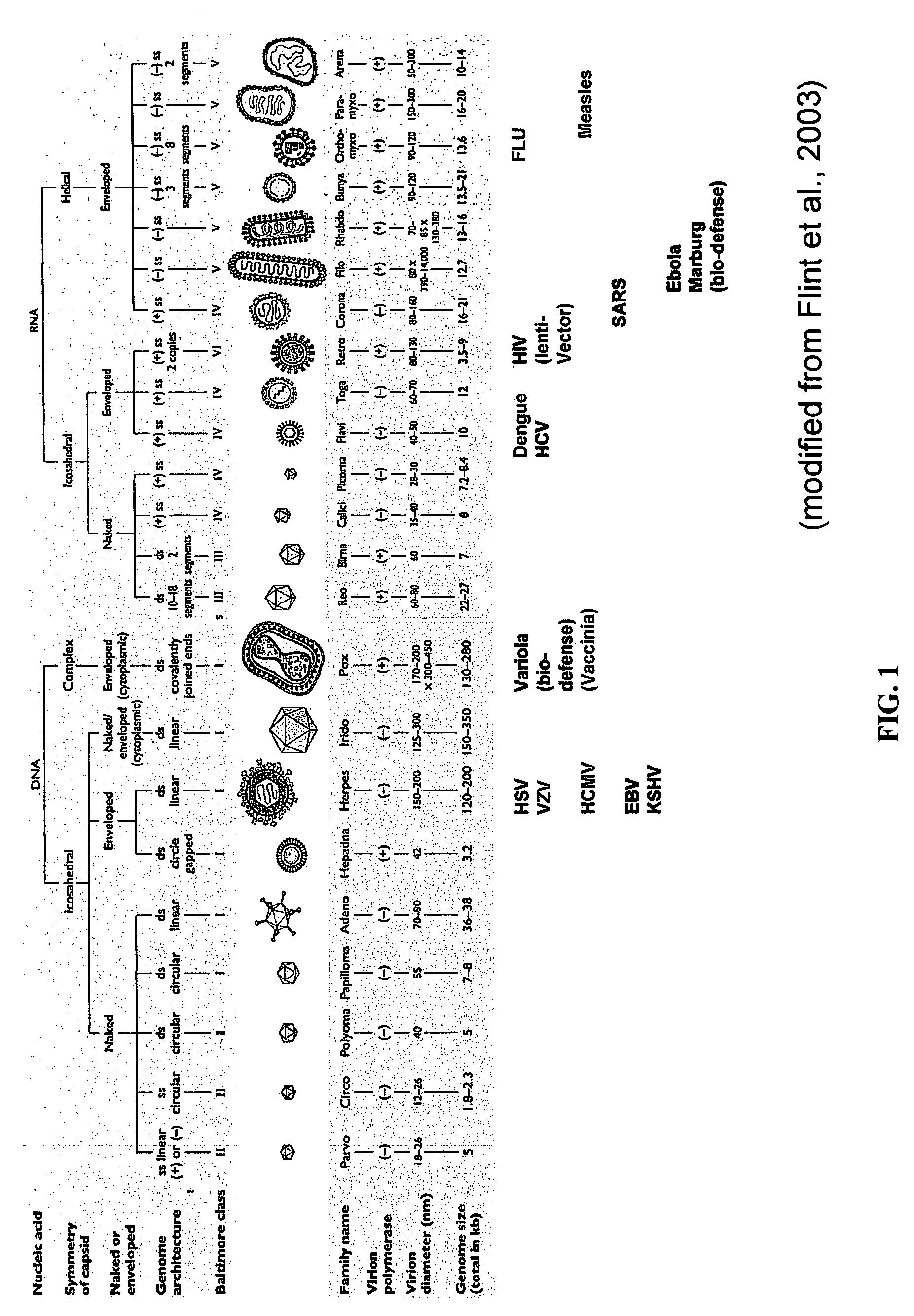
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
208results about How to "Lower titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
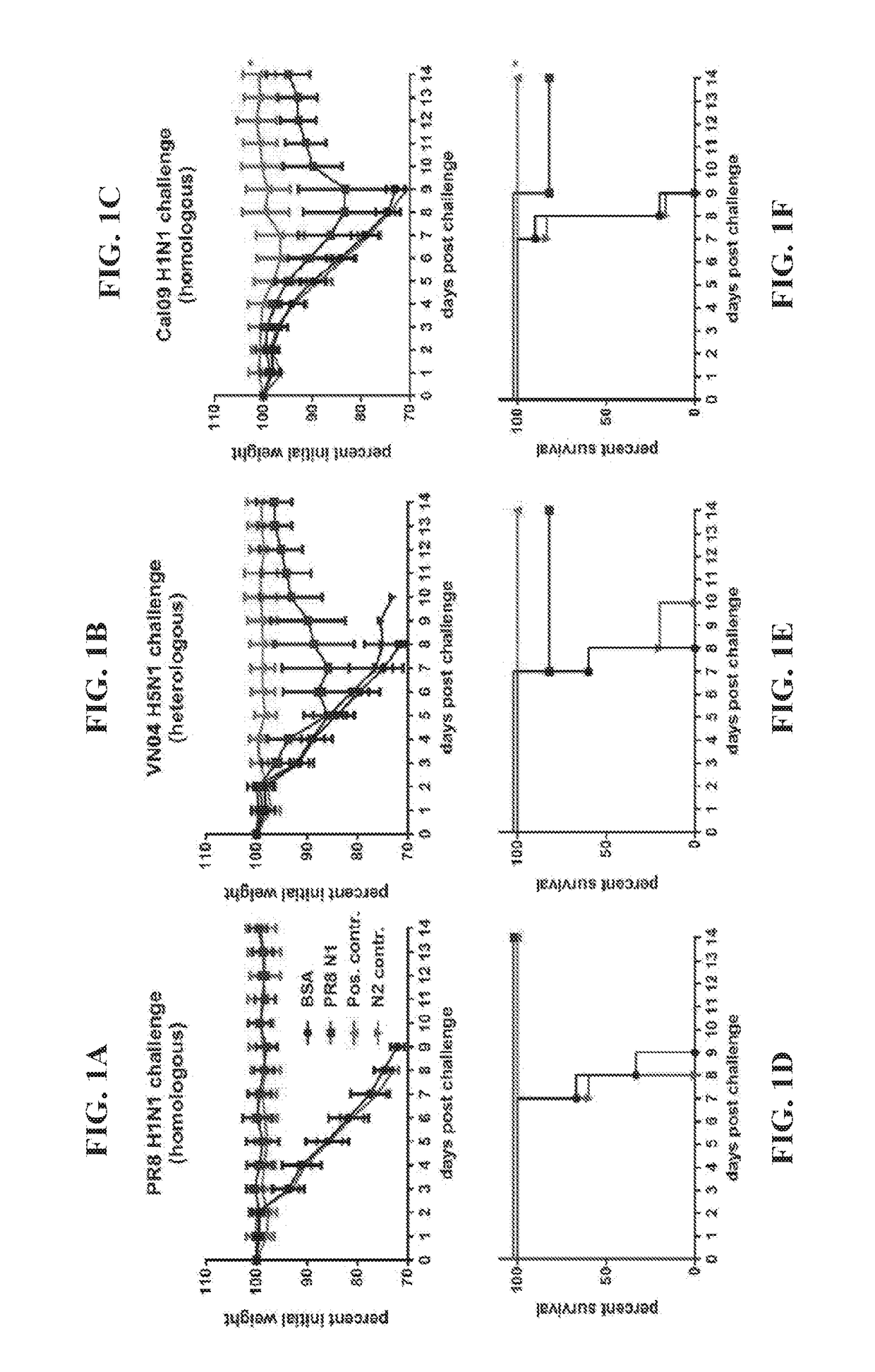
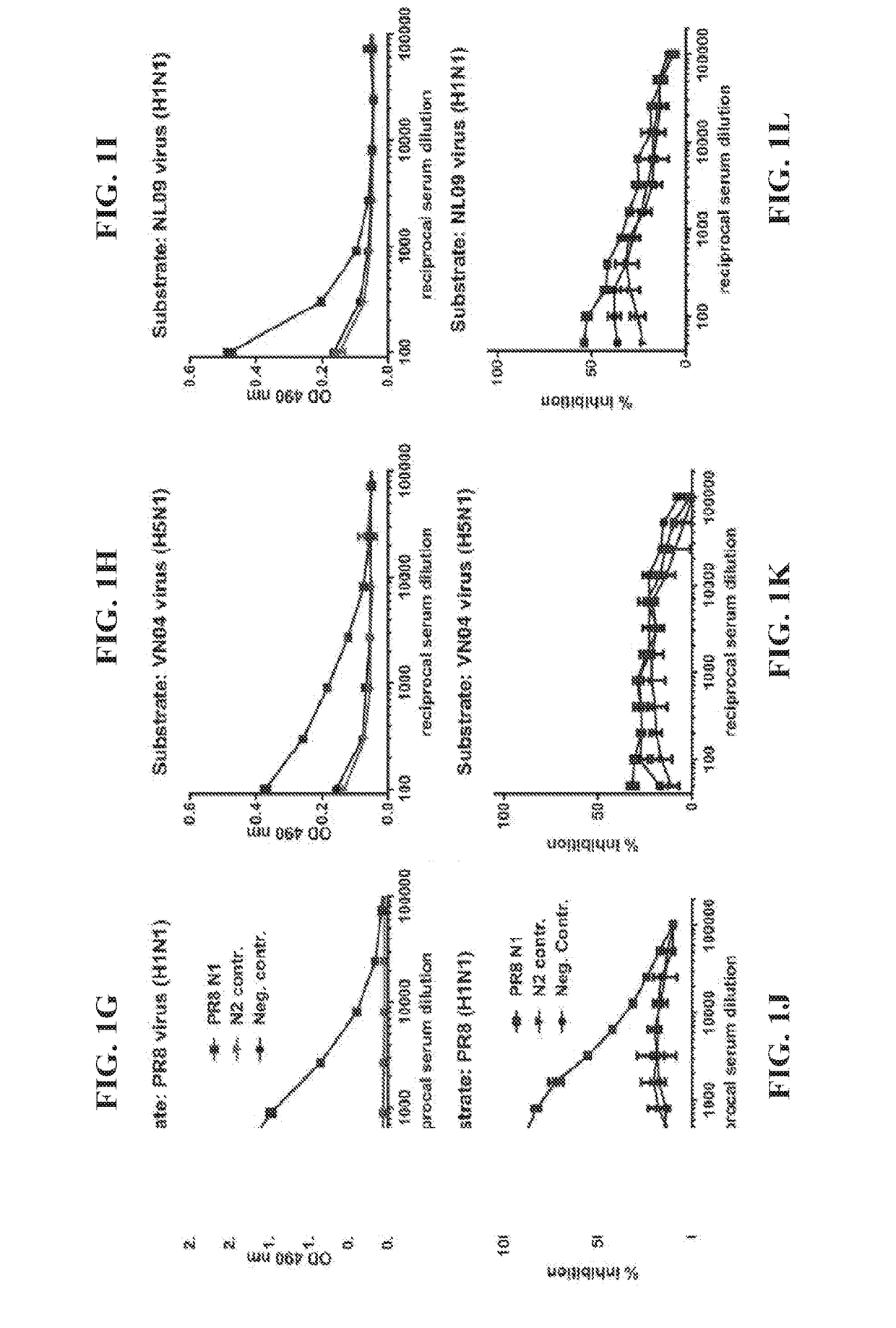
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
Pseudorabies virus SA215, pseudorabies virus polygene deletion bacterin and preparation method thereof
ActiveCN101186902ALow costConvenient researchViruses/bacteriophagesAntibody medical ingredientsGenetic engineeringPolygene
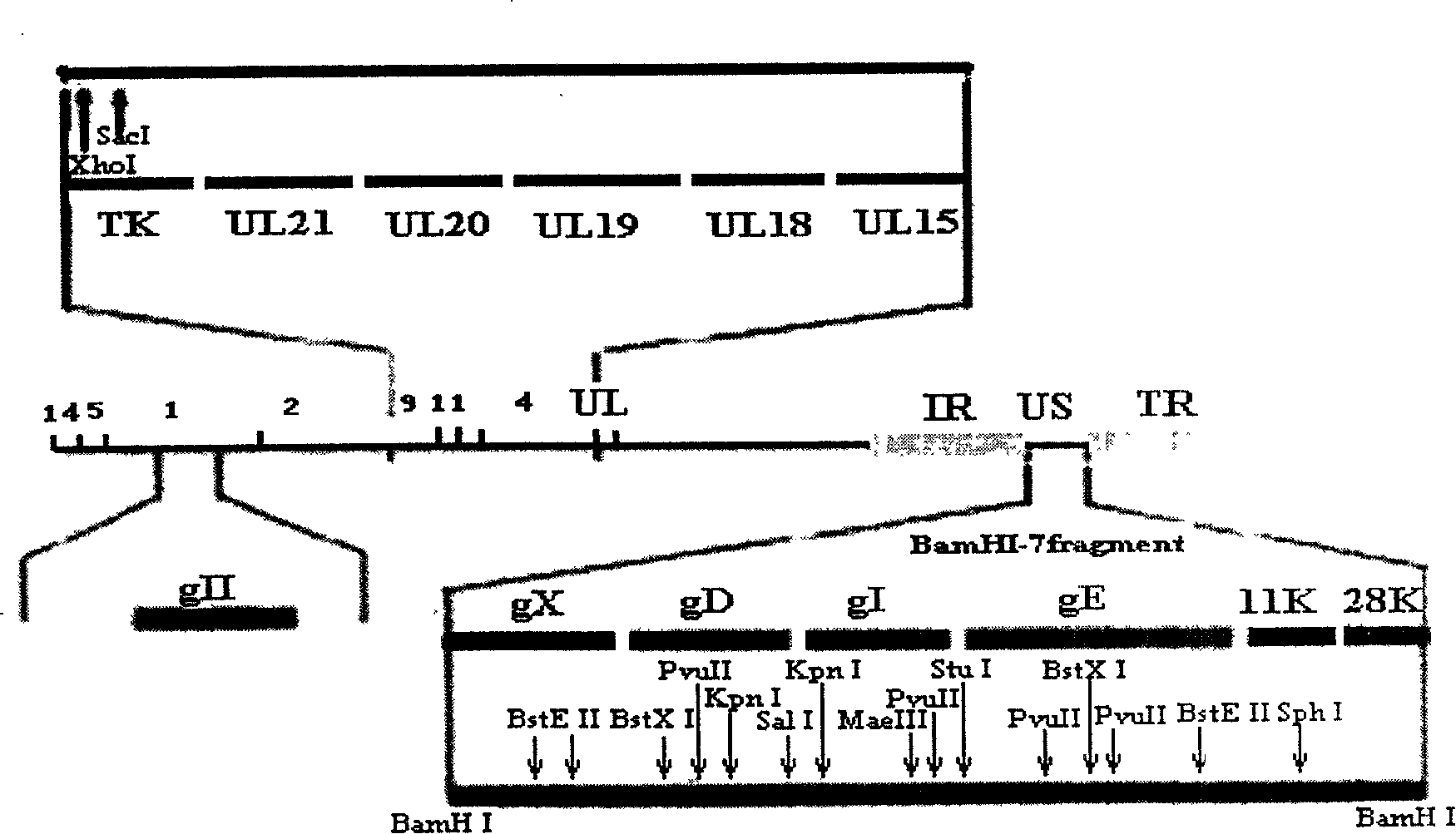
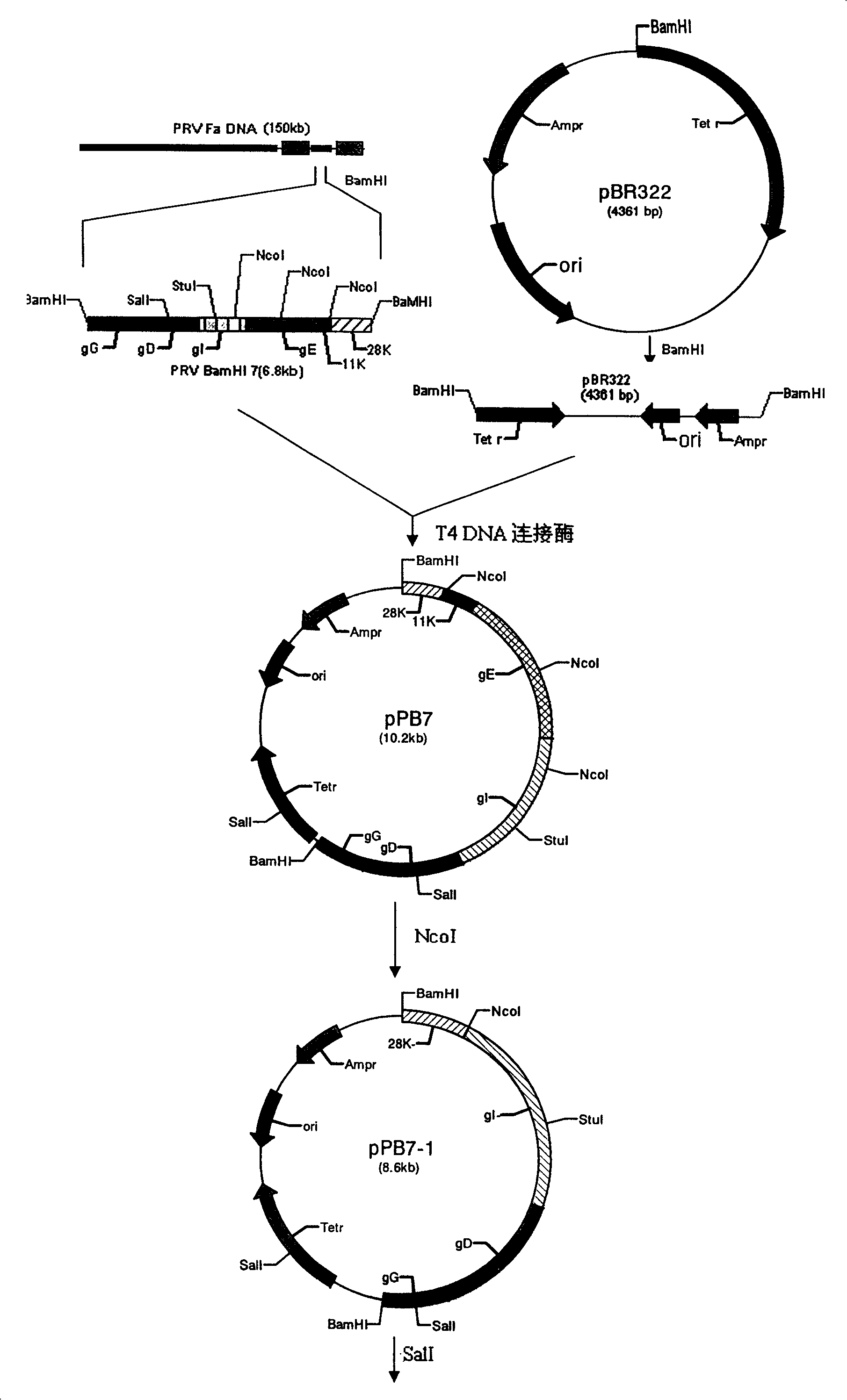
The invention belongs to the technical field of animal virology and genetic engineering, relating to pseudorabies virus which lacks parts of virulence gene of min strain A of pseudorabies virus, and the process for preparing vaccine with the virus. The DNA recombinant technology is employed to artificially remove all or parts of albumen code area of a plurality of genes of TK, gE, gI, 11K, and 28k which are unnecessary genes and duplicate virus of viruses and influence the virulence in the pseudorabies virus, thereby lowering the virulence of the pseudorabies virus, achieving attenuated vaccine strains SA215 of which the attenuation genetic engineering is lack, preparing vaccine with SA215 virus, and immunizing animals to effectively prevent and control the occurrence and prevalence of the pseudorabies.
Owner:SICHUAN AGRI UNIV
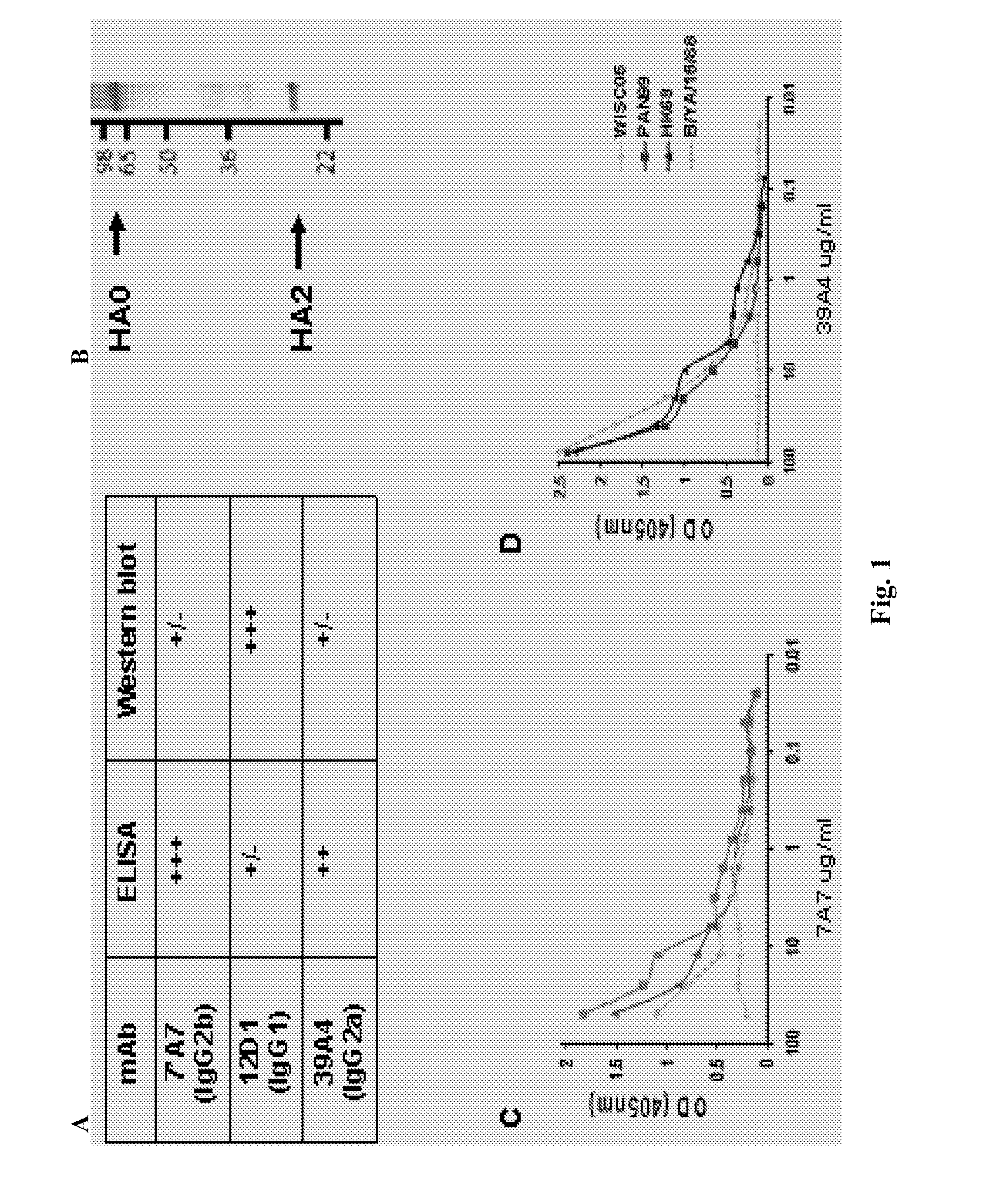
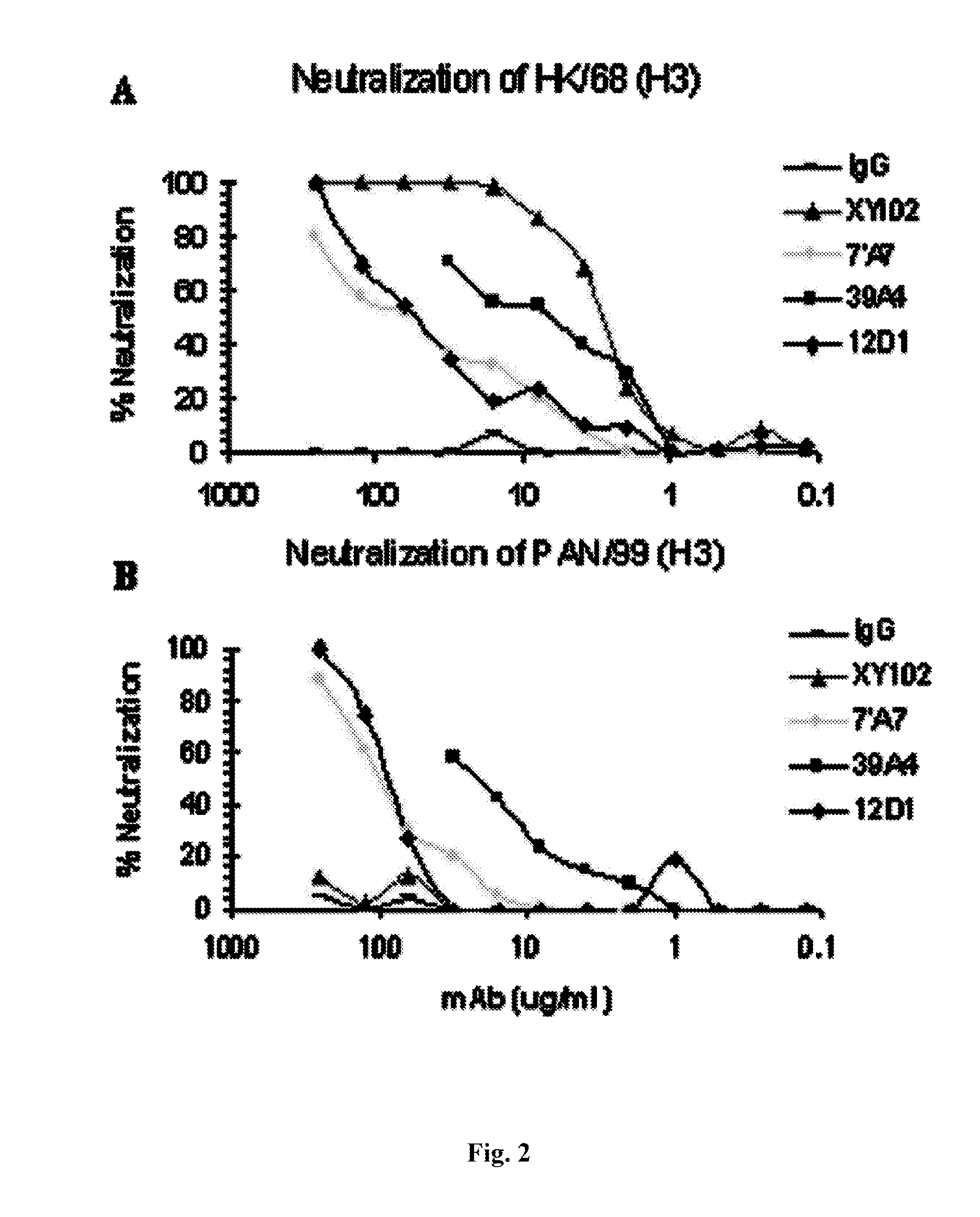
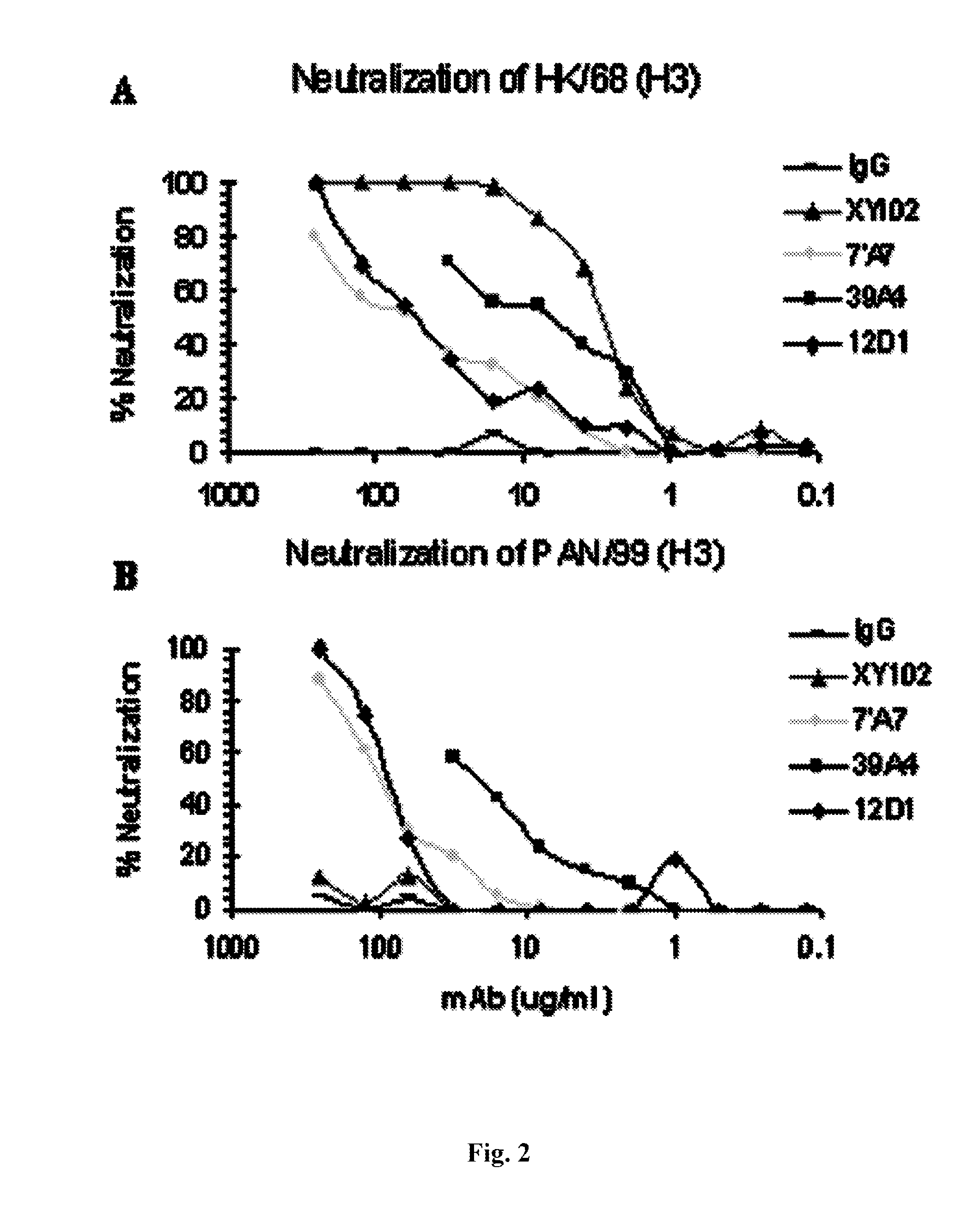
Monoclonal antibodies against influenza virus generated by cyclical administration and uses thereof
