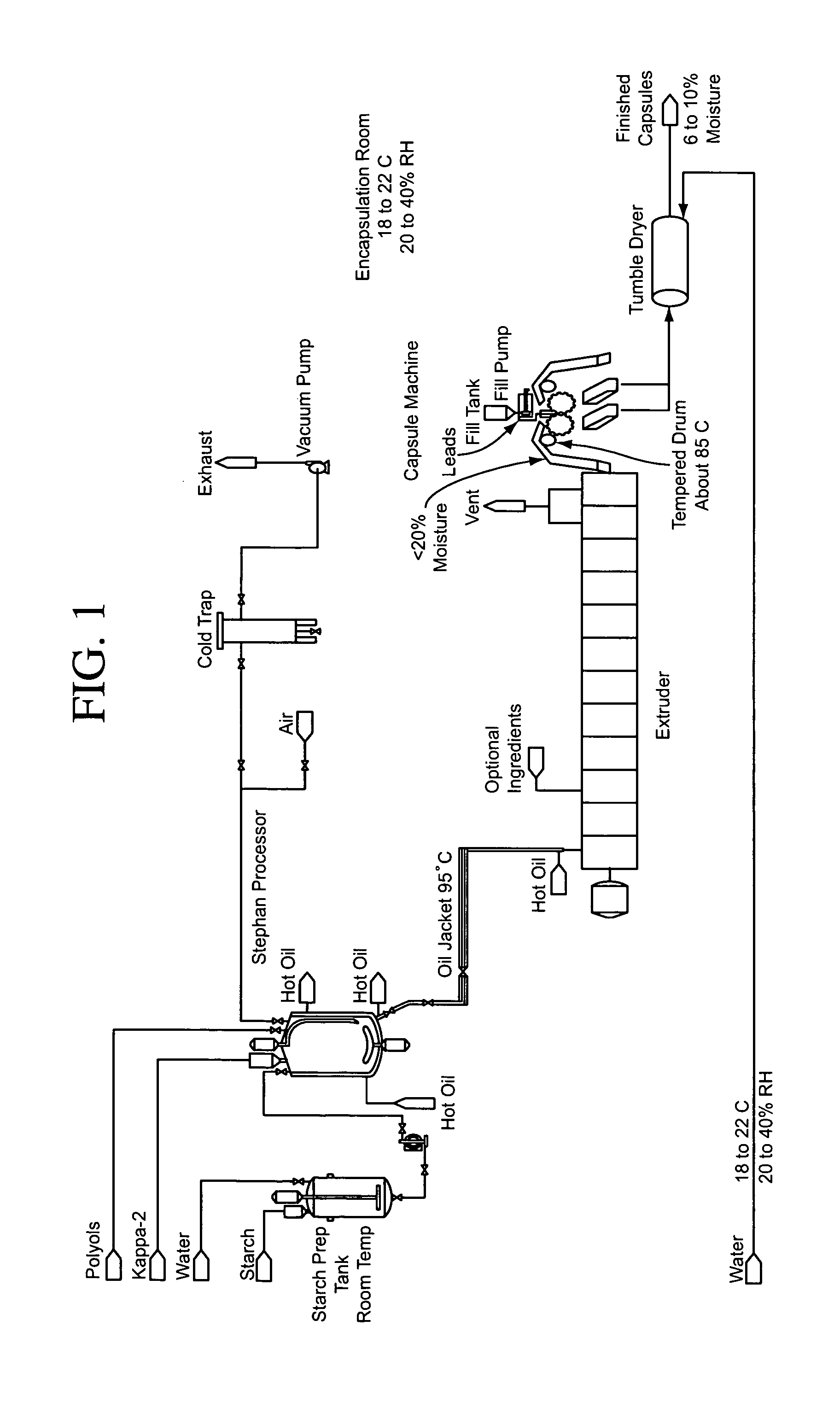
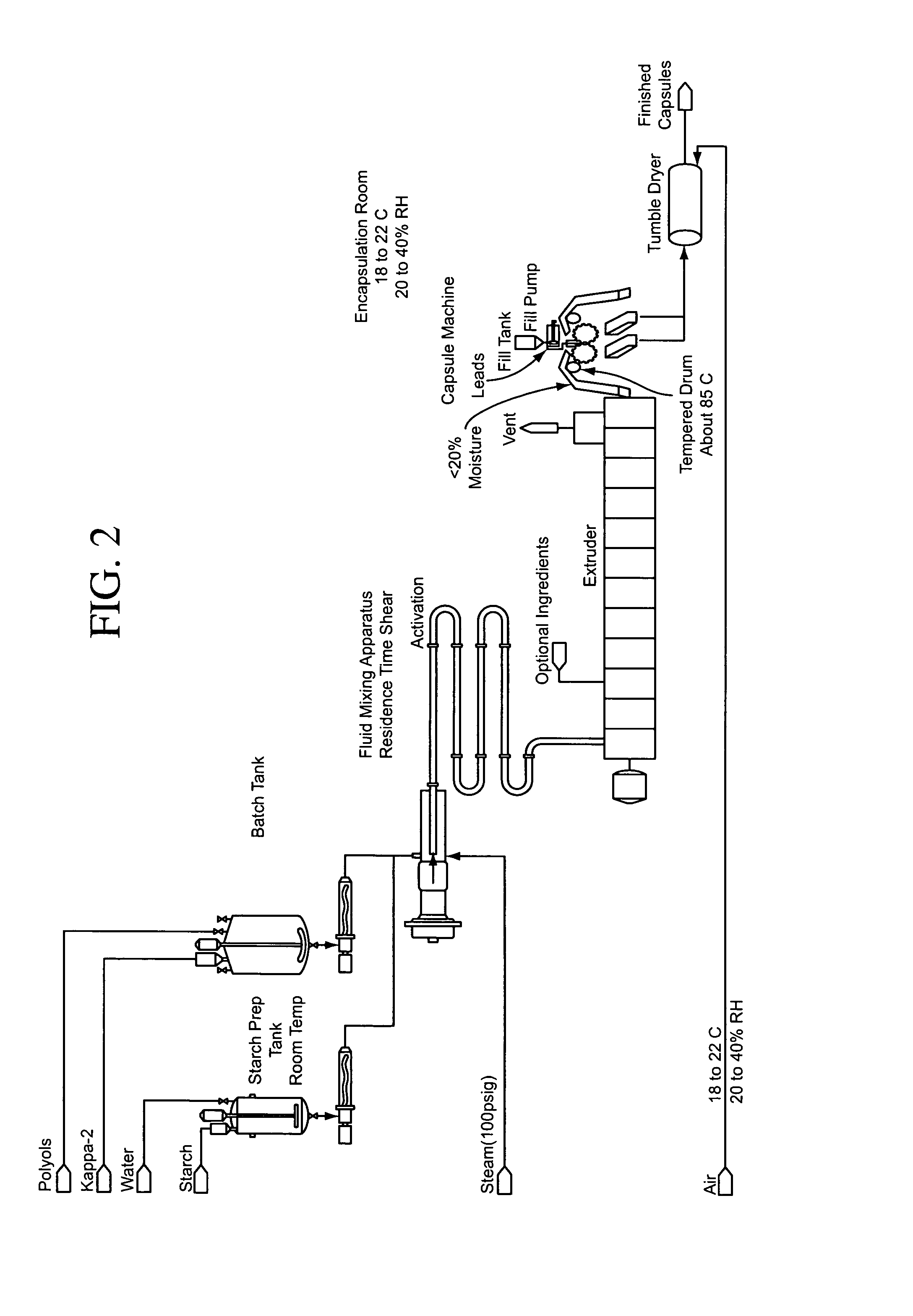
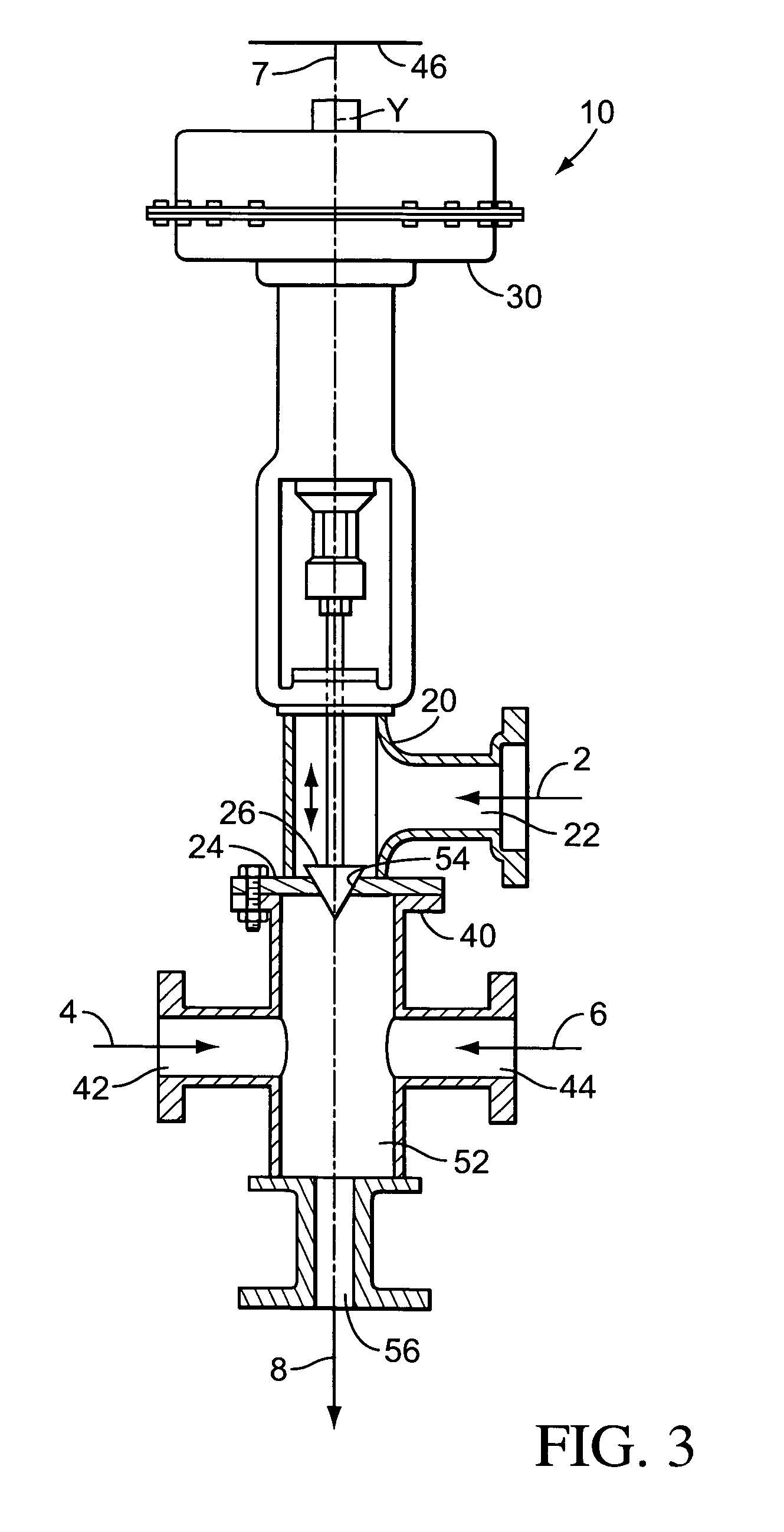
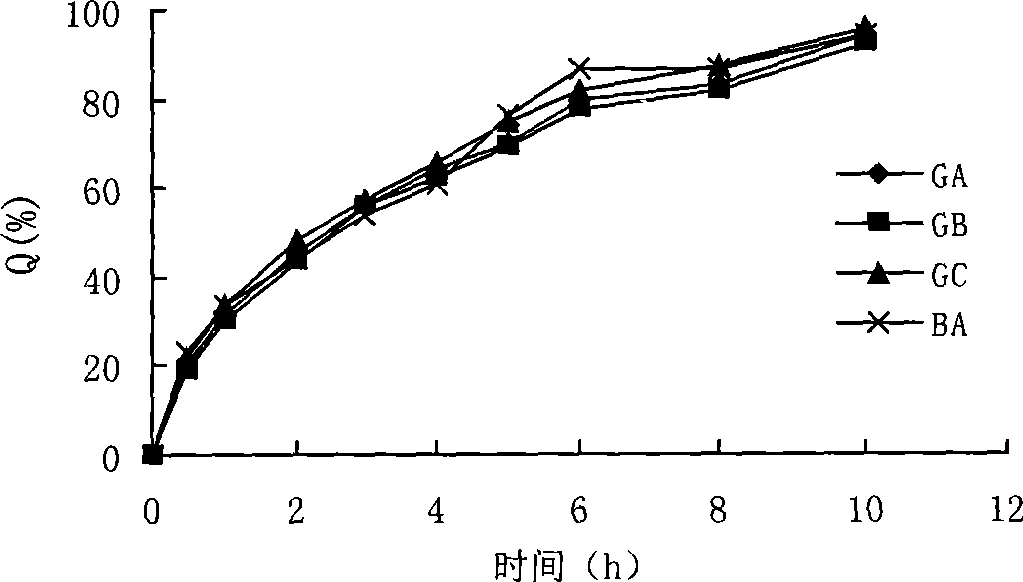
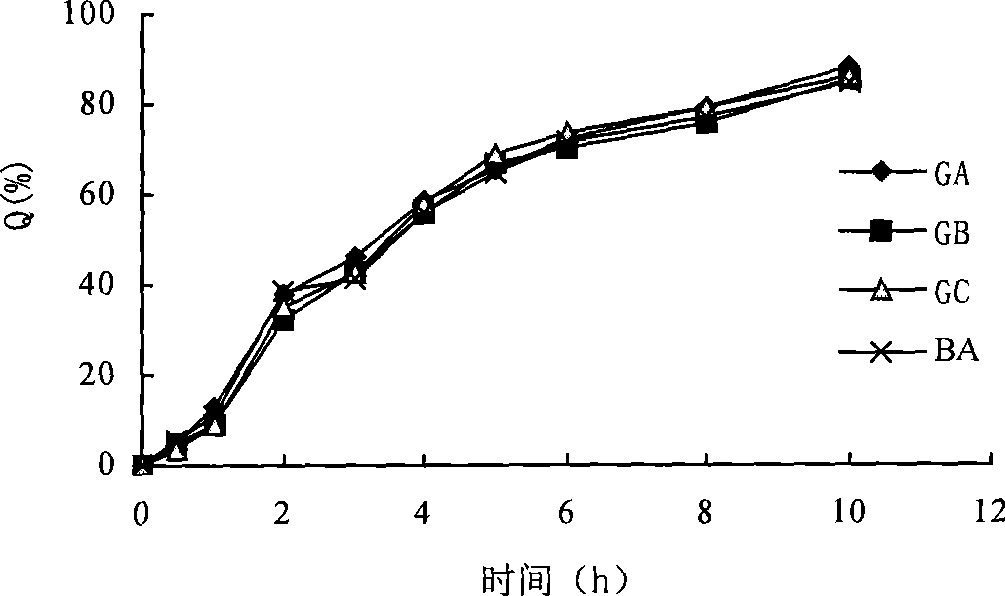
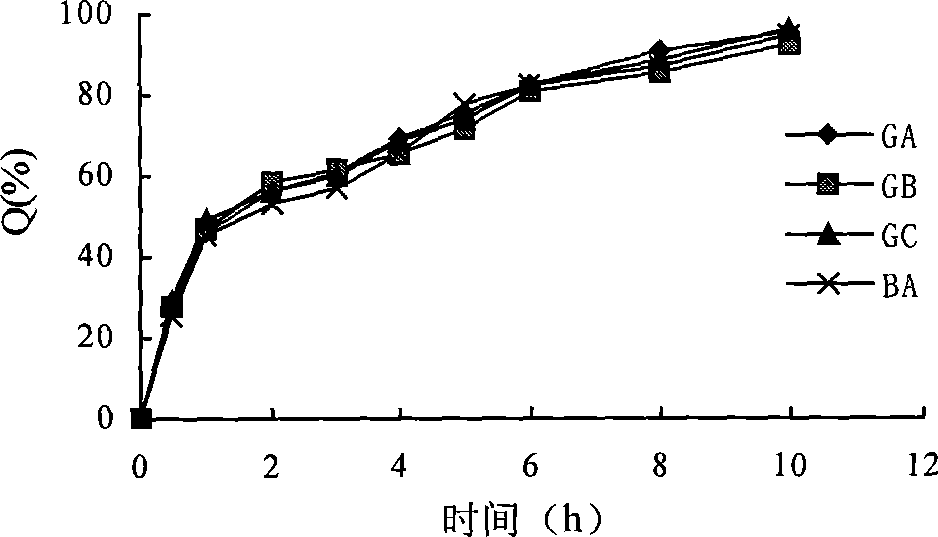
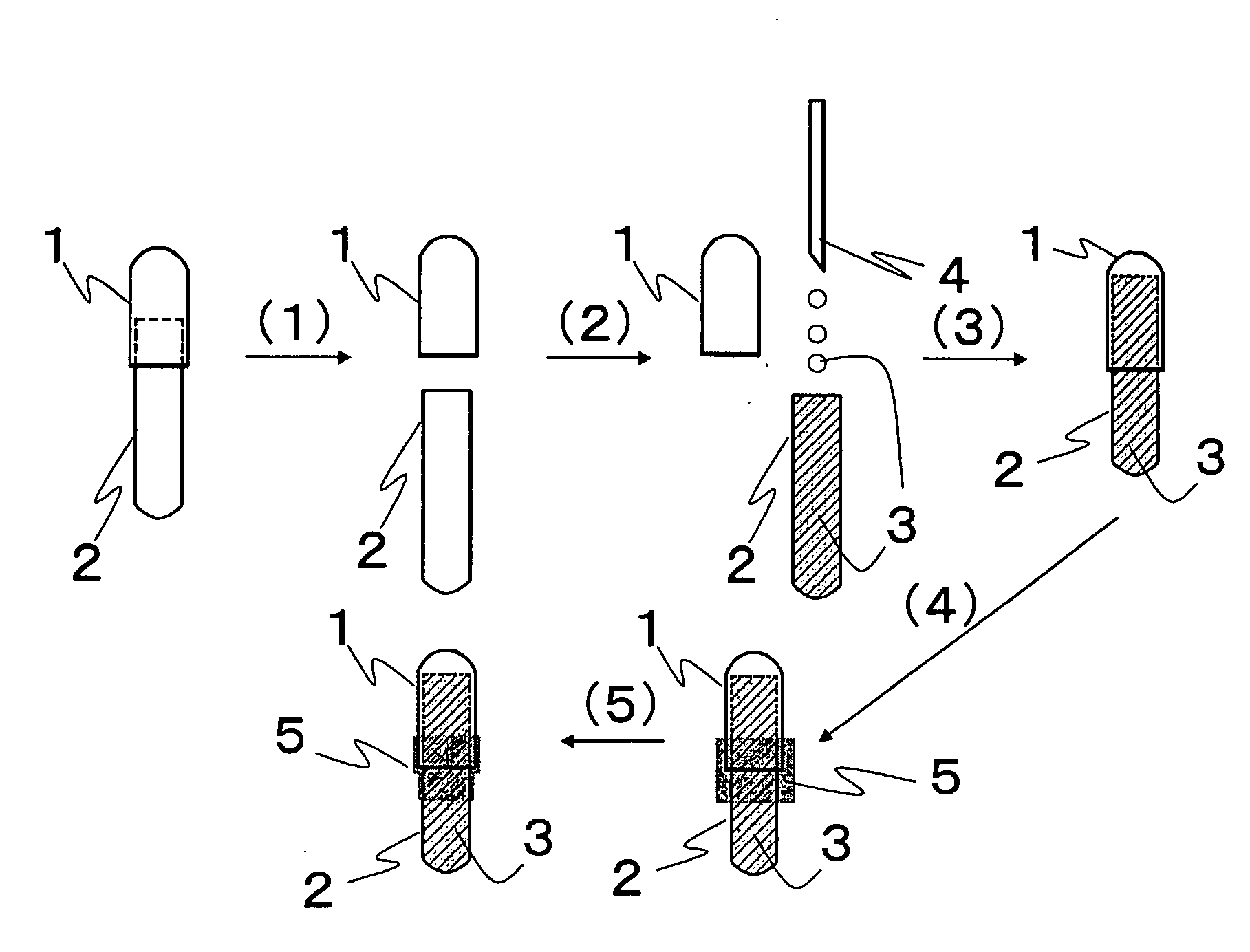
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
901 results about "Hard Capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
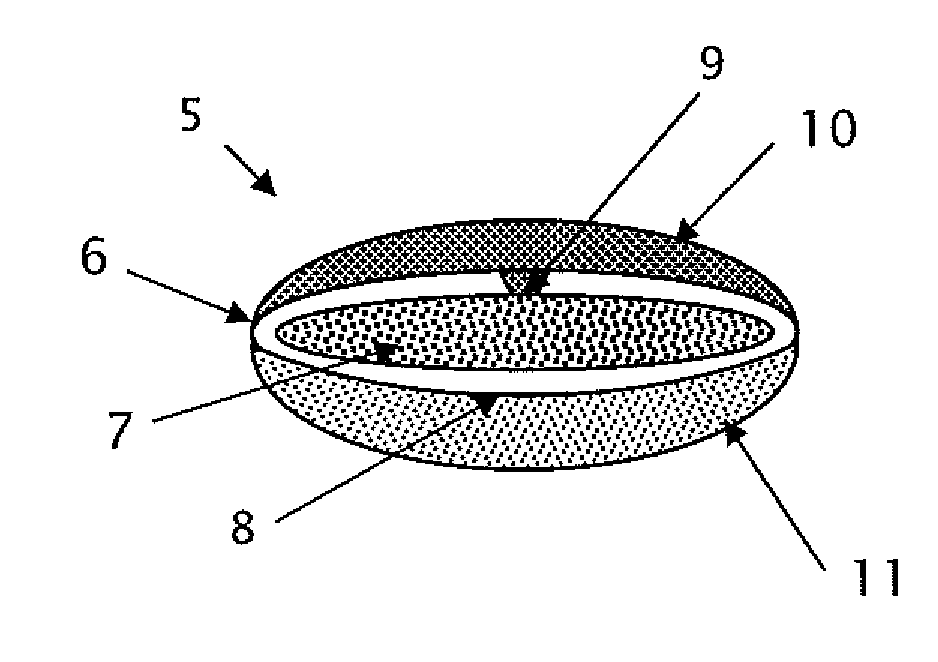
Triple Combination Release Multi-Layered Tablet
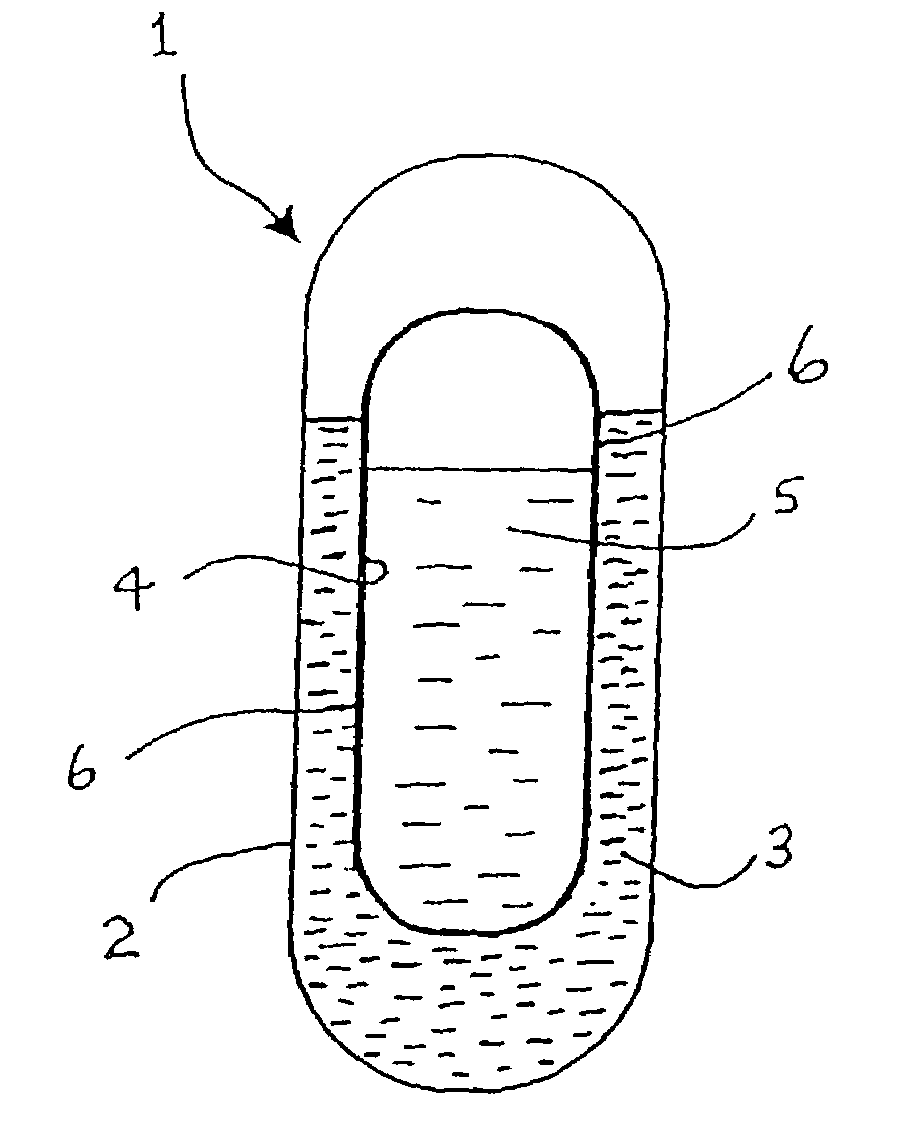
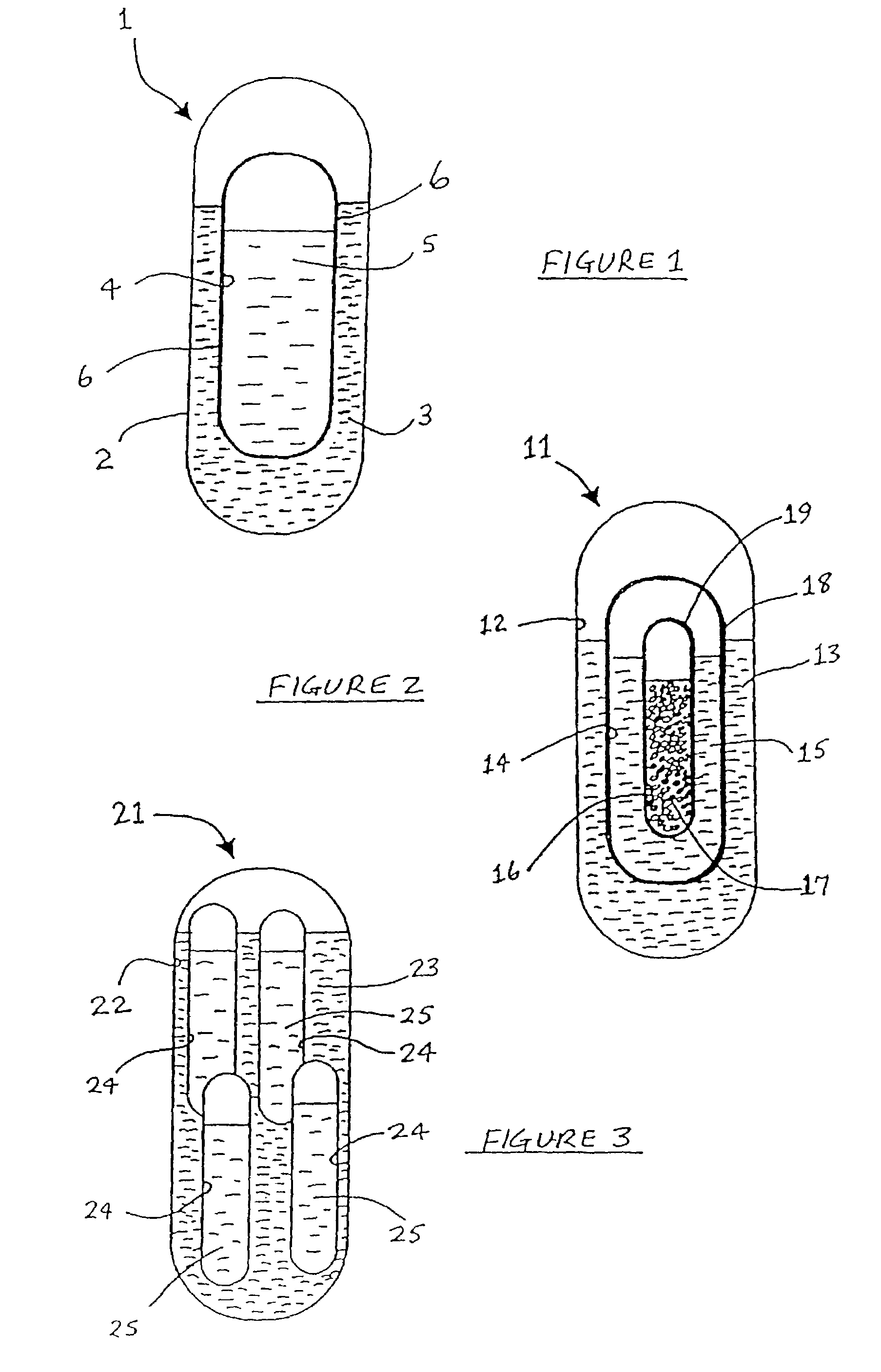
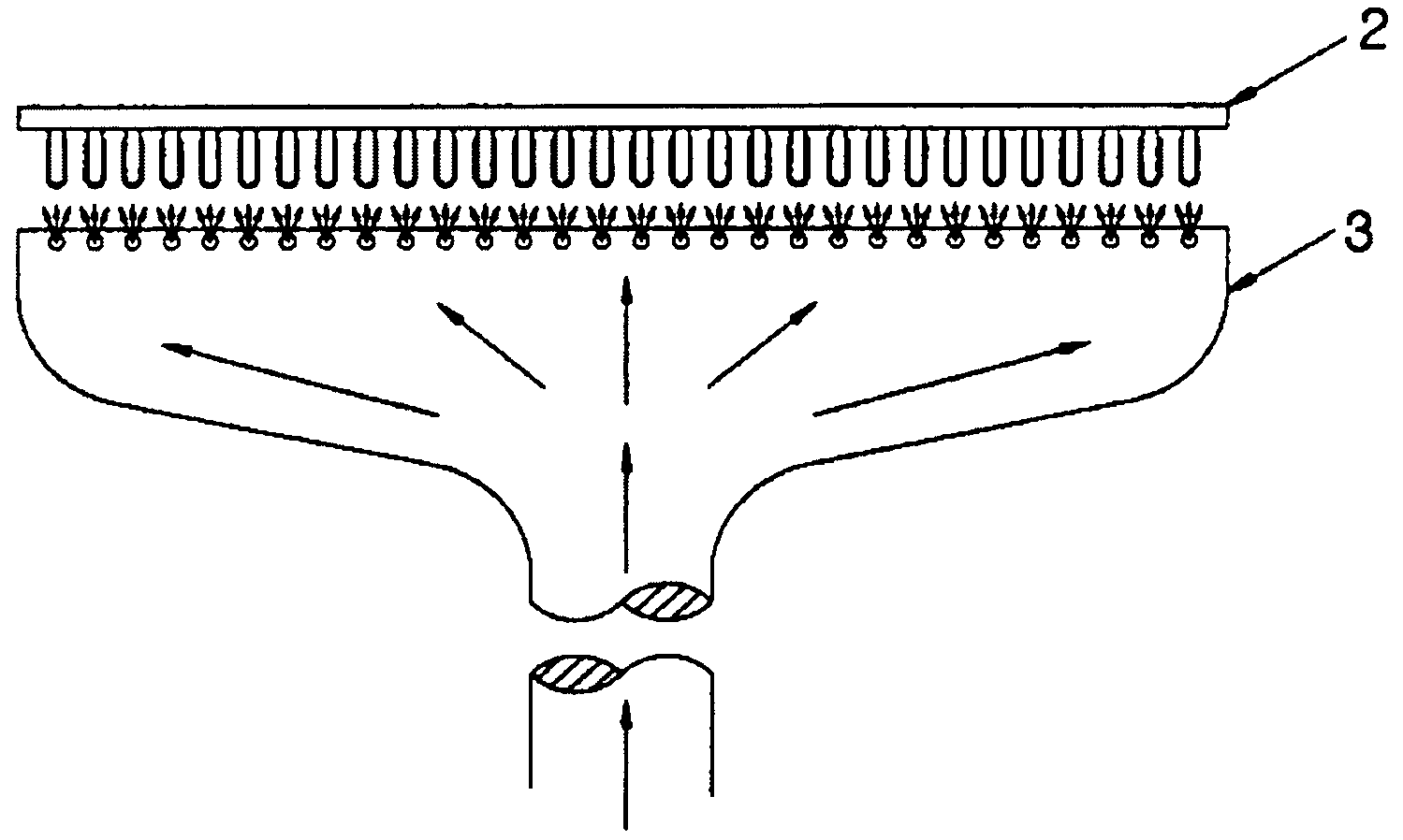
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
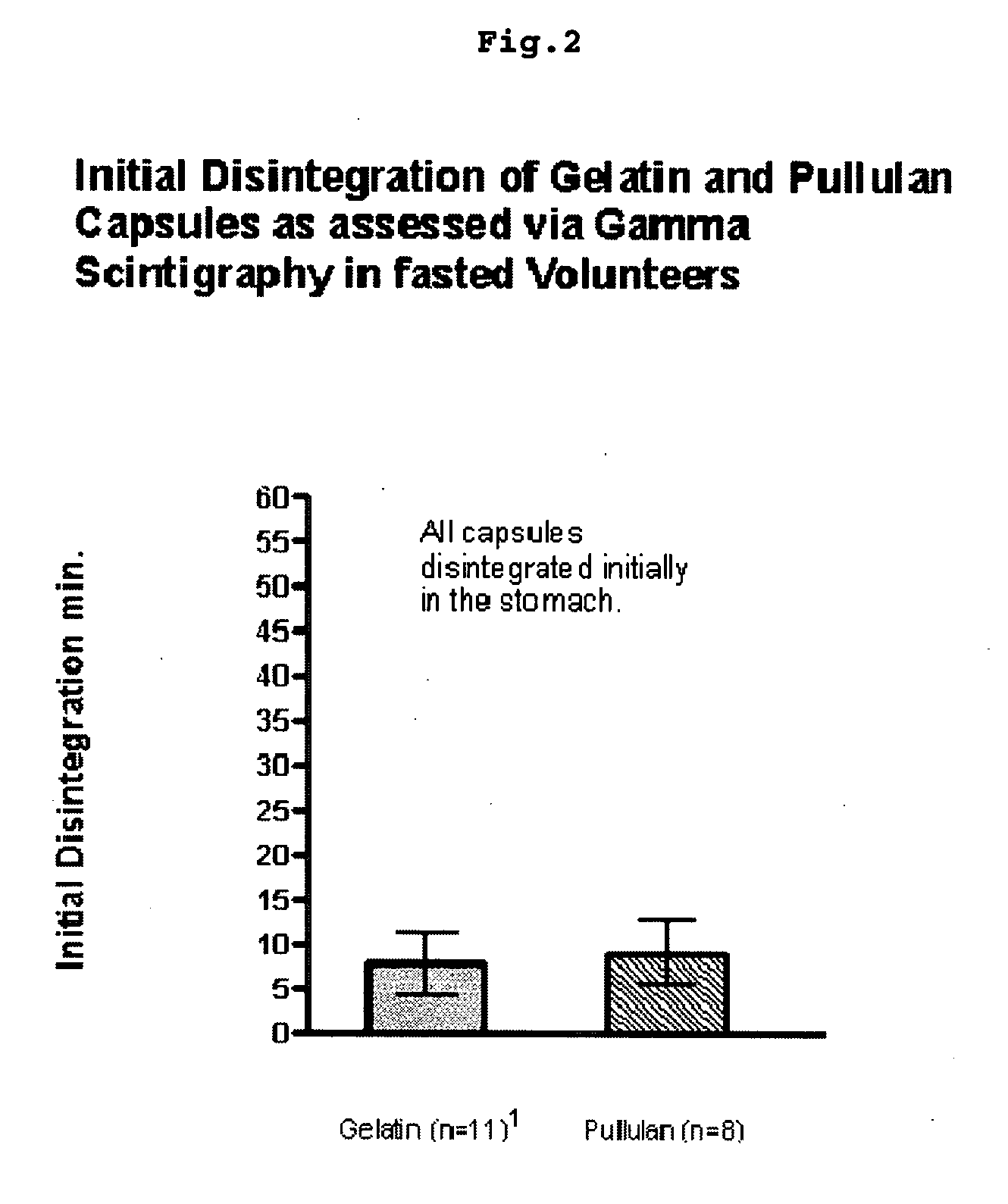
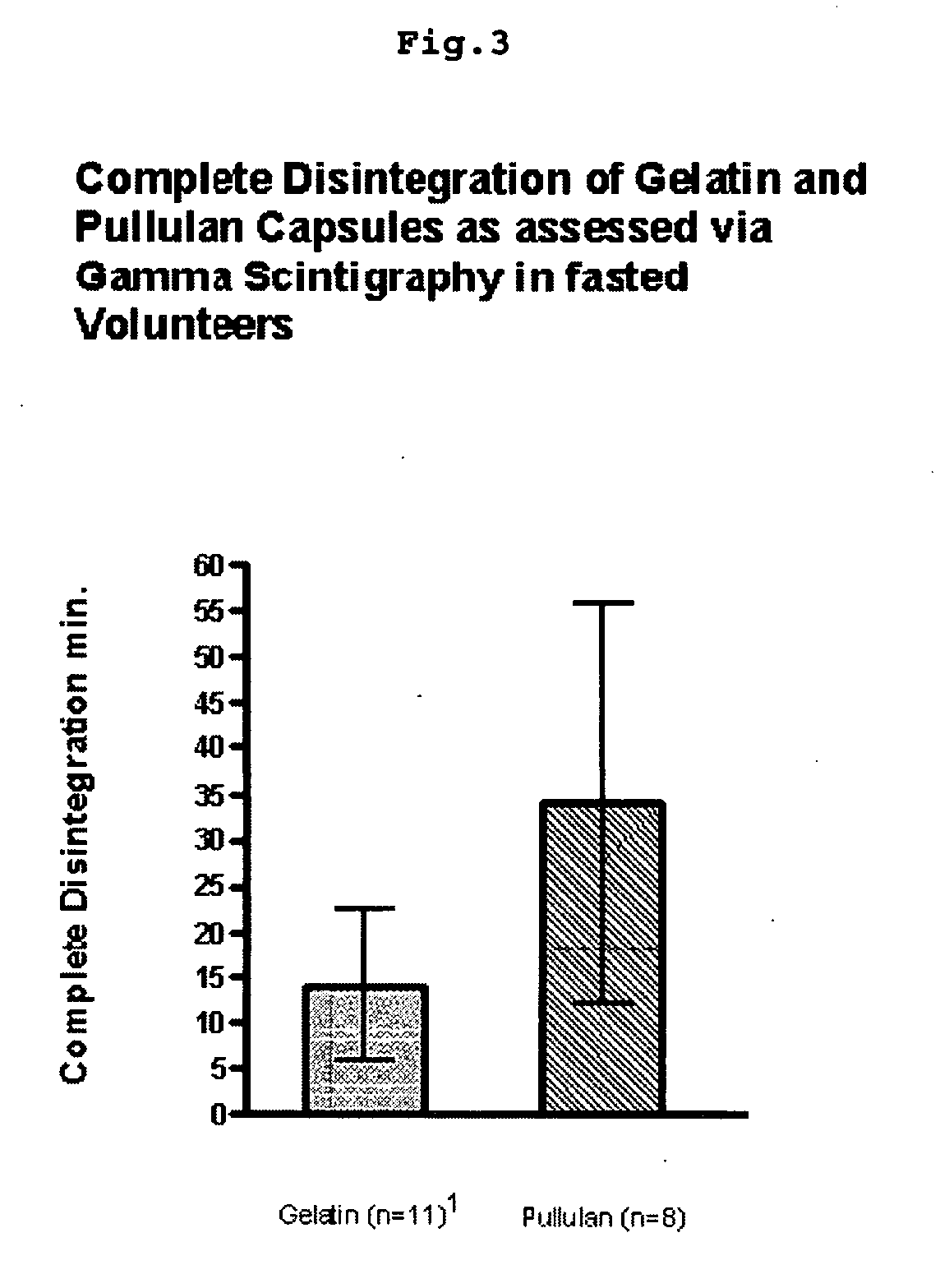
Pullulan capsules
The invention is a hard capsule comprising pullulan in an amount of 85% to 90% by weight, potassium chloride in an amount of 1.0% to 1.5% by weight, carrageenan in an amount of 0.1% to 0.4% by