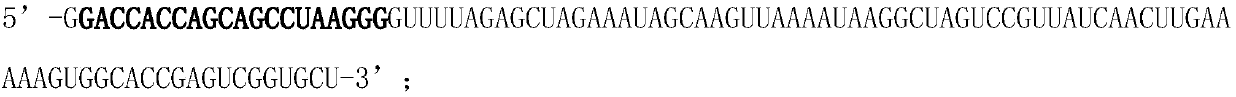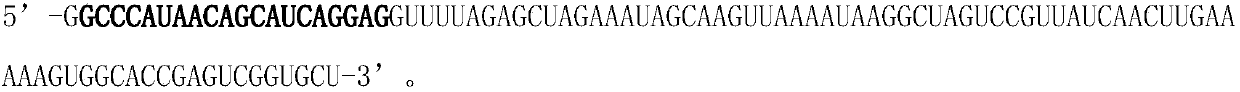Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Beta globin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
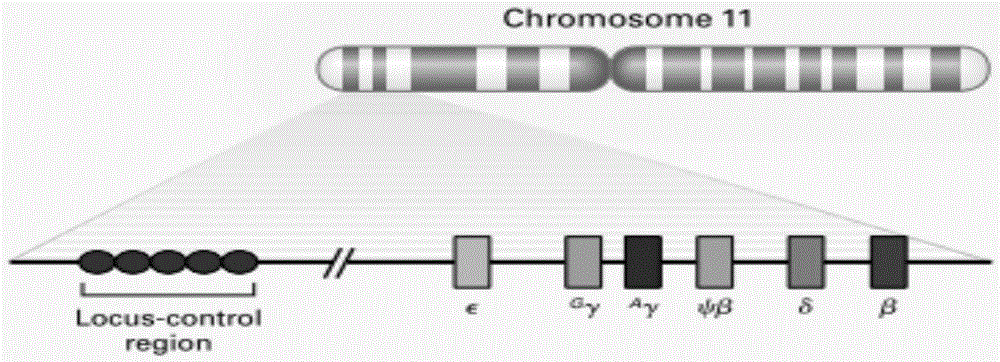
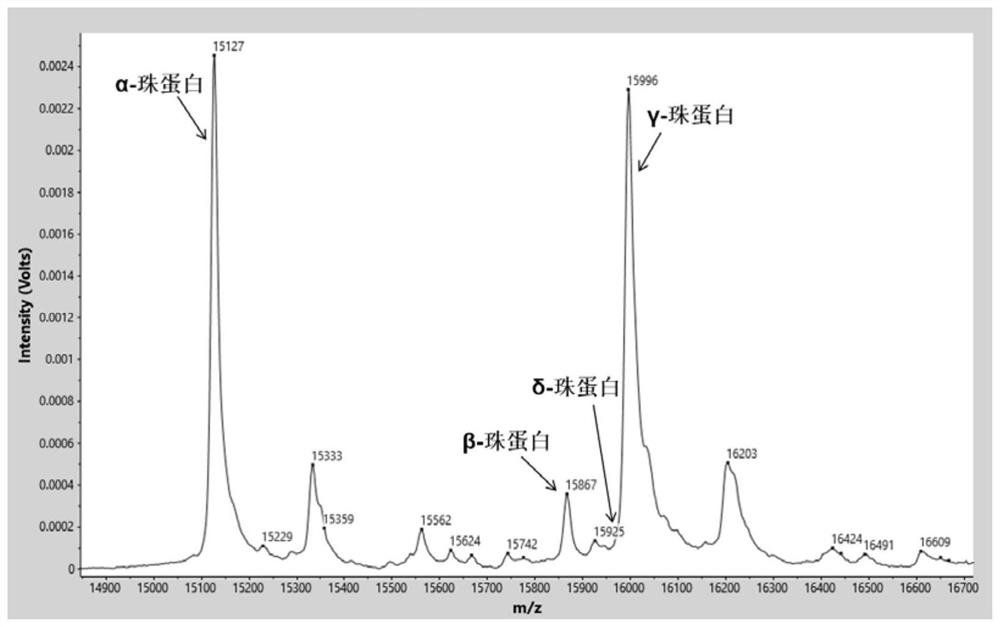
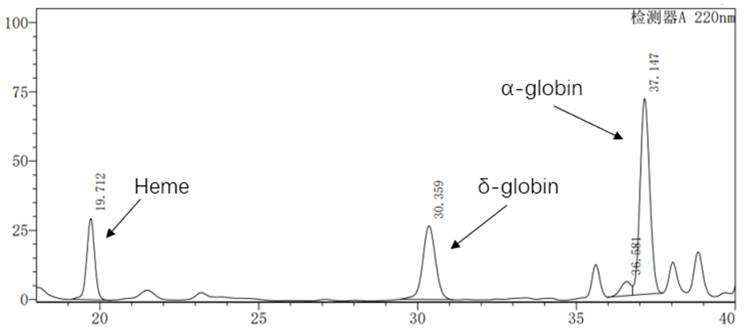
Beta globin (also referred to as HBB, β-globin, haemoglobin beta, hemoglobin beta, or preferably haemoglobin subunit beta) is a globin protein, which along with alpha globin (HBA), makes up the most common form of haemoglobin in adult humans, the HbA. It is 147 amino acids long and has a molecular weight of 15,867 Da. Normal adult human HbA is a heterotetramer consisting of two alpha chains and two beta chains.
Method to diagnose or screen for inflammatory diseases
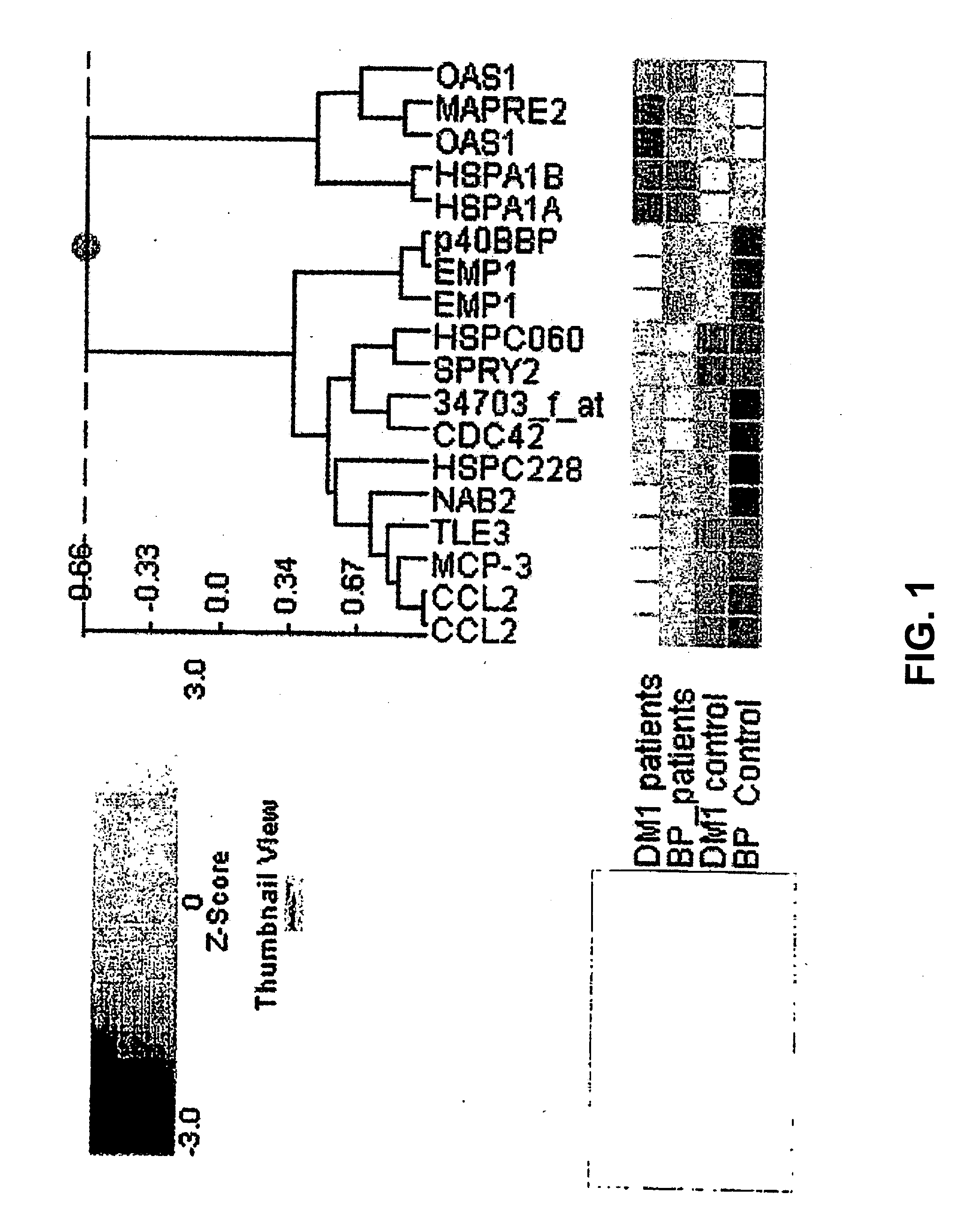
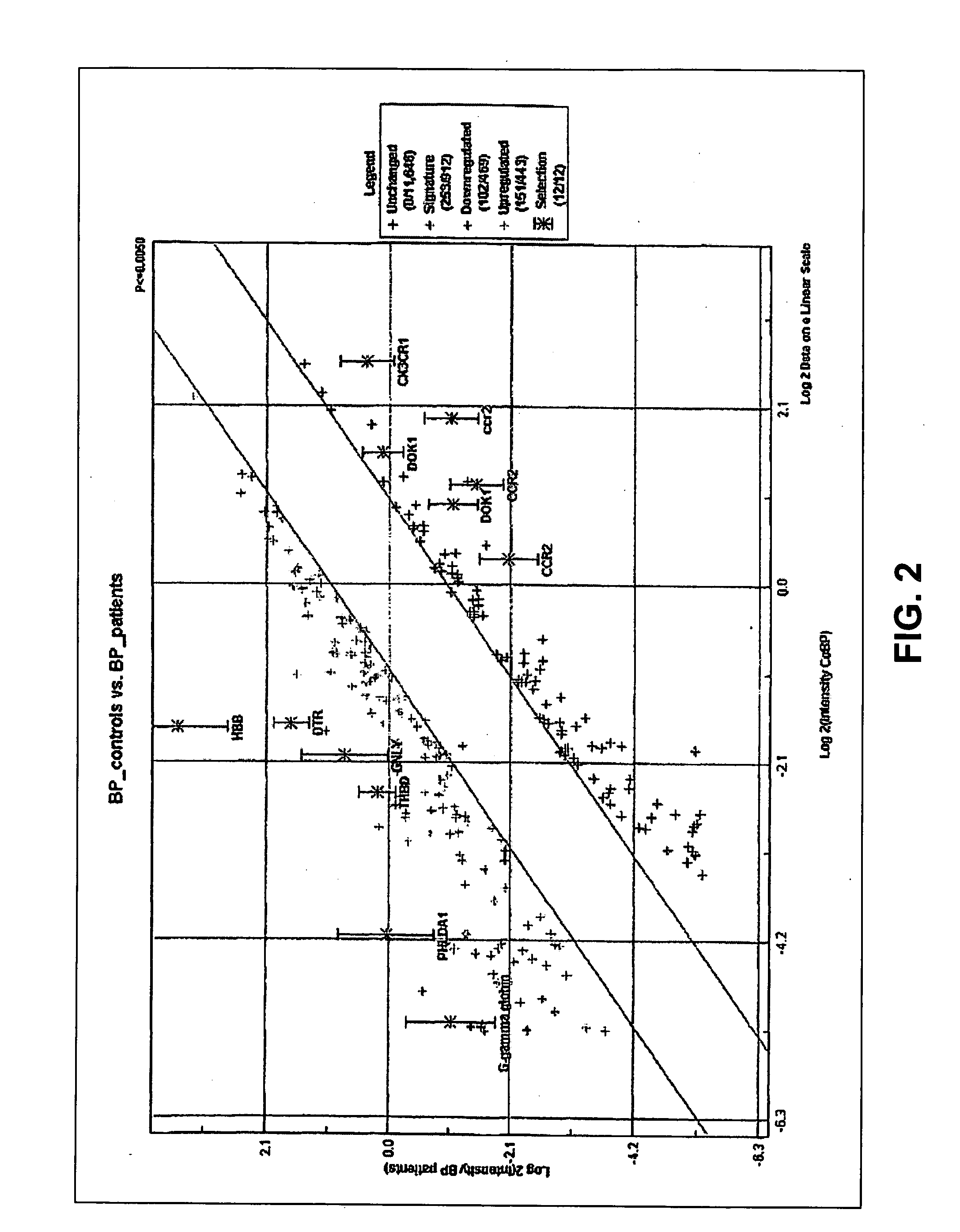
The invention relates to the field of medical diagnostics. More specifically, the invention relates to methods to diagnose or screen for inflammatory conditions or disease, including auto-inflammatory disease and affective disorder, in a subject, preferably a human subject, by assaying for a marker for an inflammatory disease. Provided is a method to diagnose, screen for or predict the development of an affective disorder (AD), preferably bipolar disorder (BP), in a subject, the method comprising determining the level of at least one, preferably at least two, more preferably at least three, most preferred at least four, AD-specific gene product(s) in a biological sample isolated from the subject, preferably peripheral blood monocytes, wherein the gene is selected from the group comprising ATF3, phosphodiesterase 4 B, CXCL2, BCL2-related protein A2, Dual specificity phosphatase 2, TNFα-induced protein 3 / A20, BTEB1 CXCL3, Chemokine CCL-3 like, CCL-4, CCL20, CX2CR1, Amphiregulin, Thrombomodulin, Heparin-binding EGF-like growth factor, DNA-damaged inducible transcript, V28 chemokine-like receptor, TRAIL. MAPK6, B4BP4, PBEF1, Thrombospondin 1, MAFF, HSP70, CCL2, MCP-3, CCR2, CX3CR1, DOK1, HBB, G-gamma globin, THBD, PHLDA1, DTR and GNLY.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Vector for mediating high-efficiency expression of exogenous gene in cells of mammal and use thereof
InactiveCN102250952AImprove output efficiencyImprove output stabilityVector-based foreign material introductionForeign genetic material cellsBeta globinMammal
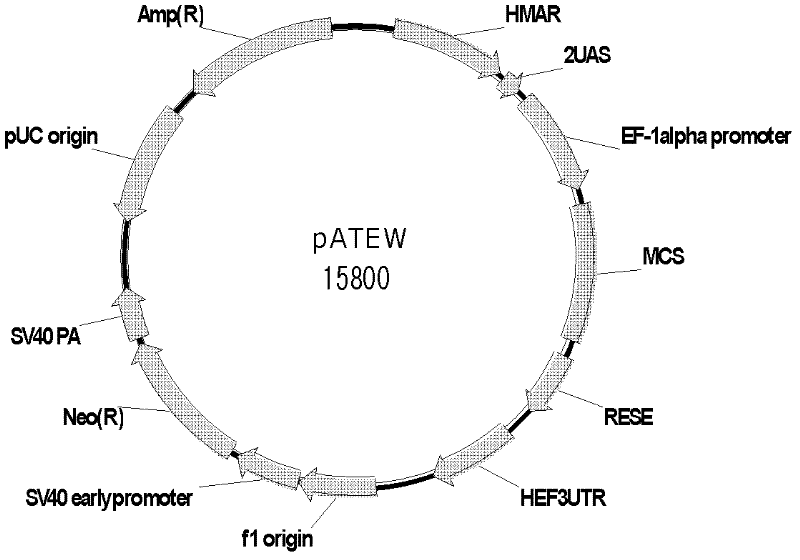
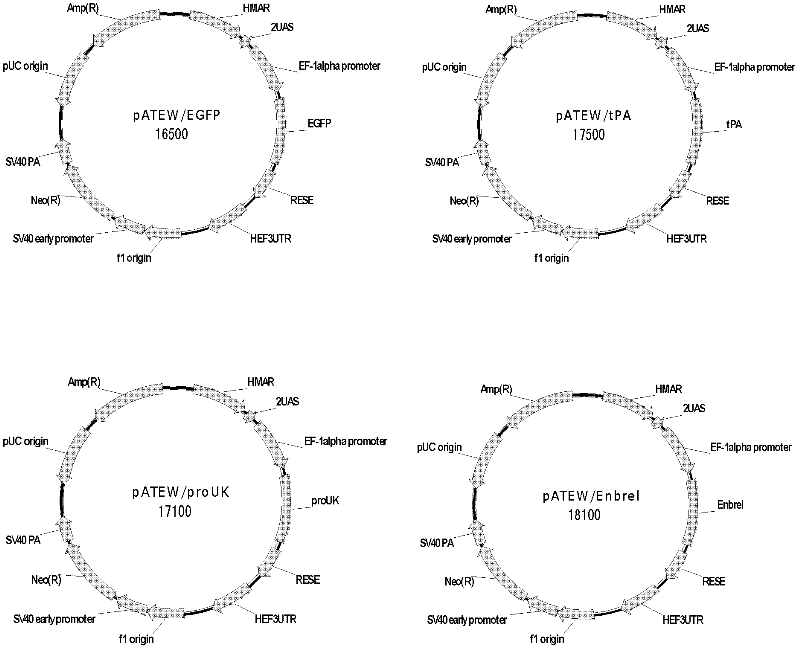
The invention discloses a combined vector for mediating the high-efficiency expression of an exogenous gene in cells of a mammal, which comprises an exogenous gene high-efficiency expression vector pATEW and an artificial transcription factor pcDNA3.1(+) / Hy / GVP4. The exogenous gene high-efficiency expression vector pATEW combines gene expression regulatory elements including a human beta-globin MAR sequence, a hEF-1a genetic transcription regulatory sequence, an after-transcription regulatory element WPRE and the like and an artificial transcription factor combined site sequence. The artificial transcription factor expression vector (pcDNA3.1(+) / Hy / GVP4 is an artificial transcription factor GVP4 which is formed by connecting two parts: four tandem repeats of 12 peptides (DALDDFDLDMLG) in VP16, which serves as the functional structural region of the artificial transcription factor; and the nuclear localization sequence of SV40. By co-transfecting cells of the mammal with the pATEW carrying the exogenous gene and the pcDNA3.1(+) / Hy / GVP4, the high-efficiency expression of the exogenous gene in the cells of the mammal can be realized.