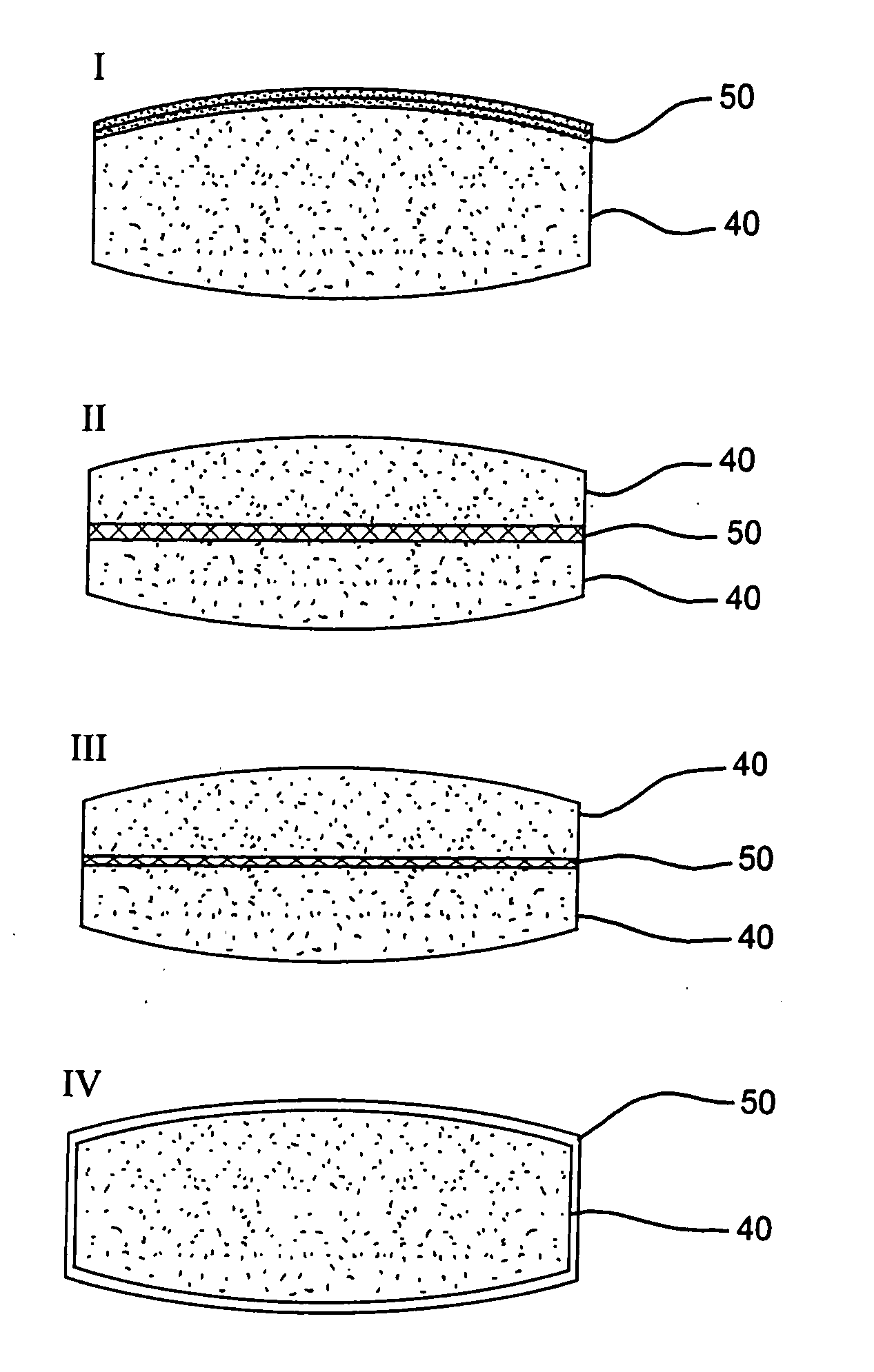
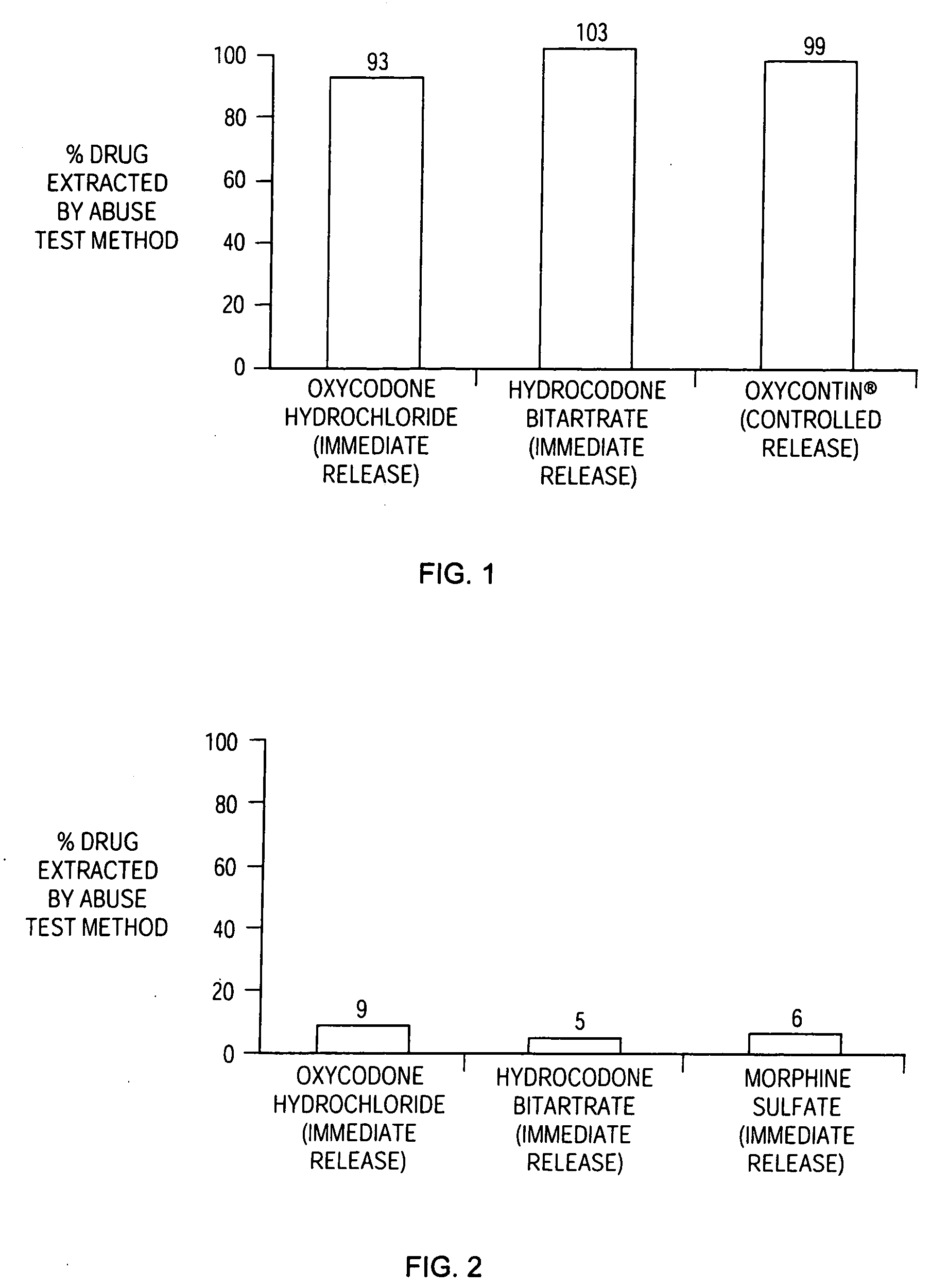
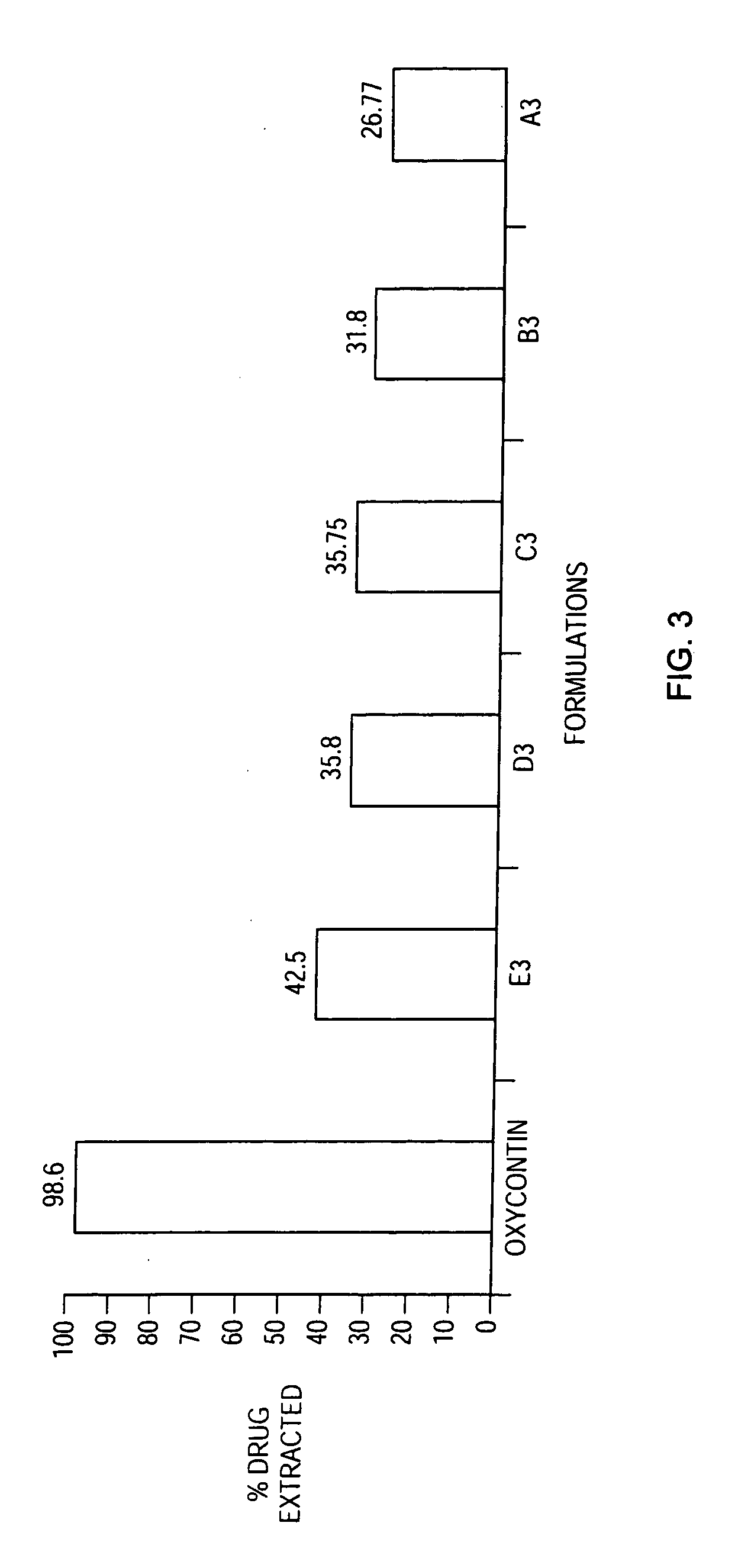
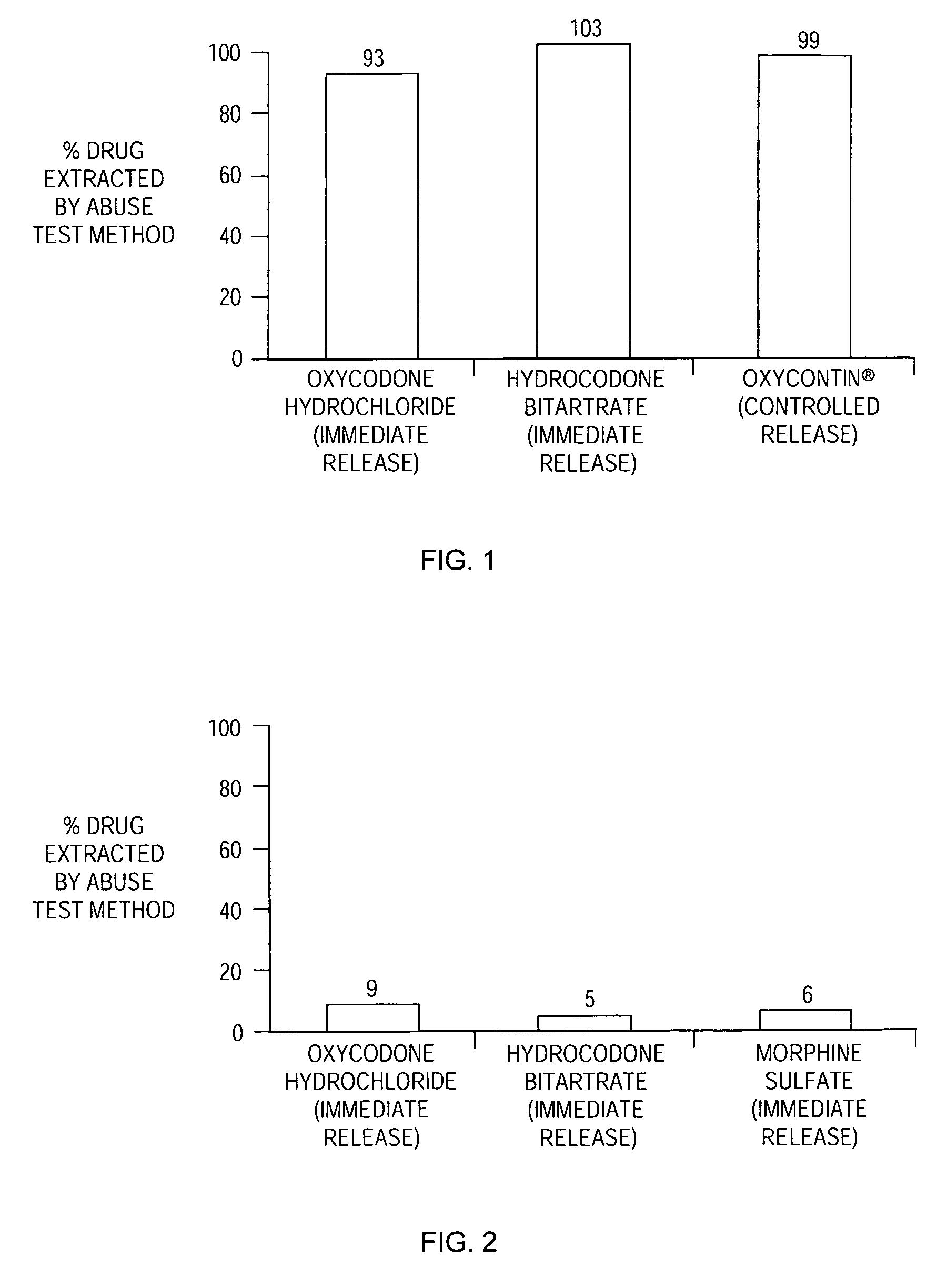
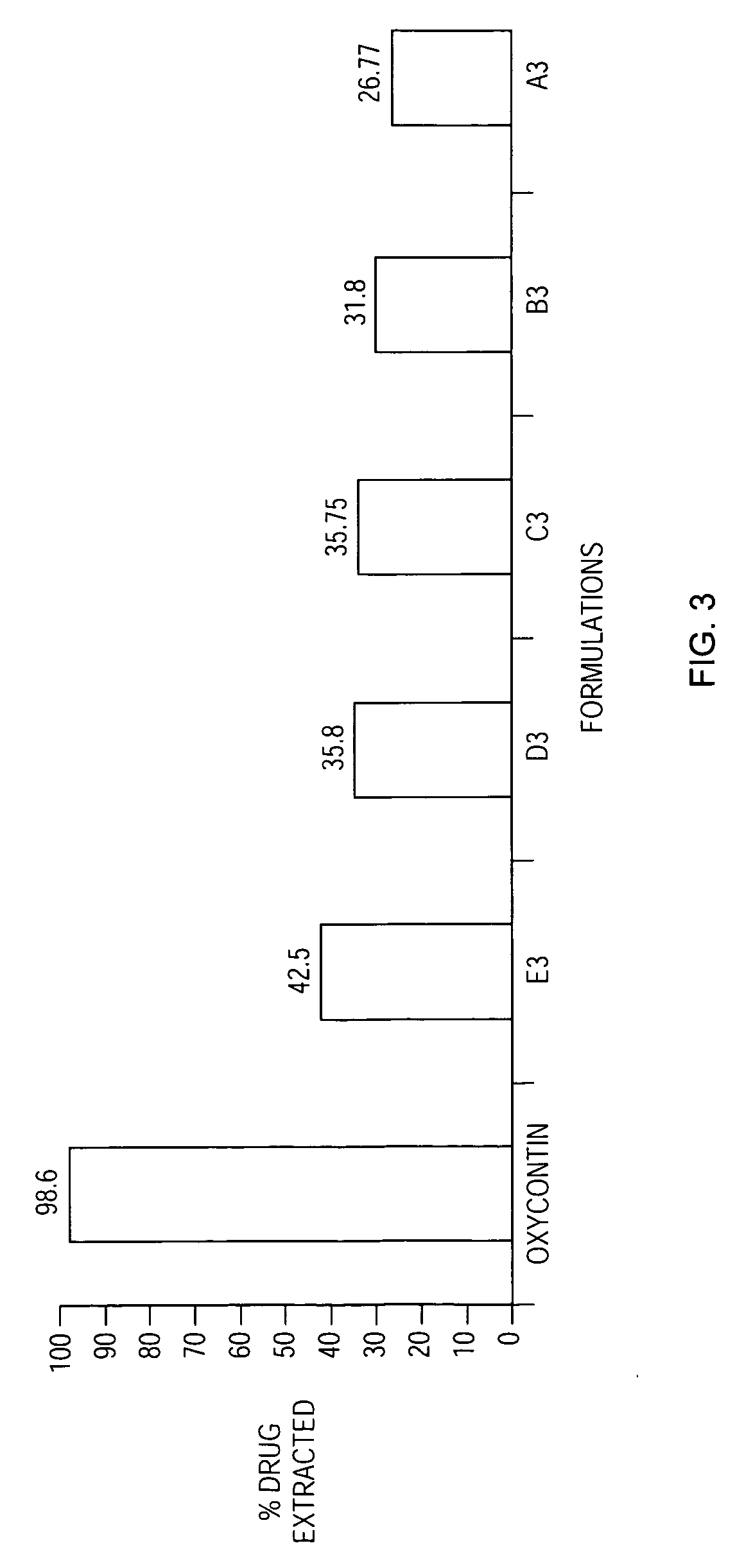
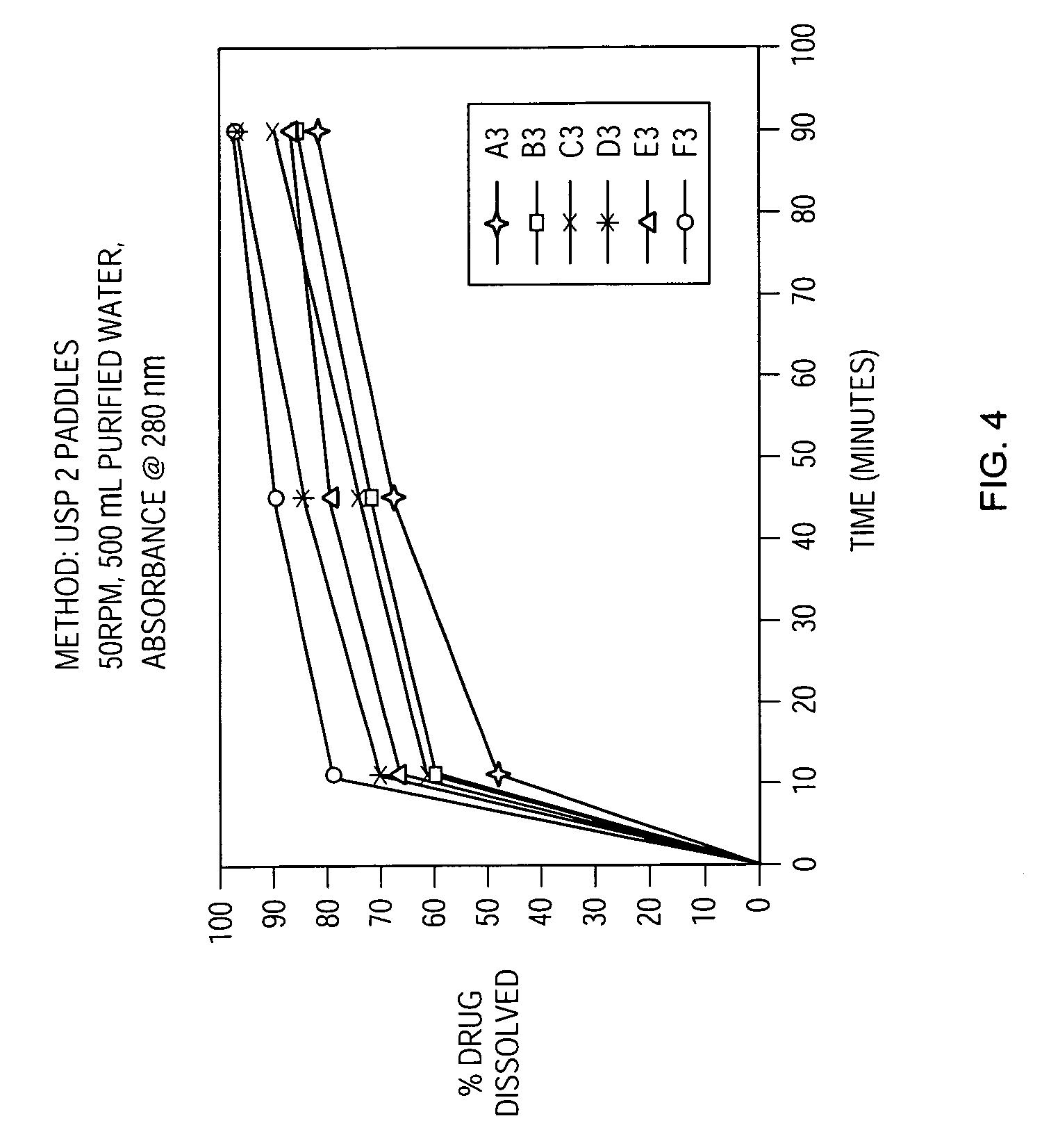
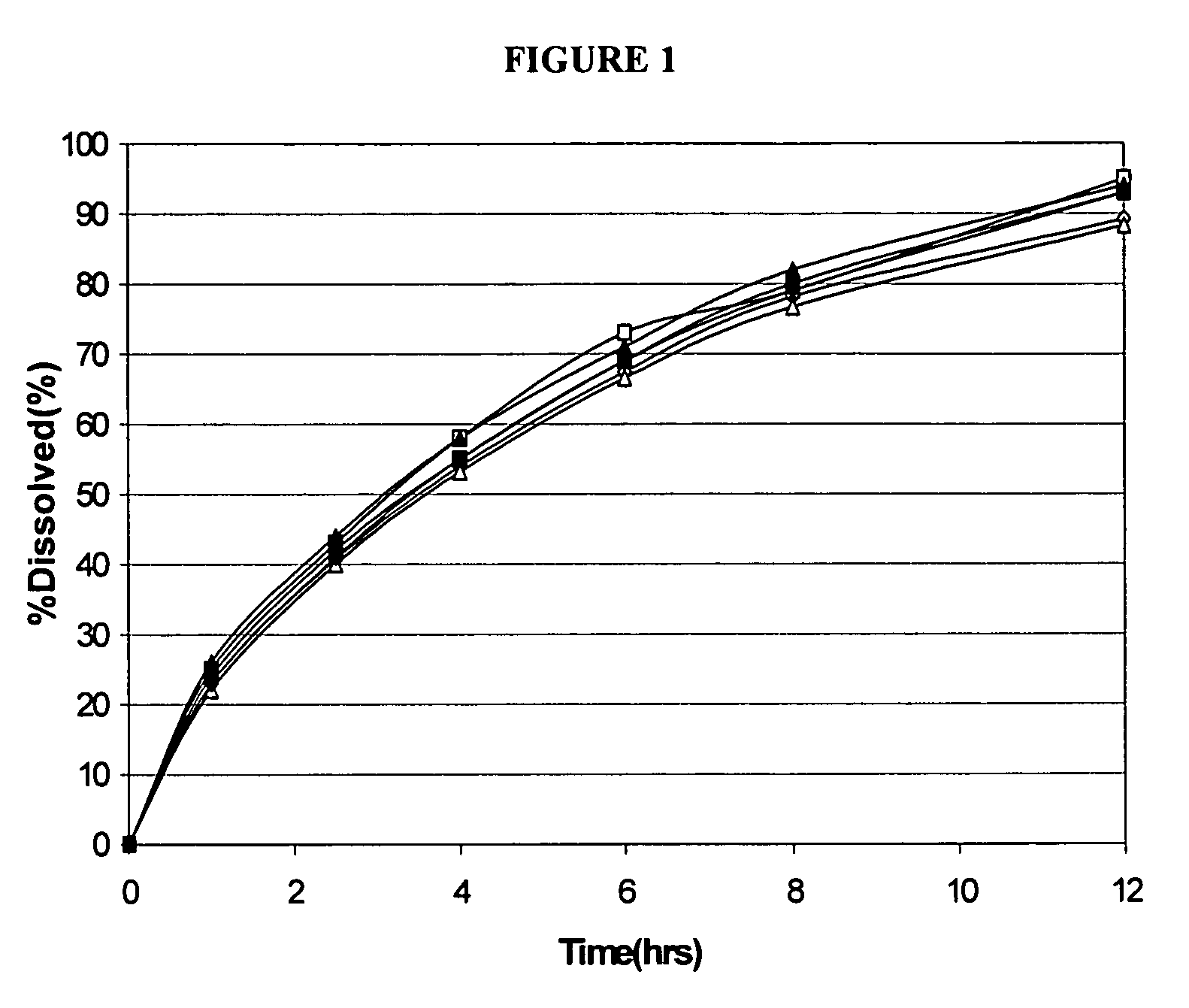
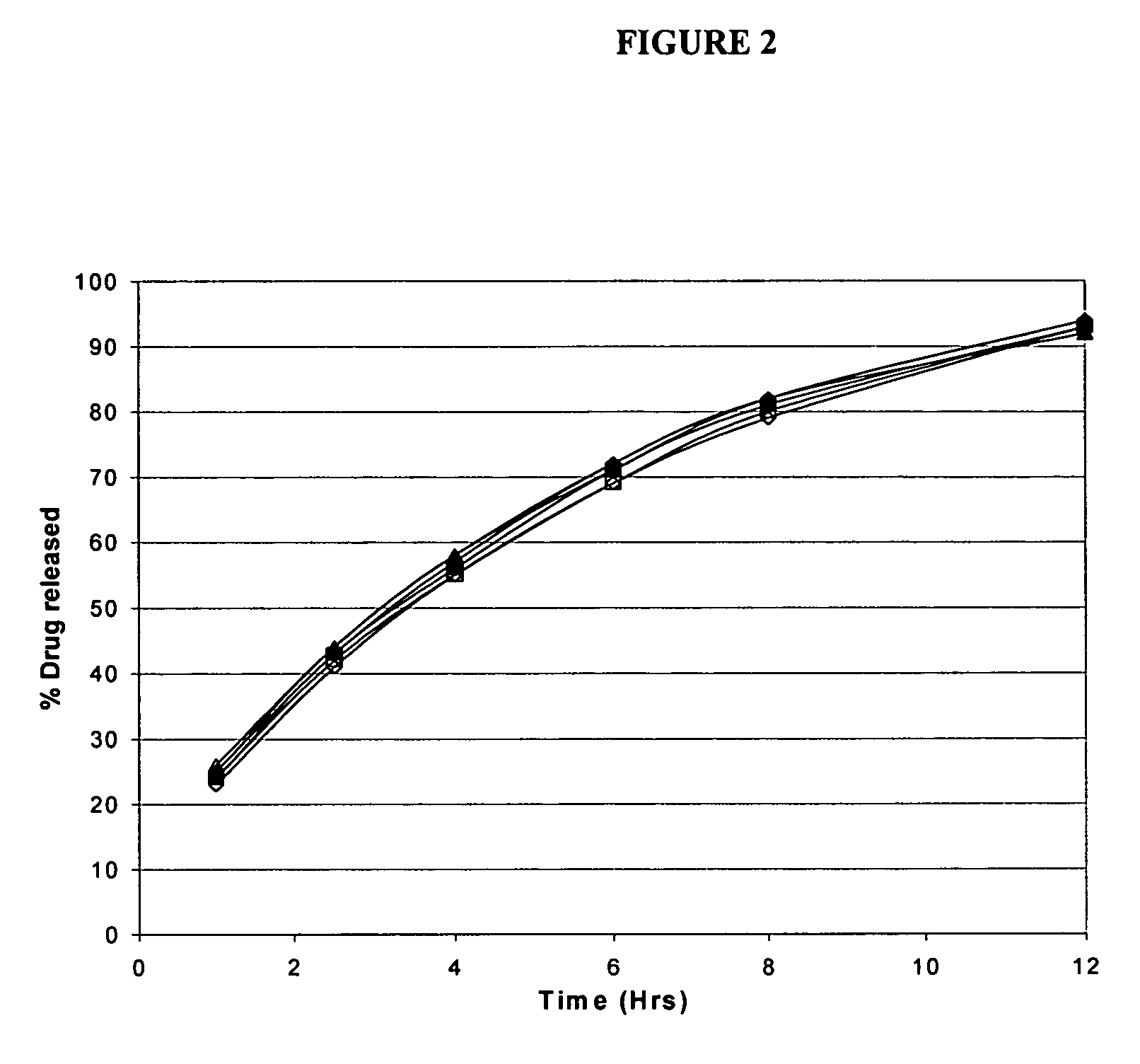
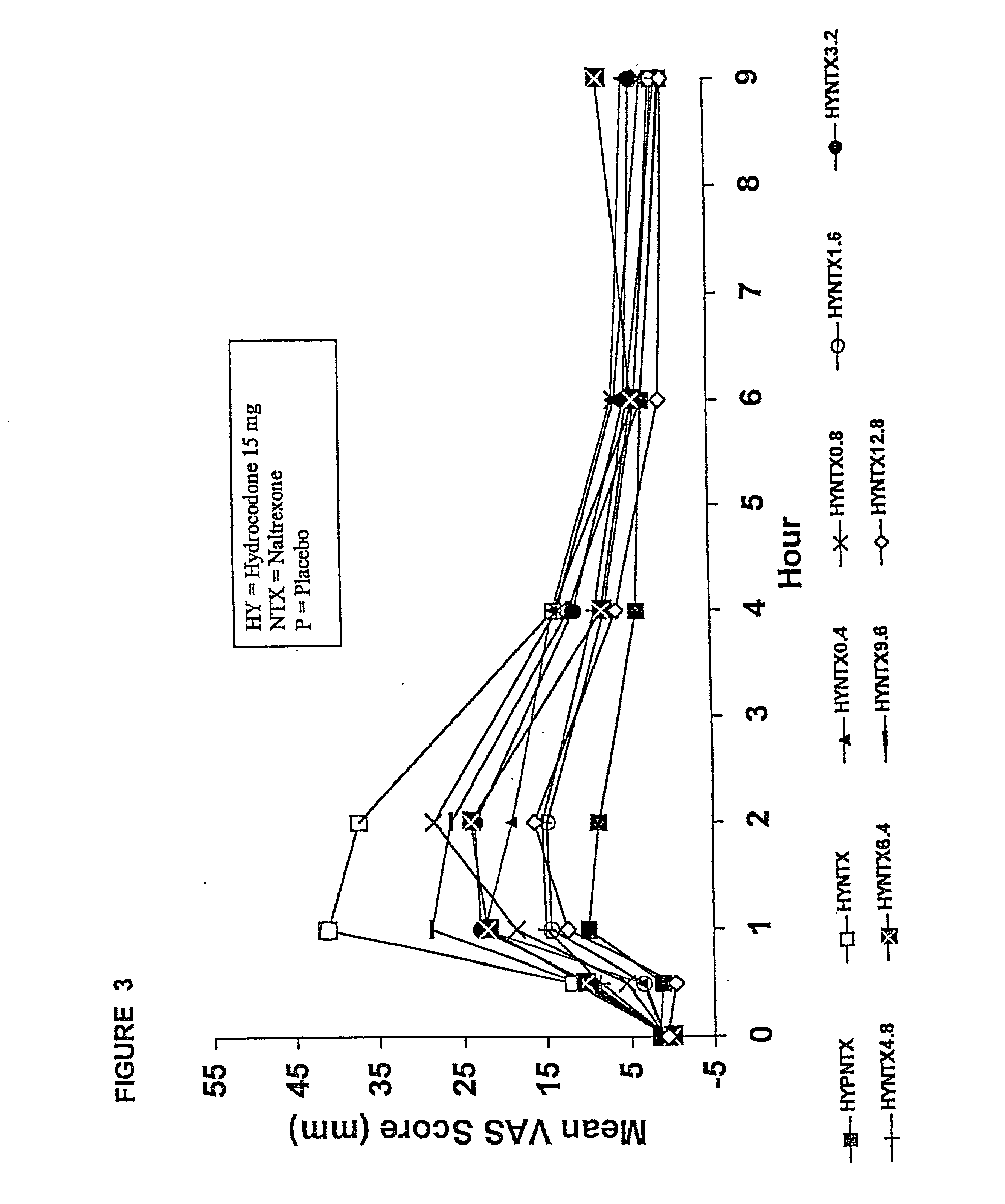
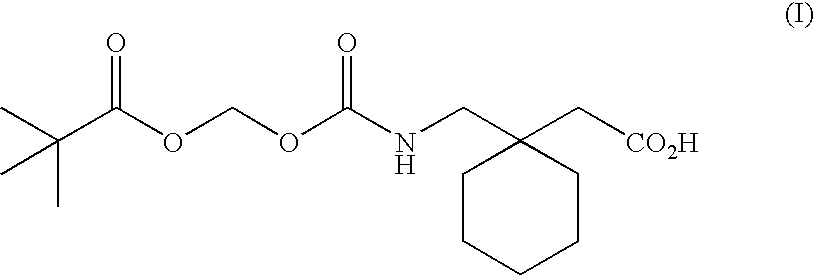
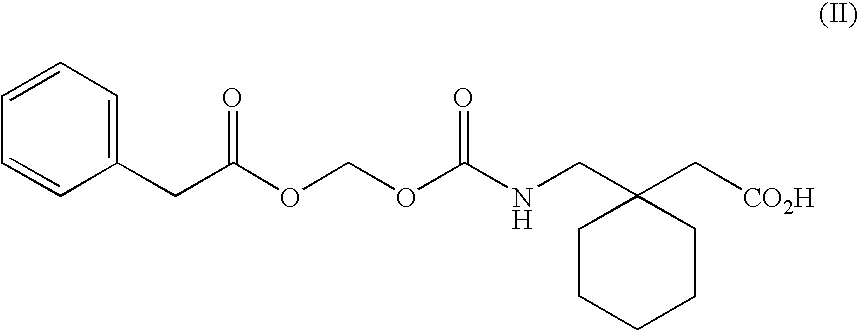
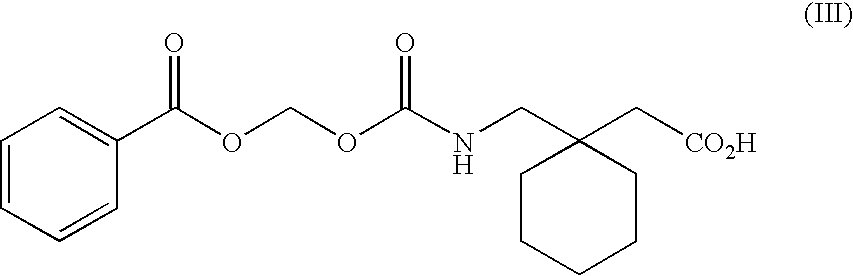
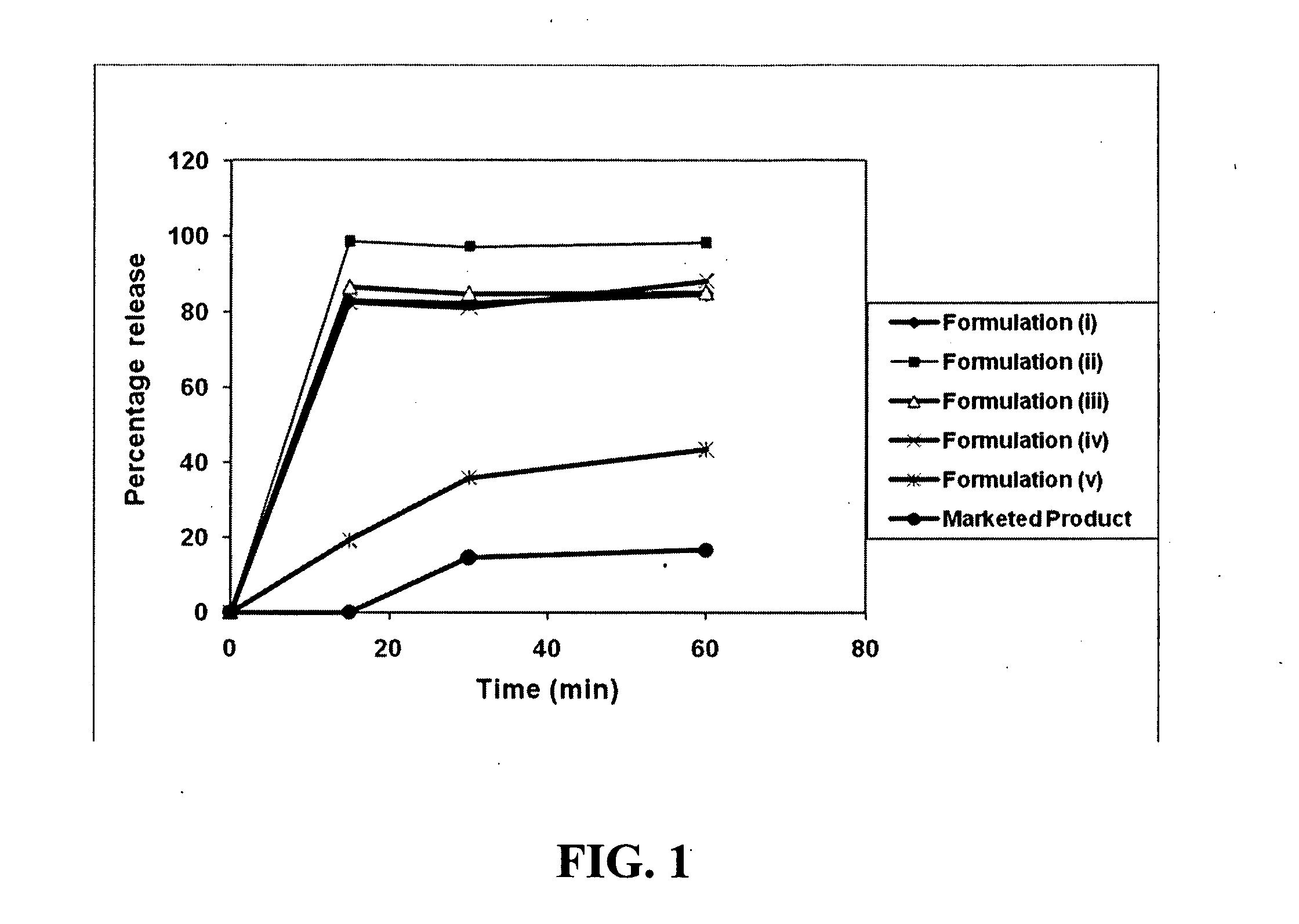
