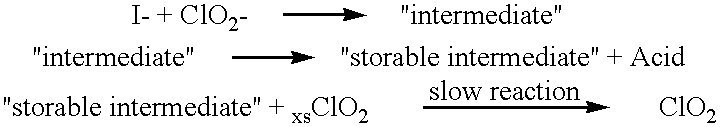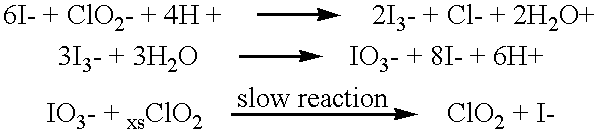Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
523 results about "Rash" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
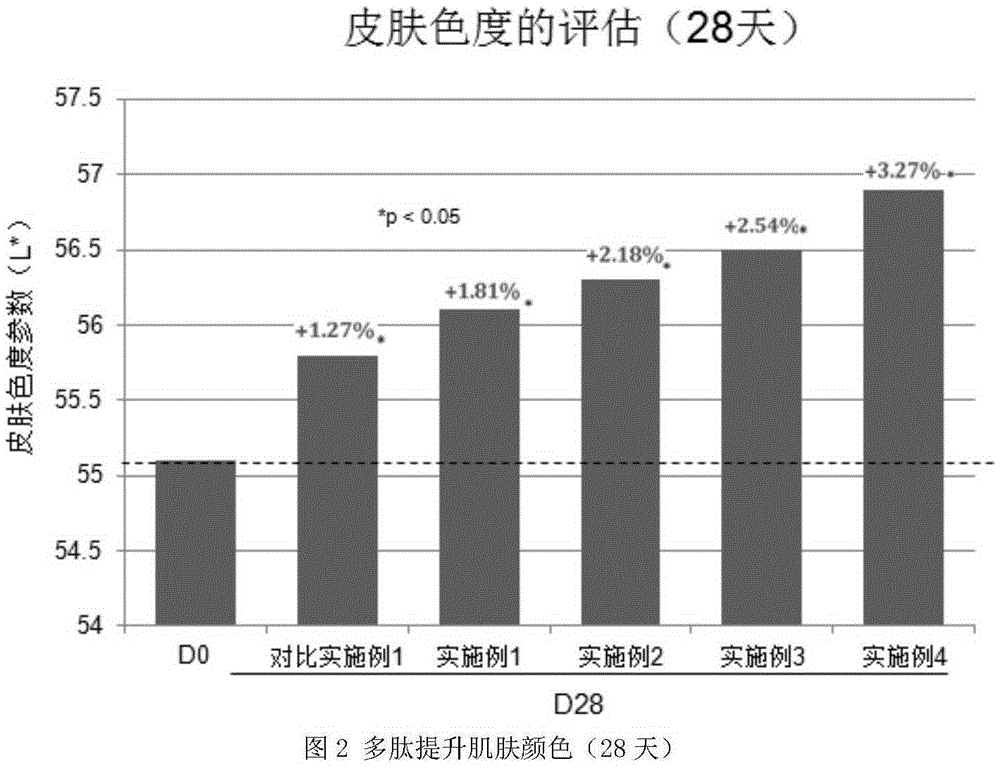
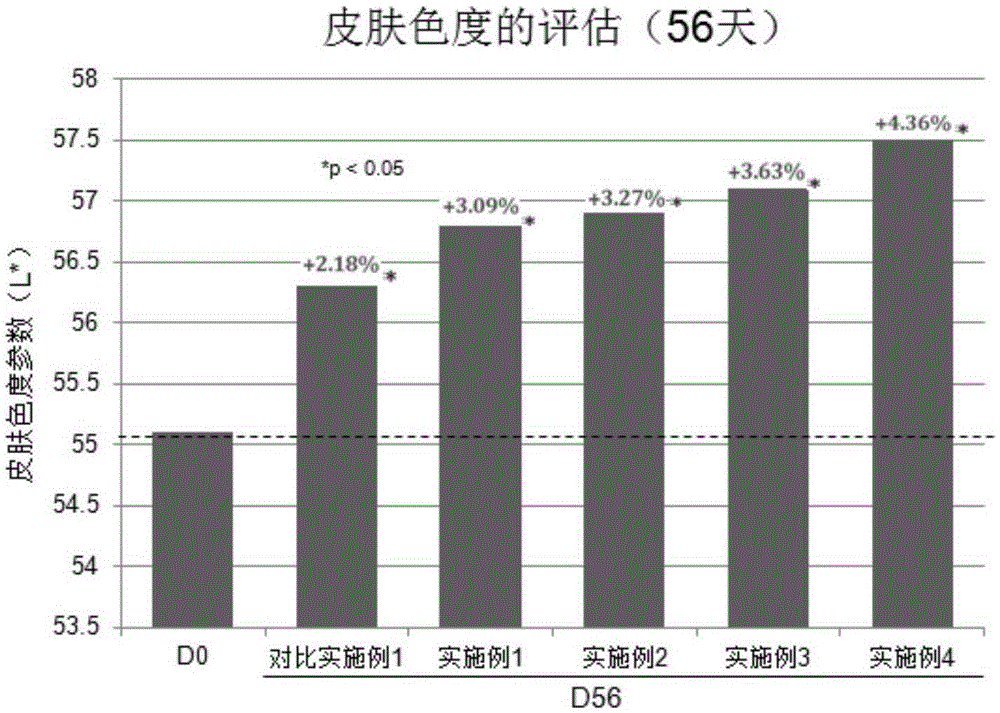
Noticeable change in the skin in terms of texture and color.
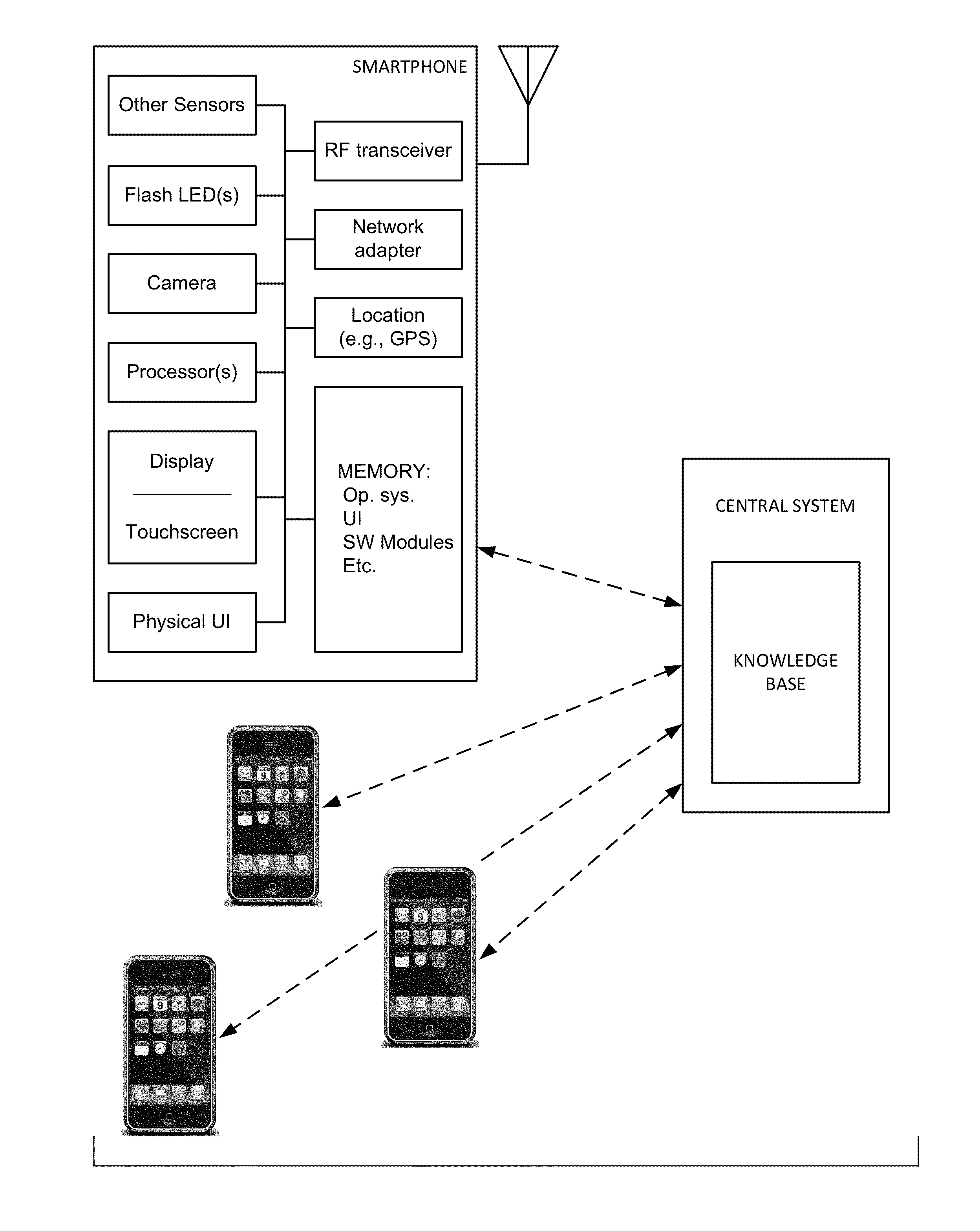
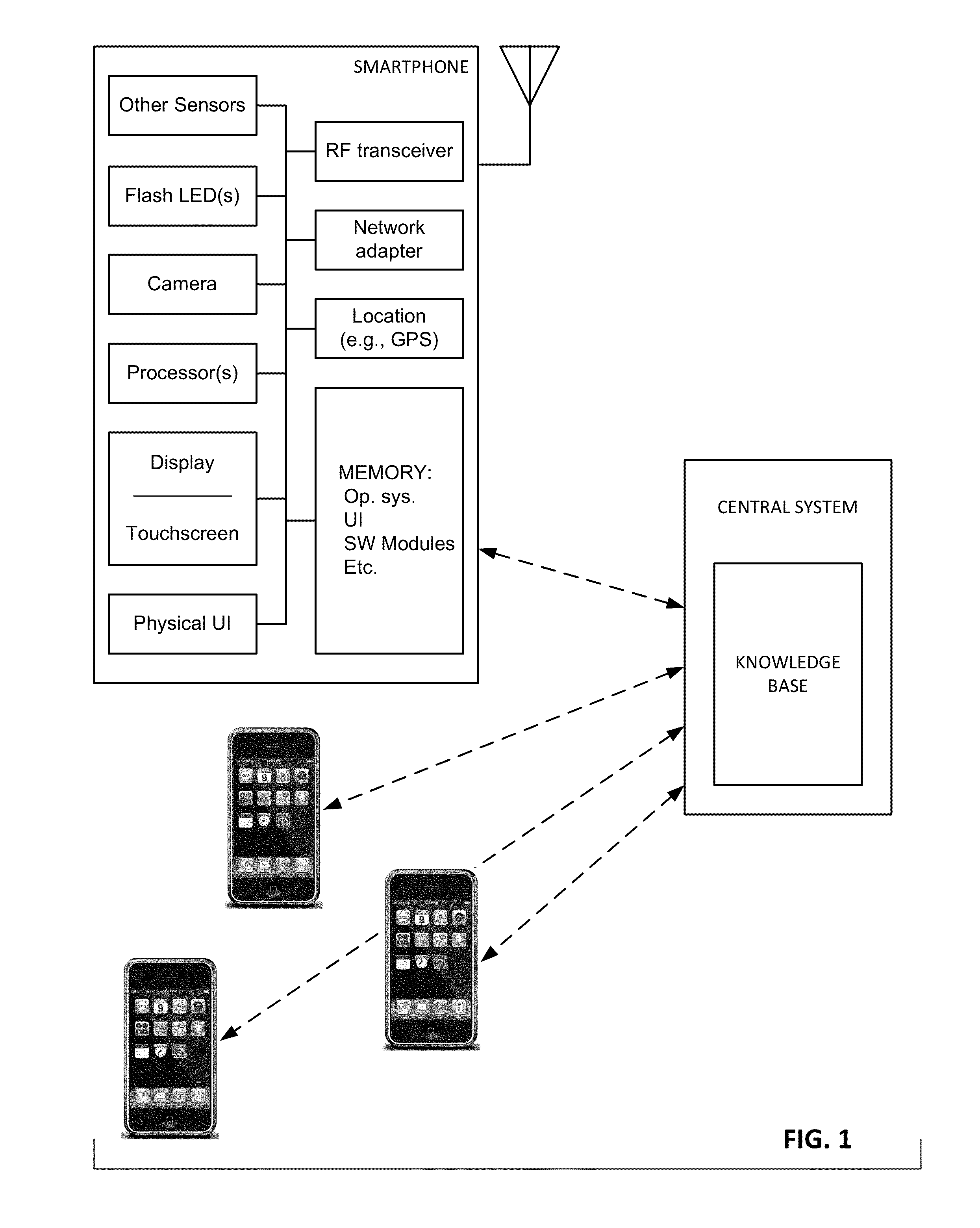
Physiologic data acquisition and analysis
InactiveUS20140378810A1Lower cost of careImage enhancementMedical imagingPattern recognitionImaging analysis
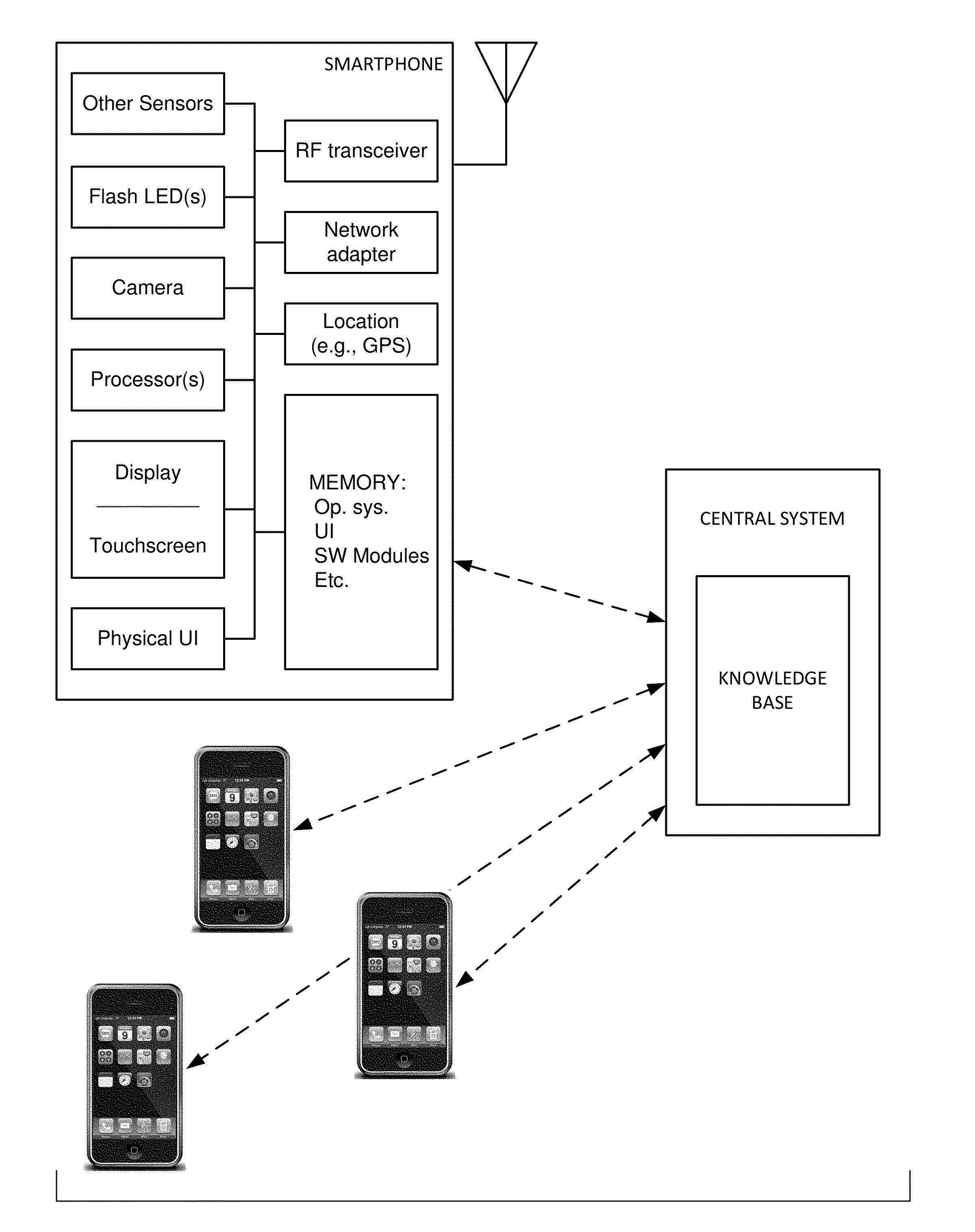
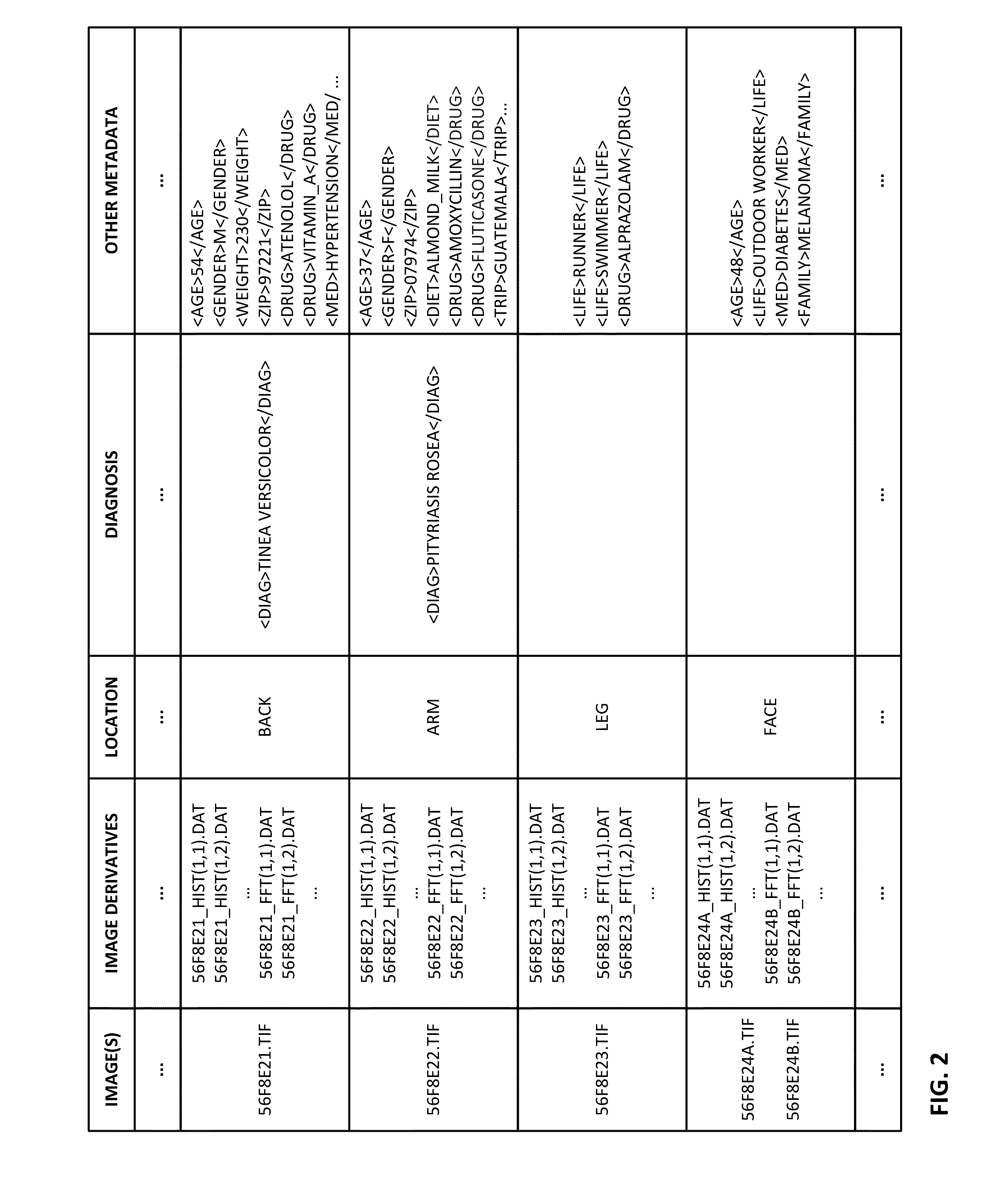
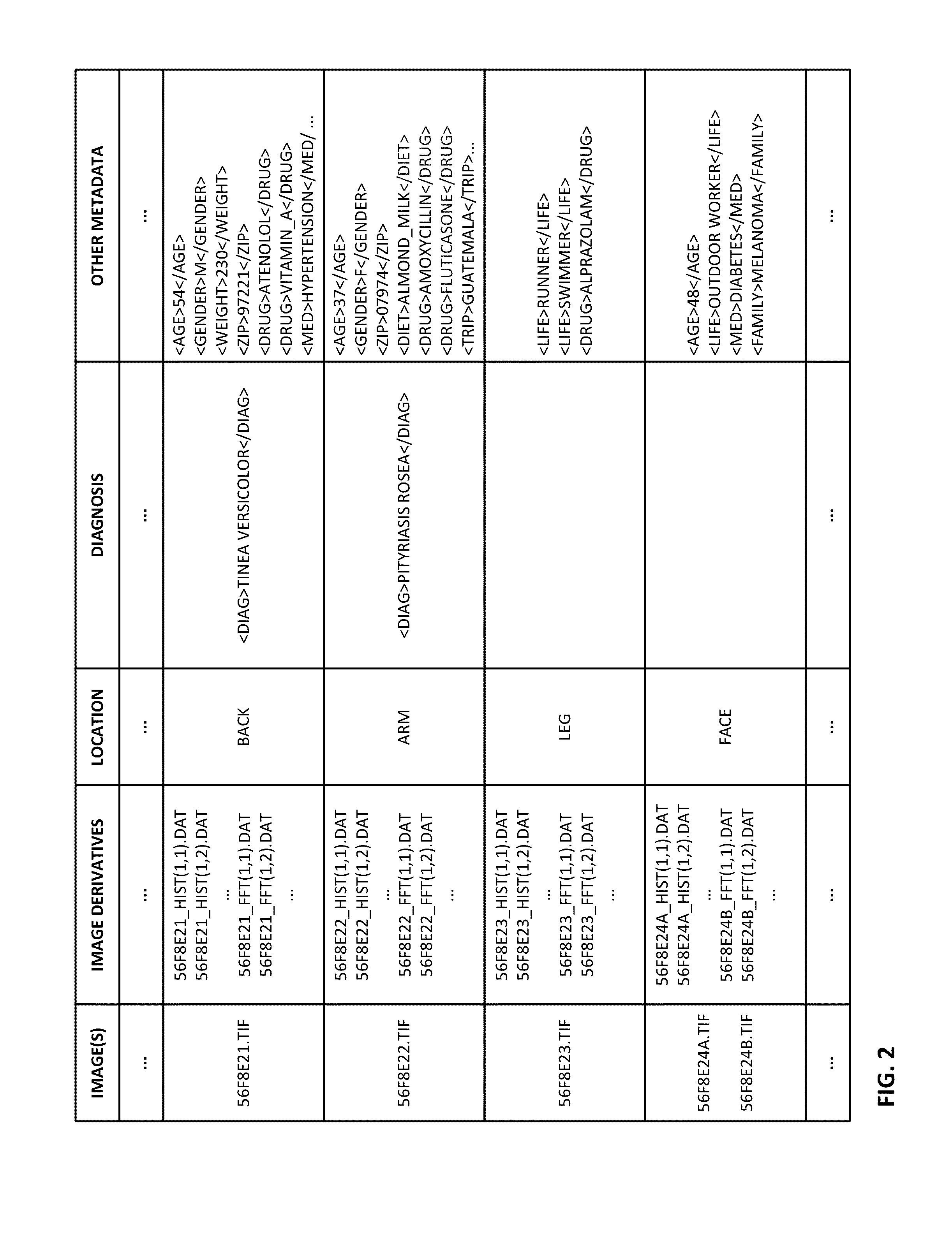
The availability of high quality imagers on smartphones and other portable devices facilitates creation of a large, crowd-sourced, image reference library that depicts skin rashes and other dermatological conditions. Some of the images are uploaded with, or later annotated with, associated diagnoses or other information (e.g., “this rash went away when I stopped drinking milk”). A user uploads a new image of an unknown skin condition to the library. Image analysis techniques are employed to identify salient similarities between features of the uploaded image, and features of images in this reference library. Given the large dataset, statistically relevant correlations emerge that identify to the user certain