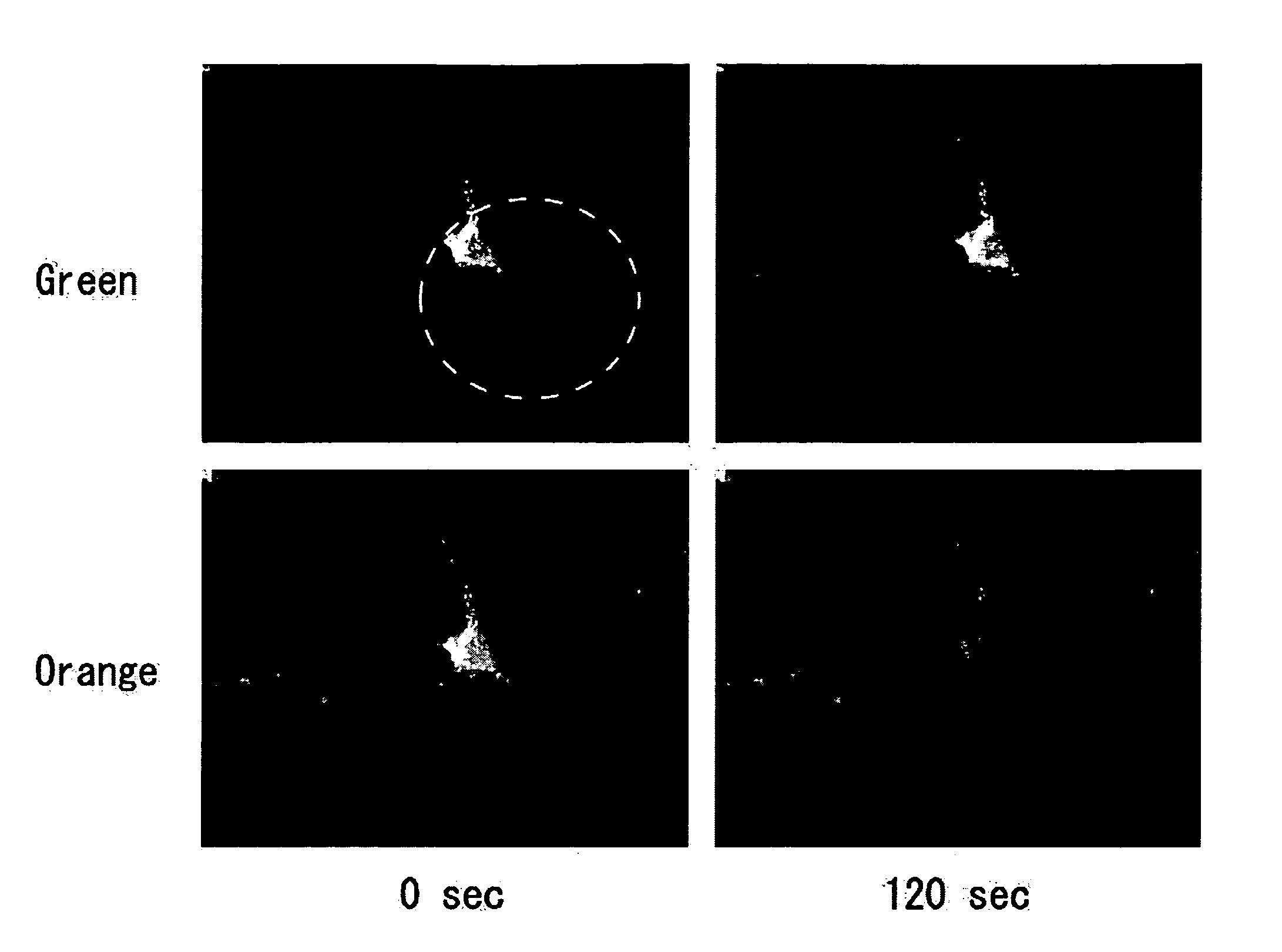
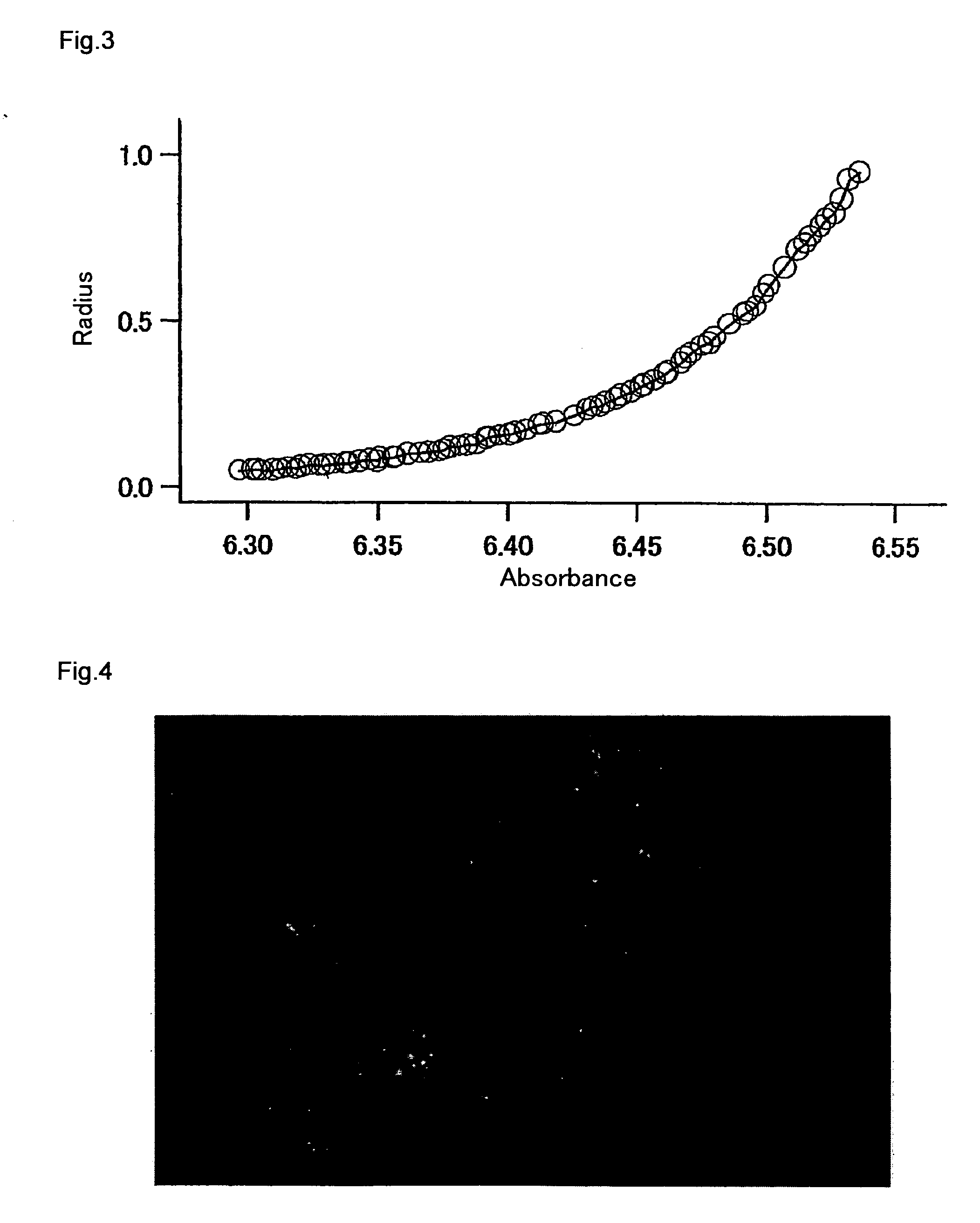
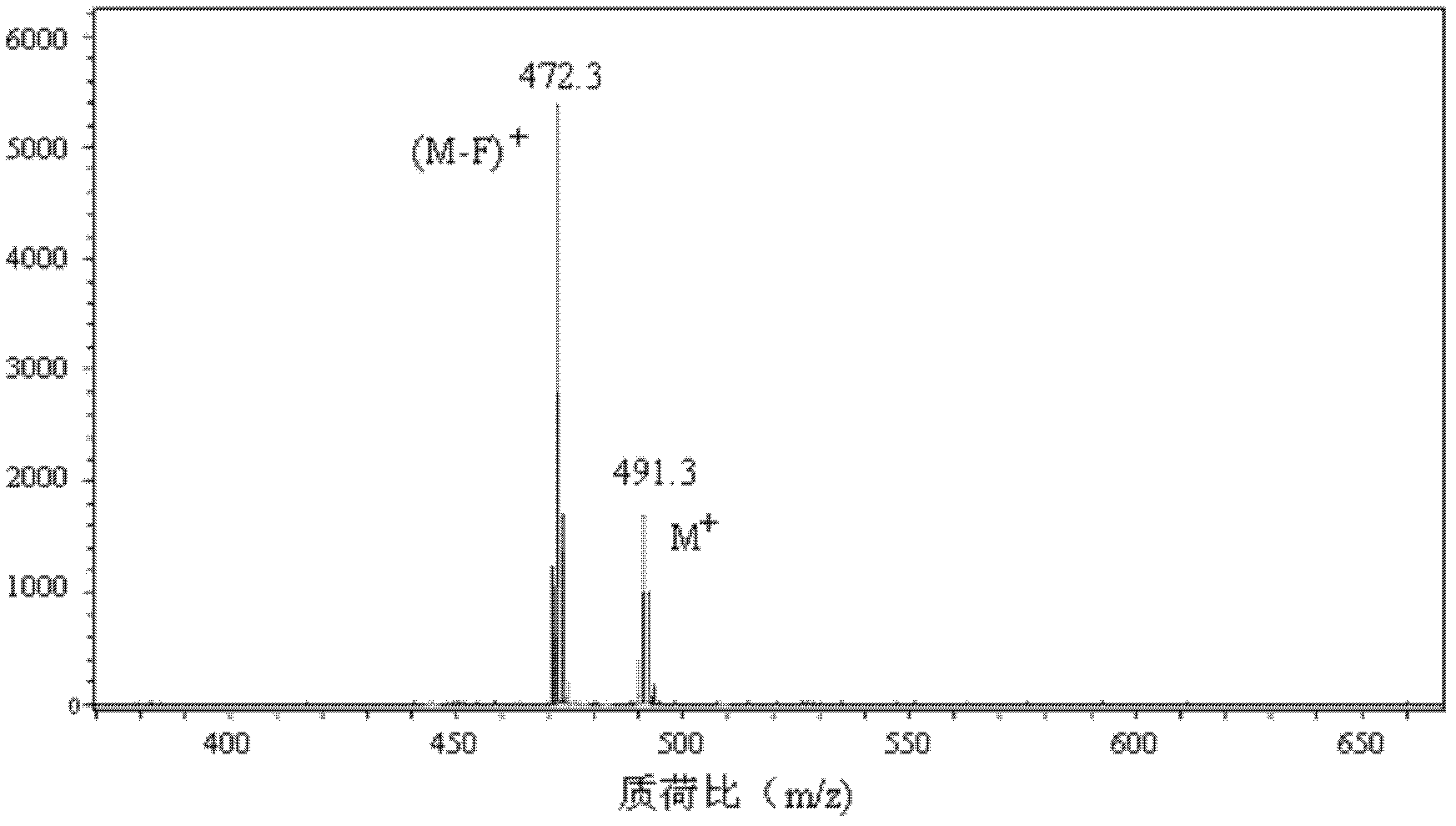Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
363 results about "Stokes shift" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
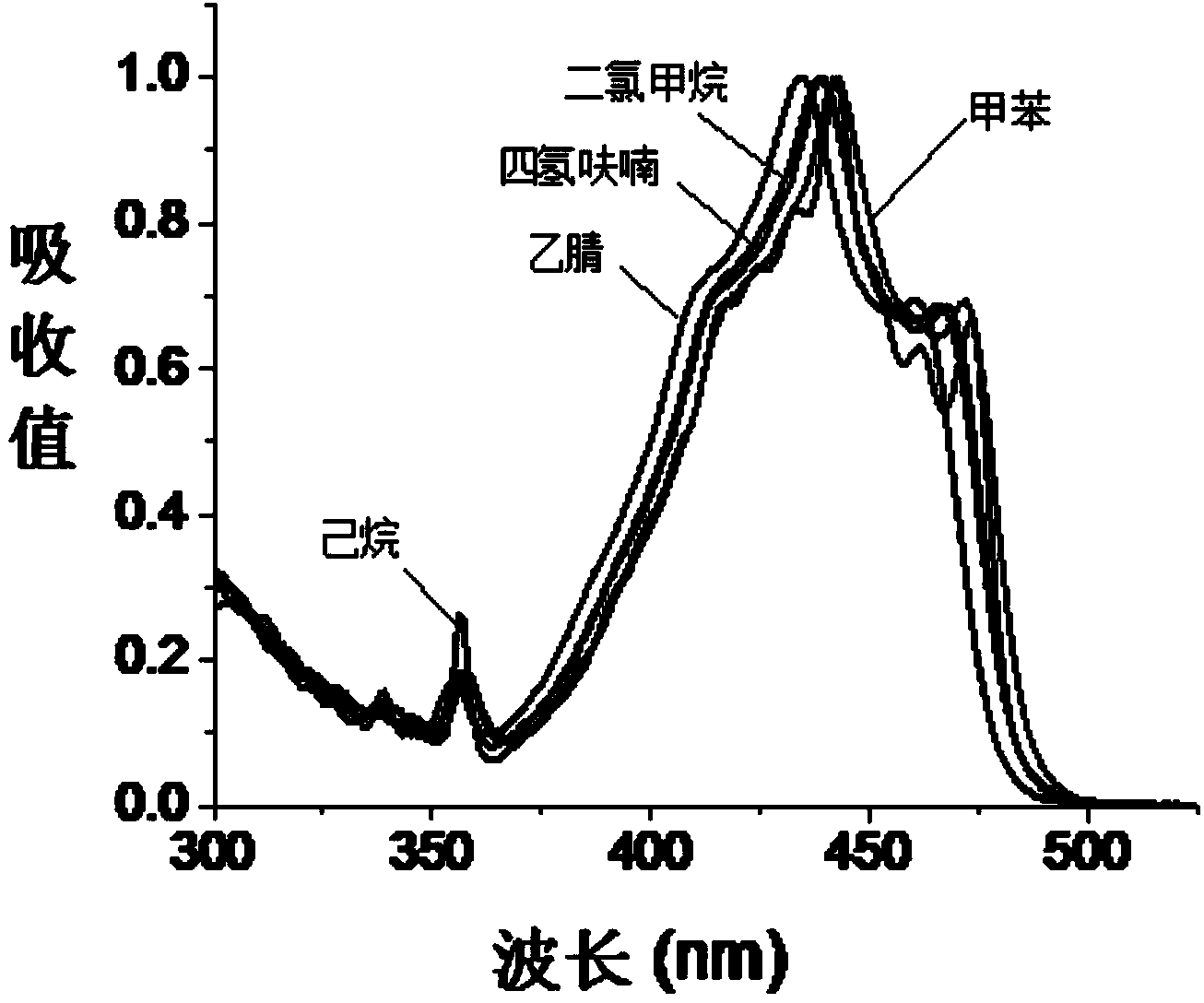
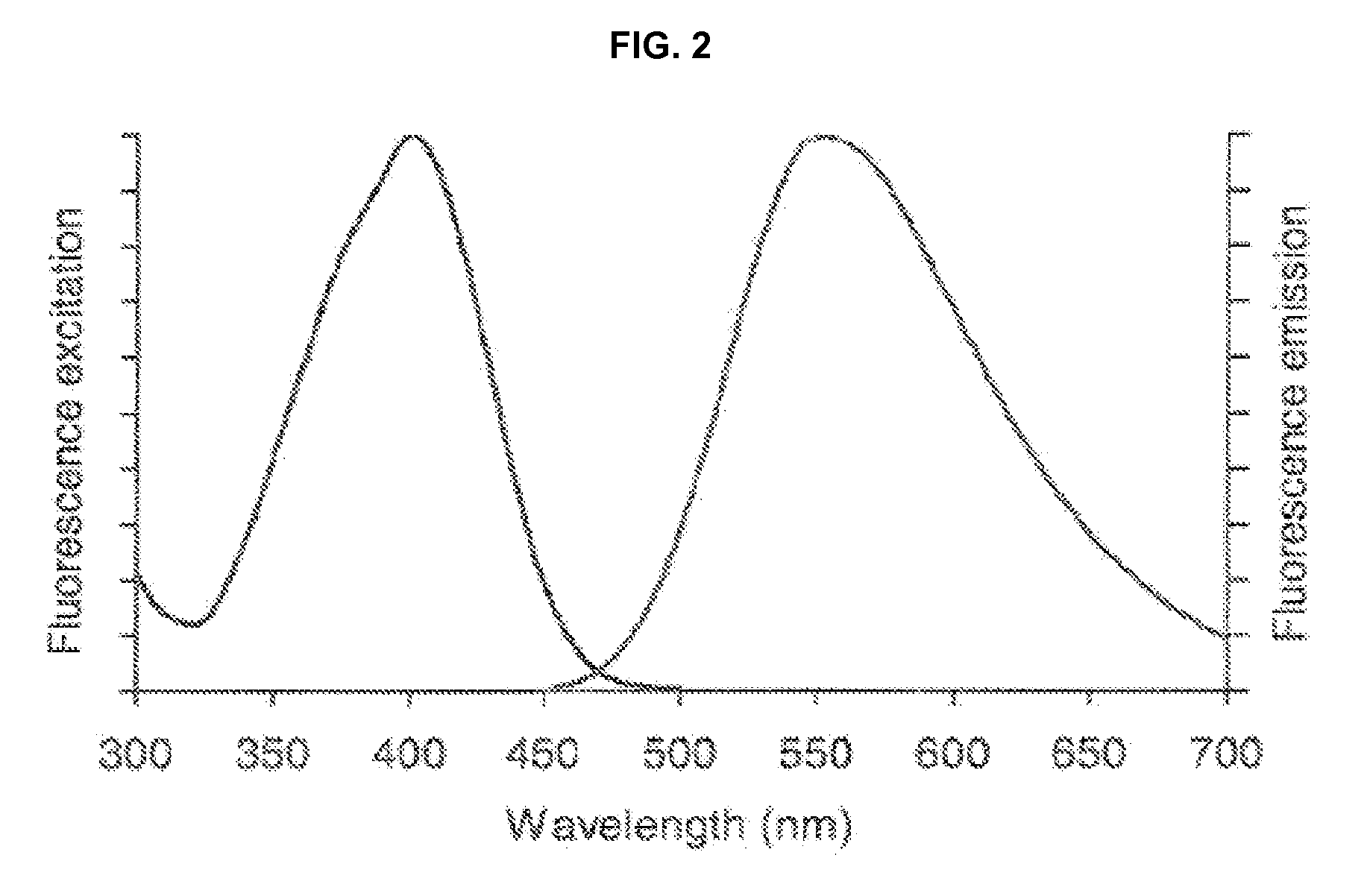
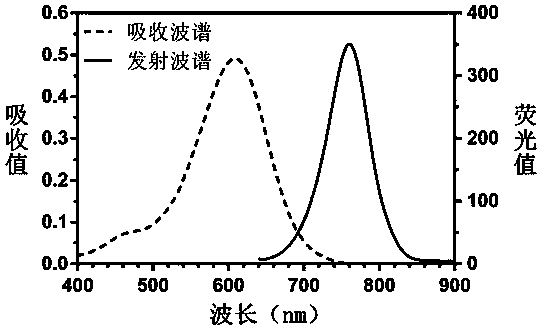
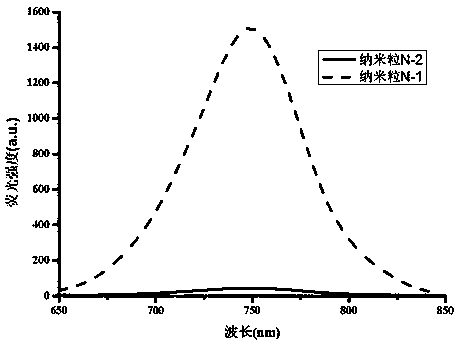
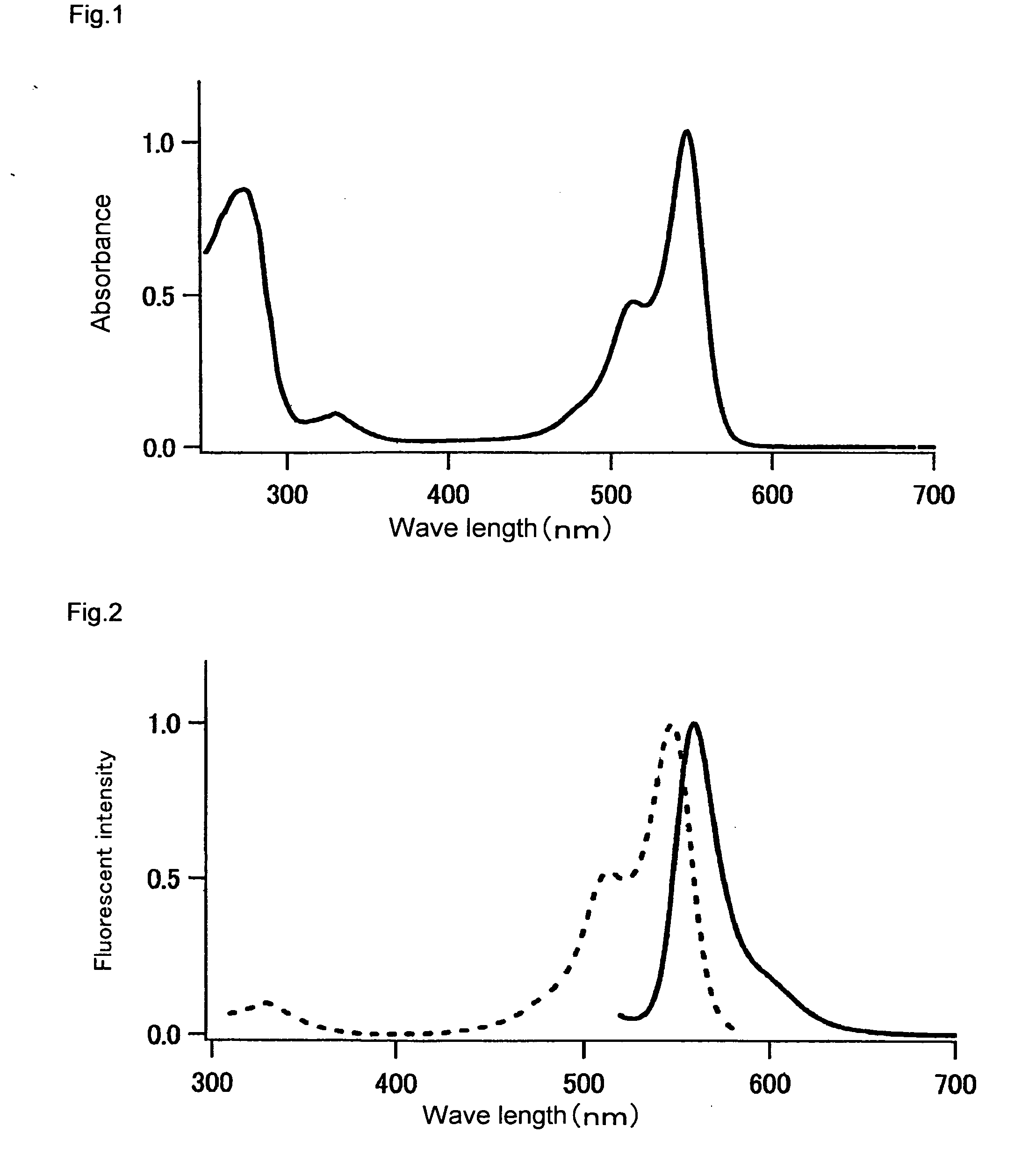
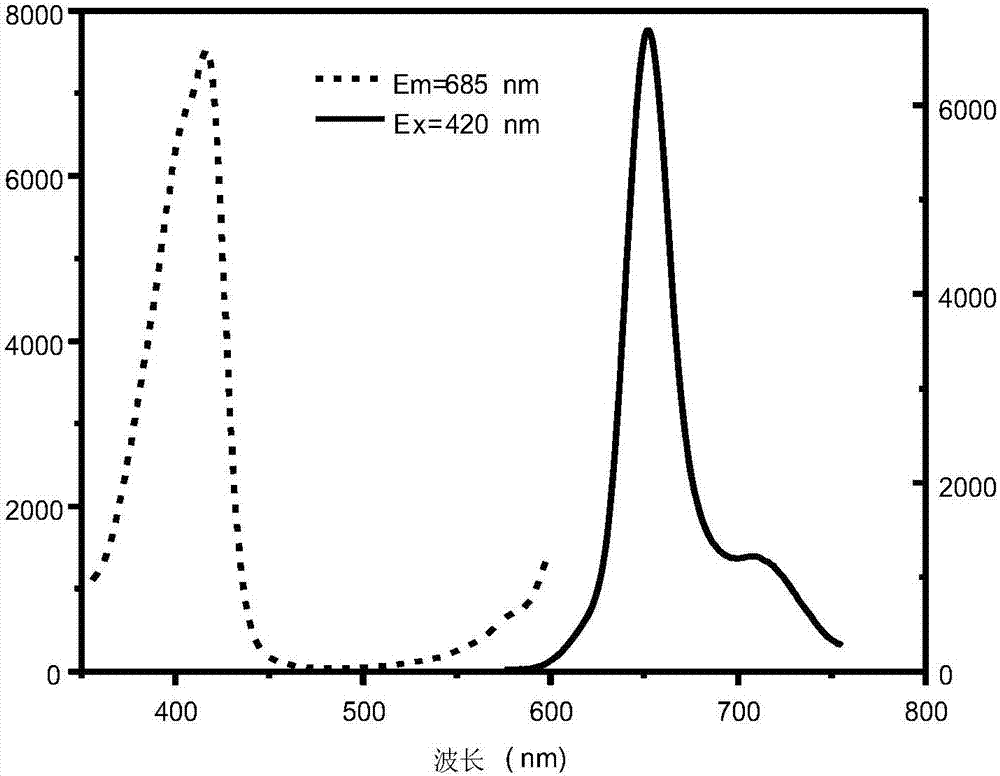
Stokes shift is the difference (in energy, wavenumber or frequency units) between positions of the band maxima of the absorption and emission spectra (fluorescence and Raman being two examples) of the same electronic transition. It is named after Irish physicist George Gabriel Stokes. Sometimes Stokes shifts are given in wavelength units, but this is less meaningful than energy, wavenumber or frequency units because it depends on the absorption wavelength. For instance, a 50 nm Stokes shift from absorption at 300 nm is larger in terms of energy than a 50 nm Stokes shift from absorption at 600 nm.
