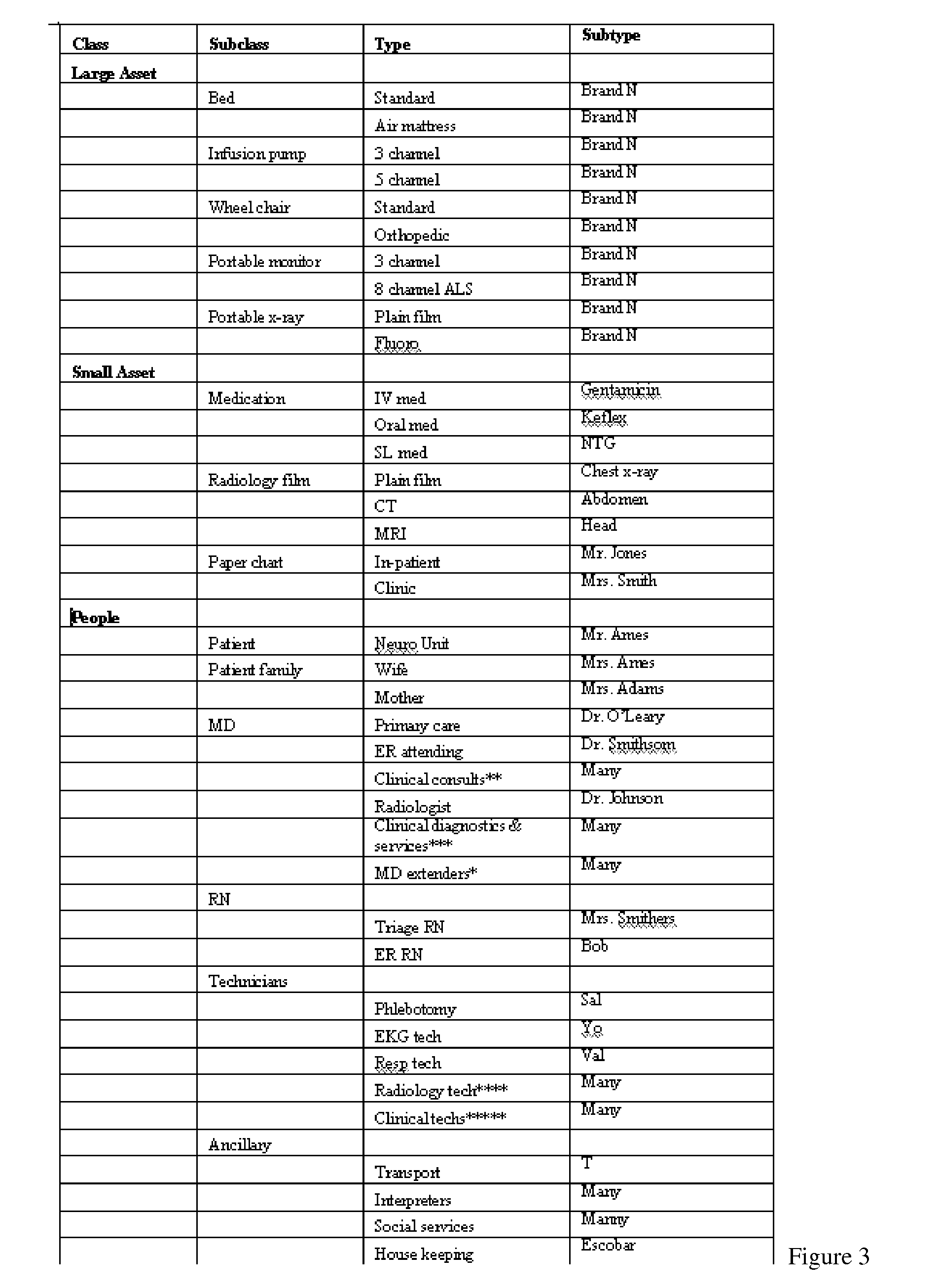
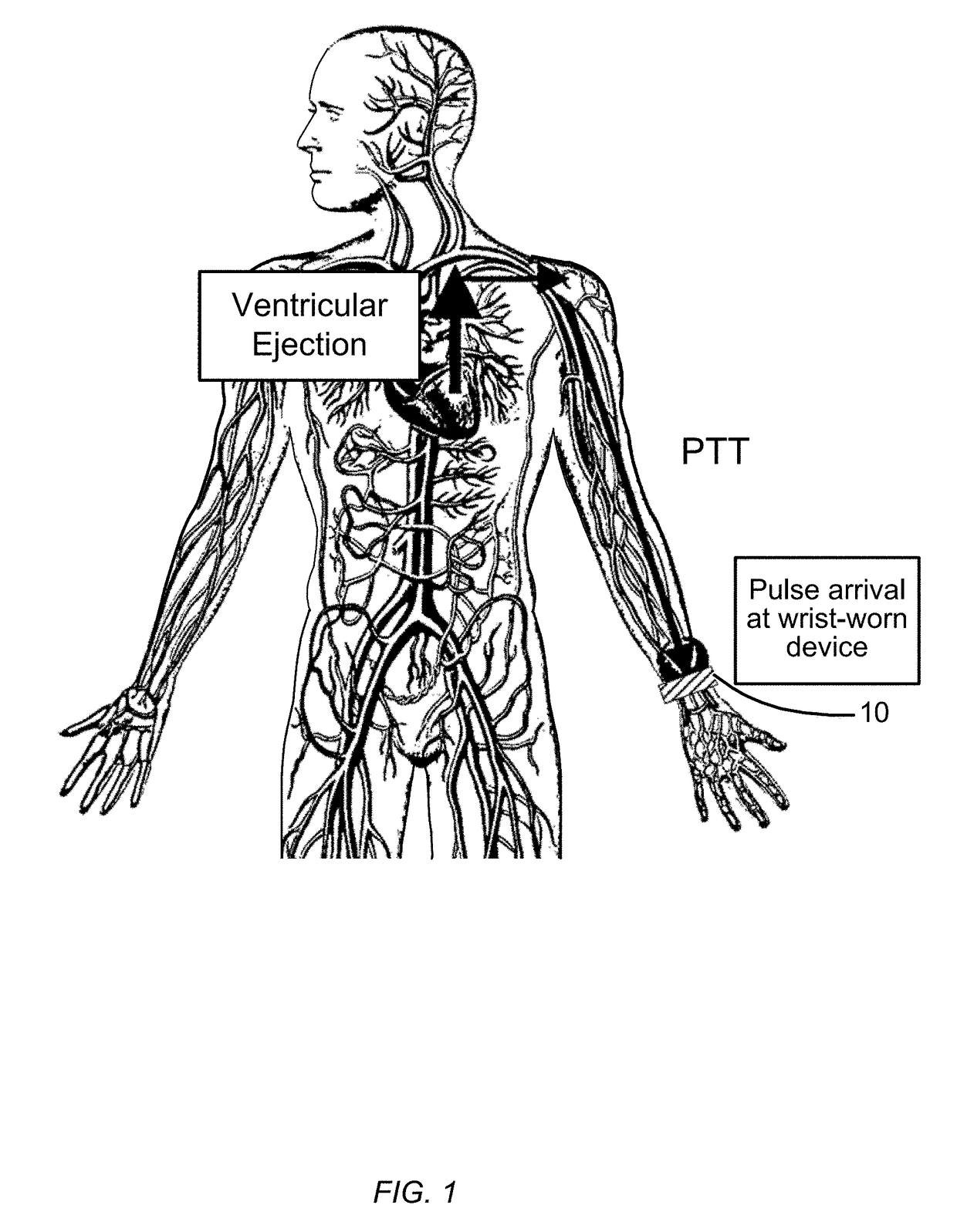
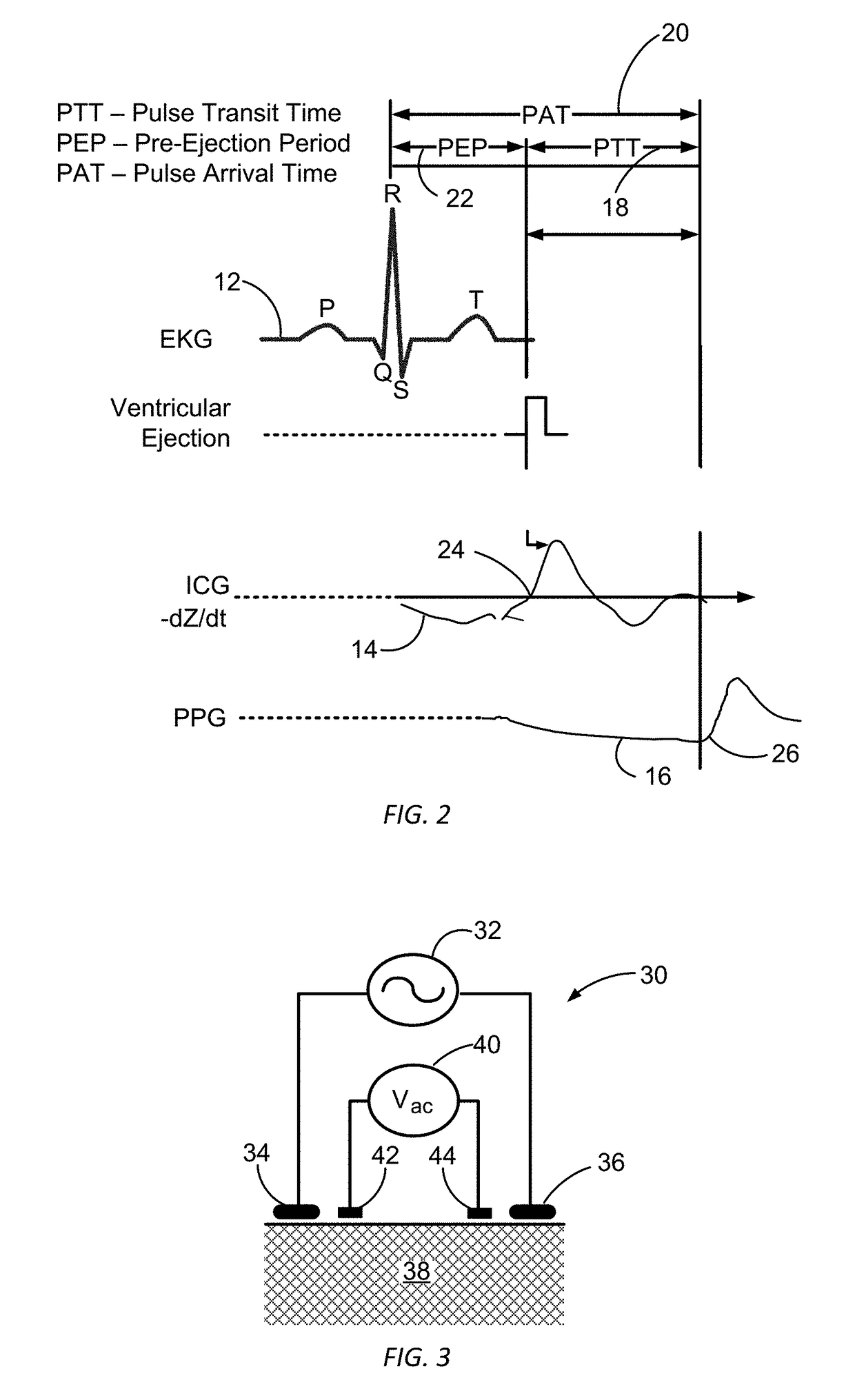
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
215results about How to "Lower cost of care" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
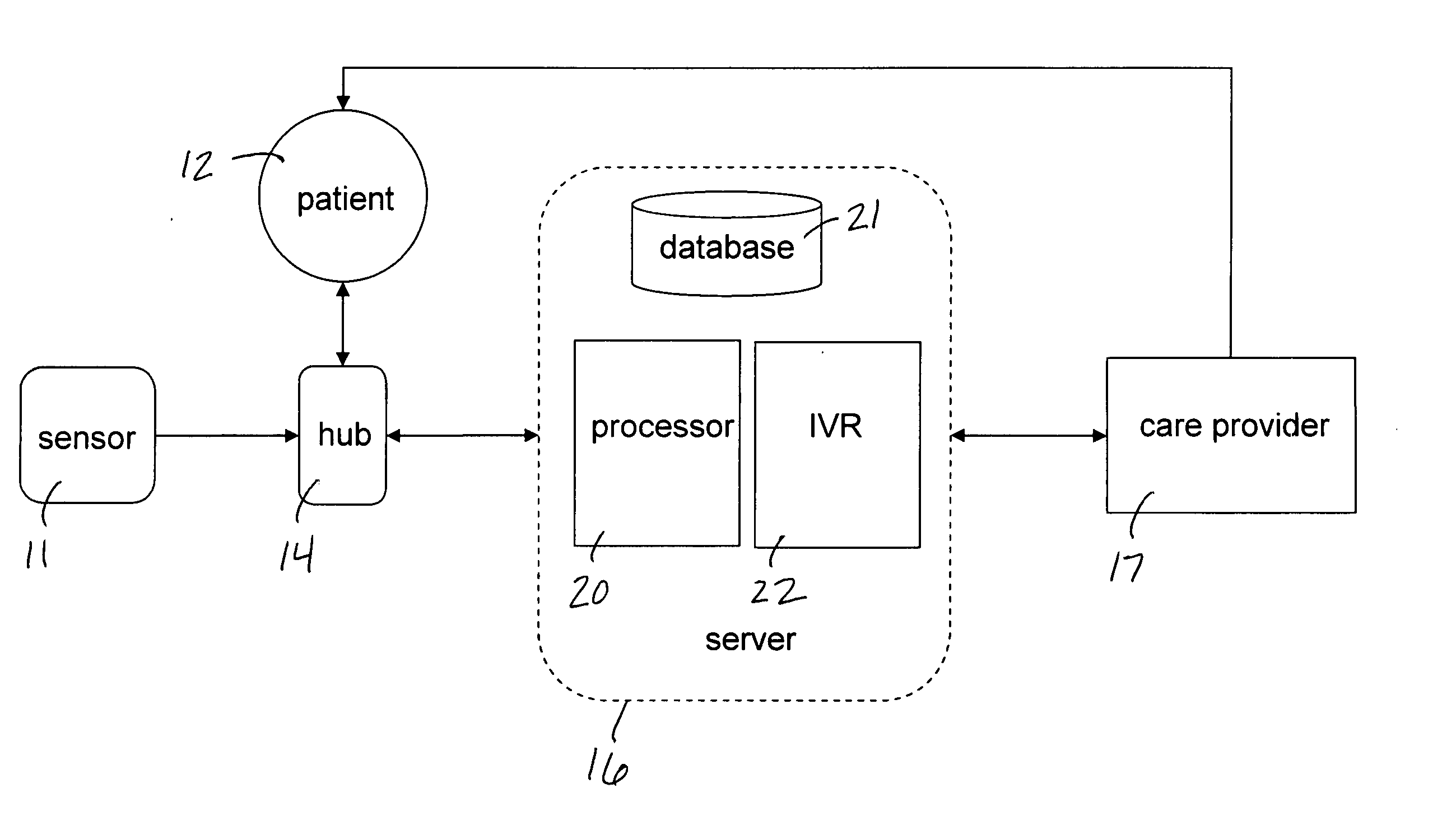
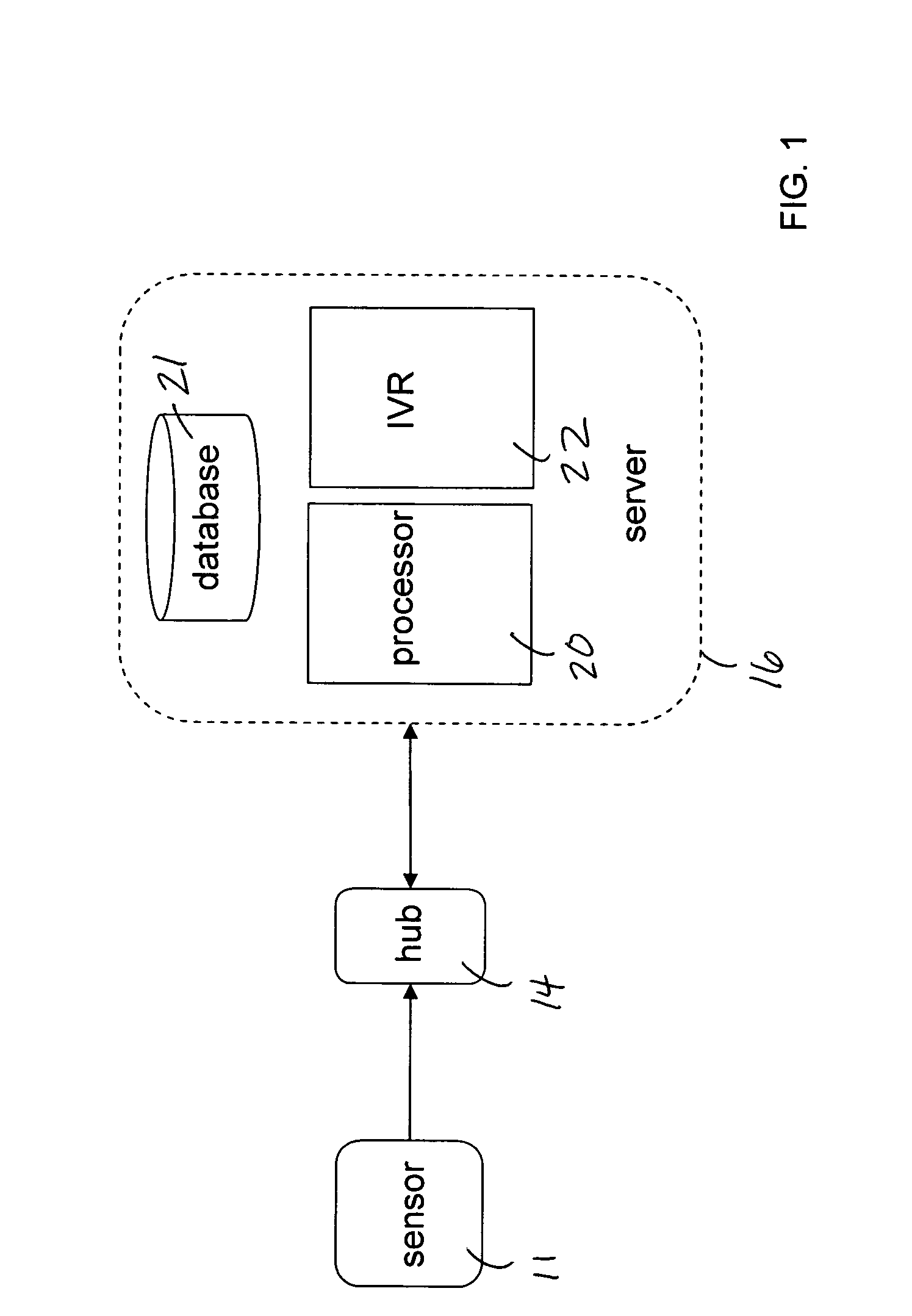
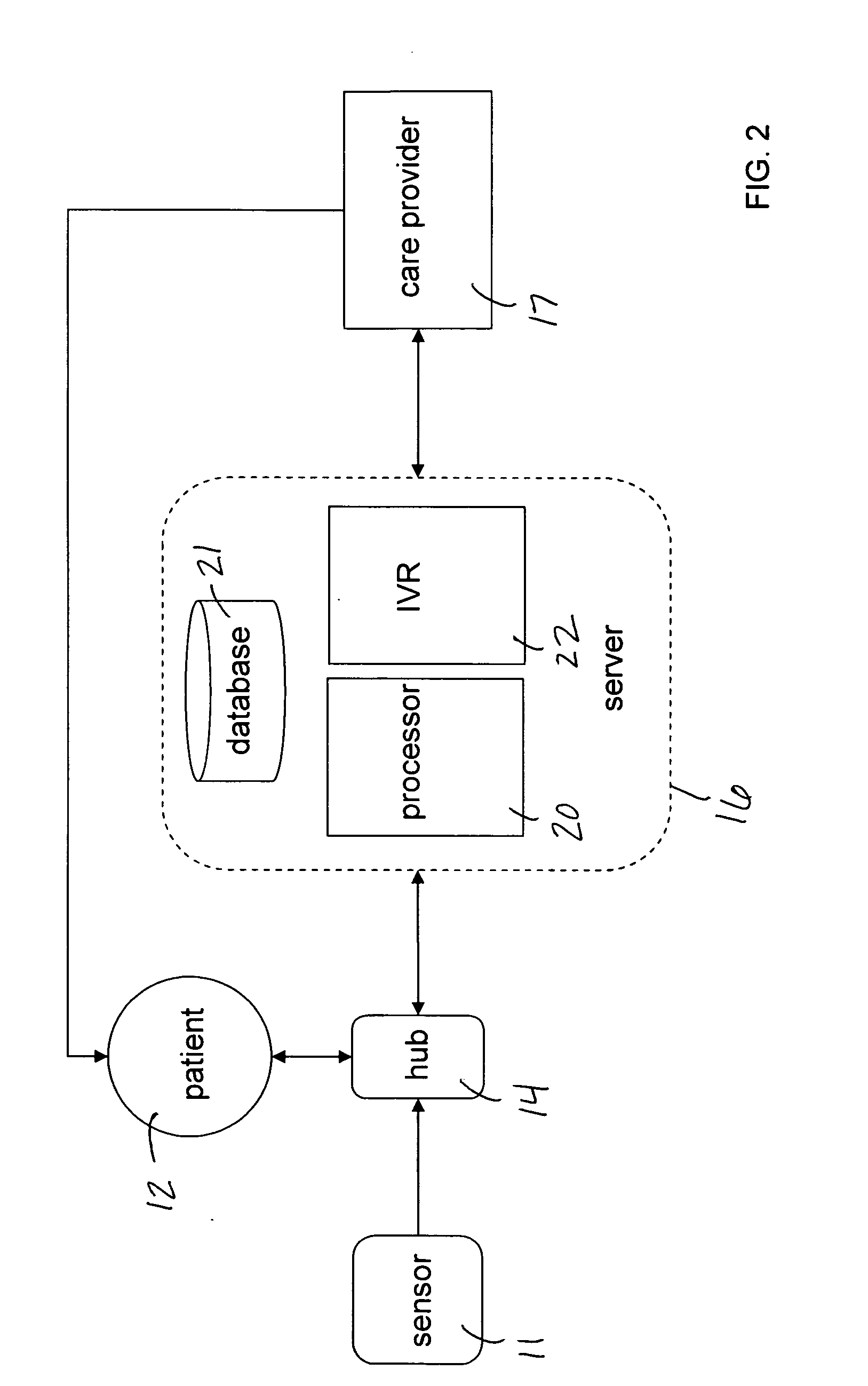
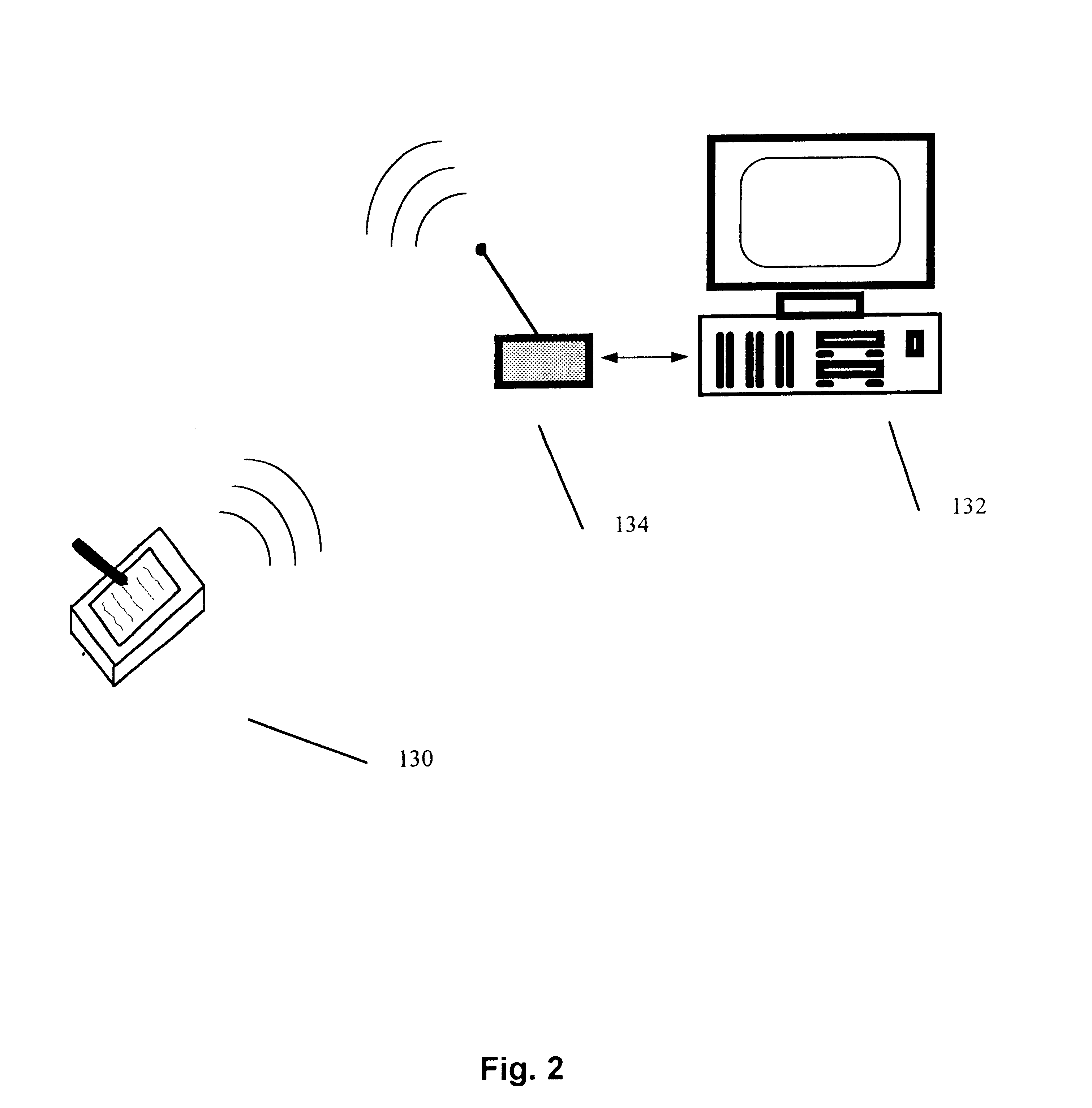
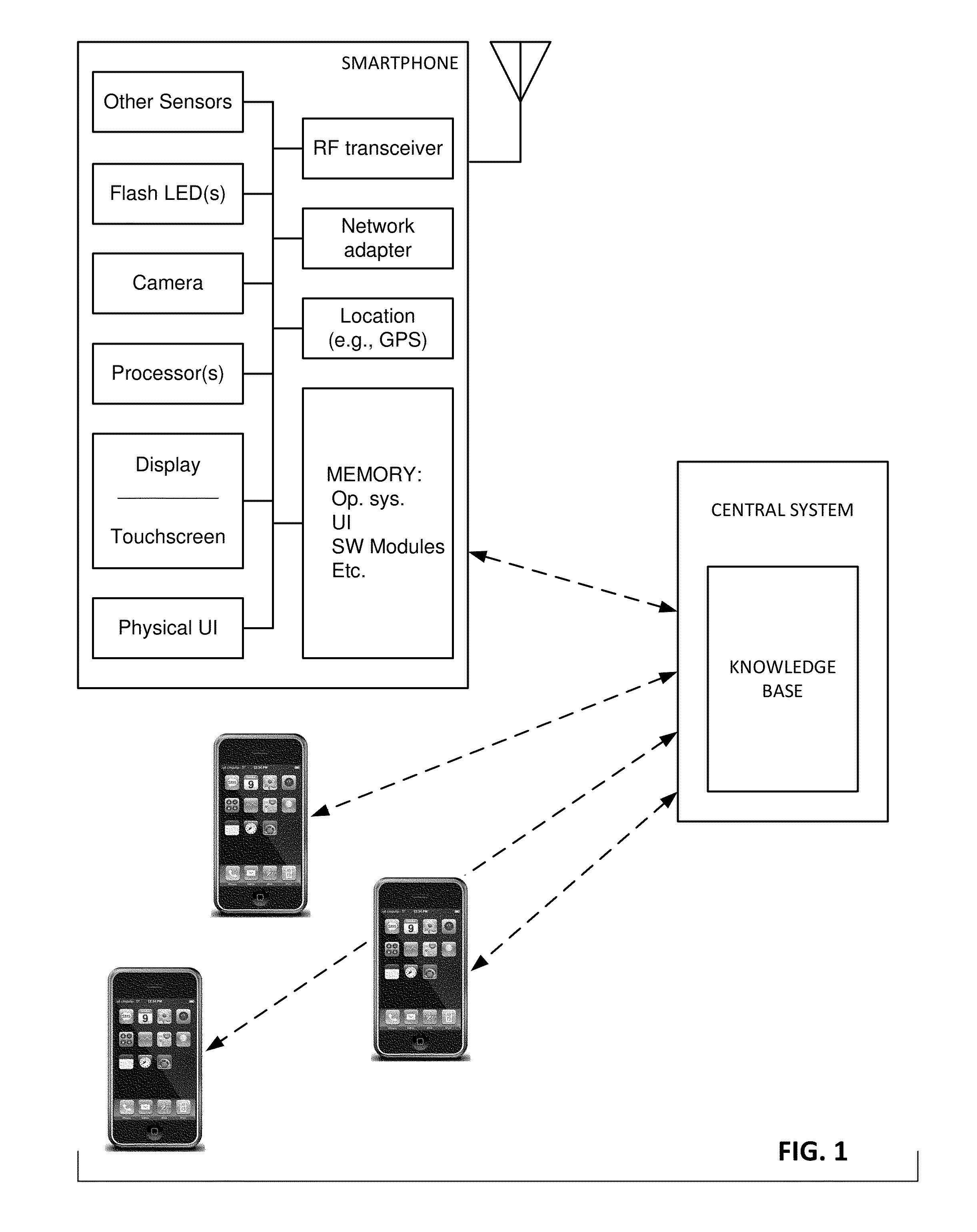
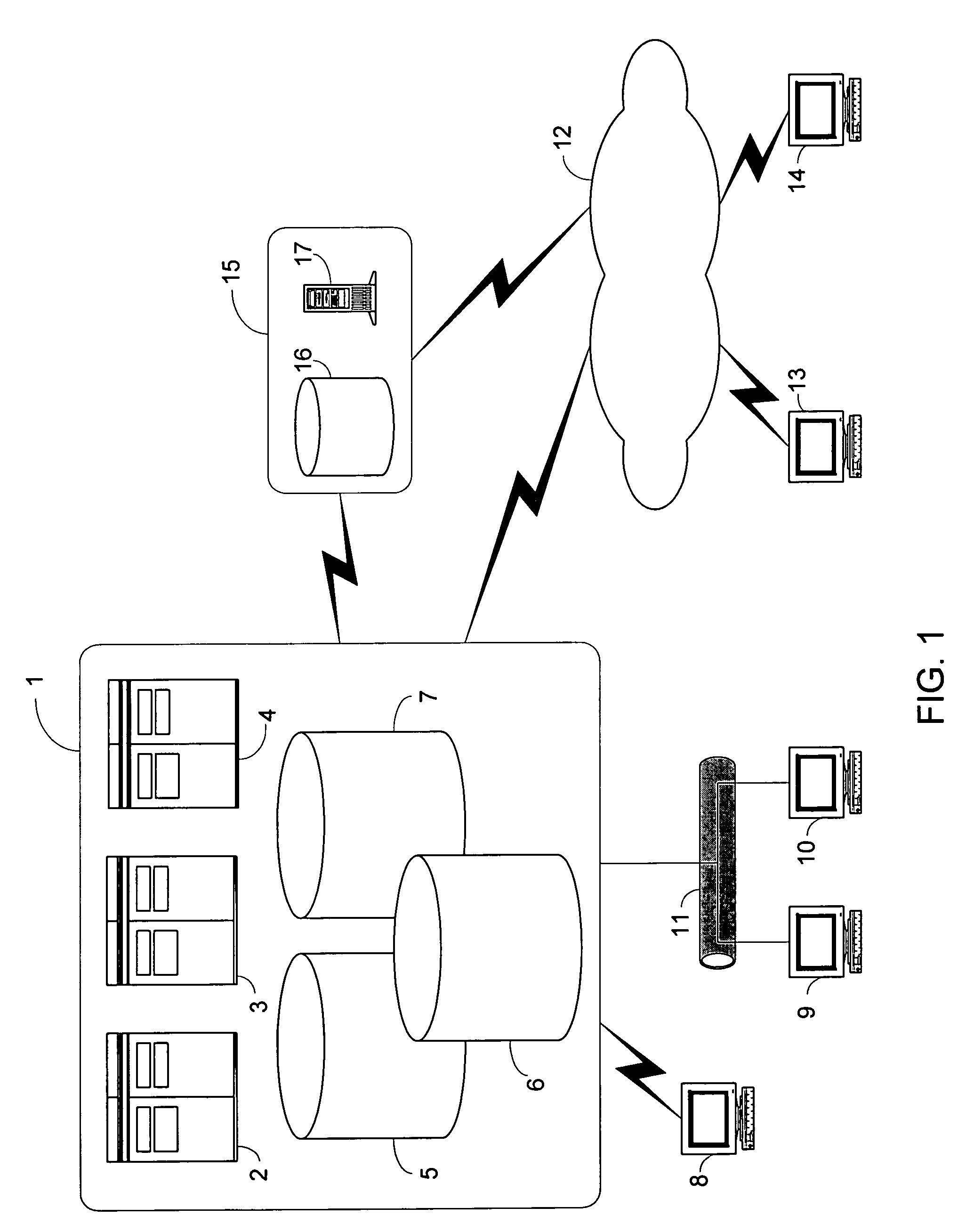
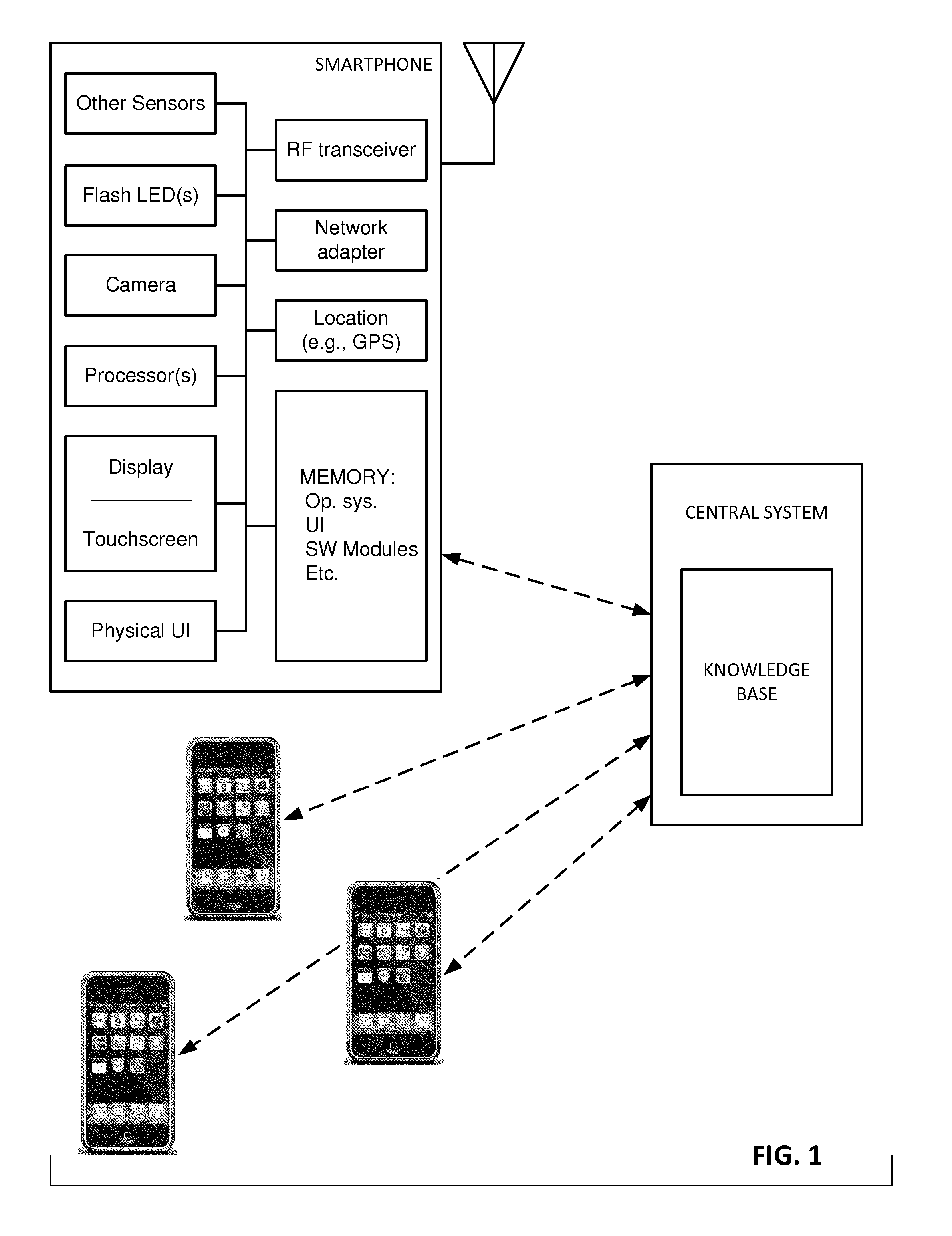
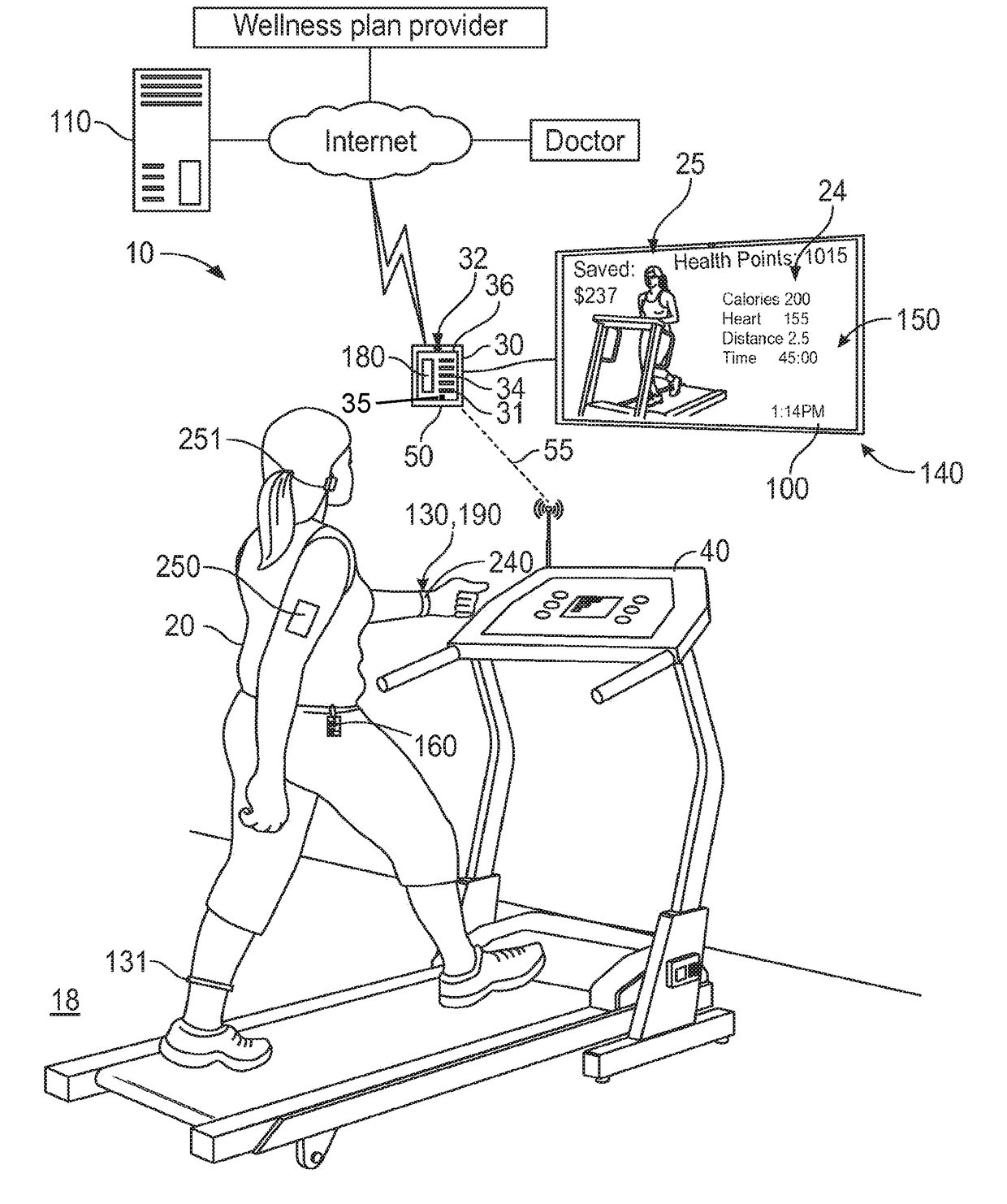
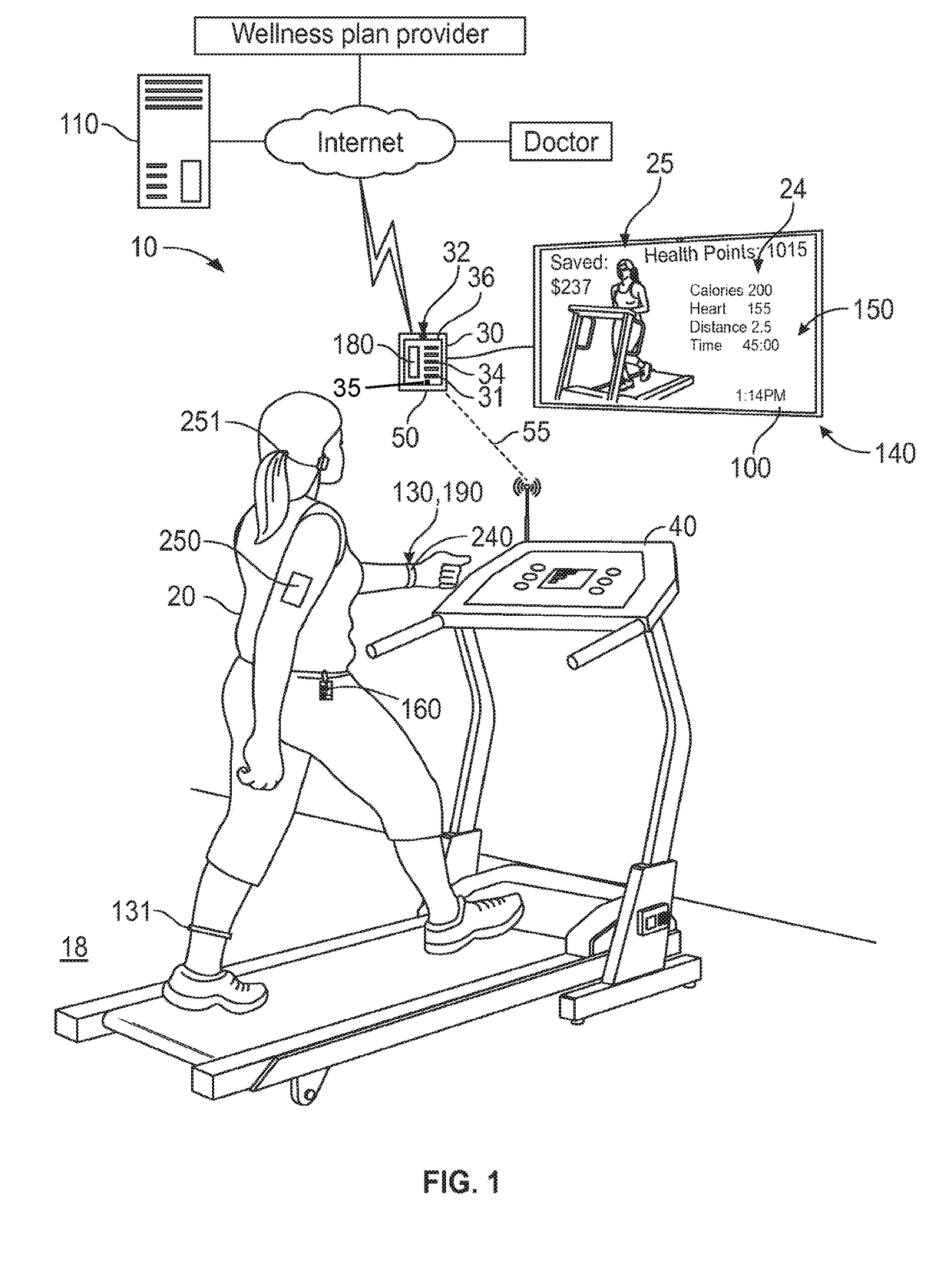
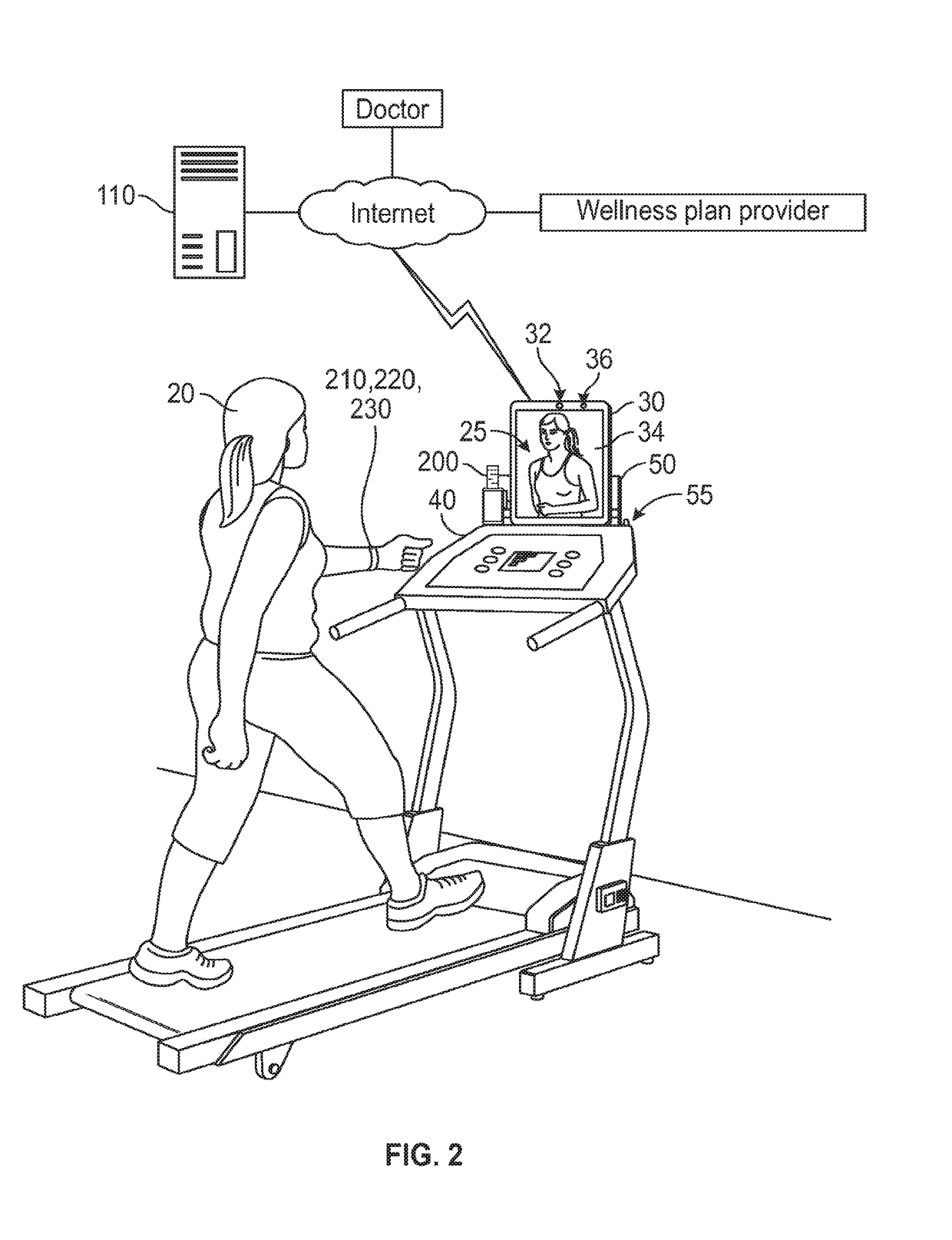
Mobile patient monitoring system with automatic data alerts
InactiveUS20060089542A1Improve complianceLower cost of careMedical communicationSurgeryPatient complianceEmergency medicine
A system to increase compliance with patient monitoring protocols for patients with chronic disease. The system uses a wireless telecommunication device as the hub of the system. The hub is configured to increase patient compliance with a monitoring protocol by being integrated with a mobile device, such as a cellular phone or PDA, that the patient normally carries or wears. The hub is further configured to increase compliance by displaying games that incorporate monitored conditions and providing rewards to the patient when he complies with the monitoring protocol. The hub receives physiological