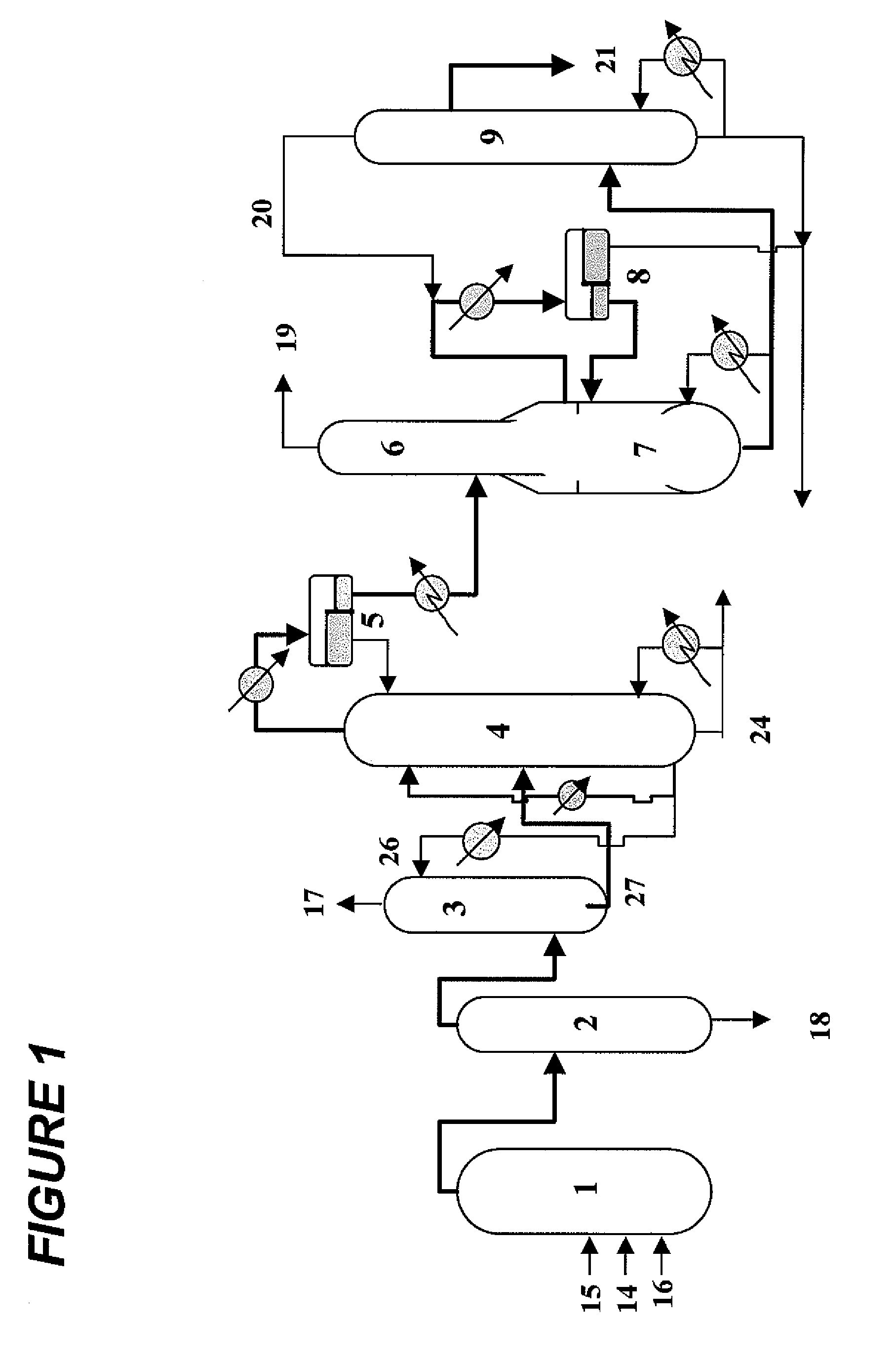
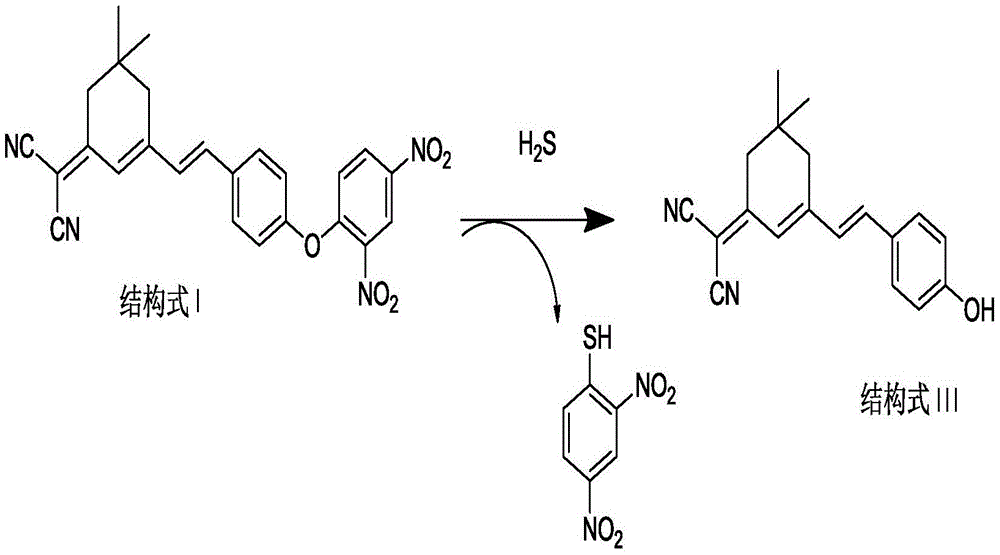
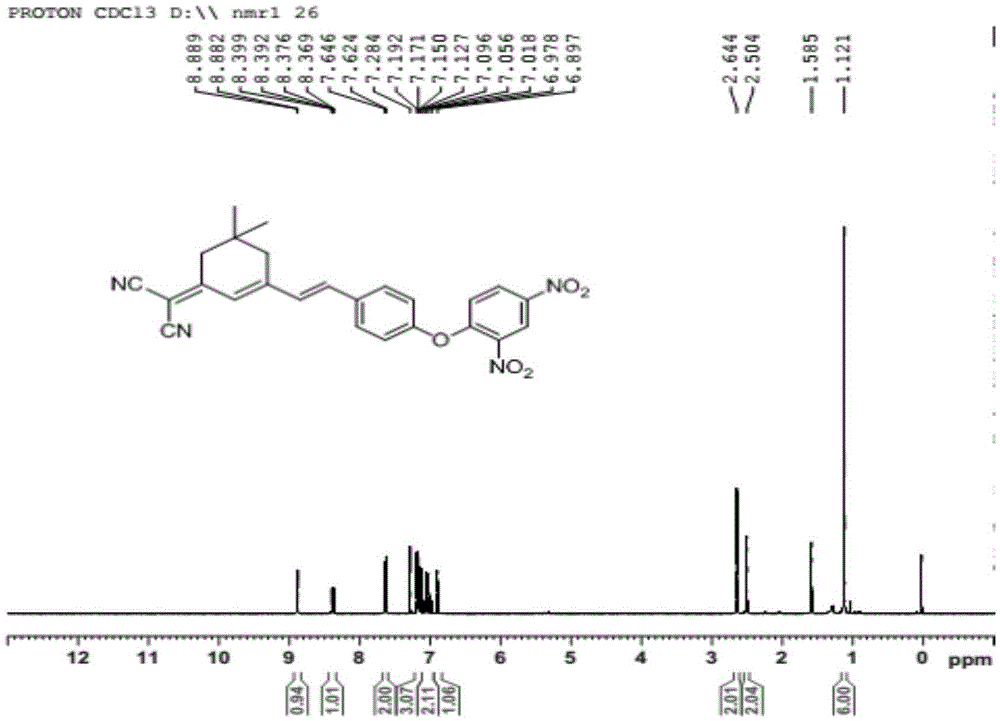
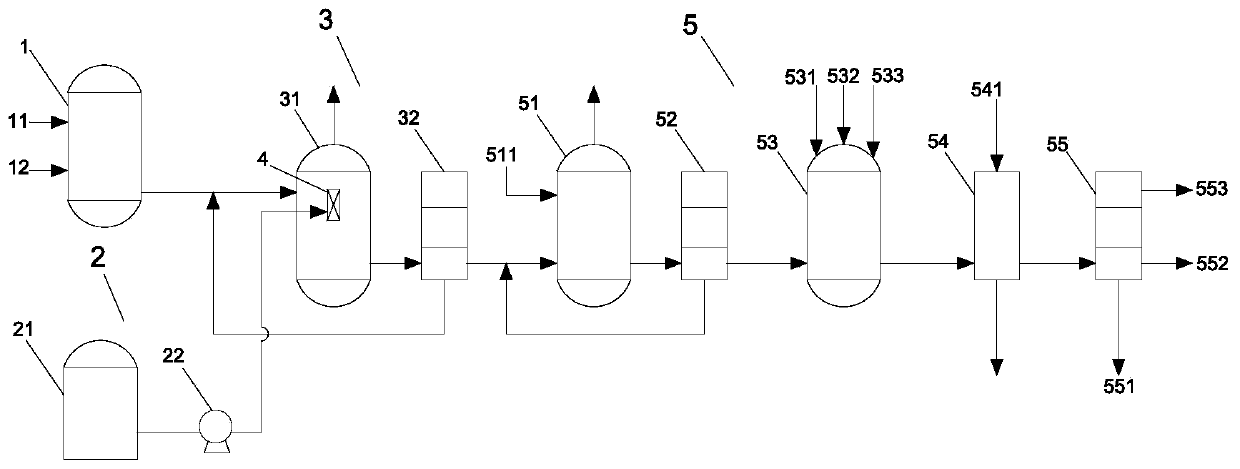
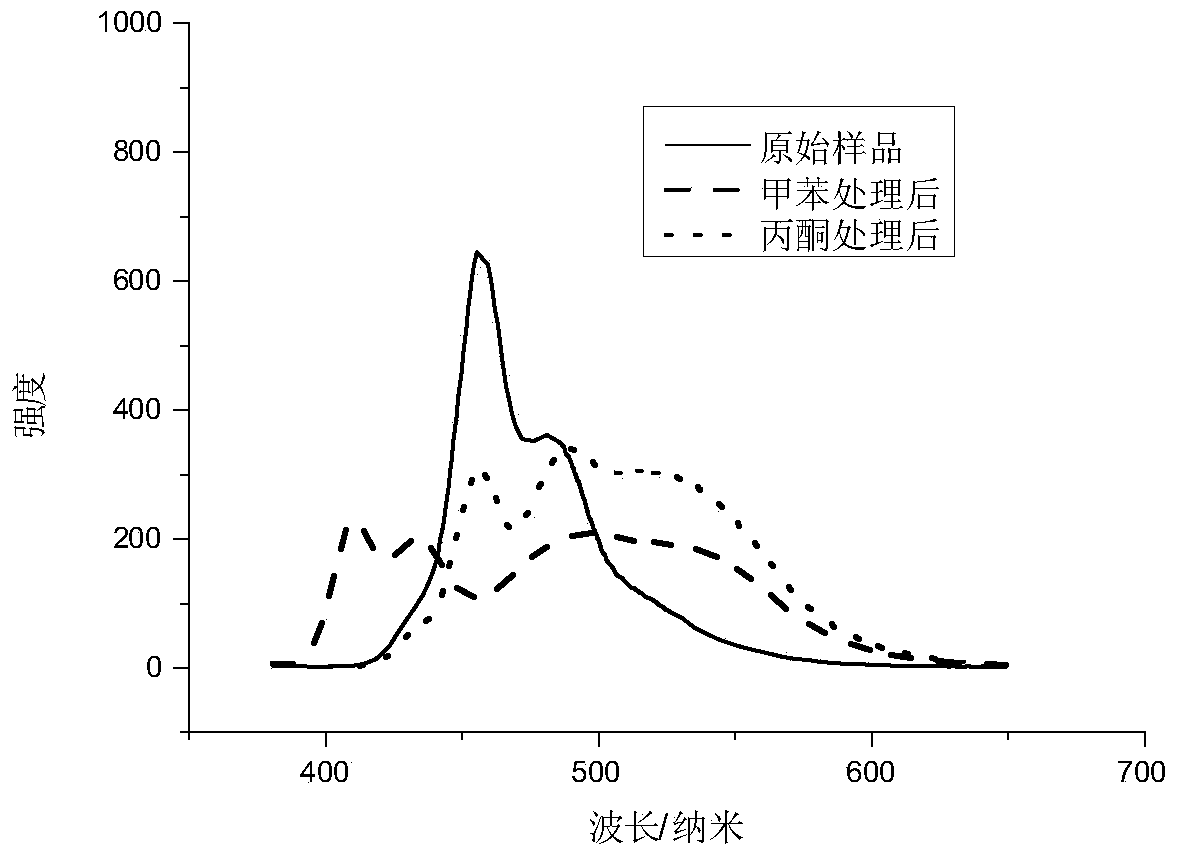
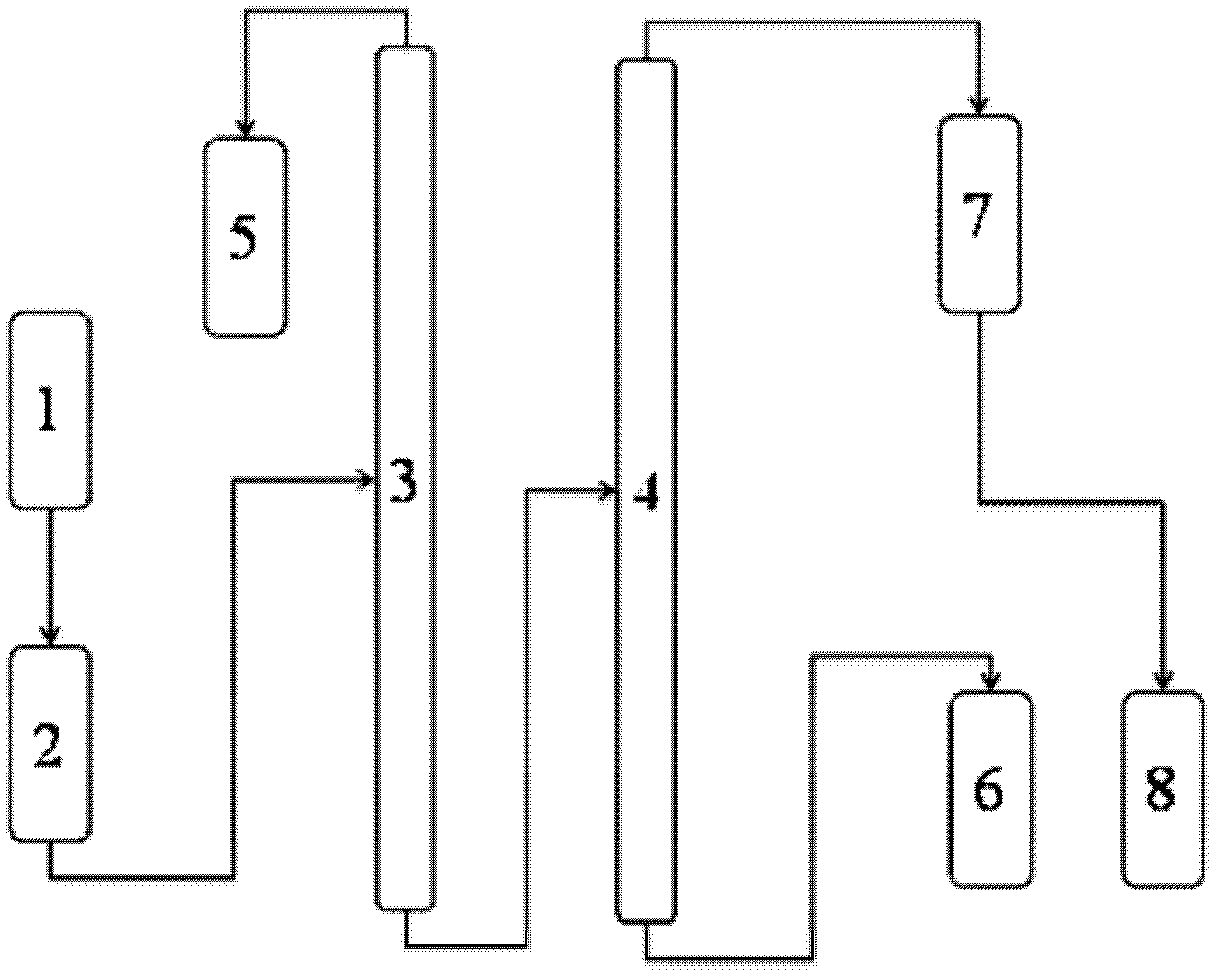
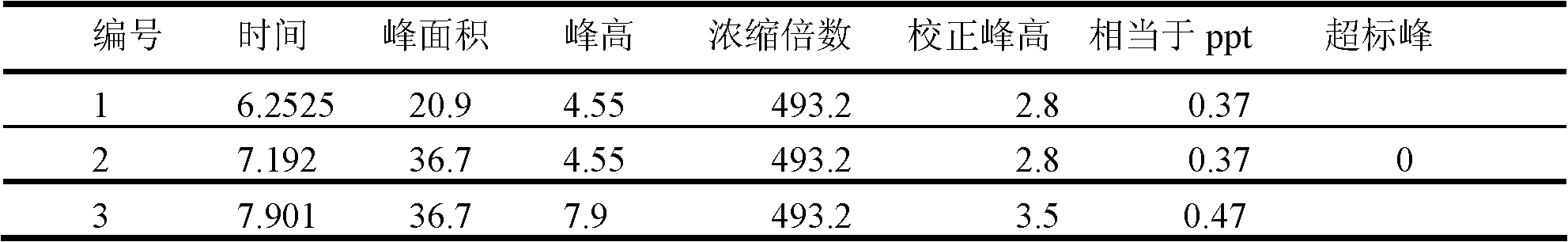
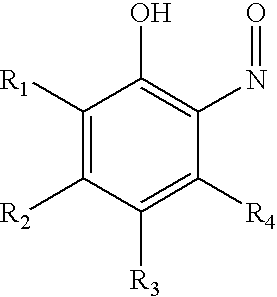
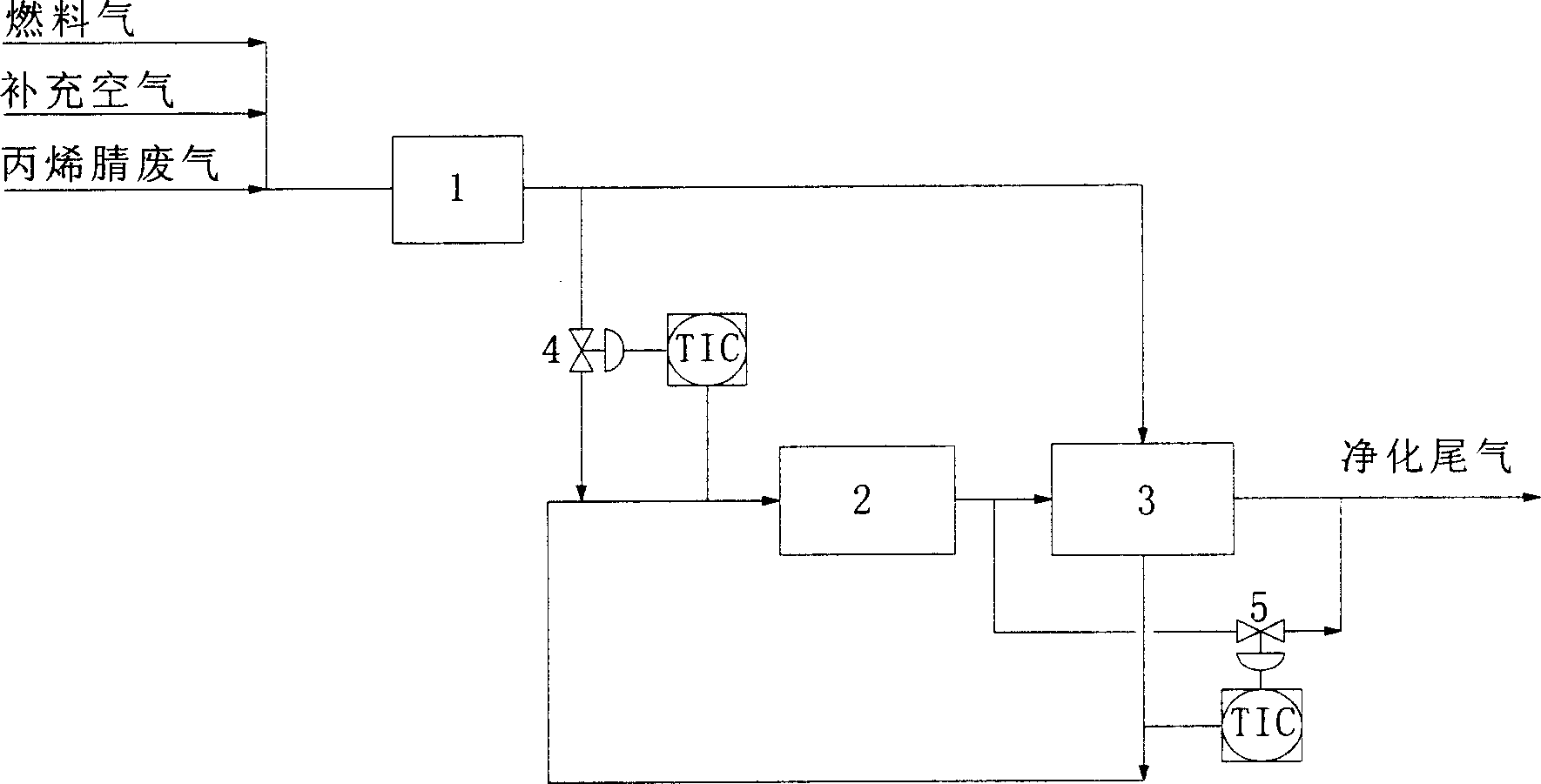Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1073results about "Carboxylic acid nitrile purification/separation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
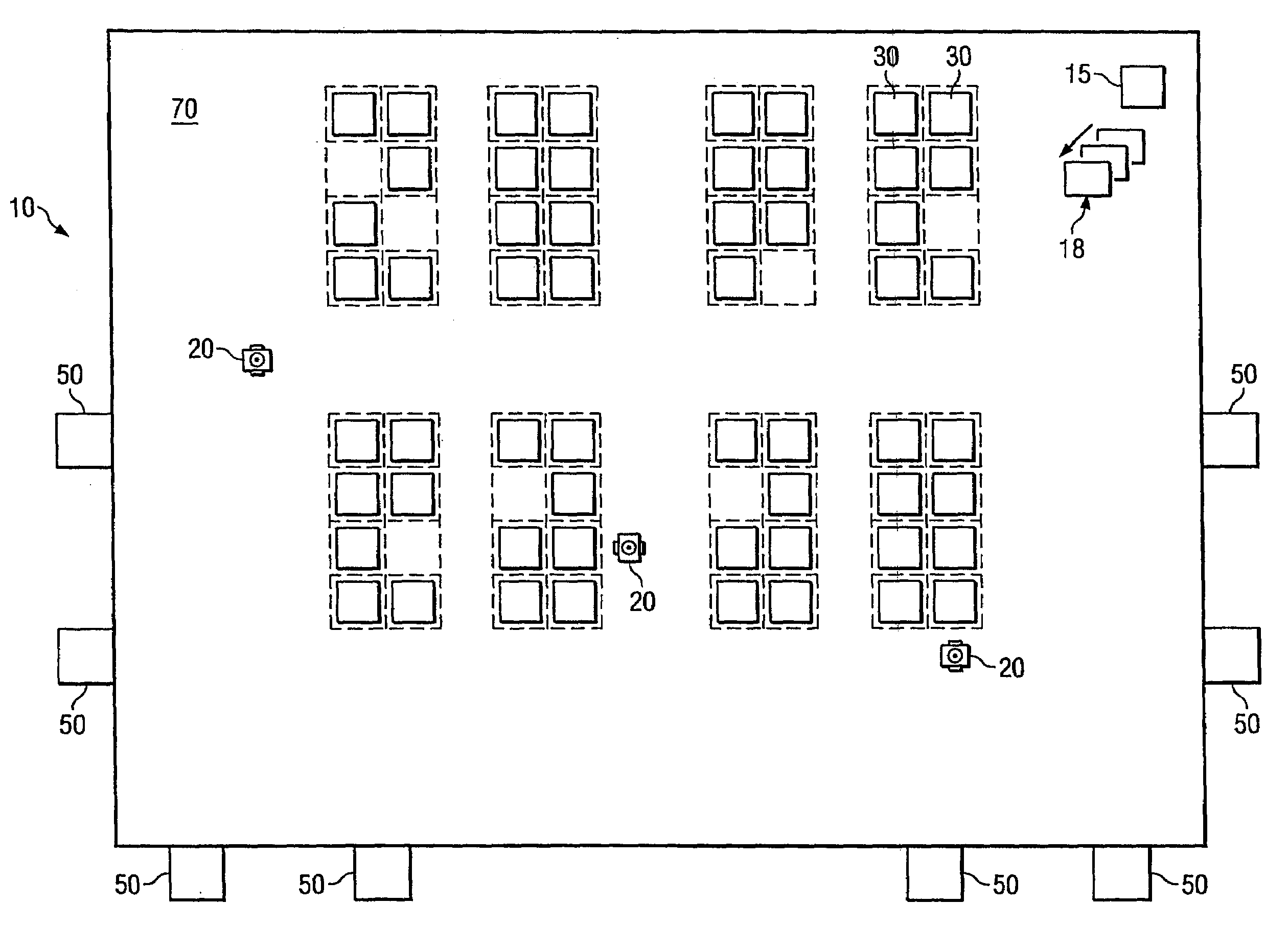
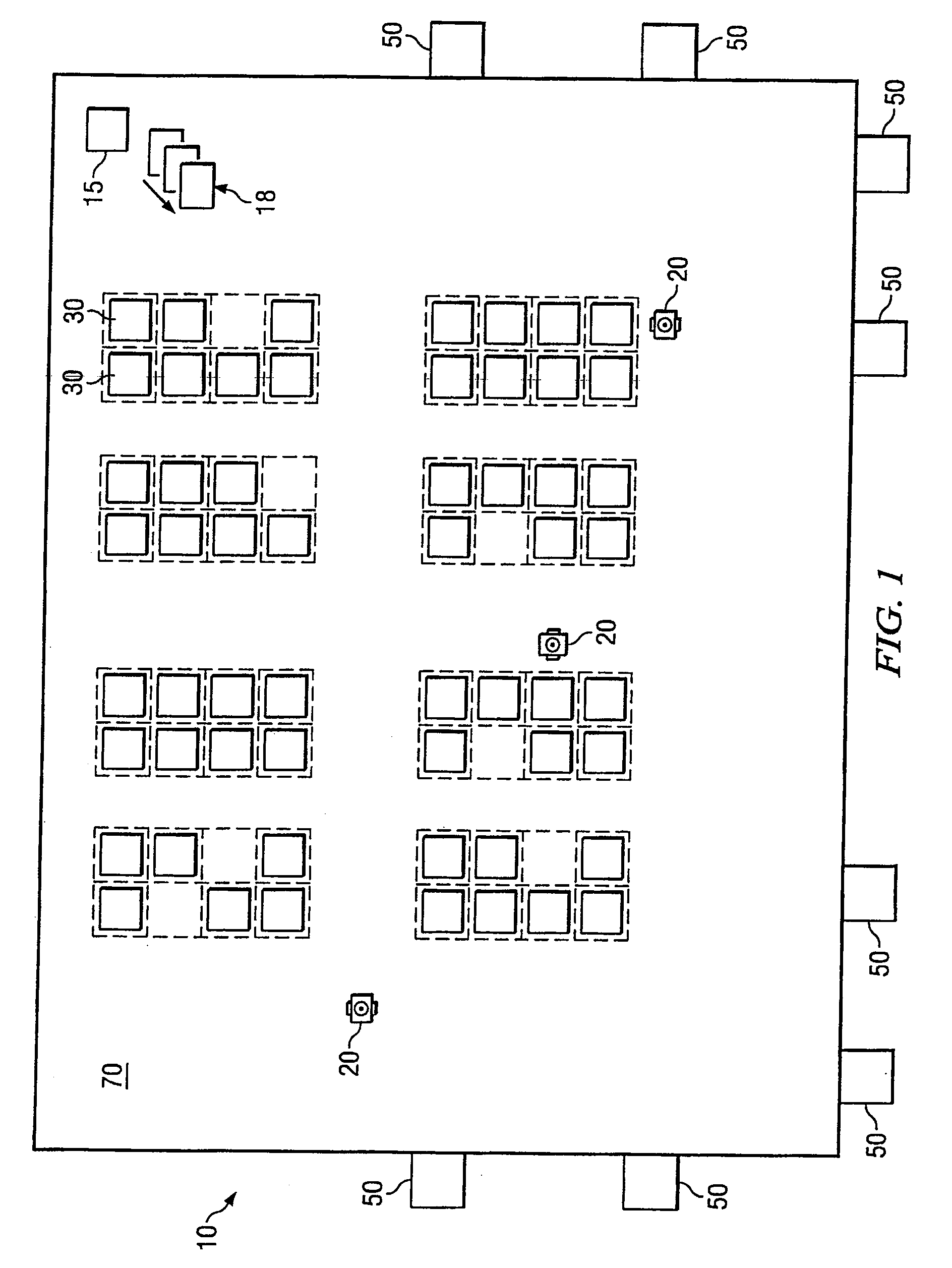
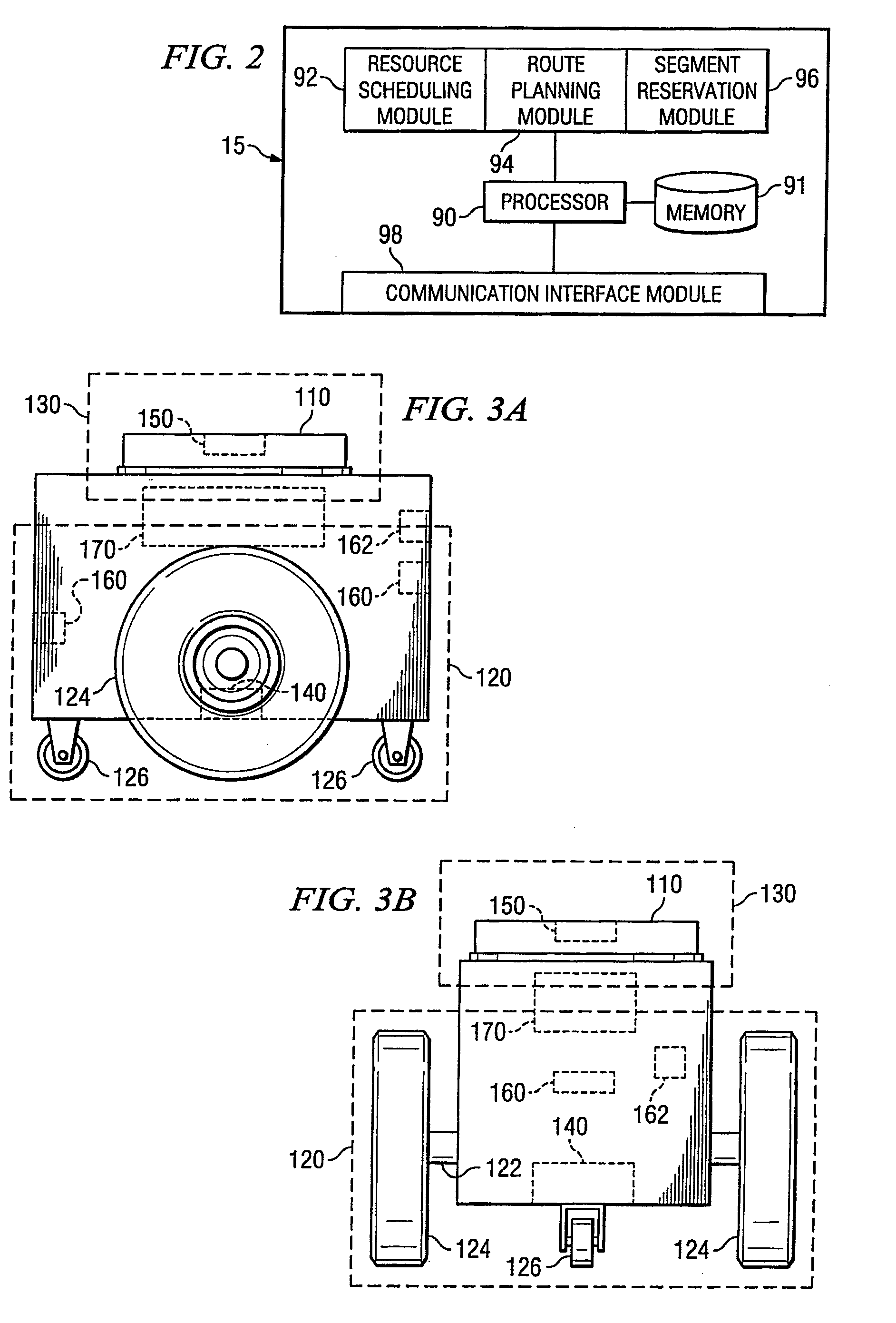
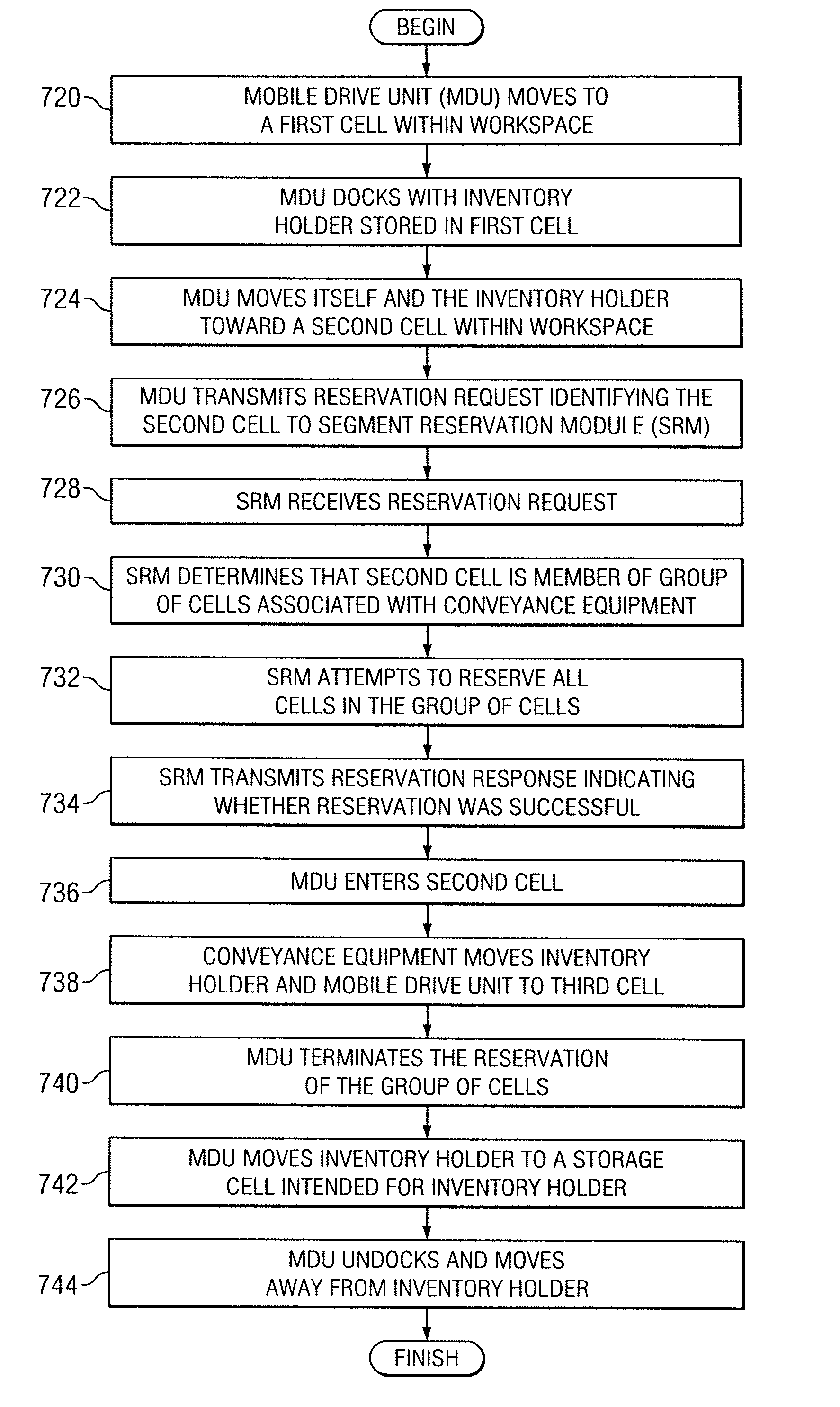
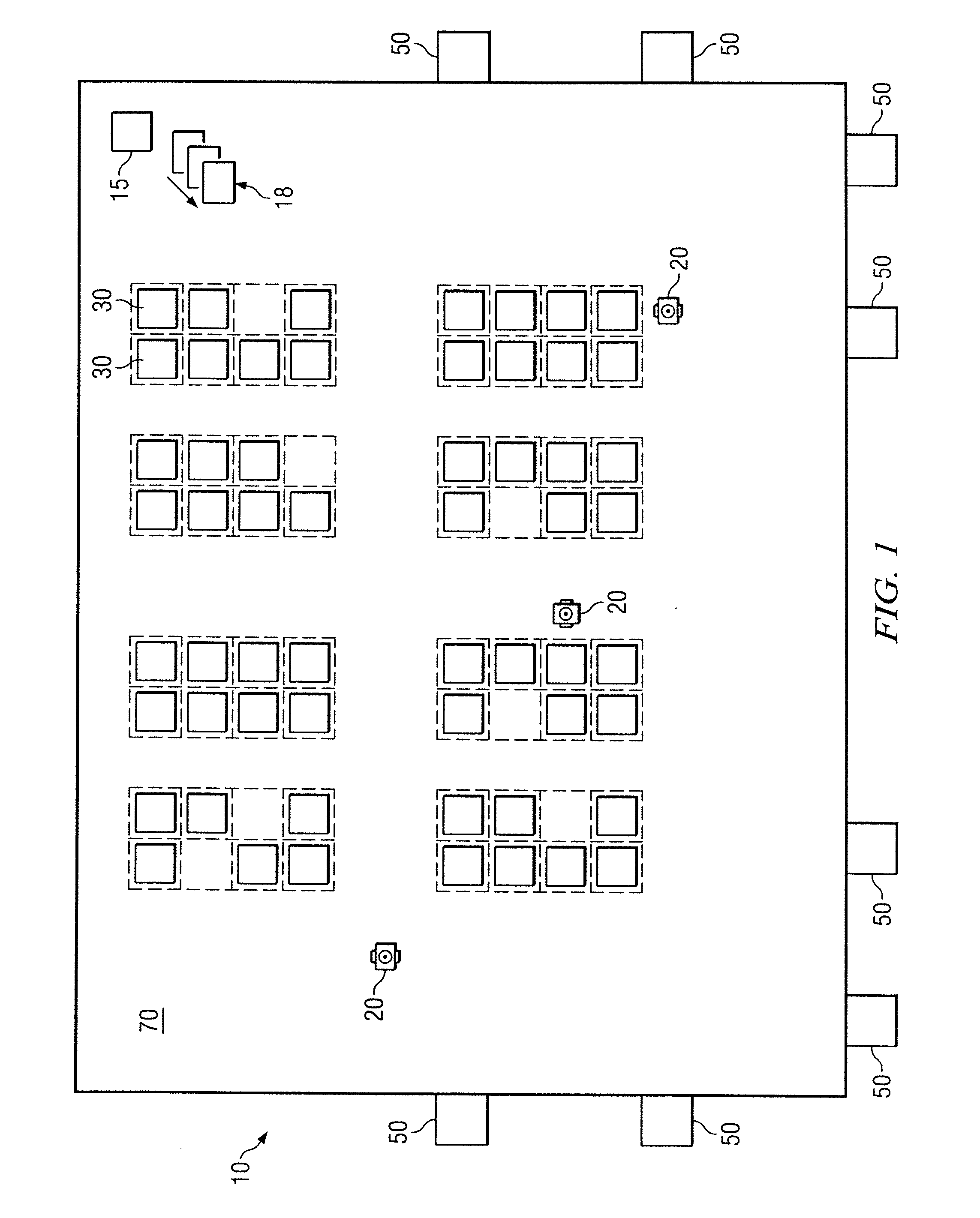
System and method for transporting inventory items
ActiveUS20070293978A1Disadvantages and reduced eliminatedInventory reduced eliminatedProgramme controlDigital data processing detailsWorkspaceTransport engineering
A method for transporting inventory items includes moving a mobile drive unit to a first point within a workspace. The first point is a location of an inventory holder. The method further includes docking the mobile drive unit with the inventory holder and moving the mobile drive unit and the inventory holder to a second point within the workspace. The second point is associated with conveyance equipment. The method further includes moving the inventory holder to a third point within the workspace using the conveyance equipment.
