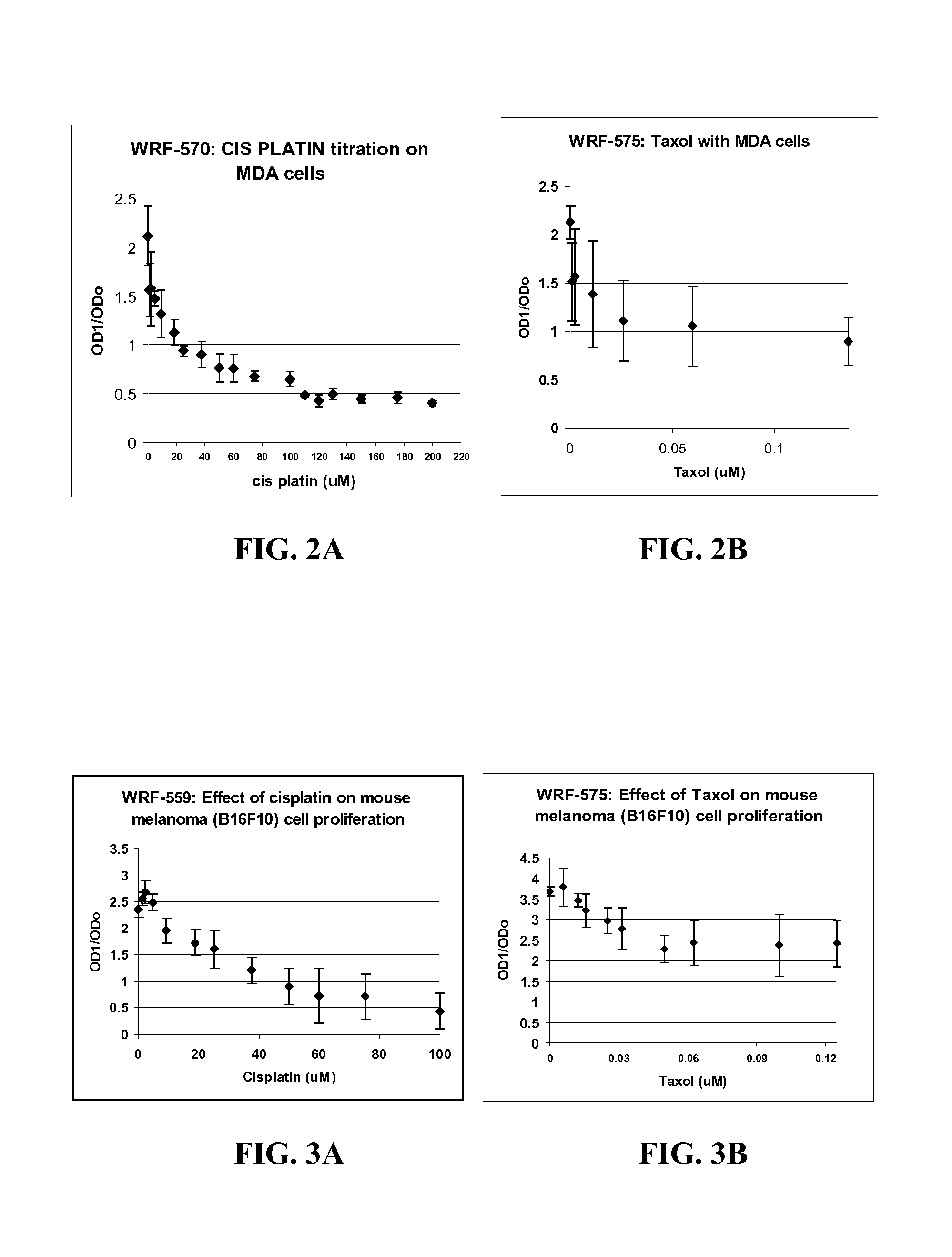
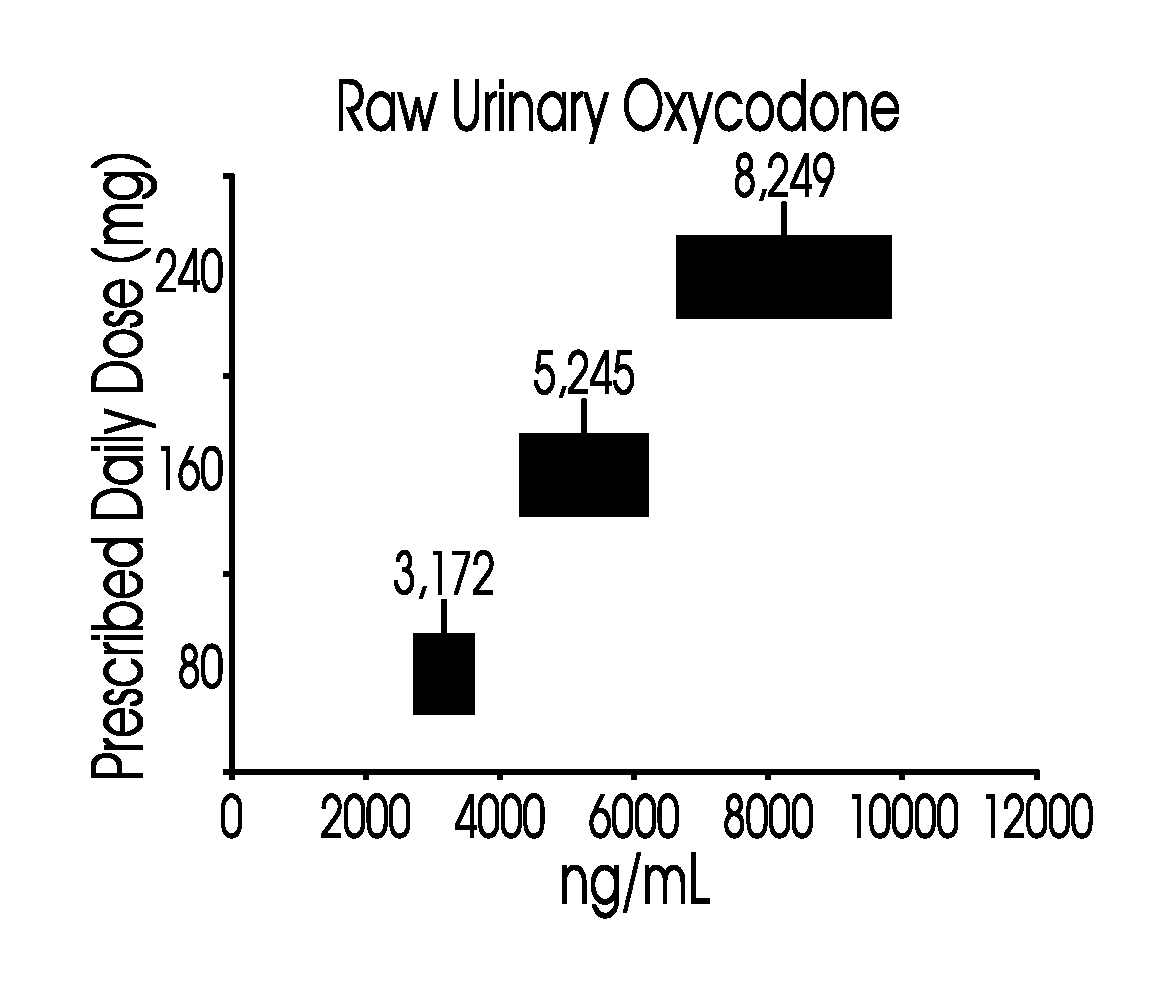
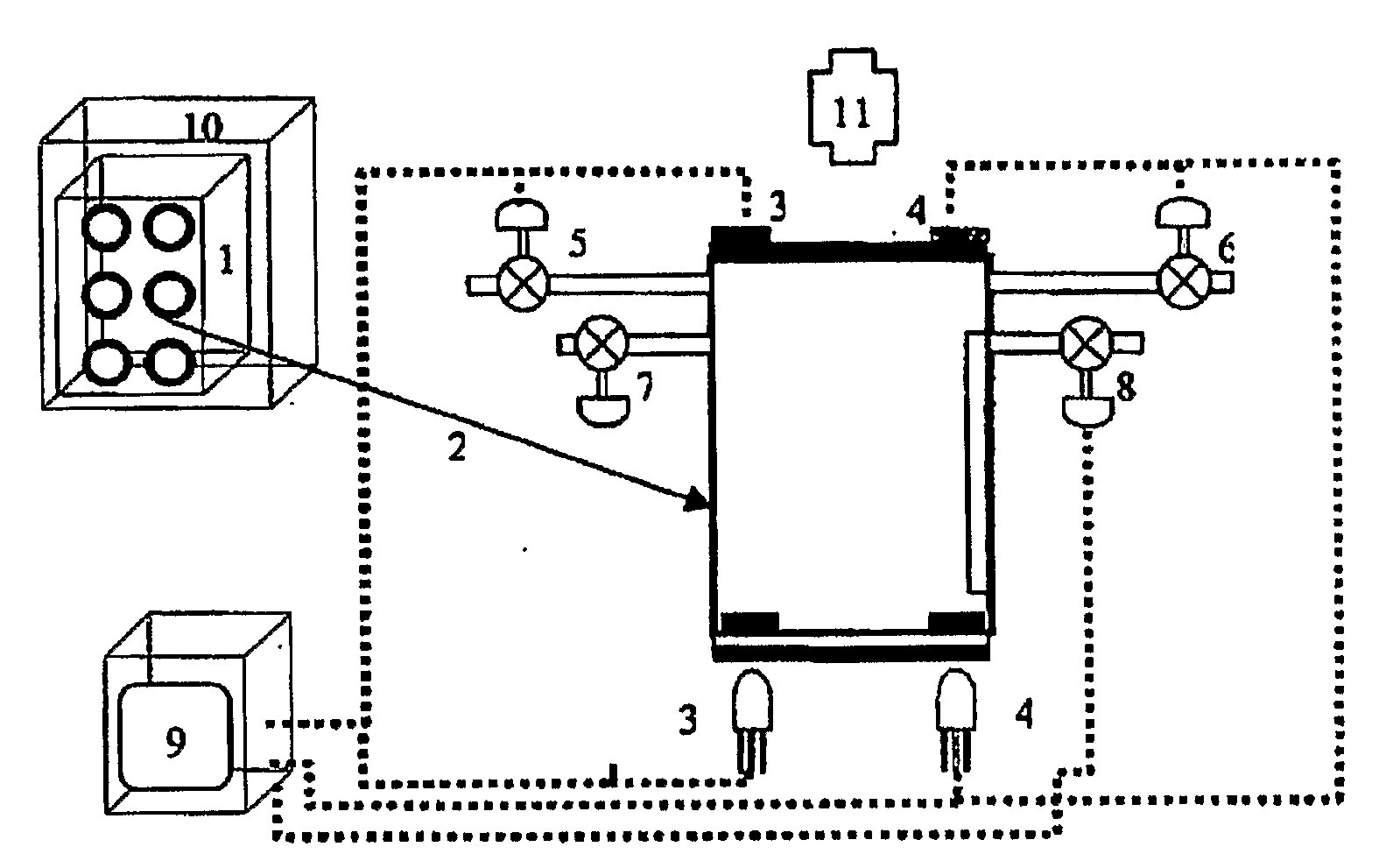
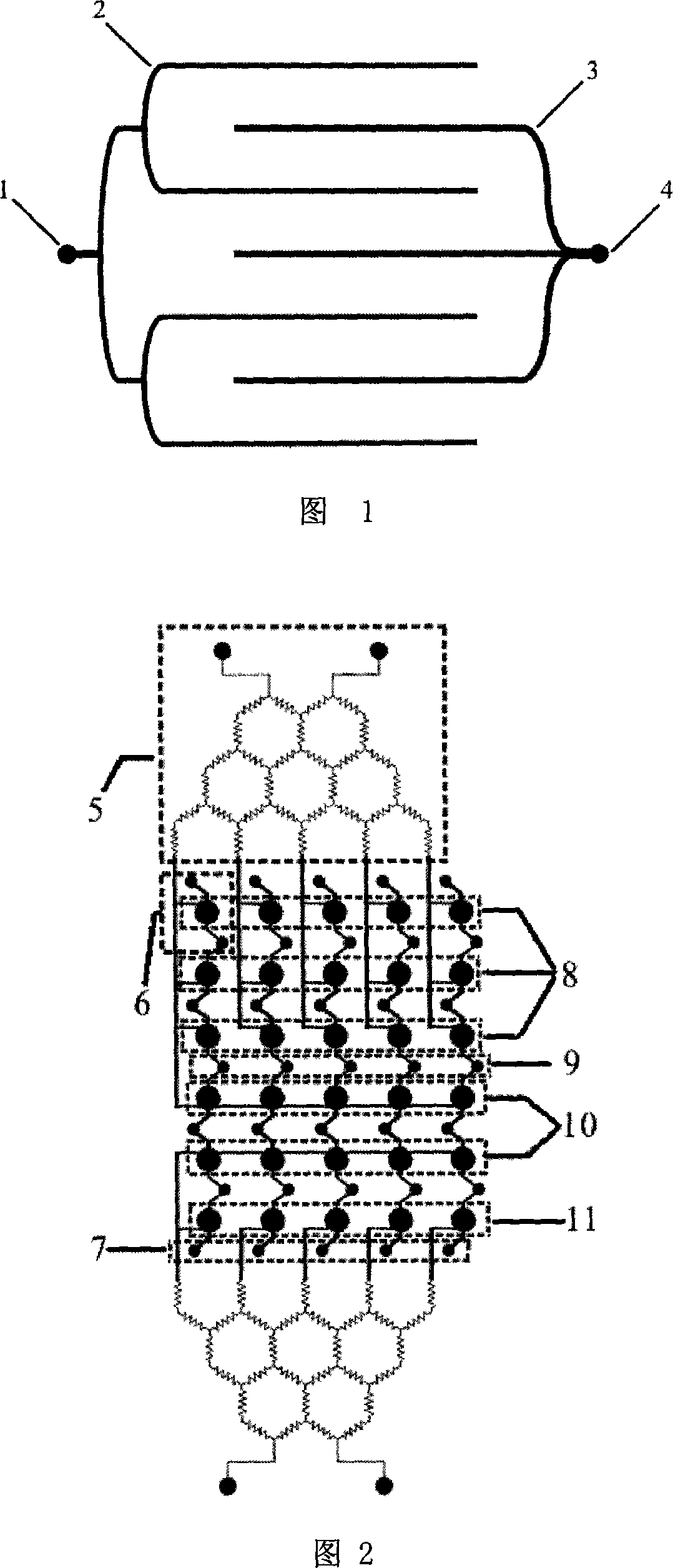
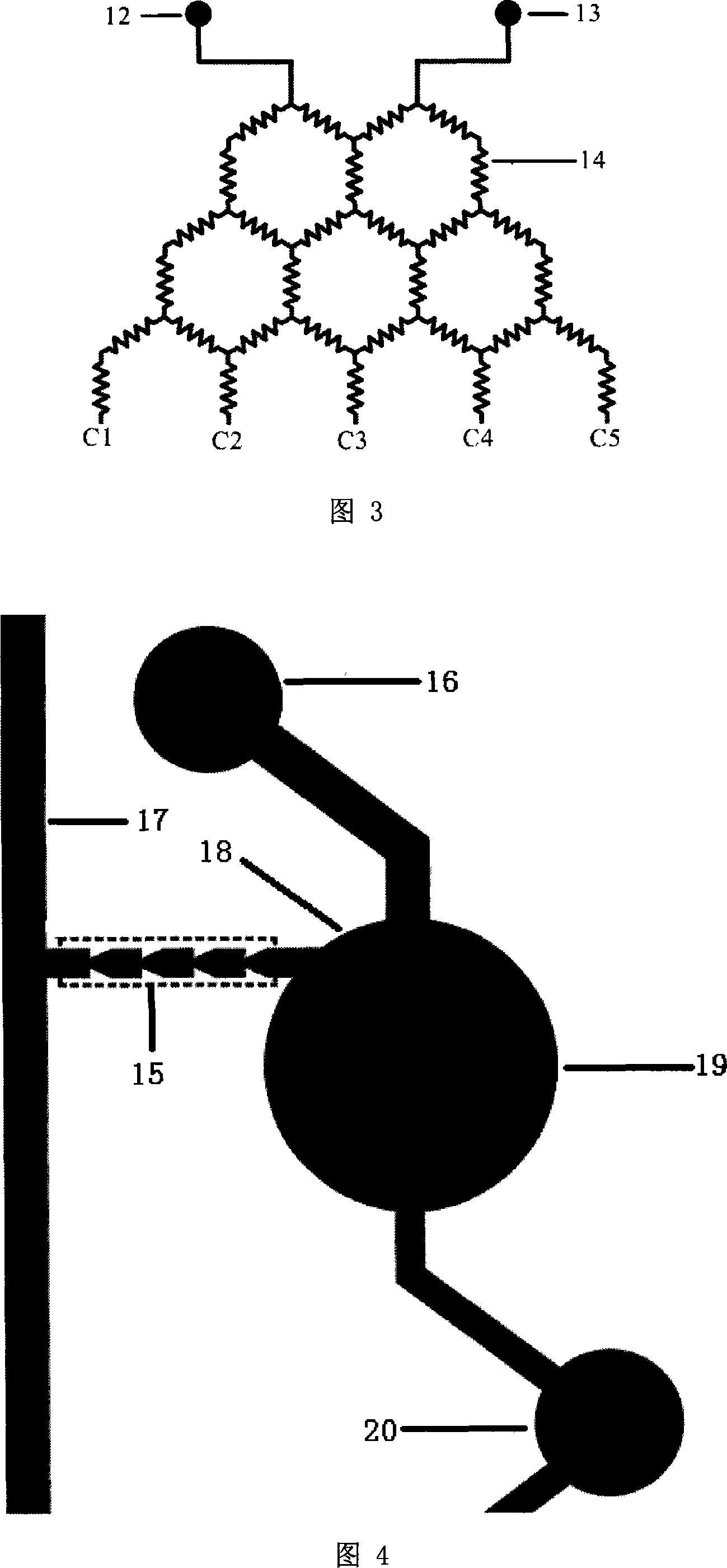
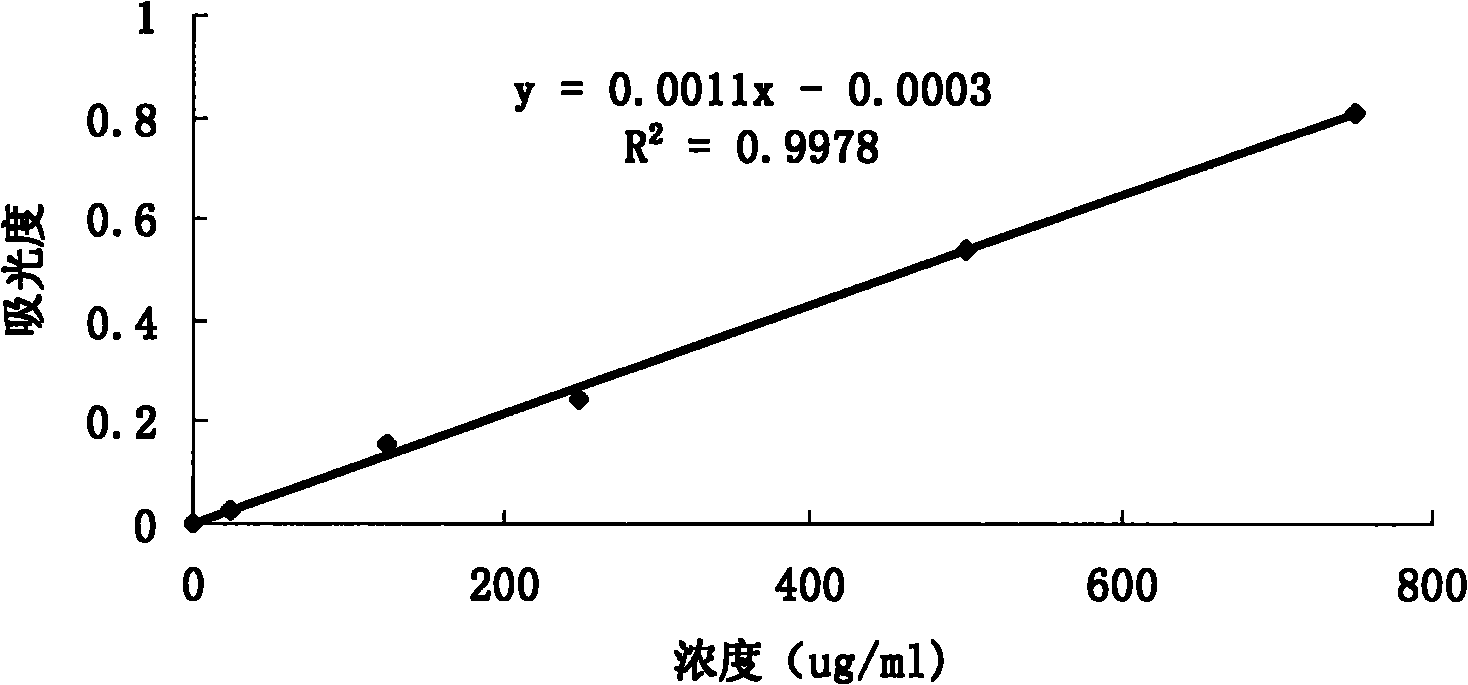
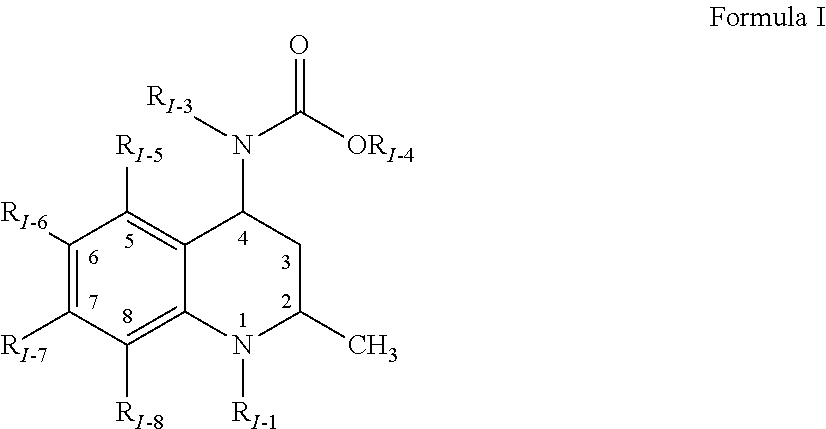
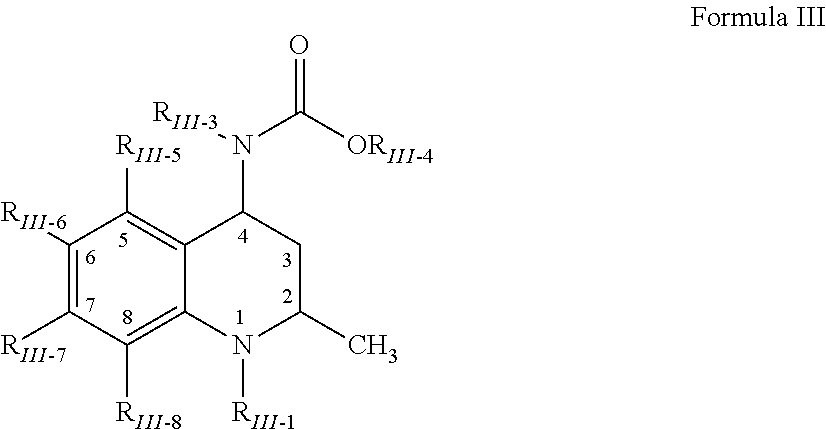
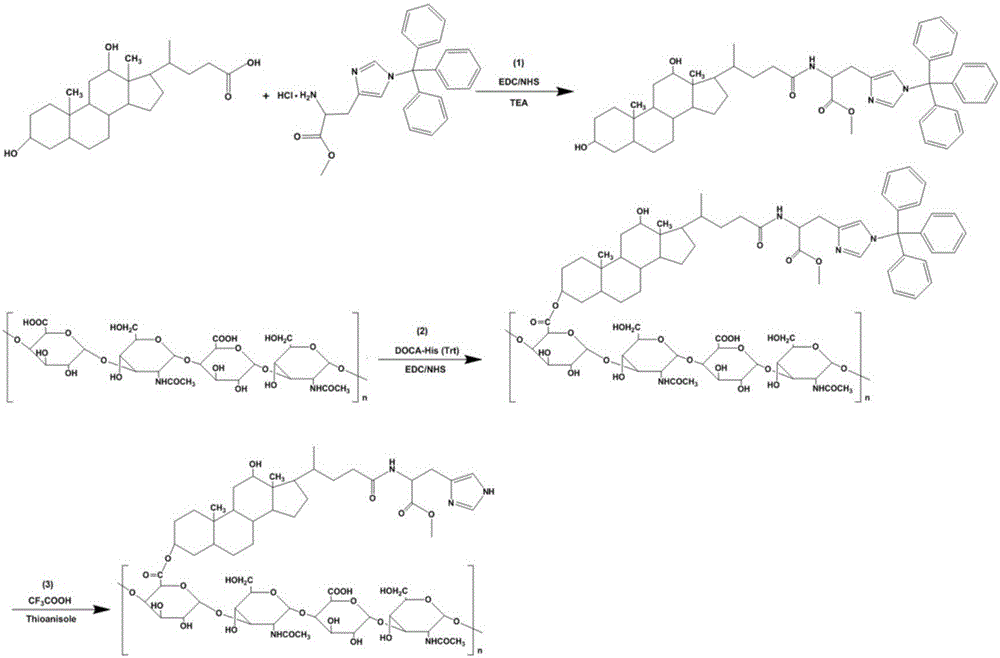
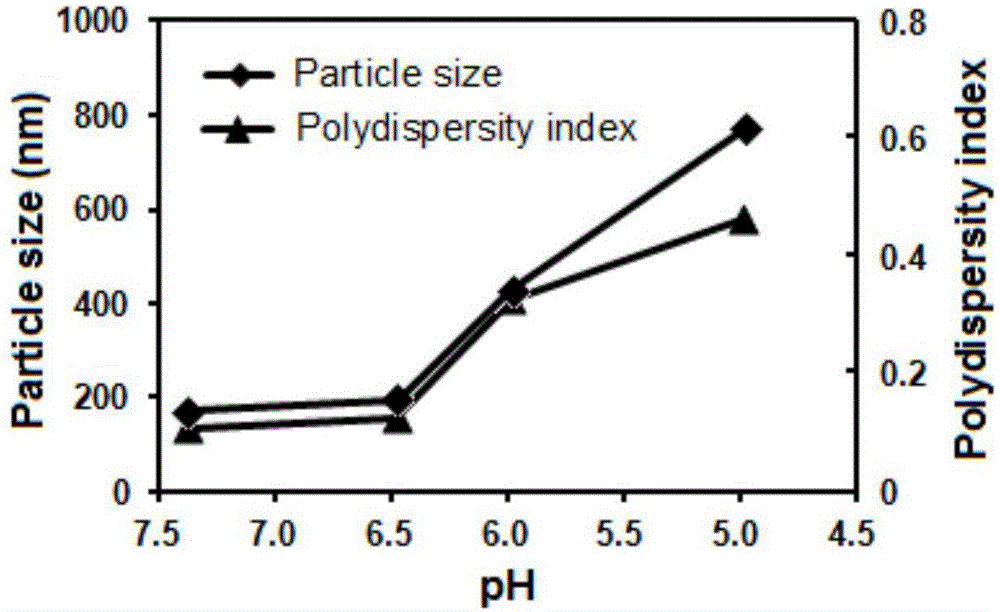
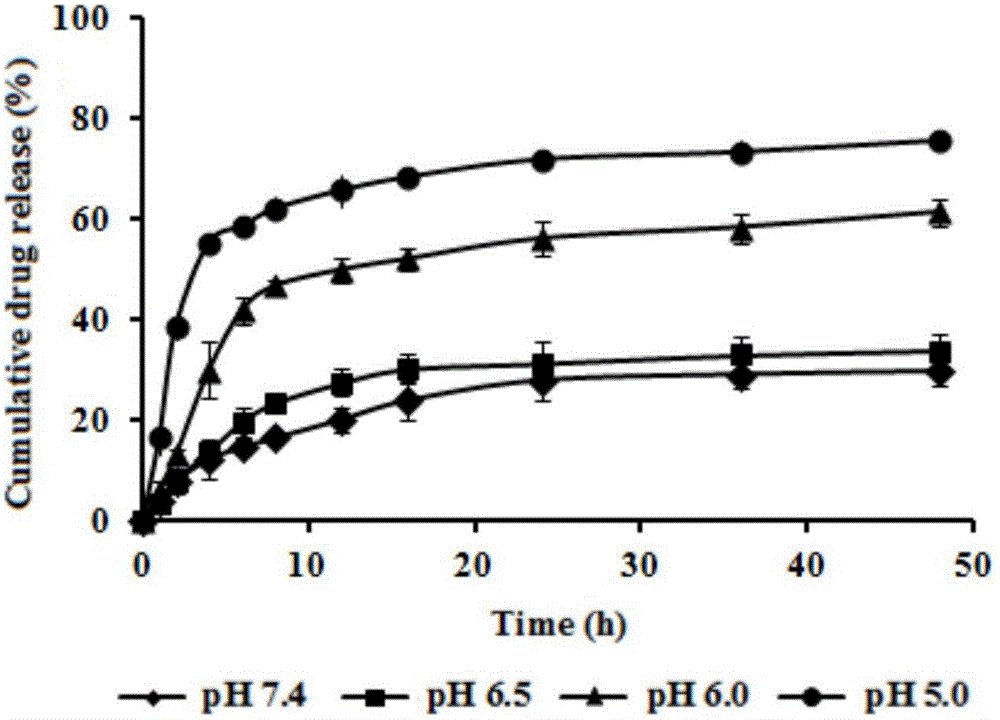
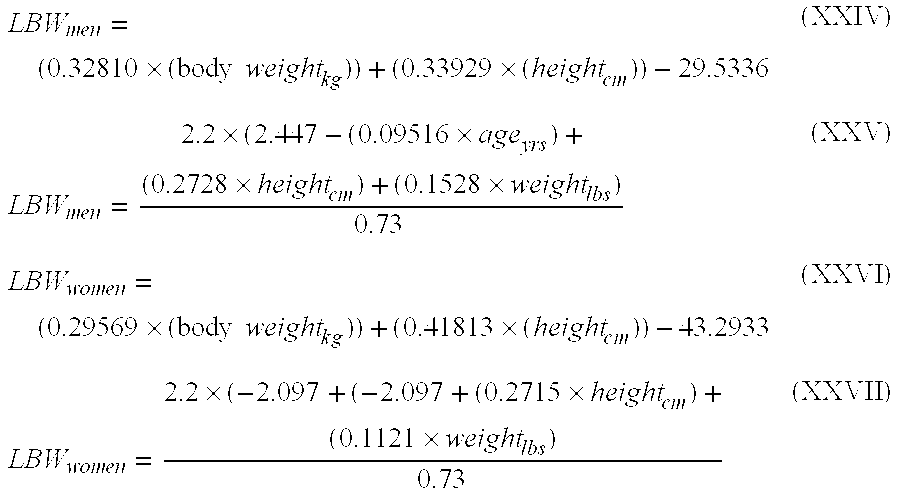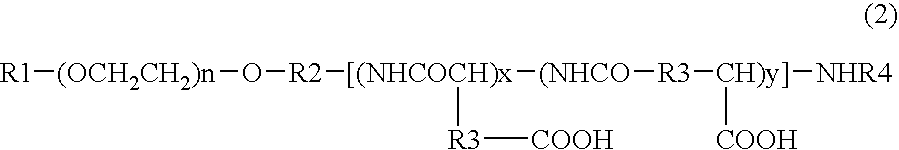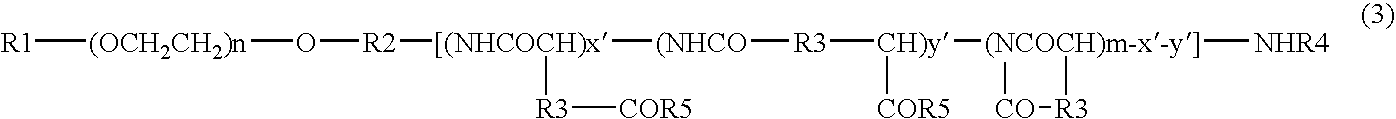Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1199 results about "Drug concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
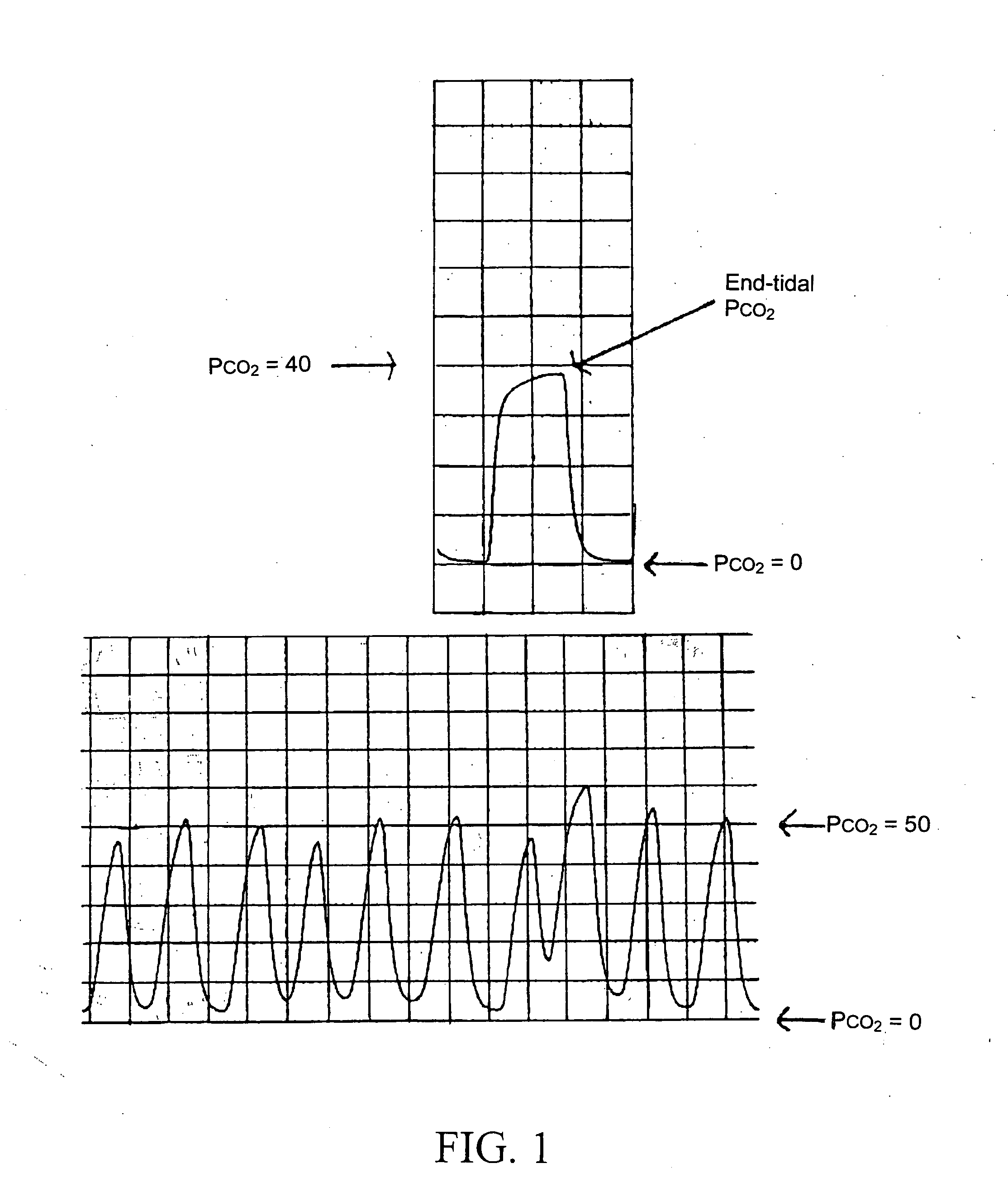
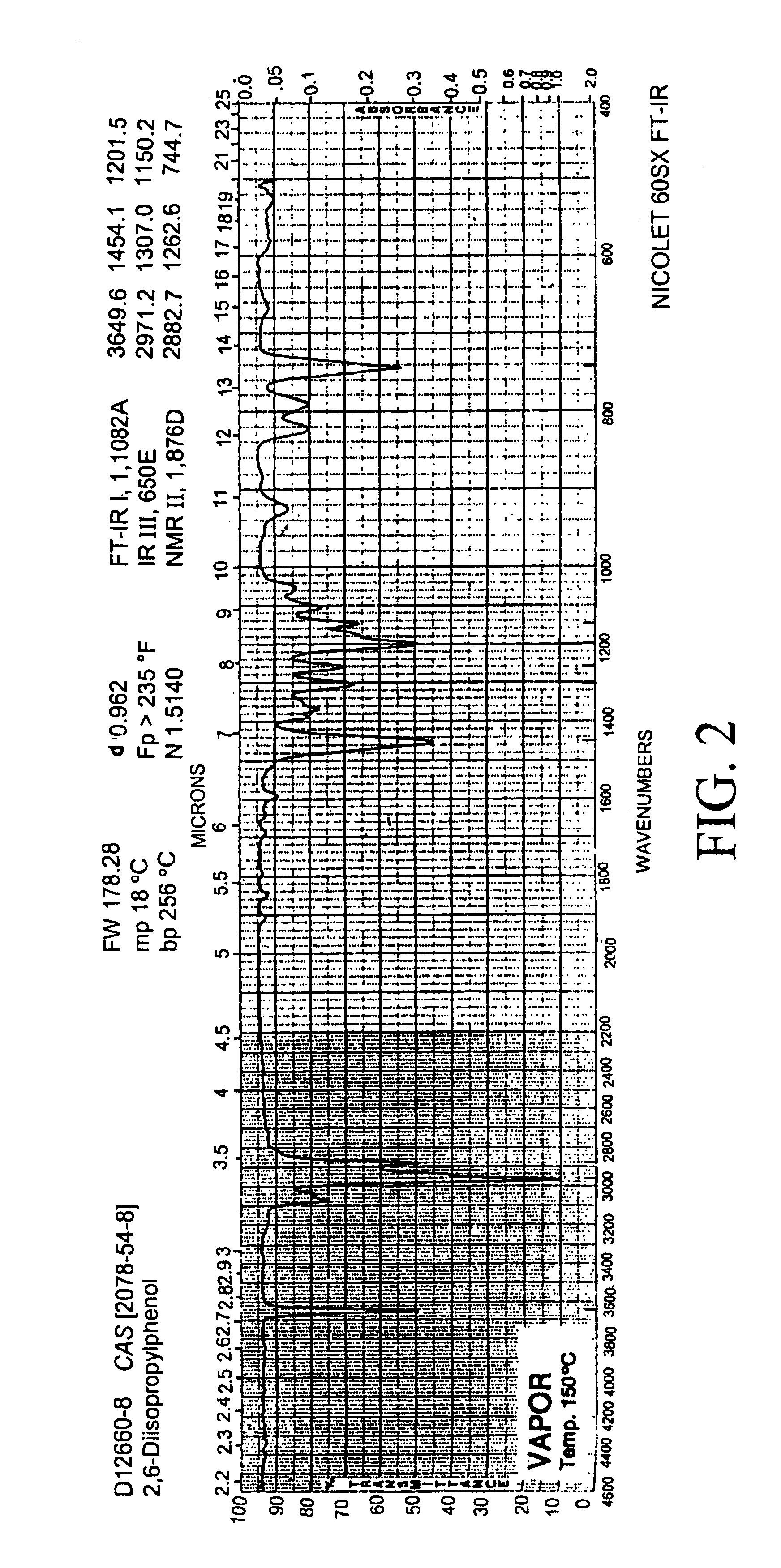
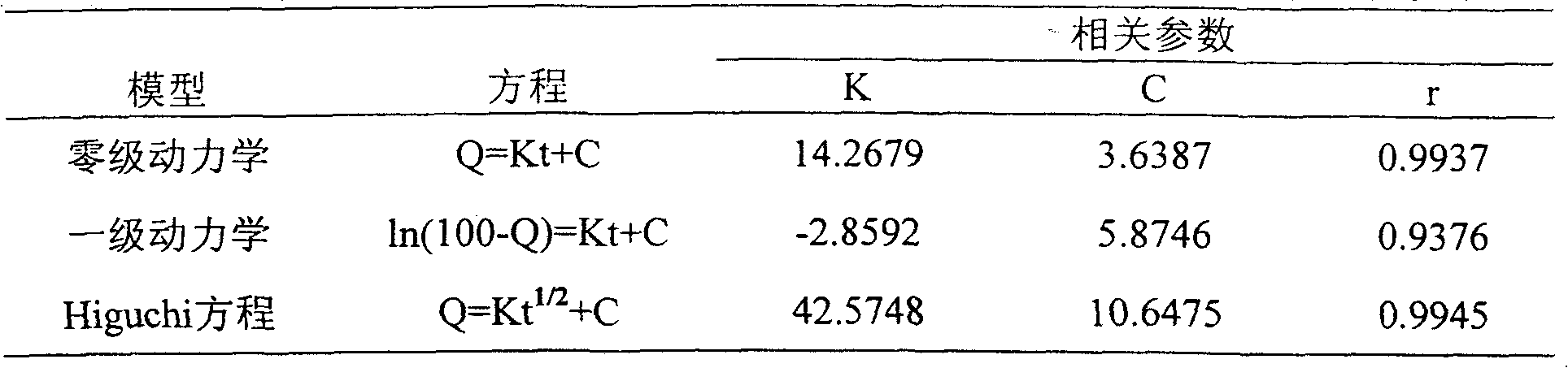
Drug concentration. the amount of drug in a given volume of plasma (e.g., number of micrograms per milliliter). Toxic drug levels may be observed when the body's normal mechanisms for metabolizing and excreting drugs are impaired, as commonly occurs in patients with liver or kidney disorders and in infants with immature organs.