ActiveUS20110027270A1Reduce in quantityLower titerSsRNA viruses negative-senseVirus peptidesMonoclonal antibodyVirus diseases
Provided herein are methods of producing neutralizing monoclonal antibodies, by cyclical immunization, that cross-react with strains of Influenza virus of the same subtype or different subtypes. Also provided herein are compositions comprising such antibodies and methods of using such antibodies to diagnose, prevent or treat Influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
Antibodies for depletion of ICOS-positive cells in vivo
ActiveUS8318905B2Extended half-lifeReduce deliveryNervous disorderPeptide/protein ingredientsDiseaseT cell
The present invention relates generally to binding agents useful in the selective depletion of T cells in vivo. More specifically, the invention relates to ICOS-binding agents which once bound to ICOS expressed on the surface of cells, in particular ICOS-bearing activated T cells, result in the in vivo depletion of cells to which they are bound. Methods of treating T cell related diseases using said ICOS-binding agents, and pharmaceutical compositions comprising said ICOS-binding agents, a method of identifying an ICOS-binding agent, and monoclonal anti-ICOS antibodies capable of eliminating cells in vivo which express ICOS on their surface are also provided.
Owner:BUNDESREPUBLIK DEUT IETZVERTRETEN DURCH DAS ROBERT KOCH INST VERTRETEN DURCH SEINEN PRASI
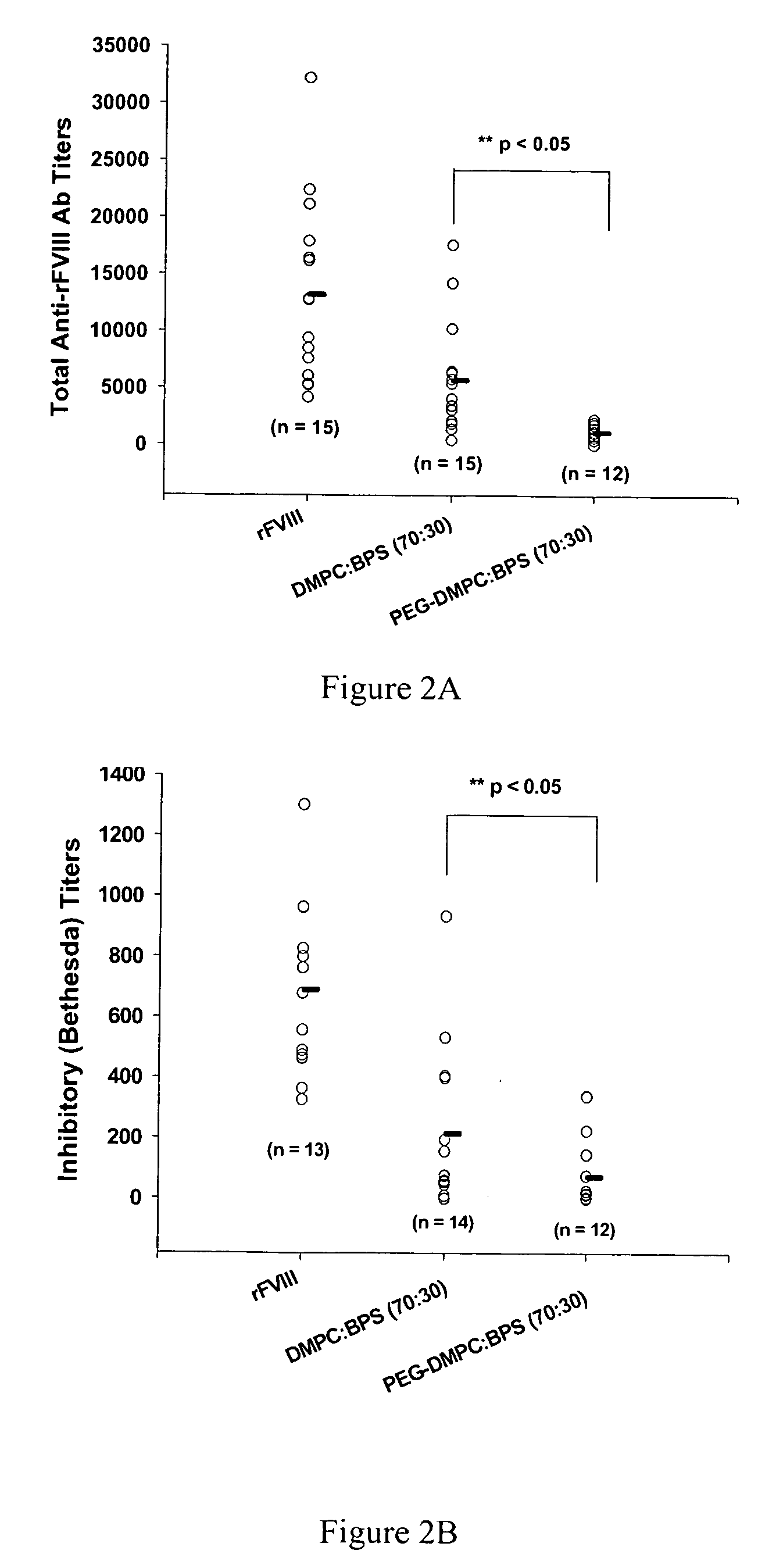
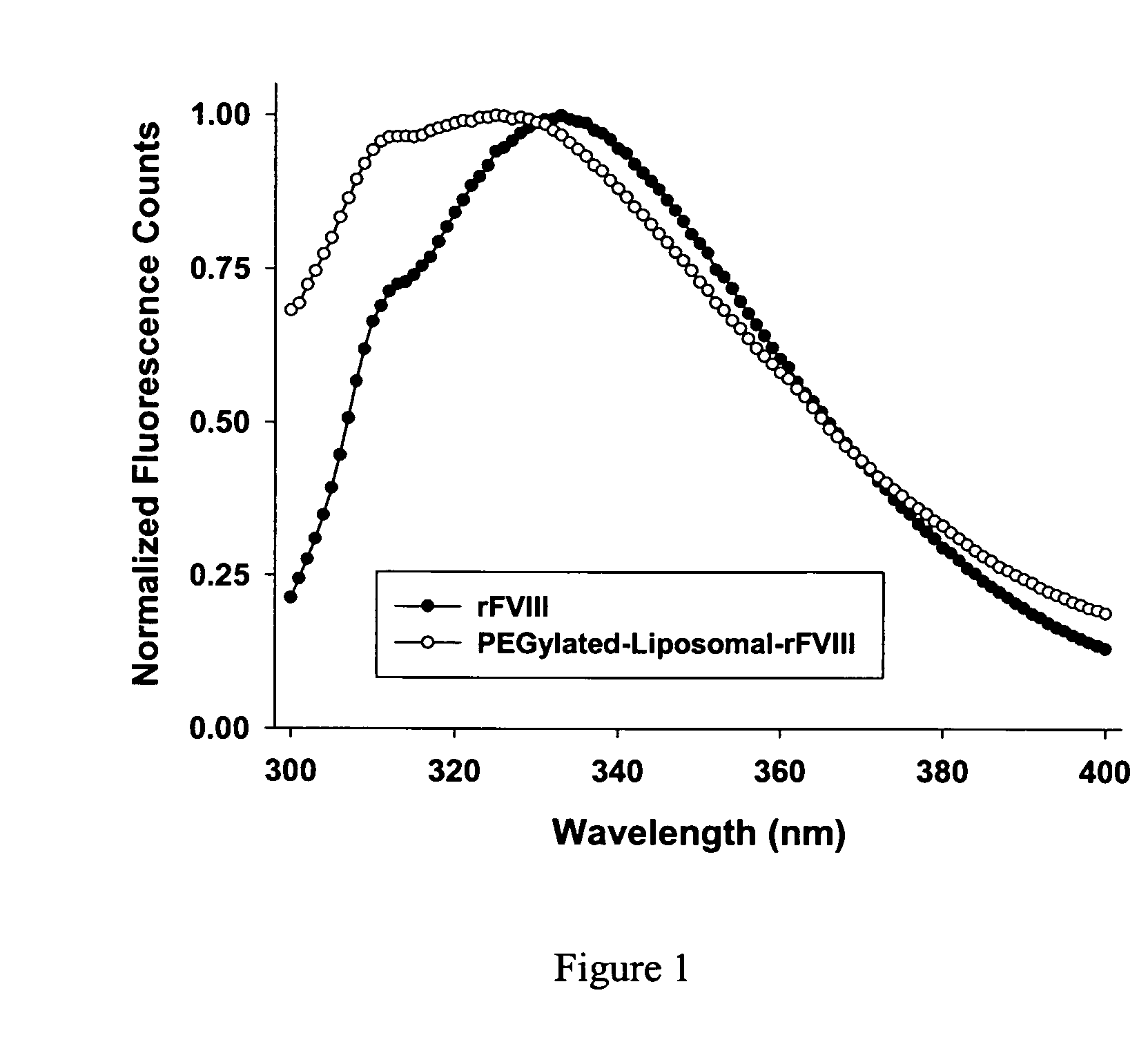
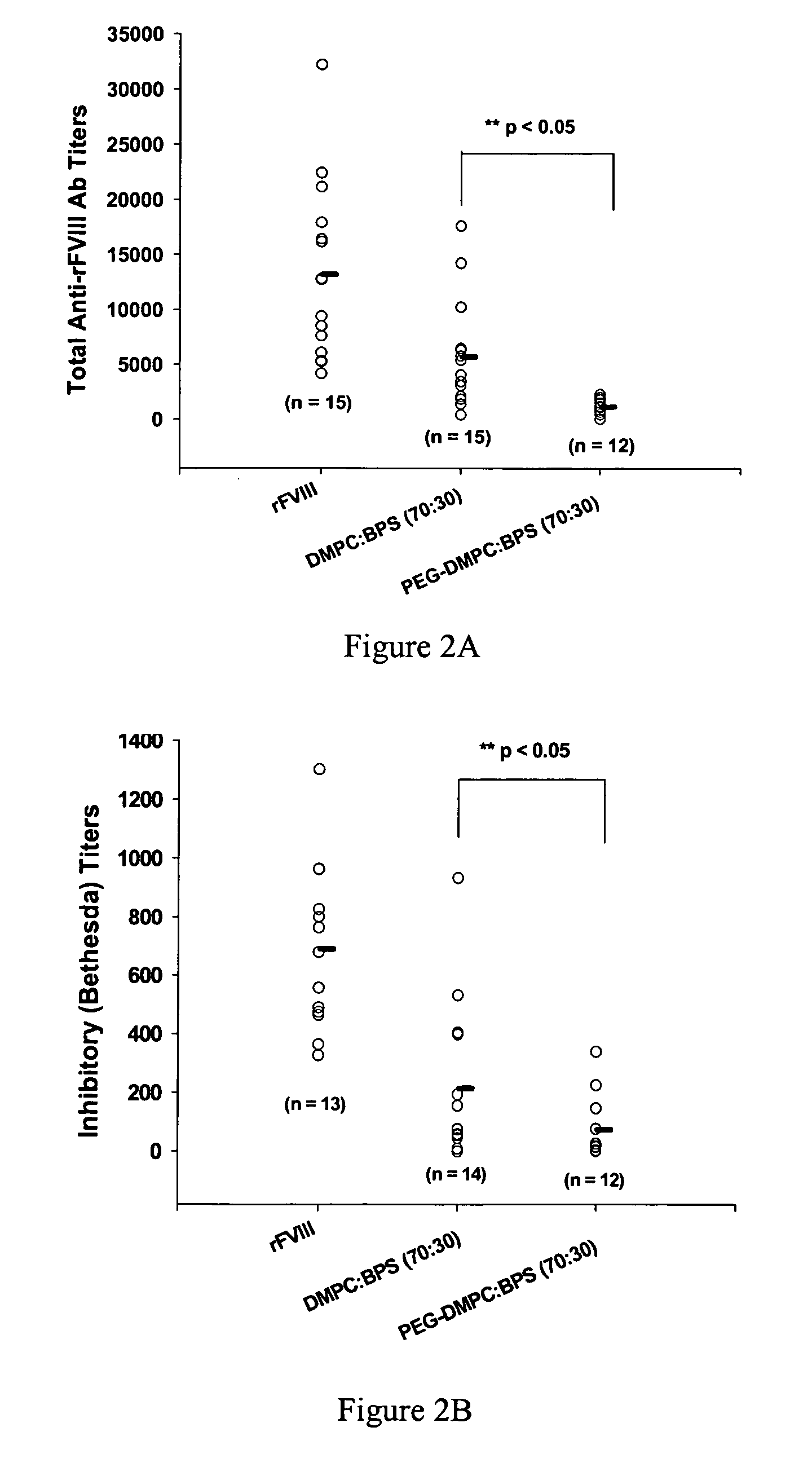
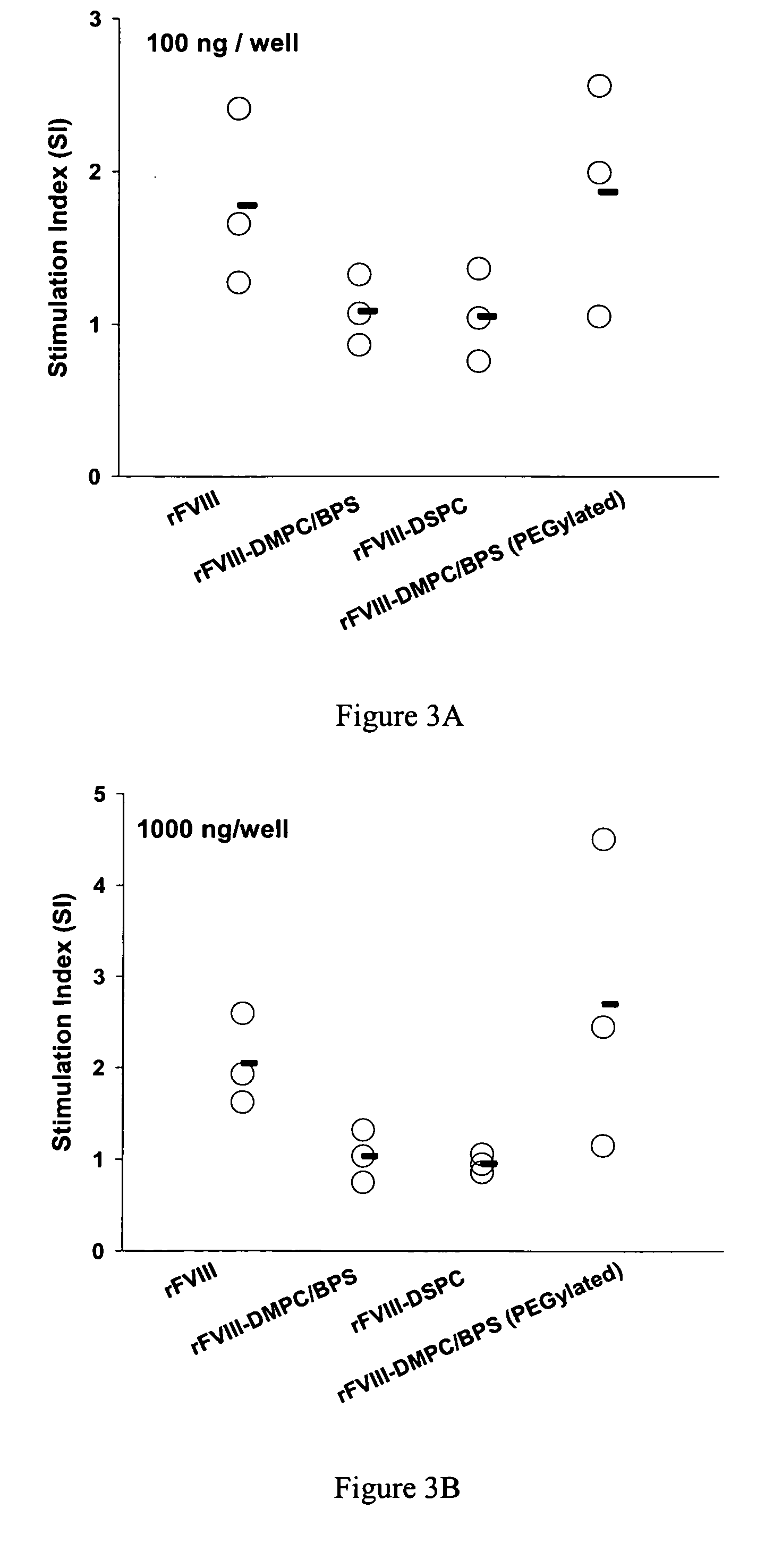
