Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Mycobacterial disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
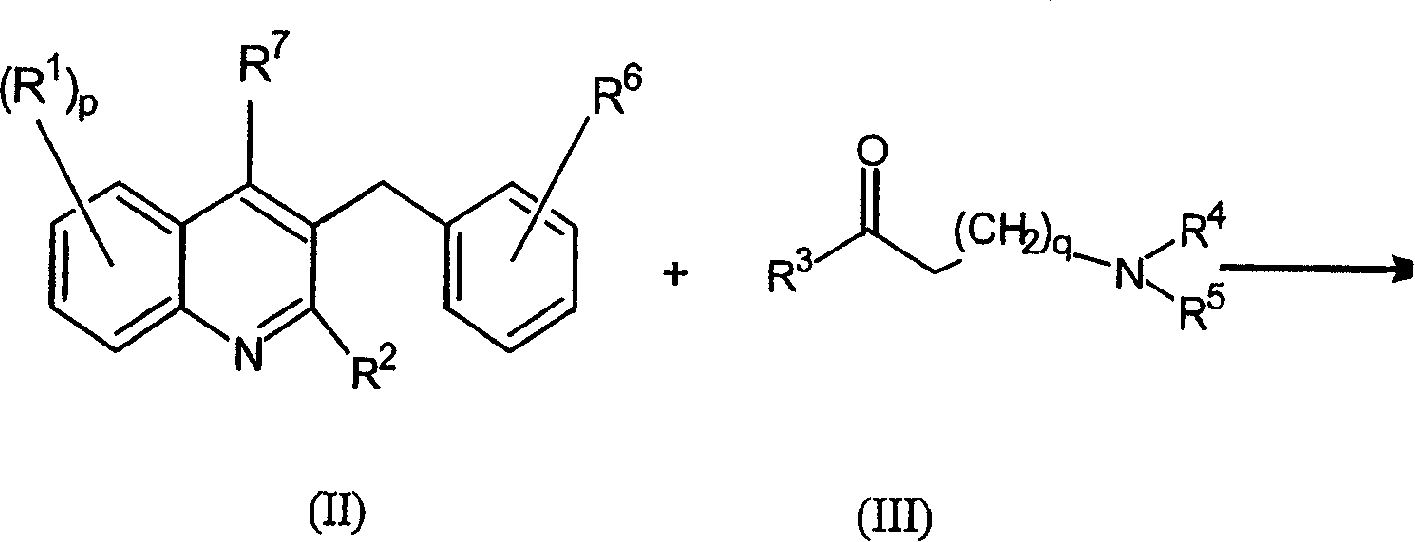
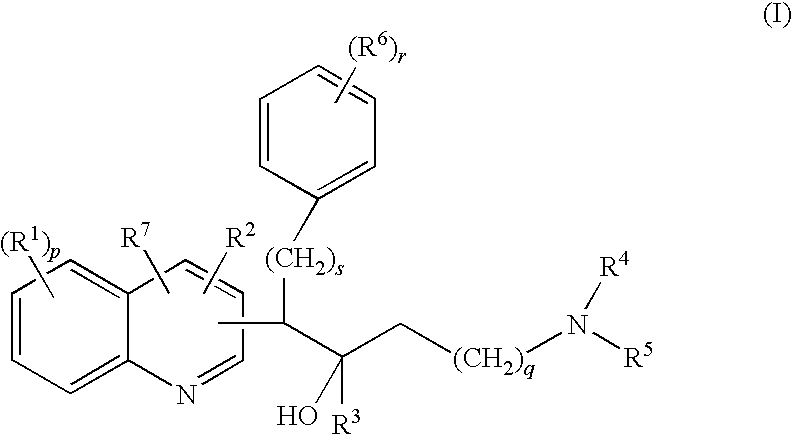
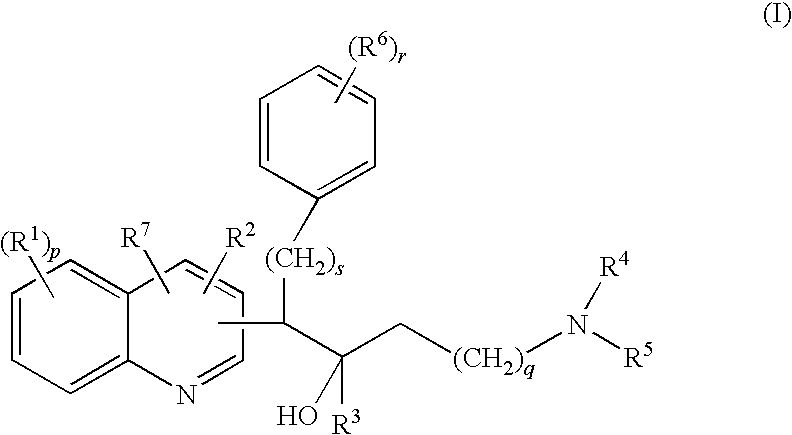
Quinoline derivatives and their use as mycobacterial inhibitors
Owner:JANSSEN PHARMA NV
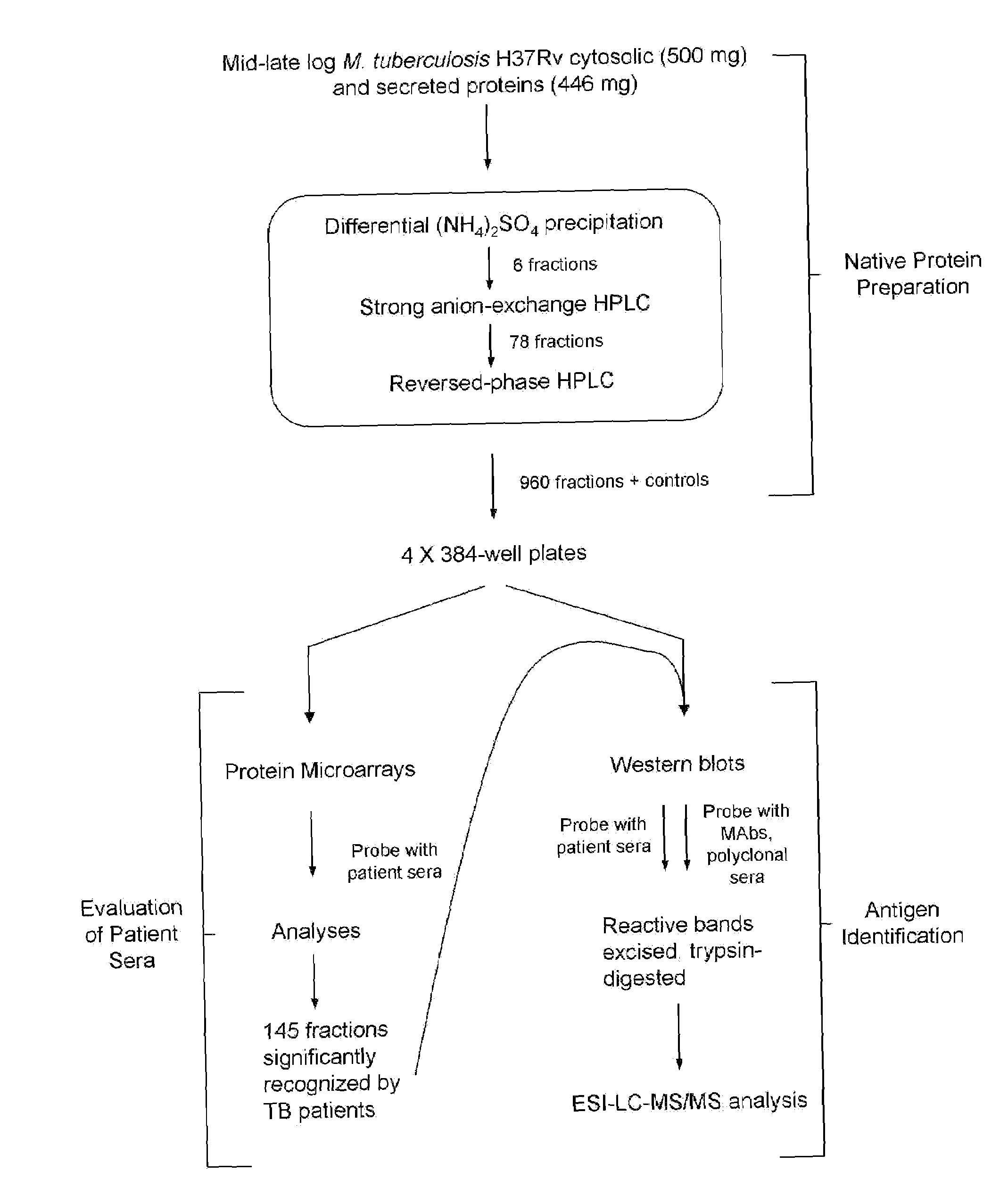
Biomarkers of tuberculosis that distinguish disease categories: use as serodiagnostic antigens
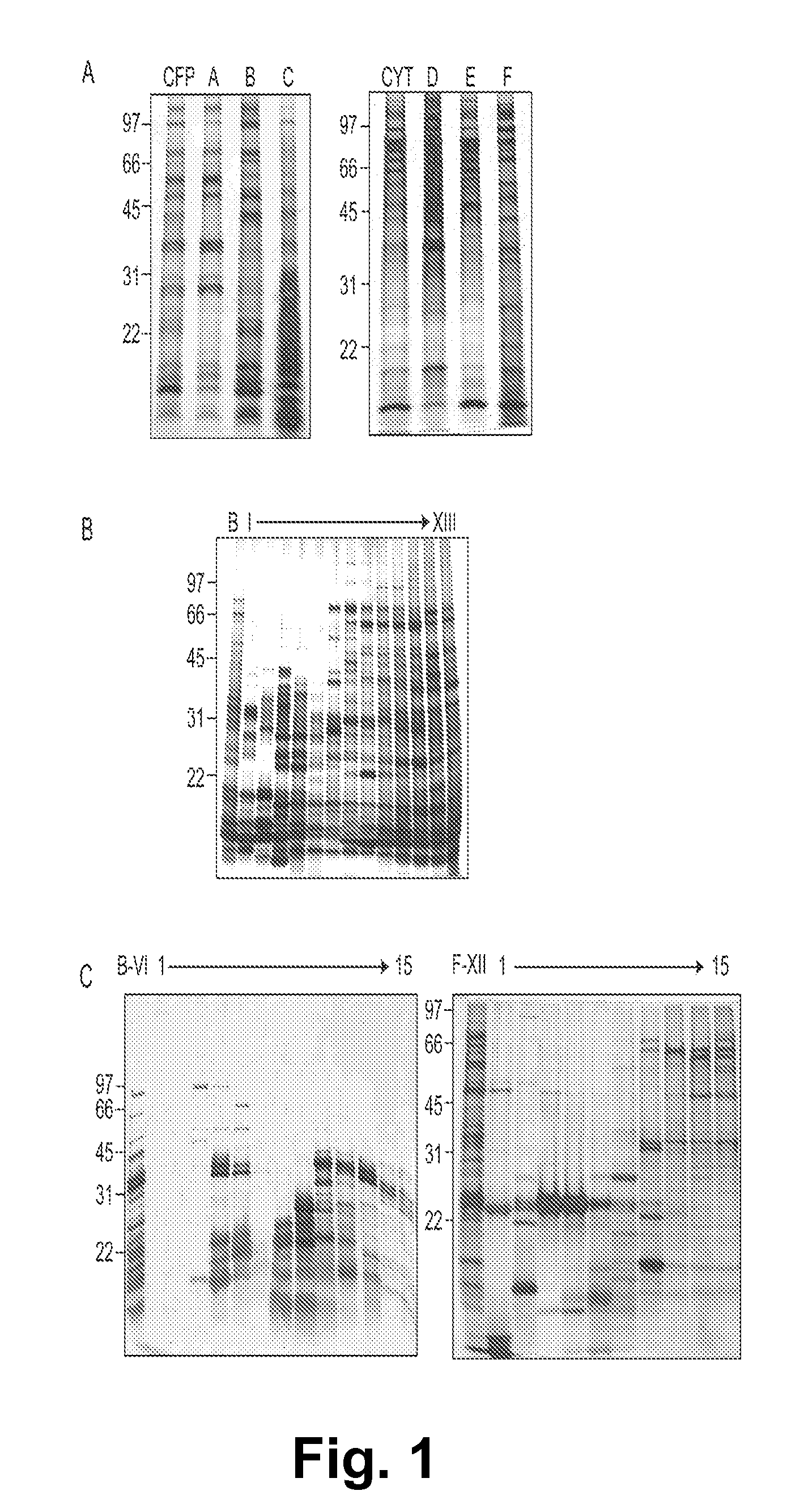
Mycobacterial proteins from culture filtrate or cytosol are disclosed as being useful B cell antigens for early diagnosis of mycobacterial disease, particularly in humans. These proteins include four that had not previously been recognized as B cell antigens (LppZ protein encoded by Mtb gene Rv3006; SodC protein encoded by Mtb gene Rv0432; BfrB protein encoded by Mtb gene Rv3841 and TrxC protein encoded byMtb gene Rv3914). Antigenic compositions include these proteins and / or peptide fragments thereof, in various combinations with each other or with one or more of a set of 10 additional Mtb proteins known to be antigens (in paricular early antigens. Methods and kits for using these antigenic composition for early diagnosis of mycobacterial infection and disease are also disclosed.
Owner:NEW YORK UNIV +1
Combination therapy to treat mycobacterium diseases
ActiveUS20140045791A1Growth inhibitionAntibacterial agentsBiocideCombined Modality TherapyAntibacterial activity
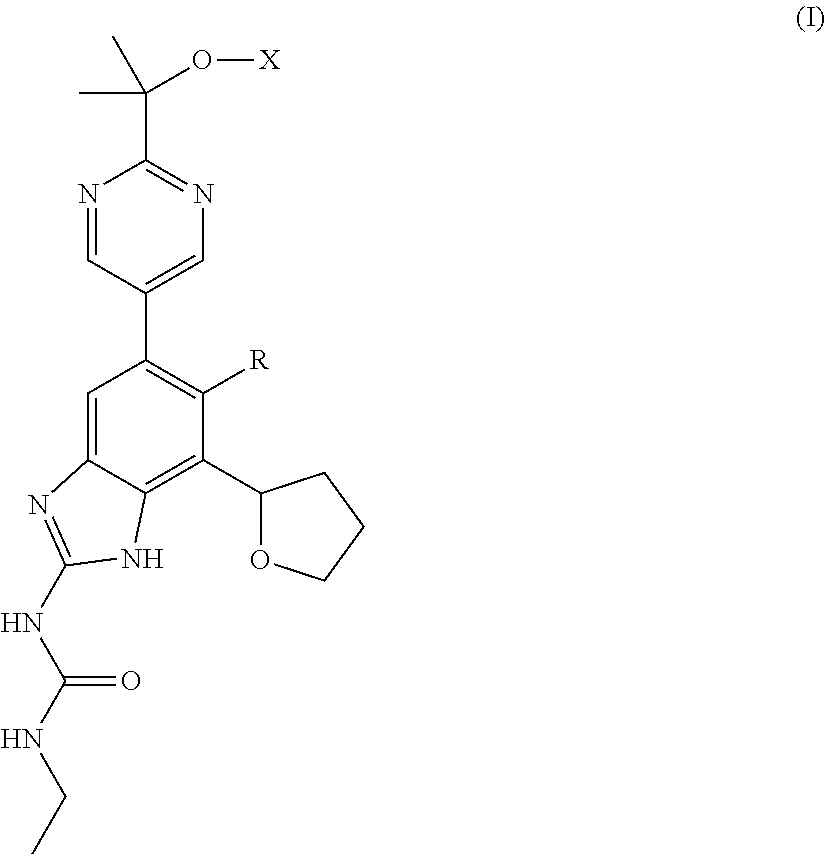
The present invention relates to a compound of formula (I)or a pharmaceutically acceptable salt thereof wherein X and R are as defined herein. The compounds of formula (I) are useful as gyrase and / or topoisomerase IV inhibitors for treating bacterial infections. The compounds of formula (I) either possess a broad range of anti-bacterial activity and advantageous toxicological properties or are prodrugs of compounds having said activity.
