Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
448 results about "Diagnostic test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medicine, a diagnostic test is any kind of medical test performed to aid in the diagnosis or detection of disease. For example, such a test may be used to confirm that a person is free from disease, or to fully diagnose a disease, including to sub-classify it regarding severity and susceptibility to treatment. Companion diagnostics have also been developed to preselect patients for specific treatments based on their own biology, where such targeted therapy may hold promise in personalized treatment of diseases such as cancer. A drug test can be a specific medical test to ascertain the presence of a certain drug in the body.
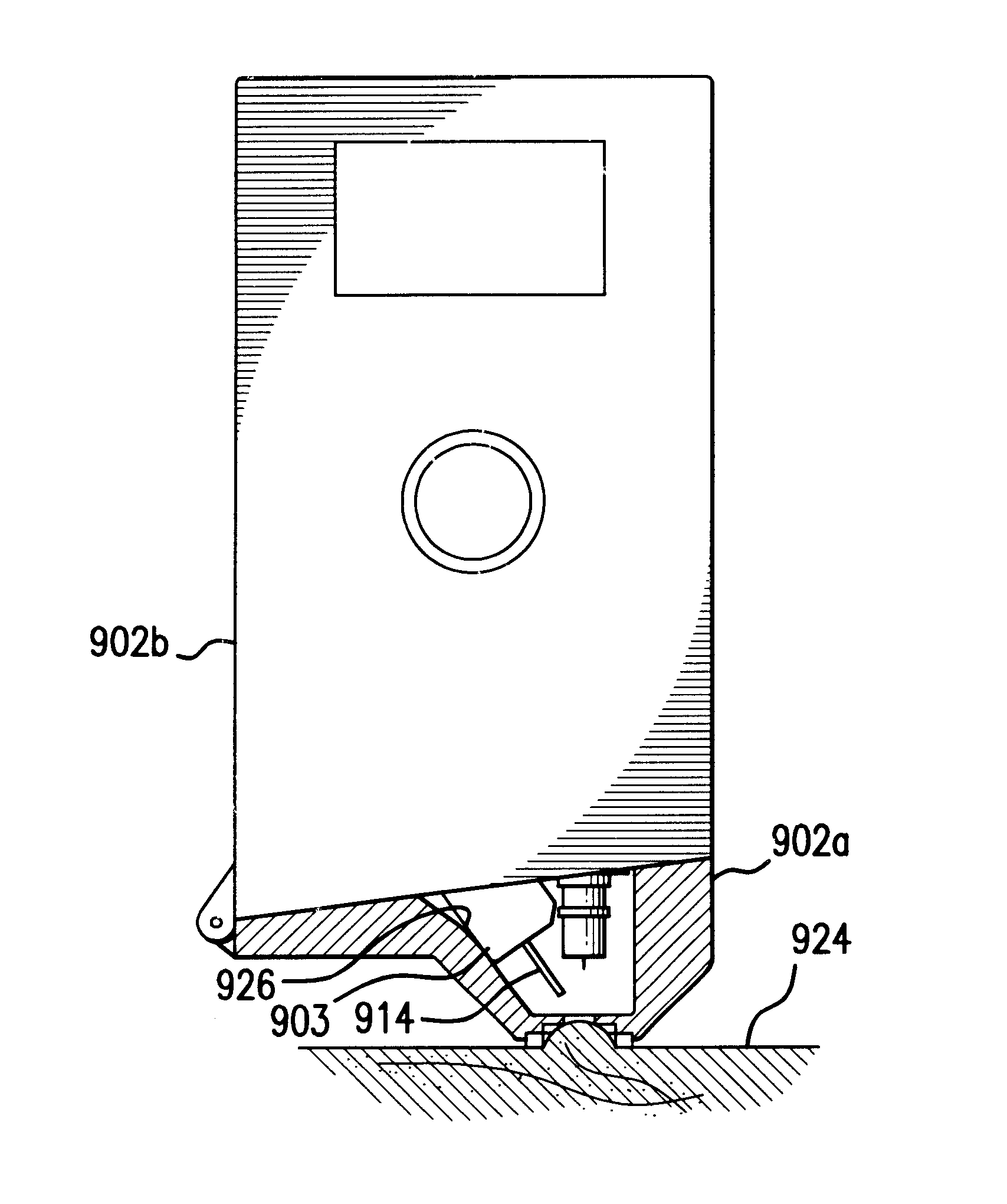
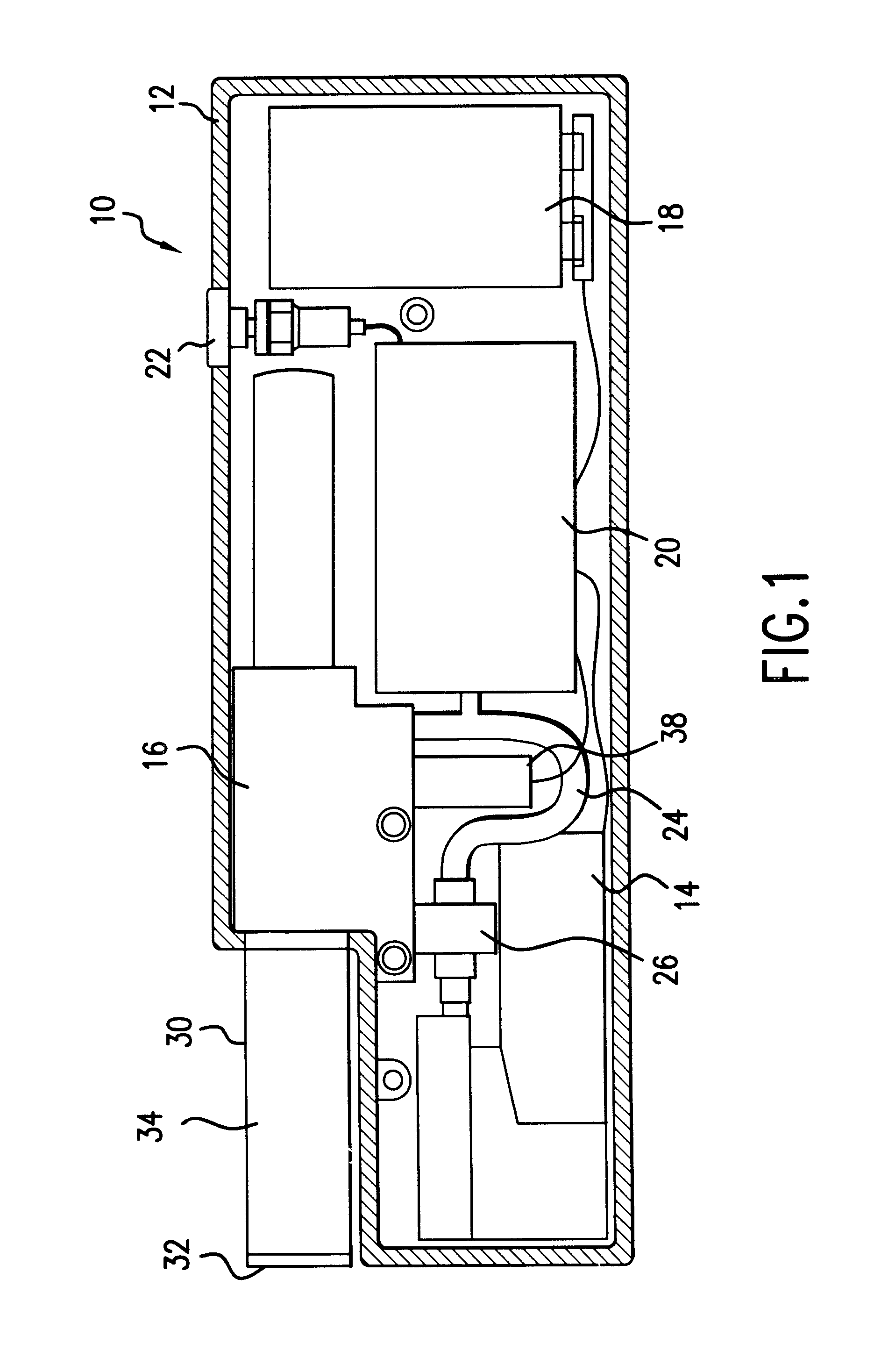
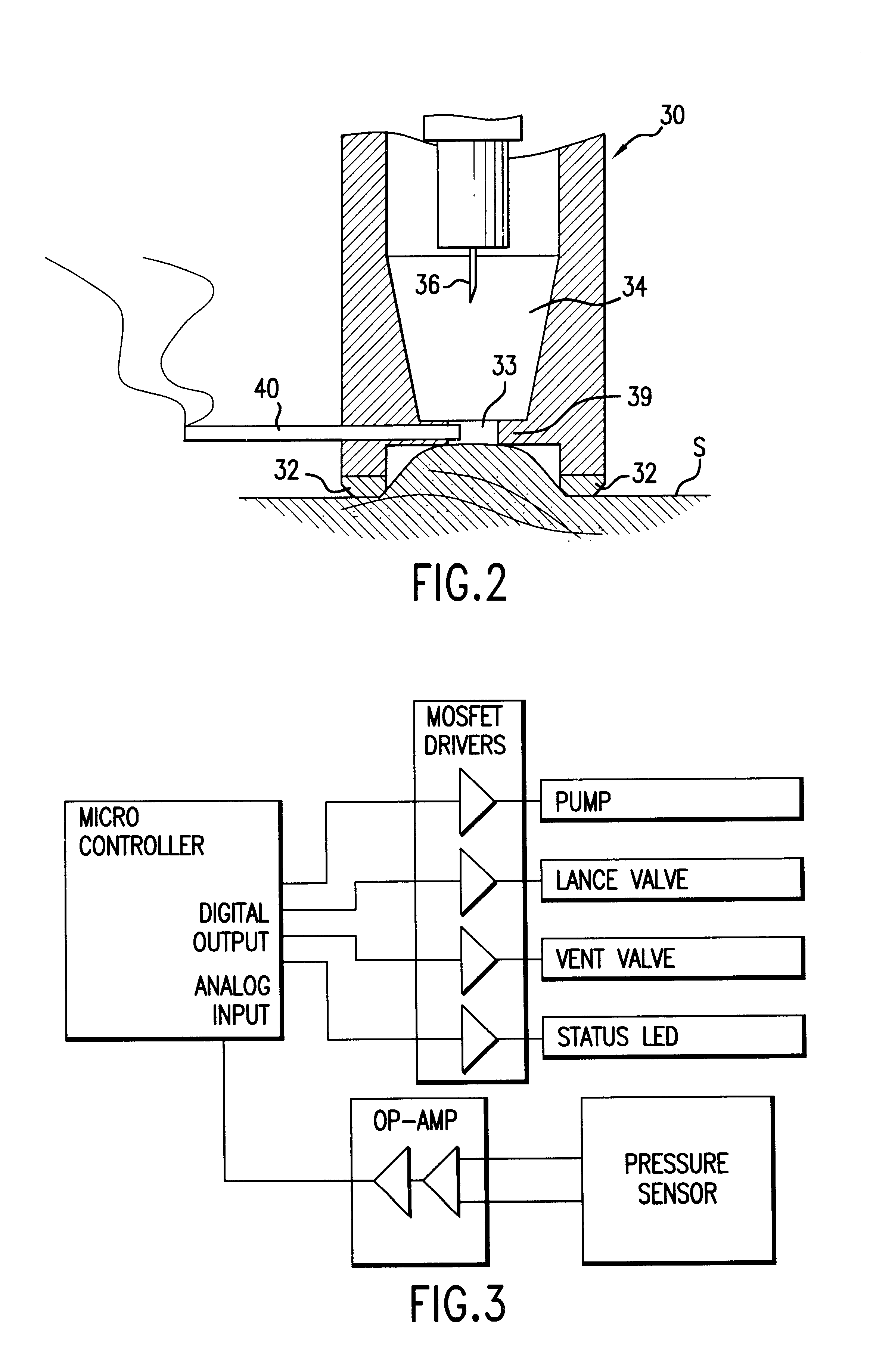
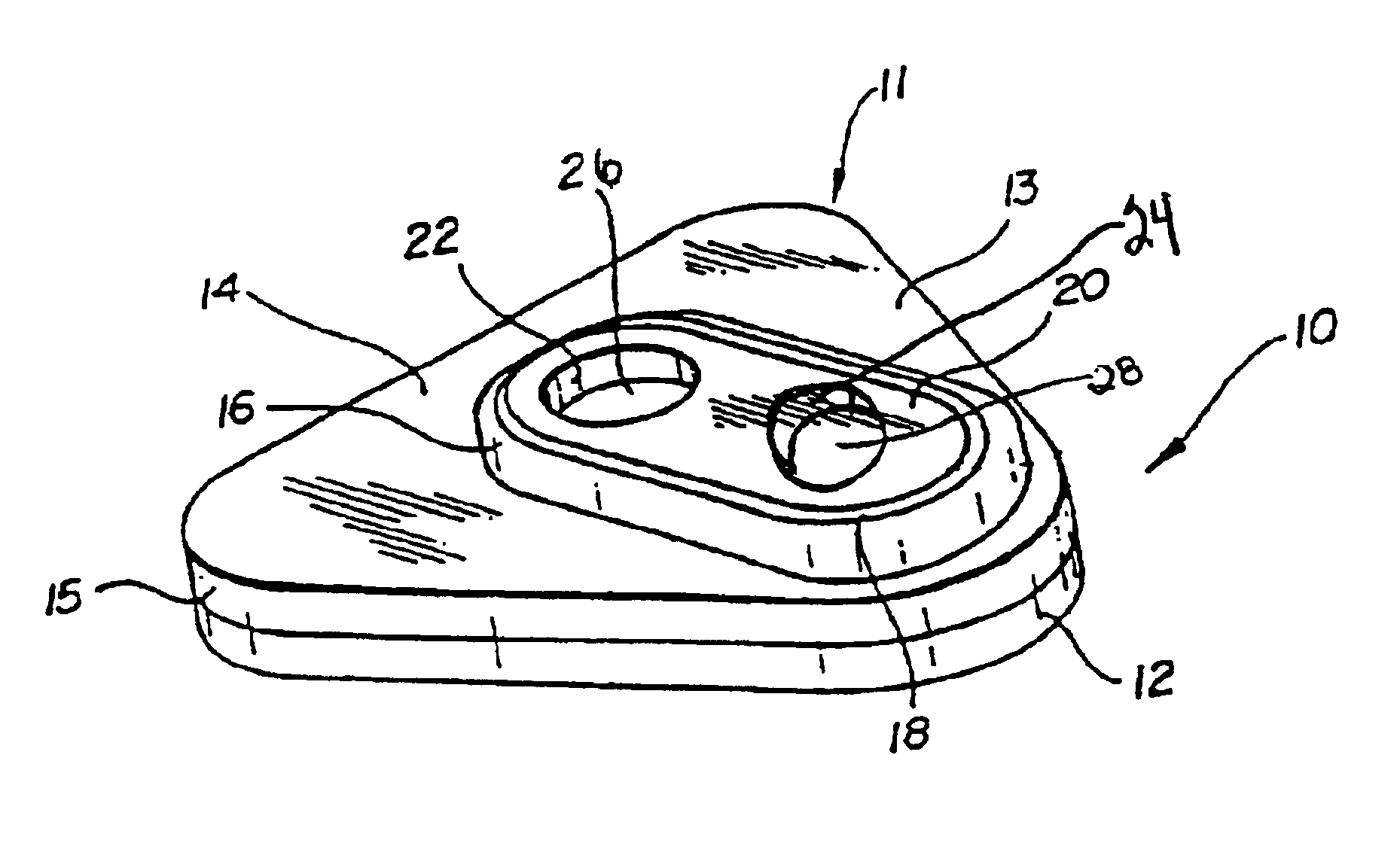
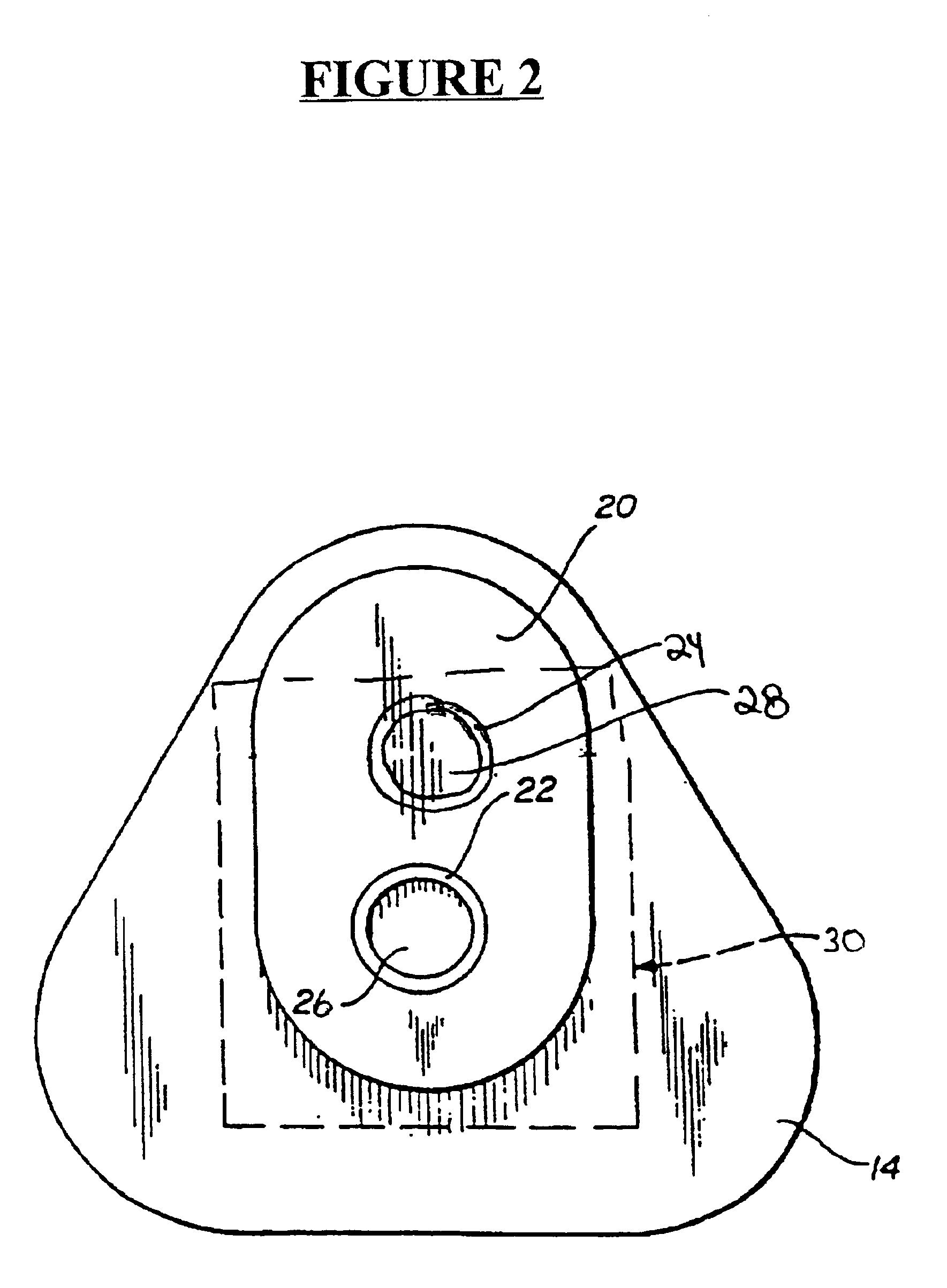
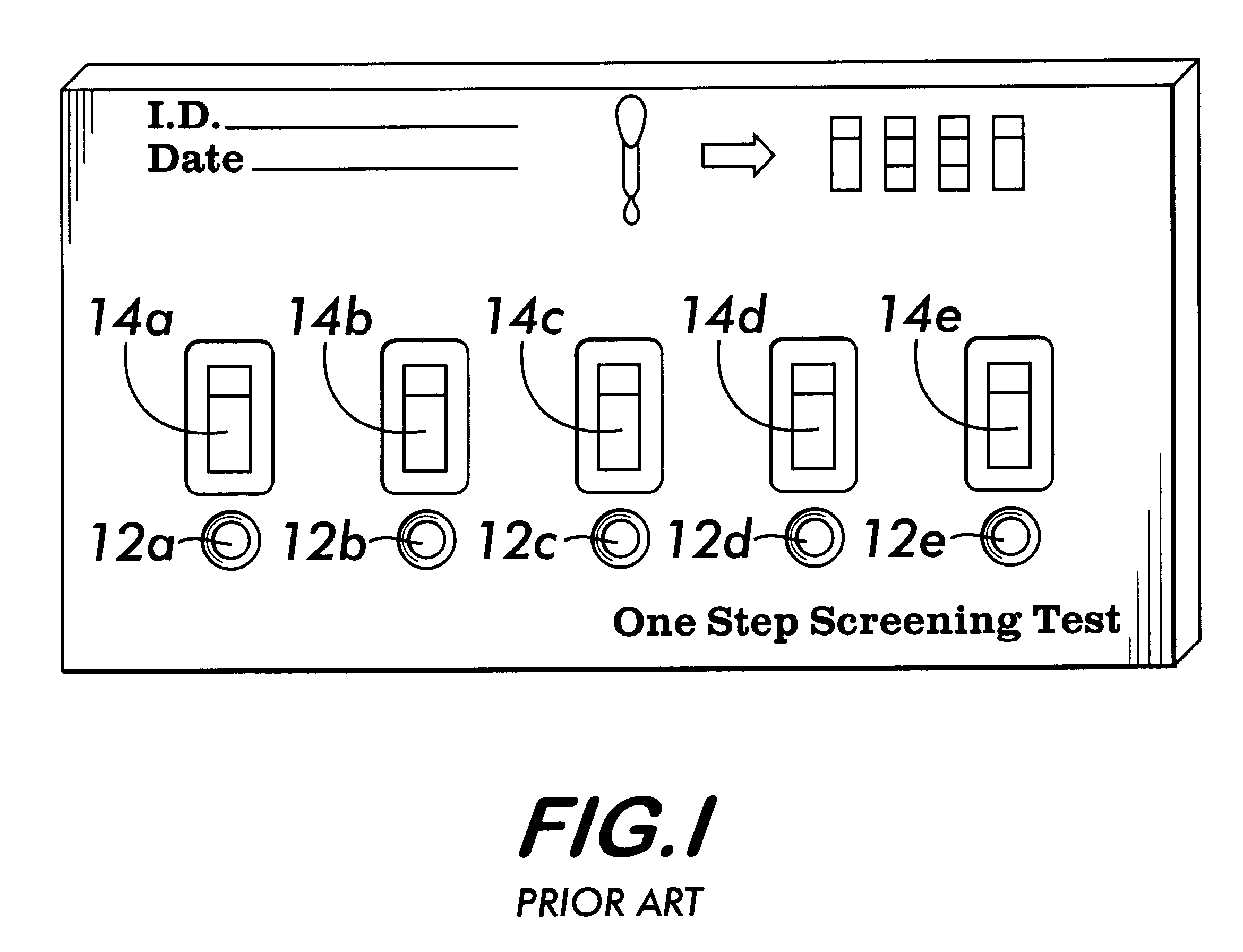
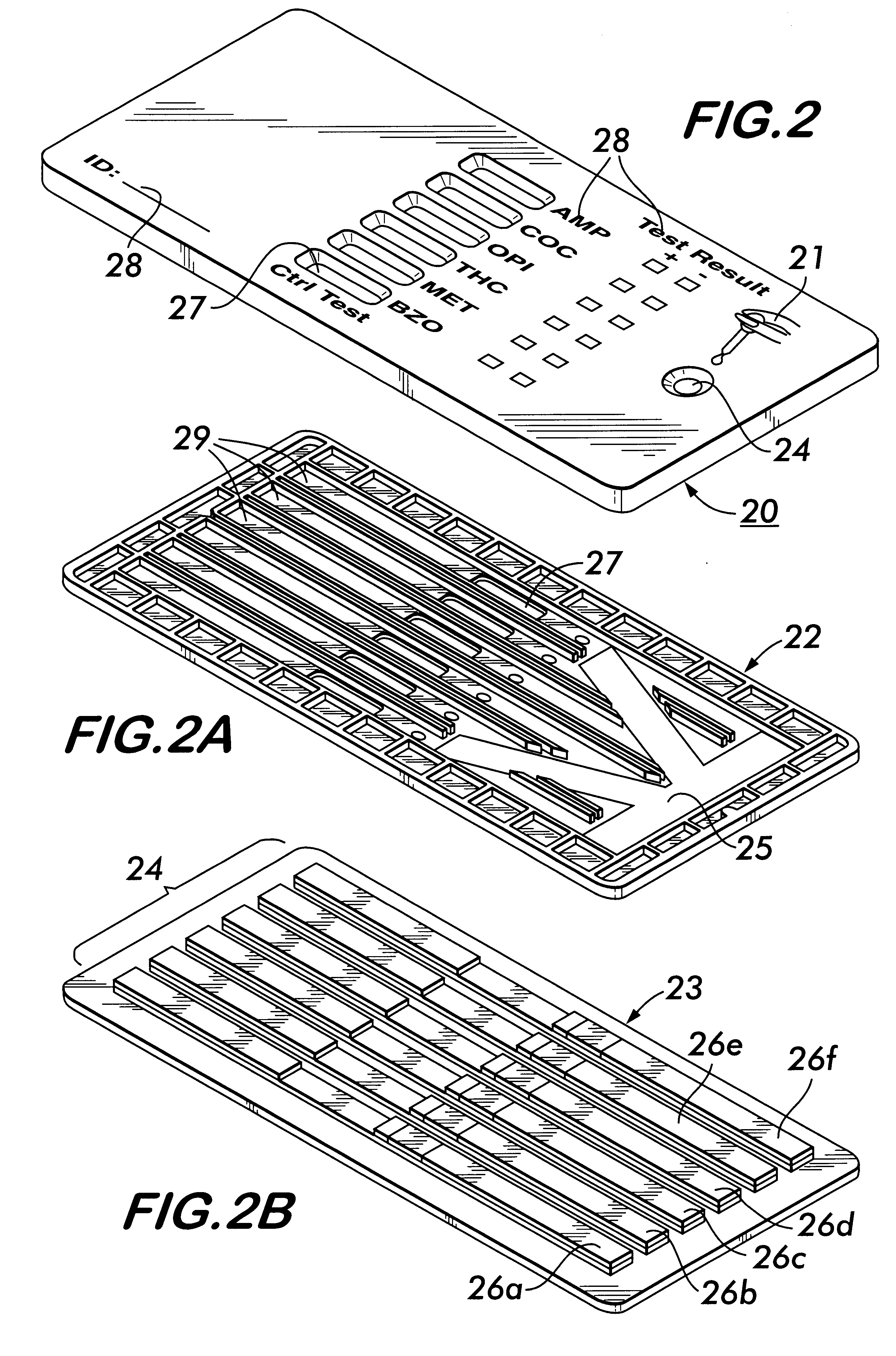
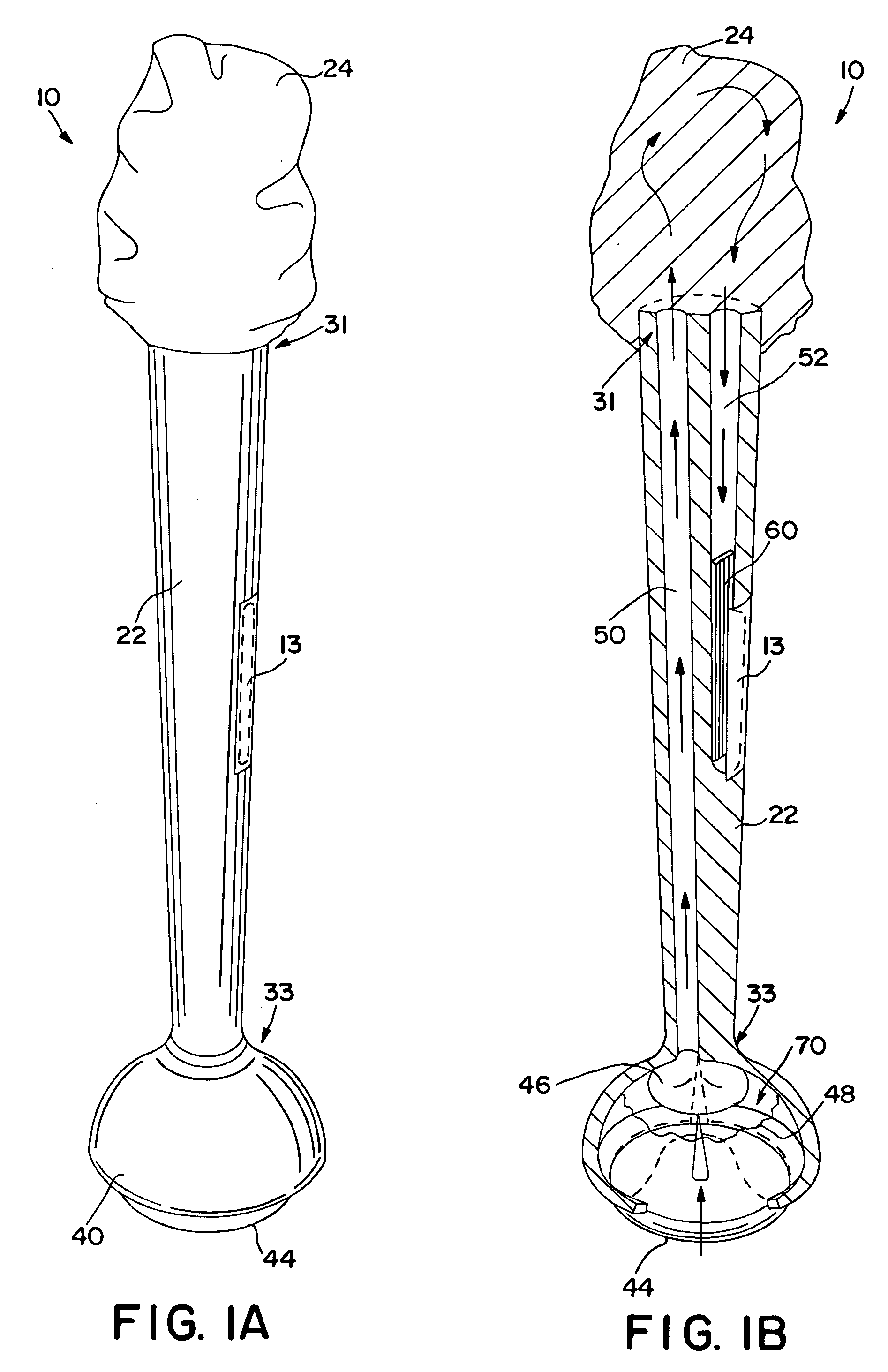
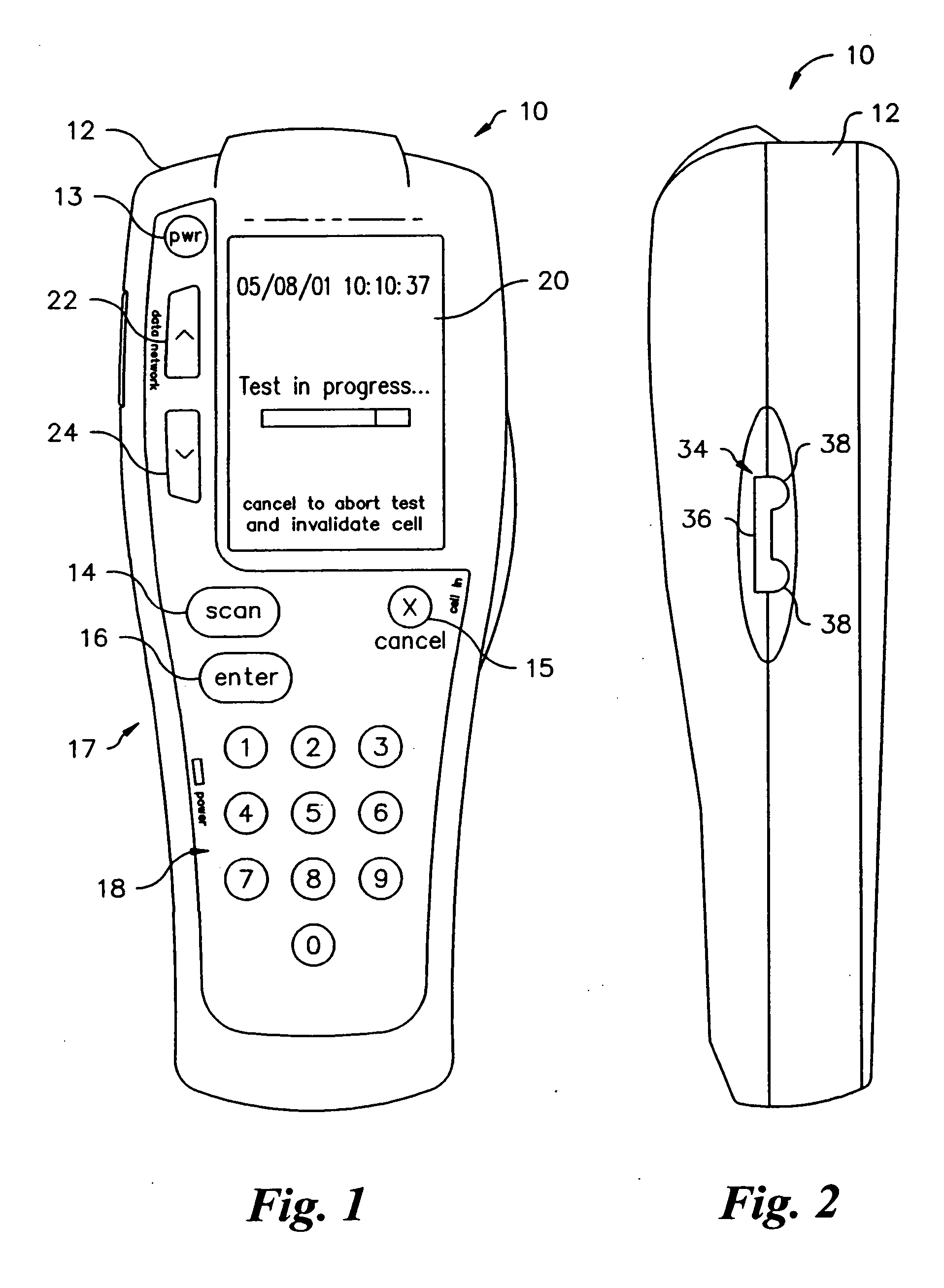
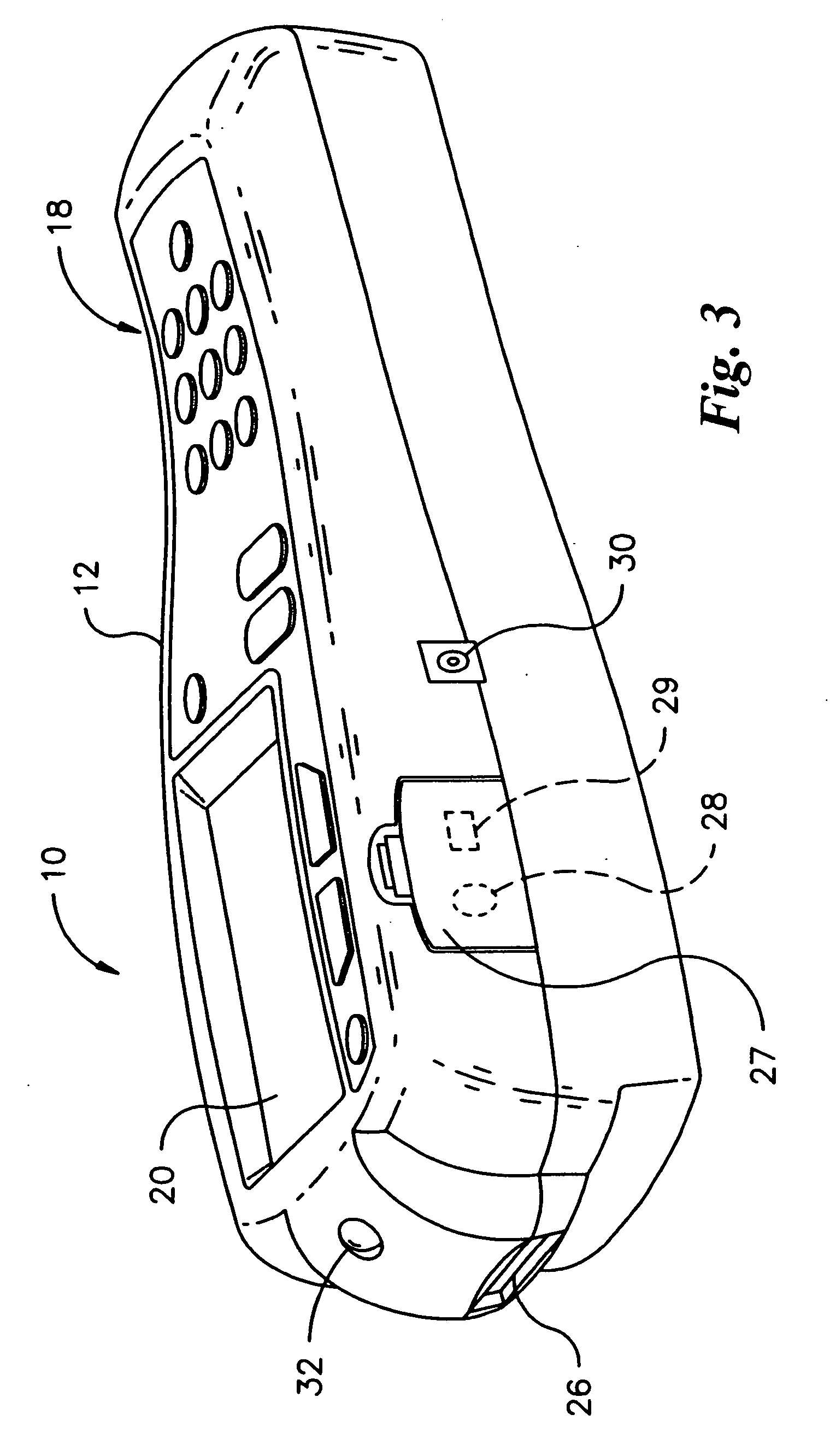
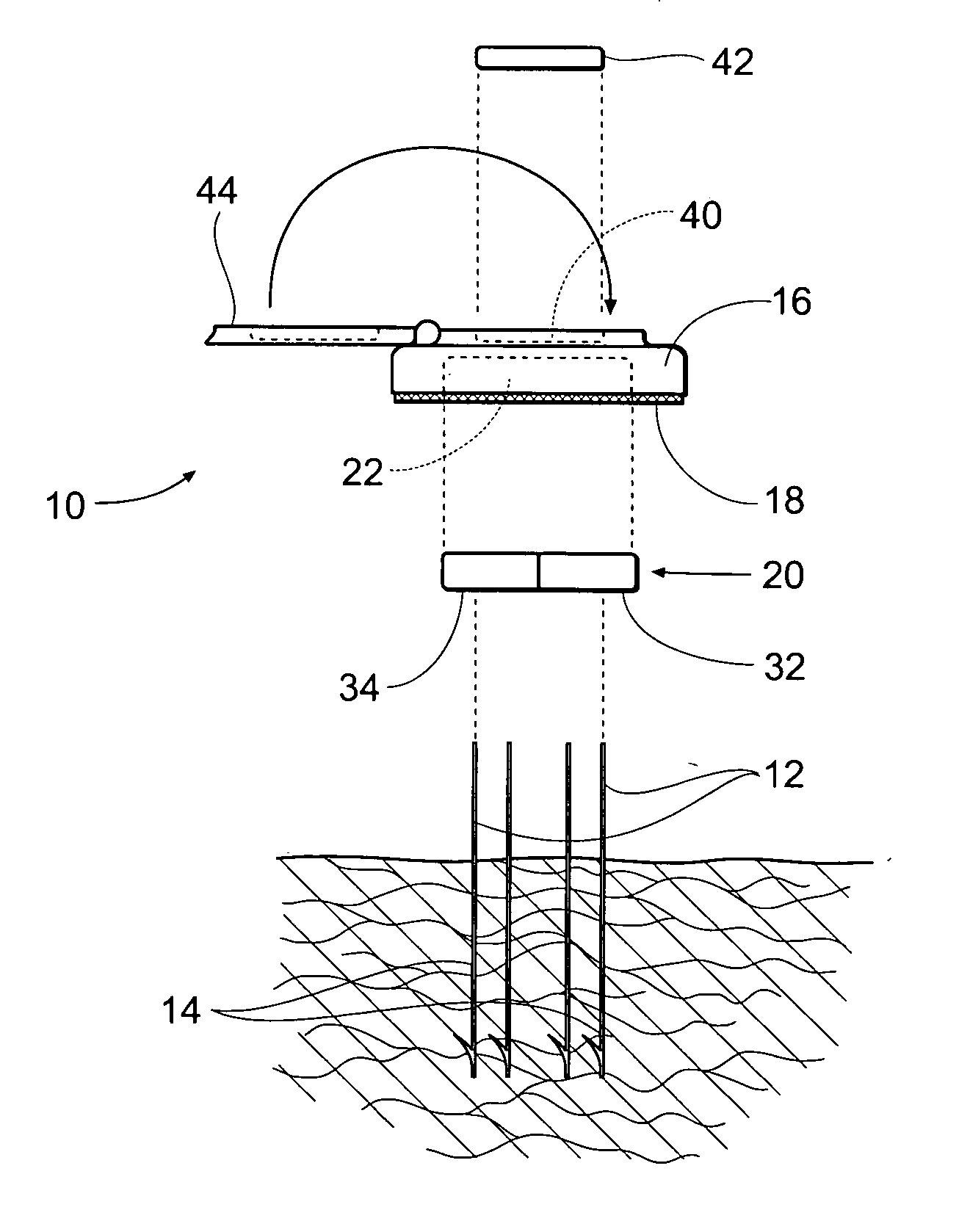
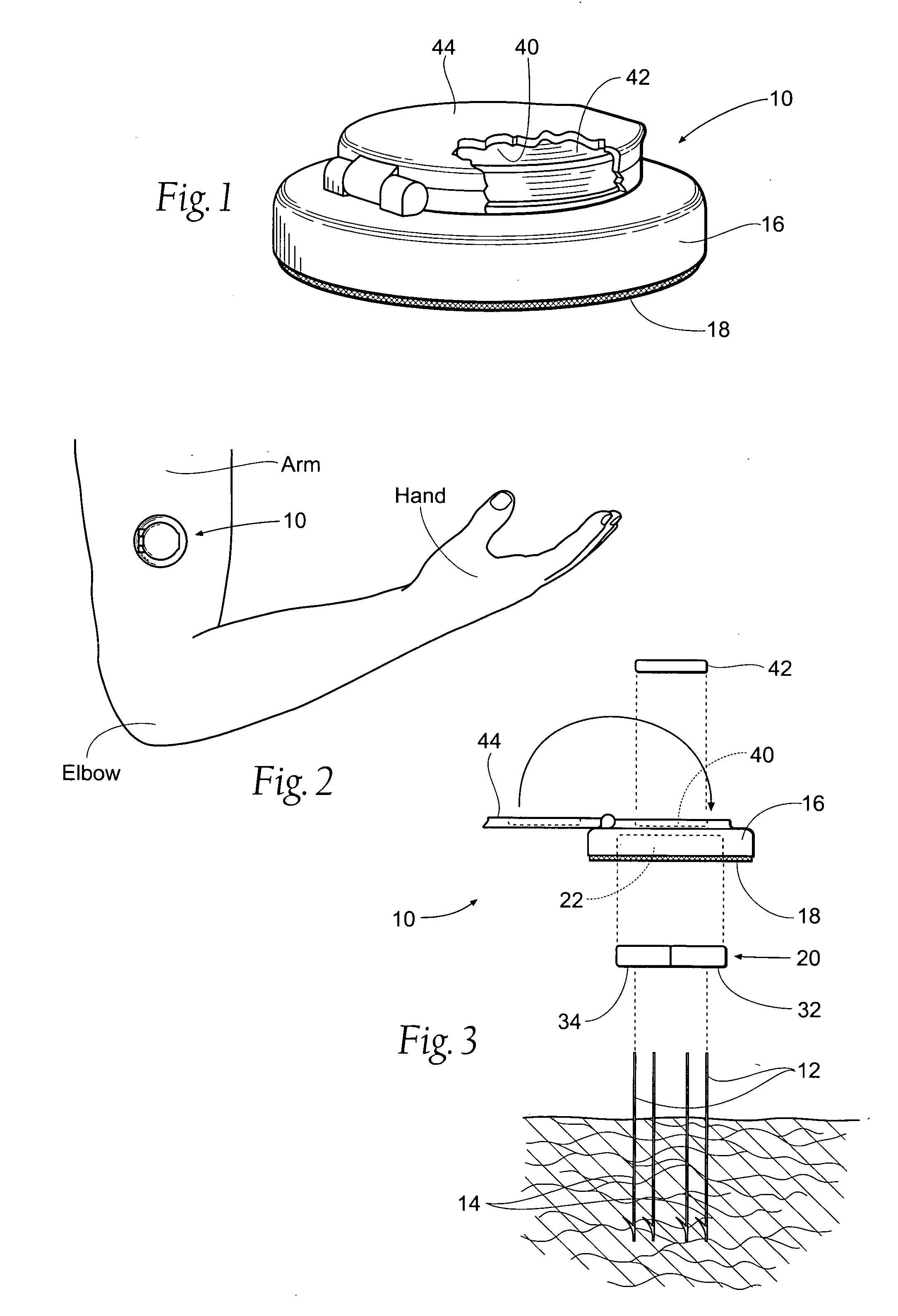
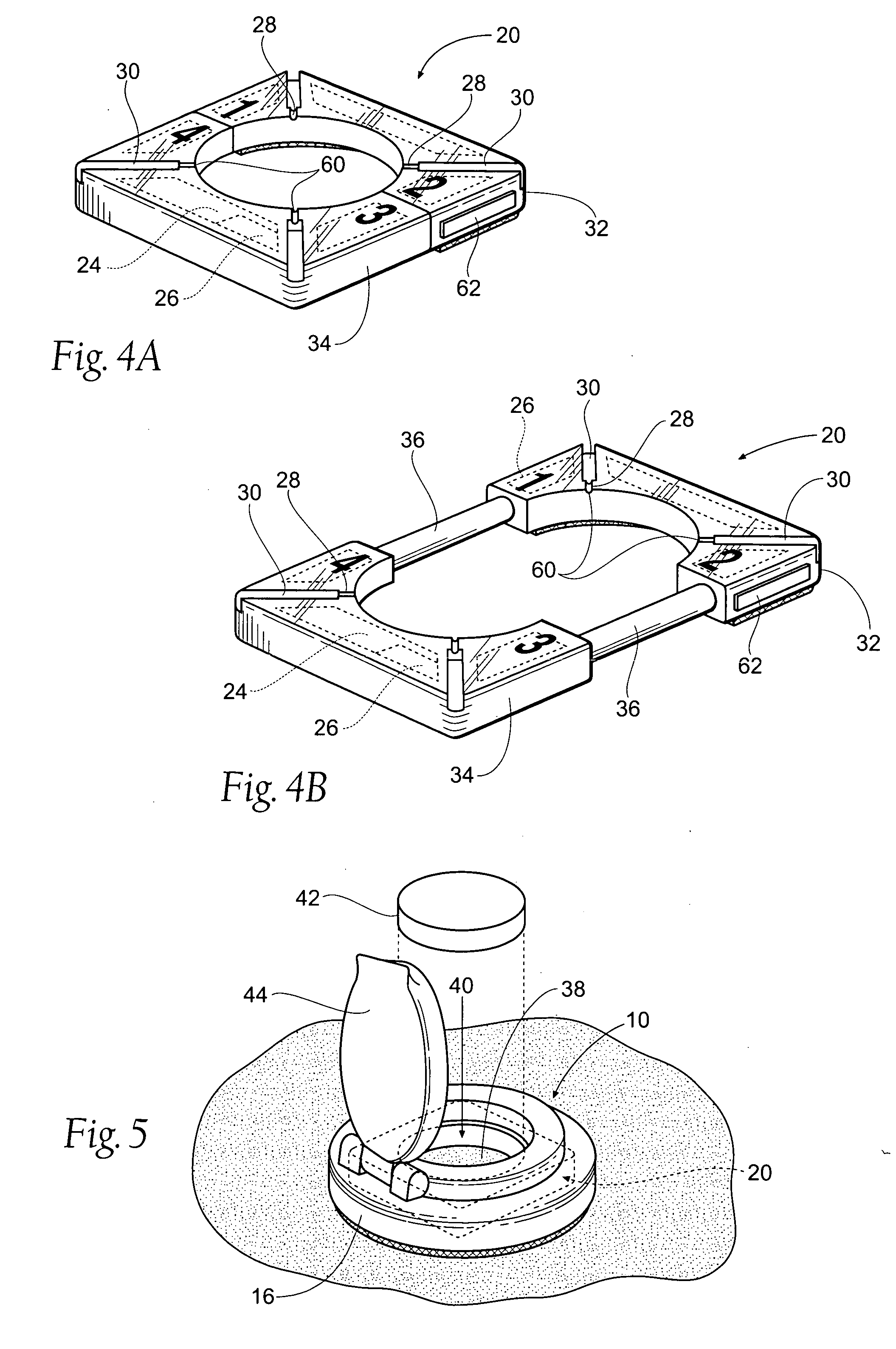
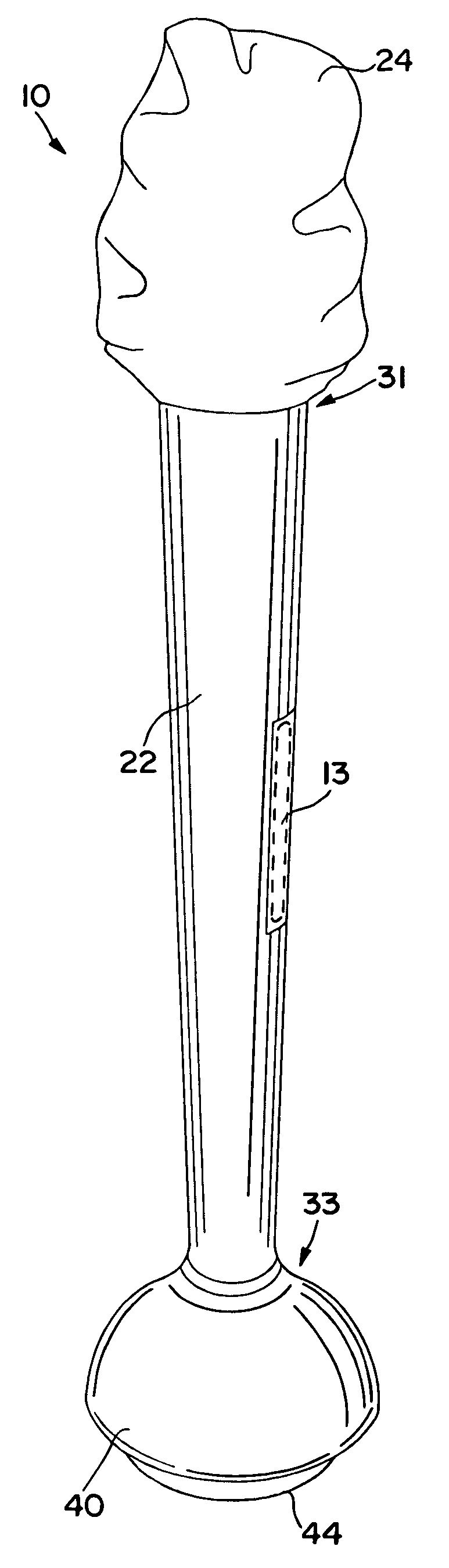
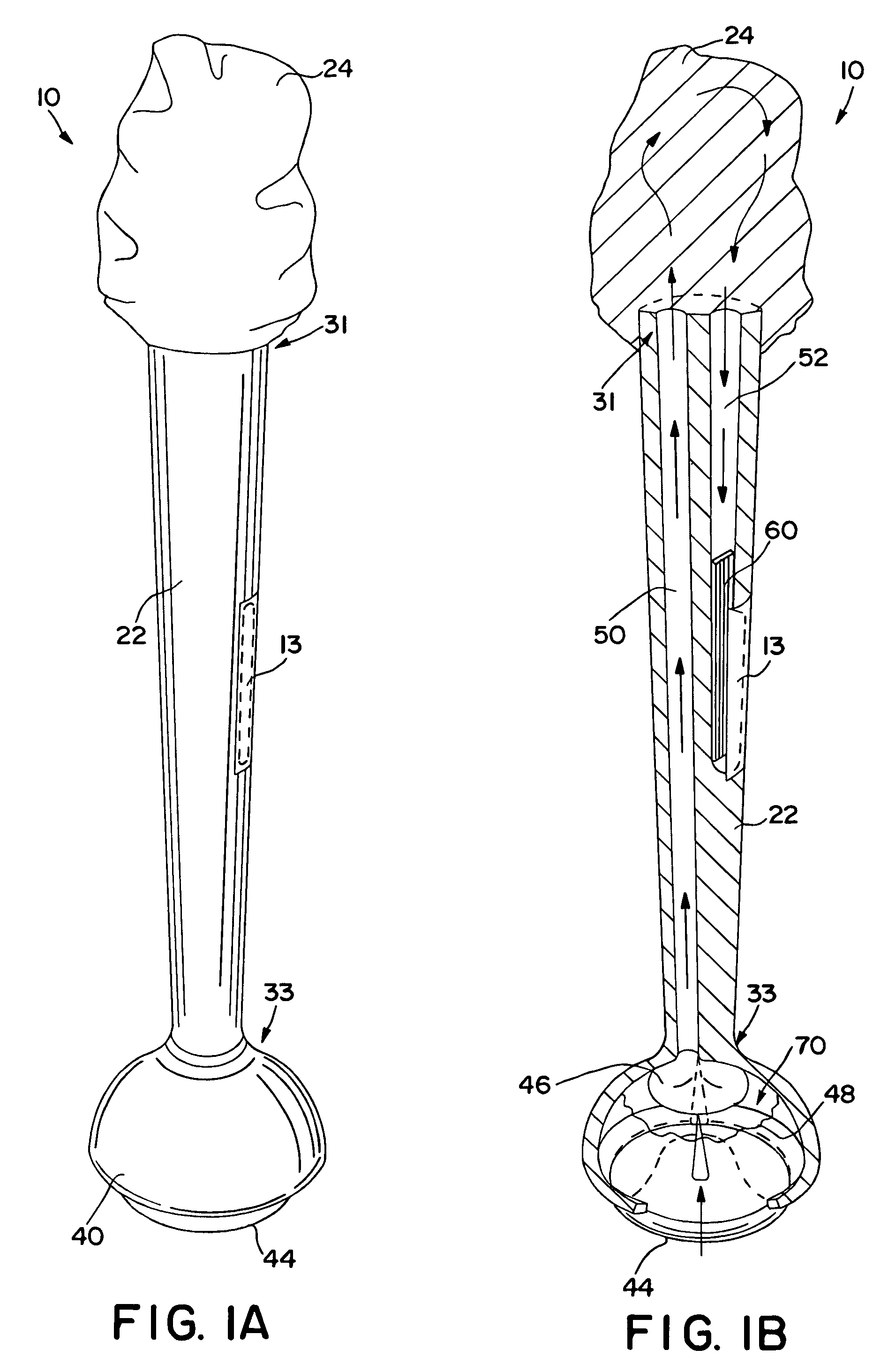
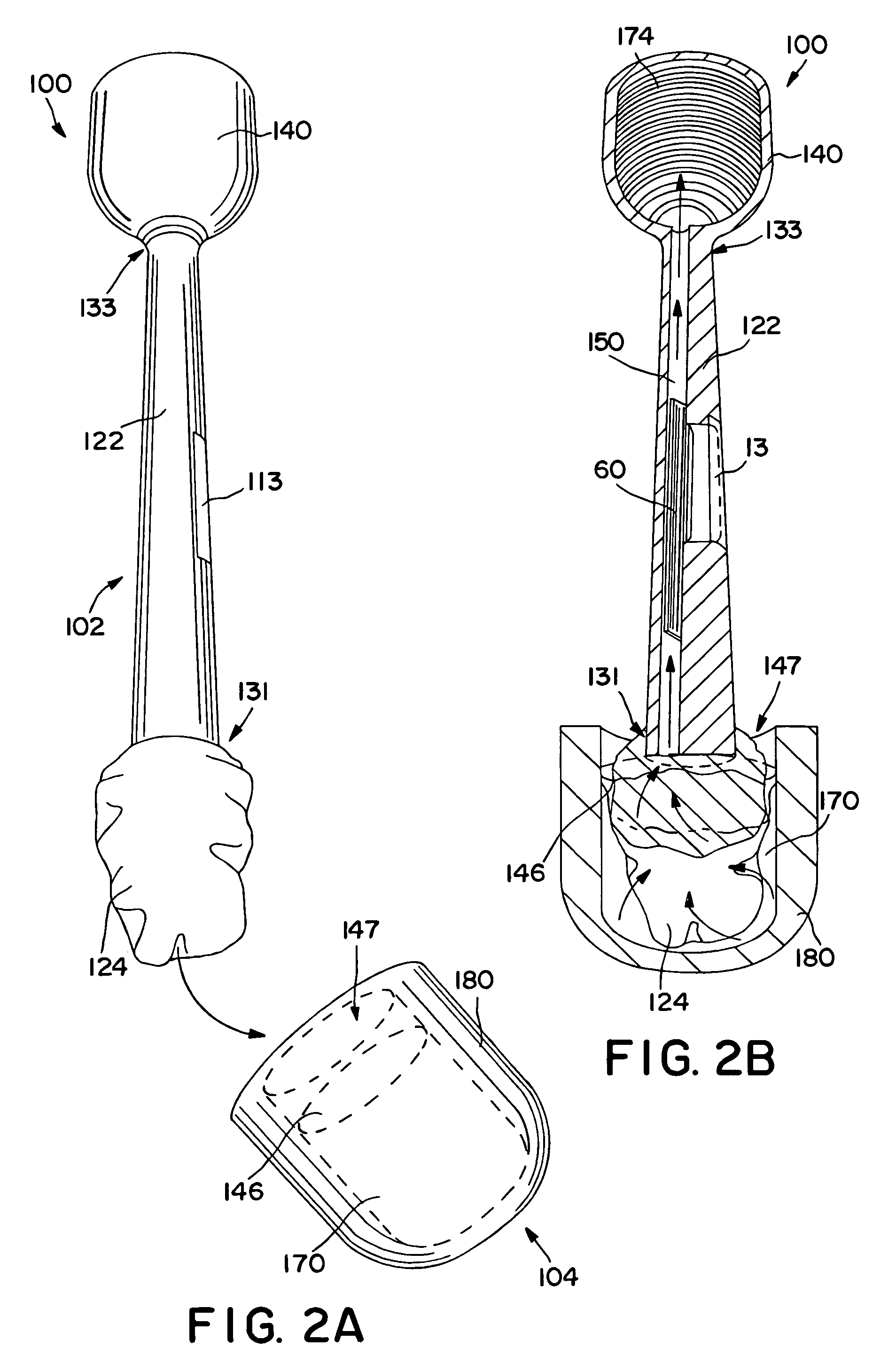