weight, one or more surfactants in an amount of 0.1% to 0.2% by weight and water in an amount of 10% to 15% by weight. Additionally the invention is related to new uses of pullulan containing containers.
Owner:WARNER-LAMBERT CO
Enteric composition for the manufacture of soft capsule wall
ActiveUS20060165778A1Loss of strengthLoss of viscosityOrganic active ingredientsPeptide/protein ingredientsHard CapsuleBiomedical engineering
Owner:PATHEON SOFTGELS INC
Hard capsules
InactiveUS20050196437A1Reduce brittlenessConvenience filmBiocideOrganic active ingredientsPlasticizerHard Capsule
The present invention pertains to a blend of a physically induced starch hydrolysate, a plasticizer, and a gelling agent. Such blend produces an excellent film with low brittleness. Further, essentially gelatin-free hard capsules may be made with such blends.
Owner:NAT STARCH & CHEM INVESTMENT HLDG CORP
Physically/molecularly distributed and/or chemically bound medicaments in empty, hard capsule shells
The present invention incorporates medicaments in the empty hard capsule shells (body and cap). The medicament is either physically / molecularly distributed and / or chemically bound to the polymer matrix of the capsule shell composition. Other medicaments in the form of drug-loaded matrices (powders, granules, beads, pellets, mini-tablets, and mini-capsules) can be filled in the drug-loaded empty, hard capsule shells. The same capsule dosage form contains medicaments in the core matrix and in the shell.
Owner:JOSHI HEMANT N +1
Maca, ginseng and medlar composition and application thereof
ActiveCN102716263AReduce hyperplasiaImprove sexual functionAlcoholic beverage preparationUrinary disorderSexual functionSpermatorrhea
The invention discloses a maca, ginseng and medlar composition and an application thereof. The composition is prepared by mixing the following components in percentage by weight: 30-85% of maca, 10-40% of ginseng and 5-30% of medlar. The composition