Owner:SPERO THERAPEUTICS INC
Early detection of mycobacterial disease using peptides
InactiveUS20090280140A1Reduce distractionsUse toolAntibacterial agentsPeptide/protein ingredientsMycobacteriumProtein C
A number of protein and glycoprotein antigens secreted by Mycobacterium tuberculosis (Mtb) have been identified as “early” Mtb antigens on the basis early antibodies present in subjects infected with Mtb prior to the development of detectable clinical disease. Epitope-bearing peptide fragments of these early Mtb antigens, in particular of an 88 kDa secreted protein, GlcB (SEQ ID NO:106) and of Mtb antigen MPT51 (SEQ ID NO:107) have been identified. These peptides, variants thereof, peptide multimers thereof that include two or more repeats of one or more of the peptides, and fusion polypeptides that include early Mtb antigenic proteins, peptides or both, are useful in immunoassay methods for early, rapid detection of TB in a subject. Preferred immunoassays detect the antibodies in the subject's urine. Also provided are antigenic compositions, kits and methods to useful for detecting an early Mtb antibodies. The antigenic proteins and peptides are also used in vaccine compositions.
Owner:NEW YORK UNIV +1
Mycobacterial inhibitors
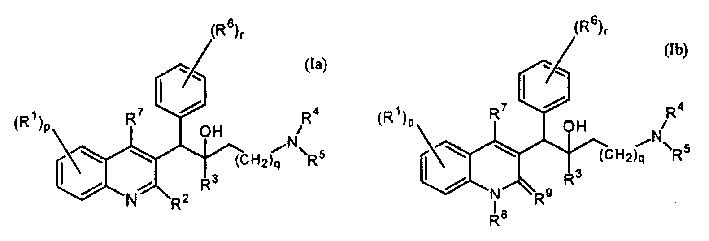
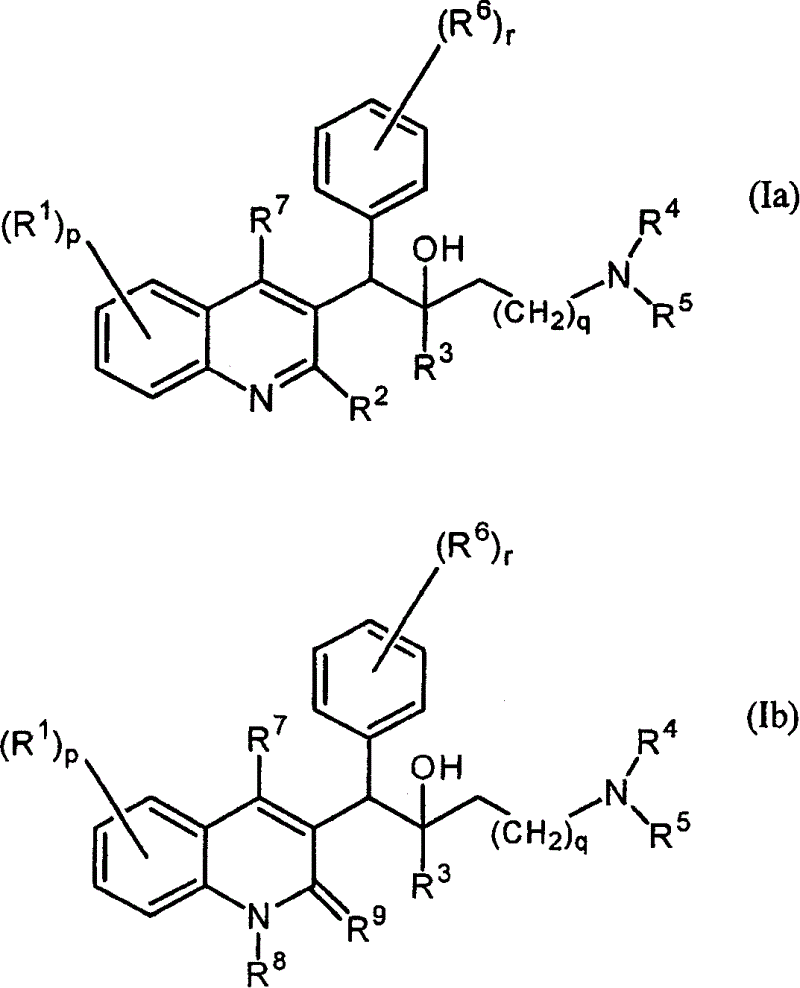
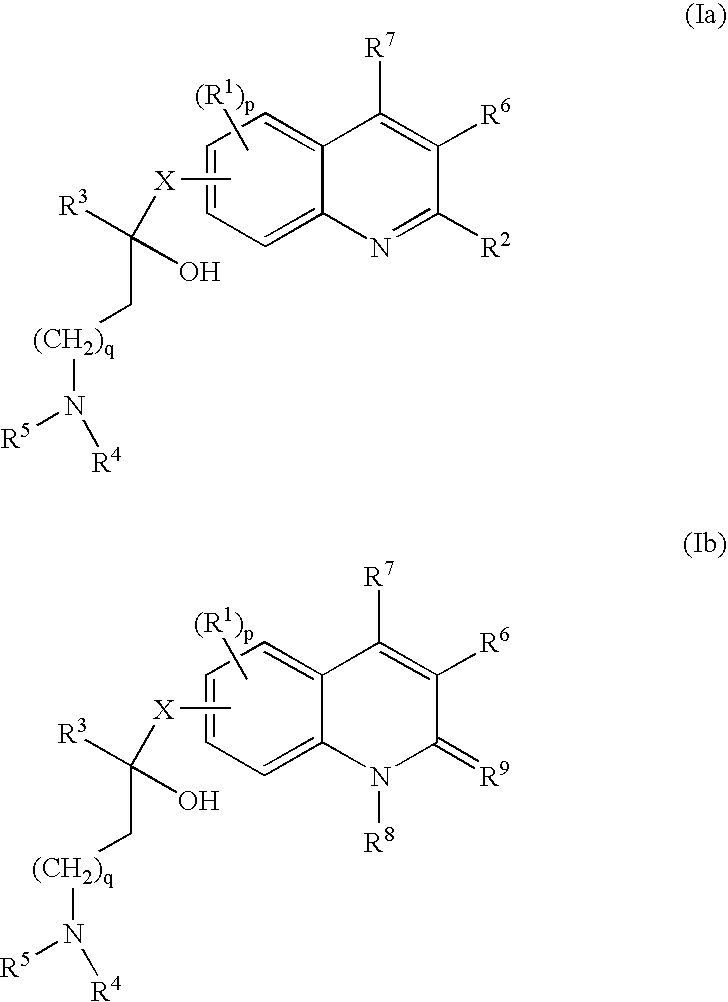
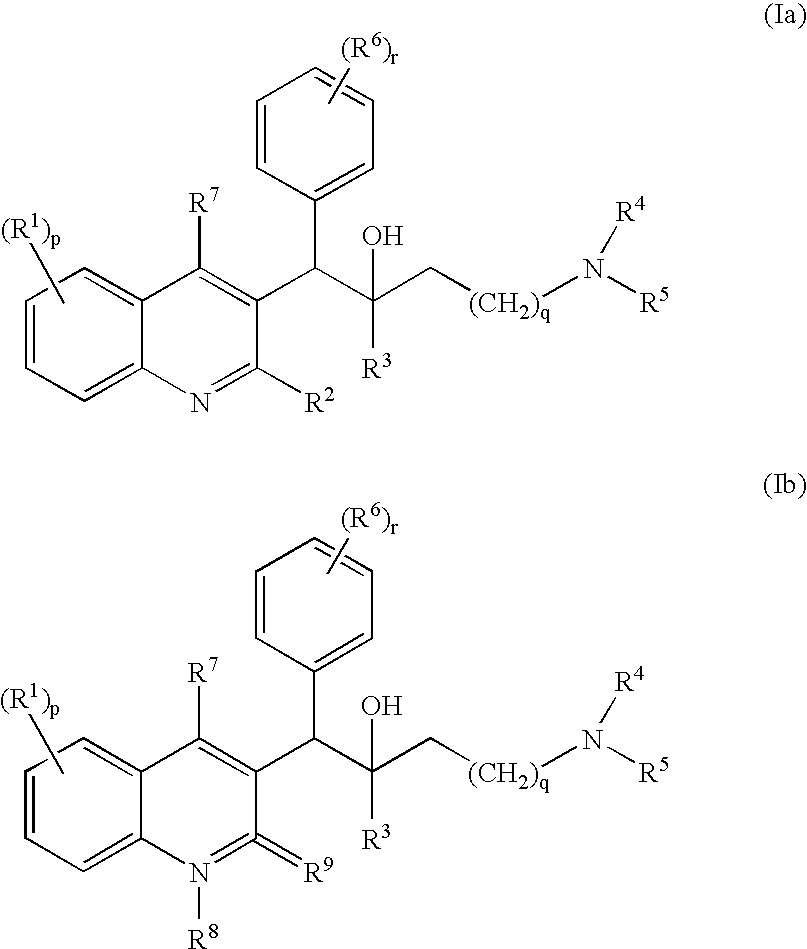
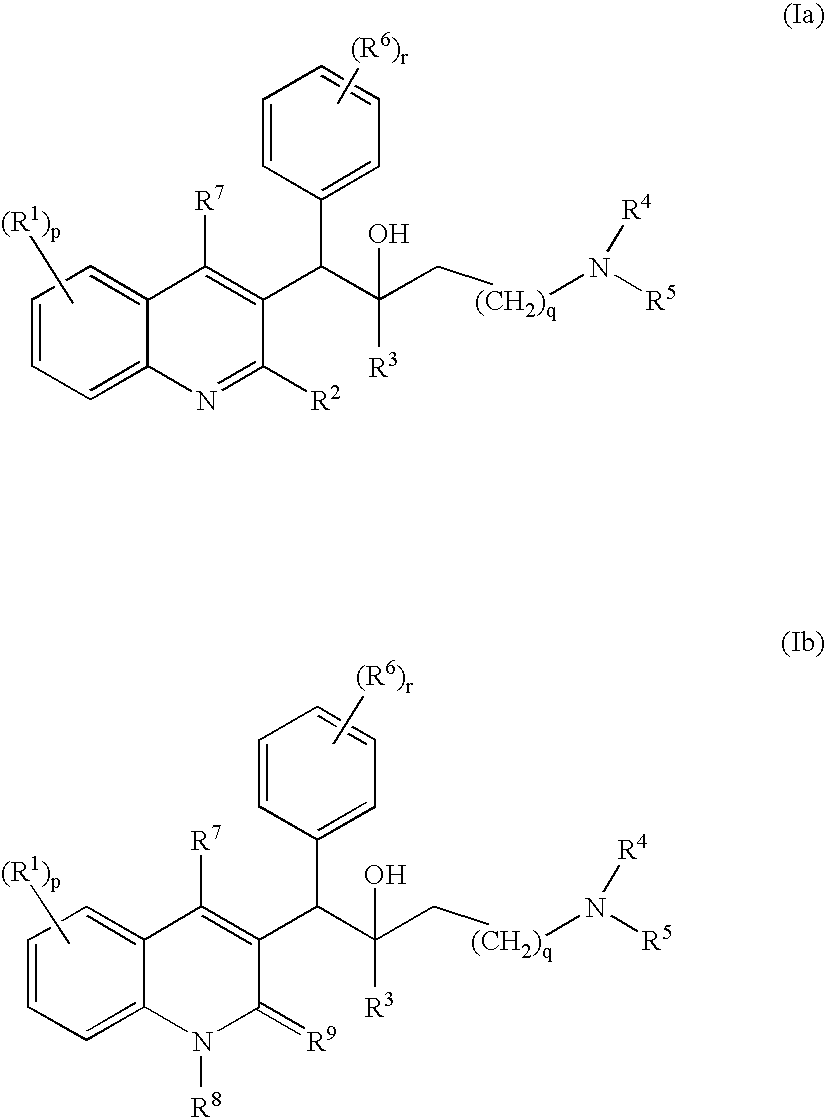
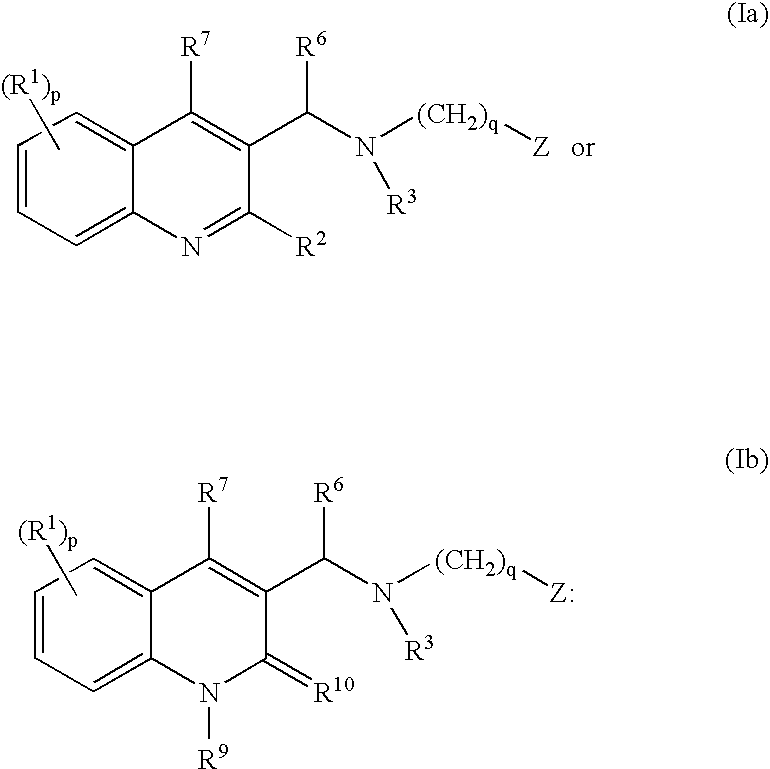
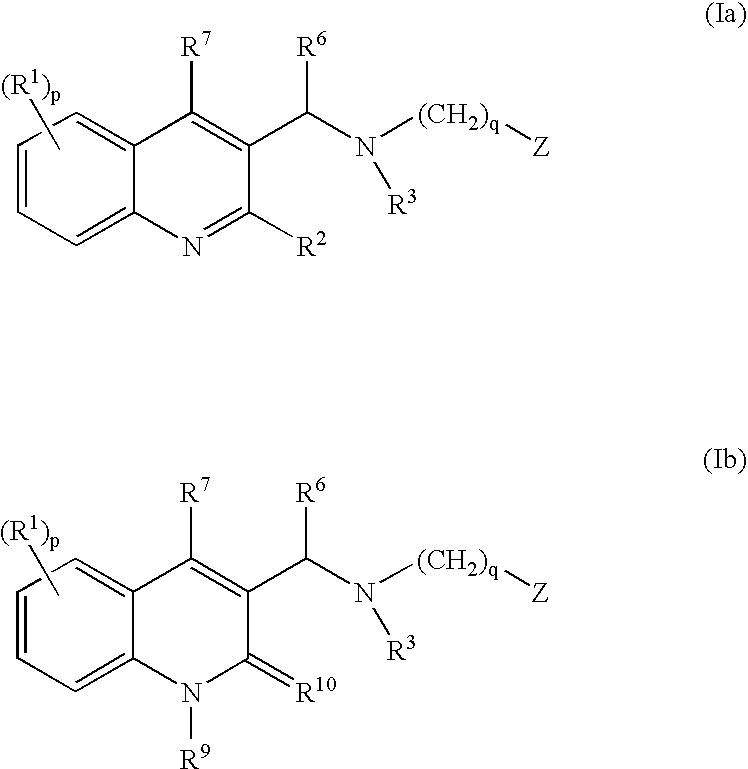
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or the general Formula (Ib)the pharmaceutically acceptable acid or base addition salts thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of mycobacterial diseases, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. In particular, compounds are claimed in which, independently from each other, R1 is bromo, p=1, R2 is alkyloxy, R3 is optionally substituted naphthyl or phenyl, q=1, R4 and R5 each independently are hydrogen, methyl or ethyl, R6 is hydrogen, r is equal to 0 or 1 and R7 is hydrogen. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of mycobacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Mycobacterial inhibitors
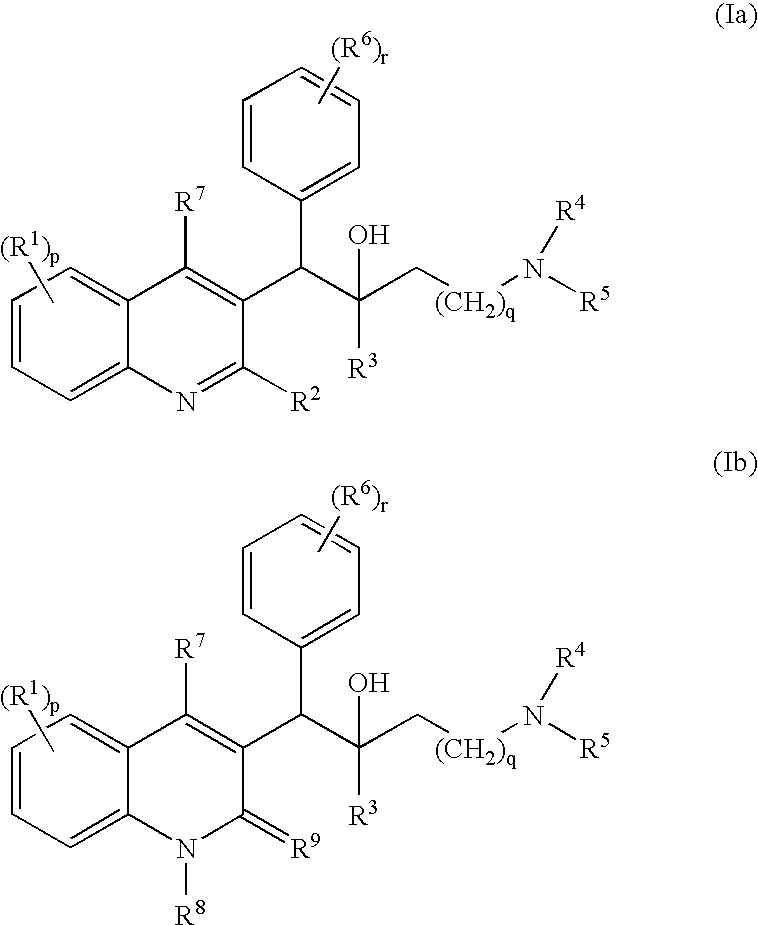
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or the general Formula (Ib)the pharmaceutically acceptable acid or base addition salts thereof, the quaternary amines thereof, the stereochemically isomeric forms thereof the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of mycobacterial diseases. In particular, compounds are claimed in which, independently from each other, R1 is halo; p=1; R2 is optionally substituted alkyloxy, alkyl, Ar, Het, or a radical of formulaR3 is optionally substituted Ar or Het; q=1, R4 and R5 each independently are alkyl; R6 is hydrogen or a radical of formular is equal to 0 or 1 and R7 is hydrogen or Ar. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of mycobacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Novel mycobacterial inhibitors
