Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Totivirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Totivirus is a genus of viruses, in the family Totiviridae. Fungi serve as natural hosts. There are currently seven species in this genus including the type species Saccharomyces cerevisiae virus L-A.
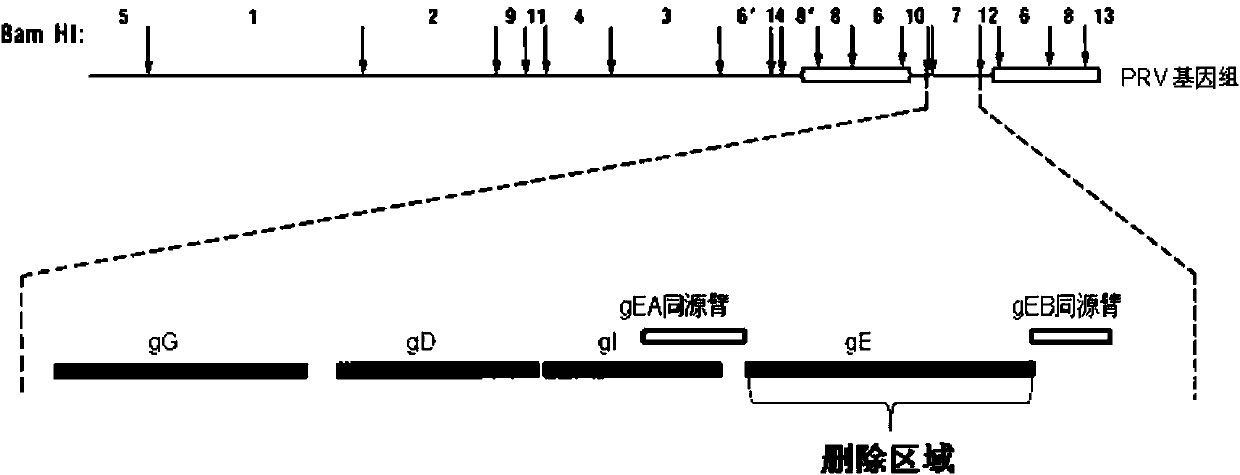
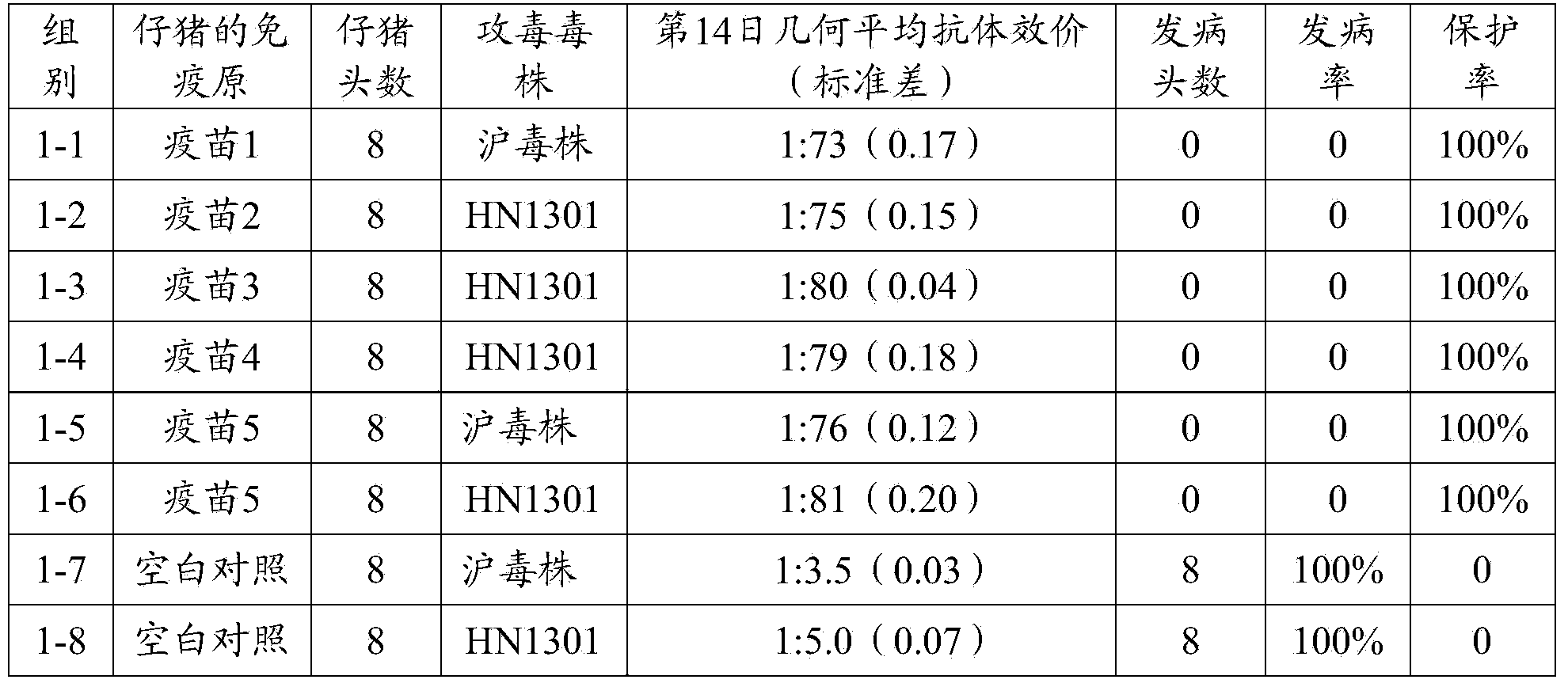
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
ActiveCN103923884ASymptoms relieved or improvedMicroorganism based processesAntiviralsVirus antigenTGE VACCINE
The invention provides a porcine pseudorabies virus gene deletion strain, a vaccine composition, and a preparation method and an application of the vaccine composition. The vaccine composition comprises an immunizing dose of an attenuated livetotivirus antigen and an inactivated totivirus antigen of the porcine pseudorabies virus gene deletion strain or its culture. The vaccine composition can effectively induce the antibody production, can effectively protect pigs, and can be used as a marking vaccine to effectively differentiate wild strains and vaccine strains.
Owner:PU LIKE BIO ENG
Preparation and application of fusion protein and vaccine composition thereof
ActiveCN104262488AShort timeEase of mass productionAntiviralsPharmaceutical non-active ingredientsDiseaseImmunoglobulin Fc Fragments
The invention provides a fusion protein, a porcine epidemic diarrhea vaccine composition containing the fusion protein and application thereof. The fusion protein contains porcine epidemic diarrhea virus antigenic protein and immunoglobulin Fc segment, wherein the porcine epidemic diarrhea virus antigenic protein contains a protein formed by series combination of porcine epidemic diarrhea virus S protein segments. The invention also provides a porcine epidemic virus vaccine composition which contains the fusion protein and a carrier. The invention also provides a preparation method of the vaccine composition and application of the vaccine composition in preparing drugs for preventing and / or treating diseases initiated by porcine epidemic diarrhea virus. The vaccine composition prepared from the fusion protein avoids the technical problem that the porcine epidemic diarrhea virus totivirus can not be easily separated and cultured in the traditional vaccine inactivation process. The fusion protein can utilize the gene engineering technique to perform abundant recombinant expressions, has the advantage of short time consumption, and is convenient for large-scale production.