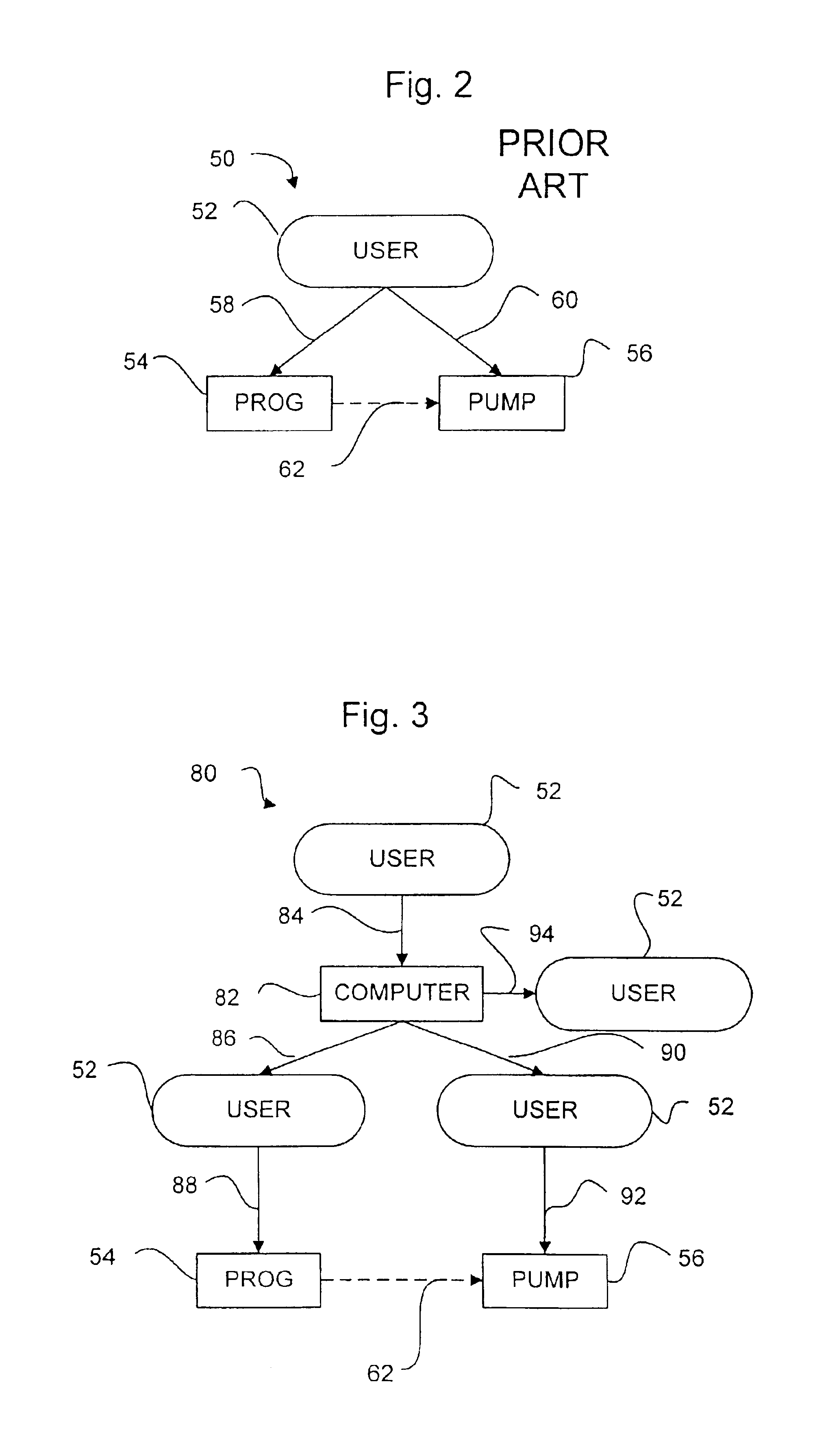
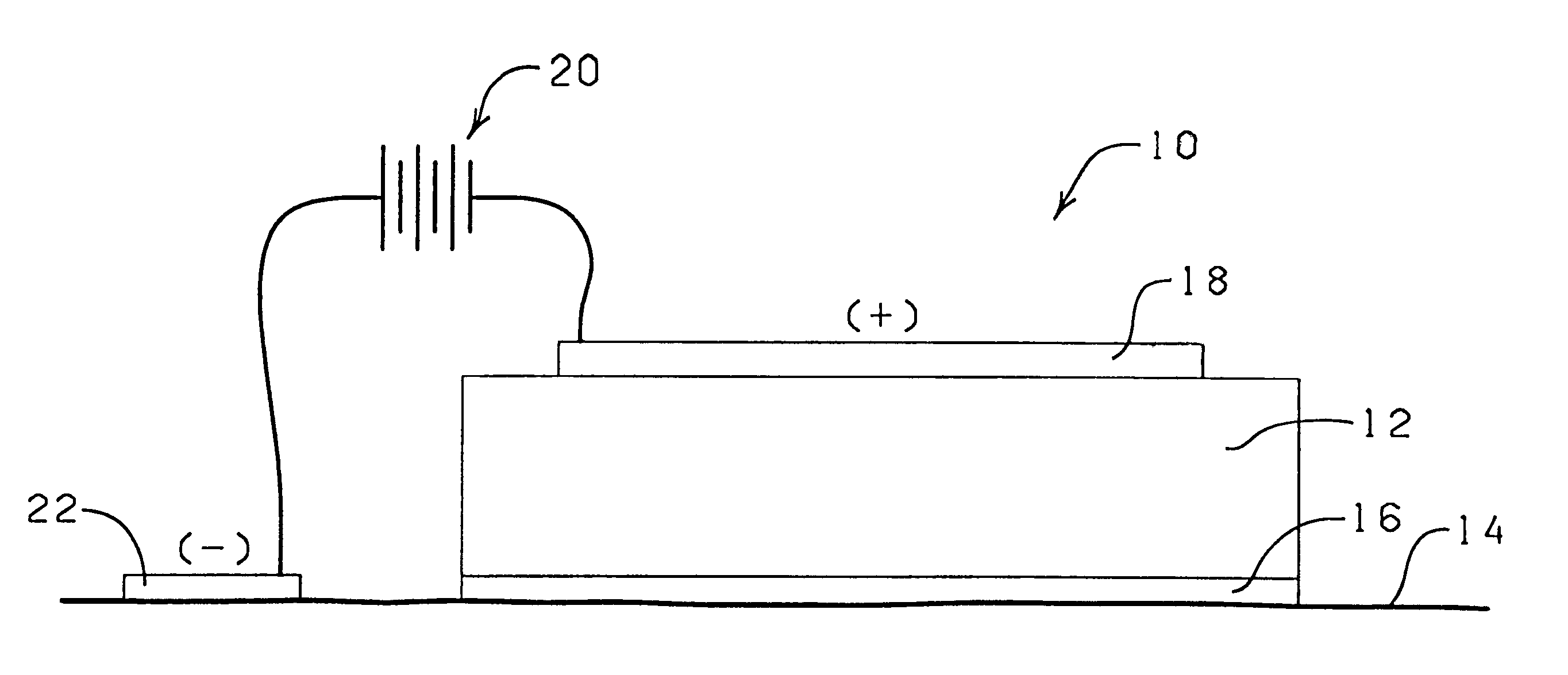
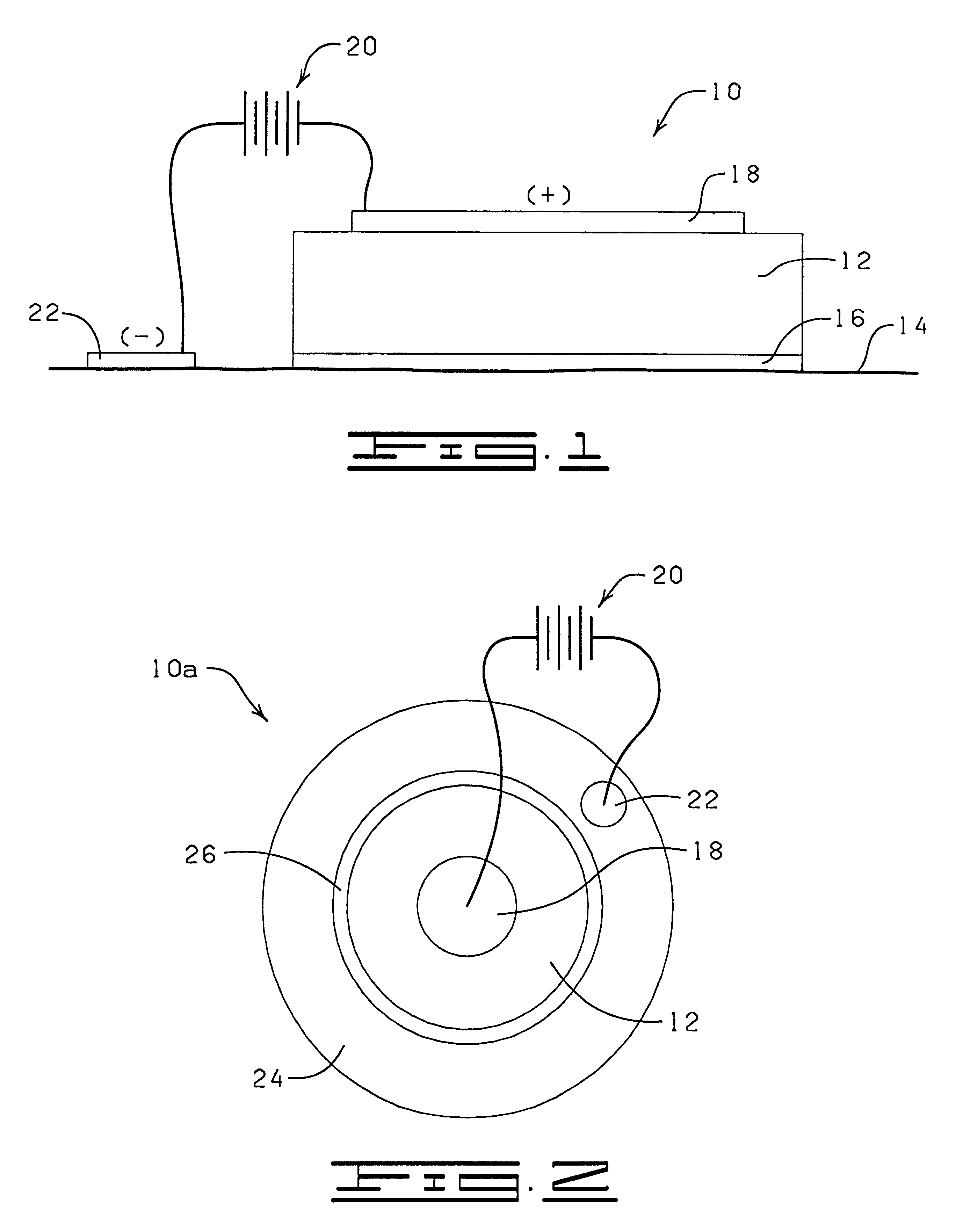
System and method for therapeutic drug monitoring
InactiveUS20050054942A1Accurate assessmentCost-effective and frequentNervous disorderElectrotherapyNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA
Decision information system for drug delivery devices
ActiveUS6928338B1Sampled-variable control systemsControlling ratio of multiple fluid flowsDoses rateGeneral purpose computer
Decision information systems, methods, and computer programs for better informing decisions to use multiple drugs in drug delivery devices, including implantable devices, for drug administration. Executable computer programs and logic embodying methods of the invention can calculate consistent multiple drug mixture amounts and drug delivery flow rates. One program accepts user input indicating a desired first drug dose rate, an initial first drug concentration, a desired second drug dose rate, an initial second drug concentration, and the reservoir size of the drug delivery device. The program method calculates a first drug amount and a second drug amount to combine in a mixture as well as a first drug true concentration in the mixture. The drugs can be mixed consistent with the physician's instructions using the program output. The first drug true concentration can be entered into a programmer device as the only drug concentration entered. Another program calculates a consistent first drug, second drug, and diluent amount to be added to a mixture for injection into a fixed flow rate, implantable drug delivery device. Methods preferably output true concentrations and dose rates for all drugs to be added and most preferably show all calculations used to arrive at the flow rate and mixture amount calculations. Yet another program receives a new desired drug dose rate for a previously filled device. The program accepts the existing mixture volume and true drug concentrations for a partially depleted device and calculates a new mixture flow rate to achieve the desired dose rate using the existing mixture. The methods can be implemented as executable computer programs in programmer devices, general purpose computers, servers, handheld computers, and personal digital assistants.