data about the patient from a medical sensor then collates the sensed data with certain data input by the patient. The reading is transmitted to a server that uses a software application to automatically examine and interpret the data. Alerts are sent to the health care provider only when the reading is outside specified parameters. The health care provider may contact the patient about the outlying event via the network.
Owner:SAFE N SOUND SOLUTIONS
Sensor Systems for Drug Delivery Devices
ActiveUS20180236181A1Reduce care costsReducing its effectivenessAutomatic syringesDiagnosticsDrugEmbedded system
Systems for drug delivery devices include a temperature control system and an identification system. A temperature control system configured to sense and control temperature of a cartridge containing a drug includes a heater, one or more temperature sensors, and a control unit. An identification system configured to identify a cartridge containing a drug includes a control unit and a tag sensor that is electrically coupled to the control unit, wherein the tag sensor is activated upon detecting a presence of the cartridge. A drug delivery device includes both the temperature control system and the identification system such that the control unit of the device may process the information of the drug that is received from the tag sensor of the identification system, and based on at least a portion of the processed information, determine and control the temperature of the drug within a cartridge.
Owner:UN HOLDINGS LLC
Method for production of medical records and other technical documents
InactiveUS6684188B1Improve overall utilizationEffective use timeData processing applicationsSurgeryMedical recordDocument preparation
A computer implemented system for the production of medical records, legal documents and other frequently produced semi-technical documents. This is accomplished by generating an intelligent computer-guided interview and the use of serialized scriptable objects. Major program elements include a knowledge base text file, a parse engine, and an execution module. The knowledge base uses a unique rule syntax. The parse engine converts the textual knowledge base file to a compiled binary representation which can then be interpreted by the execution module. The execution module leads the user through the interview by generating a series of questions and presents possible answers in the form of pick lists. The data is recorded with a computer pen and collated into a document file. This file is coded in binary format and can be written to and recalled from disk. If the file is recalled from disk the user can continue to answer questions or change answers previously given. The result is a mobile computing system whereby data is input in a structured format. When the program is executed the user is prompted for answers to questions and, based upon the user's response, the final document can