Owner:PU LIKE BIO ENG
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
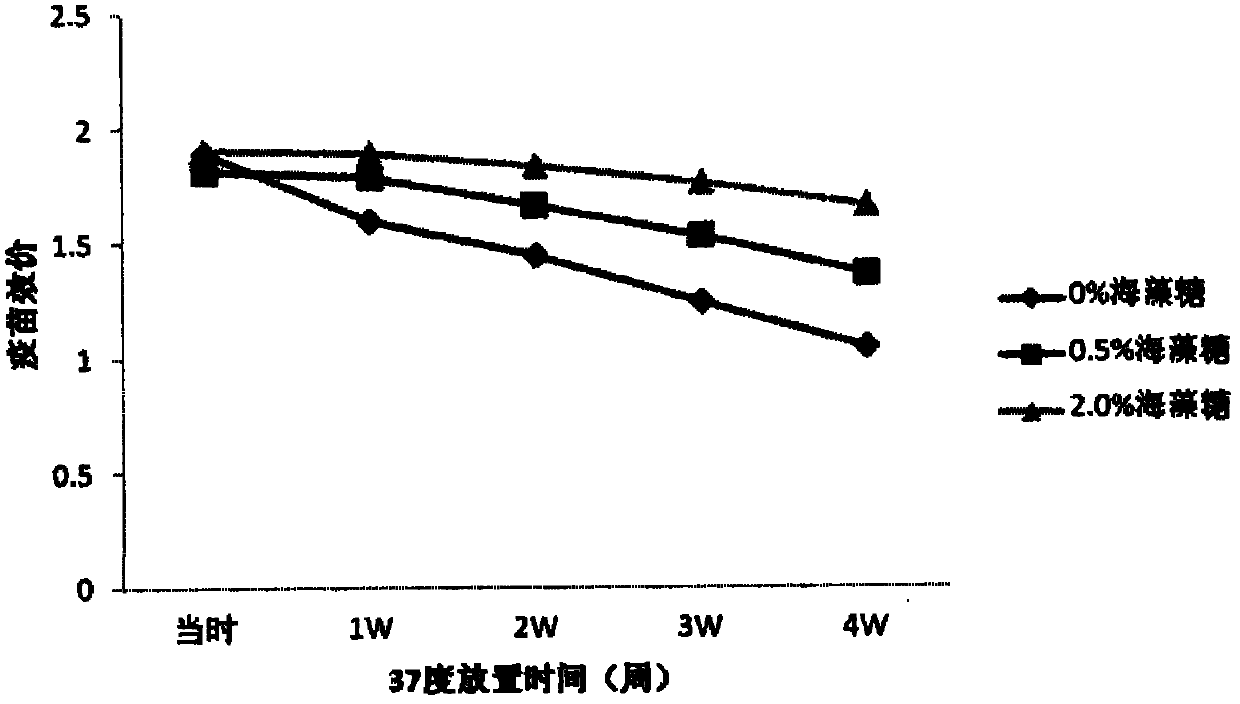
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
InactiveCN101732711ANo side effectsAntiviralsViruses/bacteriophagesVirus-like particleMultivalent Vaccine
The invention discloses a nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and preparation method thereof. The vaccine is inactivated vaccine antigen of totivirus, lytic virus, viron or virus-like particles, flue multivalent vaccine antigen is flue pentavalent, namely H1N1, H3N2, B, H5N1 and A (H1N1) or multivalent vaccine antigen combined on the basis at will, or flue multivalent vaccine antigen obtained by containing all the combination of the HA selecting from H1, H2, H3, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15 and H16 and the NA selecting from N1, N2, N3, N4, N5, N6, N7, N8 and N9 subtypes on the basis. The content of flu multivalent inactivated vaccine antigen HA in the vaccine of the invention is 1.0-15.0 Mug / 0.2ml / per person, and the vaccine of the invention can effectively prevent routine human flue, high pathogenicity H5N1 avian-human flu, influenza A (H1N1) and infection of other subtype influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
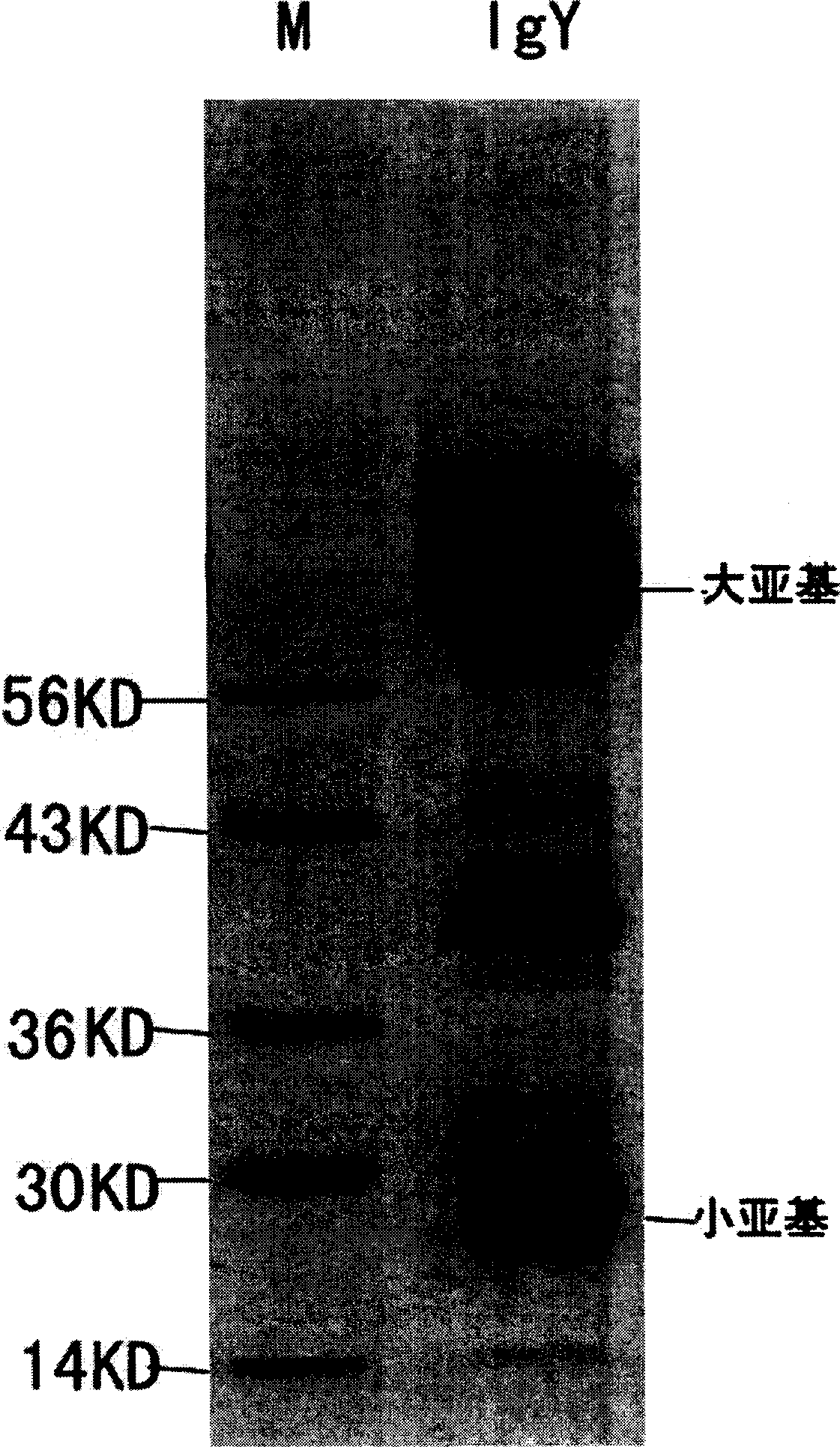
Yolk antibody of anti SARS coronavirus and its preparation method and liquid preparation
InactiveCN1556113AEasy to prepareFast preparation methodEgg immunoglobulinsImmunoglobulins against virusesYolkEpitope
A yolk antibody for SARS coronavirus is prepared through injecting the antigen, which may be recombinant genetic protein S, M, or E,or the antigen epitope for said protein able to represent SARS coronavirus, or the synthetic polypeptide of said protein, etc, in health hen for primary immunizing, booster immunizing, collecting its egg, extracting yolk, and extracting the yolk antibody from it. It can also be prepared to become liquid preparation. It can be used to prevent SARS.
