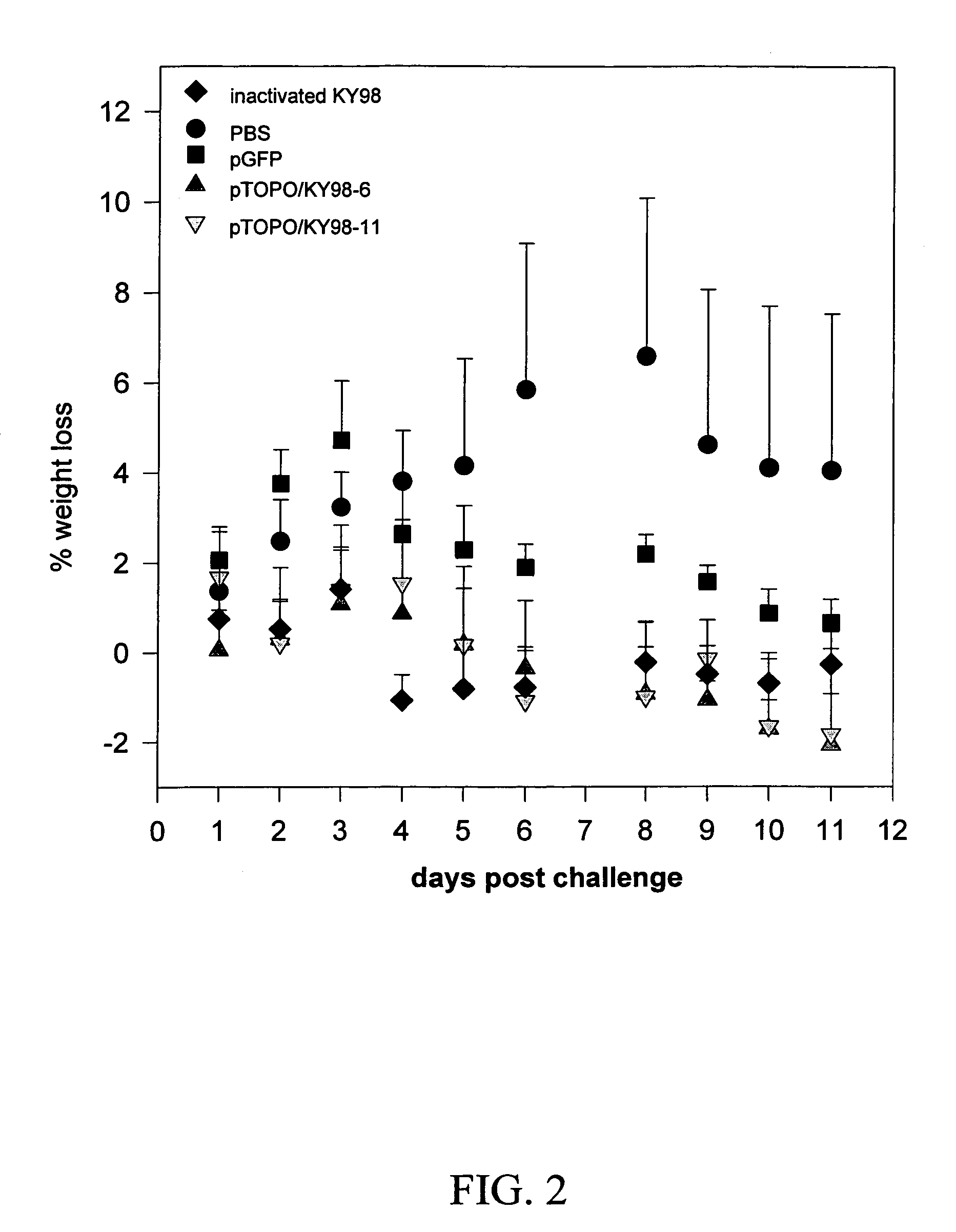
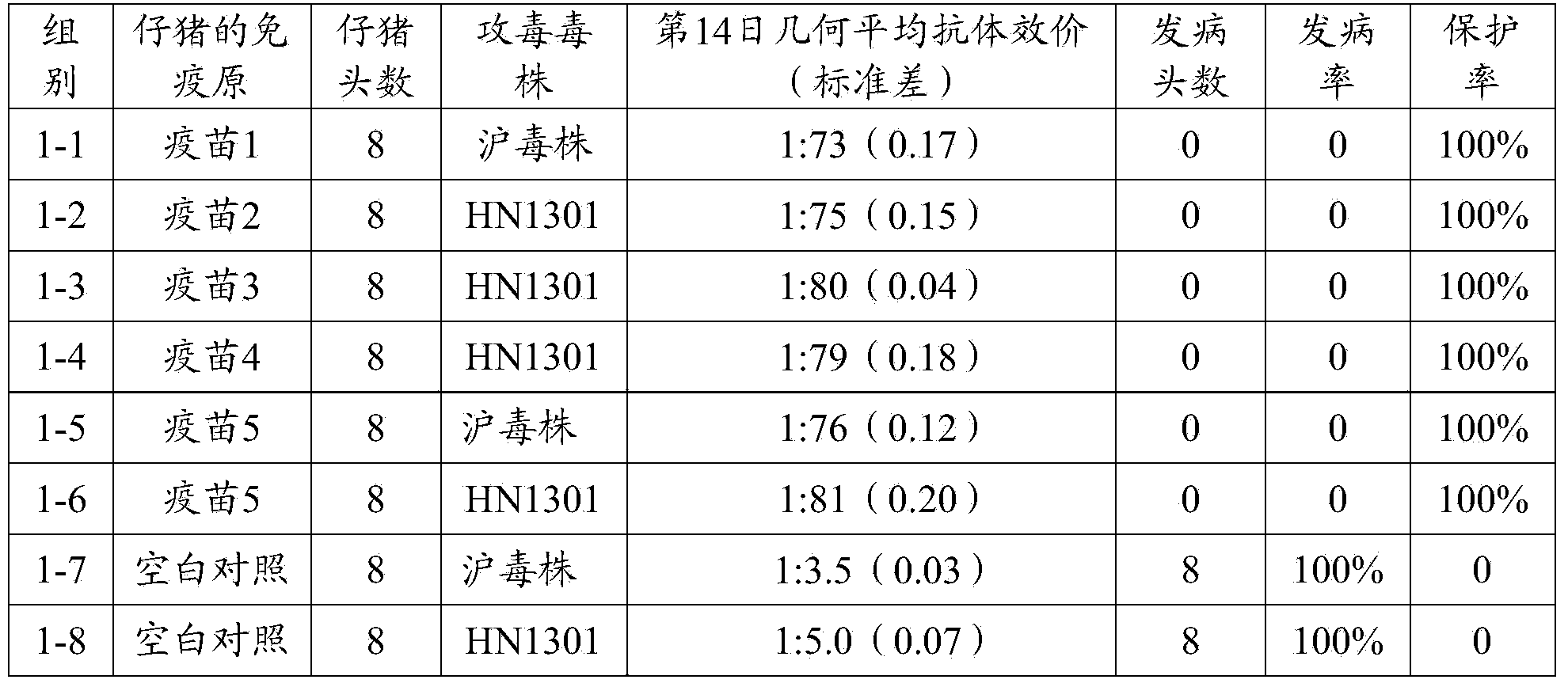
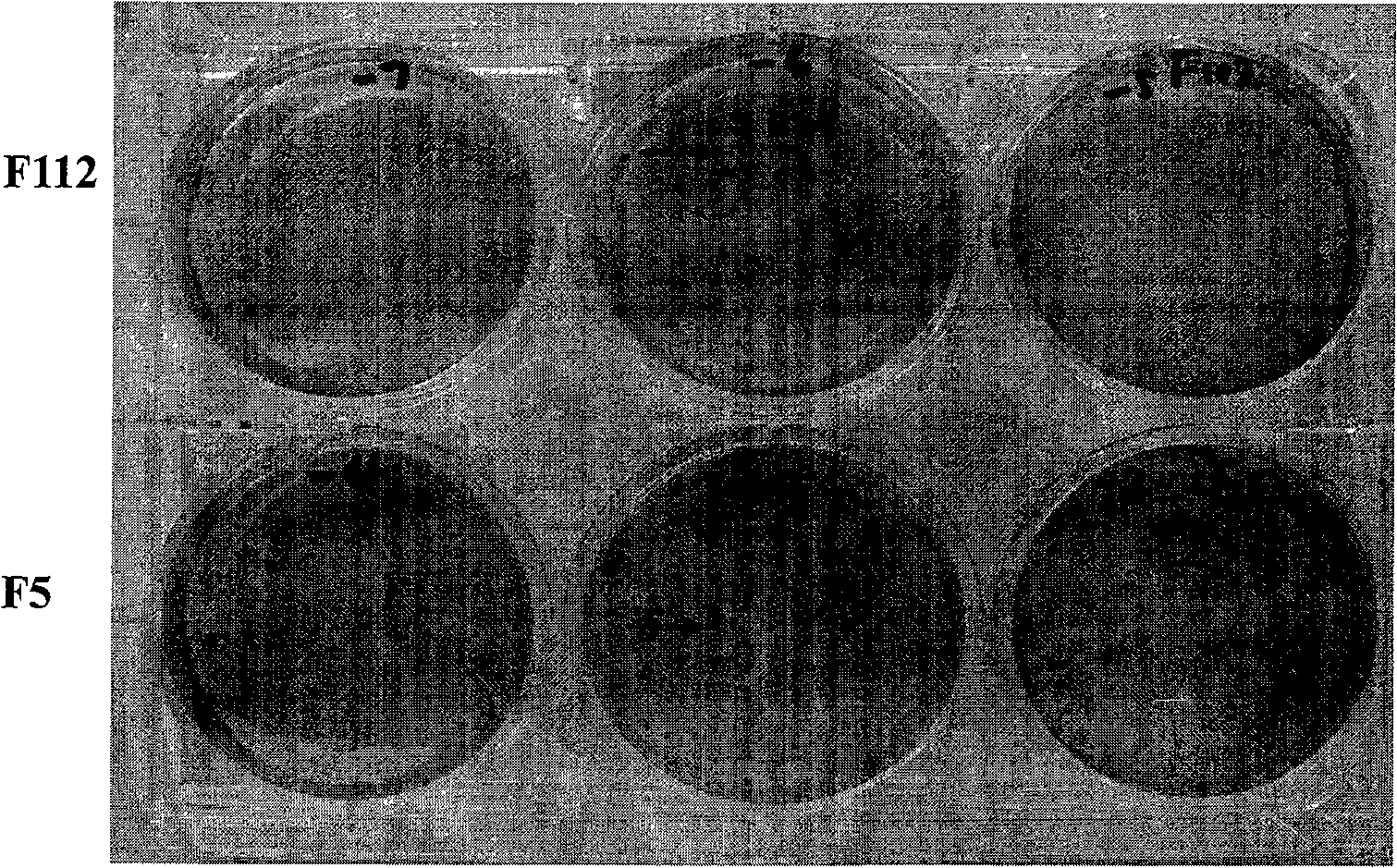Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1705 results about "Inactivated vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
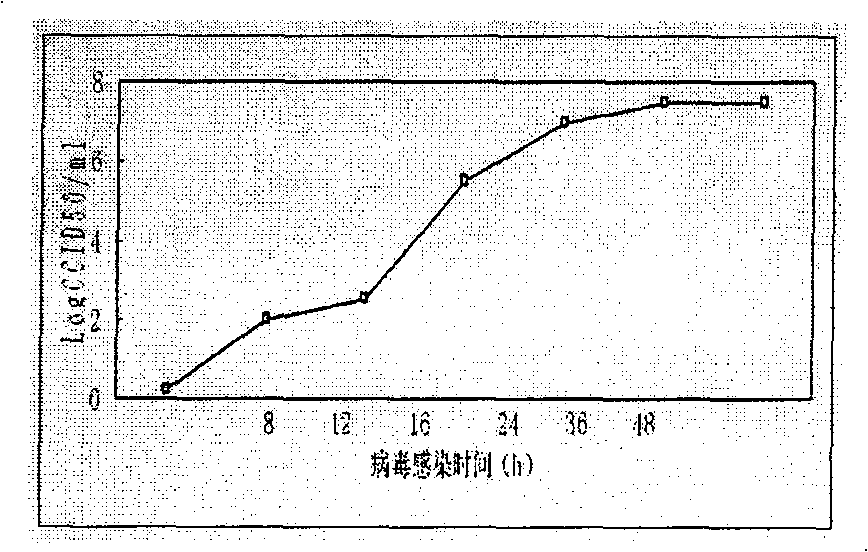
An inactivated vaccine (or killed vaccine) is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then lose disease producing capacity. In contrast, live vaccines use pathogens that are still alive (but are almost always attenuated, that is, weakened). Pathogens for inactivated vaccines are grown under controlled conditions and are killed as a means to reduce infectivity (virulence) and thus prevent infection from the vaccine. The virus is killed using a method such as heat or formaldehyde.
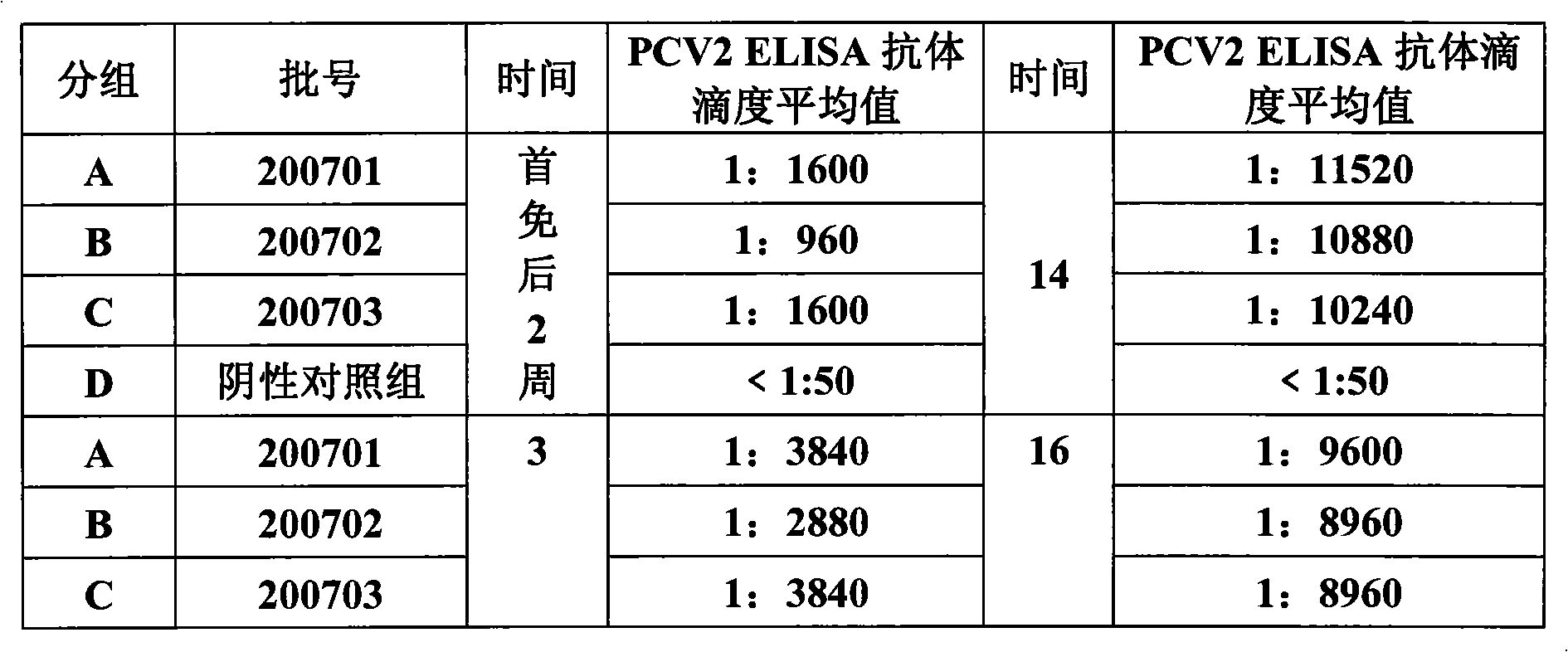
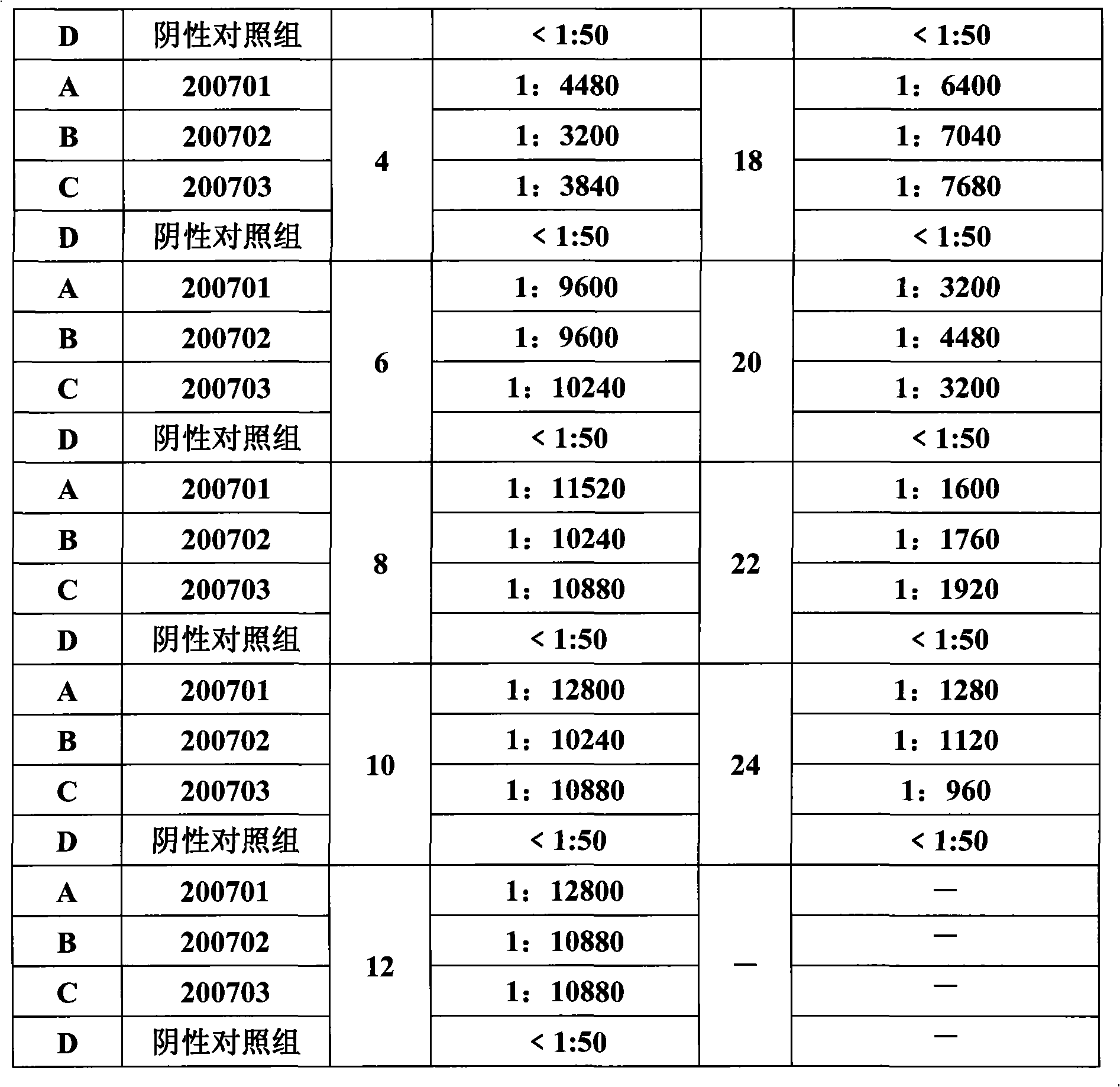
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
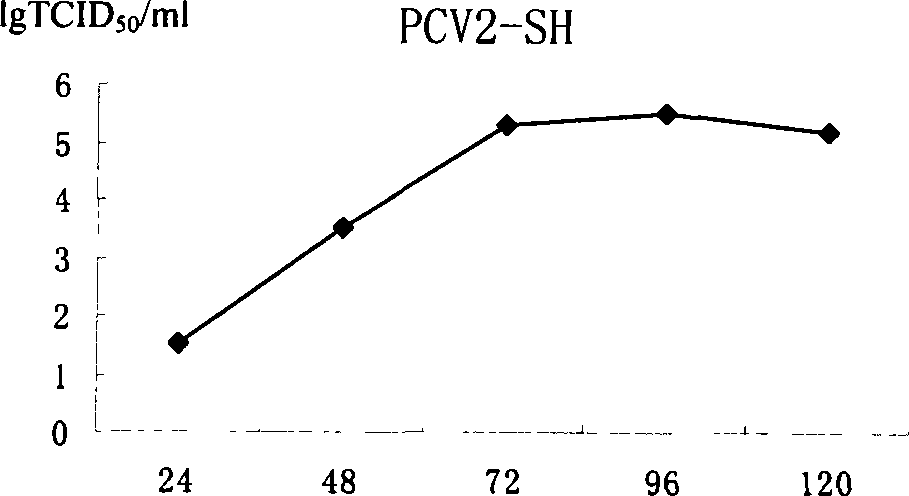
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Porcine pseudorabies virus virulent strain, and gene deletion vaccine strain thereof and applications thereof
ActiveCN102994458AEffective preventionEffective therapeuticMicroorganism based processesAntiviralsRabiesMicrobacterium
The invention discloses a porcine pseudorabies virus virulent strain, and a gene deletion vaccine strain thereof and applications thereof. The porcine pseudorabies virus virulent strain is named as HeN1, the microbial preservation number of the porcine pseudorabies virus virulent strain is CGMCC NO.6656, the deleted gE gene obtaines the gene deletion vaccine strain rPRV-gE-EGFP+ on the basis of the virulent strain HeN1, and the microbial preservation number is CGMCC NO.6657. The virulent strain can be prepared into inactivated vaccine (single vaccine or combined