change considerably. Depending upon each answer, the program may change the subsequent questions being asked, change the list associated with a question, change the text being generated, or change the entire structure of the document. The data collected may be also be stored in a database for analysis at a later date. Finally, the data collected is output in a narrative text format which can be tailored according to the traditions and expectations of the user's profession. The program will output the text via a printer or it can be transmitted via electronic means.
Owner:MITCHELL GEOFFREY C +1
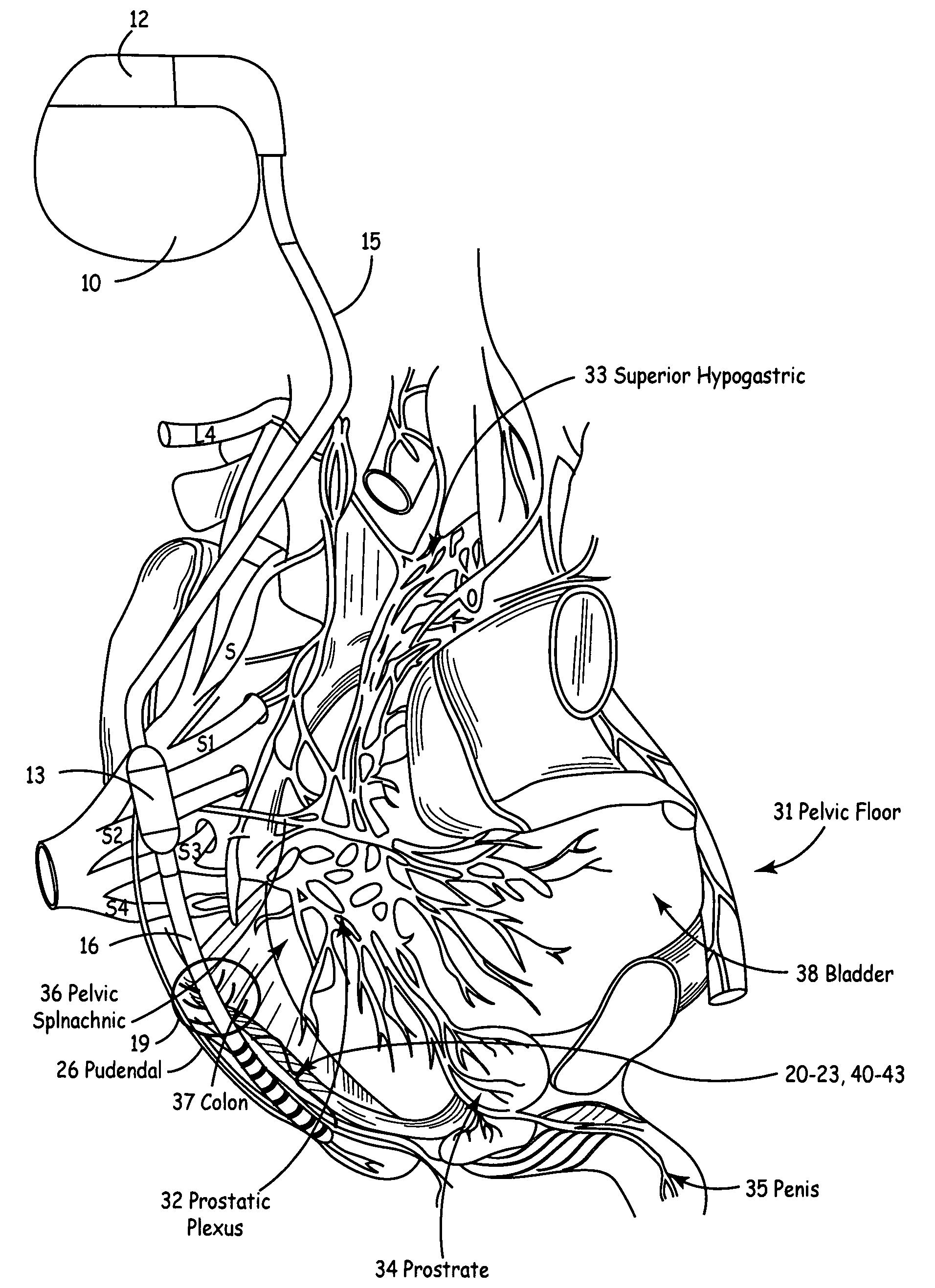
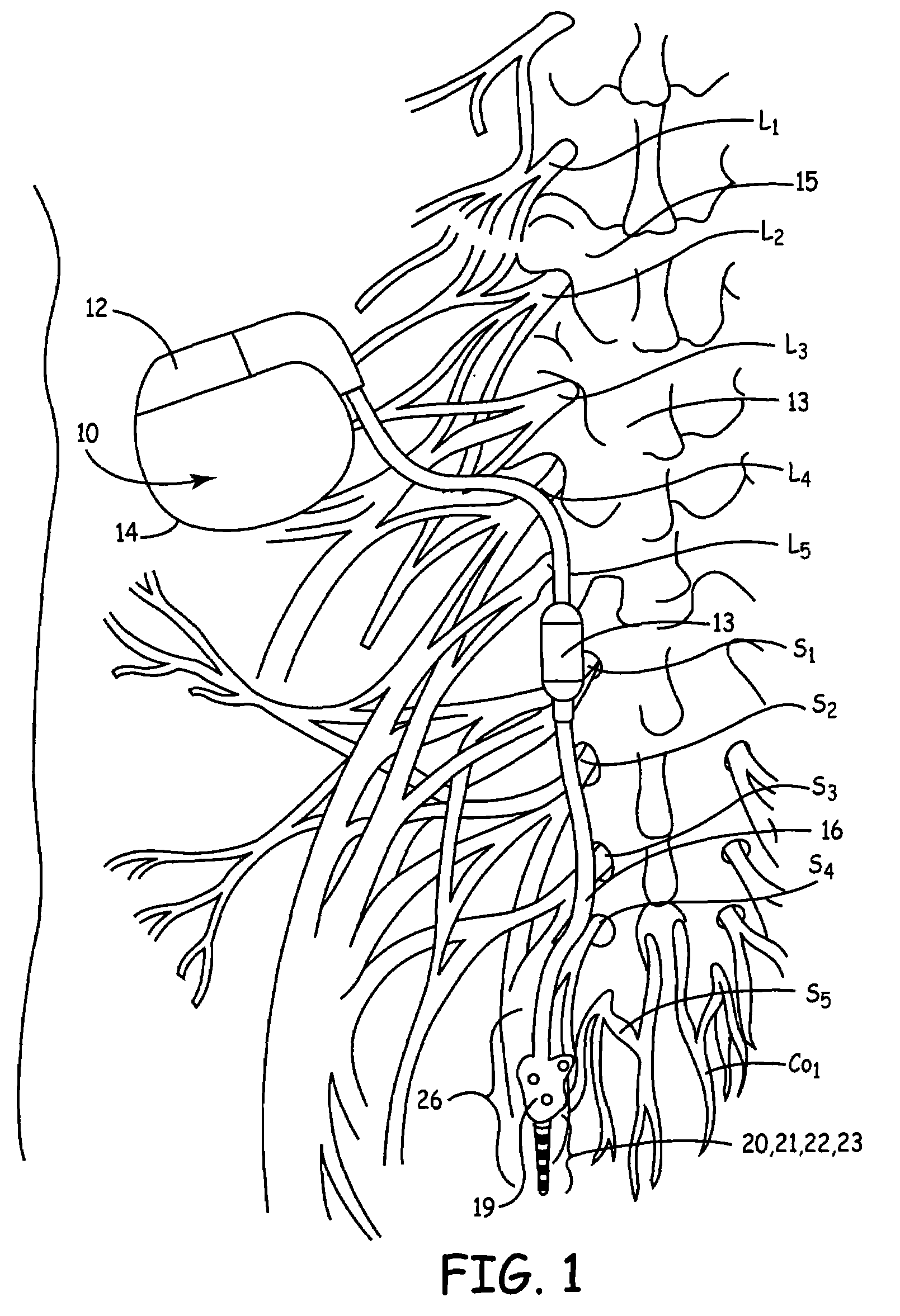
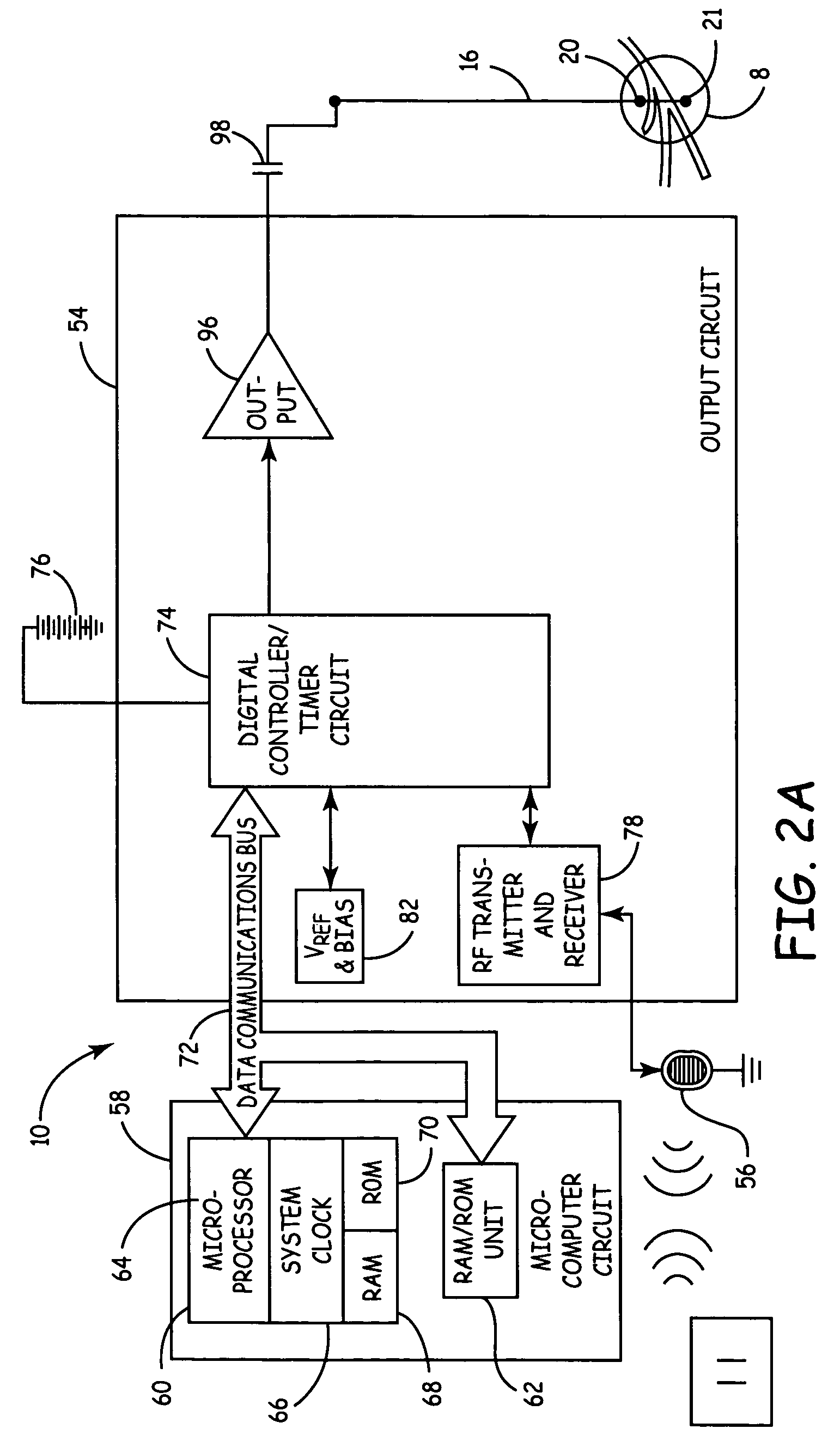
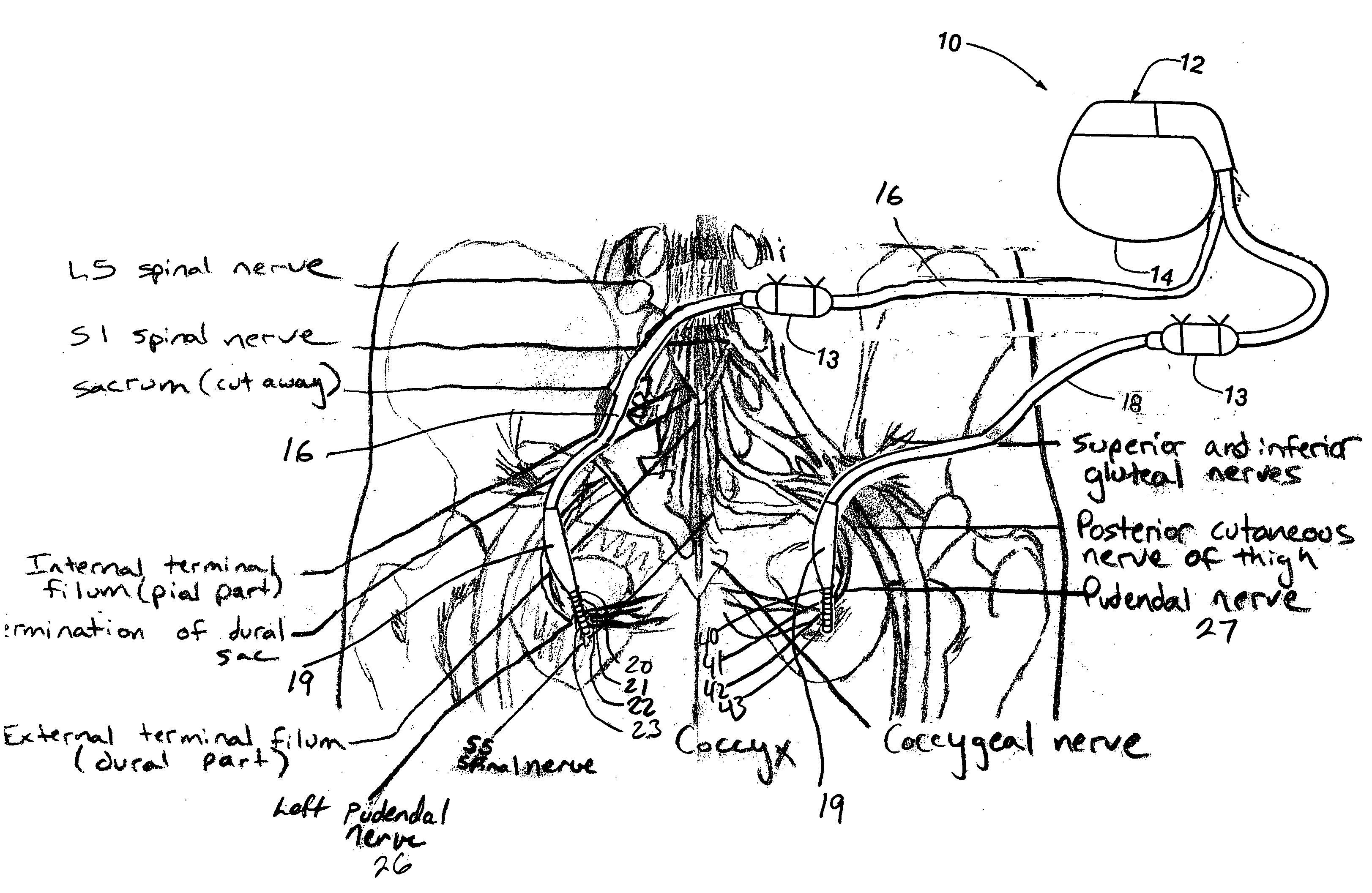
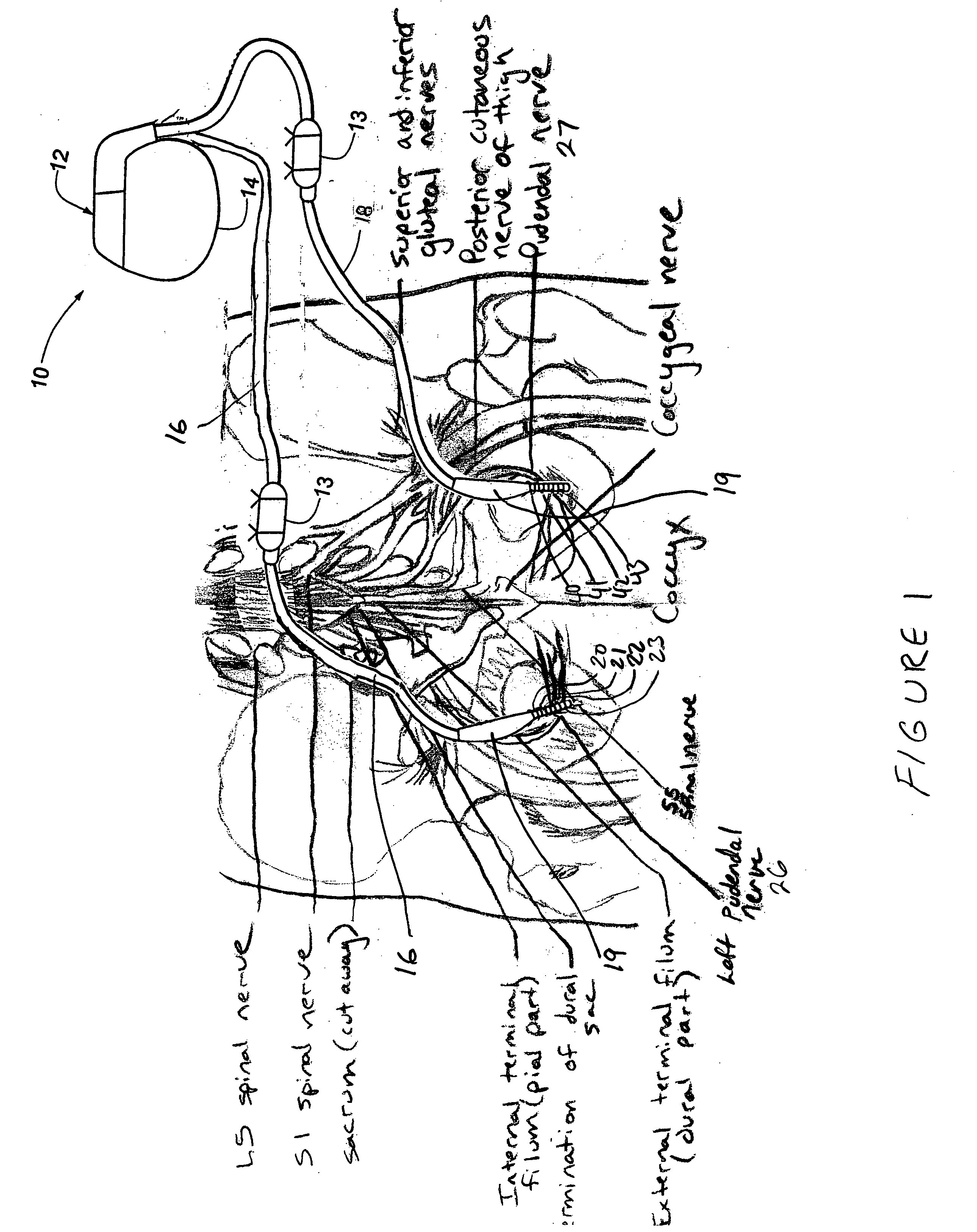
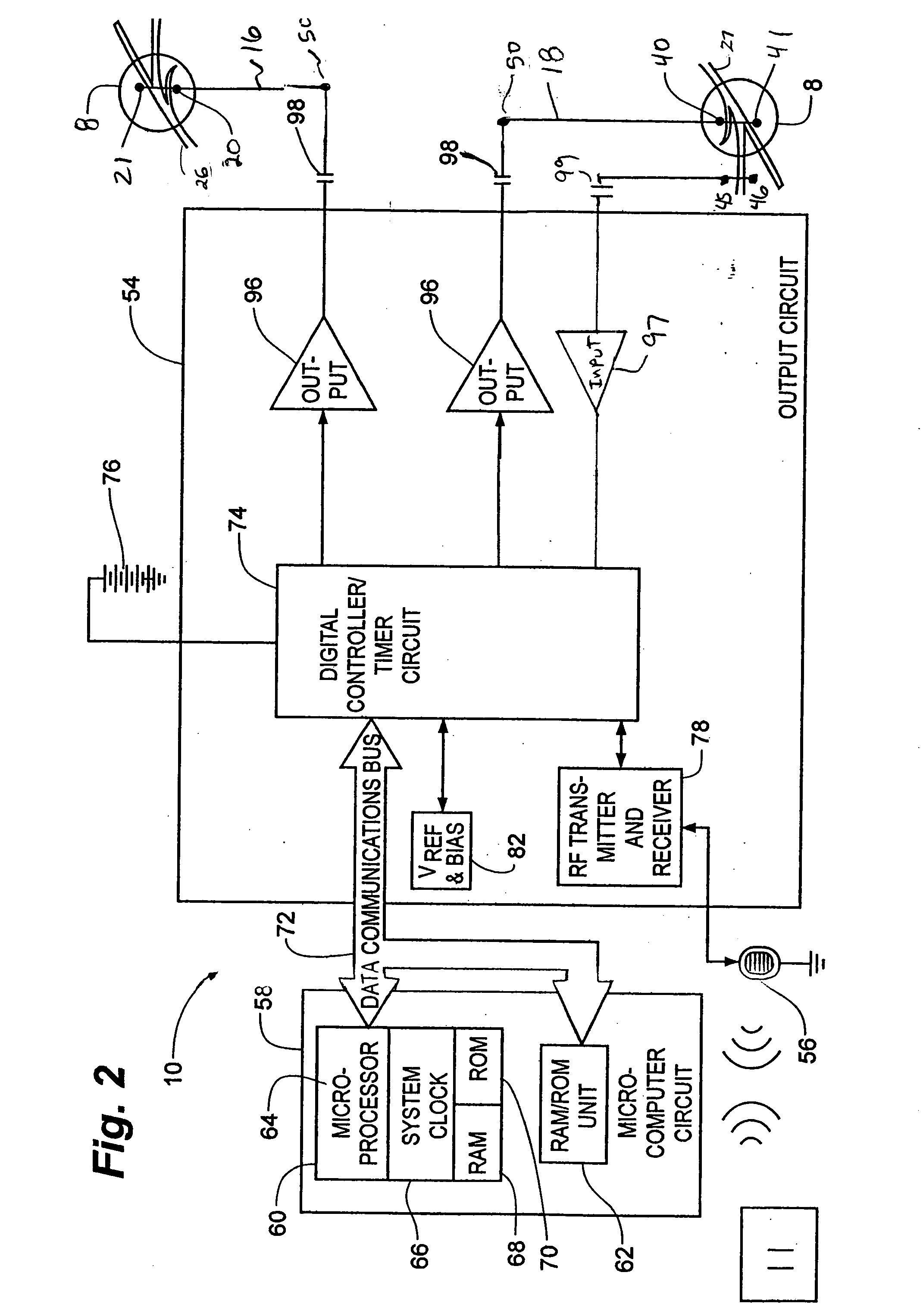
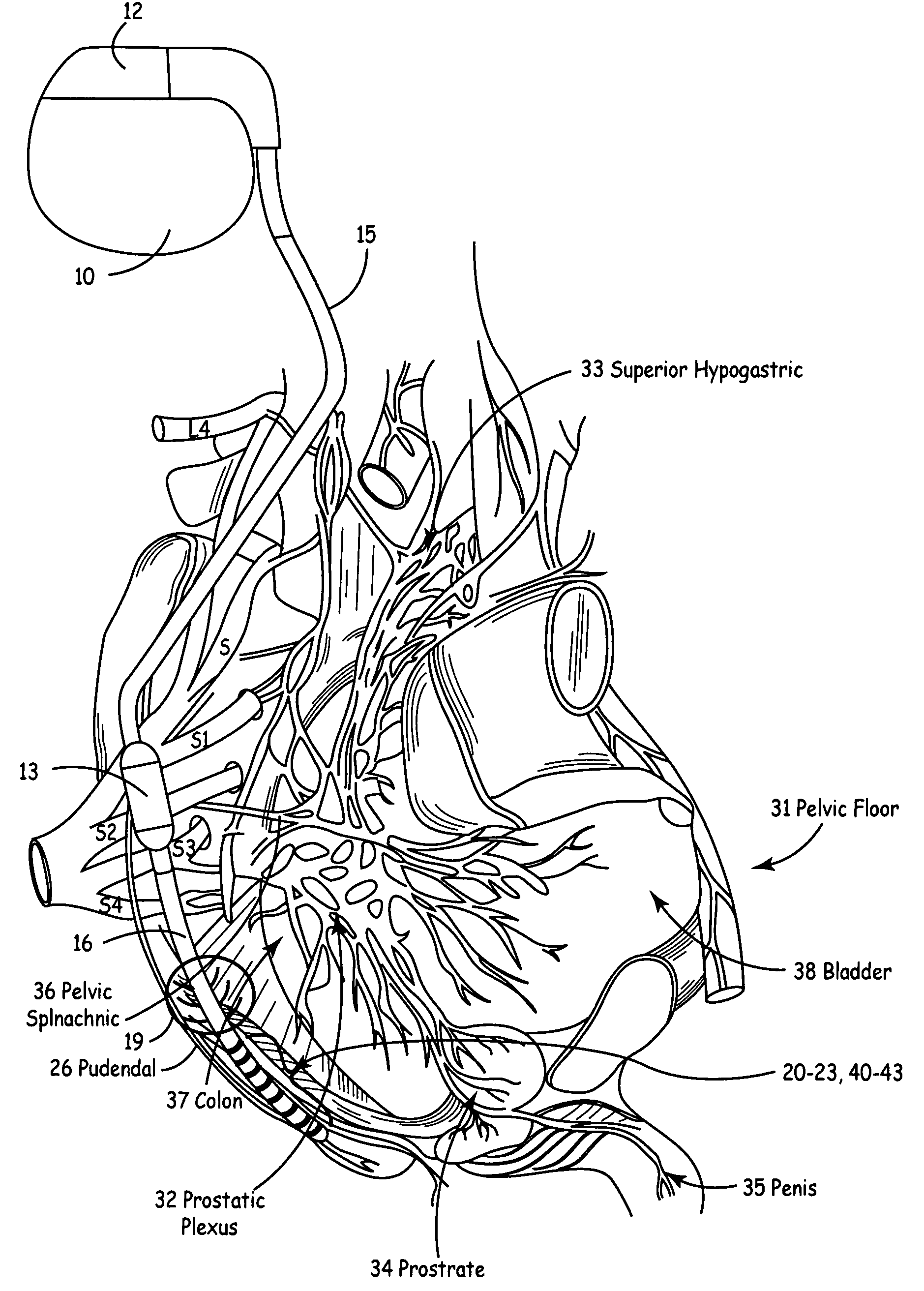
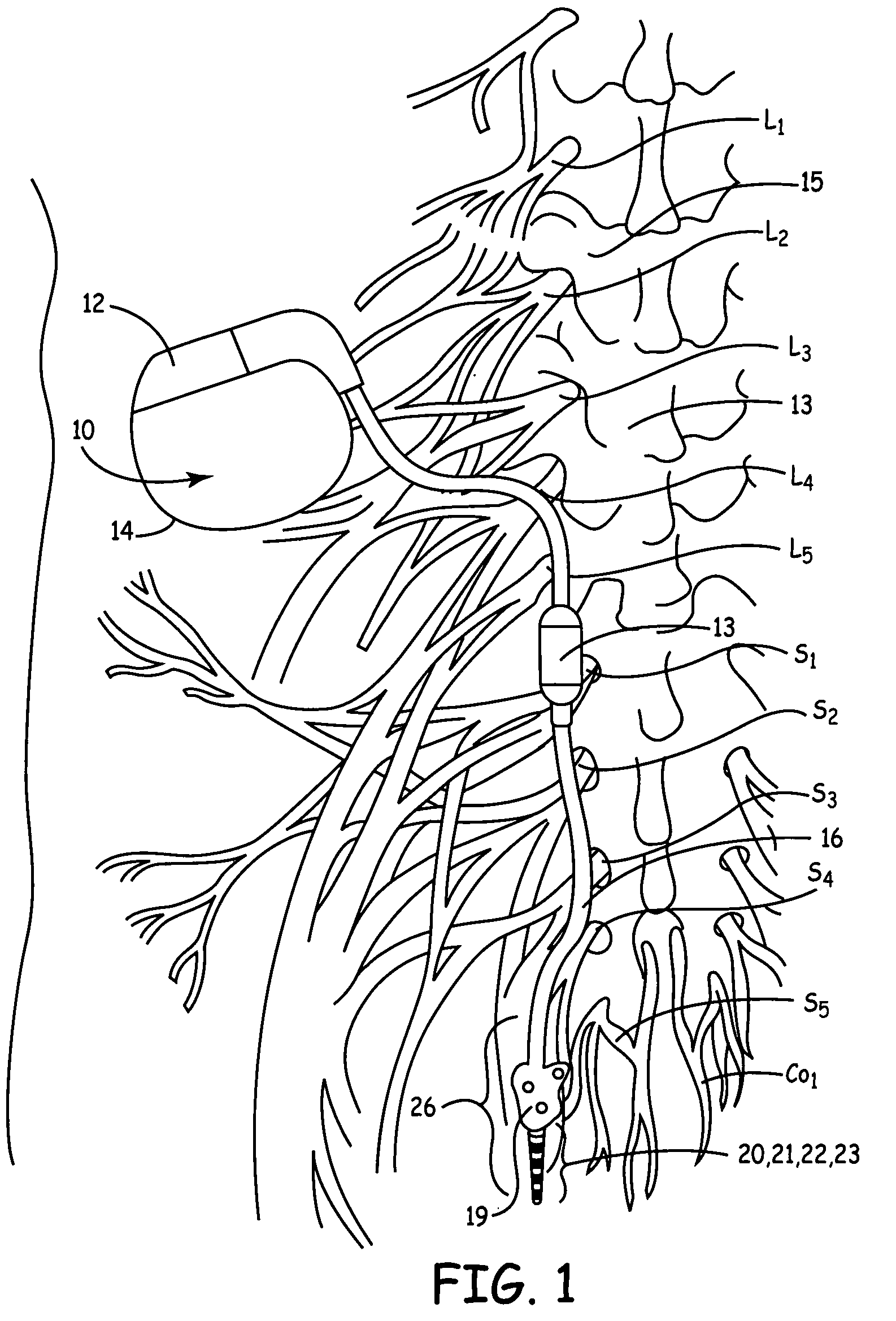
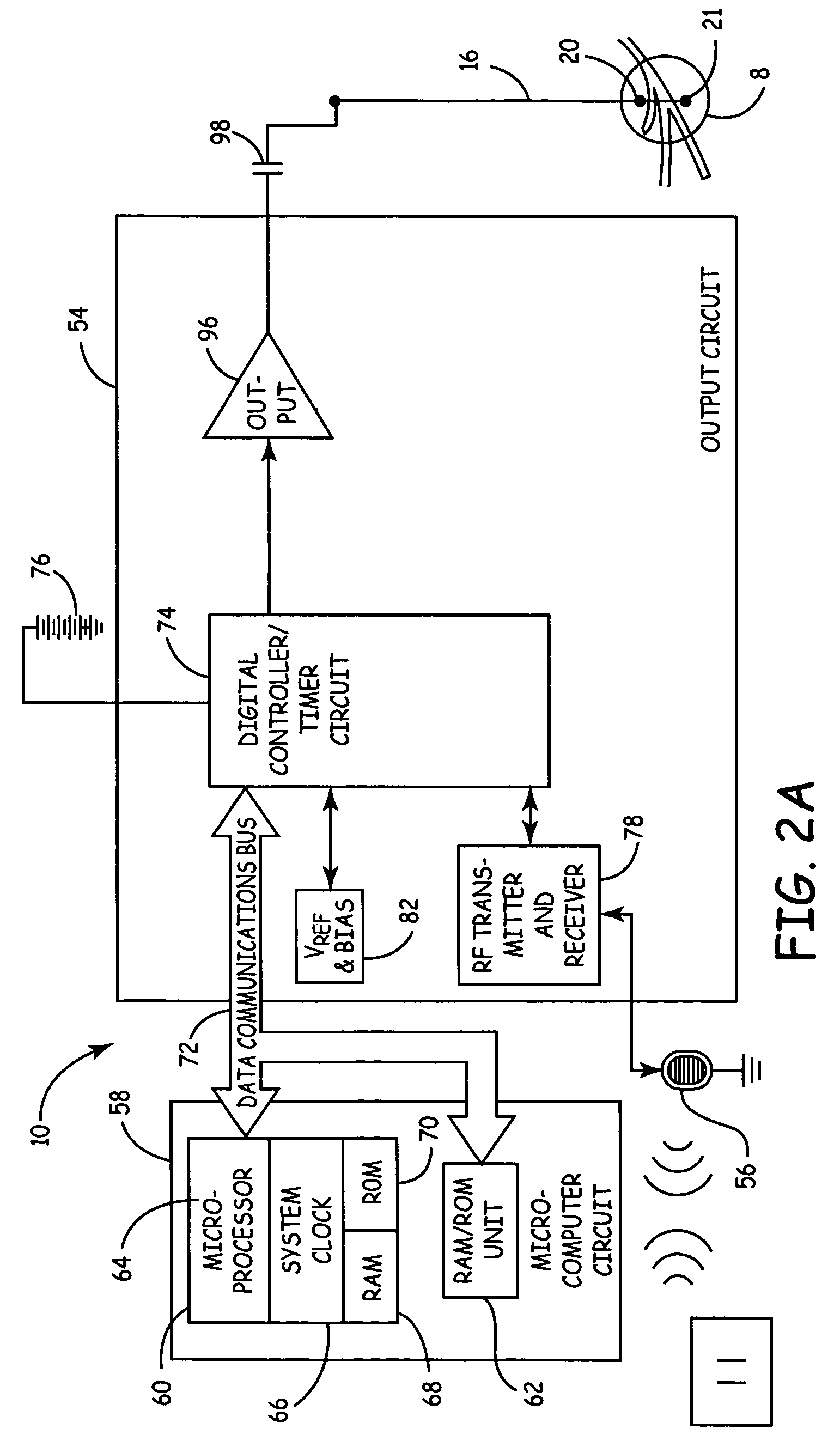
Method, system and device for treating disorders of the pelvic floor by means of electrical stimulation of the pudendal and associated nerves, and the optional delivery of drugs in association therewith
ActiveUS7328068B2Undesirable side effects of sacral nerve stimulation may be avoided or minimizedUndesirable side-effectDigestive electrodesGenital electrodesDiseaseProstatalgia
