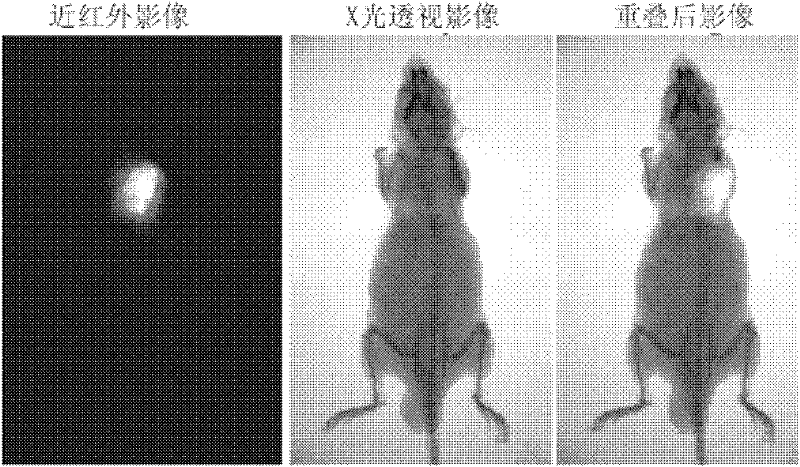
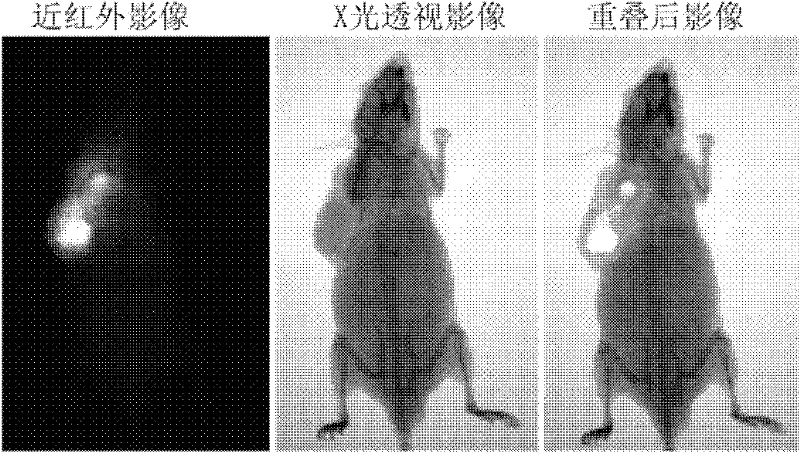
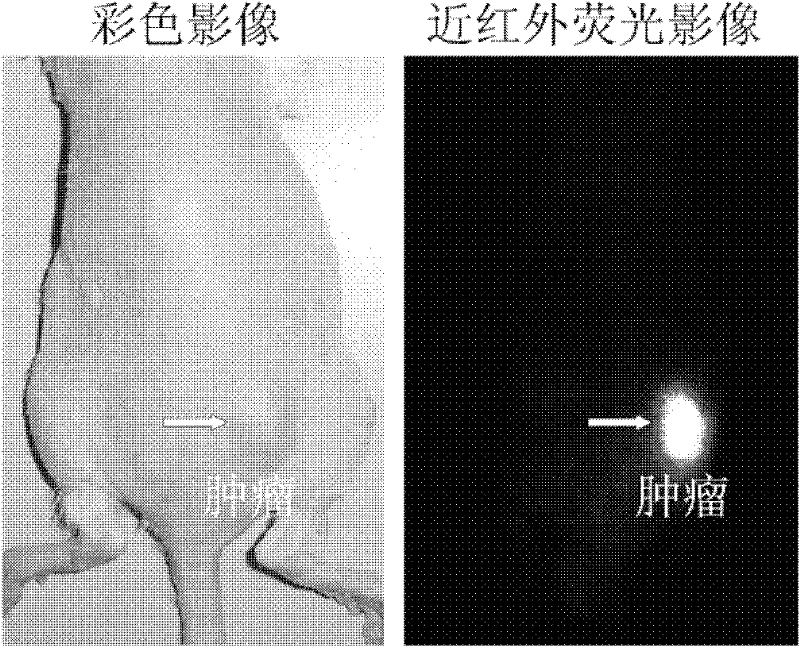
Heptamethanindocyanine dye and its synthesis method and application
A technology of indole cyanine and dyes, applied in the direction of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of limiting the application of anti-tumor therapy, not having direct killing of tumor cells, etc. Easy handling and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment 1 dialdehyde chlorocyclohexene (2-chloro-3-hydroxymethane-1-formyl cyclohexene)
[0082]
[0083] Control the internal temperature at 0-5°C, and slowly add 20ml (freshly evaporated to dry) POCl to the reactor containing 60ml (freshly evaporated to dry) DMF under stirring 3 After dropping, keep stirring at 0-5°C for another 30 minutes, then slowly rise to room temperature. 5ml of cyclohexanone was added, and the temperature was raised to 50°C for 6h. Stop the reaction, pour the reaction solution into water, and stir for 3 hours. A large number of yellow solids precipitated, filtered with suction, and the filter cake was washed with cold water until neutral. The crude product was refined with ethyl acetate to obtain 5.2 g of yellow granular crystals, with a yield of 65.3%. Store away from light and low temperature (4°C). Melting point: 130-131°C (literature melting point: 130-131°C) detection by thin layer chromatography: cyclohexane: e...
Embodiment 2
[0084] The preparation of embodiment 2N-hexanoic acid indole quaternary ammonium bromide (2,3,3-trimethyl-1-hexanoic acid-3H-indole bromide)
[0085]
[0086] Add 2,2,3-trimethyl-3H indole, 6-bromohexanoic acid and 1,2-o-dichlorobenzene (or DMF, or DMSO or toluene or ethylene glycol dimethyl ether) in the reactor, Reaction at 110°C for 12h. Check the reaction solution with fluorescence or bromocresol chloride, 6-bromohexanoic acid is fully involved in the reaction. Stop the reaction, cool to room temperature, and let stand for 10 hours. A large amount of yellow-brown solid precipitated out of the system, which was filtered with suction, and the filter cake was repeatedly washed with acetone until it became an off-white solid. The crude product does not need to be further purified, and can be directly carried out in the next step. Melting point range: 90-92°C; Thin-layer chromatography detection: methanol: chloroform (1:5), Rf value is about 0.3, inspected under 254nm ult...
Embodiment 3
[0087] The synthesis (29,34) of the heptamethine indocyanine dye of embodiment 3 double n-butyl ester or monobutyl ester side chain
[0088]
[0089] Add 2.780g indole quaternary ammonium salt 25 (7.809mmol) in the 500ml single port reaction flask, 0.645g condensing agent 29 (3.728mmol), use 250ml n-butanol alone, or with benzene (or toluene or hexanaphthene) mixed solution ( 7:3) was used as a solvent, a water separator and a condenser were installed, and under the protection of nitrogen, the reaction was refluxed at an external temperature of 120° C. for 5 hours, and the system turned from red to green. Stop the reaction, and distill under reduced pressure (0.098Mp, 60°C) to remove the solvent benzene and n-butanol to obtain 3.395g bright yellow solid (dark green), and obtain 3.395g bright yellow solid (dark green), through silica gel column chromatography Compound 29 (yield 60%) and compound 34 (yield 30%) were obtained respectively.
[0090] Structural analysis data of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com