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or the general Formula (Ib) the pharmaceutically acceptable acid or base addition salts thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of mycobacterial diseases, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. In particular, compounds are claimed in which, independently from each other, R1 is bromo, p=1, R2 is alkyloxy, R3 is optionally substituted naphthyl or phenyl, q=1, R4 and R5 each independently are hydrogen, methyl or ethyl, R6 is hydrogen, r is equal to 0 or 1 and R7 is hydrogen. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of mycobacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Preparation and application of compound disinfectant
The invention relates to a disinfectant, in particular to a preparation and application of a compound disinfectant containing various disinfection components, and the compound disinfectant comprises the following components by weight percent: 0.01%-5% of guanidine compound, 0.01%-10% of quaternary ammonium salt compound, 0.01%-2% of trilon B, 0.01%-2% of 2-bromo-2-nitro-1,3-propanediol, 0.01%-10% of monohydric alcohol, 0%-30% of dihydric alcohol, 0%-30% of polyhydric alcohol and the balance of water. The invention further provides the application of the compound disinfectant composition, the compound disinfectant composition can be applied in disinfection of object surfaces, air-conditioning systems, environments and air in the field of public health, the field of food processing and the filed of medical sanitation through a multifunctional atomizing machine for effectively preventing and killing vegetative forms of bacteria, mycobacteria, viruses, fungi and spores thereof, and the like, can be applied in the disinfection of the air in automobiles, the object surfaces and the air-conditioning systems through the multifunctional atomizing machine for effectively preventing and killing the vegetative forms of the bacteria, the mycobacteria, the viruses and the like, can be applied in the disinfection of the air in train carriages, cabins and the automobiles and the disinfection of objects and the environments through the multifunctional atomizing machine for effectively preventing and killing the vegetative forms of the bacteria, the mycobacteria, the viruses and the like, can be further applied in the disinfection of the environments, indoor spaces and the object surfaces in the field of livestock and poultry breeding through the multifunctional atomizing machine for effectively preventing and killing the vegetative forms of the bacteria, the mycobacteria, the viruses, the fungi and the spores thereof, and the like.
Owner:李新建
Attenuated strains of mycobacteria
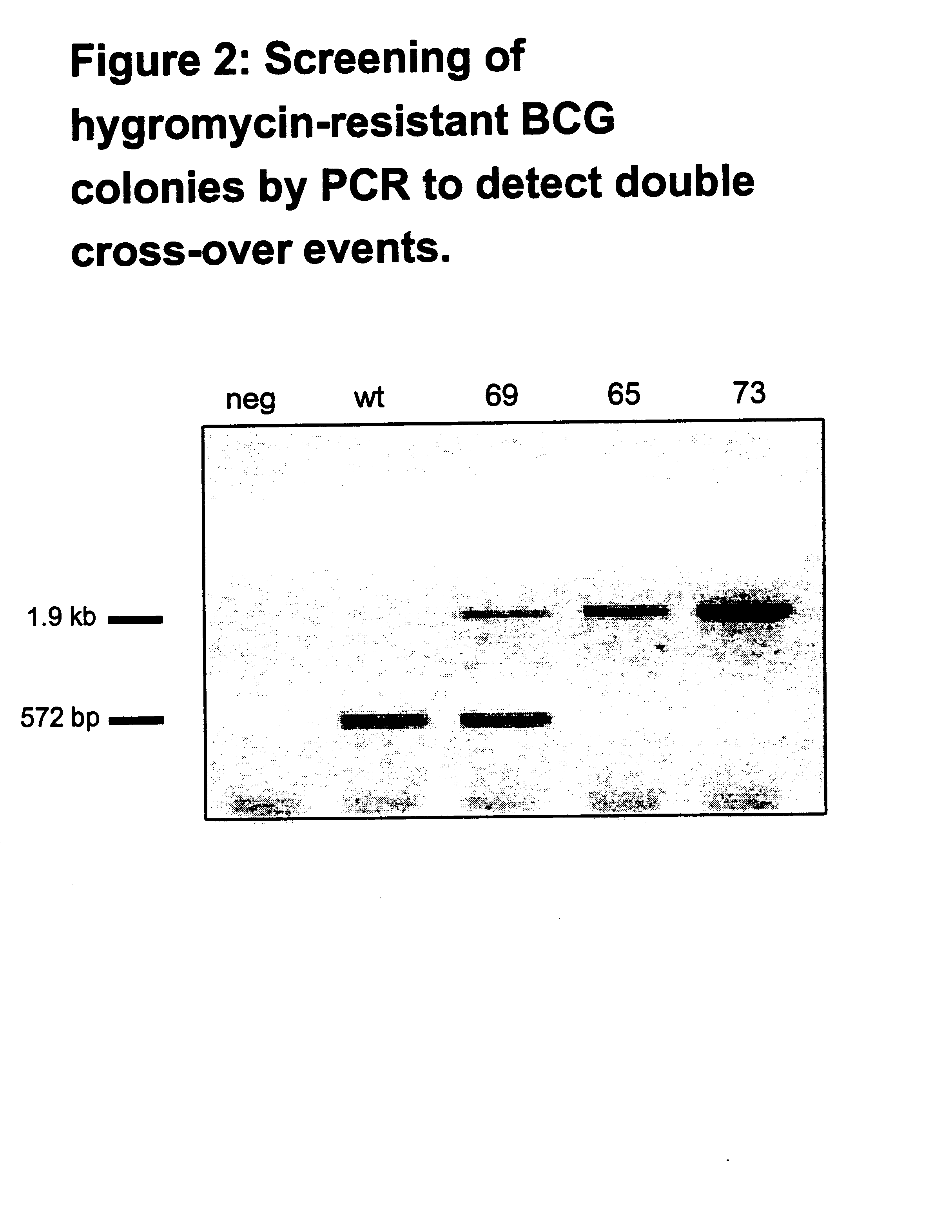
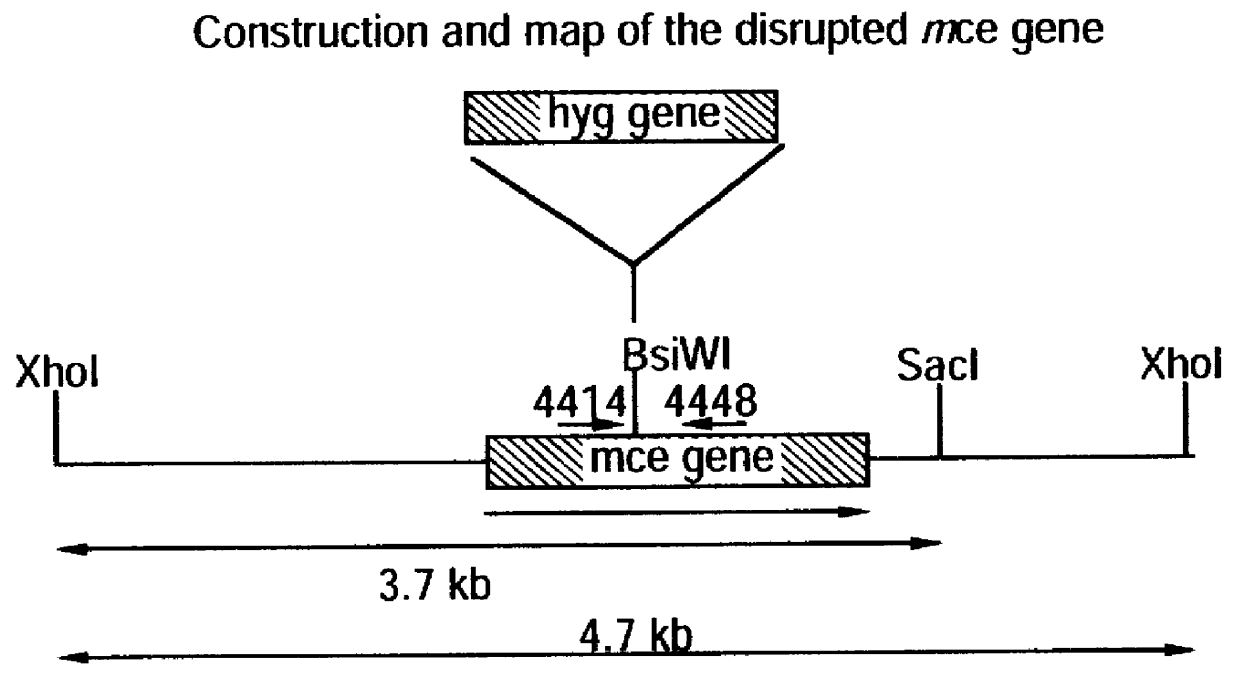
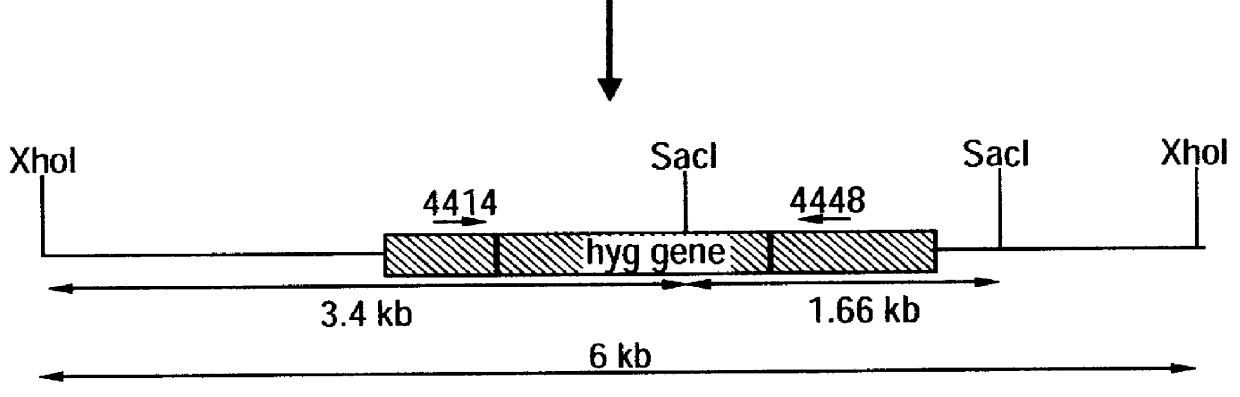
Attenuated strains of Mycobacterium, particularly species of the tuberculosis complex, have the mycobacterial cell entry (mce) gene functionally disabled. The gene may be disabled by an insertion into the gene which disrupts the mycobacterial cell entry function thereof of a selectable marker which is used for screen for homologous recombinants in which a double cross-over event has been effected. The attenuated strains may be used in the immunization of hosts against Mycobacterium disease.