Owner:BIOINFORBODY
Enterovirus 71 type specific recombinant protein antigen and application thereof
InactiveCN102558313AIncreased sensitivityImprove featuresBacteriaMicroorganism based processesSerodiagnosesType specific
Owner:SOUTHEAST UNIV
Large-scale full-suspension culture method of porcine circovirus type 2
ActiveCN107312746AReduce dosageImprove immunityViral antigen ingredientsMicroorganism based processesEngineeringPorcine kidney
The invention discloses a large-scale full-suspension production method of a porcine circovirus type 2. The inventors of the invention domesticate a porcine kidney cell adaptable to large-scale serum-free full-suspension culture, which is named as sPK15-YP, is collected in the China General Microbiological Culture Collection Center and has the culture collection number of CGMCC NO.13846. The sPK15-YP cell adaptable to full-suspension culture is used for achieving serum-free large-scale culture of the porcine circovirus type 2 (PCV2), so that the conventional spinner bottle culture technology is replaced, the human resource is reduced, the product quality is improved, and the bottleneck that the virus content is low during PCV2 full-virus culture is solved; by a full-suspension sPK15-YP cell technology, a high-potency PCV2 semifinished product is prepared; by a hollow fiber method, a PCV2 virus culture solution is concentrated and purified to obtain a more pure PCV2 virus concentrated antigen. By the large-scale full-suspension production method, a solid foundation is laid for studying a PCV2 multivalent vaccine, the dosage of the vaccine is reduced, the stress of a swine herd is reduced and the immune level of the swine herd is enhanced.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Reagent kit for LgG immunoblotting diagnosis of SARS coronavirus antibody
The present invention relates reagent kit for IgG immunoblotting diagnosis of SARS coronavirus antibody. The reagent kit consists of solid film, antiantibody of enzyme label and corresponding chromogenic substrate. The solid film is obtained through purifying SARS coronary totivirus lysate liquid antibody or recombining mixed antigen, gel electrophoresis to separate and transferring to film. The antiantibody of enzyme label is compounded with rabbet's antihuman IgG labeled with horseradish peroxidase or sheep's antihuman IgG labeled with alkaline phosphatase, 0.01 % concentration thimerosal as protecting agent, ox serum protein in 1 wt% and PBS in pH 7.4 in 0.01 M. The present invention may be used in detecting several kinds of antigen and antibody in clinical test department and sanitary epidemic prevention department.
Owner:GUANGZHOU WONDFO BIOTECH
Antigen-antibody complex for preventing and/or treating avian influenza
ActiveCN101732716AChange submission pathEnhance immune functionAntiviralsAntibody ingredientsAvian influenza virusOrganism
The invention provides an antigen-antibody complex for preventing and / or treating avian influenza, which comprises inactivation avian influenza totiviruses which are used as antigen and an immune body thereof, and the mass concentration ratio of the antigen and the immune body is more than 1. After entering organisms to perform initial immunization, the antigen-antibody complex stimulates the organisms again to induce immunoreaction, and immune response is quick in speed and strong in reaction; the antigen-antibody complex used as a carrier is more favorable for capturing and presenting antigen presenting cells and can also strengthen a breeder reaction of T cells effectively; purified totiviruses used as the antigen increase the molecular weight of the complex, are more favorable for ingestion of immunocyte of the organisms on the antigen, cause the more extensive immunoreaction quickly, and have a significance for preventing avian influenza viruses of which the antigen is easy for variation. A preparation of the antigen-antibody complex does not need to use solid carriers, does not cause intense stimulus on the organisms or initiates the organisms to generate an adverse reaction, and is simple to prepare and safe and convenient to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Kit for detecting capripoxvirus serum antibody based on synthetic peptide
The invention discloses a kit for detecting a capripoxvirus serum antibody based on a synthetic peptide. A preparation method of the kit comprises the following steps of 1, designing a short-peptide aiming at a capripoxvirus main immunogene P32, 2, synthesizing a peptide fragment by a conventional peptide synthesis technology, 3, preparing the kit for detecting a capripoxvirus serum antibody based on a synthetic peptide, and 4, optimizing conditions and finishing the preparation of the kit for detecting a capripoxvirus serum antibody. A coating antigen adopted by the kit is an external synthetic immunoactive peptide fragment, reduces the danger of totvirus use and prevents virus diffusion and escape. The synthetic peptide adopted by the kit has high purity, has immunological characteristics similar to capripoxvirus particles, and can replace capripoxvirus particles and be used as an antigen for detection and thus the kit realizes sensitive, specific, safe and reliable detection of a capripoxvirus serum antibody.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Visible protein chip for detecting poultry disease serum antibody, its preparation method and application
The invention discloses a visual protein chip for detecting serum antibody of new-castle disease virus of chickens, infectious bronchitis virus of chickens, avian influenza virus and infectious bursal disease virus of chickens , which is prepared by the following steps: purifying and diluting whole proteins of the four virus respectively; pointing samples of the positive control serum, the negative control serum and the four virus proteins onto a chip carrier respectively; drying, fixing, sealing and washing the samples to obtain the visual protein chip. The visual protein chip uses the purified whole proteins as capturing antigens to detect the virus-specific antibodies in chicken serum so as to simplify the preparation technology and reduce the production cost, and the visual protein chip has better specificity but no cross, has high reliability of results and has the advantages of quickness, simplicity and convenience, high sensitivity, good specificity and the like. When the serum is diluted by 6,400 times, the visual protein chip still can detect the antibodies, the sensitivity is 400 times of that of the prior AGP detection method. According to the detection to serum samples in-place, the detection rate of the visual protein chip is higher than the proir AGP method remarkably.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Lyophilized inactivated Japanese encephalitis vaccine
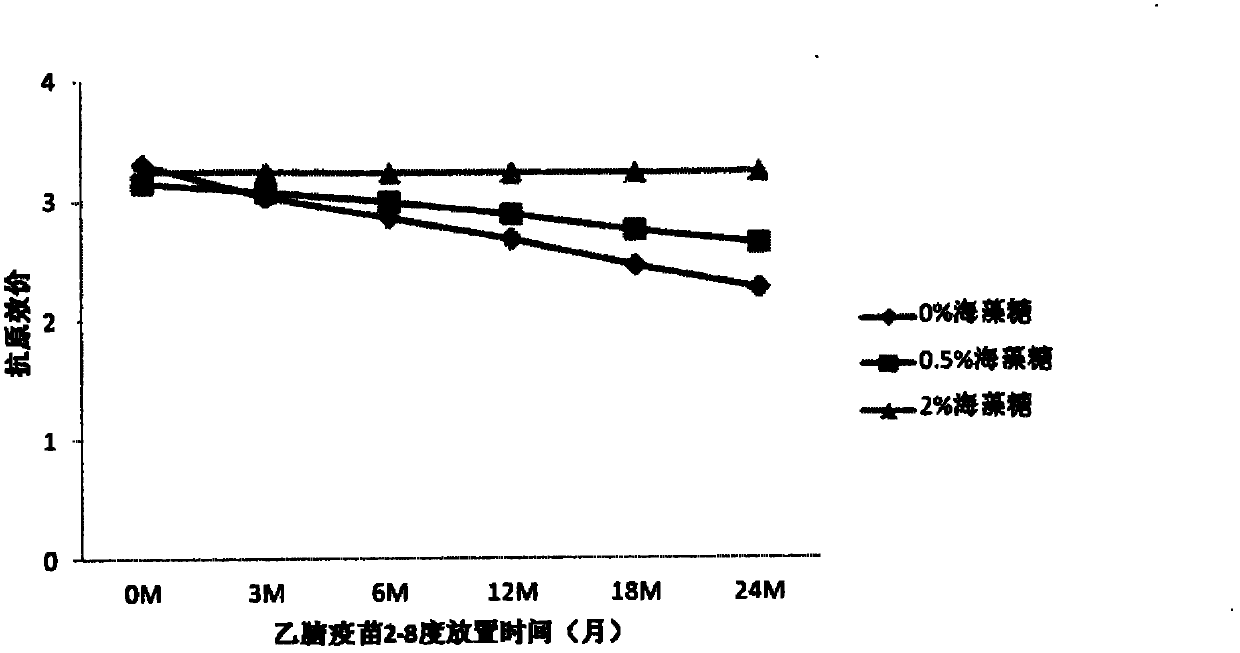
ActiveCN102631672AMeet quality requirementsQuality improvementPowder deliveryViral antigen ingredientsJapanese encephalitis vaccineLactose
The invention provides a lyophilized inactivated Japanese encephalitis vaccine, comprising: (a) inactivated Japanese encephalitis totivirus; (b) stabilizer, which comprises maltose, lactose, and optionally mycose, mannite, sorbitol and / or amino acid; and optionally (c) buffer, surfactant, isotonic regulator and / or chelating agent.