diagnoses that may be considered, other diagnoses that may likely be ruled-out, and / or anecdotal information about similar skin conditions from other users. Similar arrangements can employ audio and / or other physiologically-derived signals. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
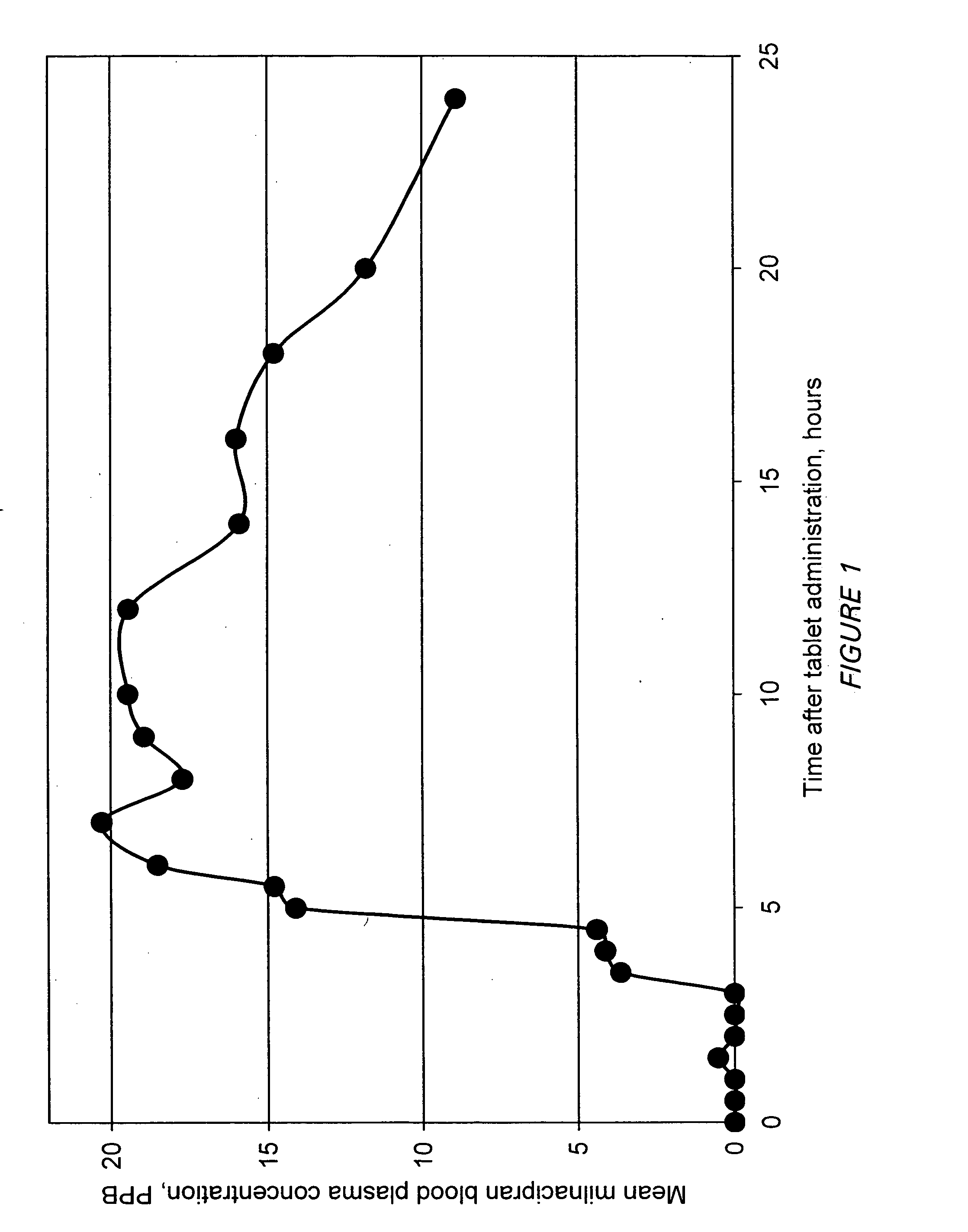
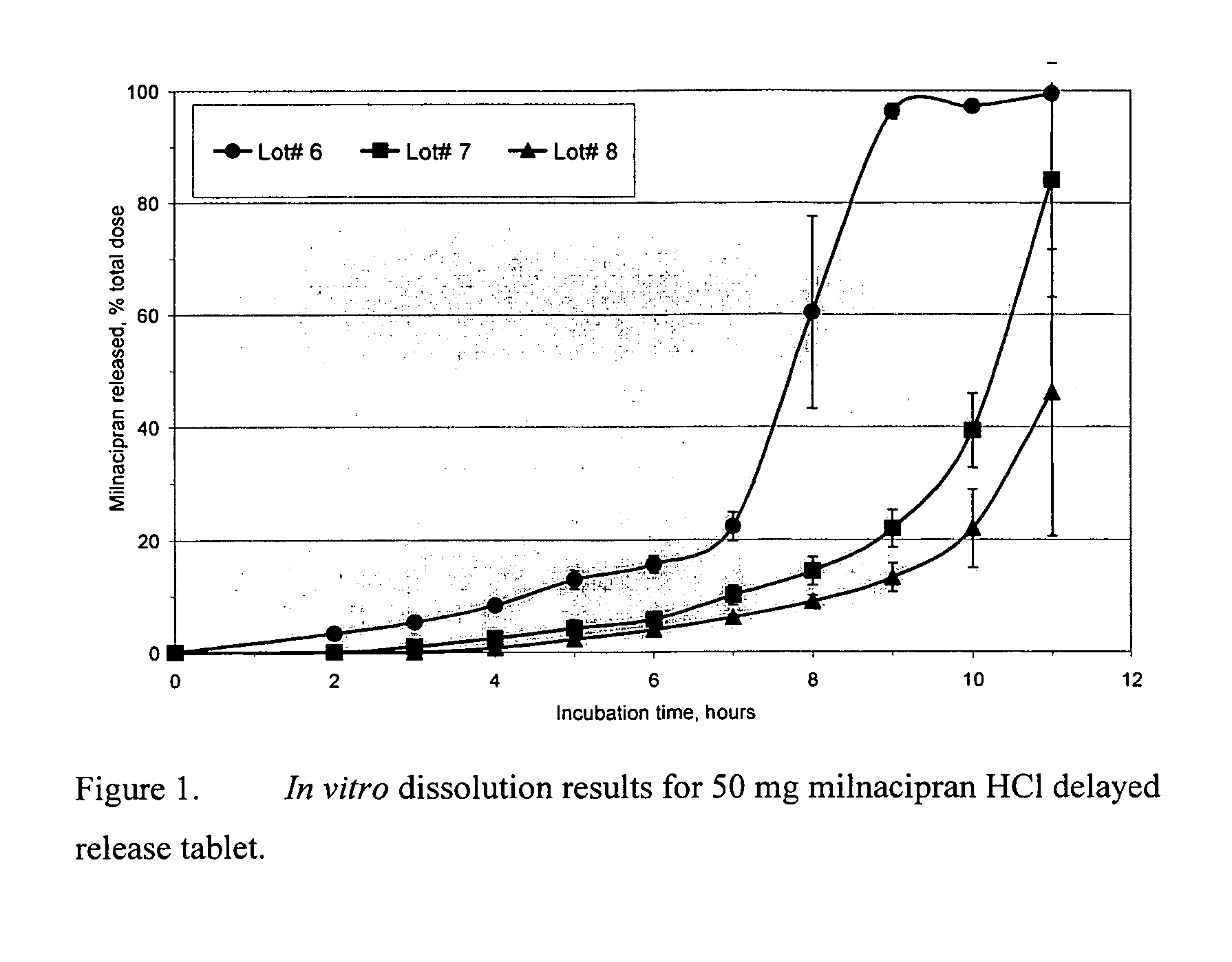
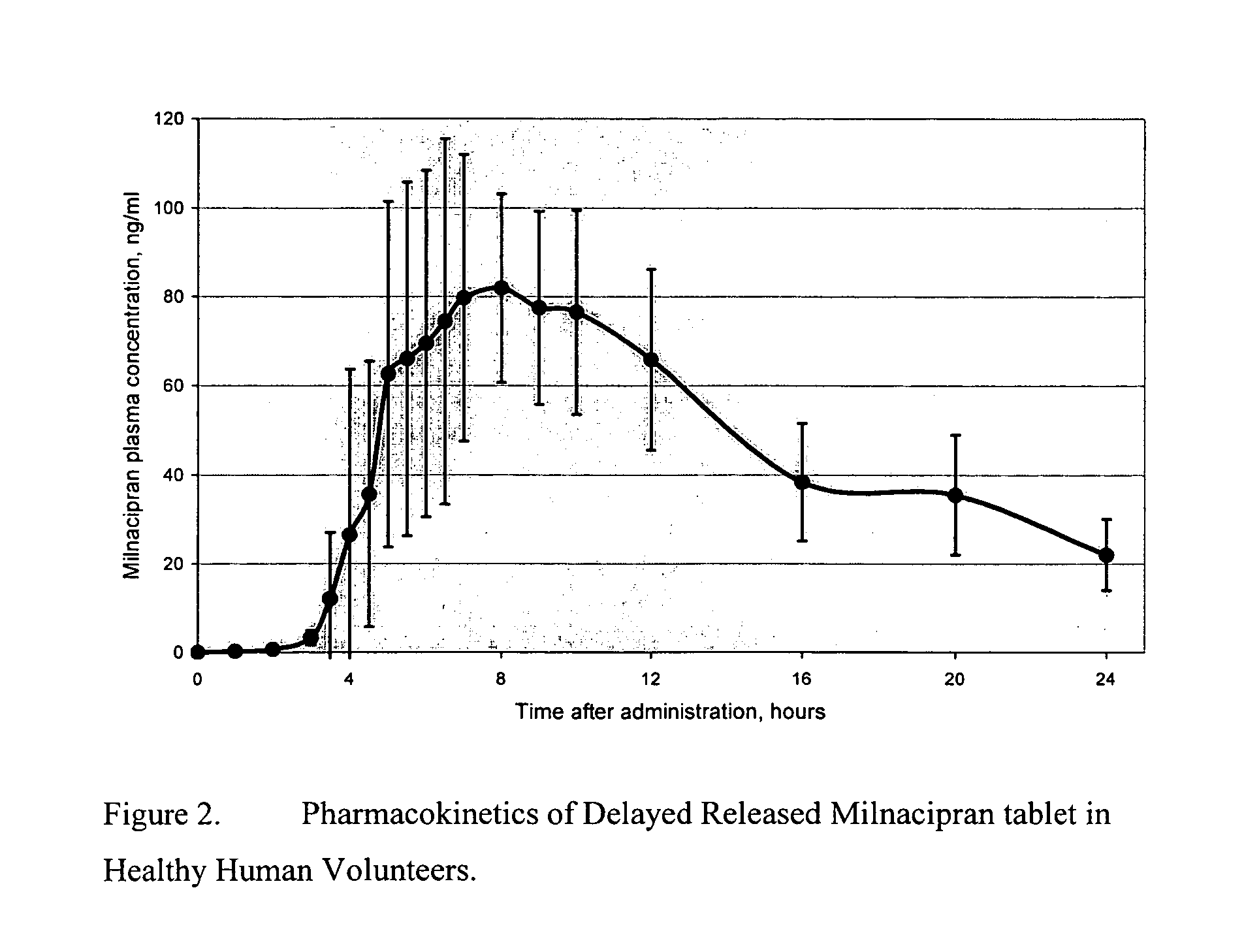
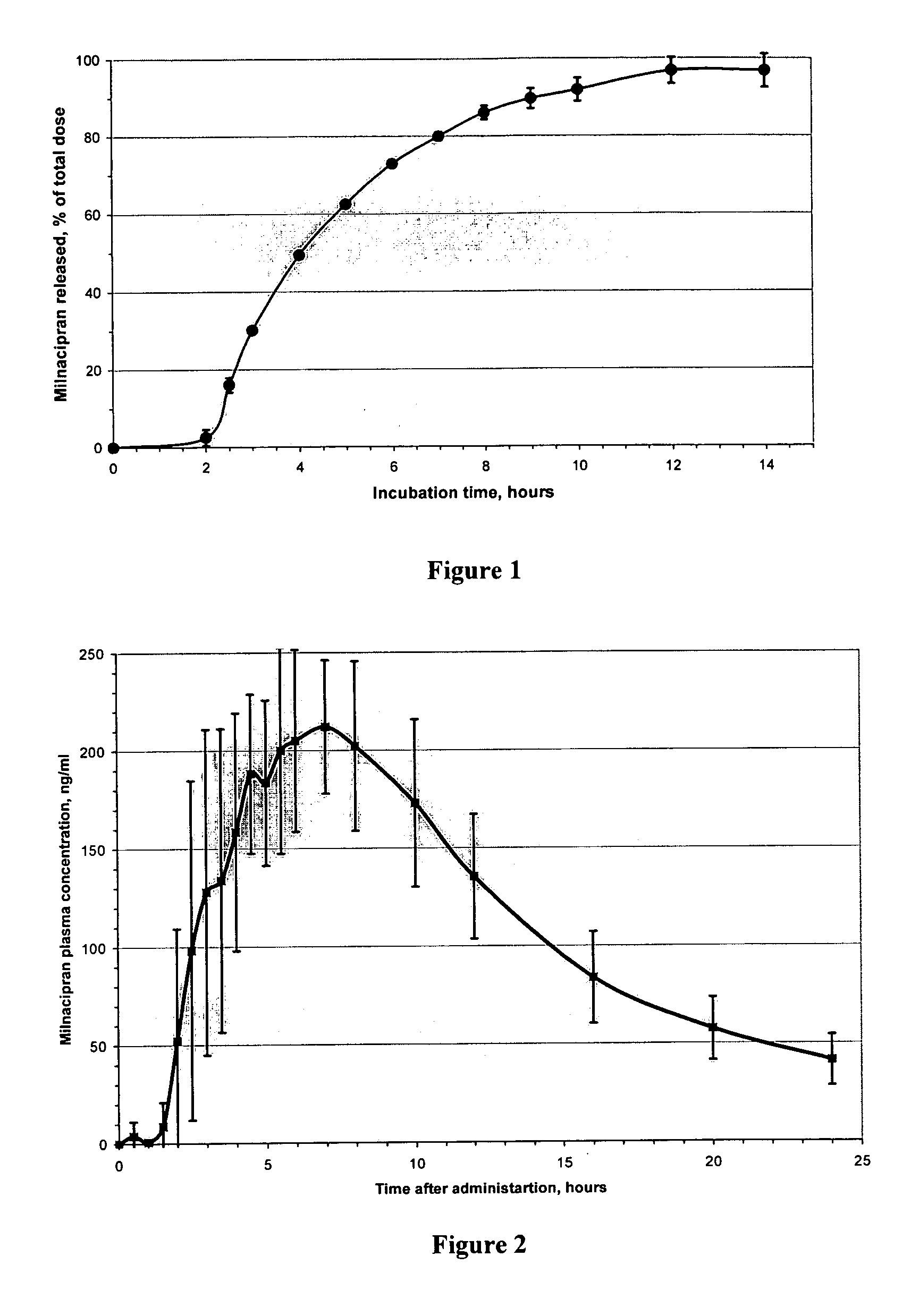
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Method of making molecular chlorine dioxide
A method for manufacturing molecular chlorine dioxide, by the addition of potassium iodide to a solution of alkali metal chlorite. The metal chlorite and the potassium iodide are kept separate, until the need for the generation of chlorine dioxide arises-to ensure long-shelf life. After initiation or activation of the chlorite anion to form chlorine dioxide, the beneficial properties of chlorine dioxide can be used, for different health and cosmetic purposes. Such uses include the treatment of herpes, dandruff, acne, skin rashes (e.g. poison ivy), ulcers, bed sores, warts, nail fungus, athletes foot, sun burn and gum disease; and as an antiseptic, disinfectant, and general deodorant form refrigerator sprays to oral mouthrinses.
Owner:MADRAY GEORGE
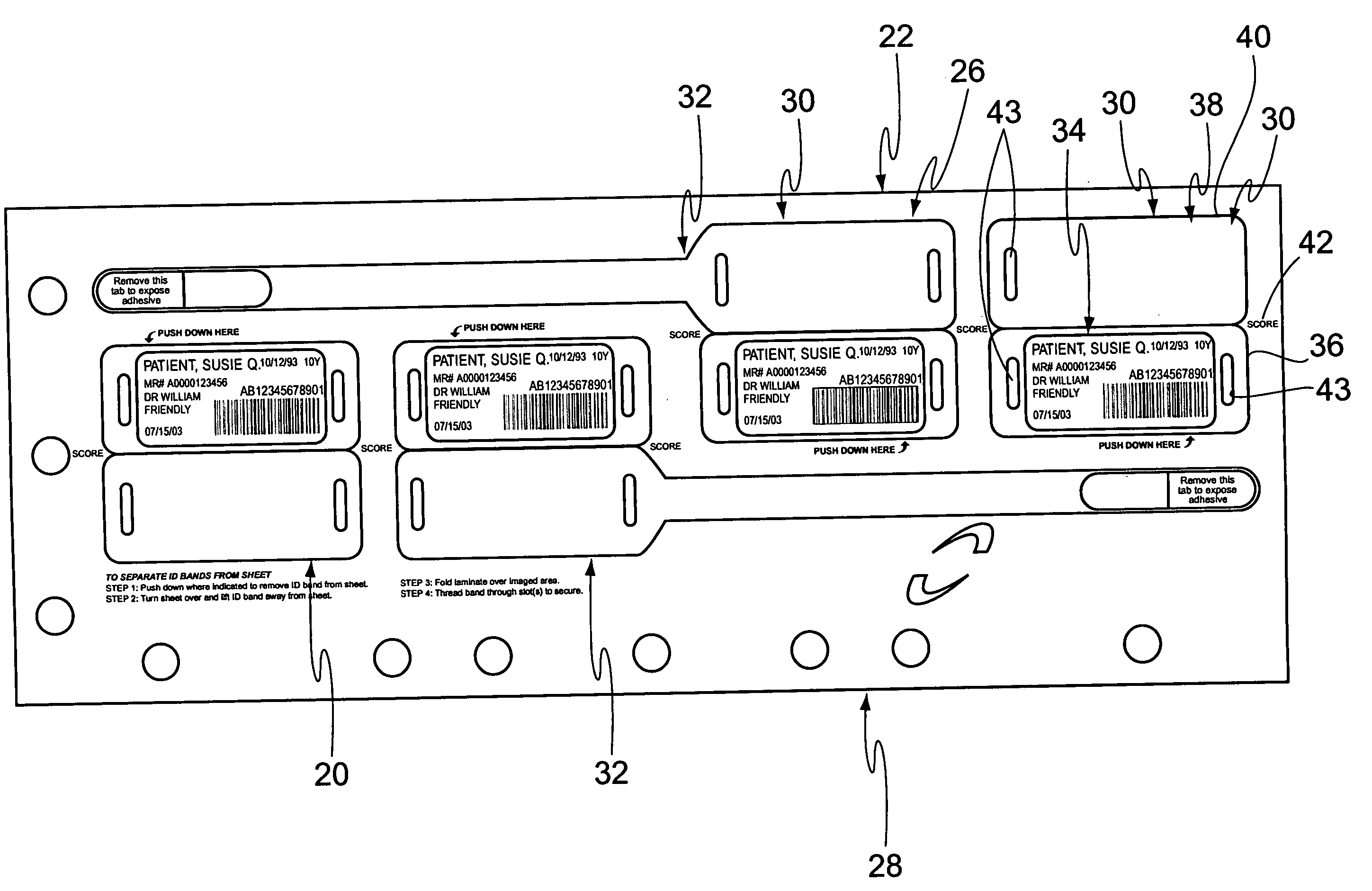
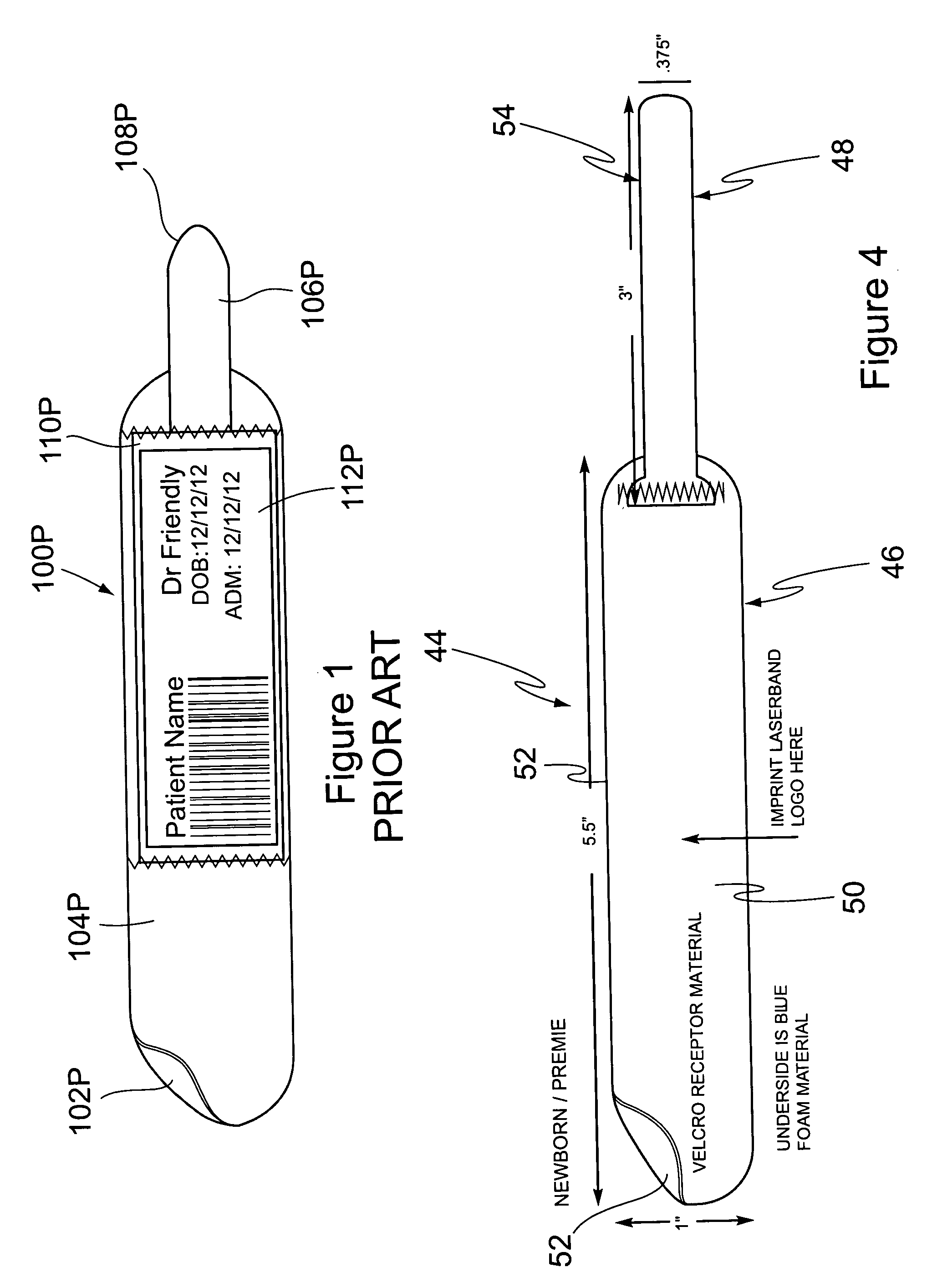
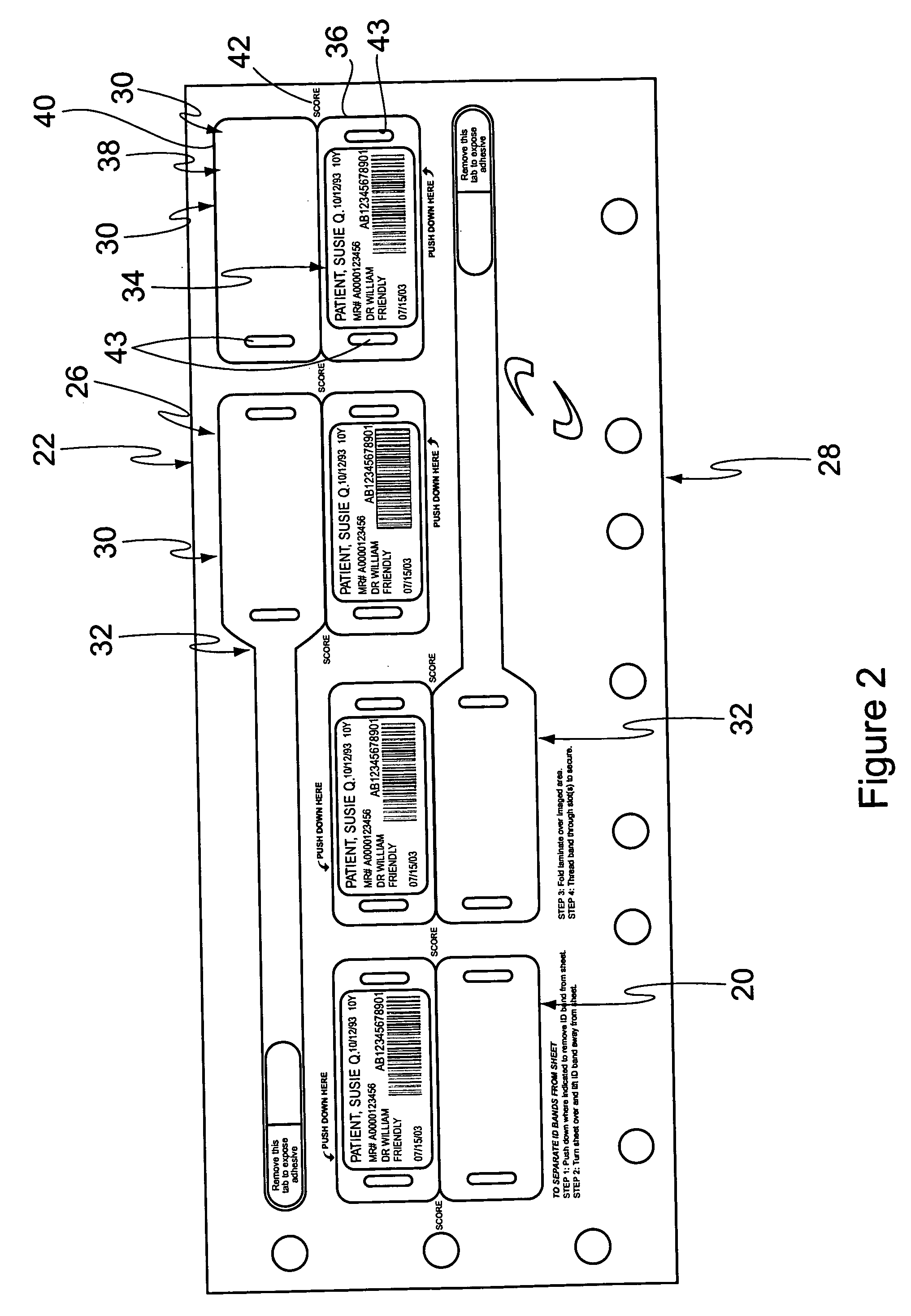
