Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Cause of death" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
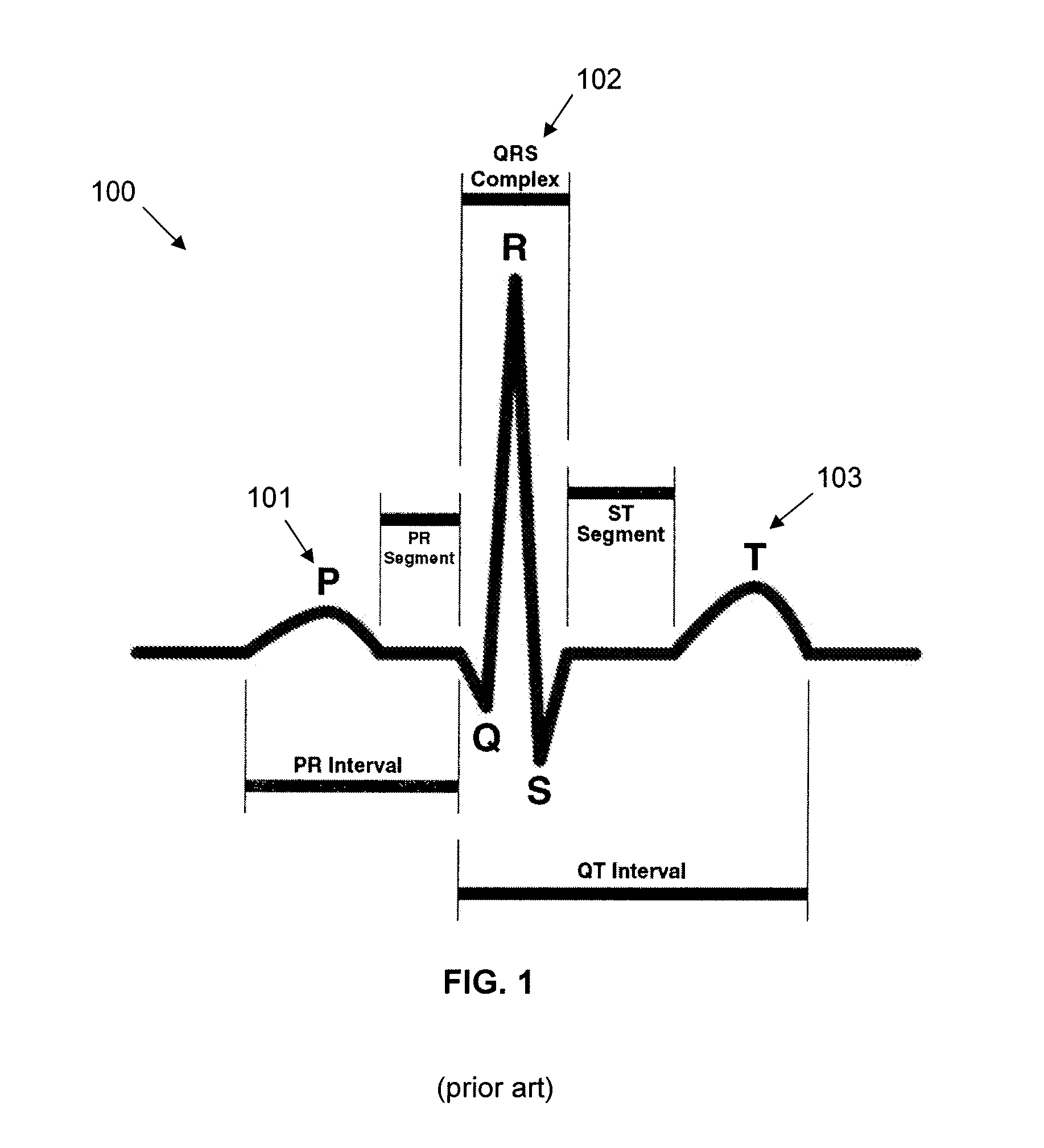
In law, medicine, and statistics, cause of death is an official determination of conditions resulting in a human's death, which may be recorded on a death certificate. A cause of death is determined by a medical examiner. The cause of death is a specific disease or injury, in contrast to the manner of death which is a small number of categories like "natural", "accident", and "homicide", which have different legal implications.
Compositions for control of drug abuse
InactiveUS20130295170A1Challenge can be overcomePrevent degradationBiocidePowder deliveryBenzodiazepinePersulfate
Opiates, amphetamines, barbiturates and other drugs such as benzodiazepines are extensively abused or misused and are frequently the cause of death by overdosing. These drugs are also prone to oxidation and the final degradation products depend on the reactants and the reaction conditions. This invention describes the use of inactivating agents such as permanganates, peroxides, persulfates, bismuthates, periodates or other oxidants in a dosage form as an approach to minimize abuse and overdose. The product is designed such that the inactivating agent is released if there is an attempt to extract the drug from the formulation or in cases of overdose. Once released, the inactivating agent quickly degrades the drug and converts it into inactive compounds. Since the reactants (drug and inactivating agent) are incompatible in situations of normal drug usage, they are kept separated within the vehicle of the invention, but released for interaction in case of misuse. A catalyst may be included in the formulation to facilitate the reaction.
Owner:KYDES PHARMA
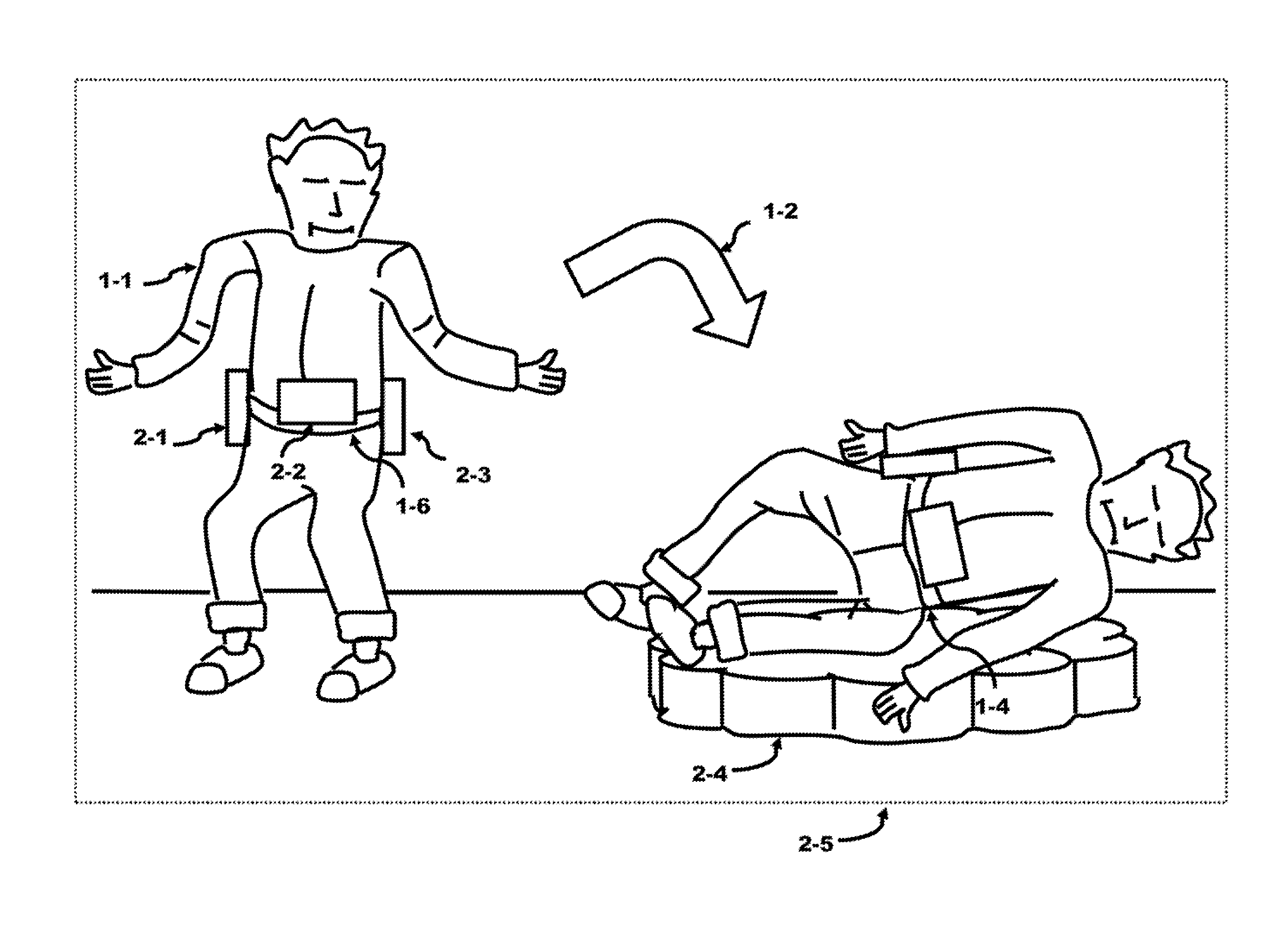
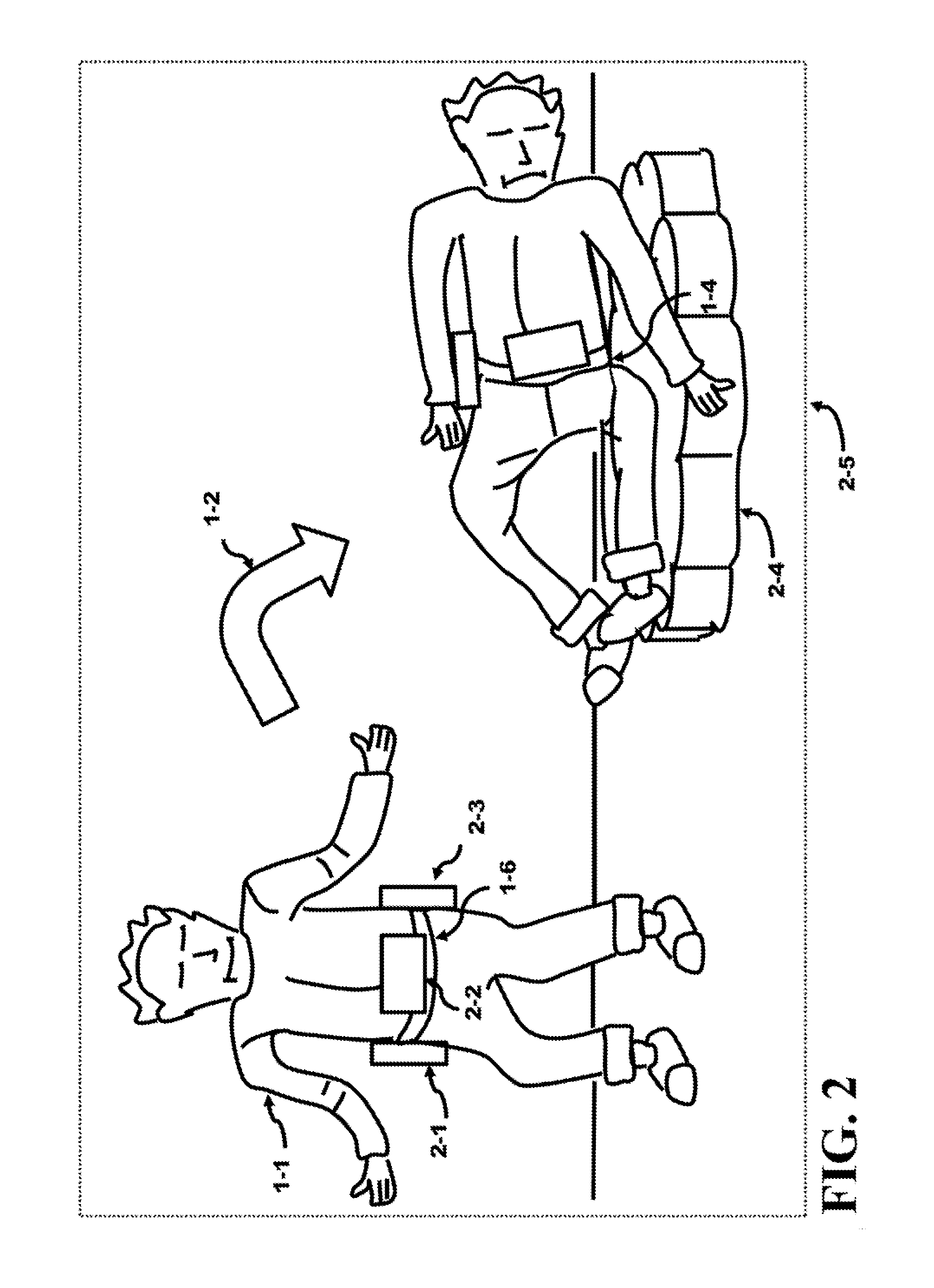
Method and Apparatus of Preventing a Fall or Minimizing the Impact of the Fall of an Individual
InactiveUS20150101112A1Minimize impactIncrease in sizeChemical protectionHeat protectionCause of deathEngineering
Falling causes serious injury to the body and is a leading cause of death in the elderly. An external belt placed over the waist comprises an inertia system and expandable modules. These modules contain packed air bags and can be expanded in size by orders of magnitude using an air tank. The modules are selected by the inertia system that senses the direction of the fall. The selected module is filled with air from the air tank generating a protective mattress which can be used to cushion their fall. Serious injury to the body can be reduced or even prevented. The module instead can contain an expandable support structure to reach the floor and act as a “third leg”. This support structure may be used by the individual to gain control of the individual's balance and stabilize the individual allowing a re-capture of balance and preventing the fall from occurring.
