Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1572 results about "Peptide fragment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptide fragments refer to fragments of proteins that are used to identify or quantify the source protein. Often these are the products of enzymatic degradation performed in the laboratory on a controlled sample, but can also be forensic or paleontological samples that have been degraded by natural effects.
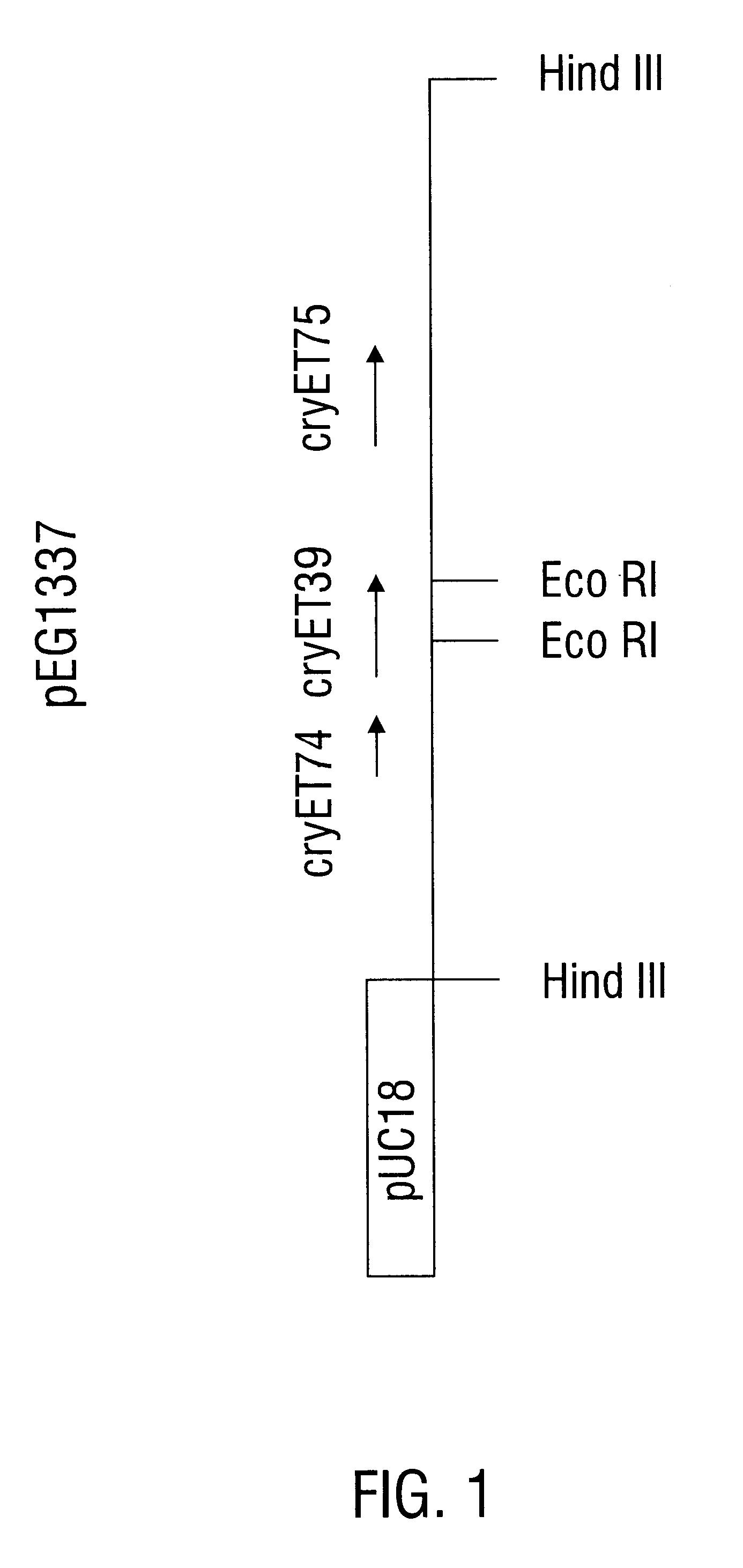
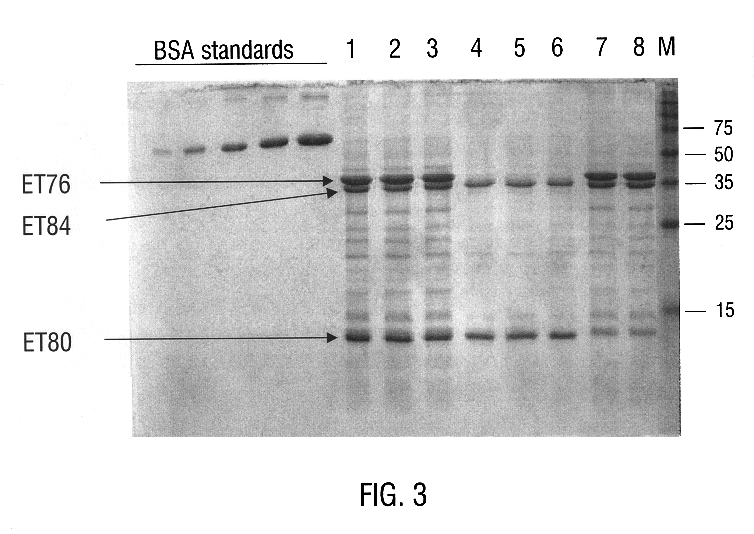
Polypeptide compositions toxic to diabrotic insects, and methods of use
InactiveUS6468523B1Easy to storeInhibit microbial growthBiocidePeptide/protein ingredientsDelta endotoxinPolynucleotide
Disclosed is a novel Lepidopteran- and Coleopteran-active delta-endotoxin polypeptide, and compositions comprising the polypeptide, peptide fragments thereof, and antibodies specific therefor. Also disclosed are vectors, transformed host cells, and transgenic plants that comprise nucleic acid segments encoding the polypeptide. Also disclosed are methods of identifying related polypeptides and polynucleotides, methods of making and using transgenic cells comprising the novel sequences of the invention, as well as methods for controlling an insect population, such as the Western Corn Rootworm and Colorado potato beetle, and for conferring to a plant population resistance to the target insect species.
Owner:MONSANTO TECH LLC
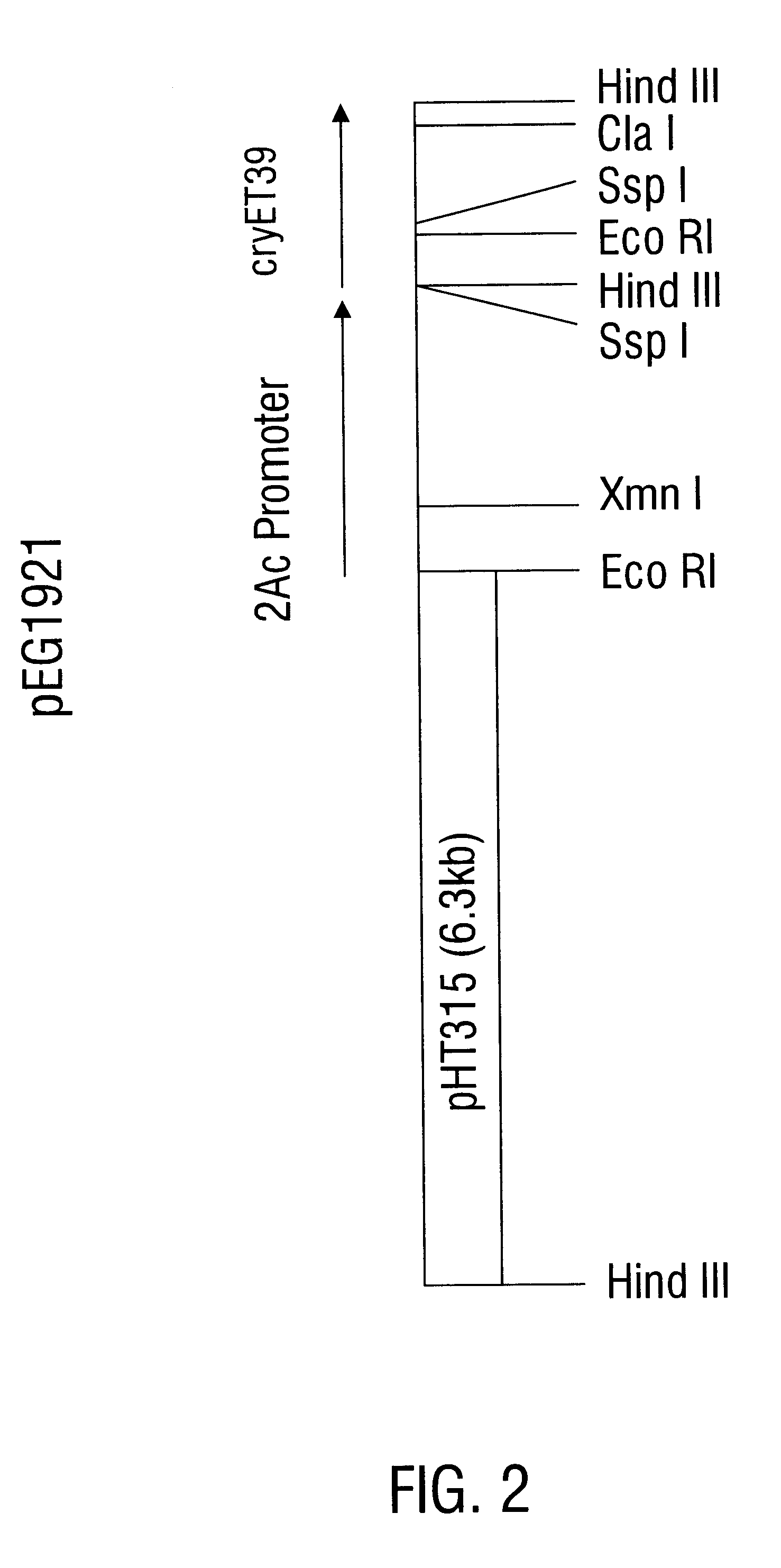
Coleopteran-toxic polypeptide compositions and insect-resistant transgenic plants
InactiveUS6555655B1Easy to storeInhibit microbial growthBiocideBacteriaDelta endotoxinPolynucleotide
Disclosed are novel insecticidal polypeptides, and compositions comprising these polypeptides, peptide fragments thereof, and antibodies specific therefor. Also disclosed are vectors, transformed host cells, and transgenic plants that contain nucleic acid segments that encode the disclosed delta-endotoxin polypeptides. Also disclosed are methods of identifying related polypeptides and polynucleotides, methods of making and using transgenic cells comprising these polynucleotide sequences, as well as methods for controlling an insect population, such as Colorado potato beetle, southern corn rootworm and western corn rootworm, and for conferring to a plant resistance to a target insect species.
Owner:MONSANTO TECH LLC
High sensitivity quantitation of peptides by mass spectrometry
InactiveUS20040072251A1Loss of substantial specific binding capacityReduce complexitySamplingComponent separationChemical structureProtein target
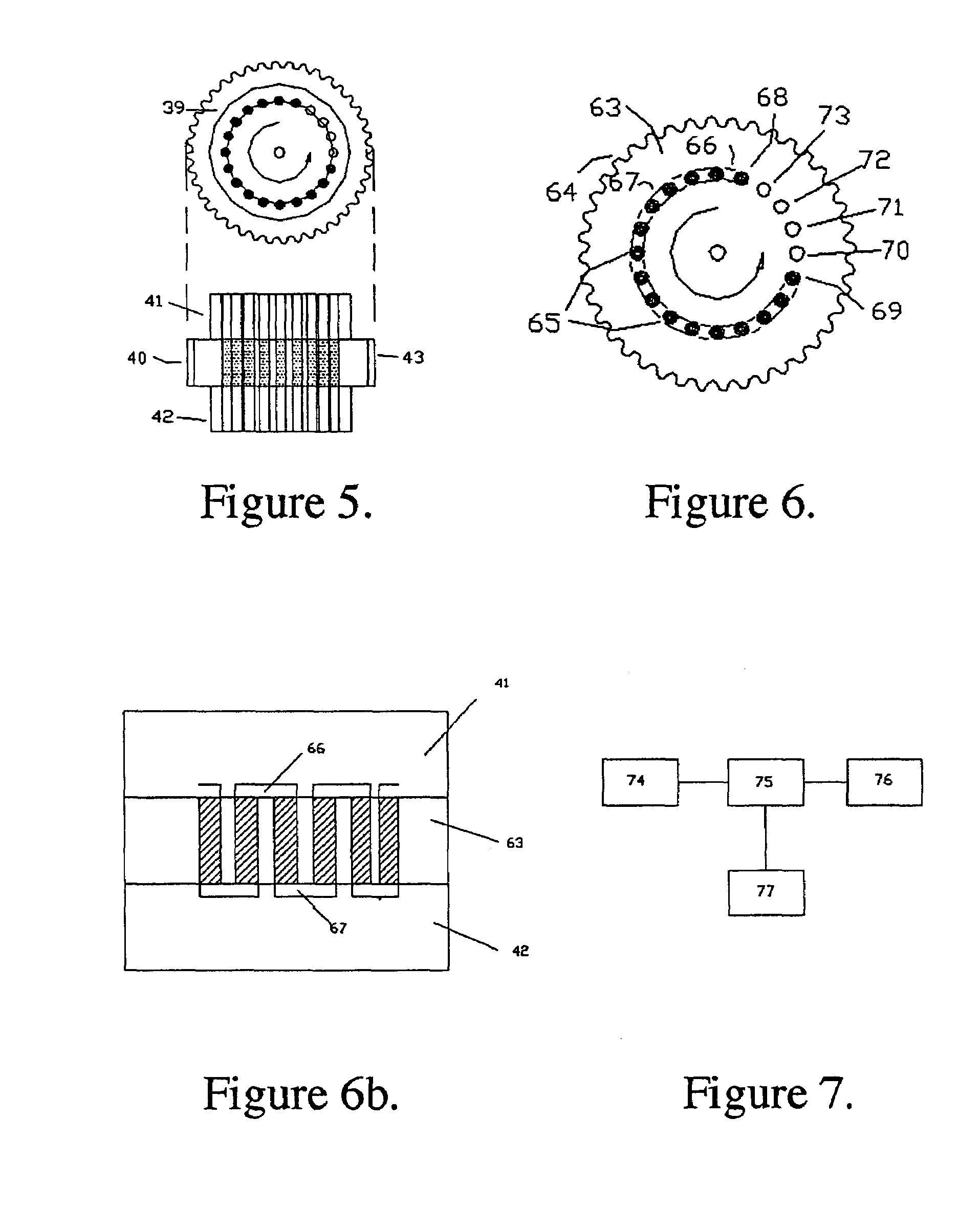
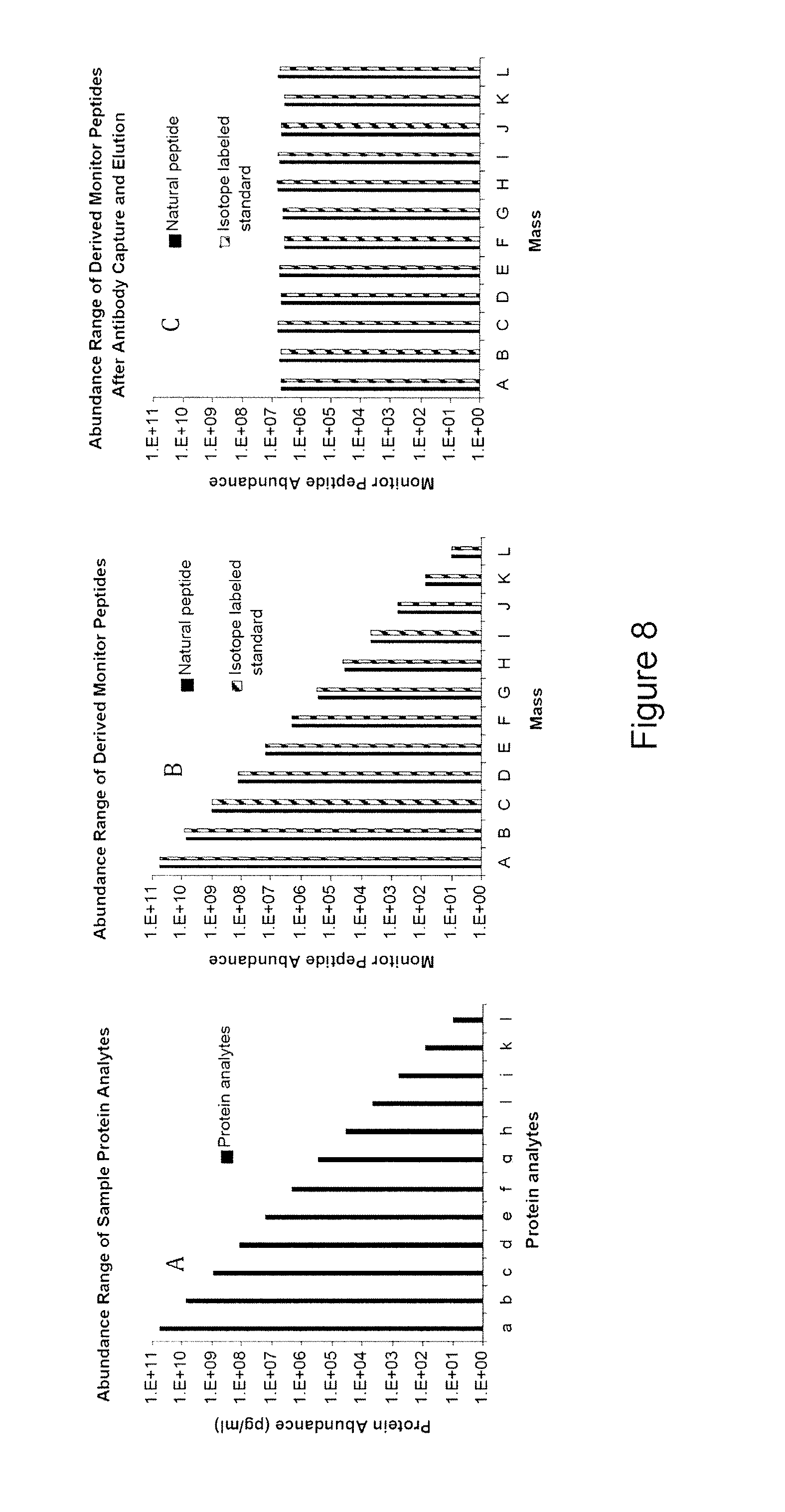
The instant invention provides an economical flow-through method for determining amount of target proteins in a sample. An antibody preparation (whether polyclonal or monoclonal, or any equivalent specific binding agent) is used to capture and thus enrich a specific monitor peptide (a specific peptide fragment of a protein to be quantitated in a proteolytic digest of a complex protein sample) and an internal standard peptide (the same chemical structure but including stable isotope labels). Upon elution into a suitable mass spectrometer, the natural (sample derived) and internal standard (isotope labeled) peptides are quantitated, and their measured abundance ratio used to calculate the abundance of the monitor peptide, and its parent protein, in the initial sample
Owner:ANDERSON FORSCHUNG GROUP
Human BNP-specific antibodies
Owner:SCIOS
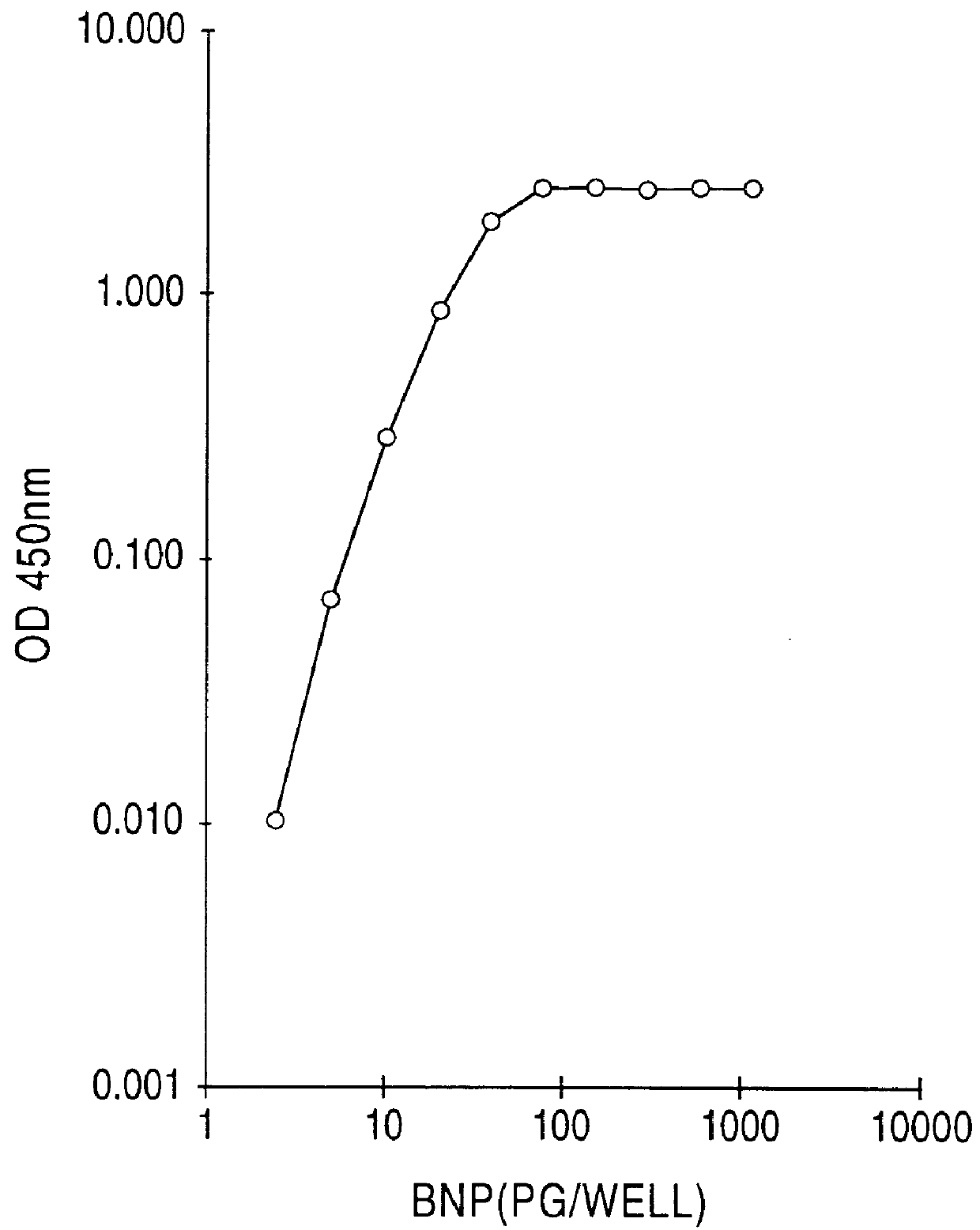
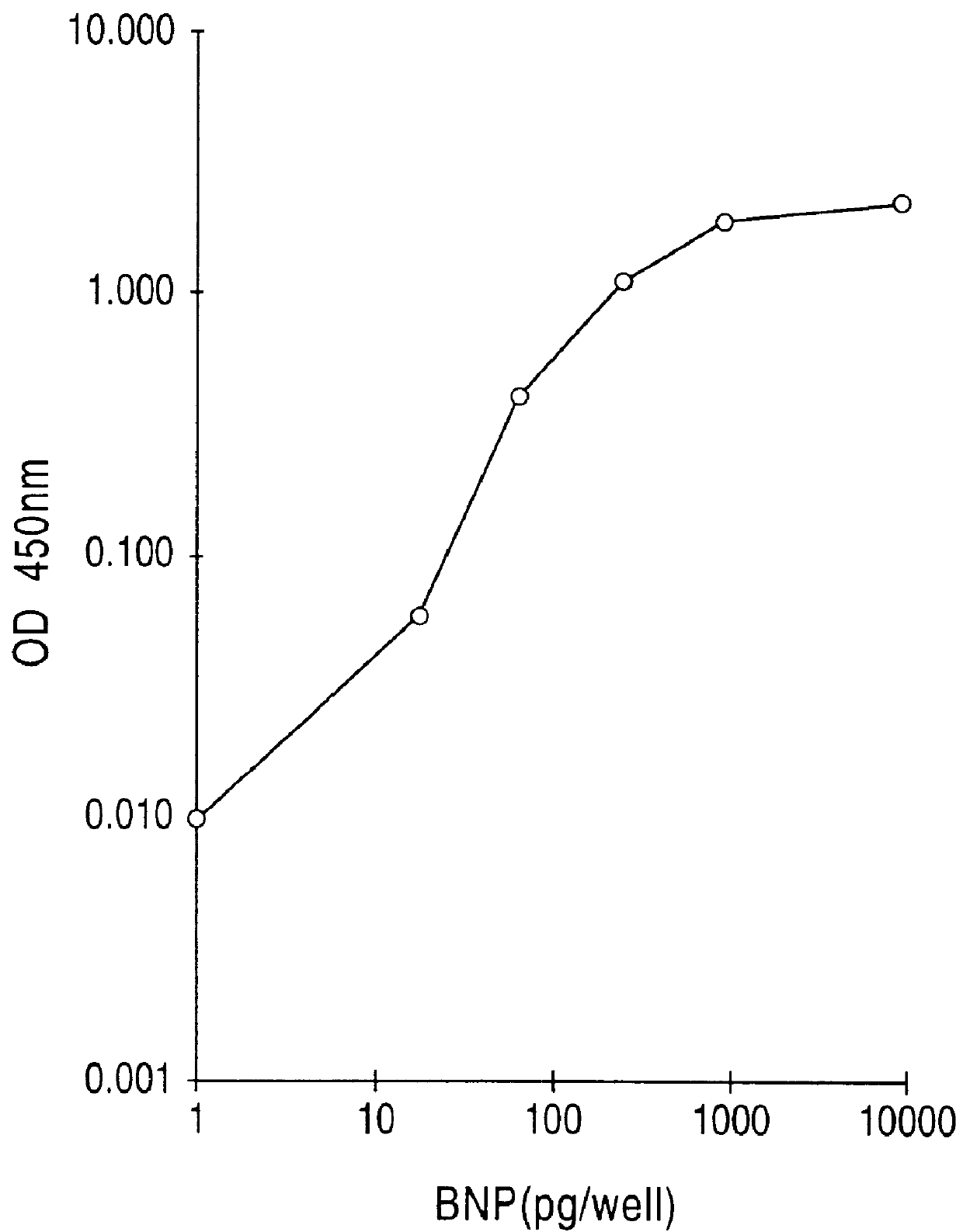
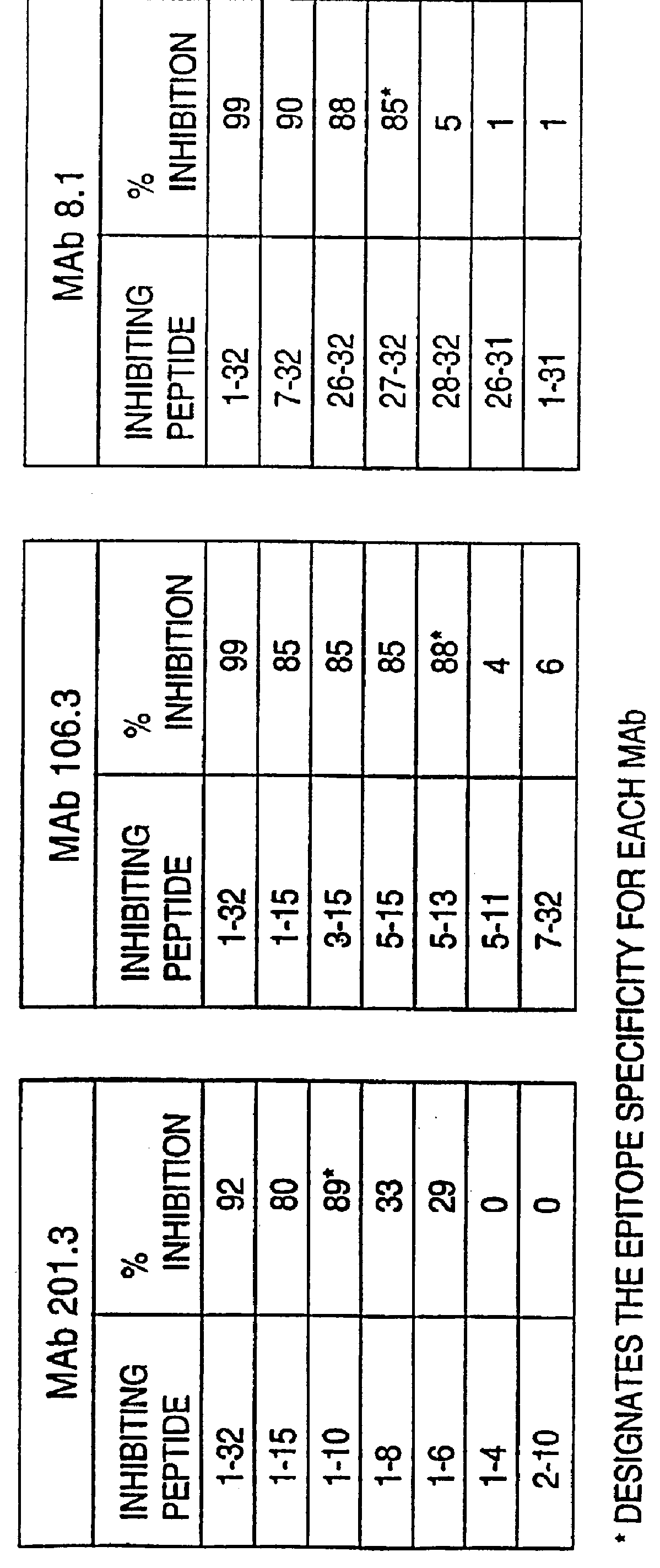
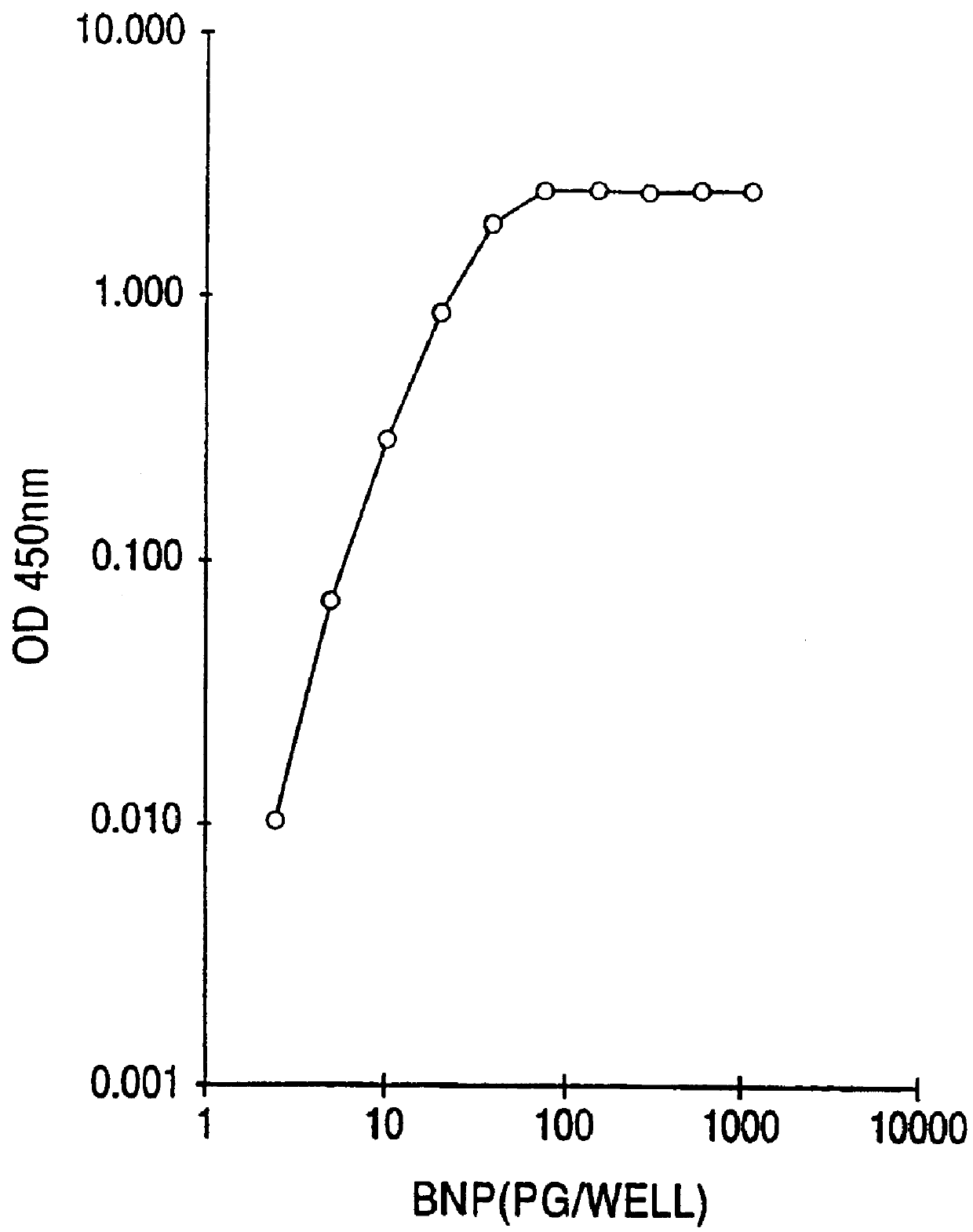
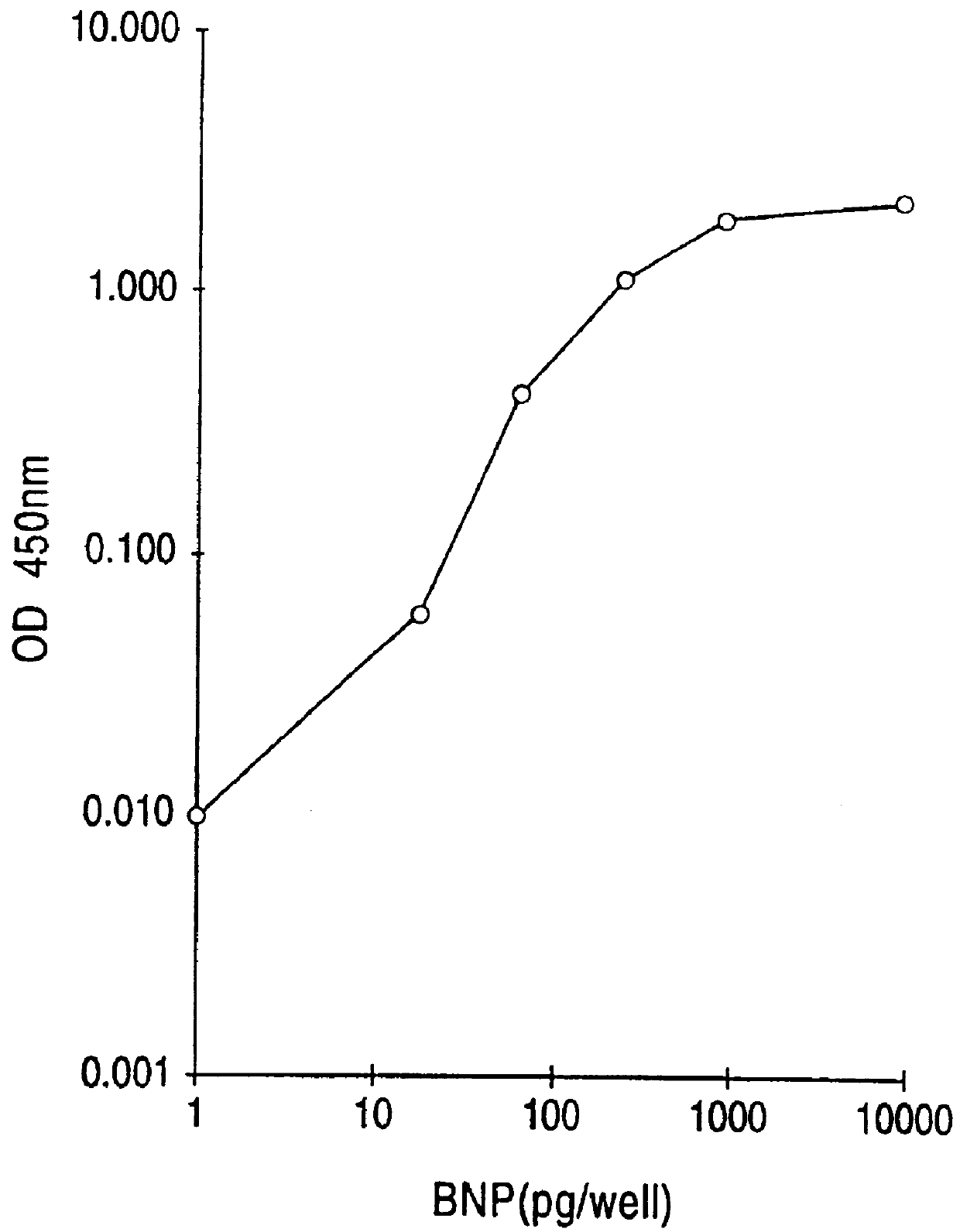
Human brain natriuretic peptides
The present invention provides reagents and assays for the quantification of hBNP in biological fluid samples such as plasma or serum. Antibodies are provided which are monospecific to epitopes comprising the amino acid sequences 5-13, 1-10 and 15-25 of hBNP. These antibodies, and peptide fragments containing these sequences, can be employed in the assays of the invention, which may be carried out in a sandwich format or a competition format.
