Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1450 results about "Ulcerative colitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition where inflammation and ulceration of the colon and rectum is observed.
Method of treating inflammatory intestinal diseases containing as the ingredient IL-6 receptors antibodies
A preventive or therapeutic agent for treating bowel disease, including Crohn's disease and ulcerative colitis, where the agent has as an active ingredient an antibody directed against IL-6 receptor which is an interleukin-6 antagonist.
Owner:CHUGAI PHARMA CO LTD +1
Method for treatment of disorders of the gastrointestinal system
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Buffered nicotine containing products
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucosal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least one amino acid, preferably at least one endogenous amino acid. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of said oral formulation for obtaining transmucosal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation as per above for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
Preventive or therapeutic agent for inflammatory bowel disease comprising IL-6 antagonist as an active ingredient
A preventive or therapeutic agent for inflammatory bowel disease such as Crohn's disease and ulcerative colitis said agent comprising as an active ingredient an interleukin-6 (IL-6) antagonist such as an antibody directed against IL-6 receptor.
Owner:CHUGAI PHARMA CO LTD
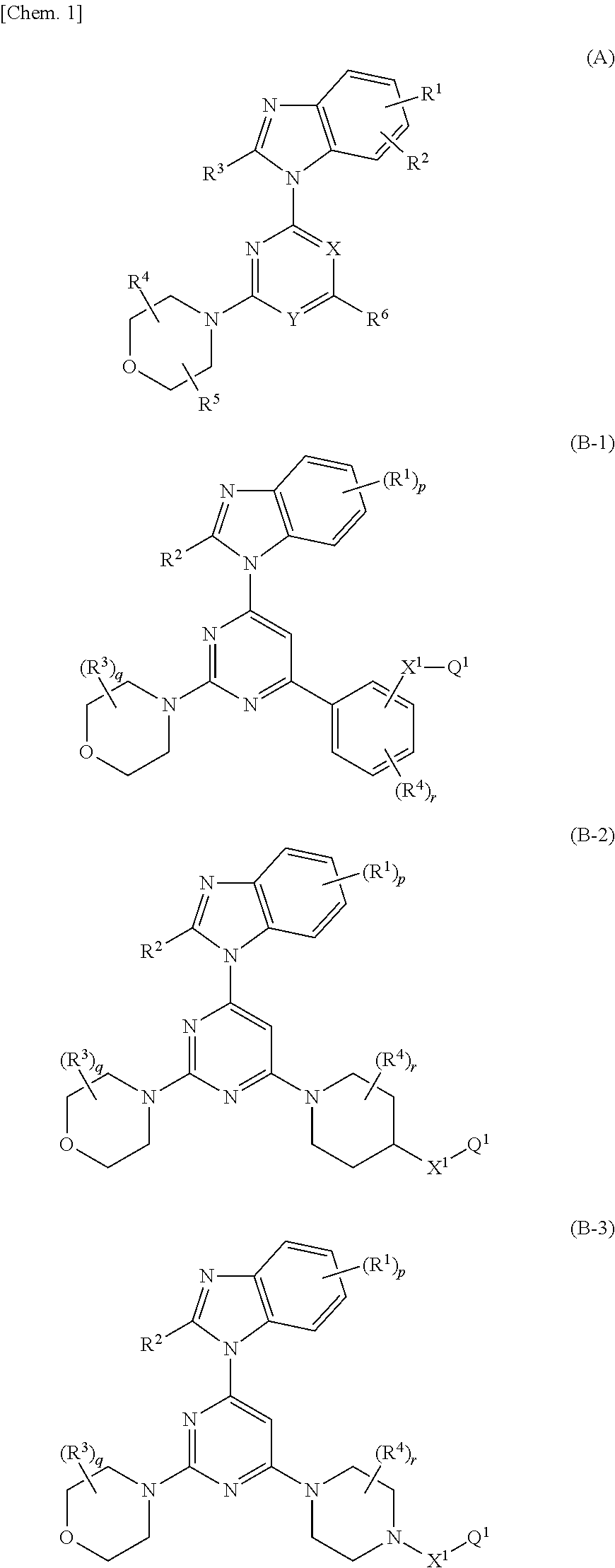
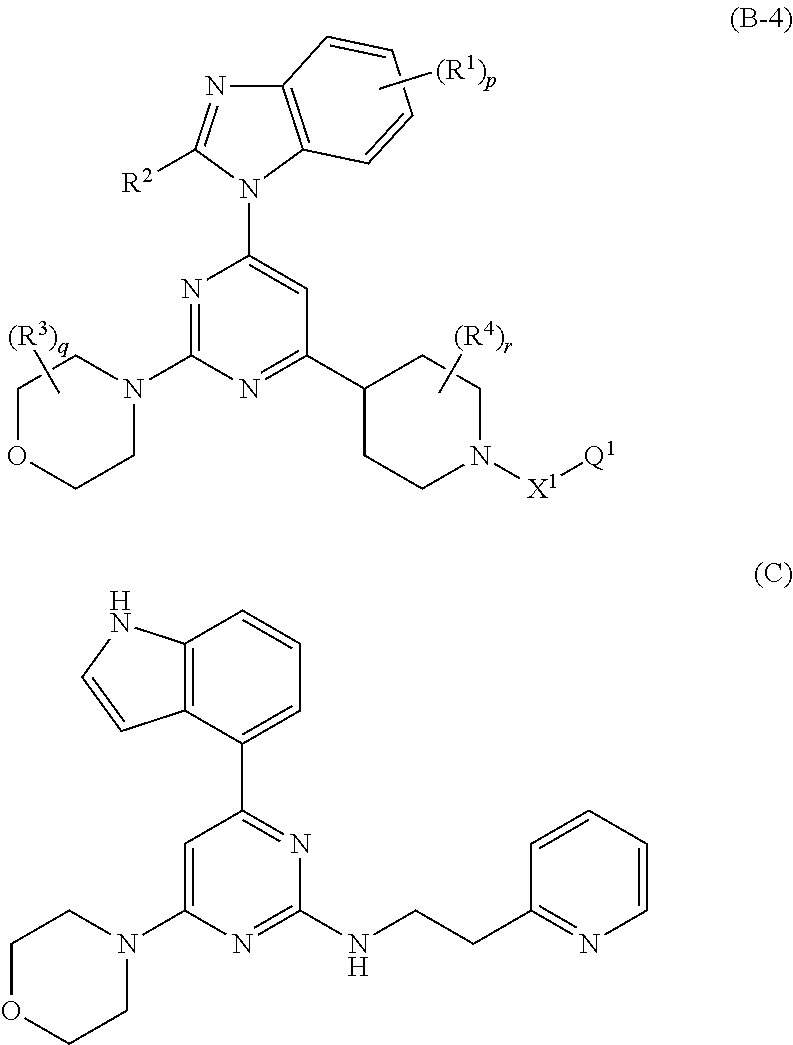
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
Treatment of gastrointestinal disorders
The present invention relates to the use of a therapeutic or nutraceutical composition for the treatment of a gastrointestinal disorder, namely ulcerative colitis. In addition, the present invention provides methods of treating subjects suffering from gastrointestinal disorders such as ulcerative colitis.
Owner:UNIV COURT OF THE UNIV OF DUNDEE
Method and compositions for the treatment of gastrointestinal disorders
ActiveUS20060094658A1Reduce accumulationIncrease gastrointestinal motilityPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
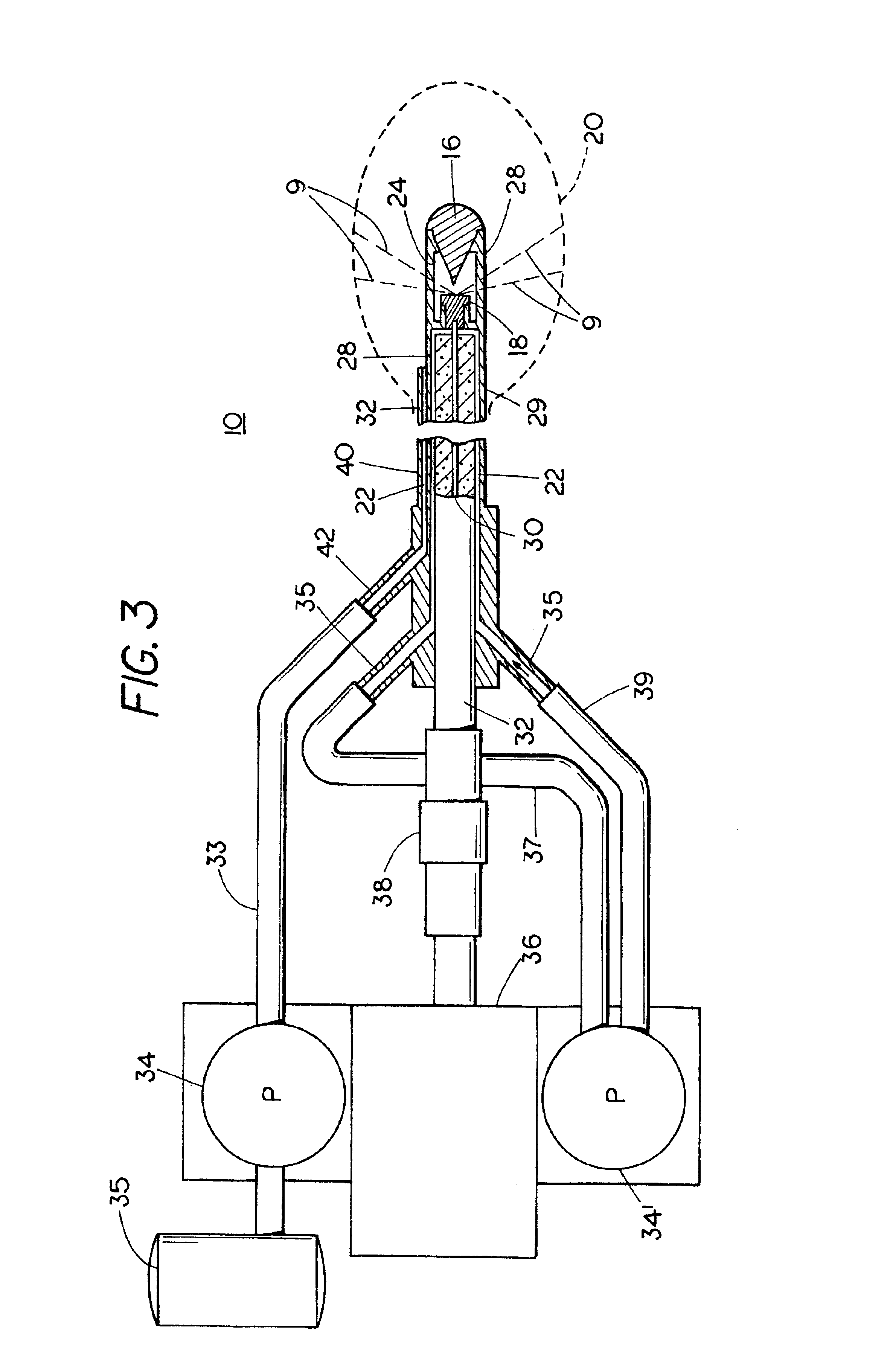
Apparatus and method for debilitating or killing microorganisms within the body
A surgical apparatus has a body portion that includes a shaft terminating in a distal head or tip for directing light radiation from the apparatus onto the lining of a body cavity for treating an ailment in a body cavity of a patient as for example a gastrointestinal ailment of a patient such as gastritis, gastric ulcer, duodenal ulcer, gastric cancer, gastric lymphoma, ulcerative colitis, or Crohn's disease as well as for treating diseases of the circulatory system, urogenital systems and other body cavities. During use, the shaft of the apparatus is inserted into a body cavity, e.g., stomach or colon, of the patient to place the distal tip of the shaft in the desired position. The body cavity of the patient is then irradiated with light radiation so as to kill or debilitate microorganisms lining the body cavity without serious destruction of the body tissue of the patient to thereby improve or alleviate one or more of the symptoms of the ailment. A probiotic comprising innocuous bacteria can be administered to the patient to reestablish the growth of normal microbial flora when treating the gastrointestinal tract.