Cushioned wristband with self-laminating identity tag
ActiveUS20050279001A1Reliable identificationWithout any discomfortStampsOther printing matterEngineeringAppendage
A cushioned wristband includes a carrier having a multi-layered band portion and a strap sewn to the back of the band portion of the carrier. The band has a surface with loop material and the strap has a surface of hook material, the hook and loop surfaces being intended to secure the band in place as it is wrapped about a patient's appendage. The strap is appropriately sized to insert through one or both of two cinch slots of a self-laminating hang tag which may be printed with patient information and separated from a sheetlet or page sized business form processed through a printer for imaging of patient information thereon. The band portion of the carrier includes a cushioned layer of soft foamy or spongy material for contacting a patient's skin to thereby substantially eliminate any possibility for abrasion, rash, or other irritation or injury to the patient through wearing of the wristband.
Owner:ZEBRA TECH CORP
Method of producing an oil extract from seeds of plants via a binary azeotropic solvent mixture
The invention describes a method of producing extracts from the seeds of meadowfoam, brassicas and crambe plants. A number of subsidiary processes and steps are shown in order to extract differing fractions of oil. Products produced from the above method are also described including uses and methods of these products which include a variety of skin conditions including eczema, facial eczema, dermatitis, external ulcers, welts, rashes, insect bites, allergic reactions and other irritations, burns, wounds, psoriasis, acneiform eruptions, dryness, dry skin, irritation, skin atrophy, secondary infections and the like. The extracts are also described as being a useful compound for treatment of the symptoms of such skin conditions as described above. In particular the use and extraction of glucosinolate (GSL), thiocyanates (TCL) and isothiocyanates (ITCL) is described.
Owner:NEW ZEALAND BOTANICAL OILS
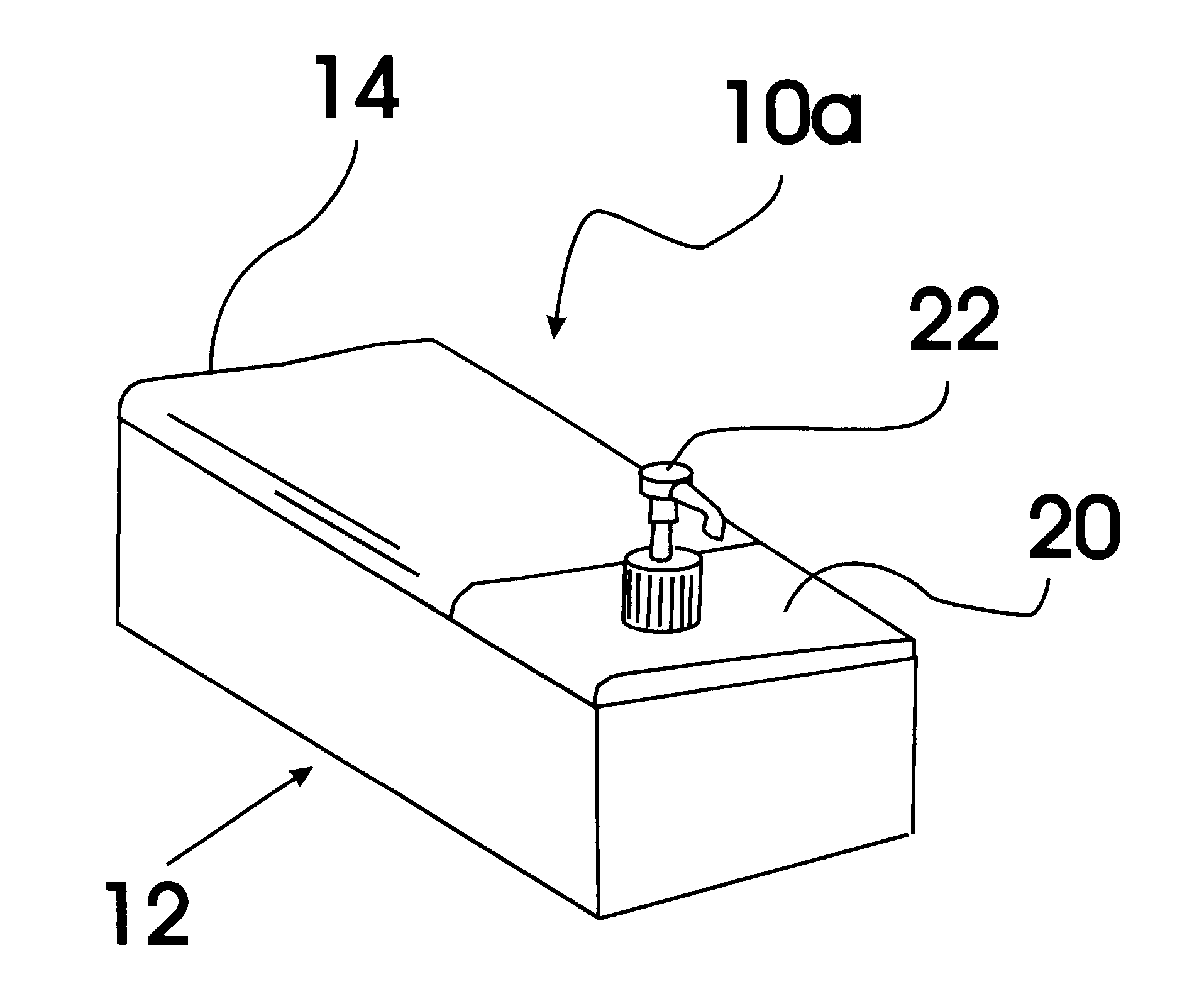
Baby wipe/rash cream dispenser
A baby wipe / rash cream dispenser that allows the user to retrieve baby wipes and rash medication with one hand while using the other hand to maintain the safety of the baby. The baby wipe / rash cream dispenser comes in a first embodiment having a moisture tight seal and two compartments one for baby wipes and one for rash cream which are each accessible when the lid is open. The second embodiment includes a housing having a sealable lid compartment for baby wipes and a separate lid compartment for rash cream that is provided with a pump mechanism for allowing the user to pump the rash cream from the reservoir without the need for opening the lid.
