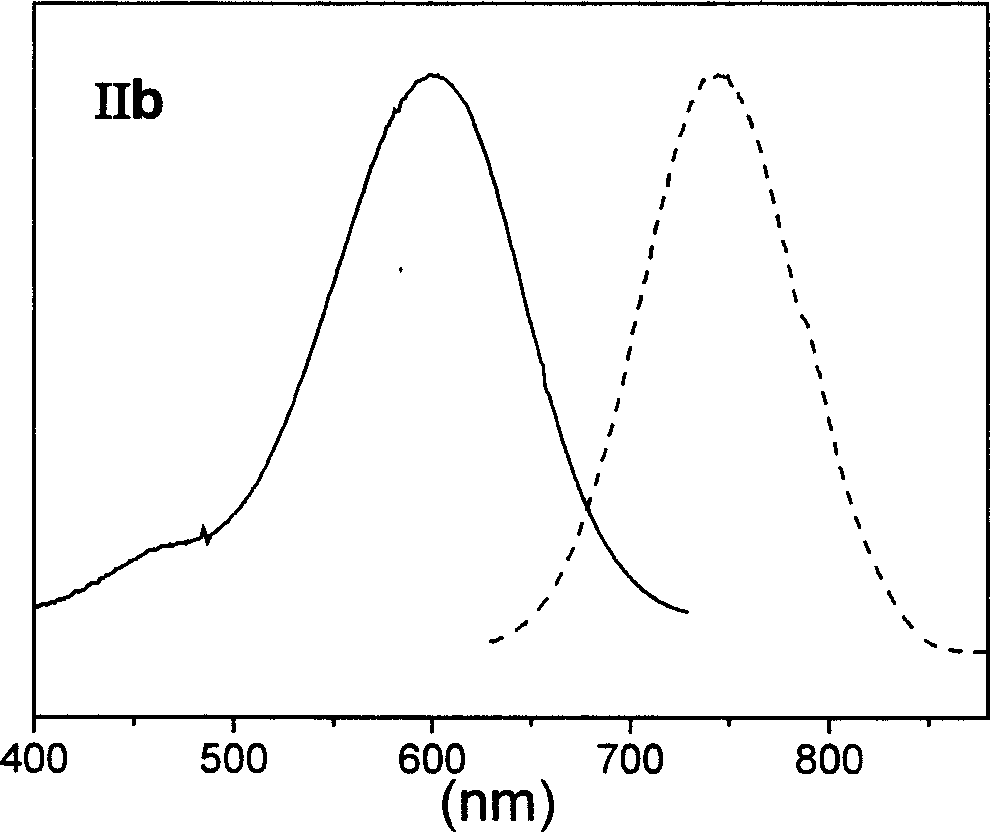
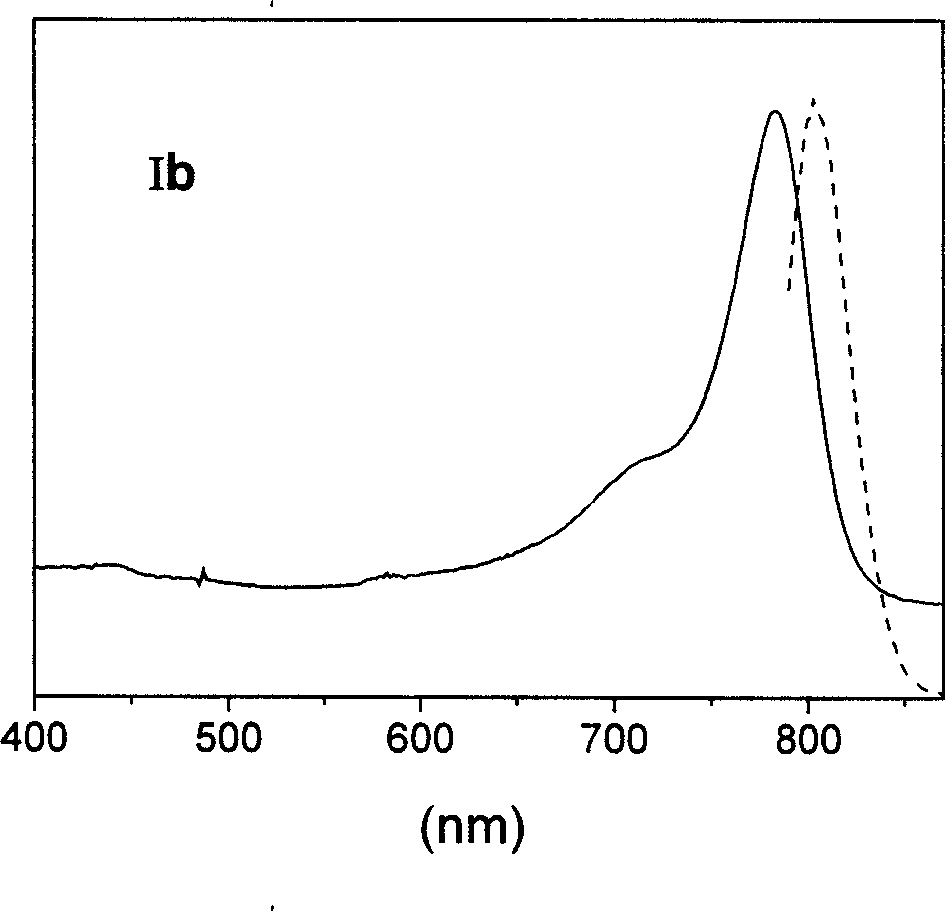
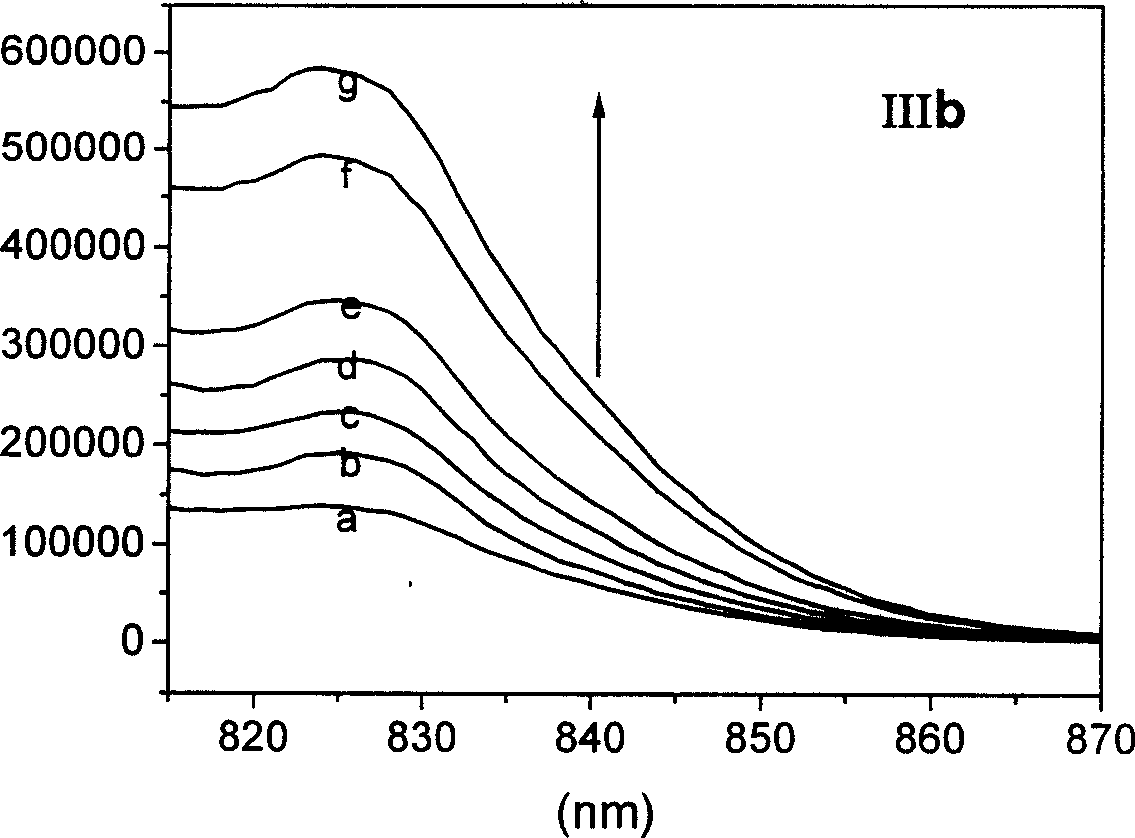
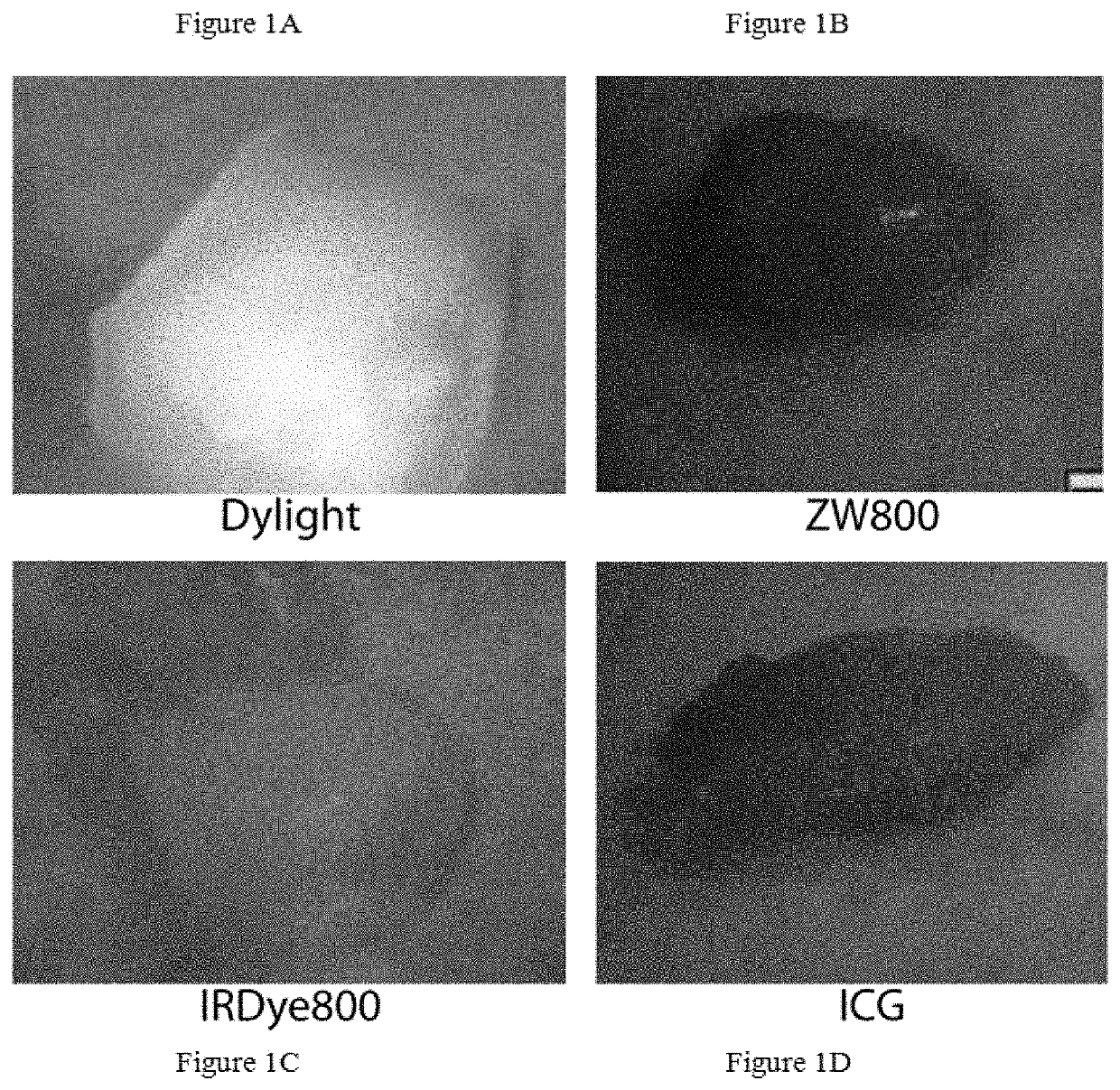
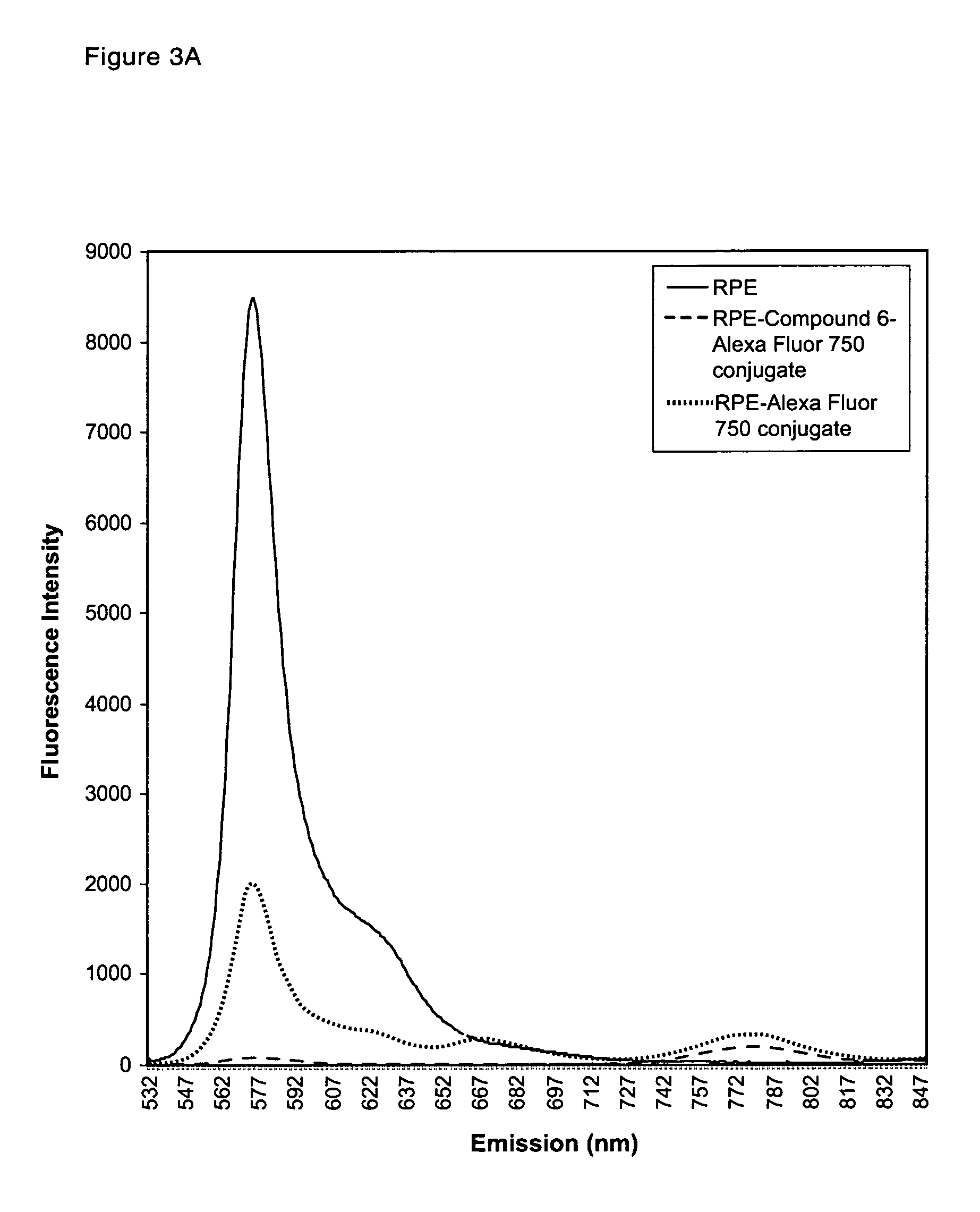
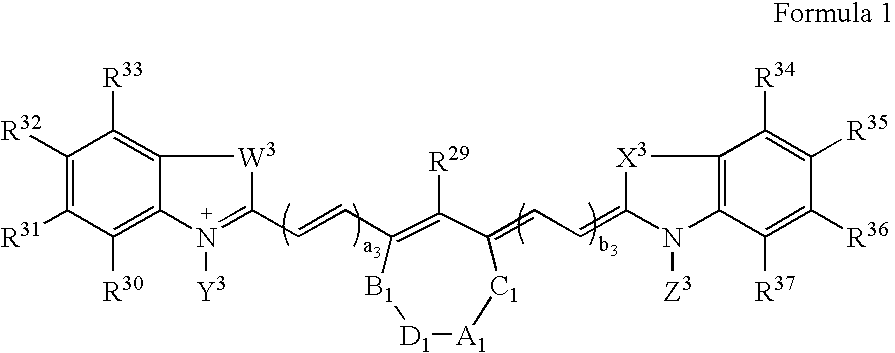
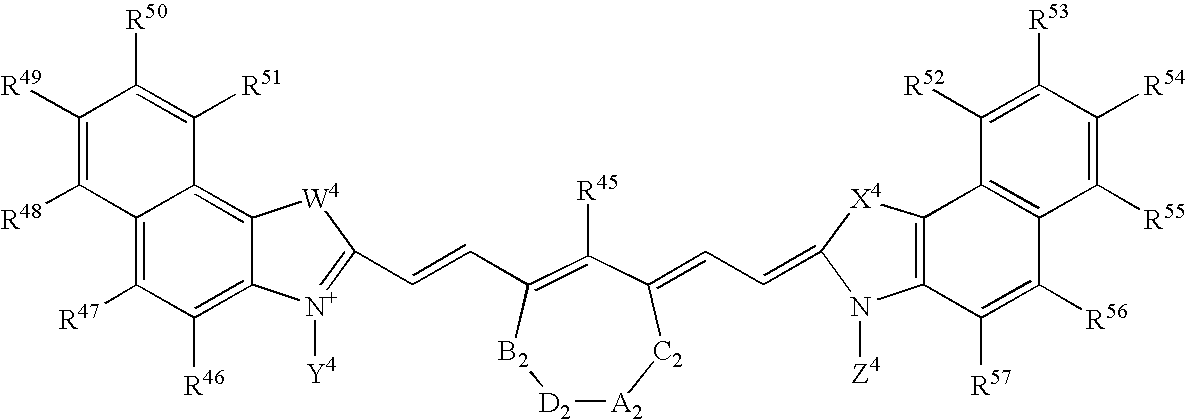
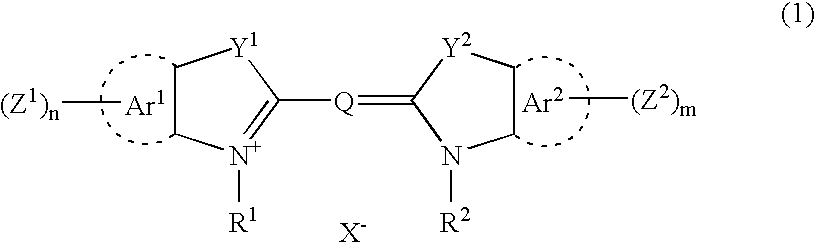
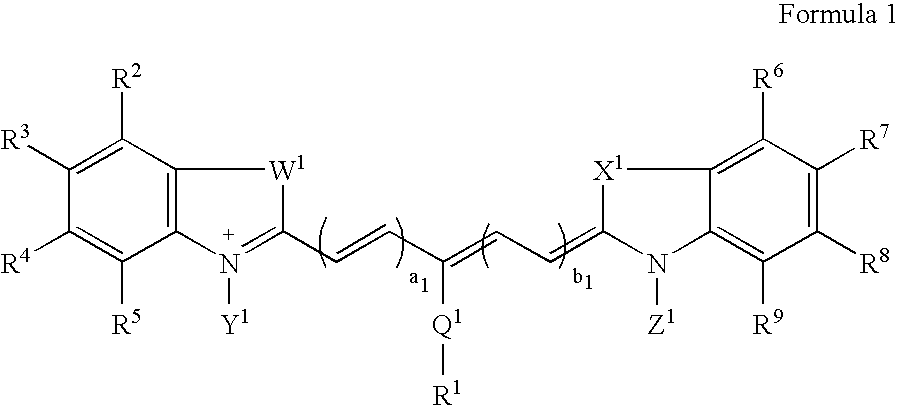
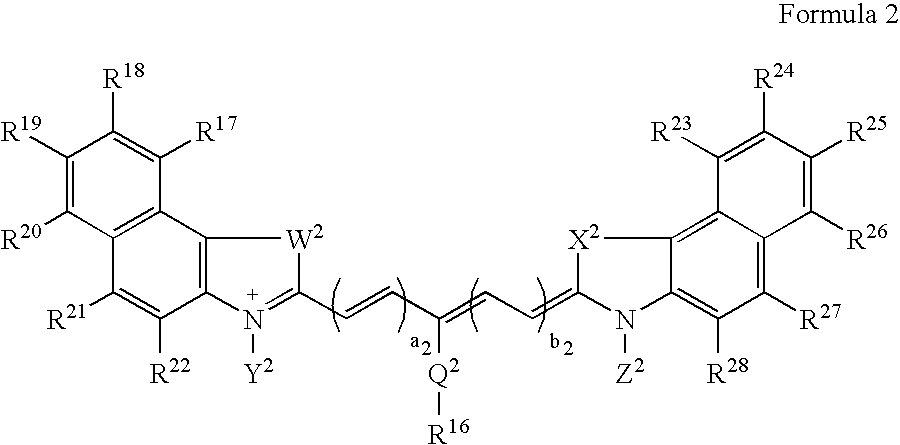
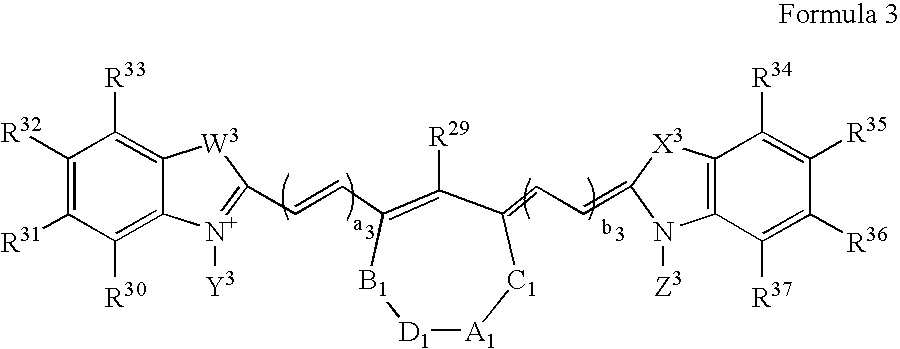
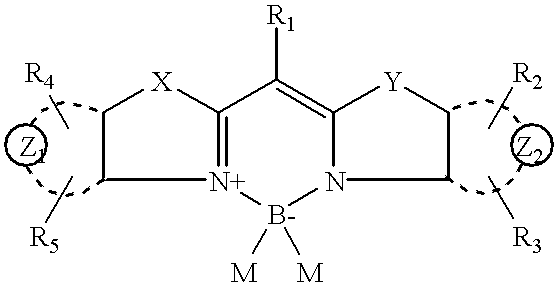
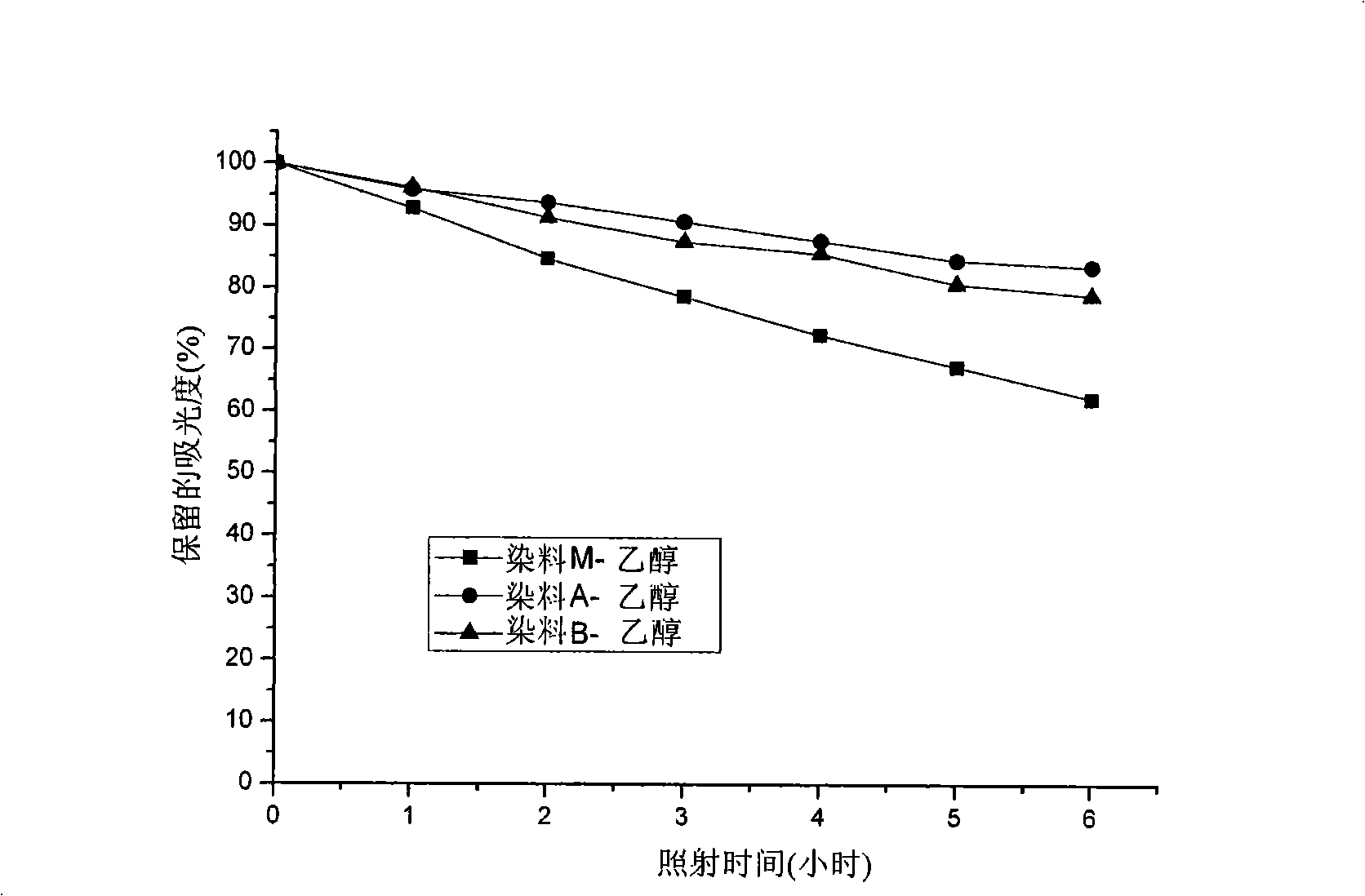
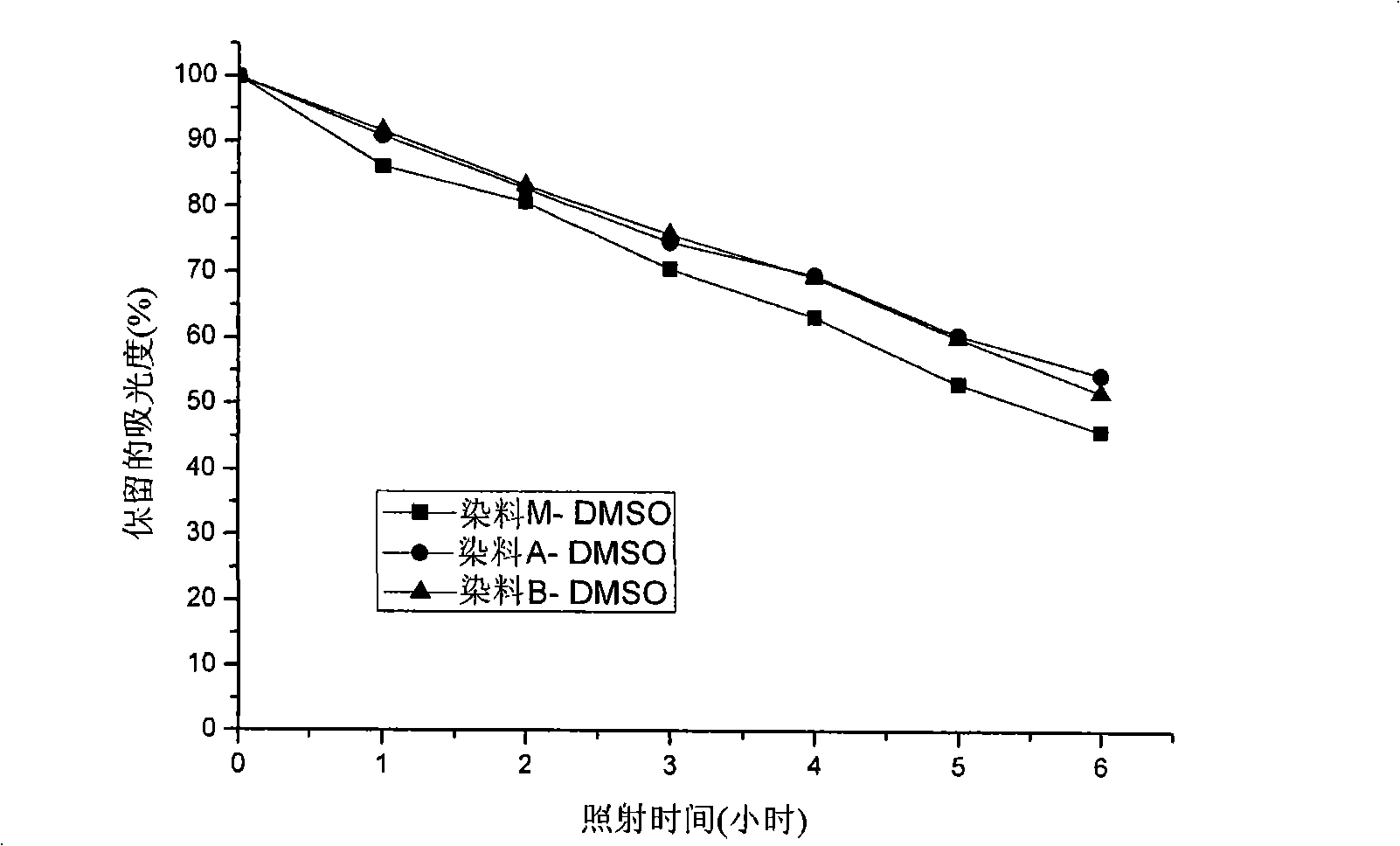
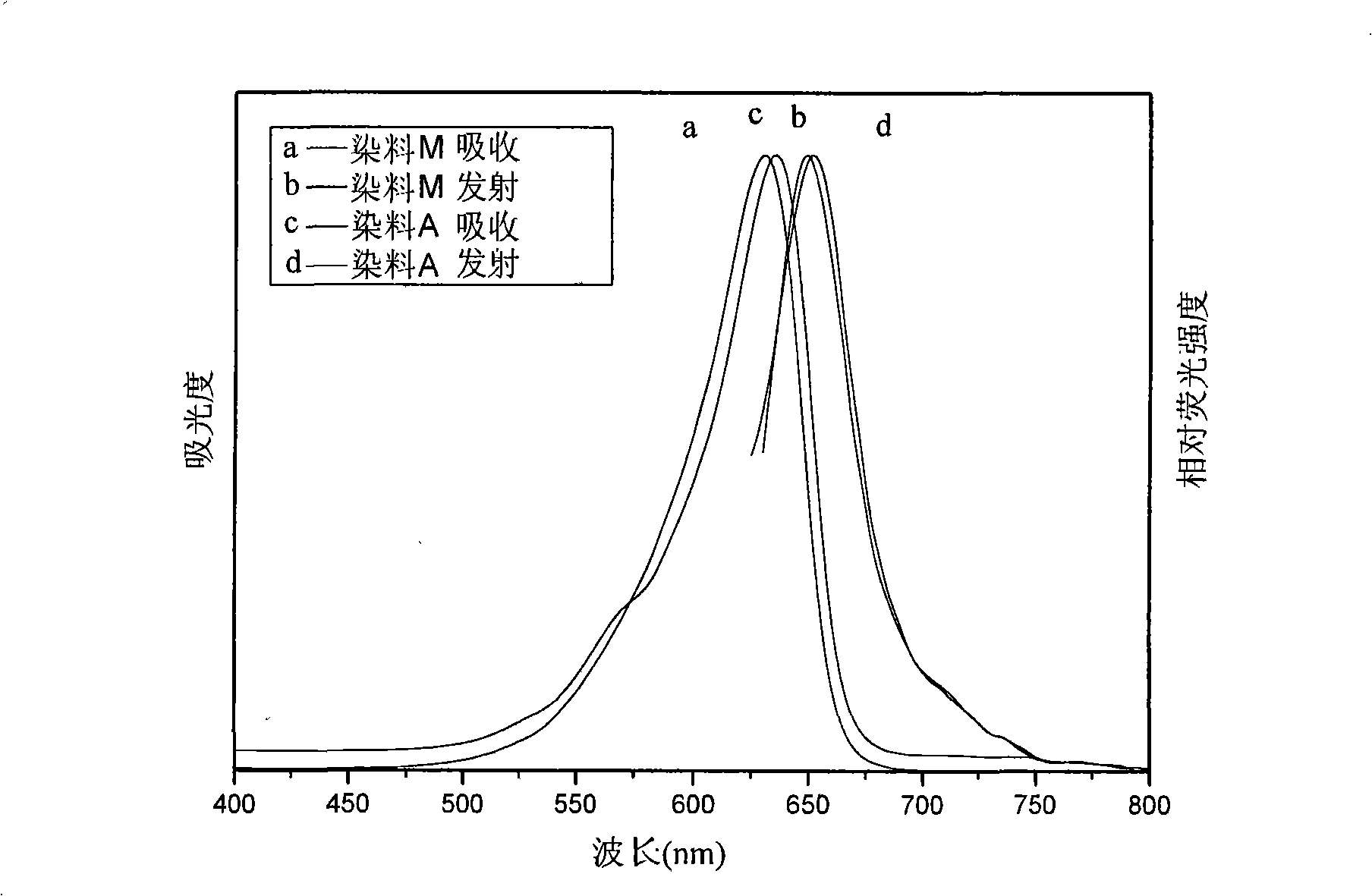
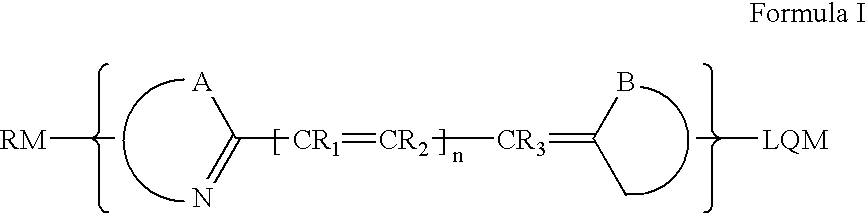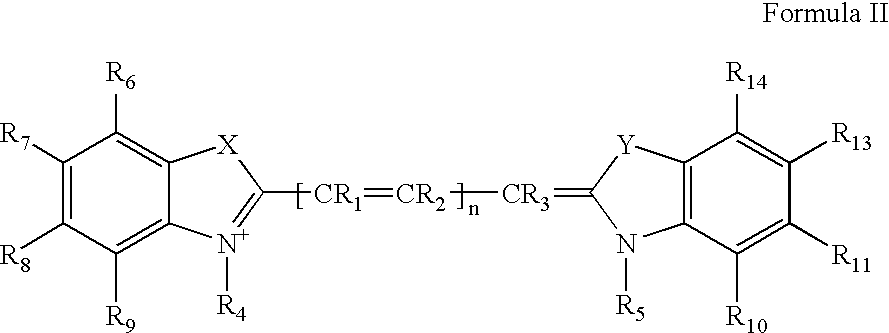Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
796 results about "Cyanine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyanine is the non-systematic name of a synthetic dye family belonging to polymethine group. The word cyanin is from the English word "cyan", which conventionally means a shade of blue-green (close to "aqua") and is derived from the Greek κυάνεος/κυανοῦς kyaneos/kyanous which means a somewhat different color: "dark blue".
Cyanine dyes as labeling reagents for detection of biological and other materials by luminescence methods
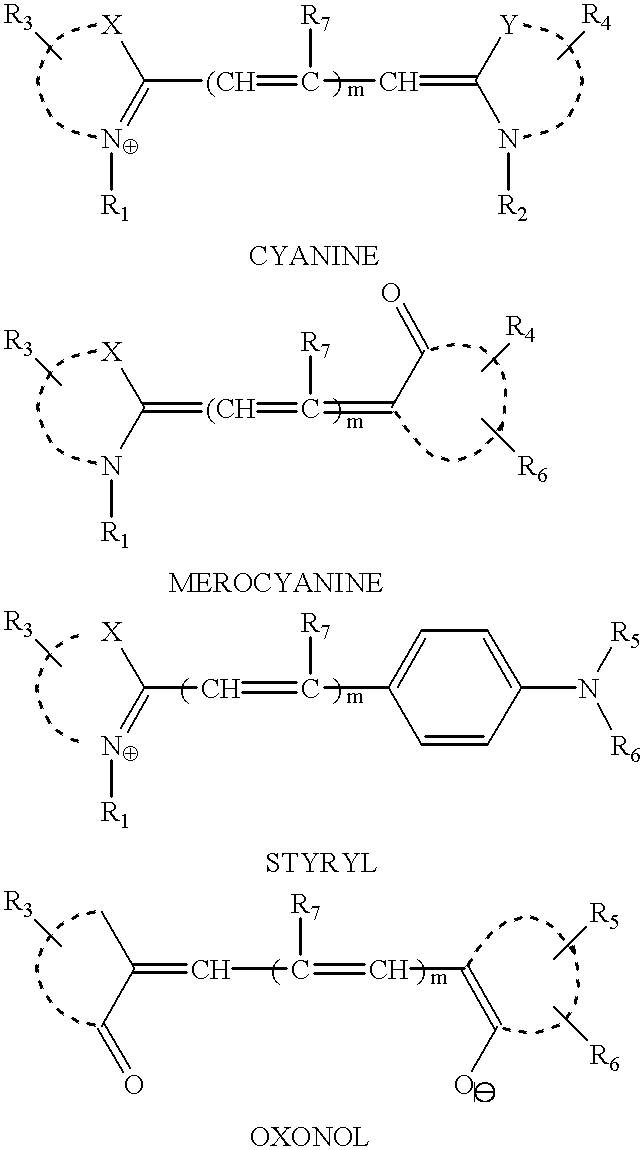
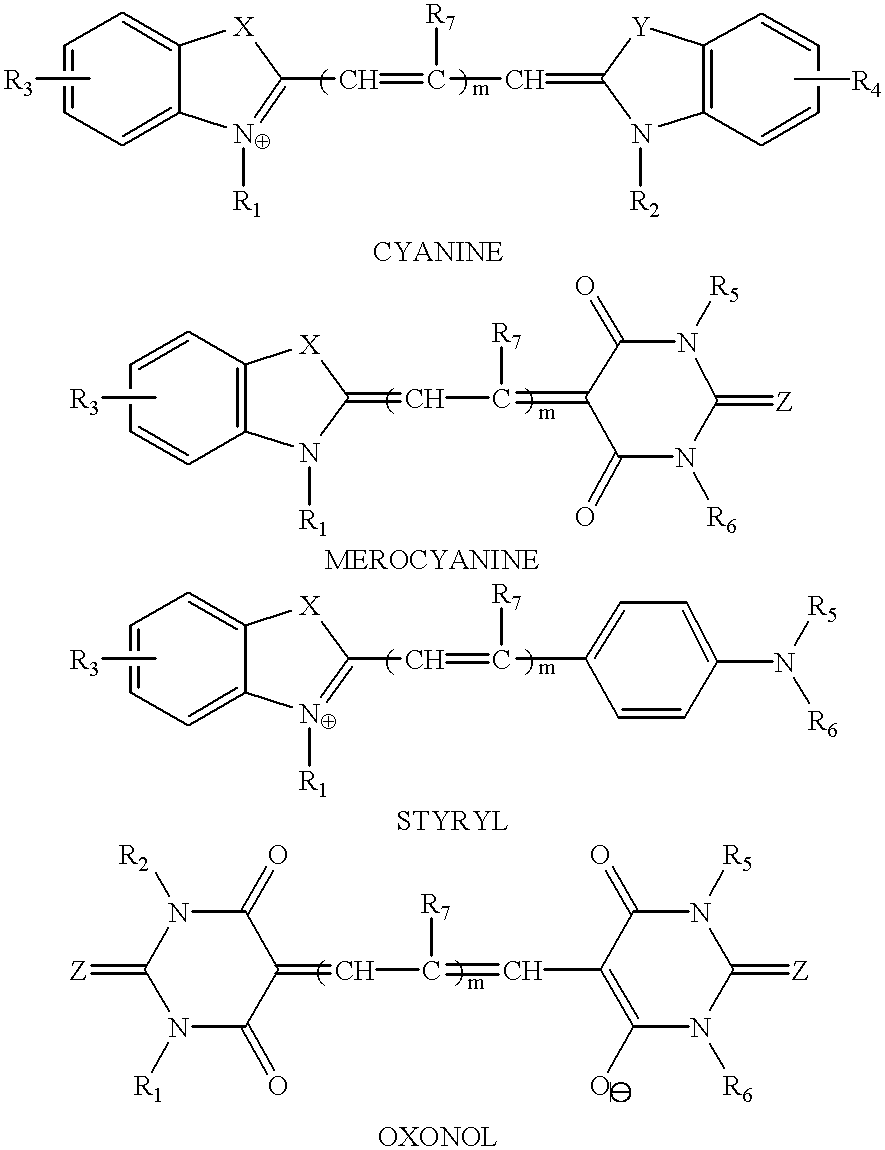
