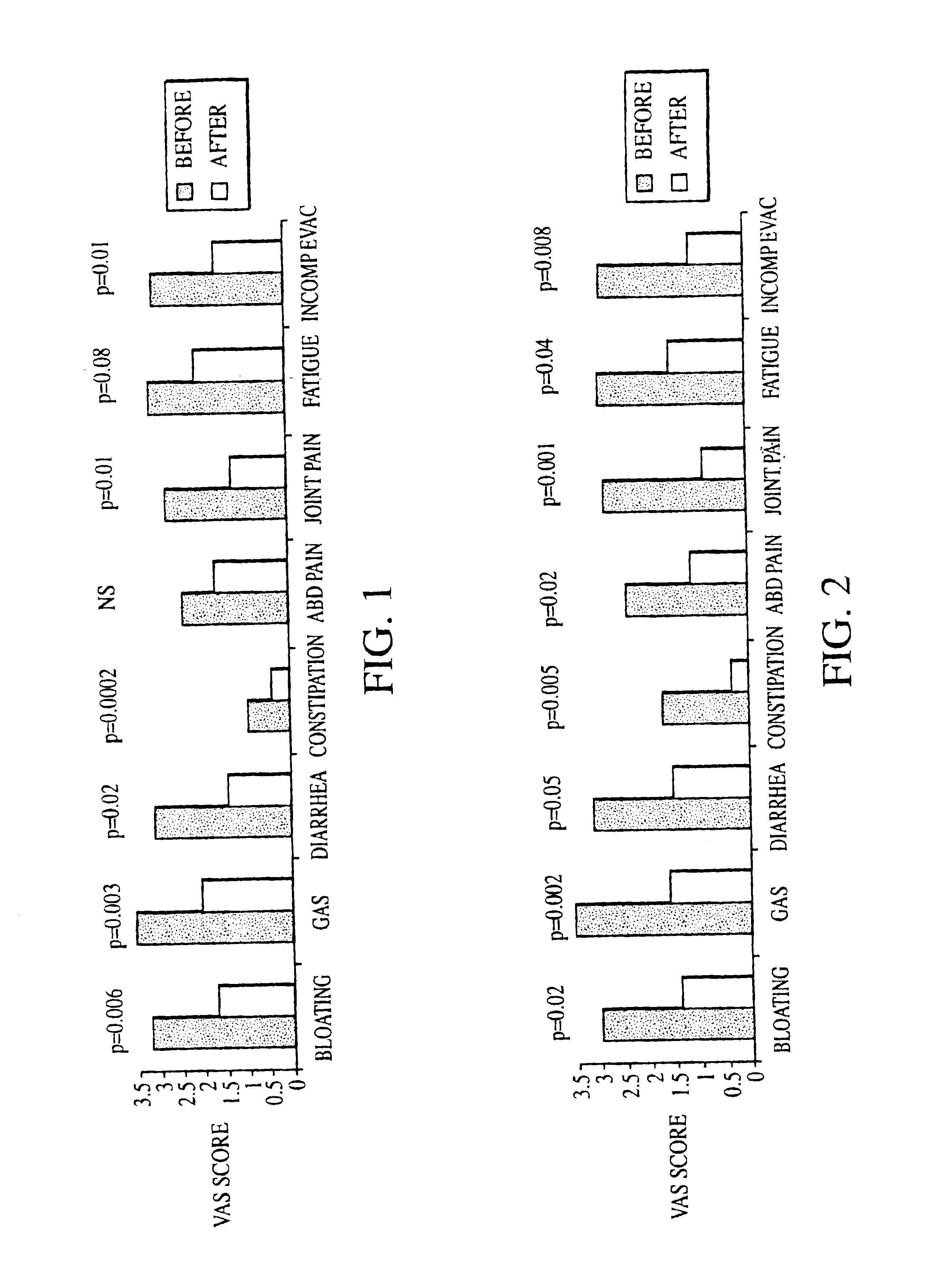
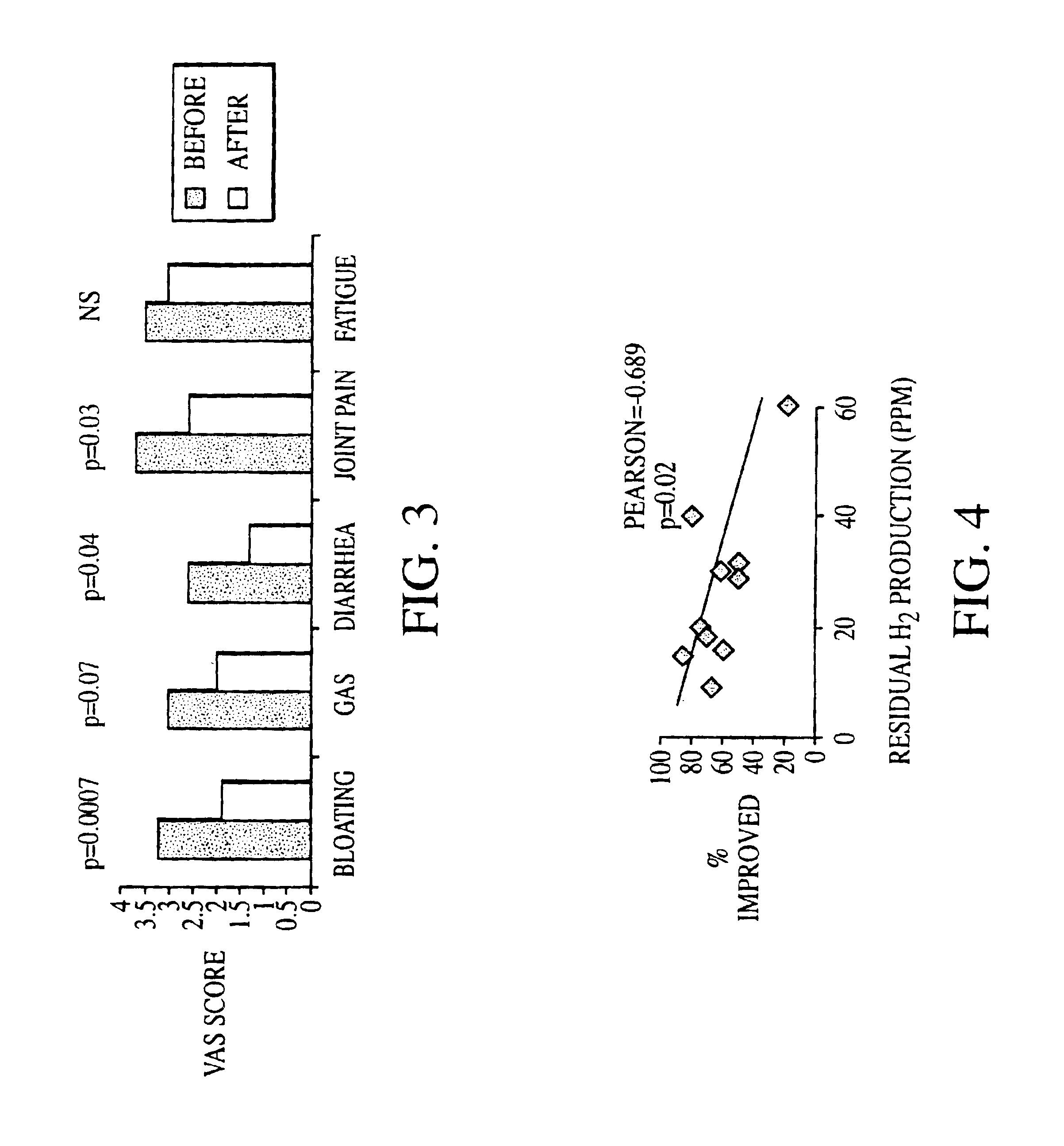
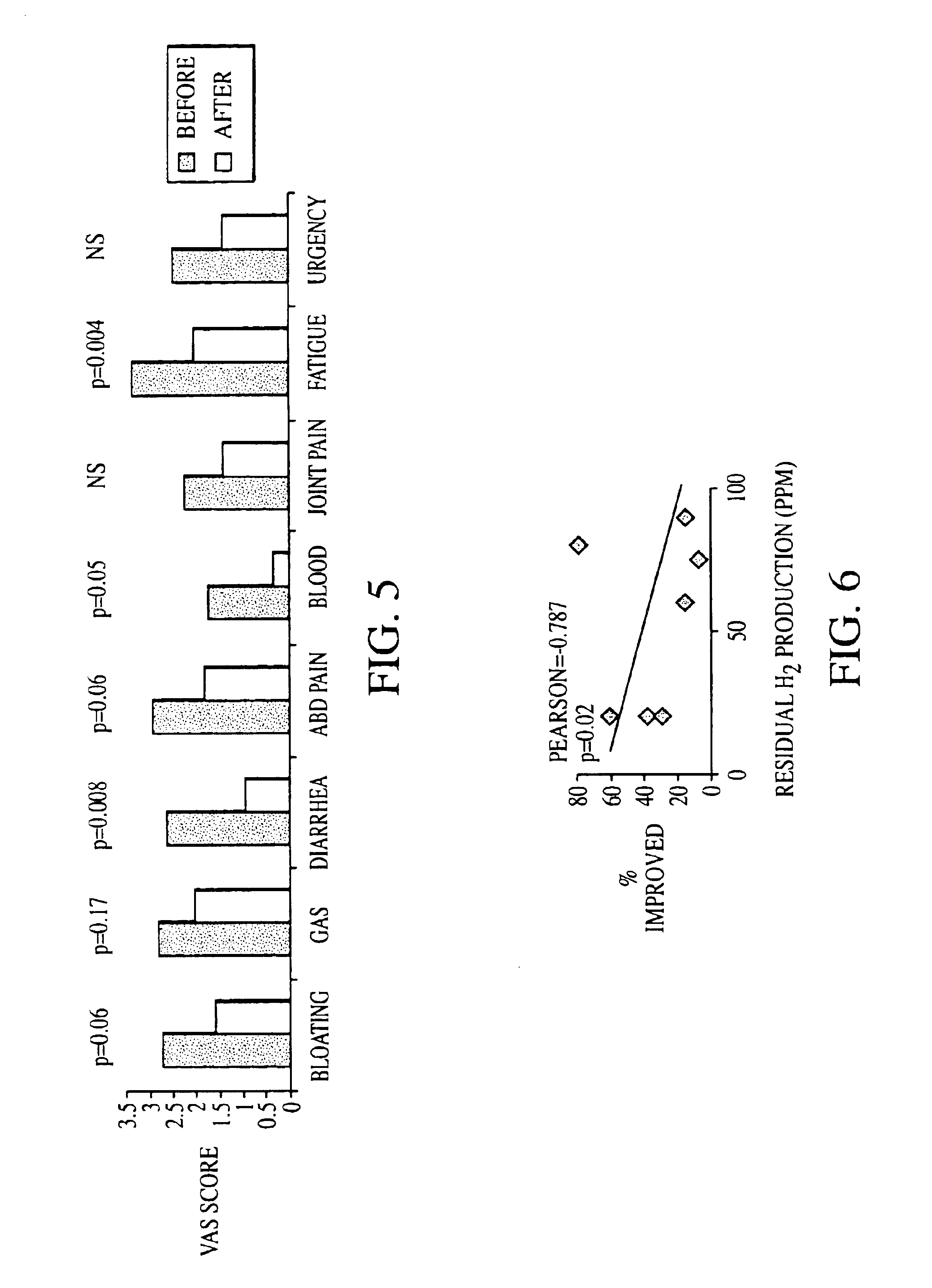
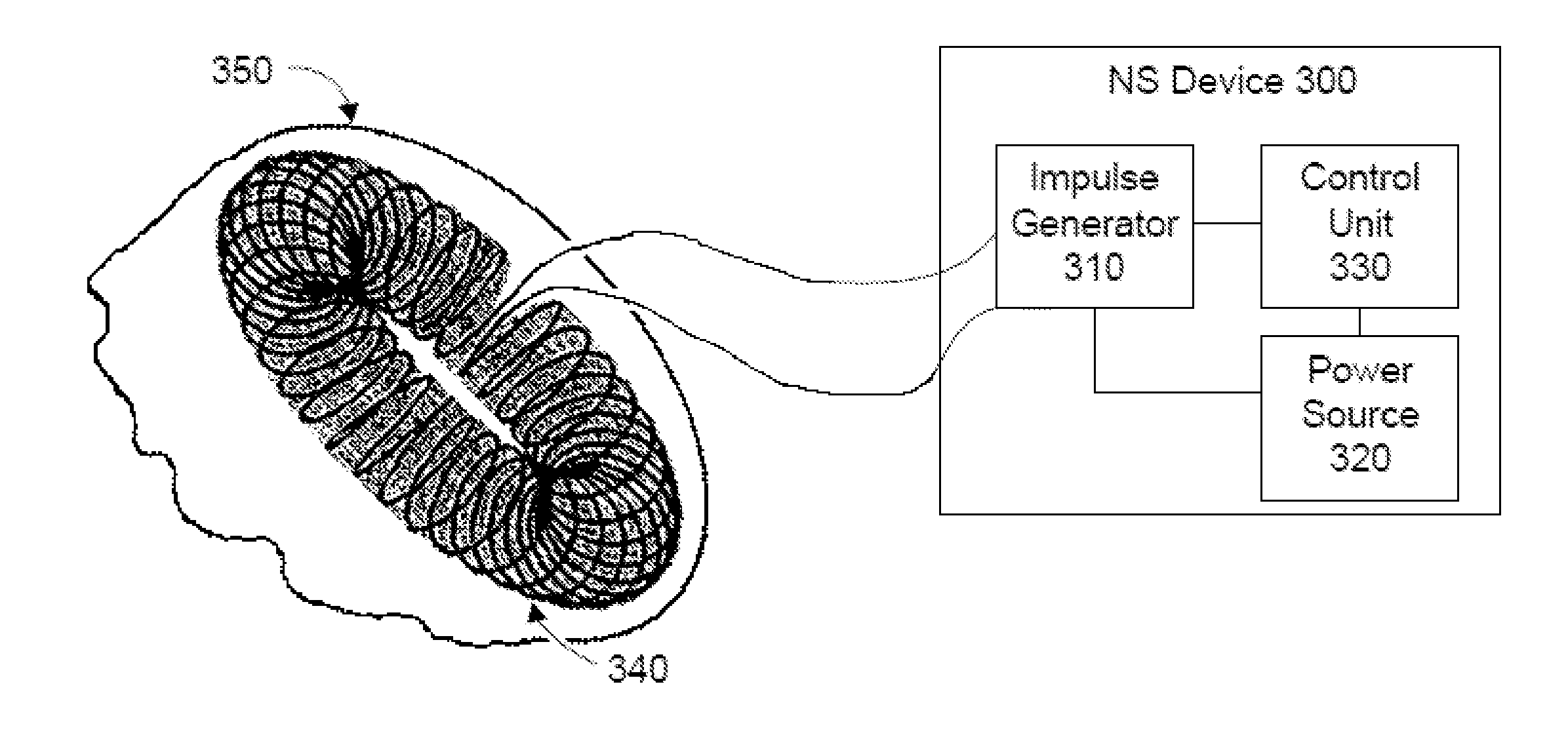
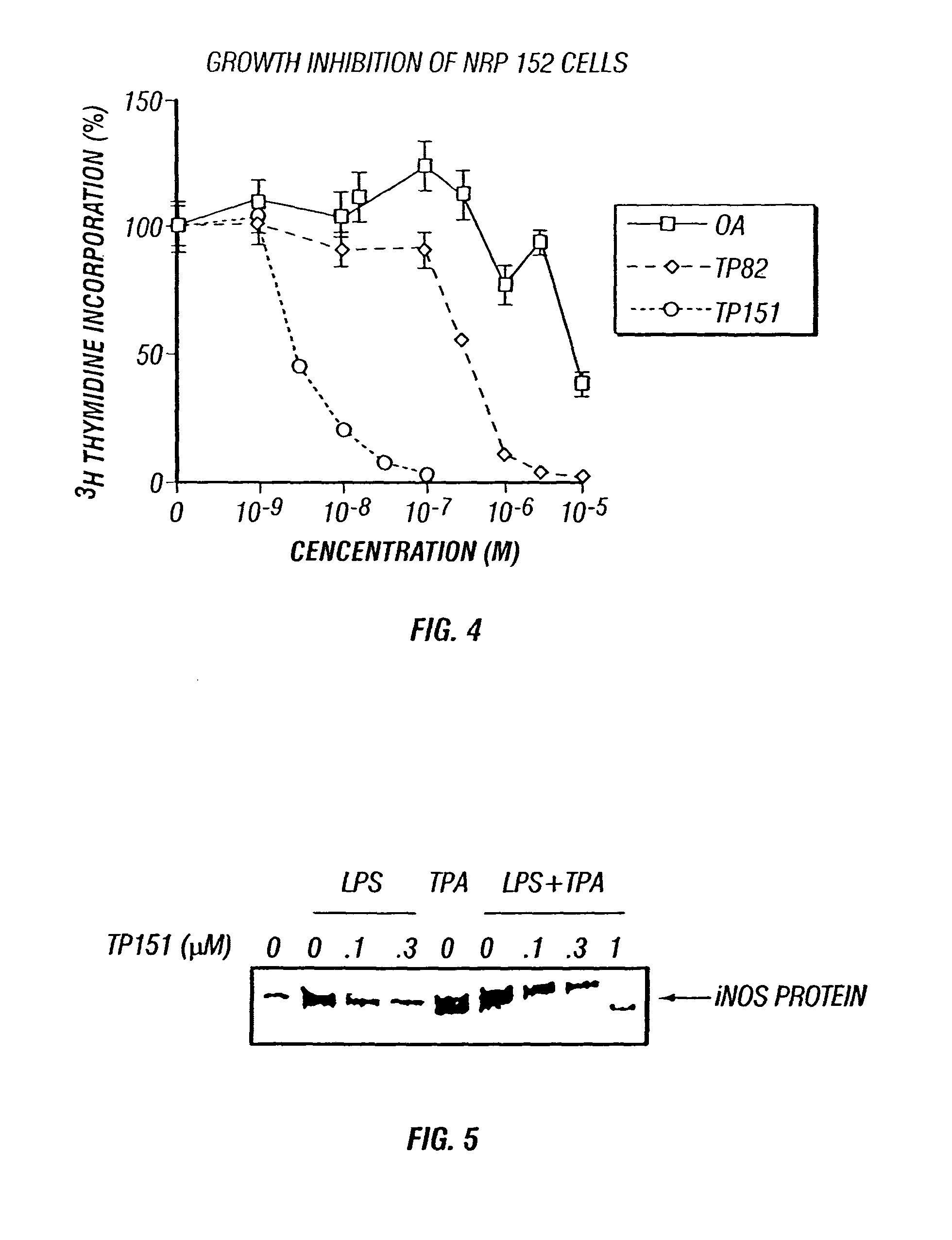
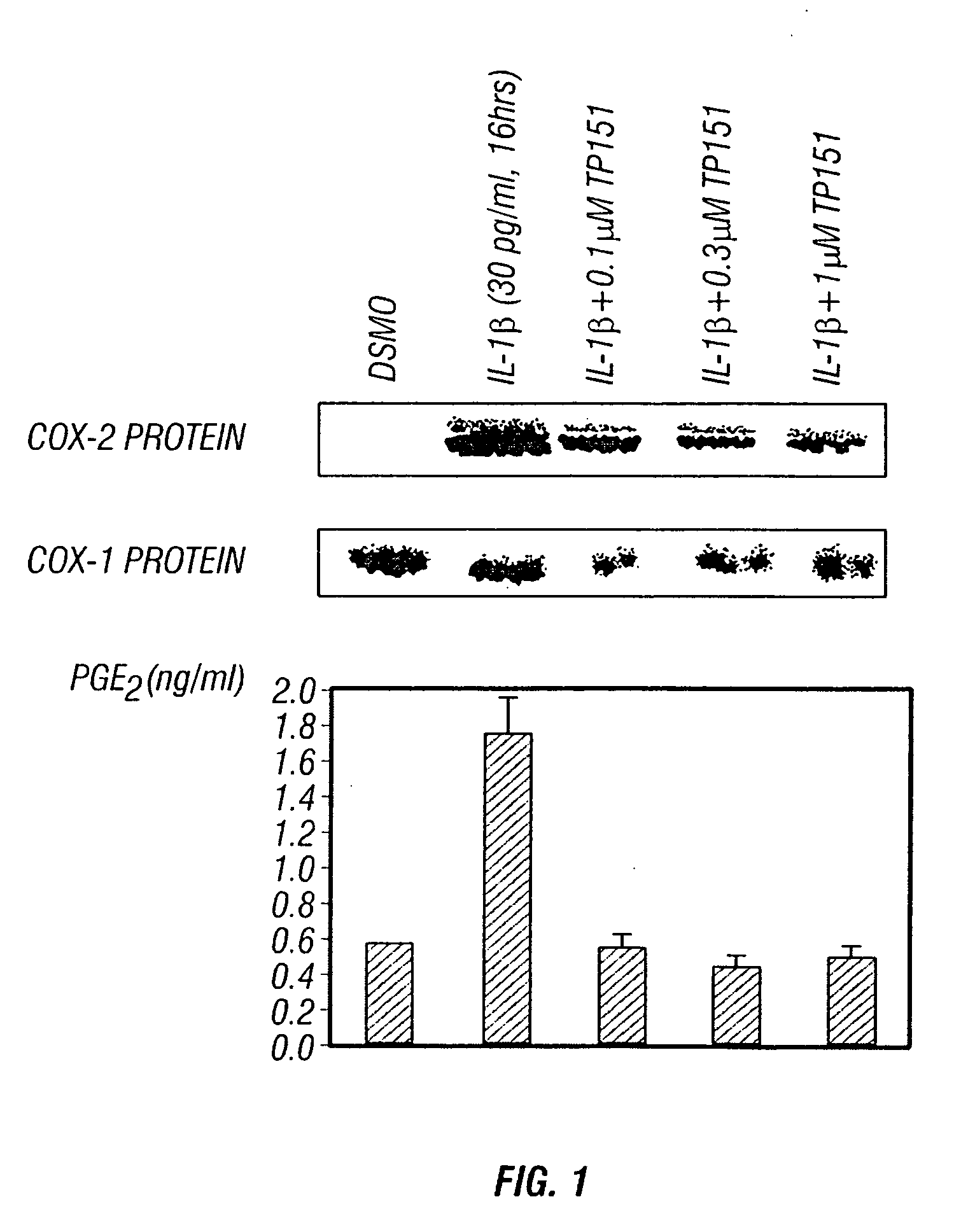
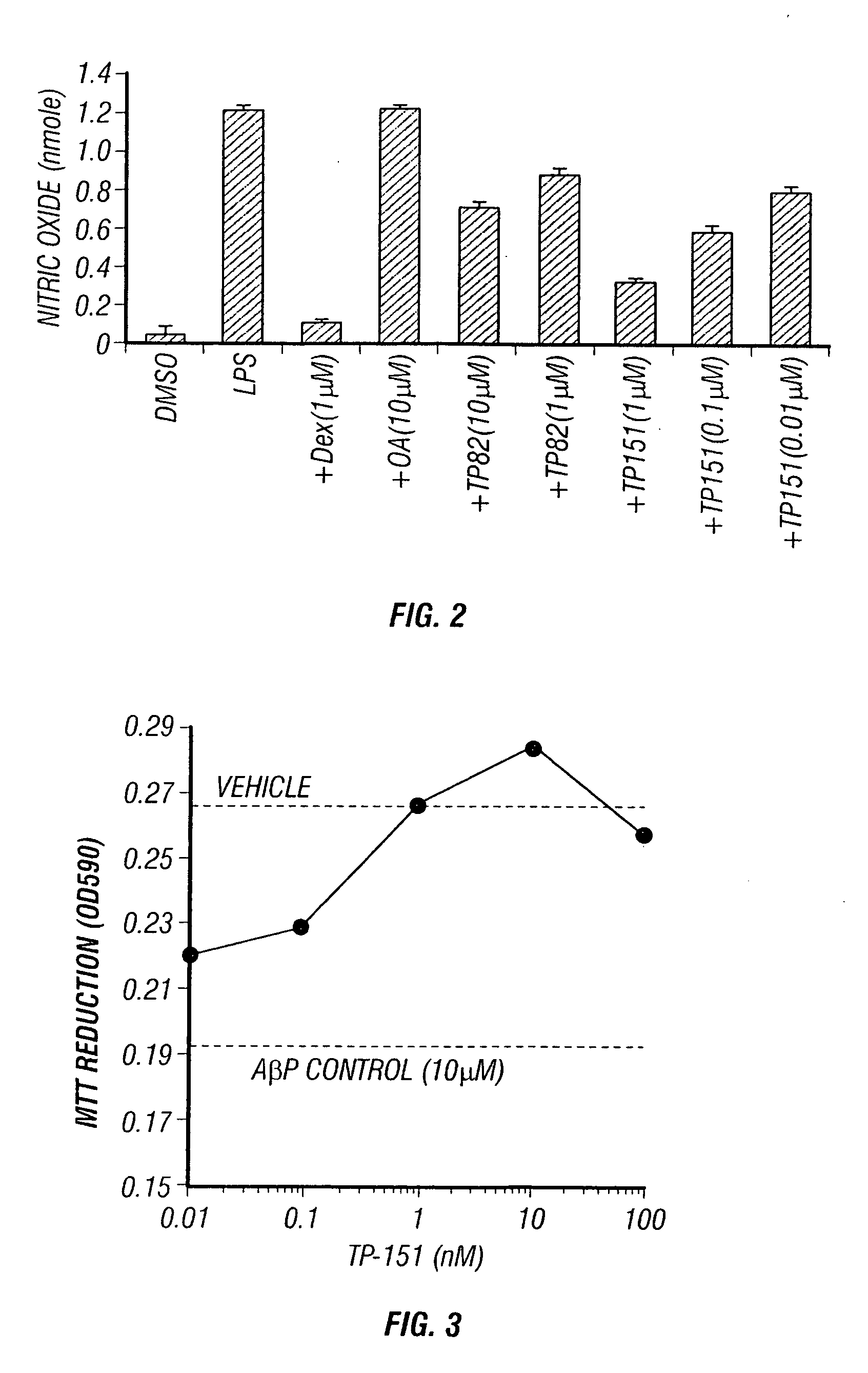
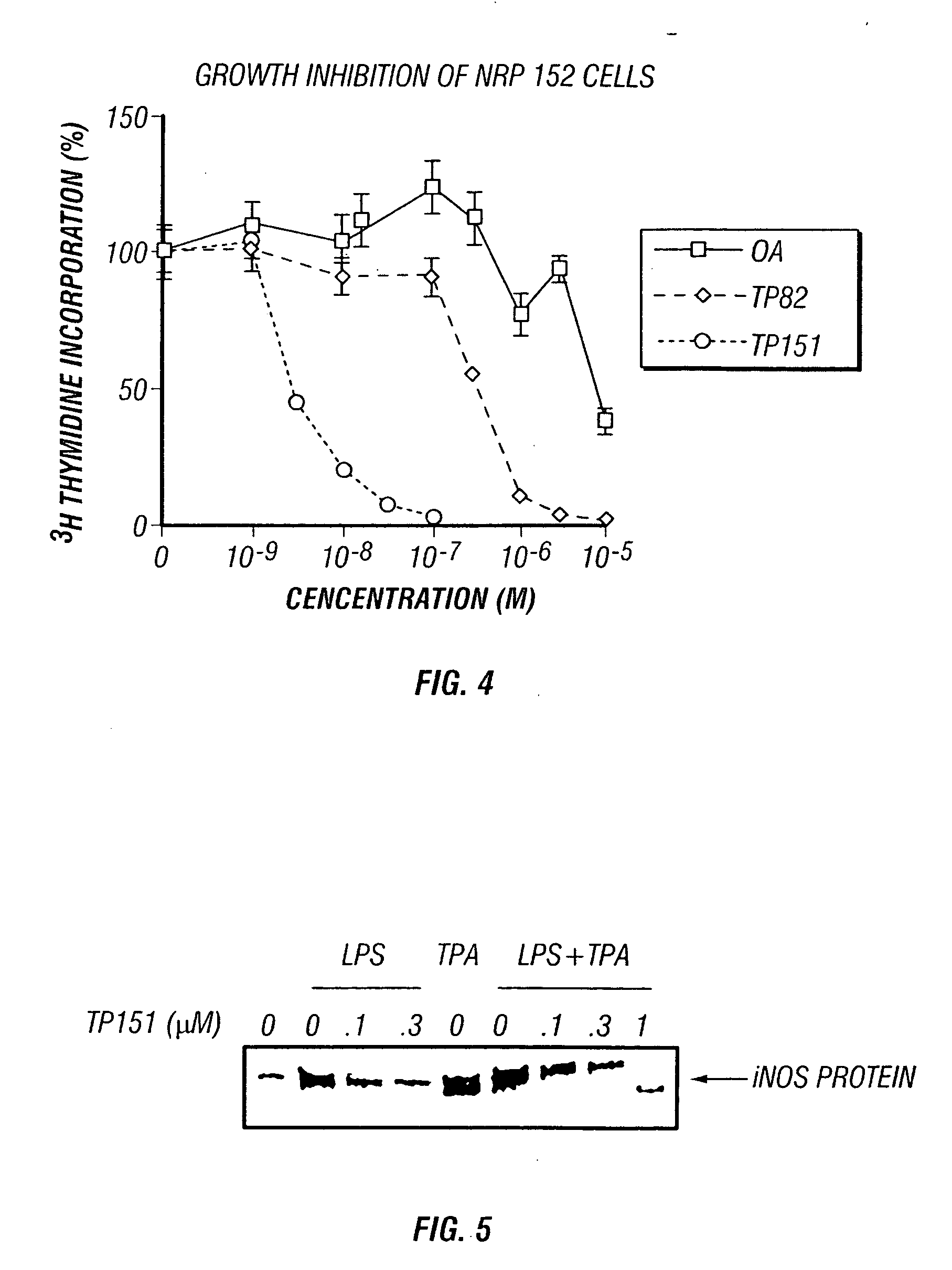
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1778 results about "Multiple sclerosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A disease that affects central nervous system.
Methods of diagnosing or treating irritable bowel syndrome and other disorders caused by small intestinal bacterial overgrowth
InactiveUS6861053B1Eradicate small intestinal bacterial overgrowthSymptoms improvedAntibacterial agentsOrganic active ingredientsBacteroidesAutoimmune responses
Disclosed is a method of diagnosing irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus, or Crohn's disease, which involves detecting the presence of small intestinal bacterial overgrowth (SIBO) in a human subject having at least one symptom associated with a suspected diagnosis of any of those diagnostic categories. Also disclosed is a method of treating these disorders, and other disorders caused by SIBO, that involves at least partially eradicating a SIBO condition in the human subject. The method includes administration of anti-microbial or probiotic agents, or normalizing intestinal motility by employing a prokinetic agent. The method improves symptoms, including hyperalgesia related to SIBO and disorders caused by SIBO. Also disclosed is a kit for the diagnosis or treatment of irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, or Crohn's disease.
