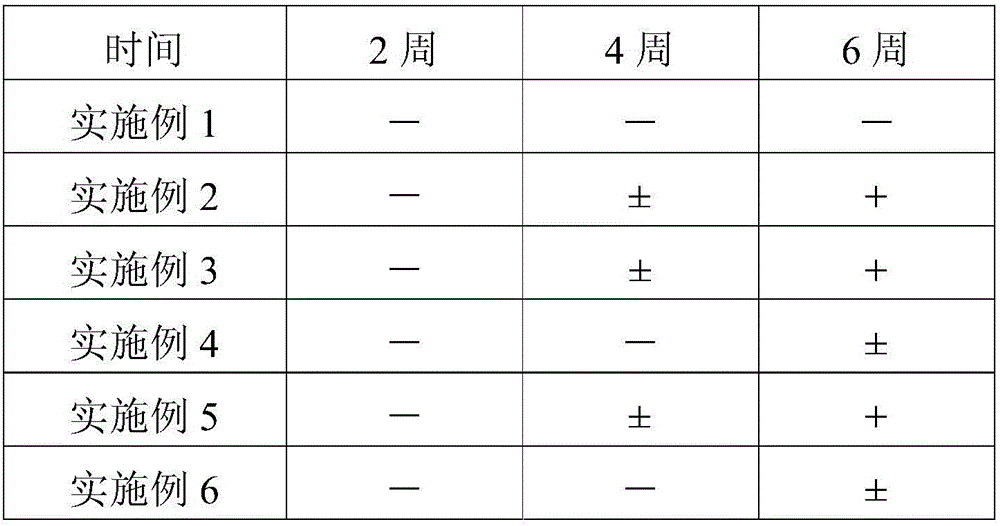
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Japanese encephalitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Japanese encephalitis (JE) is an infection of the brain caused by the Japanese encephalitis virus (JEV). While most infections result in little or no symptoms, occasional inflammation of the brain occurs. In these cases, symptoms may include headache, vomiting, fever, confusion and seizures. This occurs about 5 to 15 days after infection.
Mosquito-repelling bactericide
InactiveCN104585255AInhibition of abnormal fermentationPromote peristalsisBiocideAntisepticsDiseaseAdditive ingredient
The invention relates to a mosquito-repelling bactericide. The raw material formula comprises main ingredients, wherein the main ingredients comprise the following components: clove, folium artemisiae argyi, honeysuckle, elsholtzia, radix angelicae, purple perilla, calamus, mint and agastache rugosus. The mosquito-repelling bactericide disclosed by the invention has the beneficial effects that according to the common sense and principle that mosquitoes bite to suck blood and spread diseases, the bactericide has the main effect of repelling mosquitoes, and harmful pathogenic bacteria and bacteria in air can be killed by virtue of the flavor volatilized by the traditional Chinese medicines, so that the family members are prevented from being troubled by the mosquitoes and are even prevented from suffering from various diseases due to mosquito bites, and people are kept away from diseases such as malaria, filariasis, epidemic encephalitis B and dengue fever.
Owner:刘向上
Kit for detecting mosquito borne pathogens and detection method thereof
InactiveCN103911463AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic encephalitisMultiplex polymerase chain reaction
The invention discloses a kit for detecting a plurality of mosquito borne pathogens and a detection method thereof. The plurality of mosquito-borne infectious pathogens are detected by using multiplex polymerase chain reaction (PCR) combined with a liquid chip, and a yellow fever virus (YFV), dengue fever viruses (DV) I-IV type, epidemic encephalitis B (japanese encephalitis) virus (JEV), plasmodium (Plasmodium), (including plasmodium vivax (PV), plasmodium falciparum (PF), plasmodium malariae (PM), plasmodium ovale (PO)), a west nile virus (MNV) and a chikungunya virus (CHIKV) can be detected in parallel one time. The kit has the advantages of being large in flux, high in specificity and sensitivity, stable in result, good in repeatability, simple to operate and fast in detection speed.