Owner:SANDLER KIMBERLY L
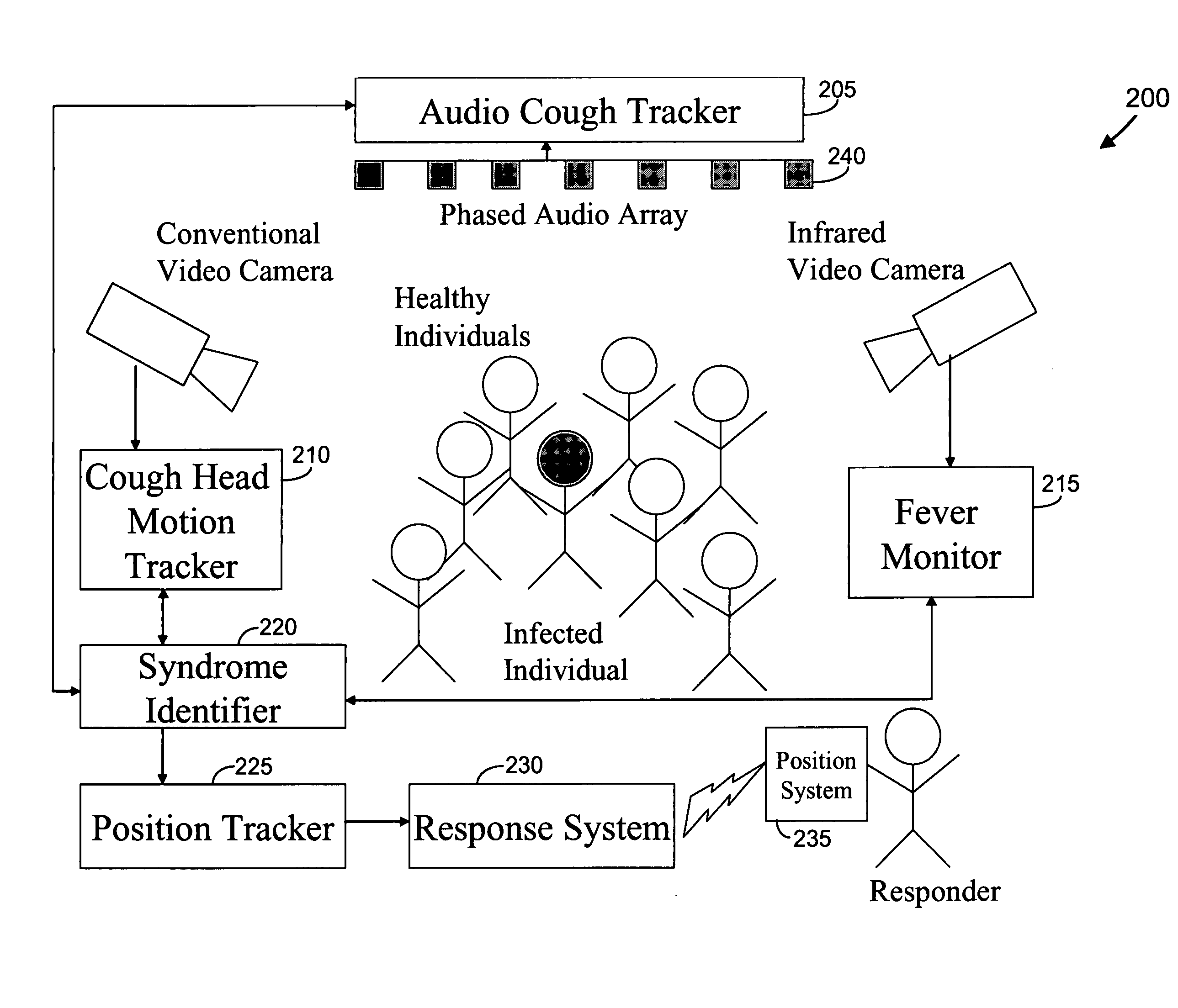
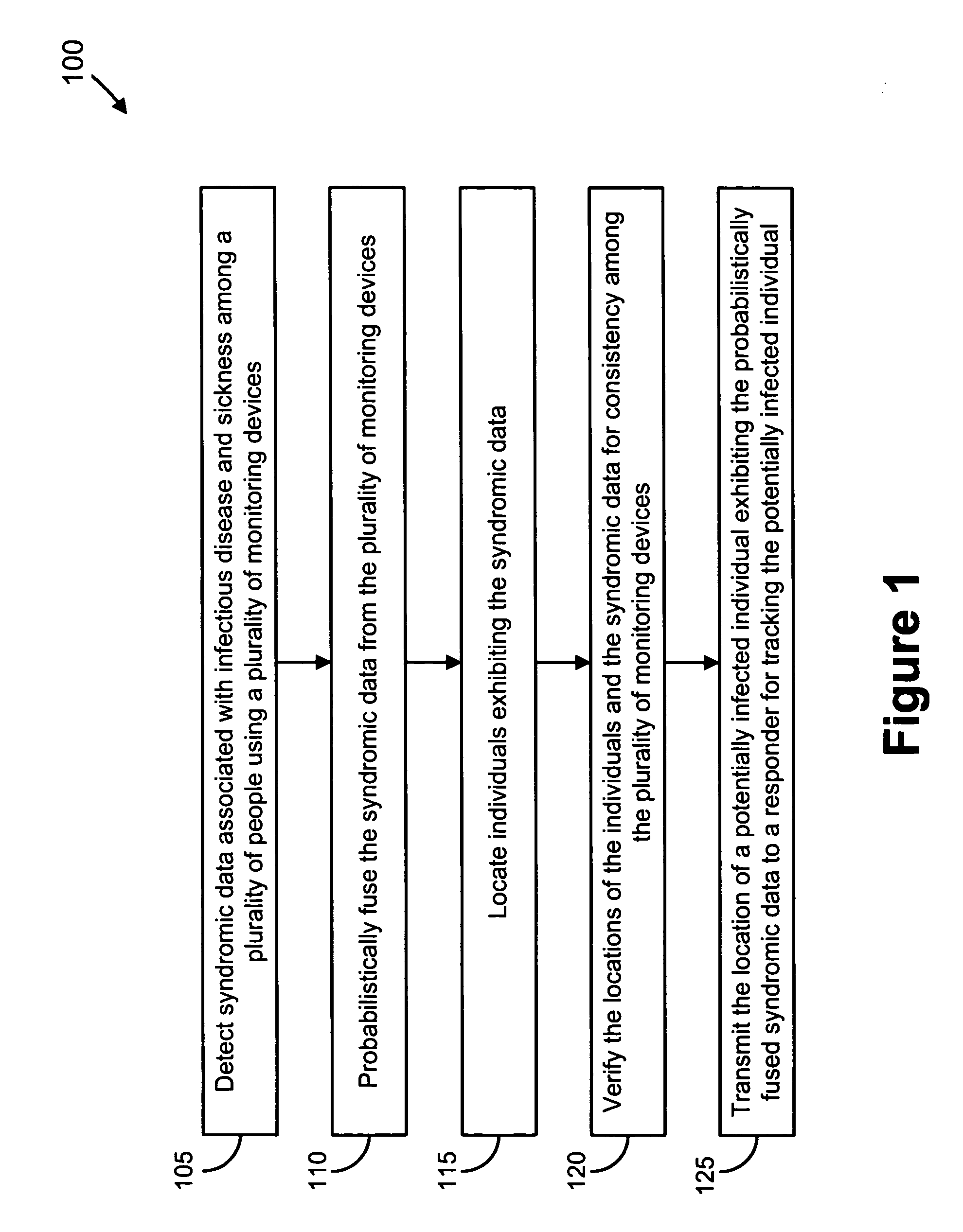
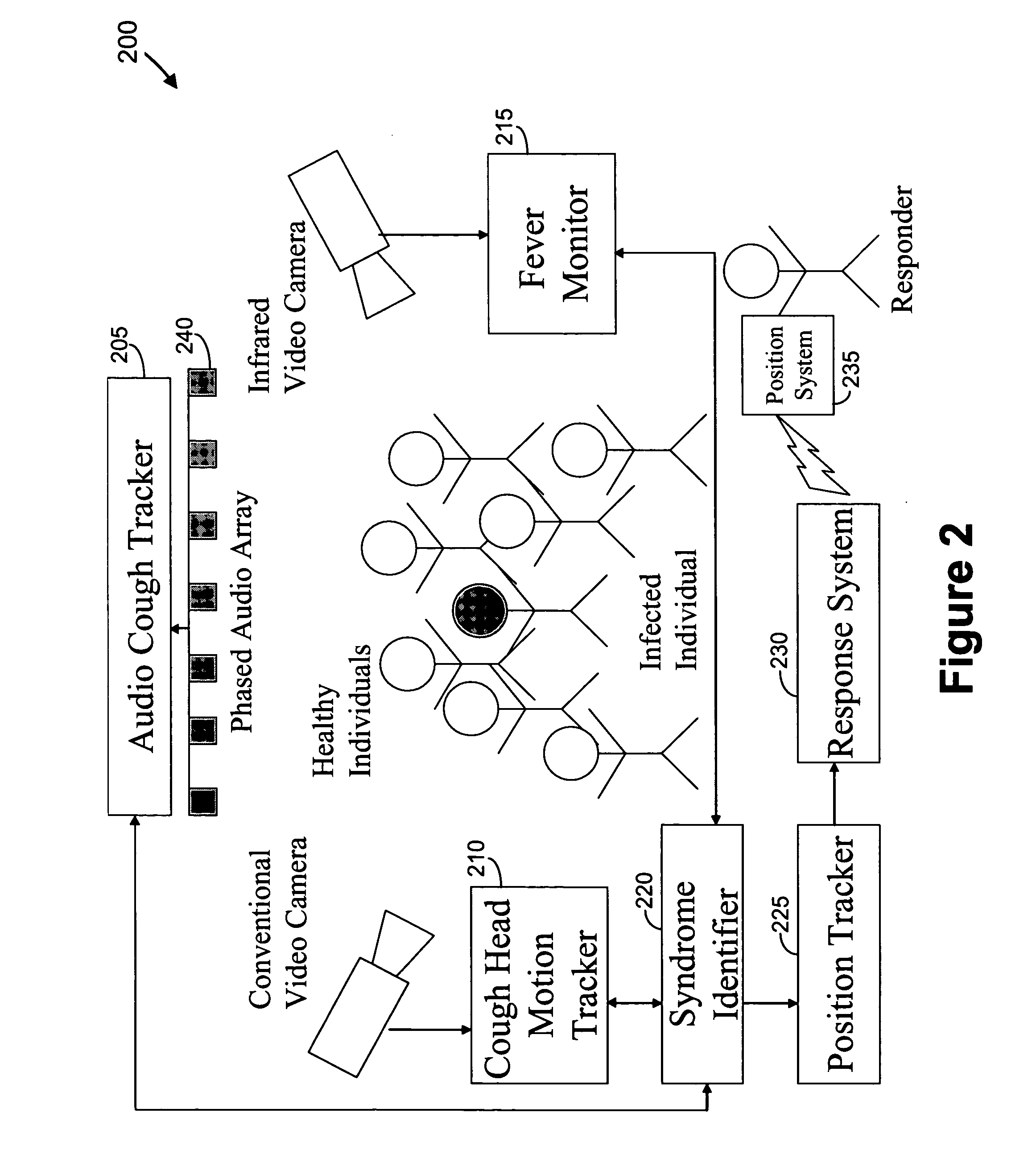
Video and audio monitoring for syndromic surveillance for infectious diseases
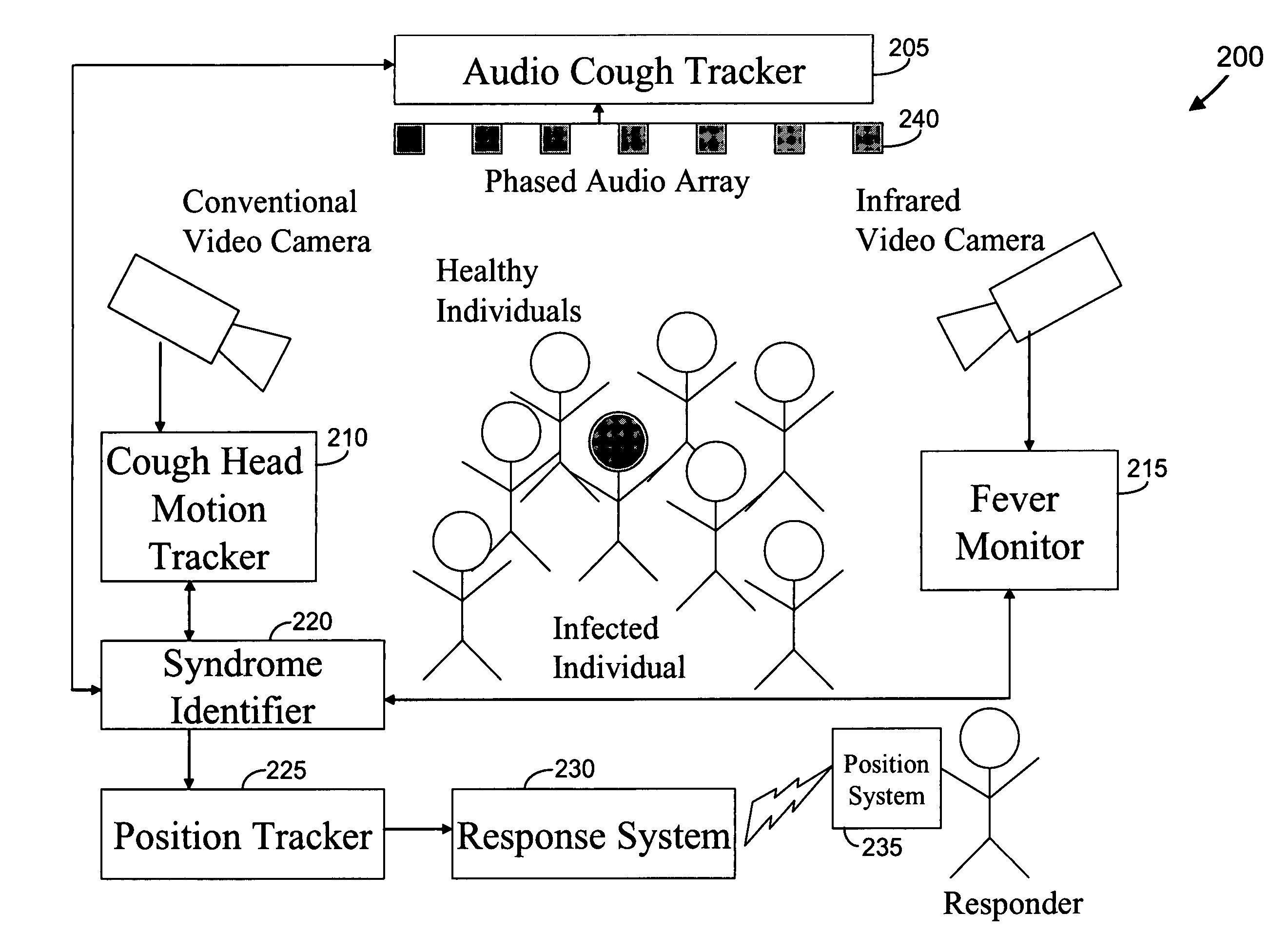
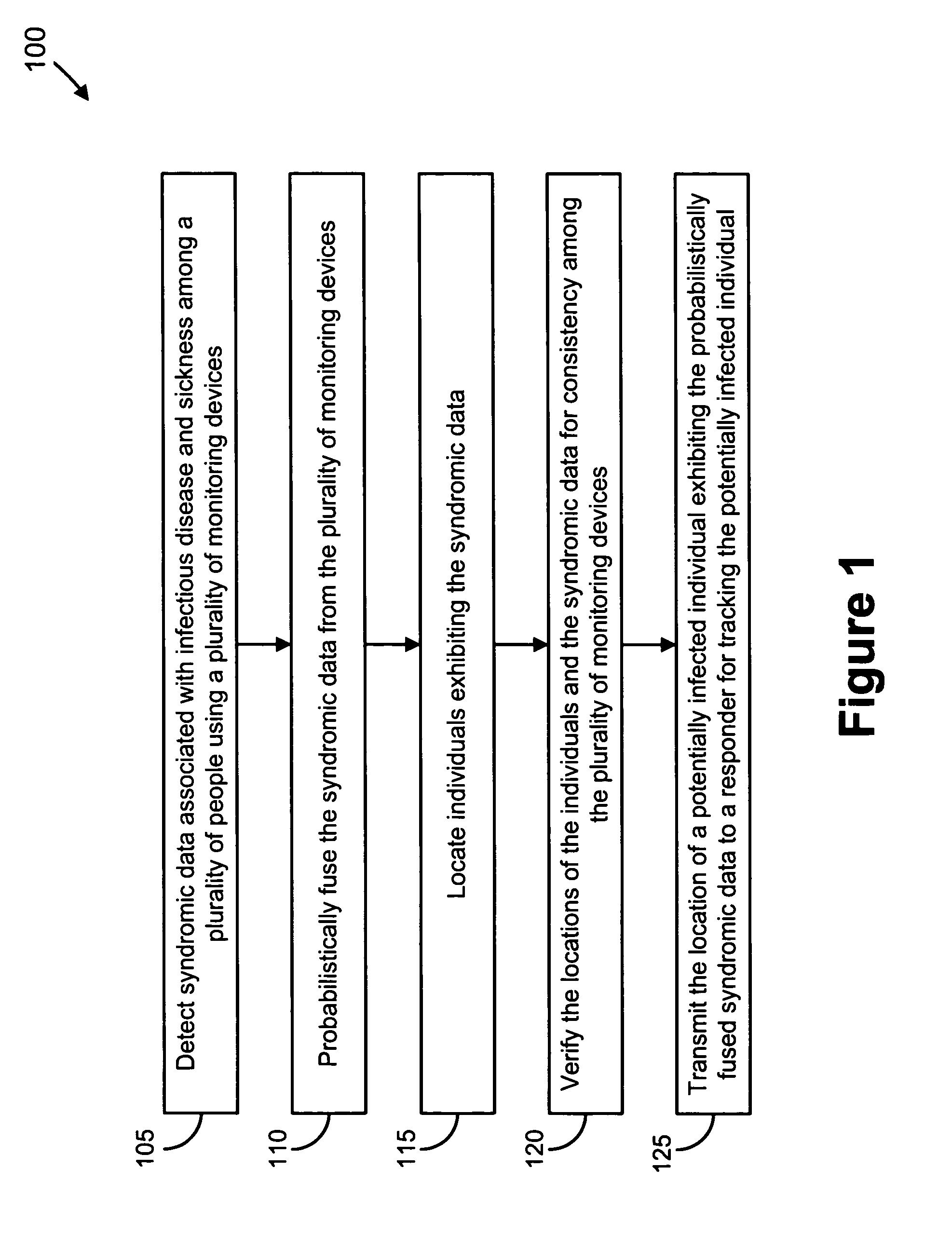
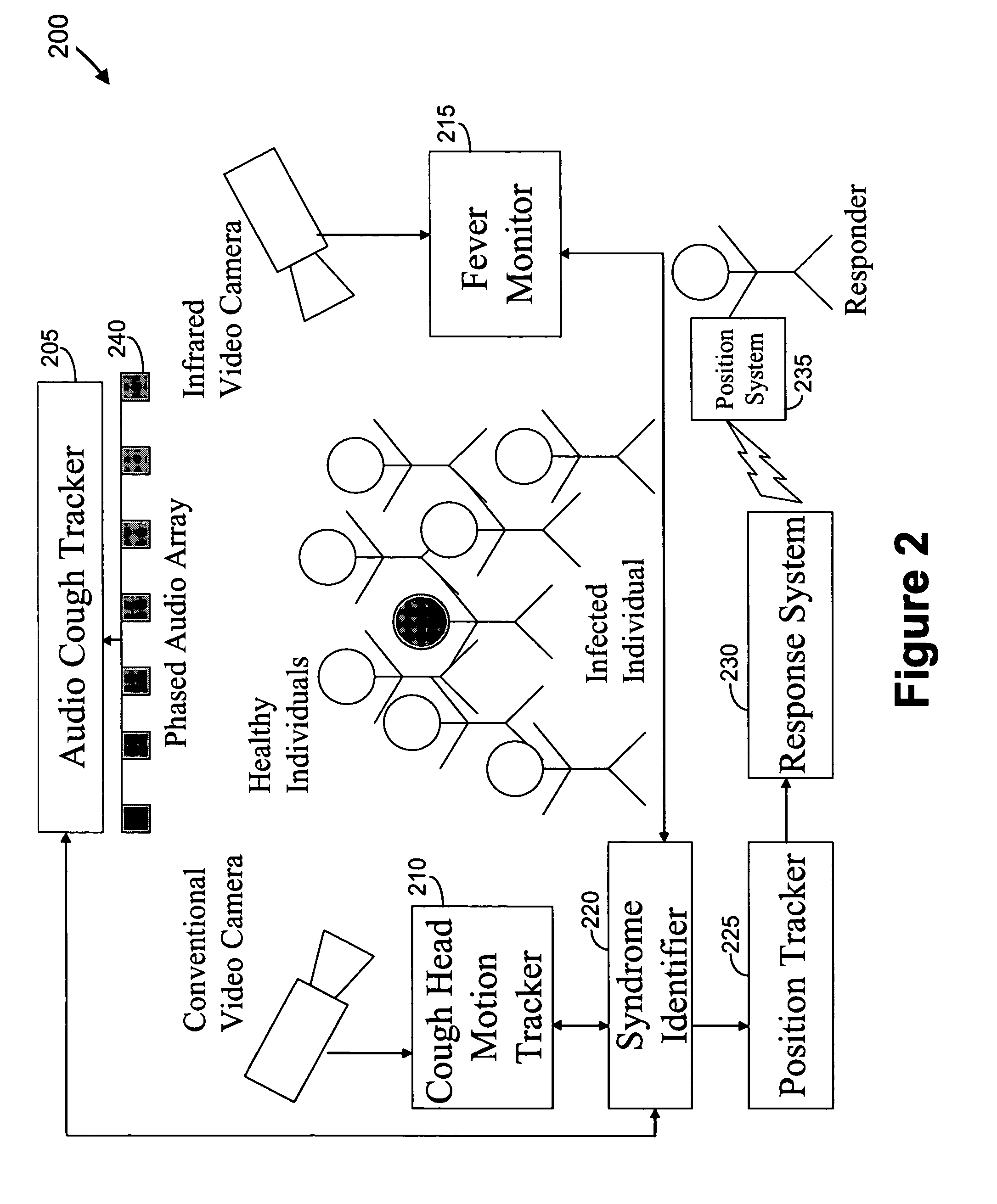
We present, in exemplary embodiments of the present invention, novel systems and methods for syndromic surveillance that can automatically monitor symptoms that may be associated with the early presentation of a syndrome (e.g., fever, coughing, sneezing, runny nose, sniffling, rashes). Although not so limited, the novel surveillance systems described herein can be placed in common areas occupied by a crowd of people, in accordance with local and national laws applicable to such surveillance. Common areas may include public areas (e.g., an airport, train station, sports arena) and private areas (e.g., a doctor's waiting room). The monitored symptoms may be transmitted to a responder (e.g., a person, an information system) outside of the surveillance system, such that the responder can take appropriate action to identifying, treat and quarantine potentially infected individuals, as necessary.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Topical application of ivermectin for the treatment of dermatological conditions/afflictions
Dermatological conditions / afflictions such as rosacea, common acne, seborrheic dermatitis, perioral dermatitis, acneform rashes, transient acantholytic dermatosis, and acne necrotica miliaris, most notably rosacea, are treated by topically applying onto the affected skin area of an individual in need of such treatment, a topical pharmaceutical composition which comprises a thus effective amount of ivermectin.