Cyanine and related dyes, such as merocyanine, styryl and oxonol dyes, are strongly light-absorbing and highly luminescent. Cyanine and related dyes having functional groups make them reactive with amine, hydroxy and sulfhydryl groups are covalently attached to proteins, nucleic acids, carbohydrates, sugars, cells and combinations thereof, and other biological and nonbiological materials, to make these materials fluorescent so that they can be detected. The labeled materials can then be used in assays employing excitation light sources and luminescence detectors. For example, fluorescent cyanine and related dyes can be attached to amine, hydroxy or sulfhydryl groups of avidin and to antibodies and to lectins. Thereupon, avidin labeled with cyanine type dyes can be used to quantify biotinylated materials and antibodies conjugated with cyanine-type dyes can be used to detect and measure antigens and haptens. In addition, cyanine-conjugated lectins can be used to detect specific carbohydrate groups. Also, cyanine-conjugated fragments of DNA or RNA can be used to identify the presence of complementary nucleotide sequences in DNA or RNA.
Owner:CARNEGIE MELLON UNIV
Infrared absorbing N-alkylsulfate cyanine compounds
Owner:KODAK POLYCHROME GRAPHICS
Cyanine dyes as labeling reagents for detection of biological and other materials by luminescence methods
Cyanine and related dyes, such as merocyanine, styryl and oxonol dyes, are strongly light-absorbing and highly luminescent. Cyanine and related dyes having functional groups make them reactive with amine, hydroxy and sulfhydryl groups are covalently attached to