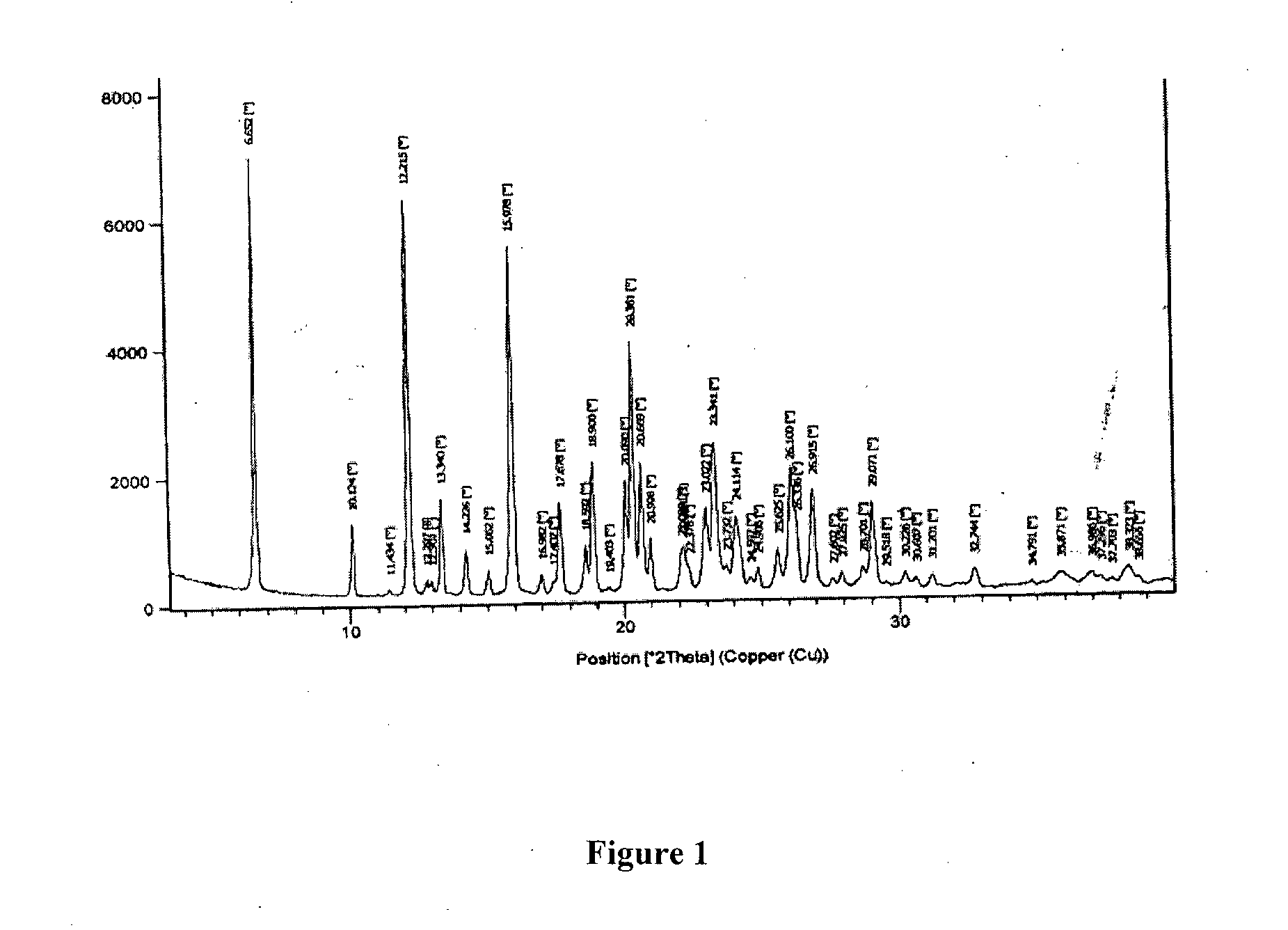
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
789 results about "Methyl benzoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl benzoate is an organic compound. It is an ester with the chemical formula C₆H₅CO₂CH₃. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.
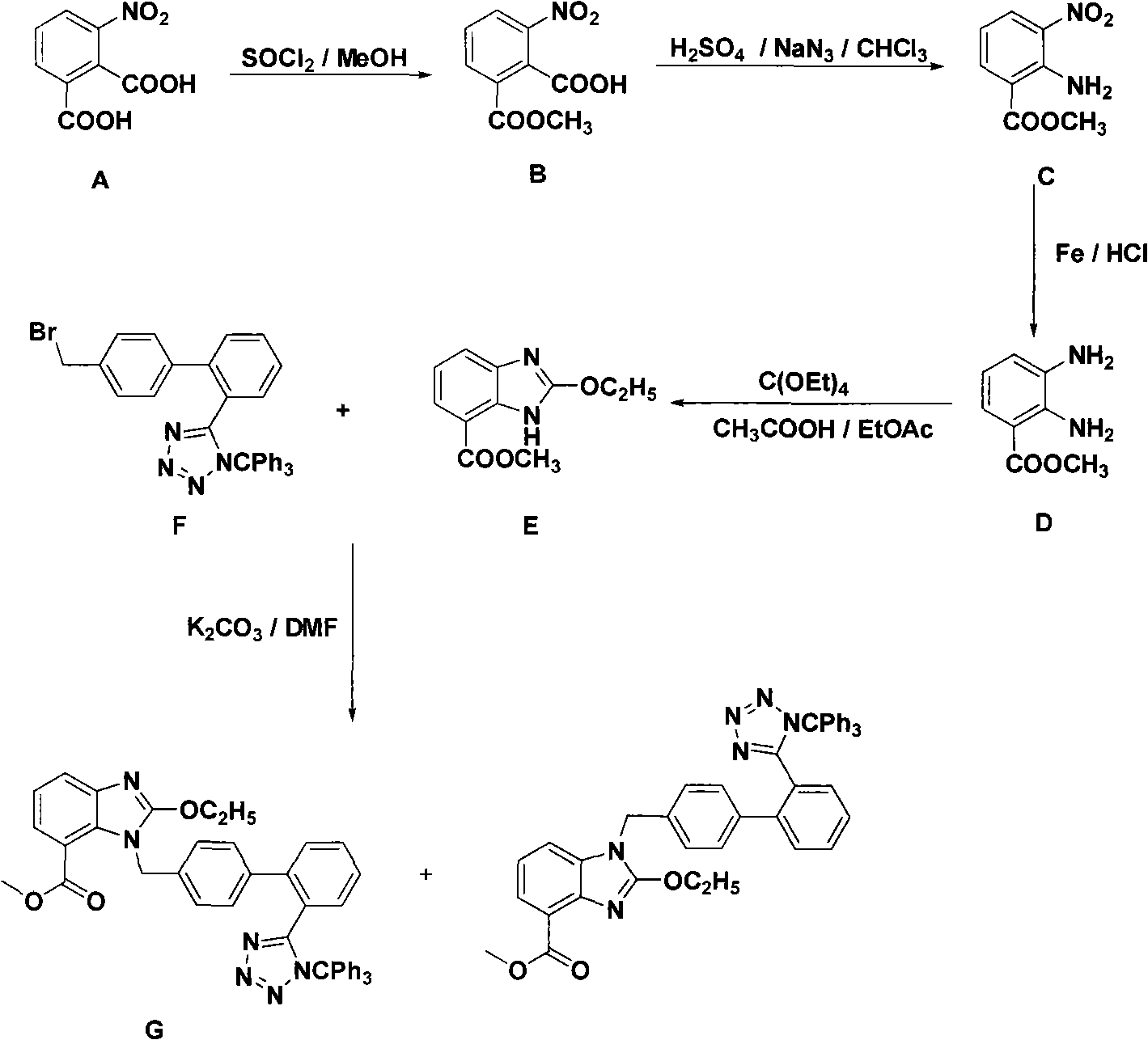
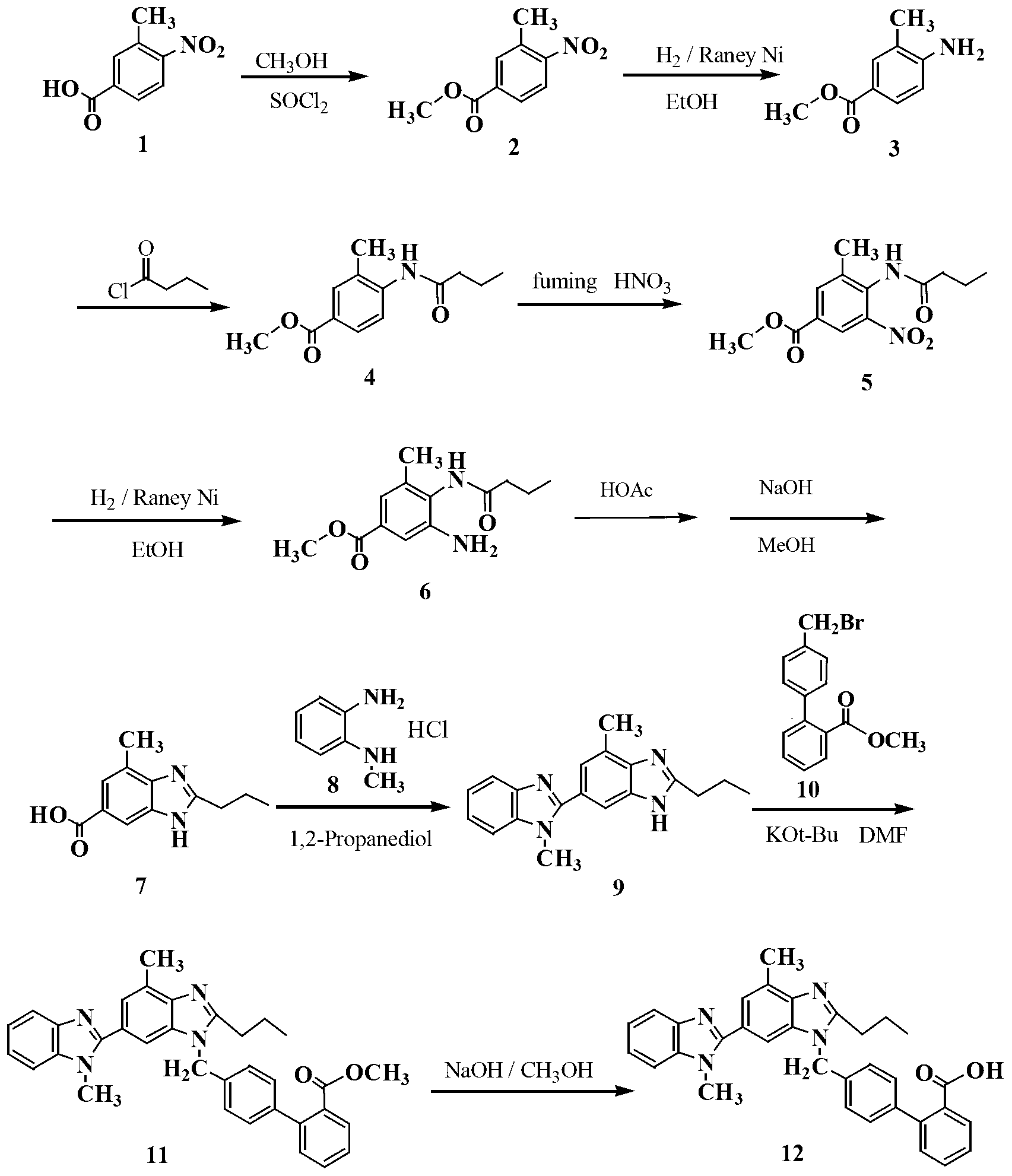
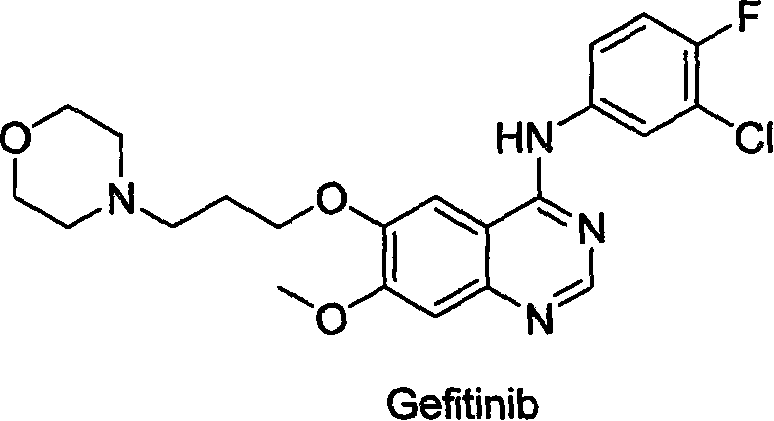
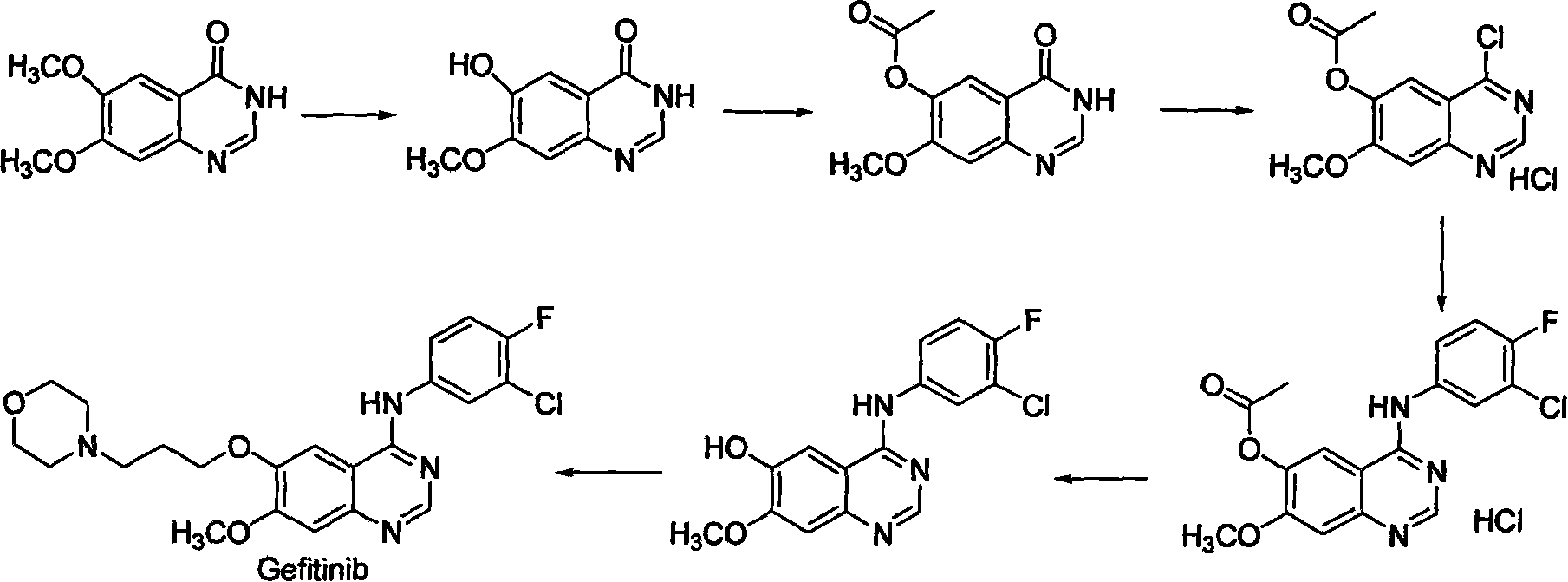
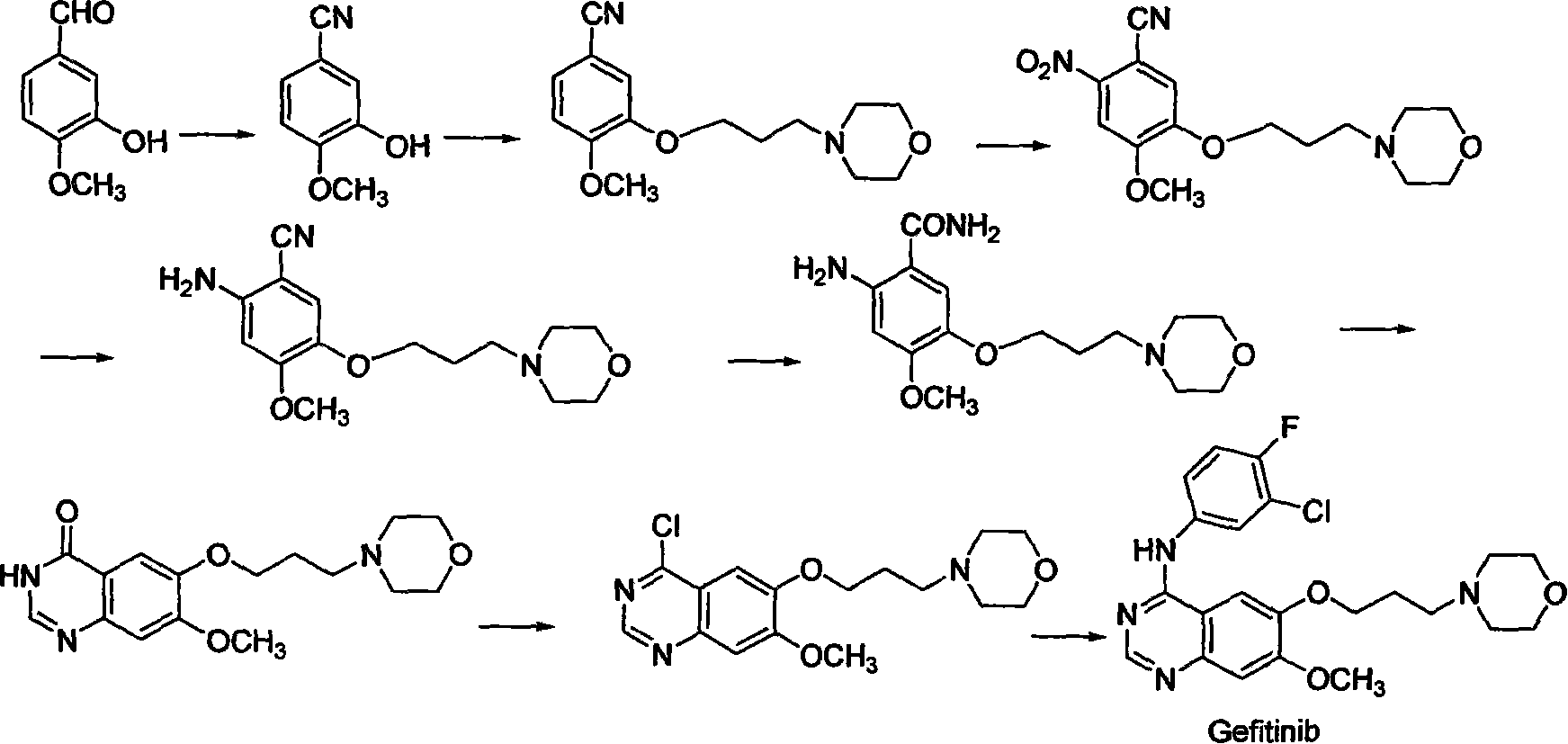
Preparing method for gefitinib
InactiveCN101148439AHigh yieldReduce manufacturing costOrganic chemistryMorpholine3-chloro-4-fluoroaniline
The process of preparing gefitinib, 4-(3-chloro-4-fluorophenylamido)-7-methoxyl-6-[3-(4- morpholinyl) propoxy] quinazoline, includes following steps: 1. reaction of 3-hydroxy-4-methoxy methyl benzoate as material and 4-(3-chloropropyl) morpholine to obtain 4- methoxyl-3-[3-(4- morpholinyl) propoxy] methyl benzoate; 2. nitration to obtain 2-nitro-4- methoxyl-5-[3-(4- morpholinyl) propoxy] methyl benzoate; 3. reduction to obtain 2-amino-4- methoxyl-5-[3-(4- morpholinyl) propoxy] methyl benzoate; 4. closing cycle to create 7- methoxyl-6-[3-(4- morpholinyl) propoxy] quinazoline-4(3H)-one; 5. chlorinating to obtain 4-chloro-7- methoxyl -6-[3-(4-morpholinyl) propoxy] quinazoline; and 6. reaction to 3-chloro-4-fluoroaniline to obtain gefitinib.
Owner:SOUTHEAST UNIV
Antidaudruff hair conditioning composition
Disclosed is hair conditioning compositions comprising antidandruff agent wherein the hair conditioning composition is substantially free of the group selected from a chelating agent, methylchloroisothiazolinone, and methylisothiazolinone. The compositions comprise by weight: (a) from about 0.1 % to about 15 % of a high melting point fatty compound; (b) compounds selected of; (b1a) from about 0.1 % to about 10 % of an amidoamine having the following general formula: R<1> CONH (CH2)m N(R<2>)2 wherein R<1> is a residue of C11 to C24 fatty acids, R<2> is a C1 to C4 alkyl, and m is an integer from 1 to 4; (b1b) an acid selected from the group consisting of 1 glutamic acid, lactic acid, hydrochloric acid, malic acid, acetic acid, fumaric acid, 1 glutamic acid hydrochloride, tartaric acid, and mixtures thereof, at a level such that the mole ratio of amidoamine to acid is from about 1:0.3 to about 1:1; or (b2) the combination of; (b2a) from about 0.1% to about 10% of a cationic conditioning agent; and (b2b) from about 0.1% to about 10% of a low melting point oil having a melting point of less than 25 DEG C; (c) a safe and effective amount of an antidandruff agent; (e) a preservative system comprising, by weight of the entire composition, from about 0.1% to about 1.0% of benzyl alcohol, from about 0.1 % to about 1.0 % of phenoxy ethanol, from about 0.05 % to about 1.0 % of methyl paraben, and from about 0.05 % to about 1.0 % of mettryl paraben, and from about 0.01 % to about 1.0 % of propyl paraben; and (f) an aqueous carrier.
