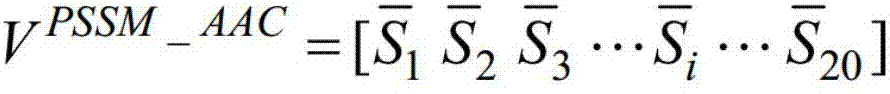Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
173 results about "Env Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
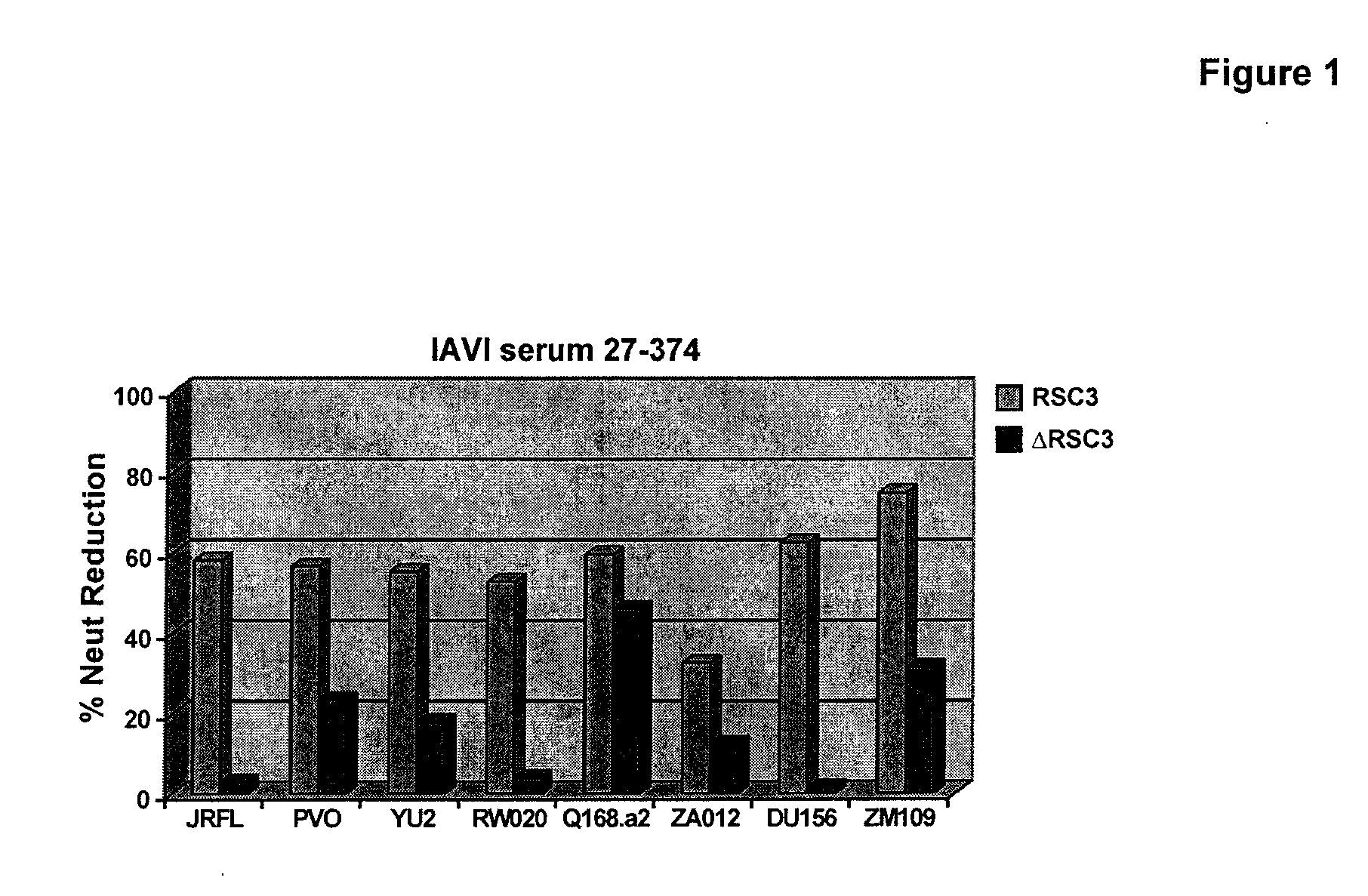
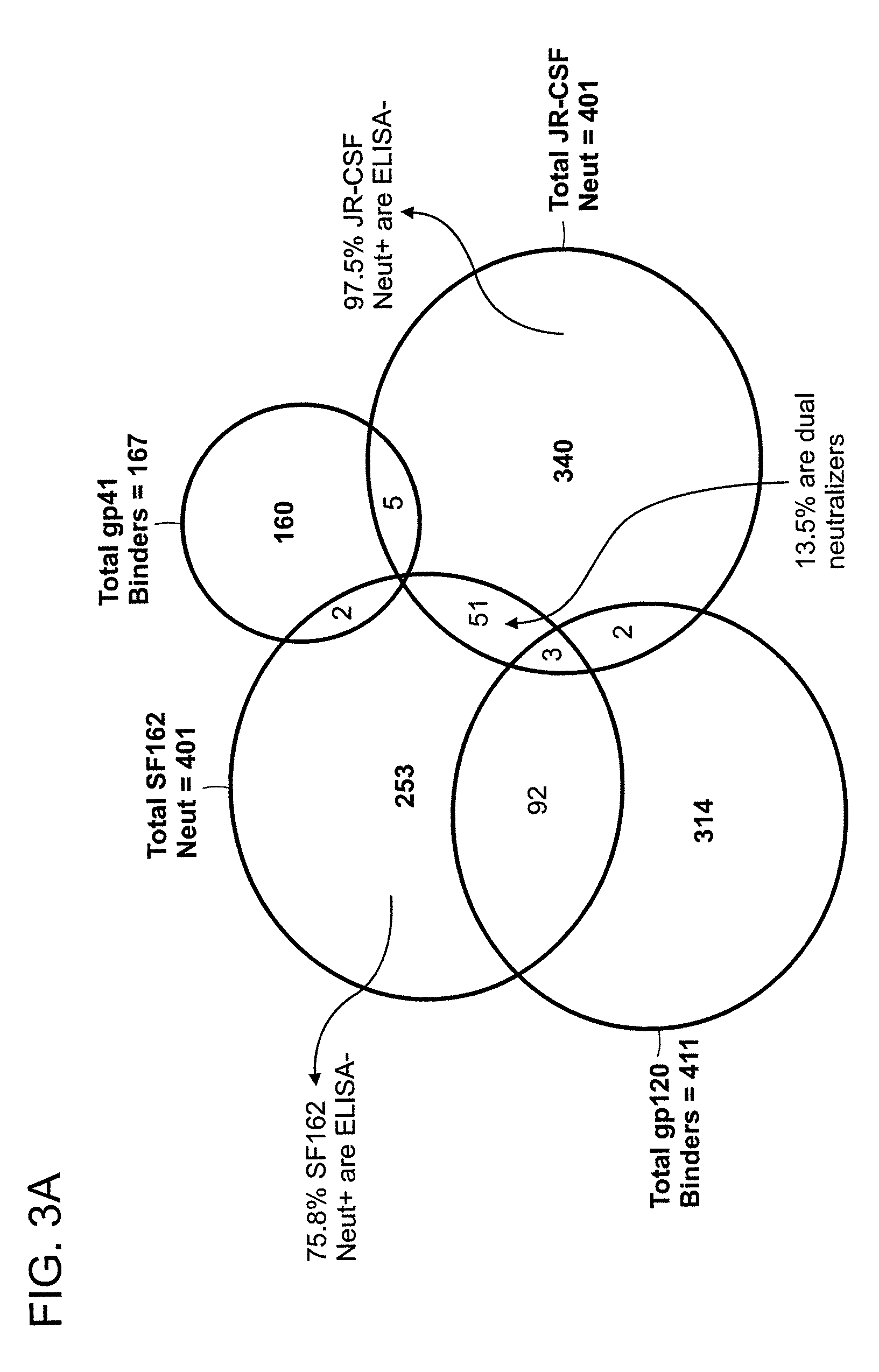
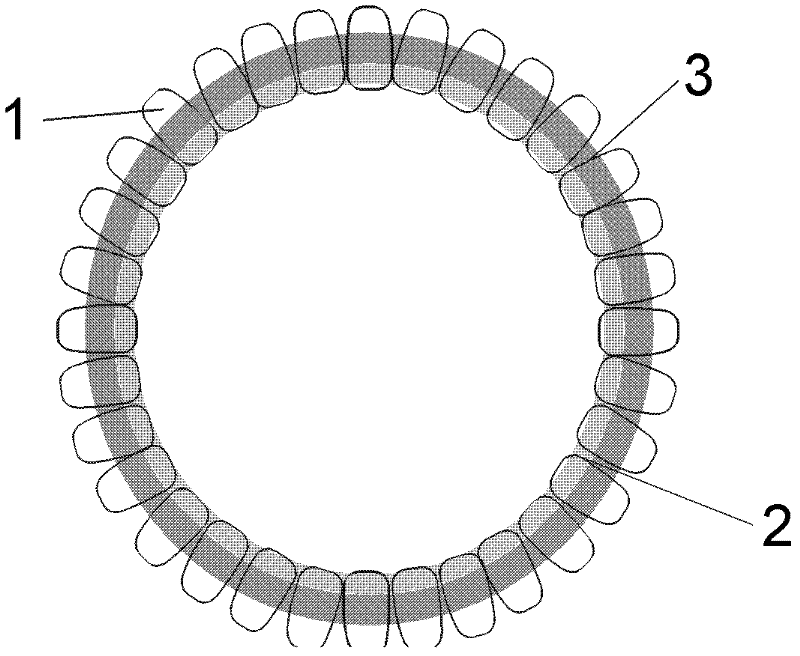
INDUCTION OF BROADLY REACTIVE NEUTRALIZING ANTIBODIES BY FOCUSING THE IMMUNE RESPONSE ON V3 EPITOPES OF THE HIV-1 gp120 ENVELOPE
InactiveUS20080279879A1Vigorous Ab responseViral antigen ingredientsAntibody mimetics/scaffoldsHeterologousNeutralizing antibody
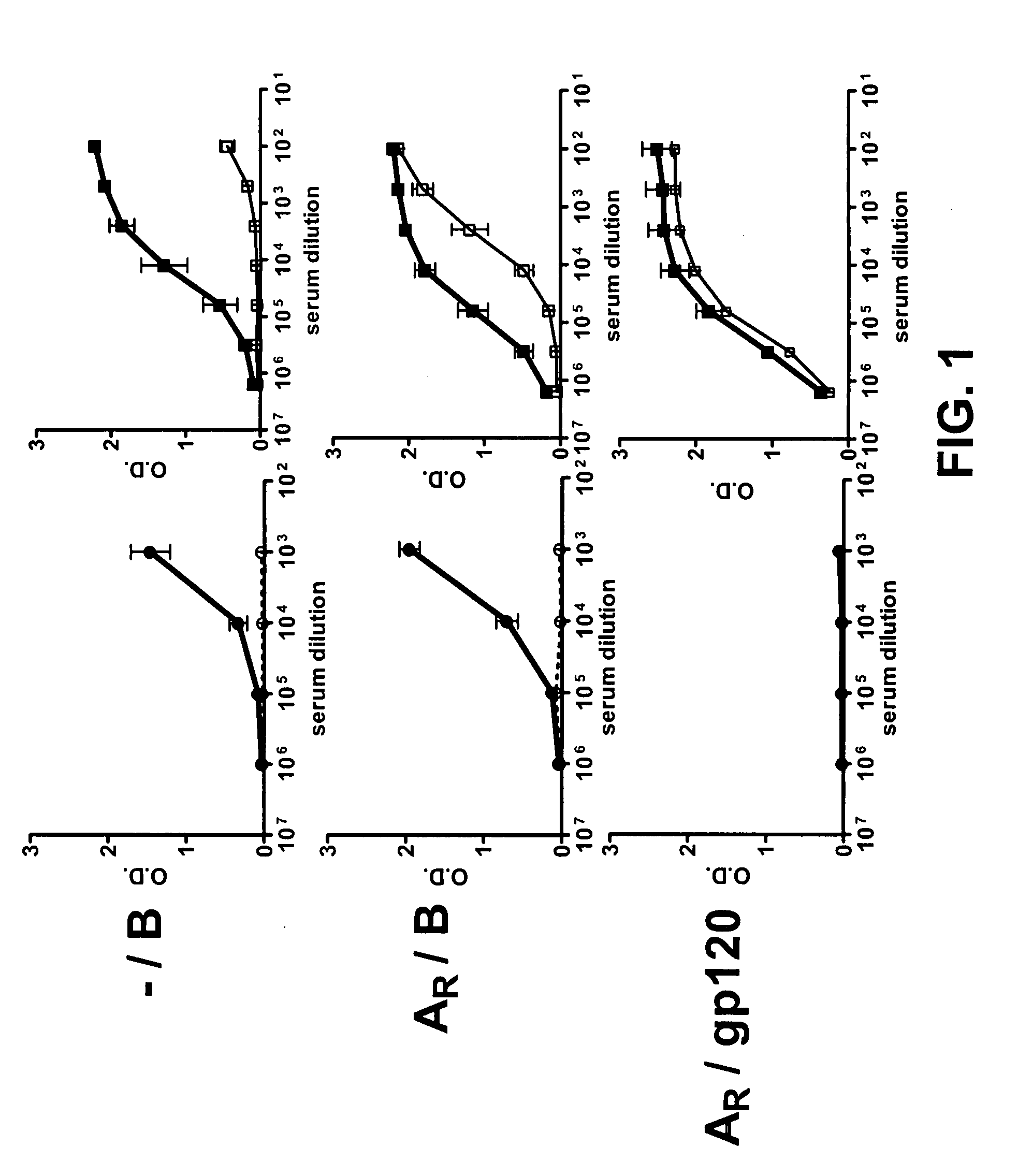
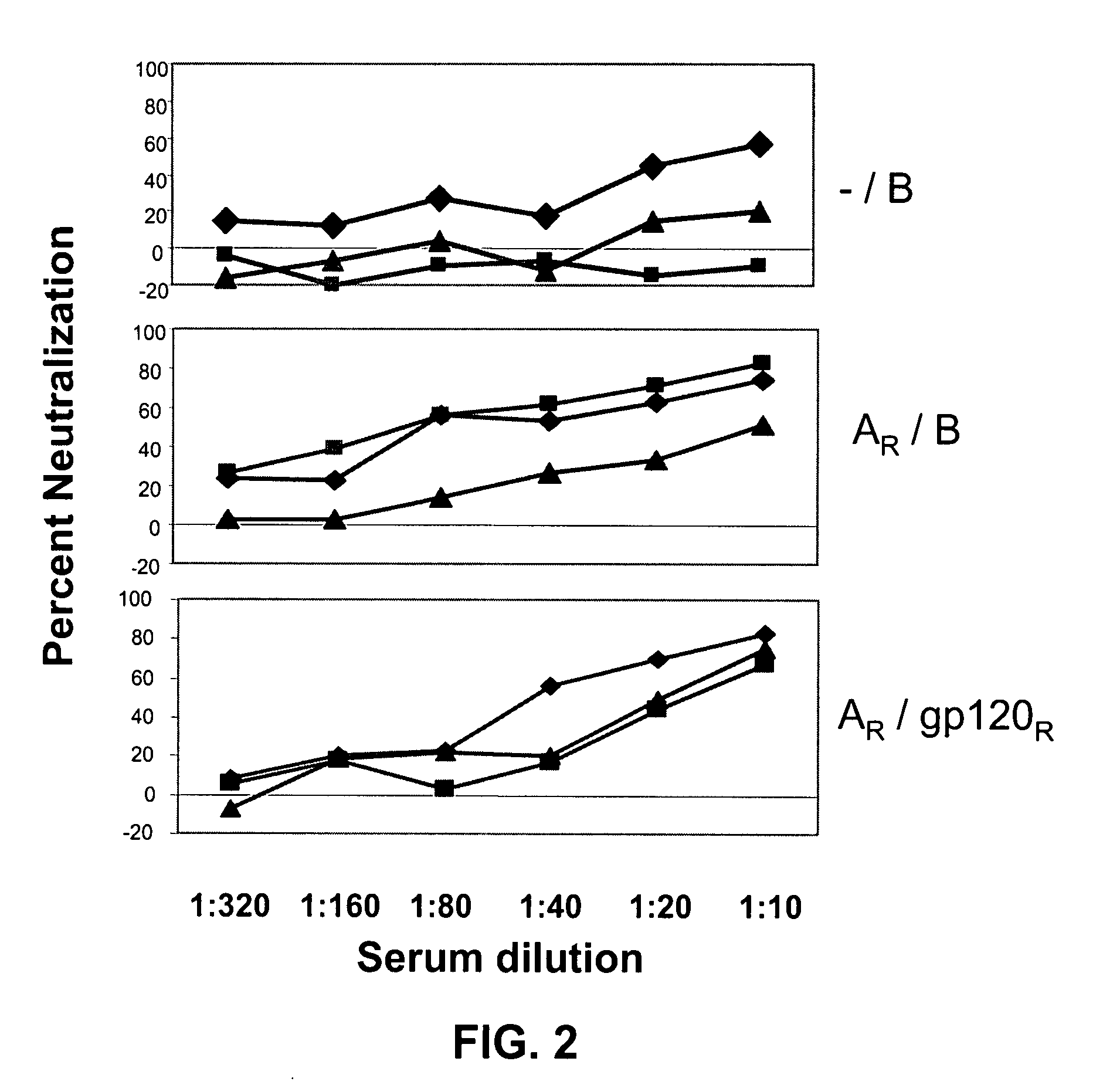
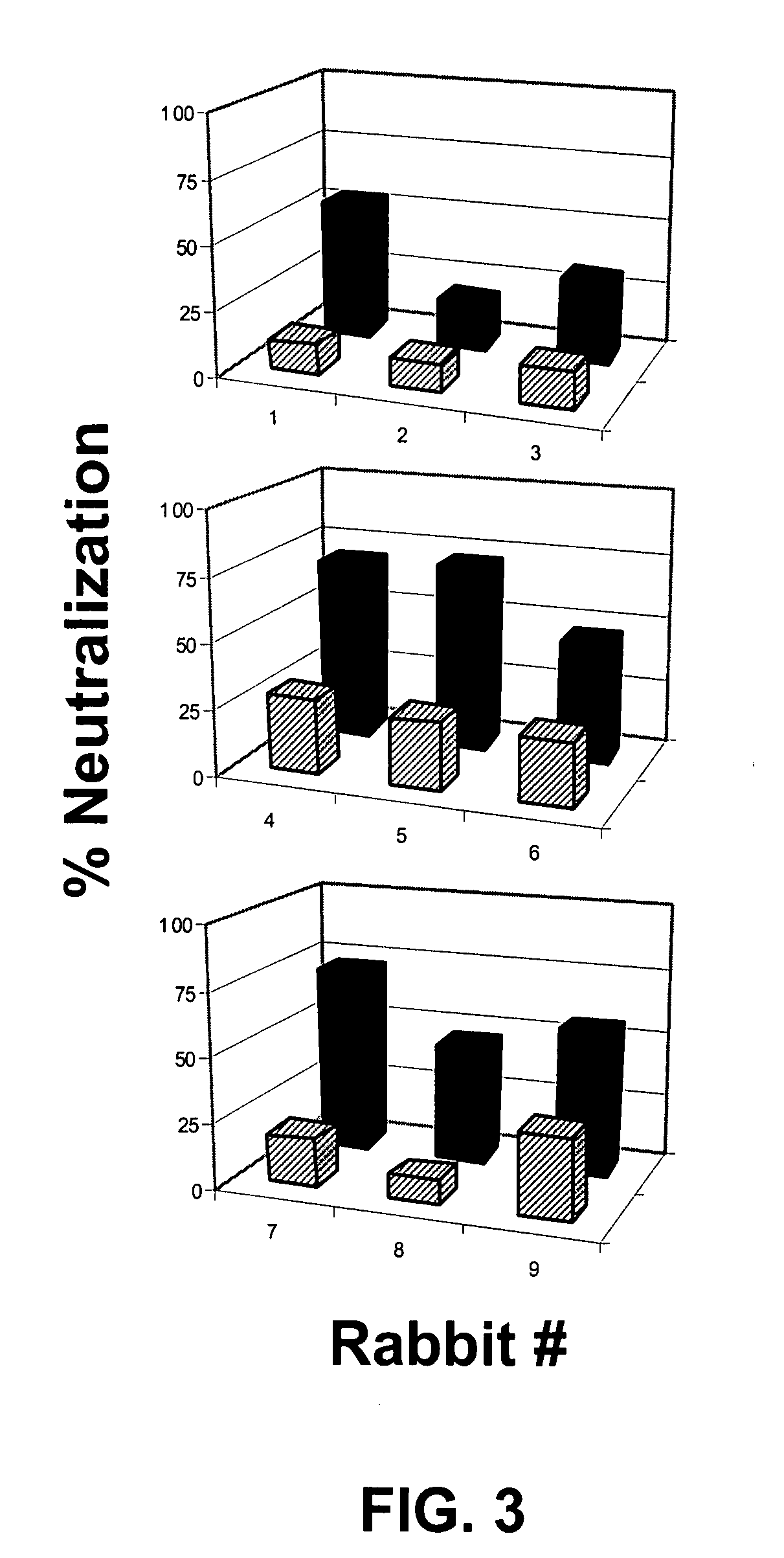
Compositions, kits and methods for boosting, or for priming and boosting, high titer broadly neutralizing cross-clade antibody responses focused on single HIV-1 neutralizing epitopes are disclosed. gp120 DNA plasmids comprising HIV env genes are used to prime the antibody response. Primed subjects are immunized with recombinant fusion proteins that comprise a “carrier” protein fusion partner, preferably a truncated form of the MuLV gp70 Env protein, and a desired HIV neutralizing epitopes. Preferred epitopes are epitopes of V3 from one or more HIV clades. Immune sera from such immunized subjects neutralized primary isolates from virus strains heterologous to those from