Method and apparatus for obtaining blood for diagnostic tests
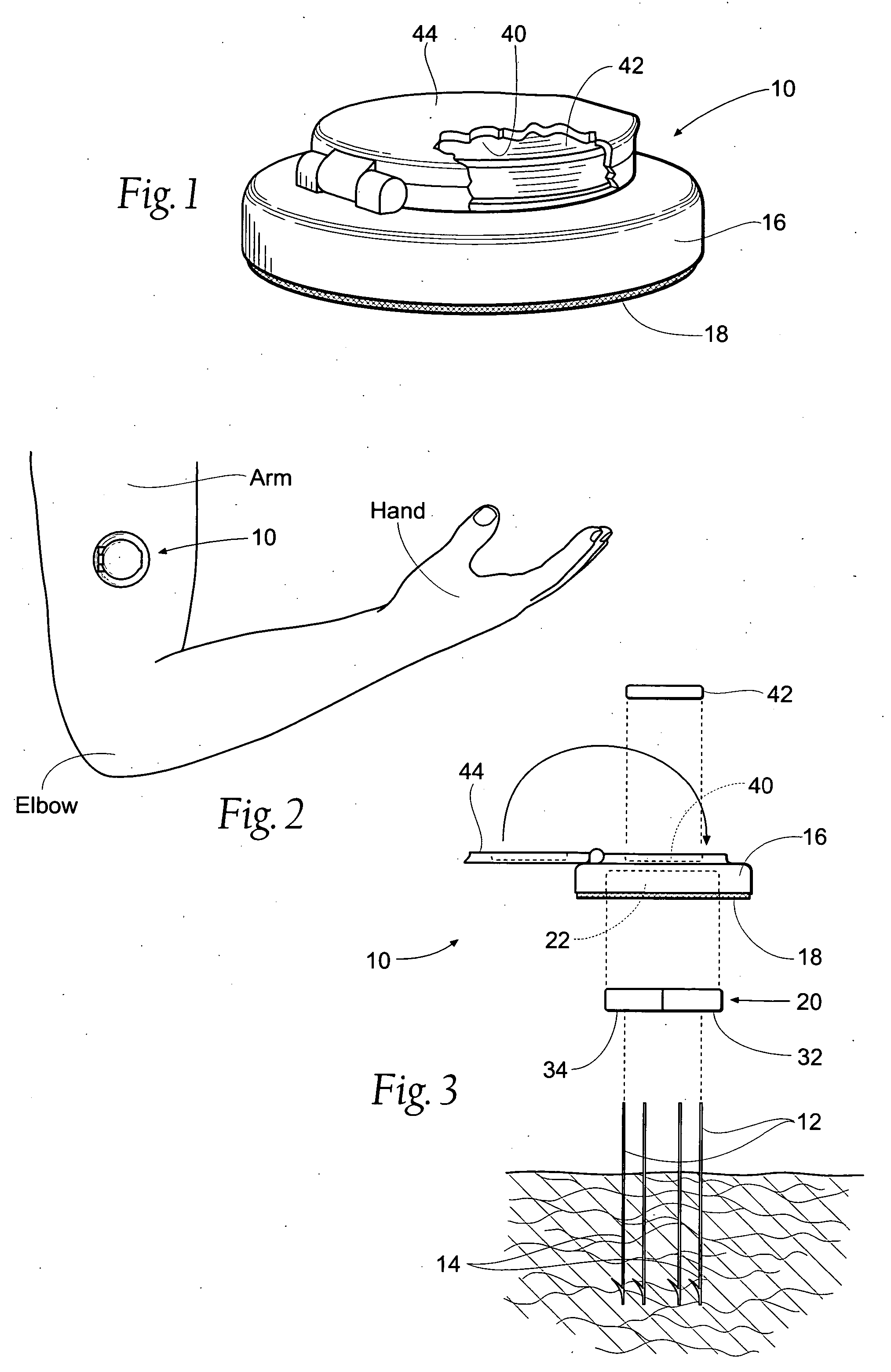
Method and apparatus for obtaining a sample of blood from a patient for subsequent diagnostic tests, e.g., glucose monitoring. In one aspect of the invention, the method comprises the steps of:(a) placing a blood collection device over a region on the surface of the skin from which said sample is to be obtained,(b) forming a seal between said blood collection device and said surface of the skin,(c) creating a vacuum sufficient to result in said surface of the skin becoming stretched and engorged with blood,(d) triggering a lancing assembly and causing a lancet to penetrate said skin,(e) retracting said lancet,(f) withdrawing blood toward and onto a fluid collector, and(g) releasing the vacuum.In another aspect of the invention, an apparatus for carrying out the method described previously is provided. The apparatus comprises:(a) a housing having a sealable chamber located therein and a sealable opening in fluid communication with said sealable chamber,(b) a power source,(c) a vacuum pump operably connected to said power source, said vacuum pump in communication with said sealable chamber,(d) a lancing assembly positioned within said housing, said lancing assembly capable of moving a lancet towards said sealable opening, and(e) a fluid collector positioned in said sealable chamber, said fluid collector in fluid communication with said sealable opening.