Owner:河北国际旅行卫生保健中心
'Xingnaojing' dripping pills for treating cephalitis and hepatic coma and preparation process thereof
InactiveCN1634145AQuality improvementDefinite curative effectMammal material medical ingredientsDrug compositionsJapanese encephalitisDisease
The invention discloses a pharmaceutical composition for treating diseases including epidemic type B encephalitis and hepatic coma, which has the advantages of wherein the bolus has the advantages of high biological availability, quick-speed medicine release, quick-speed effect, less toxic and side effects, higher medicinal content, smaller amount of administration, accurate administration dosage, easy administration, low price, and facilitated carrying. The preparation is prepared through the conventional injection extracting processes.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
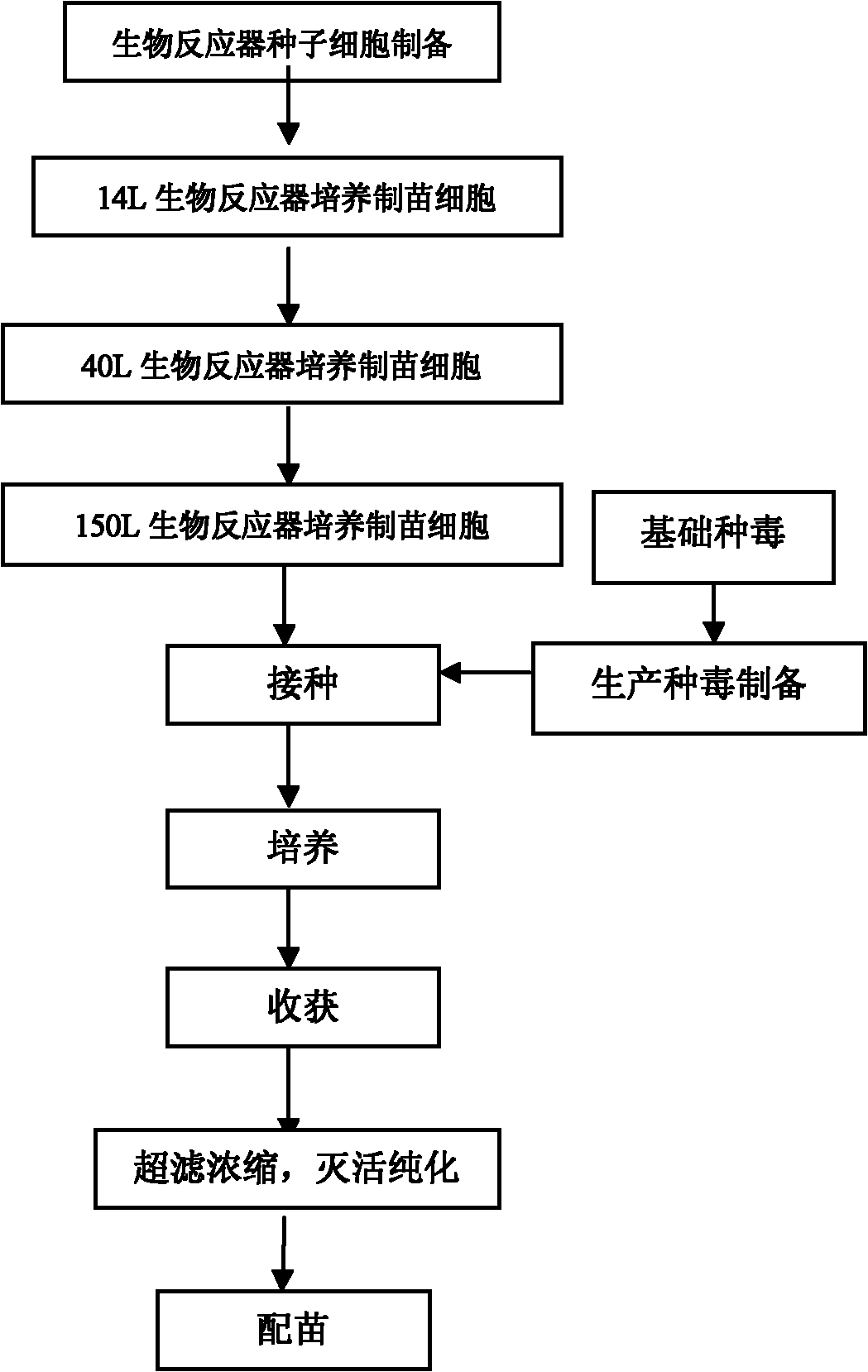
Method for industrially producing porcine Japanese encephalitis (JE) vaccines by utilizing bioreactor
InactiveCN102038943AHigh titerHigh degree of automation controlViral antigen ingredientsMicroorganism based processesAutomatic controlBottle
The invention provides a method for industrially producing porcine Japanese encephalitis (JE) vaccines by utilizing a bioreactor. The method comprises the following steps: (1) sterilizing a microcarrier and the bioreactor, then inoculating cells for vaccine preparation for culturing, inoculating JE viruses when dense monolayers are formed on the cells on the microcarrier, and continuing culturing to reproduce the viruses; (2) stopping culturing and harvesting virus suspension when cytopathy reaches more than 80%; (3) carrying out ultrafilter concentration as well as virus inactivation on the harvested virus suspension; and (4) purifying the inactivated viruses by adopting column chromatography to prepare inactivated vaccines. In the method, the technology of using the microcarrier in the bioreactor for culture is adopted to carry out high density culture of the cells to produce the porcine JE vaccines. Compared with the traditional spinner bottle production method, the method has the following advantages: the automatic control degree is high, so production can be monitored in real time; the labour is saved, thus reducing the cost; the land for production is few, thus being easy to enlarge the scale of production; and the produced viruses have high titer, so the batch-to-batch variation is small, the product quality is stable and the side reaction is low.
Owner:WUHAN CHOPPER BIOLOGY
Japanese encephalitis/dengue chimeric virus and application thereof
InactiveCN101560520AStable passageViral antigen ingredientsAntiviralsJapanese encephalitisWestern blot
The invention provides a Japanese encephalitis / dengue chimeric virus, which is obtained by replacing a prM / E gene in Japanese encephalitis cDNA clone with the prM / E gene of dengue virus type II. IFA and Western blot confirm that the chimeric virus can express E protein of a dengue virus and can stabilize passage in C6 / 36 cells. The invention lays a foundation for developing a dengue virus vaccine. The chimeric virus can be taken as a vaccine to immunize animals so as to enable the animals to obtain immune protection against the dengue virus.