Owner:MEDTRONIC INC
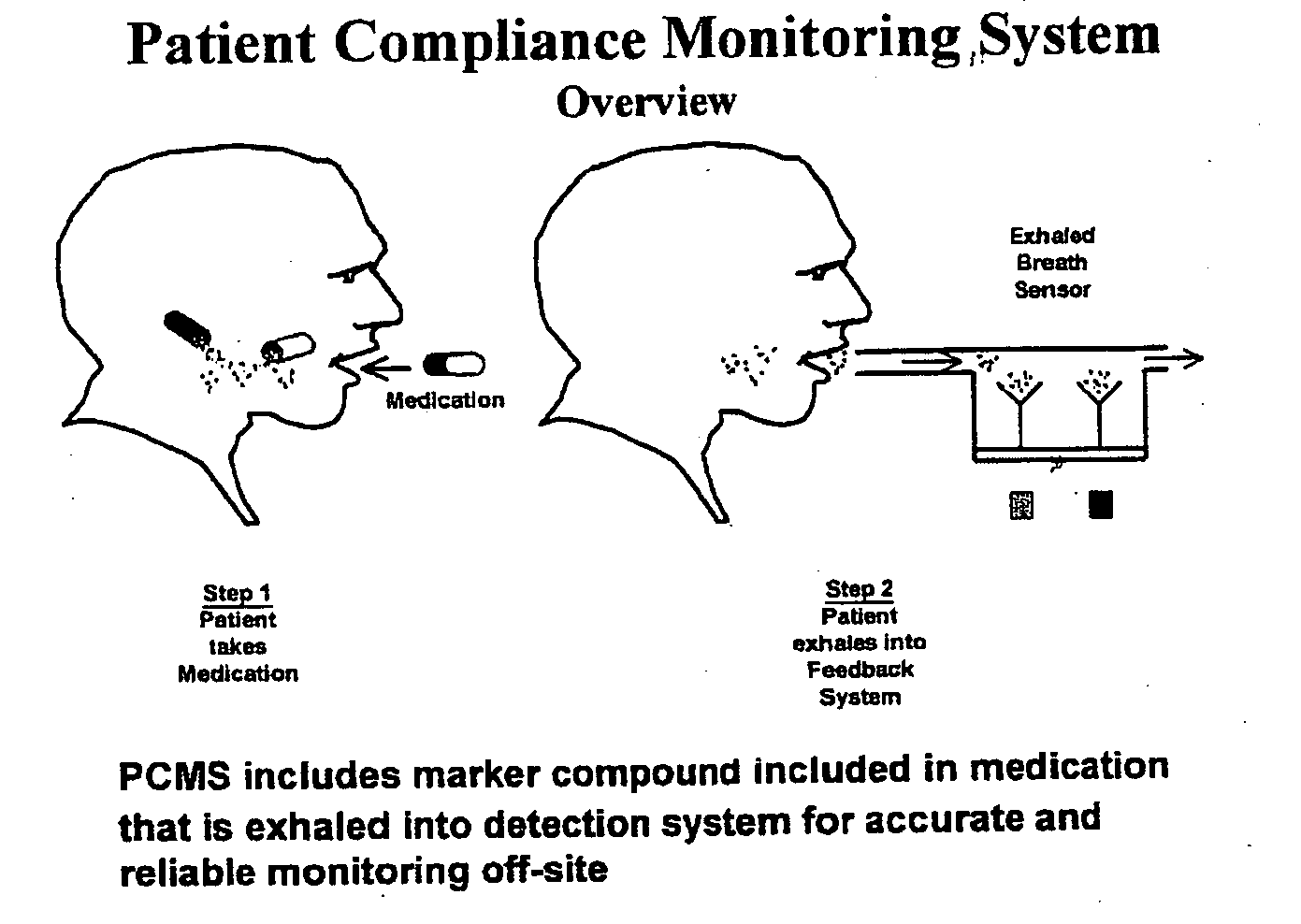
Marker detection method and apparatus to monitor drug compliance
InactiveUS20050233459A1Accurate assessmentPatient complianceDiagnostic recording/measuringSensorsNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
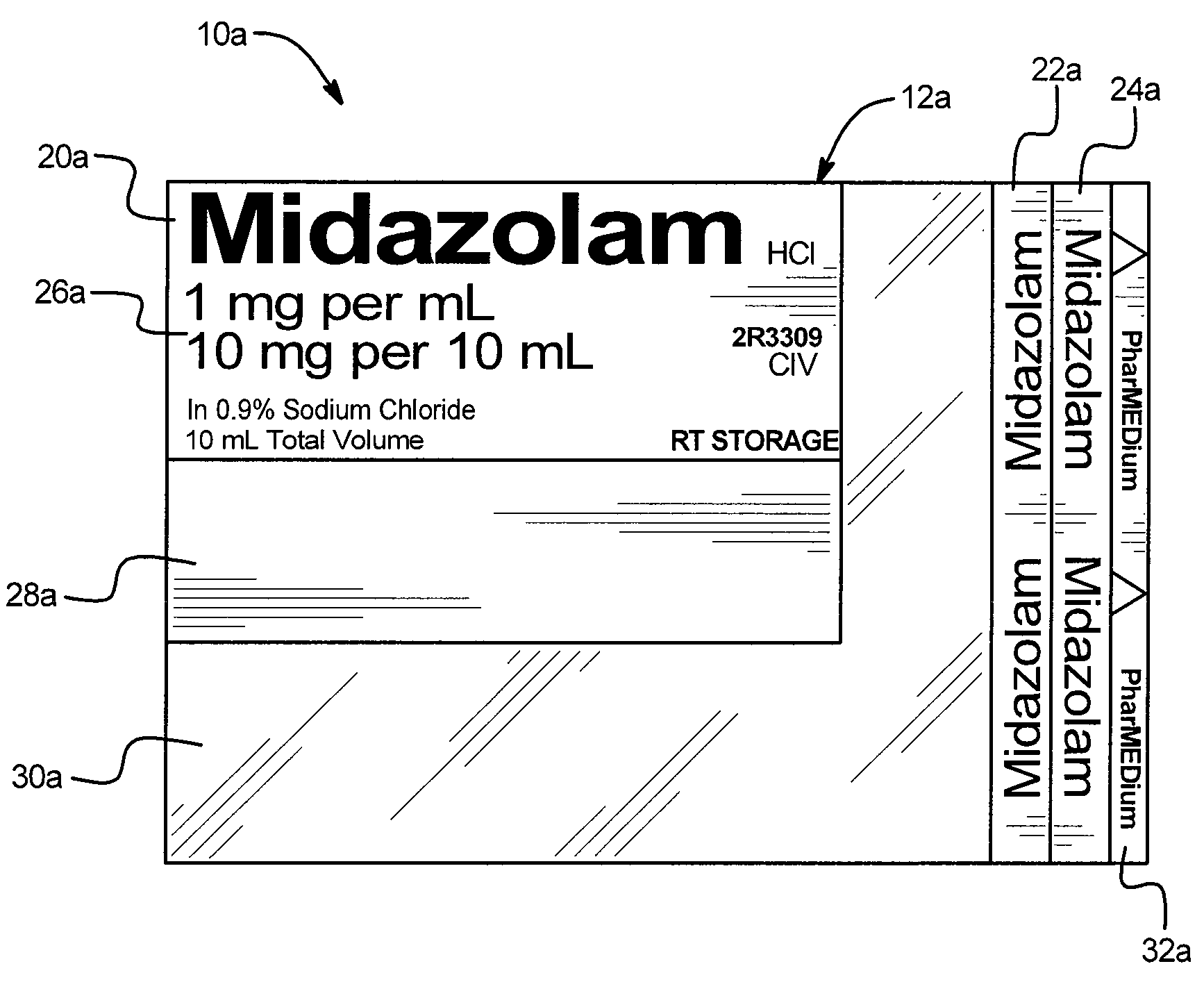
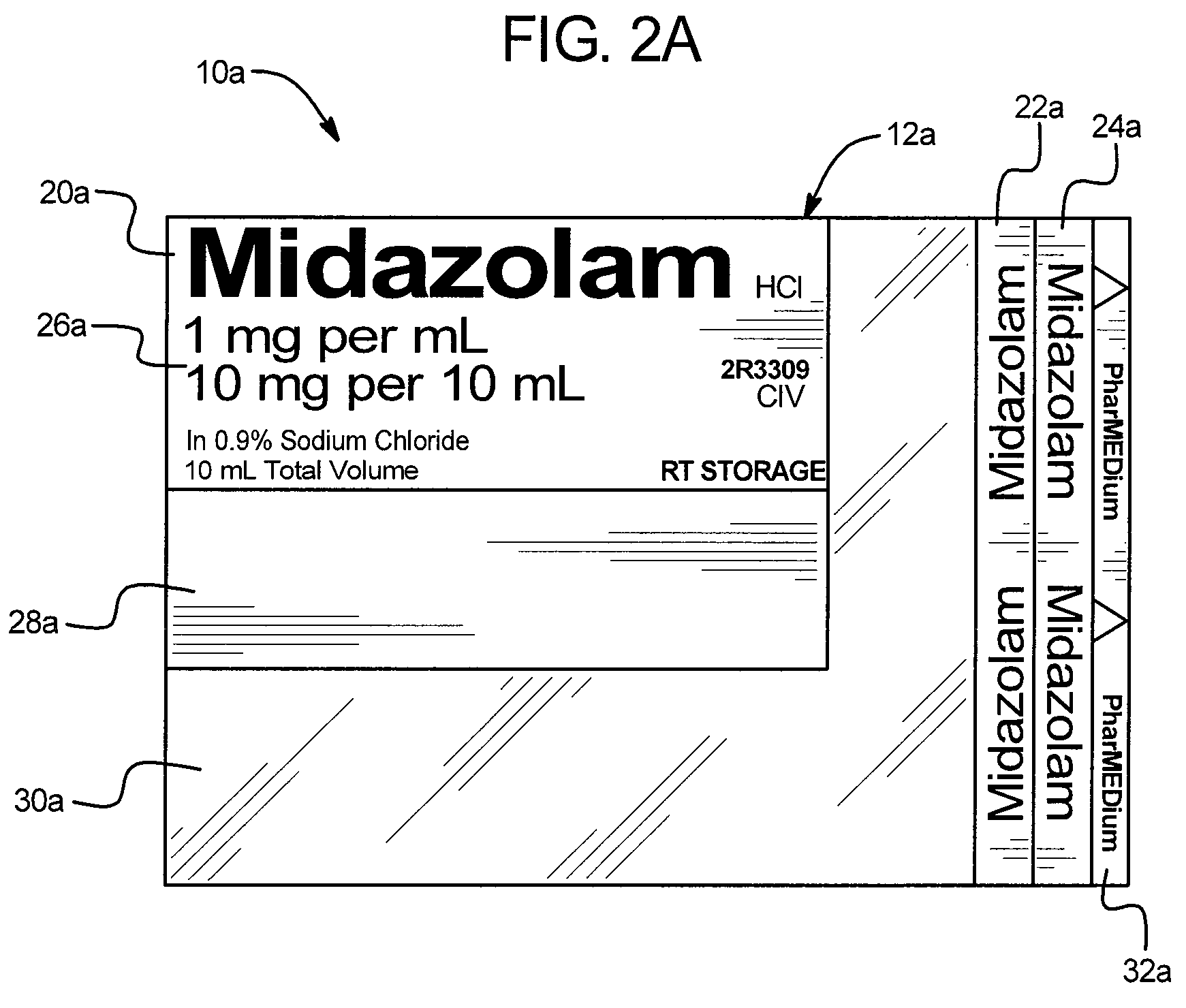
Safety device for drug delivery devices and containers
A drug administration safety device having a label configured to be attached to a drug container such as a syringe or IV bag, an adhesive on the back face of the label, and a backing or substrate for holding the label and protecting the adhesive prior to the application of the label to the drug container. In one embodiment, the label includes a first drug name section in a first orientation, a second drug name section in a second orientation, a third drug name section in a third orientation, a drug concentration section, a variable information section, and a gradiation viewing section. The first orientation, second orientation, and third orientation are different from each other to enable a user to readily see the drug name regardless of the position and orientation of the drug container.