Owner:ABBOTT LAB INC
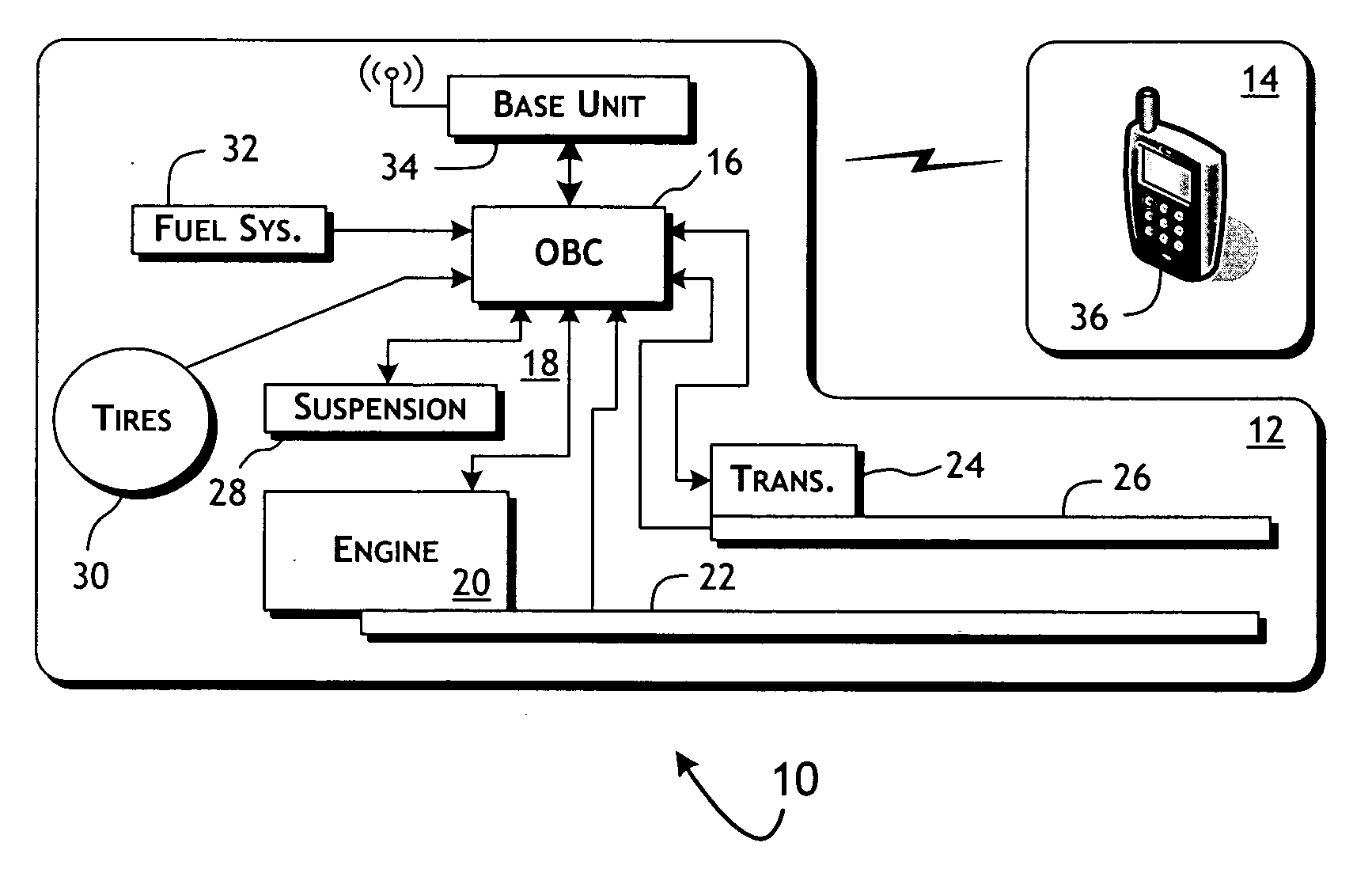
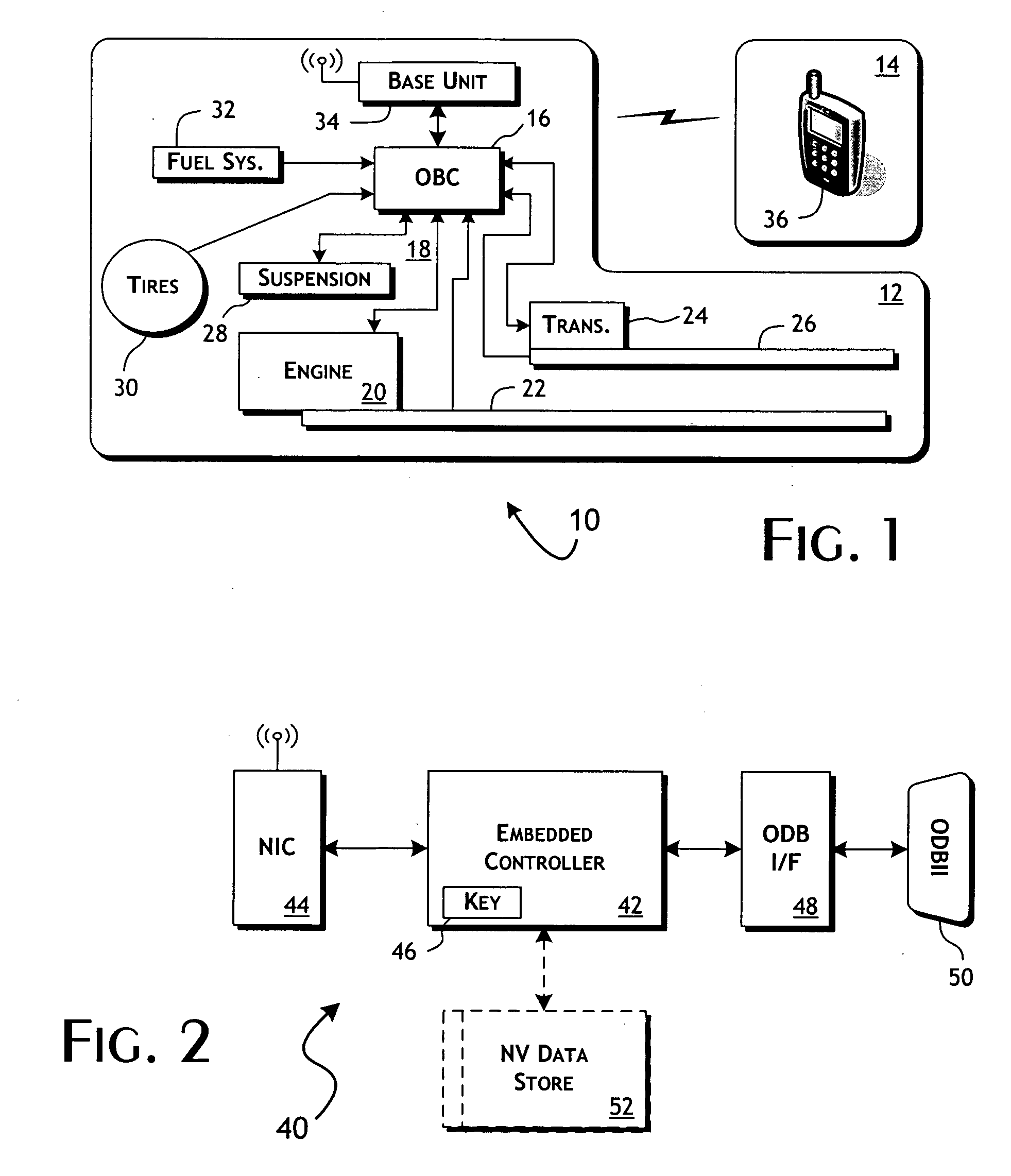
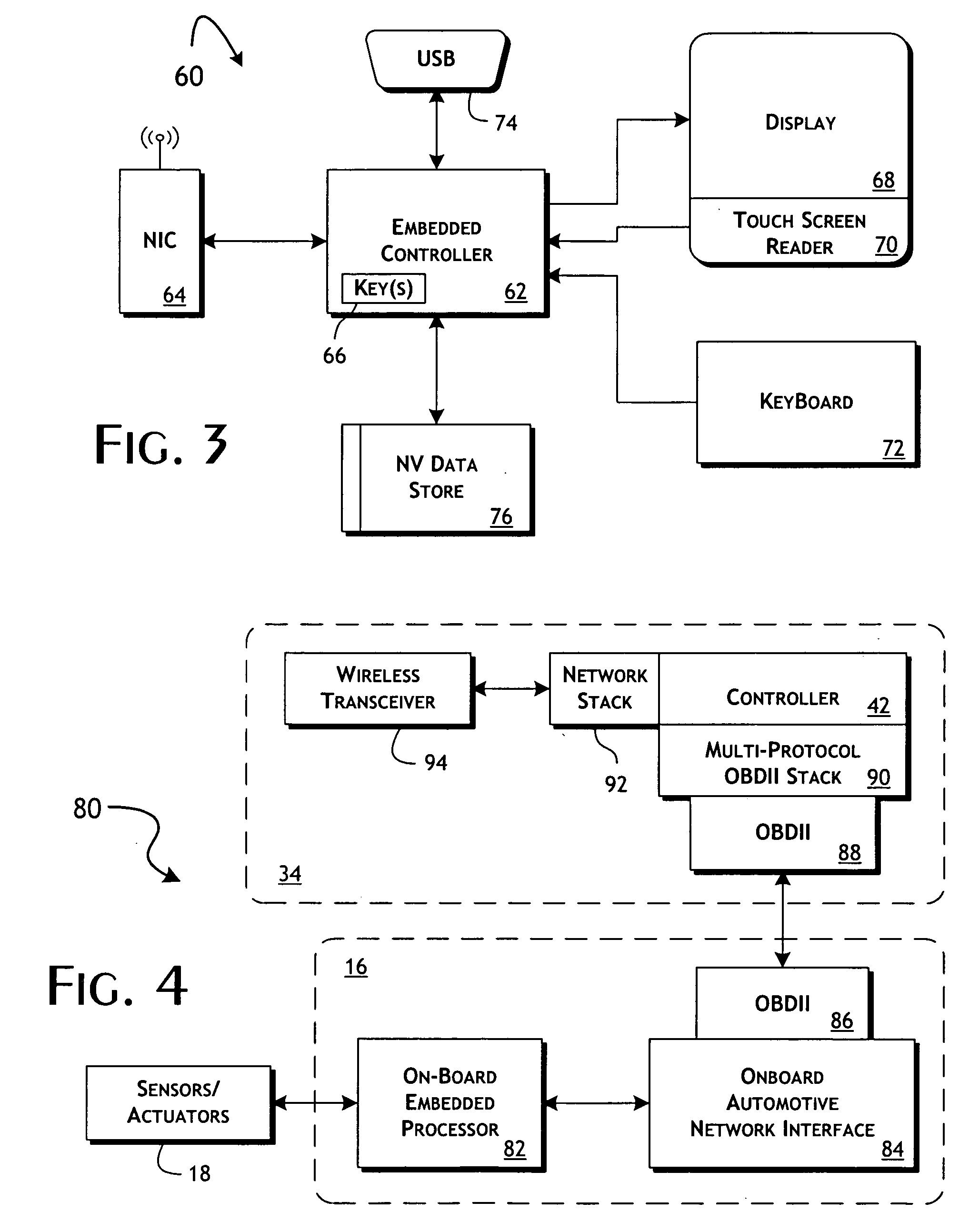
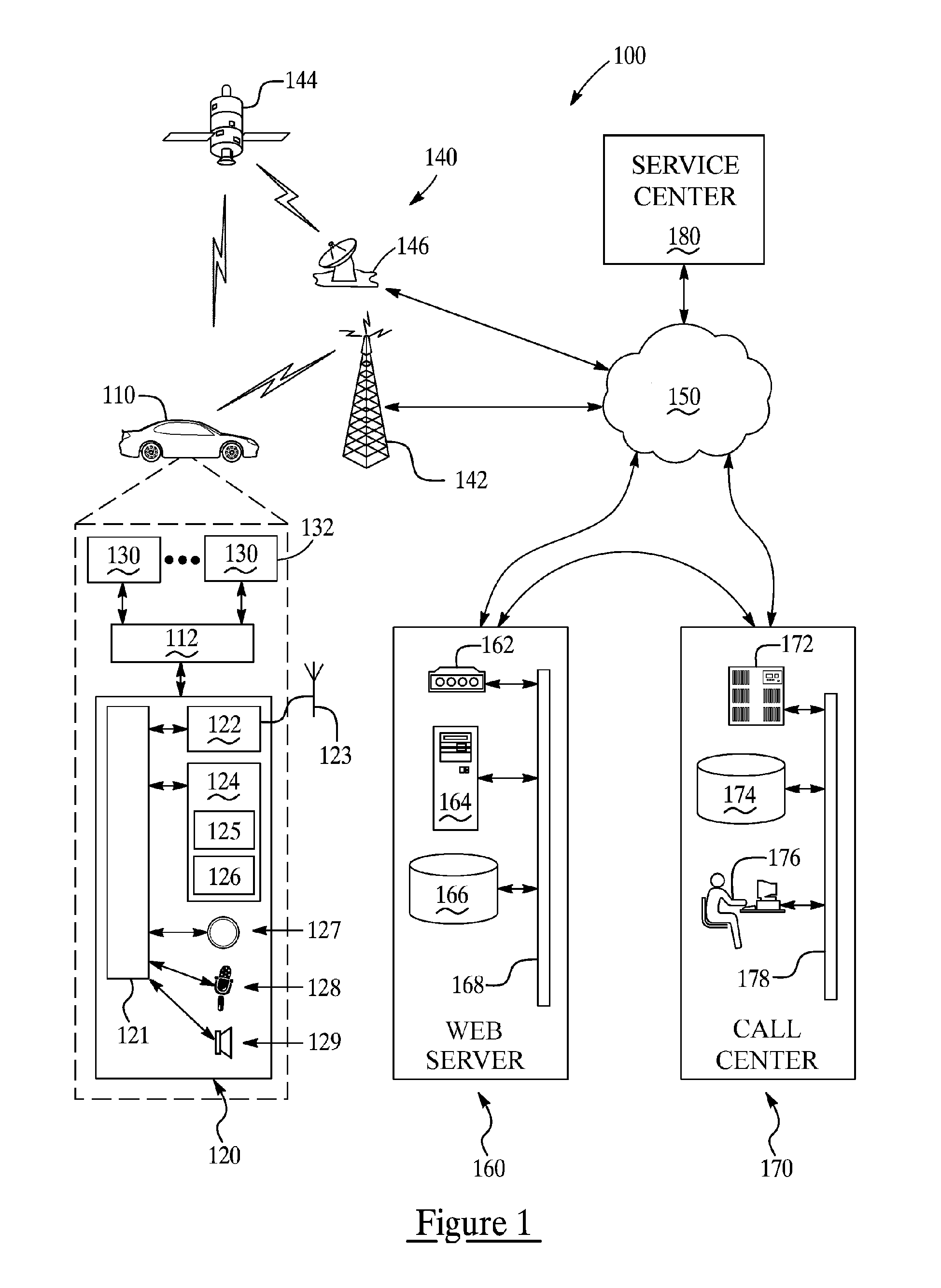
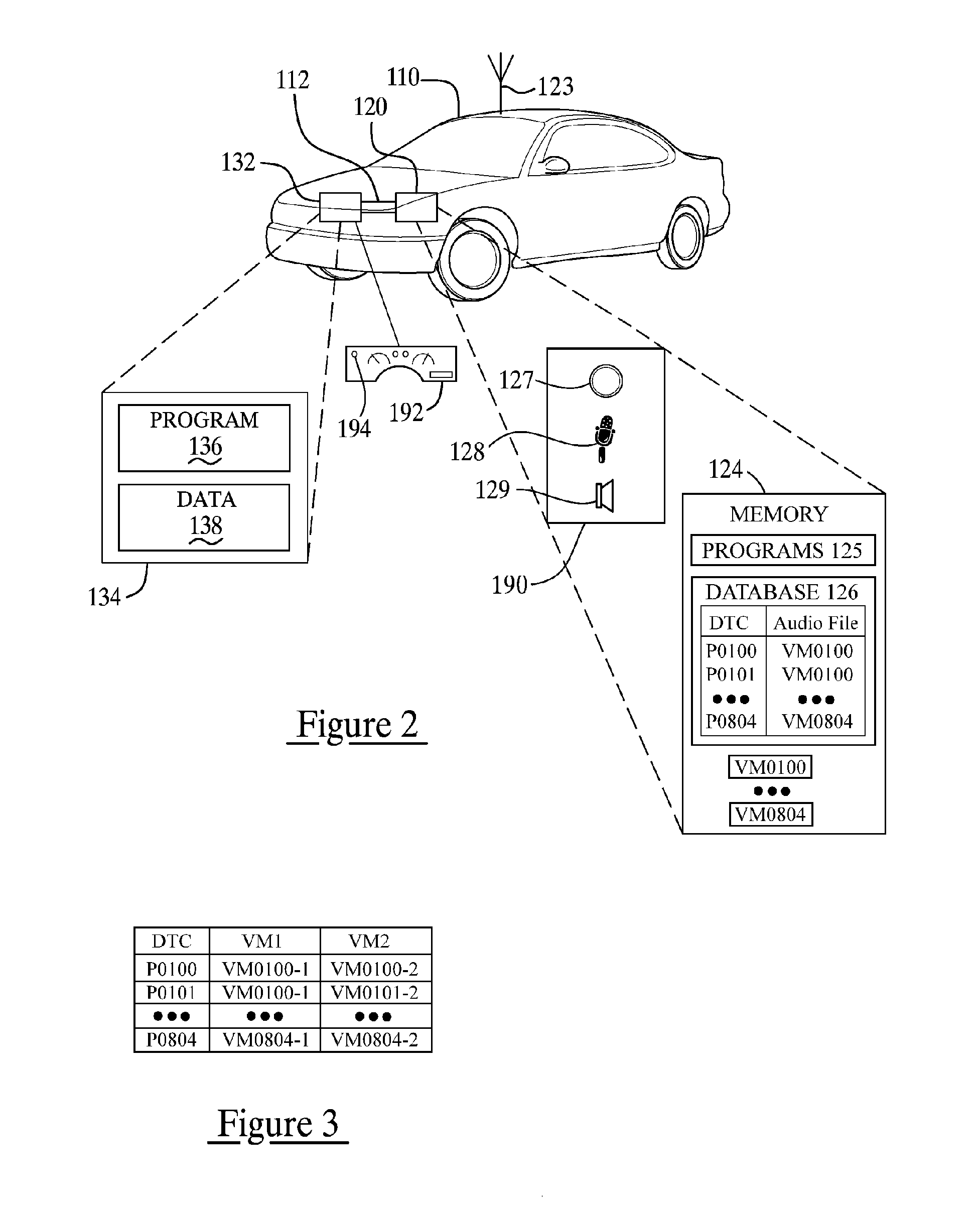
System and methods of performing real-time on-board automotive telemetry analysis and reporting
InactiveUS20060229777A1Readily and safely performedImprove abilitiesVehicle testingInternal-combustion engine testingDiagnostic testControl system
Active diagnosis of current operating and potential fault conditions in the operation of the vehicle is implemented using a diagnostic controller interoperating with an on-board vehicle control system as installed within a vehicle. The diagnostic controller supports autonomous execution of diagnostic tests initiated dependent on the operational state of the vehicle. The control system includes a diagnostics control manager that autonomously selects test routines for execution at defined operational states, including in-service operational states, a monitor, responsive to sensor data retrieved in real-time from the on-board vehicle control system, operative to detect a current instance of the in-service operational state of the vehicle, and a diagnostic test scheduler operative to initiate execution of the diagnostic test routine upon detection of the current instance of the in-service operational state of the vehicle.
Owner:HUDSON MICHAEL D +1
Point of care diagnostic systems
InactiveUS6867051B1Accurate concentrationAccurately presenceComputer-assisted medical data acquisitionMedical imagesPoint of careDiagnostic test
Systems and methods for medical diagnosis or risk assessment for a patient are provided. These systems and methods are designed to be employed at the point of care, such as in emergency rooms and operating rooms, or in any situation in which a rapid and accurate result is desired. The systems and methods process patient data, particularly data from point of care diagnostic tests or assays, including immunoassays, electrocardiograms, X-rays and other such tests, and provide an indication of a medical condition or risk or absence thereof. The systems include an instrument for reading or evaluating the test data and software for converting the data into diagnostic or risk assessment information.
Owner:CYTYC CORP
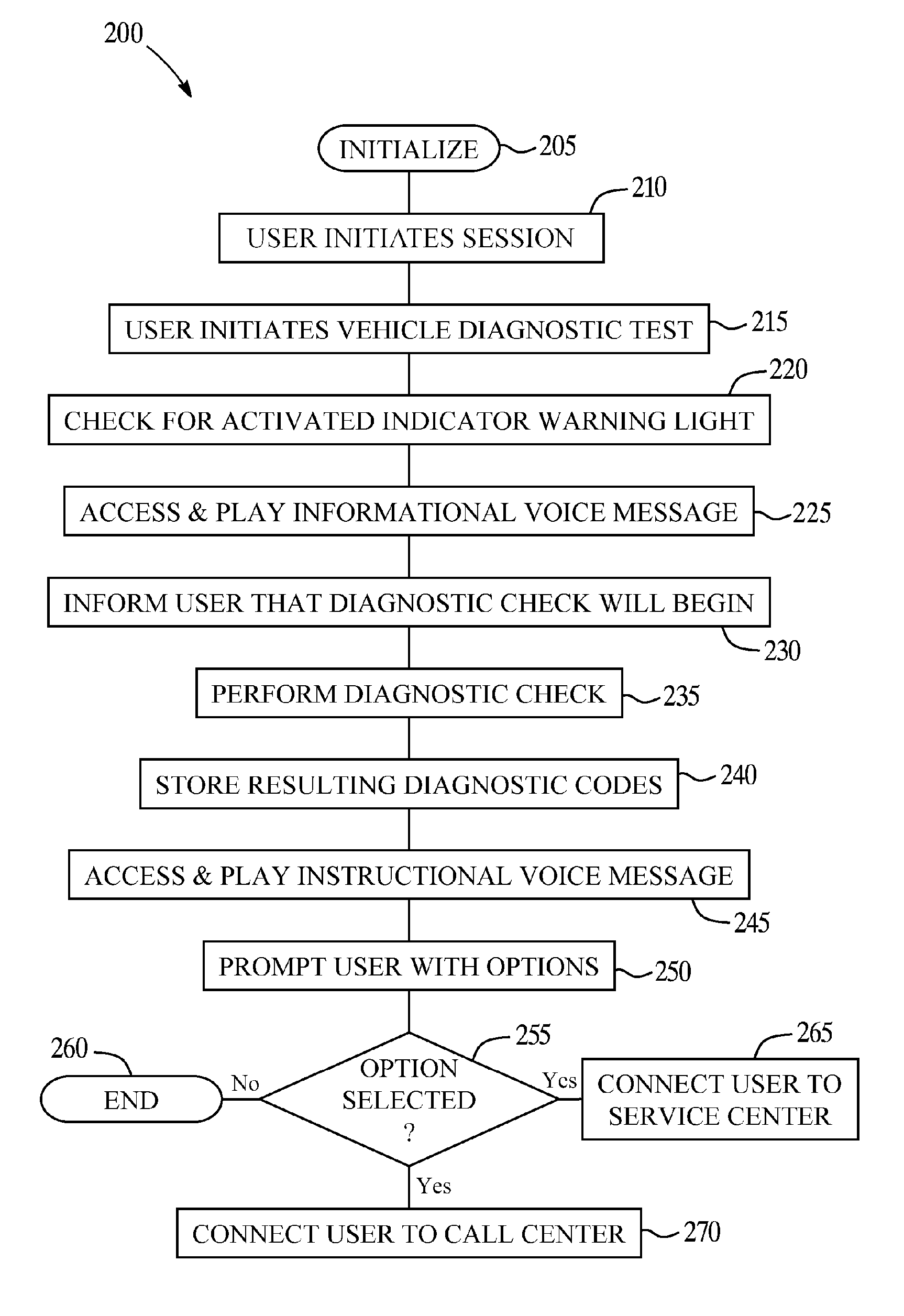
Vehicle diagnostic test and reporting method
ActiveUS20070093947A1Vehicle testingRegistering/indicating working of vehiclesInformation processingDriver/operator
A system and method for providing user-initiated vehicle diagnostic testing and reporting in a telematics-enabled vehicle. In the method, a request for a vehicle diagnostic test is received from the driver through a user interface of a telematics unit on the vehicle. A simplified initial diagnostic check is made and a first voice message is played for the driver that provides information concerning any detected vehicle problem. The method then undergoes a more complete diagnostic check and the resulting diagnostic information is used to select and play a second voice message that provides instructions for taking corrective action to fix the detected problem. Communication with a live advisor is also provided by way of a cellular or other wireless carrier system.
Owner:GENERA MOTORS LLC
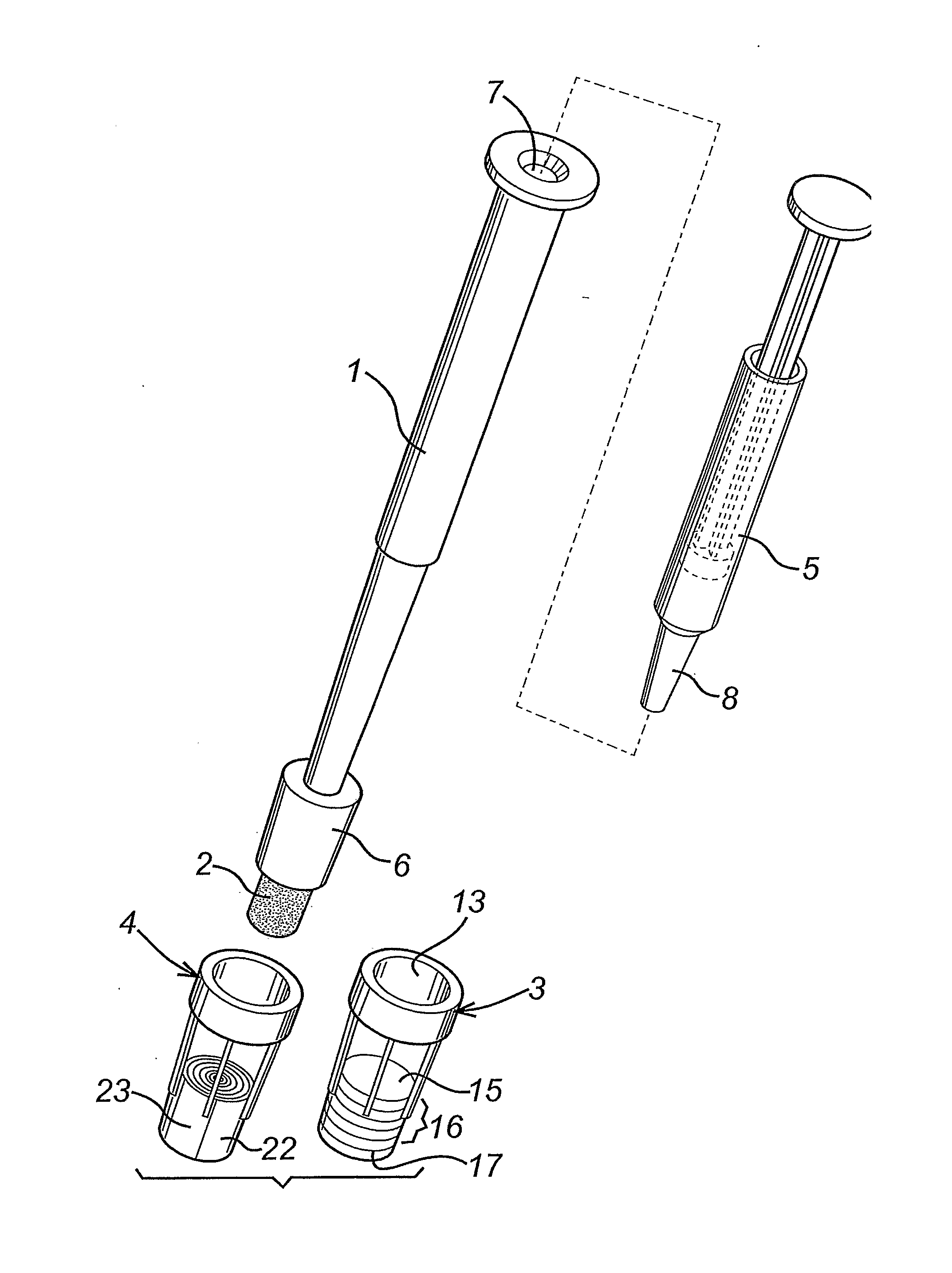
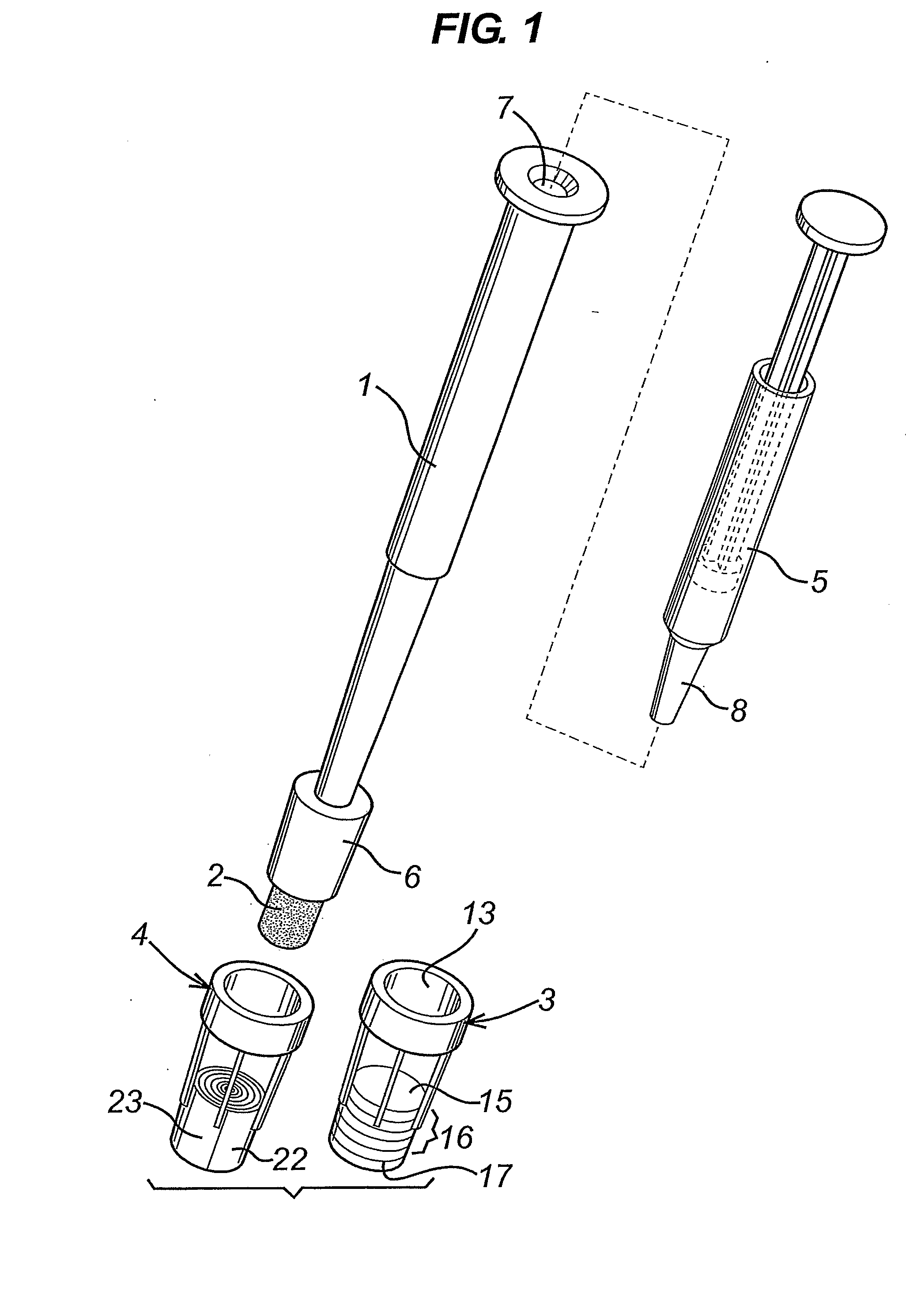
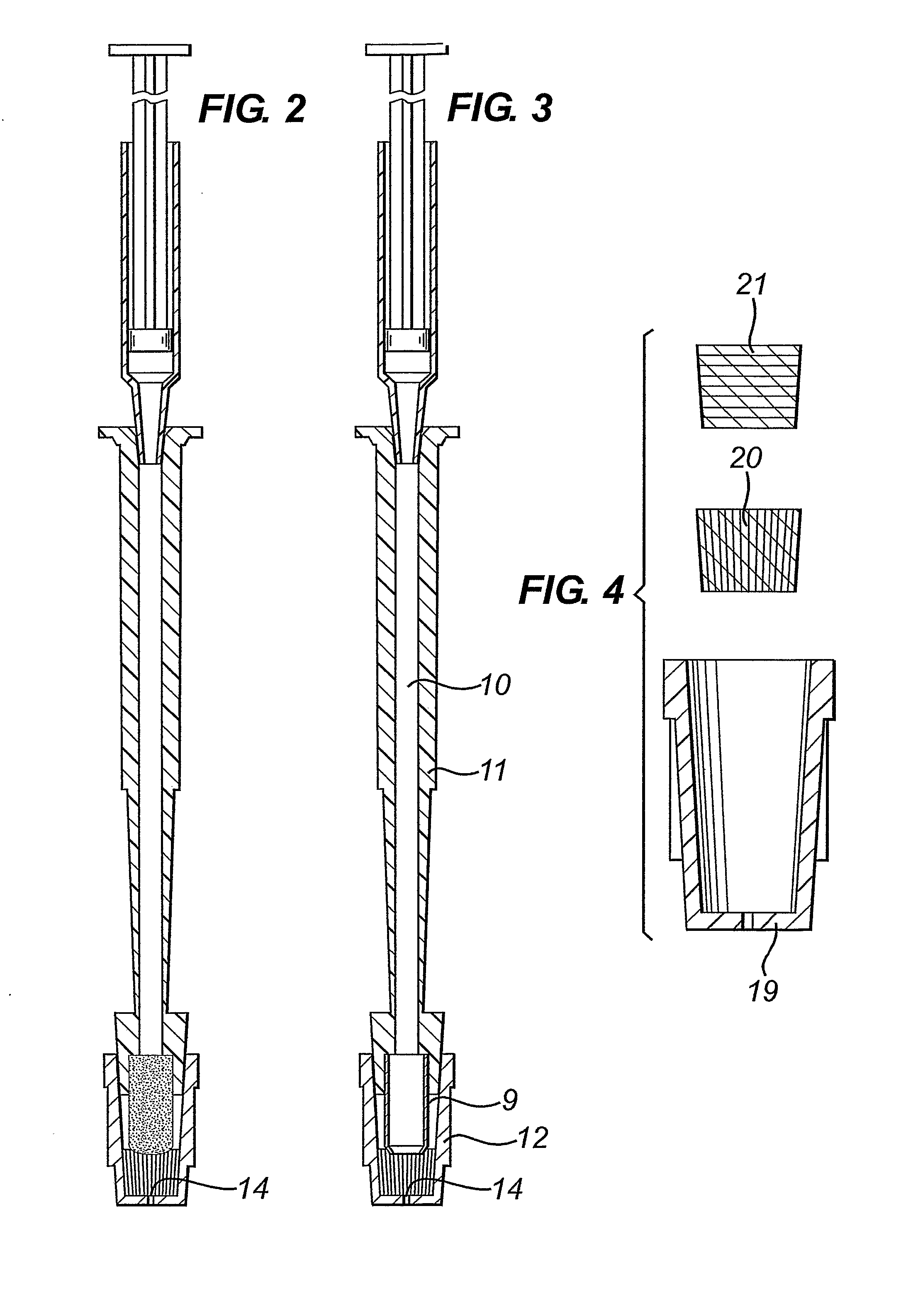
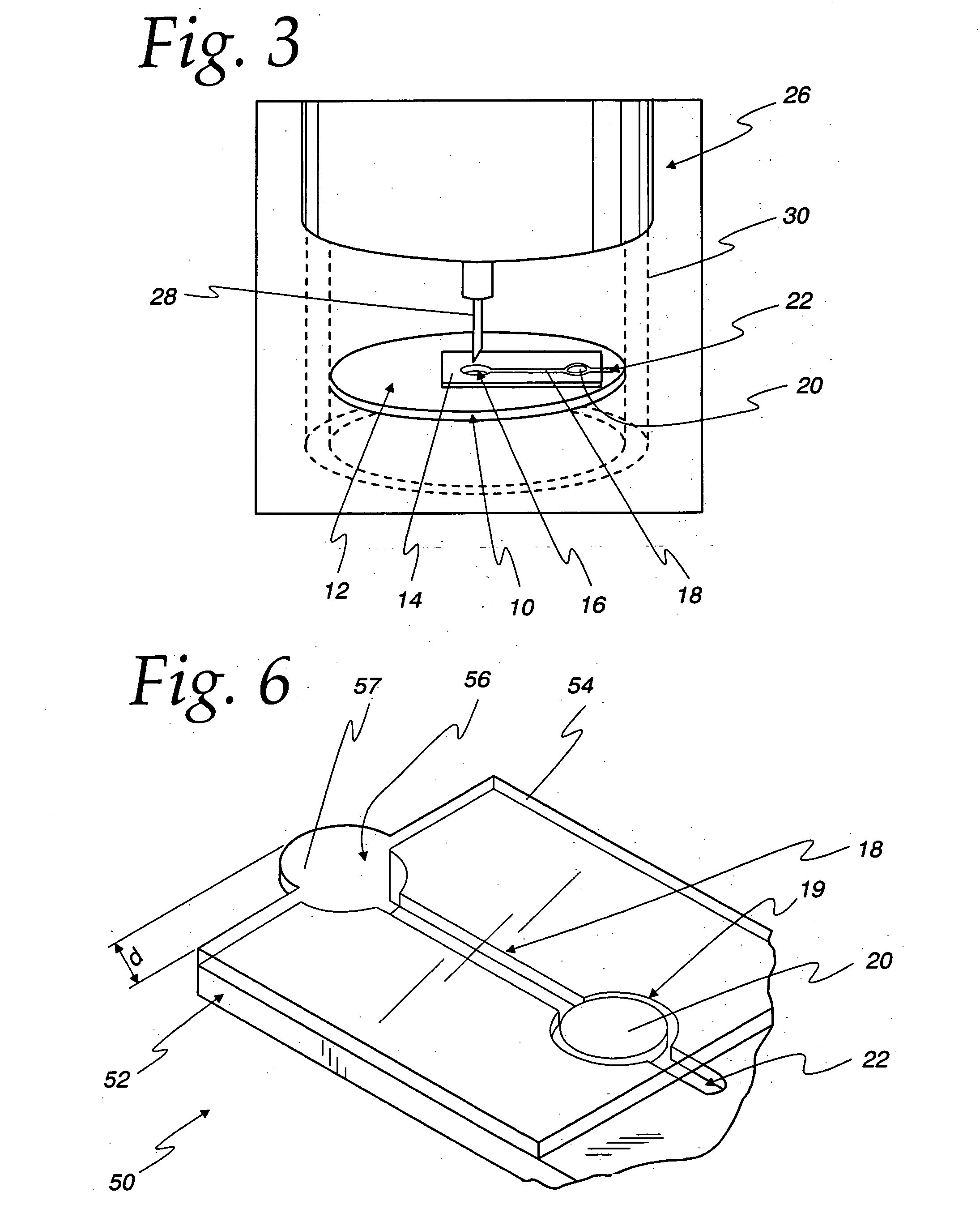
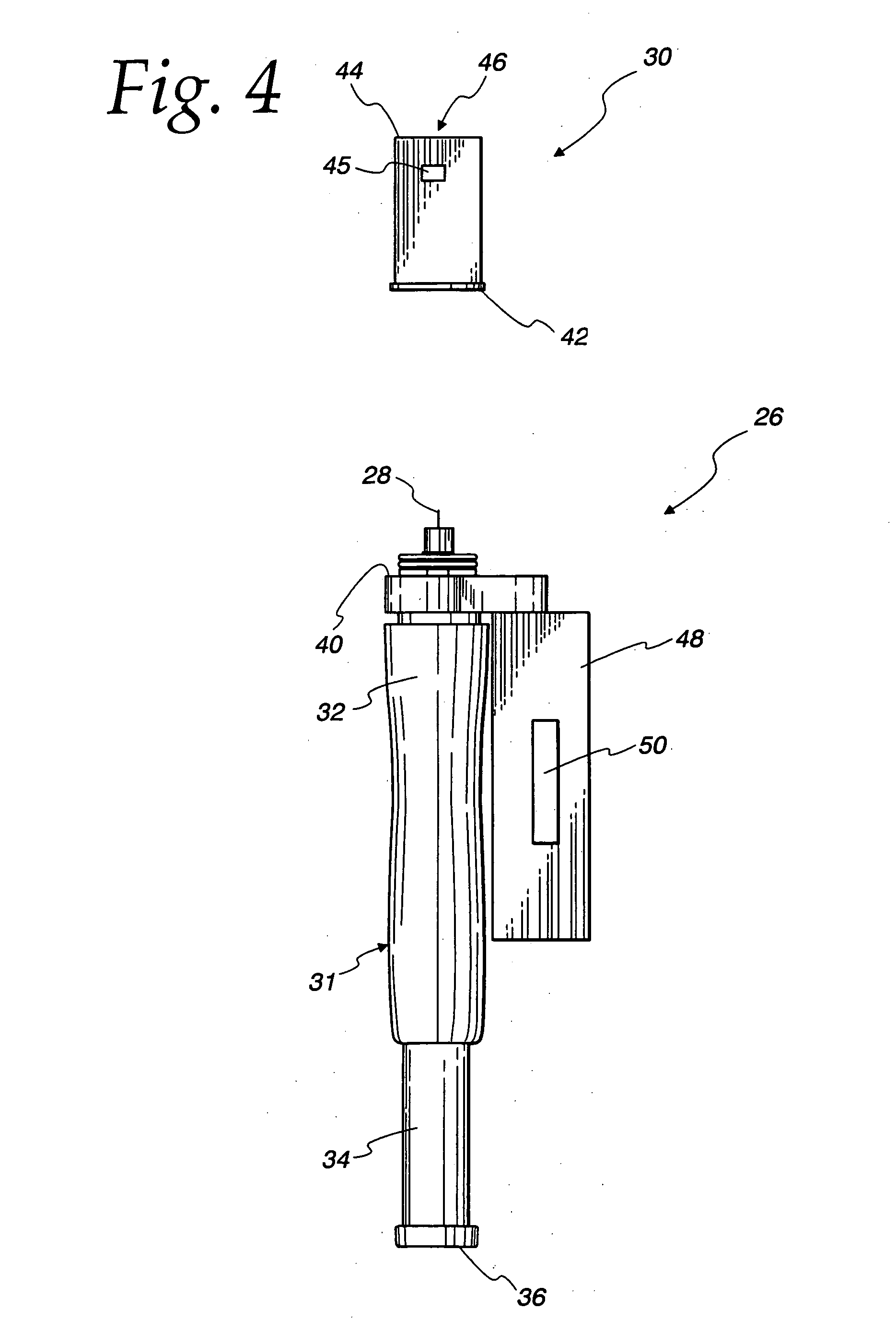
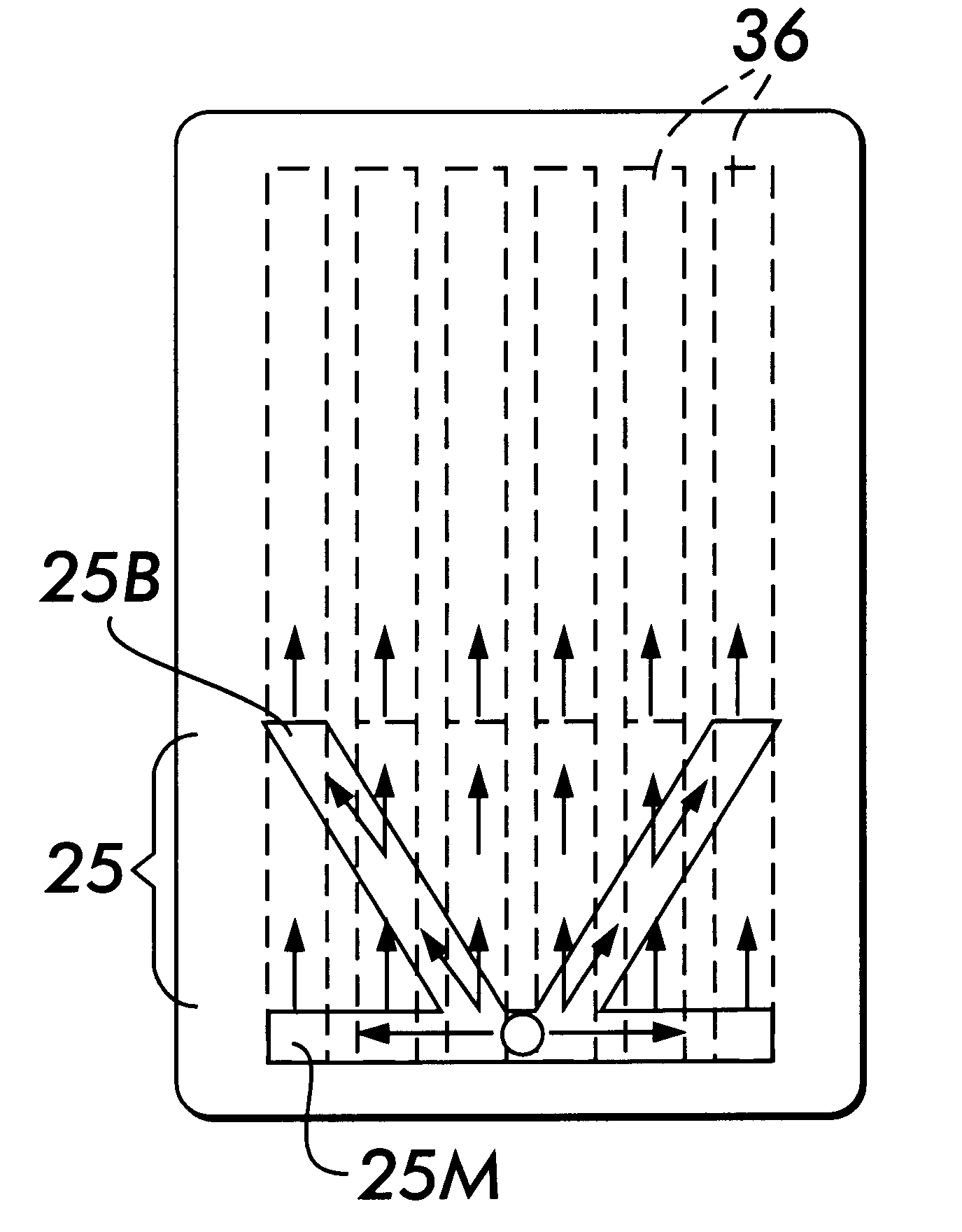
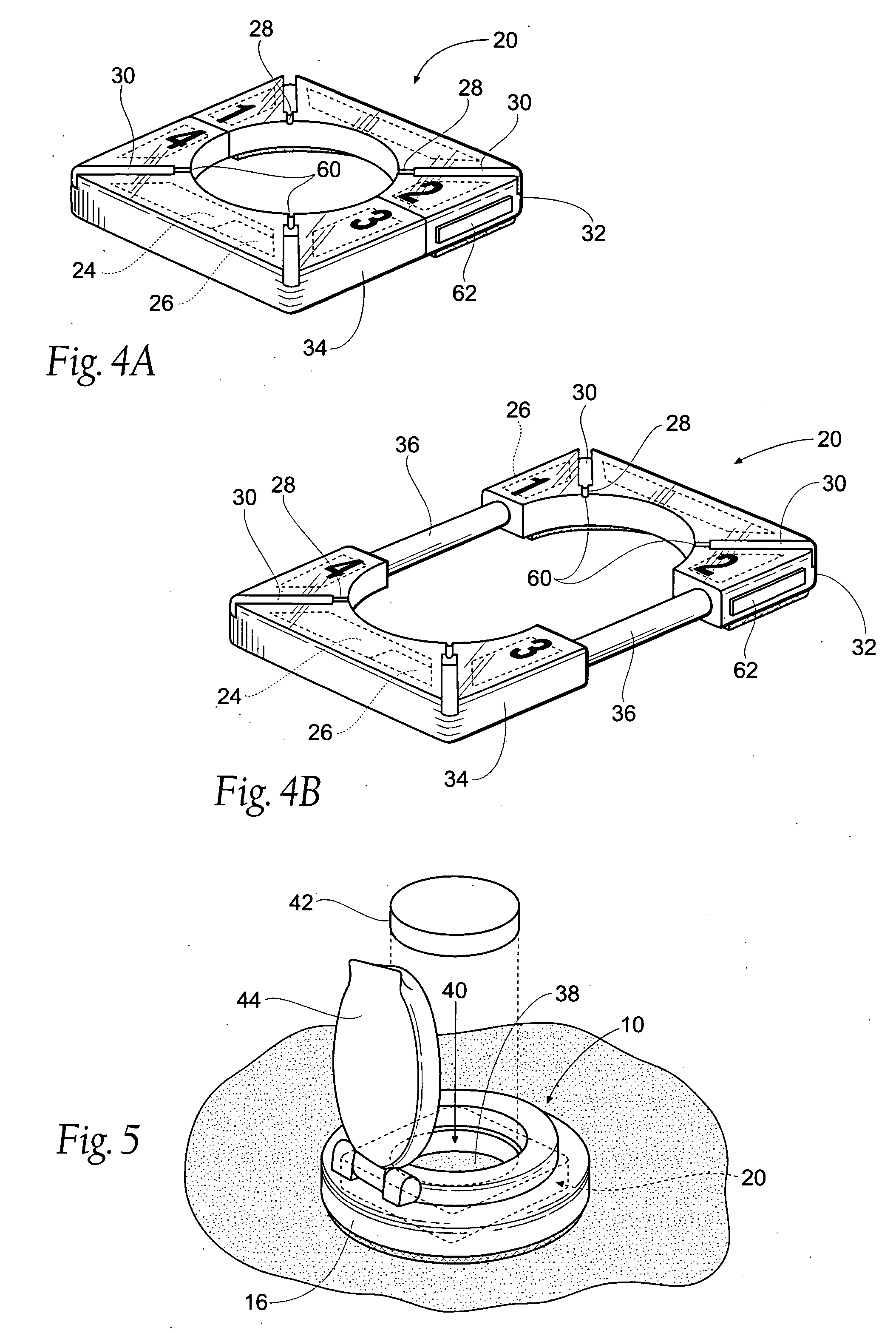
Diagnostic Test Devices
InactiveUS20070244368A1Increase rangeBioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testProximate
A diagnostic test apparatus comprising: a shaft having a first end and a second end; a swab or a biopsy punch mounted on the first end of a shaft; and a cap for fitting over the first end of the shaft, said cap containing at least one diagnostic test reagent; wherein the shaft comprises at least one cap engagement element located proximate to the first end, said element extending radially outwardly of the swab or the biopsy punch for engagement with the cap to retain the cap on the shaft. Also provided are diagnostic caps for use in the test apparatus having a small internal volume and at least one diagnostic test reagent located in or on an absorbent plug inside the cap. Also provided are diagnostic caps for use in the test apparatus having a housing extending from a sample receiving port, said housing defining a lateral flow path for the sample. Also provided is a test system comprising a swab shaft, a biopsy punch shaft and a diagnostic cap in accordance with the invention, wherein the cap can be used interchangeably between the two different shafts.
Owner:WOUNDCHEK LAB US
System and Methods for Improved Diabetes Data Management and Use Employing Wireless Connectivity Between Patients and Healthcare Providers and Repository of Diabetes Management Information
ActiveUS20100069730A1Shorten development timeExtended service lifePhysical therapies and activitiesDrug and medicationsDiseaseInformation repository
Methods, devices and a system for disease management are provided that employ diagnostic testing devices (e.g., blood glucose meters) and medication delivery devices (e.g., insulin delivery devices) for providing data to a repository in real-time and automatically. Repository data can be analyzed to determine such information as actual test strip use, patient health parameters to outside prescribed ranges, testing and medication delivery compliance, patient profiles or stakeholders to receive promotional items or incentives, and so on. Connected meters and medication delivery devices and repository data analysis are also employed to associate a diagnostic test to a mealtime based on timing of a therapeutic intervention performed by an individual.
Owner:EMBECTA CORP
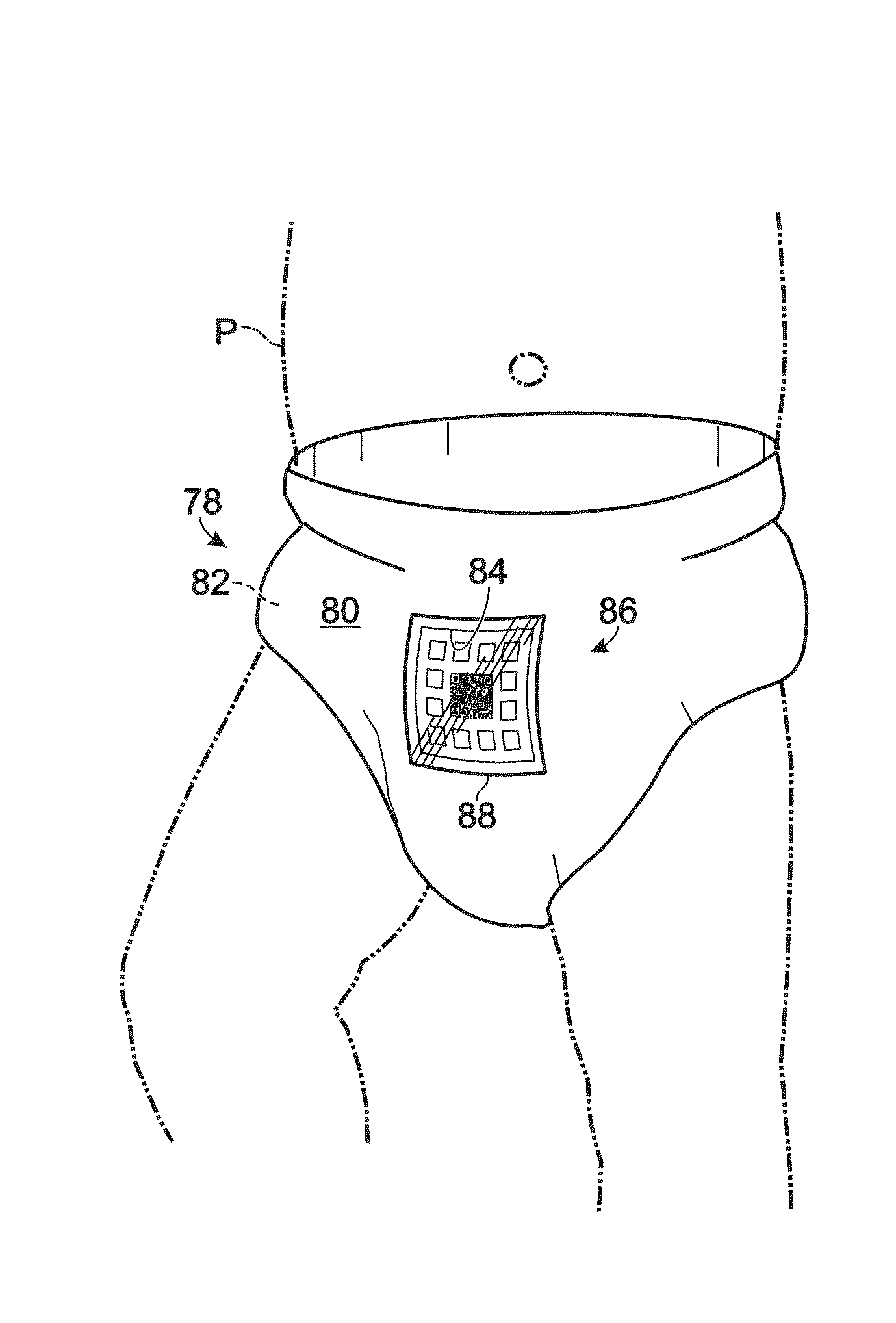
Health diagnostic systems and methods