Owner:BALBIEN JOEL ABE +1
System and method to aid diagnoses using cross-referenced knowledge and image databases
ActiveUS8538770B2Data processing applicationsLocal control/monitoringCause of deathRelational database
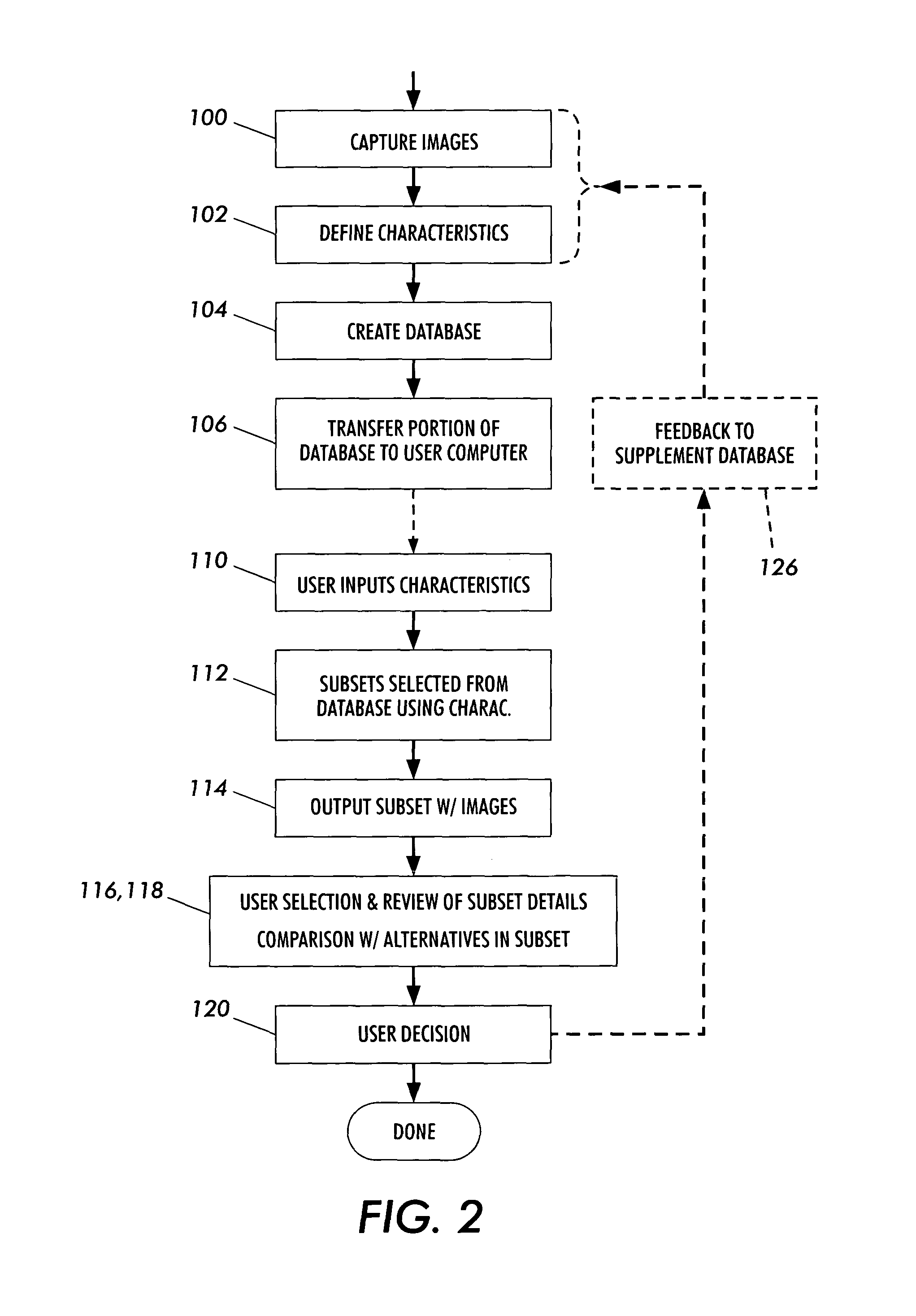
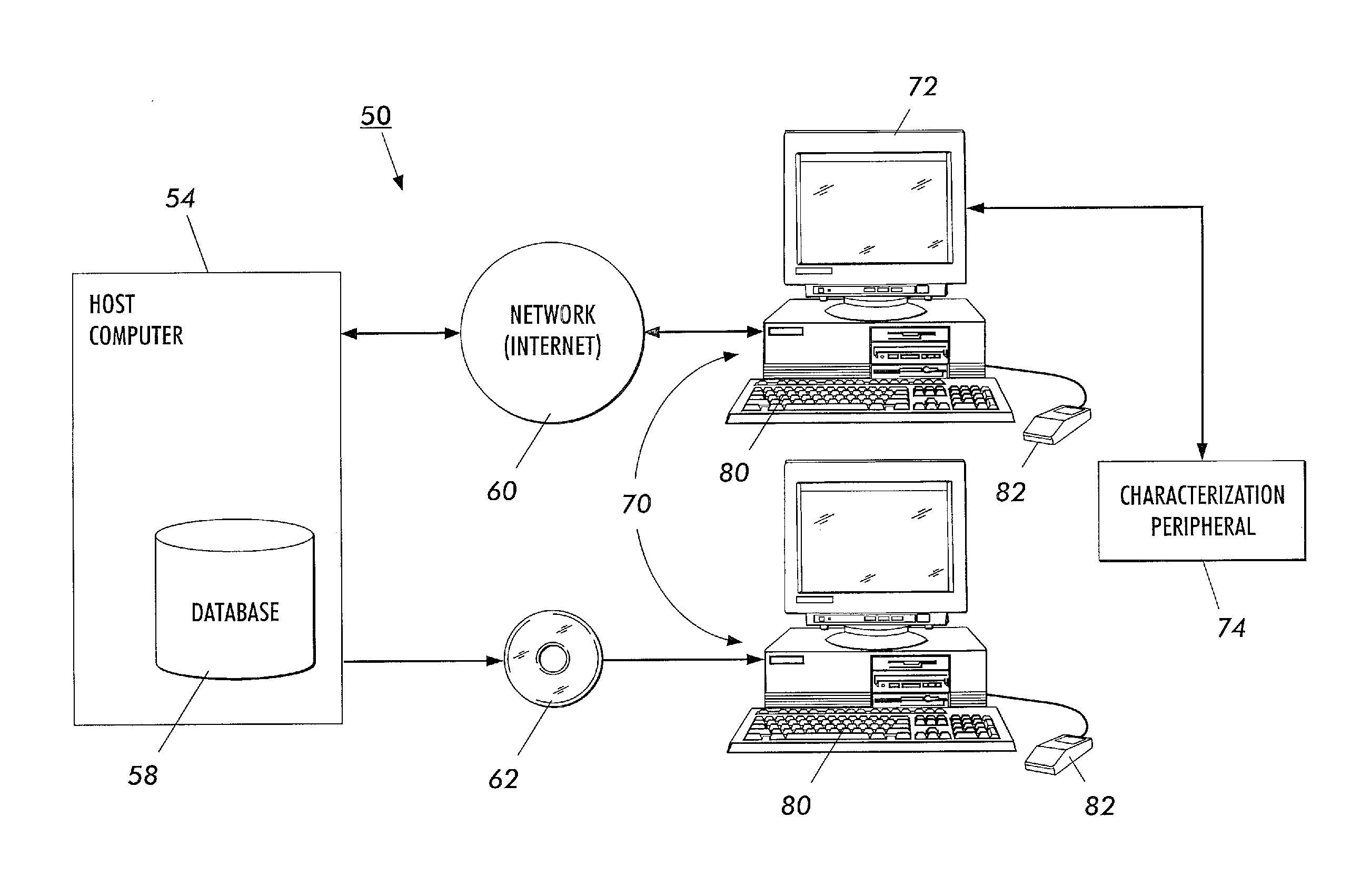
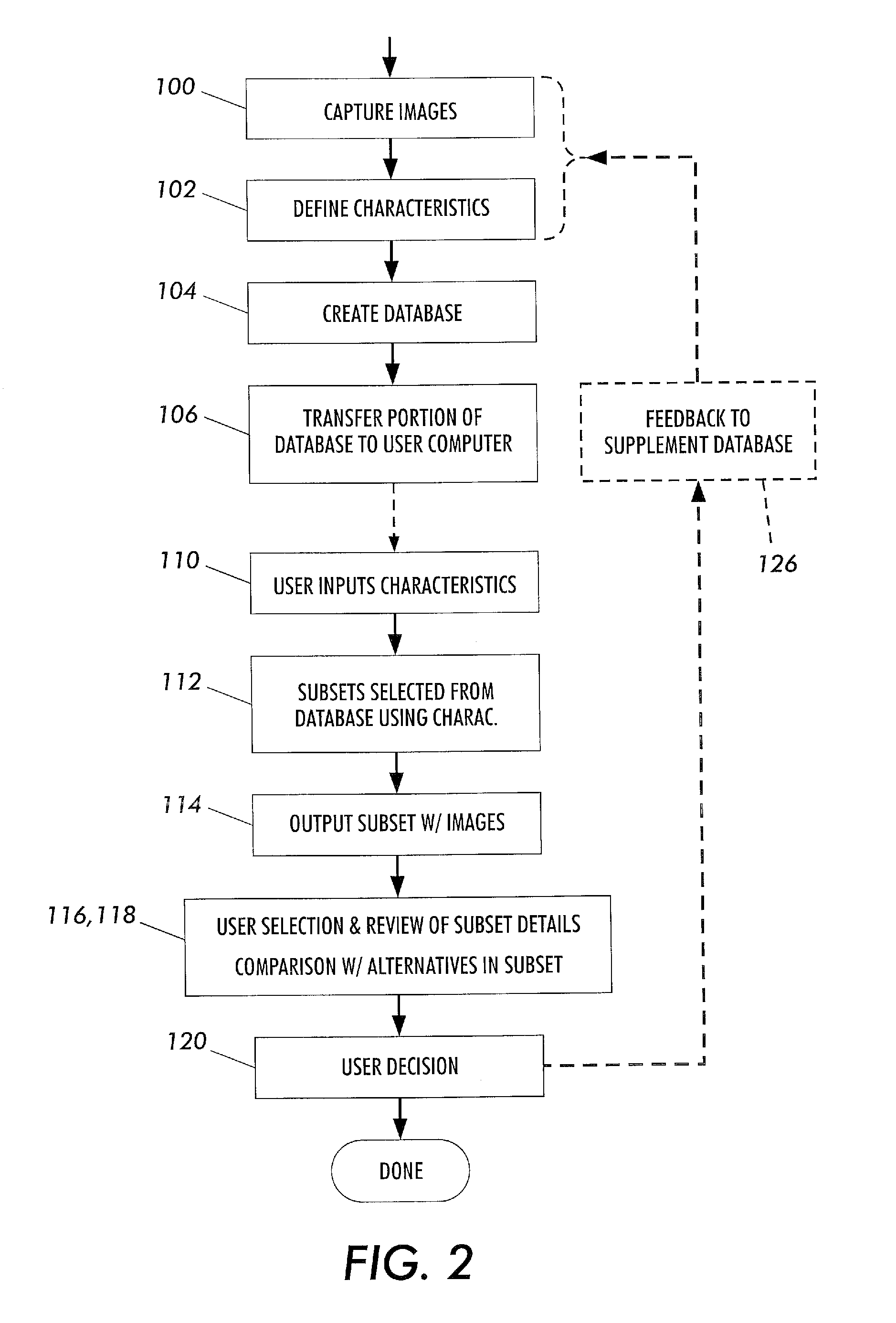
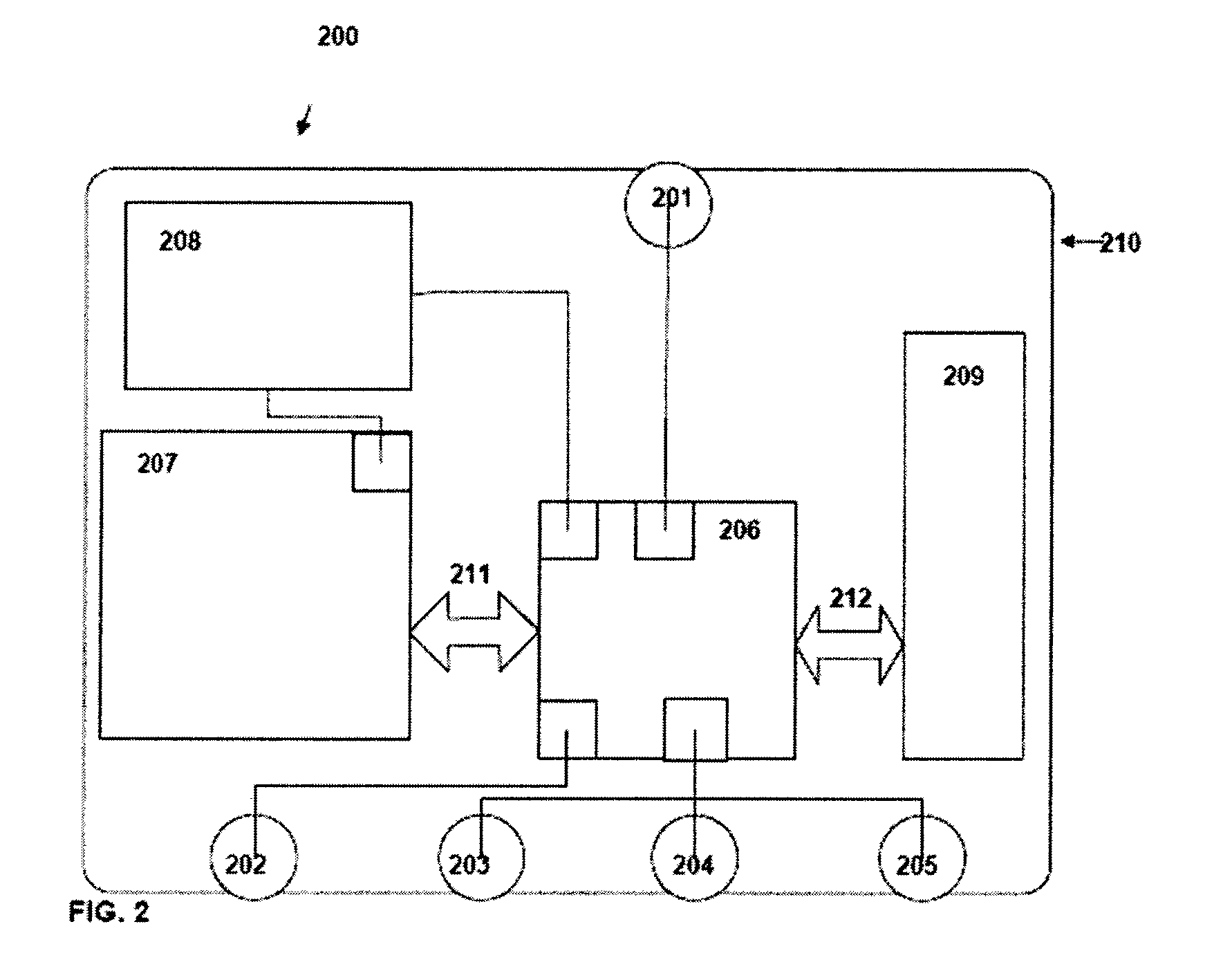
The present invention is a method and apparatus for increasing the usefulness of visual knowledge in a number of applications. It distills the relationships between characteristics and hypotheses into database form, thereby organizing visual information in a manner suitable to aid the user in the investigation of the various hypotheses (medical diagnosis, pill identification, plant / animal identification, cause of death, cause of accident, etc.). The invention sidesteps unresolved issues around knowledge engineering by not automating a decision making process. Rather, the present invention utilizes a relational database to dynamically respond to textual and visual findings as an aid to assist a user reaching a reasoned conclusion based upon information available by direct observation and comparison with stored image and textual data.
Owner:LOGICAL IMAGES
Method for breeding maggots using raw animal material
The invention relates to breeding field and especially relates to a method for breeding maggots using raw animal material. Dead bodies of poultries or livestock, leftovers from bird hatching are cut into small pieces or crushed with water content of 60% to 70%, and the raw animal material comprises dead bodies as well as internal organs, excreta, blood, hairs and bones; other raw material is mixed and then fermentation is carried out on the mixture; the fermented raw material is loaded in plates and then placed at outdoor, breeding houses or fields to attract wild flies to produce maggots under 30 to 35 DEG C with the plate-in-plate mode. Every year, rotten fish and shrimps from different causes of death, eggshells of water eggs and unhatched eggs from bird hatching, dead animal bodies (bones blood, hair, leftovers from restaurants), and house refuse are treated through fly maggot bio-functional quick harmless treatment so that pollution is eliminated and waste is changed into valuables; the method provides a good maggot protein fodder for breeding industry; pollution-free, organic and green food is produced which benefits the nation and the people.