vaccine), the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into activated vaccine or inactivated vaccine (single vaccine or combined vaccine) and the like, so that porcine pseudorabies can be effectively prevented or cured, or the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into a diagnosis reagent for diagnosing the porcine pseudorabies. The gene deletion vaccine strain rPRV-gE-EGFP+ has the advantages of being good in safety, high in protection efficiency, beneficial to differential diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Feline vaccines against avian influenza
InactiveUS20080107687A1Elicit immune responseSsRNA viruses negative-senseViral antigen ingredientsEpitopeViral Vaccine
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a recombinant poxvirus vaccine or an inactivated vaccine. The invention also encompasses recombinant poxvirus vectors encoding and expressing avian influenza antigens, epitopes or immunogens which can be used to protect animals, in particular felids, against avian influenza.
Owner:MERIAL LTD
Vaccines and methods to treat canine influenza
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Porcine circovirus II-type recombinant baculovirus as well as preparation method and application thereof
ActiveCN103122352AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliSpecific immunity
The invention discloses porcine circovirus II-type recombinant baculovirus as well as a preparation method and application thereof. ORF2 gene is artificially synthesized by referring to a PCV2b isolated strain ORF2 gene sequence; the synthesized ORF2 gene is connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHm 30RF2 is obtained. The baculovirus transfer vector pFBDPHm30RF2 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant rod granule rBac-PVR30RF2; the rod granule is transferred