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Kit for simultaneously detecting thalassemia mutant type and deletion type and application thereof
PendingCN108796054AEfficient detectionLow costNucleotide librariesMicrobiological testing/measurementBeta globinThalassemia
Owner:BGI BIOTECH WUHAN CO LTD +1
Method and kit for detecting alpha and beta thalassemia point mutation based on next generation sequencing technology
ActiveCN106755329AWide detection rangeHigh sensitivityMicrobiological testing/measurementLibrary creationBeta globinBeta thalassemia
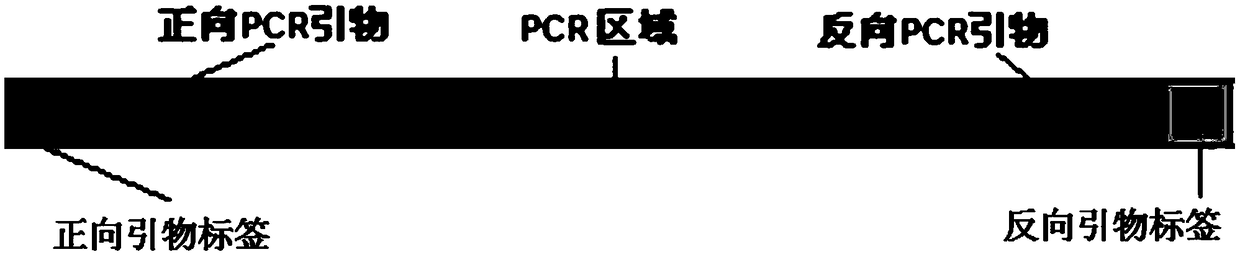
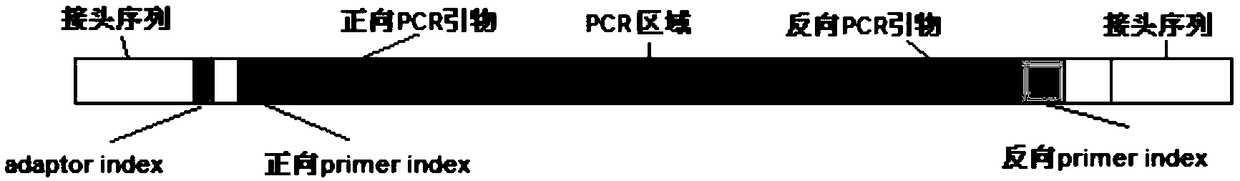
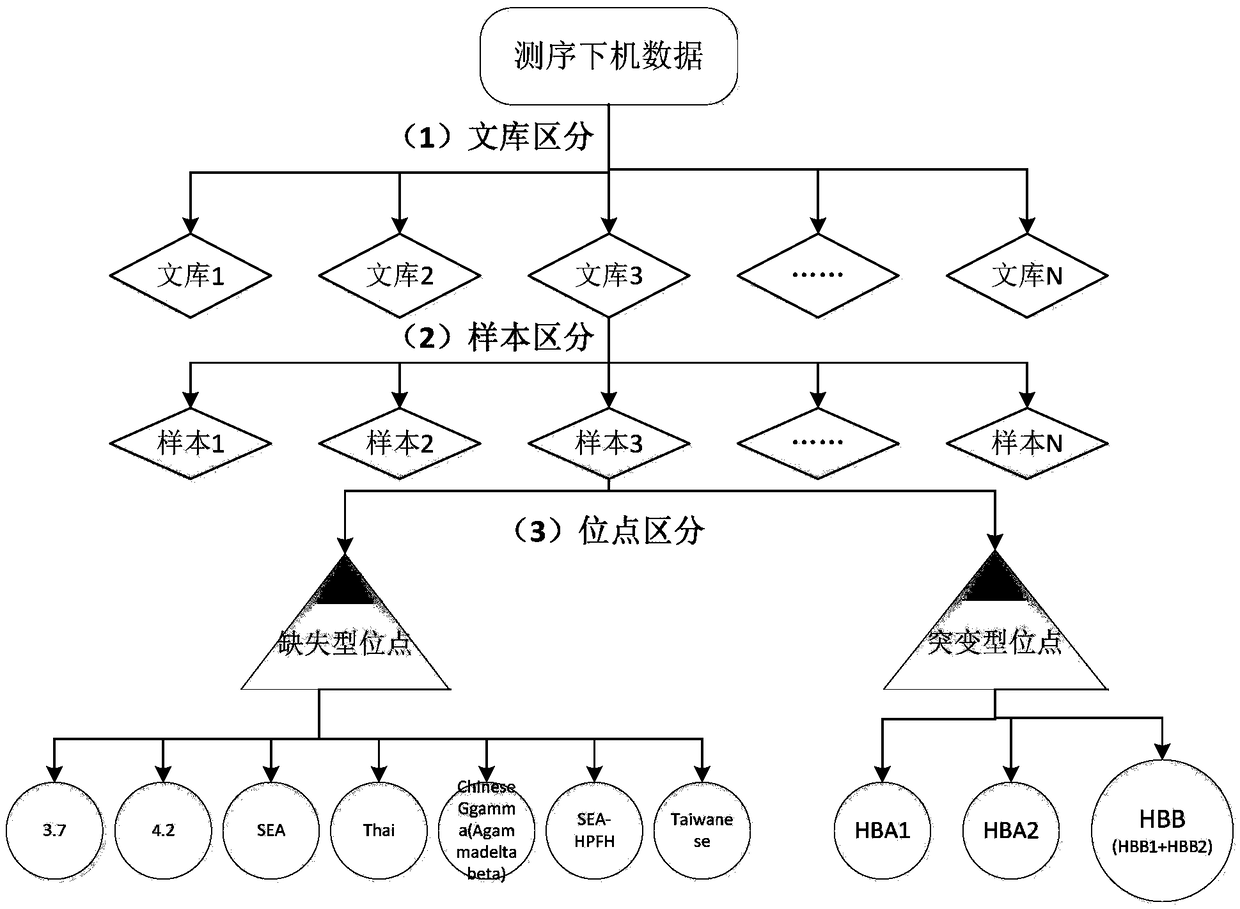
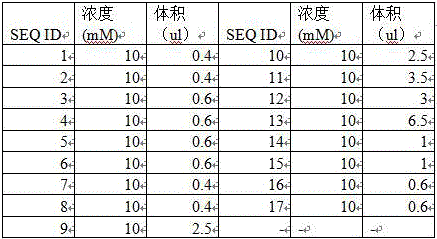
The invention relates to a method and kit for detecting alpha and beta thalassemia point mutation based on a next generation sequencing technology .In the detection method, PCR (polymerase chain reaction) amplification primer sequences SEQ ID NO1-17 in exon, regulation and transcription regions of HBA1, HBA2 and HBB genes, PCR marker primer sequences SEQ ID NO18-115 at the 5'-terminal and PCR marker primer sequences SEQ ID NO116-213 at the 3'-terminal involved with alpha and beta thalassemia are included. The detection method comprises the following detection steps: (1) constructing a next generation sequencing library; (2) purifying the next generation sequencing library; (3) sequencing through a next generation sequencer; and (4) performing bioinformatic analysis to obtain a result. The kit for detection comprises the above-mentioned primer sequences and the next generation sequencer solexa. The detection method provided by the invention has the characteristics of simple operation steps, high detection specificity, wide detection range, low cost and the like.
Owner:MATERNAL & CHILD HEALTH HOSPITAL OF GUANGXI ZHUANG AUTONOMOUS REGION GUANGXI ZHUANG AUTONOMOUS REGION
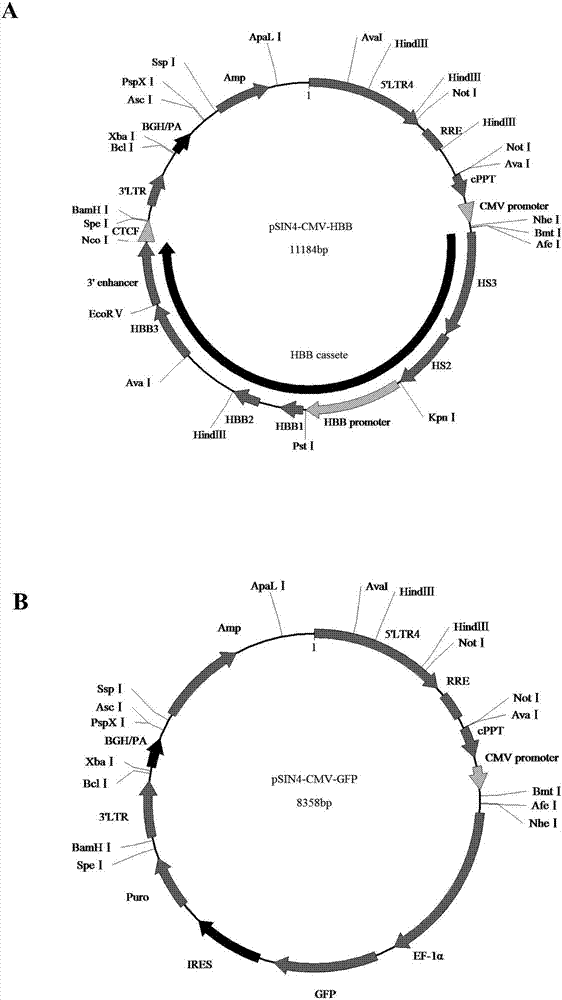
Beta-globin recombinant lentiviral vector and application thereof
Owner:济南赛尔生物科技股份有限公司
Kit and method for detecting mutation of thalassemia-related gene and use thereof
The invention provides a kit and method for detecting mutation of a thalassemia-related gene and a use thereof and relates to the technical field of medical genetics. The kit for detection is used for detecting many types of patients such as people needing premarital checkup, people in gestation, newborns, people in a zone having high incidence of thalassemia and people having thalassemia family heredity history, can realize accurate, comprehensive, visual and simple detection of mutation of a thalassemia-related gene, has a detection mutation range comprising HBA1, HBA2 and HBB genes in the genome and neighbouring zones and has the characteristics of simpleness, accuracy, good repeatability and promotion and use easiness.