A health monitoring system, and methods of use and manufacture thereof are disclosed. The health monitoring system may include a computing system and a diagnostic test coupled to a diaper. The diagnostic test may include one or more sensors configured to produce a visual indication of one or more analytes contained in a sample produced by a subject. The diagnostic test may include a machine-readable code. The computing system may be configured to read the machine-readable code to allow an application running on the computing system to automatically perform at least one task related to a production of a data point based on the visual indication. The health monitoring system may aid in identifying a potential abnormal health condition of the subject by providing automatic longitudinal analysis of analytes contained in samples produced by the subject over a period of time.
Owner:ANAVAH HEALTH LLC
Dispenser for flattened articles
InactiveUS20050281706A1Reduce amountKeep the environmentAnalysis using chemical indicatorsCoin-freed apparatus detailsUser needsDiagnostic test
A substantially moisture-proof, airtight dispenser for both storing and dispensing several flattened articles such as diagnostic test strips. The inventive dispenser includes a novel pivotable housing that a user need merely grab and squeeze to eject a test strip. Independent movement of the user's fingers to push a button or turn a knob is unnecessary to dispense a strip, which makes the present invention well suited for diabetics suffering from nerve damage in their extremities and other complications resulting from the disease. The invention includes a novel flexible arm member and pusher head that engage and push an article from the dispenser as the two parts of the housing are pivoted together. The articles are dispensed through an exit that is configured with a novel flexible seal that maintains the dispenser substantially airtight. Several inventive seal embodiments and methods of making the same are disclosed.
Owner:ROCHE DIABETES CARE INC
Detection of multiple analytes from a single sample using a multi-well, multi-analyte flow-through diagnostic test device
InactiveUS7052831B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleDiagnostic test
The present invention provides a method and device for conducting a rapid in vitro enzyme immunoassay test for the direct and qualitative detection of two or more viral antigens from specimens of symptomatic patients. The method for immunoassay of viral antigens is performed on a membrane. Non-immunological capture of viral antigens takes place by absorption onto the membrane. Captured antigen binds to a detection reagent that includes a label conjugated to a specific antibody. The test is a differentiated test such that two or more viral antigens may be distinguished from each other in a single test. The invention also includes a kit for performing an assay in accordance with the method of the present invention, wherein the kit comprises the device of the present invention.
Owner:BECTON DICKINSON & CO
Point of care diagnostic systems
InactiveUS6936476B1Accurate concentrationAccurately presenceScattering properties measurementsComputer-assisted medical data acquisitionPoint of careDiagnostic test
Systems and methods for medical diagnosis or risk assessment for a patient are provided. These systems and methods are designed to be employed at the point of care, such as in emergency rooms and operating rooms, or in any situation in which a rapid and accurate result is desired. The systems and methods process patient data, particularly data from point of care diagnostic tests or assays, including immunoassays, electrocardiograms, X-rays and other such tests, and provide an indication of a medical condition or risk or absence thereof. The systems include an instrument for reading or evaluating the test data and software for converting the data into diagnostic or risk assessment information.
Owner:CYTYC CORP
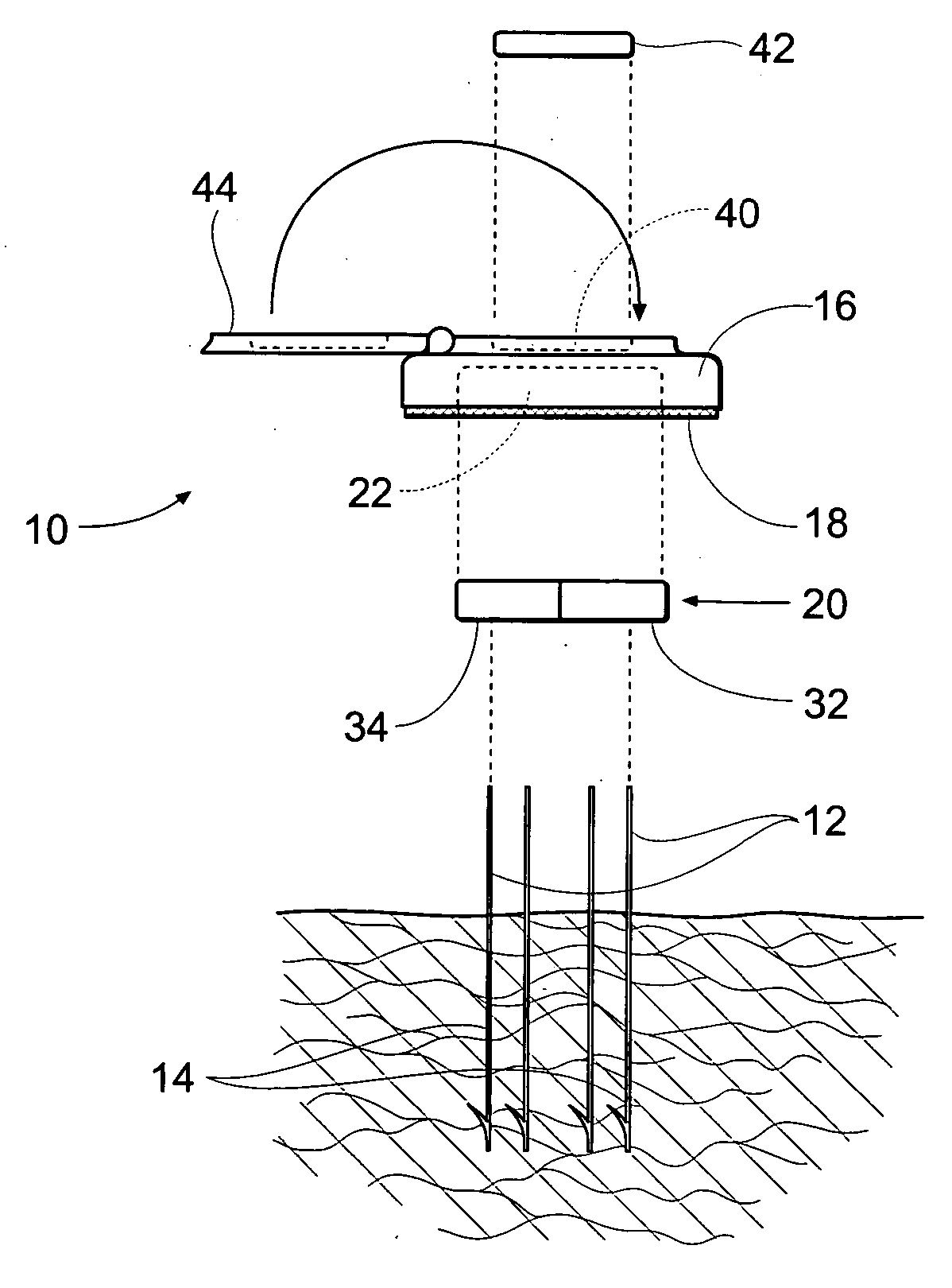
Diagnostic test strip for collecting and detecting an analyte a fluid sample and method for using same
ActiveUS20050136501A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementAnalyteDiagnostic test
A test strip for use of the determination of an analyte in a fluid sample according to one embodiment of the present invention is disclosed. The test strip comprises a base having a top and a bottom, a collection chamber that extends between the top and the bottom of the base, a containing ring that is disposed on the bottom of the base and surrounds the collection chamber, and a capillary channel formed in top of the base that has an inlet fluidly coupled to the collection chamber, a test element disposed within the capillary channel. A lid is attached to the top of the base and covers the collection chamber, the test membrane, and at least a portion of the capillary channel.
Owner:ASCENSIA DIABETES CARE HLDG AG
Fluid sample distriution system for test device
InactiveUS6203757B1Evenly distributedAnalysis using chemical indicatorsLaboratory glasswaresDistribution systemTest fixture
Diagnostic products having multiple test strips within a unitary diagnostic test device, or test icon, are described herein. In the preferred embodiments of the diagnostic test device of this invention, a fluid sample distribution system is provided wherein a sample collection and distribution port is provided in the housing for receipt of a biologic fluid sample and the channeling of such sample onto a sample receiving web. The sample receiving web, which is located within the test device, is in fluid communication with an array of test strips, and is configured to deliver an aliquot of biologic fluid sample to the test site of each such test strip at essentially the same rate. In the preferred embodiments of this invention, the sample receiving web comprises at least one base segment and at least one branched segment. Each of the base and branched segments can be formed or cut from a common sheet of material or from separate sheet material and thereafter placed in contiguous relationship one another. The relative placement of the sample receiving web within the test device is coincident with a portion of each test strip and designed to effect the balanced distribution and delivery of an aliquot of the biologic fluid sample to the test site of each of the test strips within the test device.