with a sf9 cell to obtain the recombinant baculovirus QP-Ac-30RF2. The recombinant baculovirus can be used for efficiently expressing the PCV20RF2 protein and forming virus-like particles. The VLP which is expressed and packaged by the recombinant baculovirus disclosed by the invention is used for preparing inactivated vaccine, and the organism is induced to generate specific immunity response after a 28-day-aged piglet is immunized, and the pig body can be completely protected from virulent attacks of the porcine circovirus.
Owner:HUAZHONG AGRI UNIV
Influenza virus vaccine composition and methods of use
InactiveUS20070286869A1Enhance transfection expression efficacyLow costSsRNA viruses negative-senseOrganic active ingredientsEpitopeInfluenza virus vaccine
The present invention is directed to enhancing the immune response of a human in need of protection against IV infection by administering in vivo, into a tissue of the human, at least one polynucleotide comprising one or more regions of nucleic acid encoding an IV protein or a fragment, a variant, or a derivative thereof. The present invention is further directed to enhancing the immune response of a human in need of protection against IV infection by administering, in vivo, into a tissue of the human, at least one IV protein or a fragment, a variant, or derivative thereof. The IV protein can be, for example, in purified form or can be an inactivated IV, such as those present in inactivated IV vaccines. The polynucleotide is incorporated into the cells of the human in vivo, and an immunologically effective amount of an immunogenic epitope of an IV, or a fragment, variant, or derivative thereof is produced in vivo. The IV protein (in purified form or in the form of an inactivated IV vaccine) is also administered in an immunologically effective amount.
