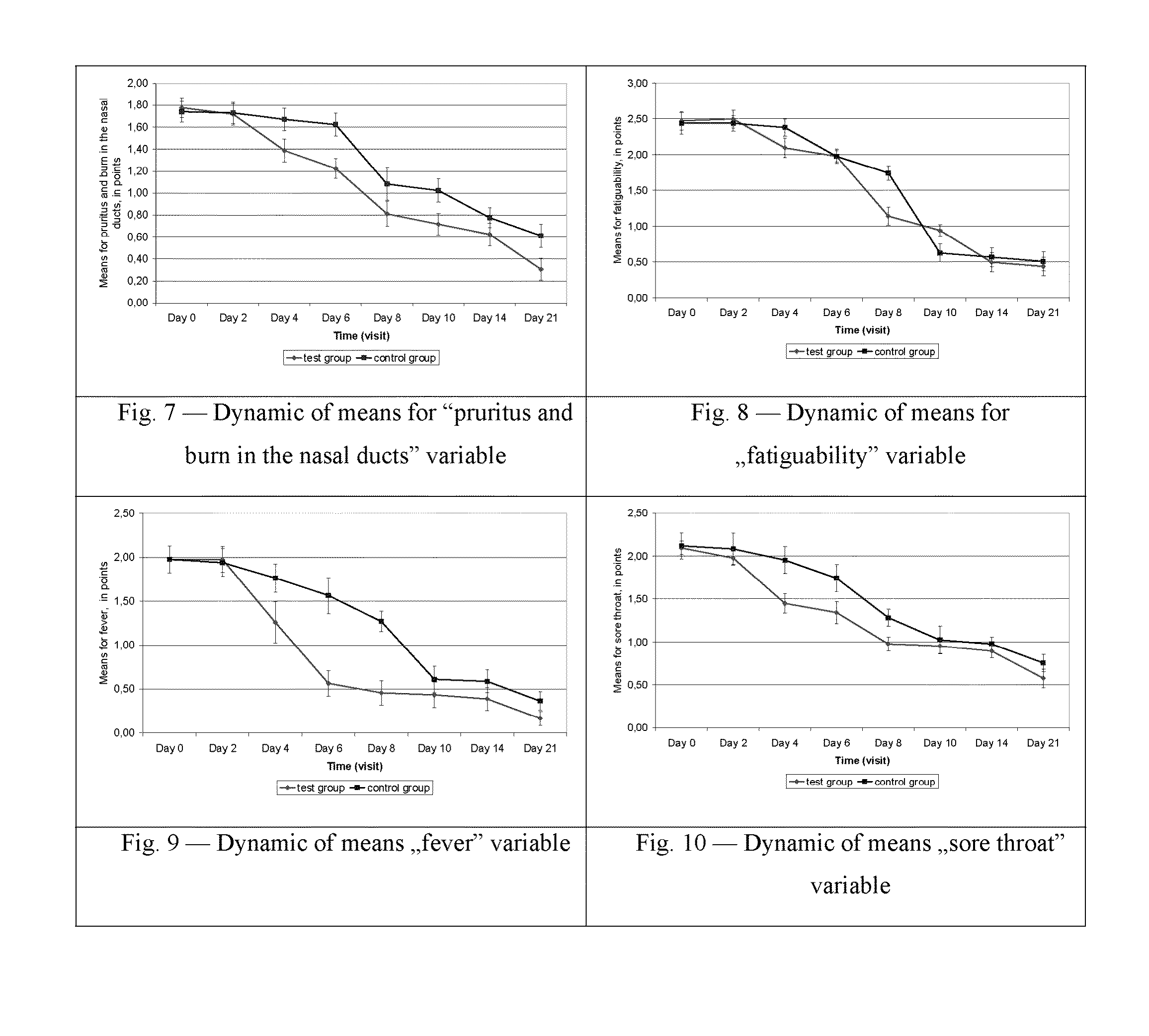
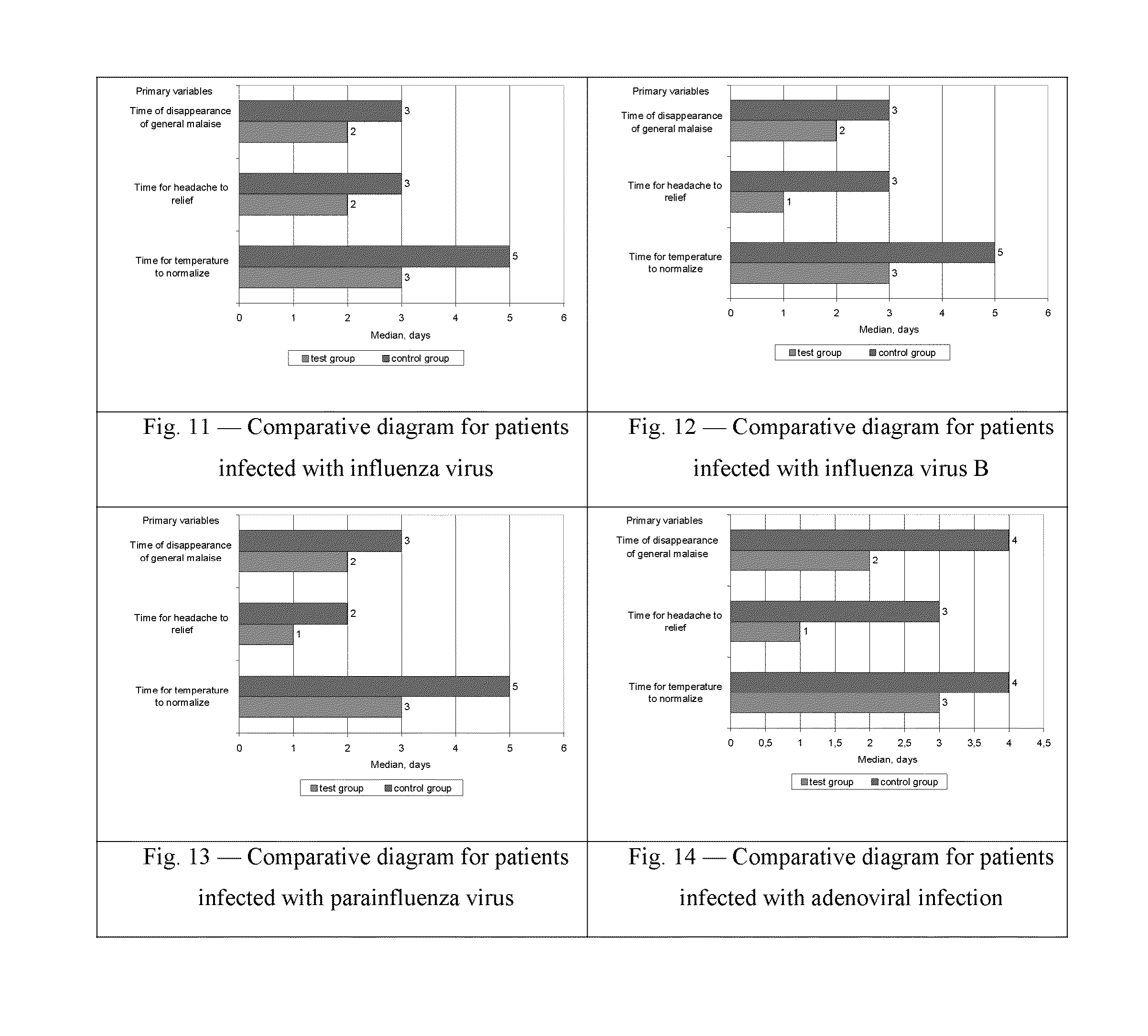
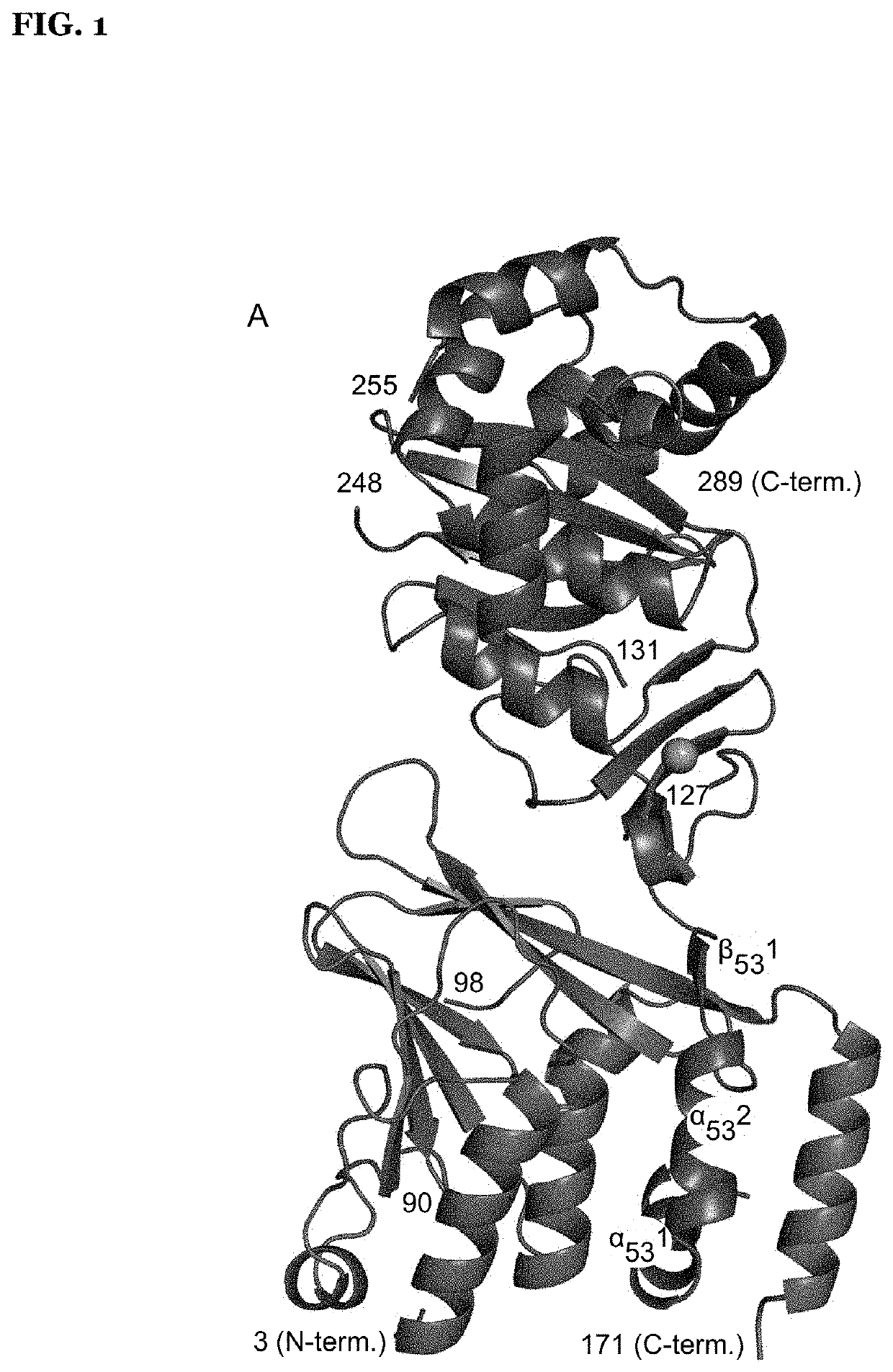
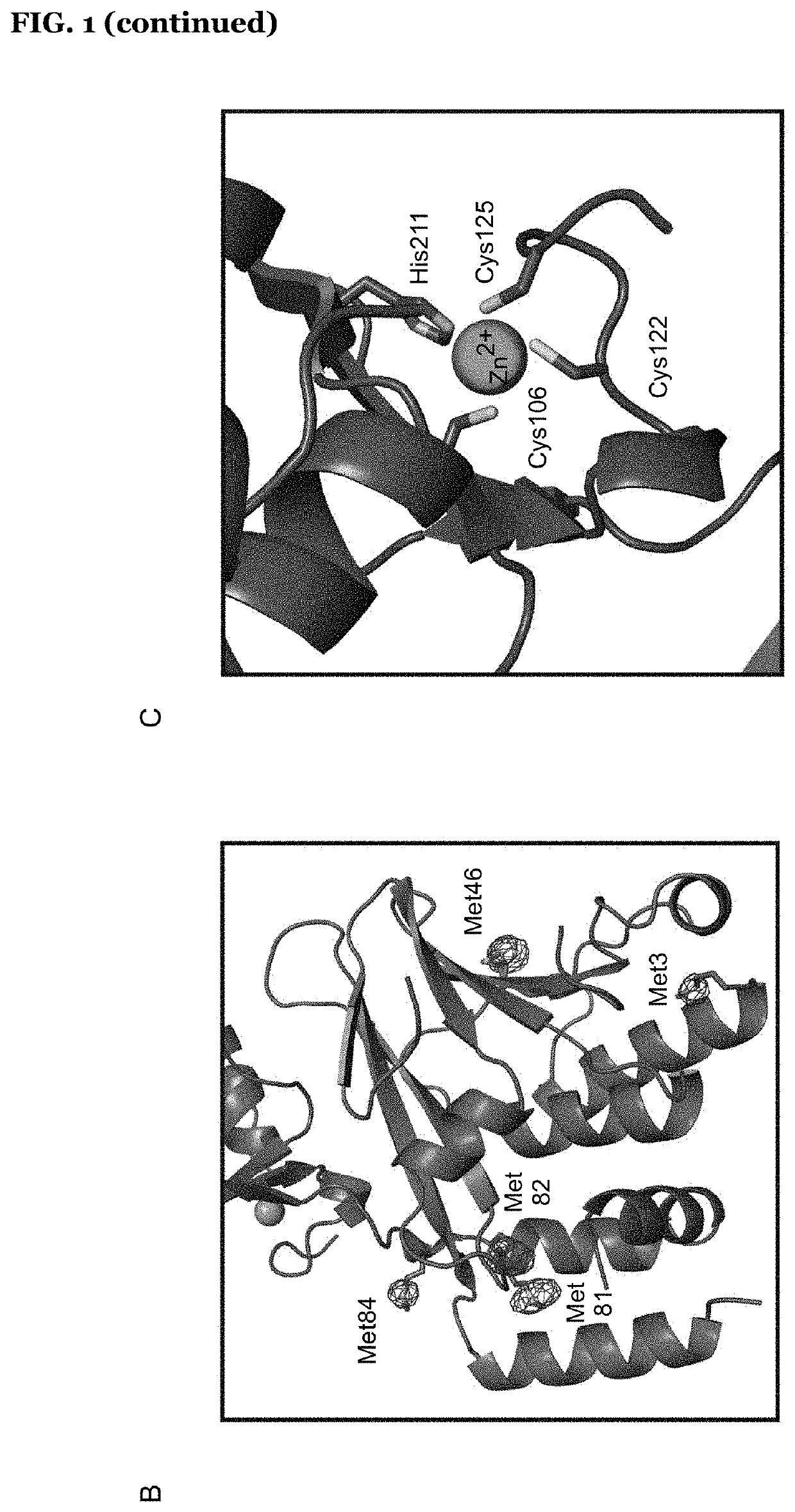
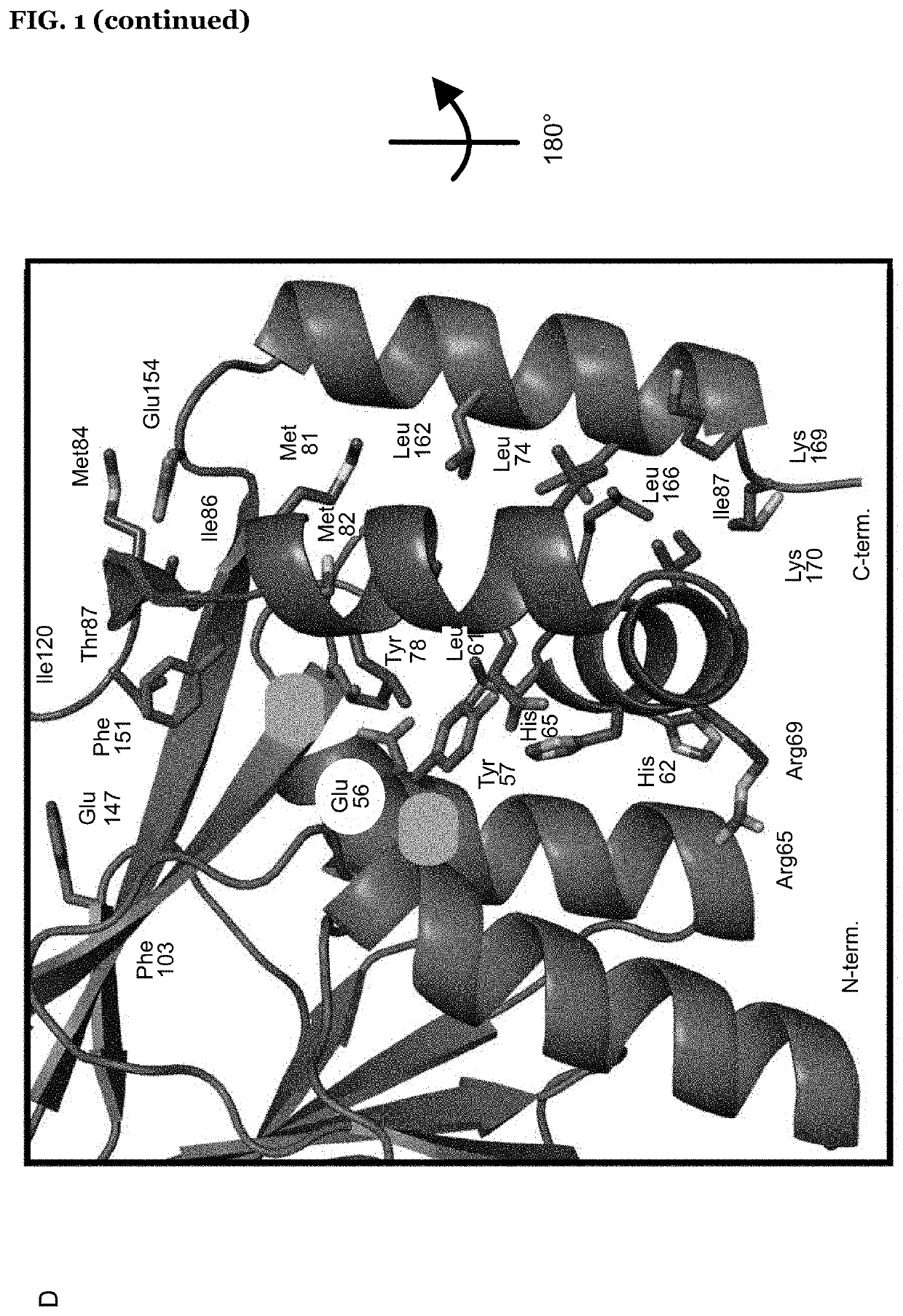
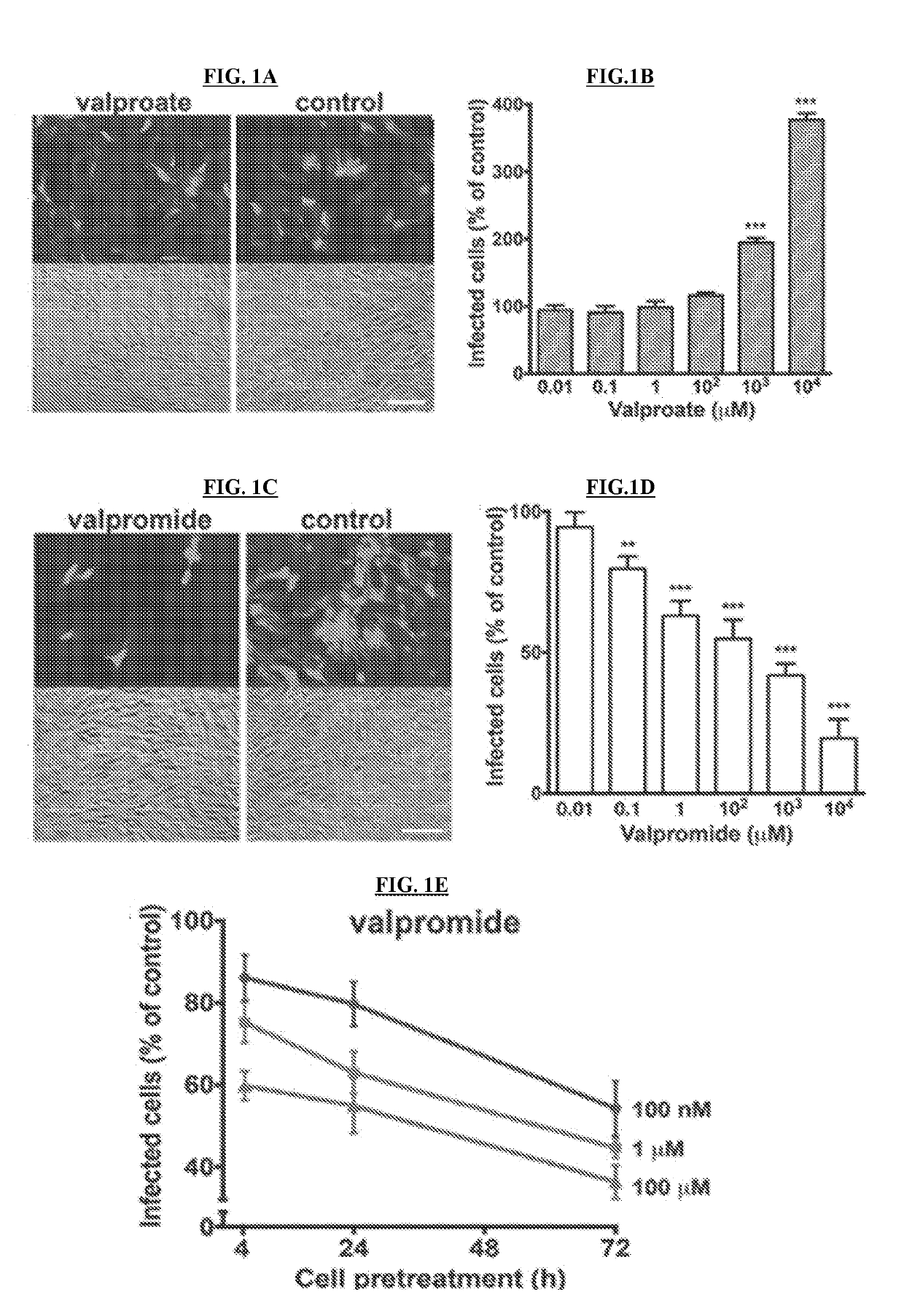
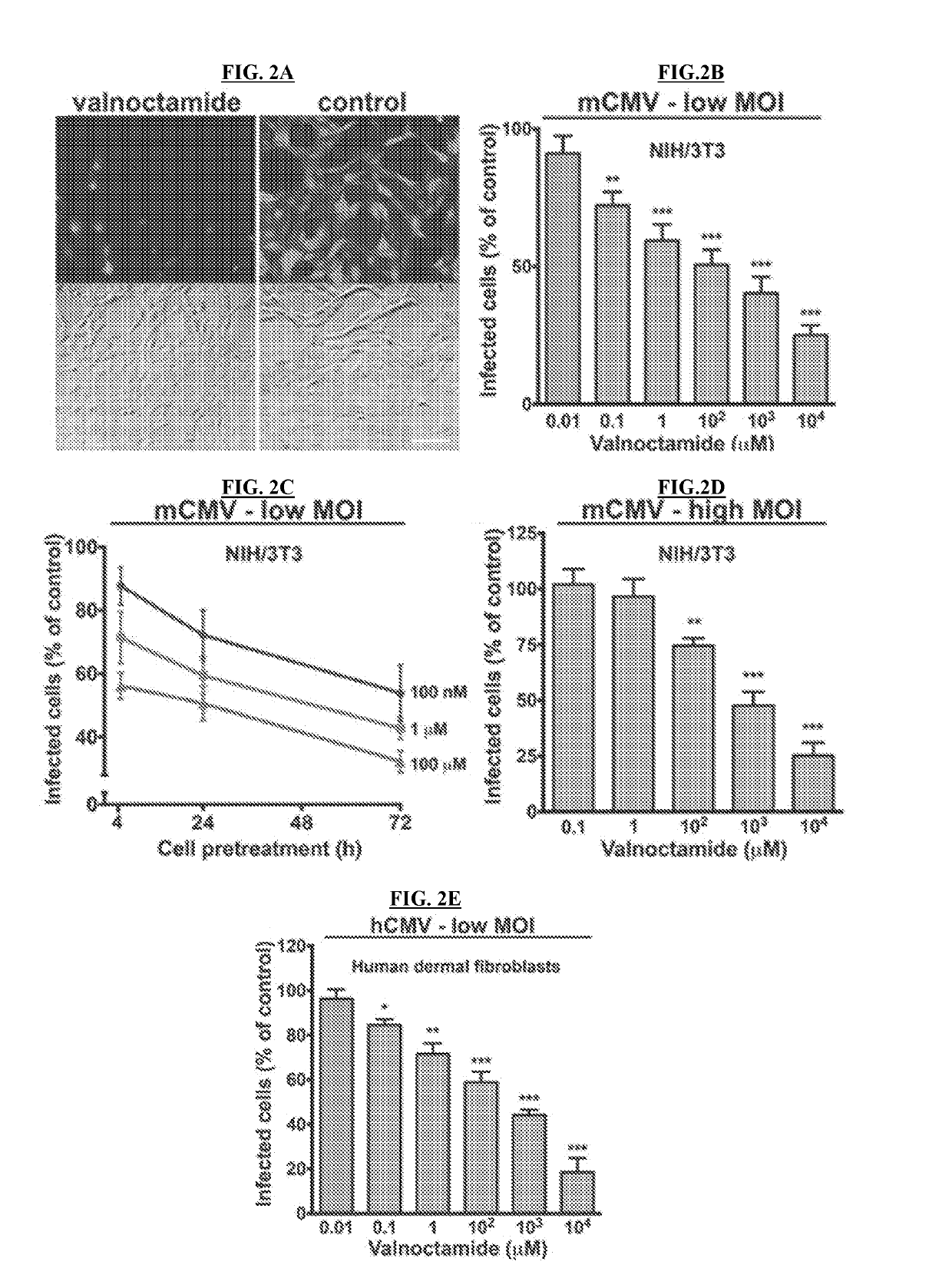
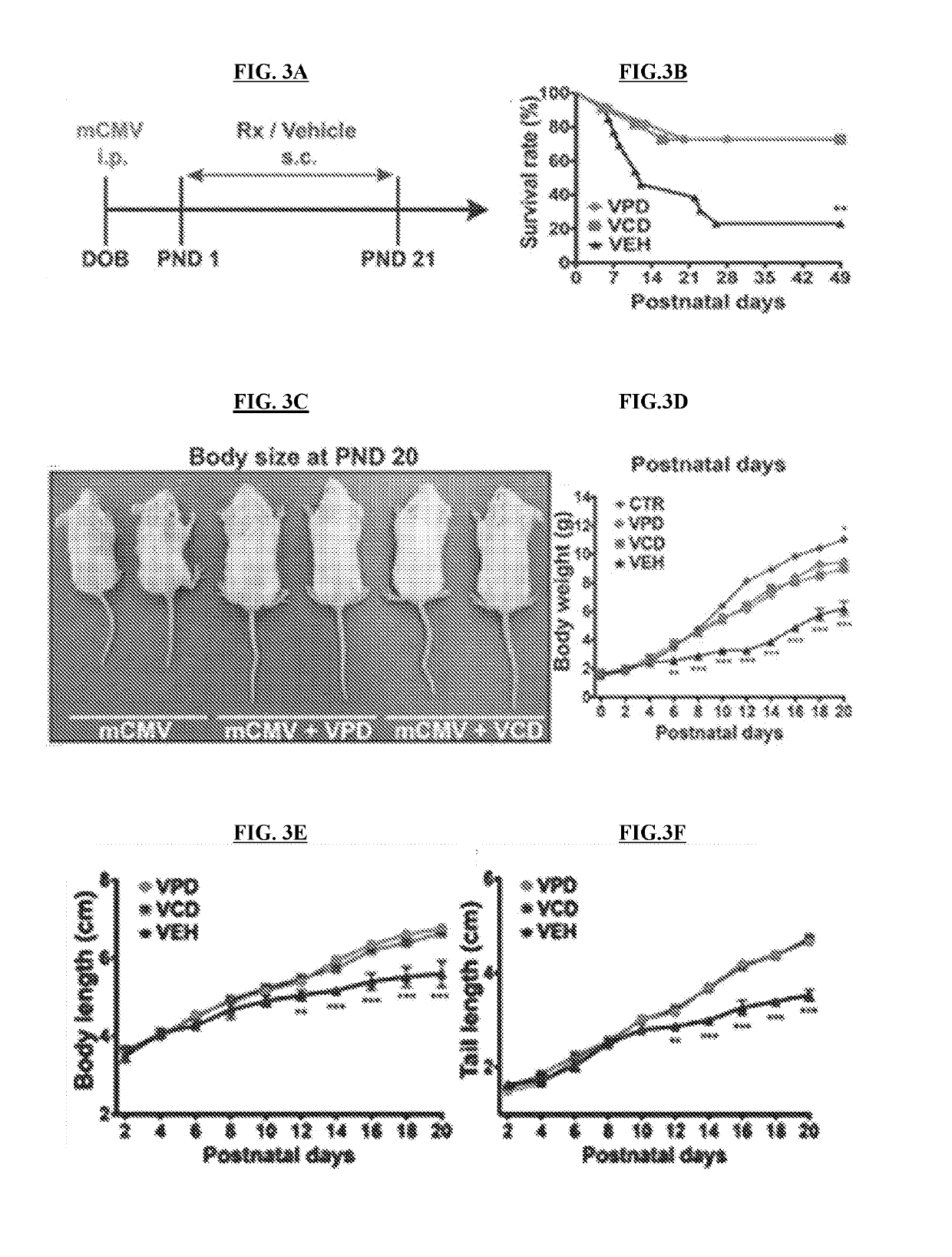
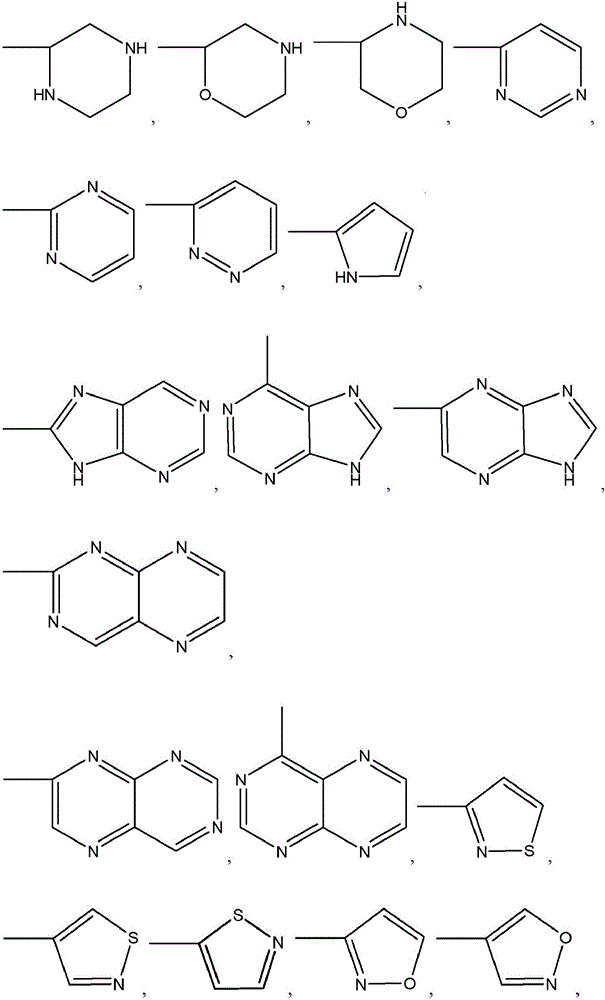
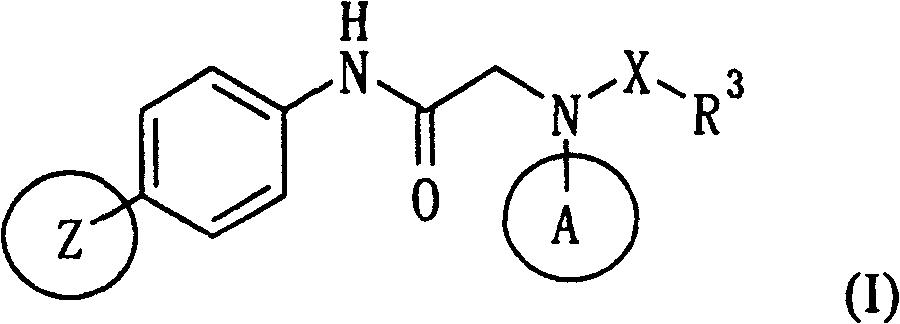
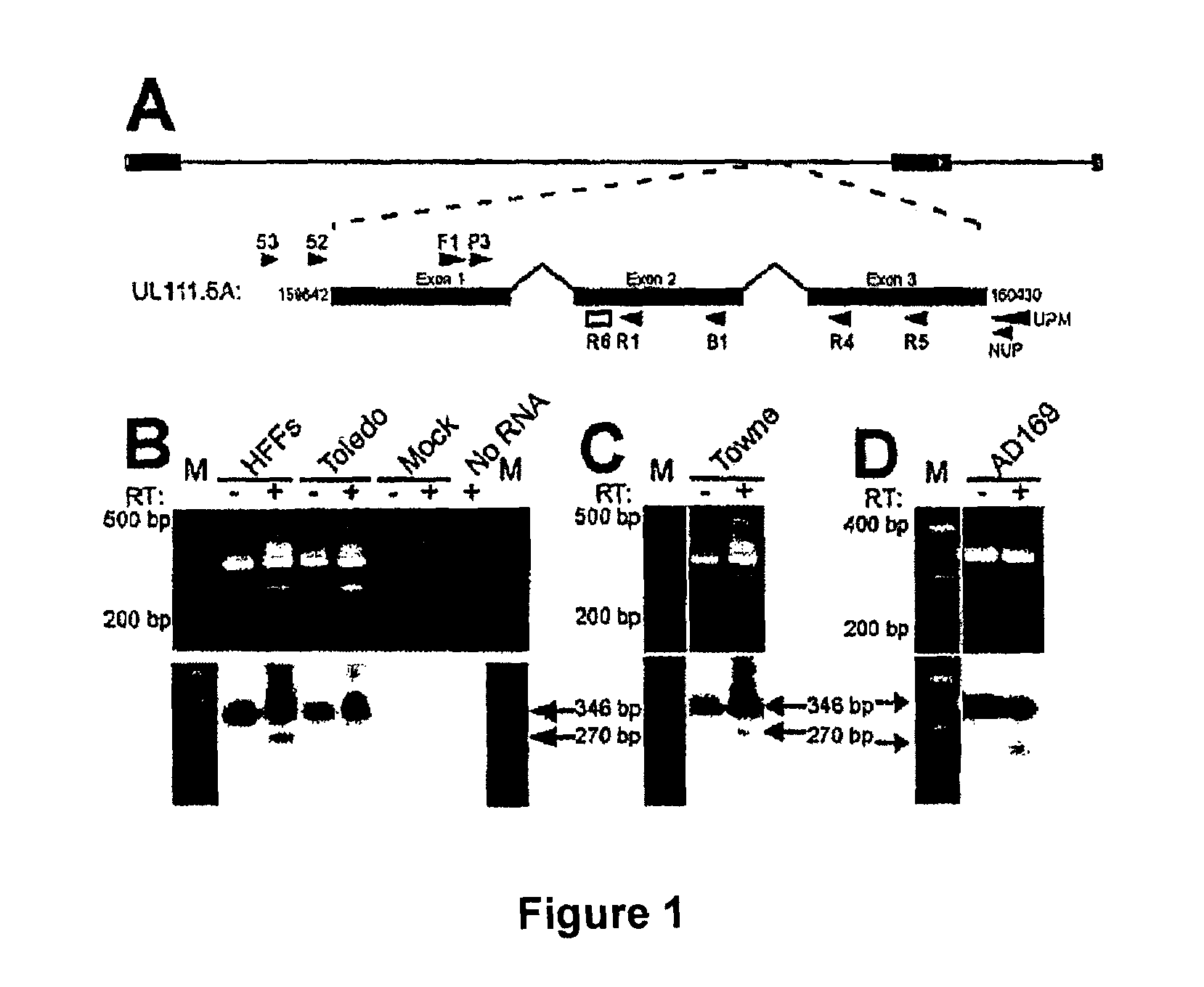
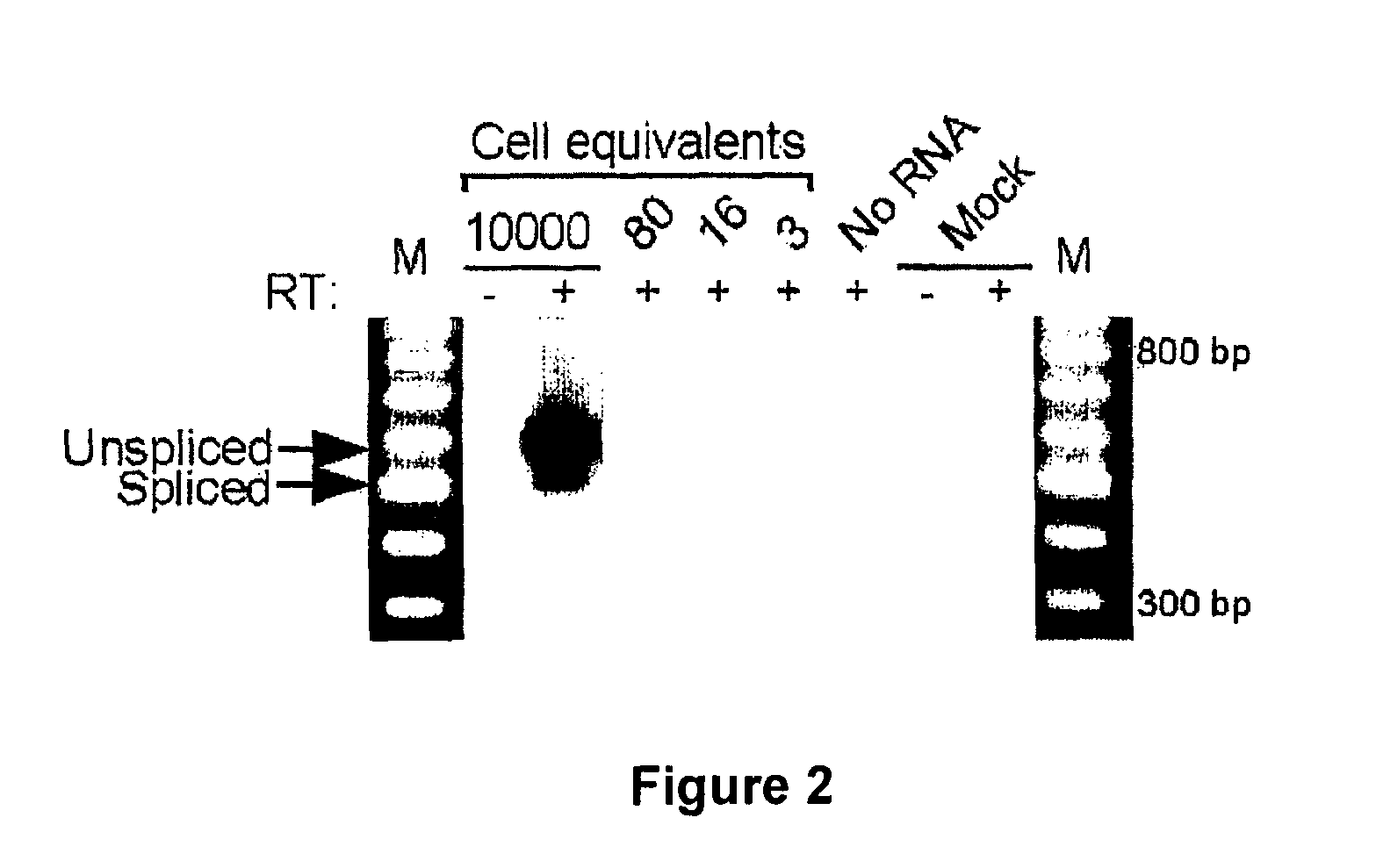
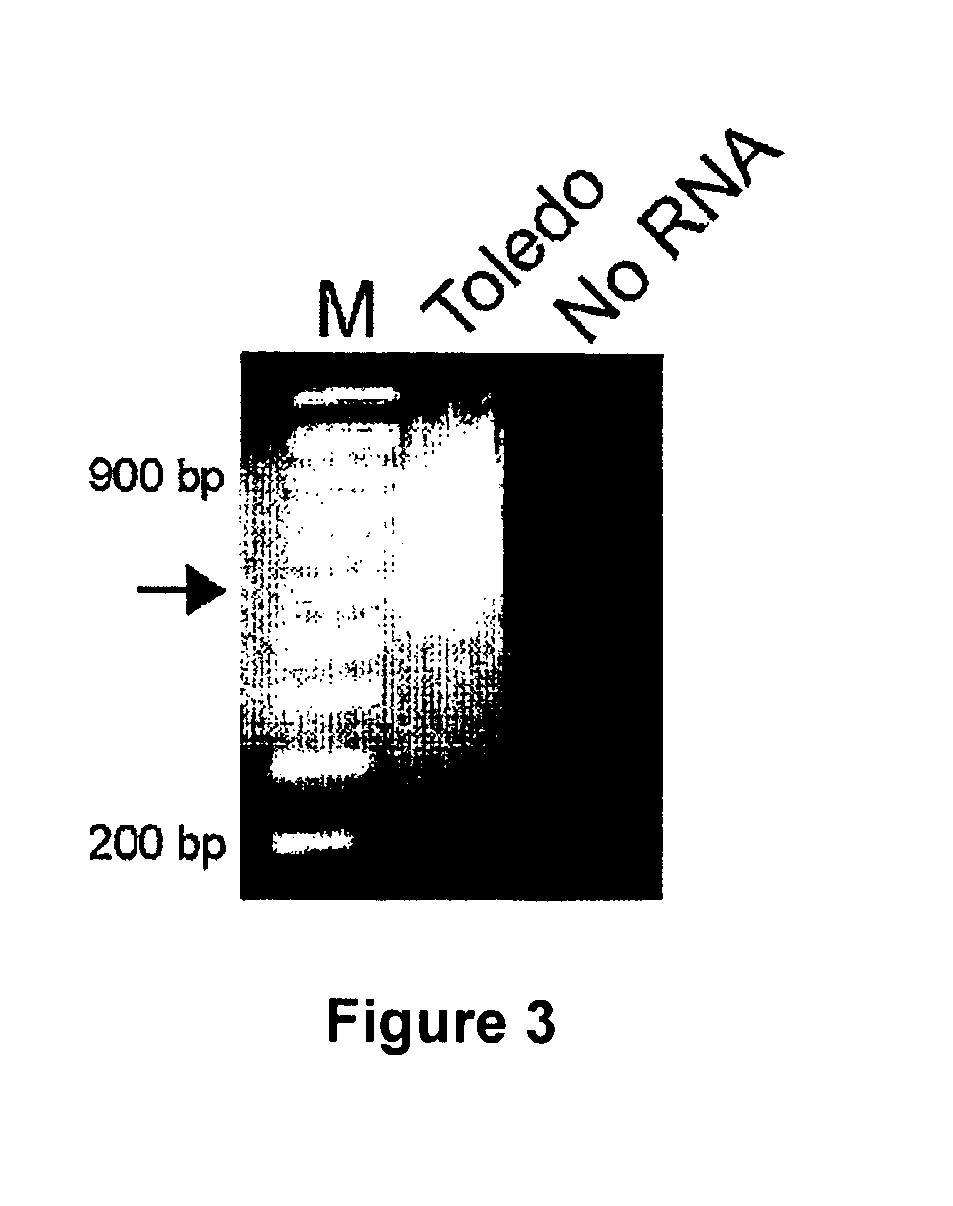
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Herpesviridae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word herpein ("to creep"), referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. Latent, recurring infections are typical of this group of viruses, though the family name does not refer to latency. Herpesviridae can cause latent or lytic infections.
Latent phase viral interleukin-10-(VII-10) and uses thereof
InactiveUS20090214463A1Reduced activityOrganic active ingredientsPeptide/protein ingredientsInterleukin 10Nucleic acid sequencing