Owner:中国疾病预防控制中心病毒病预防控制所
Japanese encephalitis (JE) vaccine composition and preparing method thereof
InactiveCN105688202AImproving immunogenicityImprove protectionSsRNA viruses positive-senseViral antigen ingredientsWater basedJapanese encephalitis
The invention provides Japanese encephalitis (JE) vaccine composition. The JE vaccine composition comprises a JE virus antigen of immunization effective quantity and water based vaccine adjuvant. The invention further provides a preparing method and application of the JE vaccine composition. The vaccine composition has good immunogenicity and immunizing protection. The immune effect of the JE vaccine composition is remarkably superior to that of single pig JE live vaccine and other live vaccine diluted by immunologic adjuvant. The clinical application prospects are good.
Owner:SICHUAN AGRI UNIV
Element fabric health-improving protective clothing capable of killing and repelling mosquitoes
The invention relates to element fabric health-improving protective clothing capable of killing and repelling mosquitoes, which belongs to the technical field of the production of healthy textile multifunctional textile fabric clothing. More than 360 types of mosquitoes are recorded in China. The mosquitoes are fond of sucking blood of warm-blooded animals, infect malaria, filariasis, yellow fever, dengue, epidemic encephalitis B and even H1N1 flu and threaten human health. The element fabric health-improving protective clothing, which is produced by performing nanometered disposal on mixed materials of a plurality of trace elements needed by a human body, a broad-spectrum powerful silver antibacterial agent, an anti-mosquito finishing agent SCJ-951, mosquito-killing pyrethrin and the like and adding the mixture into spinning solution of cotton, flax, fur, polypropylene fiber, polyamide fiber, acrylic fiber and the like, kills and repels mosquitoes and improves health.
Owner:陈欣荣
Mosquito repellent
The invention discloses mosquito repellent. The mosquito repellent is made of raw materials including, by weight, 5-15 parts of flos caryophylli, 10-15 parts of folium artemisiae argyi, 8-20 parts of flos lonicerae, 5-7 parts of herba moslae, 10-12 parts of herba menthae, 6-9 parts of agastache rugosa and 10-30 parts of realgar. The mosquito repellent has the advantages that the mosquito repellent is prepared from purely natural Chinese herbal medicines and chemical products, mosquitoes mainly can be expelled according to mosquito biting and blood sucking and disease spreading common sense and principles, harmful germs, bacteria and the like in air can be killed by odor volatilized from the traditional Chinese medicines, accordingly, family members can be guaranteed against being disturbed by the mosquitoes and can be prevented from suffering from diversified diseases due to mosquito biting, effects of keeping the family members away from diseases such as malaria, filariasis, epidemic encephalitis and dengue fever can be realized, methods for manufacturing the mosquito repellent are simple, raw materials for the mosquito repellent are easily available, and the like.
Owner:GUANGXI UNIV FOR NATITIES
Method for preparing swine epidemic encephalitis B inactivated vaccine
InactiveCN104208668APrice stabilizationStable immunogenicityViral antigen ingredientsAntiviralsFiberSide effect
The invention provides a method for preparing a swine epidemic encephalitis B inactivated vaccine. According to the method, a Chinese hamster lung cell adaptive virus screened by bacteriophage plaque cloning and purification is capable of generating cytopathic effect, the virulence is stabilized at 107.0-108.0TCID50 / mL, and the immunogenicity is stable; the swine epidemic encephalitis B virus is multiplied by utilizing the hamster pulmonary cell system has good virus adaptability and high virus virulence of prepared vaccine. According to the method, a virus concentrating process technology which sequentially performs separating and concentrating is adopted, and virus solution and cell debris can be separated by utilizing a hollow fibrous membrane clarifying and purifying system, so that the problems that the membrane is easily blocked, is sensitive to shearing force and easily aggregated when virus particles are concentrated can be solved, side effects of an inactivated vaccine finished product can be effectively reduced, and the safety of the inactivated vaccine can be improved. Binary ethyleneimine is used as an inactivator instead of formaldehyde and has small destruction on virus antigen, so that the completeness of effective immunogen in the antigen can be guaranteed.
Owner:TIANJIN RINGPU BIO TECH
Sweet wormwood ointment with anti-mosquito and anti-malaria effects
InactiveCN107802755AAvoid bitesAvoid spreadingHydroxy compound active ingredientsAerosol deliveryNepetaEugenol
The invention relates to a sweet wormwood ointment with anti-mosquito and anti-malaria effects. The ointment is mainly prepared from the following raw materials: nepeta oil, menthol, dementholized peppermint oil, sweet wormwood oil, blumea oil, lemongrass oil, eugenol, artesunate, alpha-bisabolol and an ointment excipient, prevent mosquito bite well, and further prevent rapid spread of diseases which mainly take mosquitos as media such as malaria, dengue fever, filariasis or epidemic encephalitis B. The sweet wormwood ointment further has effect of rapidly relieving itching and removing swelling on itching and swelling which are caused by mosquito bite.
