Preparation method of vaccine for epidemic encephalitis B
A Japanese encephalitis, epidemic technology, applied in the direction of resistance to vector-borne diseases, recovery/purification, inactivation/attenuation, etc. problem, to achieve the effect of good inactivation effect, small virus damage and mild nature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] Below in conjunction with embodiment the invention will be further described:
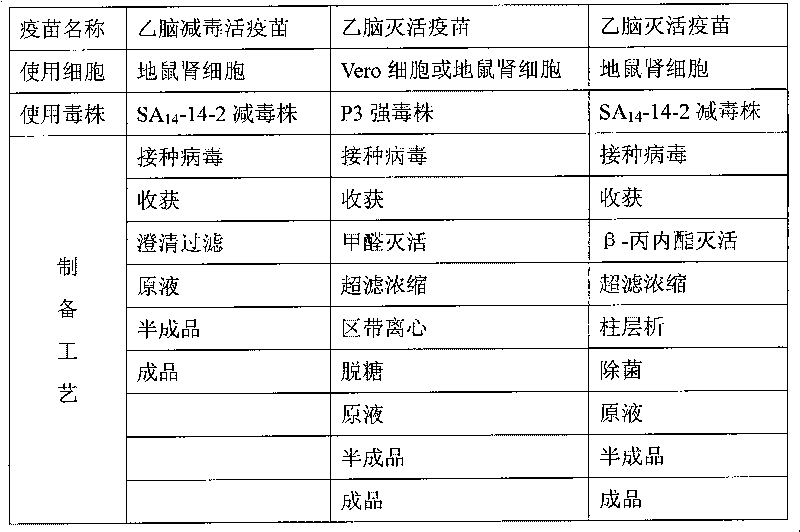
[0015] This patent uses SA 14 -14-2 attenuated strain produces purified Japanese encephalitis vaccine, which is an inactivated vaccine. The mother strain virus is the JEV SA14 strain isolated from Xi'an mosquito larvae in 1954. Through the method of multiplying the extraneural tissues of animals to improve immunity, a production strain with low virulence and high immunity (SA 14 -14-2). SA 14 The -14-2 attenuated strain has high thermal stability, and the virus is not completely inactivated when heated at 50°C for 2 to 4 hours, which is no different from the original strain of SA14. Its genotype is also quite stable, and it remains stable in the 17th passage of hamster kidney cell virus.
[0016] The present invention relates to the method for epidemic Japanese encephalitis vaccine, its steps are as follows:
[0017] Choose healthy hamsters aged 10-14 days and weighing 12-15 grams, kill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com