Owner:BIONIKE
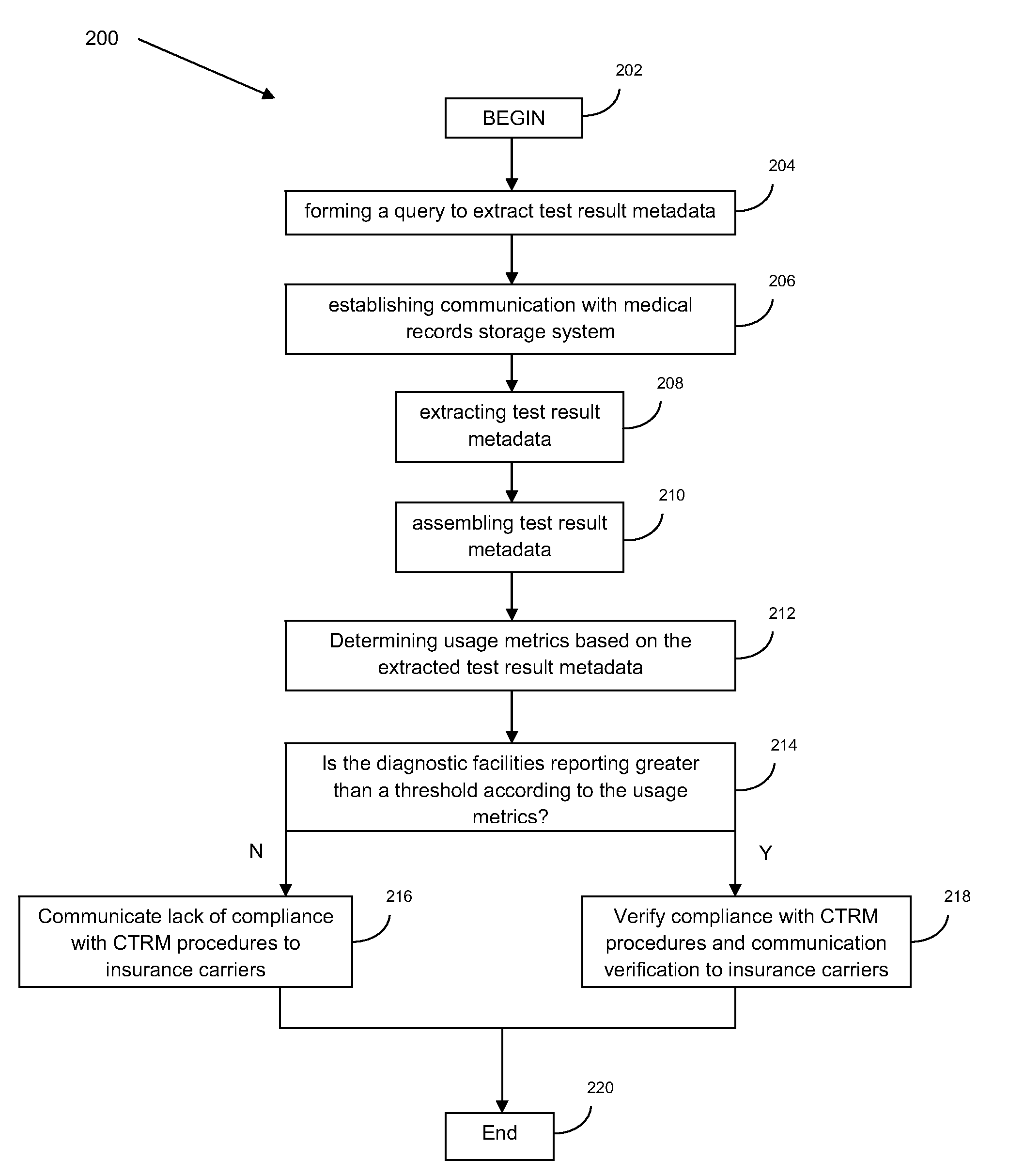
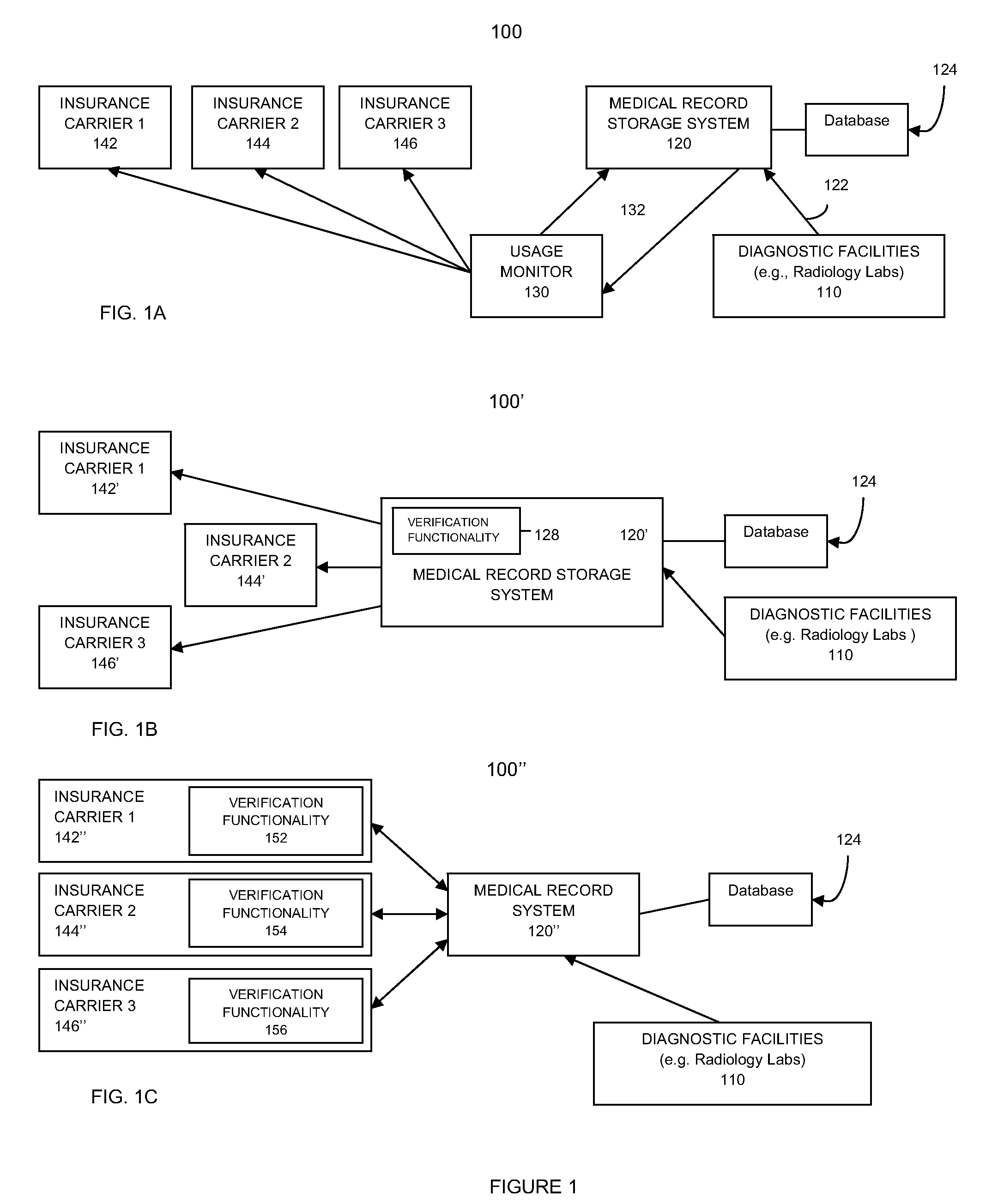
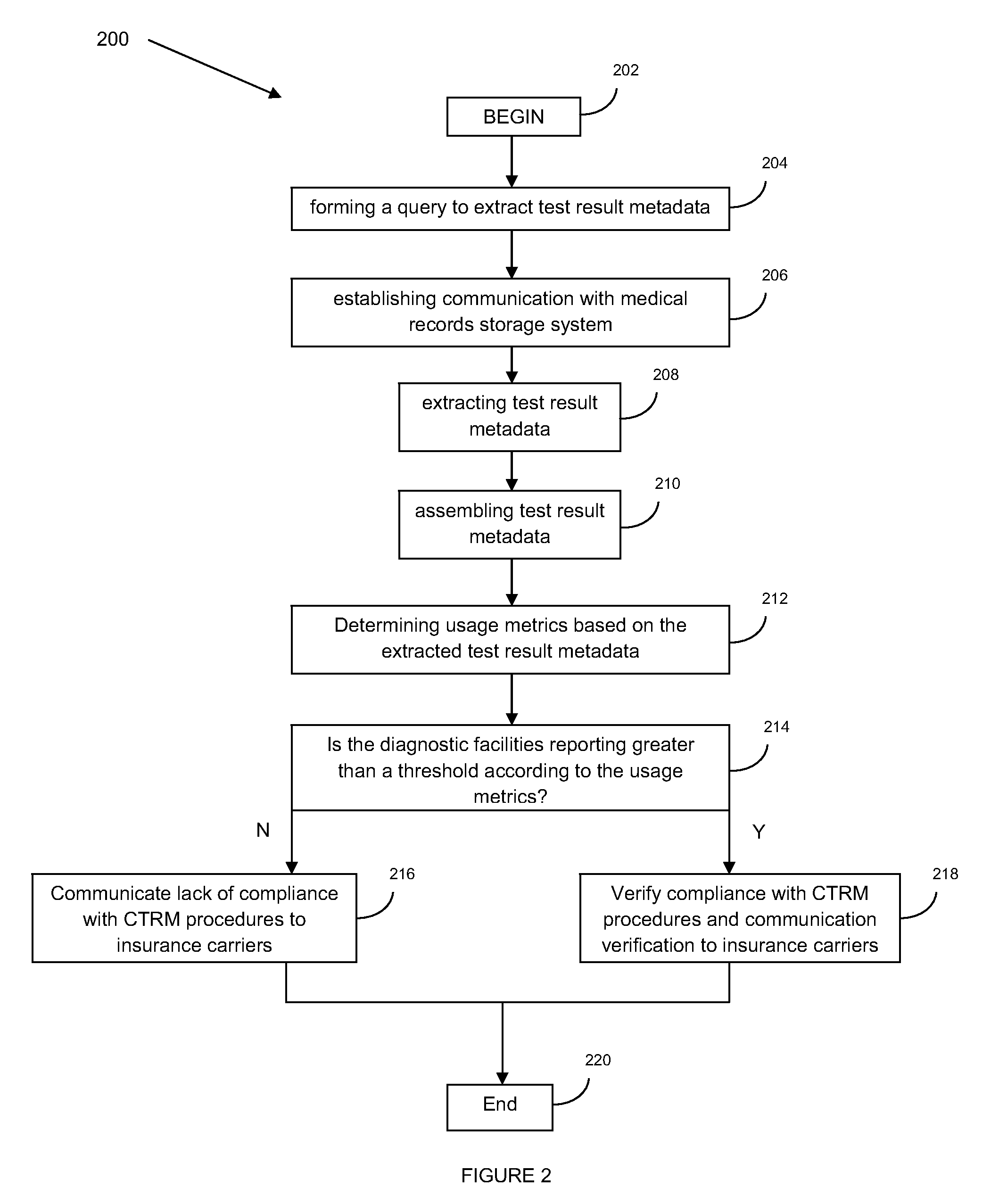
Verification monitor for critical test result delivery systems
PendingUS20100088232A1Reduce riskQuick analysisFinanceLocal control/monitoringComputer hardwareDiagnostic test
A system and method for verification monitoring of a critical test result management (CTRM) system is provided. In one embodiment, the method includes receiving test result metadata pertaining to test result messages provided to a CTRM system by a diagnostic test facility, verifying compliance of the diagnostic test facility with prescribed usage of the CTRM system using the test result metadata, and sending a message to an interested party regarding whether or not compliance of the diagnostic test facility has been verified.
Owner:BAKER SCOTT HLDG
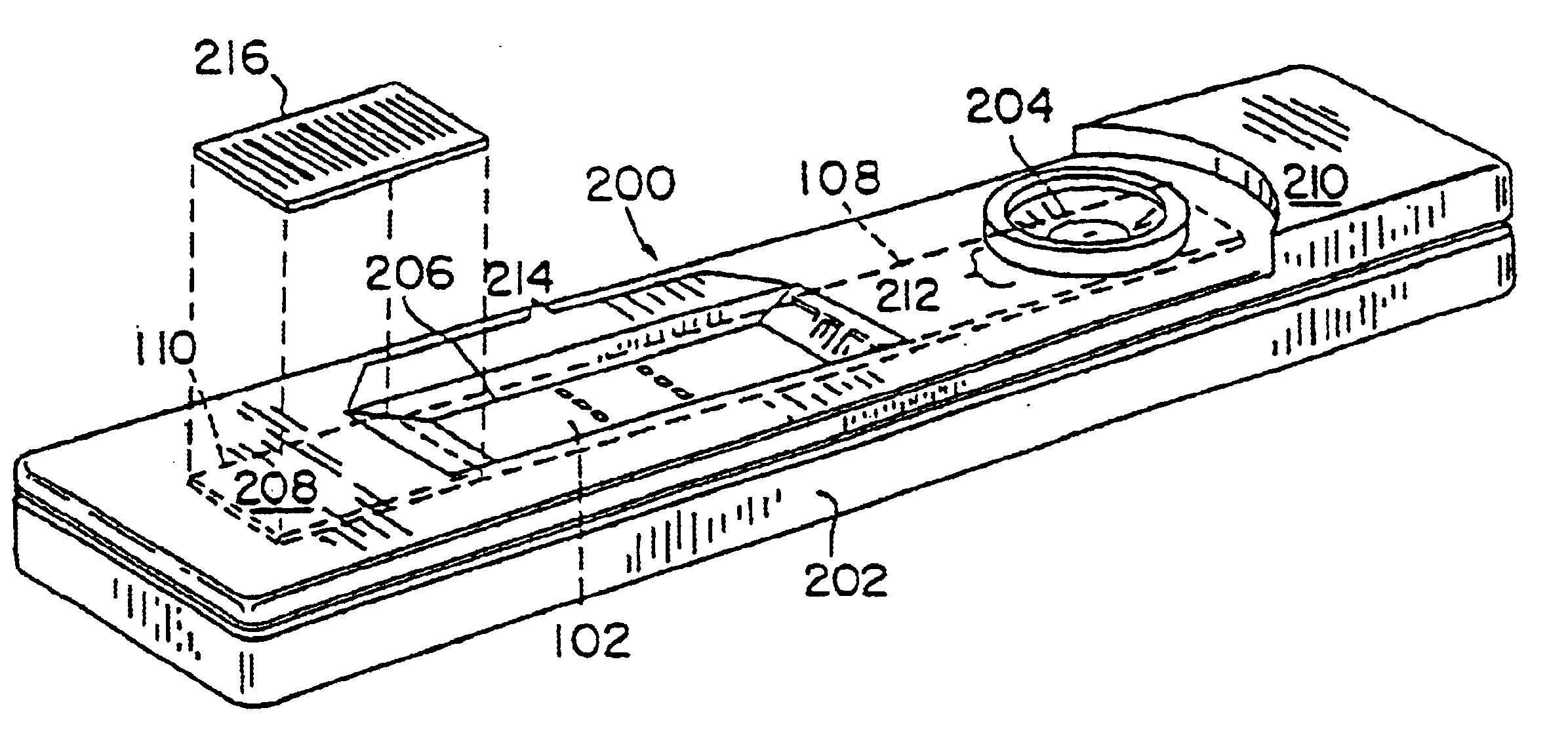
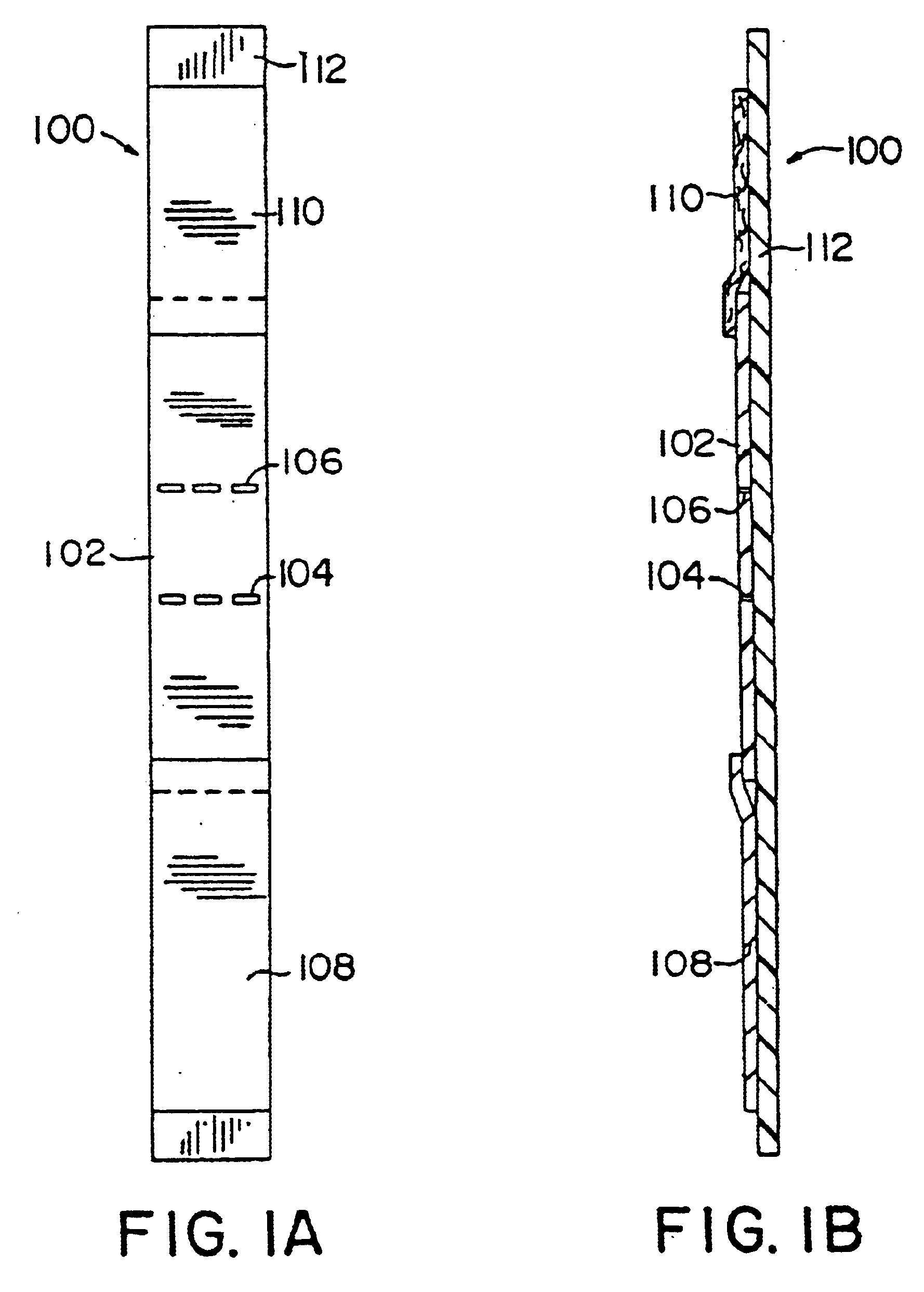
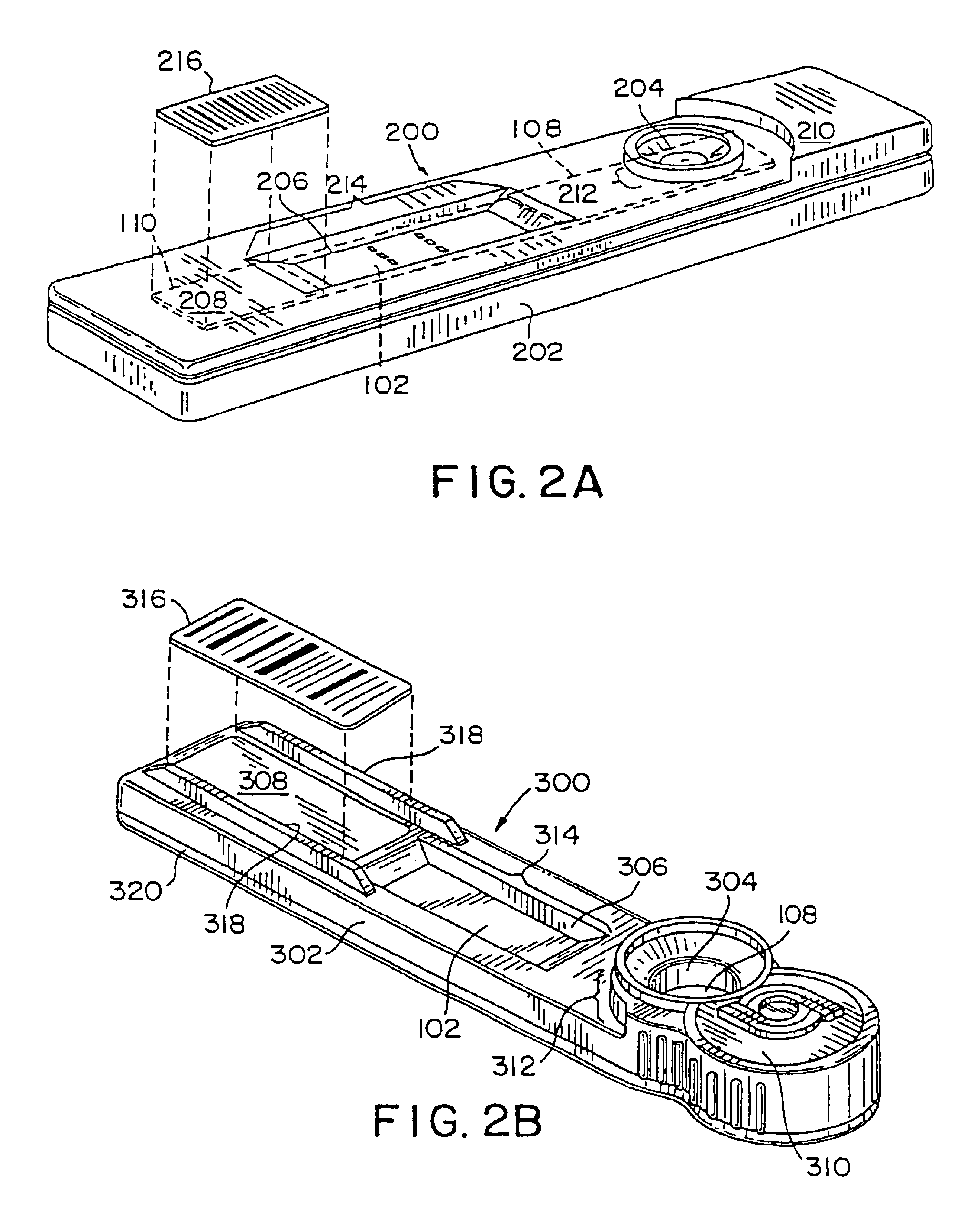
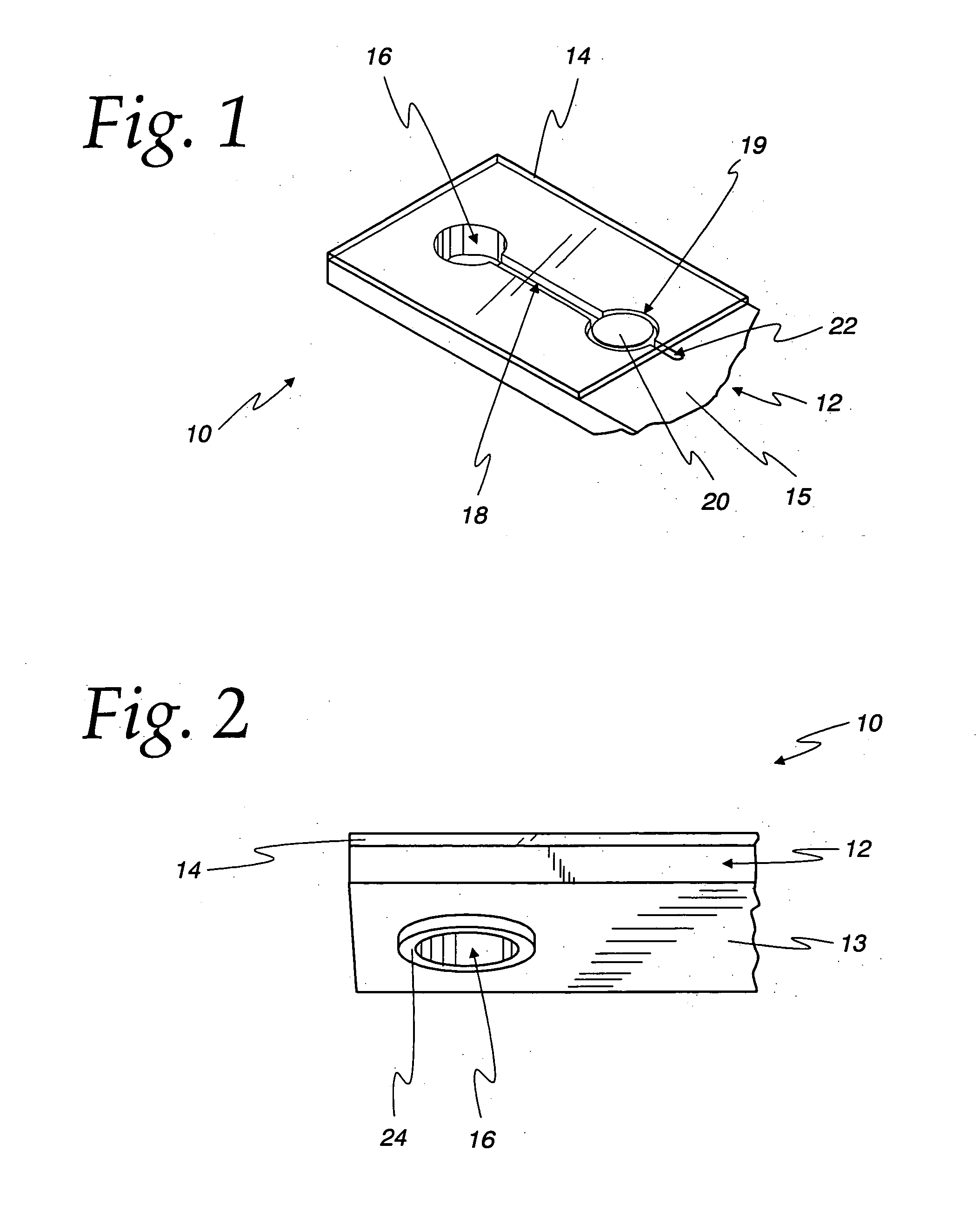
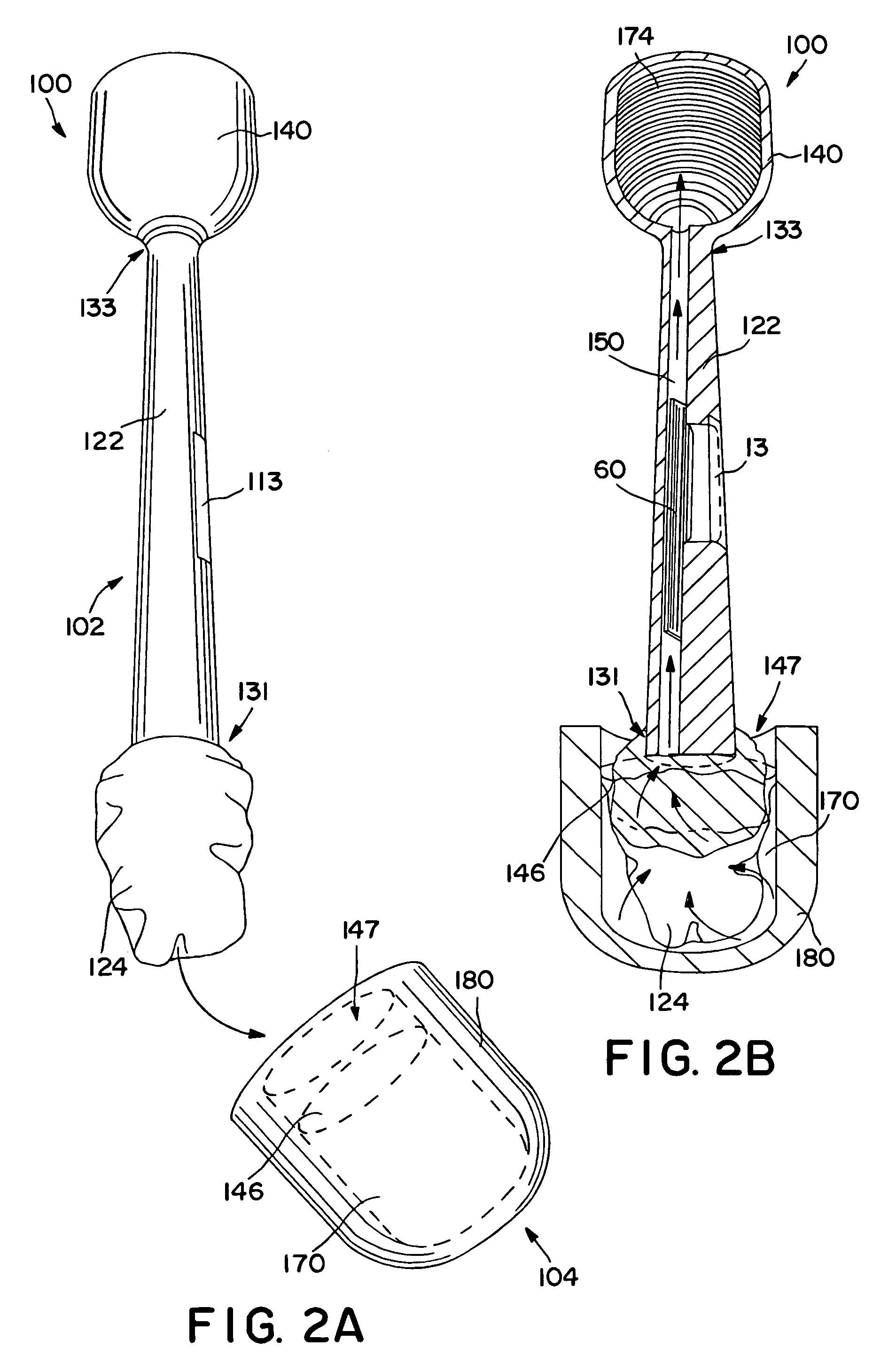
Diagnostic test device and method of using same
InactiveUS20050084842A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testEngineering
A self contained diagnostic test device is provided for use in the collection and detection of a biological specimen or the like. The device comprises a tubular swab and reagent dispensing cap component for receiving specimens. The reagent dispensing cap component includes barrel, reagent chamber, and results window subcomponents, and delivers one or more selected reagents to a specimen testing chamber for contacting the collected specimen, upon the rotation of the reagent chamber component.