Owner:GALDERMA HLDG SA
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Skin imaging and applications
InactiveUS20140316235A1Lower cost of careMedical data miningMedical automated diagnosisPattern recognitionImaging analysis
The availability of high quality imagers on smartphones and other portable devices facilitates creation of a large, crowd-sourced, image reference library that depicts skin rashes and other dermatological conditions. Some of the images are uploaded with, or later annotated with, associated diagnoses or other information (e.g., “this rash went away when I stopped drinking milk”). A user uploads a new image of an unknown skin condition to the library. Image analysis techniques are employed to identify salient similarities between features of the uploaded image, and features of images in this reference library. Given the large dataset, statistically relevant correlations emerge that identify to the user certain diagnoses that may be considered, other diagnoses that may likely be ruled-out, and / or anecdotal information about similar skin conditions from other users. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
Video and audio monitoring for syndromic surveillance for infectious diseases
We present, in exemplary embodiments of the present invention, novel systems and methods for syndromic surveillance that can automatically monitor symptoms that may be associated with the early presentation of a syndrome (e.g., fever, coughing, sneezing, runny nose, sniffling, rashes). Although not so limited, the novel surveillance systems described herein can be placed in common areas occupied by a crowd of people, in accordance with local and national laws applicable to such surveillance. Common areas may include public areas (e.g., an airport, train station, sports arena) and private areas (e.g., a doctor's waiting room). The monitored symptoms may be transmitted to a responder (e.g., a person, an information system) outside of the surveillance system, such that the responder can take appropriate action to identifying, treat and quarantine potentially infected individuals, as necessary.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Patch agent
InactiveUS6914169B1Prevent peelingSuppresses rashes in the skin and pains upon peelingAdhesive dressingsAbsorbent padsSynthetic fiberRash
The patch agent of the present invention is a patch agent comprising a support made of a synthetic fiber and an adhesive layer mounted on the support, and having a bending resistance of 10 to 30 mm and a probe tack value of 0.25 to 1.2 N. The patch agent of the present invention can fully be prevented from peeling off at the time of application and can fully suppress the rash of skin and the pain upon peeling.
Owner:HISAMITSU PHARM CO INC
Polypeptide composition for skin whitening and spot relieving
ActiveCN105362088AInhibitory activityGood effectCosmetic preparationsToilet preparationsMedicineMedical product
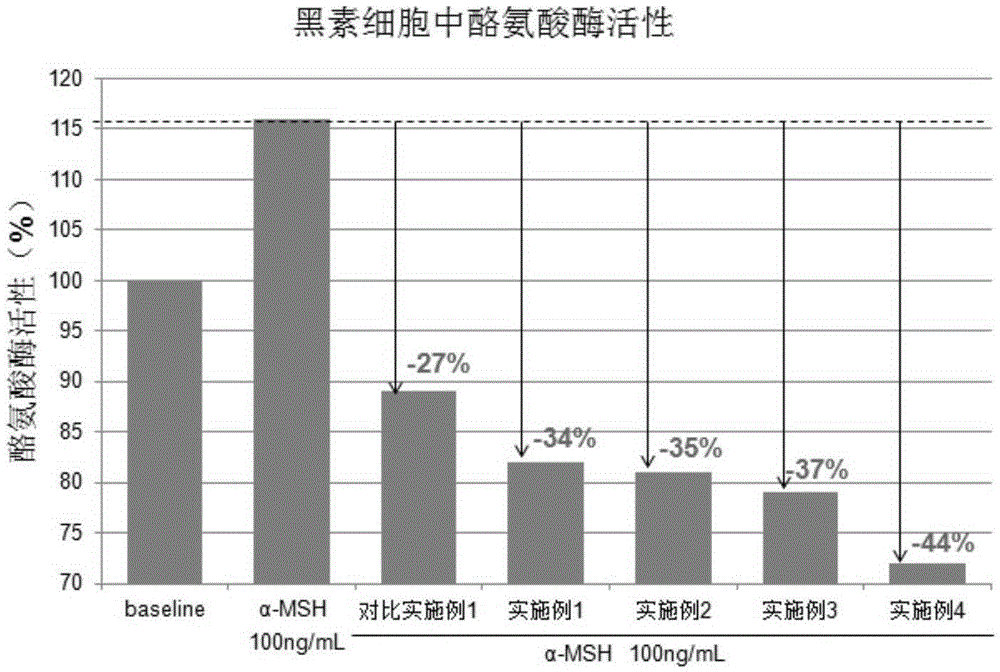
The invention provides a polypeptide composition for skin whitening and spot relieving. The polypeptide composition comprises an MC1-R antagonist (namely an alpha-MSH competitive antagonist) and / or a composition of polypeptides inhibiting alpha-MSH expression and / or a tyrosinase inhibitor. The polypeptide composition can reduce the generation of melanin, and contributes to skin brightening, so as to achieve the purposes of relieving color spots (chloasma, butterfly rash, freckle and the like), improving sallow tone and whitening skin. The concentration of each polypeptide is 0.0001-5%. The polypeptide composition provided by the invention can be prepared into various beauty and skin-care products as well as medical products.
Owner:宇肽生物(东莞)有限公司
Procyanidins for treatment and prevention of enzymatic irritation to the skin
This invention relates to methods and compositions for preventing and treating skin rash, such as perineal dermatitis, associated with enzymatic dermatitis. More particularly, this invention relates to compounds containing procyanidins, which possess trypsin and / or chymotrypsin inhibitory activity and are suitable for use in compositions for preventing and treating skin irritation caused by protease exposure, such as perineal dermatitis.
Owner:JOHNSON & JOHNSON CONSUMMER COMPANY
Modified release compositions of milnacipran
InactiveUS20060024366A1Reduce incidenceDiminished decreased intensityCoatingsDrageesPalpitationsRash
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Composition
The invention describes a method of producing extracts from the seeds of meadowfoam, brassicas and crambe plants. A number of subsidiary processes and steps are shown in order to extract differing fractions of oil. Products produced from the above method are also described including uses and methods of these products which include a variety of skin conditions including eczema, facial eczema, dermatitis, external ulcers, welts, rashes, insect bites, allergic reactions and other irritations, burns, wounds, psoriasis, acneiform eruptions, dryness, dry skin, irritation, skin atrophy, secondary infections and the like. The extracts are also described as being a useful compound for treatment of the symptoms of such skin conditions as described above. In particular the use and extraction of glucosinolate (GSL), thiocyanates (TCL) and isothiocyanates (ITCL) is described.
Owner:NEW ZEALAND BOTANICAL OILS
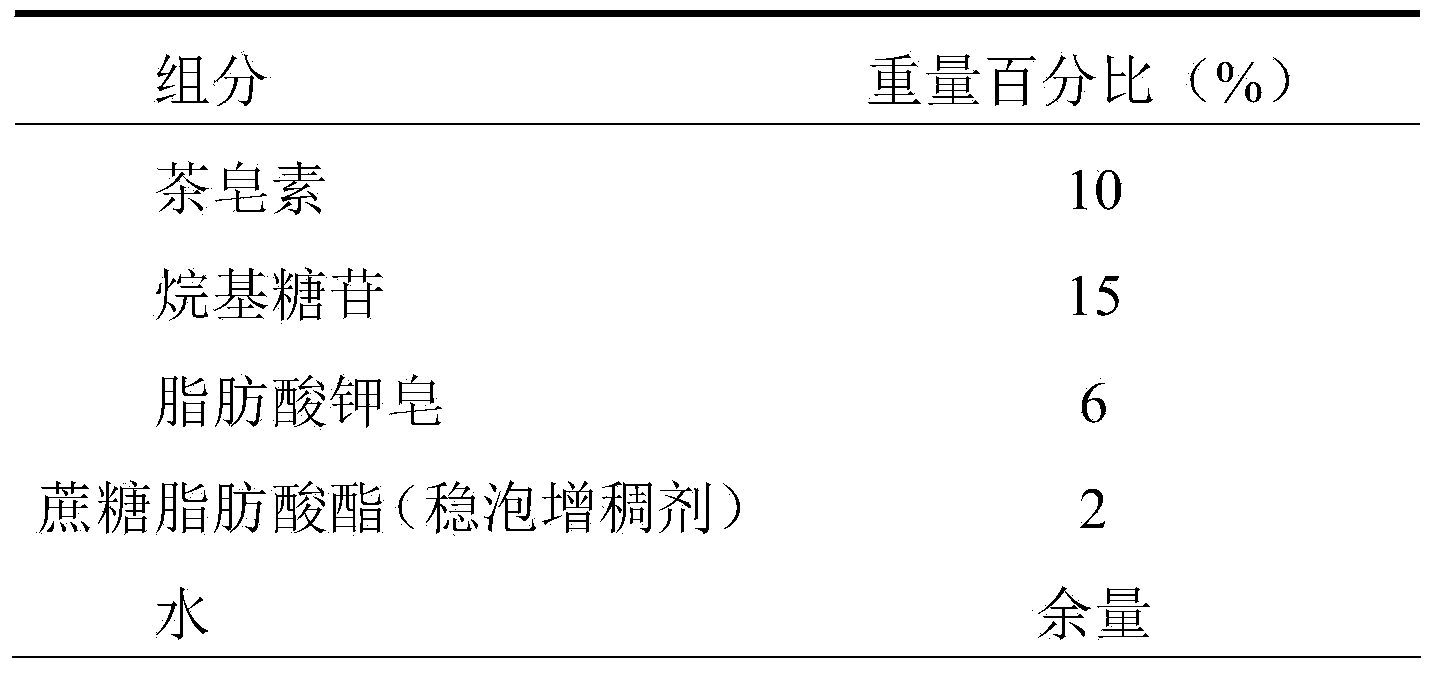
Shower gel
ActiveCN103637963ANo side effectsReduce drynessAntibacterial agentsCosmetic preparationsEthylenediamineSide effect
The invention relates to a shower gel. The shower gel provided by the invention is prepared from green and safe surfactants, namely tea saponin and alkyl glycoside, safe and mild surfactants, namely lauramidopropylamine oxide, soap of potassium aliphatate and cationic guar gum, assistants, namely sucrose fatty acid ester (a foam stabilizing and thickening agent) and aloe extraction solution, and other safe assistants, such as ethylenediamine tetraacetic acid disodium salt, a preservative and deionized water. Compared with an existing shower gel product, the shower gel provided by the invention has the advantages of reasonable formula, very rich raw material sources, property of belonging to renewable resources and no toxic or side effects to human bodies, and the shower gel is green, ecological and environment-friendly, and can relieve dry and tight feeling of skin, effectively inhibit itching, nourish and moisturize the skin, enable the skin to be tender, smooth and elastic by long-term use, realize the effects of sterilizing, inhibiting bacteria, diminishing inflammation and relieving itching and realize an auxiliary treatment effect on rash and prickly heat.
Owner:安徽优琦润健康科技有限公司
Compositions expressing a pressure of carbon dioxide for improved healing of wounds
Compositions that express a pressure of carbon dioxide are beneficial when used for the care of wounds, and when externally applied to skin damaged by cuts, burns, abrasions, excessive radiation, or from rashes and similar dysfunctions.
Owner:SWENSON RUSSELL H +1
Topical application of ivermectin for the treatment of dermatological conditions/afflictions
Dermatological conditions / afflictions such as rosacea, common acne, seborrheic dermatitis, perioral dermatitis, acneform rashes, transient acantholytic dermatosis, and acne necrotica miliaris, most notably rosacea, are treated by topically applying onto the affected skin area of an individual in need of such treatment, a topical pharmaceutical composition which comprises a thus effective amount of ivermectin.