Owner:周辛平
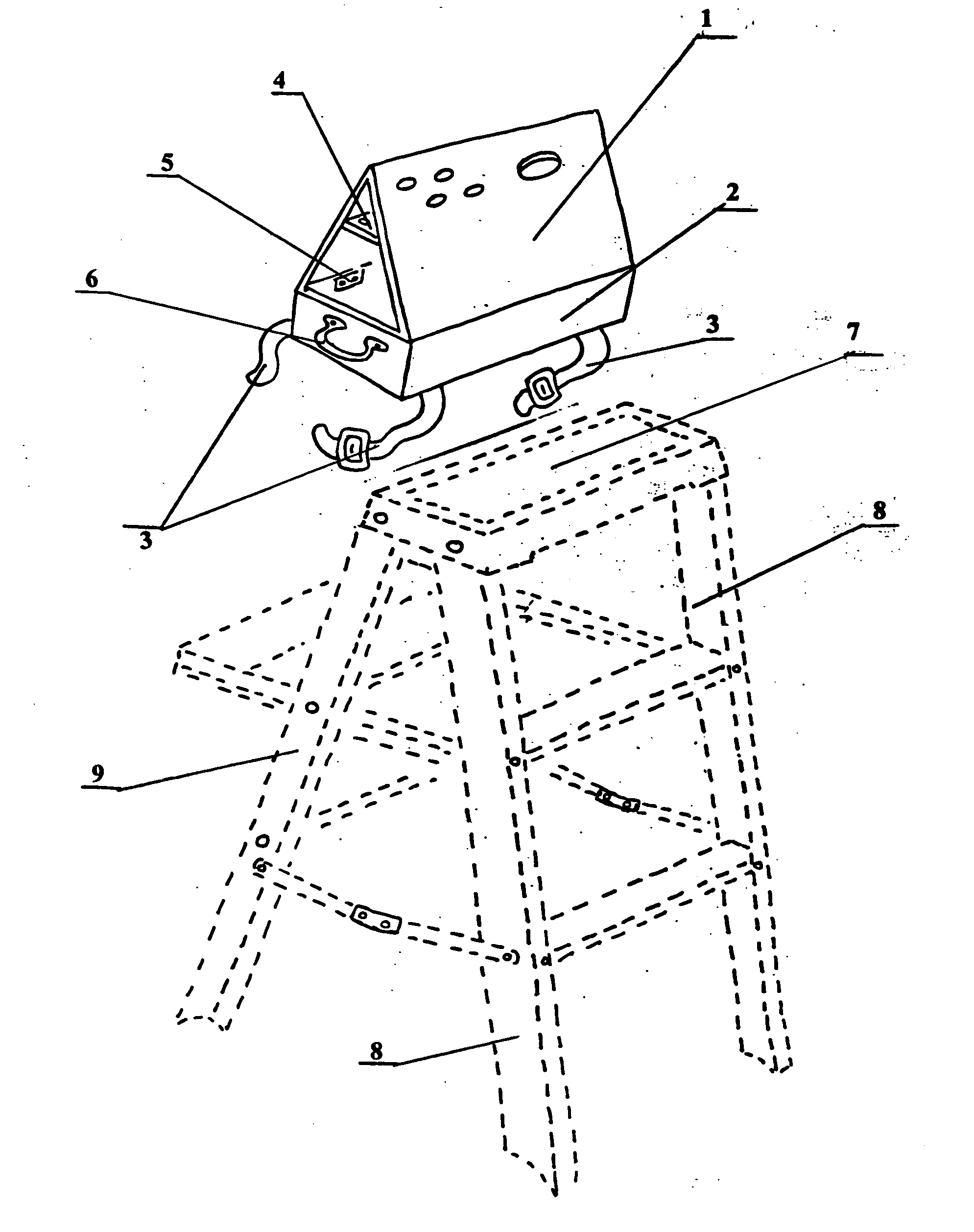
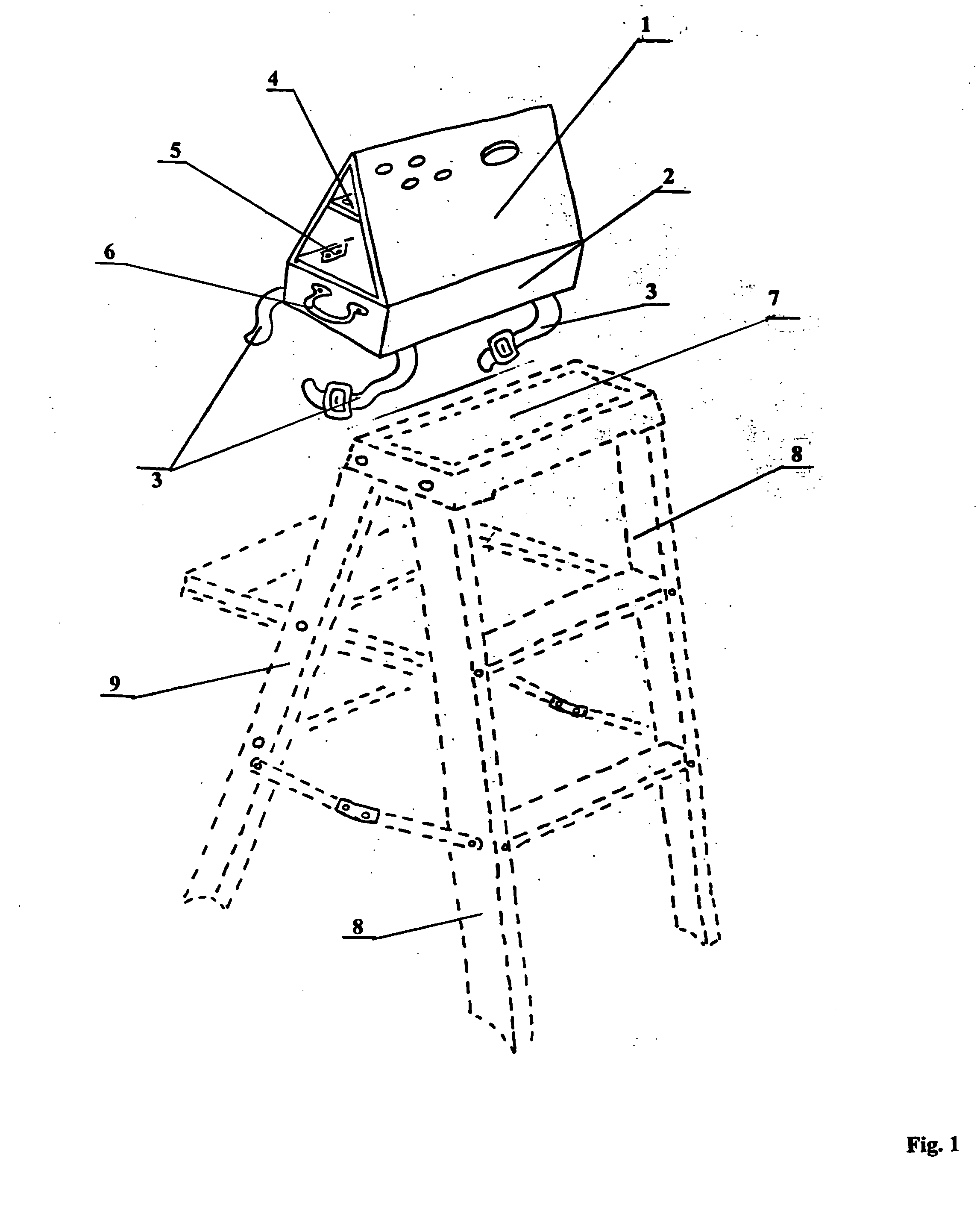
Step ladder safety device
InactiveUS7156205B2Improve efficiencyReduce in quantityOther accessoriesLidsCause of deathEngineering
A novel safety device for step ladders. This safety device is fabricated and positioned so as to deter, dissuade and deny the ladder user access to the unsupported top step of a step ladder while allowing the user to an aloft tool organizer for pre-loading job materials thereby eliminating the need to repeatedly climb up and down the ladder carrying materials. More people are killed doing construction work every year than in any other occupation and falls are the biggest killer of employees in the construction industry, many of these falls involve the improper use of ladders. Falls are the leading cause of death in and around the home, according to the National Safety Council, there are thousands of people die from falls around the home each year and many suffer disabling injuries many of these also involve ladders. At work or at home, these accidents invariably occur because the victims violate the basic rules of ladder safety.
Owner:CUSTOM PRODS ENTERPRISES
System and method to aid diagnoses using cross-referenced knowledge and image databases
InactiveUS20140037162A1Medical automated diagnosisCharacter and pattern recognitionRelational databaseCause of death
Owner:LOGICAL IMAGES
Step ladder safety device
InactiveUS20050150723A1Improve efficiencyReduce in quantityOther accessoriesLidsCause of deathEngineering
A novel safety device for step ladders. This safety device is fabricated and positioned so as to deter, dissuade and deny the ladder user access to the unsupported top step of a step ladder while allowing the user to an aloft tool organizer for pre-loading job materials there-by eliminating the need to repeatedly climb up and down the ladder carrying materials. More people are killed doing construction work every year than in any other occupation and falls are the biggest killer of employees in the construction industry, many of these falls involve the improper use of ladders. Falls are the leading cause of death in and around the home, according to the National Safety Council, there are thousands of people die from falls around the home each year and many suffer disabling injuries many of these also involve ladders. At work or at home, these accidents invariably occur because the victims violate the basic rules of ladder safety.
Owner:CUSTOM PRODS ENTERPRISES
Laser autopsy and cremation
Owner:HIERHOLZER LAWRENCE J
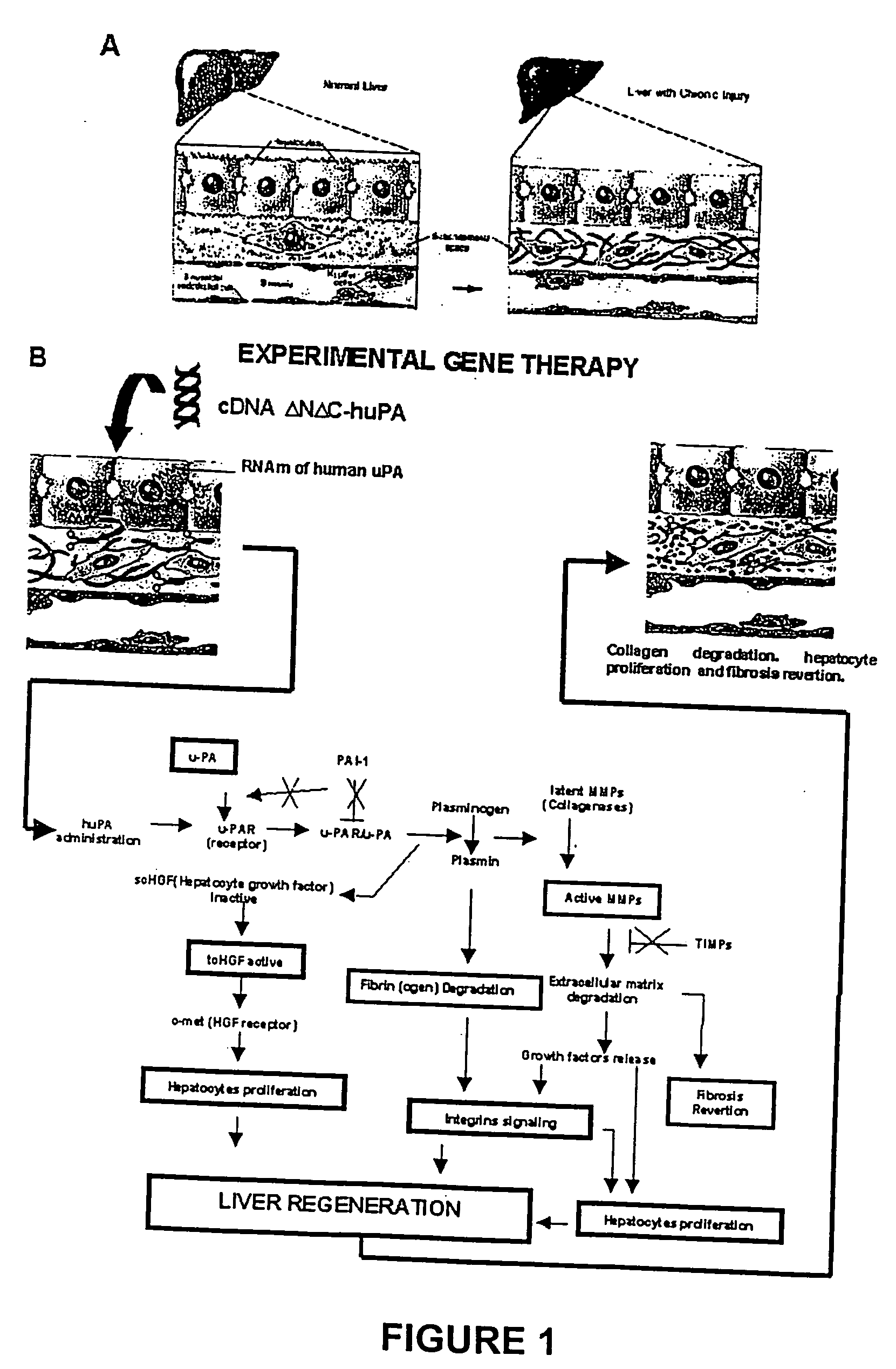
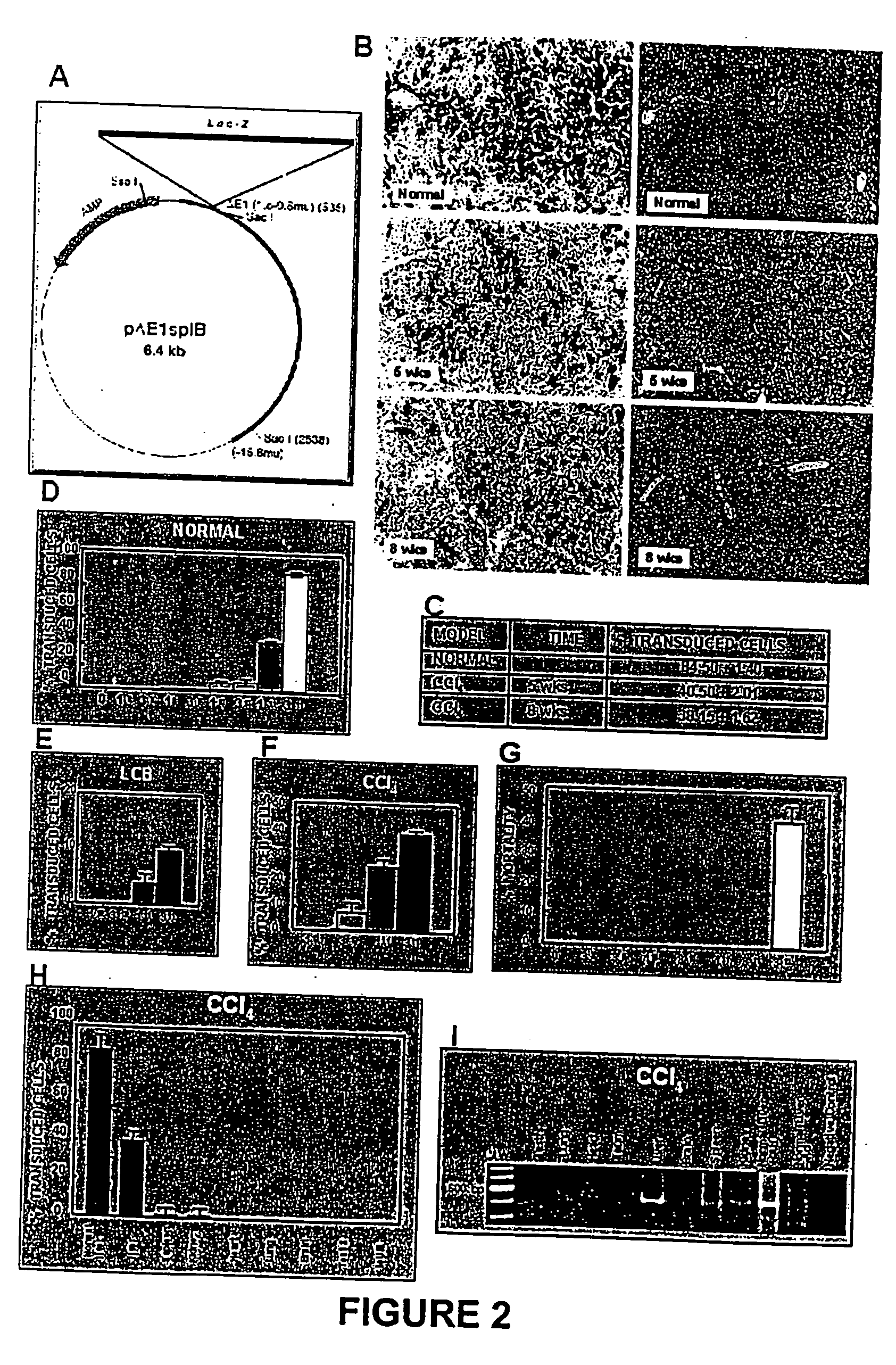
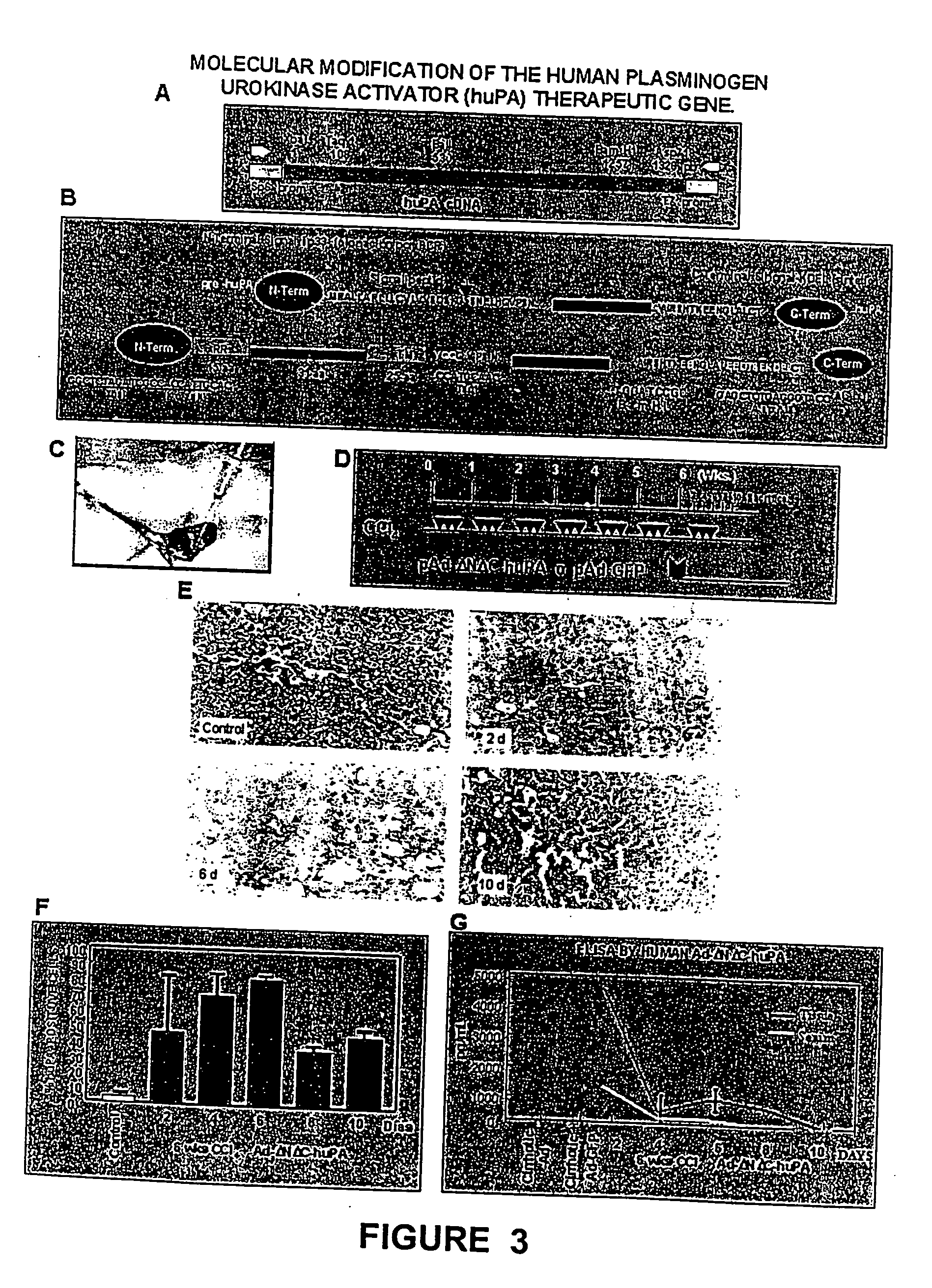
Recombinant viral and non-viral vectors containing the human urokinase plasminogen activator gene and its utilization in the treatment of various types of hepatic, pulmonary, pancreatic and cardiac fibrosis and hypertrophic scars
InactiveUS20040097455A1Reestablishment of liver functionFacilitated DiffusionPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCause of death
Hepatic cirrhosis is considered a severe health problem in Mexico, since it is the third mortality cause in working-age people and there is no 100% effective treatment. Cirrhosis is characterized by an exacerbated increase of collagen in liver parenchyma, replacing the hepatocytes and thus provoking liver failure. This is one of the reasons why we have used a gene therapy through specific delivery to cirrhotic livers of the gene of human urokinase plasminogen activator (huPA), which activates mechanisms that induce the degradation of excess cellular matrix and stimulate hepatocyte proliferation, obtaining thus a fast re-establishment of the liver function. In the instant invention, the modified human uPA gene was inserted in the adenoviral vector (pAd-DeltahuPA), because it is not secreted and does not provoke hypercoagulation or spontaneous internal bleedings. Moreover, data from the bio-distribution essay with an adenoviral vector with reporter gene beta-gal have shown liver specificity as the target organ of the vector. Using ELISA, huPa protein was detected in liver homogenates (4500 pg / ml) in animals treated with pAd-DeltahuPA and was also intracellularly detected through immunochemistry in liver cuts (80% positive cells). huPa induced a dramatic fibrosis reduction (85%) on day 10 of vector administration, compared to control cirrhotic rats and 55% hepatocyte proliferation increase. Liver function tests (ALT, AST, alkaline phosphatase and bilirubin) dropped to nearly normal levels and hepatocyte proliferation was observed. Because of the two beneficial event cascades, gene therapy with modified huPA can be developed as a definite potential treatment for patients with liver cirrhosis.
