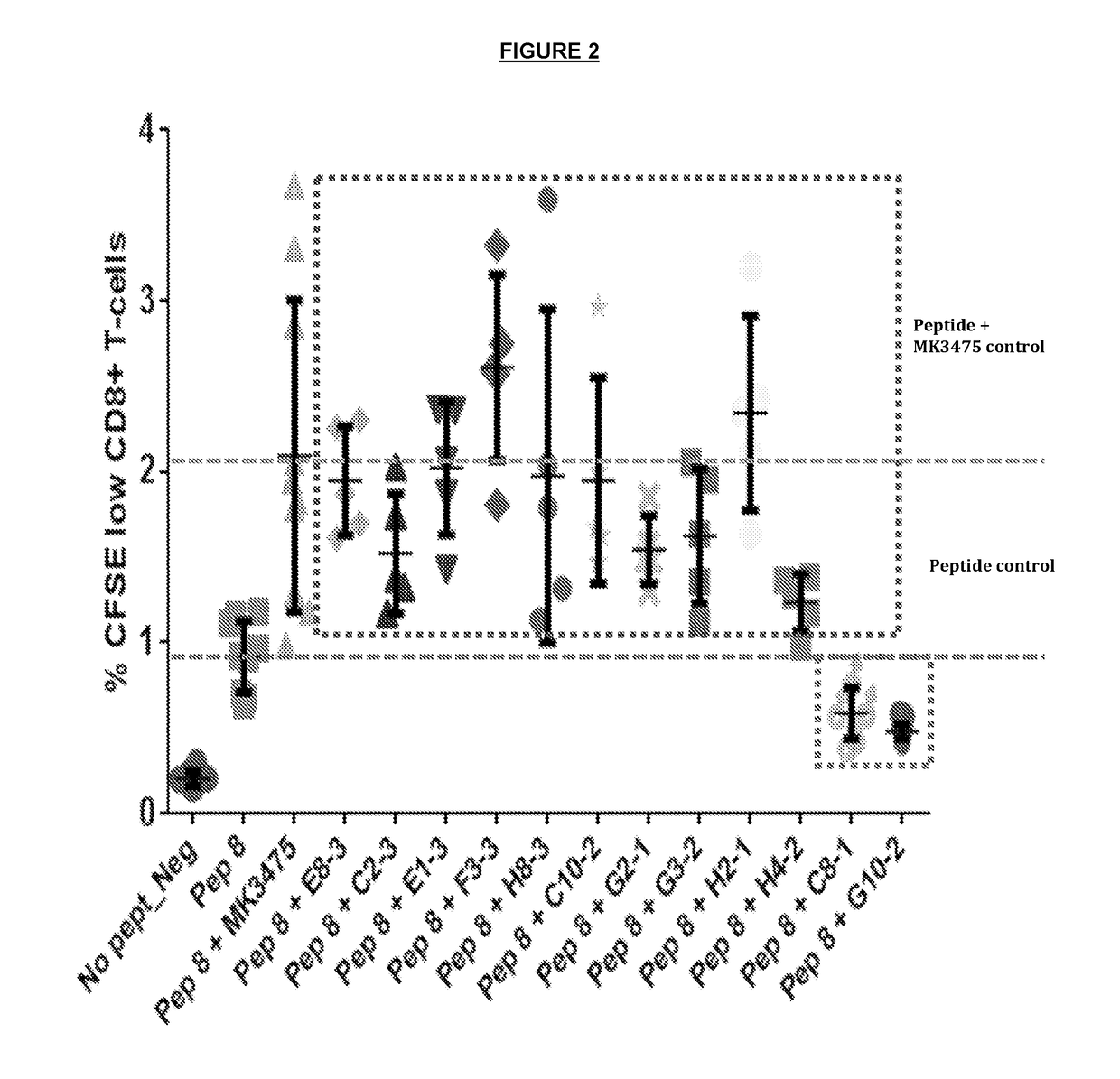
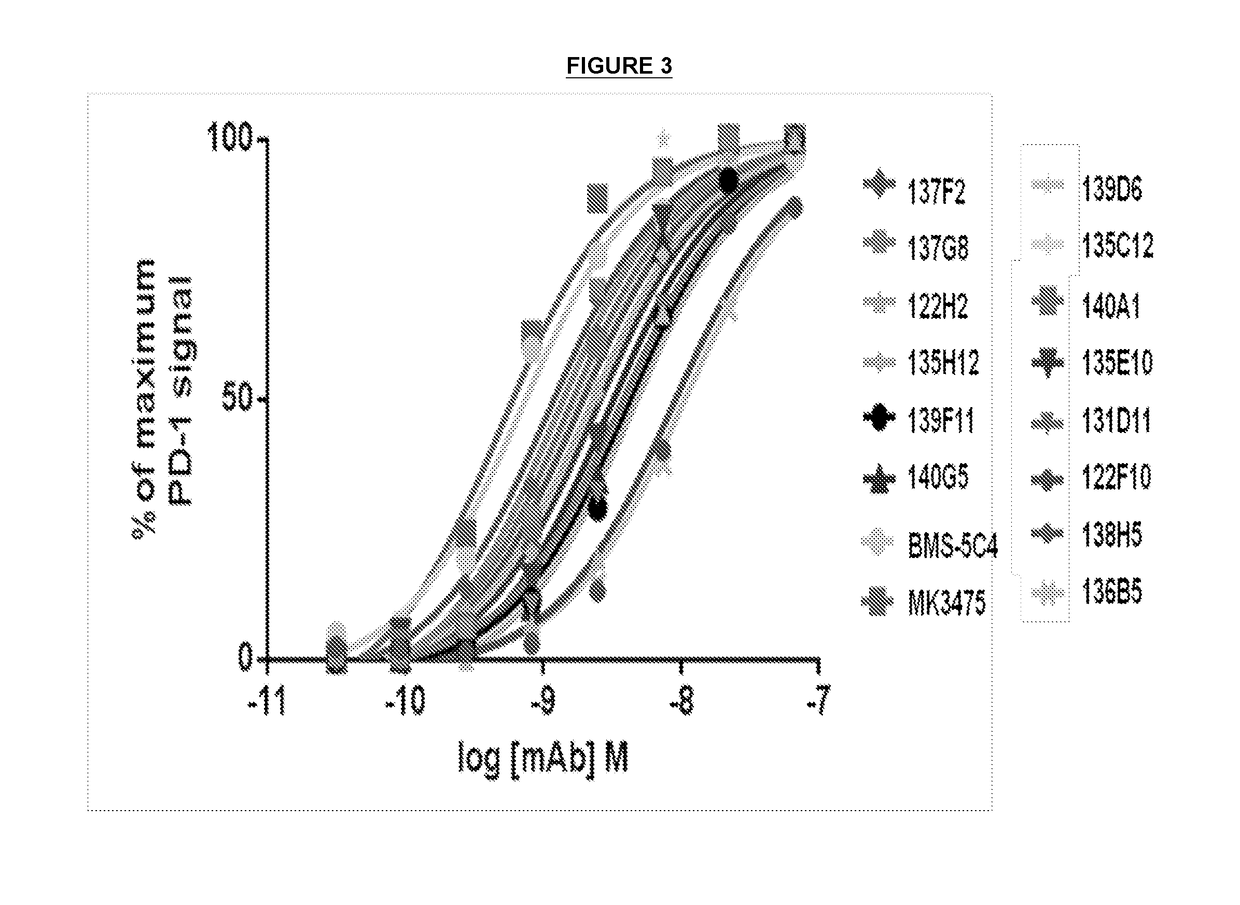
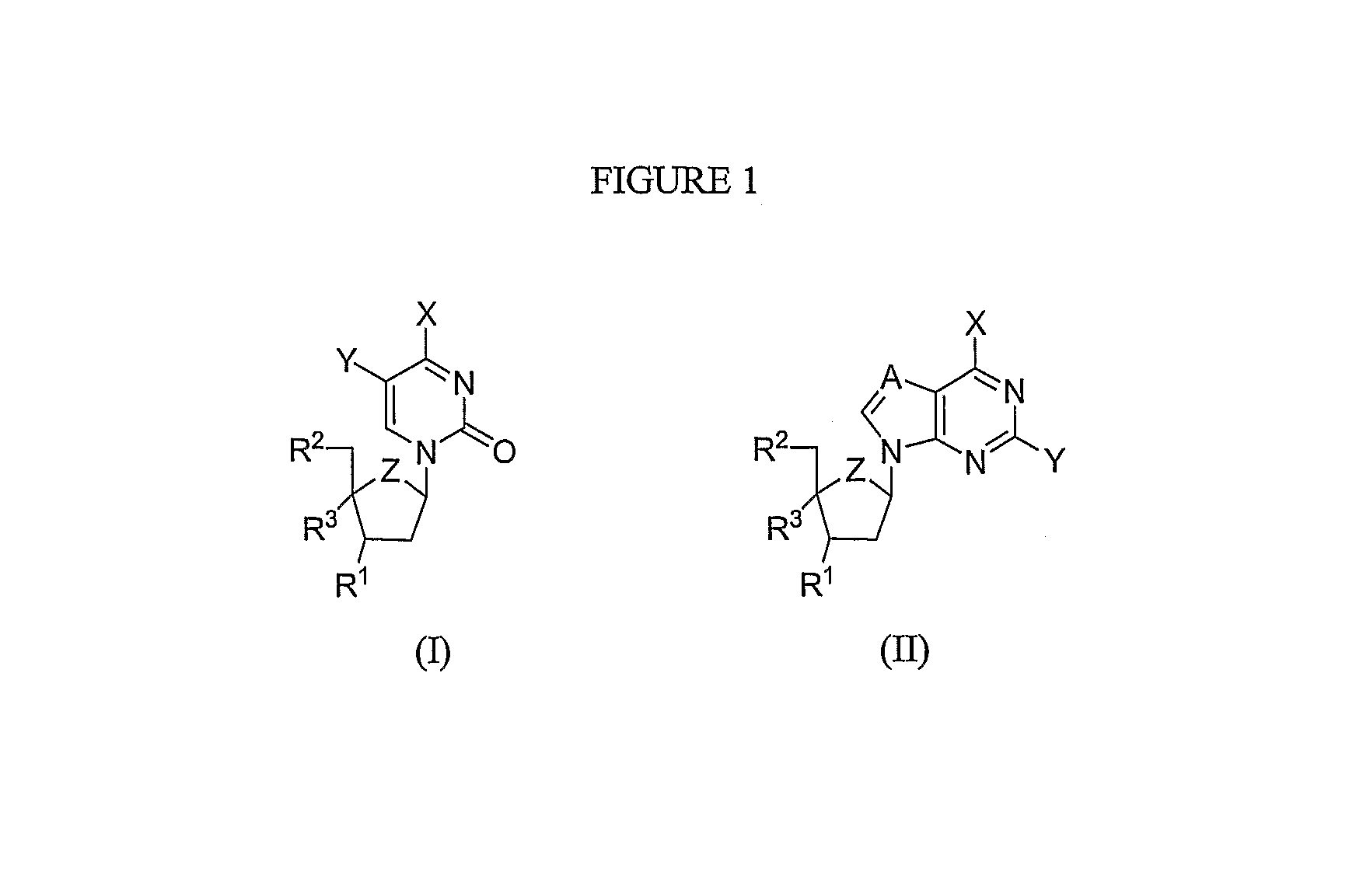
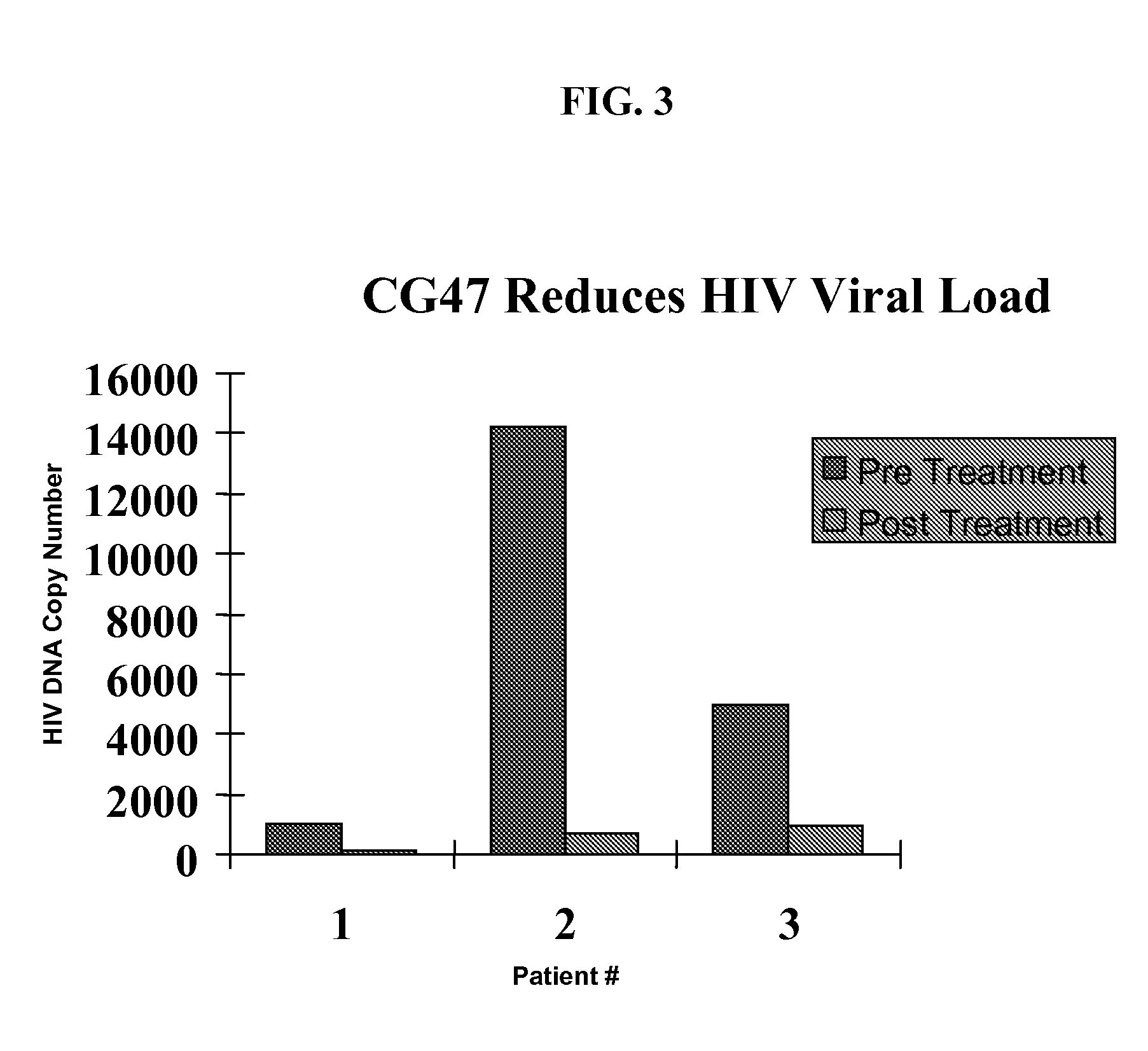
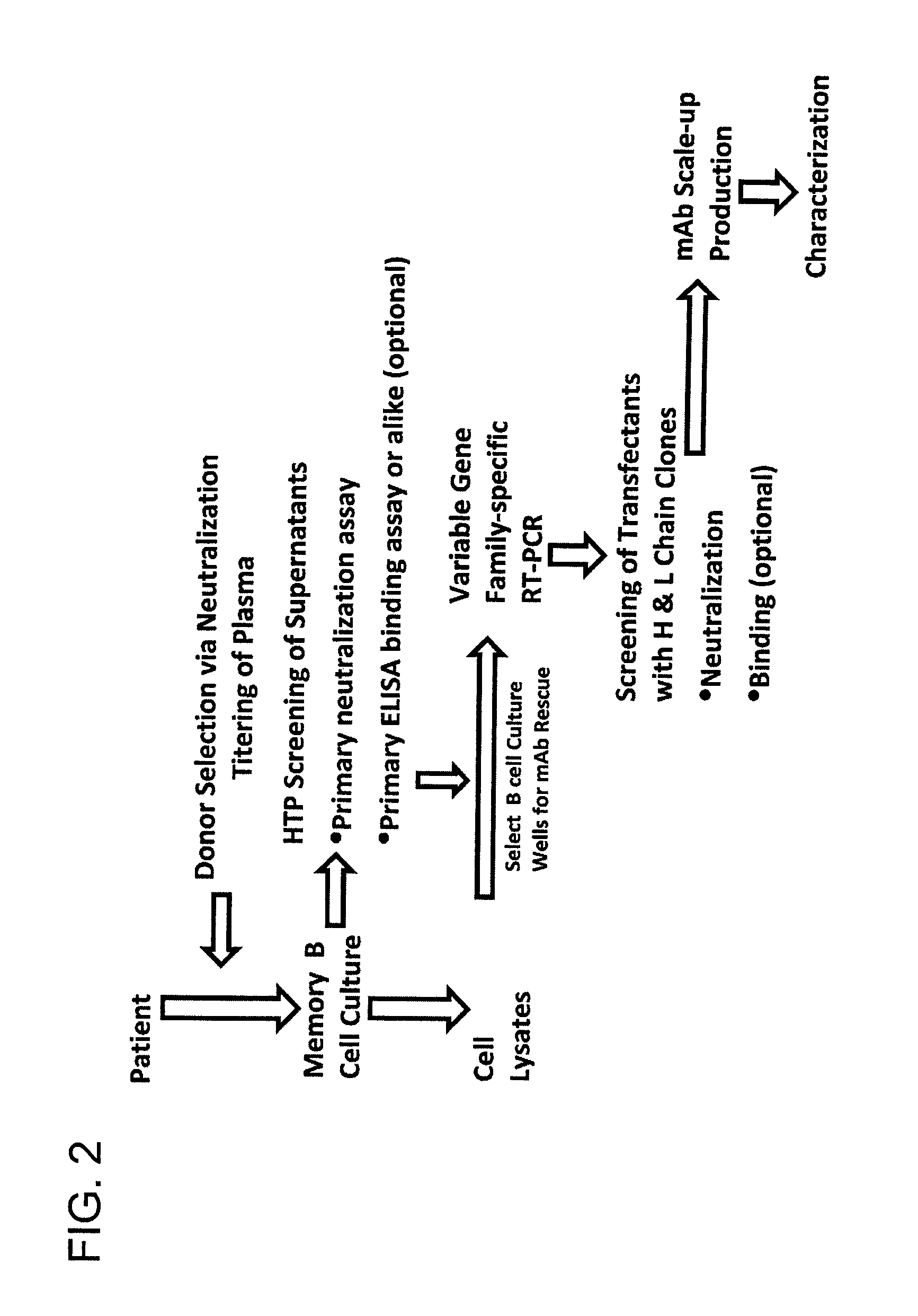
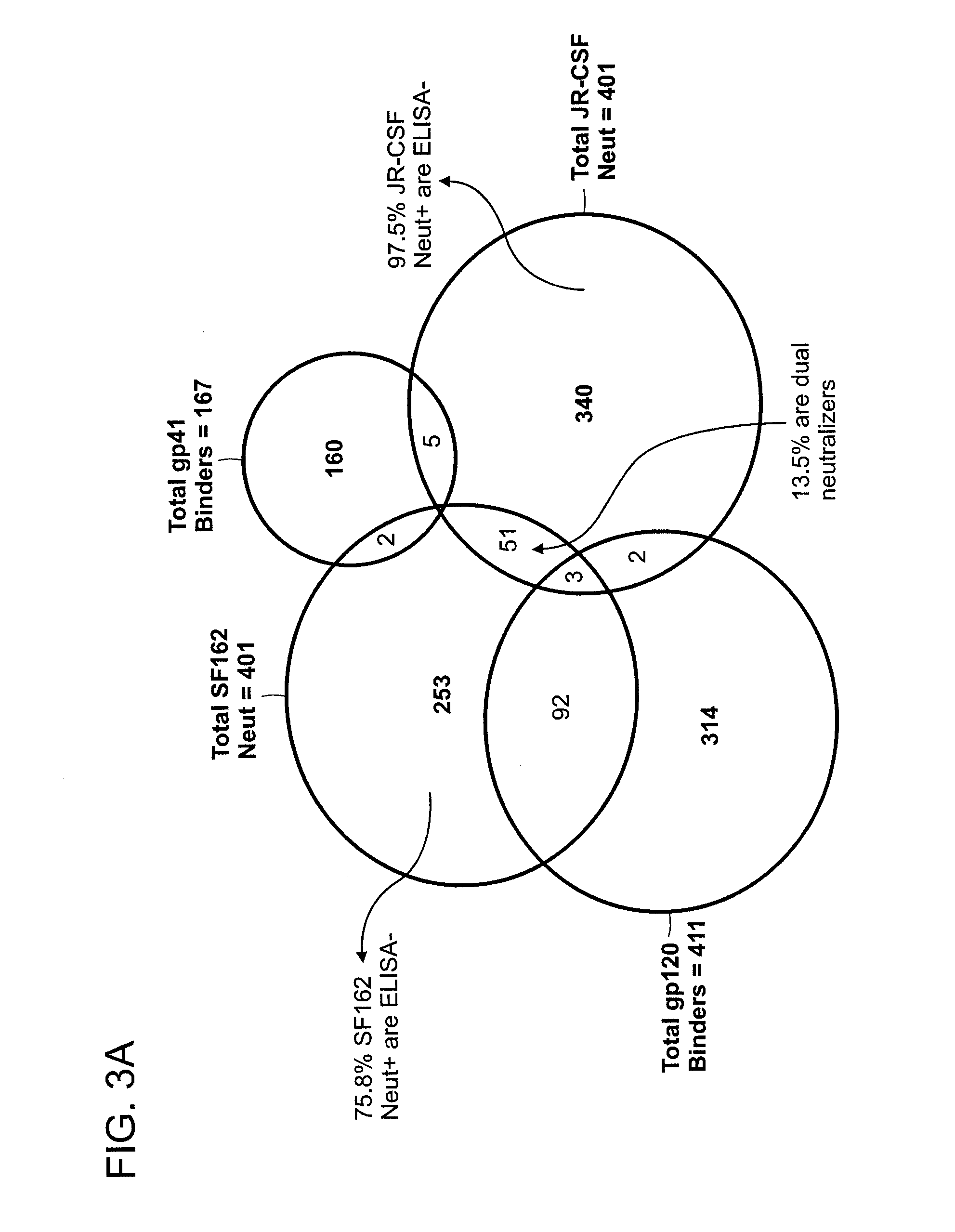
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
334 results about "Human immunodeficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human immunodeficiency virus (HIV) is a virus that weakens the body’s immune system and its ability to fight off diseases and other infections.