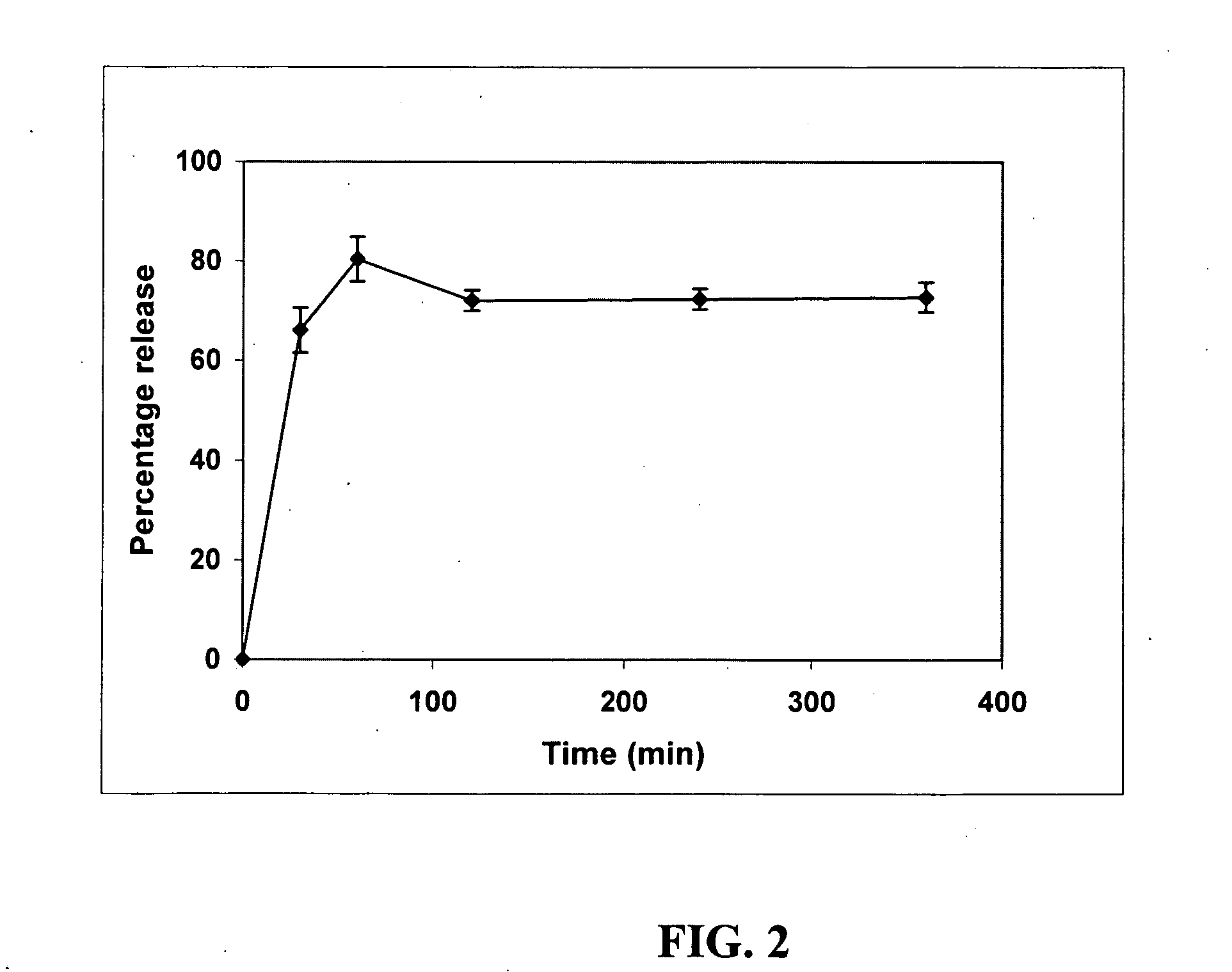
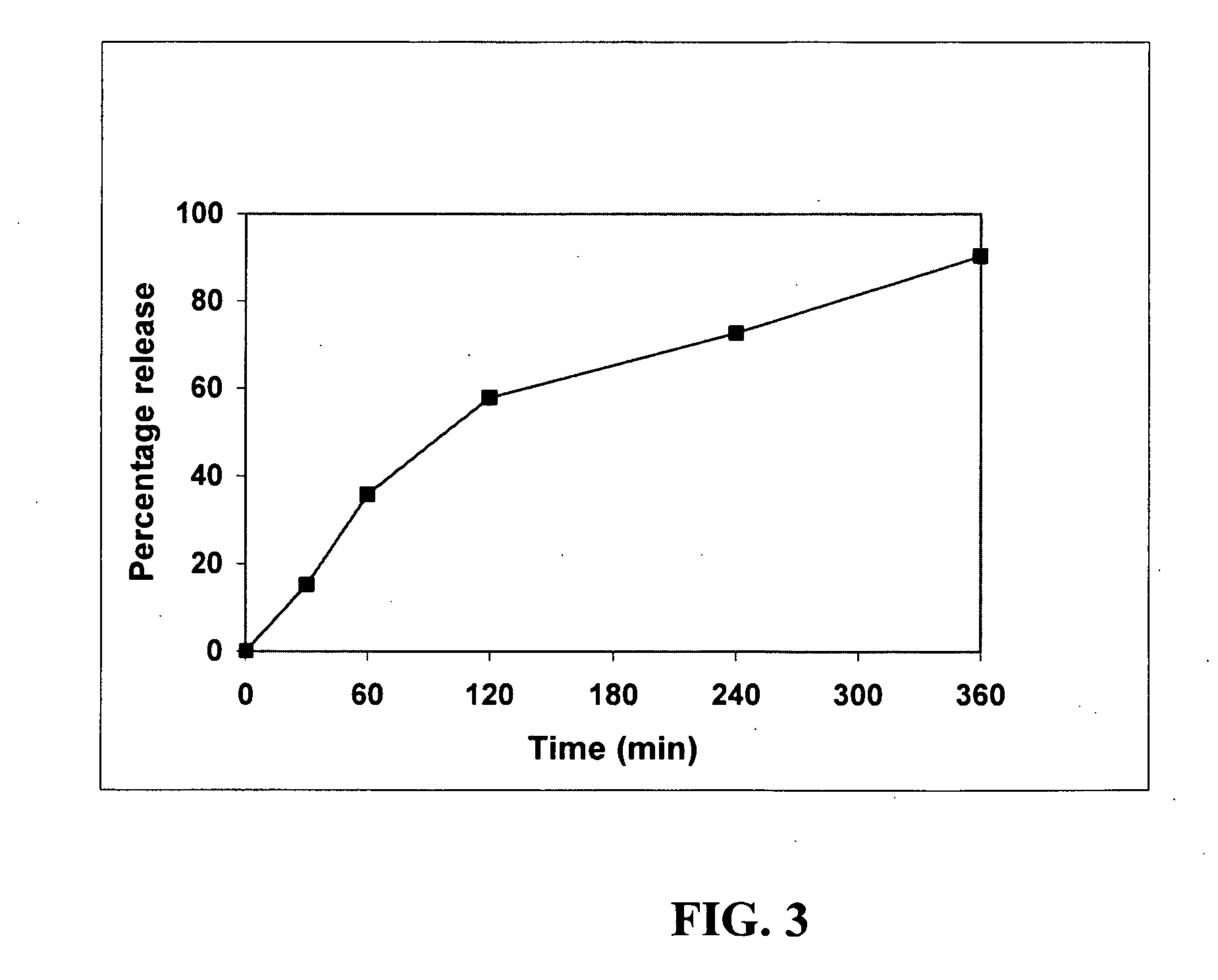
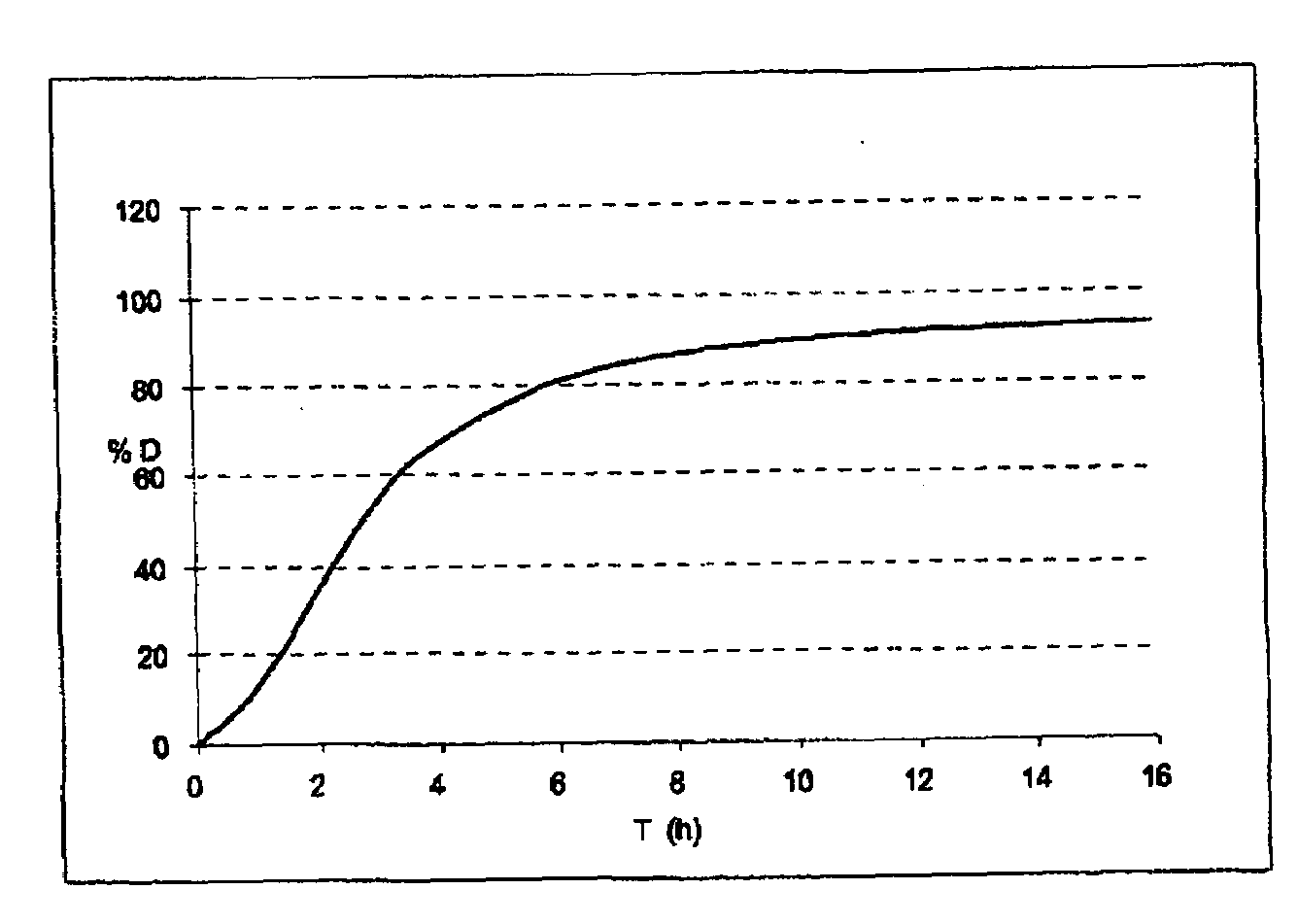
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
592 results about "Oral agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Descriptions. Oral cholecystographic agents are radiopaque agents. Radiopaque agents are drugs used to help diagnose certain medical problems. These agents contain iodine, which blocks x-rays. Depending on how the radiopaque agent is given, it localizes or builds up in certain areas of the body.