Owner:CEDARS SINAI MEDICAL CENT
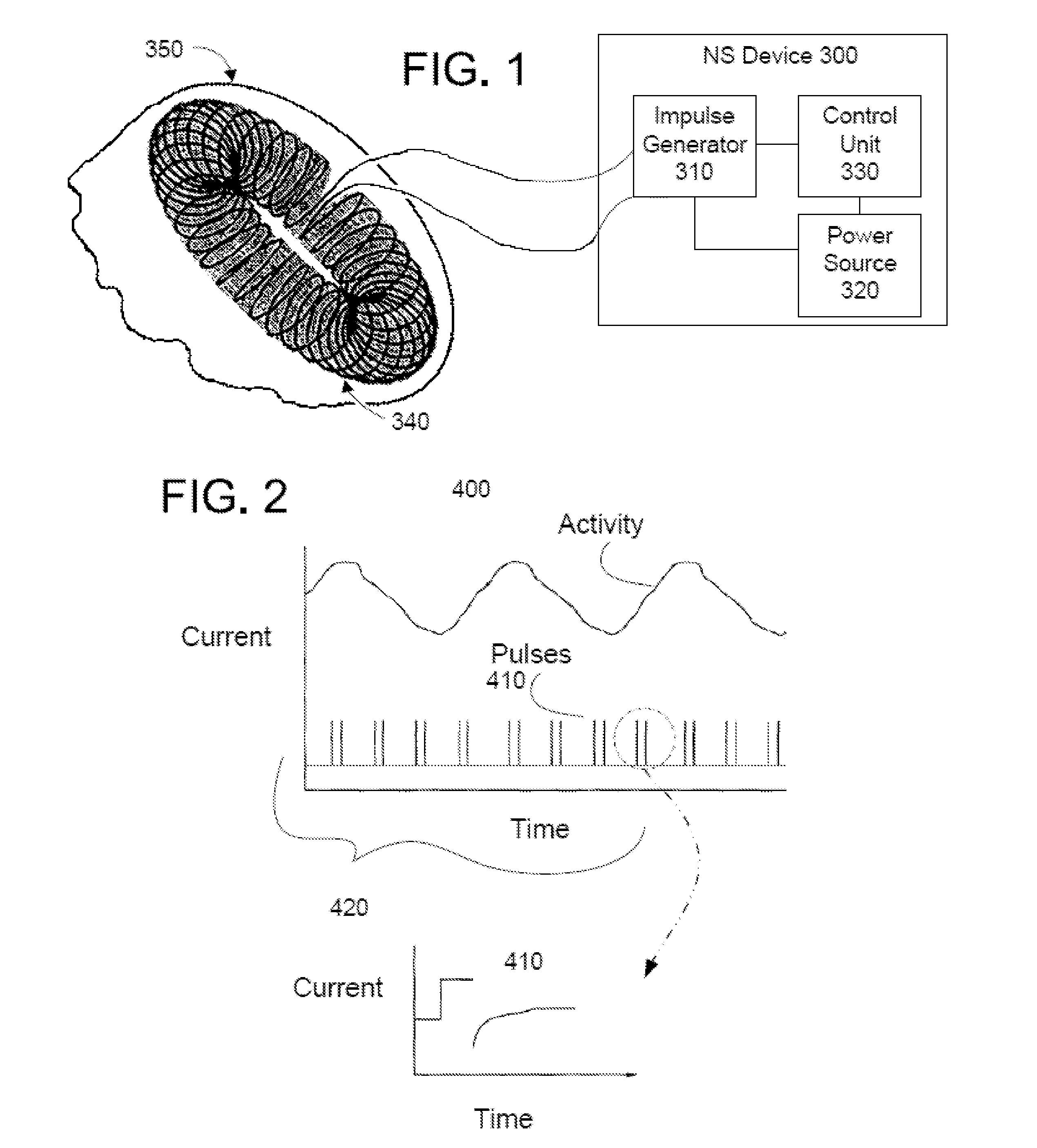
Non-invasive treatment of neurodegenerative diseases
ActiveUS20110152967A1Reduce neuroinflammationReduce inflammationElectrotherapyMagnetotherapy using coils/electromagnetsRetinoidPostoperative cognitive dysfunction
Methods and devices are disclosed for the non-invasive treatment of neurodegenerative diseases through delivery of energy to target nervous tissue, particularly the vagus nerve. The devices include a magnetic stimulator having coils with toroidal windings, which are in contact with an electrically conducting medium that is adapted to conform to the contour of a target body surface of a patient. The coils induce an electric current and / or an electric field within the patient, thereby stimulating nerve fibers within the patient. The stimulation brings about reduction of neuroinflammation in patients suffering from conditions comprising Alzheimer's Disease, Parkinson's Disease, Multiple Sclerosis, postoperative cognitive dysfunction and postoperative delirium. Reduction in inflammation is effected by enhancing the anti-inflammatory competence of cytokines such as TGF-beta, wherein a retinoid or components of the retinoic acid signaling system provide an anti-inflammatory bias, by enhancing anti-inflammatory activity of a neurotrophic factor such as NGF, GDNF, BDNF, or MANF, and / or by inhibiting the activity of pro-inflammatory cytokines such as TNF-alpha.