Owner:AMAZON TECH INC
System and Method for Transporting Inventory Items
ActiveUS20110060449A1Disadvantages and reduced eliminatedInventory reduced eliminatedProgramme controlDigital data processing detailsTransport engineering
A method for transporting inventory items includes moving a mobile drive unit to a first point within a workspace. The first point is a location of an inventory holder. The method further includes docking the mobile drive unit with the inventory holder and moving the mobile drive unit and the inventory holder to a second point within the workspace. The second point is associated with conveyance equipment. The method further includes moving the inventory holder to a third point within the workspace using the conveyance equipment.
Owner:AMAZON TECH INC
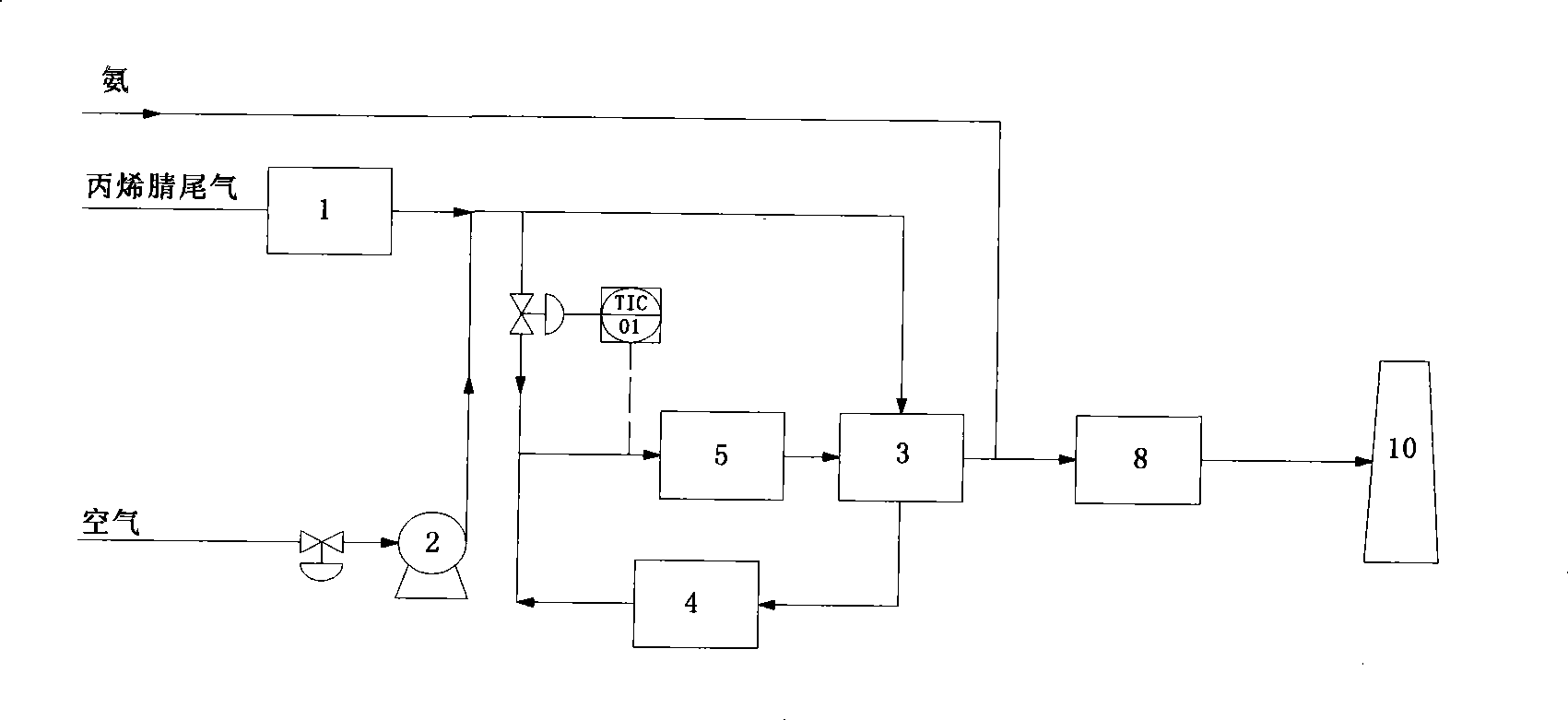
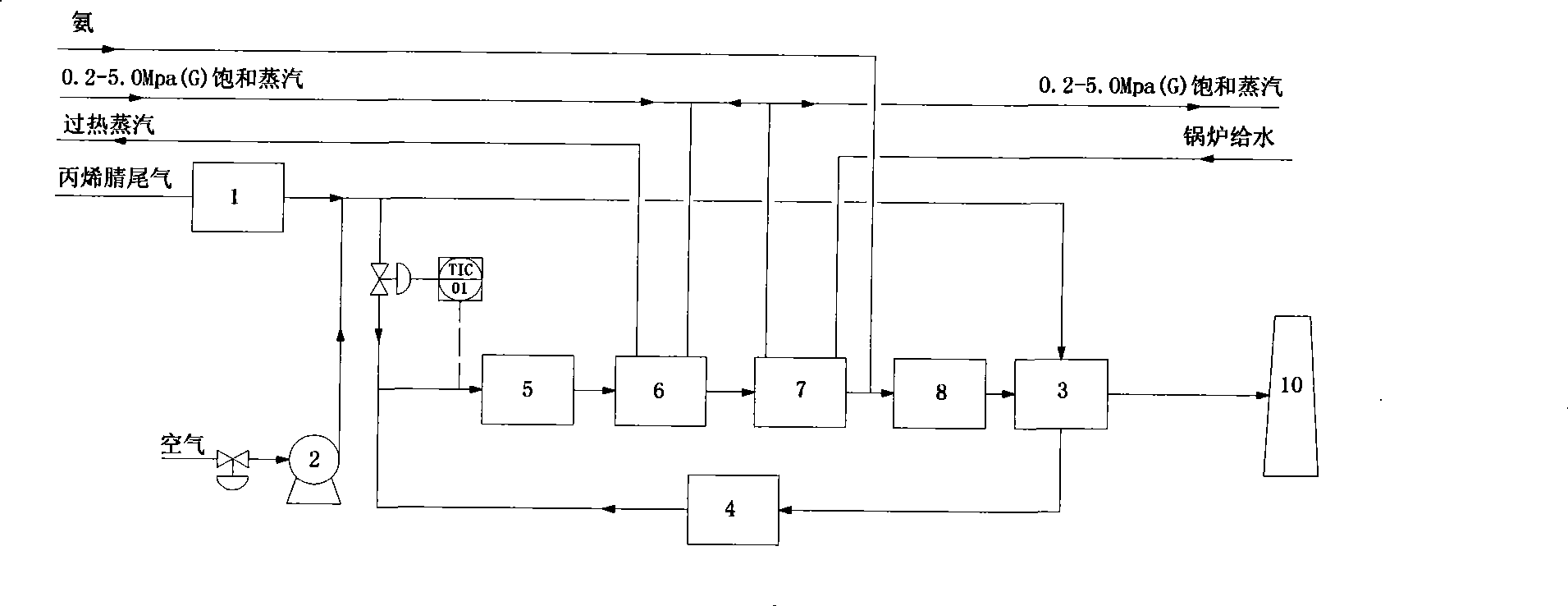
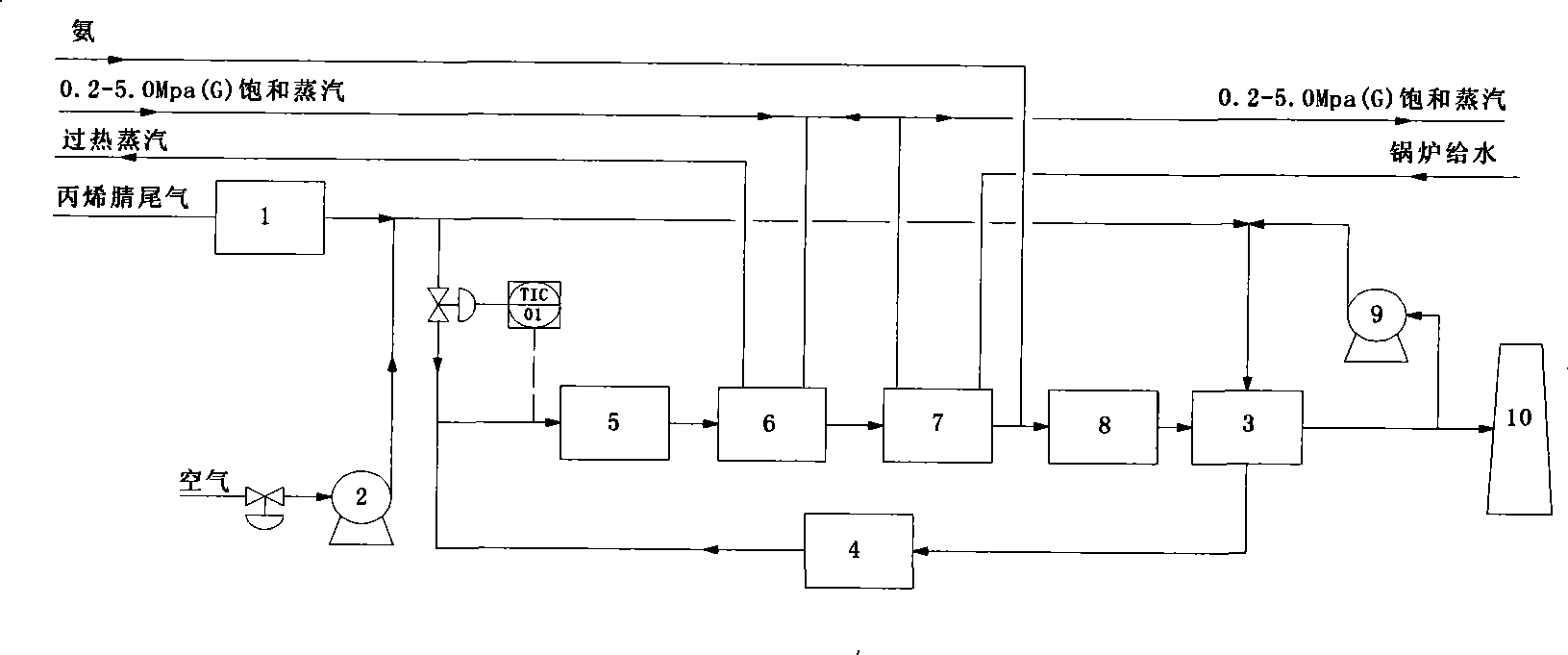
Acrylonitrile device tail-gas treatment technique
InactiveCN101362051AHigh mechanical strengthExtended service lifeChemical industryDispersed particle separationAcrylonitrileCatalytic oxidation
