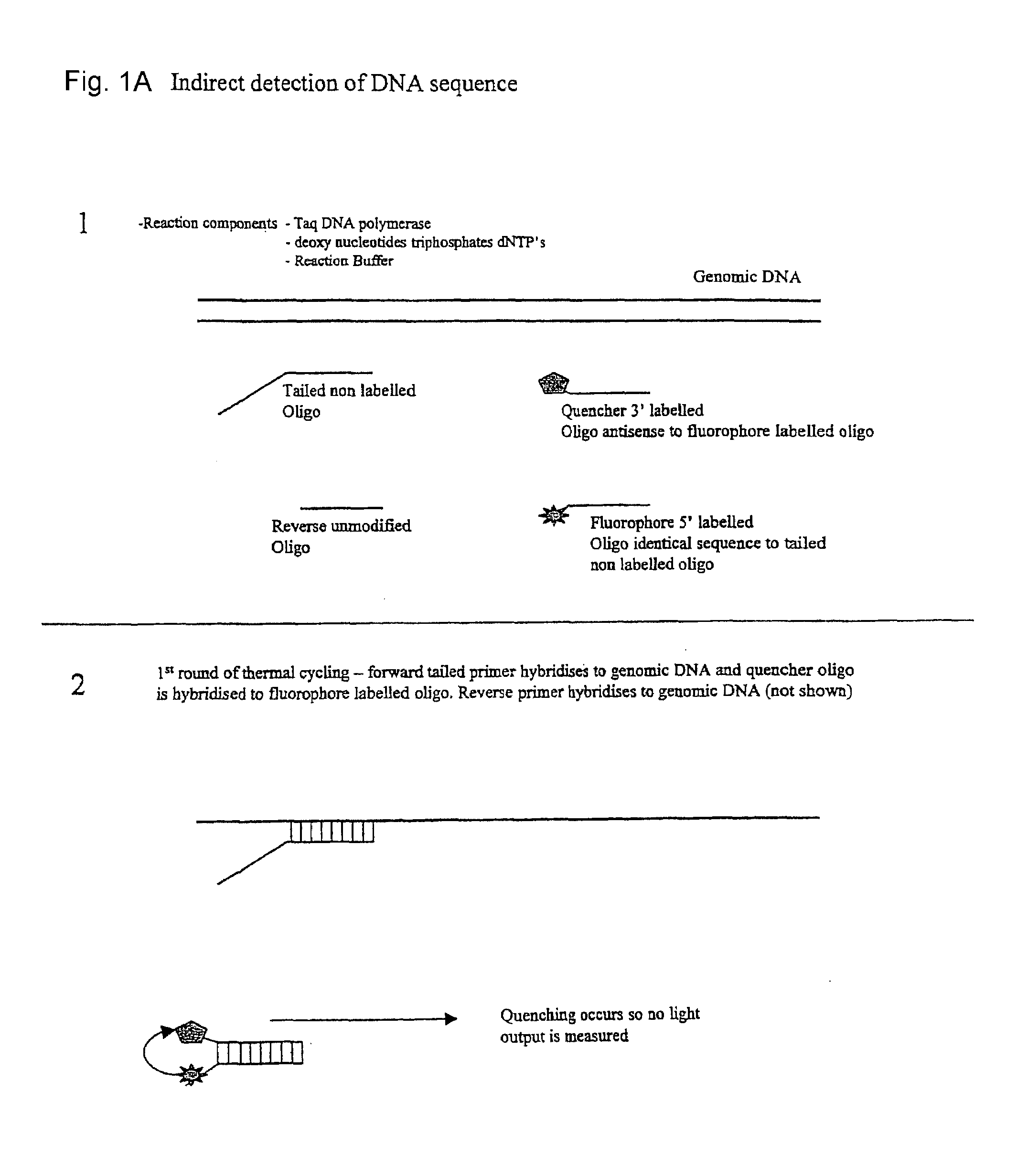
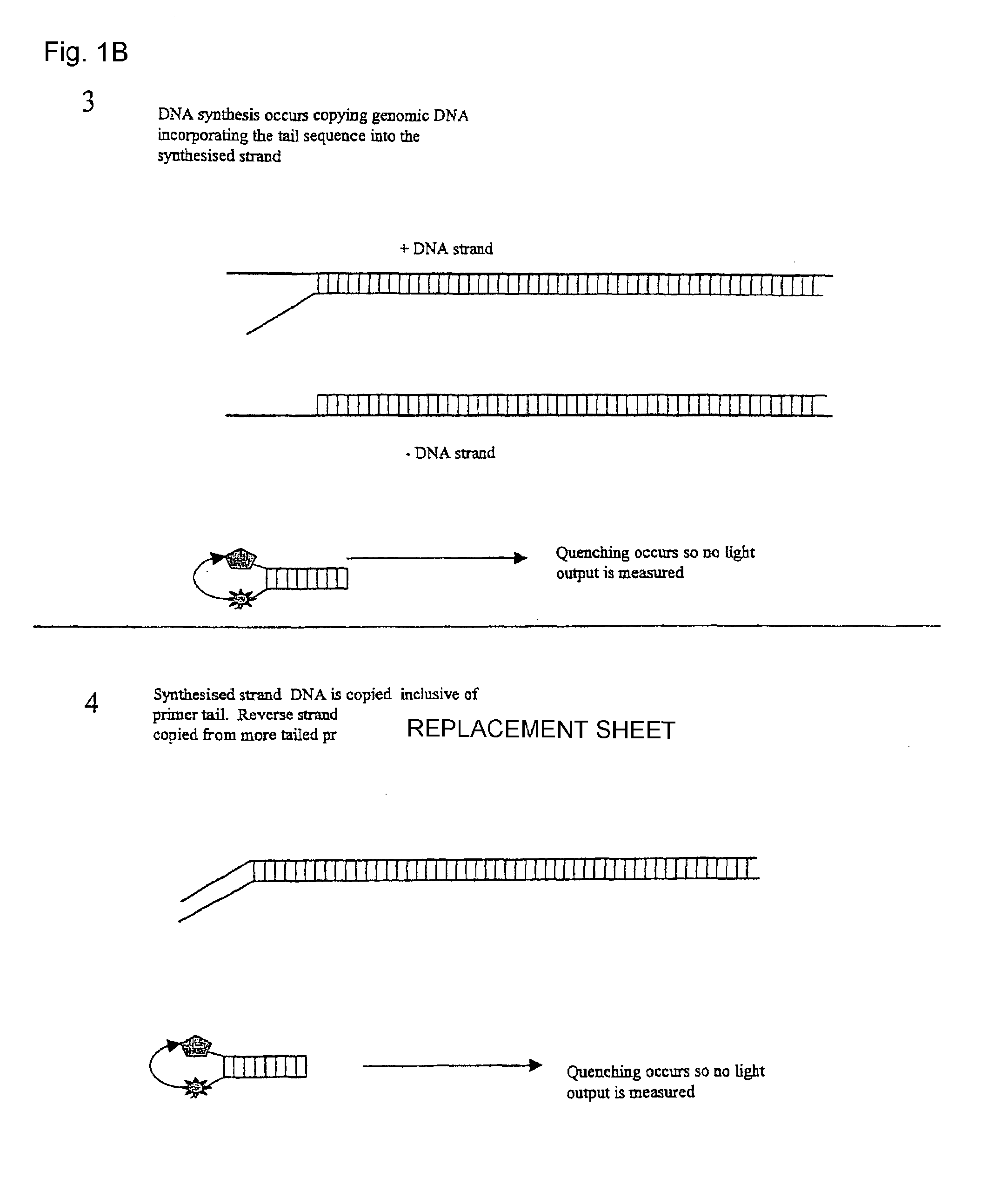
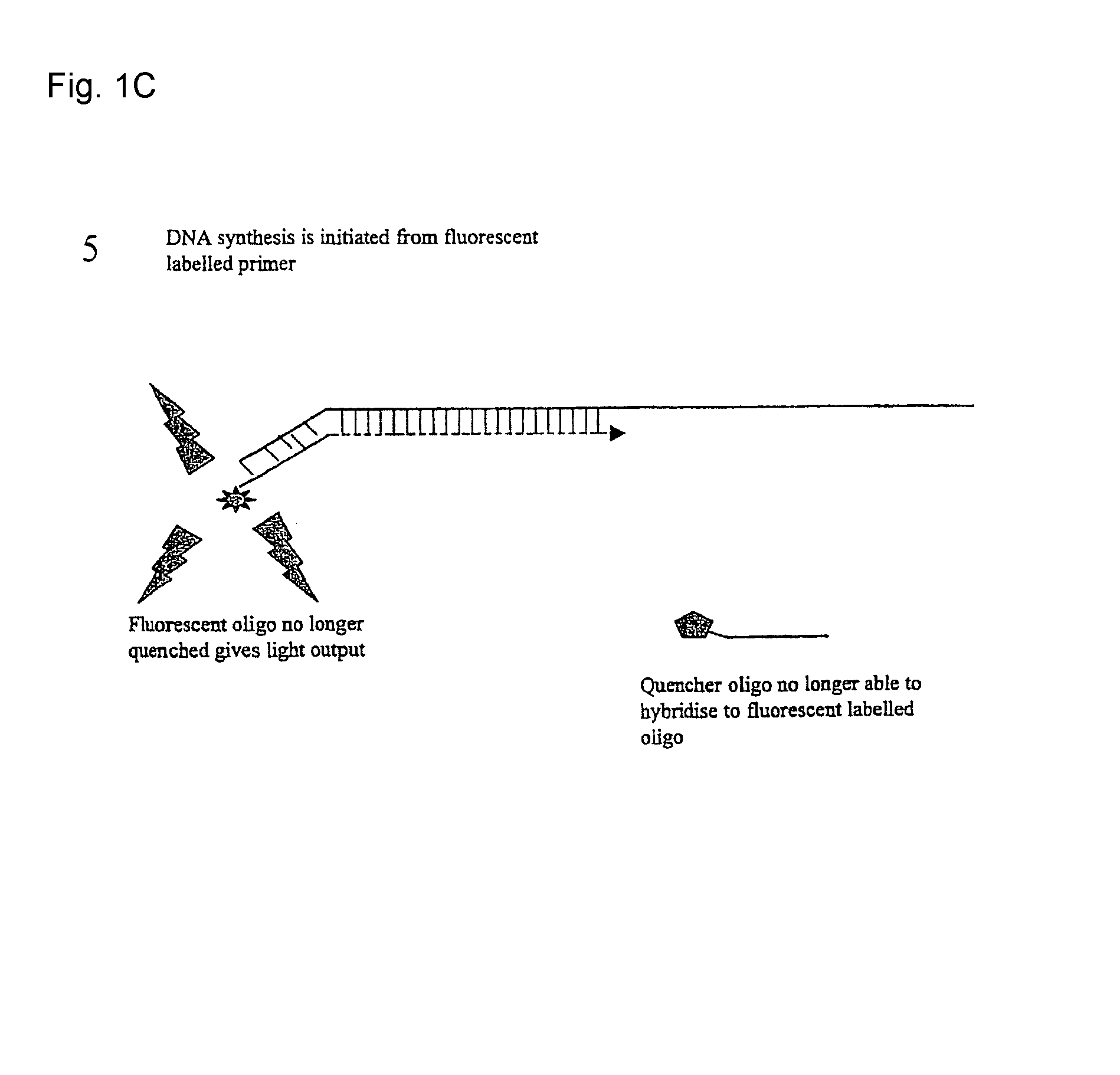
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
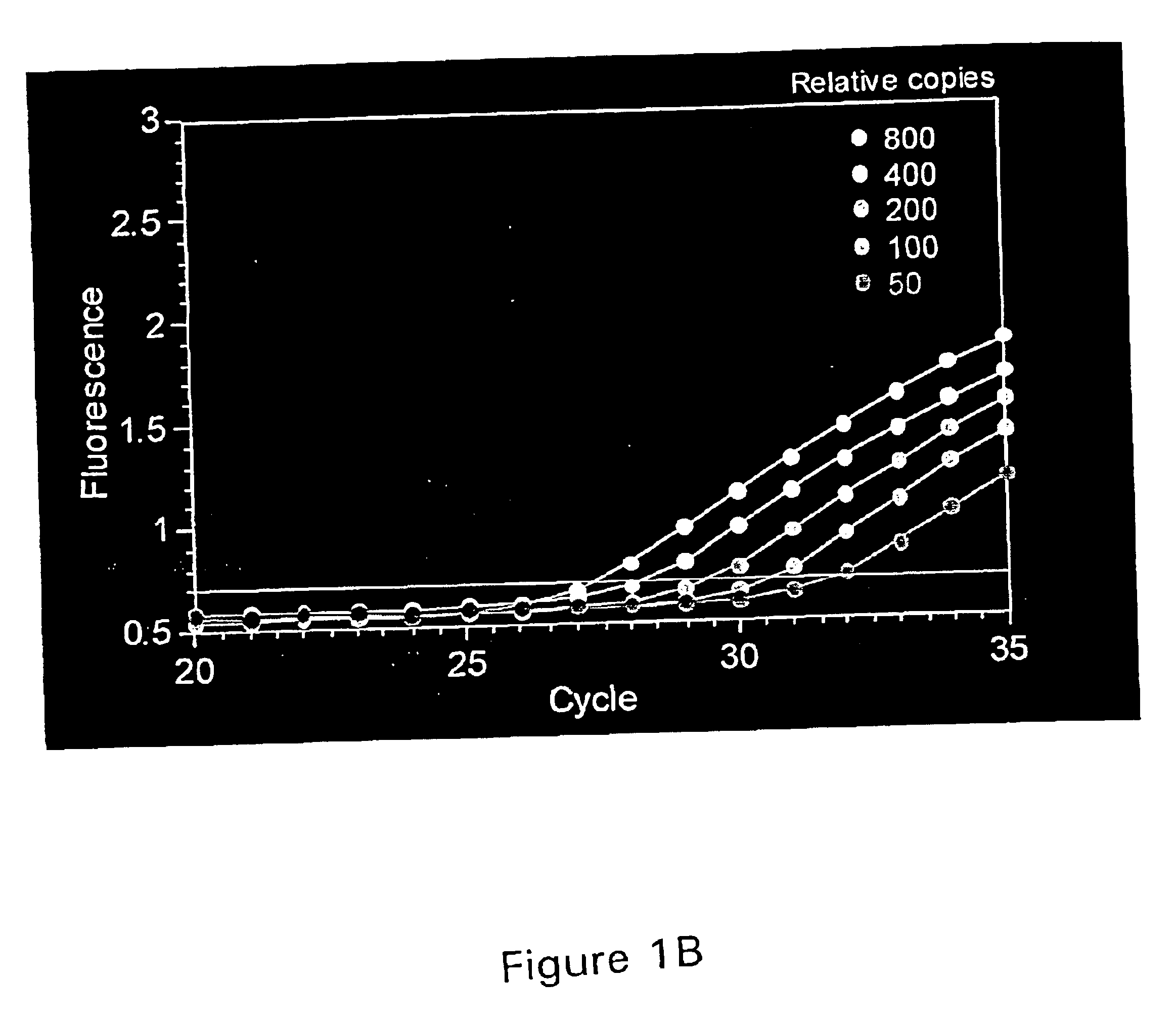
221 results about "Pcr assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
PCR-ELISAs (also known as PCR ELOSA) are a capture assay for nucleic acids that mimic enzyme linked immunosorbant assays. In this assay, PCR products hybridized to an immobilized capture probe.
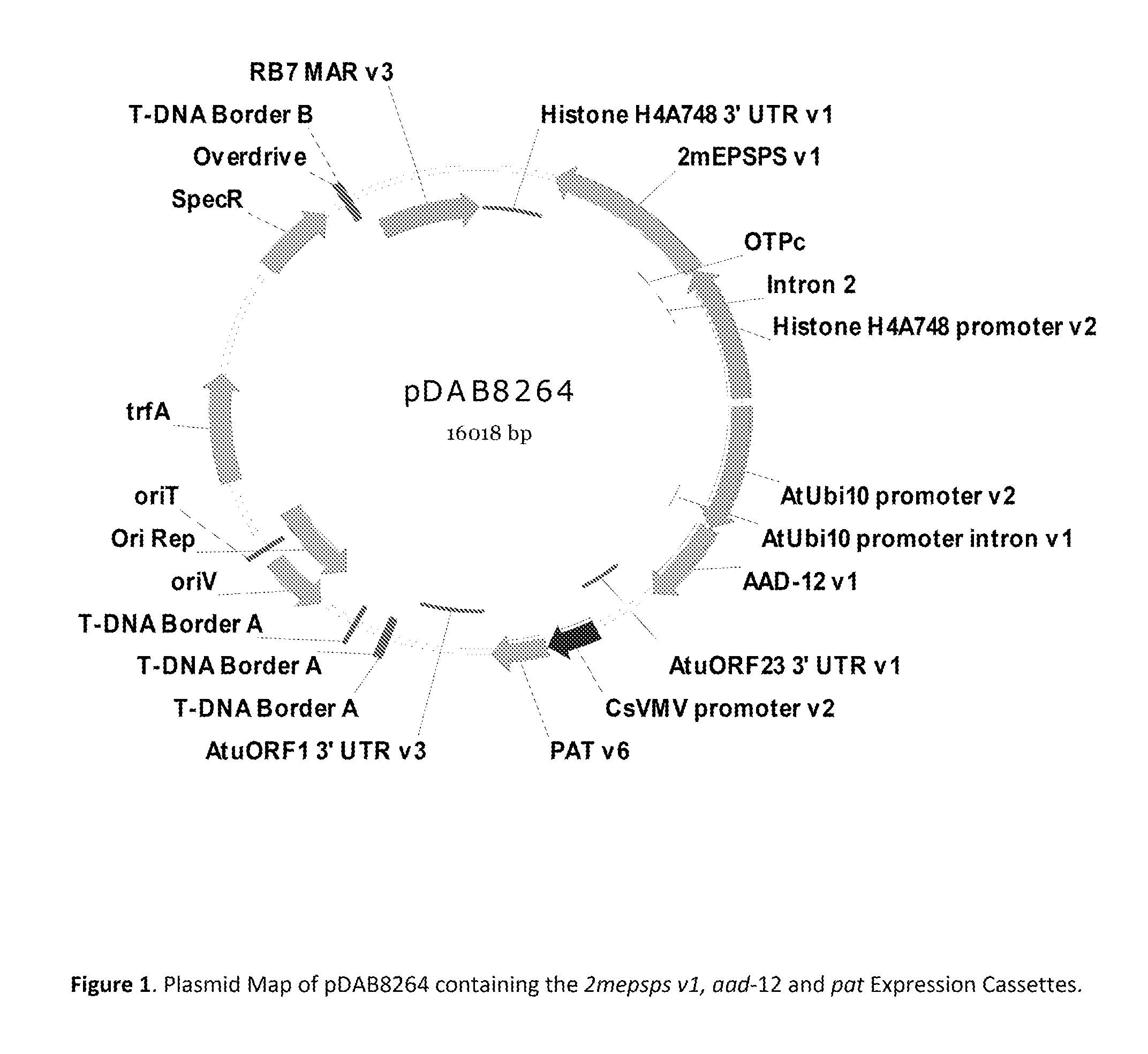
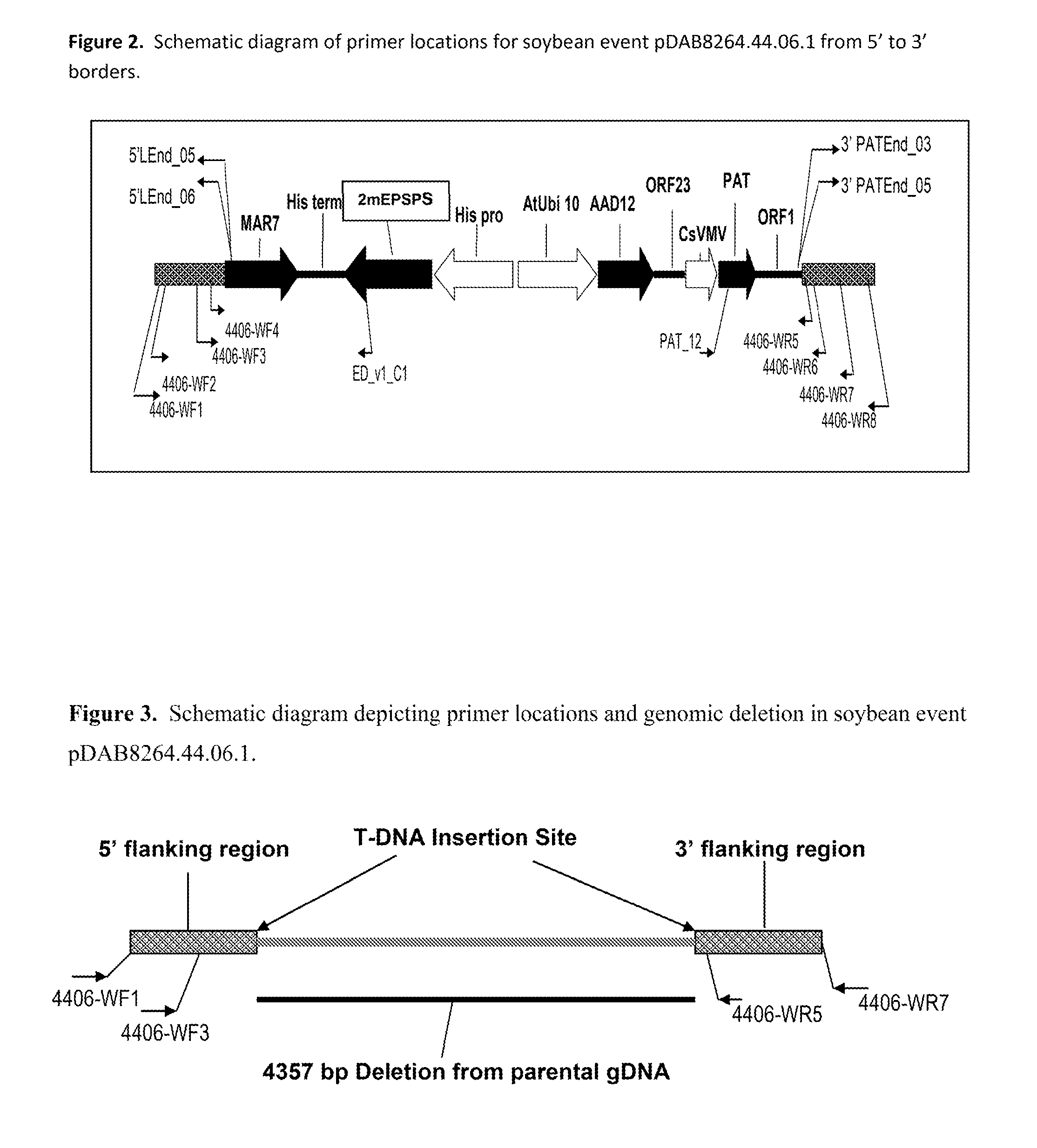
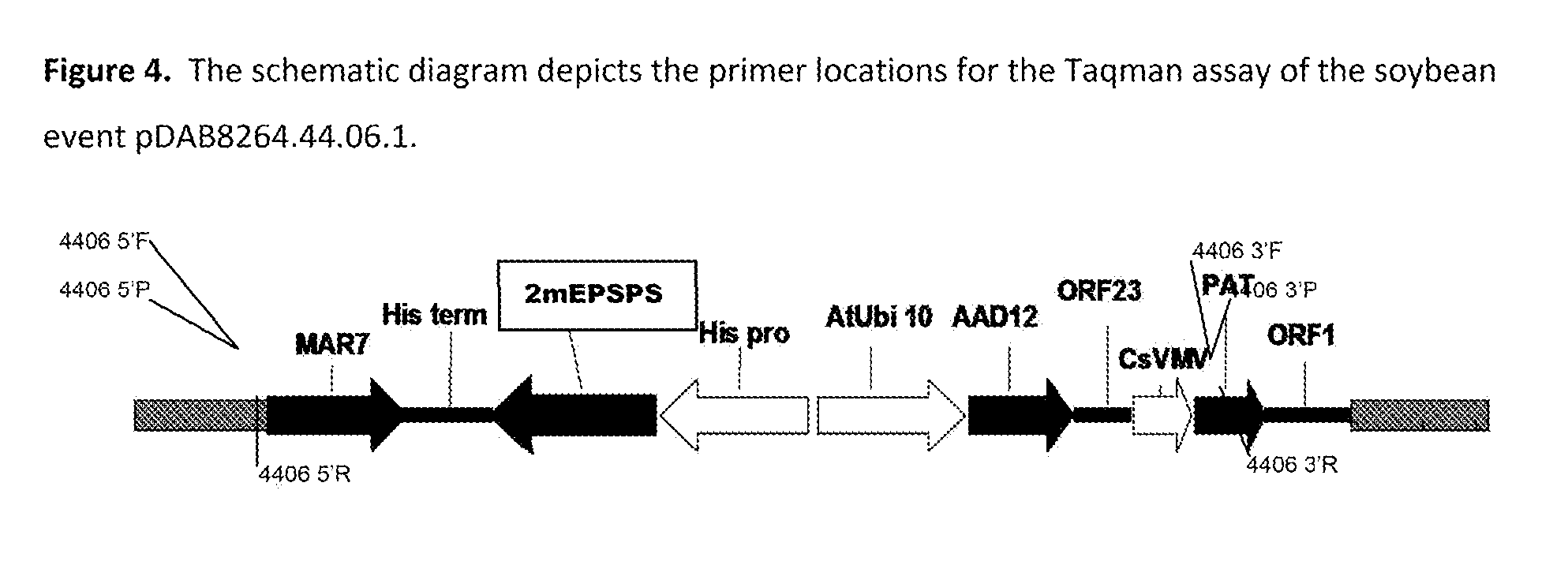
Stacked herbicide tolerance event 8264.44.06.1, related transgenic soybean lines, and detection thereof
ActiveUS9540655B2Preserve usefulnessIncrease flexibilityBiocideMicrobiological testing/measurementPcr assayMultiple traits
Owner:M S TECH +1
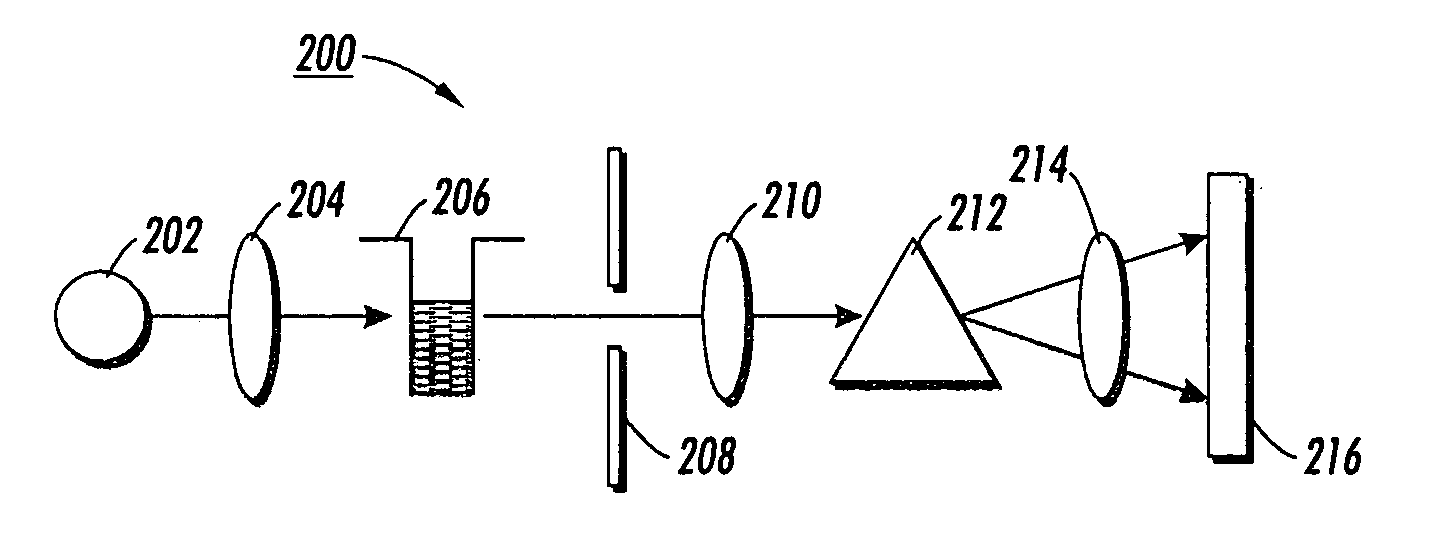
LED or laser enabled real-time PCR system and spectrophotometer
InactiveUS7122799B2Easy to useReduce entirely avoid costRadiation pyrometryHeating or cooling apparatusPhotodetectorUltraviolet
A system for conducting a polymerase chain reaction (PCR) assay upon a collection of samples is disclosed. The PCR assay is performed by absorption detection. The system includes a multi-well plate which is adapted to retain a collection of sample wells. This system includes a thermal cycler for the multi-well plate. The system additionally includes a collection of photodetectors, and a corresponding number of light sources. The light sources are positioned such that light emitted from each of the respective light sources passes through a corresponding well retained in the multi-well plate and to a corresponding photodetector. The system also includes a processor or other means for analyzing the output signals from the photodetectors. In certain versions of the system, ultra-violet light is used.