proteins, nucleic acids, carbohydrates, sugars, cells and combinations thereof, and other biological and nonbiological materials, to make these materials fluorescent so that they can be detected. The labeled materials can then be used in assays employing excitation light sources and luminescence detectors. For example, fluorescent cyanine and related dyes can be attached to amine, hydroxy or sulfhydryl groups of avidin and to antibodies and to lectins. Thereupon, avidin labeled with cyanine type dyes can be used to quantify biotinvlated materials and antibodies conjugated with cyanine-type dyes can be used to detect and measure antigens and haptens. In addition, cyanine-conjugated lectins can be used to detect specific carbohydrate groups. Also, cyanine-conjugated fragments of DNA or RNA can be used to identify the presence of complementary nucleotide sequences in DNA or RNA.
Owner:CARNEGIE MELLON UNIV
Cyanine dyes
ActiveUS20120052506A1Versatile assay designImprove analytical performanceMethine/polymethine dyesSugar derivativesSolubilityCyanine
The invention provides a novel class of cyanine dyes that are functionalized with sulfonic acid groups and a linker moiety that facilitates their conjugation to other species and substituent groups which increase the water-solubility, and optimize the optical properties of the dyes. Also provided are conjugates of the dyes, methods of using the dyes and their conjugates and kits including the dyes and their conjugates.