Owner:AVENTIS PASTEUR LTD
Biologically active substance on the basis of tetracyclic nitrogen heterocycles of pyrimidine row
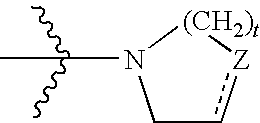
PCT No. PCT / RU97 / 00098 Sec. 371 Date Mar. 17, 1998 Sec. 102(e) Date Mar. 17, 1998 PCT Filed Apr. 2, 1997 PCT Pub. No. WO98 / 43982 PCT Pub. Date Aug. 10, 1998Biologically active substance on the basis of tetracyclicnitrogen heterocycles of pyrimidine row for treating tuberculosis, mycobacteriousis, viral diseases, infections caused by chlamydias, and also diseases which are accompanied by immunodeficiency, in particular malignant neoplasm, has high antimicrobial activity, in particular to strains of mycobacteria which are resistant to the prototype-isoniazid, and simultaneously possess antiviral activity (relative to herpes simplex viruses), antichylamidial activity and also stimulate production of endogenic interferons in organisms. It represents a derivative of 5-oxo-5H-[1]-benzopyrano-[5,6-b]-4-oxo-4H-[1,2]-pyrimido-1,4,5,6-tetrahydro-1,3-thiazine (1) of general formula(1). (I-X) where: R1-H or halogen; R2-H, or halogen, or nitro-group, or hydroxy-group or methoxy-group.
Owner:NATURAL DRUG SCI
Use of Substituted Quinoline Derivatives for the Treatment of Drug Resistant Mycobacterial Diseases
The present invention relates to the use of a substituted quinoline derivative for the preparation of a medicament for the treatment of an infection with a drug resistant Mycobacterium strain wherein the substituted quinoline derivative is a compound according to Formula (Ia) or Formula (Ib) the pharmaceutically acceptable acid or base addition salts thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the above compounds and one or more other antimycobacterial agents.
Owner:JANSSEN PHARMA NV
Biomarkers of tuberculosis that distinguish disease categories: use as serodiagnostic antigens
Mycobacterial proteins from culture filtrate or cytosol are disclosed as being useful B cell antigens for early diagnosis of mycobacterial disease, particularly in humans. These proteins include four that had not previously been recognized as B cell antigens (LppZ protein encoded by Mtb gene Rv3006; SodC protein encoded by Mtb gene Rv0432; BfrB protein encoded by Mtb gene Rv3841 and TrxC protein encoded by Mtb gene Rv3914). Antigenic compositions include these proteins and / or peptide fragments thereof, in various combinations with each other or with one or more of a set of 10 additional Mtb proteins known to be antigens (in particular early antigens. Methods and kits for using these antigenic composition for early diagnosis of mycobacterial infection and disease are also disclosed.
Owner:NEW YORK UNIV +1
Vesicular formulations containing organic acid prodrugs, process for their preparation
InactiveUS20100221315A1Suitable for treatmentImprove stabilityAntibacterial agentsOrganic active ingredientsOrganic acidPyridazine
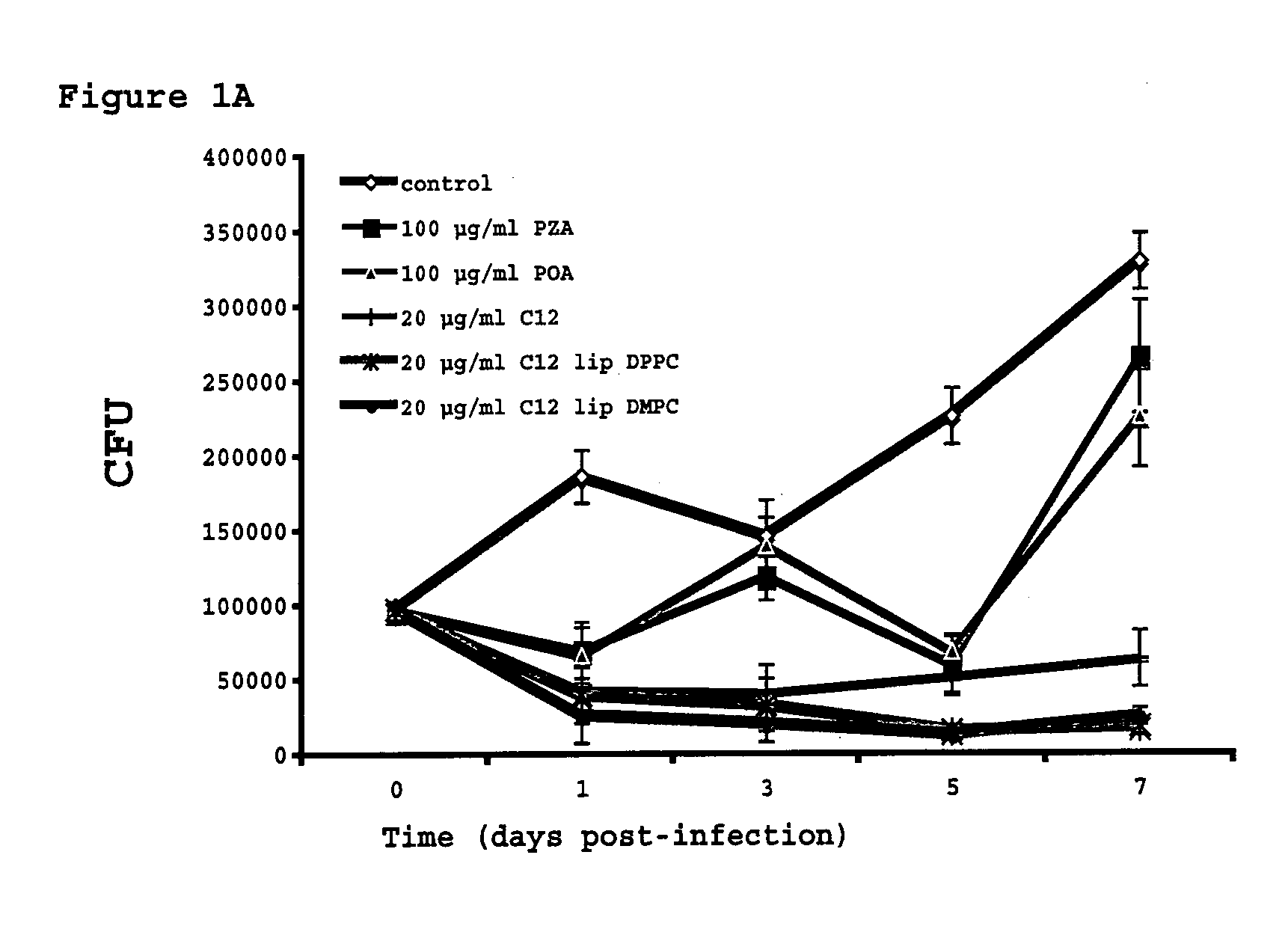
The present invention relates to vesicular formulations containing a prodrug, characterized for comprising the combination of a prodrug of weak organic acids having the following general formula: R1COOH (I) or R1SO2H (II) wherein R1 is preferably selected from the group containing a benzenic, pyridinic, pyrazinic or pyrimidinic aromatic ring, or a linear chain substituted or unsubstituted, saturated or unsaturated, such as benzoic, benzenesulphinic, cinnamic, salicylic, pyrazinoic, nicotinic, carboxylic pyridazine and carboxylic pyrimidine, caproic, caprylic, capric, lauric, myristic, palmitic and estearic acids; with a liposomal or micellar carrier, which protects the prodrug from plasma degradation. The invention further relates to the process of preparation of liposomal formulations, novel prodrugs and pharmaceutical compositions intended for use in the treatment of tuberculosis and other mycobacterioses.
Owner:UNIV DE LISBOA
Mycobacterial inhibitors