Owner:ELECTROCORE
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
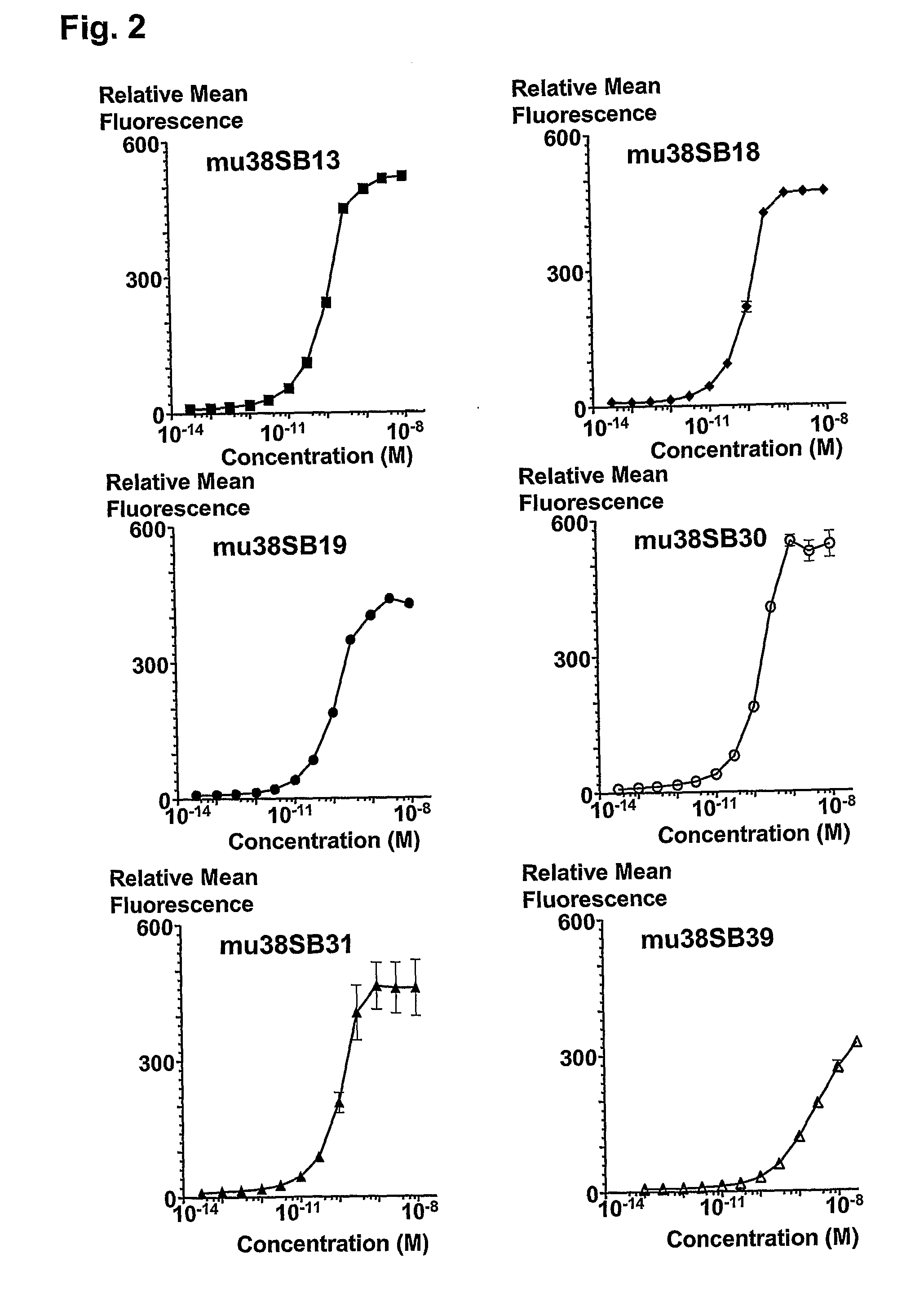
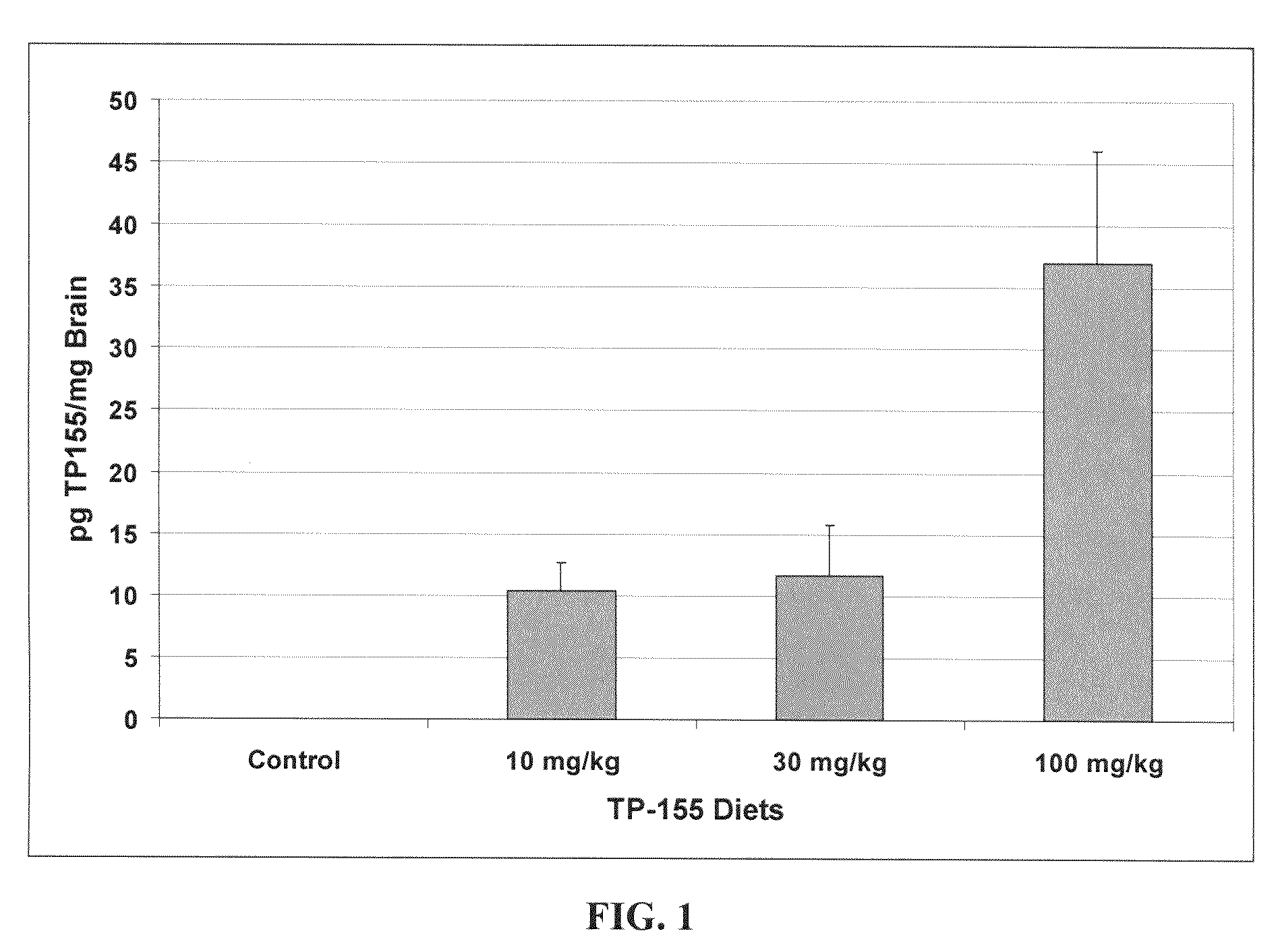
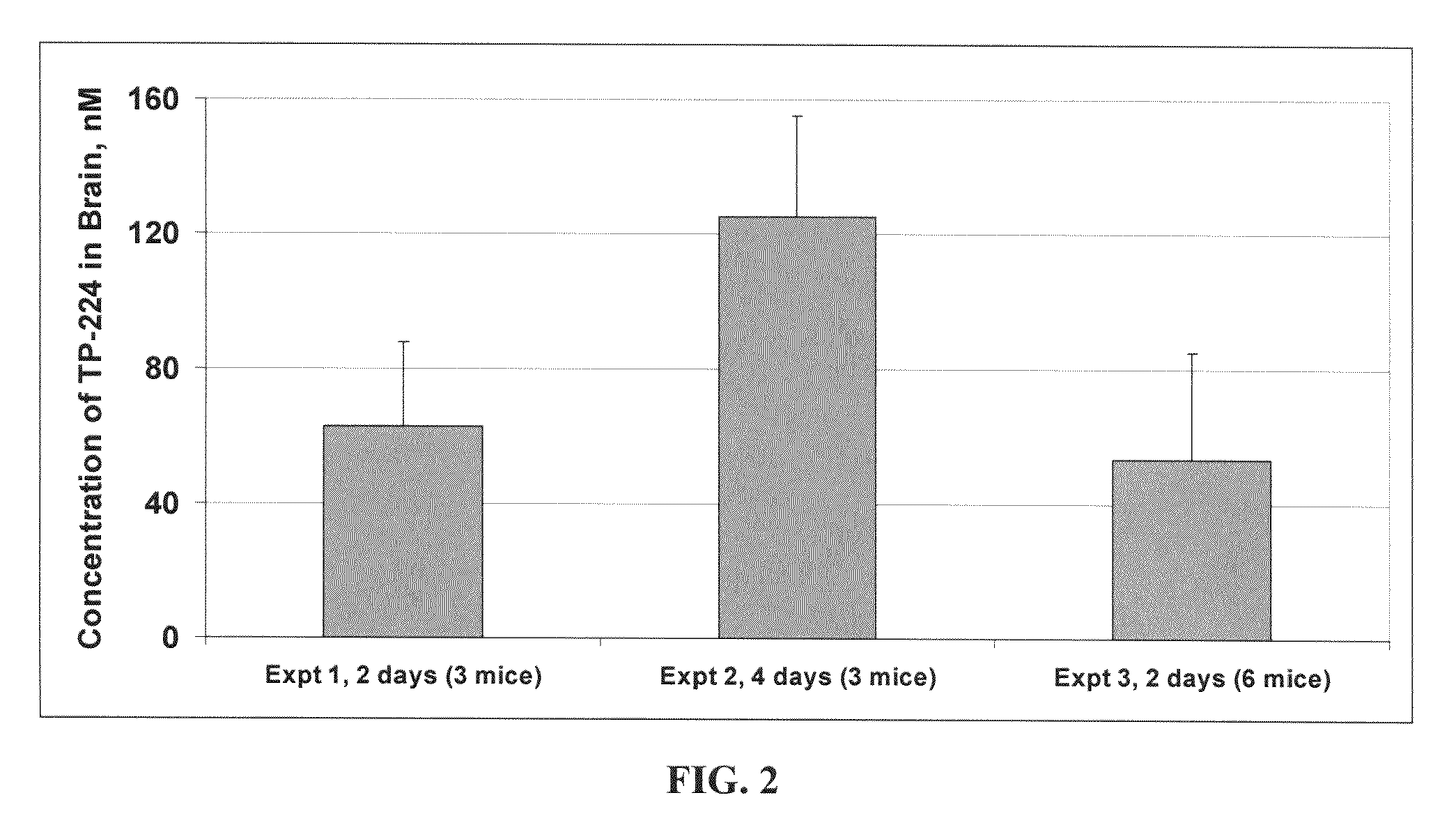
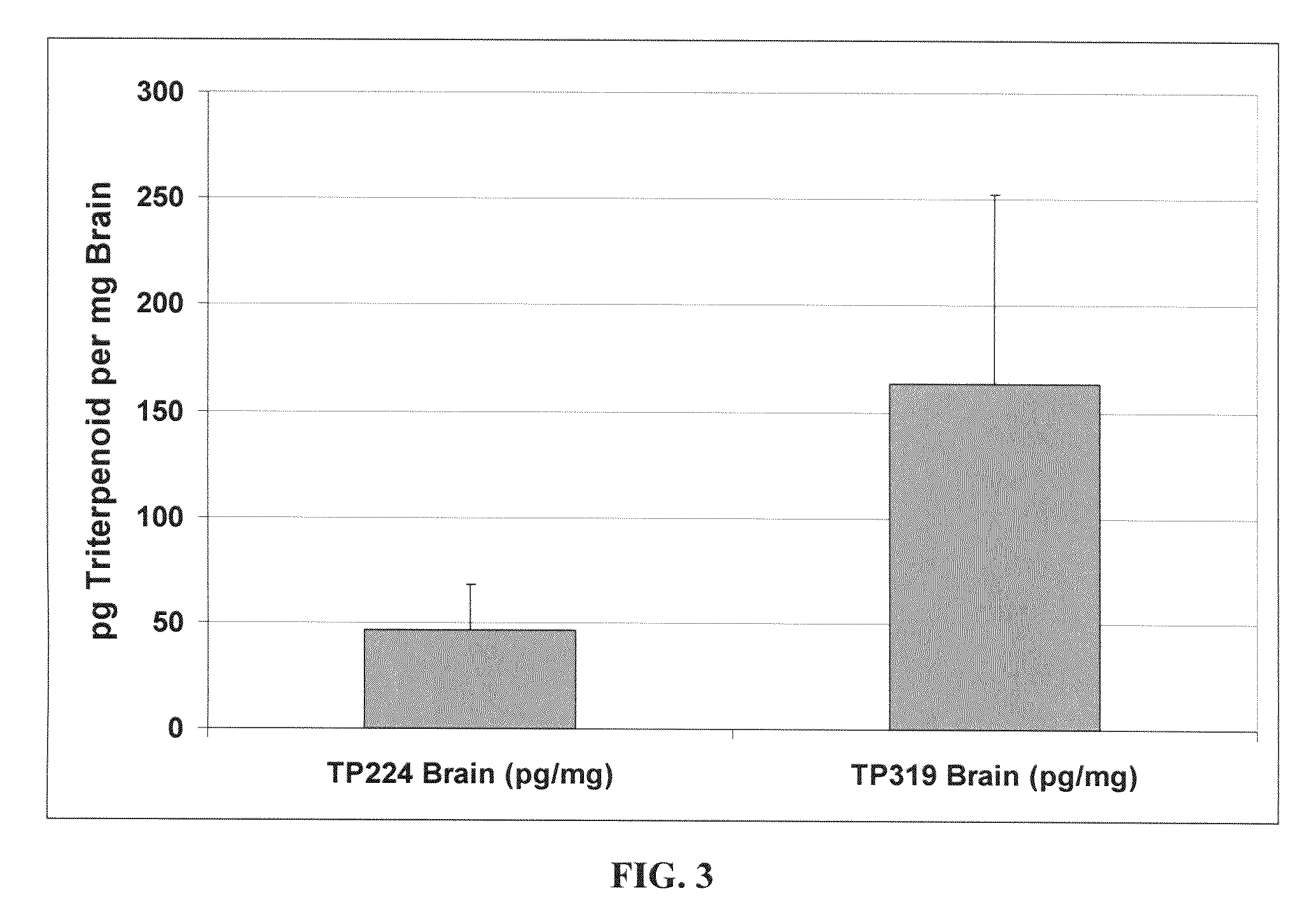
Novel synthetic triterpenoids and methods of use in the treatment and prevention of multiple scleroris
InactiveUS20090060873A1Improving glomerular filtration rateImproving creatinine clearanceBiocideSalicyclic acid active ingredientsDiseaseBipolar mood disorder
The present invention overcomes limitations of the prior art by providing new compounds and methods for the treatment of conditions, such as neurodegenerative diseases (e.g., multiple sclerosis), psychiatric disorders (e.g., psychosis, bipolar disorder, depression, neuropathic pain), conditions involving CNS-mediated chronic pain, spinal cord injuries, and other diseases or injuries.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE +1
Amine Compounds
InactiveUS20080200535A1Potent immunosuppressive actionBiocideSenses disorderUveitisAutoimmune disease
There is provided a compound exhibiting an activity of suppressing immune response with reduced adverse drug reactions, which compound is useful in the chemotherapy for preventing or treating, for example, a wide range of various autoimmune diseases including systemic erythematodes, chronic rheumatoid arthritis, Type I diabetes, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis or other disorders, or chronic inflammatory diseases, or cancers, lymphoma or leukemia, or resistance to organ or tissue transplantation or rejection against transplantation.Novel amine compounds having an S1P1 / Edg1 receptor agonist effect, possible stereoisomers or racemic bodies of the compounds, or pharmacologically acceptable salts, hydrates or solvates of the compound, the stereoisomers or the racemic bodies, or prodrugs of the compounds, the stereoisomers, the racemic bodies, the salts, the hydrates or the solvates, are provided.
Owner:ASAHI KASEI PHARMA
Inhibitors and methods of use thereof
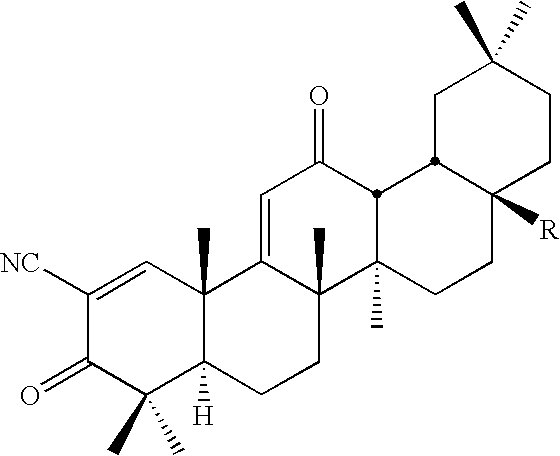
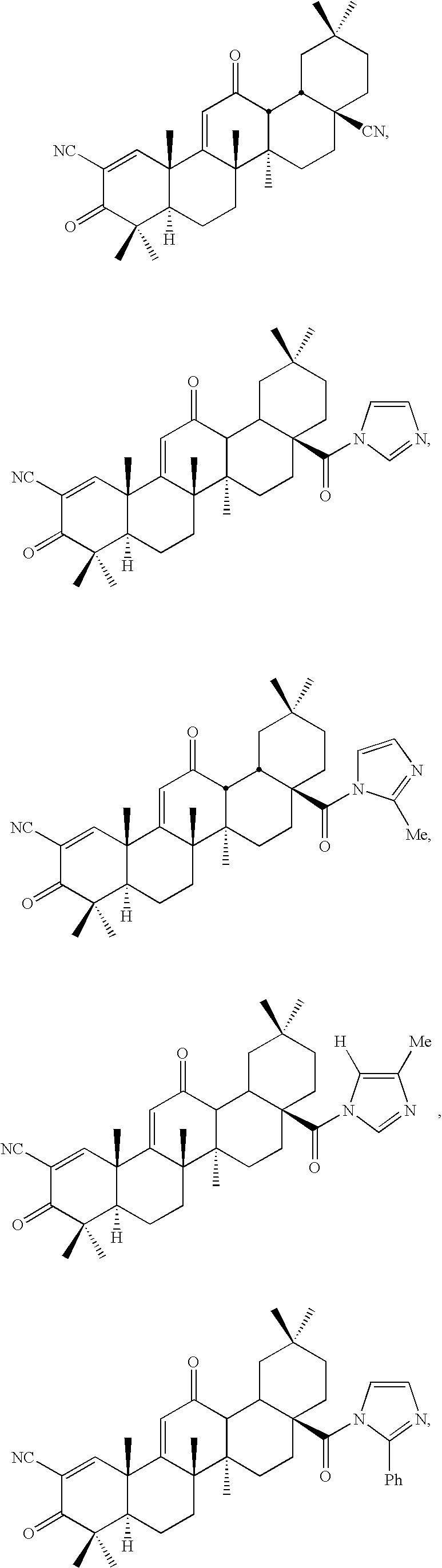
New triterpenoid derivatives with various substituents at the C-17 position of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were synthesized. Among them, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (CNDDO), 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl) imidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-2-methylimidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-4-methylimidazole show extremely high inhibitory activity (IC50=0.01-1 pM level) against production of nitric oxide induced by interferon-γ in mouse macrophages. These compounds can be used in the prevention or treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, multiple sclerosis, rheumatoid arthritis, and other inflammatory diseases. All the new triterpenoid derivatives are more potent than previously known CDDO.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Fully human antibody Fab fragments with human interferon-gamma neutralizing activity
InactiveUS7084257B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsDNA-binding domainAntigen binding
Selective binding agents of interferon-gamma (IFNγ) are provided by the invention. More particularly, the invention provides for antibodies and antigen binding domains which selectively bind to IFNγ and may be used to prevent or treat conditions relating to autoimmune and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis. Nucleic acid molecules encoding said antibodies and antigen binding domains, and expression vectors and host cells for the production of same are also provided.