Owner:PACIFIC BIOSCIENCES
Cyanine dyes and methods of use
ActiveUS20070042398A1Good water solubilityLittle and no intrinsic fluorescenceUltrasonic/sonic/infrasonic diagnosticsMethine/polymethine dyesCyanineLength wave
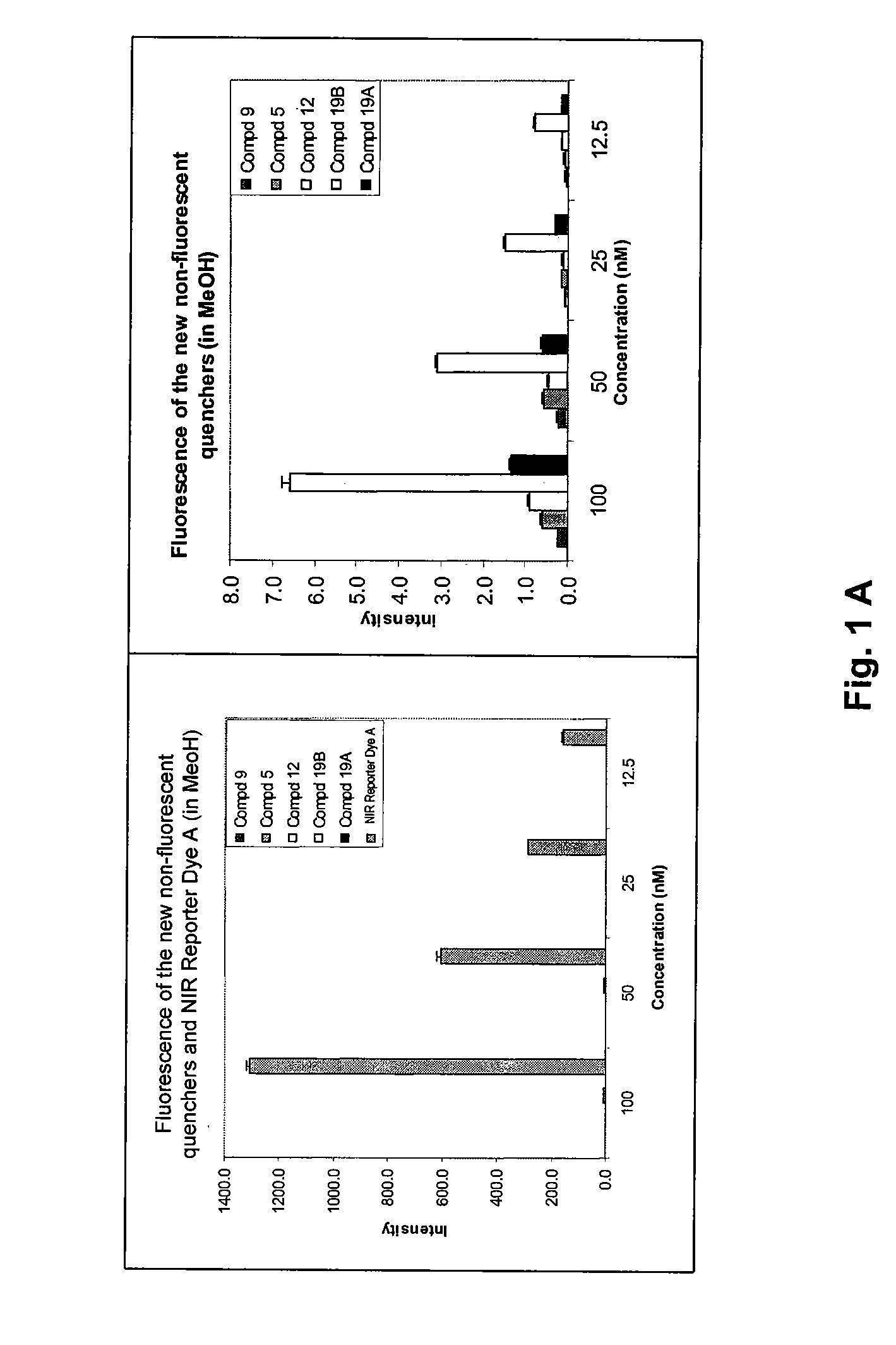
The present invention provides for cyanine dyes as near IR quenchers. The cyanine dyes have absorption wavelengths in the near-infrared region of about 650-900 nm and are essentially non-fluorescent. The dyes of the invention have at least one linking group. The present invention also provides substantially non-fluorescent, NIR probes. Biological assays based on these novel, substantially non-fluorescent, NIR probes are also provided.
Owner:LI COR
Functionalized cyanine dyes (PEG)
ActiveUS20120058469A1Good adhesionVersatile assay designMethine/polymethine dyesSugar derivativesCyanineMerocyanine dye
Owner:PACIFIC BIOSCIENCES
Functionalized cyanine dyes (PEG)
ActiveUS20120058482A1Good adhesionVersatile assay designMethine/polymethine dyesSugar derivativesCyanineMerocyanine dye
The invention provides a novel class of cyanine dyes that are functionalized with a linker moiety that facilitates their conjugation to other species and substituent groups which increase the water-solubility, and optimize the optical properties of the dyes. Also provided are conjugates of the dyes, methods of using the dyes and their conjugates and kits including the dyes and their conjugates.
Owner:PACIFIC BIOSCIENCES
Mobility-Modifying Cyanine Dyes
InactiveUS6716994B1Easy to getGood water solubilityMethine/polymethine dyesSugar derivativesCyanineNucleotide
The present invention provides a novel class of fluorescent cyanine dye compounds that are modified at one of the hetercyclic ring nitrogen atoms with a mobility-modifying moiety that permits the electrophoretic mobilities of polynucleotides labeled with the mobility-modifying cyanine dyes to be adjusted or tuned in a predictable fashion while retaining enzymatic activity. The ability to predictably tune the relative electrophoretic mobilities of the dyes permits the creation of sets of mobility-matched fluorescent dyes of a variety of structures for a variety of applications, including fluorescence-based 4-color nucleic acid sequencing reactions.
Owner:APPL BIOSYSTEMS INC
Fluorescent imaging with substituted cyanine dyes
Compounds and methods are disclosed that are useful for noninvasive imaging in the near-infrared spectral range. The cyanine compounds of Formula I are presented:wherein Q is a portion of a polymethine bridge selected from the group consisting of:Also included are bioconjugates of the compounds of Formula I, methods of labeling biomolecules with the compounds, and methods of imaging.
Owner:LI COR
Lithographic printing plate precursor, lithographic printing method, and novel cyanine dye
InactiveUS20070056457A1Improve visibilityExcellent in on-machine development propertyMethine/polymethine dyesPlate printingCyanineImage recording
A lithographic printing plate precursor comprising a support and an image-recording layer which contains (A) a cyanine dye including a solvent-soluble group and having an absorption maximum in an infrared wavelength region and is capable of being removed with at least one of printing ink and an aqueous component.
Owner:FUJIFILM CORP
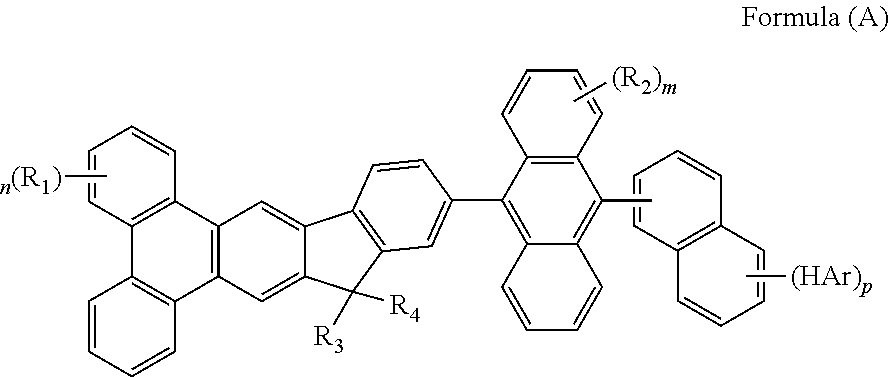
Organic compound for organic electroluminescent device
ActiveUS20140209866A1Short half-lifeDrive voltage highOrganic chemistrySolid-state devicesGroup systemPhotochemistry
The present invention discloses a novel organic compound is represented by the following formula(A), the organic EL device employing the compound as blue emitting layer can lower driving voltage, prolong half-lifetime and increase the efficiency.Wherein m represent an integer of 0 to 8, n represent an integer of 0 to 10, p represent an integer of 0 to 7, HAr represent a hydrogen, a halide, a cyanine group, a substituted or unsusbstituted heteroaryl group system having 5 to 6 aromatic ring atoms, R1 to R4 are identical or different. R1 to R4 are independently selected from the group consisting of a hydrogen atom, alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aryl group having 6 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 6 to 30 carbon atoms.
Owner:NINGBO LUMILAN NEW MATERIAL CO LTD
Differential blood count reagent, kit and method of differential blood count
ActiveCN101750274AThe classification processing effect is goodLow costMethine/polymethine dyesOrganic chemistryCyanineDifferential blood count
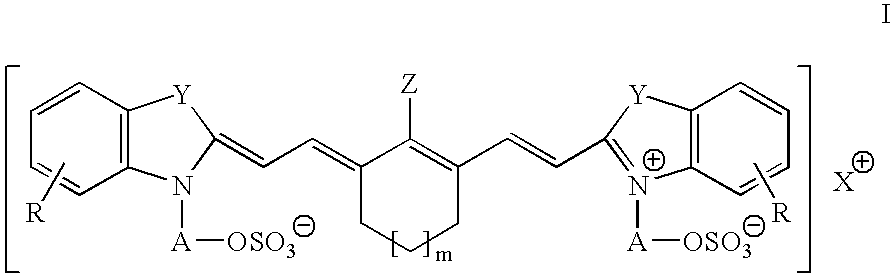
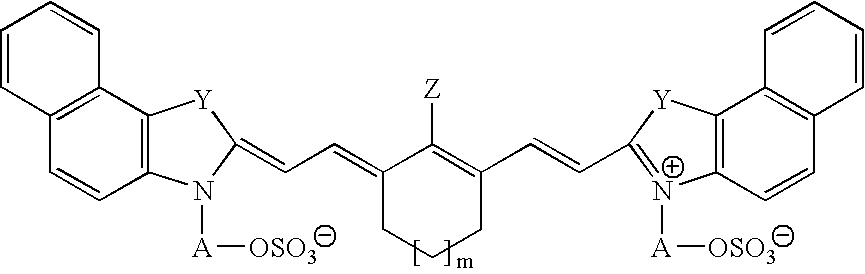
The invention discloses a differential blood count reagent which comprises the following components: (1) a cyanine anion compound selected form compounds expressed by the formula I or formula II, (2) an anode surfactant expressed by the formula III, IV or V and (3) at least one nonionic surfactant. The invention also discloses a kit containing the differential blood count reagent and a method of the differential blood count. By utilizing the reagent, the kit and the method, five subclasses of leucocytes can be quickly identified within a very short time, four-classification count is at least carried out, and immaturate cells and abnormal cells can be distinguished.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
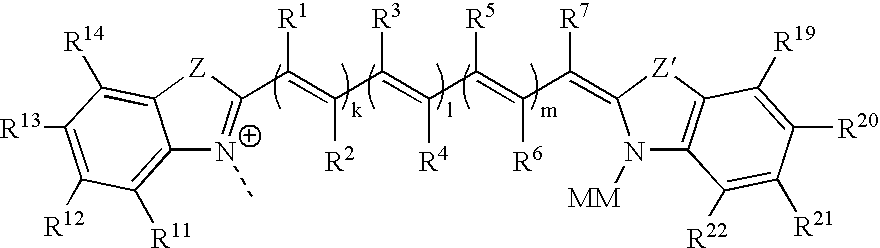
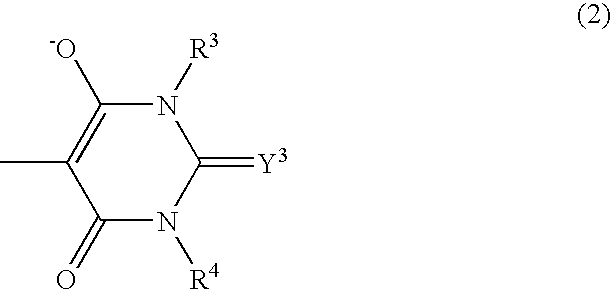
Cyanine derivatives, fluorescent conjugates containing same and use thereof
InactiveUS20100143960A1Microbiological testing/measurementPhosphorus organic compoundsCyanineSubject matter
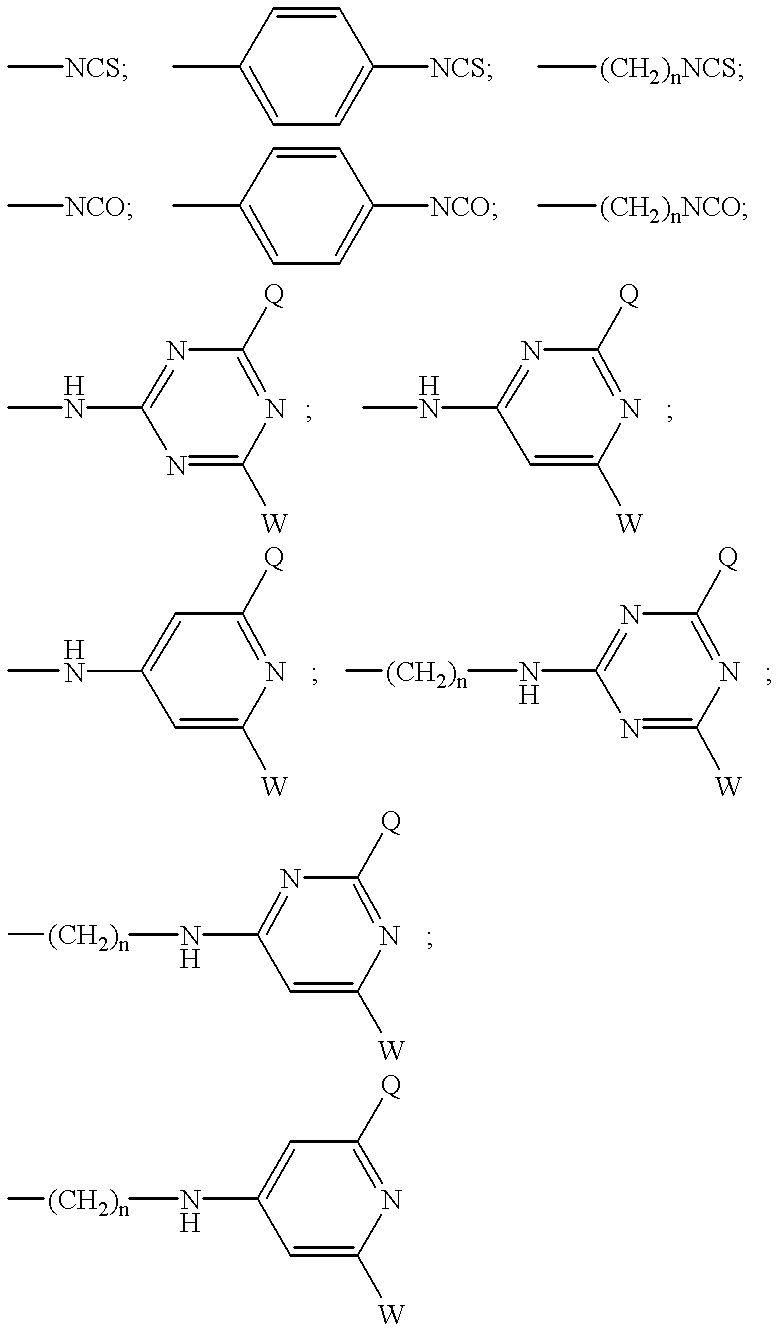
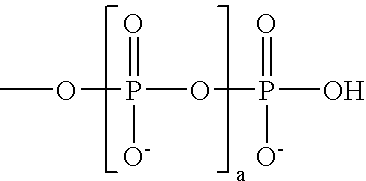
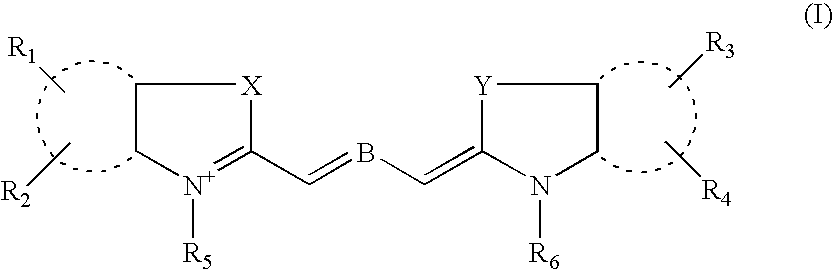
A subject matter of the invention is cyanine derivatives of formula:in which the dotted lines represent the atoms necessary for the formation of one or two fused aromatic rings, each ring comprising 5 or 6 carbon atoms;R1, R2, R3 and R4 represent, independently of one another: H; substituted or unsubstituted C1-C15 alkyl; C1-C6 alkoxy; (C2-C12)dialkylamino; C1-C6 alkoxycarbonyl; di(C2-C12)alkylamido; a substituted or unsubstituted aryl, arylalkyl or aryloxy group; a halogen atom; a nitro; an L1-W, L2-M, L2-A or L2-G group;R5 and R6 represent, independently of one another: substituted or unsubstituted C1-C15 alkyl; a substituted or unsubstituted aryl or arylalkyl group; an L1-W, L2-M, L2-A or L2-G group;X is chosen from: O, S or CR7R8; Y is chosen from: O, S or CR9R10;R7, R8, R9 and R10 independently represent: substituted or unsubstituted C1-C15 alkyl; substituted or unsubstituted aryl, arylalkyl or aryloxy; an L1-W, L2-M, L2-A or L2-G group;R7 and R8 and / or R9 and R10 can also together form a ring comprising 5 or 6 atoms or a heterocycle comprising 4 to 5 carbon atoms and an oxygen atom;B represents a polymethine bridge comprising 1 to 5 methine groups, said groups being in particular individually unsubstituted or substituted by a substituted or unsubstituted C1-C15 alkyl; a substituted or unsubstituted aryl, arylalkyl or aryloxy group; a nitro group; an L1-W, L2-M, L2-A or L2-G group;L1 and L2 are connecting arms; G is a reactive group; A is a coupling agent; M is a conjugated molecule, W is a phosphate or phosphonate ester (preferably diester), with the proviso that the cyanine derivative comprises at least one L1-W group and at least one L2-A, L2-G or L2-M group.