Owner:深圳市龙华区人民医院 +1
Recombinant adeno-associated virus vector as well as preparation method and application thereof
InactiveCN109055428AImprove survival rateInfection effect is goodSenses disorderGenetic material ingredientsBeta globinIntraocular pressure
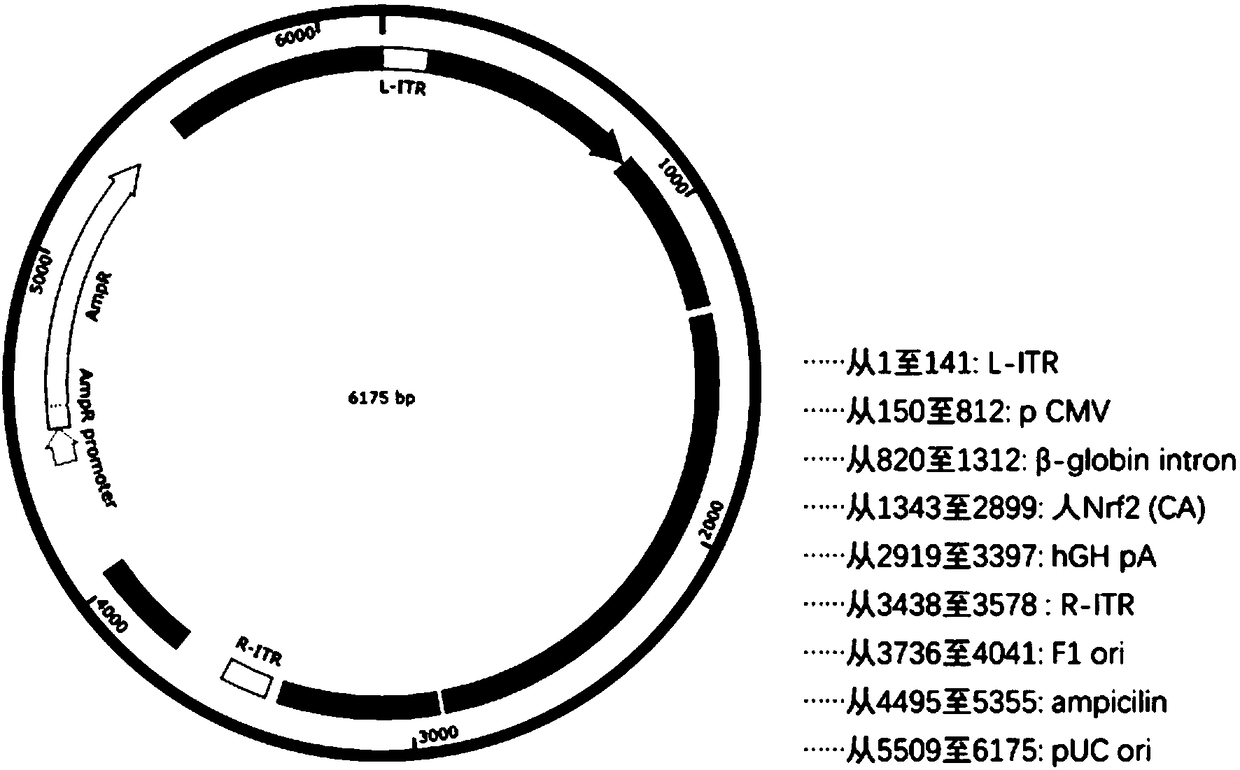
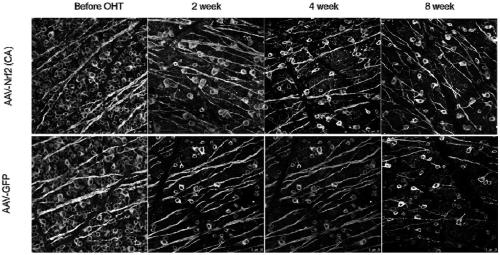
The invention relates to a recombinant adeno-associated virus vector. The recombinant adeno-associated virus vector comprises constitutively active human Nrf2 (Nrf2 (CA)), a CMV promoter / enhancer, beta-globin intron for increasing gene expression, human growth hormone poly (A) tail sequence, etc. The invention also relates to a preparation method of the recombinant adeno-associated virus vector and the application of the recombinant adeno-associated virus vector or a composition containing the recombinant adeno-associated virus vector in preparing a medicine for treating glaucoma retinal ganglion cell denaturation. The recombinant adeno-associated virus vector provided by the invention can obviously reduce the intraocular pressure of a mouse, increase the survival rate of RGCs of the mouse, and effectively treat glaucoma retinal ganglion cell pathological changes.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Human MTHFR and MTRR gene polymorphism detection primer, probe, test kit and method
ActiveCN106591473AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationReference genesBeta globin
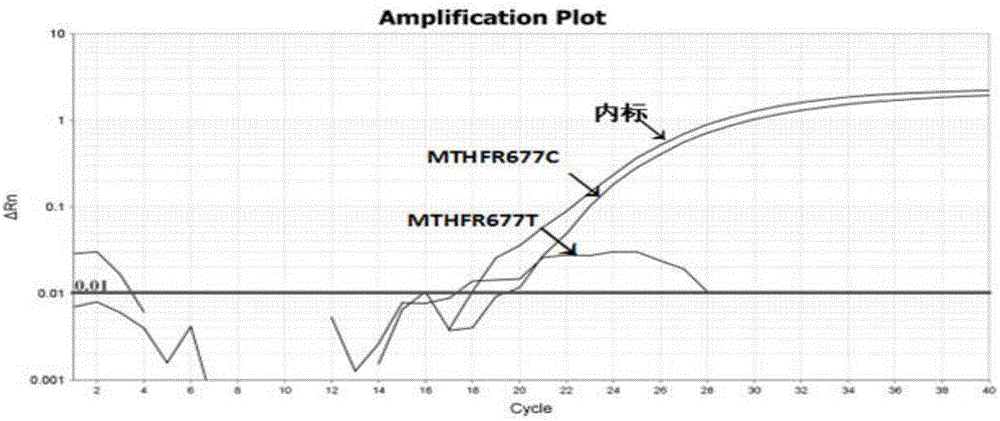
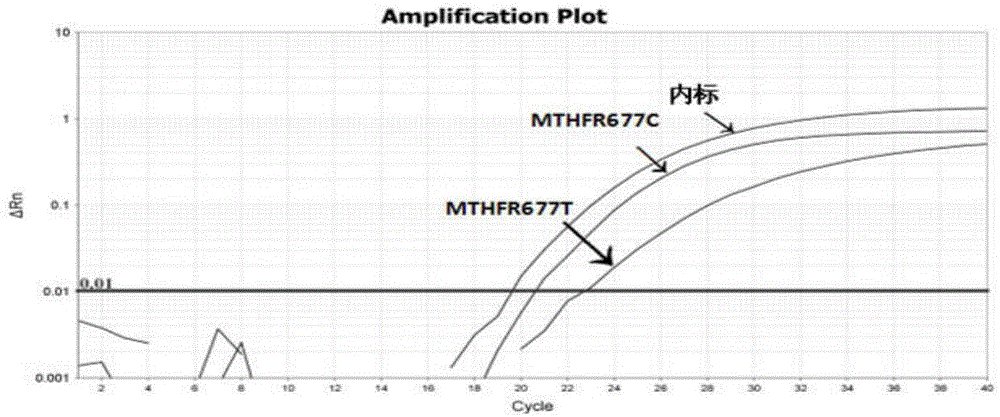
The invention discloses a human MTHFR and MTRR gene polymorphism detection primer, probe, test kit and method and belongs to the technical field of in-vitro nucleic acid detection. The two most common single nucleotide polymorphisms 677C>T and 1298A>C of the MTHFR and the common mutation site 66A>G of the MTHFR are designed for qualitative detection, and a specific primer containing reference genes beta globin and a probe are designed. The test kit includes a detection primer and probe combination, a reference substance, a PCR reaction liquid and the like. Three independent multiplex PCR reactions are conducted for qualitative detection for the three gene polymorphism sites, namely, MTHFR677C>T, MTHFR1298A>C, and MTHFR 66A>G respectively. The detection primer, the probe, the test kit and the method are strong in specificity, high in sensitivity, simple, rapid and convenient for large-scale application and popularization.
Owner:广州达晖生物技术股份有限公司
Screening Methods and Transgenic Animals for the Treatment of Beta-Globin Related Disease and Conditions
InactiveUS20080008651A1High expressionIncrease transcriptionBiocideMicrobiological testing/measurementDevelopmental stageBeta globin
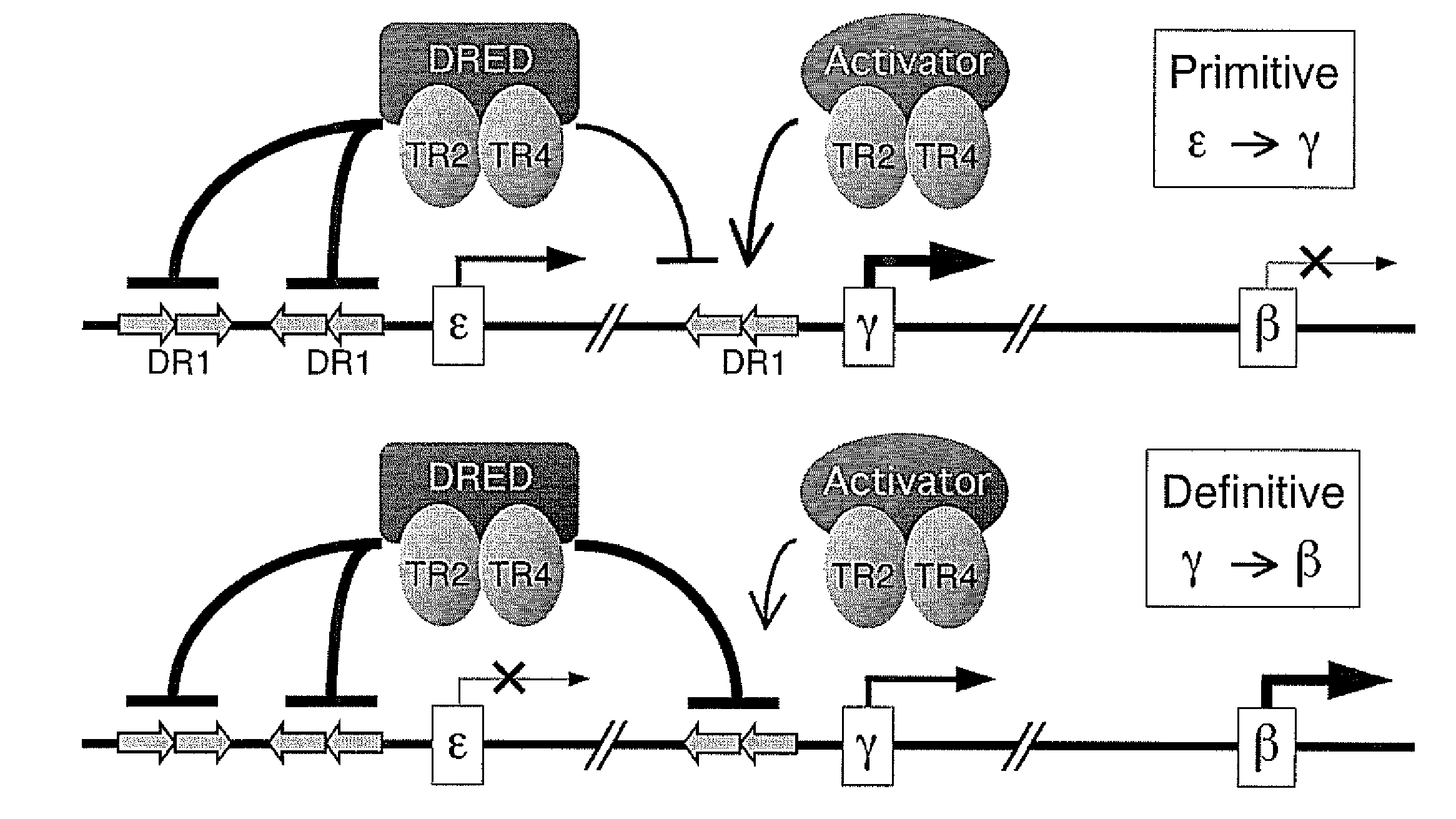
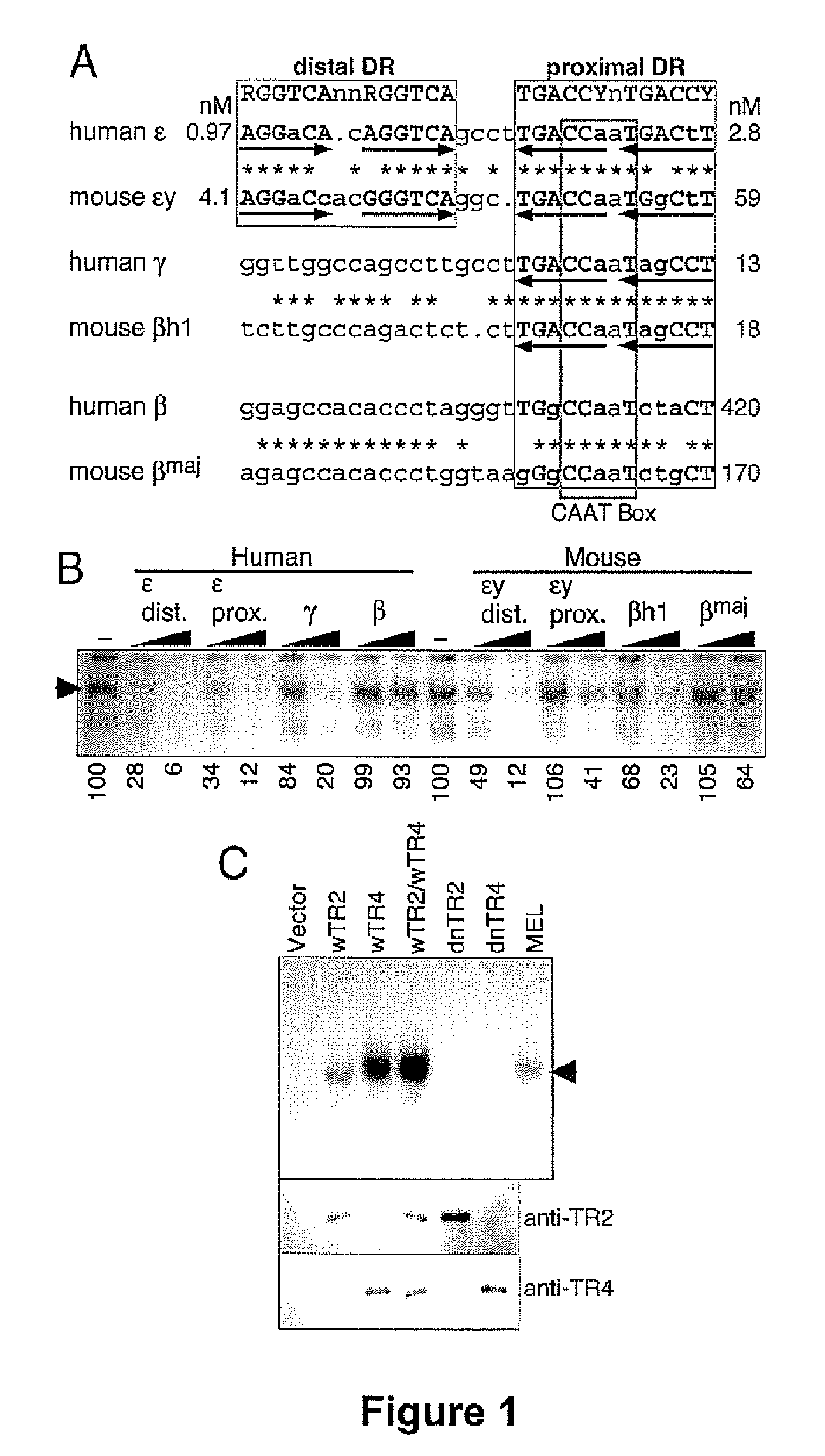
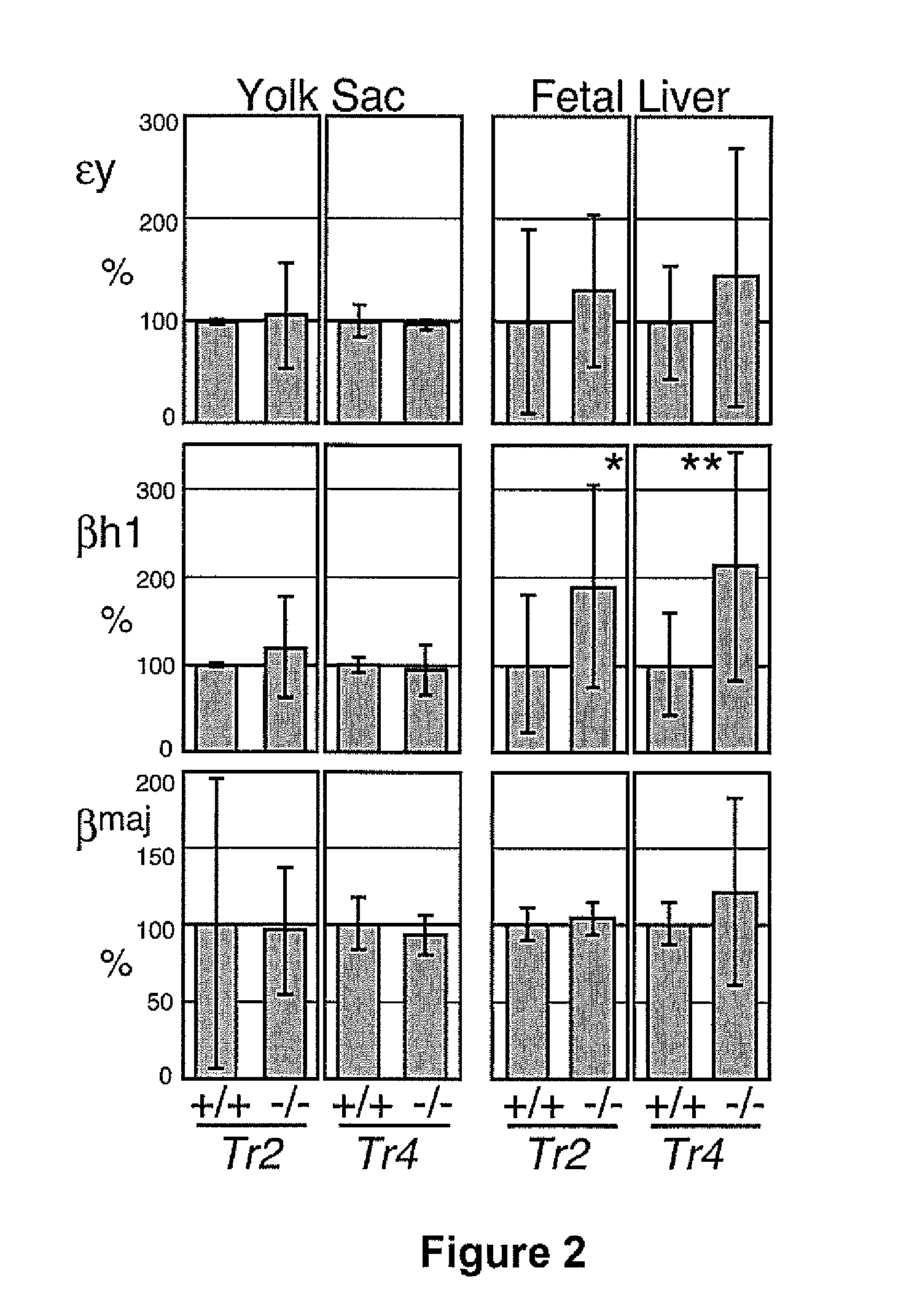
The orphan nuclear receptors TR2 and TR4 together constitute the DNA binding core of the 540 kDa DRED complex, a putative repressor of the human embryonic ε- and fetal γ-globin genes. Here the functional consequences of TR2 and TR4 germ line loss of function were examined, transgenic gain of function and dominant negative gain of function on human and murine β-type globin gene expression throughout development. ε-globin transcription responded in a manner consistent with the hypothesis that TR2 / TR4 is a constitutive erythroid ε-globin repressor. In contrast, parallel experiments show that TR2 / TR4 is a definitive stage-selective γ-globin repressor. This developmental stage-specific, gene-selective repression of the ε- and γ-globin genes by TR2 / TR4 establishes, when considered in concert with the competition hypothesis, a coherent molecular rationale for hemoglobin switching (temporally specific, sequential activation of all the β-type globin genes) during vertebrate development.
Owner:RGT UNIV OF MICHIGAN
Hematopoietic stem cell gene modification method for targeting hemoglobin HBB mutant gene