Owner:LUMERX
Methods and compositions for the treatment of gastrointestinal disorders
InactiveUS20070010450A1Increase gastrointestinal motilityReduce inflammationPeptide/protein ingredientsMetabolism disorderIntestinal tract diseasesGastroparesis
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
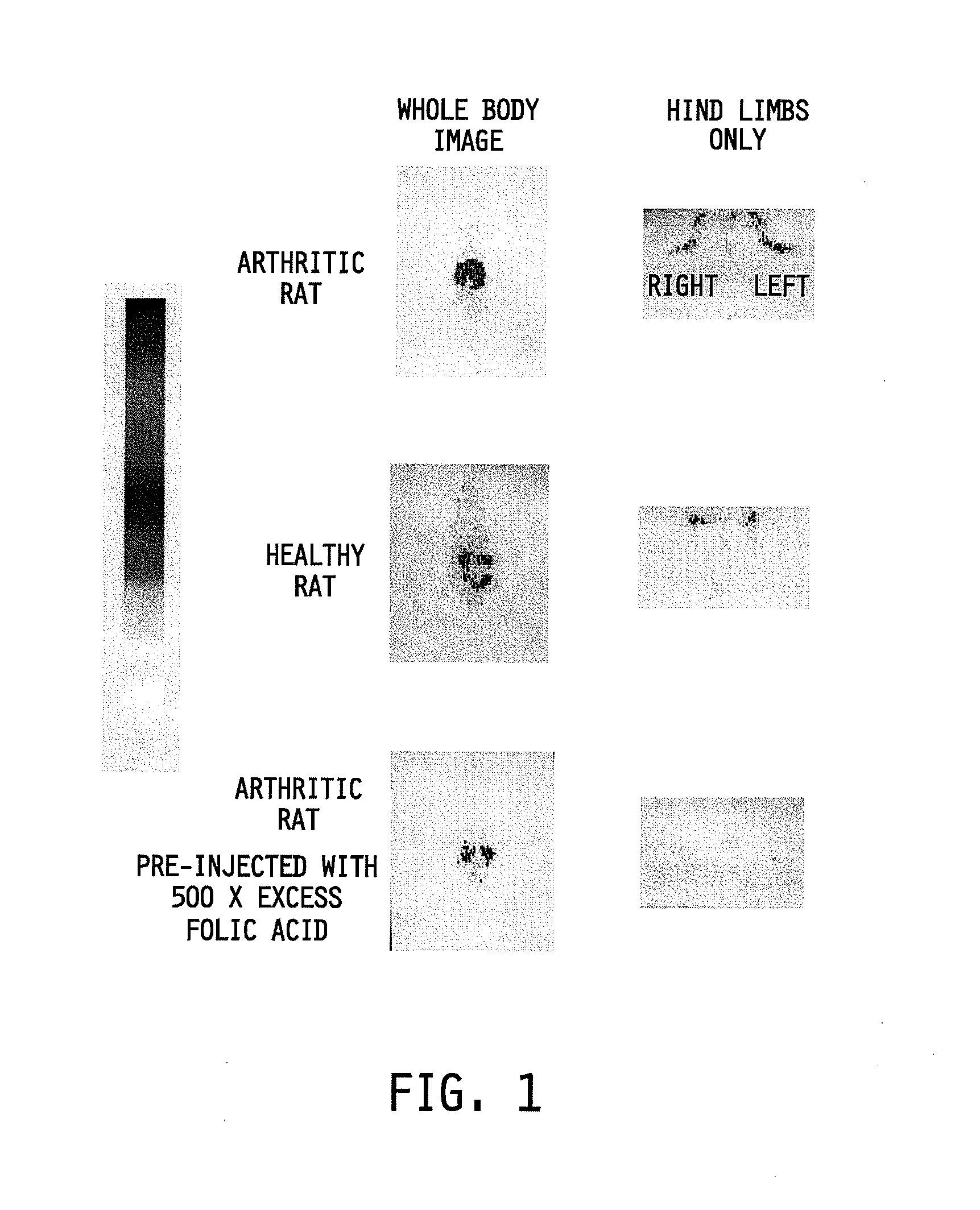
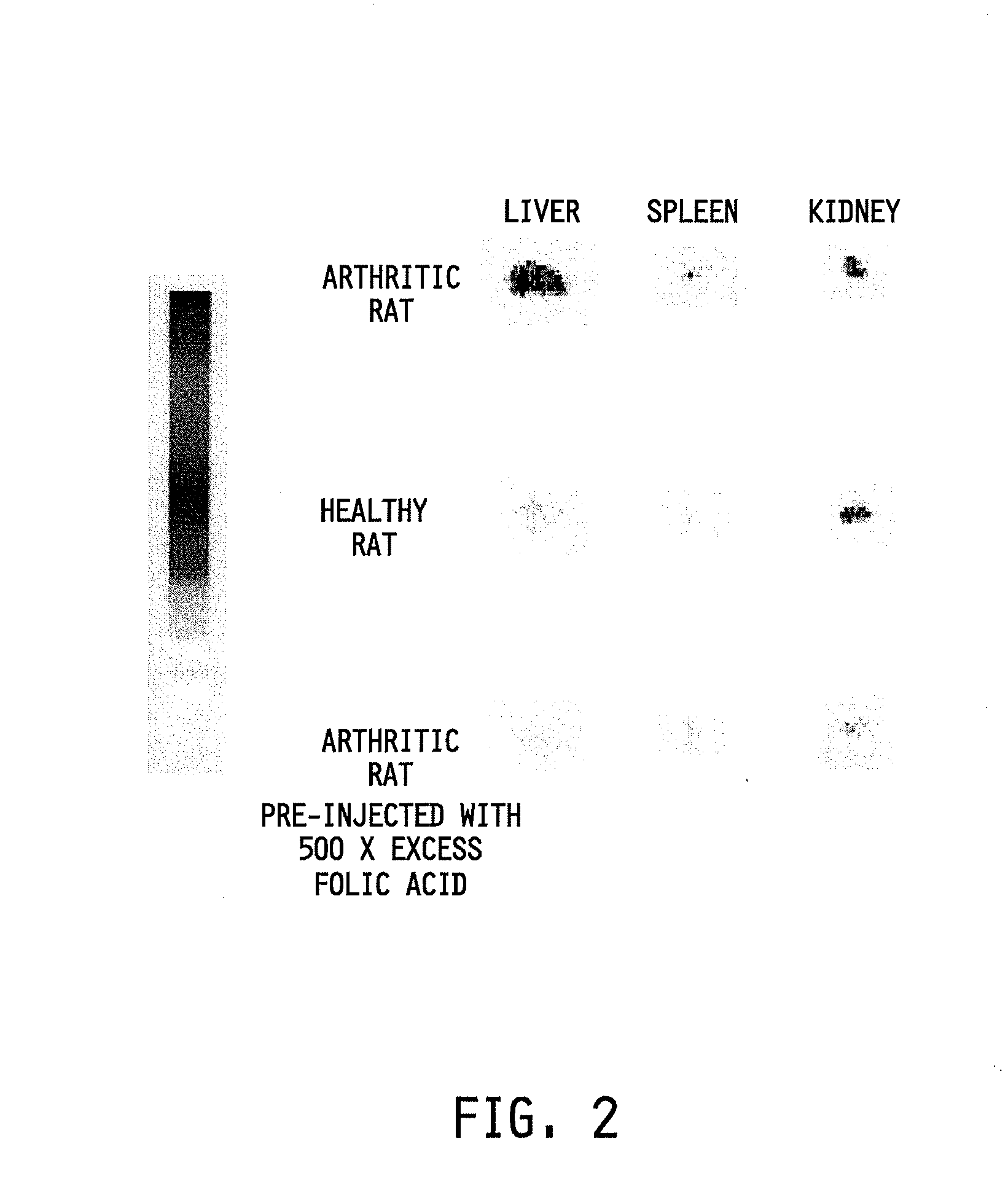
Treatment and diagnosis of macrophage mediated disease
The invention relates to a method of treating or monitoring / diagnosing a disease state mediated by activated macrophages. The method comprises the step of administering to a patient suffering from a macrophage mediated disease state an effective amount of a composition comprising a conjugate or complex of the general formulaAb−Xwhere the group Ab comprises a ligand capable of binding to activated macrophages, and when the conjugate is being used for treatment of the disease state, the group X comprises an immunogen, a cytotoxin, or a compound capable of altering macrophage function, and when the conjugate is being used for monitoring / diagnosing the disease state, X comprises an imaging agent. The method is useful for treating a patient suffering from a disease selected from the group consisting of rheumatoid arthritis, ulcerative colitis, Crohn's disease, inflammation, infections, osteomyelitis, atherosclerosis, organ transplant rejection, pulmonary fibrosis, sarcoidosis, and systemic sclerosis.
Owner:LOW PHILIP S +1
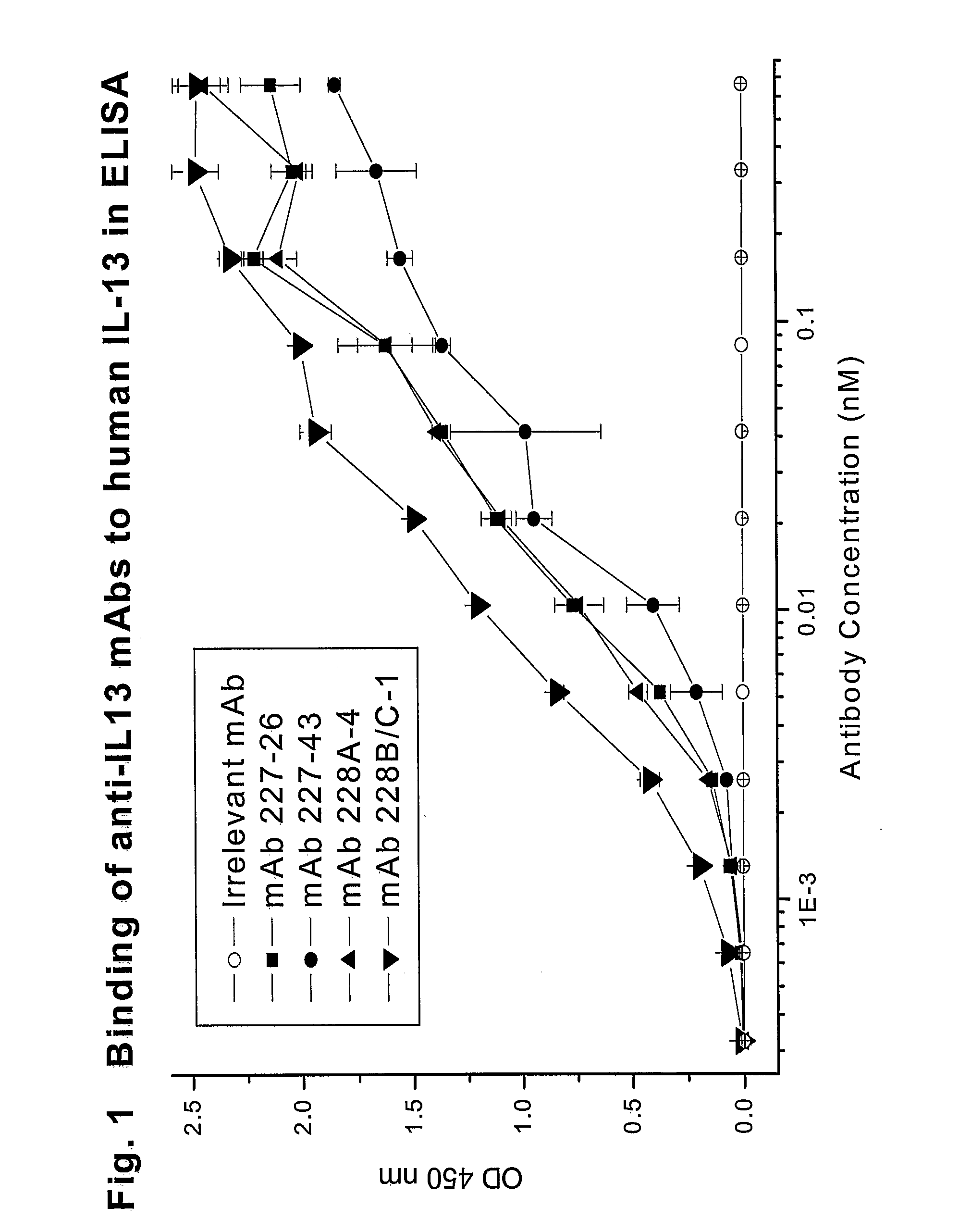
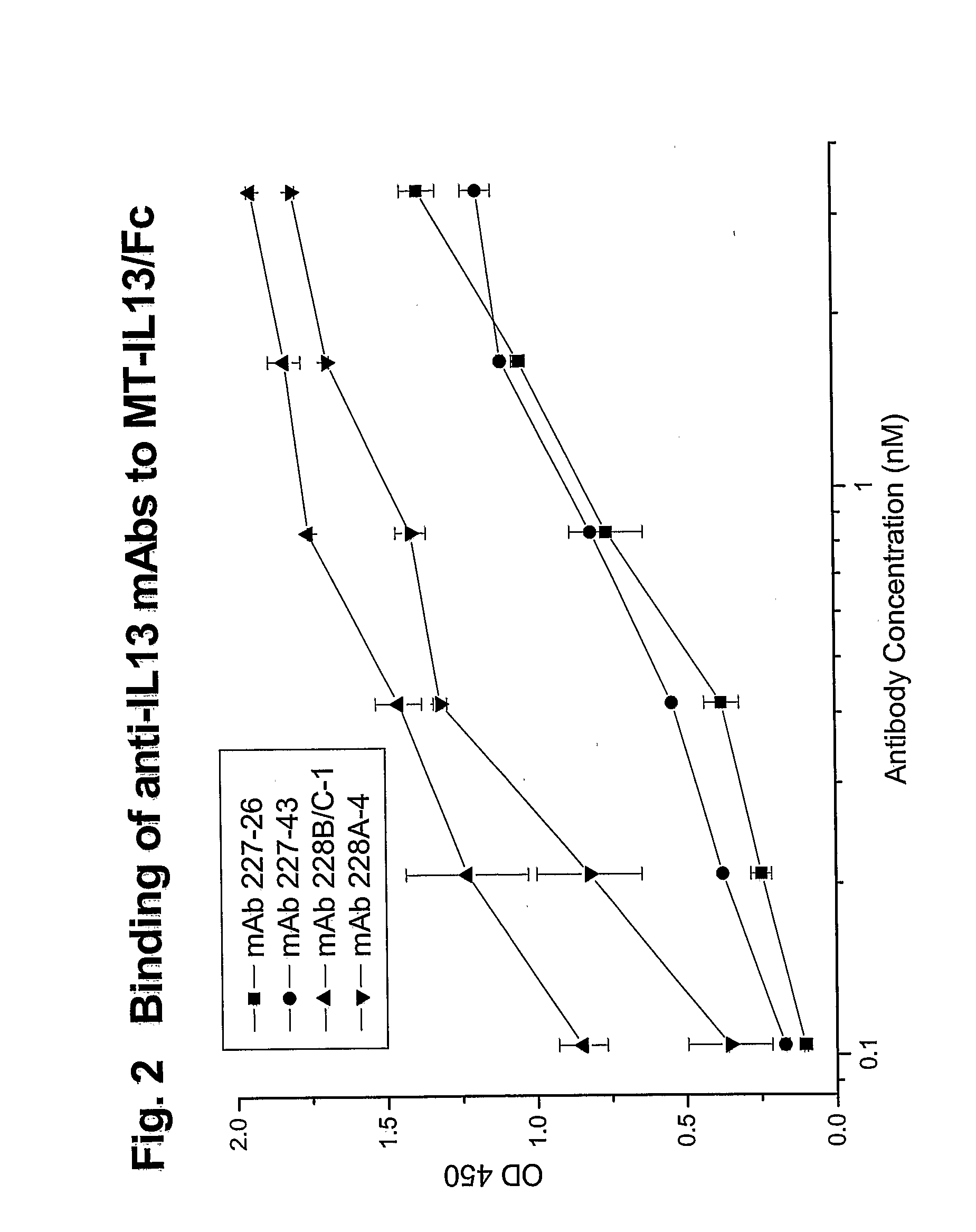
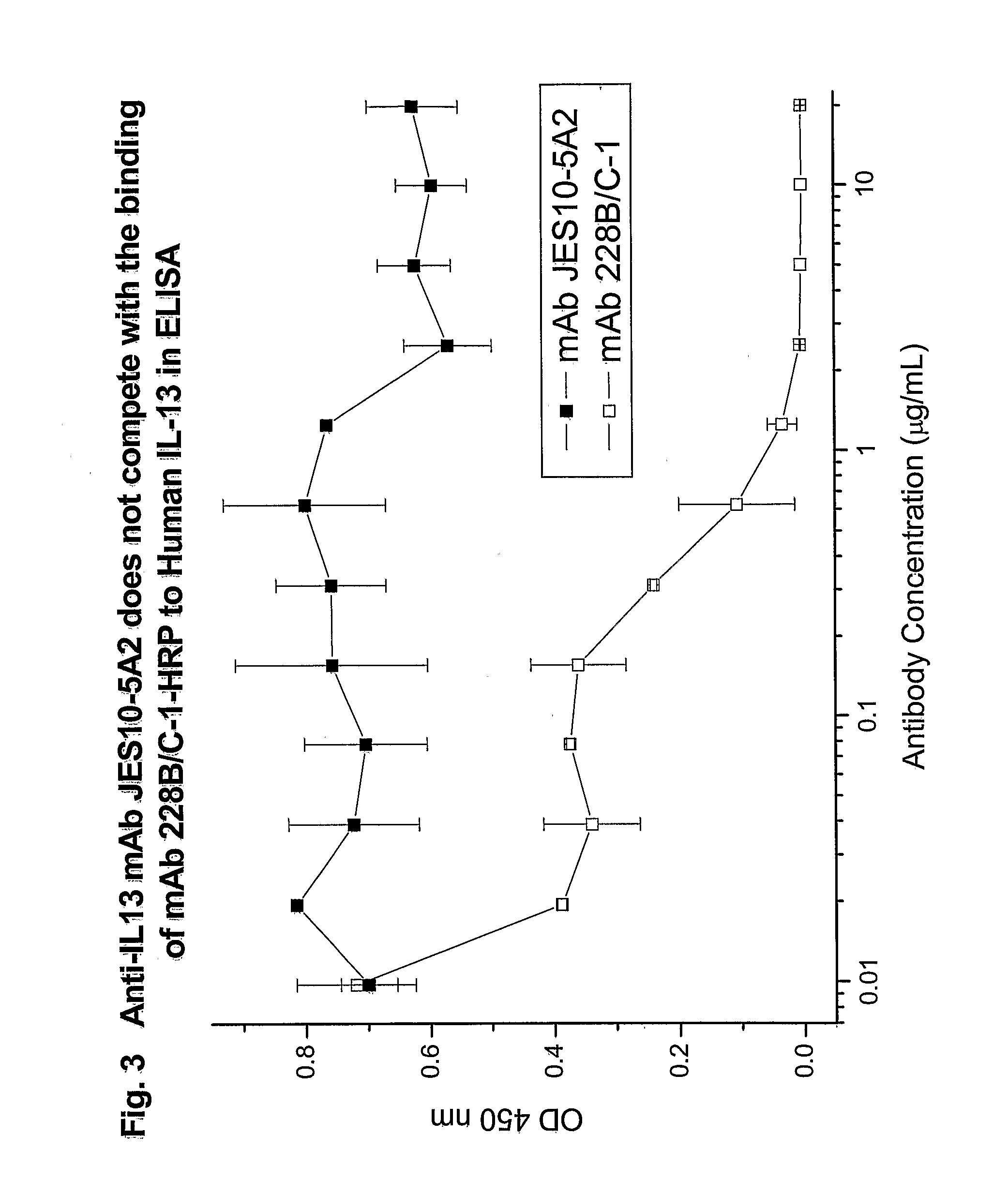
Novel anti-IL13 antibodies and uses thereof
ActiveUS20090214523A1Inhibiting antibody productionRelieve symptomsSenses disorderAntipyreticUveitisNonallergic rhinitis
The present invention relates to anti-IL13 antibodies that bind specifically and with high affinity to both glycosylated and non-glycosylated human IL13, does not bind mouse IL13, and neutralize human IL13 activity at an approximate molar ratio of 1:2 (MAb:IL13). The invention also relates to the use of these antibodies in the treatment of IL13-mediated diseases, such as allergic disease, including asthma, allergic asthma, non-allergic (intrinsic) asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, eczema, urticaria, food allergies, chronic obstructive pulmonary disease, ulcerative colitis, RSV infection, uveitis, scleroderma, and osteoporosis.
Owner:GENENTECH INC
Agonists of Guanylate Cyclase Useful for the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100069306A1Maintain good propertiesIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS7879802B2Excellent propertyIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