Immunological reagents
ActiveUS9982053B2Immunoglobulins against virusesAntiviralsAutoimmune responsesHuman immunodeficiency
Owner:MABQUEST
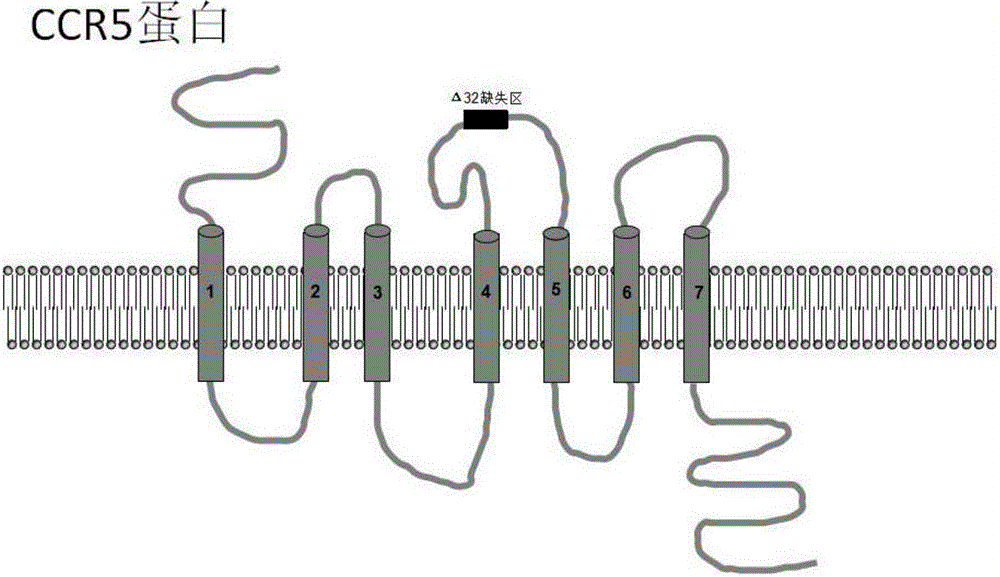
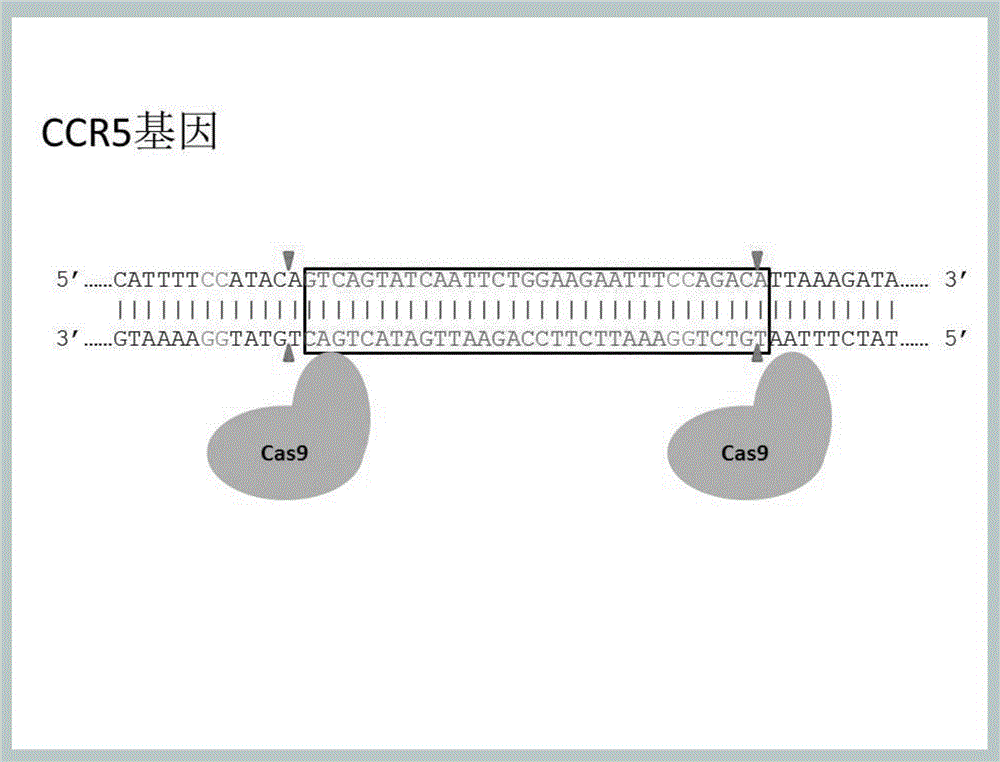
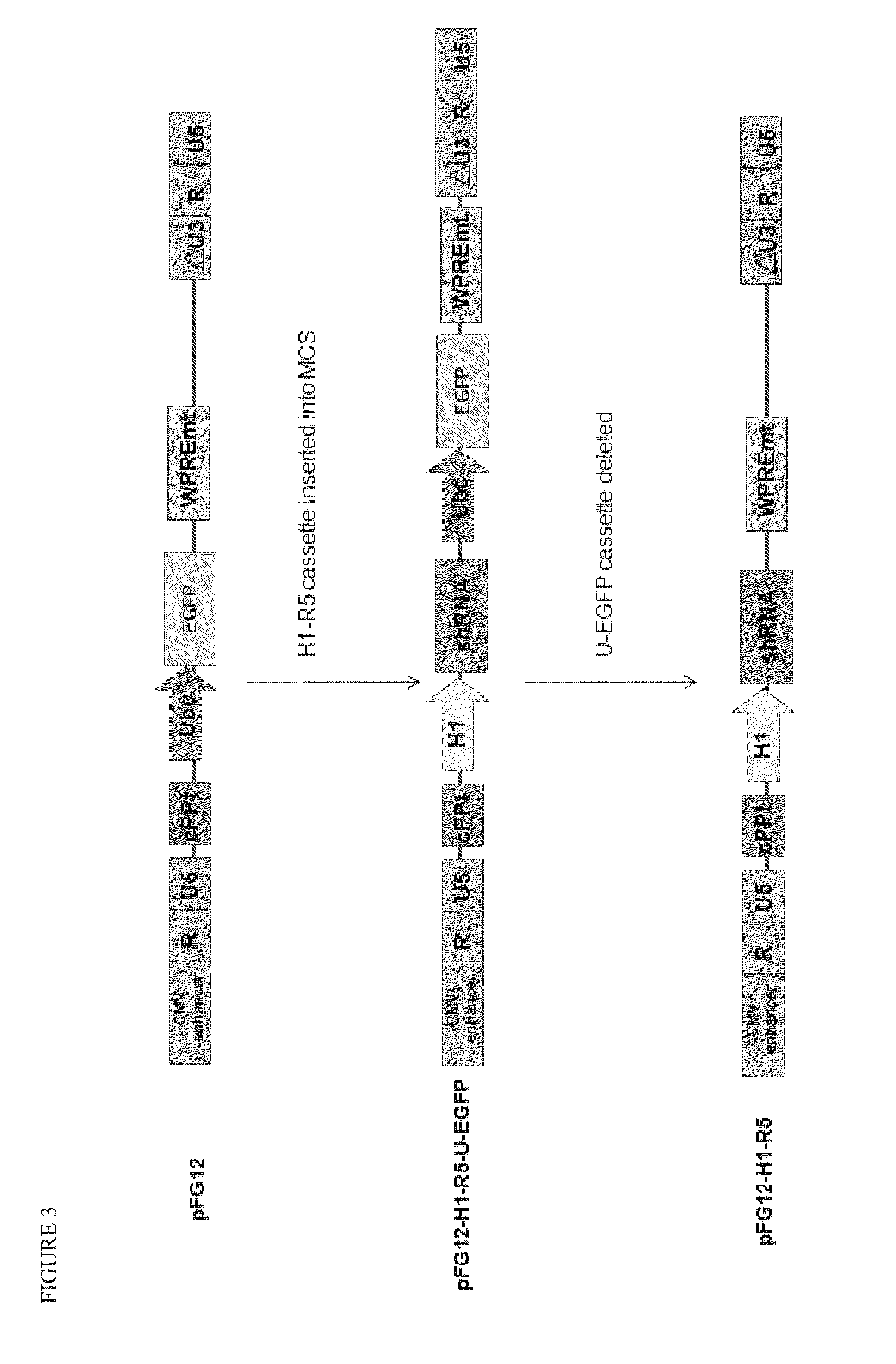
Method for inducing CCR5-delta32 deletion with genome editing technology CRISPR-Cas9
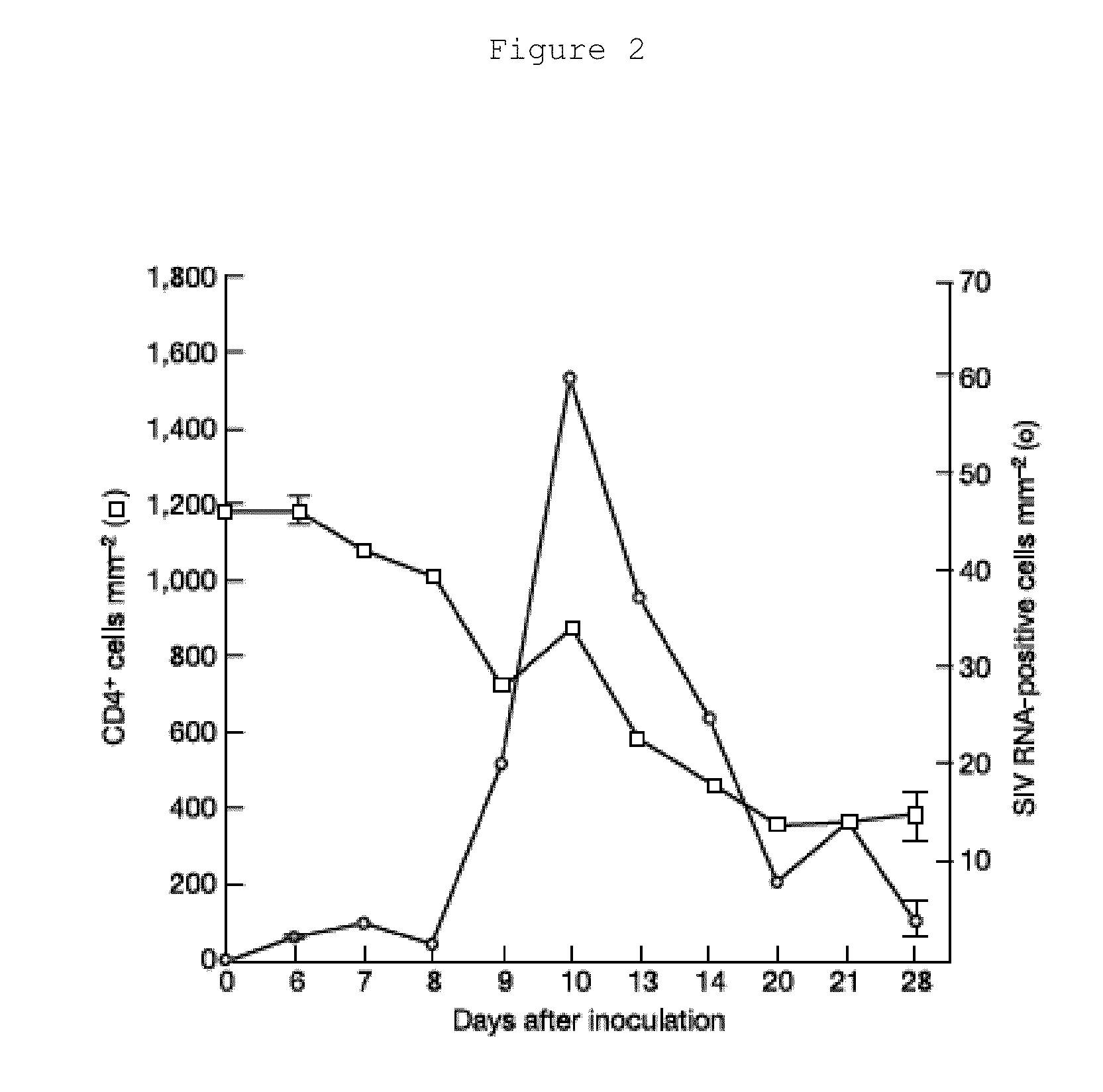
The invention relates to a method for successfully inducing cell chemokine receptor CCR5 genes to be mutated into CCR5-delta32 deletion-type genes with a new genome editing technology CRISPR-Cas9. CCR5 is an important receptor for human immunodeficiency viruses (HIV) to invade personal host cells. CCR5-delta32 deletion means deletion of 32 basic groups occurs in a CCR5 coding region, so that the sequence after the 185th amino acid is changed, and early termination occurs. CCR5-delta32 biallelic-gene homozygous deletion has natural resistance to HIV infection, and can not be infected by HIV. By means of the method, a slow virus packaging system and the CRISPR technology are used at the same time; as the slow virus infecting host range is wide, the method can be applied to cells such as bone marrow stem cells and CD4T cells, and the CCR5-delta32 deletion-type genes hopefully become medicine for treating acquired immune deficiency syndrome or other diseases.