The invention discloses a hematopoietic stem cell gene modification method for targeting a hemoglobin HBB mutant gene. The method 1 comprises the following steps: electro-transforming gRNA1, gRNA2 andthe mRNA of spCas9-2.0 into CD34+ cells containing the hemoglobin HBB mutant gene; and culturing the cells for one day, and transferring rAAV-HBB into the CD34+ cells, and harvesting the cells. The method 2 comprises the following steps: mixing and recombining a Cas9 protein with the gRNA1 and the gRNA2, and electro-transforming the recombinant Cas9 protein into the CD34+ cells; and culturing thecells for one day, and transferring the rAAV-HBB into the CD34+ cells, and harvesting the cells. The CD41 / 42 (-CTTT) in the most common HBB mutation in Guangxi province and Guangdong province in China is targeted, no other mutation or insertion sequences are left in chromosomes after gene editing is completed, and the gene editing efficiency can reach 15-20%.
Owner:YINFENG BIOLOGICAL GRP +1
Basic group editing system and method for specifically repairing HBB gene mutation, kit and application of system and method and kit in human genital system
The invention provides a basic group editing method for specifically repairing HBB gene mutation in a human genital system. The method comprises the steps of delivering a basic group editing system used for specifically repairing HBB gene mutation so that the basic group editing system is close to relevant sequences of HBB mutant genes, and obtaining repaired HBB genes, wherein the basic group editing system comprises a basic group editing enzyme and gRNA, the basic group editing enzyme is fusion protein, and the fusion protein includes effect protein structural domains, cytidine deaminase structural domains and uracil DNA glycosylase inhibitor structural domains of a CRISPR / Cas system. The gene editing system is guided into a human germ cell, a human fertilized ovum or a human embryo, A>Gpathogenic mutation can be precisely repaired, and thus beta-mediterranean anemia is cured. The basic group editing method has a wide application prospect in the field of genetic therapy.
Owner:SUN YAT SEN UNIV
Reagent kit for detecting HBB gene mutation and HLA genotyping
ActiveCN105420233AImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationBeta globinBeta thalassemia
The invention provides a method for detecting HBB gene mutation and HLA genotyping based on the high throughput sequencing technology and a corresponding reagent kit. An adopted primer composition comprises a primer of closely-linked single nucleotide polymorphisms (SNP) within the 1 Mb range of the up stream and the down stream of the specific amplification human embryo beta-thalassemia HBB gene and primers of the closely-linked single nucleotide polymorphisms (SNP) within the ranges at the up stream of the LHA-A gene, between the HLA-A gene and the HLA-B gene, between the HLA-B gene and the HLA-DRA gene, between the HLA-DRA gene and the HLA-DQB1 gene and at the downstream of the HLA-DQB1 gene of the specific amplification human leucocyte antigen system. The method has the advantages of university, single nucleotide polymorphisms (SNP) sequencing, high throughput, low cost, high flexibility and strong specificity.
Owner:海南医学院附属医院 +1
System for detecting multiple quantitative and virulent genes of H.pylori as well as kits and applications of system
ActiveCN105506160AThe test result has no impurityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBeta globinVirulent characteristics
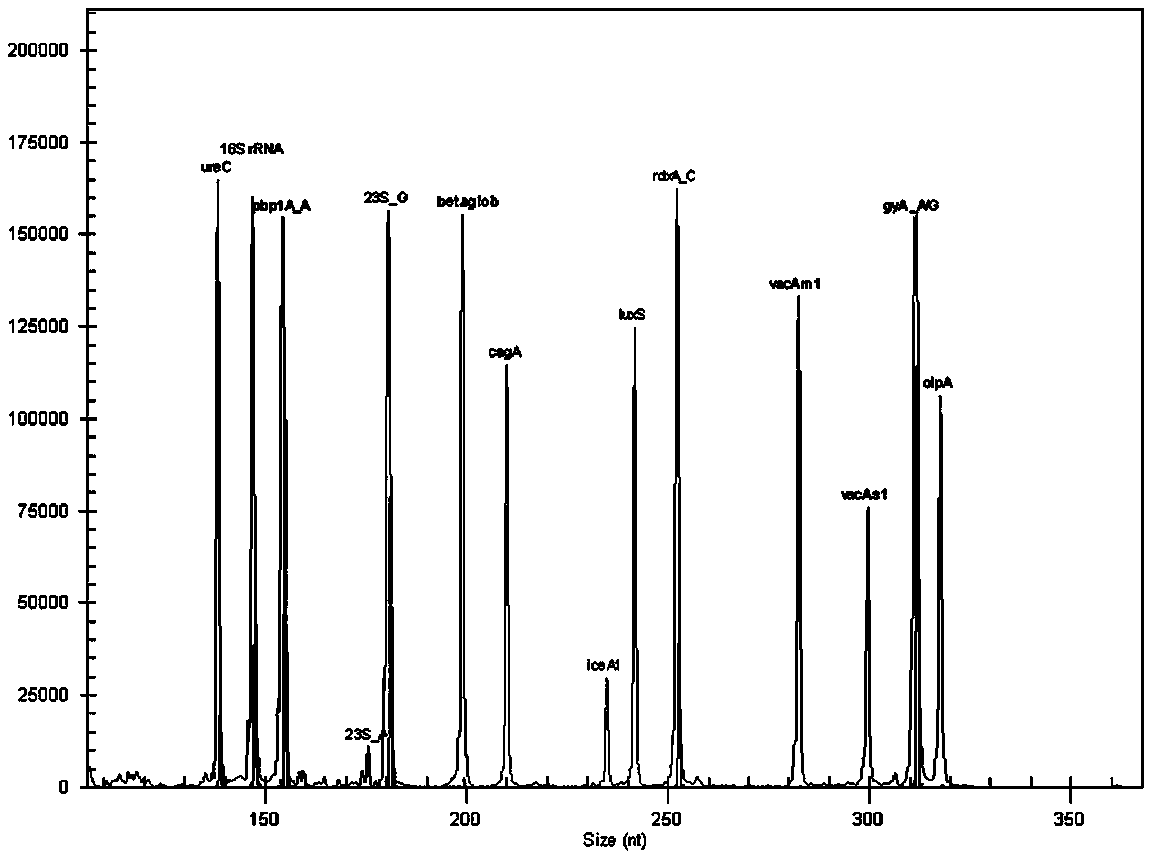
The invention relates to a system for detecting multiple quantitative and virulent genes of H.pylori as well as kits and applications of the system. The system for detecting multiple quantitative and virulent genes of H.pylori comprises multiple pairs of primers, each for strain identification genes (16S rRNA), quantitative analysis genes ureCand Beta-globin, as well as virulent genes (cagA, vacA-s1, vacA-s2, vacA-m1, vacA-m2, iceA1, iceA2, dupA, oipA and luxS). The system for detecting multiple quantitative and virulent genes of H.pylori and the kits of the system can directly perform synchronous detection and analysis on the strain identification, quantification and virulence of a tissue sample in a same reaction system without adopting a conventional culture step or other steps, remedy defects that a conventional detection method is low in throughput, time-consuming and low in detection rate, obviously improve the accuracy of detection results, immediately provide a comprehensive, accurate and low-cost etiological diagnosis for clinic, and provide an important reference for the accurate diagnosis, differential diagnosis and disease prognosis of H.pylori infection.