which the immunogens were constructed. Neutralizing activity was primarily due to V3-specific antibodies and cross-clade neutralizing Abs were present. This approach results in more potent and broader neutralizing antibody levels, a result of “immunofocusing” the humoral immune response on neutralizing epitopes such as V3.
Owner:NEW YORK UNIV
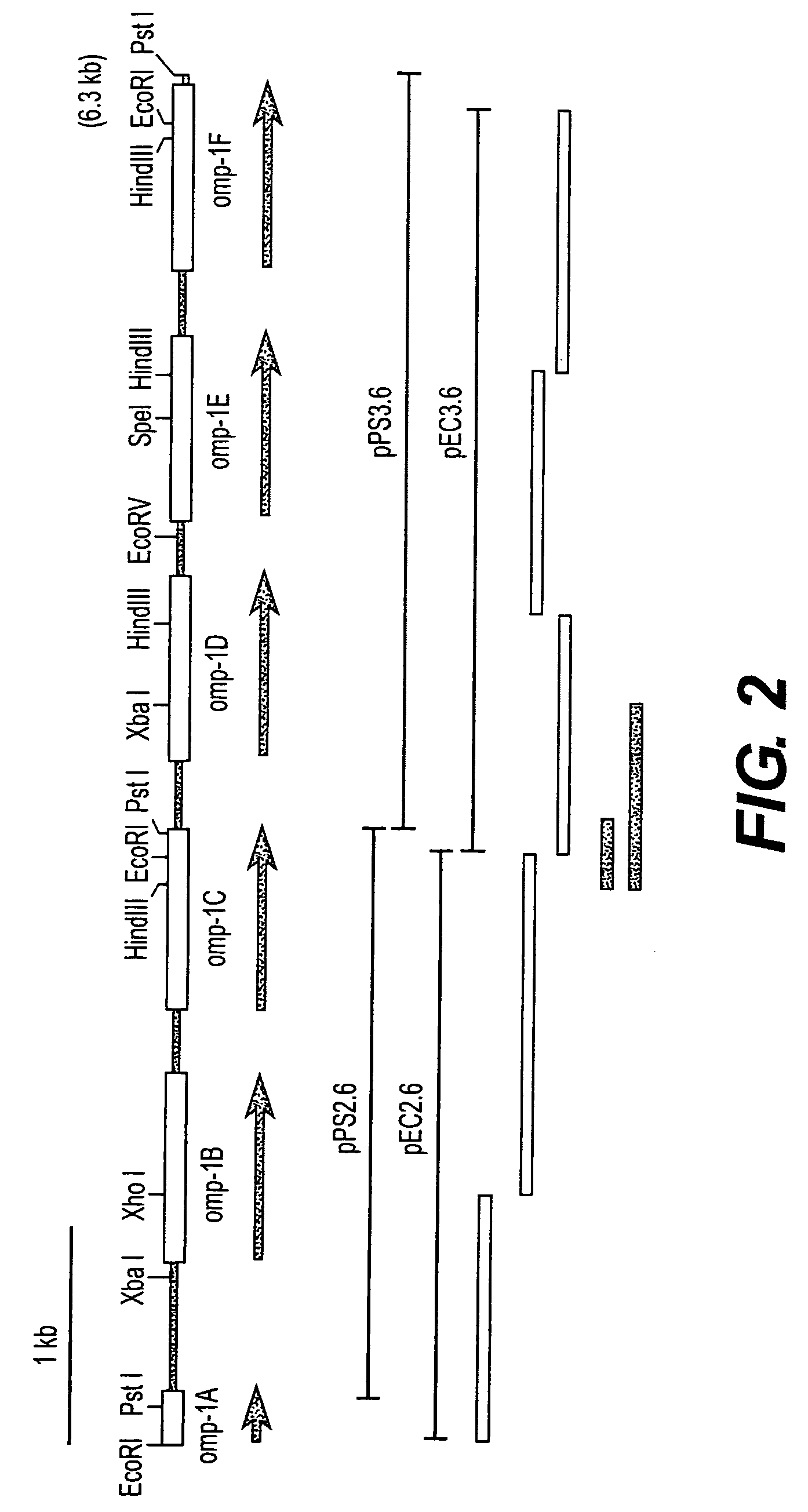
Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family
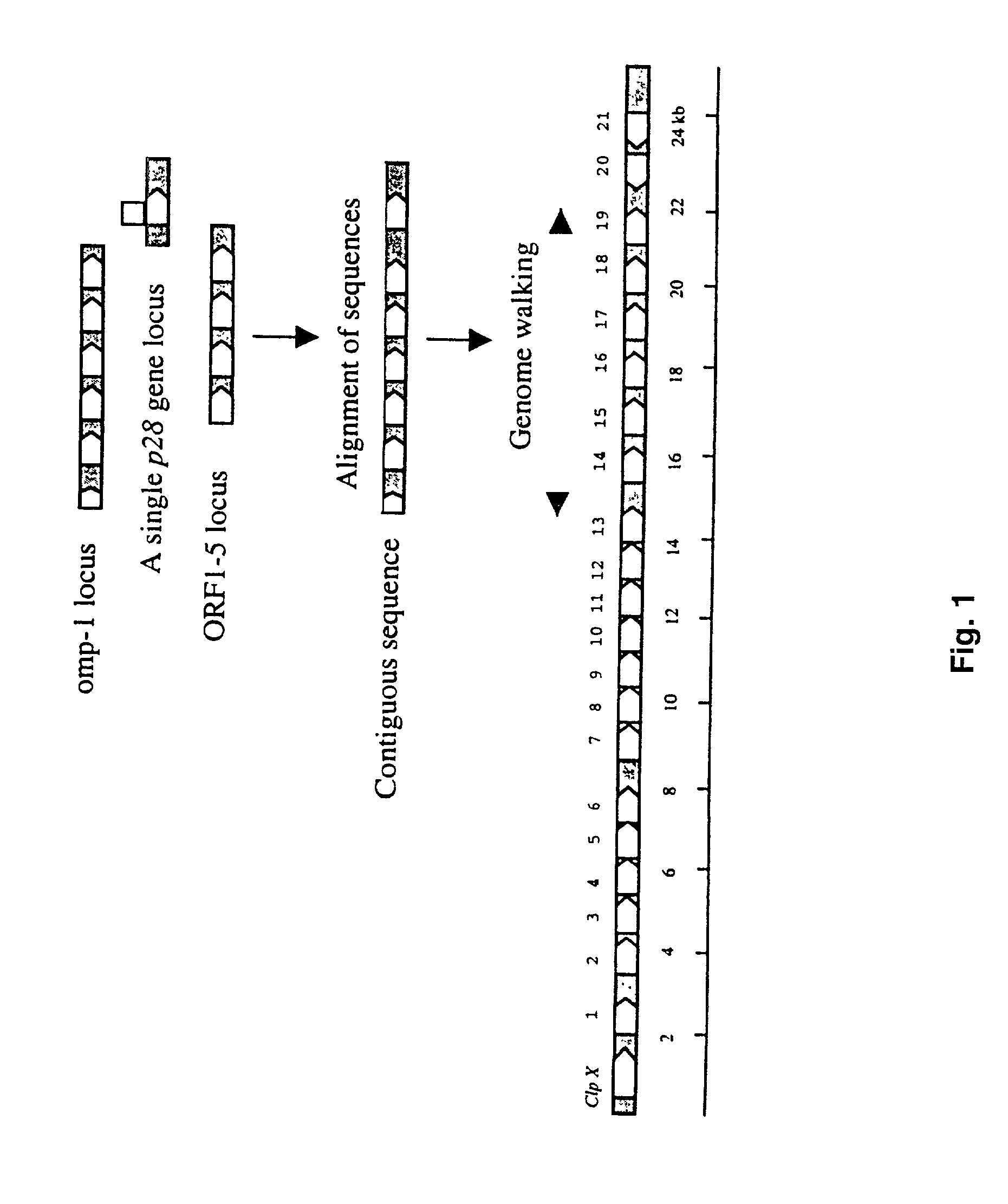
The 28-kDa outer membrane proteins (P28) of Ehrlichia chaffeensis are encoded by a multigene family consisting of 21 members located in a 23-kb DNA fragment in the genome of E. chaffeensis. Fifteen of these proteins are claimed herein as novel sequences. The amino acid sequence identity of the various P28 proteins was 20-83%. Six of 10 tested p28 genes were actively transcribed in cell culture grown E. chaffeensis. RT-PCR also indicated that each of the p28 genes was monocistronic. These results suggest that the p28 genes are active genes and encode polymorphic forms of the P28 proteins. The P28s were also divergent among different isolates of E. chaffeensis. The large repertoire of the p28 genes in a single ehrlichial organism and antigenic diversity of the P28 among the isolates of E. chaffeensis suggest that the P28s may be involved in immune avoidance.