is added with edible accessory and prepared into tablets, hard capsules, granules, soft capsule, medicinal liquor, oral liquid and beverage. According to the composition disclosed by the invention, the maca, ginseng and medlar are matched and combined according to the functional characteristics thereof; the ginseng has the effects of nourishing and strengthening and tonifying middle and replenishing qi, the maca has the effects of improving sexual function, reducing benign prostatic hyperplasia and improving the fertility, and the medlar has the effects of preventing consumptive disease, essence deficiency, impotence, spermatorrhea and the like; and the three complement one another and realize a synergistic effect in improving the sexual function, reducing benign prostatic hyperplasia and improving the fertility.
Owner:YUNNAN DIANLONG PHARMA
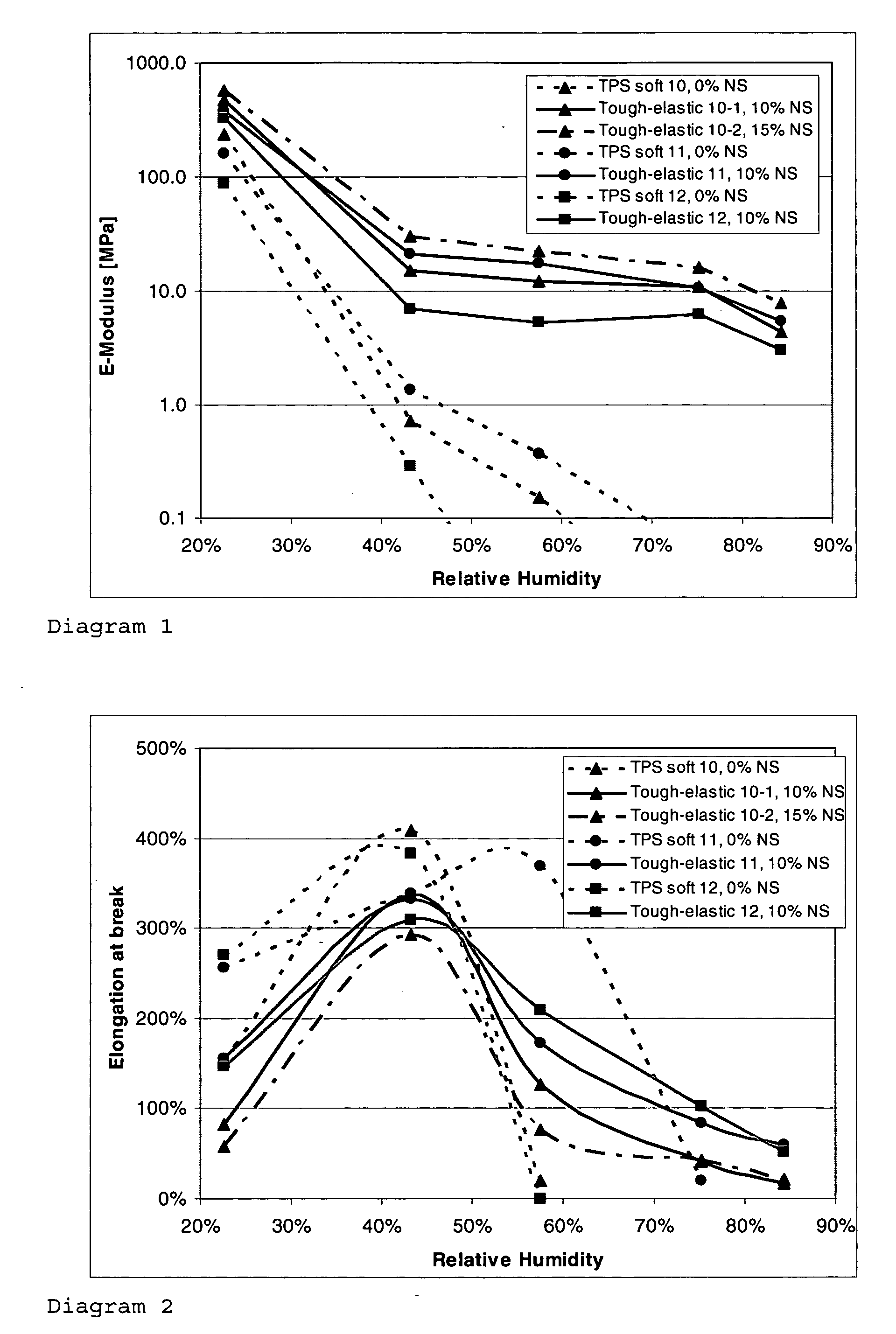
Viscoelastic material
InactiveUS20060004193A1Increase chain lengthSimplify discussionCapsule deliveryHigh humidityHard Capsule
