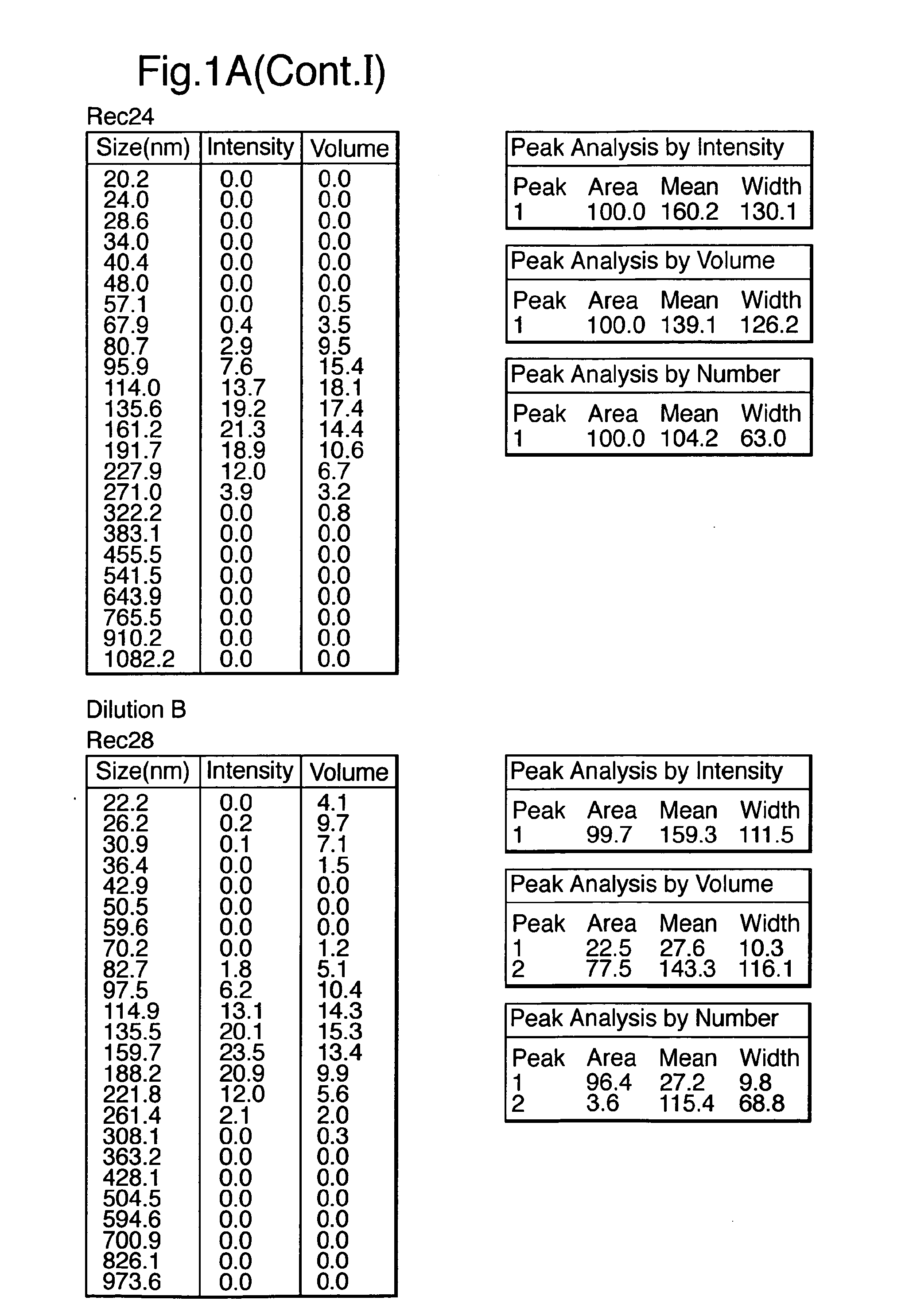
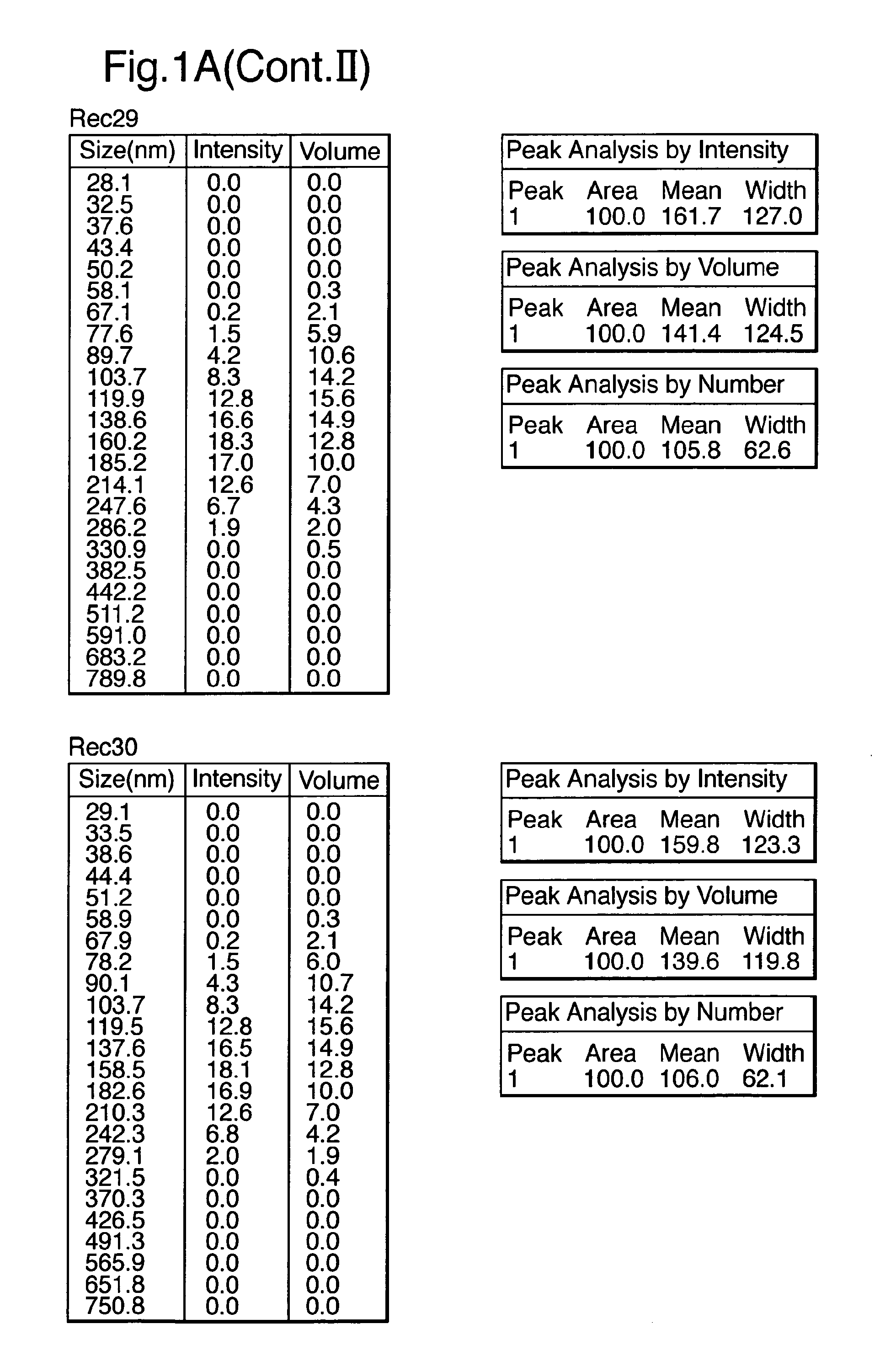
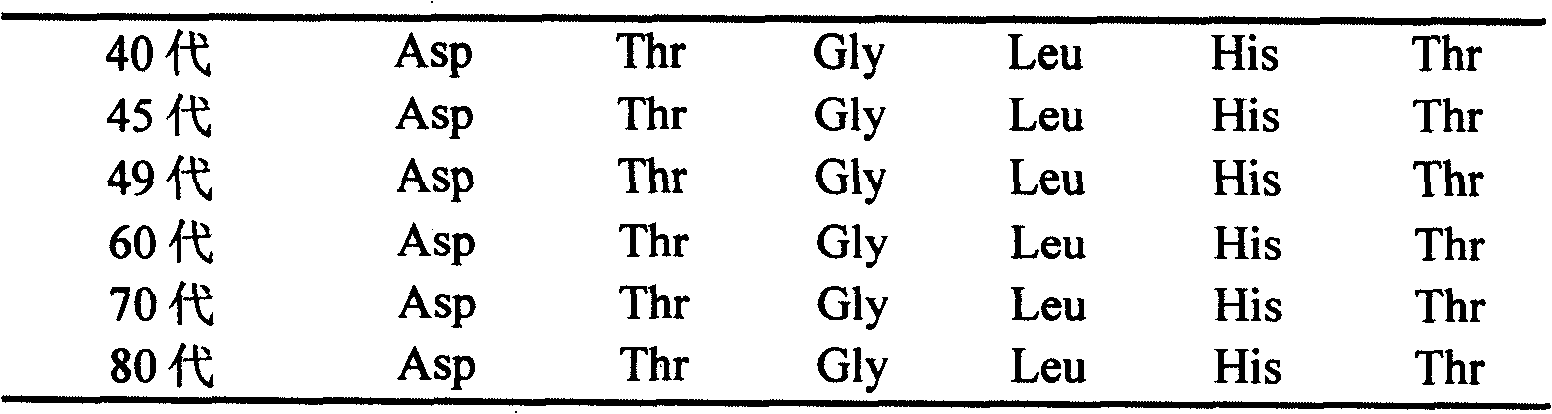Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
987 results about "Virus strain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sterilization system with ultraviolet emitter for eradicating biological contaminants
ActiveUS20120223216A1Effectively destroyEffective sterilizationAutomatic obstacle detectionTravelling automatic controlFluorescenceUltraviolet lights
An exemplary sterilization system includes a self-propelled robotic mobile platform for locating and eradicating infectious bacterial and virus strains on floors (and objects thereon), walls, cabinets, angled structures, etc., using one or more ultraviolet light sources. A controller allows the system to adjust the quantity of ultraviolet light received by a surface by, for example, changing the intensity of energy input to a ultraviolet light source, changing a distance between a ultraviolet light source and a surface being irradiated, changing the speed / movement of the mobile platform to affect time of exposure, and / or by returning to contaminated areas for additional passes. The mobile platform may include a sensor capable of detecting fluorescence of biological contaminants irradiated with ultraviolet light to locate contaminated areas. The system is thus capable of “seek and destroy” functionality by navigating towards contaminated areas and irradiating those areas with ultraviolet light accordingly.