Owner:SCIOS
High sensitivity quantitation of peptides by mass spectrometry
InactiveUS7632686B2Reduce complexityOverwhelm resolutionSamplingComponent separationChemical structureProtein target
The instant invention provides an economical flow-through method for determining amount of target proteins in a sample. An antibody preparation (whether polyclonal or monoclonal, or any equivalent specific binding agent) is used to capture and thus enrich a specific monitor peptide (a specific peptide fragment of a protein to be quantitated in a proteolytic digest of a complex protein sample) and an internal standard peptide (the same chemical structure but including stable isotope labels). Upon elution into a suitable mass spectrometer, the natural (sample derived) and internal standard (isotope labeled) peptides are quantitated, and their measured abundance ratio used to calculate the abundance of the monitor peptide, and its parent protein, in the initial sample.
Owner:ANDERSON FORSCHUNG GROUP
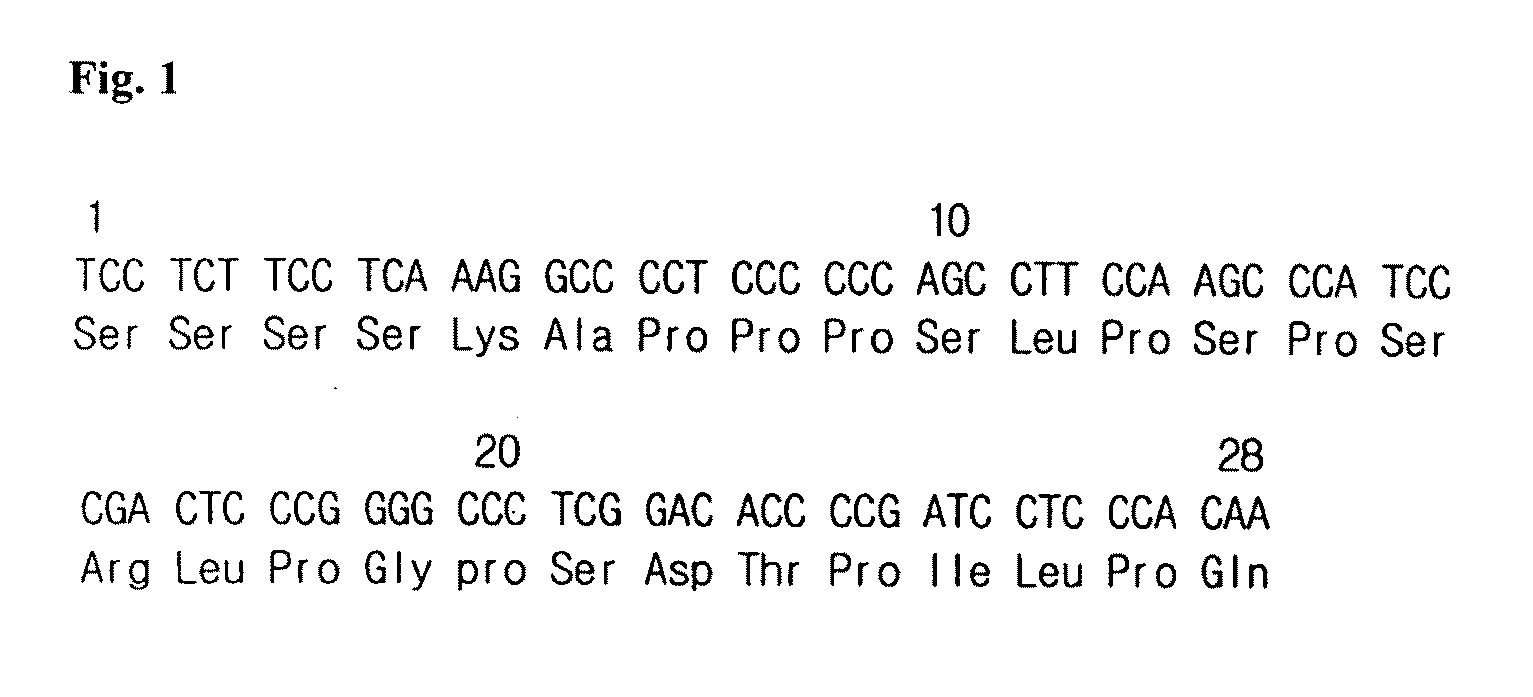
Compositions and Methods for Use of Pigment Epithelial Derived Factor (PEDF) Peptide Fragments
InactiveUS20090069241A1Organic active ingredientsNervous disorderPIGMENT EPITHELIUM-DERIVED FACTORPeptide fragment
The invention provides PEDF peptides which retain the biological activity of full-length PEDF. Fusion proteins comprising a PEDF peptide are also provided. The invention further provides a codon-optimized PEDF coding sequence and method of expressing it in bacteria. Compositions, methods of use and kits are also provided.
Owner:YALE UNIV +1
Platelet aggregation inducing substance
ActiveUS20100022755A1Low yieldLow costPeptide-nucleic acidsConnective tissue peptidesBULK ACTIVE INGREDIENTPeptide fragment
A platelet aggregation inducing substance containing as an active ingredient a polypeptide having a peptide fragment represented by formula (1) (component A):-(Pro-X-Gly)n- (1)wherein X represents Pro or Hyp; and n represents an integer of from 20 to 5,000.
Owner:JNC CORP
Recombinant human collagen and production method thereof
ActiveCN103122027ASuitable for large-scale scale-upReduce manufacturing costConnective tissue peptidesBacteriaEscherichia coliChain structure
The invention discloses a recombinant human collagen and a production method thereof and belongs to the technical field of genetic engineering. The structure of the recombinant human collagen is a single-chain structure; the basic repeating unit is gergapgfrgpagpngipgekgpagergap which is a human collagen III peptide fragment; the terminal sequence is GPPGPCCGGG which is a human collagen II peptide fragment. The production method of the recombinant human collagen comprises the following steps of: constructing escherichia coli genetically engineered bacterium; fermenting and cultivating the escherichia coli genetically engineered bacterium; inducing and expressing the recombinant human collagent; and purifying the recombinant human collagen. According to the recombinant human collagen and the production method thereof disclosed by the invention, the escherichia coli expression system is adopted for being amplified in a large-scale manner, the production cost is low and the yield is high.
Owner:SHANXI JINBO BIO PHARMA CO LTD
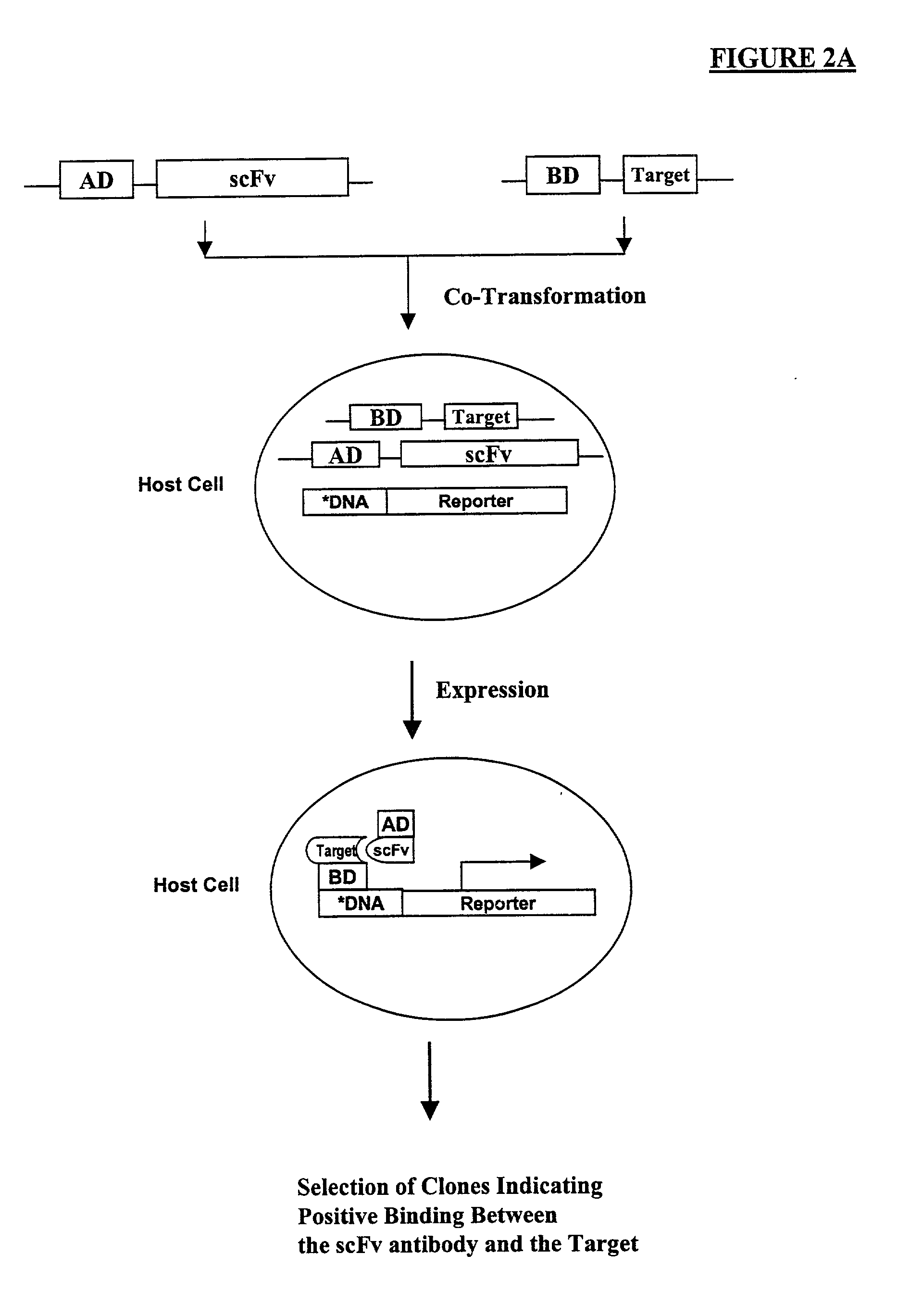
High throughput generation of human monoclonal antibody against peptide fragments derived from membrane proteins
InactiveUS20030165988A1FungiAntibody mimetics/scaffoldsHigh-Throughput Screening MethodsSingle-Chain Antibodies
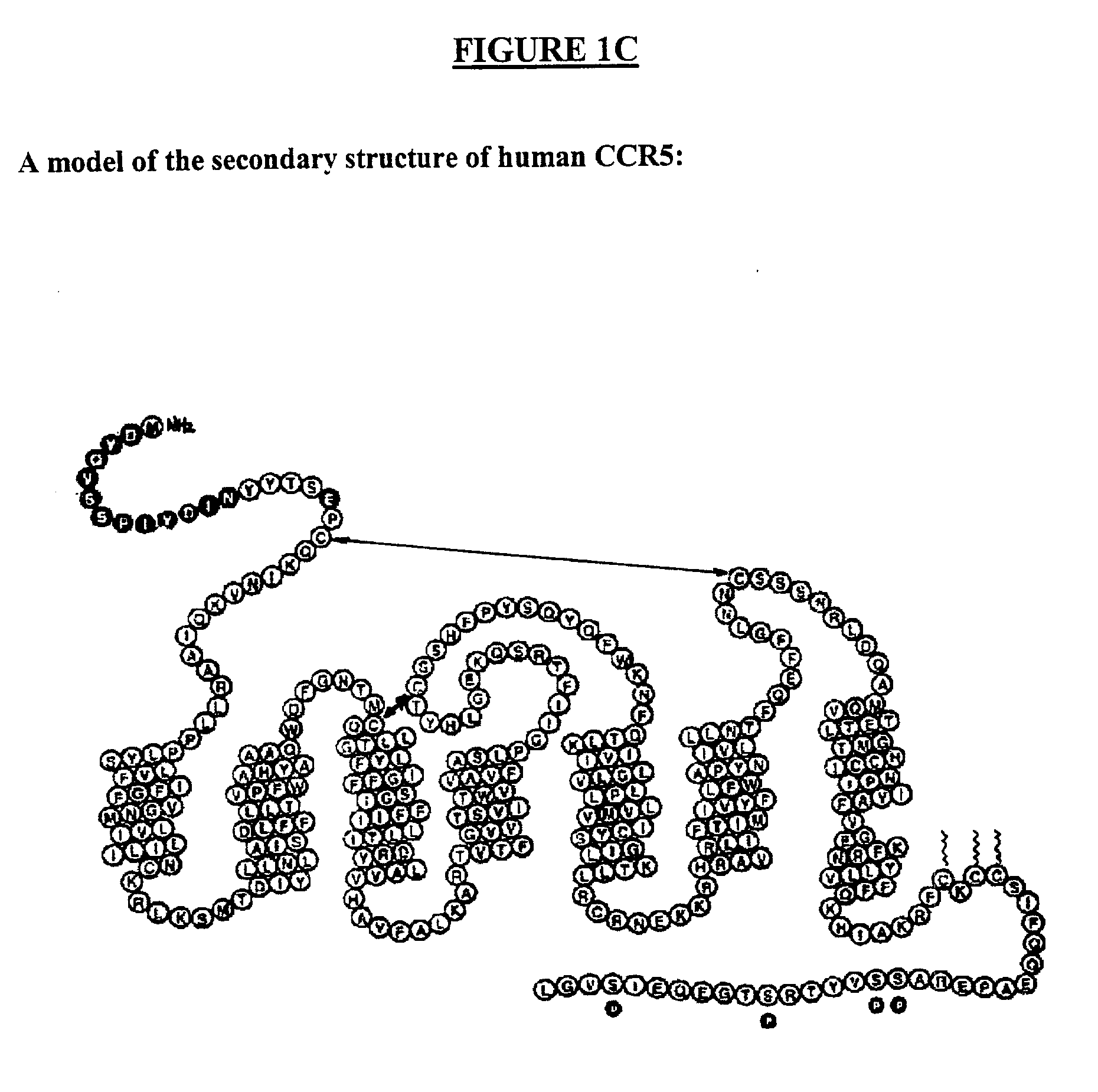
Methods are provided for efficient, high throughput screening of antibody libraries against proteins targets, especially membrane proteins. In particular, methods are provided for screening a fully human antibody library against membrane proteins such as HIV coreceptors in yeast. More particularly, a library of human single chain antibodies is screened against peptide fragments derived from extracellular domains of human CCR5 and high affinity monoclonal antibodies against CCR5 are selected.