Compound, especially marker-dye on the basis of polymethines
InactiveUS20040260093A1Improve light resistanceLong storage periodMethine/polymethine dyesOrganic chemistryQuantum yieldFluorophore
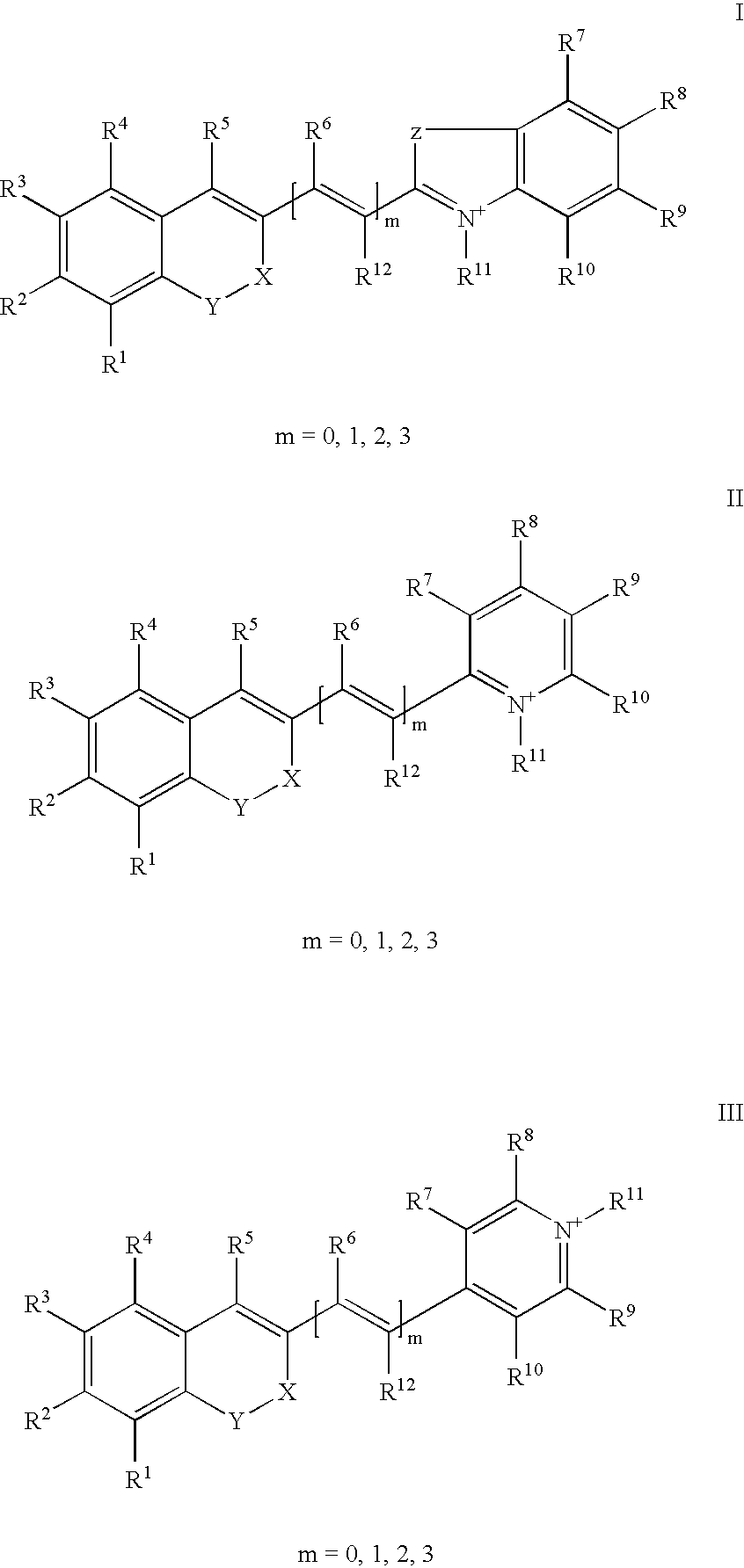
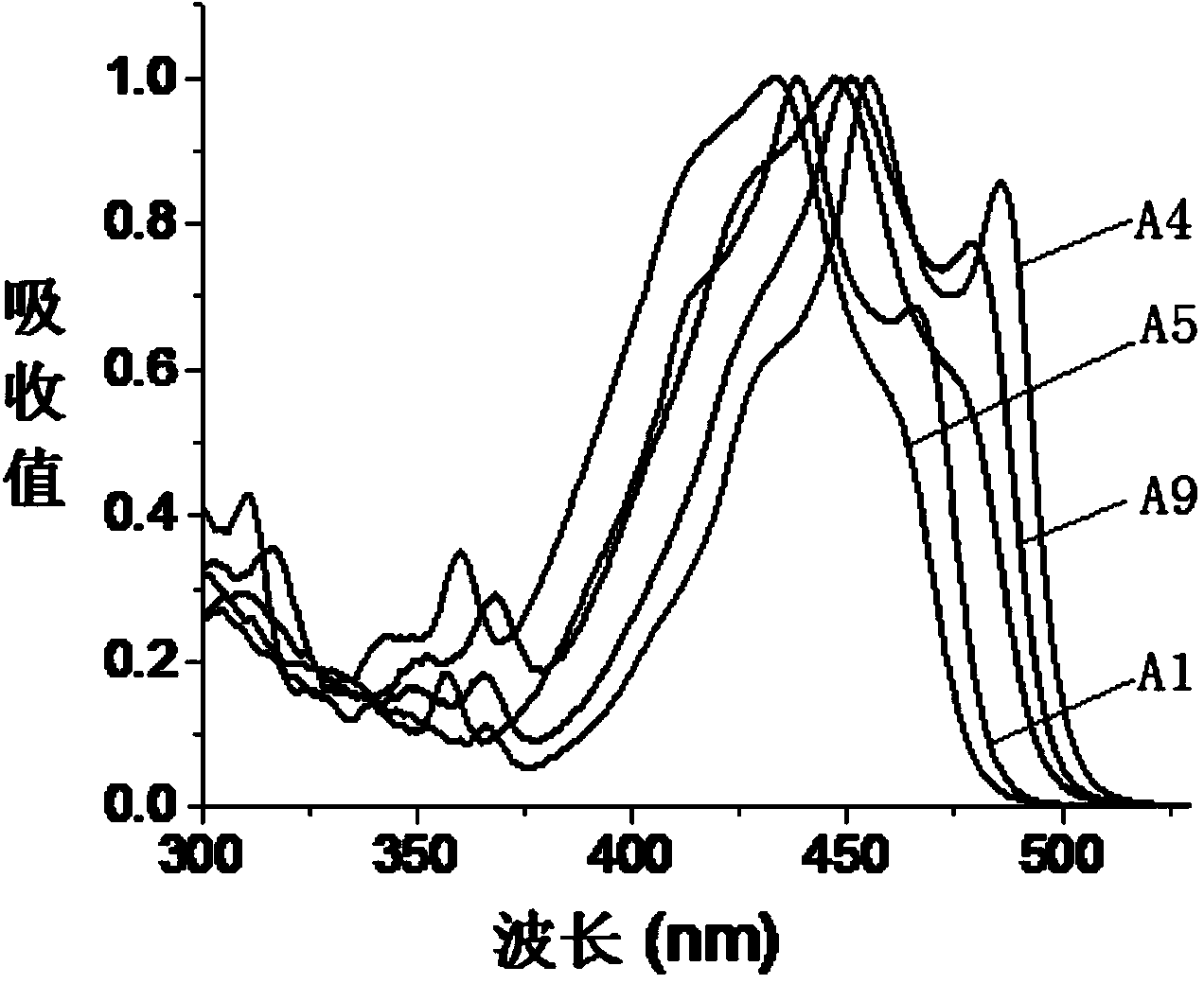
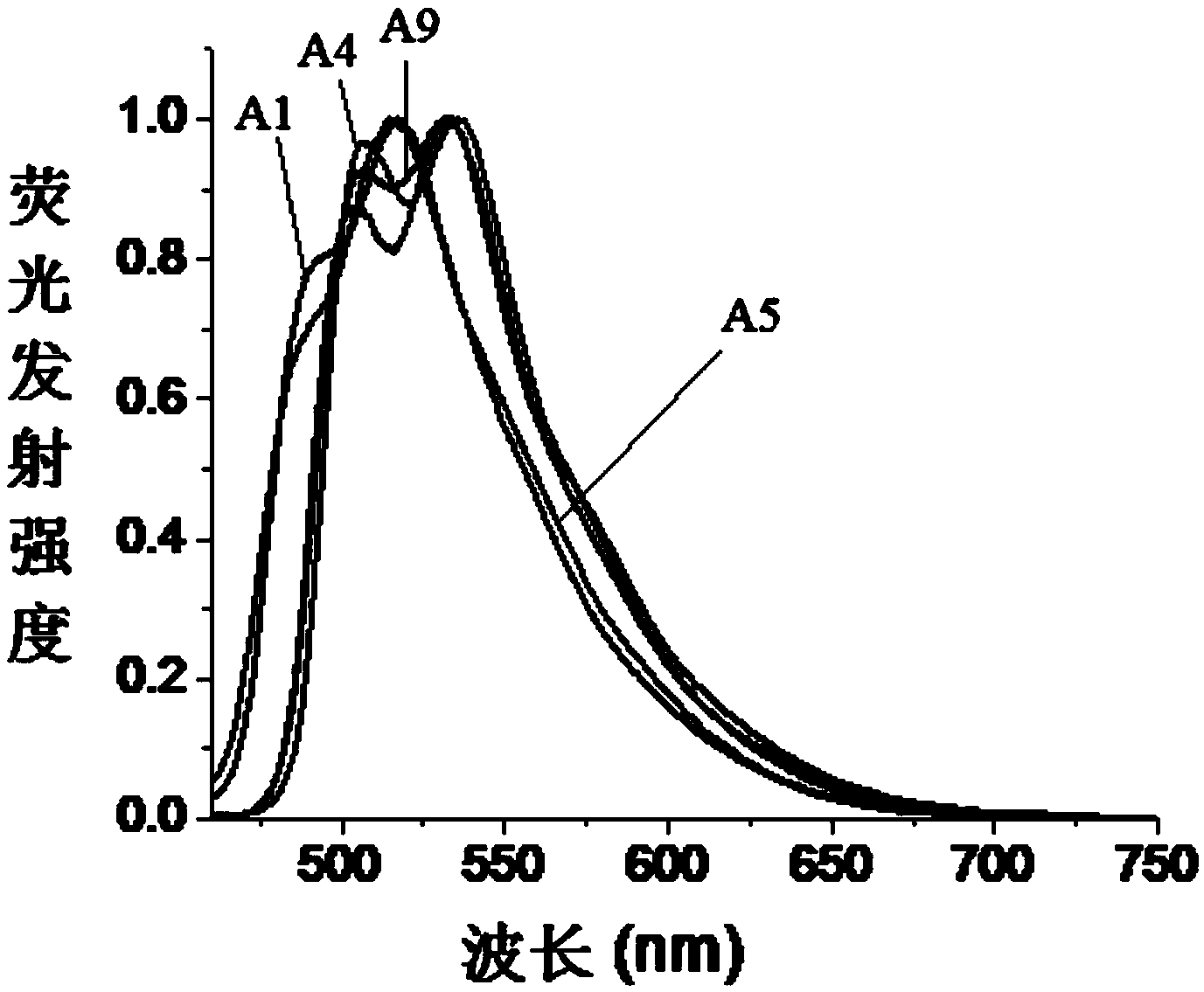
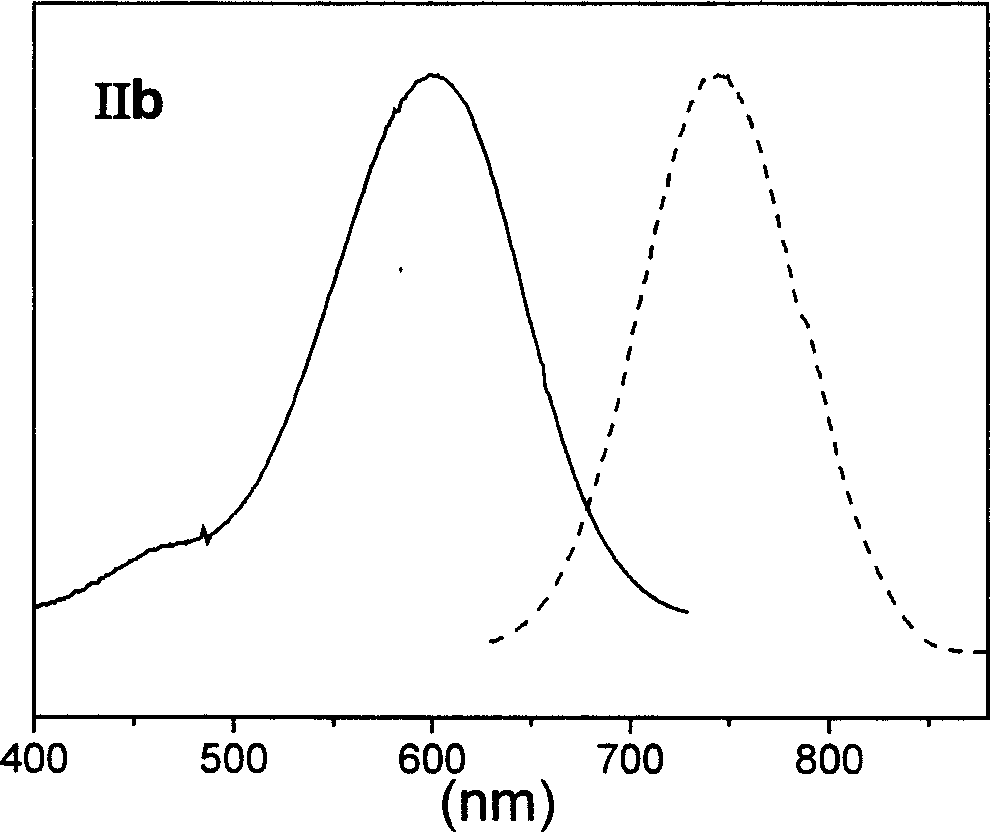
The invention relates to fluorescent dyes (fluorophores) based on polymethines for use in optical measurement and detection procedures, in particular those employing fluorescence, for example in medicine, in pharmacology and in the biological, materials and environmental sciences. The objective was to create fluorophores based on polymethines that have a large Stokes shift, high photostability, long storage life and a high fluorescent quantum yield, and that can be excited in the simplest possible manner by white-light sources or laser radiation in the UV, visible or NIR spectral region. According to the invention dyes on the basis of polymethines having the general formulas I, II or III are employed.
Owner:DYOMICS
Fluorine-boron fluorescent dye as well as preparation method and application thereof
InactiveCN103865290ANarrow absorbencyNarrow emission peakAzo dyesGroup 3/13 element organic compoundsQuantum yieldHalogen
The invention discloses a fluorine-boron fluorescent dye as well as a preparation method and application thereof, wherein the structure of the fluorine-boron fluorescent dye is shown as a formula (I) or a formula (II), in the formula (I) and the formula (II), R1 is H or halogen; R2 is CN; R3, R4, R5 and R6 are independently selected from H, halogen, C1-C6 alkyl or C1-C6 alkoxy; V, W, X, Y and Z are independently CH or N, and when V, W, X, Y or Z is N, N has no substituent group. According to the fluorine-boron fluorescent dye and the preparation method thereof, the maximal fluorescence emission wavelength of the fluorine-boron fluorescent dye is 518-600nm, and the fluorine-boron fluorescent dye also has excellent fluorescence quantum yield and Stokes shift, which shows that the fluorine-boron fluorescent dye has good application prospect in the bioanalysis fields of fluorescence labeling, bioimaging and so on; meanwhile, the preparation method is simple in steps, and raw materials can be obtained easily.
Owner:ANHUI NORMAL UNIV
Violet laser excitable dyes and their method of use
The present invention provides dye compounds optimally excited at about 400 nm and have a Stokes shift of at least about 80 nm. These dyes find use in detection of analyte in a sample and the preparation of dye-conjugates.
Owner:LIFE TECH CORP
Fluorescent probes for labelling and/or detecting lipid droplets in cells as well as preparation and applications of fluorescent probes
InactiveCN108069902ARaw materials are cheap and easy to getSpecific recognition abilityOrganic chemistryFluorescence/phosphorescenceFluorophoreLipid Body
The invention belongs to the field of biological analysis and detection and relates to fluorescent probes for labelling and / or detecting lipid droplets in cells as well as preparation and applicationsof the fluorescent probes. The probes are synthesized with 4-bromo-1,8-naphthalic anhydride as an initial material and take 1,8-naphthalimide as a fluorophore, a C12 alkyl long chain as a specific lipid droplet positioning group and an N-containing group on the 4th site of a naphthalene ring as a fluorescence enhanced and stabilized group. The probes are simple to prepare and have high yield. Compared with existing Nile red and boron dipyrromethene lipid droplet fluorescent probes, the probes have high light stability and large stokes shift and are more suitable for fluorescence imaging. Theprobes have very important application value in cell labelling fluorescent imaging and biomedical fields.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Near infrared meso-position nitrogen and sulfur substituted hepta-methyl-cyanine fluorochrome for bioanalysis
InactiveCN1702118ANo energy transferAvoid quenching effectMethine/polymethine dyesBiological testingFluorochrome DyePhotochemistry
The invention relates to a near-infrared new methenyl fluorescence dye, which is made by displacement at mid-position of methine colors by nucleophilic reagent containing nitrogen and sulfur. Mid-position nitrogen derivative replaces methine colors to generate ICT effect, Stokes displacement (70-170nm) and fine fluorescence. It can be used as high fidelity fluorescence labeling probe of biomolecules such as protein, sugar and DNA. When mid-position sulfer derivative replaces methine colours, PET phenomenon occurs in molecule.
Owner:DALIAN UNIV OF TECH
Gold nanocluster preparation method and application thereof
InactiveCN103264987ALarge Stokes shiftLarge emission wavelength Stokes shiftMaterial nanotechnologyNanostructure manufactureMaterials scienceReducing agent
The invention provides a gold nanocluster preparation method and an application thereof, and belongs to the technical field of nano materials. The problem of high cost of gold nanoparticle compositing in the prior art is solved. The gold nanocluster preparation method includes the steps: a, mixing polypeptide water solution with HAuCl4 solution in a reaction vessel to prepare solution A; and b, adding NaBH4 solution into the solution A for reduction, and reacting for a certain time to obtain fluorescent gold nanocluster products. A series of gold nanoclusters with different maximum emission wavelengths are composited successfully by means of taking different polypeptides as templates and sodium borohydride as a reducer. The composited gold nanoclusters have the advantages of large Stokes shift, long fluorescence lifetime, good stability and the like, can be used for detecting mercury ions (Hg2+) in water samples, and is high in detection sensitivity.