Owner:HUADONG HOSPITAL +1
Gene vector and gene therapy drug for treating retina ganglion cell denaturation
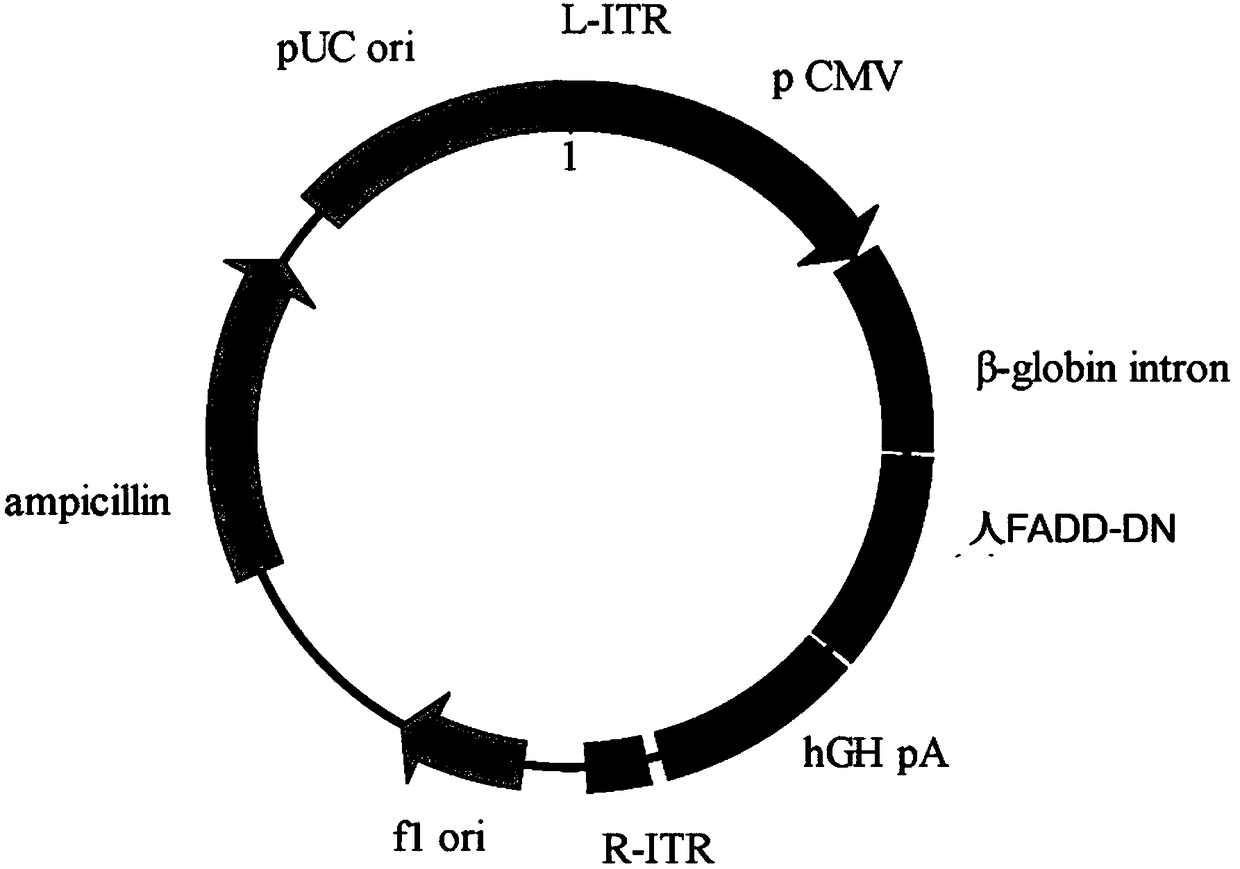
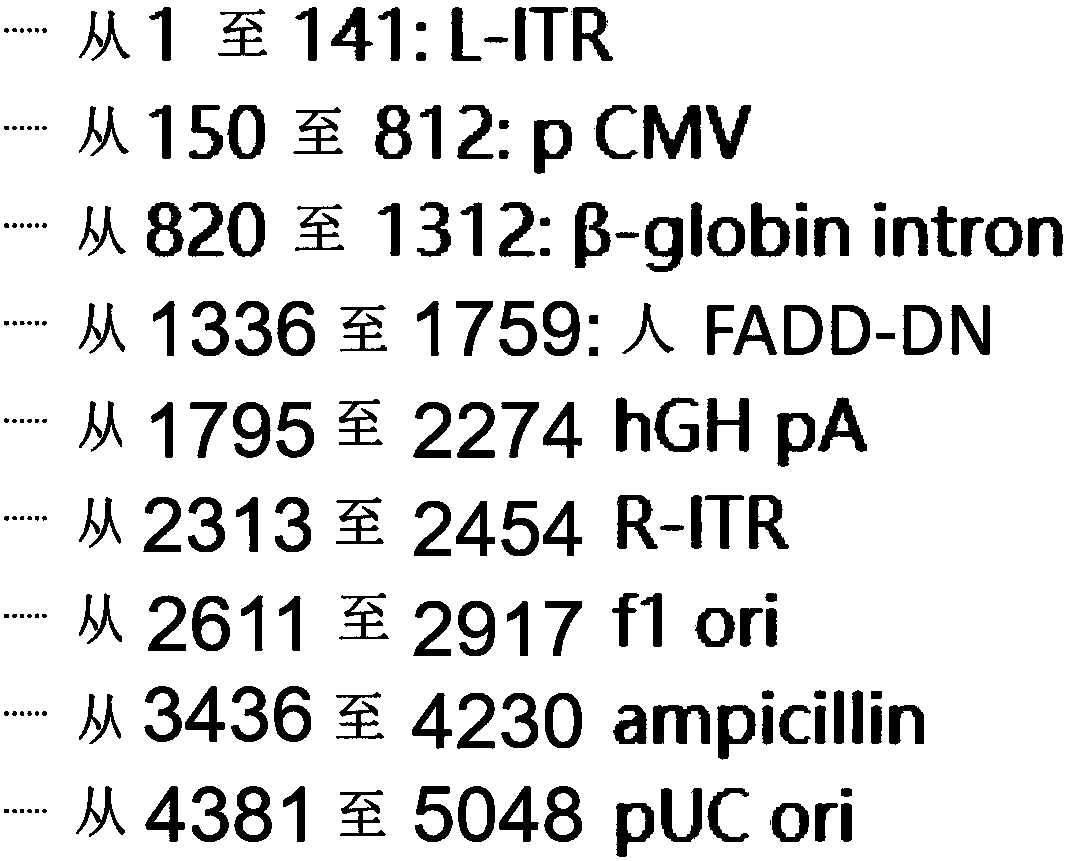
The invention relates to a gene vector and a gene therapy drug for treating retina ganglion cell denaturation. The gene vector comprises dominant negative human Fas-associated protein with death domain (FADD-DN), an enhancer / promoter, a beta-globin intron capable of enhancing gene expression, a human growth hormone poly(A) tail sequence and the like. The invention further relates to application ofthe gene vector or a composition containing the gene vector to preparation of a drug for treating the retina ganglion cell denaturation. The gene vector provided by the invention has the advantages that the gene therapy vector provided by the invention can be used for treating or preventing retina ganglion cell pathological changes caused by glaucoma and the like.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
HBB gene kit for correcting autologous hematopoietic stem cell of patient suffering from servious beta-thalassemia
InactiveCN106497976AAchieving a cureComplete operation processGenetically modified cellsUnknown materialsBeta globinBeta thalassemia
The invention discloses an HBB gene kit for correcting autologous hematopoietic stem cells of a patient suffering from servious beta-thalassemia. The kit consists of a set of reagents for preparing a specific HBB-101 HIV slow virus and a set of reagents for HIV slow virus infection hematopoietic stem cells. The invention further discloses application of the kit in converting the autologous hematopoietic stem cells of the patient suffering from servious beta-thalassemia into hematopoietic stem cells with normal beta-globin synthesis functions. Experiment shows that the kit disclosed by the invention is simple to operate and relatively high in virus titer, and has wide development prospects in clinical study and treatment application, the virus infection efficiency is as high as 56.99%, and the purpose of treating the patient suffering from servious beta-thalassemia can be achieved through venous re-transfusion after PCR identification on corrected hematopoietic stem cells is implemented and the result of DNA sequencing identification is positive.
Owner:广东铱科基因科技有限公司
HPV gene chip as well as preparation method and application thereof
ActiveCN109112184AFit large scaleImprove throughputNucleotide librariesMicrobiological testing/measurementBeta globinHuman DNA sequencing
The invention discloses an HPV gene chip. The gene chip can be individually or simultaneously perform accurate detection, screening and typing on one or more HPV subtypes, can have human genome beta globin and an HPV primer amplification quality control point to monitor the whole detection process and thus being capable of having high sensitivity and specificity. The invention also discloses a preparation method of the HPV gene chip. By utilizing PCR reversal point hybridization method to screen a probe with strong specificity and optimizing the concentration of a spotting probe of the HPV gene chip, the quality and the efficiency of hybridization are improved; and in addition, the gene chip with good optimized condition is further verified with a clinical sample. The HPV gene chip provided by the invention has a better clinical application prospect.
Owner:GUIZHOU MEDICAL UNIV
Positive quality control product for detecting HBB/GJB2/ATP7B/PAH genetic disease genes and preparation method of positive quality control product
ActiveCN105925672AEasy accessEasy to manufactureMicrobiological testing/measurementBeta globinHuman DNA sequencing
The invention relates to a positive quality control product for detecting HBB / GJB2 / ATP7B / PAH genetic disease genes and a preparation method of the positive quality control product. A DNA sequence, which includes four mutant gene segments, namely HBB_CD41-42, GJB2_235delC, ATP7B_2333G>T and PAH_728G>A, is synthesized, and the sequence is interpolated into a plasmid vector and is mixed with wild-type human genome DNA according to a copy number of 2 to 1, so that the positive quality control product is obtained. The positive quality control product prepared by the invention, as a positive internal standard quality control material of a kit for detecting HBB / GJB2 / ATP7B / PAH gene mutation related to genetic diseases, can be used for accurately monitoring an entire experimental process of detecting the related genetic diseases with the adoption of the detection kit; therefore, the positive quality control product can serve as a standard for judging whether an experiment is successful or not.
Owner:GUANGZHOU DARUI BIOTECH +1
Lentiviral vector applicable to gene therapy of thalassemia and sickle anemia
PendingCN114410687AIncrease gene expressionImprove packaging efficiencyVectorsHydrolasesBeta globinBeta thalassemia
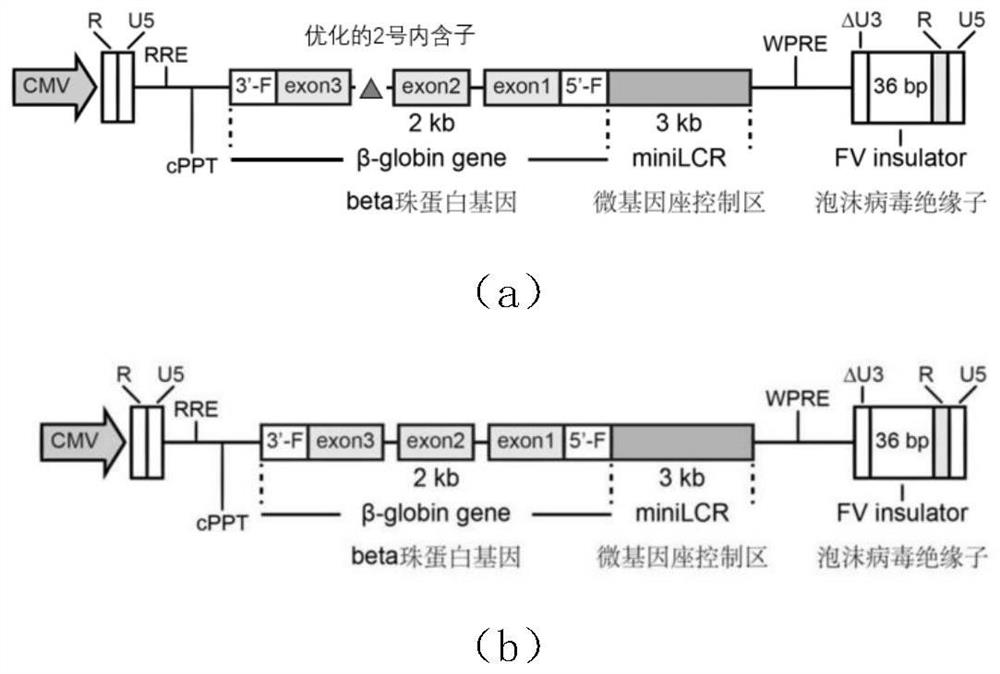
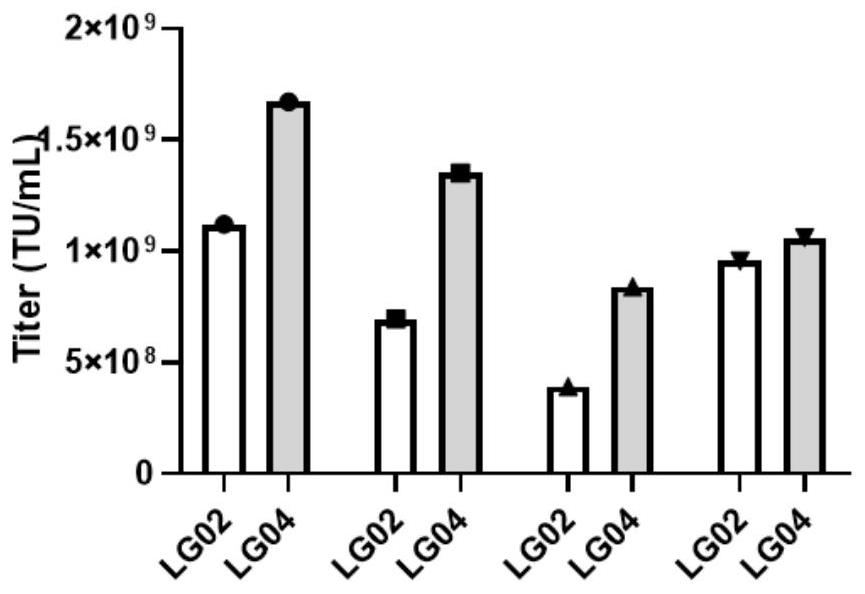
The invention discloses a lentiviral vector suitable for gene therapy of thalassemia and sickle anemia. The system comprises: a, a micro locus control region, which is a micro regulatory element which is screened from a locus control region of beta globin 16kb and does not contain an HS1 region; b, a gene sequence of beta globin, wherein the gene sequence is as shown in SEQ ID NO.3; c, a promoter sequence of an upstream flanking of the gene sequence of beta globin; d, a flanking sequence at the downstream of the gene sequence of beta globin; and e, an insulator sequence from a Foamy virus (Foamy virus). Compared with the prior art, the invention optimizes the structure of the vector, and finds that after the second intron of the Beta globin gene (HBB) is optimized, the expression of the gene can be improved to a certain extent, and the packaging yield of the virus can be greatly improved, so that the clinical use and industrialization of the Beta globin gene become possible.
Owner:SHANGHAI BDGENE TECH CO LTD
Method of performing gene correction on hemopoietic stem cells of patient with Mediterranean anemia
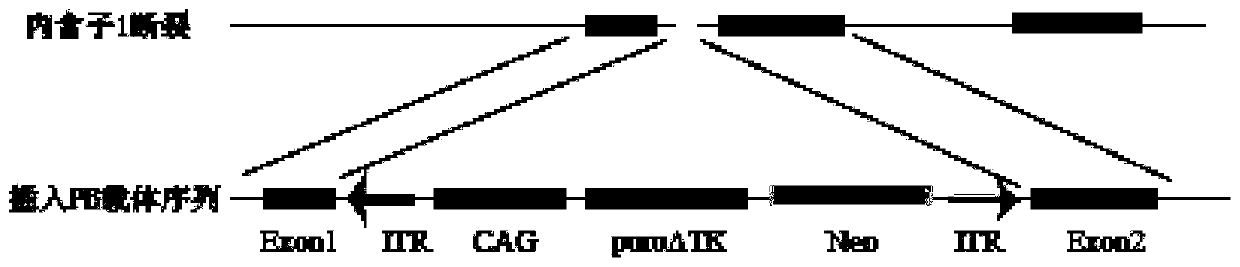
InactiveCN109971791AEfficient transfectionEffective correctionHaemoglobins/myoglobinsStable introduction of DNABeta globinCell culture media
The invention discloses a method of performing gene correction on hemopoietic stem cells of a patient with Mediterranean anemia. The method is characterized by comprising the steps of I, selecting CRISPR target near an HBB gene-28 (A / G) site, and designing a gRNA sequence; II, constructing a template plasmid, and inserting a dual-ITR (inverted terminal repeat) sequence of piggyBac between upstreamand downstream 500bp sequences of the HBB gene CRISPR target; III, co-transfecting the hemopoietic stem cells of the patient with Mediterranean anemia through a transfection reagent, the template plasmid, gRNA and Cas9 carrier, and performing puromycin screening on a cell medium; IV, authenticating and verifying a resistant gene. The CRISPR / Cas9 system and piggyBac are jointly utilized; the hemopoietic stem cells of the patient with Mediterranean anemia can be transfected effectively; homologous recombination efficiency can be improved; -28(A / G) mutation site of the patient with Mediterraneananemia can be corrected effectively.