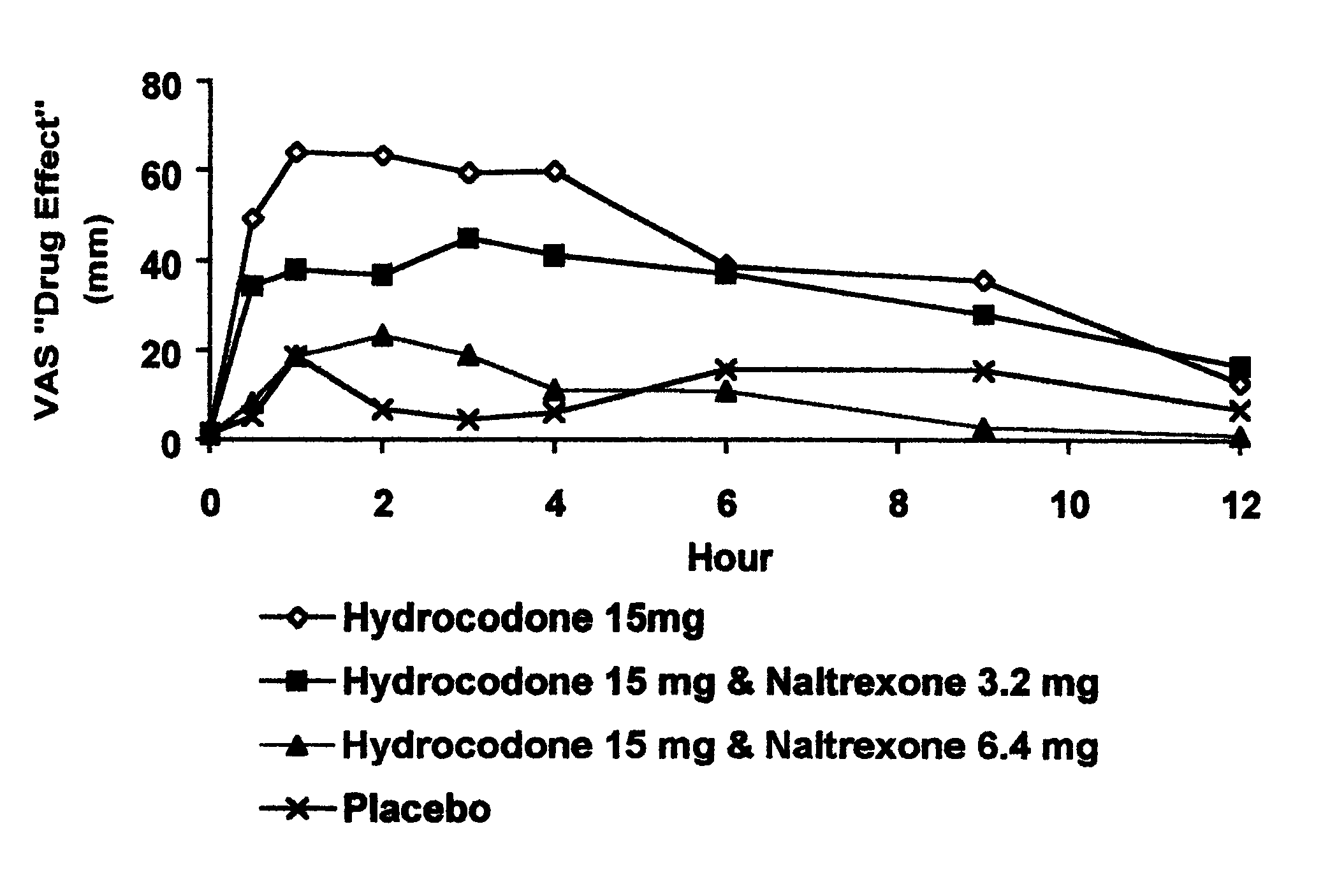
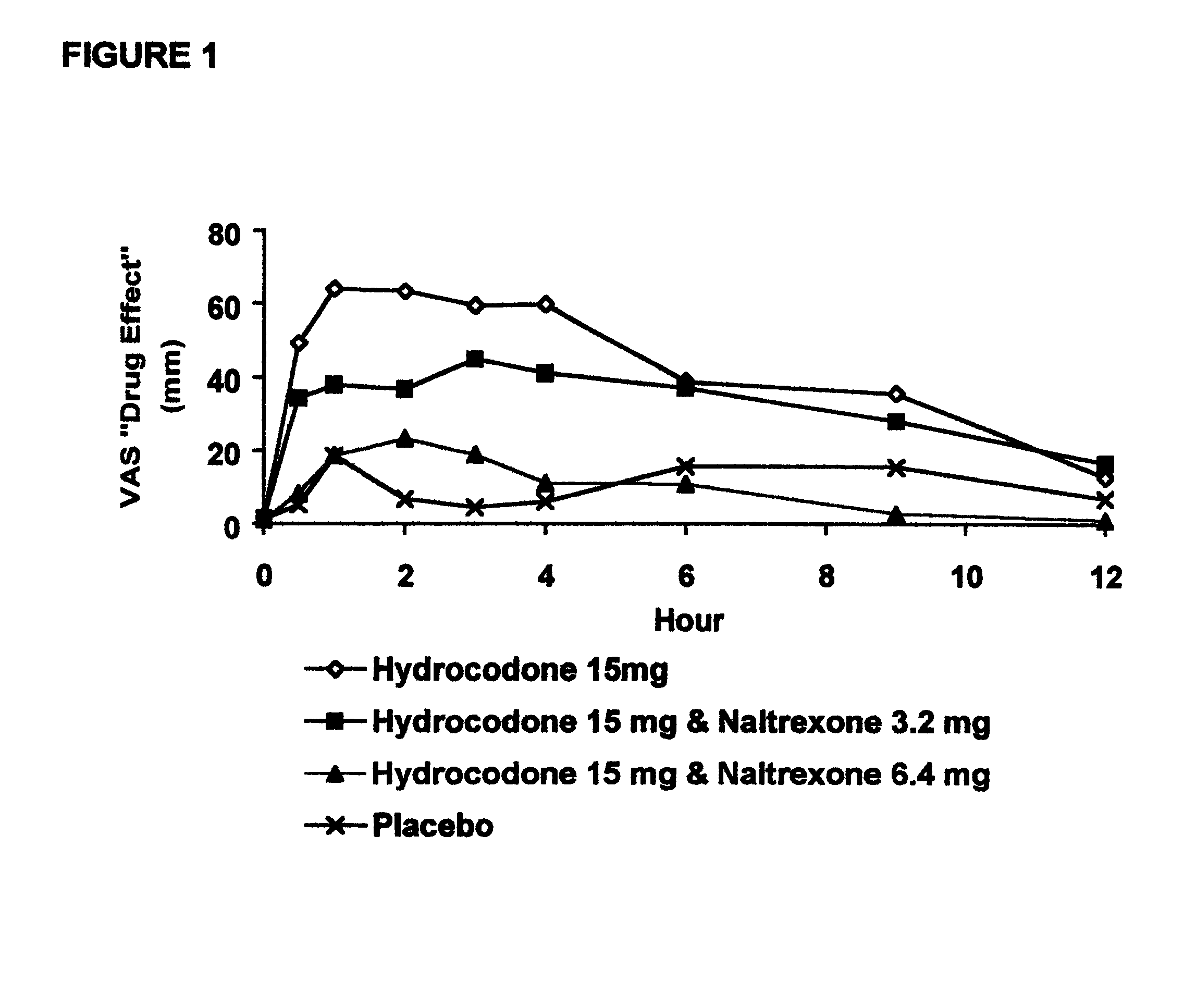
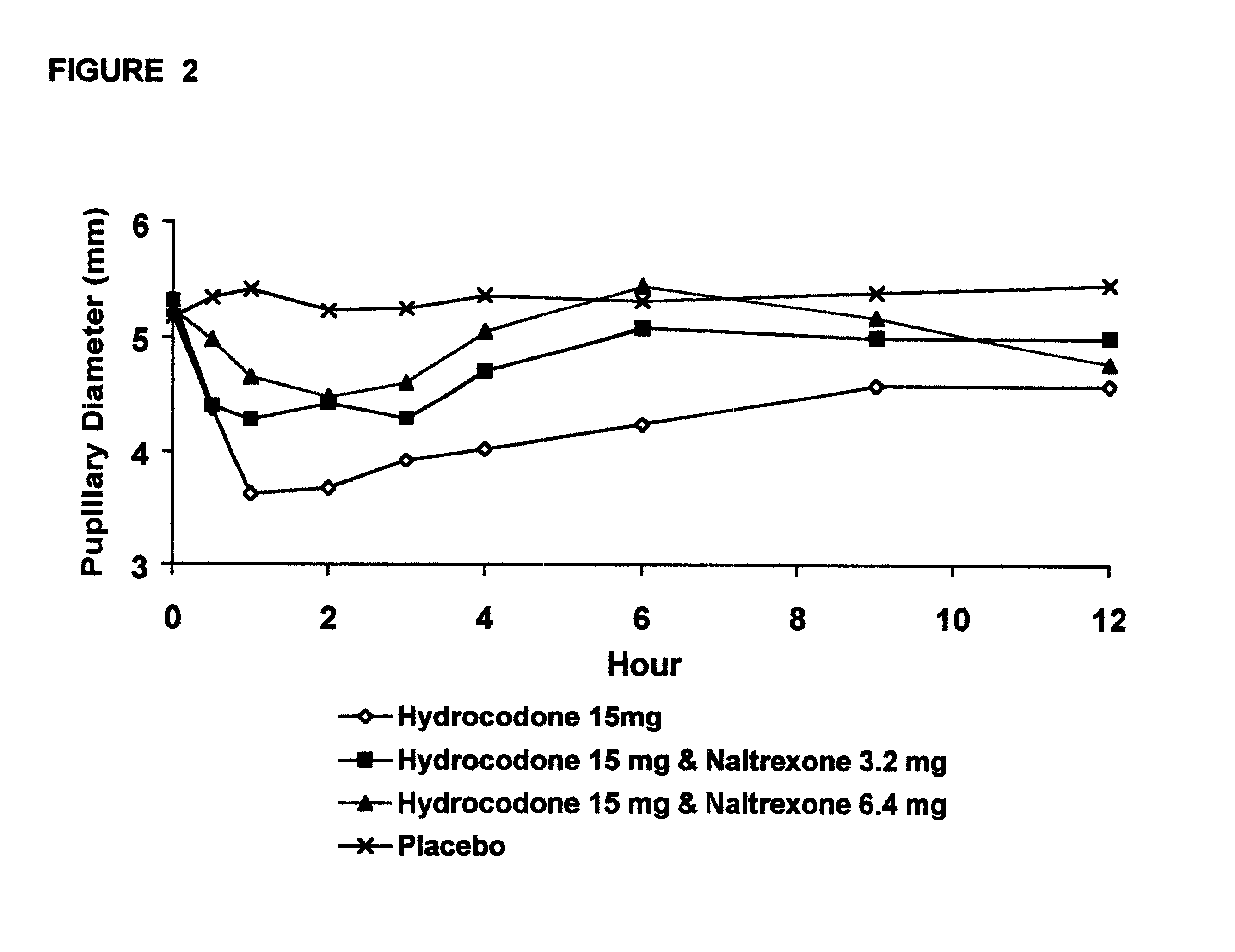
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
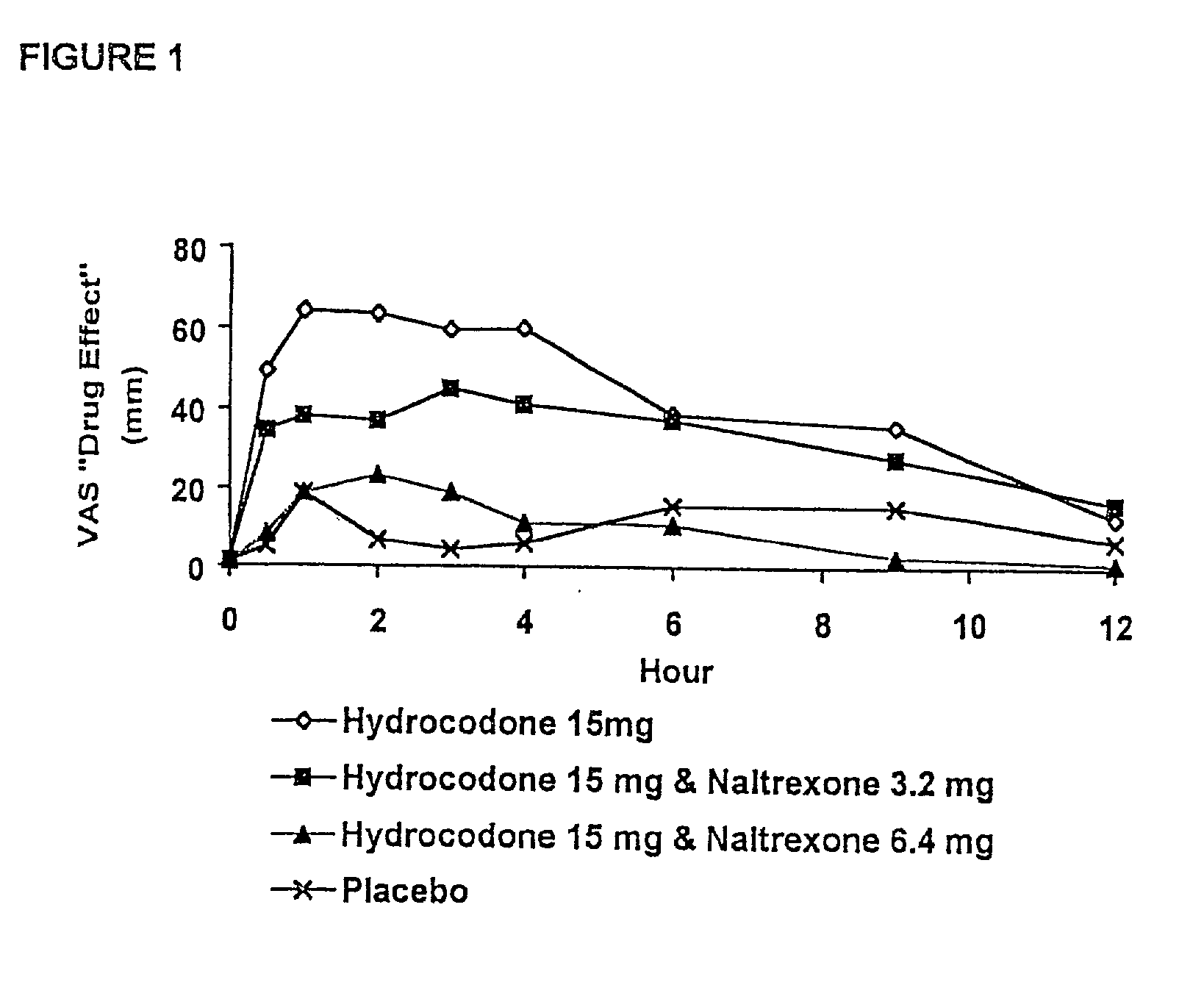
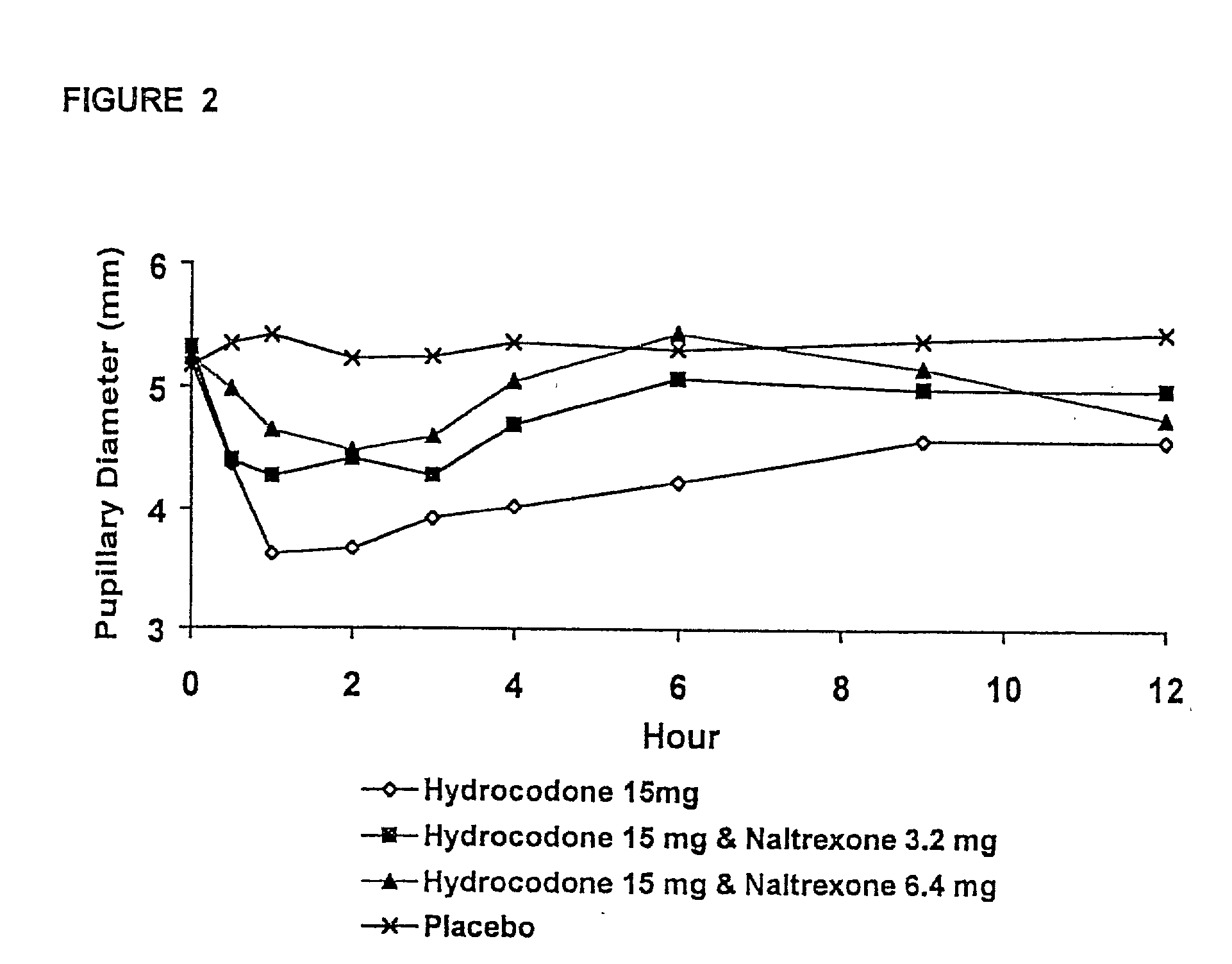
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
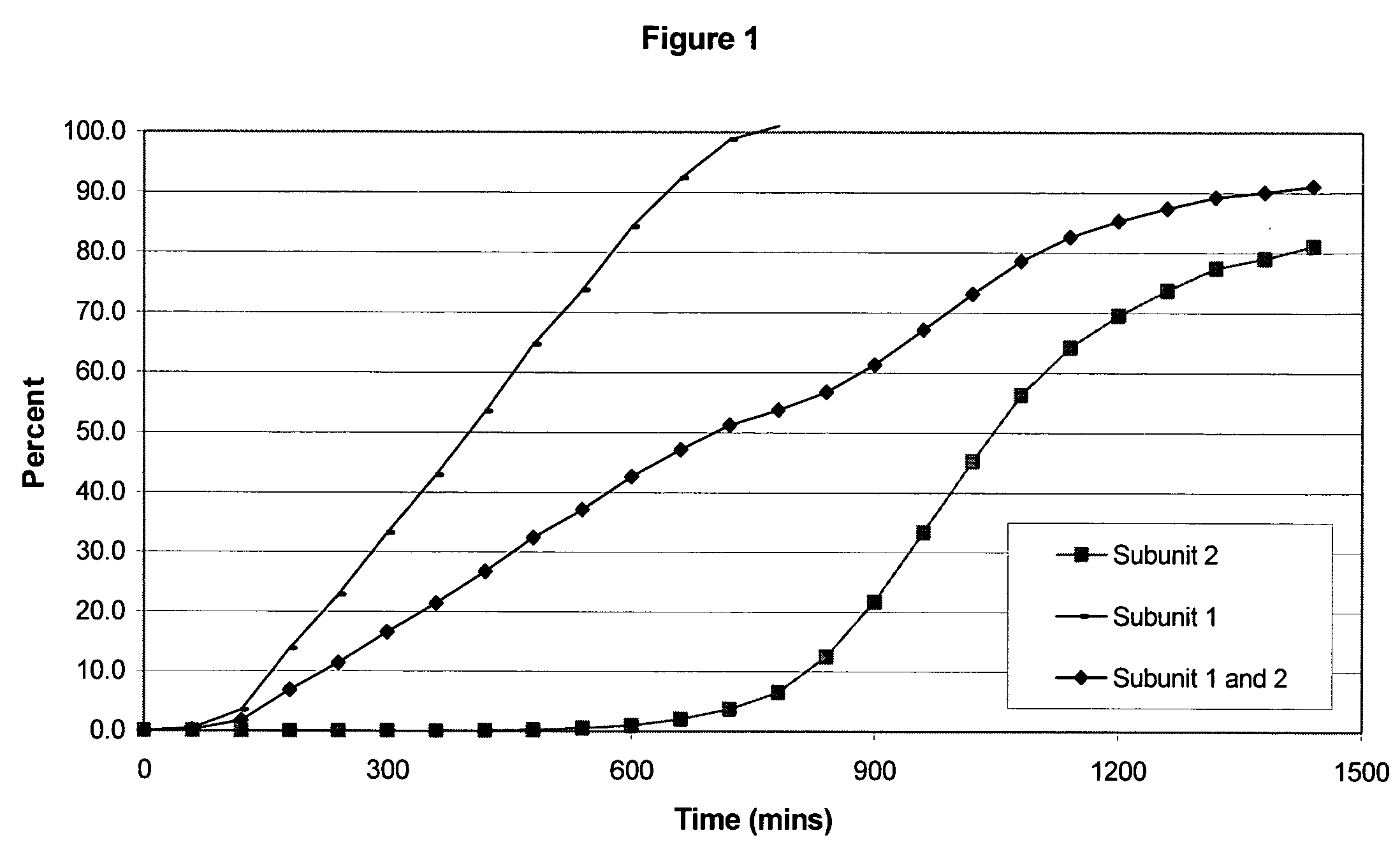
Sustained release opioid formulations and method of use
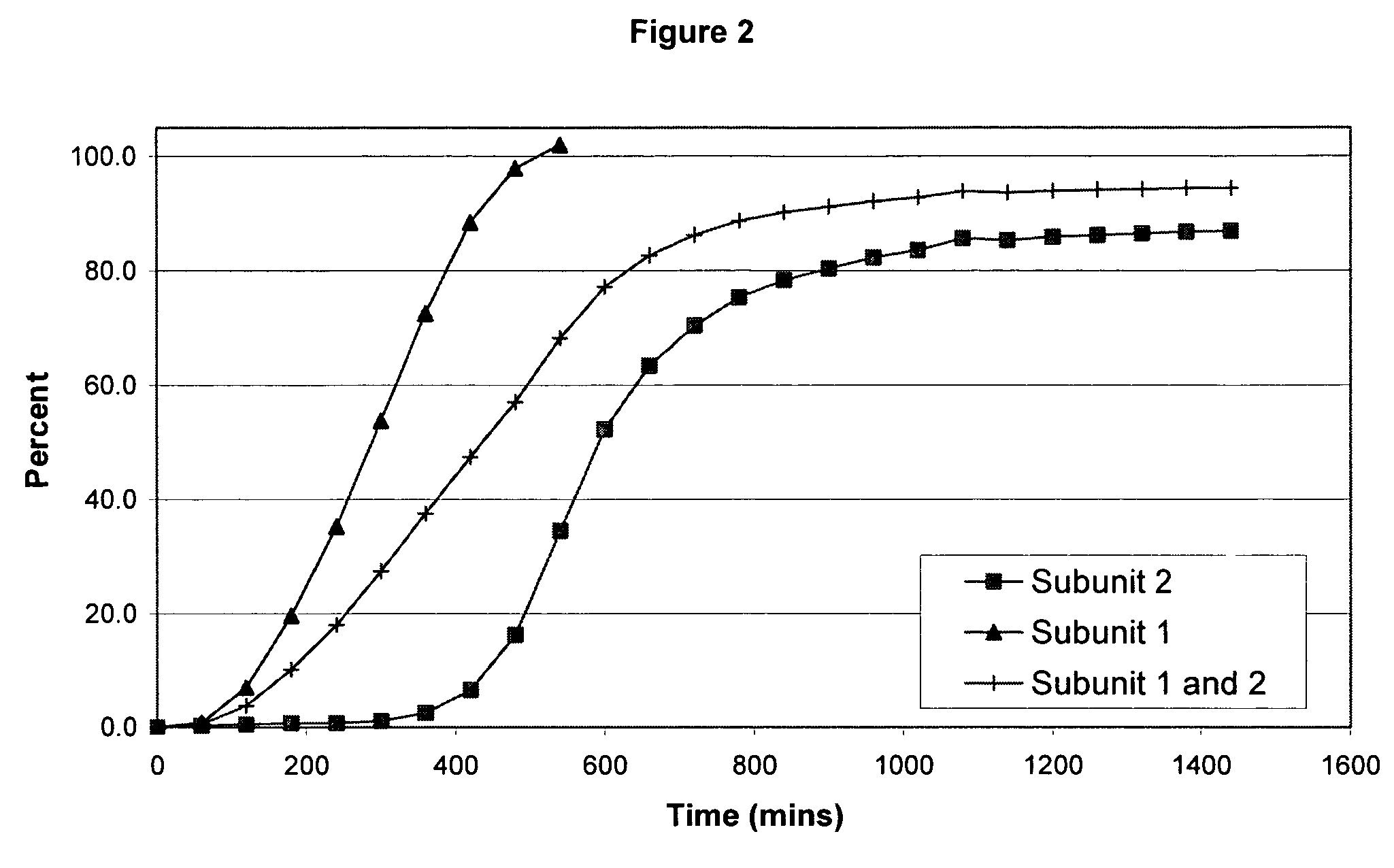
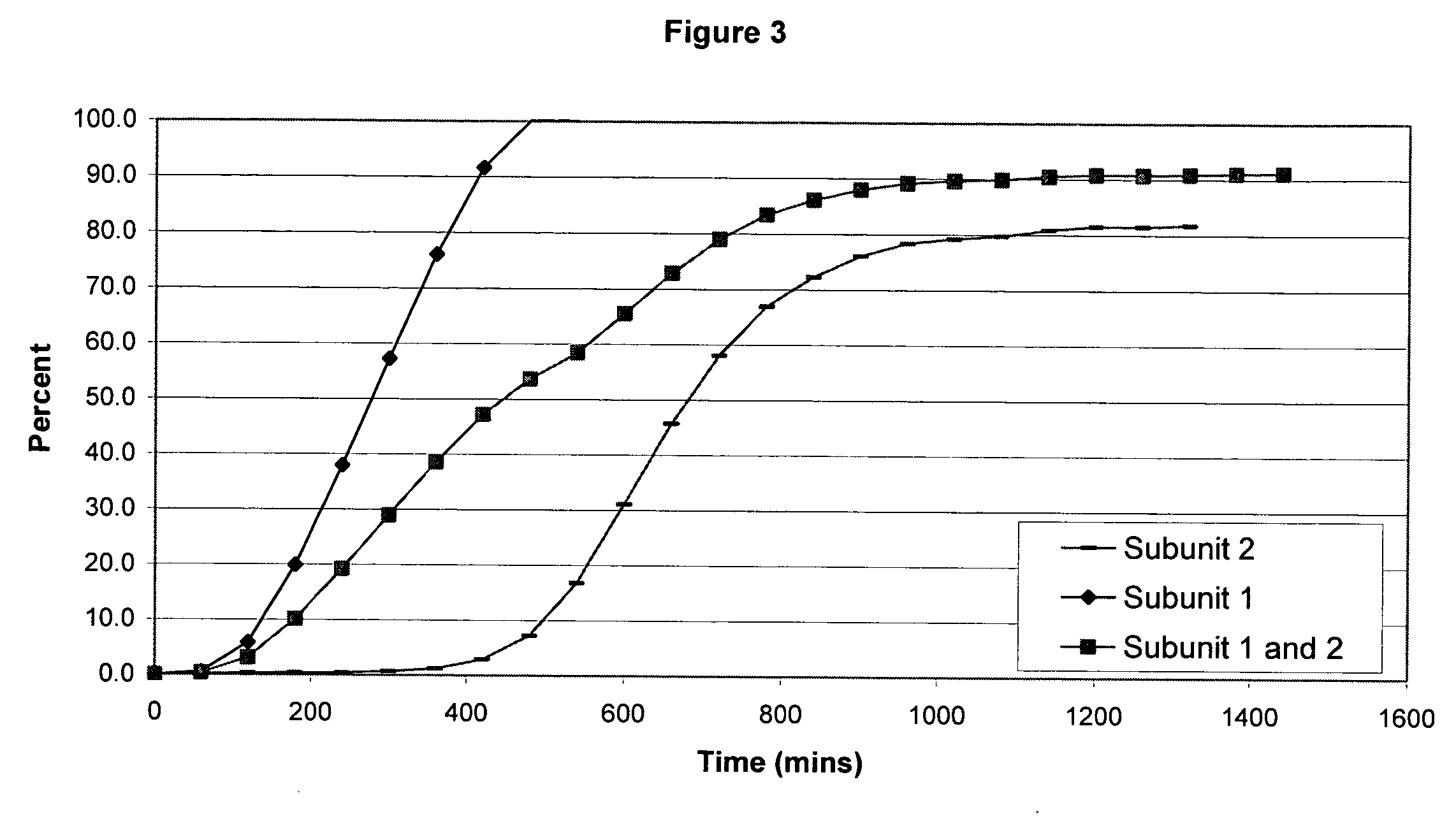
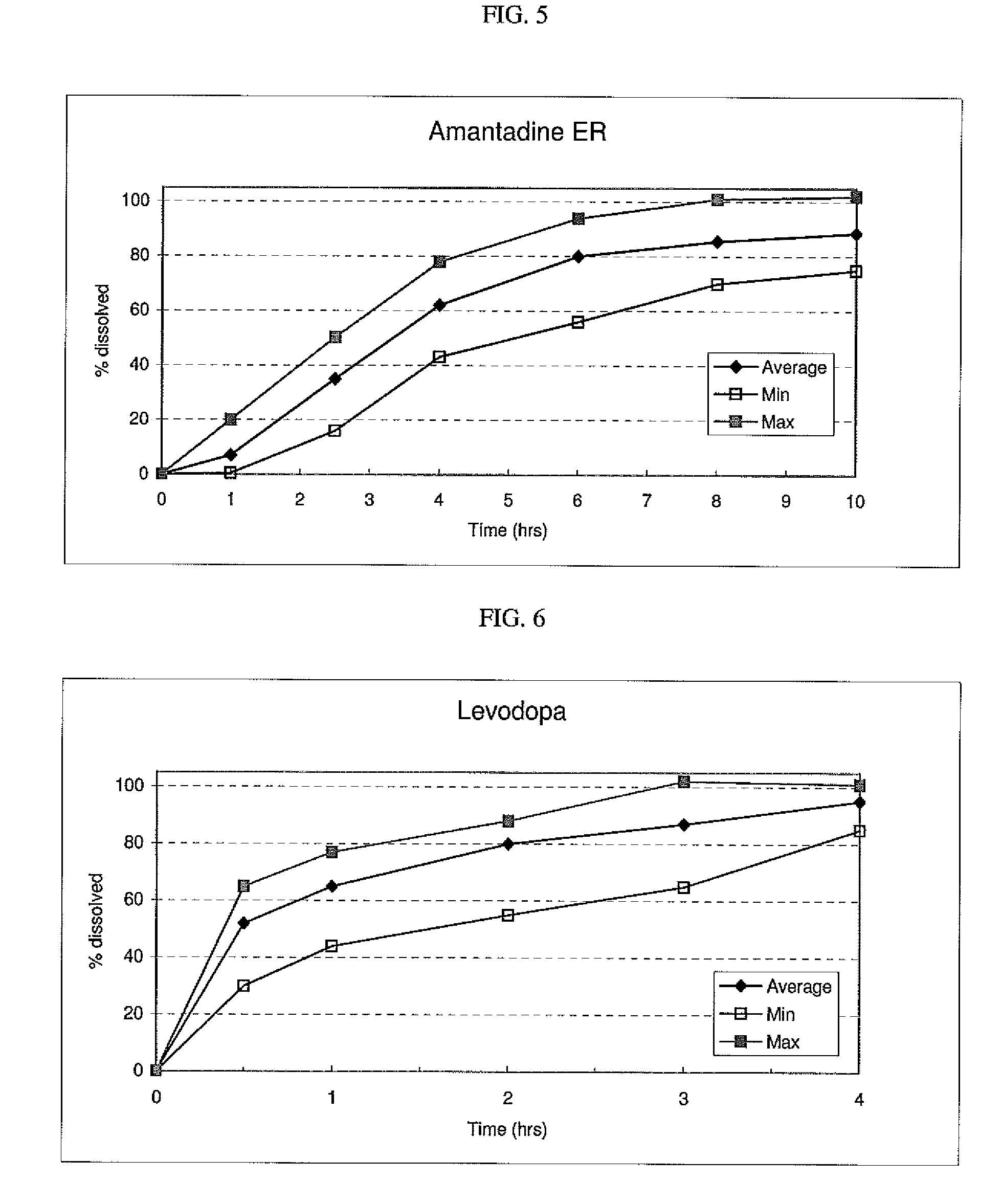
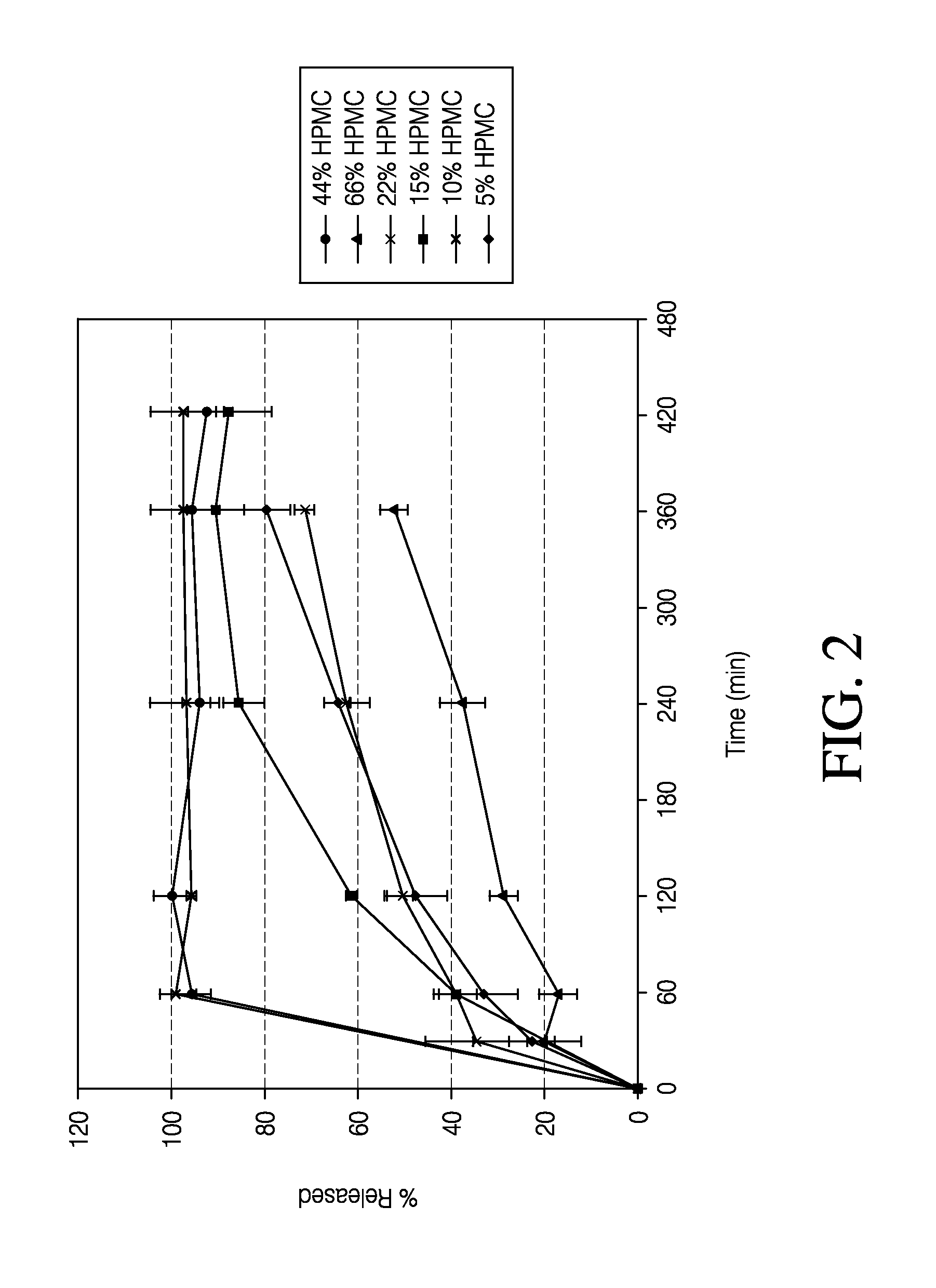
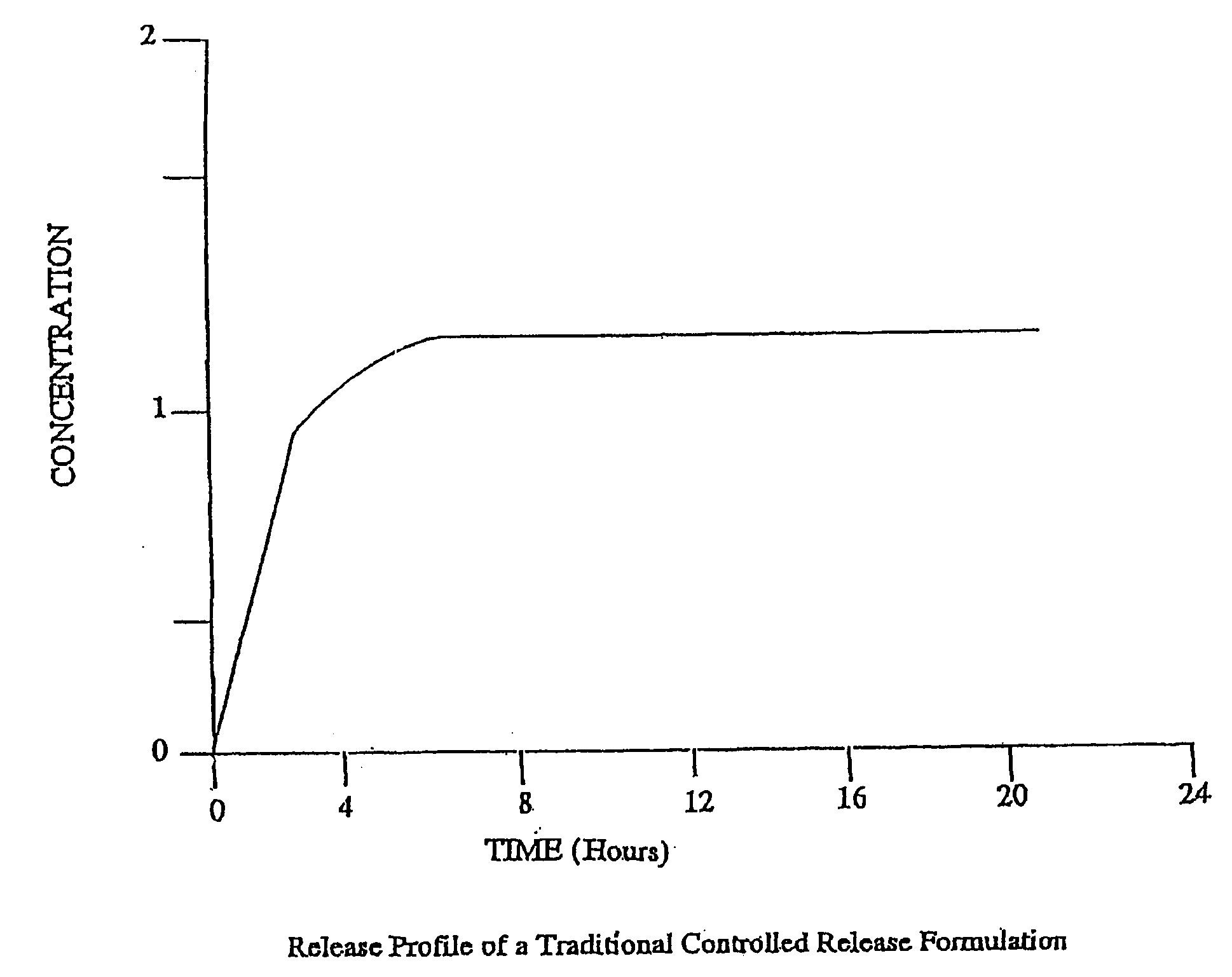
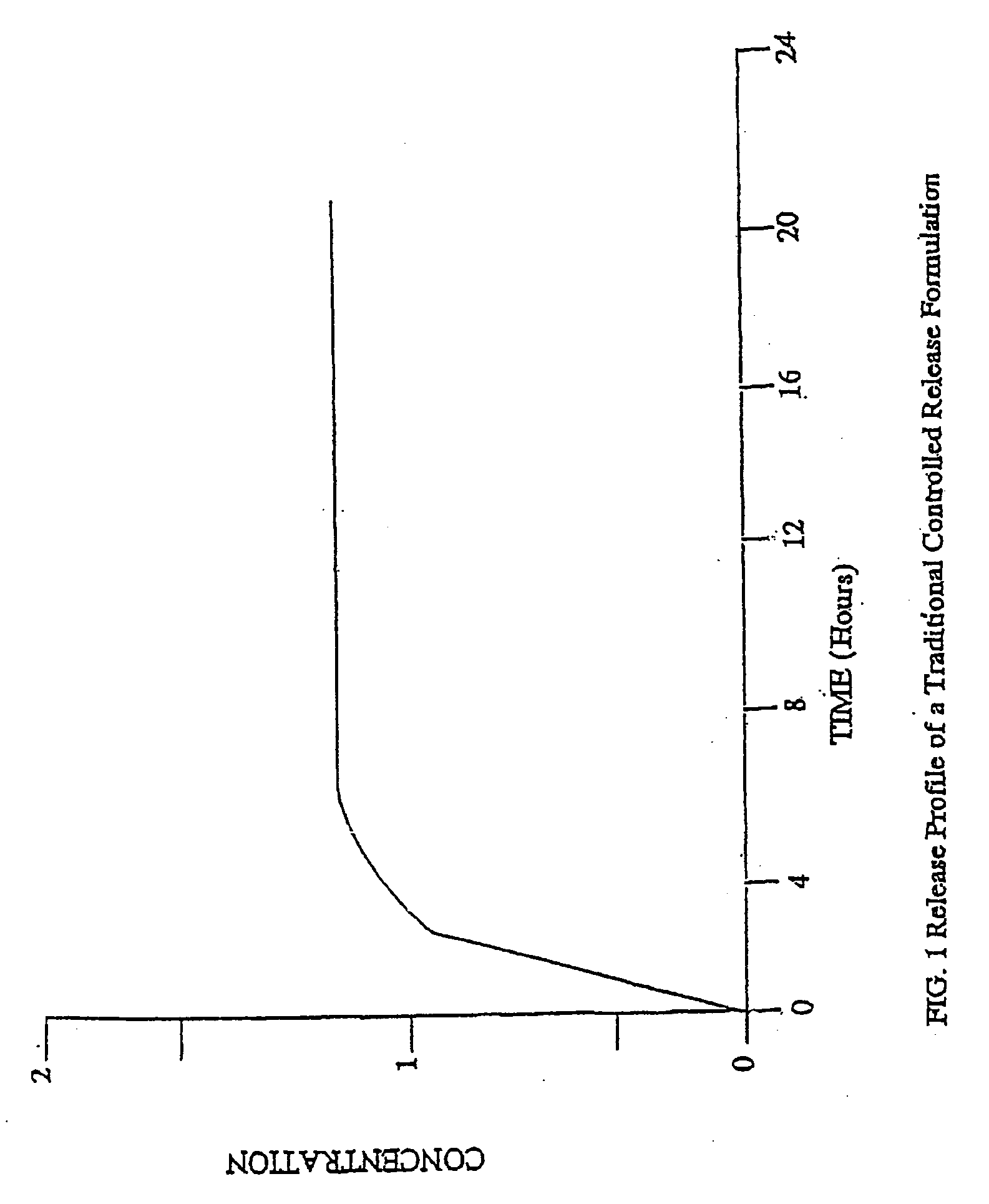
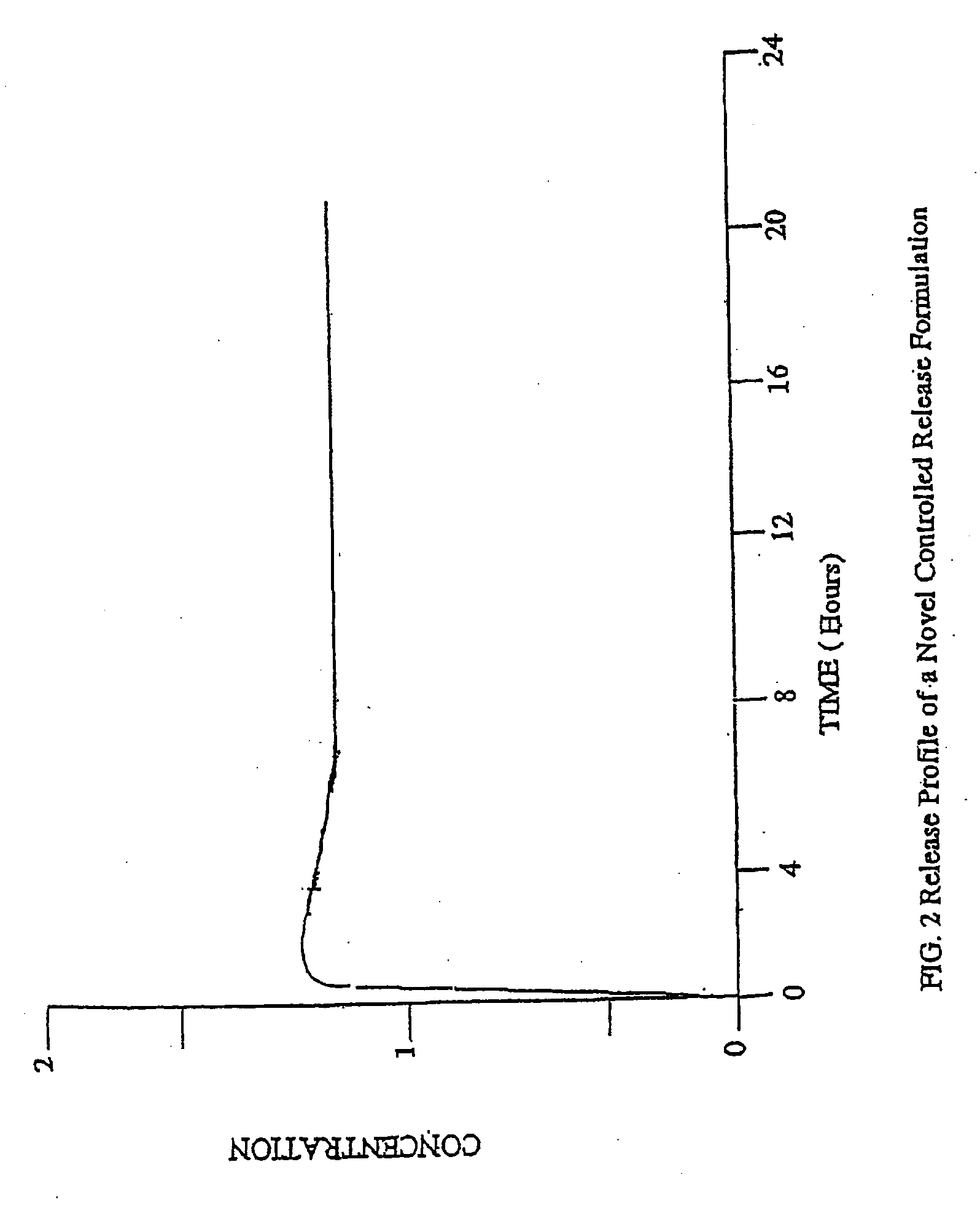
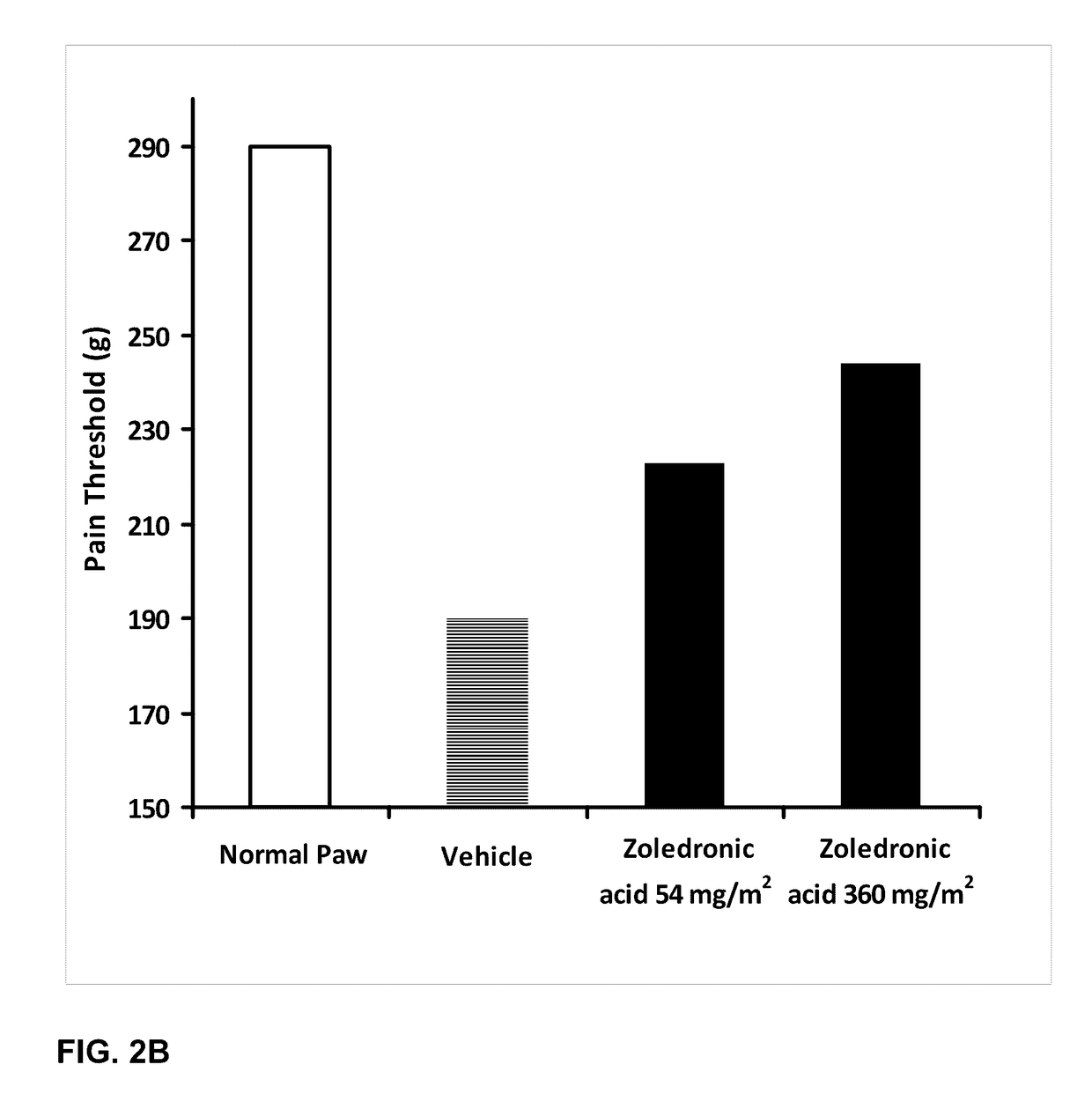
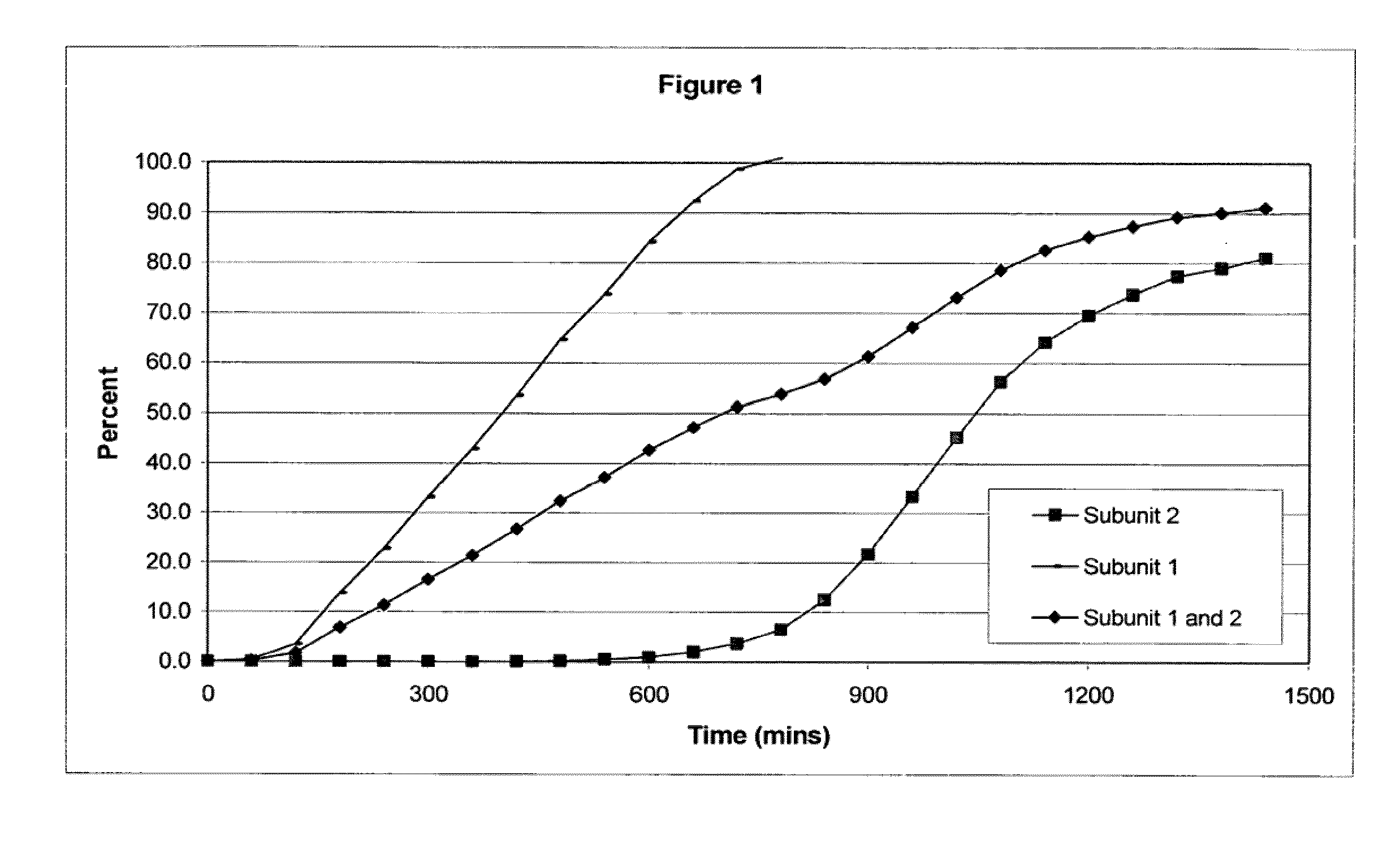
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Triple Combination Release Multi-Layered Tablet
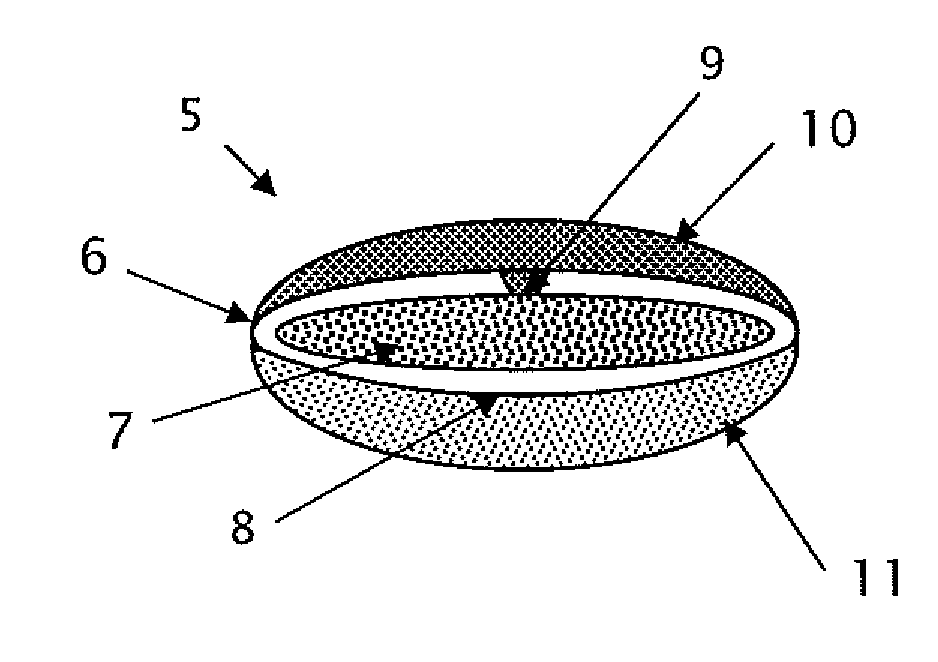
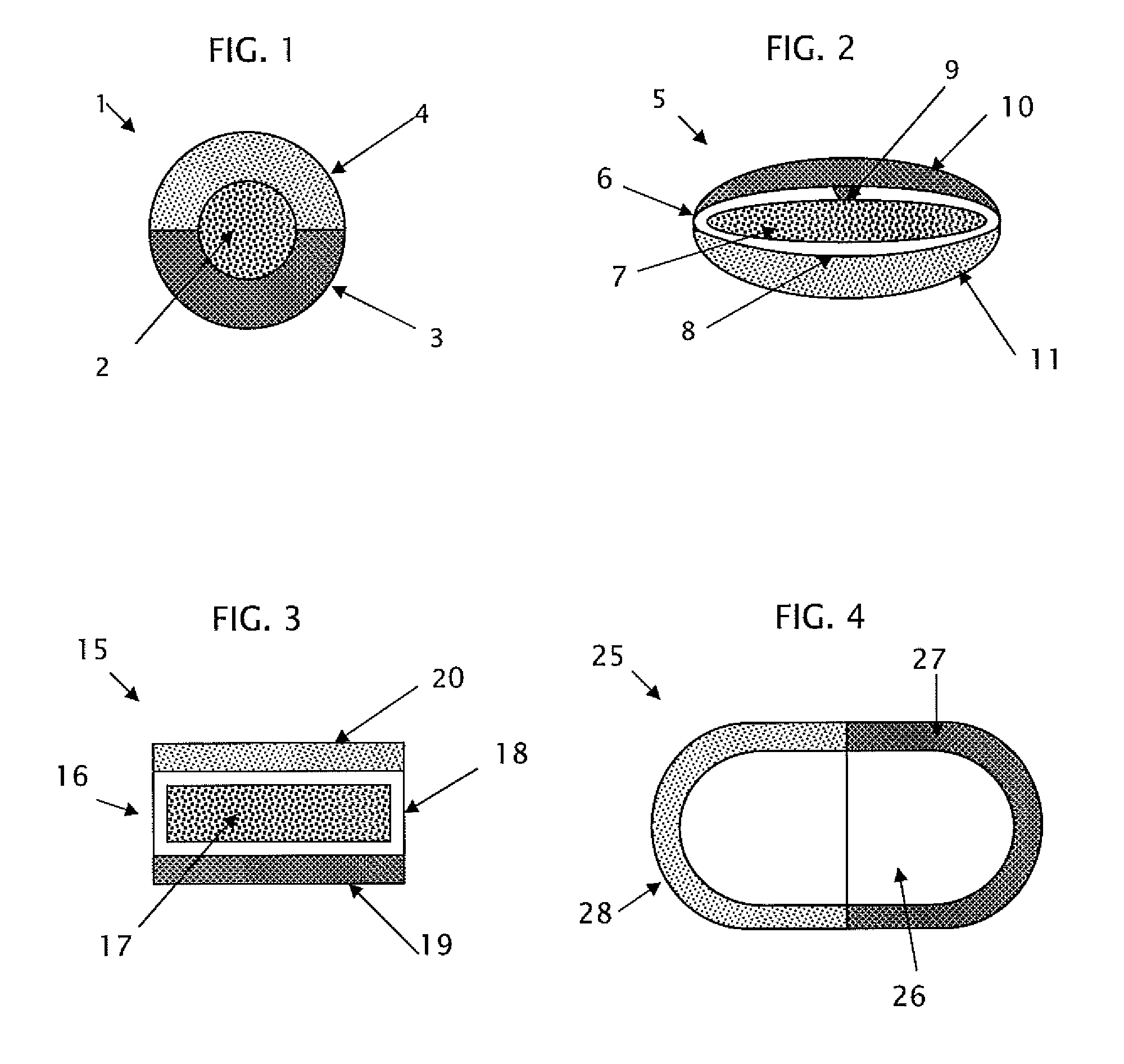
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
Oral dosage form comprising a therapeutic agent and an adverse-effect agent