The invention discloses a technique for treating the tail gas of an acrylonitrile device, which is suitable for treating acrylonitrile tail gas discharged by the acrylonitrile device. The technique is characterized in that: after free water is separated out by a gas-liquid separator, the acrylonitrile tail gas is mixed with air, a noble metal monolithic catalyst is taken as a catalyst and catalytic oxidation reaction is carried out to turn harmful volatile organic compounds into carbon dioxide and water; then a selective reduction monolithic catalyst is taken as a catalyst and selective catalytic reduction reaction with added ammonia is carried out to reduce nitrogen oxide in the tail gas into nitrogen and water. The technique has the advantages of simple technique, turning the harmful volatile organic compounds and nitrogen oxide in the tail gas into carbon dioxide, nitrogen and water, without secondary pollution, the test results totally meeting the environmental protection control requirements of the State Standard of China, adopting a tail gas heat exchanger to recycle reaction heat to heat input tail gas, needing no additional fuel during the normal operation process, small system resistance and low operation cost.
Owner:SHANGHAI DONGHUA ENVIRONMENT ENG
Method for producing 3-pentenenitrile
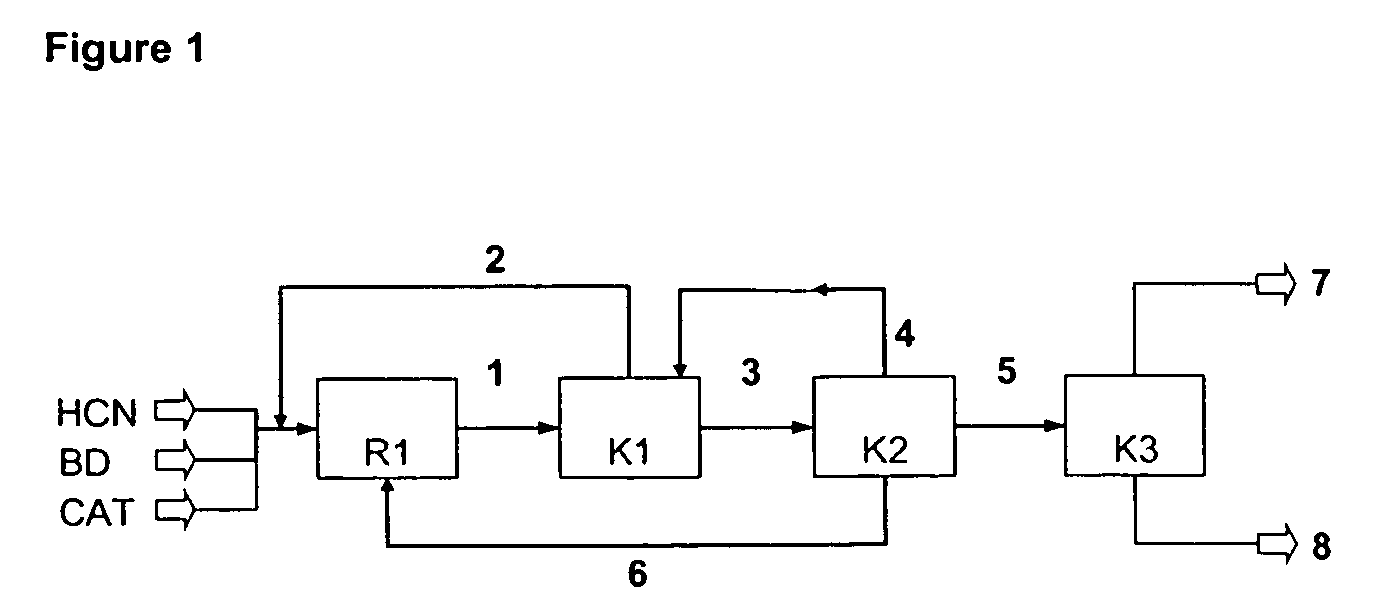
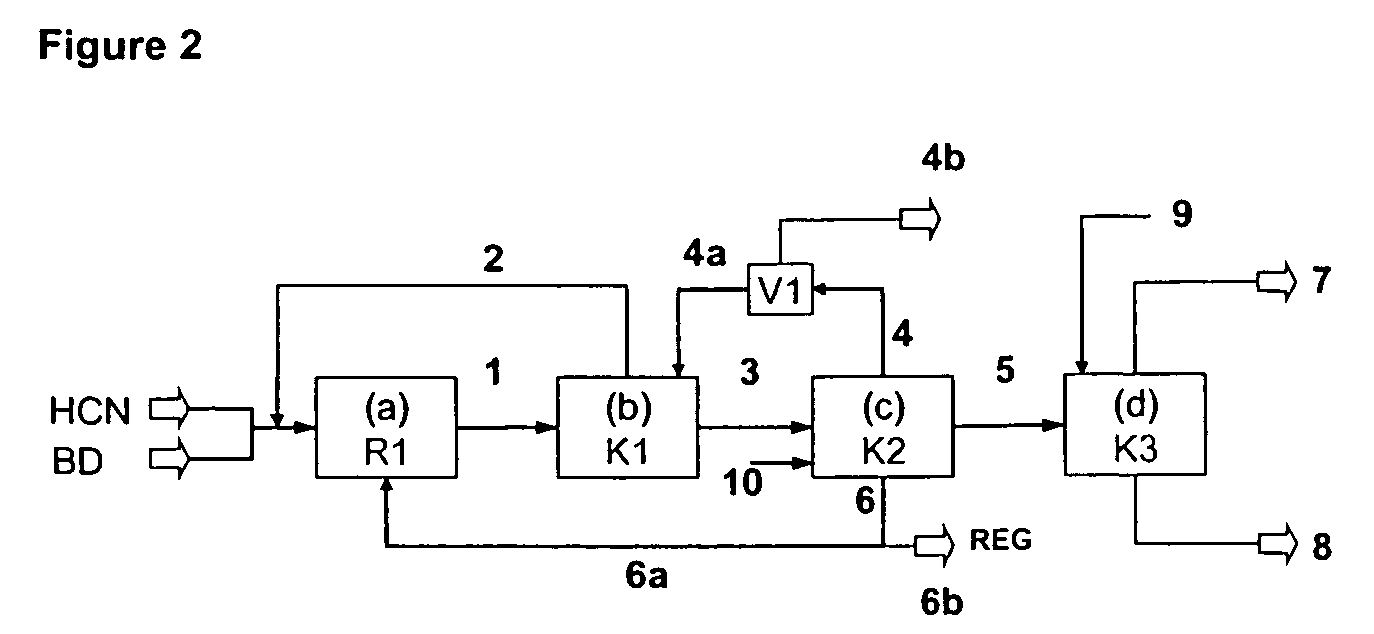
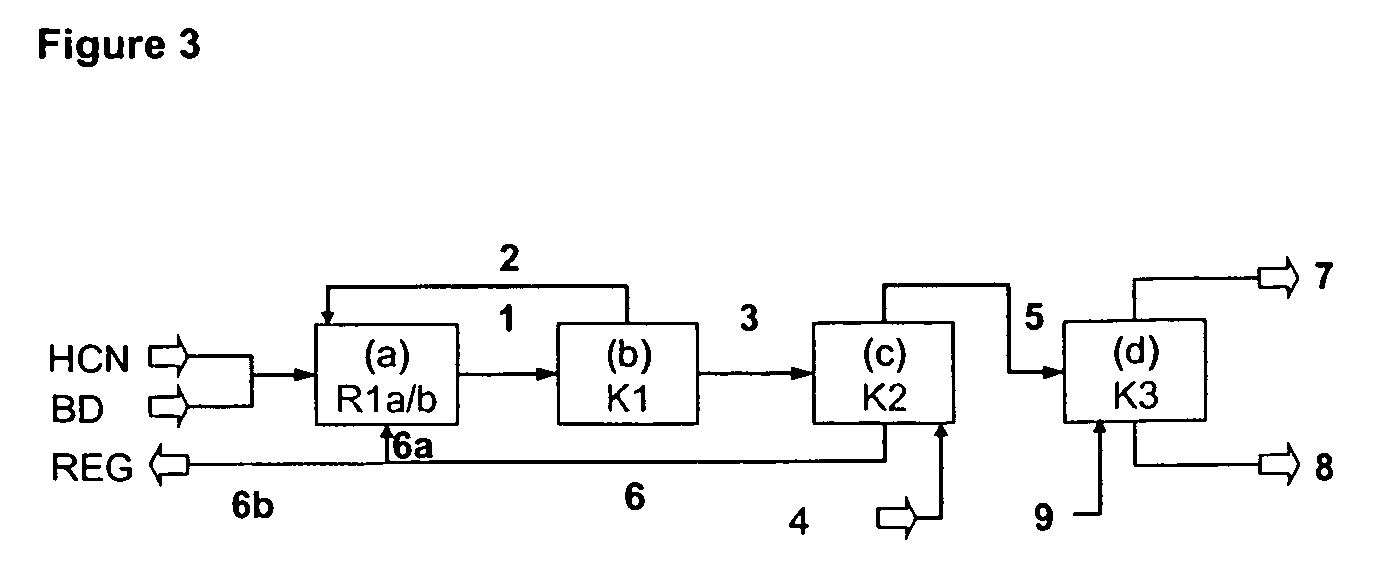
InactiveUS7541486B2Improve processing yieldReduce thermal stressOrganic compound preparationPreparation by hydrogen cyanide additionPtru catalystButadiene Dioxide