Owner:BAUSCH HEALTH IRELAND LTD
Materials and methods for treatment of gastrointestinal disorders
InactiveUS7312243B1Reduced activityIncrease incidenceBiocideHeavy metal active ingredientsColonic epitheliumUlcerative colitis
The subject invention pertains to materials and methods for the prevention and treatment of gastrointestinal diseases, including inflammatory bowel diseases such as Crohn's disease and ulcerative colitis. Therapeutic compositions of the invention include compositions that can neutralize hydrogen peroxide, such as reducing agents and oxidizing agents. In one embodiment, a therapeutic composition of the invention comprises a reducing agent such as sodium thiosulfate. Therapeutic compositions of the invention can optionally include compounds with antibacterial activity, compositions that inhibit bacterial adherence to cells and tissue, compositions that inhibits epithelial lipid peroxidation, compositions that add viscosity to a solution, compositions that inhibit most cells, and / or compositions that help to seal or repair tight junctions between cells of the colonic epithelium of the gastrointestinal tract. Methods of the invention include administration of compounds or compositions of the invention. In one embodiment, compounds or compositions of the invention are rectally instilled in a patient.
Owner:THERAPEUTIC RES
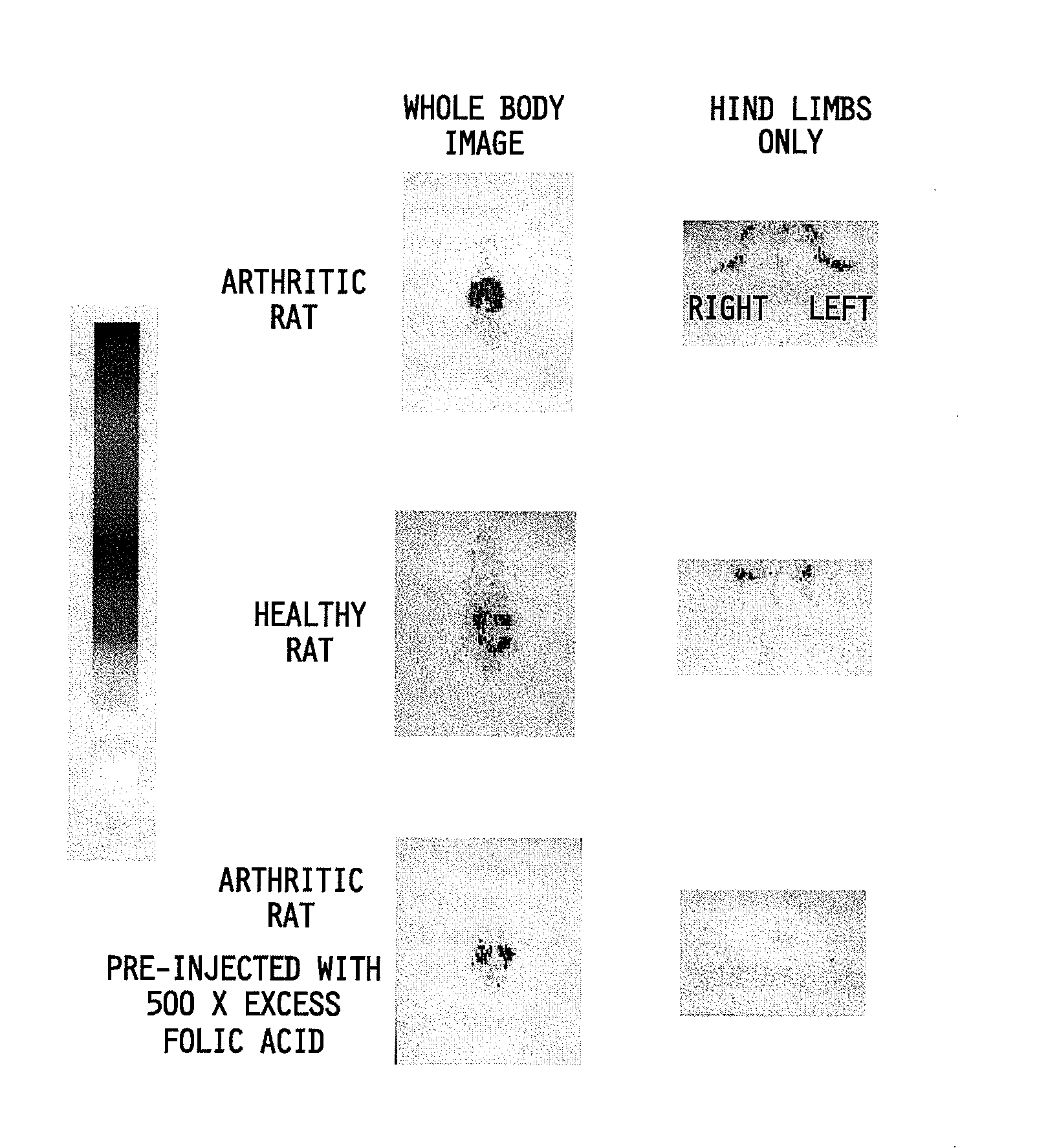
Diagnosis of macrophage mediated disease
InactiveUS20070231266A1Reduce inflammationReduce retentionAntibacterial agentsNervous disorderUlcerative colitisImaging agent
The invention relates to a method of treating or monitoring / diagnosing a disease state mediated by activated macrophages. The method comprises the step of administering to a patient suffering from a macrophage mediated disease state an effective amount of a composition comprising a conjugate or complex of the general formula Ab-X where the group Ab comprises a ligand capable of binding to activated macrophages, and when the conjugate is being used for treatment of the disease state, the group X comprises an immunogen, a cytotoxin, or a compound capable of altering macrophage function, and when the conjugate is being used for monitoring / diagnosing the disease state, X comprises an imaging agent. The method is useful for treating a patient suffering from a disease selected from the group consisting of rheumatoid arthritis, ulcerative colitis, Crohn's disease, inflammation, infections, osteomyelitis, atherosclerosis, organ transplant rejection, pulmonary fibrosis, sarcoidosis, and systemic sclerosis.