The present invention relates to a purified nucleic acid sequence encoding a homologue of human interleukin 10 (IL-10), wherein said IL-10 homologue is expressed during the latent phase of infection by a virus of the herpesvirideae group. The present invention also relates to uses of this polypeptide, in particular for diagnosing disease states and screening for modulator and inhibitor compounds of such polypeptides and in turn the virus itself, screening for infection in vertebrates and biological tissue, cleansing of infected biological tissues, and in the treatment and / or prophylaxis and / or diagnosis of disease caused by a virus of the herpesvirideae group.
Owner:THE UNIV OF SYDNEY
Recombinant porcine pseudorabies virus strain and preparation method thereof
InactiveCN103952379AMicroorganism based processesViruses/bacteriophagesMultiple cloning siteTransfer vector
The invention discloses a recombinant porcine pseudorabies virus strain (belonging to Herpesviridae) and a preparation method thereof. The recombinant porcine pseudorabies virus strain has the accession number of CCTCC NO: V201345. Based on porcine pseudorabies virus vectors, green fluorescent protein labels are used so that green fluorescent protein expression gG-less universal transfer vector PG is constructed, and the recombinant porcine pseudorabies virus strain contains a pseudorabies virus self-late gene gG promoter, a CMV promoter and SV40Poly(A), has upstream flanks of 0.8kb and downstream flanks of 1.7kb and completely satisfies homologous recombination demands. Enhanced green fluorescent protein (EGFP) can be used as a gene expression indicator and comprises porcine IL-18 gene inserted into multiple cloning sites of the EGFP so that connection construction of different expression cassettes by a common vector is realized and two expression cassettes are individually expressed. The exogenous gene expression quantity is closely related to the connection direction of the two expression cassettes.