Owner:RES DEVMENT FOUND
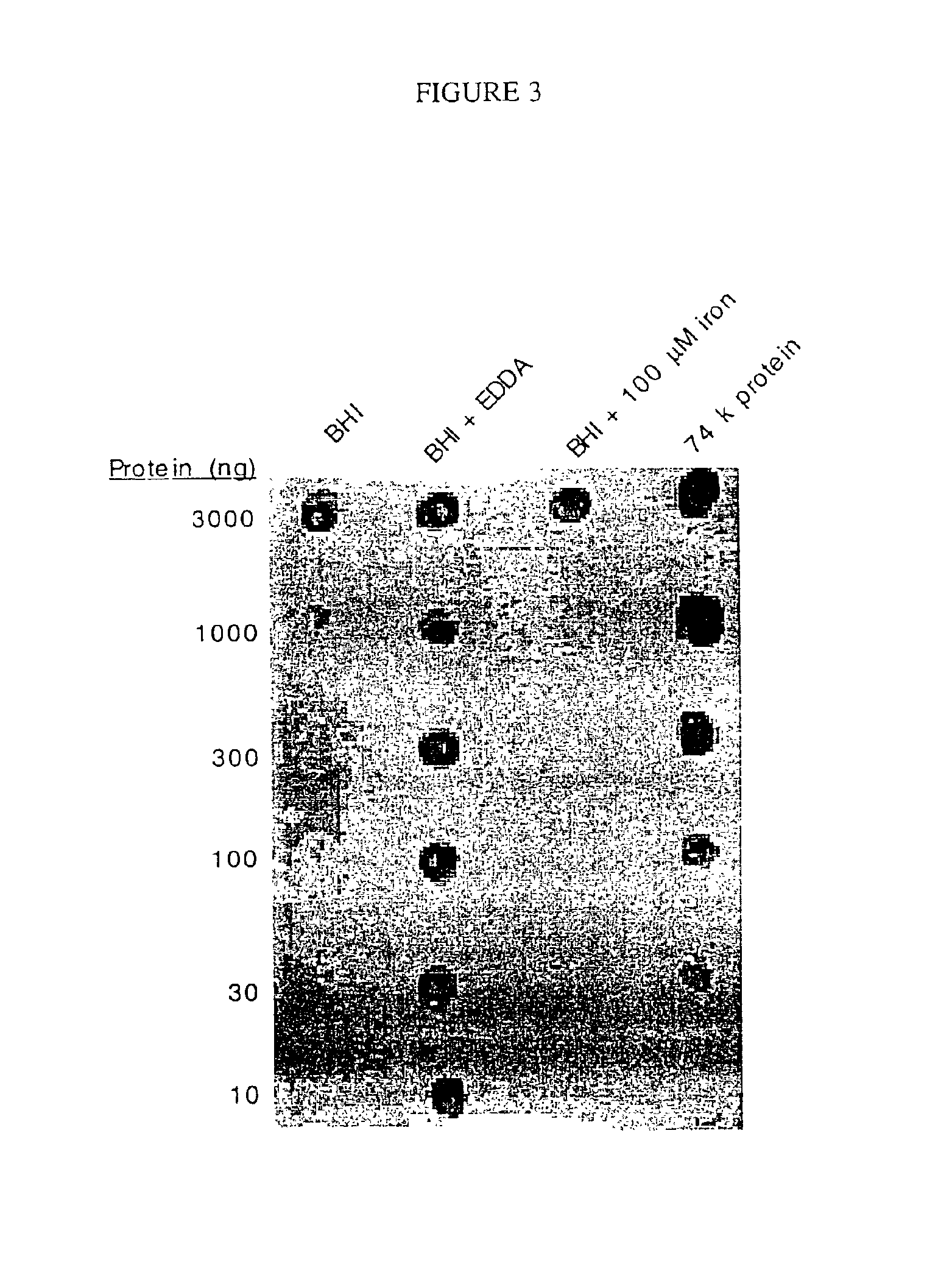
74-kilodalton outer membrane protein from Moraxella catarrhalis
InactiveUS6899885B1Enhance antibody responseLower titerBacterial antigen ingredientsBacteriaDiseaseKilodalton
A protein from M. catarrhalis, designated the 74 kD protein, is isolated and purified. The 74 kD protein has an amino-terminal amino acid sequence which is conserved among various strains of M. catarrhalis. The protein has a molecular weight of approximately 74.9 kD as measured on a 10% SDS-PAGE gel, while its molecular weight as measured by mass spectrometry is approximately 74 kD. The 74 kD protein is used to prepare a vaccine composition which elicits a protective immune response in a mammalian host, to protect the host against disease caused by M. catarrhalis.
Owner:WYETH HOLDINGS CORP
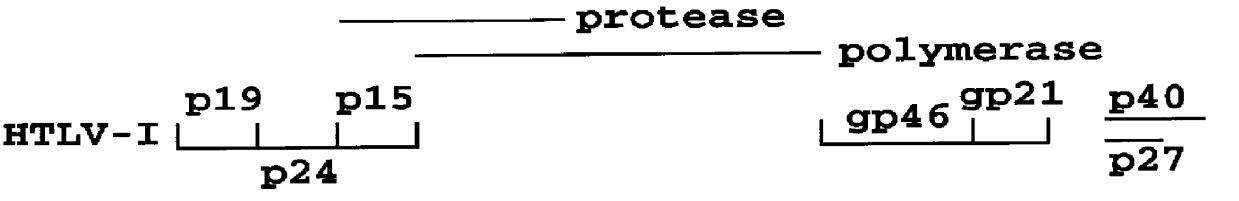
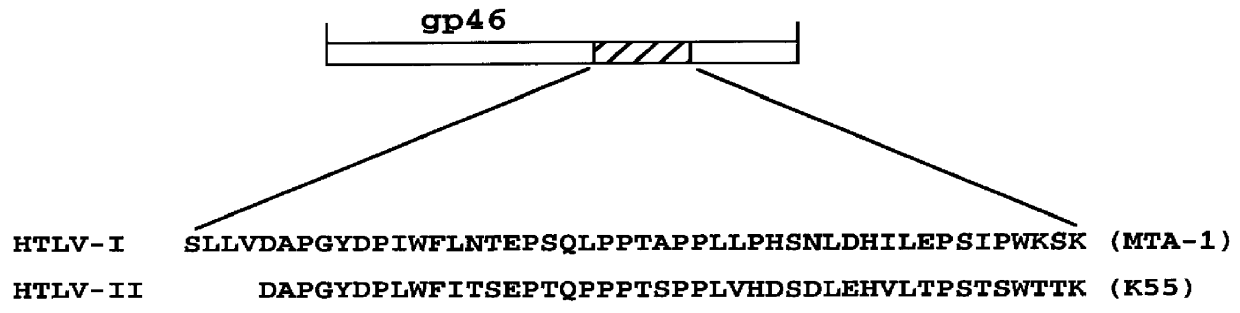
HTLV-I/HTLV-II assay and method
InactiveUS6110662AMicrobiological testing/measurementBiological material analysisPeptide antigenSerum samples
Method and assay kit for positively identifying HTLV-I and HTLV-II infection from human serum samples. The kit includes peptide antigens from the C-terminal regions of HLTV-I p19 and HTLV-II p21 gag proteins, and peptide antigens from the HLTV-I and HTLV-II env proteins immobilized on a solid support. After reaction of the serum sample with the solid support, an antibody-detection reagent in the kit is added to the support, to detect binding of human serum antibodies to each of the peptide antigens separately. The test allows positive identification of HTLV-I or HTLV-II when antibody binding to each HTLV-I or HTLV-II gag and env peptide antigen, respectively, is observed. Also disclosed is a kit for screening human sera for evidence of HTLV-I or HTLV-II infection.
Owner:GENELABS TECH INC +1
Novel HIV -1 broadly neutralizing antibodies
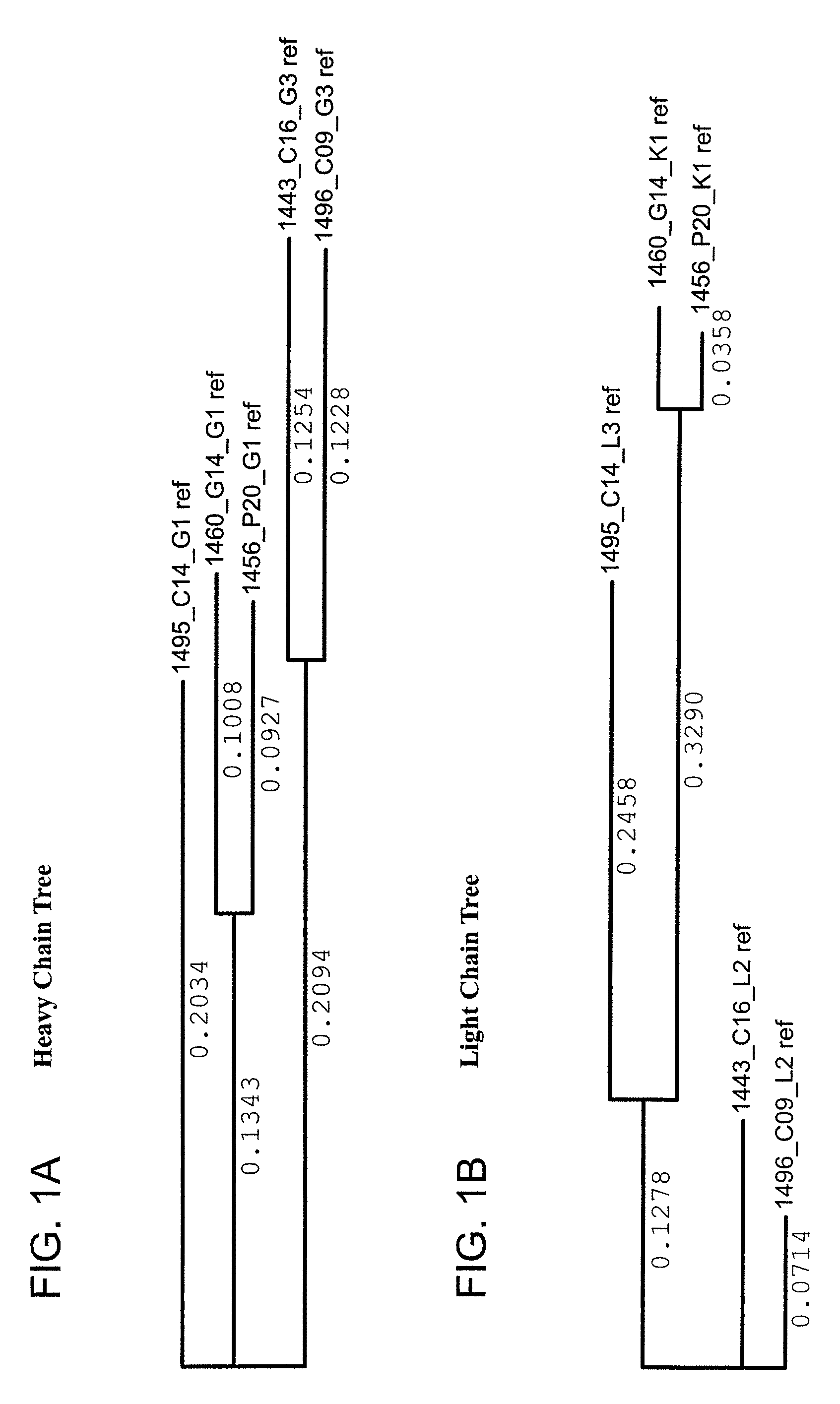