Owner:GALDERMA HLDG SA
Cover and applicator for a portion of a mammalian body
InactiveUS7487779B2Reduce the amount requiredFreedom of movementBreast bandagesAnaesthesiaAnesthetic AgentInjury mouth
One aspect of the present invention relates to a covering member for a protruding portion of a patient's body. This cover is flexible and collapsible so that it conforms to the shape of the encased portion of the body. This prevents the cover from being noticed while it is worn under clothing. The cover can contain a medicament or agent for pretreating a portion of the body before an examination or procedure. When the cover is used to apply a topical anesthetic to a portion of the body for a recommended period of time prior to the procedure or examination commencing, the amount of discomfort experienced by the patient can be significantly reduced compared to the amount experienced by the patient who has the anesthetic applied for just a few moments before the procedure begins. Alternatively, the cover can be used to hold a medicament or agent on the intended portion of the body in order to heal a wound or cure a condition such as a skin rash or the like.
Owner:DR SUSAN LOVE RES FOUND
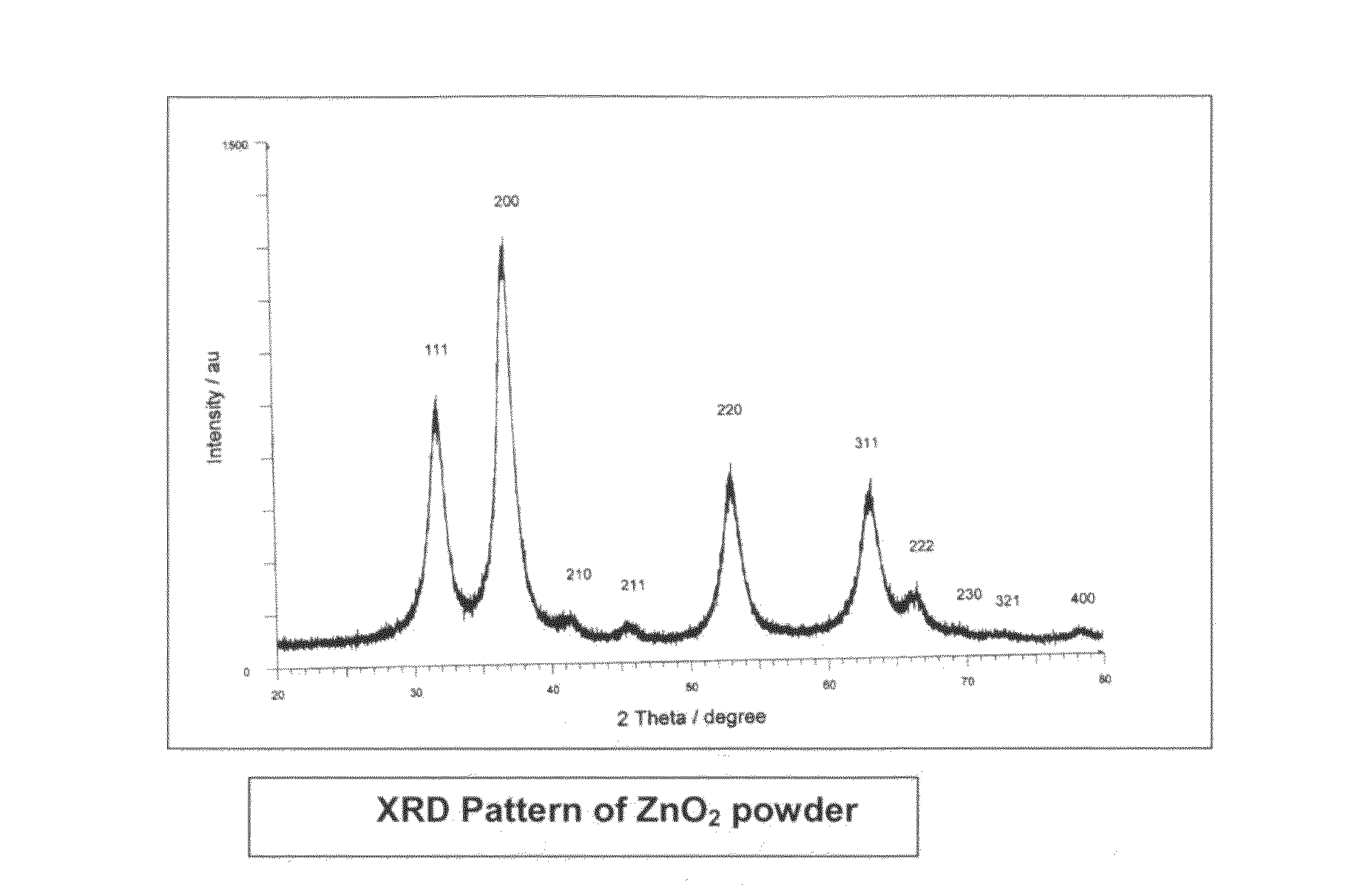
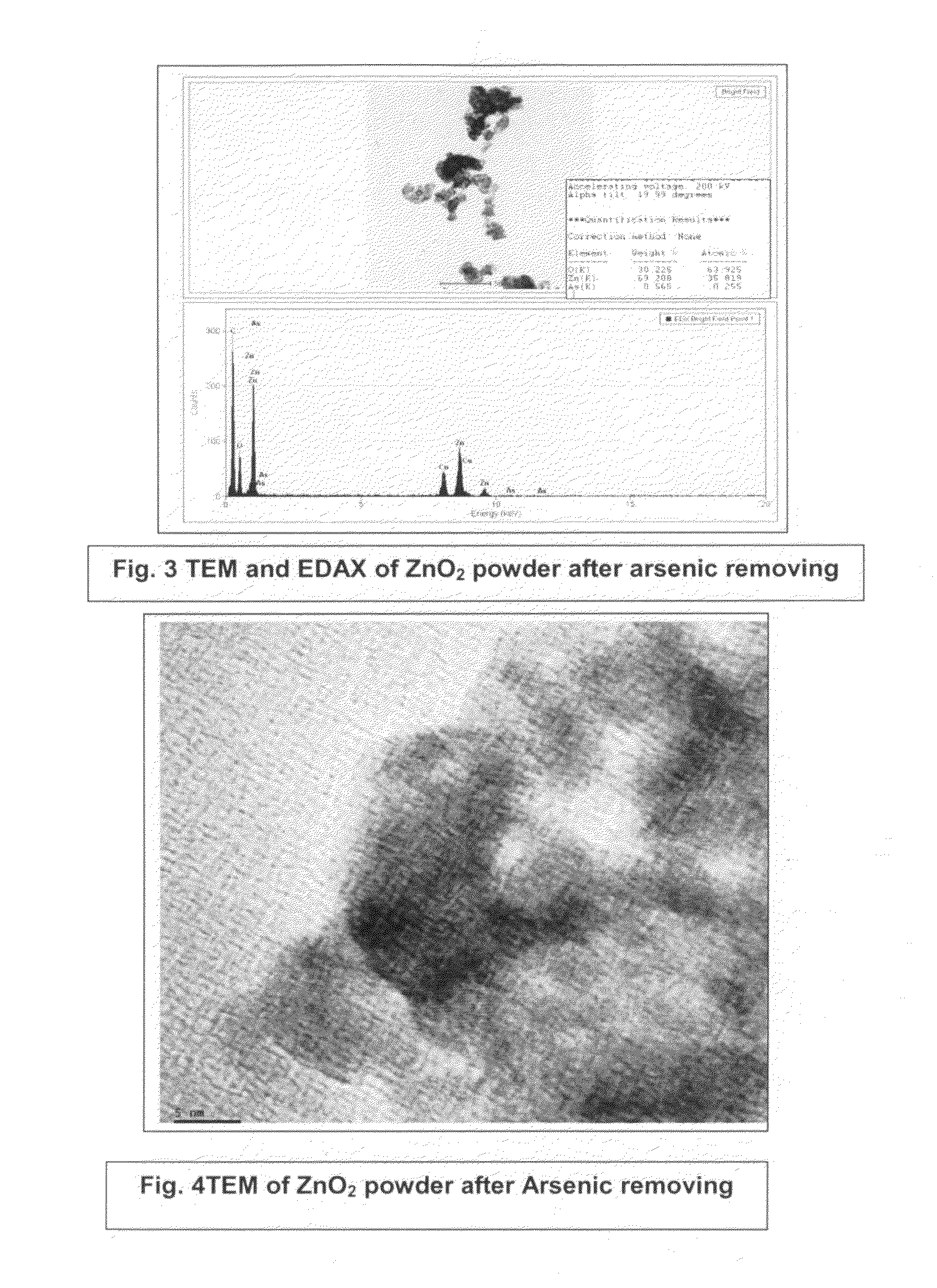
Process for the removal of arsenic and chromium from water
InactiveUS20110220577A1Low costSmall sizeMaterial nanotechnologyWater treatment compoundsZinc peroxideGlycerol
The present invention provides low cost and highly effective method for the removal of arsenic and Cr(III&VI) from contaminated water using zinc peroxide nanoparticles (20±5 nm) capped with glycerol / PVP / TEA upto the permissible range of drinking water. As Arsenic and chromium occurs naturally in the earth's crust. When rocks, minerals, and soil erode, they release arsenic and chromium into groundwater. Arsenic and chromium occurs naturally in varying amounts in groundwater in various parts of country from ppb level to ppm level. The average concentration of arsenic and chromium as per USEPA standard in drinking water it is 10 parts per billion and 0.05 ppm (50 ppb) respectively. In drinking water the level of chromium is usually low as well, but contaminated water may contain the dangerous Cr(III&VI). Although Cr(III) is an essential nutrient for humans and shortages may cause heart problems, disruptions of metabolisms and diabetes. But the uptake of too much Cr(III) can cause health effects as well, for instance skin rashes. Cr(VI) is known to cause various health effects Skin rashes, upset stomachs, respiratory problems, weakened immune systems, kidney and liver damage and lung cancer The persons who are drinking water having upto 50 ppb of arsenic and 0.05 ppm chromium over for many years could experience skin damage or problems with their circulatory system, and may have an increased risk of getting cancer. Keeping the above facts we developed a cost effective nanoparticles for the removal of Arsenic and Cr(III&VI) from potable water upto potable range.
Owner:COUNCIL OF SCI & IND RES
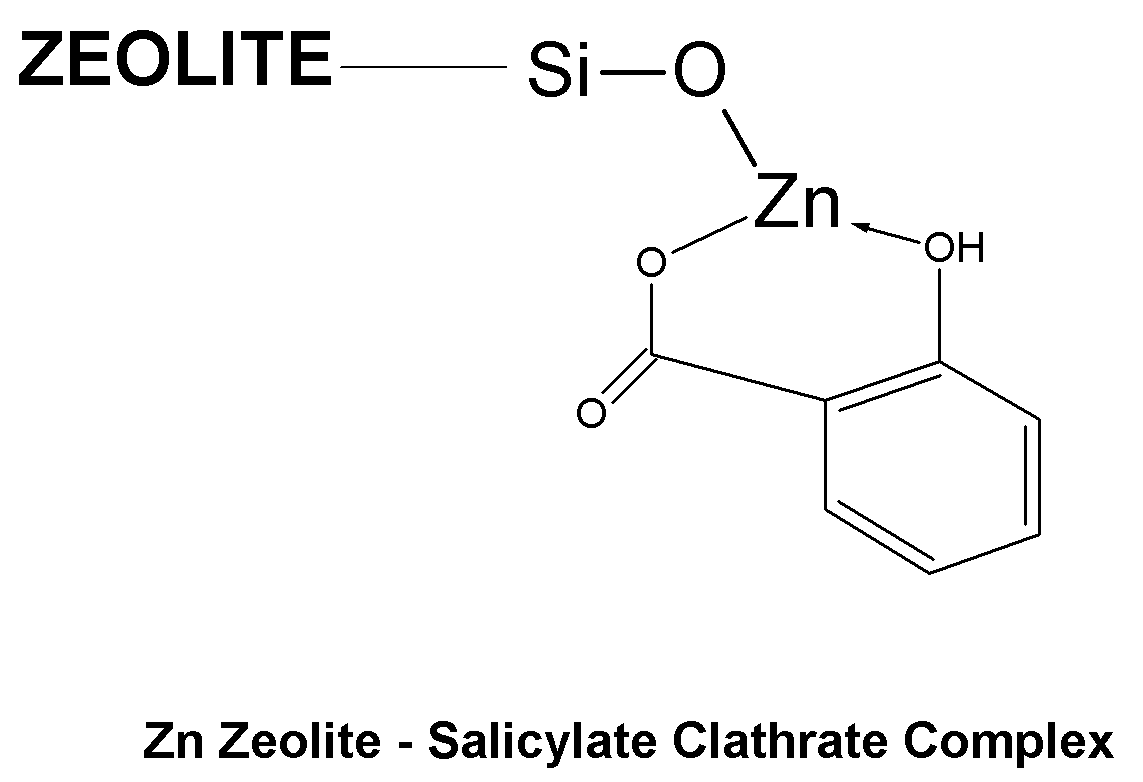
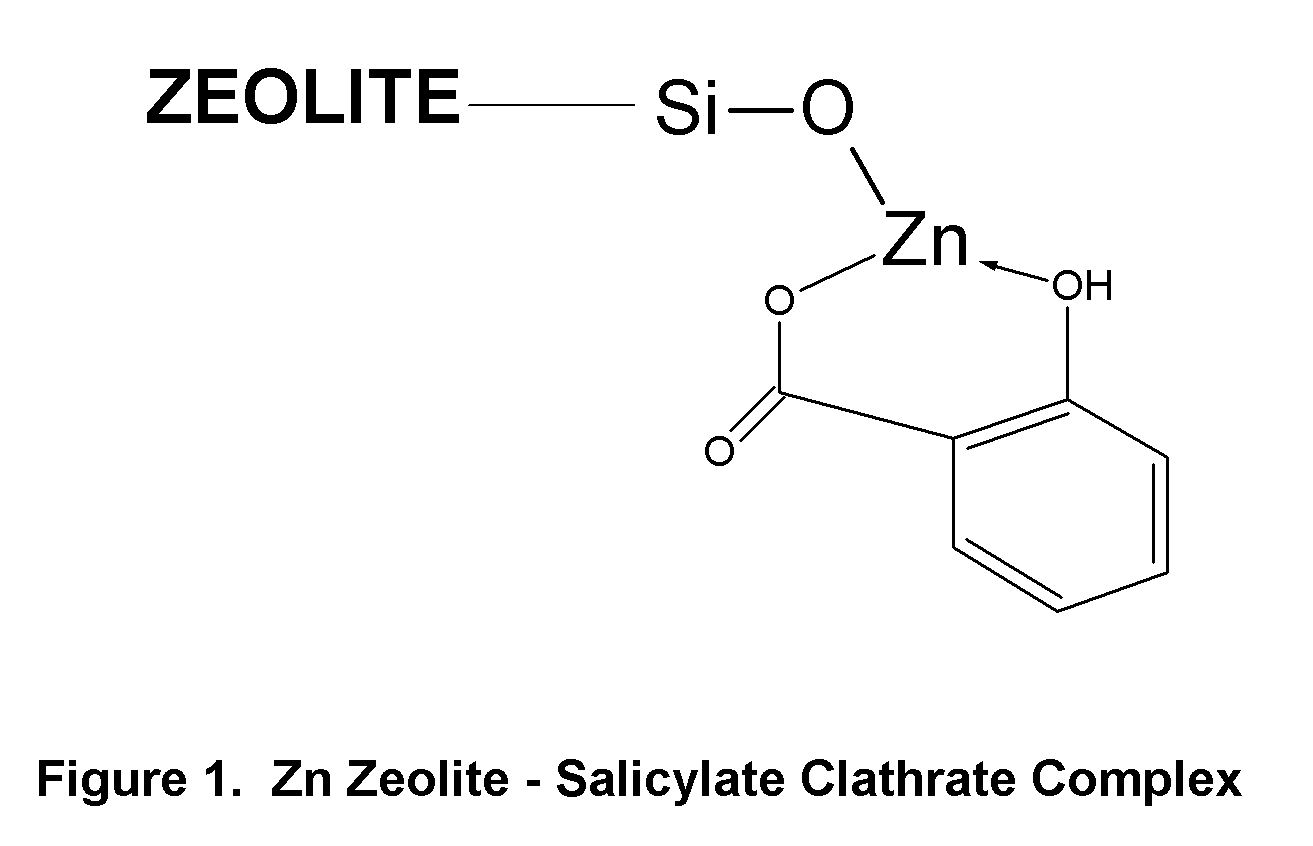
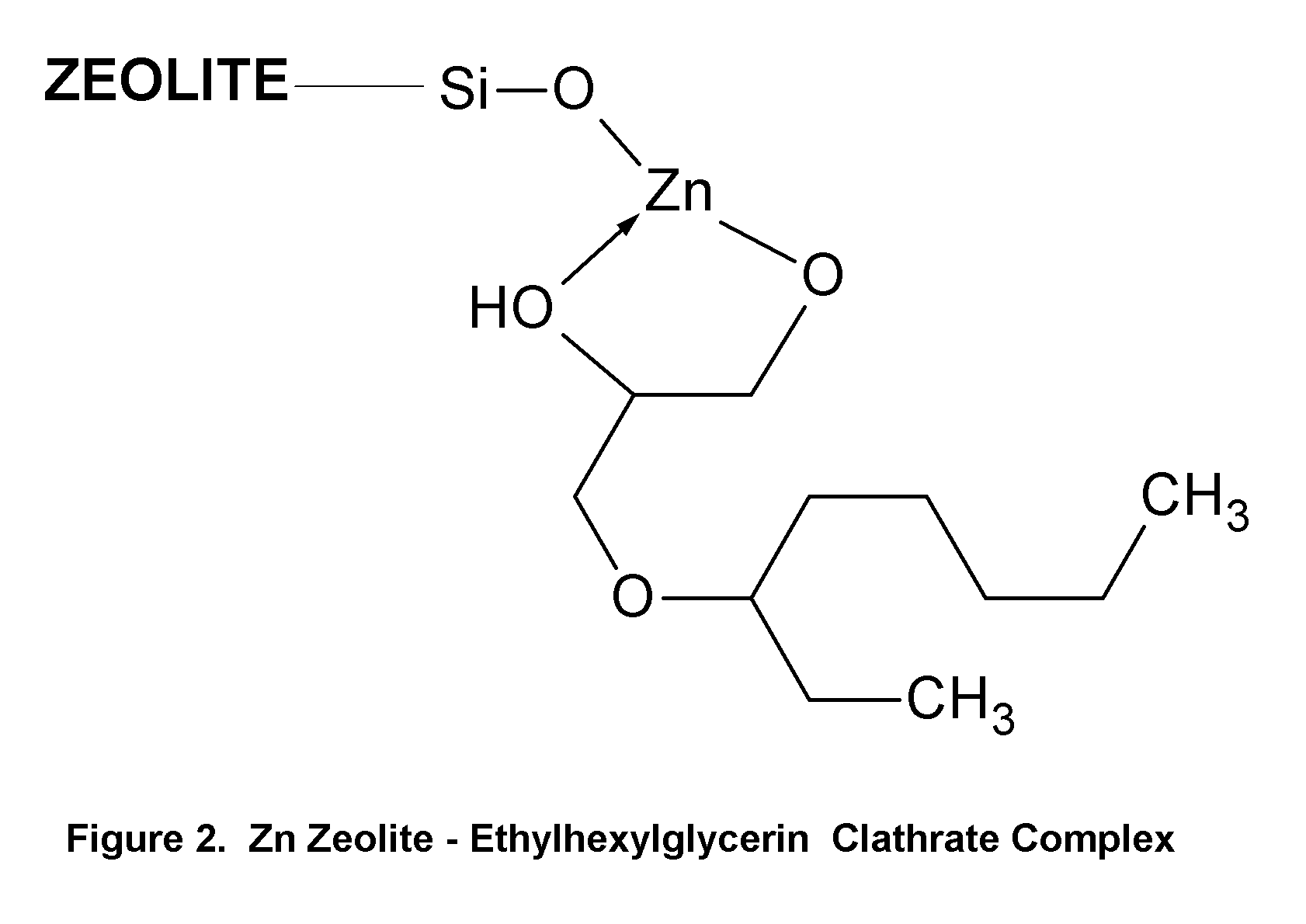
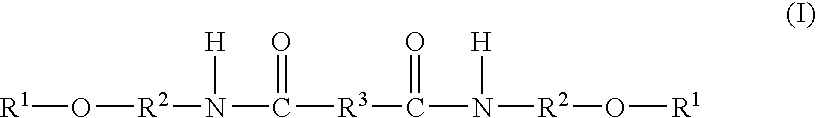
Topical Delivery of Biological and Cosmetic Agents by Zeolites
The present invention discloses certain di- and polyvalent metal zeolite compounds (formula I) for topical delivery of biological and skin and hair care agents. The method of treating skin and hair condition via topical application of said zeolite compounds is also disclosed. The said method provides a treatment for topical condition, which includes alleviation of skin conditions such as skin rash including diaper rash, dry skin, scalp dandruff, broken or chafed skin, sunburn, skin damage from UV, skin irritation, acne including excess facial oil and facial pore size; darkened skin including age spots, dark circles around the eyes, and discoloration of skin from stretch marks; skin aging including wrinkles and fine lines; loss of collagen including thinning skin and loss of skin pliability; body odor, including oral cavity odor, arm-pit odor, and incontinence odor; cellular inflammation including intracellular and extra cellular inflammation; premature hair aging including premature hair loss hair graying; malfunction of tyrosinase group of enzymes, malfunction of matrix metalloprotease group of enzymes; and combinations thereof. The said method also provides topical delivery of certain metals, including trace metals, and certain zirconium aluminum amino acids that provide antiperspirant benefits;
Owner:BIODERM RES
Topical Copper Ion Treatments and Methods of Treatment Using Topical Copper Ion Treatments in the Dermatological Areas of the Body
ActiveUS20140271797A1Good lookingImprove skin appearanceSuture equipmentsBiocideDiseaseWound dressing
Owner:CDA RES GROUP
Pure traditional Chinese medicine mask with effects of spot removing, acne removing, wrinkle removing and whitening
InactiveCN103462875ACosmetic preparationsAnthropod material medical ingredientsTreatment effectSide effect
The present invention relates to a pure traditional Chinese medicine mask with effects of spot removing, acne removing and whitening, wherein the mask comprises mung bean, ma-yuen jobstears seed, seaweed, lemon, aloe, cordyceps sinensis, pangolin piece, pearl, dangshen, peppermint, safflower, peach seed, almond, angelica archang lica, largehead atractylodes rhizome, common bletilla tuber, baikal skullcap root, dan-shen root, silkworm larva, common motherwort herb, fragrant solomonseal rhizome, smilax china, ginkgo leaf, fortune firethorn fruit and root, and other main raw materials, and is a full-nature nutritional substance with no toxic-side effect. With the mask, a conditioning effect is provided based on skin detoxification cycle, skin metabolism cycle is promoted, pores are cleaned and minimized, problems of dull complexion, large pores, dark yellow complexion, oiliness, acne, comedo, yellow qi and the like of the whole skin can be conditioned and cleaned, significant treatment effects are provided for acne, rash, comedo and the like, and functions of nutrition, skin health care and whitening are provided. In addition, the traditional Chinese medicine mask is used in more than 200 beautifying institutions in the region, and is compensatedly used in more than 600 national beautifying institutions according to contract agreements, wherein more than 80 beautifying chain institutions are included.