The present invention provides an oral dosage form comprising a first composition and a second composition. The first composition comprises an effective amount of a therapeutic agent and the second composition comprises an effective amount of an adverse-effect agent. The adverse-effect agent is covered with a coating that is substantially insoluble in the gastrointestinal tract. In one embodiment, the adverse-effect agent is coated with an outer base-soluble layer and an inner acid-soluble layer. The therapeutic agent can be uncoated or can be coated with a coating having an outer acid-soluble layer and an inner base-soluble layer. The dosage form discourages administration of the therapeutic agent by other than oral administration.
Owner:PURDUE PHARMA LP
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Modified release formulations of memantine oral dosage forms
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
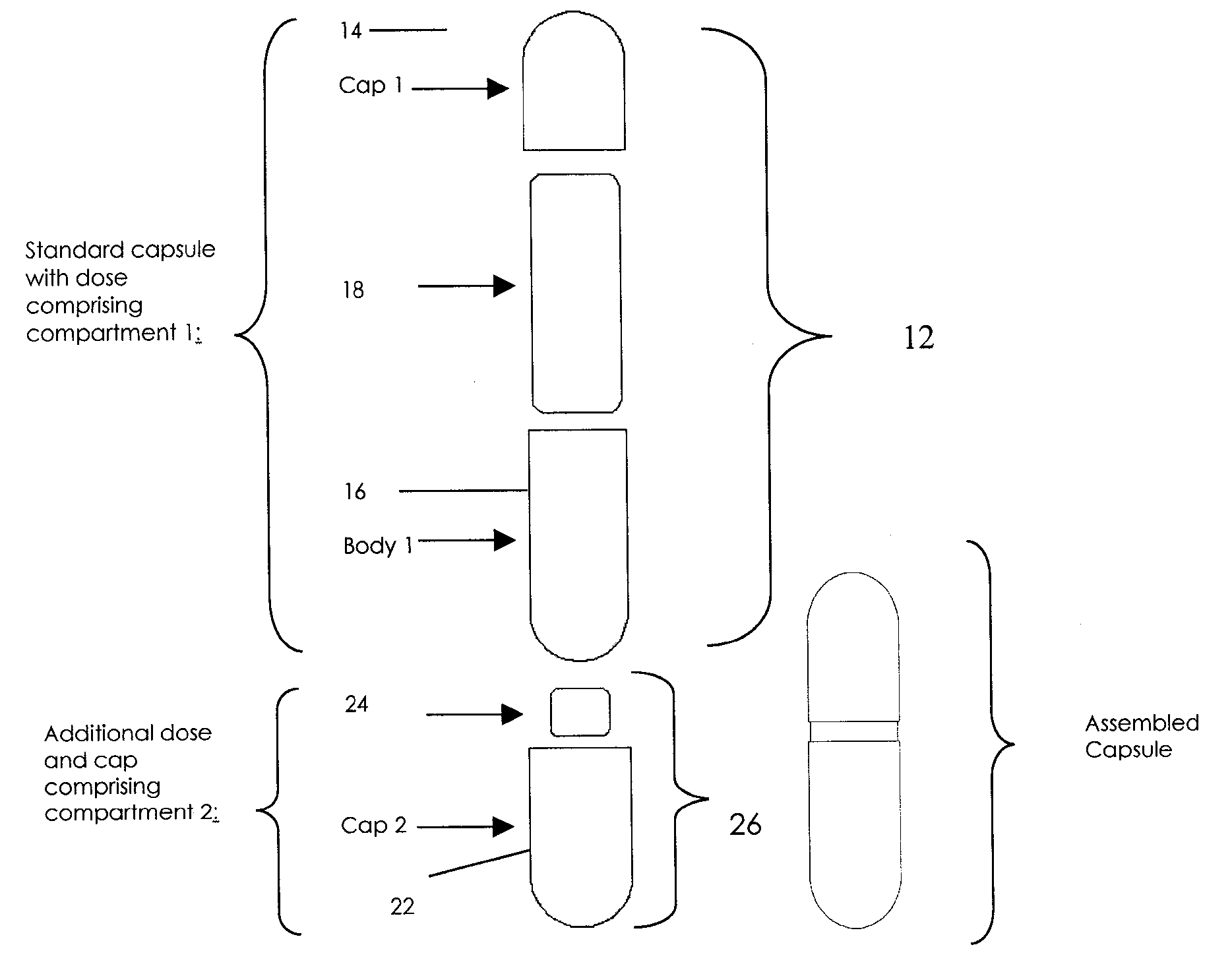
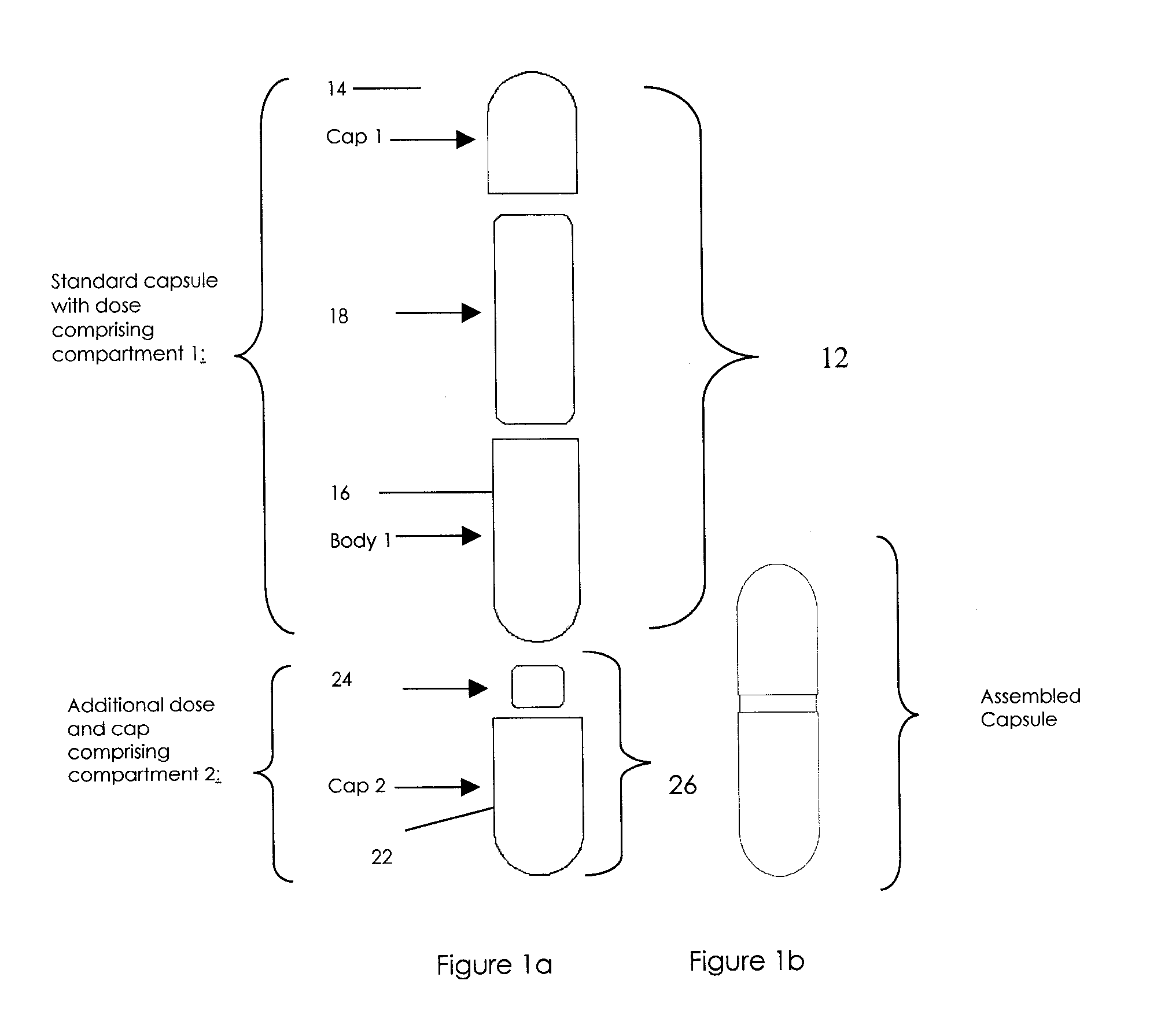
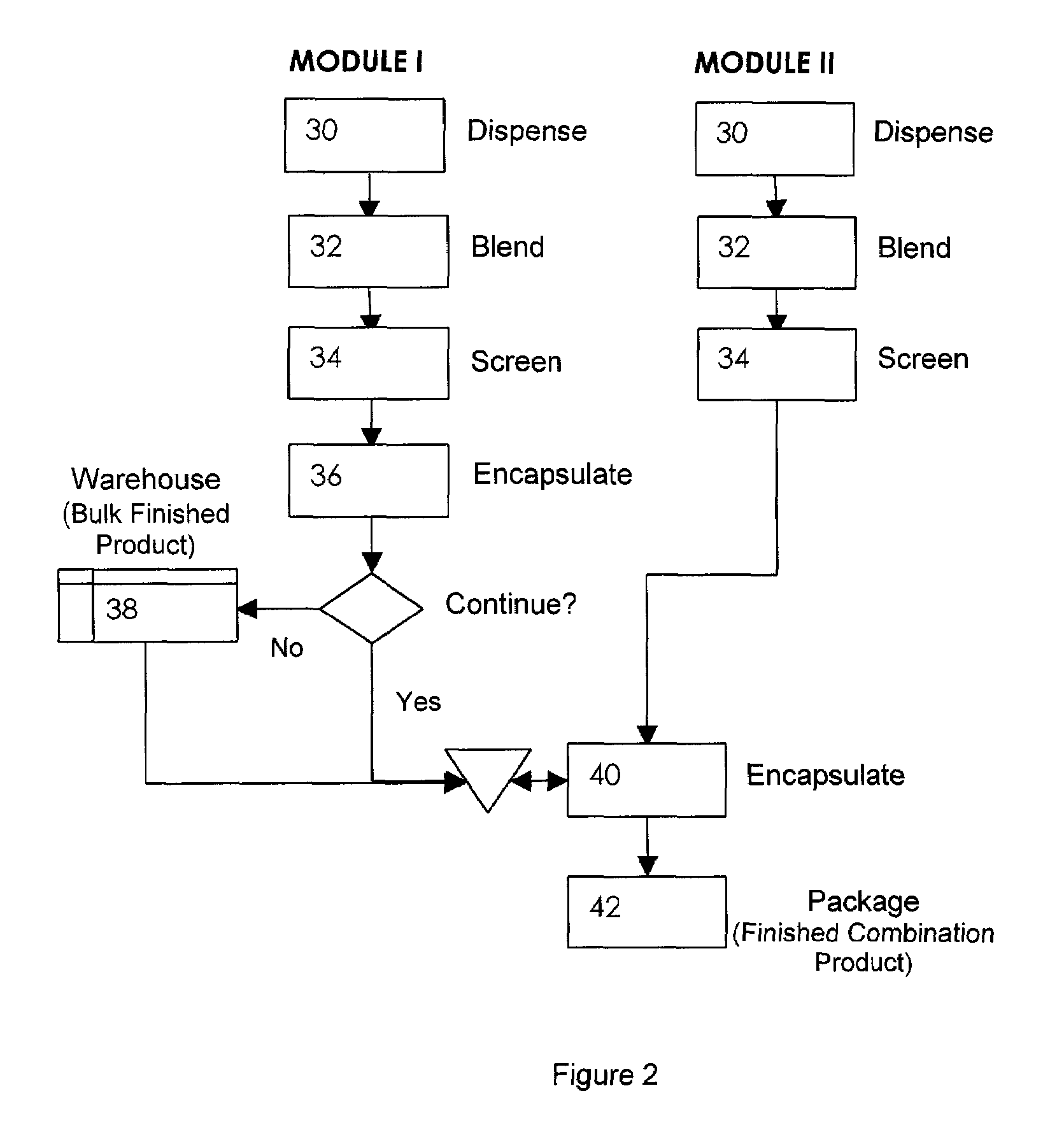
Oral dosage combination pharmaceutical packaging
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
Method for improving the bioavailability of orally delivered therapeutics
The disclosed invention is a method and composition for improving the bioavailability of a pharmaceutically active ingredient comprising an oral dosage form consisting essentially of a granulation of active ingredient, amino acid, and hydrophilic polymer, wherein the granulation is dispersed in an immediate release or extended release excipient.