Owner:THE PROCTER & GAMBLE COMPANY
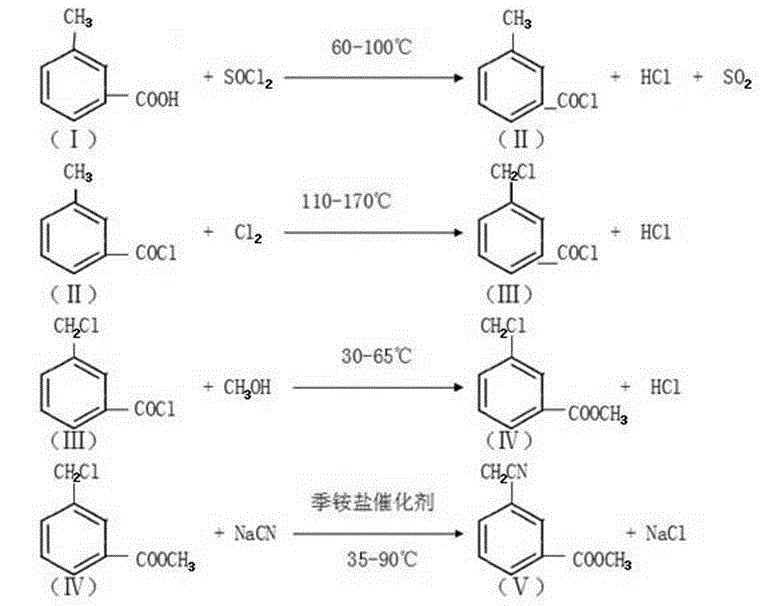
Synthetic system of 3-cyanomethylbenzoic acid methyl ester and method thereof
InactiveCN110002993AIncrease the mass transfer area of the phase boundaryImprove mass transfer efficiencyOrganic compound preparationPreparation by cyanide reactionChlorideMethyl benzoate
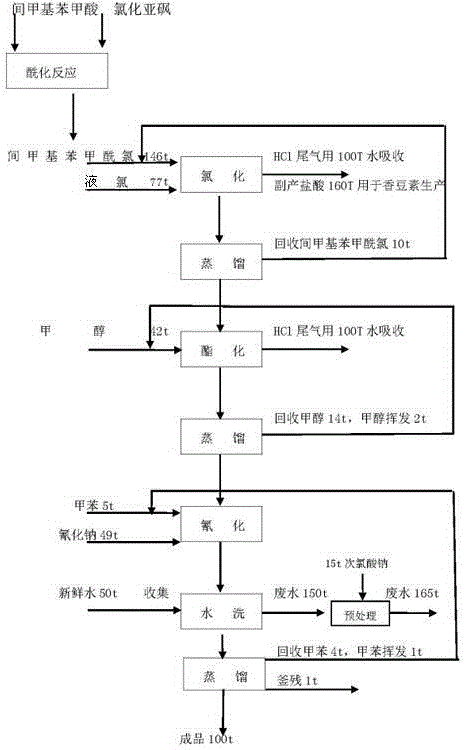
The invention provides a synthetic system of 3-cyanomethylbenzoic acid methyl ester and a method thereof. The system comprises an acylation reaction vessel used for preparation of m-Toluoyl chloride;a liquid chlorine feeding unit used for storing and conveying liquid chlorine; a chlorination reaction unit which is connected with the acylation reaction vessel and used as a chlorination reaction chamber of m-Toluoyl chloride; a micro-interface generator which is respectively connected with the liquid chlorine feeding unit and the chlorination reaction unit and used for receiving liquid chlorineconveyed by the liquid chlorine feeding unit, crushing the liquid chlorine into micro-droplets with the diameter being micron level and conveying the micro-droplets to the chlorination reaction unitafter the crushing; and an esterification and cyanation reaction unit which is connected with the chlorination reaction unit. In the prior art, m-Toluoyl chloride and liquid chlorine cannot be fully mixed when m-Toluoyl chloride and liquid chlorine react such that yield of 3-cyanomethylbenzoic acid methyl ester is reduced. The above problem is solved by the synthetic system and the method of the invention.
Owner:NANJING UNIV
Patterning process
ActiveUS20120009529A1High dissolution contrastIncrease contrastPhotomechanical exposure apparatusMicrolithography exposure apparatusResistMeth-
A pattern is formed by applying a resist composition comprising a (meth)acrylate copolymer comprising both recurring units having an acid labile group-substituted carboxyl group and recurring units having a lactone ring, an acid generator, and an organic solvent onto a substrate, prebaking the composition to form a resist film, exposing the resist film to high-energy radiation, baking, and developing the exposed film with a developer. The developer comprises at least 40 wt % of an organic solvent selected from methyl benzoate, ethyl benzoate, phenyl acetate, benzyl acetate, methyl phenylacetate, benzyl formate, phenylethyl formate, methyl 3-phenylpropionate, benzyl propionate, ethyl phenylacetate, and 2-phenylethyl acetate.
