Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Cutaneous T-cell lymphoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cutaneous T-cell lymphoma is a rare type of cancer that begins in the white blood cells and attacks the skin. Cutaneous T-cell lymphoma is one of several types of lymphoma collectively called non-Hodgkin lymphoma.
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Owner:TOPOTARGET GERMANY AG
Histone deacetylase inhibitors
ActiveUS20100056588A1Reduce systemic toxicityUseful in treatmentBiocideOrganic chemistryHypopigmentationHalf-life
In recognition of the need to develop novel therapeutic agents, the present invention provides novel histone deacetylase inhibitors. These compounds include an ester bond making them sensitive to deactivation by esterases. Therefore, these compounds are particularly useful in the treatment of skin disorders. When the compounds reaches the bloodstream, an esterase or an enzyme with esterase activity cleaves the compound into biologically inactive fragments or fragments with greatly reduced activity Ideally these degradation products exhibit a short serum and / or systemic half-life and are eliminated rapidly. These compounds and pharmaceutical compositions thereof are particularly useful in treating cutaneous T-cell lymphoma, neurofibromatosis, psoriasis, hair loss, skin pigmentation, and dermatitis, for example. The present invention also provides methods for preparing compounds of the invention and intermediates thereto.
Owner:DANA FARBER CANCER INST INC +1
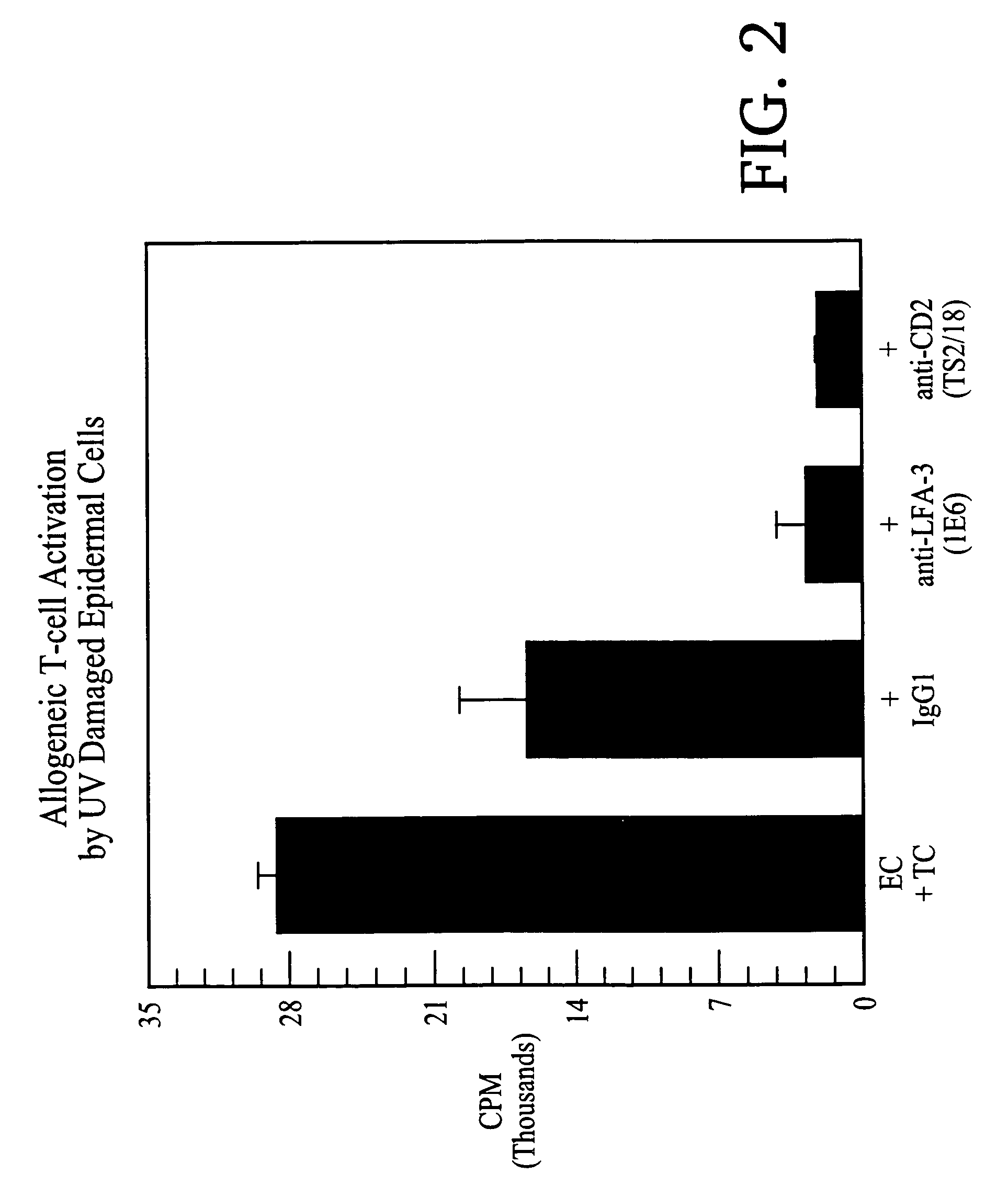
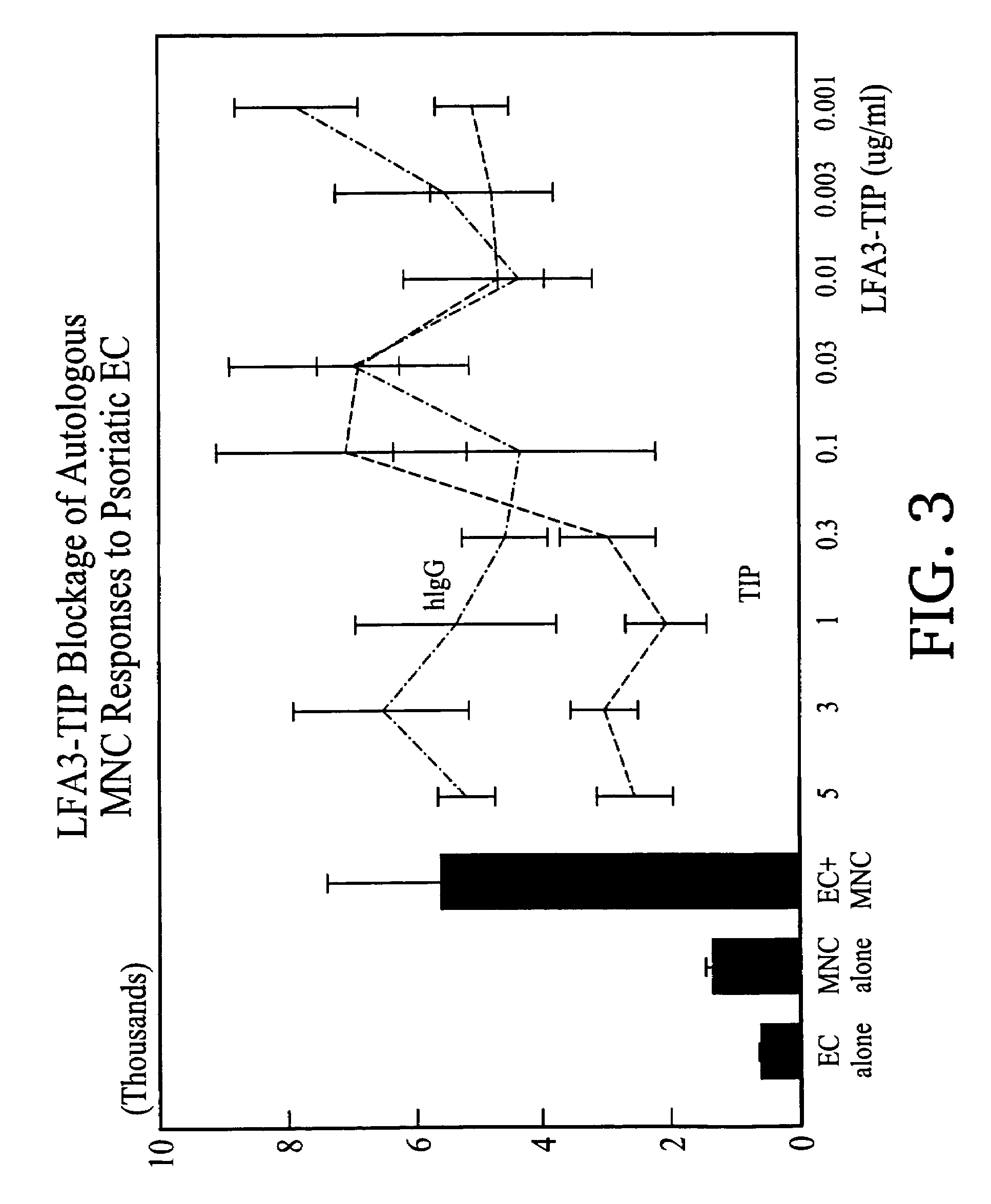
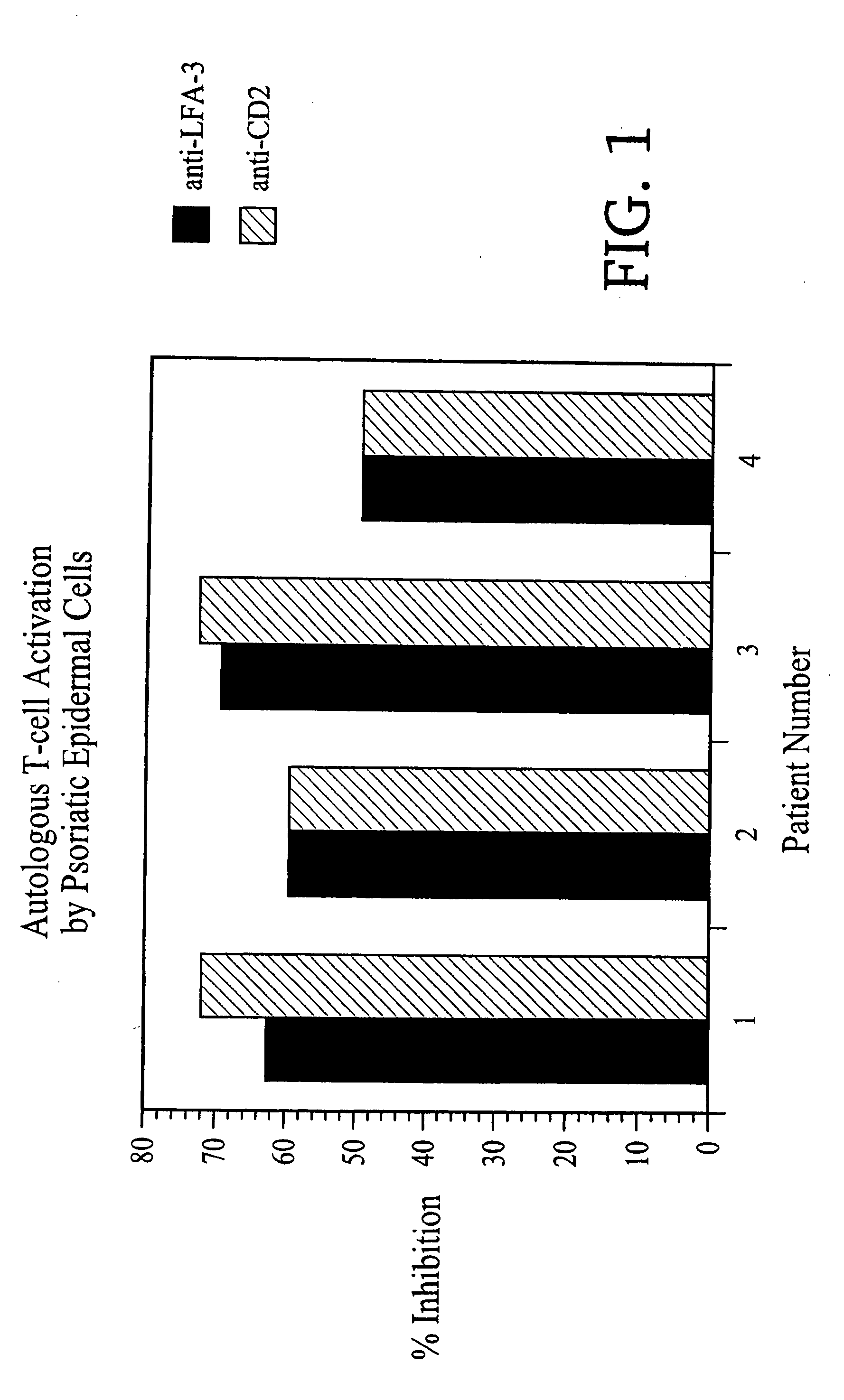
Methods of treating skin conditions using inhibitors of the CD2/LFA-3 interaction
InactiveUS7323171B2Increased activationImprove presentationCompound screeningApoptosis detectionAntigenMammal
Methods of using inhibitors of the CD2 / LFA-3 interaction in treating skin conditions characterized by increased T cell activation and abnormal antigen presentation in the dermis and epidermis in mammals, including humans. Such conditions include psoriasis, UV damage, e.g., photoaging, atopic dermatitis, cutaneous T cell lymphoma such as mycosis fungoides, allergic and irritant contact dermatitis, lichen planus, alopecia areata, pyoderma gangrenosum, vitiligo, ocular cicatricial pemphigoid, and urticaria.
Owner:ASTELLAS US
Methods of treating diseases which are mediated by cutaneous lymphocyte antigen positive cells
ActiveUS8388964B2Improves and prevents and inhibits and reduces skinImproves and prevents and inhibits and reduces pruritisCompounds screening/testingLuminescence/biological staining preparationDiseaseContact dermatitis
The present invention relates to methods of treating patients suffering from itching and puritis mediated by cutaneous lymphocyte antigen positive T cell. In particular, diseases or disorders including contact dermatitis, drug induced delayed type cutaneous allergic reactions, toxic epidermal necrolysis, cutaneous T cell lymphoma, bullous pemphigoid, alopecia aereata, vitiligo, acne rosacea, prurigo nodularis, and herpes simplex virus, or combination thereof will benefit from the administration of an IL-31 antagonist. The invention also includes methods of predicting a therapeutically responsive patient population.
Owner:ZYMOGENETICS INC +1
Methods for treatment of cutaneous T-cell lymphoma
InactiveUS7052685B1Relieve symptomsPeptide/protein ingredientsWhite blood cellCutaneous T-cell lymphoma
A method and composition for treatment of advanced cutaneous T cell lymphoma is provided which involves administration of recombinant interleukin-12.