Owner:TGT LAB DE C V
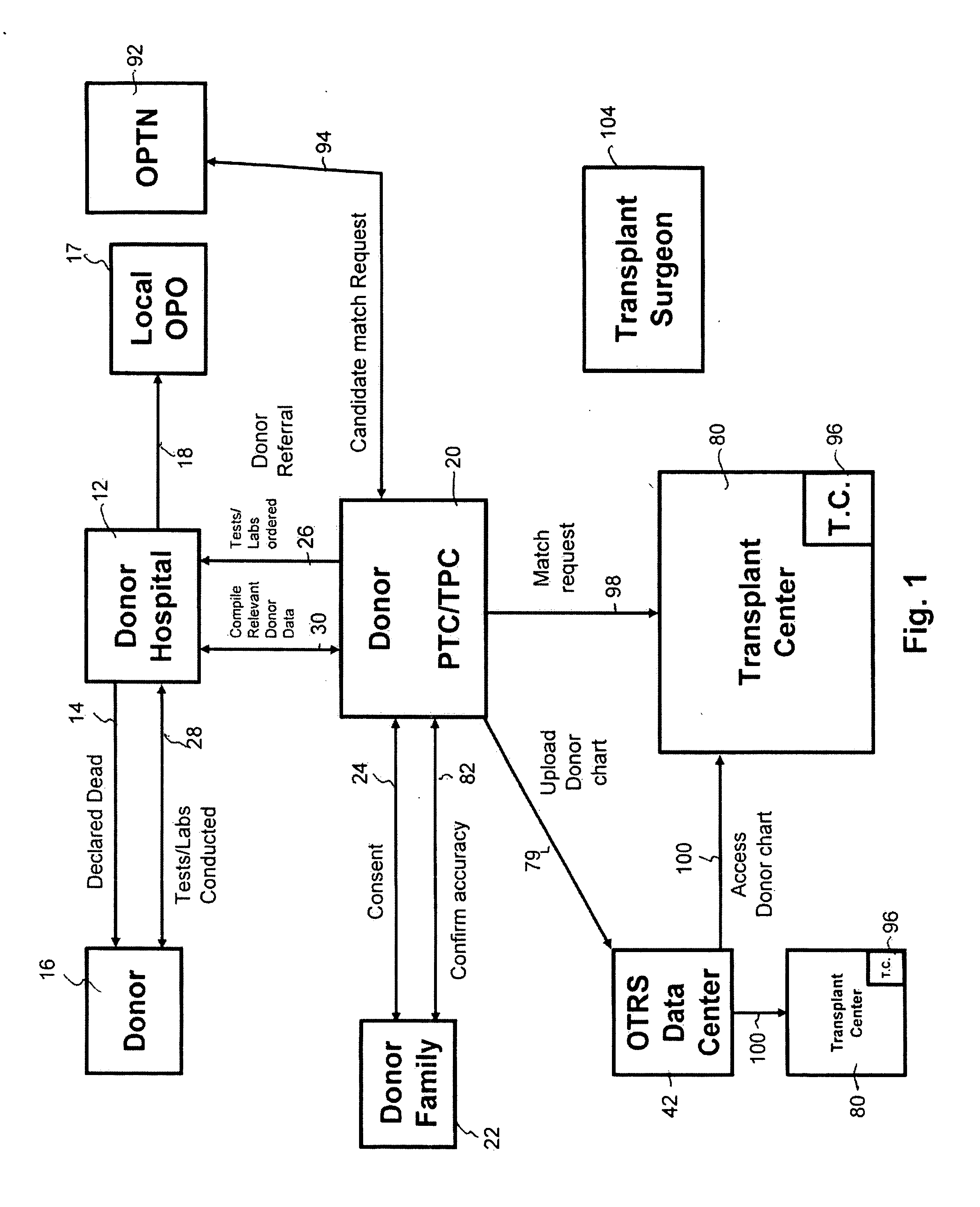
Secure network gateway for accessible patient data and transplant donor data
InactiveUS20050102161A1Shorten the timeQuality improvementSpecial service provision for substationTelemedicineCause of deathPatient data
A method of accessing transplant donor data from a remote location, the method comprising the steps of: (a) accessing a database over a network containing transplant donor data that includes information specific to a potential transplant donor; (b) reviewing at least one of information specific to the potential transplant donor, cause of death of the potential transplant donor, and time of death of the potential transplant donor; and (c) acting on the reviewed transplant donor data to establish qualification to at least one of an organ and a tissue available for transplant.
Owner:KALTHOFF ROBERT MICHAEL +1
Liver cancer metastasis therapeutic device
ActiveCN104207926ASuitable for recovery, recovery and survivalIn line with the laws of natureBlood stagnation preventionDevices for pressing relfex pointsHuman bodyCancer cell
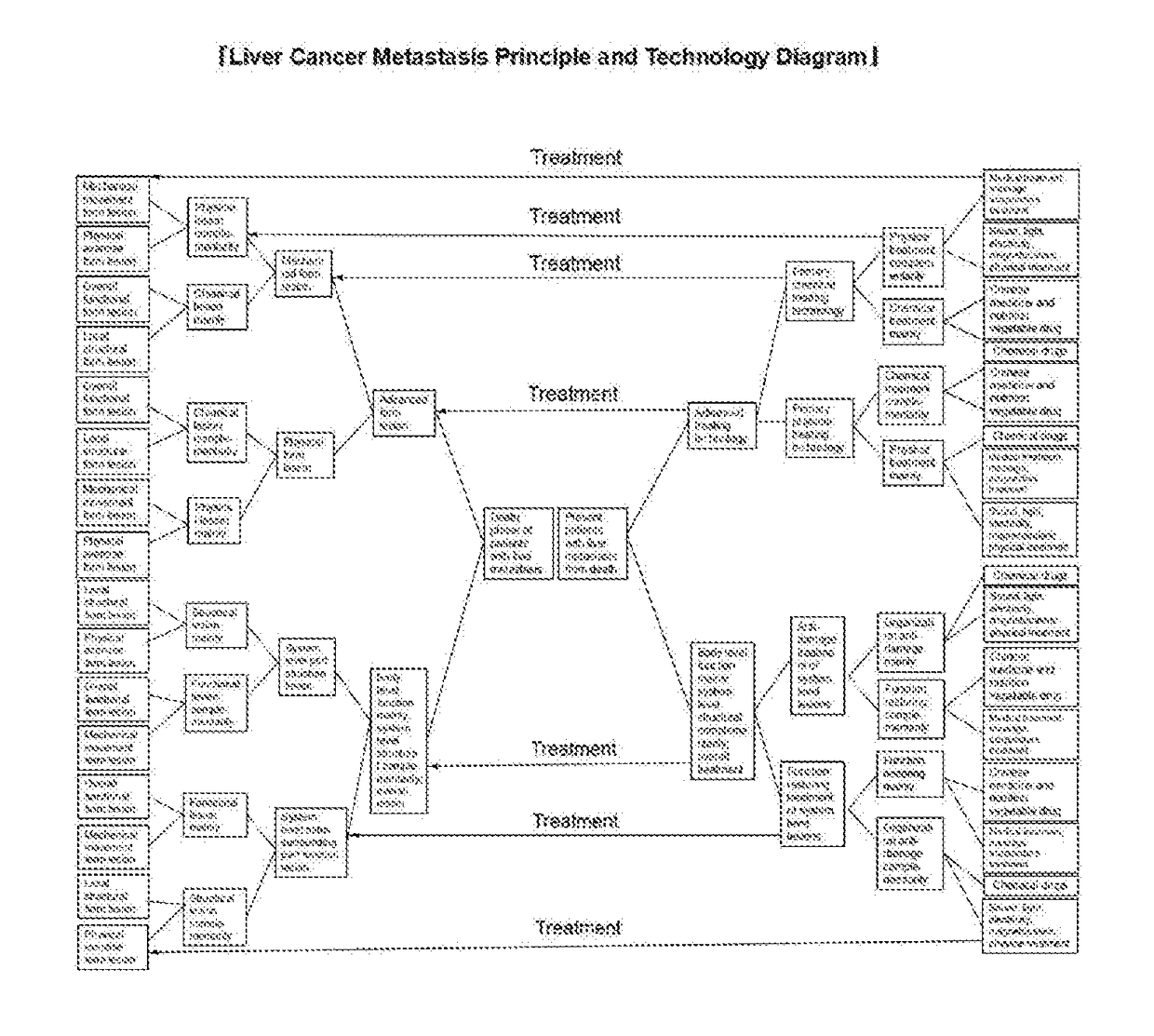
The invention discloses a liver cancer metastasis therapeutic device, which is composed of a base plate, a prone support, a belly medicine massager, a chain wheel, a rotating wheel and a handheld massager, and used for recovering functions of a whole body. As shown in Schematic Diagram of Liver Cancer Metastasis Generation and Development Principle, Schematic Diagram of Liver Cancer Metastasis Therapeutic Principle and Technology, and Schematic Diagram of Death Causes and Death Process Change Rules of Patients with Liver Cancer Metastasis in a specification, in the focus generation period of cancerometastasis, cancer cells are killed mainly for eliminating metastasis focuses, attenuating symptom and delaying development of the focuses; in the death period of cancerometastasis, the functions of the whole body are recovered mainly for controlling development trend of the lesion and preventing death; functional lesion of the whole human body is the main factor for death, and systematic cancerometastasis focuses are secondary factors for death. The liver cancer metastasis therapeutic device is focused on recovering the functions of the whole human body with supplement of performing surgery and chemoradiotherapy for killing cancer cells to perform therapy aiming at causes of death, and thereby the target of significantly reducing a death rate of patients with metastasis of liver cancers and digestive organ cancers is achieved.
Owner:李复生
Big data traditional Chinese medicine disease prevention management system
InactiveCN107153766AEasy to useImprove efficiencySpecial data processing applicationsCause of deathStatistical analysis
The invention provides a big data traditional Chinese medicine disease prevention management system. The system comprises a cloud server, a hospital data interface, an external data interface and a user data interface, wherein the cloud server comprises a data receiving and classified processing module, a resident health archive module, a chronic disease management module, a death cause management module, a community archive management module and a traditional Chinese medicine constitution identification module. The system can obtain health condition data from hospitals, other coherent units and patients, perform analysis and classified processing on the obtained data, judge the constitutions of the patients according to the situations of the patients, and is convenient to use and high in efficiency.