Owner:SHIN ETSU CHEM IND CO LTD
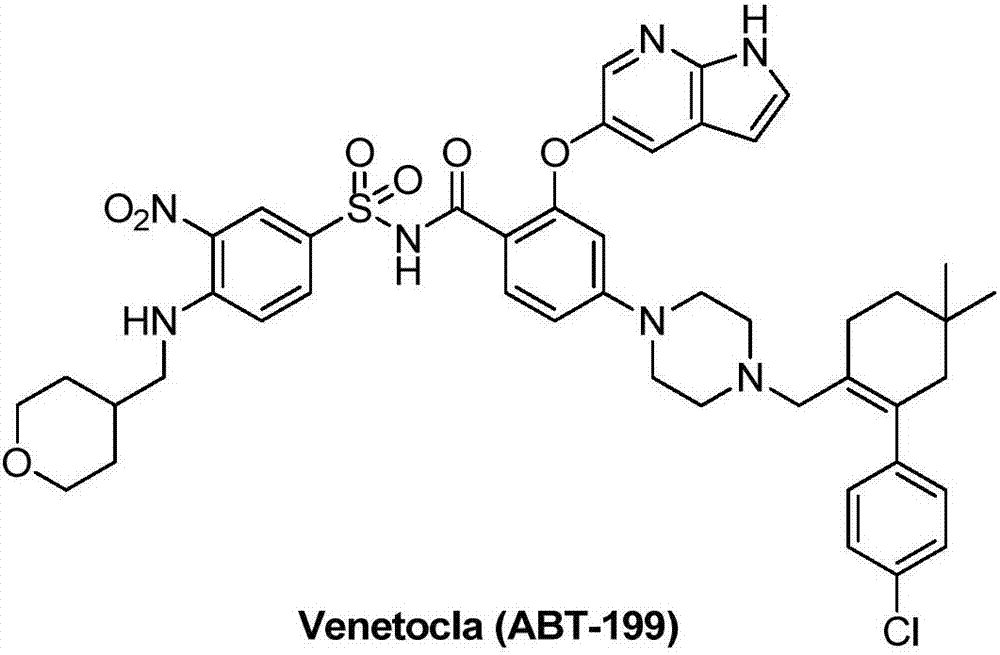
Synthesis method for BCL-2 inhibitor Venetoclax
InactiveCN107089981AFollow-up response is simpleHas industrial application valueOrganic chemistrySynthesis methodsReaction intermediate
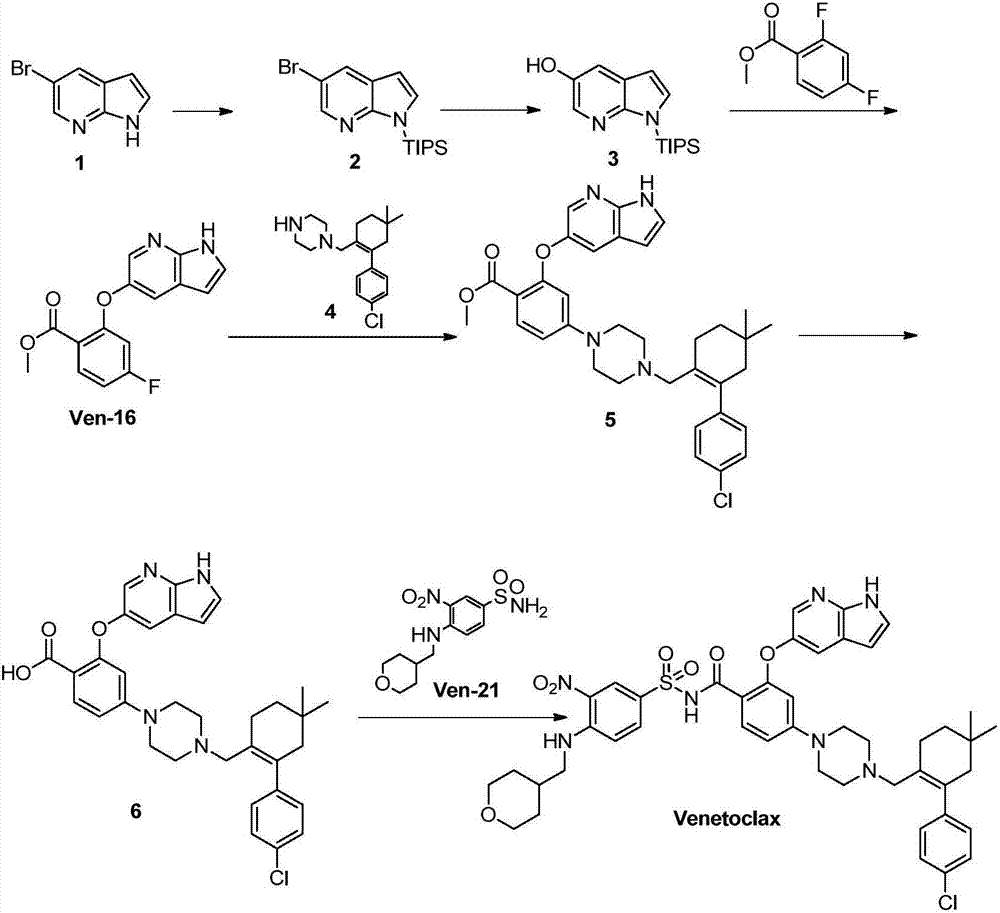
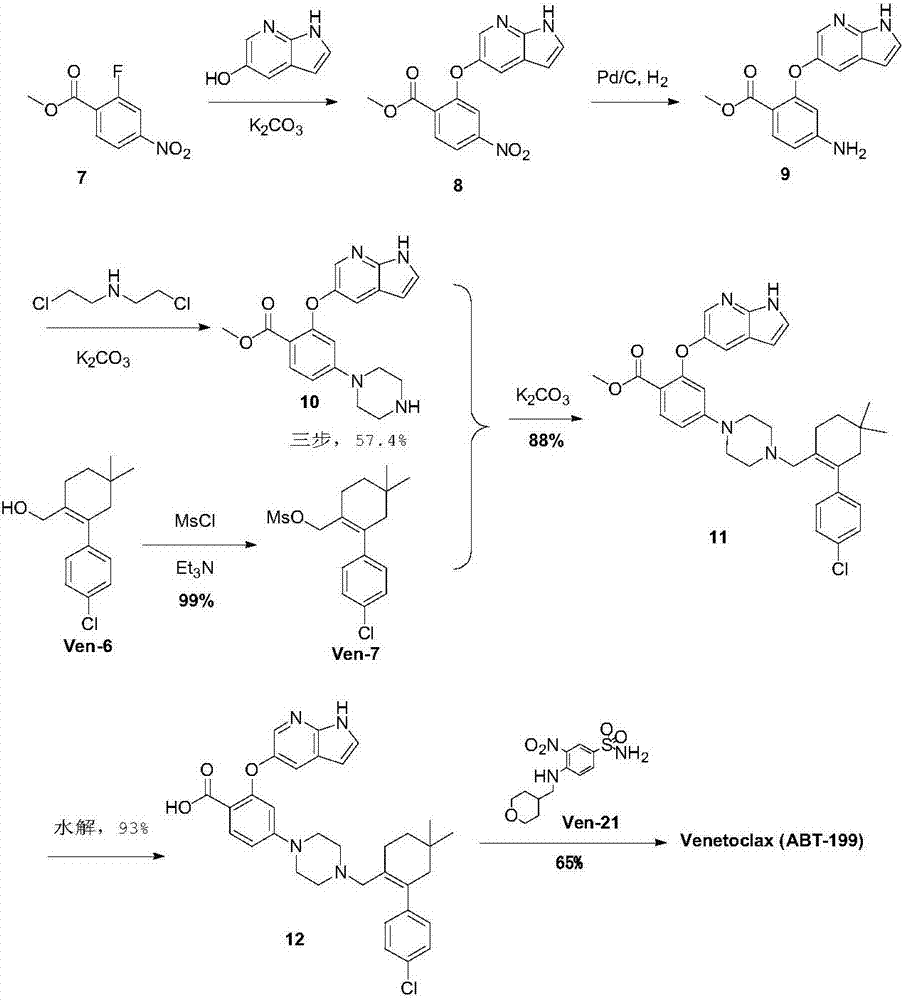
The invention discloses a new synthesis method for a BCL-2 inhibitor Venetoclax. The synthesis method includes the steps that 2,4-difluoro methyl benzoate serves as a raw material, the 2,4-difluoro methyl benzoate and 5-hydroxy-7-azaindole are subjected to a condensation reaction, Ven-16 is obtained, and 4-substituted isomer and other impurities are removed through recrystallization; the product and N-Boc-piperazine are reacted and hydrolyzed, and Ven-18 is obtained; Ven-18 and Ven-21 are condensed, Boc of trifluoroacetic acid is removed, and Ven-20 is obtained; the product and Ven-7 are reacted, reaction intermediates in all the steps have the good crystal forms, purification can be carried out through crystal to achieve the center control requirements of the technology, and a final product Venetoclax can also be subjected to recrystallization to obtain the product Venetoclax, wherein the HPLC purity of the Venetoclax is larger than 99.5%, and the individual impurity of the Venetoclax is smaller than 0.1%; the Venetoclax has the industrialized application value.
Owner:杭州科耀医药科技有限公司
Compound fruit fragrance and flower fragrance essence for daily chemicals and preparation method thereof
InactiveCN105316109AImprove stabilityMeet the needs of material and cultural lifeEssential-oils/perfumesBenzoic acidDamascone
Owner:广东铭康香精香料有限公司
Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION
Disclosed herein are mixed metal-organic frameworks, Zn3(BDC)3[Cu(SalPycy)] and Zn3(CDC)3[Cu(SalPycy)], wherein BDC is 1,4-benzenedicarboxylate, CDC is 1,4-cyclohexanedicarboxylate, and SalPyCy is a ligand of the formula:These are useful for applications such as selective gas storage, selective molecular separations, and selective detection of molecules, including enantioselective applications thereof.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
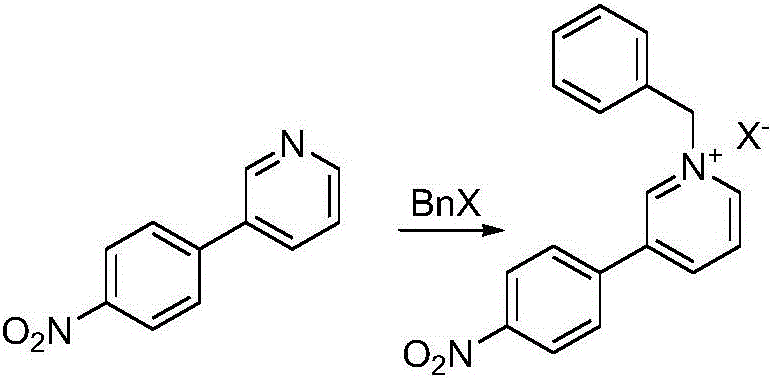
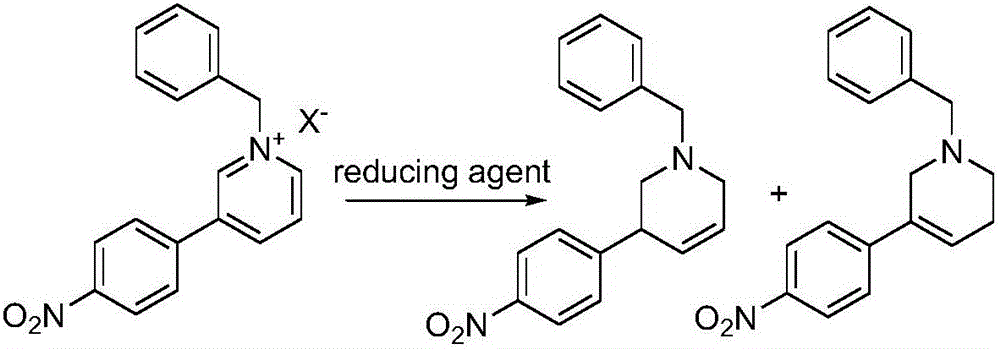
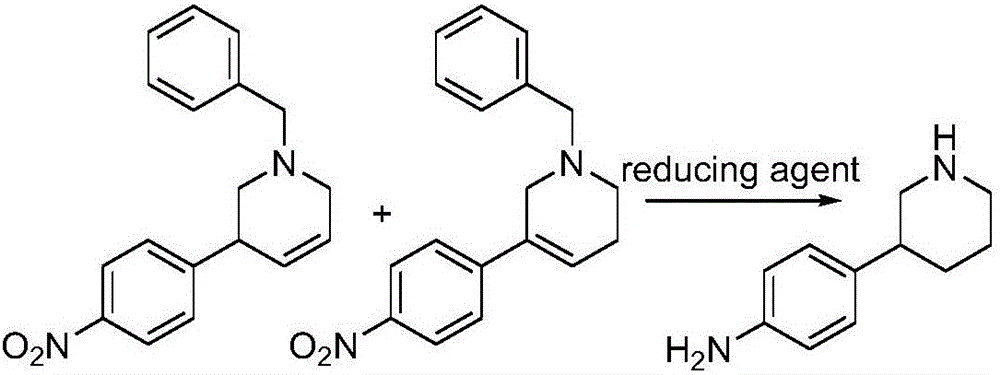
Preparation method of Niraparib
The invention discloses a preparation method of a compound 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide; through the reaction of 4-nitryl phenylpyridine and benzyl halides, the benzyl quaternary ammonium salt is generated; the pyridine quaternary ammonium salt is restored selectively through sodium borohydride; under the effect of palladium reagent, the 3-(4- aminophenyl)- piperidine is obtained the (S)-3-(4- halogenated phenyl) piperidine is obtained through manually splitting the reagent, and then condensed with 3- formyl group-2- nitrobenzene methyl benzoate and forms pyrazol ring under the effect of sodium azide; through aminolysis, the Niraparib (molecule entity is: 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide) is prepared.
Owner:NANJING CORE TECH CO LTD
Method for preparing phenylformic acid through liquid phase oxidation of methylbenzene
InactiveCN101613269AEliminate attachmentImprove reaction efficiencyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHigh concentrationBenzoic acid
The invention relates to a process of preparing phenylformic acid through liquid phase oxidation of methylbenzene, in particular to preparation of the phenylformic acid by taking a complex of one or more metal ions of cobalt, manganese, copper and cerium as a catalyst, taking molecular oxygen as an oxidant, taking ester compounds containing aromatic rings as a solvent, and performing catalytic selective oxidation on the methylbenzene. The process has the advantages that: (1) the process adopts a homogeneous complex catalyst, and has high conversion rate of the methylbenzene; (2) the process has mild oxidation reaction conditions, and the methylbenzene has high selectivity to generate a phenylformic acid target product; (3) the process uses the air to oxidize the methylbenzene, takes the ester compounds containing the aromatic rings such as methyl formate or nzyl benzoate as the solvent, has high concentration of oxygen in the system, and is favorable for the oxidation of the methylbenzene; and (4) a product mixture mainly contains the phenylformic acid and the ester compounds taken as the solvent, and has simple product separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Rare earth complex and process for preparing the same
InactiveCN1803804AHigh strengthSimple preparation processGroup 3/13 element organic compoundsRare earthMethyl benzoate
The related rare-earth complex has structural formula as Ln2M1(M2)2R with two trivalent Ln as central ions and ligand M contained M1 as 3,5-bi(4-carboxylbenzoate)phenyl benzoate and M2 as 1, 10-orthophenanthroline(phen) and R as solvent. Wherein, the reaction needs 5-20h at 20-80Deg. This invention can be used as luminous complex with high efficiency, intensity and stability.