Owner:GENETASTIX CORP
Methods to identify growth differentiation factor (GDF) receptors
InactiveUS20040077053A1Compound screeningOrganic active ingredientsGrowth differentiation factor-9Agonist
The present invention provides receptors for the growth differentiation factor (GDF) family of growth factors and methods of identifying such receptors. Also included are methods of identifying antibodies which bind to the receptors, peptide fragments of the receptor which inhibit GDF binding, GDF receptor-binding agents capable of blocking GDF binding to the receptor. The receptors of the invention allow the identification of antagonists or agonists useful for agricultural and human therapeutic purposes.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
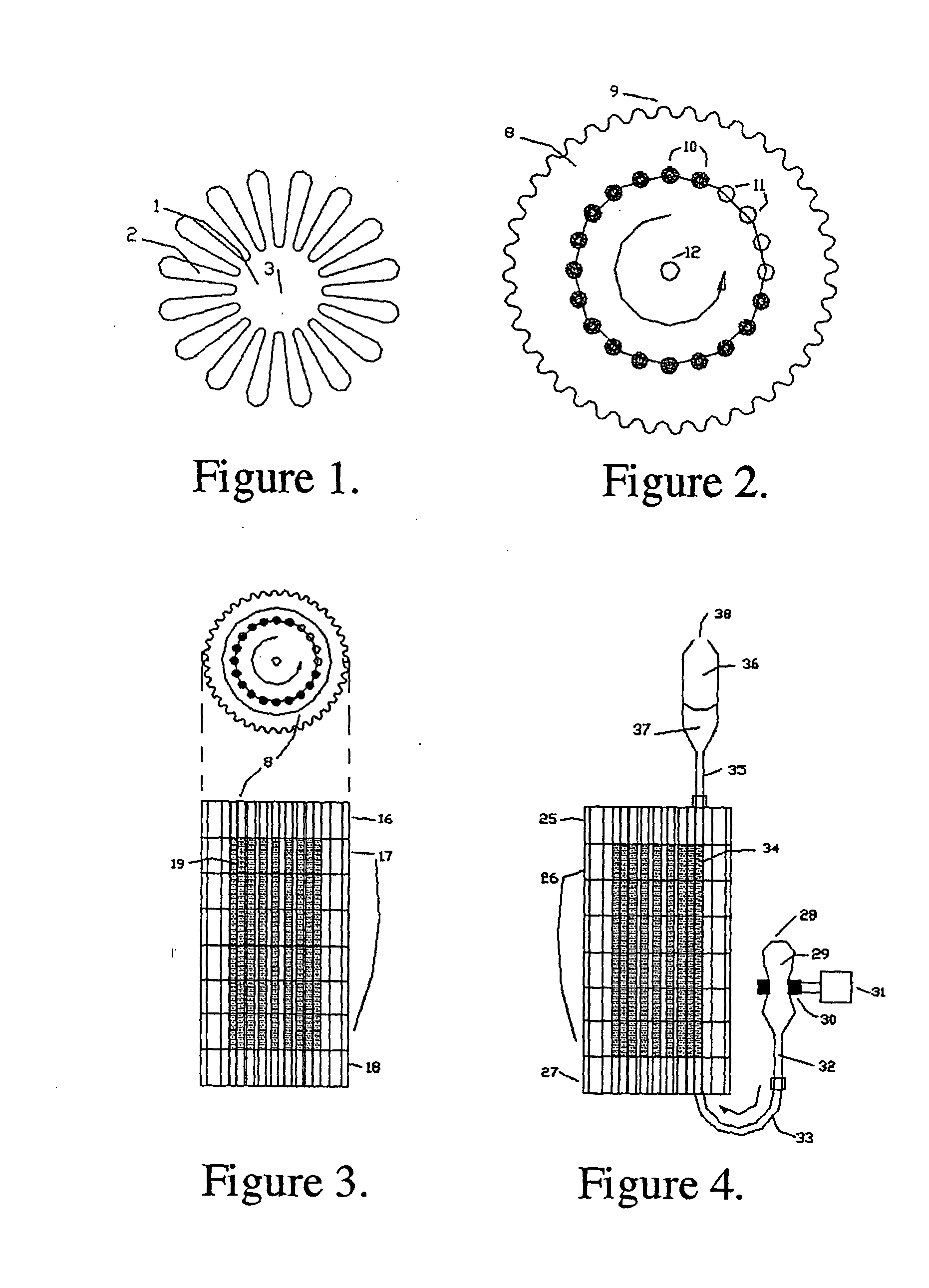
Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material
InactiveCN103143331AGood magnetic responseReduce adsorptionOther chemical processesComponent separationMicrosphereMetal-organic framework
The invention relates to a synthetic method for a magnetic metal organic framework composite material coated by [Cu2(btc)2] on surfaces of ferroferric oxide microspheres and application of the composite material. The method comprises the following steps of: firstly synthesizing ferroferric oxide microspheres by a hydrothermal synthesis method; dispersing magnetic spheres in an ethanol liquid of mercaptoacetic acid, wherein hydroxyls are formed on the surface of the spheres; dispersing mercaptoacetic acid modified magnetic spheres to an ethanol liquid of copper acetate, reacting for 15 minutes at 70 DEG C, and then dispersing the product in an ethanol liquid of trimesic acid and reacting for 30 minutes at 70 DEG C; and performing alternate reaction of magnetic spheres with copper acetate and the ethanol liquid of trimesic acid to finally, obtain the magnetic metal organic framework composite material with a core-shell structure. The material has a metal organic framework shell layer and can be coordinated with peptide fragments with amino groups and carboxylic group so as to enrich low concentration peptide. Meanwhile, the enriching and separating process is fast, simple and convenient due to high paramagnetism of ferroferric oxide. The synthetic method is simple and low in cost, and can be used for enrichment and separation of low abundance peptide fragments less than 1nM and MALDI-TOFMS (Matrix-Assisted Laser Desorption Ionization-Time Of Flight Mass Spectrometer) detection.
Owner:FUDAN UNIV
Method of epitope discovery
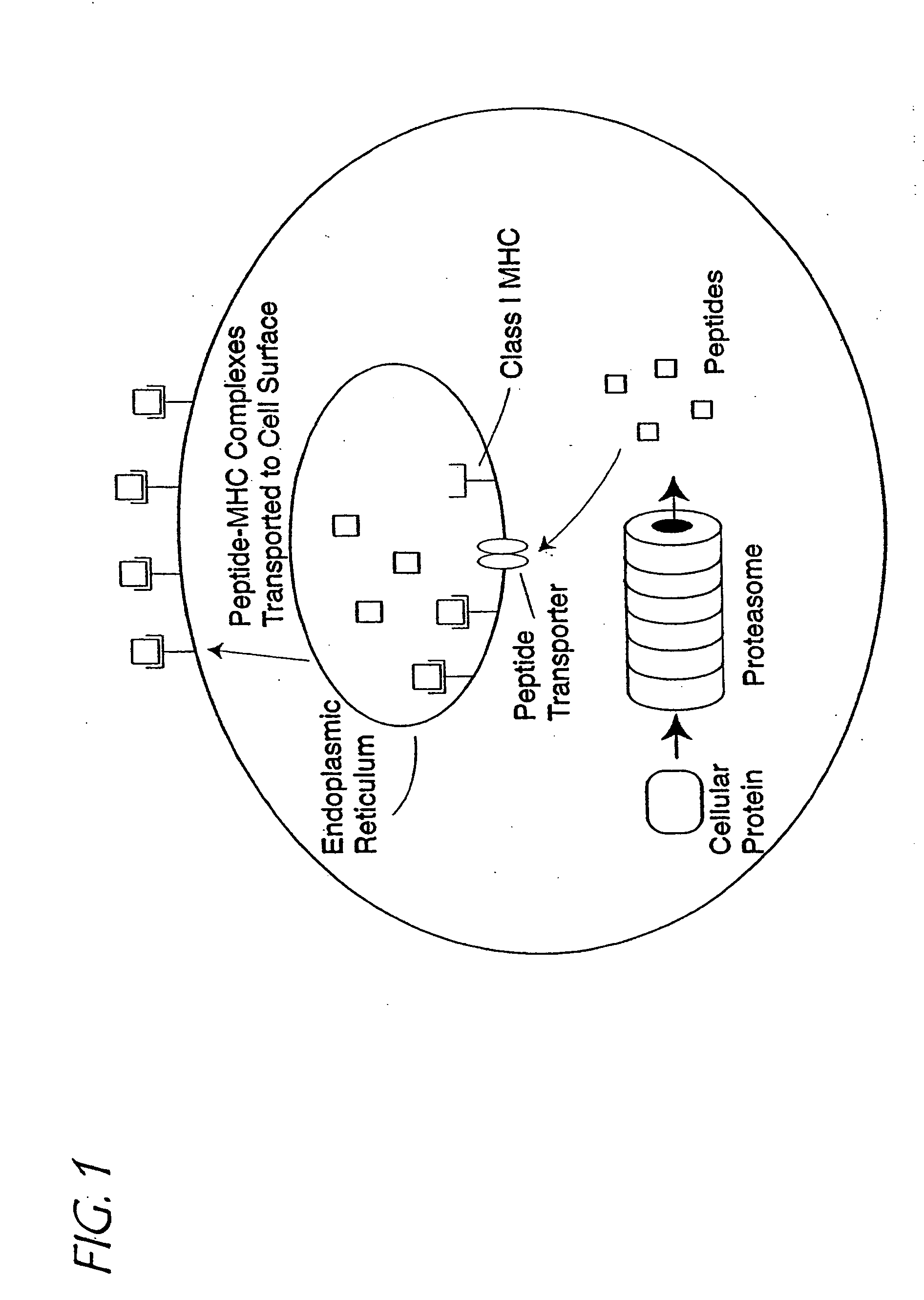
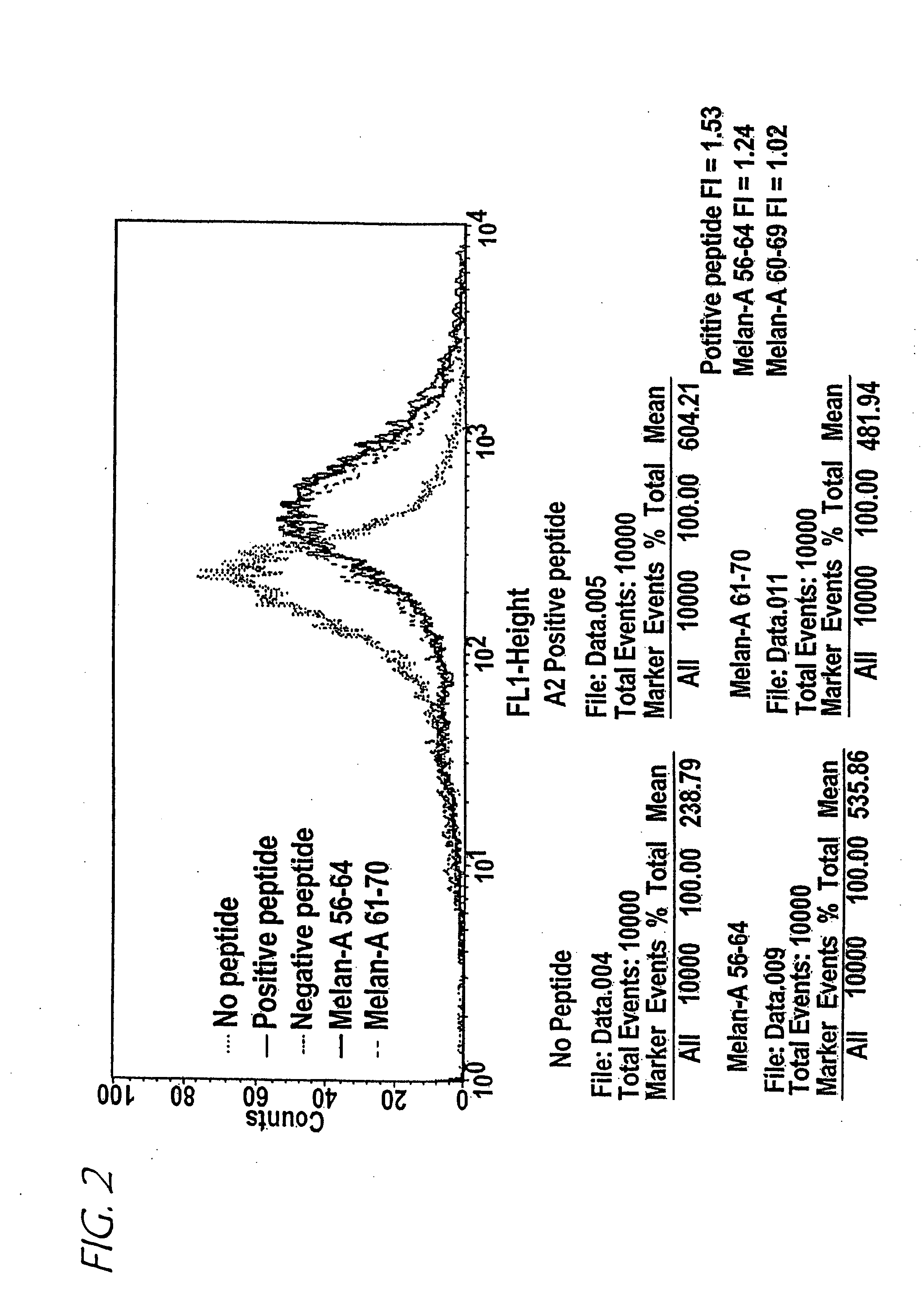
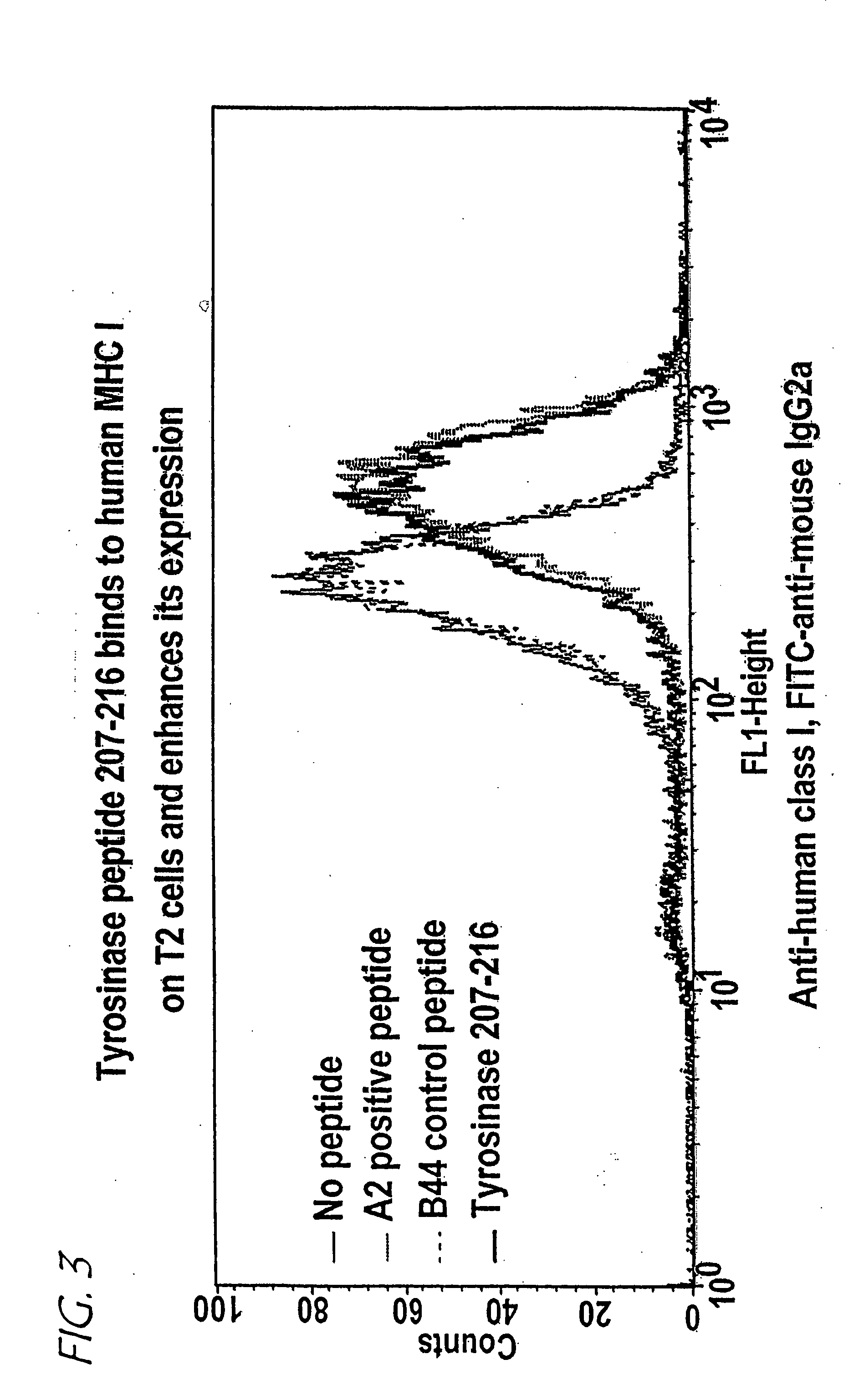
A method of epitope discovery comprising the step of selecting an epitope from a population of peptide fragments of an antigen associated with a target cell, wherein the fragments have a known or predicted affinity for a major histocompatibility complex class I receptor peptide binding cleft, wherein the epitope selected corresponds to a proteasome cleavage product of the target cell.
