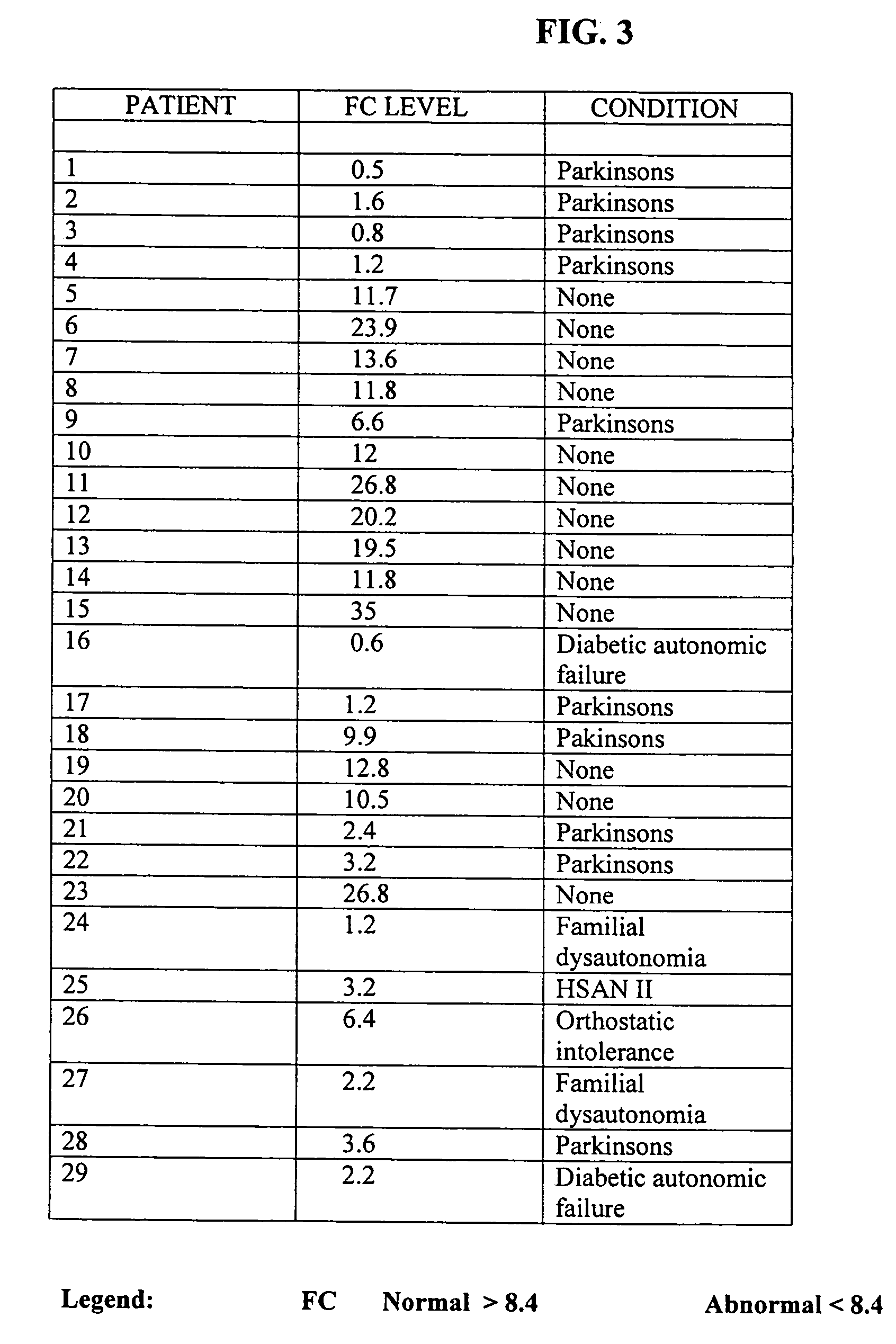
Methods for diagnosing and treating dysautonomia and other dysautonomic conditions
Methods for aiding in the diagnosis of dysautonomic disorders and dysautonomic conditions and methods for treating individuals diagnosed as having a dysautonomic disorder or a dysautonomic condition. In one aspect, a diagnosis method comprising analyzing a stool sample of an individual for the presence of a biological marker wherein the quantity of the biological marker is an indication of whether the individual has, or can develop, a dysautonomic disorder or dysautonomic condition, as well as a therapeutic method for treating a dysautonomic disorder or dysautonomic condition by administration of, e.g., secretin, neuropeptides, peptides and / or digestive enzymes.