Owner:NANKAI UNIV
Dock-and-lock (DNL) constructs for human immunodeficiency virus (HIV) therapy
ActiveUS8481041B2Reduce eliminateReduce and eliminate focusOrganic active ingredientsImmunoglobulins against virusesAntigenAntibody fragments
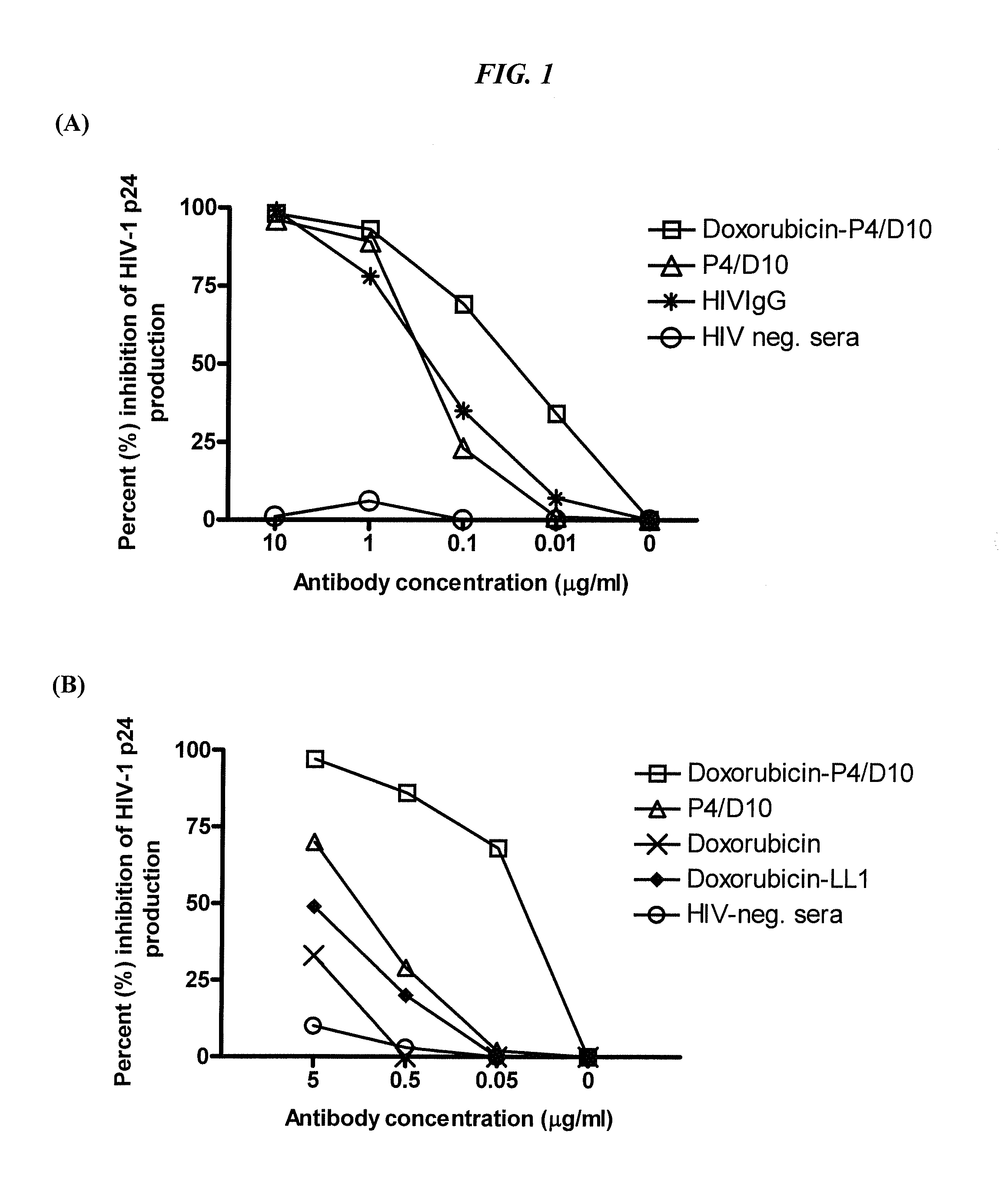
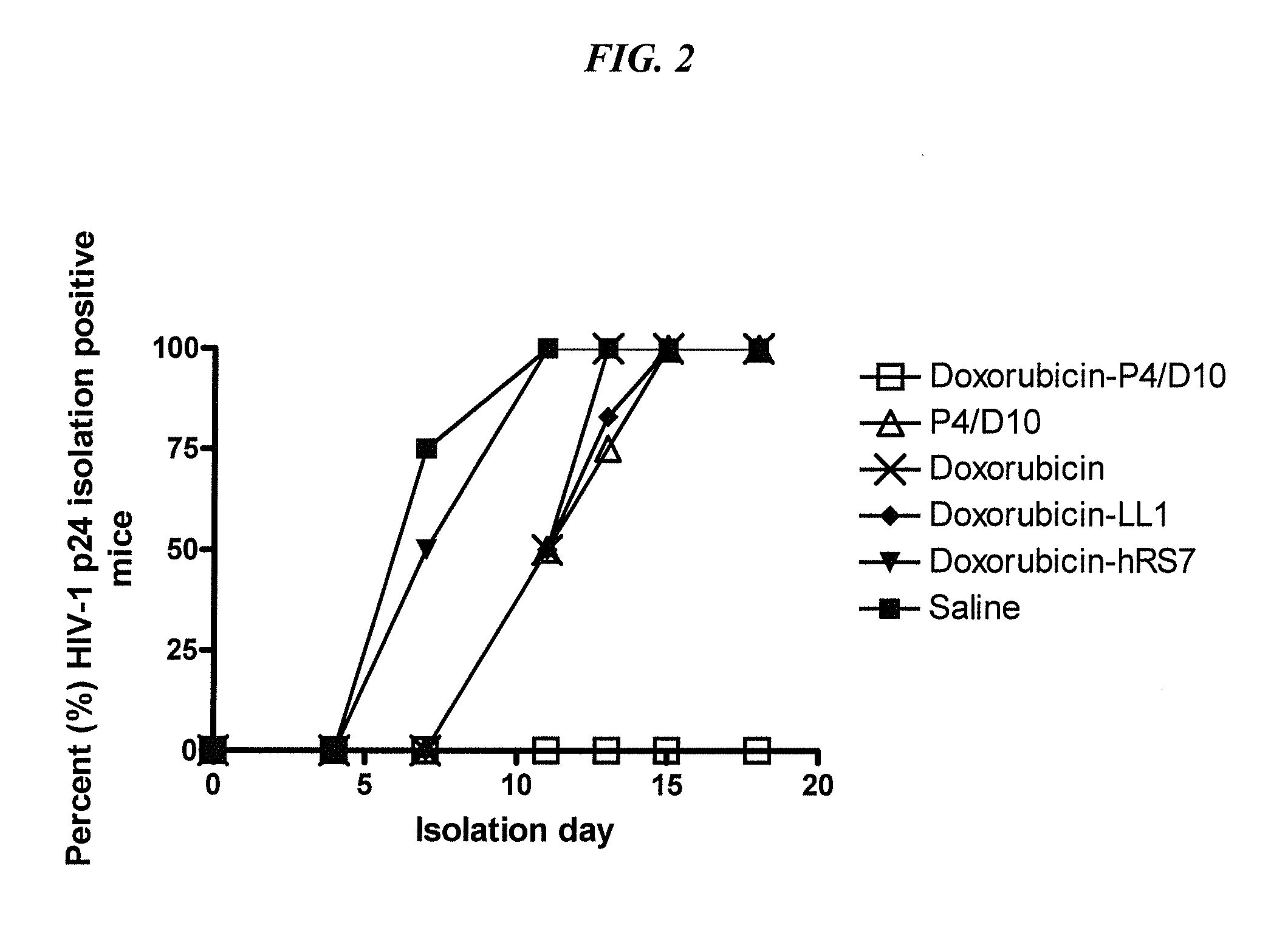
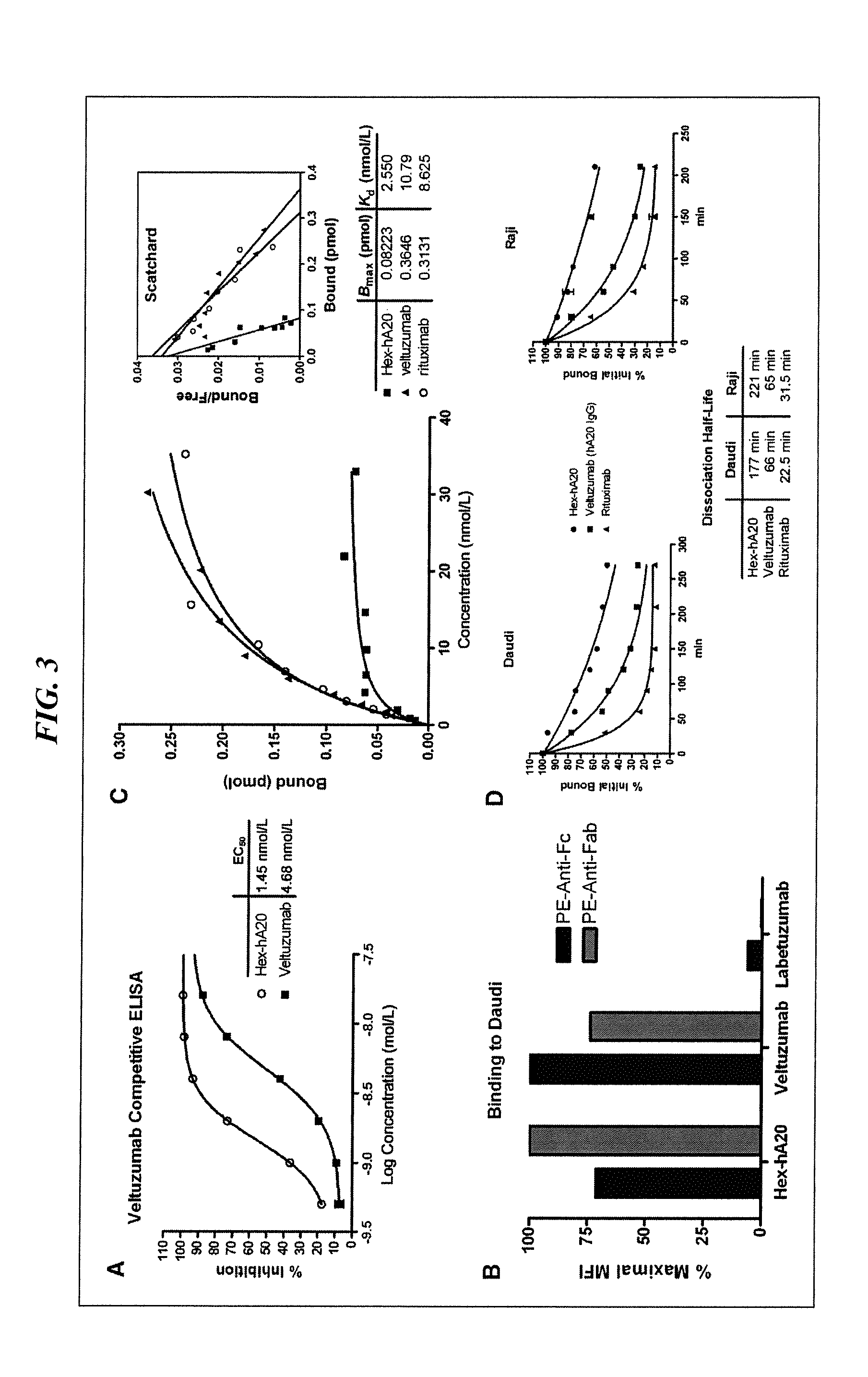
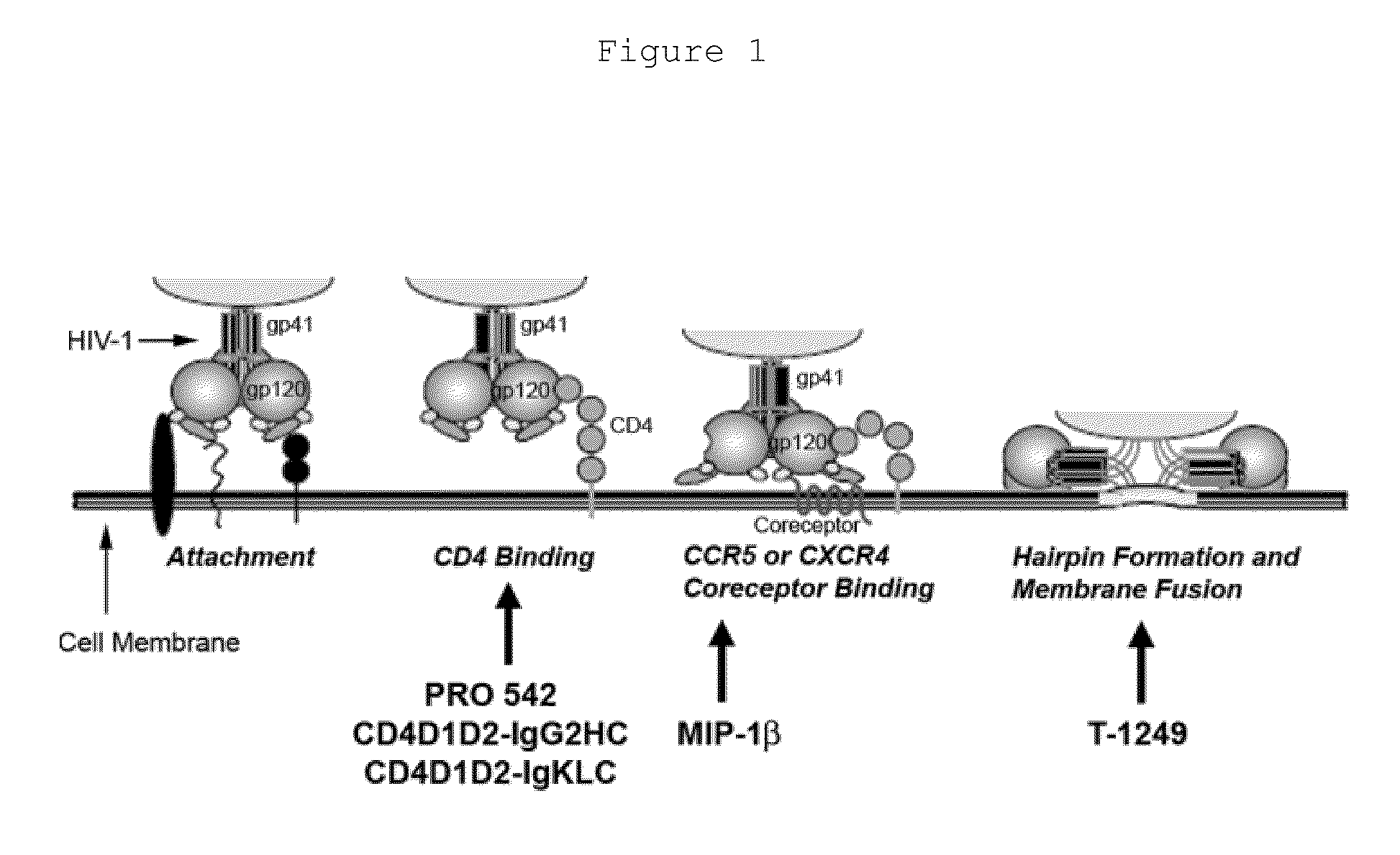
The present invention concerns methods and compositions for treatment of HIV infection in a subject, utilizing a DNL complex comprising at least one anti-HIV therapeutic agent, attached to an antibody, antibody fragment or PEG. In a preferred embodiment, the antibody or fragment binds to an antigen selected from gp120, gp41, CD4 and CCR5. In a more preferred embodiment the antibody is P4 / D10 or 2G12, although other anti-HIV antibodies are known and may be utilized. In a most preferred embodiment, the anti-HIV therapeutic agent is a fusion inhibitor, such as T20, T61, T651, T1249, T2635, CP32M or T-1444, although other anti-HIV therapeutic agents are known and may be utilized. The DNL complex may be administered alone or may be co-administered with one or more additional anti-HIV therapeutic agents.
Owner:IBC PHARMACEUTICALS INC
Dual vector for inhibition of human immunodeficiency virus
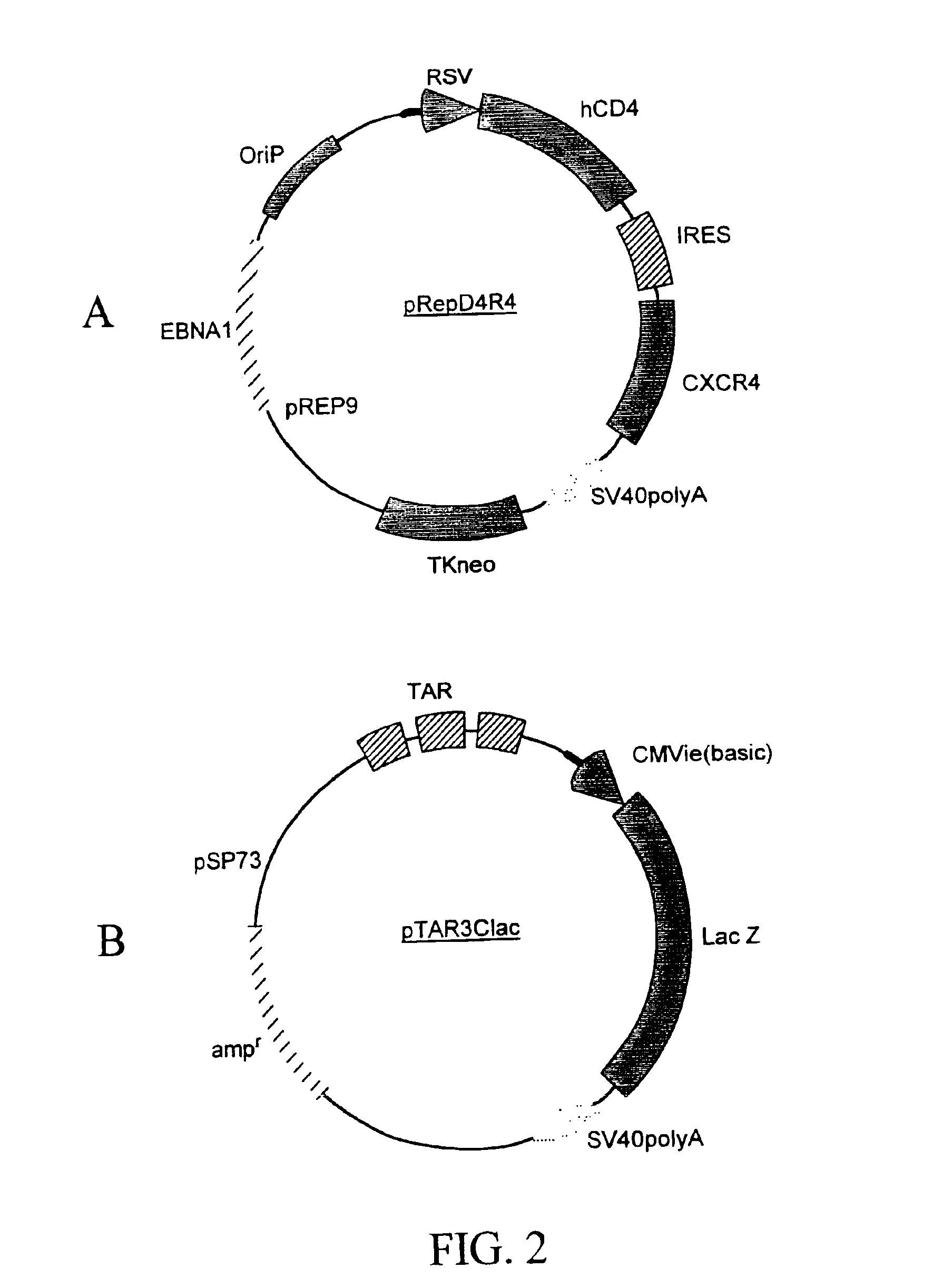
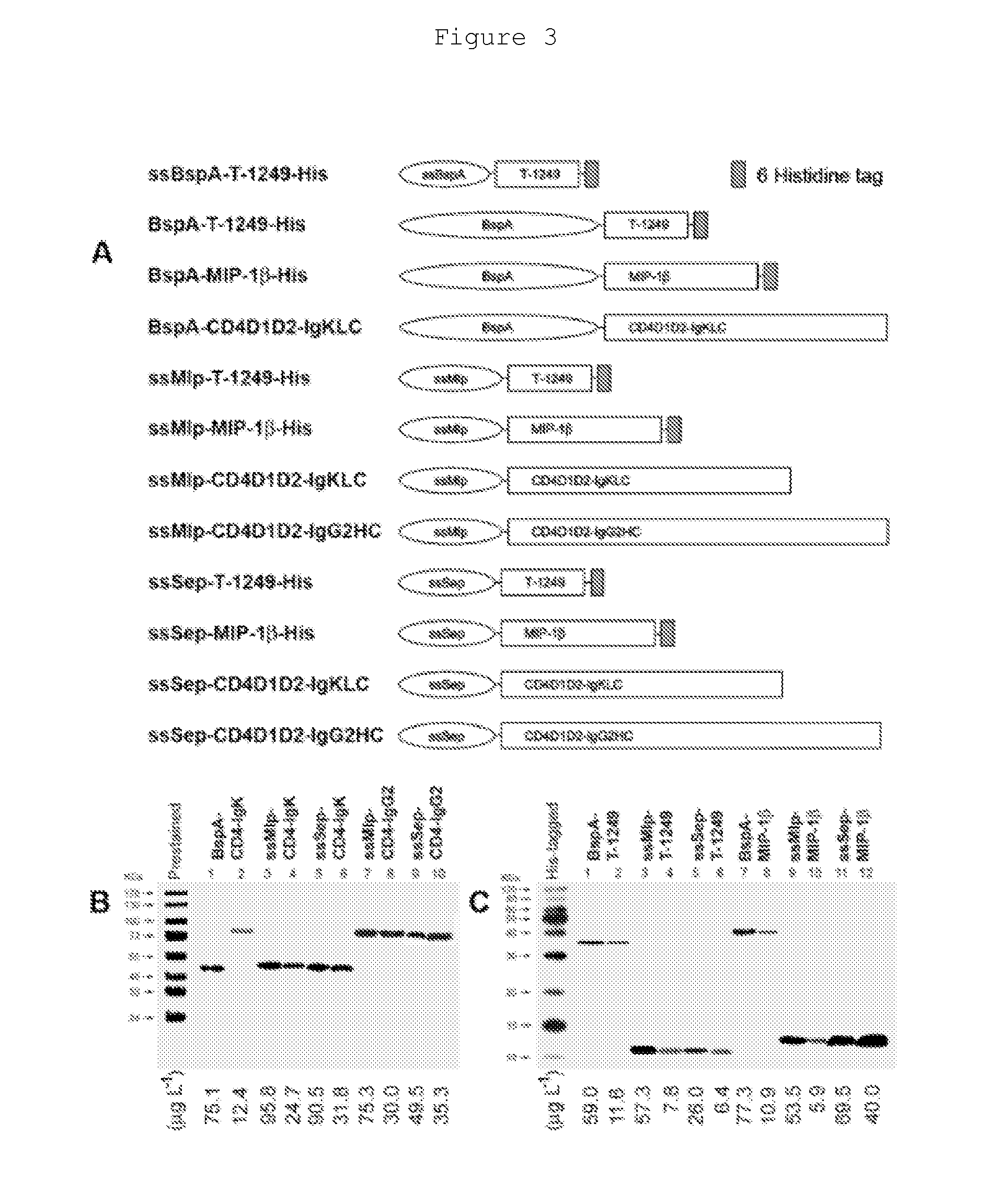
The present invention provides an expression vector for preventing or inhibiting HIV entry, fusion or replication in mammalian cells. In particular, the invention provides a recombinant retroviral vector that encodes an inhibitor of a HIV co-receptor, such as CCR5 or CXCR4, and a protein that inhibits HIV fusion to target cells and / or HIV replication. Pharmaceutical compositions comprising such constructs and methods of use thereof to prevent or treat HIV infection in a patient are also disclosed.