Owner:江西龙卿堂科技有限公司
Gene chip and kit for detecting pig epidemic type B encephalitis virus and/or pig porcine reproductive and respiratory syndrome virus
ActiveCN104293976AEfficient detectionQuick checkNucleotide librariesMicrobiological testing/measurementJapanese encephalitisEncephalitis Viruses
The invention discloses a gene chip and a kit for detecting a pig epidemic type B encephalitis virus and / or pig porcine reproductive and respiratory syndrome virus. The gene chip and the detection kit disclosed by the invention can accurately and effectively detect the pig epidemic type B encephalitis virus and the pig porcine reproductive and respiratory syndrome virus and are high in specificity, high in sensitivity, short in time, fast in detection speed and good in application prospect.
Owner:SICHUAN AGRI UNIV
Gene chip and kit for detecting swine epidemic encephalitis B viruses (SEEBVs) and/or swine fever viruses (SFVs)
InactiveCN104293981AEfficient detectionHigh sensitivityNucleotide librariesMicrobiological testing/measurementJapanese encephalitisSwine Fever Virus
The invention discloses a gene chip and kit for detecting swine epidemic encephalitis B viruses (SEEBVs) and / or swine fever viruses (SFVs). The gene chip comprises a solid phase carrier and a probe immobilized on the solid phase carrier, wherein the probe comprises any one or two oligonucleotide fragments shown in SEQ ID NO:1 or 2 and / or any one or two oligonucleotide fragments shown in SEQ ID NO:3 or 4. The kit comprises the gene chip and reagents for amplifying genes of the SEEBVs and / or the SFVs. The gene chip and the detection kit can be used for accurately and effectively detecting the SEEBVs and the SFVs, have strong specificity, high sensitivity and good application prospects, consume short time and are fast in detection.
Owner:SICHUAN AGRI UNIV
Method for producing Yujin composition and medicament containing the same
InactiveCN101152324AQuality improvementGood curative effectAntibacterial agentsAntiviralsSide effectHouttuynia
The invention relates to an oral preparation of a drug combination of heartleaf houttuynia and honeysuckle, including the volatile component, the water soluble component and the spirit soluble component of the heartleaf houttuynia and the honeysuckle. The drug of the invention is mainly used for the symptoms of the upper respiratory infection, the bronchial pneumonia, the acute epidemic type-B encephalitis, the viral infection, the gynecological inflammation, the fever after the operation etc. The invention also relates to the extraction method of the main components which contain the drug combination and the preparation method of the drug preparation. The drug nature test indicates that compared with the market injection preparation, the drug of the invention has the advantages of good curative effect, low side effect, convenient use, cheap price etc.
Owner:SHANDONG BUCHANG PHARMA
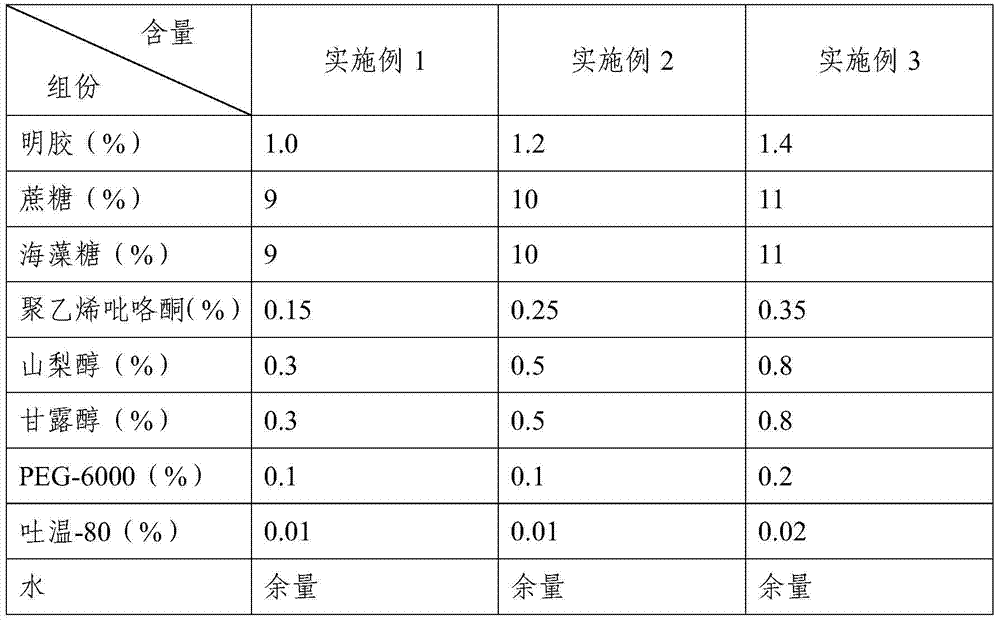
Porcine Japanese encephalitis live vaccine heat-resistant lyophilized protective agent as well as preparation method and application thereof
ActiveCN103656661AAvoid damageReduce loss ratePowder deliveryViral antigen ingredientsProtective antigenSucrose
The invention provides a porcine Japanese encephalitis live vaccine heat-resistant lyophilized protective agent. The protective agent comprises the following components in percentage by weight: 1.0-1.4% of gelatin, 9-11% of sucrose, 9-11% of trehalose, 0.15-0.35% of polyvinylpyrrolidone, 0 .3-0.8% of sorbitol, 0.3-0.8% of mannitol, 0.1-0.2% of PEG-6000, and 0.01-0.02% of tween-80, the pH value of the protective agent is 7.5, and the components are added with water to be dissolved and sterilized at high temperature to obtain the protective agent.The lyophilized protective agent disclosed by the invention is mixed with a porcine Japanese encephalitis live vaccine virus solution, and freeze-drying is carried out to obtain a lyophilized vaccine. The heat-resistant lyophilized protective agent disclosed by invention is simple in formulation, easy in preparation, suitable for large-scale production, has good protection efficacy for vaccines, and can ensure that the vaccines can be preserved for 10 days effectively at the normal temperature, even at the temperature of 37 DEG C, and preserved for 24 months at the temperature of 2-8 DEG C.