Owner:UNIV OF PENNSYLVANIA THE TRUSTEES OF THE
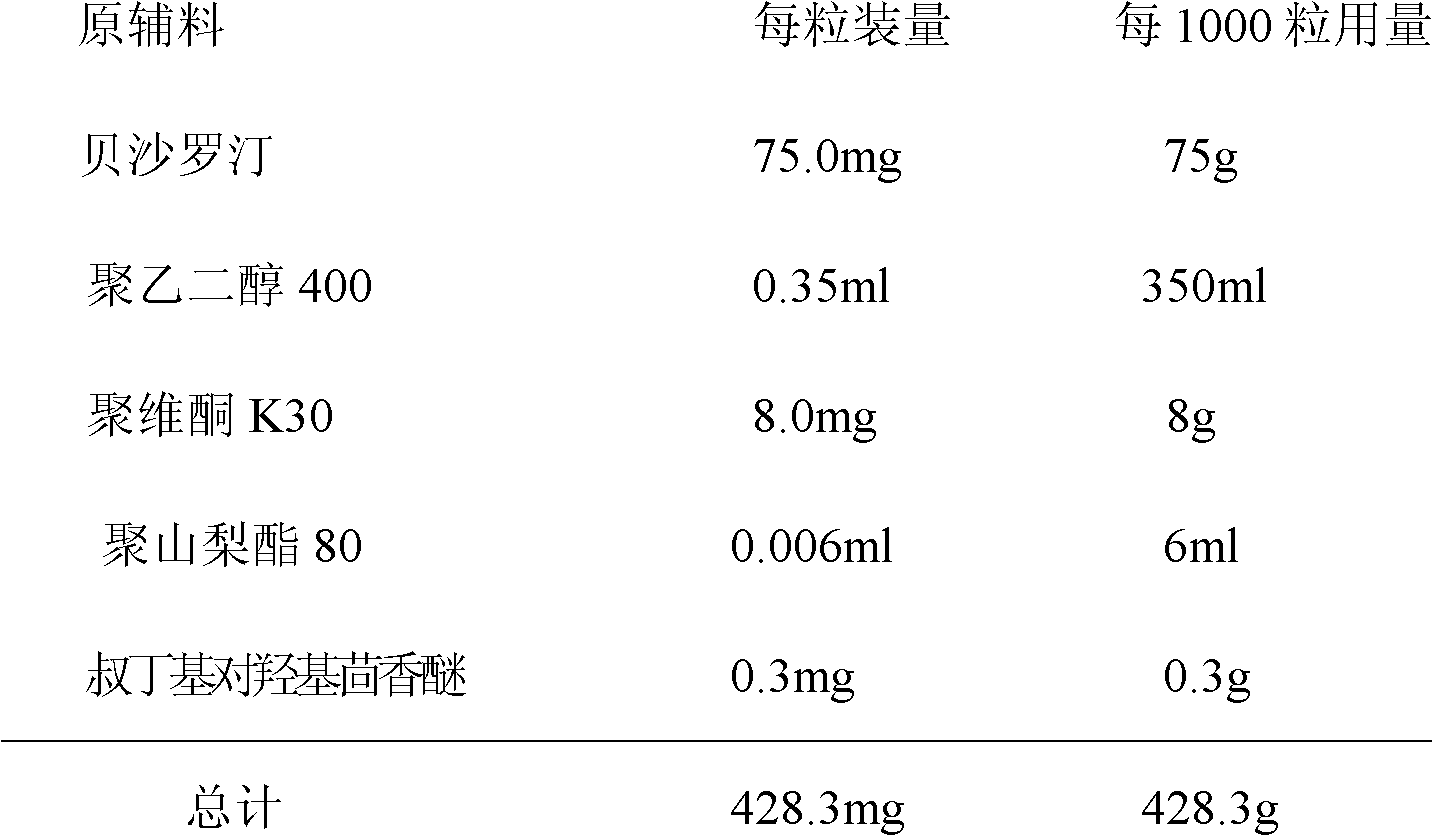
Bexarotene soft capsules and preparation method thereof
InactiveCN103181912AQuality improvementEvenly dispersedOrganic active ingredientsPharmaceutical non-active ingredientsButylated hydroxyanisoleSide effect
The invention discloses bexarotene soft capsules for treating cutaneous T-cell lymphoma and a preparation method thereof. The bexarotene soft capsules consist of bexarotene, polyethylene glycol 400, polysorbate 80, povidone K30 and butylated hydroxyanisole. The soft capsules can cover up bitterness of drug and discomfortable odor, make up the defects of other solid preparations and improve drug stability. Application of the single bexarotene soft capsules improves effectiveness, safety and medication compliance of clinical medication and greatly reduces toxic or side effects of combined medication.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
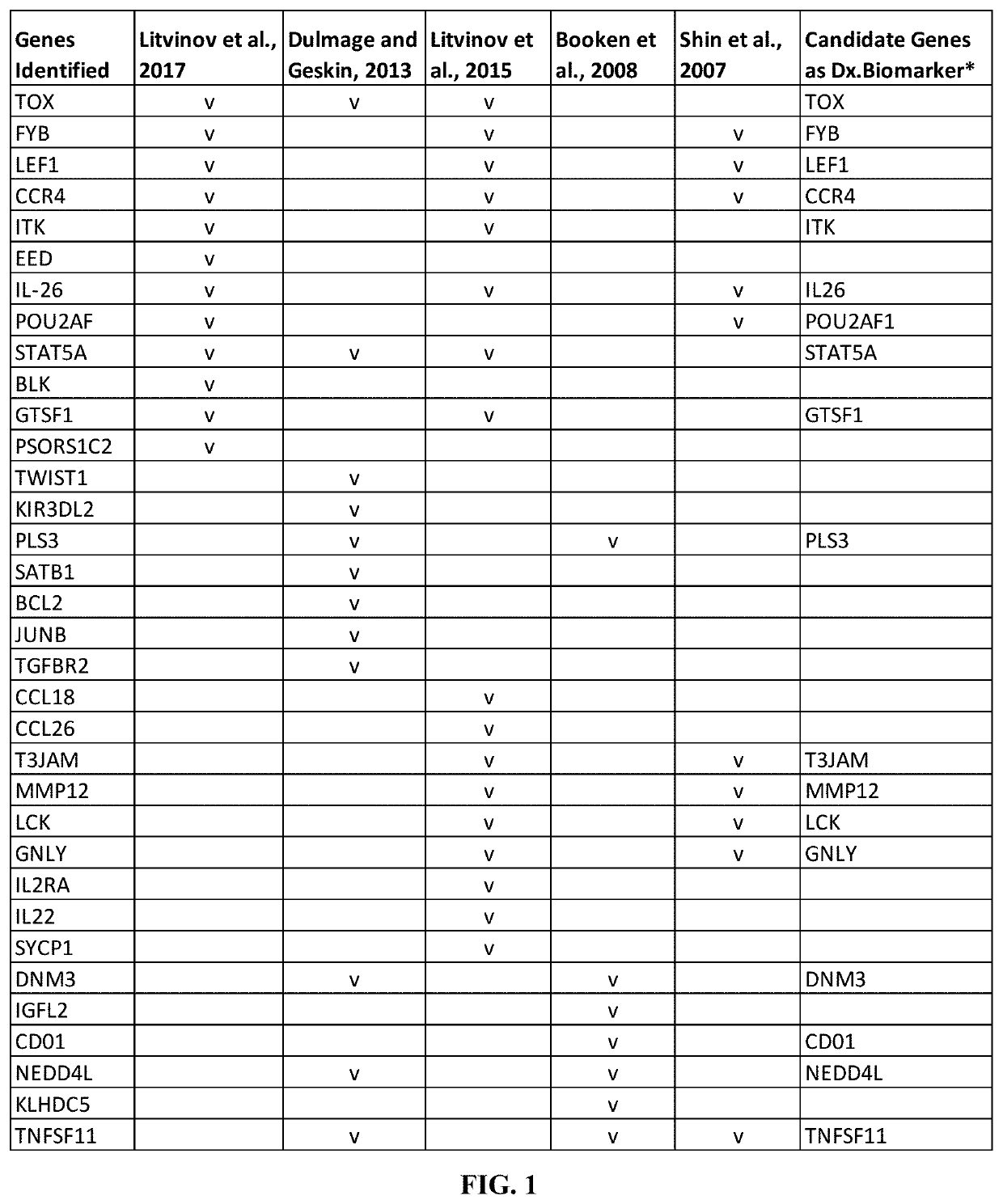
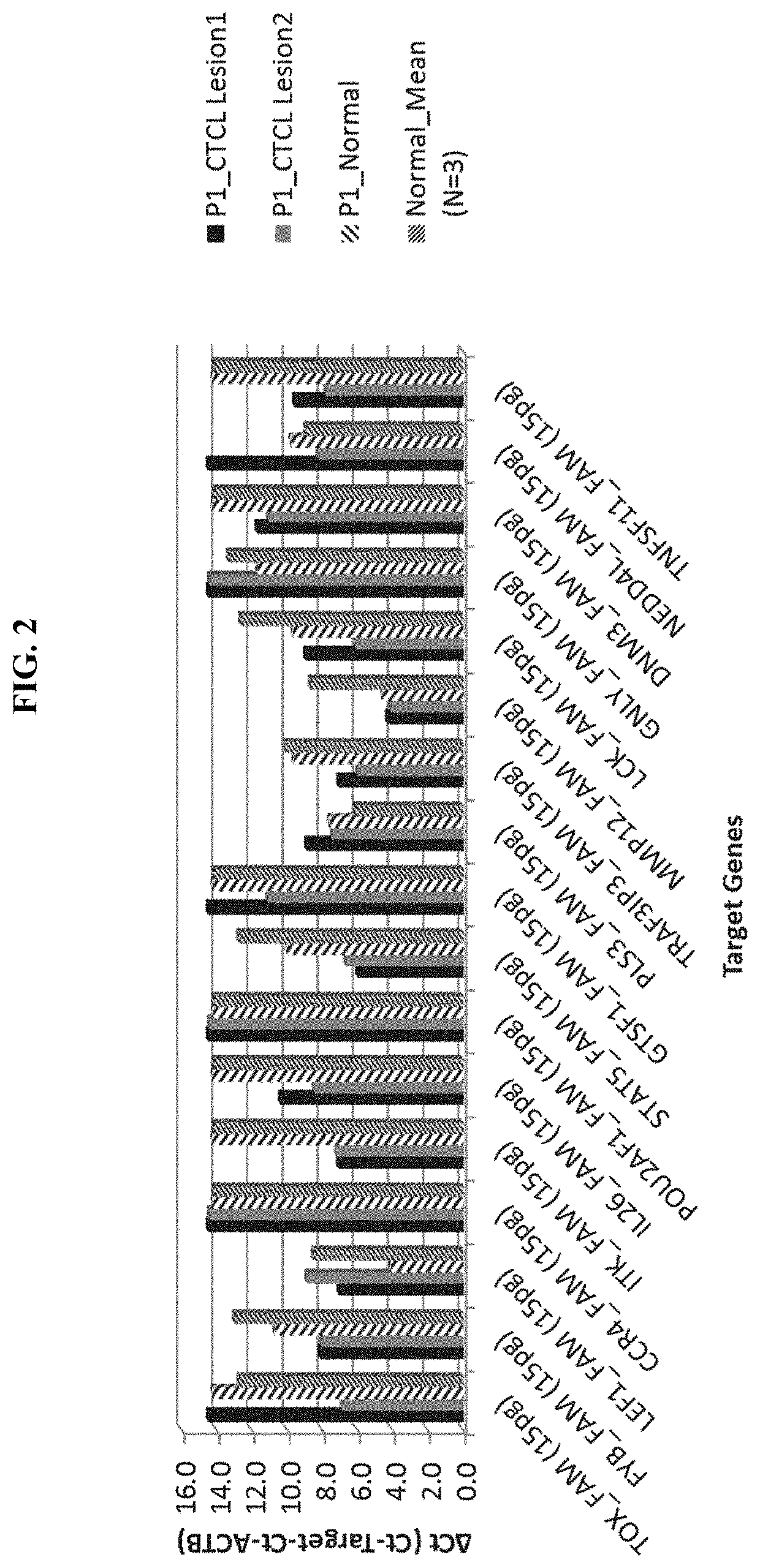
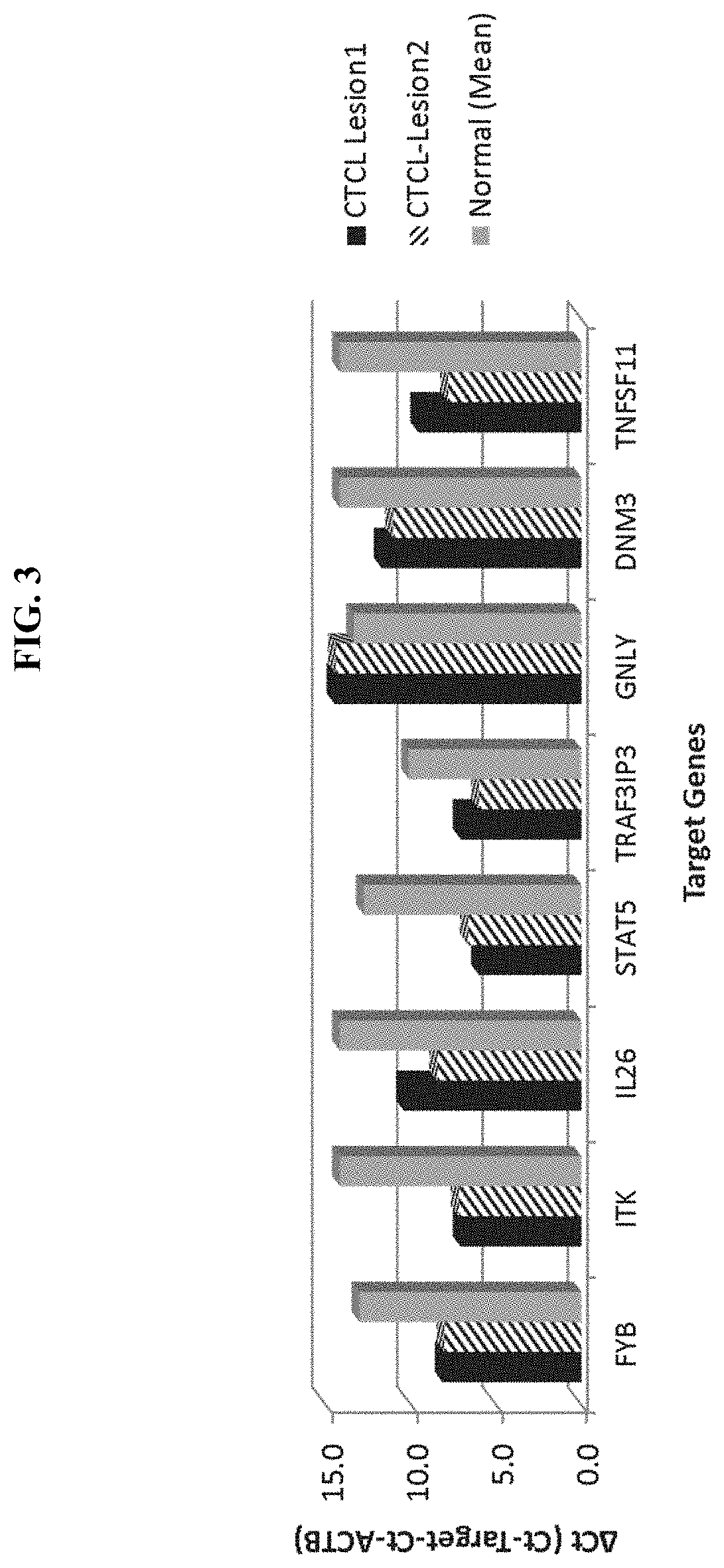
Novel gene classifiers and uses thereof in skin cancers
ActiveUS20200308657A1Organic active ingredientsPeptide/protein ingredientsGranulomaCutaneous T-cell lymphoma
Owner:DERMTECH INC
Methods for diagnosis and treatment of cutaneous t cell lymphomas
InactiveUS20120288499A1Microbiological testing/measurementLibrary screeningAbnormal expressionCutaneous T-cell lymphoma
The present invention relates to a method for diagnosing a cutaneous T cell lymphoma (CTCL) in a patient, detecting the abnormal expression of NKp46 receptor on malignant T cells and to a kit for diagnosing a CTCL in a patient, said kit comprising an antibody capable of detecting NKp46 receptor and an antibody capable of detecting T cells. Furthermore, the invention refers to an anti-NKp46 antibody for use in the treatment of CTCL and a pharmaceutical composition for the treatment of CTCL comprising said anti-NKp46 antibody.
