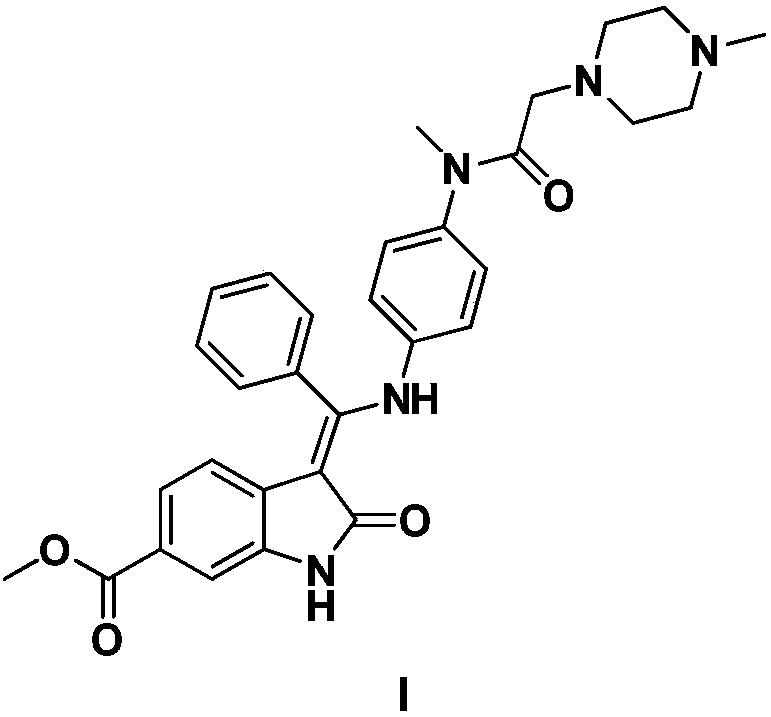
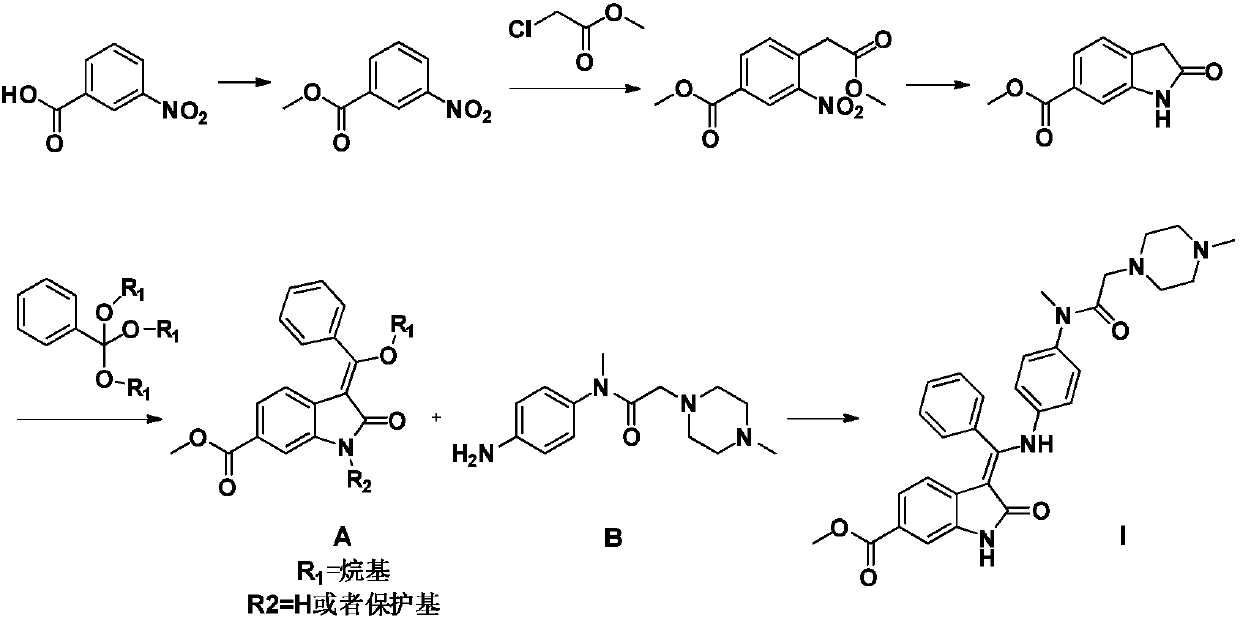
Preparation method and key intermediates of Nintedanib
A technology for nintedanib and a compound is applied in the field of preparation of the drug nintedanib, which can solve the problems of unsuitability for industrial scale-up and low yield, and achieve the effects of easy availability of raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Methyl 4-(1-methoxy-1,3-dioxo-3-phenylpropan-2-yl)-3-nitrobenzoate (IV)
[0024] Add 160 mL of N,N-dimethylformamide and 18.8 g of 4-chloro-3-nitro-benzoic acid methyl ester into a 500 mL reaction flask, and stir until it dissolves at 25°C. Then 17.1 g of 3-oxo-3-phenylpropionate and 24.1 g of anhydrous potassium carbonate were added in sequence, and the temperature of the reaction was raised to 80-90 to start the reaction for 4-6 hours until TLC showed that the raw materials disappeared. Add glacial acetic acid to the reaction solution to adjust the pH to neutral, add 600mL water and extract with 200mL*3 times of ethyl acetate. The combined ethyl acetate layers were washed with 300 mL*2 times of 5% NaCl solution. The ethyl acetate layer was dried over anhydrous magnesium sulfate, and the solvent was spin-dried to obtain 28.7 g of compound IV, with a yield of 92.1%. 1 H NMR (400MHz, CDCl 3 ):δ8.38-8.33(m,2H),7.98-7.93(m,2H),7.79(d,J=7.6,1H),7.64-7.56(m,1H)...
Embodiment 2
[0025] Example 2: Methyl 3-benzoyl-2-oxoindoline-6-carboxylate (V)