Owner:RGT UNIV OF CALIFORNIA +1
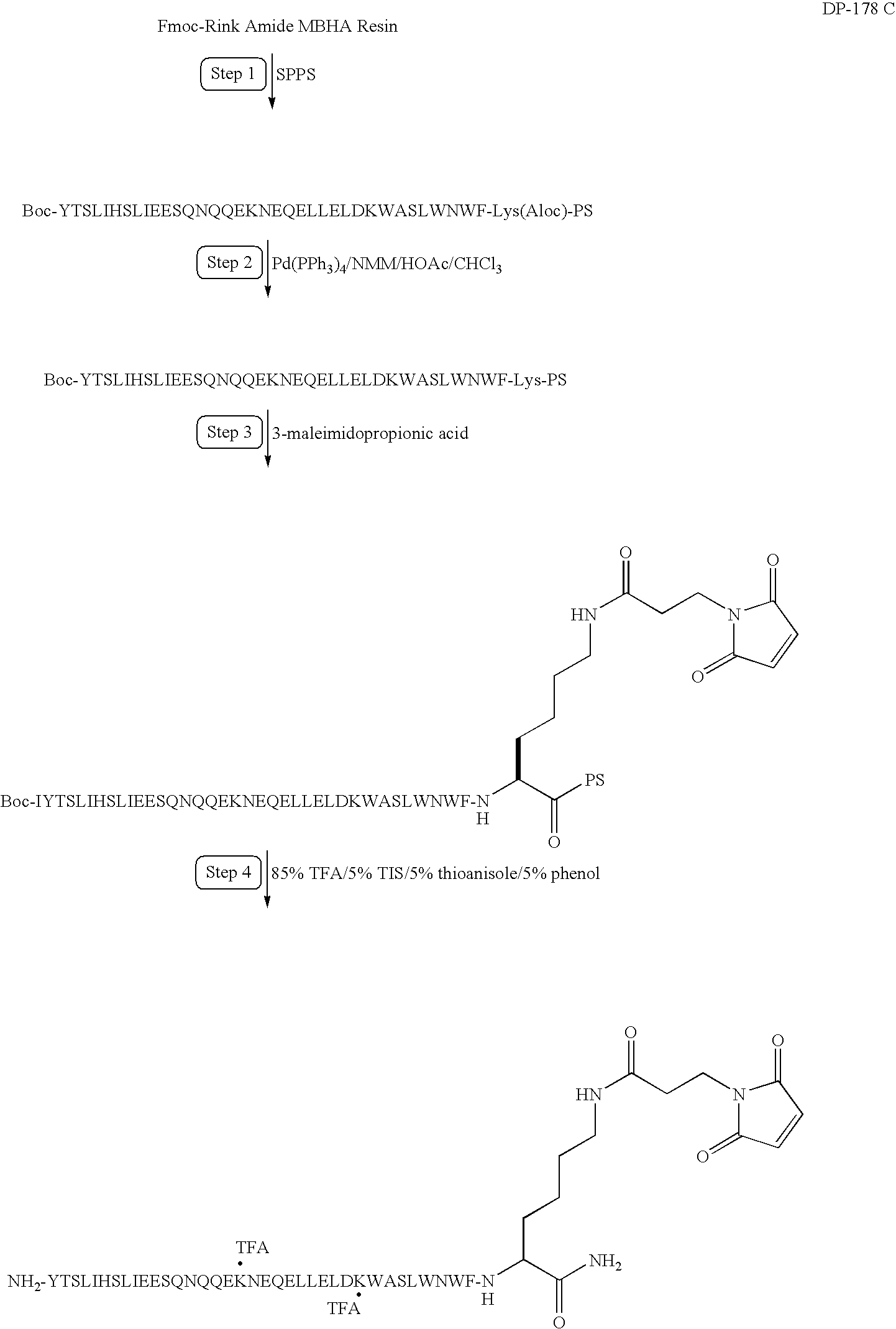
Long-lasting antiviral fusion inhibitor peptide conjugates comprising albumin and human immunodeficiency virus (HIV) peptides
InactiveUS7582301B1Reduce sensitivityImprove in vivo stabilityPeptide/protein ingredientsVirus peptidesHalf-lifeImmunodeficiency virus
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
Stabilized human immunodeficiency virus (HIV) envelope (ENV) trimer vaccines and methods of using same
ActiveUS20140302080A1Reduce dissociationImprove stabilitySugar derivativesViral antigen ingredientsImmunodeficiency virusHuman immunodeficiency
The invention features stabilized human immunodeficiency virus (HIV) envelope (Env) trimers. The invention also features vaccines, nucleic acids, and vectors to deliver and / or facilitate production of the stabilized HIV Env trimers. In addition, the invention features methods of making and using the stabilized HIV Env trimers of the invention.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
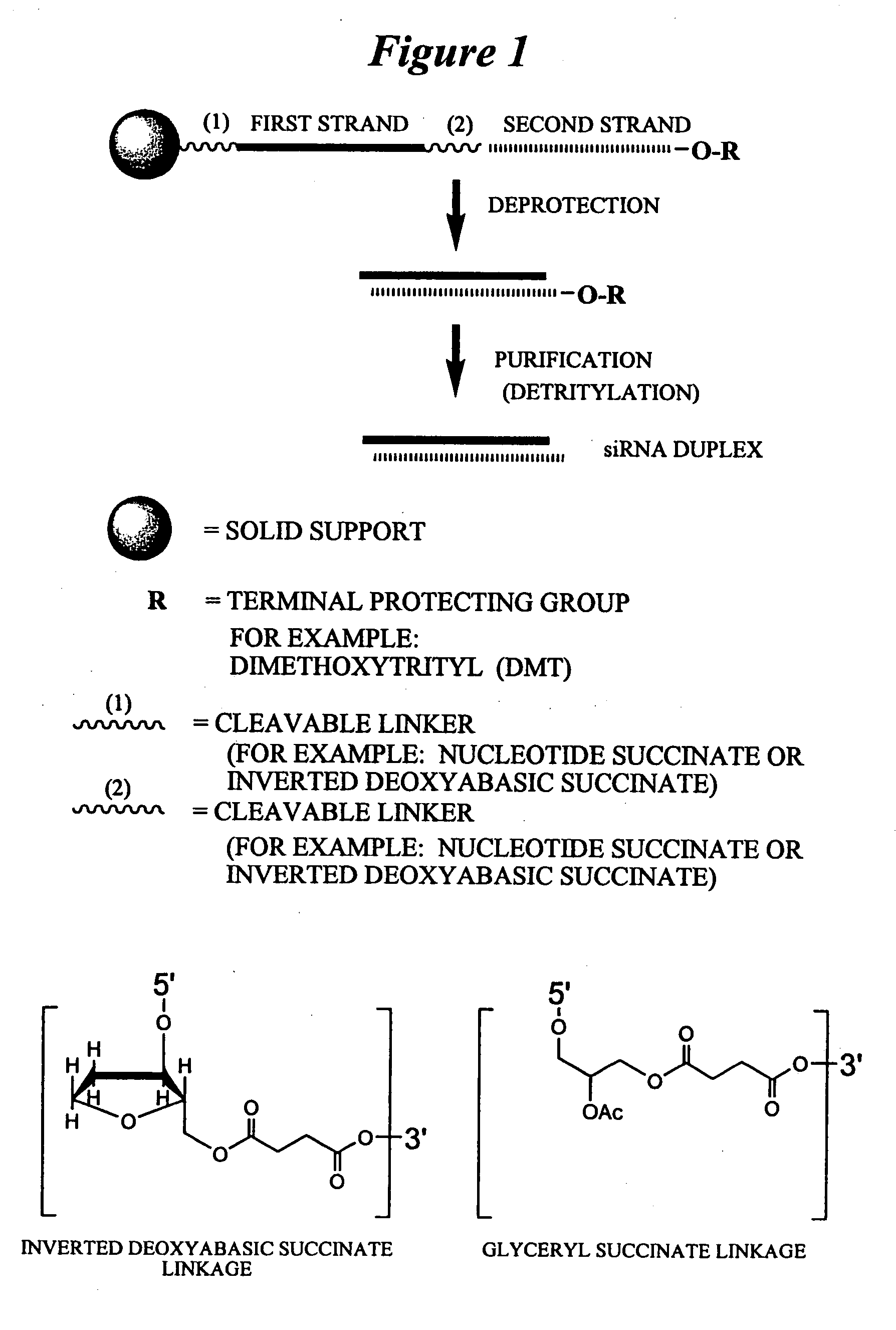
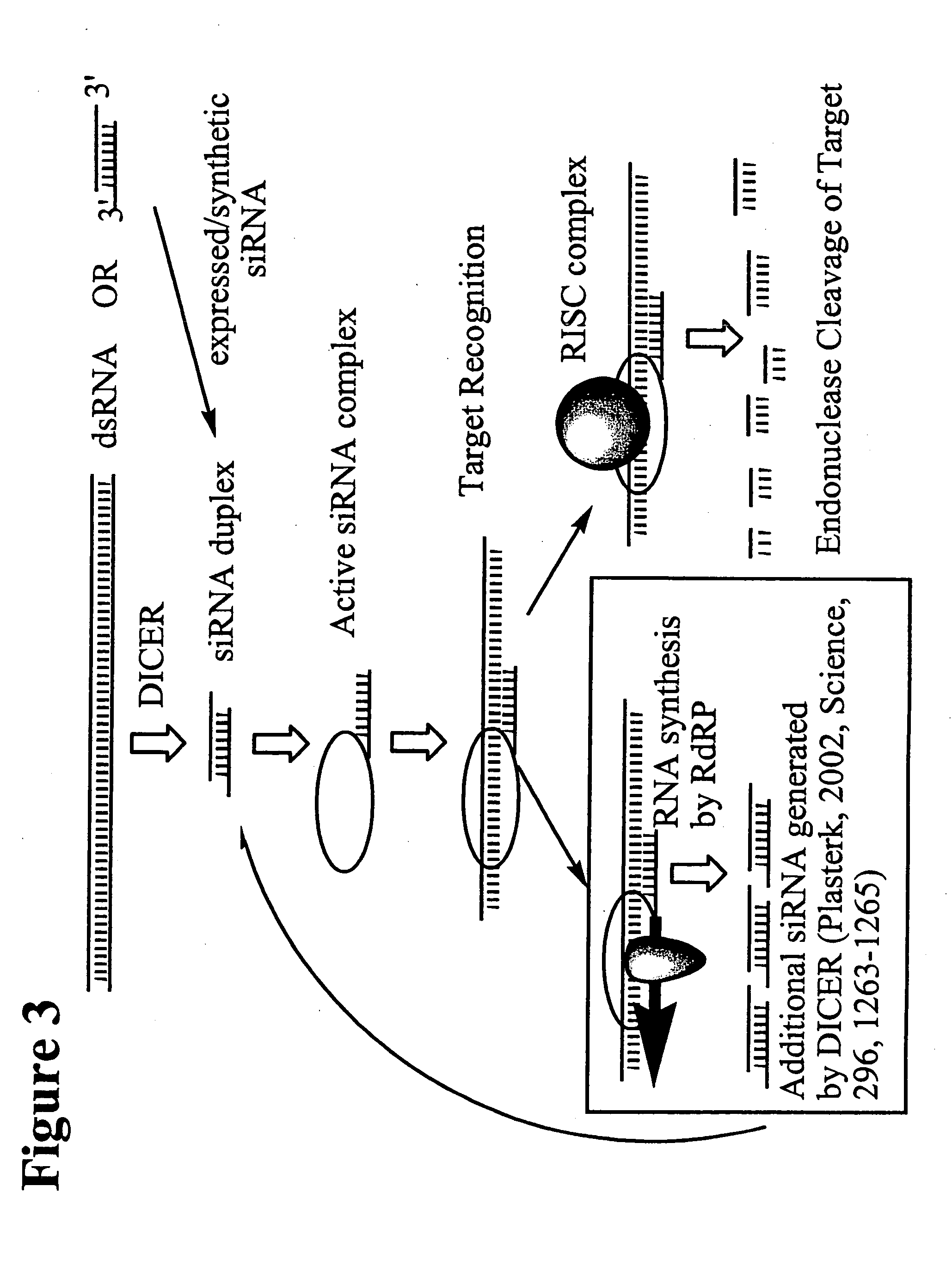
RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050191618A1Improves various propertyImprove the immunitySugar derivativesMicrobiological testing/measurementDiseaseBiological body
This invention relates to compounds, compositions, and methods useful for modulating human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of human immunodeficiency virus (HIV) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of HIV genes. The small nucleic acid molecules are useful in the treatment of HIV infection, AIDS, and / or diseaes and conditions related to HIV infection and / or AIDS in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
Human immunodeficiency virus affinity adsorption column, and preparation method and uses thereof
InactiveCN102631891ARich sourcesQuality improvementIon-exchange process apparatusOther blood circulation devicesMicrosphereHuman immunodeficiency
The invention discloses a human immunodeficiency virus (HIV) affinity adsorption column, comprising a column body and at least one activated affinity microsphere located in the column body, wherein the activated affinity microsphere is connected with a human immunodeficiency virus affinity protein, and the affinity protein can be bound with human immunodeficiency virus. The affinity protein comprises a main receptor CD4 molecule, a gp120 antibody, an auxiliary receptor CXC chemokine receptor 4 (CXCR-4) and a CC chemokine receptor 5 (CCR-5). The affinity microsphere may be a glass microsphere with the size of 1mm, a chitosan crosslinking microsphere with the diameter more than 500 microns, or a gluosan microsphere. The human immunodeficiency virus affinity adsorption column disclosed by the invention is applicable for eliminating the human immunodeficiency virus in the blood of HIV patients, and relieving and treating the immunodeficiency syndrome of HIV patients; and compared with the traditional treatment methods, the immunoadsorption column has high safety, good specificity, small toxic or side effects and good operation simplicity.