Owner:ZHEJIANG NORMAL UNIVERSITY
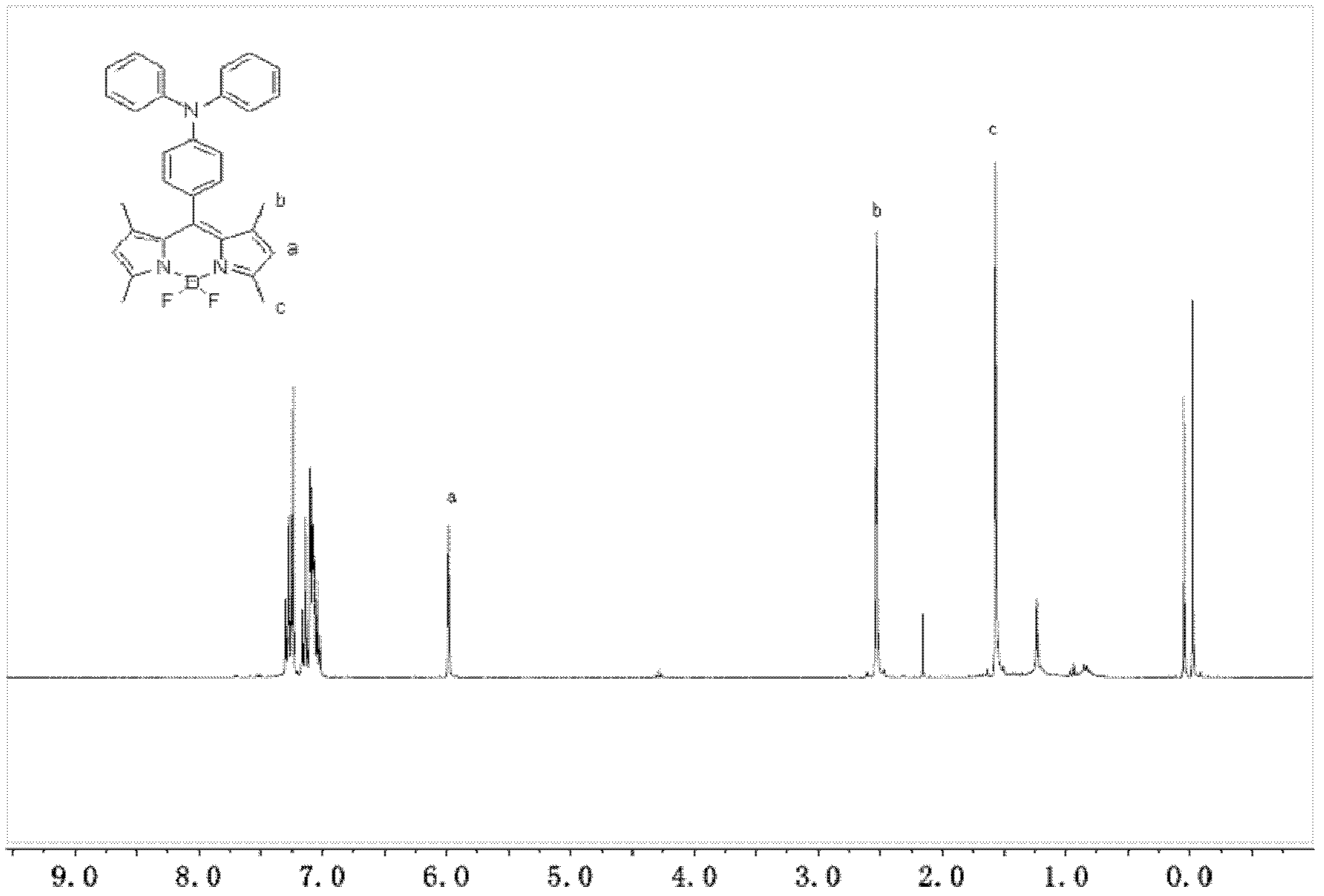
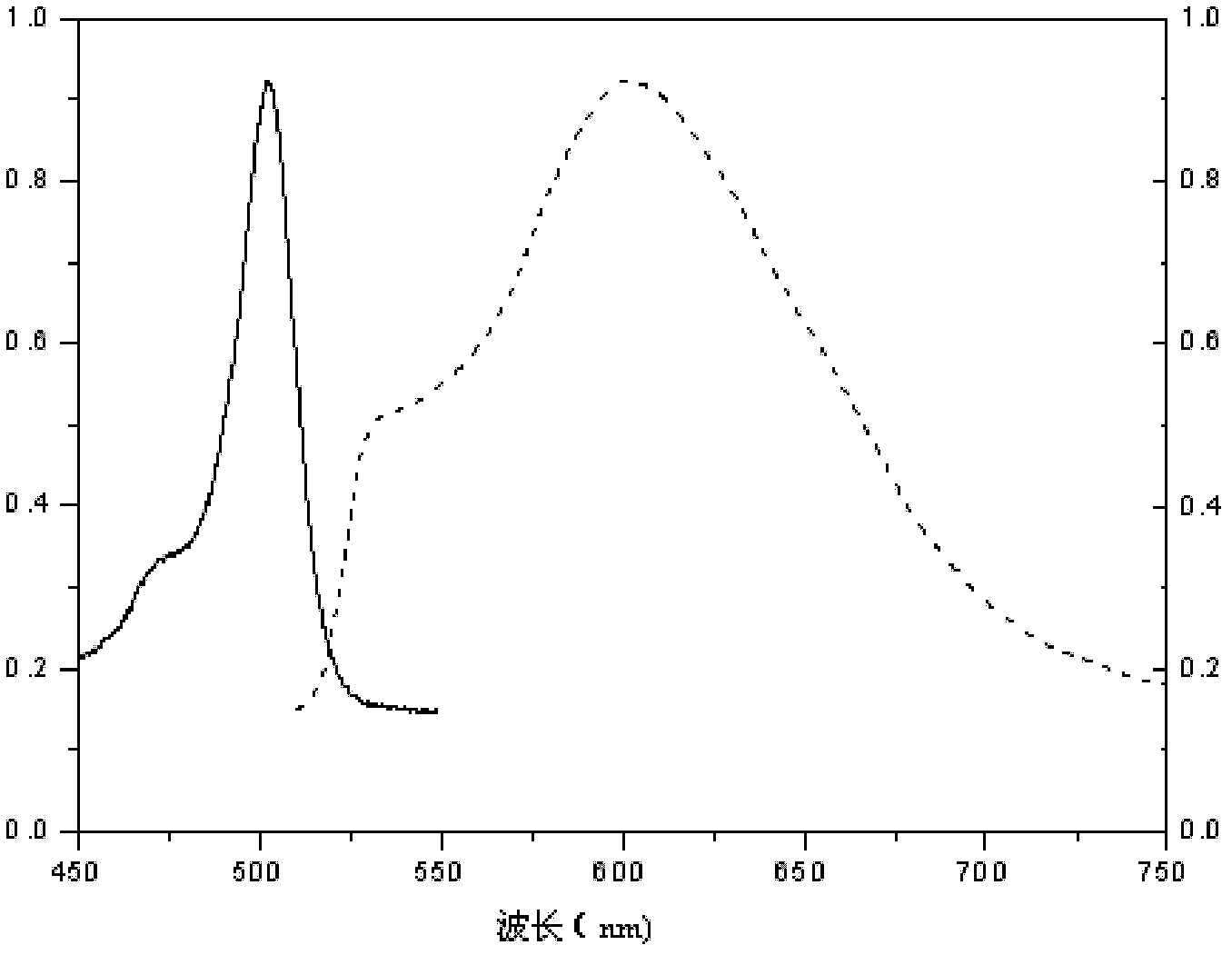
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Functional nano-rare earth fluorescent micro particle and its preparation and application
InactiveCN1493647ASolve problems with very strong stray lightElimination of Assay EffectsLuminescent compositionsNanoparticleLanthanide
A functional lanthanide fluorescence nanoparticle (LFNP) is prepared from the strong-fluorescent RE matches as luminous center through chemically wrapping by silica gel. Its advantages are long fluorescence lift, big stoke shift, narrow emitting peak and strong specific signal. It can be used as the fluorescent marker for biological detection.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Isophorone fluorescence probe, and preparation method and application thereof
ActiveCN105524612AGood practical valueAvoid interferenceOrganic compound preparationFluorescence/phosphorescenceIsophoroneCytotoxicity
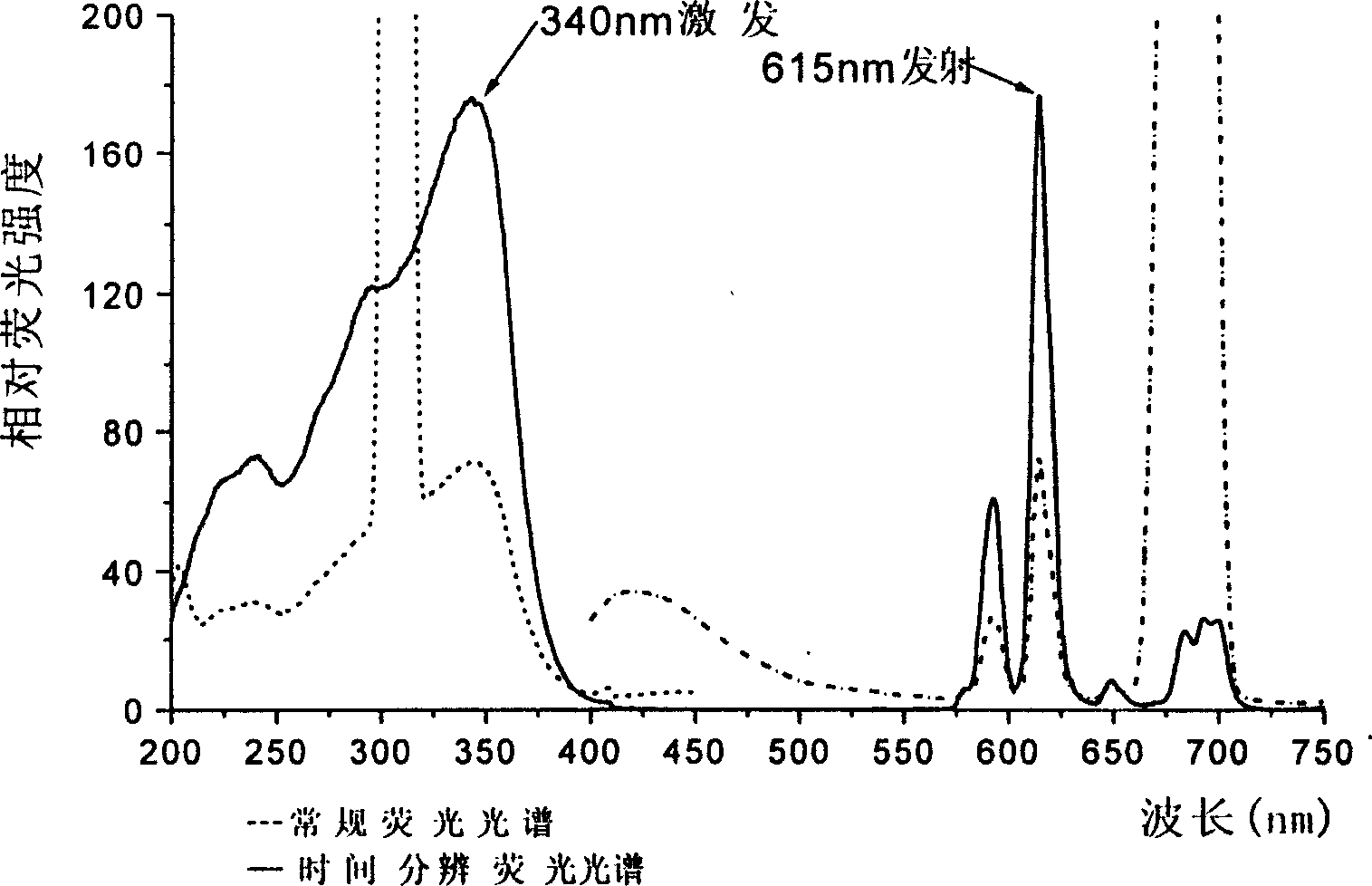
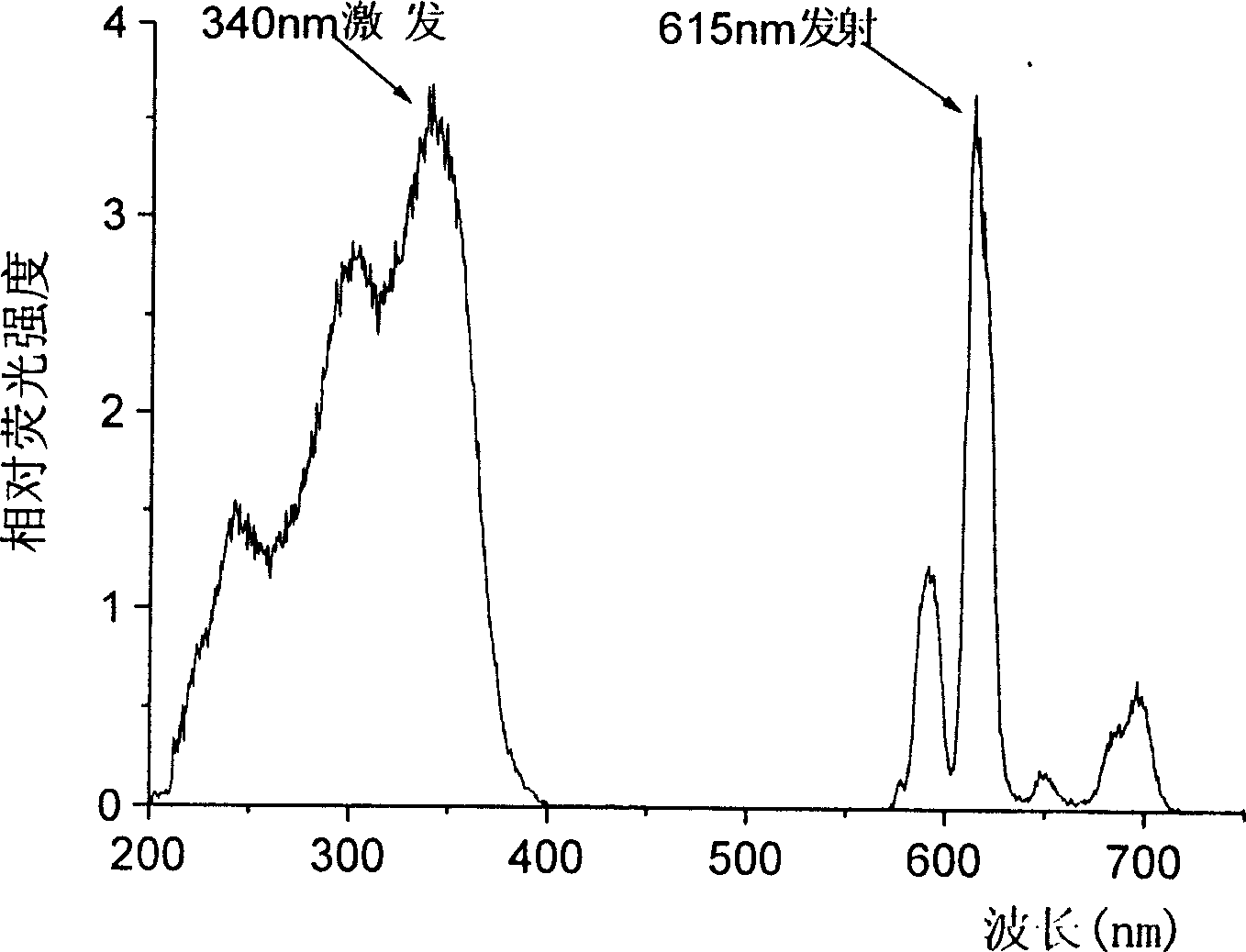
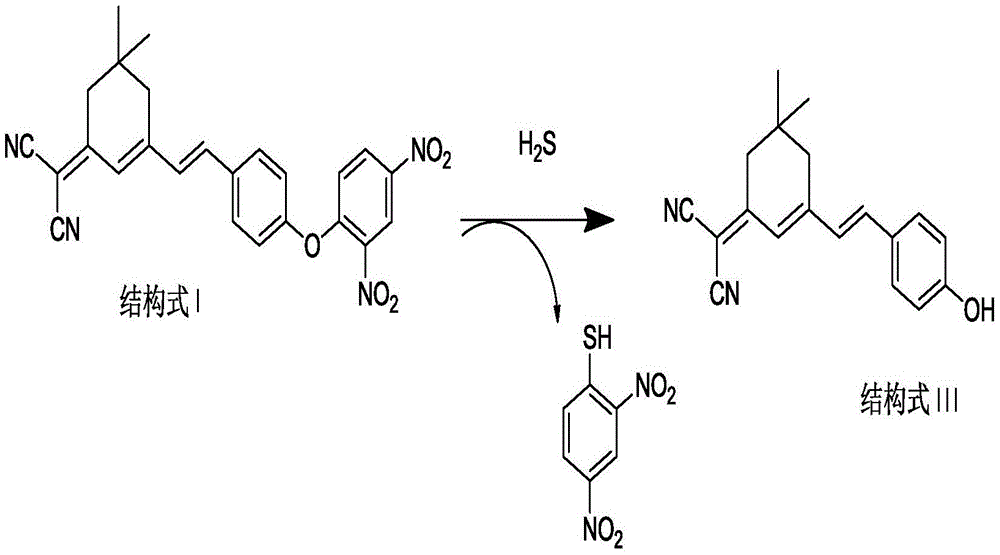
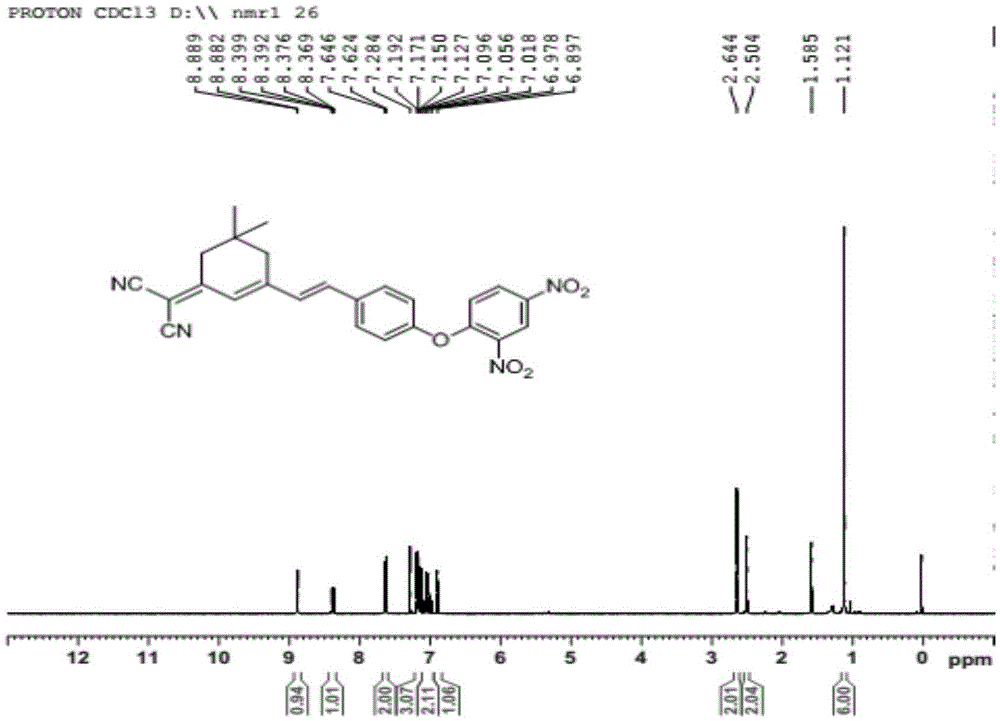
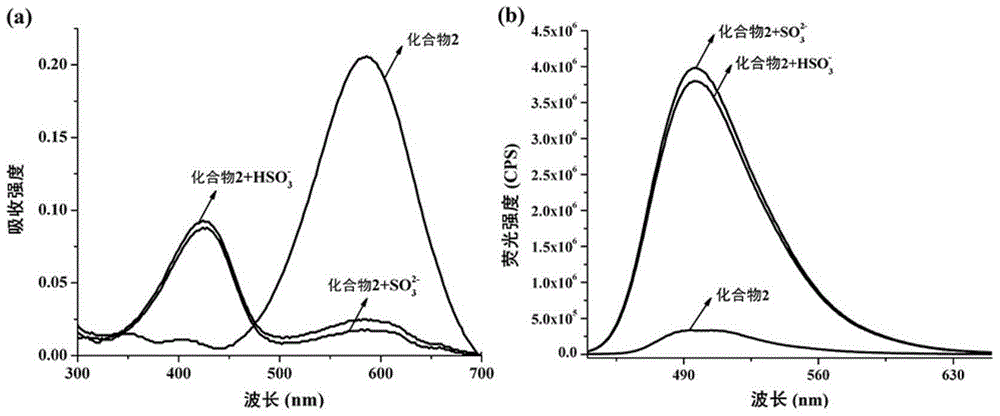
The invention discloses an isophorone fluorescence probe, and a preparation method and an application thereof. The structure of the fluorescence probe is represented by formula I. The preparation method comprises the following steps: adding isophorone, malononitrile and pyridine to a reaction bottle, carrying out heating refluxing under the protection of an inert gas for 18-24h, purifying, adding the above obtained pure product, p-hydroxybenzaldehyde and pyridine to the reaction bottle, carrying out heating refluxing under the protection of the inert gas for 4-18h, purifying, adding the obtained pure product, 2,4-dinitrofluorobenzene and triethylamine to the reaction bottle, stirring above materials for 8-36h, and purifying the obtained mixture to obtain the fluorescence probe. The fluorescence probe has large Stokes shift (182nm), long fluorescence emission wavelength (592nm), high sensitivity and good selectivity, can specifically detect hydrogen sulfide in organisms, environment and foods, has good biomembrane permeability and low cytotoxicity, and also has the advantages of simple synthesis route, high yield and very large practical values.