Compositions and methods for less immunogenic protein-lipid complexes
InactiveUS20090053297A1Lower titerLow immunogenicityPeptide/protein ingredientsMicroencapsulation basedLipid formationHalf-life
The present invention provides compositions and methods for reducing the immunogenicity and increasing the circulating half-life of therapeutic proteins such as Factor VIII. The compositions comprise lipidic structures such as liposomes, micelles and cochleates comprising a negatively charged lipid and polyethylene glycol derivatized phosphatidyl ethanolamine.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Influenza virus vaccines and uses thereof
ActiveUS20140328875A1Highly potent and broadly neutralizing antibodiesStimulate immune responseSsRNA viruses negative-senseBacteriaHemagglutininInfluenza virus vaccine
Provided herein are chimeric influenza hemagglutinin (HA) polypeptides, compositions comprising the same, vaccines comprising the same, and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
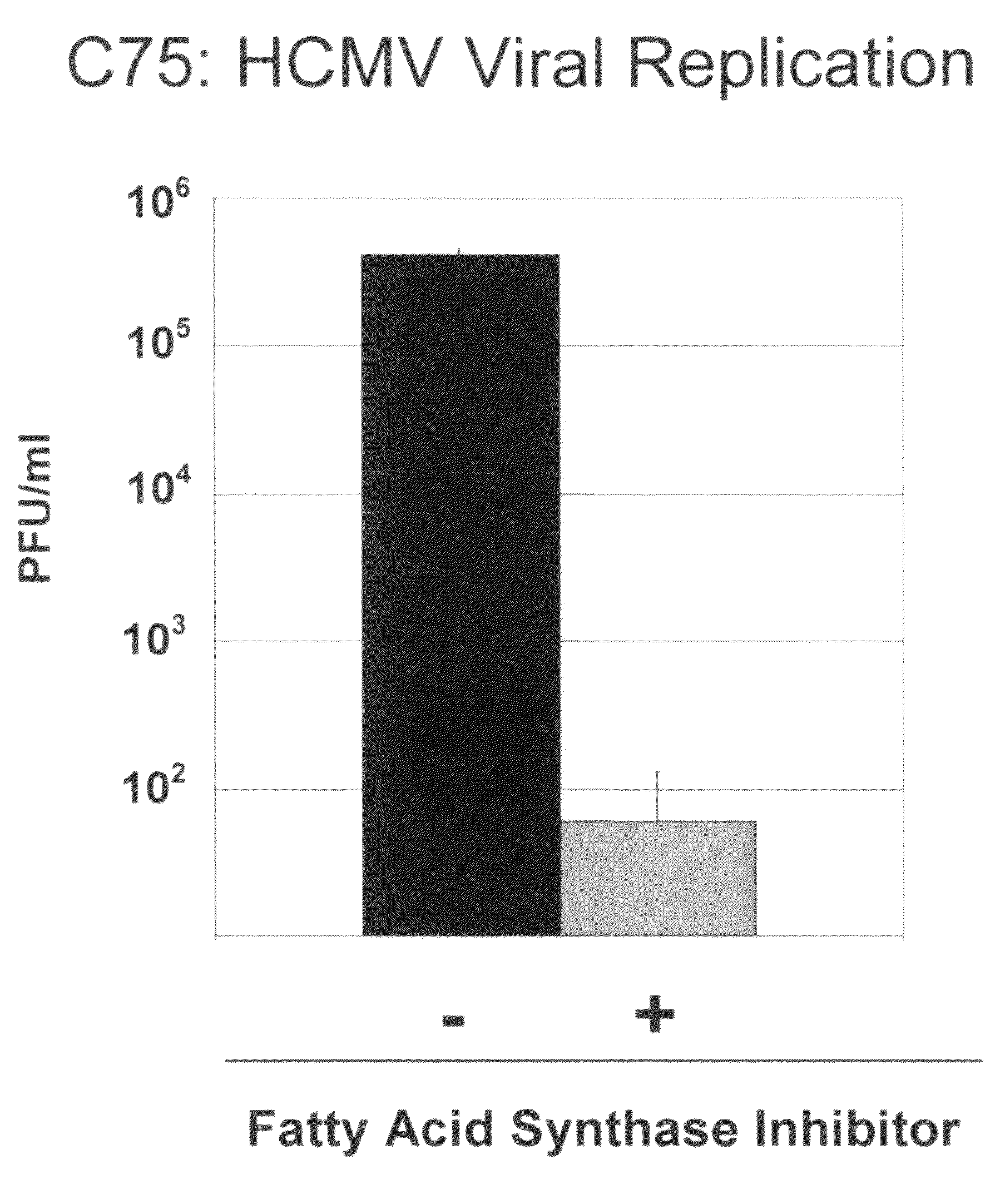
Treatment of viral infections by modulation of host cell metabolic pathways
InactiveUS8158677B2Reduce severityImprove survivalBiocideDigestive systemCritical control pointMetabolite
Owner:THE TRUSTEES FOR PRINCETON UNIV
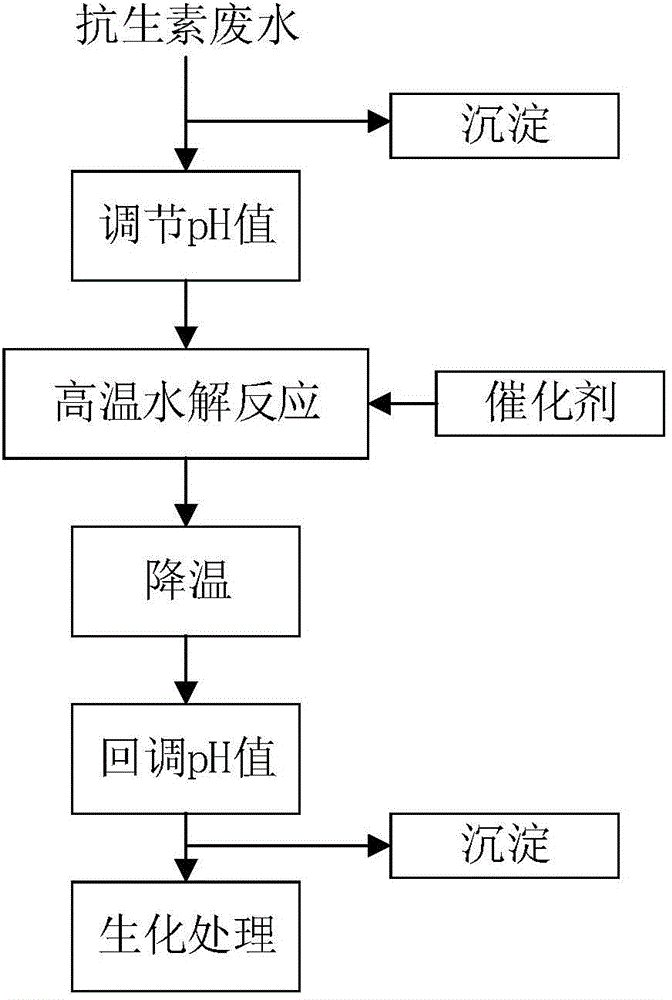
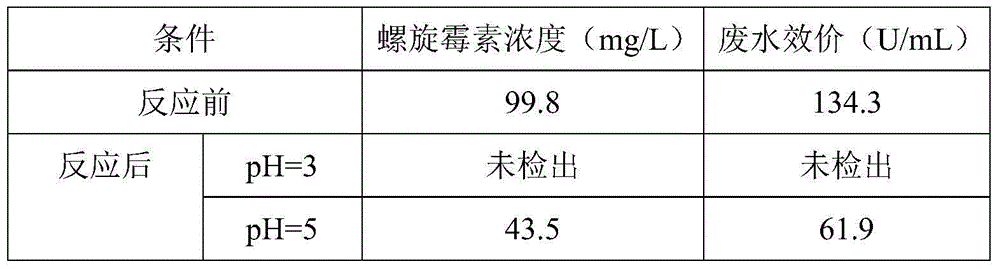
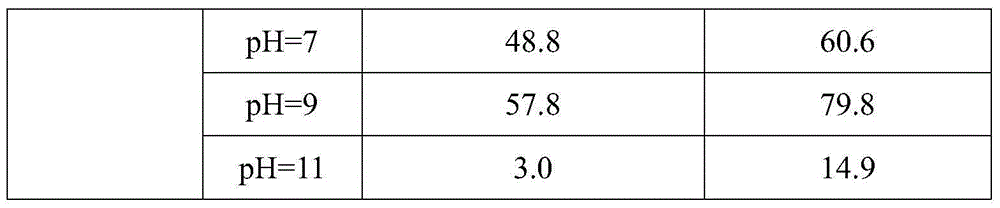
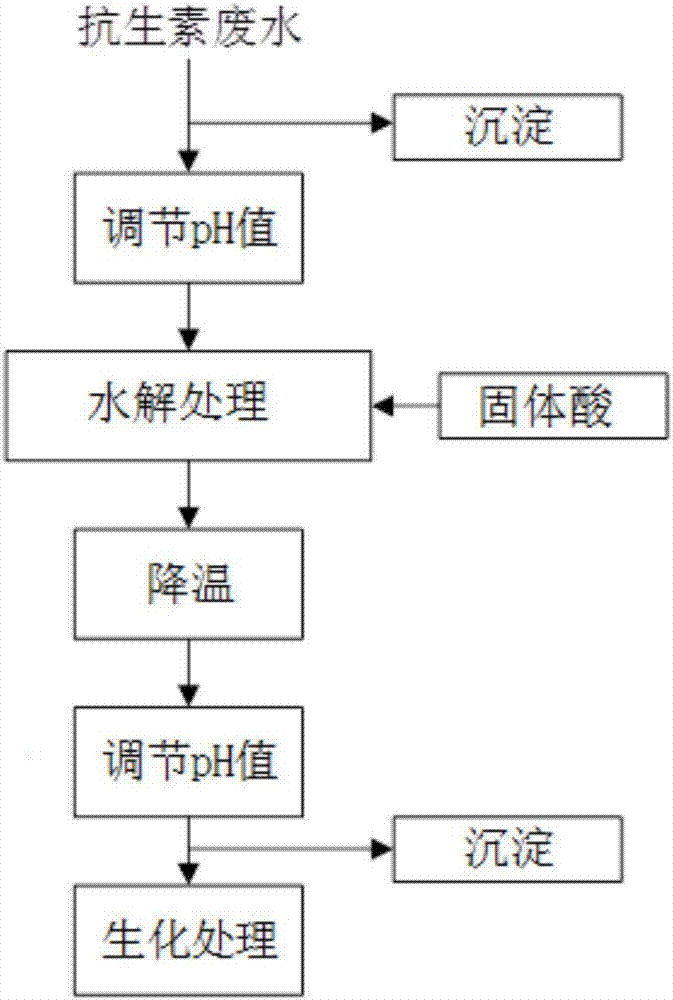
Pretreatment method for removing antibiotics in ferment antibiotic pharmaceutical wastewater
ActiveCN105084442ALower titerEfficient decompositionMultistage water/sewage treatmentWater/sewage treatment by heatingMicroorganismResistant bacteria