Owner:杭州荣耀星泽医药有限公司
Beta-globin recombinant lentiviral vector and application thereof
ActiveCN114438130APacked with high titerReduce manufacturing costPeptide/protein ingredientsHaemoglobins/myoglobinsBeta globinThalassemia
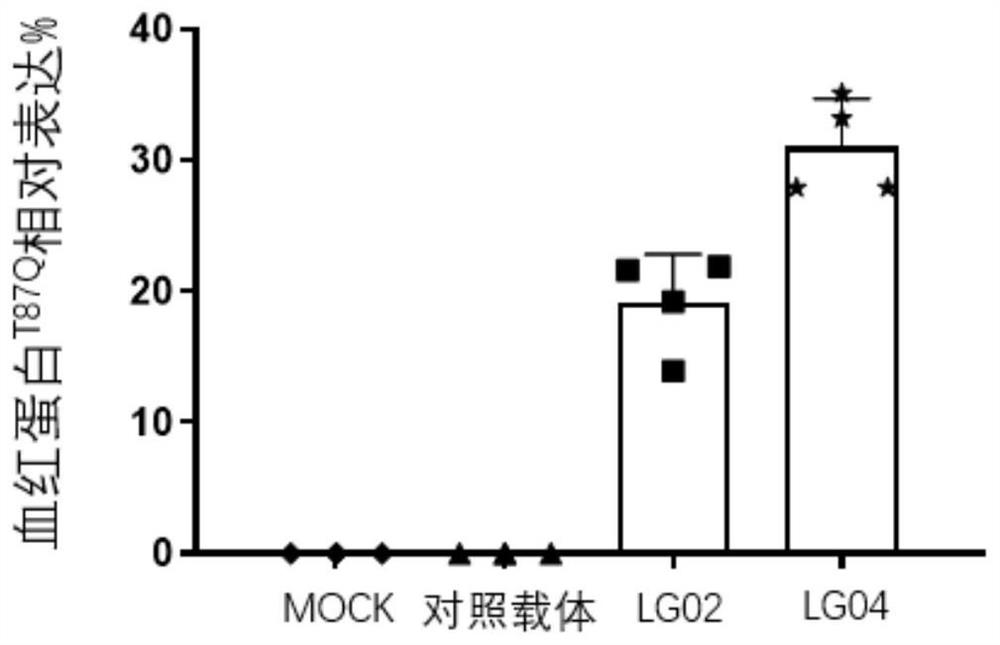
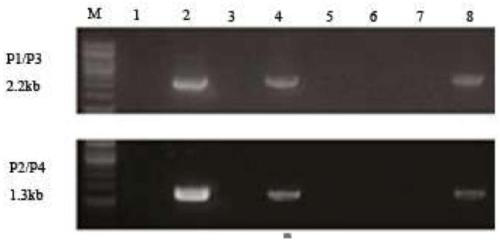
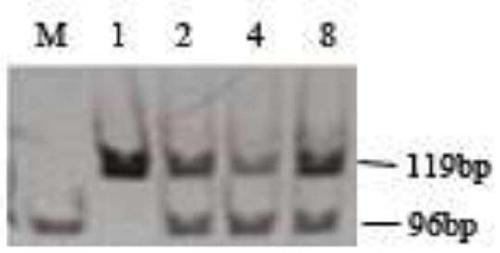
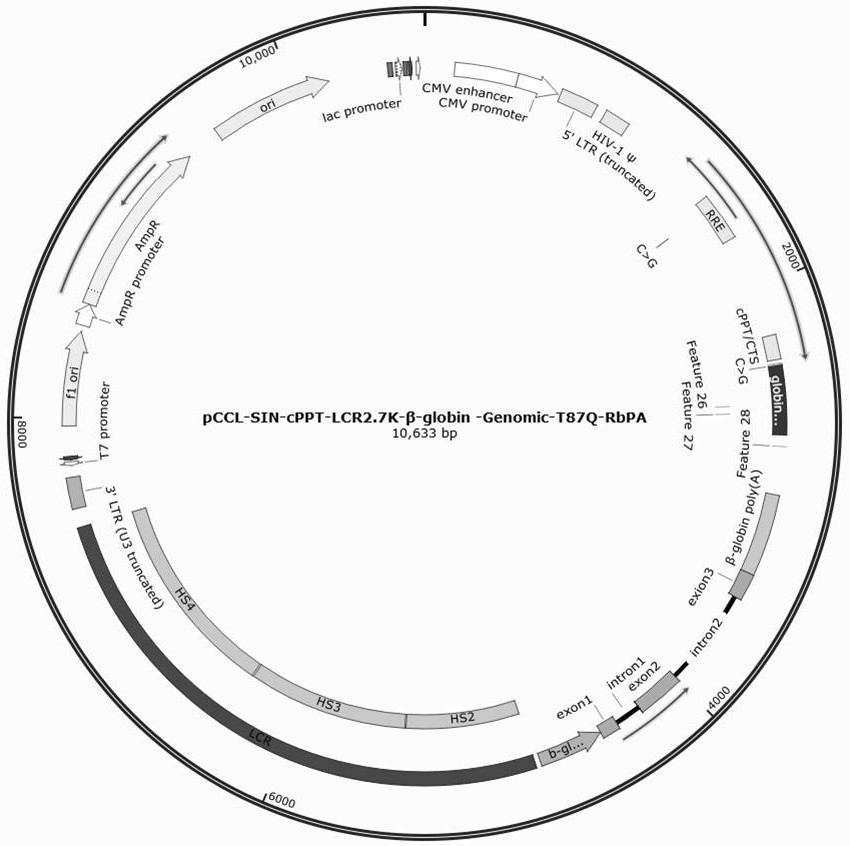
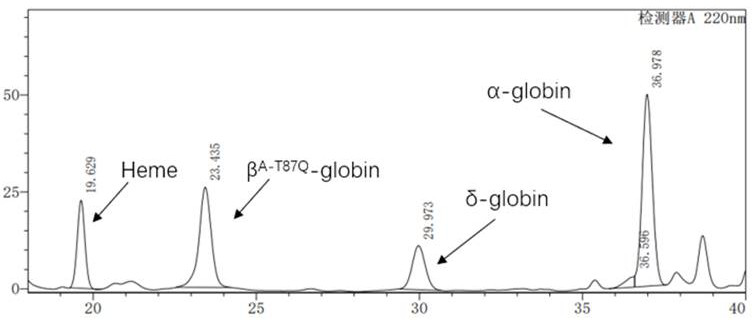
The invention discloses a beta-globin recombinant lentiviral vector and application of the beta-globin recombinant lentiviral vector, the beta-globin recombinant lentiviral vector is pCCL-SIN-cPPT-LCR < 2.7 > K-beta-globin-Genomic-T87Q-RbPA, the beta-globin recombinant lentiviral vector comprises a pCCL-SIN-cPPT-MCS-RbPA skeleton, a 2.7 kb beta-LCR regulatory sequence and an expression cassette for expressing beta globin Hb beta A-T87Q with mutant amino acid at the 87th position, and the beta-LCR is a abbreviation of beta-globin chloride regulation. The lentiviral vector disclosed by the invention is high in packaging titer and low in vector production cost, the potential carcinogenic risk of the viral vector on tested cells is reduced, the Hb betaA-T87Q is expressed more efficiently and stably, and an important basis is provided for curing blood transfusion dependent type beta-thalassemia patients.
Owner:GENMEDICN BIOPHARMA INC
Microarray for detection of mutations in beta-globin genes and detection method thereof
InactiveCN104204227APracticalImprove usabilityBioreactor/fermenter combinationsBiological substance pretreatmentsBeta globinGlobulin
An objective of the present invention is to provide a microarray for detection of mutations in beta-globin genes with which a large number of mutations (subject for detection) can be simply and quickly detected. The present invention provides a probe group for detection of mutations in beta-globin genes including genes represented by SEQ ID NOs: 3, 4, 7, 8, 11, 12, 17, 18 and 25 to 66, a microarray in which the probe group is immobilized, a method for detection of mutations in beta-globin genes using the microarray, and a kit for detection of mutations in beta-globin genes using the microarray and primers.
Owner:MITSUBISHI CHEM CORP
Biomarker for epilepsy diagnosis
InactiveCN108117591AImprove consistencyImprove stabilityComponent separationDisease diagnosisDiseaseBeta globin
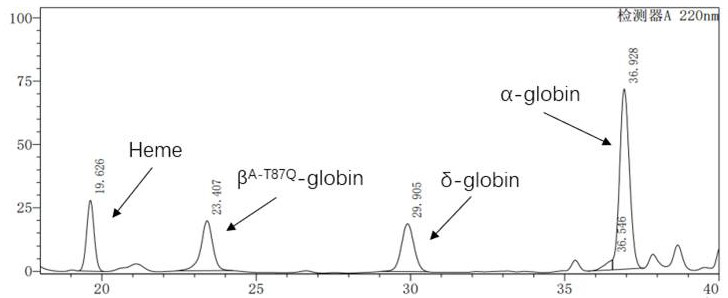
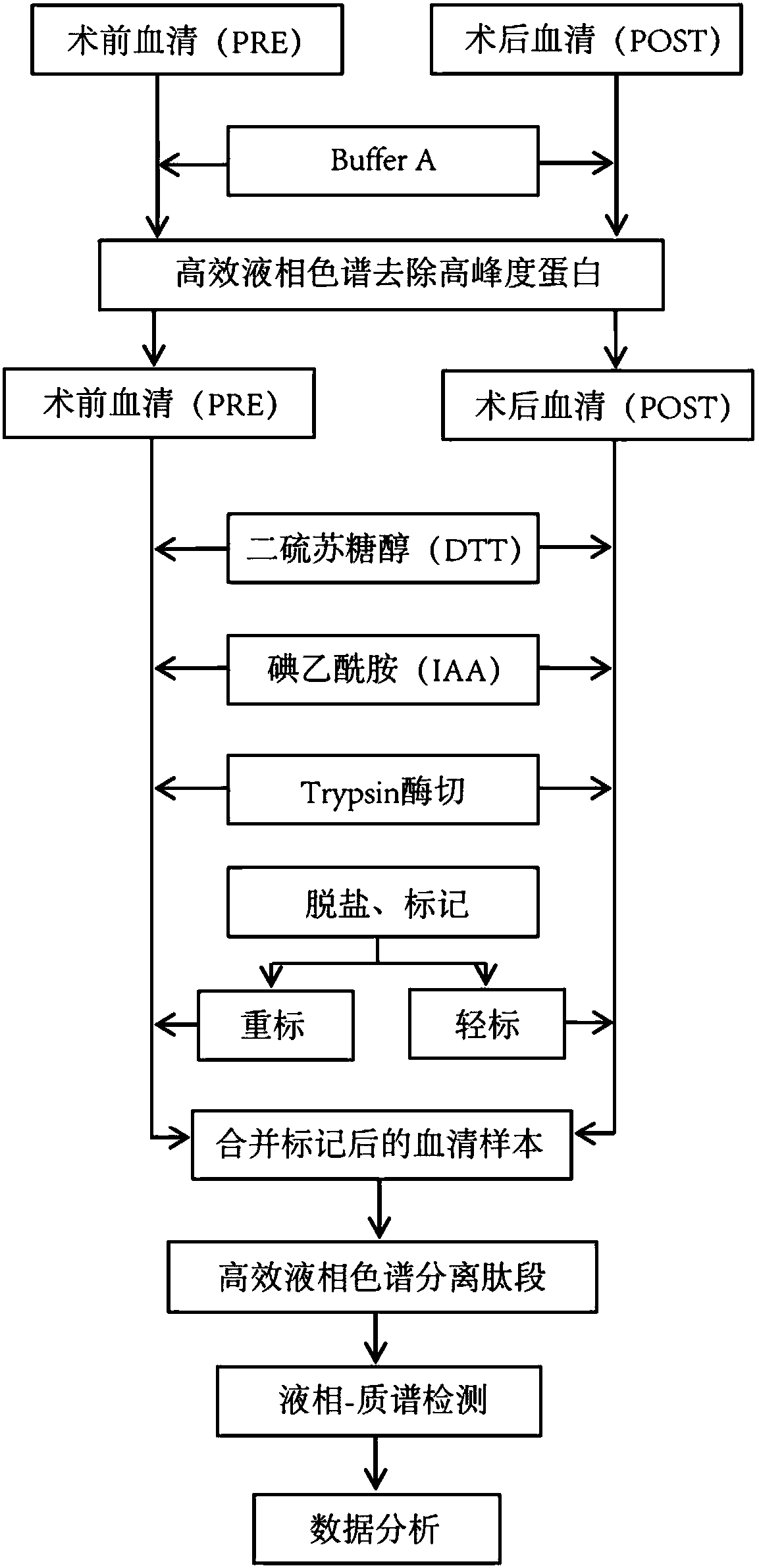
The invention discloses a biomarker or a biodegradation product thereof for epilepsy prediction, diagnosis, treatment and prognosis evaluation. The protein is named as Hemoglobin subunit beta, and theencoding gene of the biomarker is HBB. Particularly, the invention relates to a proteomic screening and detection method for a Hemoglobin subunit beta protein or a biodegradation product thereof. Results of the method show that before and after epilepsy primary lesion resection operation of a patient suffering from epilepsy, the contents of Hemoglobin subunit beta or biodegradation products thereof in serum are remarkably different (as shown in the figure 1 accompanying the abstract). The biomarker protein Hemoglobin subunit beta or the biodegradation product thereof disclosed by the invention has great significances in fields such as epilepsy research, prediction, diagnosis, treatment and prognosis evaluation.
