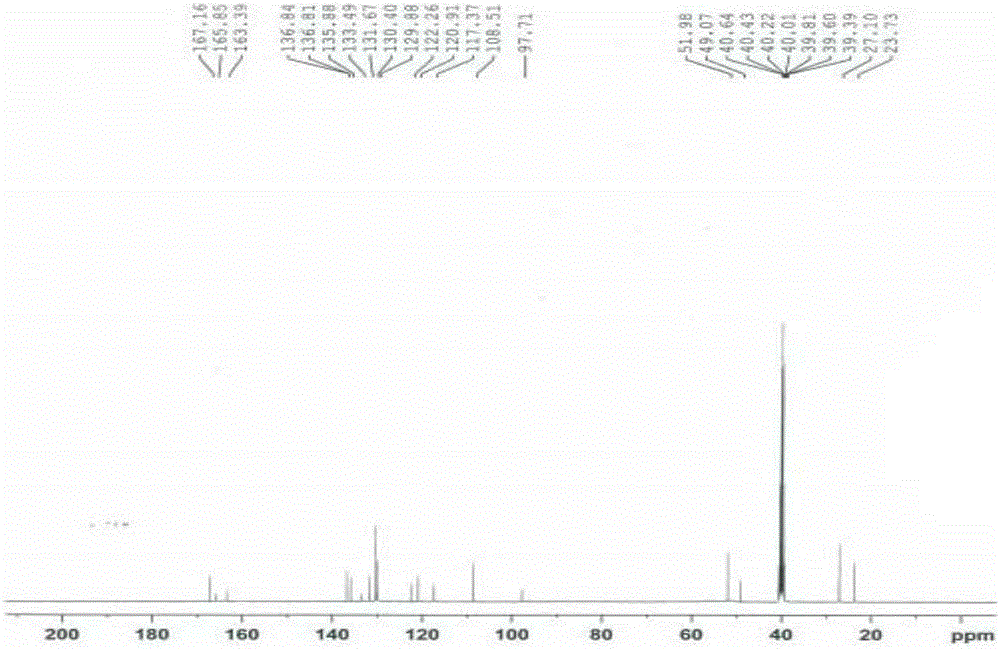
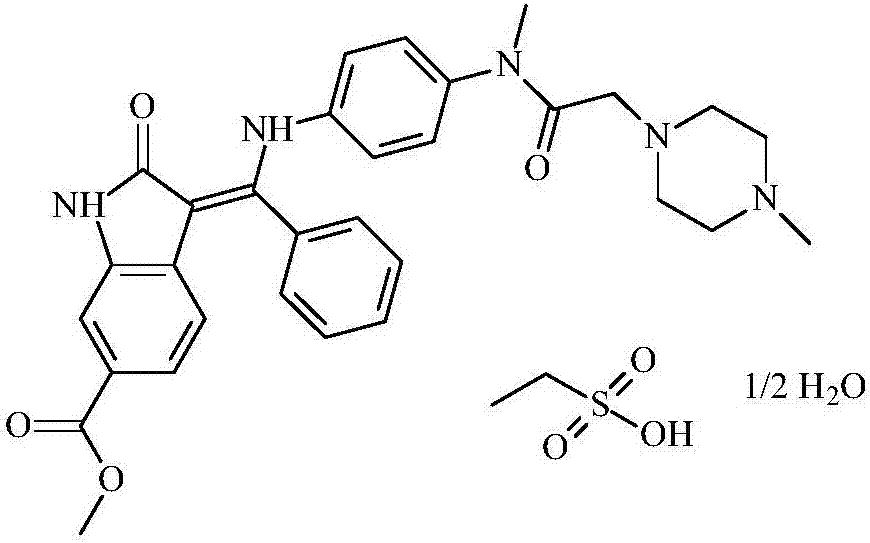
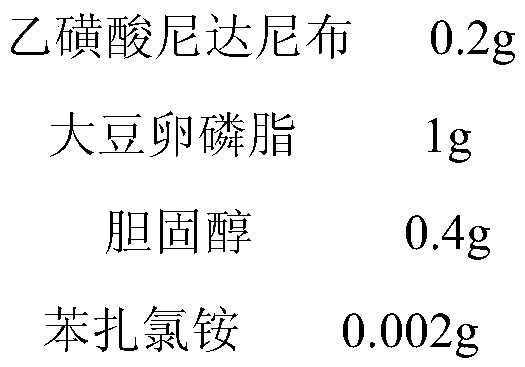
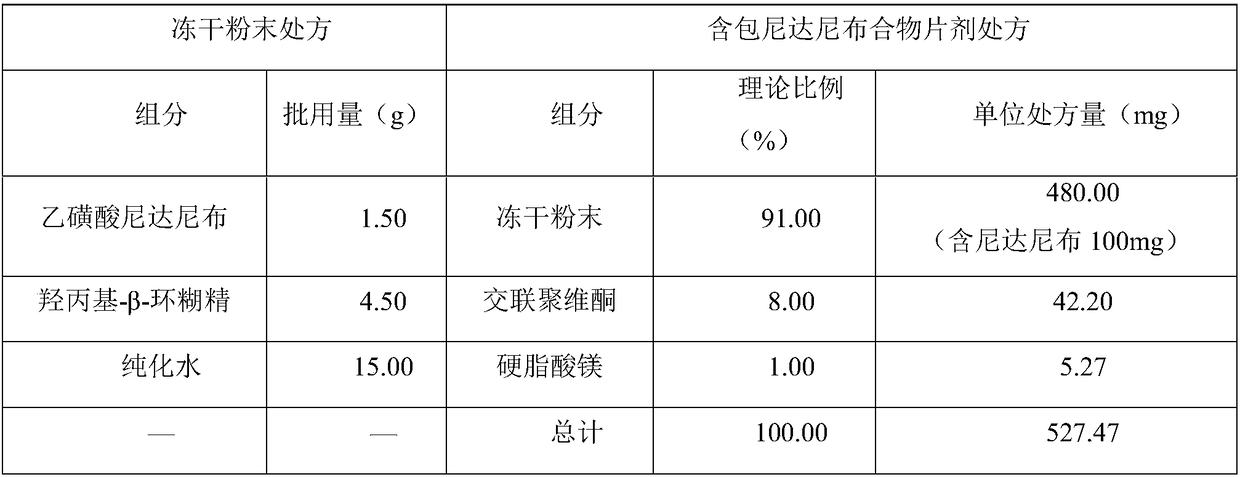
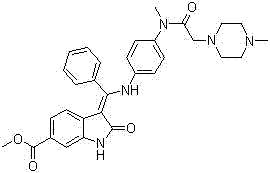
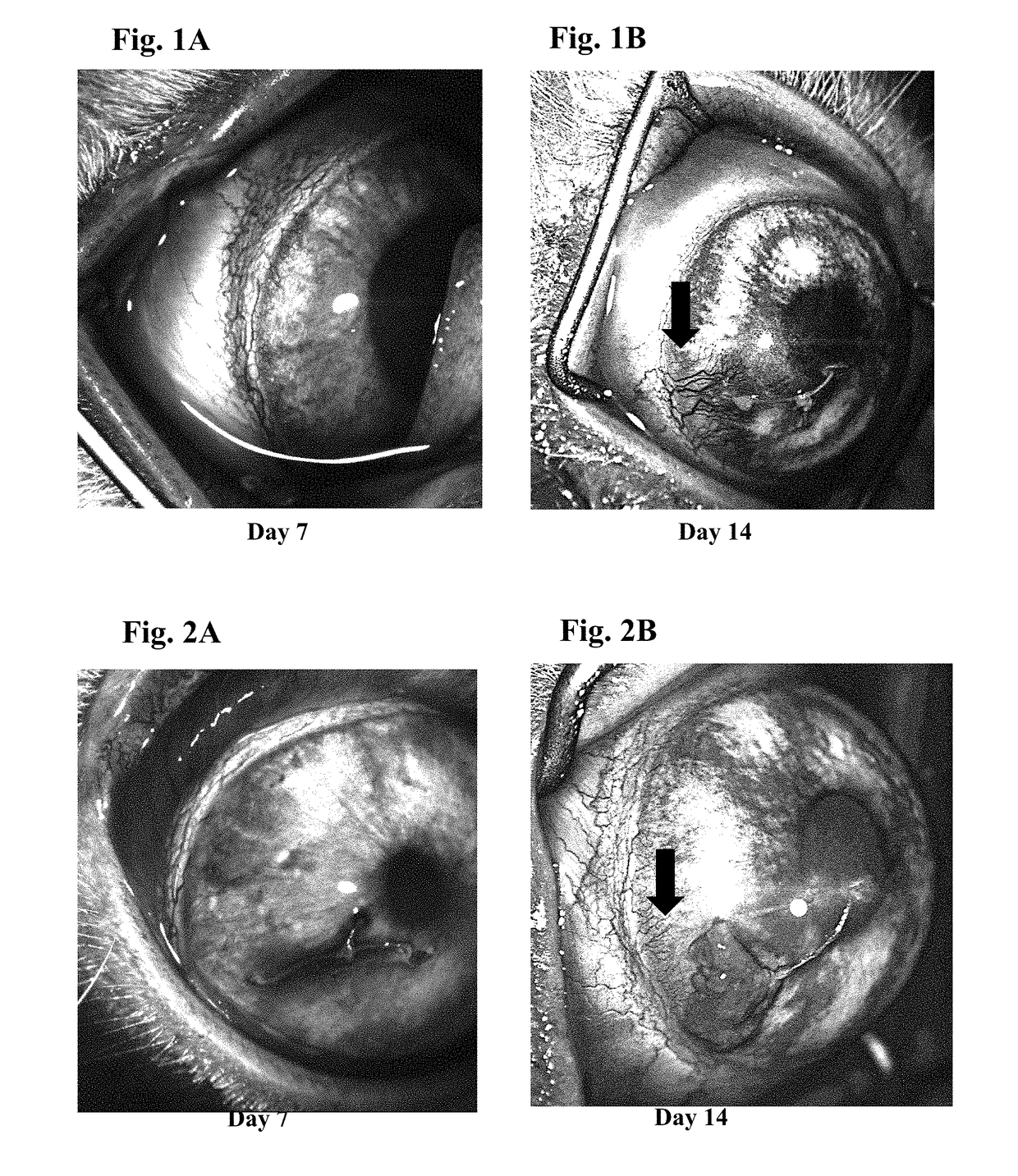
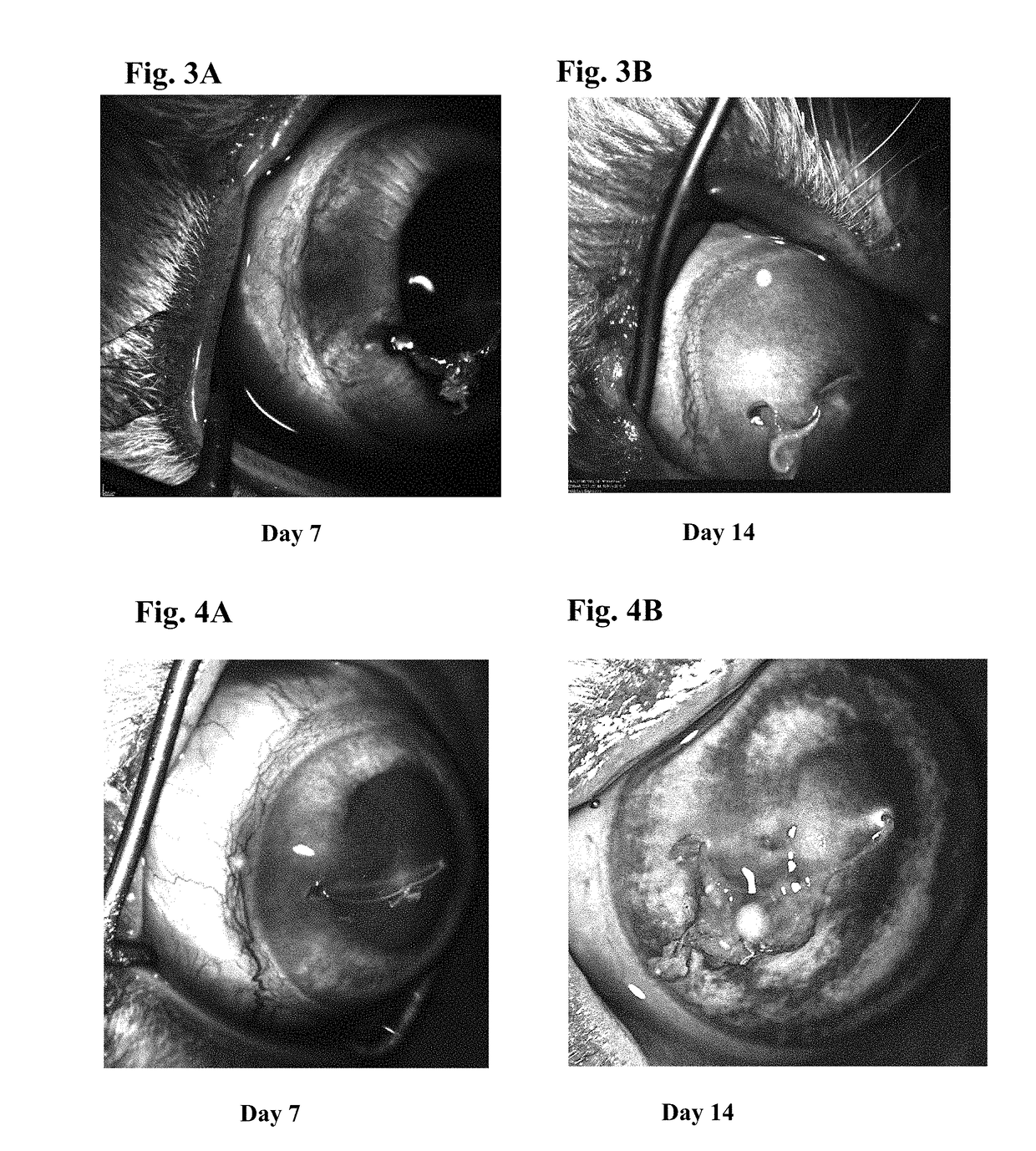
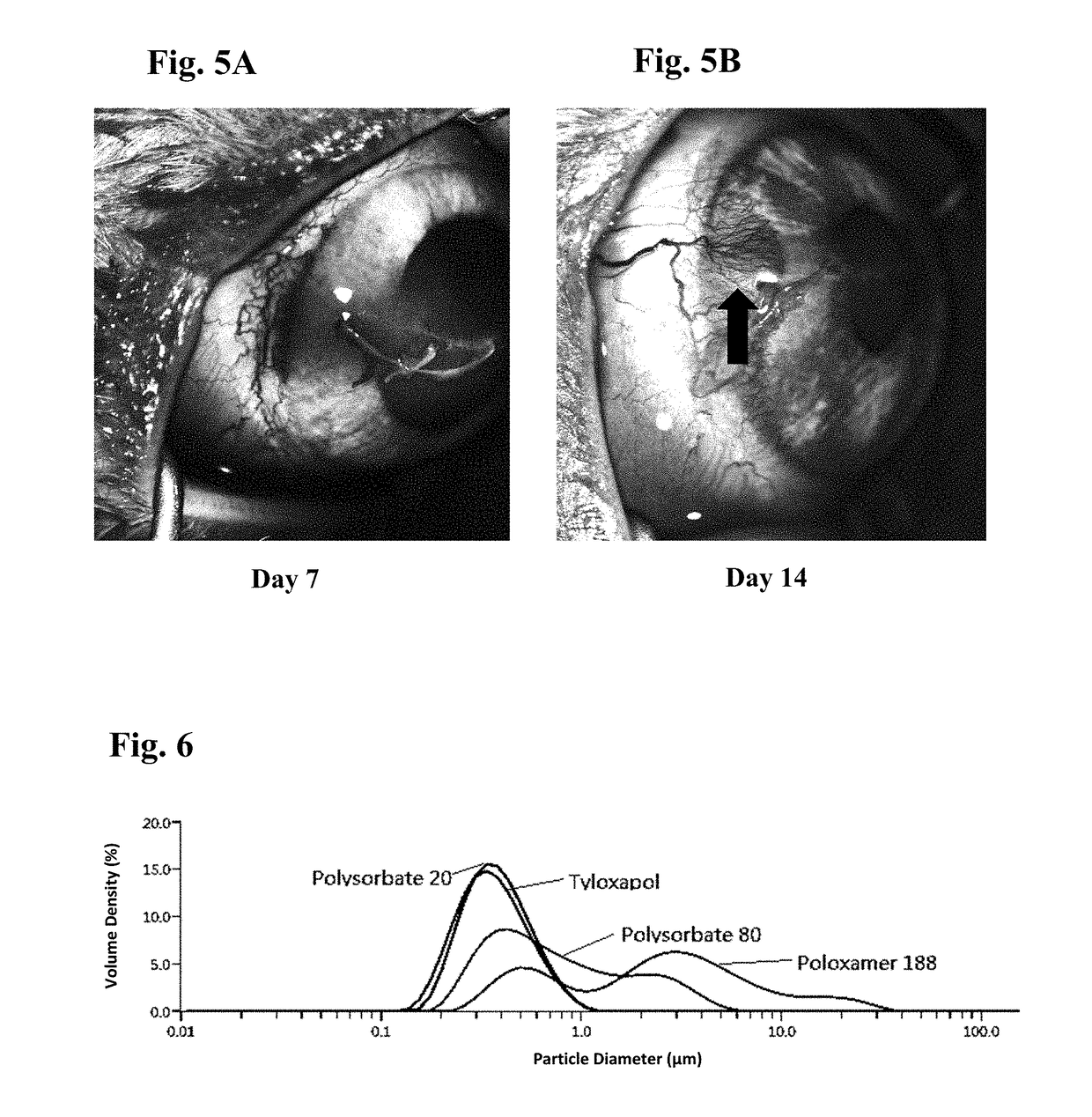
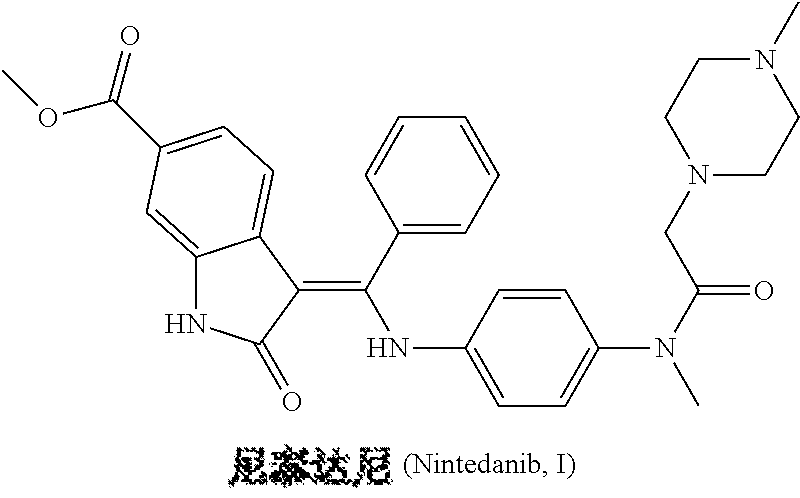
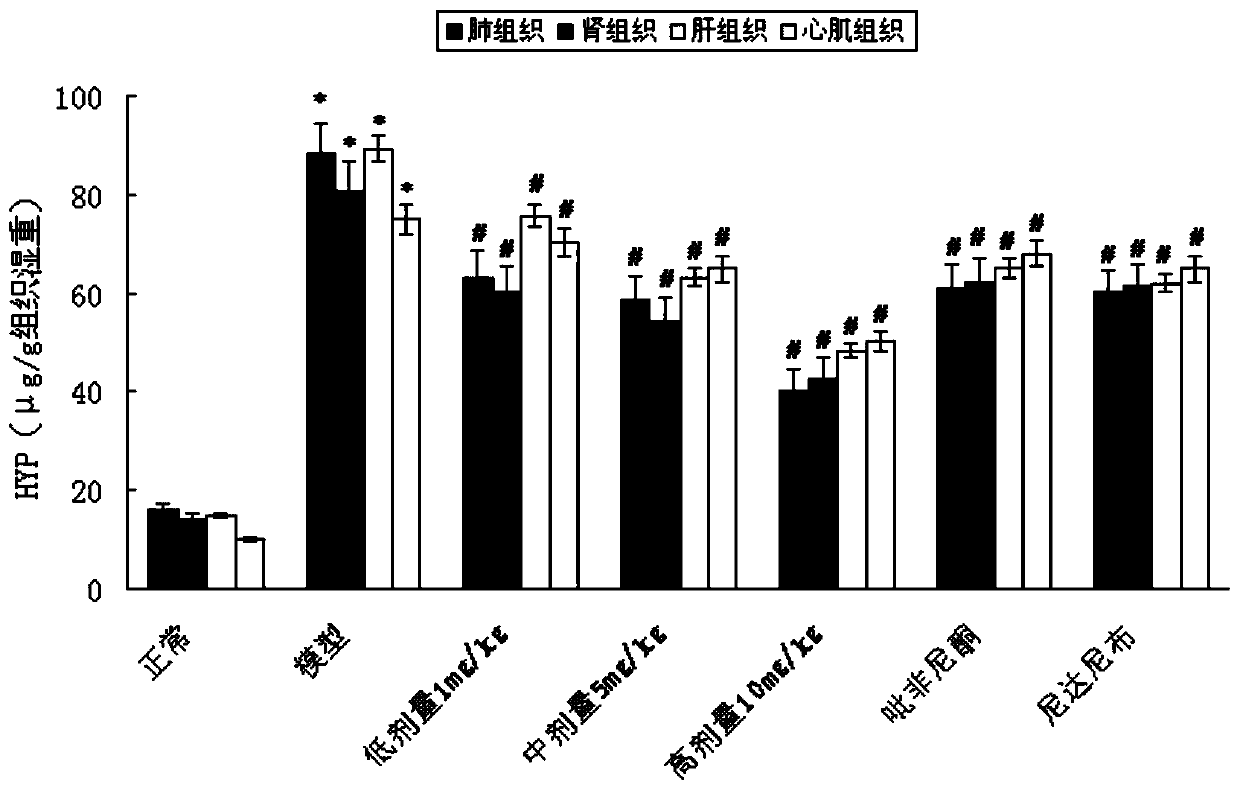
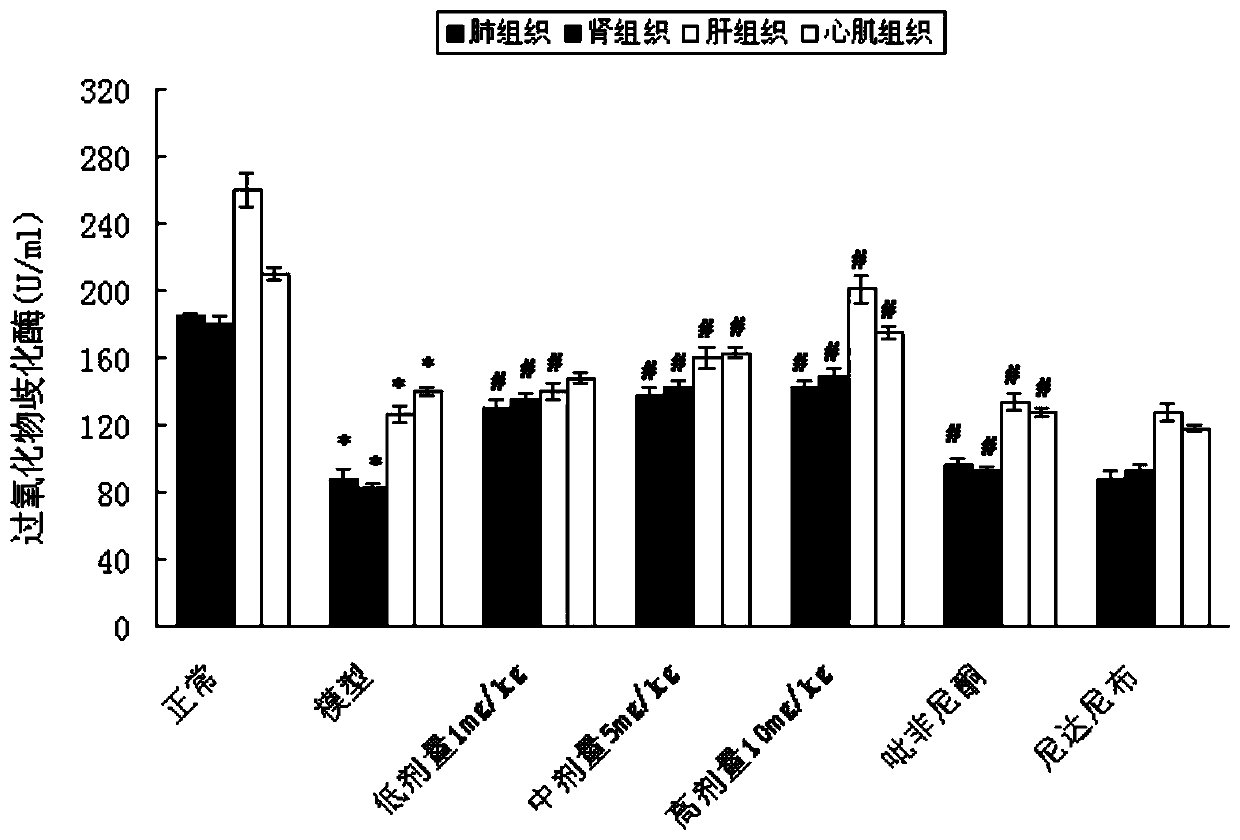
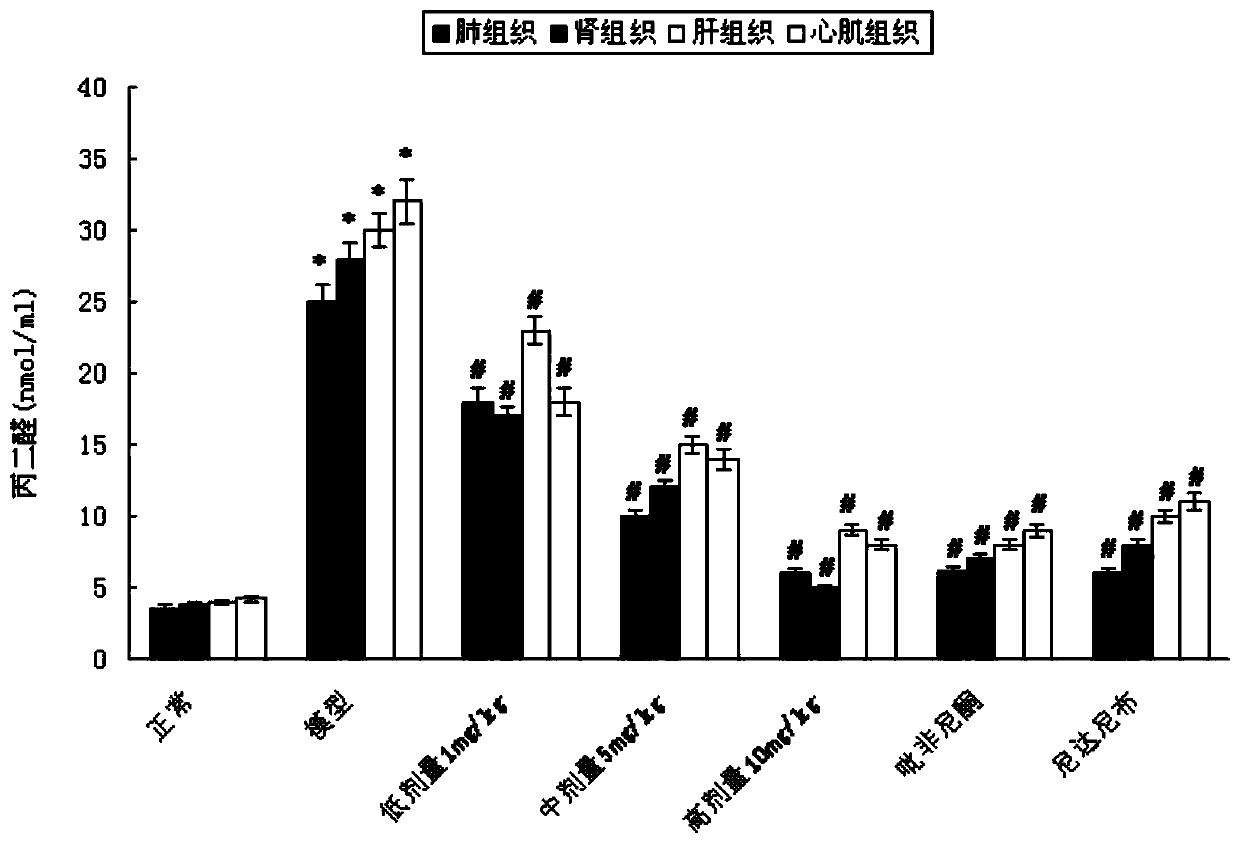
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Nintedanib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a certain lung disease (idiopathic pulmonary fibrosis- IPF).
Pharmaceutical composition for prevention or treatment of fibrotic diseases and application thereof