Owner:CIS BIO INT
Preparation method and application of zinc oxide visible-light-induced photocatalyst sensitized by squarylium cyanine
InactiveCN103071535AEfficient degradationImprove photocatalytic activityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsCarbon compositesMicrosphere
The invention relates to a preparation method of a zinc oxide visible-light-induced photocatalyst sensitized by squarylium cyanine. The preparation method comprises the following steps: dissolving Zn(Ac)22H2O and glucose into deionized water; carrying out ultrasonic processing to form a settled solution; after transferring the solution into a liner of a polytetrafluoroethylene autoclave, performing hydrothermal reaction for 12 to 24 hours at a temperature of 140 to 180 DEG C; after cooling to room temperature, carrying out centrifugal washing on the obtained black powder respectively with the deionized water and absolute ethyl alcohol; drying at a temperature of 60 DEG C to obtain a zinc oxide and carbon composite precursor; placing the precursor into a muffle furnace to calcine for 3 hours at a temperature of 400 to 600 DEG C, so as to obtain zinc oxide microspheres; mixing the zinc oxide microspheres and the squarylium cyanine as well as an organic solvent; at a temperature of 30 to 60 DEG C, carrying out ultrasonic processing on the mixture for 0.5 to 2 hours; then removing the organic solvent under the condition of pressure reduction; and finally, drying the obtained solid at a temperature of 60 DEG C. The zinc oxide visible-light-induced photocatalyst sensitized by the squarylium cyanine can be used for photocatalysis processing on organic pollutants in air, soil and sewage.
Owner:常州浩瀚万康纳米材料有限公司
Ink and ink set for ink jet printing and method of ink jet printing
InactiveUS20050178288A1Improve spraying effectFastness to light and heatInksPorphines/azaporphinesCyanineHeat resistance
An inkjet recording ink composition comprising an aqueous medium having dissolved and / or dispersed therein at least one dye, wherein at least one betaine-type surfactant is contained in the ink composition. The dye used is preferably a magenta dye having a specific azo structure or a cyanine dye having a specific phthalocyanine structure. By virtue of such a constitution, an inkjet recording ink composition excellent in ejection property, light fastness, heat fastness and oxidation resistance and causing less dye bleeding can be provided.
Owner:FUJIFILM HLDG CORP +1
Heptamethanindocyanine dye and its synthesis method and application
ActiveCN102268191AEasy post-processingHigh yieldOrganic active ingredientsMethine/polymethine dyesCyanineSide chain
The invention relates to a heptamethine indocyanine dye containing N-fatty esters or N-fatty amide side chains, a synthetic method thereof and applications thereof in tumor targeting imaging and treatment. The heptamethine indocyanine dye which possesses or individually possesses near infrared absorption, fluorography and antineoplastic activity allows present research and development ideas and treatment levels of tumor targeting treatment medicaments to be greatly improved, and has great significances to aspects of early discovery, control and the like of various tumors.
Owner:ARMY MEDICAL UNIV
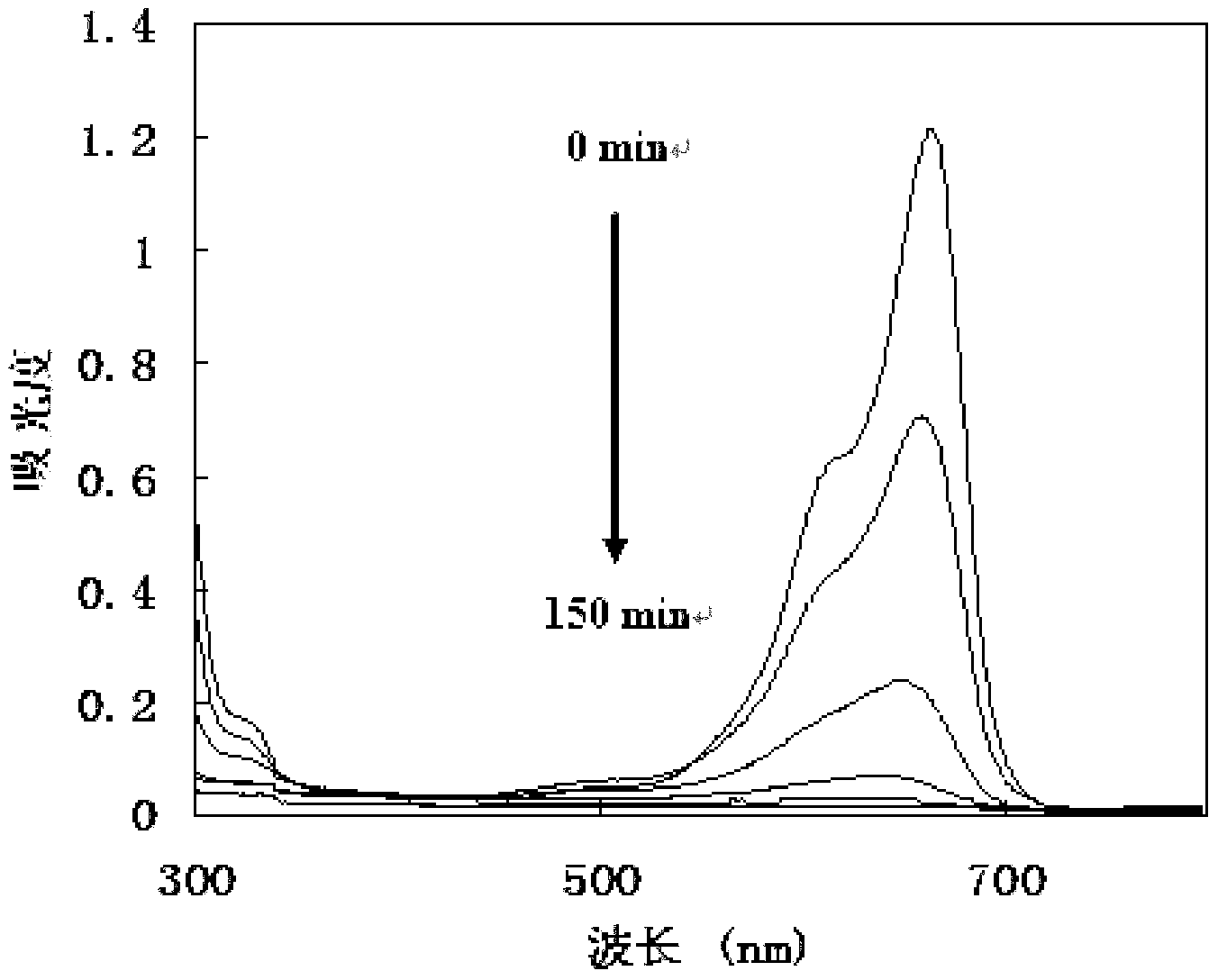
Use of cyanine dyes for the diagnosis of proliferative diseases
InactiveUS20100166659A1Strong specificityHigh sensitivityIn-vivo testing preparationsCyanineBody weight
The present invention concerns the use of the cyanine dye SF64 for the diagnosis of proliferative diseases upon administration of less than 5 mg / kg body weight.
Owner:SCHERING AG
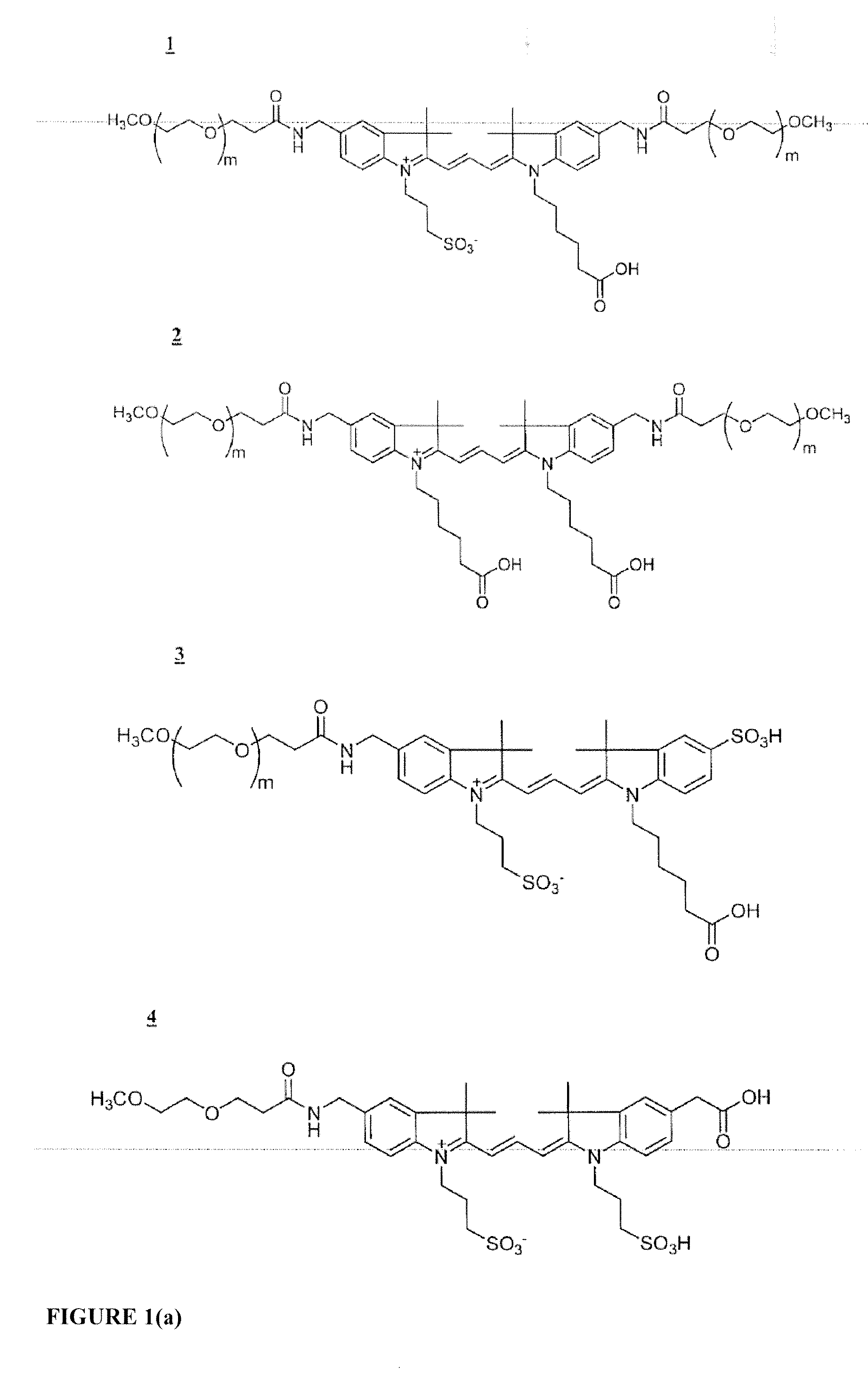