Owner:AMGEN INC
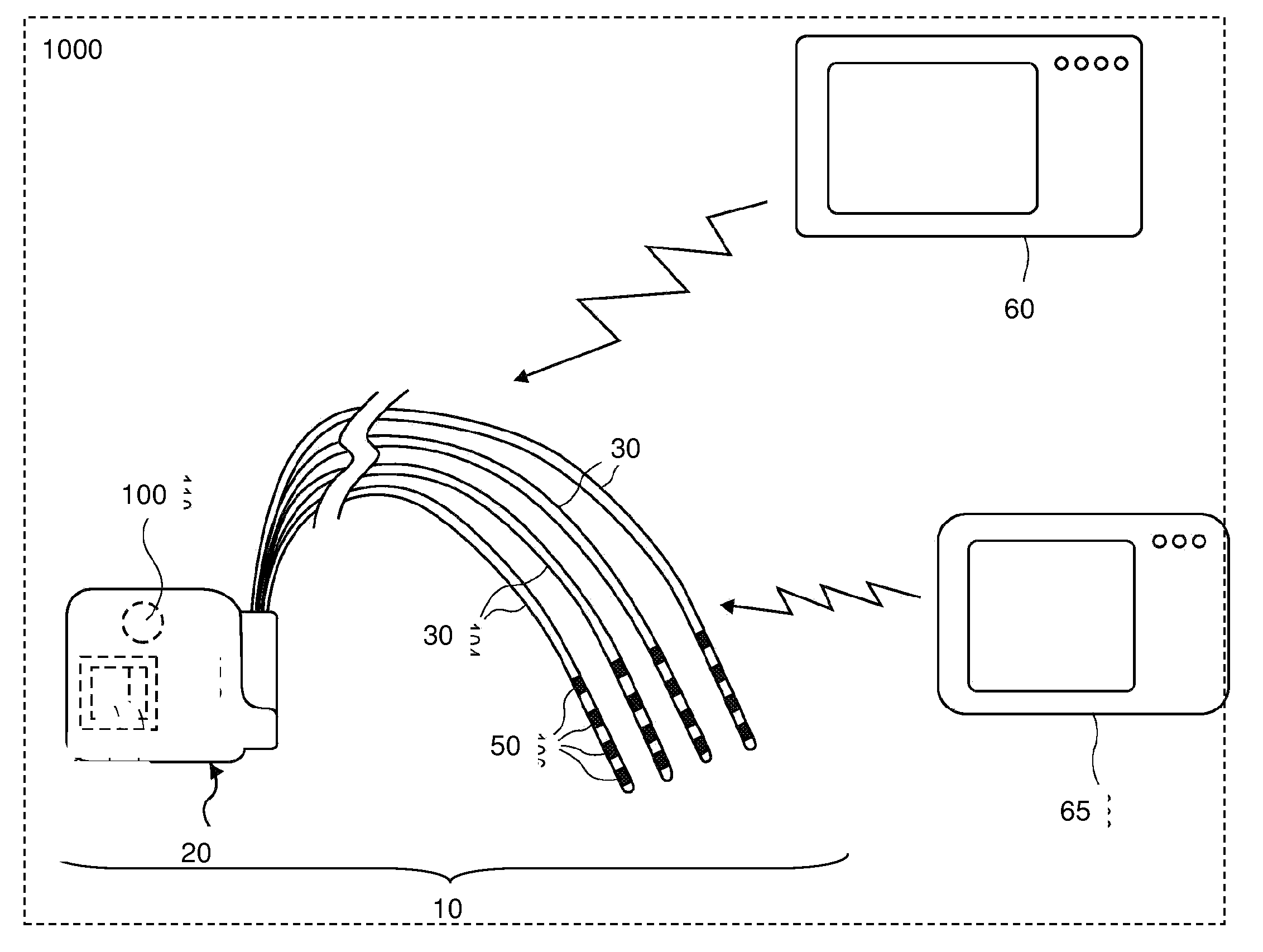
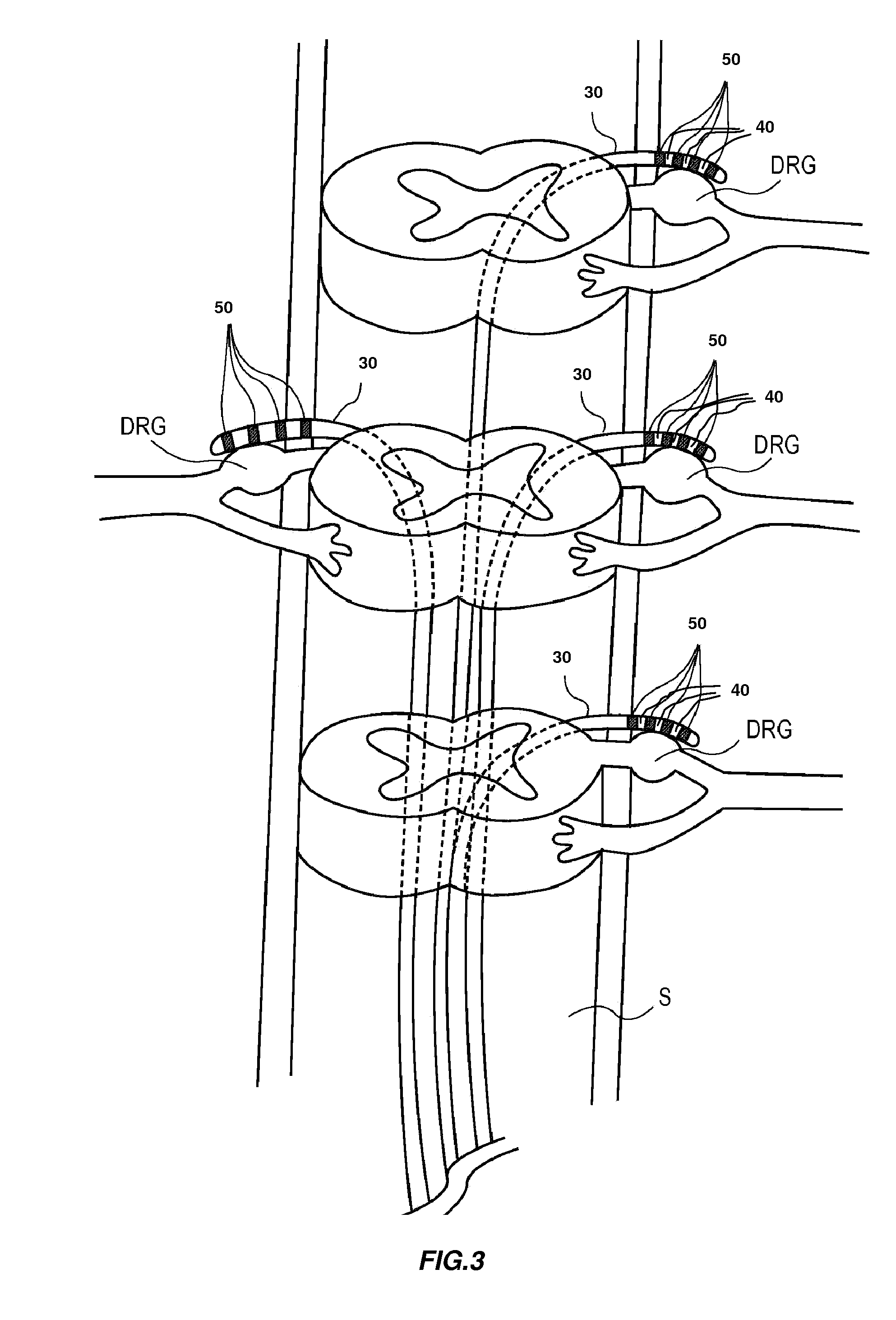
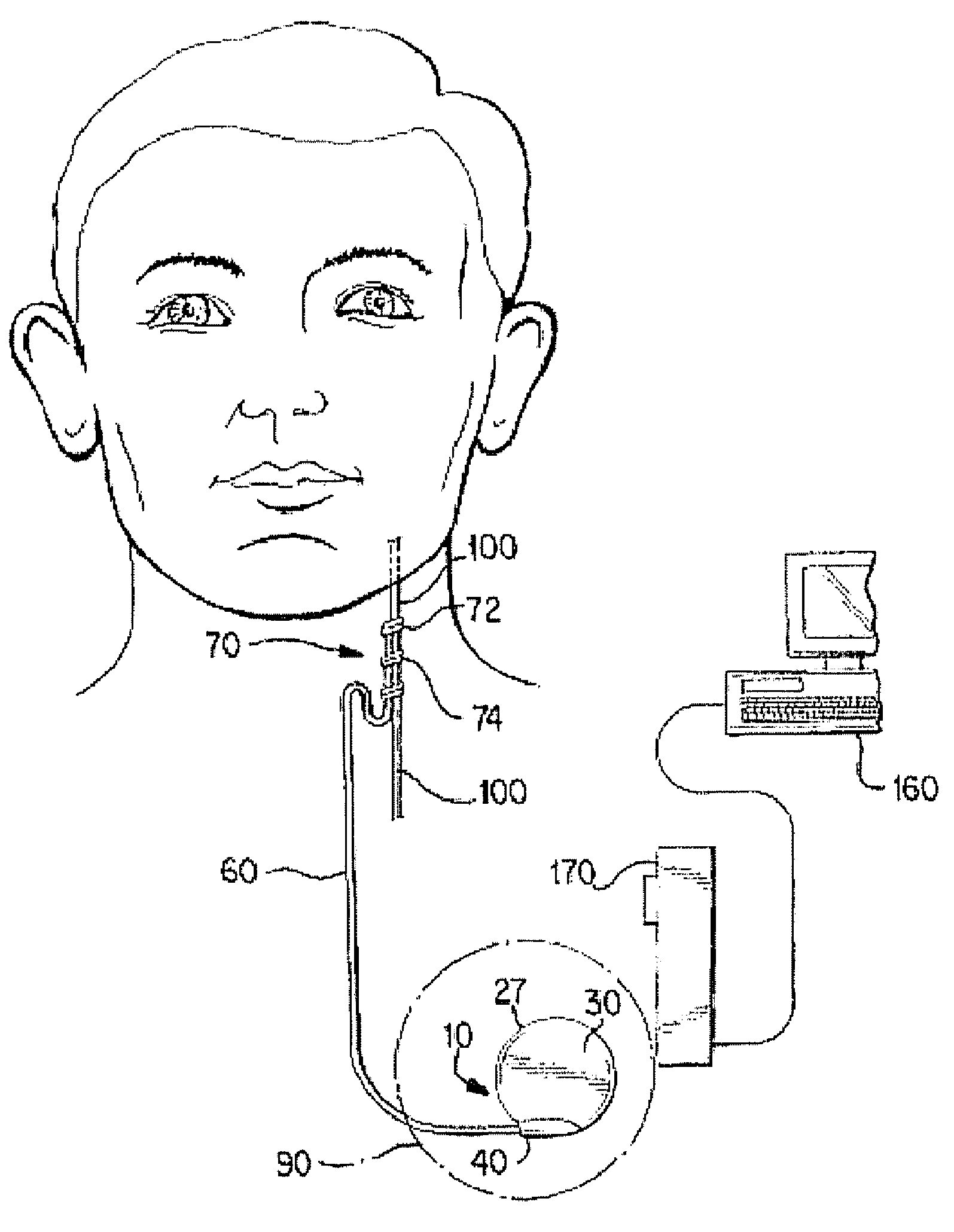
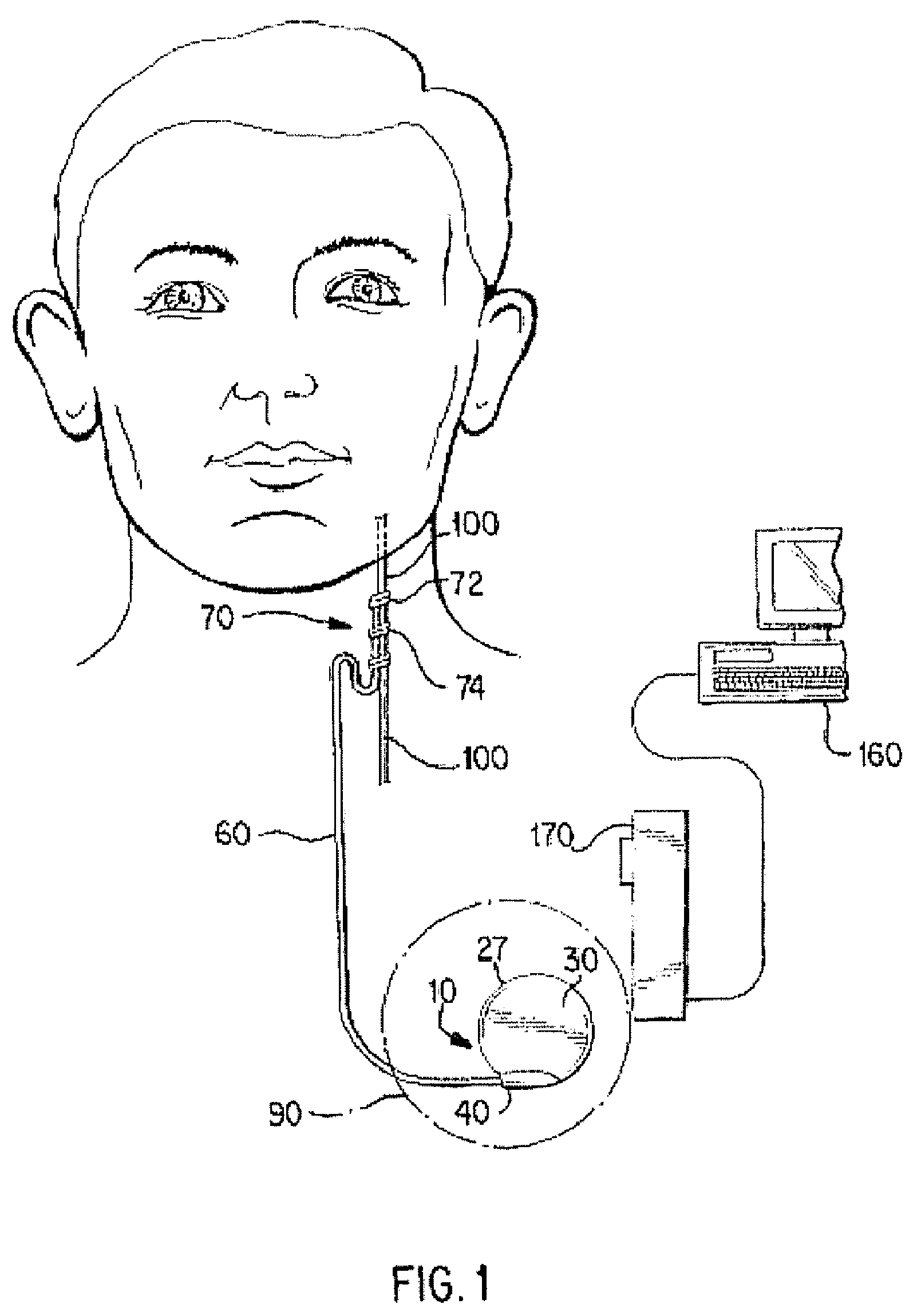
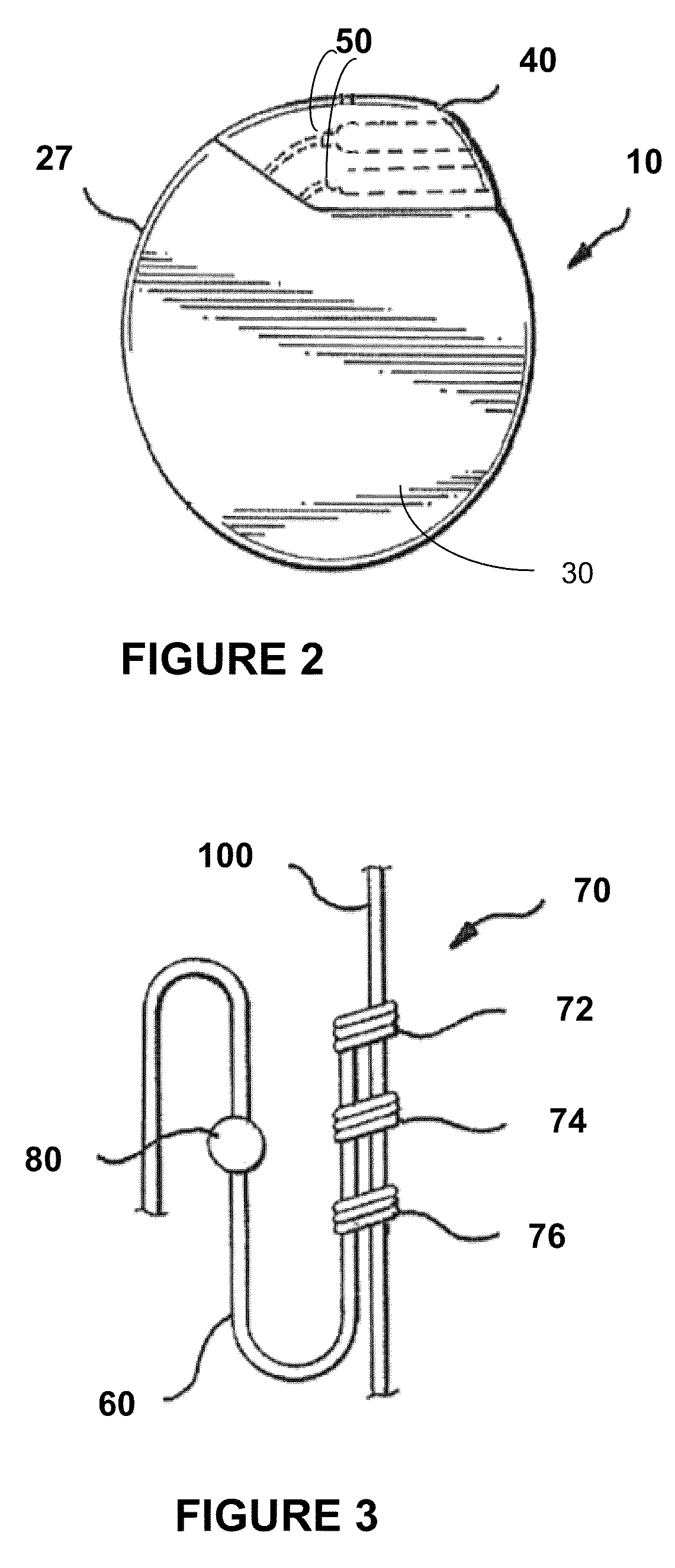
Directed delivery of agents to neural anatomy
InactiveUS20120310140A1Effective pain managementAdequate pain reliefSpinal electrodesPharmaceutical delivery mechanismDiseaseAutomatic control
The present invention is directed generally to systems, devices and methods for direct delivery of agents, e.g., pharmaceutical agents, to target spinal and neuronal anatomies, e.g., the dorsal root ganglia (DRG), for the treatment of various disorders, particularly pain and pain related disorders, such as chronic itch, sensory disorders, multiple sclerosis, post-herpetic neuralgia and the like. The system, devices and methods of the invention encompass the agents to be delivered to the target anatomy alone or in combination with electrical stimulation. The delivery device and systems and methods as disclosed herein place the distal end of the delivery element, which comprises at least one agent delivery structure, and optionally at least one electrode, in close proximity, or in contact with or next to the target spinal anatomy, e.g., DRG. A variety of agents can be delivered using the device, including sodium channel blockers, biologics, neuroinflammatory modulators, toxins etc., to selectively neuromodulate the neurons. Agent delivery and / or electrical stimulation can be automated and / or can be controlled automatically or by a pre-determined program, or by a patient control pump (PCA).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
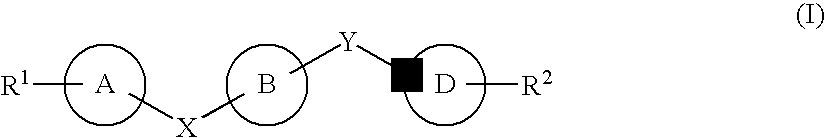
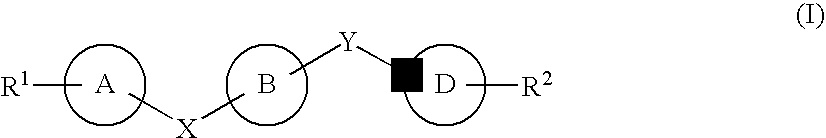
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
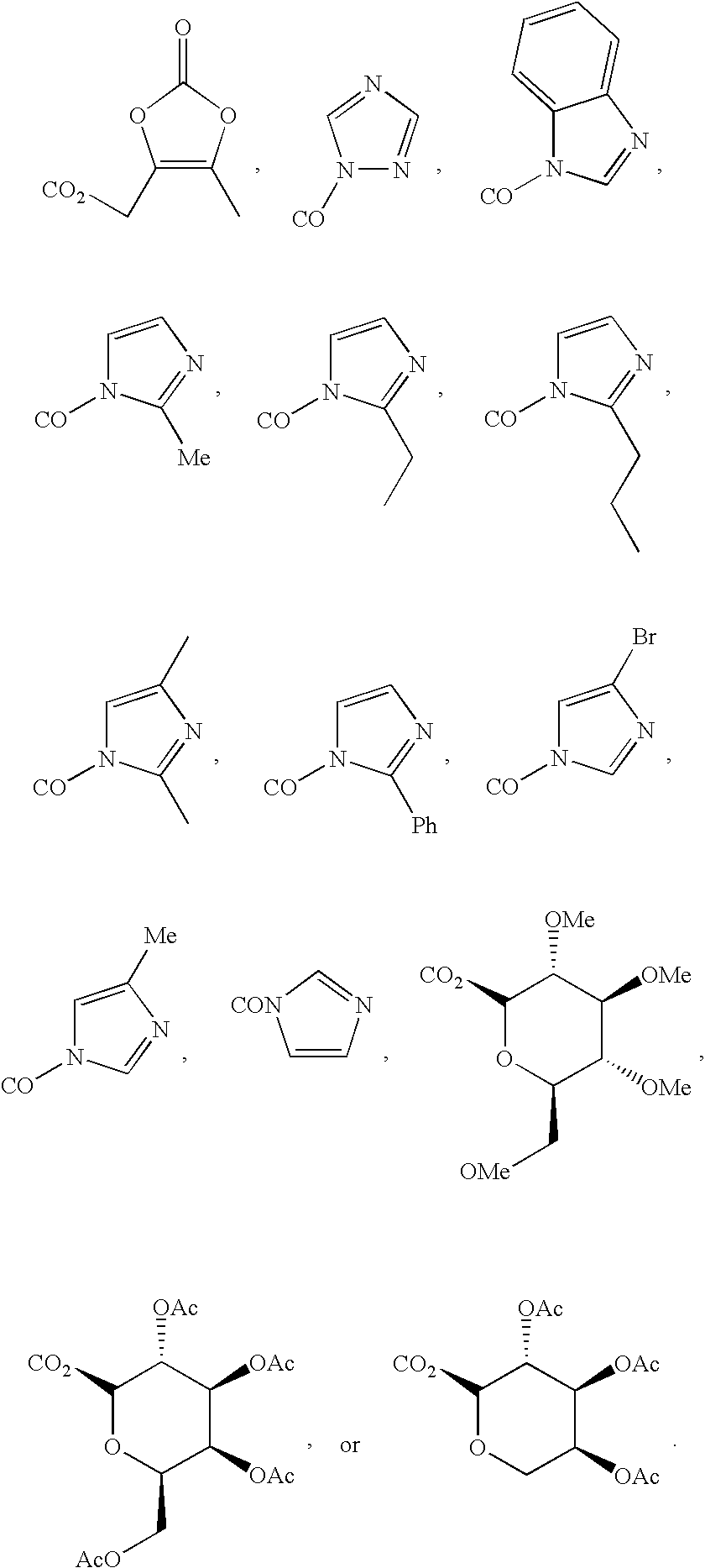
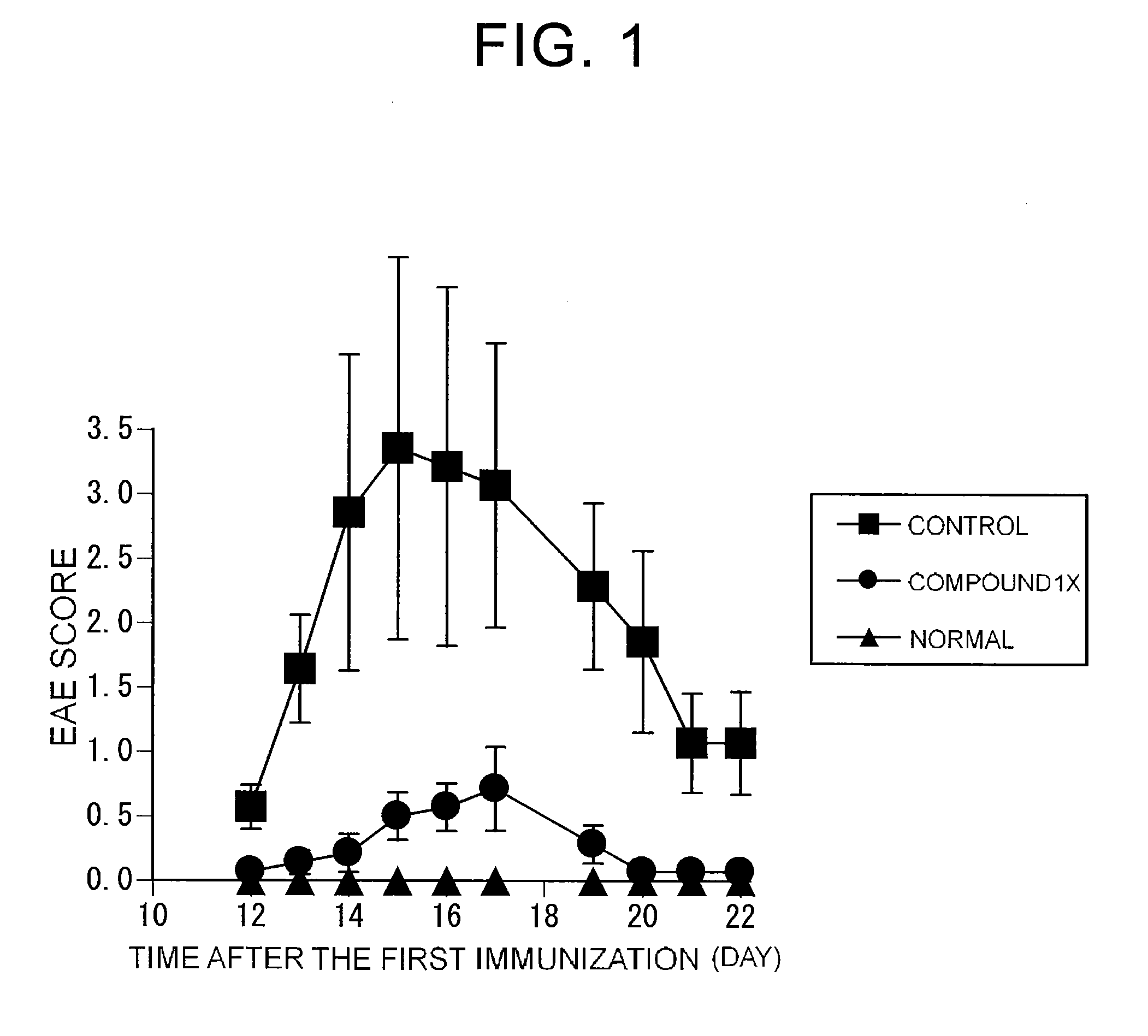
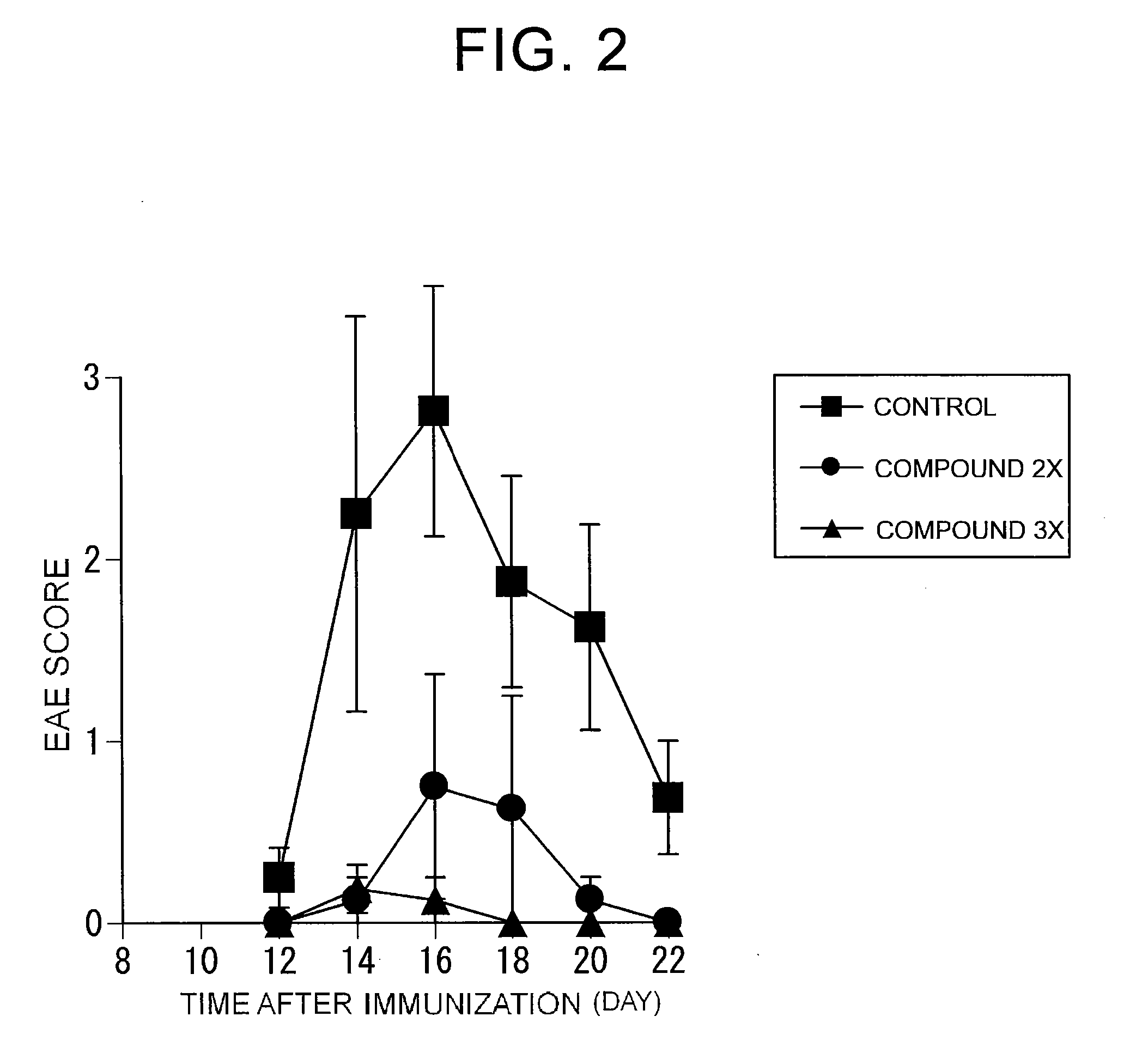
Preventive or therapeutic agents for multiple sclerosis
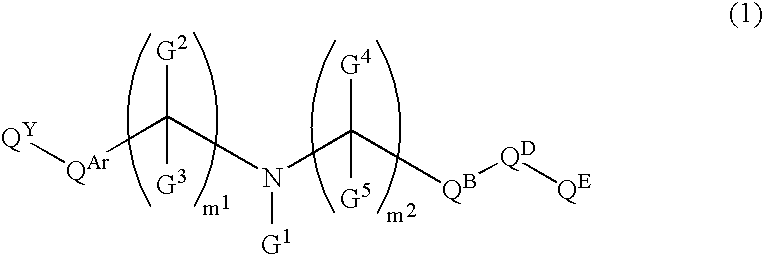
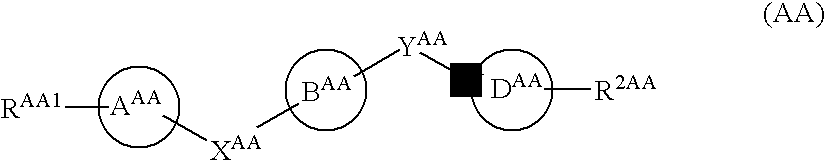
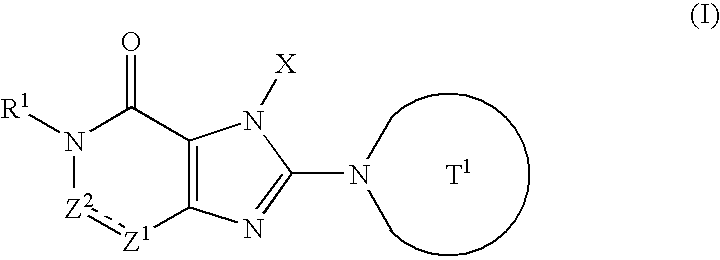
The preventive or therapeutic agents of the present invention for multiple sclerosis comprise compounds represented by the following formula (I), or salts or hydrates thereof, [wherein, T1, X, Z1, Z2, and R1 have the same meaning as T1, X, Z1, Z2, and R1 in this application].
Owner:EISIA R&D MANAGEMENT CO LTD
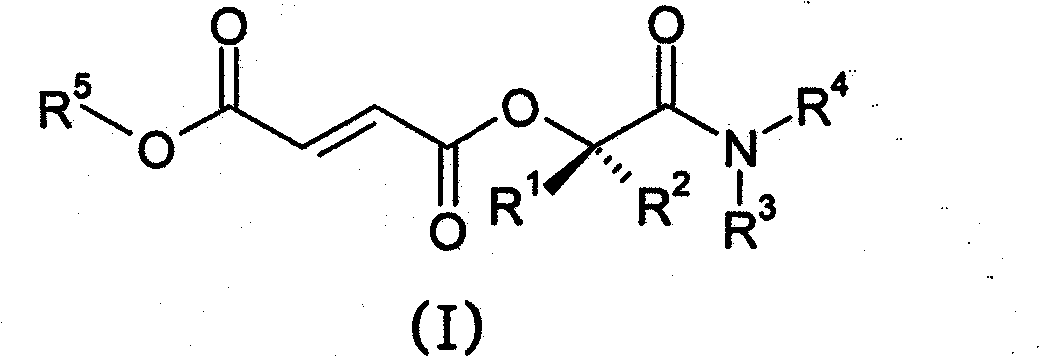
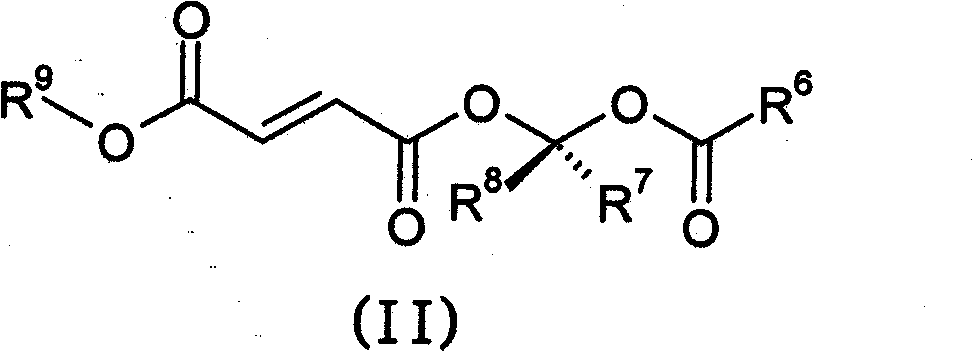