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal or other nerves, and optional means for delivering drugs in association therewith. A method of precisely positioning and implanting a medical electrical lead so as to provide optimal stimulation of the pudendal nerve or a portion thereof is also described. Placement of a stimulation lead next to or on the pudendal nerve may be performed using conventional prior art techniques through gross anatomical positioning, but usually does not result in truly optimal lead placement. One method of the present invention utilizes neurophysiological monitoring to assess the evoked responses of the pudendal nerve, and thereby provide a method for determining the optimal stimulation site. Additionally, one or more electrical stimulation signals are applied, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Method, system and device for treating various disorders of the pelvic floor by electrical stimulation of the left and right pudendal nerves
InactiveUS20040193228A1Directly and effectively stimulatingUndesirable side effects of sacral nerve stimulation may be avoided or minimizedElectrotherapyDiseaseProstatalgia
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal and sacral nerves, or portions thereof, and optional means for delivering drugs in association therewith. Two or more electrical stimulation regimes are applied on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve and / or sacral nerve, or portions thereof, in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:GERBER MARTIN T
Physiologic data acquisition and analysis
InactiveUS20140378810A1Lower cost of careImage enhancementMedical imagingPattern recognitionImaging analysis
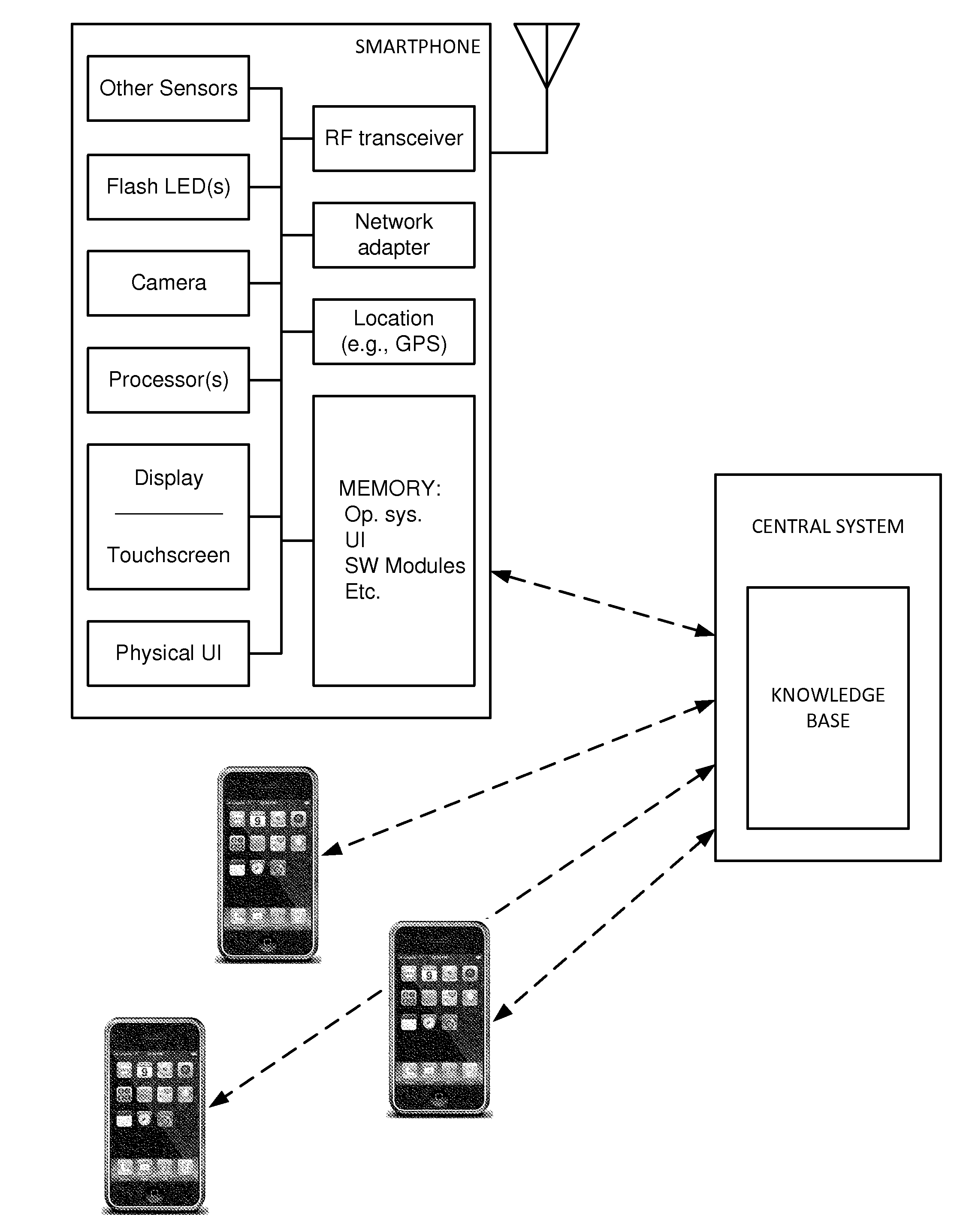
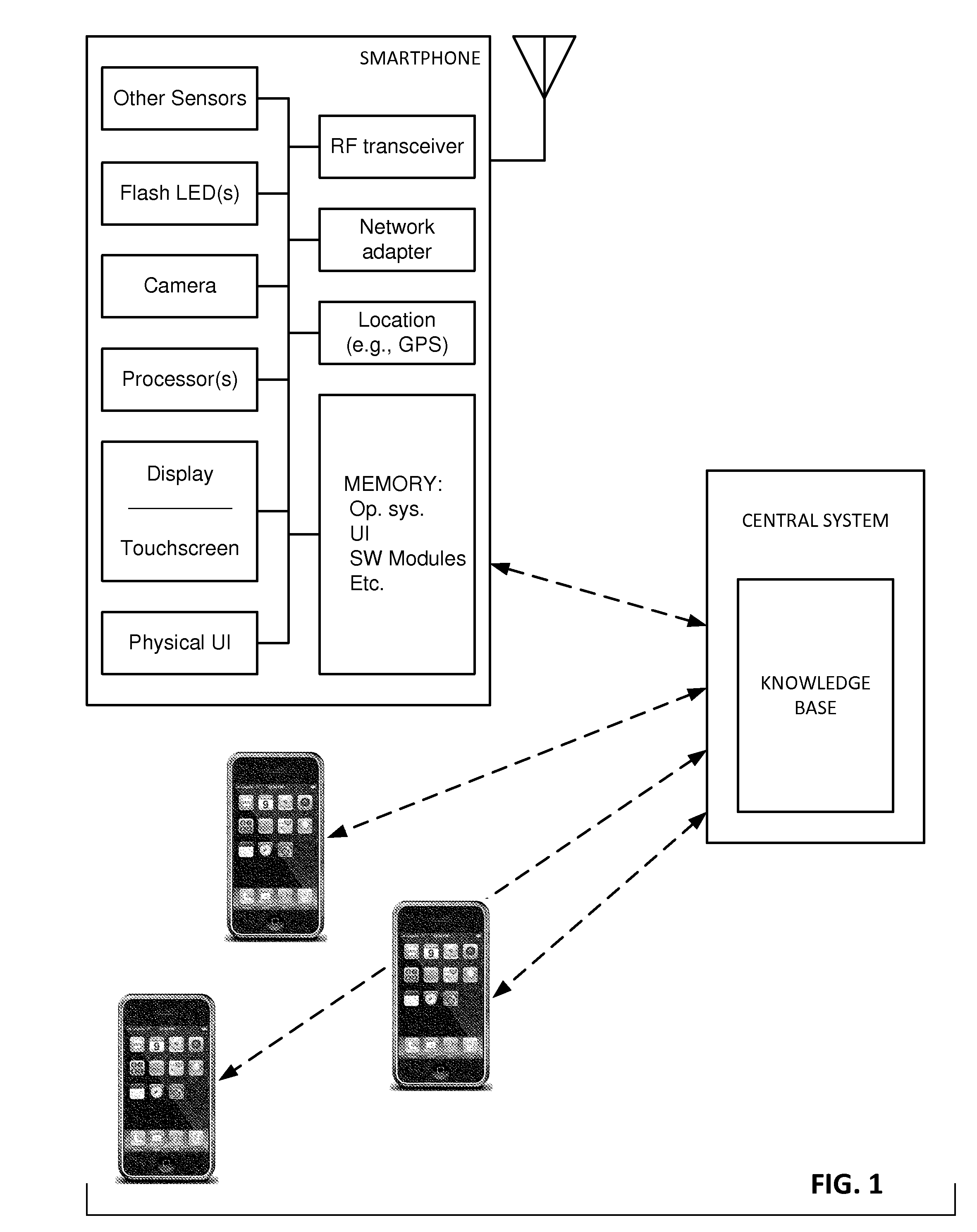
The availability of high quality imagers on smartphones and other portable devices facilitates creation of a large, crowd-sourced, image reference library that depicts skin rashes and other dermatological conditions. Some of the images are uploaded with, or later annotated with, associated diagnoses or other information (e.g., “this rash went away when I stopped drinking milk”). A user uploads a new image of an unknown skin condition to the library. Image analysis techniques are employed to identify salient similarities between features of the uploaded image, and features of images in this reference library. Given the large dataset, statistically relevant correlations emerge that identify to the user certain diagnoses that may be considered, other diagnoses that may likely be ruled-out, and / or anecdotal information about similar skin conditions from other users. Similar arrangements can employ audio and / or other physiologically-derived signals. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
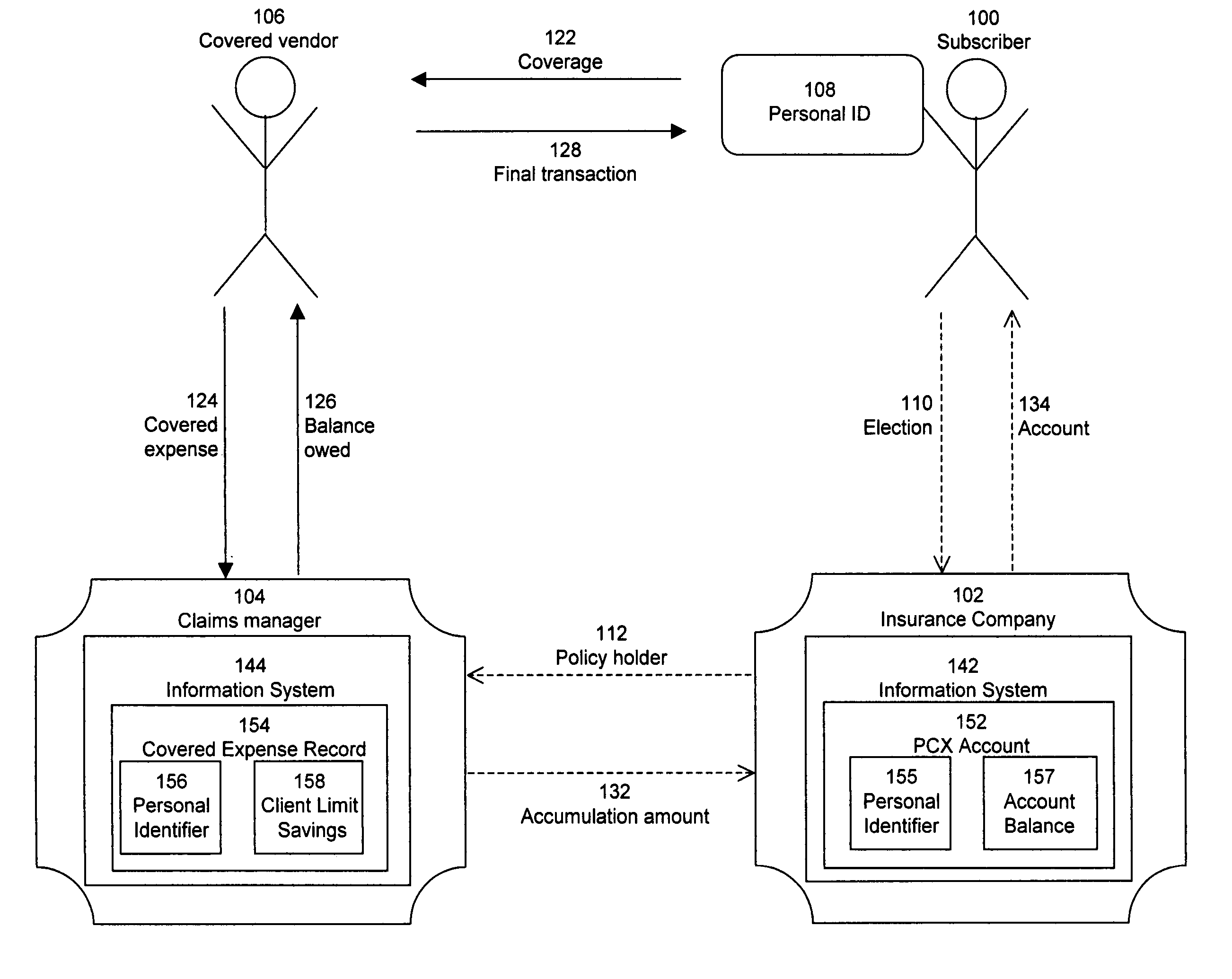
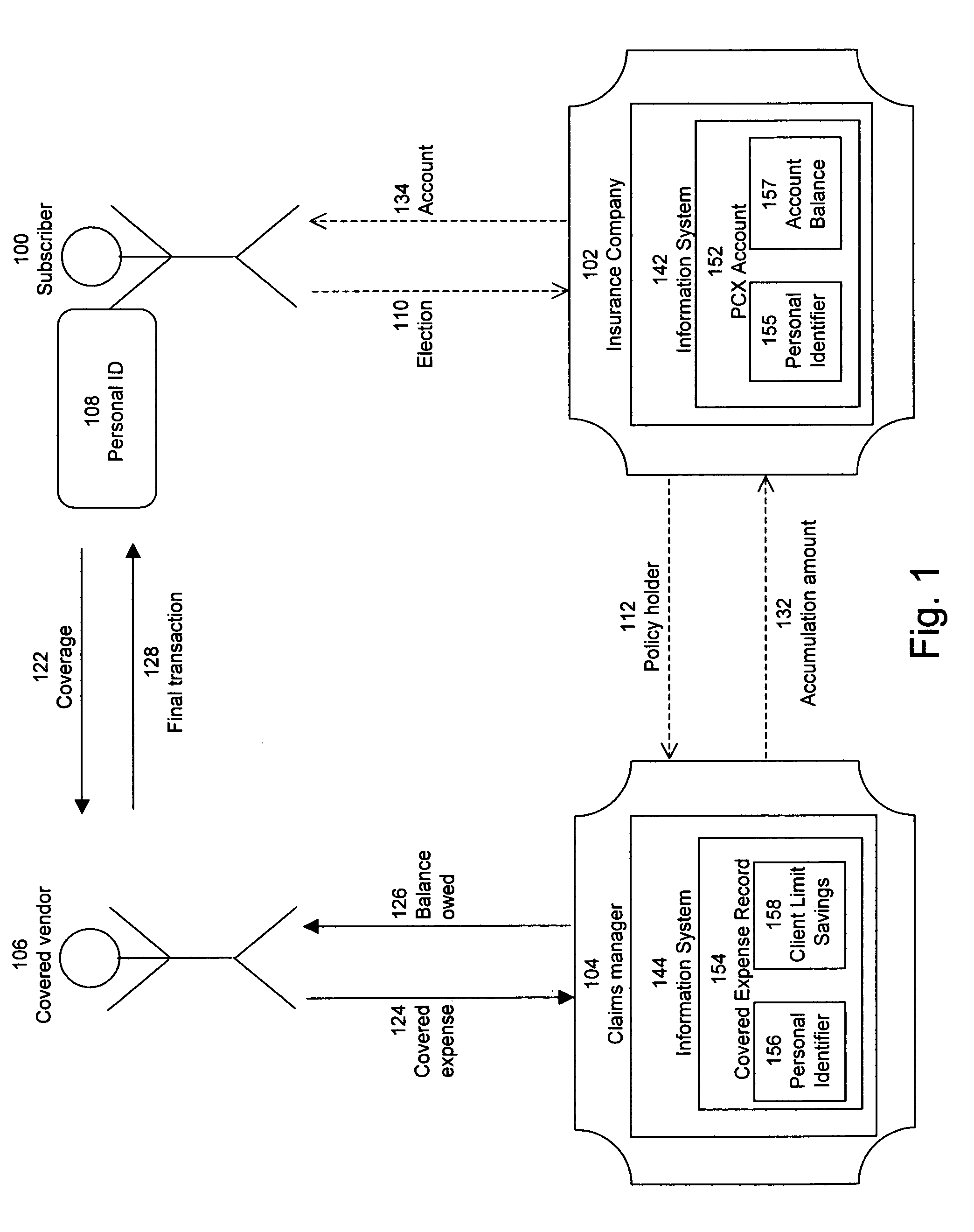
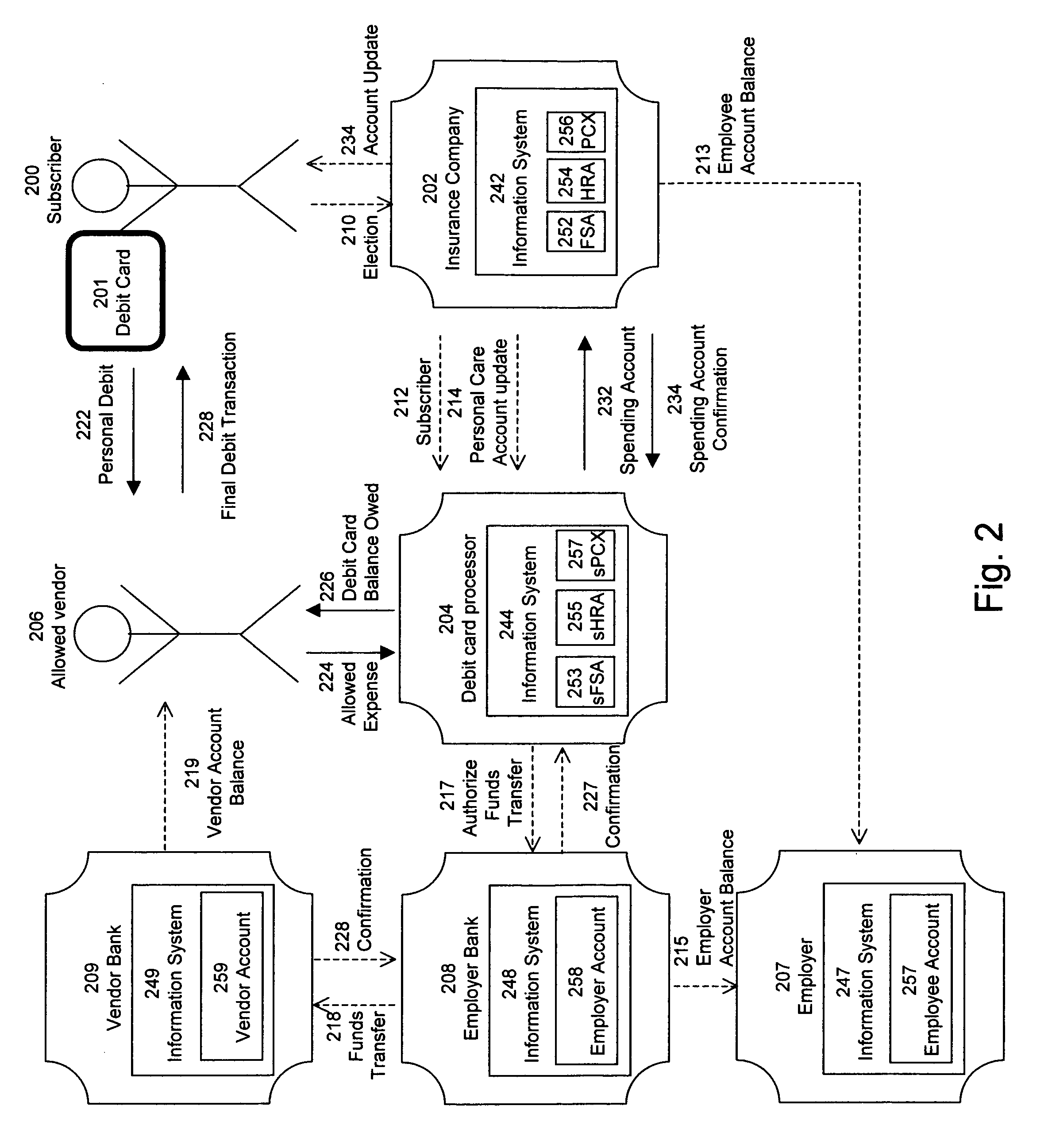
Pharmacy personal care account