Owner:LOW PHILIP S +1
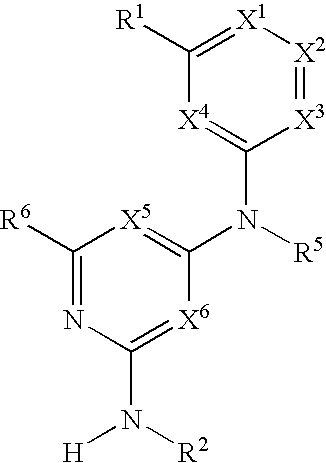
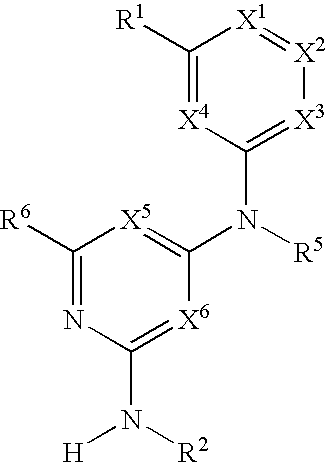
Hetero ring derivative
InactiveUS20120165309A1Enhanced inhibitory effectBiocideOrganic chemistryAutoimmune conditionAutoimmune disease
[Object]A novel and excellent method for preventing or treating rejection in the transplantation of various organs, allergy diseases, autoimmune diseases, hematologic tumor, or the like, based on a PI3Kδ-selective inhibitory action and / or an IL-2 production inhibitory action, and / or a B cell proliferation inhibitory action (including an activation inhibitory action), is provided[Means for Solution]It was found that a 3-substituted triazine or 3-substituted pyrimidine derivative exhibits a PI3Kδ-selective inhibitory action, and / or an IL-2 production inhibitory action, and / or a B cell proliferation inhibitory action (including an activation inhibitory action), and can be an agent for preventing or treating rejection in the transplantation of various organs, allergy diseases (asthma, atopic dermatitis, etc.), autoimmune diseases (rheumatoid arthritis, psoriasis, ulcerative colitis, Crohn's disease, systemic lupus erythematosus, etc.), hematologic tumor (leukemia etc.), or the like, thereby completing the present invention.
Owner:ASTELLAS PHARMA INC
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8034782B2Excellent propertyIncrease resistanceAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Lanthionine synthetase component c-like proteins as molecular targets for preventing and treating diseases and disorders
ActiveUS20110275558A1Organic active ingredientsPeptide/protein ingredientsThiazolidinedioneAutoimmune disease
The present invention relates to the field of medical treatments for diseases and disorders. More specifically, the present invention relates to the use of the lanthionine synthetase component C-like (LANCL) proteins as therapeutic targets for novel classes of anti-inflammatory, immune regulatory and antidiabetic drugs. This includes but it is not limited to abscisic acid (ABA), ABA analogs, benzimidazophenyls, repurposed drugs or drug combinations, including thiazolidinediones (TZDs); naturally occurring compounds such as conjugated diene fatty acids, conjugated triene fatty acids, isoprenoids, and natural and synthetic agonists of peroxisome proliferator-activated receptors that activate this receptor through an alternative mechanism of action involving LANCL2 or other membrane proteins to treat or prevent the common inflammatory pathogenesis underlying type 2 diabetes, atherosclerosis, cancer, some inflammatory infectious diseases such as influenza and autoimmune diseases including but not limited to inflammatory bowel disease (Crohn's disease and Ulcerative colitis), rheumatoid arthritis, multiple sclerosis and type 1 diabetes and other chronic inflammatory conditions.
Owner:VIRGINIA TECH INTPROP INC
Drug Identification and Treatment Method
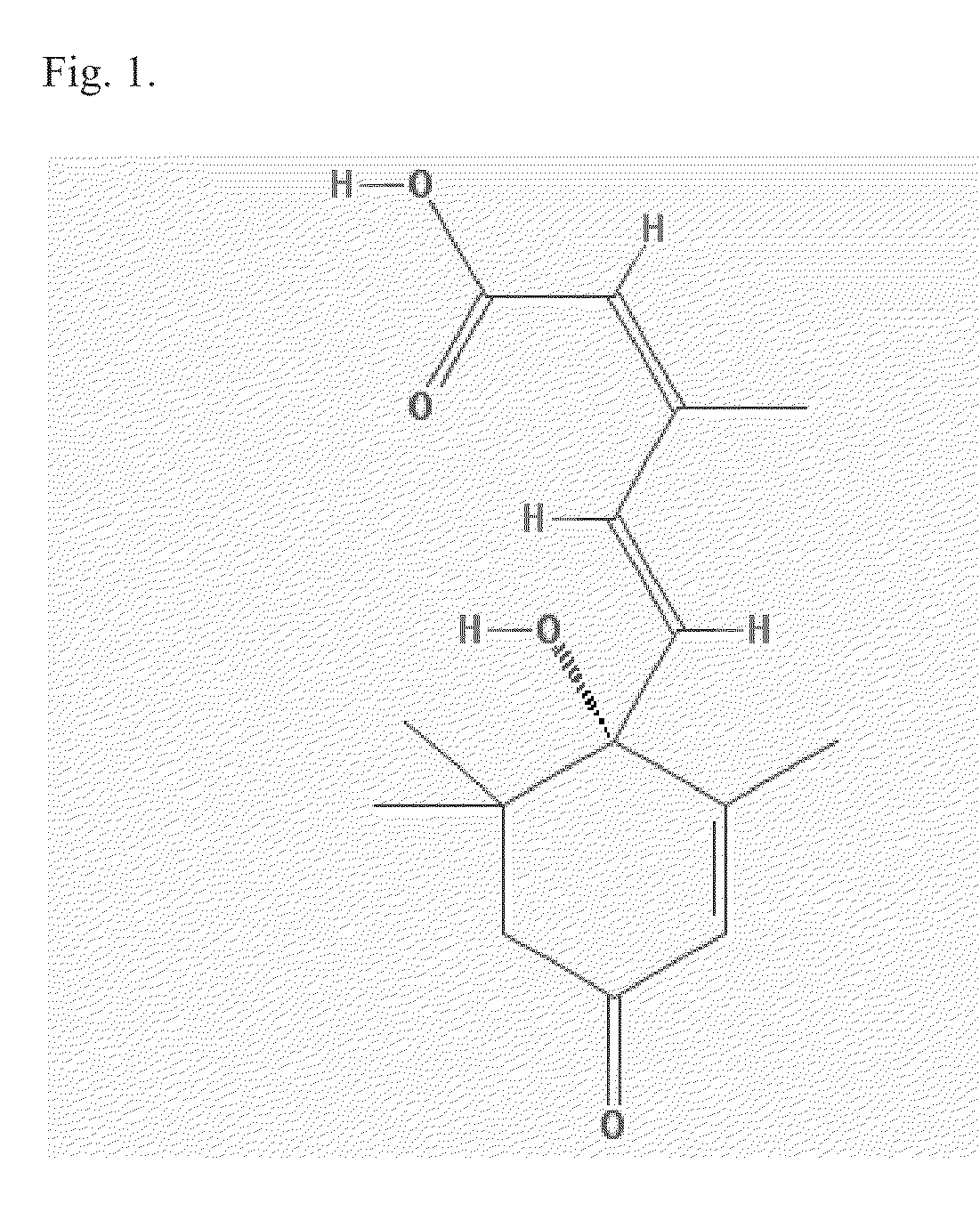
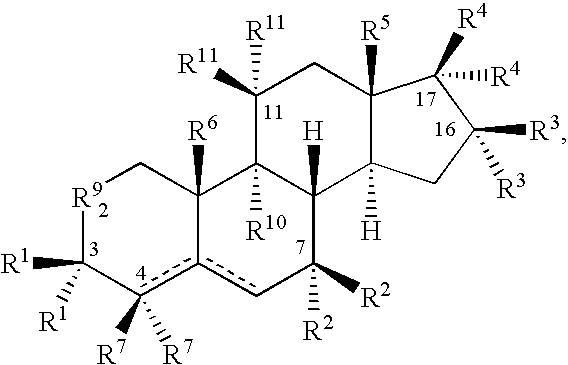
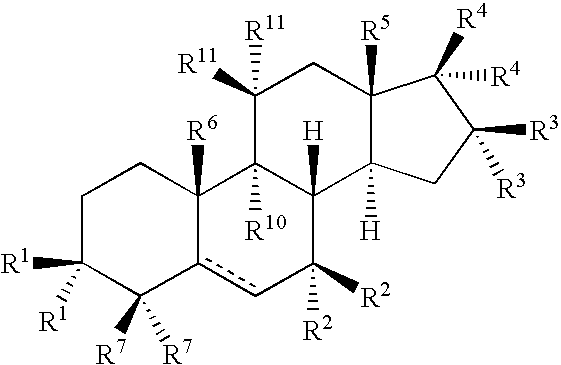
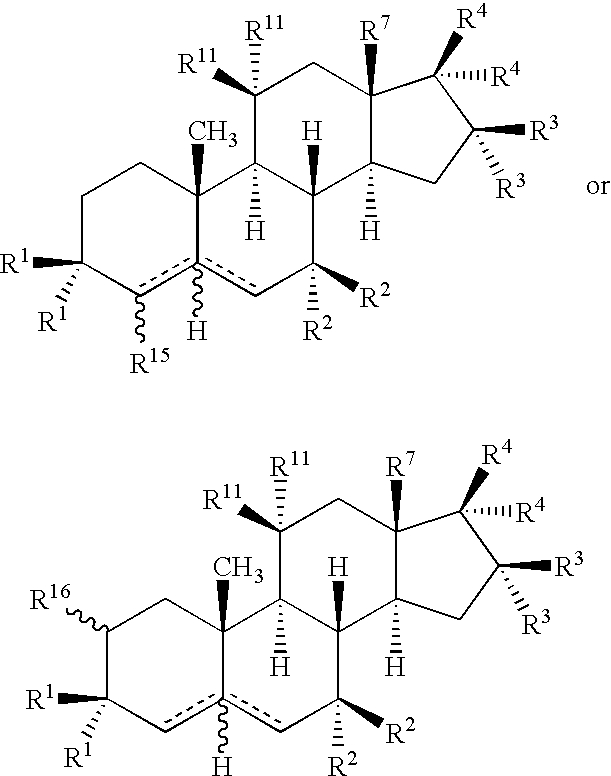
The invention relates to methods to identify compounds that can treat autoimmune conditions and treat specified clinical disorders such as multiple sclerosis, ulcerative colitis or arthritis. Compounds include 17α-ethynylandrost-5-ene-3β,15β,7α,17β-tetrol, 4α-acetoxy-17α-ethynylandrost-5-ene-3β,7β,17β-triol, 17α-ethynylandrost-5-ene-3β,4β,7α,17β-tetrol, 17α-ethynylandrost-5-ene-3α,4β,7α,17β-tetrol and 17α-ethynylandrost-5-ene-3α,4β,17β-triol-7-one.