The invention provides a pretreatment method for removing antibiotics in ferment antibiotic pharmaceutical wastewater. The method removes antibiotics in the ferment antibiotic pharmaceutical wastewater mainly through adjustment of the pH value of the wastewater and high-temperature catalytic hydrolysis, and the treated pharmaceutical wastewater without antibiotics can enter a subsequent biochemical treatment process for treatment. The method provided by the invention can basically remove antibiotics in the pharmaceutical wastewater, reduce inhibition effect of high-concentration antibiotics on microbes, lower down difficulty in treatment of the wastewater by using the subsequent biochemical process and decrease generation of drug-resistant bacteria and drug-resistant genes in subsequent biochemical treatment.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Influenza virus vaccines and uses thereof
ActiveUS9051359B2Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Monoclonal antibodies against influenza virus generated by cyclical administration and uses thereof
ActiveUS20140170163A1Reduce in quantityLower titerSsRNA viruses negative-senseAnimal cellsMonoclonal antibodyVirus diseases
Provided herein are methods of producing neutralizing monoclonal antibodies, by cyclical immunization, that cross-react with strains of Influenza virus of the same subtype or different subtypes. Also provided herein are compositions comprising such antibodies and methods of using such antibodies to diagnose, prevent or treat Influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
Duck plague yolk antibody freeze-dried powder and preparation method thereof
The invention relates to duck plague yolk antibody freeze-dried powder, which comprises the following components in percentage by weight: 87 to 90 percent of duck plague yolk antibody, 0.8 to 1.0 percent of octanoic acid, 0.2 to 0.3 percent of formaldehyde, 0.01 to 0.02 percent of thimerosal, 3 to 4 percent of glucose, 1 to 2 percent of sorbitol, 2 to 3 percent of glycine and 2 to 3 percent of mannitol. The freeze-dried powder has the advantages of long storage time, quick dissolution, high antibody valence and high bioactivity, and the purity of the antibody of the freeze-dried powder is higher than that of the conventional product in market. The invention also discloses a preparation method for the duck plague yolk antibody freeze-dried powder. The preparation method comprises the following steps of: separating strains to prepare vaccines, immunizing healthy ducks to obtain high-immunity eggs, performing sterilization, acidification, octanoic acid treatment, refining, extraction, purification, preparation, freeze drying and the like on the high-immunity eggs, and thus obtaining the duck plague high-immunity yolk antibody freeze-dried powder. According to the preparation method, yolk liquid is heated by using the temperature of pasteurization during acidification and water treatment, so that a sterilization effect is achieved, and the recovery rate of the antibody and the clarity of the solution during acidification can be improved at the same time; and the prepared yolk antibody has the advantages of high bioactivity, long storage time, retarded valence reducing speed and the like.