Owner:CHINA ANIMAL HUSBANDRY IND
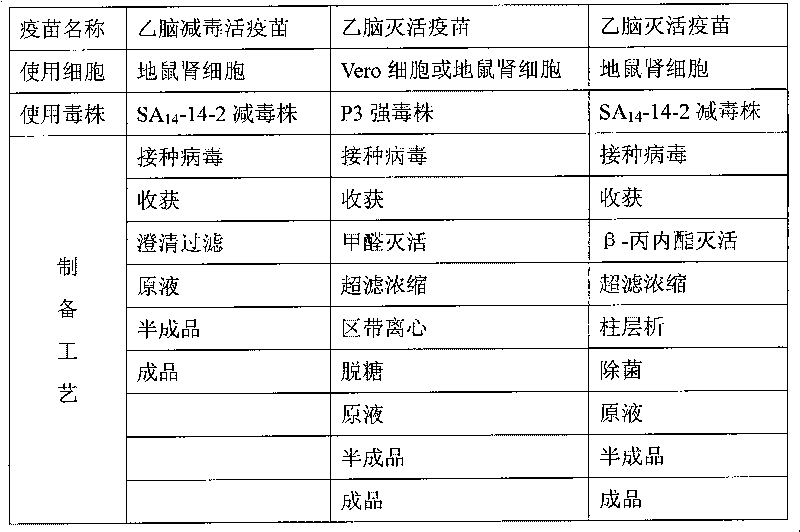
Preparation method of vaccine for epidemic encephalitis B
InactiveCN101732708AImprove securityAvoid damageViral antigen ingredientsInactivation/attenuationSodium bicarbonateViral culture
The invention relates to a preparation method of a vaccine for epidemic encephalitis B, which comprises the processes of cell culture, virus propagation and vaccine preparation. After cells overgrow with a single layer, a seed culture of viruses and a culture solution are inoculated, and the use quantity of the seed culture of the viruses is that every 100cm<2> of cells is inoculated with 1ml of seed culture of the viruses. The culture solution of the viruses mainly comprises 90-95% of MEM culture solution, 3-5% of calf serum and 7.5% of sodium bicarbonate solution the pH of which is regulated to 7.2-7.6. The vaccine is prepared by adding human serum albumin with the final concentration of 0.3% after being clarified and filtered. The invention adopts an SA14-14-2 attenuated strain to produce an encephalitis purified vaccine. Compared with a vaccine produced by a P3 strain, the attenuated strain is safer and more effective. The invention has the advantages that: (1) the attenuated strain is used for preparing the purified vaccine, which has good safety; (2) the inactivation effect of inactivating the viruses by using beta-propiolactone is good, and an inactivator is naturally degraded and has no residue; and (3) the column chromatography purification property is mild, and the injury to the viruses is small.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
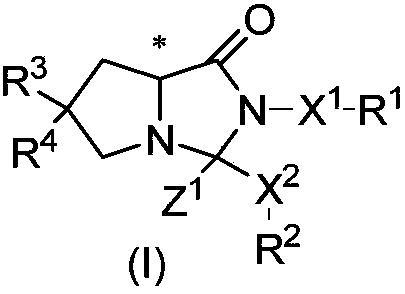
Cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof
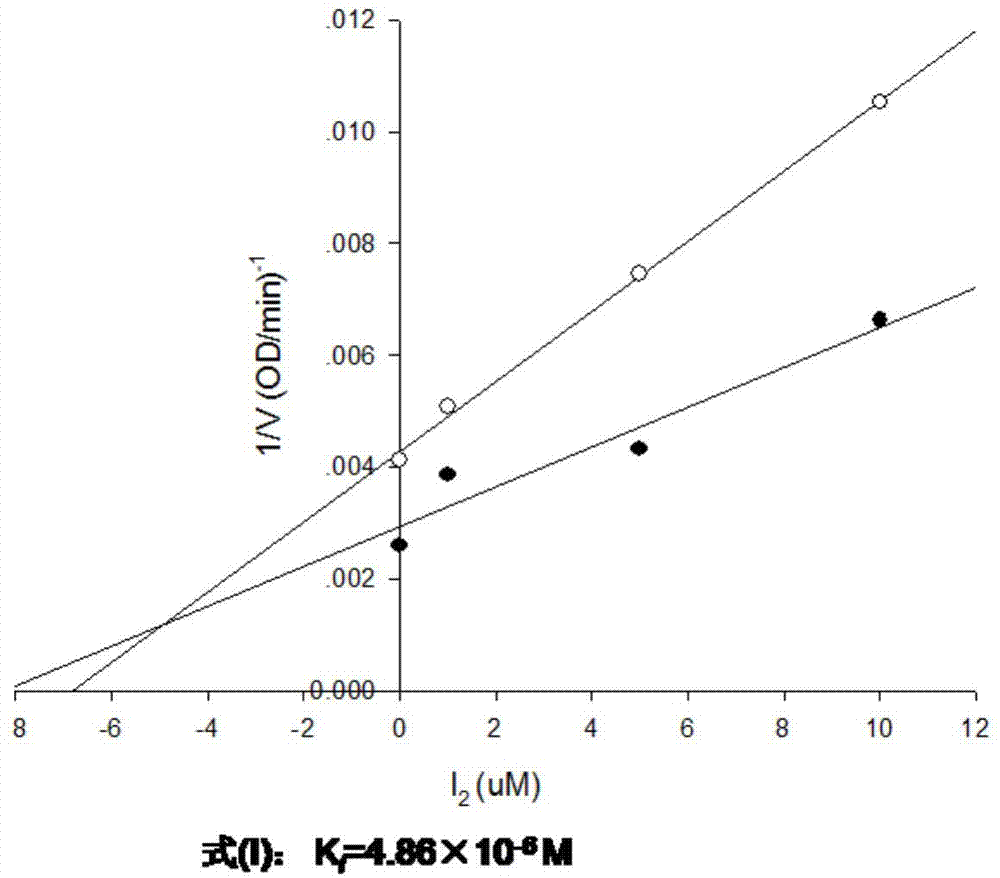
The invention discloses a cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof. The cyclocompound is a compound shown in a formula (I), an isomer or pharmaceutically acceptable salt thereof. The compound, isomer or pharmaceutically acceptable salt thereof can be applied to preparation of medicines used for preventing or treating related diseases (such as dengue fever, dengue hemorrhagic fever, dengue shock syndrome, Zika, Chikungunya, Japanese encephalitis, yellow fever, hepatitis C and West Nile disease) caused by a dengue fever virus and related viruses. (The formula (I) is described in the specification).