Owner:PALO ALTO RES CENT INC
PCR assay
InactiveUS6927024B2Equal and improved sensitivityImprove dynamic rangeSugar derivativesMicrobiological testing/measurementPcr assayBiology
An oligonucleotide assay is useful for the detection of target compounds in samples which may contain the target compound.
Owner:GENENTECH INC
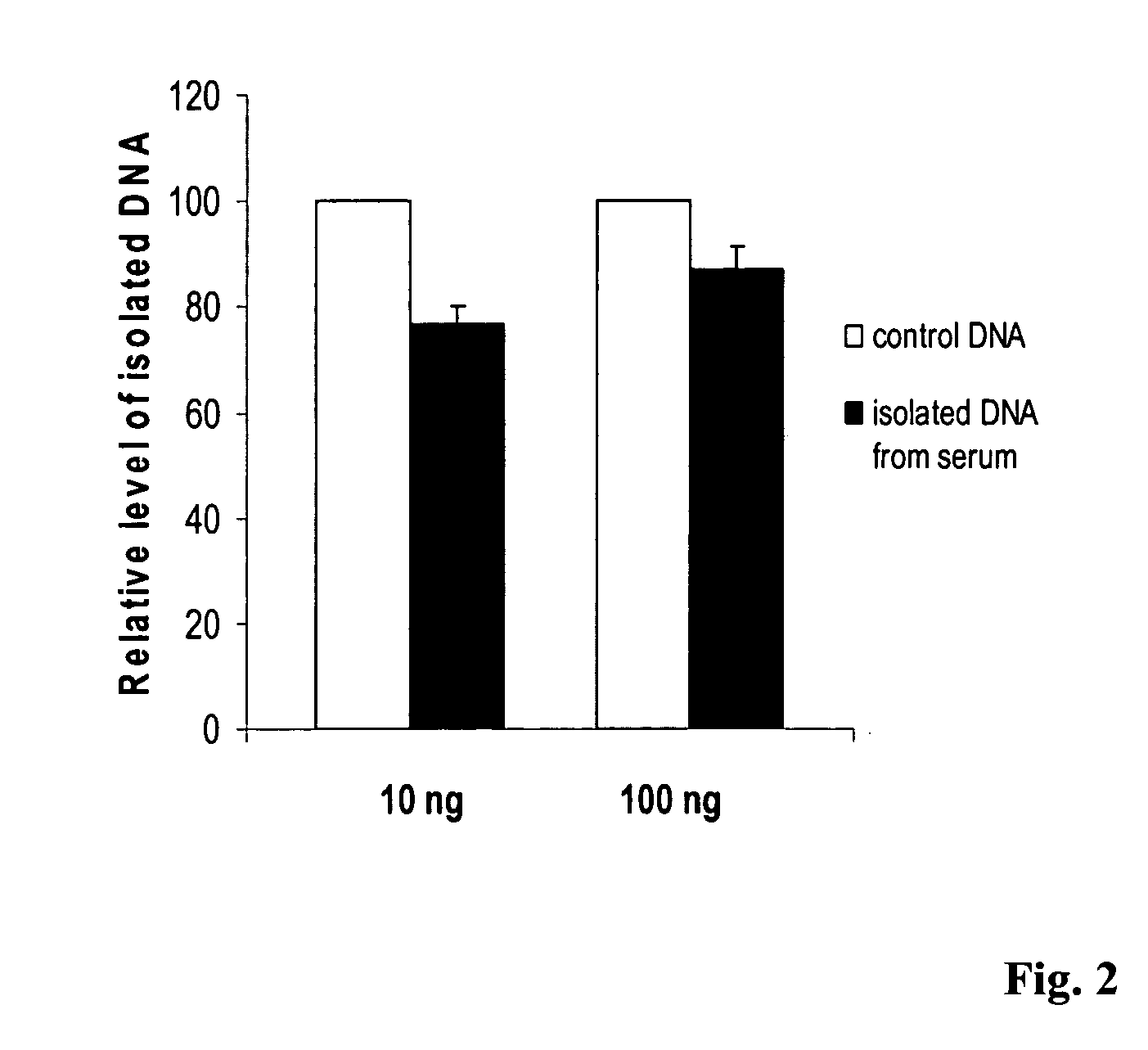
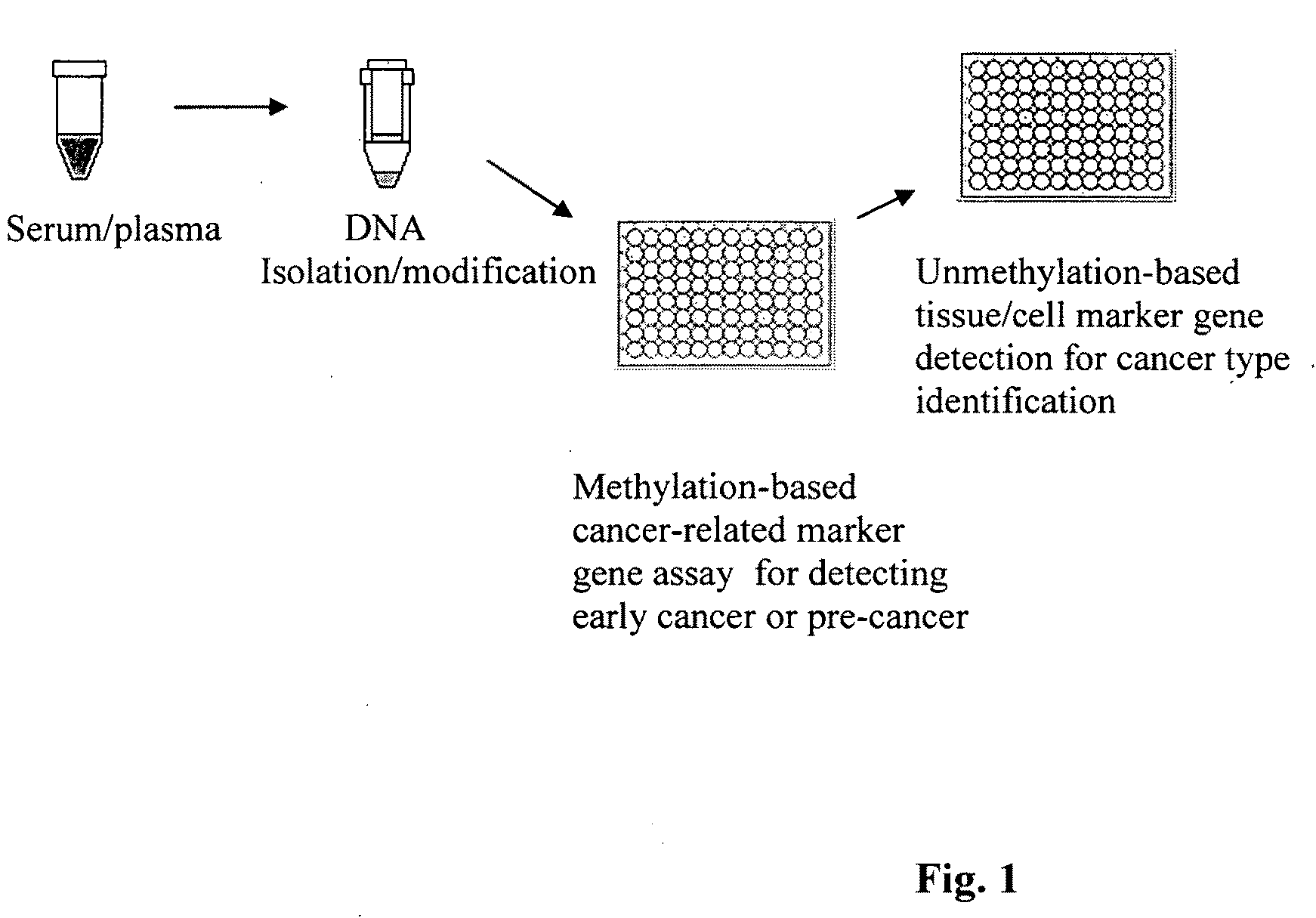
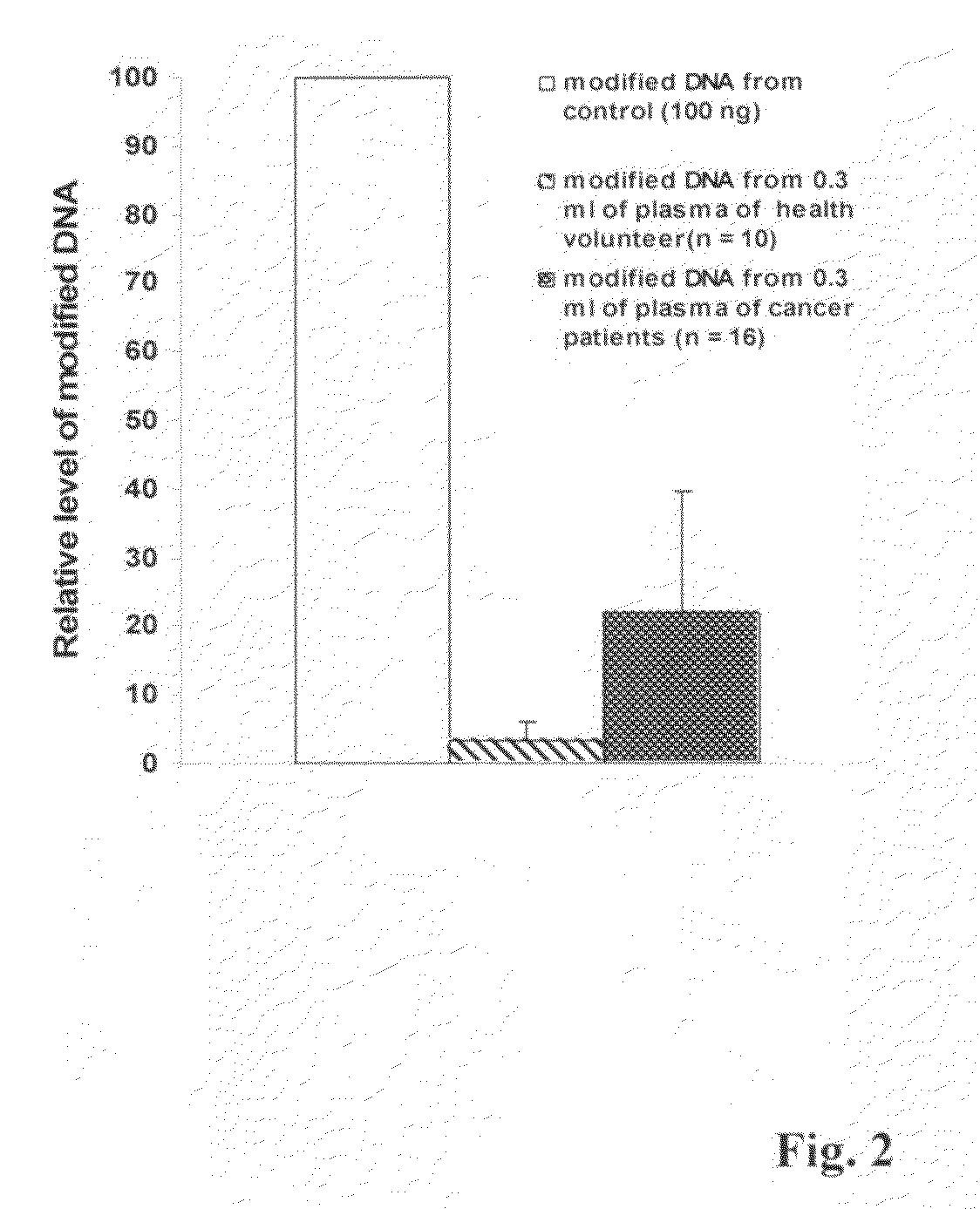
Method for isolating and modifying DNA from blood and body fluids
InactiveUS20070042384A1Highly efficient and fast isolation and modificationEasily and quickly isolatedSugar derivativesMicrobiological testing/measurementModified dnaPcr assay
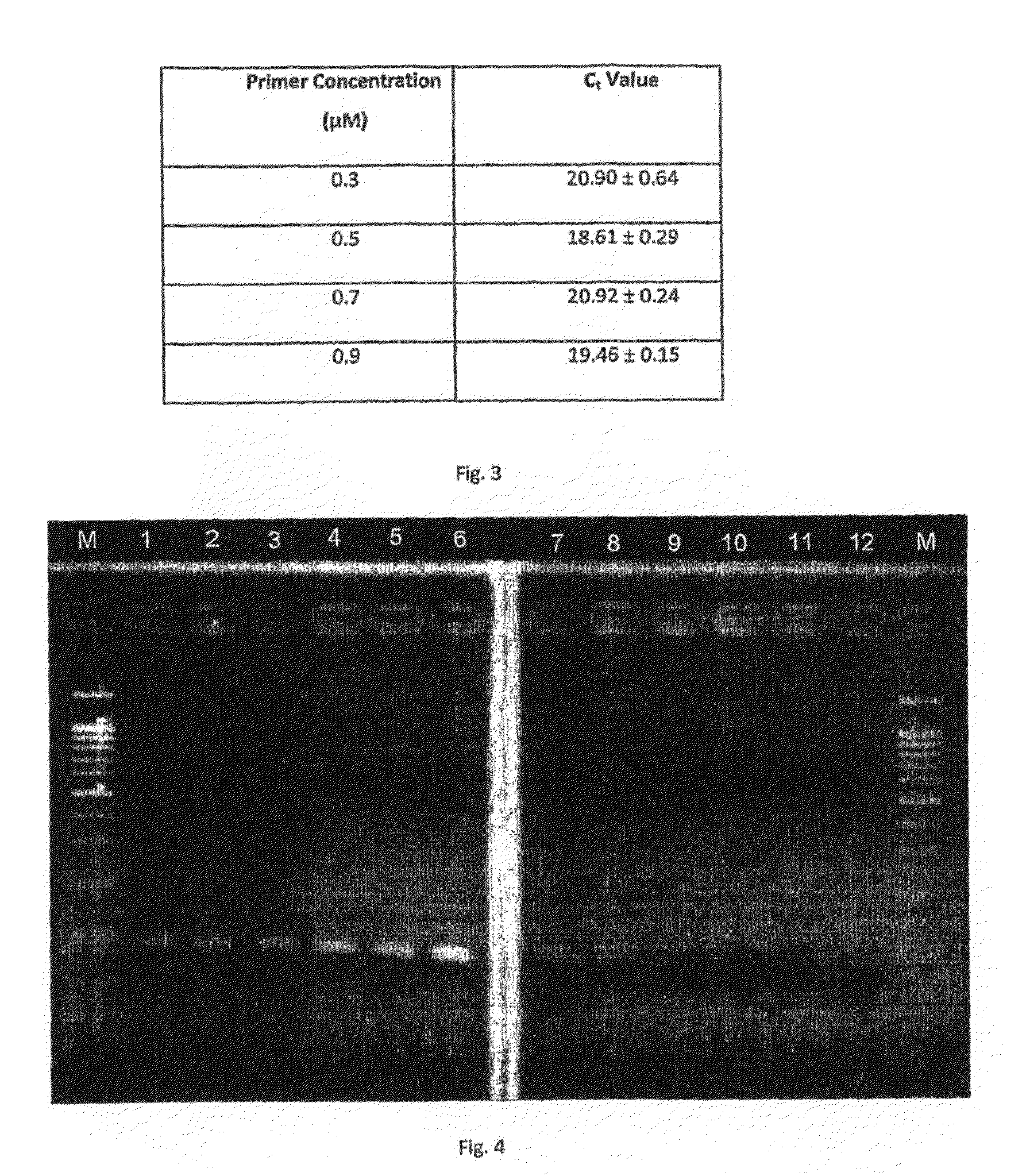
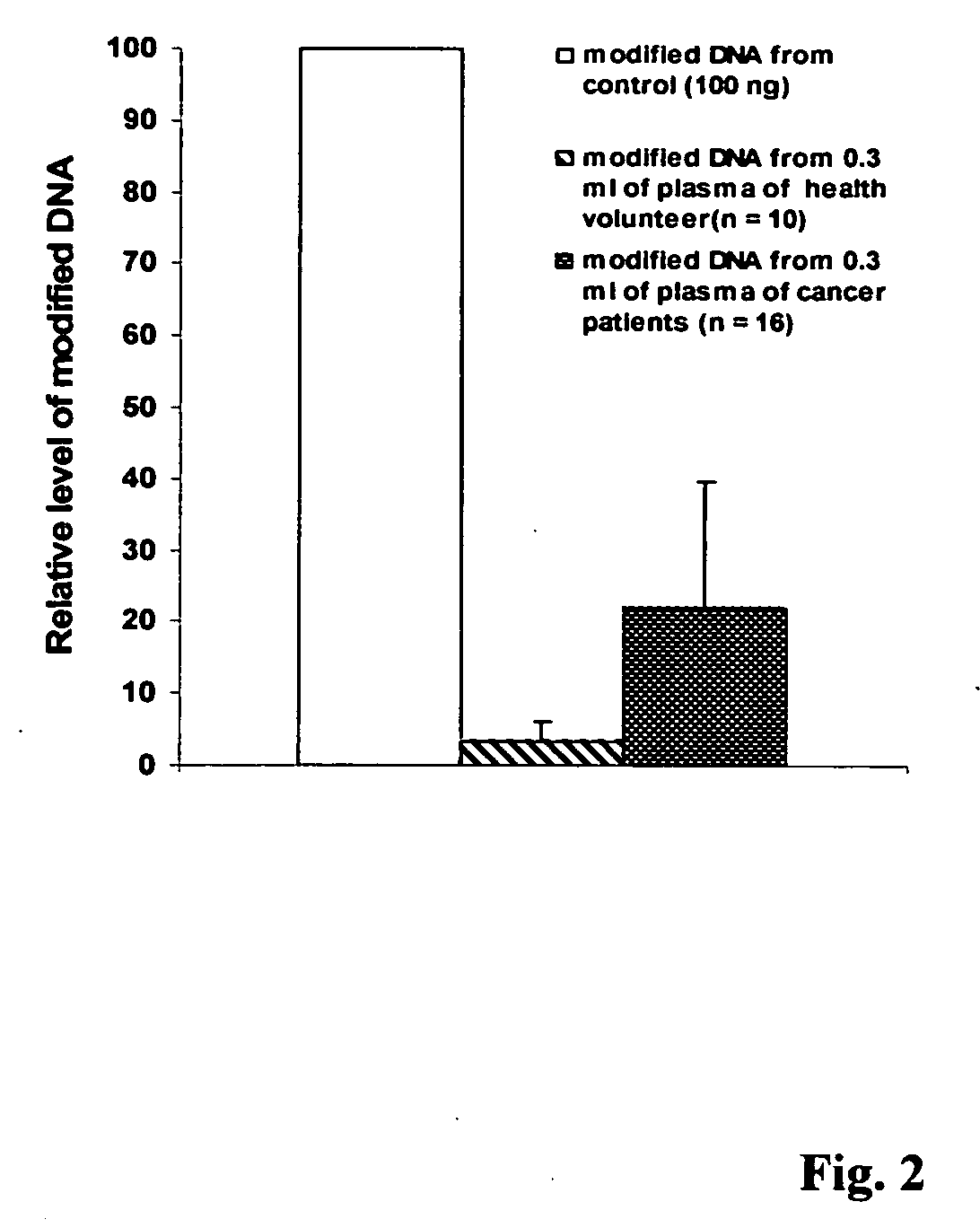
This invention is related a method for rapidly isolating and modifying DNA from plasma / serum and body fluids. This invention provides a procedure and composition to obtain a high yield of modified DNA for methylation-specific PCR assay by coupling DNA isolation and modification courses.