Owner:HENAN AGRICULTURAL UNIVERSITY
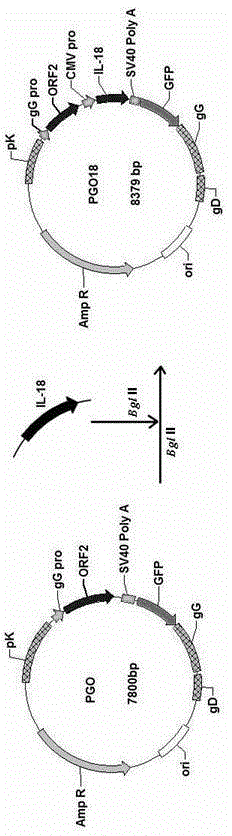
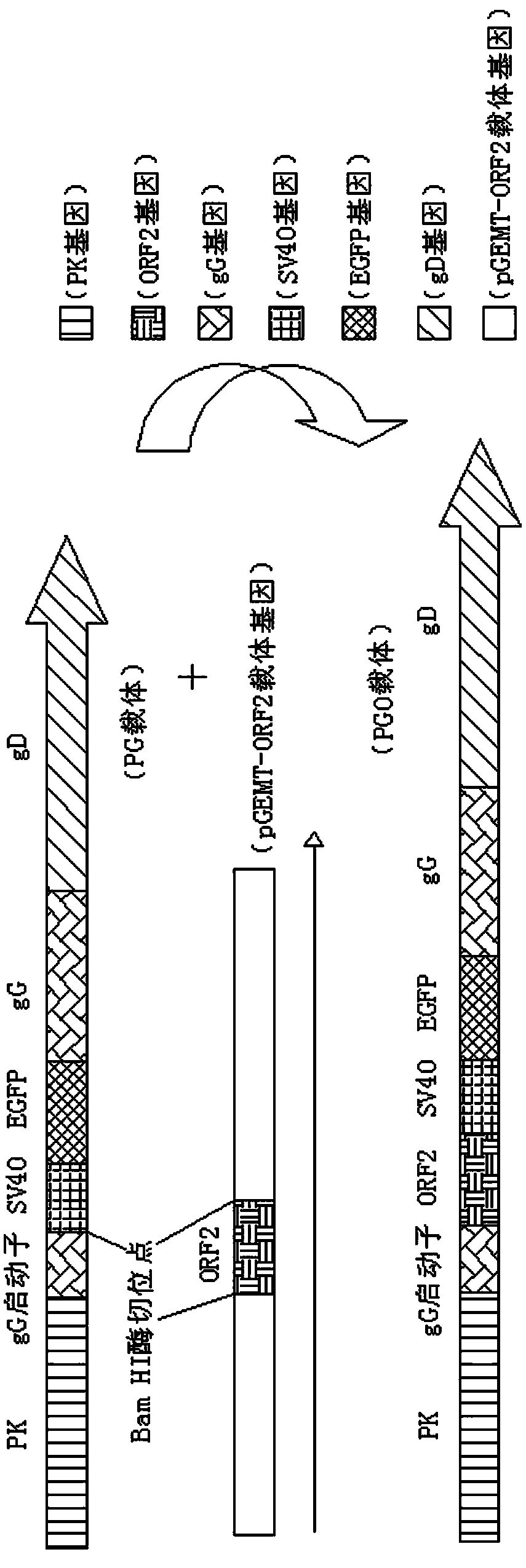
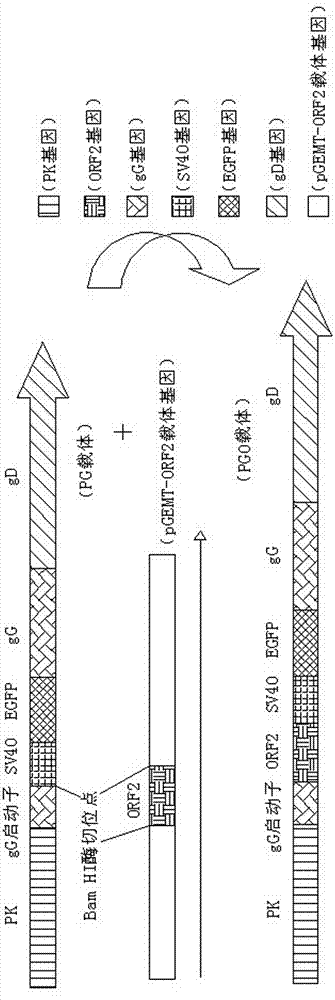
Recombinant porcine pseudorabies virus strain used for expression of porcine circovirus type II (PCV2) ORF2 gene, and preparation method thereof
ActiveCN103509761AHigh antibody titerResist attackMicroorganism based processesViruses/bacteriophagesCircovirusMouse Lymphocyte
The invention discloses a recombinant porcine pseudorabies virus (Herpesviridae) strain used for expression of porcine circovirus type II ORF2 gene. Preservation number of the recombinant porcine pseudorabies virus strain is CCTCC No.V201315. According to the preparation method, PCV2ORF2 gene is inserted into common carrier PG so as to construct transferring plasmid PGO; monolayer ST cells are inoculated with PRVTK<-> / gG<-> / gE<-> virus by 2h of adsorption; ST cells are transfected with plasmid PGO, wherein fusion degree of the monolayer ST cells is 80 to 90%; the recombinant porcine pseudorabies virus PGO strain is obtained by plaque purification, and is used for immunization of mouse. It is shown by results that commercial PCV2 inactivated vaccine and a group immunized by the recombinant porcine pseudorabies virus PGO strain are both capable of inducing specific humoral immune response of mouse, antibody titers of both the commercial PCV2 inactivated vaccine and the group are obviously higher than that of a group immunized by DMEM medium, and difference is significant (p<0.05). It is shown by the results of mouse lymphocyte proliferation test that specific ceullar immune response caused by the group immunized by the recombinant porcine pseudorabies virus PGO strain is more obvious than that caused by the commercial PCV2 inactivated vaccine and the group immunized by DMEM medium, and difference is obvious (p<0.05). In addition, the group immunized by the recombinant porcine pseudorabies virus PGO strain is capable of resisting severe attack by PCV2. Therefore application of the recombinant porcine pseudorabies virus strain in development of a novel PCV2 vaccine is possible.
Owner:HENAN AGRICULTURAL UNIVERSITY
Varicella attenuation live vaccine
ActiveCN101161286AImprove securityHigh viral titerAntiviralsAntibody medical ingredientsInfected cellKanamycin
The present invention relates to herpes virus family medical microorganism. The present invention is prepared by the following method: (1) serial passage MRC-5 cell; (2) inoculating to cell and continuous infecting; (3) adding culture solution; (4) washing cell surface; (5) eluting infected cell, centrifugal separating, adding vaccine fluid containing stabilizing agent to refrigerate; (6) ultrasonic crashing and centrifugal separating to acquire virus stock solution; (7) combining and diluting, and at once processing to pack and freeze-dry, preparing attenuated pox vaccine. The stabilizing agent is prepared by dissolving NaCl, KCl, KH2PO4, Na2HPO4.12H2O, saccharose, sodium glutamate, kalamycin, erythromycin and deionized water into the water with weight ratio 3:0.1:0.2:3.3:50:0.4:0.1:0.03:1000. The present invention has good advantages of safety and immunogenicity.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
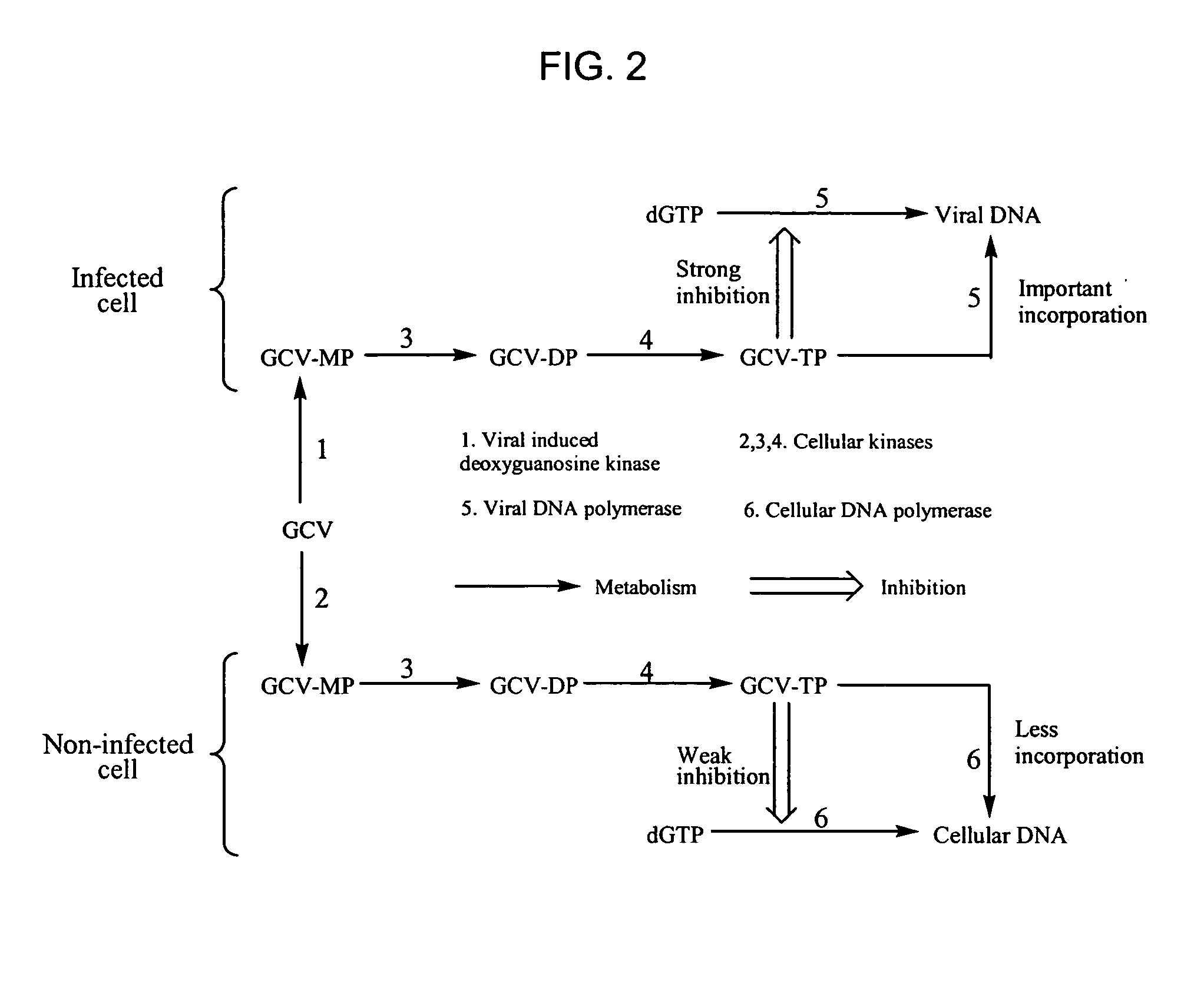
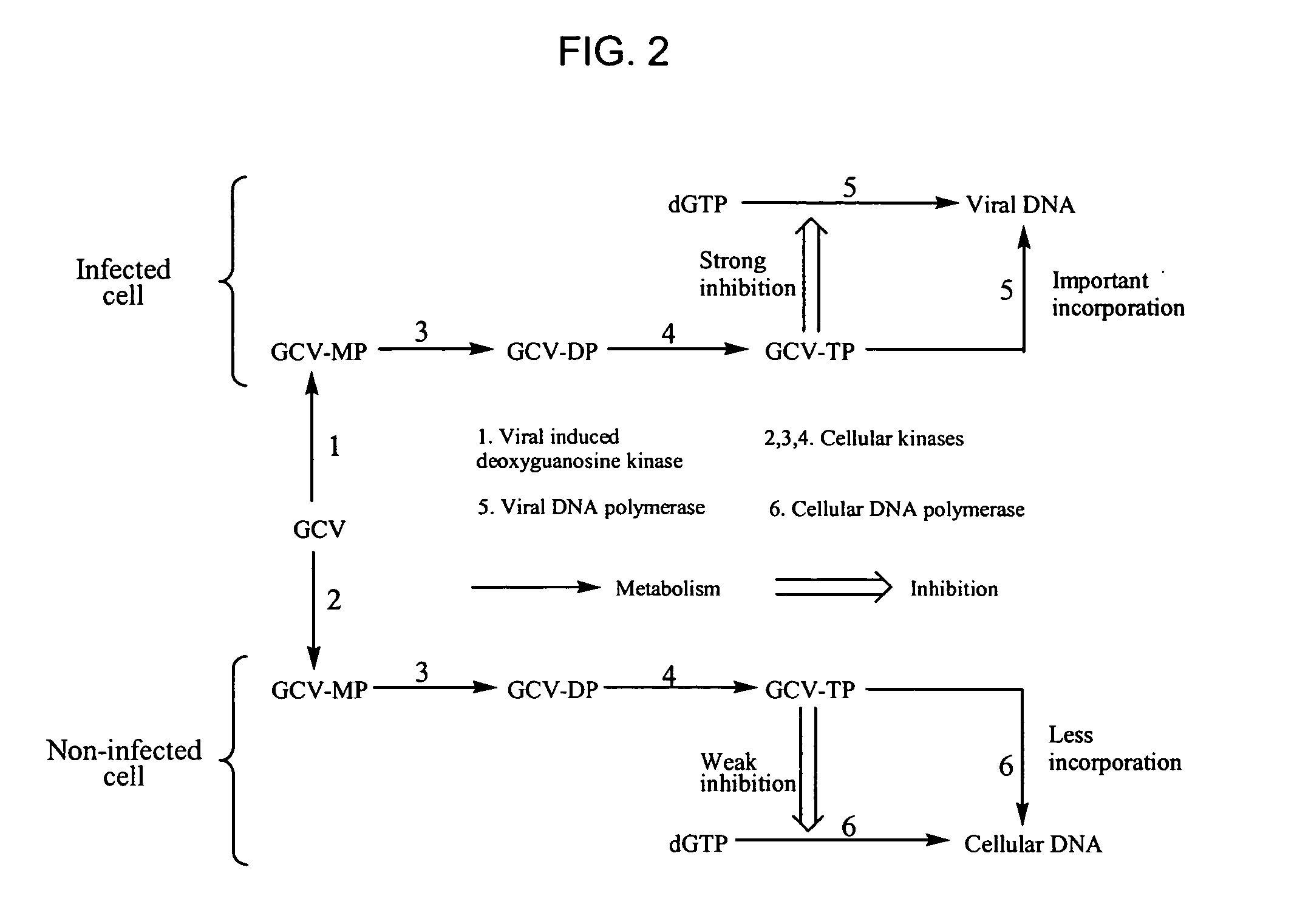
Thymidine Kinase Mutants and Fusion Proteins Having Thymidine Kinase and Guanylate Kinase Activities
InactiveUS20090298156A1Improve biological activityAntibacterial agentsOrganic active ingredientsBinding siteMutant
The present invention provides isolated nucleic acid molecules encoding a Herpesviridae thymidine kinase enzyme comprising one or more mutations, at least one of the mutations encoding an amino acid substitution located toward the N-terminus from a DRH nucleoside binding site which increases a biological activity of the thymidine kinase, as compared to unmutated thymidine kinase. Such mutations include amino acid substitutions within a Q substrate binding domain which increases a biological activity of the thymidine kinase, as compared to unmutated thymidine kinase. Within a further aspect, fusion proteins are provided which have both guanylate kinase and thymidine kinase biological properties. Also provided are vectors suitable for expressing such DNA molecules, as well as methods for utilizing such vectors.