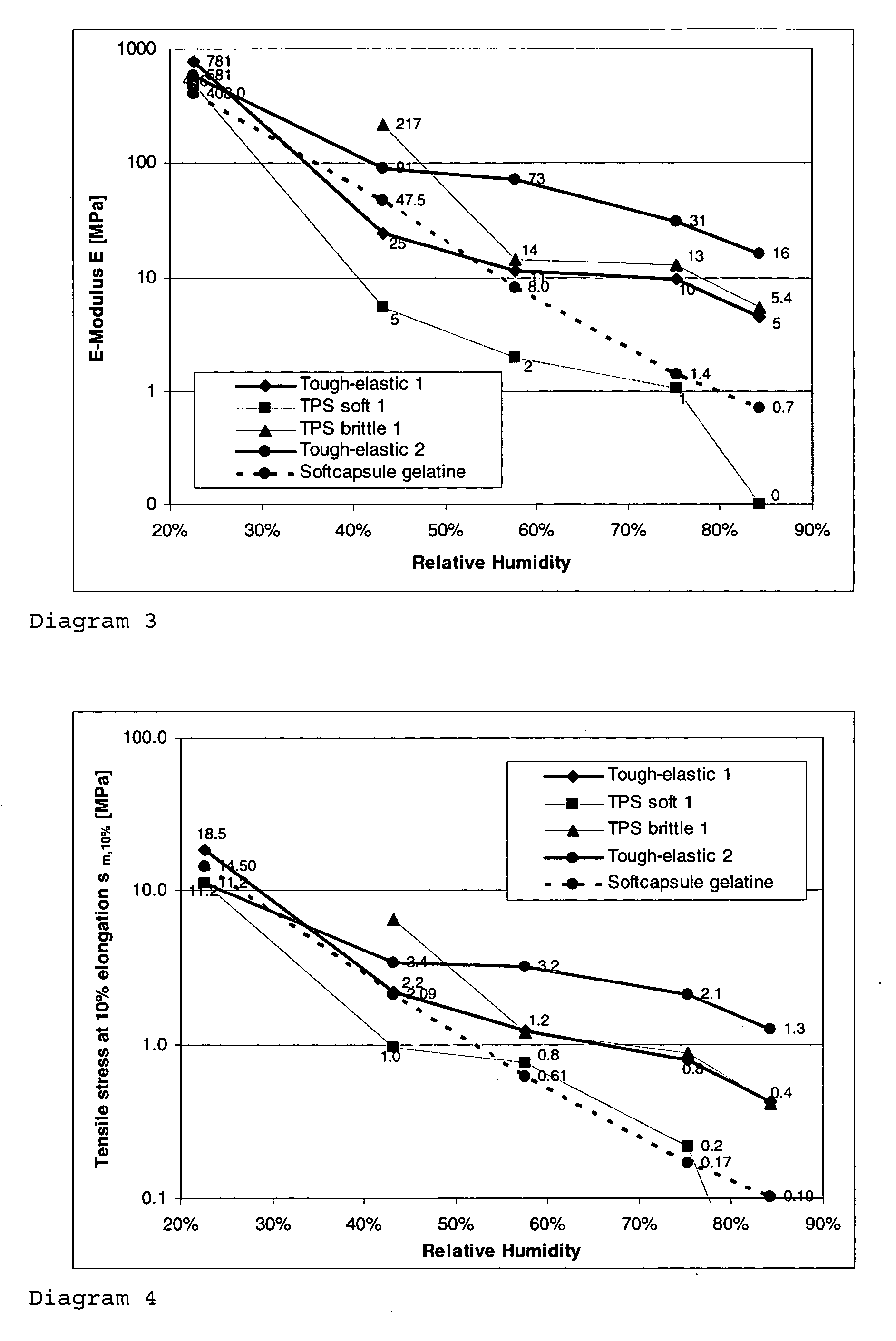
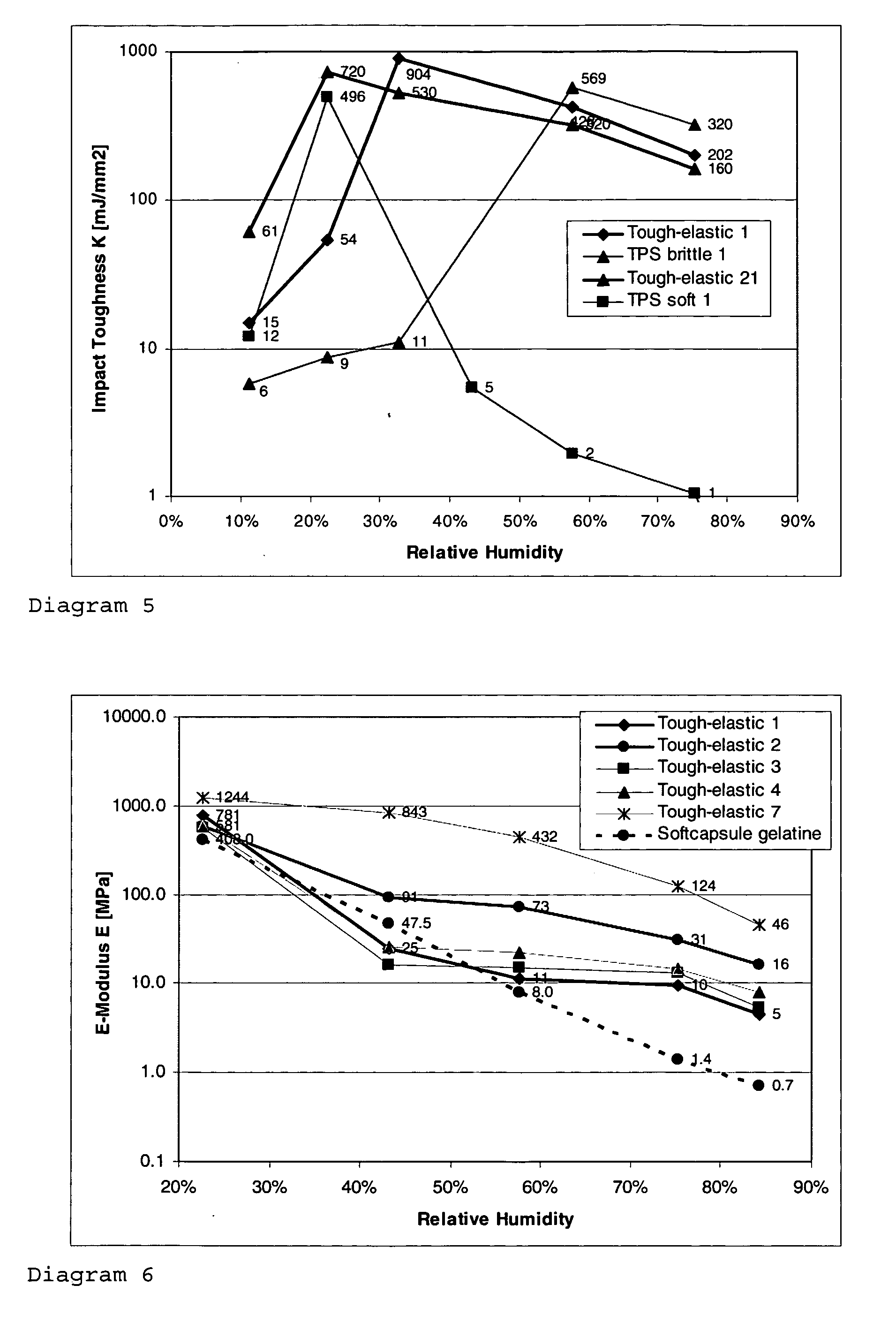
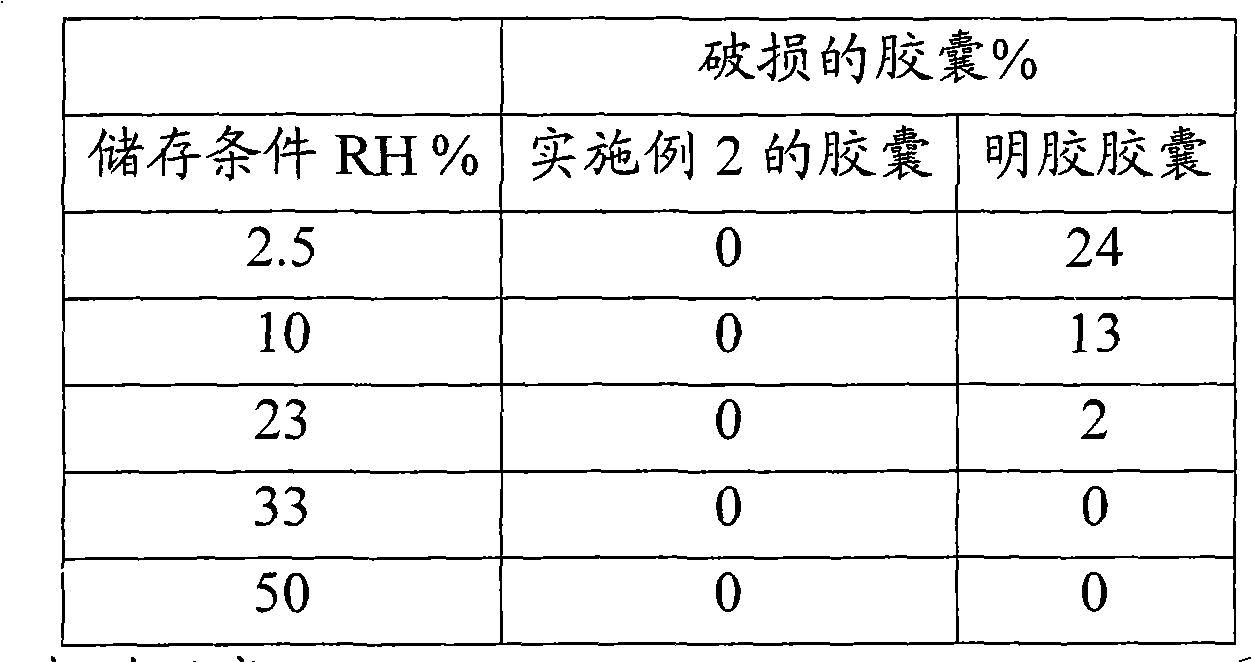
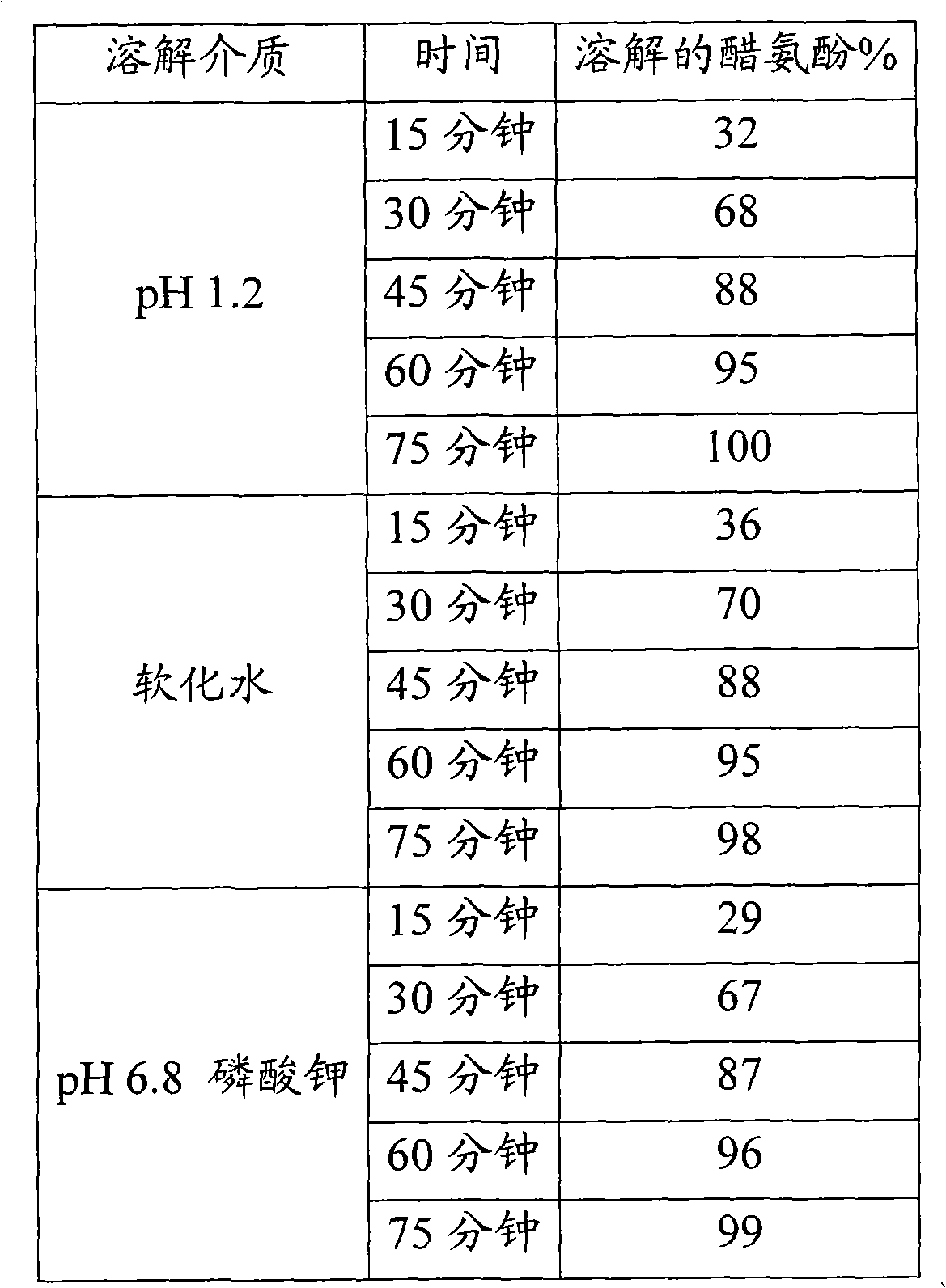
The invention relates to a tough-elastic material based on starch, which on the one hand has high impact toughness at low humidities, and on the other hand still has a high modulus of elasticity at high humidities and has a high elongation capacity in a broad range of humidities and on account of its property profile is suited to use as moulded elements such as for example for foils, films, fibres, injection-moulded articles, in particular as edible film and for the packaging of active ingredients, chemicals, aromas and perfumes as well as high-quality substitution of gelatine in the area of soft and hard capsules. The tough-elastic material can be obtained transparent and adjusted such that it dissolves on swelling in water or respectively disintegrates or remains intact.