Owner:DONGHUA UNIV
Preparation method for trapping agent for bauxite flotation
The invention relates to a preparation method for a trapping agent for bauxite flotation. The method is characterized by comprising the following steps of: undergoing a hydroxyl oximation reaction on a caustic soda solution, a hydroxylamine sulfate solution and methyl benzoate serving as raw materials to obtain a mixed solution containing sodium benzohydroxamic acid, sodium sulfate, methanol and water; and diluting the obtained mixed solution with water. The KL trapping agent prepared with the method has the advantages of high trapping capability, small using amount, good separating effect, effective reduction in chloride ions which are introduced into an alumina production flow, and effective reduction in corrosion of stainless steel equipment in ore dressing and the alumina production flow.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
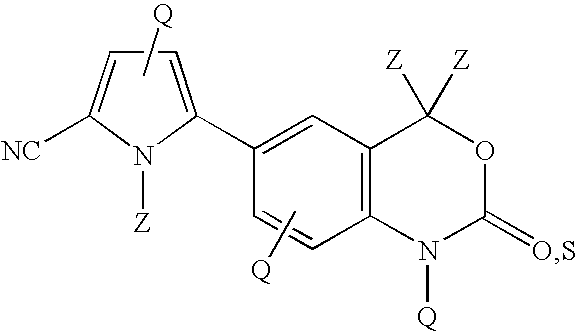
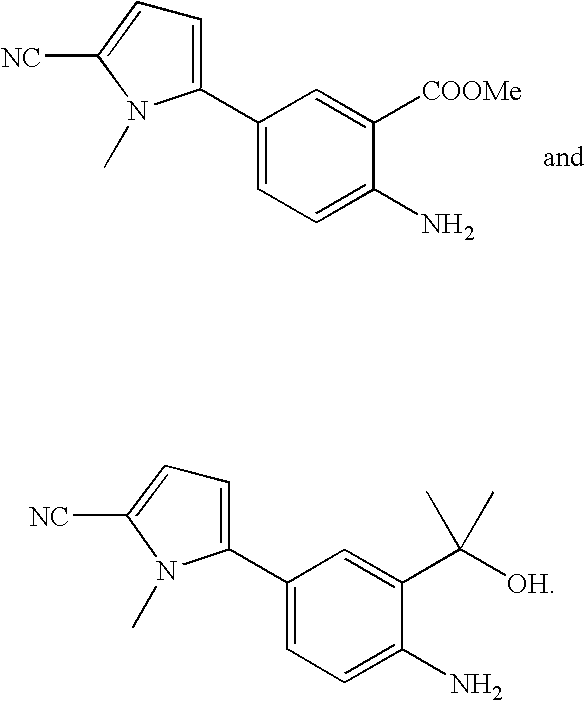
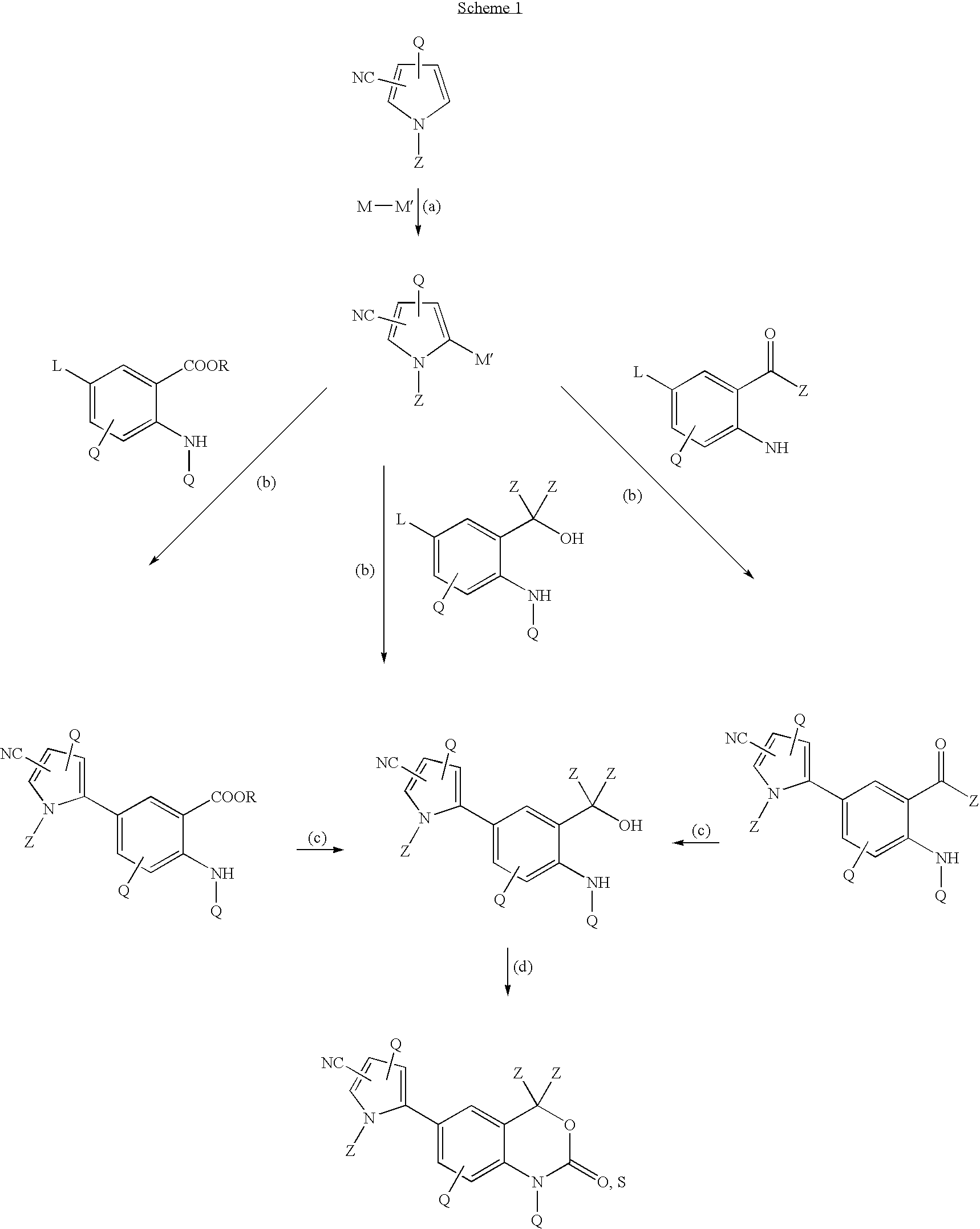
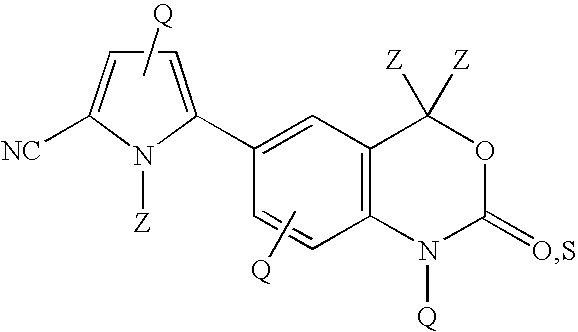
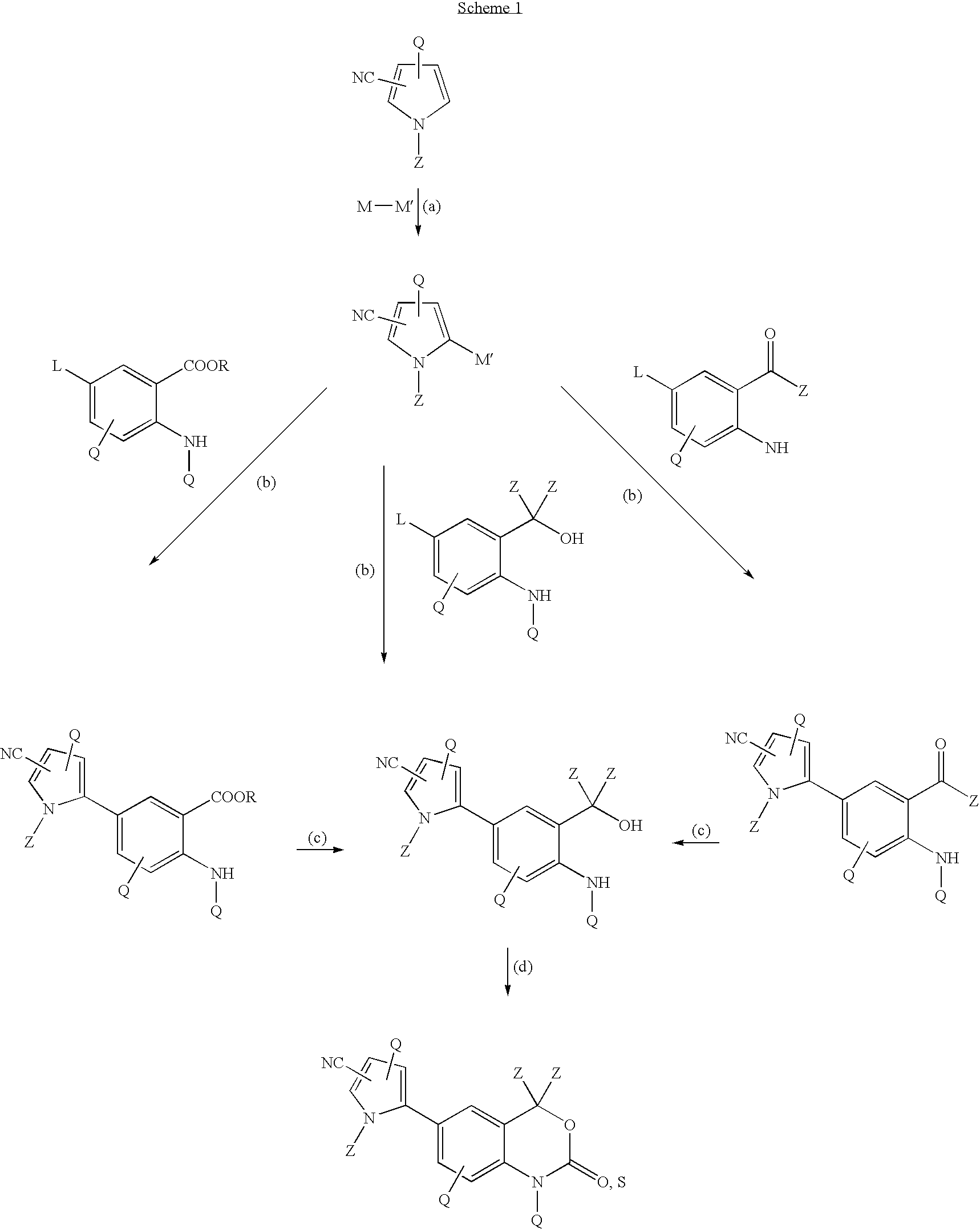
Cyanopyrrole-containing cyclic carbamate and thiocarbamate biaryls and methods for preparing the same
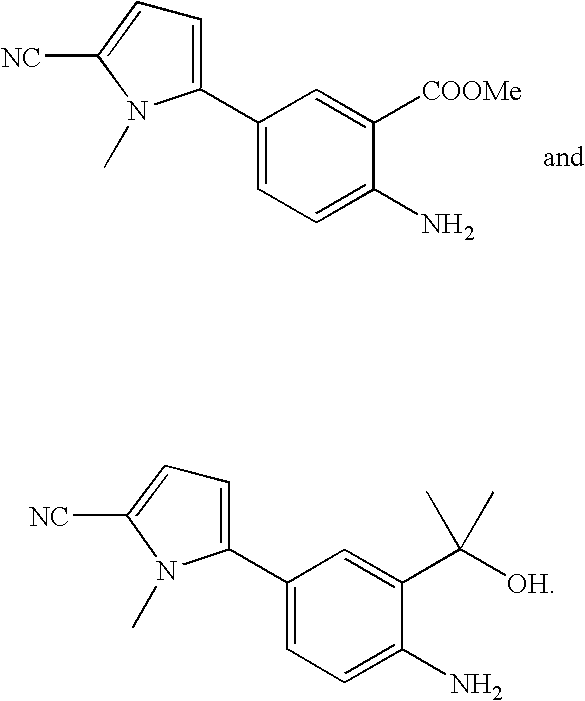
Methods for preparing cyclic carbamates and thiocarbamates containing cyanopyrrole moieties and of the formula are provided. Z are the same or different and are H, optionally substituted C1 to C6 alkyl, or CORA; RA is H, optionally substituted C1 to C6 alkyl, optionally substituted C1 to C6 alkoxy, or optionally substituted C1 to C6 aminoalkyl; Q are the same or different and are H, OH, NH2, CN, halogen, optionally substituted C1 to C6 alkyl, optionally substituted C2 to C6 alkenyl, optionally substituted C1 to C6 alkynyl, optionally substituted C1 to C6 alkoxy, optionally substituted C1 to C6 aminoalkyl, or CORB; and RB is H, optionally substituted C1 to C6 alkyl, optionally substituted C1 to C6 alkoxy, or optionally substituted C1 to C6 aminoalkyl. Compounds including 2-amino-5-(5-cyano-1-methyl-1H-pyrrol-2-yl) benzoic acid methyl ester, 5-[4-amino-3-(1-hydroxy-1-methyl-ethyl)-phenyl]-1-methyl-1H-pyrrole-2-carbonitrile, and 2-amino-5-(5-cyano-1-methyl-1H-pyrrol-2-yl)-phenyl-ethanone, or pharmaceutically acceptable salts thereof, and the uses thereof are also provided.