Owner:MANNKIND CORP
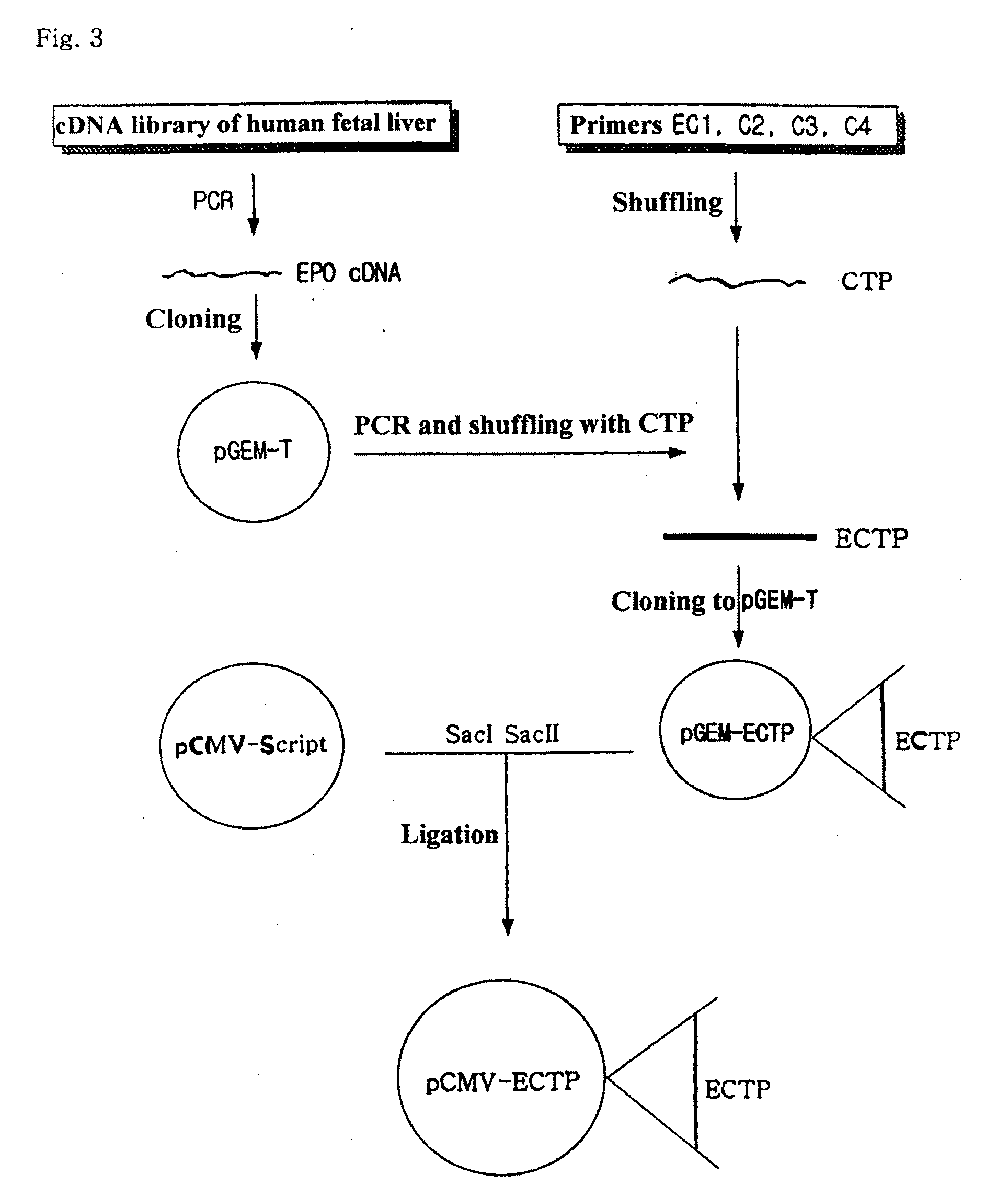
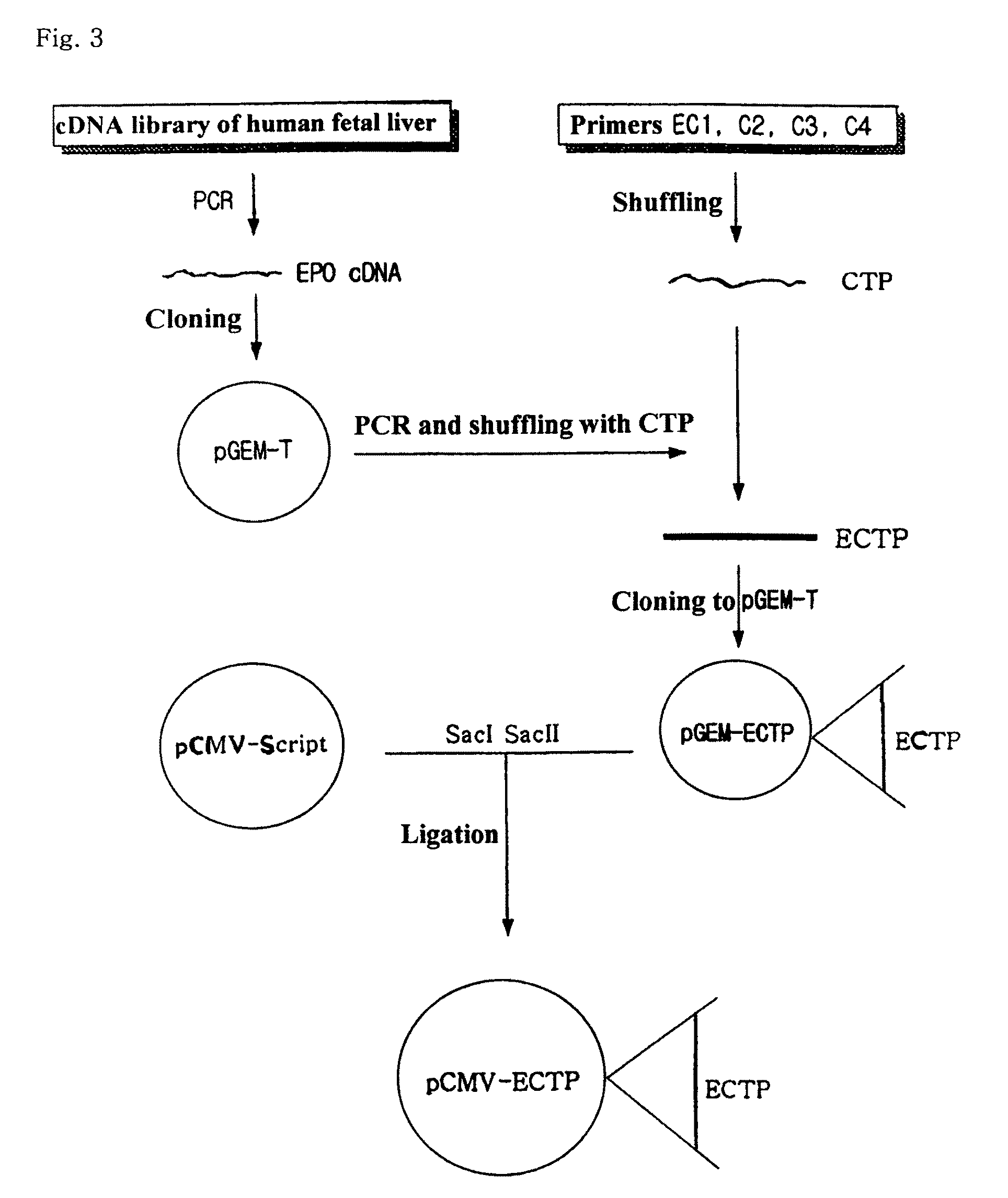
Fusion protein having the enhanced in vivo activity of erythropoietin
InactiveUS20090221037A1High activityImprove in vivo activitySugar derivativesAntibody mimetics/scaffoldsΒ subunitRed blood cell
The present invention relates to a fusion protein in which a carboxy terminal of human erythropoietin (EPO) is fused with a carboxy terminal peptide fragment of β subunit of human chorionic gonadotropin (HCG), to DNA encoding the fusion protein, and to a method for preparation of the fusion protein. The fusion protein has the enhanced in vivo activity of erythropoietin.
Owner:CJ HEALTHCARE CORP
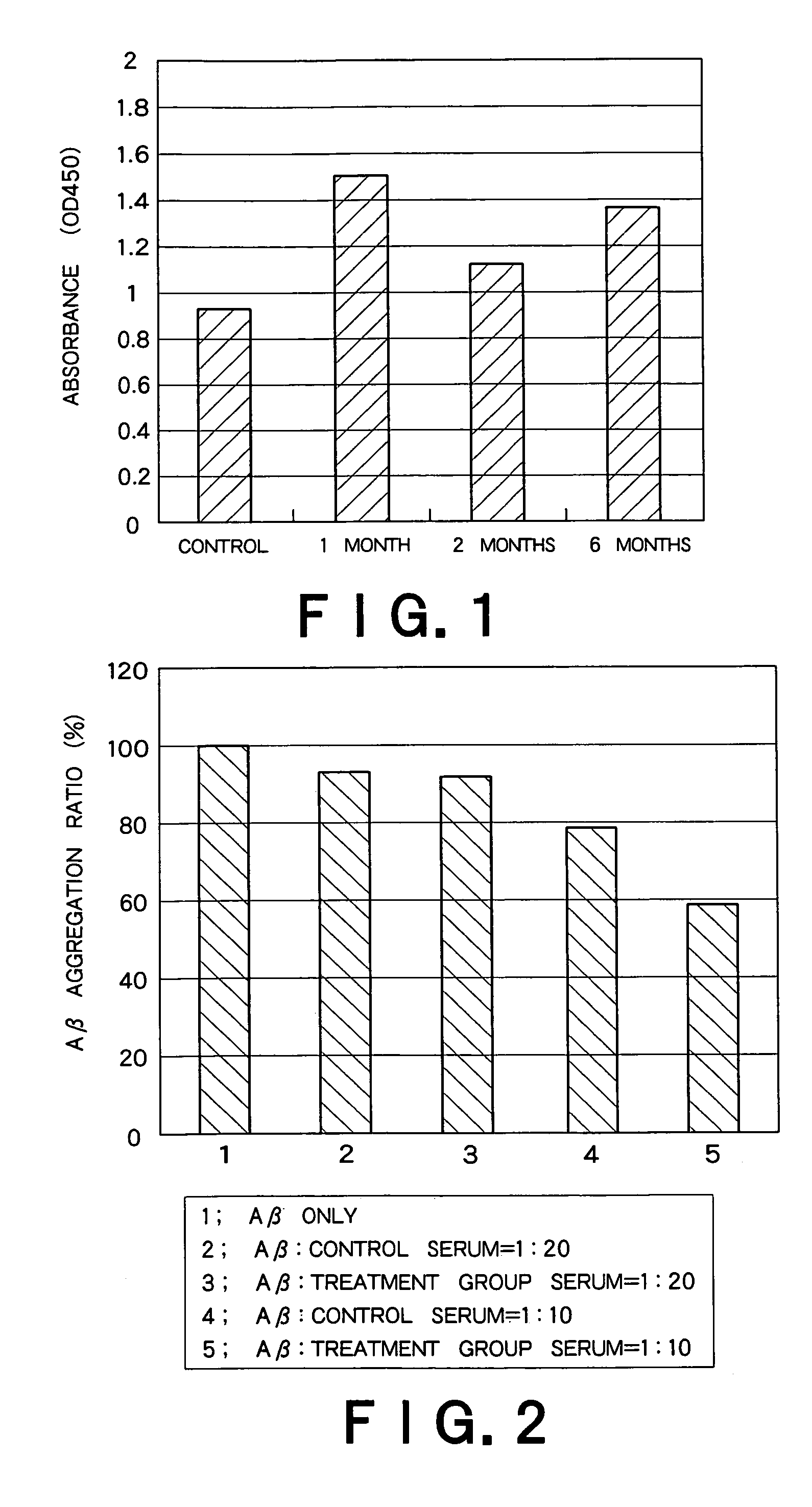
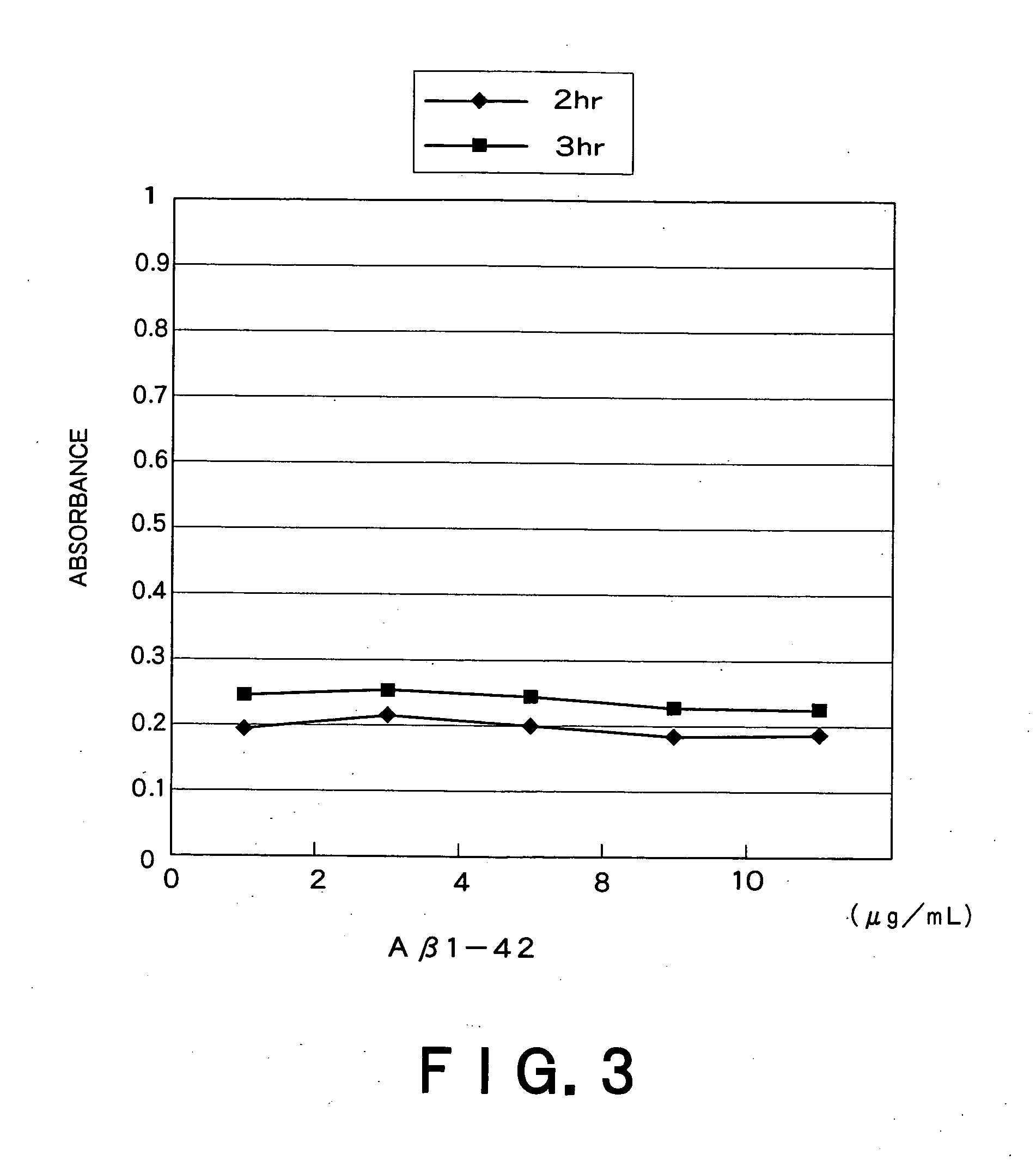
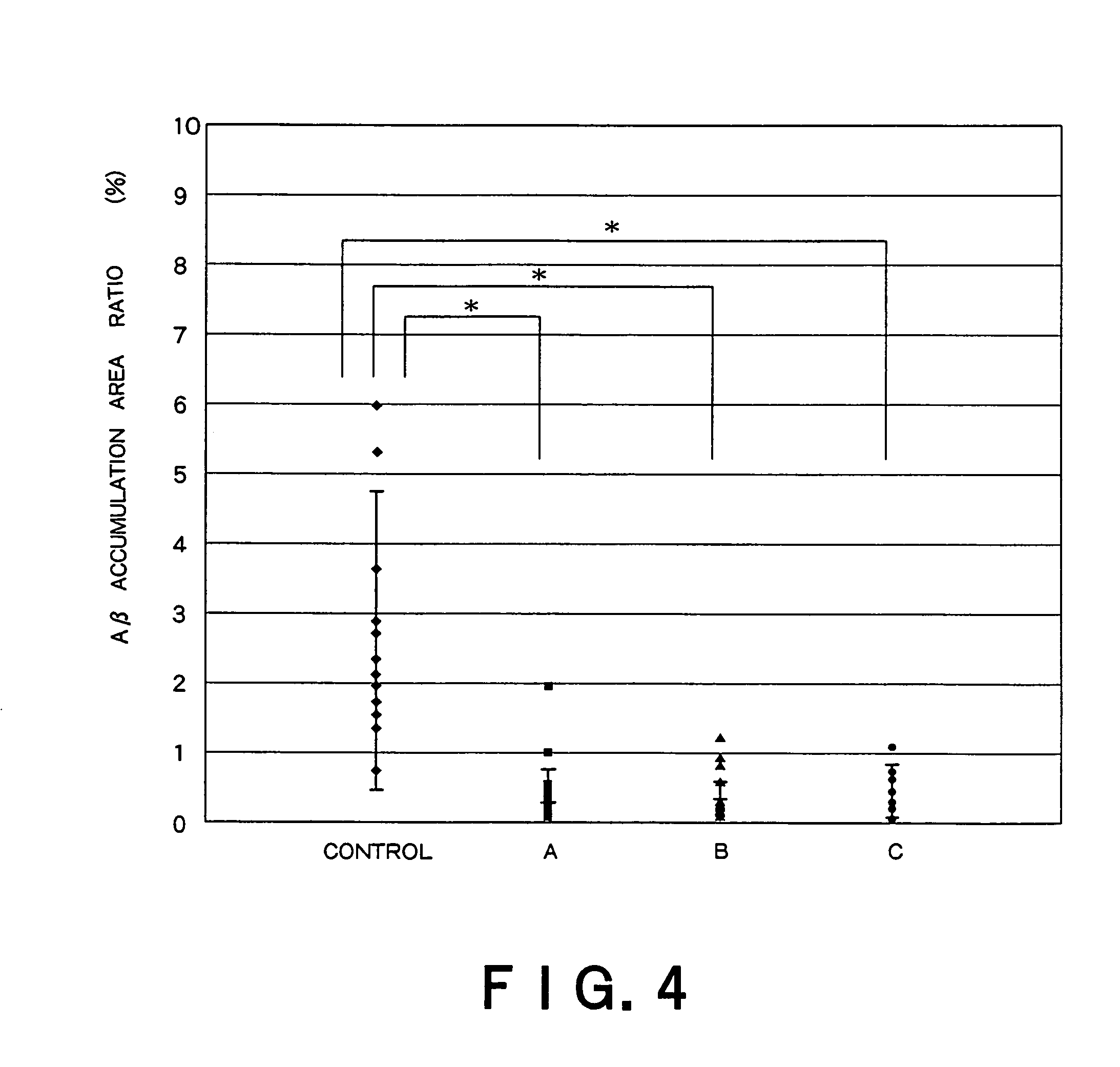
Recombinant adeno-associated virus vector for treatment of Alzheimer disease
ActiveUS8318687B2Highly safe treatmentReduce concentrationNervous disorderVirusesAdeno associate virusDna encoding
Owner:TABIRA TAKESHI +1
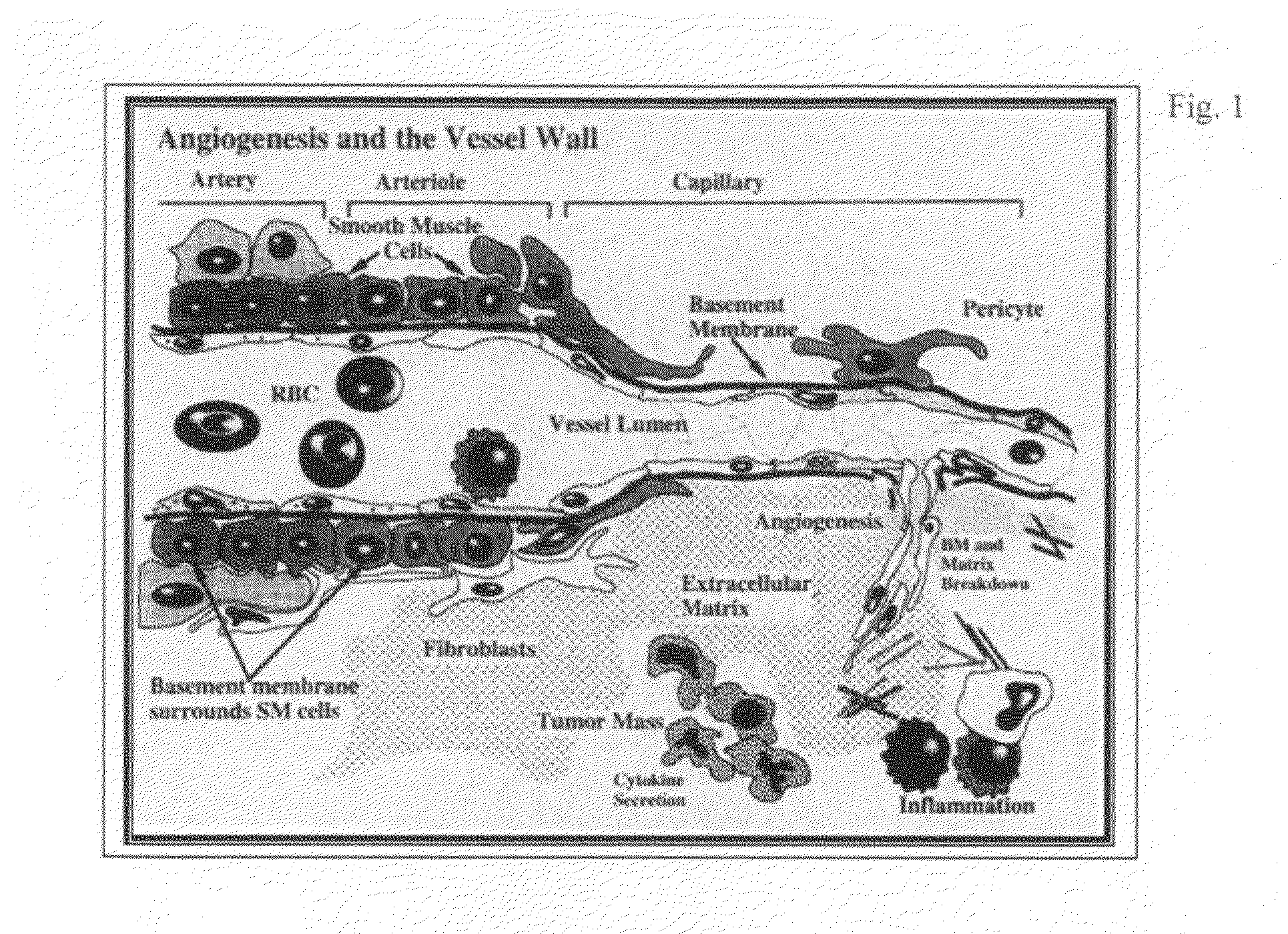
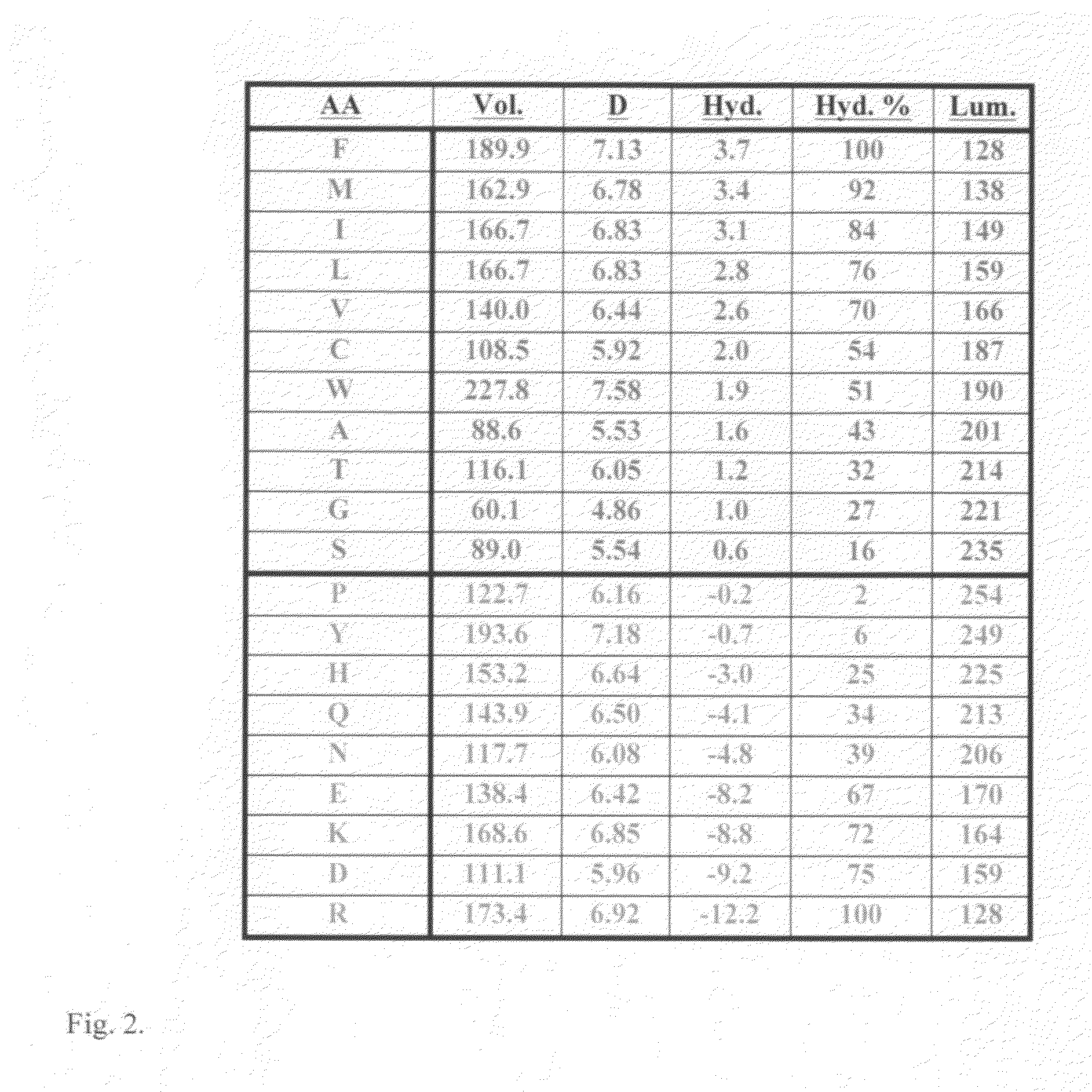
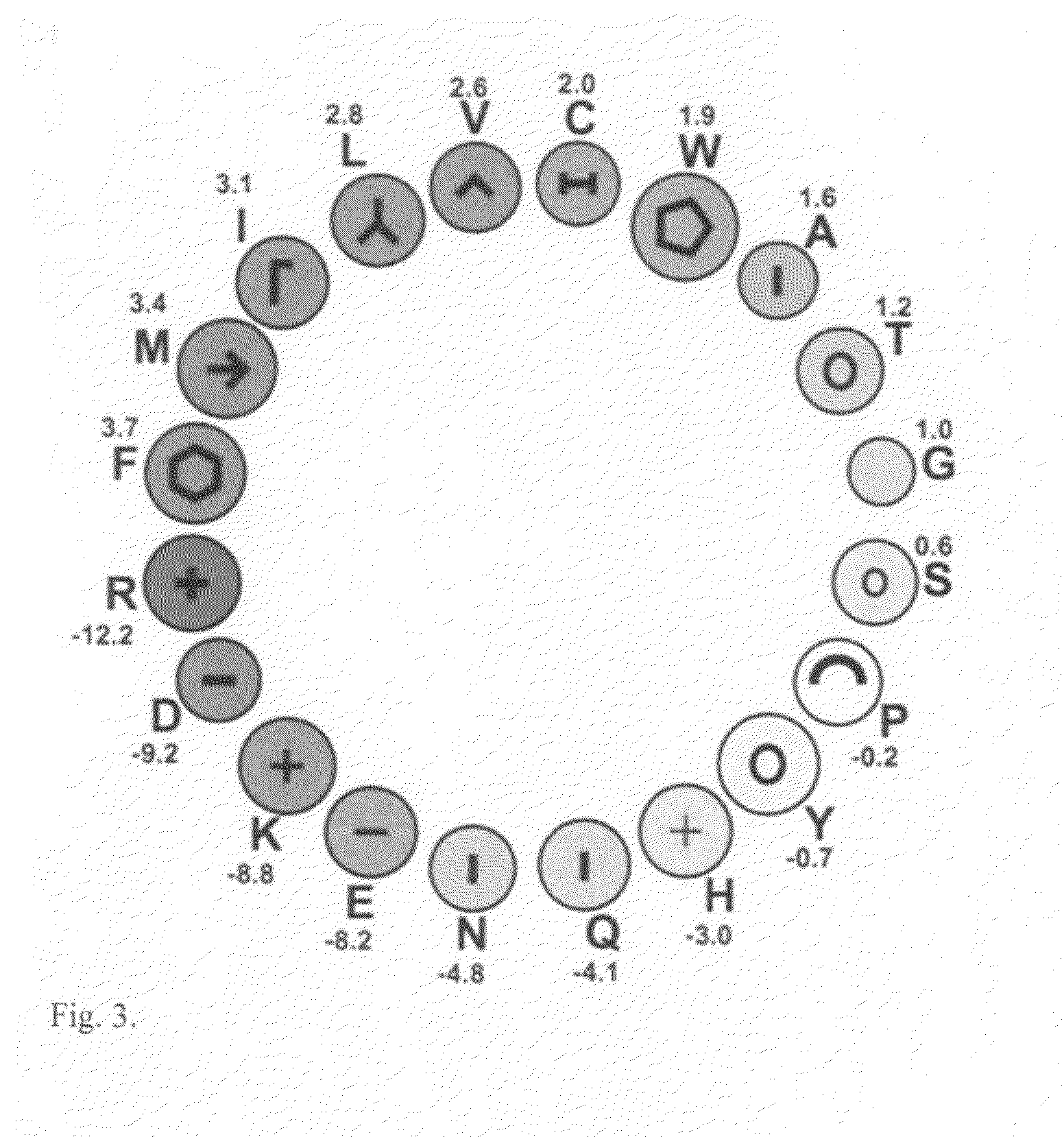
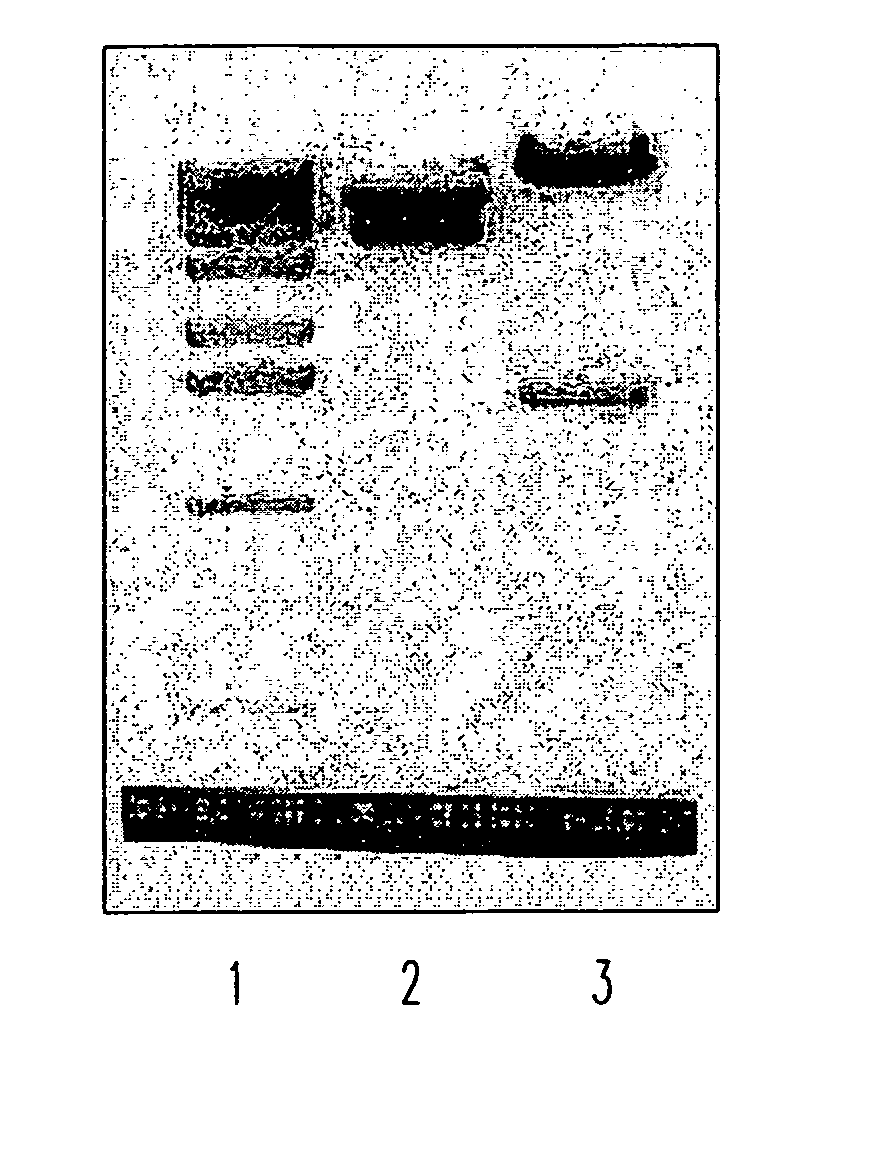
Anti-angiogenic peptides and their uses
InactiveUS20120270770A1Enhance and inhibit angiogenesisEffective therapySenses disorderNervous disorderLytic peptideDisease