Owner:WUHAN UNIV
Modified 4'-nucleosides as antiviral agents
Owner:GILEAD SCI INC
Methods for treating viral infections using polyamine analogs
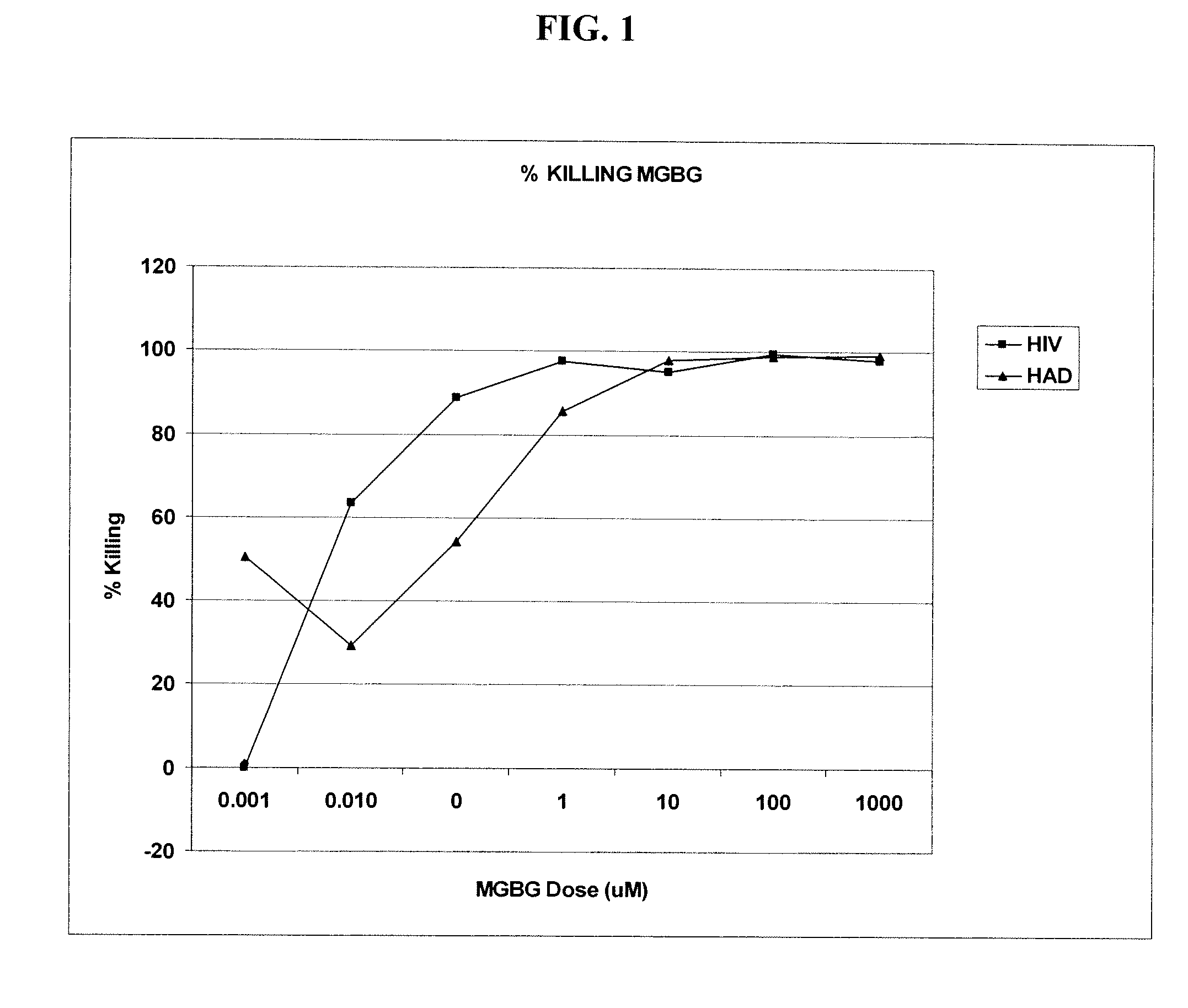
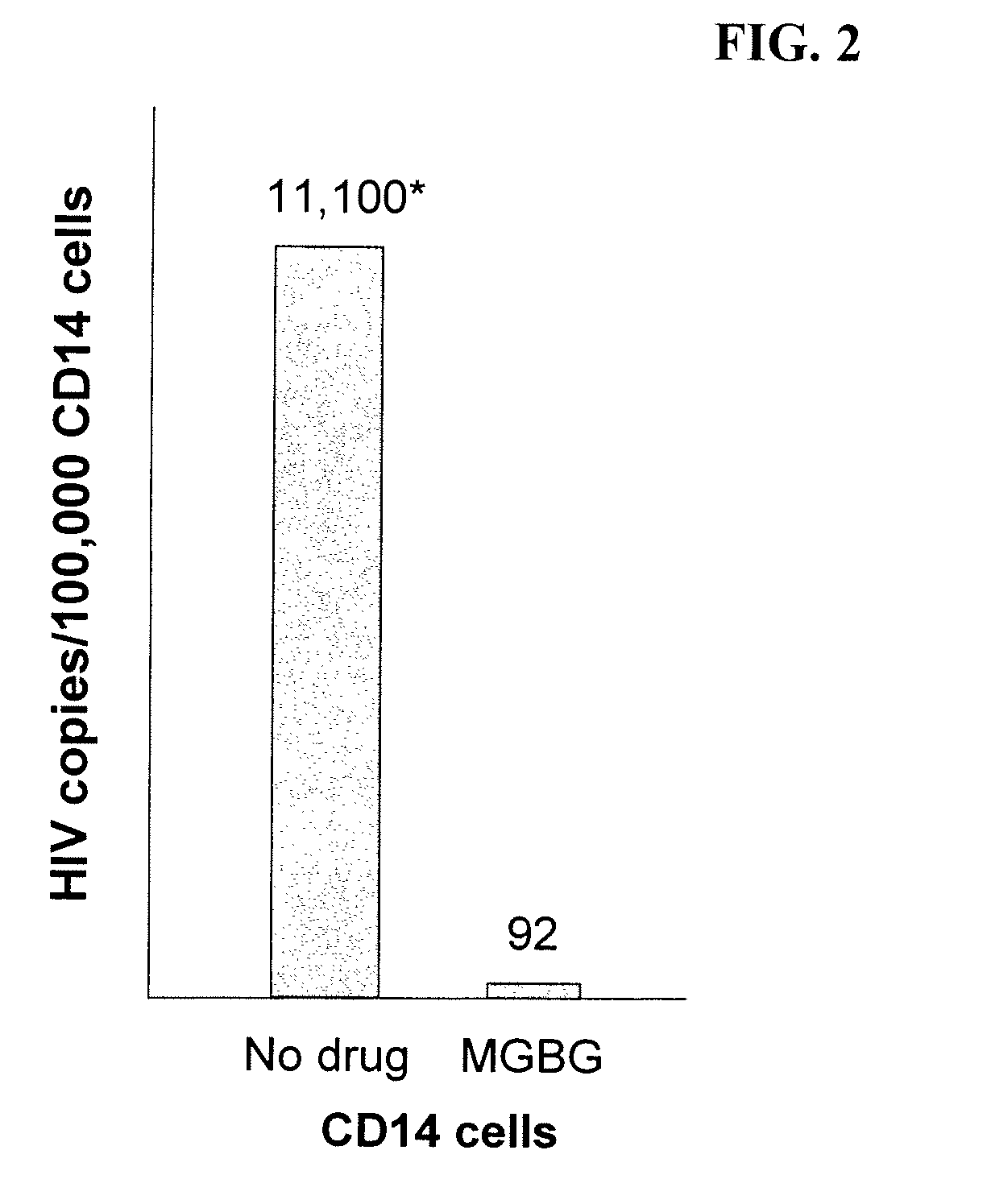
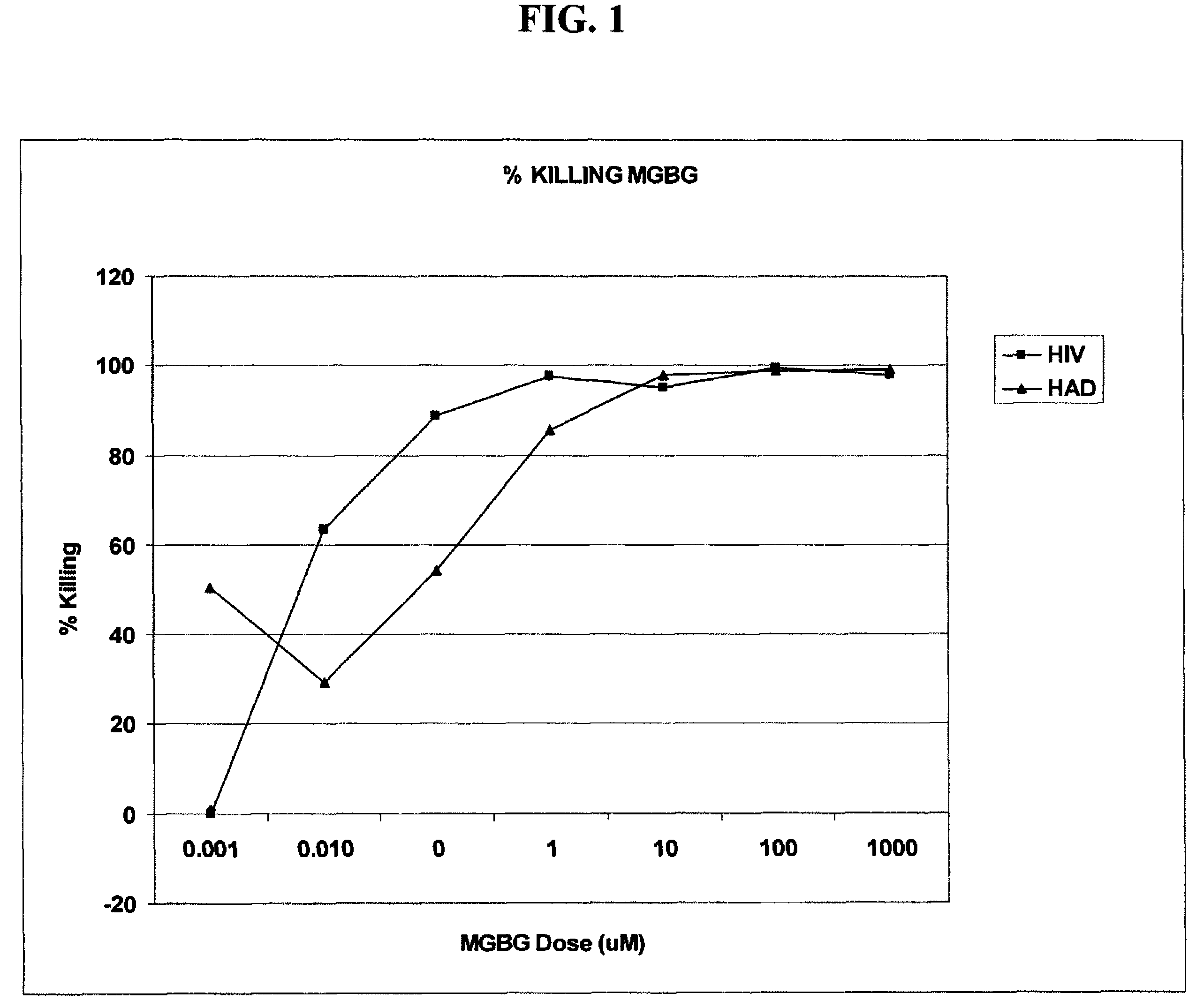
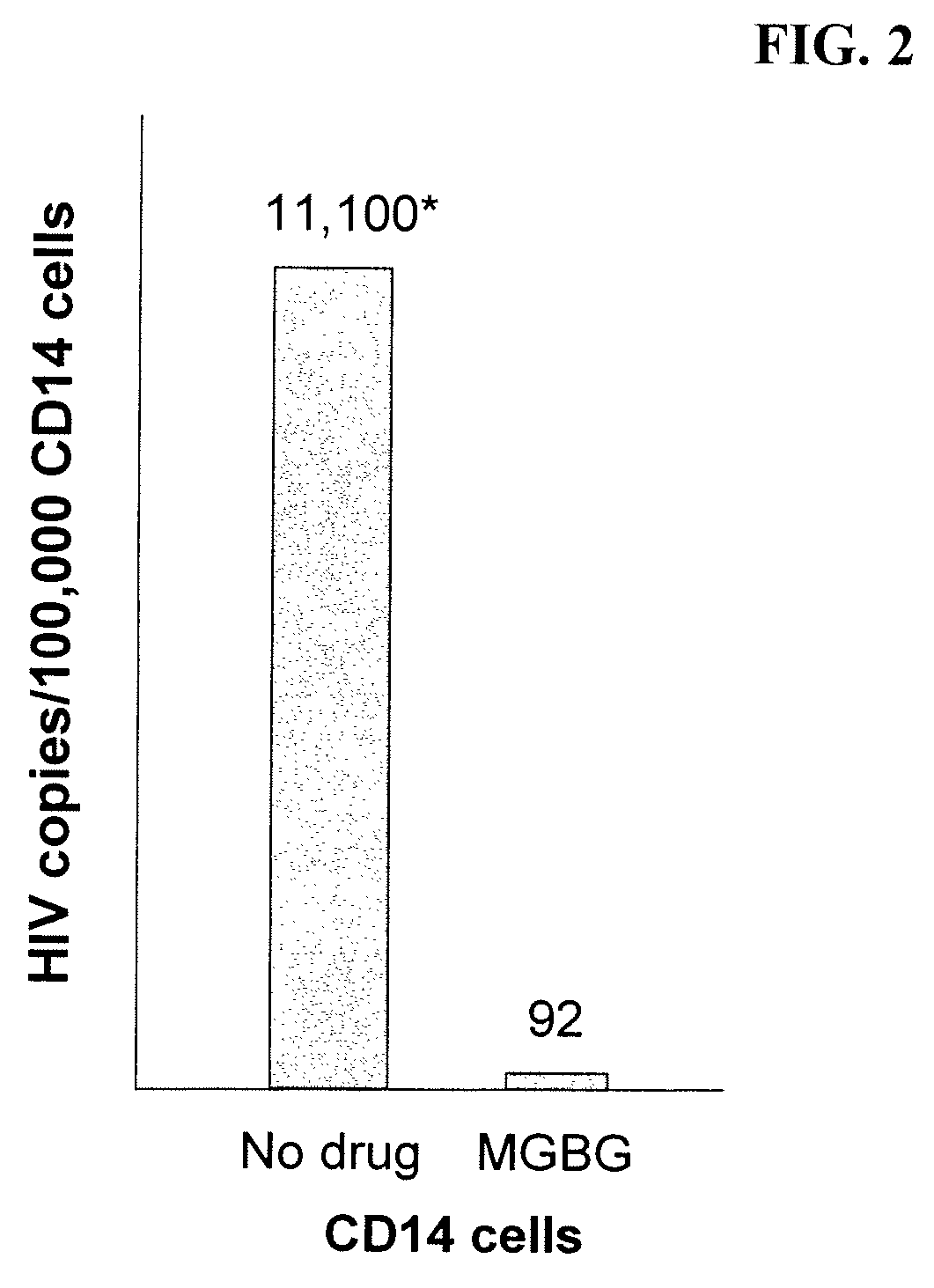
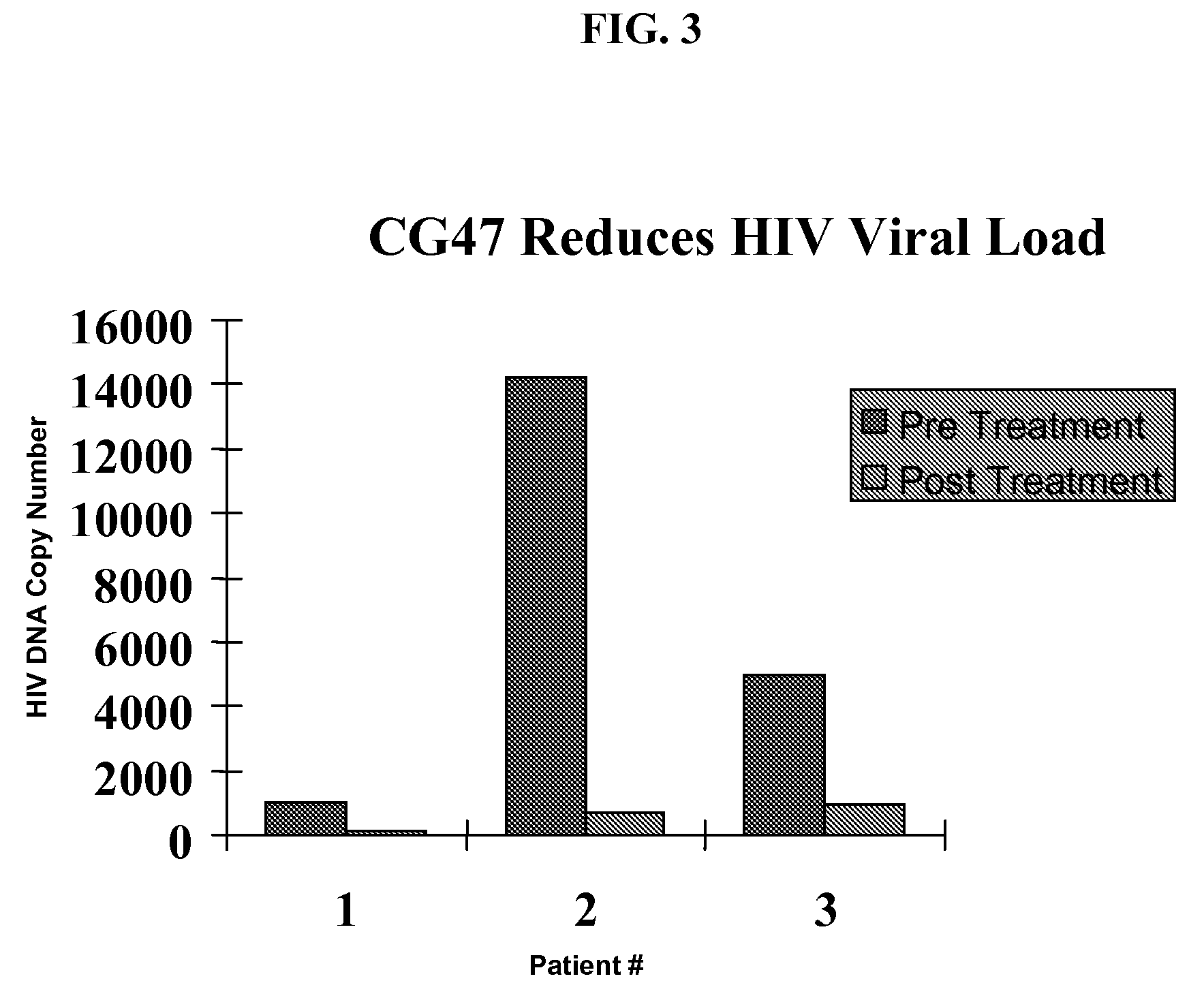
InactiveUS20070078187A1Avoid seizuresReducing viral load of an infected subjectBiocideAntiviralsAbnormal tissue growthPresent method
Methods for treating viral infections using polyamine analogs, including mitoguazone (MGBG), are provided. In these methods, polyamine analogs destroy macrophages that act as viral reservoirs, facilitating the destruction of the viruses that dwell within the macrophages. Examples of viral infections that may be treated with the present methods include, but are not limited to, infections from human immunodeficiency viruses. These methods differ from previous methods of treatment using polyamine analogs, wherein the polyamine analogs were administered only as anti-tumor agents.
Owner:PATHOLOGICA
Human immunodeficiency virus (HIV)-neutralizing antibodies
ActiveUS20110044994A1Sugar derivativesImmunoglobulins against virusesVaccinationImmunodeficiency virus
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
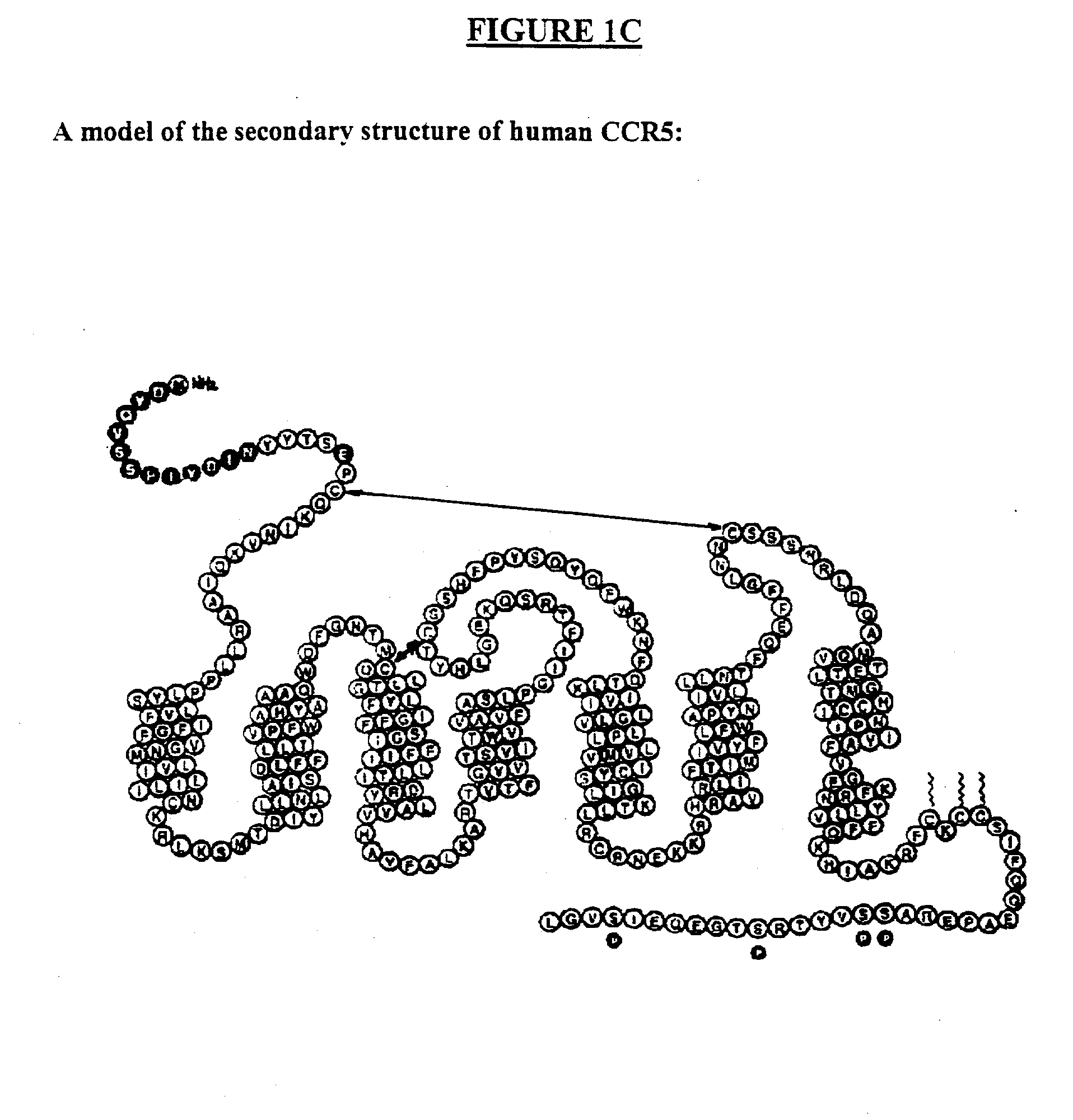
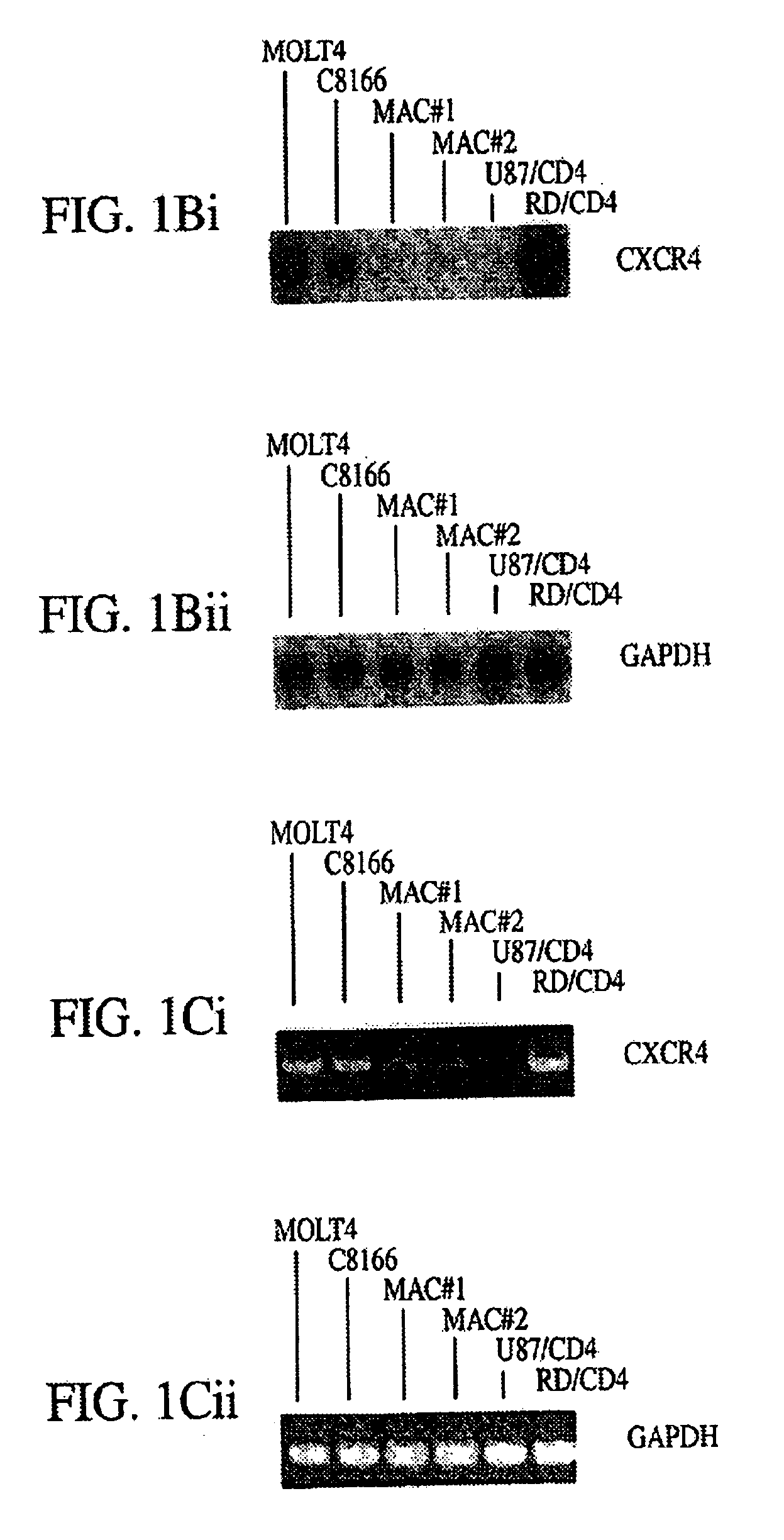
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS7005503B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Immunodeficiency virus
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
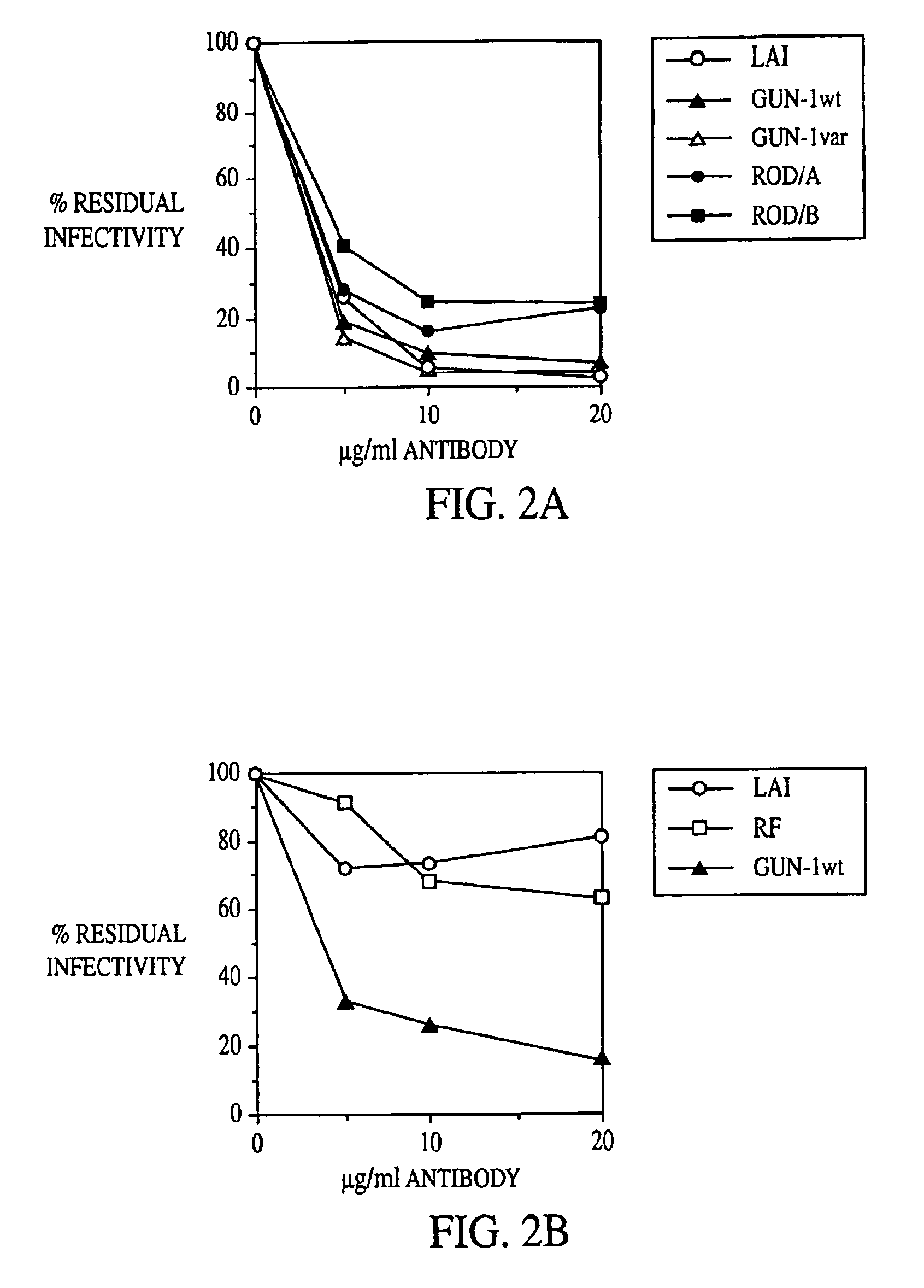
Broadly cross-reactive neutralizing antibodies against human immunodeficiency virus selected by Env-CD4-co-receptor complexes
InactiveUS7223844B2Increase exposurePeptide/protein ingredientsAntibody mimetics/scaffoldsAntibody fragmentsMammal
The present invention features antibodies and antibody fragments that specifically bind a CD4-inducible HIV gp120 epitope that is enhanced by binding a co-receptor for HIV, such as CCR5 or CXCR4, and pharmaceutical compositions comprising the antibodies or antibody fragments. The invention also features nucleic acids encoding the antibodies or antibody fragments, pharmaceutical compositions comprising the nucleic acids encoding the antibodies or antibody fragments, vectors comprising the nucleic acids, and cells comprising the vectors. The invention further features methods of identifying antibodies or antibody fragments with broadly neutralizing activity against HIV. The invention also features methods of inhibiting HIV entry into cells and methods of inhibiting replication of HIV in mammals, using the antibodies and nucleic acids of the invention.