Owner:LI WEIWEI +1
Led or laser enabled real-time PCR system and spectrophotometer
InactiveUS20050133724A1Easy to useReduce entirely avoid costRadiation pyrometryHeating or cooling apparatusPcr assayPhotovoltaic detectors
A system for conducting a polymerase chain reaction (PCR) assay upon a collection of samples is disclosed. The PCR assay is performed by absorption detection. The system includes a multi-well plate which is adapted to retain a collection of sample wells. This system includes a thermal cycler for the multi-well plate. The system additionally includes a collection of photodetectors, and a corresponding number of light sources. The light sources are positioned such that light emitted from each of the respective light sources passes through a corresponding well retained in the multi-well plate and to a corresponding photodetector. The system also includes a processor or other means for analyzing the output signals from the photodetectors. In certain versions of the system, ultra-violet light is used.
Owner:PALO ALTO RES CENT INC
qRT-PCR assay system for gene expression profiling
InactiveUS20050095634A1High precisionSensitive highMicrobiological testing/measurementFermentationMulti analytePcr assay
The invention concerns an integrated, qRT-PCR-based system for analyzing and reporting RNA expression profiles of biological samples. In particular, the invention concerns a fully optimized and integrated multiplex, multi-analyte method for expression profiling of RNA in biological samples, including fixed, paraffin-embedded tissue samples. The gene expression profiles obtained can be used for the clinical diagnosis, classification and prognosis of various pathological conditions, including cancer.
Owner:GENOMIC HEALTH INC
Evaluating genetic disorders
ActiveUS7702468B2Digital data processing detailsMicrobiological testing/measurementPcr assayGenetics
Owner:POPULATION BIO INC
Use of whole blood in PCR reactions
ActiveUS20090170060A1Avoid mixingMicrobiological testing/measurementTransferasesPcr assayPolymerase L
A method of obtaining DNA amplification of a nucleic acid target from a volume of whole blood comprising performing DNA amplification in a PCR assay mixture with a blood-resistant polymerase.
Owner:DNA POLYMERASE TECH
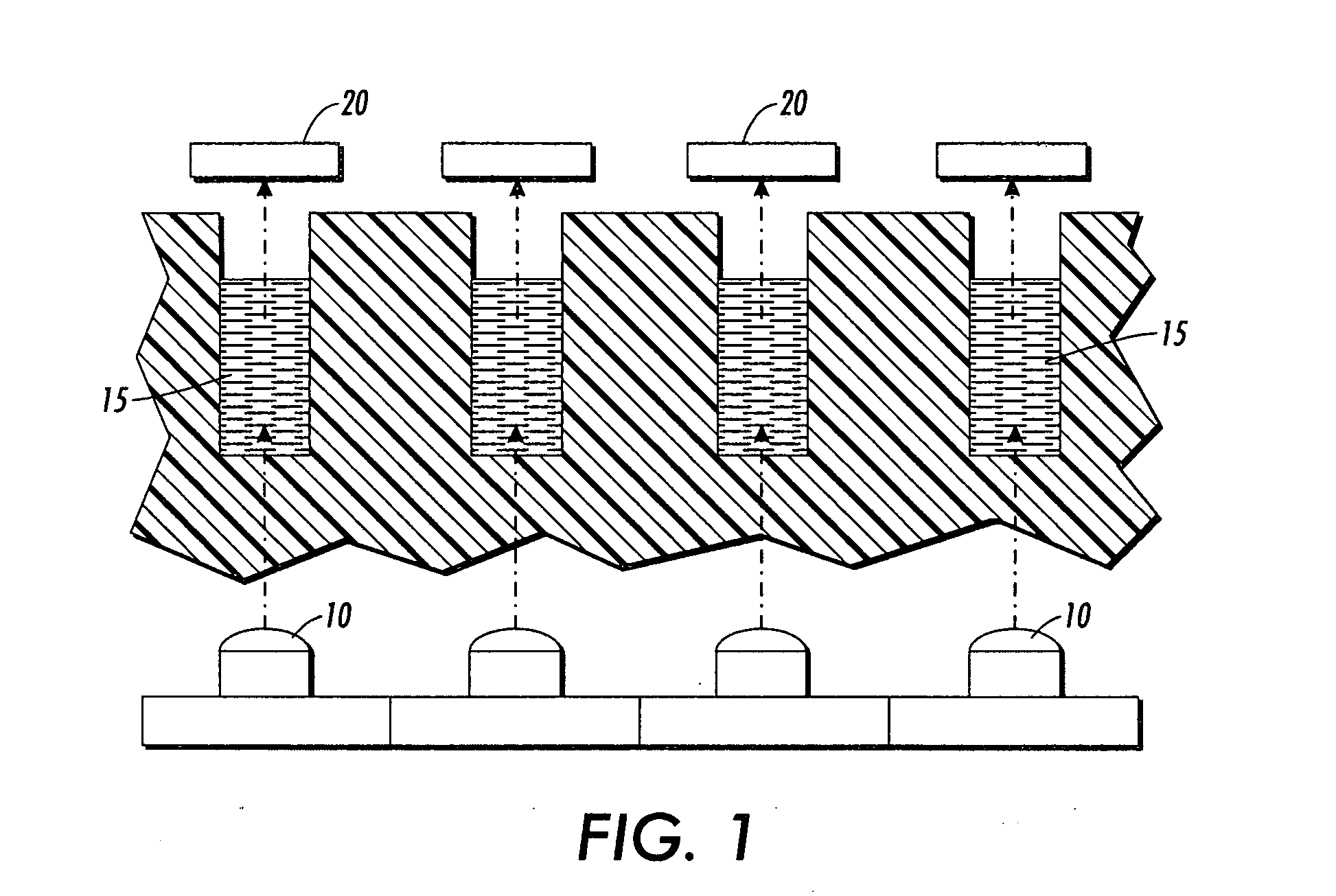
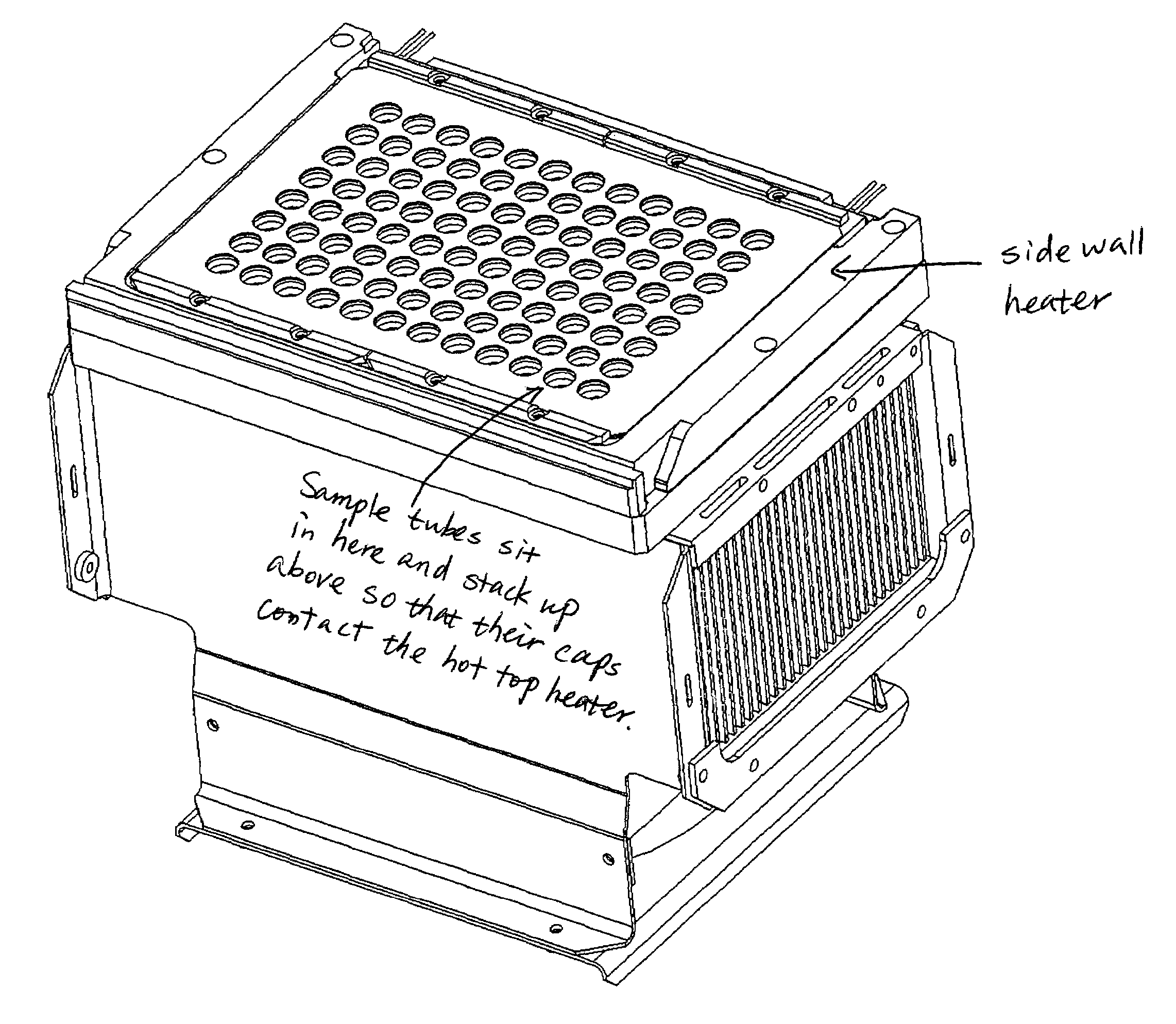
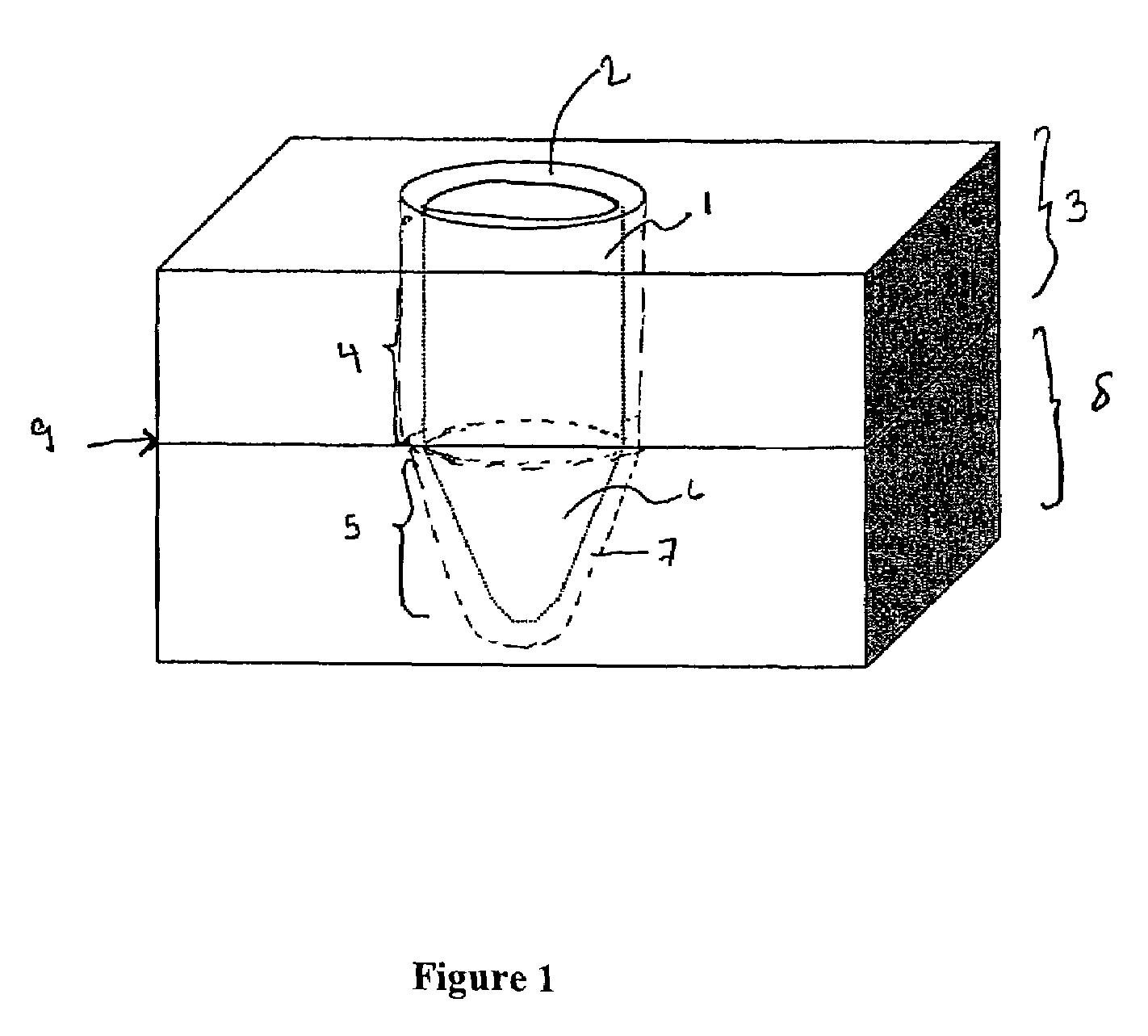
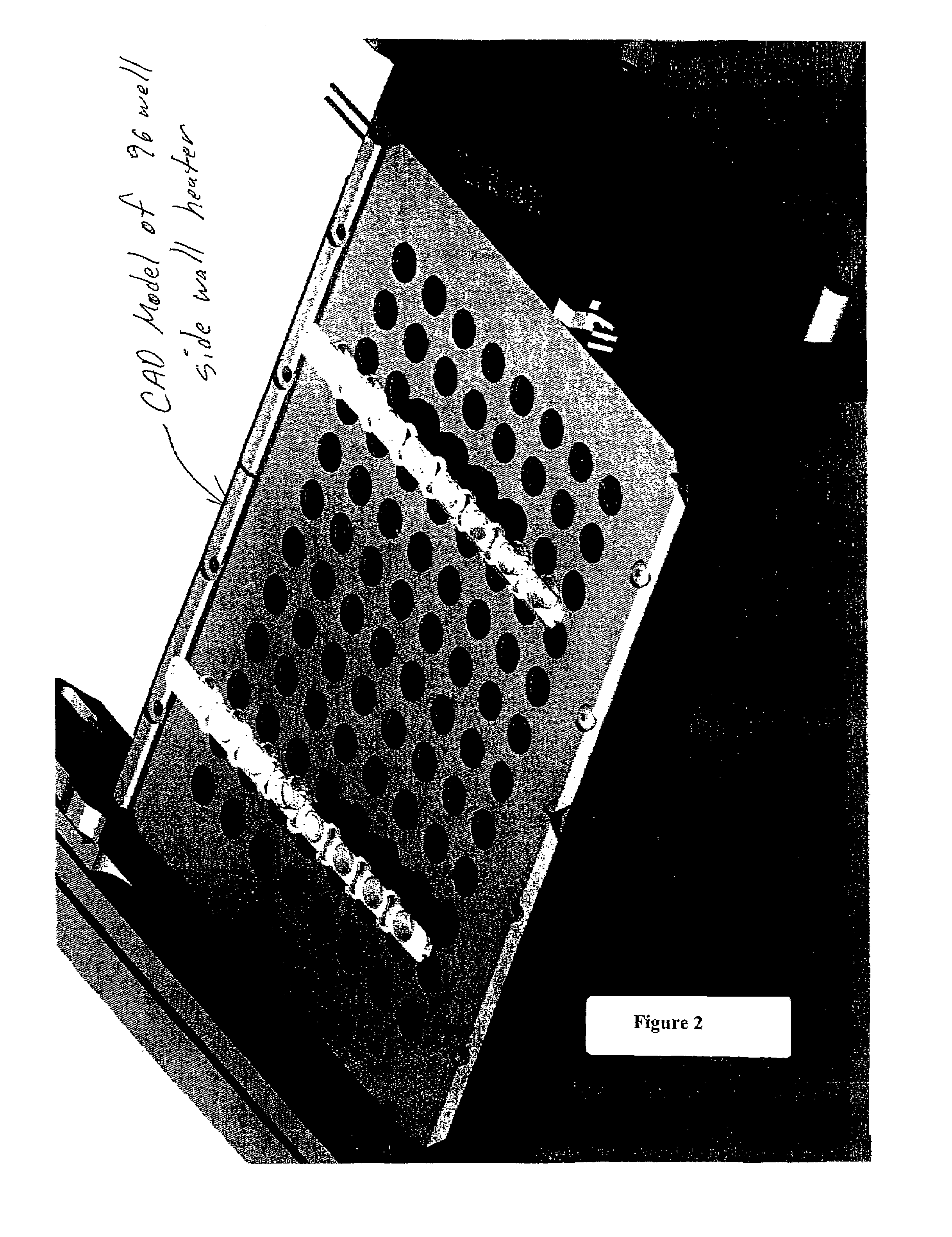
Side-wall heater for thermocycler device
InactiveUS7459302B2Avoid condensationImprove uniformityBioreactor/fermenter combinationsBiological substance pretreatmentsPcr assaySignal strength
The invention relates to an apparatus and methods for preventing condensation on the interior surfaces of sample tubes which are being exposed to temperature cycles, such as during a PCR amplification reaction. In particular, the invention relates to apparatus comprising a sample block comprising a plurality of sample wells for receiving sample tubes and heating elements disposed in the sample wells for heating at least a portion of the sides of sample tubes (e.g., at least the portion which forms the head space after a tube is filled with a PCR reaction mixture). In a preferred aspect, the sample block is part of a thermocycling device for performing PCR and the side-wall heater is used to enhance uniformity and speed of amplification reactions. For example, by decreasing or eliminating condensation, signal strength jumps in a real-time PCR assay can be minimized as can reaction non-homogeneity.
Owner:AGILENT TECH INC
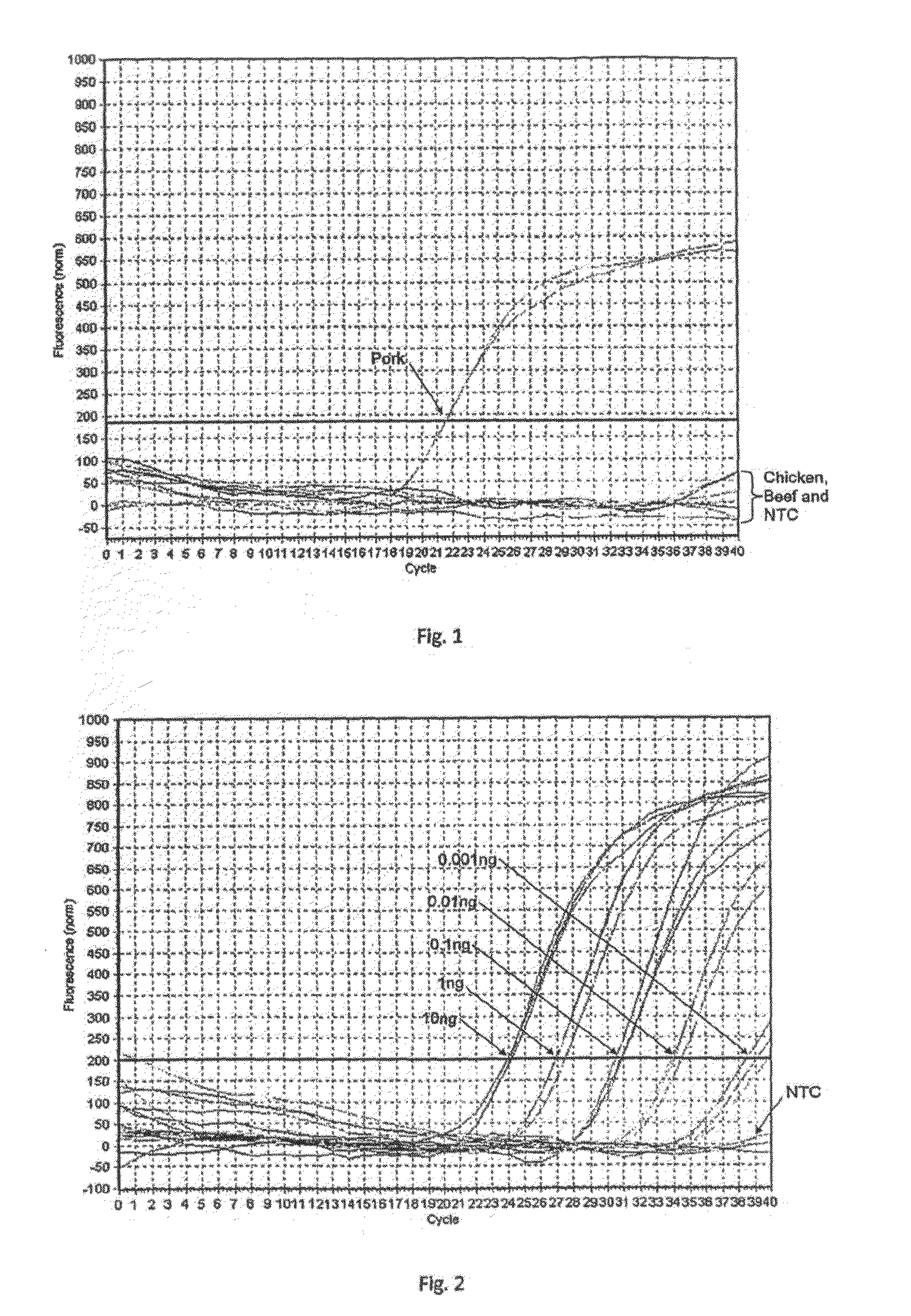
Method for identifying a pork content in a food
InactiveUS20100216136A1Microbiological testing/measurementDNA/RNA fragmentationBiotechnologyPcr assay
Pork-specific PCR assay is performed for Halal authentication, by detecting porcine DNA in food products. DNA from raw meat samples is extracted. The extracted DNA is tested using primers that react by amplifying pork DNA but not beef and chicken DNA. The real-time PCR assay is sensitive with a low detection limit when using samples that can be obtained from food products. The methods described herein can have a sensitivity threshold as low as 0.001 ng pork DNA or lower, whereas convention techniques typically do not have a detection limit lower than 0.1 ng pork DNA.