Owner:DARWIN MOLECULAR CORP
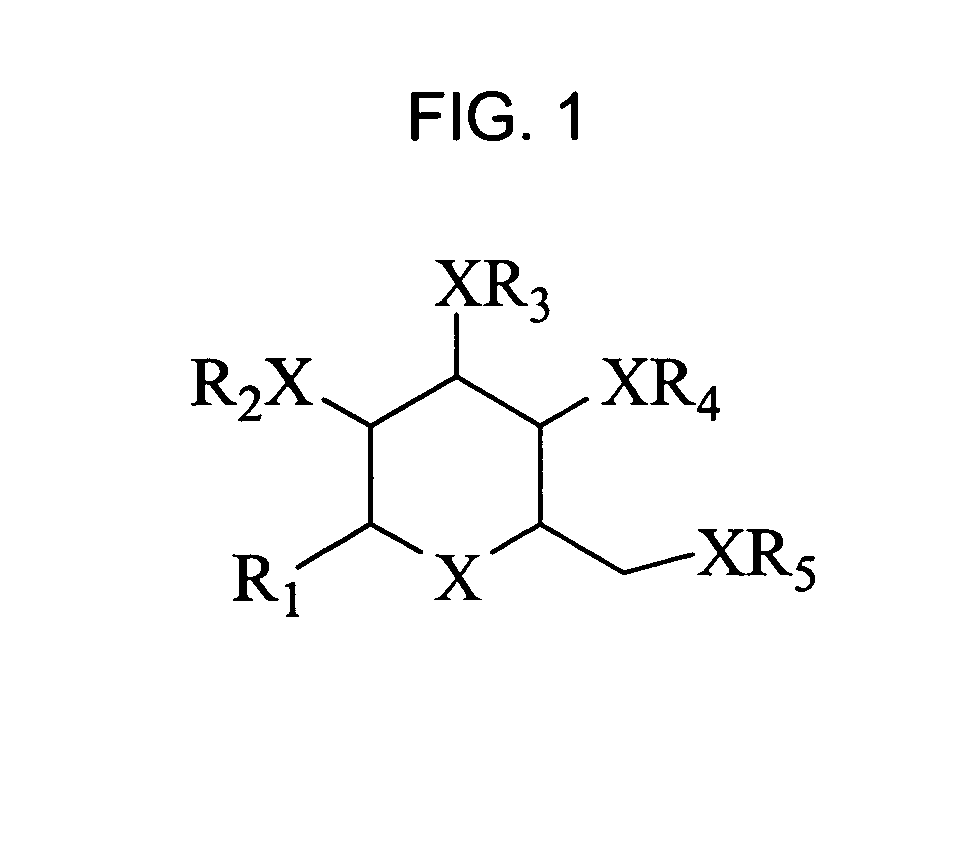
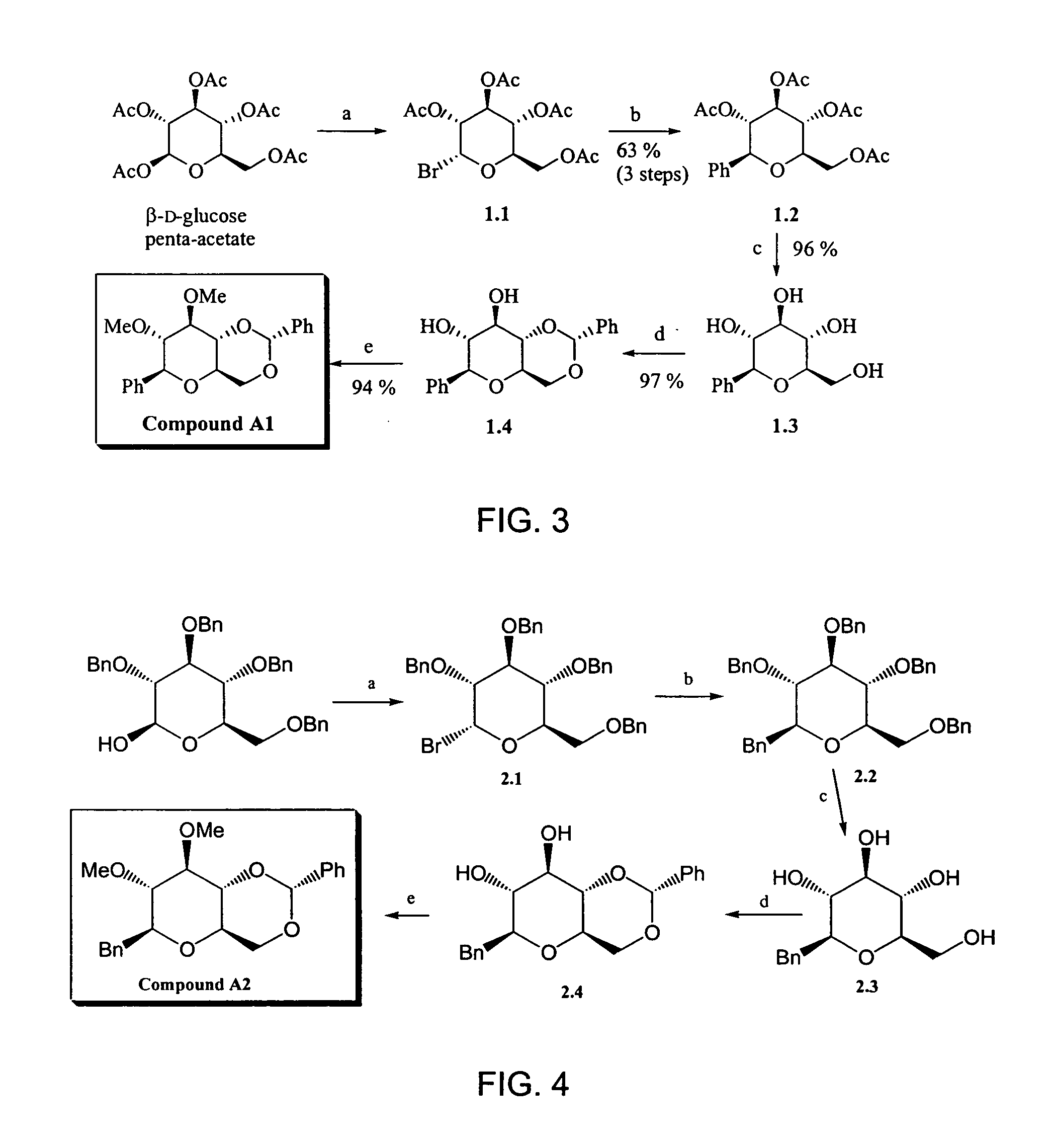
Bicyclic carbohydrate compounds useful in the treatment of infections caused by herpesviridae
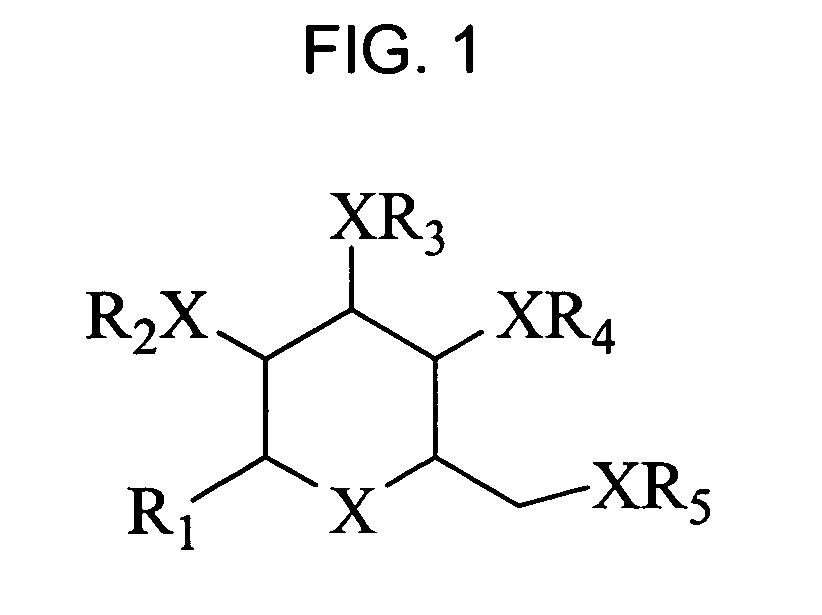
Bicyclic carbohydrates for the treatment of infections caused by herpseviridae, and in particular cytomegalovirus. The invention consists of the novel bicyclic carbohydrates the generic structure of which is: wherein R1 is either -Bn or -Ph; R2 and R3 are either -alkyl, -aryl, -allyl, or —H; R4 and R5 form a ring and are either —CH(Ph)- or —CH(aryl)- and X is either O, N or S.
Owner:KEMIN PHARMA EUROPE B.V.B.A.
Use of Carex rigescens in preparing antivirus medicament
The invention discloses an application of carex rigescens in preparing antivirus medicine. The testing shows that the carex rigescens has significant inhibitory effects to HSV-1, HSV-2, HCMV, RSV and flu A, which implicates that the carex rigescens has potential application value in remedying various virus infectious disease fields. The carex rigescens has especially important therapeutic significance for infectious diseases of herpesviridae (HSV-1, HSV-2, HCMV). The application provides an experimental basis for the carex rigescens clinically applying in remedying virus infectious diseases, provides a certain guide significance for developing medicines which can against HSV-1, HSV-2, HCMV, RSV AND Flu A virus, and has significant reference value.
Owner:高川
Preventive or therapeutic agent for herpesvirus-related disease
InactiveCN101111247AImprove securityReduce adverse effectsOrganic chemistryAntiviralsDiseaseMedicine
To provide a medicine, particularly a pharmaceutical composition useful for preventing or treating a variety of diseases caused by infection of a virus of the Herpesviridae family. [MEANS FOR SOLVING PROBLEMS] The present invention relates to an anti-herpesvirus agent characterized by combining a helicase-primase inhibitor with a polymerase inhibitor. By combining the helicase-primase inhibitor with the polymerase inhibitor having different action mechanisms, the anti-herpesvirus agent of the present invention achieves a dramatically superior anti-herpesvirus action compared with conventional administration of a polymerase inhibitor alone. Therefore, it is particularly effective in a case in which a sufficient therapeutic effect cannot be achieved only with a polymerase inhibitor. Further, the doses of both agents can be kept low, therefore it is also possible to perform treatment by lowering the effect of an adverse reaction to be concerned.
Owner:ASTELLAS PHARMA INC
A method of treatment or prophylaxis of symptoms of herpes viral infection
A method is provided for the treating or prophylaxis of one or more symptoms of viral infection. The method involves the administration of citrate and / or succinate salts. The present invention also encompasses the use of citrate and / or succinate salts for the treatment or prevention of lesions or blisters, or other symptoms of infection by members of the herpes virus family.
Owner:PENAM INVESTMENTS PTY LTD
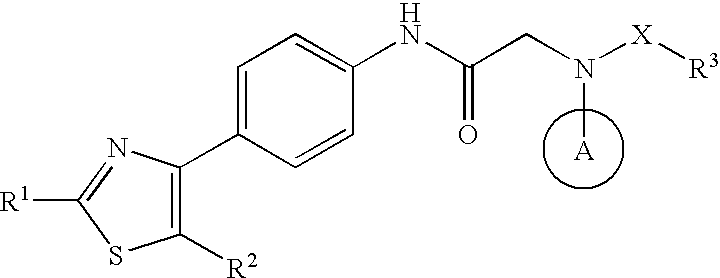
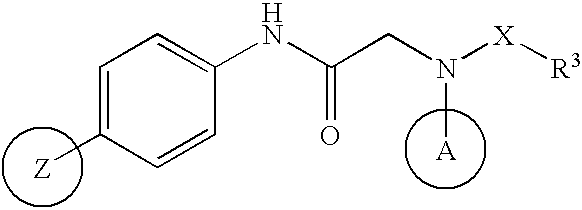
Amide derivative
InactiveUS6949543B2High activityEasy to produceCosmetic preparationsBiocideRecurrent infectionsVaricella-zoster virus infection
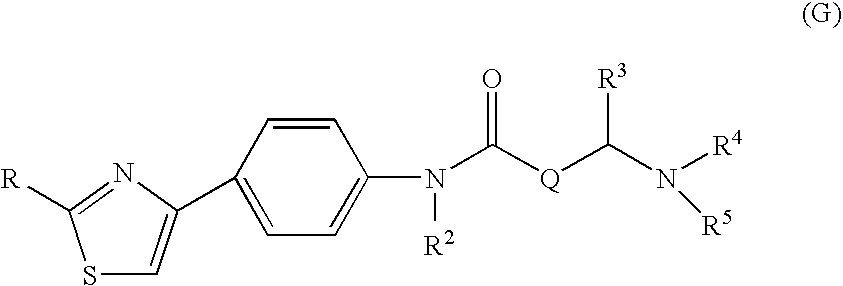
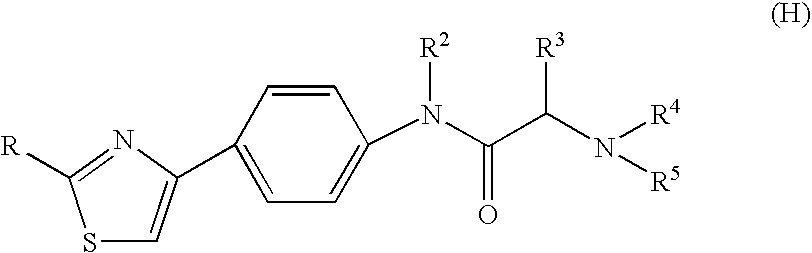
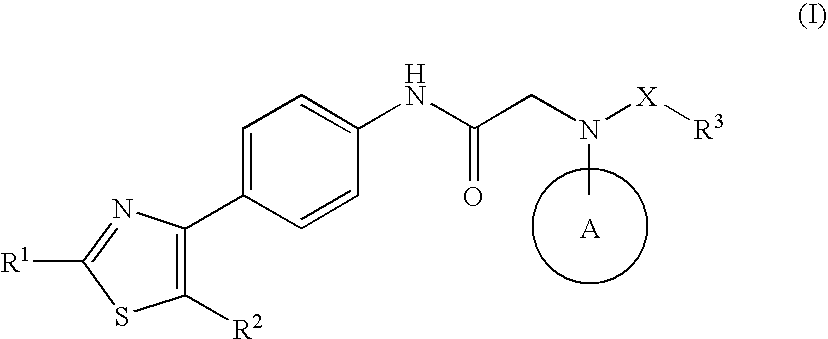
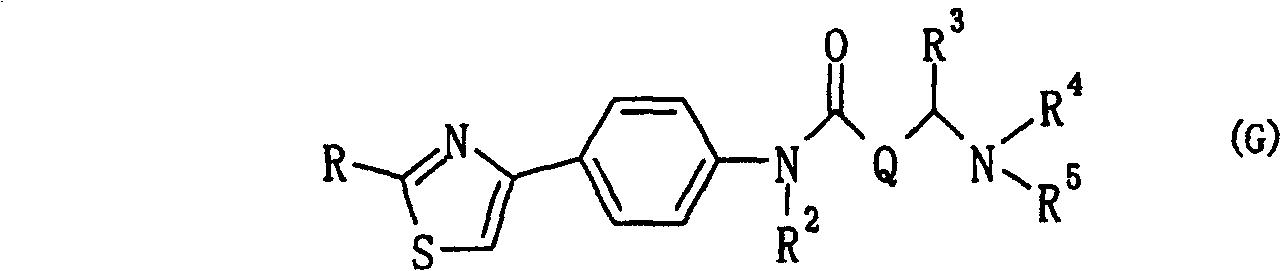
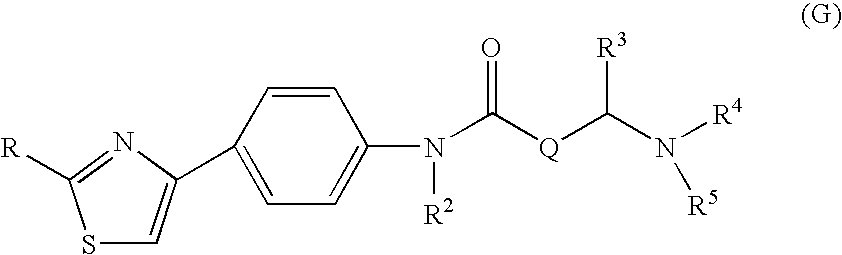
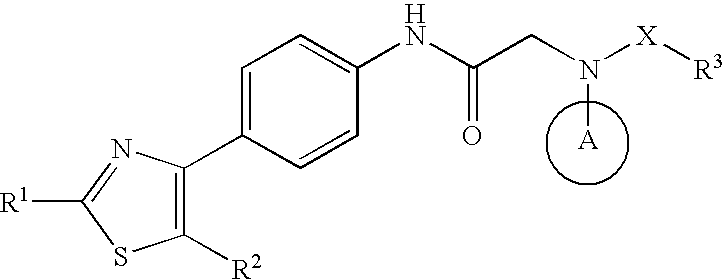
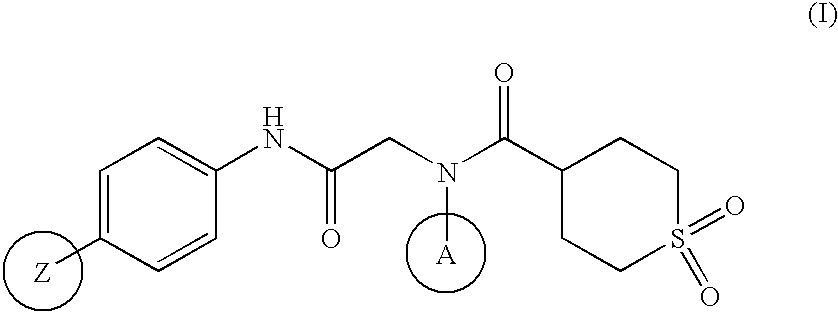
The invention relates to a novel amide derivative which is an N-({[4-(substituted thiazol-4-yl)phenyl]carbamoyl}methyl)amide derivative having a characteristic in that an aryl or heteroaryl group as an aromatic ring group is directly substituted on the N atom of amido group. Since said amide derivative has excellent anti-herpesvirus action, it is useful as medicaments and antiviral agents, particularly as preventive or therapeutic agents for various diseases accompanied by Herpesviridae virus infections, illustratively, varicella (chickenpox) accompanied by varicella zoster virus infection, shingles accompanied by the recurrent infection of latent varicella zoster virus, labial herpes and herpes encephalitis accompanied by HSV-1 infection, genital herpes accompanied by HSV-2 and the like various herpesvirus infections.