The invention discloses application of nintedanib, its salt and solvate in preparation of drugs for prevention or treatment of fibrotic diseases and a pharmaceutical composition for prevention or treatment of fibrotic diseases. The pharmaceutical composition comprises an active component and excipients, wherein the active component is selected from one or more of nintedanib, its salt and solvate. The nintedanib preparation provided by the invention can achieve treatment or prevention of fibrotic diseases on VEGF, PDGF and FGF angiogenesis receptors, and can provide an effective drug concentration, thus reaching a good control effect.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
Preparation method of nintedanib
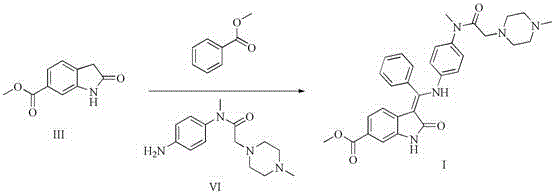
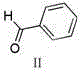
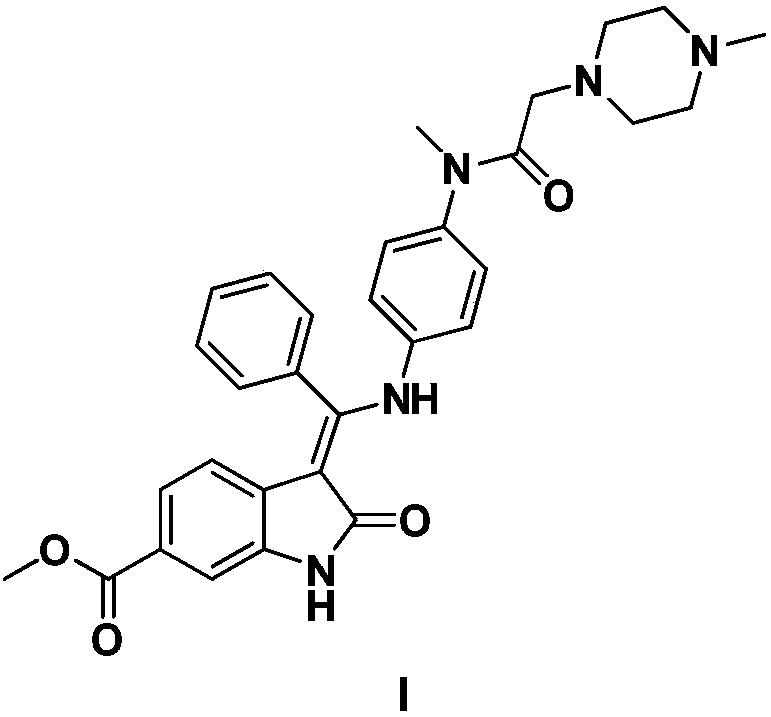
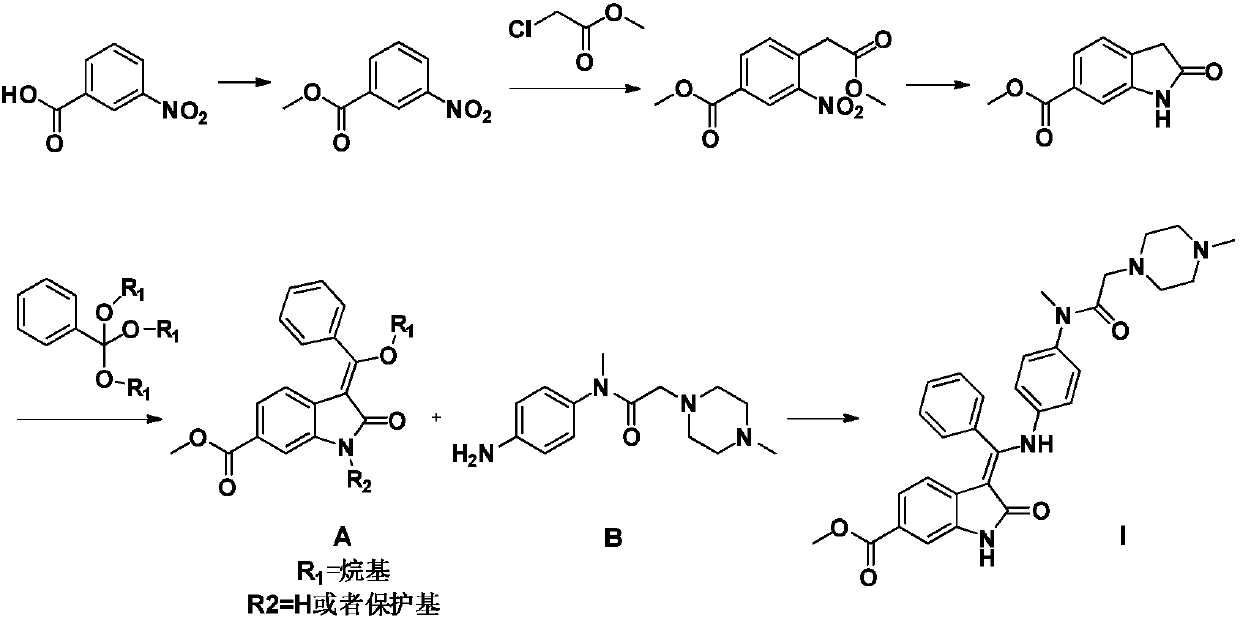
The invention relates to a preparation method of nintedanib (I). The preparation method comprises the steps that 2-oxoindole-6-methyl formate (III) and benzaldehyde (II) are used as raw materials to generate a condensation reaction, so as to obtain a compound IV; then halogen or halogenating reagents are added to generate a substitution reaction, so as to obtain a compound V; the compound V and the compound IV are condensed under the action of alkali, so as to obtain the nintedanib (a compound I). The method has the advantages of short reaction time, low cost, high yield and environmental friendliness of used reagents and is suitable for industrial production. The structural formulas are shown in the description.