Owner:天津嘉诚顺隆商贸有限公司
Method for detecting classical swine fever virus specific antibody in pig saliva
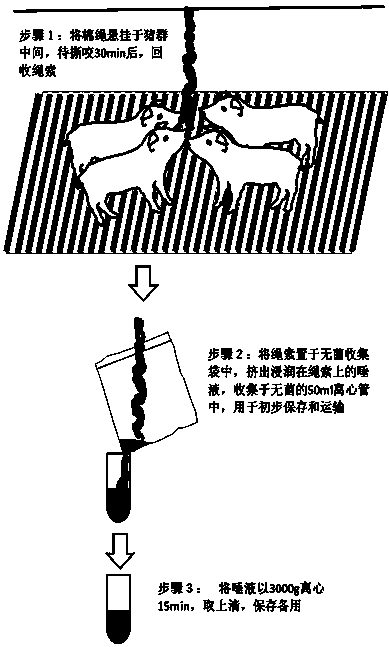
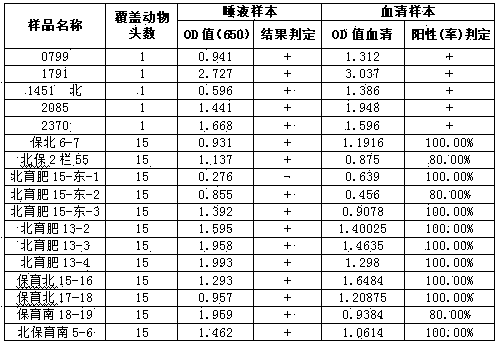
InactiveCN103995119AAvoid the problem of blood samplingPerform large area samplingMaterial analysisClassical swine fever virus CSFVBlood collection
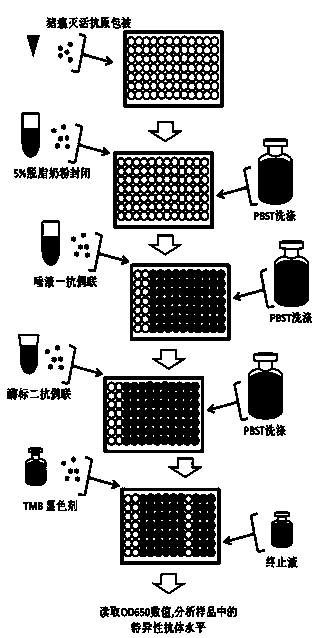
The present invention discloses a method for detecting classical swine fever virus specific antibody in pig saliva. The method comprises: 1) preparing classical swine fever totivirus antigen, inactivating, and coating a 96-well polystyrene reaction plate; 2) adopting a cotton rope suspending method, collecting saliva, and treating to obtain a saliva first antibody; 3) adding the saliva first antibody to an antigen coated reaction plate according to the number, carrying out a 22-28 DEG C saturated humidity reaction for 1 h, and washing; 4) sequentially adding horseradish peroxidase-labeled goat anti-pig IgG-FC antibody to the reaction plate, carrying out a 22-28 DEG C saturated humidity reaction for 30 min under a dark condition, and washing; 5) mixing TMB coloring agents solution A and solution B according to a ratio of 1:1, sequentially adding to the reaction plate, carrying out a reaction under a dark condition for 15 min, and adding a termination solution; and 6) placing into an enzyme-labeling measuring instrument, reading the OD650 data, and determining the result. According to the present invention, the problem of single animal blood collection is avoided, and the method is suitable for large scale investigation of classical swine fever antibody in pig herd.
Owner:杭州贝尔塔生物技术有限公司
Recombinant classical swine fever virus NS3 protein, ELISA kit and application of kit
The invention provides a recombinant classical swine fever virus (CSFV) NS3 protein, an ELISA kit and the application of the kit. The recombinant CSFV NS3 protein is prepared by the following steps: firstly, cloning the NS3 coding sequence in a complete genome sequence of CSFV into a pET32a-Intein-ELK16 plasmid vector, expressing, then performing inductive cutting, and obtaining an recombinant CSFV NS3 protein by collecting supernatant in a centrifugation manner, wherein the amino acid sequence of the recombinant CSFV NS3 protein is shown in SEQ ID NO: 1; secondly, establishing an ELISA kit based on the recombinant CSFV NS3 protein so as to realize the application of identification of a CSFV vaccine and a recombinant adenovirus vaccine. The recombinant CSFV NS3 protein is high in protein purification efficiency, good in reactivity, low in cost, and simple in operating process, can realize large-scale production, is matched with recombinant adenovirus for use, so as to distinguish antibodies generated by totivirus and a recombinant adenovirus stimulated body, which has an important significance of rapid detection of NS3 antibodies.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
Hepatitis C virus and its external cell culture method
An external culture system of hepatitis C totivirus for culturing complete multiplicative HCV virus by the molecular biology and gene recombination technique is disclosed. Its culture steps include amplifying the full-length genom containing 98 nucleotides after 3' PolyA in the serum of hepatitis C patient; artificial site-directed mutation to NS5A and NS5B in HCV genon; inserting the expression box of marker gene IRES-GFP at NS5B3' terminal in the mutated HCV genom; transfecting sensitive cell and culturing to obtain progeny HCV virus with infection activity.
Owner:西安金昊科技投资管理有限公司
Porcine pseudorabies virus gene-deleted strain, vaccine composition and their preparation method and application
InactiveCN105368791ASymptoms relieved or improvedMicroorganism based processesAntiviralsRabiesMarker vaccine
The invention provides a vaccine composition, comprising porcine pseudorabies virus gE gene-deleted strain of immunizing dose, or an attenuated live totivirus antigen or inactivated totivirus antigen of the culture thereof. The vaccine composition is effective in inducing generation of antibodies to provide effective protection for swine and can be used as a marker vaccine to effectively distinguish wild strains and vaccine strains.
Owner:PULIKE BIOLOGICAL ENG INC
Rabies virus monoclonal antibody and application thereof
InactiveCN103954777AHigh neutralization potencyHigh detection sensitivityBiological material analysisBiological testingVaccine ProductionGlycoprotein G
The invention relates to a rabies virus monoclonal antibody with neutralization activity and capability of specifically recognizing rabies virus glycoprotein G. The monoclonal antibody is authenticated to be IgG1-subtype. According to the rabies virus monoclonal antibody disclosed by the invention, rabies virus inactivation totivirus is taken as an immunogen for immunizing a mouse, an antibody with neutralization activity is screened, the neutralization titer is high, the secretion of hybridoma for the antibody is stable, and the antibody is easy to store; and an in-vitro preparation link for glycoprotein is avoided, and innovativeness is achieved. A colloidal gold test paper card or test paper strip prepared from the antibody is high in test sensitivity and capable of achieving 0.03 IU / mL, capable of being used for test on human rabies virus or quality control in a vaccine production process, and high in application value.