This invention relates to novel synthetic lytic peptide fragments of full-length peptides with the capacity to modulate angiogenic activity in mammals. The invention also relates to the use of such peptides in pharmaceutical compositions and in methods for treating diseases or disorders that are associated with angiogenic activity.
Owner:JAYNES JESSE MICHAEL
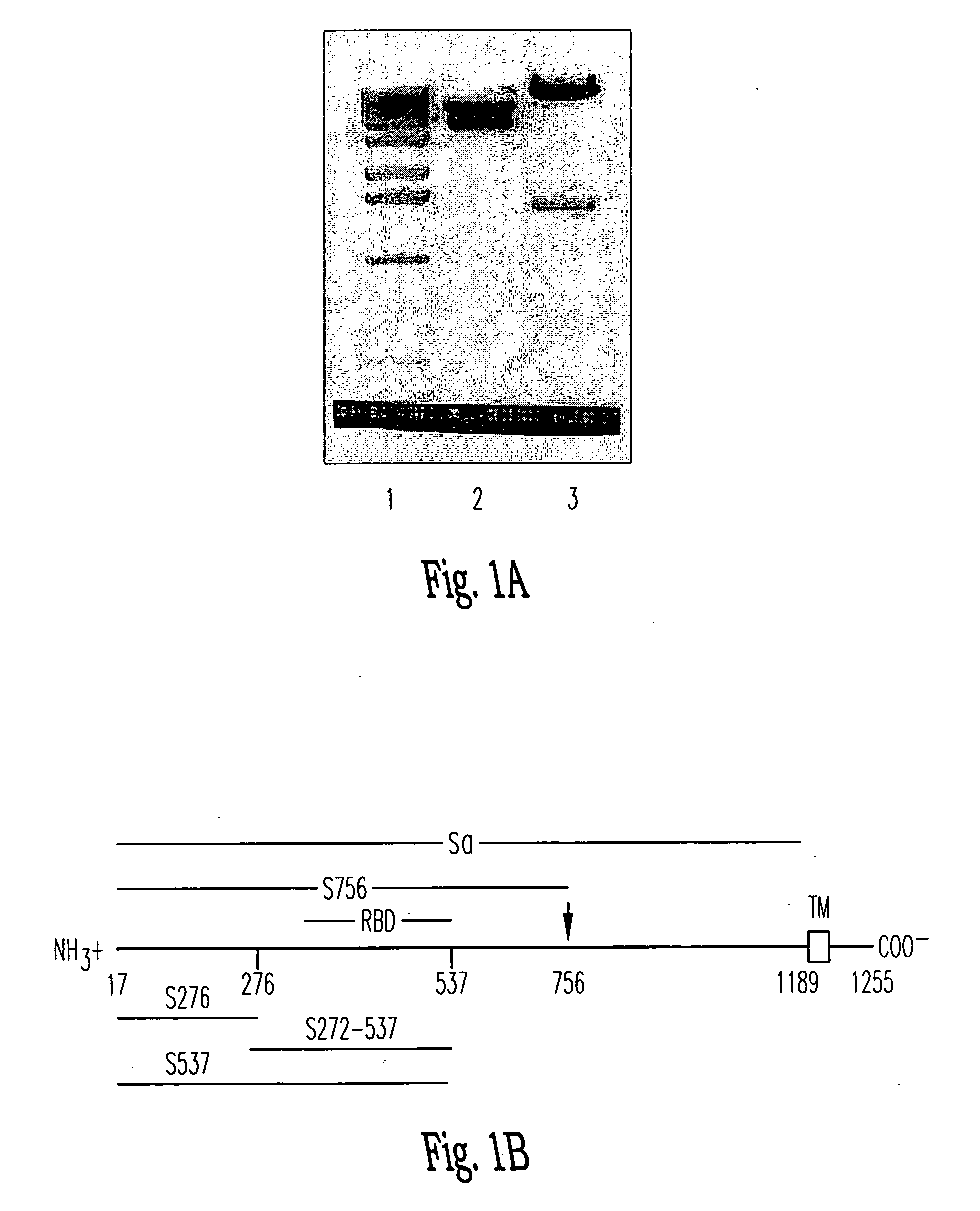
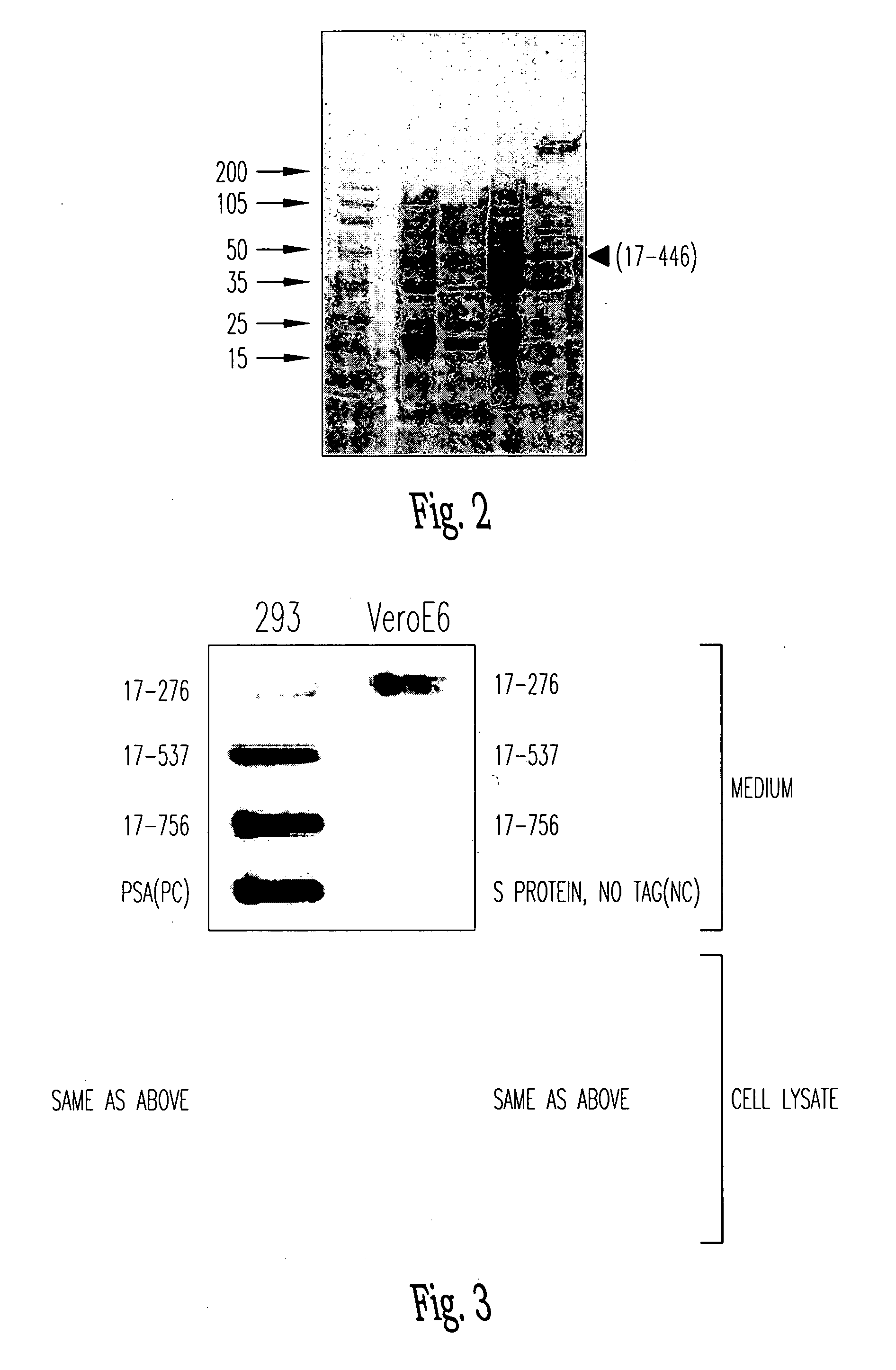
Soluble fragments of the SARS-CoV spike glycoprotein
InactiveUS20060240515A1Easy to produceImprove resistance to degradationSsRNA viruses positive-senseAntibody mimetics/scaffoldsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF US SEC THE DEPT OF
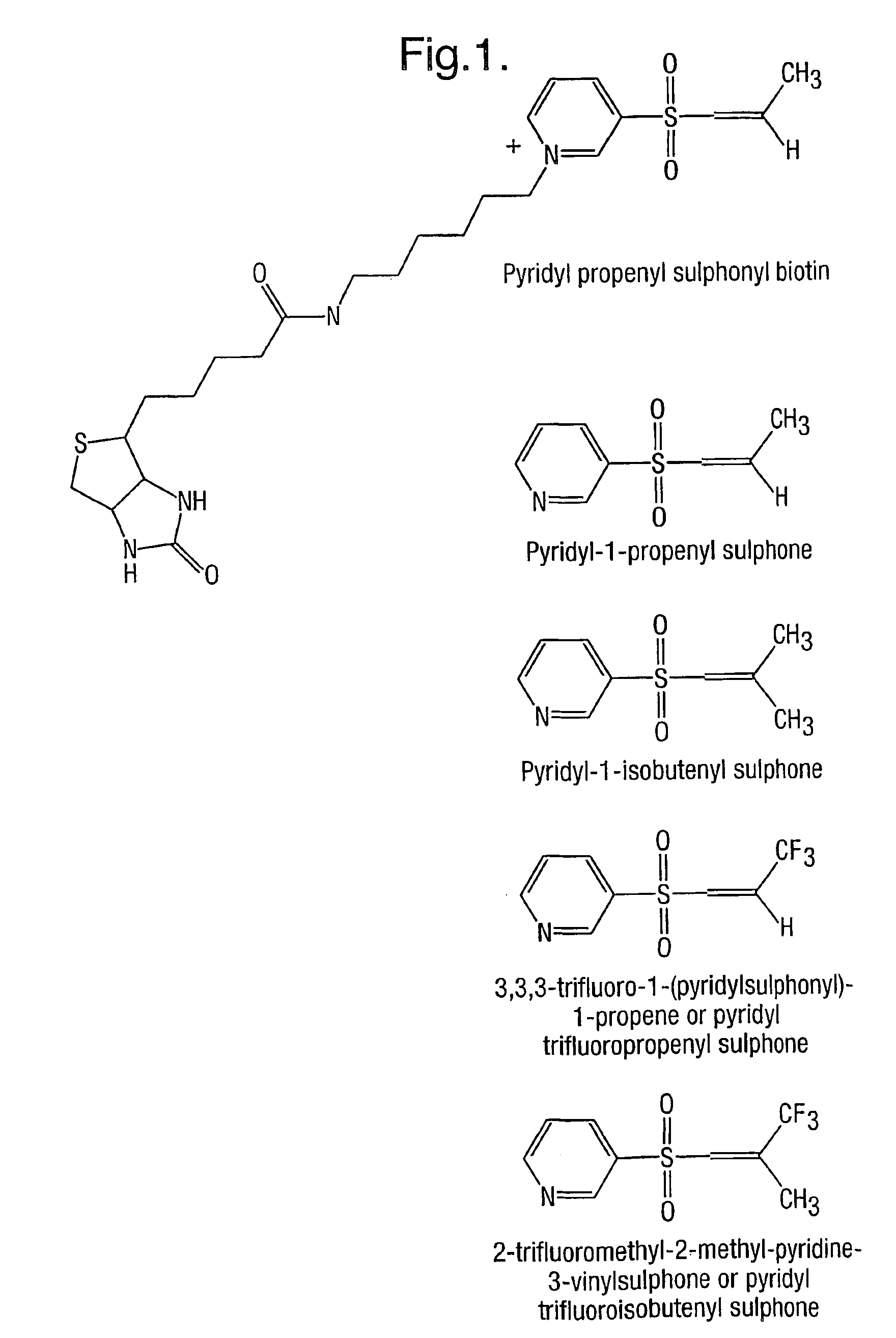
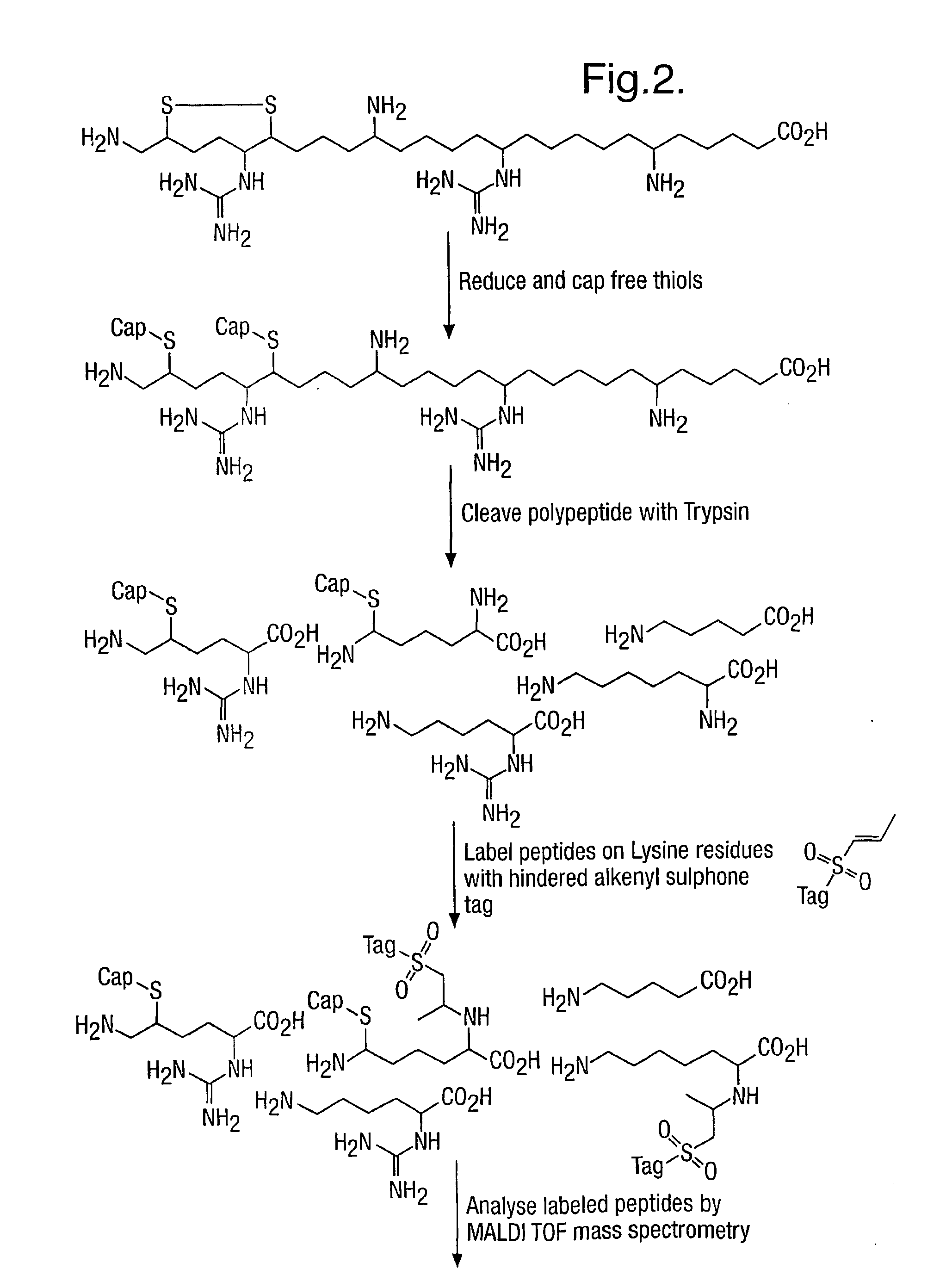
Method for characterizing polypeptides
InactiveUS7163803B2Component separationMicrobiological testing/measurementCysteine thiolateMass Spectrometry-Mass Spectrometry
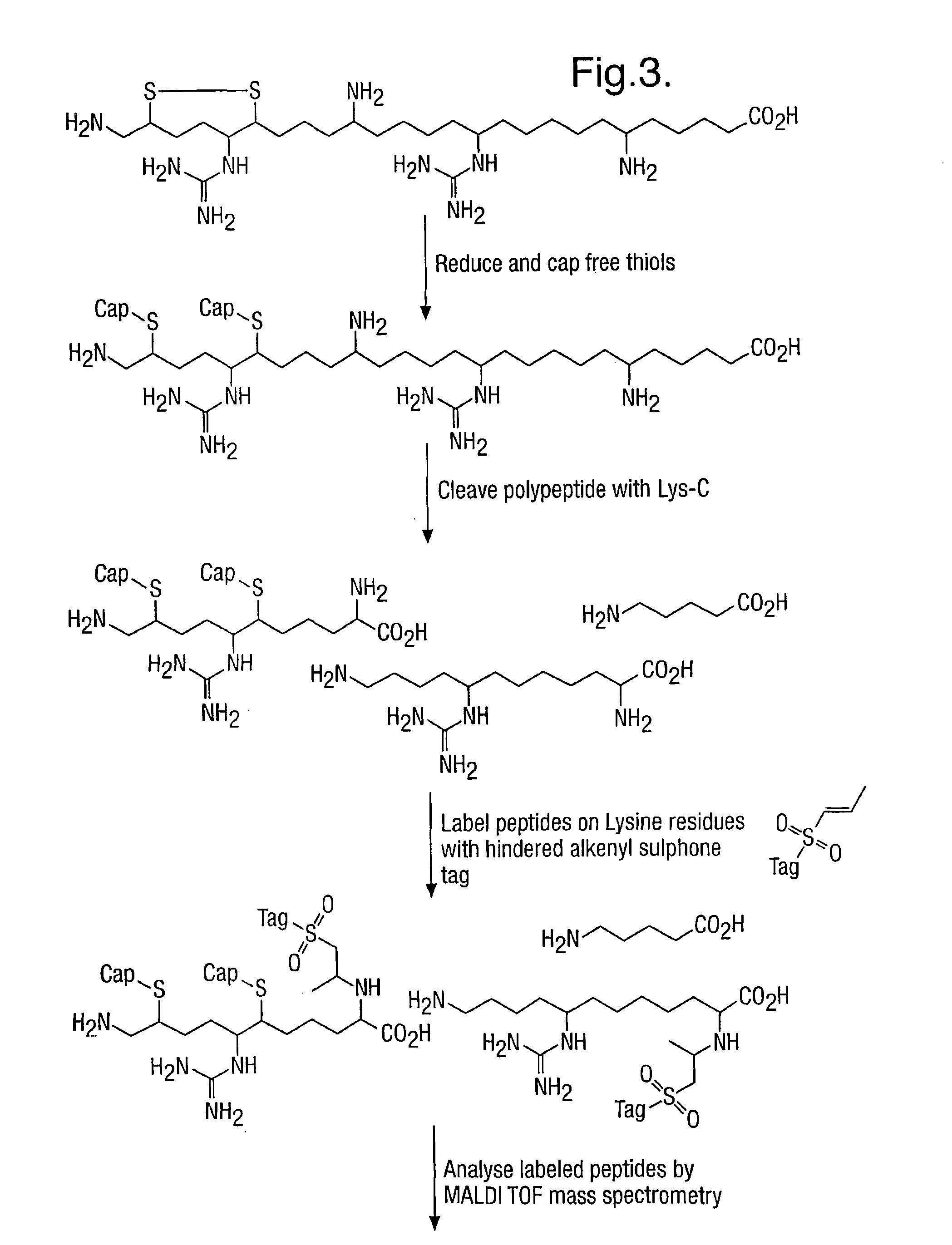
Provided is a method for characterizing a polypeptide, which method comprises the steps of: (a) optionally reducing cysteine disulphide bridges in the polypeptide to form free thiols, and capping the free thiols; (b) cleaving the polypeptide with a sequence specific cleavage reagent to form peptide fragments; (c) optionally deactivating the cleavage reagent; (d) capping one or more ε-amino groups that are present with a lysine reactive agent; (e) analyzing peptide fragments by mass spectrometry to form a mass fingerprint for the polypeptide; and (f) determining the identity of the polypeptide from the mass fingerprint.