Owner:UNIVERSITI PUTRA MALAYSIA
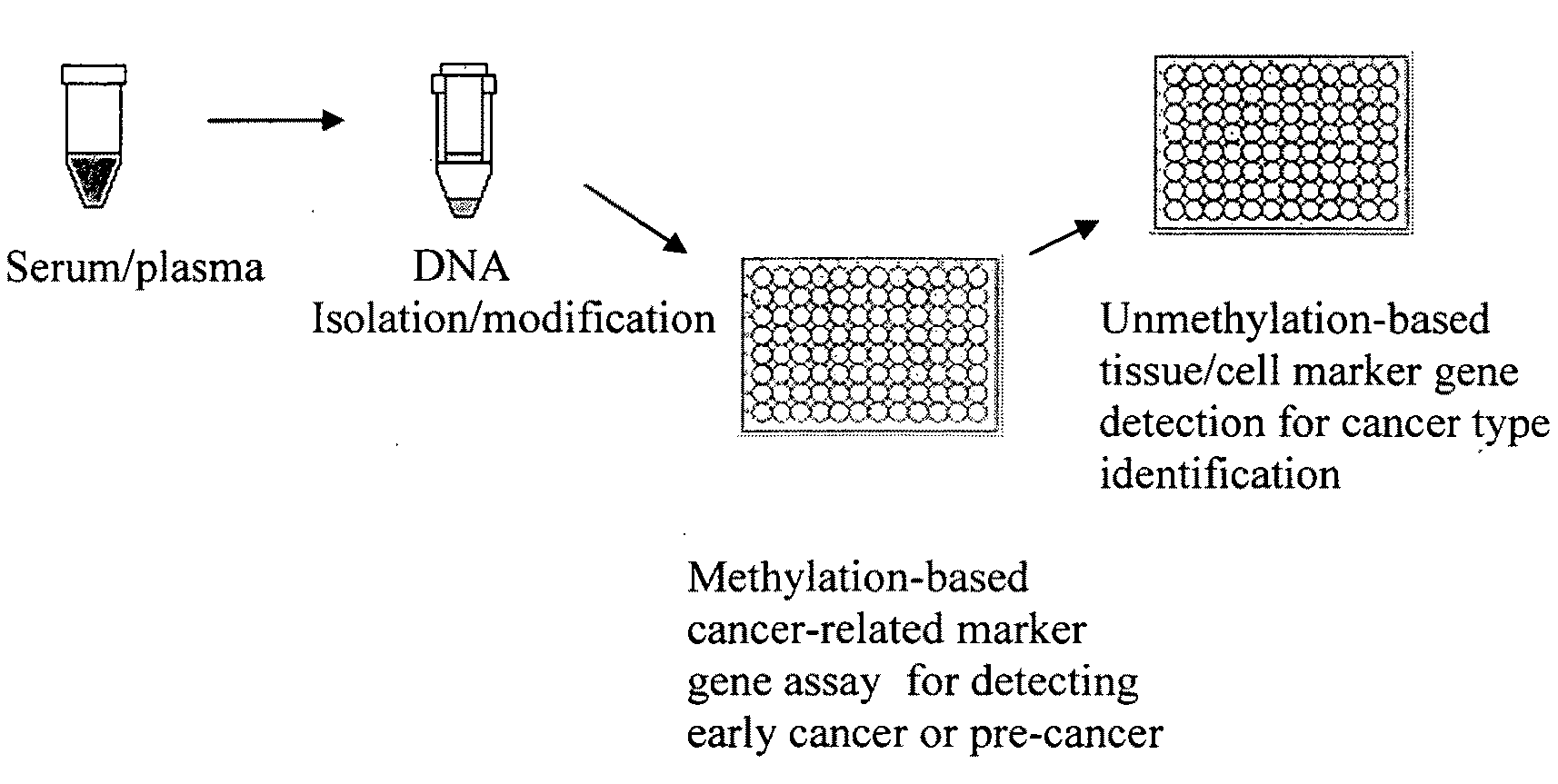
Method and kit for detection of early cancer or pre-cancer using blood and body fluids
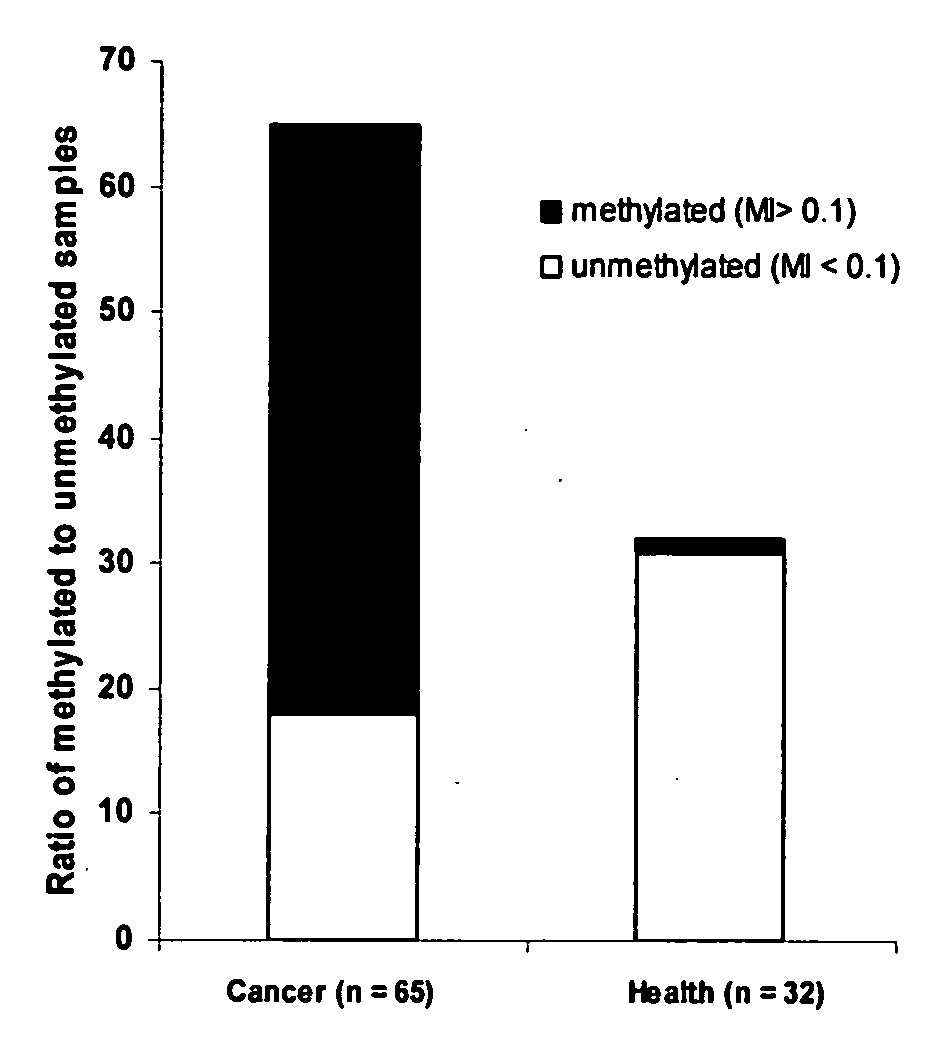
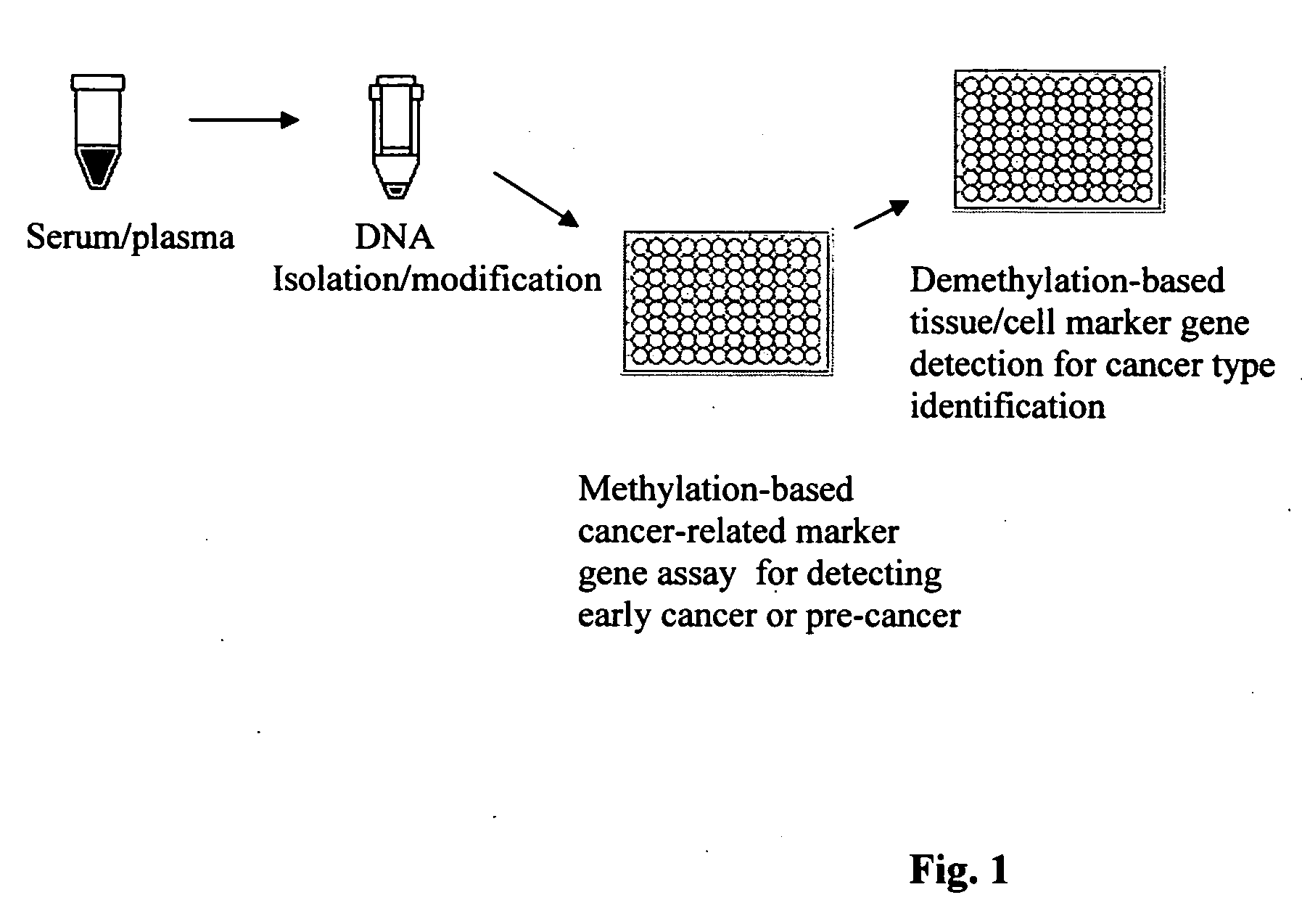
This invention is related to a method for the detection of early or pre-cancer using DNA isolated from blood and body fluids. This invention provides an improved methylation-based PCR assay, a panel of methylated-based cancer-related gene markers for the detection of general cancer and a panel of demethylation-based tissue- or cell-specific gene markers for discriminating different type of cancer detected from blood samples. This method couples a sequential cancer-related gene marker detection and tissue or cell-specific gene marker assay and is particularly useful as a simultaneous screening test for following type of cancer: lung, breast, ovarian, colon, stomach, prostatic, pancreatic and liver cancer.
Owner:LI WEIWEI +1
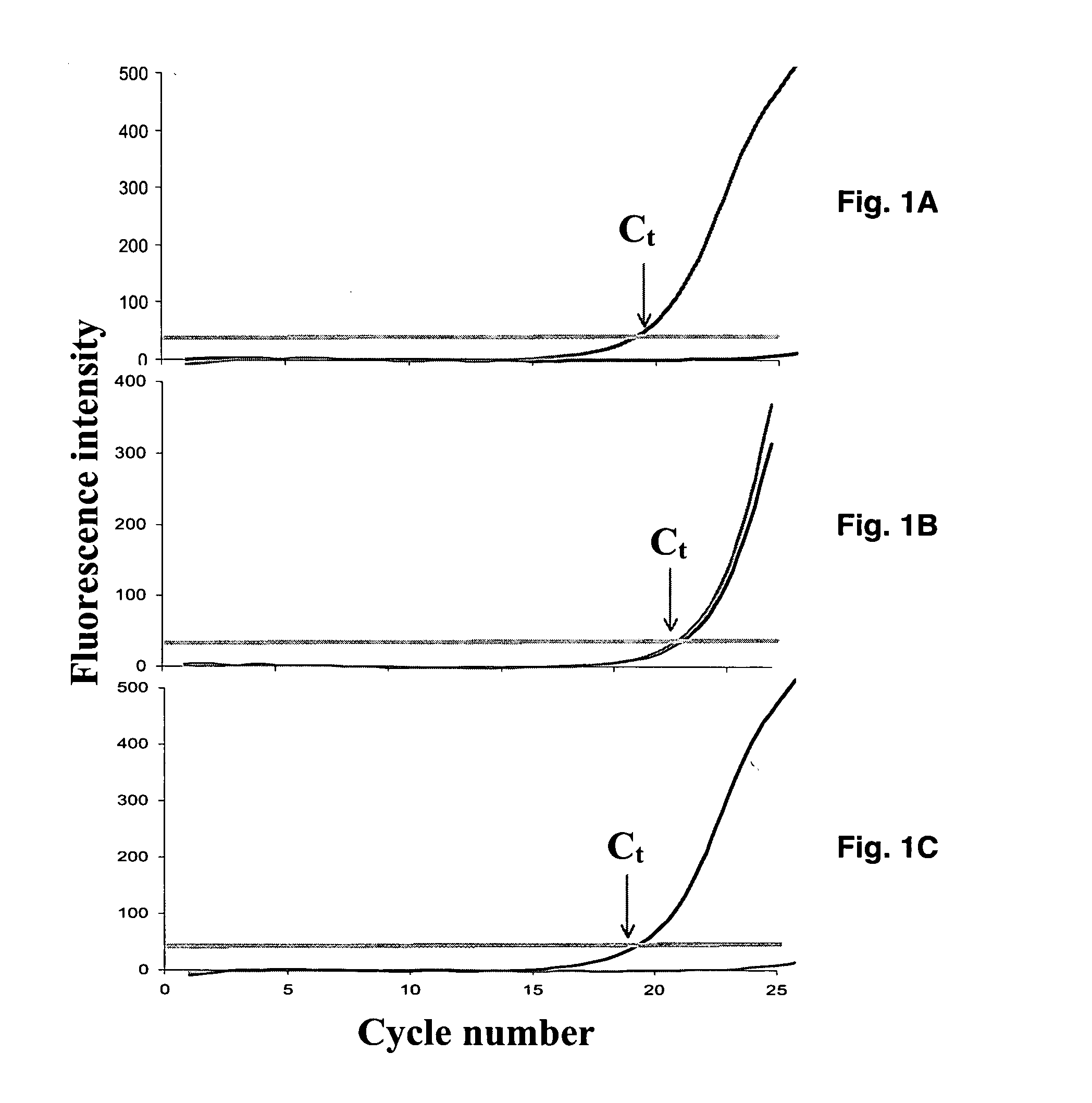
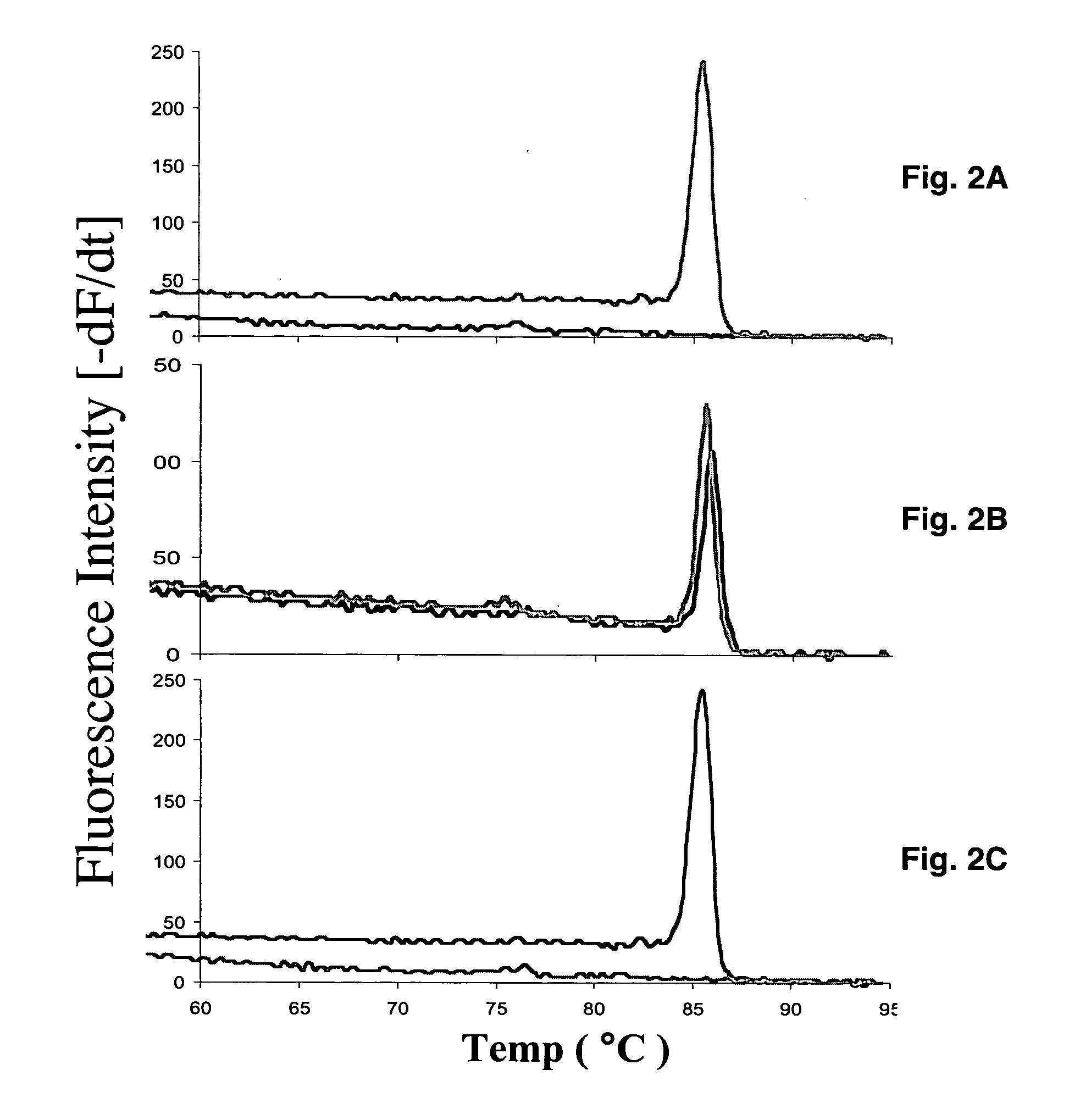
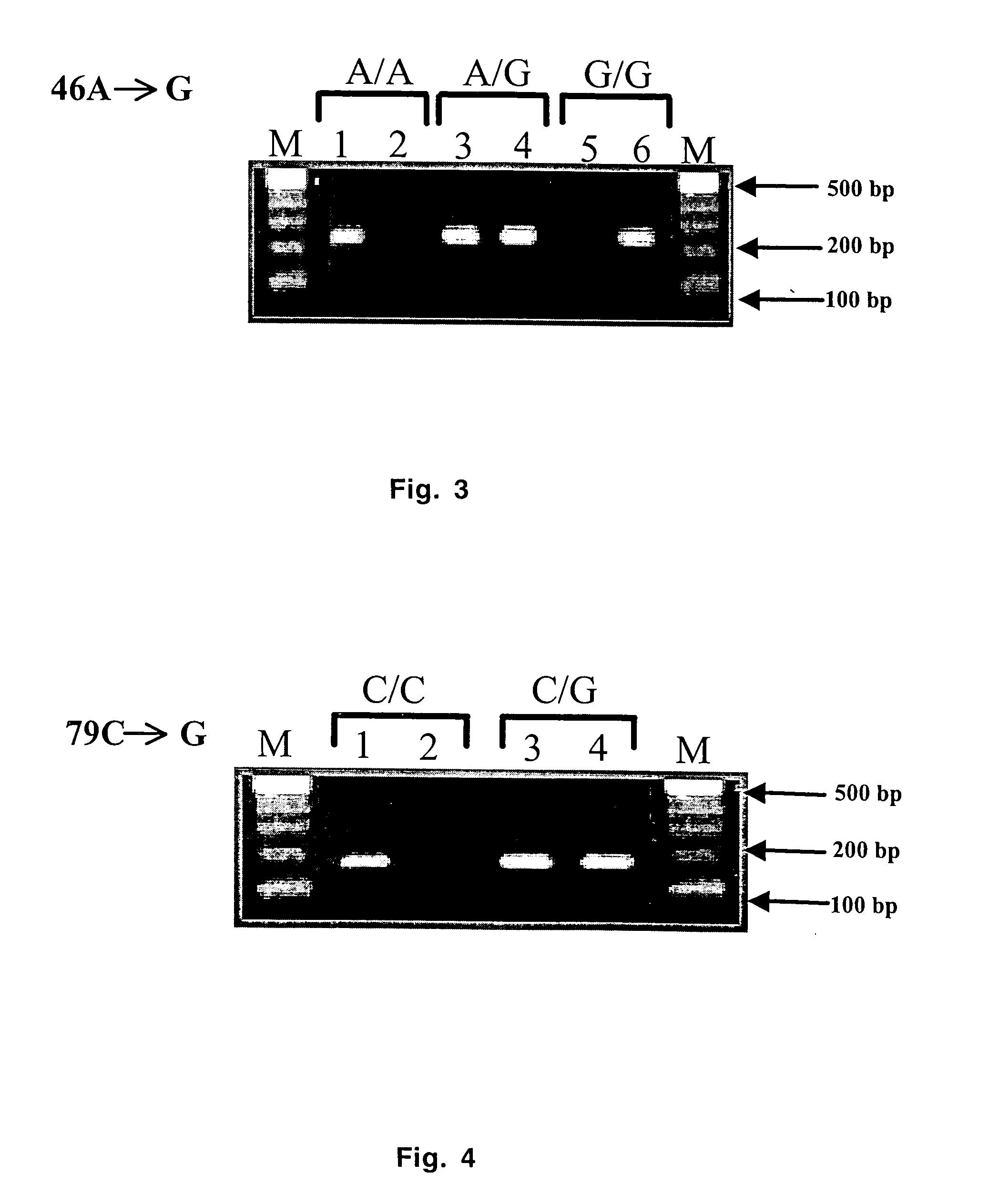
Real-time polymerase chain reaction-based genotyping assay for beta2-adrenergic receptor single nucleotide polymorphism
InactiveUS20050153353A1Rapid and inexpensive and robust chain reactionGrowth inhibitionSugar derivativesMicrobiological testing/measurementGenotype AssayReal-Time PCRs
The present invention provides fluorescence-based real-time PCR assays for the rapid detection of β2-adrenergic receptor single nucleotide polymorphisms (SNPs). The genotyping assay can be used to detect SNPs of human β2-adrenergic receptor (β2-AR) single nucleotide polymorphisms A46G and C79G.
Owner:UNIV OF TENNESSEE RES FOUND
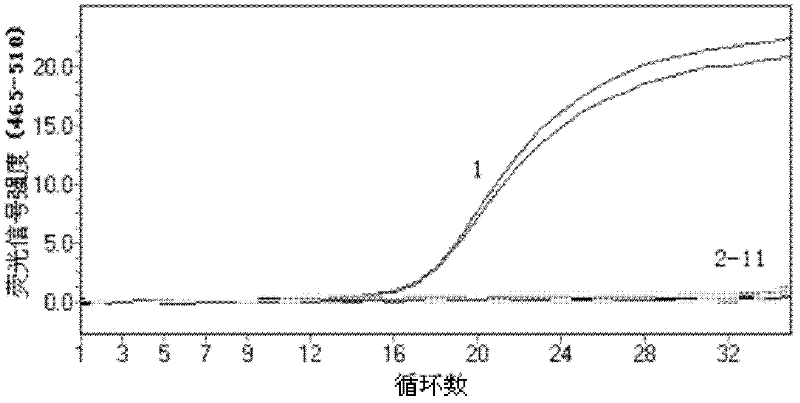
Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
ActiveUS7267942B2Sugar derivativesMicrobiological testing/measurementPcr assaySevere acute respiratory syndrome
The present invention relates to a diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a real-time quantitative PCR assay for the detection of hSARS virus using reverse transcription and polymerase chain reaction. Specifically, the quantitative assay is a TaqMan® assay using the primers and probes constructed based on the genome of the hSARS virus. The invention further relates to a diagnostic kit that comprises nucleic acid molecules for the detection of the hSARS virus.
Owner:HONG KONG THE UNIV OF +1
Evaluating genetic disorders
The present invention relates to genetic analysis and evaluation utilizing copy-number variants or polymorphisms. The methods utilize array comparative genomic hybridization and PCR assays to identify the significance of copy number variations in a human, non-human animal, and plant subject or subject group.