Owner:北京凯思百奥科技发展有限公司
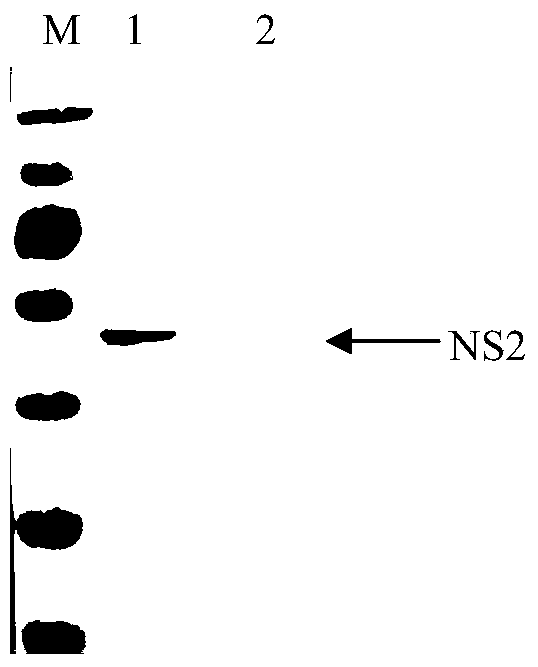
Bluetongue virus NS2 protein monoclonal antibody BTV-4D4, B cell epitope recognized thereby and applications
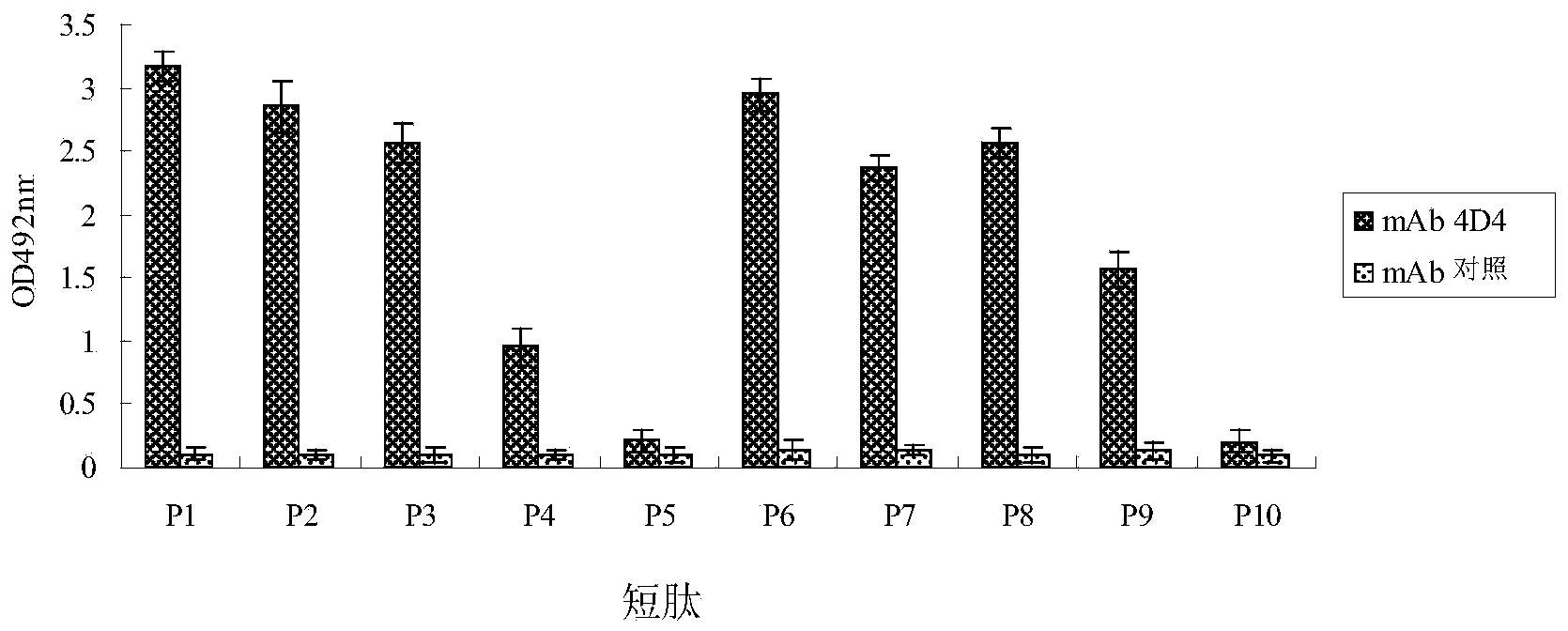
The invention discloses a kind of bluetongue virus (BTV) NS2 protein monoclonal antibody BTV-4D4, a B cell epitope recognized thereby and applications. BALB / c mice are subjected to totivirus immunization by utilization of bluetongue virus serotype 8 type (BTV8), splenic lymphocytes of the mice after immunization and SP2 / 0 cells are fused. A hybridoma cell strain BTV-4D4 secreting anti-BTV-NS2 protein monoclonal antibodies steadily is obtained after screening with BTV 8 as a coating antigen through an indirect ELISA method. The monoclonal antibodies secreted from the hybridoma cell strain and the BTV-NS2 protein are subjected to a specific reaction. The B cell epitope recognized by BTV-4D4 is 149RSNYDV154 after identification by utilization of a phage display technology. The monoclonal antibody and the BTV-NS2 protein B cell epitope recognized by the monoclonal antibody can be used for diagnosis or prevention of bluetongue virus infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of soluble I-type duck hepatitis virus 3D protein to preparation of ELISA reagent and ELISA kit
ActiveCN104297493AStrong specificityGood reproducibilityBiological testingDuck hepatitis A virusAntigen
The invention discloses an application of a soluble I-type duck hepatitis virus 3D protein to preparation of an ELISA reagent and an ELISA kit. The ELISA kit comprises a soluble I-type duck hepatitis virus 3D protein coated reaction plate, an enzyme-labeled antibody, a developing solution and a stop solution. When being used for detecting I-type duck viral hepatitis antibodies, the ELISA kit is strong in specificity, good in reproducibility and high in sensitivity, and has a higher agreement rate than the ELISA kits taking totivirus as coating antigen in the aspect of detection, so that the ELISA kit can be used for the detection of clinical samples.
Owner:SICHUAN AGRI UNIV
Fusion protein sHA1-Fc and application
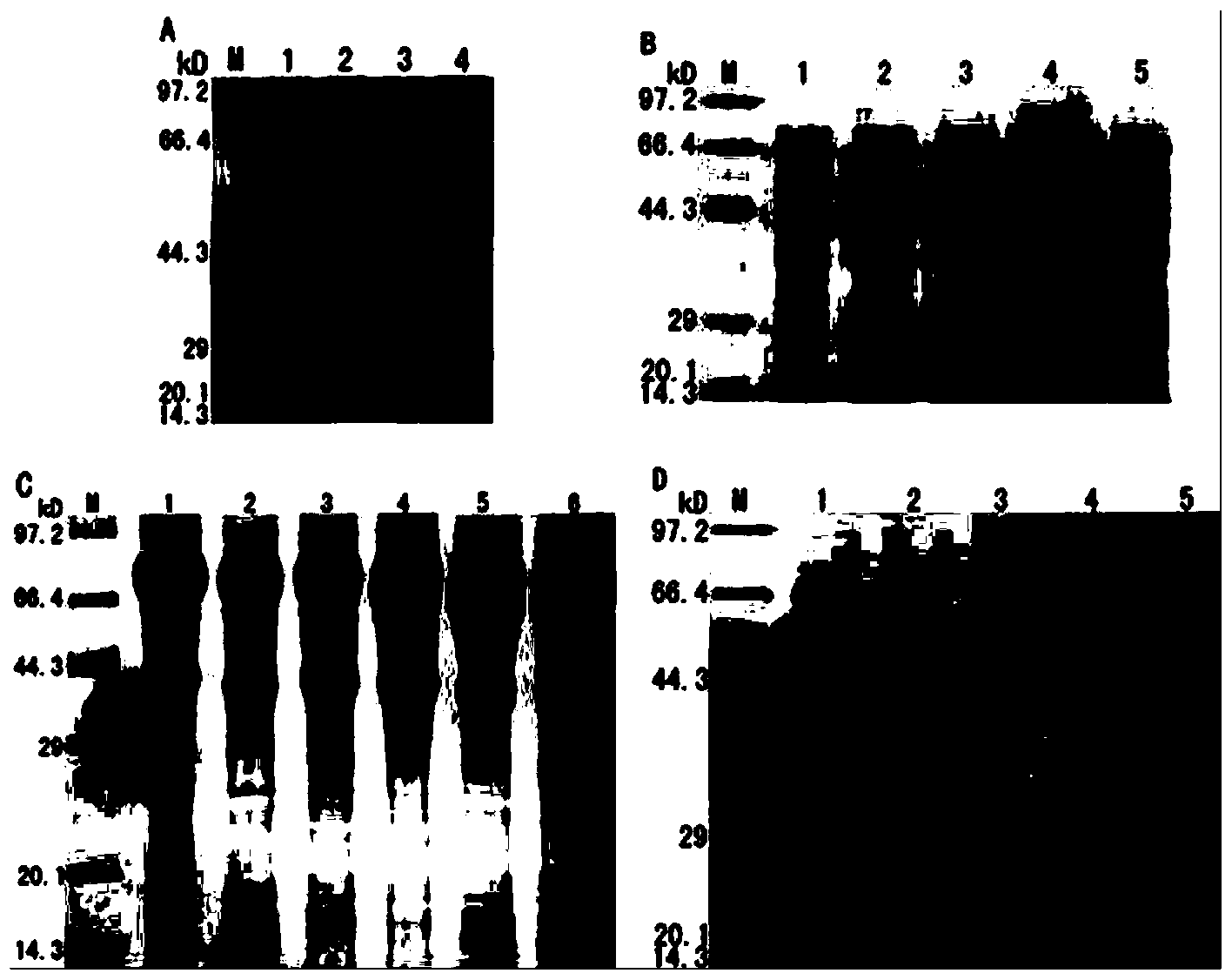
InactiveCN106117369AProtect animal organismsSimple and efficient operationSsRNA viruses negative-senseAntibody mimetics/scaffoldsSpecific immunityFc fragment
The invention discloses fusion protein sHA1-Fc and an application. The fusion protein sHA1-Fc of a highly pathogenic AIV (avian influenza virus) H5N1 and a chicken IgY Fc fragment is expressed by a Bacto-Bac expression system, has a molecular structure similar to IgY, and can penetrate a respiratory tract mucosal barrier of a chicken to enter the body under transfer of FcRy expressed in epithelial cells. A sequence of an sHA1-Fc fusion gene is represented as SEQ ID NO:1. The fusion protein is supplemented with a mucosal adjuvant and inoculates the chicken through nasal inhalation, thereby being capable of triggering an organism to generate effective mucosal immunity and triggering systemic immunity of the organism. Therefore, the organism is enabled to obtain anti-AIV local specific immunity and systemic immunity, the immune effect of the fusion protein is better than that of a commercial inactivated virus vaccine, and a new strategy is provided for development of a novel mucosal vaccine for poultry bird flu and other infectious diseases.
