Imidazolopyrimidine derivatives for preventing and treating pulmonary fibrosis and application of imidazolopyrimidine derivatives
A technology for pulmonary fibrosis and its derivatives, applied in organic chemistry, drug combination, respiratory system diseases, etc., can solve the problems of pharmacokinetic performance to be improved, toxic and side effects, etc., to delay the progress of pulmonary fibrosis, low toxicity , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In this example, the steps of preparing imidazopyrimidine derivatives for preventing and treating pulmonary fibrosis are as follows:
[0020] Take 1.36g of hypoxanthine, 3mL of iodoisopropane (1.703g / mL) and 0.24g of sodium hydride, put them into a flask containing 300mL of DMSO, stir and react at 80°C for 3h, then add acid to quench, and spin dry under reduced pressure to get Isopropyl modified hypoxanthine. Take 1.5 g of isopropyl-modified hypoxanthine and 8.08 g of Lawesson reagent (Lawesson reagent), add it to a flask filled with 500 mL of toluene, heat to reflux for 3 hours, then spin dry under reduced pressure, and pass through a silica gel column to prevent and treat pulmonary fibrosis The imidazopyrimidine derivative CSA.
[0021] Carry out nuclear magnetic spectrum and nuclear magnetic carbon spectrum detection to CSA, the result is: 1 H NMR (500MHz, Chloroform-d) δ8.57(s,1H),7.76(s,1H),5.26(hept,J=6.2Hz,1H),4.86(hept,J=6.2Hz,1H),1.46 (d, J=6.1 Hz, 6H), 1.23...
Embodiment 2
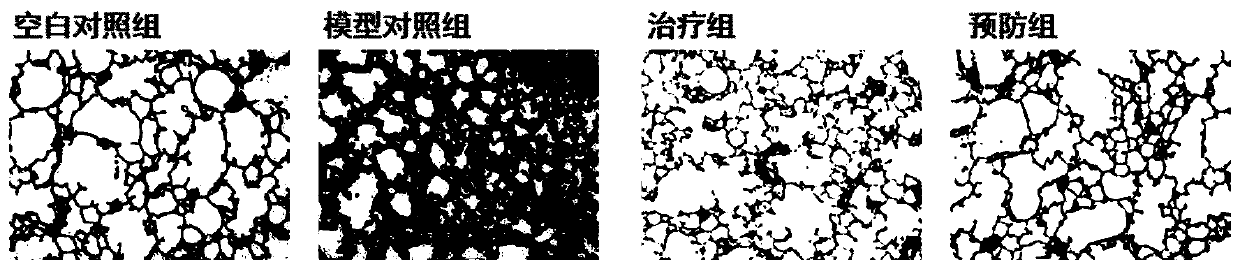
[0023] In this example, the anti-pulmonary embolism activity of the CSA prepared in Example 1 was tested.
[0024] 1. Test method
[0025] (1) Animals: SPF grade c57bl / 6 mice, 25g±2g.
[0026] (2) Grouping
[0027] The animals were divided into blank control group, model control group, prevention group and treatment group, with 10 animals in each group.
[0028] The prevention group received drug intervention 14 days in advance, with a dose of 50 mg / kg / d. After modeling, the treatment group was administered with an intervention dose of 50 mg / kg / d.
[0029] (3) Inject mice intraperitoneally with 1% sodium pentobarbital at a dose of 50 mg / kg, anesthetize them, place them supine on the operating table, expose the trachea with tweezers (pull out the tongue), and slowly inject 5 mg / kg bleomycin , and then quickly erected the rat board, rotated for 5 minutes, so that the drug was evenly distributed in the lungs.
[0030] The blank control group was given intragastric administra...
Embodiment 3
[0047] In this example, a toxicity test is performed on CSA.
[0048] C57 mice were given the compound CSA by intragastric administration at a dose of 50 mg / kg / d for 180 consecutive days, and the blank control group was intragastrically administered an equal volume of isotonic saline. One hour after the last administration, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg / kg), placed on a constant temperature hot plate at 37±1°C, and blood was collected by cardiac puncture. Blood routine, liver and kidney function, electrolytes and other blood pathological indicators were measured according to the operating instructions of the instrument, and the results are shown in Table 3. It can be seen from Table 3 that CSA has no obvious adverse effects on the liver and kidney functions of mice, and has the characteristics of low toxicity.
[0049] Table 3 Mouse liver and kidney function test results (values are expressed as mean ± SED, n=9-10)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com