Owner:ASTELLAS PHARMA INC
Method for optimized preparation of inactivated vaccine and/or attenuated live vaccine
InactiveCN105349498AOptimizing Viral TitersHigh potencyMicroorganism based processesViruses/bacteriophagesCandidate Gene Association StudyViral Vaccine
The present invention provides a method for optimized preparation of an inactivated vaccine and / or an attenuated live vaccine, wherein in host cells, the cancer inhibition candidate gene 2 of glioma is subjected to over-expression to optimize the inside-host-cell replication environment for preparing the inactivated vaccine and / or attenuated live vaccine so as to improve the titers of 4 categories of viruses (including coronavirus, Paramyxoviridae, Orthomyxoviridae and herpes virus) by 1-3 lg unit. According to the present invention, the new strategy and the feasible basis are provided for the optimization of the whole virus vaccine virus titer, and important application prospects and innovative significance are provided in the biomedical field.
Owner:王晓佳
Methods and pharmaceuticals for treatment of viral infections of the eye
Viral infections of the eye, and particularly viral infections in the Herpesviridae and Adenoviridae families, can be treated by administration of a pharmaceutical made up of an enzymatically active ribonuclease and a vehicle. Advantageously, the enzymatically active ribonuclease is ranpirnase, the '805 variant, rAmphinase 2, and Amphinase 2, and the vehicle is an aqueous solution.
Owner:ORGENESIS INC
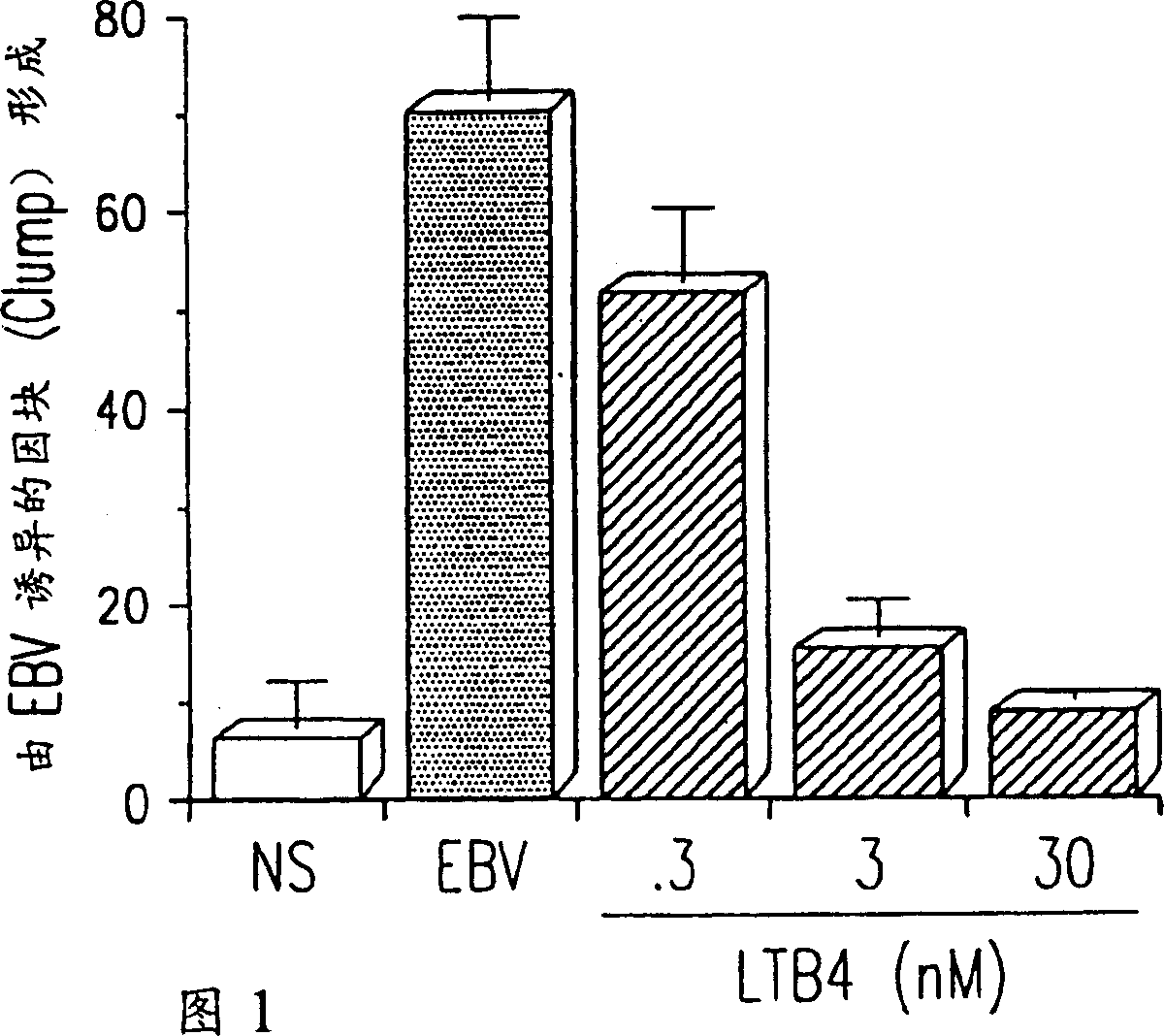
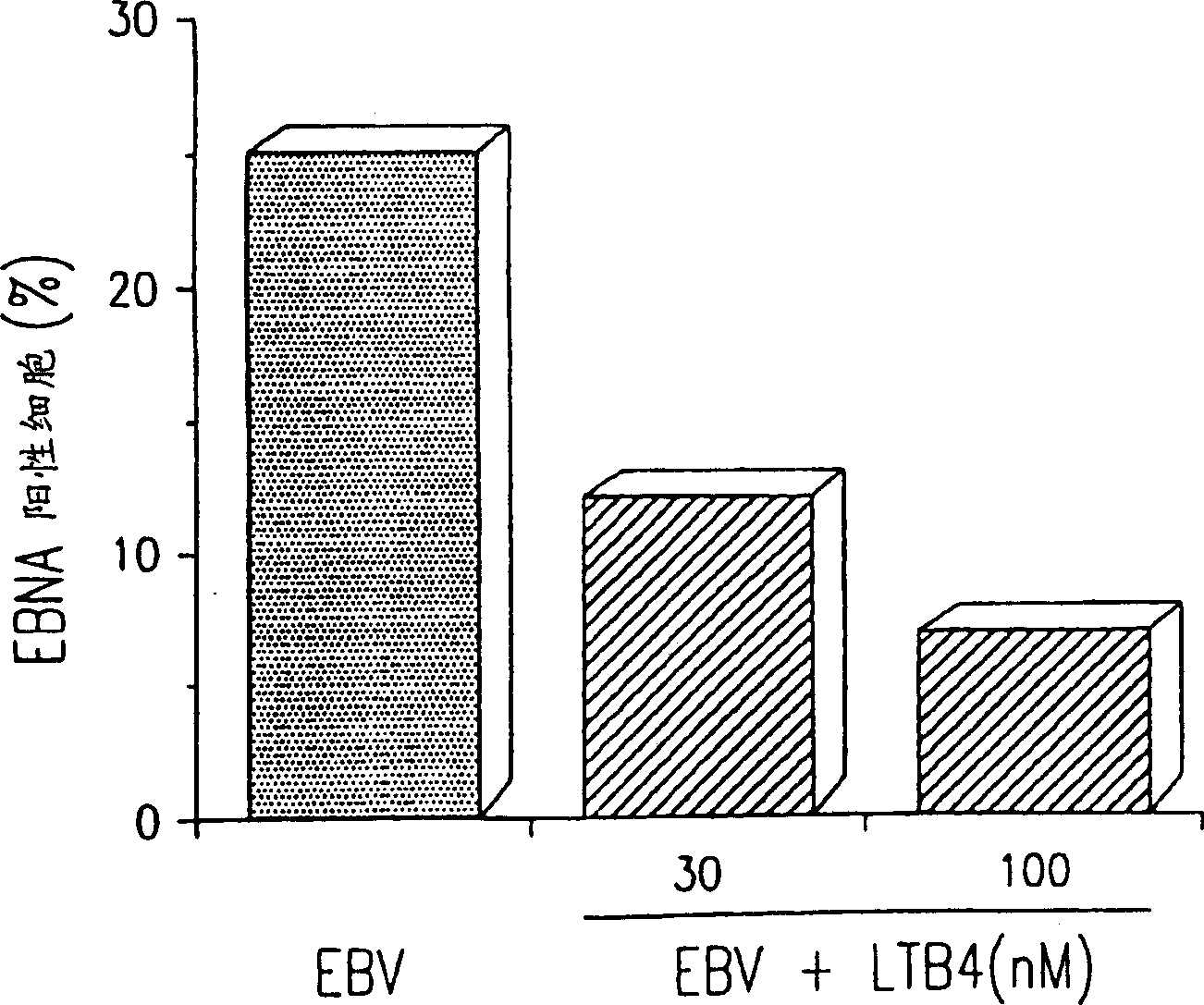
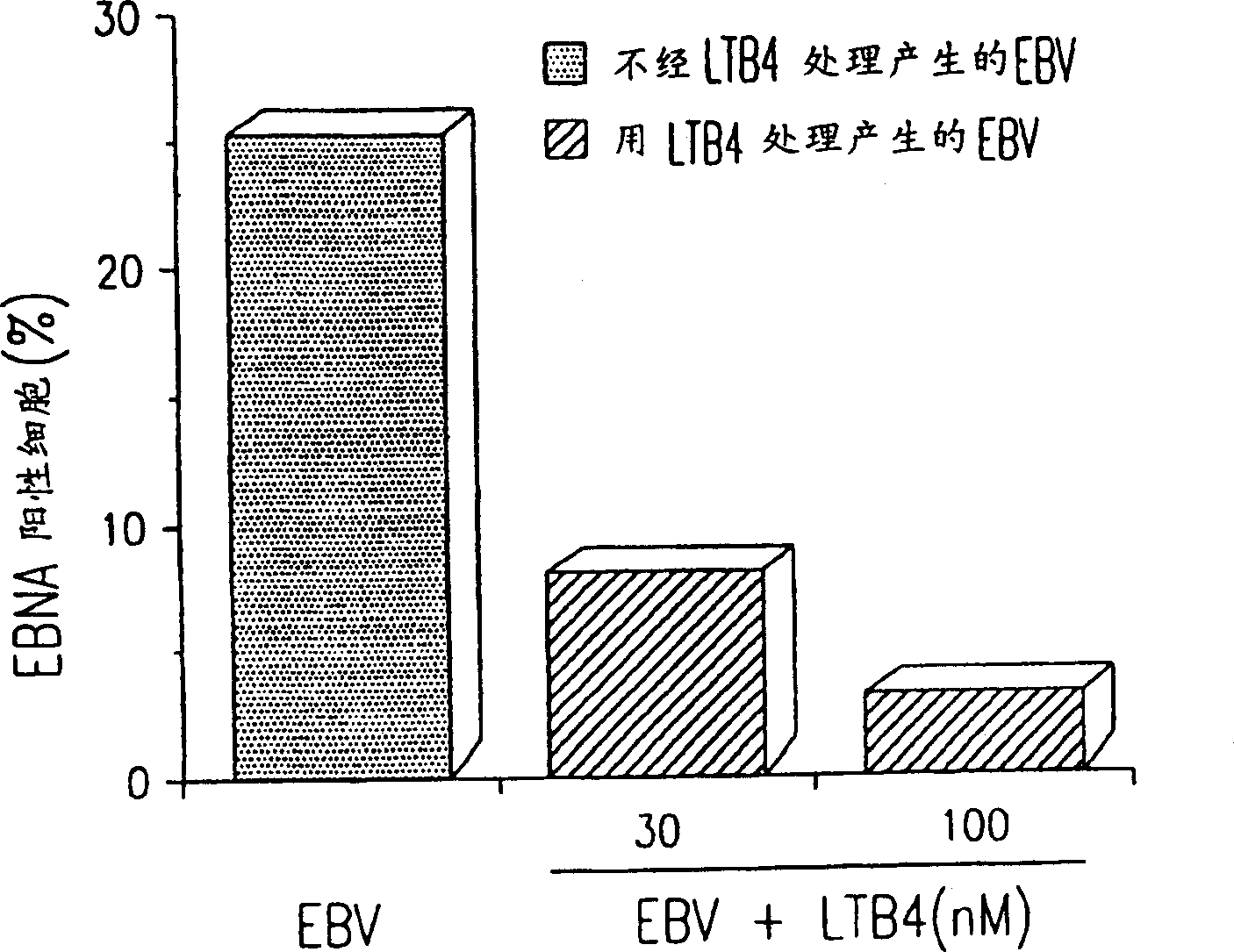
Use of leukotriene B4 or its analogues as antiviral and antineoplastic agents
InactiveCN1113653CEliminate side effectsElcosanoid active ingredientsPeptide/protein ingredientsDiseaseAnimal virus
The present invention relates to the use of leukotriene B4 (LTB4), variants and derivatives thereof as a therapeutic agent in the treatment or prophylaxis of viral infections caused by human and animal viruses. The present invention also relates to the use of LTB4, variants and derivatives thereof as an anti-neoplastic agent in the prophylaxis and treatment of cancers induced by tumor viruses and in other neoplastic diseases. The human and animal viruses are DNA viruses, such as parvoviridae, papovaviridae, adenoviridae, herpesviridae, poxviridae and hepadnaviridae; RNA viruses, such as picornaviridae, togaviridae, orthomyxoviridae, paramyxoviridae, coronaviridae, reoviridae, oncornaviridae and filoviridae in general, and Retroviridae such as HIV-1 and HIV-2.