Owner:HANGZHOU SINOPEP ALLSINO PHARMA TECH DEV CO LTD
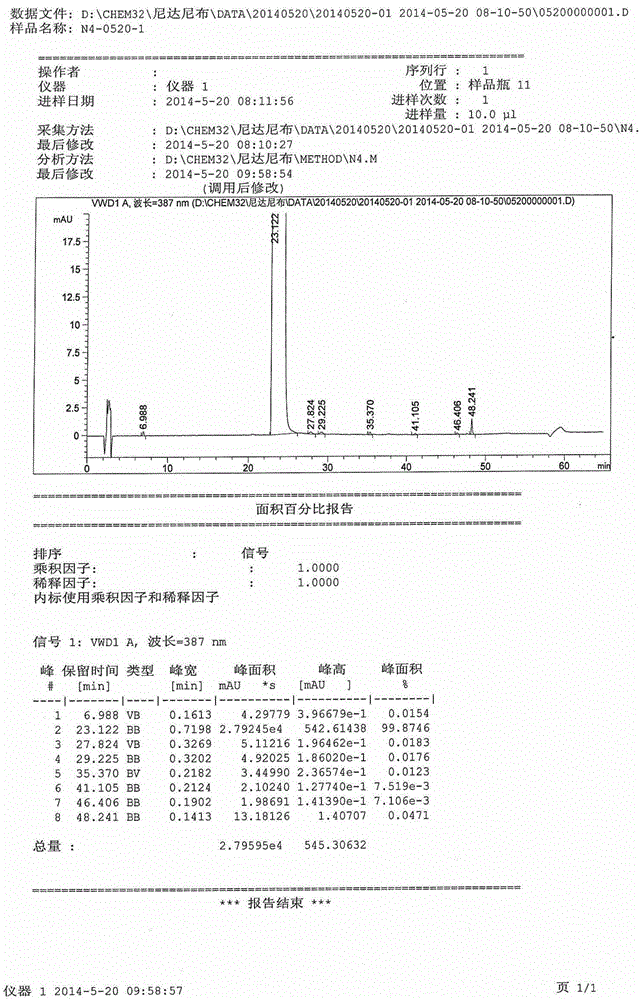
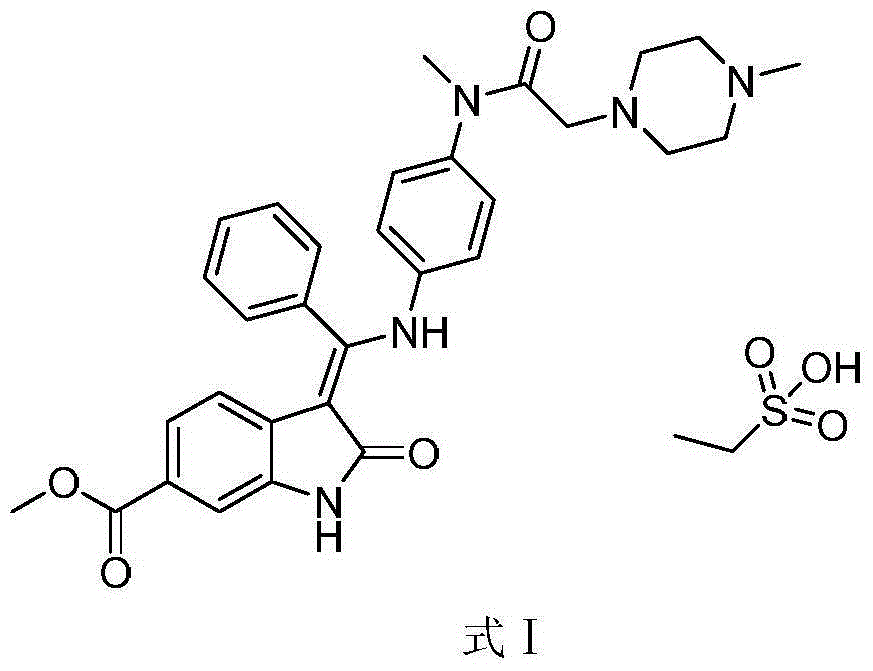
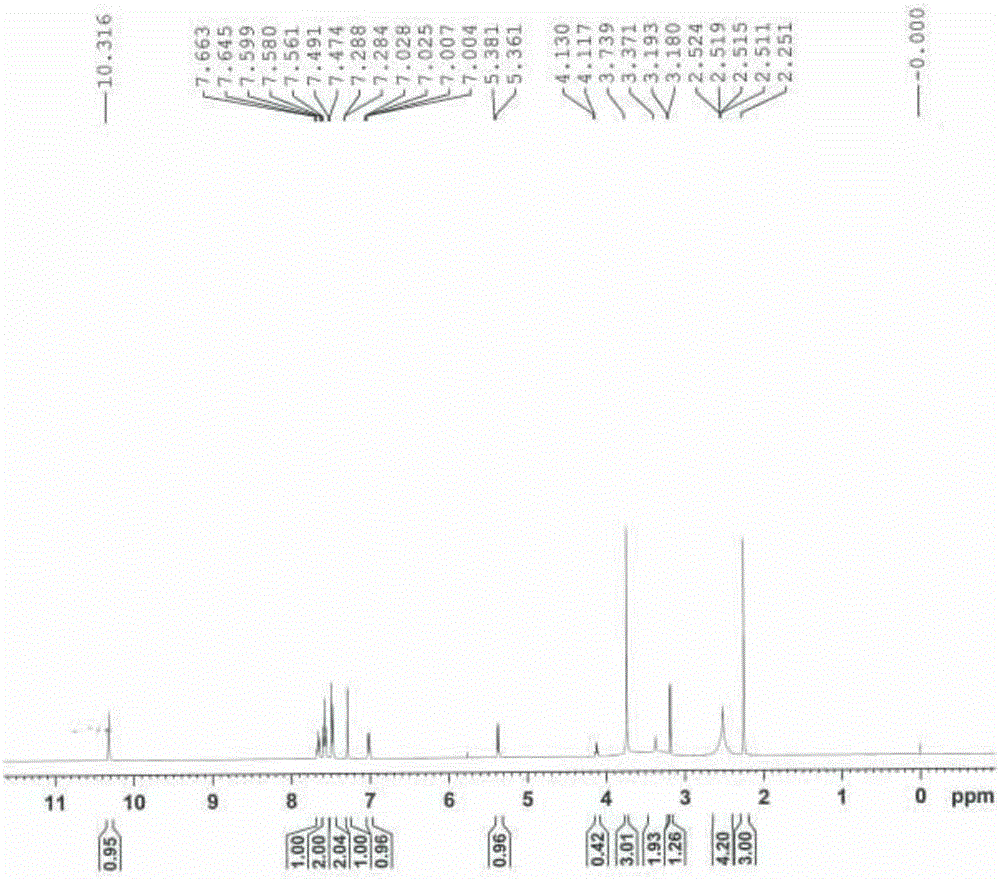
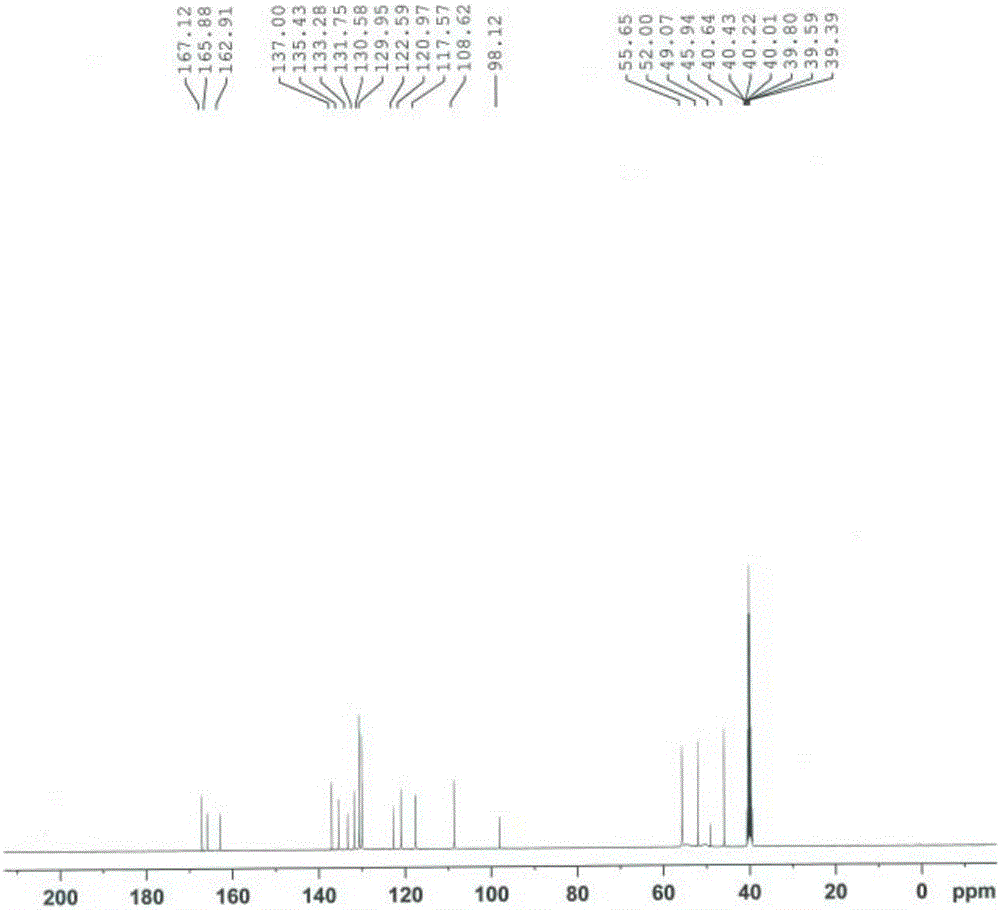
Method for preparing high-purity ethanesulfonic acid nintedanib
The invention discloses a refining method for preparing ethanesulfonic acid nintedanib. Through a refining mode of combining recrystallization and backflow washing, the impurity content in a finished product is reduced remarkably, so that the medication safety of medicine is guaranteed, and the refining method is simple in process and suitable for industrialized batch production.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
A synthetic method of Nintedanib and an intermediate of Nintedanib
ActiveCN105837493AReasonable designLow reaction temperatureOrganic chemistryAcetic acidChloroacetic acids
A synthetic method of Nintedanib and an intermediate of Nintedanib are disclosed. The method includes reacting a compound shown as a formula (II) and a compound shown as a formula (I) under acidic conditions to produce a compound shown as a formula (III), removing t-butyloxycarboryl of the compound shown as the formula (III) with an acid, adding an alkali, reacting to produce a compound shown as a formula (V), reacting the compound shown as the formula (V) with an activated derivative of chloroacetic acid to produce a compound shown as a formula (VI), and reacting with N-methyl piperazine to produce the Nintedanib. The novel synthetic method with mild reaction conditions for the Nintedanib is provided. The intermediate for synthesizing the Nintedanib is also provided.