Owner:XUZHOU MEDICAL COLLEGE
Heptamethine cyanine active fluorescent probe and preparation method and application thereof
InactiveCN108033907AImprove stabilityReduce background fluorescenceOrganic chemistryFluorescence/phosphorescenceChemical reactionStructural formula
The invention relates to a heptamethine cyanine active fluorescent probe and a preparation method and application thereof. The structural formula of the heptamethine cyanine active fluorescent probe is as shown in the specification, wherein X=II-IX; each of R1 and R2 is (CH2)mCH3, (CH2)nOH, (CH2CH2O)pCH3 and CH2C6H5; each of R3 and R4 is H, SO3H, SO3Na and SO3K; each of a, b, c, d, e, f and g is 2-8; each of n, m and p is 1-10. The heptamethine cyanine active fluorescent probe has the advantages that the heptamethine cyanine active fluorescent probe is based on near-infrared long-wave heptamethine cyanine dye, indoline is selected as the aroma parent nucleus to increase fluorescence intensity, and methenyl chain intermediate cyclohexene rigid bridging enhances stability; nitrogen derivatives with chemical reactivity sites are used to perform nucleophilic substitution on the meso-position of the heptamethine cyanine parent dye, and accordingly Stokes shift and active chemical groups areincreased greatly to facilitate the fluorescent labeling of various substances; the fluorescent probe is of a symmetrical structure, preparation and purification processes are simplified, and cost islowered favorably; the probe can be used as the fluorescent labeling probe of biological molecules such as high-sensitivity protein, sugar and DNA and nano carriers to perform cell or living-body horizontal fluorescence imaging, and the like.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Cascaded Raman Fiber Laser System Based on Filter Fiber
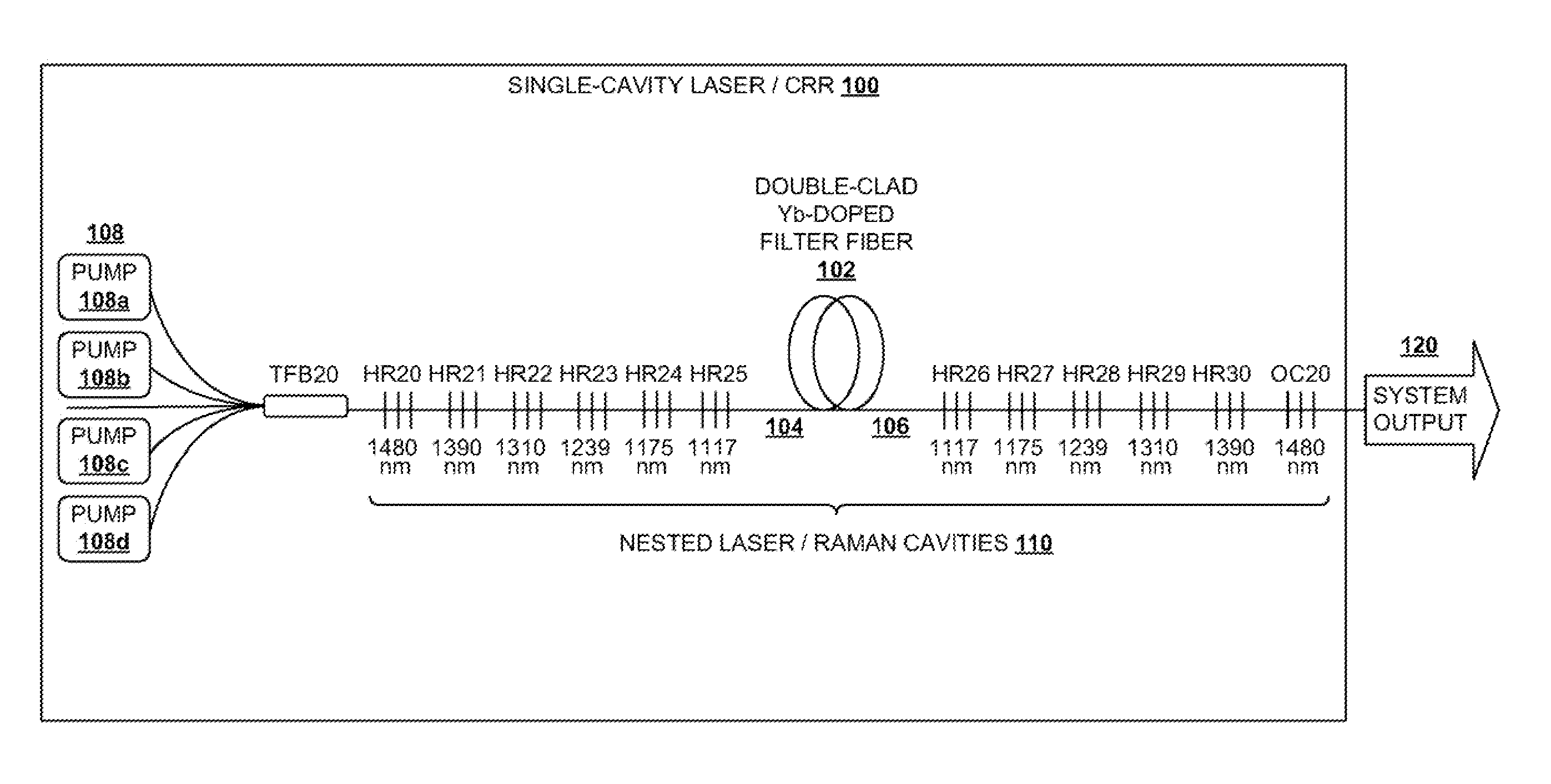
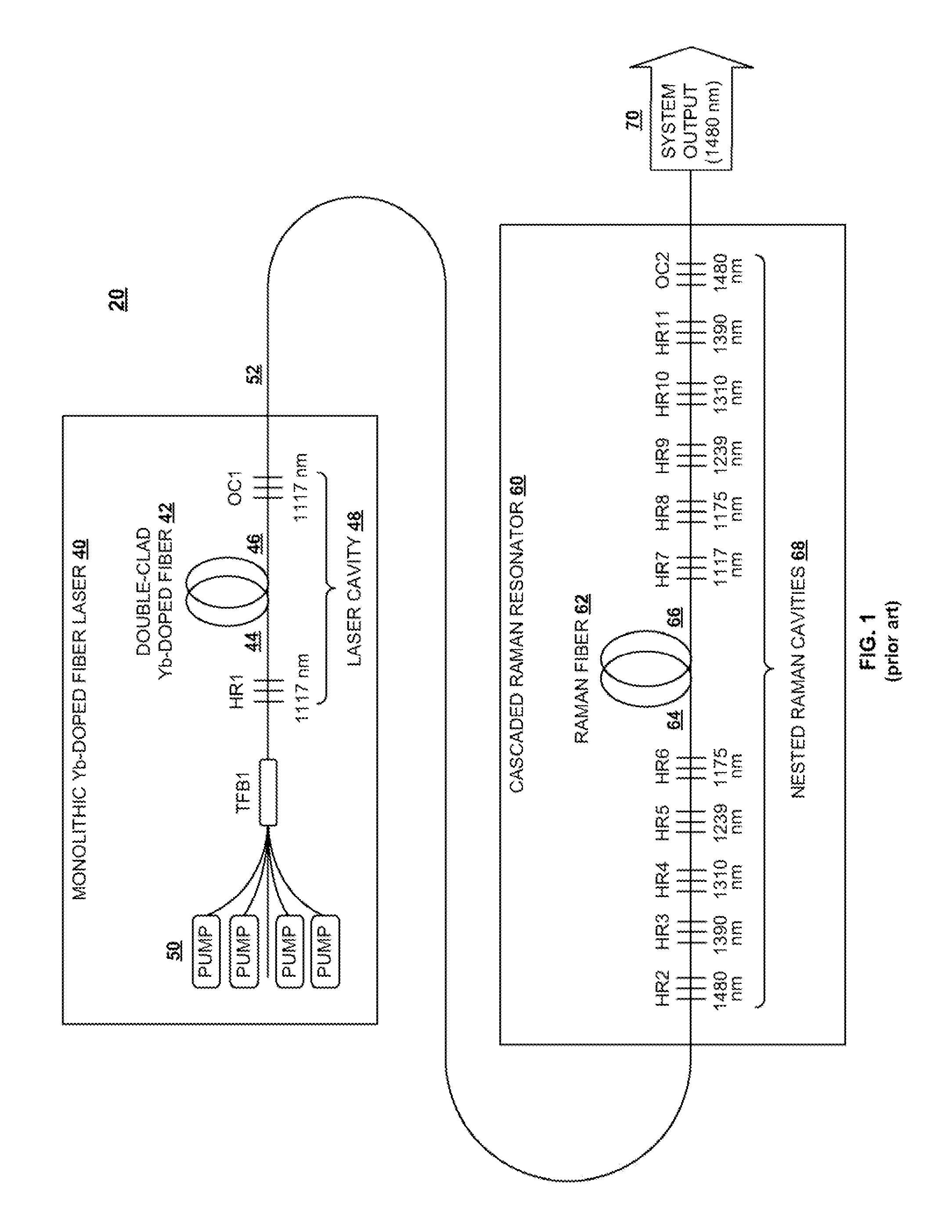
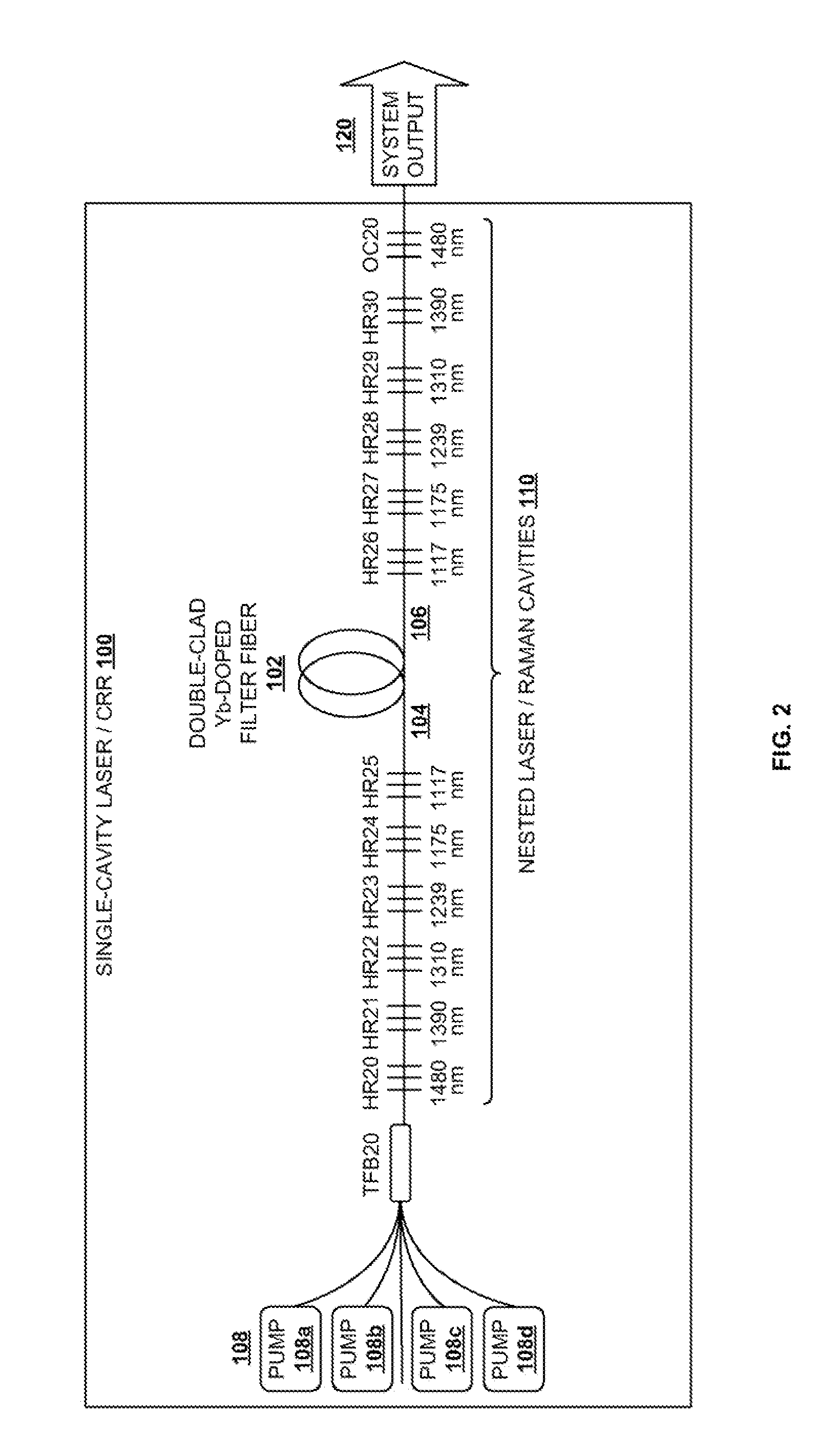
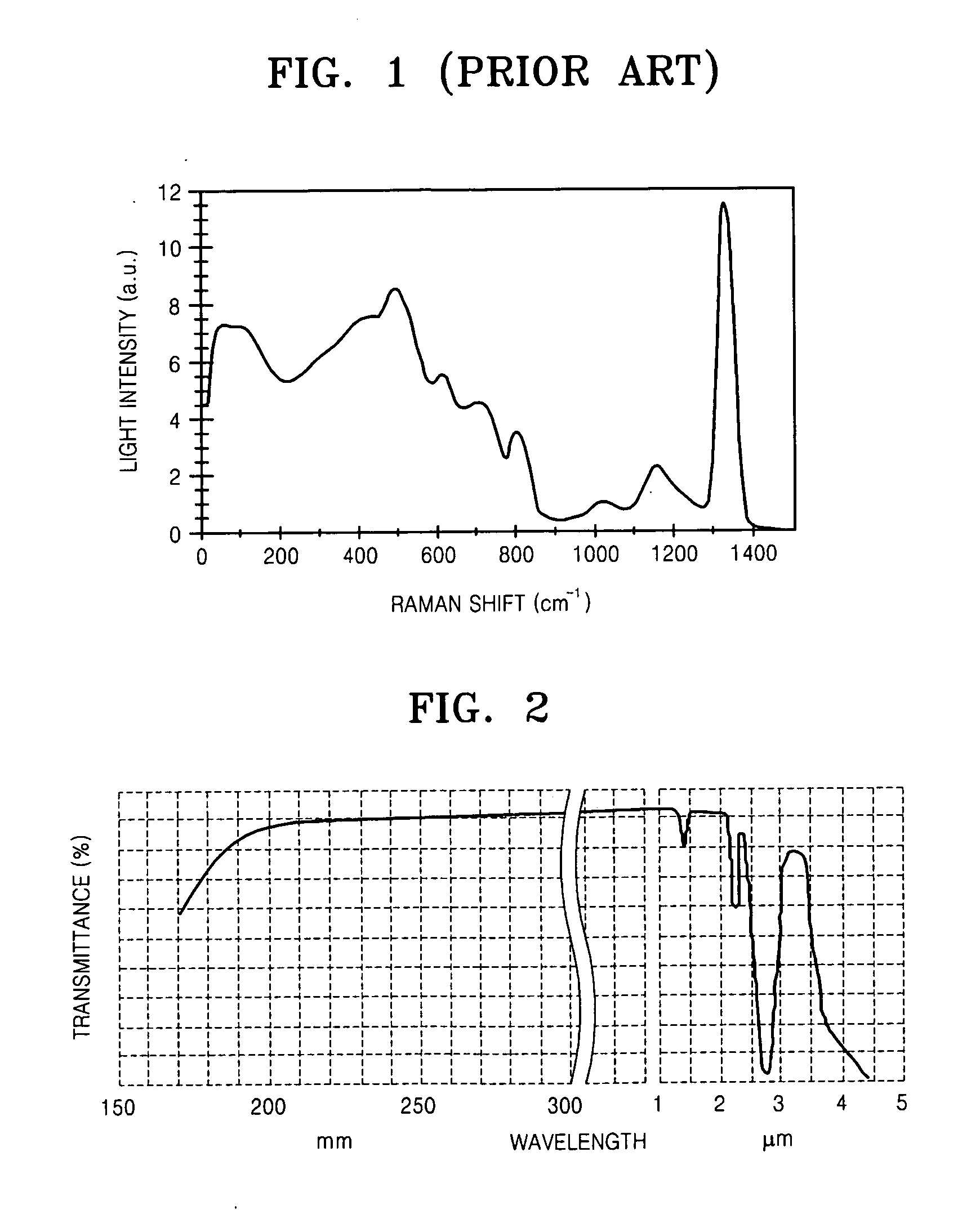
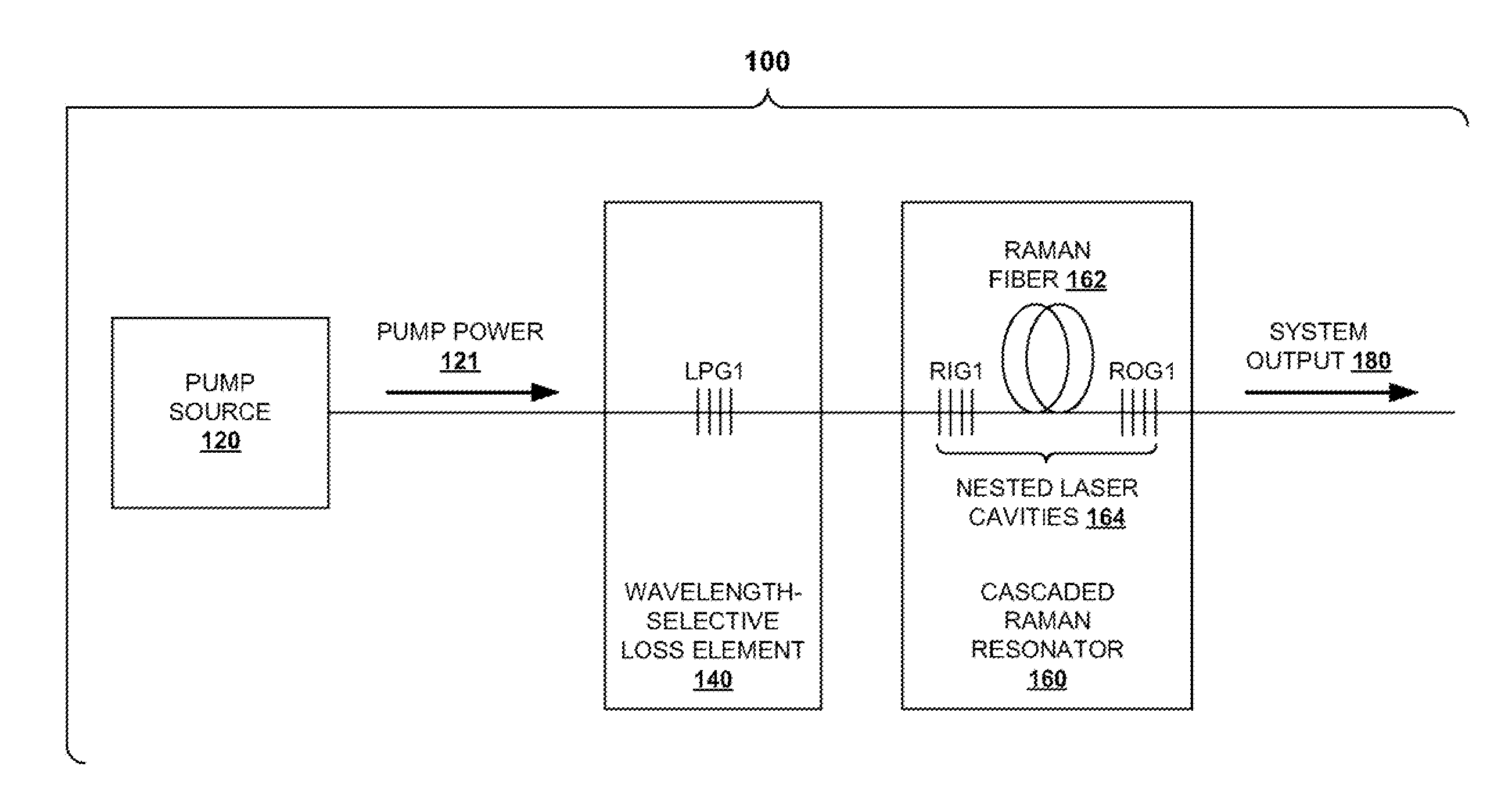
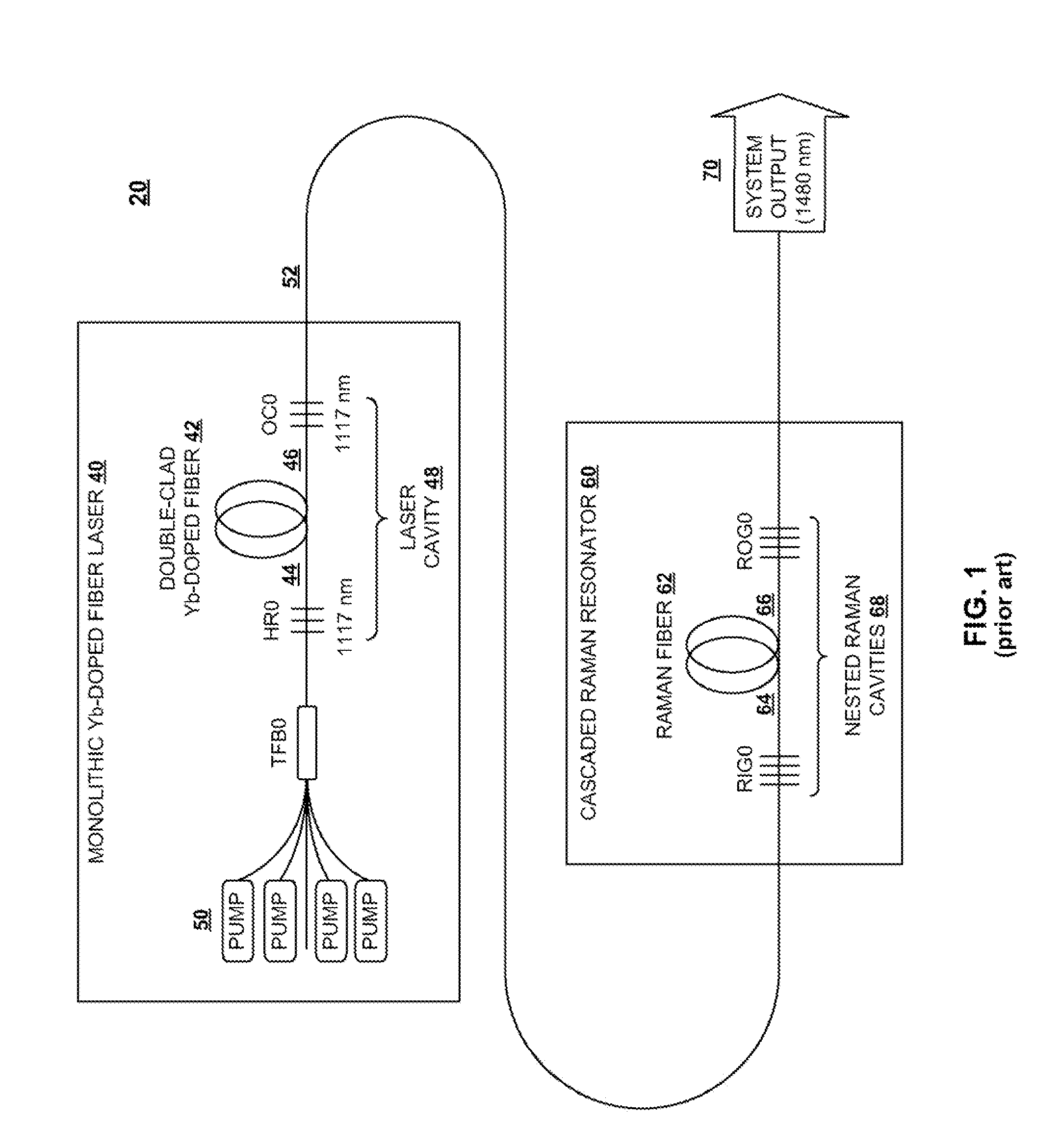
InactiveUS20100290106A1Reduce stepsImprove efficiencyLaser using scattering effectsFibre transmissionLength waveFiber laser
A light generation and amplification system includes a length of laser-active filter fiber having a refractive index profile that suppresses unwanted Stokes orders at wavelengths longer than a target wavelength and that has normal dispersion over its operating wavelength. A nested series of reflectors is provided at the fiber's input and output ends, and are configured to provide a nested series of Raman cavities, separated in wavelength by approximately the respective Stokes shifts. The first cavity in the series is a combined cavity that provides laser oscillation due to a combination of ionic gain and feedback at a selected first wavelength and that provides Raman gain to light at the first Stokes shift of the first wavelength when light at the first wavelength has an energy exceeding a Raman scattering threshold. The Raman cavities provide a stepwise transition between the first wavelength and the target wavelength.