A process is described for preparing 3-pentenenitrile, characterized by the following process steps:(a) reacting 1,3-butadiene with hydrogen cyanide over at least one catalyst to obtain a stream 1 which comprises 3-pentenenitrile, 2-methyl-3-butenenitrile, the at least one catalyst and 1,3-butadiene,(b) distilling stream 1 in a column to obtain a high-1,3-butadiene stream 2 as the top product and a low-1,3-butadiene stream 3 as the bottom product which comprises 3-pentenenitrile, the at least one catalyst and 2-methyl-3-butenenitrile,(c) distilling stream 3 in a column to obtain a stream 4 as the top product which comprises 1,3-butadiene, a stream 5 which comprises 3-pentenenitrile and 2-methyl-3-butenenitrile at a side draw of the column, and a stream 6 as the bottom product which comprises the at least one catalyst,(d) distilling stream 5 to obtain a stream 7 as the top product which comprises 2-methyl-3-butenenitrile, and a stream 8 as the bottom product which comprises 3-pentenenitrile.
Owner:BASF AG
Dispersant antifoulant for acrylonitrile
A styrene sulfonate polymer which is highly effective at preventing fouling of equipment used in the manufacturing process of acrylonitrile. The styrene sulfonate polymer is particularly effective when introduced into the quench column, the recovery stage and the wastewater processing section of the acrylonitrile manufacturing process.