Owner:UNIV PARIS DIDEROT PARIS 7 +2
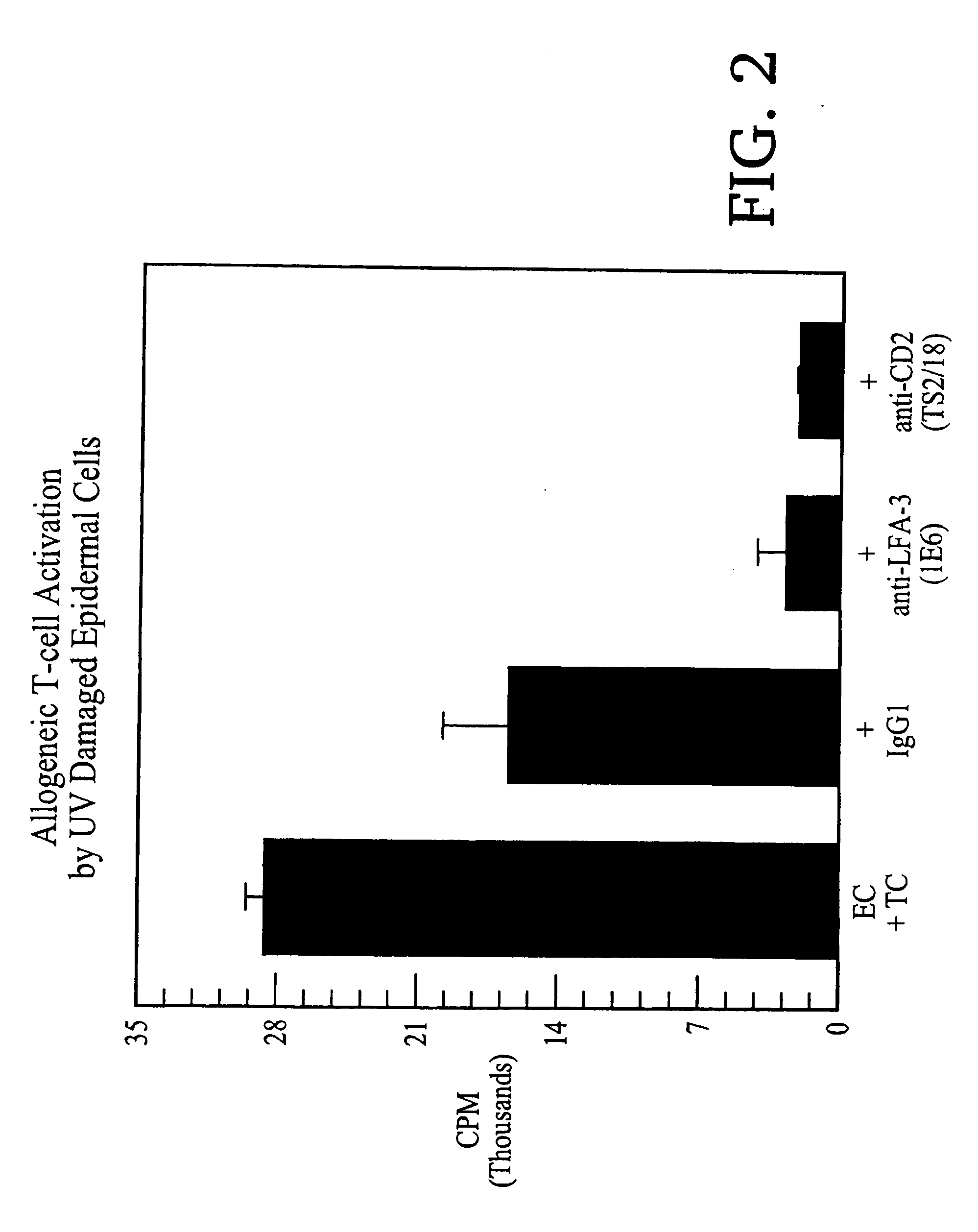
Methods of treating skin conditions using inhibitors of the CD2/LFA-3 interaction
InactiveUS20060084107A1Increased activationImprove presentationCompound screeningImmunoglobulin superfamilyAntigenMammal
Methods of using inhibitors of the CD2 / LFA-3 interaction-in treating skin conditions characterized by increased T cell activation and abnormal antigen presentation in the dermis and epidermis in mammals, including humans. Such conditions include psoriasis, UV damage, e.g., photoaging, atopic dermatitis, cutaneous T cell lymphoma such as mycosis fungoides, allergic and irritant contact dermatitis, lichen planus, alopecia areata, pyoderma gangrenosum, vitiligo, ocular cicatricial pemphigoid, and urticaria.
Owner:ASTELLAS US
Method for treating cell proliferation disorders
InactiveUS20150313896A1Reduction of cell proliferation disorderReduce proliferationBiocideCosmetic preparationsAdrenergicCutaneous T-cell lymphoma
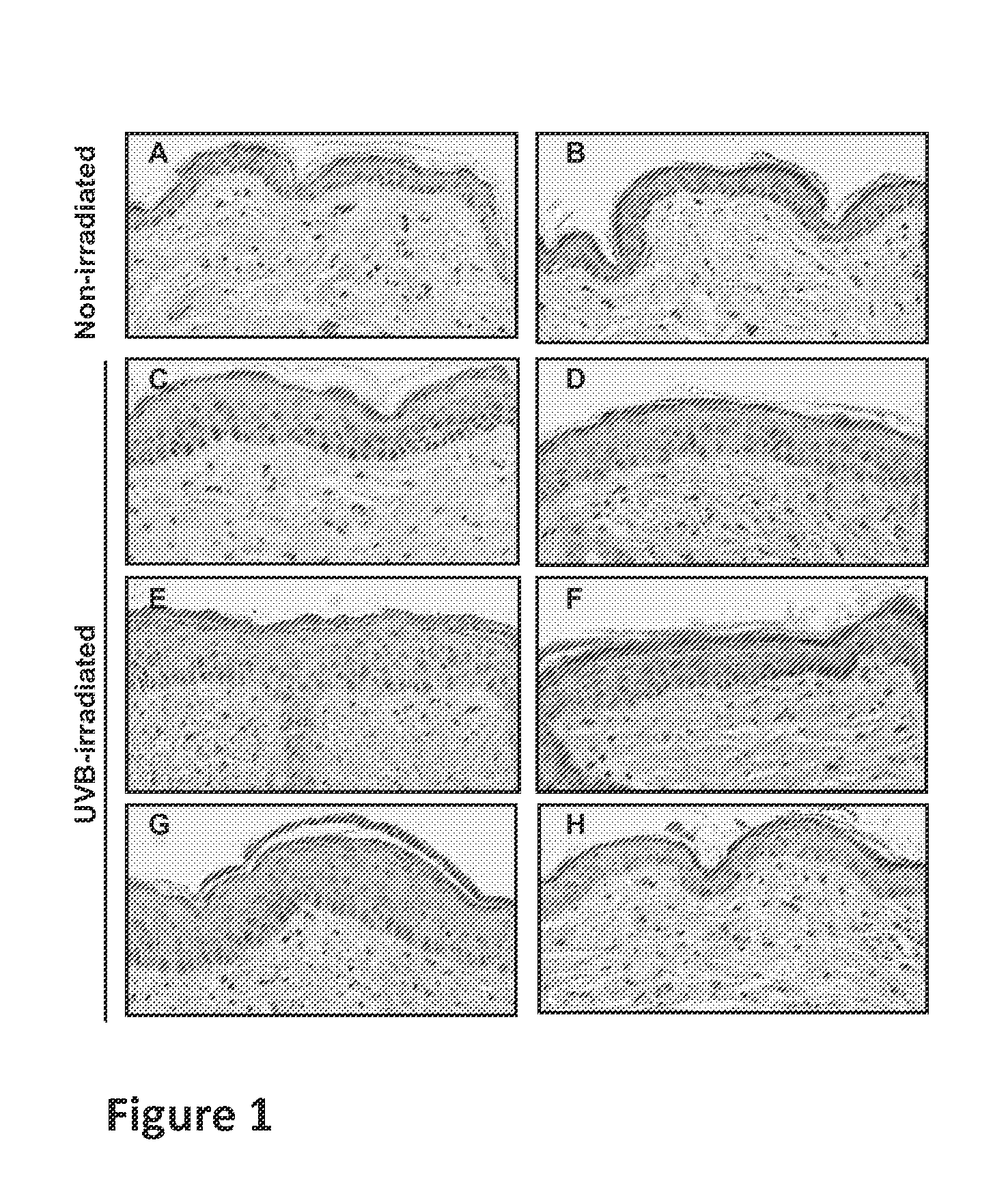
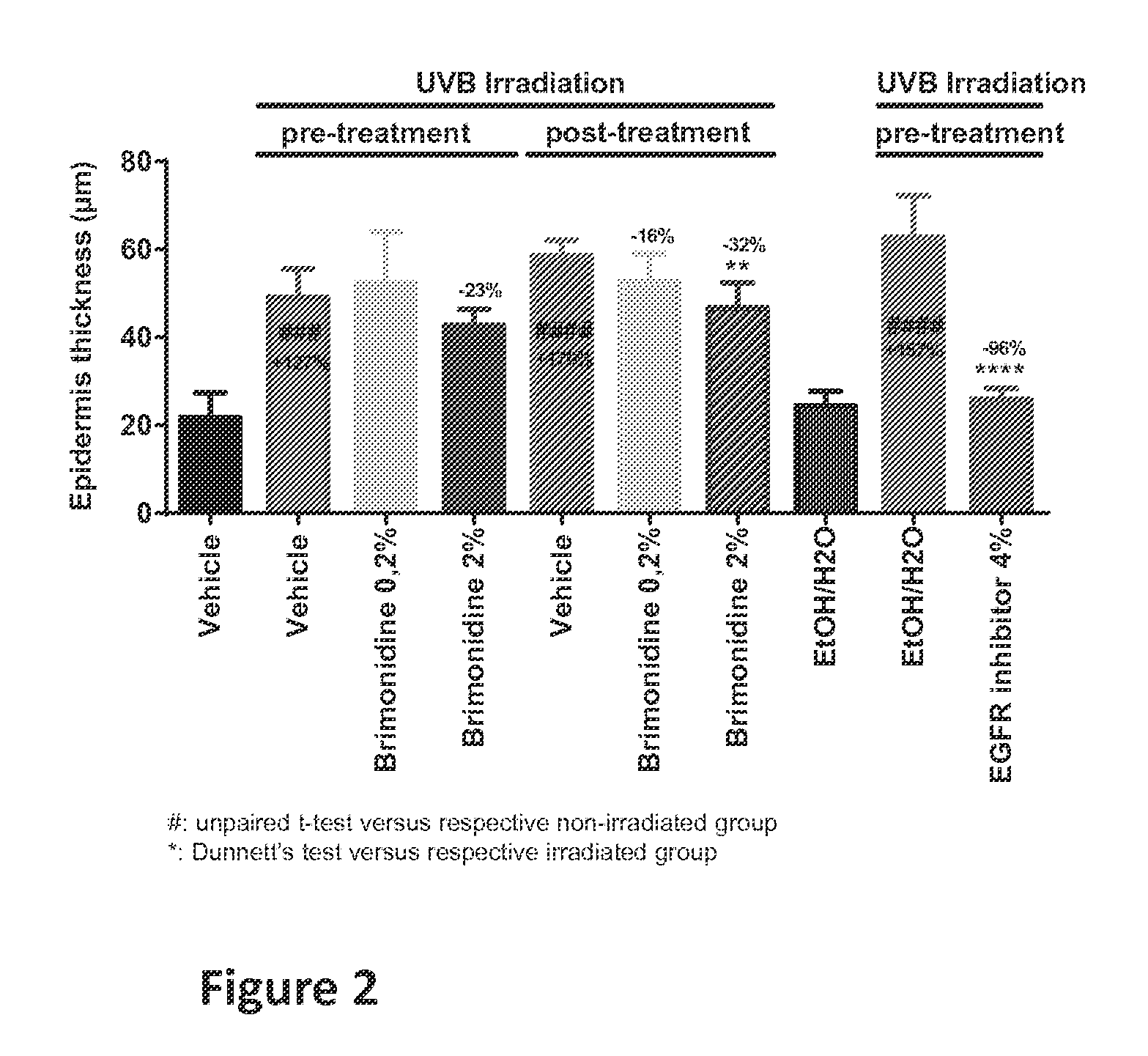
Methods, compositions and products for treating or reducing a cell proliferation disorder, such as hand and foot syndrome or cutaneous T cell lymphoma, in a subject in need thereof are described. The methods involve topically administering to the subject a composition containing an α adrenergic receptor agonist, such as brimonidine.