ActiveUS20130251726A1Reduce the impactMicrobiological testing/measurementImmunoglobulins against virusesHeavy chainNeutralising antibody
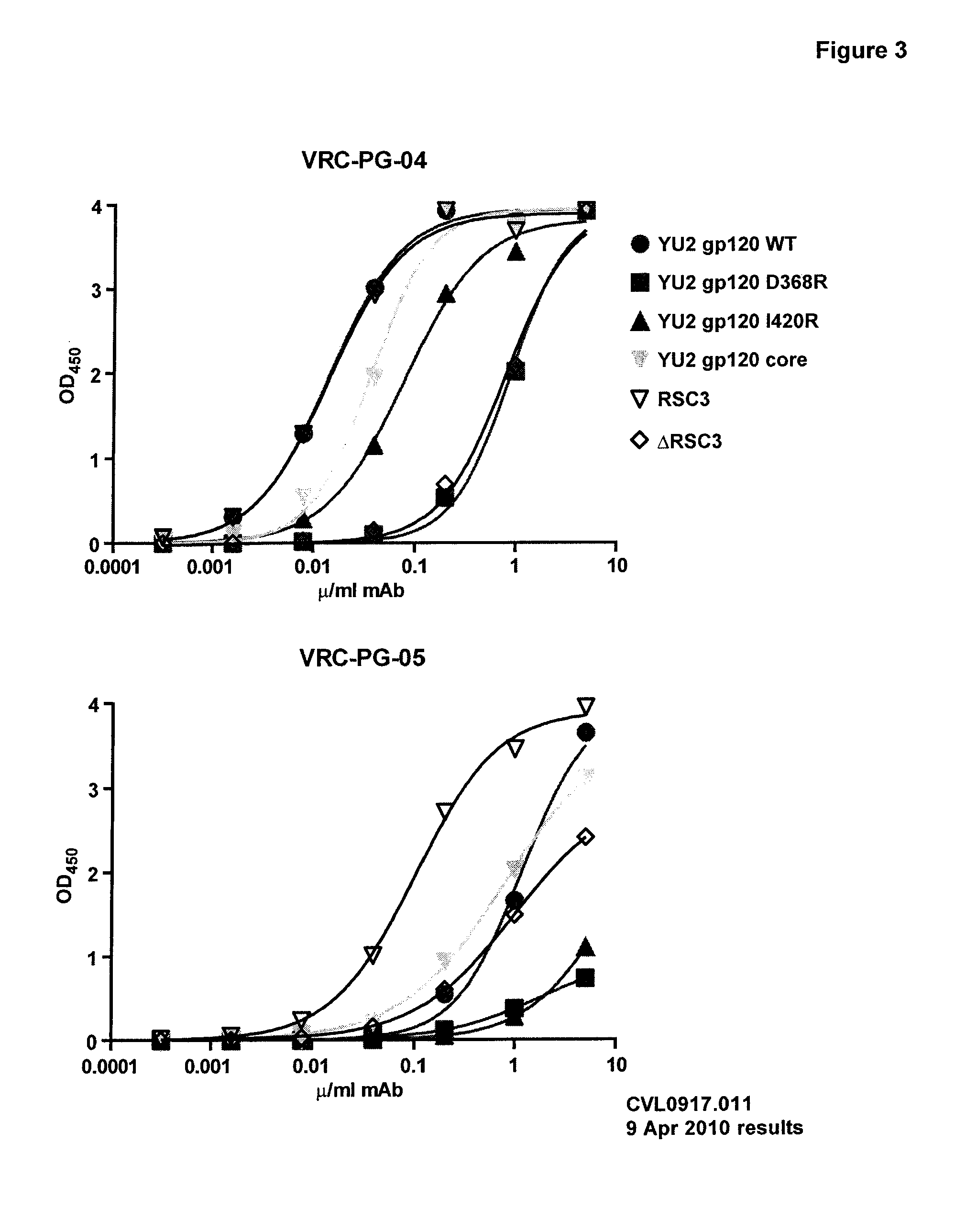
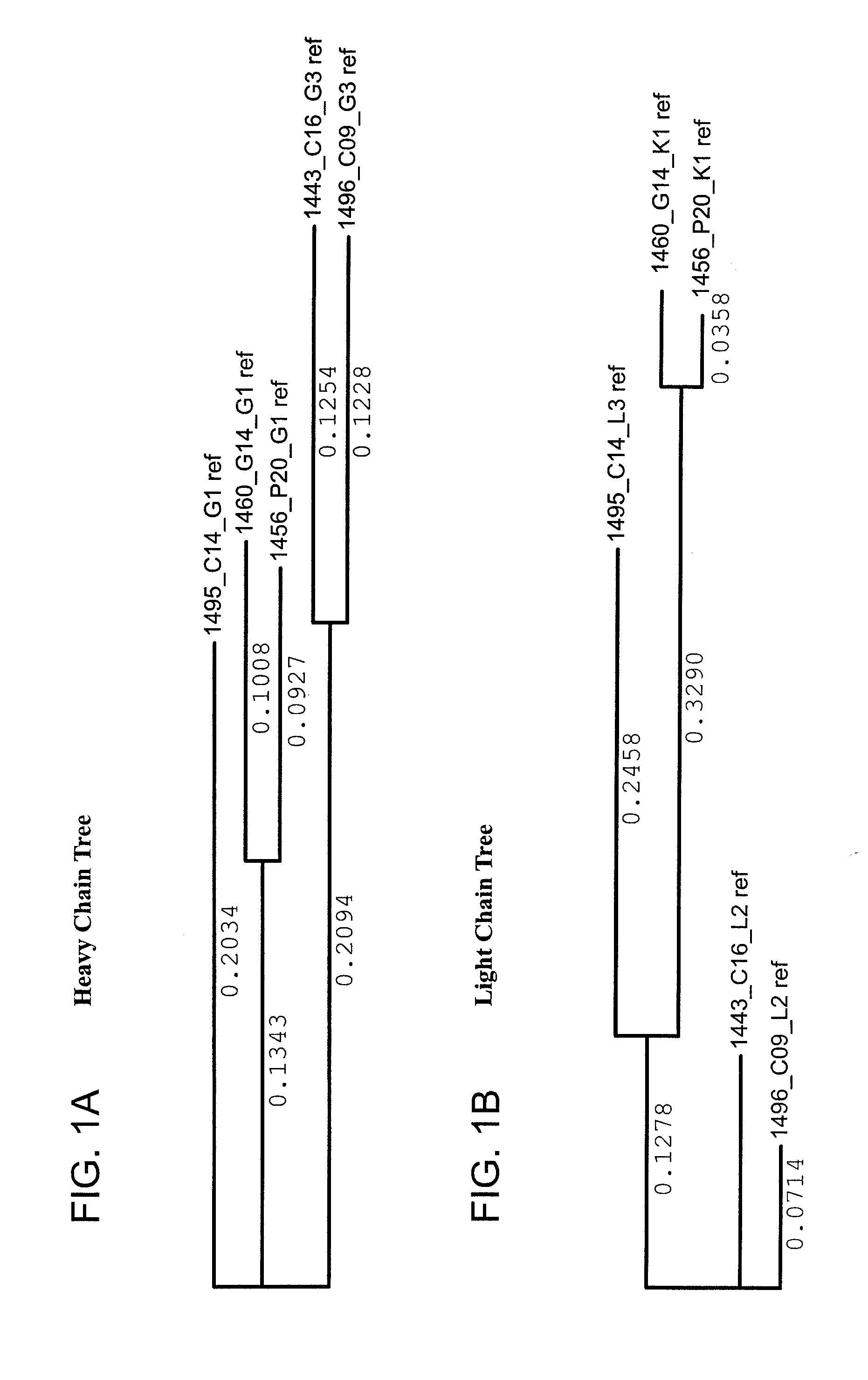
The present application relates novel HIV-1 broadly neutralizing antibodies. The antibodies of the present invention are further characterized by their ability to bind epitopes from the Env proteins. The invention also provides light and heavy chain variable region sequences. Compositions for prophylaxis, diagnosis and treatment of HIV infection are provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
Human immunodeficiency virus (HIV)-neutralizing antibodies
ActiveUS20110044994A1Sugar derivativesImmunoglobulins against virusesVaccinationImmunodeficiency virus
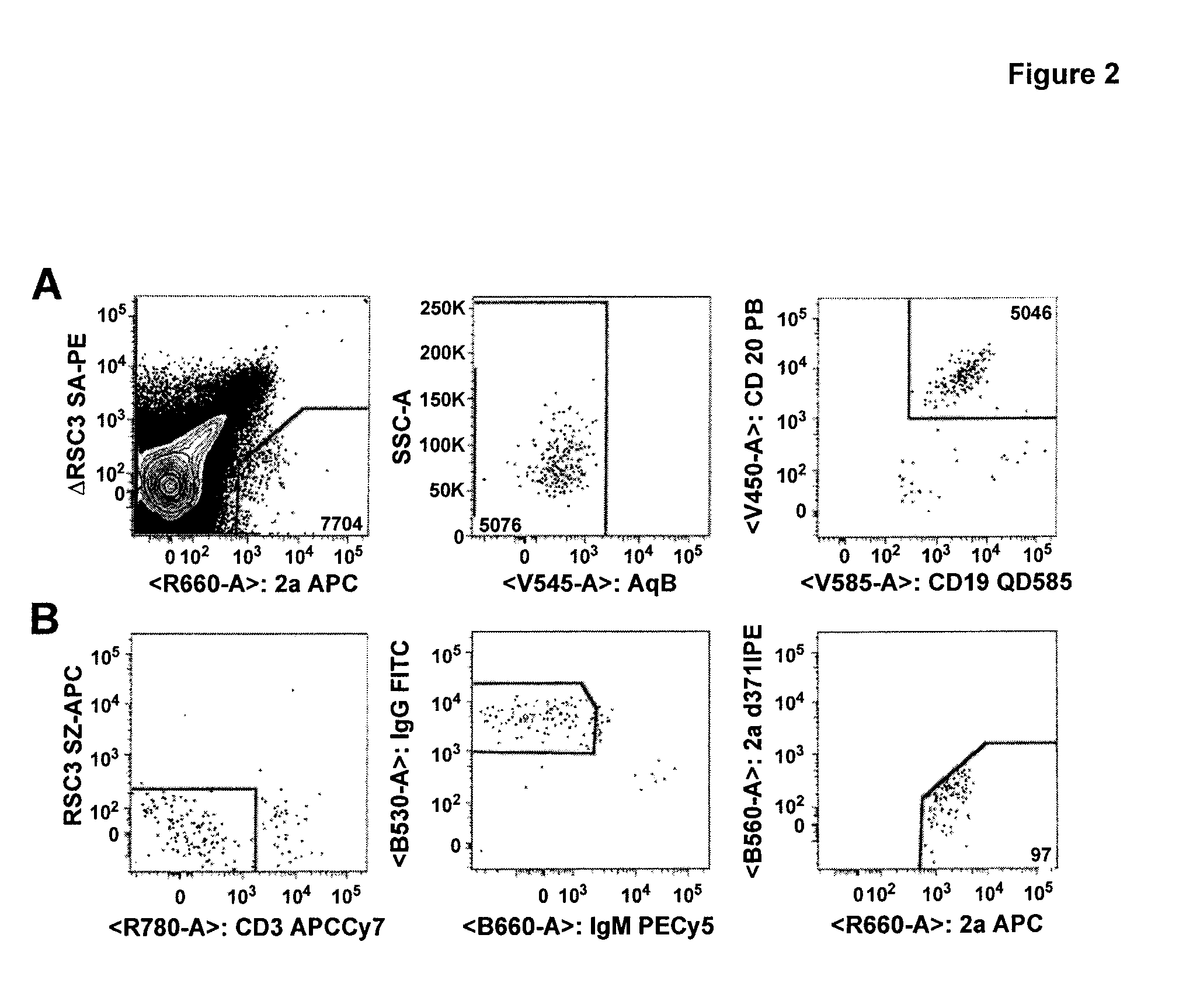
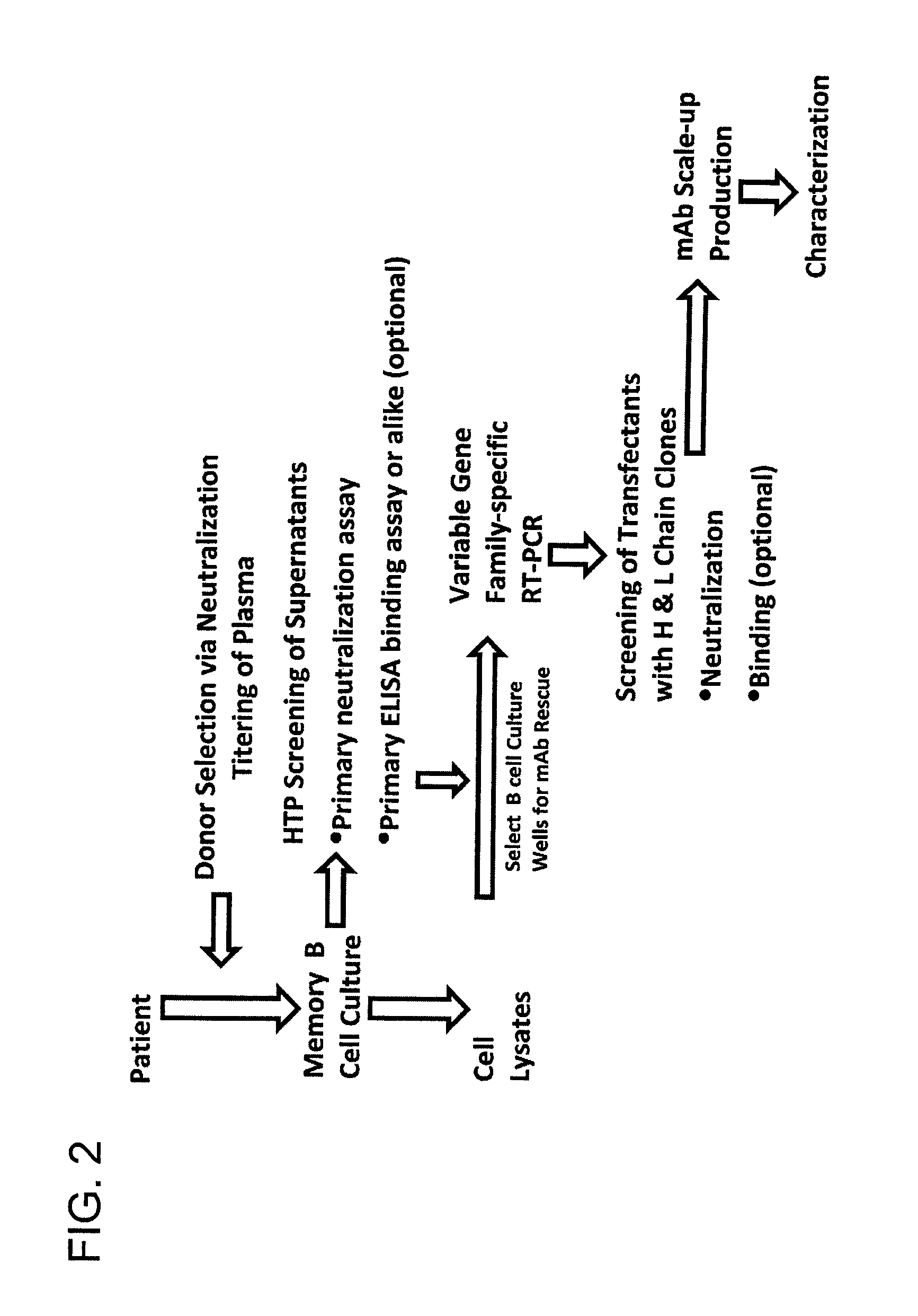
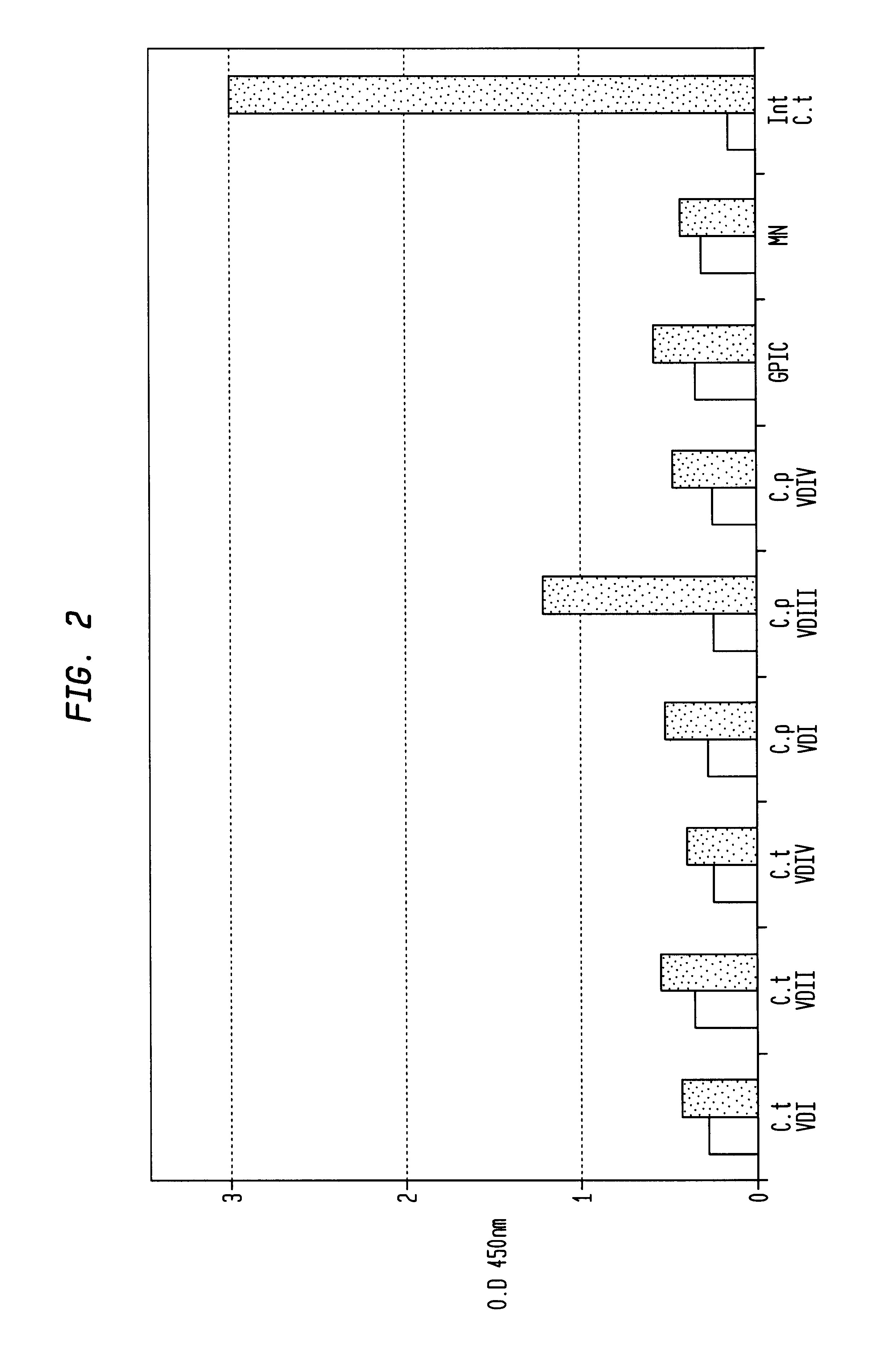
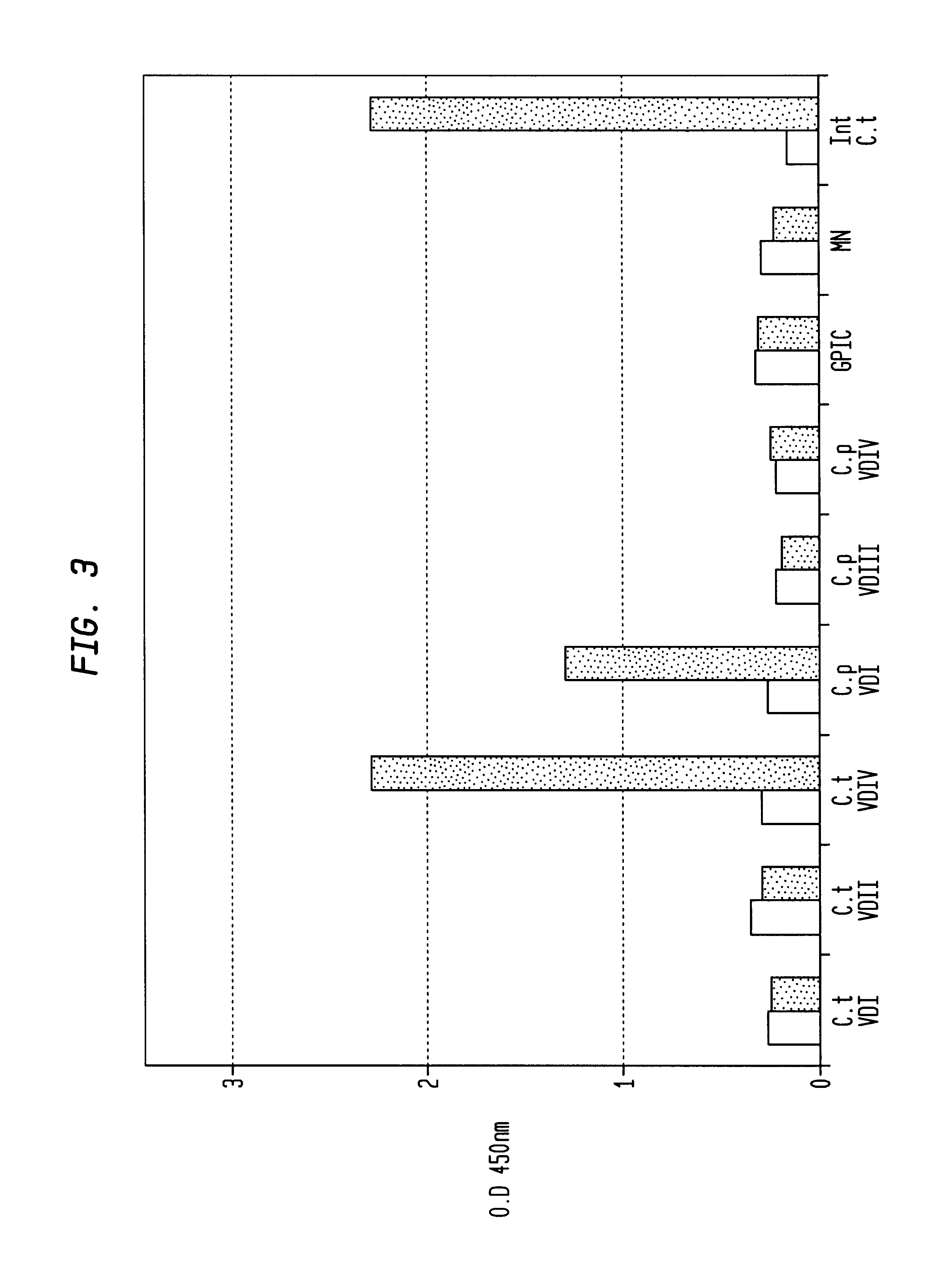
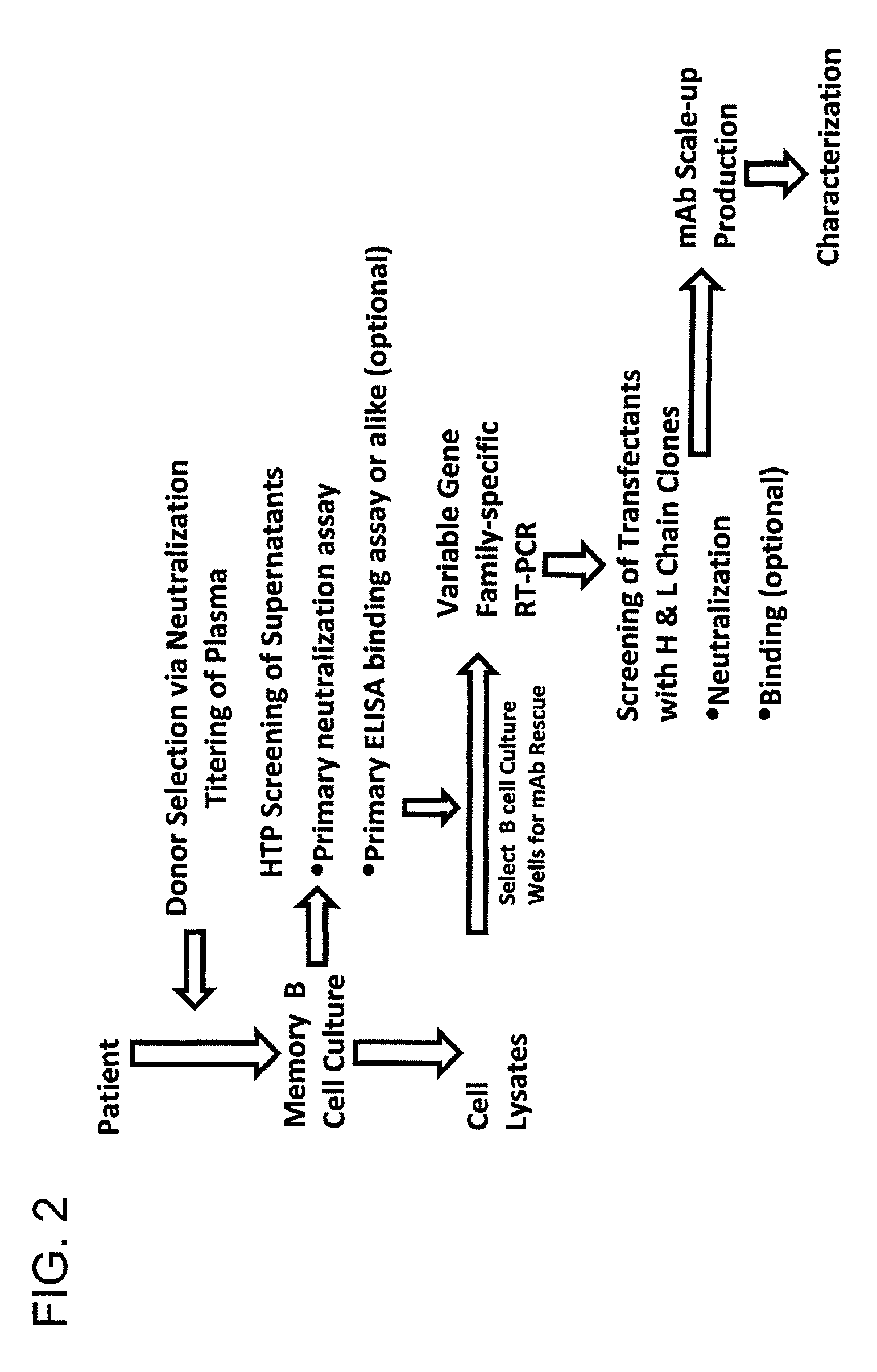
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
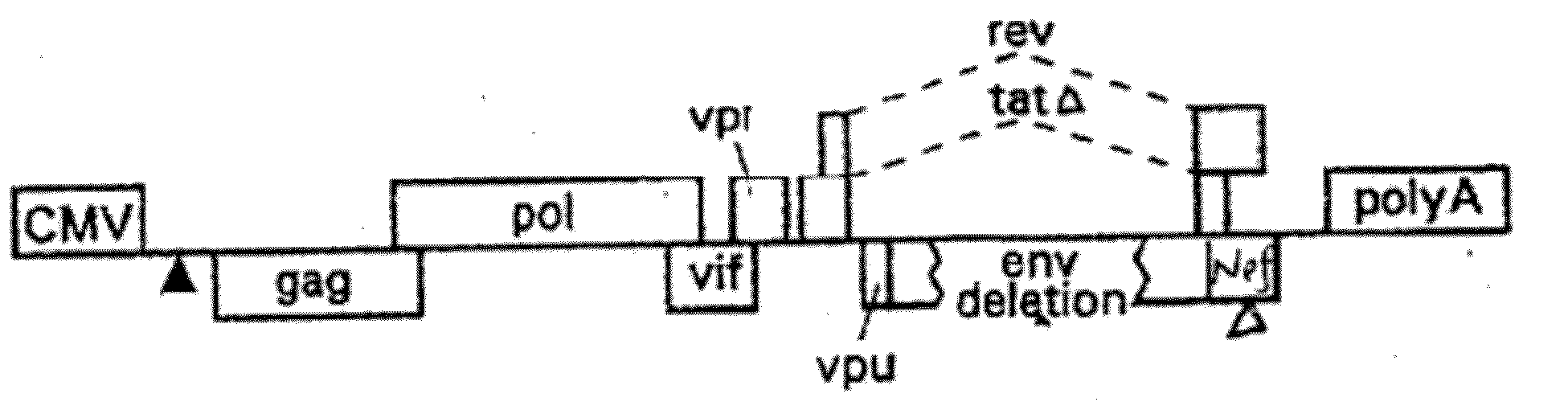
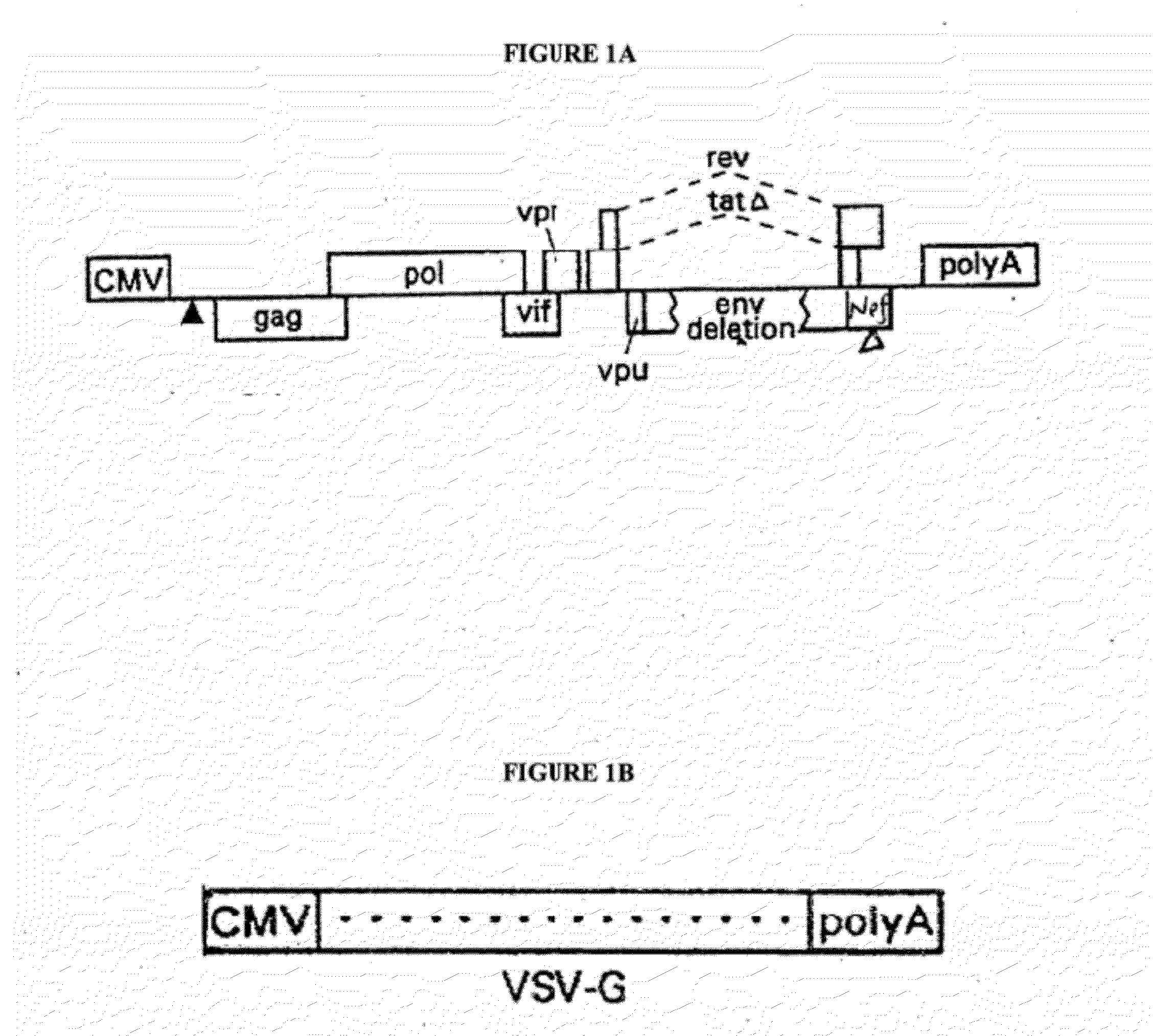
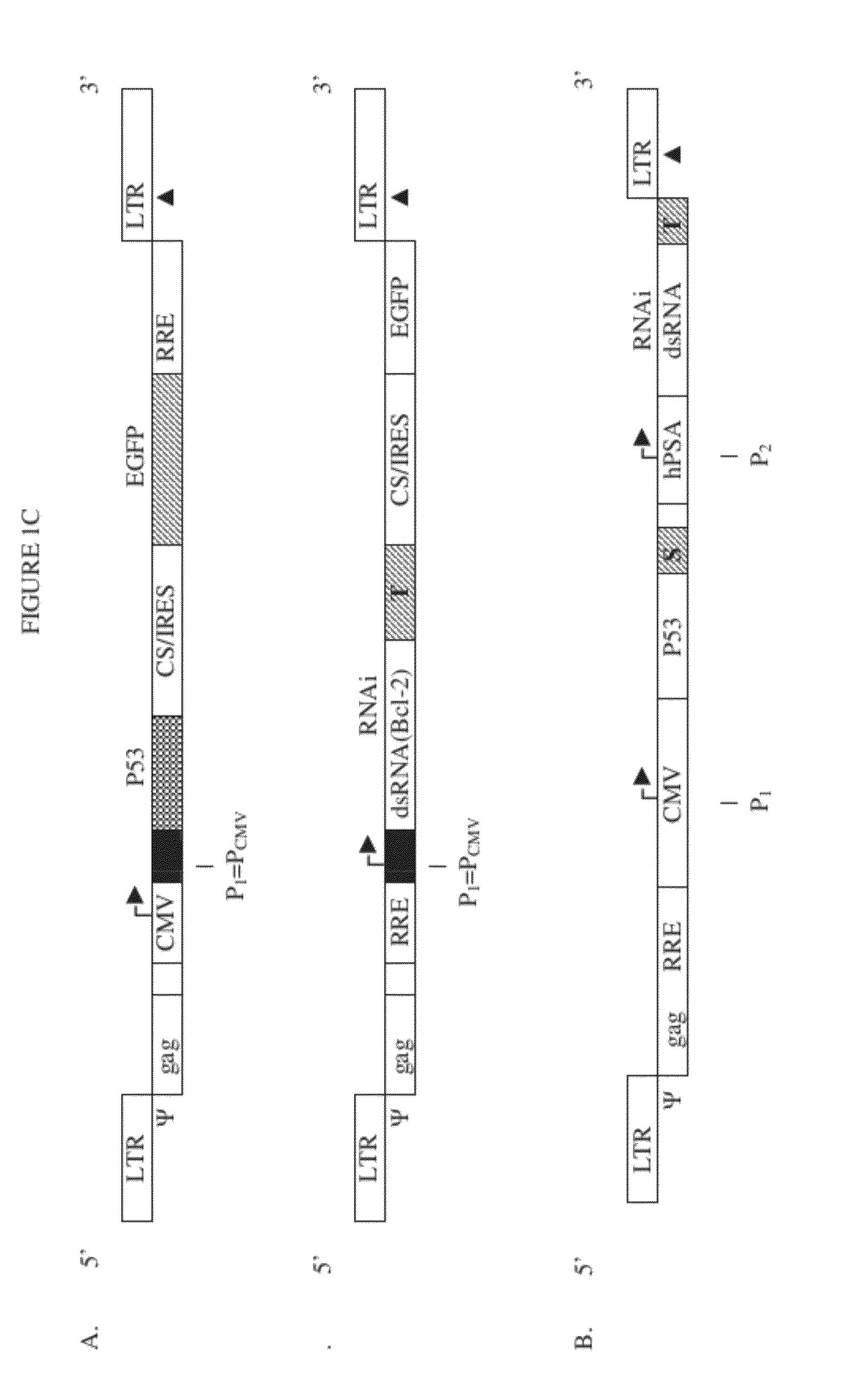
Lentiviral packaging cells and uses therefor
InactiveUS6955919B2Improve securityRule out the possibilitySugar derivativesGenetic material ingredientsPol genesVpr Protein
Owner:GENETIX PHARMA
Safe lentiviral vectors for targeted delivery of multiple therapeutic molecules
Owner:AMERICAN GENE TECH INT
Nontypeable Haemophilus influenzae virulence factors
InactiveUS7306805B2Translation is prevented and reducedAvoid stickingAntibacterial agentsSenses disorderVirulent characteristicsOperon
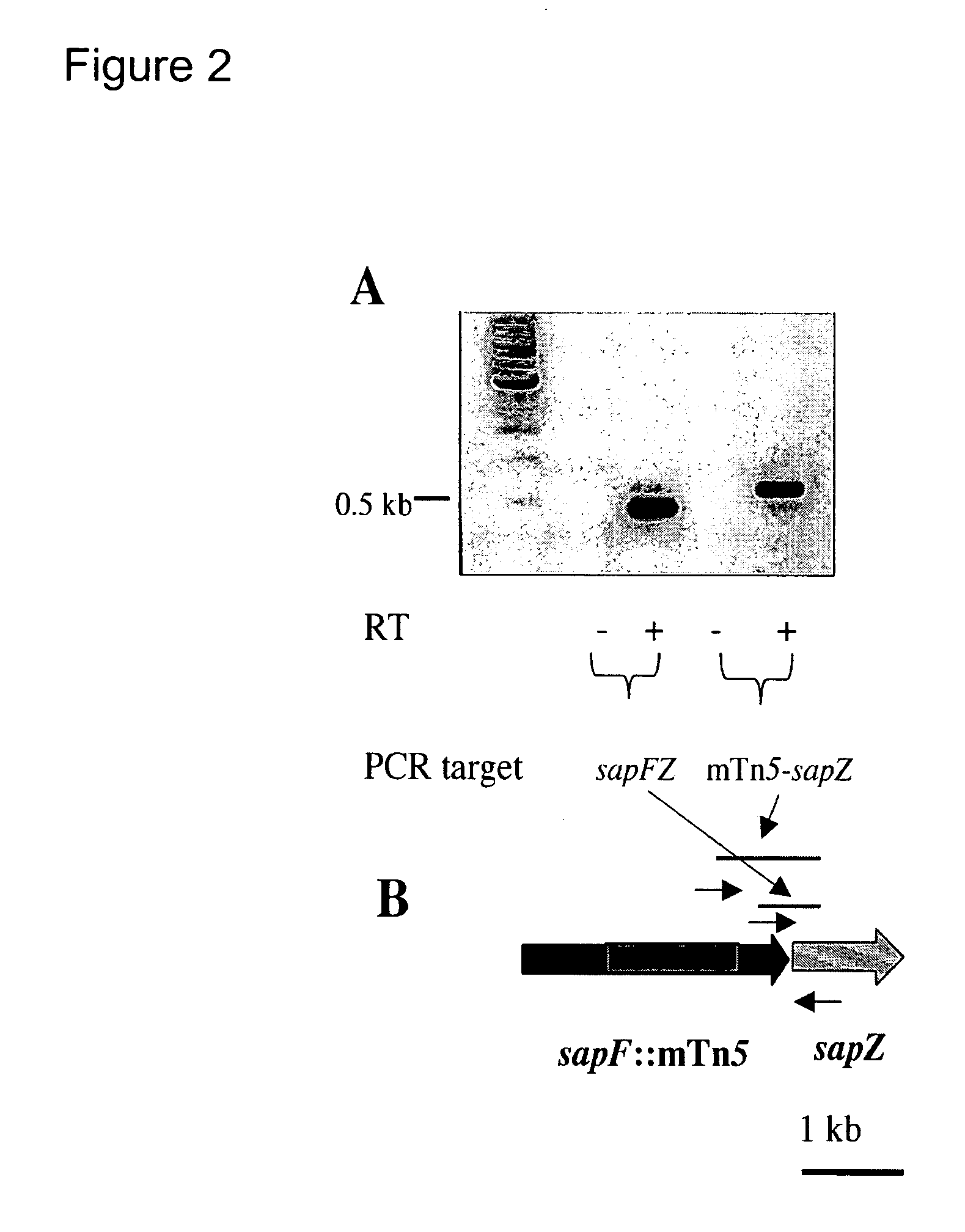
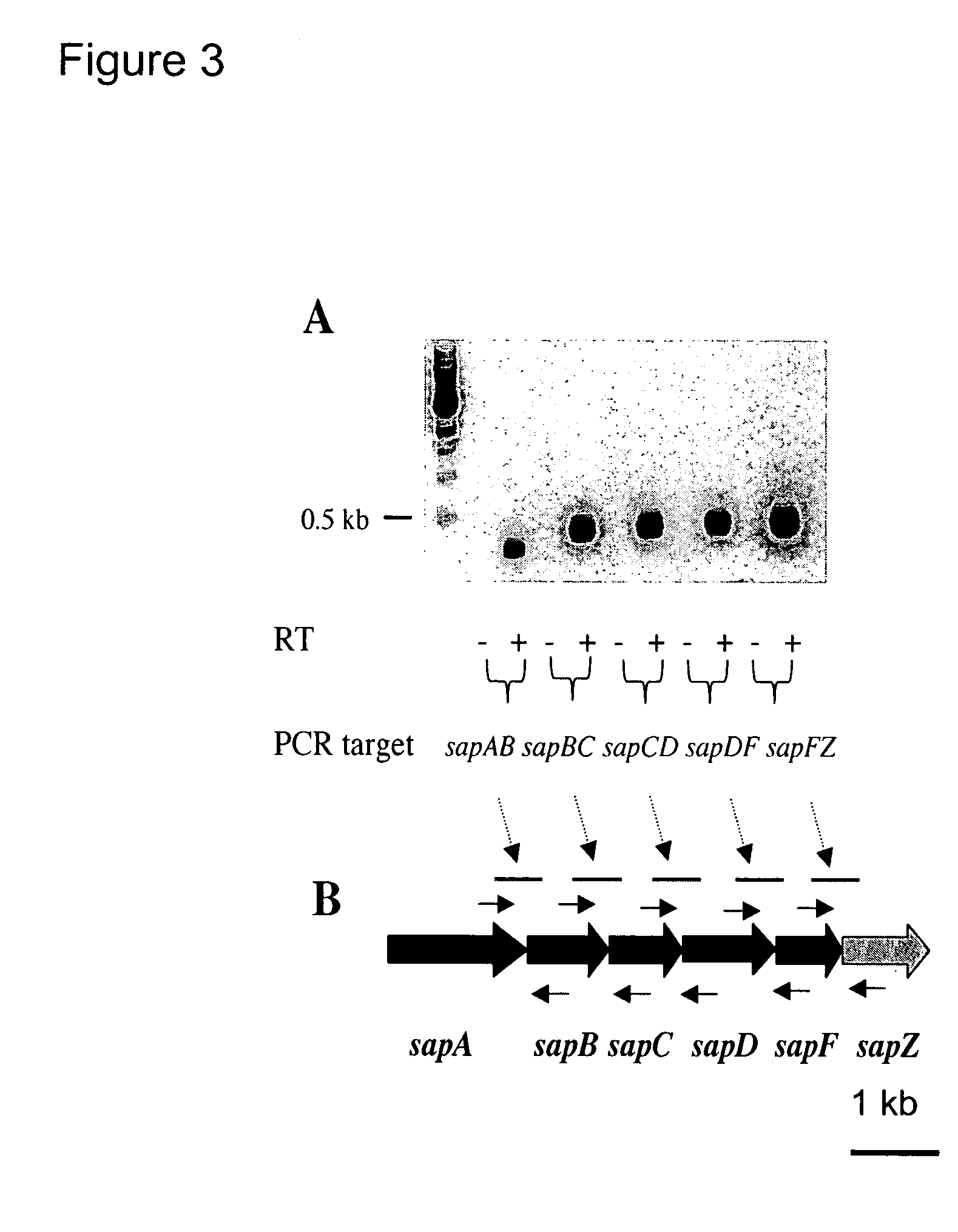