Owner:WYETH LLC
Catalyst prepared by using M/Mn/Al hydrotalcite as precursor for producing benzyl formaldehyde by gaseous phase hydrogenation of benzoic acid or methoylbenzoatc
InactiveCN1473810APreparation by hydrogenolysisMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidHydrogen
The present invention relates to the method of applying catalyst prepared with M / Mn / Al hydrotalcite as precursor in gaseous phase hydrogenation of benzoic acid or methyl benzoate to produce benzyl formaldehyde. The M / Mn / Al hydrotalcite precursor prepared through co-precipitation process is roasted in air or nitrogen atmosphere to obtain catalyst with high dispersivity and high specific surface area. Under the catalysis of the catalyst containing Mn, Al, M (M is Mg, Zn, Pb, Ni or Cr) and K elements, the temperature of 350-500 deg.c and pressure 0.1-1.0 MPa, proper reactant ratio and materialfeeding rate, benzoic acid or methyl benzoate is gaseous phase hydrogenated to produce benzyl formaldehyde at conversion rate and selectivity over 90 %.
Owner:FUDAN UNIV
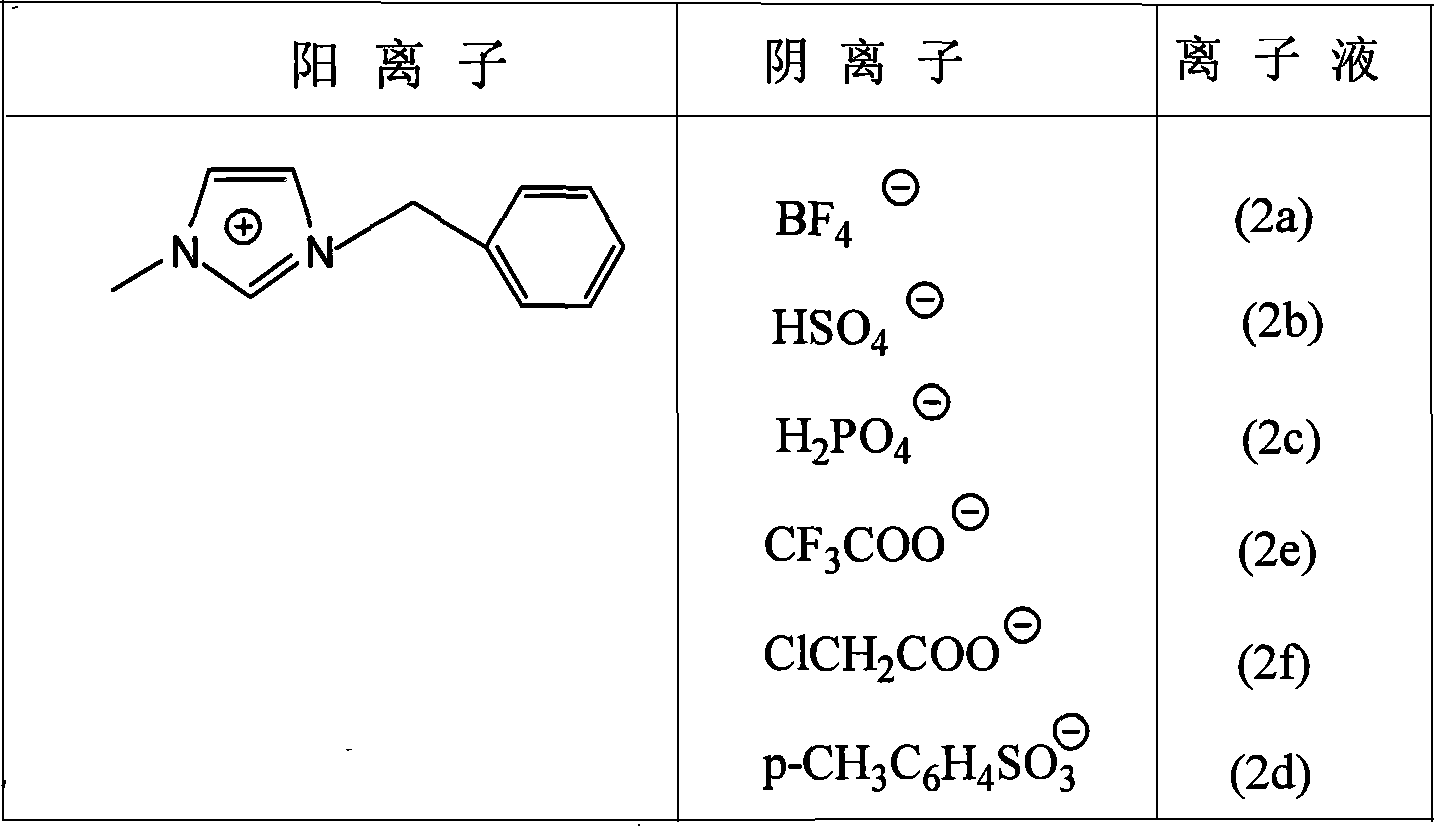
Acidic ionic liquid based on 1-methyl-3-benzyl imidazole cation, synthetic method and use
InactiveCN101058560AAchieve green synthesisEasy to separateOrganic compound preparationCarboxylic acid esters preparationInto-structureSolvent
The invention discloses an acid ion liquid and synthesizing method and application based on 1-methyl-3-benzyl imidazole cation, which is 1-methyl imidazole benzyl methylation organic cation, wherein the inorganic / organic anion is BF4<->,HSO4<->, H2PO4<->, p-CH3C6H4SO<3->, CF3COO<-> and ClCH2COO<->, wherein the ion liquid is liquid phase under indoor temperature, which can repair structure as mother compound due to leading functional group into structure, such as benzene ring, methylene and so on; the BrPhinsted acid can be repeating ring, reacting dielectric and reacting catalytic, which can be used to synthesize important perfume and solvent.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Sheet substrates impregnated with aromatic releasing compositions and a method of delivery of aromatic releasing compositions
An article of manufacture and a method directed to application of aromatic releasing compositions impregnated within substrates such as non-woven paper materials (e.g., wipes, paper towels) and dispensable cloth materials (e.g., gauze or a thin fabric of silk, linen, or cotton materials) for providing relief from cold, allergies, sinus and symptoms associated with respiratory disorders, the aromatic releasing compositions including the following: Menthol; Camphor; Eucalyptus oil; Cedarleaf Oil; Myristica Oil; Peppermint Oil; Lavender oil; Methyl Salicylate; Naproxen; Nutmeg Oil and Thymol; Beclometasone dipropionate; Benzethonium chloride with base solution consisting of Emollients, Emulsifiers and Moisturizer; Deionized Water; Vegetable Oil; Dicaprylyl Carbonate; Glyceryl Oleate; Polyglyceryl-2 Dipolyhydroxystearate; Cetearyl Isononanoate; Ceteareth-20; Cetearyl Alcohol; Glyceryl Stearate; Glycerin; Cetyl Palmitate; Ceteareth-12, Lauryl Glucose Carboxylate; Lauryl Glucoside; Sodium Citrate; Citric Acid; Benzethonium Chloride 0.05%; Ethylene diamine tetra acetic acid; Phenoxyethanol; Methylparaben; Propylparaben; 2-bromo-2-nitropropane-1,3-diol; and subcombinations thereof. In further embodiments, the compositions impregnated within substrate further include one or more topical actives, and are useful for providing relief from cold, allergies, sinus and symptoms associated with respiratory disorders, as well as repelling common virus and bacteria.
Owner:ADELAKUN OLUFEMI
Method for comprehensive recycling of crude terephthalic acid (CTA) residue
ActiveCN102206156AOrganic compound preparationPreparation by ester-hydroxy reactionBenzoic acidSocial benefits
The invention discloses a method for comprehensive recycling of crude terephthalic acid (CTA) residue. The method comprises: esterifying the CTA residue to obtain mixed diisooctyl phthalate, isooctyl methyl benzoate and isooctyl benzoate; and separating to obtained mixed diisooctyl phthalate, which serves as a heavy component, and a mixture of isooctyl methyl benzoate and isooctyl benzoate, whichserves as a light component, wherein the mixed diisooctyl phthalate is a plasticizer that can be used directly; and the mixture of isooctyl methyl benzoate and isooctyl benzoate can be formed into a mixture of (methyl) benzoic acid diethylene glycol ester by interesterification; the mixture of (methyl) benzoic acid diethylene glycol ester is also a high-performance plasticizer; and thus, all CTA residue is used for producing plastic products, the comprehensive recycling of the CTA residue is realized, and high economic benefit and social benefit are created.
Owner:FUJIAN TIANDA CHEM
Chemical crossbred agent composition, its using method and application
InactiveCN1524417AEfficient inductionImprove sterilityBiocideAnimal repellantsBiotechnologyBenzoic acid
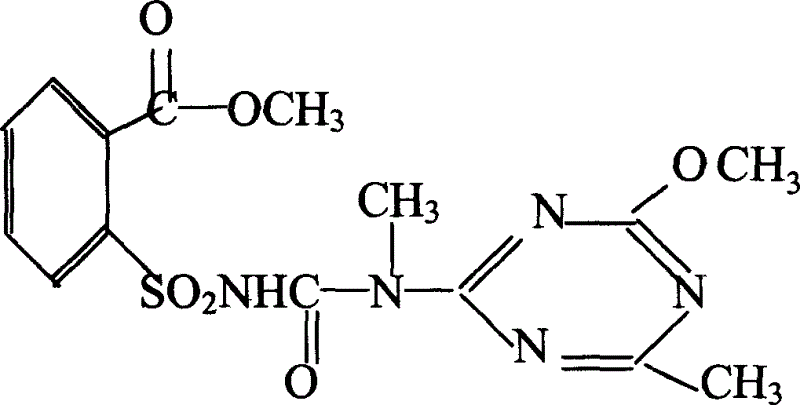
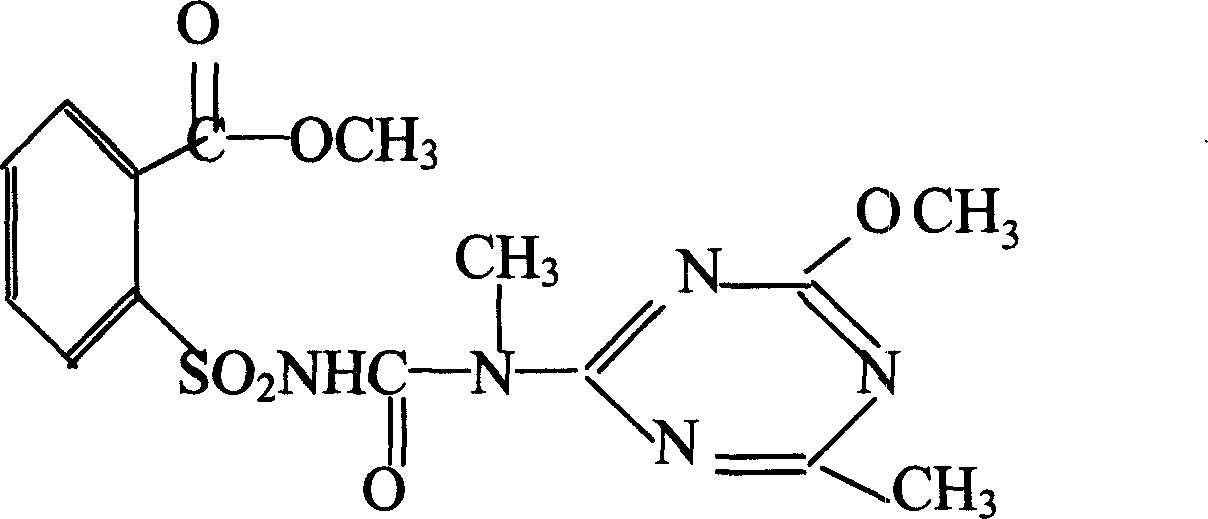
The invention relates to a rape chemical hybridization agent composition and uses thereof, wherein the composition (SX-1) contains active constituent of 2-[3-(4- methoxy-6- methyl-1,3,5- triazine-2-group)-3- methyl carbamido sulfonyl] methyl benzoate, aerosol, emulsifying agent, thickening agent, crumbling agent and soluble starch. The invention also relates to the method of use and application in rape chemical hybridization of the composition.
Owner:陕西省杂交油菜研究中心
Novel preparation of trityl group candesartan cilexetil intermediate
ActiveCN101323610AThe synthesis process is simpleLow investment costOrganic chemistryBenzoic acidCandesartan
The invention discloses a novel technology for synthesizing an intermediate of trityl candesartan; the synthesis steps thereof comprise: (1) a preparation method of 2-menthyl formate-6-nitryl-benzoic acid; (2) a preparation method of 2-amido-3-nitryl-methyl benzoate; (3) a preparation method of 2, 3-diaminobenzene menthyl formate; (4) a preparation method of 2-oxethyl-4-menthyl formate-3-H-benzimidazole; and (5) the preparation method of the intermediate of trityl candesartan.
Owner:APELOA PHARM CO LTD +1
Method for methyl esterification recovery and recycle of PTA oxidation residue
InactiveCN105017022ARealize resource utilizationHigh purityOrganic compound preparationCarboxylic acid esters preparationDimethyl terephthalateMethyl benzoate
The invention discloses a method for methyl esterification recovery and recycle of PTA oxidation residue. The method comprises 1, primary oxidation treatment, 2, secondary methyl esterification treatment, and 3, tertiary rectification treatment, wherein methyl benzoate is recovered by primary reduced pressure rectification, methyl toluate is recovered by secondary reduced pressure rectification, mixed benzenedicarboxylic acid dimethyl ester is recovered by tertiary reduced pressure rectification, and dimethyl terephthalate, dimethyl isophthalate and dimethyl phthalate are respectively recovered by mixed benzenedicarboxylic acid dimethyl ester crystallization treatment. The method effectively recovers BA, IPA, TA, PT, 4-CBA, 4-HBA, OPA and TMA of the PTA oxidation residue, can produce high purity methyl benzoate, mixed benzenedicarboxylic acid dimethyl ester and trimethyl trimellitate products and realizes separation and recovery of DMT, DMI and DMO products from mixed benzenedicarboxylic acid dimethyl ester by crystallization.