Owner:GALDERMA RES & DEV SNC
miR-155 inhibitors for treating cutaneous T cell lymphoma (CTCL)
InactiveUS9771585B2Reducing and inhibiting proliferationReduced activitySugar derivativesGenetic material ingredientsMedicineCutaneous T-cell lymphoma
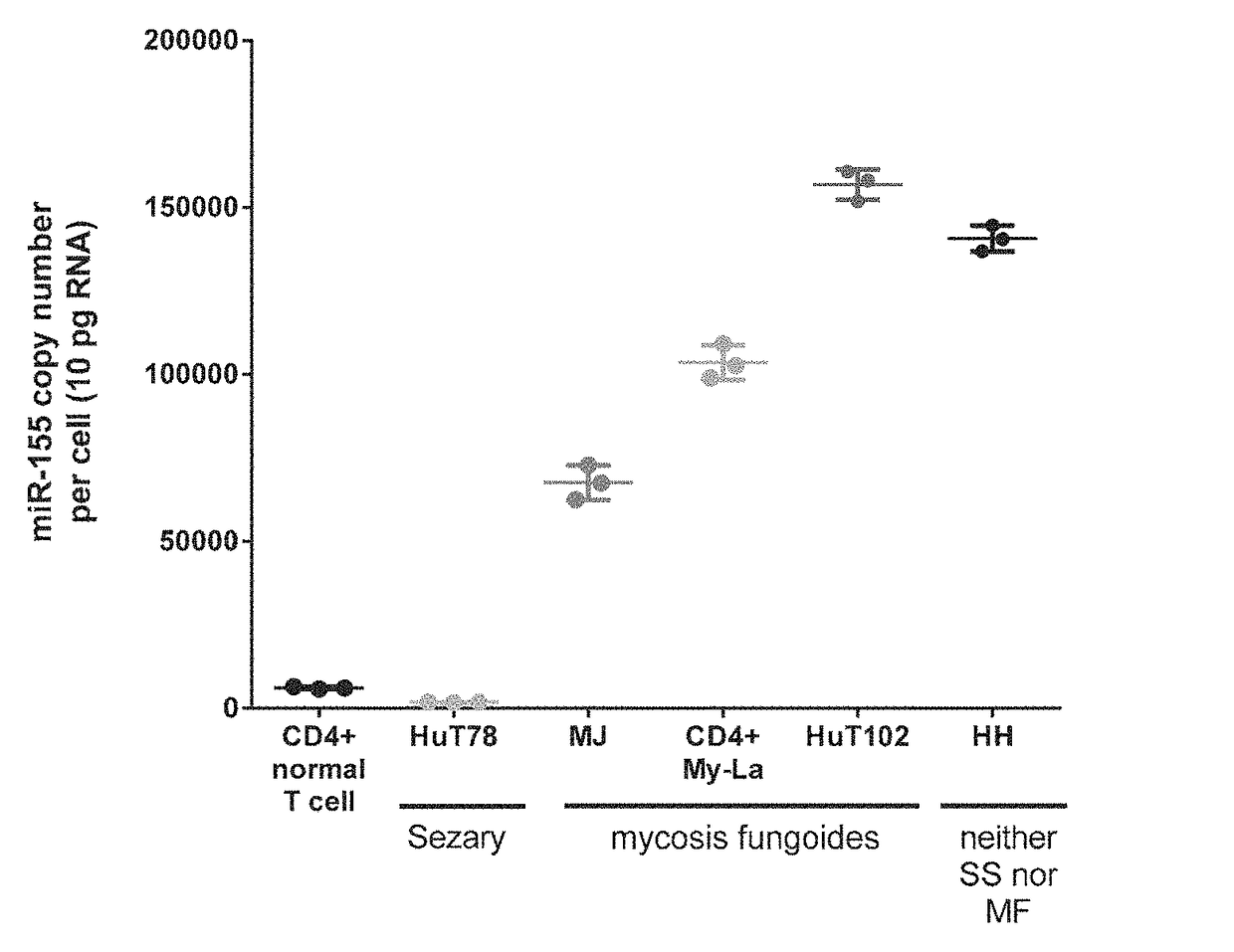
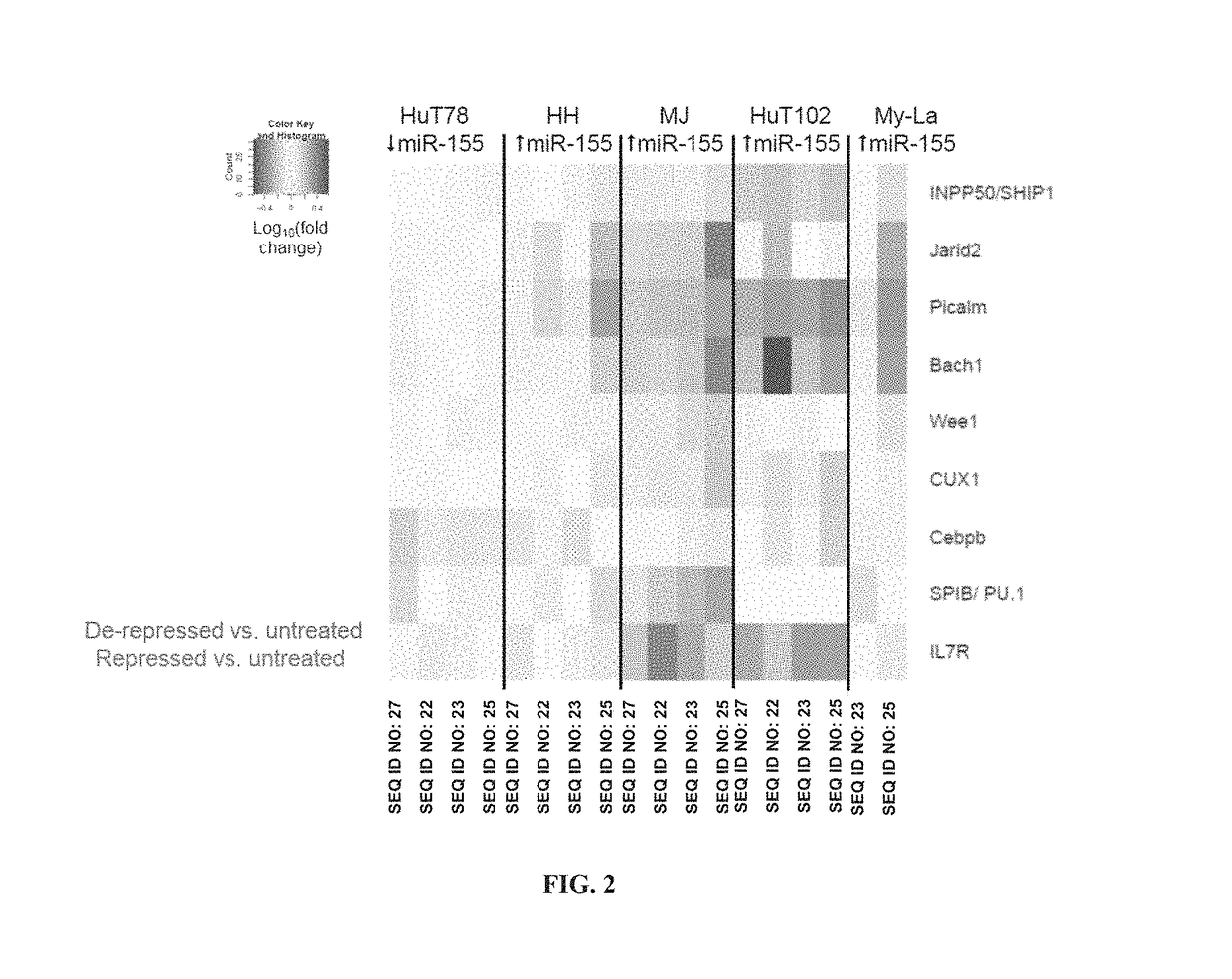
The present invention provides oligonucleotide inhibitors of miR-155 and compositions thereof. The invention further provides methods for treating cancer such as a T cell lymphoma in a subject by administering to the subject an oligonucleotide inhibitor of miR-155. The invention also provides methods for reducing or inhibiting the proliferation of malignant T cells by administering an oligonucleotide inhibitor of miR-155.
Owner:MIRAGEN THERAPEUTICS
Methods of treating diseases which are mediated by cutaneous lymphocyte antigen positive cells
ActiveUS20110212093A1Improves and prevents and inhibits and reduces skinImproves and prevents and inhibits and reduces pruritisCompounds screening/testingLuminescence/biological staining preparationContact dermatitisCutaneous T-cell lymphoma
The present invention relates to methods of treating patients suffering from itching and puritis mediated by cutaneous lymphocyte antigen positive T cell. In particular, diseases or disorders including contact dermatitis, drug induced delayed type cutaneous allergic reactions, toxic epidermal necrolysis, cutaneous T cell lymphoma, bullous pemphigoid, alopecia aereata, vitiligo, acne rosacea, prurigo nodularis, and herpes simplex virus, or combination thereof will benefit from the administration of an IL-31 antagonist. The invention also includes methods of predicting a therapeutically responsive patient population.
Owner:ZYMOGENETICS INC +1
Dosing regimens for treatment of proliferative disorders comprising administration of sapacitabine
ActiveUS8536188B2Maximize efficiencyMinimising adverse side effectOrganic active ingredientsBiocideSapacitabineDosing regimen
One aspect of the present invention relates to the use of sapacitabine, or a metabolite thereof, in the preparation of a medicament for treating a proliferative disorder, wherein the sapacitabine or metabolite thereof is administered in a dosing regimen comprising at least one treatment cycle, wherein said treatment cycle comprises administering a therapeutically effective amount of sapacitabine or metabolite thereof for about 2 to about 6 days per week, for 2 weeks out of 3 weeks. Another aspect of the invention relates to the use of sapacitabine, or a metabolite thereof, in the preparation of a medicament for treating cutaneous T-cell lymphoma (CTCL).
Owner:CYCLACEL
Use of neurokinin-1 antagonists to treat a variety of pruritic conditions
InactiveUS20190216779A1Shorten the progressReduce severityDigestive systemBoron compound active ingredientsDiseasePrurigo
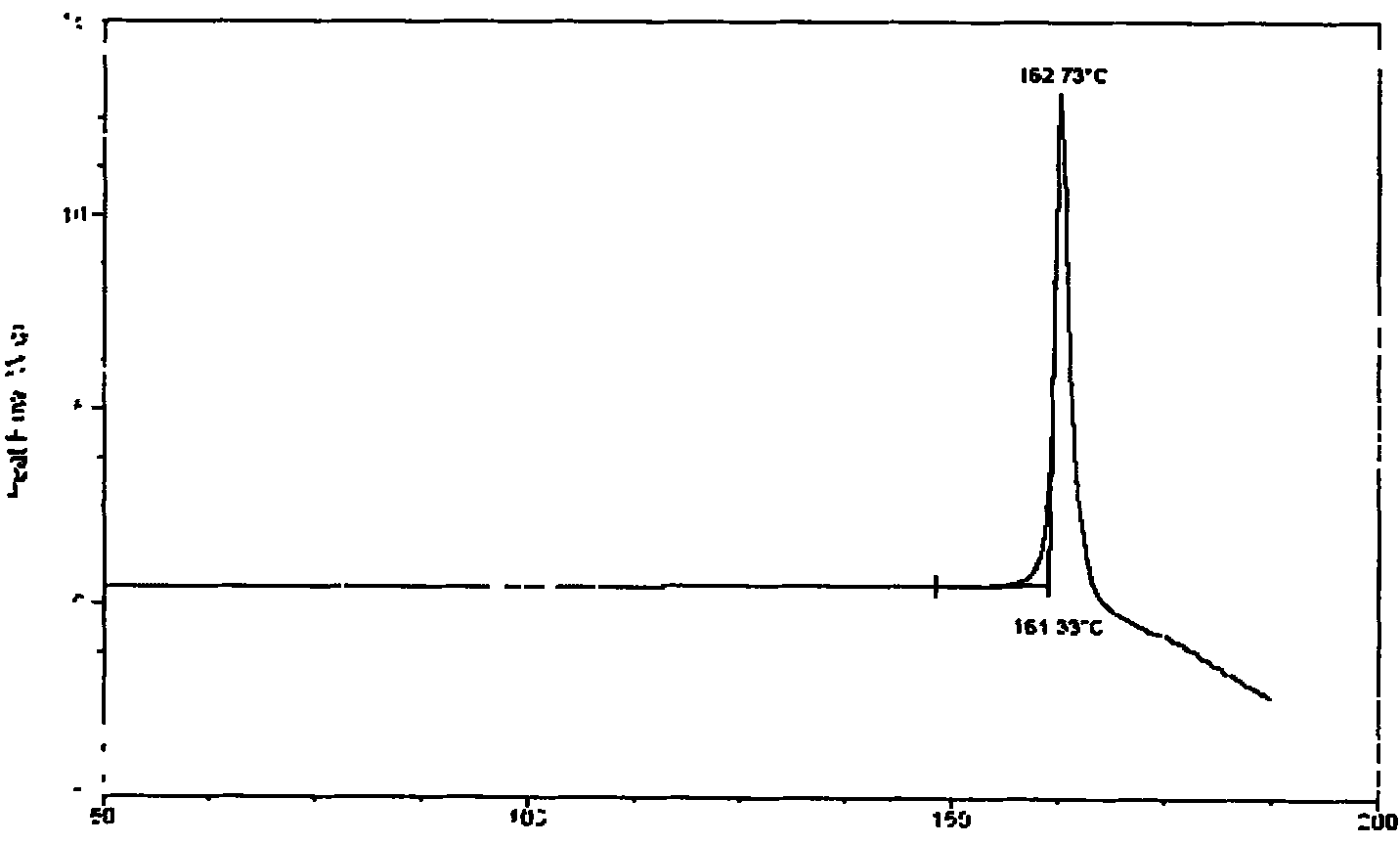
The disclosure relates to the use of neurokinin-1 (NK-1) antagonists, such as serlopitant, in treating acute or chronic pruritus associated with a variety of medical conditions, including dermatitis / eczema, psoriasis, prurigo, urticaria, cutaneous T-cell lymphoma, epidermolysis bullosa, bums and hepato-biliary diseases, or / and treating the medical conditions themselves. One or more additional antipruritic or therapeutic agents can optionally be used in combination with an NK-1 antagonist to treat acute or chronic pruritus associated with a medical condition or / and the medical condition itself.
Owner:VYNE THERAPEUTICS INC
Dosing regimens for treatment of proliferative disorders comprising administration of sapacitabine
ActiveUS20100125084A1Good antitumor activityImproved ease of handlingOrganic active ingredientsBiocideDosing regimenSapacitabine
One aspect of the present invention relates to the use of sapacitabine, or a metabolite thereof, in the preparation of a medicament for treating a proliferative disorder, wherein the sapacitabine or metabolite thereof is administered in a dosing regimen comprising at least one treatment cycle, wherein said treatment cycle comprises administering a therapeutically effective amount of sapacitabine or metabolite thereof for about 2 to about 6 days per week, for 2 weeks out of 3 weeks. Another aspect of the invention relates to the use of sapacitabine, or a metabolite thereof, in the preparation of a medicament for treating cutaneous T-cell lymphoma (CTCL).
Owner:CYCLACEL
Preparation method and preparation of vorinostat I crystal form large grains
ActiveCN102643214AGood dissolution effectHigh commercial application valueOrganic active ingredientsOrganic chemistryMedicineCutaneous T-cell lymphoma
The invention provides a preparation method of vorinostat I crystal form large grains as a medicament for treating cutaneous T cell lymphoma and a preparation prepared from vorinostat obtained by the preparation method. The preparation method can crystallize in place once, is simple, effective and suitable for large-scale industrial production and further has greater commercial application value. The obtained preparation is good in dissolution effect.
Owner:杭州容立医药科技有限公司
Combination comprising CNDAC (2′-cyano-2′-deoxy-N4-palmitoyl-1-beta-D-arabinofuranosyl-cytosine) and a cytotoxic agent
A first aspect of the invention relates to a combination comprising 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A second aspect relates to a pharmaceutical product comprising (i) 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and (ii) a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect relates to a method of treating a proliferative disorder, said method comprising simultaneously, separately or sequentially administering to a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A fourth aspect of the invention relates to the use of a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating cutaneous T-cell lymphoma (CTCL).