Owner:VICAL INC
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
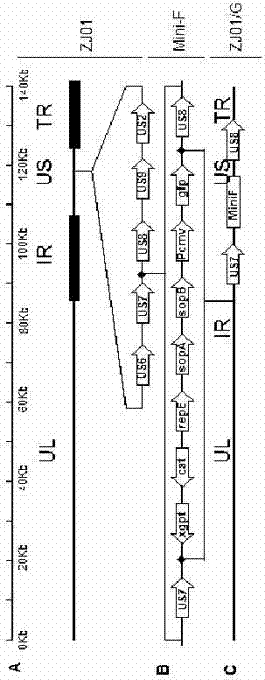
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
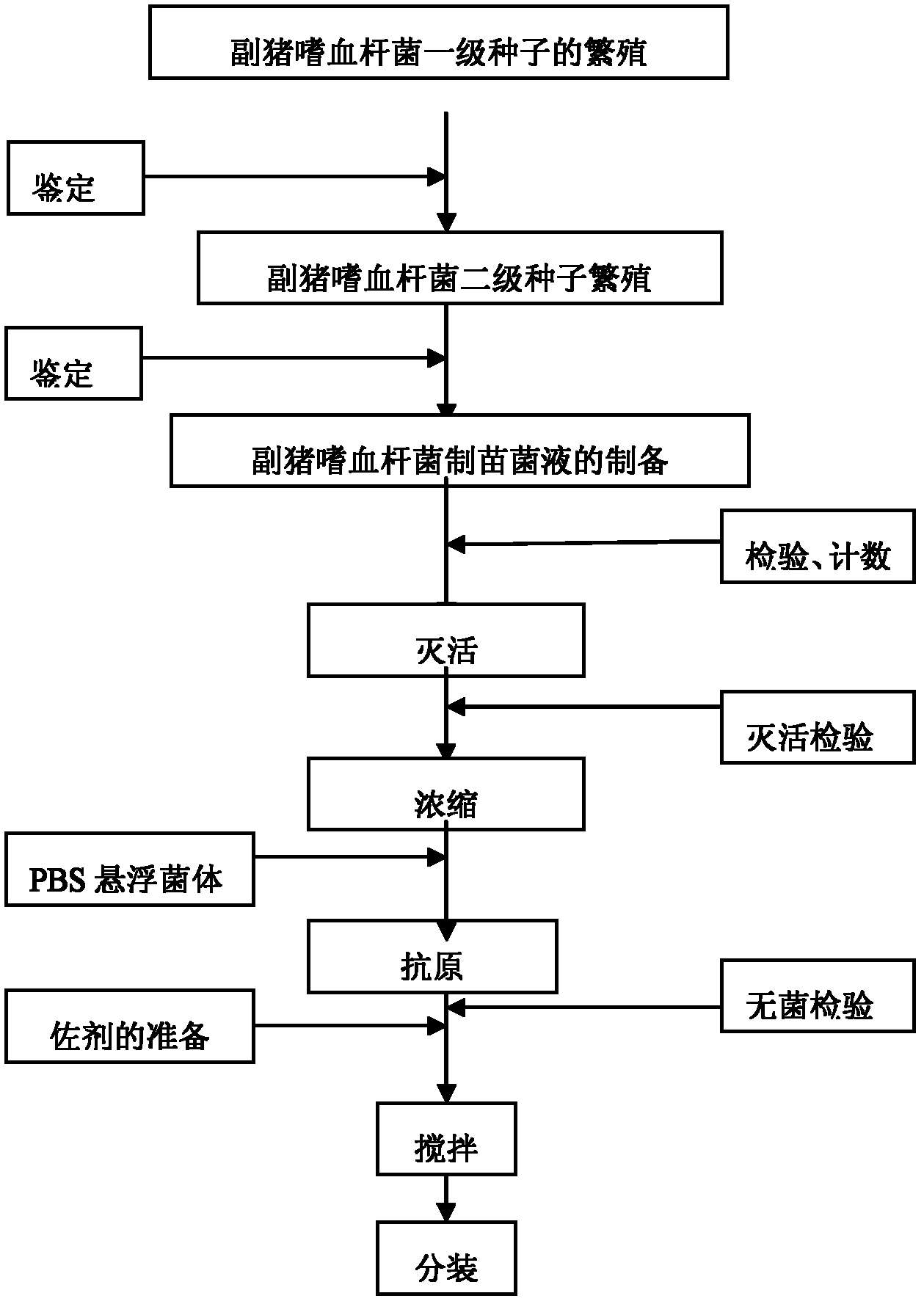
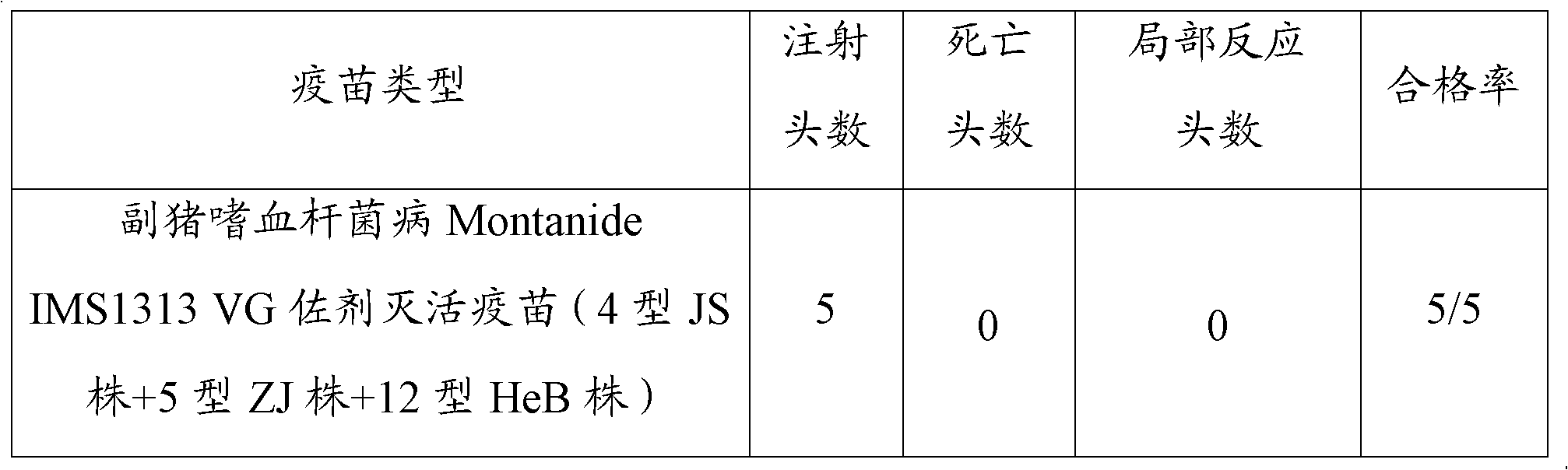
Novel haemophilus parasuis disease trivalent inactivated vaccine and preparation method thereof
ActiveCN102908615ASolve preparation difficultiesImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The invention provides a novel haemophilus parasuis disease trivalent inactivated vaccine and a preparation method thereof. The vaccine comprises equal proportions of antigens of: inactivated haemophilus parasuis serotype 4 JS strain, inactivated haemophilus parasuis serotype 5 ZJ strain, and inactivated haemophilus parasuis serotype 12 HeB strain. According to the invention, the haemophilus parasuis serotype 4, serotype 5, and serotype 12 strains are obtained by separation. Concentration contents and relative proportions of the antigens prepared from the three strains are subjected to large amounts of woks and practices, such that the trivalent vaccine with an appropriate antigen ratio and an appropriate concentration is obtained. The trivalent vaccine has good preventing and treating effects against haemophilus parasuis diseases caused by various epidemic haemophilus parasuis serotypes in our nation. Especially, the trivalent vaccine can solve a problem of poor treatment effect of existing vaccines caused by novel haemophilus parasuis pathogens.