Owner:江阴市中医院
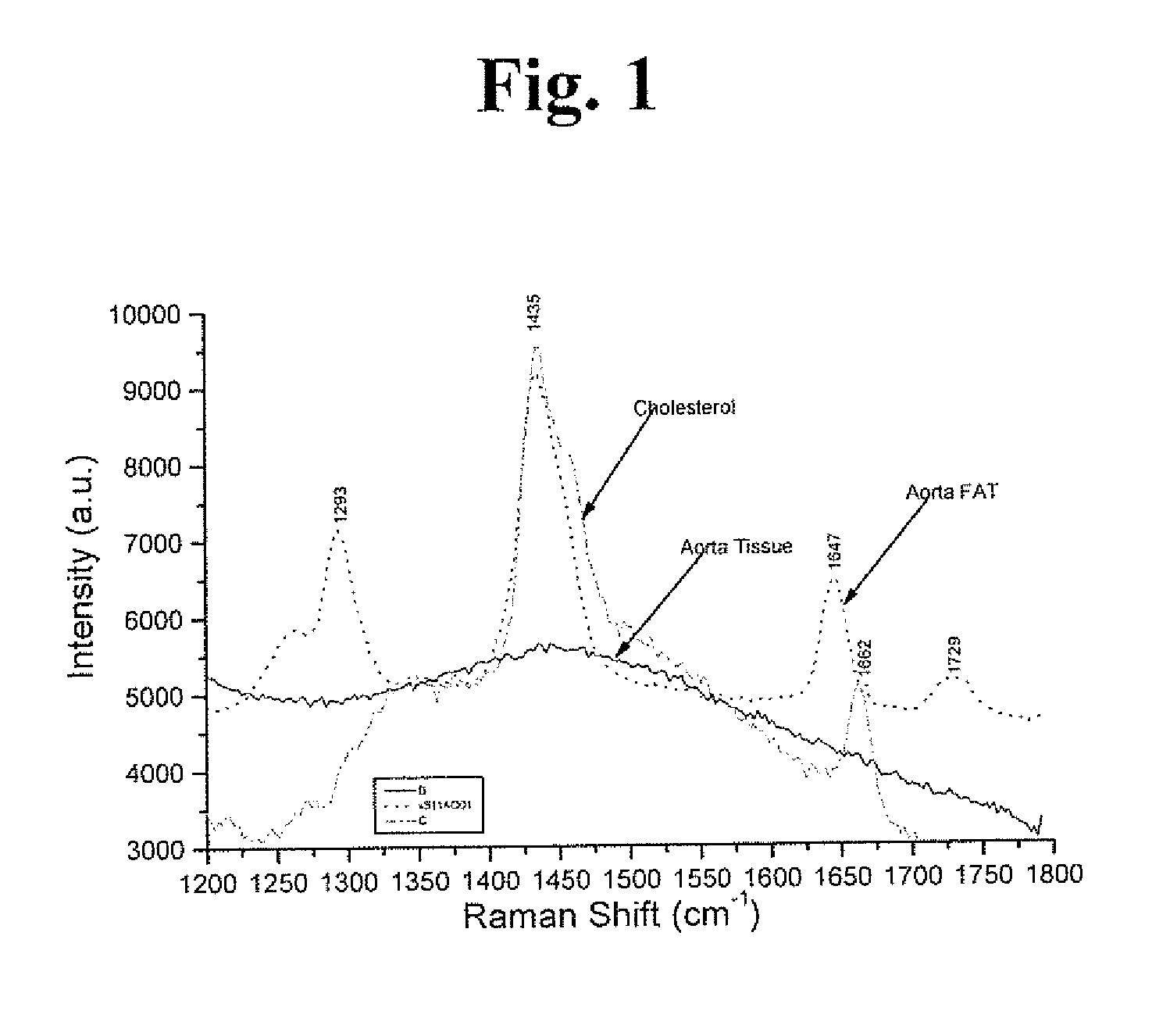
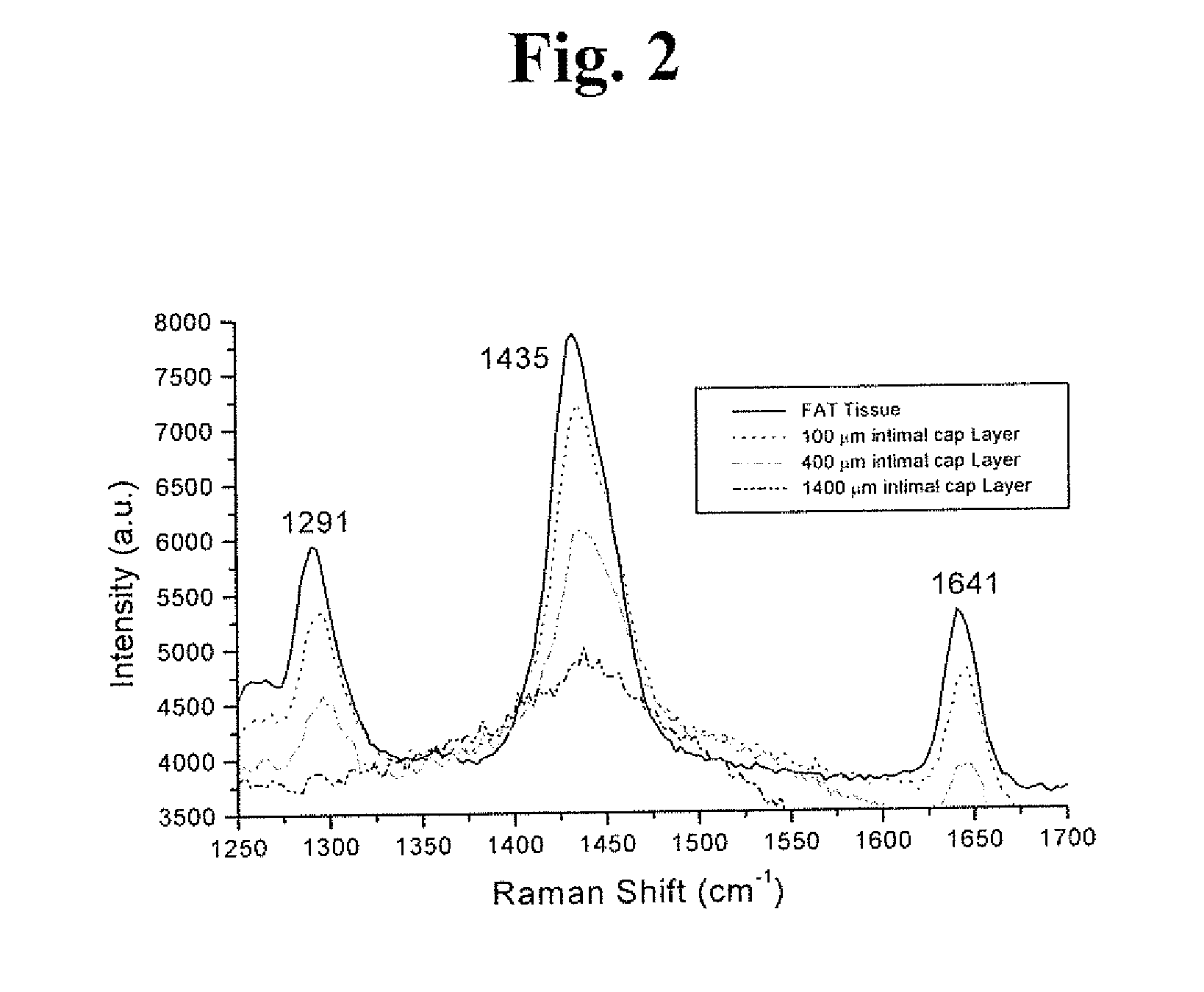
Raman and resonant raman detection of vulnerable plaque optical analyzer and imager
InactiveUS20170049328A1Increase scatteringIncreased peak intensityDiagnostics using spectroscopyCatheterVulnerable plaqueMedicine
Vulnerable plaque (VP) is the main cause of death from heart attacks. All currently available methods developed to diagnose VP lack sensitivity and or specificity and are still unable to identify VP. Our patent addresses the problem to diagnose VP in arteries. The teachings here disclose a vulnerable plaque optical analyzer (VPOA) and Imager (VOPAI) for monitoring arterial walls by measuring whether the fingerprint Raman spectrum of adipose (lipid) tissue using Resonance Raman (RR) and common Raman(R) signals of aortic intimal wall layer. The RR and R lines of lipid determine presentation of VP.
Owner:ALFANO ROBERT R +2
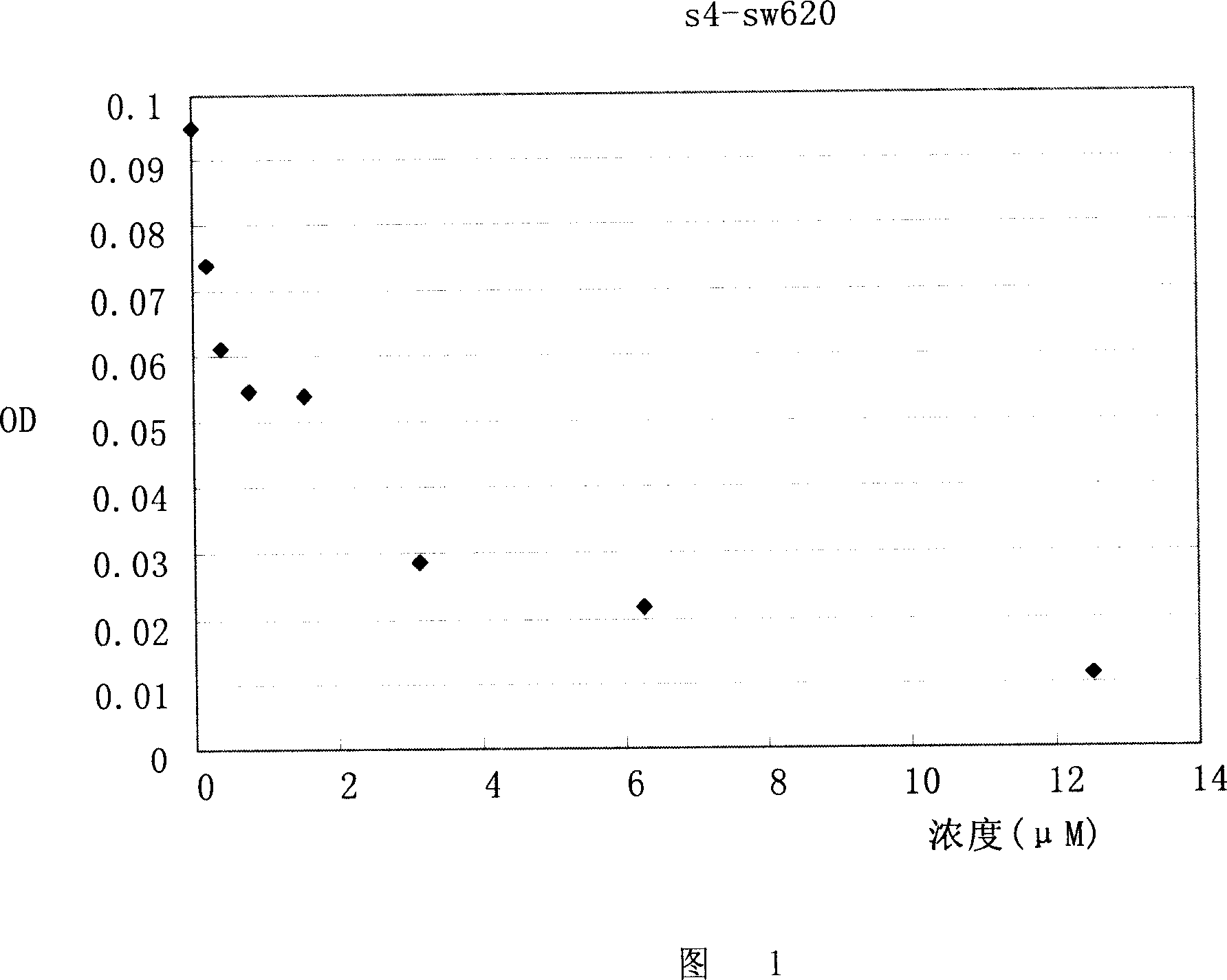
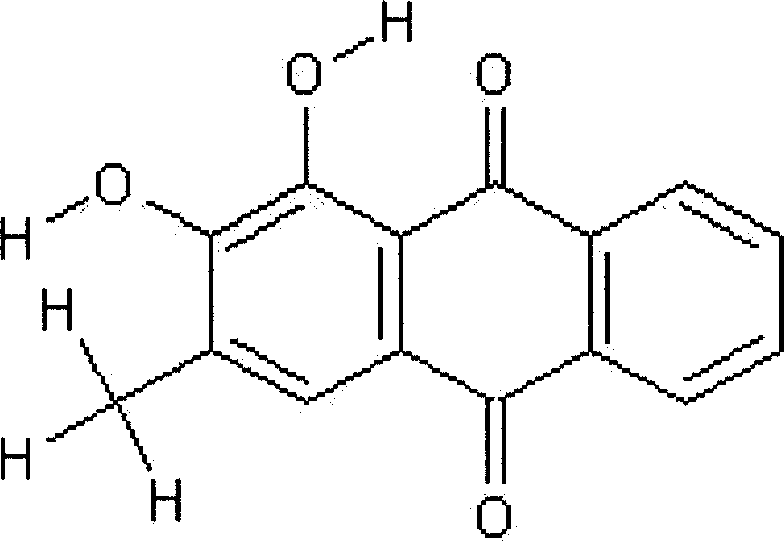
Application of compound s4 in the preparation of anti-cancer medicine
InactiveCN101112368AGrowth inhibitionPowder deliveryOrganic active ingredientsAbnormal tissue growthCancer cell
The present invention relates to the field of cell biology, and provides a compound s4 and the application in the preparation of anti-cancer drugs. Cancer becomes a first cause of death, and the substances which can kill the tumor and the growth of the cancer cells also become the hot spot in research in the current biomedical field. The s4 of the present invention can kill or inhibit a variety of tumor cells and cancer cells; at the same time, the compound s4 of the present invention also has the inhibition effect of phosphate kinase Aurora-B. All these probe that the compound s4 of the present invention has the inhibition effect of malignant tumors. The compound s4 of the present invention can be linked with the suitable pharmaceutical acceptable carriers to prepare the anti-cancer drugs, so as to inhibit the continuous growth of the tumors.