Owner:CYCLACEL
Compositions, methods and kits for diagnosis and treatment of Chlamydia pneumoniae infections of the skin and those associated with cutaneous T-cell lymphoma
InactiveUS7282343B1Energy modified materialsTetracycline active ingredientsAntigenCutaneous T-cell lymphoma
This invention provides methods of diagnosing and treating any C. pneumoniae infection of the skin including C. pneumoniae-associated diseases such as cutaneous T-cell lymphoma (CTCL), mycosis fungoides, Sézary syndrome, lymphomatoid papillosis, Ki-1 lymphoma, exfoliative exematous rash, and digitate parapsoriasis. This invention provides kits that are useful in the methods of the invention as well as for identifying new anti-chlamydial agents in treating skin infections. This invention includes a pharmaceutical vaccine composition comprising an antigen, including a full length antigenic determinant, such as any SAF positive determinant or portion thereof, which produces a detectable immune, humoral and / or cellular response to C. pneumoniae. The invention also includes a method of treating an active CTCL in a living being comprising delivering a therapeutically effective amount of a vaccine, wherein the vaccine comprises an agent, such as an inactivated C. pneumoniae material that produces a detectable immune, humoral and / or cellular response.
Owner:BRILLMAN CINDY +1
Il-21 antagonists
ActiveUS20090075341A1Increasing in vivo serum half-lifeModulate antibody responseAntipyreticAntibody mimetics/scaffoldsAutoimmune conditionAnkylosing spondylitis
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
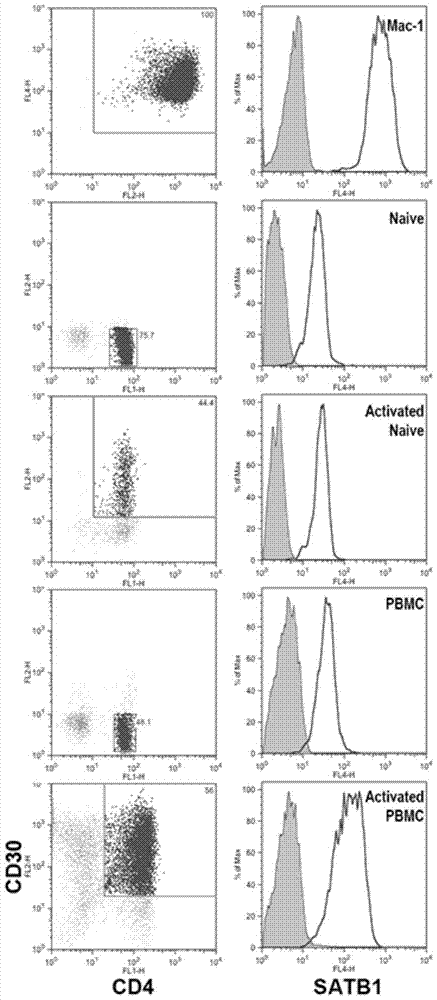
Application of SATB1 to treatment of cutaneous T cell lymphomata
ActiveCN103920150ABlock cyclePromote apoptosisGenetic material ingredientsAntibody ingredientsSATB1Nucleotide
The invention discloses application of SATB1 to treatment of cutaneous T cell lymphomata. The invention discloses application of a product taking the following any one substance as a target to preparation of medicaments for preventing and / or treating cutaneous T cell lymphomata: (1) a protein shown in SEQ ID No.1; (2) a DNA molecule shown in SEQ ID No.2; (3) a DNA molecule shown in nucleotides from 1736th to 4024th beginning from the 5'-end in SEQ ID No.2. SATB1 protein is specifically expressed in CD30+ tumorous T cells in cutaneous T cell lymphomata, the expression up-regulation of the SATB1 protein plays a major role in attack and development of the cutaneous T cell lymphomata through promoting the cell cycle development, and SATB1 serving as a target has a bright application prospect in treatment of cutaneous T cell lymphomata.
Owner:PEKING UNIV FIRST HOSPITAL
Mir-155 inhibitors for treating cutaneous t cell lymphoma (CTCL)
InactiveUS20160355814A1Reducing and inhibiting proliferationReduced activityGenetic material ingredientsMicrobiological testing/measurementCutaneous T-cell lymphomaT cell
The present invention provides oligonucleotide inhibitors of miR-155 and compositions thereof. The invention further provides methods for treating cancer such as a T cell lymphoma in a subject by administering to the subject an oligonucleotide inhibitor of miR-155. The invention also provides methods for reducing or inhibiting the proliferation of malignant T cells by administering an oligonucleotide inhibitor of miR-155.
Owner:MIRAGEN THERAPEUTICS
Butyroyloxymethyl diethyl phosphate compounds and uses thereof
InactiveUS20170189376A1Improved therapeutic responseGood curative effectOrganic active ingredientsButyroyloxymethyl-diethyl phosphateCutaneous T-cell lymphoma
Methods for treating ameliorating, reducing and / or preventing a cutaneous T-cell lymphoma in a subject in need thereof, comprising administration butyroyloxymethyl diethyl phosphate (AN-7) alone or combined with an additional anti-cancer therapy.
Owner:MOR RES APPL LTD +1
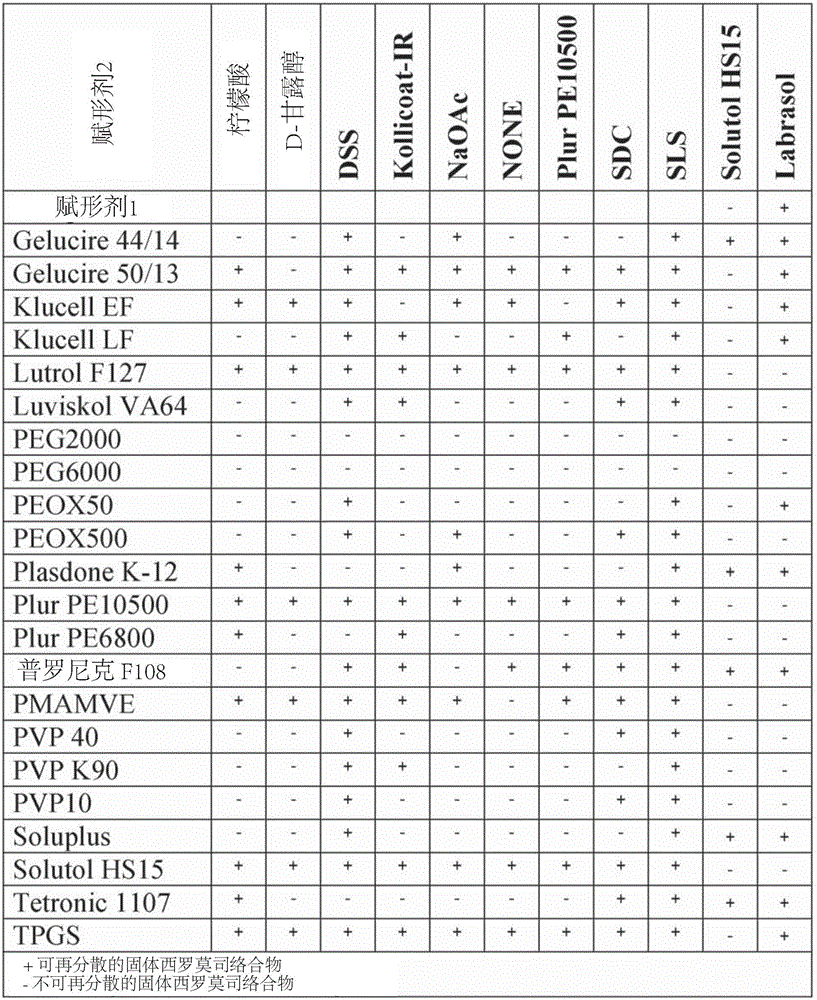
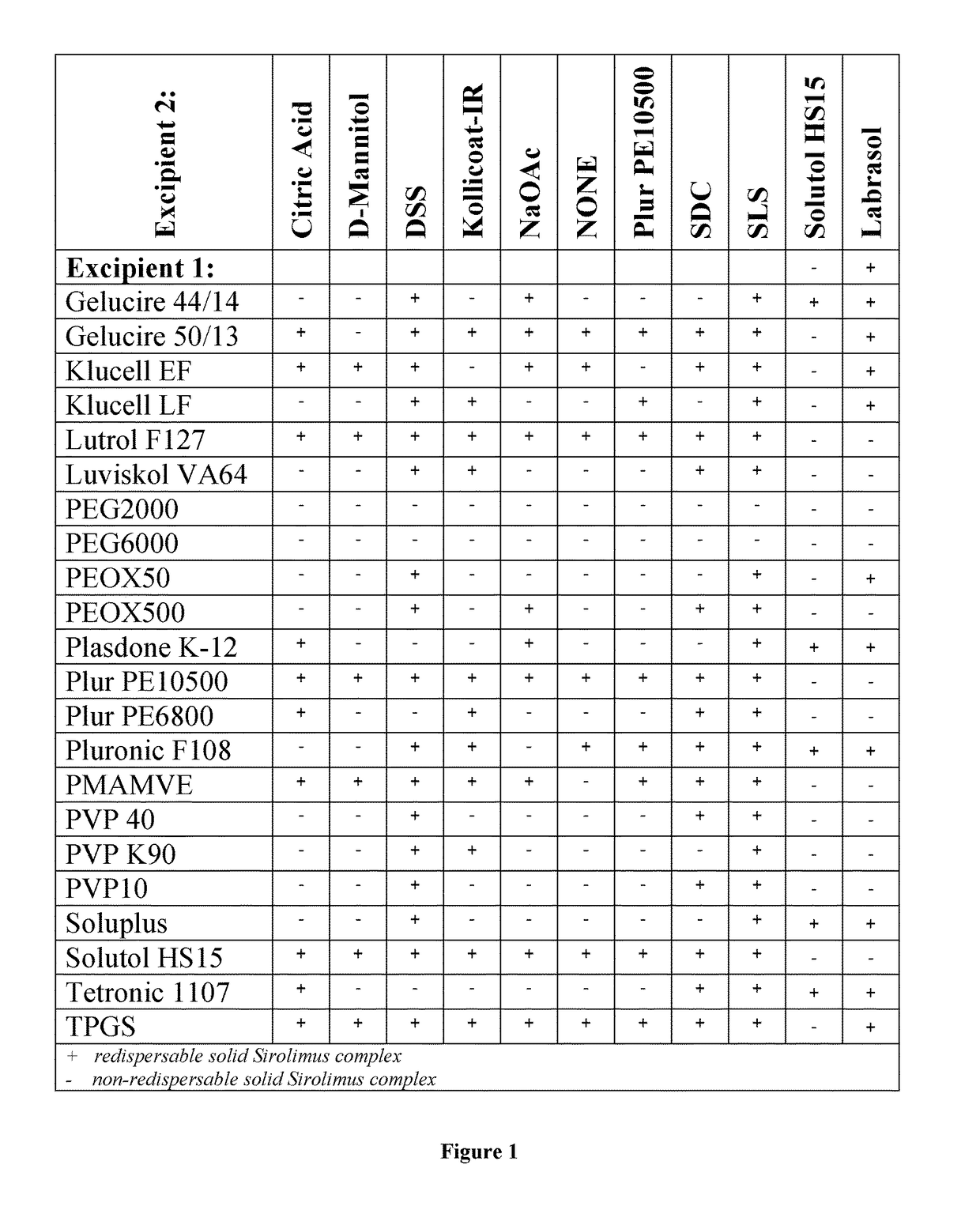
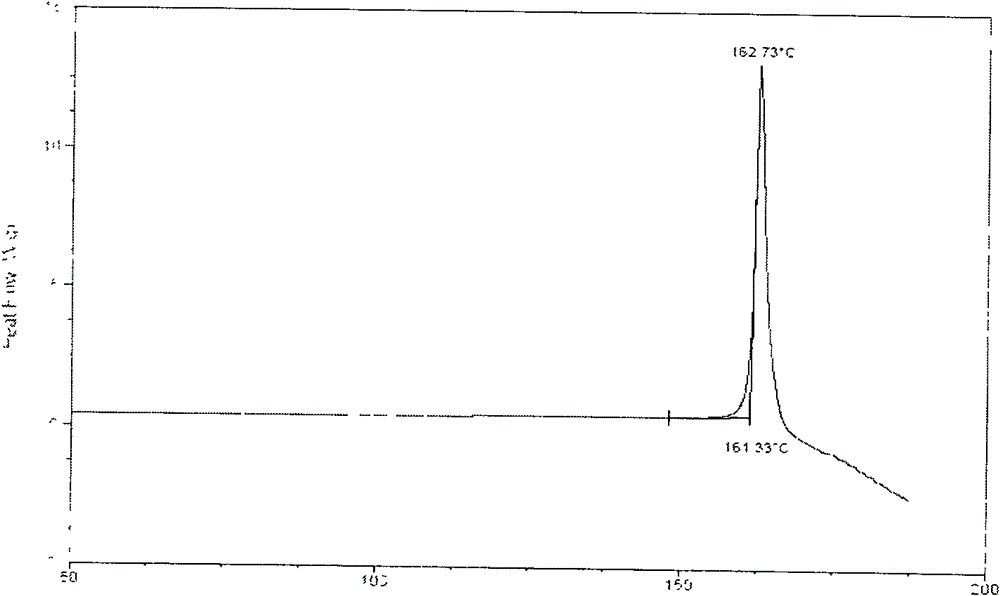
Complexes of sirolimus and its derivatives, process for the preparation thereof and pharmaceutical compositions containing them
InactiveCN106456780AImprove solubilityImprove permeabilityOrganic active ingredientsPowder deliverySolubilityRenal transplant
Owner:DRUGGABILITY TECH IP HOLDCO
Methods for use of TARGRETIN in organ transplant rejection, autoimmune diseases and cancer
InactiveUS20050209335A1Raise countBoosting immune systemBiocideAnimal repellantsCo administrationAutoimmune condition
Methods are provided for treatment of graft versus host disease and other autoimmune diseases, organ transplant rejection, acute myeloid leukemia and T-cell non-Hodgkin's lymphoma other than cutaneous T-cell lymphoma in patients by administration of TARGRETIN. Also provided are methods for increasing neutrophil count and boosting the immune system of patients by administration of TARGRETIN and methods for enhancing efficacy of a cancer chemotherapeutic agent by co-administration of the cancer chemotherapeutic agent with TARGRETIN.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method of treatment using il-13r antibody
InactiveUS20200165347A1Extended half-lifeLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCutaneous T-cell lymphomaAnti antibody
The present disclosure provides a method of treating cutaneous T cell lymphoma (CTCL), in particular mycosis fungoides and / or Sézary syndrome, comprising administering a therapeutically effective amount of an antagonist antibody or binding fragment thereof specific to the IL-13 receptor to a patient in need thereof.