Owner:HUAZHONG AGRI UNIV
H3N2 and H3N8 subgenetype dog influenza divalent inactivated vaccine and preparation method and application thereof
ActiveCN110680912ASsRNA viruses negative-senseViral antigen ingredientsVirus influenzaVaccine antigen
The invention discloses an H3N2 and H3N8 subgenetype dog influenza divalent inactivated vaccine and a preparation method and application thereof, and provides a dog influenza virus vaccine. The vaccine comprises a vaccine antigen which is totivirus bivalent inactivated vaccine antigen consisting of (1) or (2): (1) A / canine / Quanzhou / 1224-1109 / 2018(H3N2)CGMCCNO.18180 and an H3N8 subgenetype dog influenza virus influenza virus strain; and (2)a recombinant virus bivalent inactivated vaccine antigen consisting of a recombinant H3N2 influenza virus obtained by A / canine / Quanzhou / 1224-1109 / 2018(H3N2)CGMCC and a recombinant H3N8 subgenetype influenza virus strain obtained by the H3N8 subgenetype influenza virus strain. The H3N2 and H3N8 subgenetype dog influenza totivirus bivalent inactivated vaccine and the recombinant virus(H3N2 / PR8+H3N8 / PR8) bivalent inactivated vaccine both have favorable protective effects on different branch H3N2 subgenetype dog influenza virus and H3N8 subgenetype dog influenza virus.
Owner:CHINA AGRI UNIV
Adenovirus vector bivalent vaccine for simultaneously preventing avian influenza H5 and H9 subtypes and preparation method of adenovirus vector bivalent vaccine
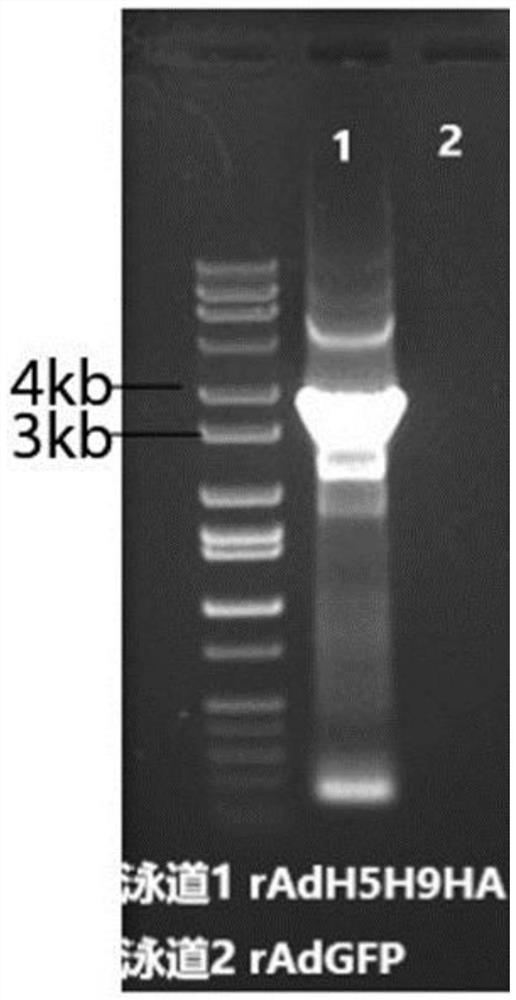
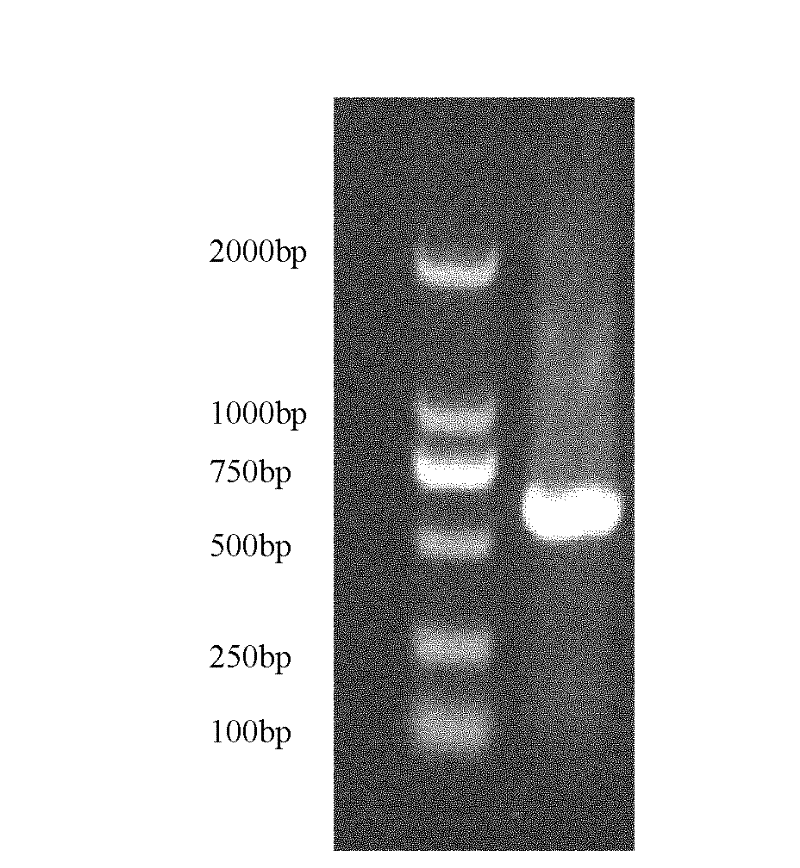
ActiveCN112111503AEfficient use ofShorten the production cycleSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininShuttle vector
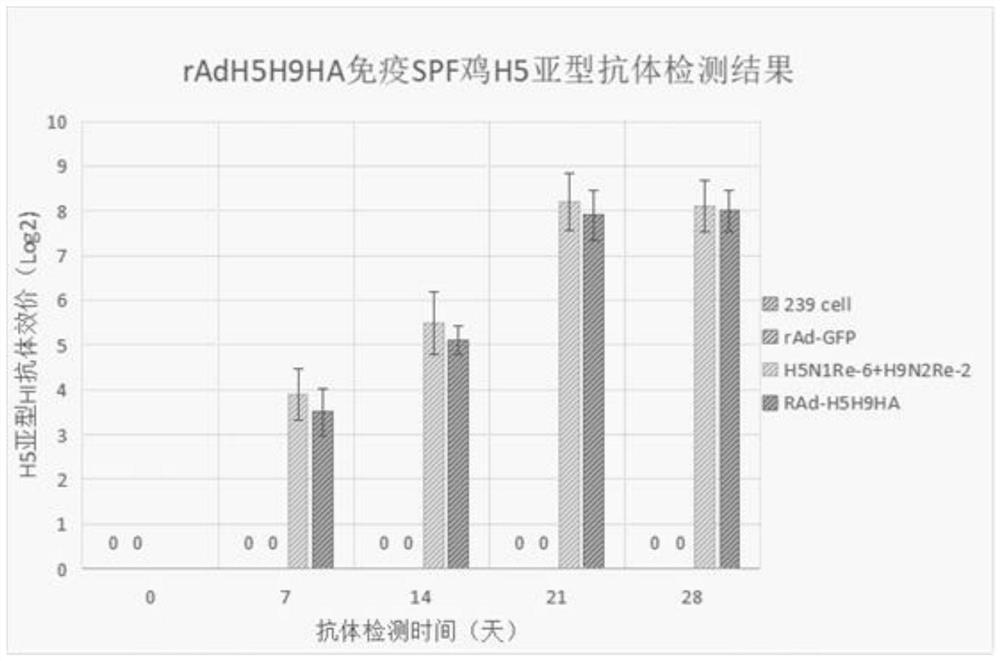
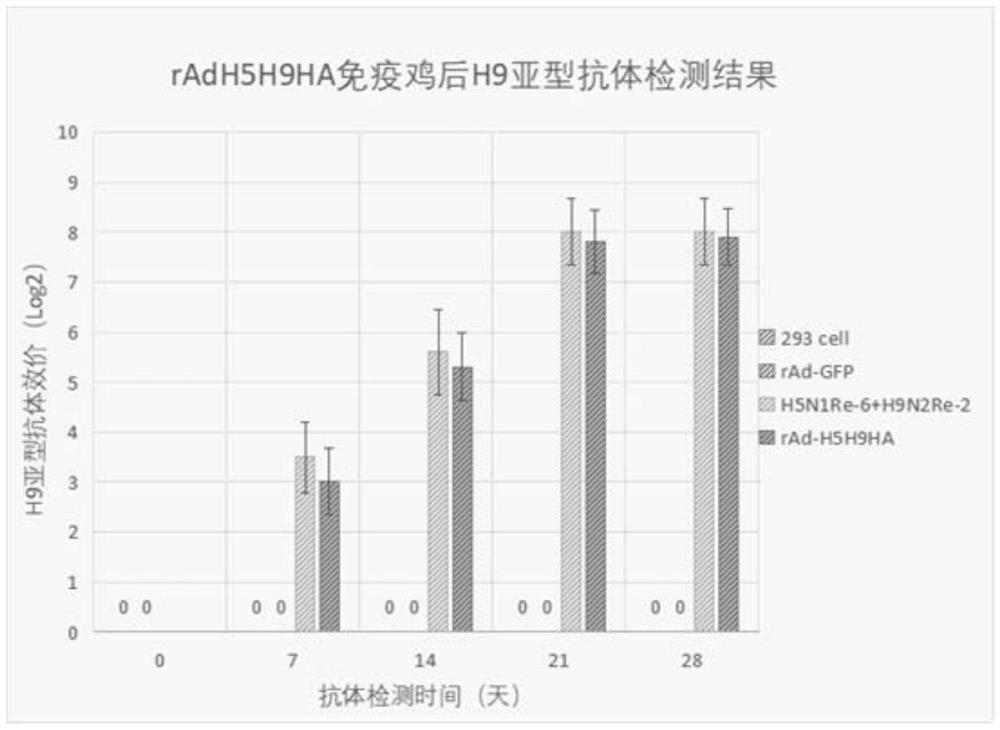
The invention provides an adenovirus vector bivalent vaccine for simultaneously preventing avian influenza H5 and H9 subtypes and a preparation method of the adenovirus vector bivalent vaccine. Hemagglutinin antigens of the avian influenza H5 subtype and H9 subtype are used as core antigens to synthesize a fusion gene H5H9HA, an adenovirus is used as a vector, the fusion gene H5H9HA is cloned to an adenovirus shuttle vector pDC315, then the fusion gene H5H9HA and skeleton plasmids pBHGlox (delta) E1 and 3Cre are co-transfected into 293 cells to complete recombinant adenovirus production; and then through amplification and purification, the high-purity recombinant adenovirus rAd-H5H9HA carrying the hemagglutinin antigens is obtained, and through freeze-drying and packaging post-treatment, an adenovirus vector bivalent vaccine capable of preventing avian influenza H5 and H9 subtypes at the same time is obtained. The vaccine can induce a high-level H5 subtype specific antibody and a high-level H9 subtype specific antibody, the level of the antibodies is consistent with that of an existing commercial totivirus inactivated vaccine and exceeds the immune standard specified by China, andthe vaccine has huge application prospects and popularization value in the field of avian influenza immunoprophylaxis.
Owner:河北省动物疫病预防控制中心 +1
Restrictive replicated west nile virus system for expressing green fluorescent protein and application thereof
ActiveCN106754982AStrong indicatorImprove securityCompound screeningSsRNA viruses positive-senseAnti virusAntiviral drug