Owner:ECOLAB USA INC
Method for synthesizing acetonitrile through one-step ammoniating method with acetic acid
ActiveCN101830829AReduce typesReduce contentPreparation by ammonia-carboxylic acid reactionCarboxylic acid nitrile purification/separationChemical treatmentSynthesis methods
The invention discloses a method for synthesizing acetonitrile through a one-step ammoniating method with acetic acid. The method comprises the following steps of: (1) reacting acetic acid with liquid ammonia in a fixed-bed reactor provided with a catalyst of aluminum oxide to generate a mixed gas containing the acetonitrile; and (2) continuously refining the gas mixture to obtain an acetonitrileproduct. By adopting the method, the acetonitrile product is relatively pure, the purity can reach more than 99.9%, the separation process is relatively simple, the process is short, the energy consumption in the production process is low, the energy consumption of a unit product is about 40% of the energy consumption with a by-product method, and the cost is lower. The separation process adopts a physical separation method, and avoids the problem of generating a great deal of wastewater and waste gas in the chemical process in the by-product method. Meanwhile, because the synthesis method isadopted, the limitation that the yield is restricted by the main product can be thoroughly avoided.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY
Method for preparing high-purity acetonitrile from acetic acid and ammonia by two steps
ActiveCN101891648AReduce typesReduce contentPreparation by ammonia-carboxylic acid reactionCarboxylic acid nitrile purification/separationChemical treatmentSynthesis methods
The invention discloses a method for preparing high-purity acetonitrile from acetic acid and ammonia by two steps, which comprises the following steps of: (1) neutralizing acetic acid and ammonia to generate ammonium acetate; (2) mixing aqueous solution of ammonium acetate and gaseous ammonia, preheating, and making the mixture enter a fixed bed reactor filled with a catalyst aluminium oxide for reaction to generate acetonitrile-containing mixed gas; and (3) continuously refining the mixed gas to obtain the acetonitrile. The 70 to 80 weight percent acetic acid is taken as a raw material, and the produced acetonitrile product has the advantages of less varieties and low content of impurities, high purity of over 99.9 percent, simple separation process, shorter flow, low energy consumption only about 40 percent of that of a byproduct method in unit product, and lower cost. The separation process is a physical separation method, and avoids generating a great amount of wastewater and waste gas in the chemical treatment process of the byproduct method. Meanwhile, a synthesis method is adopted, so the limitation that the yield is limited by a main product can be radically eliminated.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY
Isophorone fluorescence probe, and preparation method and application thereof
ActiveCN105524612AGood practical valueAvoid interferenceOrganic compound preparationFluorescence/phosphorescenceIsophoroneCytotoxicity
The invention discloses an isophorone fluorescence probe, and a preparation method and an application thereof. The structure of the fluorescence probe is represented by formula I. The preparation method comprises the following steps: adding isophorone, malononitrile and pyridine to a reaction bottle, carrying out heating refluxing under the protection of an inert gas for 18-24h, purifying, adding the above obtained pure product, p-hydroxybenzaldehyde and pyridine to the reaction bottle, carrying out heating refluxing under the protection of the inert gas for 4-18h, purifying, adding the obtained pure product, 2,4-dinitrofluorobenzene and triethylamine to the reaction bottle, stirring above materials for 8-36h, and purifying the obtained mixture to obtain the fluorescence probe. The fluorescence probe has large Stokes shift (182nm), long fluorescence emission wavelength (592nm), high sensitivity and good selectivity, can specifically detect hydrogen sulfide in organisms, environment and foods, has good biomembrane permeability and low cytotoxicity, and also has the advantages of simple synthesis route, high yield and very large practical values.
Owner:XUZHOU MEDICAL COLLEGE
Synthetic system of 3-cyanomethylbenzoic acid methyl ester and method thereof
InactiveCN110002993AIncrease the mass transfer area of the phase boundaryImprove mass transfer efficiencyOrganic compound preparationPreparation by cyanide reactionChlorideMethyl benzoate
The invention provides a synthetic system of 3-cyanomethylbenzoic acid methyl ester and a method thereof. The system comprises an acylation reaction vessel used for preparation of m-Toluoyl chloride;a liquid chlorine feeding unit used for storing and conveying liquid chlorine; a chlorination reaction unit which is connected with the acylation reaction vessel and used as a chlorination reaction chamber of m-Toluoyl chloride; a micro-interface generator which is respectively connected with the liquid chlorine feeding unit and the chlorination reaction unit and used for receiving liquid chlorineconveyed by the liquid chlorine feeding unit, crushing the liquid chlorine into micro-droplets with the diameter being micron level and conveying the micro-droplets to the chlorination reaction unitafter the crushing; and an esterification and cyanation reaction unit which is connected with the chlorination reaction unit. In the prior art, m-Toluoyl chloride and liquid chlorine cannot be fully mixed when m-Toluoyl chloride and liquid chlorine react such that yield of 3-cyanomethylbenzoic acid methyl ester is reduced. The above problem is solved by the synthetic system and the method of the invention.
Owner:NANJING UNIV
Selective Manufacture of N,N'-BIS(Cyanoethyl)-1,2-Ethylenediamine and N, N'-BIS(3-aminopropyl)-1,2-Ethylenediamine
InactiveUS20080194857A1High selectivityOrganic compound preparationAmino compound preparationEthylenediamineAcrylonitrile
A method for making N,N′-bis(3-aminopropyl)-1,2-ethylenediamine which comprises reacting acrylonitrile and ethylenediamine in about a 2:1 molar ratio to make a N,N′-bis(cyanoethyl)-1,2-ethylenediamine reaction product and hydrogenating the reaction product, the improvement for improving the selectivity of the reactions to N,N′-bis(2-cyanoethyl)-ethylenediamine and to N,N′-bis(3-aminopropyl)-1,2-ethylenediamine which comprises reacting acrylonitrile and ethylenediamine in the presence of 2-30 wt % water, based on total reactants.
Owner:AIR PROD & CHEM INC
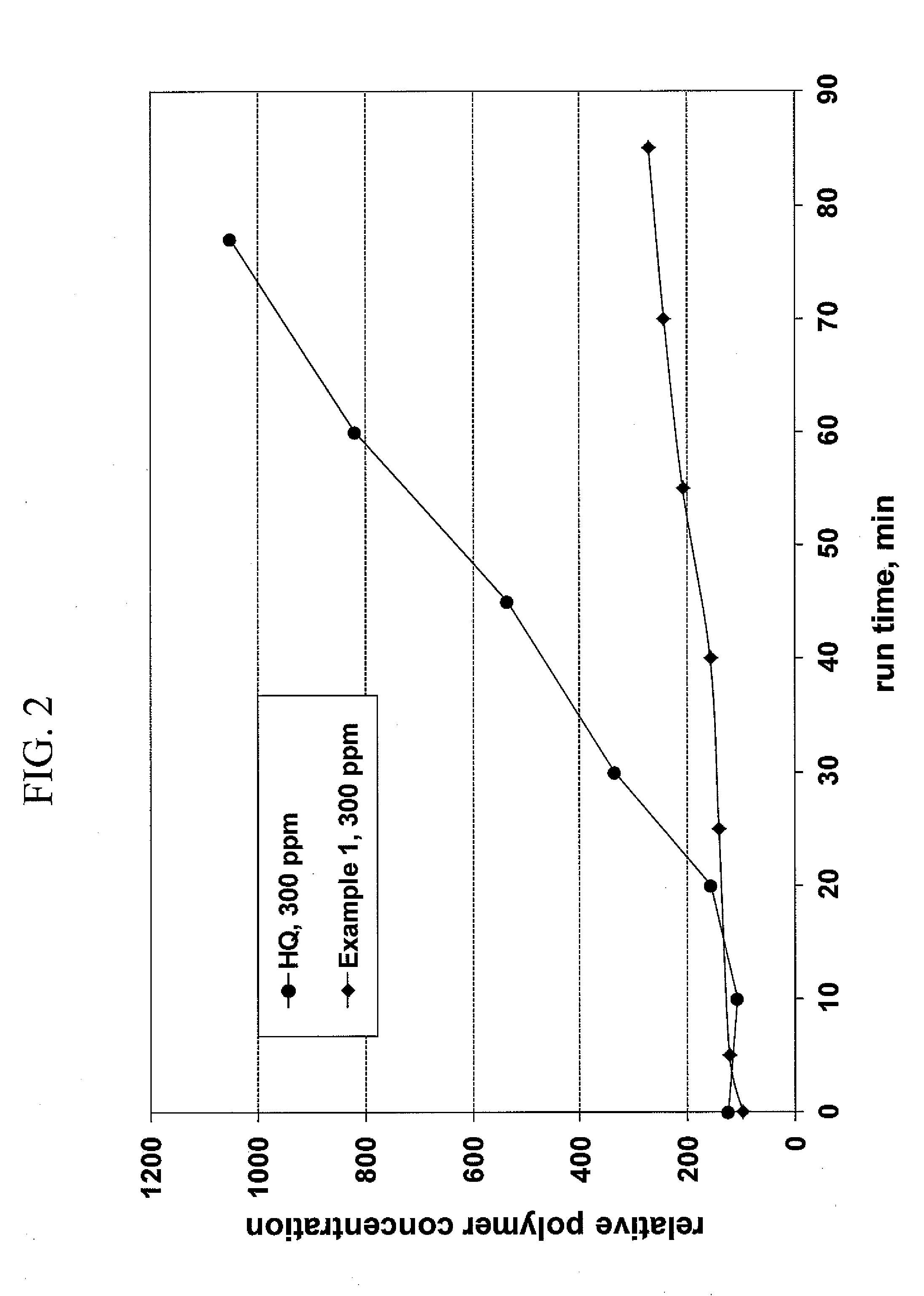
Inhibition of polymerisation
InactiveUS20100168434A1Inhibition of polymerizationGood miscibilityOrganic compound preparationOther chemical processesUnsaturated monomerChemistry
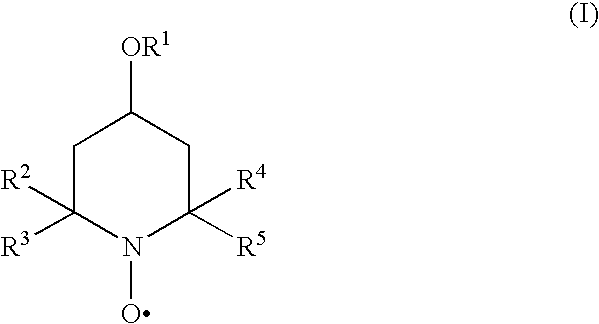
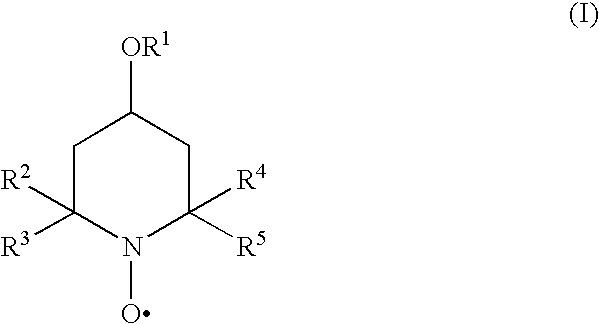
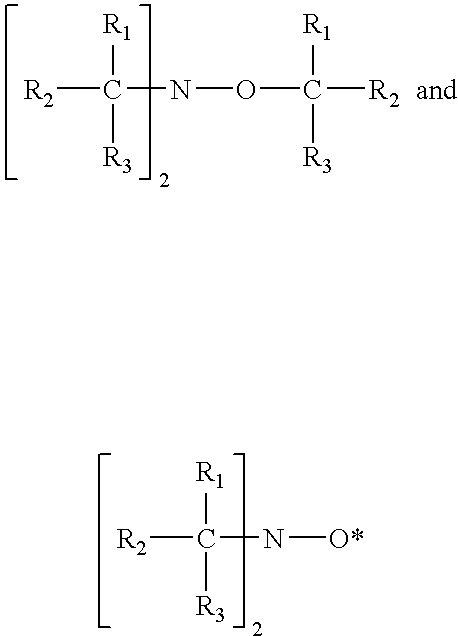
The present invention provides methods and compositions for inhibiting polymerisation of ethylenically unsaturated monomers, which involve the use of nitroxide compound of formula (I):whereinR1 is C4-20 hydrocarbyl; andR2, R3, R4 and R5 are independently each C1-6 alkyl.
Owner:NUFARM LTD
Preparation method for liquid chromatography mass spectrometry (LC-MS)-grade high-purity acetonitrile reagents
InactiveCN102746187AHigh recovery rateSimple and fast operationCarboxylic acid nitrile purification/separationGas chromatography–mass spectrometrySorbent
The invention relates to a preparation method for liquid chromatography mass spectrometry (LC-MS)-grade high-purity acetonitrile reagents. The method includes steps: subjecting raw acetonitrile to adsorption by the aid of adsorbents such as activated carbon and activated alumina, oxidizing for purification, rectifying, dehydrating and drying by the aid of a molecular sieve, filtering by the aid of a microfiltration membrane, filling nitrogen for packaging, and obtaining the LC-MS-grade high-purity acetonitrile reagents. By the method, the recovery rate is more than 98%, the yield is more than 95%, the purity reaches to more than 99.99%, application requirements of clients on scientific researches and subject studies of the LC-MS-grade high-purity acetonitrile reagents can be met, domestic vacancies of production of the LC-MS-grade high-purity acetonitrile reagents can be filled, dependency on foreign reagents is lowered, high-quality reagents are provided for growing wide application of LC-MS quantitative analysis in high-tech fields such as biomedical research and development, clinic, food safety detection, pesticide residue analysis, veterinary drug residues, legal examiners, criminal investigations, doping detection, drug testing, agriculture and environment protection.
Owner:天津康科德医药化工有限公司
Catalytic oxidation process for treating tail gas from absorption tower of acrylonitrile installation
A process for treating the acrylonitrile contained waste gas exhausted by the acrylonitrile apparatus includes such steps as mixing said waste gas with air, heating, catalytic oxidizing reaction to decompose the harmful volatile organic substance to CO2 and H2O, recovering heat and exhausting.
Owner:SHANGHAI DONGHUA ENVIRONMENT ENG
Method for purifying high-purity organic solvent acetonitrile for research
ActiveCN101570497ATo achieve the purpose of purificationHigh purityCarboxylic acid nitrile purification/separationFiltrationPesticide residue
The invention relates to a method for purifying high-purity organic solvent acetonitrile for research, including the steps as below: acetonitrile which is used as a raw material reacts with solid alkali at low temperature so as to eliminate unsaturated nitrile; impurities absorbed by high waveband ultraviolet are removed by the adsorption of zeolite and polymeric adsorbent; and a 3A molecular sieve is used for drying and dehydration, and the operations of rectification, filtration and encapsulation are carried out to obtain the product of chromatographic grade, pesticide residue grade and gradient elution grade chromatographic acetonitrile. By the method, the product of chromatographic pure acetonitrile with purity of more than 99.9 percent can be obtained, and can meet the requirement of the pesticide residue grade and gradient elution grade chromatographic acetonitrile, thereby meeting the demand of customers on application of high efficiency liquid chromatography (HPLC) in pesticide residues, filling in the gap of domestic preparation of pesticide residue grade and gradient elution grade chromatographic reagents, reducing the dependency on abroad reagents, and providing the qualified reagents for the inspection of the pesticide residues and the separation and the nature determination of complex medicines.
Owner:天津市康科德科技有限公司
Technological method for separating methanol, acetonitrile and benzene tertiary mixture
InactiveCN103214345AAchieve separationOrganic compound preparationDistillation purification/separationBenzeneDistillation
The invention relates to a technological method for separating a methanol, acetonitrile and benzene tertiary mixture. According to the method, a preliminary separating column is arranged, and methanol is introduced to change a distillation area of the mixture so that the distillation end point develops towards a methanol-benzene binary mixture, wherein the acetonitrile content of the mixture is nearly 0 when the mixture enters a difficult separating area, and thus methanol-benzene is extracted from a column top and methano-acetonitrile is extracted from a column bottom. By utilizing the method, a tertiary material system is decomposed into two binary material systems under the condition of not introducing a fourth material; the methanol-benzene mixture which is extracted from the column top can be decomposed so as to respectively obtain over 99.0% of the methanol and over 99.0% of benzene through pressure-swing distillation; and a methano-acetonitrile mixture which is extracted from the column bottom can be decomposed so as to respectively obtain over 99.0% of the methanol and over 99.0% of acetonitrile through pressure-swing distillation.