Owner:HIKMA PHARMA USA INC
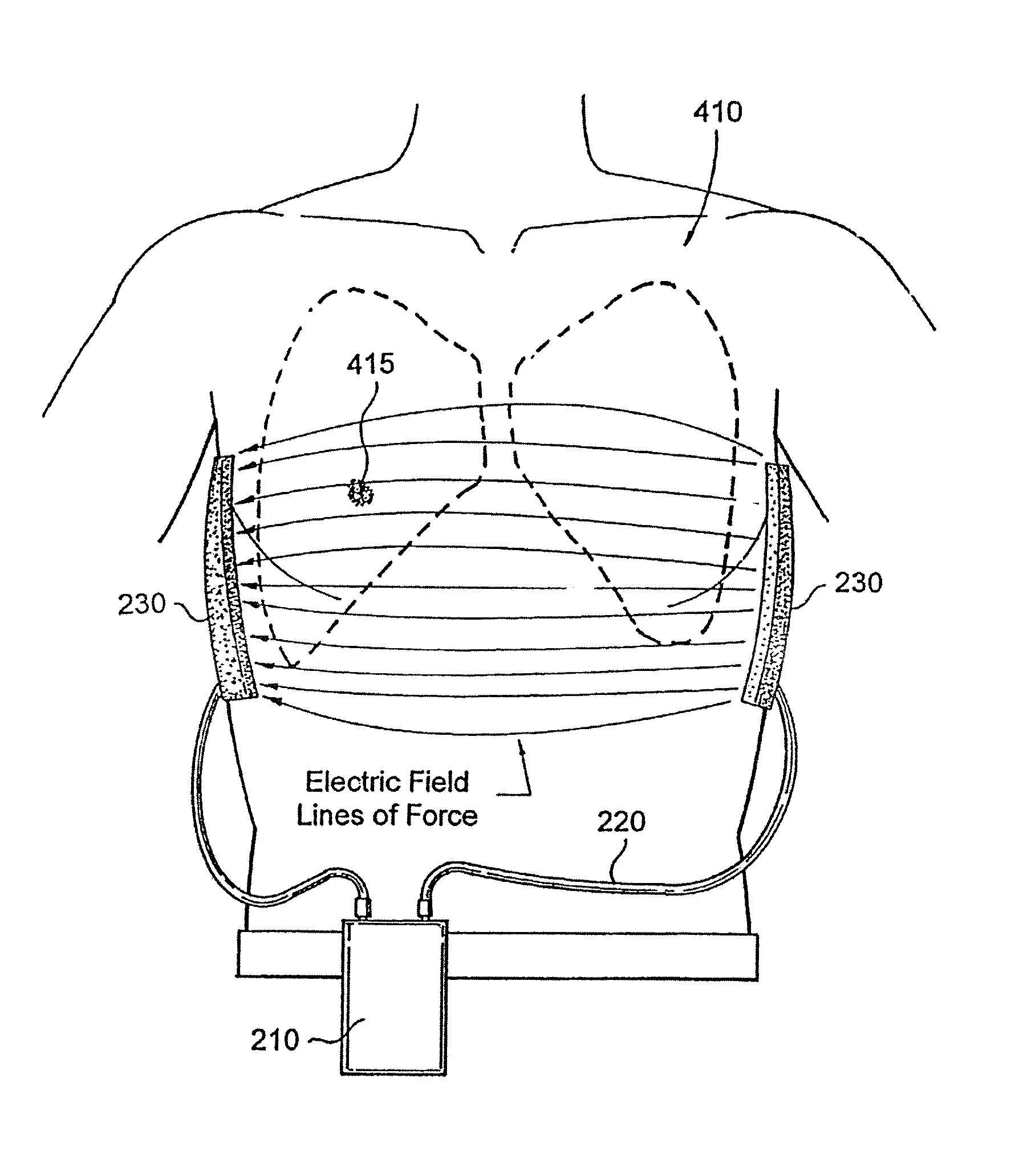
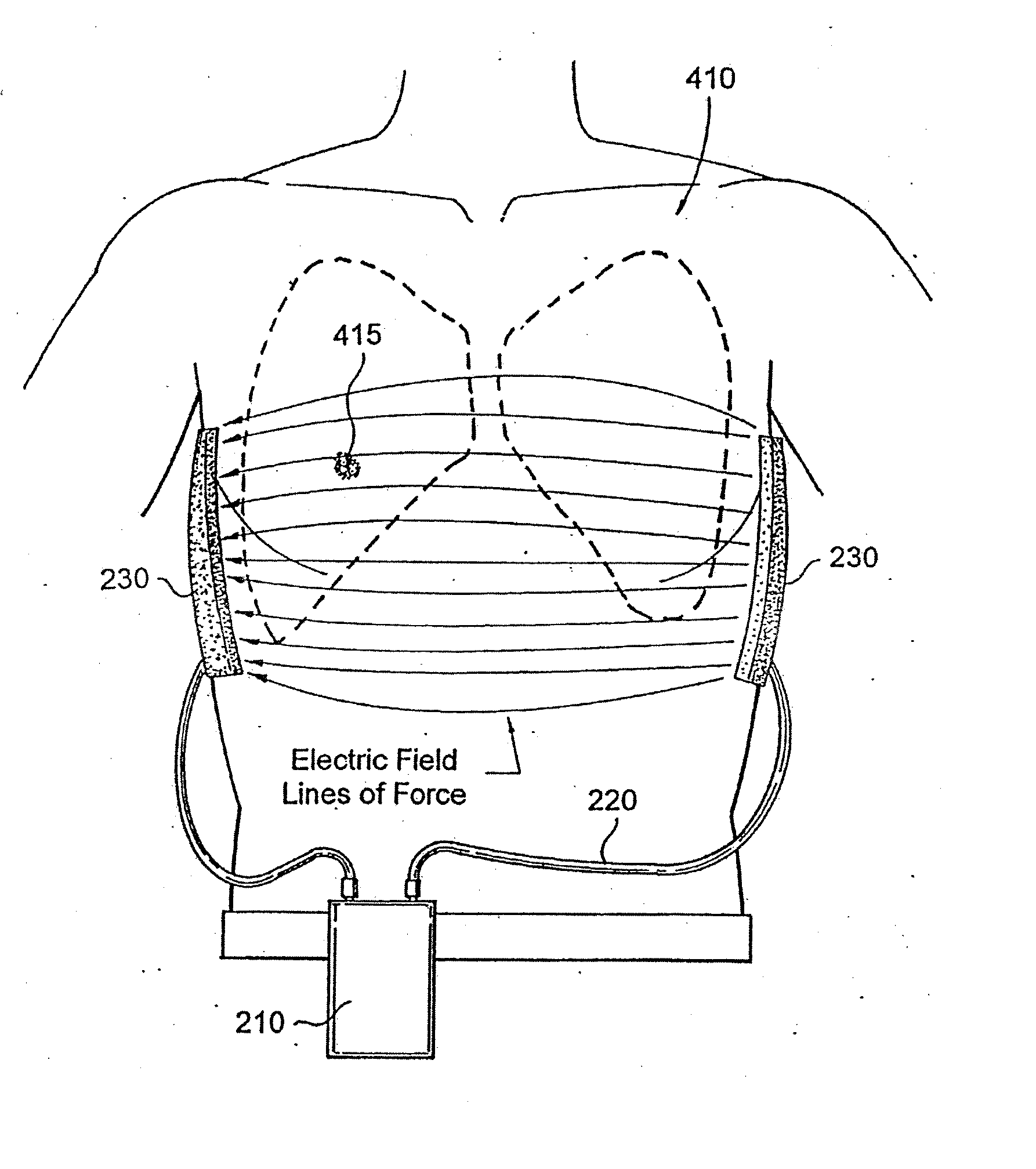
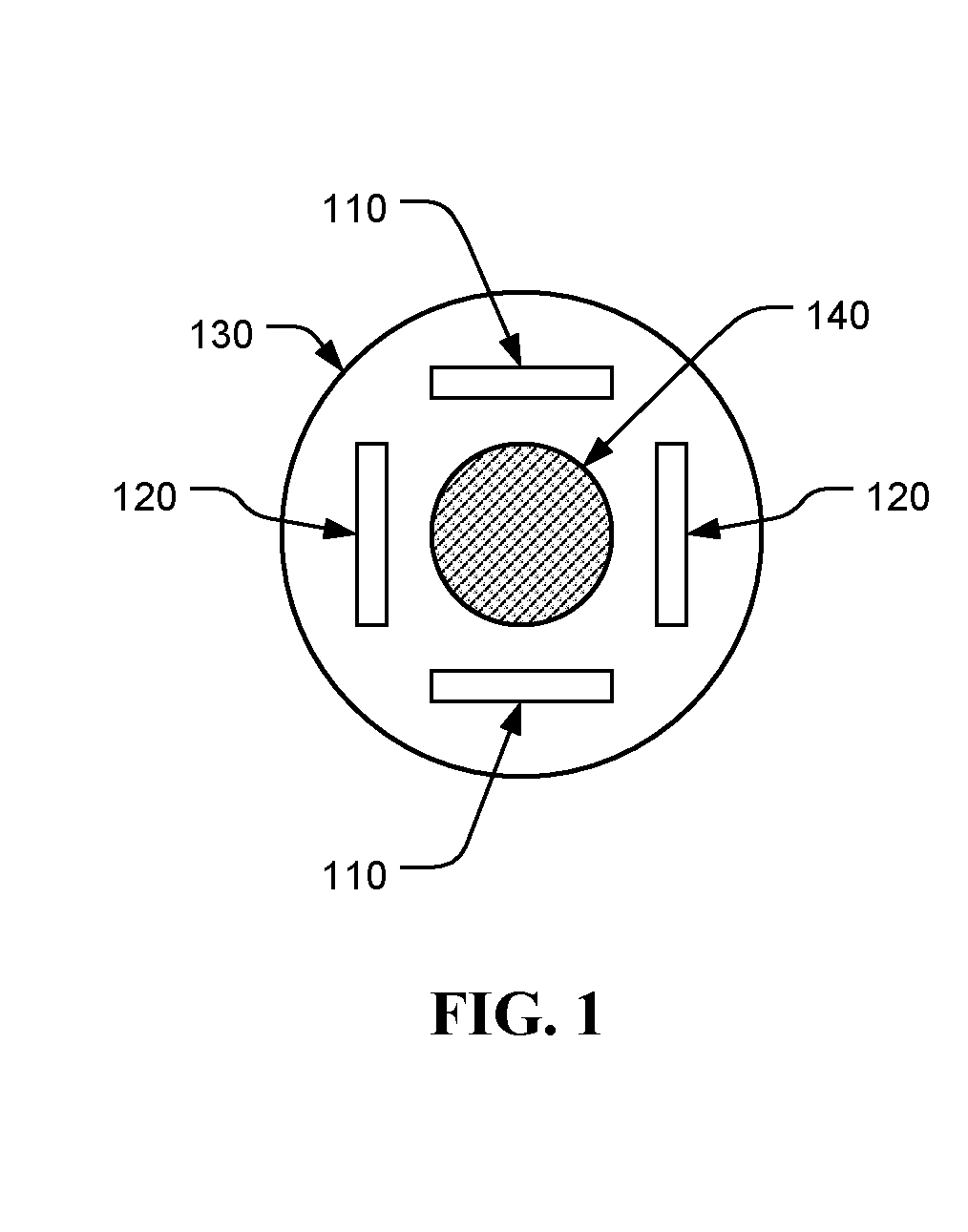
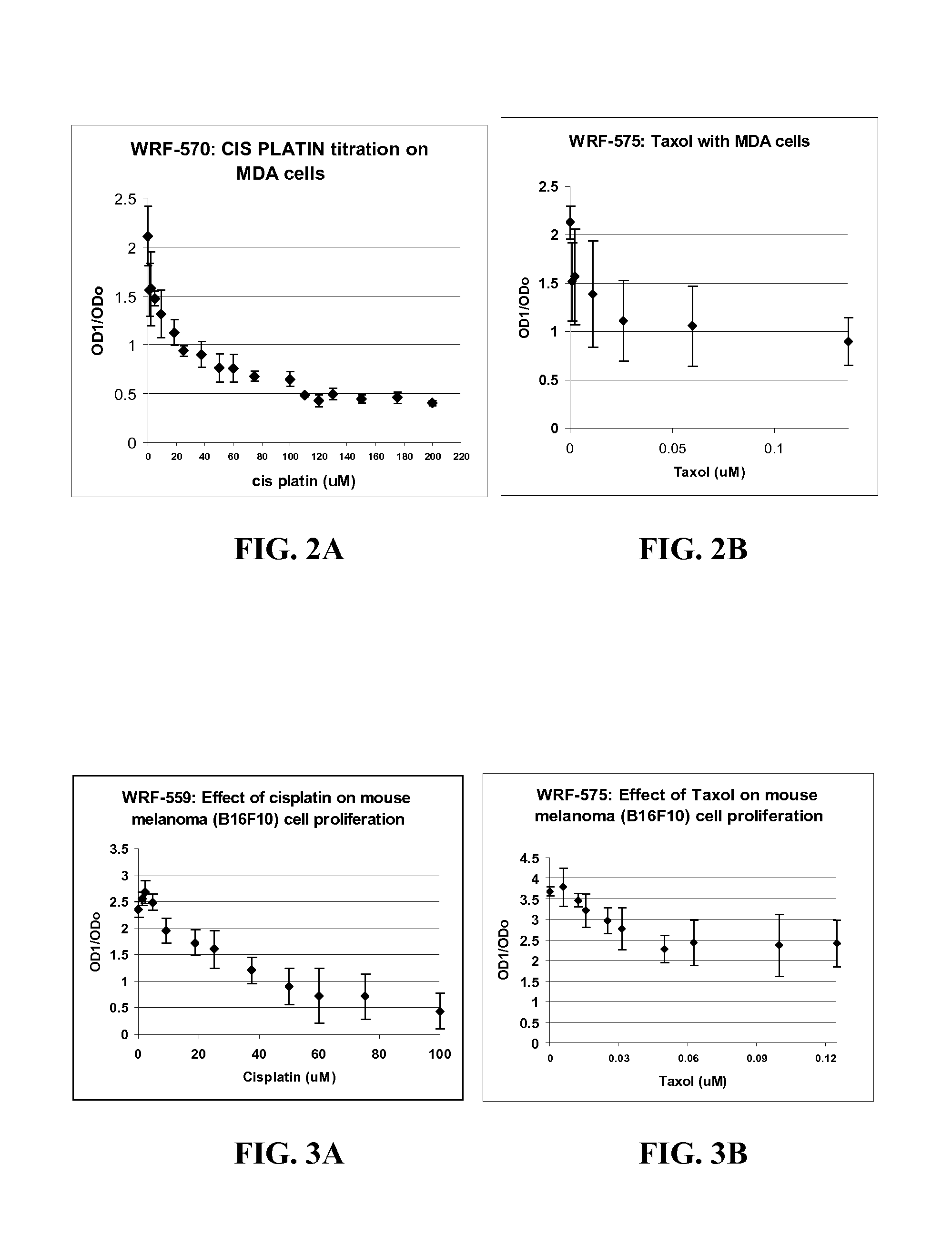
Treating cancer using electromagnetic fields in combination with other treatment regimens
Chemotherapeutic treatment for certain cancers may be combined with low intensity, intermediate frequency alternating electric fields that are tuned to a particular type of target cell. When the tuned fields were combined with Paclitaxel, Doxorubicin or Cyclophosphamide, excellent results were obtained against human breast cancer cells (MDA-MB-231) and non-small cell lung (H1299) carcinomas in culture. More specifically, cell proliferation inhibition similar to that obtained by drug alone was reached by exposure to the combined treatment at drug concentrations between one and two orders of magnitude lower than for drug-only regimens of treatment.
Owner:NOVOCURE GMBH
Methods of normalizing measured drug concentrations and testing for non-compliance with a drug treatment regimen
Methods for monitoring subject compliance with a prescribed treatment regimen are disclosed. In one embodiment, the method comprises measuring a drug level in fluid of a subject and normalizing said measured drug level as a function of one or more parameters associated with the subject. The normalized drug level is compared to a reference value and associated confidence intervals or to a concentration range. The reference value and associated confidence intervals and / or the concentration range may be normalized based on one or more parameters associated with subjects in a reference population.
Owner:AMERITOX LLC
Temperature-regulated culture plates
InactiveUS20100009335A1Eliminate needEliminate condensationBioreactor/fermenter combinationsBiological substance pretreatmentsOn boardBiology
Described herein are environmentally isolated tissue culture devices that may be used for cell culture, as well as systems including these devices and methods for using them. These devices may include control features for regulating the micro-environment within a well or wells of the device. For example, on-board features may regulate the temperature, humidity, pH, media level, media composition, CO2 / O2 / N2 levels, drug concentration, cell density, byproduct (or product) production, and mixing of materials within the chamber. Material may be added to or withdrawn from the wells of the device without opening the device. Also described herein are controllers for analyzing and controlling the micro- environment within the well. Thus, the plates described herein may be used without requiring a separate incubator, allowing cells to be analyzed (e.g., imaged) continuously, allowing real-time reactions while monitoring under a microscope for hours, days or even weeks.
