Methods of Treating Sickle Cell Disease and Related Disorders Using Fumaric Acid Esters
a technology of fumaric acid and esters, which is applied in the field of methods of treating sickle cell disease and related disorders using fumaric acid esters, can solve the problems of unresponsive or inability to respond well to hu treatment alone, and achieve the effects of increasing hfb expression, improving hu uptake, and improving hu uptak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Monomethylfumarate (MMF) Induces γ-Globin (Hbf) Gene and Protein Expression in Cells of Erythroid Lineage
Materials and Methods
[0191]Pharmaceutical Agents
[0192]The fumaric acid esters dimethylfumarate (DMF) and monomethylfumarate (MMF) are the primary constituents of Fumaderm and BG00012, drugs currently marketed for treatment of psoriasis. BG00012 is also completing phase III clinical testing for treatment of multiple sclerosis. MMF is the major bioactive component of each.
[0193]Cell Culture
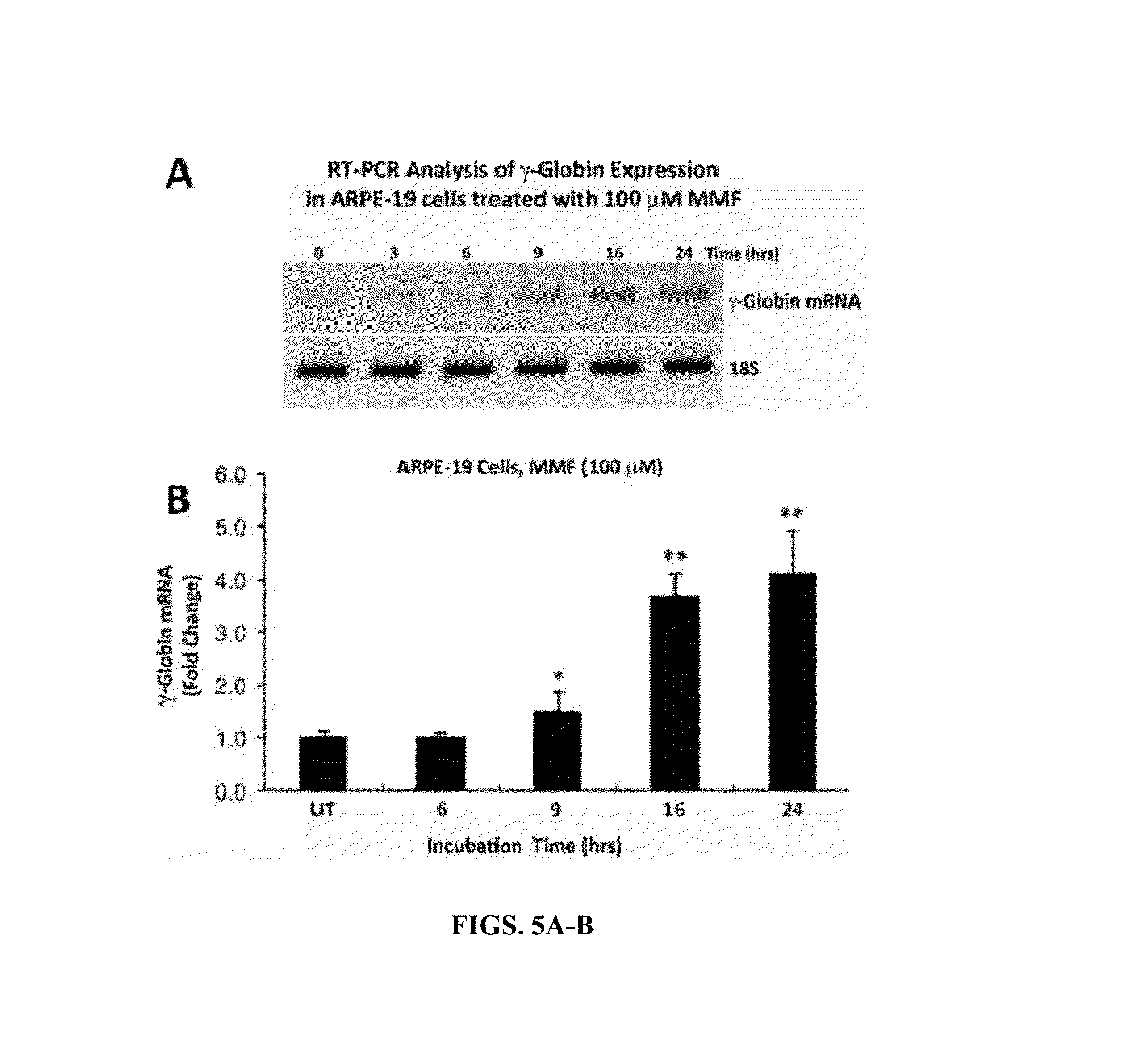
[0194]KU812, a human leukemic cell line that expresses the fetal γ-globin and adult β-globin genes, is a commonly used system for screening and discovery of novel HbF inducers; this is because of comparable globin gene response patterns in KU812 and primary erythroid cells after treatments with drug inducers.