Owner:ASLAN PHARMA PTE LTD +1
Combination comprising cndac (2'-cyano-2'-deoxy-n4-palmitoyl-1-beta-d-arabinofuranosyl-cytosine) and a cytotoxic agent
ActiveUS20100069291A1Low toxicityGood effectBiocidePeptide/protein ingredientsMetaboliteCytotoxicity
A first aspect of the invention relates to a combination comprising 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A second aspect relates to a pharmaceutical product comprising (i) 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and (ii) a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect relates to a method of treating a proliferative disorder, said method comprising simultaneously, separately or sequentially administering to a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from: (a) a HDAC inhibitor; and (b) a topoisomerase inhibitor selected from etoposide, topotecan and SN-38, or a prodrug thereof. A fourth aspect of the invention relates to the use of a subject 2′-cyano-2′-deoxy-N4-palmitoyl-1-β-D-arabinofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating cutaneous T-cell lymphoma (CTCL).
Owner:CYCLACEL
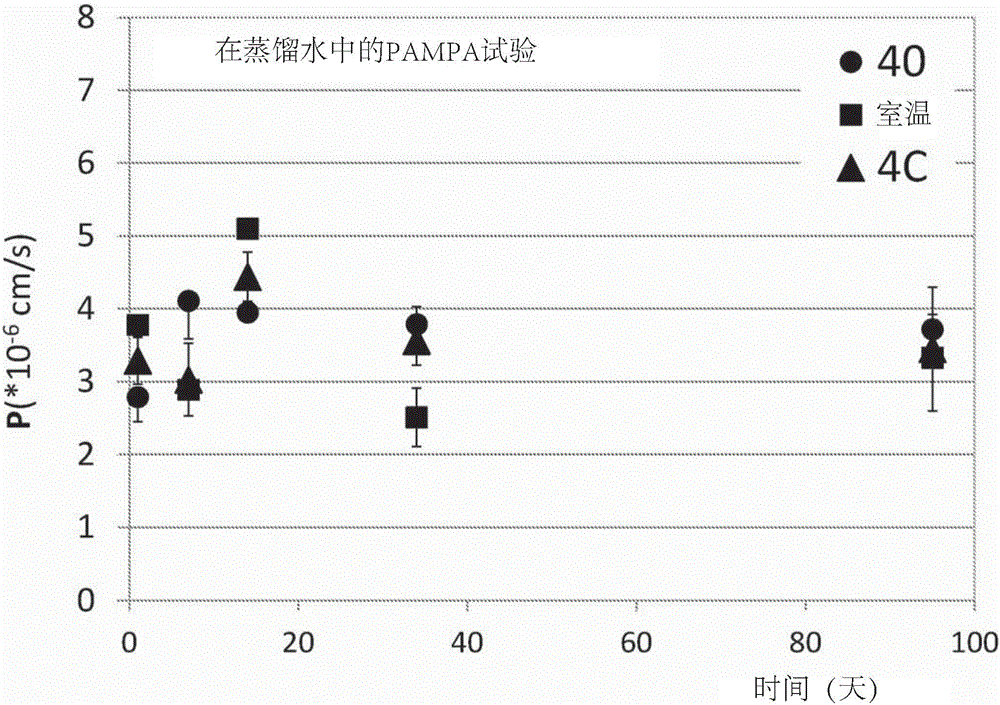
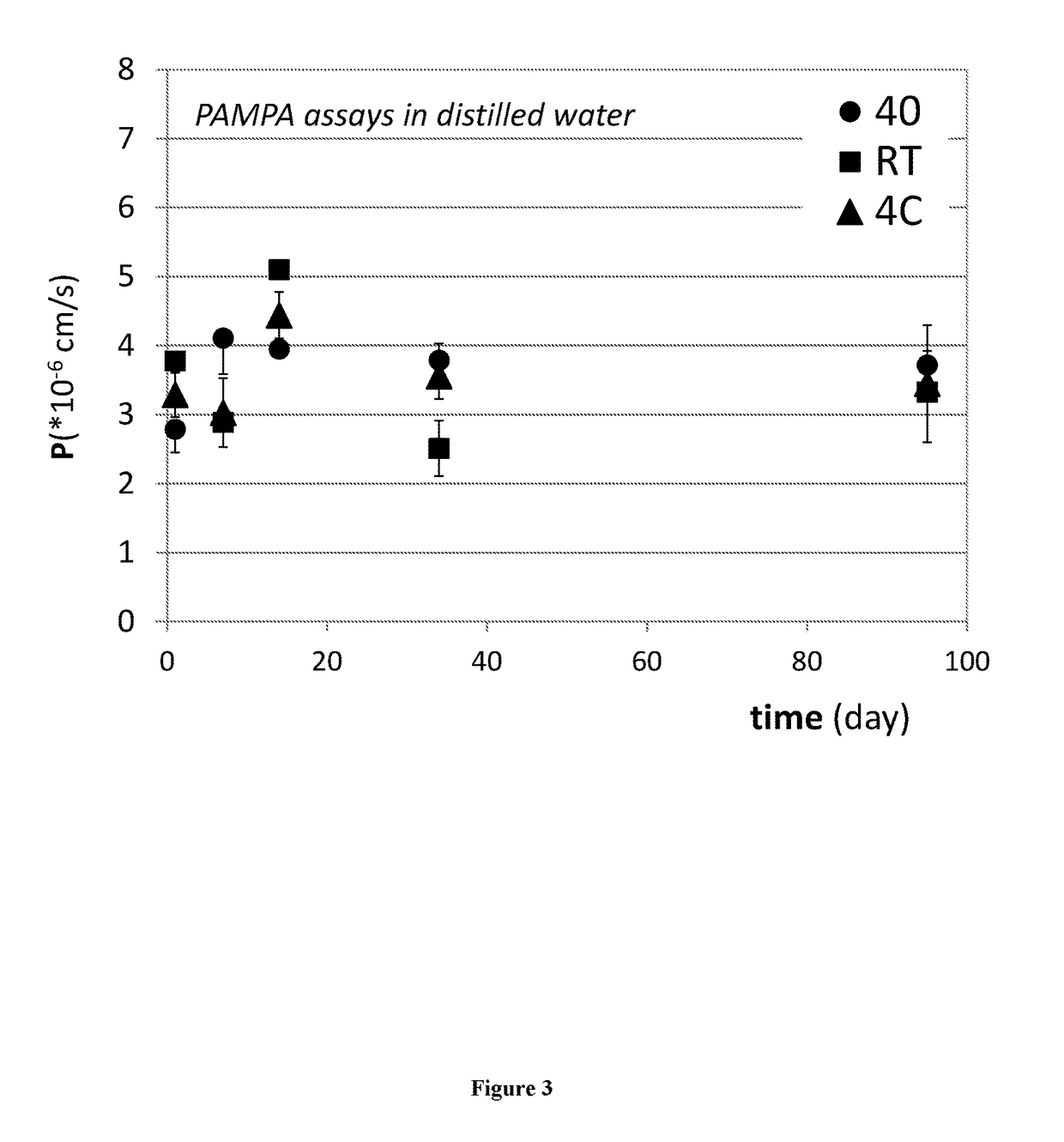
Complexes of sirolimus and its derivatives, process for the preparation thereof and pharmaceutical compositions containing them
InactiveUS20170165237A1Increase exposureReduce dosePowder deliveryOrganic active ingredientsSolubilityRenal transplant