Owner:JOSEPH VICTOR +2
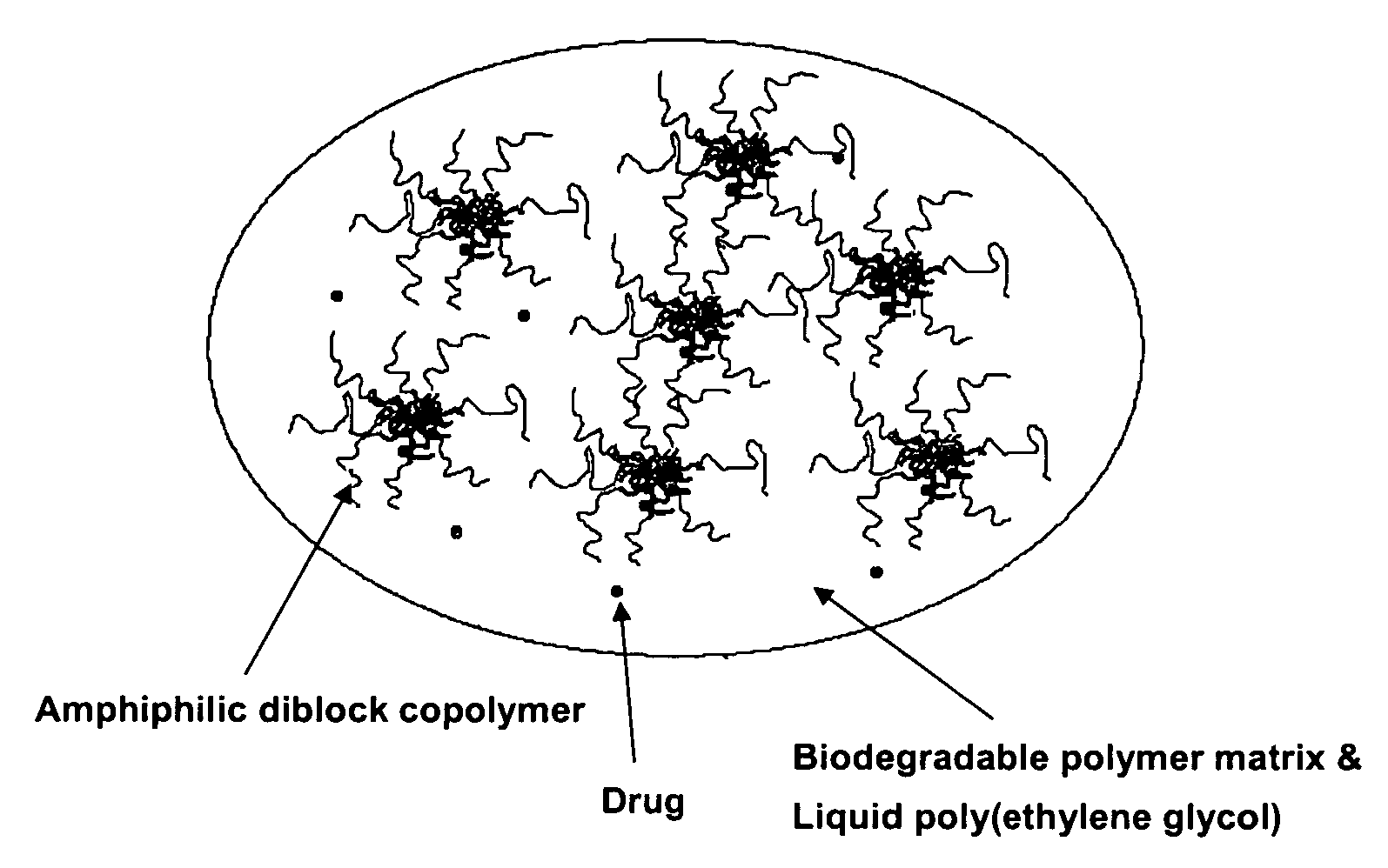
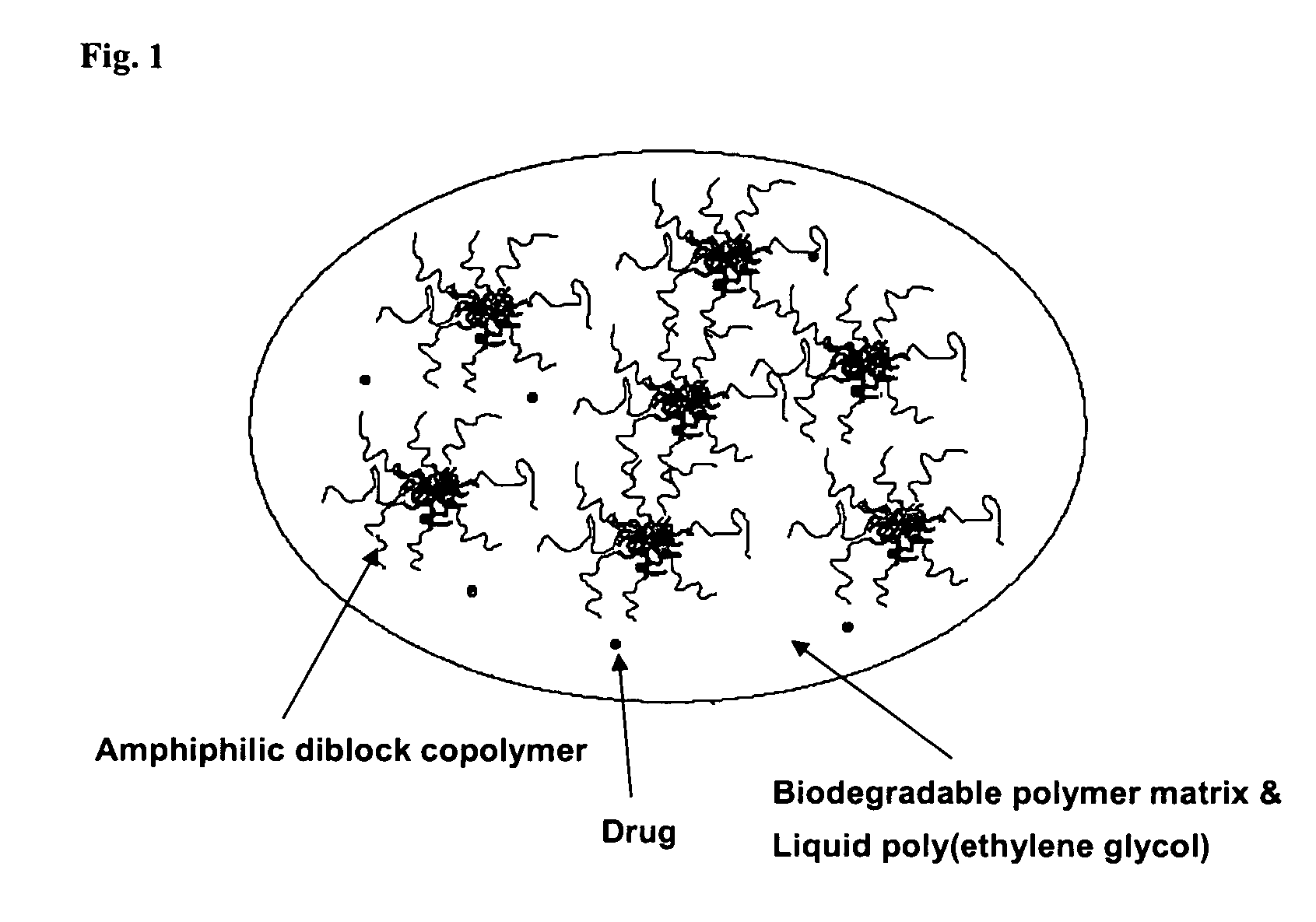
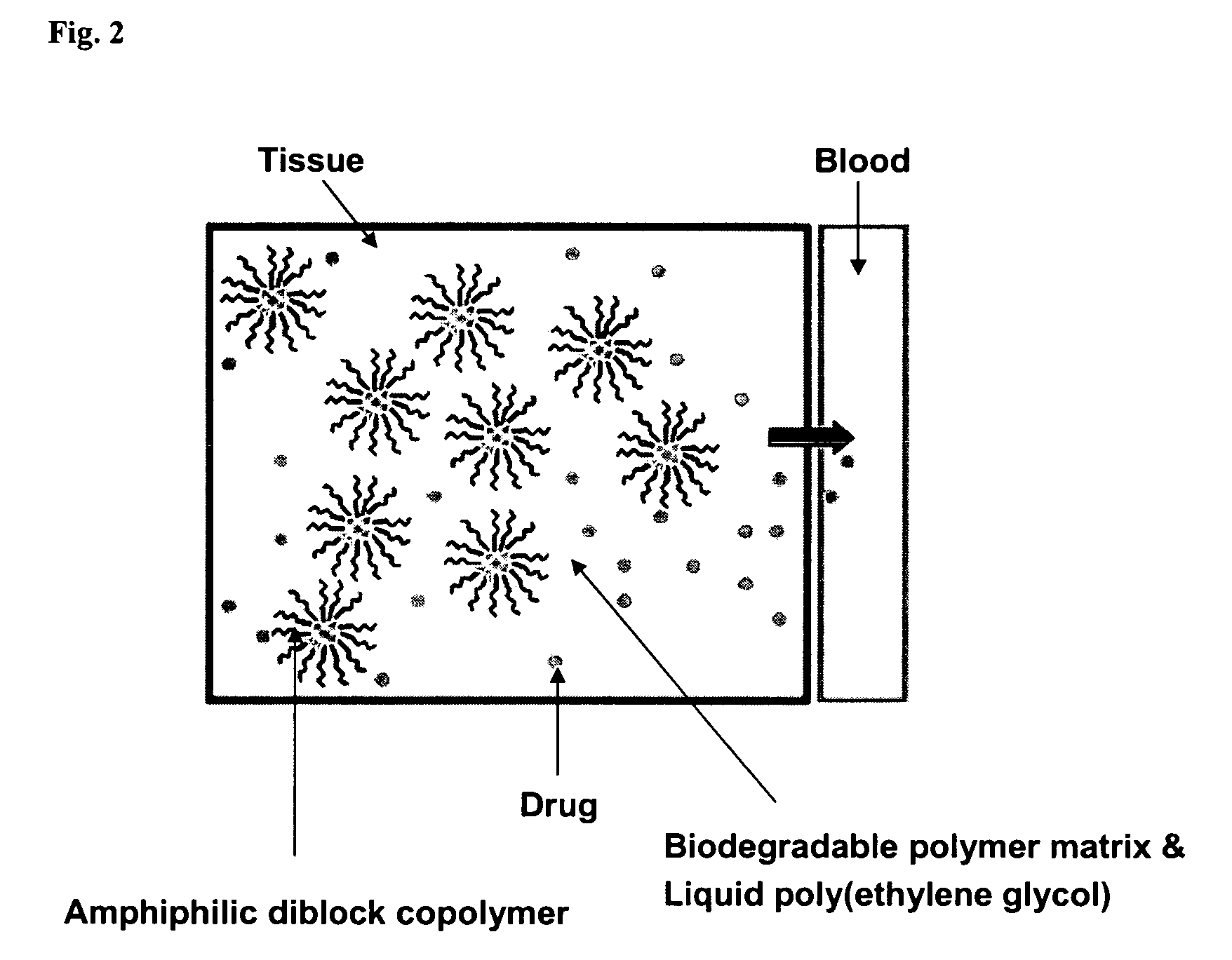
Composition for sustained delivery of hydrophobic drugs and process for the preparation thereof
InactiveUS7153520B2Enhance pharmacological effectsPowder deliverySolution deliveryPolythylene glycolPharmaceutical Substances
A composition for the sustained delivery of a drug comprising an amphiphilic diblock copolymer; a poorly water-soluble drug; a biodegradable polymer; and liquid poly(ethylene glycol) or functional derivatives thereof and a process for preparing the composition are disclosed. When administered into a particular body site, the composition forms an implant containing the drug and drug containing polymeric micelles, which are slowly released from the implant to maintain a constant drug concentration for an extended period of time.
Owner:SAMYANG HLDG CORP
Method and apparatus for monitoring intravenous (IV) drug concentration using exhaled breath
InactiveUS7104963B2Good correlationCost-effective and frequentRespiratorsRespiratory organ evaluationAnesthetic AgentMetabolite
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Phase-transition polymeric microneedles
ActiveUS20110195124A1Easy yet multi-functional fabrication processPowder deliveryPeptide/protein ingredientsOrganic solventMicrofabrication
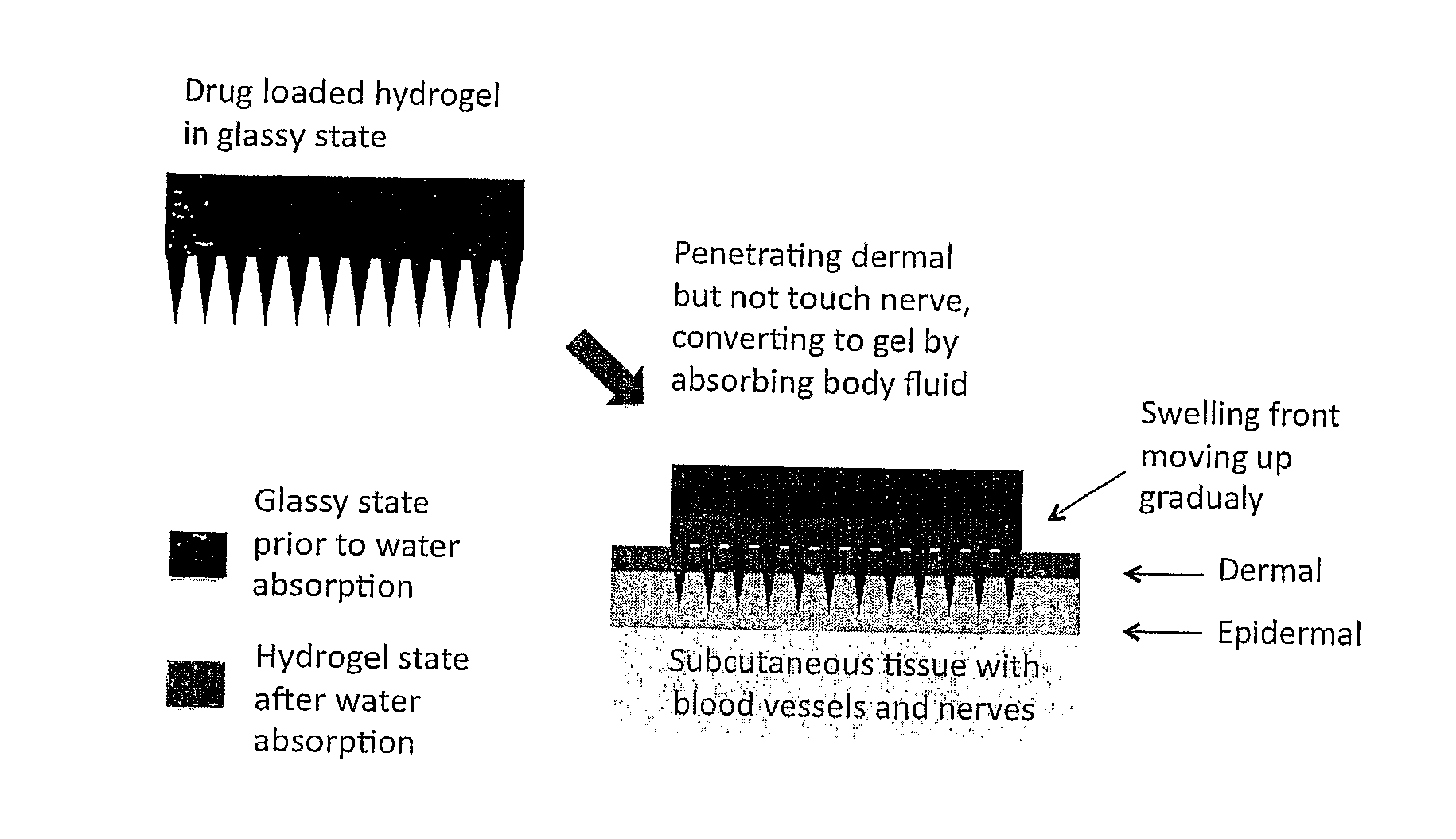
This invention discloses a novel microneedle system, phase-transition microneedle patch, which overcomes all the limitations that existing microneedles encountered. The microneedle patch is formed of an integrated polymeric piece consisting of a microneedle array and a plate (called holding plate) on which the needles stand. The microneedles of the patch are hard and strong enough to penetrate epidermis at dry state but turn to be hydrogel state soft and permeable to hydrophilic agents when absorbing body fluid. The hydrogel state of the patch is a hydrophilic network held by physical or chemical cross-linking junctions. The pores of the network are opened up by body fluid for drugs and macromolecules to diffuse through. The polymeric materials used to form the microneedle patch have been used in the pharmaceutical field for years and have proven compatibility with the skin and with proteins. The drugs may be stored in the matrix of the microneedle array as well as the holding plate so that the requirement for high dose applications may be full filled. In addition, molding (casting) of this type of microneedle patch is simple, easy to achieve and needs no microfabrication systems and organic solvents. By a programmed molding (casting), the patch may be assembled in a layered structure with desired drug concentration in each layer, respectively. Due to this design, a programmed pulse or a zero order release of drugs may easily be achieved. In addition, delicate proteins loaded in the patch are kept in a dry and hydrophilic glassy state before being released, the most favored state for protein storage. Finally, during the swelling-based drug release, the microneedle patch increases their thickness gradually between the skin and the back cover (which holds the needles) lo create a sustained pressure to ensure good contact of the microneedles inside epidermis.
Owner:JIN TUO
Treating cancer using electromagnetic fields in combination with other treatment regimens
ActiveUS20070239213A1Growth inhibitionEnhanced inhibitory effectOrganic active ingredientsElectrotherapyRegimenIntermediate frequency
Chemotherapeutic treatment for certain cancers may be combined with low intensity, intermediate frequency alternating electric fields that are tuned to a particular type of target cell. When the tuned fields were combined with Paclitaxel, Doxorubicin or Cyclophosphamide, excellent results were obtained against human breast cancer cells (MDA-MB-231) and non-small cell lung (H1299) carcinomas in culture. More specifically, cell proliferation inhibition similar to that obtained by drug alone was reached by exposure to the combined treatment at drug concentrations between one and two orders of magnitude lower than for drug-only regimens of treatment.
Owner:NOVOCURE GMBH
Method and apparatus for monitoring intravenous (IV) drug concentration using exhaled breath
A method and system is provided for detecting the depth of anesthesia wherein at least one anesthetic agent is absorbed in a patient's bloodstream during the administration of anesthesia, which includes sampling a patient's expired breath; analyzing the breath for concentration of at least one substance indicative of the anesthetic agent using sensor technology such as free (unmetabolized) anesthetic agent or its metabolites; determining the effect of the agent based on that concentration; and determining depth of anesthesia based thereon. The method also detects endogenous compounds such as ketones and ammonia in exhaled breath as well as other pathologic organisms.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
M-diamide compound and preparation method and application thereof
ActiveCN109497062AReduce harmResidue reductionBiocideOrganic chemistryOrganic chemistryDrug concentration
Owner:CAC NANTONG CHEM
Polymer stent
A polymer intravascular stent that is resorable over a period of time containing a drug, which is delivered to the vascular wall during the absorption of the polymer material. A gradient of drug concentration may be established in the polymer.
Owner:ADVANCED BIO SURFACE
Three-dimensional high-flux medicaments sifting chip and manufacture method thereof
InactiveCN101245311ALow costShort cycleBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringPolydimethyl siloxane
The invention discloses a three-dimensional high-flux drug screening PDMS-glass chip and the preparation method thereof, which makes uses of micro-electro processing and micro-plastic film technologies to produce the three-dimensional PDMS-glass chip with a two-layer micro-channel structure. A cell injection and waste liquid output channel of a first layer of PDMS chip is bonded with another layer of polydimethyl siloxane chip together, a drug concentration gradient channel and a quantitative cell culture chamber are arranged on the other layer of PDMS chip, the quantitative cell culture chamber is connected with an outlet channel of the drug concentration gradient channel, the quantitative cell culture chamber is respectively connected with a cell sample injection hole drilling position and a waste liquid outlet hole drilling position by the channel; the three-dimensional high-throughput drug screening PDMS-glass chip can overcome the shortcomings of heavy workload, great reagent and drug consumption, long screening period and high development cost of the traditional drug screening technology. The three-dimensional high-flux drug screening PDMS-glass chip can realize miniaturization of the chip, high flux, low cost, small reagent consumption, accurate and reliable test data and the simple design requirements of the operational process in the drug screening application on cell level.