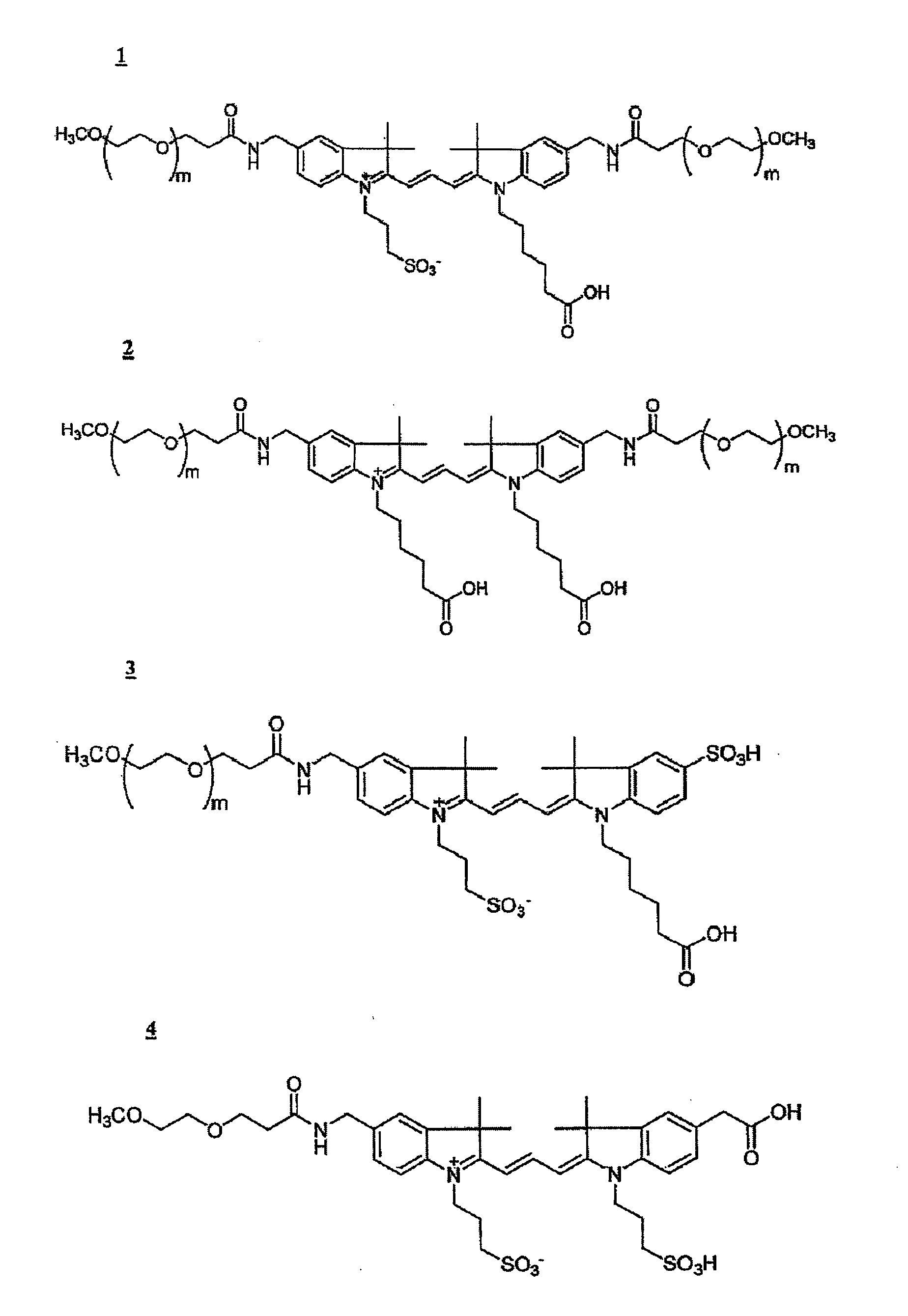
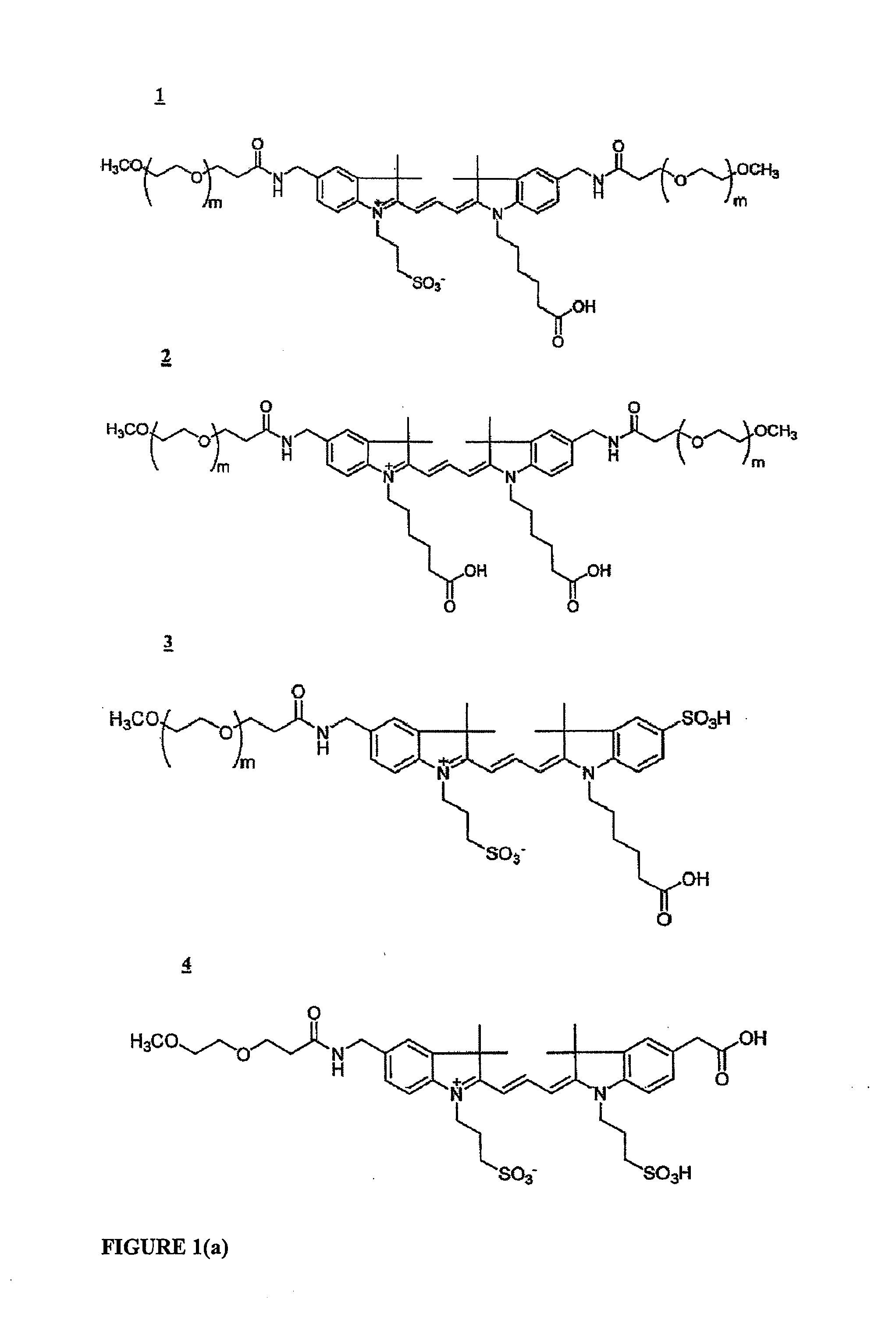
Cyanine dyes and their applications as luminescence quenching compounds
ActiveUS20060223076A1Improve solubilityMethine/polymethine dyesSugar derivativesEnergy transferCyanine
The quenching compounds of the invention are weakly luminescent cyanines that are substituted by one or more heteroaromatic quenching moieties. The quenching compounds of the invention exhibit little or no observable luminescence and efficiently quench a broad spectrum of luminescent compounds. The chemically reactive quenching compounds possess utility for labeling a wide variety of substances, including biomolecules. These labeled substances are highly useful for a variety of energy-transfer assays and applications.
Owner:ANASPEC
Fluorescence probe for detecting intracellular hydrogen sulfide and preparation method and application of fluorescence probe
ActiveCN103160274AEasy to detectStrong penetrating powerOrganic chemistryFluorescence/phosphorescenceChromatographic columnCyanine
The invention relates to a fluorescence probe for detecting intracellular hydrogen sulfide and a preparation method and application of the fluorescence probe. Cy7 fluorochrome and sodium acetate are dissolved in anhydrous dimethylformamide (DMF), heating reflux is carried out for 2-4 hours under protection of Ar gas, cooling and suction filtration are carried out on an obtained mixture, mother liquor is crystallized under the low temperature of minus 4-20 DEG C, and keto form cyanine reddish brown crystals are obtained after the suction filtration; toluoyl benzoic acid and oxalyl chloride are dissolved in anhydrous CH2Cl2, then a bit of DMF is added, mixing is carried out for 2-6 hours under the temperature of 0 DEG C, and toluoyl benzoyl chloride is obtained; and keto form cyanine and triethylamine are dissolved in the anhydrous CH2Cl2 and are cooled to be 0 DEG C, the toluoyl benzoyl chloride is added dropwise, mixing is carried out for 10-30 minutes under the temperature of 0 DEG C, the mixture is heated to be in the room temperature, mixing is carried out overnight, suction filtration and spinning drying are carried out, separation is carried out through a chromatographic column, green solids are obtained after the spinning drying, and the structural formula is as follows. The fluorescence probe is simple in composition, high in detection speed, free of long-term incubation in a detection process, high in sensitivity, and capable of detecting intracellular endogenic hydrogen sulfide.
Owner:SHANDONG NORMAL UNIV
Near infrared meso-position nitrogen and sulfur substituted hepta-methyl-cyanine fluorochrome for bioanalysis
InactiveCN1702118ANo energy transferAvoid quenching effectMethine/polymethine dyesBiological testingFluorochrome DyePhotochemistry
The invention relates to a near-infrared new methenyl fluorescence dye, which is made by displacement at mid-position of methine colors by nucleophilic reagent containing nitrogen and sulfur. Mid-position nitrogen derivative replaces methine colors to generate ICT effect, Stokes displacement (70-170nm) and fine fluorescence. It can be used as high fidelity fluorescence labeling probe of biomolecules such as protein, sugar and DNA. When mid-position sulfer derivative replaces methine colours, PET phenomenon occurs in molecule.
Owner:DALIAN UNIV OF TECH
Compositions of Near IR Closed Chain, Sulfo-Cyanine Dyes and Prostate Specific Membrane Antigen Ligands
Owner:INTUITIVE SURGICAL OPERATIONS INC
Cyanine compounds and their application as quenching compounds