Owner:FUDAN UNIV
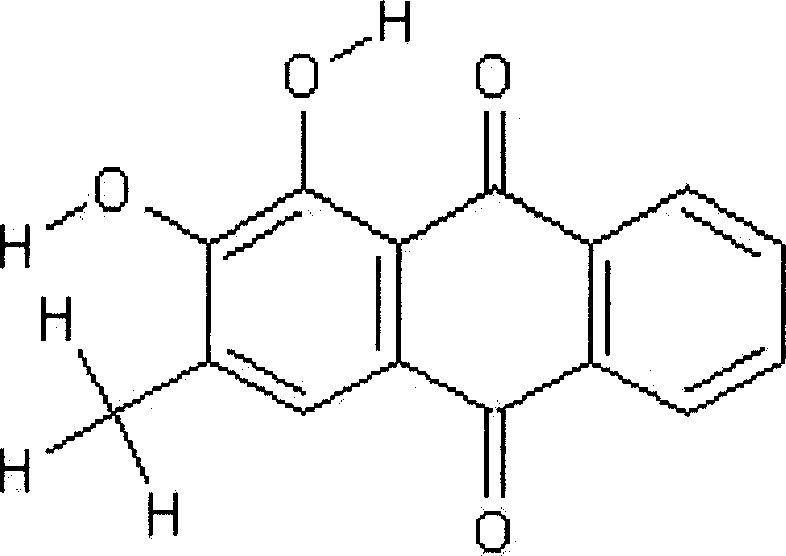
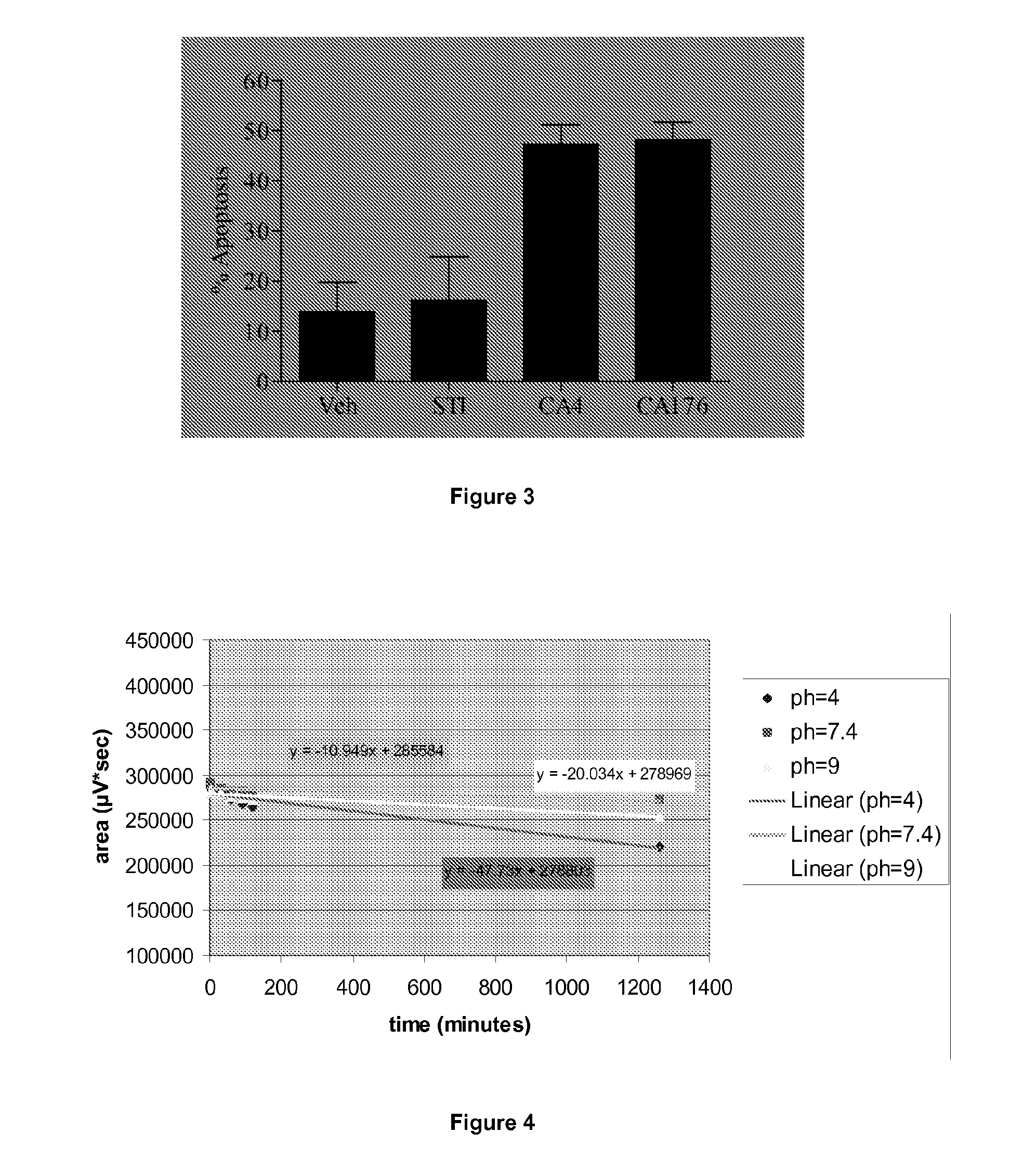
Combretastatin derivatives and uses therefor
InactiveUS20120309734A1Low toxicityInhibit angiogenesisBiocideOrganic active ingredientsCause of deathNitrogen
Cancer is one of the major causes of death worldwide. Although many advances have been made in the treatment and management of the disease, the existence of chemotherapy-resistance means there is still a great need to develop new strategies and drugs for its treatment. Provided herein are synthetic derivatives of combretastatin A-4, in particular those in which the aromatic rings are locked into a non-isomerisable active conformation, thus resulting in improved, stable compounds. The novel compounds are structurally related to combretastatin A-4 (CA-4) and lock the rings into the known active conformation by means of a four membered nitrogen containing heterocyclic ring, such as a beta-lactam ring, incorporated into the standard CA-4 structure. The compounds exhibit potent anti-cancer activity.
Owner:TRINITY COLLEGE DUBLIN
Early stage prostate cancer semi-quantitative diagnosis method utilizing double indication line immunity chromatography
InactiveCN102221617ADetection object is singleStrong targetingBiological testingPregnancyCause of death
The invention discloses a detection method in the technical field of biological engineering, and specifically provides an early stage prostate cancer semi-quantitative diagnosis method utilizing double indication line immunity chromatography. Prostate cancer is a frequently-occurring disease among middle-aged and old males. According to statistics, prostate cancer has the highest incidence of disease in male cancer patients, and is the third cause of death following lung cancer and colorectal cancer. Currently, a PSA detection method used clinically is an ELISA and a CLIA method, etc. the above methods are time-consuming, energy-consuming and is not conducive to popularization and is expensive. An immunization colloidal gold technology has developed rapidly and has been applied widely to detections of infectious disease, early pregnancy and cancer, etc. The invention employs a colloidal gold immunity chromatography to establish a PSA rapid detection method which can realize a semi-quantitative determination of 4ng / mL-10ng / mL PSA ambiguous region and guide a prostate biopsy according to age.
Owner:正元盛邦(天津)生物科技有限公司
CaMKII inhibitors and uses thereof
The present invention provides compounds useful as inhibitors of Ca2+ / calmodulindependent protein kinase (CaMKII), compositions thereof, and methods of using the same. Cardiovascular disease remains the number one cause of death in developed countries. Furthermore, incidence of cardiovascular disease has increased dramatically in developing countries. Although cardiovascular disease usually affects older adults, the antecedents of cardiovascular disease, notably atherosclerosis, begin in early life, making primary prevention efforts necessary from childhood.
Owner:ALLOSTEROS THERAPEUTICS
Electronic device and system for detecting rejection in transplant recipients
The current invention relates to an implantable device, system, method, and computer readable medium comprising computer executable components for detection of rejection of a cardiac allograft. Acute rejection and chronic rejection are the leading cause of death in heart transplant recipients post transplant. The current invention seeks to address this problem by providing methods, devices, systems and computer readable media comprising computer executable components to monitor the efficacy of immunosuppressant therapy and evaluate the degree of rejection in a cardiac allograft. To provide this functionality, an implantable device comprises sensors which measure physiologic parameters at specified intervals after the transplant procedure. These measured parameters are subsequently compared with baseline parameter data to detect rejection of the cardiac allograft.
Owner:CEDARS SINAI MEDICAL CENT
Portable, rapid, and inexpensive diagnostic tests for cardiac disease risk
InactiveUS9885663B2Material analysis by observing effect on chemical indicatorBiological testingDisease riskRapid screening test
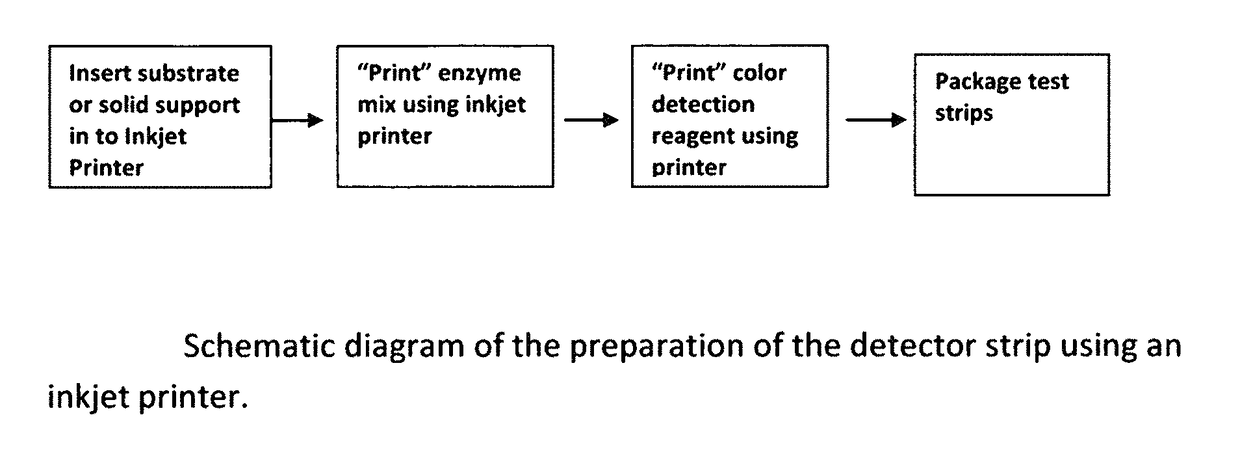
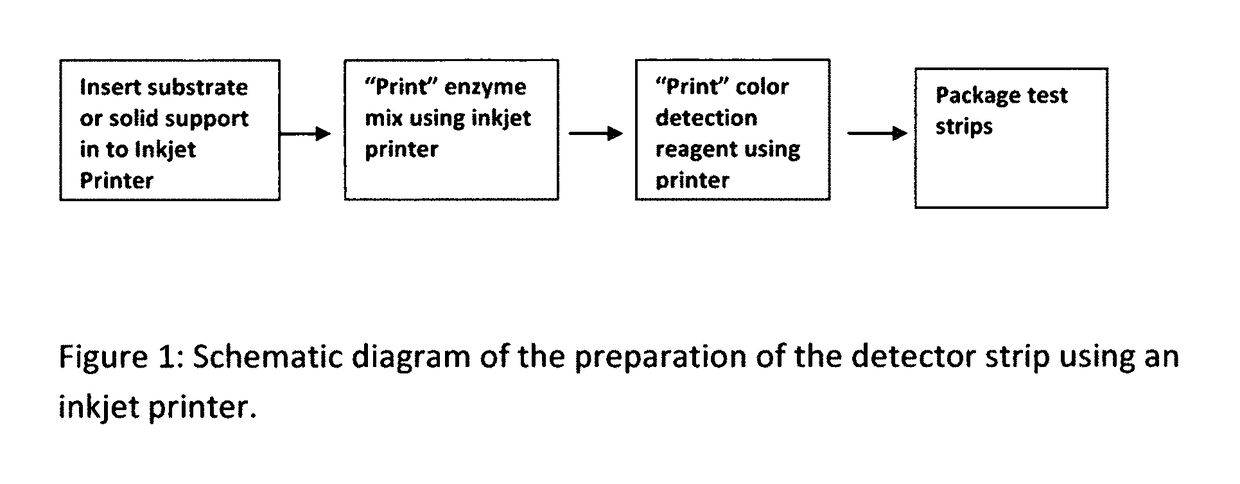
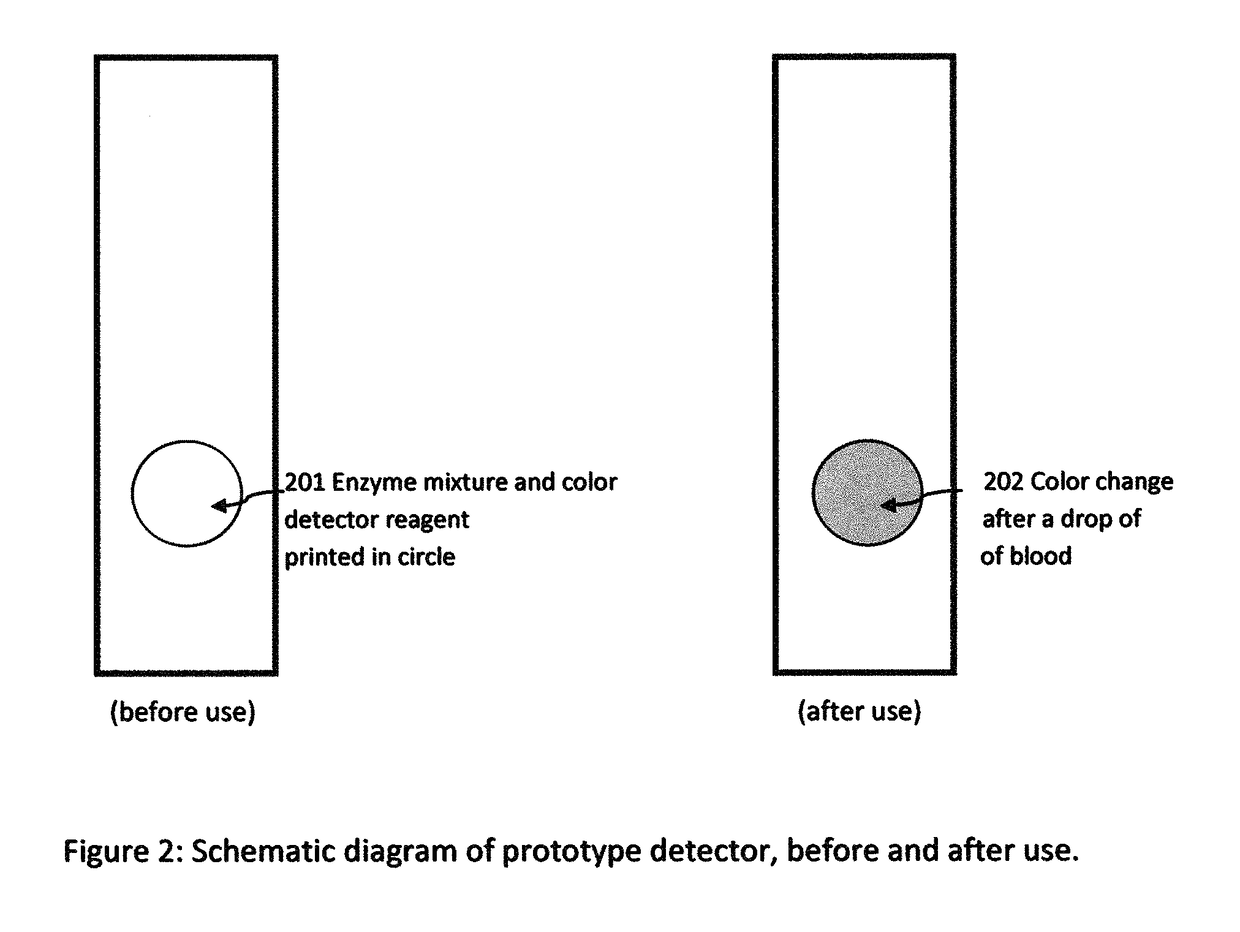
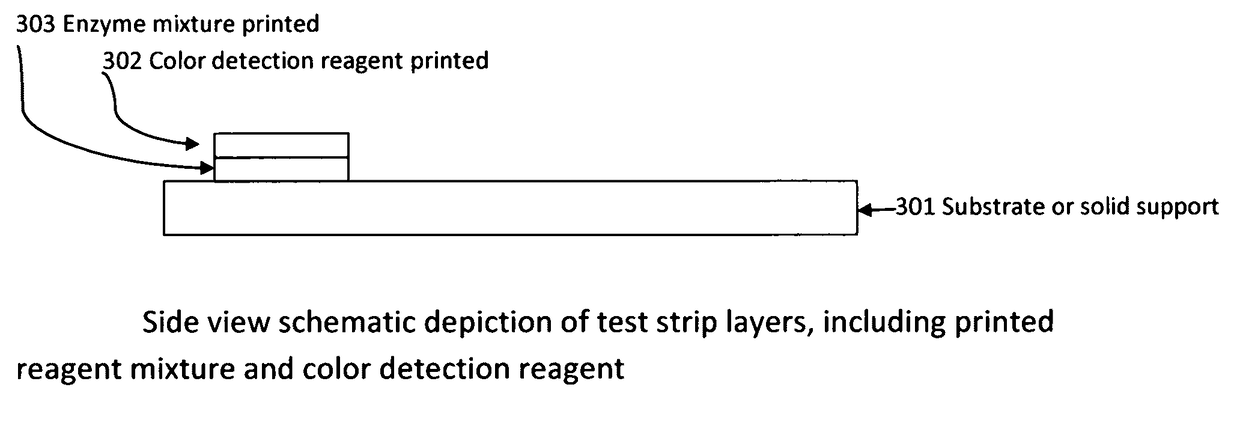
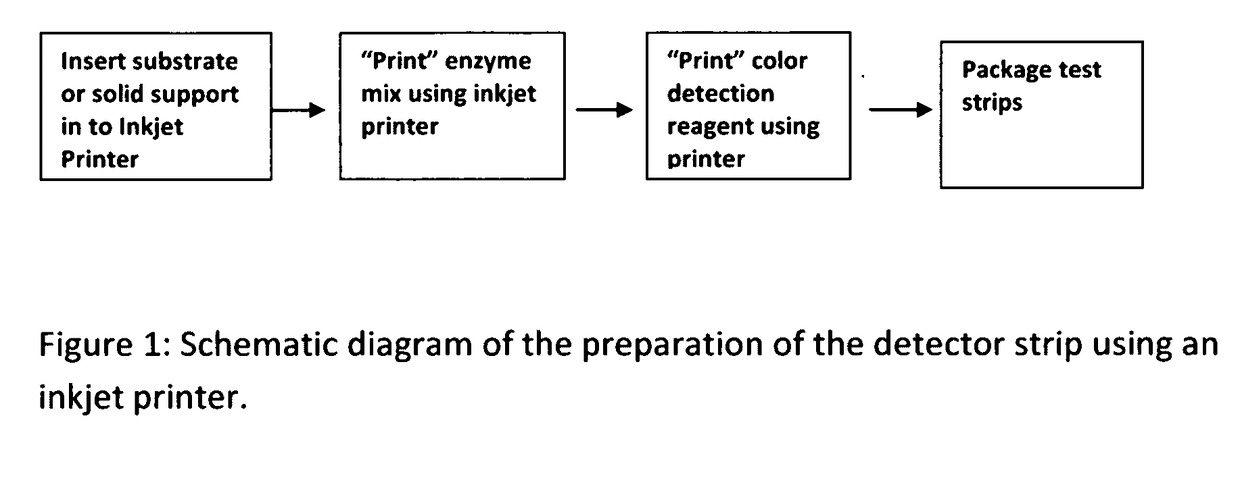
Cardiac disease is the world's leading cause of death. Cardiac Disease's pathogenesis is largely attributed to cholesterol, predominantly carried by low-density lipoproteins (LDL). However, recent research has found that oxidized-LDL is significantly more potent in the initiation and development of atherosclerosis. Tests for both cholesterol and ox-LDL tests are lab-based and expensive. With every third American at risk for Cardiac Disease, this invention discloses an on-site, ultra-low cost, rapid diagnostic test. The diagnostic test disclosed will enable a holistic assessment of Cardiac Disease risk. Furthermore, portability and ultra low-cost of the test put vital health data in the hands of patients and doctors. Additionally, the inkjet printing system disclosed can be used to mass produce the test strips on demand.