Owner:LTB4 SWEDAN AB
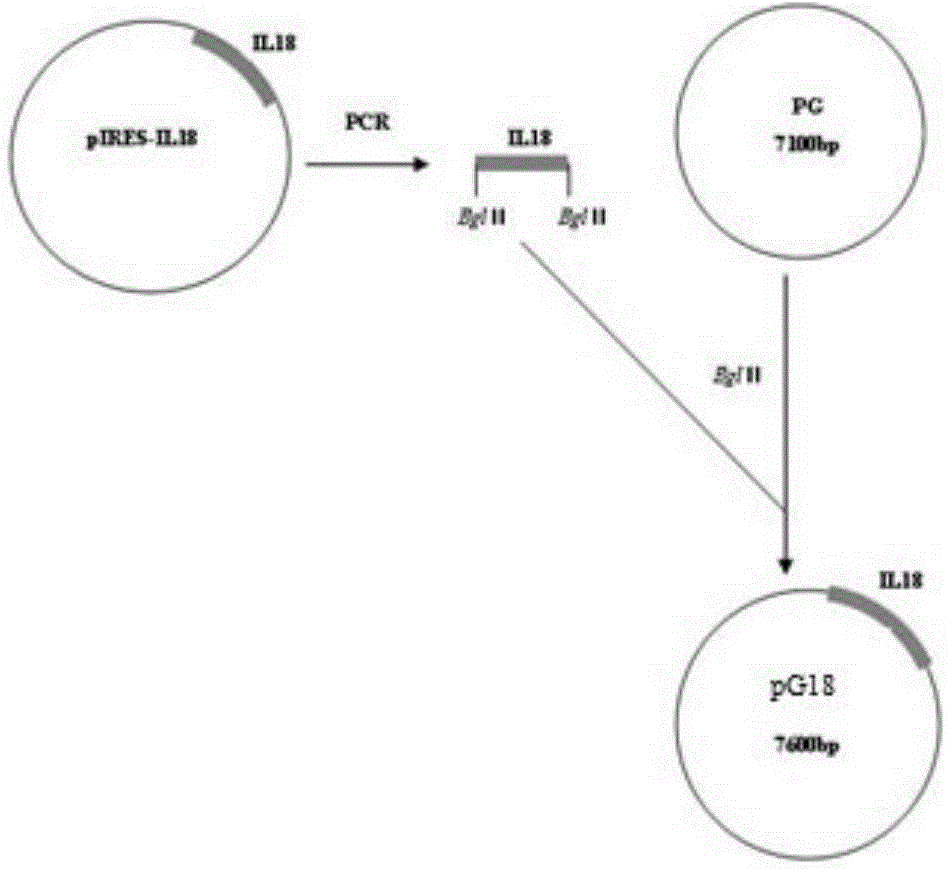
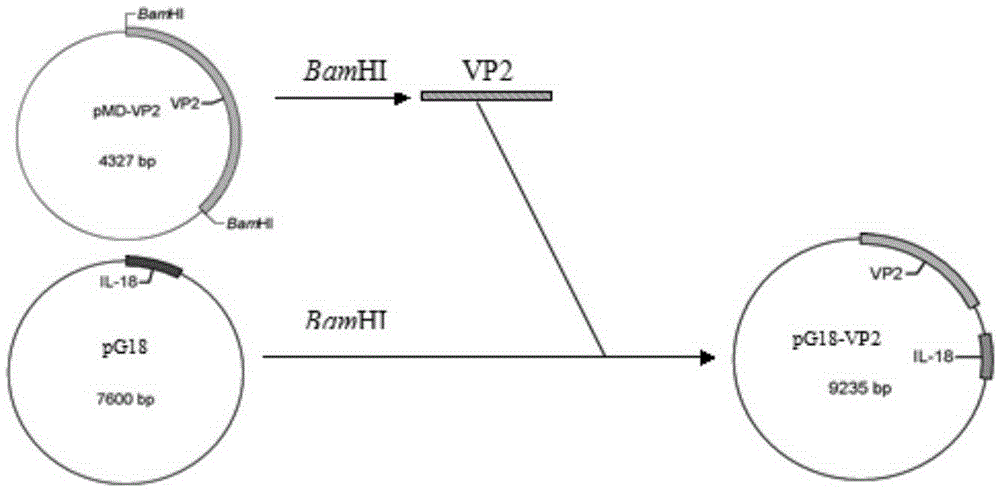
Recombinant porcine pseudorabies virus strain expressing PPV VP2 gene and porcine IL-18 gene
InactiveCN105368796ANon-toxicNo pollution in the processMicroorganism based processesViruses/bacteriophagesPositive controlRabies virus strain
The invention discloses a recombinant porcine pseudorabies virus strain expressing a PPV VP2 gene and a porcine IL-18 gene. The recombinant porcine pseudorabies virus (Herpesviridae) strain is numbered as CCTCC NO.V201347. The recombinant porcine pseudorabies virus PGVP218 established by the recombinant porcine pseudorabies virus strain and an IL-18-free recombinant pseudorabies virus rPRV-VP2 established in a laboratory respectively immunize rats, meanwhile a DMEM culture solution is used as a negative control and a PPV HB98 vaccine and a PRV inactivated vaccine are used as a positive control group to immunize the rats, and the immunogenicity of the recombinant virus is studied through PPV specific antibody detection, a PRV antibody neutralization test, peripheral blood T lymphocyte subpopulation determination and a attacking protection test. Results show that the recombinant virus PGVP218 is safe and effective to the rats, can induce the rats to produce specific PPV and PRV antibodies, and the number of CD4+ / CD8+T lymphocyte subpopulations is increased.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiantivirus compound, composition and method for treatment of virus diseases
ActiveUS20120232129A1Action potentialSugar derivativesHydroxy compound active ingredientsAnti virusDisease
A method for obtaining a new antiviral compound with multiple action against many viruses, comprising modified highly purified yeast RNA, a pharmaceutical composition comprising such RNA, and a method for the treatment and prevention of viral disease comprising administering to a patient a composition comprising an amount effective to ameliorate the symptoms of viral disease of ribonucleic acid. The exogenous modified yeast RNA has a pronounced multiple anti-virus action in a wide range of concentrations. The modified yeast RNA is capable of inhibiting the reproduction of viruses from Orthomyxoviridae, Paramyxovirus, Hepatitis, Herpesviridae families, enterovirus and adenovirus. Also, the modified yeast RNA is capable of inhibiting the reproduction of influenza viruses, hepatitis C virus, genital herpes, human immunodeficiency virus and Coxsackie B virus.
Owner:BIOCELL LAB
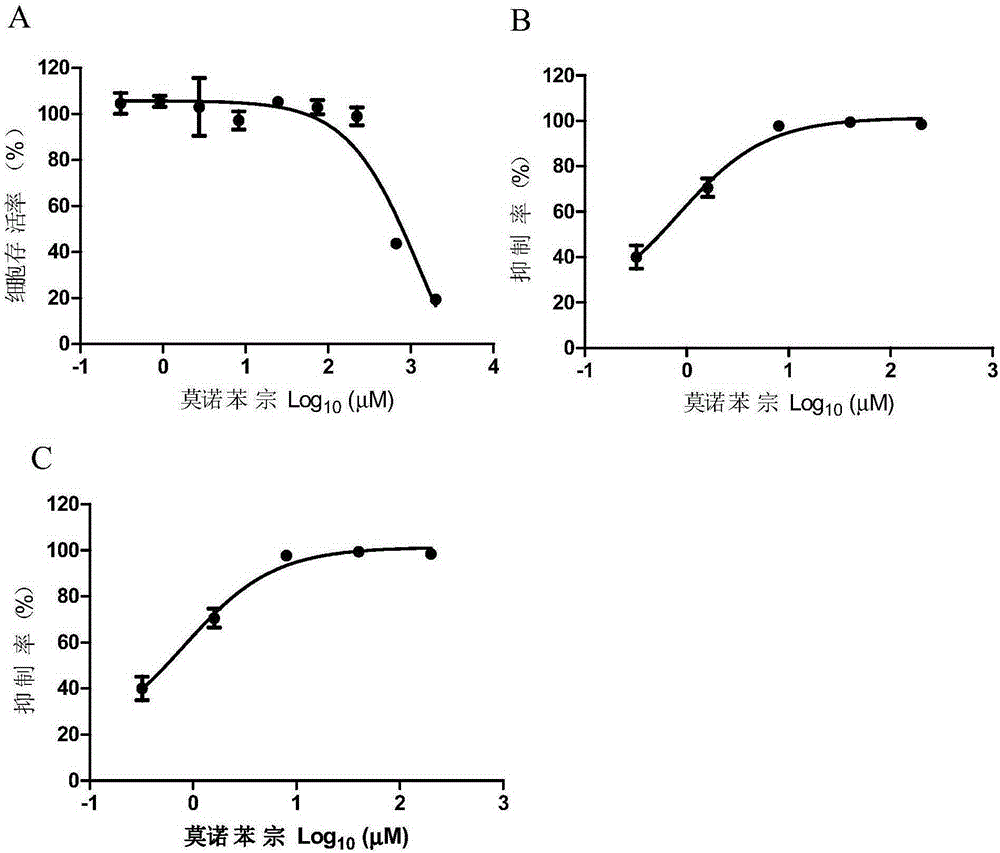
Application of Monobenzone to preparation of medicaments
InactiveCN106580934AAvoid infectionGood inhibitory effectOrganic active ingredientsAntiviralsMedicineHerpesviridae
The invention provides an application of Monobenzone to preparation of medicaments, and the medicament is used for treating or preventing Herpesviridae. According to the embodiment, Monobenzone can be effectively applied to treatment or prevention of infection of herpes virus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
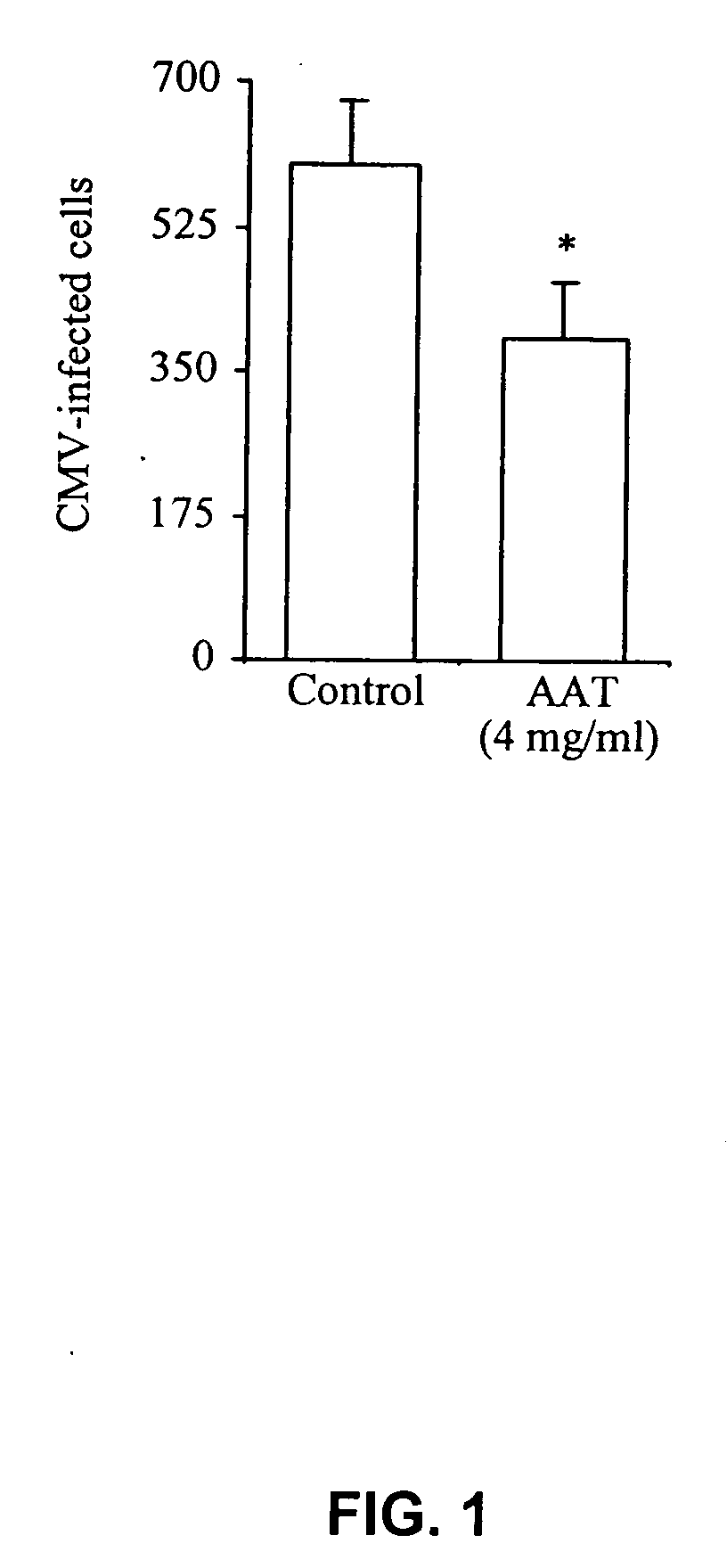
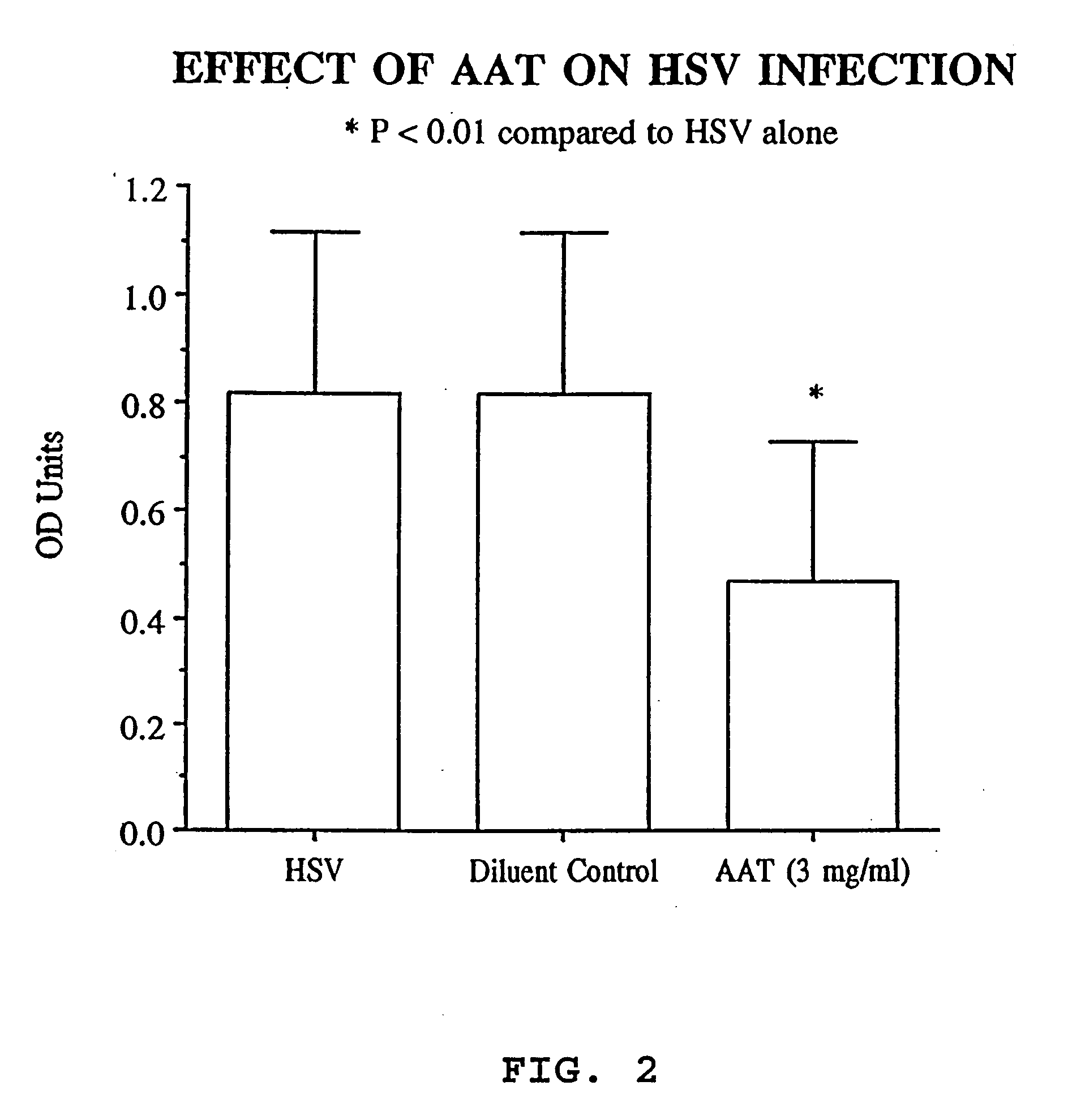
Inhibitors of serine protease activity, methods and compositions for treatment of herpes viruses
InactiveUS20080051330A1Reduce the possibilityReduce viral infectionAntibacterial agentsBiocideProteinase activityHerpesviridae
Novel compositions and methods of treating and preventing a viral infection are provided. A method of blocking a viral infection facilitated by a serine proteolytic (SP) activity is disclosed, which involves administering to a subject suffering or about to suffer from a viral infection a therapeutically effective amount of a substance having serine protease inhibitory activity or serpin activity. Among the substances found to be useful are α1-antitrypsin (AAT), peptide derivatives from the carboxy terminal end of AAT and synthetic drugs mimicking the action of such substances. The invention is particularly well suited for checking a viral infection mediated by members of herpesviridae family.