Owner:NANJING TECH UNIV
Phlegm eliminating and convulsion arresting powder and application thereof
InactiveCN102335357AReasonable formulaGood effectAntibacterial agentsInorganic boron active ingredientsConvulsionJapanese encephalitis
The invention discloses phlegm eliminating and convulsion arresting powder and application thereof. The phlegm eliminating and convulsion arresting powder is prepared from roasted scorpion, centipede, defatted croton seed powder, bezoar, borax, realgar, arisaema cum bile, unibract fritillary bulb, tabasheer and musk. The formula is reasonable, and the powder has a good treatment effect, and can effectively treat diseases such as Japanese encephalitis, pneumonia, epidemic cerebrospinal meningitis, toxic bacillary dysentery, pertussis encephalopathy and the like.
Owner:朱良春
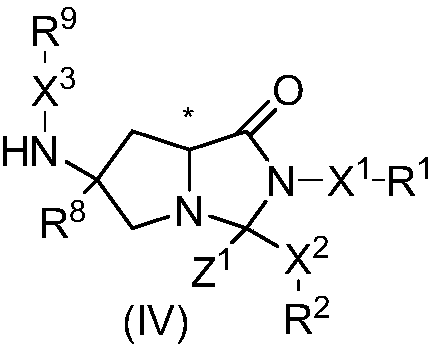
Use of dipeptide analogue
The invention discloses use of a dipeptide analogue, and particularly relates to use of compounds in formulas (I) and (II), a stereisomer or a pharmaceutically acceptable salt thereof in pharmaceutical aspects. The compounds shown in the formulas (I) and (II) can be utilized as drugs for treating and preventing a disease caused by a dengue fever virus, and simultaneously can become the drugs for treating and preventing the disease caused by other flaviviruses, such as a yellow fever disease, hepatitis c, Japanese encephalitis, forest encephalitis, a disease caused by west nile virus infection, or aids caused by a human immunodeficiency virus (HIV) and the like.
Owner:NANJING TECH UNIV
Recombinant E protein ELISA kit for detecting antibody against Japanese encephalitis in pig
InactiveCN1766620AStrong specificityGood reactogenicityColor/spectral properties measurementsJapanese encephalitisAntigen
The invention relates to a recombinant E protein agent box for detecting Japanese cerebritis antibody in the field of biology technology. The agent comprises: ELISA batten which uses the recombinant E protein as the coated antibody, PBS solution, blood dilution solution, color developing base material, ending solution, JEV anodic serum and JEV cathodic serum and so on. The method can be used in other serum sample detection after peculiar, sensitive and repeating detection.