Owner:张淯河
Method of Treating Skin Condition Including Acne, Skin Aging, Body Odor & Diaper Rash by Zinc Zeolite Clathrates
Owner:BIODERM RES
Absorbent article
The present invention provides an absorbent article capable of suppressing rash of skin in regions which come into contact with a gather portion of the absorbent article, by applying a water-soluble skin care agent and oily skin care agent on the gather portion to be contacted with the skin of a wearer in wearing the absorbent article, and allowing at least a part of the above mentioned oily skin care agent to be transferred to the skin of a wearer when wearing before the above mentioned water-soluble skin care agent is transferred to the skin of a wearer.
Owner:KAO CORP
Biodegradable filling and making up injection material and preparation method thereof
InactiveCN106176292AReduce dosageReduce erythemaCosmetic preparationsToilet preparationsWrinkle skinRash
The invention relates to the technical field of medical plastic surgery, in particular to the technical field of facial filling and anti-wrinkle, in particular, it is an injection filling cosmetic modification anti-aging, skin beautification and facial rejuvenation material. The preparation formula of the present invention includes hyaluronic acid, collagen, vitamin E and PLGA. The preparation method comprises: 1) preparing high-viscosity gel; 2) preparing PLGA nano-microspheres; 3) fully mixing high-viscosity gel and PLGA nano-microspheres; 4) sterilizing and filling. Compared with the prior art, the present invention has the beneficial effect that: the combined use of collagen and hyaluronic acid reduces skin erythema, swelling, hardening or itching, muscle pain, urticaria or Other symptoms such as rash. It reduces the probability of severe complications such as peripheral blood circulation embolism, skin necrosis, retinal artery embolism, and cardiocerebral artery embolism that may be caused by simple hyaluronic acid injection, and the safety of product use is improved.
Owner:江苏华亿美素生物组织工程有限公司
Composition and Method for the Topical Treatment of Dermatitis
InactiveUS20130243888A1Quick and exceptional treatmentShorten the construction periodOrganic active ingredientsBiocideTopical treatmentPoison oak
A composition for topical treatment of dermatitis is provided. The composition includes one or more anti-histamines or a pharmaceutically acceptable salt thereof; one or more polysaccharides; and one or more Group 1, 2, or 13 metal hydroxides. The dermatitis may be a poison ivy, poison oak, poison sumac, diaper rash, eczema, lichen simplex chronicus, rashes, dermatoses, seborrheic dermatitis, psoriasis, atopic dermatitis, or the combination thereof A method of treatment of dermatitis is also provided.
Owner:FORD SARA BETH
Method of controlling rash advance at tail end of route and vehicle equipment of controlling rash advance at tail end of route
ActiveCN107284477AFulfil requirementsInfluence of Ensuring Positioning AccuracyAutomatic initiationsSatellite radio beaconingCurrent velocityEngineering
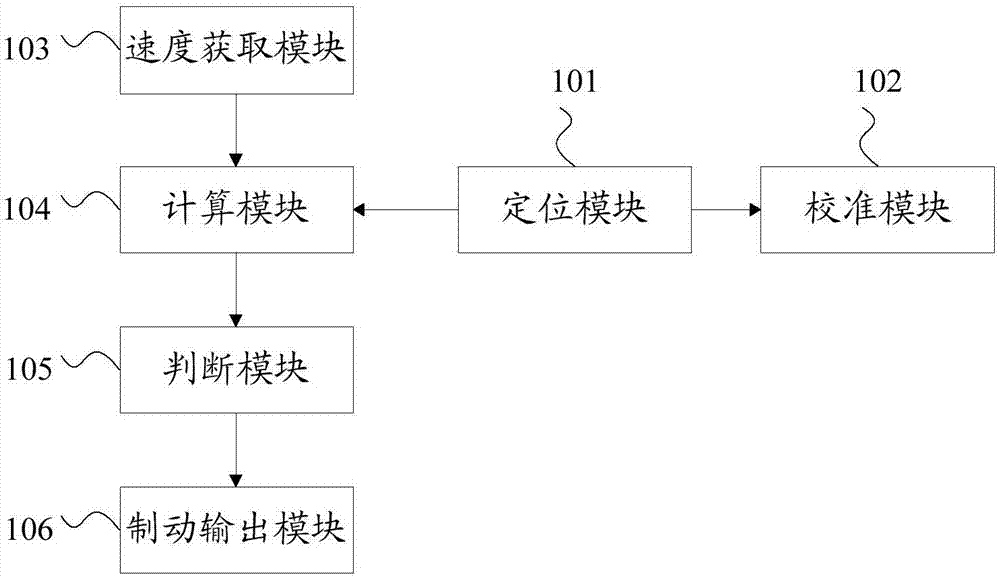
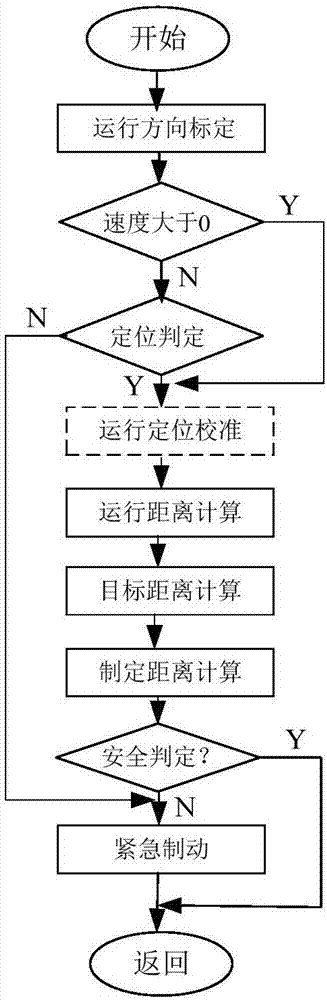
The invention discloses vehicle equipment for controlling the rash advance at the tail end of a route. The vehicle equipment comprises a positioning module, a calibration module, a speed acquisition module, a calculation module, a judging module, and a braking output module; the positioning module is used for obtaining the satellite positioning of a vehicle, the calibration module is used for positioning and calibrating the satellite positioning and the actual location of the vehicle when the vehicle speed is at zero; the speed acquisition module is used for real-time acquisition of the current speed of the vehicle and using the current speed as the calculation speed; the calculation module is used for calculating the distance of the vehicle currently apart from the end and the distance of braking corresponding to the current velocity; the judging module is used for determining whether the difference between the distance of the vehicle currently apart from the end and the distance of braking is less than the preset safety margin; the braking output module is used for issuing a braking instruction to a braking system when the difference between the distance of the vehicle currently apart from the end and the distance of braking is smaller than the preset safety margin. The invention also discloses a method for controlling the rash advance at the tail end of the route. By the adoption of the vehicle equipment for controlling the rash advance at the tail end of the route, the positioning precision is high, the structure is simple, the maintenance cost is low, and the requirements of a test route can be met.
Owner:ZHUZHOU ELECTRIC LOCOMOTIVE CO LTD
Infant diaper rash cream and preparation process thereof
ActiveCN109044915AExcellent anti-inflammatoryExcellent Pain Relief and SwellingCosmetic preparationsAntipyreticButtocksEthylhexyl palmitate
The embodiment of the invention discloses infant diaper rash cream. The infant diaper rash cream comprises a skin conditioning agent, emollient, humectant, absorbent, emulsifier, antioxidant, a thickening agent and deionized water; the skin conditioning agent comprises allantoin, bisabolol, borneol, and witchhazel extract; the emollient comprises ethylhexyl palmitate, polydimethylsiloxane, cyclopentasiloxane, cyclohexasiloxane, synthetic beeswax, camellia-seed oil, and deep-sea crambe abyssinica seed oil. Various ingredients of the diaper rash cream are reasonably compounded, besides the moisturizing effect of the existing diaper rash cream, multiple pure natural plant extracts are adopted so as to form a layer of protective film on the surface of the skin, the skin friction is reduced, the excrement can be isolated, thereby effectively preventing the infant buttock; the product further has bacteriostatic and anti-inflammatory effects, the skin can be further relieved, so as to furtherachieve the purposes of improving the treating the read buttock. The diaper rash cream disclosed by the invention is stable in performance, safe and non-irritant, and is an expected and healthy infant skin protection product.
Owner:北京纯粹主义科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com