The present invention relates to novel substituted quinoline derivatives according to the general Formula (I)and pharmaceutically acceptable addition salts thereof, wherein the variable moieties are as defined in the specification. The invention also relates to a method of treating of mycobacterial diseases through administration of the claimed compounds and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Novel antimycobacterial compounds
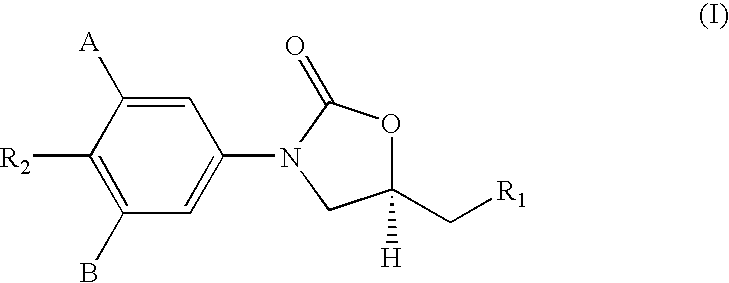
Novel compounds belonging to the class of oxazolidinones possessing potent antimycobacterial properties especially useful in the treatment of acid fast organisms such as Mycobacterium tuberculosis, Mycobacterium avium-intracellular complex, M. fortuitum and M. kansai. The compound and its pharmaceutically acceptable salts thereof act as antibacterial agents. Also disclosed is a method for inhibiting growth of mycobacterial cells as well as a method of treating mycobacterial conditions such as Mycobacterium tuberculosis, drug resistant Mycobacterium tuberculosis, Mycobacterium avium-intracellular complex, M. fortuitum and M. kansai, comprising administering an antimycobacterially effective amount of the said compound and / or pharmaceutically acceptable salts thereof. There is also disclosed a process for the manufacture of the said compound or its pharmaceutically acceptable salts.
Owner:LUPIN LTD
Early detection of mycobacterial disease using peptides
InactiveUS7807182B2Reduce distractionsUse toolAntibacterial agentsPeptide/protein ingredientsSecretory proteinTGE VACCINE
A number of protein and glycoprotein antigens secreted by Mycobacterium tuberculosis (Mtb) have been identified as “early” Mtb antigens on the basis early antibodies present in subjects infected with Mtb prior to the development of detectable clinical disease. Epitope-bearing peptide fragments of these early Mtb antigens, in particular of an 88 kDa secreted protein, GlcB (SEQ ID NO:106) and of Mtb antigen MPT51 (SEQ ID NO:107) have been identified. These peptides, variants thereof, peptide multimers thereof that include two or more repeats of one or more of the peptides, and fusion polypeptides that include early Mtb antigenic proteins, peptides or both, are useful in immunoassay methods for early, rapid detection of TB in a subject. Preferred immunoassays detect the antibodies in the subject's urine. Also provided are antigenic compositions, kits and methods to useful for detecting an early Mtb antibodies. The antigenic proteins and peptides are also used in vaccine compositions.
Owner:NEW YORK UNIV +1
Methods and compositions for detection and diagnosis of infectious diseases
InactiveUS20050106107A1Easy to manageSensitive highUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingAntigenMedicine
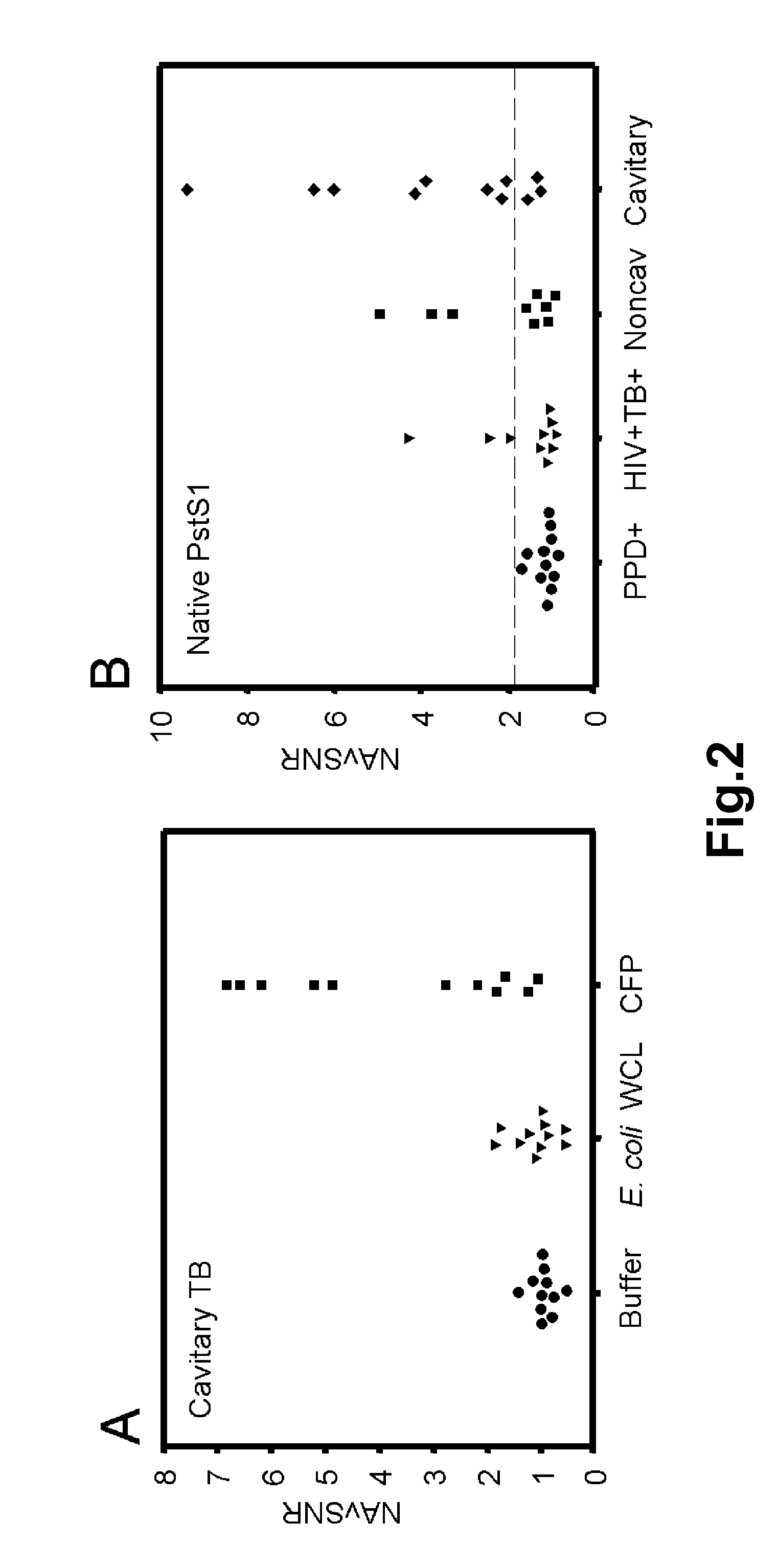
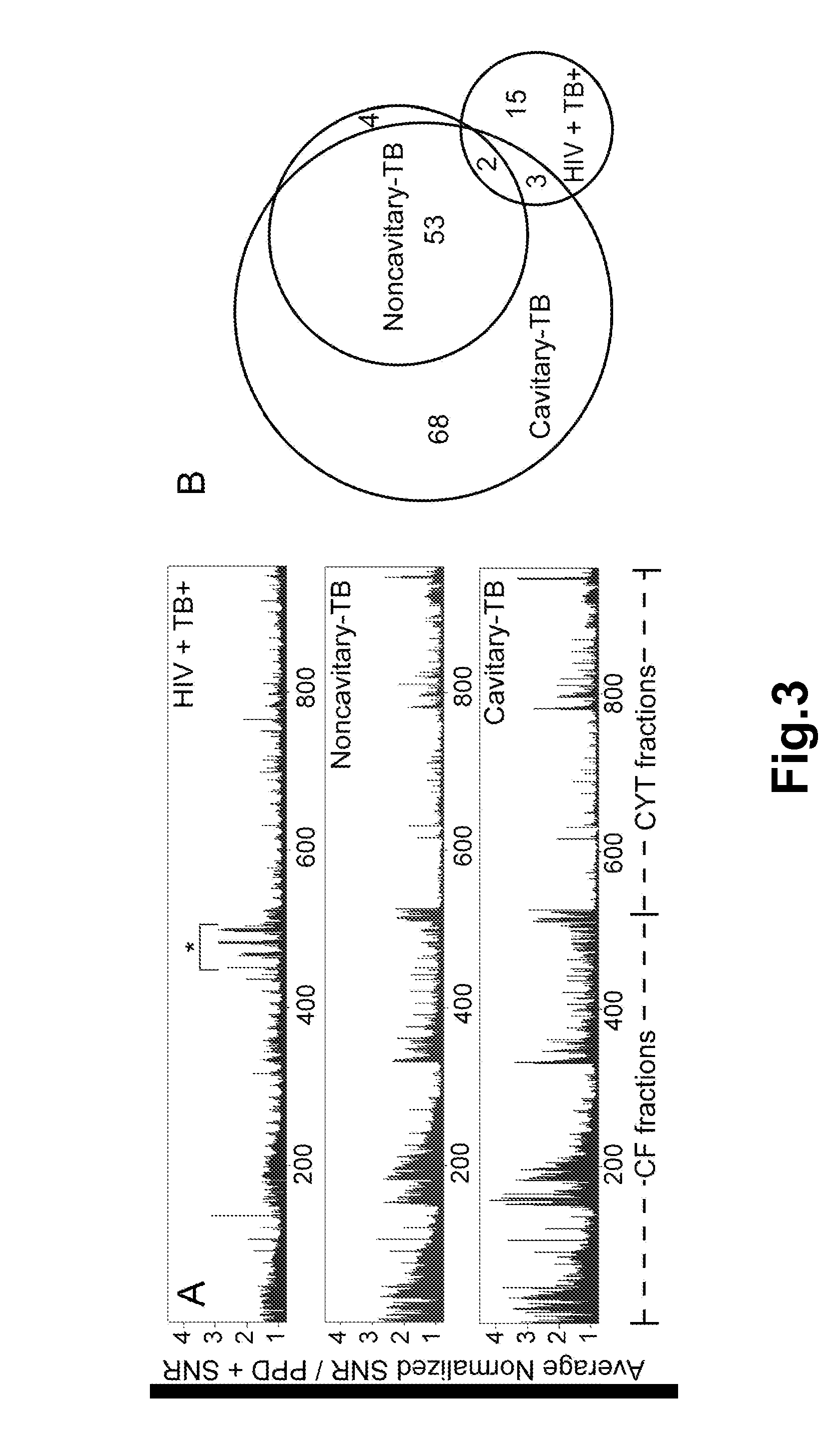
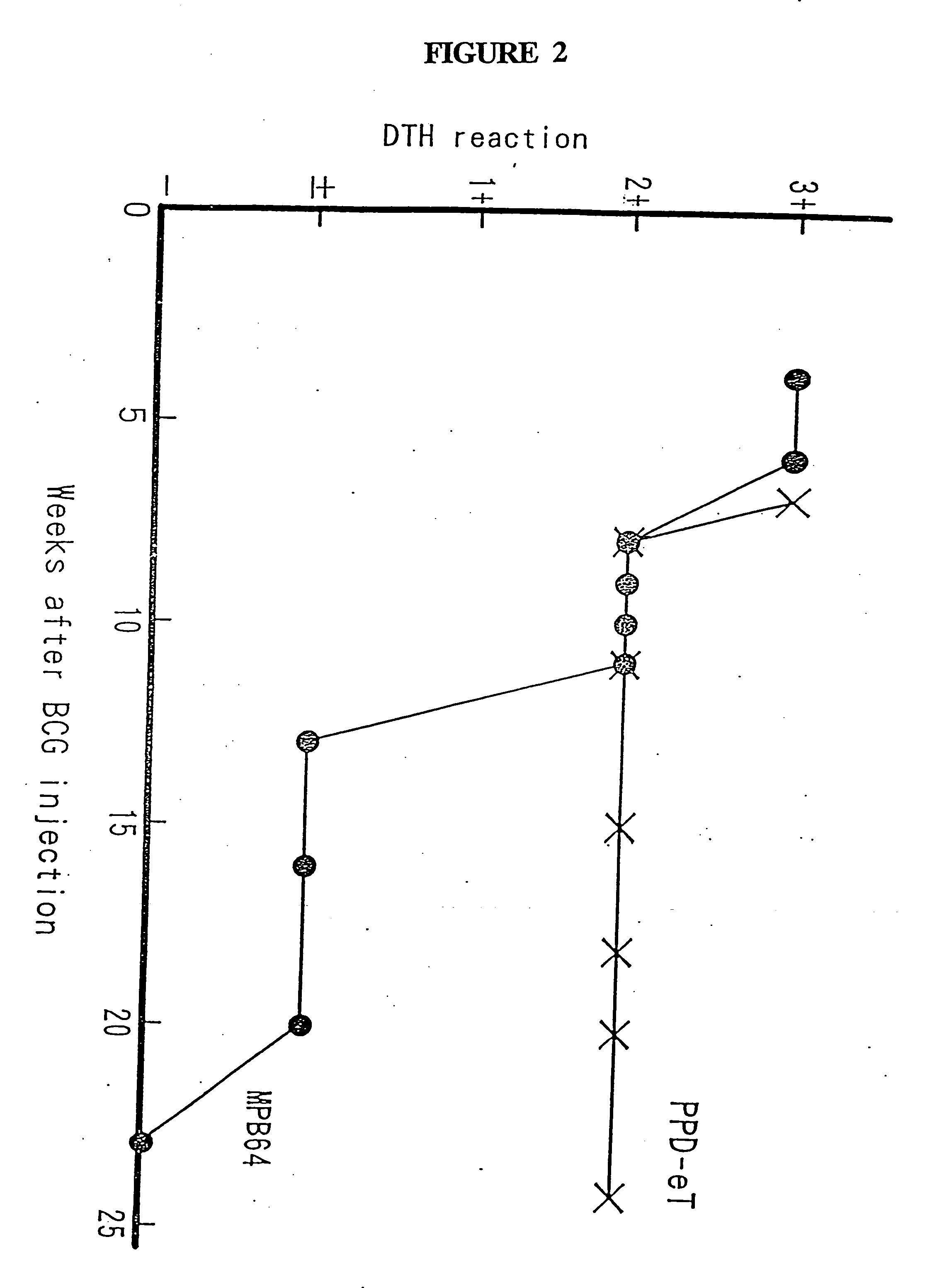
Methods and compositions for the detection and diagnosis of infectious diseases are provided. In particular, efficient and sensitive methods and compositions for the detection of active mycobacterial disease are provided for distinguishing between individuals having active disease, and individuals who have been immunologically exposed, such as those infected with a mycobacterium but are without active disease, or those who have been vaccinated with BCG. The methods comprise topical application of antigen compositions for transdermal delivery.
Owner:JAPAN BCG LAB
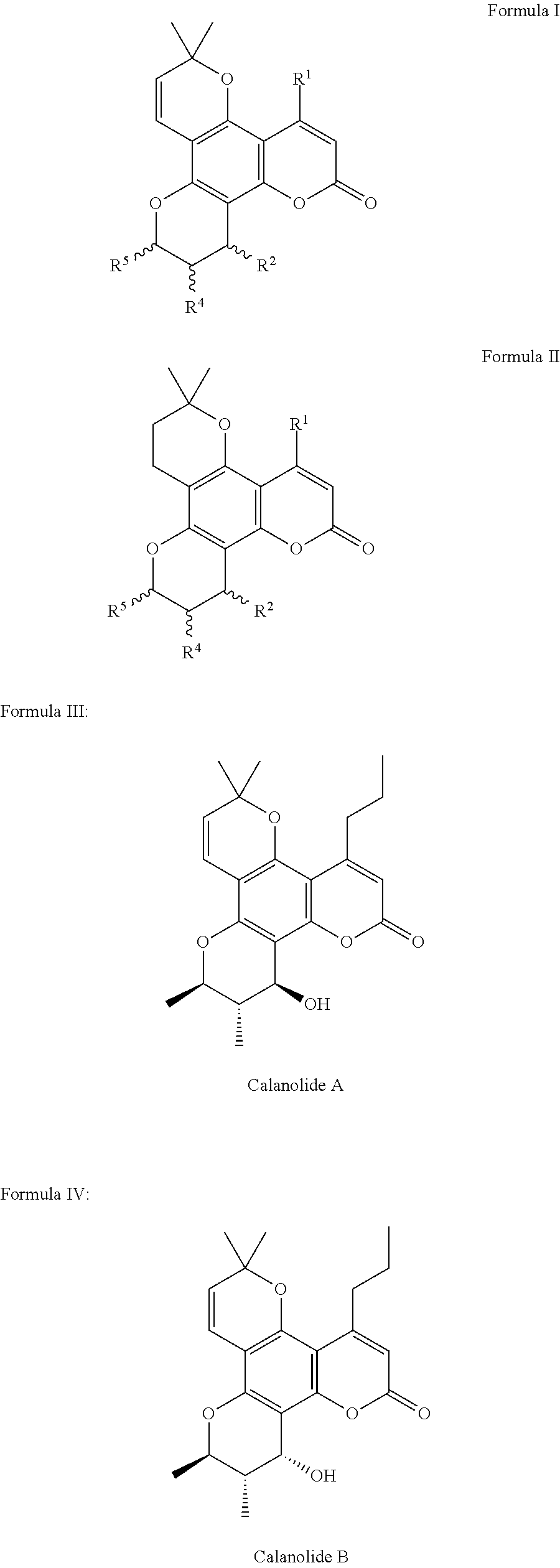
Pharmaceutical compositions for calanolides, their derivatives and analogues, and process for producing the same
InactiveUS20130183383A1Improve solubilityImprove bioavailabilityAntibacterial agentsBiocideSolubilityImmunodeficiency
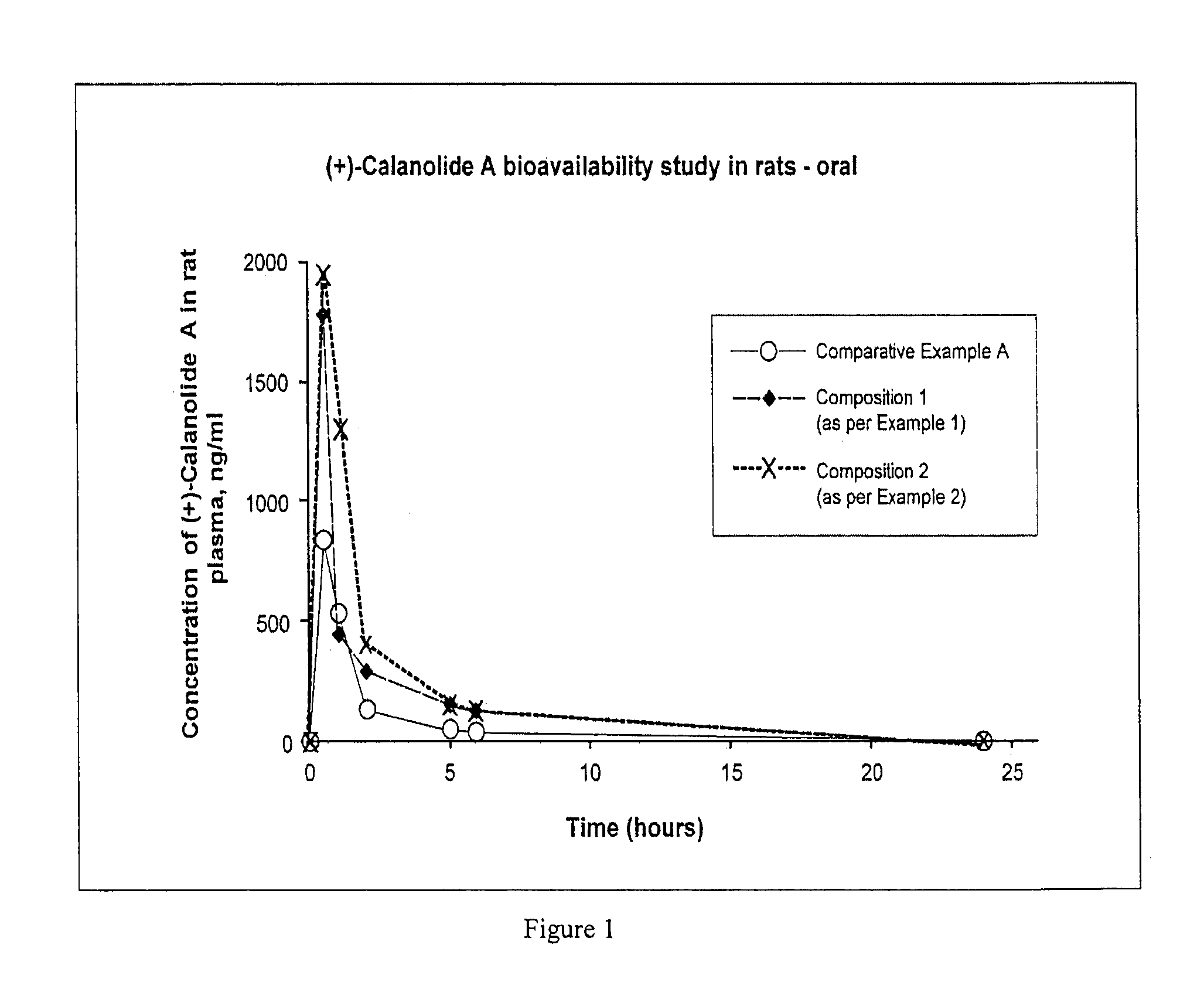
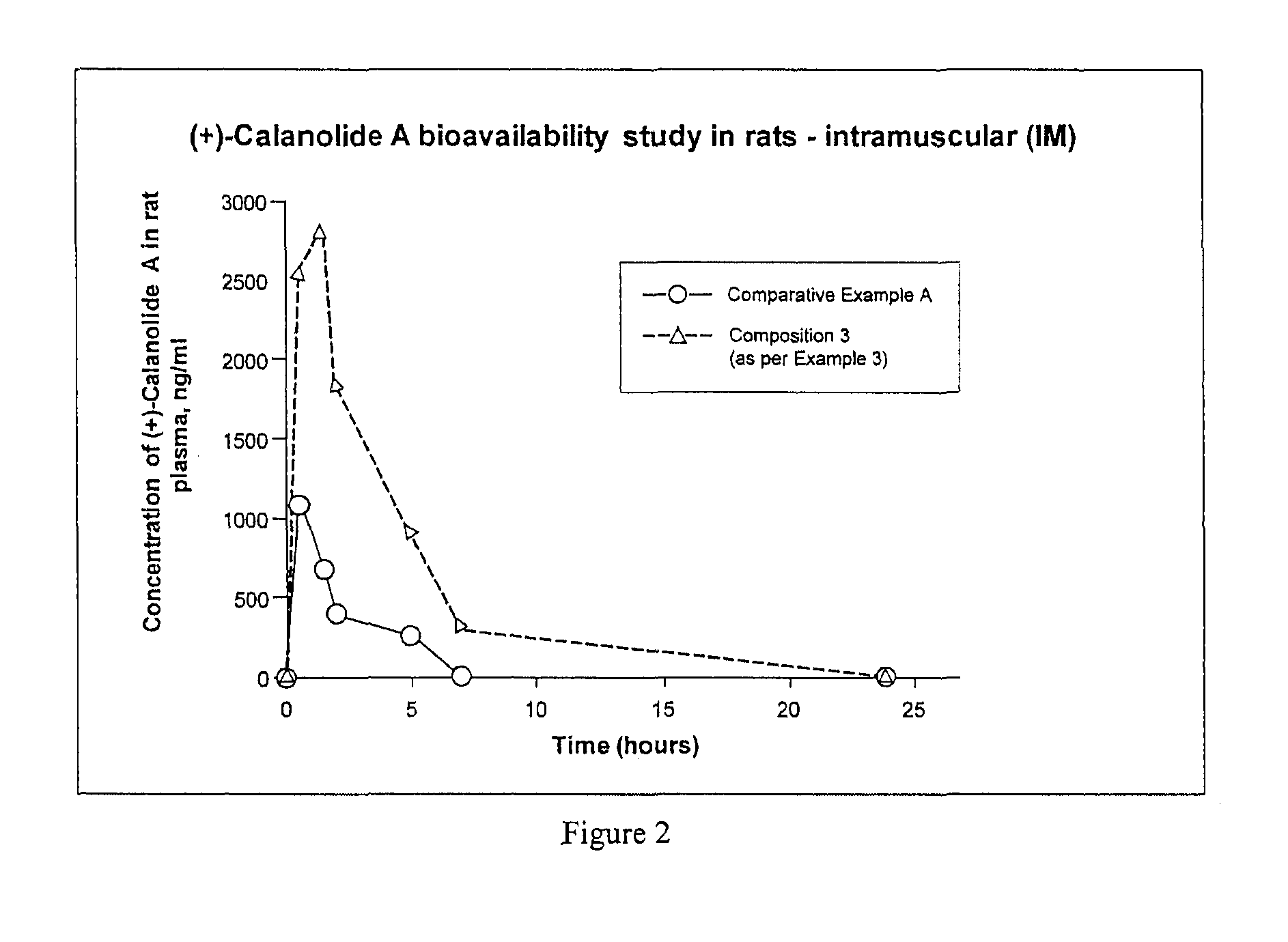
The present invention relates to pharmaceutical compositions of calanolides, their derivatives and analogues, and process for producing the same having enhanced solubility and bioavailability for oral or parenteral administration. The invention further provides for a method of using the disclosed compositions for the treatment and prevention of retroviral diseases such as human immunodeficiency, specifically HTV-1 and mycobacterial diseases especially tuberculosis infections in mammals, particularly humans.