Owner:SOUTHEAST UNIV
Potential impurity compound of Nintedanib, as well as preparation method, application and detection method of potential impurity compound
InactiveCN106748960ARaise quality standardsEnsure medication safetyOrganic chemistryComponent separationImpurityQuality standard
The invention relates to a potential impurity compound of Nintedanib, and a preparation method, application and detection method of the potential impurity compound. The structure of the potential impurity compound is shown in the formula (as shown in the specification). The invention aims to improve the quality standard of Nintedanib through study on impurities to guarantee the safe medication of Nintedanib. According to the preparation method disclosed by the invention, the pure potential impurity compound of Nintedanib is obtained, so that a reference substance for qualitative and quantitative analysis is provided for the detection of a finished Nintedanib product.
Owner:REYOUNG PHARMA
Nintedanib self-microemulsion preparation and soft capsule thereof and preparation method
ActiveCN107184549AImprove bioavailabilityHigh dissolution rateOrganic active ingredientsCapsule deliveryIrritationOil phase
The invention provides a Nintedanib self-microemulsion preparation and a soft capsule thereof and a preparation method. The concentration of nintedanib in the nintedanib self-microemulsion preparation is 2%-3% by mass; the proportion of oil phase in the nintedanib self-microemulsion preparation is 20%-40% (w / w); the proportion of co-emulsifier in the nintedanib self-microemulsion preparation is 20%-60% (w / w); the proportion of stabilizer in the nintedanib self-microemulsion preparation is 0-3% (w / w). by means of the self-microemulsion technology, the prepared Nintedanib self-microemulsion preparation is used to produce the soft Nintedanib self-microemulsion capsule. Since the Nintedanib self-microemulsion preparation can be quickly emulsified after encountering with gastrointestinal aqueous environment, the irritation generated after long contact of drugs and gastrointestinal tracts is reduced; meanwhile, the self-microemulsion drug delivery system can not only improve the dissolution rate of drugs, but increase the absorption rate and degree of drugs, and therefore, the bioavailability of drugs is higher.
Owner:JIANGSU UNIV
Impurity compound of Nintedanib, as well as preparation method, application and detection method of impurity compound
InactiveCN106748961ARaise quality standardsEnsure medication safetyOrganic chemistryComponent separationImpurityQuality standard
The invention relates to an impurity compound of Nintedanib, as well as a preparation method, application and detection method of the impurity compound. The structure of the impurity compound is shown in the following formula I (as shown in the specification), or in the following formula II (as shown in the specification). The invention aims to improve the quality standard of Nintedanib through study on impurities to guarantee the safe medication of Nintedanib. According to the preparation method disclosed by the invention, the pure impurity compound of Nintedanib is obtained, so that a reference substance for qualitative and quantitative analysis is provided for the detection of a finished Nintedanib product.
Owner:REYOUNG PHARMA
Ethanesulfonic acid nintedanib preparation and application thereof
InactiveCN105902507AOrganic active ingredientsInorganic non-active ingredientsEthanesulfonic acidPharmaceutical drug
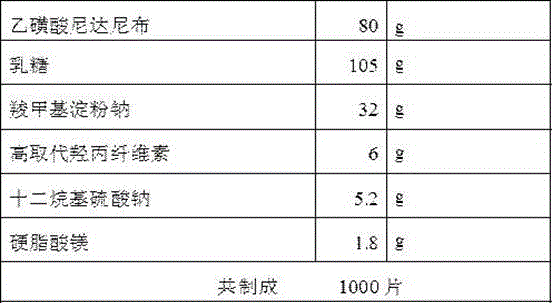
The invention discloses an ethanesulfonic acid nintedanib preparation and application thereof. The ethanesulfonic acid nintedanib preparation is prepared from ethanesulfonic acid nintedanib, lactose, microcrystalline cellulose and a pharmaceutically-acceptable carrier, and the ethanesulfonic acid nintedanib preparation with the good flowability, stability and dissolution rate can be obtained, so that the ethanesulfonic acid nintedanib preparation is suitable for large industrial production. The ethanesulfonic acid nintedanib preparation is the pharmaceutical composition for treating IPF, the compatibility is reasonable, medicine can be rapidly released, and the good treatment effect on the disease can be generated.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
Preparation method of nintedanib ethyl sulfonate
InactiveCN107011241AReduce pollutionLow reaction temperatureSulfonic acids salts preparationEthanesulfonic acidSulfonate
The invention discloses a preparation method of nintedanib ethyl sulfonate. The method comprises the steps of reacting by taking 2-oxoindole-6-carboxylic acid methyl ester, triethyl orthobenzoate and acetic anhydride as starting materials to obtain a compound; and carrying out condensation on (Z)-1-acetyl-3-(ethoxy (phenyl) methylene)-2-oxo indoline-6-carboxylic acid methyl ester and the starting material N-(4-aminophenyl)-N-methyl-2-(4-methyl piperazine-1-yl) acetamide to obtain nintedanib free alkali and carrying out salt-forming reaction of ethanesulfonic acid to prepare the nintedanib ethyl sulfonate. The method is short in step and easy to treat, and a high-quality nintedanib ethyl sulfonate crystal product is obtained without purification.
Owner:常州佳德医药科技有限公司
Application of nintedanib to prevention and treatment of ocular diseases
InactiveCN108295072AEffective drug concentrationGood control effectOrganic active ingredientsSenses disorderDiseaseTherapeutic effect
The invention discloses application of nintedanib to prevention and treatment of ocular diseases, specifically to application of nintedanib and a salt or polymorph thereof and a nintedanib hydrate orsolvate as active ingredients in drugs used for prevention and treatment of ocular diseases. The related pharmaceutical compositions of nintedanib in the invention can treat ocular diseases directed at three angiogenesis receptors including VEGF, PDGF and FGF; and the application frequency of the pharmaceutical compositions is no more than 5 times a day, and the pharmaceutical compositions can provide an effective drug concentration, so good prevention and treatment effects are obtained.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
Method for utilizing microchannel reactor for synthesizing Nintedanib intermediate
InactiveCN107674043AHigh temperature degradation impurities are reducedReduce energy consumptionOrganic chemistryChemical/physical/physico-chemical microreactorsSynthesis methodsHydrogen combustion
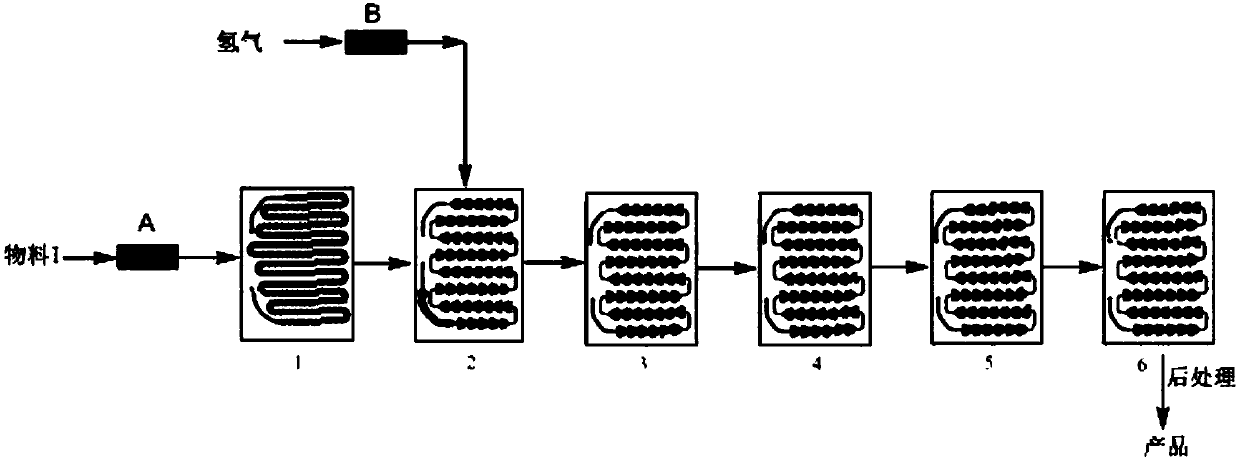
The invention discloses a method for synthesizing a nintedanib intermediate by using a microchannel reactor, and belongs to the technical field of synthesis of antitumor drugs. The inventive method is that N-(4-nitrophenyl)-N,4-dimethyl-1-piperazine acetamide is added in an organic solvent, and then the active carbon catalyst of loading 10% Pd is added as material I, and The material I is sent to the preheating module of the microchannel reactor for preheating; the preheated material I is sent to the reaction module group of the microchannel reactor, and the hydrogen is directly sent to the reaction module group of the microchannel reactor , make hydrogen and material I carry out reductive hydrogenation reaction, collect the reaction liquid that flows out from microchannel reactor outlet, aftertreatment obtains N-(4-aminophenyl)-N-4-dimethyl-1-piperazine B amides. The synthesis method of the invention can effectively shorten the reaction time, greatly reduce the potential safety hazard of hydrogen leakage, combustion and explosion, and is suitable for synthesizing the nintedanib intermediate.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Ethanesulfonic acid nintedanib dispersible tablet and preparation method thereof
InactiveCN105963268ADisintegrates quicklyFast absorptionOrganic active ingredientsPill deliveryEthanesulfonic acidIdiopathic pulmonary fibrosis
The invention discloses an ethanesulfonic acid nintedanib dispersible tablet used for treating idiopathic pulmonary fibrosis (IPF). Ethanesulfonic acid nintedanib serves as the raw material, auxiliary materials are added, and the ethanesulfonic acid nintedanib dispersible tablet is prepared. The ethanesulfonic acid nintedanib dispersible tablet is rapid to disintegrate and absorb, high in bioavailability, convenient to take, less in intestinal tract residue, few in side effect and sweet in taste and has fragrance, medicine taking compliance of patients is particularly easy to improve, the taste of the preparation is improved, the sticking phenomenon cannot occur in the tabletting process, and industrial production is promoted.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
Method for preparing Nintedanib ethylsulfonate
ActiveCN109988094AHigh purityHigh yieldOrganic compound preparationSulfonic acids salts preparationPyrrolidineSolvent
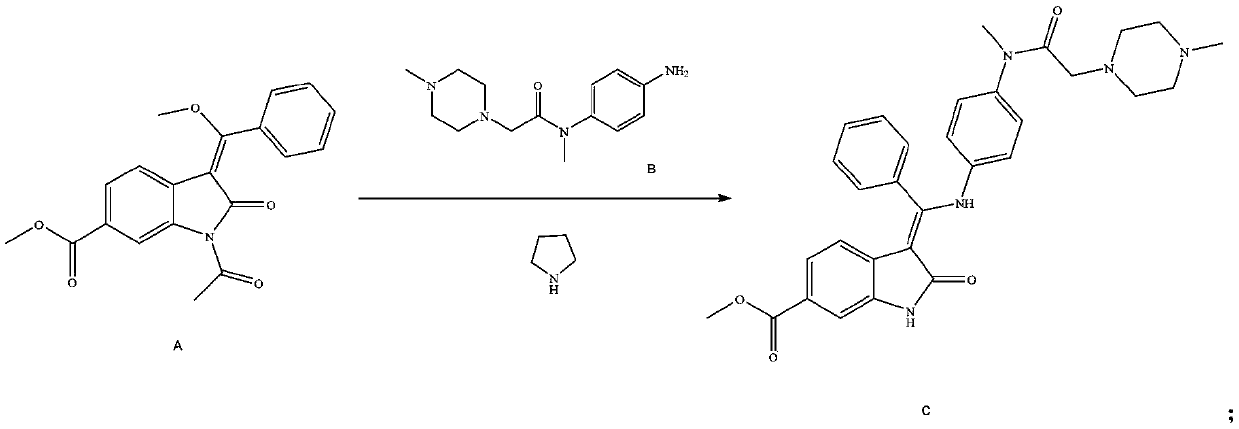
The invention relates to a method for preparing Nintedanib ethylsulfonate. The method comprises the following steps: (1) adding 1-acetyl-3-[methoxy(phenyl)methylene]-2-oxo-indolinyl-6-methyl formate (compound A) into a reaction solvent to react with N-(4-amino phenyl)-N,4-dimethyl-1-piperazinyl acetamide (compound B), adding pyrrolidine for continuous reaction after the reaction ends up, carryingout crystallization, carrying out stirring washing with a mixed solvent, and carrying out drying, so as to produce (3Z)-3-{[(4-{methyl-[(4-methyl piperazin-1-yl)acetyl]amino}phenyl)amino]-(phenyl)methylene} -2-oxo-2,3-indolinyl-6-methyl formate (compound C); and (2) subjecting the compound C to a reaction with ethyl sulfonic acid, carrying out crystallization, carrying out filtering, and carryingout drying, thereby producing the Nintedanib ethylsulfonate. According to the method, the Nintedanib ethylsulfonate with high purity, good yield and low impurity level is obtained through reactions oftwo steps.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Nintedanib inclusion compound and preparation and preparation method thereofof preparation
PendingCN108144069AImprove solubilityImprove bioavailabilityOrganic active ingredientsPill deliveryTherapeutic effectPharmaceutical Aids
The invention provides a Nnintedanib inclusion compound and preparation and a preparation method thereofof the preparation. The inclusion compound comprises a main drug and an inclusion material, wherein the main drug is Nnintedanib or pharmaceutically acceptable salt in pharmacy, and the inclusion material is cyclodextrin or a derivative of cyclodrxtrin. The inclusion compound can sharply improvethe drug solubility of a simulated intestinal solution, the probability of the drug being separated out in the intestinal canal is lowered, and accordingly the bioavailability of the drug is improved. The inclusion compound and the preparation prepared from the inclusion compound and pharmaceutically acceptable ingredients in the pharmacy can outstandingly improve the dissolution degree and the dissolution rate of the drug in the simulated intestinal solution, the bioavailability of the drug is improved, meanwhile, the inclusion compound and the preparation have good stability, thus the flexibility and compliance of drug taking of patients are improved, and accordingly the treatment effect of the drug is improved. In addition, the invention provides a preparation method of the medicinal preparation of the Nnintedanib inclusion compound, no specific equipment is needed, the technology is simple, the production cost is low, and the preparation method is suitable for industrialized production.