Owner:SCOLR PHARMA
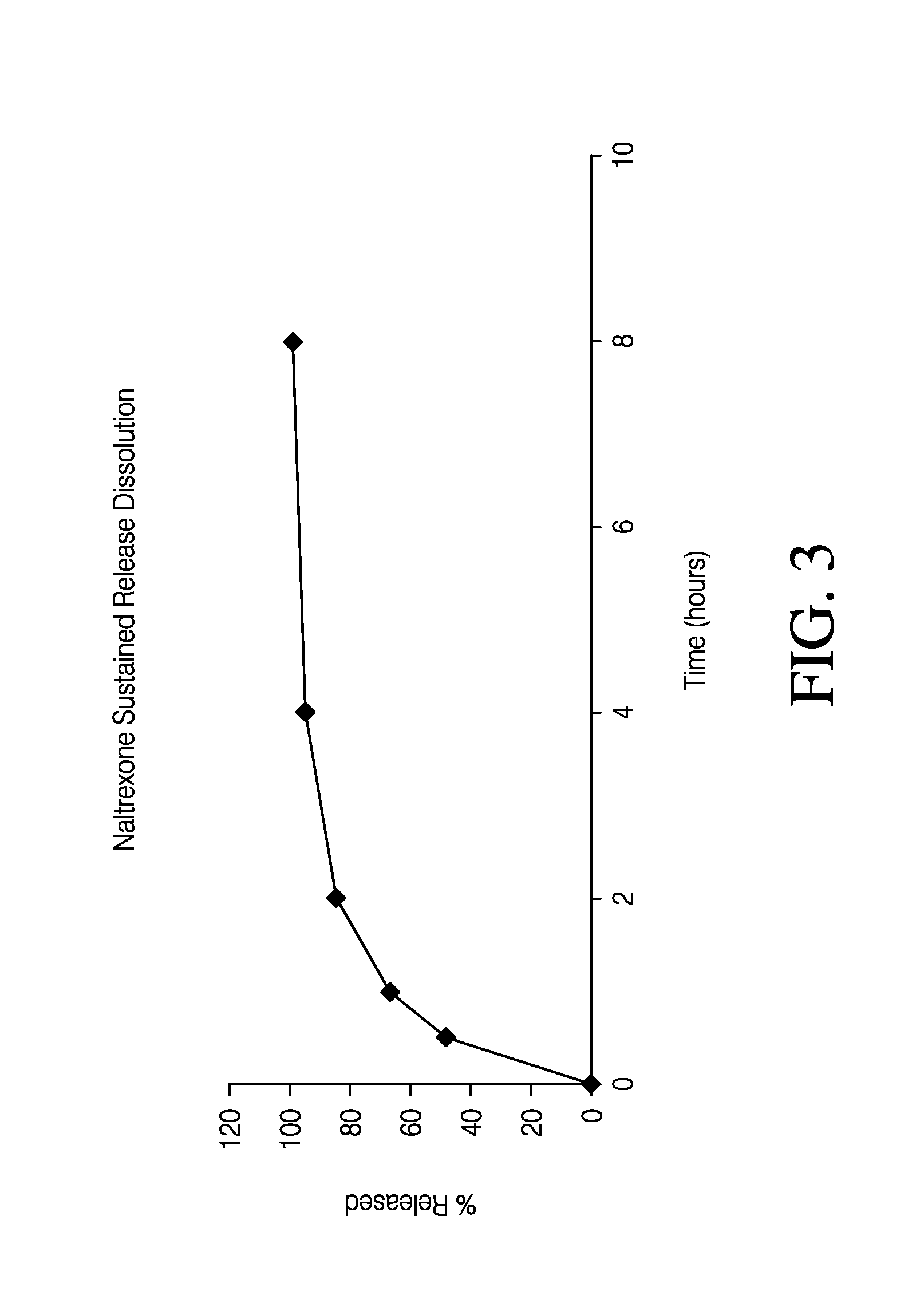
Sustained release formulation of naltrexone
ActiveUS20070281021A1Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectCompound (substance)
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Engineering absorption of therapeutic compounds via colonic transporters
Methods of modifying therapeutic compounds such as drugs to be substrates for active transporters expressed in epithelial cells lining the lumen of the human colon are disclosed. The transporters expressed in the human colon include the sodium dependent multi-vitamin transporter (SMVT), and monocarboxylate transporters 1 and 4 (MCT 1 and MCT 4). The modified compounds can themselves be pharmacologically active, or upon cleavage of a chemical moiety after uptake from the colon, can be metabolized to form a compound that is pharmacologically active (e.g., a prodrug). The modified compounds disclosed herein are suitable for use in extended release oral dosage forms, particularly those that release drug over periods of greater than about 2-4 hours following administration.