Owner:INNOGEL AG
Hard capsule
InactiveUS20050186268A1Improve stabilityMaintain good propertiesDrug compositionsPharmaceutical non-active ingredientsPolymer sciencePolyvinyl alcohol
A method of manufacturing a hard capsule made mainly of a polymer or copolymer obtained includes polymerizing or copolymerizing at least one polymerizable vinyl monomer in the presence of polyvinyl alcohol and / or a derivative thereof. Unlike conventional hard capsules, this hard capsule can be filled with a solvent (e.g., polyethylene glycol) for a sparingly soluble drug ingredient.
Owner:HOSHI NOBORU +2
Vegetative hard capsule casing material and its prodn. method
A vegetative shell of capsule is prepared from hydrophilic vegetative gel, adhesive, plasticizer and water. Its preparing process is also disclosed.
Owner:上海慧源植物胶囊股份有限公司
Hydroxypropyl methyl cellulose hard capsules and process of manufacture
ActiveCN101595133ASolubility effectCost effectivePowder deliveryCapsule deliveryPolymer scienceHard Capsule
A composition for manufacture of hard hydroxypropyl methyl cellulose capsules comprising a film forming material of hydroxypropyl methyl cellulose having a methoxy content of 27.0-30.0% (w / w), and a hydroxypropoxy content of 4.0 - 7.5% and as a 2% weight solution, a viscosity of 3.5 - 6.0 cPs at 20 DEG C, dipping compositions, process for manufacture of hard hydroxypropyl methyl cellulose capsules according to a dip coating process and hard capsule shells.
Owner:CAPSUGEL BELGIUM NV
Single flower selfing isolation method for small chili
The invention belongs to the technical field of plant breeding, and in particular relates to a single flower selfing isolation method for small chili. The method comprises the following steps: (1) selecting an excellent single plant, namely in the flowering period, selecting the excellent single plant according to a breeding objective, and marking; (2) preparing a capsule shell, namely separating a shell cap from a shell body of a hollow hard capsule shell; (3) selecting a flower bud, namely selecting a large flower bud which is expected to bloom in the next day; (4) treating the flower bud, namely holding the flower bud to be treated by one hand, holding the capsule shell, which is matched with the flower bud in size, by the other hand, slightly sleeving the flower bud with the lower opening of the capsule shell over the widest part of the flower bud, and marking a flower stalk; (5) managing after the treatment in a method which is the same as a conventional field management method for the production of small chili. The capsule shell used in the method is low in cost and does not hurt the flower bud and the flower stalk when being used, the operation is convenient and easy, and the work efficiency is high; moreover, the capsule shell with certain elasticity and permeability does not influence the cracking of anther and pollination, and thus the selfing setting rate of the small chili is high.
Owner:NANTONG SCI & TECH VOCATIONAL COLLEGE
Delivery device, method of using and method of manufacturing
An active principle delivery device (1) comprising an inner capsule (4) within an outer capsule (2), the inner and outer capsules (4,2) containing the same active principle (5,3), with at least the outer capsule (2) being a hard capsule and the active principle (3,5) in at least one of the capsules (2,4), comprising a fluid. Also provided is a method of fabricating such a delivery device (1), as well as a method of controlling the pharmaco-kinetic profile of an active principle.
Owner:MW ENCAP
Health-care food with functions of improving skin moisture and reinforcing immunity
The invention discloses a health-care food with functions of improving skin moisture and reinforcing immunity. The health-care food comprises the following components in percentage by weight: 1.0 to 80.0 percent of collagen peptide powder, 0.1 to 40.0 percent of glucosamine, 0.1 to 30.0 percent of aloe extract, 0.1 to 50.0 percent of vitamin C (ascorbic acid), and 0.0 to 90.0 percent of auxiliarymaterials. The health-care food can be made into tablets, powder, particles, hard capsules, soft capsules, oral liquids and other oral preparations with various flavors. By virtue of a scientific formula, the raw materials with exact efficacies are compatible reasonably, the collagen peptide powder, the glucosamine, the aloe extract and the vitamin C (ascorbic acid) are selected as main raw materials, and the corresponding auxiliary materials can be selected according to different oral preparations. Furthermore, the selection, purchase, preparation and other aspects of the raw materials are completely operated according to related regulations of health-care foods, so the advancement and stability of the product are ensured; and the health-care food is safe, efficient and stable, and has controllable quality by adopting a modern preparation process matched with a strict quality control method. Toxicological safety tests and function tests prove that the health-care food can be used foreffectively improving the human skin moisture and reinforcing the human body immunity.
Owner:PERFECT CHINA +1
Enteric composition for the manufacture of soft capsule wall
ActiveUS8685445B2Loss of strengthLoss of viscosityOrganic active ingredientsPeptide/protein ingredientsHard CapsuleBiomedical engineering
Owner:PATHEON SOFTGELS INC
Pullulan polysaccharide hollow hard capasule and its preparing method
InactiveCN101069677ASmall range of molecular weight variationProduct quality is easy to controlPharmaceutical non-active ingredientsCapsule deliveryPullulanHard Capsule
The present invention discloses a hollow hard capsule and its preparation method. It is made up by adopting prolanpolysaccharide as main body material and adding gelling agent, coagulant aids, surfactant and humectant through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:广东强基药业有限公司
Health food with function of relieving visual fatigue and preparation method thereof
The invention relates to a health food with a function of relieving visual fatigue and a preparation method thereof, belonging to the technical field of health foods. The health food with the function of relieving visual fatigue is characterized in that active ingredients and additives are used for preparing the health food into soft capsules, hard capsules, oral liquid or tablets, wherein the active ingredients comprise the following raw materials: flaxseed oil or perilla oil, beta-carotene or vitamin A, lutein, zeaxanthin, vitamin E, ginkgo leaf extract and grape pip extract or cowberry extract; when the health food is prepared into the soft capsules, an emulsifying agent is used as the additive; when the health food is prepared into the hard capsules, a diluting agent is used as the additive; when the health food is prepared into oral liquid, a preservative, a flavoring agent, an emulsifying agent and water are used as the additives; and when the health food is prepared into the tablets, a diluting agent and a disintegrating agent are used as the additives. The health food prepared by the invention has the function of relieving visual fatigue.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
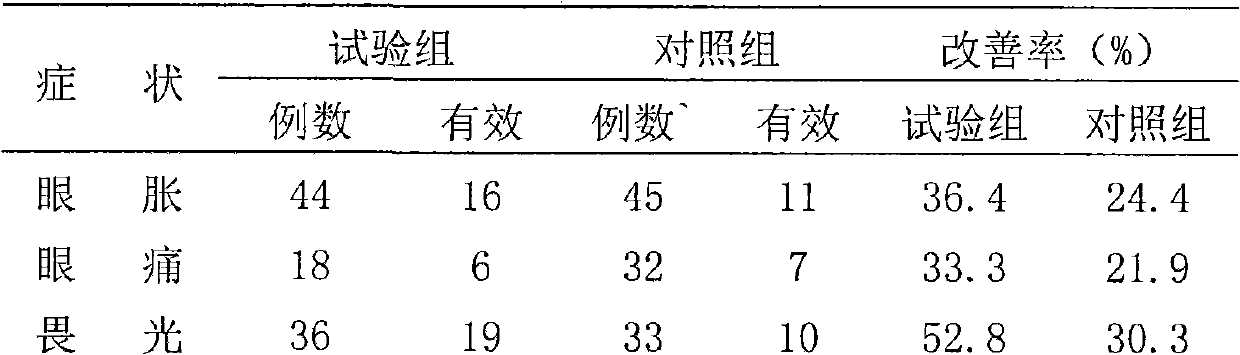
Hard Capsules
Publicly available pullulan hard capsule shells present certain drawbacks, notably a non fully satisfactory shell mechanical strength (i.e. shell brittleness) at shell low LOD. Improving this property is a particularly desirable goal for pullulan hard capsule shells. The present invention solves this and other objects by providing new hard pullulan capsule shells and capsules comprising (I) moisture, (II) a mono-, di-, and oligosaccharides free pullulan and (III) a setting system. Also provided are an aqueous composition and a dip-molding manufacturing method for the manufacture of such shells and capsules.
Owner:CAPSUGEL BELGIUM NV
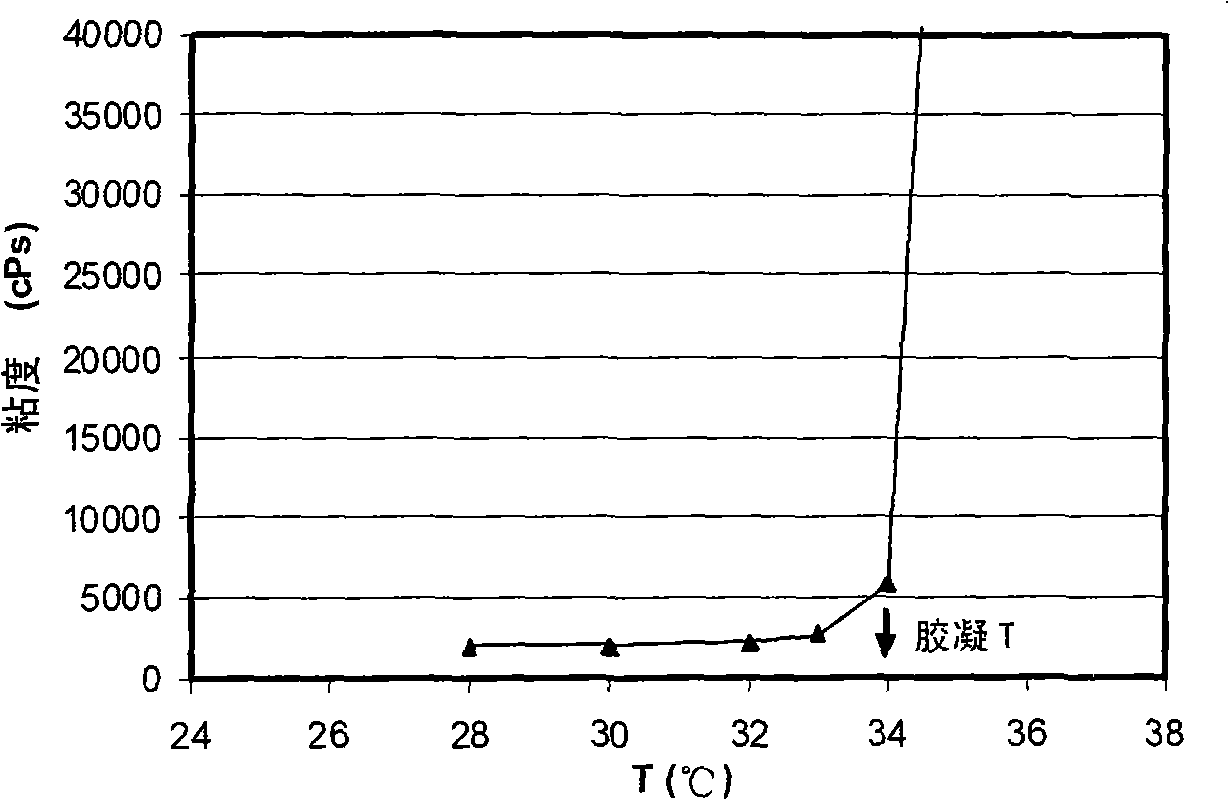
Homogeneous, thermoreversible gel containing reduced viscosity carrageenan and products made therefrom
The present invention is directed to a homogeneous, thermoreversible gel comprising carrageenan wherein the carrageenan has a viscosity of less than 10 cP at 75° C. when measured in a 0.10 molar aqueous sodium chloride solution containing 1.5% by weight of the carrageenan based on the weight of all components in the solution, and optionally at least one of a plasticizer, a second film former, a bulking agent, and a pH controlling agent, wherein the gel has a solids content of at least 40%. The present invention is also directed to processes for the preparation thereof, as well as to variety of products containing the gel including edible products, soft capsules, hard capsules and solid forms encapsulating powders, tablets, caplets, etc.