Owner:THE SCRIPPS RES INST +1
Compositions and methods for treating obesity, obesity related disorders and for inhibiting the infectivity of human immunodeficiency virus
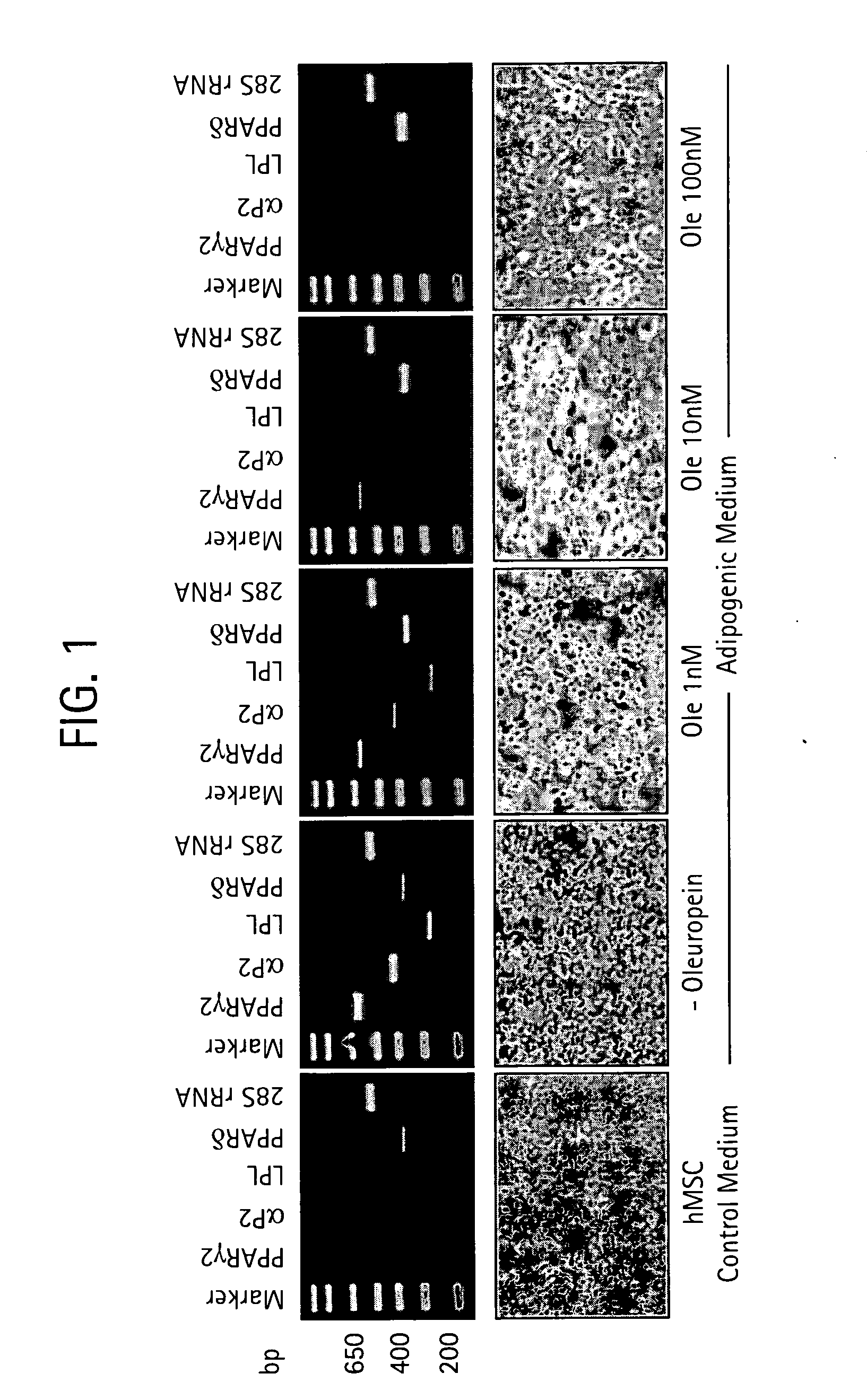
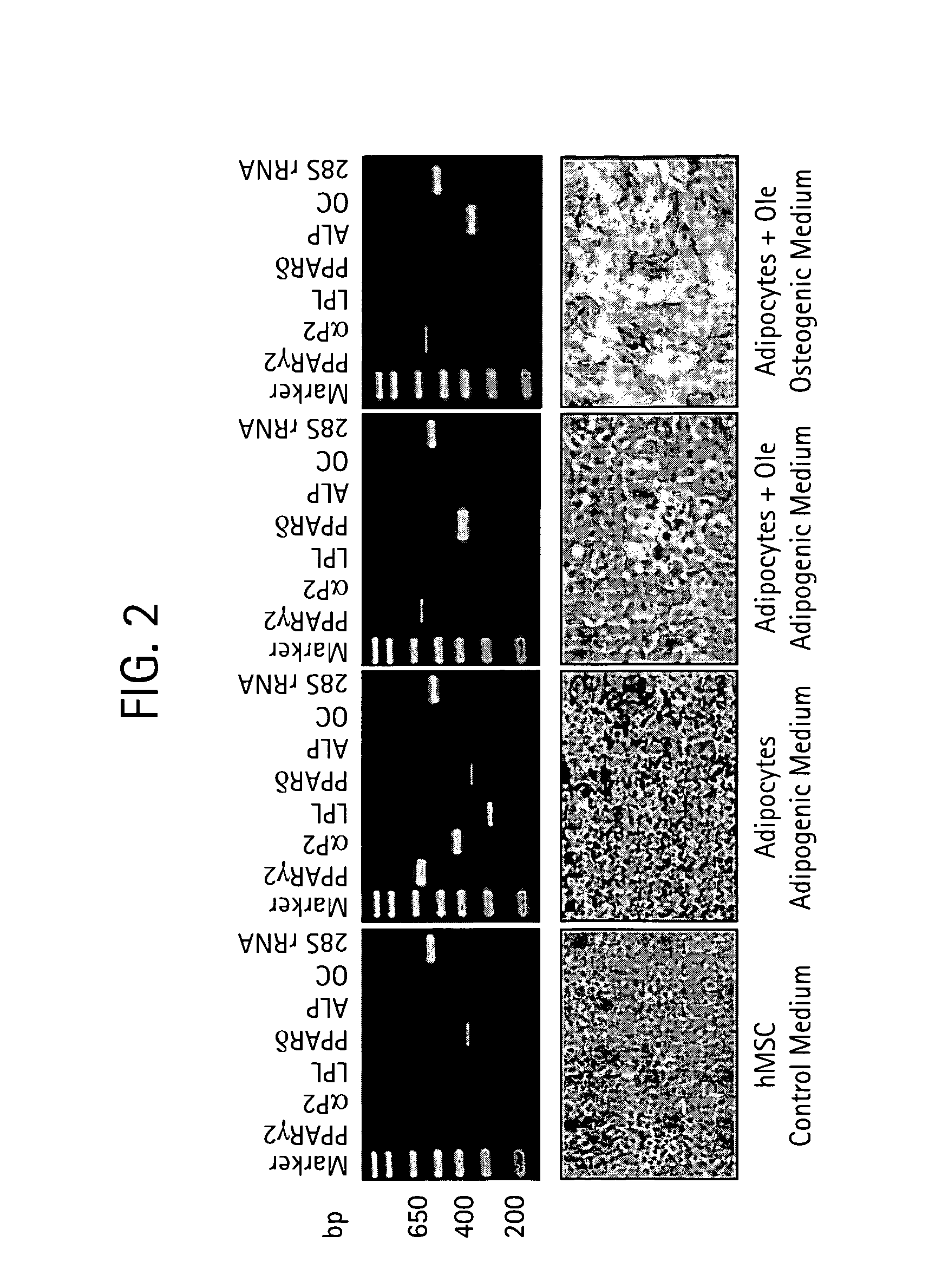
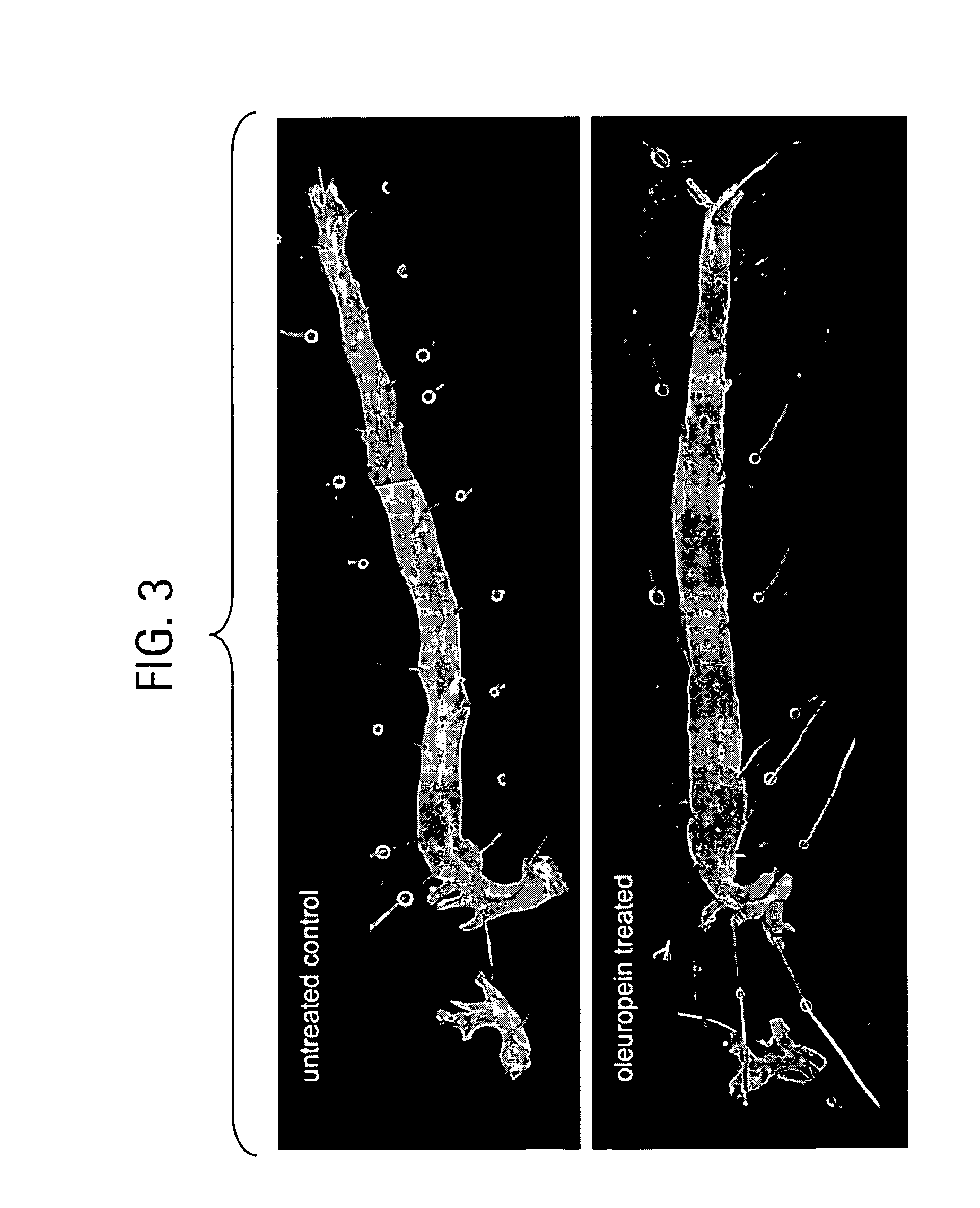
InactiveUS20090061031A1Modulate adipogenesisModulate lipodystrophyBiocideHydroxy compound active ingredientsMetaboliteSecoiridoid Glycosides
The present invention relates to methods and pharmaceutical compositions for treating obesity or obesity-related disorders in a subject suffering from or predisposed to developing obesity or an obesity-related disorder, or for inhibiting the infectivity of HIV, by administering oleuropein, an analogue or derivative thereof, or the major metabolites of oleuropein including oleuropein aglycone, hydroxytyrosol, and elenolic acid or their analogues, or derivatives thereof, an iridoid glycoside, or a secoiridoid glycoside or analogues or derivatives thereof, or any combination of the foregoing including olive leave extract. The invention also relates to methods for screening / diagnosing a subject having, or predisposed to having obesity or a related disorder by measuring the expression profiles of an adipogenic gene selected from PPARγ2, LPL and αP2 gene and gene product, or other adipogenic, lipogenic, or lipolytic genes and gene products in an individual. The invention further provides for screening for novel oleuropein analogues.