The invention relates to a mutation within the sap operon of an avirulent clone of a nontypeable strain of Haemophilus influenzae (NTHi). The invention also relates to the NTHi sap operon genes and the polypeptides encoded by these polynucleotide sequences. The invention also relates to a novel 110 kDa NTHi outer membrane protein and the polynucleotide that encodes this outer membrane protein. Methods of screening for NTHi infection, and treating and preventing NTHi related disorders are also contemplated.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Methods and compositions for improved retroviral gene and drug delivery
InactiveUS20070003522A1Improve abilitiesReduce deliveryBiocideGenetic material ingredientsLiposomeRecombinant virus
The present invention provides recombinant viral particles for gene therapy and liposome compositions for drug delivery comprising an env protein of MMTV. The invention also provides retroviral or lentiviral env proteins comprising a mutation in a receptor-binding motif. The invention also provides nucleic acids, proteins, and compositions comprising the recombinant viral particles, nucleic acids, and proteins. The invention also provides methods for enhancing delivery of a gene or compound of interest to a target cell, and methods for targeting a compound of interest to an acidified compartment of a cell.
Owner:UNIV OF TENNESSEE RES FOUND
Method for predicting outer membrane protein of germs on basis of machine learning technique
InactiveCN108009405AQuick forecastEasy to identifyBiostatisticsCharacter and pattern recognitionFeature vectorSupport vector machine
The invention discloses a method for using a machine learning technique for predicting outer membrane protein coded on genomes of germs. The method includes the steps of utilizing a PSI-BLAST algorithm to calculate a location specificity feature vector of the protein, adopting an autocorrelation function for conducting feature conversion, building a classification device based on a supporting vector machine, conducting classification on the outer membrane protein and non-outer membrane protein, receiving a protein sequence input by a user through a local computer