Owner:FLAHERTY KAREN
Influenza vaccine
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:SMITHKLINE BEECHAM PHARMA GMBH +1
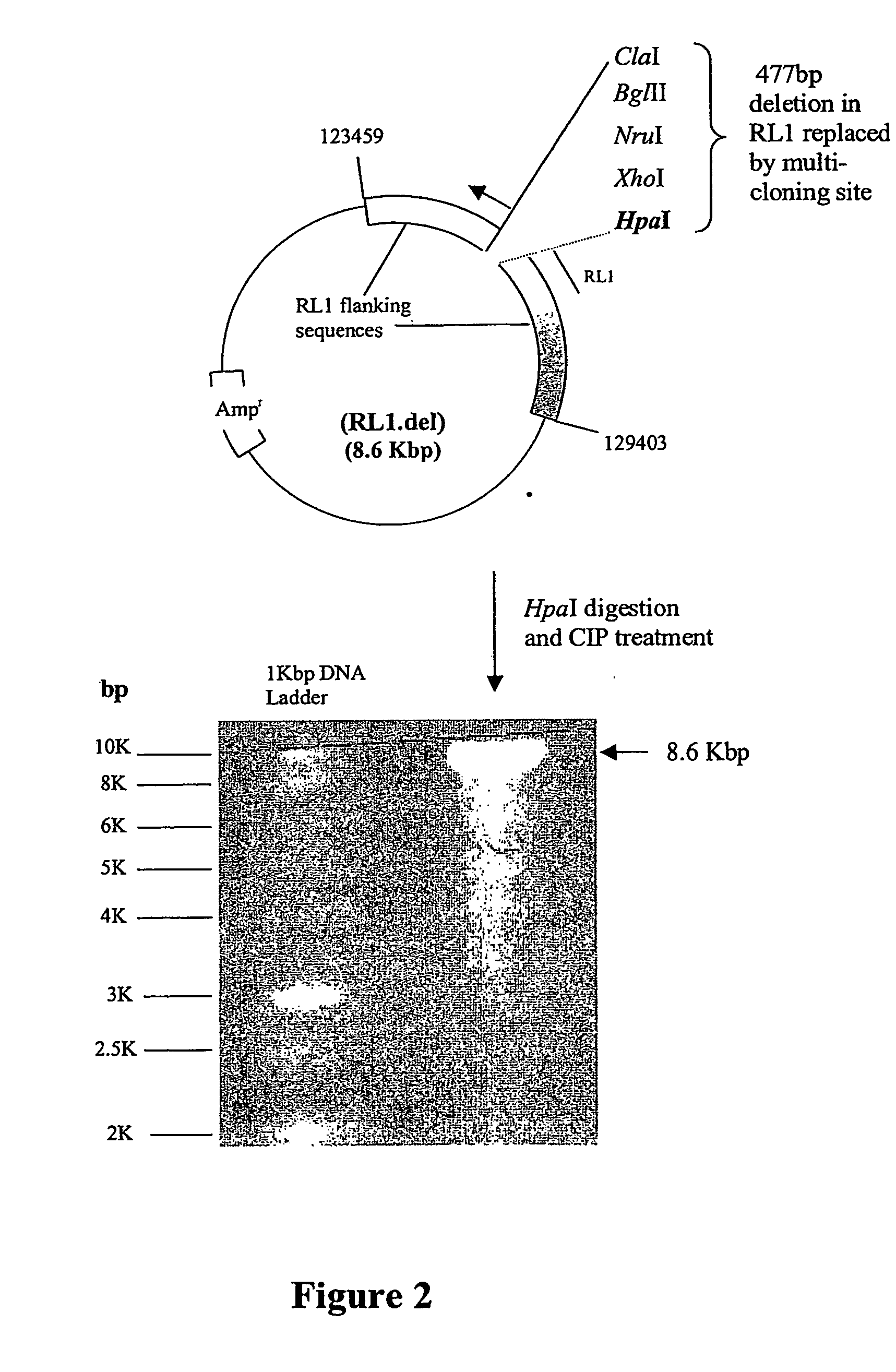
Gene deletion attenuated African swine fever virus and application thereof as vaccine
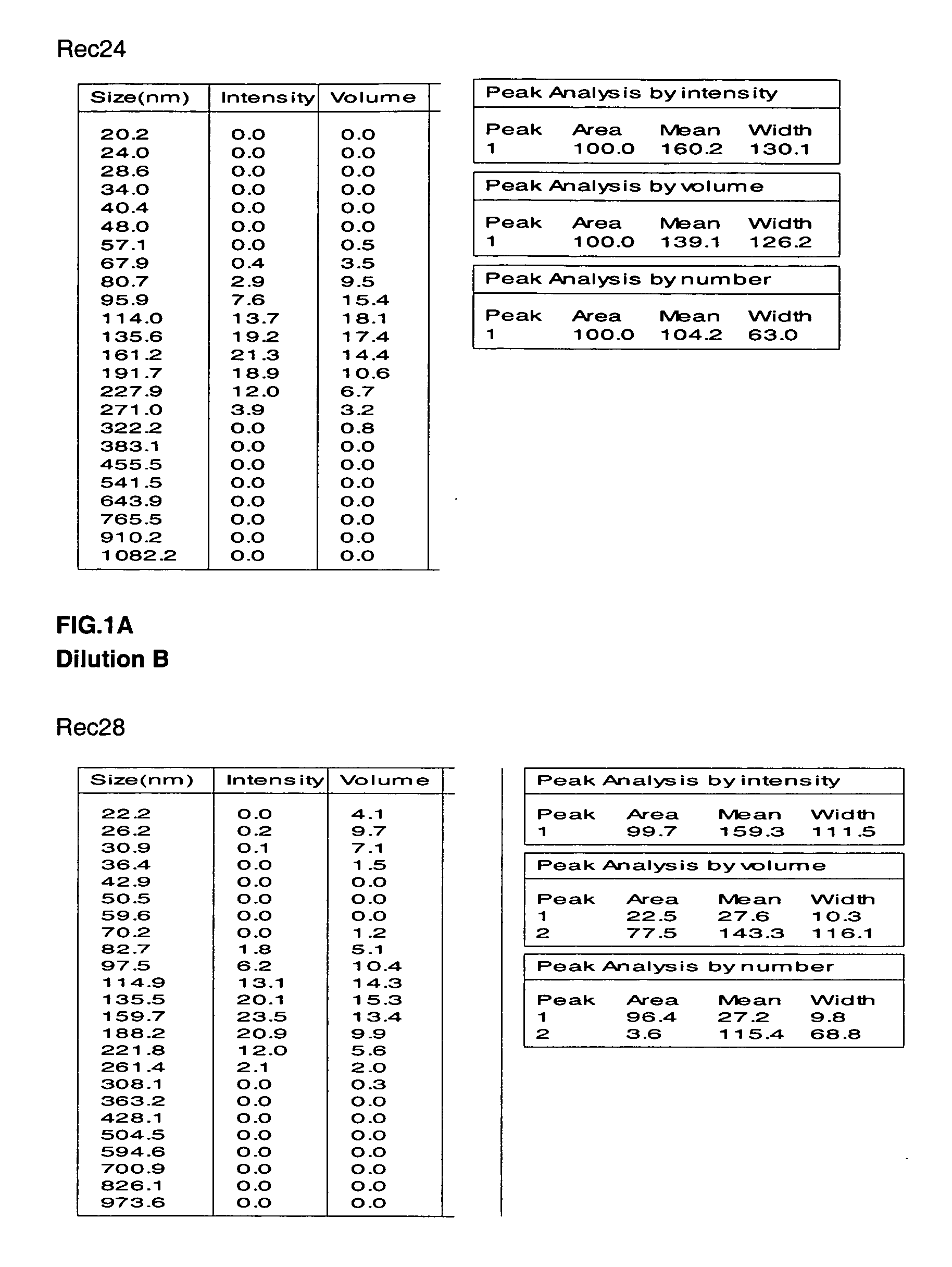
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Vectors, mutant viruses and methods for generating mutant viruses
InactiveUS20070110720A1Easy to identifyEnhanced cytotoxic potentialBiocideGenetic material ingredientsHeterologousBinding site
A nucleic acid vector comprising first and second nucleotide sequences corresponding to nucleotide sequences flanking an insertion site in the genome of a selected herpes simplex virus strain; and a cassette located between said first and second nucleotide sequences comprising nucleic acid encoding: (a) one or a plurality of insertion sites and / or a nucleotide sequence of interest; and (b) a ribosome binding site or a regulatory nucleotide sequence; and (c) a marker is disclosed. Herpes simplex viruses generated using said vector, methods for their generation and herpes simplex viruses having a genome comprising heterologous nucleic acid are also disclosed.
Owner:CRUSADE LAB
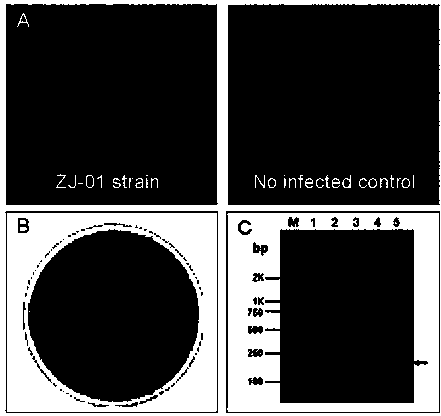
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Influenza vaccine
InactiveUS20090263422A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alphatocopherol, and an emulsifying agent.
Owner:HANON EMMANUEL JULES +1
INDUCTION OF BROADLY REACTIVE NEUTRALIZING ANTIBODIES BY FOCUSING THE IMMUNE RESPONSE ON V3 EPITOPES OF THE HIV-1 gp120 ENVELOPE
InactiveUS20080279879A1Vigorous Ab responseViral antigen ingredientsAntibody mimetics/scaffoldsHeterologousNeutralizing antibody
Compositions, kits and methods for boosting, or for priming and boosting, high titer broadly neutralizing cross-clade antibody responses focused on single HIV-1 neutralizing epitopes are disclosed. gp120 DNA plasmids comprising HIV env genes are used to prime the antibody response. Primed subjects are immunized with recombinant fusion proteins that comprise a “carrier” protein fusion partner, preferably a truncated form of the MuLV gp70 Env protein, and a desired HIV neutralizing epitopes. Preferred epitopes are epitopes of V3 from one or more HIV clades. Immune sera from such immunized subjects neutralized primary isolates from virus strains heterologous to those from which the immunogens were constructed. Neutralizing activity was primarily due to V3-specific antibodies and cross-clade neutralizing Abs were present. This approach results in more potent and broader neutralizing antibody levels, a result of “immunofocusing” the humoral immune response on neutralizing epitopes such as V3.