Owner:OFS FITEL LLC
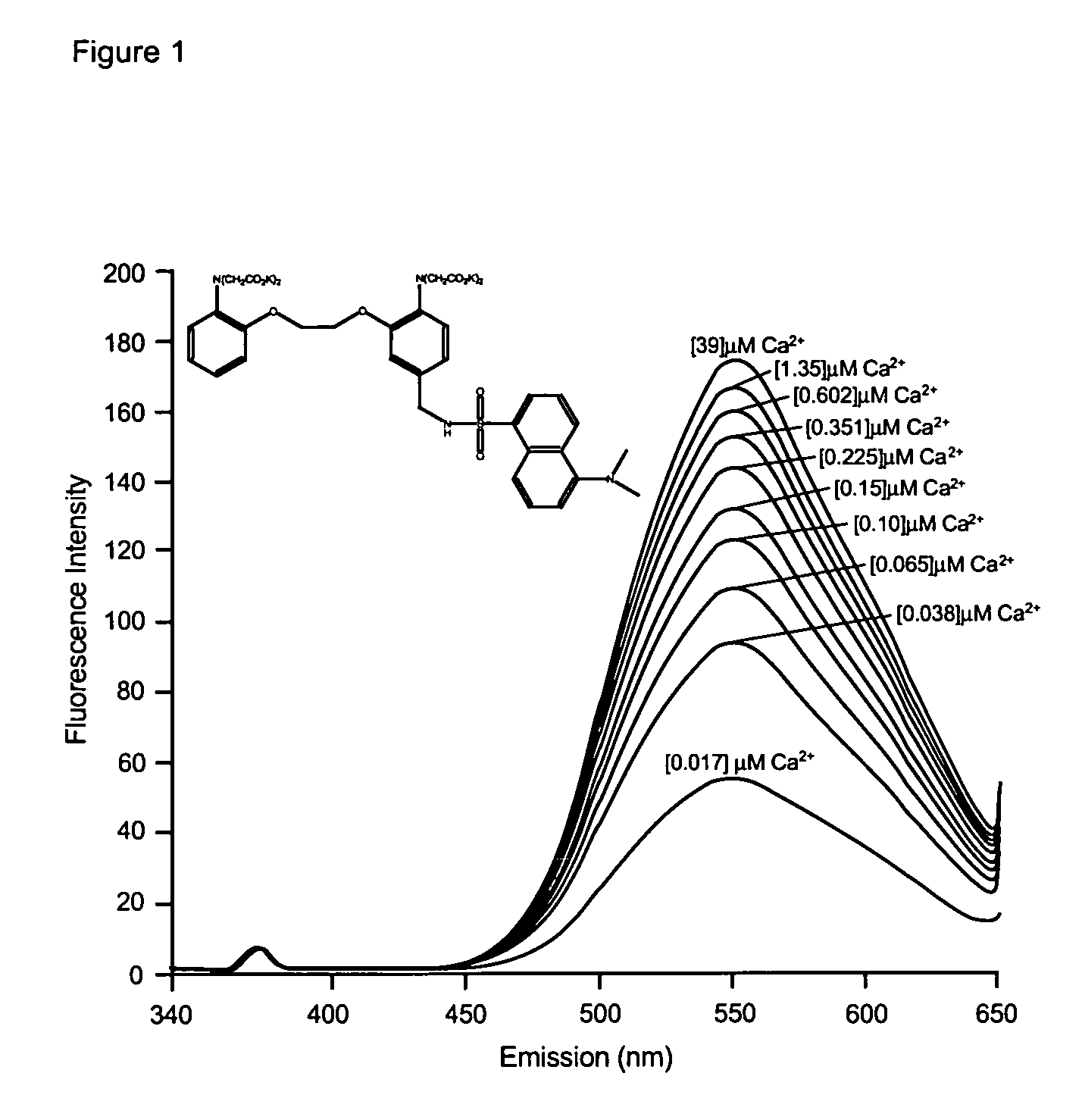
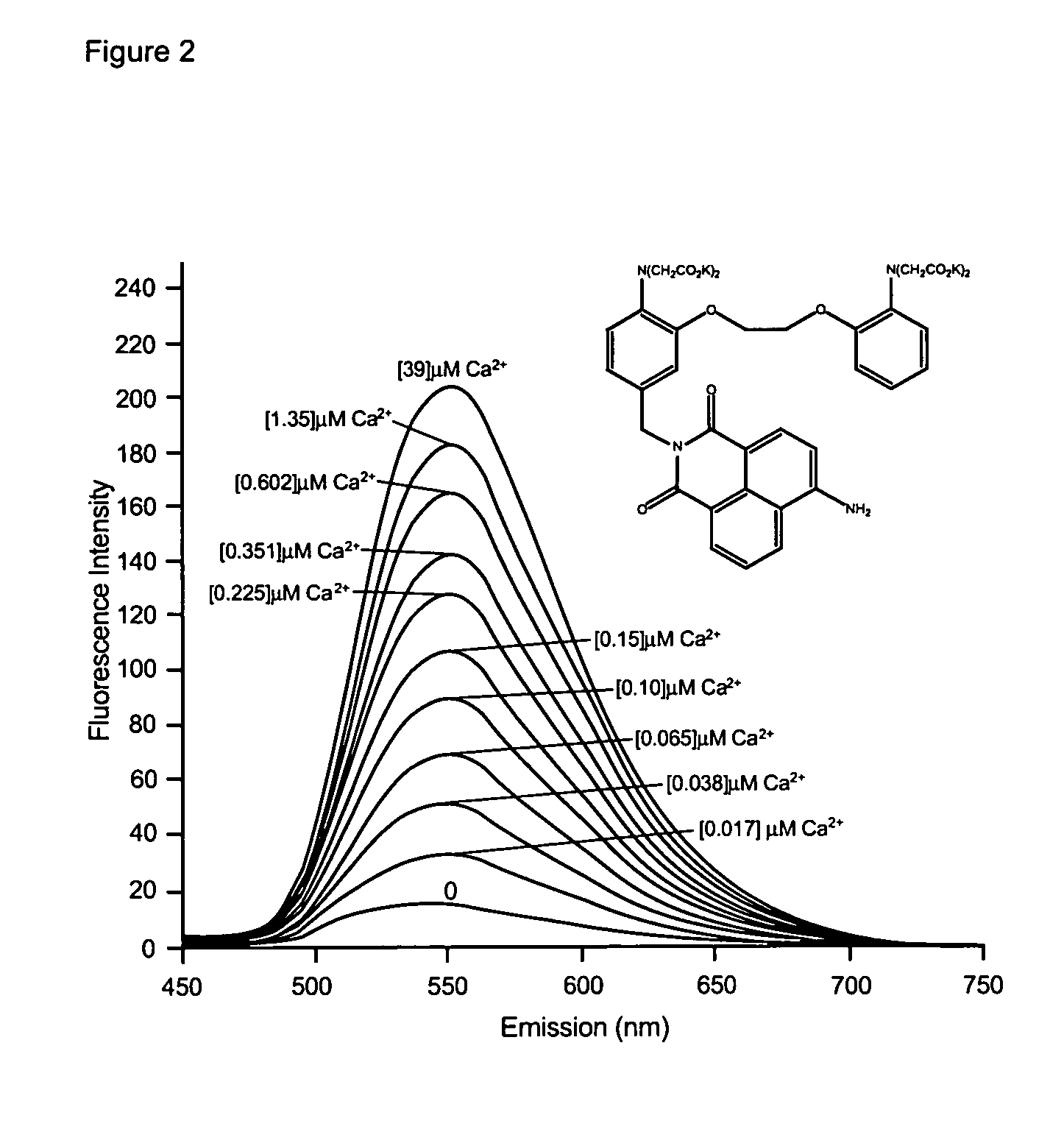
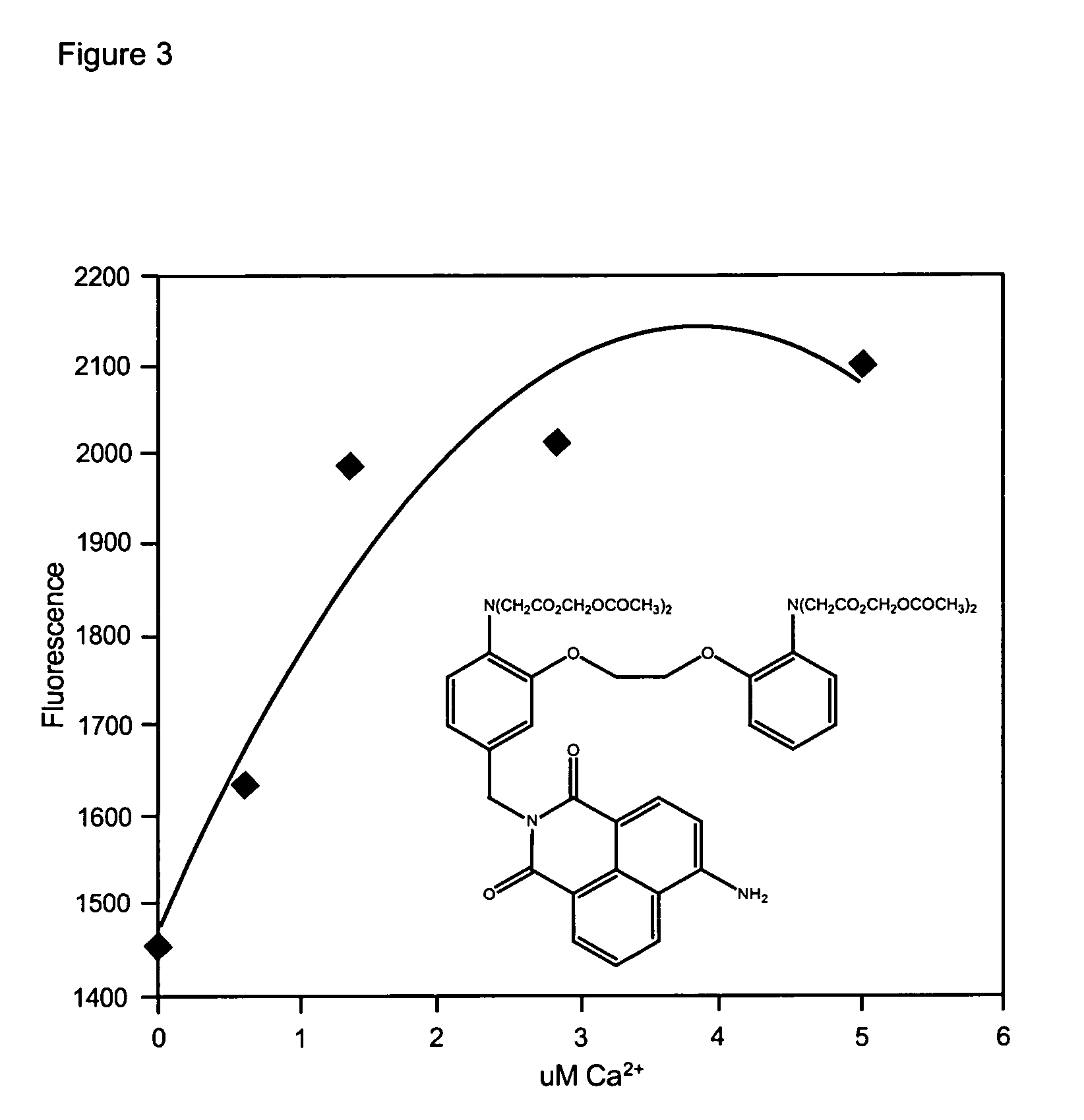
Fluorescent metal ion indicators with large stokes shift
InactiveUS20060024833A1Naphthalimide/phthalimide dyesAnalysis by subjecting material to chemical reactionPhotoinduced electron transferCompound (substance)
The present invention provides fluorogenic compounds for the detection of target metal ions wherein the compounds exhibit a Stokes shift greater than 50 nm and the detectable signal is modulated by photoinduced electron transfer (PET). The present compounds consist of three functional elements, the ion sensing moiety (chelating moiety), the reporter moiety (fluorophore or fluorescent protein) and spacer or linker between the sensing and reporter moieties of the present compound that allows for PET upon binding of a metal ion and excitation by an appropriate wavelength.
Owner:MOLECULAR PROBES
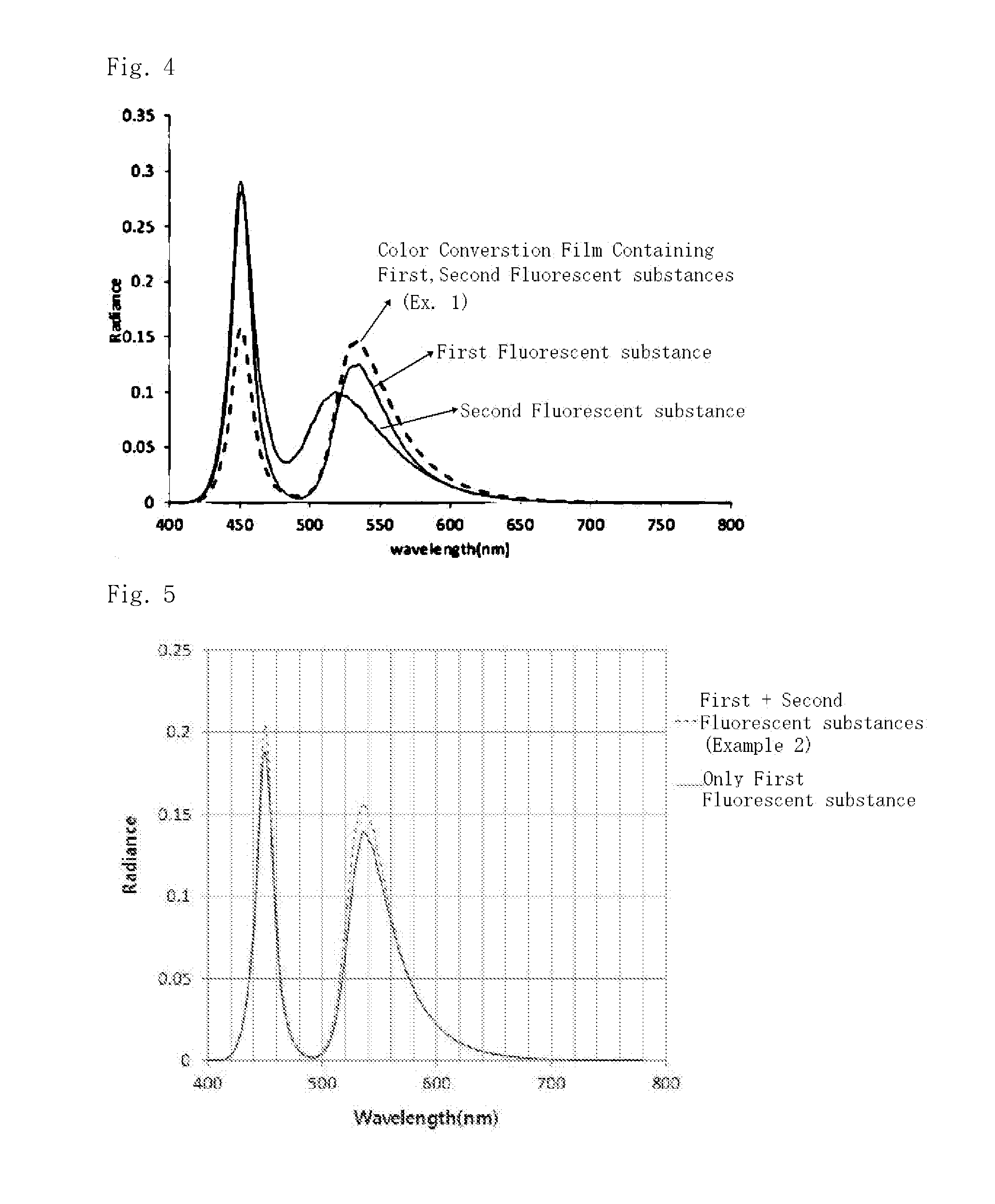
Color conversion film and back light unit and display apparatus comprising the same
ActiveUS20160223162A1Improved light emissionImprove color gamutLuminescent compositionsSpectral modifiersResin matrixLight emission
The invention described in the present specification relates to a color conversion film including a resin matrix; and an organic fluorescent substance, wherein the organic fluorescent substance includes a first fluorescent substance and a second fluorescent substance, and the first fluorescent substance has a light emission peak with FWHM of 60 nm or less and a Stokes shift of 30 nm or less when irradiating light having a light emission peak at 450 nm, a FWHM of 40 nm or less and monomodal light emission intensity distribution, a method for preparing the same, and a back light unit including the color conversion film.