Prodrugs of fumarates and their use in treating various diseases
The present invention provides compounds of formula (I),wherein:R1 is unsubstituted C1-C6 alkyl;La is substituted or unsubstituted C1-C6 alkyl linker, substituted or unsubstituted C3-C10 carbocycle, substituted or unsubstituted heterocycle comprising one or two 5- or 6-member rings and 1-4 heteroatoms selected from N, O and S, or substituted or unsubstituted heteroaryl comprising one or two 5- or 6-member rings and 1-4 heteroatoms selected from N, O and S; andR2 and R3 are each, independently, H, substituted or unsubstituted C1-C6 alkyl, or substituted or unsubstituted C6-C10 aryl;or alternatively, R2 and R3, together with the nitrogen atom to which they are attached, form a substituted or unsubstituted heteroaryl comprising one or two 5- or 6-member rings and 1-4 heteroatoms selected from N, O and S or a substituted or unsubstituted heterocycle comprising one or two 5- or 6-member rings and 1-4 heteroatoms selected from N, O and S.The invention also provides pharmaceutical compositions and methods for treating neurological diseases, such as multiple sclerosis.
Owner:ALKERMES PHARMA IRELAND LTD
Methods for promoting nerve regeneration and neuronal growth and elongation
ActiveUS20070239080A1Increase amplitudeUltrasonic/sonic/infrasonic diagnosticsChiropractic devicesNervous systemRisk stroke
A method of enhancing the regeneration of injured nerves has the step of administering an effective exposure of pressure pulses or acoustic shock waves in a pulse or wave pattern to the zone of injury of the nerve during the regeneration process. The inventive method may include enhancing the stimulation of neuronal cell growth or regeneration by administering an effective exposure of pressure pulses or acoustic shock waves in a pulse or wave pattern to stimulate neuronal cell growth or regeneration, wherein the administering of the treatment is applied to a patient who has a pathological condition where neuronal repair can be facilitated including peripheral nerve damage caused by injury or disease such as diabetes, brain damage associated with stroke, and for the treatment of neurological disorders related to neurodegeneration, including Parkinson's disease, Alzheimer's disease and amyotrophic lateral, sclerosis multiple sclerosis and disseminated sclerosis. The treatment is ideally suited for neural regeneration after a degenerative condition due to any neurological infections or any other pathological condition.