Owner:NEW YORK UNIV
Influenza vaccine
InactiveUS20080014217A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Surface enhanced raman spectroscopy (SERS) systems and methods of use thereof
ActiveUS7583379B2Radiation pyrometryMicrobiological testing/measurementSurface-enhanced Raman spectroscopyOblique angle
Surface-enhanced Raman spectroscopic (SERS) systems and methods for detecting biomolecules of interest, such as a virus, bacterium, or other infectious agent, are provided. A spectroscopic assay based on surface enhanced Raman scattering (SERS) using a silver nanorod array substrate fabricated by oblique angle deposition has been developed that allows for rapid detection of trace levels of viruses or bacteria with a high degree of sensitivity and specificity. This novel and improved SERS assay can detect minor spectral differences within strains of a single virus type such as respiratory syncytial virus or influenza virus in the presence of biological media. The method provides rapid diagnostics for direct molecular and structural characterization of virus strains and virus gene deletion mutants generating reproducible viral spectra without viral manipulation.
Owner:UNIV OF GEORGIA RES FOUND INC
Vaccines Including Antigen From Four Strains of Influenza Virus
InactiveUS20140178429A1Reduce in quantityFacilitate matterSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:SEQIRUS UK LTD
DNA prime/activated vaccine boost immunization to influenza virus
InactiveUS20110177122A1Enhance immune responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininEpitope
The present invention relates to a combination of a priming composition and a boosting composition to prime and boost an immune response in a subject whereby the immune response resulting from administration of the priming composition to the subject is capable of being boosted. The priming composition comprises a DNA plasmid that comprises a nucleic acid molecule encoding an influenza virus hemagglutinin (HA) or an epitope-bearing domain thereof. The boosting composition comprises an influenza vaccine. The present invention also relates to a method to use such a combination to vaccinate a subject and to enhance an immune response to an influenza vaccine administered alone. Such a combination can elicit an immune response not only against at least one influenza virus strain from which the priming composition or boosting composition is derived but also to at least one heterologous influenza virus strain.