Owner:FMC CORP
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Triple combination release multi-layered tablet
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
Film-forming composition suitable for hard capsules and method for preparing the same
The present invention relates to a film-forming composition suitable for hard capsules, and a preparation method thereof. More particularly, the invention relates to a film-forming composition for hard capsules, comprising 7-12% by weight of starch, 1-6% by weight of a plasticizer, 0.7-3% by weight of a gelling agent, and 79-91.3% by weight of water, and a preparation method thereof. Also, the invention relates to films and hard capsules comprising the composition. The inventive composition, and the hard capsules and films comprising the same, will be useful in various industrial fields, including pharmaceutical field and food field.
Owner:CHA YOUNG SOO +1
Process for preparing gelatin from enzyme degradation bone collagen
InactiveCN1473901AShorten the production cycleReduce water consumptionGlue/gelatin preparationHard CapsuleGranularity
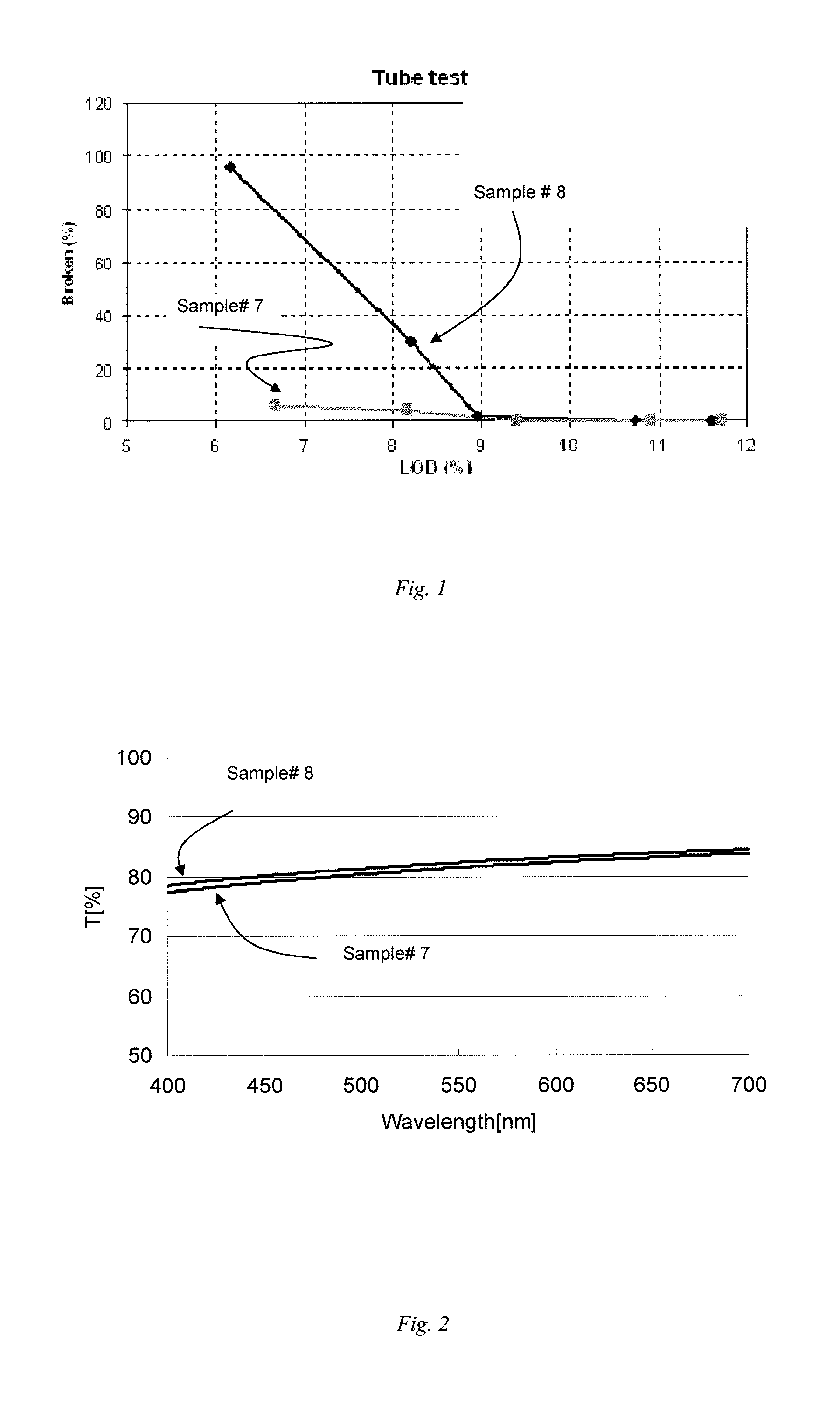
The present invention relates to gelatin preparation, and is especially the enzyme degradation process of preparing gelatin with bone collagen. The process includes milling the mixing of defatted bone material and water into bone slurry with bone size of 1-5 mm; regulating pH value to 1.5-4 with acid or to 7-8 with alkali and adding acid or alkali proteinase in 0.2-0.8 wt% of bone slurry to control the degradation of collagen; reaction at room temperature for 7-10 hr, regulating pH 5.0-6.5 with alkali or acid and heating to 70-85 deg.c for extraction; separating the turbid gelatin obtained via extraction; filtering gelatin liquid to obtain high purity hard capsule gelatin solution. The dried gelatin has molecular weight distribution of alpha=48 % and beta=18 %; gelatin jelly strength over 240 g; viscosity 3.0 mPs; transparency 90 % at 620 nm and 71 % at 450 nm.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Composition for preparing plant hollow hard capsule and preparation method of capsule
ActiveCN101167705AHigh glossImprove toughnessInorganic non-active ingredientsCapsule deliveryHard CapsulePlasticizer
The invention relates to a composition for preparing plant hollow hard capsules and a preparation method of the capsules. In parts by weight, the composition includes: 2-10 parts of coagulant, 40-60 parts of filler, 1-20 parts of plasticizer, 2-15 parts of defoamer and 10-100 parts of water. The composition may also include 0.02-1 parts by weight of coagulant aid and / or 1-5 parts by weight of dispersant. The present invention also relates to a preparation process for preparing a plant-derived hard hollow capsule from the above-mentioned composition for preparing a plant-derived hard hollow capsule. Compared with the prior art, the advantages of the formula and production process of the present invention are that the transparency of the product and the glossiness of the capsule surface are also increased, so that the capsules made are more aesthetically pleasing; the capsules obtained by the present invention have good stability and can Adapts well to changes in temperature and humidity.
Owner:北京长征天民高科技有限公司
Hard capsule
InactiveUS20060153909A1Impair stability of drugImpair propertyOrganic active ingredientsBiocideWater activityHard Capsule
Owner:WAKUNAGA PHARMA CO LTD
Band-Seals For Hard Capsules
InactiveUS20080008750A1Low production costShorten production timeN-vinyl-pyrrolidone polymer adhesivesCapsule deliveryPullulanMedicine
The present invention has as its object to provide a band-seal to manufacture a leak-tight, stable hard capsule preparation not being susceptible to deformation after having filled pharmaceuticals, foods, healthy foods, etc. in a hard capsule comprising a polyvinyl alcohol copolymer or pullulan as a base, and in more particular, relates to a band-seal intended for use in the hard capsule comprising a polyvinyl alcohol copolymer or pullulan as a base, characterized in that the band-seal is incorporated with at least one or not less than two of the below-described (a) to (c): (a) A polyvinylpyrrolidone with a molecular weight of 100,000 to 4,000,000, (b) A polyvinylpyrrolidone with a molecular weight of 10,000 to 80,000 in the proportion of not more than 90% by weight against the total weight of the band-seal; and (c) A copolymer from 1-vinyl-2-pyrrolidone and vinyl acetate.
Owner:QUALICAPS CO LTD
Cellulose hard capsule enhancing mechanical film strength
InactiveUS20080134937A1Enhancing mechanical film strengthCapsule deliveryCellulose adhesivesAcetic acidCellulose
This invention provides a process for preparing a cellulose capsule enhancing mechanical film strength comprising the steps of: i) preparing 100 wt part of an aqueous solution containing 18˜21 wt part of solubilized cellulose; ii) adding 0.1˜0.5 wt part of sucrose fatty acid ester, 0.05˜0.3 wt part of potassium pyrophosphate and 0.01˜0.2 wt part of glacial acetic acid to 100 wt part of an aqueous solution of solubilized cellulose; iii) adding a mixed solution of 0.1˜1.0 wt part of iota-carageenan and 0.02˜0.5 wt part of agar to the resulting admixutre; and iv) allowing obtained product to stand, adjusting its viscosity and forming a capsule from it, wherein said forming step comprises i) dipping the mold pin into the obtained cellulose mixture at the dipping pan, ii) molding the film of the cellulose capsule, iii) cooling said film of the cellulose capsule using 15˜18° C. cooling air for 10˜20 seconds at the bottom film cooling device, iv) cooling said film of the cellulose capsule again using 15˜18° C. cooling air for 70˜100 seconds at the upper film cooling device in order to control the flow of film.