program, and predicting whether or not the protein sequence belongs to the outer membrane protein. By means of the method, calculation prediction can be conducted on the protein sequence coded by the whole genome of the germs, thesensitivity is high, the calculation speed is high, and an effective tool is provided for fast identification and screening of inner and outer membrane protein of the genomes of the germs. The methodis an accurate and effective screening method for the outer membrane protein and can be widely applied to identification of the outer membrane protein of new sequencing genomes of germs.
Owner:上海韦翰斯生物医药科技有限公司
Outer membrane protein of Ehrlichia canis and Ehrlichia chaffeensis
InactiveUS7888491B2Lower Level RequirementsPeptide/protein ingredientsMicroorganismsSerodiagnosesNucleotide
Diagnostic tools for for serodiagnosing ehrlichiosis in mammals, particularly in members of the Canidae family and in humans are provided. The diagnostic tools are a group of outer membrane proteins of E. chaffeensis and variants thereof, referred to hereinafter as the “OMP proteins”, a group of outer membrane proteins of E. canis and variants thereof referred to hereinafter as the “P30F proteins”, and antibodies to the OMP proteins and the P30F proteins. The OMP proteins of E. chaffeensis encompass OMP-1, OMP-1A, OMP1-B, OMP-1C, OMP1-D, OMP1-E, OMP1-F, OMP1-H, OMP-1R, OMP-1S, OMP-1T, OMP-1U, OMP-1V, OMP-1W, OMP-1X, OMP-1Y and OMP-1Z. The P30F proteins of E. canis encompass P30, P30a, P30-1, P30-2, P30-3, P304, P30-5, P30-6, P30-7, P30-8, P30-9, P30-10, P30-11, and P30-12. Isolated polynucleotides that encode the E. chaffeensis OMP proteins and isolated polynucleotides that encode the E. canis P30F protein are also provided. The present invention also relates to kits containing reagents for diagnosing human ehrlichiosis and canine ehrlichiosis, and to immunogenic compositions containing one or more OMP proteins or P30F proteins.