Owner:PU LIKE BIO ENG
A type foot-and-mouth disease recombinant vaccine strains and preparation method and application thereof
ActiveCN103266091AMultiple phenotype improvements and enhancedPhenotype Improvement and EnhancementMicroorganism based processesAntiviralsAntigenDisease
The invention relates to A type foot-and-mouth disease recombinant vaccine strains prepared by using a reverse genetic manipulation technology and a preparation method and application of the strains. One strain is an A type foot-and-mouth disease recombinant vaccine strain with high titer, antigen matching property and immune protection rate, and the other strain is an A type foot-and-mouth disease recombinant non-pathogenic vaccine strain with high titer, antigen matching property and immune protection rate and without pathogenicity for a host; an antigen nucleotide sequence of each of the vaccine strains is shown as SEQ ID NO: 1; eukaryotic plasmids of viruses can be saved by using a reverse genetic manipulation system; after pigs and cattle are immunized by using the inactivated vaccines prepared from the prepared recombinant vaccine strains, the bodies can be effectively stimulated to produce immune response, and an immune protection effect is provided for the bodies of the pigs and the cattle; through a 10,000-times cattle median infectious dose (BID50) challenge assay of A type AISA topological strains, the immune protection rate reaches 100 percent, and the median protective dose (PD50) is 10.81 to 13.59; and the recombinant vaccine strains can be applied to prevention and control of A type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
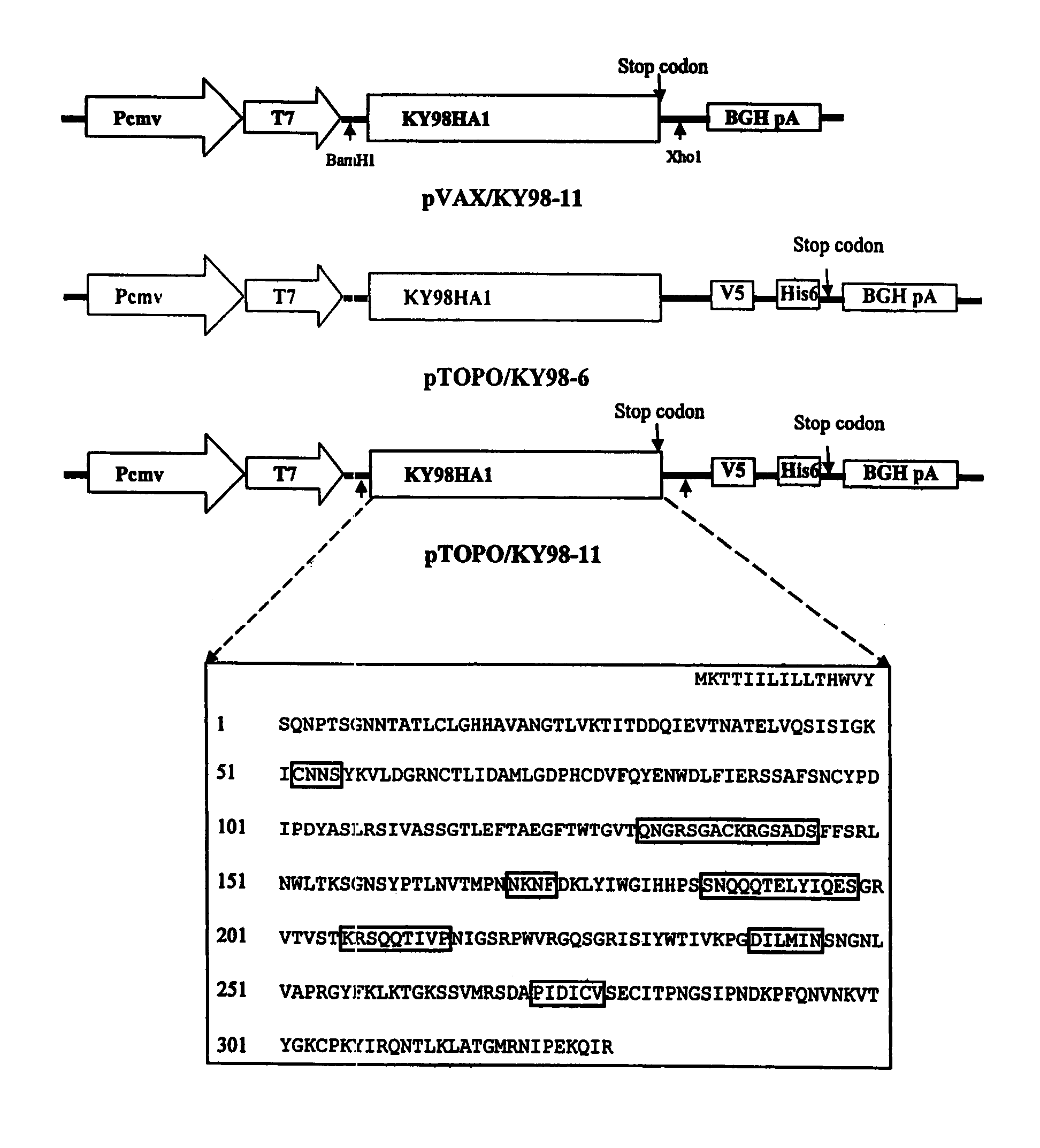
DNA vaccine expressing HA1 of equine-2 influenza virus
InactiveUS7244435B2Reduce riskReduce dosageSsRNA viruses negative-senseViral antigen ingredientsHemagglutininA-DNA
The invention is for a DNA vaccine expressing the hemagglutinin (HA1) gene of equine-2 influenza virus. By engineering a stop codon within HA1, expression of HA1 is ensured. By encapsulation of the DNA vaccine in liposome and by intranasal inoculation, it is sufficient to elicit protective immunity at a significantly lower dosage compared to a DNA vaccine expressing the full length HA gene. Lower dosage reduces the risk of induction of anti-DNA antibodies. Intranasal inoculation directly to the respiratory epithelial cells reduces the risk of DNA integration. The inventive vaccine is advantageous over current inactivated or live attenuated vaccines, as updating of the vaccine requires only the replacement of the encoding sequence with the new virus.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Virus velogen strain for porcine reproductive and respiratory syndrome, attenuated vaccine strain thereof and application thereof
ActiveCN101280292AImprove securityNo side effectsViral antigen ingredientsMicrobiological testing/measurementDiseaseMicroorganism
The invention discloses a virulent strain HuN4 for porcine reproductive and respiratory syndrome, and an attenuated vaccine strain HuN4-F112 (the collection number of microorganism: CGMCC No.2484) attenuated because of virulent strain HuN4 passage, and also discloses the application of the attenuated vaccine strain in preventing or curing the highly pathogenic blue-ear disease, which belongs to the biomedical field. The attenuated vaccine strain HuN4-F112 can be prepared into single-vaccine or combined strain (live vaccine or inactivated vaccine), can effectively prevent or cure the highly pathogenic blue-ear disease, and also can be prepared into diagnostic reagent for the highly pathogenic blue-ear disease diagnosis. The attenuated vaccine strain HuN4-F112 of the invention has the advantages of good security and high protection efficiency.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Development of a Marker Foot and Mouth Disease Virus Vaccine Candidate That is Attenuated in the Natural Host
ActiveUS20120315295A1Easy to replaceQuick changeSsRNA viruses positive-senseSugar derivativesSerotypeViral Vaccine
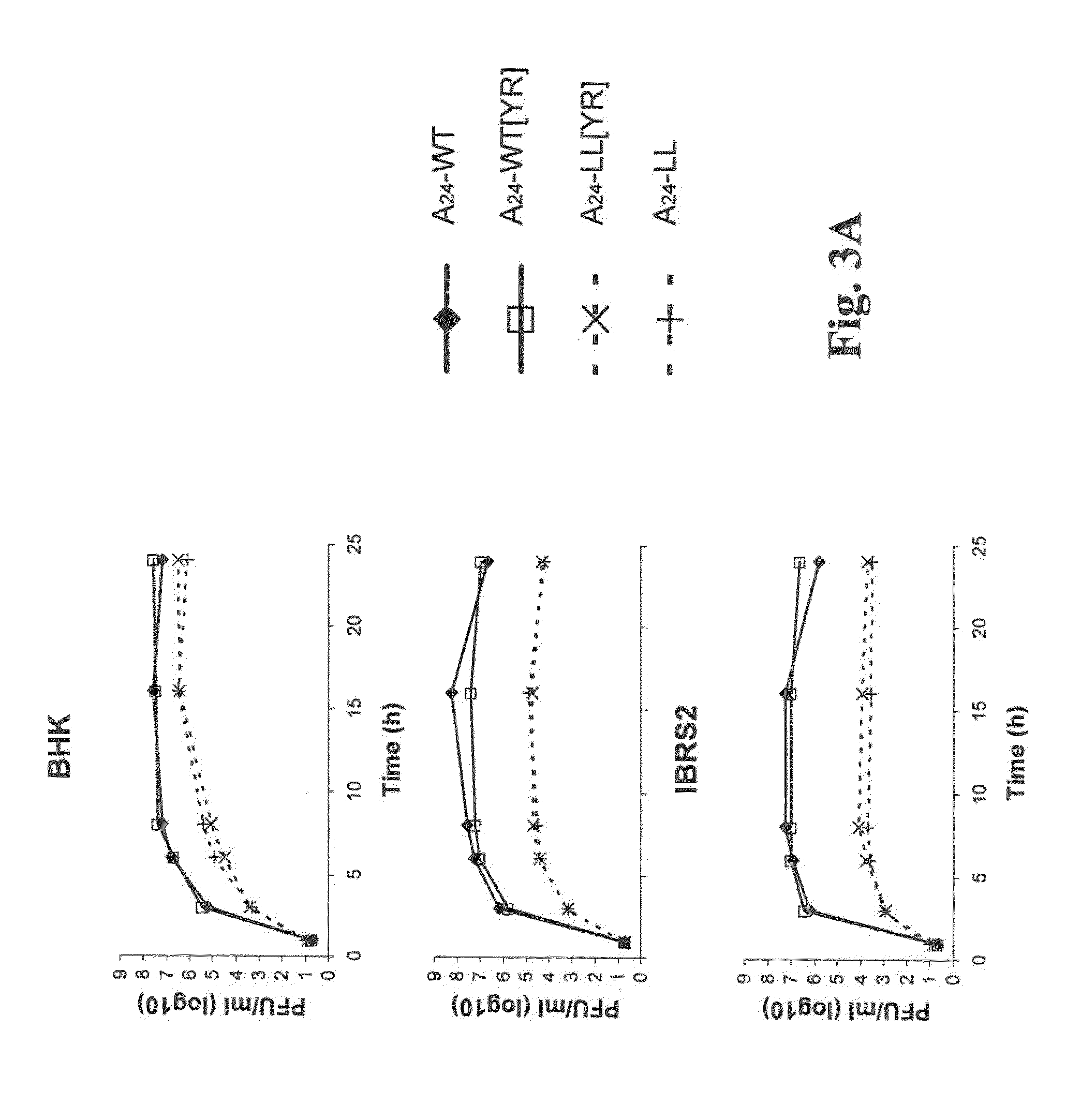
We have generated novel molecularly marked FMDV A24LL3DYR and A24LL3BPVKV3DYR vaccine candidates. The mutant viruses contain a deletion of the leader coding region (LL) rendering the virus attenuated in vivo and negative antigenic markers introduced in one or both of the viral non-structural 3Dpol and 3B proteins. The vaccine platform includes unique restriction endonuclease sites for easy swapping of capsid proteins for different FMDV subtypes and serotypes. The mutant viruses produced no signs of FMD and no shedding of virulent virus in cattle. No clinical signs of disease or fever were observed and no transmission to in-contact animals was detected in pigs inoculated with live A24LL3DYR. Cattle immunized with chemically inactivated vaccine candidates showed an efficacy comparable to a polyvalent commercial FMDV vaccine. These vaccine candidates used in conjunction with a cELISA provide a suitable target for DIVA companion tests.
Owner:US SEC AGRI
Pseudo-rabies gE/gI-gene loss poison strain, killed vaccine containing it and use
ActiveCN1940063AStrong targetingFacilitate chemical processingViral antigen ingredientsViruses/bacteriophagesRabiesPseudorabies Virus PRV
A recombinant Pseudorabies virus PrV gene engineering strain WKQ-001, inactivated vaccine containing the poisonous strain and its use are disclosed. It can be used to discriminate and diagnose artificial immunity pig or natural infectious pig. It's safe and doesn't contain exogenous gene.