Owner:XENOPORT
Oral pharmaceutical dosage forms
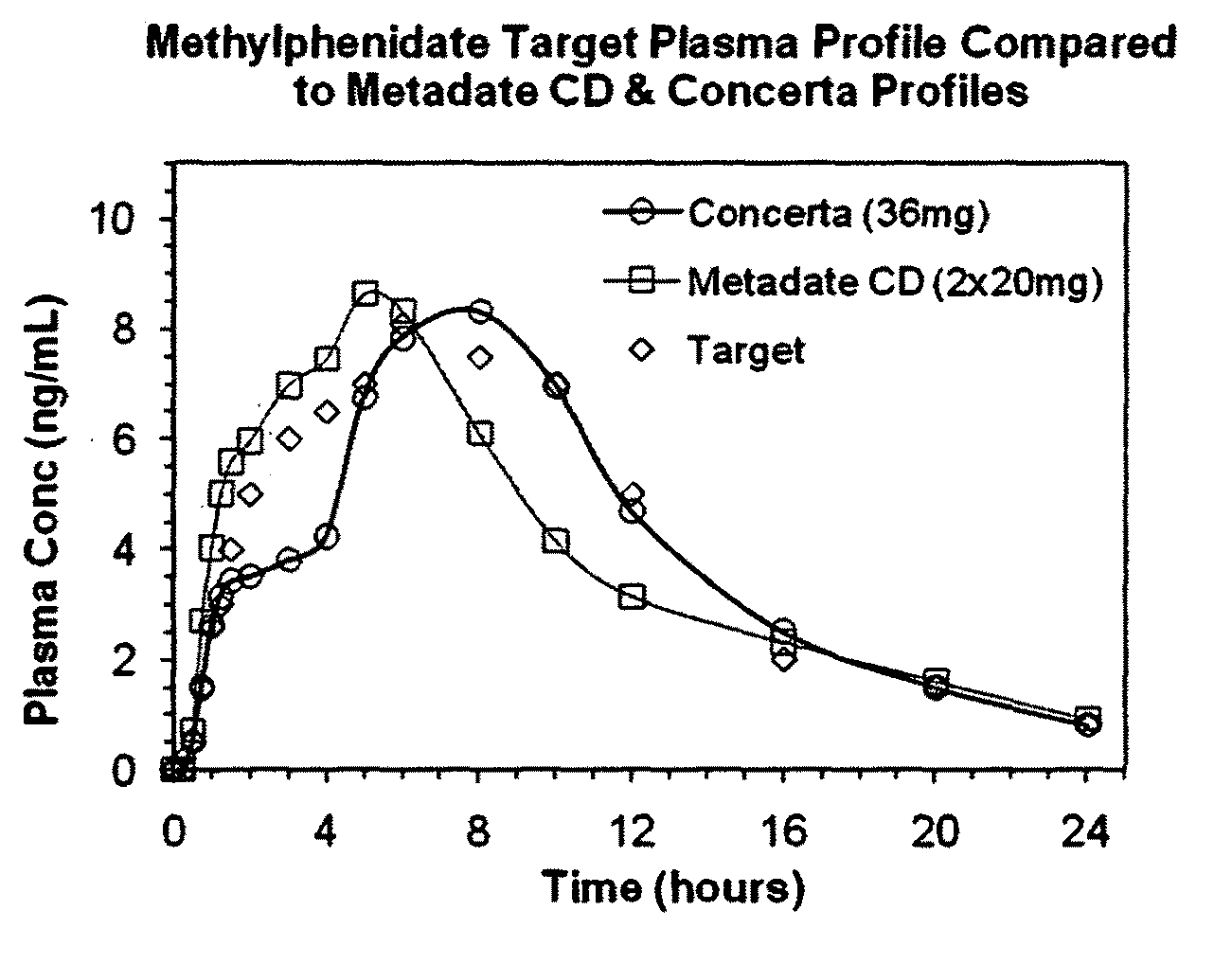
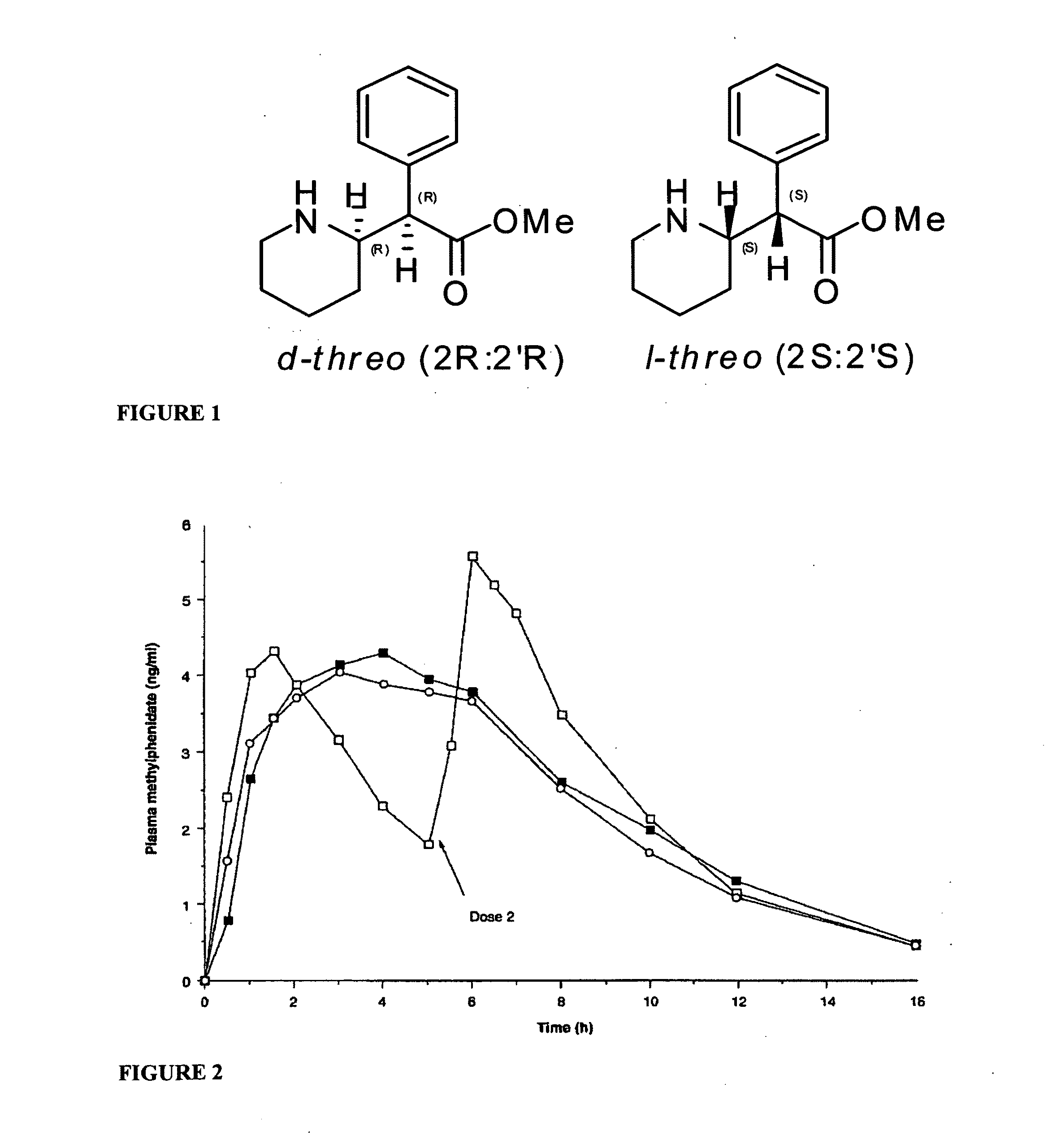
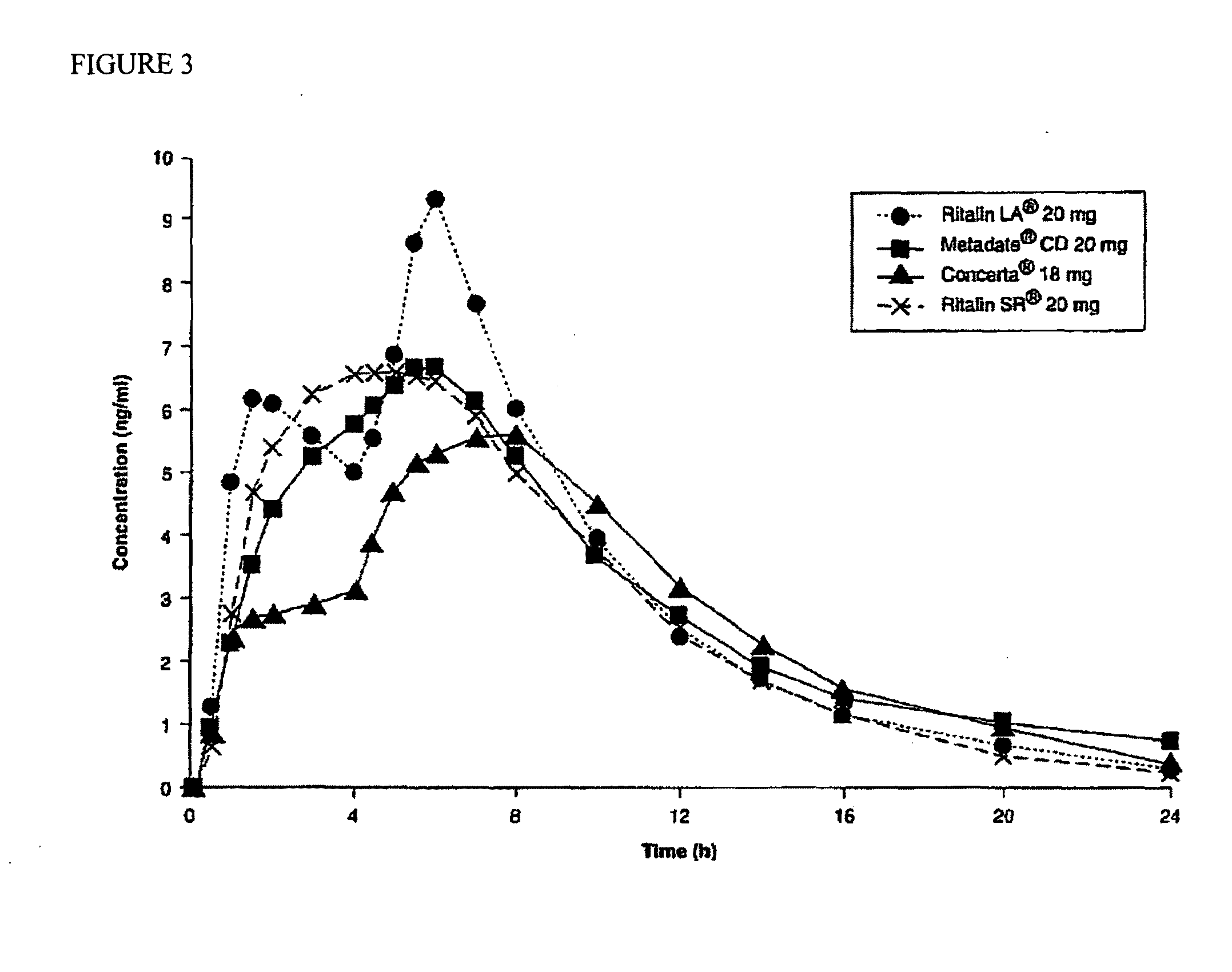
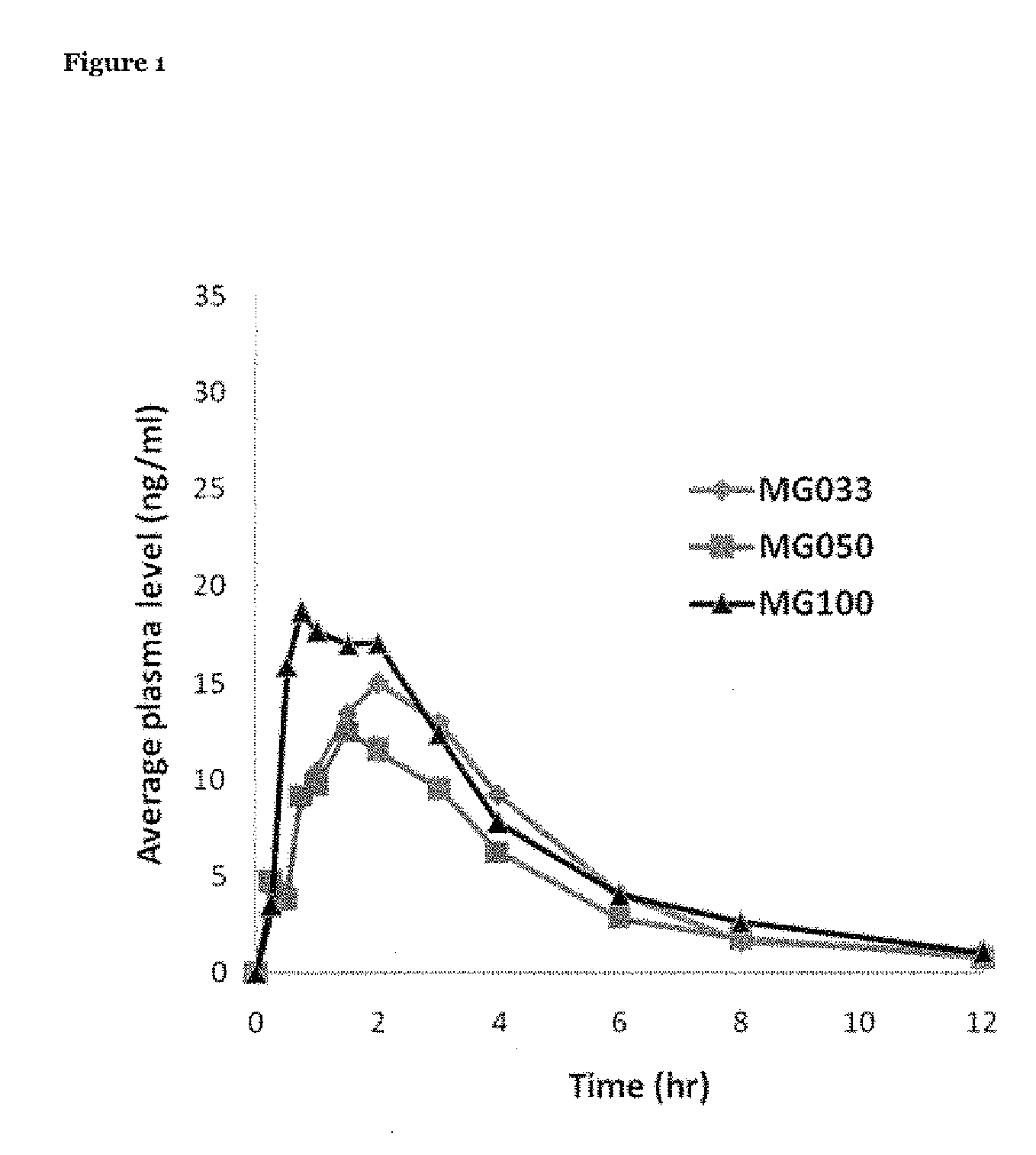
InactiveUS20100260844A1Enhanced delivery kineticsEasy constructionBiocidePowder deliveryControlled releaseMethylphenidate
Controlled release oral dosage forms suitable for administration of methylphenidate are provided. Abuse-resistant controlled release oral dosage forms suitable for administration of methylphenidate are also provided. Methods of treating ADD and ADHD using the oral dosage forms are also provided.
Owner:DURECT CORP
Dosage Form For Insertion Into The Mouth
InactiveUS20100112050A1Efficient deliveryImproved bioavailability and deliveryPowder deliveryBiocideActive agentMigraine
Oral dosage forms as a biodegradable, water soluble film for delivering pharmaceutically active agents, particularly anti-migraine agents to patients through insertion into the mouth of patient and methods for administering pharmaceutically active agents to patients by insertion into the mouth to provide selective uptake of said agents through the mucosa and thus avoiding the gastrointestinal tract.
Owner:NAL PHARM LTD
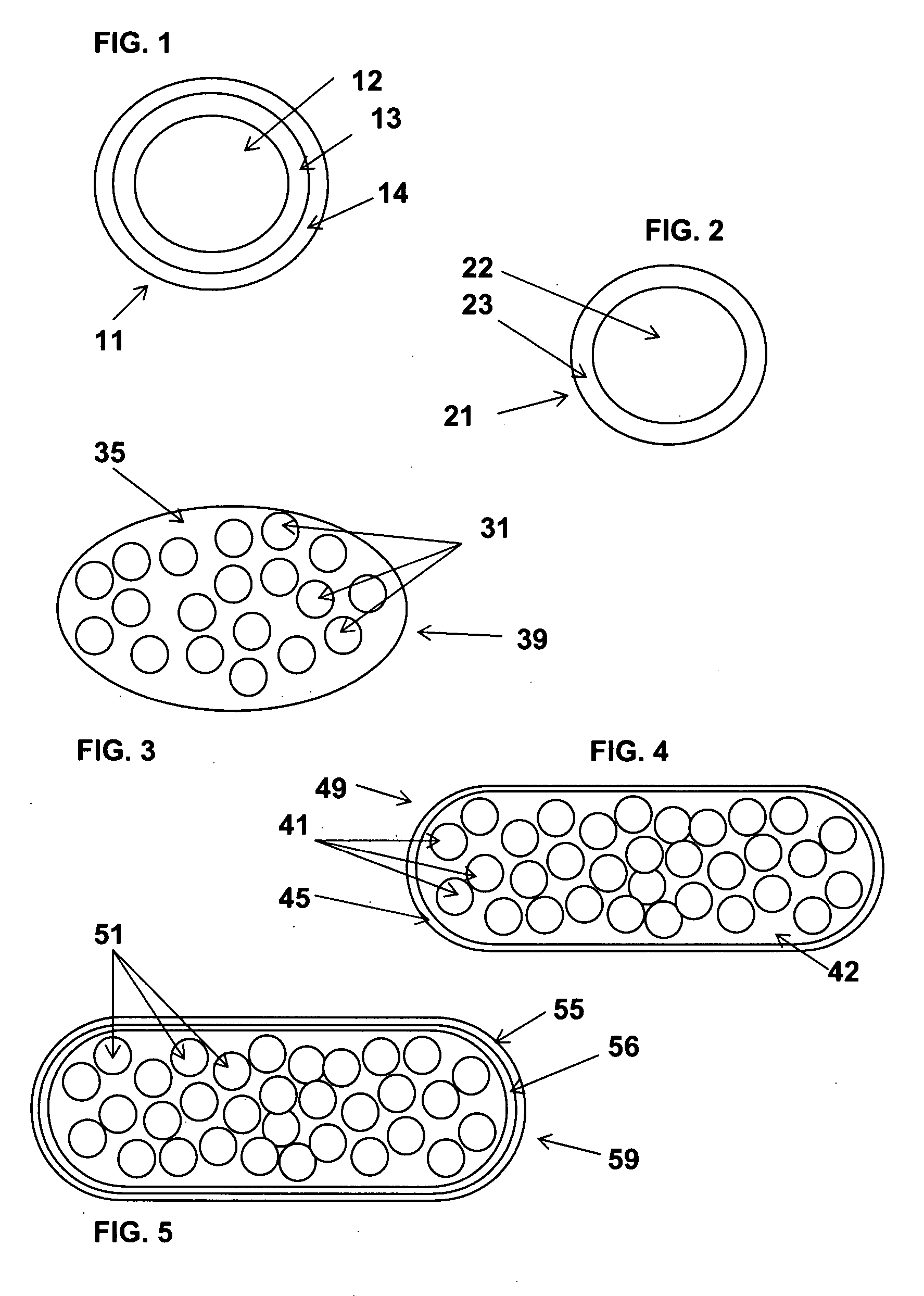
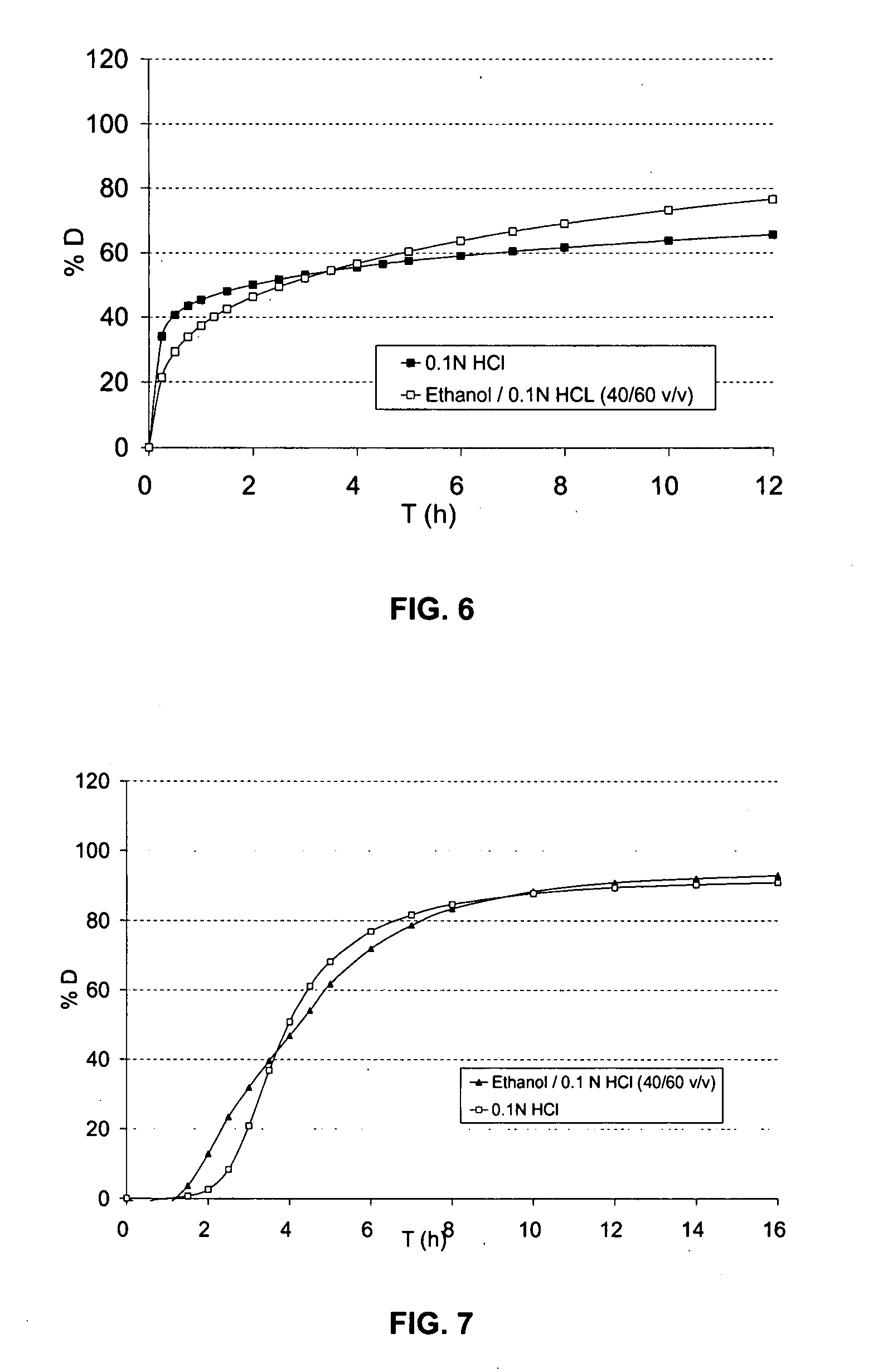
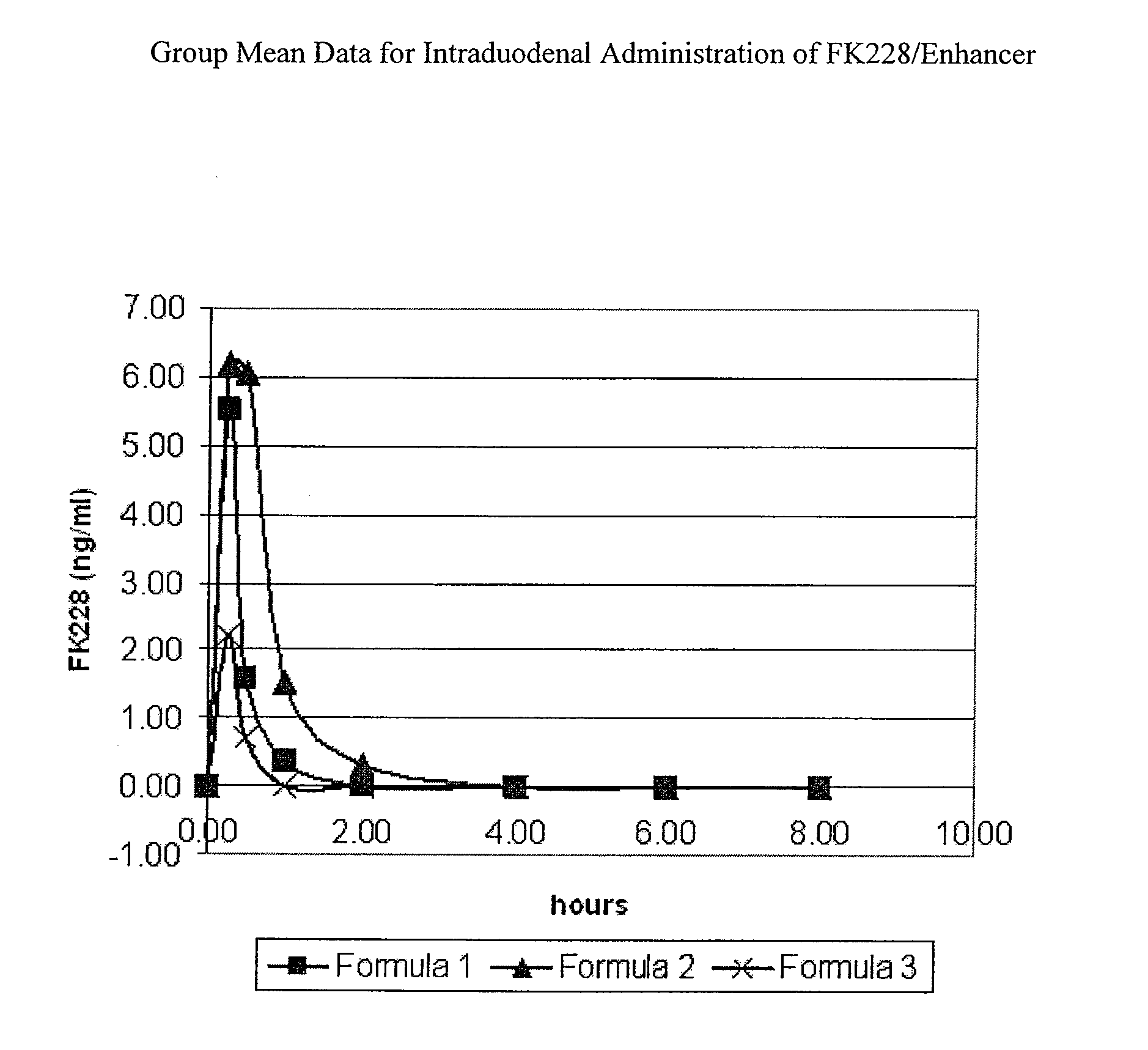
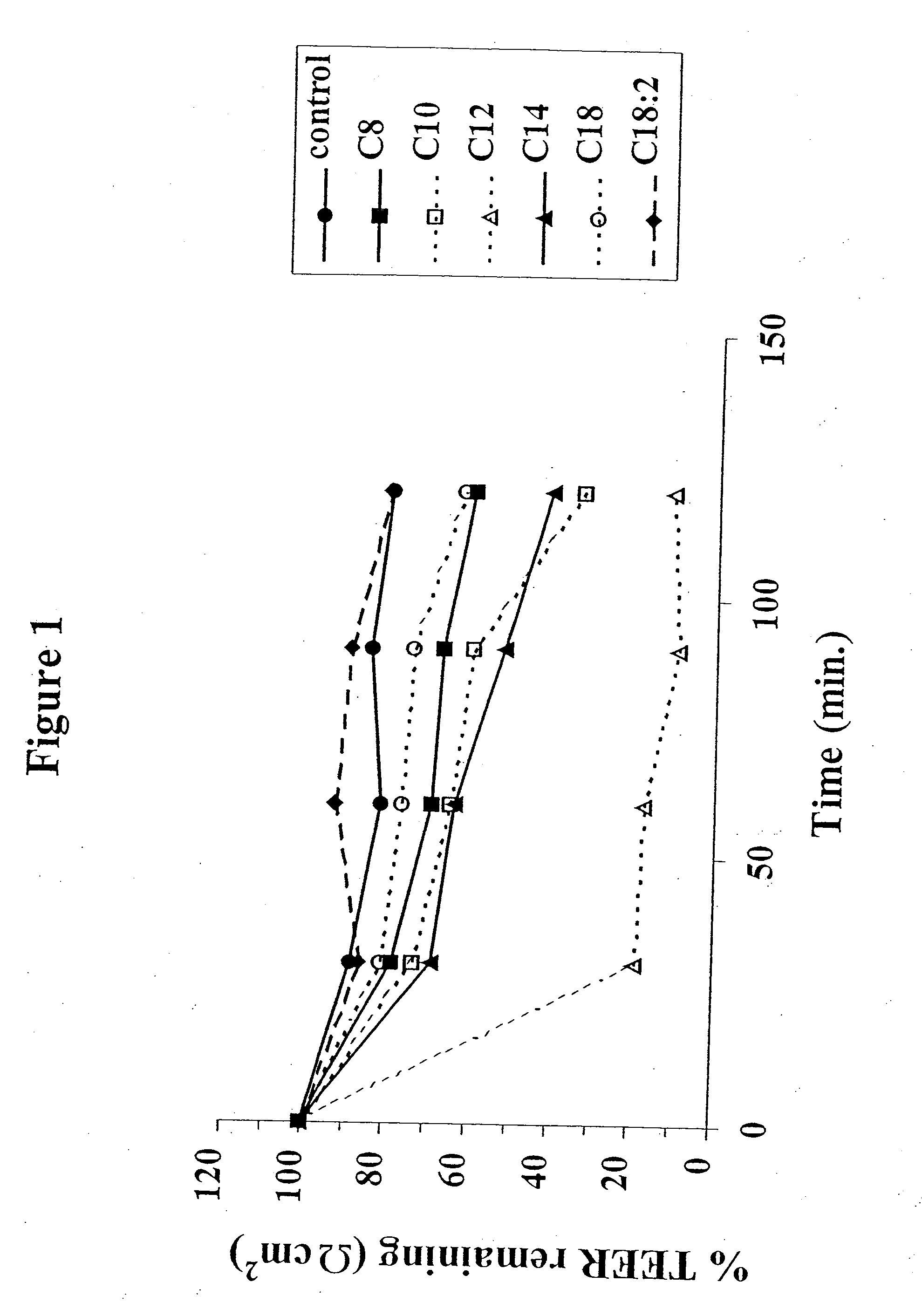
Solid oral dosage form containing an enhancer
InactiveUS20070148228A1BiocideCyclic peptide ingredientsDelayed Release Dosage FormPharmaceutical drug
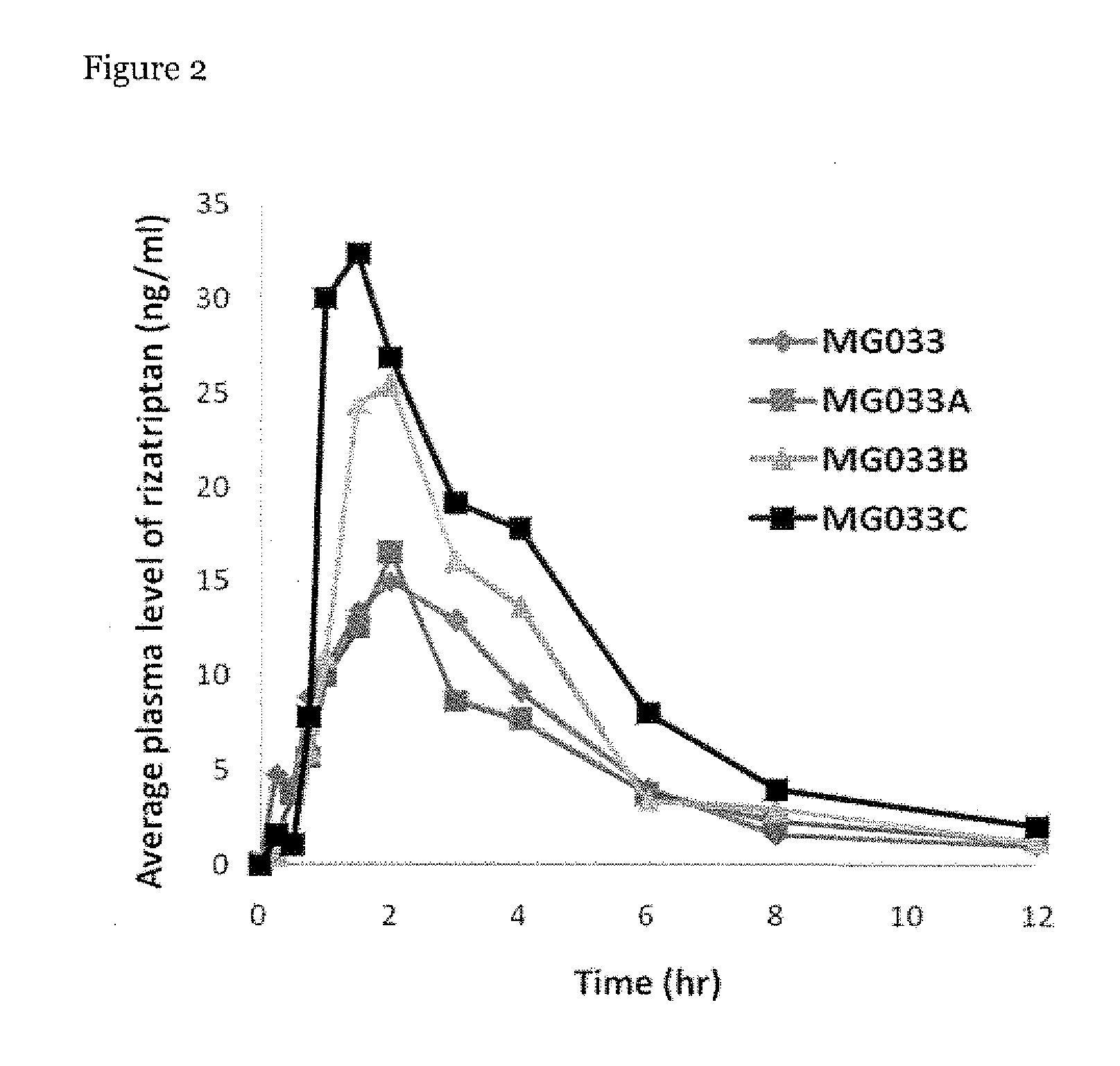
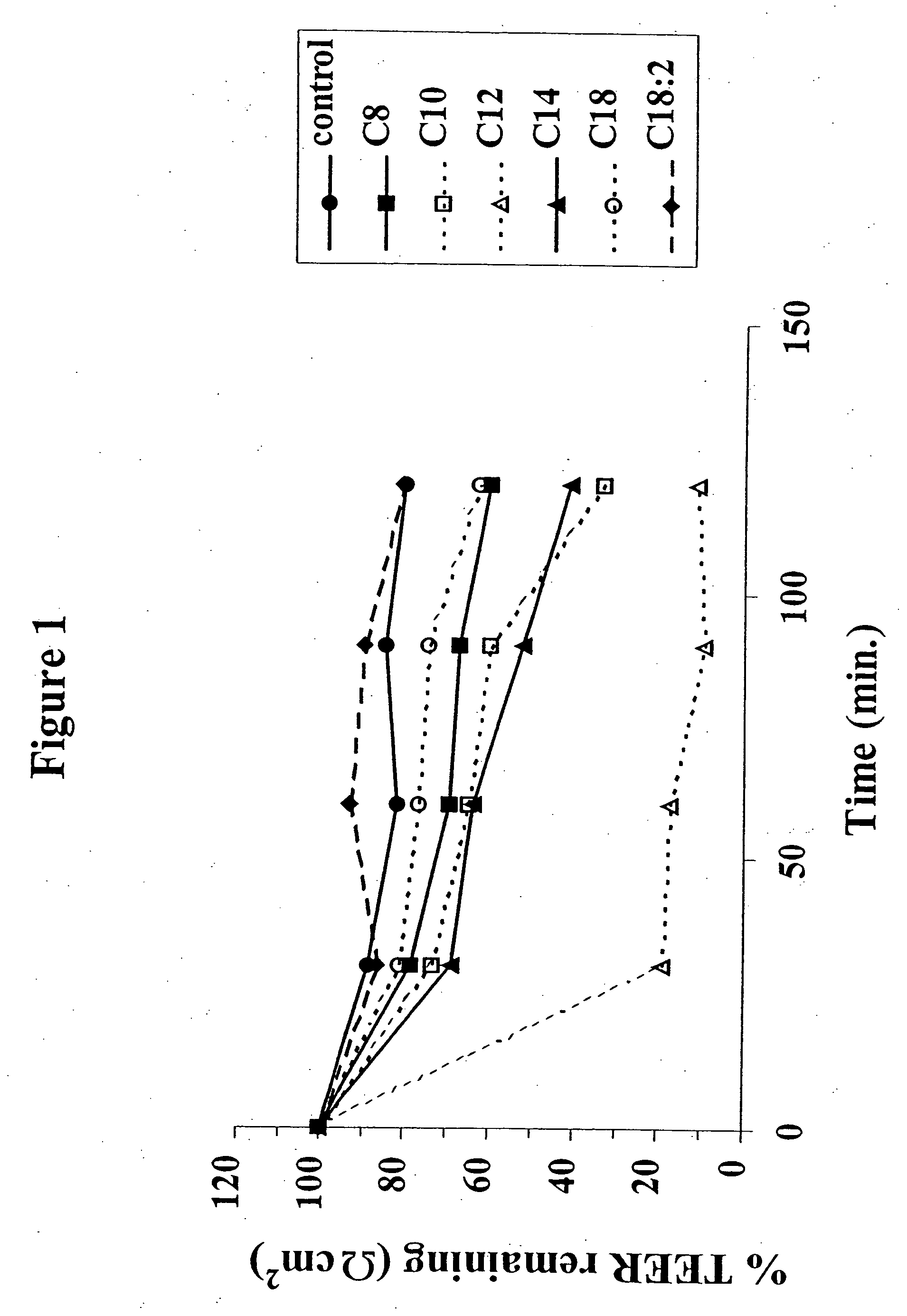
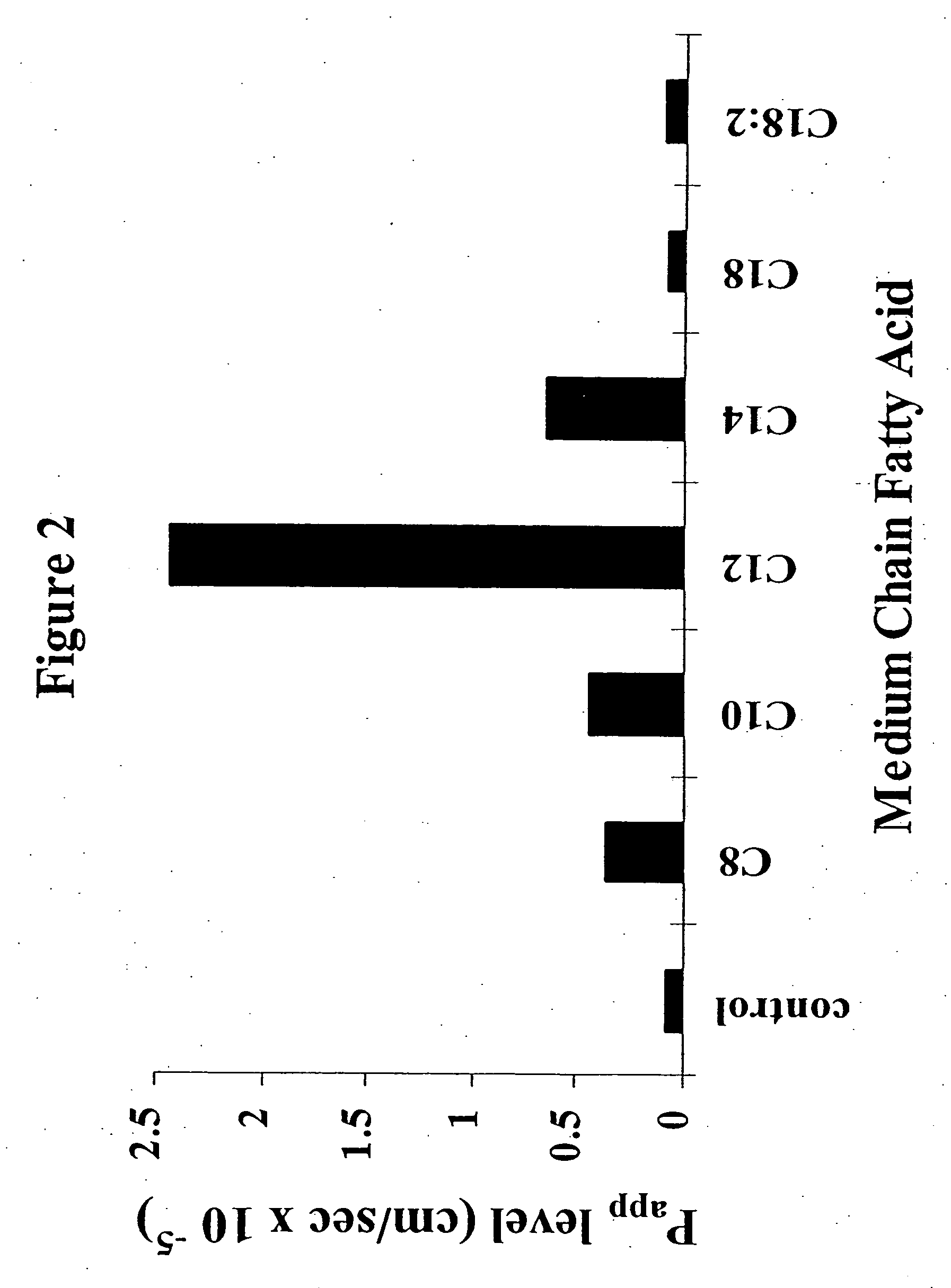
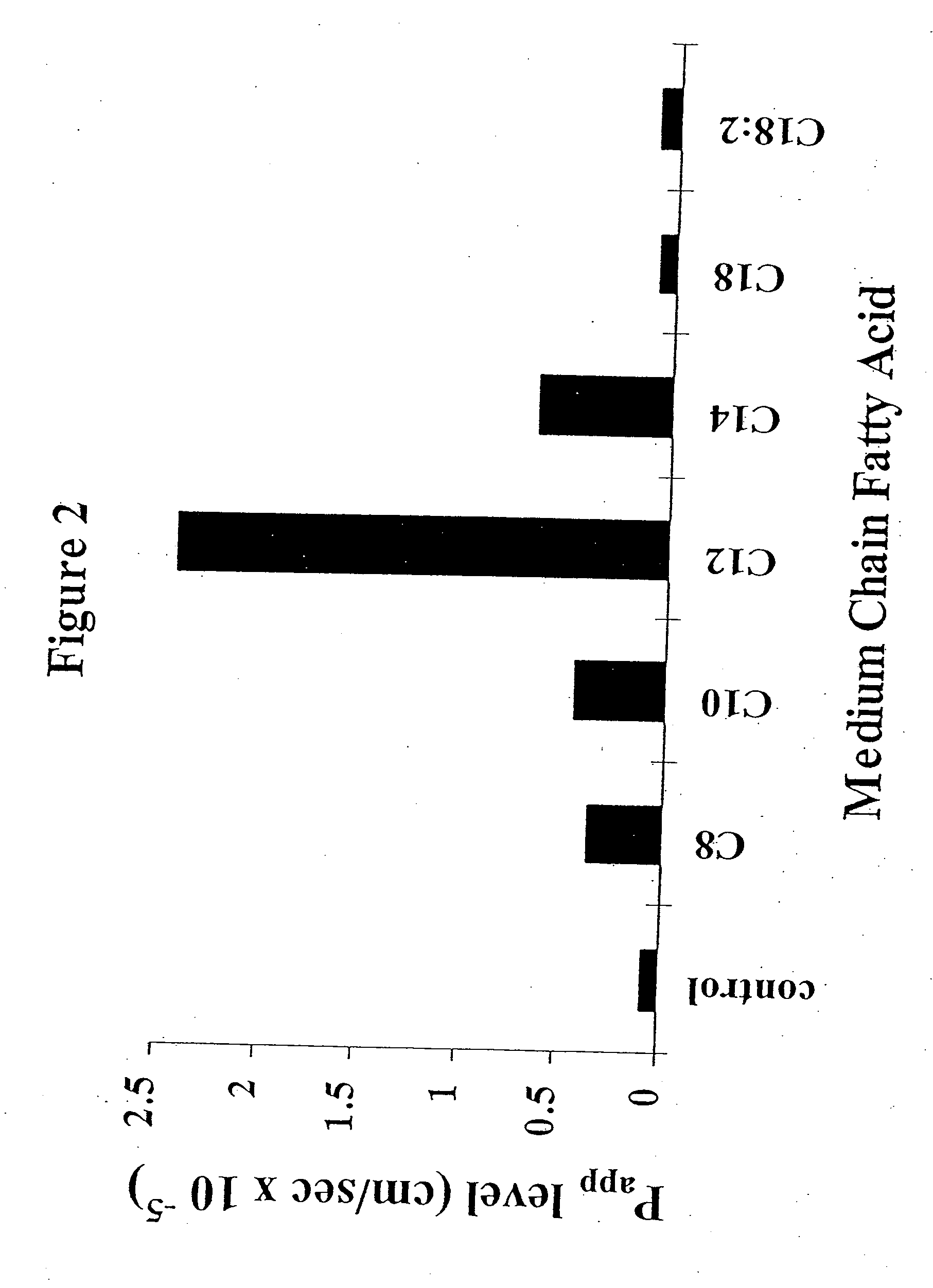