Owner:UNITED STATES OF AMERICA
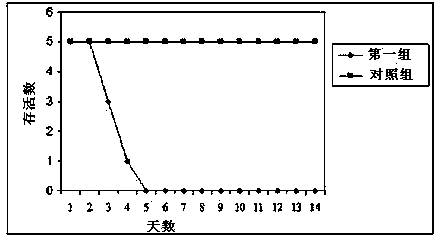
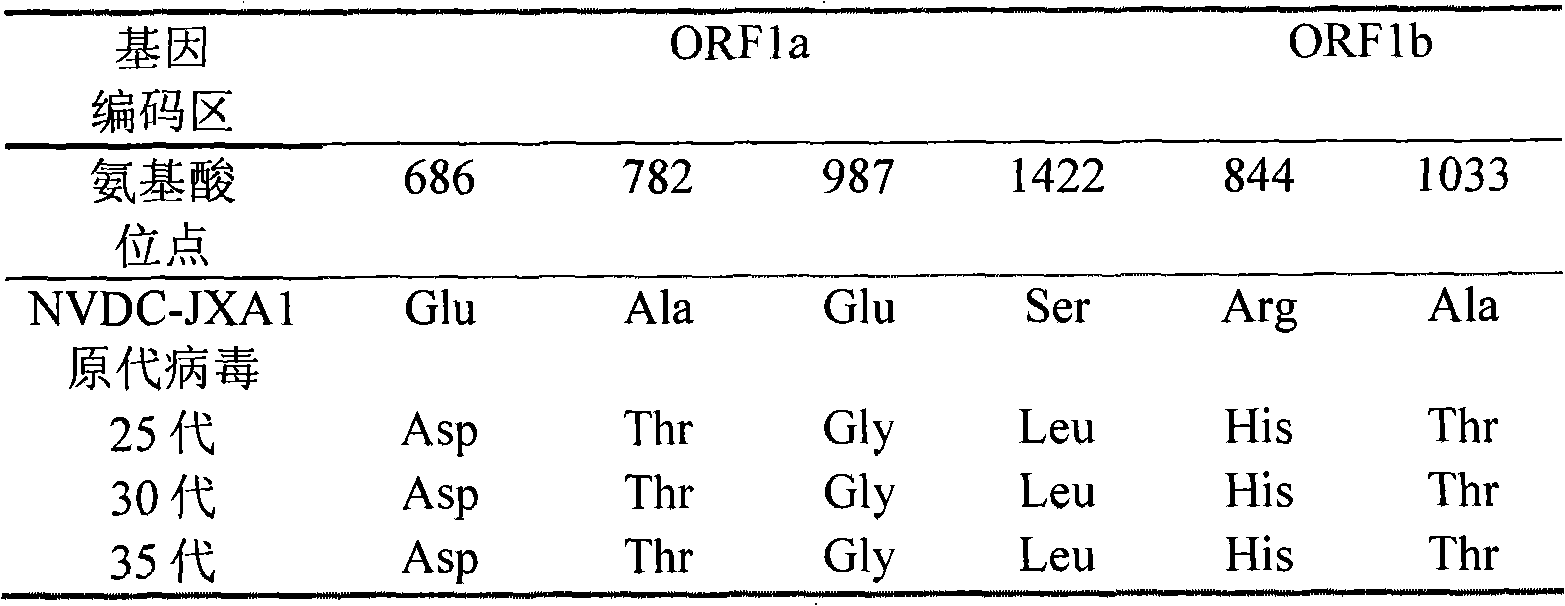
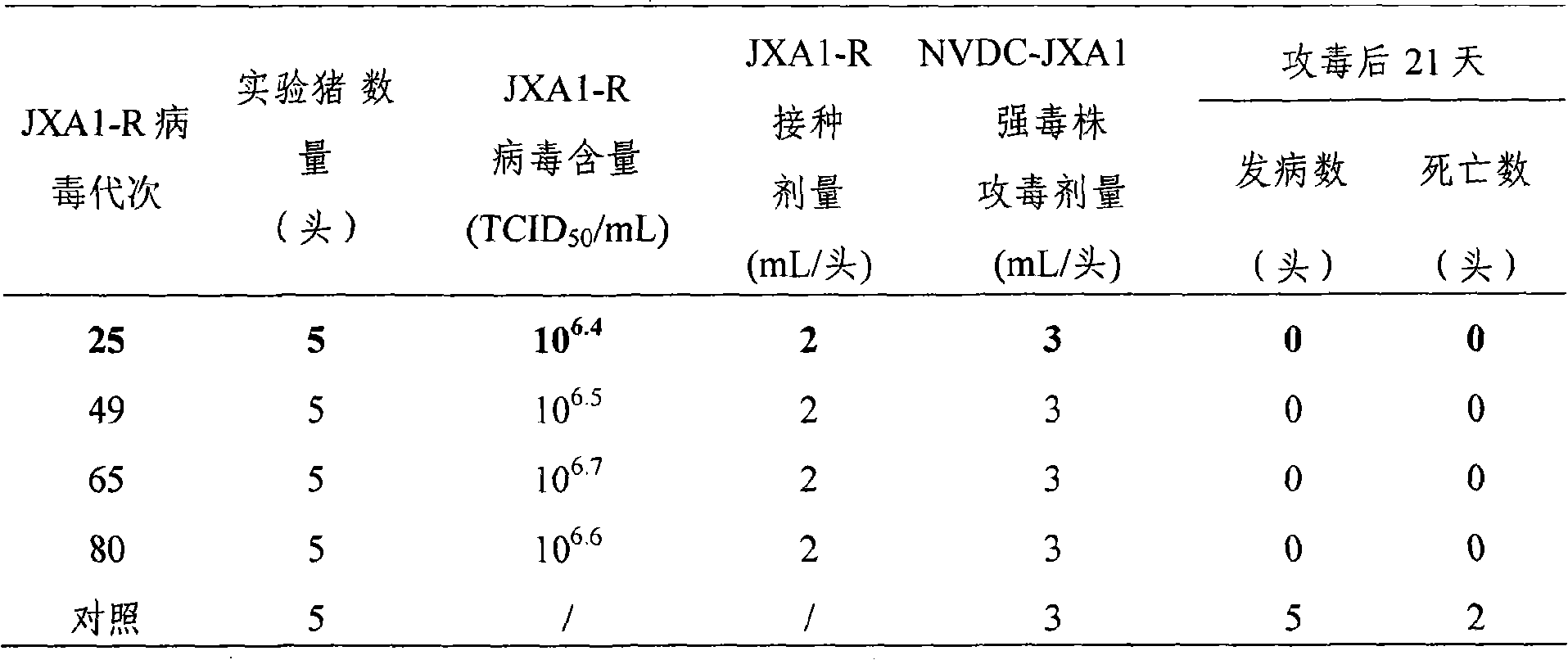
Low virulent strain of porcine reproductive and respiratory syndrome virus, immunogenicity immunogenicity material and vaccine
The application relates to a domesticated porcine reproductive and respiratory syndrome virus low virulent strain (JXA1-R strain), immunogenicity substance containing the viral strain, and a porcine reproductive and respiratory syndrome virus low virulent live vaccine developed through making use of the strain. The live vaccine is used to prevent the porcine reproductive and respiratory syndrome, in particular the highly pathogenic blue-ear porcine disease.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
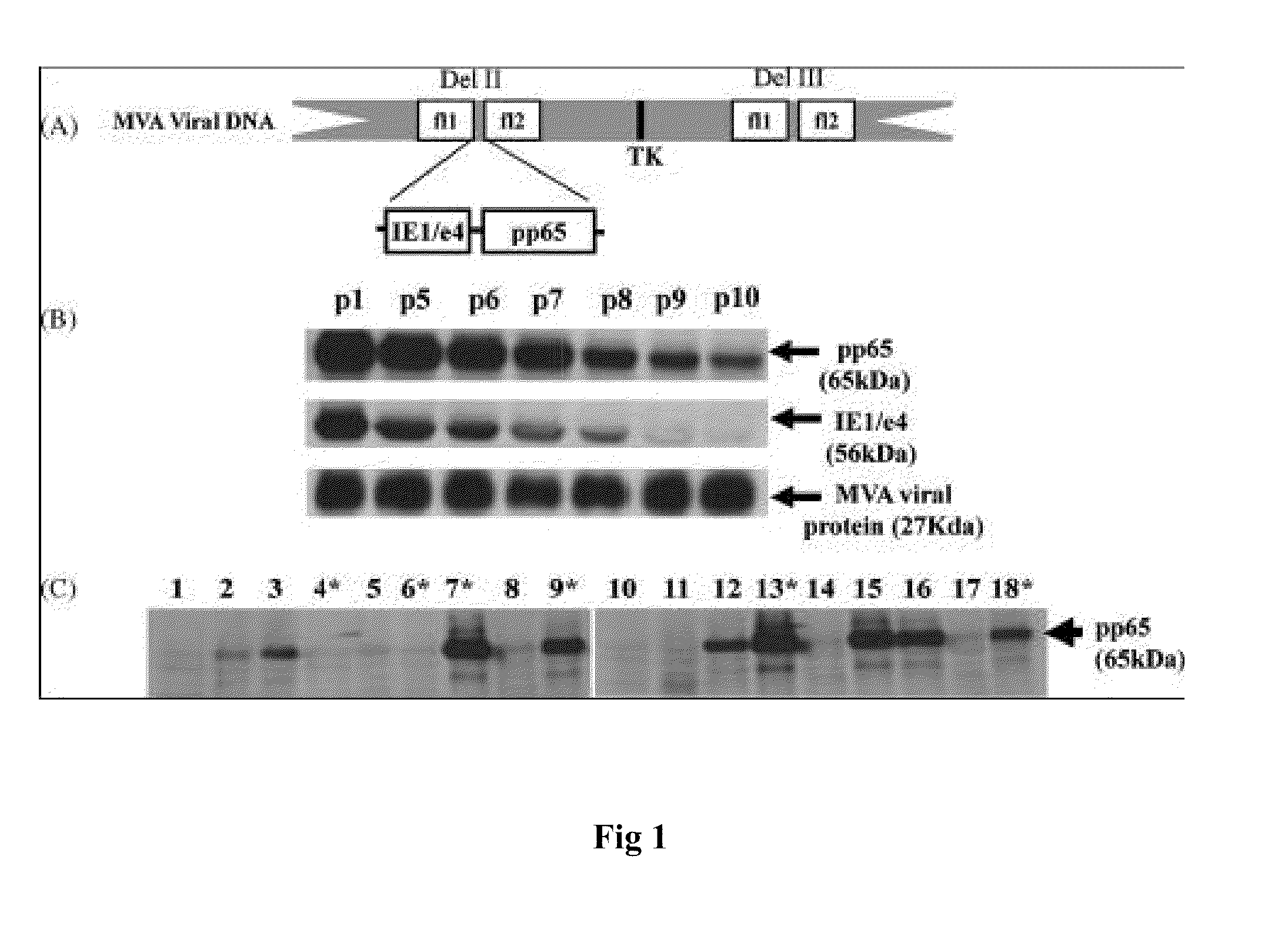
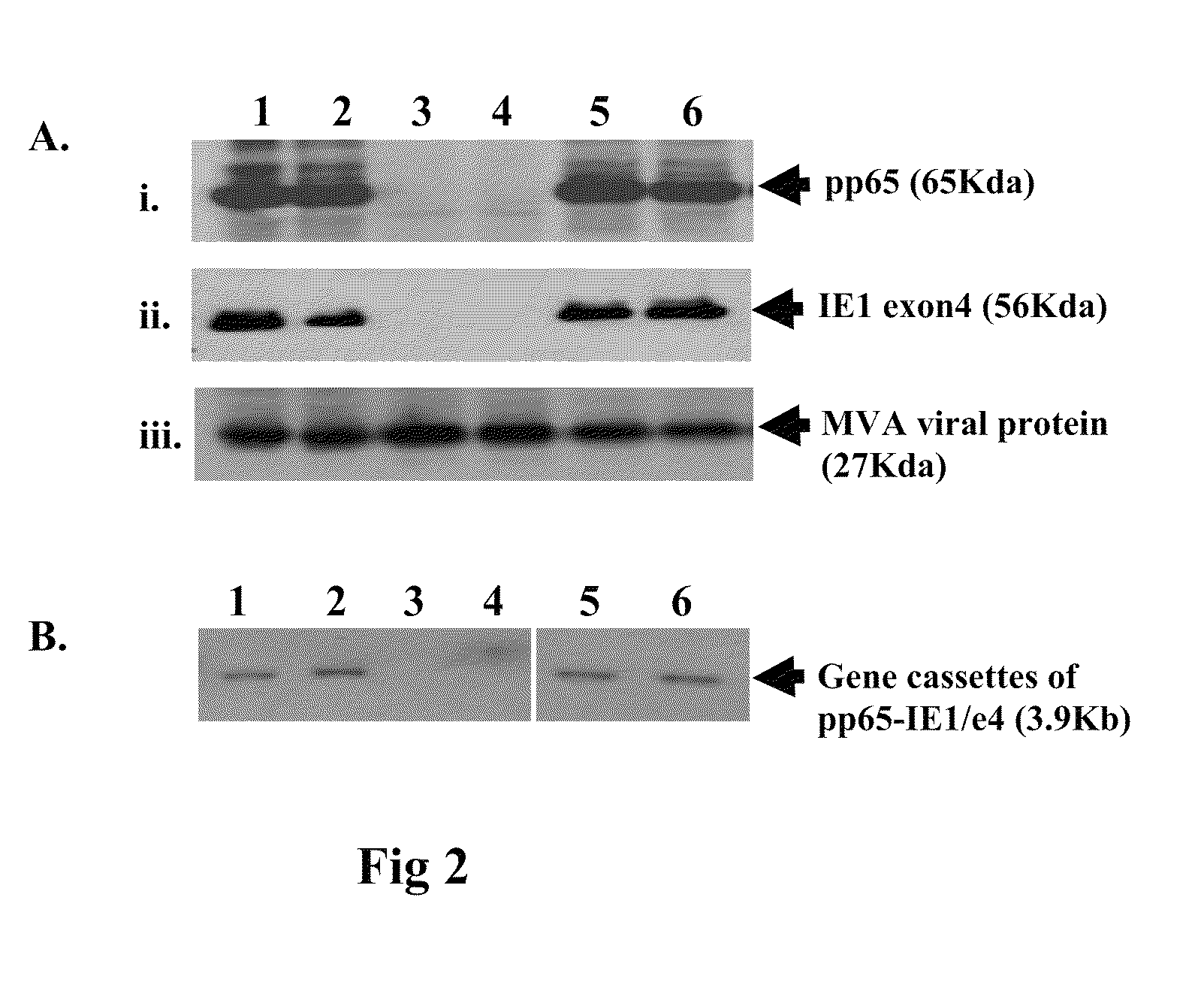
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