Owner:HEBEI UNIV OF TECH
High-purity acetonitrile and process for producing the same
ActiveCN101171233AReduce contentLow absorbanceCarboxylic acid nitrile purification/separationDistillationPropionitrile
The present invention relates to high-purity acetonitrile and its production method. The object of the present invention is to provide a kind of method of manufacturing high-purity acetonitrile, wherein, the absorbance of high-purity acetonitrile under the wavelength 200nm is low, and this method is that the used energy of purification is few and the technique of purification operation is simple, obviously reduces the content in acetonitrile of propionitrile. The invention relates to a method for producing high-purity acetonitrile, which is characterized in that the crude acetonitrile with water is mixed with an alkali, separated into an acetonitrile phase and a water phase, and then the water phase is removed, and the obtained acetonitrile phase is subjected to a distillation process to obtain purified acetonitrile, The resulting purified acetonitrile is passed through a cation exchange resin to obtain high-purity acetonitrile.
Owner:ASAHI KASEI KK
Multi-component polymerization inhibitors for ethylenically unsaturated monomers
ActiveUS20120203020A1Inhibition of polymerizationOrganic compounds purification/separation/stabilisationOrganic compound preparationArylHydroxylamine
The invention provides a composition and a method of using the composition for inhibiting the unwanted polymerization of ethylenically unsaturated monomers. The composition is prepared by reducing a nitroxide stable free radical to its corresponding hydroxylamine through reaction with a dialkyl / aryl hydroxylamine and subsequent addition of a polymerization prevention component selected from phenolic antioxidants, phenylenediamine and phenylenediamine derivatives, or phenothiazine and phenothiazine derivatives targeted towards ethylenically unsaturated monomers. The conversion of the nitroxide by the dialkyl / aryl hydroxylamine is to prevent it or the polymerization prevention component from being “spent” by reaction with each other, impurities or any other incompatible components. This allows previously incompatible combinations to now work effectively. In fact, the combination is more efficacious as the combination exerts a synergy due to the presence of various polymerization prevention reagents. The composition is a synergistic combination of various polymerization inhibition compounds for the inhibition of the unwanted polymerization of ethylenically unsaturated monomers.
Owner:ECOLAB USA INC
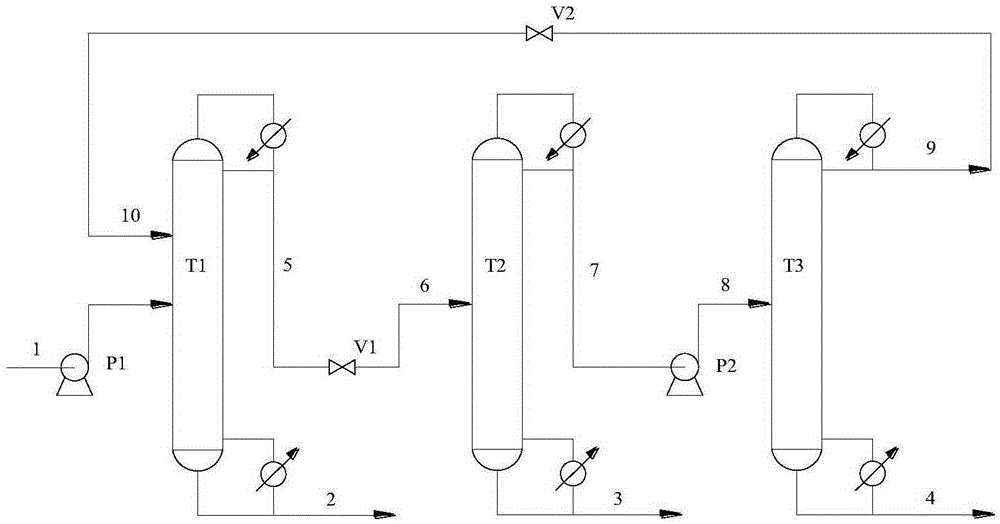
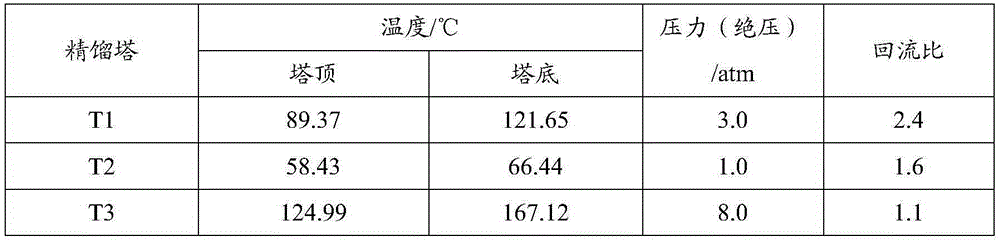
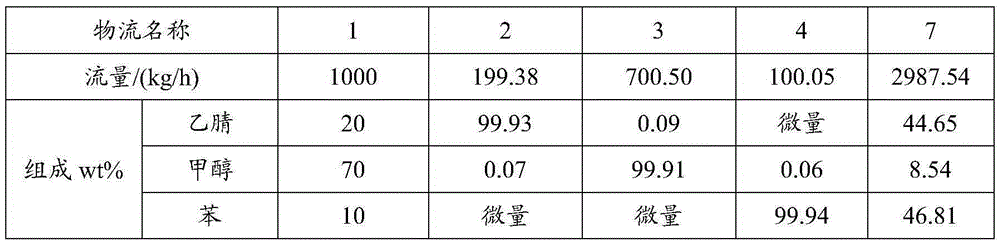
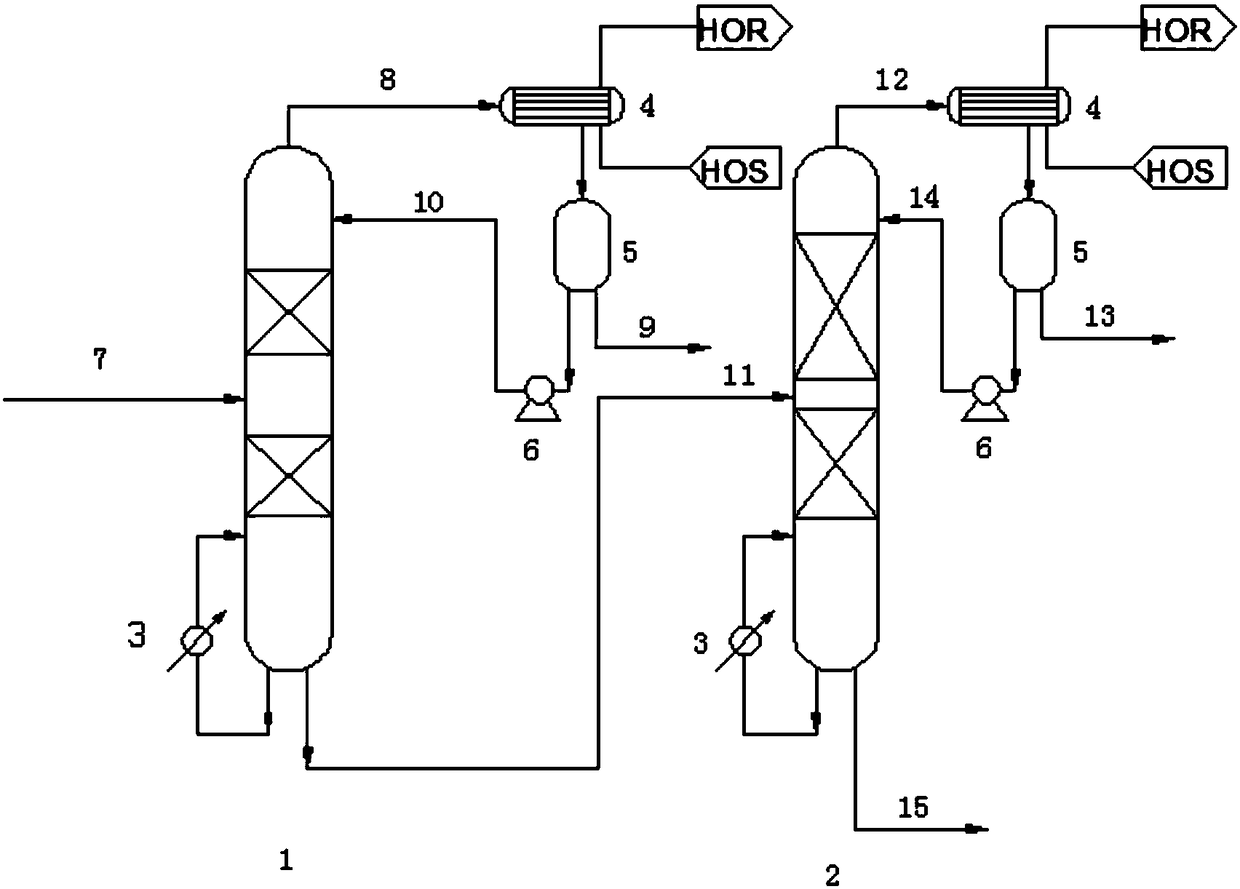
Method for separating acetonitrile-methanol-benzene ternary azeotrope through three-tower pressure-swing distillation
ActiveCN105254532ALow investment costReduce performanceOrganic compound preparationDistillation purification/separationBenzeneDistillation
The invention relates to a method for separating an acetonitrile-methanol-benzene ternary azeotropic system through three-tower pressure-swing distillation. An acetonitrile-methanol-benzene raw material mixed liquor 1 is delivered to an acetonitrile tower T1 via a pressure pump P1 so as to obtain an acetonitrile product 2 at the bottom of the tower, steam at the top of the tower is condensed by a condenser, a part of the steam flows back to the acetonitrile tower T1, and a part of a material flow 5 is conveyed a methanol tower T2 after pressure reduction by a pressure-reducing valve V1; a methanol product 3 is obtained at the bottom of the methanol tower T2, steam at the top of the tower T2 is condensed by a condenser, a part of the steam flows back to the methanol tower T2, and a part of a material flow 7 is conveyed to a benzene tower T3 after pressurization by a pump P2; a benzene product 4 is obtained at the bottom of the benzene tower T3, steam at the top of the tower T3 is condensed by a condenser, a part of the steam flows back to the benzene tower T3, and a part of material flow 9 cyclically returns to the acetonitrile tower T1 via a pressure-reducing valve 2; and the purities and the recovery rates of the acetonitrile product, the methanol product and the benzene product are all greater than 99.90 wt% and 99.50 wt%, respectively. The method provided by the invention can substantially reduce energy consumption in separation process, improves product purity and yield, is simple and uses reasonable apparatuses.
Owner:QINGDAO UNIV OF SCI & TECH
Separation method of ammoniation dehydration product of caprolactam and synthesis method of hexamethylenediamine
ActiveCN111574400ARealize separation and purificationThe separation method is simpleOrganic compound preparationCarboxylic acid amides preparationHexamethylenediamineProcess engineering
The invention provides a separation method of an ammoniation dehydration product of caprolactam and a synthesis method of hexamethylenediamine. The separation method comprises the following steps: S1,carrying out ammoniation and dehydration reaction on caprolactam and ammonia gas to obtain a product system; S2, carrying out primary condensation treatment on the product system to obtain a caprolactam-containing fraction and a primary gas-phase component; and S3, carrying out secondary condensation treatment on the primary gas-phase component to obtain a 6-aminocapronitrile fraction and a secondary gas-phase component, wherein the temperature of the secondary condensation treatment is lower than that of the primary condensation treatment. According to the separation method, the product system obtained through the reaction is directly subjected to condensation treatment, secondary heating is not needed, continuous operation of the reaction and product separation can be achieved, waste heat can be fully utilized, the separation efficiency is high, the production intensity is high, the separation energy consumption is low, and therefore the whole process is more energy-saving.
Owner:JIANGSU YANGNONG CHEM GROUP +2
Novel energy-saving three-tower continuous extractive distillation technology and extractive distillation system thereof
ActiveCN104230657AIncrease production capacitySimple processOrganic compound preparationChemical industryExtractive distillationReactive distillation
The invention relates to a novel energy-saving three-tower continuous extractive distillation technology and an extractive distillation system thereof. The novel energy-saving three-tower continuous extractive distillation technology comprises the steps as follows: a preheated azeotrope system enters a tower body, a preheated extracting agent enters an extractive distillation tower body, raw materials enter the tower body from the tower bottom, infinite reflux operation is performed, a high-purity and light-component product is produced from the tower top, a tower bottom produced liquid enters an extracting agent recovery tower from a sixth tower plate of the extracting agent recovery tower, infinite reflux operation is performed, a high-purity extracting agent is produced from the tower bottom and mixed with a fresh extracting agent and enters the extractive distillation tower, a tower top produced liquid enters an azeotropic distillation tower, infinite reflux operation is performed for one hour, a heavy-component product is produced from the tower bottom, the tower top produced liquid circularly returns to an azeotrope feeding pipe of the extractive distillation tower, is mixed with the raw materials and enters the extractive distillation tower, and production is performed continuously. The novel energy-saving three-tower continuous extractive distillation technology and the extractive distillation system thereof have the advantages of simple technological process, easiness in operation, little investment, high efficiency, short construction period, consumption reduction, remarkable energy saving and obvious increase of azeotrope light-component productivity.
Owner:SHANDONG ZHONGSHAN PHOTOELECTRIC MATERIAL CO LTD
Activated carbon immobilized ionic liquid catalyst and application thereof
InactiveCN104549495AEasy to separateCatalytic activity did not decreaseOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingActivated carbonAfter treatment
The invention discloses an activated carbon immobilized ionic liquid catalyst and application thereof. The activated carbon immobilized ionic liquid catalyst is prepared from the following steps: performing oxidation treatment and chlorination on activated carbon sequentially, so as to obtain oxidized and chlorinated activated carbon; performing refluxing on the oxidized and chlorinated activated carbon, bromized 1-(2-amino ethyl hydrobromic acid)-3 methylimidazole onium salt ionic liquid and organic amine in tetrahydrofuran for 20-30 h, filtering, and washing and drying filter cakes B, so as to obtain solid products; adding the solid products into a potassium hydroxide solution, stirring for 20-30 h at the temperature of 0-5 DEG C, filtering, washing filter cakes C and drying, so as to obtain the activated carbon immobilized ionic liquid catalyst. The activated carbon immobilized ionic liquid catalyst provided by the invention can be used for catalyzing of knoevenagel condensation, during after-treatment, the activated carbon immobilized ionic liquid catalyst can be separated from reaction mixed liquor through simple centrifuging or filtering only, and the activated carbon immobilized ionic liquid catalyst can be used repeatedly.
Owner:ZHEJIANG UNIV OF TECH
Diphenylethylene type co-crystallization materials with multi-stimulus fluorescence response property and preparation method thereof
InactiveCN103642484AOptical function optimization or changeChanged crystallographic propertiesTenebresent compositionsHalogenated hydrocarbon preparationBenzeneObject structure
The invention discloses a series of cyano-substituted diphenylethylene type co-crystallization compounds with multi-stimulus fluorescence response property and a preparation method thereof, belonging to the field of crystalline materials with subject and object structures. The novel crystalline compounds are obtained by selecting a cyano-substituted diphenylethylene compound as a subject, taking a series of organic small molecular compounds with hydroxyl, carboxyl and other groups or halogenated benzene and the like as objects, and performing co-crystallization by subject and object identification and intermolecular interaction. The compounds disclosed by the invention are characterized in that the selected cyano-substituted diphenylethylene compound has excellent optical property, the co-crystallization materials can be obtained by the action of frictional force, the ultrasonic action, solvent volatilization and other methods, and the precise crystal structures can be determined; and simultaneously, the series of the co-crystallization materials have reversible fluorescence response against a variety of external stimuli (such as light, pressure, organic solvents and the like). The compounds have application prospects in the fields of manufacturing of optical anti-counterfeiting materials, fluorescent sensors and optoelectronic devices, and the like.