Owner:THE OHIO STATE UNIV RES FOUND
Immunogenic compositions for protection against Chlamydial infection
InactiveUS20050065106A1Improve efficacySugar derivativesChlamydiaceae ingredientsNucleotideProtection sex
Owner:MURDIN ANDREW +1
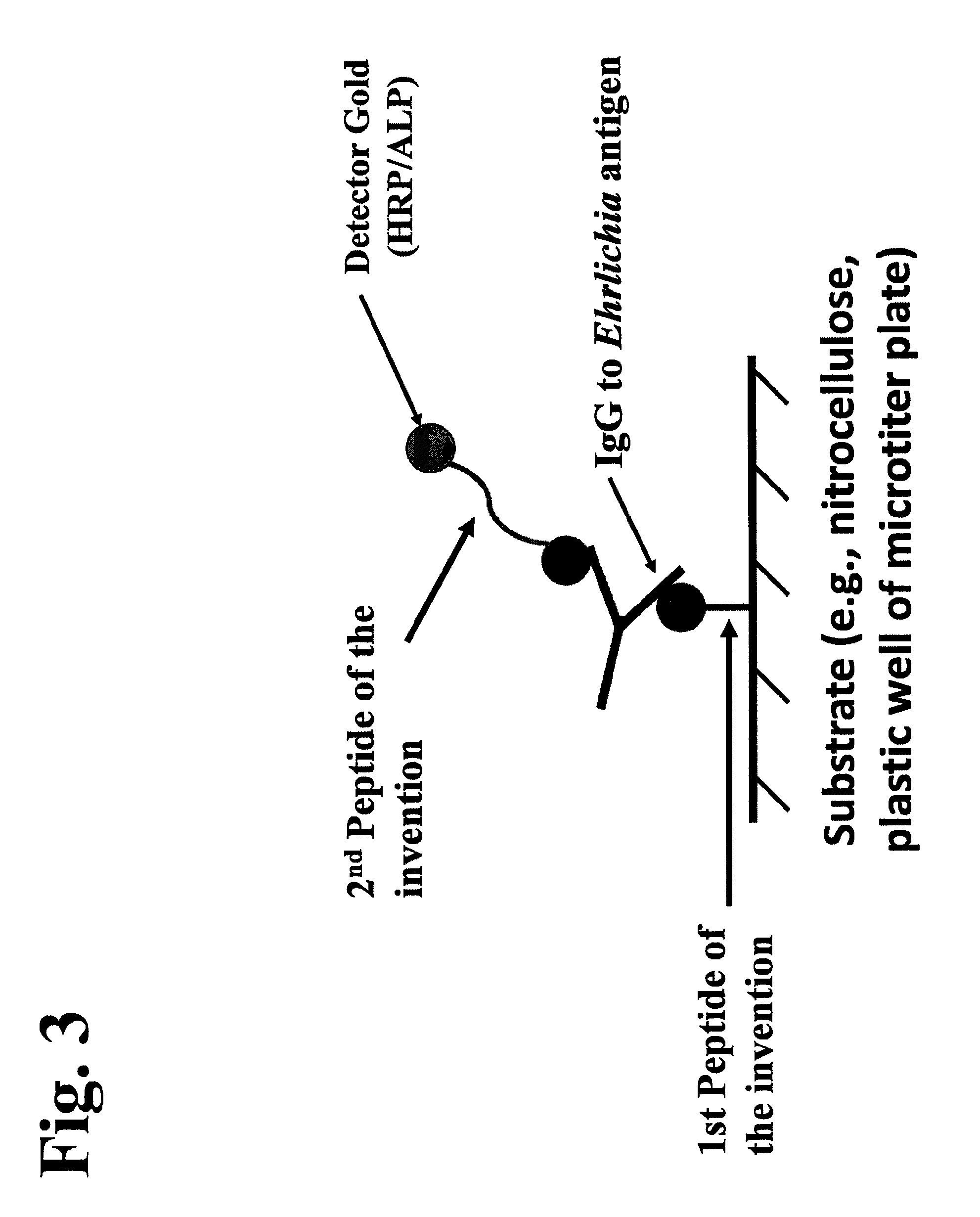
Peptides, devices, and methods for the detection of ehrlichia antibodies
ActiveUS8828675B2Simple and inexpensive and rapid and sensitive and accurate detectionAvoid serologic cross-reactivityPeptide/protein ingredientsBiological material analysisCell Membrane ProteinsMonocyte
Owner:ZOETIS SERVICE LLC
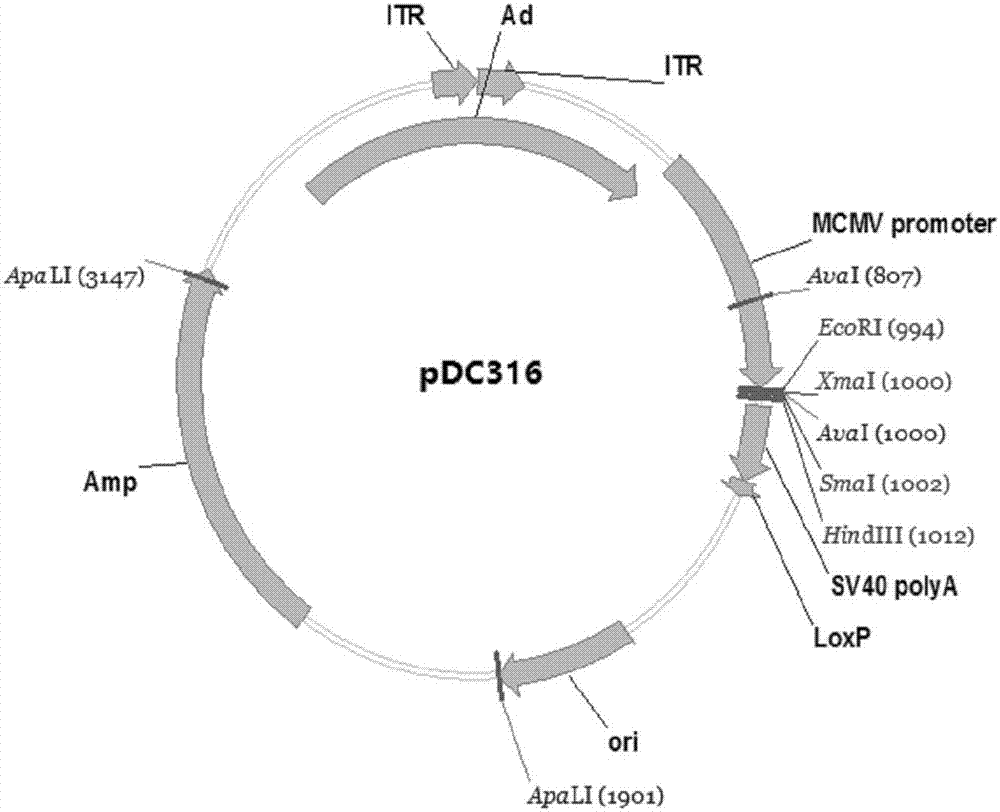
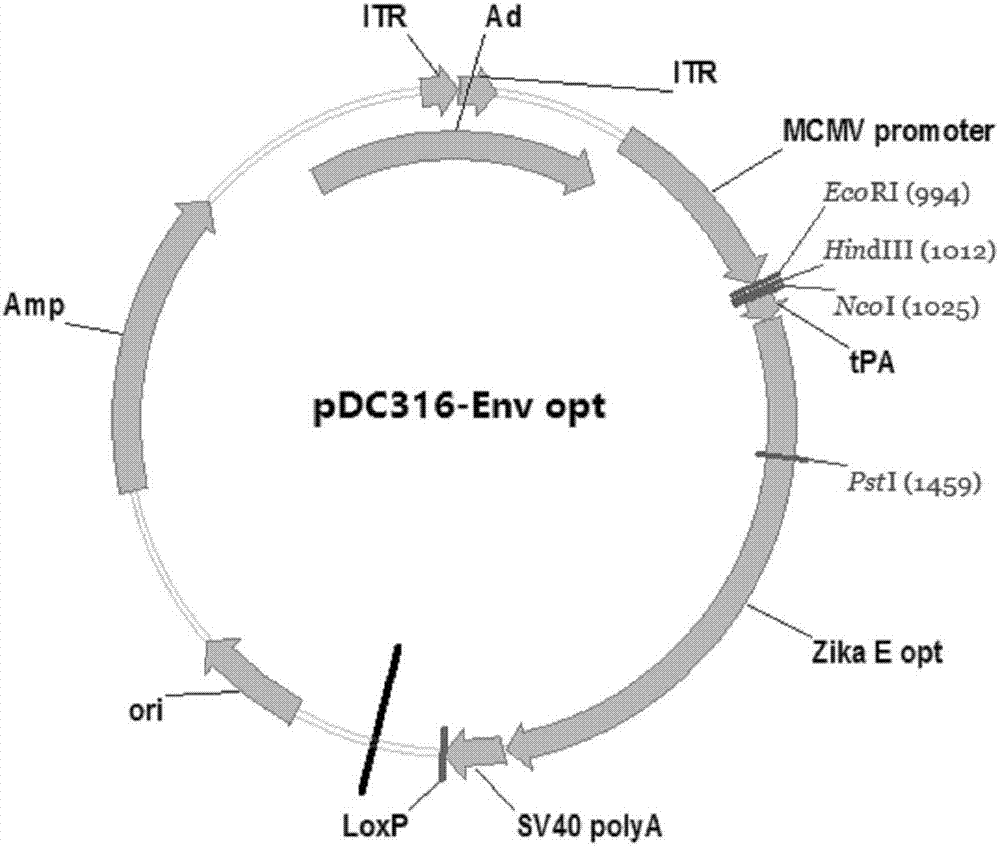
Zika virus disease vaccine taking human Ad5 replication-defective adenovirus as vector
ActiveCN107190013ARapid responseRapid immune responseSsRNA viruses positive-senseViral antigen ingredientsInfected cellShuttle vector
The invention discloses a codon-optimized nucleotide sequence capable of expressing an Env protein of a zika virus. The sequence can be fused with a protein prM and a protein prM endogenous signal peptide of a codon-optimized zika virus; after the sequence is inserted into a shuttle vector pDC316, the sequence and an auxiliary vector pBHGlox_E1, 3Cre realize cotransfection of a cell HEK293, so as to package an E1 and E3 combined missing-recombinant adenovirus taking a replication-defective human type-5 adenovirus as a vector; the recombinant adenovirus vector can efficiently express an envelope protein of the zika virus in an infected cell. After the nucleotide sequence-inserted recombinant adenovirus serving as a vaccine is immune to an animal for single time, strong humoral immune and cellular immune responses can be quickly induced. A zika vaccine taking the recombinant adenovirus as the vector is suitable for large-scale and rapid preparation and can be used for emergent vaccination for a large scale of people in a zika outbreak and prophylactic immunization for people at ordinary times.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Hivcon: an HIV immunogen and uses thereof
InactiveUS20080089901A1Elicit immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHIV ProteinsA-DNA
The present invention provides artificial fusion proteins (AFPs) designed to elicit an anti-HIV immune response, as well as nucleic acid molecules and expression vectors encoding those proteins. The AFPs of the invention may comprise domains from various HIV proteins, such as Gag, Pol, Vif, and Env proteins, which are partial sequences. HIVCON is an AFP in which the HIV domains are from several HIV clade consensus sequences and which optionally contains additional domains which may be useful, for example, in monitoring expression levels or laboratory animal immune responses. Other aspects of the invention may include compositions and methods for inducing an anti-HIV immune response in a subject, preferably with a DNA prime-MVA boost strategy, and to induce a cell-mediated immune response.
Owner:MEDICAL RESEARCH COUNCIL
Chlamydia trachomatis specific peptides and their use in diagnostic assays
InactiveUS6699678B1High sensitivityStrong specificityPeptide/protein ingredientsMicroorganismsAntiendomysial antibodiesDrug biological activity
Disclosed are peptides or a mixture of peptides, or analogs thereof, derived from the variable domains of the Chlamydia trachomatis (C. trachomatis) immunodominant major outer membrane protein (MOMP). The peptides or mixtures of peptides are characterized by having specificity only to C. trachomatis anti-MOMP antibodies and being non-cross reactive with anti-MOMP antibodies of other Chlamydia species Specific peptides are described (SEQ ID Nos. 1 to 8) as well as their analogs, which have essentially the same biological activity.
Owner:SAVYON DIAGNOSTICS
Monoclonal antibodies directed against trimeric forms of the HIV-1 envelope glycoprotein with broad and potent neutralizing activity
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
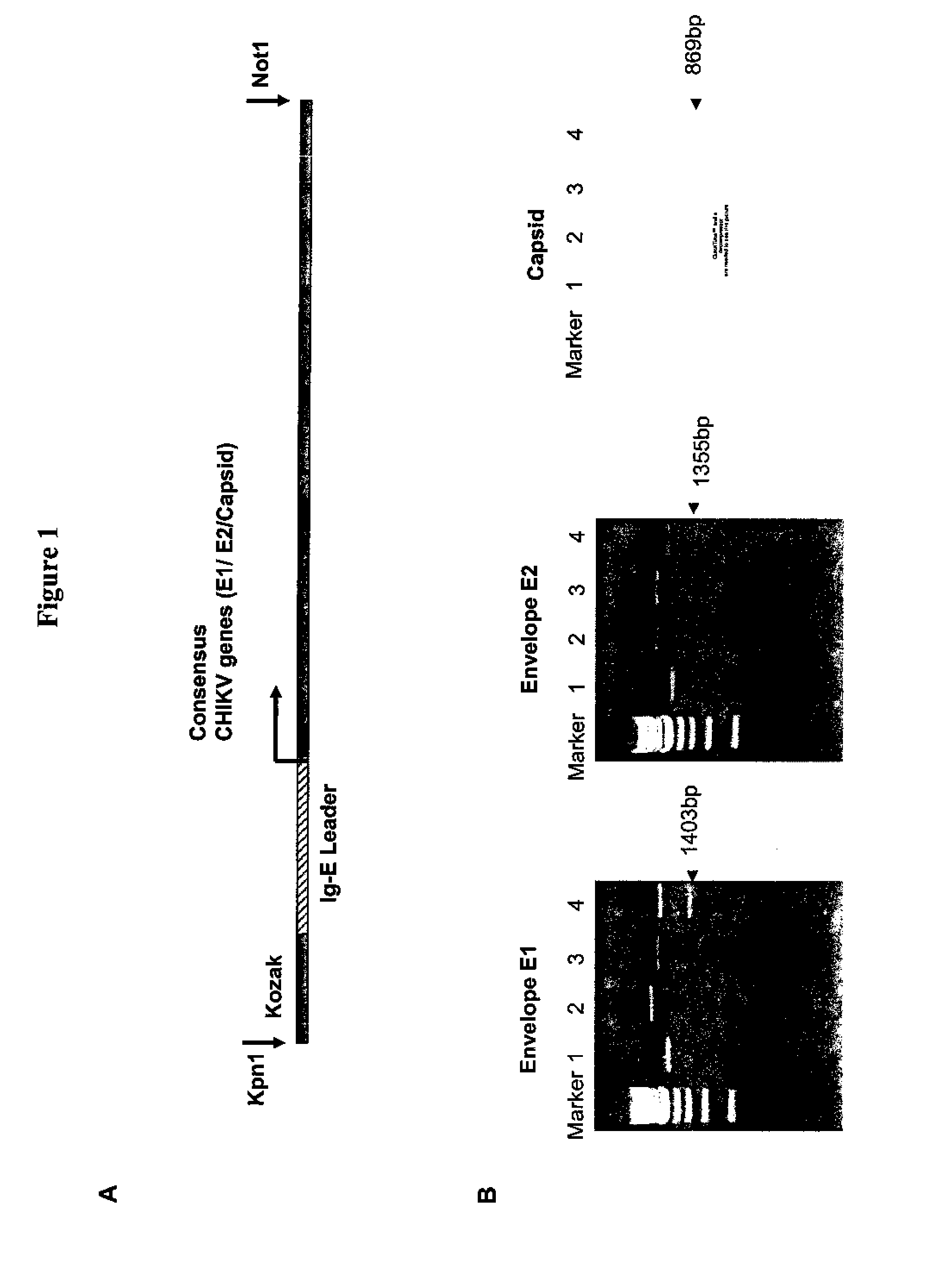
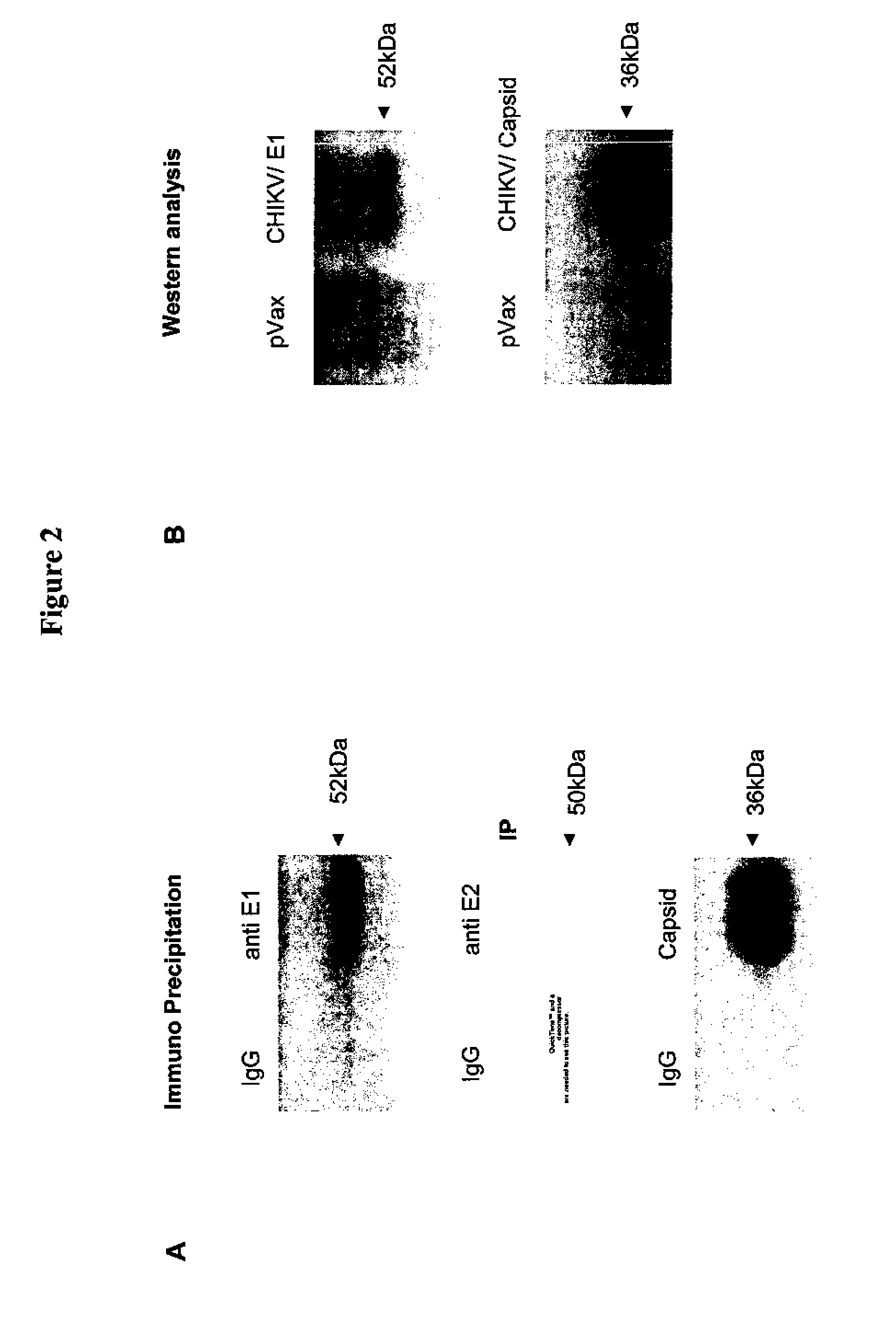
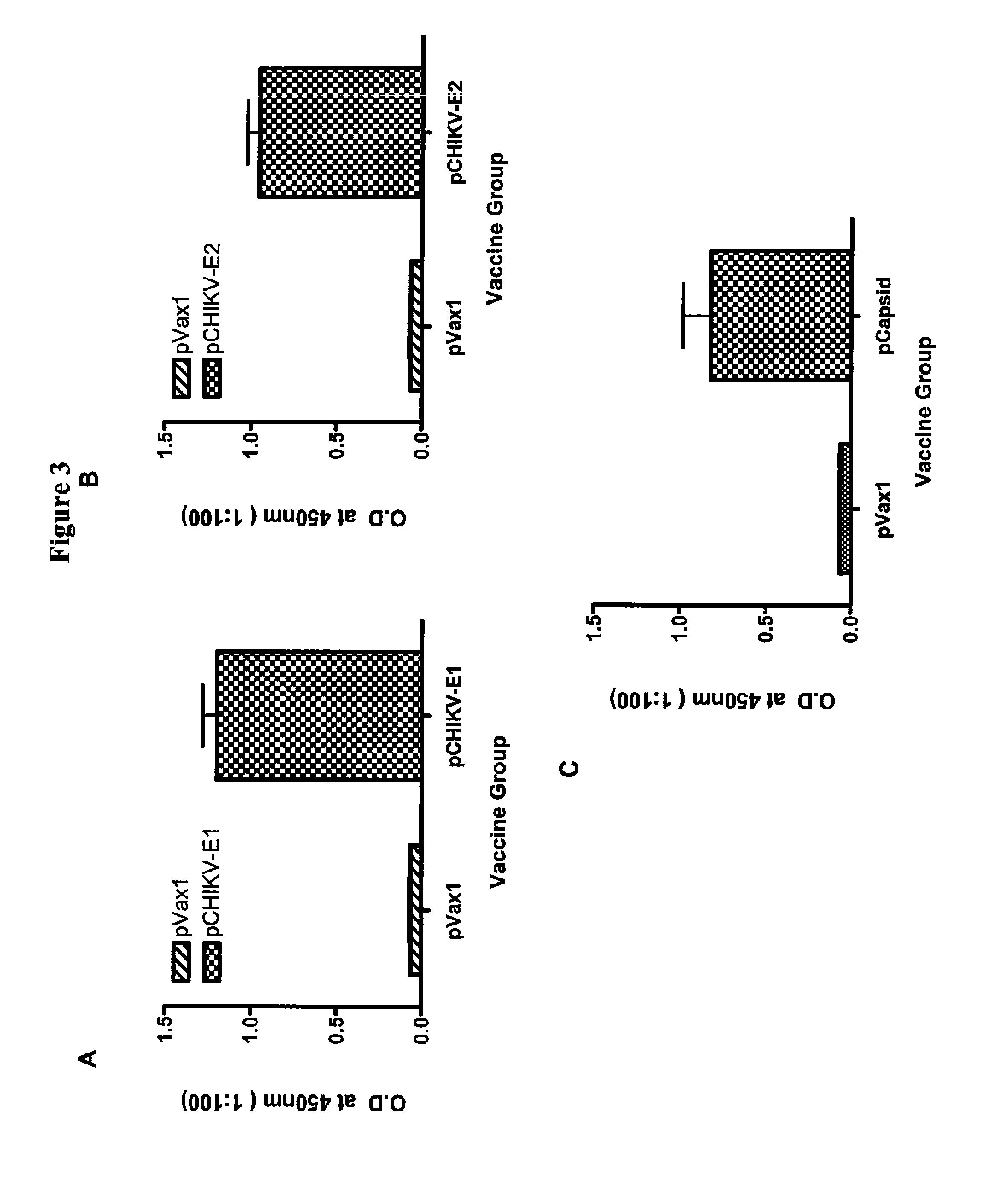
Consensus sequences of chikungunya viral proteins, nucleic acid molecules encoding the same, and compositions and methods for using the same
Consensus CHIKV E1 protein, consensus CHIKV E2 protein, consensus CHIKV capsid protein, or fragments and homologues thereof, and nucleic acid molecules that encode the same are disclosed. A consensus CHIKV Env protein which includes CHIKV E1 consensus protein, CHIKV E2 consensus protein, CHIKV E3 consensus protein, or fragments and homologues thereof and nucleic acid molecules that encode the same are also disclosed. Compositions and recombinant vaccines comprising CHIKV consensus proteins, and methods of using them are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Anti-Pseudomonas aeruginosa medicine screening model using Pseudomonas aeruginosa efflux pump outer membrane protein MEXAB-OPRM as target
The invention relates to an antibacillus pyocyaneus drug screening model which selects bacillus pyocyaneus external discharge pump adventitial protein MexAB-OprM as bull's-dot and to a method of screening antibacillus pyocyaneus drug using the said screening model. The invention also relates to a recombination bacillus pyocyaneus of excess expression MexAB-OprM protein which can be used in the said screening model and the screening method. The invention also relates to the antibacillus pyocyaneus drug obtained by the said screening model and method.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Vibrio parahaemolyticus tunica externa protein ompK subunit vaccine and preparation method thereof
InactiveCN101172157APreserve immunogenicityDefense against invasionAntibacterial agentsDigestive systemEscherichia coliHaemolysis
The invention discloses protein ompK subunit vaccine of assistant haemolysis vibrio extine and a preparation method thereof. The vaccine is PBS solution which converts the recombination protein of the coliform bacteria of recombination prokaryon expression plasmid pET30a-ompK expressed by inducing and after being purified; the concentration of the PBS solution is 0.25-0.5mg / ml. The method has the steps that: firstly, the extraction of an assistant haemolysis vibrio full gene group, the overall length of extine protein ompK DNA and the clone of a mature peptide coded sequence; secondly, the construction of a prokaryon expression plasmid of the ompK mature peptide coded sequence, thirdly, the obtaining way of the recombination ompK protein; fourthly, the detection to the immunity way and the immunity effect of a large yellow croaker with recombination ompK protein. The invention provides the preparation method of the assistant haemolysis vibrio ompK protein subunit vaccine, and simultaneously provides the detection method of the immunity effect of the large yellow croaker with recombination ompK protein, the preparation method is simple, and the usage is convenient.
Owner:ZHEJIANG UNIV
Diagnosis method and therapeutic method for cancer
InactiveCN102215870AOrganic active ingredientsGenetic material ingredientsDiagnosis methodsEnvelope Gene