The invention is directed to a stable complex with controlled particle size, increased apparent solubility and increased dissolution rate comprising as active compound Sirolimus or derivatives thereof, which is useful in the prophylaxis of organ rejection in patients receiving renal transplants, in the treatment of psoriasis, facial angiofibromas associated with tuberous sclerosis, fibrofolliculomas found in Birt-Hogg-Dubé Syndrome, chronic erosive oral lichen planus, Early Stage Cutaneous T-cell Lymphoma, Treatment of Autoimmune Active Anterior Uveitis, dry eye syndrome, age-related macular degeneration, diabetic macular edema, noninfectious uveitis, telangiectasia, inflammatory skin diseases (dermatitis, including psoriasis and lichen ruber planus), Pachyonychia Congenita and in the suppression of angiogenesis pathways. More specifically, the complex of the present invention possesses increased apparent solubility, permeability and enhanced biological performance including significantly improved exposure, earlier tmax, higher Cmax and higher trough concentrations at 24 hours which will allow the reduction of the dose.
Owner:TAVANTA THERAPEUTICS HUNGARY INC
Preparation method and preparation of large crystalline form of vorinostat I
ActiveCN102643214BGood dissolution effectHigh commercial application valueOrganic active ingredientsOrganic chemistryCutaneous T-cell lymphomaDissolution
The invention provides a method for preparing large crystalline vorinostat I crystal grains for treating skin T-cell lymphoma and a preparation made of vorinostat obtained therefrom. The above preparation method can crystallize in place at one time, is simple and effective, is suitable for large-scale industrial production, and has great commercial application value. The obtained preparation has good dissolution effect.