Owner:LG CHEM LTD
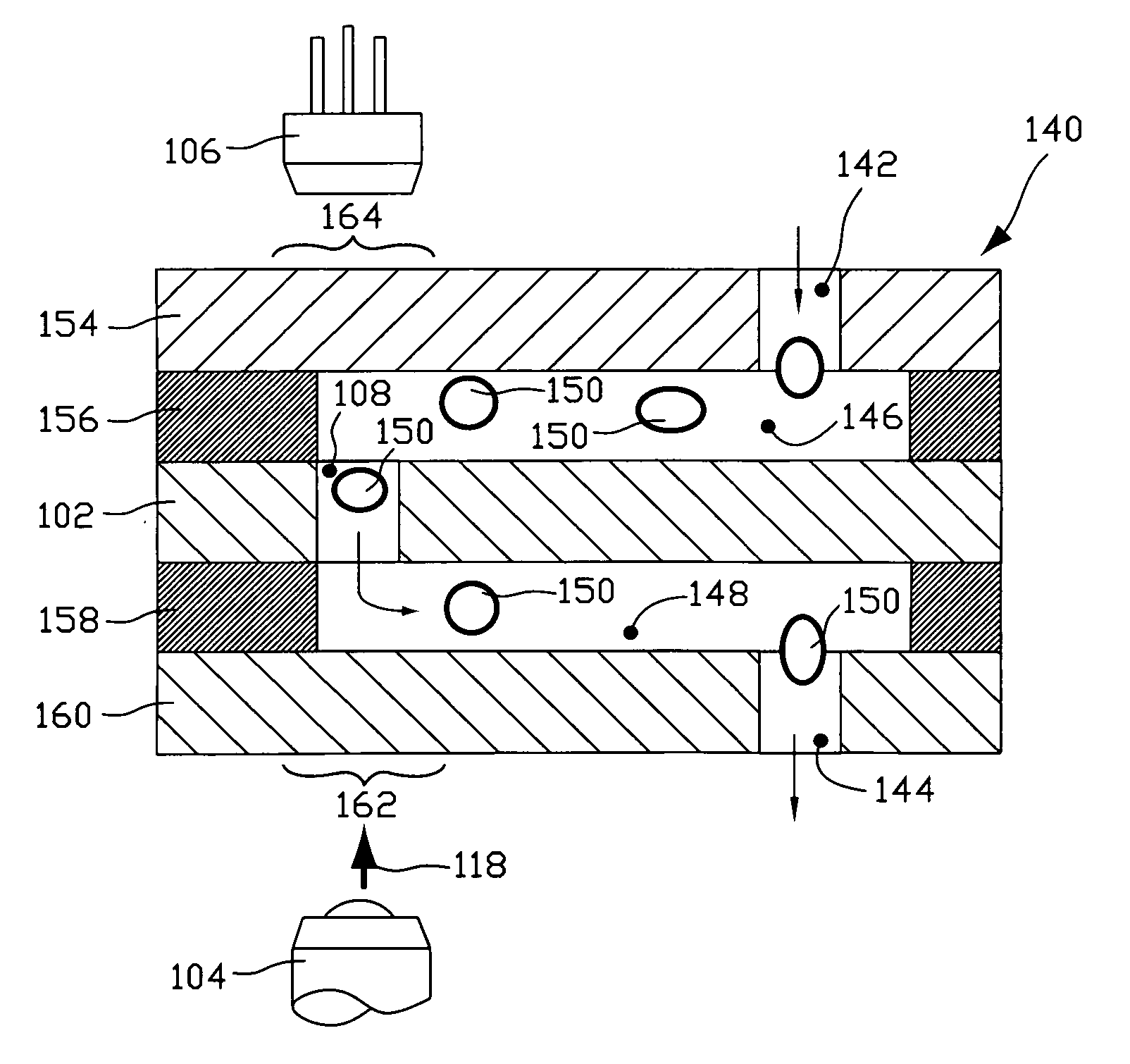
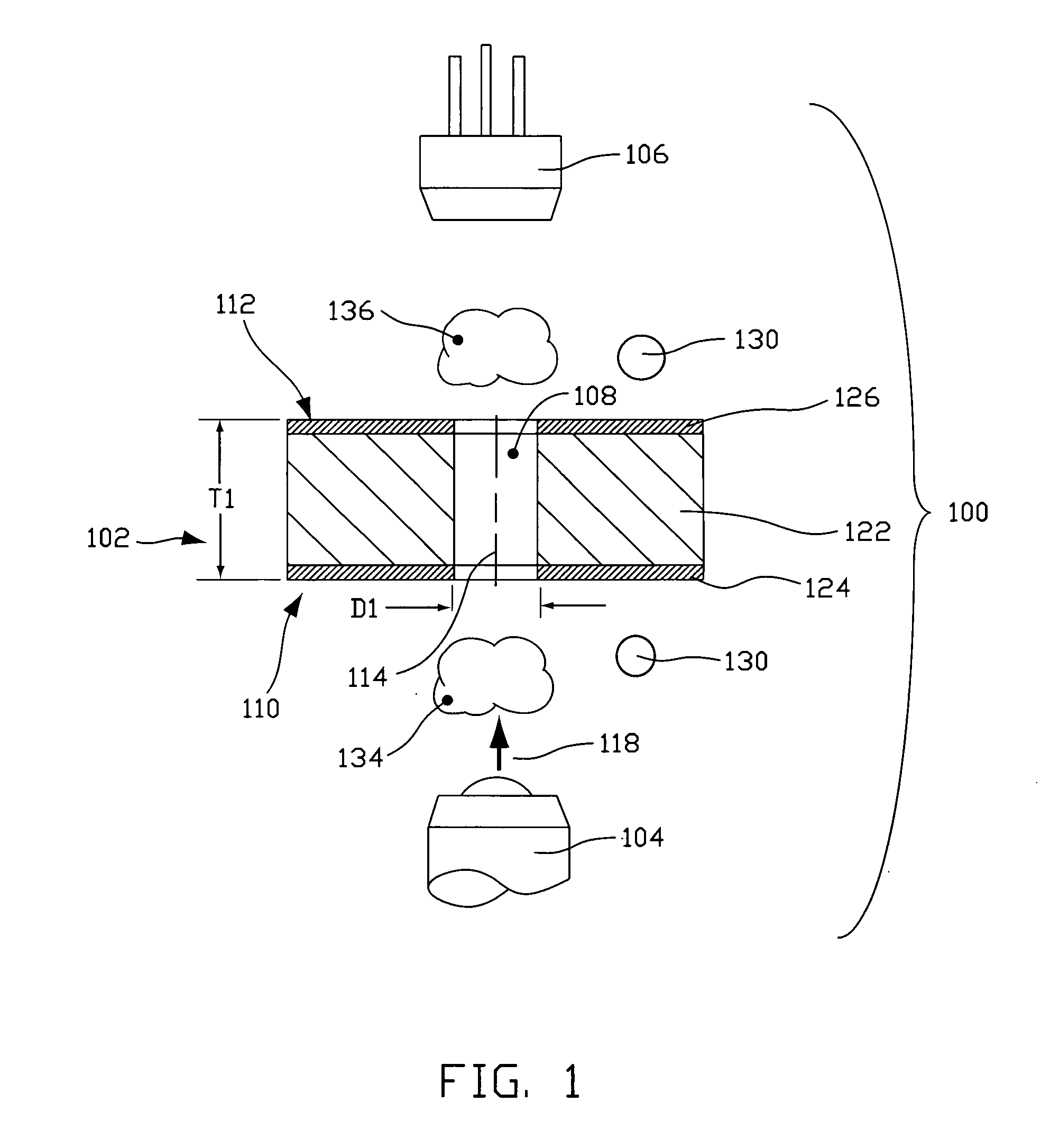
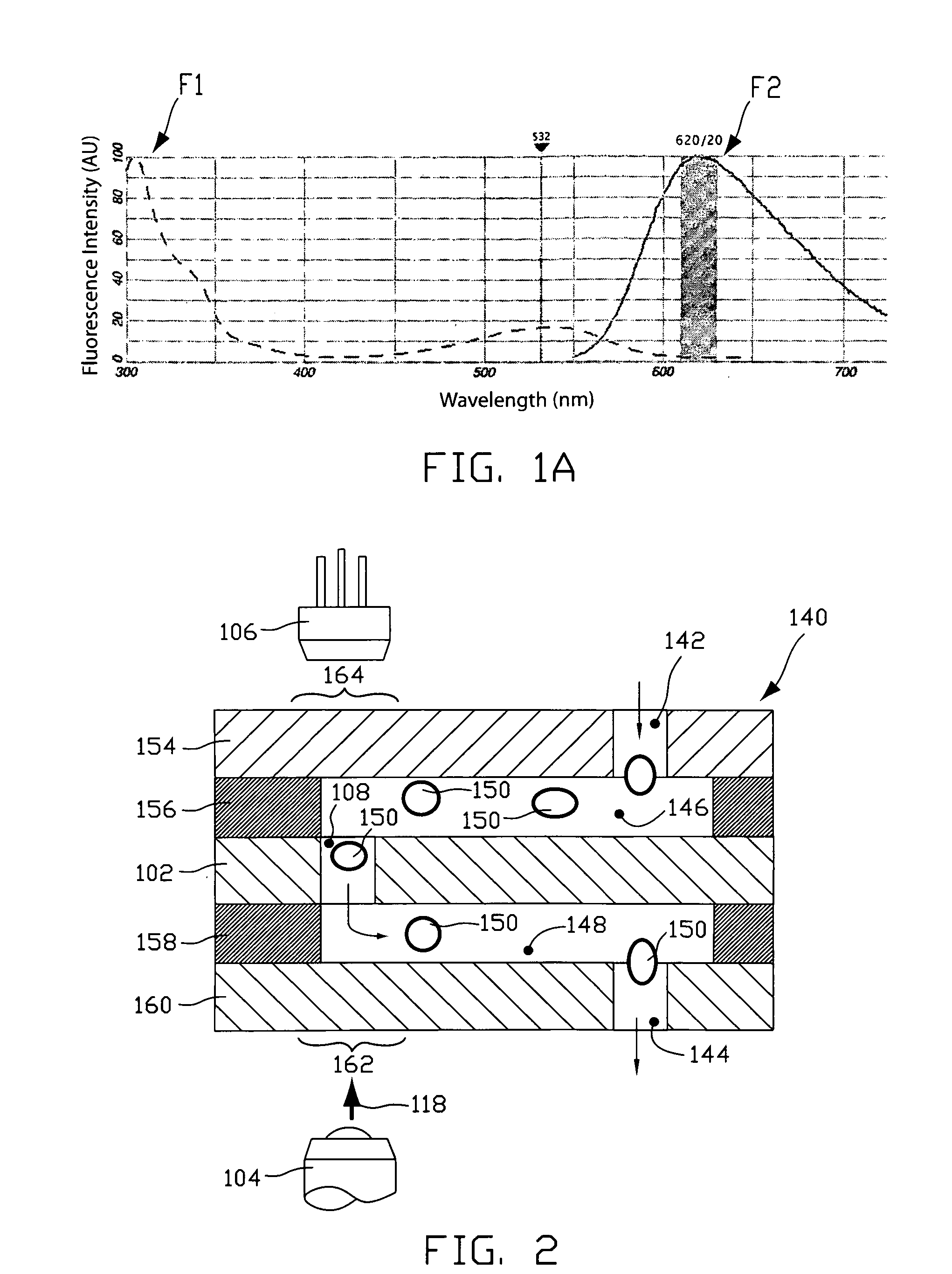
Fluorescence-based pipette instrument
An improved pipette tip (276) including an elongate body stretching between a proximal end (277) and a distal end. The body typically includes a plurality of thin film layers (e.g. 154, 156, 102, 158, 202) configured and arranged to provide a fluid path extending from the distal end toward the proximal end (277). The improved pipette tip (276) includes an interrogation zone in which to interrogate fluid flowing along the fluid path. One operable interrogation arrangement, generally (100), includes structure configured to permit detection of radiation resulting from a Stokes-shift. Optionally, a sensor component may include one or more electrode (e.g. 248, 250) that is disposed in the fluid path to contact fluid therein for electrically-based interrogation. A pipette tip (276) may be embodied to: count particles, verify sample integrity (e.g. freedom from bubbles), monitor sample flow rate, and confirm an inspired volume, among other uses.
Owner:ORFLO TECH LLC
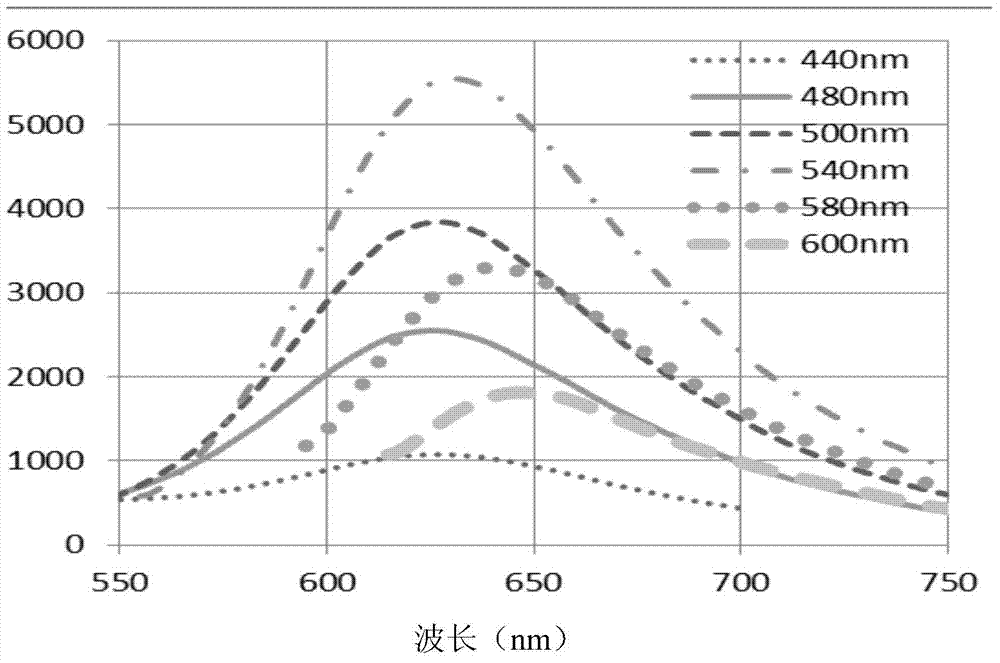
Mid-infrared raman fiber laser system
Provided is a mid-infrared Raman optical fiber laser system that oscillates in a 3.0 to 4.0 μm range using a high power laser in a near-infrared wavelength region and a silica optical fiber as a pump light source and a gain medium, respectively. The system includes a plurality of pairs of FBGs (Fiber Bragg Gratings) that are disposed at either end of an optical fiber inducing Raman scattering and obtains a lasing wavelength using a plurality of stokes shifts generated by energy decrease of about 1330 cm−1 during the Raman scattering; and a pump light source that is disposed at one end of FBG pair and induces the Raman scattering in the optical fiber.
Owner:ELECTRONICS & TELECOMM RES INST
Red-light emission fluorescent carbon dot with up and down conversion function and preparation method of red-light emission fluorescent carbon dot
ActiveCN104263366AHigh fluorescence quantum yieldHigh-resolutionLuminescent compositionsQuantum yieldOrganic compound
The invention discloses a red-light emission fluorescent carbon dot with an up and down conversion function and a preparation method of the red-light emission fluorescent carbon dot. The particle size of the carbon dot is 10-50nm; the position of a fluorescence emission peak generated under the irradiation of exciting lights with different wavelengths is 600-670nm; the fluorescence quantum yield is greater than 40%; and the preparation method comprises the following steps: dissolving a carbon precursor into a liquid organic compound to form a mixed reaction solution; heating and carrying out heat preservation to form a brownish red solution; and washing the solid matters separated from the brownish red solution, so as to form the carbon dot. The carbon dot disclosed by the invention has the advantages of up and down conversion and red-light emission, high fluorescence quantum yield and relatively large stokes shift, and has a wide application prospect in the fields such as fluorescent tag imaging, drug delivery, disease diagnosis, analysis detection and the like; and meanwhile, the preparation process is simple and rapid, convenient to operate, high in yield, free of complicated and expensive equipment, low in cost, and easily achieves large-scale production.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Compound with aggregation induced luminescence property and preparation method and application thereof
ActiveCN106565606AHigh photoactivation efficiencyImprove signal-to-noise ratioOrganic chemistryFluorescence/phosphorescenceCancer cellCytotoxicity
The invention discloses a compound with an aggregation induced luminescence property and a preparation method and application thereof in lipid droplet targeting light activating fluorescence imaging. The structure of the compound and the structure of an intermediate product of the compound are represented as the formula I and the formula II, and the formula I can be converted to generate the formula II under the light condition. The compound in the formula I is prepared through the following steps that a compound in the formula III and a compound in the formula IV are dissolved in acetonitrile under the protection of nitrogen, light avoiding reaction is conducted, and the 1,2-dihydro-2-diphenyleneimine ketone compound in the formula I is generated. The novel compound with the aggregation induced luminescence property has the aggregation induced luminescence advantage and can effectively overcome the aggregation induced quenching defect of traditional fluorescent dye, and thus lipid droplet targeting specificity light activating fluorescence imaging in a living cell can be achieved; in addition, the compound has the advantages that the light activating efficiency and the signal-to-noise ratio are high, the cytotoxicity is small, the Stokes shift is large, and the capability of the compound to enter cells is high; and cancer cells and normal cells can be effectively distinguished.
Owner:SOUTH CHINA UNIV OF TECH
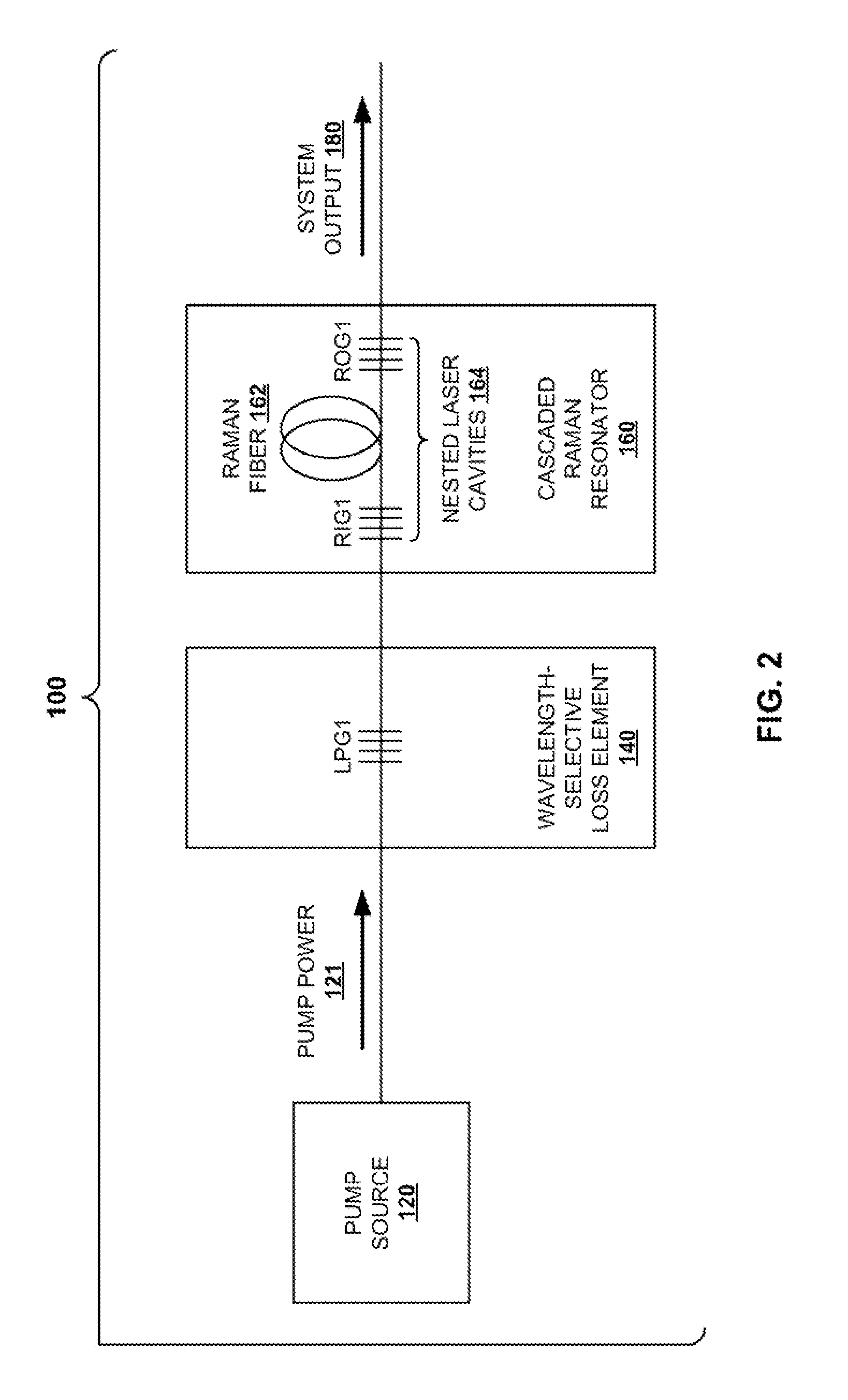
Systems and Techniques for Suppressing Backward Lasing in High-Power Cascaded Raman Fiber Lasers
InactiveUS20100284061A1Prevent buildupReduce lossLaser using scattering effectsActive medium shape and constructionLength waveResonator
In a light amplification system and technique, a pump source provides pump power at a source wavelength. The pump power is launched as an input into a cascaded Raman resonator. A wavelength-dependent loss element is connected such that it precedes the cascaded Raman resonator. The wavelength-dependent loss element is configured to transmit light power at the source wavelength with low loss, and to provide high loss at the first Stokes shift. The wavelength-dependent loss element prevents buildup of light power between the pump source and the cascaded Raman resonator, thereby preventing backward propagation of light power back into the pump source.
Owner:OFS FITEL LLC
Cu-doped Type-II core-shell structure white light quantum dot material and preparation method thereof
InactiveCN103952136AStrong chemical stabilityImprove antioxidant capacityEnergy efficient lightingLuminescent compositionsQuantum dot laserSpatial distribution
The invention discloses a Cu-doped Type-II core-shell structure white light quantum dot material and a preparation method thereof, which belong to the technical field of semiconductor nanometer material preparation. The core in a quantum dot structure is a Cu-doped CdS quantum dot, the core is coated with a ZnSe quantum wall to form a type-II core-shell structure which is then coated with a broad-band gap ZnS passivation protective layer, and the final quantum dot is a Cu:CdS / ZnSe / ZnS structure. The quantum dot material has continuous spectrum white light, large Stokes shift, a low self-absorption factor, high fluorescence quantum efficiency and a high color rendering index; the distribution of light colors in space is uniform; different chromaticity coordinates and color temperature can be adjusted by means of adjusting the size of the inner core CdS, the thickness of the water outer ZnSe and the doping concentration of Cu. The quantum dot material does not have light color distortion within a certain temperature range; besides, the quantum dot still has excellent light stability after being excited for a long time by blue light.