Owner:HUAZHONG AGRI UNIV
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695570AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent inactivated vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: screening vaccine strains for the hand-foot-and-mouth disease; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; collecting virus suspension; inactivating the virus; ultra-filtering, concentrating and purifying the virus suspension to obtain vaccine stock solution; and finally preparing the univalent and bivalent inactivated vaccine. The univalent and bivalent inactivated vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Preparation and application of fusion protein and vaccine composition thereof
ActiveCN104262488AShort timeEase of mass productionAntiviralsPharmaceutical non-active ingredientsDiseaseImmunoglobulin Fc Fragments
The invention provides a fusion protein, a porcine epidemic diarrhea vaccine composition containing the fusion protein and application thereof. The fusion protein contains porcine epidemic diarrhea virus antigenic protein and immunoglobulin Fc segment, wherein the porcine epidemic diarrhea virus antigenic protein contains a protein formed by series combination of porcine epidemic diarrhea virus S protein segments. The invention also provides a porcine epidemic virus vaccine composition which contains the fusion protein and a carrier. The invention also provides a preparation method of the vaccine composition and application of the vaccine composition in preparing drugs for preventing and / or treating diseases initiated by porcine epidemic diarrhea virus. The vaccine composition prepared from the fusion protein avoids the technical problem that the porcine epidemic diarrhea virus totivirus can not be easily separated and cultured in the traditional vaccine inactivation process. The fusion protein can utilize the gene engineering technique to perform abundant recombinant expressions, has the advantage of short time consumption, and is convenient for large-scale production.
Owner:PU LIKE BIO ENG
Porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and preparation method thereof
ActiveCN102329746AReduce stressEasy to useAntibacterial agentsBacterial antigen ingredientsHaemophilusAluminium stearate
The invention discloses a porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and a preparation method thereof. The preparation method comprises the following steps of: a, respectively carrying out enrichment culture on a porcine streptococcus strain, a haemophilus parasuis strain and a haemophilus parasuis strain to obtain a porcine streptococcus strain bacterial solution, a haemophilus parasuis strain bacterial solution and a haemophilus parasuis strain bacterial solution; b, respectively adding a formaldehyde solution into the porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, and inactivating; c, mixing the collected porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, adding Tween-80 for preparing a water phase, preparing white oil, Span-80 and aluminium stearate into an oil phase, mixing the water phase with the oil phase to prepare a uniform emulsion, i.e. an oil emulsion inactivating vaccine; and 4, sub-packaging the oil emulsion inactivating vaccine. The porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine can effectively prevent the porcine streptococcus disease and haemophilus parasuis disease, does not have hidden danger of scattering viruses and is safe and reliable; and the immunization is realized by one vaccine, thus the cost is reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
EV-71 virus seed, inactivated vaccine for human and method of producing the same
ActiveCN101402944AEffective immunogenicityGood growth and replication abilityInactivation/attenuationAntiviralsAntigenVaccine Immunogenicity
The invention discloses an EV-71 virus species, a human inactivated vaccine and a preparation method thereof. The classification name of the virus species is intestinal virus 71 with a storage number of CGMCC No.2701. The prepared human EV-71 inactivated vaccine comprises the following components: 100 Mu g / ml of EV-71 alive purified antigen, 0.8-1mg / ml of aluminum hydroxide, and 0.05-0.1mg / ml of merthiolate. The experiments show that the vaccine products have good immunogenicity and safety.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Emulsion breaking method for aftosa oil emulsion inactivated vaccine
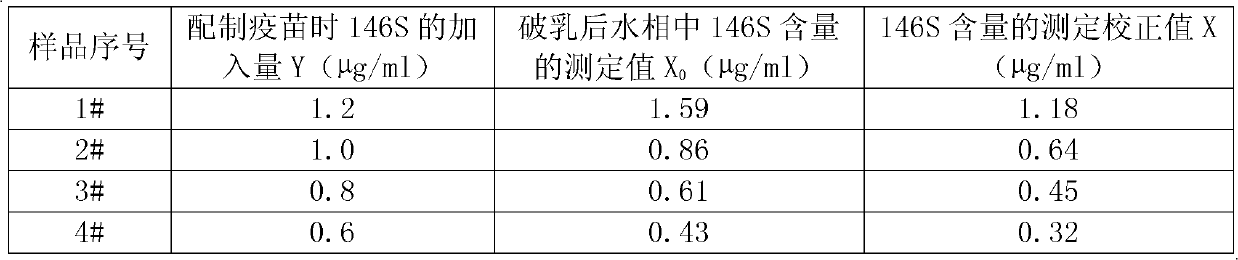
InactiveCN102380232AEasy to separateHigh recovery rateNon-miscible liquid separationAntigenDemulsifier
The invention relates to an emulsion breaking method for an aftosa oil emulsion inactivated vaccine used in an aftosa vaccine quality detection technology. The method comprises the following steps of: fully and uniformly mixing an emulsion breaking agent with an aftosa oil emulsion inactivated vaccine; standing; separating oil from water; and distributing aftosa virus particles into a water phase, wherein the emulsion breaking agent is selected from any of benzyl alcohol, n-amyl alcohol, chloroform and hexyl alcohol serving as organic solvents. Due to the adoption of the method, the completeness of aftosa virus particles 146S is not broken basically, emulsion is broken rapidly and simply, the antigen 146S recovery rate is high, and the stability is high; and the method is particularly suitable to be taken as a method for monitoring the quality of commercial aftosa vaccines for aftosa vaccine producing enterprises and the national quality test departments.