Owner:BIO HLDG
Multiantivirus compound, composition and method for treatment of virus diseases
A method for obtaining a new antiviral compound with multiple action against many viruses, comprising modified highly purified yeast RNA, a pharmaceutical composition comprising such RNA, and a method for the treatment and prevention of viral disease comprising administering to a patient a composition comprising an amount effective to ameliorate the symptoms of viral disease of ribonucleic acid. The exogenous modified yeast RNA has a pronounced multiple anti-virus action in a wide range of concentrations. The modified yeast RNA is capable of inhibiting the reproduction of viruses from Orthomyxoviridae, Paramyxovirus, Hepatitis, Herpesviridae families, enterovirus and adenovirus. Also, the modified yeast RNA is capable of inhibiting the reproduction of influenza viruses, hepatitis C virus, genital herpes, human immunodeficiency virus and Coxsackie B virus.
Owner:BIOCELL LAB
A strain of porcine pseudorabies virus
ActiveCN105200015BHighly toxicStrong resistanceMicroorganism based processesViruses/bacteriophagesWater bathsHigh resistance
The invention discloses a porcine pseudorabies virus strain, which is characterized in that: porcine pseudorabies virus (Herpesviridae) strain, CCTCC NO: V201314. The present invention passes the PRV / HN2012 strain to the 7th generation, and carries out the virus titer TCID to the 7th generation virus 50 The results of the determination of TCID 50 for 10 9.0 . The PRV / HN2012 strain was inoculated into mice. After 24 hours of challenge, the mice in the PRV / HN2012 strain-infected group developed neurological symptoms. After 48 hours of challenge, all the mice died, indicating that the strain has strong toxicity. Based on this, through the research on the physical and chemical characteristics of PRV / HN2012 strain, the results show that the strain is sensitive to analytical pure chloroform and belongs to enveloped virus; it has strong resistance to acid and alkali, hydrochloric acid at pH 3.0, and It was inactivated by NaOH treatment for 1 h; it had strong resistance to heat and could be inactivated by treatment in a water bath at 56°C for 1 h; it was sensitive to trypsin and had no obvious effect on its infectivity after being treated with ultraviolet light for 30 min.
Owner:HENAN AGRICULTURAL UNIVERSITY
Methods for screening and identifying agents that inhibit or modulate the nuclear egress complex of herpesviruses
The present invention generally provides for a novel NEC-targeted strategy for the development of antiherpesviral drugs as well as for a novel antiviral strategy targeting the viral-cellular nuclear egress complex (NEC) for a small molecule-based therapy or prophylaxis to control infections with human cytomegalovirus or other pathogenic viruses of the group of the Herpesviridae. Methods for screening agents / compounds / small molecules modulating / inhibiting the nuclear egress complex of Herpesviridae are provided as well. Specifically novel drug targets of the viral nuclear egress complex of viruses of the Herpesviridae are provided.
Owner:AICURIS GMBH & CO KG +1
Compositions and Methods for Treating Viral Infection in Mammals
The present invention provides compositions and methods useful for treating and / or preventing Herpesviridae viral infections in a mammal. In certain embodiments, the virus comprises human cytomegalovirus.
Owner:YALE UNIV
Amide compounds and methods for the production and use thereof
This invention relates to the field of medicine and concerns a method for the prophylaxis or treatment of diseases caused by RNA- and DNA-containing viruses, and concomitant diseases, which envisages the use of an effective amount of a compound of general formula I or a pharmaceutically acceptable salt thereof. The invention also relates to methods for producing the aforesaid compounds, and to pharmaceutical compositions for the prophylaxis or treatment of diseases caused by RNA- and DNA-containing viruses, which contain an effective amount of a compound of general formula I or a pharmaceutically acceptable salt thereof. The invention solves the problem of providing a novel agent effective in the treatment of diseases caused by RNA-containing viruses belonging to the enterovirus genus, the metapneumovirus genus, the pneumovirus genus, the respirovirus genus or the alphacoronavirus genus, and DNA-containing viruses belonging to the adenovirus family and the herpesvirus family, and also effective in the prophylaxis and treatment of asthma exacerbations, chronic obstructive pulmonary disease, cystic fibrosis, conjunctivitis, gastroenteritis, hepatitis and myocarditis, and in the prophylaxis and treatment of rhinorrhea, acute and infectious rhinitis, pharyngitis, nasopharyngitis, tonsillitis, laryngitis, laryngotracheitis, laryngotracheobronchitis, bronchitis, bronchiolitis, pneumonia or obstructive airways syndrome.
Owner:OBSCHESTVO S OGRANICHENNOI OTVETABTVENNOSTIYU PHARMENTERPRISES
Amide derivative
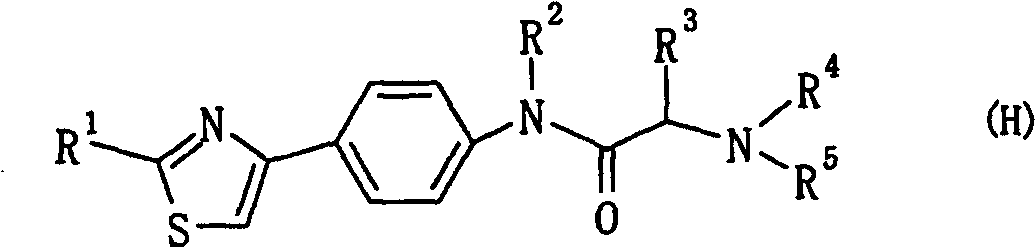
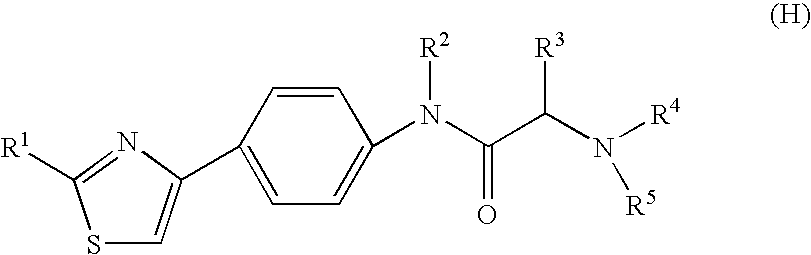
The present invention provides a medicament, in particular, a novel compound for preventing or treating various infections caused by herpesviridae viruses, especially infections caused by various herpes viruses, such as those caused by varicella zoster virus. Varicella (chickenpox), varicella zoster caused by multiple infections of latent varicella-zoster virus, cold sores and herpes encephalitis caused by HSV-1 infection, and genital herpes caused by HSV-2 infection. The present invention provides N-{2-[(4-substituted phenyl)amino]-2-oxoethyl}tetrahydro-2H-thiopyran-4-carbamoyl derivatives and salts thereof, wherein, phenyl The 4-position is substituted by a specific 5- or 6-membered heteroaryl group, it has effective antiviral activity, and it can be administered orally at a low dose to treat the above-mentioned diseases.
Owner:ASTELLAS PHARMA INC
Preventive or therapeutic agent for herpesvirus-releated disease
InactiveUS20090042915A1Superior anti-herpesvirus activityLowering undesirableBiocideOrganic chemistryDiseaseTreatment effect
[Problems] To provide a medicament, particularly a pharmaceutical composition useful for preventing or treating a variety of diseases caused by infection of a virus of the Herpesviridae family.[Means for Solving Problems] The present invention relates to an anti-herpesvirus agent characterized by combining a helicase-primase inhibitor with a polymerase inhibitor. By combining the polymerase inhibitor with the helicase-primase inhibitor having different functional mechanisms, the anti-herpesvirus agent of the present invention achieves an extremely superior anti-herpesvirus activity compared with conventional administration of a polymerase inhibitor alone. Therefore, it is particularly effective in a case in which a sufficient therapeutic effect cannot be achieved only with a polymerase inhibitor. Further, since the doses of both agents can be kept low, it is also possible to perform treatment by lowering the effect of an adverse reaction to be concerned.
Owner:ASTELLAS PHARMA INC
Thymidine Kinase Mutants and Fusion Proteins Having Thymidine Kinase and Guanylate Kinase Activities
InactiveUS20100112685A1Improve biological activityAntibacterial agentsOrganic active ingredientsBinding siteMutant
The present invention provides isolated nucleic acid molecules encoding a Herpesviridae thymidine kinase enzyme comprising one or more mutations, at least one of the mutations encoding an amino acid substitution located toward the N-terminus from a DRH nucleoside binding site which increases a biological activity of the thymidine kinase, as compared to unmutated thymidine kinase. Such mutations include amino acid substitutions within a Q substrate binding domain which increases a biological activity of the thymidine kinase, as compared to unmutated thymidine kinase. Within a further aspect, fusion proteins are provided which have both guanylate kinase and thymidine kinase biological properties. Also provided are vectors suitable for expressing such DNA molecules, as well as methods for utilizing such vectors.
Owner:DARWIN MOLECULAR CORP
Bicyclic carbohydrate compounds useful in the treatment of infections caused by herpesviridae
Bicyclic carbohydrates for the treatment of infections caused by herpseviridae, and in particular cytomegalovirus. The invention consists of the novel bicyclic carbohydrates the generic structure of which is: wherein R1 is either -Bn or -Ph; R2 and R3 are either -alkyl, -aryl, -allyl, or —H; R4 and R5 form a ring and are either —CH(Ph)- or —CH(aryl)- and X is either O, N or S.
Owner:KEMIN PHARMA EUROPE B.V.B.A.
Agent for prevention/treatment of disease caused by acyclovir-resistant herpesvirus
InactiveUS20090048310A1Excellent anti-viral activityImprove pharmacokineticsBiocideOrganic chemistryRecurrent infectionsVaricella-zoster virus infection
[Problems] To provide an agent useful for the prevention or treatment of various diseases associated with the infection with acyclovir-resistant viruses of the family Herpesviridae, specifically various infectious caused by herpes viruses, such as varicella associated with varicella-zoster virus infection, herpes zoster associated with recurrent infection with latent varicella-zoster virus, and herpes labialis, herpes encephalitis and genital herpes associated with HSV-1 and HSV-2 infection, and the like.[Means for Solving Problems] An N-[2-[(4-substituted phenyl)amino]-2-oxoethyl]tetrahydro-2H-thiopyran-4-carboxamide derivative in which the phenyl group is substituted at position 4 by a specific 5- or 6-membered heteroaryl group. This derivative has an excellent anti-viral activity against acyclovir-resistant herpes viruses and, therefore, is effective for the treatment of the diseases as mentioned above.
Owner:ASTELLAS PHARMA INC
Recombinant porcine pseudorabies virus strain used for expression of porcine circovirus type II (PCV2) ORF2 gene, and preparation method thereof
ActiveCN103509761BHigh antibody titerResist attackMicroorganism based processesViruses/bacteriophagesMouse LymphocyteOrf2 gene
The invention discloses a recombinant porcine pseudorabies virus (Herpesviridae) strain used for expression of porcine circovirus type II ORF2 gene. Preservation number of the recombinant porcine pseudorabies virus strain is CCTCC No.V201315. According to the preparation method, PCV2ORF2 gene is inserted into common carrier PG so as to construct transferring plasmid PGO; monolayer ST cells are inoculated with PRVTK<-> / gG<-> / gE<-> virus by 2h of adsorption; ST cells are transfected with plasmid PGO, wherein fusion degree of the monolayer ST cells is 80 to 90%; the recombinant porcine pseudorabies virus PGO strain is obtained by plaque purification, and is used for immunization of mouse. It is shown by results that commercial PCV2 inactivated vaccine and a group immunized by the recombinant porcine pseudorabies virus PGO strain are both capable of inducing specific humoral immune response of mouse, antibody titers of both the commercial PCV2 inactivated vaccine and the group are obviously higher than that of a group immunized by DMEM medium, and difference is significant (p<0.05). It is shown by the results of mouse lymphocyte proliferation test that specific ceullar immune response caused by the group immunized by the recombinant porcine pseudorabies virus PGO strain is more obvious than that caused by the commercial PCV2 inactivated vaccine and the group immunized by DMEM medium, and difference is obvious (p<0.05). In addition, the group immunized by the recombinant porcine pseudorabies virus PGO strain is capable of resisting severe attack by PCV2. Therefore application of the recombinant porcine pseudorabies virus strain in development of a novel PCV2 vaccine is possible.
Owner:HENAN AGRICULTURAL UNIVERSITY
Latent phase viral interleukin-10-(VII-10) and uses thereof
InactiveUS8133700B2Reduced activityBiocidePeptide/protein ingredientsInterleukin 10Nucleic acid sequencing
The present invention relates to a purified nucleic acid sequence encoding a homologue of human interleukin 10 (IL-10), wherein said IL-10 homologue is expressed during the latent phase of infection by a virus of the herpesvirideae group. The present invention also relates to uses of this polypeptide, in particular for diagnosing disease states and screening for modulator and inhibitor compounds of such polypeptides and in turn the virus itself, screening for infection in vertebrates and biological tissue, cleansing of infected biological tissues, and in the treatment and / or prophylaxis and / or diagnosis of disease caused by a virus of the herpesvirideae group.
Owner:THE UNIV OF SYDNEY
Live attenuated varicella vaccine
ActiveCN101161286BImprove securityHigh viral titerAntiviralsAntibody medical ingredientsKanamycinSucrose
The invention relates to a medical microorganism of the herpesviridae family, in particular to a live attenuated varicella vaccine. The present invention is prepared by the following method: (1) continuously passaging MRC-5 cells; (2) inoculating into cells and continuously infecting; (3) adding culture medium; (4) washing the cell surface; (5) washing out the infection The cells are separated by centrifugation, and the vaccine solution containing a stabilizer is added for freezing; (6) the virus stock solution is obtained by ultrasonic crushing and centrifugation; (7) combined and diluted, and immediately subpackaged and freeze-dried to obtain a live attenuated varicella vaccine. The stabilizer is NaCl, KCl, KH2PO4, Na2HPO4 12H2O, sucrose, sodium glutamate, kanamycin, erythromycin and deionized water in a weight ratio of 3: 0.1: 0.2: 3.3: 50: 0.4: 0.1: 0.03:1000 dissolved in water prepared. The invention has the advantages of good safety and immunogenicity.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com