The present invention provides methods and non-fluorescent carbocyanine quencher compounds having the general formula:Wherein the A moiety is a substituted pyridinium, unsubstituted pyridinium, substituted quinolinium, unsubstituted quinolinium, substituted benzazolium, unsubstituted benzazolium, substituted indolinium, or unsubstituted indolinium. The invention further provides luminescent donor molecule-quencher pairs and luminescent donor molecule-quencher-luminescent acceptor molecule conjugates wherein the quencher is a cyanine compound of the present invention. The energy transfer pairs are used to detect an analyte of interest in a sample.
Owner:MOLECULAR PROBES
Hydrophilic cyanine dyes
InactiveUS7011817B2Enhance tumor detectionPreserve fluorescence efficiencyUltrasonic/sonic/infrasonic diagnosticsMethine/polymethine dyesCyaninePhotoacoustic imaging in biomedicine
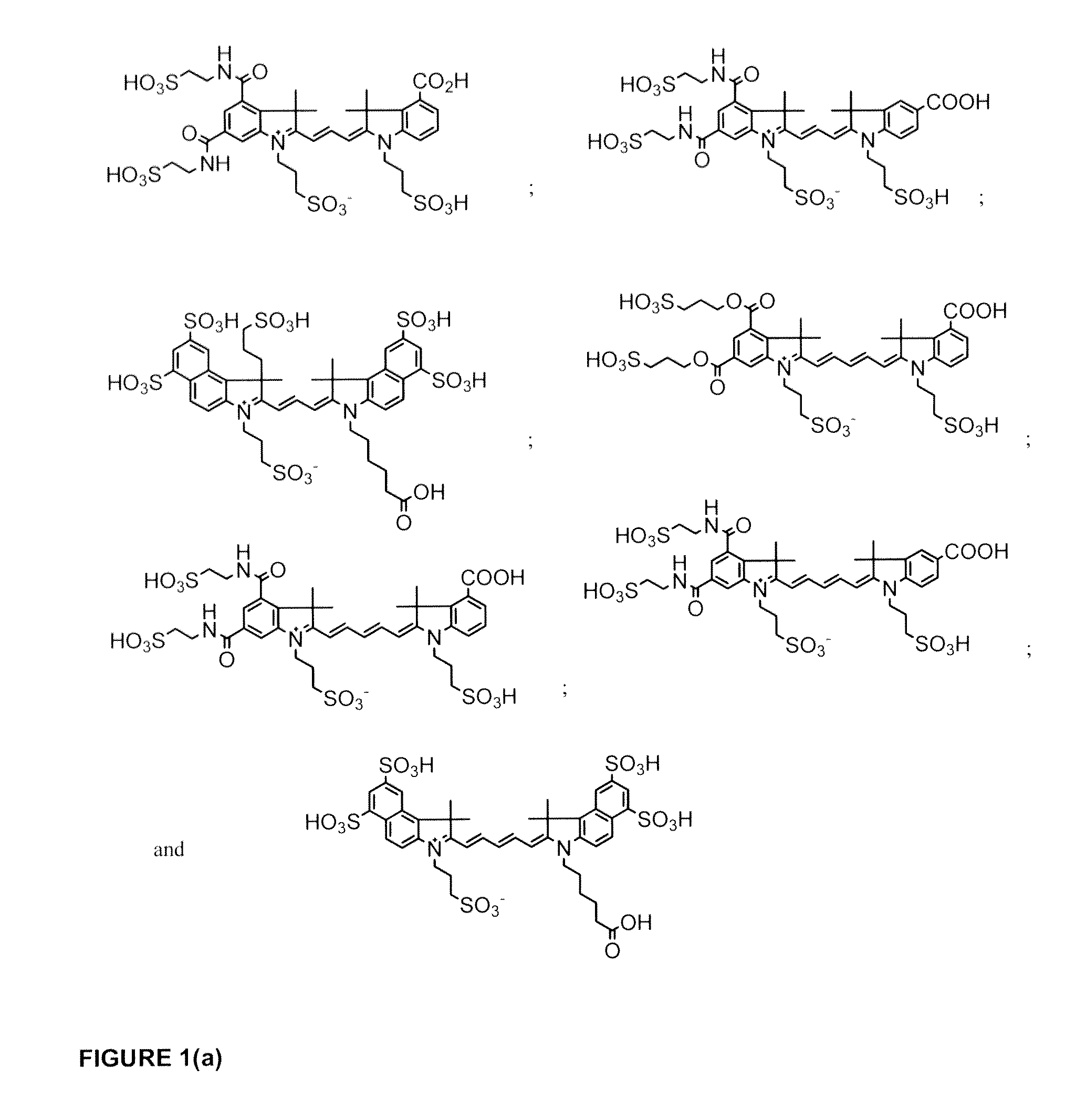
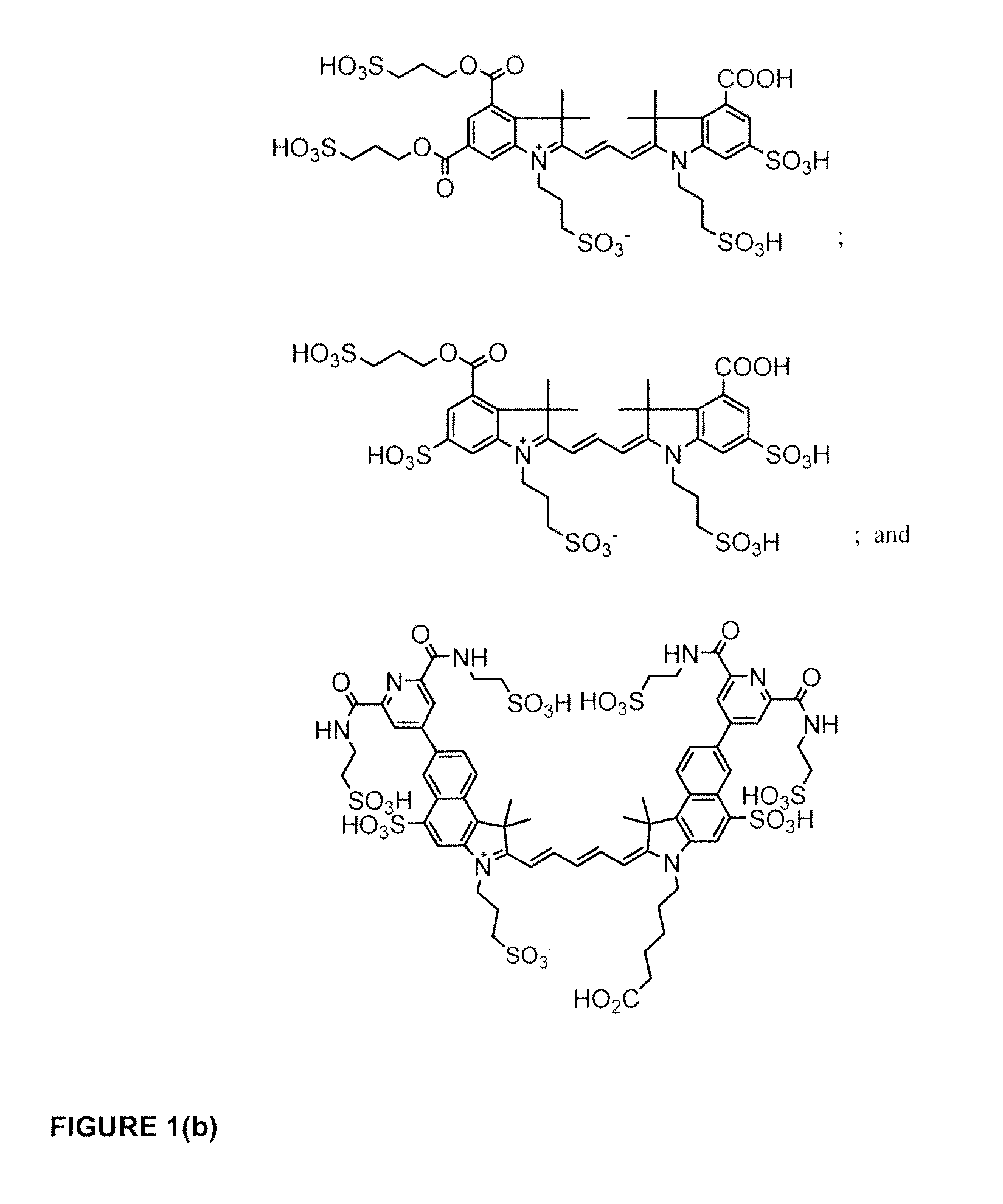
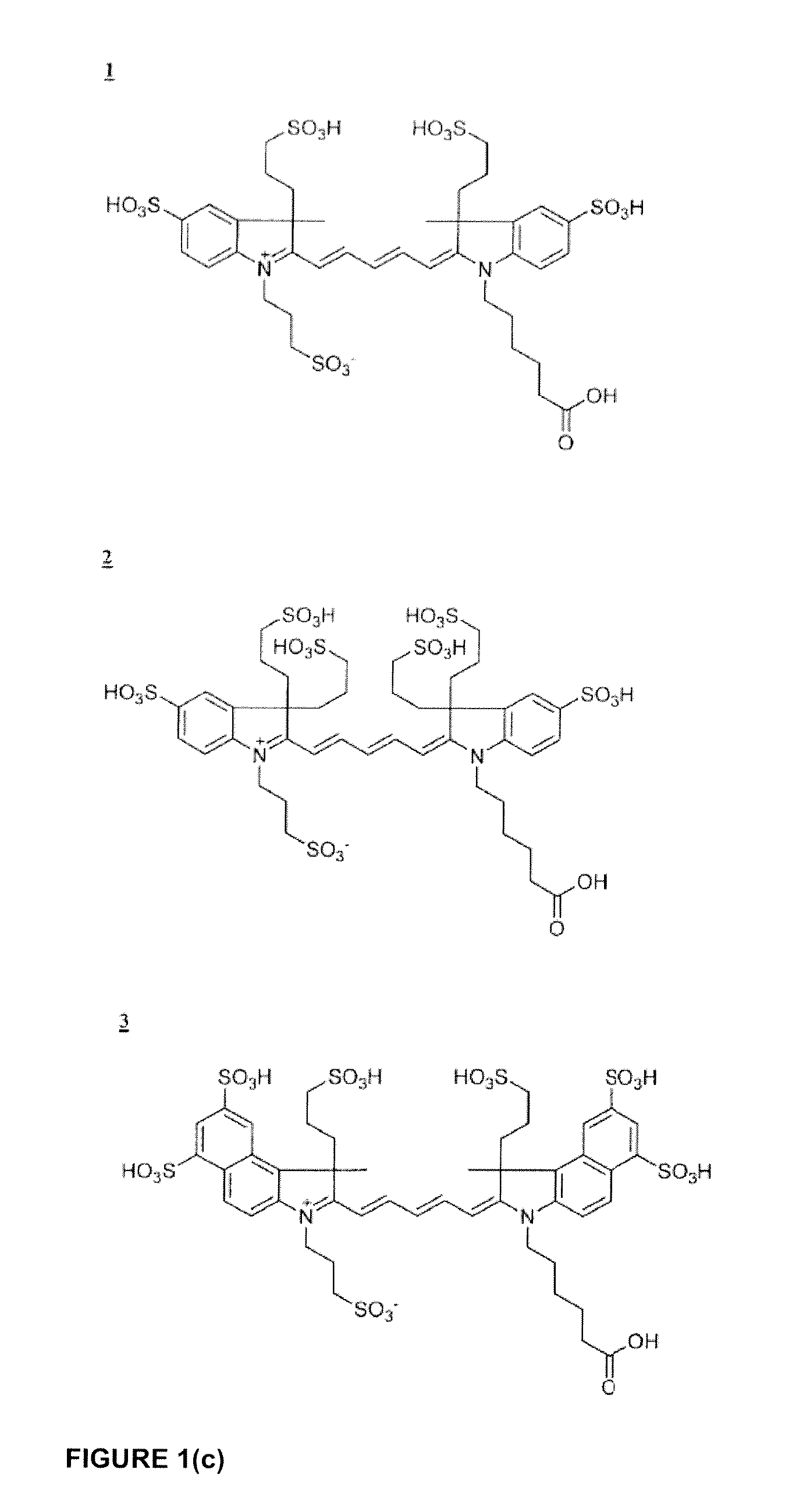
Cyanine dye bioconjugates useful for diagnostic imaging and therapy are disclosed. The conjugates include several cyanine dyes with a variety of bis- and tetrakis (carboxylic acid) homologues. The compounds may be conjugated to bioactive peptides, carbohydrates, hormones, drugs, or other bioactive agents. The small size of the compounds allows more favorable delivery to tumor cells as compared to larger molecular weight imaging agents. The various dyes are useful over the range of 350-1300 nm, the exact range being dependent upon the particular dye. Use of dimethylsulfoxide helps to maintain the fluorescence of the compounds. The inventive compounds are useful for diagnostic imaging and therapy, in endoscopic applications for the detection of tumors and other abnormalities, for localized therapy, for photoacoustic tumor imaging, detection and therapy, and for sonofluorescence tumor imaging, detection and therapy.
Owner:MALLINCKRODT INC
Lithographic printing method and presensitized plate
ActiveUS20040197701A1Practical amount of energyIncrease impressionRadiation applicationsPhotomechanical apparatusDigital dataNitrogen
Disclosed is a presensitized plate composed of a support having thereon an image recording layer which includes: an infrared absorber (A) that is a cyanine dye having at least one fused ring composed of a nitrogen-containing heterocycle in combination with an aromatic ring or a second heterocycle, and having on the aromatic ring or second heterocycle an electron-withdrawing group or a heavy atom-containing group, a radical generator (B), and a radical-polymerizable compound (C), and which is removable with printing ink and / or dampening water. The presensitized of the present invention can be imaged with an infrared light-emitting laser to directly record an image from digital data on a computer or the like and is then subjected to on-machine development without carrying out a development step, which is capable of providing a large number of good impressions with a practical amount of energy.
Owner:FUJIFILM CORP
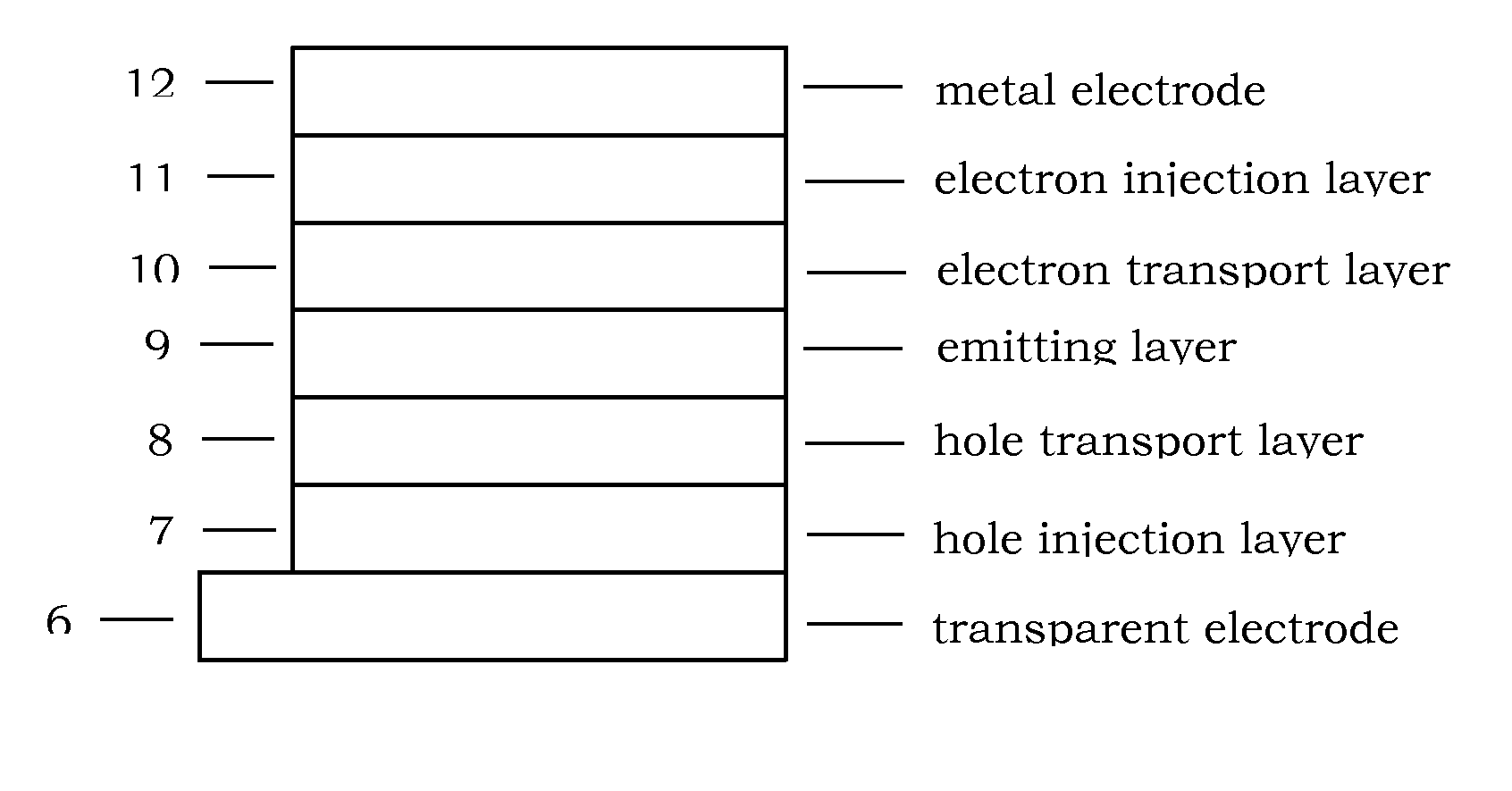
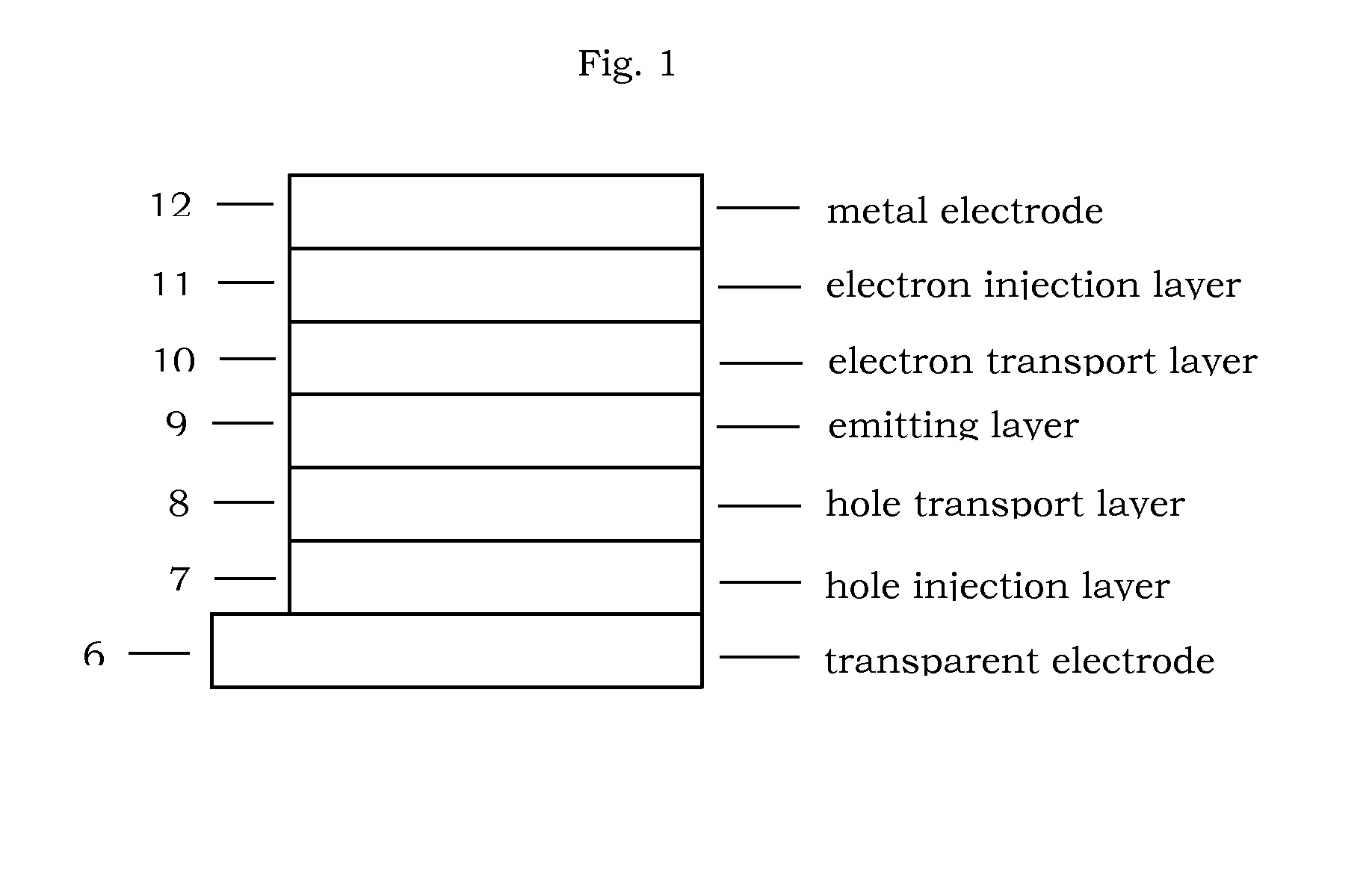
Photoelectric conversion device
InactiveUS20080236663A1Improve conversion efficiencyMethine/polymethine dyesElectrolytic capacitorsCyaninePhotoelectric conversion
Provided is a photoelectric conversion device capable of improving a conversion efficiency. In a dye-sensitized photoelectric conversion device including a working electrode and a facing electrode, and an electrolyte inclusion, a dye is carried on a metal-oxide semiconductor layer of the working electrode. The dye includes cyanine dye having a benzyl group and an indolenine skeleton. Therefore, crystallization of the dye on the surface of the metal-oxide semiconductor layer is suppressed.