Owner:POPULATION BIO INC
Detection system for PCR assay
ActiveUS7615620B2High of synthesisImprove throughputSugar derivativesMicrobiological testing/measurementPcr assayFluorescence
Owner:LGC GENOMICS
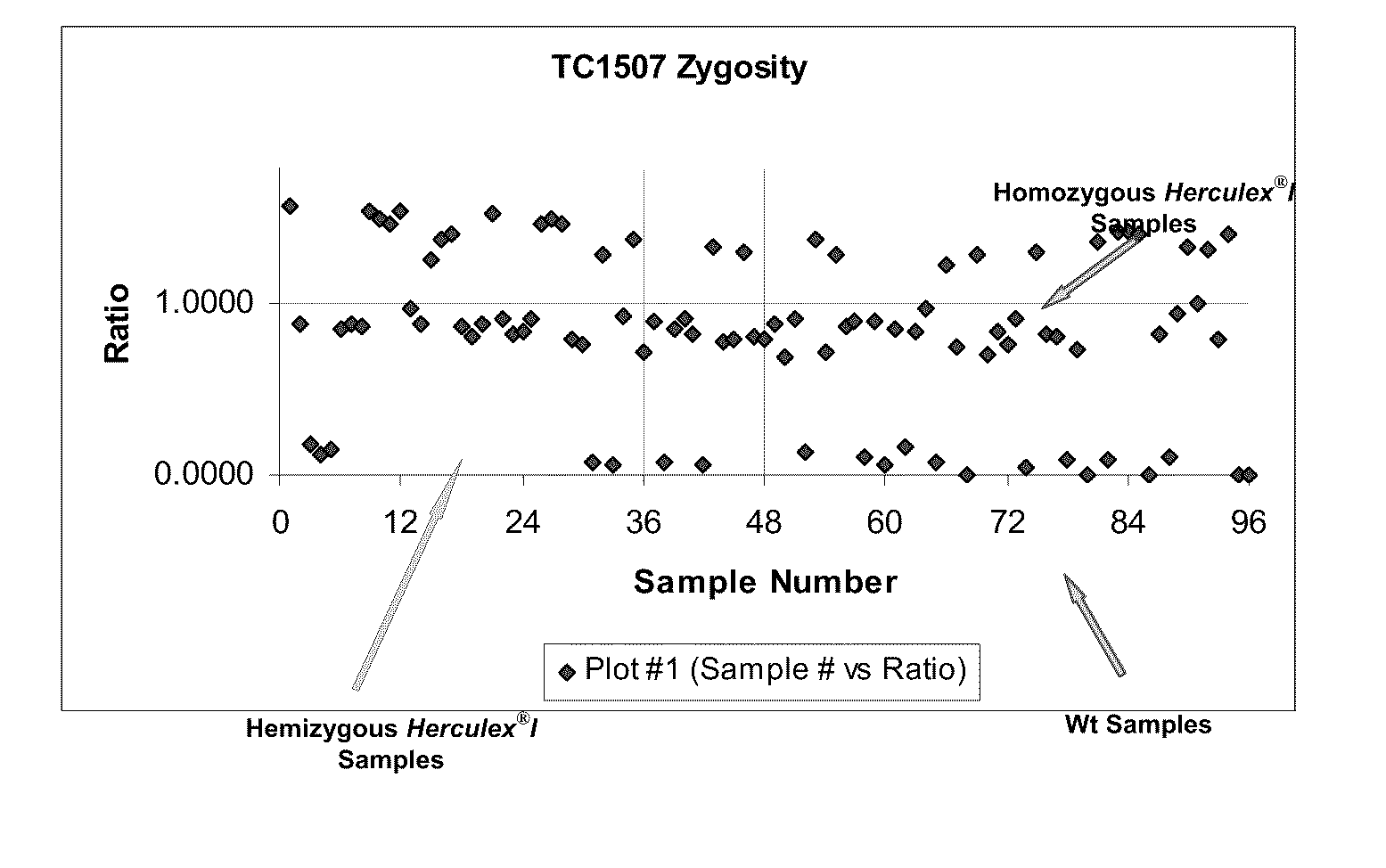
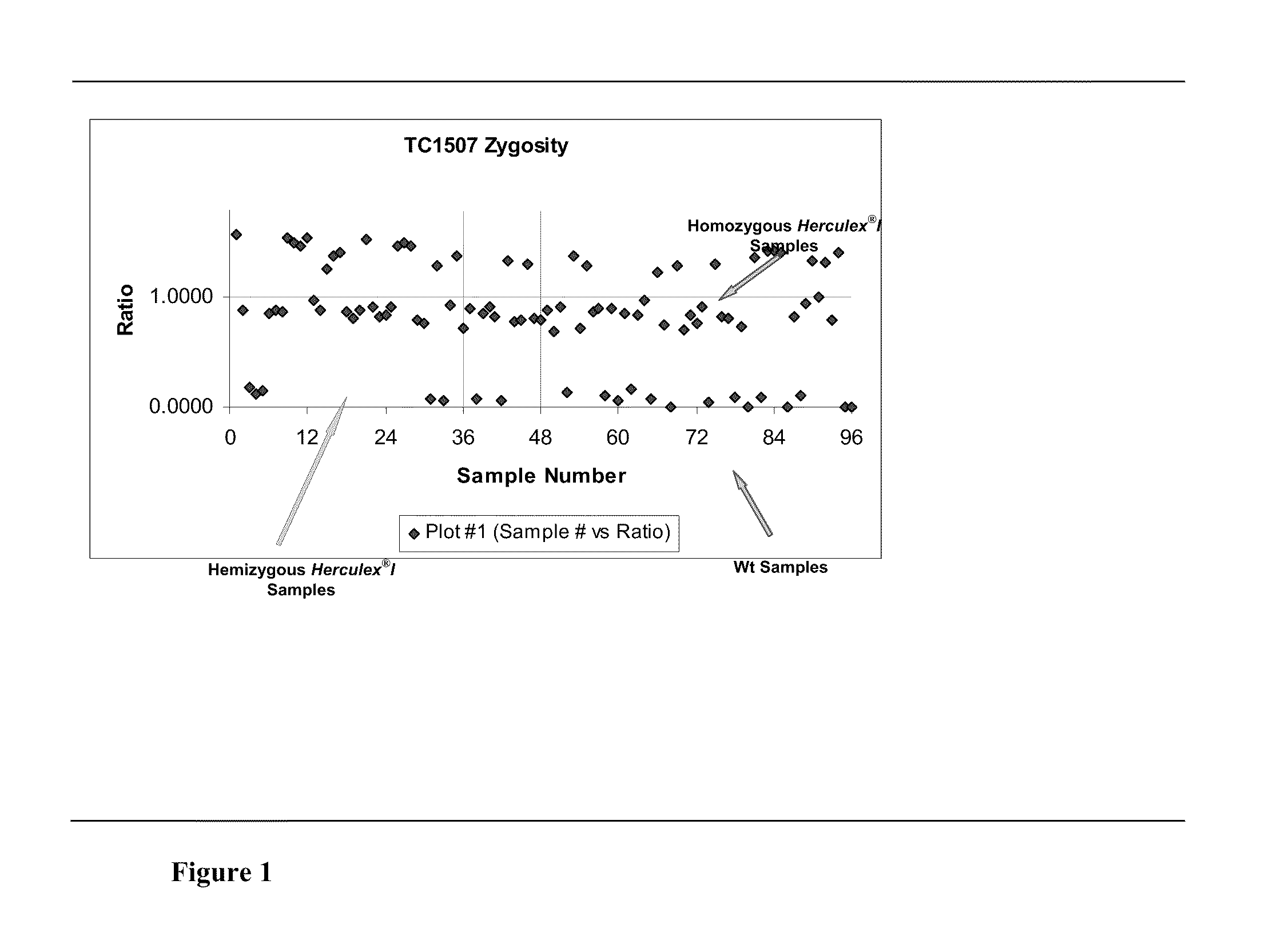
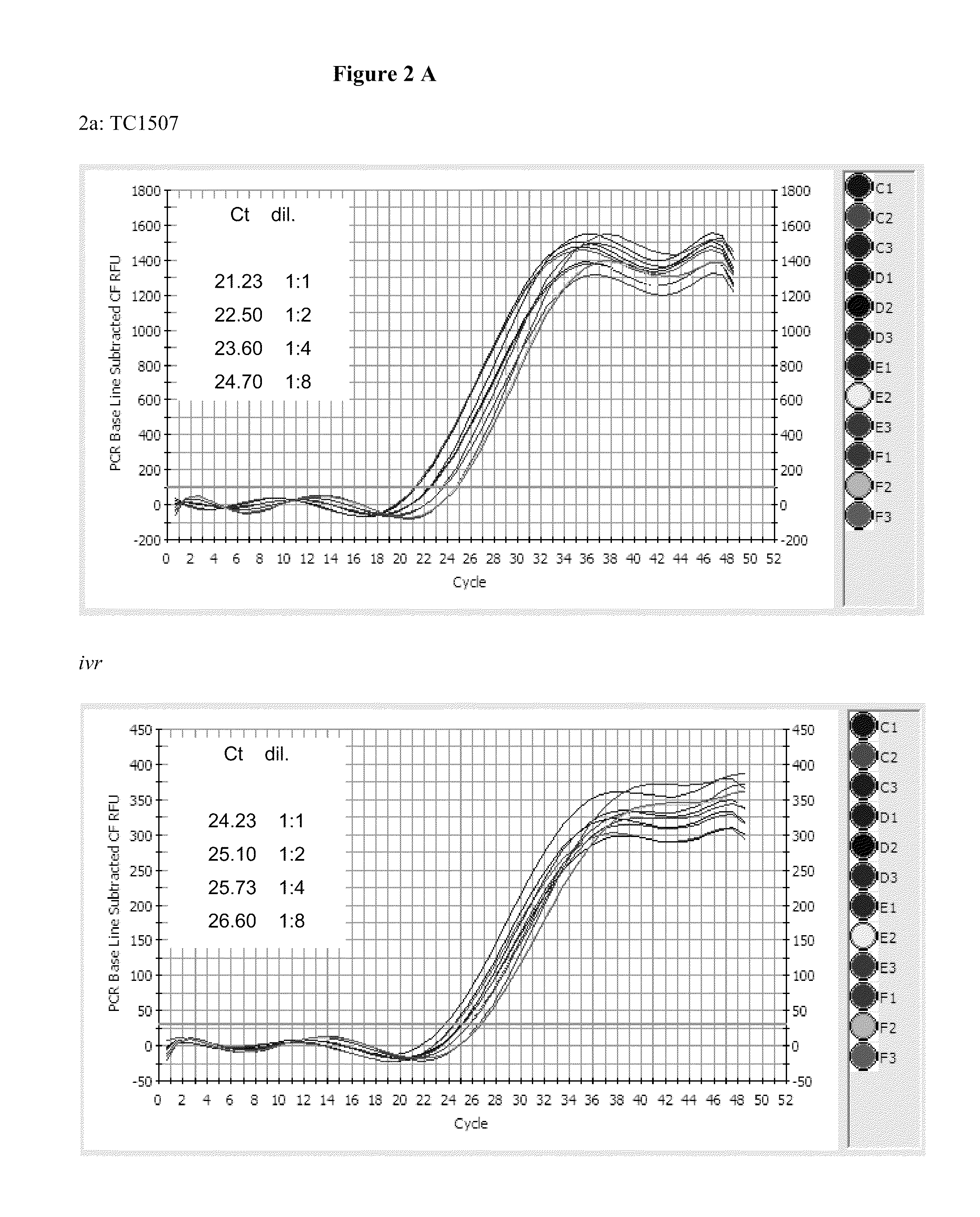
Endpoint taqman methods for determining zygosity of corn comprising tc1507 events
InactiveUS20110151441A1High throughput zygosity analysisSugar derivativesMicrobiological testing/measurementReference genesPcr assay
A method for zygosity analysis of the maize Cry1F event TC1507 is provided. The method provides TC1507 event-specific and maize endogenous reference gene-specific primers and TaqMan probe combinations for use in an endpoint biplex TaqMan PCR assay capable of producing robust genotype calls for assisting in molecular breeding of TC1507.
Owner:DOW AGROSCIENCES LLC
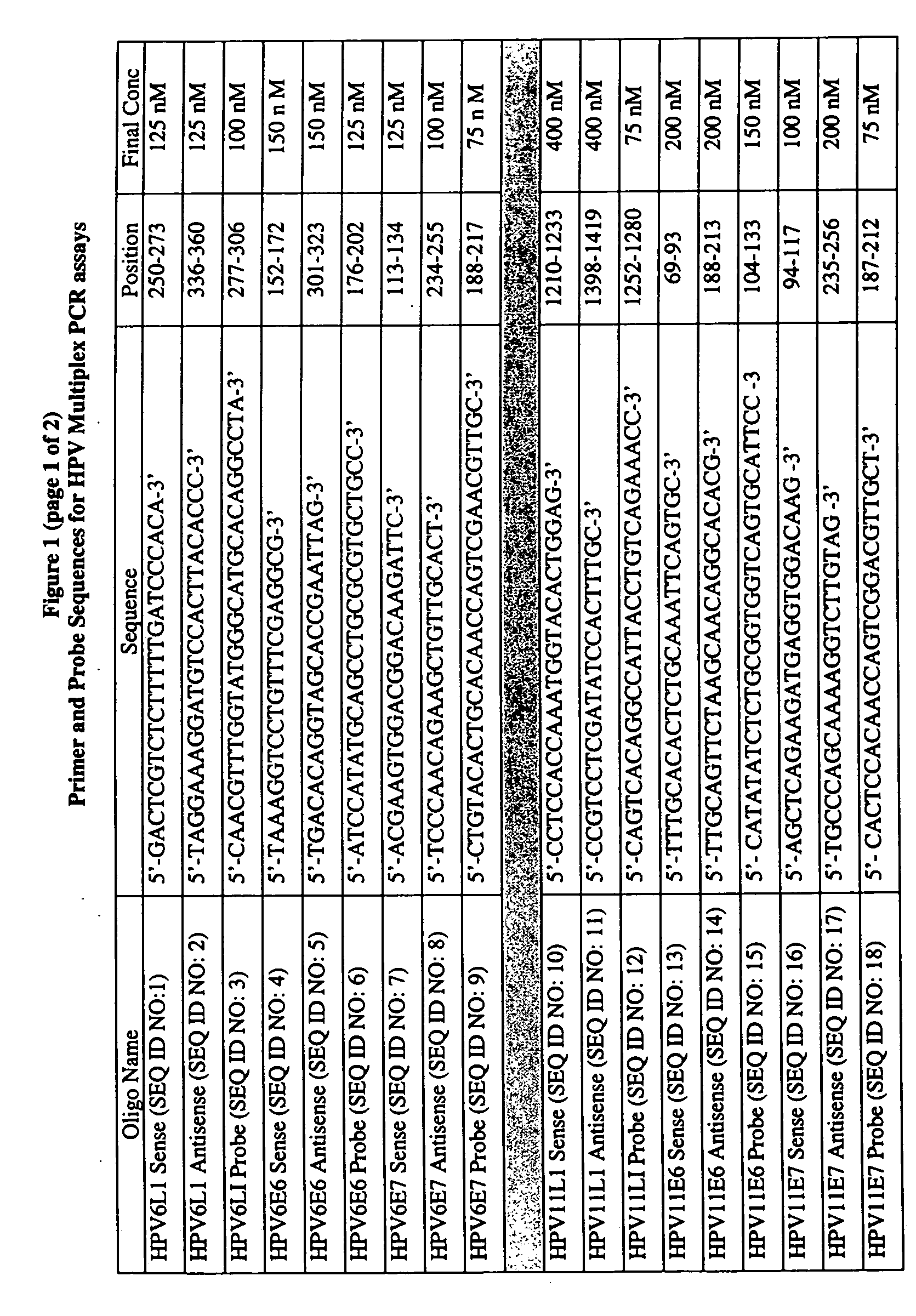
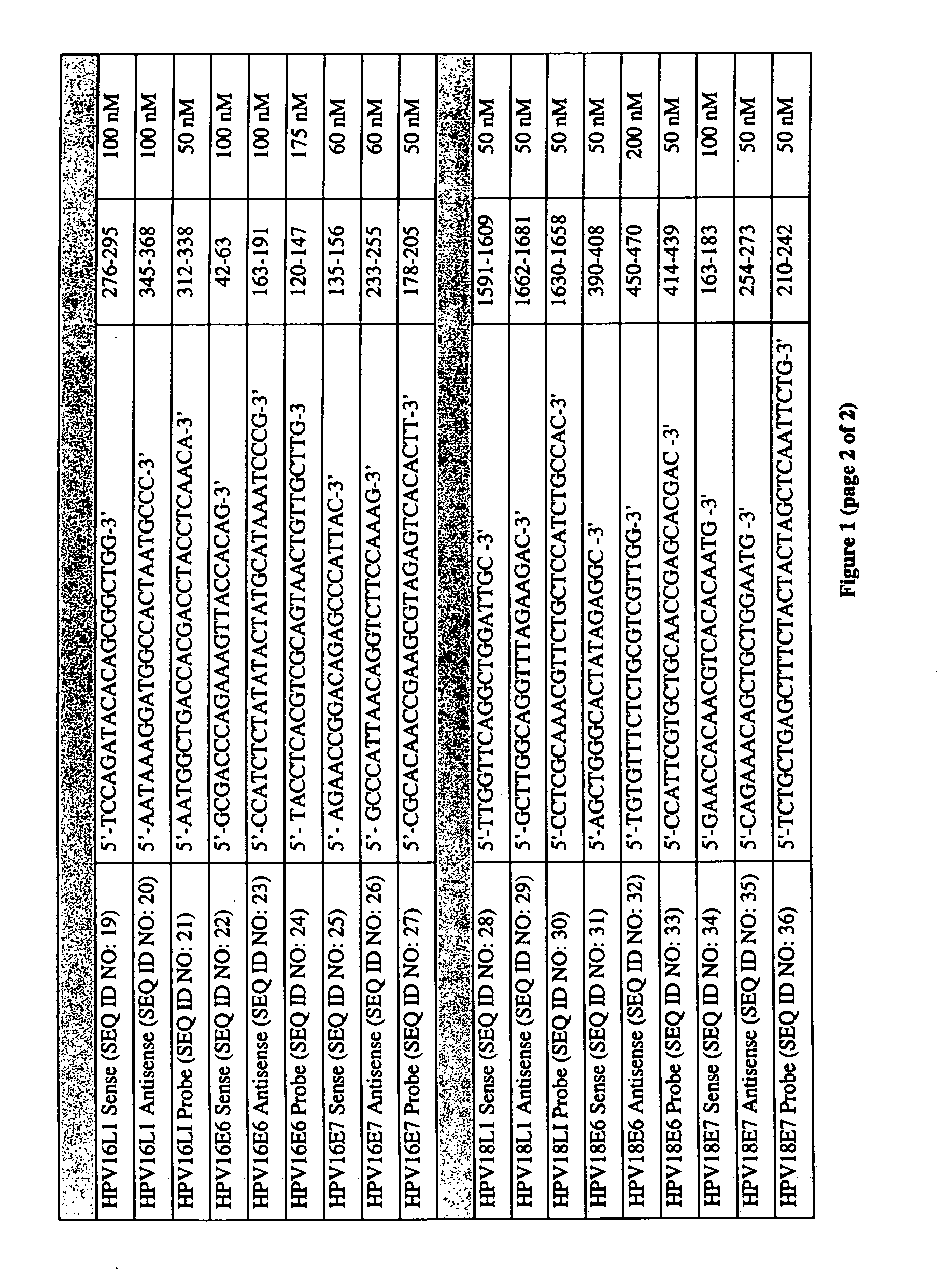
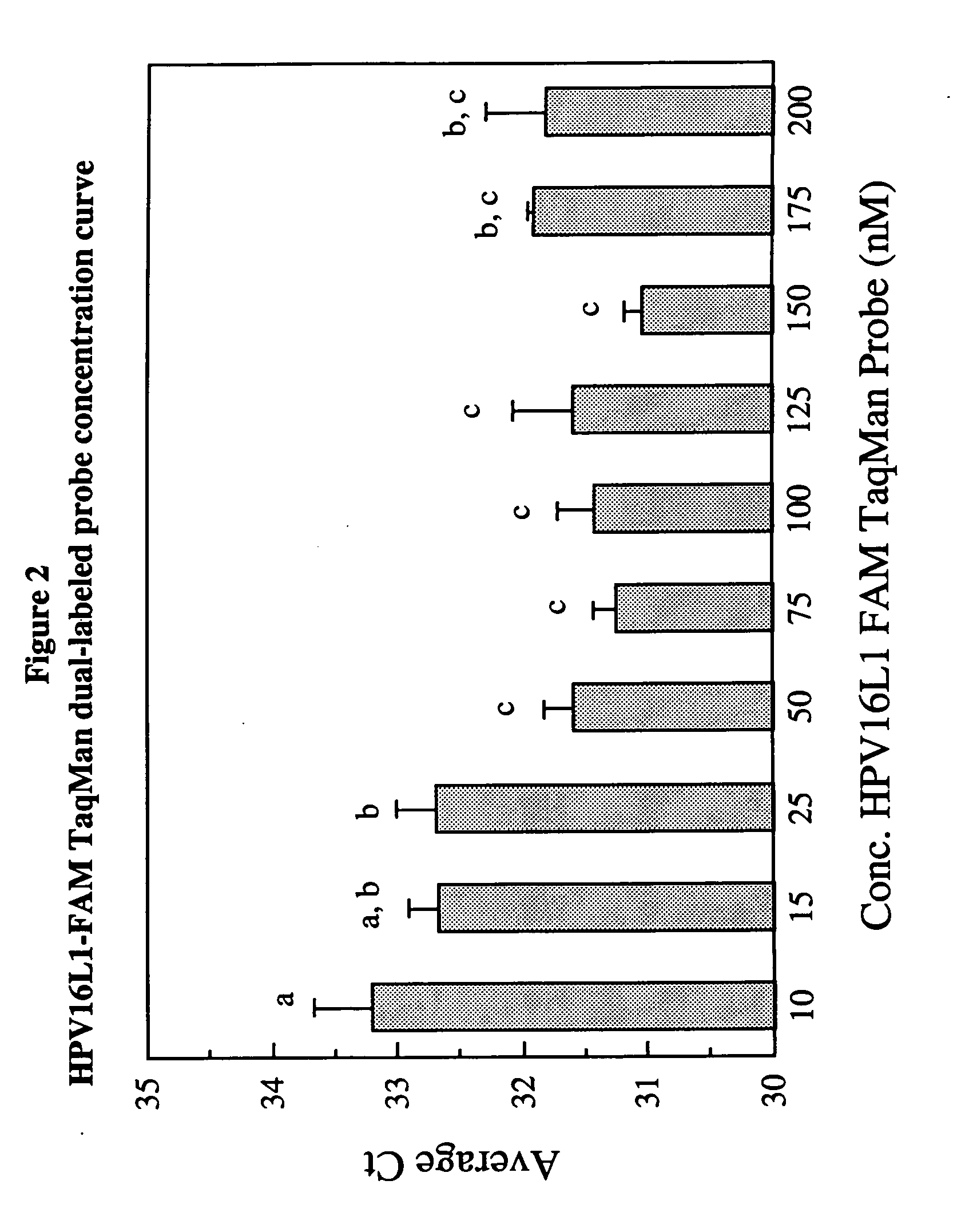
Fluorescent multiplex hpv pcr assays using multiple fluorophores
InactiveUS20050175987A1Microbiological testing/measurementRecombinant DNA-technologyPcr assayFluorophore
The present invention relates a fluorescent multiplex PCR assay for detecting the presence of an HPV subtype in a sample using multiple fluorophores to simultaneously detect a plurality of HPV genes of the same HPV subtype. The present invention also relates to primer pairs and probes specific to HPV subtypes for use in the methods of the present invention.
