Synthetic system of 3-cyanomethylbenzoic acid methyl ester and method thereof
A technology of methyl m-cyanomethylbenzoate and methyl m-cyanomethylbenzoate, which is applied in the field of synthetic systems of methyl m-cyanomethylbenzoate, can solve the problem of reducing the yield of methyl m-cyanomethylbenzoate, Problems such as inability to fully mix m-toluoyl chloride, etc., achieve the effects of environmental friendliness, low cost, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
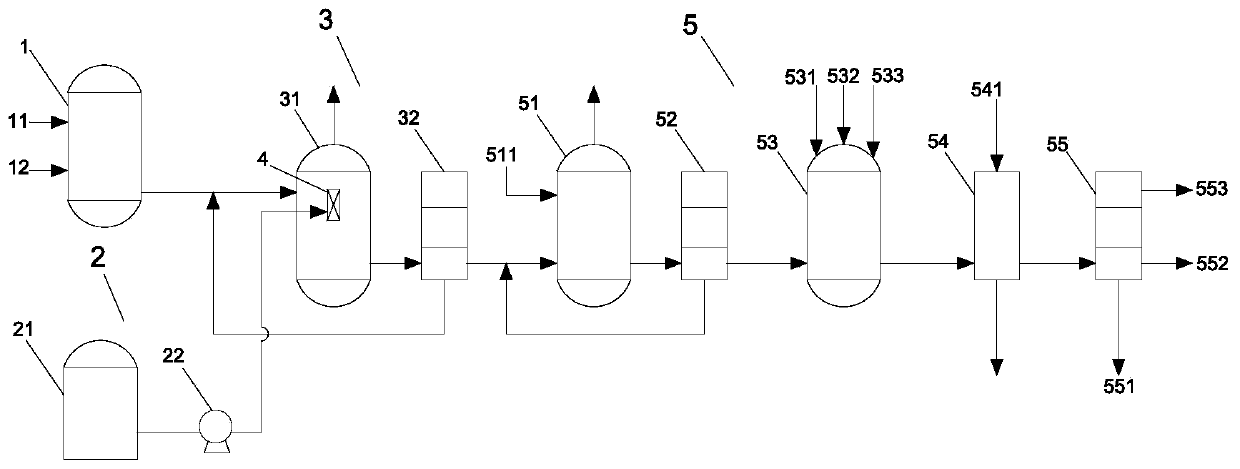
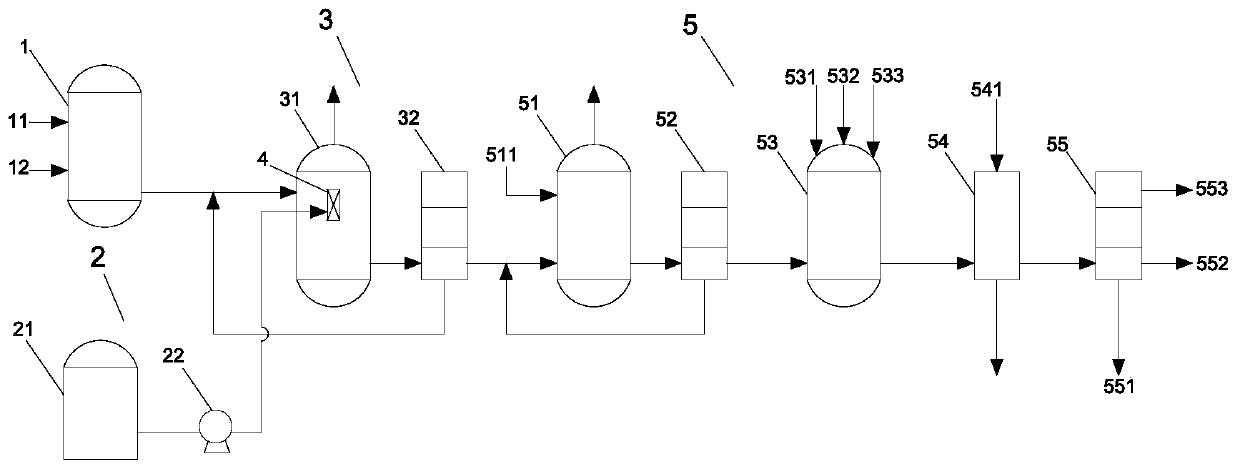
[0045] A synthetic method for methyl m-cyanomethylbenzoate, comprising:
[0046] Pump m-toluic acid into the acylation reaction kettle, add excess thionyl chloride to generate m-toluoyl chloride, and transport m-toluoyl chloride to the chlorination reaction kettle, at the same time, liquid chlorine The storage tank transports liquid chlorine to the inside of the micro-interface generator;
[0047] The micro-interface generator breaks the liquid chlorine into micron-scale micro-droplets, and transports the micro-droplets to the chlorination reactor, so that m-toluyl chloride undergoes a chlorination reaction in the chlorination reactor to obtain m- Chloromethylbenzoyl chloride;
[0048] Pump m-chloromethylbenzoyl chloride into the esterification reaction kettle and add dropwise anhydrous methanol to generate methyl m-chloromethylbenzoate. At the same time, add solvent methanol, quaternary ammonium salt catalyst and sodium cyanide to cyanide In the reaction kettle, the tempera...
Embodiment 1
[0054] a): 350 parts of m-toluic acid are pumped into the acylation reaction kettle, 400 parts of thionyl chloride are added, and m-toluic acid chloride is produced by acylation reaction at 65° C.;
[0055] b): m-toluoyl chloride is pumped into the chlorination reaction kettle, and 5% liquid chlorine by weight of m-toluoyl chloride is passed into it under the condition of maintaining 120° C., so that the acylate is converted into a chloride. The reacted m-toluyl chloride is chlorinated again in the chlorination reactor until finally obtaining high-quality m-chloromethylbenzoyl chloride;
[0056] c): The chloride is pumped into the esterification reaction kettle, and 80 parts of anhydrous methanol are added dropwise, and the esterification reaction is carried out at 45° C. to form an esterified product. After the esterification reaction is completed, excess methanol is distilled off;
[0057] d): Add the solvent methanol, 20 parts of trioctylmethyl ammonium chloride, and 150 pa...
Embodiment 2
[0060] a): 350 parts of m-toluic acid are pumped into the acylation reaction kettle, 350 parts of thionyl chloride are added, and m-toluic acid chloride is produced by acylation at 75° C.;
[0061] b): pump m-toluoyl chloride into the chlorination reaction kettle, and feed liquid chlorine with 10% weight of m-toluoyl chloride under the condition of 130°C to convert the acylate into chloride, and recover the non-participated by distillation The reacted m-toluyl chloride is chlorinated again in the chlorination reactor until finally obtaining high-quality m-chloromethylbenzoyl chloride;
[0062] c): The chloride is pumped into the esterification reaction kettle, and 80 parts of anhydrous methanol are added dropwise, and the esterification reaction is carried out at 50° C. to form an esterified product. After the esterification reaction is completed, excess methanol is distilled off;
[0063] d): Add the solvent methanol, 20 parts of trioctylmethyl ammonium chloride, and 150 part...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com