Owner:CUREMARK
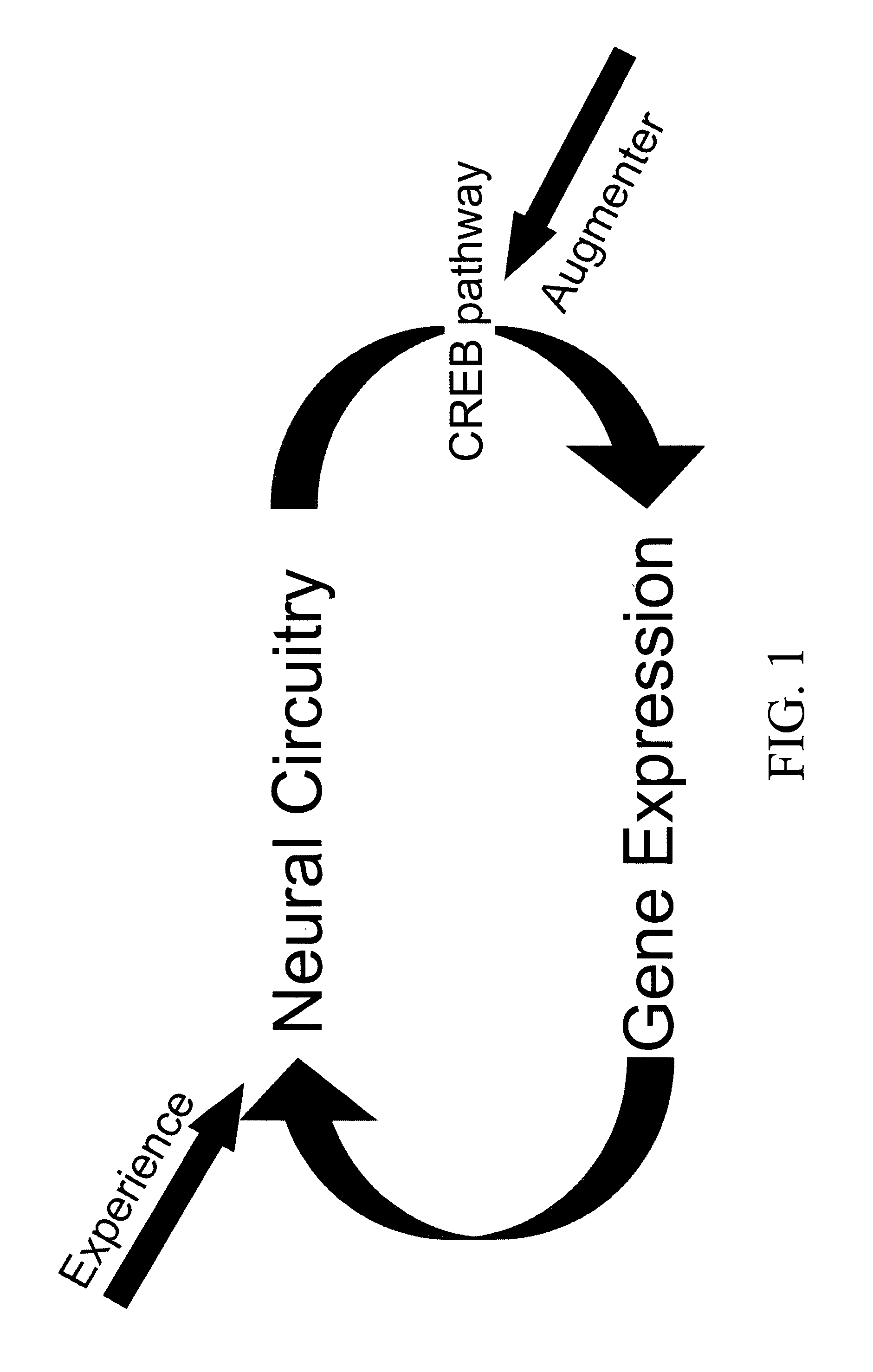
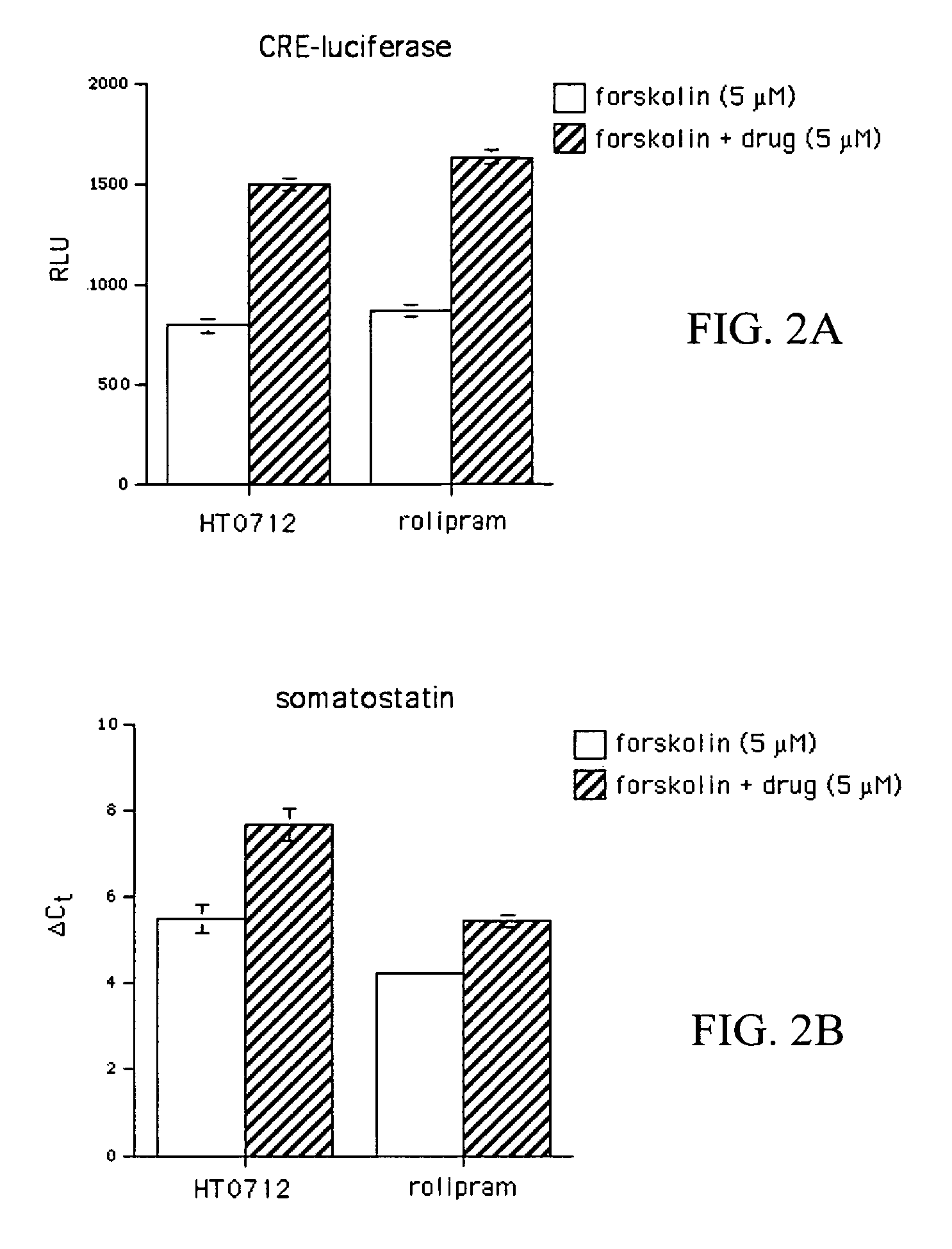
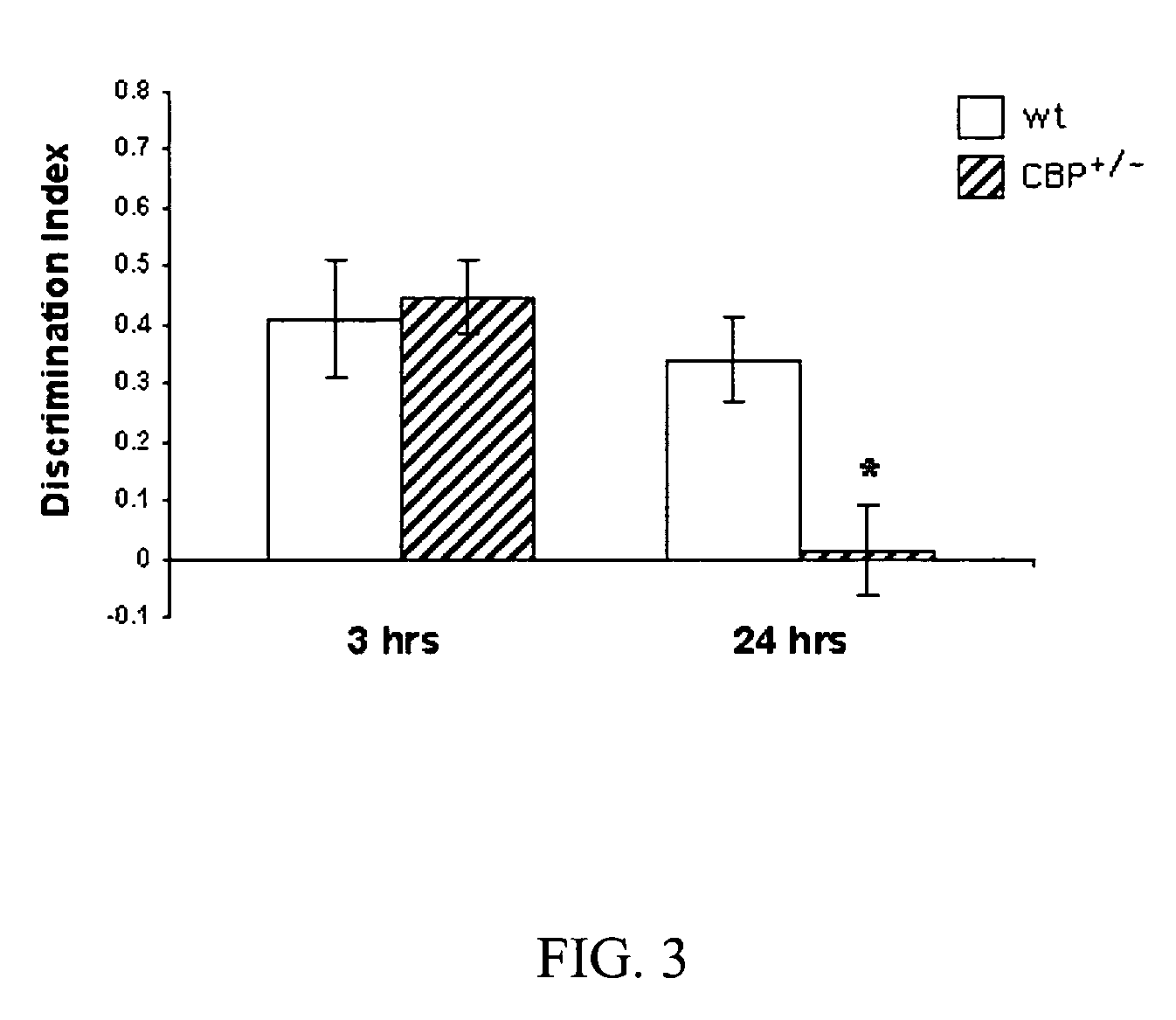
Phosphodiesesterase 4 inhibitors for the treatment of a cognitive deficit
InactiveUS7868015B2Normally performanceVarious formsPhysical therapies and activitiesBiocidePhosphodiesterase-4Psychiatry
The present invention provides methods of treating cognitive deficits associated with mental retardation. The methods comprise combining cognitive training protocols and a general administration of phosphodiesterase 4 inhibitors.