Owner:KIMBERLY-CLARK WORLDWIDE INC
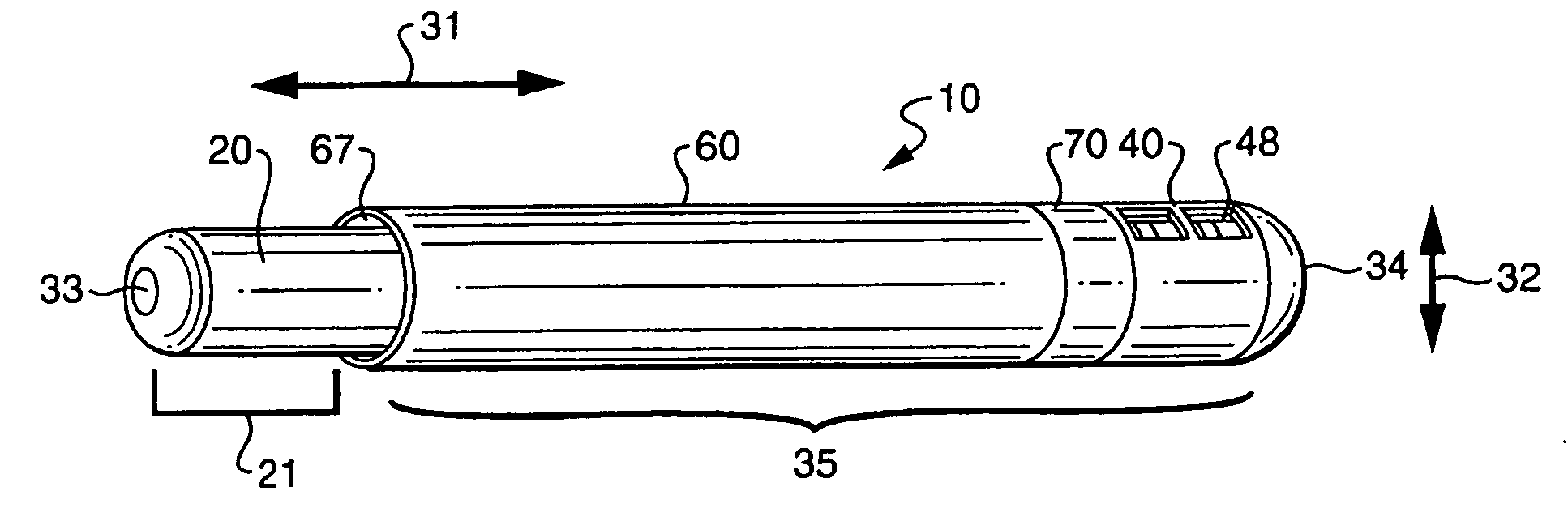
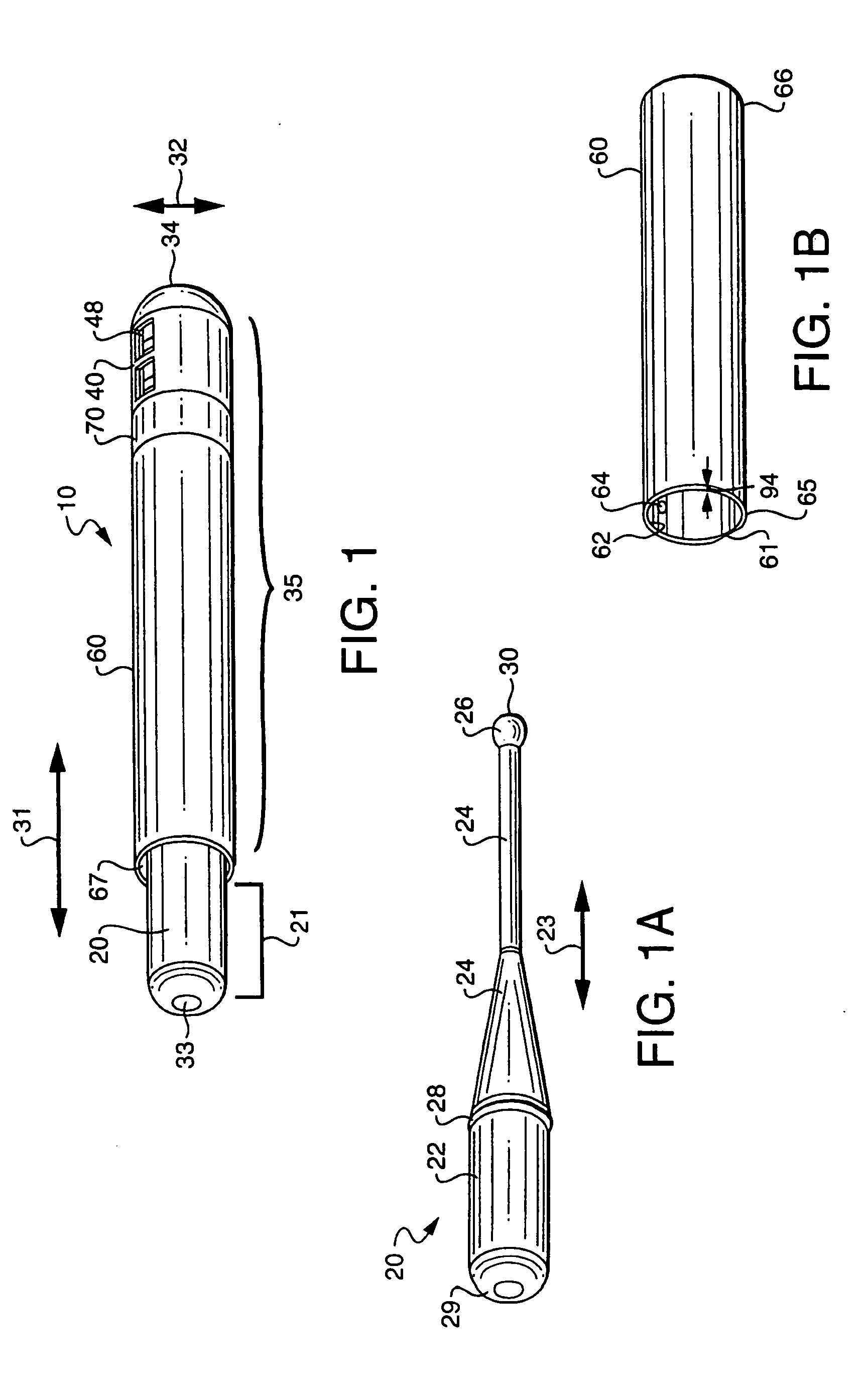
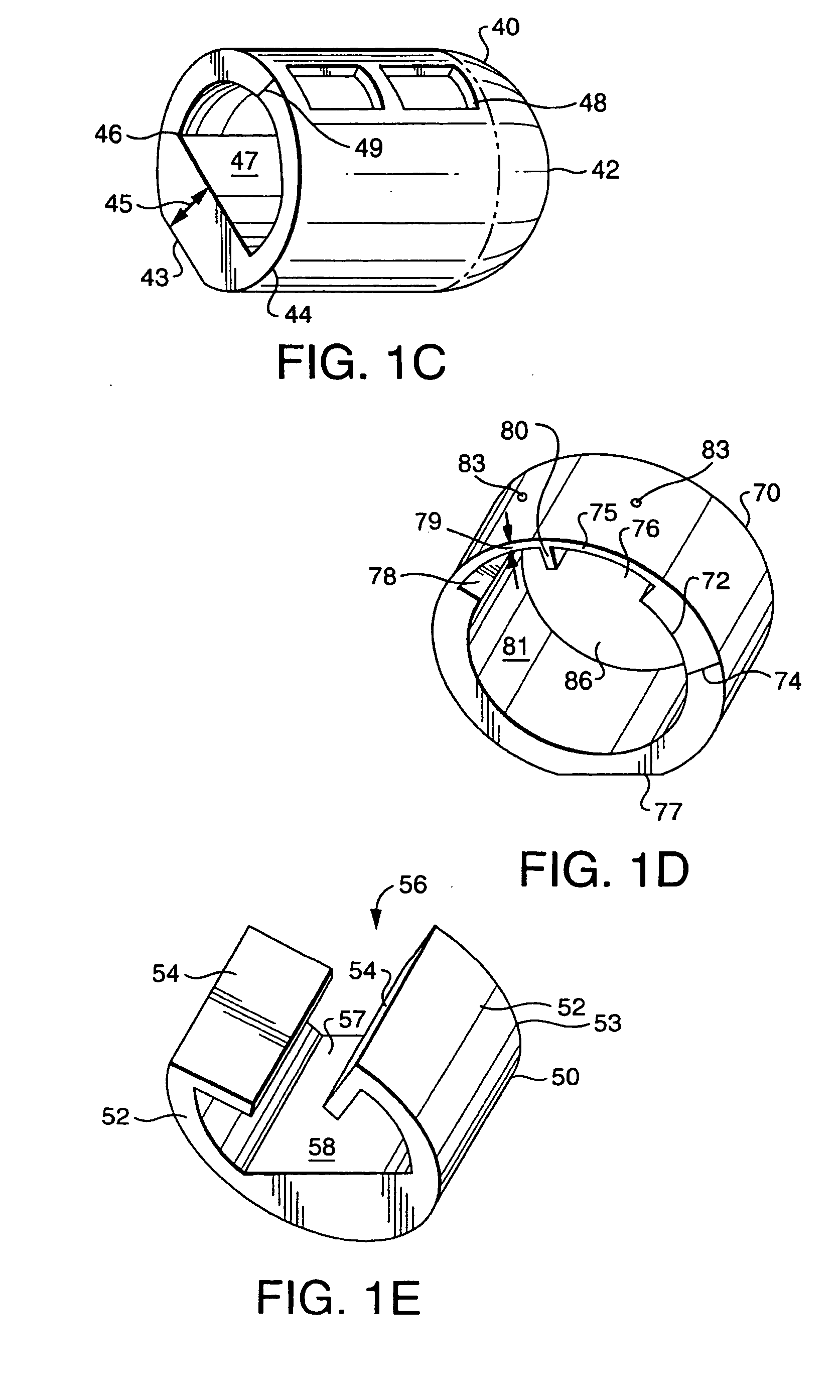
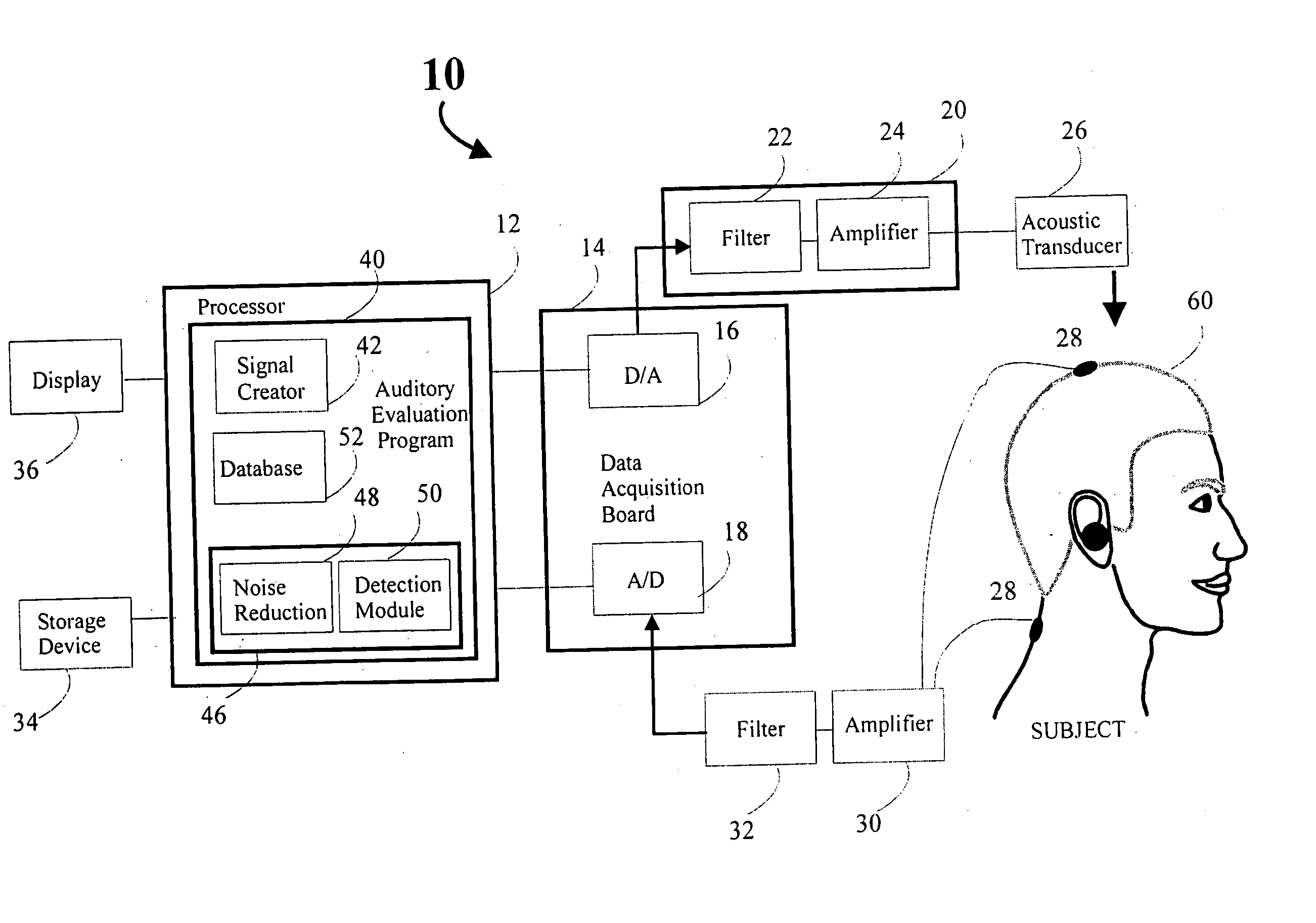
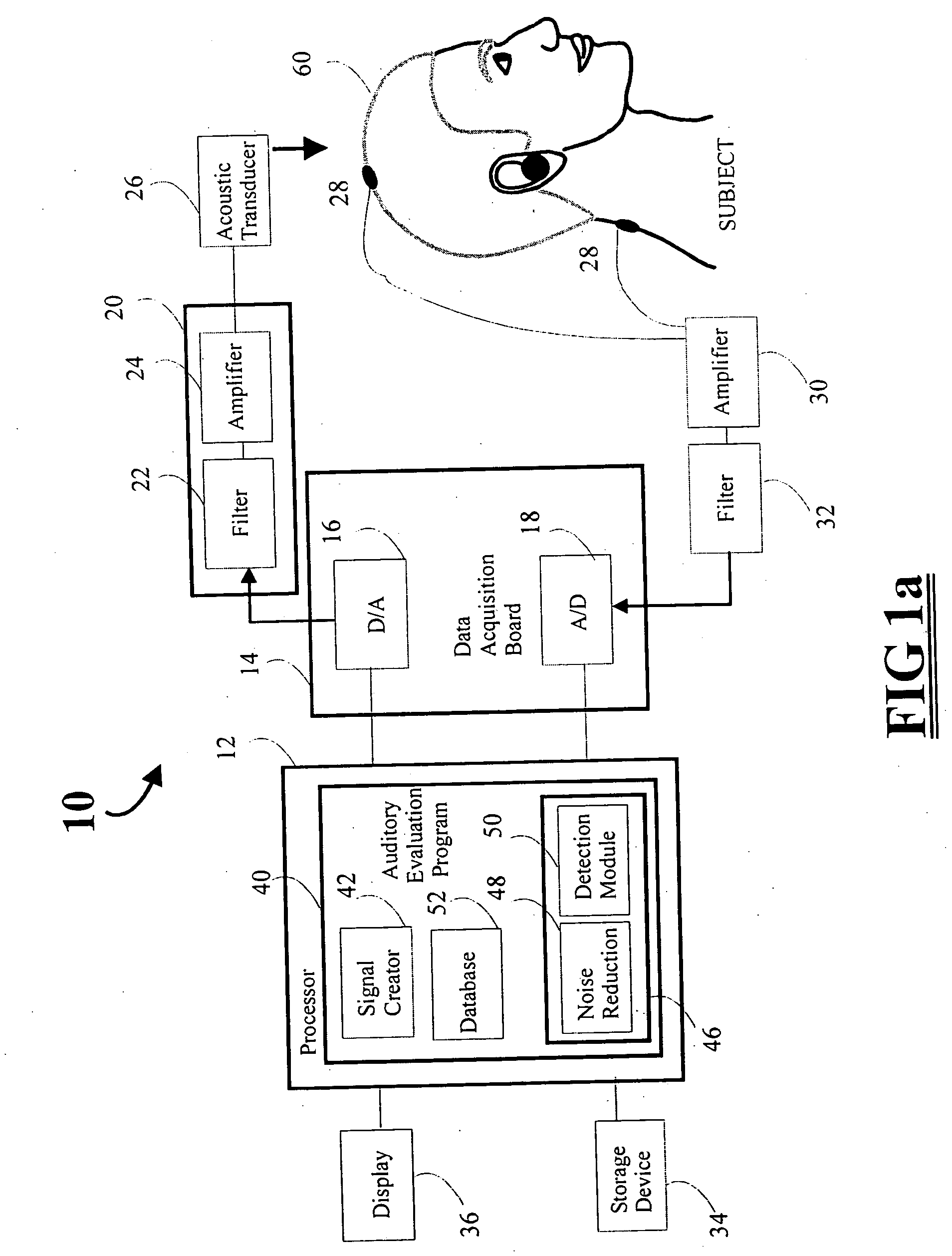
Rapid screening, threshold, and diagnostic tests for evaluation of hearing
Owner:JOHN MICHAEL SASHA
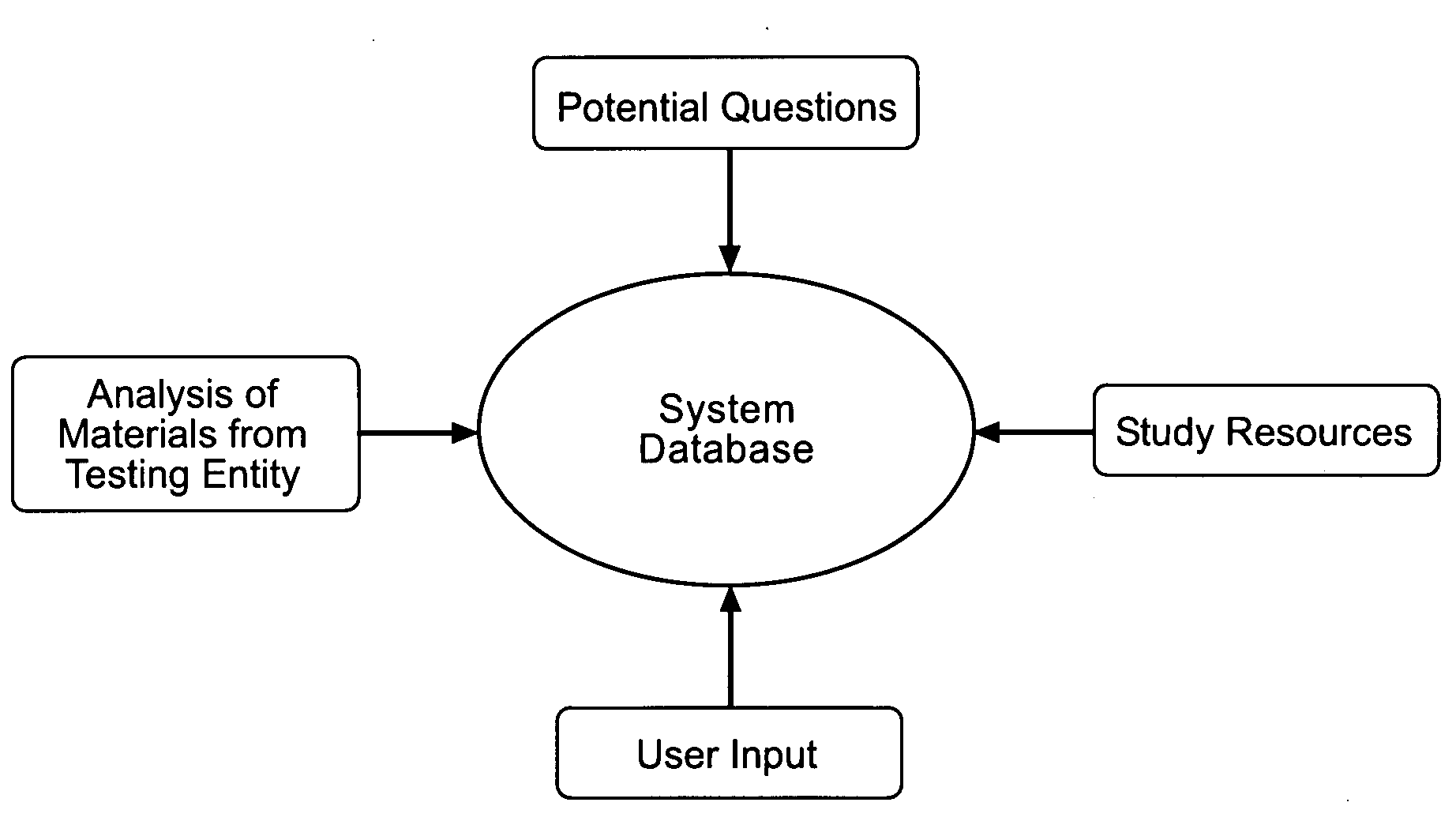
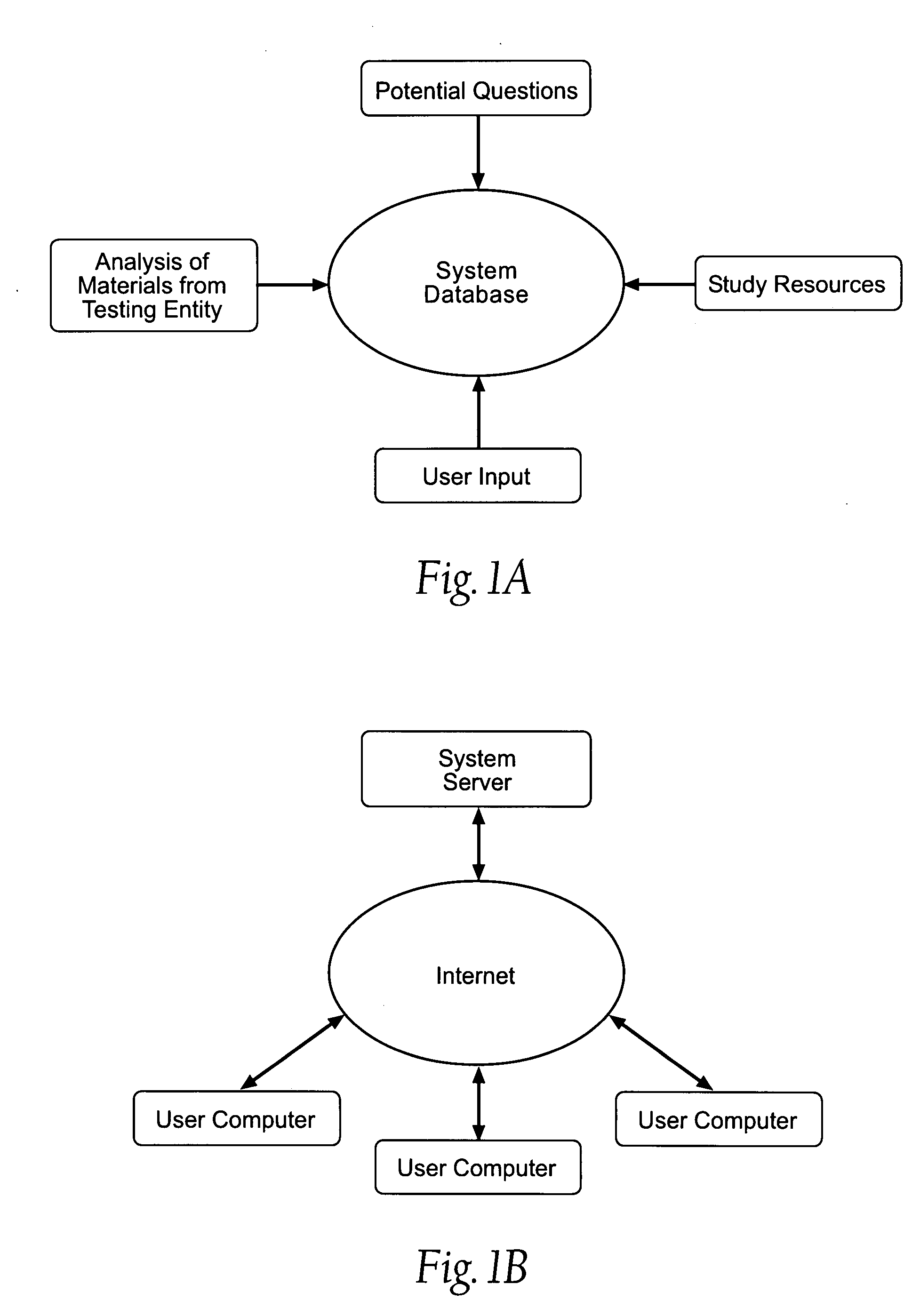
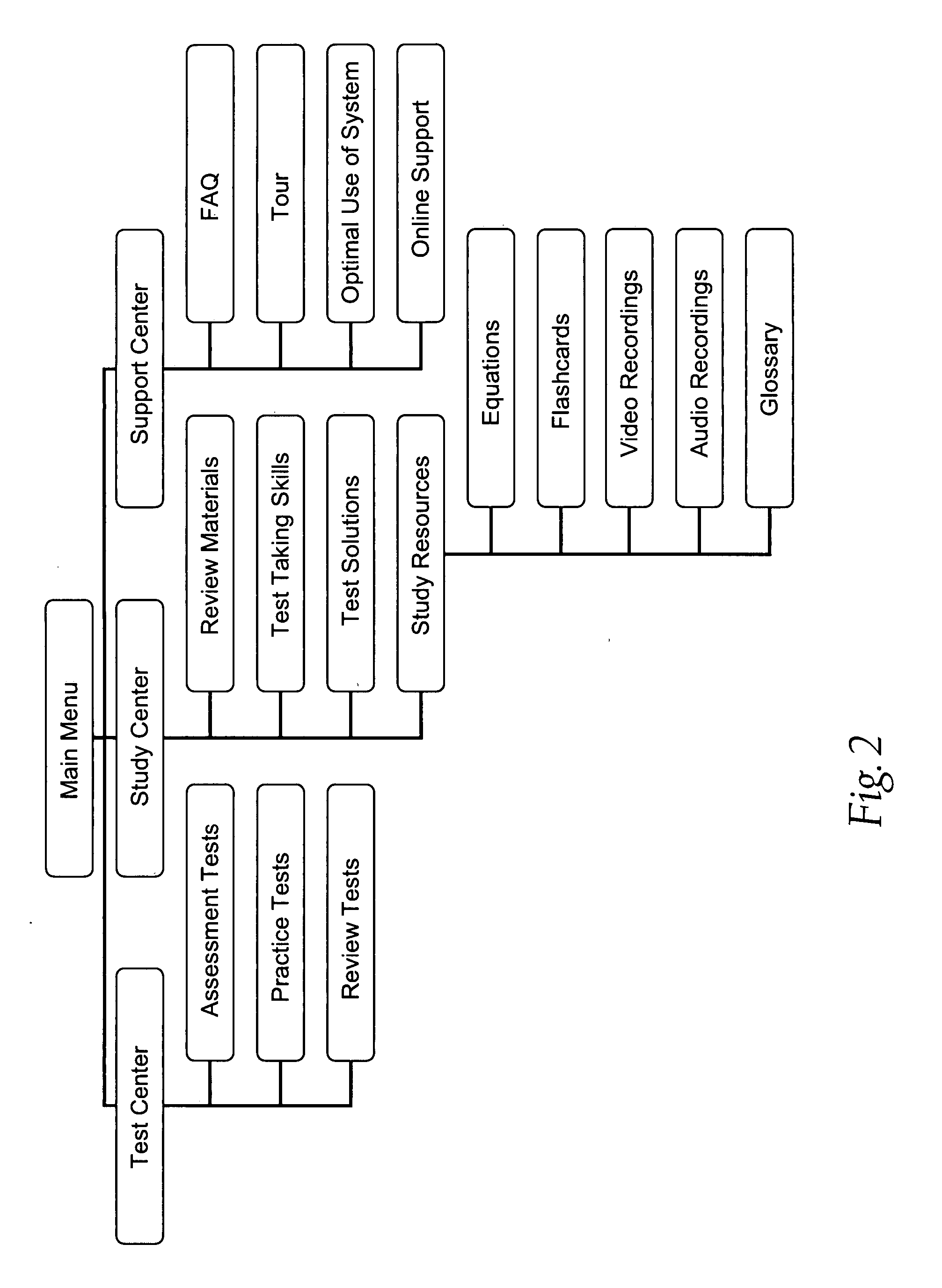
E learning platform for preparation for standardized achievement tests
InactiveUS20070269788A1Improve performanceElectrical appliancesMechanical appliancesPersonalizationDiagnostic test
A database driven system for preparing for a standardized test. The database includes an analysis of previous tests, a Question Pool, and study resources. Based on the analysis of previous tests, the system pulls questions from the Question Pool to create diagnostic tests. The diagnostic tests are administered to the user. Based on the results of the diagnostic tests and the student's goals, the system develops an individualized Study Plan for the student. The Study Plan can be based on an analysis of previous tests, the student's performance on the diagnostic test, the student's goals, or any combination thereof. The Study Plan identifies the areas that are of most importance for the student to learn. The student may they use the study resources based on the results of the Study Plan. The system uses a variety of tools to make the study process more efficient and motivate the student.
Owner:DR FLOWERS TEST PREP LLC
Nanoparticles in diagnostic tests
InactiveUS20090291508A1Long lastingLong shelf lifeMaterial nanotechnologyAnalysis using chemical indicatorsNanoparticleBiology
Owner:RAPID PATHOGEN SCREENING INC
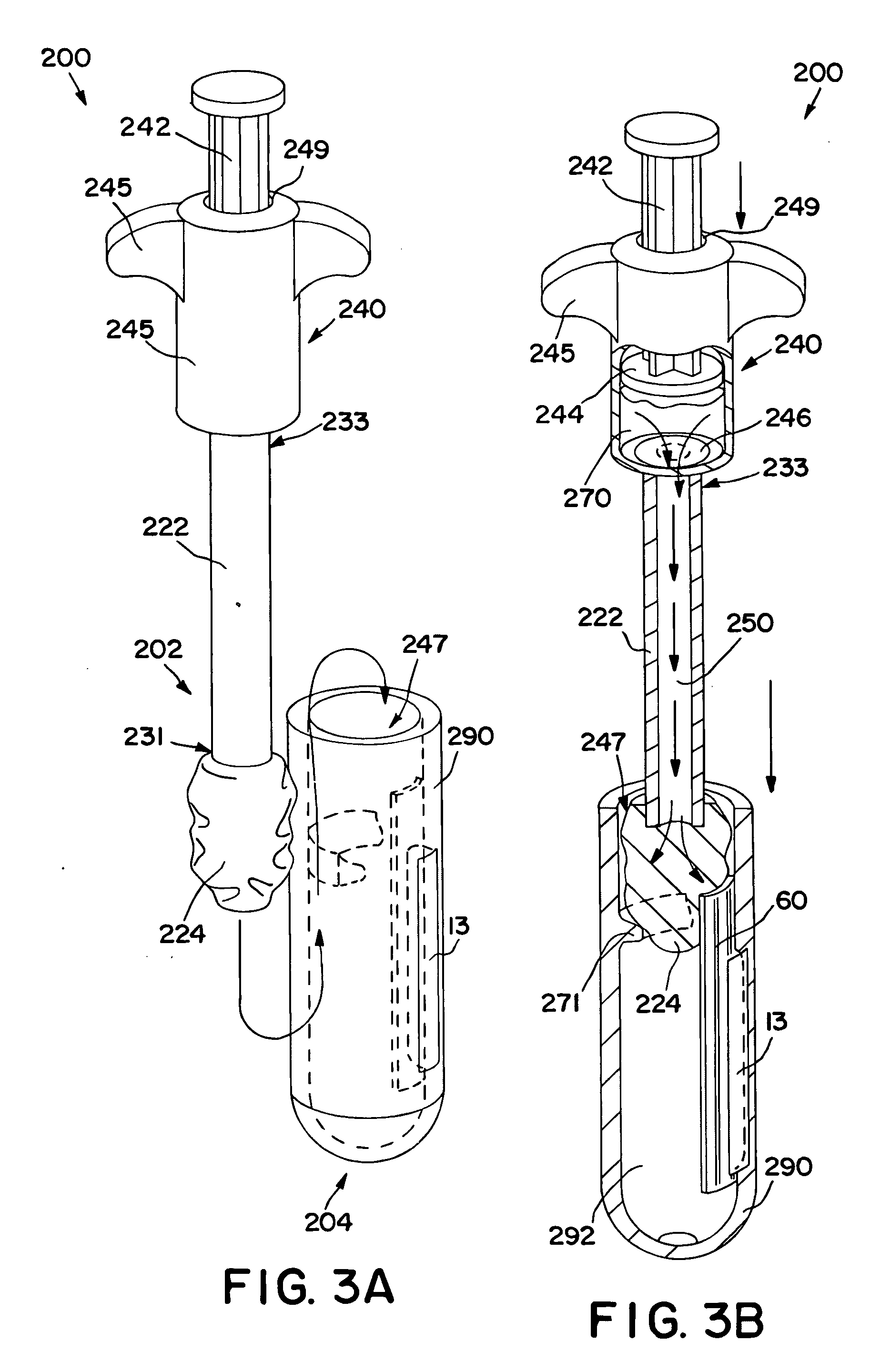
Self-contained swab-based diagnostic systems
InactiveUS20050136553A1Prevent leakageBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDiagnostic test
A diagnostic test unit is provided. The test unit comprises a stem having a first end and a second end, the stem defining at least one flow channel extending between the first end and the second end. A swab is disposed at the first end of the stem, the swab being configured to collect a test sample derived from a biological source that is suspected of containing an analyte. The test unit also comprises a fluid chamber configured to contain a fluid, wherein the fluid chamber is in fluid communication with the swab via the flow channel. The test unit also comprises a rupturable seal that inhibits leakage of the fluid from the fluid chamber prior to use, and an assay for detecting the presence or absence of the analyte. The assay is in fluid communication with the swab, the flow channel, and the fluid chamber.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Portable assemblies, systems and methods for providing functional or therapeutic neuromuscular stimulation
ActiveUS20050182457A1Simplifies meeting power demandEasy to carrySpinal electrodesElectricityMedicine
Neuromuscular stimulation assemblies, systems, and methods make possible the providing of short-term therapy or diagnostic testing by providing electrical connections between muscles or nerves inside the body and stimulus generators or recording instruments mounted on the surface of the skin outside the body. Neuromuscular stimulation assemblies, systems, and methods may include a steerable introducer that defines an interior lumen sized and configured to shield a percutaneous electrode from contact with tissue during advancement to a desired position within tissue.