[0026] Dissolve 24.0g of compound IV in 240mL of ethyl acetate in a 500mL reaction flask, add 2.4g of 10% palladium carbon catalyst, place the reaction in a hydrogen atmosphere of 3 atmospheres and react at 20-30 degrees for 16 hours until TLC shows the raw material Complete conversion leads to intermediates. Then the temperature of the reaction was raised to 80-90°C for 4-6 hours until TLC showed that the intermediate was completely converted. After cooling down to room temperature, the palladium-carbon catalyst was removed by filtration, and the ethyl acetate solution was evaporated to dryness, replaced with methyl tert-butyl ether to recrystallize the product to obtain 14.2 g of white crystals, with a yield of 71.6%. 1 H NMR (400MHz, CDCl 3 )δ8.55(br,1H),8.13(s,1H),8.09–8.01(m,2H),7.88(dd,J=7.6,1.6Hz,1H),7.76–7.65(m,2H),7.52 –7.40(m,2H),5.33(s,1H),3.87(s,3H).Mass:296.0[M+H + ].
Embodiment 3
[0027] Embodiment 3: nintedanib (I)
[0028] Dissolve 21.2g of compound V in 210mL of toluene in a 500mL reaction flask, add 20.7g of compound IV and 2.47g of p-toluenesulfonic acid, heat the reaction to reflux at 100-110 degrees and use Dean-Stark to separate the generated water. After 28 hours of reaction, TLC showed complete conversion of starting material. After cooling down to room temperature, wash once with 5% NaHCO3 solution and twice with 5% NaCl solution, then dry with 10 g of anhydrous magnesium sulfate. After the toluene was evaporated to dryness, 120 mL of methanol / n-heptane was added for recrystallization to obtain 24.7 g of the product nintedanib, with a yield of 63.8%. 1 H NMR (400MHz, DMSO-d 6 ):12.17(s,1H),11.03(s,1H),7.64–7.59(t,J=7.6Hz,2H),7.56–7.52(t,J=7.6Hz,2H),7.50–7.45(d, J=7.6Hz, 1H), 7.43–7.40(d, J=1.6Hz, 1H), 7.21–7.17(d, J=8.3Hz, 1H), 7.15–7.07(m, 2H), 6.82–6.77(m ,2H),5.85–5.83(d,J=8.3Hz,1H),3.79(s,3H),3.11–3.04(m,3H),2.75–2.66(m,2H),2.27–2.19(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com