Owner:ZHEJIANG UNIV
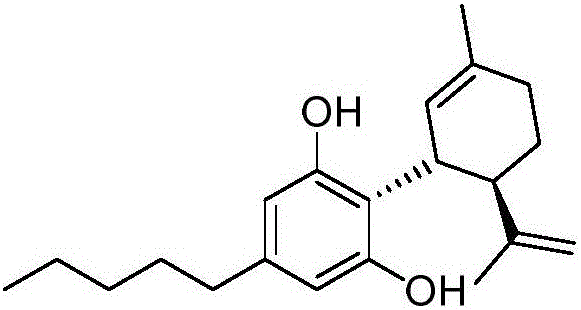
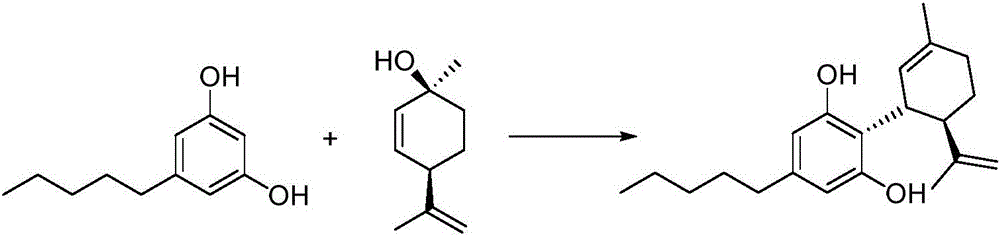
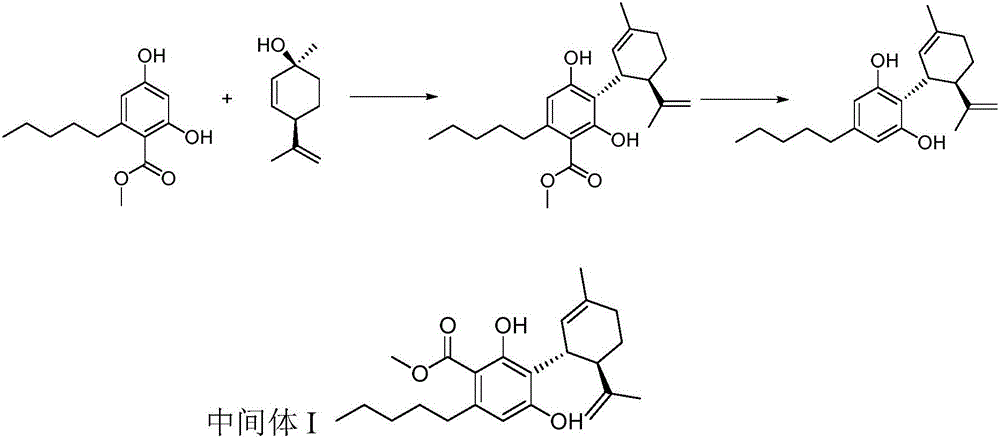
Cannabidiol synthesis method
ActiveCN106810426ALow priceCommercially availableOrganic compound preparationOrganic chemistry methodsSynthesis methodsCyclohexene
The invention provides a cannabidiol synthesis method. The method includes: taking 2,4-dyhydroxy-6-pentyl methyl benzoate as a raw material, and subjecting to ester exchange with N,N-dialkyl alcohol amine under potassium hydroxide catalysis; subjecting to coupled reaction with (1S, 4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-alcohol under Lewis acid catalysis; performing acid-base extraction and recrystallization to obtain a high-purity key intermediate product; subjecting to hydrolysis decarboxylation to obtain crude cannabidiol, and subjecting the crude cannabidiol to once recrystallization to obtain cannabidiol according with raw medicine quality requirements. The cannabidiol synthesis method has advantages that raw materials and reagents are cheap and commercially easy to acquire, the total yield of the finally prepared raw medicine in qualified purity reaches 35-40%, the process is improved evidently, and the method has a promising industrial application prospect.
Owner:暨明医药科技(苏州)有限公司
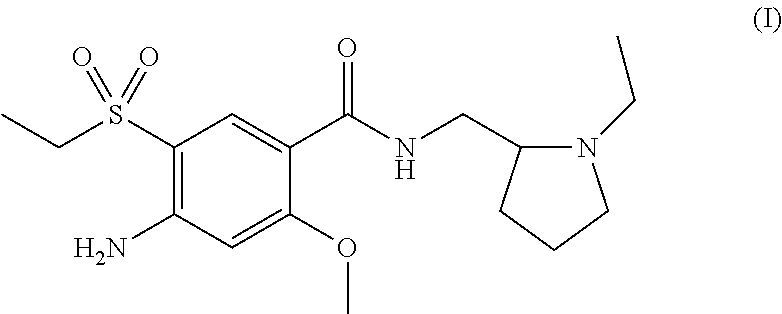
Process for preparation of amisulpride
The present invention is related to a novel process for the preparation of amisulpride (I) which involves: methylation of 4-amino-salicylic-acid (VI) with dimethyl sulphate and base, optionally in presence of TBAB to obtain 4-amino-2-methoxy methyl benzoate (VII) and (ii) oxidation of 4-amino-2-methoxy-5-ethyl thio benzoic acid (IX) or 4-amino-2-methoxy-5-ethyl thio methyl benzoate (X) with oxidizing agent in the presence of sodium tungstate or ammonium molybdate to give 2-methoxy-4-amino-5-ethyl-sulfonyl benzoic acid (IV) or 2-methoxy-4-amino-5-ethyl-sulfonyl methyl benzoate (XI) respectively.
Owner:LUPIN LTD
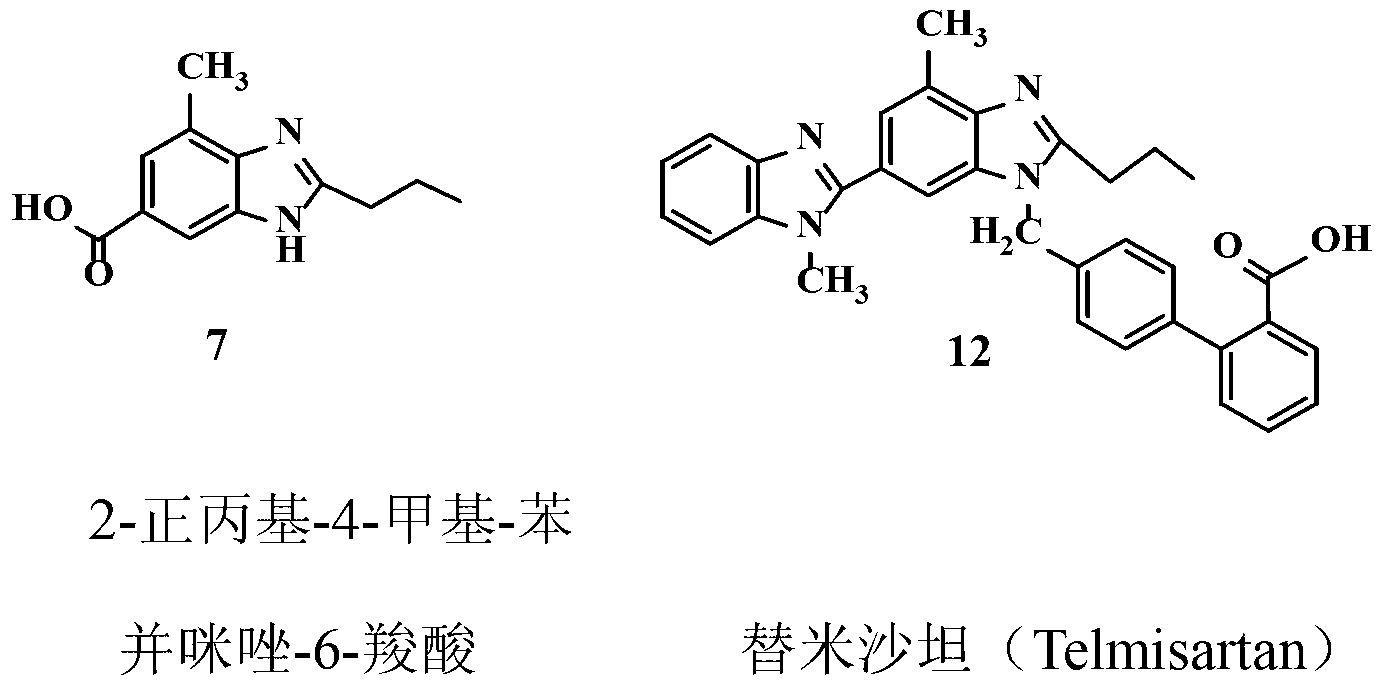
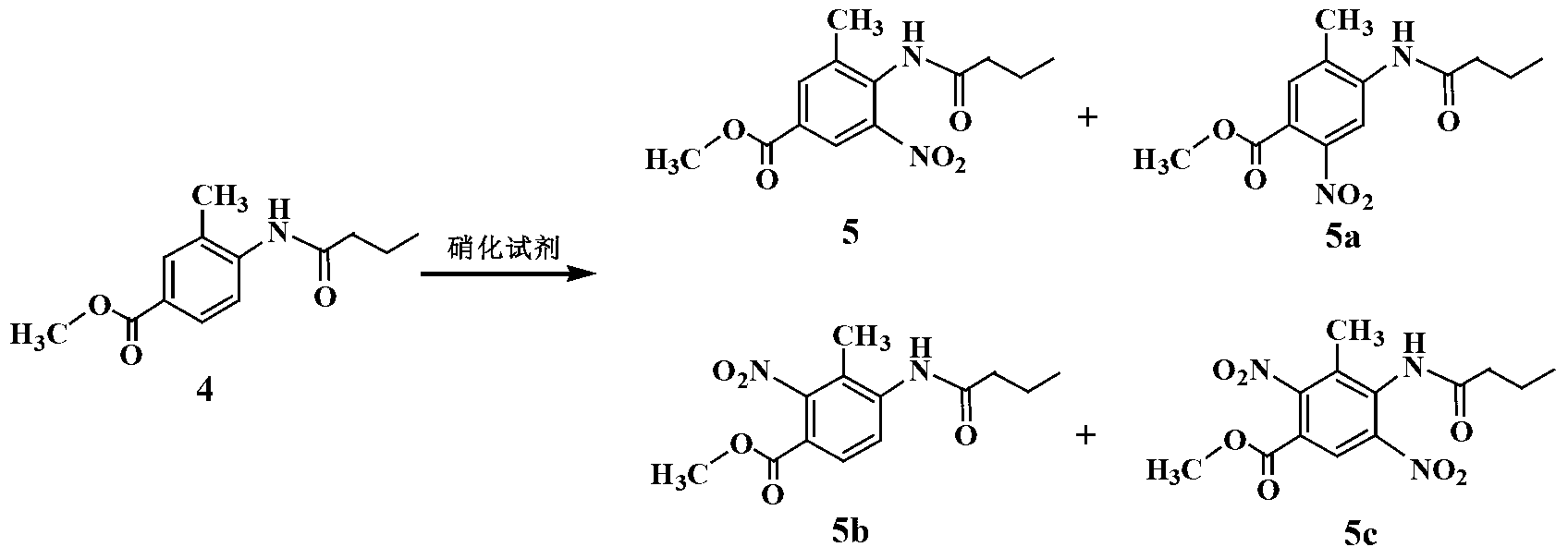
Improved telmisartan preparation process
InactiveCN103319414AEasy to operateShorten the production cycleOrganic chemistryChromatographic separationNitration
The invention provides an improved process for preparing a telmisartan raw material medicament. The improved process comprises the following step: preparing an intermediate 2-n-propyl-4-methyl-benzimidazole-6-carboxylic acid by using methyl 4-butylacetamino-3-methylbenzoate as a raw material through four steps, namely nitration, reduction, cyclization and hydrolysis. According to the process, crude products in nitrification, reduction and cyclization reactions are not subjected to purification and separation, and a target product can be separated by regulating the pH of a reaction liquid obtained in a hydrolysis reaction to be between 6 and 7, so that the problems of complicated operation and yield loss caused by separation of nitrified and reduced products by recrystallization and chromatographic separation of a cyclized product by a column are solved. Therefore, the purity of the prepared telmisartan crude product is over 99 percent after recrystallization. The improved process is simple to operate, high in yield and low in cost, and is advantageous to industrial production.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
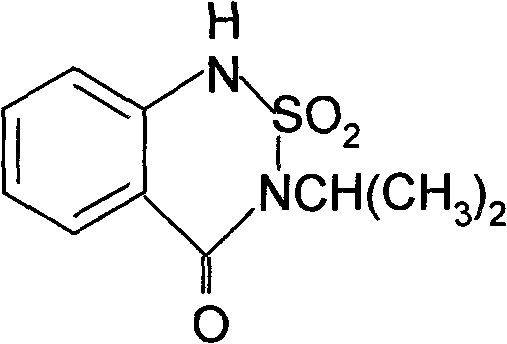
Synthetic method of bentazone
InactiveCN101863858ALow priceIncreased cyclization yieldOrganic chemistrySulfonyl chlorideMethyl anthranilate
The invention discloses a synthetic method of bentazone, comprising the following steps: taking phthalic anhydride as a main raw material; aminating, degrading and esterifying the phthalic anhydride to obtain methyl anthranilate; taking the methyl anthranilate, chlorosulfonic acid, isopropyl amine and phosphorus oxychloride as raw materials and triethylamine as an acid binding agent to prepare o-isopropylamide sulfonamido-methyl benzoate by a one-pot method; and finally carrying out cyclization and acidizing reaction on the o-isopropylamide sulfonamido-methyl benzoate by utilizing sodium alkoxide to synthesize the bentazone. In the method, the phthalic anhydride with cheap price and wide source is taken as the raw material instead of isopropylamide sulfonyl chloride, the o-isopropylamide sulfonamido-methyl benzoate is directly synthesized by a one-step method and meanwhile a catalyst is added to improve the cyclization yield; the synthetic method has the characteristics of easy operation, less three wastes, high yield, low cost and the like; and the final bentazone product has the yield up to over 85% and the purity up to over 98%.