Owner:CRAUN RES
Method and compositions for detection and diagnosis of infectious diseases
InactiveUS6979450B2Sensitive highEfficient detectionUltrasonic/sonic/infrasonic diagnosticsBiocideAntigenActive disease
Methods and compositions for the detection and diagnosis of infectious diseases are provided. In particular, efficient and sensitive methods and compositions for the detection of active mycobacterial disease are provided for distinguishing between individuals having active disease, and individuals who have been immunologically exposed, such as those infected with a mycobacterium but are without active disease, or those who have been vaccinated with BCG. The methods comprise topical application of antigen compositions for transdermal delivery.
Owner:JAPAN BCG LAB
Attenuated strains of mycobacteria
Attenuated strains of Mycobacterium, particularly species of the tuberculosis complex, have the mycobacterial cell entry (mce) gene functionally disabled. The gene may be disabled by an insertion into the gene which disrupts the mycobacterial cell entry function thereof of a selectable marker which is used for screen for homologous recombinants in which a double cross-over event has been effected. The attenuated strains may be used in the immunization of hosts against Mycobacterium disease.
Owner:CONNAUGHT LAB
Kit for identifying nucleic acid of mycobacterium pathogeny through multiple PCR (polymerase chain reaction)
InactiveCN104388575AEasy to identifyFast and accurate identificationMicrobiological testing/measurementMicroorganism based processesMicrobiologyMultiplex pcrs
The invention provides a kit for identifying nucleic acid of mycobacterium pathogeny through multiple PCR (polymerase chain reaction). The kit comprises primer composition as follows: 5'-acggtgggtactaggtgtgggtttc-3', 5'-tctgcgattagcgactaagacttca-3', 5'-gcgttgaccgagatggattat-3', 5'-gctcatctcacccagttggc-3', 5'-ttccgaatcccttgtga-3', 5'-ggagagcgccgttgta-3' or 5'-agtcgccgtggcttctctttta-3'. The kit has high sensitivity and high specificity, is simple to operate and can realize rapid and large-flux detection for virulent and avirulent mycobacterium.
Owner:YANGZHOU UNIV
Prokaryotic proteasomal proteases of Mycobacterium tuberculosis (MTB) as targets for antibiotic therapy
The present invention relates to methods of treating Mycobacterium pathogen infection in a subject that involve: inhibiting proteasomal activity in a pathogen under conditions effective to make the pathogen susceptible to antibacterial host defenses; inhibiting enzyme activity in a pathogen under conditions effective to make the pathogen susceptible to antibacterial host defenses, where the enzyme is a DNA repair enzyme or a flavin-like co-factor synthesis enzyme, or inhibiting proteasomal and enzyme activity under conditions to make the pathogen susceptible to antibacterial host defenses. The present invention also relates to methods for screening compounds that inhibit proteasomal and protease activity, DNA repair enzyme activity, or flavin-like co-factor synthesis enzyme activity, where the inhibitory compounds have an ability to sensitize bacteria to the antibacterial effects of oxidative / nitrosative stress.
Owner:CORNELL RES FOUNDATION INC
Novel live recombinant booster vaccine against tuberculosis
ActiveUS20150056242A1Limited efficacyPreventing and reducing possibilityBacterial antigen ingredientsAntimycoticsMycobacteriumMycobacterial disease
Owner:RGT UNIV OF CALIFORNIA