Owner:BEIJING UNIV OF CHEM TECH
Method and device for preparing mass spectrum level acetonitrile
InactiveCN102432498AMeet the requirements of high-purity solventsEasy to operateCarboxylic acid nitrile purification/separationPesticide residuePotassium hydroxide
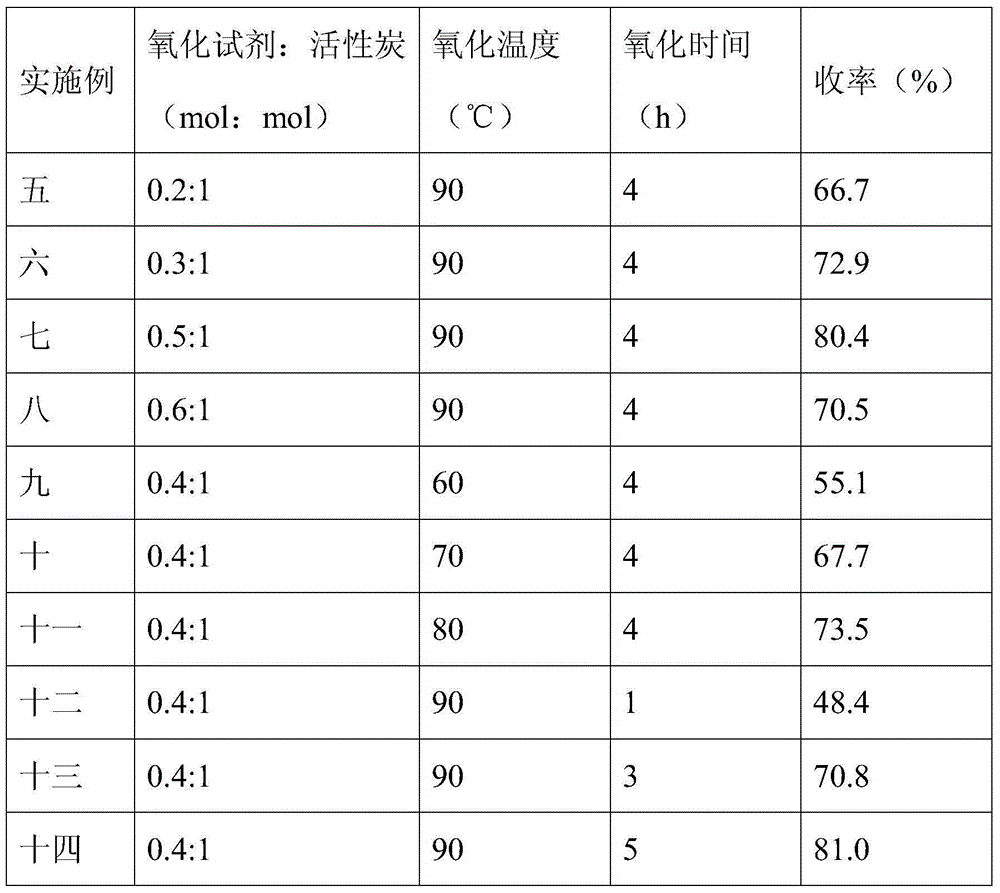
The invention relates to a method and a device for preparing mass spectrum level acetonitrile. The method comprises the following steps of: performing oxidation reaction on acetonitrile to remove impurities, rectifying, and filtering to obtain the mass spectrum level acetonitrile, wherein an oxidant used in the oxidation reaction is potassium permanganate, sodium hydroxide, potassium hydroxide, calcium hydroxide and the like; the impurities such as residual trace hydrocyanic acid and acrylonitrile and the like in industrial acetonitrile are mainly removed; the using amount of the oxidation reaction agent is 1 to 50 weight percent of that of the acetonitrile; and oxidation reaction time is 3 to 24 hours. The impurity content of the mass spectrum level acetonitrile product prepared by the method is lower than 10 to 12, the requirement of pesticide residue analysis can be met, the method and the device are easy to operate, cost is saved, and wide market application prospects are achieved.
Owner:NAT INST OF METROLOGY CHINA
Ortho-nitrosophenols as polymerization inhibitors
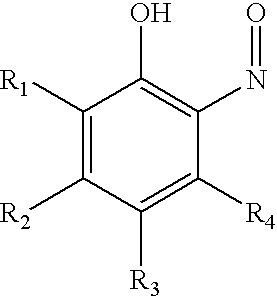
InactiveUS20060283699A1Prevent premature polymerizationEffective inhibiting amountGroup 4/14 element organic compoundsOrganic compounds purification/separation/stabilisationNitrosoHalogen
Disclosed herein is a method for inhibiting the premature polymerization of ethylenically unsaturated monomers comprising adding to said monomers an effective amount of at least one nitroso inhibitor of the structure: wherein R1, R2, R3, and R4 are independently selected from the group consisting of hydrogen, nitro, nitroso, halogen, COOR wherein R is hydrogen or alkyl, alkyl, and heteroatom-substituted alkyl; or adjacent groups R1, R2, R3, and R4 can be taken together to form a substituted or unsubstituted fused six-membered ring. Also disclosed is a composition of matter comprising: A) an ethylenically unsaturated monomer and B) at least one nitroso compound of the above-described structure.
Owner:ADDIVANT USA
Preparation method for 2-(cyclohexenyl) malonic acid derivative and application thereof
ActiveCN108264469AEfficient synthesisHigh reaction yieldBiocideOrganic compound preparationArylMalonic acid
The invention provides a preparation method for a 2-(cyclohexenyl) malonic acid derivative and application thereof. The method takes polylefin and a 2-substituted malonic acid derivative as raw materials, the two materials are subjected to a cyclization reaction in the presence of catalyzer, and the 2-(cyclohexenyl) malonic acid derivative is obtained. Compared with the prior art, the preparationmethod adopts a completely different strategy and has the special functions that (1) a 2-(2, 6-disubstituted cyclohexenyl) malonic acid derivative can be more efficiently synthesized; (2) the reactionyield is high, the reaction conditions are mild, the three wastes are less, and the method is beneficial to industrial production. More importantly, the method expands the application of the 2-(cyclohexenyl) malonic acid derivative in organic synthesis, especially the synthesis of a 2-aryl malonic acid derivative and corresponding pesticides such as a herbicide of pinoxaden.
Owner:ORIENTAL LUZHOU AGROCHEM
Method for producing adiponitrile with adipic acid liquid-phase method
ActiveCN106146345AImprove dehydration ratePhysical/chemical process catalystsCarboxylic acid nitrile purification/separationSynthesis methodsDiluent
The invention discloses a method for producing adiponitrile with an adipic acid liquid-phase method. A solid phosphoric acid catalyst is adopted, adipic acid and a diluent are added into a reaction still, heating and raw material stirring are conducted during reaction, ammonia gas is supplied after temperature reaches a certain value, and the product is generated after reaction is conducted for a while. A novel environment-friendly adiponitrile synthesis method is adopted. By the adoption of the solid phosphoric acid catalyst, the defects of traditional catalysts are overcome, and catalysis effect is improved.
Owner:PETROCHINA CO LTD
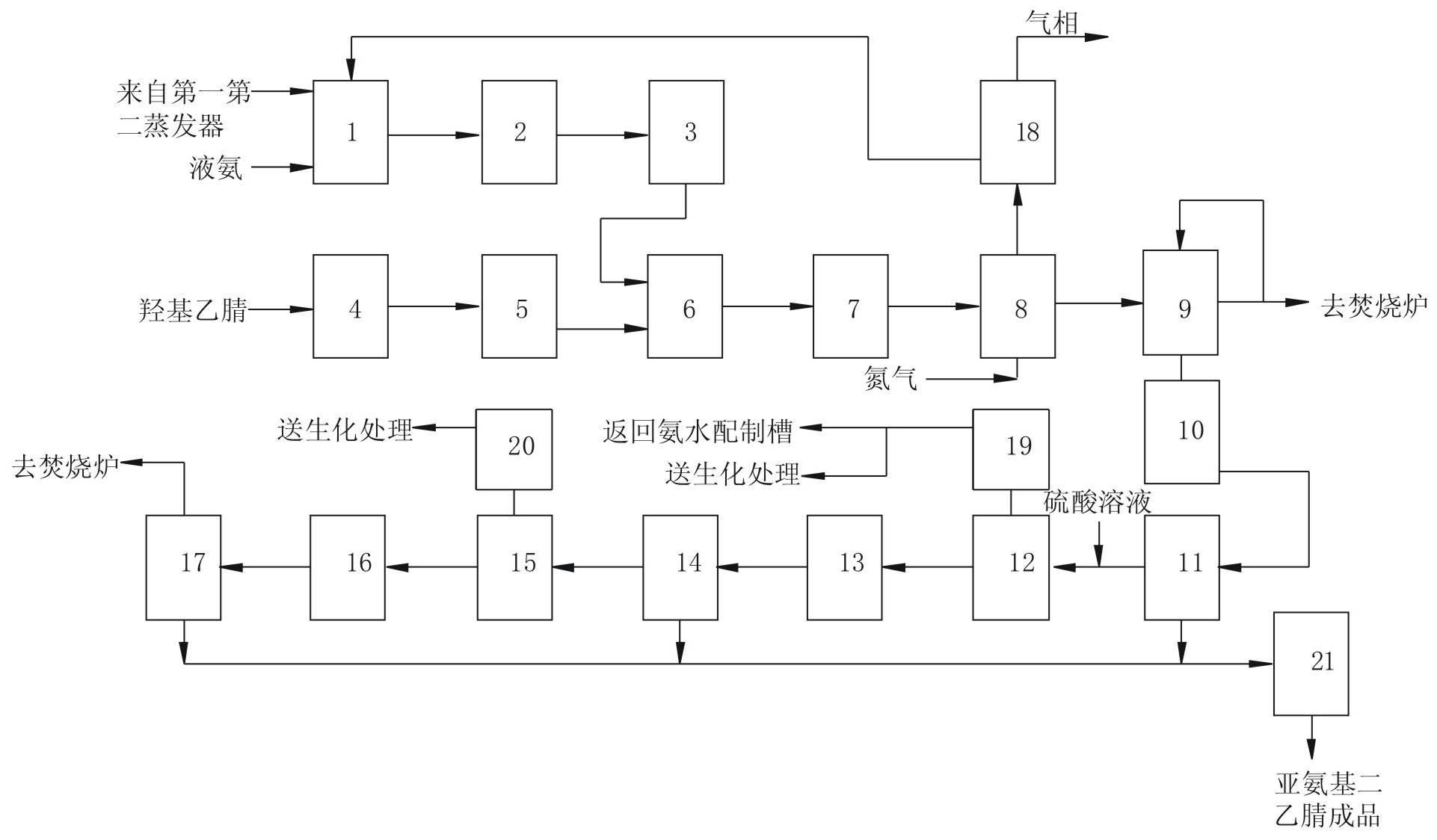
New process for producing high-purity iminodiacetonitrile
ActiveCN101914037AImprove conversion rateHigh yieldOrganic compound preparationCarboxylic acid nitrile purification/separationAcetonitrileWaste treatment
The invention relates to a new process for producing high-purity iminodiacetonitrile. The new process is characterized in that: high-purity reaction liquid is obtained by using a process for synthesizing iminodiacetonitrile at high conversion rate; and white or pale yellow high-purity iminodiacetonitrile with iminodiacetonitrile content of over 99 percent is produced by using a synthesis reaction liquid decolorizing process, an impurity removal process and three crystallization processes, wherein a part of thin waste liquid produced during the production is returned to a raw material preparation system, the rest part of waste liquid is conveyed to a biochemical device for processing, and the produced thick waste liquid is directly conveyed to an incinerator for processing, so that pollution-free production of the entire process is realized. The new process has the advantages of high product quality, white color, purity of over 99 percent high product yield, iminodiacetonitrile yield of about 90 percent during the entire production process and low three-waste treatment difficulty.