Owner:江苏绿利来股份有限公司 +1
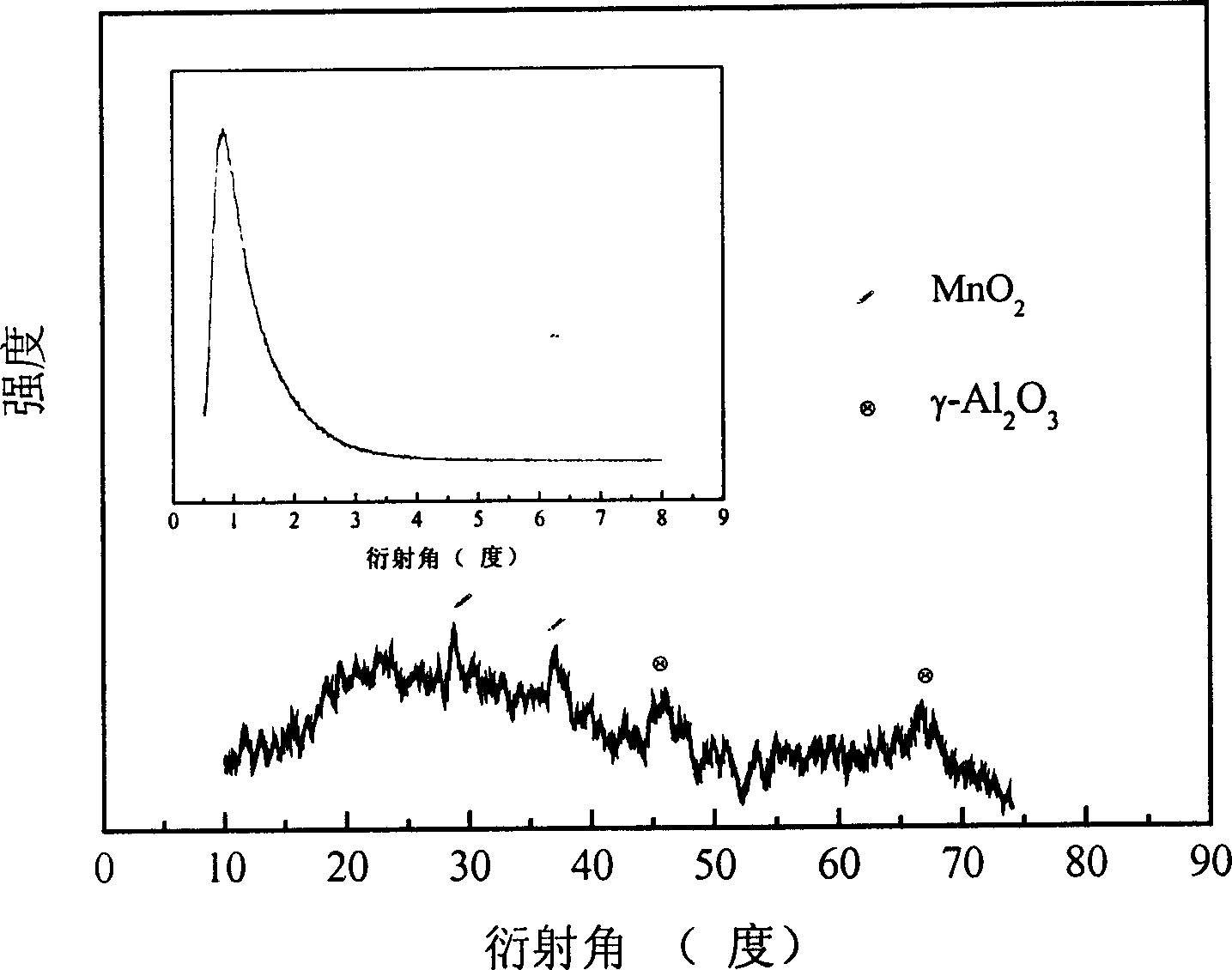
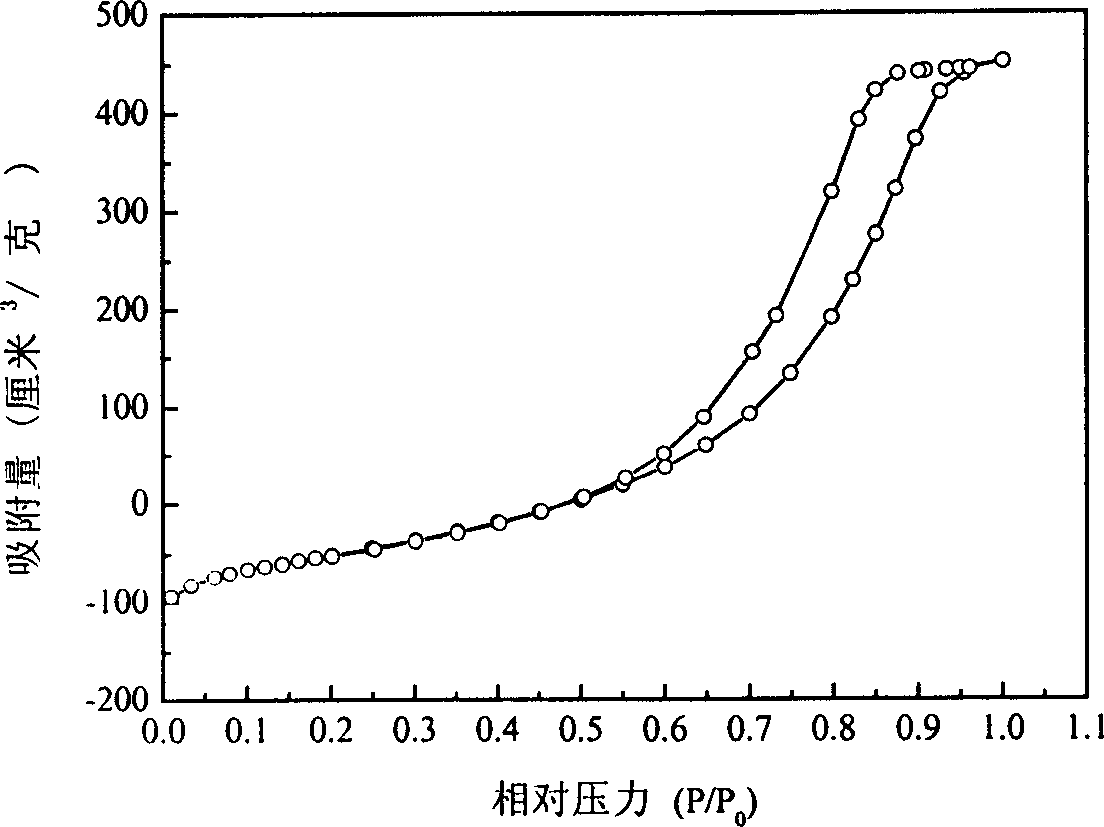
Mesoporous Mn/Al oxide catalyst, and its preparation method and use
InactiveCN1579619ALow reaction temperatureReduce energy consumptionCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsGas phaseHydrogenation reaction
The invention relates to a bore Mn / Al oxide catalyst and its producing method. The catalyst is one of the most effective compounds. Adopting bore alumina as carrier, producing process is simple and convenient for using isometric dipping method. The production has characters of bore construction, high decentralization and high specific surface. It has a remarkable effect to use the catalyst in hydrogenation reaction. In temperature of 350-450deg.C and pressure of 0.1-1.0 Mpa, conversion of methyl benzoate gas phase hydrogenation reaction is over 99 percent through selecting proper reactant ratio and feeding rate.
Owner:FUDAN UNIV
Cyanopyrrole-containing cyclic carbamate and thiocarbamate biaryls and methods for preparing the same
Methods for preparing cyclic carbamates and thiocarbamates containing cyanopyrrole moieties and of the formula are provided.Z are the same or different and are H, optionally substituted C1 to C6 alkyl, or CORA; RA is H, optionally substituted C1 to C6 alkyl, optionally substituted C1 to C6 alkoxy, or optionally substituted C1 to C6 aminoalkyl; Q are the same or different and are H, OH, NH2, CN, halogen, optionally substituted C1 to C6 alkyl, optionally substituted C2 to C6 alkenyl, optionally substituted C1 to C6 alkynyl, optionally substituted C1 to C6 alkoxy, optionally substituted C1 to C6 aminoalkyl, or CORB; and RB is H, optionally substituted C1 to C6 alkyl, optionally substituted C1 to C6 alkoxy, or optionally substituted C1 to C6 aminoalkyl.Compounds including 2-amino-5-(5-cyano-1-methyl-1H-pyrrol-2-yl) benzoic acid methyl ester, 5-[4-amino-3-(1-hydroxy-1-methyl-ethyl)-phenyl]-1-methyl-1H-pyrrole-2-carbonitrile, and 2-amino-5-(5-cyano-1-methyl-1H-pyrrol-2-yl)-phenyl-ethanone, or pharmaceutically acceptable salts thereof, and the uses thereof are also provided.
Owner:WYETH LLC
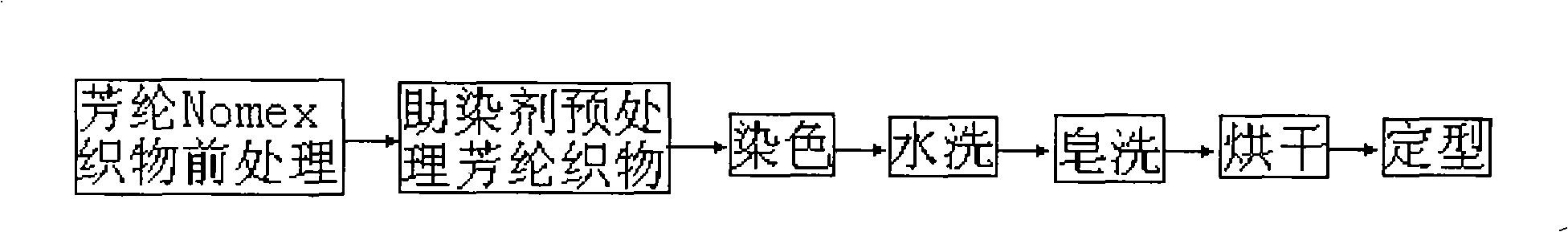
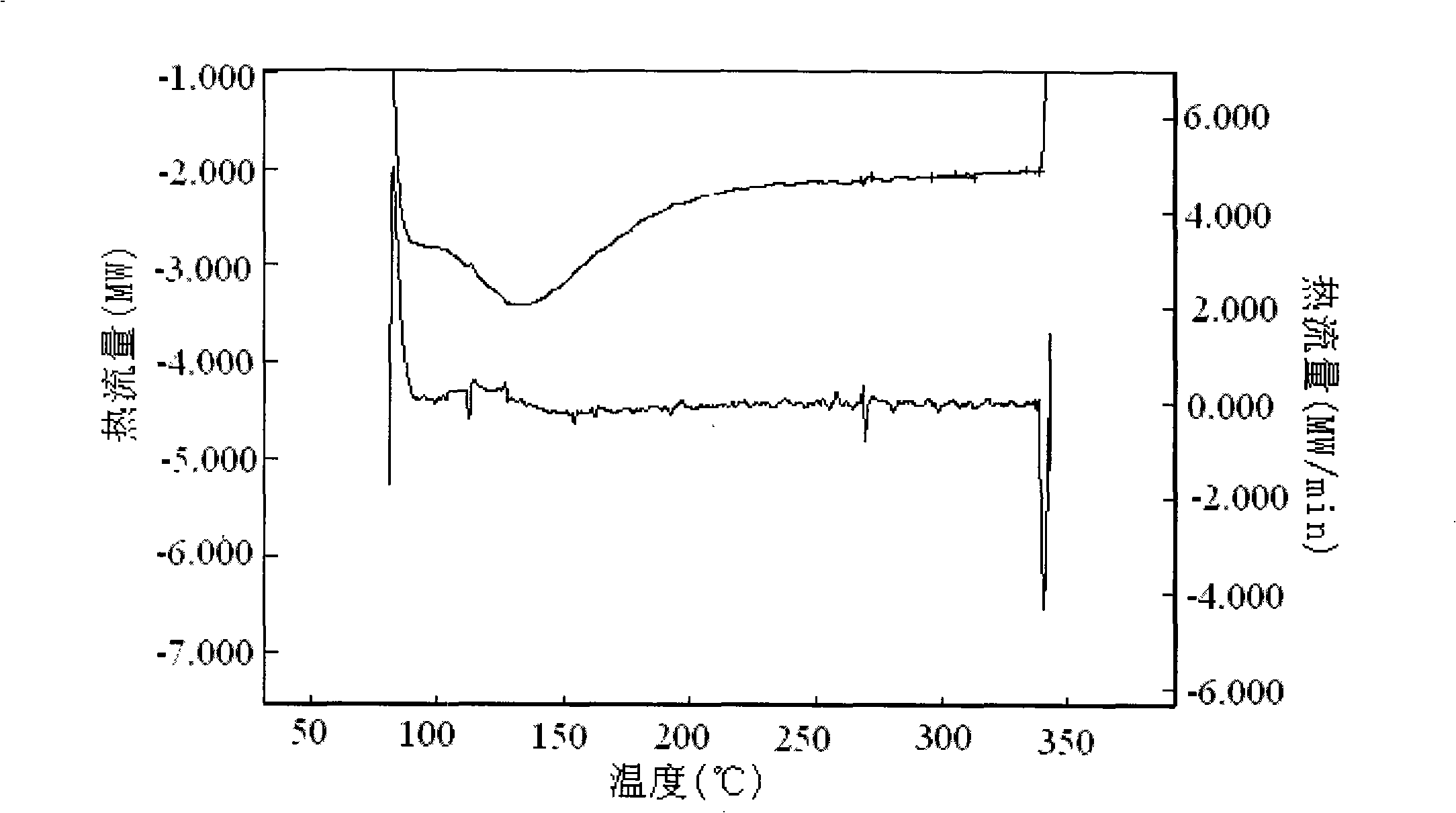
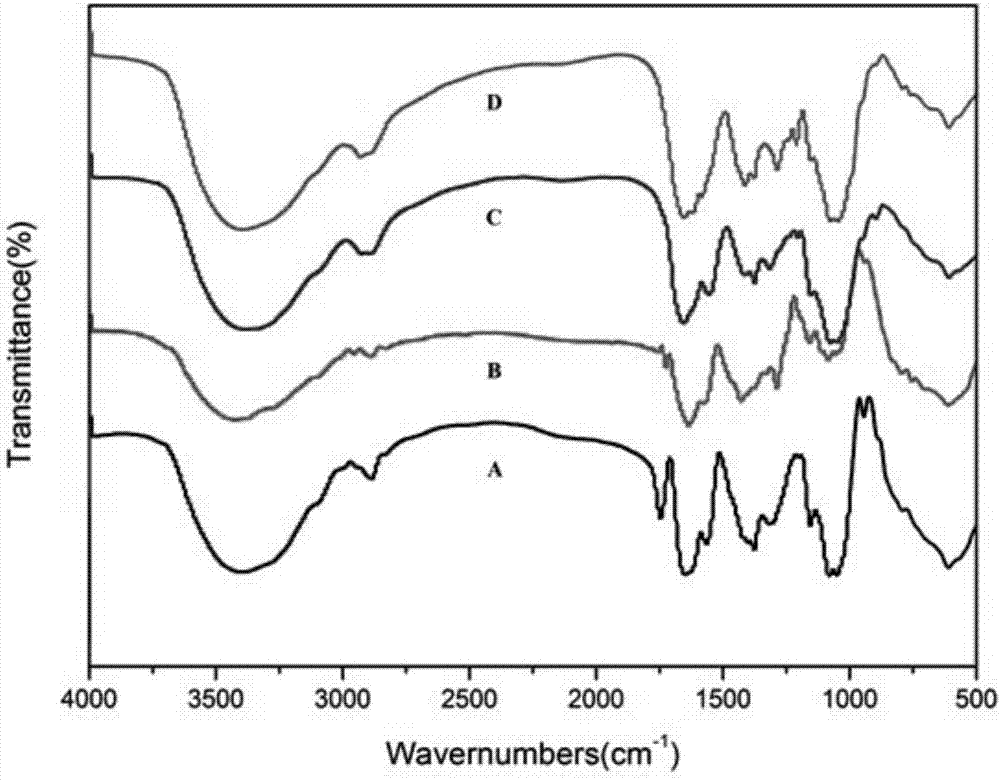
Dyeing-assist agent used for aramid fiber dyeing, preparation method and dyeing method thereof
InactiveCN101319462ALower glass transition temperatureSolve the problem of difficult dyeingFibre typesDyeing processSulfolaneVitrification
The invention discloses a dyeing assistant for aramid dyeing, consisting of the following components in percentage by mass: 8-12 percent of methyl benzoate, 4-6 percent of sulfolane, 1-3 percent of OP-10, 1-3 percent of sodium dodecyl sulfonate and 80-82 percent of water, and the total quantity of the components is 100 percent. A method of putting the raw materials into a high shear mulser for emulsification is adopted to prepare the dyeing assistant. A dyeing method for aramid comprises the following steps that: an aramid fabric is pretreated and then pretreated by using the dyeing assistant; then dyeing, washing, soaping, drying and shaping are carried out to the treated fabric in a conventional method, thus the aramid fabric is dyed. By lowering the vitrification temperature of the aramid, the dyeing method solves the problem that the aramid is difficult to dye and cannot has the dyeing depth reach medium deep color in a dyeing process, is novel in dyeing technique, short in technological process and simple to operate, causes no pollution to environment, and has extensive economic benefit and market benefit.