Disclosed are a diagnosis method and a therapeutic method for cancer, both of which utilize HERV-H env gene and protein. Particularly, cancer can be diagnosed by detecting the expression of HERV-H env gene, and a reagent used for the detection of the expression can be used as a diagnosing agent. Cancer can be treated by inhibiting the function of HERV-H env gene, and a reagent used for the inhibition of the function can be used as an anti-cancer agent. Alternatively, cancer can be treated by administering a peptide having a specific sequence contained in HERV-H env protein or the like, and the peptide or the like can be used as a cancer vaccine.
Owner:KEIO UNIV
Method for the rapid taxonomic identification of pathogenic microorganisms and their toxic proteins
InactiveUS20060257929A1Rapid detection of lowImprove efficiencyBiological testingOptical absorbanceBinding pattern
The present invention describes a method for the rapid binding of pathogenic microorganisms and their toxic proteins with ligands that have been covalently tethered at some distance from the surface of a substrate. Ligands directed to microbes are covalently attached to the substrate surface by tethers that are between 35 Å and 50 Å in length for optimal binding efficacy. Ligands directed to capture and concentrate proteinaceous materials are covalently attached to the substrate surface by tethers that are between 35 Å and 50 Å in length for optimum assay kinetics. The ligands described herein include heme compounds, siderophores, polysaccharides, and peptides specific for toxic proteins, outer membrane proteins and conjugated lipids. Non-binding components of the solution to be analyzed are separated from the bound fraction and binding is confirmed by detection of the analyte via microscopy, fluorescence, epifluorescence, luminescence, phosphorescence, radioactivity, or optical absorbance. By patterning numerous ligands in an array on a substrate surface it is possible to taxonomically identify the microorganism by analysis of the binding pattern of the sample to the array.
Owner:MICROBIOSYST
Chimeric HIV Env proteins comprising CD4 mini-proteins or CD4 mimetics that are capable of inducing neutralizing antibody responses against cryptic Env epitopes
Env-CD4 complexes and hybrids are disclosed that expose cryptic epitopes that are important in virus neutralization. Methods of diagnosis, treatment and prevention using the polynucleotides and polypeptides are also provided.
Owner:NOVARTIS AG +1
Truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi strains Karp, Kato and Gilliam and its use in antibody based detection assays and vaccines
A recombinant, refolded non-fusion polypeptide expressed from a truncated r56 gene of the causative agent of scrub typhus, Orientia tsutsugamushi for the Karp, Kato and Gilliam strains has been produced. The invention is useful for detecting prior exposure to scrub typhus, screening for and / or identification of at least one infectious strain-similarity (i.e. a Karp-like, Kato-like or Gilliam-like strain) based on its strength of reaction toward a truncated protein and as a component in vaccine formulations and production of immune globulins for passive prophylaxis and immunity in subjects.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Virus-like particles for pseudorabies virus and preparation method for same
ActiveCN102533680AAvoid quality risksEasy to design and manufactureInactivation/attenuationAntiviralsRabiesGlycoprotein G
The invention provides virus-like particles for pseudorabies virus. The virus-like particles consist of pseudorabies virus glycoprotein G and pseudorabies virus matrix protein M, and have stable and homogenous spatial structures; and the outer membrane protein of the virus-like particles contains all or partial fragments of the pseudorabies virus glycoprotein G, and can induce an organism to generate protective-level cellular immunity and humoral immunity. The virus-like particles do not comprise any nucleic acid component of chromosome of the pseudorabies virus, and avoid quality risks caused by inactivator addition. The virus-like particles can be conveniently purified by the conventional purification technology, and the quality risks possibly caused by inactivator addition in the traditional inactivated vaccines are avoided on principle. Meanwhile, due to a simple and quick construction and preparation mode, novel vaccines aiming at new strains can be conveniently and quickly designed and prepared. Based on the principle, novel polyvalent vaccines and multi-vaccines are easily formed on the basis of the particles; and the particles can widely replace related critical raw materials in the conventional practical technologies such as related antigen and antibody detection, functional protein vectors and the like.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
High molecular weight major outer membrane protein of moraxella
InactiveUS20020068070A1Improving immunogenicitySlow and sustained releaseAntibacterial agentsVirusesDiseaseIn vivo
An isolated and purified outer membrane protein of a Moraxella strain, particularly M. catarrhalis, having a molecular mass of about 200 kDa, is provided. The about 200 kDa outer membrane protein as well as nucleic acid molecules encoding the same are useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the about 200 kDa outer membrane protein or produces a protein capable of inducing antibodies in a host specifically reactive with the about 200 kDa outer membrane protein.
Owner:AVENTIS PASTEUR LTD
Method of using adenoviral vectors to induce an immune response
InactiveUS20090286860A1Organic active ingredientsViral antigen ingredientsViral vectorBioinformatics
The invention provides a method of inducing an immune response against a human immunodeficiency virus (HIV) in a mammal. The method comprises administering to the mammal an adenoviral vector composition comprising one or more adenoviral vectors encoding two or more different HIV antigens, the production of which induces an immune response against HIV in the mammal. The invention also provides an adenoviral vector composition comprising four adenoviral vectors encoding an HIV clade A Env protein, an HIV clade B Env protein, an V clade C Env protein, and a fusion protein comprising an HIV clade B Gag protein and Pol protein, respectively.
Owner:GEN VEC INC +1
Pharmaceutical composition containing antibodies directed against the herv-w envelope
InactiveUS20100074894A1Nervous disorderImmunoglobulins against virusesBinding siteBULK ACTIVE INGREDIENT
A pharmaceutical composition that contains, as an active ingredient, at least one antibody directed against the HERV-W envelope protein, except for any antibody specifically directed against the binding site between said env protein and the hASCT1 or hASCT2 receptor.
Owner:GENEURO SA
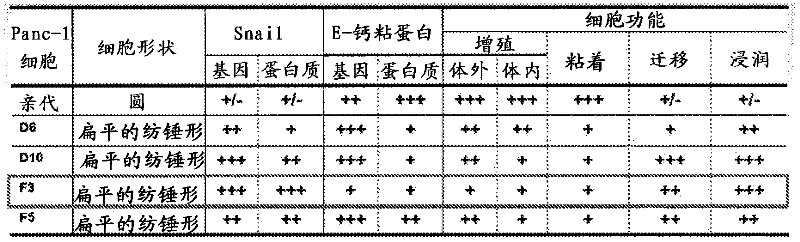
DNA immunization against chlaymdia infection
InactiveUS6344202B1Effectively induces protective immunityGood curative effectOrganic active ingredientsVirusesNucleotideDna immunization
Nucleic acid, including DNA, for immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com