The invention relates to a pharmaceutical composition and oral dosage forms comprising an HDAC inhibitor in combination with an enhancer to promote absorption of the HDAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Solid Oral Dosage Form Containing an Enhancer
The invention relates to a pharmaceutical composition, particularly oral dosage forms, comprising a DAC inhibitor in combination with an enhancer to promote absorption of the DAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or derivative thereof having a carbon chain length of from 6 to 20 carbon atoms. In certain embodiments, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Pharmaceutical composition for oral use with improved absorption
InactiveUS7008640B2Promote absorptionDifficult to absorbBiocideNervous disorderBULK ACTIVE INGREDIENTAcid substances
The present invention presents a pharmaceutical composition for oral use with improved absorption, which comprises drug, aminoalkyl methacrylate copolymer E, and acidic substance and is obtained by bringing said 3 components together and uniformly mixing at least this polymer and this acidic substance, and a method of improving oral absorption by using this pharmaceutical composition. Moreover, the present invention presents an agent for improving oral absorption that increases drug permeability of the digestive tract mucous membrane and / or mucous layer present on the surface of this membrane, whose active ingredient is aminoalkyl methacrylate copolymer E. In addition, the present invention presents an oral agent for improving absorption by increasing drug permeability of the digestive tract mucous membrane and / or the mucous layer distributed over this mucous membrane, whose effective component is aminoalkyl methacrylate copolymer E.