Owner:SOFTWAVE TISSUE REGENERATION TECH LLC
Topical treatments for pain
InactiveUS20130029989A1Increase blood flowGood analgesic effectBiocideNervous disorderPhosphodiesteraseNitric oxide
Owner:MCGILL UNIV
Compositions and methods for treating or preventing inflammatory diseases
Methods and compositions for treating or preventing inflammatory diseases such as psoriasis or multiple sclerosis are provided, comprising the step of delivering to the site of inflammation an anti-microtubule agent, or analogue or derivative thereof.
Owner:ANGIOTECH INT AG (CH)
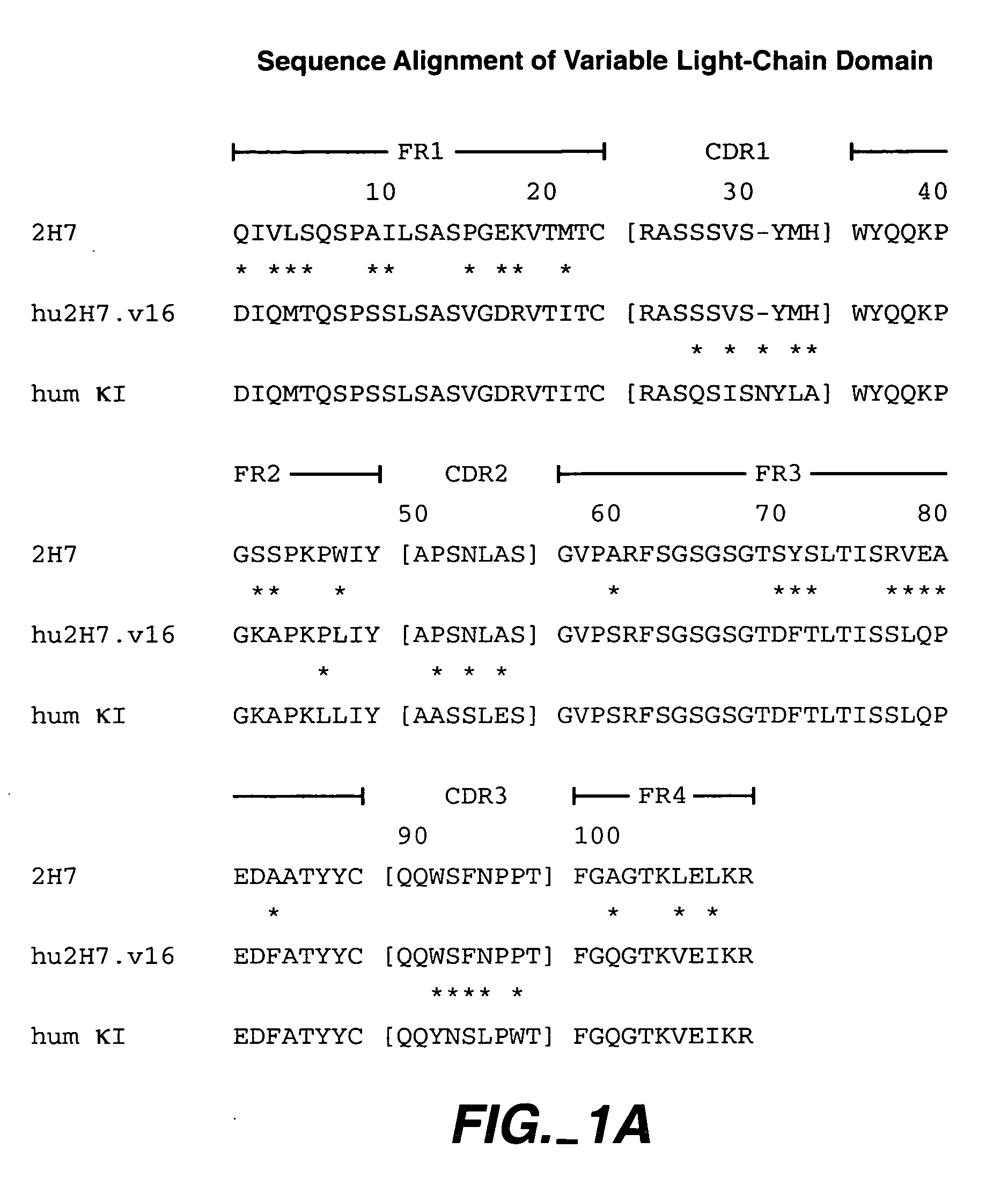
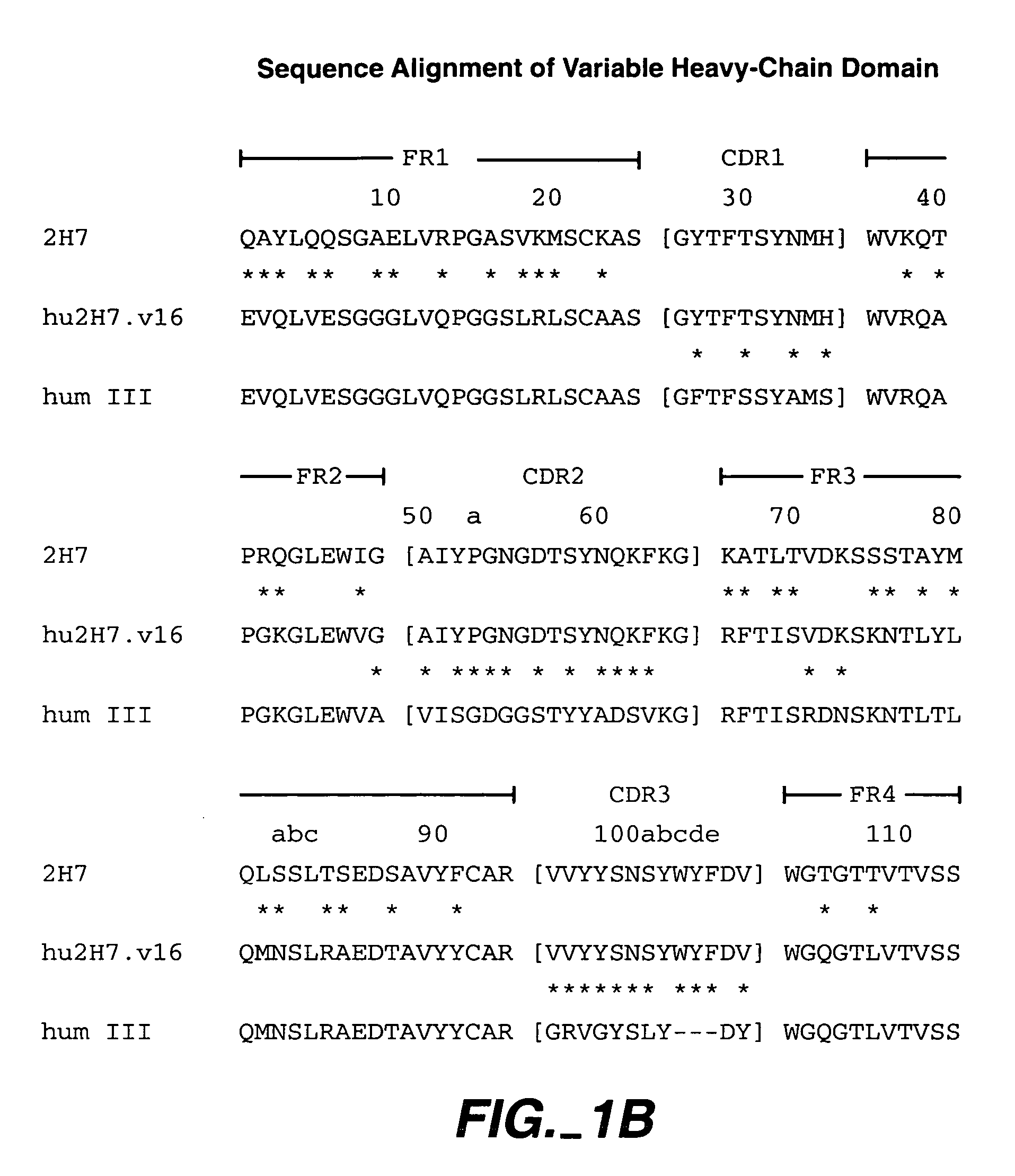
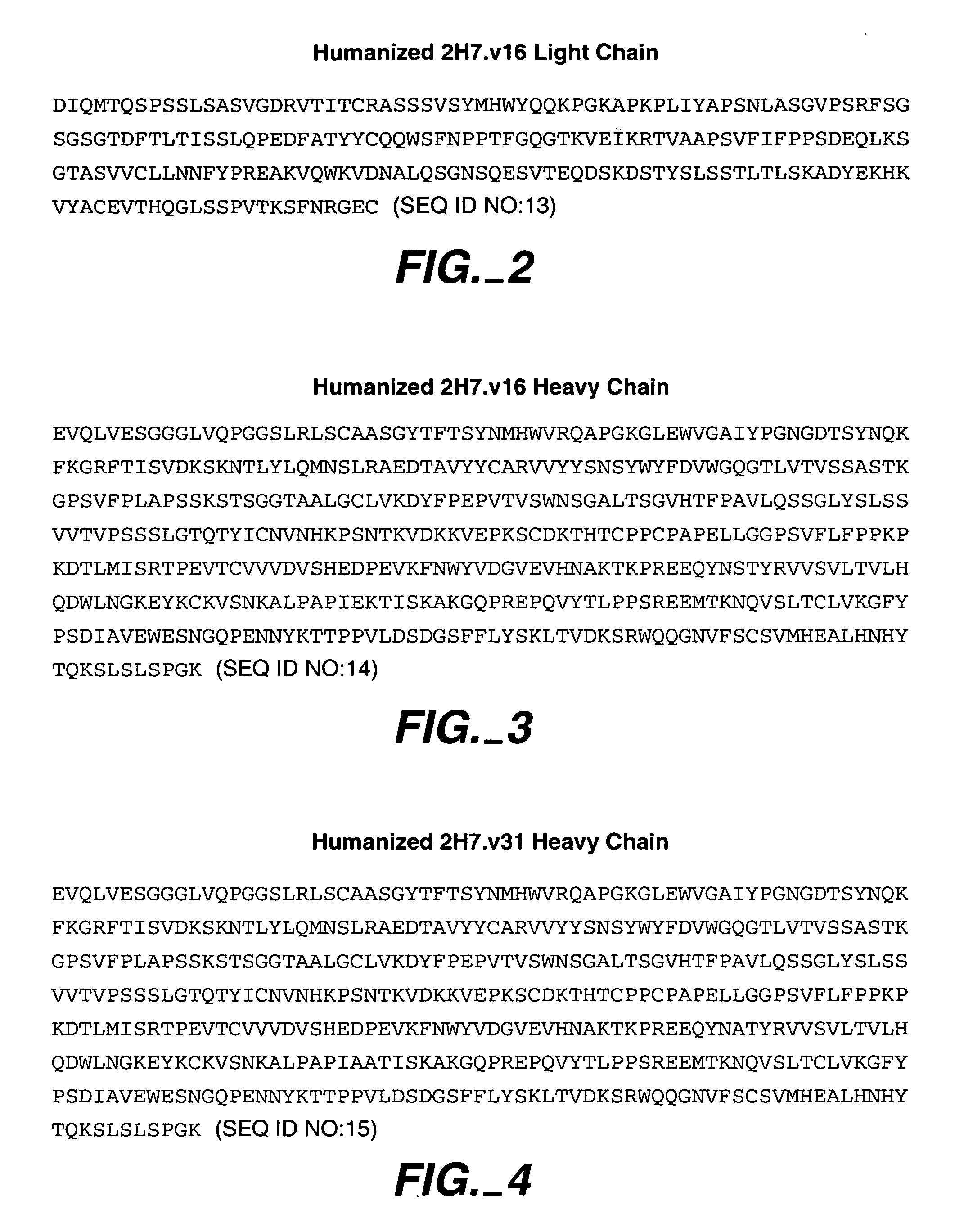
Method for treating multiple sclerosis
Methods for treating multiple sclerosis (MS) with a CD20 antibody using special dosing regimens and protocols are described. Articles of manufacture for use in such methods are also described.
Owner:GENENTECH INC
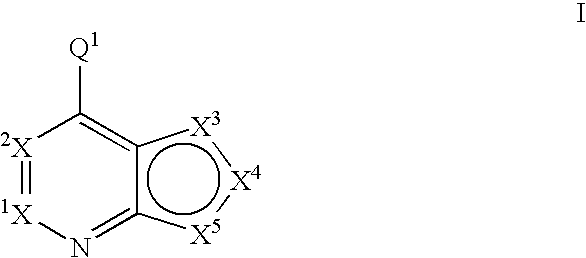
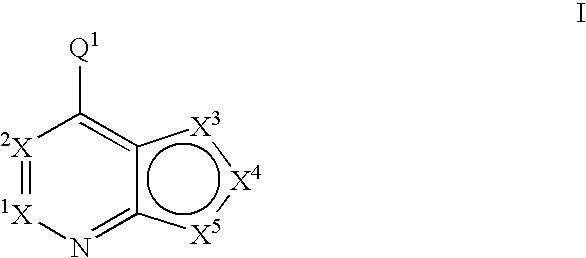
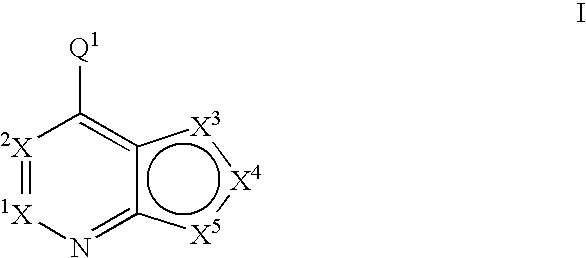
Fused heterobicyclic kinase inhibitors
InactiveUS20070208053A1Low toxicityDisrupting virus life-cycleAntibacterial agentsBiocideNervous systemAllergy
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X5, X7, R1, and Q1 are defined herein, inhibit kinase enzymes and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer. The compounds are also useful in the treatment of inflammation, allergy, asthma, disease and conditions of the immune system, disease and conditions of the nervous system, cardiovascular diseases, disease and conditions of the eye, dermatological diseases, osteoporosis, diabetes, multiple sclerosis, and infections.
Owner:OSI PHARMA INC
Therapeutic compositions and methods of use
Compounds and methods useful for chemopreventative treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, inflammatory bowel diseases, and multiple sclerosis.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Treatment of disease states and adverse physiological conditions utilizing anti-fungal compositions
InactiveUS20060177424A1Effectively ameliorateEffective regulationBiocideBacteria material medical ingredientsSinusitisVaginal Yeast Infections
A method for treatment or prophylaxis of a disease state or other physiological condition, e.g., autism, delayed development, acid reflux disease, vaginal yeast infections, impaired hearing, chronic ear infections, seasonal allergies, Fibromyalgia syndrome, Crohn's disease, colitis, irritable bowel syndrome, interstitial cystitis, acne, sinusitis, rheumatoid arthritis, chronic fatigue syndrome, asthma, attention deficit disorder, attention deficit / hyperactivity disorder, rosacea, multiple sclerosis, hyperglycemia, or Ménière's disease, by administration of an anti-fungal composition that includes at least one of the bacilli (1) Bacillus subtilis, (2) Lactobacillus sporogenes, and (3) Streptococcus faecalis.
Owner:COBB AND CO
Therapeutic compositions and methods of use
Compounds and methods useful for chemopreventative treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, inflammatory bowel diseases, and multiple sclerosis.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Compositions and methods for treating or preventing inflammatory diseases
Methods and compositions for treating or preventing inflammatory diseases such as psoriasis or multiple sclerosis are provided, comprising the step of delivering to the site of inflammation an anti-microtubule agent, or analogue or derivative thereof.
Owner:ANGIOTECH INT AG (CH)
Non-invasive treatment of neurodegenerative diseases
ActiveUS8868177B2Inhibit inflammationOffset and reduce effectElectrotherapySurgeryPostoperative cognitive dysfunctionNeuro-degenerative disease
Methods and devices for the non-invasive treatment of neurodegenerative diseases through delivery of energy to target nervous tissue, particularly the vagus nerve. In certain embodiments, the devices include a magnetic stimulator having coils with toroidal windings, which are in contact with an electrically conducting medium that is adapted to conform to the contour of a target body surface of a patient. The coils induce an electric current and / or an electric field within the patient, thereby stimulating nerve fibers within the patient. The stimulation brings about reduction of neuroinflammation in patients suffering from conditions comprising Alzheimer's Disease, Parkinson's Disease, Multiple Sclerosis, postoperative cognitive dysfunction and postoperative delirium.
Owner:ELECTROCORE
Multimeric VLA-4 antagonists comprising polymer moieties
InactiveUS20060013799A1Minimize degradationSenses disorderNervous disorderLymphatic SpreadWhite blood cell
Disclosed are conjugates which bind VLA-4. Certain of these conjugates also inhibit leukocyte adhesion and, in particular, leukocyte adhesion mediated by VLA-4. Such conjugates are useful in the treatment of inflammatory diseases in a mammalian patient, e.g., human, such as asthma, Alzheimer's disease, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, tissue transplantation, tumor metastasis and myocardial ischemia. The conjugates can also be administered for the treatment of inflammatory brain diseases such as multiple sclerosis.
Owner:ELAN PHARM INC
Substituted aryl amides
Novel compounds of structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as psychotropic drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as, the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK & CO INC
Use of glutaminyl cyclase inhibitors
ActiveUS20090068699A1BiocideOrganic active ingredientsMild cognitive impairment (MCI)Percent Diameter Stenosis
An inhibitor of a glutaminyl peptide cyclotransferase, and use thereof for the treatment and / or prevention of a disease or disorder selected from the group consisting of inflammatory diseases selected froma. neurodegenerative diseases, e.g. mild cognitive impairment (MCI), Alzheimer's disease, neurodegeneration in Down Syndrome, Familial British Dementia, Familial Danish Dementia, multiple sclerosis,b. chronic and acute inflammations, e.g. rheumatoid arthritis, atherosclerosis, restenosis, pancreatitis,c. fibrosis, e.g. lung fibrosis, liver fibrosis, renal fibrosis,d. cancer, e.g. cancer / hemangioendothelioma proliferation, gastric carcinomas,e. metabolic diseases, e.g. hypertension,f. and other inflammatory diseases, e.g. neuropathic pain, graft rejection / graft failure / graft vasculopathy, HIV infections / AIDS, gestosis, tuberous sclerosis.Additionally disclosed are a respective diagnostic method, assay and kit.