Results
[0195]KU812 cells were cultured in the presence or absence of MMF for time periods ranging from 0-24 hours and evaluated for changes in γ-globin gene expression relative to 18S ribosom...
example 2
Monomethylfumarate (MMF) Drives Expression by Activation the γ-Globin (Hbf) Gene Promoter
Materials and Methods
[0197]From MaKala et al., Anemia, Volume 2012, Article ID 428137) (2012)
[0198]KU812 Stable Lines
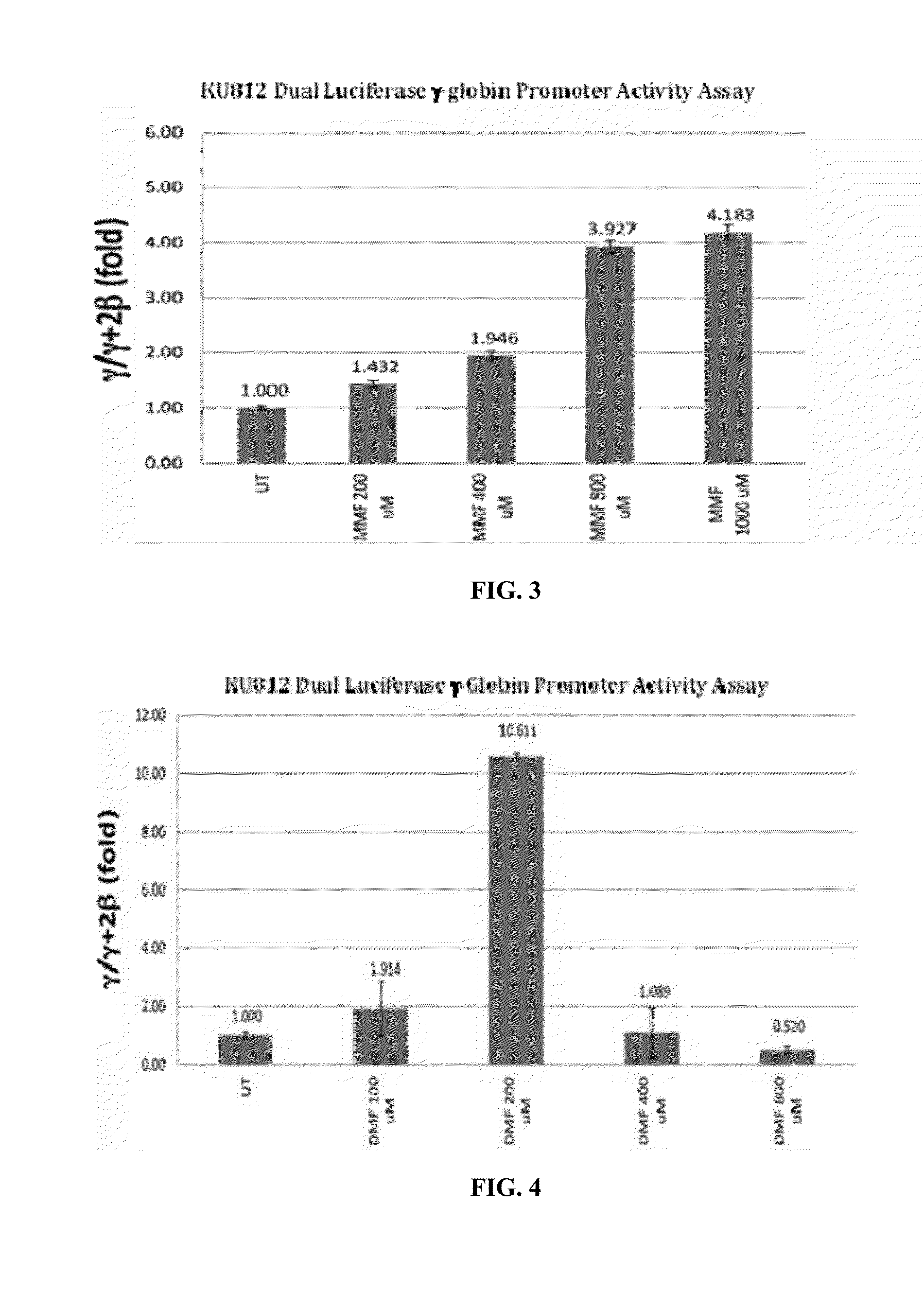
[0199]KU812 stable cell lines were created by co-transfecting wild-type KU812 cells with pEGFP-NI (G418 selectable marker) and the μLCRβprRluc AγprFluc dualreporter a kind gifts from Dr. George Stamatoyannopoulos (University of Washington). Briefly, the 315-bp human β-globin gene promoter sequence was inserted upstream of the Renilla along with a polyadenylation signal downstream to create PβprRluc. Likewise, 1.4 kb of human Aγ-globin promoter was inserted upstream of firefly luciferase to create AγprFluc. The μLCR (locus control region), PβprRluc, and AγprFluc fragments were subsequently cloned into the mammalian vector, pRL-null. The dual-luciferase reporter lines were produced using 10 μg each of linearized μLCRβprRluc AγprFluc and pEGFP-NI plasmids co-transfected into KU812 ce...
example 3
Dimethylfumarate (DMF) Drives Expression by Activation the γ-Globin (Hbf) Gene Promoter
[0204]MMF is the primary bioactive metabolite derived from metabolism of FUMADERM and BG00012, the fumaric acid ester drugs presently used clinically, and DMF is the primary ingredient. Therefore, the effectiveness of DMF was tested as an inducer of γ-globin gene expression under experimental conditions identical to those described above in Example 2. As with MMF, treatment of cells with DMF also induced γ-globin promoter activity significantly (FIG. 4). At concentrations in the 100-200 μM range, induction of γ-globin promoter activity increased ˜2-10 fold in comparison to control, untreated (UT) cells. However, at higher concentrations the effectiveness of DMF as an inducer of γ-globin promoter activity declined substantially. Trypan blue exclusion revealed that this decrease was likely due to an increase in cellular toxicity as indicated by a decrease in cell viability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| aerodynamic diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com