Owner:COLD SPRING HARBOR LAB INC
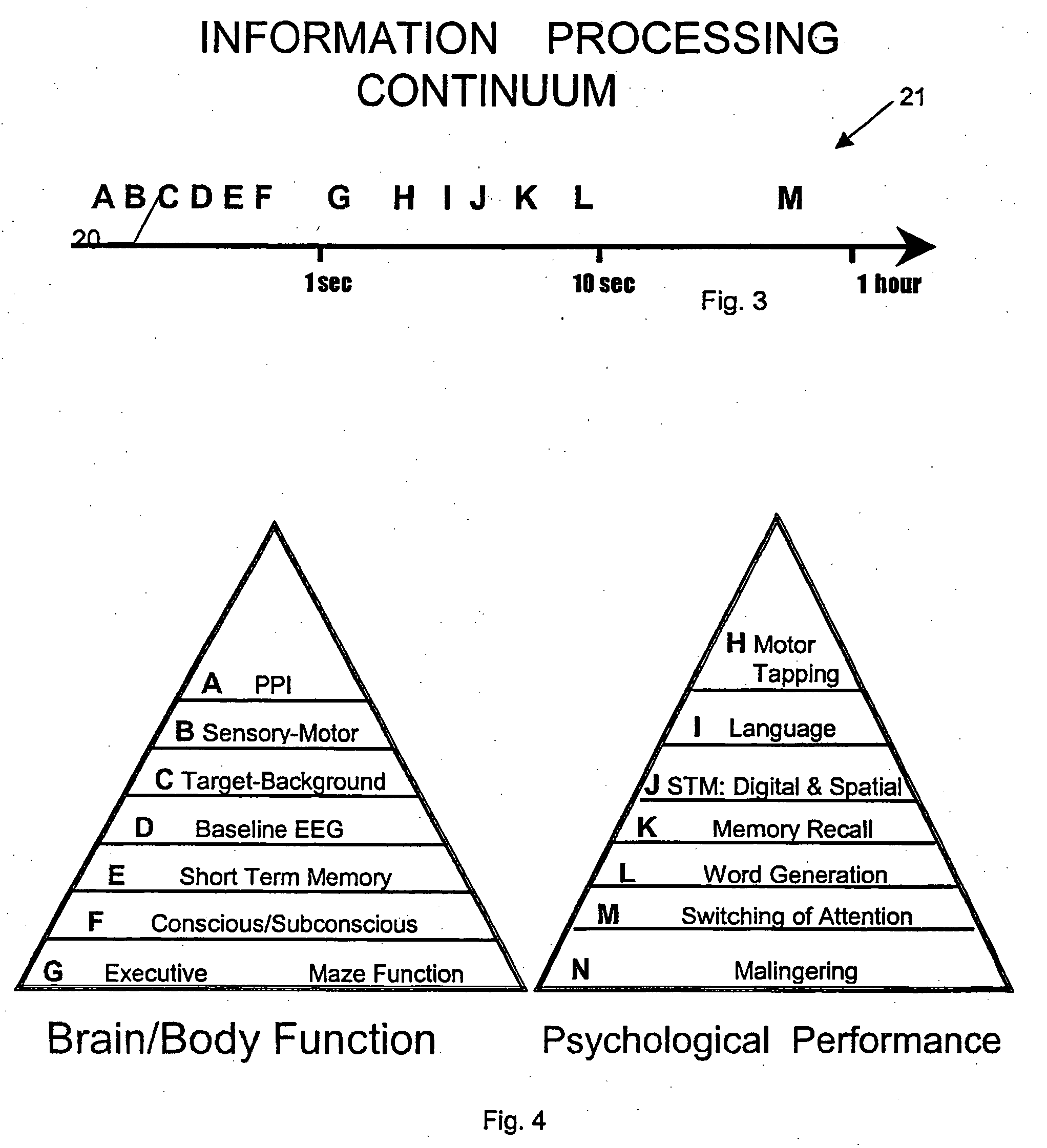
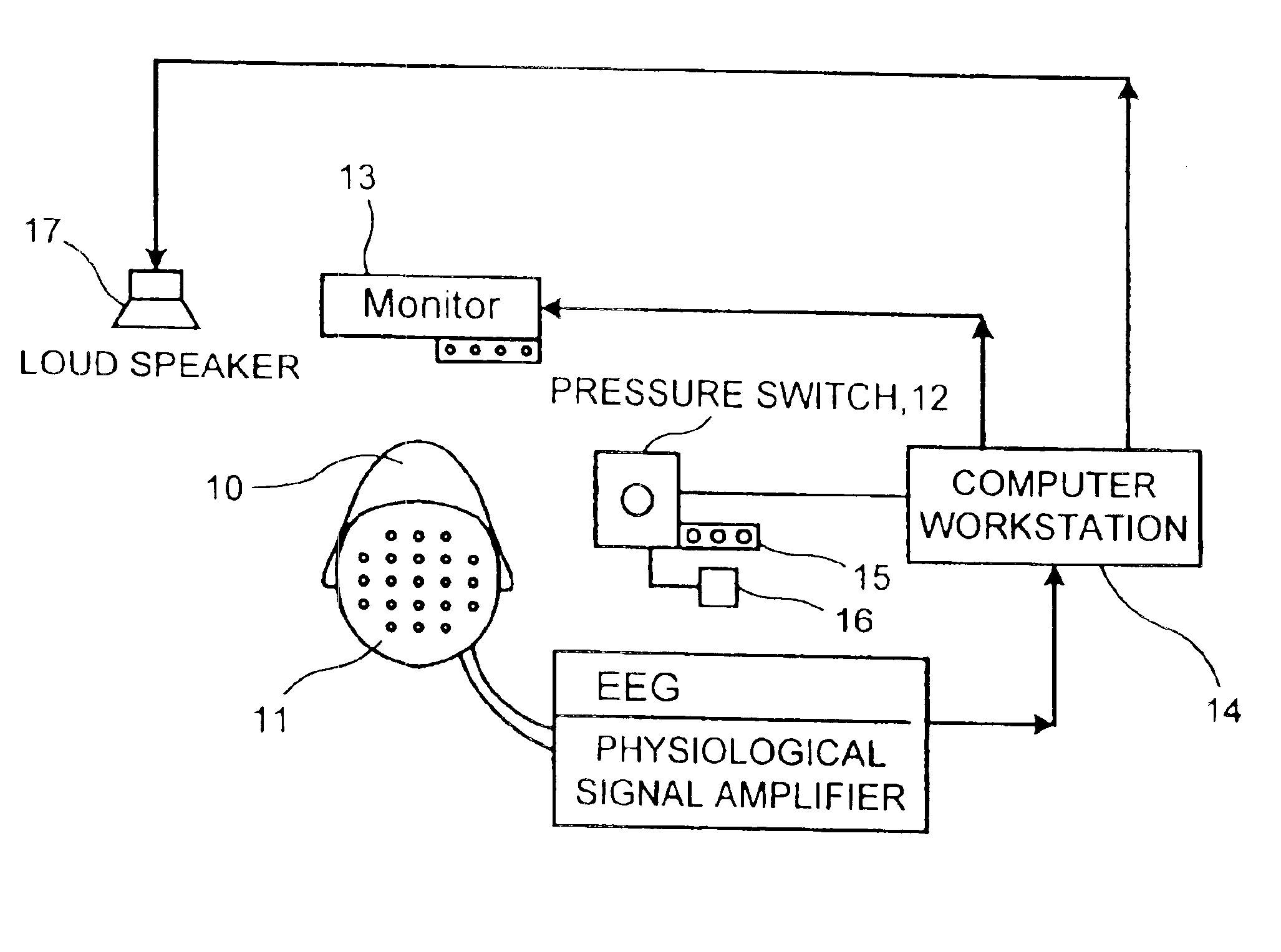
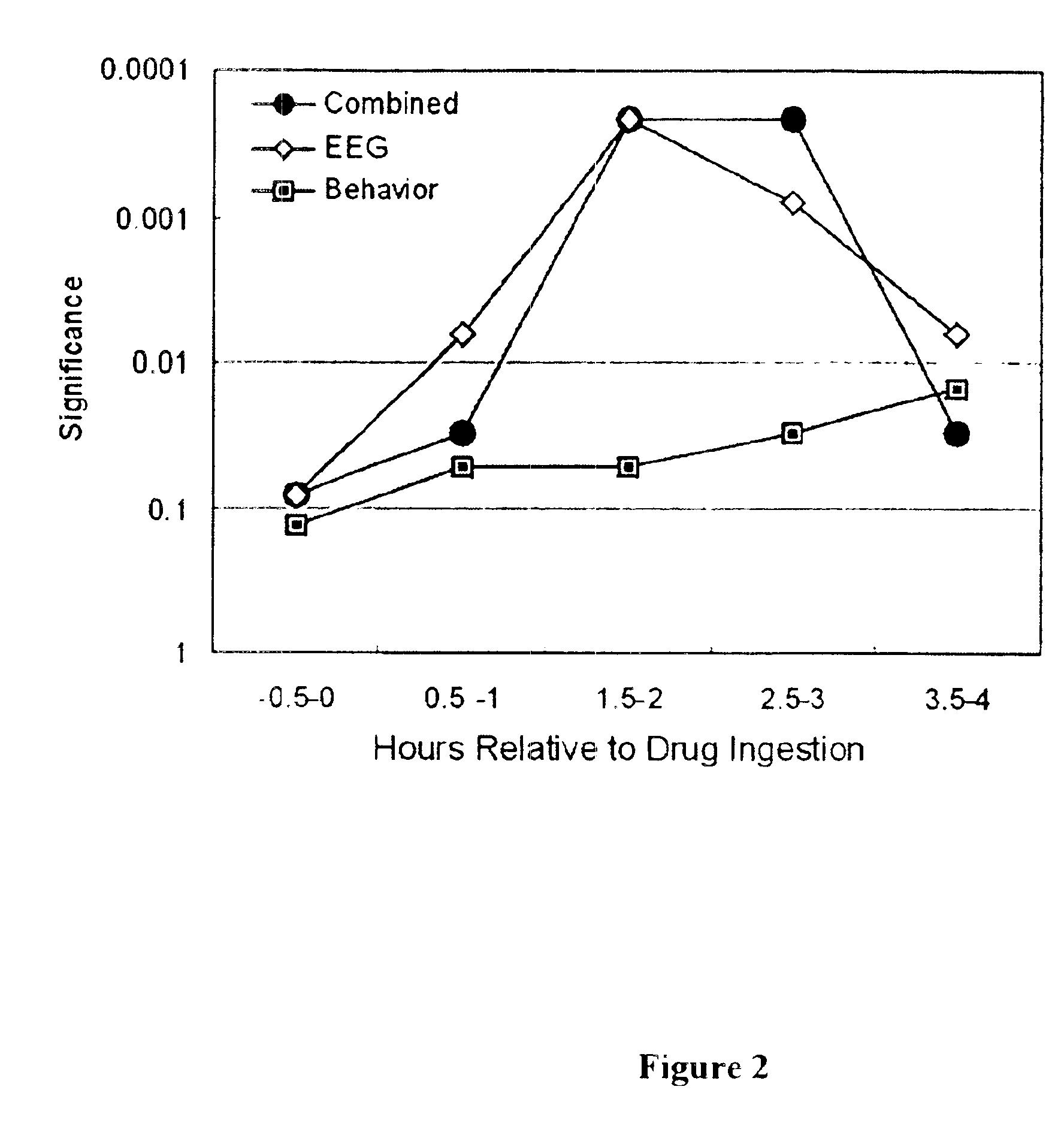
Neurocognitive function EEG measurement method and system
InactiveUS6947790B2Sufficient sensitivityHigh sensitivityElectroencephalographyHealth-index calculationDiseaseDrug approval
An efficient, objective testing method and system for evaluating changes in mental function is described. The method and system are based on measuring an individual's behavioral responses and brain function during a brief cognitive test battery and passive control conditions. The method and system is designed to assess an individual's fundamental cognitive functions, and whether those functions have been significantly affected by a variety of factors such as progressive disease processes, medication, stress, fatigue, training, or the passage of time. The method and system can