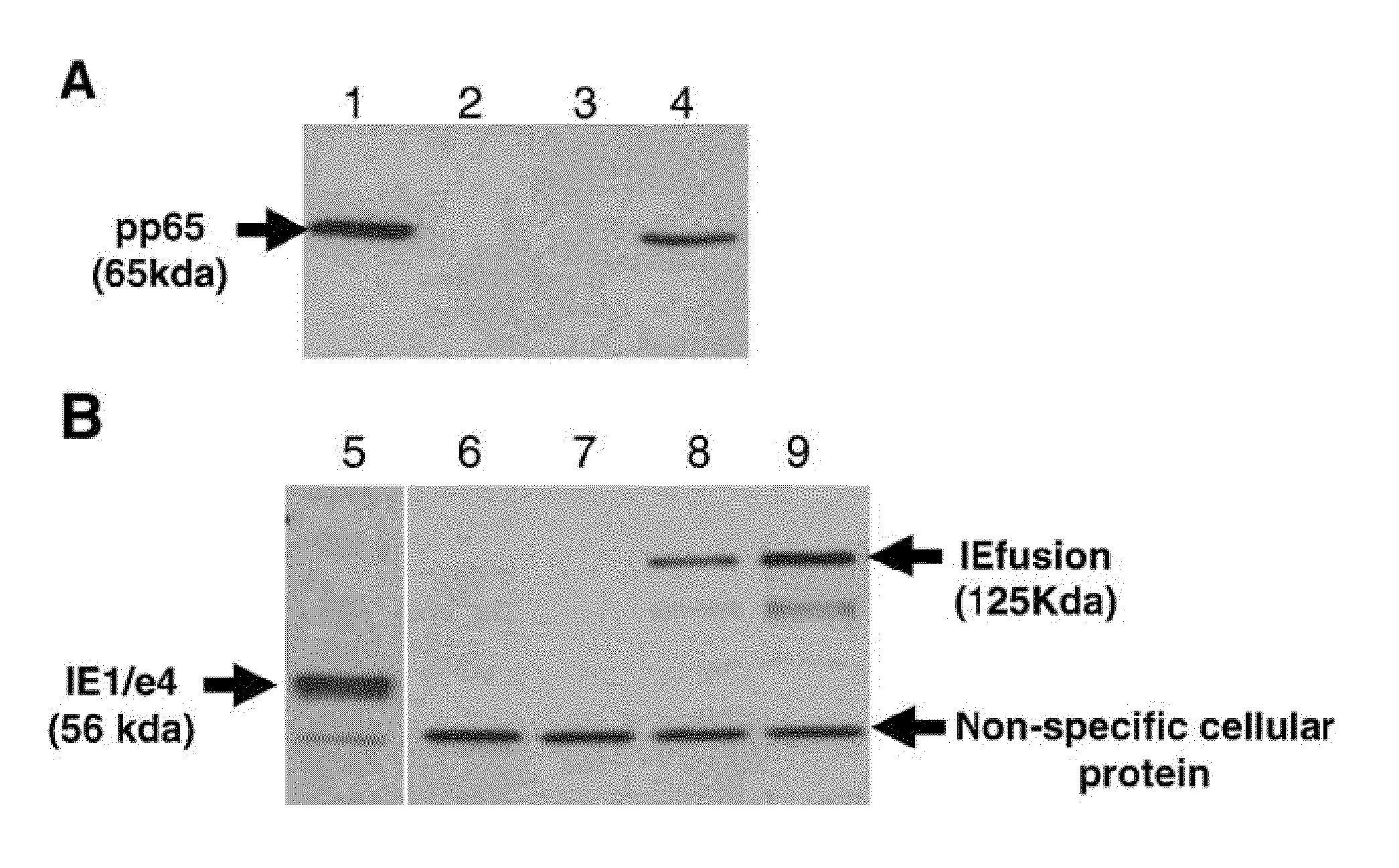
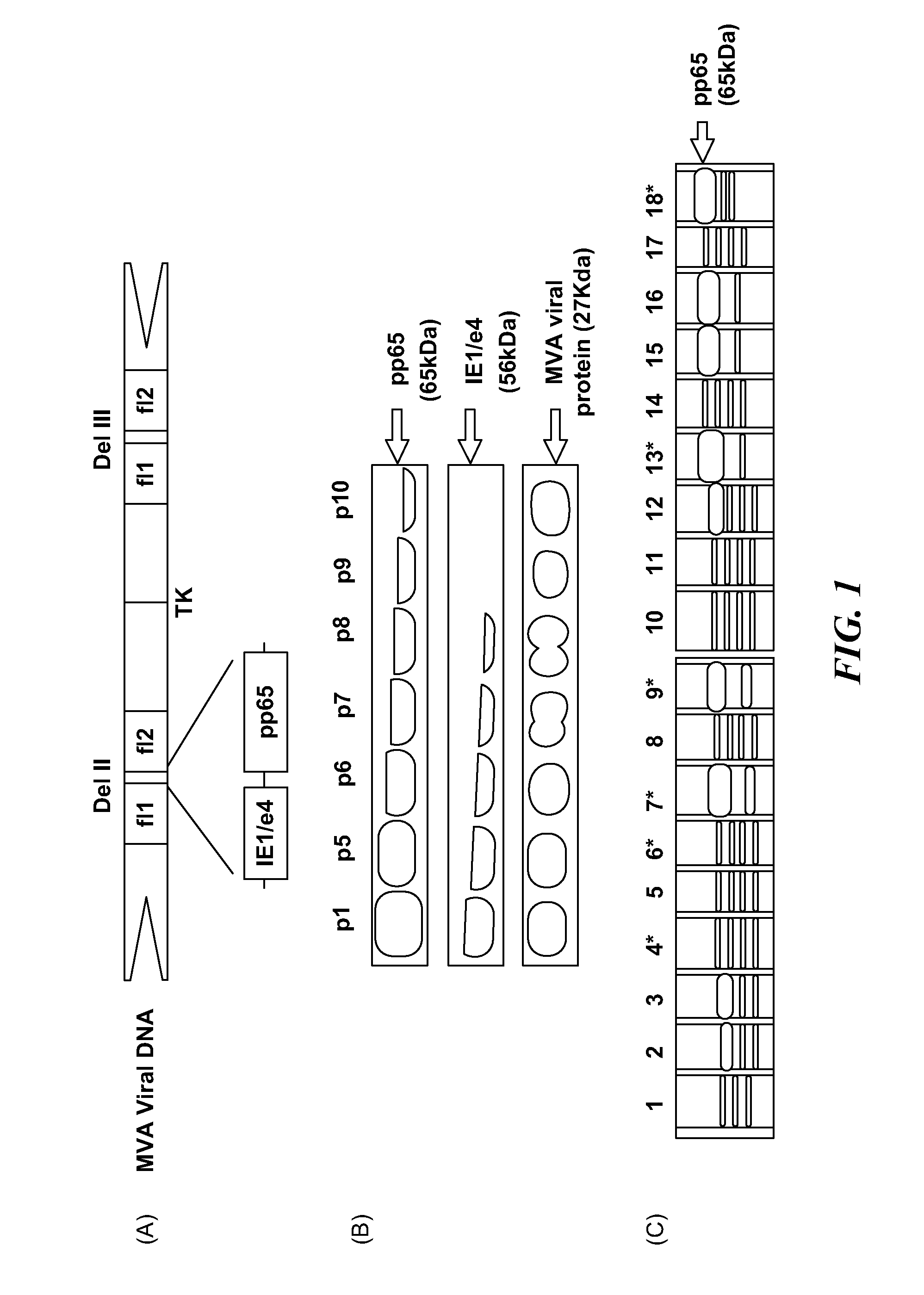
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Porcine epidemic diarrhea virus, and culture method and application thereof
ActiveCN103756974APerfect infection monitoring systemMicroorganism based processesAntiviralsSerum freeTGE VACCINE
The invention discloses a porcine epidemic diarrhea virus, and a culture method and an application thereof. The porcine epidemic diarrhea virus is called as coronaviridae coronavirus porcine epidemic diarrhea virus GDSJ / 2012, is preserved in China center for type culture collection on May 15th, 2013, and has the preservation number of CCTCC NO:V201309. The culture of the virus strain requires a serum-free DMEM culture solution containing trypsin and magnesium chloride. The virus strain can be used for preparation of a PEDV diagnostic kit and a PEDV vaccine, has good immunogenicity, and can make up for the deficiency of few conventional vaccine types.