The invention discloses a restrictive replicated west nile virus system for expressing green fluorescent protein (GFP) and an application thereof. The restrictive replicated west nile virus system for expressing the green fluorescent protein, disclosed by the invention, comprises a west nile virus restrictive replicon plasmid and a recombinant cell line capable of stably expressing west nile virus C-prM-E protein. A restrictive replicated west nile virus (deltaWNV) obtained by the system is obtained, and the virus has properties similar with a WNV whole virus in a BWNV-CME cell line; the GFP can be expressed and the system has a visual effect; more importantly, the virus only can be replicated in the system; when other cells are infected, progeny viruses are not generated, so that the safety is extremely high; and the restrictive replicated west nile virus system can be used for detecting a WNV neutralizing antibody and screening an anti-virus medicine. According to the restrictive replicated west nile virus system, the safety and convenience of WNV related operation are improved, and the system does not depend on BSL-3 high-grade biological safety condition; and a good technical means is provided for WNV vaccine evaluation, anti-virus medicine screening and researches of a virus pathogenic mechanism.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antigen protein and encoding gene and applications thereof
InactiveCN102174088AImprove featuresIncreased sensitivityVirus peptidesMicroorganism based processesDiseaseInclusion bodies
The invention discloses an antigen protein and encoding gene and applications thereof. The amino acid sequence of the antigen protein is shown in the SEQ ID NO.1. The antigen protein relates to deficiency virus antigen, has good safety and does not contain unrelated impurity protein; the antigen protein is only bound with the antibody of the rabbit hemorrhagic disease virus (RHDV) in serum and can not perform cross reaction with the positive serums of other rabbit diseases, thus the antigen protein has higher sensitivity and specificity. By adopting the genetic engineering means, the antigen protein is obtained through cloning and expression; and in the expression process, the antigen protein is accumulated in the host strain in an inclusion mode and is easy to separate and purify, thus the industrial production can be performed.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Preparation method and application of anti-PCV2 monoclonal antibody
ActiveCN108752471AStrong specificityHigh monoclonal antibody purityImmunoglobulins against virusesHybrid cell preparationEtiologyHeavy chain
The invention discloses a preparation method and application of an anti-PCV2 monoclonal antibody. The anti-PCV2 monoclonal antibody, and DNA sequences and amino acid sequences of the heavy chain variable region and the light chain variable region of the antibody are disclosed. The preparation method of the antibody is designed. The antibody is applied in an antigen / antibody detection kit, antigen / antibody immunochromatography test paper, IFA, IPMA and a PCV2 or PCV2 Cap immunoaffinity column. The antibody can simultaneously recognize two major epidemic subtypes which are PCV2a and PCV2b, and has good reactogenicity with PCV2 totivirus and PCV2 Cap protein, but is free of cross reaction with PCV1 and PCV3 subtypes and porcine viruses, and lays a foundation for the study of etiology and pathogenesis of PCV2 and clinical detection of PCV2 pathogens.