Owner:SUHEUNG CAPSULE CO LTD
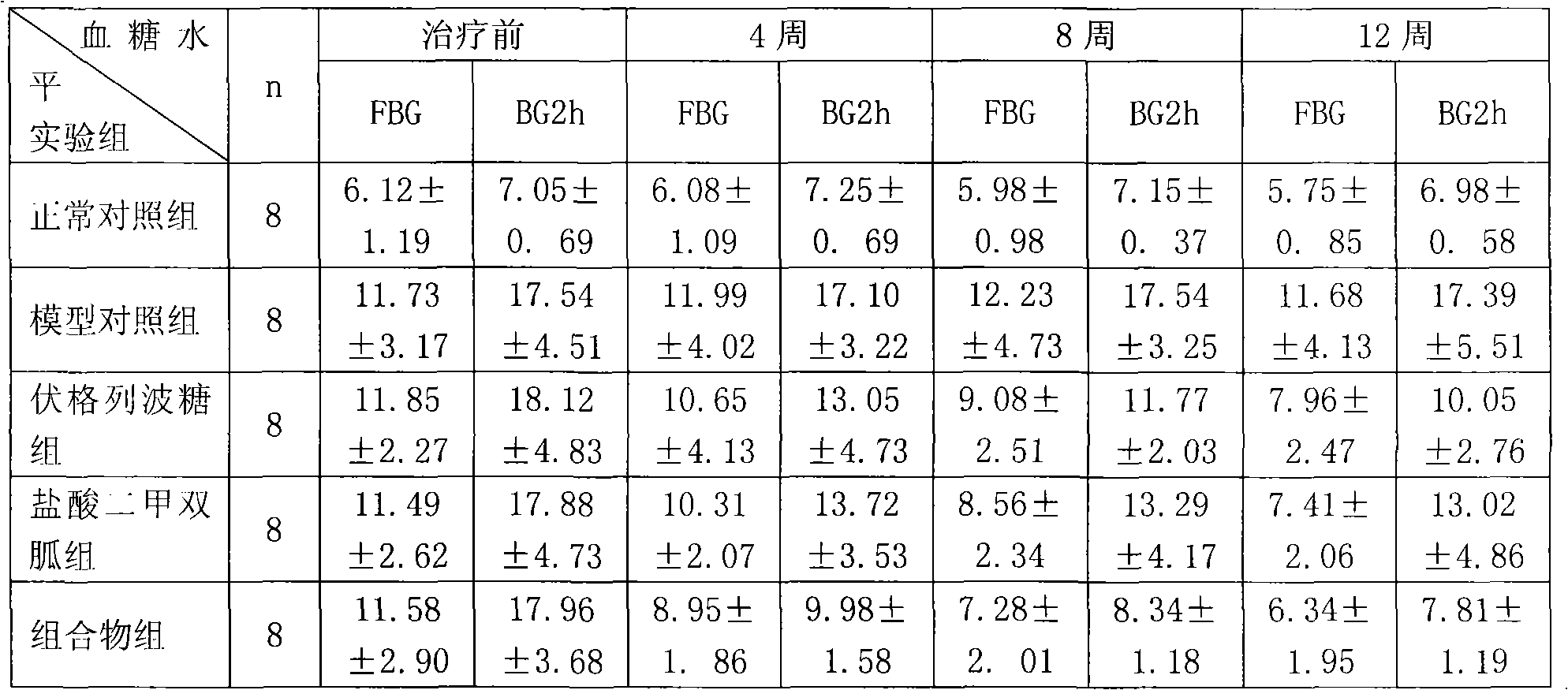
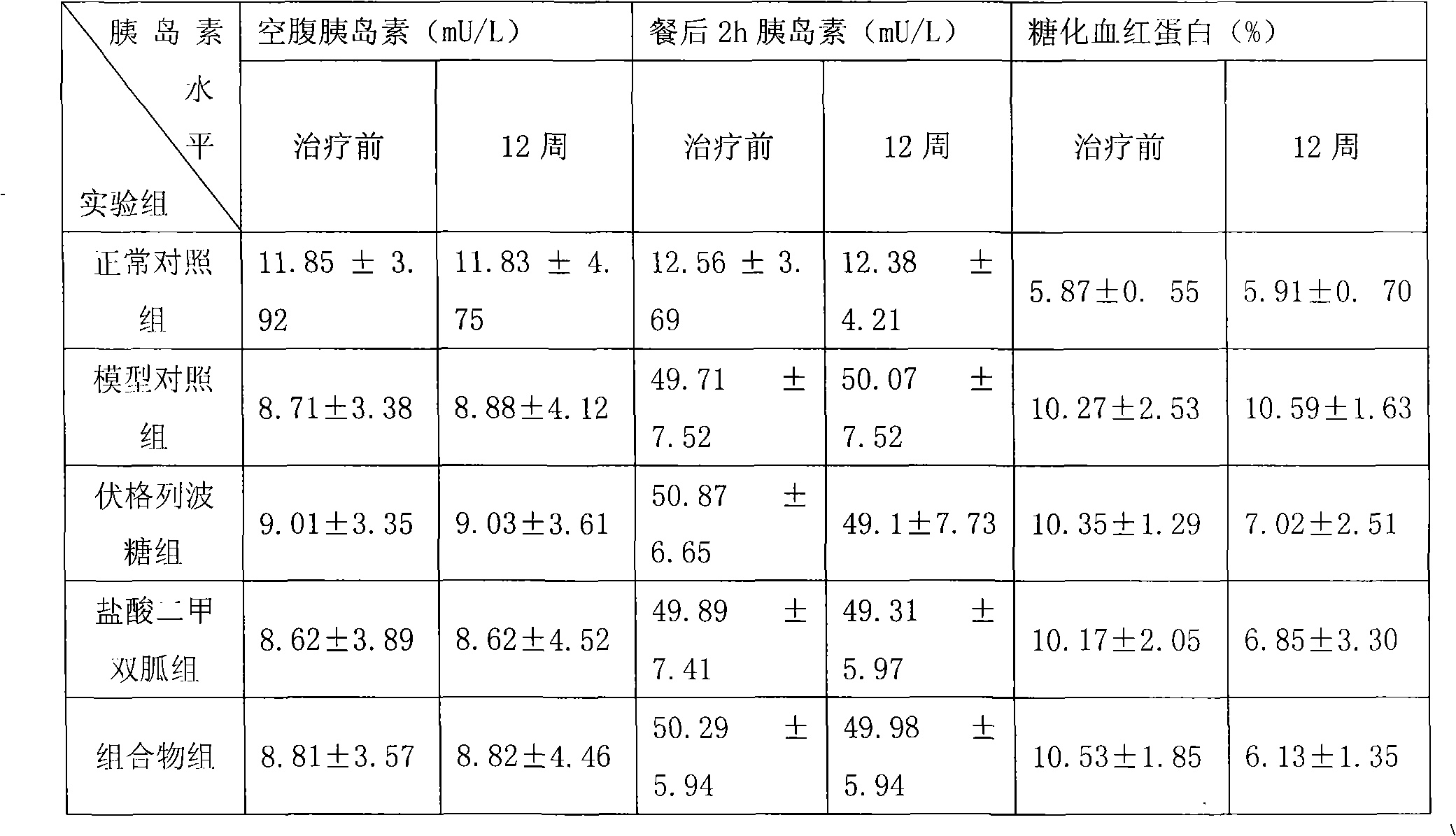
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
Coated pharmaceutical capsule dosage form
InactiveUS20100291201A1Improve oral bioavailabilityImprove solubilityBiocideNervous disorderAdditive ingredientCapsule Dosage Form
Owner:CEROVENE
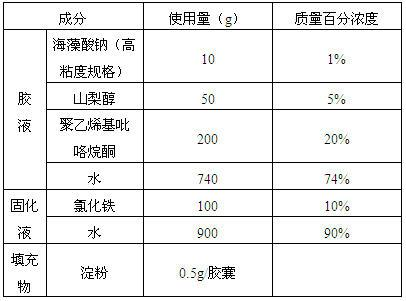
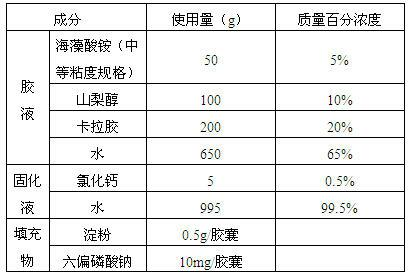
Alginate hard capsule disintegrable at different positions in gastrointestinal tract
ActiveCN102657869AGood heat and humidity stabilityEasy to preparePharmaceutical non-active ingredientsCapsule deliveryHard CapsuleMedicine
Disclosed is an alginate hard capsule disintegrable at different positions in gastrointestinal tract. Processing steps include preparing glue solution, glue solution dipping, solidifying, drying, drawing out, cutting, fastening, optical testing and sterilizing of glue solution and capsule filling. The glue solution is prepared by dissolving univalent alginate, plasticizer and pore former in water, wherein the pore former is a polymer film-forming material, glycerin and sorbitol serve as the plasticizer; the solidifying is that dipped gel solution is made into alginate gel capsule by means of ionic cross-linking or acidulating, wherein solidifying solution is multivalent metal ion water solution or acid solution; and finally active ingredients and disintegrating accelerator are filled in hard capsule shells to obtain finished products, wherein the disintegrating accelerator is a substance which can be in precipitation reaction or complexation with multivalent metal ions. The alginate hard capsule is widely applied to different disintegrating environments in the gastrointestinal tract and disintegrable in vivo at different speeds to release the active ingredients and has the advantages of high hydrothermal stability, simple preparation method and low cost.
Owner:南京健辉生物科技有限公司
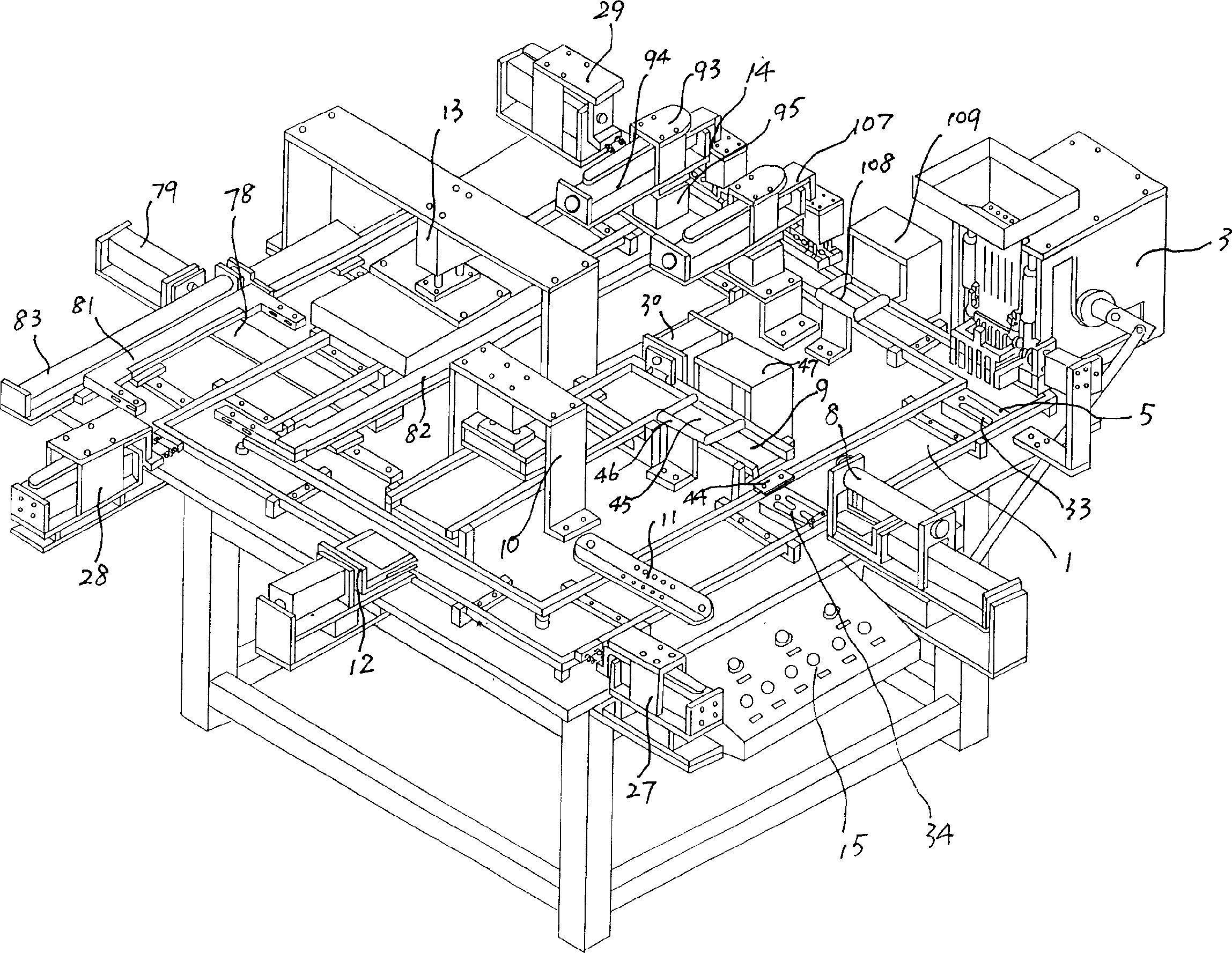
Full automatic hard gelatin capsule liquid and soft-body filler
ActiveCN1607161ALow quality stability requirementsIncrease productivityCapsule deliveryLiquid materialHard CapsuleSoft materials
A hard capsule filling machine for low or intermediate viscosity liquid and soft material consists of the first and second rail, pouring apparatus, gluing device for gluing inner joining area of capsule cap, negative pressure sleeve join device, upper and lower mold block circular moving in rail, every join mold can hold multiple capsule simultaneously, capsule can be joined under vacuum condition. Said invention has higher productivity and yield. When defect capsule occurred and contaminated mold, the cleaned or new mold can be replaced at any position in the first rail.
Owner:广东强基药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com