Owner:JINYUBAOLING BIO PHARMA CO LTD
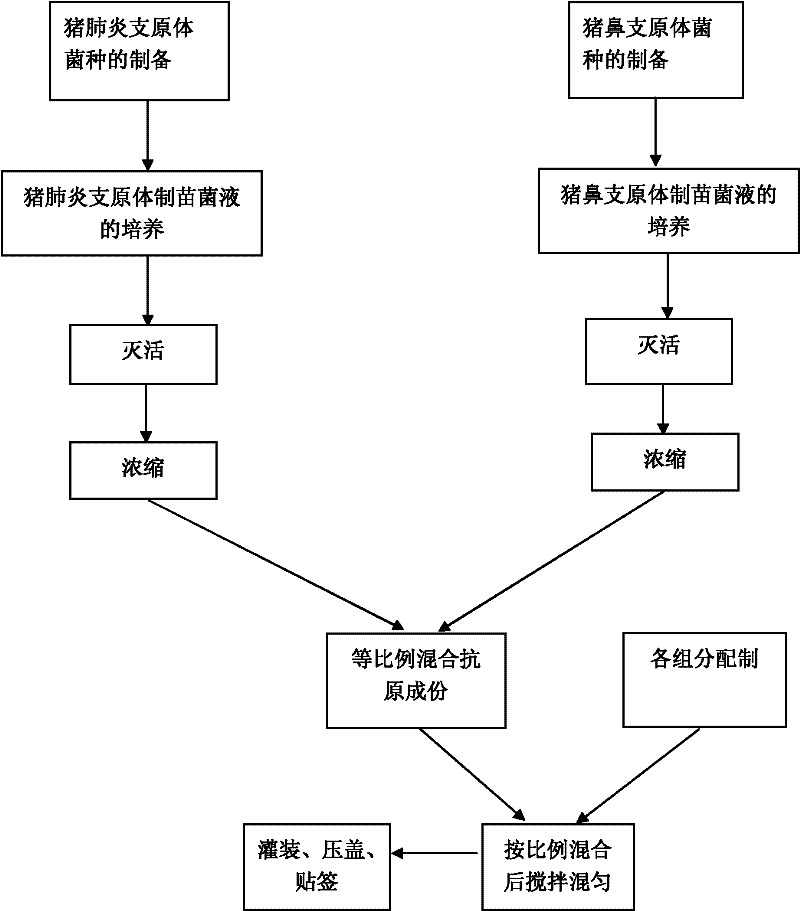
Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
ActiveCN102258776AStrong specificityGood immune effectAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
The invention provides a combined inactivated vaccine against mycoplasma hyopneumoniae (MHP) and mycoplasma hyorhinis. The combined inactivated vaccine contains the MHP and the mycoplasma hyorhinis with preferred content as well as a Carbomer adjuvant with concentration of 10% (V / V). The invention further provides a preparation method of the combined inactivated vaccine against the MHP and the mycoplasma hyorhinis. The preparation method comprises the following steps: preparing production strains; preparing bacterium solution for producing seedlings; and inactivating, concentrating and blending to obtain the vaccine. The combined inactivated vaccine has the advantages of strong specificity and good immunity, thus solving the problem of specific infection caused by the MHP in the current domestic breeding farm and obtaining the mycoplasma hyorhinis vaccine under a blank state at home and abroad at present. The combined inactivated vaccine has the beneficial effects that the step of vaccine inoculation is simplified, trouble caused by a plurality of inoculation and easily produced side effects are avoided, and vaccine cost is saved, thus being especially applicable to preventing andtreating mixed infection diseases in the breeding farms at home and abroad and the like; and compared with the existing single vaccine, application range is widened and immune effect is enhanced.
Owner:PU LIKE BIO ENG
Rhodococcus ruber and application of same as immunologic adjuvant in preparing vaccine
ActiveCN109576180AWill not cause accidental infectionReduce pollutionBacteriaMicroorganism based processesSide effectShort terms
The invention discloses rhodococcus ruber and application of same as an immunologic adjuvant in preparing vaccine. The rhodococcus ruber is also called rhodococcus ruber RDC-01, and the preservation number is CGMCC (China General Microbiological Culture Collection Center) NO. 16640. The rhodococcus ruber disclosed by the invention has the function of increasing and regulating the body immunity andis capable of nonspecifically enhancing the activity of TB (Tuberculosis) lymphocyte, macrophagocyte and NK cells and inducing multiple cell factors such as interferon, and the rhodococcus ruber canbe used as the immunologic adjuvant after being inactivated so as to be added in an oil-adjuvant inactive vaccine, so that generation of an animal antibody induced by the vaccine can be obviously promoted; compared with single use of the oil-adjuvant inactive vaccine, a high-titre antibody can be generated, the use is safe, long-term and short-term toxic and side effects are not generated, and anapplication prospect in the field of preparation of vaccines for animals is good.
Owner:北京利昂盛生物技术有限公司
Porcine epizootic diarrhea virus strain and vaccine composition, preparation method and application thereof
ActiveCN106148287AImprove immune efficiencyImprove securityDigestive systemAntiviralsBiologyDiarrhea
The invention discloses a porcine epizootic diarrhea virus strain with good immunogenicity and an inactivated vaccine prepared through the porcine epizootic diarrhea virus strain. The porcine epizootic diarrhea virus strain is a current epidemic strain, and good immunogenicity and stability are achieved; compared with a commercially available vaccine strain, the vaccine prepared through the strain has the advantages of being good in safety, high in immune protective capability, high in immune efficacy and the like, and porcine epizootic diarrhea can be comprehensively and effectively prevented and treated.