Antibacterial Quinoline Derivatives
ActiveUS20080182855A1Ease of administrationImprove uniformityAntibacterial agentsBiocideMycobacterium marinumQuinoline
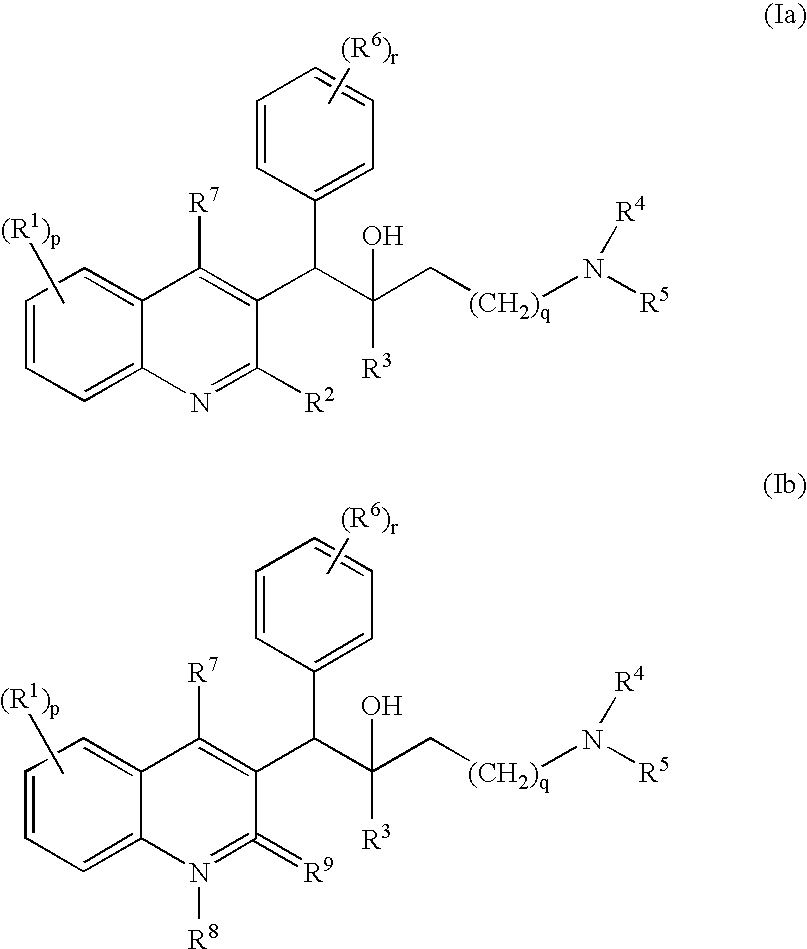
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or Formula (Ib):the pharmaceutically acceptable acid or base addition salts thereof, the quaternary amines thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of a bacterial disease including a mycobacterial disease, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of bacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Biomarker of mycobacteriosis or mycobacterial infection
The present invention provides a solid carrier capable of easily and accurately evaluating the activity of mycobacteriosis or the presence or absence of mycobacterial infection. The solid carrier for detecting mycobacteriosis or mycobacterial infection includes at least one probe including a polynucleotide consisting of a base sequence complementary to all or a part of a base sequence having 85% or more identity with respect to a base sequence set forth in SEQ ID NO: 1, which is immobilized on a surface thereof.
Owner:KEIO UNIV +1
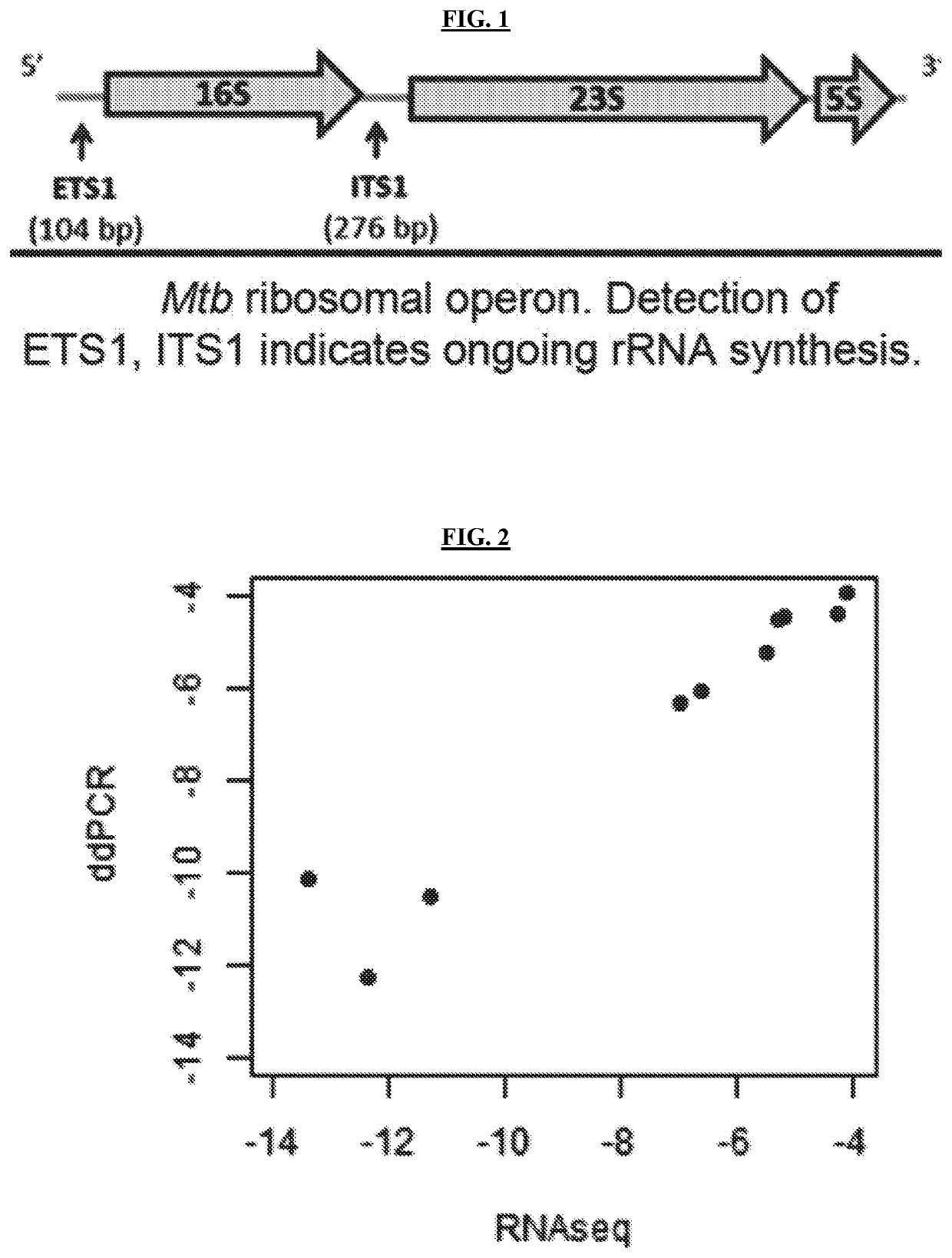
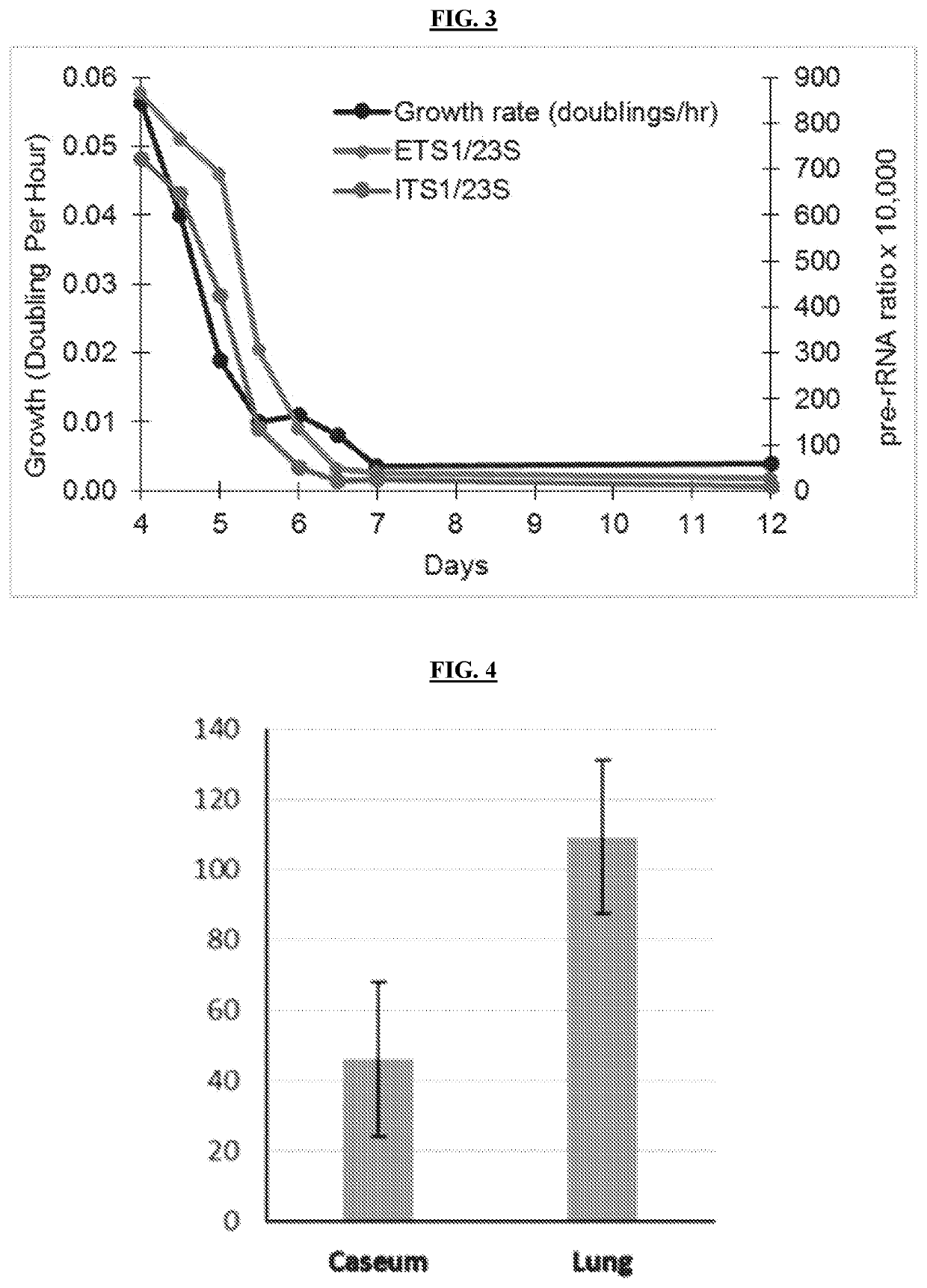
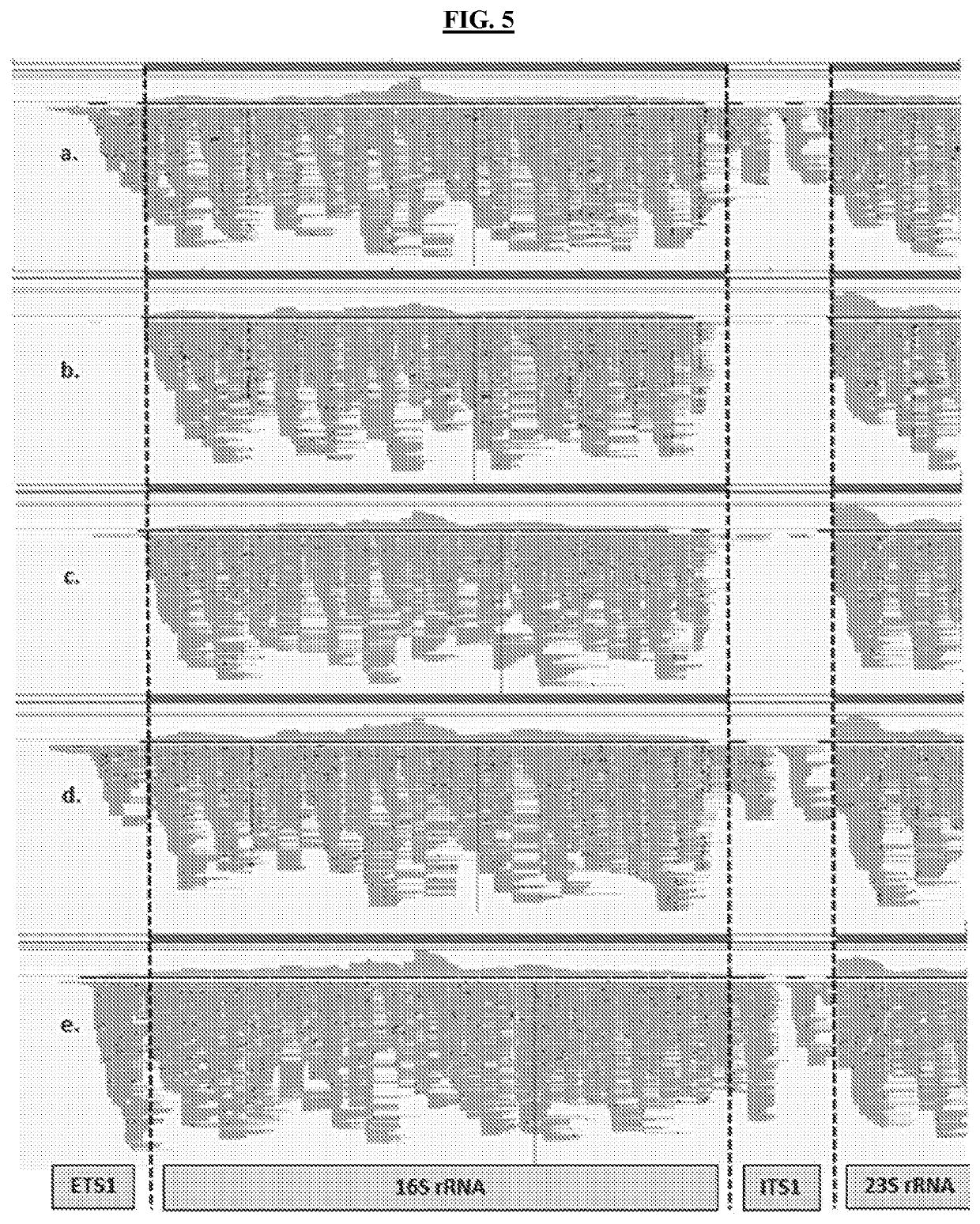
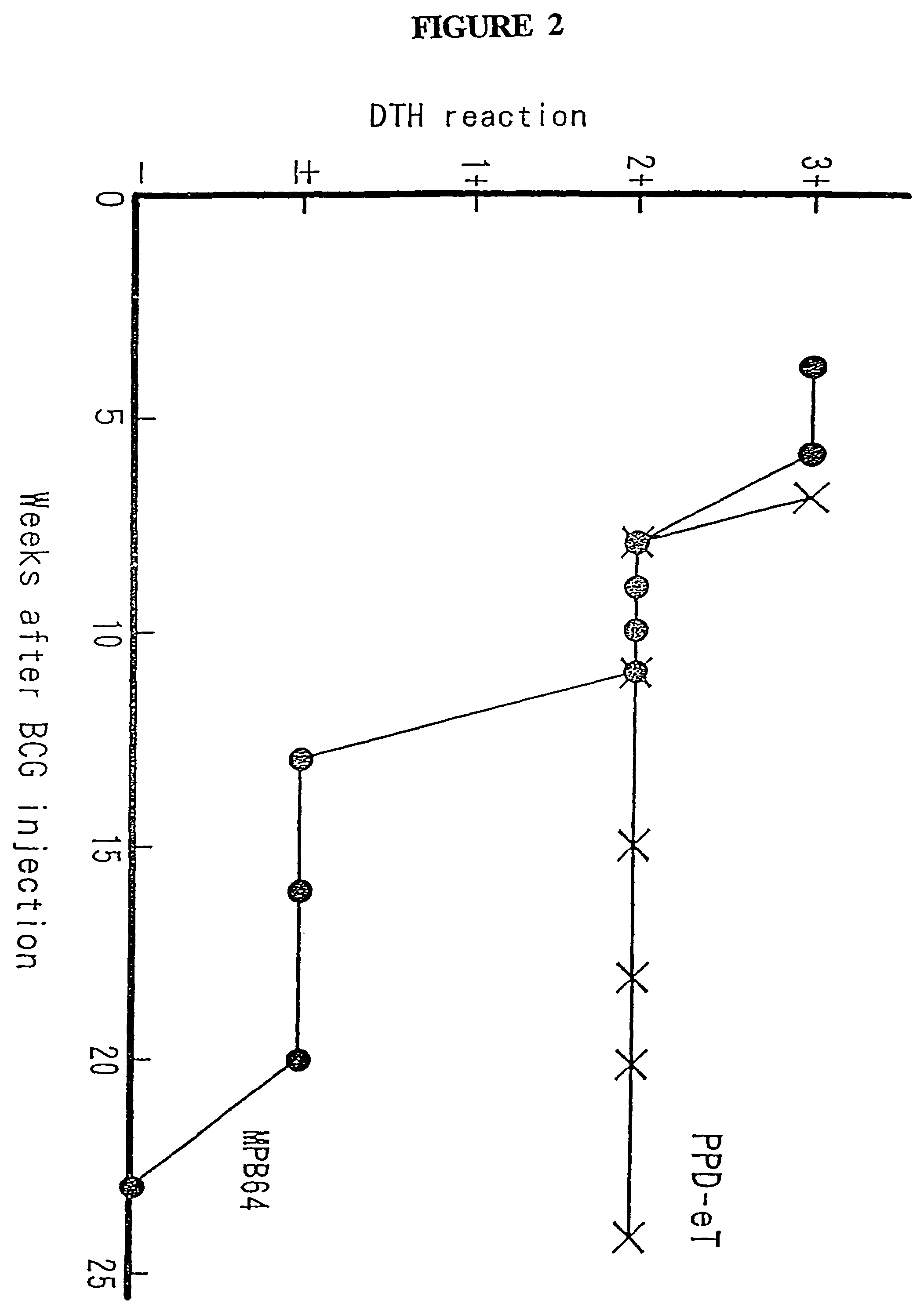
Methods of Evaluating Treatment Efficacy and/or Treatment Duration in Mycobacterial Diseases
ActiveUS20200248259A1Exact correlationMicrobiological testing/measurementDisease diagnosisTherapeutic effectBiomedical engineering
The present invention provides novel markers of treatment response in a subject infected with Mycobacterium, which allow for quantifying treatment impact on the physiologic state of the Mycobacterium.
Owner:COLORADO STATE UNIVERSITY +3
Methods and compositions for detection and diagnosis of infectious diseases
InactiveUS7655218B2Sensitive highEfficient detectionUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingAntigenMedicine
Methods and compositions for the detection and diagnosis of infectious diseases are provided. In particular, efficient and sensitive methods and compositions for the detection of active mycobacterial disease are provided for distinguishing between individuals having active disease, and individuals who have been immunologically exposed, such as those infected with a mycobacterium but are without active disease, or those who have been vaccinated with BCG. The methods comprise topical application of antigen compositions for transdermal delivery.
Owner:JAPAN BCG LAB
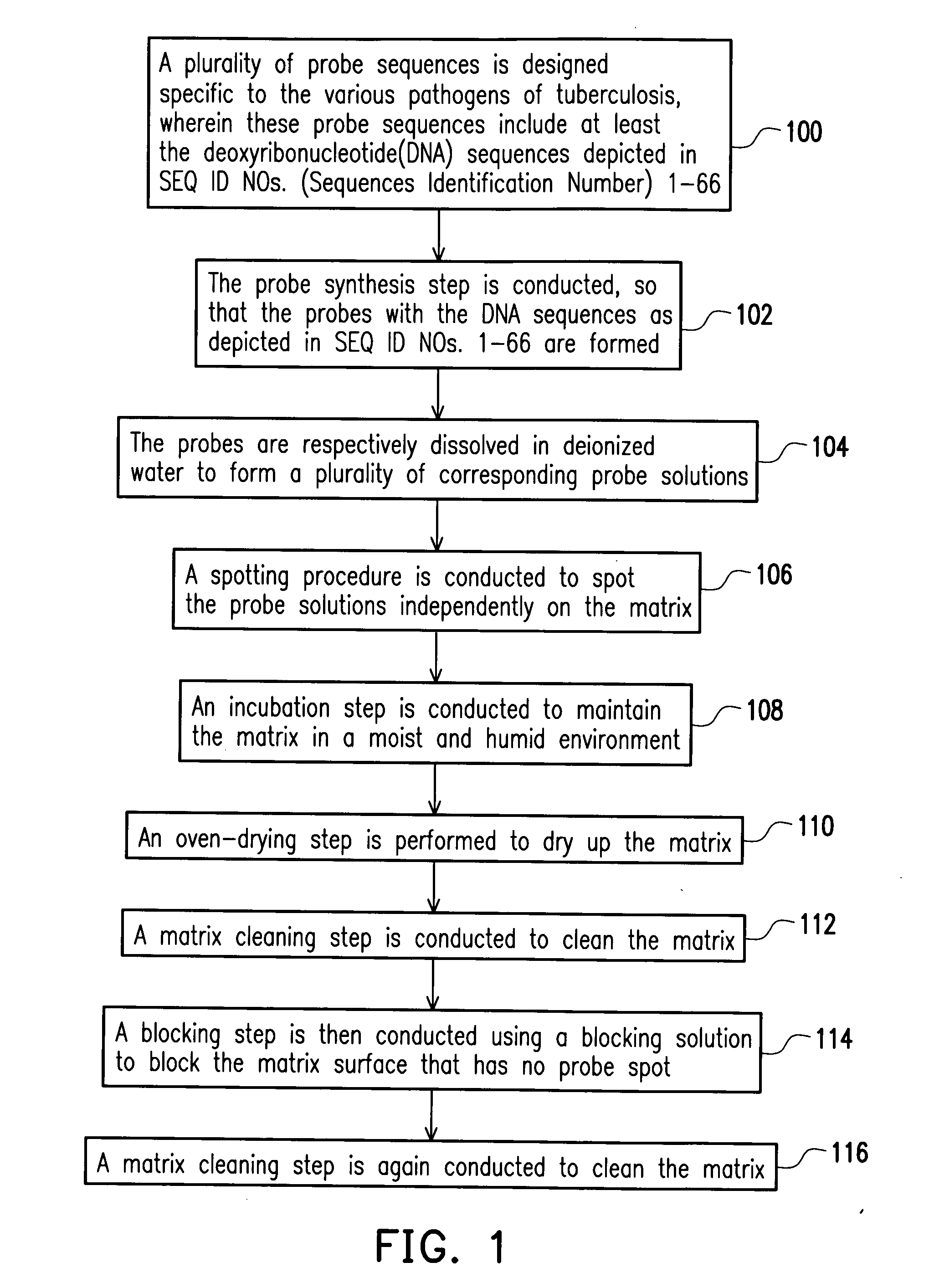
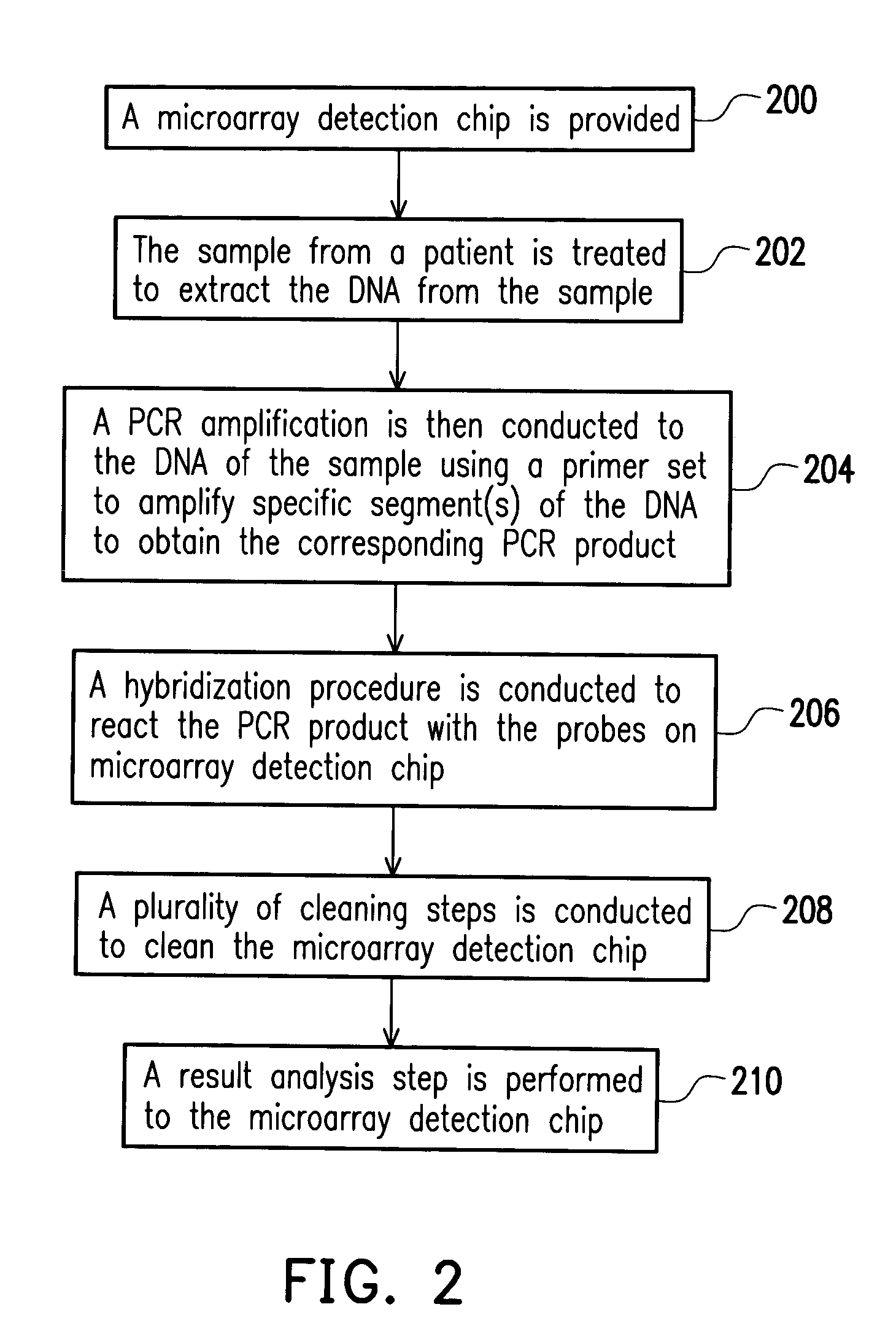
Mycobacterial disease detection chip and fabrication method thereof and method of detecting mycobacterial disease and primer set for mycobacterial disease and drug resistance detection
InactiveUS20050136432A1The process is fast and accurateHigh-precision detectionBioreactor/fermenter combinationsBiological substance pretreatmentsDrug resistanceMycobacterium
A mycobacterial disease microarray detection chip includes a plurality of probes immobilized on a matrix, wherein each of the probe is selected from the group of deoxyribonucleotide sequences depicted in the SEQ ID NOs. 1 to 66. Since these probes are formed with deoxyribonucleotide sequences specific to mycobacterial disease, they can be used to detect whether the patient has contracted mycobacterial disease and the mycobacterial disease pathogen infected the patient has drug resistance.