Owner:NEW YORK UNIV +1
Diagnostic kits comprising genetically engineered human immunodeficiency virus-like particles containing heterologous antigenic markers
InactiveUS6342228B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesHeterologous
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
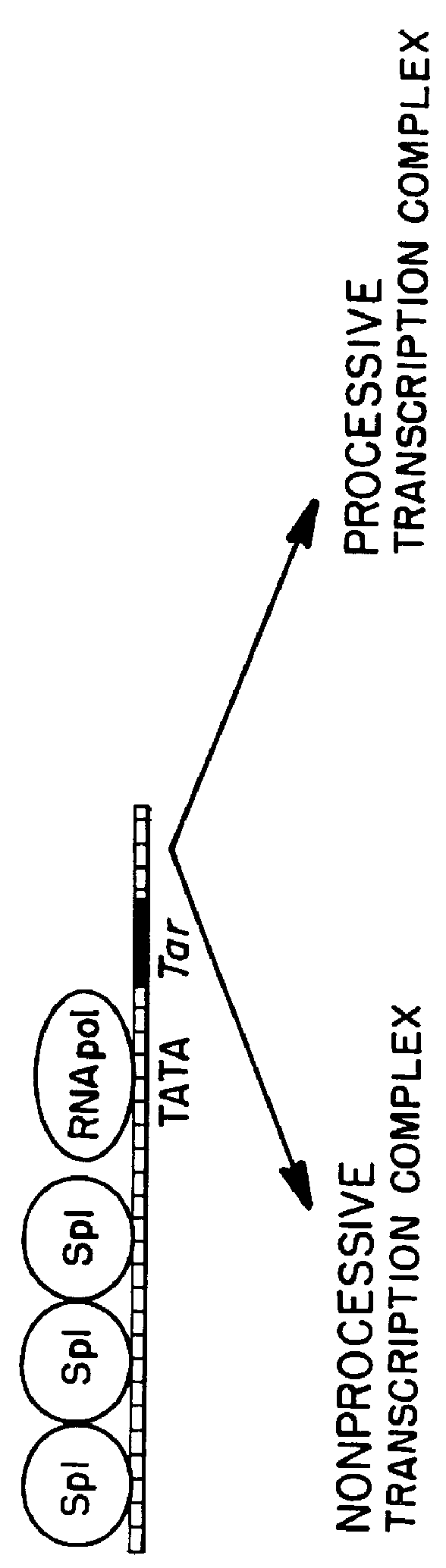
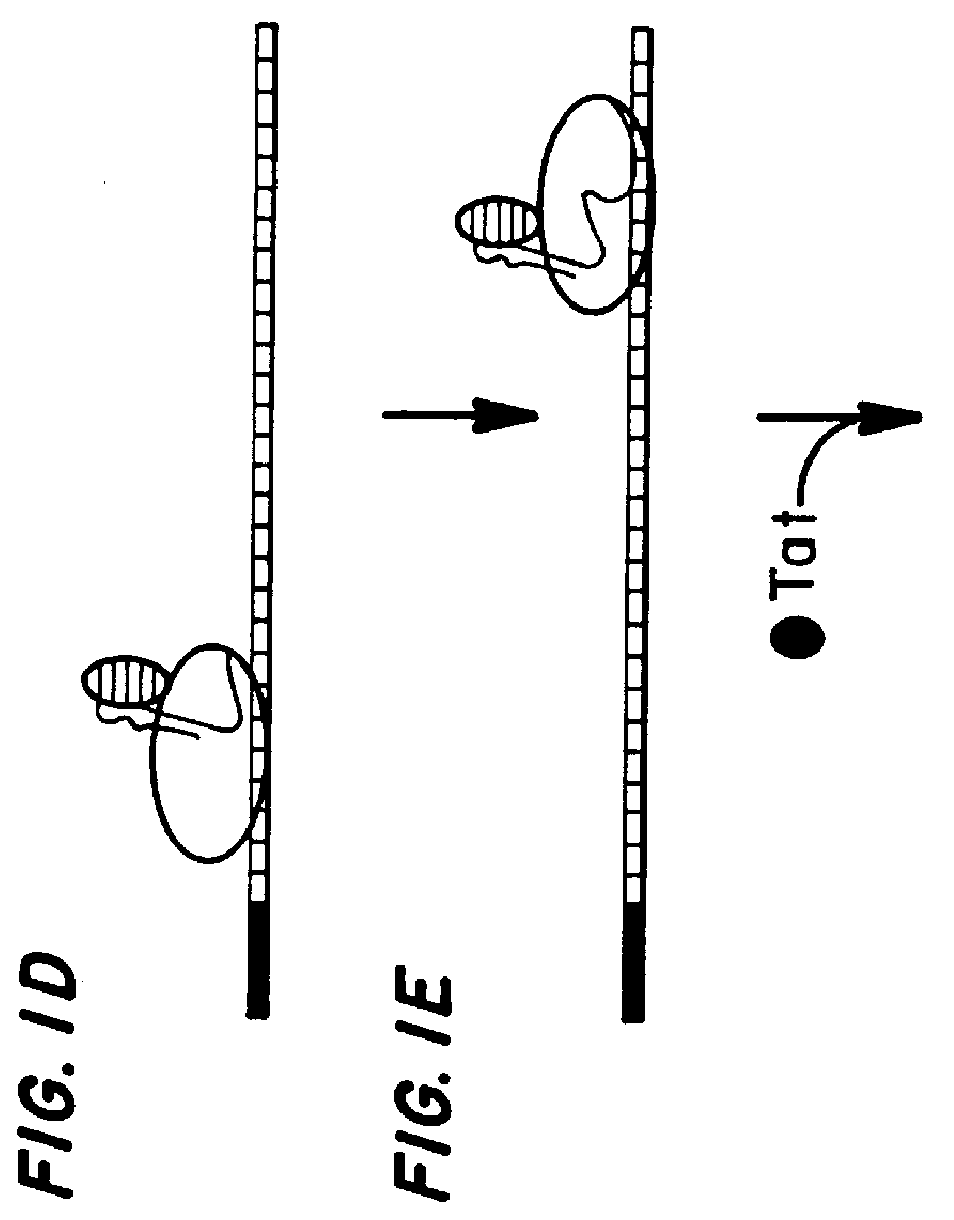
Marker, method and an assay for detection and monitoring of latency and activation of human immunodeficiency virus
A TAR marker, method for detection and monitoring of HIV latency and activation and an assay for detection of the marker. The assay sensitively detects HIV transcription and monitors HIV transcriptional activity by detecting the presence of TAR fragments and full length transcripts, quantifying both and determining the ratio of short to long transcripts. A low ratio correlates with a latent-type transcriptional activity of HIV whereas the appearance of long transcripts signifies increased efficiency of transcriptional activity of HIV and the transition from latency to activation. The size difference between the TAR fragments appearing predominantly in latency and the full length transcripts appearing predominantly during the HIV activation is detected by RT-PCR assay that utilizes novel primers and probes. The obtained ratio is a sensitive tool in detection of HIV infection, the analysis of load of latent and active virus and monitoring the transition from the latent to active state of HIV replication.
Owner:RGT UNIV OF CALIFORNIA
Dual vector for inhibition of human immunodeficiency virus
InactiveUS20160243169A1Organic active ingredientsPeptide/protein ingredientsCXCR4Immunodeficiency virus
The present invention provides an expression vector for preventing or inhibiting HIV entry, fusion or replication in mammalian cells. In particular, the invention provides a recombinant retroviral vector that encodes an inhibitor of a HIV co-receptor, such as CCR5 or CXCR4, and a protein that inhibits HIV fusion to target cells and / or HIV replication. Pharmaceutical compositions comprising such constructs and methods of use thereof to prevent or treat HIV infection in a patient are also disclosed.
Owner:RGT UNIV OF CALIFORNIA +1
Compositions and methods for detecting human immunodeficiency virus
InactiveUS6900010B2Robust and efficient and sensitive in detectionEarly diagnosisBiocideGenetic material ingredientsHeterologousInfected cell
Owner:MUSC FOUND FOR RES DEV
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS20030152913A1Microbiological testing/measurementImmunoglobulins against virusesCXCR4Immunodeficiency virus
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
Nucleic acids encoding modified human immunodeficiency virus type 1 (HIV-1) group M consensus envelope glycoproteins
The present invention relates, in general, to an immunogen and, in particular, to an immunogen for inducing antibodies that neutralizes a wide spectrum of HIV primary isolates and / or to an immunogen that induces a T cell immune response. The invention also relates to a method of inducing anti-HIV antibodies, and / or to a method of inducing a T cell immune response, using such an immunogen. The invention further relates to nucleic acid sequences encoding the present immunogens.
Owner:UNIVERSITY OF ALABAMA +2
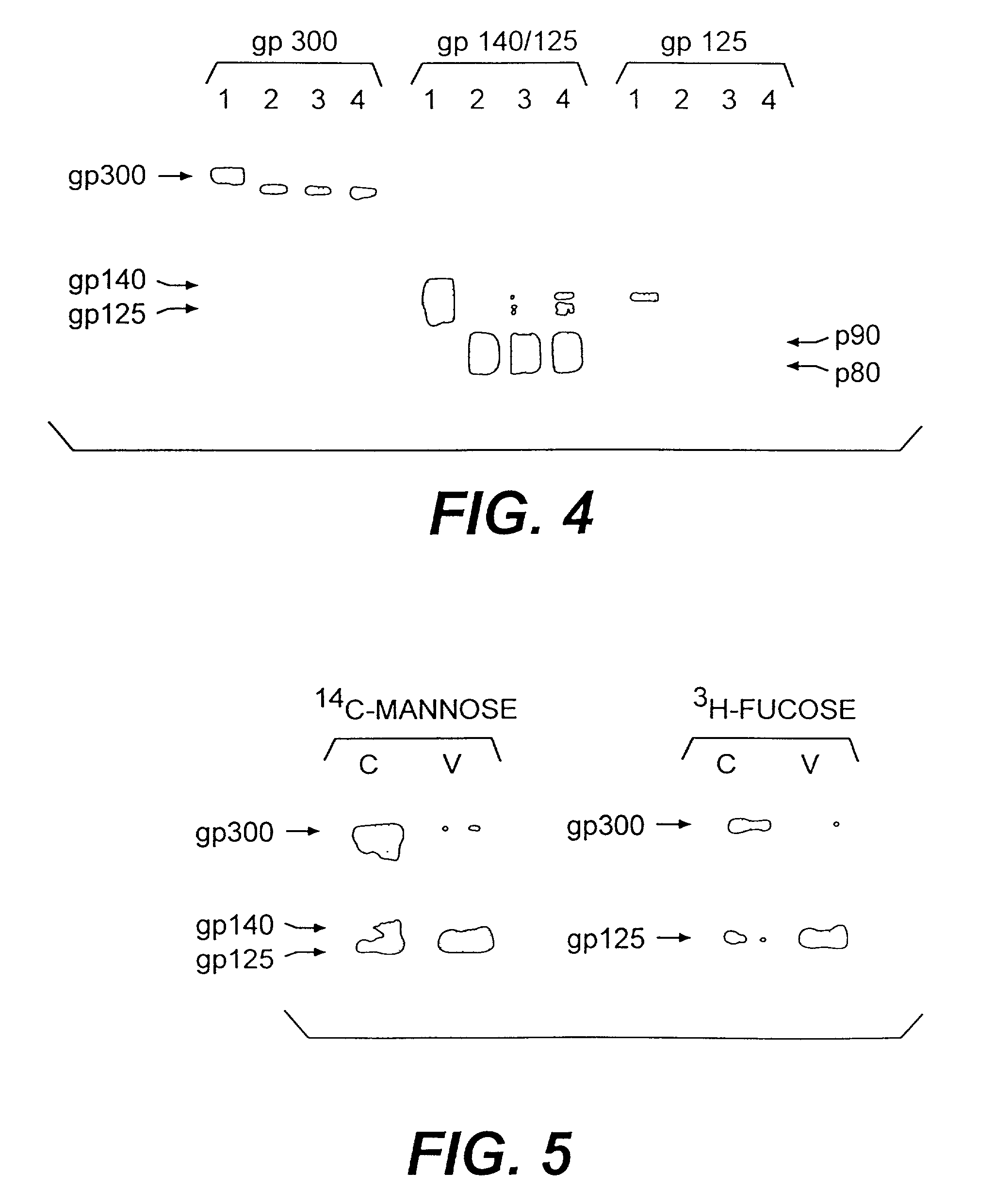
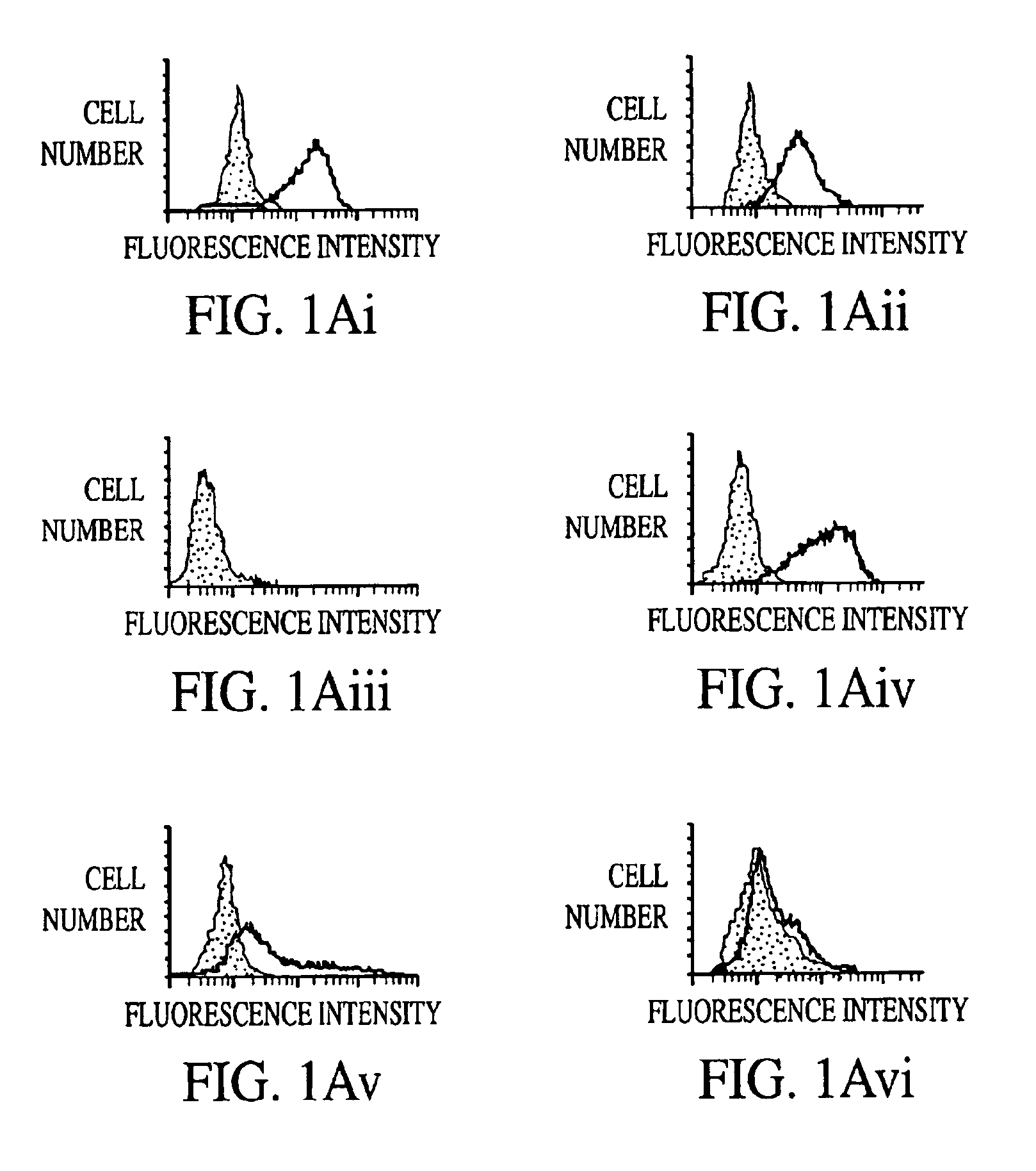
Immunological reagents and diagnostic methods for the detection of human immunodeficiency virus type 2 utilizing multimeric forms of the envelope proteins GP300, P200, and P90/80
InactiveUS6984721B2Reduced viabilityUse of compositionViral antigen ingredientsMicrobiological testing/measurementImmunodeficiency virusHuman immunodeficiency
Owner:INST PASTEUR
Antibodies directed against cellular coreceptors for human immunodeficiency virus and methods of using the same
InactiveUSRE39057E1Avoid infectionPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunodeficiency virusEphA Receptors
The invention relates to an anti-immunodeficiency virus antibody which binds to a cellular protein and diagnostic and therapuetic methods of using the same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Treatment of HIV and aids using probiotic lactobacillus reuteri
InactiveUS20100143305A1Reducing and preventing depletionReducing and preventing HIV replicationBiocidePeptide/protein ingredientsImmunodeficiency virusHuman immunodeficiency
A method for treating or preventing a Human Immunodeficiency Virus (HIV) infection, or treating or preventing Acquired Human Immunodeficiency Syndrome (AIDS), in a subject in need thereof, is disclosed. The method involves colonizing a genetically modified probiotic Lactobacillus reuteri RC-14 in the gastrointestinal tract of a subject, wherein the probiotic is able to secrete two or more fusion inhibitors that decrease or prevent HIV production and CD4+T cell depletion in the gastrointestinal tract.