Owner:ELECTROPHORETICS LTD
Method for preparing Semaglutide through solid and liquid combination
ActiveCN108059666AReduce generationAvoid it happening againPeptide preparation methodsBulk chemical productionDipeptideSide chain
The invention relates to a method for preparing Semaglutide through solid and liquid combination, and solves the technical problems that in the process for synthesizing long-sequence polypeptide by the existing technology, the synthesis period is long; the purification difficulty is high; the yield is low. The method for preparing Semaglutide through solid and liquid combination provided by the invention is characterized in that firstly, Lys and resin are condensed in an Alloc-Lys(Fmoc)-OH form by adopting a solid phase synthesis method; Fmoc protecting groups on epsilon-NH2 are removed; sidechain connection is performed; cracking is performed to obtain Alloc-Lys(PEG-PEG-gamma-Glu(OtBu)-Monobutyl octadecanate)-OH; meanwhile, 10 dipeptide or tripeptide or tetrapeptide fragments are simultaneously synthesized by a liquid phase synthesis method; then, the condensation reaction of the synthesized peptide fragments and single amino acid is performed by using the resin as a carrier; the 15-step solid phase condensation reaction is reduced in the process; the generation of lacked peptide impurities is reduced; the product purity and the yield are improved; meanwhile, the generation of the impurities of [+Gly]-Semaglutide and [+Ala]-Semaglutide is effectively avoided; the purification difficulty is greatly reduced. The method is widely applied to the technical field of polypeptide medicine preparation.
Owner:润辉生物技术(威海)有限公司
Rapid quantitative analysis of proteins or protein function in complex mixtures
InactiveUS7544518B2Facilitates quantitative determinationFacilitates quantitative determination of the absolute amountsComponent separationMaterial analysis by electric/magnetic meansIsotopic labelingProtein expression profile
Analytical reagents and mass spectrometry-based methods using these reagents for the rapid, and quantitative analysis of proteins or protein function in mixtures of proteins. The methods employ affinity labeled protein reactive reagents having three portions: an affinity label (A) covalently linked to a protein reactive group (PRG) through a linker group (L). The linker may be differentially isotopically labeled, e.g., by substitution of one or more atoms in the linker with a stable isotope thereof. These reagents allow for the selective isolation of peptide fragments or the products of reaction with a given protein (e.g., products of enzymatic reaction) from complex mixtures. The isolated peptide fragments or reaction products are characteristic of the presence of a protein or the presence of a protein function in those mixtures. Isolated peptides or reaction products are characterized by mass spectrometric (MS) techniques. The reagents also provide for differential isotopic labeling of the isolated peptides or reaction products which facilitates quantitative determination by mass spectrometry of the relative amounts of proteins in different samples. The methods of this invention can be used for qualitative and quantitative analysis of global protein expression profiles in cells and tissues, to screen for and identify proteins whose expression level in cells, tissue or biological fluids is affected by a stimulus or by a change in condition or state of the cell, tissue or organism from which the sample originated.
Owner:UNIV OF WASHINGTON
Fusion protein having the enhanced in vivo activity of erythropoietin
InactiveUS8008454B2Improve in vivo activityExtended half-lifeSugar derivativesAntibody mimetics/scaffoldsΒ subunitDna encoding
The present invention relates to a fusion protein in which a carboxy terminal of human erythropoietin (EPO) is fused with a carboxy terminal peptide fragment of β subunit of human chorionic gonadotropin (HCG), to DNA encoding the fusion protein, and to a method for preparation of the fusion protein. The fusion protein has the enhanced in vivo activity of erythropoietin.
Owner:CJ HEALTHCARE CORP
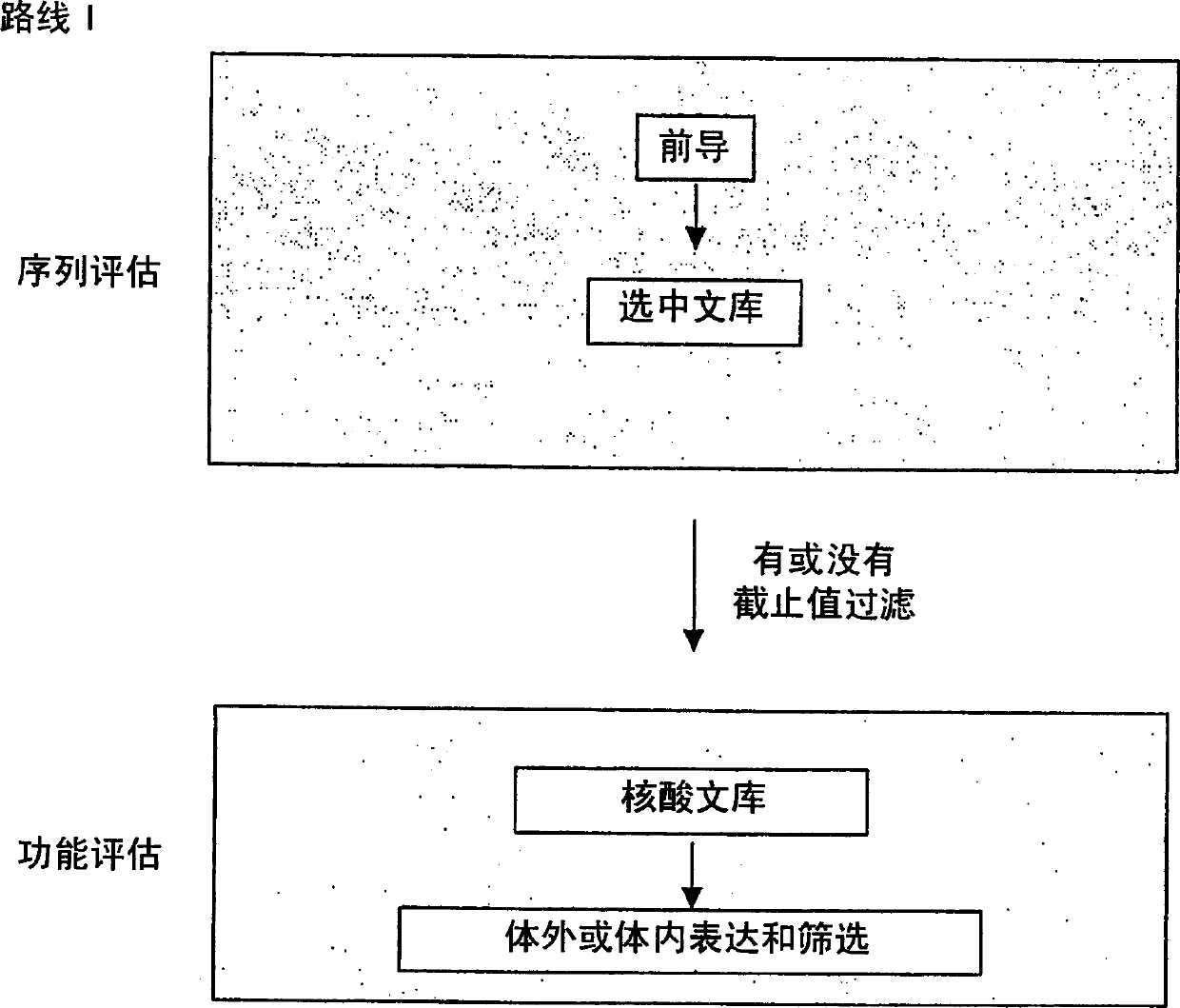
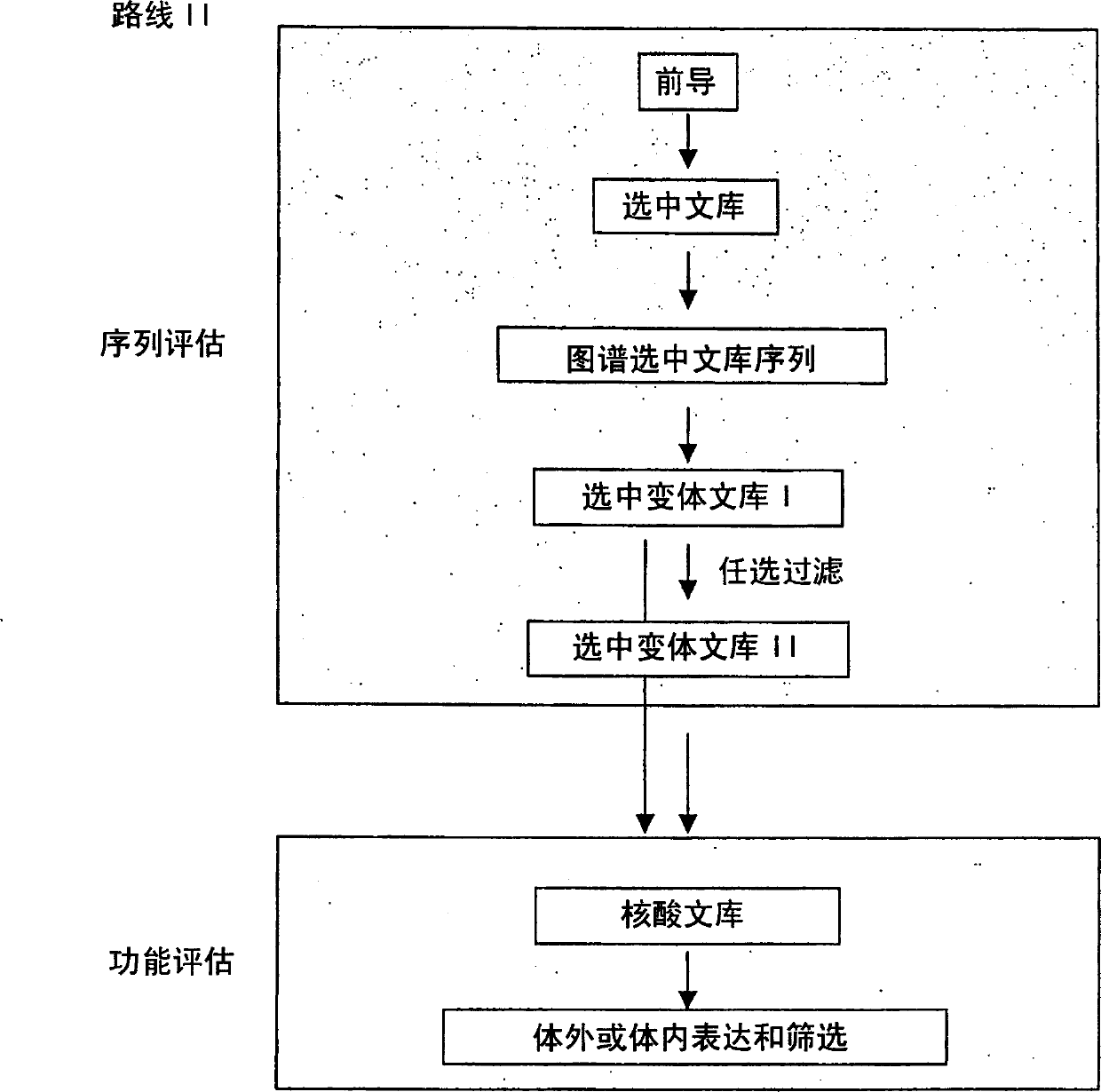
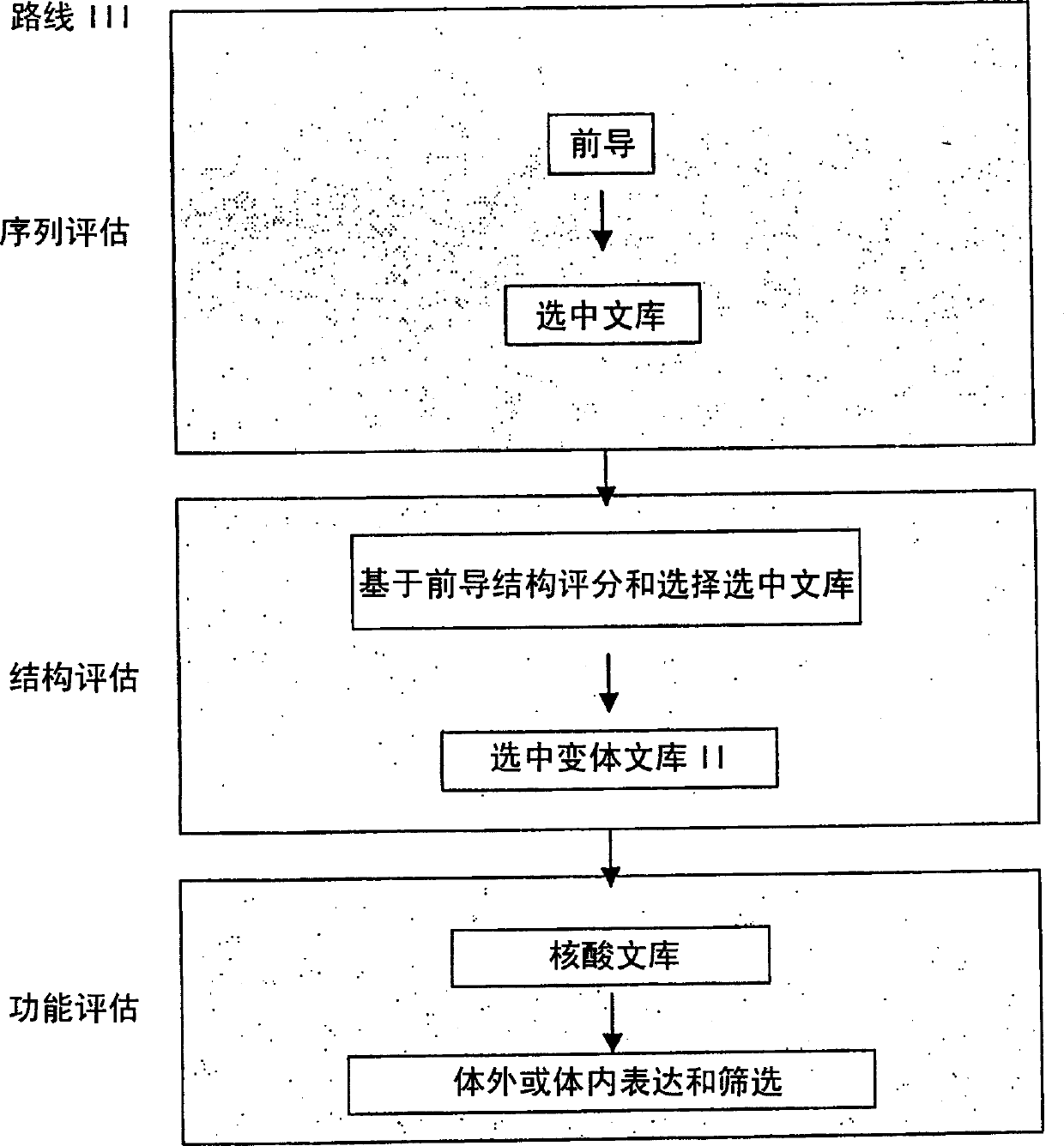
Generation and selection of protein library in silico
The present invention provides methods for efficiently generating and screening protein libraries for optimal proteins with desired biological functions, such as improved binding affinity for biologically and / or therapeutically important target molecules. The method is performed in silico in a high-throughput fashion by mining the ever-expanding database of protein sequences in all living things, especially humans. In one embodiment, a method of constructing a designer protein library comprises the steps of: providing an amino acid sequence derived from a lead protein, referred to as a lead sequence; comparing the lead sequence with a plurality of test protein sequences; Select at least two peptide fragments having at least 15% sequence identity with the leader sequence from each test protein sequence, and the selected peptide fragments form a selection library; a library of designed proteins is formed by replacing the leader sequence with the selection library. Libraries of designed proteins can be expressed in vitro or in vivo to generate libraries of recombinant proteins that can be screened for new or improved functions relative to lead proteins, such as antibodies against a therapeutically important target.