Owner:PU LIKE BIO ENG
Bactrian camel heavy-chain (HC) variable-domain antibody resisting porcine circovirus 2 as well as preparation method and application thereof
ActiveCN102766207AMicrobiological testing/measurementMicroorganism based processesSingle-domain antibodyAmino acid
The invention discloses a bactrian camel heavy-chain (HC) variable-domain antibody resisting a porcine circovirus 2 as well as a preparation method and an application thereof, and belongs to the field of a biotechnology. A PCV2 (Porcine Circovirus Virus) inactivated vaccine immunized bactrian camel is firstly utilized and a phage display technology is utilized to establish a PCV2 immune VHH antibody base; and a high-specificity variable domain antibody of heavy chains of HCAbs (VHH) which has a good affinity with the PCV, particularly a PCV2 virus antigen; and the antibody disclosed by the invention has an amino acid sequence shown as SEQ (sequence) ID NO.1 or an amino acid sequence which is shown as SEQ ID NO.2 and has the sequence isogeny being more than 95%. The PCV2 heavy-chain variable-domain antibody disclosed by the invention has the very important meanings on researching porcine circovirus, particularly researching a porcine circovirus 2-type infection mechanism and establishing rapid antigen detection with high sensitivity and specificity or diagnosing a reagent.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
Canine vaccine for protection against ehrlichiosis
ActiveUS20060188524A1Antibacterial agentsBacterial antigen ingredientsAdjuvantCell mediated immunity
The present invention provides a safe and effective vaccine composition which comprises: an effective immunizing amount of an inactivated Ehrlichia canis bacterin; a pharmacologically acceptable carrier; and an immunogenically stimulating amount of an adjuvant system consisting essentially of an antibody response inducing agent and a cell-mediated immunity response inducing agent. The present invention also provides a method for the prevention or amelioration of canine ehrlichiosis in dogs.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
InactiveCN101732711ANo side effectsAntiviralsViruses/bacteriophagesVirus-like particleMultivalent Vaccine
The invention discloses a nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and preparation method thereof. The vaccine is inactivated vaccine antigen of totivirus, lytic virus, viron or virus-like particles, flue multivalent vaccine antigen is flue pentavalent, namely H1N1, H3N2, B, H5N1 and A (H1N1) or multivalent vaccine antigen combined on the basis at will, or flue multivalent vaccine antigen obtained by containing all the combination of the HA selecting from H1, H2, H3, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15 and H16 and the NA selecting from N1, N2, N3, N4, N5, N6, N7, N8 and N9 subtypes on the basis. The content of flu multivalent inactivated vaccine antigen HA in the vaccine of the invention is 1.0-15.0 Mug / 0.2ml / per person, and the vaccine of the invention can effectively prevent routine human flue, high pathogenicity H5N1 avian-human flu, influenza A (H1N1) and infection of other subtype influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
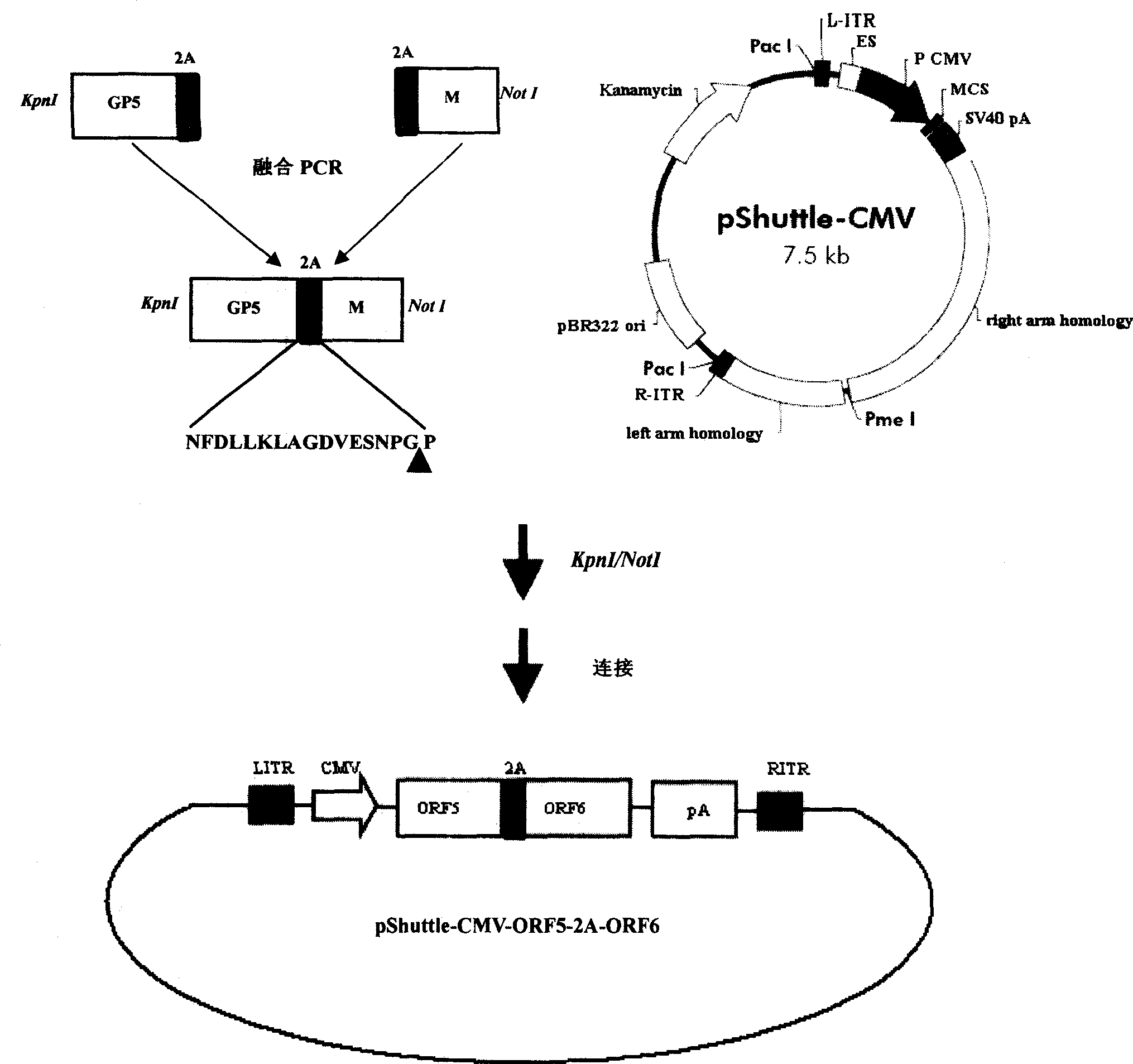
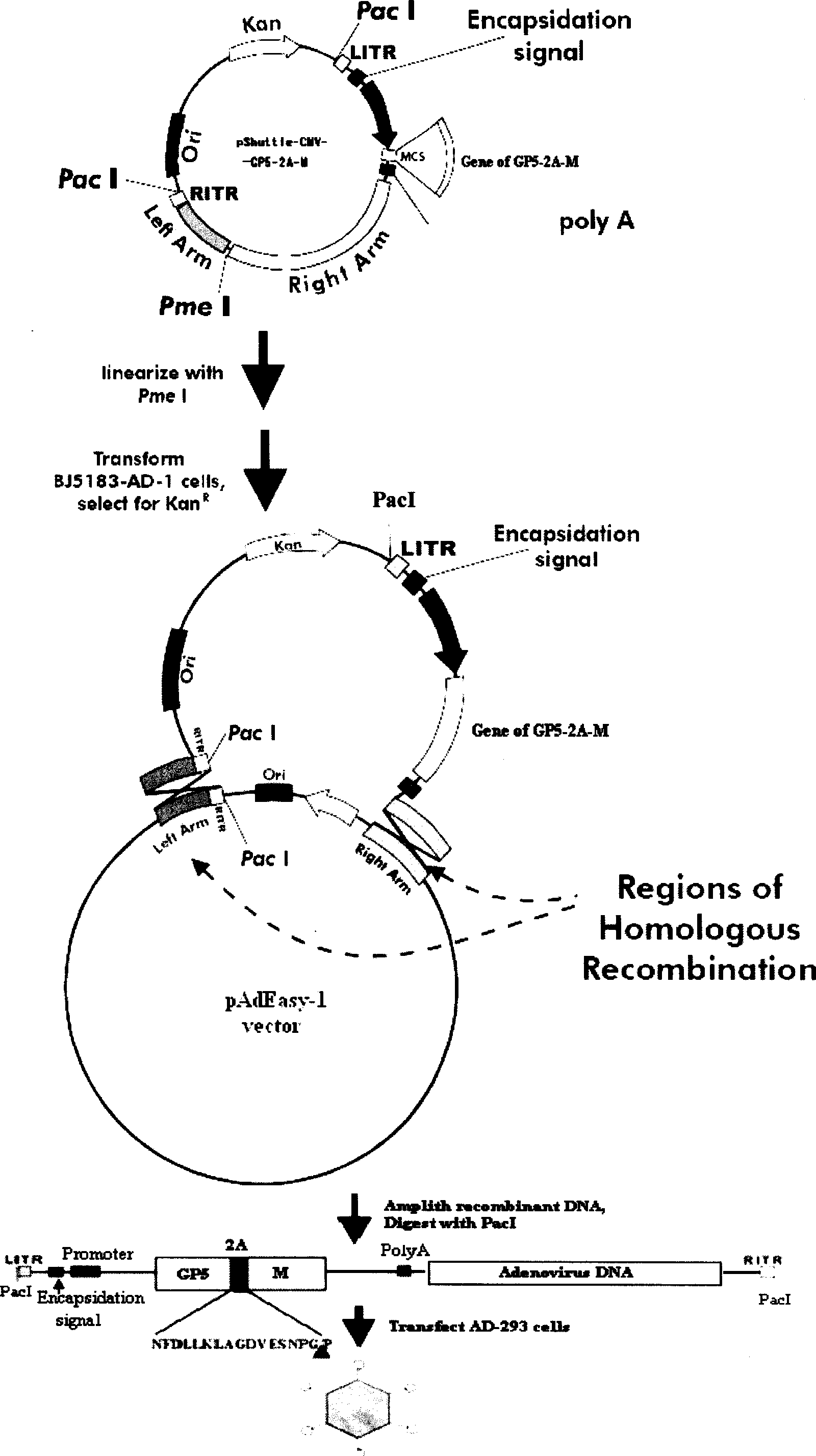
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151AStrengthen cellsEnhance humoral immune stimulationAntibacterial agentsBacterial antigen ingredientsCholesterolVaccine antigen
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com