Owner:SUMATHIPALA ADRIEL
Portable, Rapid, and Inexpensive Diagnostic Tests for Cardiac Disease Risk
InactiveUS20170115228A1Liquid surface applicatorsMaterial analysis by observing effect on chemical indicatorDisease riskRapid screening test
Cardiac disease is the world's leading cause of death. Cardiac Disease's pathogenesis is largely attributed to cholesterol, predominantly carried by low-density lipoproteins (LDL). However, recent research has found that oxidized-LDL is significantly more potent in the initiation and development of atherosclerosis. Tests for both cholesterol and ox-LDL tests are lab-based and expensive. With every third American at risk for Cardiac Disease, this invention discloses an on-site, ultra-low cost, rapid diagnostic test. The diagnostic test disclosed will enable a holistic assessment of Cardiac Disease risk. Furthermore, portability and ultra low-cost of the test put vital health data in the hands of patients and doctors. Additionally, the inkjet printing system disclosed can be used to mass produce the test strips on demand.
Owner:SUMATHIPALA ADRIEL
Glycopyrronium bromide compound
The invention belongs to the technical field of medicines, and particularly relates to a glycopyrronium bromide compound. COPD (chronic obstructive pulmonary disease) is a common respiratory disease,morbidity and mortality of the COPD are increasing year by year in China, about 210 million people in the world are affected, and the disease is predicted to be the third leading cause of death in 2020. Glycopyrronium bromide has an improvement effect on patients with the COPD, and the pharmacokinetics of glycopyrronium bromide within one week after treatment reaches a steady state.
Owner:SICHUAN HAISCO PHARMA CO LTD
Hepatocarcinoma metastasis therapy apparatus and method for using hepatocarcinoma metastasis therapy apparatus
ActiveUS20170304142A1Insufficient functionRelieve symptomsBlood stagnation preventionDevices for pressing relfex pointsLymphatic SpreadCancer cell
A liver cancer metastasis therapeutic device, consisting of a base plate, a prone support, an abdominal medicine massager, a chain wheel, a rotating wheel and a handheld massager, and being used in a treatment for restoring functions of a whole body; and the therapeutic device is focused on restoring the functions of the whole human body and complementarily performs chemoradiotherapy and surgery for killing cancer cells to treat causes of death, thus significantly reducing a death rate of patients due to metastasis of liver cancers and digestive organ cancers.
Owner:LI FUSHENG
Electronic device and system for detecting rejection in transplant recipients
The current invention relates to an implantable device, system, method, and computer readable medium comprising computer executable components for detection of rejection of a cardiac allograft. Acute rejection and chronic rejection are the leading cause of death in heart transplant recipients post transplant. The current invention seeks to address this problem by providing methods, devices, systems and computer readable media comprising computer executable components to monitor the efficacy of immunosuppressant therapy and evaluate the degree of rejection in a cardiac allograft. To provide this functionality, an implantable device comprises sensors which measure physiologic parameters at specified intervals after the transplant procedure. These measured parameters are subsequently compared with baseline parameter data to detect rejection of the cardiac allograft.
Owner:CEDARS SINAI MEDICAL CENT
Application of compound s2 in the preparation of anti-cancer medicine
InactiveCN101112371AGrowth inhibitionPowder deliveryOrganic active ingredientsAbnormal tissue growthCancer cell
The invention relates to the field of cell biology and provides the application of a compound s2 in the preparation of anti-cancer drugs. Cancer becomes a first cause of death, and the substances which can kill the tumor and the growth of the cancer cells also become the hot spot in research in the current biomedical field. The s2 of the invention can kill or inhibit a variety of tumor cells and cancer cells; at the same time, the compound s2 of the invention also has the inhibition effect of phosphate kinase Aurora-B. All these probe that the compound s2 of the invention has the inhibition effect of malignant tumors. The compound s2 of the invention can be linked with the suitable pharmaceutical acceptable carriers to prepare the anti-cancer drugs, so as to inhibit the continuous growth of the tumors.
Owner:FUDAN UNIV
Non-diagnostic method for preliminarily inferring cause of death based on endogenous metabolite and method for detecting endogenous metabolite
The invention belongs to the technical field of forensic medicine, and particularly relates to a method for preliminarily deducing a dead cause based on endogenous metabolites. According to the method, acetylcarnitine, propionyl carnitine and succinic acid in a blood sample are taken as differential metabolites, preliminary deduction of lethal reasons of a main body of an unknown blood sample is realized by combining quantitative analysis with mathematical modeling, and the method can be used for preliminarily judging whether the lethal factors of a death case are poisoning caused by excessive use of antipsychotic drugs or not; and the related antipsychotic drug comprises one or more of chlorpromazine, perphenazine, olanzapine and clozapine. The invention further relates to a method for detecting the endogenous metabolites. The method is used for detecting the endogenous metabolites generated after chlorpromazine, perphenazine, olanzapine and clozapine are taken in a blood sample.