Owner:PEKING UNIV
Gene expression cassette, lentiviral vector and application of lentiviral vector in treatment of beta thalassemia
InactiveCN113699186AGood treatment effectOrganic active ingredientsPeptide/protein ingredientsBeta globinPolyadenylation
The invention relates to the field of biomedical treatment, in particular to a gene expression cassette, a lentiviral vector and application of the lentiviral vector in treatment of beta thalassemia. The gene expression cassette comprises a promoter, a beta globin gene, an intron-BCL11A-shRNAmir and a polyadenylation signal which are sequentially connected; and the promoter is a type II promoter with erythroid cell specificity. The gene expression cassette can specifically up-regulate expression of beta globin and gamma globin in erythroid cells, and the two mechanisms have a synergistic effect, so that the treatment effect is remarkably enhanced.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Library building method for screening large samples of thalassemia based on high-throughput sequencing
InactiveCN108728903AFull-length sequence testingReduce complexityMicrobiological testing/measurementLibrary creationGenes mutationBeta globin
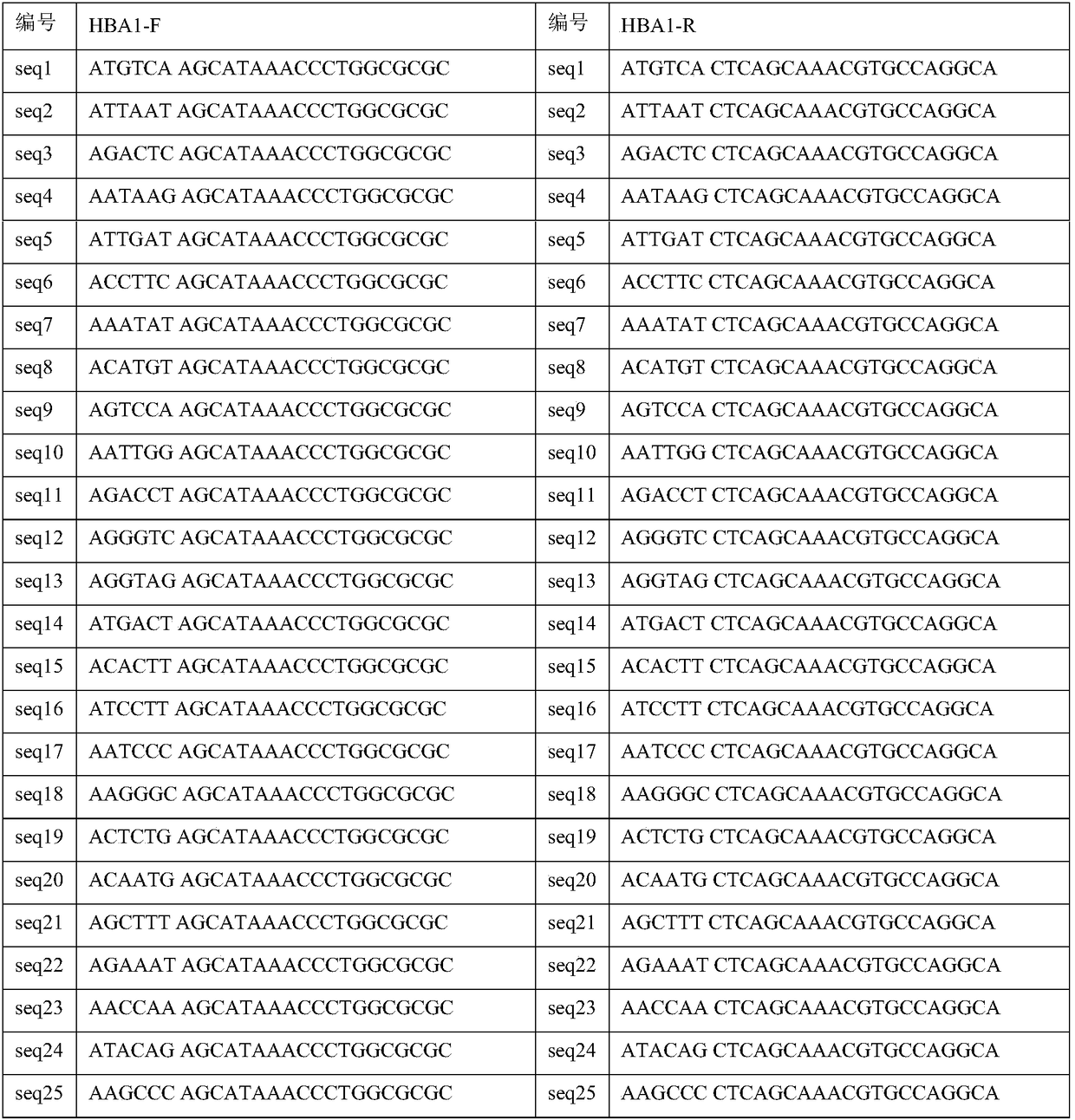
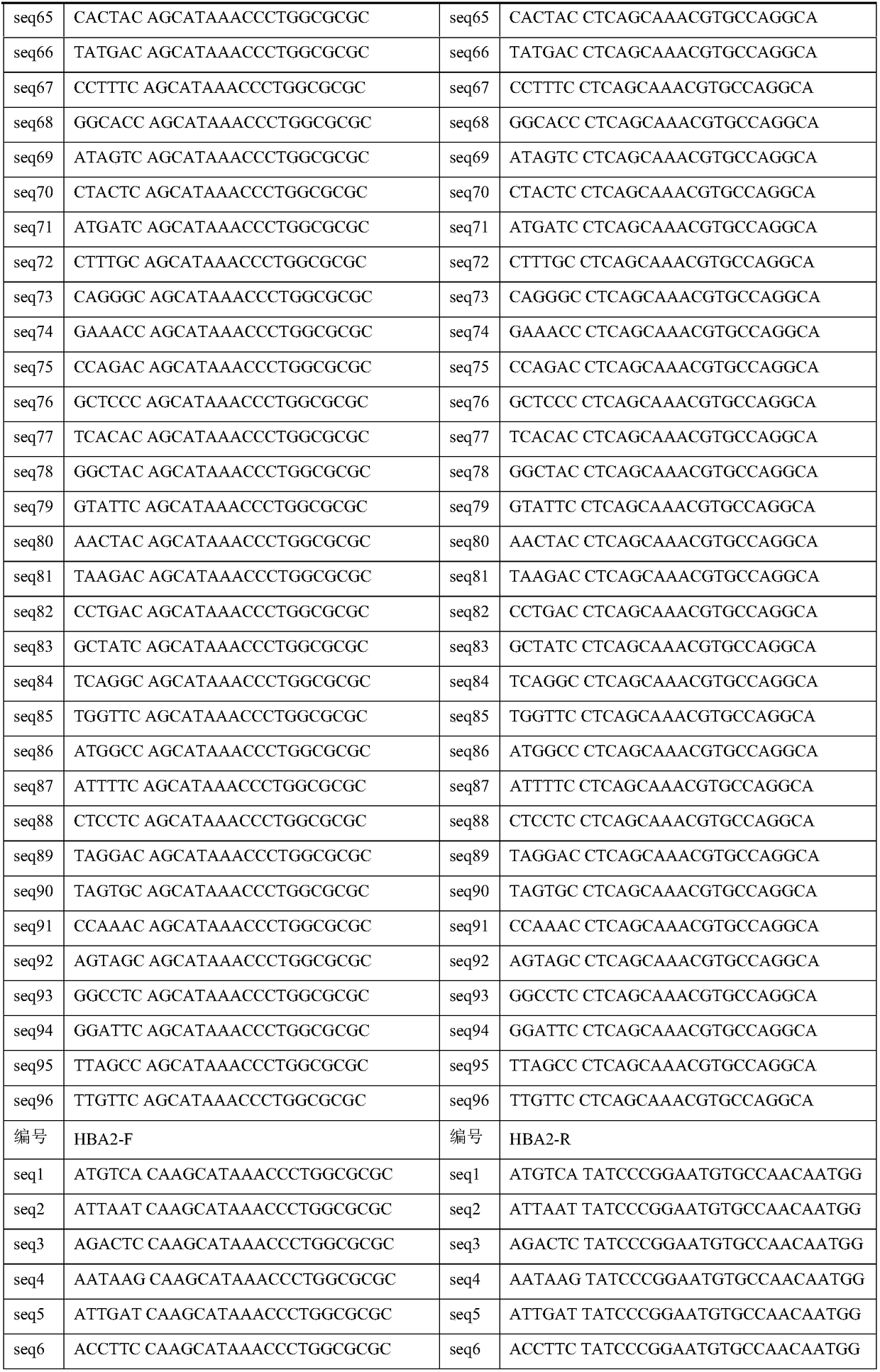
The invention discloses a library building method for screening large samples of thalassemia based on high-throughput sequencing. The library building method comprises the following steps: respectively amplifying HBA1, HBA2 and HBB genes by using specific primers with tag sequences, wherein the tag sequences are used for distinguishing different samples; mixing amplification products with same genes from different samples and then mixing the mixed amplification products with different genes; purifying the mixed products; incompletely interrupting the purified products; carrying out 5' phosphorylation blunt end repair and 3' end plus A to obtain a DNA segment with 5' phosphorylation and 3' viscous end A; ligating the DNA fragment with a connector containing a unique barcode sequence for distinguishing the library; purifying a ligation product to obtain an upper computer library suitable for the high-throughput sequencing. The method disclosed by the invention has the advantages that 245kinds of HBB gene mutation types and 93 kinds of HBA gene mutation types which are currently known and related to the thalassemia can be covered; meanwhile, novel types in the target area range can also be detected.
Owner:CHEERLAND BIOTECH CO LTD
siRNA sequence for targeted inhibition of human alpha-globin gene expression
InactiveCN104480113AImprove synthesis imbalanceHigh expressionOrganic active ingredientsBlood disorderBeta globinBeta thalassemia
The invention provides a siRNA sequence for targeted inhibition of human alpha-globin gene expression. The siRNA sequence is a HBA2 siRNA1 sequence and comprises a sense strand and an antisense strand; the nucleotide sequence of the sense strand is shown by SEQ ID No.1; and the nucleotide sequence of the antisense strand is shown by SEQ ID No.2. The siRNA sequence can specifically and efficiently inhibit the expression of the alpha-globin gene so as to improve the synthesis imbalance of alpha / beta globin of beta thalassemia sufferers and lay a foundation for establishing a medicine and technology system for treating beta thalassemia through siRNA. Moreover, the siRNA-mediated RNAi technology is efficient, specific and convenient and has a good application prospect.
Owner:广东省计划生育科学技术研究所
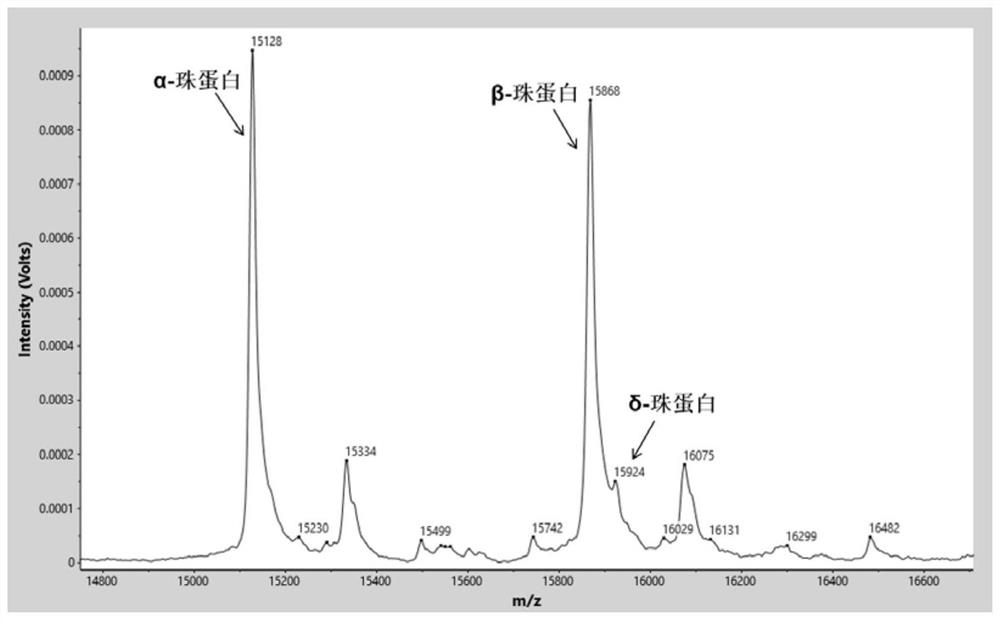
Characteristic protein marker composition for screening thalassemia, mass spectrum model and application thereof
ActiveCN111948404AEasy to handleLess consumablesMaterial analysis by electric/magnetic meansDisease diagnosisBeta globinBeta thalassemia
The invention discloses a characteristic protein marker composition for screening thalassemia, a mass spectrum model and application thereof. The invention firstly discloses a characteristic protein marker composition for screening thalassemia. The composition comprises alpha globin, beta globin, delta globin, gamma globin and / or carbonic anhydrase I, and the sequences of the alpha globin, the beta globin, the delta globin, the gamma globin and / or the carbonic anhydrase I are respectively shown as SEQ ID NO.1-5. The invention further discloses a mass spectrum model containing the marker composition and application of the mass spectrum model in preparation of products for screening thalassemia. Based on the MALDI-TOF mass spectrum technology, the method can screen beta thalassemia by analyzing the mass spectrum peak areas and ratios of different characteristic proteins, carries alpha and beta thalassemia, alpha thalassemia and abnormal hemoglobin samples, and has the characteristics ofsimple sample pretreatment, high detection flux, less reagent consumables, low cost, less sampling amount, high sensitivity and the like; and large-scale population screening can be carried out in regions with high occurrence rate of thalassemia.