Owner:SICHUAN LUTIANHUA
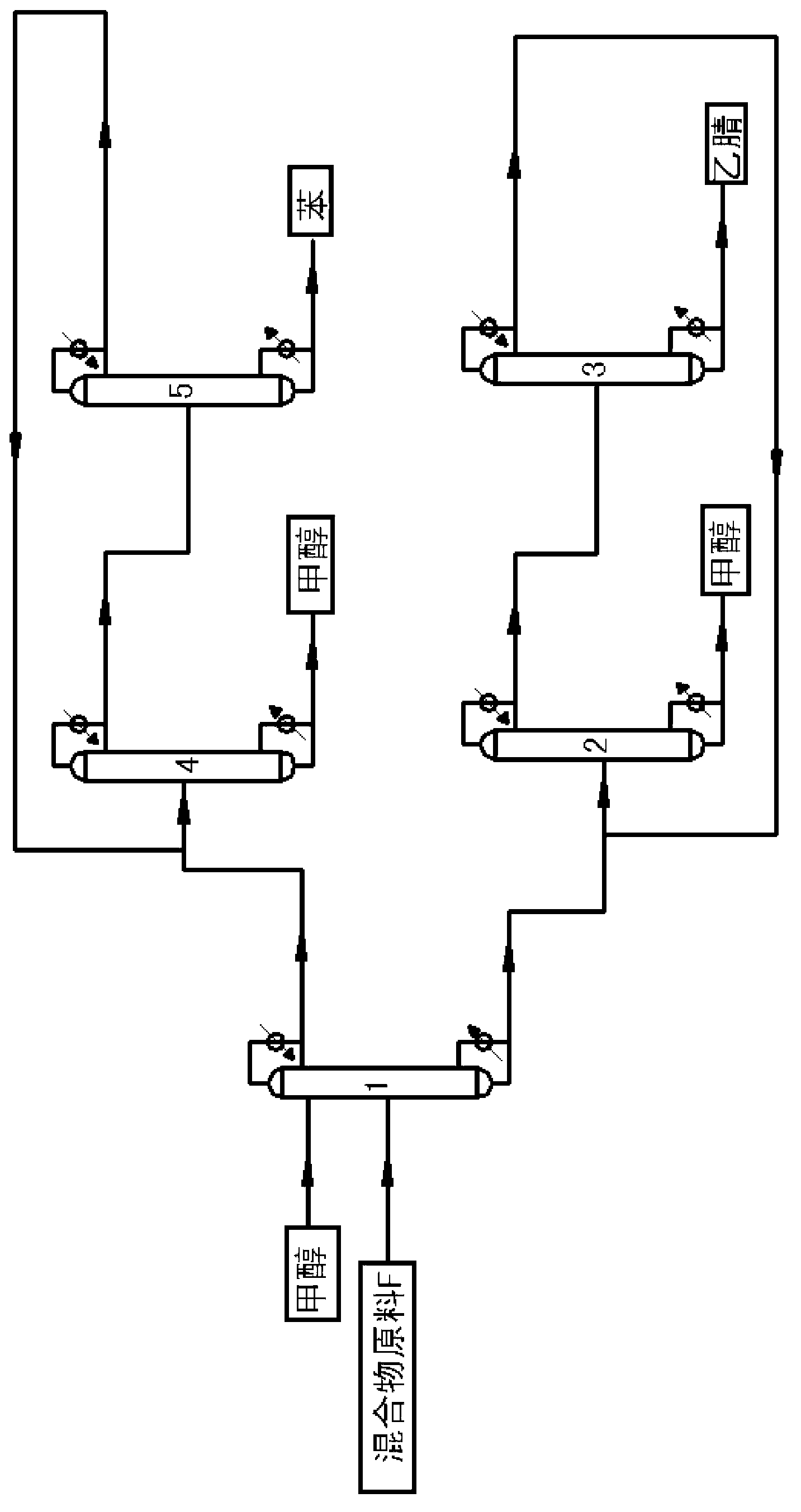
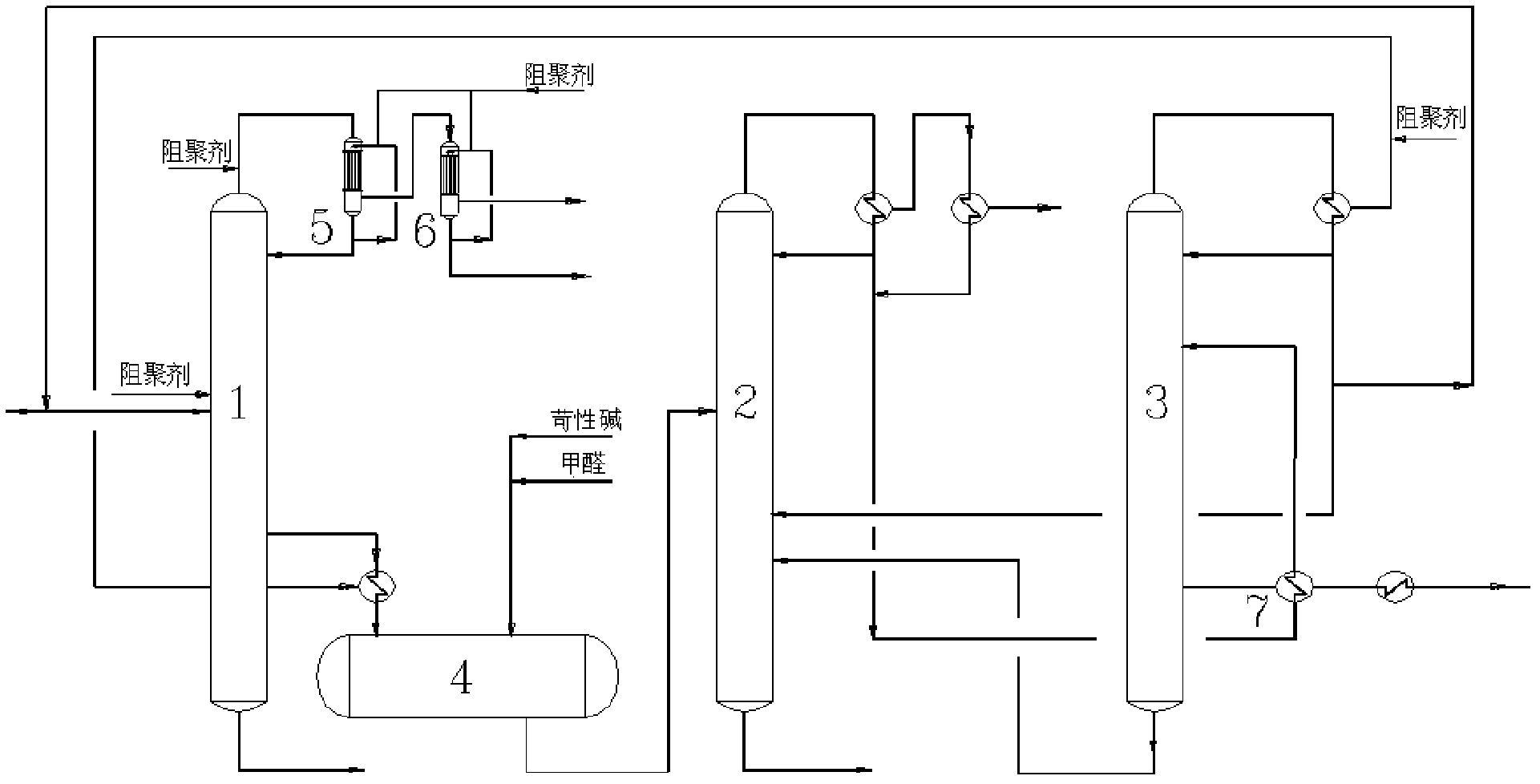
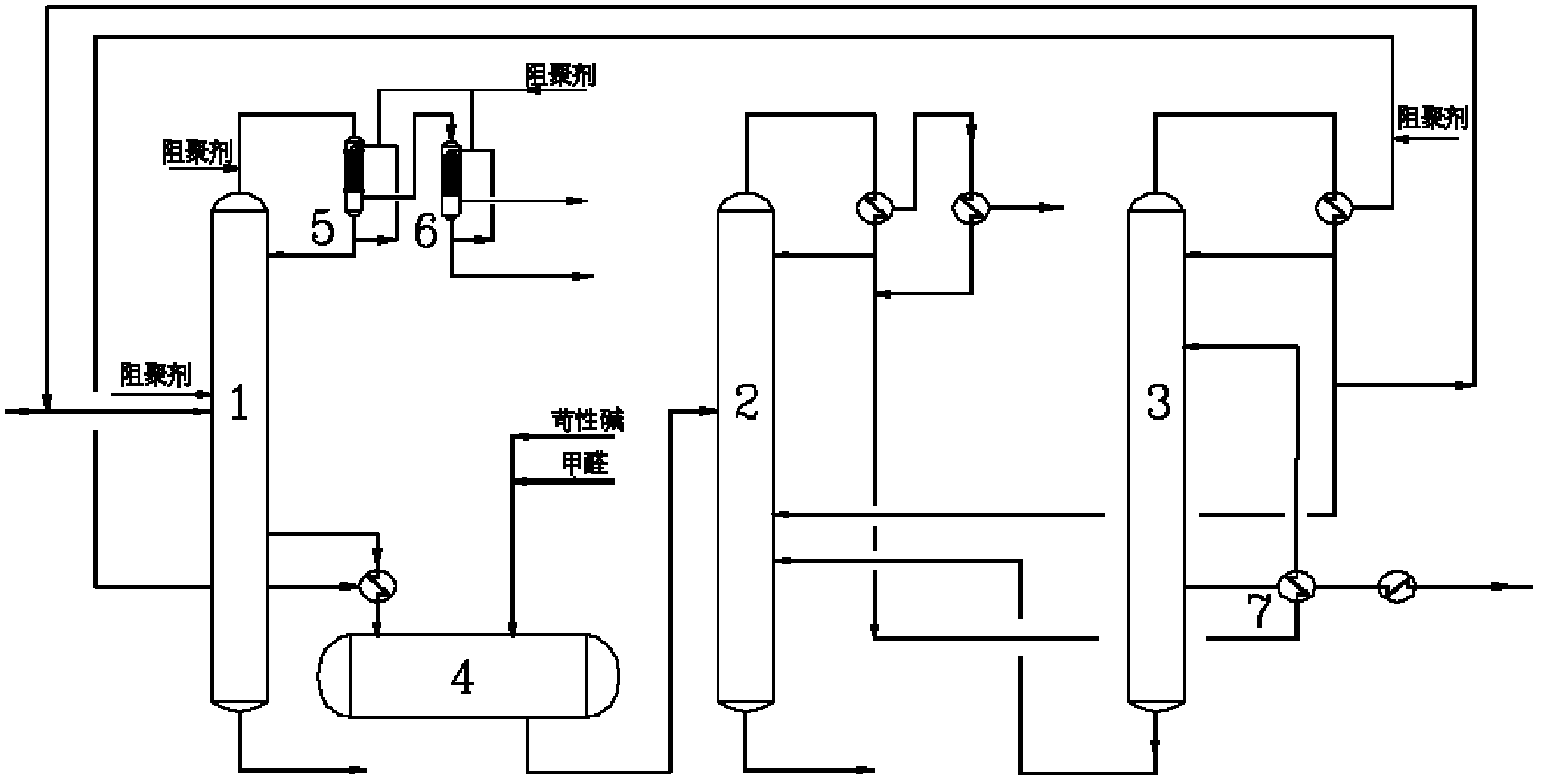
Continuous purification method for crude acetonitrile
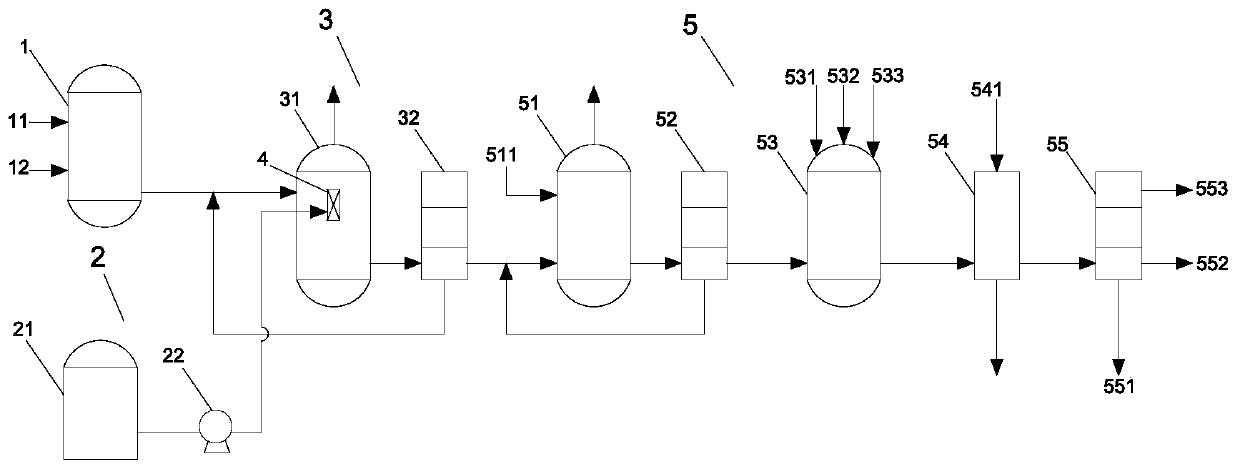
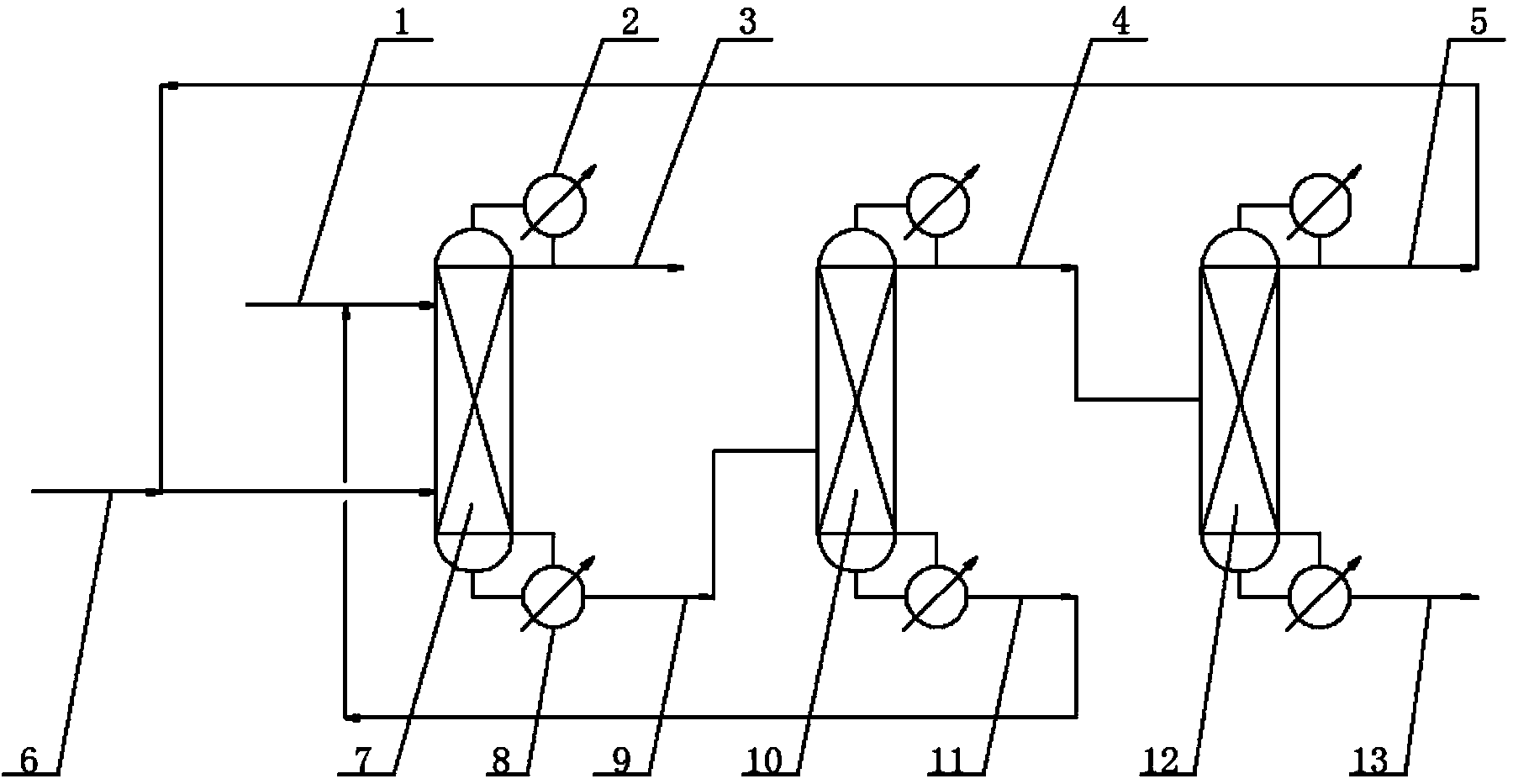
ActiveCN102633679AReduce the amount of refrigerantReduce consumptionCarboxylic acid nitrile purification/separationPurification methodsReboiler
The invention provides a continuous purification method for crude acetonitrile. A lateral line condenser is arranged at a third rectifying tower, so that the heat energy of an acetonitrile final product can be adequately recovered, the load of a reboiler at the bottom of the third rectifying tower and the refrigerant quantity of an acetonitrile final product cooler can be effectively reduced, the energy consumption can be saved, and 500-600MJ of energy can be saved for each ton of the acetonitrile product; and distillate is returned from the top of the third rectifying tower, so that the accumulation of oxazole in a system can be reduced, the yield of the acetonitrile can be guaranteed as well, and the problem that the operation load of a first rectifying tower can be obviously increased since all the distillate on the top of the third rectifying tower returns to the first rectifying tower in the prior art can be improved. Furthermore, a corresponding pipeline for adding a polymerization inhibitor is increased on the conventional three-tower and one-kettle refining technology, so that the problem that the exposed devices are easy to polymerize in the existing acetonitrile purification process in China can be solved, and the continuous purification method has the advantages of being high in industrial application value, and high in acetonitrile final product purity.
Owner:CHINA TIANCHEN ENG +3
Process method for producing high-purity chlorothalonil
The invention provides a process method for producing high-purity chlorothalonil. The process method has the characteristics of comprising the steps of: sending raw materials containing chlorothalonilm-phthalodinitrile, trichloro-m-phthalodinitrile, pentachloro benzonitrile, hexachloro benzene and water to a light-component removing tower, collecting a mixture of the chlorothalonil containing light components with a mass fraction of <= 0.01% with heavy components from the tower bottom of the light-component removing tower, sending the mixture to a product tower, and collecting the chlorothalonil product with a mass fraction of >= 99.5% from the tower top of the product tower. According to the process method for producing the high-purity chlorothalonil, a rectification process method is adopted to refine the chlorothalonil, the operation process is simple, and the chlorothalonil product with a mass fraction of 99.5% or above can be obtained.
Owner:TIANJIN SEPTECH SCI & TECH
Method for separating carbinol acetonitrile azeotrope
InactiveCN101134736AShort processLess investmentOrganic compound preparationHydroxy compound preparationTheoretical plateRaoult's law
The present invention relates to method of separating methanol-acetonitrile azeotrope. By means of the feature of methanol-acetonitrile azeotrope system with positive deviation to Raoult's law, the pressure of the system is lowered or raised to shift the binary azeotropic point and alter the composition of the azeotrope system, so as to reach the separation aim. The decompressing rectification tower has a theoretical plate number of at least 40 and top pressure of 0.3-0.5atm, and compressing rectification tower has a theoretical plate number of at least 25 and top pressure of 5-10 atm. The method can obtain separated methanol and acetonitrile products of purity over 99.5 wt%, has short technological process, easy control and other advantages, and is suitable for separating methanol-acetonitrile solution in different ratio.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com