Owner:埃博马可西斯公司
Protein quantitative method utilizing equiponderance dimethylation marking
ActiveCN104076098AAchieve large scaleImprove accuracyComponent separationQuantitative accuracyIsotope
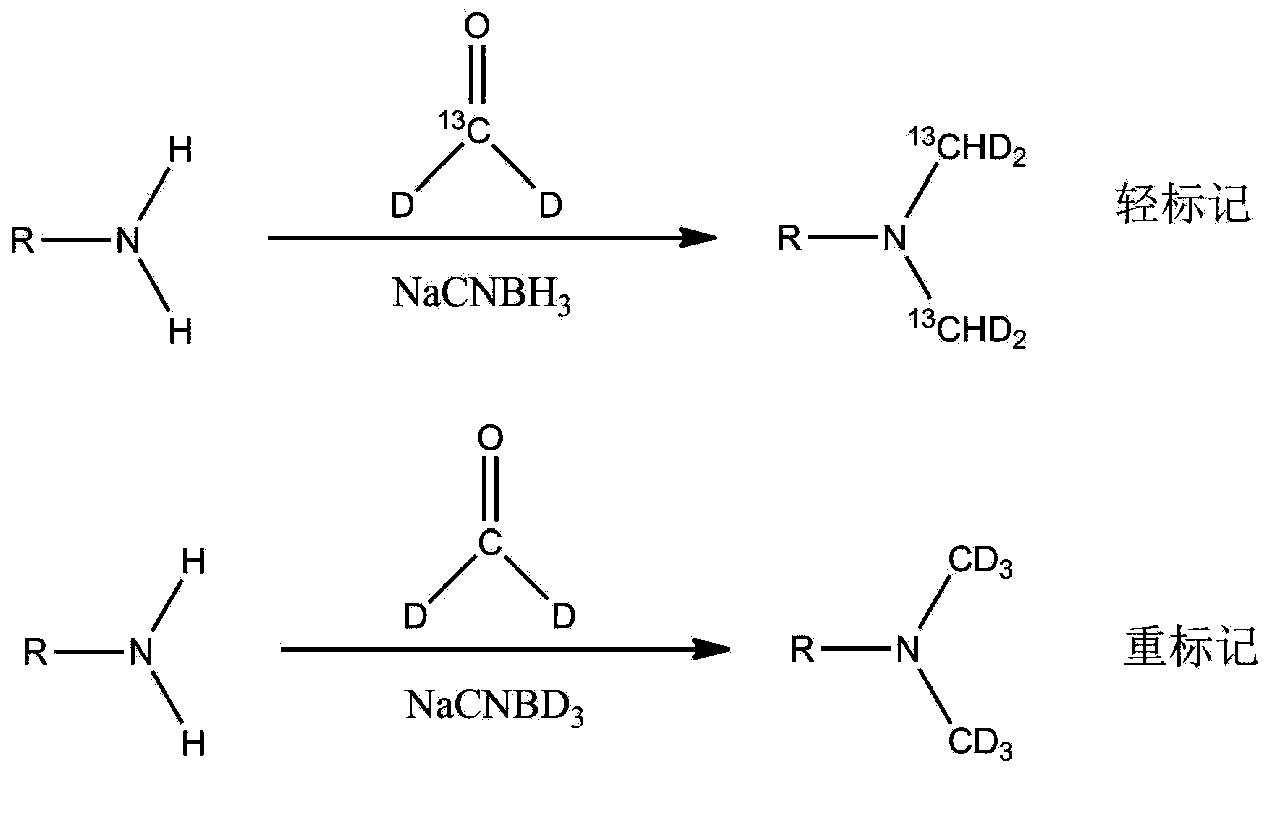
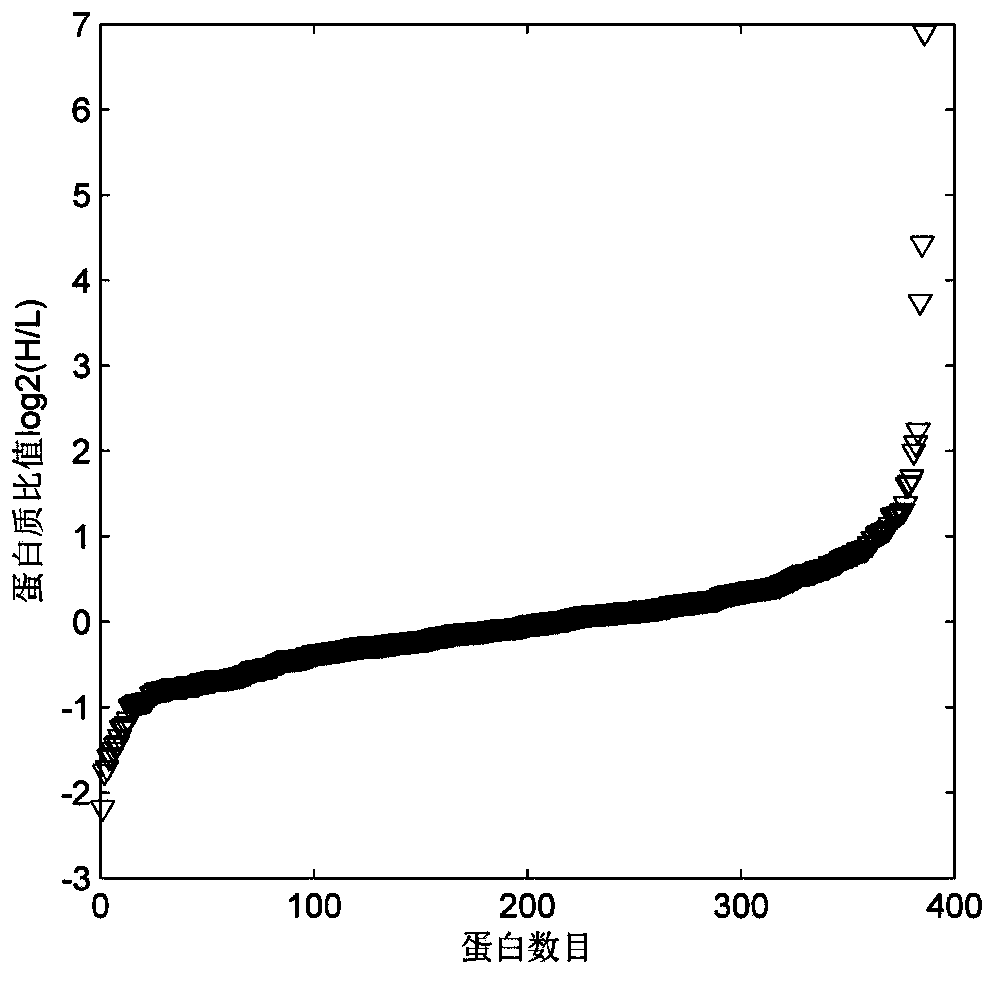
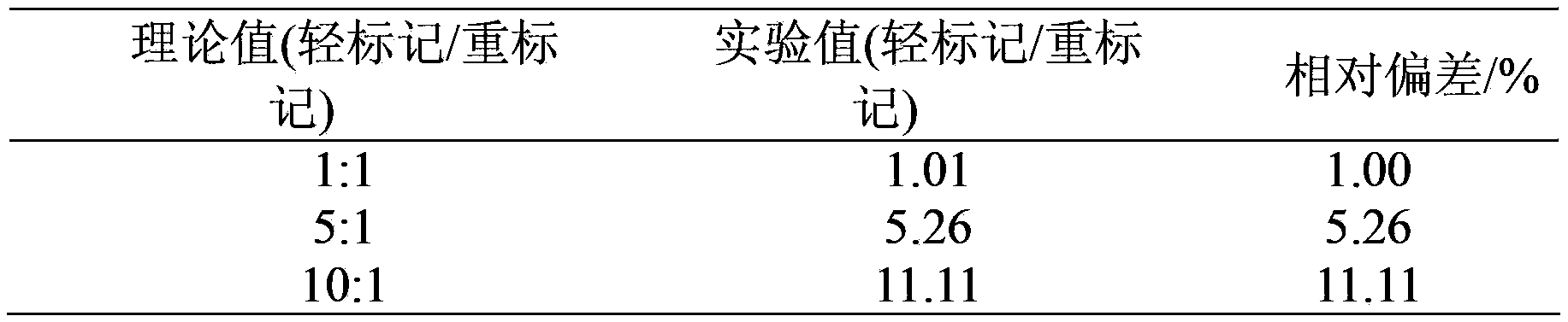
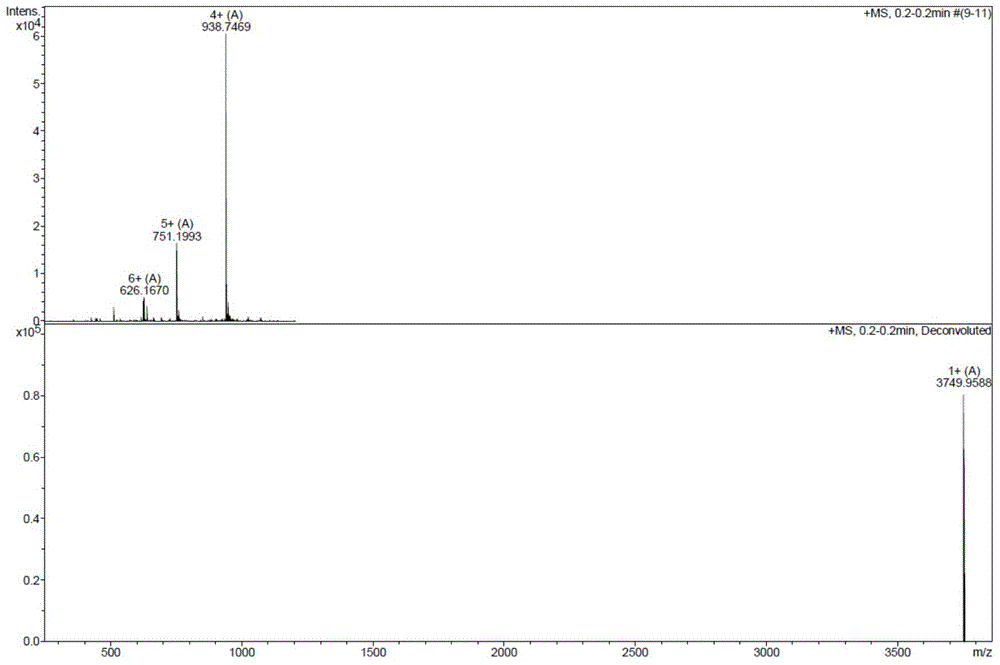
The invention relates to a new protein quantitative method, in particular to a quantitative method utilizing equiponderance dimethylation marking. The method utilizes the organic combination of methanal and isotope of the methanal, and sodium cyanoborohydride and isotope of the sodium cyanoborohydride for marking peptide fragments, so that the peptide fragments have the similar mass-to-charge ratio on the primary spectrum of the mass spectrum and have the different mass-to-charge ratios on the secondary spectrum of the mass spectrum, and the protein quantitative analysis is achieved on the secondary spectrum. Compared with other quantitative methods, the method has the advantages of being high in quantitative accuracy and precision, and wide in dynamic range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
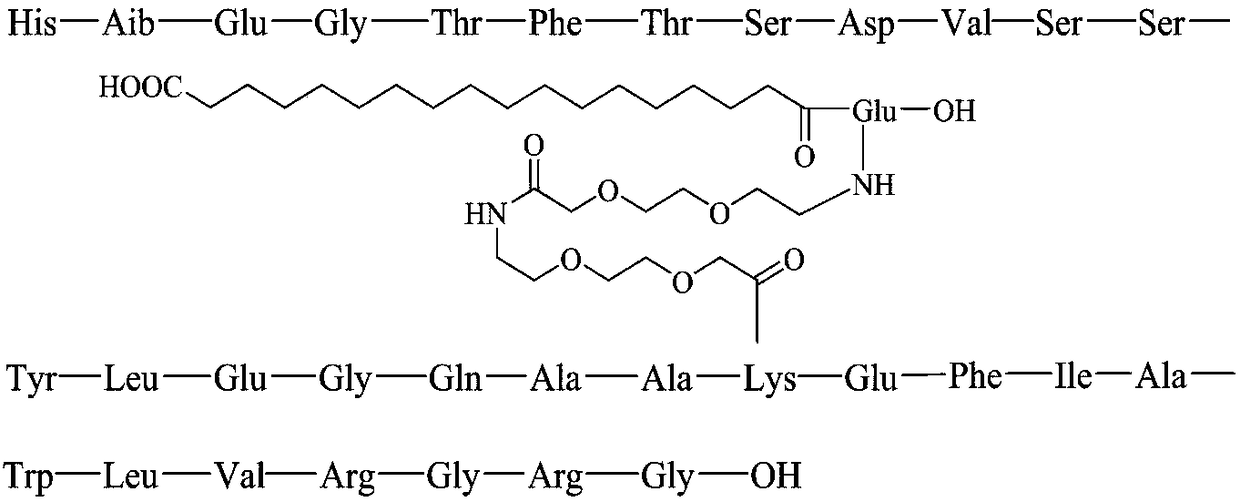
Method for preparing liraglutide by convergent synthesis
ActiveCN104650219AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionSide chainPeptide fragment
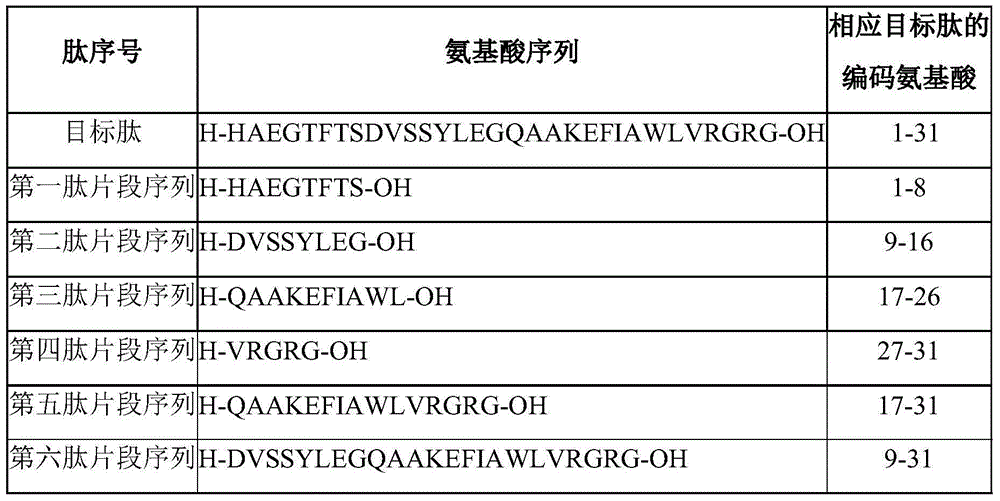
The invention discloses a method for preparing liraglutide by convergent synthesis. The method comprises the steps of performing solid phase synthesis to obtain four side chain protected peptide fragment sequences, gradually coupling the peptide fragments in a solution system to obtain an all-protected liraglutide straight chain polypeptide, removing the side chain protection of the 20th Lys, performing modification to form all-protected liraglutide, then cracking to remove protecting groups to obtain a crude liraglutide peptide, purifying and exchanging salt to obtain liraglutide; wherein in the four peptide fragment sequences, the first peptide fragment sequence is 1st to 8th amino acids in the liraglutide sequence, the second peptide fragment sequence is 9th to 16th amino acids in the liraglutide sequence, the third peptide fragment sequence is 17th to 26th amino acids in the liraglutide sequence, and the fourth peptide fragment sequence is 27th to 31st amino acids in the liraglutide sequence. By adopting the method, the yield is improved, and the synthesis cost is greatly reduced; and the method is favorable for large-scale and industrialized production.
Owner:LANZHOU UNIVERSITY
Post translational modification pattern analysis
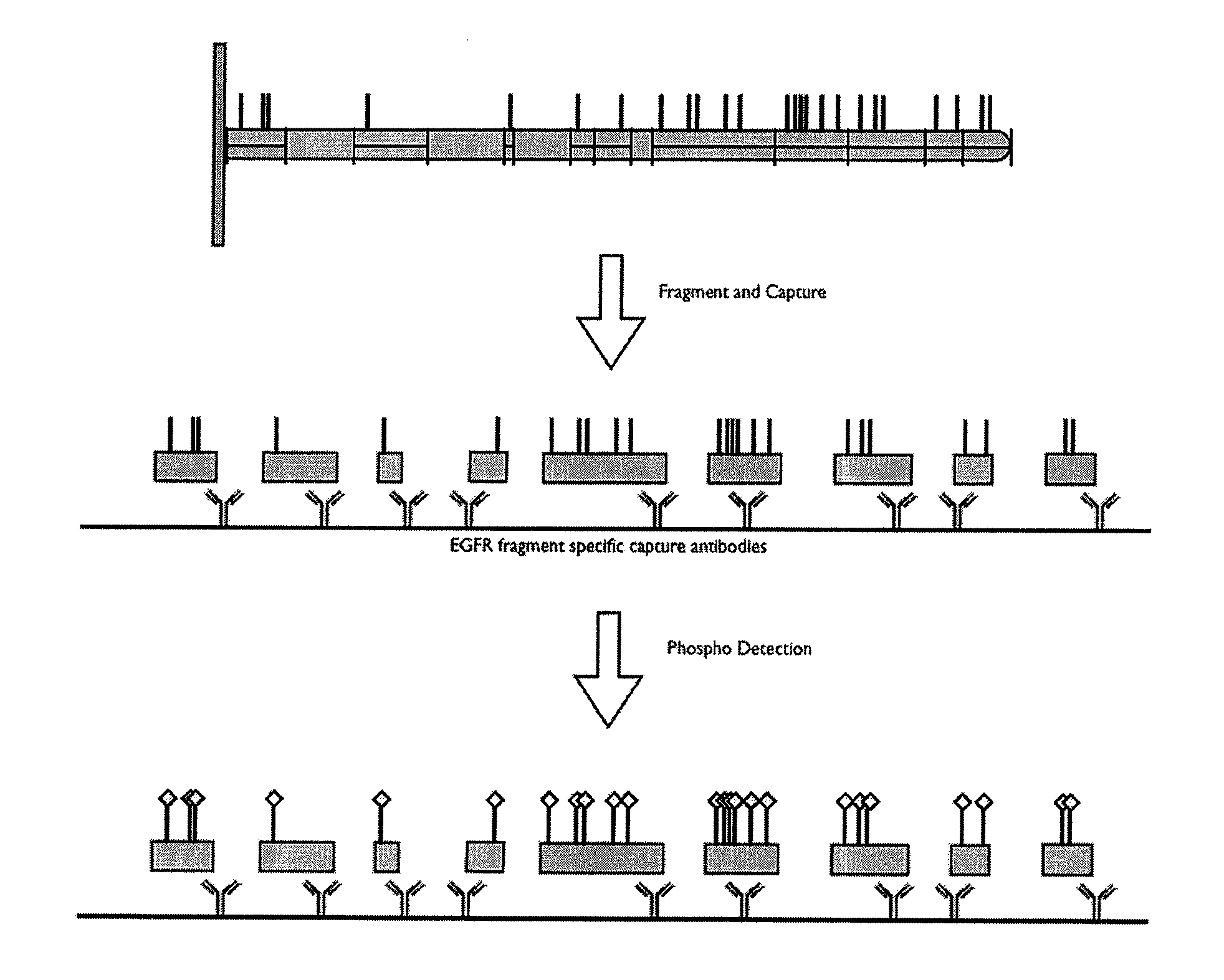
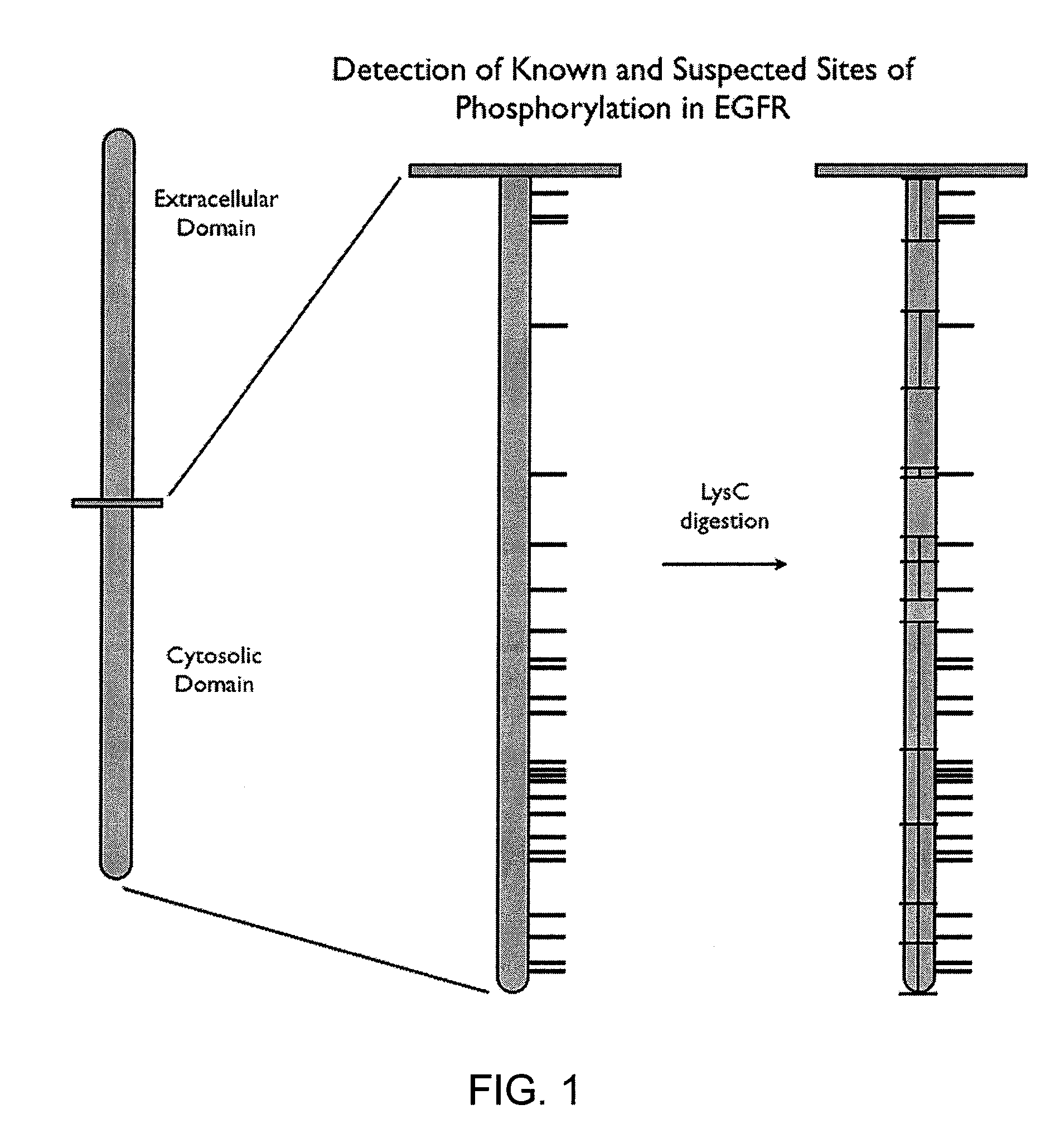
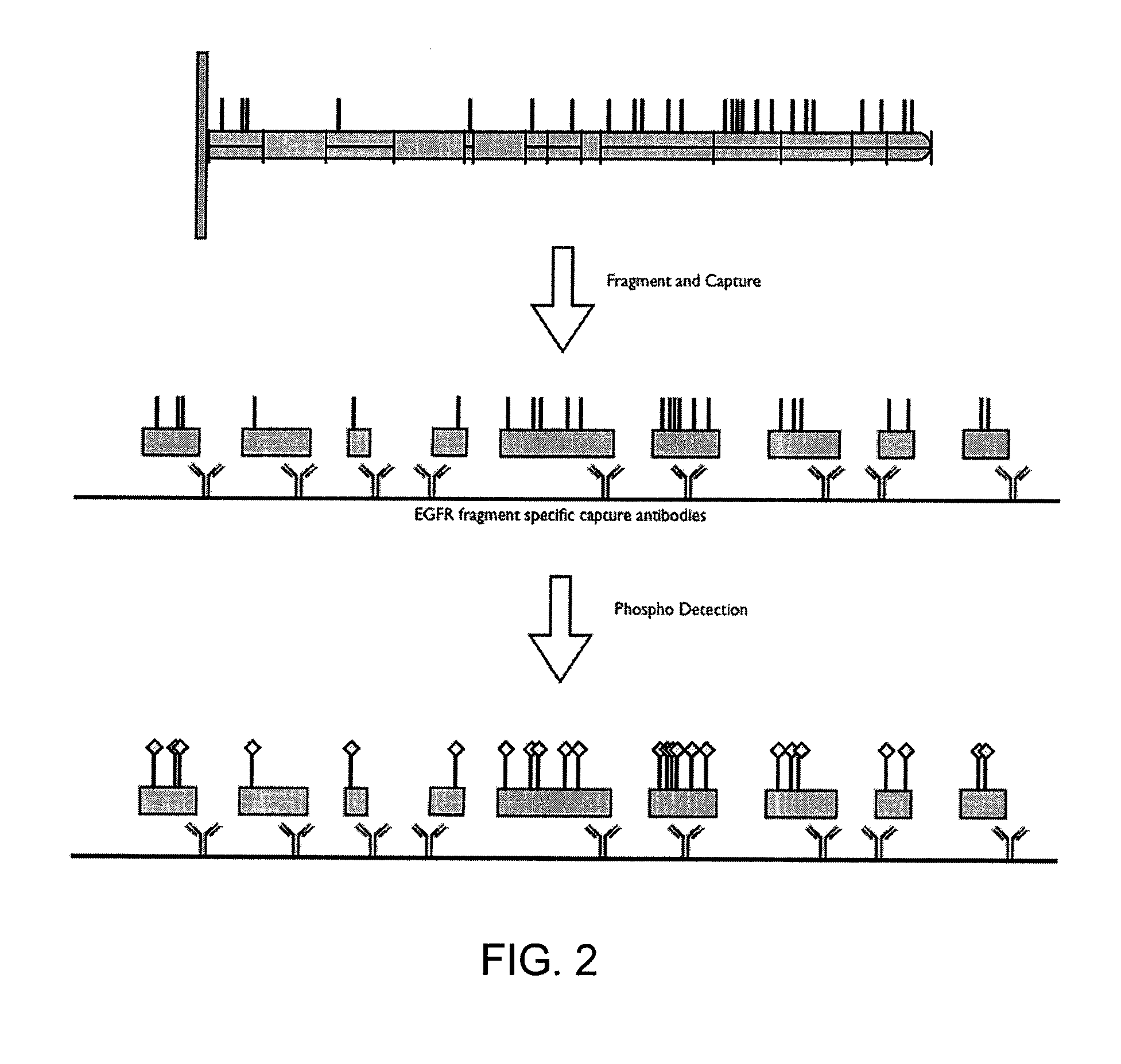
InactiveUS20100331200A1Increase flexibilityReliably retrievedLibrary screeningBiological testingPost translationalProtein target
This invention relates to methods and apparatus for detecting the pattern of post translational modification in a protein or in a plurality of proteins in a sample. One or more target proteins are subjected to predetermined proteolysis to yield plural peptide fragments comprising potential post translational modification sites. The fragments and the state of such sites are analyzed to yield a post translational pattern for the protein or proteins.