Owner:JANSEN KATHRIN +3
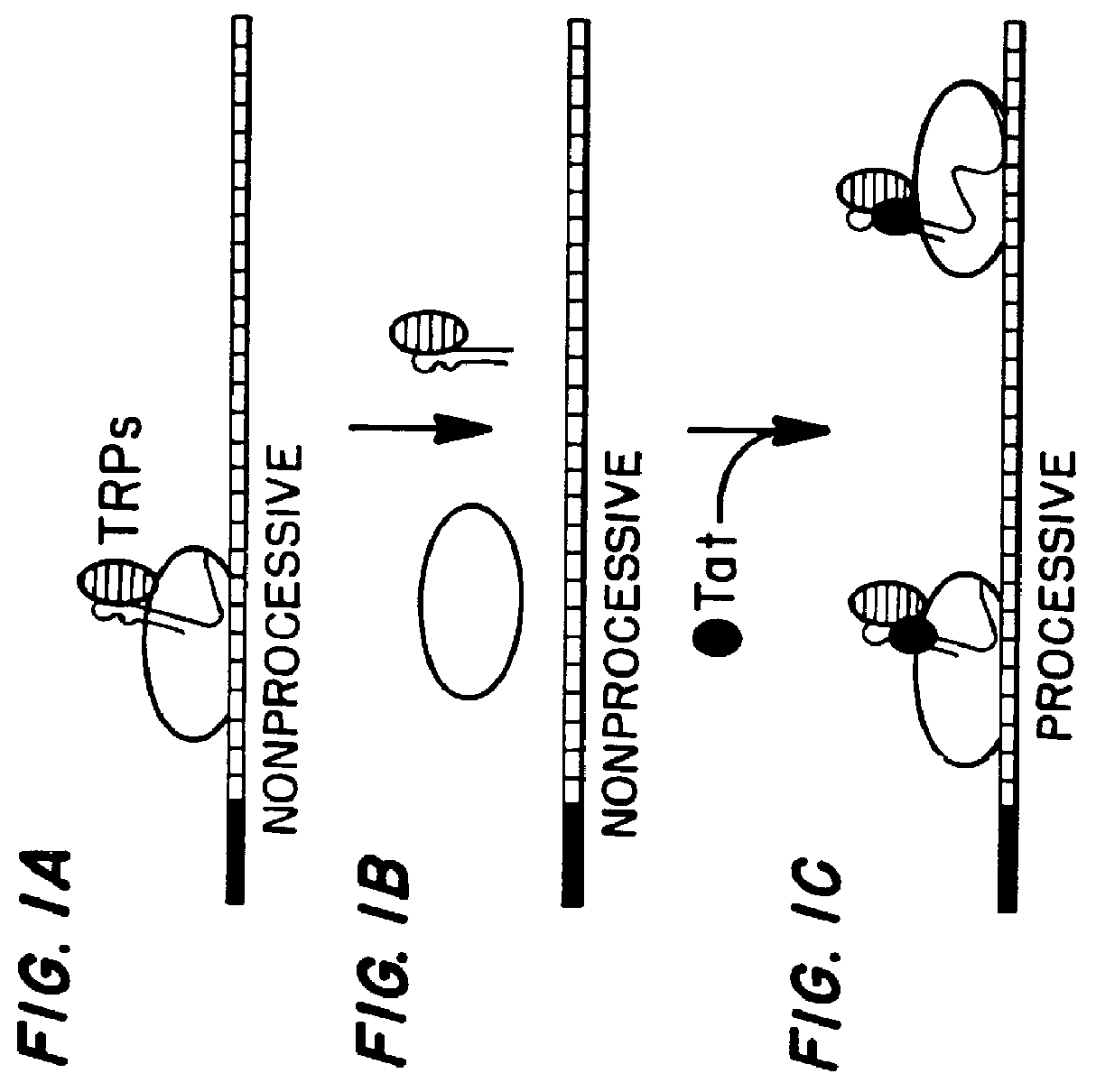
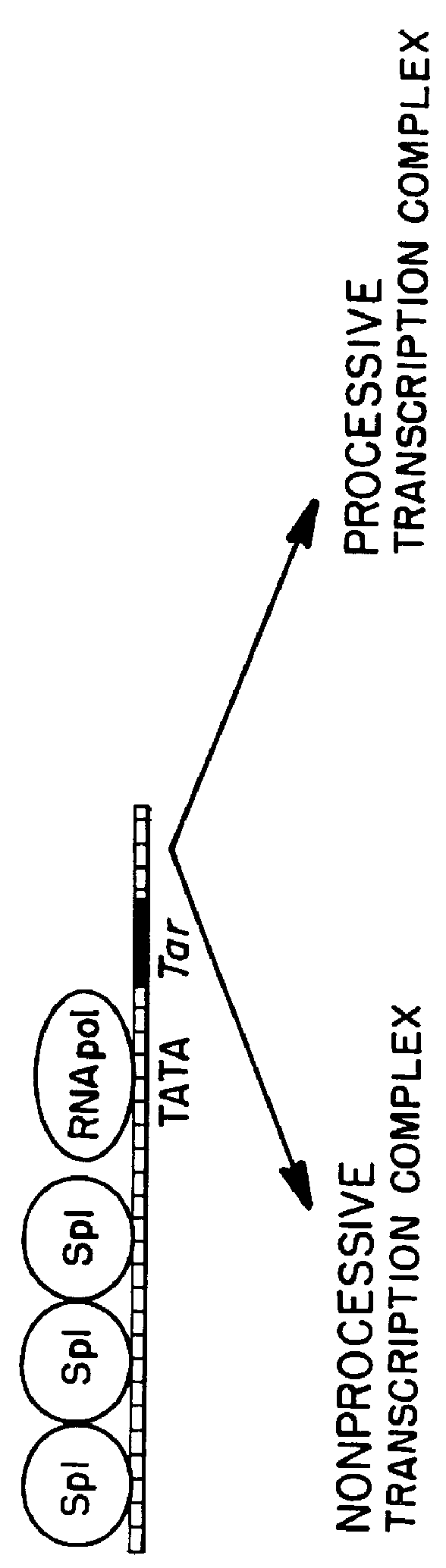
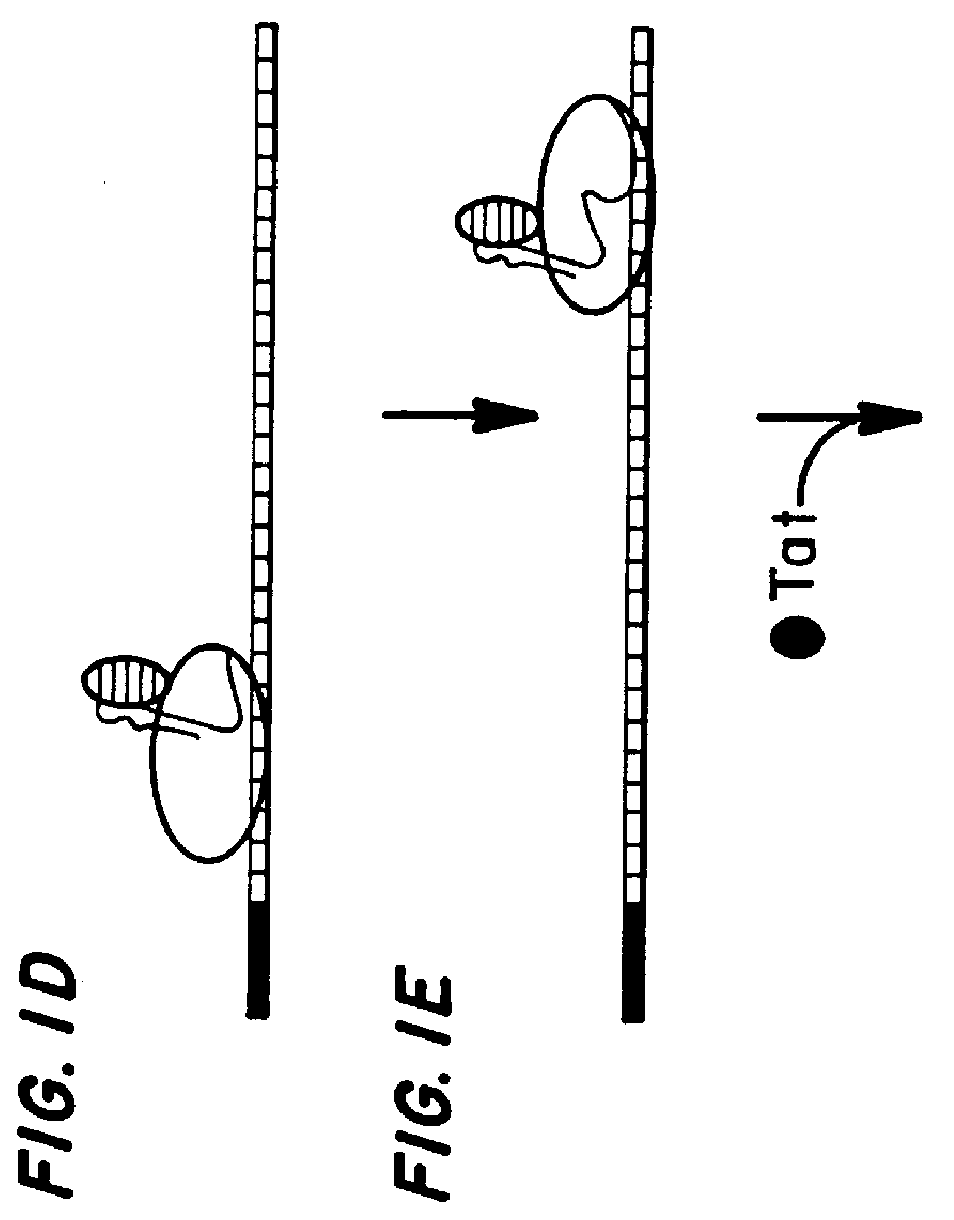
Marker, method and an assay for detection and monitoring of latency and activation of human immunodeficiency virus
A TAR marker, method for detection and monitoring of HIV latency and activation and an assay for detection of the marker. The assay sensitively detects HIV transcription and monitors HIV transcriptional activity by detecting the presence of TAR fragments and full length transcripts, quantifying both and determining the ratio of short to long transcripts. A low ratio correlates with a latent-type transcriptional activity of HIV whereas the appearance of long transcripts signifies increased efficiency of transcriptional activity of HIV and the transition from latency to activation. The size difference between the TAR fragments appearing predominantly in latency and the full length transcripts appearing predominantly during the HIV activation is detected by RT-PCR assay that utilizes novel primers and probes. The obtained ratio is a sensitive tool in detection of HIV infection, the analysis of load of latent and active virus and monitoring the transition from the latent to active state of HIV replication.
Owner:RGT UNIV OF CALIFORNIA
Non-invasive detection of fish viruses by real-time PCR
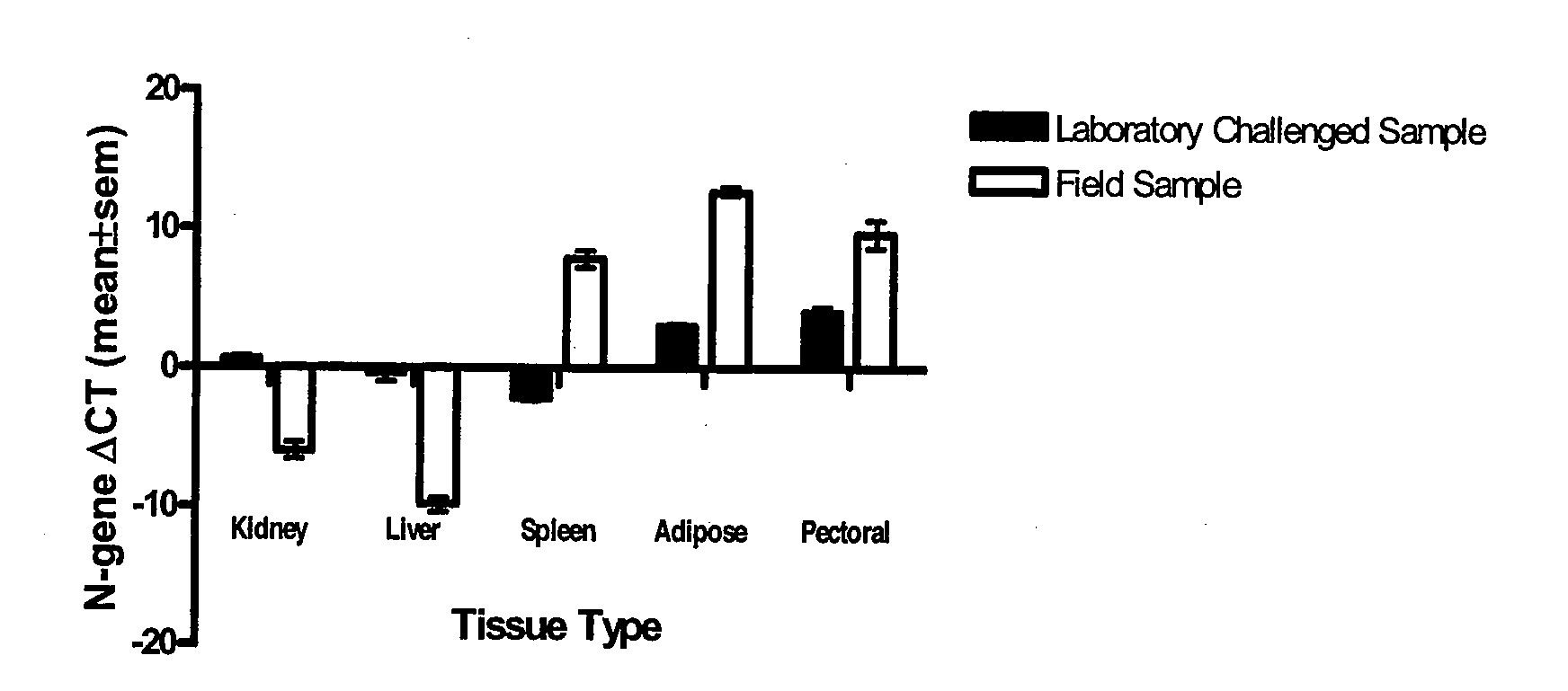
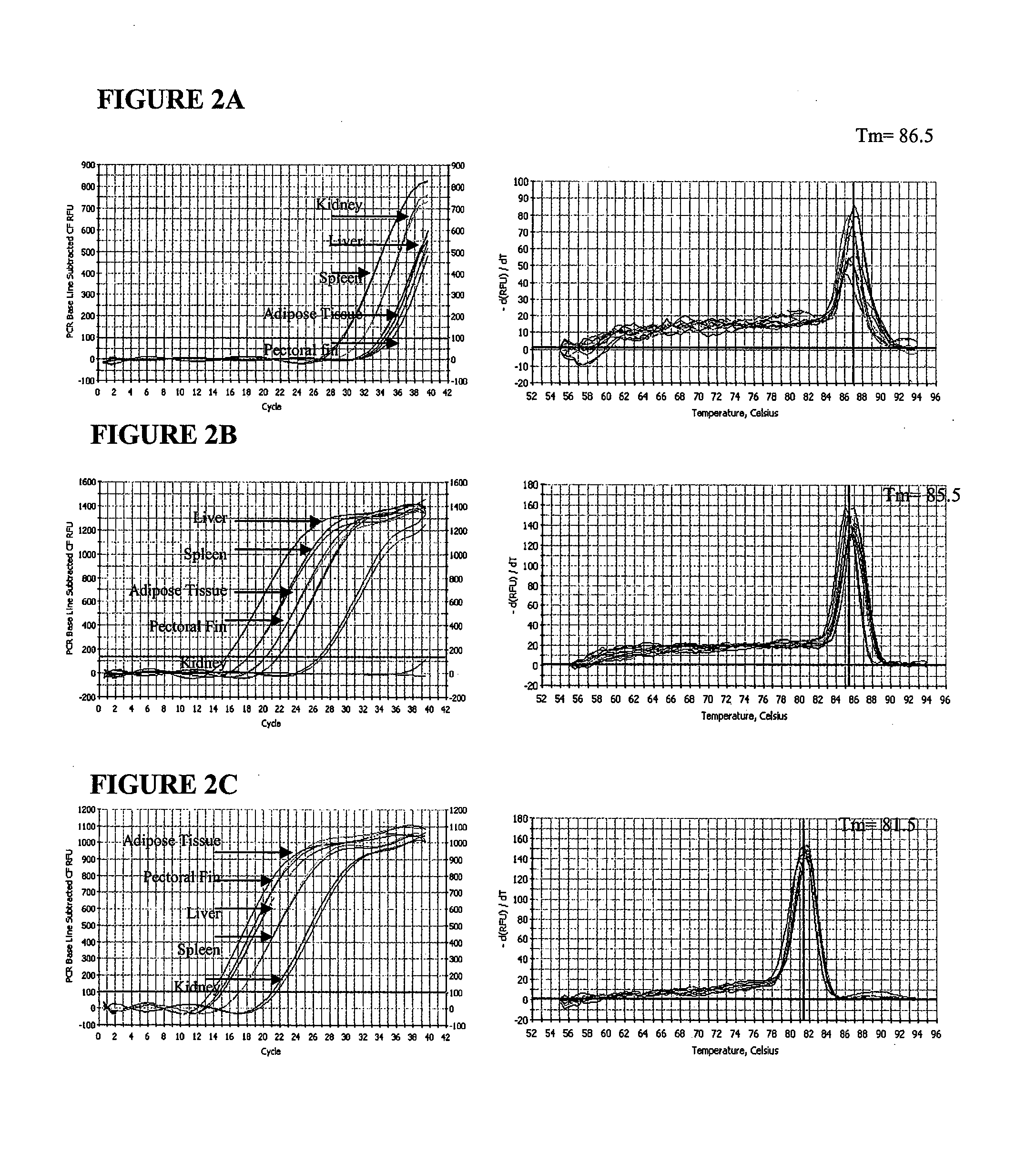
A real-time assay coupled with a non-invasive tissue sampling was developed for the detection and quantification of fish viruses. As a proof of principles, data were presented for the detection and quantification of infectious hypodermal necrosis virus (IHNV) in trout. The primers were designed for IHNV nucleocapsid (N), and surface glycoprotein (G) genes, and trout &bgr;-actin and elongation factor-l&agr; (EF-I &agr;) were used as internal control for the assay. The reaction conditions for the real-time RT-PCR were optimized using cDNA derived from IHNV-infected Epithelioma papulosum cyprinid (EPC) cells. Using both N- and G-gene primers, IHNV was successfully detected in liver, kidney, spleen, adipose tissue and pectoral fin samples of laboratory-challenged and wild samples. The dissociation curves with a single melting peak at expected temperature (85° C. for the N-gene and 86.5° C. for the G-gene) confirmed the specificity of the N- and G-gene amplicons. The IHNV N- and the G-gene expression levels in different tissues of laboratory challenged samples were in the order of spleen, liver, kidney, adipose tissue and pectoral fin, however in the field-collected samples the order of gene expression was liver, kidney, pectoral fin, adipose tissue, and spleen. The N- and G-gene expressions in spleen were found to be dramatically lower in the field-collected samples compared to the laboratory-challenged samples indicating a potential difference in the IHNV replication in the laboratory as opposed to field conditions. The real-time PCR assay was found to be rapid, highly sensitive, and reproducible. Based upon the ability to detect the virus in pectoral fins a non-invasive detection method for IHNV and other fish viruses is developed. Such a non-invasive tissue sampling coupled with real-time PCR assay is very valuable for large-scale virus screening of fish in aquaculture facilities as well as for epidemiological studies.
Owner:ADVANCED BIONUTRITION CORP
Multiplexed PCR assay for detecting disseminated Mycobacterium avium complex infection
InactiveUS6465638B2Sugar derivativesMicrobiological testing/measurementPcr assayMycobacterium avium complex
Owner:ORTHO-CLINICAL DIAGNOSTICS
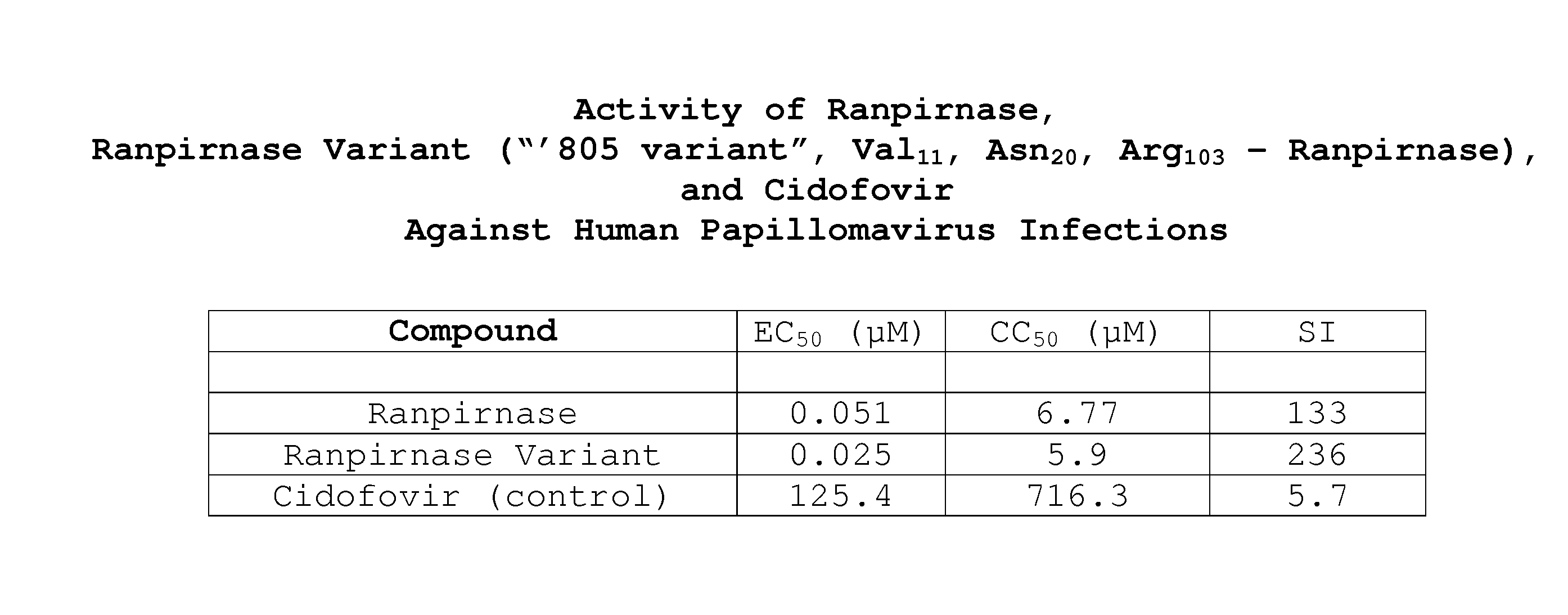
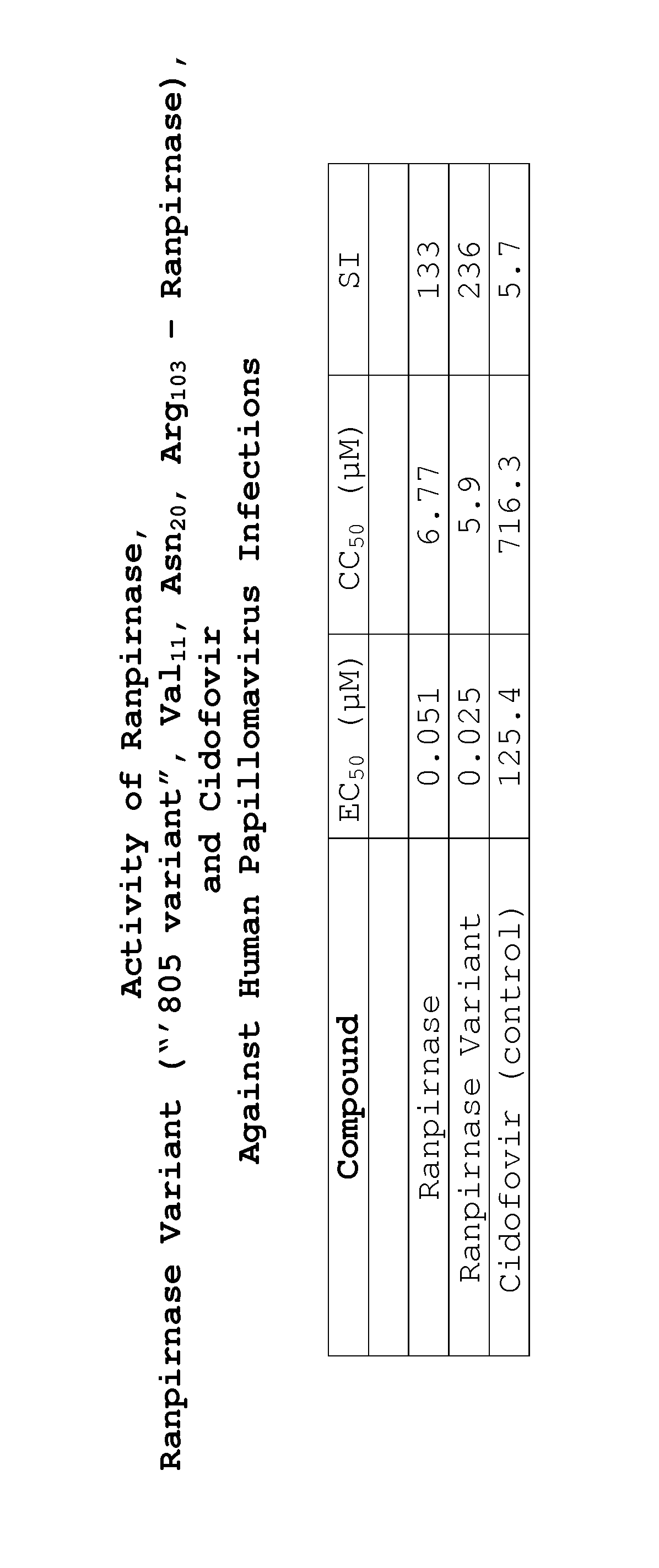
Methods of treating human papillomavirus
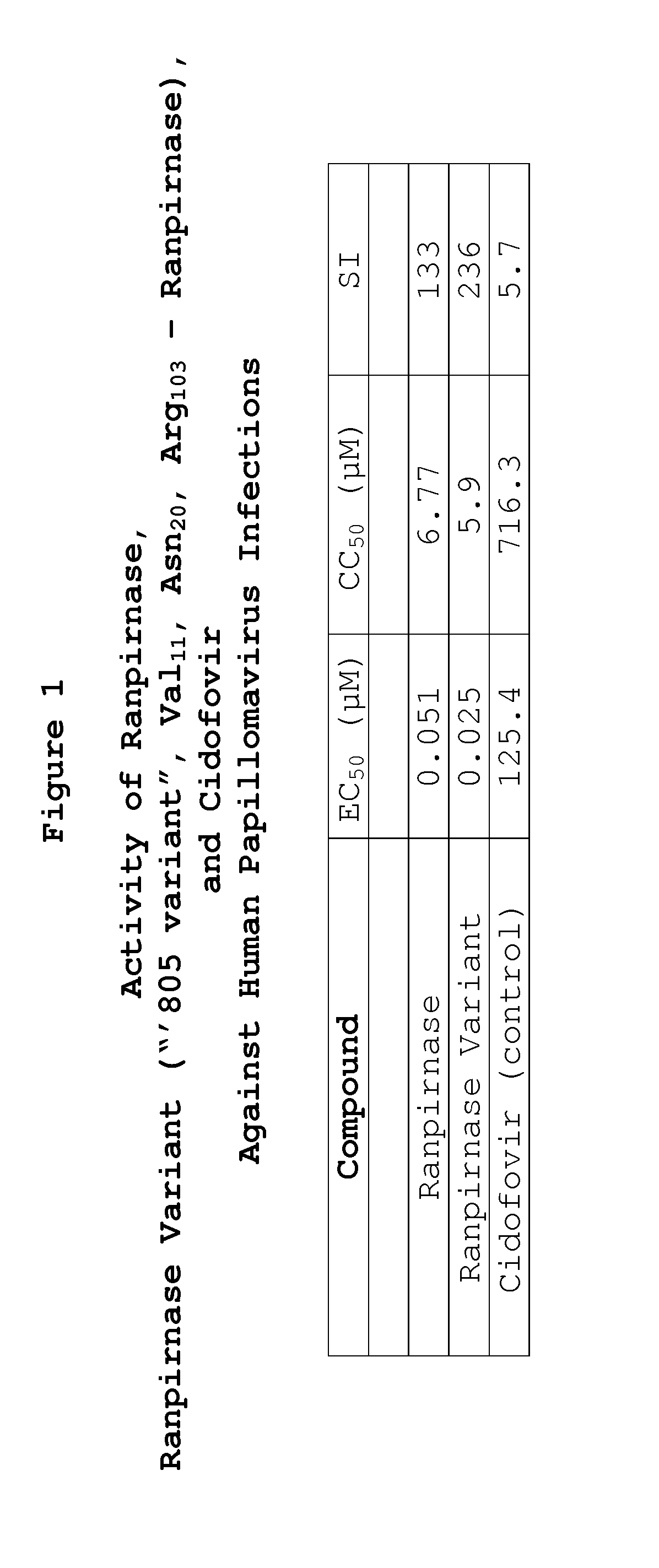
Two RNases (ranpirnase and the second embodiment disclosed in U.S. Pat. No. 5,728,805) are tested against human papillomavirus infections. QRT-PCR assays indicate that the RNases have anti-viral activity against type 11 HPV.