Owner:XI'AN POLYTECHNIC UNIVERSITY
Preparation method and application of pH-sensitive hyaluronic acid-doxorubicin nanometer prodrug
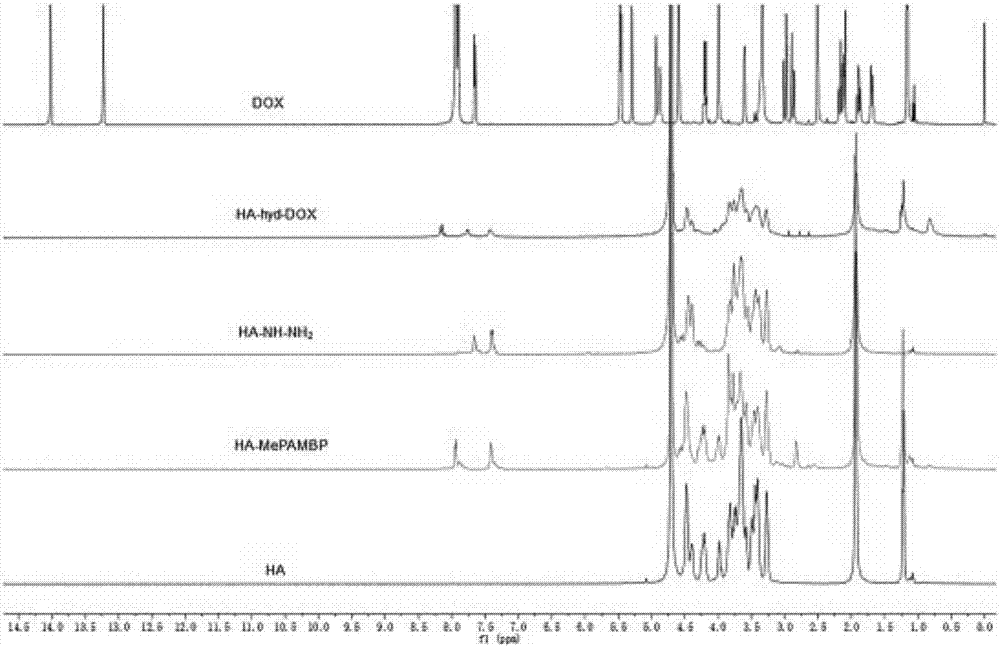
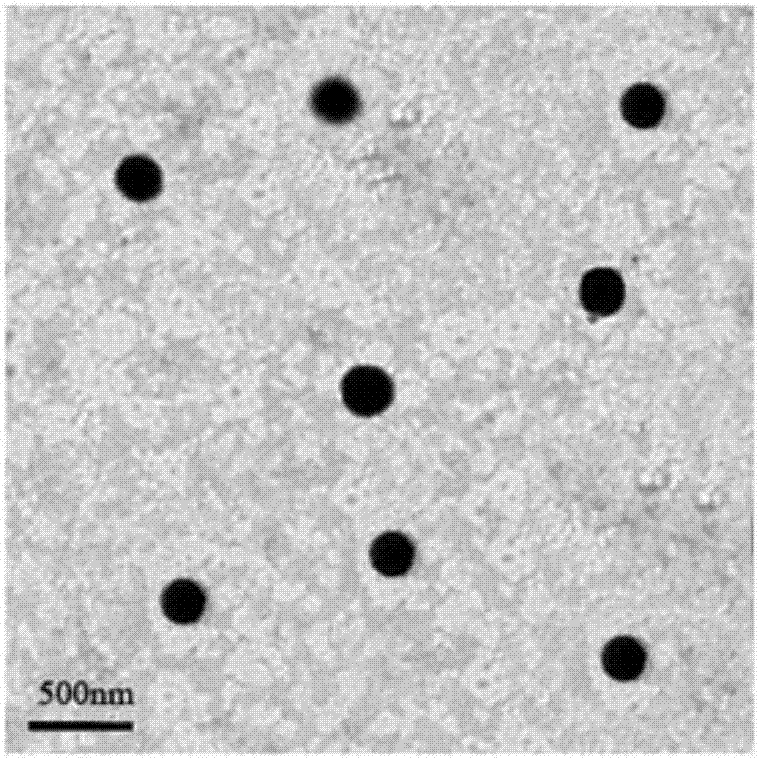
InactiveCN107096036AGood biocompatibilityImprove hydrophilic abilityOrganic active ingredientsPharmaceutical non-active ingredientsMethyl benzoateChemistry
The invention discloses a preparation method and application of a pH-sensitive hyaluronic acid-doxorubicin nanometer prodrug. The nanometer prodrug is prepared through the following preparation steps of firstly reacting amino in 4-aminomethyl methyl benzoate with carboxyl of hyaluronic acid to form an amido bond; forming hydrazine by using methoxy in a reactant in the previous step and hydrazine hydrate, and further forming a hydrazone bond by using a product and carbonyl of doxorubicin; and finally preparing nanometer prodrug particles through an ultrasonic assisted self-assembly method. The microstructure and the in vitro release property of the nanometer particles are researched, cellular uptake and a cytotoxicity test can be further carried out to prove that the macromolecular prodrug nanometer particles have obvious pH sensitivity and ideal in vitro anti-tumor activity, the problem of large toxic and side effects of the micromolecular drug doxorubicin can be solved and a basis can be provided for clinical application.
Owner:WUHAN UNIV OF TECH
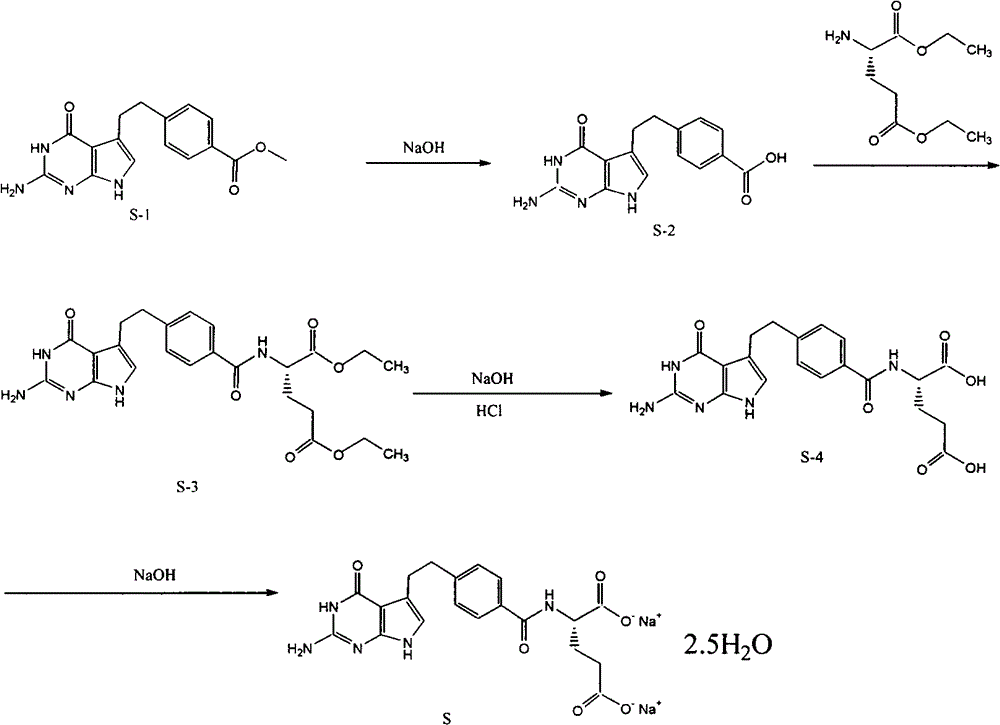
Preparation method of pemetrexed disodium
The invention provides a preparation method of pemetrexed disodium, which comprises the steps of performing hydrolysis reaction of 4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl] methyl benzoate in sodium hydroxide to generate acid, then condensing with L-glutamic acid diethyl ester to obtain N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid diethyl ester toluenesulfonate, obtaining the crude pemetrexed disodium through saponification under the action of sodium hydroxide, and finally crystallizing in a mixed solvent at room temperature to obtain the end product. With the adoption of the technical scheme, the purification and purification of the crude pemetrexed disodium can be directly performed, so that the cost can be effectively lowered. As the steps are carried out at room temperature, the oxidation of the pemetrexed disodium caused by high temperature can be effectively avoided. The purity of the end product is high and meets the national requirement on purity of chemicals.
Owner:DEZHOU DEYAO PHARMA
Methyl 3-(cyanomethyl)benzoate synthetic method
InactiveCN105130846AMild reaction conditionsEasy to operatePreparation by cyanide reactionMethyl benzoateSodium cyanide
The invention discloses a methyl 3-(cyanomethyl)benzoate synthetic method. The method comprises the following steps: taking m-toluic acid as a starting material, adding sulphone chloride for an acylation reaction, and flowing into liquid chlorine for a chlorination reaction, adding non-aqueous methanol dropwise for an esterification reaction to prepare ester, adding toluene and sodium cyanide into a cyanation reaction still, raising the temperature for backflow, adding the ester dropwise, carrying out backflow for 2 hours to prepare the methyl 3-(cyanomethyl)benzoate, and after the reaction, adding water to extract the methyl 3-(cyanomethyl)benzoate. The methyl 3-(cyanomethyl)benzoate synthetic method is high in yield, avoids serious three-waste pollution, and is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
Dimethyl terephthalate composition and process for producing the same
InactiveUS7078440B2Good colorLower levelOrganic compound preparationPreparation by ester-hydroxy reactionPolyesterDimethyl terephthalate
A dimethyl terephthalate composition includes 0.001 to 200 ppm of methyl 4-(1,3-dioxolan-2-yl)benzoate and 0 to 1 ppm of dimethyl hydroxyterephthalate contained in dimethyl terephthalate, and exhibits improved properties as a material for producing polyester.
Owner:TEIJIN FRONTIER CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00001.png)
![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00002.png)
![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00003.png)