Owner:NEURMEDIX +2
Minimal-heart-rate reduction parasympathetic stimulation
ActiveUS20080275514A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsInternal electrodesMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Treatment of inflammatory bowel disease (IBD)
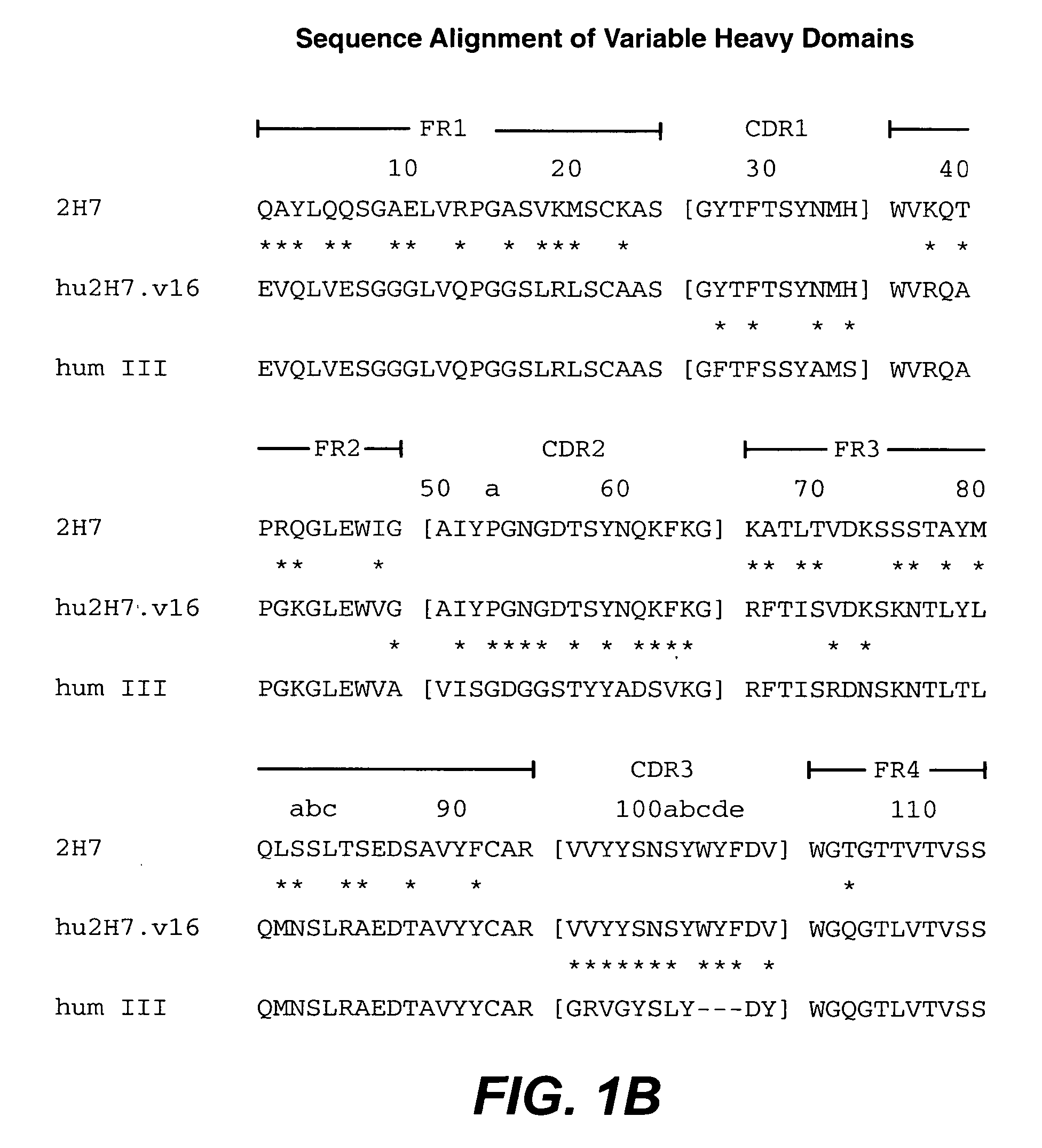
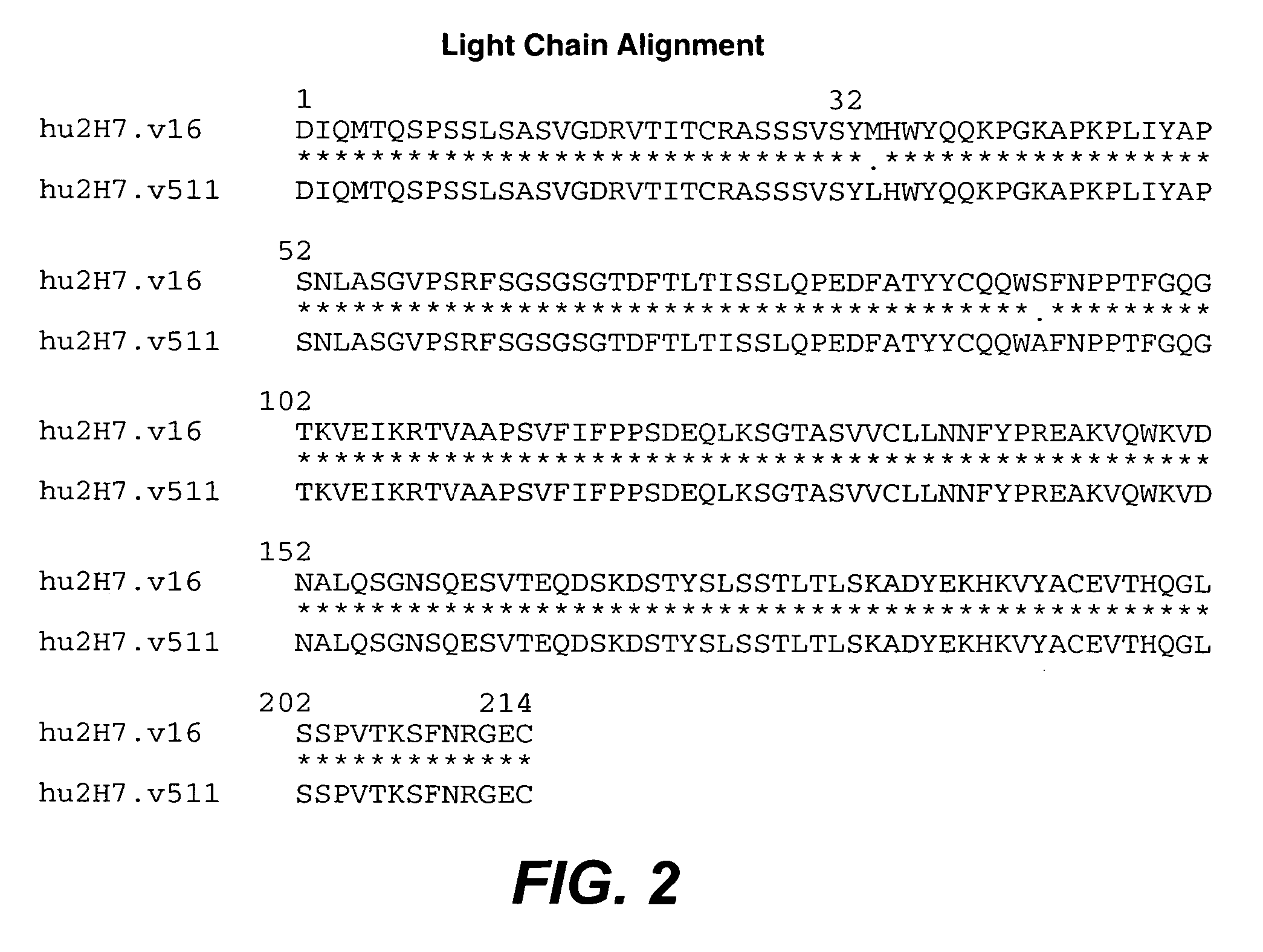
The present invention concerns treatment of IBD, especially ulcerative colitis (UC), with an antibody that binds to CD20.
Owner:GENENTECH INC
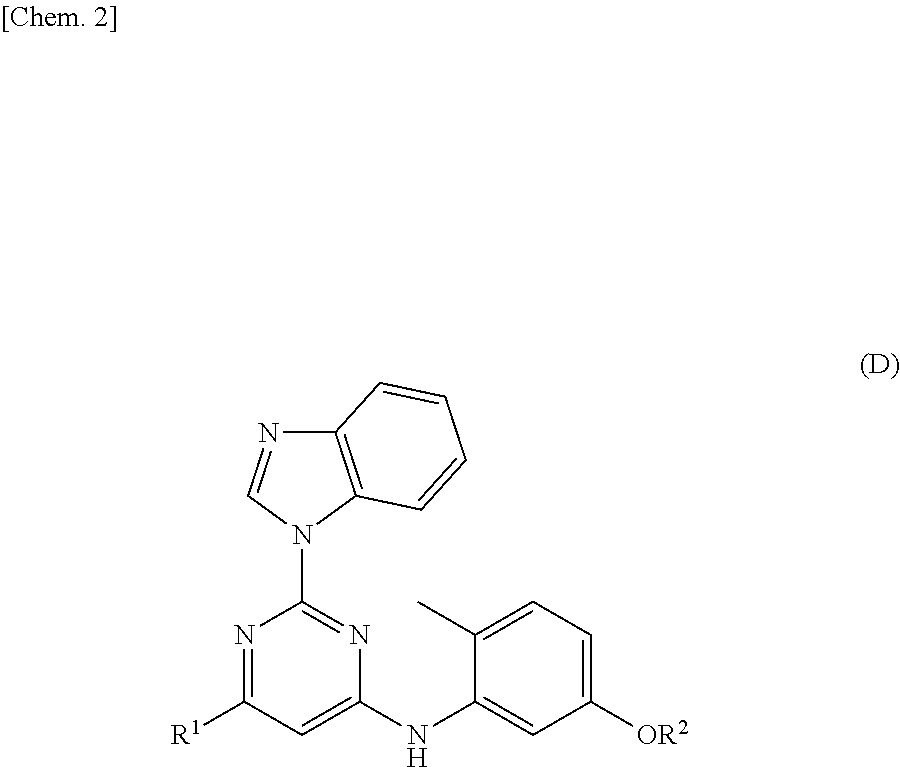
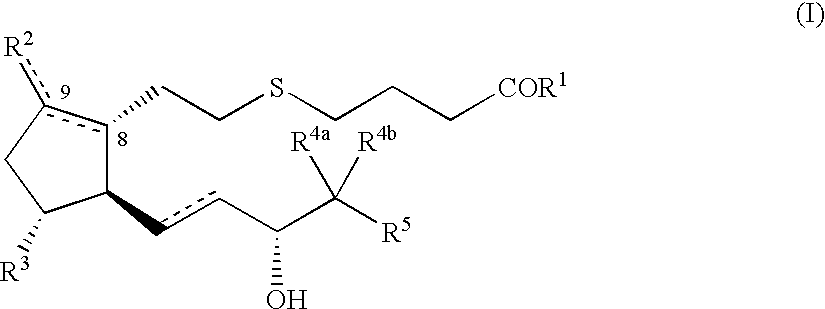
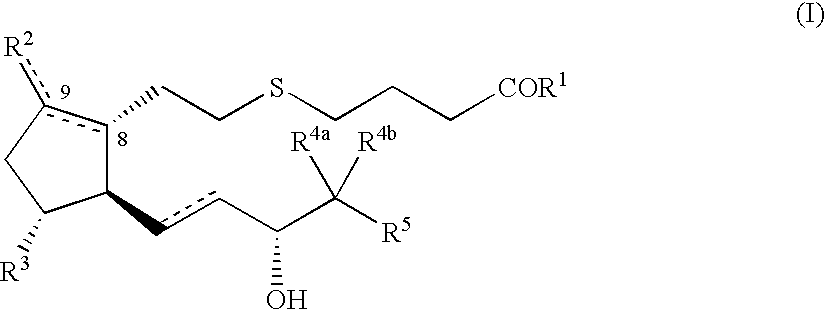
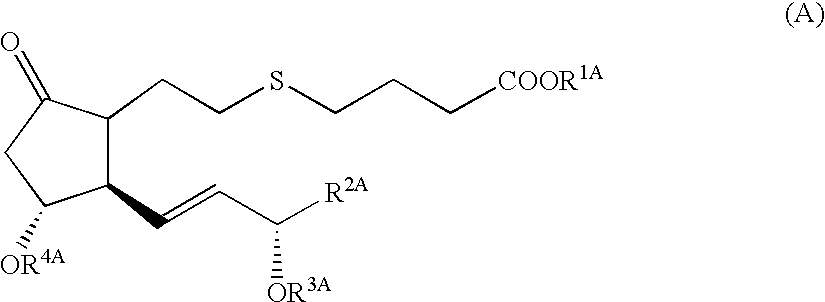
5-thia-omega-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient
The present invention relates to 5-thia-omega-substituted phenylprostaglandin E derivatives of the formula (I)(wherein, all the symbols are as defined in the specification), process for producing them and pharmaceutical compositions comprising them as active ingredient.The compounds of the formula (I) can bind to PGE2 receptors (especially, subtype EP4) strongly, so they are expected to be useful for prevention and / or treatment of immunological diseases (autoimmune diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Sjoegren's syndrome, chronic rheumarthrosis and systemic lupus erythematosus etc., and rejection after organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, lung failure, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardiac ischemia, systemic inflammatory response syndrome, ambustion pain, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, and shock etc. Further, it is thought that EP4 subtype receptor relates to sleeping disorder and blood platelet aggregation, so the compounds of the present invention are expected to be useful for the prevention and / or treatment of such diseases.