Owner:杭州容立医药科技有限公司
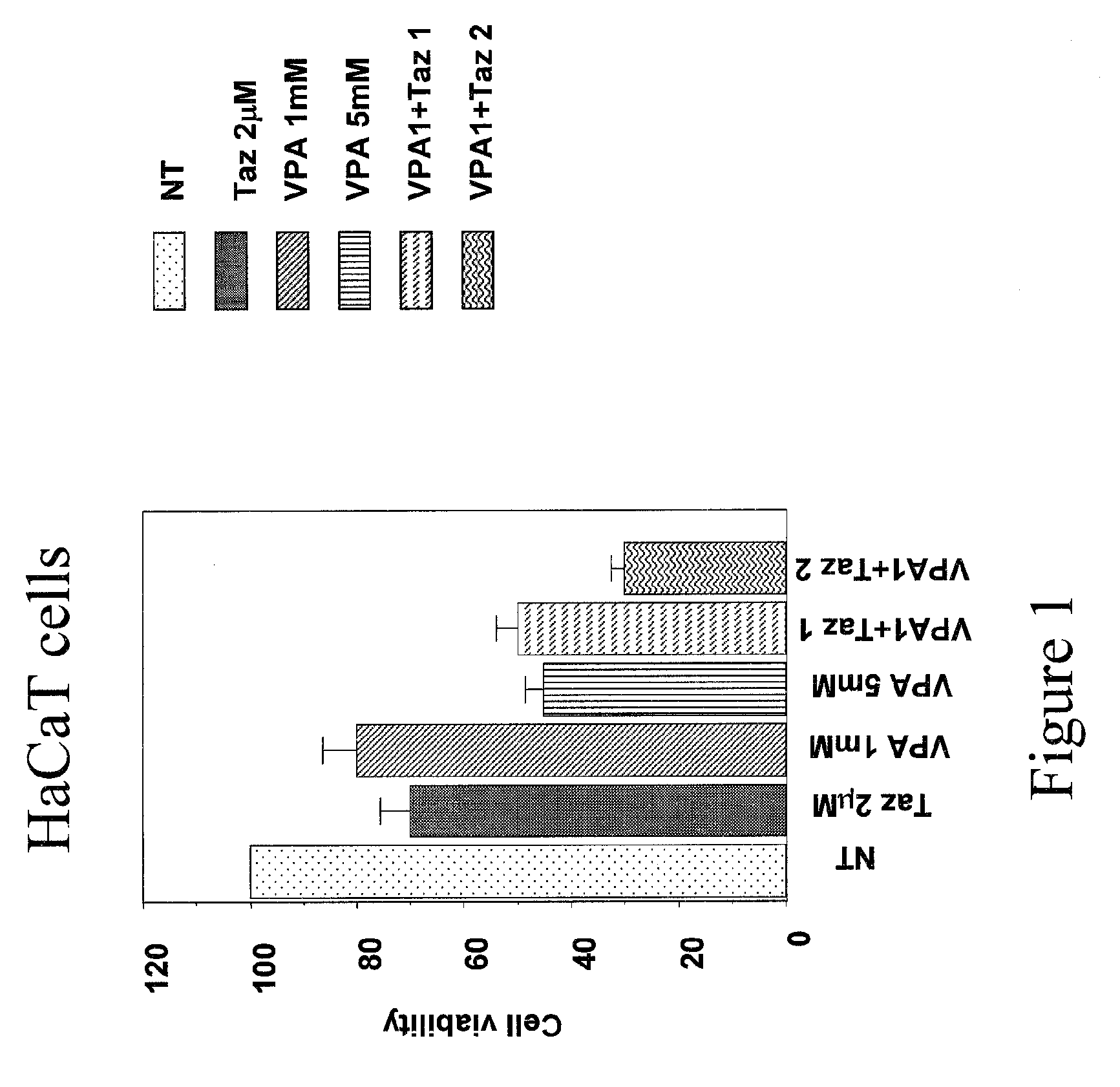
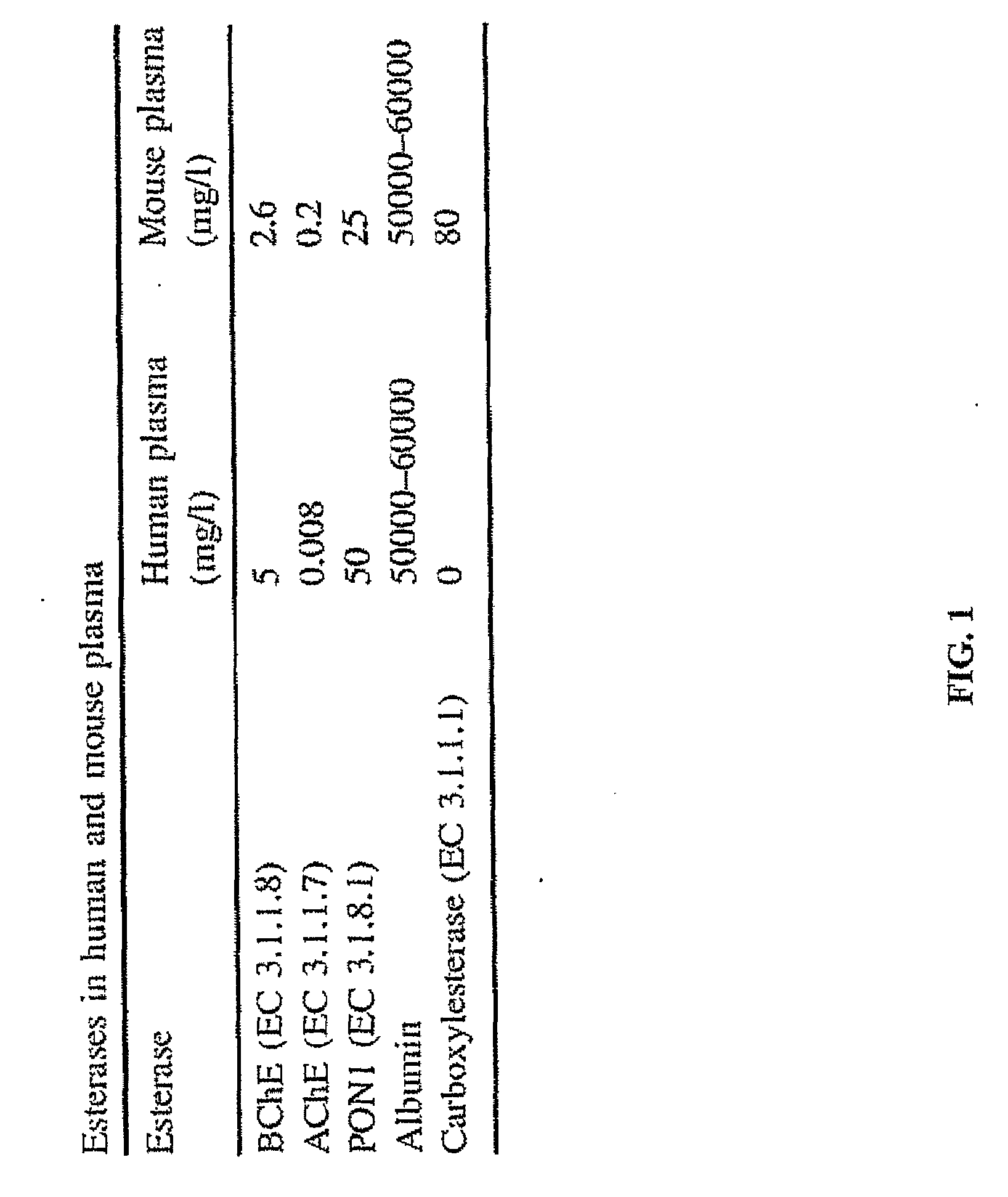
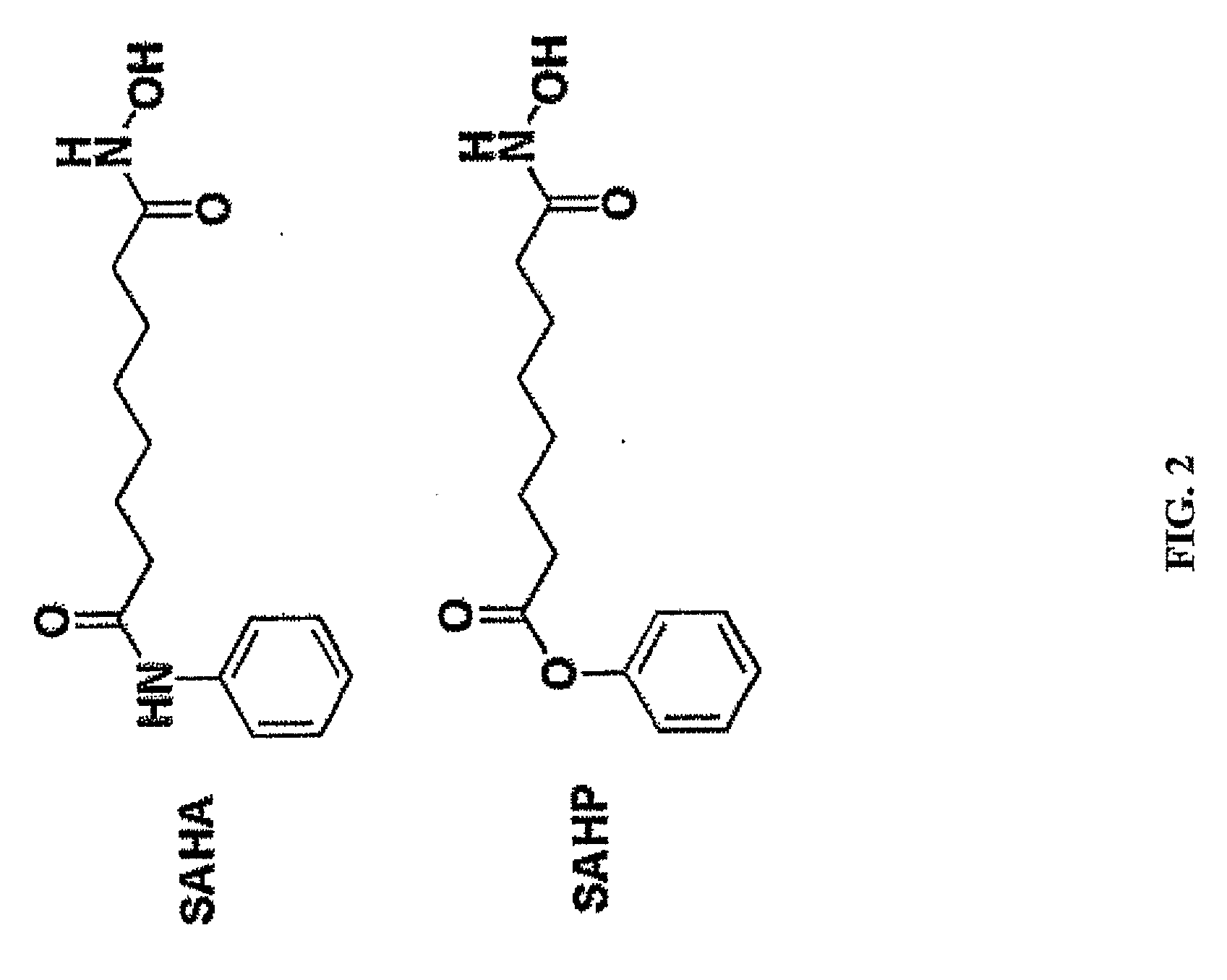
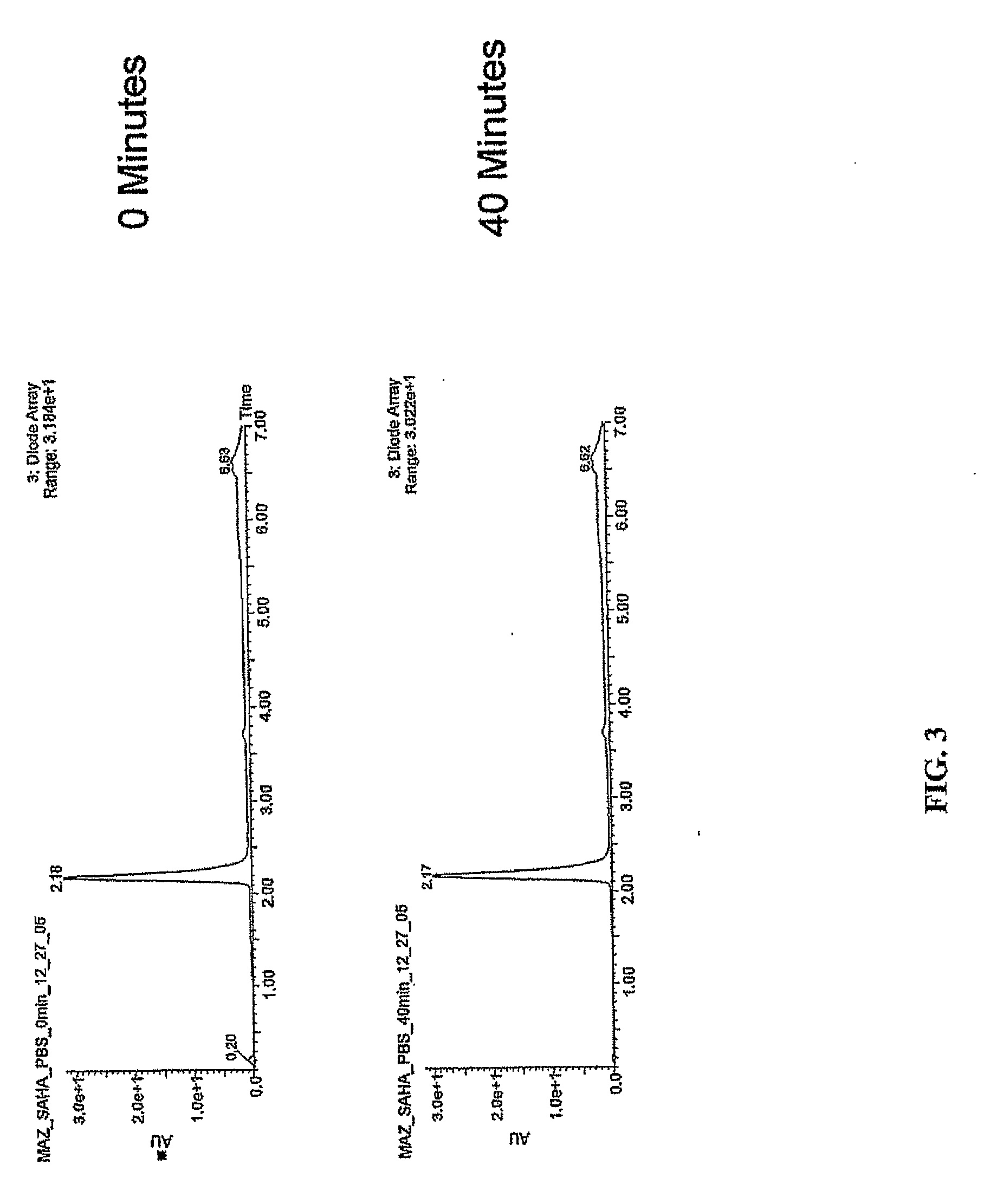
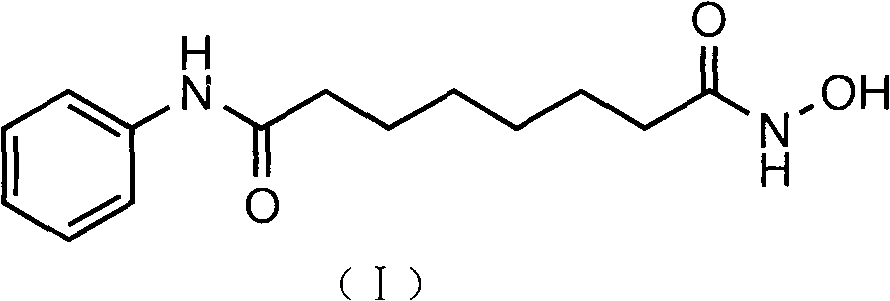
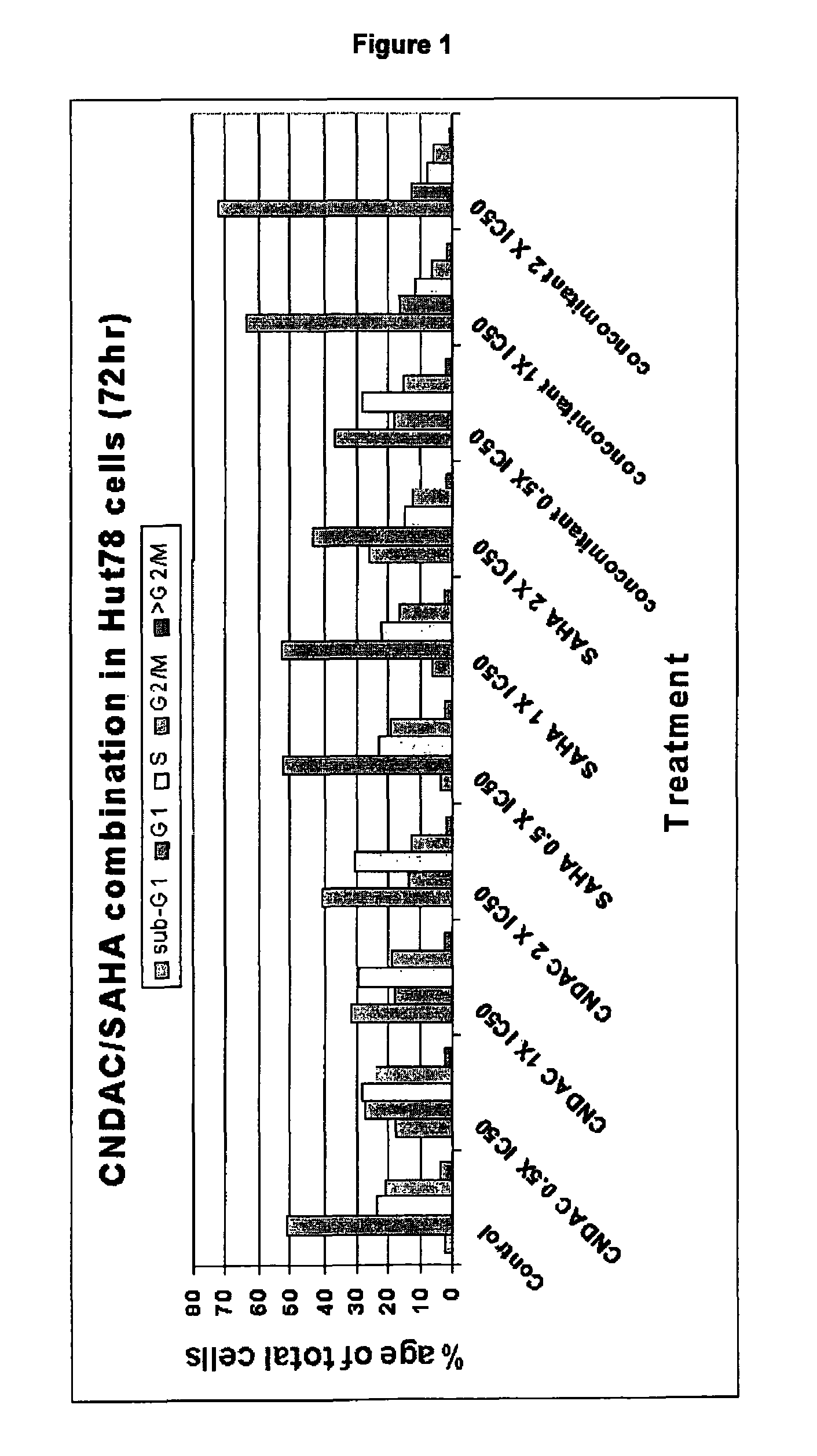
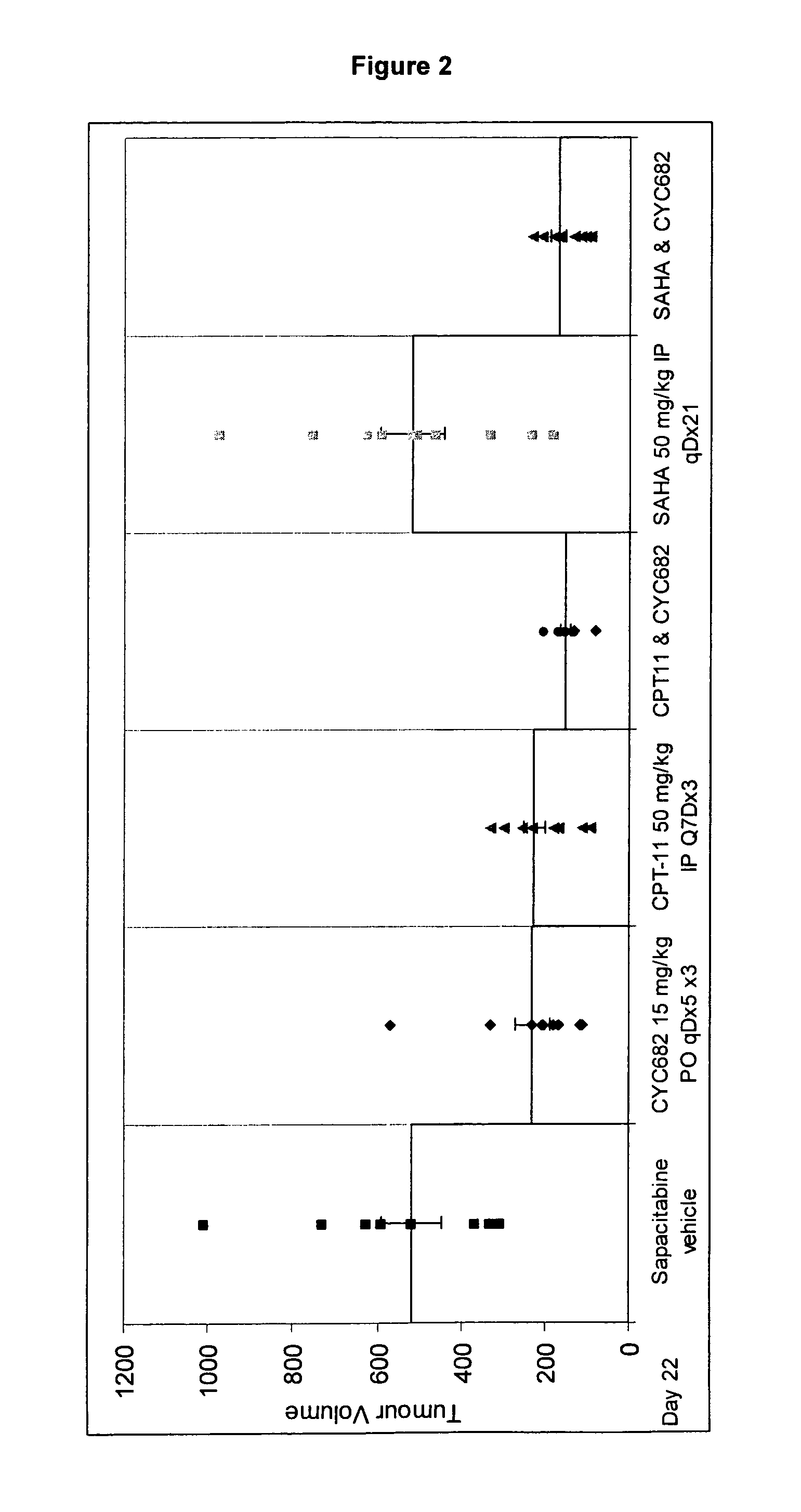
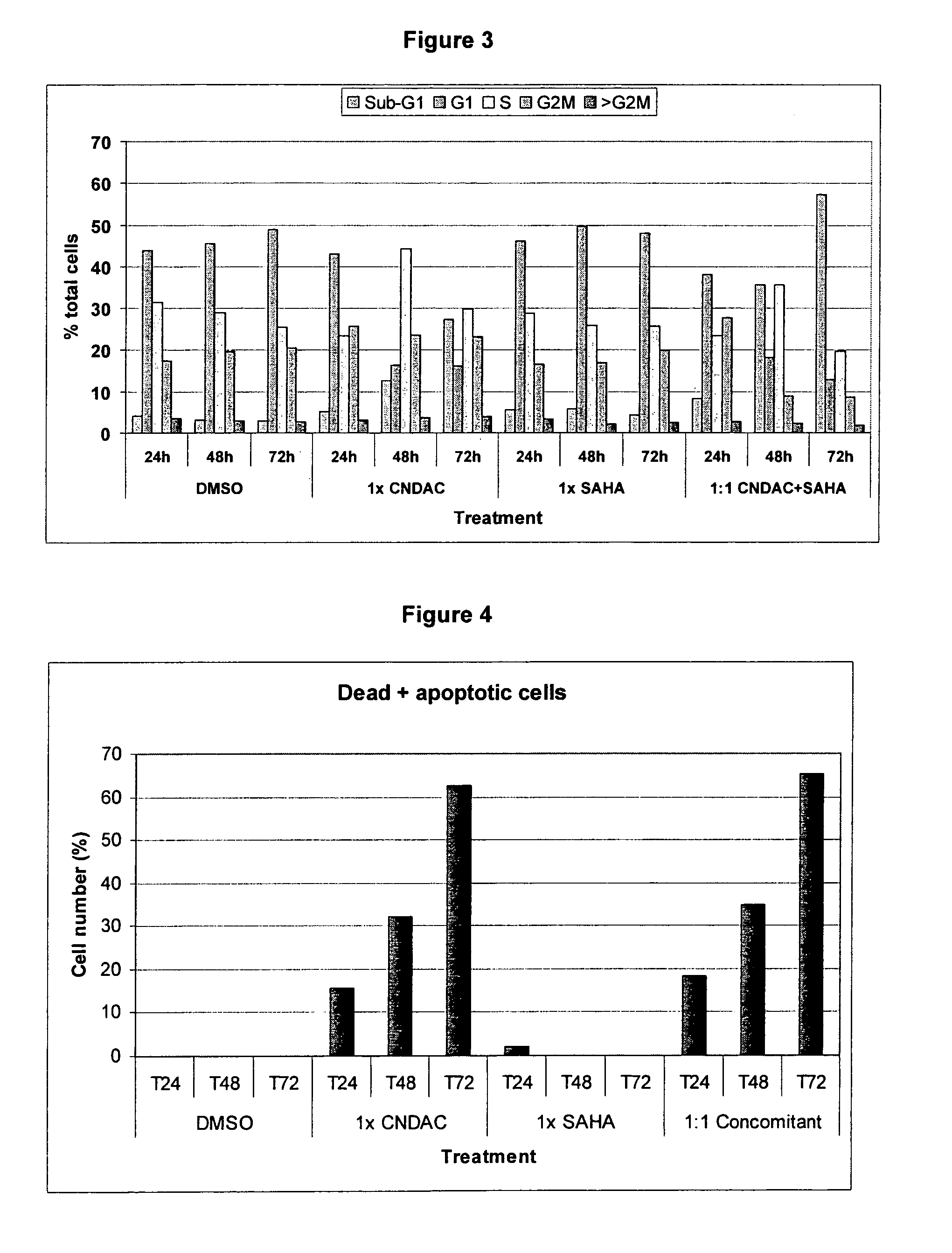
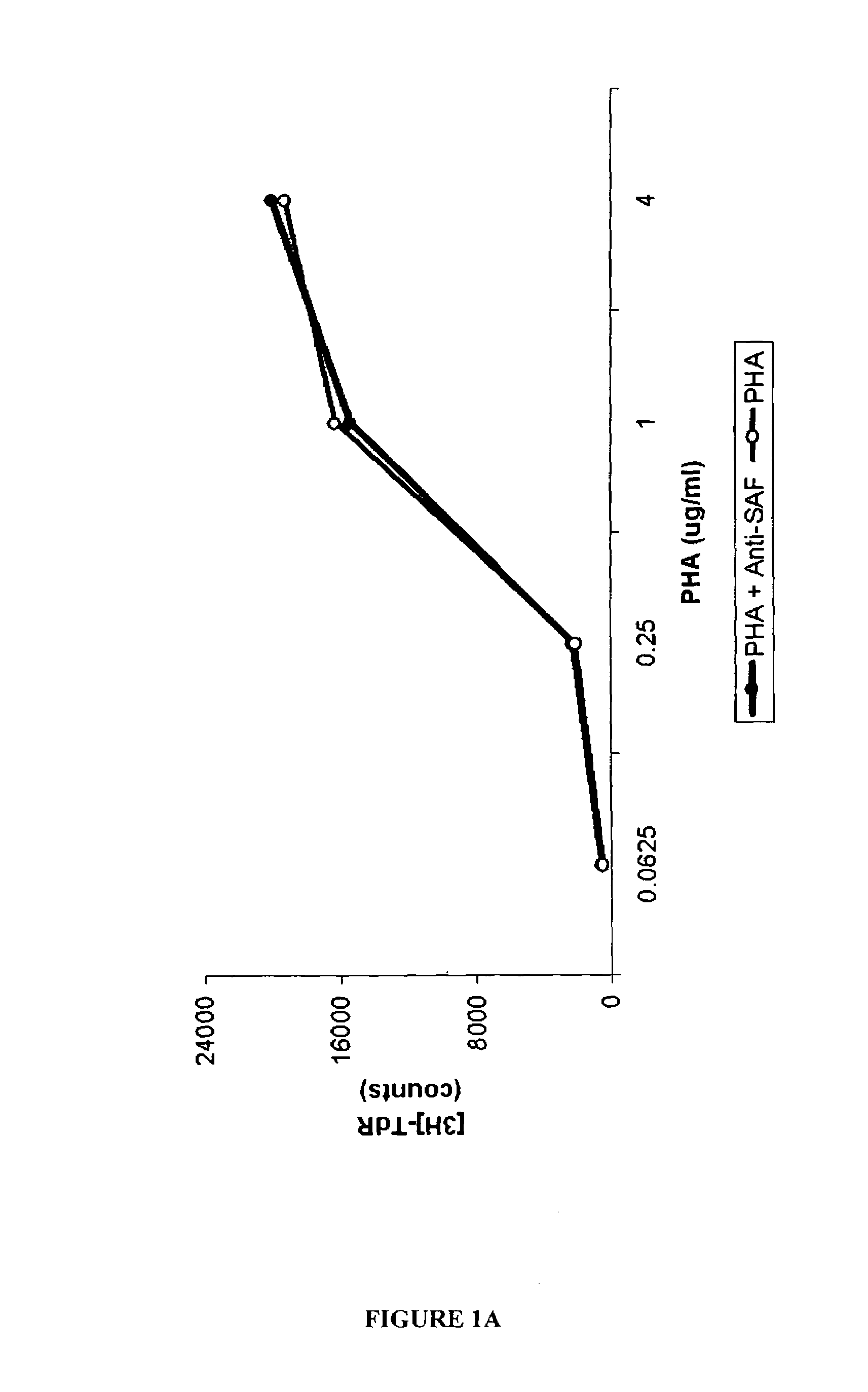
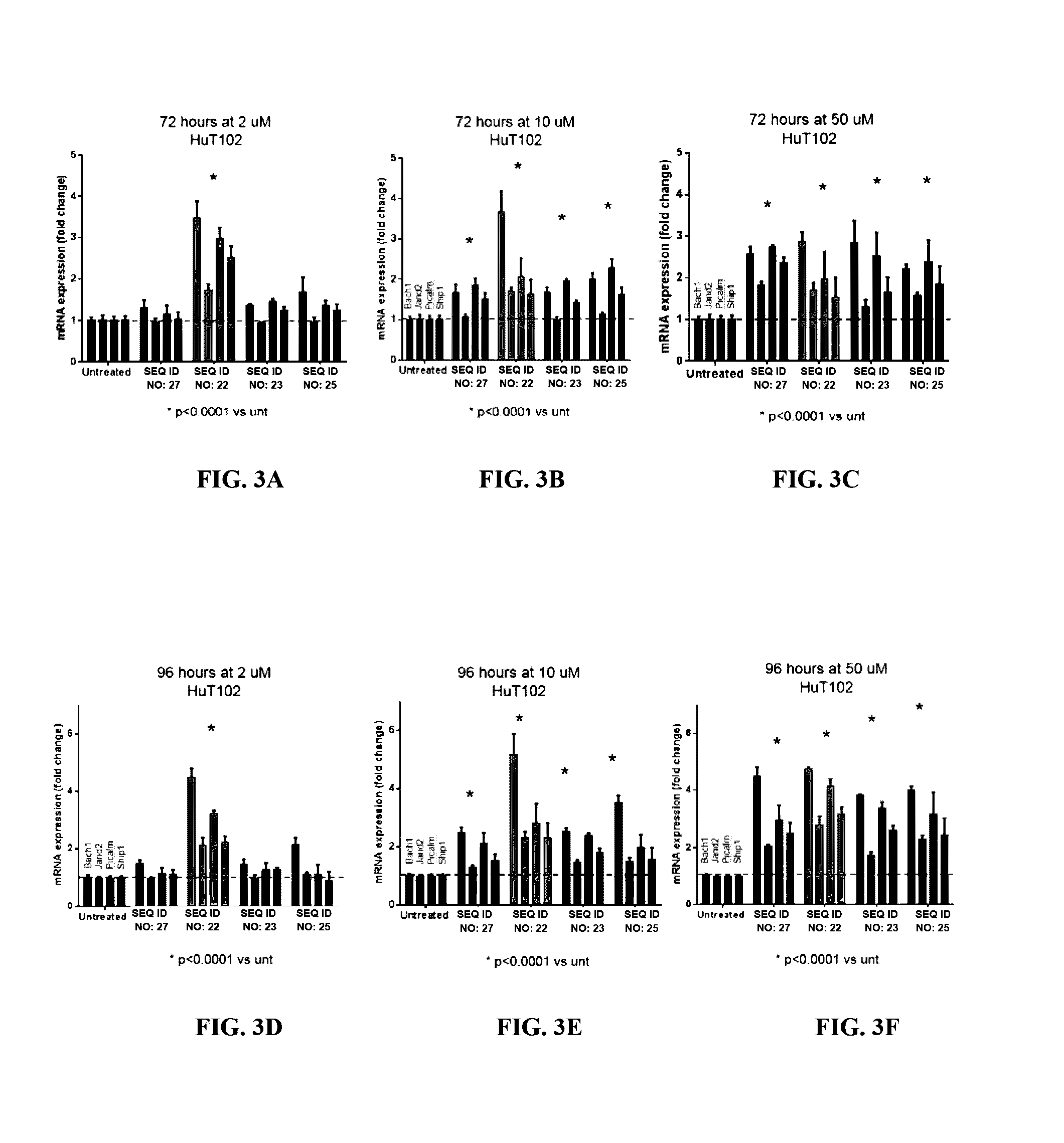
Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy
InactiveUS20130102556A1BiocideCarbohydrate active ingredientsDNA Methyltransferase InhibitorCutaneous T-cell lymphoma
Combination methods for treatment of cancer and of blood disorders, using PG-11047 ((2Z)-N1,N4-bis[3-(ethy-lammo) propyl]-2-butene-1,4-diamine) and PG-11048 ((2E)-N1,N4-bis[3-(ethylamino)propyl]-2-butene-1,4-diamine) in combination with DNA methyltransferase (DNMT) inhibitors, histone deacetylase (HDAC) inhibitors, or both DNA methyltransferase inhibitors and histone deacetylase inhibitors, are disclosed. Hematopoietic cancers, lung cancers, mesothelioma, cutaneous T-cell lymphoma (CTCL), multiple myeloma, solid tumors, and blood disorders such as myelodysplastic syndromes can be treated using the methods of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






























![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00001.png)
![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00002.png)
![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00003.png)