Owner:河南中泽生物工程有限公司
Method for preparing porcine circovirus type 2 high-sensitivity cell line
InactiveCN103497932AHigh titerFacilitate the study of pathogenic mechanismMicrobiological testing/measurementMicroorganism based processesVirus CultivationGene silencing
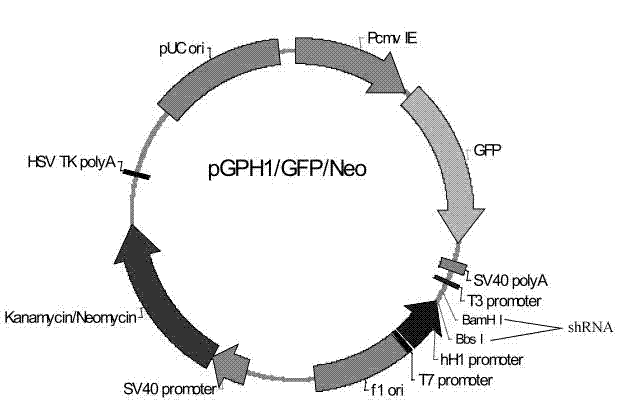
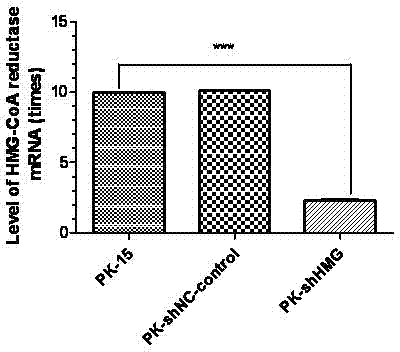
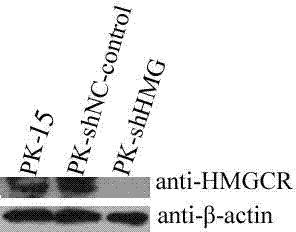
The invention provides a cell line highly sensitive to a porcine circovirus type 2 (PCV2) and discloses a preparation method of the cell line. After the PCV2 is adopted to infect PK-15 cells, expression of 3-hydroxyl-3-HMG-CoAreductase (HMGCR) is obviously changed; and after HMGCR genes are subjected to silence, TCID50 (tissue culture infectious dose 50) of the PCV2 reaches 108.5 / ml. An HMGCR gene silence cell line can be obtained by RNA (ribonucleic acid) interfering technology, and the cell line highly sensitive to the PCV2 can be finally obtained through screening. By utilizing HMGCR gene silence cells prepared by the method to culture the PCV2, high-titer viruses can be obtained, and studying on pathogenesis of the PCV2, virus culturing and development and industrial production of totivirus inactivated vaccines are facilitated.
Owner:JILIN UNIV
Method for detecting IFA neutralizing antibody of PCV2
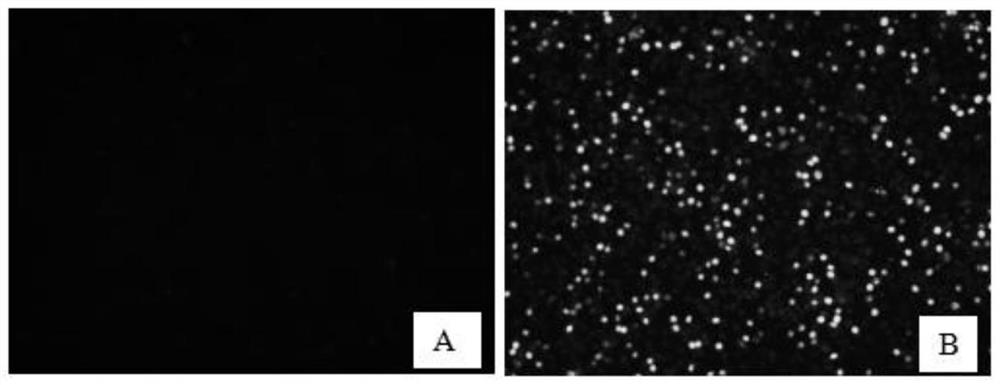
The invention discloses a method for detecting an IFneutralizing antibody of PCV2, and belongs to the technical field of biology. According to the method for detecting the IFA neutralizing antibody of the PCV2, disclosed by the invention, a cell reaction plate can be prepared in advance and can be stored for a long time at 20 DEG C; and reaction results can be observed by a fluorescence microscope. IFA detection has the characteristics of strong specificity, high sensitivity and simple operation; and in addition, the IFA method utilizes live virus inoculation, uses whole viruses, enables an antigen structure to be more complete, and enables results to be more accurate and representative.
Owner:XINXIANG UNIV
H5 subtype avian influenza vaccine strain capable of distinguishing immunized and infected animals, and preparation method and application thereof
ActiveCN108103084AImprove compatibilityEasy to copyPolypeptide with localisation/targeting motifSsRNA viruses negative-senseHighly pathogenicAnimal husbandry
The invention discloses an H5 subtype avian influenza vaccine strain capable of distinguishing immunized and infected animals, and a preparation method and application thereof. Highly-pathogenic H5 subtype avian influenza brings in great economic loss to the animal husbandry and poses a severe threat to public health. Although conventional H5 subtype avian influenza totivirus inactivated vaccineshave the advantages of definite effect, low cost, a wide application scope and the like, but cannot serologically distinguish immunized and infected animals, which brings in great obstacles for monitoring and purifying of avian influenza. According to the invention, the NA of B type influenza is used as a label so as to successfully establish the method for constructing the H5 subtype avian influenza vaccine strain capable of distinguishing immunized and infected animals. The H5 subtype avian influenza vaccine strain has critical application value and public health significance to prevention,control and purification of H5 subtype avian influenza.
Owner:ZHEJIAN DIFFERENCE BIOLOGICAL TECH CO LTD
Rabies virus antibody test paper, preparation method thereof and detection method thereof
ActiveCN109959789AEliminate distractionsAvoid false positivesBiological testingAgainst vector-borne diseasesNeutralizing antibodyRabies Virus Antigen
The invention discloses rabies virus antibody test paper and a preparation method thereof and a detection method thereof, which belong to the technical field of antibody test paper. The problems of false positive risk and safety hazard in the prior art are solved. The test paper comprises, from one end to the other end, a sample pad which coats a rabies virus antigen, a gold standard pad which coats a monoclonal antibody against the rabies virus antigen, a nitrocellulose membrane which coats a detection line and a quality control line, and an absorbent pad, wherein the sample pad, the gold standard pad, the nitrocellulose membrane and the absorbent pad overlap each other and are attached to the upper surface of a backboard. The rabies virus antigen coated by the sample pad is rabies virus-like particles. According to the invention, the used labeled antigen is the rabies virus-like particles expressed by insect cells, which avoids false positives; the monoclonal antibody against the rabies virus G protein competes with a neutralizing antibody in the blood to improve the detection accuracy and sensitivity; the virus-like particles are free of nucleic acid components; and biosafety risks produced by the use of whole viruses as marker antigens are eliminated.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Rotavirus vaccine and preparation method thereof
InactiveCN103182078ALess side effectsFast immune responseViral antigen ingredientsAntiviralsAdjuvantRotavirus RNA
The invention provides an inactivated vaccine for a human rotavirus, wherein the vaccine contains capsid protein produced from cracking of rotavirus totivirus particles, and the viral protein in each dose of the inactivated vaccine for the human rotavirus is 100-200 [mu]g, and the aluminum phosphate adjuvant is 0.5 mg per dose. Compared with an existing virus purification stoste, the impure protein in the inactivated vaccine for the human rotavirus is greatly reduced, and the rotavirus vaccine has higher immunogenicity and potency, is safer to use and is more applicable to large-scale production.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com