Owner:ONO PHARMA CO LTD
Substituted heterocyclic compounds and methods of use
The present invention relates to pyridines, pyrimidines and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Medicinal composition containing American cockroach and its ethanol extract and new use
ActiveCN1943600APromote hyperplasiaPromote repairAnthropod material medical ingredientsDigestive systemAlcoholTreatment effect
The invention relates to uses of Bombay canary and its alcohol extract in preparing medicine for treatment of inflammation, specifically, for treating cervical erosion, ulcerative colitis, fig wart after operation, said invention belongs to medicine field.
Owner:耿福能
Application of bacteroides fragilis to prevention and/or treatment of inflammatory bowel diseases (IBDs)
ActiveCN105434476AStrong resistanceEasy to solveBacteria material medical ingredientsMicroorganismsFood additiveSide effect
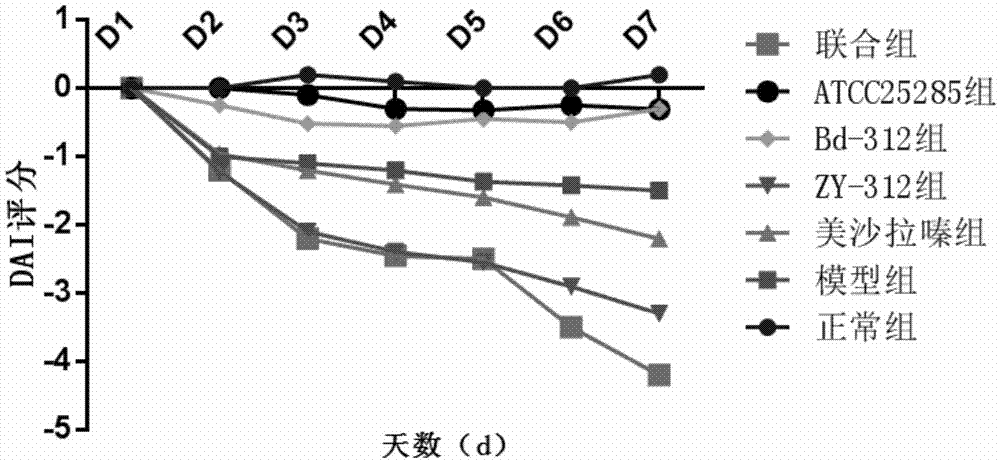
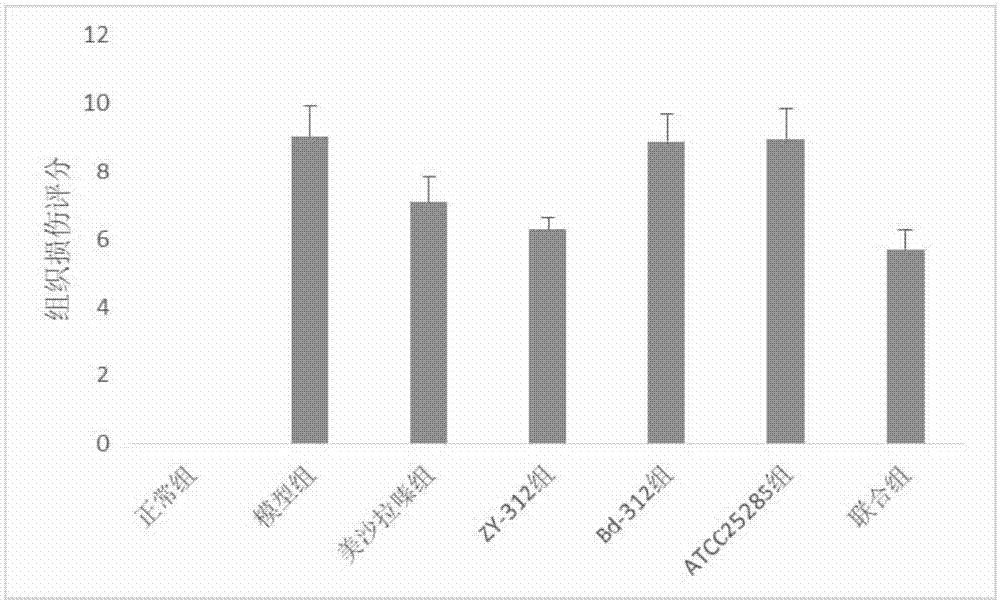
The invention provides an application of bacteroides fragilis ZY-312 to prevention and / or treatment of inflammatory bowel diseases (IBDs), including an application of bacteroides fragilis ZY-312 to preparation of medicines, medicine compositions, food, health care products, food additives, and the like for preventing and / or treating IBDs. In vivo and in vitro related experiments confirm that bacteroides fragilis ZY-312 has excellent resistance to the IBD, including ulcerative colitis or the Crohn's disease, does not have toxic or side effect and can be permanently and effectively applied to preparation of the medicines, medicine compositions, food, health care products or food additives for preventing and / or treating IBDs. The medicines, medicine compositions, food, health care products or food additives can be used for preventing and treating IBDs and have important application values.
Owner:GUANGZHOU ZHIYI PHARMA INC
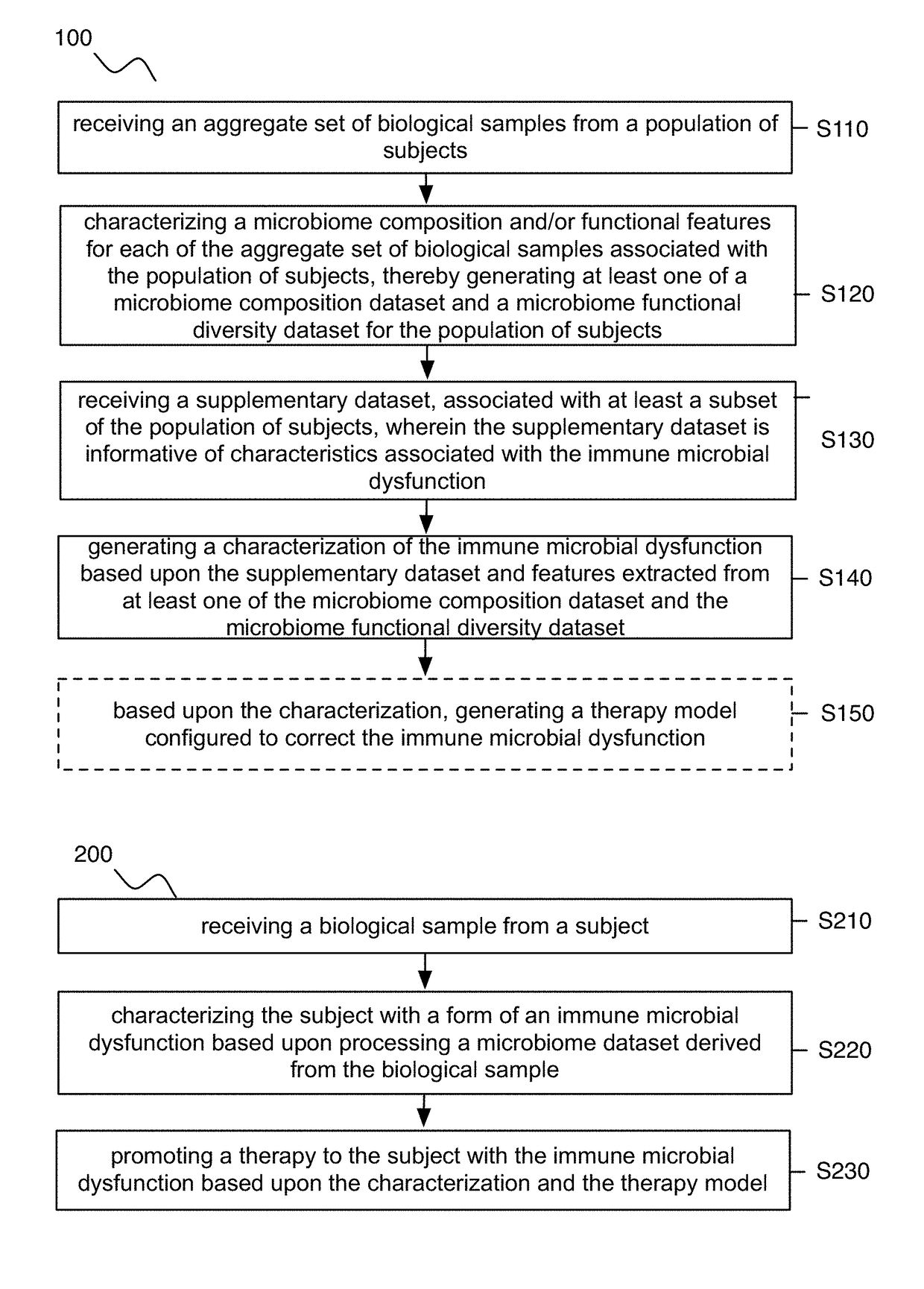
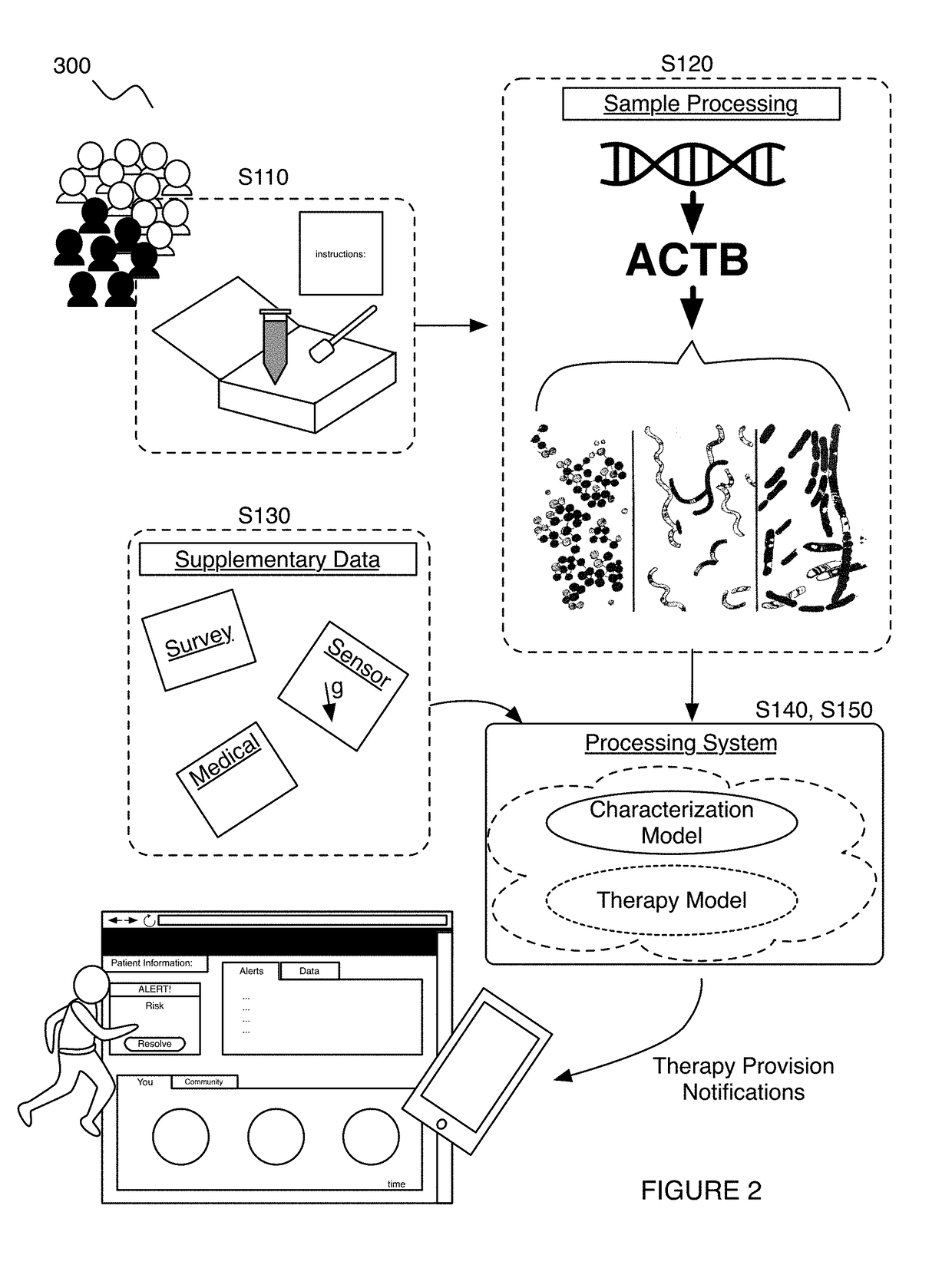
Method and system for microbiome-derived diagnostics and therapeutics
A method for diagnosing and treating an immune microbial dysfunction in a subject, the method comprising: receiving an aggregate set of biological samples from a population of subjects; generating at least one of a microbiome composition dataset and a microbiome functional diversity dataset for the population of subjects; generating a characterization of the immune microbial dysfunction based upon features extracted from at least one of the microbiome composition dataset and the microbiome functional diversity dataset, wherein the characterization is diagnostic of at least one of Crohn's disease, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), ulcerative colitis, and celiac disease; based upon the characterization, generating a therapy model configured to correct the immune microbial dysfunction; and at an output device associated with the subject, promoting a therapy to the subject based upon the characterization and the therapy model.