Owner:MILLIPORE CORP
Tumor neoantigen detection method and device based on next-generation sequencing, and storage medium
ActiveCN108796055AQuality improvementMicrobiological testing/measurementSpecial data processing applicationsMolecular identificationClonality Analysis
Owner:深圳裕策生物科技有限公司
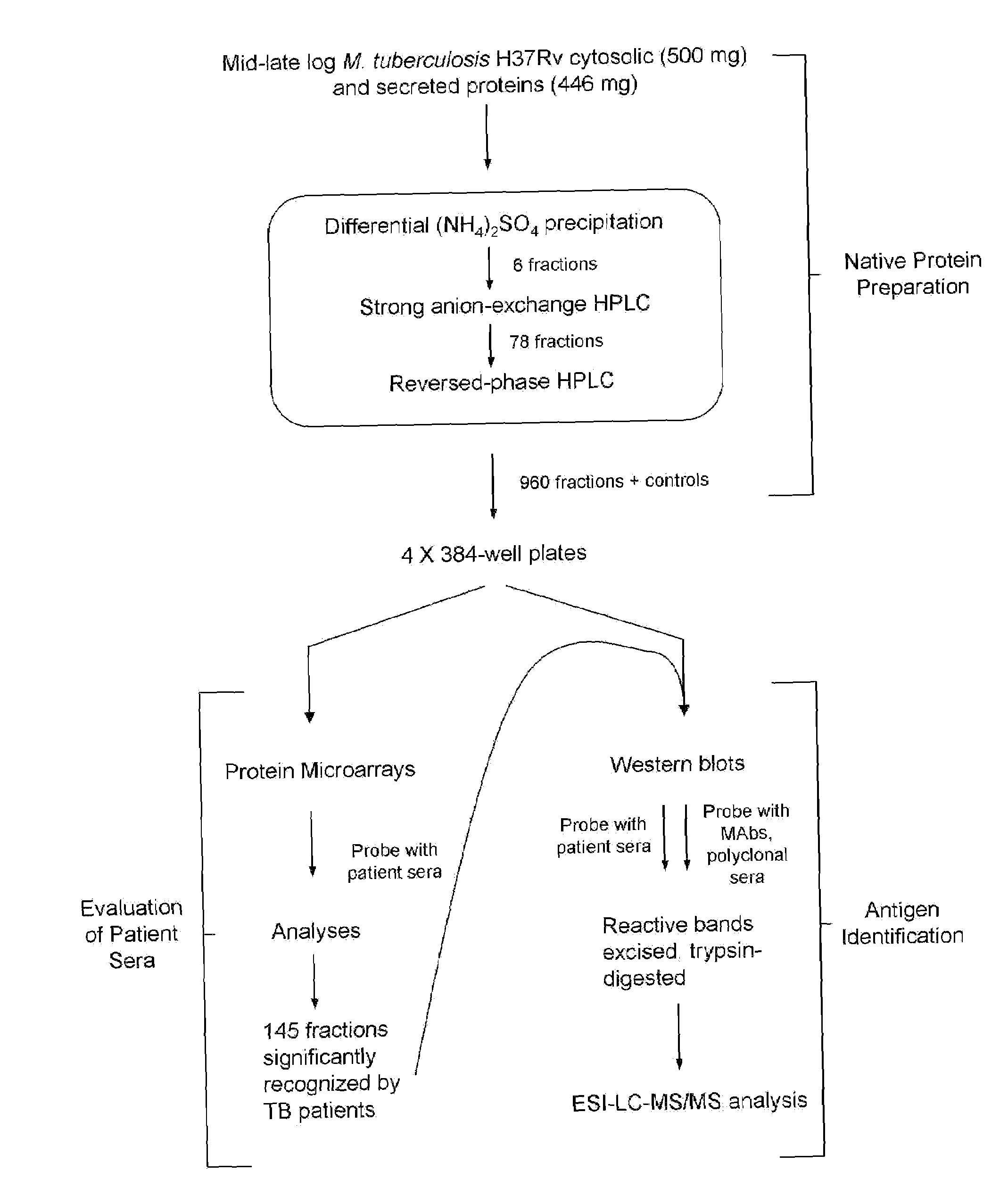
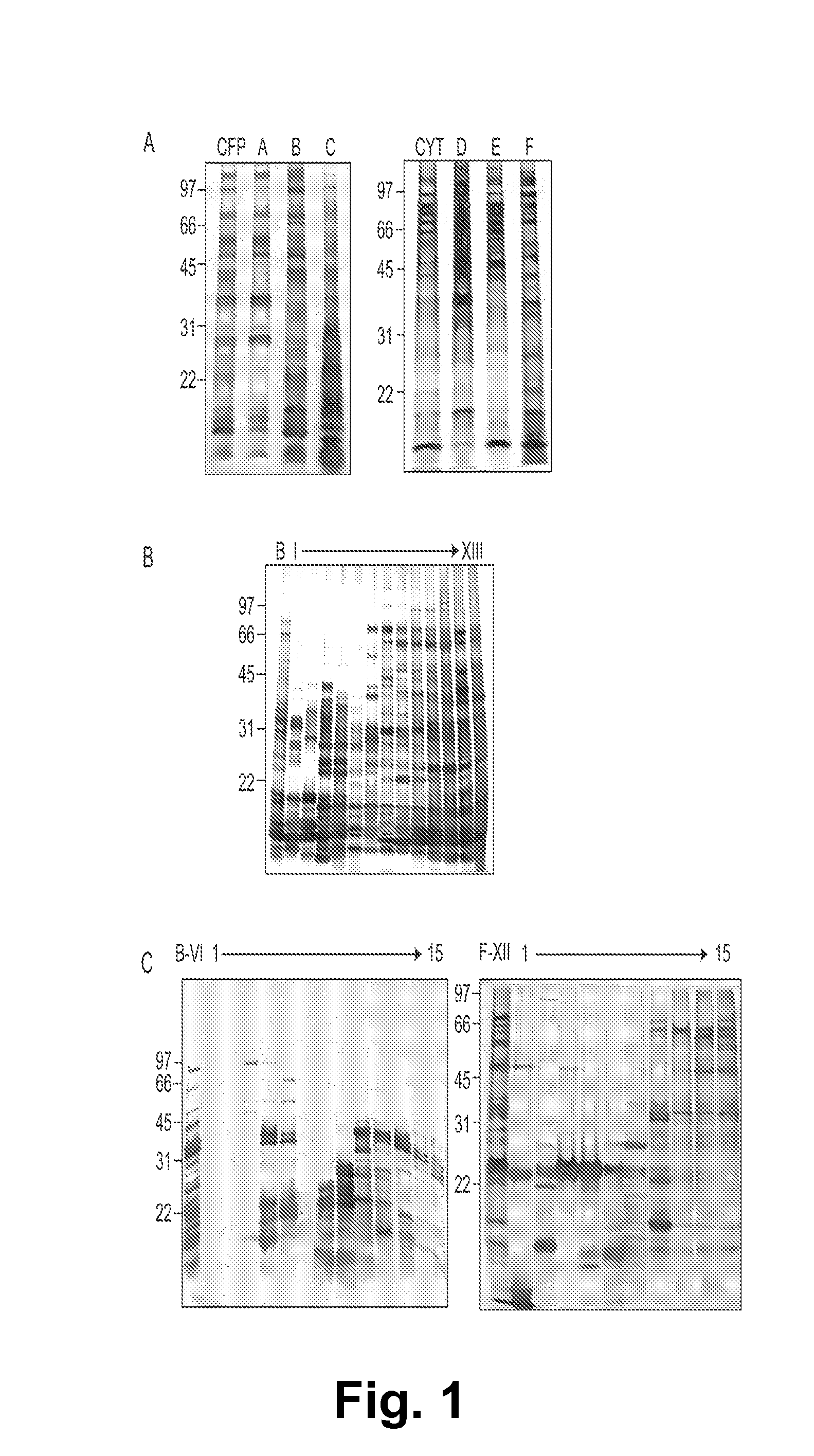
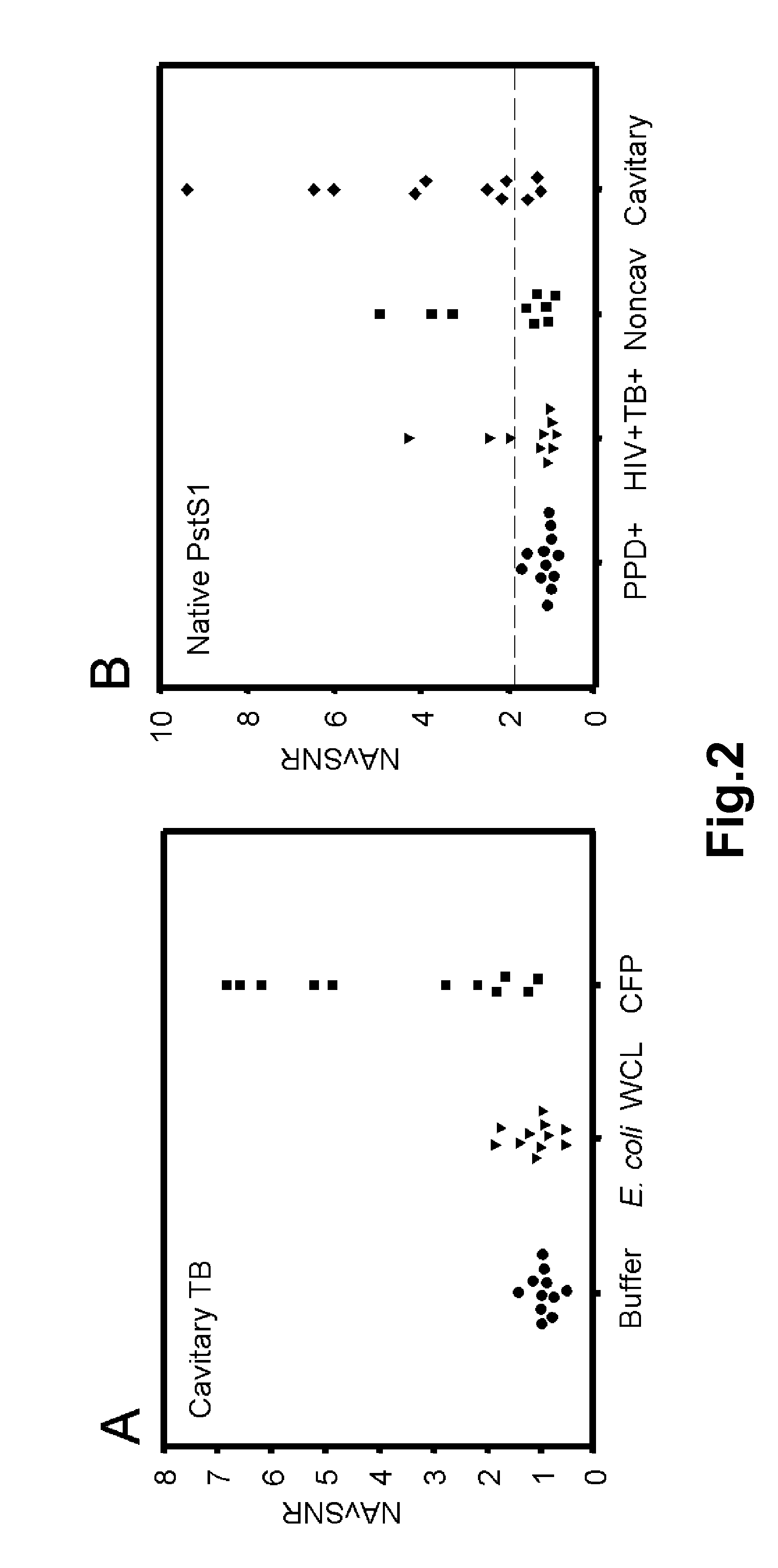
Biomarkers of tuberculosis that distinguish disease categories: use as serodiagnostic antigens
Mycobacterial proteins from culture filtrate or cytosol are disclosed as being useful B cell antigens for early diagnosis of mycobacterial disease, particularly in humans. These proteins include four that had not previously been recognized as B cell antigens (LppZ protein encoded by Mtb gene Rv3006; SodC protein encoded by Mtb gene Rv0432; BfrB protein encoded by Mtb gene Rv3841 and TrxC protein encoded byMtb gene Rv3914). Antigenic compositions include these proteins and / or peptide fragments thereof, in various combinations with each other or with one or more of a set of 10 additional Mtb proteins known to be antigens (in paricular early antigens. Methods and kits for using these antigenic composition for early diagnosis of mycobacterial infection and disease are also disclosed.
Owner:NEW YORK UNIV +1
Prevention and treatment of hypergastrinemia
Serum-associated hypergastrinemia is treated by administration of gastrin active or passive immunization. An anti-gastrin immunogenic composition containing a gastrin G17 or G34 peptide fragment which is amino acid spacer-linked to an immunogenic carrier, is administered so as to effectively neutralize the circulating gastrin hormone, and moreover, inhibit autocrine activity by progastrin such as Gly-extended G17, and amidated G17, in patients with pernicious anemia. Moreover, the method includes administration of a therapeutically effective amount of anti-G17 or anti-G34 antibodies which may be in humanized form. Finally, the method provides ameliorating treatment of hypergastrinemic effects of proton pump inhibitors or H2 histamine receptor blocking agents or antagonists, in addition to treatment of hypergastrinemia caused by diseases such as pernicious anemia.
Owner:CANCER ADVANCES INC
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
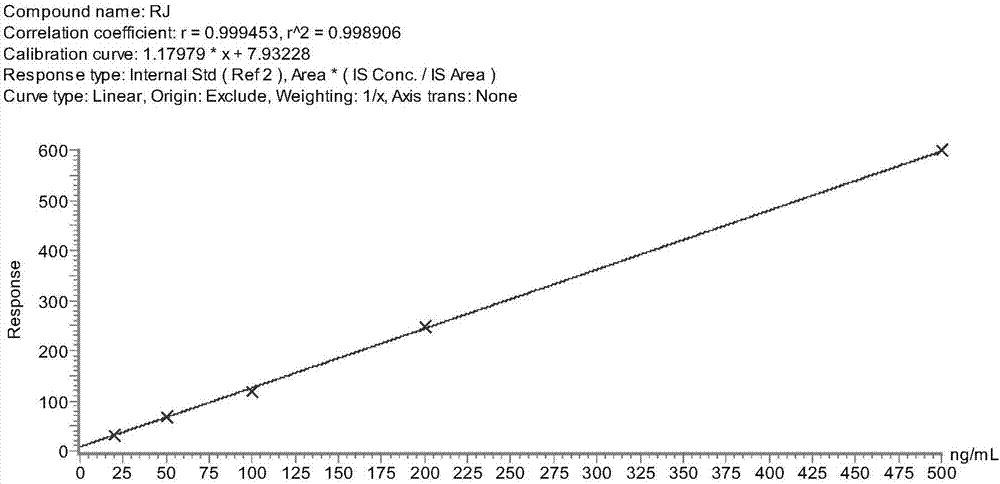
Method of establishing liquid chromatography-mass spectrometry (LC-MS) analysis of royal jelly sensitized protein
ActiveCN107290461AAccurate quantitative analysisStrong specificityComponent separationElectrophoresisMass spectrometry imaging
The invention discloses a method of establishing liquid chromatography-mass spectrometry (LC-MS) analysis of a royal jelly sensitized protein. The method comprises the following steps: carrying out electrophoresis analysis on royal jelly proteins, preprocessing the sensitized protein bands, analyzing sensitized protein bands by LC-MS, screening the specific peptide fragments of sensitized proteins; extracting proteins from a royal jelly sample, digesting the proteins by enzymes, and quantitatively measuring the sensitized proteins by LC-MS / MS. The provided sensitized protein quantitative method has the advantages of high specificity and sensitivity, good repeatability, high accuracy, and wide linear range, and is capable of precisely and quantitatively analyzing a sensitized protein in a sample.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
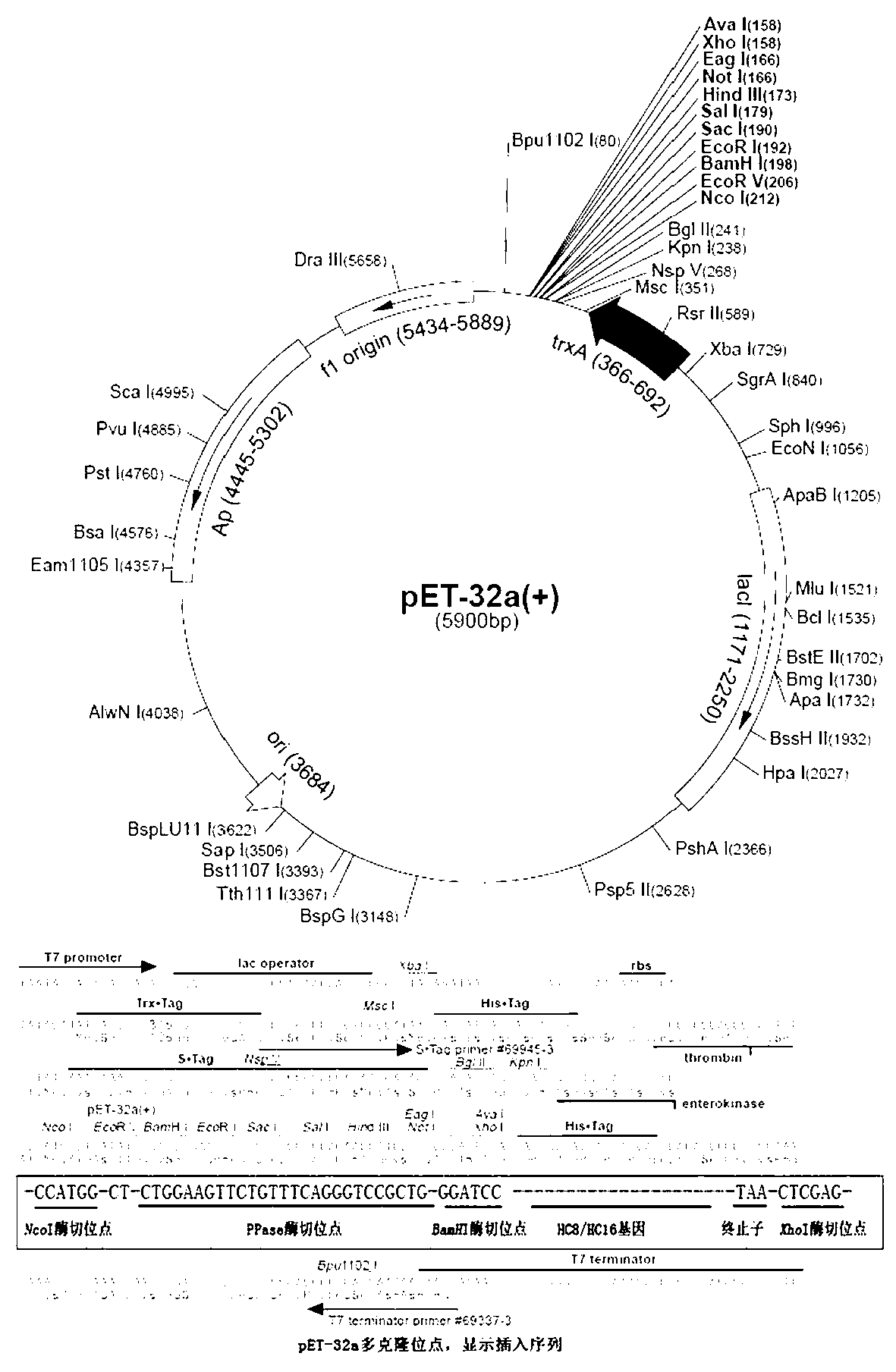




![Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02083782-a159-4611-b11f-ac69b2a26ec4/130312135516.PNG)
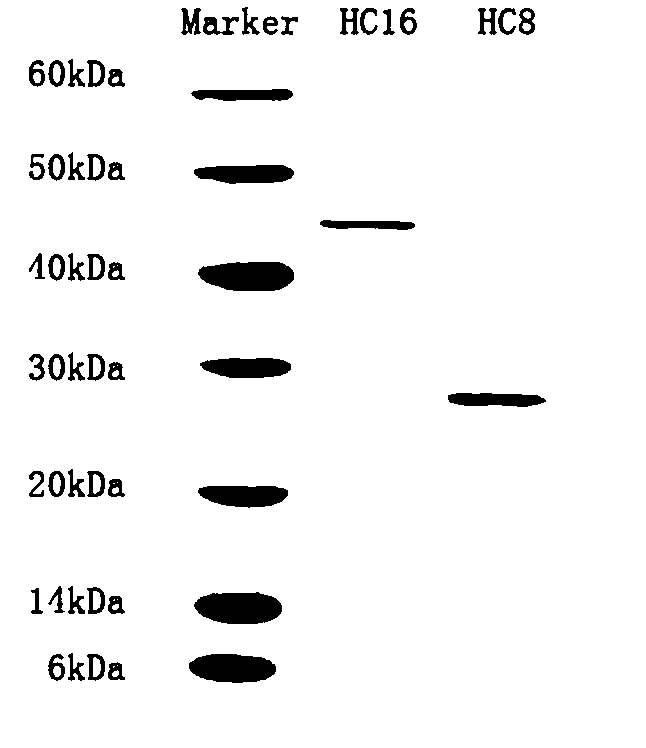
![Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02083782-a159-4611-b11f-ac69b2a26ec4/130312135520.PNG)
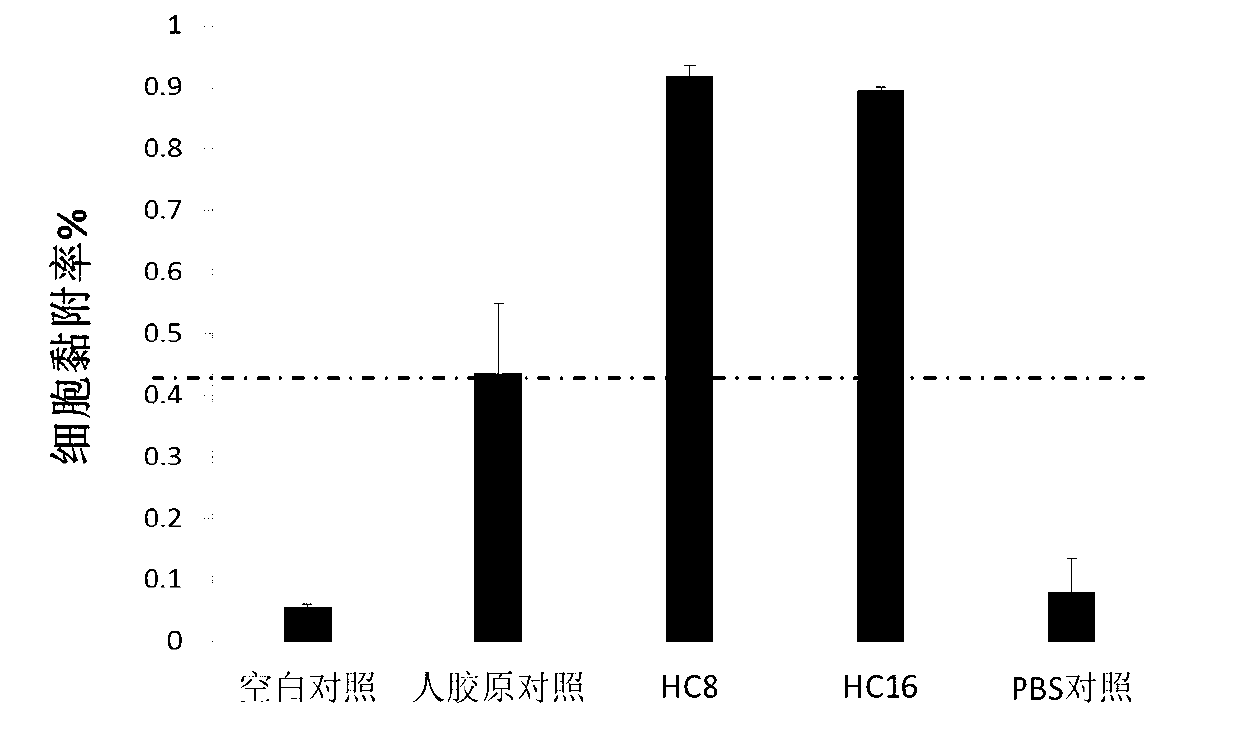
![Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material Synthetic method for magnetic metal organic framework composite material coated by [Cu3(btc)2] on surfaces of ferroferric oxide microspheres and application of composite material](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02083782-a159-4611-b11f-ac69b2a26ec4/130312135523.PNG)