Owner:WENS FOODSTUFF GRP CO LTD
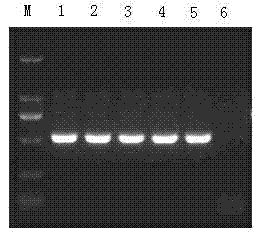
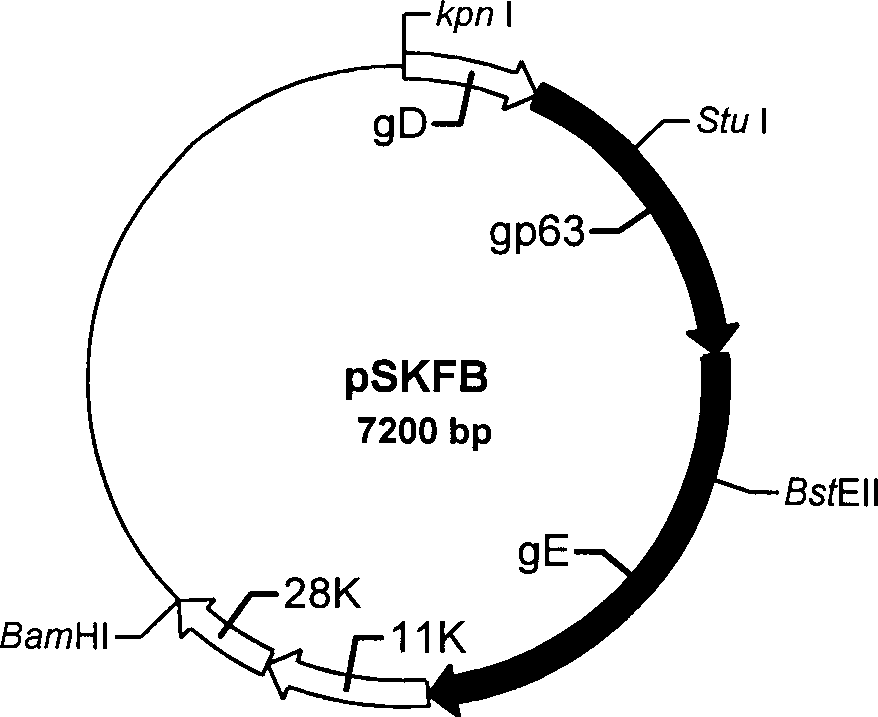
Pseudorabies TK*/gE*/gI* gene dificiency mark live vaccine and preparation method thereof
The present invention discloses a construction of three-gene defected recombinant pseudorabies virus (PrV) strain, vaccine prepared by said strain, method for constructing said strain and method for preparing said vaccine. The described recombinant pseudorabies virus strain defects genes of TK.gE ang gI, and contains no exogenous gene. The described vacine belongs to the freeze-dried live vaccine made up by virus liquid containing said invention and gelatin. Said invented live vaccine can be inoculated on the piglet, slaughter pig and sow with farrow, and can obtain obious immune effect. Said vaccine has good biological safety, and can be used for preventing and curing pseudorabies.