Owner:ZHENGZHOU HOUYI PHARMA
Process for preparing yelk antibody with high bioactivity
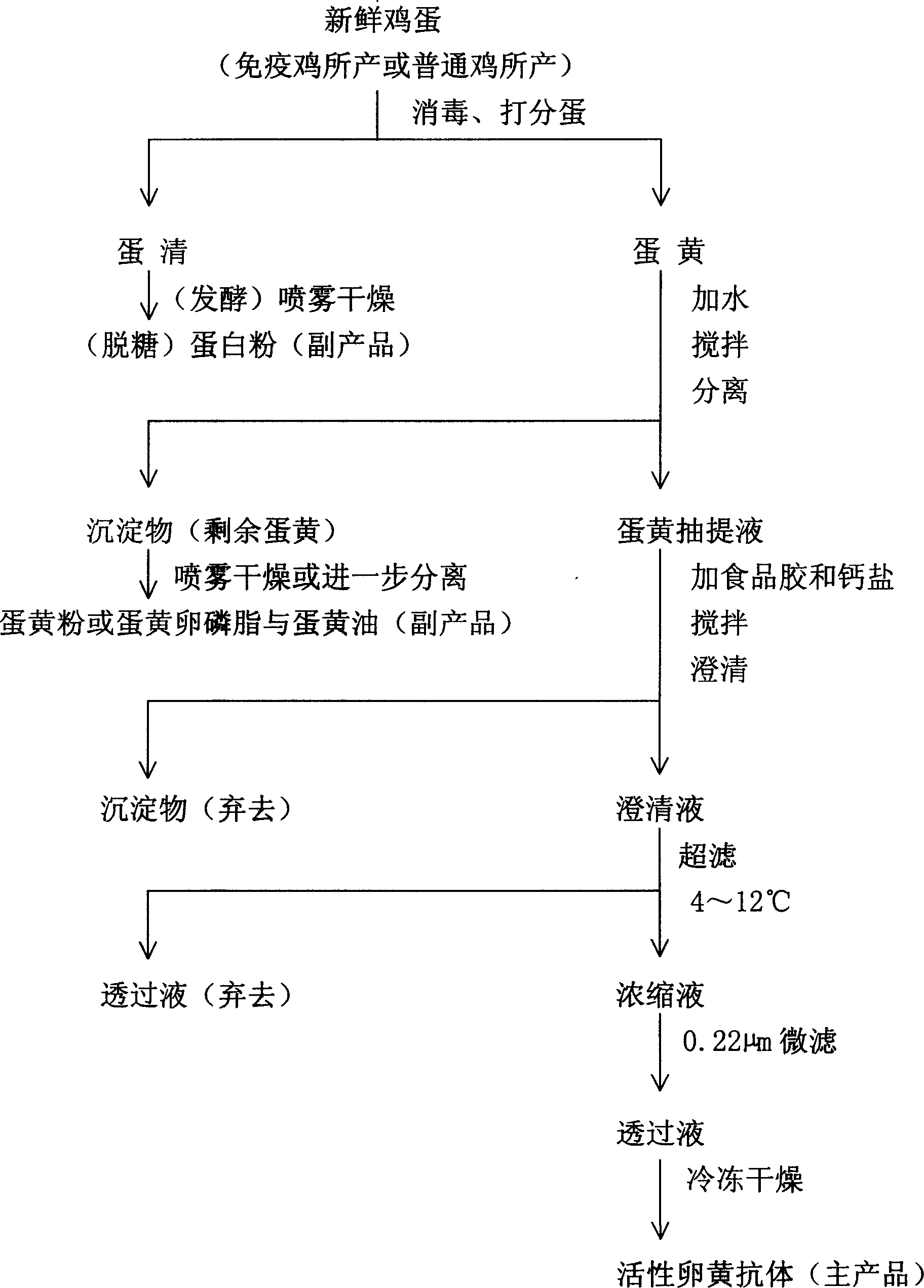
ActiveCN1844144AHigh biological potencyImprove biological activityEgg immunoglobulinsImmunoglobulins against bacteriaYolkDrug biological activity
This invention is preparation procedure of the yolk antibody of high biological activity, belonging to the field of bioproduct and industrialized isolation technique. In this invention, we adopt the natural macromolecule gel high -extractive technique and hyperfiltration to extract the yolk-antibody of high biological activity from yolk at an industrialized level, annual output is upto 10 ton. the yolk-antibody content is about 30%-50%, meanwhile we could obtain some by-products like albumen powder, yolk powder, egg oil, vitellin and so on. In the invention high technology like bioimmunology and membrane separation is adopted. The invention is high-tech, high-usage, highly-automated and typical of green food and environmental-friendly.
Owner:NANTONG KANGDE BIOLOGICAL PROD
Monoclonal antibodies against influenza virus generated by cyclical administration and uses thereof
ActiveUS8673314B2Reduce in quantityLower titerSsRNA viruses negative-senseVirus peptidesDiseaseVirus diseases
Provided herein are methods of producing neutralizing monoclonal antibodies, by cyclical immunization, that cross-react with strains of Influenza virus of the same subtype or different subtypes. Also provided herein are compositions comprising such antibodies and methods of using such antibodies to diagnose, prevent or treat Influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
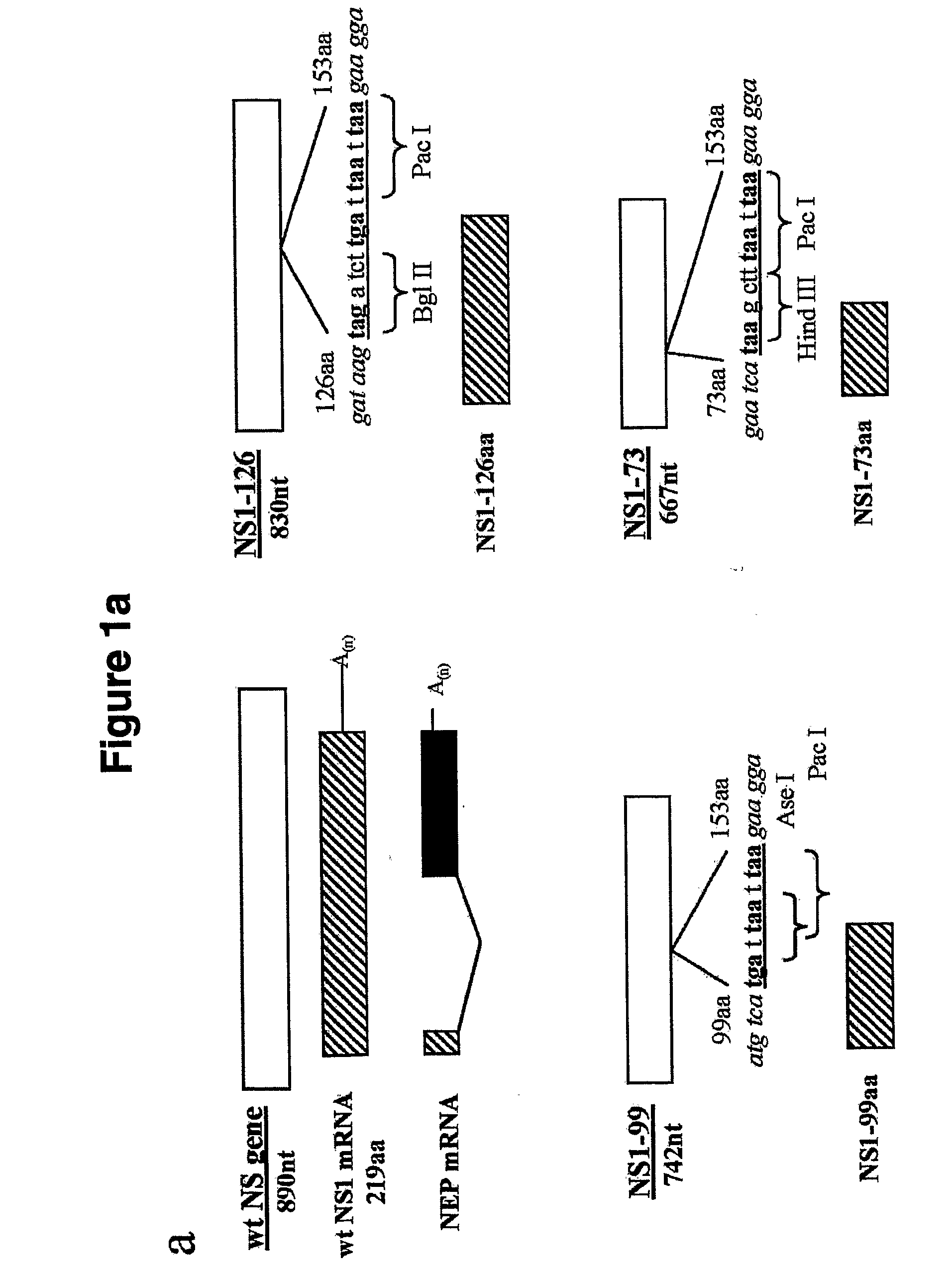
Genetically Engineered Equine Influenza Virus and Uses Thereof
ActiveUS20080254060A1Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsUltrasound attenuationVirulent characteristics
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Genetically engineered swine influenza virus and uses thereof
ActiveUS8124101B2Reduce capacityLow toxicitySsRNA viruses negative-senseViral antigen ingredientsUltrasound attenuationGene product
The present invention relates, in general, to attenuated swine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated swine influenza viruses having modifications to a swine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +2
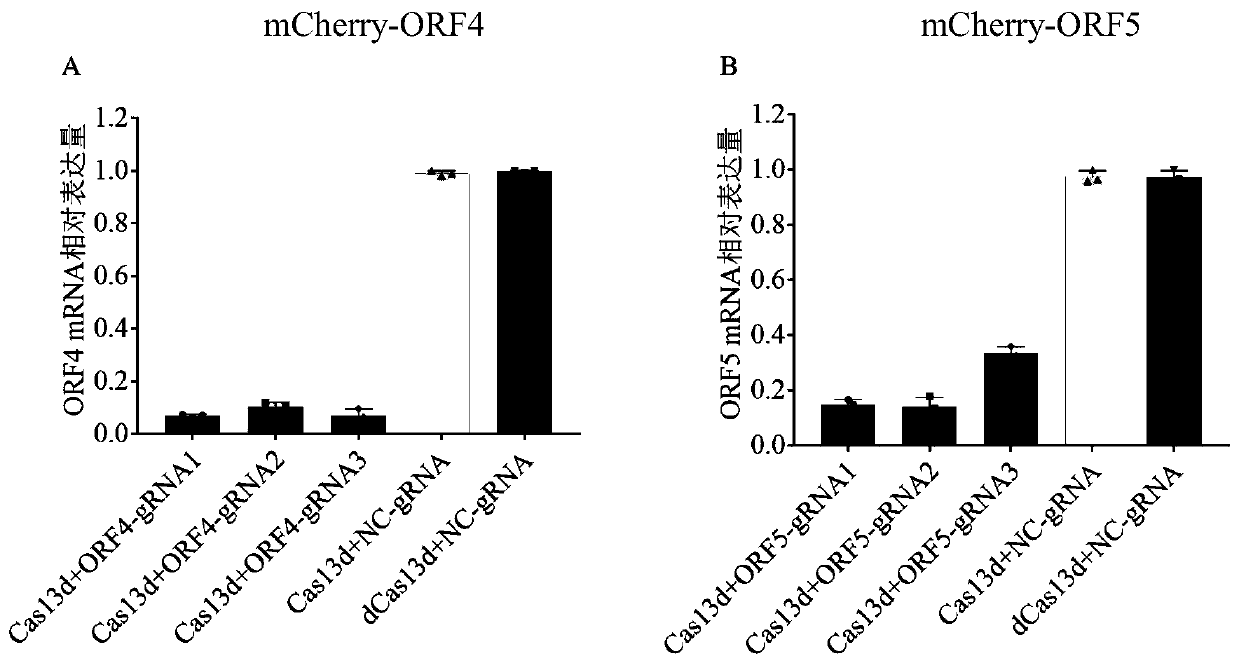
Screening method of sgRNA (small guide ribonucleic acid) efficient action target based on CRISPR-Cas13d (clustered red regularly interspaced short palindromic repeat) system and application
ActiveCN110656123AUniversalImprove efficiencyVector-based foreign material introductionDNA/RNA fragmentationVirusBioinformatics
The invention provides a screening method of a sgRNA (small guide ribonucleic acid) efficient action target based on a CRISPR-Cas13d (clustered red regularly interspaced short palindromic repeat) system and application, and particularly application in RNA virus knockdown. According to the screening method, two expression read frame sequences ORF4 and ORF5 of a PRRSV are respectively fused with a carbon end of a mCherry gene through a mCherry fluorescence report gene, a fluorescence report system for screening sgRNA efficient action targets of the CRISPR-Cas13d system is established, and by virtue of the property that mRNA is efficiently cut by CRISPR-Cas13d, rapid screening of sgRNAs with high efficiency is implemented. Additionally, PRRSV-GFP recombinant viruses are efficiently degraded by using the screened efficient targetedly combined sgRNA based on the CRISPR-Cas13d system. The RNA virus knockdown method provided by the invention has the advantages of being high in efficiency, high in precision and low in target missing rate.