Owner:HEBEI MEDICAL UNIVERSITY
Personal file collection set
The invention discloses a personal file collection set for persons. The personal file collection set is characterized in that a term of file collection is set at one or two full years of life; the file collection from 38 terms to 108 terms can be freely set; the personal file collection comprises a birth file, a childhood file, an adolescent file, an adult file, an account collection, a photo collection and a signature place for idol friends; a wedding anniversary file comprises a signature place for people congratulating the wedding, record for people congratulating the wedding, marriage record and wedding photo collection; and a legacy and testament record collection comprises legacy record, a testament record, signature of notary of testator, death time and cause of death. The invention has the benefits of unified collection, safety, stability, durability, convenience for use, attractiveness and elegance, is the optimal choice for personal file and photo collection and is a collection for your lifetime. The true value of the file is embodied after protagonists die, in particular to a celebrity file. The file belongs to an environment-friendly personal file collection set.
Owner:游玉明
Method for quantitative assessment of thymus integrity
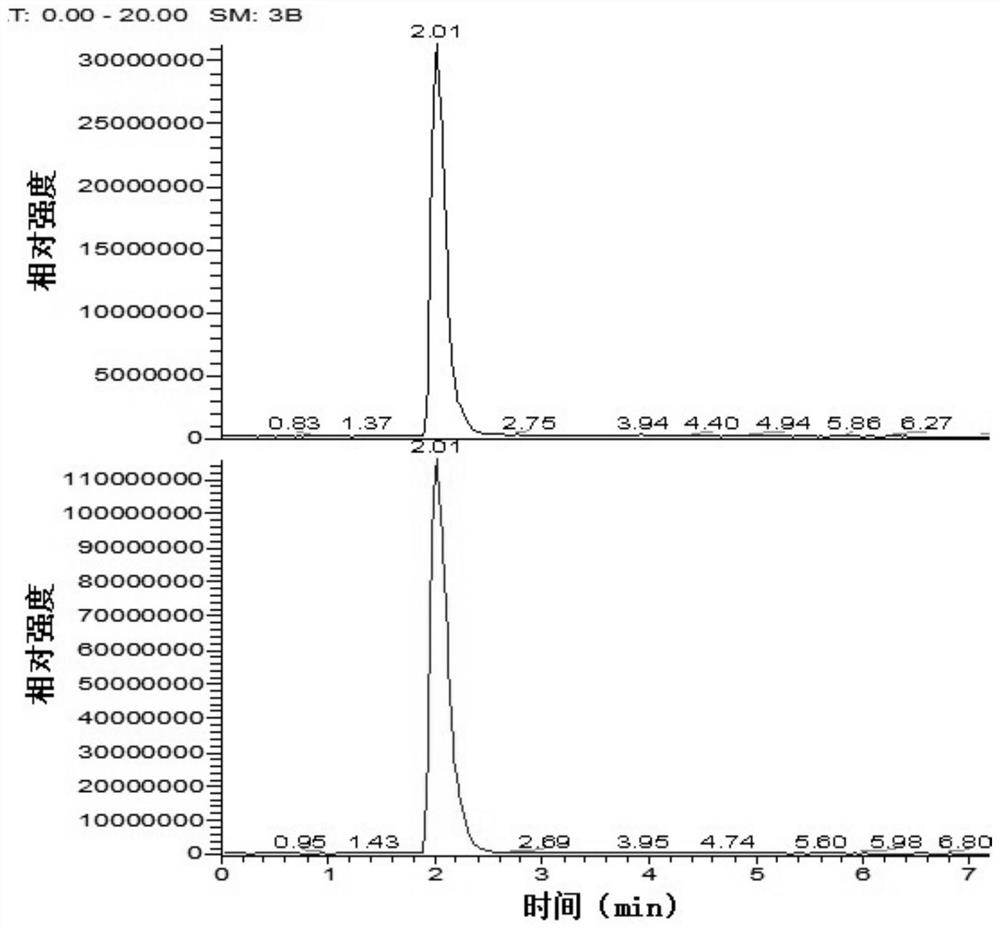
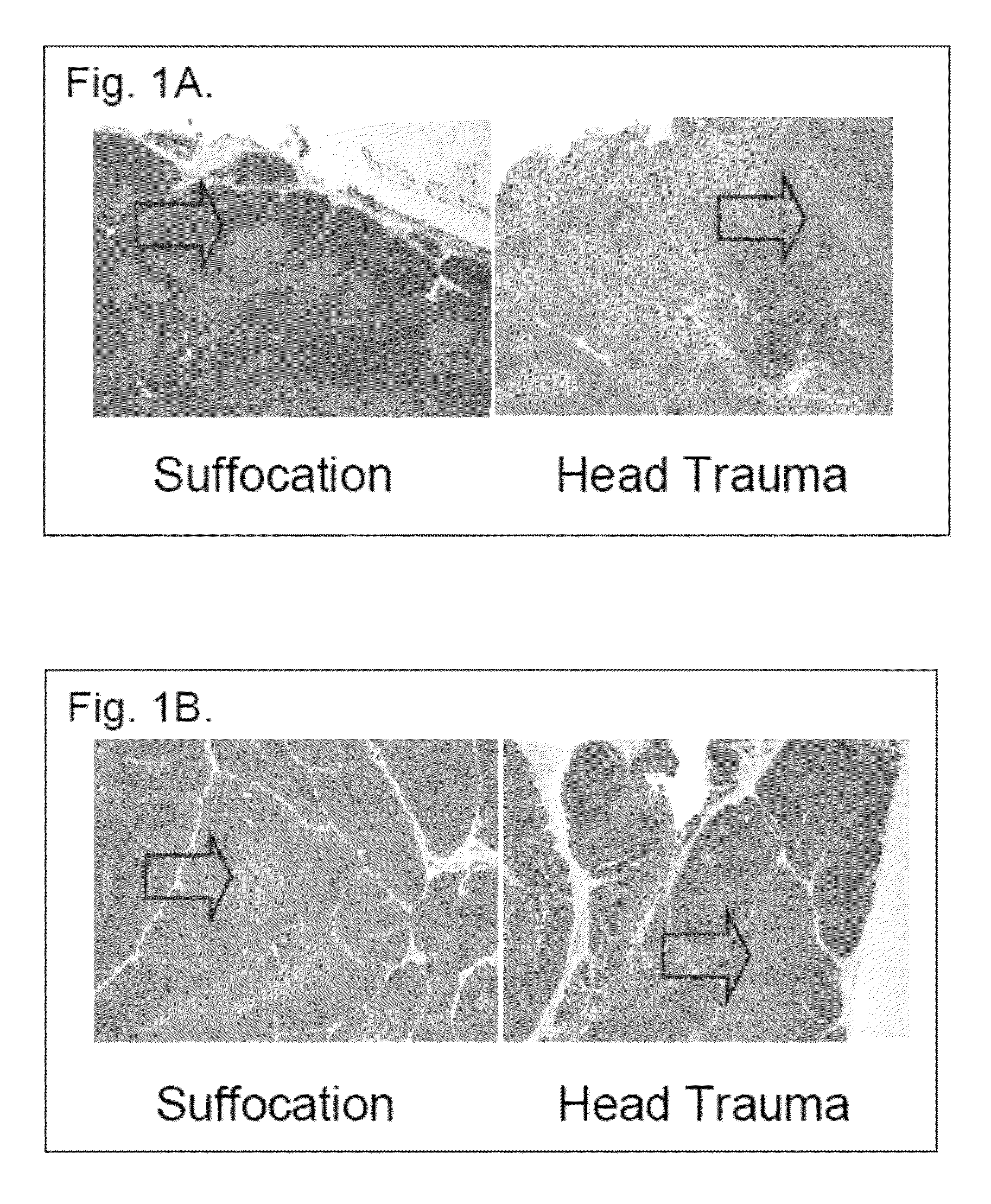
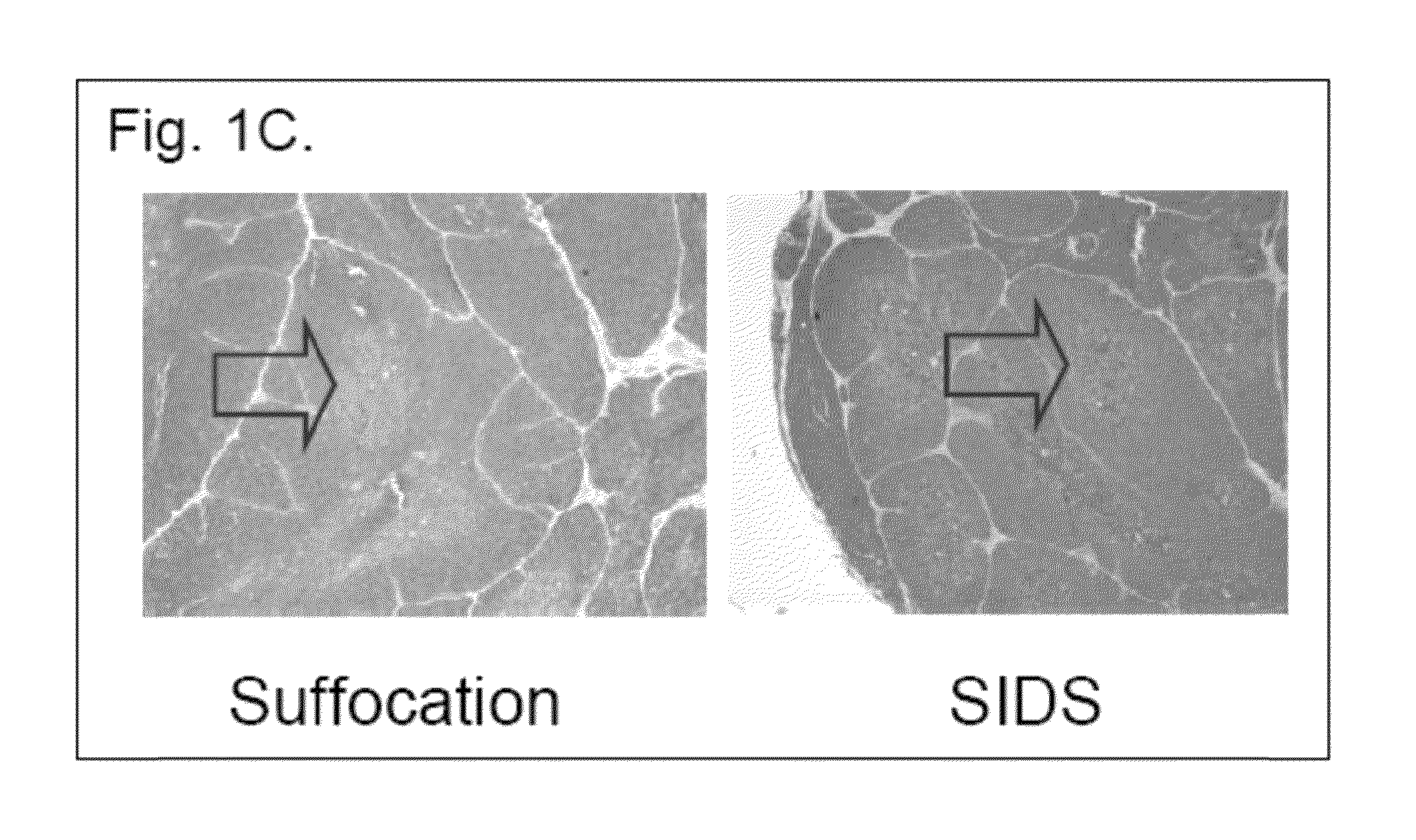
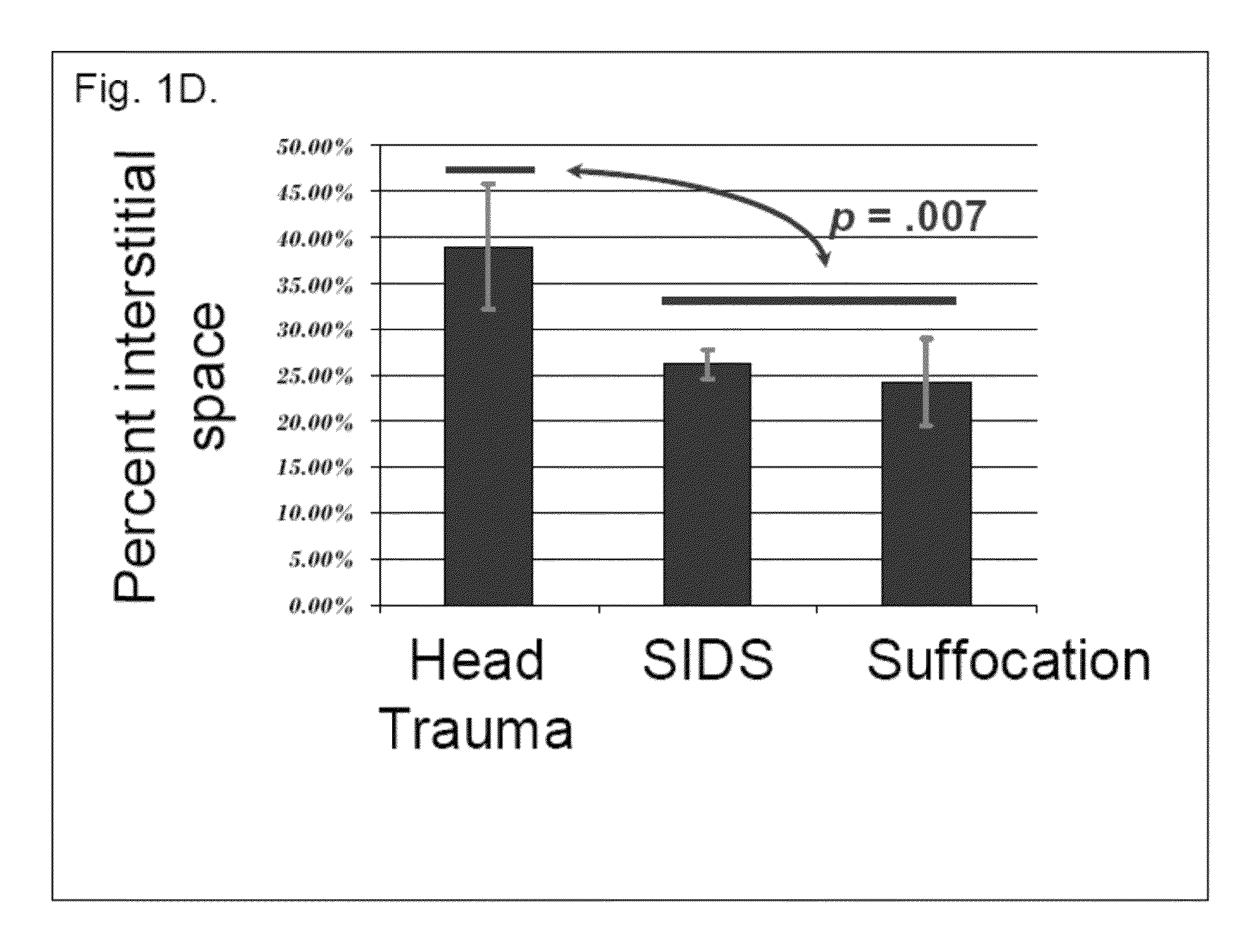
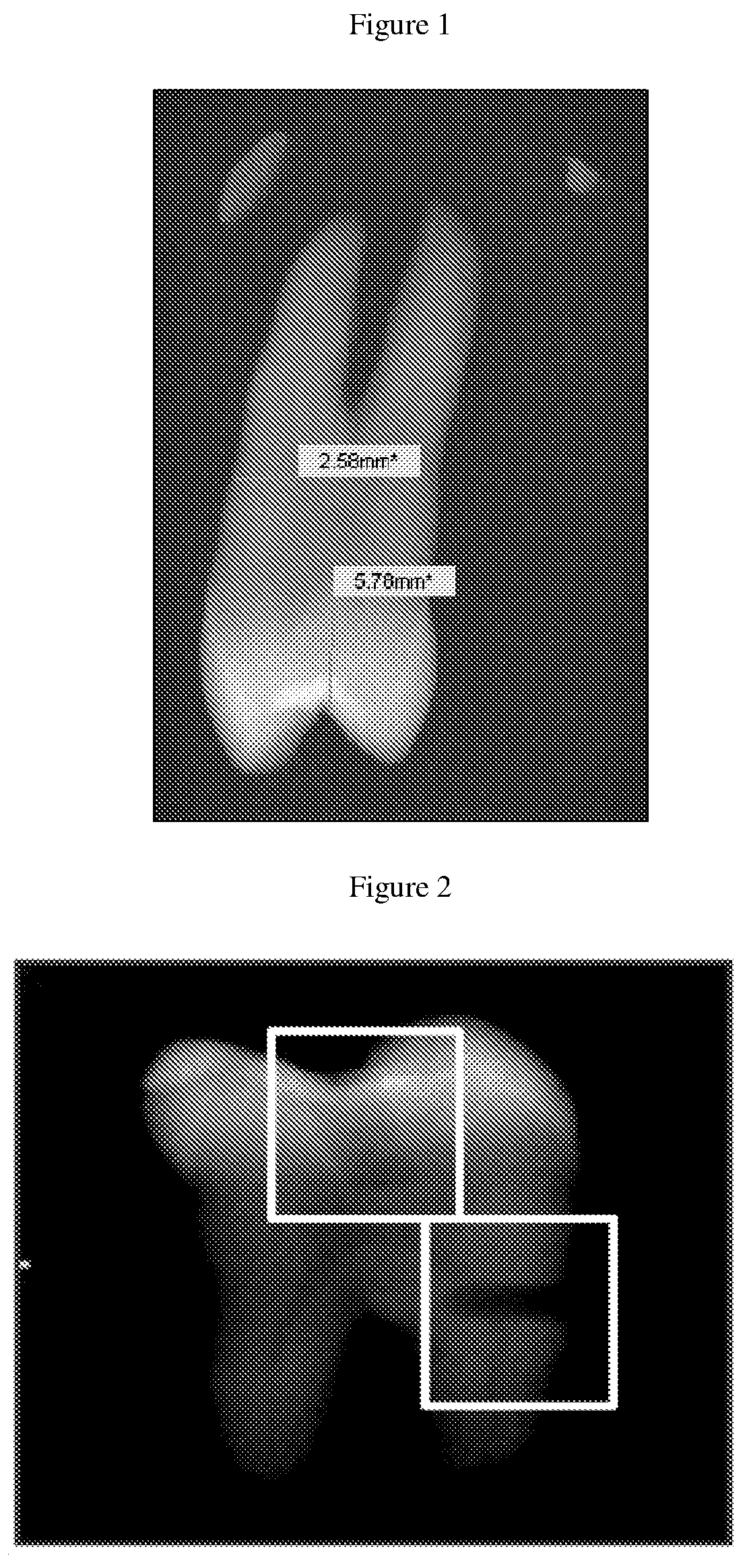
The present invention relates to a series of markers and a method of determining cause of death post-mortem by quantifying thymus integrity. An examination of thymuses from human infants suffering mortal head trauma revealed a disruption of the cortical-medullary organization of the thymus, particularly involving dissolution of the cortical-medullary border. A similar result was obtained for related mouse and rat models. The human thymuses from head trauma cases also displayed a higher percentage of Ki67-positive thymocytes.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
Deratization tracing paste and preparation method thereof
InactiveCN109430302ATo achieve the effect of rodent controlReduce contentBiocideAnimal repellantsCause of deathAdhesive
The invention discloses a deratization tracing paste and a preparation method thereof, and belongs to the field of public hygiene. The deratization tracing paste comprises 0.001-0.005% of anticoagulation powder, 0.5-5% of konjac juice, 0.05-0.5% of castor leaf juice, 5-20% of an adhesive, 0.001-0.005% of an opium poppy concentrated solution, 0.001-0.01% of amphetamine and the balance of food bait.The konjac juice and the castor leaf juice are added in the tracing paste, hairs of rats can be stimulated, thus, the rats may lick the paste and then eat the deratization tracing paste, and a deratization effect is achieved; the content of the deratization tracing paste is low, death of the rats can be delayed to prevent rats in the same nest or rat companions from discovering cause of death, resistance of the rats to the deratization tracing paste is reduced, the effect of the paste is promoted to cause nervous excitation of the rats, thus, the rats flee hither and thither to adhere the paste to the companions, and the rat kill ratio is increased.
Owner:绵阳机场蓝天环保科技有限公司
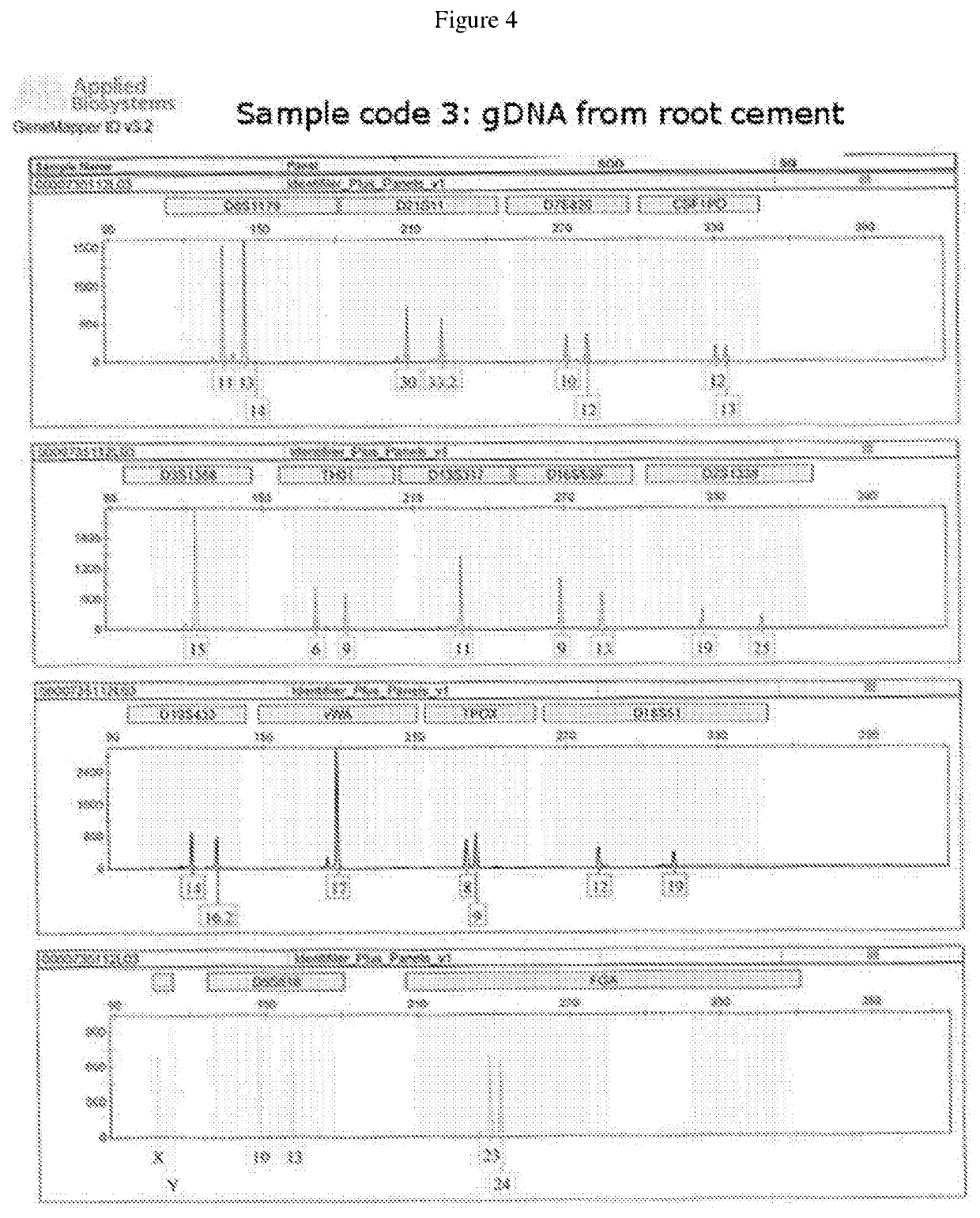
Method, systems and kit for forensic identification, post mortem interval estimation and cause of death determination by recovery of dental tissue in physiological conditions
ActiveUS10648893B2Avoid pollutionPreserve integrityMicrobiological testing/measurementWithdrawing sample devicesDigital RayForensic Pharmacy
Owner:UNIV DE LOS ANDES
Compositions for control of drug abuse
InactiveUS20170296532A1Challenge can be overcomePrevent degradationPowder deliveryOrganic active ingredientsBenzodiazepineCause of death
Opiates, amphetamines, barbiturates and other drugs such as benzodiazepines are extensively abused or misused and are frequently the cause of death by overdosing. These drugs are also prone to oxidation and the final degradation products depend on the reactants and the reaction conditions. This invention describes the use of inactivating agents such as permanganates, peroxides, persulfates, bismuthates, periodates or other oxidants in a dosage form as an approach to minimize abuse and overdose. The product is designed such that the inactivating agent is released if there is an attempt to extract the drug from the formulation or in cases of overdose. Once released, the inactivating agent quickly degrades the drug and converts it into inactive compounds. Since the reactants (drug and inactivating agent) are incompatible in situations of normal drug usage, they are kept separated within the vehicle of the invention, but released for interaction in case of misuse. A catalyst may be included in the formulation to facilitate the reaction.
Owner:KYDES PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com