Owner:SPR THERAPEUTICS
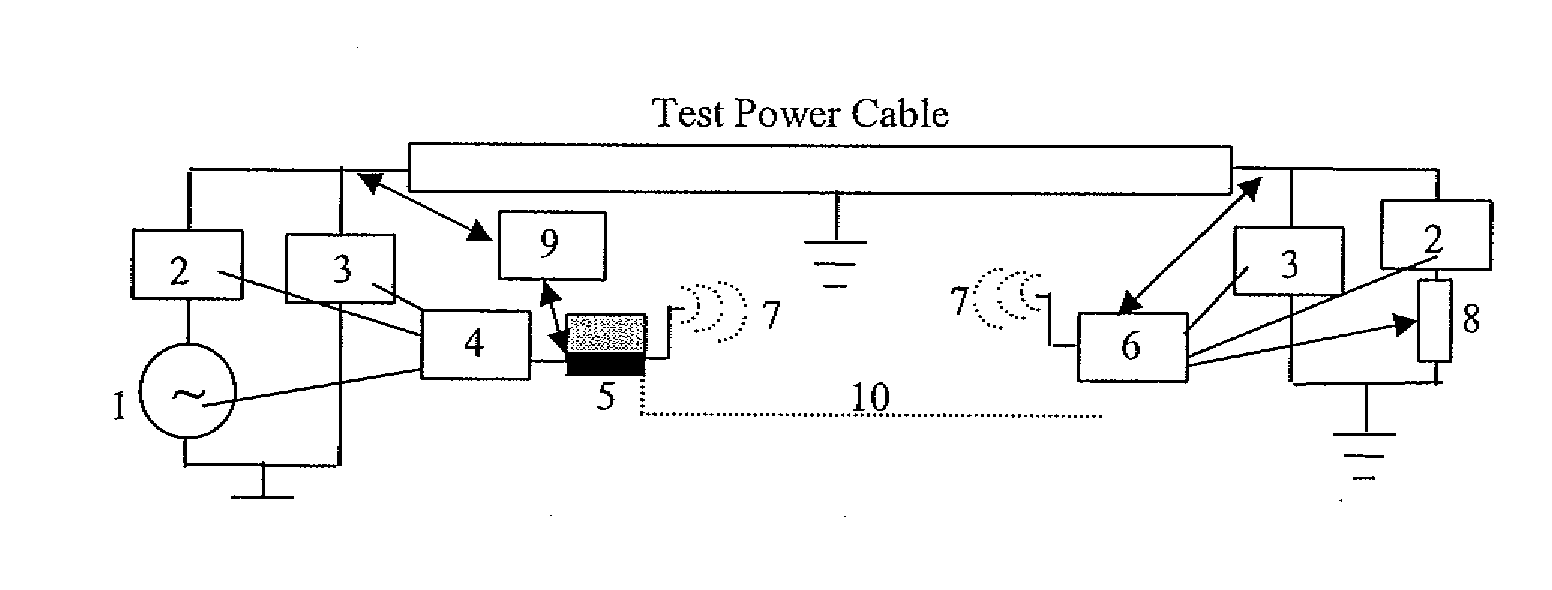
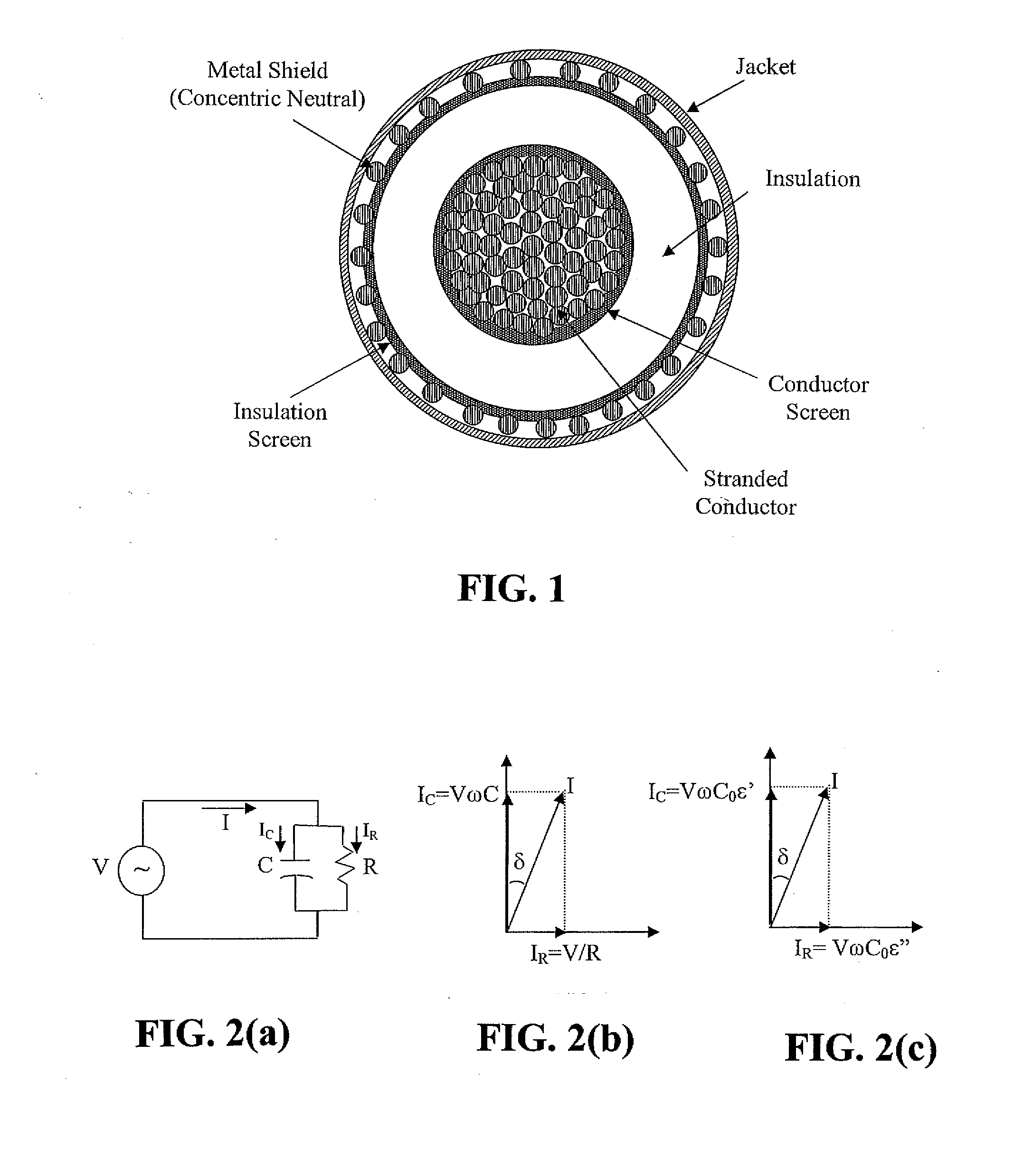
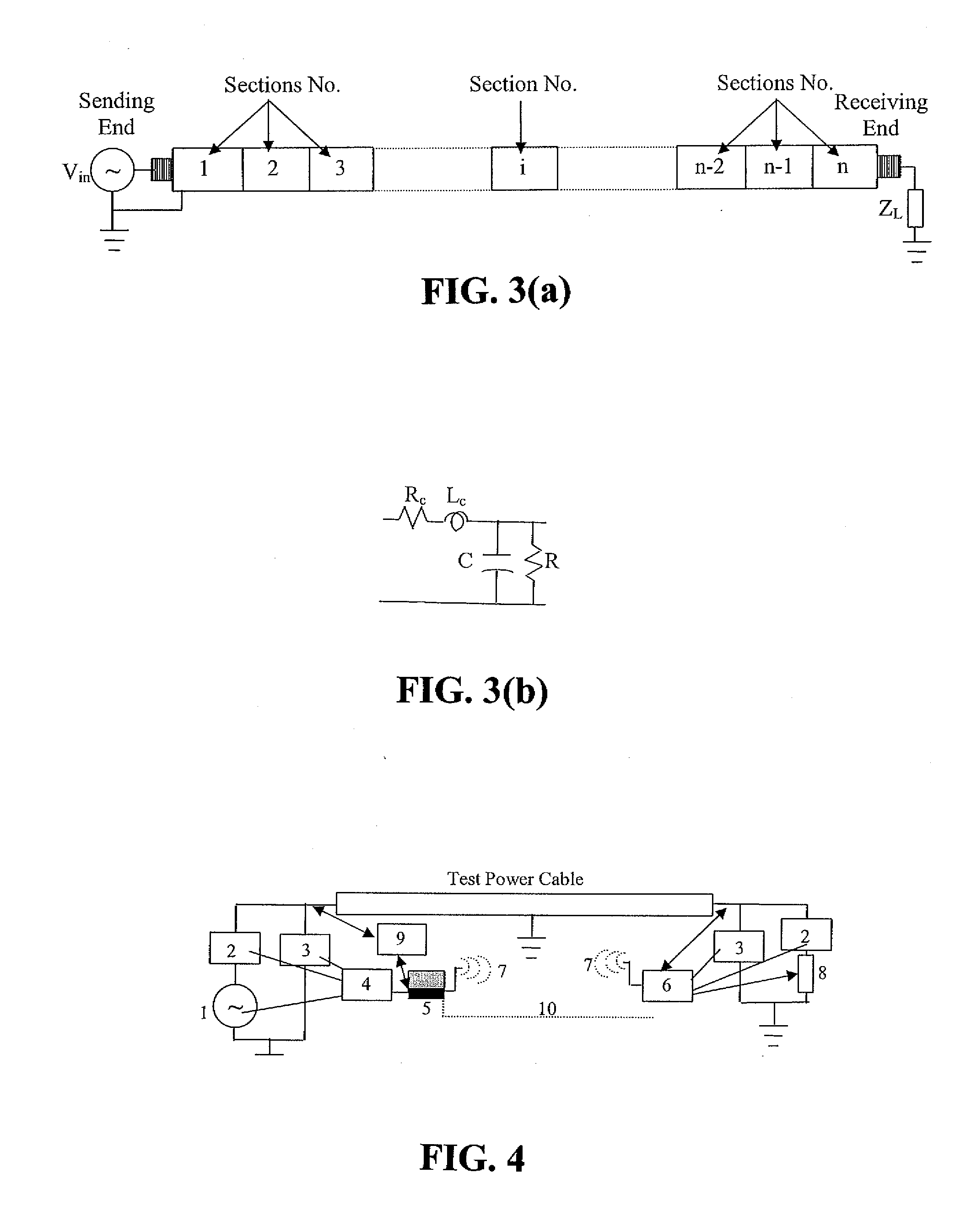
Diagnostic methods for electrical cables utilizing axial tomography
ActiveUS20080048668A1Easy to identifyPrecise positioningFault location by conductor typesFault location by pulse reflection methodsElectrical conductorEngineering
Cable diagnostic test methods, systems and apparatus are disclosed that utilize “standing wave” principles to facilitate identification and location of insulation defect(s) along a power cable. The methods / systems measure dissipation factors and dielectric constants associated with the power cable insulation and the impedance of the power cable conductor at any number of points or sections along the axial length of the cable. In an exemplary embodiment, the disclosed method involves (i) connecting an alternating voltage source to a cable at a “sending end” thereof; (ii) applying a voltage to the cable at a first frequency to set up a traveling wave along the cable that is reflected at the “receiving end” thereof; (iii) permitting a standing wave pattern to be established along the cable by the traveling wave and the reflection thereof; (iv) measuring the total complex power loss (Sin) at the sending end of the cable; (v) calculating the standing wave voltage at any point / section of the cable based on the load impedance (ZL) connected at the receiving end of the cable, and the characteristic impedance (ZO) of the cable, or the measured / calculated cable parameters for the first frequency of the voltage source, (vi) repeating the foregoing steps while one of: (1) varying at least one of: the load impedance (ZL) connected at the receiving end of the cable, the first frequency of the voltage source; the output impedance of the voltage source, a combination of the load impedance (ZL), the output impedance of the voltage source and the first frequency of the voltage source, and combinations thereof; (2) interchanging sending and receiving cable ends; and (3) a combination thereof, and (vii) determining a dissipation factor (tan δ) and a dielectric constant (∈′), for the insulation, and an impedance, for the conductor at predetermined points / sections along the axis of the cable.
Owner:INSTR MFG
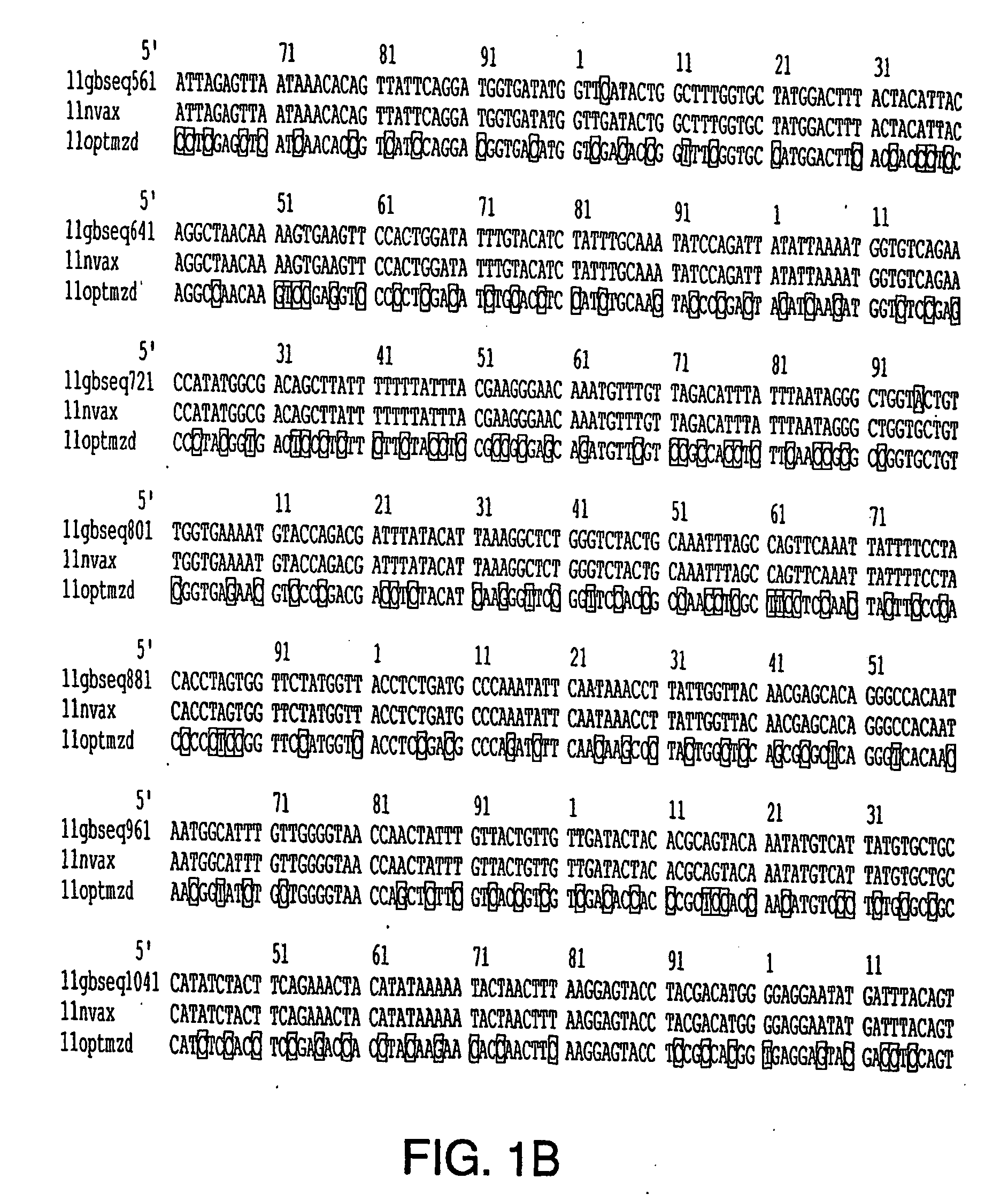
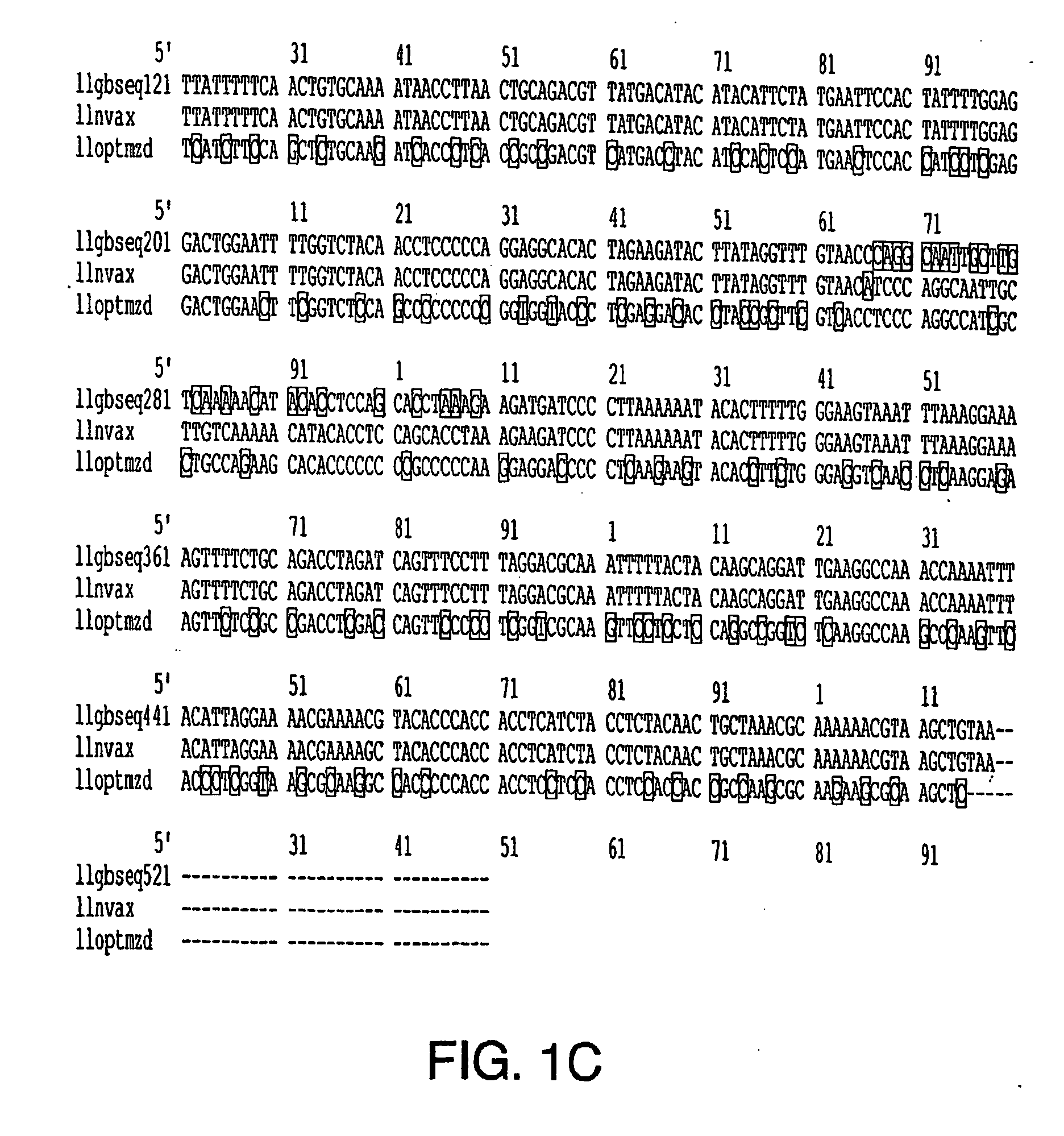
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Test cell for use with medical diagnostic instrument
A disposable, single use test cell is provided for receiving fluid to be diagnostically tested by an instrument. The test cell includes a housing sized and shaped for engagement by the instrument when a diagnostic test is to be performed, the housing including at least one chamber, at least one pair of electrodes within the chamber and in electrical contact with circuitry within the instrument when the housing is engaged by the instrument and a specimen capsule containing the fluid to be tested.
Owner:CLINICAL ANALYSIS
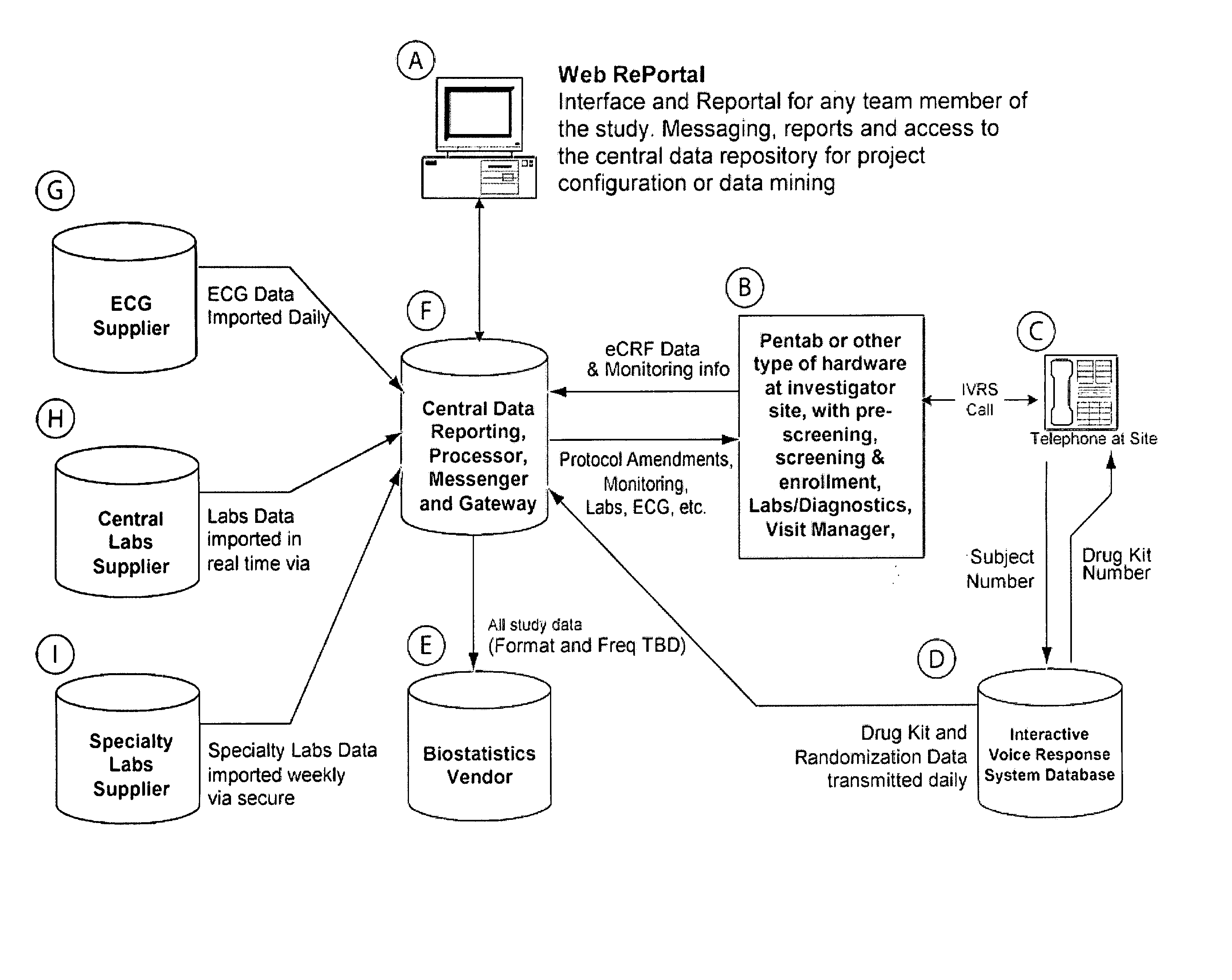
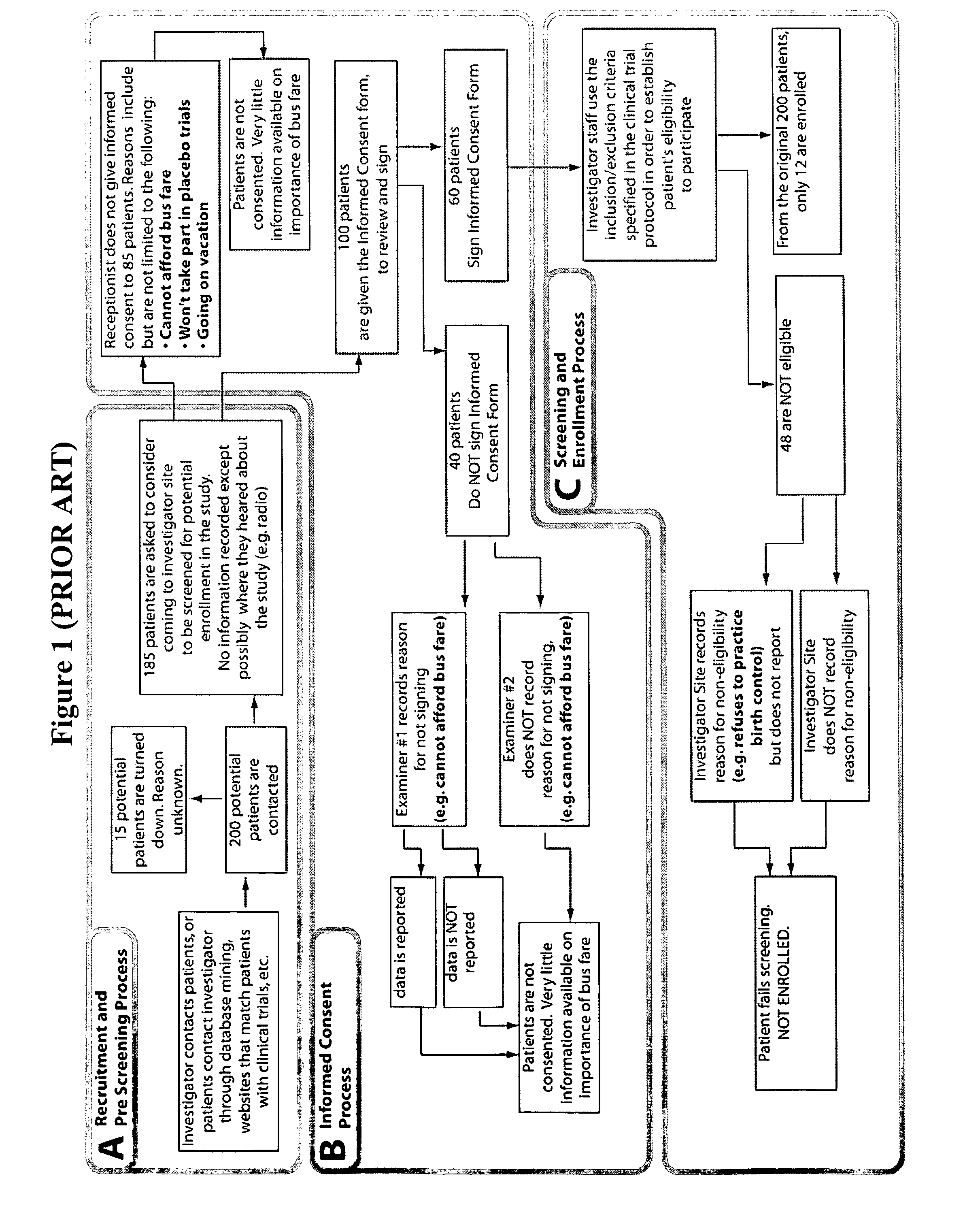
Method and apparatus for screening, enrollment and management of patients in clinical trials
InactiveUS20070067189A1Simple speedImproves of enrollment processData processing applicationsComputer-assisted medical data acquisitionDiagnostic testData center
A computer-implemented method of tracking patient data in a clinical trial is provided. The clinical trial has one or more investigative sites which perform patient screening and enrollment for the clinical trial, one or more diagnostic sites which perform analysis on one or more patient diagnostic tests ordered by an investigative site and generate analysis results, and a centralized data center in electronic communication with the one or more investigative sites and the one or more diagnostic sites. Each investigative site is provided with a user interface display screen for allowing a user at the investigative site to enter data regarding patients who have been screened for the clinical trial and patients who have been enrolled in the clinical trial. The data from each of the investigative sites is electronically communicated to the centralized data center. Also, the analysis results from each of the diagnostic sites are electronically communicated to the centralized data center. The centralized data center consolidates the data and analysis results from each of the sites and provides one or more status reports regarding the patients for whom data and analysis results were received from the one or more investigative sites and the one or more diagnostic sites.
Owner:NUMODA TECH
Portable percutaneous assemblies, systems and methods for providing highly selective functional or therapeutic neuromuscular stimulation
Neuromuscular stimulation assemblies, systems, and methods make possible the providing of short-term therapy or diagnostic testing by providing electrical connections between muscles or nerves inside the body and stimulus generators or recording instruments mounted on the surface of the skin outside the body.
Owner:SPR THERAPEUTICS
Self-contained swab-based diagnostic systems
InactiveUS7098040B2Prevent leakageBioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testAnalyte
A diagnostic test unit is provided. The test unit comprises a stem having a first end and a second end, the stem defining at least one flow channel extending between the first end and the second end. A swab is disposed at the first end of the stem, the swab being configured to collect a test sample derived from a biological source that is suspected of containing an analyte. The test unit also comprises a fluid chamber configured to contain a fluid, wherein the fluid chamber is in fluid communication with the swab via the flow channel. The test unit also comprises a rupturable seal that inhibits leakage of the fluid from the fluid chamber prior to use, and an assay for detecting the presence or absence of the analyte. The assay is in fluid communication with the swab, the flow channel, and the fluid chamber.
Owner:KIMBERLY-CLARK WORLDWIDE INC
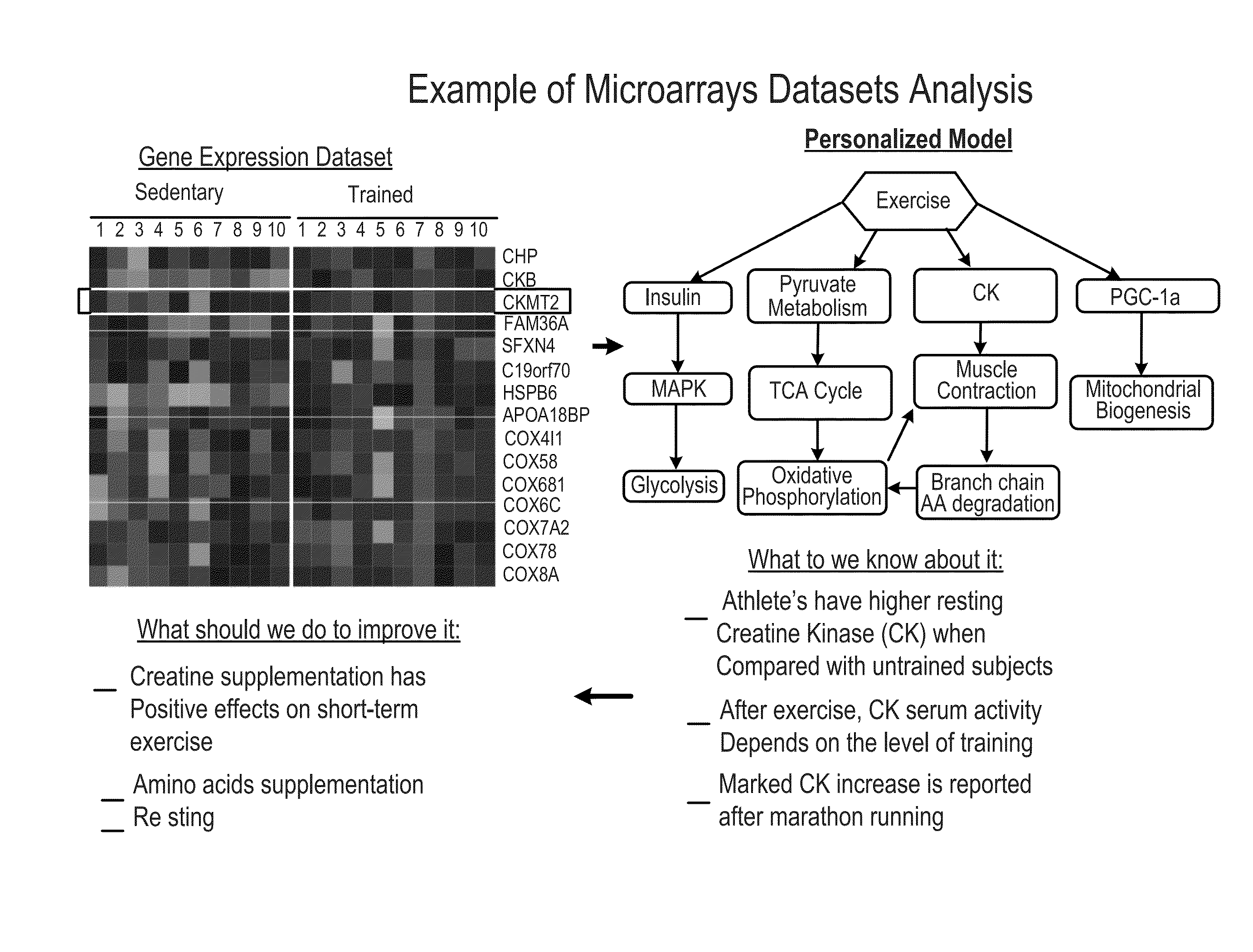
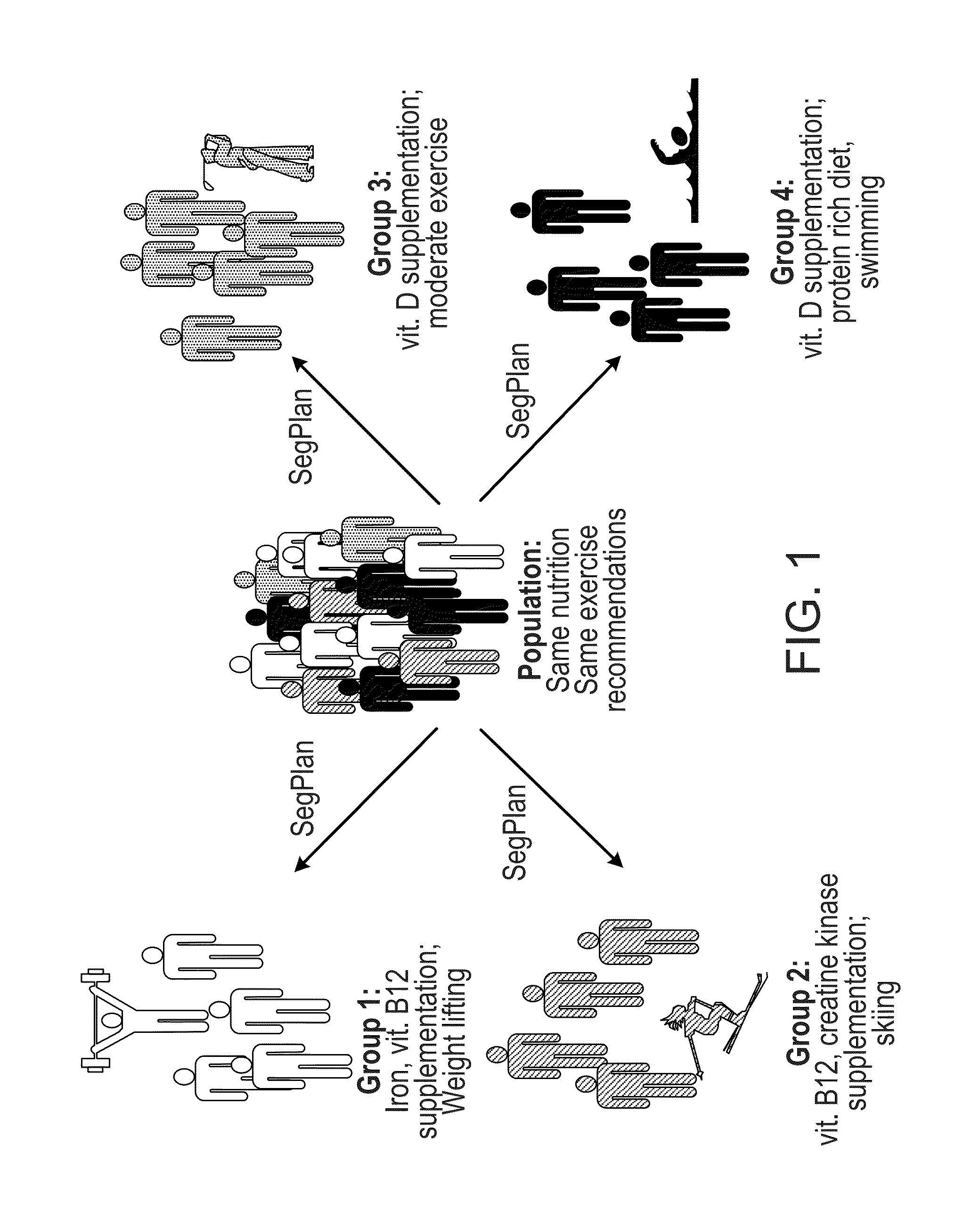
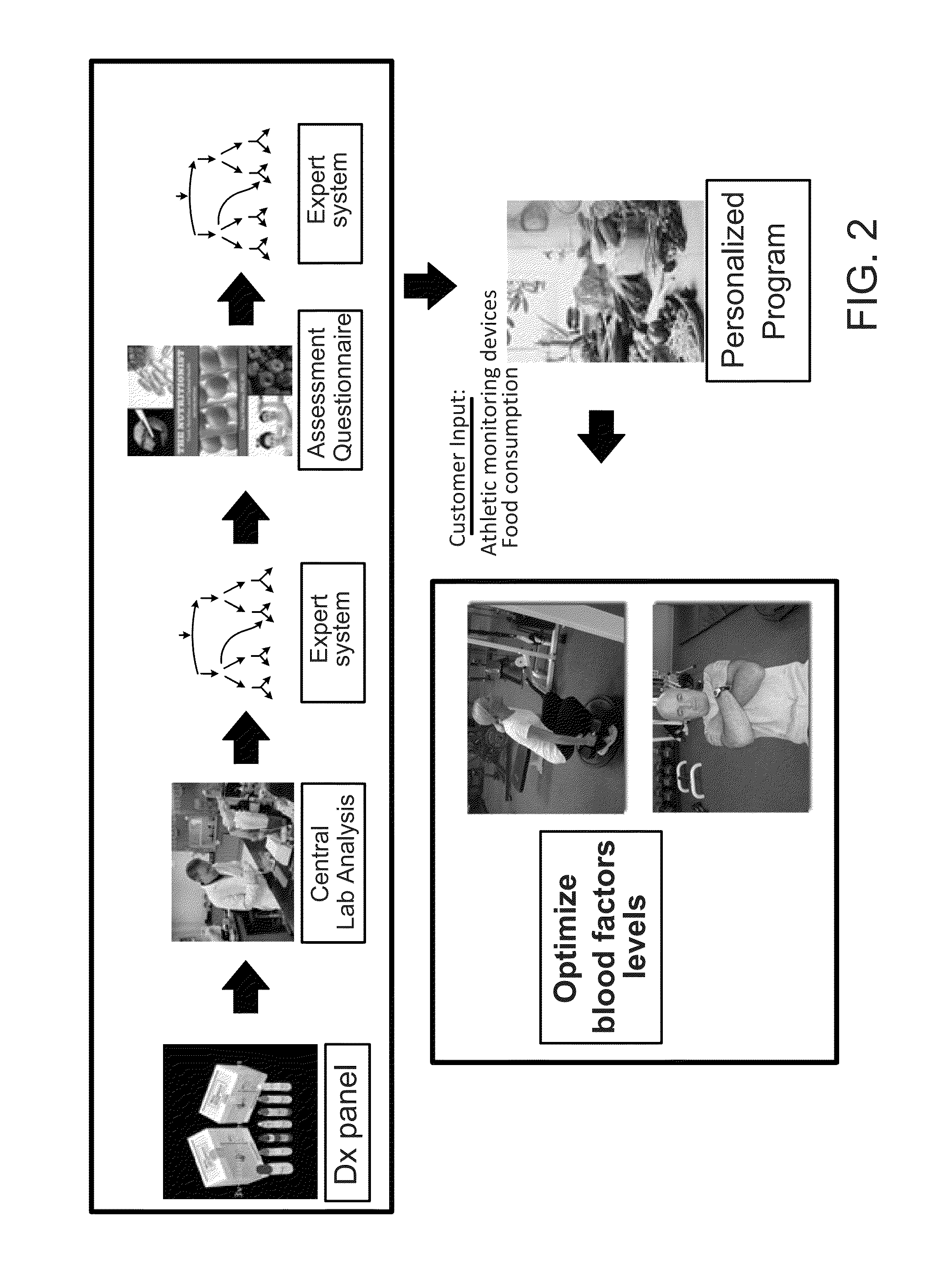
Methods and systems for generation of personalized health plans
ActiveUS8762167B2Easy to derivePhysical therapies and activitiesBiostatisticsDiagnostic testNutrition
Personalized, health and performance programs are generated for individuals based on various biomarkers and performance and lifestyle assessments. In one embodiment, a diagnostic test of blood or other biological specimen(s) is used to determine key biological marker levels. Information and assessments of the user's physical performance, life style and health and wellness goals is also collected and provided to an expert system that matches the biomarker levels and assessments to a knowledgebase of scientific knowledge about biomarker levels and health and fitness outcomes. Personal recommendations and advice on nutrition and exercise is then generated, which may be used to help individuals reach their diet, fitness, and wellness goals and improve their physical and mental performance and well being in measurable ways.