be used to determine whether drugs being evaluated to treat diseases or conditions affecting cognitive brain function have a significant positive effect on delaying or improving the symptoms of such a disease or condition, especially during clinical trials for drug approval and subsequent marketing. The method and system may also be employed as part of the successful diagnosis or ongoing treatment of neurological diseases or conditions that directly or indirectly affect human neurocognitive performance. The method and system may also be used to determine transitory changes in overall cognitive function due to emotional stress or fatigue, and more long lasting changes in overall cognitive function following training and educational programs.
Owner:SAM TECHOLOGY
Compositions and methods for improving or preserving brain function
InactiveUS20070179197A1Improve performancePrevent and reduce delayBiocideNervous disorderRegimenExercise performance
The present invention is related to mammalian nutrition and effects thereof in individuals with age associated cognitive decline such as Age Associated Memory Inpairment (AAMI) or a dementing illness such as Alzheimer's disease or related dementia, or Mild Cognitive Impairment, such as improving performance in, or reversal, prevention, reducing and delaying decline in, one or more of cognitive function, memory, behavior, cerebrovascular function, motor function, and / or brain physiology are seen. In particular, the present invention utilizes medium chain triglycerides, in one embodiment, administered as part of a long-term treatment regimen, to preserve or improve learning, attention, motor performance, cerebrovascular function, social behavior, and to increase activity levels, particularly in aging mammals.
Owner:ACCERA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com