Owner:SUNSHINE LAKE PHARM CO LTD
Application of nintedanib in external preparation drug for treating psoriasis
The invention relates to application of nintedanib or its physiologically acceptable salt in an external preparation drug for treating psoriasis. When the nintedanib or its physiologically acceptable salt is used for treating the psoriasis, a mode of locally applying an external preparation is adopted. During local application, the nintedanib or its physiologically acceptable salt can be in the form of a semisolid or liquid preparation containing the nintedanib or its physiologically acceptable salt and at least one physiologically acceptable excipient and / or adjuvant, and the preparation can be an ointment, cream, gel, a solvent agent, an emulsion, a mixed suspension or spray. An experiment shows that the nintedanib has the positive effect on the aspect of nintedanib treatment and can obviously inhibit epithelial mitosis and promote formation of a mouse tail scale epidermis particle layer.
Owner:REYOUNG PHARMA
Methods for treating fibrosis
Some embodiments of the invention include methods for treating an animal for fibrosis comprising one or more administrations of one or more compositions comprising one or more opioid receptor inhibitors. Other embodiments of the invention further include other fibrosis treatments. Still other embodiments of the invention include methods for treating a human for idiopathic pulmonary fibrosis, comprising administering one or more compositions comprising naltrexone and optionally administering one or more compositions comprising pirfenidone, nintedanib, or both. Additional embodiments of the invention are also discussed herein.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Anti-pulmonary fibrosis composition with improved dissolution property
ActiveCN111973596AGood dissolution propertiesImprove solubilityOrganic active ingredientsPowder deliveryOral medicationPharmaceutical drug
The invention relates to an anti-pulmonary fibrosis composition with an improved dissolution property. The anti-pulmonary fibrosis composition comprises a salt formed by nintedanib and an acidic polymer. The salt formed by nintedanib and the acidic polymer has higher solubility and certain stability. The composition can be orally taken and is beneficial to dissolution and absorption of drugs in gastrointestinal tracts.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
Application of nintedanib in preparing medicament for preventing and treating liver fibrosis and hepatocirrhosis
InactiveCN106692150ASignificant effectSmall toxicityOrganic active ingredientsDigestive systemLiver fibrosisHepatocyte
The invention relates to application of nintedanib in preparing medicament for preventing and treating liver fibrosis and hepatocirrhosis. The invention proves that nintedanib is capable of obviously reducing the degree of liver fibrosis or hepatocirrhosis and alleviating hepatic injury, is free from obvious hepatotoxicity and has favorable application prospect.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Preparation method and key intermediates of Nintedanib
InactiveCN107935908ARaw materials are easy to getSimple processOrganic chemistryOrganic compound preparationMethyl benzoateFormate Esters
The present invention relates to a completely-new preparation method of Nintedanib, and key intermediates thereof (such as 4-(1-alkoxy-1,3-dioxo-3-phenylpropane-2-yl)-3-nitro methyl benzoate and 3-benzoyl-2-oxodihydroindol-6-methyl formate), and a preparation method of the key intermediates. According to the present invention, the method has advantages of easily available raw materials, simple process, economy and environmental protection, and is suitable for industrial production; and the preparation method comprises that 1, 4-(1-alkoxy-1,3-dioxo-3-phenylpropane-2-yl)-3-nitro methyl benzoateis prepared from 4-halo-3-nitro-methyl benzoate and 3-oxo-3-phenylpropionate, 2, the compound reacts to generate a compound 3-benzoyl-2-oxodihydroindol-6-methyl formate, and 3, the compound reacts with N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide to generate Nintedanib (I).
Owner:SHANGHAI SYNCORES TECH INC
Imidazolopyrimidine derivatives for preventing and treating pulmonary fibrosis and application of imidazolopyrimidine derivatives
ActiveCN109912600AThe synthesis process is simpleReduce manufacturing costOrganic chemistryRespiratory disorderMedicinePathology diagnosis
The invention provides imidazolopyrimidine derivatives for preventing and treating pulmonary fibrosis and an application of the imidazolopyrimidine derivatives to preparation of drugs for preventing and treating pulmonary fibrosis. The structural formula of the derivatives is represented in the description or the derivatives are pharmaceutically acceptable salts of the compound represented as thestructural formula. The imidazolopyrimidine derivatives can obviously reduce degree of pathology of pulmonary fibrosis and delay pulmonary fibrosis development, has a remarkable function of preventingand treating pulmonary fibrosis, and has better pharmacokinetics and lower toxicity than Nintedanib as a clinical first-line anti-pulmonary fibrosis drug.
Owner:SICHUAN YIASUO PHARM TECH CO LTD
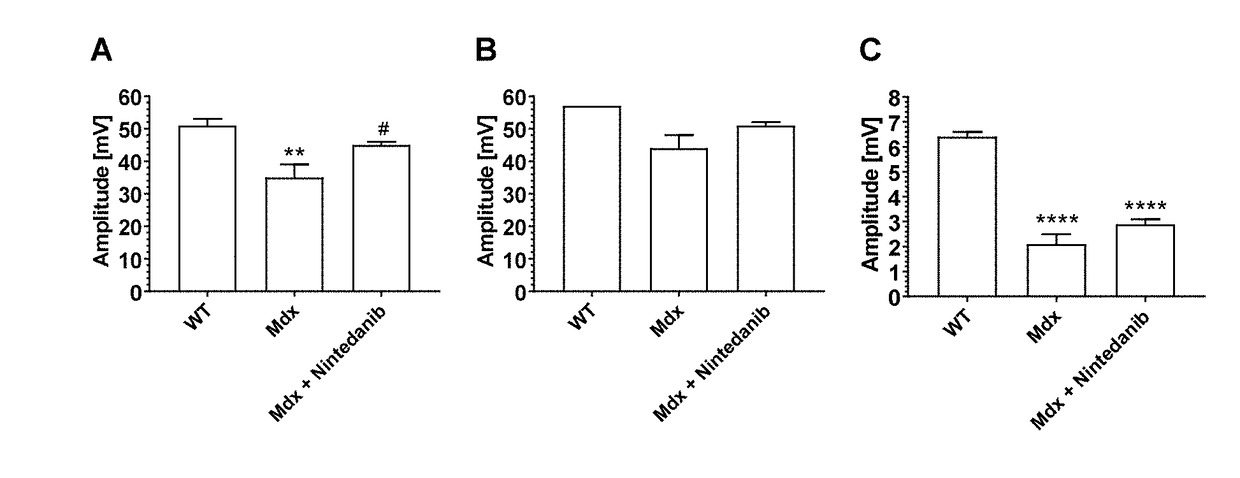
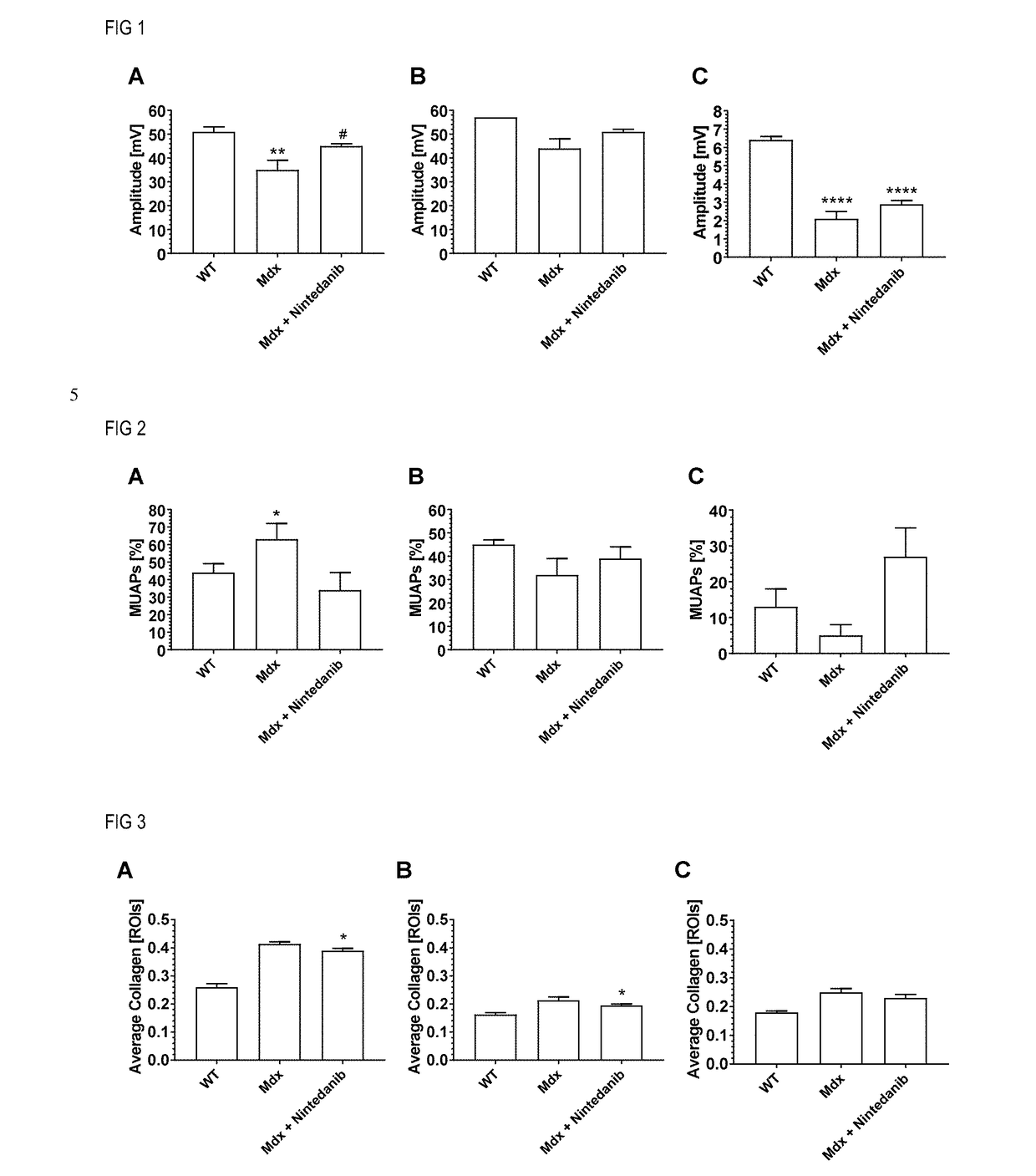
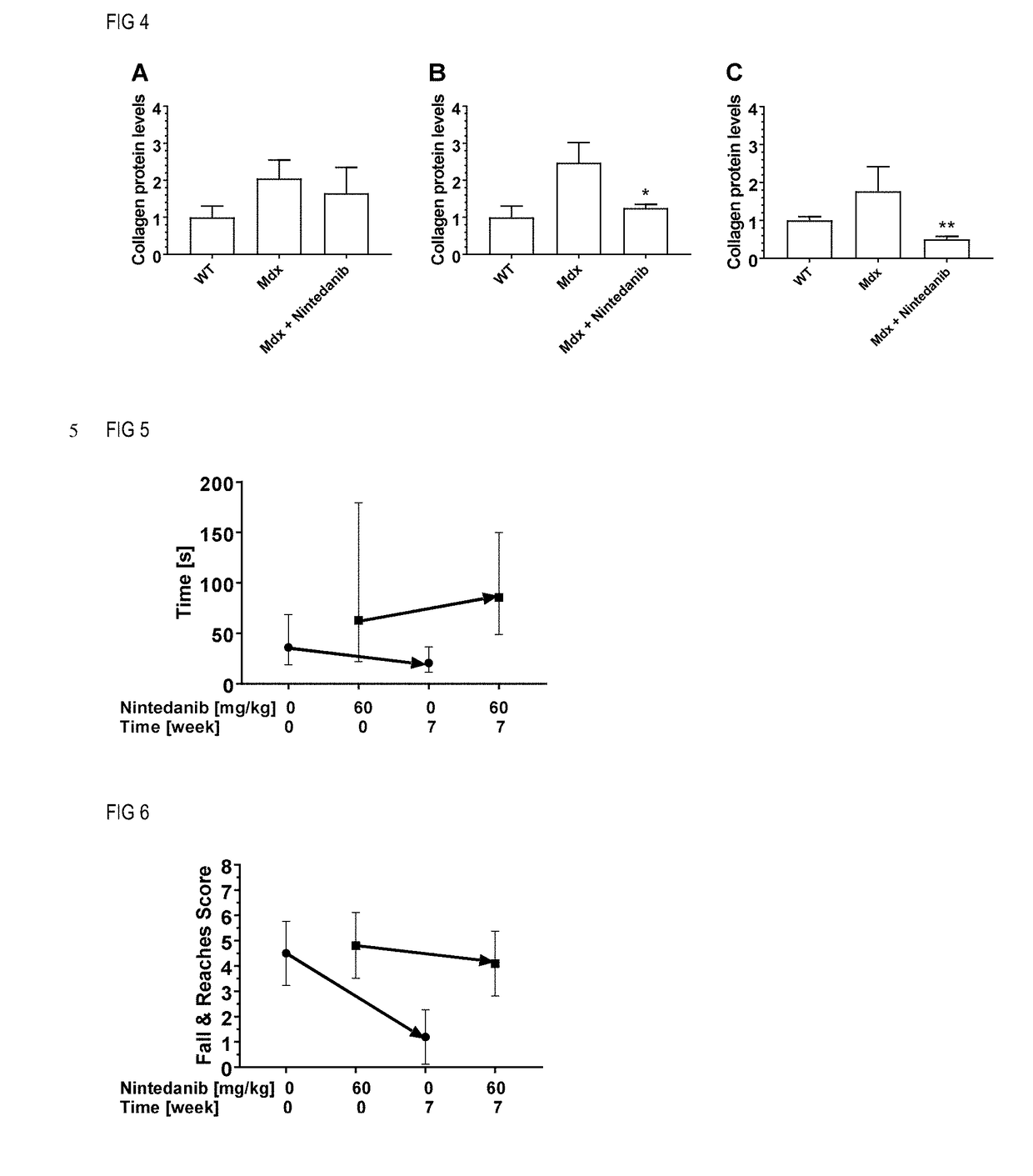
Nintedanib for use in methods for the treatment of muscular dystrophy
InactiveUS20180280385A1Powder deliveryOrganic active ingredientsDuchenne muscular dystrophyMuscular dystrophy
The invention relates to the use of tyrosine kinase inhibitors, selected from nintedanib and pharmaceutically acceptable salts thereof, for the treatment of muscular dystrophy.
Owner:BOEHRINGER INGELHEIM INT GMBH
Drug for preventing or treating choriodal neovascular