Owner:CHINA AGRI UNIV
Influenza virus vaccines and uses thereof
ActiveUS20150297712A1Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Pig pseudorabies virus natural low-virulent C strain and heatproof preservation method thereof
ActiveCN102399755AImprove stabilityImprove securityMicroorganism based processesViruses/bacteriophagesBiotechnologyMicroorganism
The invention relates to a pig pseudorabies virus natural low-virulent C strain and a heatproof preservation method thereof. The microbe collection registry number of the strain is CCTCC NO: V201114. According to the invention, various cryoprotectant substrates are mixed and blended according to a certain ratio; the cryoprotectants are respectively subject to aseptic processing; cryoprotectant components that can be subject to high-pressure sterilization are dissolved in bi-distilled water, and are sterilized for 15-30min under a temperature of 108-121 DEG C; cryoprotectant components that can not be subject to high-pressure sterilization are dissolved in bi-distilled water according to a certain formula, and are sterilized by using a filter membrane with a size of 0.22mum; all the cryoprotectant components are then mixed into a heatproof cryoprotectant; the heatproof cryoprotectant is mixed with a pseudorabies virus liquid according to a ratio of 1:1-1.2; and the mixture is lyophilized with a corresponding lyophilization curve. Compared to prior arts, the C strain provided by the invention has good stability. In a fifth generation animal of continuous back-procreation, the genetic sequence is stable, and no mutation is found. Therefore, the strain provided by the invention provides a good resource for the researches of pig pseudorabies low-virulent vaccines. The strain can bepreserved for 24 months under a temperature of 2-8 DEG C, and the virus titer is reduced by no more than 1 titer.
Owner:SHANGHAI CHUANG HONG BIOTECH
Extraction method for hyaluronidase
The invention provides an extraction method for hyaluronidase. Acid liquid is adopted to extract and ammonium sulfate is adopted to salt out in order to obtain a hyaluronidase crude product; and a hyaluronidase competitive product is obtained by implementing dialysis, pyrogen treatment and freeze-drying for the crude product. The method chooses sheep testis as a raw material, and adopts the high-speed centrifugal extraction method to separate and purify the hyaluronidase, so that the titer of the hyaluronidase can achieve 340 iu / mg.
Owner:QINGDAO JIULONG BIO PHARMA
Process for preparing straight-through 6-aminopenicillanic acid
InactiveCN101735243AAvoid disadvantagesLarge amount of deesterificationOrganic chemistryFermentationGas phasePressure reduction
The invention discloses a process for preparing straight-through 6-aminopenicillanic acid, which comprises the following steps: a, filtering and acidizing penicillin fermentation solution, extracting the penicillin fermentation solution by using butanol, and concentrating and decoloring the extract to obtain butyl ester extracting solution of penicillin; b, back extracting the butyl ester extracting solution of the penicillin by using alkali solution to obtain brine solution of penicillin (heavy phase or RB for short); c, continuously injecting the brine solution of the penicillin into a degreasing tower in a vacuum pressure reduction state to convert the butyl ester into a gas phase from the brine solution of the penicillin, discharging the degreased brine solution of the penicillin out of a pressure reduction system from the bottom of the tower to a storage tank with a cooling device, and cooling the degreased brine solution of the penicillin for later use; and d, performing enzymatic conversion on the degreased brine solution of the penicillin, then adding 6-APA crystal seeds into the solution, growing the crystals, crystallizing the solution, and drying the crystals.
Owner:NORTH CHINA PHARMA COMPANY
Pretreatment method capable of removing antibiotic in antibiotic pharmaceutical wastewater and antibiotic pharmaceutical wastewater treatment method
ActiveCN106986433ALower titerEfficient decompositionWater contaminantsMultistage water/sewage treatmentDrugChemistry
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Genetically engineered equine influenza virus and uses thereof
ActiveUS8137676B2Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsVirus influenzaIn vivo
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Influenza virus vaccination regimens
ActiveUS20180008696A1Reduce severityImprove survivalSsRNA viruses negative-senseViral antigen ingredientsHemagglutininRegimen
Provided herein are immunization regimens for inducing an immune response (e.g., an antibody response) against influenza virus. In specific aspects, the immunization regimens involve the administration of a chimeric hemagglutinin (HA), a headless HA or another influenza virus stem domain based construct (e.g., the HA stem domain or a fragment thereof) to a subject. In certain aspects, the immunization regimens also involve the administration of an influenza virus neuraminidase immunogen.
Owner:MT SINAI SCHOOL OF MEDICINE
Compositions and methods for less immunogenic protein-lipid complexes
InactiveUS20070141135A1Low titerReduce immunogenicityFactor VIIPeptide/protein ingredientsHalf-lifeTherapeutic protein
The present invention provides compositions and methods for reducing the immunogenicity and increasing the circulating half-life of therapeutic proteins such as Factor VIII. The compositions comprise lipidic structures such as liposomes, micelles and cochleates comprising a negatively charged lipid and polyethylene glycol derivatized phospatidyl ethanolamine.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
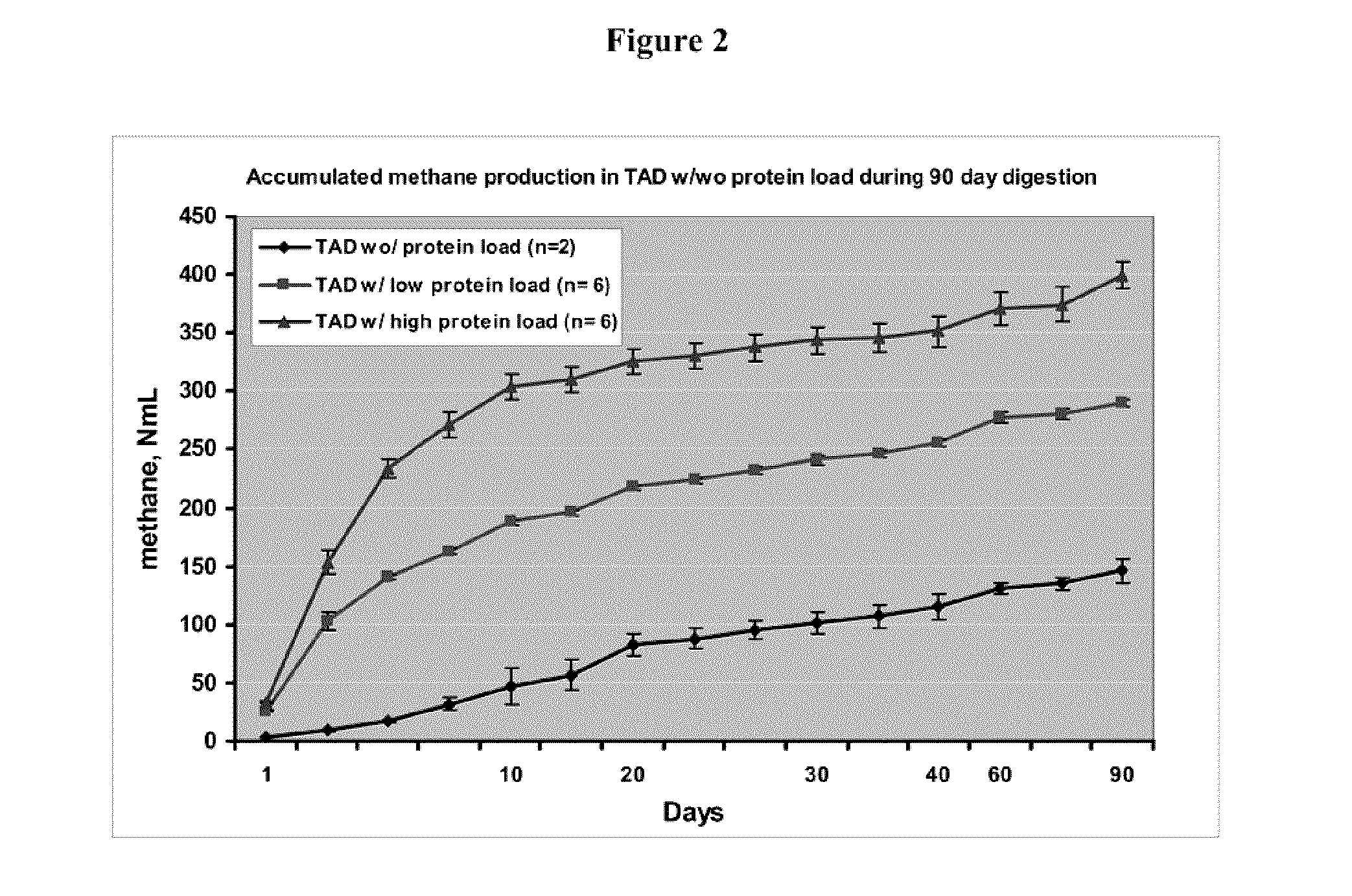
Use of anaerobic digestion to destroy antibiotics in organic waste
InactiveUS20150125921A1Lower titerStable rateSolid waste disposalScale removal and water softeningBioHazardAerobic digestion
The invention relates to systems and methods for using the anaerobic digestion (AD) process, especially thermophilic anaerobic digestion (TAD), to destroy biohazard materials including antibiotics.
Owner:HIMARK BIOGAS INC
Novel paramyxovirus and uses thereof
Described herein are isolated paramyxovirus, a morbillivirus (FmoPV), isolated nucleic acids encoding the genome of FmoPV, isolated amino acid sequences of FmoPV proteins, antibodies to FmoPV and its proteins, and uses thereof. In certain embodiments, the modified FmoPV is a feline morbillivirus. Also described herein is a recombinant FmoPV comprising a modified FmoPV gene or gene segments and the use of such a virus. The recombinant FmoPV may be used in the prevention and / or treatment of diseases related to FmoPV or as a delivery vector. Also described herein is a diagnostic assay for the FmoPV. In certain embodiments, the FmoPV causes kidney disease. In certain embodiments, the kidney disease is in felines. In certain embodiments, the kidney disease is tubulointerstitial nephritis (“TIN”). Also described herein is a quantitative assay for the detection of the FmoPV, natural or artificial variants, analogs, or derivatives thereof. In certain embodiments, the quantitative assay is reverse transcription and polymerase chain reaction (RT-PCR). Also described herein is a vaccine and a kit containing the vaccine for the prevention and treatment of FmoPV infection. Described herein is a diagnostic kit that comprises nucleic acid molecules for the detection of the FmoPV.