Owner:WUHAN UNIV +1
Pharmaceutical compositions providing enhanced drug concentrations
InactiveUS8026286B2High dissolution rateImprove drug bioavailabilityAntibacterial agentsPowder deliverySolubilityPharmaceutical drug
A drug in a solubility-improved form is combined with a concentration-enhancing polymer in a sufficient amount so that the combination provides substantially enhanced drug concentration in a use environment relative to a control comprising the same amount of the same solubility-improved form of drug without the concentration-enhancing polymer.
Owner:BEND RES
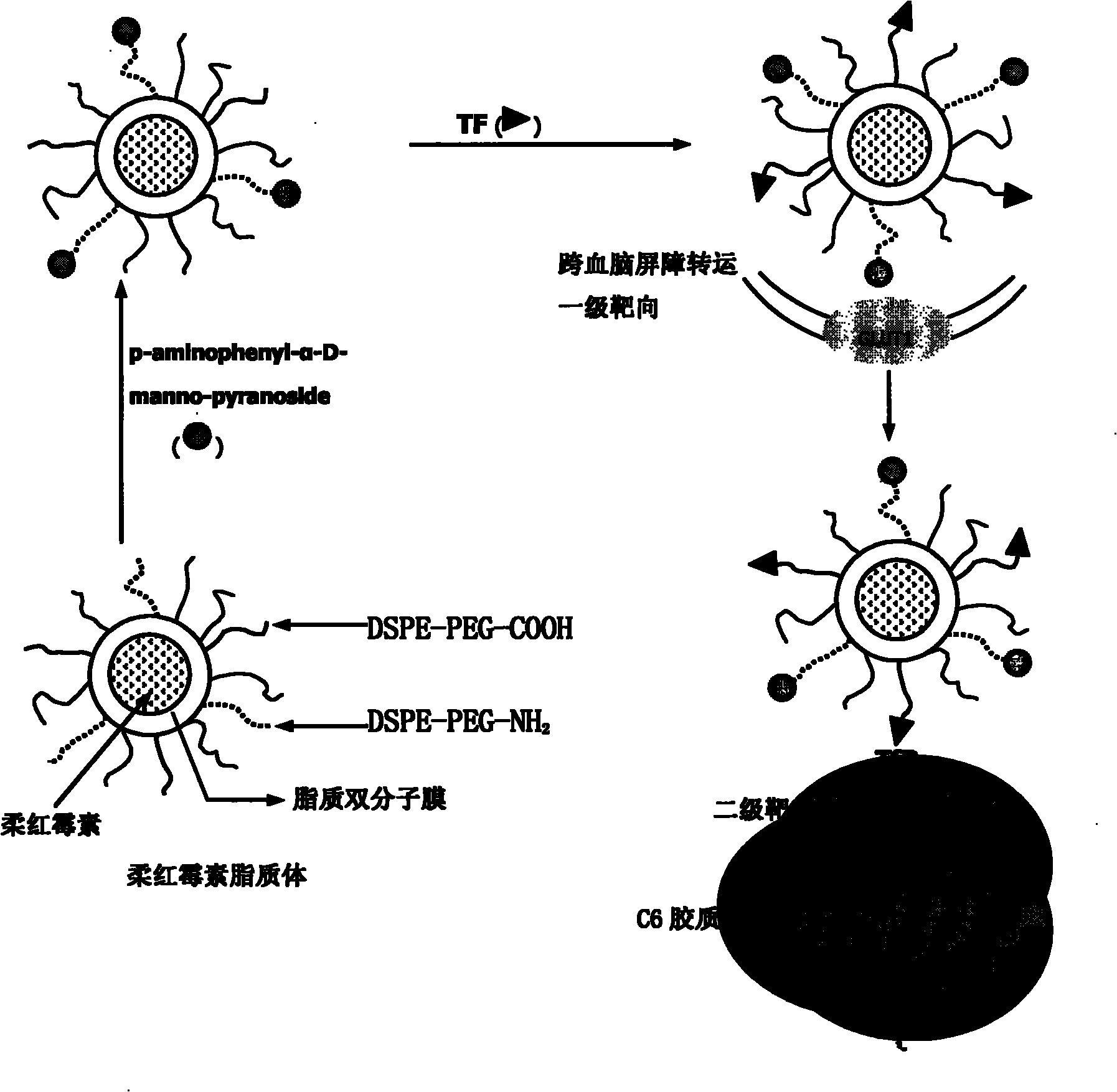
Dual target liposome and preparation method and application thereof
InactiveCN101816629AIncrease drug concentrationResearch to aid in non-invasive treatmentsOrganic active ingredientsMacromolecular non-active ingredientsDaunorubicinNon invasive
The invention discloses a dual target liposome and a preparation method and application thereof. The target liposome provided by the invention consists of liposome and modifiers on the surface of the liposome, wherein the modifiers on the surface of the liposome comprise p-aminophenyl-alpha-D-manno-pyranoside and transferrin. The invention also discloses a medicament-loaded liposome, which is obtained by wrapping daunorubicin by using the target liposome. The obtained target liposome has good capability of crossing the blood brain barrier, targets brain glioma, and can be used as a medicament carrier. The medicament-loaded liposome can target the medicament to a brain glioma site after crossing the blood brain barrier so as to greatly increase the concentration of the medicament at a tumor site and improve the effect of chemotherapy. The target liposome provides a new measure for brain glioma chemotherapy, contributes to the research of non-invasive therapy of the brain glioma, and has important theoretical meaning and clinical meaning.
Owner:PEKING UNIV
Pharmaceutical composition for prevention or treatment of fibrotic diseases and application thereof
The invention discloses application of nintedanib, its salt and solvate in preparation of drugs for prevention or treatment of fibrotic diseases and a pharmaceutical composition for prevention or treatment of fibrotic diseases. The pharmaceutical composition comprises an active component and excipients, wherein the active component is selected from one or more of nintedanib, its salt and solvate. The nintedanib preparation provided by the invention can achieve treatment or prevention of fibrotic diseases on VEGF, PDGF and FGF angiogenesis receptors, and can provide an effective drug concentration, thus reaching a good control effect.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
Novel Block Copolymer, Micelle Preparation, And Anticancer Agent Containing The Same As Active Ingredient
InactiveUS20080113028A1Good water solubilityEliminate side effectsBiocideOrganic active ingredientsSolubilityHigh concentration
A medicinal preparation is desired which has no harmful side effects such as hypersensitive reaction, heightens the water solubility of a sparingly water-soluble anticancer agent, maintains a high drug concentration in the blood, accumulates a drug in a tumor tissue at a high concentration, heightens the pharmacological effect of the sparingly water-soluble anticancer agent, and diminishes the side effects of the anticancer agent. Provided are: a novel block copolymer which can be a drug carrier having no harmful side effects such as hypersensitive reaction; a micelle preparation in which micelles are formed and which contains a sparingly water-soluble anticancer agent, especially paclitaxel, incorporated in the micelles in an amount necessary for a disease treatment without bonding it to the block copolymer and which can heighten the solubility of the drug in water; and an anticancer agent which comprises the micelle preparation as a medical ingredient, maintains a high concentration in the blood, has more potent drug activity, and is reduced in toxicity.
Owner:NIPPON KAYAKU CO LTD
Pharmaceutical compositions comprising drug and concentration-enhancing polymers
InactiveUS7887840B2High dissolution rateImprove solubilityPowder deliveryOrganic active ingredientsSolubilityPolymer
A solubility-improved drug form is combined with a concentration-enhancing polymer in a sufficient amount so that the combination provides substantially enhanced drug concentration in a use environment relative to a control comprising the same amount of the same drug form without the concentration-enhancing polymer.
Owner:BEND RES
Marker Detection Method And Apparatus To Monitor Drug Compliance
InactiveUS20140294675A1Accurate assessmentPatient complianceWithdrawing sample devicesDiagnostic recording/measuringNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Controlled-release colon targeting drug administration preparation and preparation method thereof
InactiveCN101780055AConvenient treatmentImprove complianceOrganic active ingredientsDigestive systemIntestinal tract diseasesColonic segment
The invention relates to a controlled-release colon targeting drug adminitration preparation. The forms of the preparation are colon site-specific coated tablets or colon targeting pellets. The preparation consists of a tablet core or pellet core, an isolating layer and a controlled-release coating layer, wherein the controlled-release coating layer comprises an internal coating layer and an external coating layer. By adopting the multilayer coating technology, enteric soluble acrylic resin water dispersion and osmotic acrylic resin water dispersion are used as main coating materials for carrying out coating, thereby obtaining the controlled-release colon targeting drug adminitration preparation. The preparation of the invention enables drugs to be released at a constant rate at a colon section, realizes accurate site-specific drug release, increases the concentration of the drugs at some parts of positions with pathological changes, is beneficial to treating ulcerative colitis and carcinoma of colon, avoids the stimulation of the drugs on stomaches and small intestines, achieves the goal of colon site-specific drug release, enhances the targeting site-specific curative effect on colon diseases and reduces the toxic and side effect. Compared with the common oral preparations, under the condition of the same drug adminitration dosage, the preparation of the invention can enhance the curative effect and reduce the incidence rate of untoward reactions. Compared with the enemas or the rectal suppositories, the preparation has the advantages of uniform drug distribution in the colon and good patient compliance.
Owner:ZHEJIANG UNIV
Taxine kind anti-cancer slow release injection
InactiveCN1923189AOrganic active ingredientsPharmaceutical delivery mechanismCelluloseAcetic acid ethenyl ester
The invention relates to a slow-release injection of taxine anti-cancer drug, which comprises anti-cancer drug, slow-release finding, suspension and / or solvent. Wherein, said anti-cancer drug is taxine, 2'-hydroxy Paclitaxel, etc; the slow-release finding is polymer of hydroxyl, glycollic acid and glycolic acid, one of acetic acid ethyenyl ester polymer and polyphony; the suspension is polyphenyl (sodium), and mannite; the solvent is distilled water, injection water, absolute ethyl alcohol, etc. The invention can be injected to reduce the toxicity effect of drug, and improve the density locally to strengthen the treatment effect of chemotherapy and radiation therapy.
Owner:孔庆忠
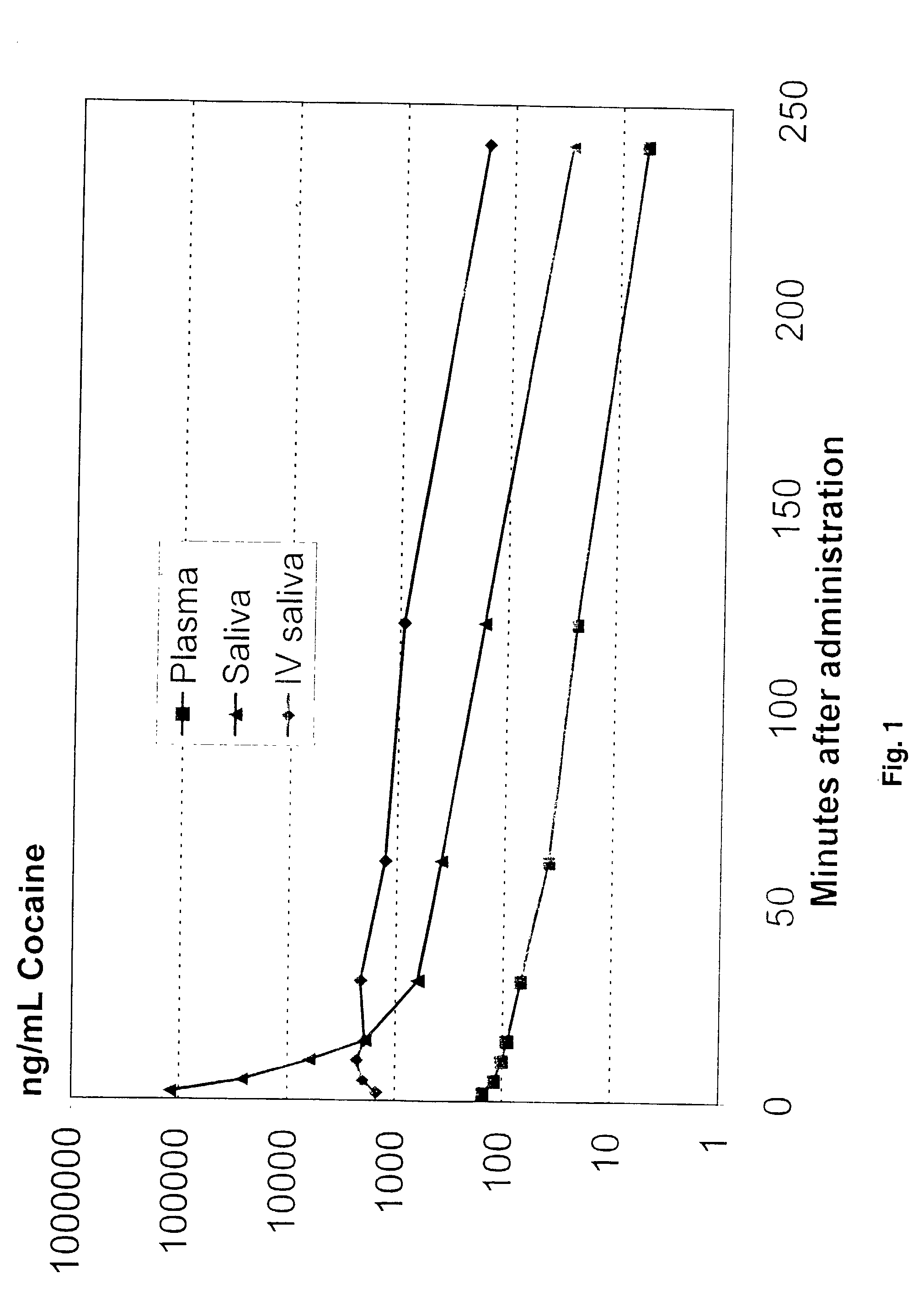
Ion selective electrodes for direct organic drug analysis in saliva, sweat, and surface wipes
InactiveUS20030121779A1Immobilised enzymesBioreactor/fermenter combinationsHydrophobic polymerMedication monitoring
A hand-held portable drug monitoring system to detect and quantitate cocaine and other organic drugs in saliva, sweat, and surface wipes by using an ion selective electrode or an array of ion selective electrodes. The ion selective electrode has a cast membrane reference electrode and a sensing electrode with a hydrophobic polymer, a plasticizer, and an ionophore selective for the organic drug to be tested. The ion selective electrode can be connected to a converter that coverts a voltage reading from the ion selective electrode to a quantitative drug concentration level. Also disclosed is the related method of using an ion selective electrode to detect an organic drug in saliva, sweat, and surface wipes, the method of testing electrical contact in an ion selective electrode, and the method of making a cast membrane reference electrode.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
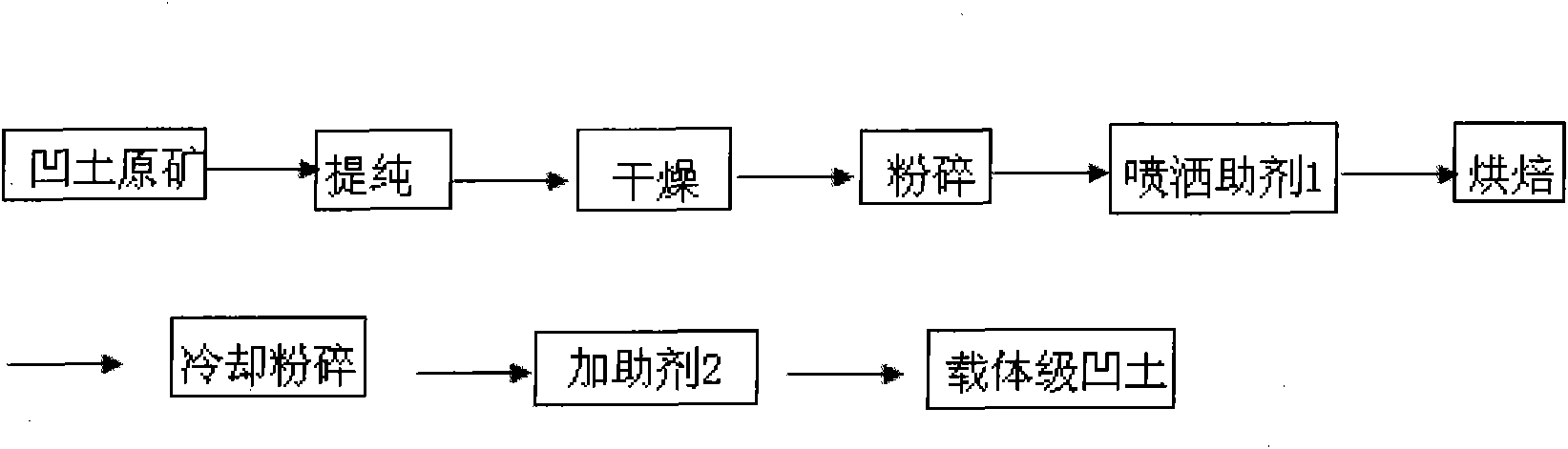
Preparation method and application of modified attapulgite clay
The invention discloses a preparation method of attapulgite high-performance pesticide carrier. The preparation method comprises the following steps: purifying attapulgite clay, drying, grinding, adding an additive, baking at a low temperature, cooling, grinding again, then adding another additive to mix evenly and prepare the pesticide carrier. The modified attapulgite obtained through a series of treatments has the original characteristics of attapulgite that the attapulgite has high absorbability and is inert to pesticide; the surface acid value of the original attapulgite can be reduced, the specific area can be increased, the water absorption rate can be reduced; the modified attapulgite can be used as pesticide carrier to replace white carbon black, thus the drug concentration can be reduced, the medical effect can be prolonged and the environmental pollutions caused by pesticide can be reduced. Therefore, the modified attapulgite has great significance in increasing the qualityof the Chinese powder pesticide, reducing the dosage of the Chinese pesticide and expanding the exports of the Chinese pesticide and carrier.