InactiveCN106902117ASignificant effectOrganic active ingredientsSenses disorderBULK ACTIVE INGREDIENTActive ingredient
The invention relates to a drug for preventing or treating choriodal neovascular. The drug comprises active ingredients which are one or more of Nintedanib and salts thereof or solvate. The Nintedanib and salts thereof or solvate can prevent or treat choriodal neovascular. The drug has remarkable efficacy on preventing or treating choriodal neovascular when the oral dosage is 1.26-32mg / person per time.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
3-vinyl indazole derivative as well as preparation method and application thereof
PendingCN111205227AEnhanced inhibitory effectPrevent proliferationOrganic active ingredientsOrganic chemistryAnticarcinogenic EffectCancer cell
The invention relates to a 3-vinyl indazole derivative as well as a preparation method and application thereof, and belongs to the field of chemical medicines. The invention provides a compound represented by a formula I, an optical isomer of the compound, and a pharmaceutically acceptable salt of the compound or the optical isomer of the compound. Biological experiments prove that the 3-vinyl indazole derivative provided by the invention has a remarkable inhibition effect on the activity of FGFR kinase, can effectively inhibit proliferation of various cancer cells such as breast cancer, lungcancer and gastric cancer, and has a broad-spectrum anti-cancer effect; besides, the 3-vinyl indazole derivative also has an obvious inhibiting effect on proliferation of fibroblasts, the effect of the 3-vinyl indazole derivative is equivalent to that of a drug Nintedanib for treating pulmonary fibrosis clinically at present, and the anti-fibrosis curative effect is remarkable. The 3-vinyl indazole derivative provides a new choice for development and application of anti-cancer and anti-fibrosis drugs.
Owner:SICHUAN UNIV +1
Nintedanib lyophilized liposome preparation for aerosol inhalation and preparing method thereof
InactiveCN109758437AAvoid first pass effectAvoid destructionPowder deliveryOrganic active ingredientsPhospholipidLiposome
The invention discloses a nintedanib lyophilized liposome preparation for aerosol inhalation and for treating pulmonary fibrosis and a preparing method thereof. The single dosage of the lyophilized liposome preparation contains 10-80 mg of nintedanib or a salt thereof (according to the amount of free nintedanib), 25-250 mg of lipid, 100-1000 mg of phospholipid, and 20-150 mg of an osmotic agent. The prepared preparation has good stability and small particle size, and is suitable for aerosol administration. At the same time, the lyophilized liposome preparation has the advantages of high efficiency, low toxicity and high safety, and greatly reduces the hepatic and renal toxicity of medication of patients.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
Ophthalmic formulations of tyrosine kinase inhibitors, methods of use thereof, and preparation methods thereof
ActiveUS20180243294A1Improve toleranceLittle to no toxicityPowder deliverySenses disorderDiseaseNanoparticle
Ophthalmic formulations containing nintedanib, or a pharmaceutically acceptable salt thereof are provided. The ophthalmic formulations can contain microparticles or nanoparticles of nintedanib. Also provided are methods of using the ophthalmic formulations for treating ocular surface diseases, such as dry eye disease.
Owner:ALLGENESIS BIOTHERAPEUTICS INC
Preparation method of nintedanib
Disclosed is a preparation method of nintedanib (I), comprising the following steps: carrying out a condensation reaction on 4-(R acetate-2-yl)-3-nitrobenzoate (II) and trimethyl orthobenzoate to obtain (E)-4-[(2-methoxybenzylidene) R acetate-2-yl]-3-nitrobenzoate (III); carrying out a substitution reaction on the compound (EI) and N-(4-aminophenyl)-N-methyl-2-(4-methyl piperazine-1-yl) acetamide (IV) under the action of an acid-binding agent to generate (Z)-4-{[2-(N-methyl-2-(4-methyl piperazine-1-yl) acetamido-aniline) benzylidene] R acetate-2-yl}-3-nitrobenzoate (V); and sequentially carrying out reduction reactions and cyc-lization reactions on the compound (V) to prepare the nintedanib (I). The preparation method has an easily obtained raw material and a simple process, is economical and environmentally friendly, and is suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Nintedanib nano lipid carrier with high bioavailability and preparation method thereof
InactiveCN110623927AIncrease intakeImprove bioavailability in vivoKetone active ingredientsRespiratory disorderWater bathsIce water
The invention discloses a nintedanib nano-lipid carrier with high bioavailability and a preparation method thereof. The nano lipid carrier comprises nintedanib, a solid lipid material, a liquid lipidmaterial, a surfactant and an absorption promoter. The preparation scheme comprises the following steps: heating solid and liquid lipids in a water bath at a certain temperature, and stirring until the solid and liquid lipids are molten; and adding a surfactant, and continuously stirring for 5 minutes; adding nintedanib, and stirring for 1h to obtain a drug-containing oil phase; adding part or allof the surfactant into a water phase, preheating at the same temperature, and adding the water phase into the drug-containing oil phase while stirring; after probe ultrasonic treatment, performing ice-water bath curing, and obtaining the product. The prepared nintedanib nano lipid carrier is simple in scheme, free of organic solvents, high in drug loading capacity, uniform in particle size and particle size distribution and good in stability, intake of nintedanib by small intestine epithelial cells can be remarkably increased, and in-vivo bioavailability is effectively improved.