Owner:HUAZHONG AGRI UNIV
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
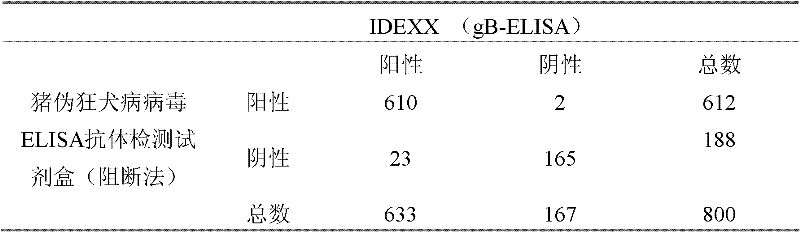
Kit for detecting pig pseudorabies virus antibodies and block enzyme-linked immuno sorbent assay (ELISA) method
The invention discloses a kit for detecting pig pseudorabies virus antibodies and a block enzyme-linked immuno sorbent assay (ELISA) method. The kit for detecting pig pseudorabies virus antibodies comprises pig pseudorabies virus monoclonal antibodies which are labelled by horseradish peroxidase, wherein the pig pseudorabies virus monoclonal antibodies are monoclonal antibodies obtained by pig pseudorabies viruses as immunogens and the pig pseudorabies viruses are pseudorabies virus strain Ea. The kit for detecting pig pseudorabies virus antibodies also comprises an enzyme label plate, a sample diluent, negative and positive contrasts, a coloured solution, a washing solution, and a stopping solution. The block ELISA method comprises the following steps of 1, taking out a detection plate pre-coated with virus antigens from the kit for detecting pig pseudorabies virus antibodies, adding diluted blood serum needing to be detected into the detection plate pre-coated with the virus antigens, and simultaneously, setting negative and positive contrast apertures, 2, shaking up the diluted blood serum in the negative and the positive contrast apertures, shaking off a solution in the negative and the positive contrast apertures, and washing the detection plate by the washing solution, and 3, adding the pig pseudorabies virus monoclonal antibodies labelled by horseradish peroxidase into the negative and the positive contrast apertures, washing, adding the colored solution into the negative and the positive contrast apertures to carry out room-temperature coloration in the dark, adding the stopping solution into the negative and the positive contrast apertures, and determining OD630nm values of the negative and the positive contrast apertures by an ELISA apparatus. The block ELISA method has the advantages of good singularity, high sensitivity, short detection time, and high accuracy because of utilization of an S / N ratio method in result determination.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Porcine reproductive and respiratory syndrome vaccine, based on isolate JA-142
InactiveUS6641819B2Improve efficiencyShorten the timeSsRNA viruses positive-senseViral antigen ingredientsPhysiologyVirus strain
Substantially avirulent forms of atypical porcine reproductive and respiratory syndrome (PRRS) virus and corresponding vaccines are provided which result from cell culture passaging of virulent forms of PRRS. The resultant avirulent atypical PRRS virus is useful as a vaccine in that PRRS specific antibody response is elicited by inoculation of host animals, thereby conferring effective immunity against both previously known strains of PRRS virus and newly isolated atypical PRRS virus strains. The preferred passaging technique ensures that the virus remains in a logarithmic growth phase substantially throughout the process, which minimizes the time required to achieve attenuation. The present invention also provides diagnostic testing methods which can differentiate between animals infected with field strains and attenuated strains of PRRSV.
Owner:US SEC AGRI +1
Porcine pseudorabies virus strain as well as inactivated vaccine and applications thereof
ActiveCN103305474APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsLaboratory cultureVirus strain
The invention discloses a porcine pseudorabies virus strain as well as an inactivated vaccine and applications thereof, belonging to the field of separation and application of the porcine pseudorabies virus strain. The invention firstly provides a porcine pseudorabies virus BJ strain separated from diseased pig tissues, and the microbial preservation number of the porcine pseudorabies virus BJ strain is CGMCC (China General Microbiological Culture Collection Center) No.7351. The invention discloses a method for preparing the inactivated vaccine by applying the porcine pseudorabies virus BJ strain. The method comprises the steps of culturing a virus strain to obtain a virus solution; adding an inactivator, and inactivating and concentrating the virus solution; and evenly mixing an adjuvant and the virus solution, and emulsifying to obtain the inactivated vaccine. The technological parameters of the inactivated vaccine preparation method are further optimized, and the immune protection efficacy and safety of the inactivated vaccine can be improved. Shown by the immune protection efficacy and safety tests, the porcine pseudorabies inactivated vaccine prepared has good immune protection efficacy and safety, and can be clinically used for preventing or treating porcine pseudorabies.
Owner:泰州博莱得利生物科技有限公司 +2
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Influenza Virus Vaccines
InactiveUS20080254065A1SsRNA viruses negative-senseViral antigen ingredientsFowlInfluenza virus vaccine
The invention provides a vaccine for protecting a human patient against infection by a human influenza virus strain, wherein the vaccine comprises an antigen from an avian influenza virus strain that can cause highly pathogenic avian influenza. The antigen can invoke an antibody response in the patient that is capable of neutralising said human influenza virus strain. Whereas the prior art used known non-pathogenic avian strains to generate antibodies in humans against known pathogenic avian strains, the invention uses known pathogenic avian strains to protect against emerging pathogenic human strains. Furthermore, whereas the prior art focused on achieving a close antigenic match between the vaccine strain and the target strain, the invention selects vaccine strains based on their pathogenicity, regardless of any perceived close antigenic relationship to the target strain. As the invention does not require detailed knowledge of an emerging strain, a vaccine can be provided further in advance to reduce the risk and potential effects of a human pandemic outbreak.
Owner:SEQIRUS UK LTD
Sterilization system with ultraviolet emitter for eradicating biological contaminants
ActiveUS8779391B2Improve sanitation and health and safetyLittle dangerAutomatic obstacle detectionTravelling automatic controlFluorescenceUltraviolet lights
An exemplary sterilization system includes a self-propelled robotic mobile platform for locating and eradicating infectious bacterial and virus strains on floors (and objects thereon), walls, cabinets, angled structures, etc., using one or more ultraviolet light sources. A controller allows the system to adjust the quantity of ultraviolet light received by a surface by, for example, changing the intensity of energy input to a ultraviolet light source, changing a distance between a ultraviolet light source and a surface being irradiated, changing the speed / movement of the mobile platform to affect time of exposure, and / or by returning to contaminated areas for additional passes. The mobile platform may include a sensor capable of detecting fluorescence of biological contaminants irradiated with ultraviolet light to locate contaminated areas. The system is thus capable of “seek and destroy” functionality by navigating towards contaminated areas and irradiating those areas with ultraviolet light accordingly.