Owner:NANJING AGRICULTURAL UNIVERSITY
Heat-resistant freeze-drying protectant for porcine Japanese encephalitis live vaccine, preparation method and application thereof
ActiveCN103656661BAvoid damageReduce loss ratePowder deliveryViral antigen ingredientsJapanese encephalitisSucrose
Owner:CHINA ANIMAL HUSBANDRY IND
RAA primer probe for detecting Nipah viruses as well as detection method
PendingCN110592285AAccurate detectionNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceHendra Virus
The invention discloses a primer and a probe for detecting Nipah viruses by an RAA fluorescence method as well as a detection method, wherein the primer and the probe are suitable for RAA fluorescencemethod detection, Nipah virus plasmid can be detected, cross reaction with Hendra virus plasmid, foot and mouth disease viruses, porcine parvoviruses, pseudorabies viruses, porcine circoviruses, epidemic type b encephalitis viruses, reproductive and respiratory syndrome viruses, hog cholera viruses and infectious gastroenteritis viruses can be avoided, and the specificity reaches to 100 percent;the detection method is rapid, high flux is easy to realize, the detection time and the detection cost are reduced; and according to the method for rapidly detecting Nipah virus DNA based on the RAA fluorescence method, the sensitivity is high, and 74 copies / reaction is realized under the probability that the reaction detection sensitivity is 95 percent.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT +1
Test strip for quickly detecting swine Japanese encephalitis antibody and preparation method of test strip
InactiveCN103116022APrevent proliferationRapid diagnosisMaterial analysisCelluloseJapanese encephalitis
The invention discloses a test strip for quickly detecting a swine Japanese encephalitis antibody and a preparation method of the test strip. The test strip consists of absorbent paper, a nitrocellulose membrane, a glass cellulose membrane, a sample pad and a support object, wherein the nitrocellulose membrane contains a detection line coated by JEV E protein and a control line coated by a goat anti-JEV antibody; and the glass cellulose membrane is combined with colloidal gold marked JEV E protein. According to the invention, by adopting a membrane chromatography indirect sandwich method, a specific antibody in Japanese encephalitis immune pig or infected pig serum can be detected. When detecting a swine Japanese encephalitis antibody, the test strip disclosed by the invention is simple, convenient and quick to operate, special instrument or professional training is not required, and the result is clear and easy to distinguish; and the test strip is easy to popularize and particularly suitable for base field test and epidemiological investigation, and plays an important auxiliary role in the diagnosis of swine Japanese encephalitis.
Owner:GUANGXI VETERINARY RES INST
Traditional Chinese medicine for treating epidemic encephalitis B
InactiveCN101152487ACompatibility is simpleLow costAnthropod material medical ingredientsAntiviralsSkin complexionDisease
A Chinese traditional medicine for curing epidemic type-B encephalitis belongs to a Chinese traditional medicine for curing type-B encephalitis. The ingredients of the medicine are 40g of plaster stone, 10g of rehmannia root, 6g of rhinoceros horn, 5g of coptis, 5g of liquorice, 5g of bamboo leaves, 8g of pittosporm roots and 8g of balloon flower roots; the auxiliary ingredients are 15g of scorpion and 15g of hooked uncaria under the situation of twich and lockjaw; 10g of tabasheer and 10g of articifical bezoar under the situation of much sputum; 12gof moutan bark under the situation of dark purple complexion or maculae on the skin. The medicines used in the prescription are all natural herbs and the Chinese traditional medicine is processed with traditional method; the fetching of material is convenient and the prescription and manufacture method are simple; the expense for manufacturing the medicine is low. The composition of the Chinese herbal medicine is simple and the medicines used in the prescription are all natural ones and the fetching and use are convenient; the manufacture method is simple and the effect is excellent; the price is low; in this way, the herbal medicine is especially fit for people living in remote villages far away from counties and towns; the curing expense for patients with the disease of epidemic type-B encephalitis is low which solves the problem that the household income is low and the life is poor and the medical conditions is deficient locally.
Owner:张贤清
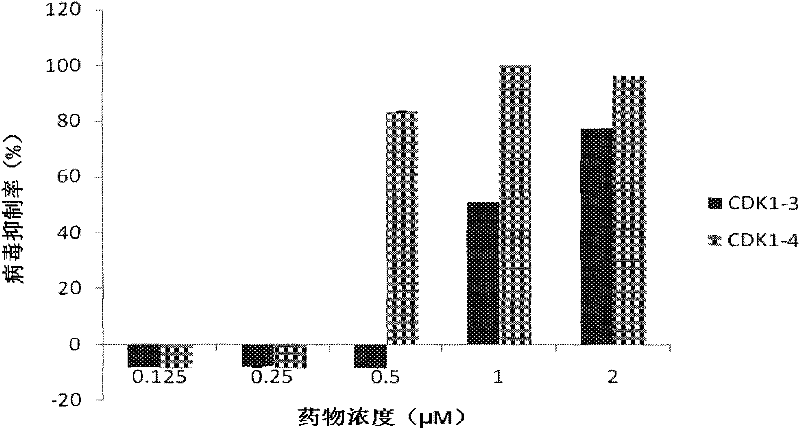
Structure and application of anti-ev71, dengue, Japanese encephalitis and influenza virus oligonucleotide targeting cdk1
InactiveCN102296067AFree from diseaseStrong specificityGenetic material ingredientsAntiviralsDiseaseVirus influenza
The invention relates to a structure and applications of antisense oligonucleotide, in particular to targeting cyclin-dependent kinase 1, a structure of antisense oligonucleotide of Enterovirus 71, Dengue virus and Japanese encephalitis resistance and applications of the antisense oligonucleotide in preparing medicines for treating Enterovirus 71, Dengue virus, Japanese encephalitis, flu virus and related diseases.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Traditional Chinese medicine for treating epidemic encephalitis B
InactiveCN101690791ARelieve symptomsEasy to preparePowder deliveryNervous disorderDiseaseSide effect
The invention discloses a traditional Chinese medicine, in particular to a traditional Chinese medicine for treating epidemic encephalitis B. The medicine comprises the following raw materials in certain weight ratio: mix-fried licorice, chrysanthemum, oriental waterplantain rhizome, amur corktree bark, turmeric root-tuber, platycladi seed, juncus effuses, fructus forsythiae, bile, vietnamese sophora root, white stiff silkworm, cynanchum glaucescens, platycodon root, Chinese magnoliavine fruit, scorpion, bamboo juice, lalang grass rhizome and common bletilla pseudobulb. The medicine is simple in preparation, good in absorption effect, obvious in curative effect, free of side effects and high in safety, and is beneficial to timely alleviating symptoms of the disease.