Owner:江苏世博生物医药科技有限公司
Nintedanib esilate impurity as well as preparation method and application thereof
InactiveCN110759848ARaise quality standardsHigh yieldOrganic chemistryUse medicationEthanesulfonic acid
The invention relates to a nintedanib esilate impurity as well as a preparation method and application thereof. By using the nintedanib esilate impurity, an analytic reference can be provided for detecting a nintedanib esilate product, the quality standard of nintedanib esilate can be improved, and the guarantee can be provided for the safe medication of nintedanib esilate.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation method of nintedanib key intermediate
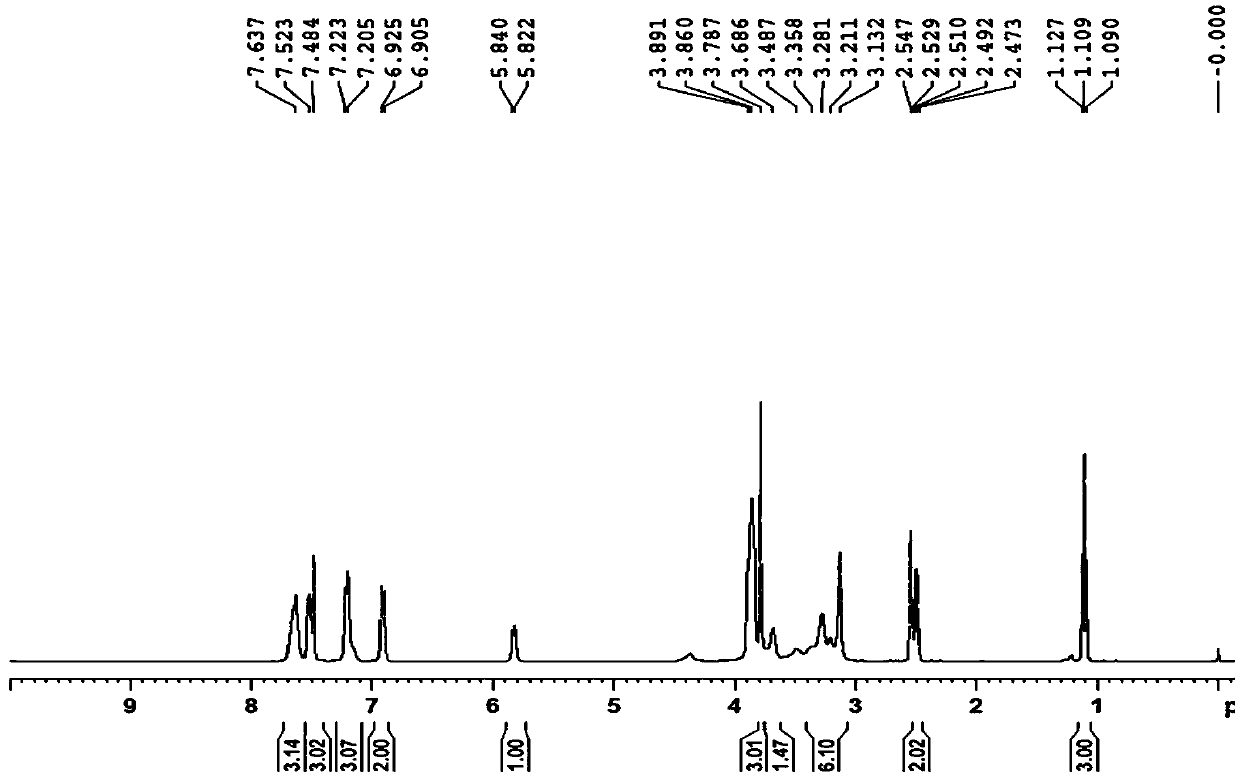
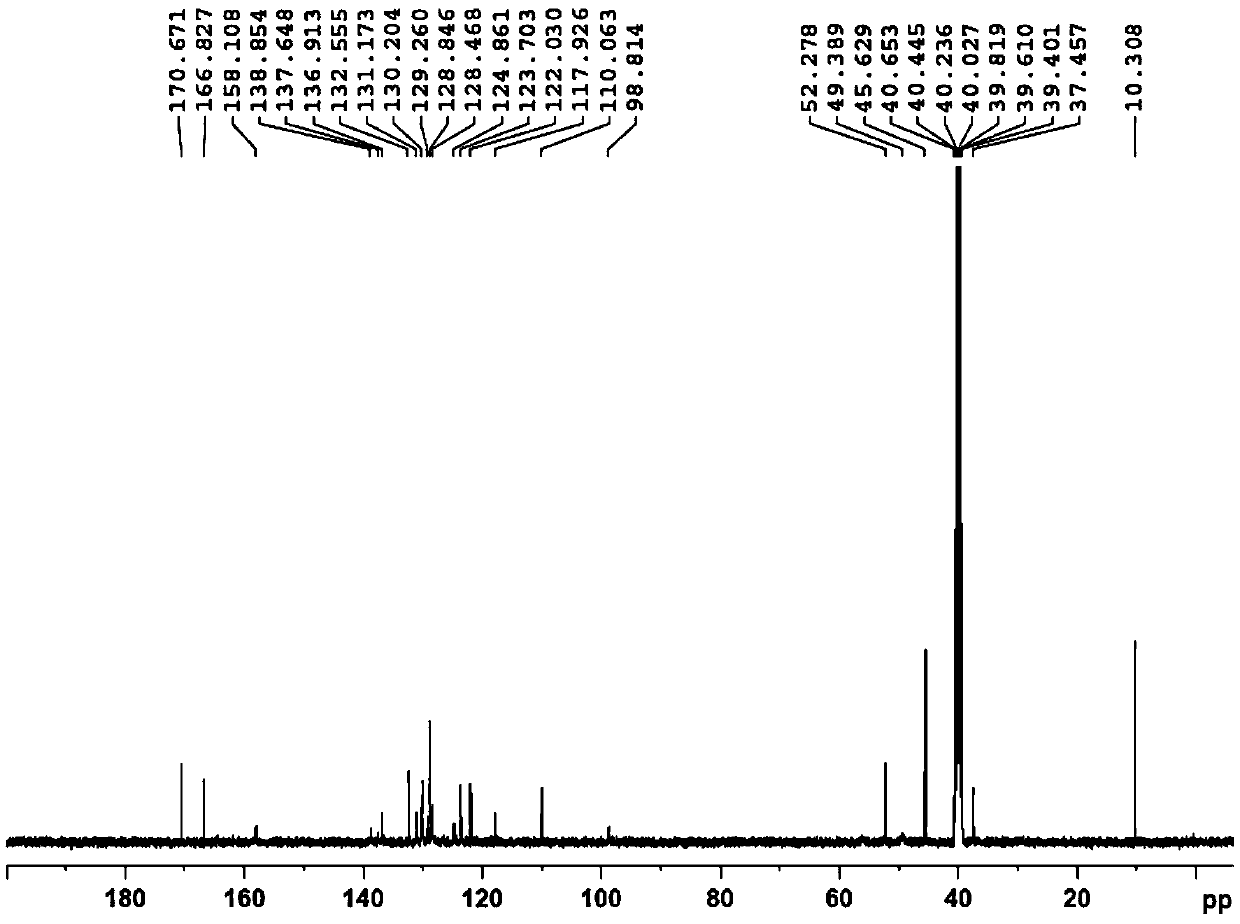
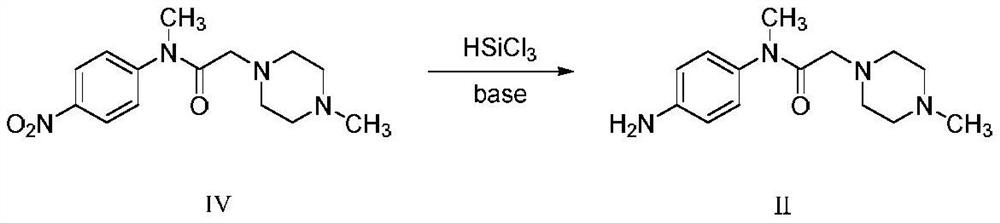
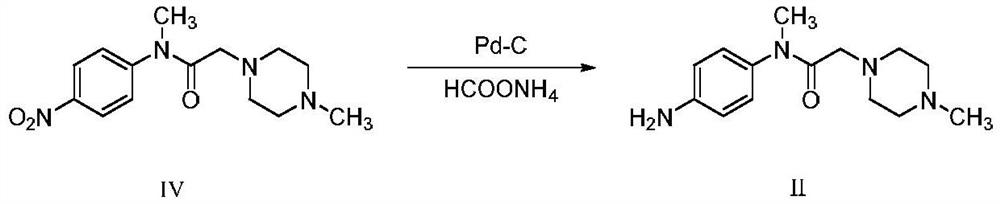
InactiveCN111777576AHigh feasibilityHigh purityOrganic chemistryBiochemical engineeringProcess engineering
The invention belongs to the technical field of pharmaceutical chemicals, and particularly relates to a preparation method of a nintedanib key intermediate compound II. A compound IV is reduced by adopting a common reaction reagent under the conditions of normal temperature and normal pressure, so that a high-purity compound II can be conveniently obtained. The adopted reaction conditions are mild, high-pressure equipment is avoided, the technical operation is simple, the conditions are mild, the process is safer and more environmentally friendly, and the feasibility of large-scale productionof nintedanib is greatly improved.
Owner:QILU PHARMA
Eucheuma muricatum extract and application of Eucheuma muricatum extract in preparation of drugs for treatment of organ fibrosis
ActiveCN110464742ASignificant efficacy in treating organ fibrosisDispersion deliveryAlgae medical ingredientsFiltrationPreservative
The invention discloses an organ fibrosis-preventing oral solution prepared from an Eucheuma muricatum extract. The oral solution comprises the following components: in percent by mass, 0.5-2% of mucilage, 0.2-1% of a preservative, 0.1-1% of a co-solvent and the balance Eucheuma muricatum extract. The preparation method of the Eucheuma muricatum extract includes the following steps: (1) washing Eucheuma muricatum; (2) taking the step-(1) washed Eucheuma muricatum with a quality of M1, adding water of a quality M2, then performing homogenizing, sterilizing, filtration and centrifugation sequentially, collecting the supernatant, and then performing ultrafiltration by using an ultrafiltration membrane system with 0.1 micron microporous membranes, molecular weight cut-off of 5,000 Daltons andoperating pressure difference of 0.1-0.5 MPa so as to obtain the Eucheuma muricatum extract Extract, wherein the ratio of M1 to M2 is 1:(8-12). The prepared oral solution has remarkable efficacy in treatment of organ fibrosis, and has a better effect on treatment of organ fibrosis than pirfenidone and Nintedanib, and in addition, compression ultrafiltration of the ultrafiltration membrane system is adopted by the preparation method of the Eucheuma muricatum extract, and the efficiency is better than that of a chromatography extraction technology.
Owner:GUANGDONG ZHANJIANG PROVINCIAL LAB OF SOUTHERN MARINE SCI & ENG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com