Owner:VIVORYON THERAPEUTICS NV
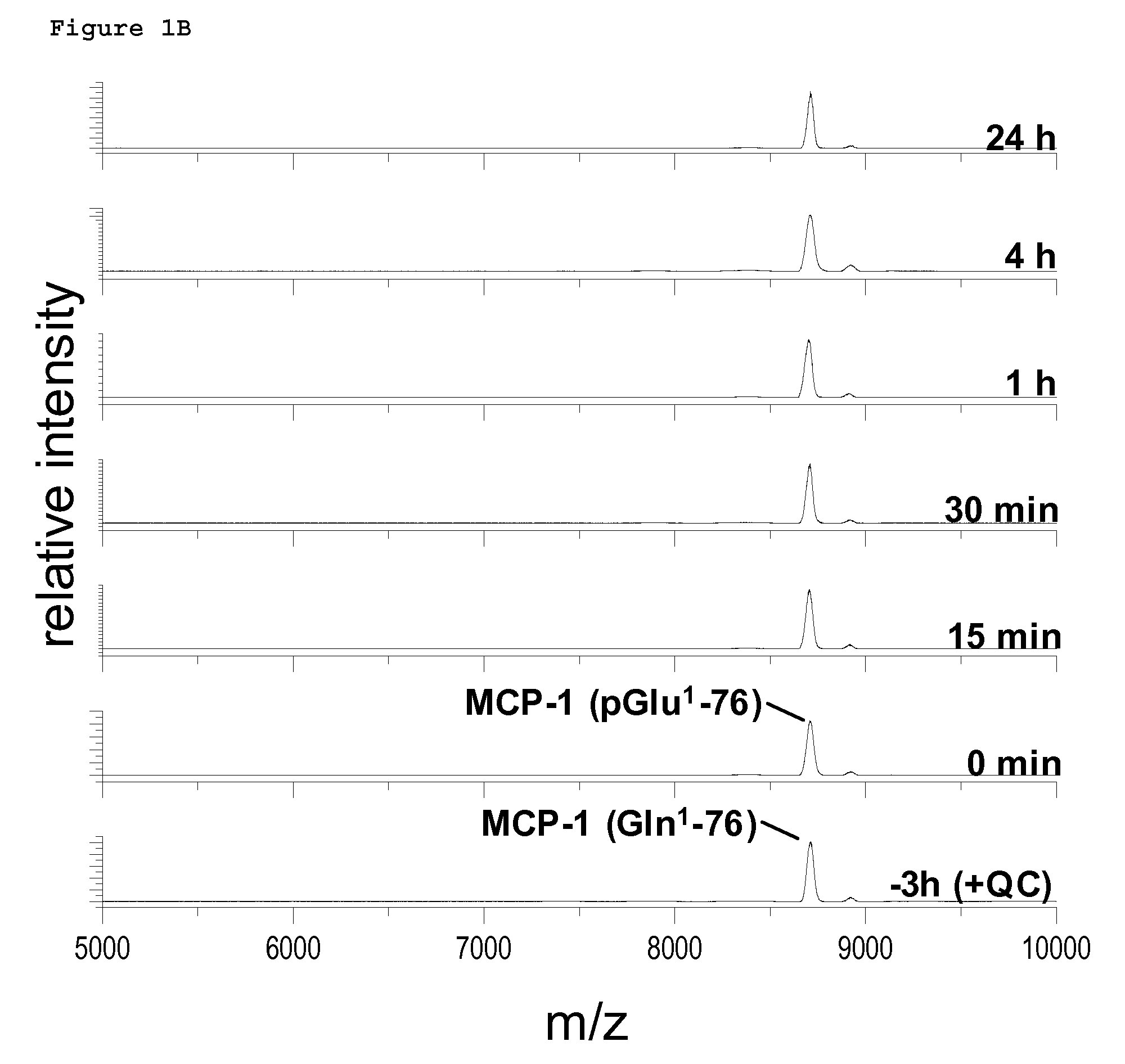
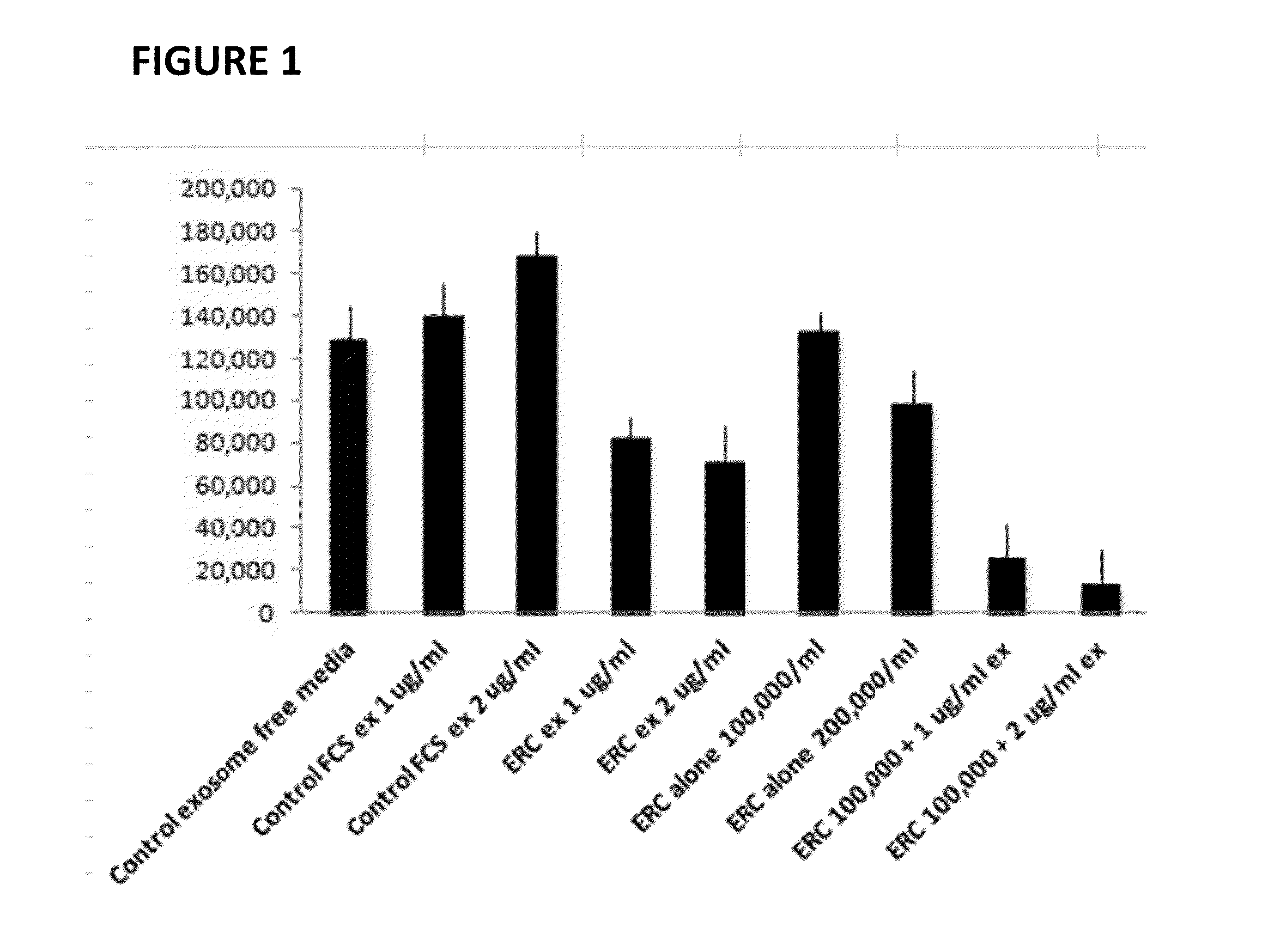
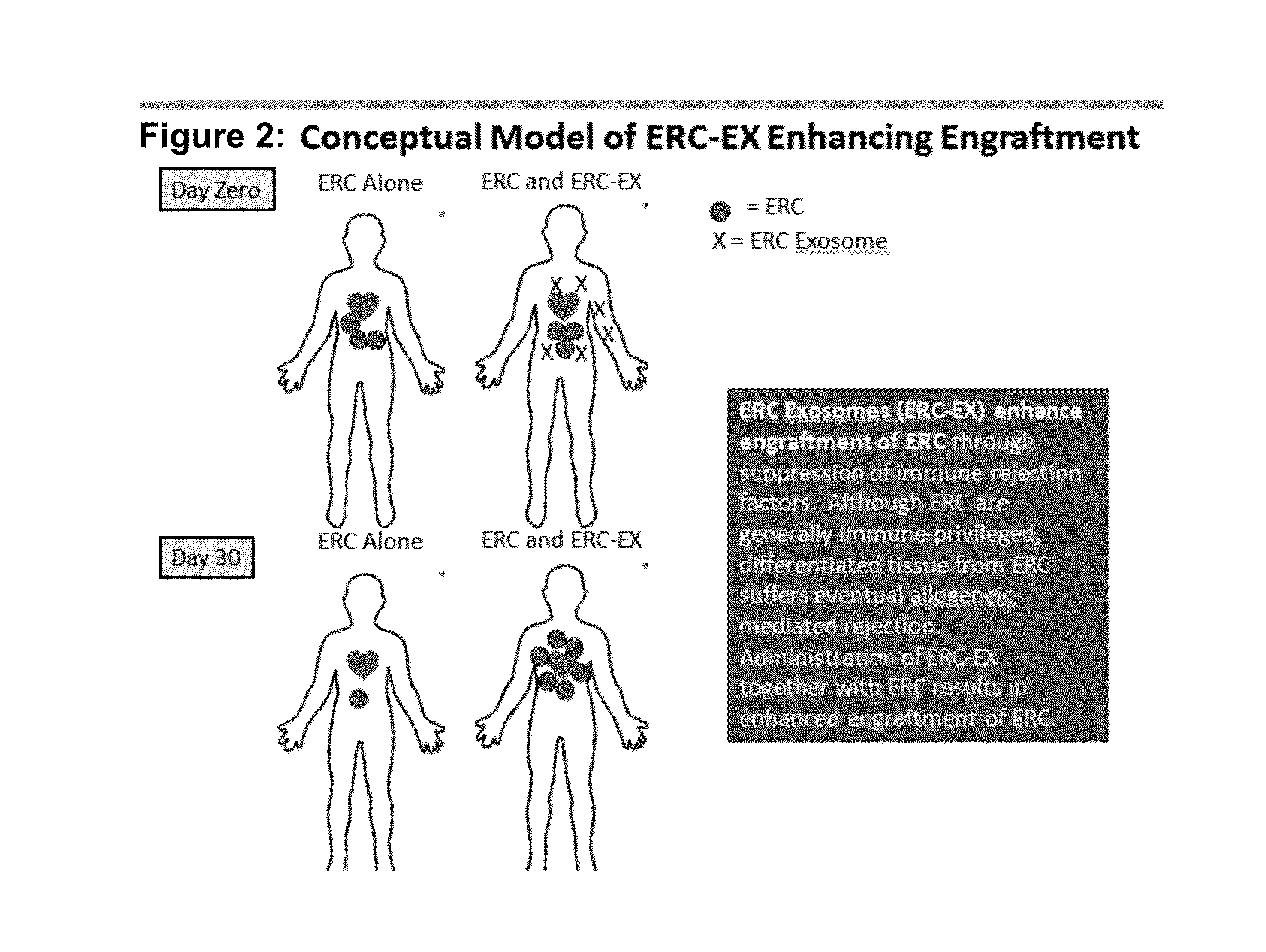
Therapeutic immune modulation by stem cell secreted exosomes
ActiveUS20130195899A1Inhibit inflammationReduce the amount of solutionGenetic material ingredientsSnake antigen ingredientsAutoimmune conditionEndometrium
Disclosed are methods, compositions of matter, and protocols useful for the induction of a therapeutic immune modulatory response through administration of exosomes derived from a stem cell source. In one embodiment, said stem cell source is endometrial regenerative cells. Specifically, in one embodiment stem cell derived exosomes are used as a method of treating an autoimmune condition such as rheumatoid arthritis, multiple sclerosis, or systemic lupus erythromatosis.
Owner:XON CELLS
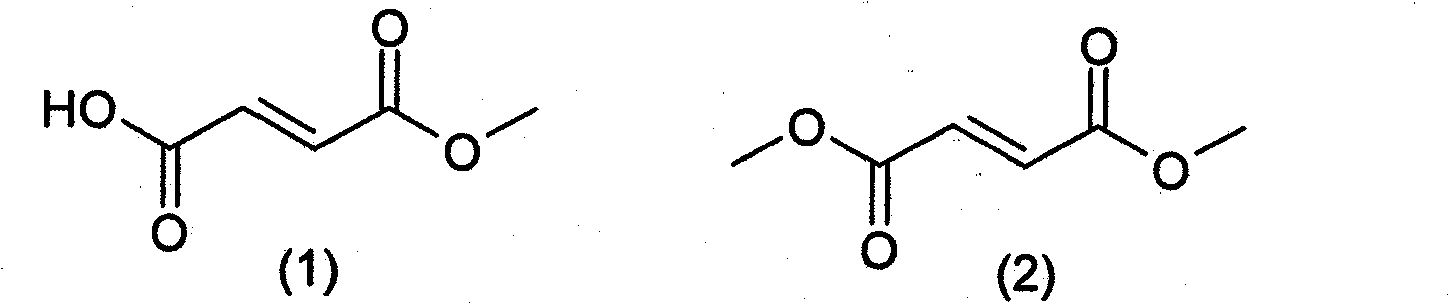
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use
InactiveCN102123763AInhibition of TNF functionNervous disorderOrganic chemistryDiseaseMS multiple sclerosis
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions comprising the prodrugs of methyl hydrogen fumarate, and methods of using the prodrugs of methyl hydrogen fumarate and the pharmaceutical compositions thereof for treating diseases such as psoriasis, asthma, multiple sclerosis, inflammatory bowel disease, and arthritis are disclosed.
Owner:XENOPORT
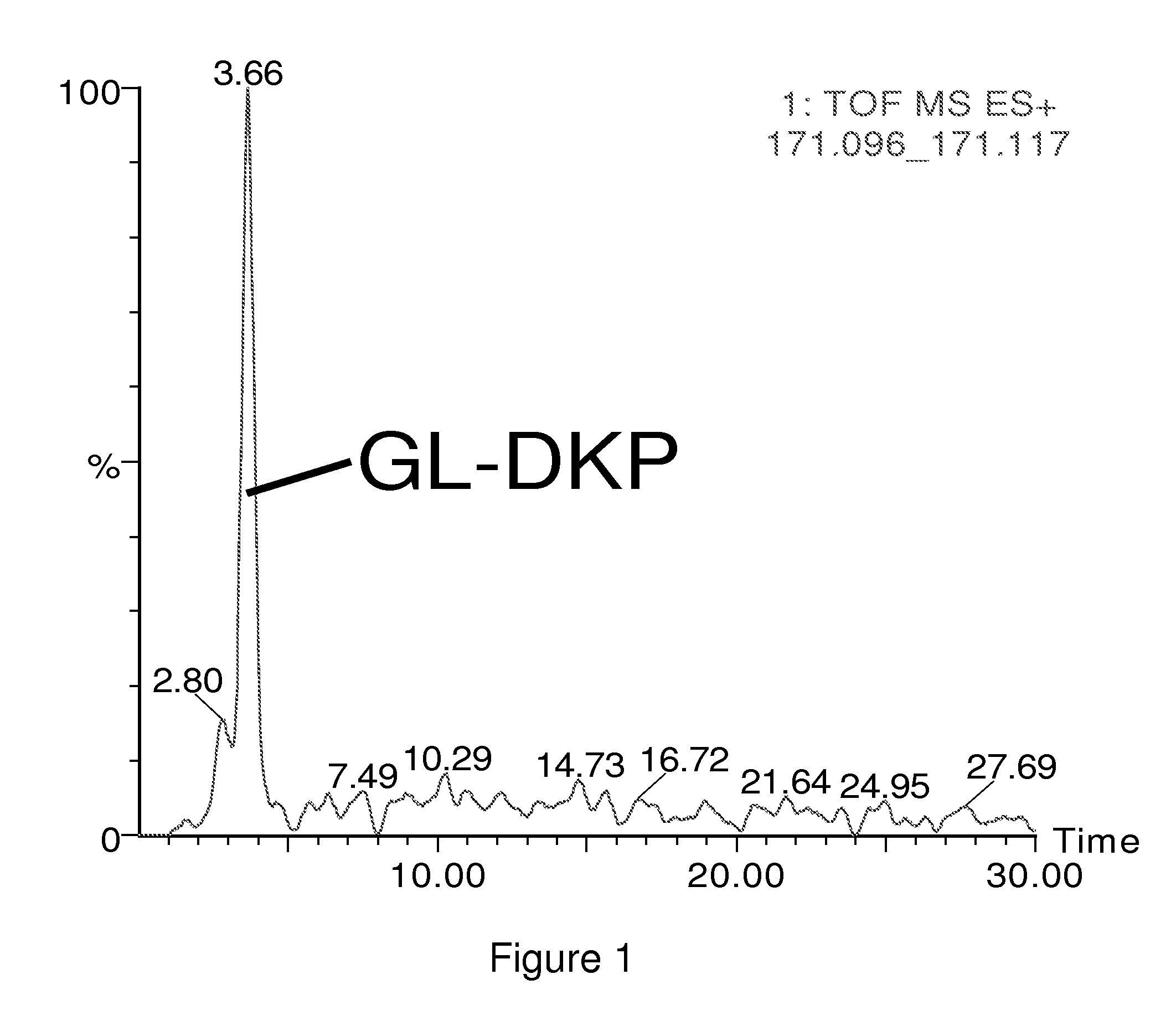
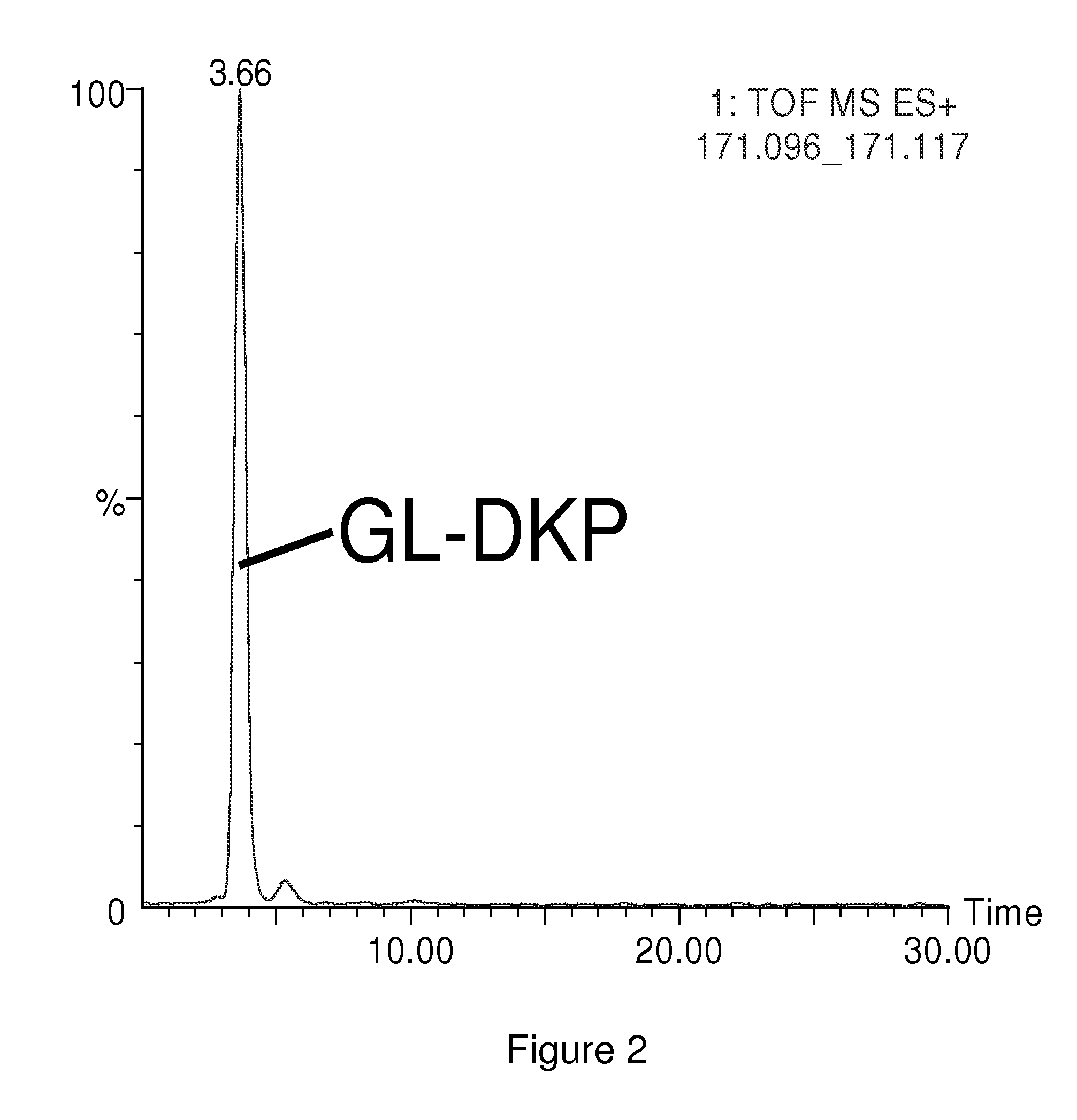
Diagnosis and monitoring of diseases
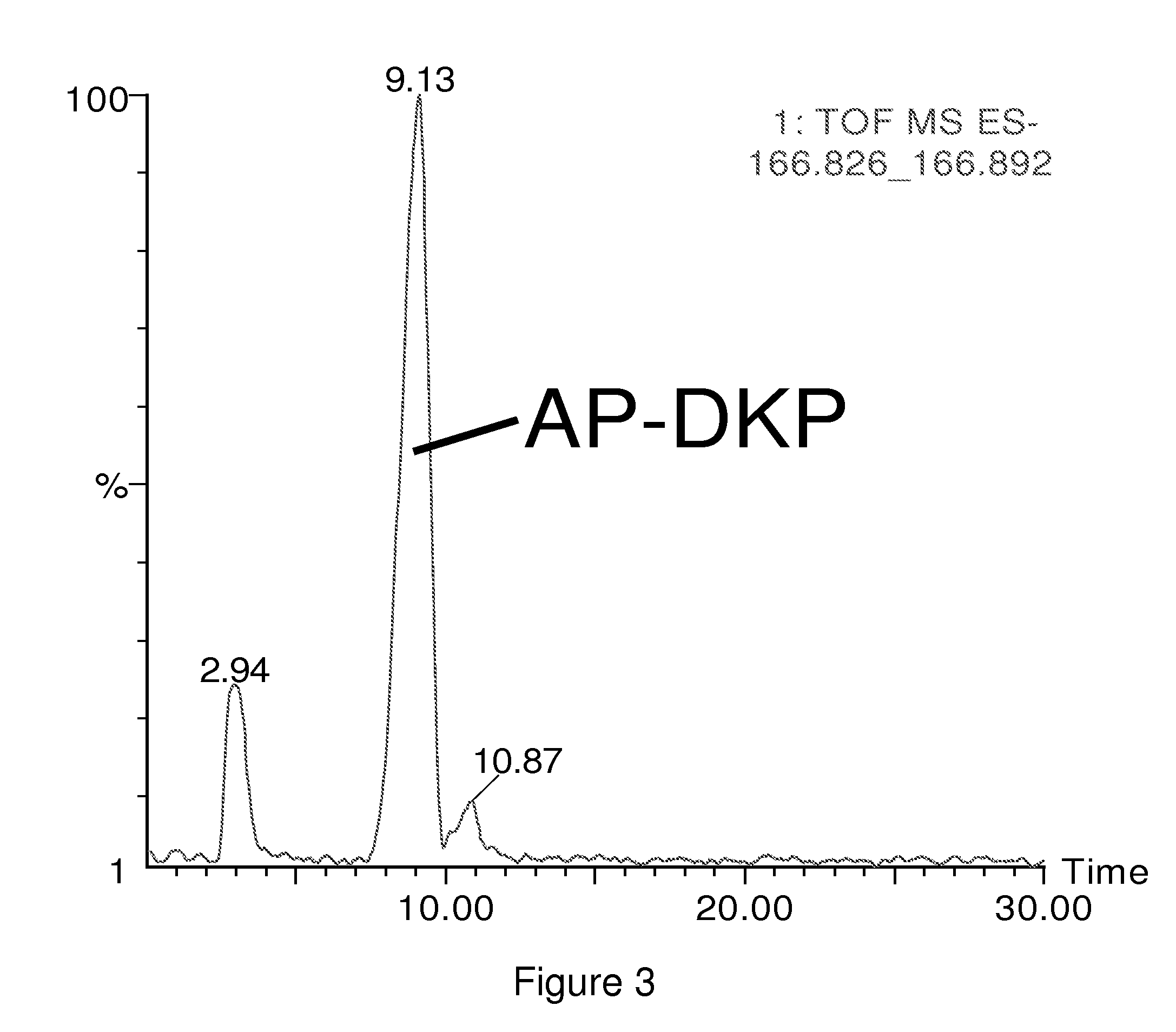
InactiveUS20100120056A1Measured rapidly and convenientlyEasy diagnosisBiocidePeptide/protein ingredientsDiketopiperazinesIschemia