Owner:融智生物科技(青岛)有限公司
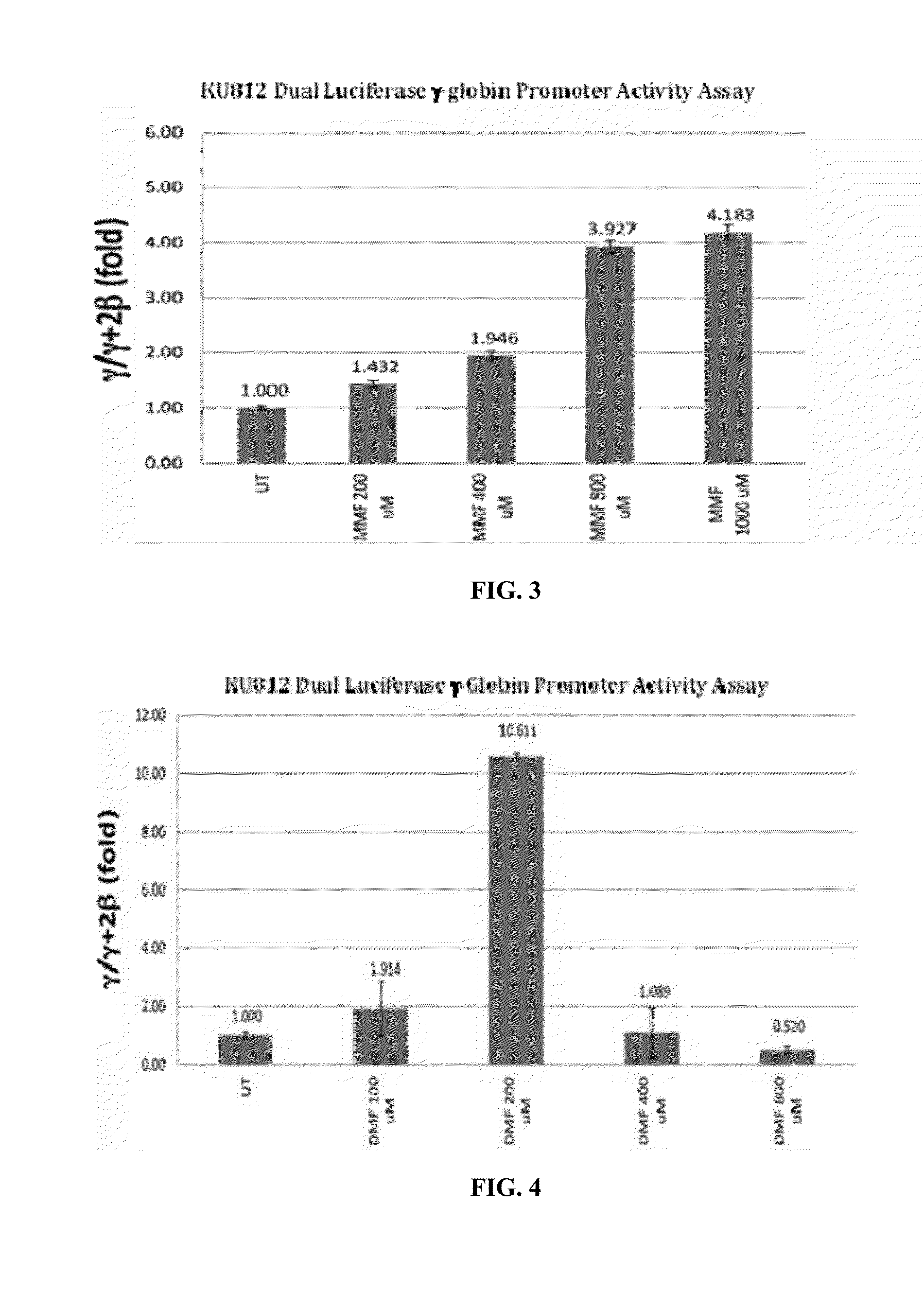
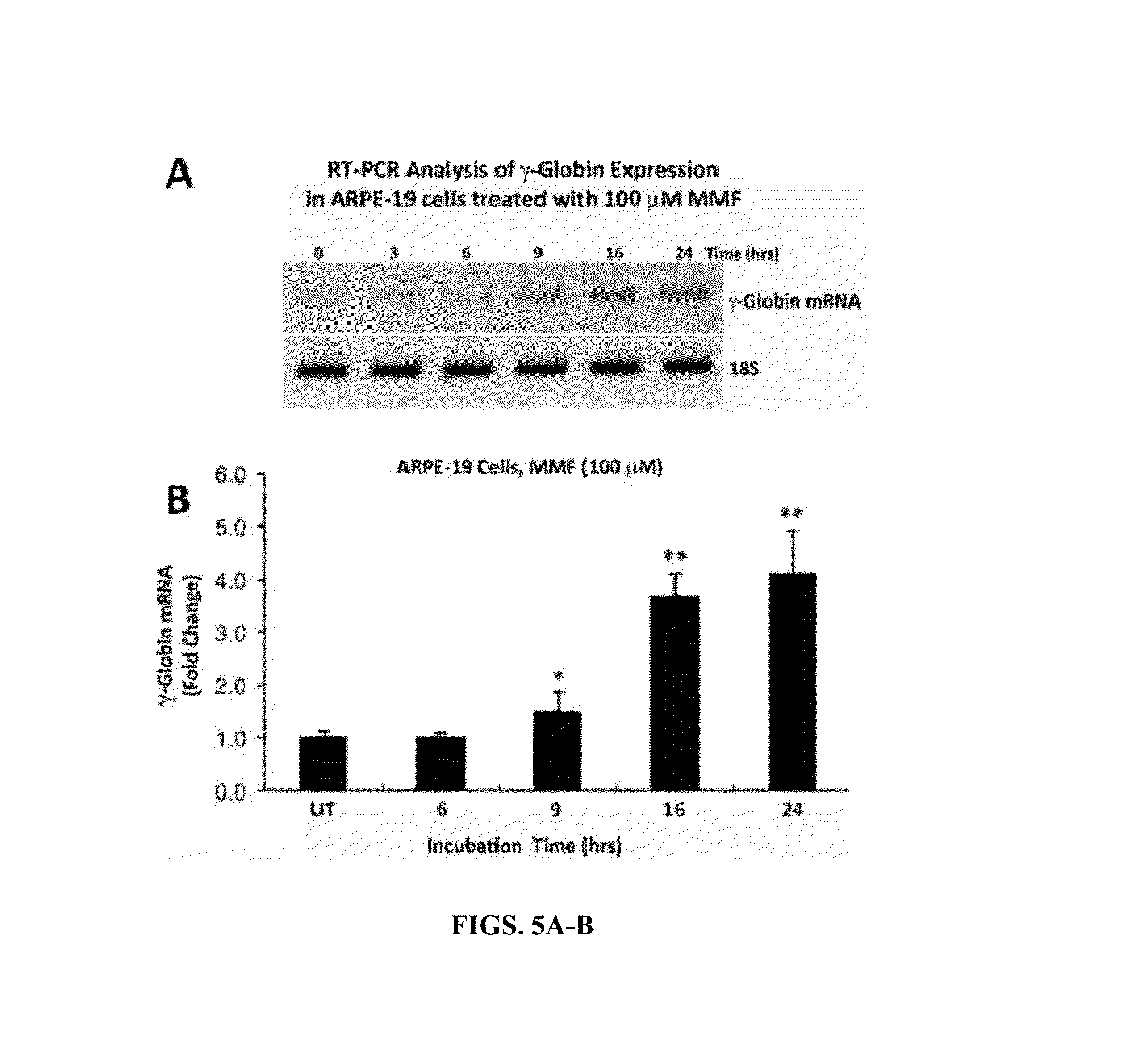
Methods of Treating Sickle Cell Disease and Related Disorders Using Fumaric Acid Esters
Methods of using one or more fumaric acid esters or pharmacologically active salts, derivatives, analogues, or prodrugs thereof to increase expression of fetal hemoglobin (HbF) are disclosed. The methods typically include administering to a subject an effective amount of one or more fumaric acid esters optionally in combination or alternation with hydroxyurea to induce HbF expression in the subject in an effective amount to reduce one or more symptoms of a sickle cell disorder, a hemoglobinopathy, or a beta-thalassemia, or to compensate for a genetic mutation is the human beta-globin gene (HBB) or an expression control sequence thereof. Pharmaceutical dosage units and dosage regimes for use in the disclosed methods are also provided.
Owner:AUGUSTA UNIV RES INST INC
Application of lentiviral vector in preparation of medicine for treating beta-thalassemia
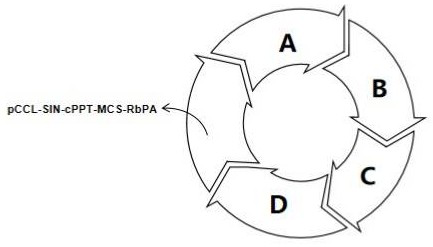
ActiveCN114457119AImprove packaging efficiencyIncrease productionPeptide/protein ingredientsHaemoglobins/myoglobinsBeta globinBeta thalassemia
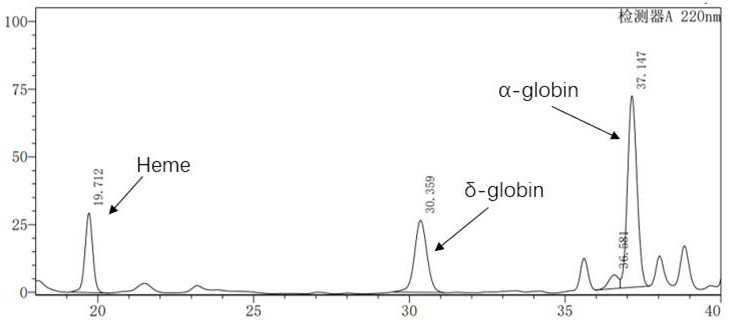
The invention discloses application of a lentiviral vector in preparation of a medicine for treating beta-thalassemia. The lentiviral vector is composed of a pCCL-SIN-cPPT-MCS-RbPA skeleton, a regulatory sequence A, a promoter sequence B, an enhancer sequence C and a gene sequence D. The lentivirus can enhance the specific expression of genes and reduce the size of the vector, so that the virus packaging efficiency is improved, the potential carcinogenic risk of the virus vector to tested cells is reduced, and the lentivirus vector infects hematopoietic stem cells (CD34 + cells) of beta-thalassemia patients, so that the beta-thalassemia patients are infected with hematopoietic stem cells (CD34 + cells). The expression level of exogenous beta globin (Hb beta A-T87Q) containing 87th amino acid mutation is quantified by high performance liquid chromatography, the exogenous beta globin (Hb beta A-T87Q) can be distinguished from wild beta globin expressed by an endogenous gene or derived from blood transfusion, and high expression of Hb beta A-T87Q is observed.
Owner:GENMEDICN BIOPHARMA INC
A kind of differential protein in the serum of dairy cows in early pregnancy stage and its application
ActiveCN104987379BWhether pregnancy is accurateEarlyHaemoglobins/myoglobinsDisease diagnosisBeta globinBiology
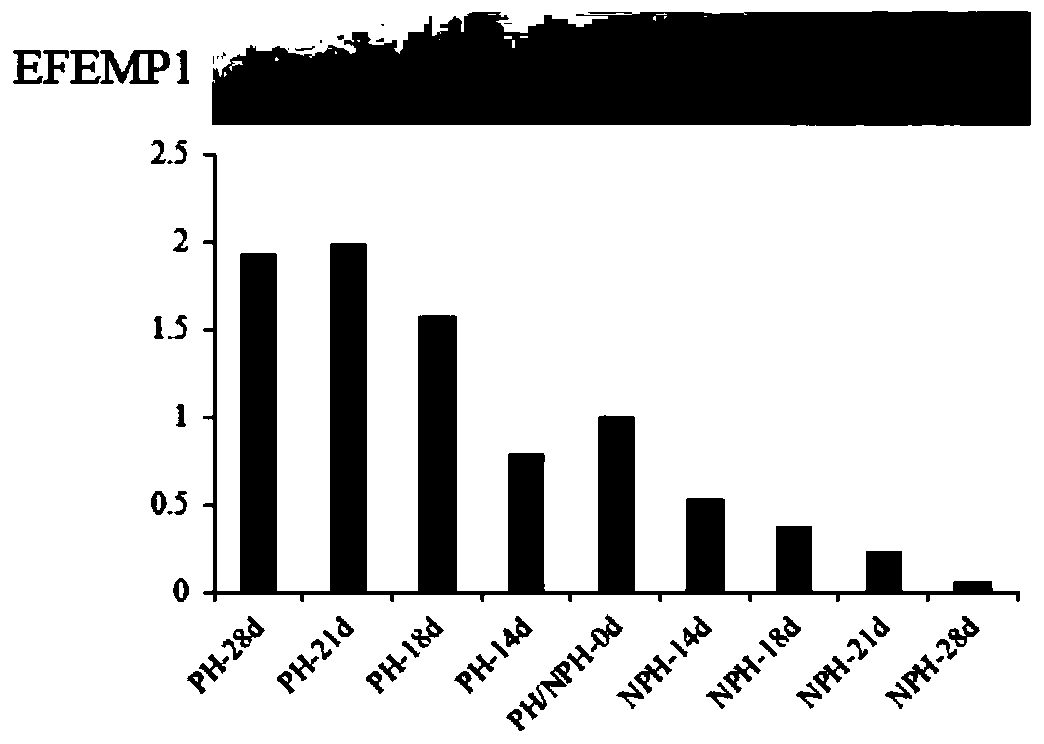
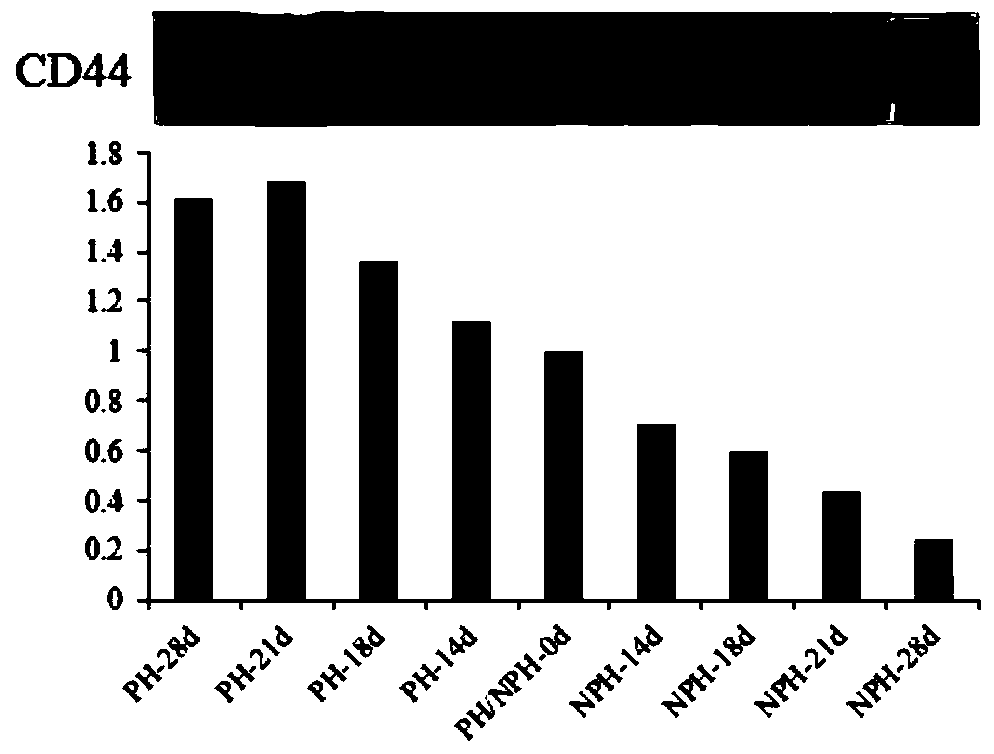
The invention discloses differential protein in serum at the early pregnancy stage of a cow and application of the differential protein, and relates to the field of cow feeding. The differential protein includes pregnancy differential protein, non-pregnant differential protein and reverse differential protein. The pregnancy differential protein comprises ARHGAP42, EFEMP1, GTF2F1, LOXL2, CPB2, LYST, RDH11, TRABD, TRIM67 and / or TRMT112. The non-pregnant differential protein comprises IGL. The reverse differential protein comprises HBB and HP. The differential protein is used for judging whether the cow is pregnant or not and whether early embryo loss exists or not or increasing the embryo survival rate. By means of the differential protein, whether the fertilized cow is pregnant or not can be judged, synchronous estrus and the second time of artificial insemination can be conducted on the non-pregnant cow in time, early embryo loss early warning and progesterone preparation can be conducted, and the pregnancy rate and the breeding efficiency of cattle can be comprehensively increased.
Owner:武汉市畜牧兽医科学研究所
Method for preparing transgenic mouse with central nervous system specific expression Cre recombinase
A process for preparing the transgenic mouse able to specifically express Cre recombinant enzyme in cental nerve system includes such steps as using the terminals' regulatory sequence of 1.8 kb GFAP gene to configure the transgenic carrier pGFAP-Cre-hGH containing two beta-glubolin insulators, GFAP5' terminal regulatory region, Cre recombinant enzyme gene and human growth hormone gene, injecting the 7.6 kbtransgenic fragment beta globin insulator-pGFAP-Cre-hGH into mouse's fertilizer eggs, transplating them in the deference duct of male mouse, and developing to obtain the target mouses.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com