Owner:刘鹏捷
Visual gene chip and kit used for detecting porcine epidemic encephalitis b viruses and/or porcine reproductive and respiratory syndrome viruses
InactiveCN105018642AAccurate detectionLow costNucleotide librariesMicrobiological testing/measurementFluorescenceJapanese encephalitis
The invention discloses a visual gene chip and kit used for detecting porcine epidemic encephalitis b viruses and / or porcine reproductive and respiratory syndrome viruses. With the visual gene chip prepared by using a specific method, detection results can be visually seen with naked eyes, and an expensive laser confocal scanner is not needed; and the visual gene chip and kit have the advantages of low cost, high sensitivity which is almost equal to sensitivity of conventional fluorescence labeling methods, good specificity, short detection time, rapid detection and good application prospects.
Owner:SICHUAN AGRI UNIV
Medicament for treating encephalitis b and preparation method thereof
ActiveCN101352571AQuality improvementHigh yieldPowder deliverySerum immunoglobulinsJapanese encephalitisWhole blood product
The invention relates to a drug used for treating Japanese encephalitis and a preparation method thereof, belonging to the field of blood products. The invention provides a drug which can effectively treat Japanese encephalitis, thus solving the technical problem. The drug for treating Japanese encephalitis of the invention is a preparation prepared by taking Japanese encephalitis human immunoglobulin as an active ingredient and adding medicinally acceptable accessories. The drug can effectively cure Japanese encephalitis and provides a novel drug choice for Japanese encephalitis patients. In addition, the Japanese encephalitis human immunoglobulin intravenous injection prepared by the method of the invention has the advantages of better quality, high yield and purification, high virus safety and great application prospect.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD +1
Visual gene chip and kit used for detecting porcine epidemic encephalitis b viruses and/or hog cholera viruses
InactiveCN105018643AAccurate detectionQuick checkNucleotide librariesMicrobiological testing/measurementJapanese encephalitisFluorescence
The invention discloses a visual gene chip and kit used for detecting porcine epidemic encephalitis b viruses and / or hog cholera viruses. With the visual gene chip prepared by using a specific method, detection results can be visually seen with naked eyes, and an expensive laser confocal scanner is not needed; and the visual gene chip and kit have the advantages of low cost, high sensitivity which is almost equal to sensitivity of conventional fluorescence labeling methods, good specificity, short detection time, rapid detection and good application prospects.
Owner:SICHUAN AGRI UNIV
Xingnaojing injection and preparation method thereof
InactiveCN106267004AImprove quality stabilityClearing heat and purging fireNervous disorderHydroxy compound active ingredientsHepatic comaAntioxidant
The invention discloses a Xingnaojing injection and a preparation method thereof. The Xingnaojing injection is prepared from raw materials in parts by weight as follows: 7.5 parts of musk, 30 parts of turmeric root tuber, 30 parts of cape jasmine fruits, 1 part of borneol, 8 parts of sodium chloride for injection, 0.1-0.5 parts of a stabilizer and 0.1-1 part of an antioxidant. According to the Xingnaojing injection and the preparation method thereof, the stabilizer and the antioxidant are added to the preparation process of the injection, the quality stability of the Xingnaojing injection is improved, the stabilizer further has a solubilization function and increases content of effective ingredients in the Xingnaojing injection, and the prepared Xingnaojing injection has functions of clearing away heat and fire, cooling blood, eliminating toxins and inducing resuscitation, and can be used for treating epidemic encephalitis, hepatic coma, the symptom of heat entering construction-blood, the symptom of invasion of pericardium by heat, high fever, dysphoria, coma and delirium, deep red tongue, rapid pulse and the like.
Owner:上海辰松新材料科技有限公司
Encephalitis-related virus detection kit and application thereof
InactiveCN105603128AReduced risk of cross-contaminationLow costMicrobiological testing/measurementDNA/RNA fragmentationJapanese encephalitisEastern equine encephalitis virus
The invention discloses an encephalitis-related virus detection kit and application thereof. The kit is used on the basis of the principles of multiplex PCR (polymerase chain reaction) combined nucleic acid intrusive reaction and nano gold color development. The kit comprises a combination of five types of primers and probes, and can be used for simultaneously detecting eastern equine encephalitis virus, western equine encephalitis virus, epidemic encephalitis b virus, West Nile virus and Nipah virus. When being used for detecting the five viruses, the kit has the advantages of high specificity and high sensitivity, and does not need any expensive apparatus; the detection result can be observed by naked eyes; and thus, the kit can be applied to the basic level.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com