Owner:VERSITECH LTD +1
Compositions and methods for prion decontamination
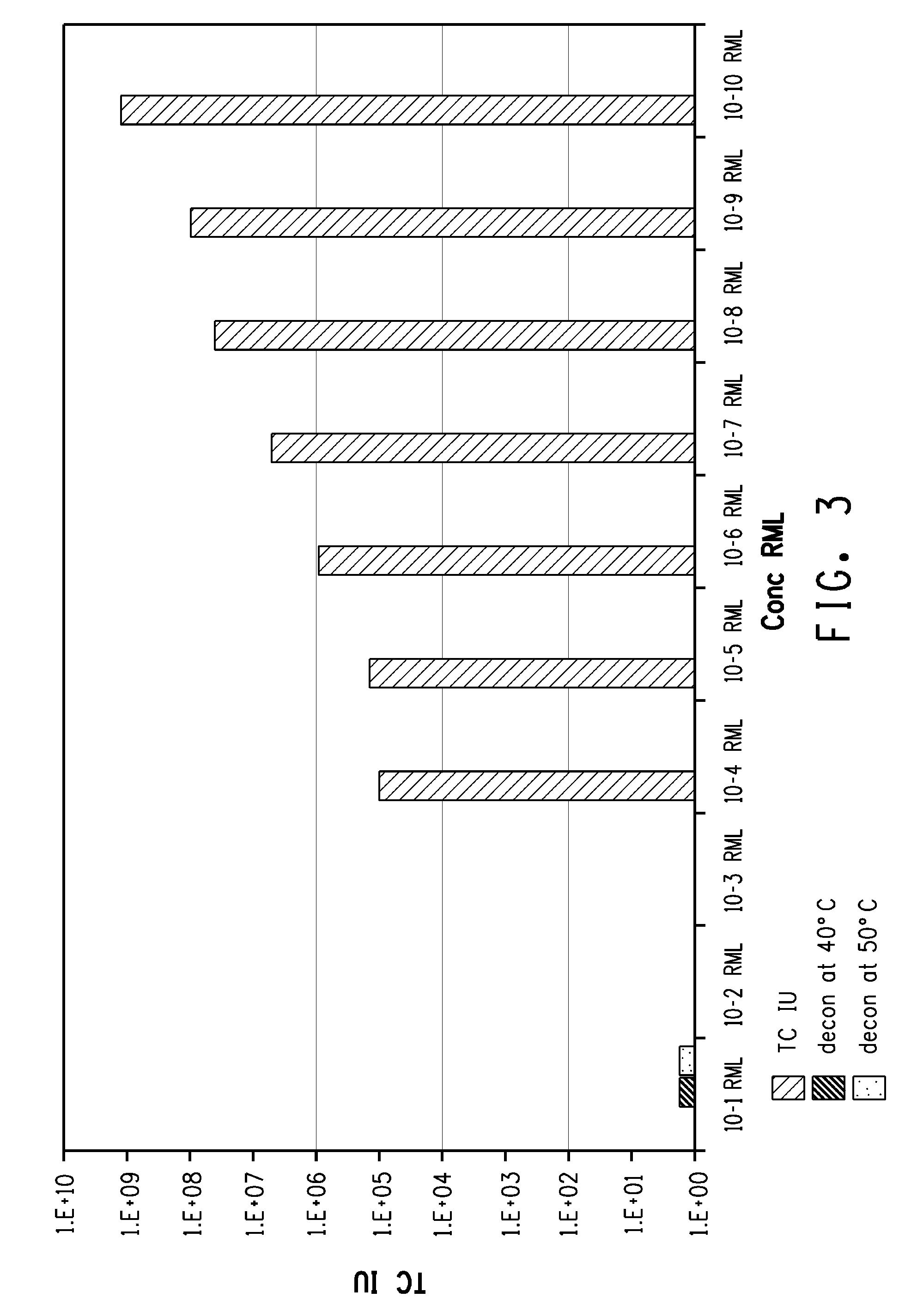
ActiveUS8034766B2Lower titerExcellent decontaminationBiocidePeptide/protein ingredientsSURFACTANT BLENDNuclear chemistry
The invention relates to compositions and methods for prion degradation, decontamination or disinfection. The composition comprises an oxidizing agent, one or more proteases and a surfactant such as an ionic surfactant / detergent. The method comprises contacting a prion contaminated entity with a prion-degrading composition comprising an effective amount of an oxidizing agent, an effective amount of at least one protease, and an effective amount of a surfactant. The components of the composition may be contacted with a prion-contaminated entity sequentially or simultaneously using an aqueous composition. Typically at least two different proteases are used for optimal efficacy. Preferably the oxidizing agent comprises peracetyl ions or a source thereof. The invention also relates to kits comprising the various reagents.
Owner:UNIV COLLEGE OF LONDON +1
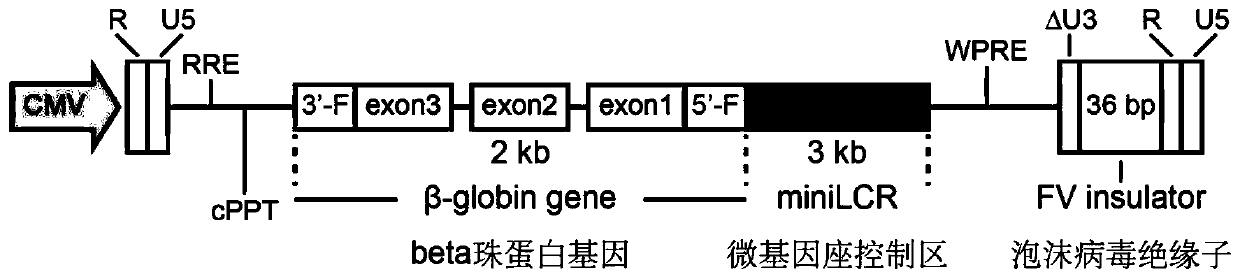
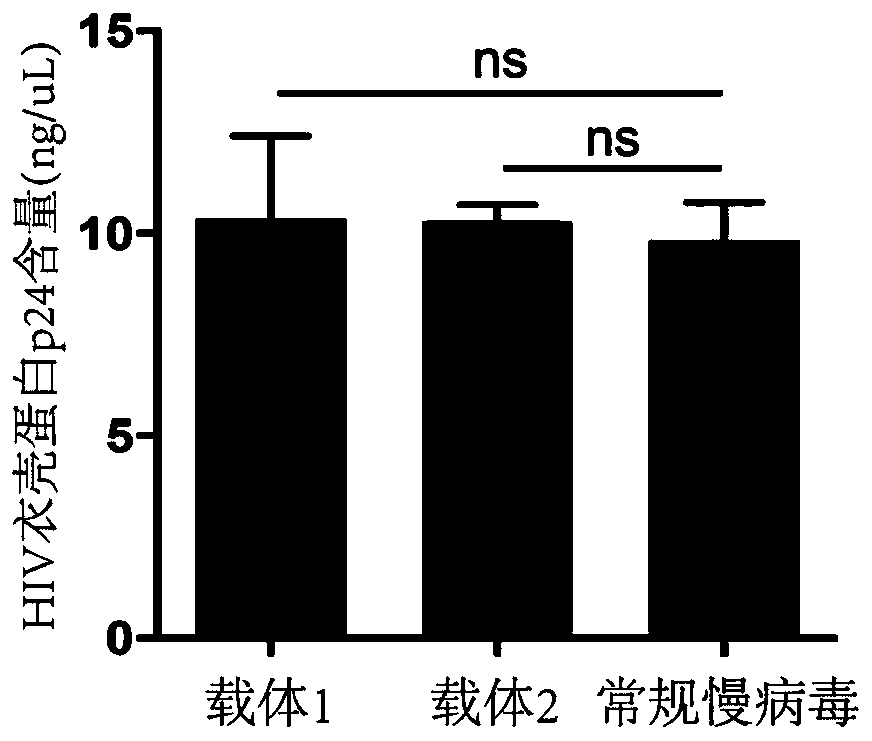
Lentiviral vector suitable for gene therapy of thalassemia and sickle anemia
ActiveCN110564770ALower titerInhibition of activationVectorsHaemoglobins/myoglobinsThalassemiaSickle cell anemia
The invention discloses a lentiviral vector suitable for gene therapy of thalassemia and sickle anemia. The lentiviral vector comprises a beta globin expression cassette; the expression cassette comprises a micro-locus control area, a gene sequence of a beta globin, a promoter sequence of a flank of the upstream of the gene sequence of the beta globin, and a flanking sequence of the downstream ofthe gene sequence of the beta globin; the micro-locus control area is a micro-control element which is screened out from the locus control area of beta globin 16kb and does not contain an HS1 area. Compared with the prior art, the screened beta globin expression cassette has both efficiency and specificity, and does not cause reduction of lentivirus titer; a screened insulator sequence which comesfrom foamy virus and is only 36 bp does not contain a hidden RNA splicing signal, and has the functions of maintaining gene expression and preventing gene activation in a region where the insulator sequence is located; the lentiviral vector can be used for gene therapy of thalassemia and sickle anemia.
Owner:SHANGHAI BDGENE TECH CO LTD
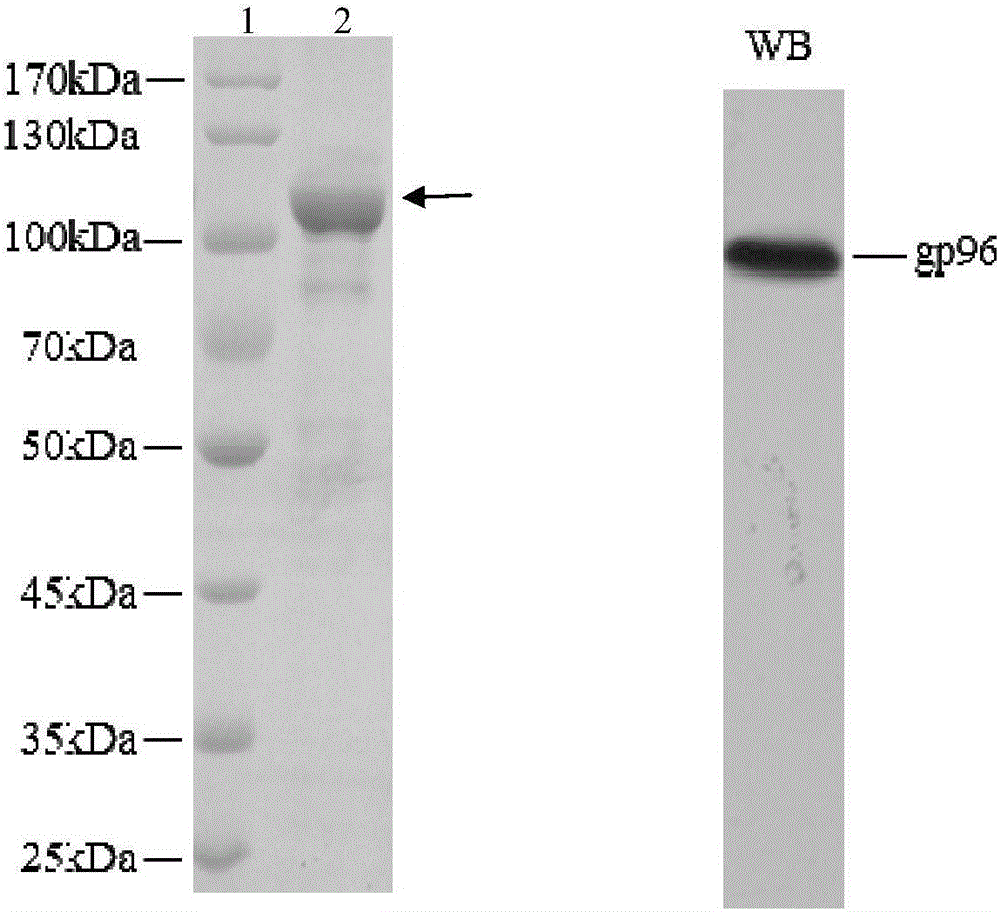
Application of heat shock protein gp96 in treatment of rheumatoid arthritis
ActiveCN106039287ALower titerReduced proliferative capacityPeptide/protein ingredientsAntipyreticEarly rheumatoid arthritisBioinformatics
The invention discloses application of a heat shock protein gp96 in the treatment of rheumatoid arthritis. The heat shock protein gp96 is (a1) or (a2) or (a3) or (a4): (a1) a protein with an amino acid sequence represented from a site 2 to a site 783 from a terminal N in a sequence 2 in a sequence table; (a2) a protein with an amino acid sequence represented by the sequence 2 in the sequence table; (a3) a fusion protein obtained by linking a label to the terminal N or / and a terminal C of (a1) or (a2); and (a4) a protein, with the same functions, obtained through substitution and / or deletion and / or addition of one or more amino acid residue from the site 2 to the site 783 from the terminal N in the sequence 2 in the sequence table or the amino acid sequence represented by the sequence 2 in the sequence table. Experiments prove that the heat shock protein gp96 has important application values in the treatment of rheumatoid arthritis and / or the relief of symptoms of the rheumatoid arthritis.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Cell-factory-based measles virus stock solution and measles-series attenuated live vaccine preparation
InactiveCN107418936AAchieve trainingSmall batch-to-batch varianceSsRNA viruses negative-senseSsRNA viruses positive-senseCell factoryCulture fluid
The invention provides a cell-factory-based measles virus stock solution and a measles-series attenuated live vaccine preparation. A preparation method of the measles virus stock solution includes selecting SPF chick-embryo cells, and adding cell culture fluid to prepare cell suspension liquid, and adding the cell suspension liquid into a cell factory; inoculating the cell factory with working seeds of measles viruses and the cell suspension liquid according to the ratio of the working seeds to the cell suspension liquid being (0.005-0.05):1, and standing and cultivating the cell factory at the temperature of 33+ / -1 DEG C for 3-4 days, pouring out archeocyte culture fluid, and replacing with fresh cell growth liquid for continuous cultivation; during cytopathy, collecting single measles virus liquid step by step. In an equal production scale, the batch number of cell dissociation is reduced, and the high-titer measles virus liquid is obtained, so that quality uniformity of measles-series vaccine products is improved effectively, and product yield is increased.
Owner:BEIJING BIOLOGICAL PROD INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com