Compound with aggregation induced luminescence property and preparation method and application thereof
The technology of a compound, azafluorenone, is applied in the application field of lipid droplet-targeted photoactivated fluorescence imaging, which can solve the problems of difficulty in introducing functional groups, limited types of photochemical reactions, aggregation-induced quenching, etc., and achieve low cytotoxicity, Overcome the effects of aggregation-induced quenching and large Stokes shift
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis of three compounds of formula I:
[0078] (1) Compound I-1: 1-(4-(Diphenylamino)phenyl)-3-morpholino-9-oxo-2,9-dihydro-1H-indeno[2,1-c ] Synthesis of pyridine-4-nitrile (R 1 = 4-(Diphenylamino)phenyl, R 2 =morpholinyl)
[0079]
[0080] 2-((Z)-2-(4-Diphenylamino)-benzylidene)-1,2-dihydro-1-oxindan-3-ylidene)-malononitrile (225mg, 0.5mmol ) And morpholine (43.5 mg, 0.5 mmol) were dissolved in 10 mL of acetonitrile, and then reacted for 12 hours at 50°C under nitrogen protection. After the reaction is completed and the temperature is restored to room temperature, the reaction solution is rotated under reduced pressure to remove the solvent, and the residue is separated by silica gel column chromatography to obtain the red solid product 1-(4-(diphenylamino)phenyl)-3-morpholine Benzyl-9-oxo-2,9-dihydro-1H-indeno[2,1-c]pyridine-4-carbonitrile (126 mg, yield 47%). The relevant structural characterization data are as follows:
[0081] 1 H NMR(CD 2 Cl 2 ,500MHz):δ7.79(d,...
Embodiment 2
[0091] Synthesis of 4 compounds of formula II:
[0092] (1) Compound II-1: 1-(4-(Diphenylamino)phenyl)-3-morpholino-9-oxo-9H-indeno[2,1-c]pyridine-4-carbonitrile Synthetic (R 1 = 4-(Diphenylamino)phenyl, R 2 =morpholinyl)
[0093]
[0094] 2-((Z)-2-(4-Diphenylamino)-benzylidene)-1,2-dihydro-1-oxindan-3-ylidene)-malononitrile (225mg, 0.5mmol ) And morpholine (43.5 mg, 0.5 mmol) were dissolved in 10 mL of acetonitrile, and then irradiated with a 7W white energy-saving lamp under dry air, and reacted under reflux for 12 hours. After the reaction is complete and the temperature is restored to room temperature, the reaction solution is evaporated under reduced pressure to remove the solvent, and the residue is separated by silica gel column chromatography to obtain the orange-red solid product 1-(4-(diphenylamino)phenyl)-3-? Linyl-9-oxo-9H-indeno[2,1-c]pyridine-4-carbonitrile (162 mg, yield 61%). The relevant structural characterization data are as follows:
[0095] 1 H NMR(CD 2 Cl 2 ...
Embodiment 3
[0109] Single crystal structure analysis of compound II-2:
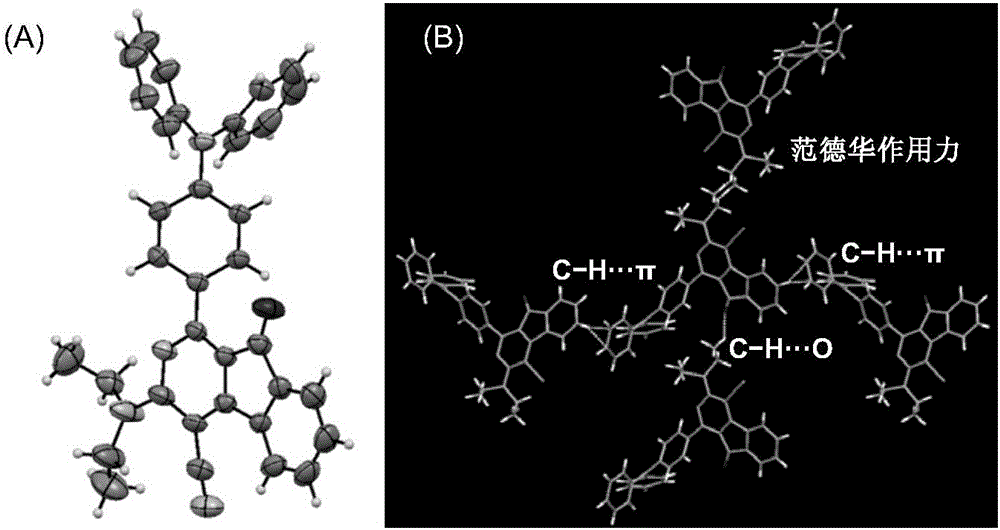
[0110] In the single crystal structure of compound II-2 (see figure 1 In ), the dihedral angle between 2-azafluorenone and 1-aromatic ring is 41.9(6)°, while the triphenylamine fragment is in the propeller conformation, and the dihedral angles between the corresponding aromatic rings are 61.3(2)°, respectively , 67.9(1)° and 73.3(6)°. The twisted molecular conformation helps the solution state to dissipate the absorbed light energy through intramolecular rotation, so that it does not emit fluorescence. In addition, in the crystal structure, there are a variety of CH···π, CH···O and van der Waals forces, which help limit the movement of molecules in the crystalline or aggregate state, prevent non-radiative transitions, and facilitate the passage of radiation The transition emits fluorescence.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity coefficient | aaaaa | aaaaa |
| Viscosity coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com