Owner:PSOMAGEN INC
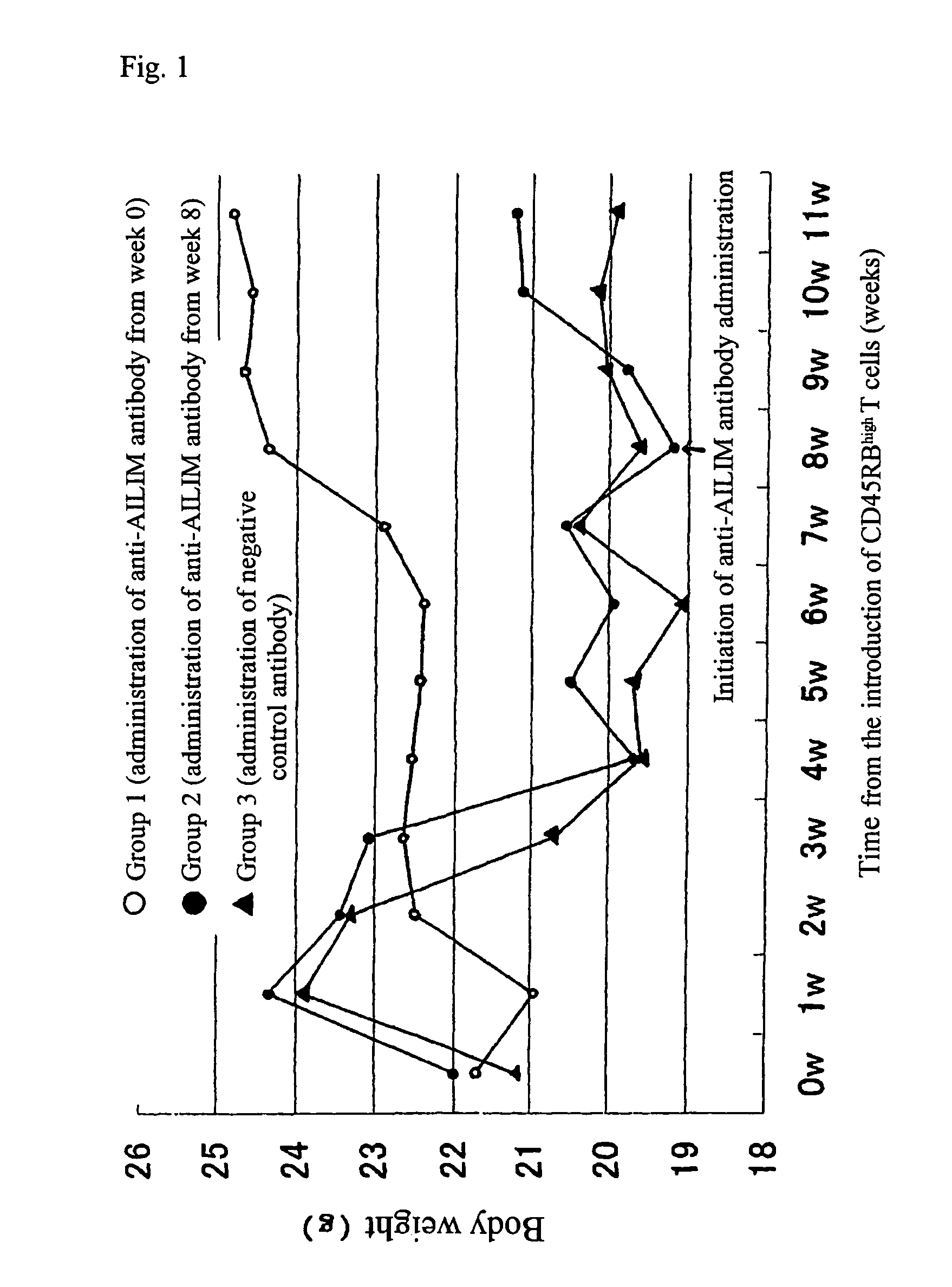
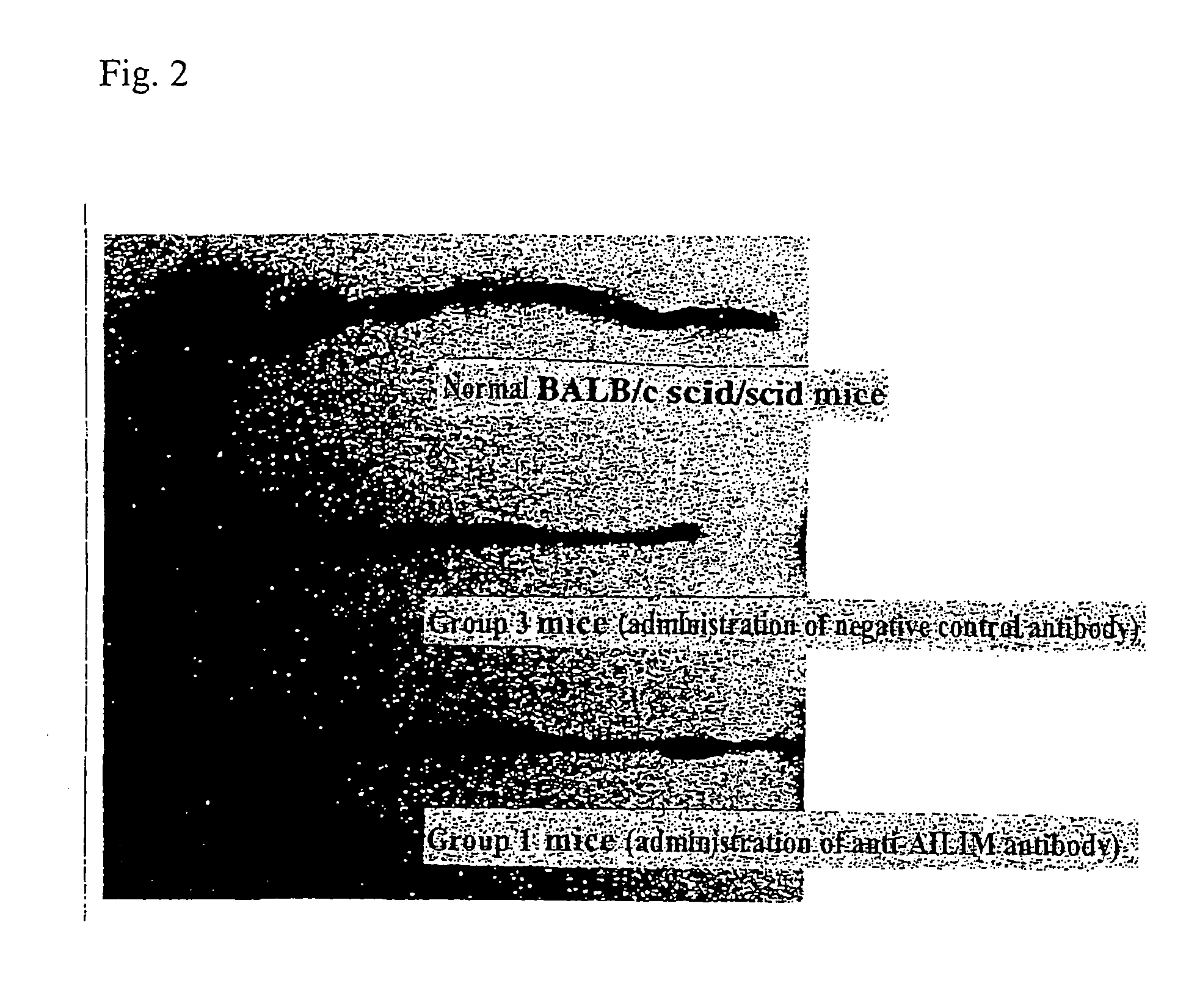
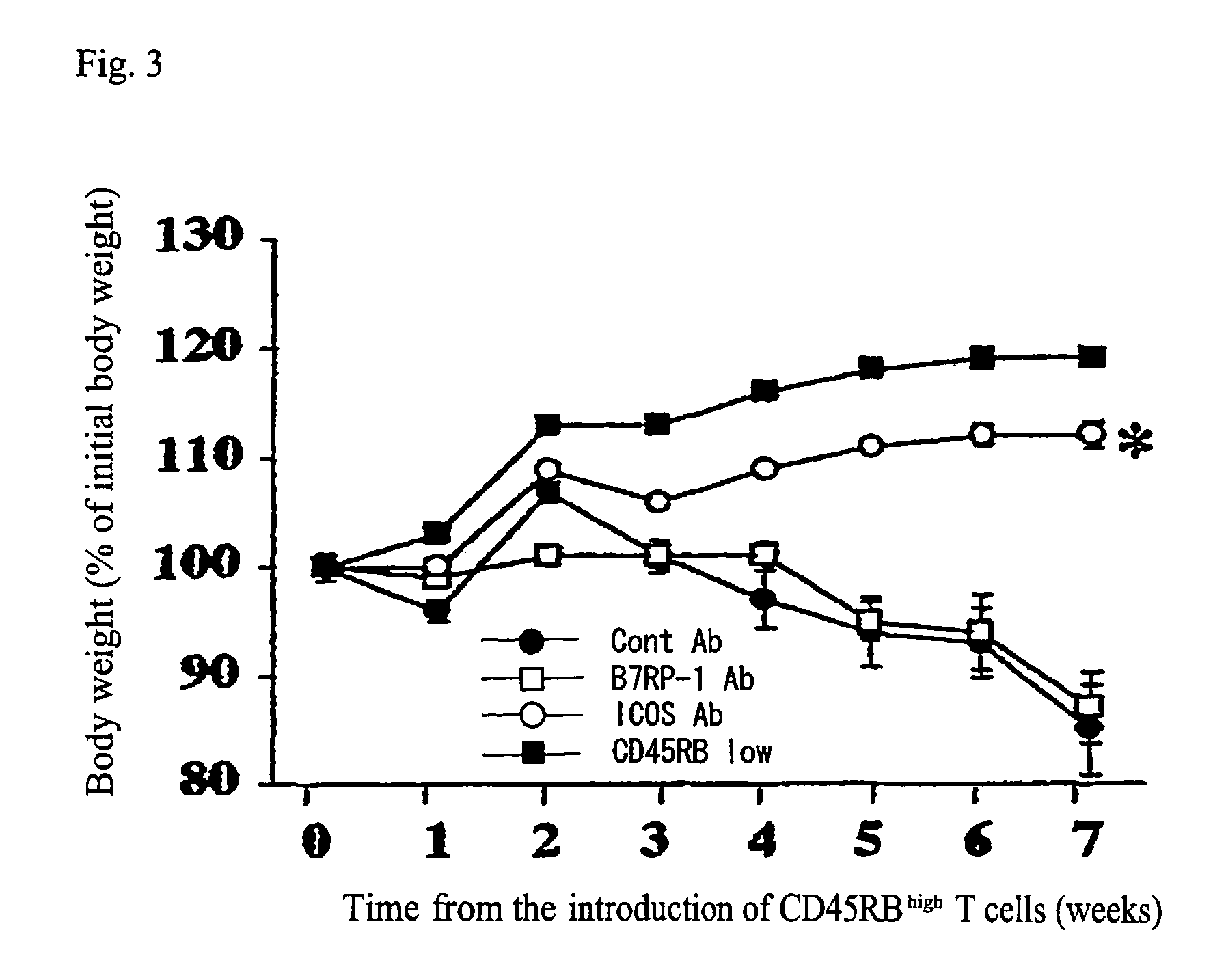
Methods of suppressing or treating an inflammatory bowel disease by administering an antibody or portion thereof that binds to AILIM
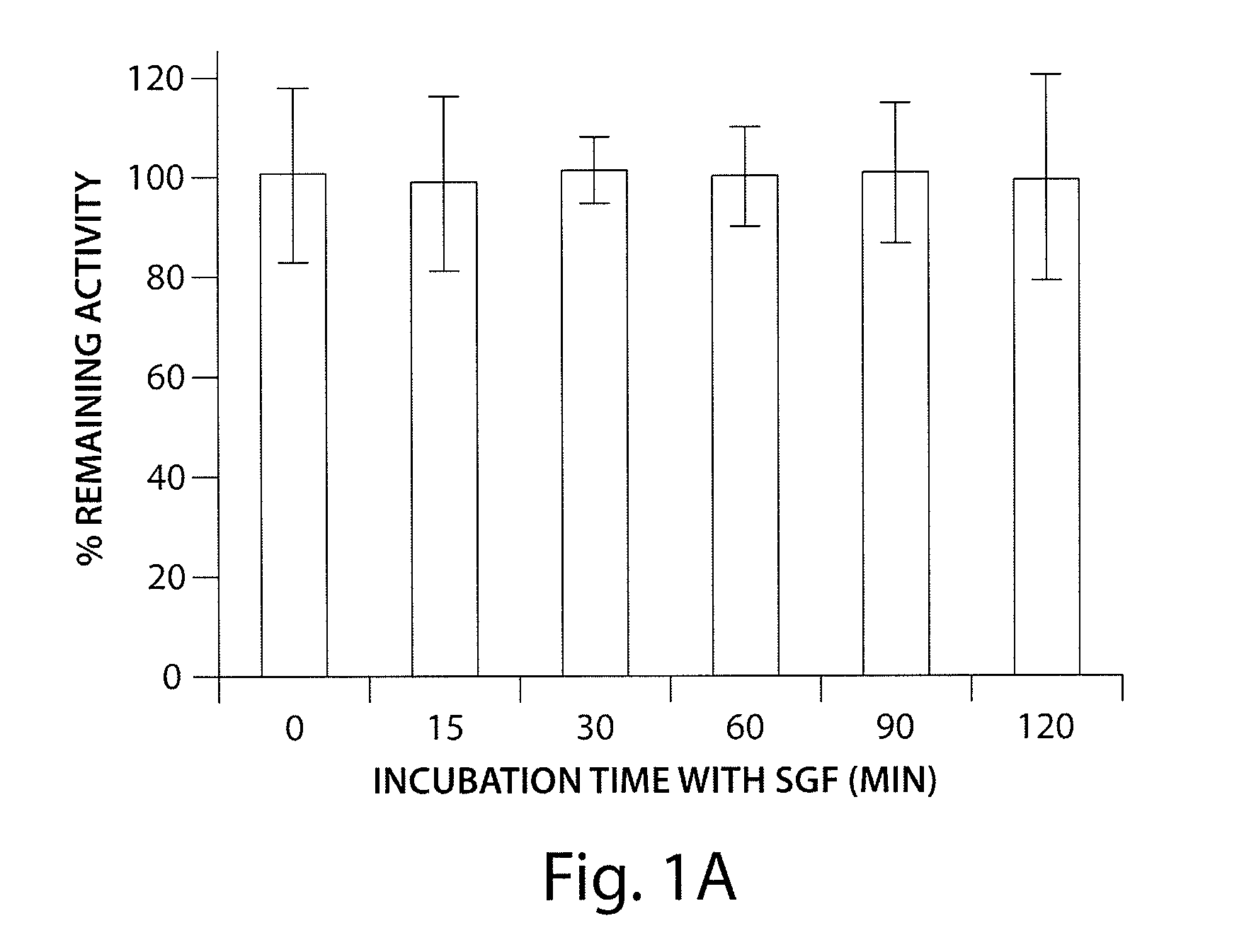
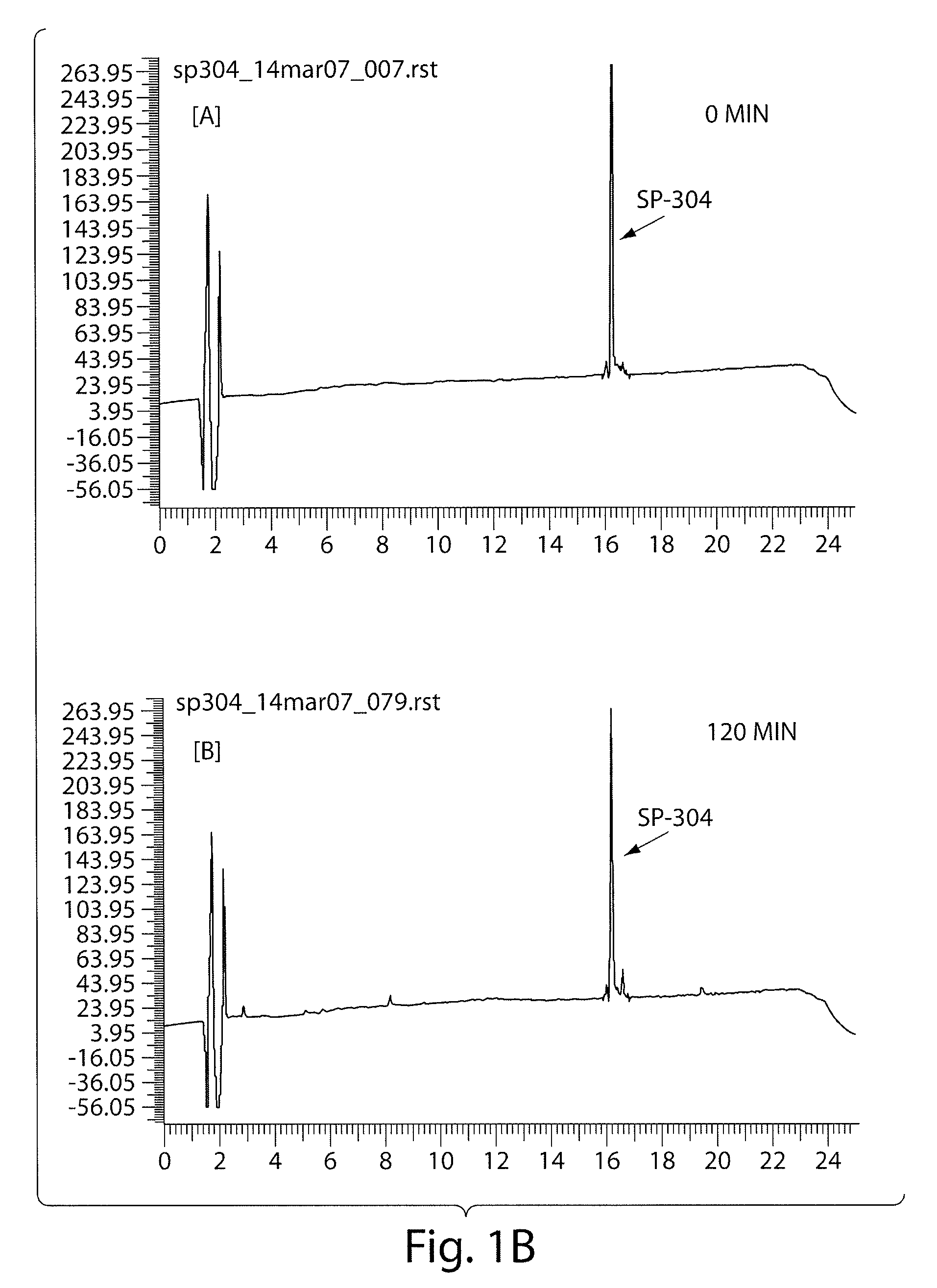
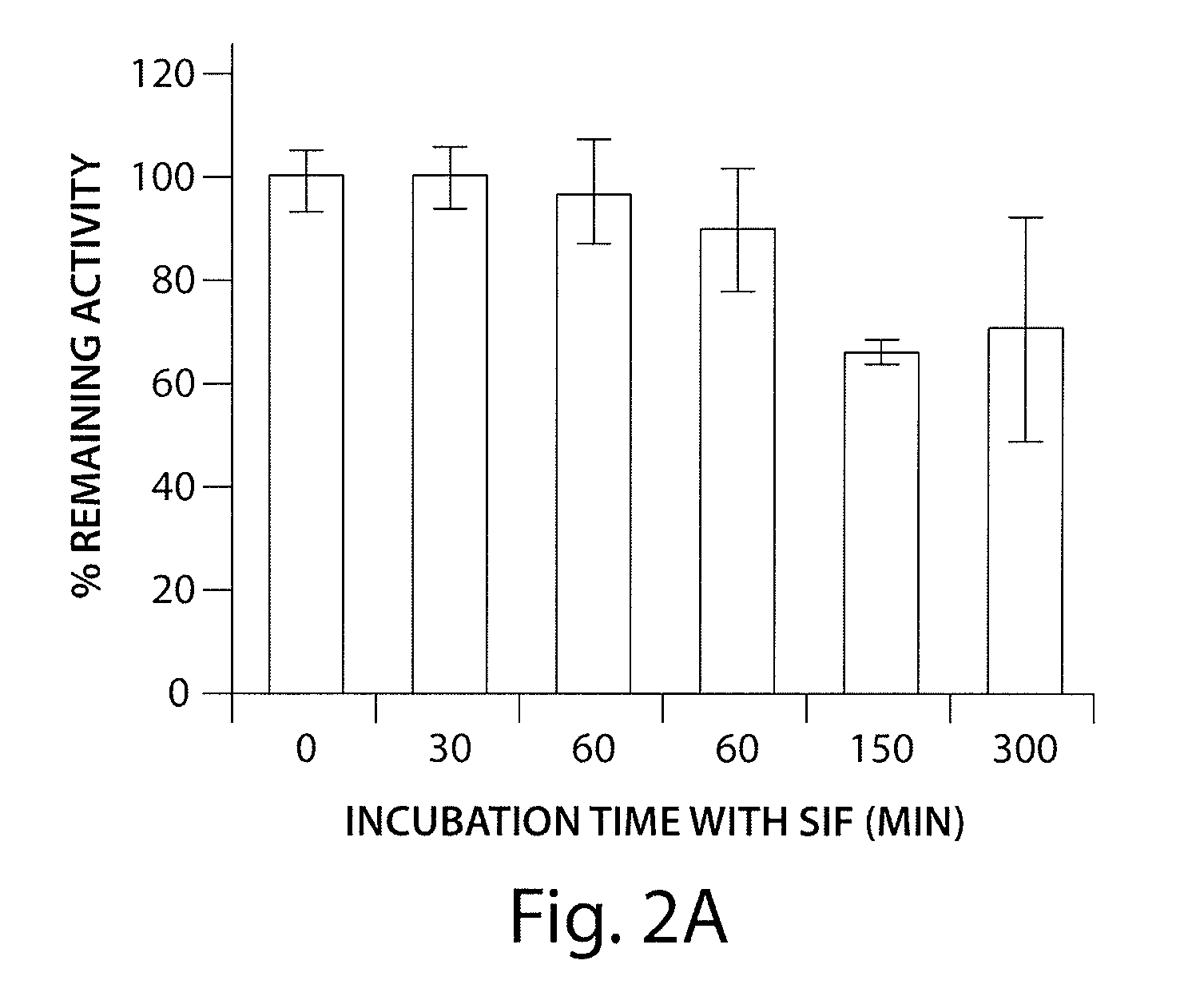
InactiveUS7465444B2Good treatment effectOrganic active ingredientsPeptide/protein ingredientsCrohn's diseaseUlcerative colitis
Antibodies against AILIM (also called ICOS and 8F4) were found to significantly suppress the onset of inflammatory bowel diseases (especially Crohn's disease and colitis (ulcerative colitis and such)), and exhibit a significant therapeutic effect against inflammatory bowel diseases.
Owner:JAPAN TOBACCO INC
Concomitant drug as therapeutic agent for inflammatory bowel disease
An object of the present invention is to provide a medicament efficacious for an inflammatory bowel disease such as ulcerative colitis or Crohn's disease. Specifically, it provides a therapeutic agent for inflammatory bowel diseases comprising active ingredient (a) consisting of at least one compound having inflammatory inhibiting activity selected from the group consisting of an aminosalicylic acid derivative, an antiinflammatory glucocorticoid, an immunosuppressive compound, an anti-TNFα antibody, a neurohypophysial hormone and an antiinfective compound, combined with active ingredient (b) consisting of at least one compound having PPARγ agonistic activity, wherein the agent is so configured that the compound (a) and the compound (b) are used simultaneously, separately or every scheduled time.
Owner:EISIA R&D MANAGEMENT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com