Owner:JILIN UNIV
Fluorescent Protein
InactiveUS20070292909A1Broad excitation and fluorescence spectrumSugar derivativesBacteriaFluorescent proteinAnalytical chemistry
An object of the present invention is to provide a red or orange fluorescent protein, which is characterized in that the difference (stokes shift) between an excitation peak value (wavelength of maximum absorption) and a fluorescence peak value (wavelength of maximum fluorescence) is greatened, so that the maximum fluorescence can be obtained by the maximum excitation. The present invention provides a novel fluorescent protein monomerized by introducing a mutation into a florescent protein derived from Fungia sp., and a novel chromoprotein and fluorescent protein derived from Montipora. sp.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD +1
Preparation and applications of novel fluorescent probe capable of specifically recognizing cysteine
InactiveCN106946801AThe synthetic route is simpleLow costOrganic chemistryFluorescence/phosphorescenceInterference resistanceFluorescence sensing
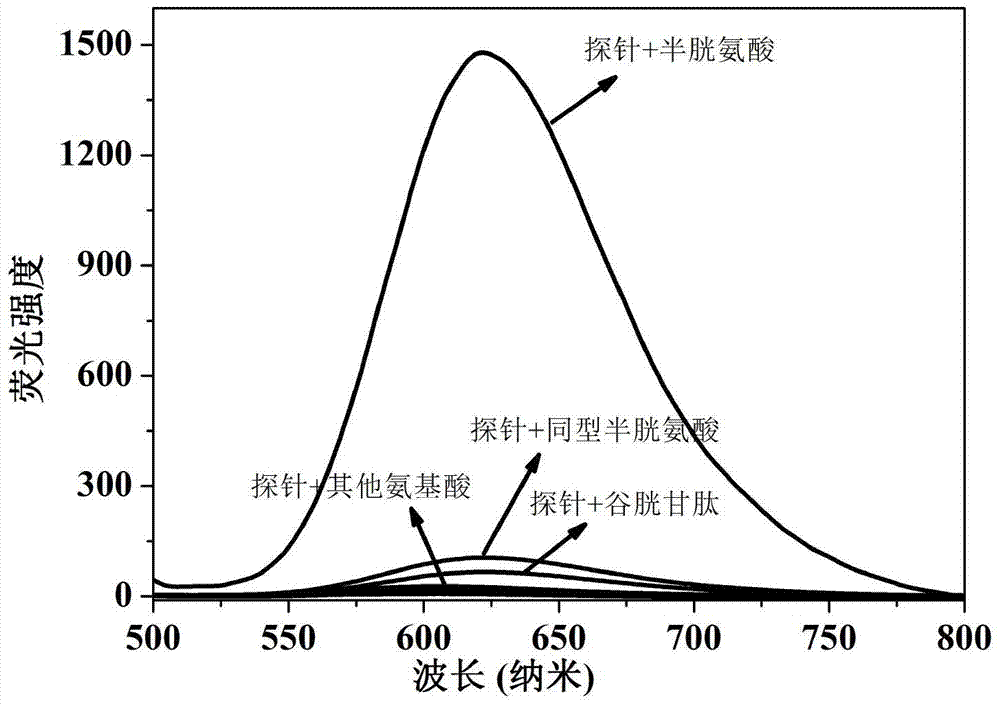
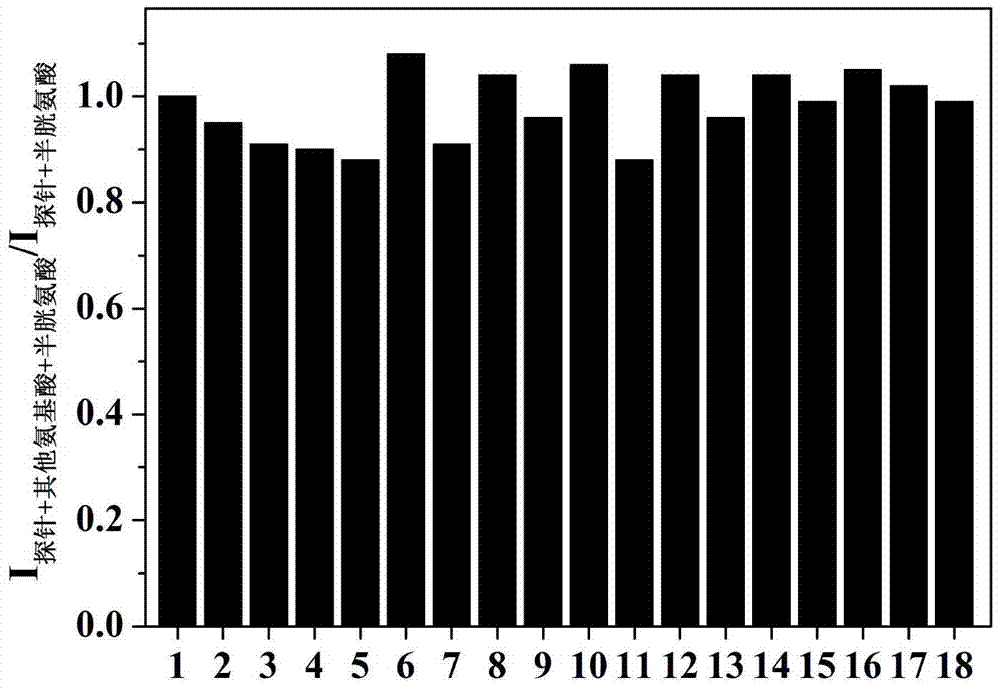
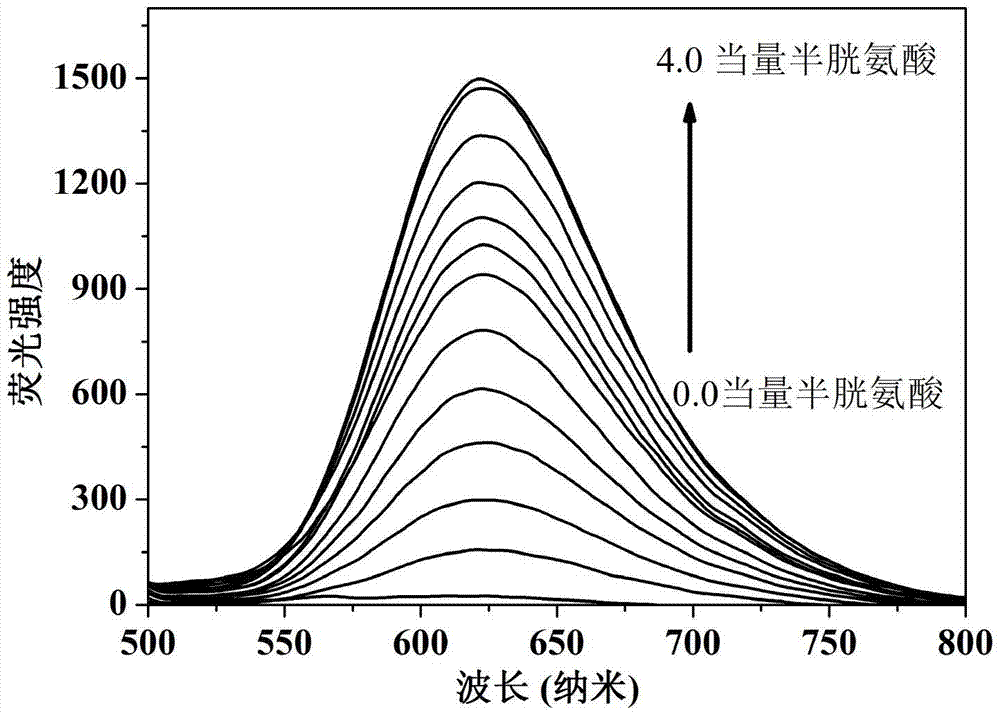
The present invention discloses a novel fluorescent probe capable of specifically recognizing cysteine, wherein the molecular structure formula is defined in the specification. According to the present invention, the fluorescent probe can distinguish the mercapto-containing cysteine from the mercapto-free amino acid region, can distinguish the cysteine from the homocystine and the glutathione having the similar structure, emits red light during cysteine detection, can reduce the background interference and the damage of light on tissue cells in biological applications, exhibits great Stokes shift, can reduce self-absorption so as to improve the sensitivity, can be used for the fluorescence sensing analysis of the cysteine in environments or biological samples, and has advantages of good selectivity to cysteine, high sensitivity, strong interference resistance, and good application prospect.
Owner:CENT SOUTH UNIV
Unsaturated dipyrromethene-boron borocarbons
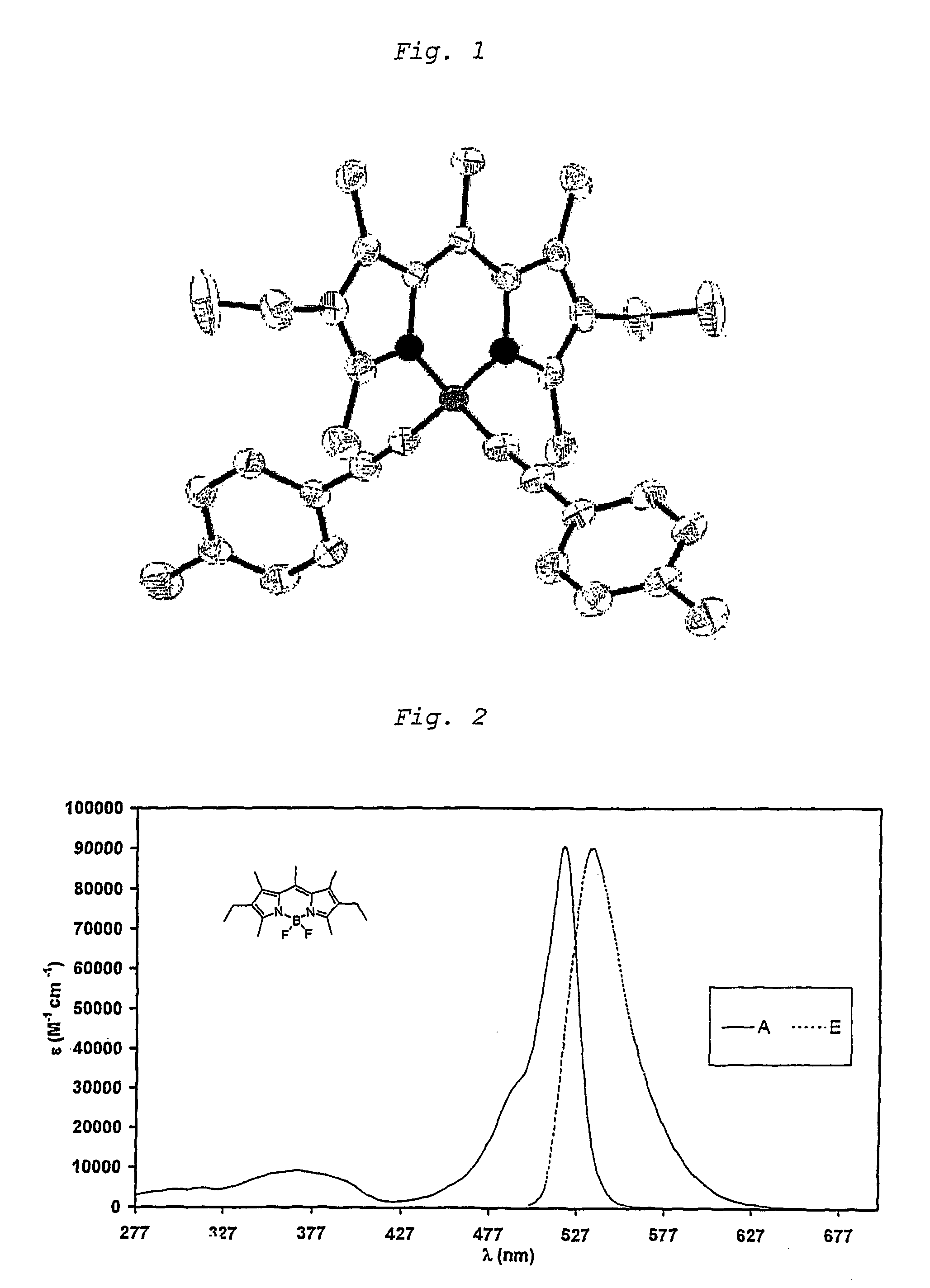
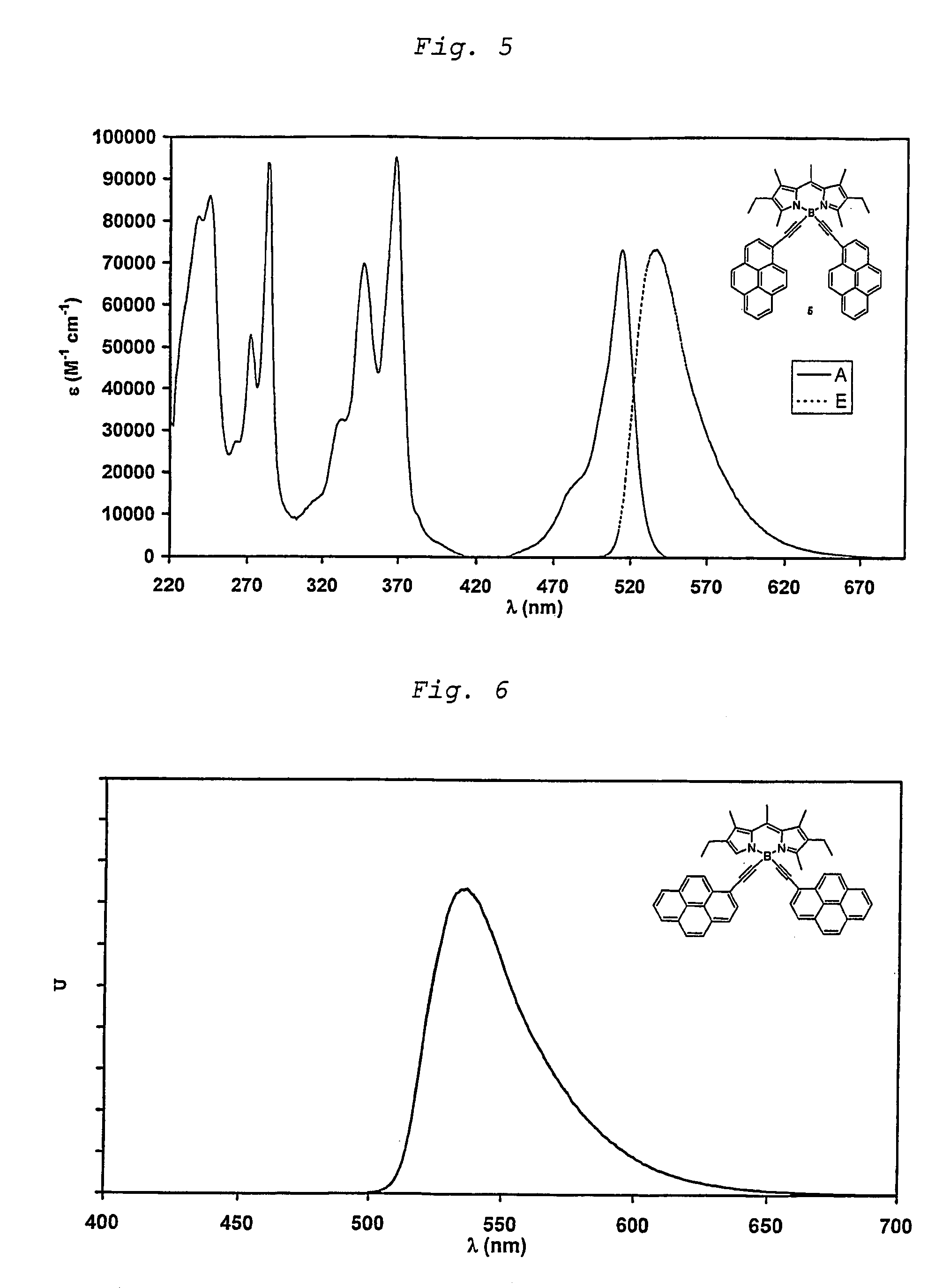
ActiveUS7897786B2Increases Stokes shiftHigher Stokes shiftSilicon organic compoundsChemiluminescene/bioluminescenceEnd-groupUltraviolet
The invention relates to unsaturated dipyrromethene-boron borocarbons of formula (I) and the use thereof for fluorescence or electroluminescent analysis. The fluorescent properties are provided by the central ring of six atoms comprising the —N—B—N— sequence, R1 to R7 permitting the modification of the compound properties (fluorescence emission wavelength, quantitative fluorescent yield), at least one of the substituents S1 and S2 has a chromophore end group which permits an excitation of the molecule at wavelengths close to those of the substituent chromophore. A preferably selected from the chromophore substituents with a wavelength close to the ultraviolet which significantly increases the Stokes displacement.
Owner:CENT NAT DE LA RECHERCHE SCI +1
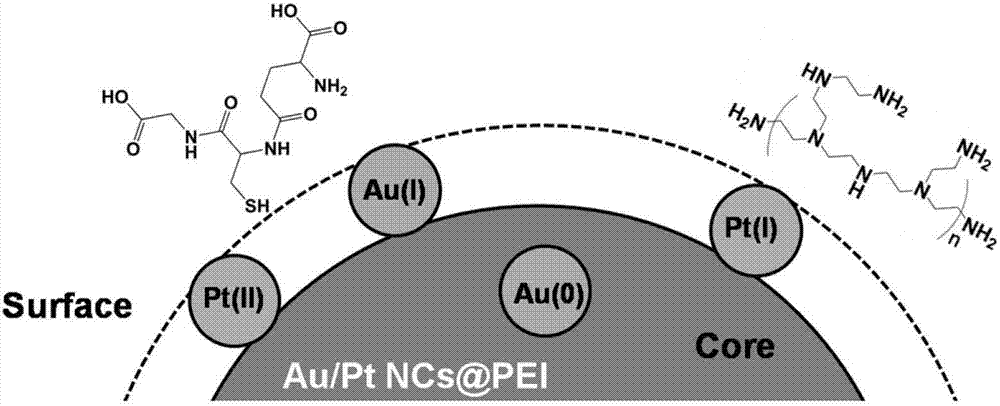
Gold/platinum bimetal nanocluster fluorescence probe based on polyethyleneimine protection, and application of fluorescence probe in aureomycin detection
InactiveCN107118758AProcess stabilityExtensiveFluorescence/phosphorescenceLuminescent compositionsPlatinum nanoclustersHydrogen
The invention discloses a gold / platinum bimetal nanocluster fluorescence probe based on polyethyleneimine protection, and an application of the fluorescence probe in aureomycin detection, and belongs to the technical field of fluorescence probes. A bimetal gold / platinum nanocluster has a great Stokes shift (about 150nm), can stably exist in a solution with extreme pH (potential of hydrogen) and high ion concentration, and releases stable fluorescence under long-time ultraviolet irradiation, so that a wide application of the nanocluster in the fields of medicines and biology is facilitated. In addition, the probe is used for detecting tetracycline antibiotics based on a mechanism of fluorescence quenching, and aureomycin can be distinguished from tetracycline by adding Al<3+> based on a mechanism of fluorescence enhancement, so that the purpose of specific aureomycin detection is achieved. The probe is used for detecting the aureomycin; a linear range is 0.5-30 micrometers; and a detection limit is 0.5 micrometers. In addition, real object detection is successfully performed on the aureomycin in milk by the fluorescence probe, and a good effect is obtained, so that the fluorescence probe can be used for detecting aureomycin residues in food.
Owner:JILIN UNIV
Benzylidene indandione compound and preparation thereof and application in specific imaging of lipid droplet
ActiveCN106674028AEasy accessEasy to manufactureOrganic chemistryOrganic compound preparationFluorochrome DyePhotochemistry
The invention belongs to the field of medical materials, and discloses a benzylidene indandione compound and a preparation thereof and an application in specific imaging of a lipid droplet. The structure of the benzylidene indandione compound is as shown in a formula I. The benzylidene indandione compound has the advantage of aggregation-induced emission, and the defects of aggregation-induced quenching of a traditional fluorescent dye can be effectively overcome, so that the specific fluorescence imaging of the lipid droplet in a living cell can be achieved; furthermore, the benzylidene indandione compound has a two-photon absorption cross-section which is high in living cell penetration rate, high in signal to noise ratio, small in cytotoxicity, large in stokes shift and large in near infrared region. The formula I is as shown in the specification.
Owner:SOUTH CHINA UNIV OF TECH
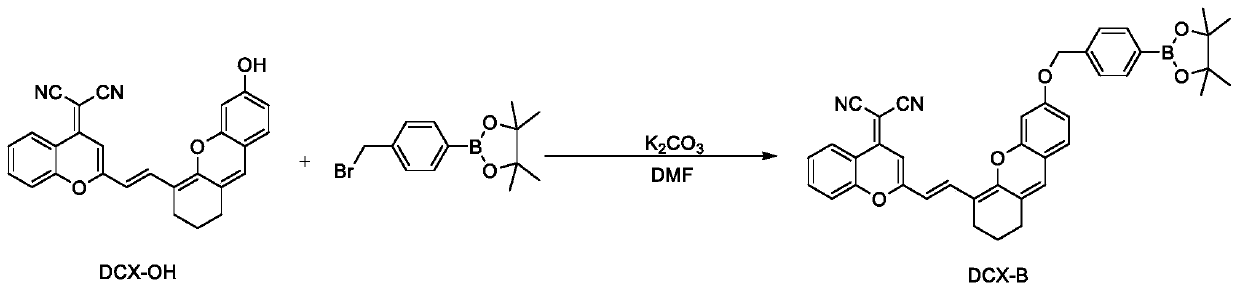
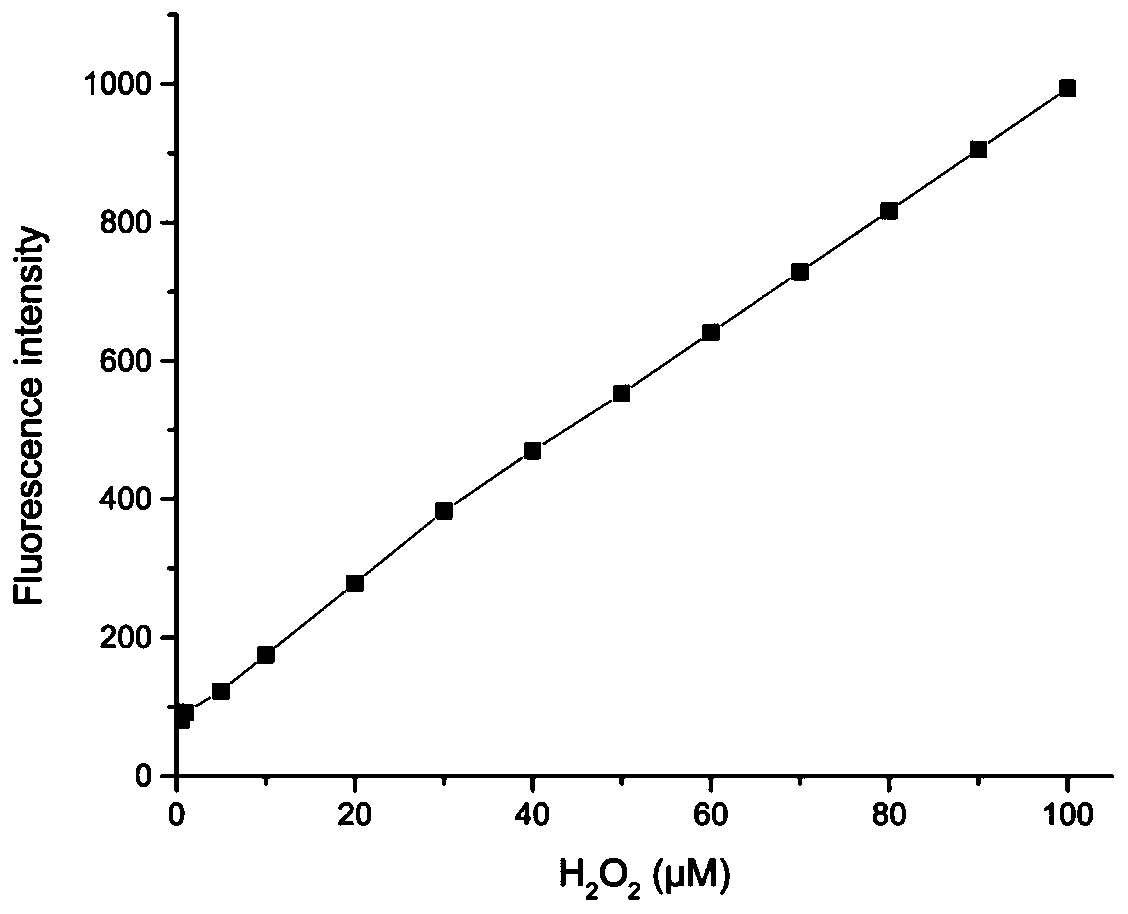
Preparation and application of hydrogen peroxide near-infrared fluorescent probe
ActiveCN110540837AGood spectral response performanceEnhanced near-infrared fluorescence intensityMethine/polymethine dyesGroup 3/13 element organic compoundsStructural formulaPotassium carbonate
The invention relates to preparation and application of a hydrogen peroxide (H2O2) near-infrared fluorescent probe. A structural formula of the probe is as shown in the specification. The invention provides a preparation method for synthesizing the fluorescent probe by using a benzopyranitrile-xanthene dye, potassium carbonate, 4-(bromomethyl)benzeneboronic acid pinacol ester and other raw materials. The fluorescent probe is a hydrogen peroxide fluorescent probe with near-infrared emission and large Stoke shift. Firstly, the probe shows high sensitivity to H2O2, and fluorescence intensity is increased by 24 times. Secondly, the fluorescent probe shows high selectivity to H2O2, and is not affected by other active oxygen, active nitrogen, various ions and biothiols. Moreover, the fluorescentprobe reacts quickly with H2O2, and a response time is within 30 min. In addition, the fluorescent probe is applied to detection of hydrogen peroxide content in living cells.