Owner:GENE TECH +1
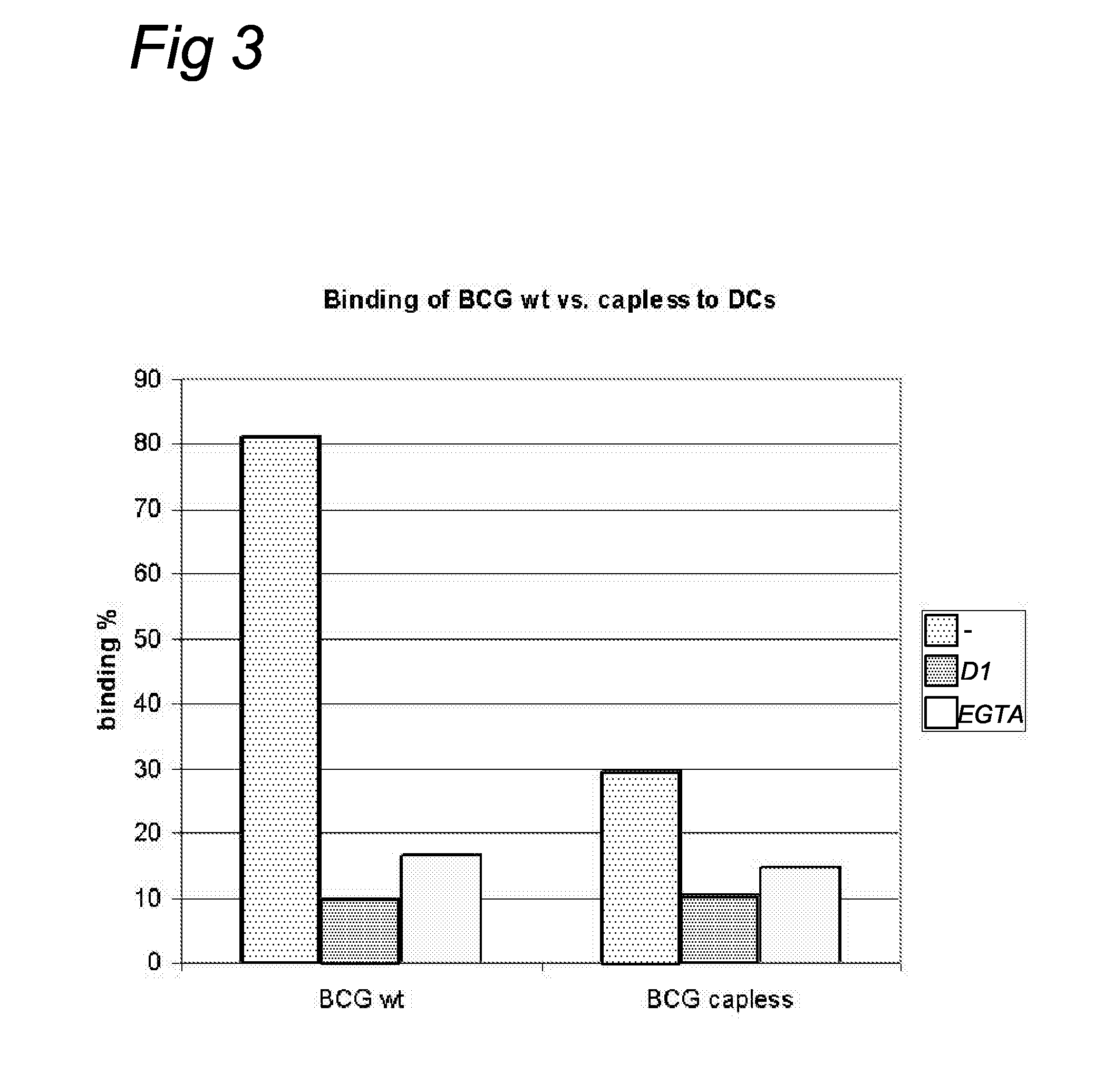
Mycobacteria with Mannose Cap-Deficient Lipoarabinomannan
InactiveUS20090017061A1Maximizing numberMinimize the numberBiocideBacteriaImmunosuppressive effectMycobacterium
The present invention relates to mycobacterial lipoarabinomannan cap-specific mannosyl transferases and nucleic acid encoding such transferases. The invention further relates to Mycobacteria in which the lipoarabinomannan cap-specific mannosyl transferases have been inactivated and that therefore express mannose cap-deficient lipoarabinomannan. Such Mycobacteria with mannose cap-deficient lipoarabinomannan may be used as more effective vaccines against mycobacterial diseases as they lack the immunosuppressive action of the mannose cap.
Owner:VER VOOR CHRISTELIJK HOGER ONDERWIJS WETENSCHAPPELIJK ONDERZOEK & PATIENTENZORG
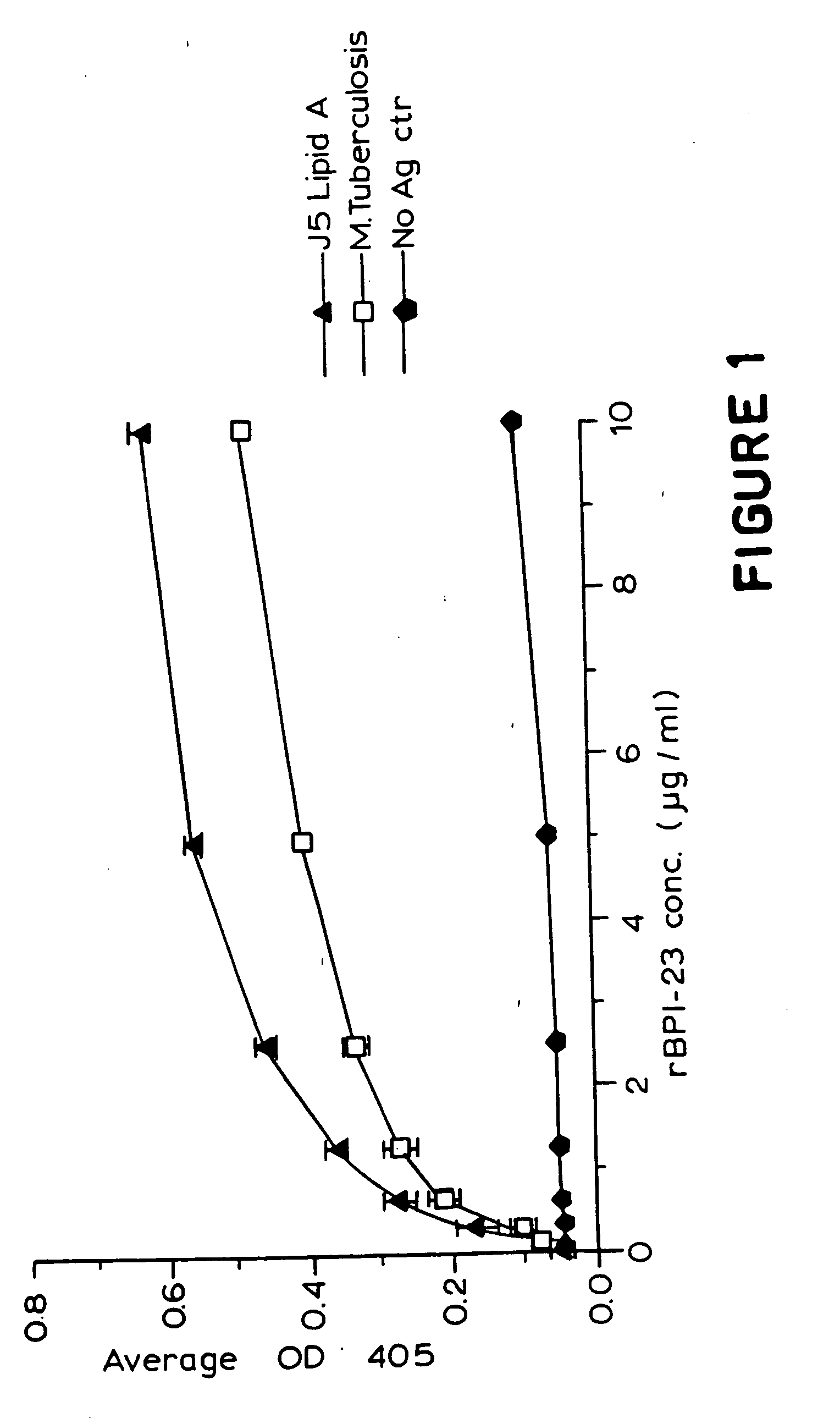
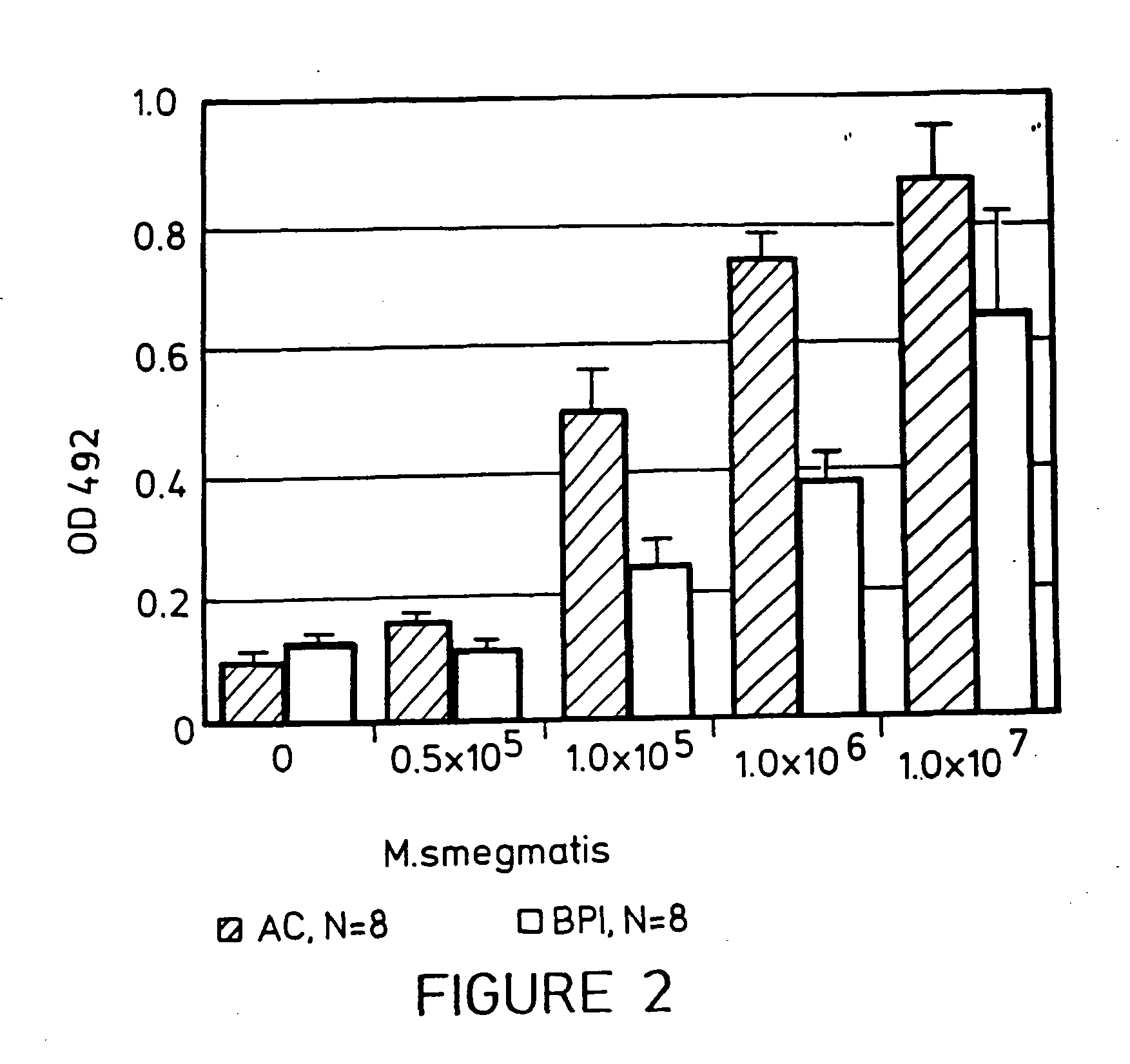
Treatment of Mycobacterial diseases by administration of bacterial/permeability-increasing protein products
InactiveUS20050118112A1Stimulating cytokine productionNeutralize effectAntibacterial agentsBiocideAnti mycobacterialAntibiotic Y
The present invention relates to methods for treating a subject suffering from infection with Mycobacteria, such as M. leprae or M. tuberculosis comprising administering to the subject a composition comprising a bactericidal / permeability-inducing (BPI) protein product alone or in combination with administration of an anti-Mycobacterial antibiotic.
Owner:XOMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com