Owner:MINGGUANG TIANJIAO TECH DEV
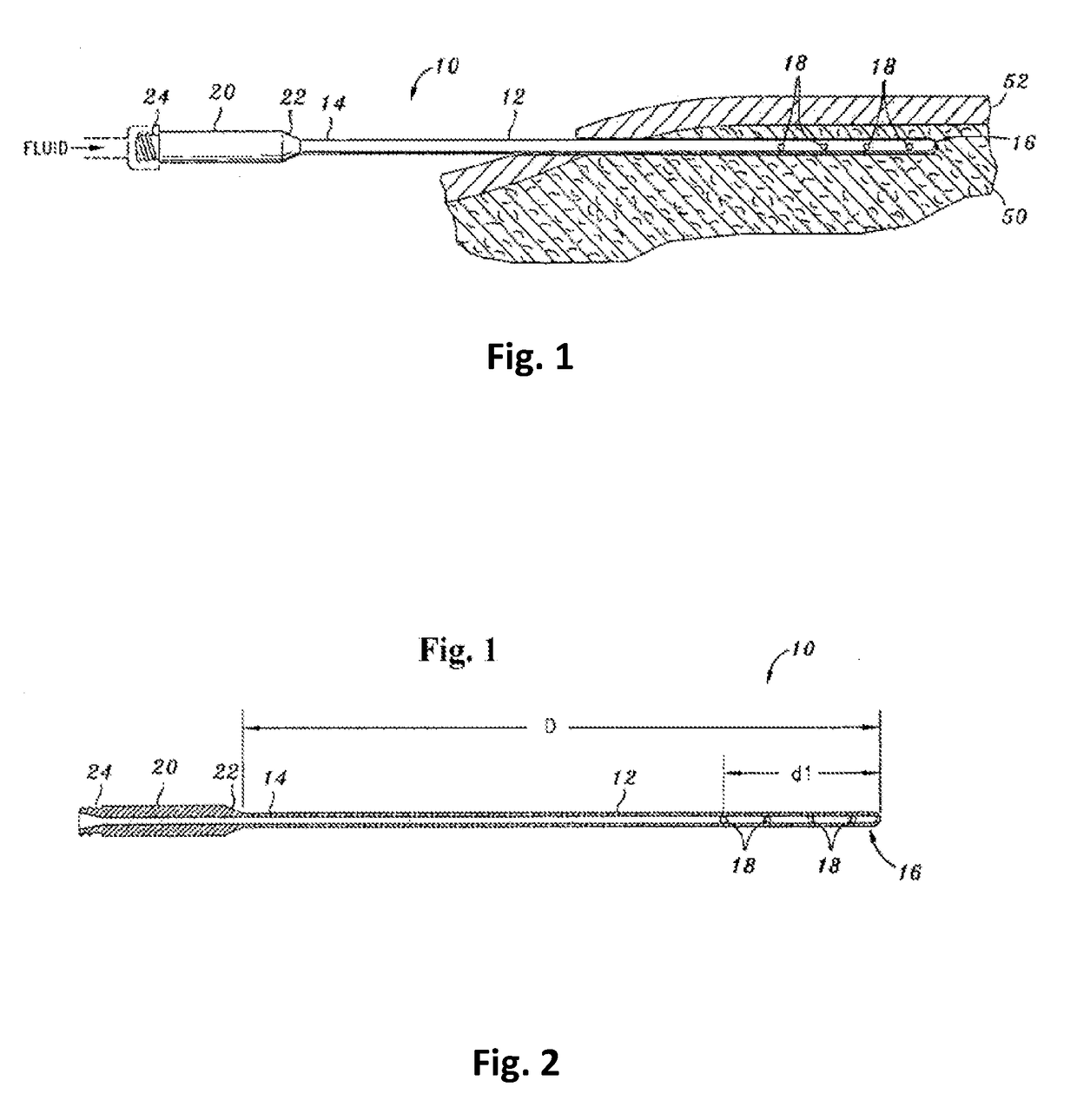
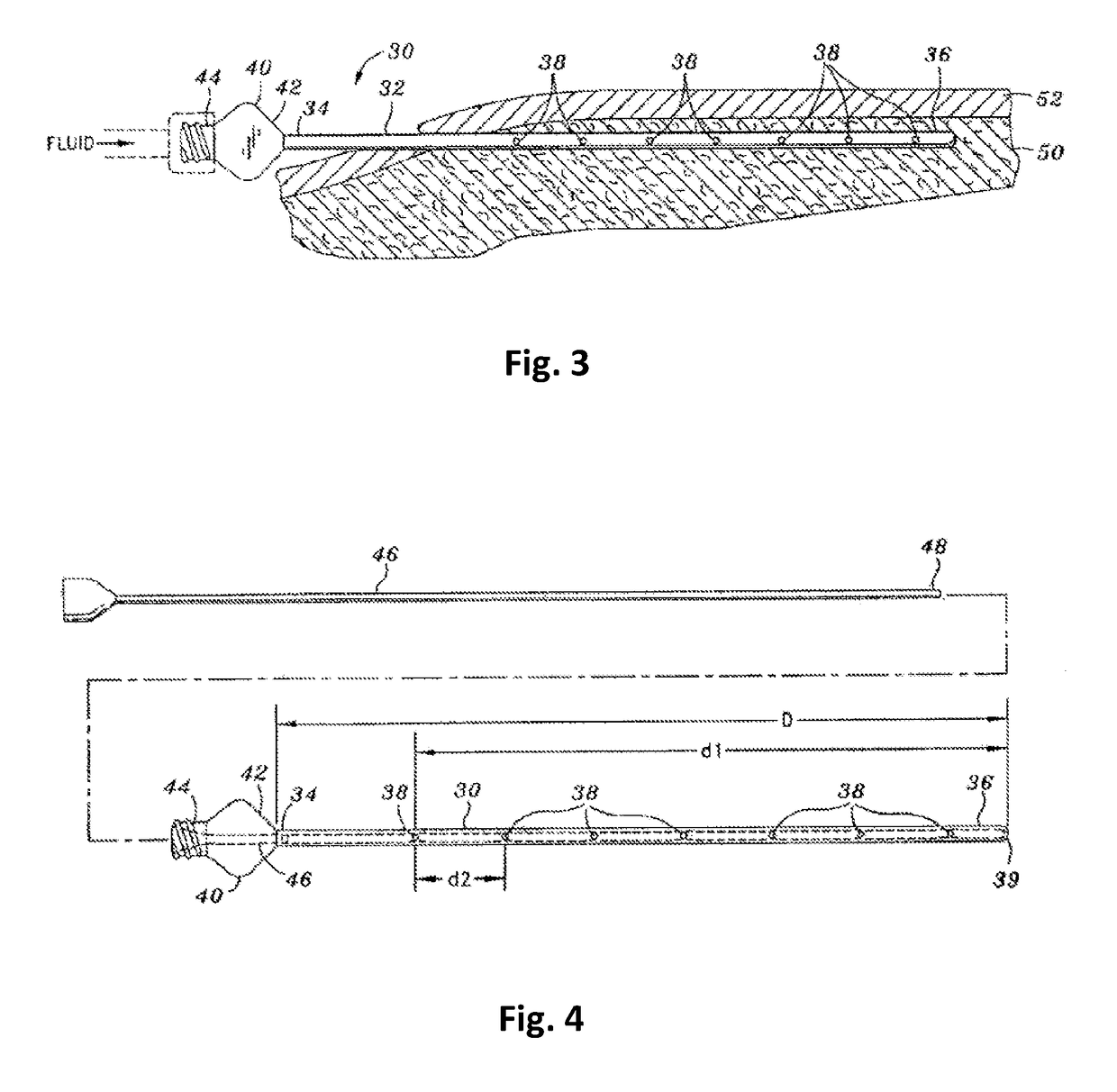
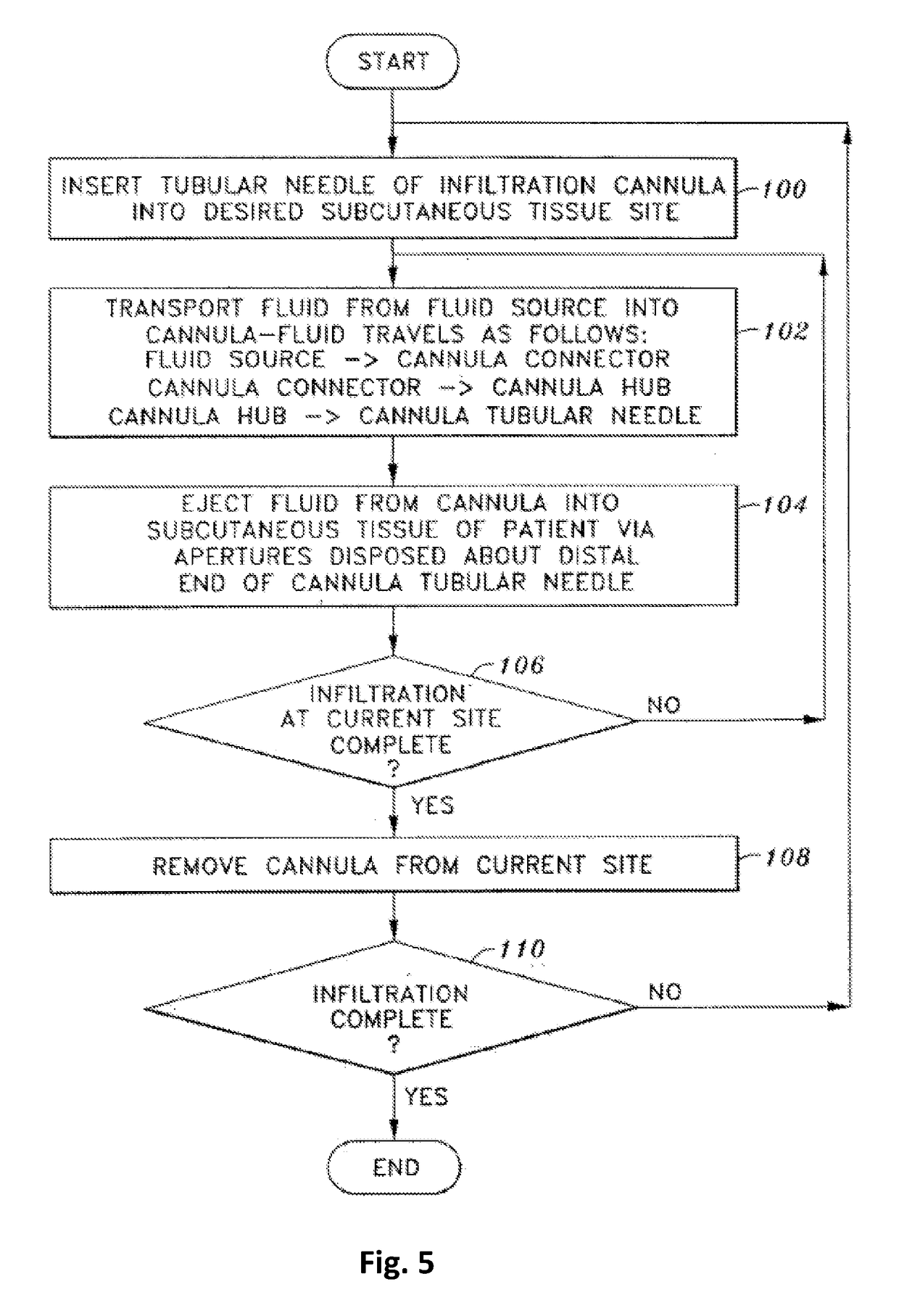
Tumescent infiltration drug delivery of high subcutaneous drug concentrations with prolonged local and systemic effects and minimal local or systemic toxicity
ActiveUS20170100331A1Increase local drug concentrationReduce blood viscosityInorganic non-active ingredientsPharmaceutical delivery mechanismTherapeutic effectVasoconstrictor Agents
Disclosed are methods of subcutaneous delivery of a drug or a therapeutic agent to a subject comprising administering to said subject a tumescent composition comprising: (a) the drug or the therapeutic agent, wherein a tumescent concentration of the drug is simultaneously: 1) below a threshold for local, subcutaneous tissue toxicity, 2) above a threshold for positive local therapeutic effect, and 3) above a concentration achievable by intravenous (IV), intramuscular (IM) or oral (PO) delivery; (b) a vasoconstrictor; and (c) a pharmaceutically acceptable carrier. Some embodiments relate to a method of treating or preventing sepsis or Systemic Inflammatory Response Syndrome (SIRS) in a subject. Some embodiments relate to a tumescent solution for treating a localized viral infection, e.g., varicella-zoster (shingles), the tumescent solution comprising an antiviral agent.
Owner:HK PHARMA INC
PH-triggering-to-release multilevel targeting polymer micelle in tumor cells and preparation method of multilevel targeting polymer micelle
ActiveCN106265510AImprove anti-tumor efficacyIncrease concentrationOrganic active ingredientsPharmaceutical non-active ingredientsCD44Drug release
The invention relates to pH-triggering-to-release multilevel targeting polymer micelle in tumor cells and a preparation method of the multilevel targeting polymer micelle. The micelle is formed by self assembly of hydrophobic modified polysaccharide polymer with an endosome pH sensitive characteristic in a water medium. A hyaluronic acid-deoxycholate-histidine polymer is used as a carrier, and caner initiatively-targeting endosome pH sensitive polymer micelle is prepared by virtue of an ultrasonic method or a dialysis method to wrap an antitumor drug difficult to dissolve. By using the synergistic mechanism of EPR-mediated passive targeting, CD44 receptor initiative targeting and pH sensitive targeting strategies, the 'whole-course targeting' guidance is performed at four medicine delivery key steps, i.e., blood long circulation, tumor tissue accumulation, cell digestion and intracellular drug release, so that multilevel targeting drug delivery is realized, the intracellular drug concentration is effectively increased, and a novel carrier and a preparation strategy are provided for improving the antitumor effect of the difficultly-soluble antitumor drug.
Owner:NINGXIA MEDICAL UNIV
Derivatives containing 2, 5-substituted heterocyclic radical sulphone and synthesis method and application thereof
The invention relates to derivatives containing 2, 5- substituted heterocyclic radical sulphone and a synthesis method and application thereof, and provides compounds which can prevent soil-borne diseases and diseases caused by migration, namely the derivatives containing 2, 5-substituted heterocyclic radical sulphone. The derivatives can be expressed by the following general expression shown in the specification, wherein the Z, X and R are defined in the specification. Parts of the compounds have good inhibition activity for the soil-borne diseases, the diseases caused by migration and otherfungus diseases. When the drug concentration reaches 50ppm, the inhibition ratio of a compound I-5 for fusarium graminearum, fusarium oxysporum f.sp.capsicum and cytospora mandshurica is 100%, 99.9% and 100% respectively, thus showing a preferable activity in preventing plant diseases.
Owner:GUIZHOU UNIV
Iontophoretic transdermal delivery device
A conducting silicone matrix incorporating a suspension of a drug in ionized and non-ionized phases in an emulsion of a hydrophobic polymer. In one version, the drug is prepared as a concentrated aqueous suspension incorporated in a silicone matrix with a silicone surfactant. An electrolyte may be incorporated into the silicone matrix for increasing its conductivity. When a current is applied, the drug in individual globules in the drug suspension migrates away from the electrode and becomes concentrated at the distal side of the globules eventually resulting in an increase in the drug concentration distal to the electrode and adjacent to the skin and thereby resulting in transfer of the active drug through the skin. This system provides a matrix with minimal electroendosmotic flow that is current efficient and provides a drug reservoir that can last for several days during application of the drug.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Anti solid tumor medicine composition
InactiveCN1628850AImprove anti-cancer effectInhibitory activityAntineoplastic agentsPharmaceutical active ingredientsHydroxylamineAdjuvant
Disclosed is an anti solid tumor medicine composition, which comprises medicinal adjuvant and DNA restoring enzyme inhibitor and / or Nitrosoureas anti-cancer drugs, wherein the DAN restoring enzyme inhibitor is selected from methoxamine, hydroxylamine and their analogues, which can effectively destroy the DNA restoring function in the tumor cells, and lower the survivability of tumor cell to Semustine anti-cancer drugs and their analogues, the medicinal adjuvant is biological compactable, degradable and absorbing macromolecular polymer, which can slowly release the DNA restoring enzyme inhibitor onto tumor partially during the degradation and absorption process, thus the whole body toxicity reaction is reduced appreciably , and the effective medicinal concentration can be sustained to the tumor partially. By dispensing the composition to the tumor partially, the whole body toxicity reaction of Nitrosoureas anti-cancer drugs and / or DNA restoring enzyme inhibitor can be lowered, selectively increase the tumor local medicinal concentration, and the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can be improved.
Owner:南京天一药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com