Owner:ASTELLAS PHARMA INC
Sustained release compositions using wax-like materials
ActiveUS20080220079A1Low costDecrease scale-up complexityAntibacterial agentsPowder deliveryWaxEngineering
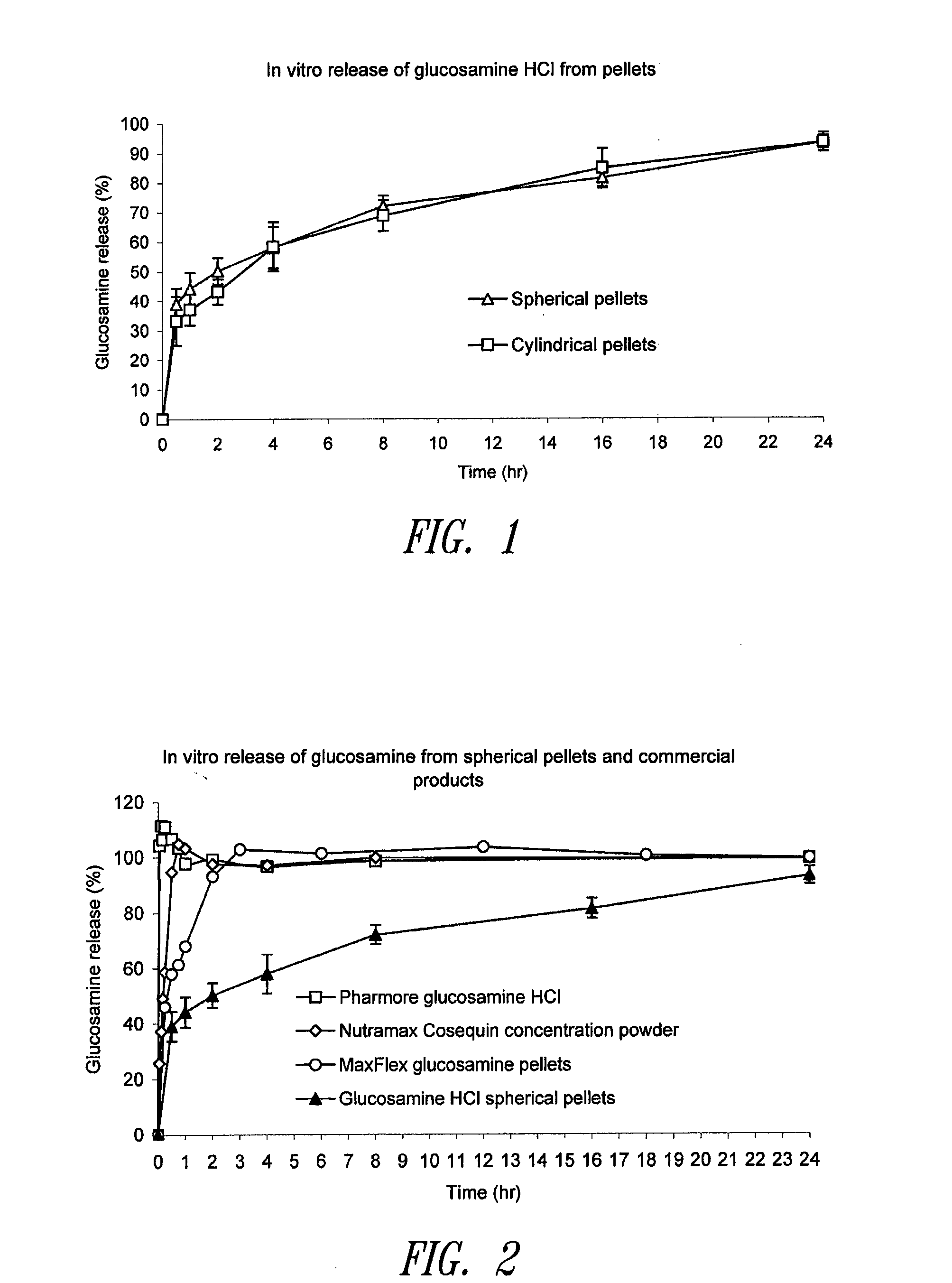
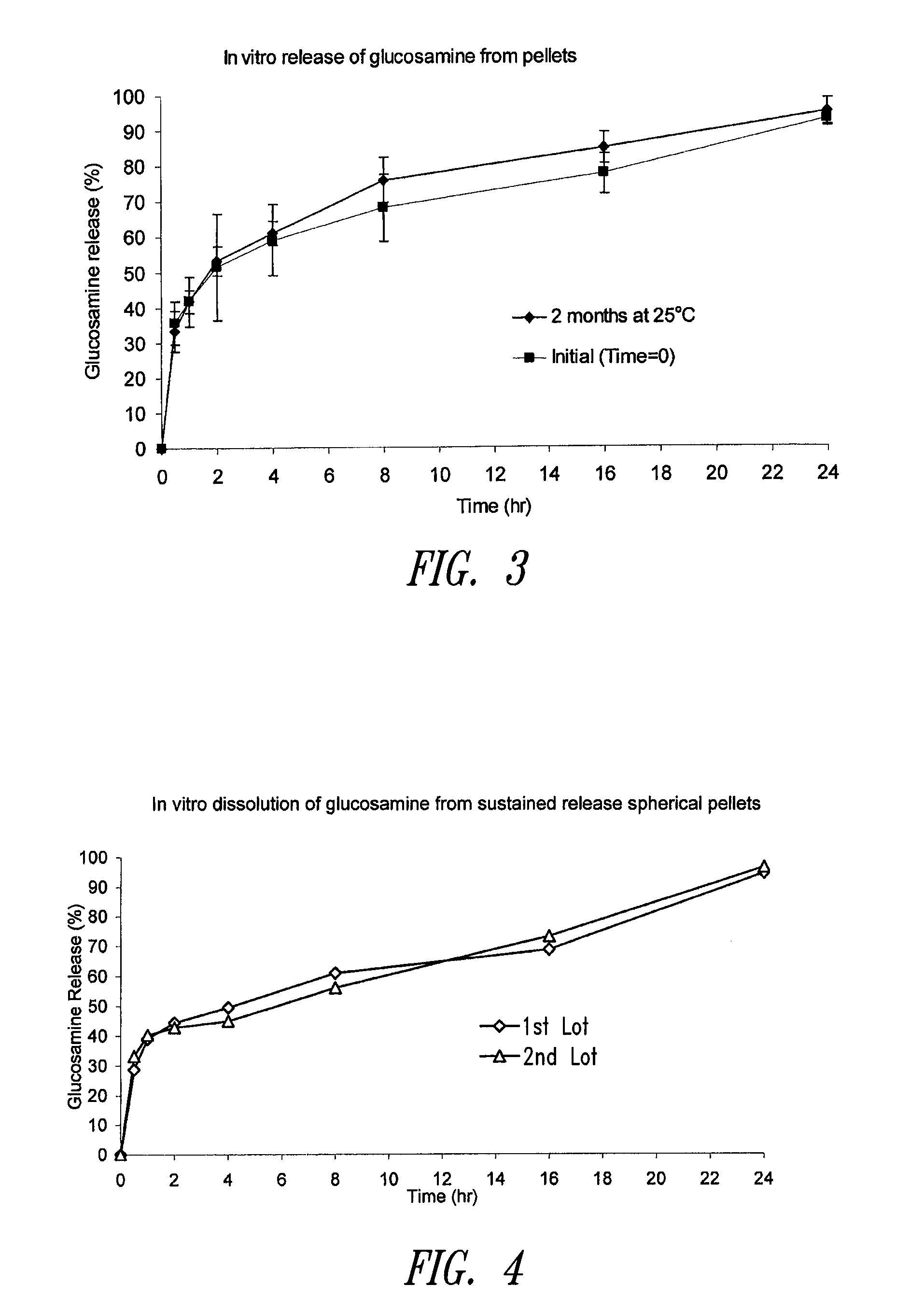
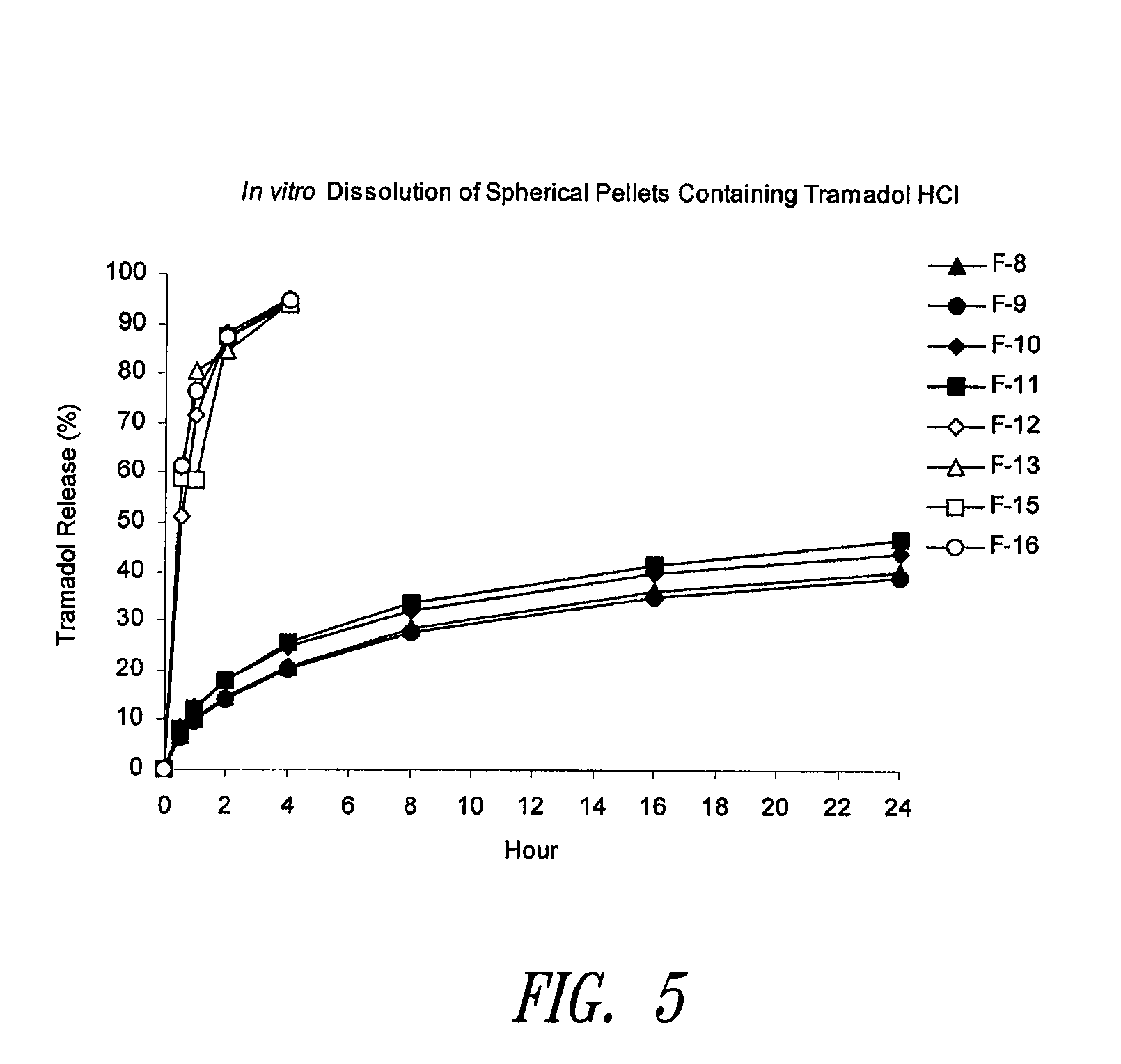
Sustained release spherical or non-spherical pellets comprising (a) an active ingredient (b) a wax-like agent, and (c) a spheronizing agent are provided. Oral dosage forms comprising said pellets and methods for preparing and using such pellets and dosage forms are also provided.
Owner:FARNAM
Drug Delivery Formulations For Targeted Delivery
The size and location of microsphere uptake / delivery are important determinants of the final biodistribution of oral microsphere systems. Formulations, kits, methods of administering the formulations, and using the kits are described herein. The formulations are oral dosage formulations. In one embodiment, the formulations contain microparticles and / or nanoparticles having a homogenous size range selected to optimize uptake in a specific region of the GI tract and target drug delivery to specific organs. In some embodiments, the dosage formulation contains an enteric coating and / or a magnetic material. In a preferred embodiment, the formulation contains a magnetic material and an active agent to be delivered, optionally the active agent is in the form of micro- or nano-particles. In some embodiments metallomucoadhesive materials and / or magnetic materials are employed as magnetic and / or mucoadhesive sources. Formulations containing magnetic materials can be localized using the kits and methods disclosed herein. In one embodiment, the method includes orally administering the formulation and applying an extracorporeal magnet to a site on the outside surface of the patient's body in an area that closely apposes the location in the gastrointestinal tract to which delivery of the formulation is desired. The extracorporeal magnet is applied for a suitable time period to allow for the drug to be released from the formulation and / or to allow for the formulation to adhere to the site. Both magnetic and mucoadhesive forces may be utilized to site-direct and retain the dosage form in the region of the gastrointestinal (GI) tract most suitable for the desired delivery.
Owner:PEROSPHERE INC
Oral Dosage Forms with Therapeutically Active Agents In Controlled Release Cores and Immediate Release Gelatin Capsule Coats
InactiveUS20080026052A1Increase release rateIncrease ratingsBiocideNervous disorderActive agentGelatin capsule
The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release gelatin capsule coats.
Owner:SCHOENHARD GRANT L
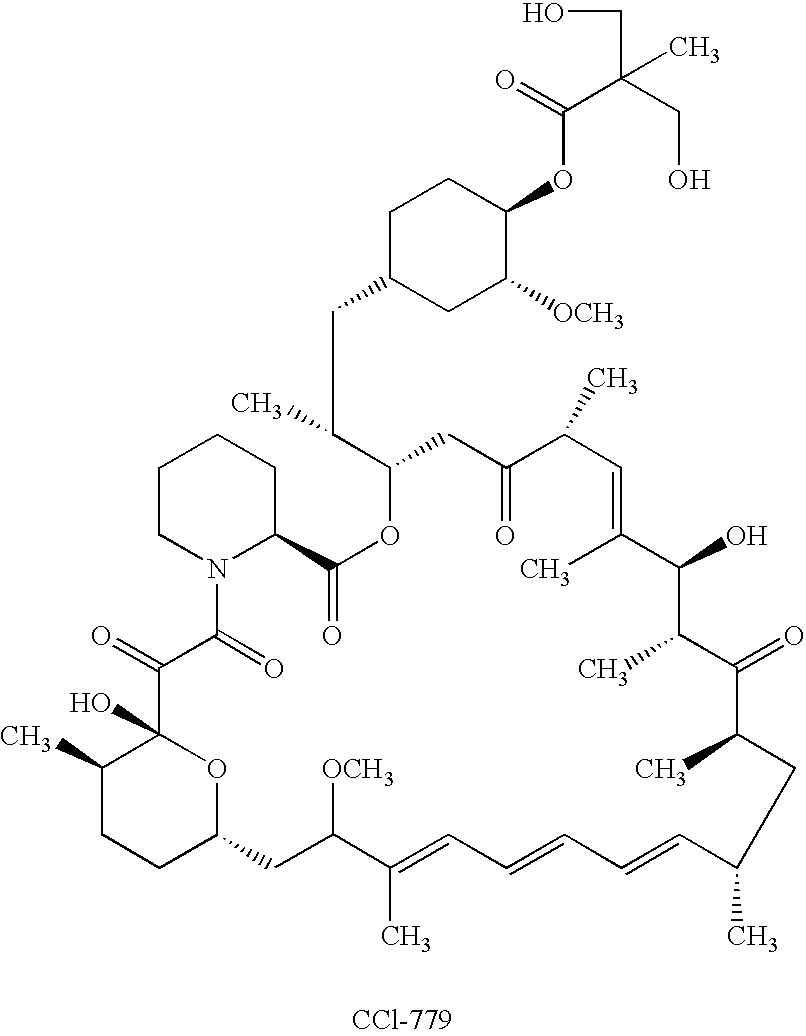
Orally bioavailable CCI-779 formulations
A CCI-779 oral dosage form is provided in which, after oral administration to a subject, the CCI-779 has a whole blood peak concentration (Cmax) of 5.4±1.8 ng / mL and an area under the curve (AUC) of about 66±about 22 ng-hr / ml and the sirolimus has a Cmax of 18.7±9.6 ng / mL and an AUC of about 600±about 228 ng-hr / ml, for a 25 mg unit dose of CCI-779. Another CCI-779 oral dosage form is provided which, after oral administration thereof to a subject, the CCI-779 has a Cmax of 5.7±1.7 ng / mL and an AUC of about 60±about 20 ng-hr / ml and the sirolimus has a Cmax of 17.1±8.1 ng / mL and an AUC of about 548±about 187 ng-hr / ml in whole blood, for a 25 mg unit dose of CCI-779. Products containing these oral dosage forms, and methods of use thereof, are also described.
Owner:WYETH LLC
Osteoclast inhibitors for joint conditions
ActiveUS9610300B2Improve bioavailabilityPhosphorous compound active ingredientsPill deliveryNitrogenAnesthesia
Oral dosage forms of osteoclast inhibitors, such as nitrogen-containing bisphosphonates, can be used to treat or alleviate pain or related conditions.
Owner:ANTECIP BIOVENTURES II
Chinese herbal preparation for treating posirasis and preparation method thereof
InactiveCN101637585ARelief of clinical symptomsAnthropod material medical ingredientsDermatological disorderHerbal preparationsBarbed Skullcap Herb
The invention provides a Chinese herbal preparation for treating posirasis, which adopts 58 raw medicinal materials such as astragalus root, suberect spatholobus stem, Job stears, glabrous greenbrierrhizome, spreading hedyotis herb, barbed skullcap herb, motherwort herb and the like to manufacture oral agent and abrasive cleaner. The manufacturing method of the invention is as follows: 1. manufacturing by using the above raw material medicines into water-decocted liquid according to the conventional method; 2. manufacturing by using the water-decocted liquid which is manufactured in step 1 into oral liquid according to the conventional method; 3. drying, grinding and sieving the water-decocted liquid which is manufactured in step 1, and taking the throughs as powder; and 4. manufacturinginto powder tablets, granular formulation, medicinal granules or capsules by utilizing step 3 according to the conventional method. The powder and the abrasive cleaner can be manufactured by the following steps: 1. grinding the above raw material medicines; 2. sieving; and 3. taking the throughs, disinfecting and sterilizing to obtain the powder. The invention has high cure rate, low recurrence rate, can treat the primary and the secondary aspects at the same time, is safe and reliable, and can strengthen the autoimmunity. The invention mainly treats various types of posirasis, can treat neurodermatitis, comedo, acne and eczema concurrently, and has effects on lupus erythematosus, syphilis, lepra and skin cancer.
Owner:卢速江
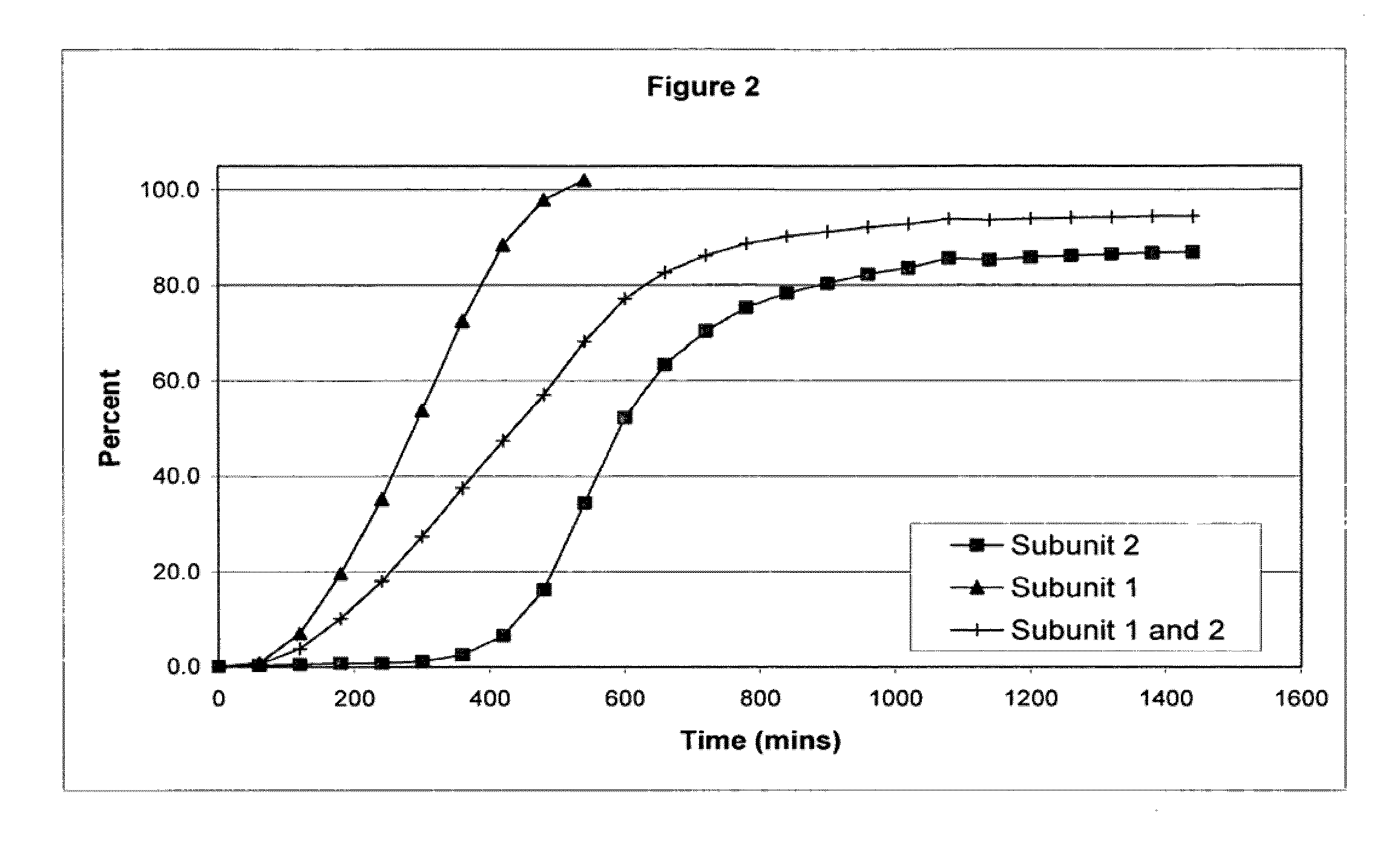
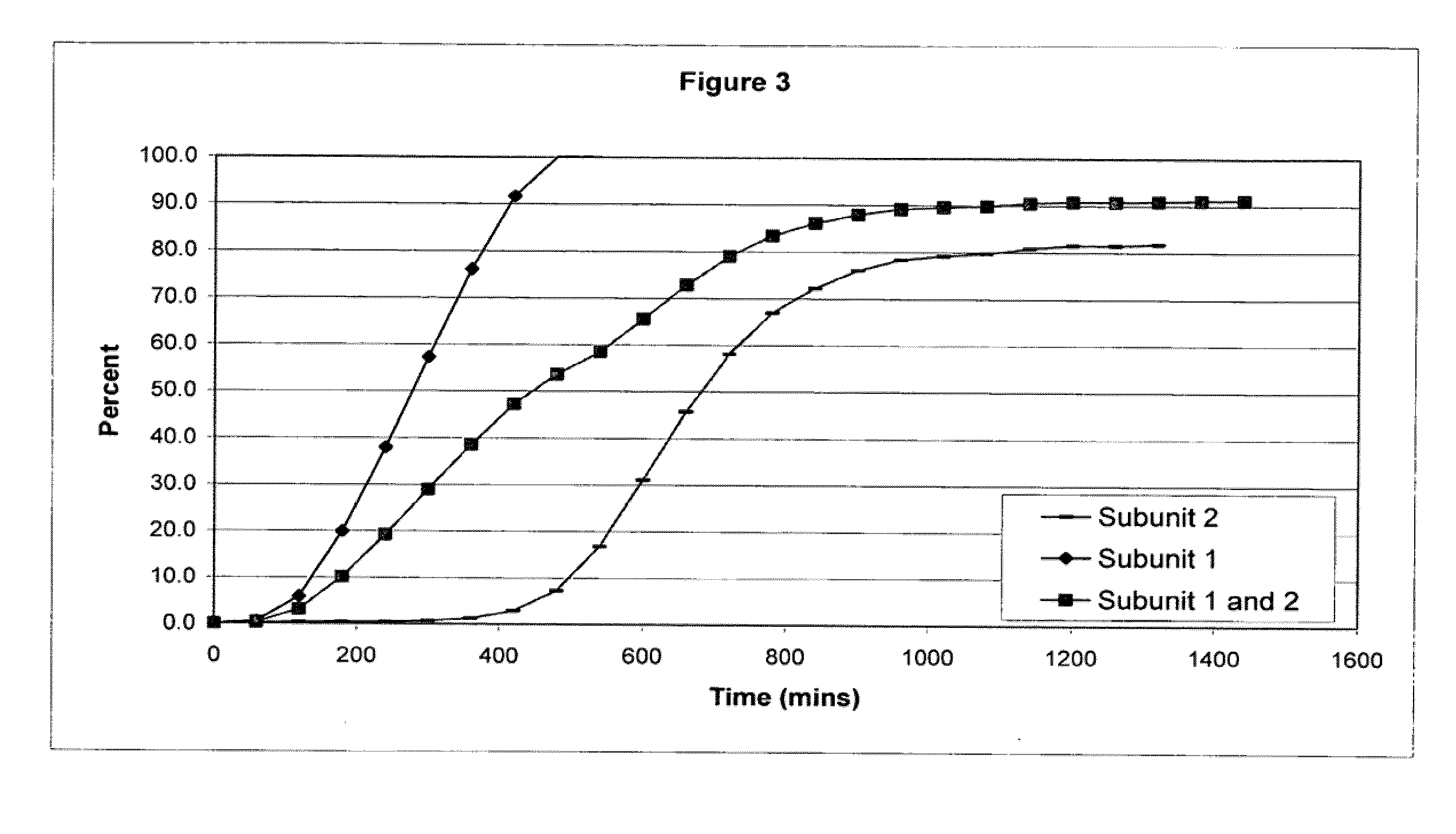
Sustained release opioid formulations and methods of use
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Oral Dosage Form Of Tetrahydrocannabinol And A Method Of Avoiding And/Or Suppressing Hepatic First Pass Metabolism Via Targeted Chylomicron/Lipoprotein Delivery
ActiveUS20110092583A1Easy to transportPromote lymphatic transportBiocideSenses disorderChylomicronCytochrome P450
Self-emulsifying drug delivery systems are provided to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron / lipoprotein delivery and optimal bioavailability through the mammalian intestinal tract. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY RAM B +1
Anti-Misuse Microparticulate Oral Drug Form
InactiveUS20090041838A1Avoid misuseAvoid fraudulent abuseOrganic active ingredientsPowder deliveryAdditive ingredientMicroparticle
The invention relates to solid microparticulate oral dosage forms having a composition that prevents the misuse of the active pharmaceutical ingredient (API) contained therein. The aim of the invention is to prevent the improper use of solid oral drugs for any use other than the therapeutic use(s) officially approved by the appropriate public health authorities. Another aim of the invention is to provide novel analgesic drugs which can be used to: prevent the misuse of, and addiction to certain analgesics and / or to control plasma concentration variability and / or to facilitate oral; administration; and / or to combine analgesics with one another and / or with one or more active ingredients in the same oral form. More specifically, the invention relates to a solid oral drug form comprising anti-misuse means and at least one active ingredient, which is characterized in that: at least part of the active ingredient is contained in microparticles; and the anti-misuse means comprise anti-crushing means (a) which enable the microparticles of the active ingredient to resist crushing, such as to prevent the misuse thereof. According to the invention, the drug form can also comprise means (b) for preventing the misuse of the active ingredient following a possible liquid extraction process.
Owner:FLAMEL TECHNOLOGIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com