The present invention relates to the diagnosis and monitoring of diseases and conditions by quantifying markers, including degradation products of disease-associated proteins, such as diketopiperazines composed of the two N-terminal amino acids or the two C-terminal amino acids of such proteins. The methods are useful for diagnosing or monitoring various diseases, including multiple sclerosis, Alzheimer's disease and ischemia. The invention further provides binding partners specific for the markers and compositions and kits for conducting the methods of the invention.
Owner:AYTU BIOSCI
Vagus nerve stimulation by electrical signals for controlling cerebellar tremor
A neurostimulator system for alleviating cerebellar tremor associated with multiple sclerosis, for instance, comprises a programmable electrical pulse generator. The programmable electrical pulse generator is programmed to generate electrical signals with the following parameters: a current magnitude of about 1 mA or less, a stimulation signal on-time to signal off-time ratio in the range of 2:1 to 1:1.8, signal on-times and off-times in the range of about 10 seconds to about 5 minutes, a signal frequency below 15 Hz, and a pulse width within the range of 50 μs to 300 μs. Other embodiments are disclosed and claimed.
Owner:CYBERONICS INC
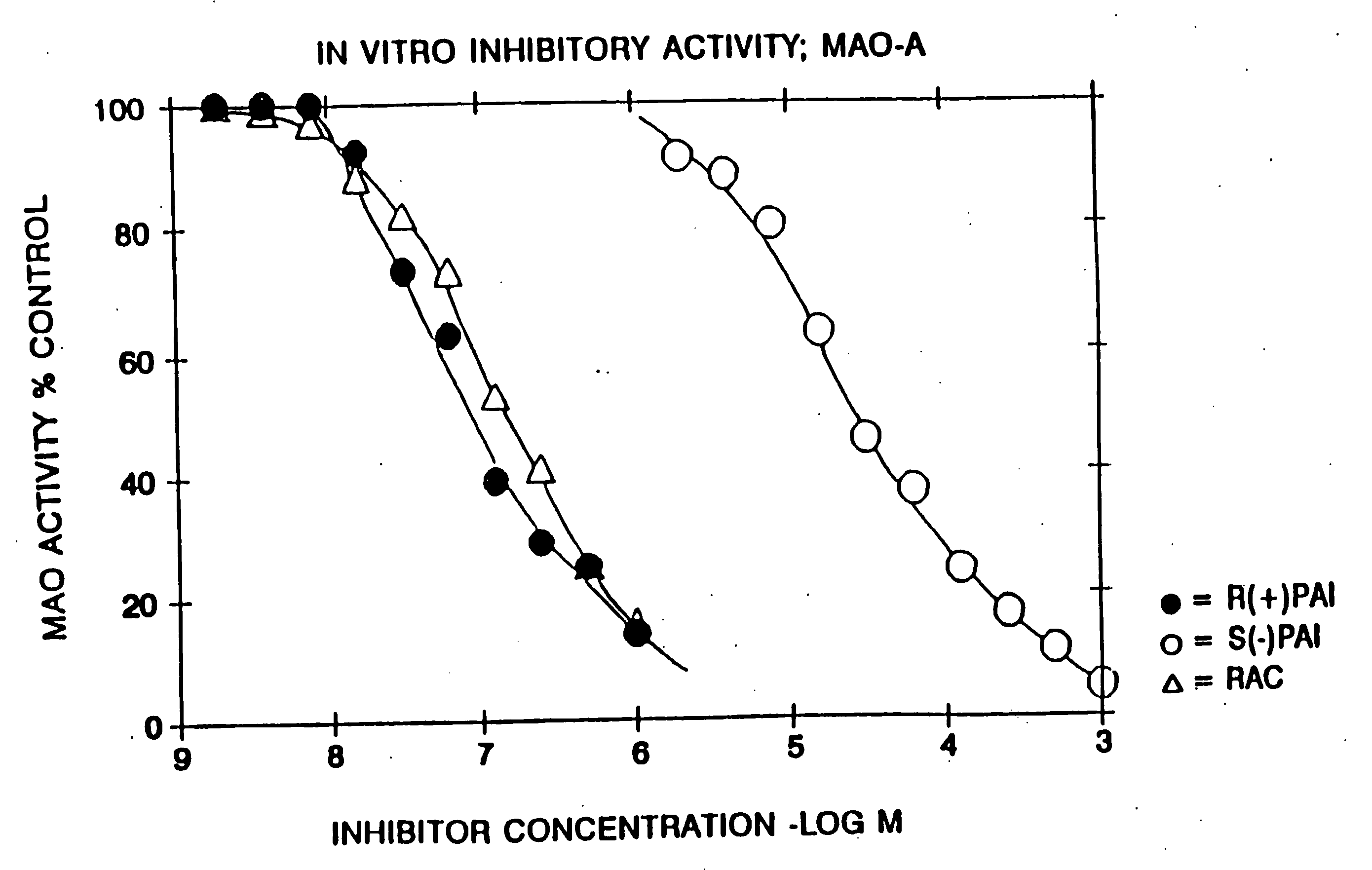
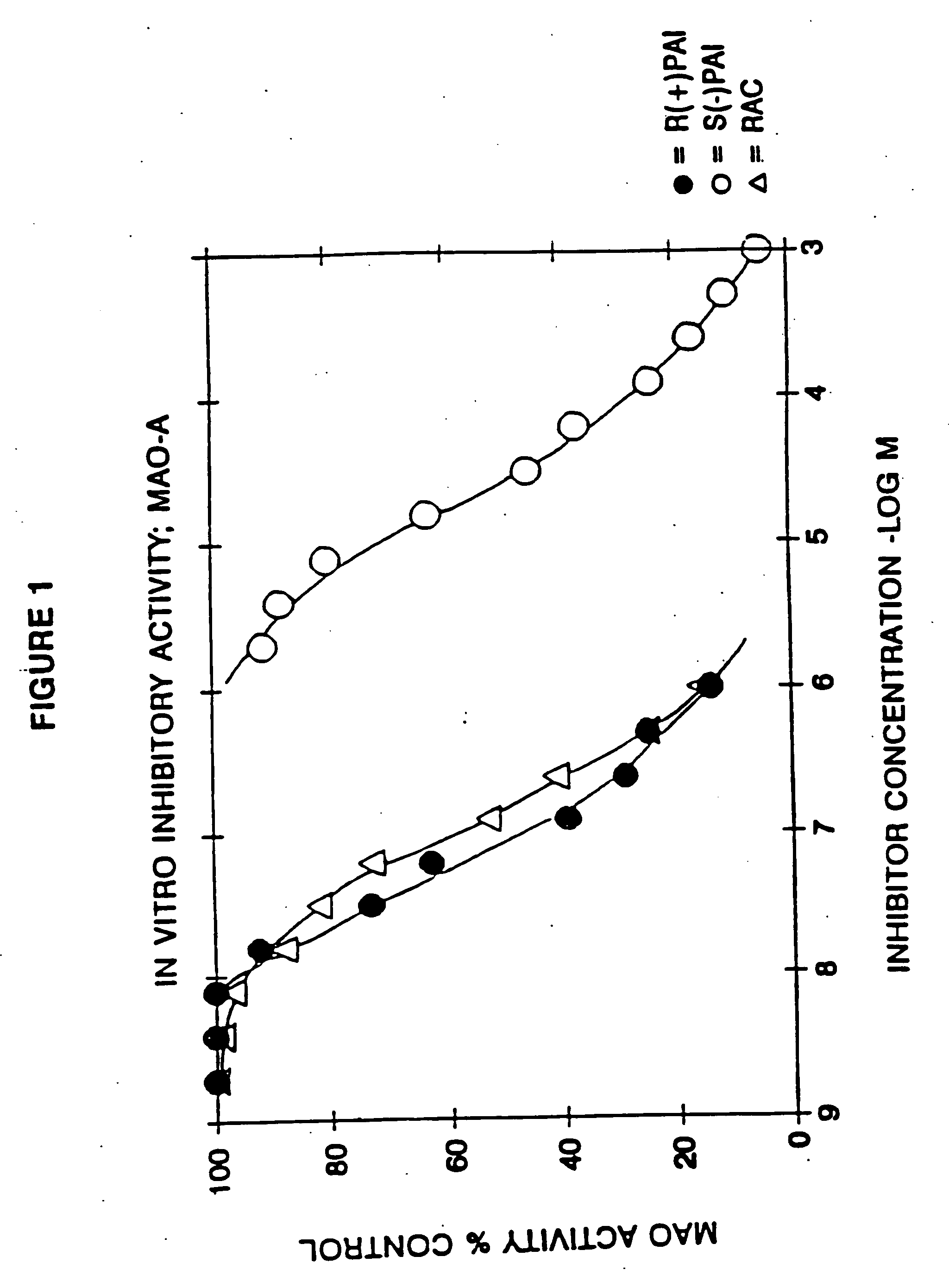
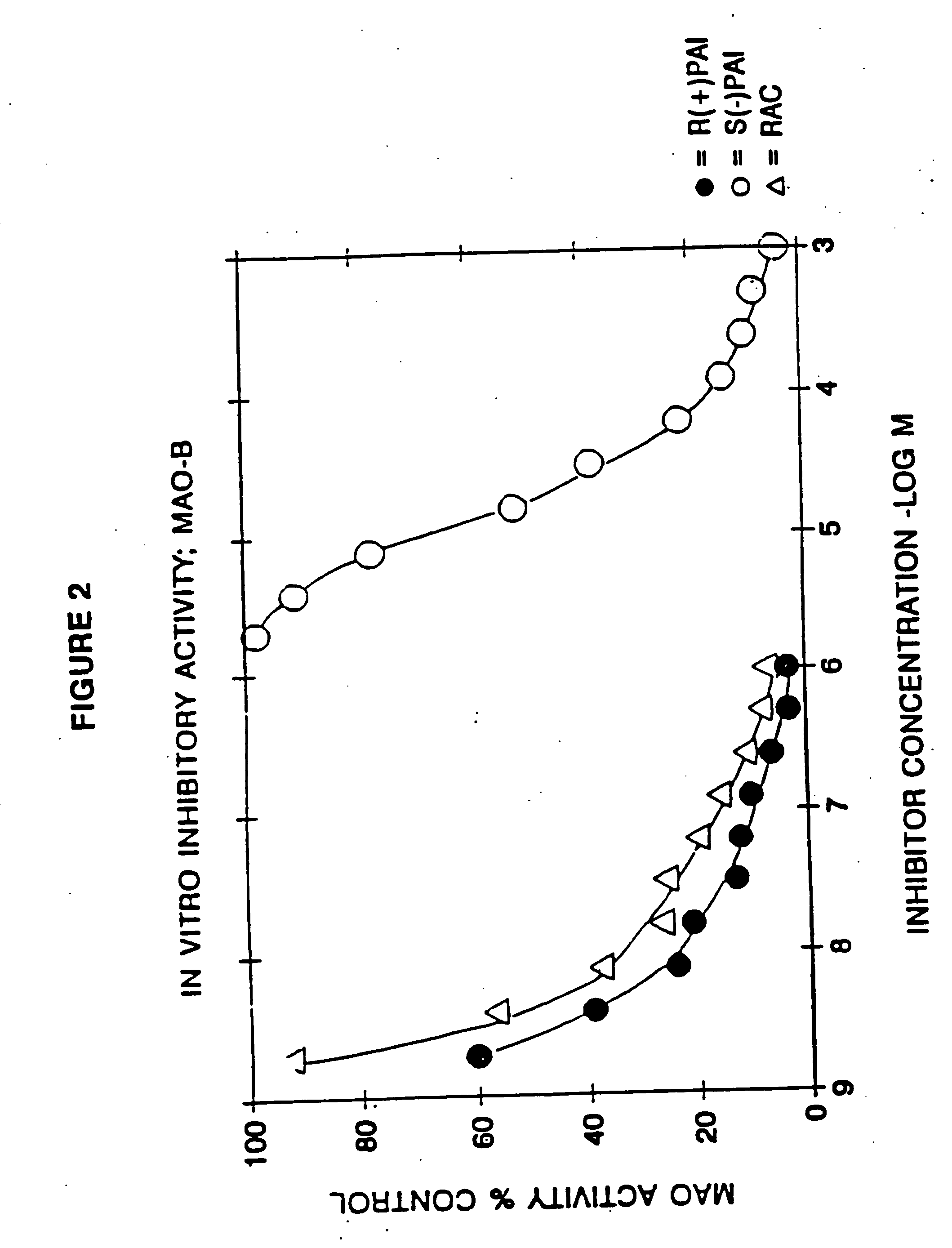
Use of R-enantiomer of N-propargyl-1-aminoindan, salts, compositions and uses thereof
InactiveUS20060094783A1Avoid nerve damageBiocideOrganic active ingredientsMemory disorderAttention deficits
The subject invention provides methods of treating a subject afflicted with Parkinson's disease, memory disorder, depression, hyperactive syndrome, Attention Deficit Disorder, dementia, brain ischemia, stroke, head trauma injury, spinal trauma injury, neurotrauma, neurodegenerative disease, neurotoxic injury, multiple sclerosis, nerve damage, affective illness, schizophrenia or symptoms of withdrawal from an addictive substance, using the mesylate salt of R(+)-N-propargyl-1-aminoindan.
Owner:TEVA PHARMA IND LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com