Owner:XIANGTAN UNIV
Near infrared emitting fluorescent carbon dot having up-conversion and down-conversion functions and preparation method thereof
ActiveCN107227152AHigh fluorescence quantum yieldHigh-resolutionNanoopticsNano-carbonInfraredQuantum yield
The invention discloses a near infrared emitting fluorescent carbon dot having up-conversion and down-conversion functions and a preparation method thereof. The particle diameter of the carbon dot is 1-50nm; and under the irradiation of exciting light with different wavelengths, the positions of produced fluorescence emission peaks are all in a range of being more than 670nm and being less than or equal to 750nm, and the fluorescence quantum yield is more than 15%. The preparation method comprises the following steps: dissolving a carbon precursor in a liquid organic compound to form a mixed reaction solution, performing heating to form a green-black solution, and washing the solid separated out from the green-black solution to obtain the carbon dot. According to the invention, the carbon dot has the effects of up-conversion and down-conversion near infrared emitting, high fluorescence quantum yield, low peak width at half height and large Stokes shift, and has wide application prospects in the fields of fluorescent label imaging, drug delivery, disease diagnosis, analysis and detection and the like; and meanwhile, the preparation process is simple, quick, convenient to operate, high in yield and low in cost, does not need complicated and expensive equipment, and can easily implement large-scale production.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Synthesis and application of novel fluorescence probe for detecting hydrogen peroxide
InactiveCN106749359AWide range of PH applicationDoes not affect detectionGroup 3/13 element organic compoundsFluorescence/phosphorescenceInterference resistanceWavelength
The invention discloses a fluorescence probe for detecting hydrogen peroxide, as well as a preparation method and application of the fluorescence probe. The chemical structural formula of the fluorescence probe is as shown in the specification. The raw materials of the probe disclosed by the invention are low in cost and readily available, and the probe is easy and convenient to synthesize. The probe does not emit fluorescence; after the probe reacts with hydrogen peroxide, the fluorescence intensity of the probe is gradually enhanced (enhanced to 77.8 times of the original fluorescence intensity to the maximum extent); the maximum emission wavelength is at the near infrared, and has the Stokes shifts which is 217 nm. The detection limit is low (6.6 nM), the sensitivity is high, and the detection range is wide; the fluorescence probe is extremely high in selectivity and interference resistance to identification of the hydrogen peroxide, can detect the hydrogen peroxide in vitro and in living cells, and has a wide application prospect in the field of chemical analysis detection.
Owner:CENT SOUTH UNIV
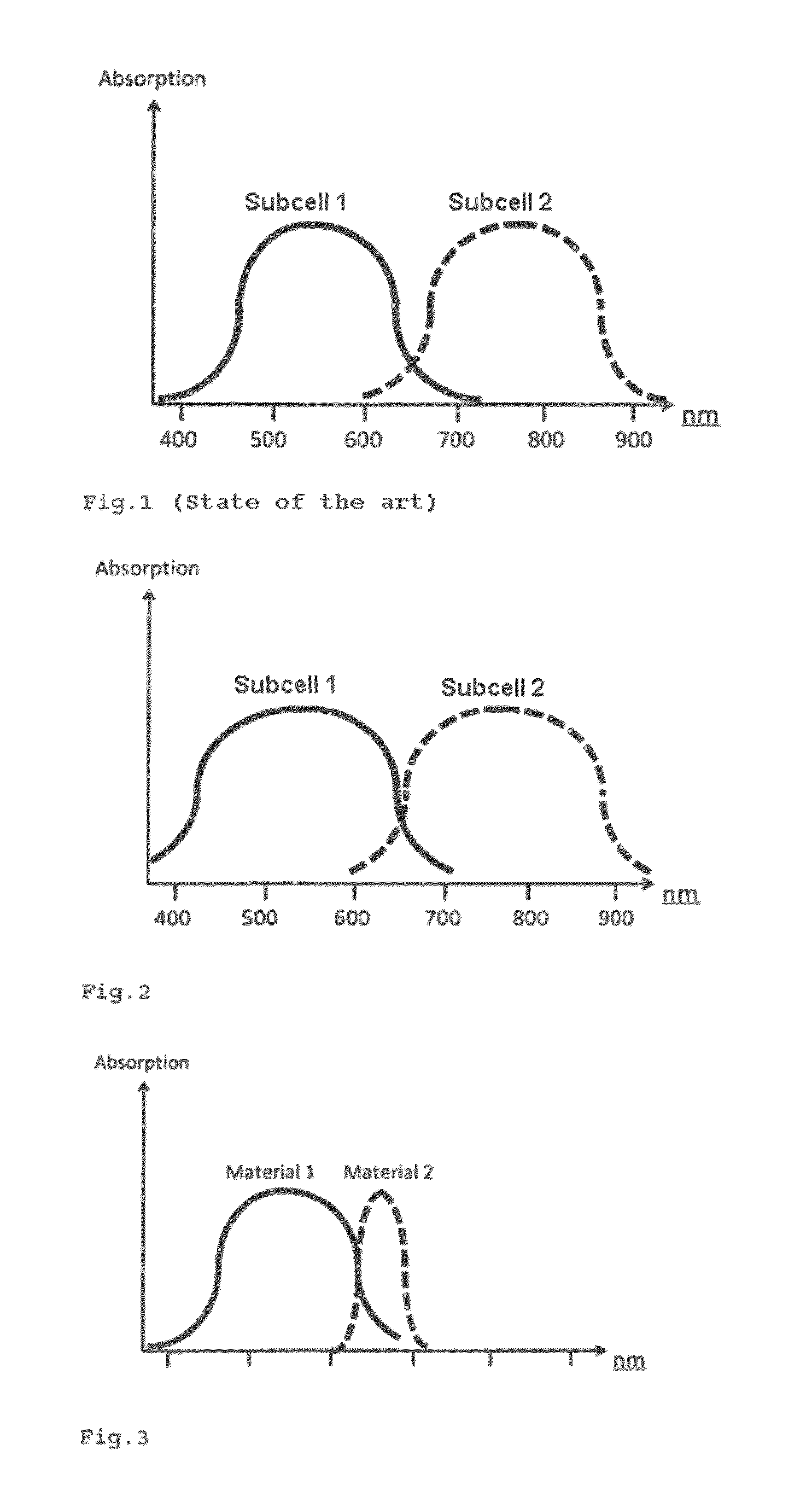
Photoactive Component Comprising Organic Layers
A photoactive component includes organic layers, including a single, tandem or multiple cell with two electrodes and, between the electrodes, a photoactive acceptor-donor layer system that includes at least three absorber materials. At least two absorber materials are donors or acceptors. One of the two absorber materials is configured as donors or acceptors absorbing at greater wavelengths than the other absorber material and one of the two absorber materials have a lower Stokes shift and / or a lower absorption width than the other absorber material.
Owner:HELIATEK GMBH
Substitution coumarin-pyridine derivative, preparing method thereof and application thereof
ActiveCN105218538ASmall side effectsLow maneuverabilityOrganic chemistryFluorescence/phosphorescenceQuantum yieldSolubility
The invention belongs to the field of organic chemistry, and particularly relates to a substitution coumarin-pyridine derivative, a preparing method thereof and an application thereof. The structural formula of the substitution coumarin-pyridine derivative is shown as the first formula (please see the specification). In addition, the invention provides the preparing method of the compound and the application of the compound to detection of endogenous SO2 in living cells. The substitution coumarin-pyridine derivative has the advantages of being good in water solubility, high in SO2 selectivity, low in detection limitation, small in toxicity, high in quantum yield, large in Stokes shifting, mild in overall reaction condition, low in cost, capable of achieving detection of the endogenous SO2 in the living cells and the like at the same time; the defects that as for an existing SO2 detection small-molecular fluorescent probe, organic solvents need to serve as cosolvents, and the quantum yield is low are overcome, and a new choice is provided for detecting the endogenous SO2 in the living cells.
Owner:SICHUAN UNIV
Luminescent converter for a phosphor-enhanced light source
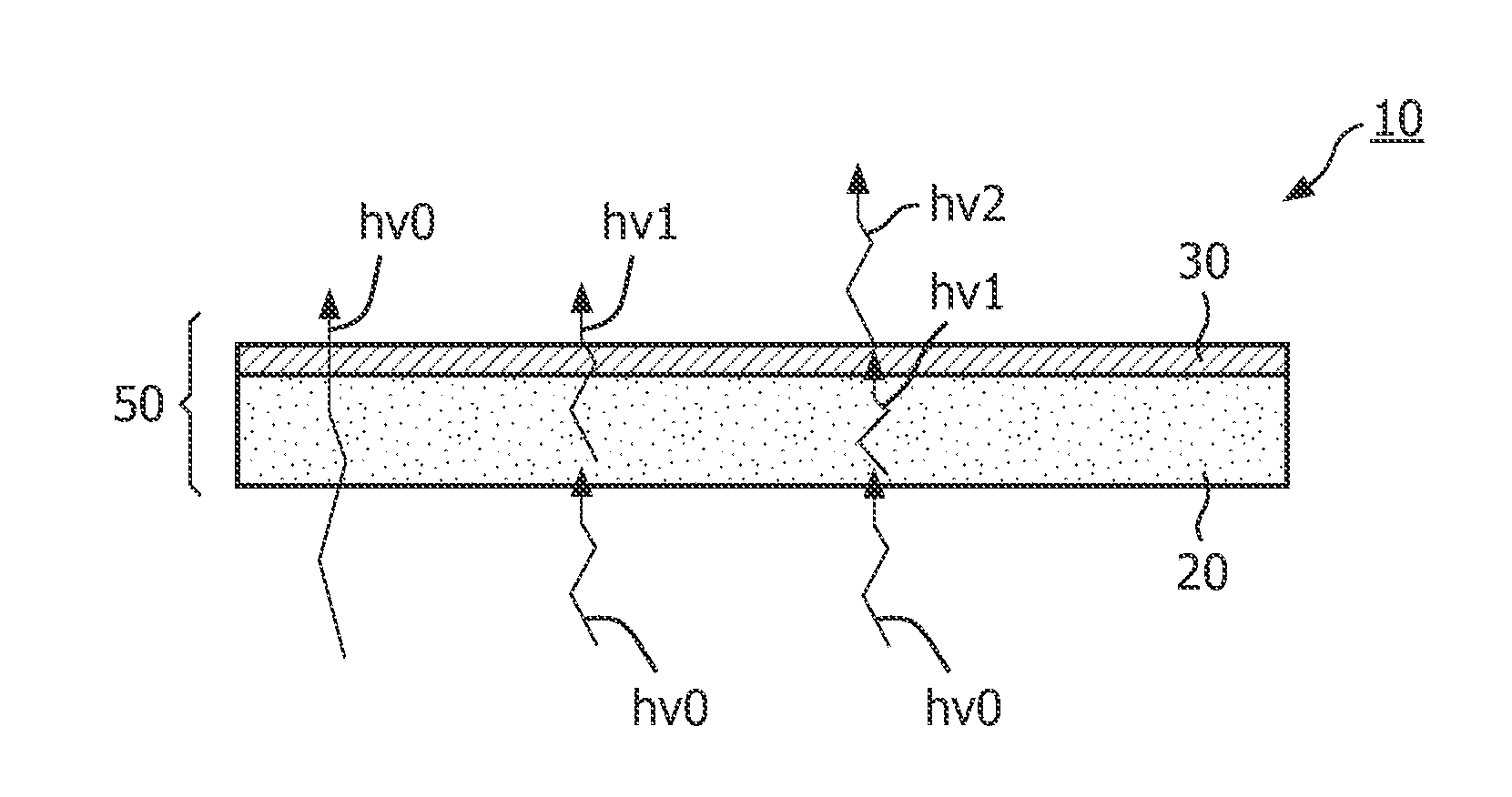
ActiveUS20120119639A1Improve efficiencyDischarge tube luminescnet screensLamp detailsPhosphorLength wave
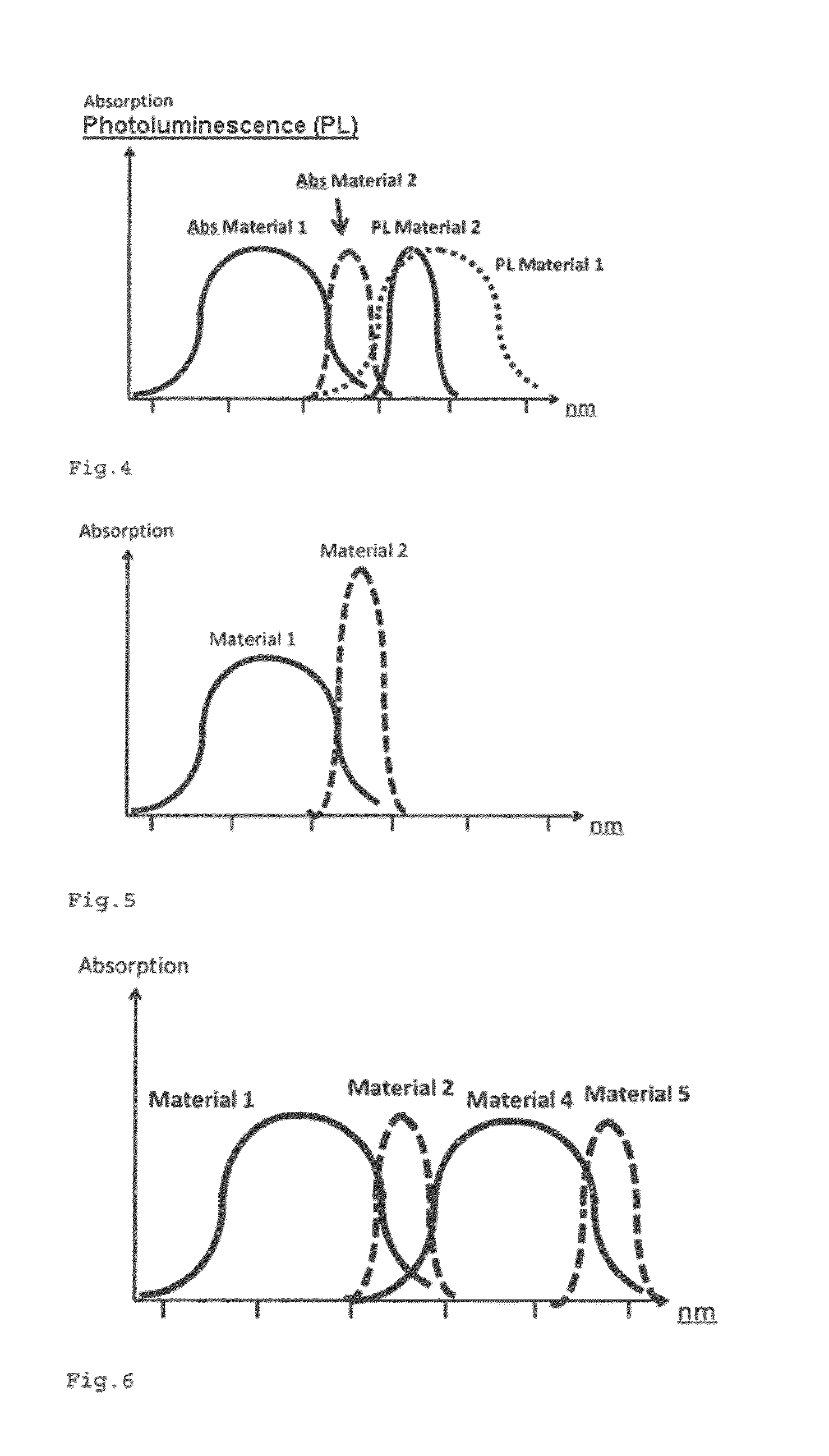
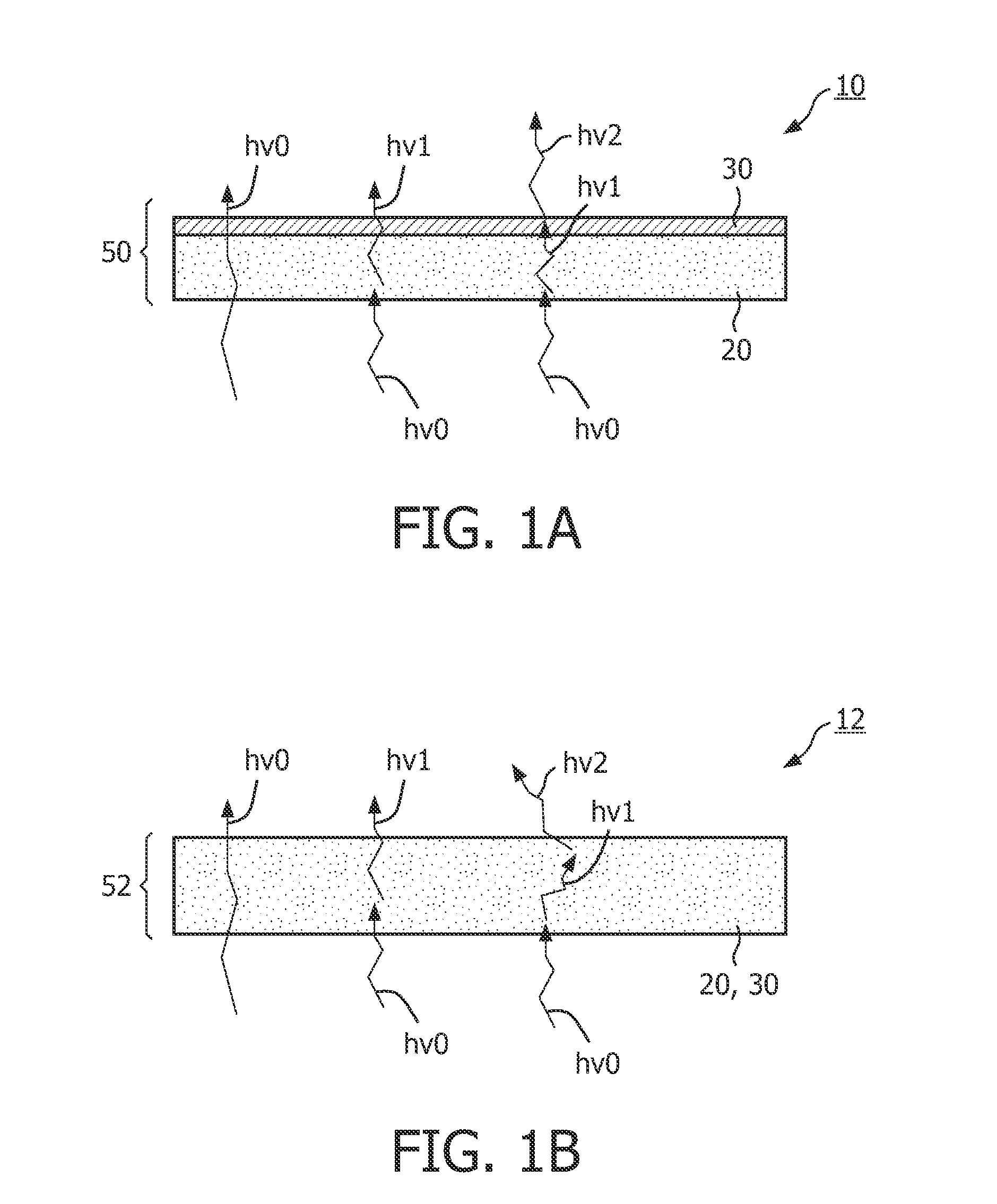
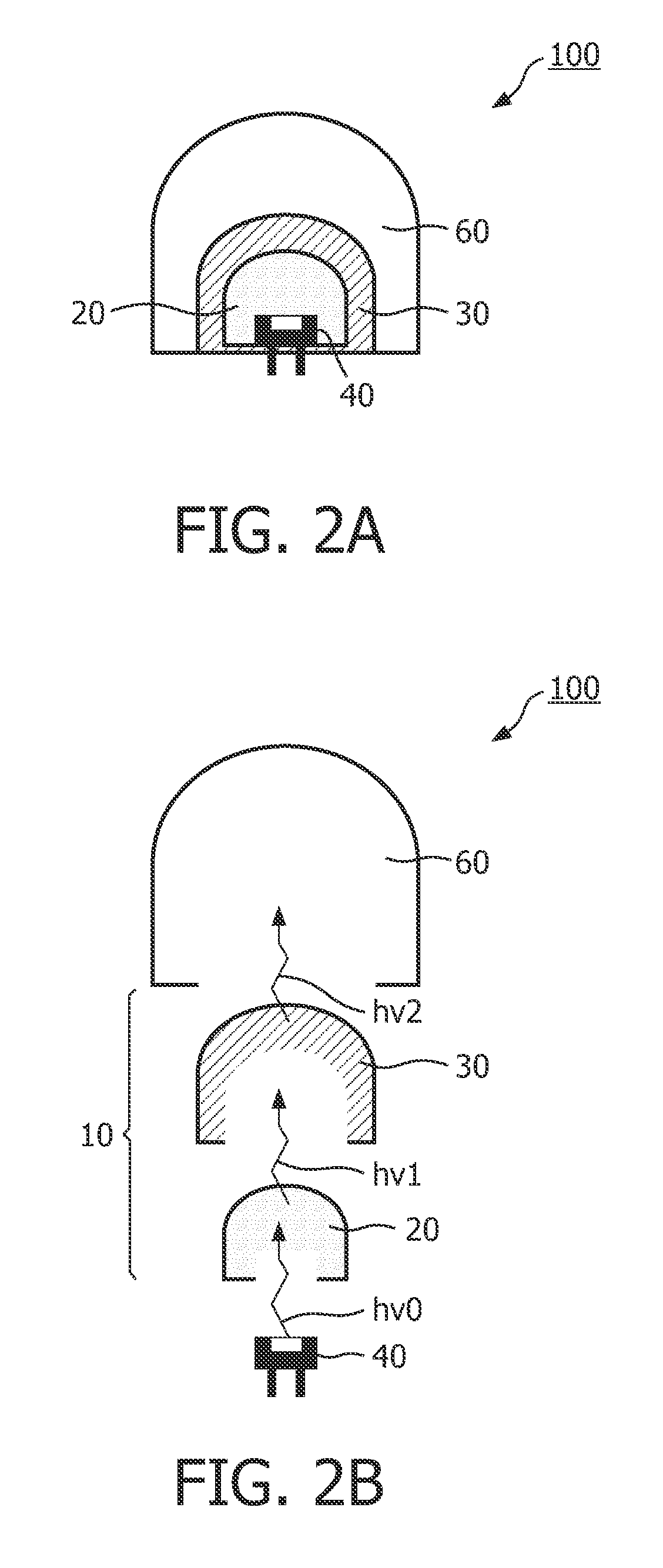
The invention relates to a luminescent converter (10, 12) for a phosphor-enhanced light source (100, 102, 104). The luminescent converter comprises a first luminescent material (20) configured for absorbing at least a part of excitation light (hv0) emitted by a light emitter (40, 42) of the phosphor-enhanced light source, and for converting at least a part of the absorbed excitation light into first emission light (hv1) comprising a longer wavelength compared to the excitation light. The luminescent converter further comprising a second luminescent material (30) comprising organic luminescent material (30) and configured for absorbing at least a part of the first emission light emitted by the first luminescent material, and for converting at least a part of the absorbed first emission light into second emission light (hv2) having a longer wavelength compared to the first emission light.An effect of the luminescent converter according to the invention is that the two-step light conversion according to the invention generates a relatively small Stokes shift of the light emitted by the organic luminescent material. The inventors have found that by reducing the Stokes shift of the organic luminescent material, the width of the spectrum of the second emission light is limited to reduce an infrared part in the emission spectrum. As such, the efficiency is improved.
Owner:SIGNIFY HLDG BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com