Owner:FLAHERTY KAREN
Method for expanding antigen spectrum of foot-and-mouth disease vaccine strain by reverse genetic operation and preparation method of vaccine
ActiveCN101948811AHigh protection rateBroad antigen spectrumVirus peptidesMicroorganism based processesImmune effectsSoutheast asia
The invention relates to a method for expanding the antigen spectrum of a foot-and-mouth disease vaccine strain by reverse genetic operation and a preparation method of a vaccine. The amino acid sequence of the VP3 and VP1 structural proteins of the foot-and-mouth disease virus strain of the invention is represented by the amino acid residues from a position 304 to a position 736 in SEQ ID No.4. Experiments show that the vaccine prepared from the mutant virus strain obtained by the invention can resist porcine epidemic viruses of China O / TL / Taiwan / 97 lineage, Pan-Asia O / China / 99 lineage and Southeast Asia Myanmar O / GS / 2010 / 98 lineage, has a characteristic of wide antigen spectrum, can immunize pigs and obviously improve the rate of protection against foot-and-mouth disease viruses which are of the same type and have antigenicity difference, achieves an immune effect of cross protection, and is expected to play an important role in the prevention and control of foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Novel coronavirus antigen epitope and application thereof
ActiveCN111848753AImproving immunogenicityHigh titerSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigen epitopeCoronavirus vaccination
The invention provides a novel coronavirus antigen epitope and application thereof. A super computer is used for simulating S, E, M and N protein structures of the novel coronavirus, and novel coronavirus epitopes SEQ ID NO 1-38 are obtained through calculation. The epitopes have very good immunogenicity; an antibody generated by inducing a part of epitope polypeptide has the effect of neutralizing the novel coronavirus; the antigen epitope can be combined with antibodies in serum of novel coronavirus patients at home and abroad, has the potential of resisting various novel coronavirus strains, and also has the potential of identifying and applying different strains. The epitope provided by the invention can be used for (1) research and development of novel coronavirus vaccines and universal coronavirus vaccines such as MERS viruses and SARS viruses; and (2) preparing a novel coronavirus antibody, and further detecting and typing viruses and treating diseases caused by the viruses. Thenovel coronavirus antigen epitope has a wide application prospect in the aspects of prevention of coronavirus and propagation and outbreak thereof, and detection and diagnosis of virus strains.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Wheat-rye T2BL.1RS translocation line germplasm breeding method
InactiveCN101263782AExcellent yield traitsPlant tissue cultureHorticulture methodsDiseaseHigh resistance
The invention provides a breeding method for the germ plasm of a new translocation line wheat-rye T2BL.1RS, which is characterized in that, distant hybridization is performed by taking common wheat variety Xiaoyan 6 as female parent and relative plant rye variety German White as male parent; seeds are acquired after immature embryo salvation culture, colchicine half root soaking method and chromosome doubling; backcross seeds are acquired after disease resistance identification, cytological identification and backcrossing utilizing the original parent Xiaoyan 6 as male parent; then after disease resistance identification and cytological identification, individual plants with excellent comprehensive characters are selected in direction for self crossing, and after continuous self cross selective breeding for six generations, the finally acquired individual plant is confirmed to be wheat-rye T2BL.1RS translocation line after identified by continuous total genome in situ hybridization and three-probe multicolor fluorescence in situ hybridization. The breeding method for the germ plasm of a new translocation line wheat-rye T2BL.1RS has the advantages that, the wheat-rye T2BL.1RS translocation line has high resistance to stripe rust, current prevalent fungus strain, powdery mildew and other important virus strains; the invention has excellent high yield character; the method lays an excellent germplasm base for the cultivation of breakthrough new wheat variety with disease resistance and high yield.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
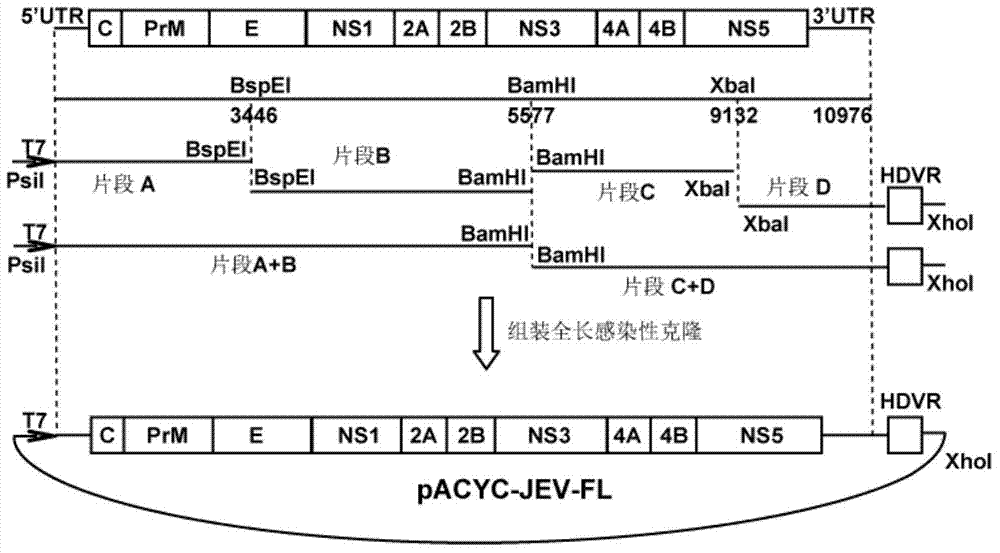
Japanese encephalitis virus (JEV) infectious clone with luciferase gene and building method and application thereof
InactiveCN103497972ASolve the phenomenon of not copyingFast growing trendMicrobiological testing/measurementFluorescence/phosphorescenceLuciferase GeneVaccine evaluation
The invention discloses a Japanese encephalitis virus (JEV) infectious clone with a luciferase gene, and a building method and application thereof. The JEV infectious clone with the luciferase gene is prepared by the following steps: (1) sectional synthesis of a JEV SA14 strain gene sequence; (2) assembling and building of the JEV infectious clone; (3) building of the JEV infectious clone with the luciferase gene. It is proved that a Rluc-JEV report virus with the same growth tendency as the JEV virus can be saved by the JEV infectious clone with the luciferase gene built by the method, and the JEV infectious clone has wide application value in the aspects of an animal model, virus replication and pathogenesis, drug screening and drug action mechanism, live animal imaging and vaccine evaluation and the like through the experiments of immunofluorescence, Rluc activity detection, virus bacteriophage plaque, drug inhibition, live animal imaging, vaccine evaluation and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Porcine seneca valley virus strain and application thereof
ActiveCN107513524AGood strain backgroundLay the material foundationSsRNA viruses positive-senseViral antigen ingredientsPig farmsDisease
The invention discloses a porcine seneca valley virus strain and an application thereof. In the invention, the seneca valley virus strain CH-FJZZ-2017 is isolated from pathological materials of porcine idiopathic vesicular disease in a pig farm in Fujian Province, with the preservation number of CGMCC No. 12160. The isolated porcine seneca valley virus CH-FJZZ-2017 in the invention is isolated from swinery newly suffering from the epidemic disease within Chinese territory, represents the current epidemic predominant virus strain in China, has a good virus strain background, and can be used as an inactivated vaccine production virus strain and a virus seed for testing, thereby providing a material for subsequent relevant experimental study, and laying a material foundation.
Owner:CHINA ANIMAL HUSBANDRY IND
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Attenuated vaccine strain for avian infectious bronchitis virus and application thereof
InactiveCN102851257AImprove securityInactivation/attenuationMicroorganism based processesInfectious bronchitisFowl
The method relates to an attenuated vaccine strain for avian infectious bronchitis virus and application thereof. According to the invention, avian infectious bronchitis virus (IBV) is attenuated, so as to successfully obtain an IBV attenuated strain CCTCC.V201232 and a derivative virus strain thereof. The attenuated strain and derivative virus strain provided by the invention can be used in preparation of a vaccine composition for prevention of infectious bronchitis. Experiments show that the attenuated strain and vaccine composition provided by the invention can be inoculated to immature birds, so as to effectively activate immune system in the birds and well prevent avian infectious bronchitis.
Owner:SHANGHAI QISHENG BIOTECH CO LTD
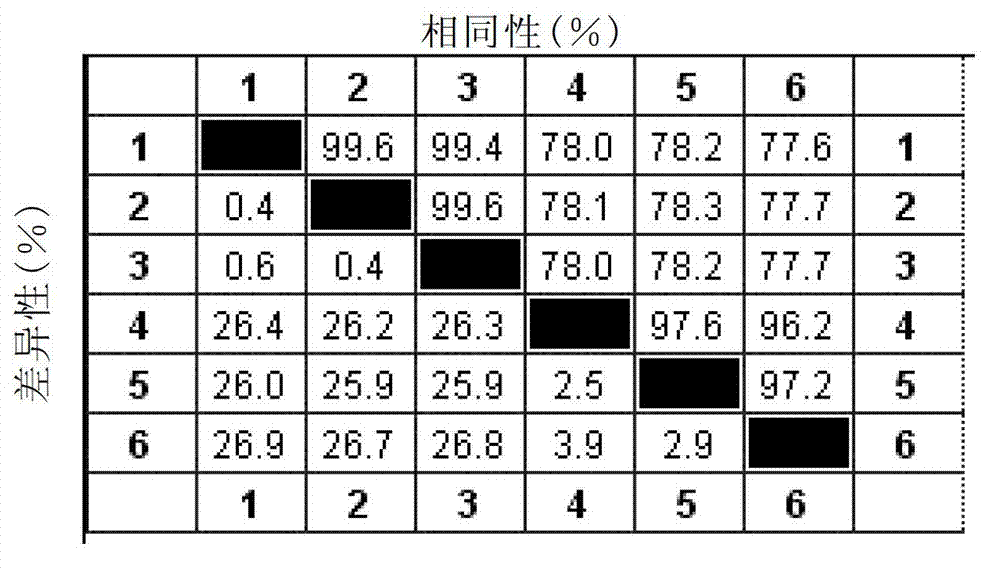
Porcine reproductive and respiratory syndrome isolates and methods of use
ActiveUS7632636B2SsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsVirus strain
A method of predicting the virulence of a new or uncharacterized PRRS virus strain is provided wherein the strain is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus strain of known virulence, as a measure of the virulence of the new or uncharacterized strain.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com