Owner:SEGTERRA
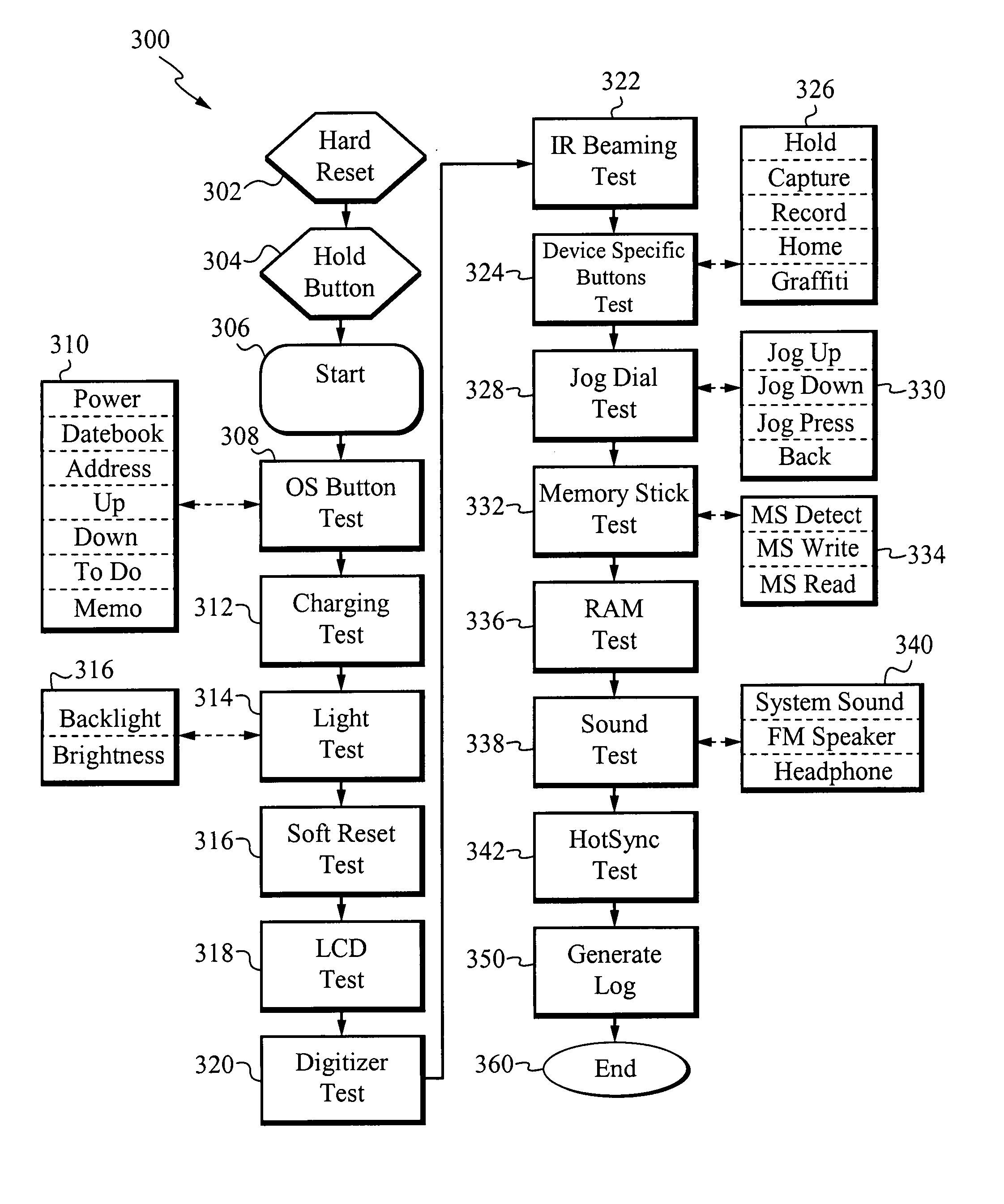
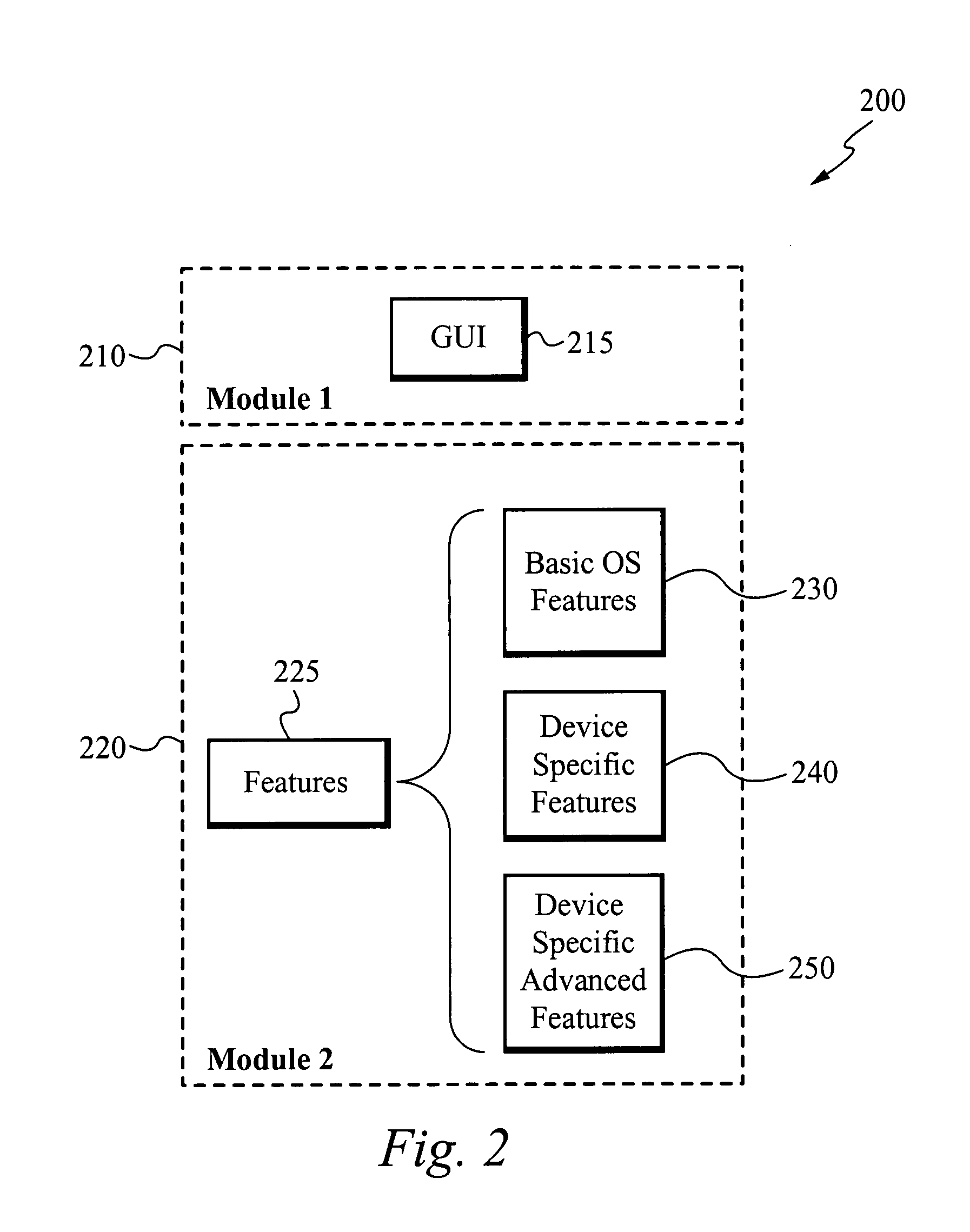
Method of, apparatus and graphical user interface for automatic diagnostics
InactiveUS20070245175A1Simple, precise, thorough, automaticDetecting faulty computer hardwareDigital computer detailsGraphicsGraphical user interface
The present invention includes a method, apparatus and graphical user interface (GUI) that allows a simple, precise, thorough, automatic and interactive diagnostic system for electronic devices. The present invention fully automates every test item, as a memory device including the diagnostic test items is inserted into the electronic device and is configured to automatically begin the diagnostic method. The present invention allows for interactive diagnostic analysis and a user is able to automatically repair many of the defects detected by the diagnostic method.
Owner:SONY CORP & SONY ELECTRONICS +1
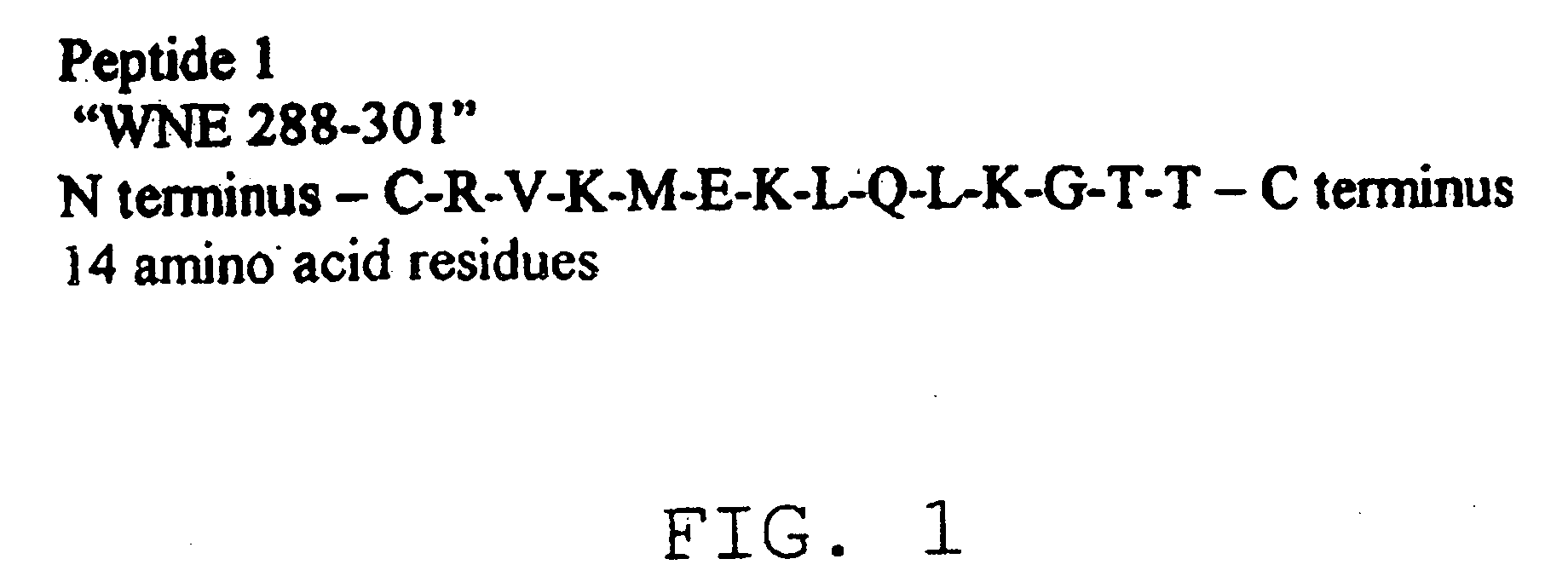
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
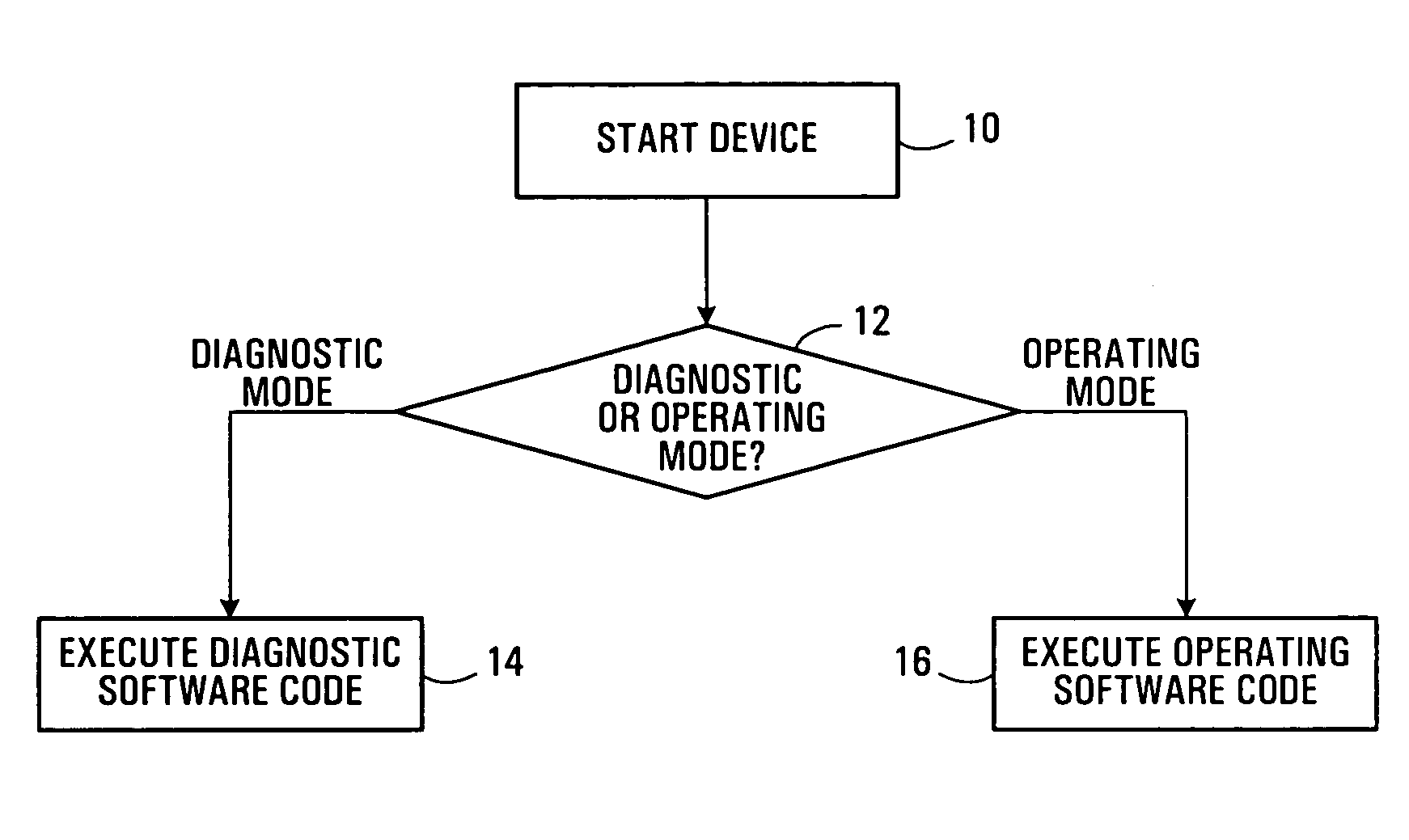
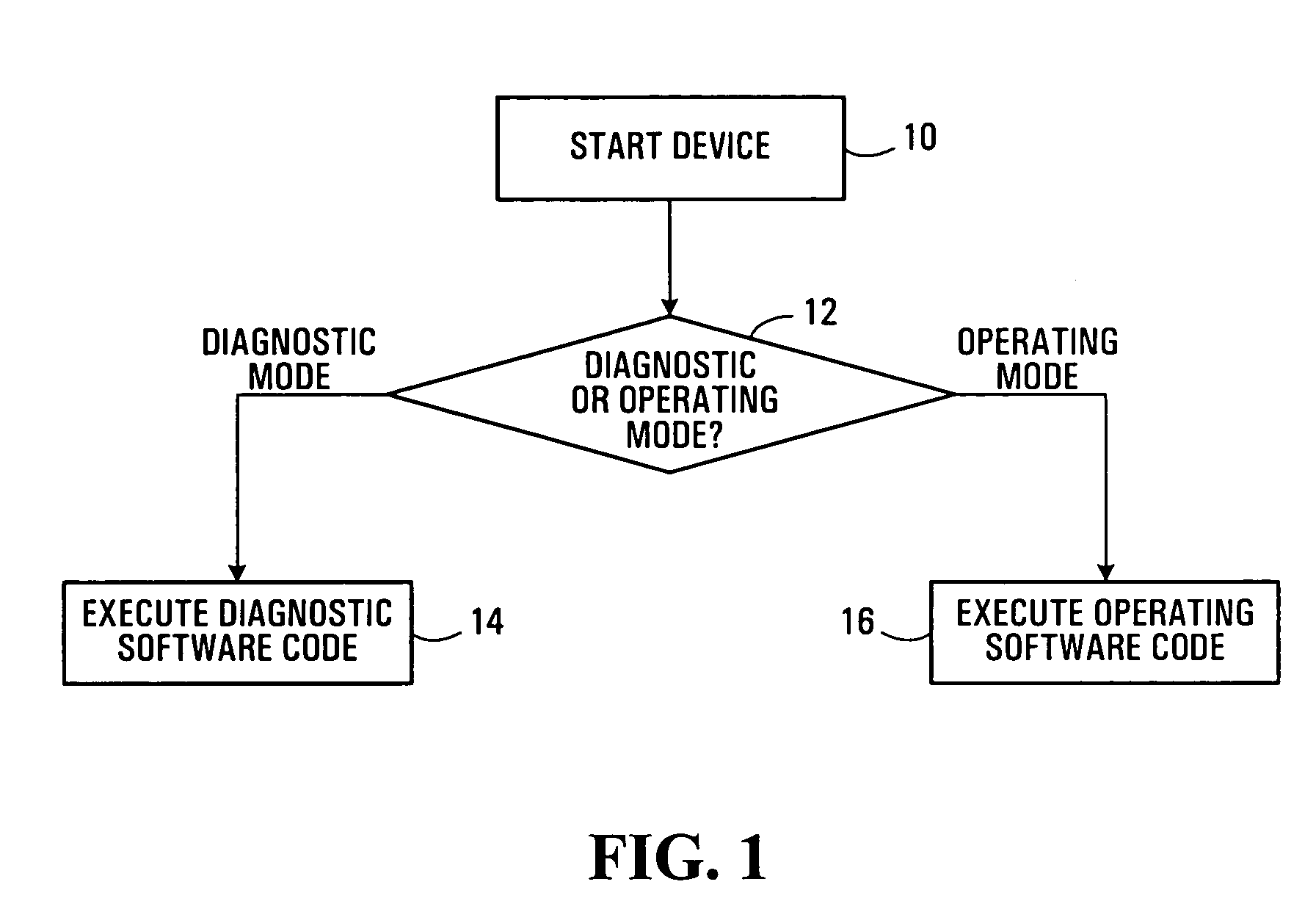
Electronic device diagnostic methods and systems
ActiveUS20050278147A1Efficient accessDigital data processing detailsElectrical testingAction CodeDiagnostic test
Diagnostic methods and systems for electronic devices are disclosed. Separate dedicated diagnostic software code and operating code are respectively executed in a diagnostic mode and an operating mode of an electronic device. A determination may be made as to whether the electronic device is to be operated in the diagnostic mode or the operating mode when the electronic device is started, for example. A transition to the diagnostic mode from the operating mode may also be made in response to errors detected by online diagnostic tests performed in the operating mode. Results of diagnostic tests are preferably stored in a non-volatile memory for subsequent access, independent of the operating software code.
Owner:CIENA
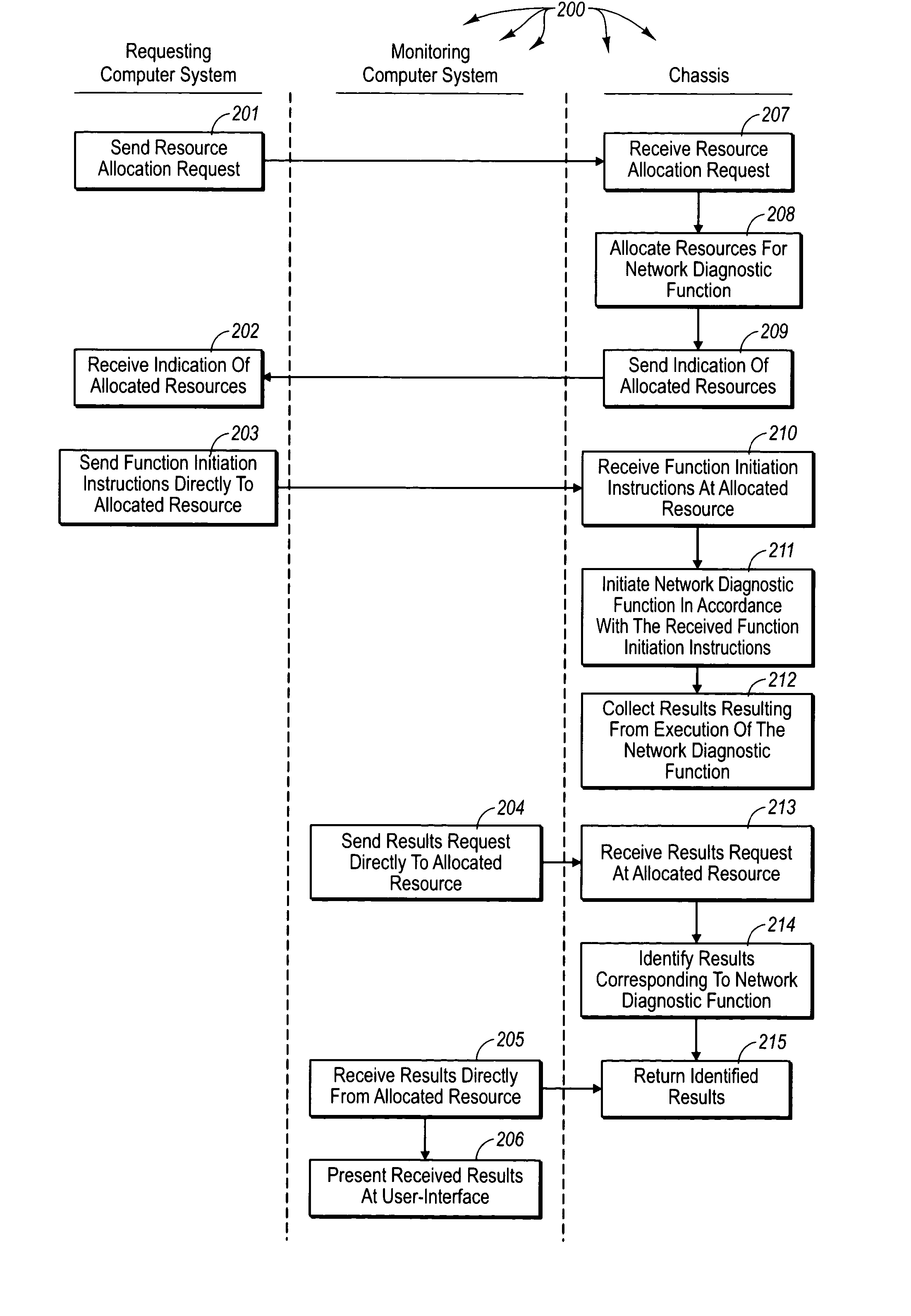
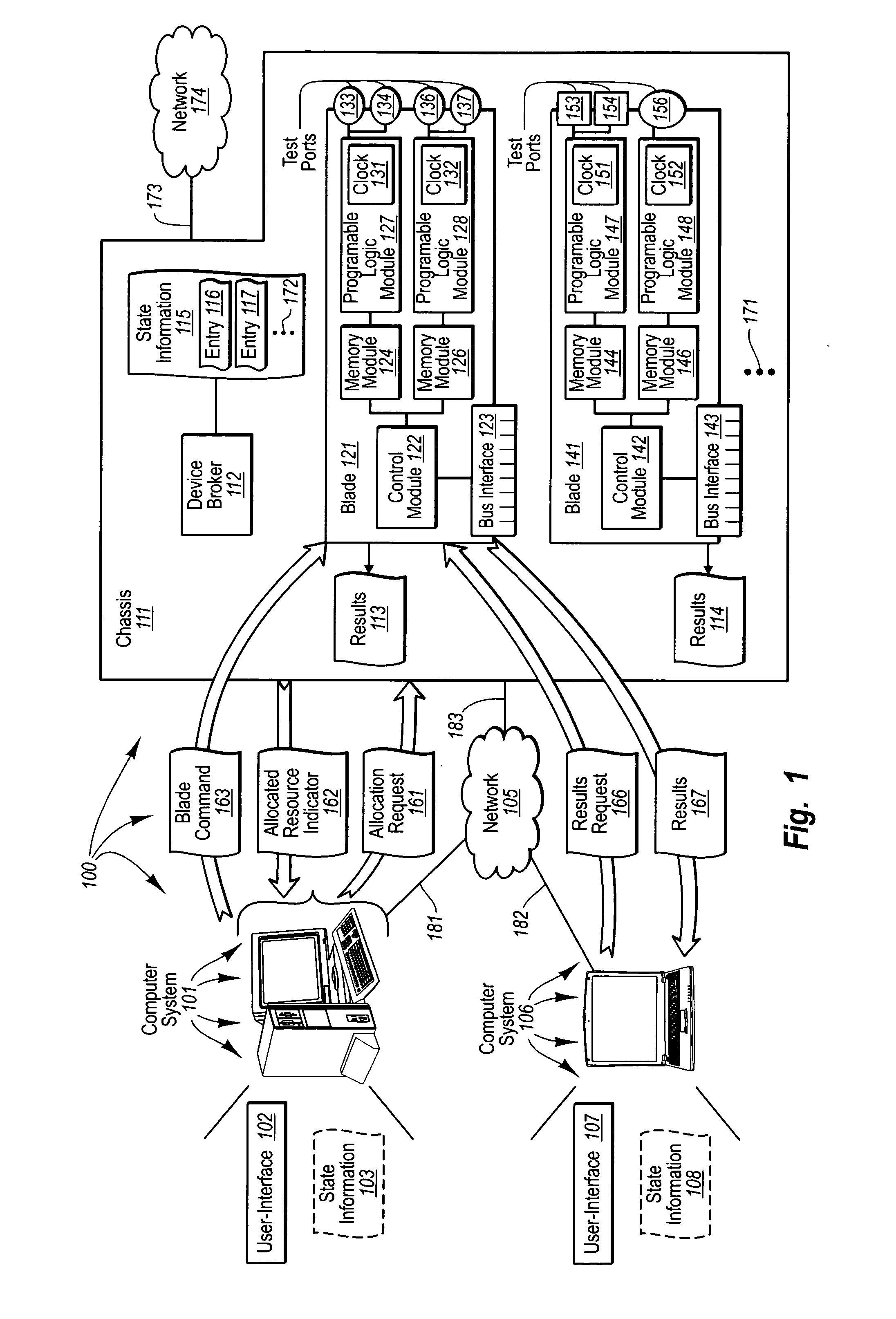
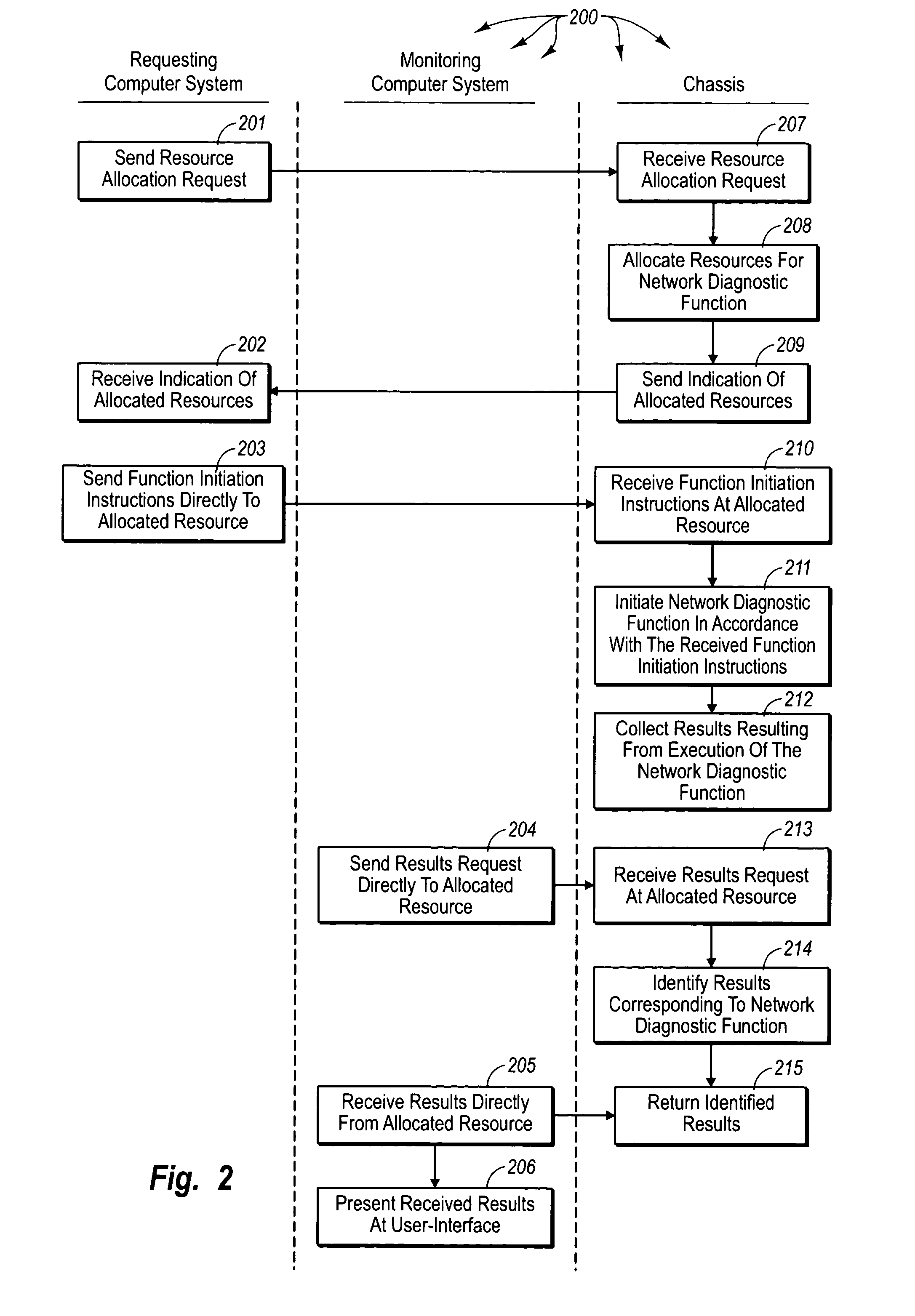
Accessing results of network diagnostic functions in a distributed system
ActiveUS20050050189A1Increase flexibilityMultiprogramming arrangementsMultiple digital computer combinationsComputer hardwareDiagnostic test
The principles of the present invention extend to accessing results of network diagnostic functions in a distributed system. A chassis allocates resources for performing a network diagnostic function directly to requesting computer system. The requesting computer system communicates directly with the allocated resources to initiate the network diagnostic function. The network diagnostic test continues to execute even if the requesting computer system subsequently malfunctions. Collected test results are stored at the chassis such that any network connectable computer system can access the collected test results. A monitoring computer system (which may or may not be the requesting computer system) requests collected test results corresponding to the network diagnostic function. The chassis identifies appropriate test results and returns the results to the monitoring computer system.
Owner:VIAVI SOLUTIONS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com