Owner:LEMKE JAMES ALLEN
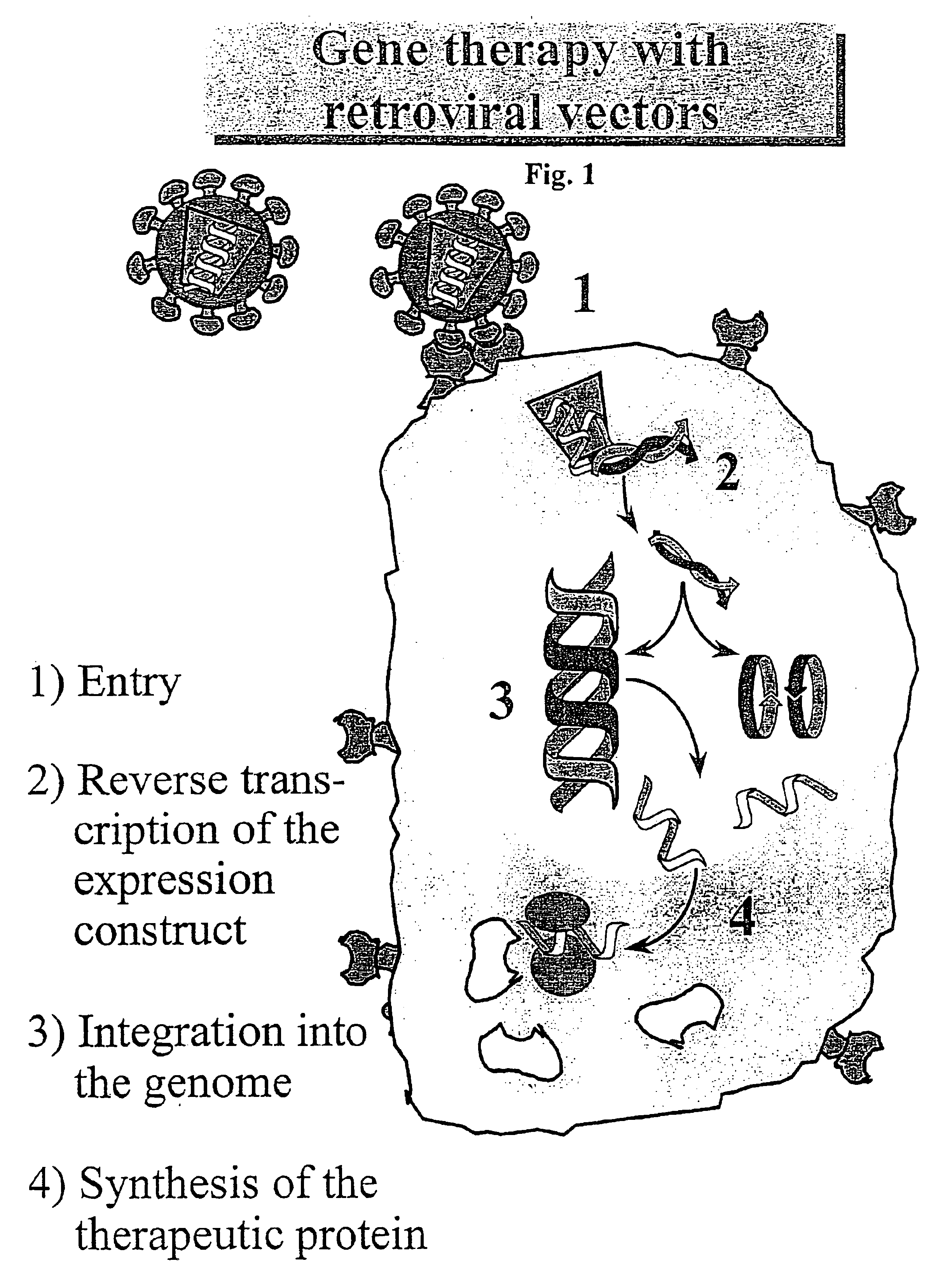
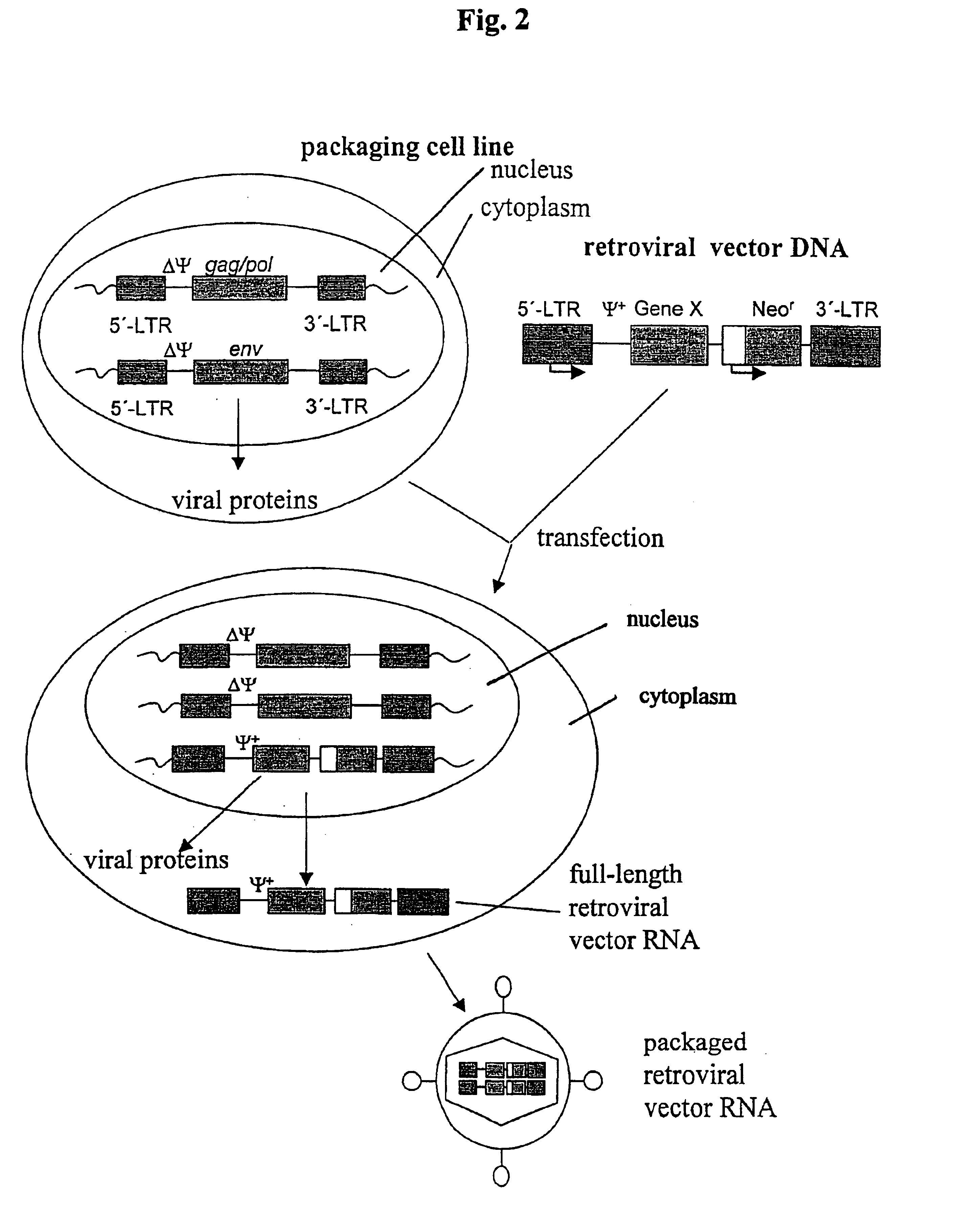
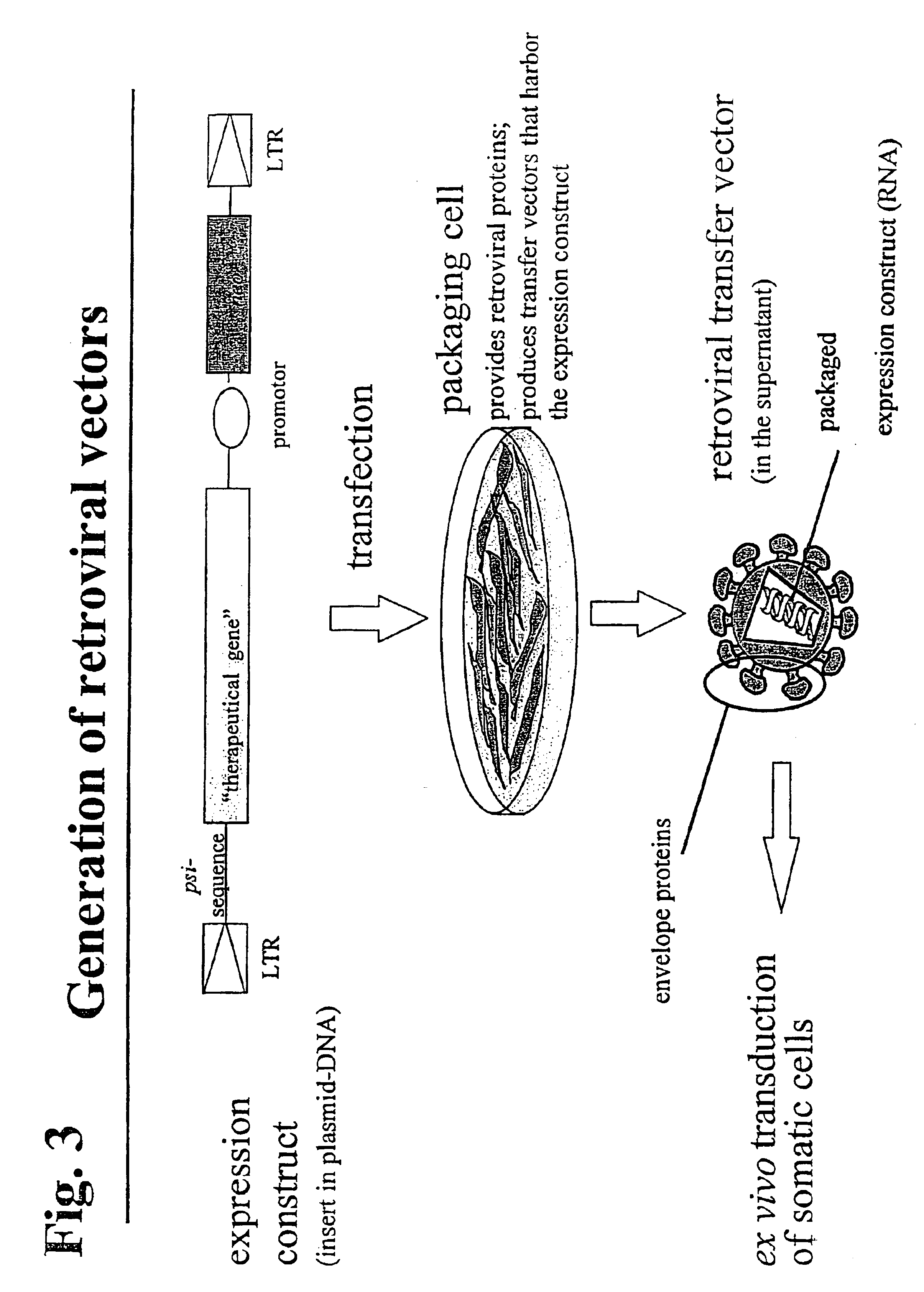
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Human immunodeficiency virus (HIV) nucleic acid detection kit
ActiveCN103074446AReduce the chance of missed detectionIncrease coverageMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionHuman immunodeficiency
The invention provides a human immunodeficiency virus (HIV) nucleic acid detection kit. The kit comprises at least two sets of primer probes which are respectively from different conservative regions. Preferably, the conservative regions comprise the relative conservation regions of HIV gene, i.e. GAG, POL and LTR regions. According to the invention, the two sets of designed and combined primer probe preparation reaction systems from two different regions jointly detect HIV-1RNA to identify the existence of positive reaction, effectively lower the probability of detection miss, can more comprehensively cover all subtypes, including all the subtypes of M, N and O groups of HIV-1, and further improve detection sensitivity and quantification accuracy, wherein the detection sensitivity can reach 50 IU / ml, and the linear range of the quantification is 50-1.0*108 IU / ml. The human immunodeficiency virus (HIV) nucleic acid detection kit provided by the invention further comprises a set of primer probe sequence, which is selected from any one of six sets of sequences including LTR1, LTR2, GAG1, GAG2, POL1 and POL2.
Owner:SANSURE BIOTECH INC
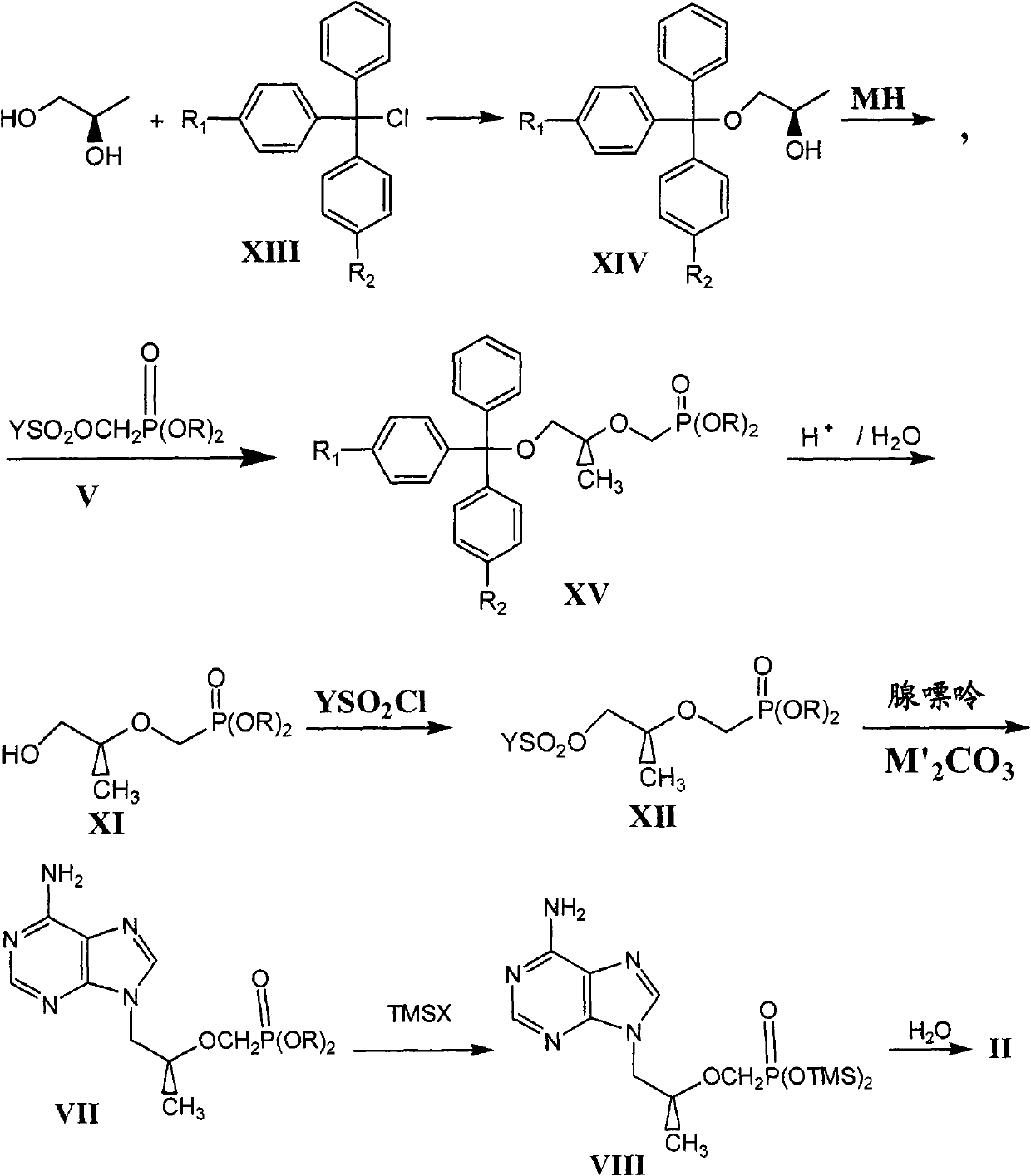
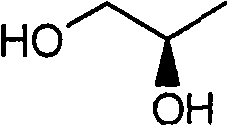
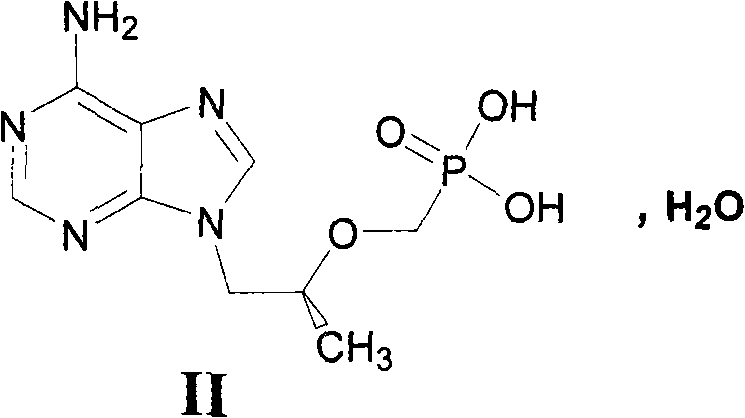
Novel method for preparing tenofovir
InactiveCN101906119AOrganic active ingredientsGroup 5/15 element organic compoundsTrimethylsilyl bromideHuman immunodeficiency
The invention relates to a novel method for preparing tenofovir, in particular to a method for preparing a tenofovir compound, in particular the monohydrate of the tenofovir for resisting hepatitis B viruses and human immunodeficiency viruses. The method comprises the following steps of: using (substituted) triphenylchloromethane XIII to protect hydroxy in the end position of (R)-1,2-propanediol,condensing the obtained product with dialkyl sulfonyloxy methyl phosphonate V to obtain XV, hydrolyzing the XV in the aqueous solution of an organic acid to remove the protective group of the hydroxyl at the end position of XV to obtain an intermediate XI, condensing sulfonate XII of the intermediate XI with adenine to obtain an intermediate VII, transforming the intermediate VII into the corresponding trimethylsilyl phosphonate VIII by trimethylsilyl bromide (TMSBr), and hydrolyzing the VIII to obtain the target product. The method has the advantages of short process route, convenient operation, low cost and easy access of raw materials and favorability for large scale production.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
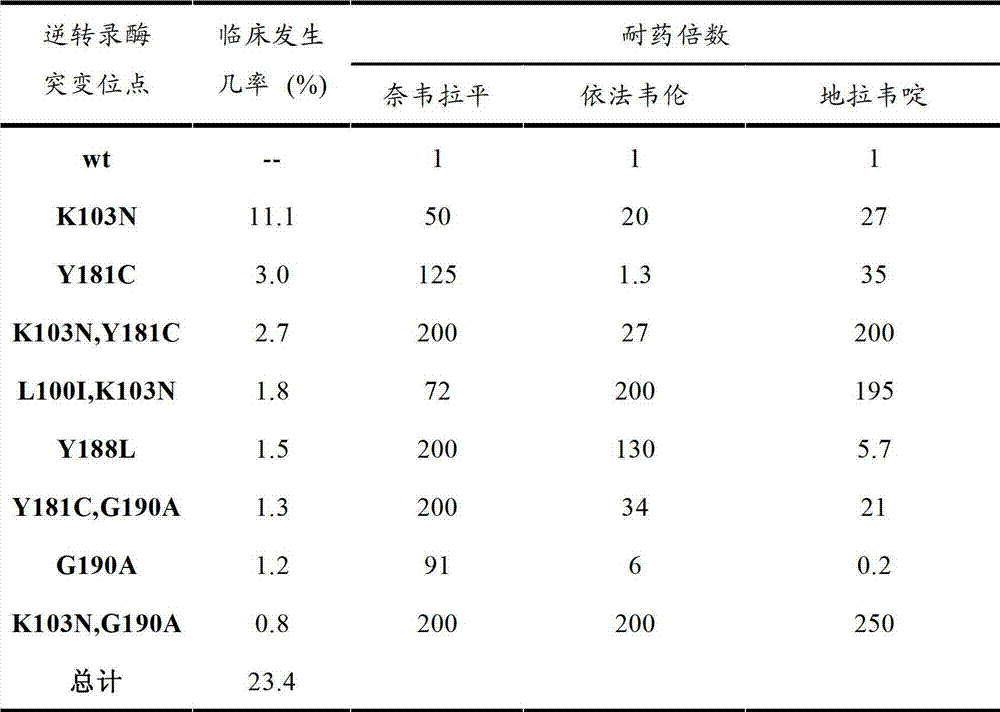
HIV-1 Protease Inhibitors
Described are novel protease inhibitors and methods for using said protease inhibitors in the treatment of human immunodeficiency virus (HIV) infection.
Owner:MASSACHUSETTS INST OF TECH +2
Methods for treating viral infections using polyamine analogs
InactiveUS7879914B2Reducing viral load of an infected subjectReduce loadBiocideAntiviralsPresent methodImmunodeficiency virus
Methods for treating viral infections using polyamine analogs, including mitoguazone (MGBG), are provided. In these methods, polyamine analogs destroy macrophages that act as viral reservoirs, facilitating the destruction of the viruses that dwell within the macrophages. Examples of viral infections that may be treated with the present methods include, but are not limited to, infections from human immunodeficiency viruses. These methods differ from previous methods of treatment using polyamine analogs, wherein the polyamine analogs were administered only as anti-tumor agents.
Owner:PATHOLOGICA
Human immunodeficiency virus (HIV) antibody detection kit and preparation method thereof
InactiveCN104090101AHigh sensitivityStrong specificityChemiluminescene/bioluminescencePositive controlTrue positive rate
The invention belongs to the technical field of immunologic diagnosis and particularly relates to a human immunodeficiency virus (HIV) antibody detection kit using a microparticle chemiluminiscence method and a preparation method of the kit. The kit is composed of magnetic microparticles for detecting an HIV antibody, a tracing conjugate for detecting the HIV antibody, a negative control, a I type positive control, a II type positive control and an analyzing buffering solution. The invention further discloses the preparation method of the detection kit, which adopts a particle chemiluminiscence immunoassay technology; compared with ELISA (Enzyme-Linked Immunosorbent Assay), the technology has higher sensitivity and specificity and is suitable for the clinical auxiliary diagnosis of HIV.
Owner:威海威高生物科技有限公司
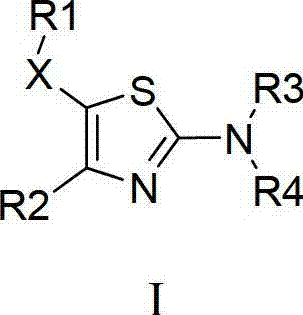
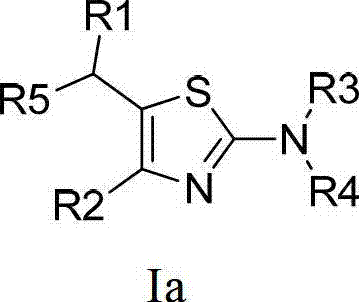
Thiazole compound capable of restraining human immunodeficiency virus (HIV) replication and effective against drug-resistant HIV virus strains and preparation method and purpose thereof
The invention provides a 2,4,5-tri-substituted thiazole compound which is in a general formula I and can serve as a human immunodeficiency virus (HIV) replication inhibitor, and all possible isomers or prodrug or medicinal salt or solvate or aquo-complex of the 2,4,5-tri-substituted thiazole compound, and further provides a preparation method for the compound in the general formula I as well as a medical composition of the compound in the general formula I, and the purpose of the compound in the medicine preparation process. The medicine is used for treating and / or preventing diseases caused by HIV infection.
Owner:CAPITAL NORMAL UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com