Owner:ORGENESIS INC
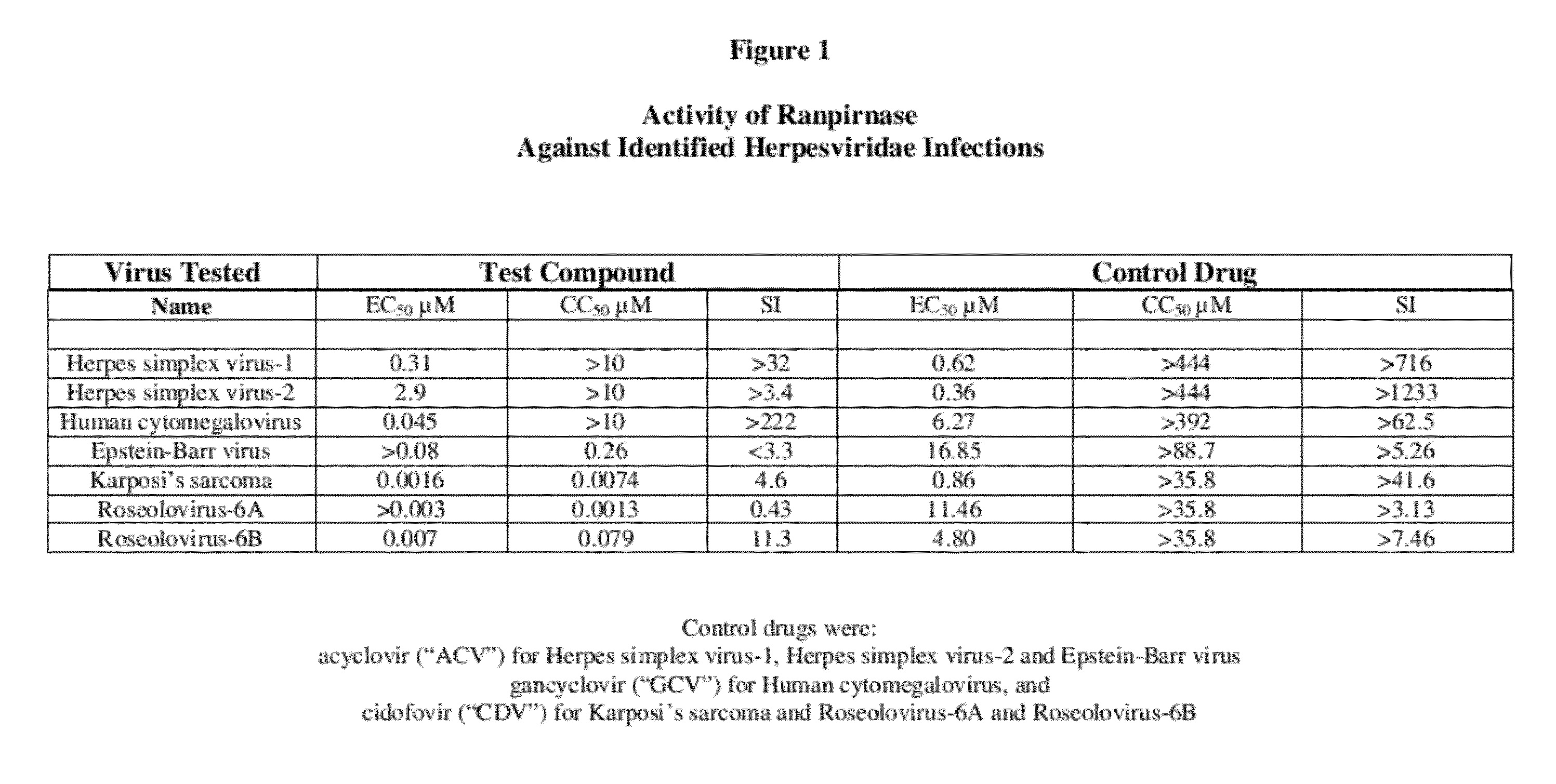
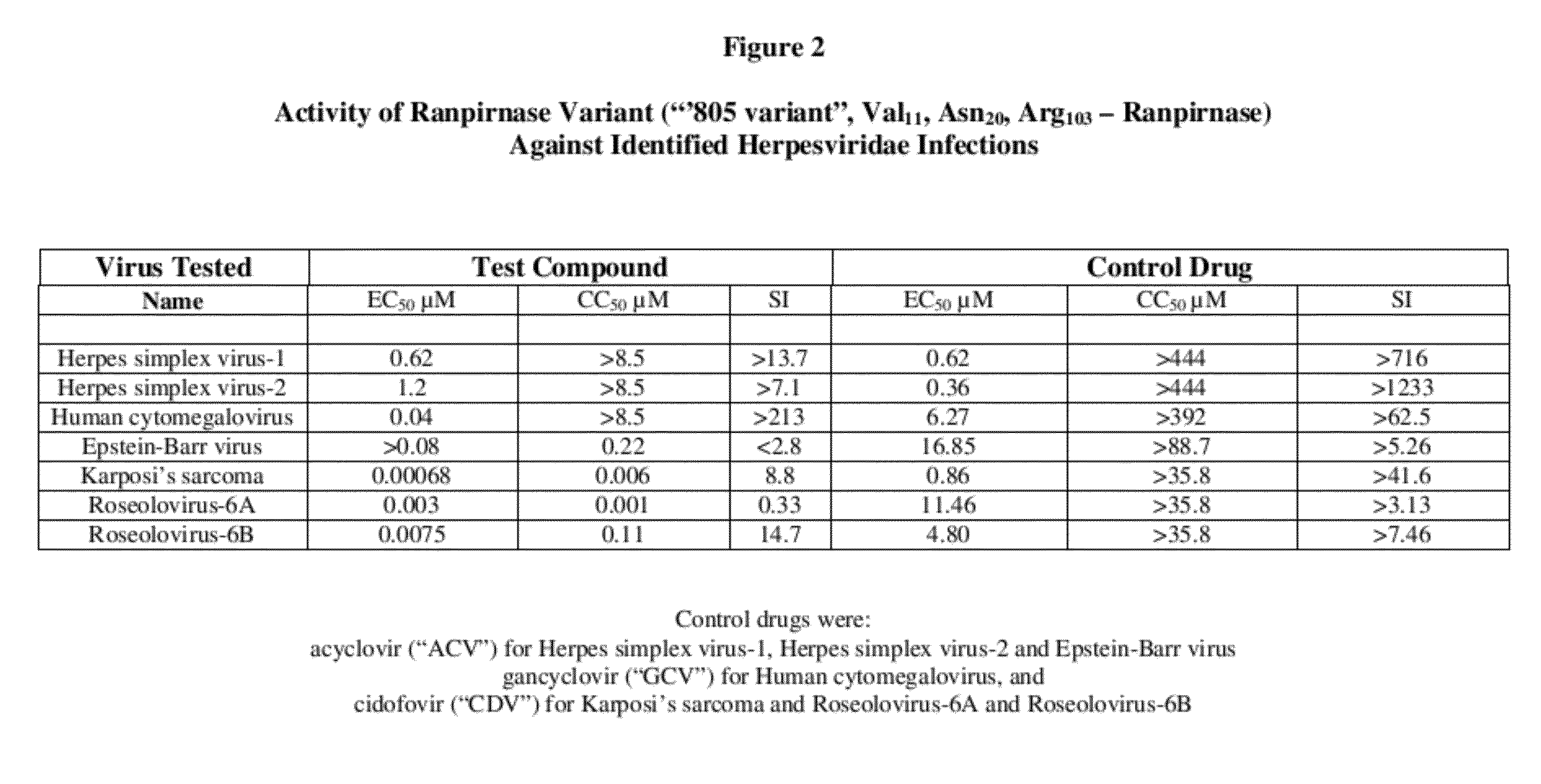
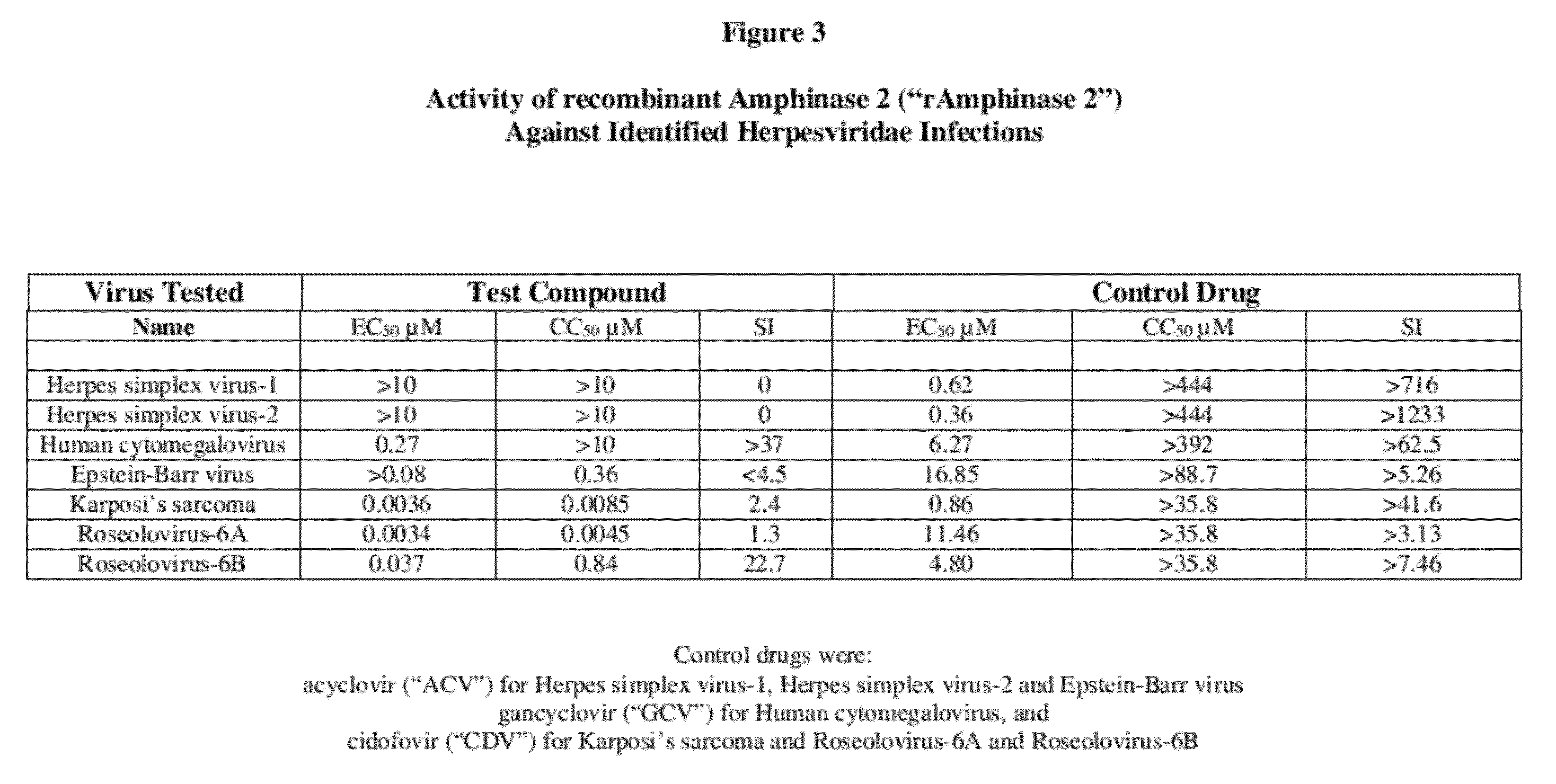
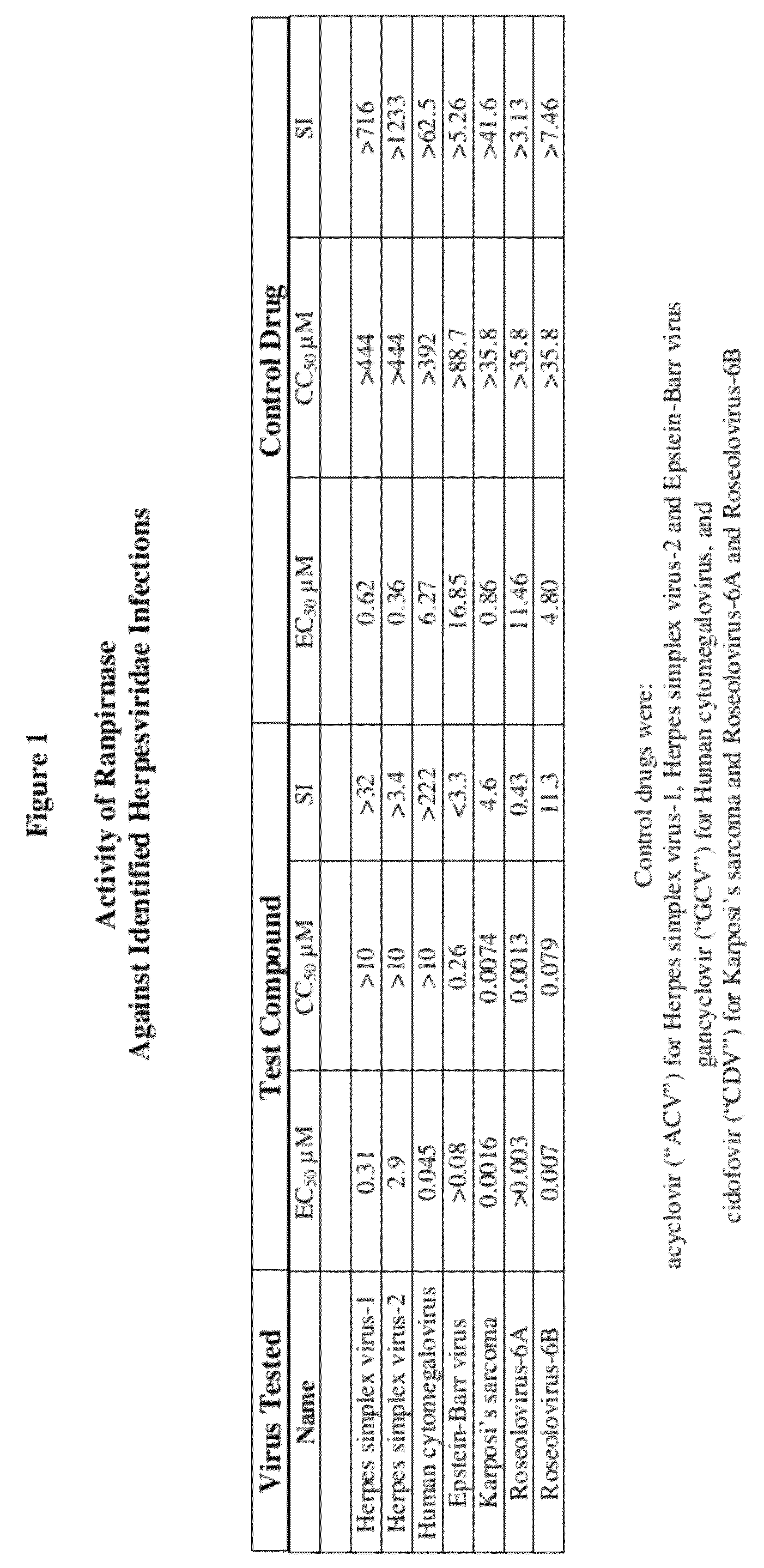
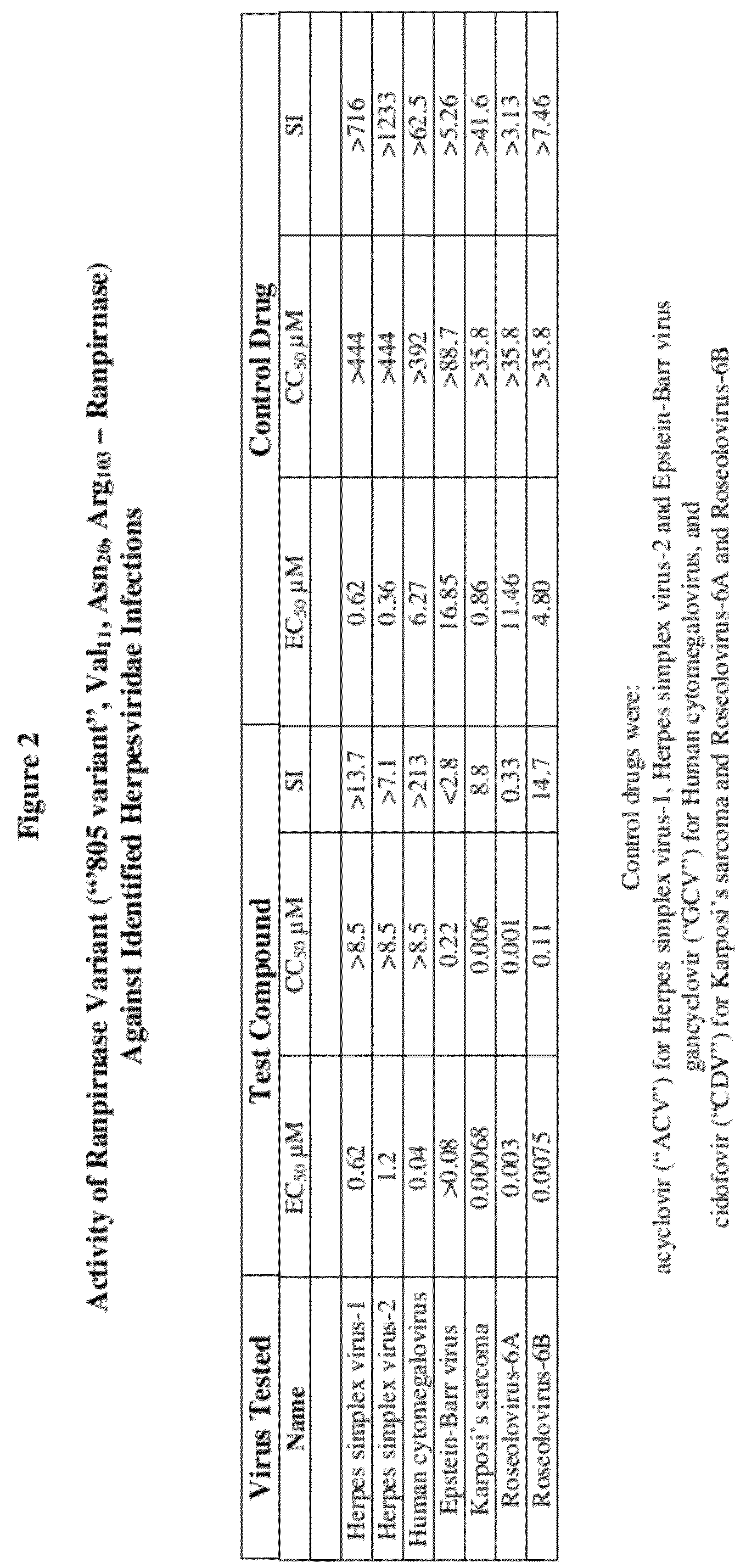
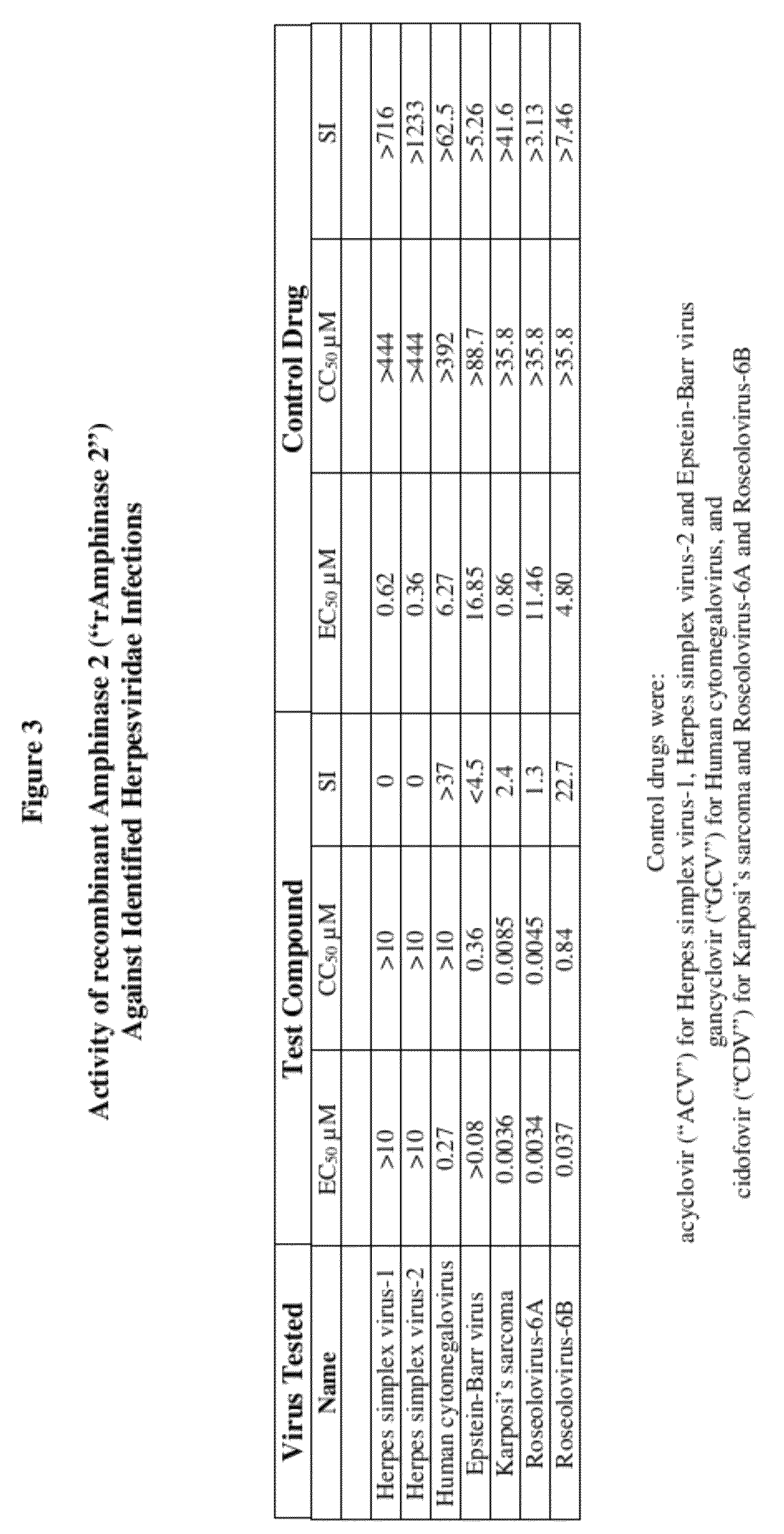
Methods of treating infections originating from viruses in the herpesviridae family
Three RNases (ranpirnase, the second embodiment disclosed in U.S. Pat. No. 5,728,805, and recombinant Amphinase-2) are tested against identified herpesviridae infections. With some exceptions, quantitative PCR assays indicate that the RNases have anti-viral activity against many of these viruses.