Owner:ADEKA CORP
Receptor-avid exogenous optical contrast and therapeutic agents
InactiveUS20050281741A1Enhance tumor detectionPreserve fluorescence efficiencyUltrasonic/sonic/infrasonic diagnosticsMethine/polymethine dyesAbnormal tissue growthImaging agent
Cyanine and Indocyanine dye compounds and bioconjugates are disclosed. The present invention includes several cyanine and indocyanine dyes, including bioconjugates of the same, with a variety of bis- and tetrakis (carboxylic acid) homologues. The compounds of the invention may be conjugated to bioactive peptides, carbohydrates, hormones, drugs, or other bioactive agents. The small size of compounds of the invention allows favorable delivery to tumor cells as compared to larger molecular weight imaging agents. Further, use of a biocompatible organic solvent such as dimethylsulfoxide may be said to assist in maintaining the fluorescence of compounds of the invention. The compounds and bioconjugates herein disclosed are useful in a variety of medical applications including, but not limited to, diagnostic imaging and therapy, endoscopic applications for the detection of tumors and other abnormalities, localized therapy, photoacoustic tumor imaging, detection and therapy, and sonofluorescence tumor imaging, detection and therapy.
Owner:MEDIBEACON
Rigidized monomethine cyanines
InactiveUS6207464B1Easy to detectMinimal levelMethine/polymethine dyesMicrobiological testing/measurementCyanineSulfur
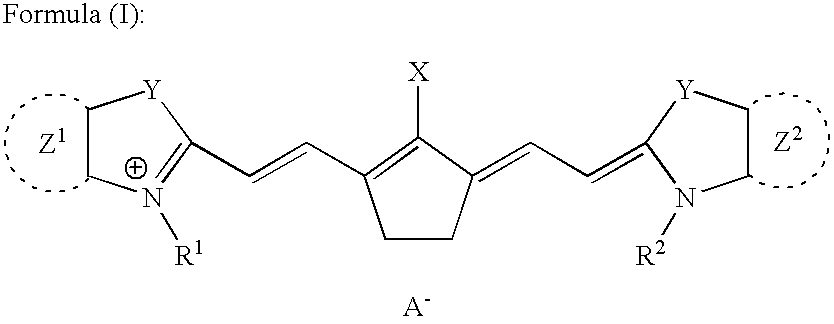
Boron complexes of certain bis-heterocyclic compounds are provided. The complexes resemble monomethine cyanines and are useful for imparting fluorescent properties to materials by covalent and noncovalent association. The compounds have the following general formula:wherein the dotted lines Z1 and Z2 represent the atoms necessary to complete a structure selected from the group consisting of one ring, two fused rings, and three fused rings, each said ring having five or six atoms, and each said ring comprising carbon atoms and, optionally, no more than two atoms selected from oxygen, nitrogen and sulfur, and R1 through R5 represent various atoms or groups and M is Cl or F.
Owner:CARNEGIE MELLON UNIV
Activatable cell penetrating peptides with quenched fluorophores
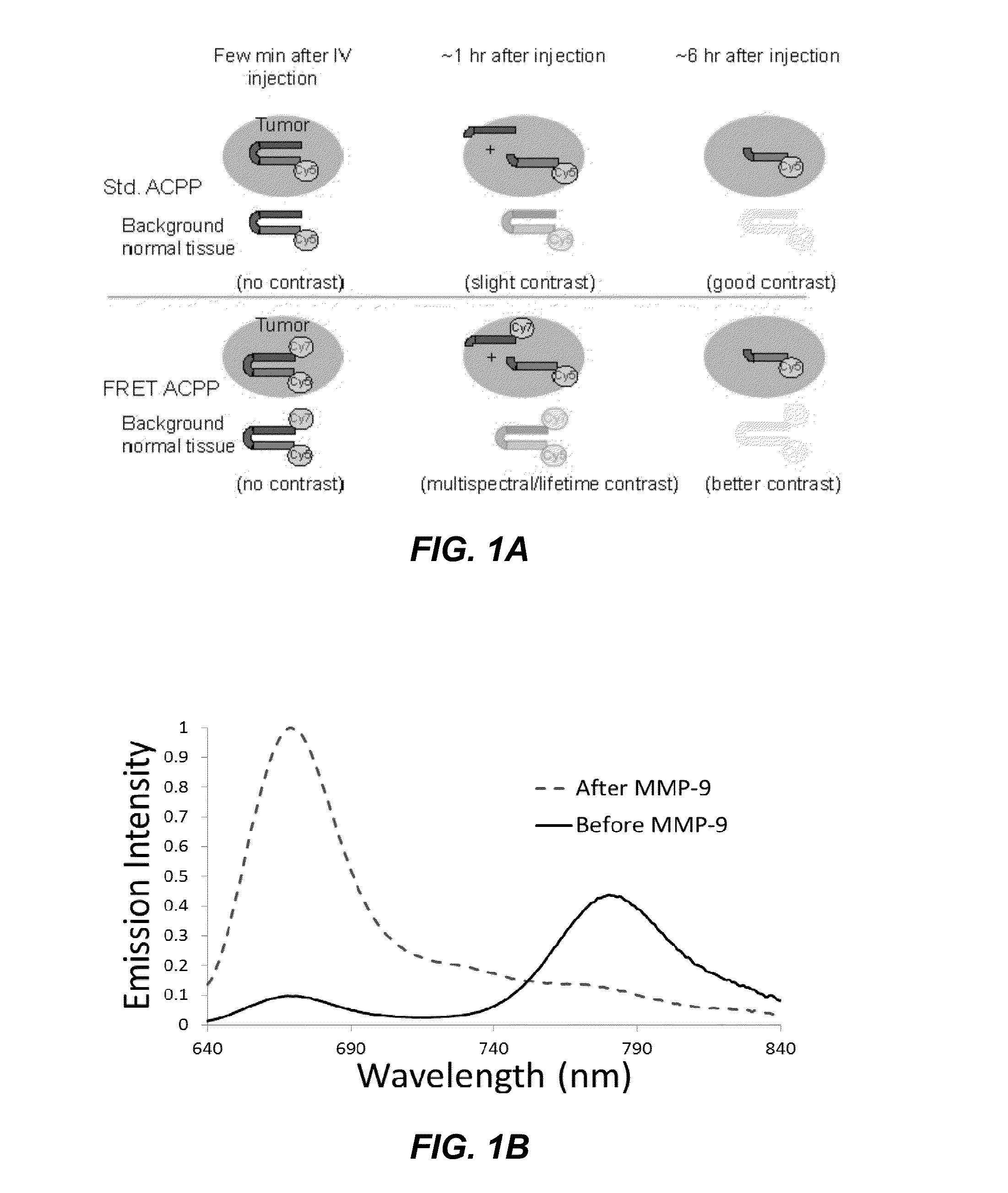
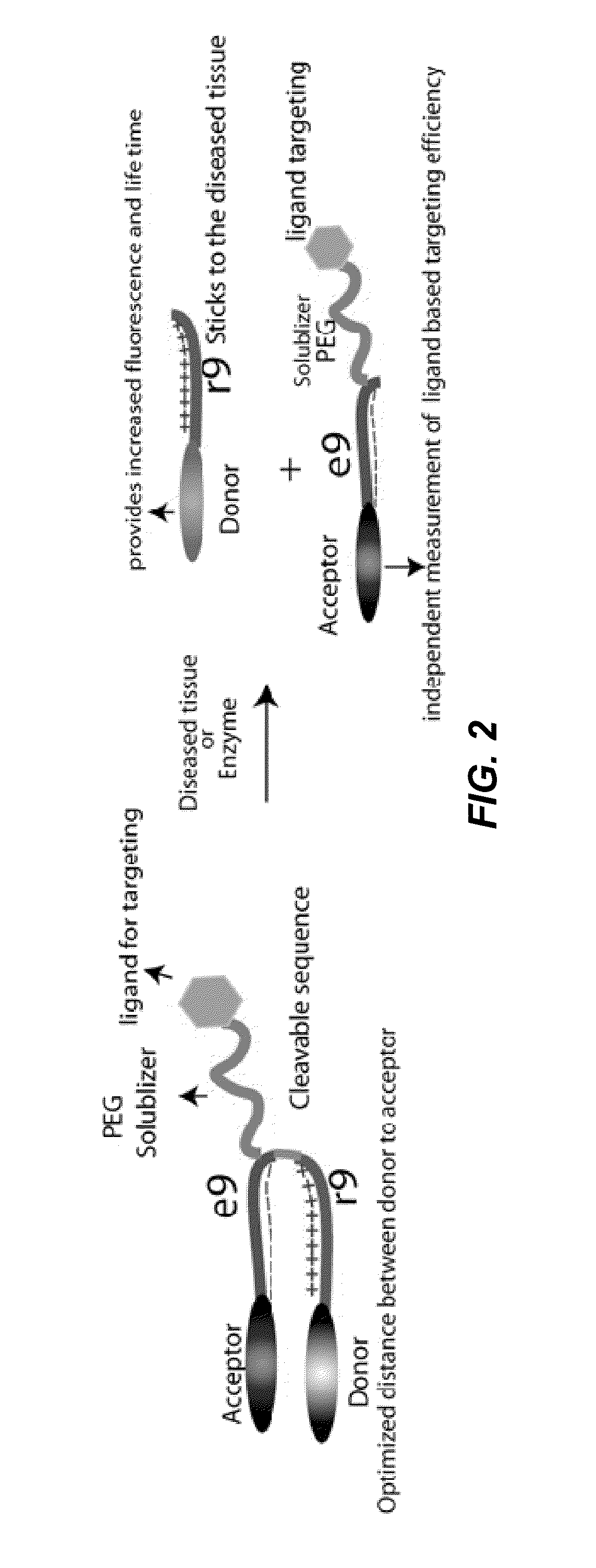
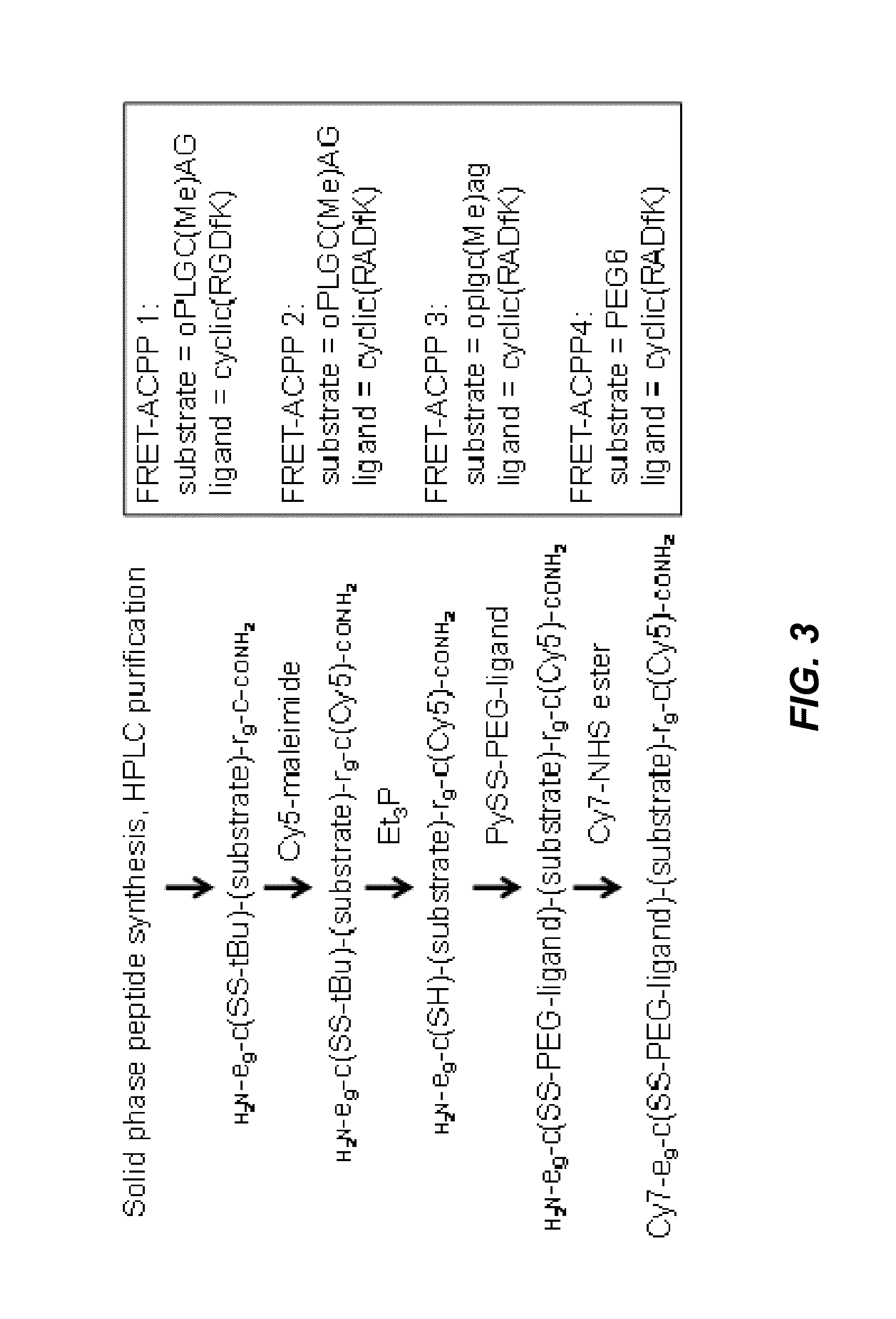
ActiveUS20130078188A1Ultrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsCyanineFluorophore
The invention provides compositions useful as molecular probes. In particular, the invention provides activatable cell penetrating peptides comprising a fluorescence donor and a fluorescence acceptor. Exemplary fluorescence donors and fluorescence acceptors include compounds derived from cyanine. Also provided are ratiometric, multispectral, and excitation lifetime imaging methods for detecting the molecular probes provided herein.
Owner:RGT UNIV OF CALIFORNIA
Hydrophilic labels for biomolecules
Compounds, compositions, and methods for optical, including fluorescence optical, determinations useful in labelling biomolecules such as protein and deoxyribonucleic acid for their detection and quantitation. The compounds are diasteromeric cyanines with high hydrophilicity and other desirable properties.
Owner:DYOMICS
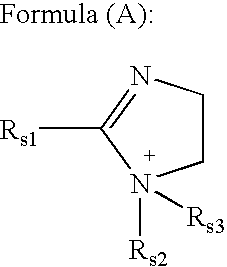
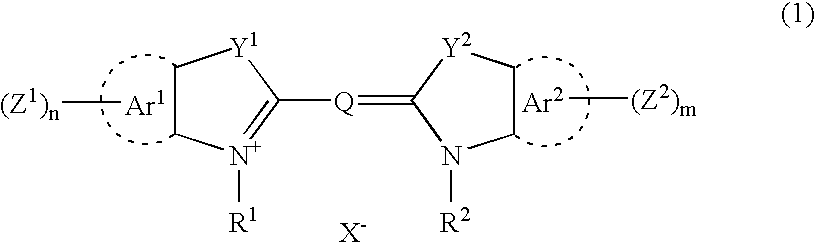
Unsymmetrical cyanines fluorochrome
The invention provides type I unsymmetrical cyanine fluorescent dye, wherein X, n, R1, R2, R3, R4, R5 and Y<-> are defined as in the instruction book. The invention also provides the conjugate and the preparation method of the fluorescent dye, the combination containing the fluorescent dye and the biological dyeing method by using the fluorescent dye or the combination.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com