Owner:ORGENESIS INC
Method and kit for detection of early cancer or pre-cancer using blood and body fluids
Owner:LI WEIWEI +1
Methods of treating human papillomavirus
Owner:ORGENESIS INC
RT-PCR-based method for determining the content of specific meat components in mixed meat products
ActiveCN102296111AHigh extraction rateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationPcr assayOligonucleotide primers
The invention relates to a RT-PCR based method for determining specific meats in mixed meat products, especially to a method for determining the component content of pork. The invention provides real time fluorescence PCR porcine specific oligonucleotide primers and probe for determining the component content of pork in mixed meat products as well as vertebrate general oligonucleotide primers and probe. Furthermore, the invention also provides a detection kit containing the above porcine specific primers and probes as well as the vertebrate general primers and probe. The method provided by the invention can be adopted to simply, rapidly and accurately determine the porcine-derived DNA content in raw meat or a cooked sample. Based on the correlation between a porcine specific primer system and a general primer system amplification curve, the method provided by the invention has a high accuracy and an anti-interference capability. In addition, the invention has such a strong portability that the component contents of other species in the mixed meat sample can be determined by replacing the specific primer system.
Owner:CHINA MEAT RES CENT
Prognostic PCR assay for severe acute respiratory syndrome (SARS)
InactiveUS20050112558A1Sugar derivativesMicrobiological testing/measurementPcr assaySARS-CoV infection
Disclosed are nucleic acid primers, probes and standard target sequences that allow for the quantitative assessment of the SARS-CoV viral titer found in a biological specimen from a patient. Quantifying the viral titer in the patient sample allows the practitioner to make a prognostic determination of the severity of the SARS-CoV infection and the necessary treatment regime.
Owner:THE CHINESE UNIVERSITY OF HONG KONG +1
Methods of treating infections originating from viruses in the herpesviridae family
Three RNases (ranpirnase, the second embodiment disclosed in U.S. Pat. No. 5,728,805, and recombinant Amphinase-2) are tested against identified herpesviridae infections. With some exceptions, quantitative PCR assays indicate that the RNases have anti-viral activity against many of these viruses.
Owner:ORGENESIS INC
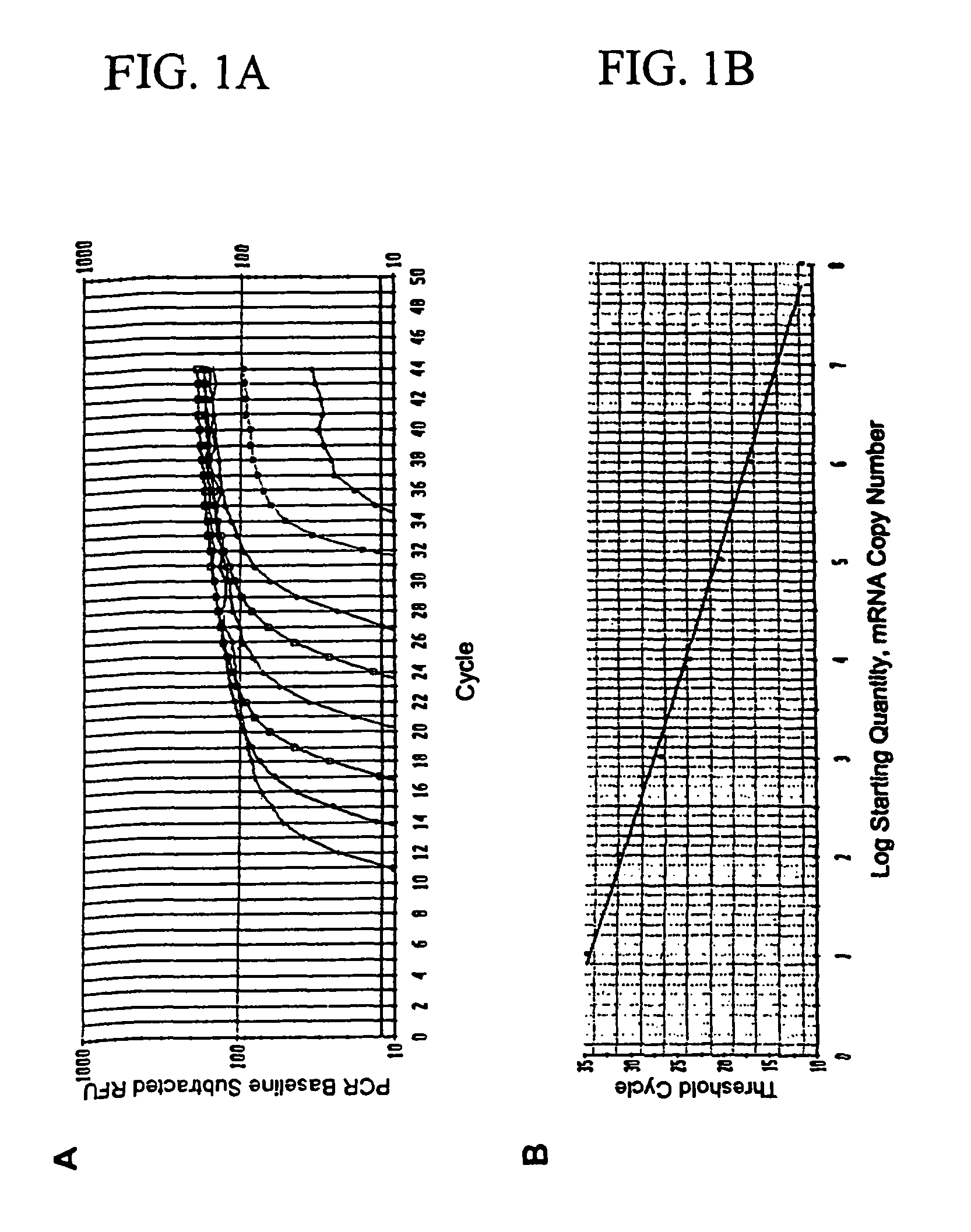
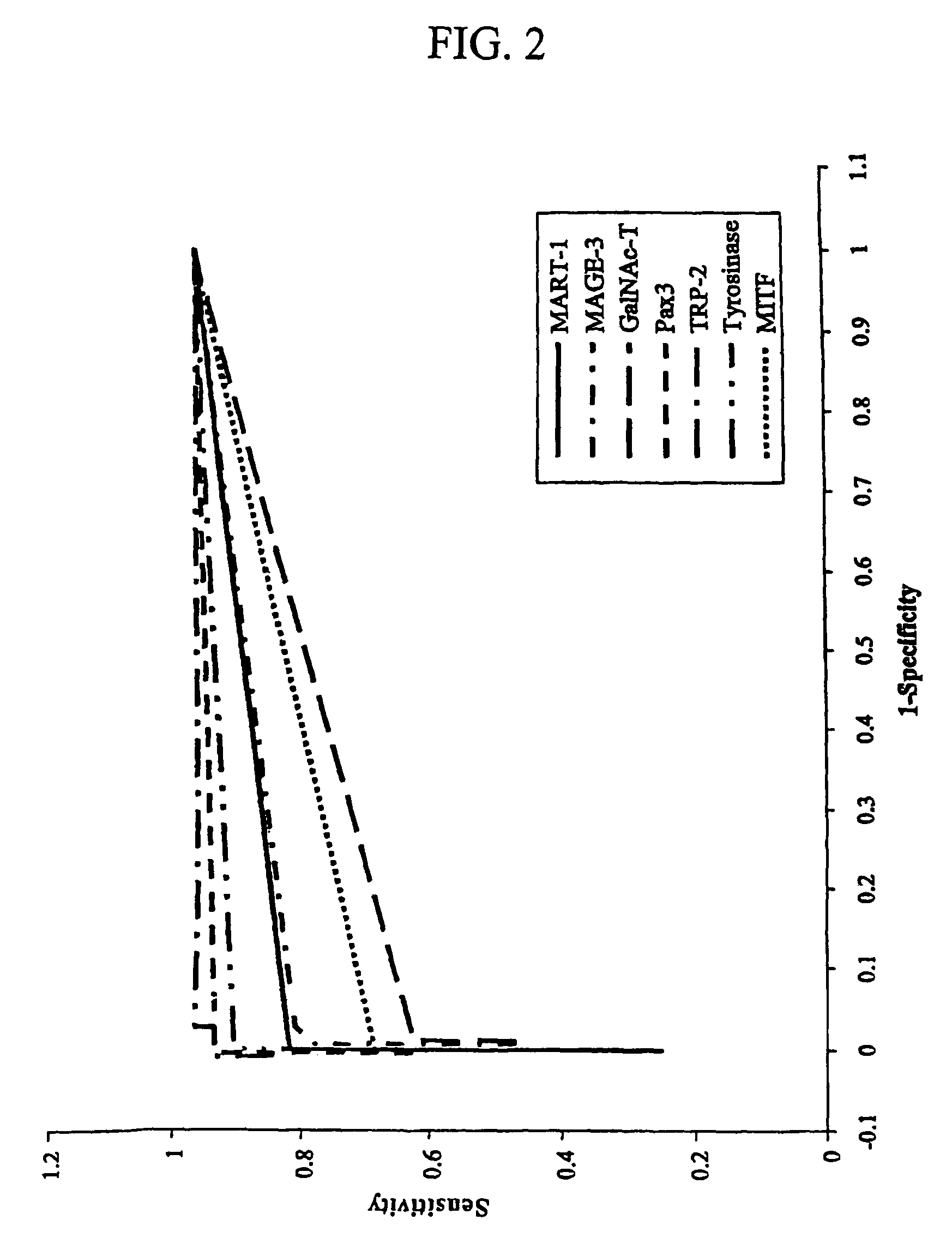
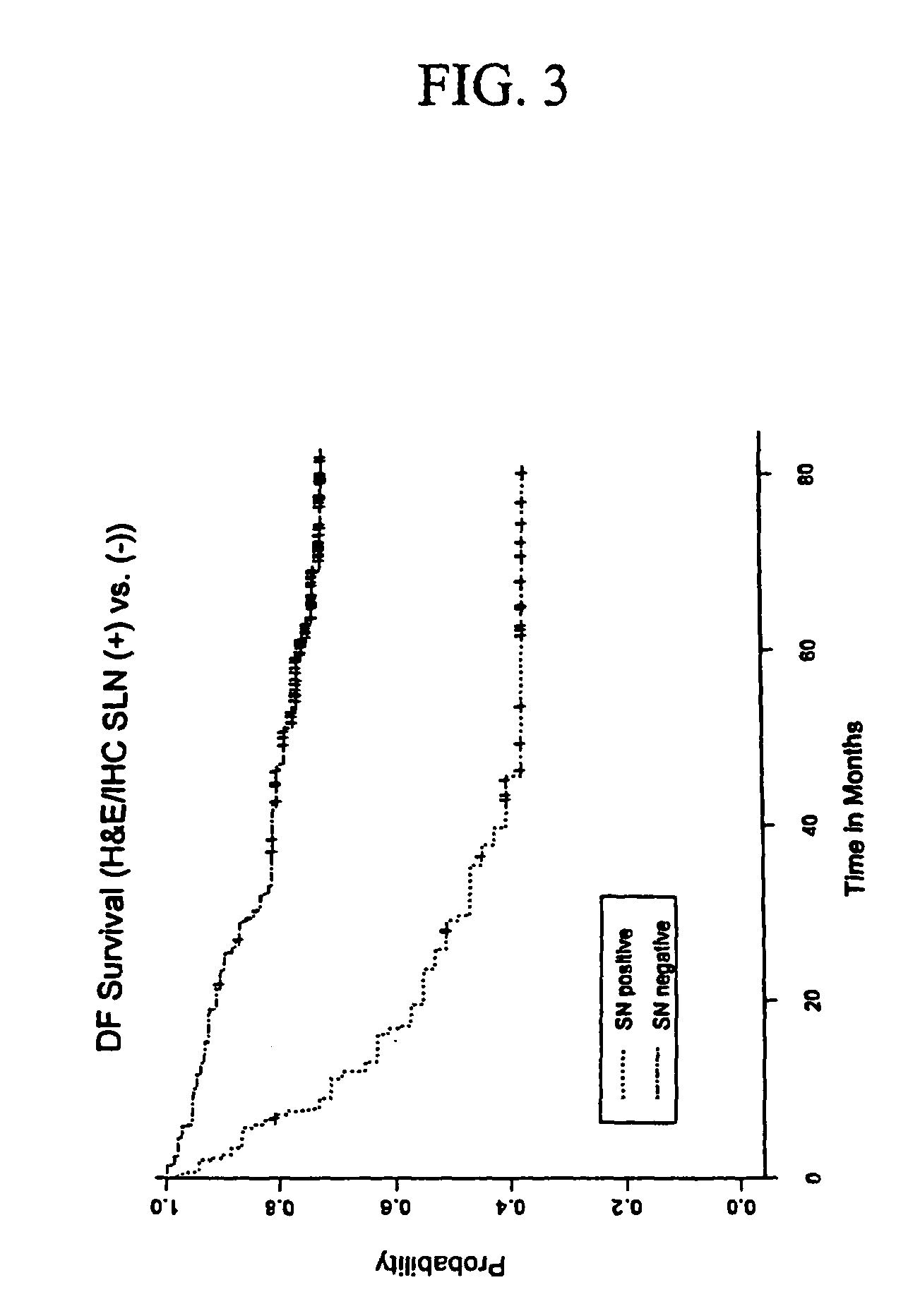
Detection of micro metastasis of melanoma and breast cancer in paraffin-embedded tumor draining lymph nodes by multimarker quantitative RT-PCR
InactiveUS7910295B2High sensitivityStrong specificityMicrobiological testing/measurementBiological testingAbnormal tissue growthMetastatic melanoma
The invention provides a quantitative realtime RT-PCR assay for detection of metastatic breast, gastric, pancreas or colon cancer cells or metastatic melanoma. The assay allows to predict disease recurrence and survival in patients with AJCC stage I and II, and III disease using multimarker panels. The method for detecting metastatic melanoma cells utilizes panels of markers selected from a group consisting of MAGE-A3, GalNAcT, MART-1, PAX3, Mitf, TRP-2, and Tyrosinase. The method for detecting metastatic breast, gastric, pancreas or colon cancer cells in paraffin-embedded samples utilizes panels of markers selected from a group consisting of C-Met, MAGE-A3, Stanniocalcin-1, mammoglobin, HSP27, GalNAcT, CK20, and β-HCG.
Owner:JOHN WAYNE CANCER INST
Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
ActiveUS20050009009A1Reduce the amount of solutionSugar derivativesMicrobiological testing/measurementPcr assaySevere acute respiratory syndrome
The present invention relates to a diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a real-time quantitative PCR assay for the detection of hSARS virus using reverse transcription and polymerase chain reaction. Specifically, the quantitative assay is a TaqMan® assay using the primers and probes constructed based on the genome of the hSARS virus. The invention further relates to a diagnostic kit that comprises nucleic acid molecules for the detection of the hSARS virus.
Owner:HONG KONG THE UNIV OF +1
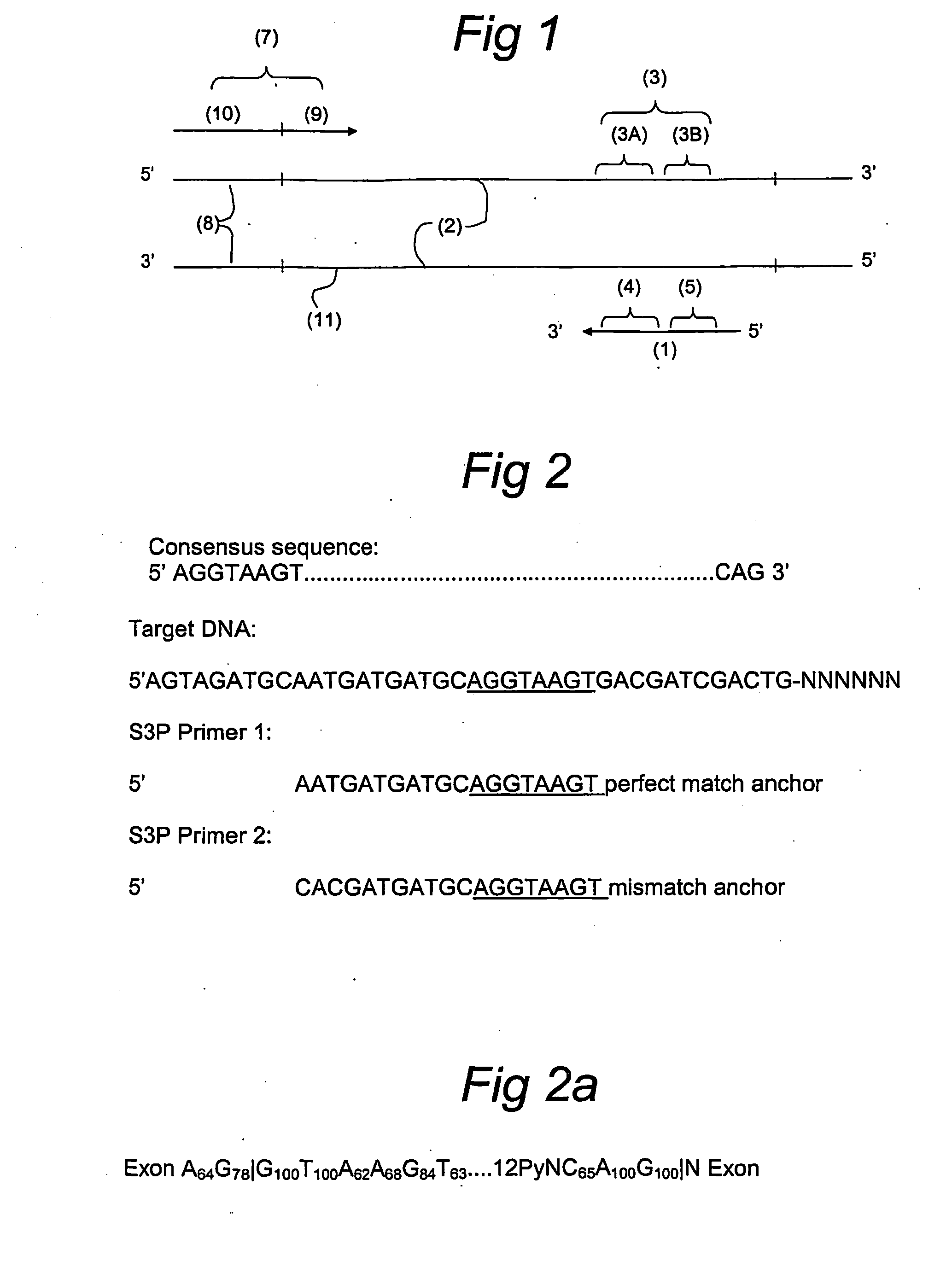
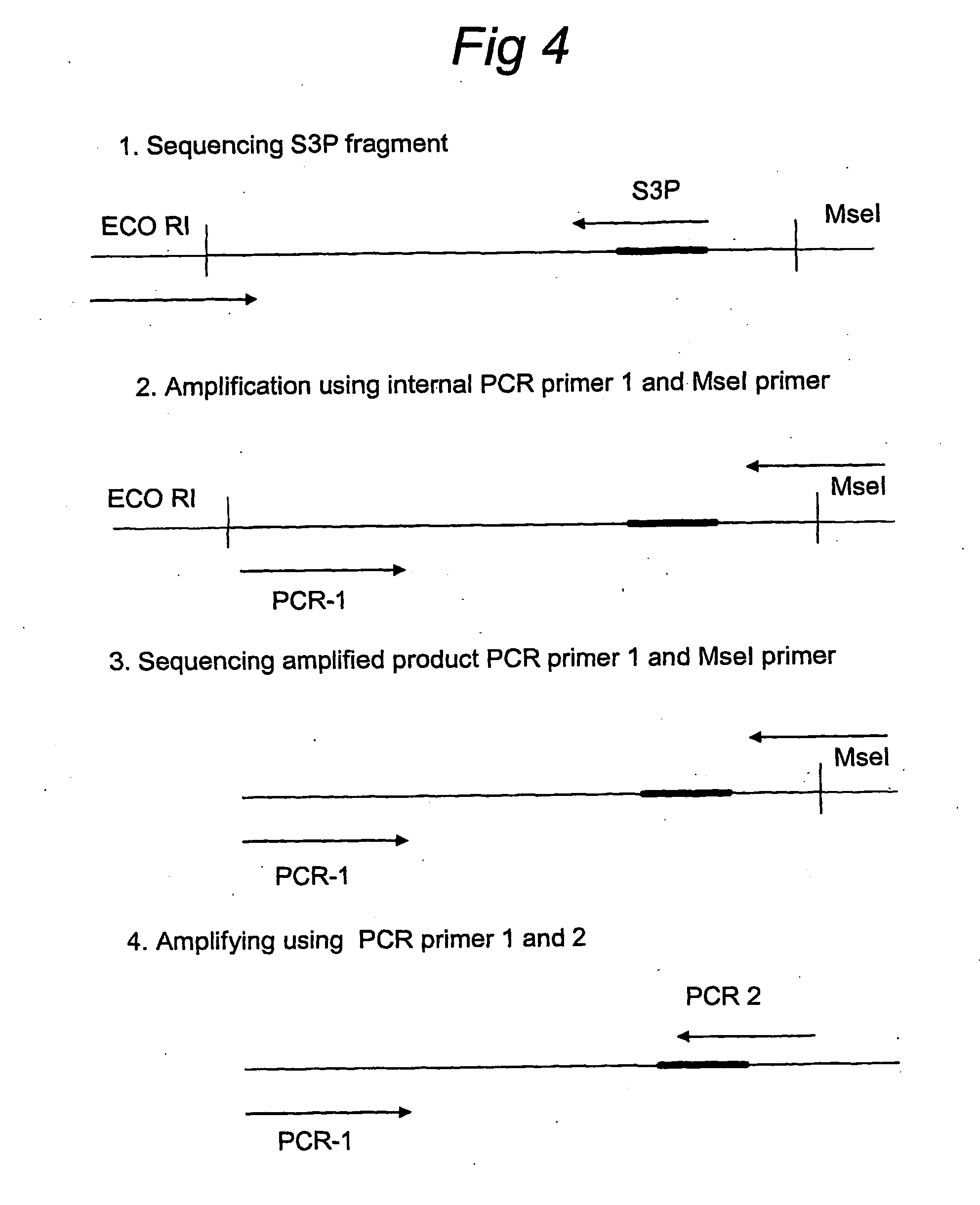
Splice site aflp
InactiveUS20060246457A1Quick identificationReliable and powerfulMicrobiological testing/measurementFermentationPcr assayGene
Splice site-specific primers for increasing the informational content of AFLP fingerprints, a method for splice site specific fingerprinting, and a method for targeting genic regions based on conserved splice sites sequences. The invention further describes the conversion into a PCR assay of the splice site-specific fragments obtained by the method of the invention.
Owner:KEYGENE NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com