A method for providing insurance coverage to a subscriber comprises offering an insurance policy to said subscriber wherein said insurance policy comprises providing allowances to pay for one or more expenses covered by said insurance policy and providing monetary credits to said subscriber for any unused portion of a given allowance where said monetary credits can be used to cover the cost of a subsequent allowed expense. The insurance policy may be a pharmacy benefits plan. The covered expenses may be pharmacy expenses. The allowed expenses may be medical expenses which qualify under section 213 of the US Internal Revenue code for payment by an employer without said subscriber having to declare said payment as taxable income.
Owner:HUMANA INC
Longitudinal dermoscopic study employing smartphone-based image registration
InactiveUS20140313303A1Lower cost of careImage enhancementTelevision system detailsHypopigmentationPattern recognition
The evolution of a skin condition over time can be useful in its assessment. In an illustrative arrangement, a user captures skin images at different times, using a smartphone. The images are co-registered, color-corrected, and presented to the user (or a clinician) for review, e.g., in a temporal sequence, or as one image presented as a ghosted overlay atop another. Image registration can employ nevi, hair follicles, wrinkles, pores, and pigmented regions as keypoints. With some imaging spectra, keypoints from below the outermost layer of skin can be used. Hair may be removed for image registration, and restored for image review. Transformations in addition to rotation and affine transforms can be employed. Diagnostic correlations with reference image sequences can be made, employing machine learning in some instances. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
Method, system and device for treating disorders of the pelvic floor by means of electrical stimulation of the pudenal and associated nerves, and the optional delivery of drugs in association therewith
ActiveUS20050113877A1Reduce traumaAvoid damageDigestive electrodesArtificial respirationDiseaseProstatalgia
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal or other nerves, and optional means for delivering drugs in association therewith. A method of precisely positioning and implanting a medical electrical lead so as to provide optimal stimulation of the pudendal nerve or a portion thereof is also described. Placement of a stimulation lead next to or on the pudendal nerve may be performed using conventional prior art techniques through gross anatomical positioning, but usually does not result in truly optimal lead placement. One method of the present invention utilizes neurophysiological monitoring to assess the evoked responses of the pudendal nerve, and thereby provide a method for determining the optimal stimulation site. Additionally, one or more electrical stimulation signals are applied, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
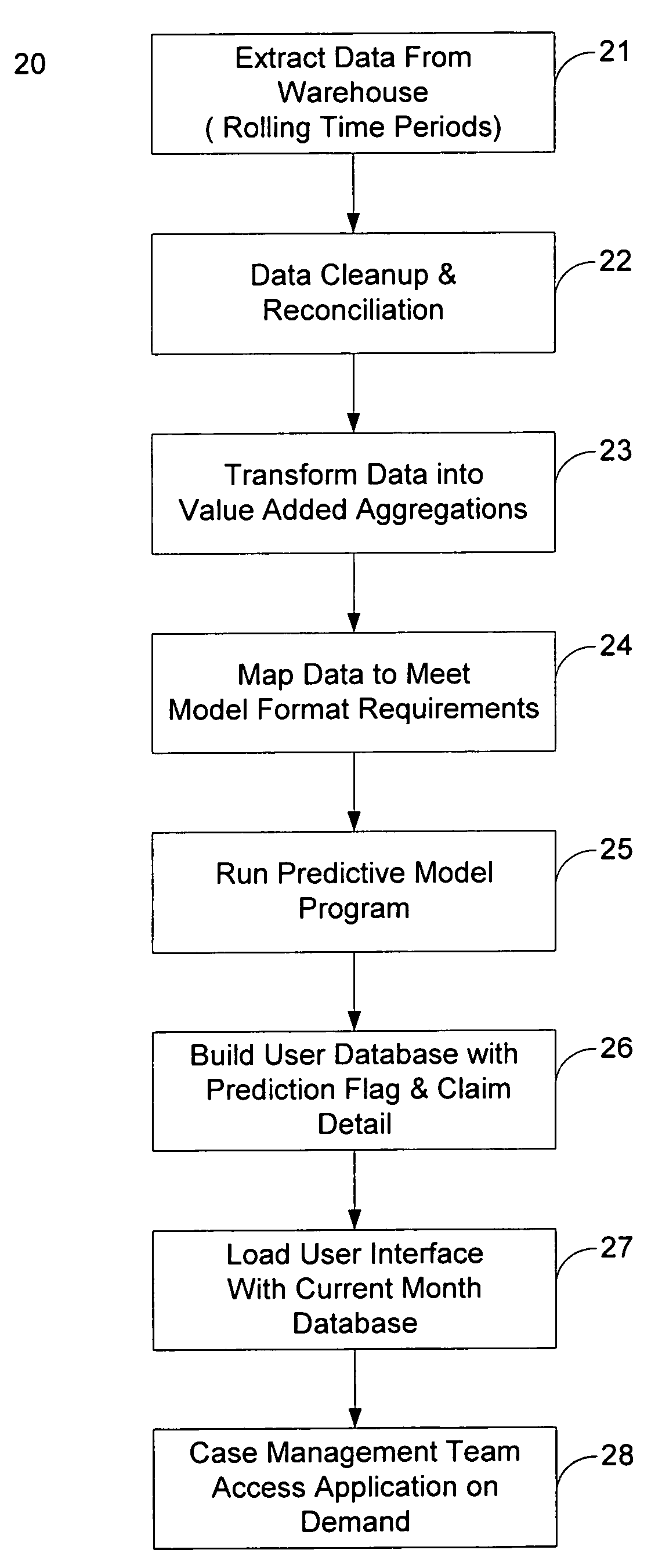
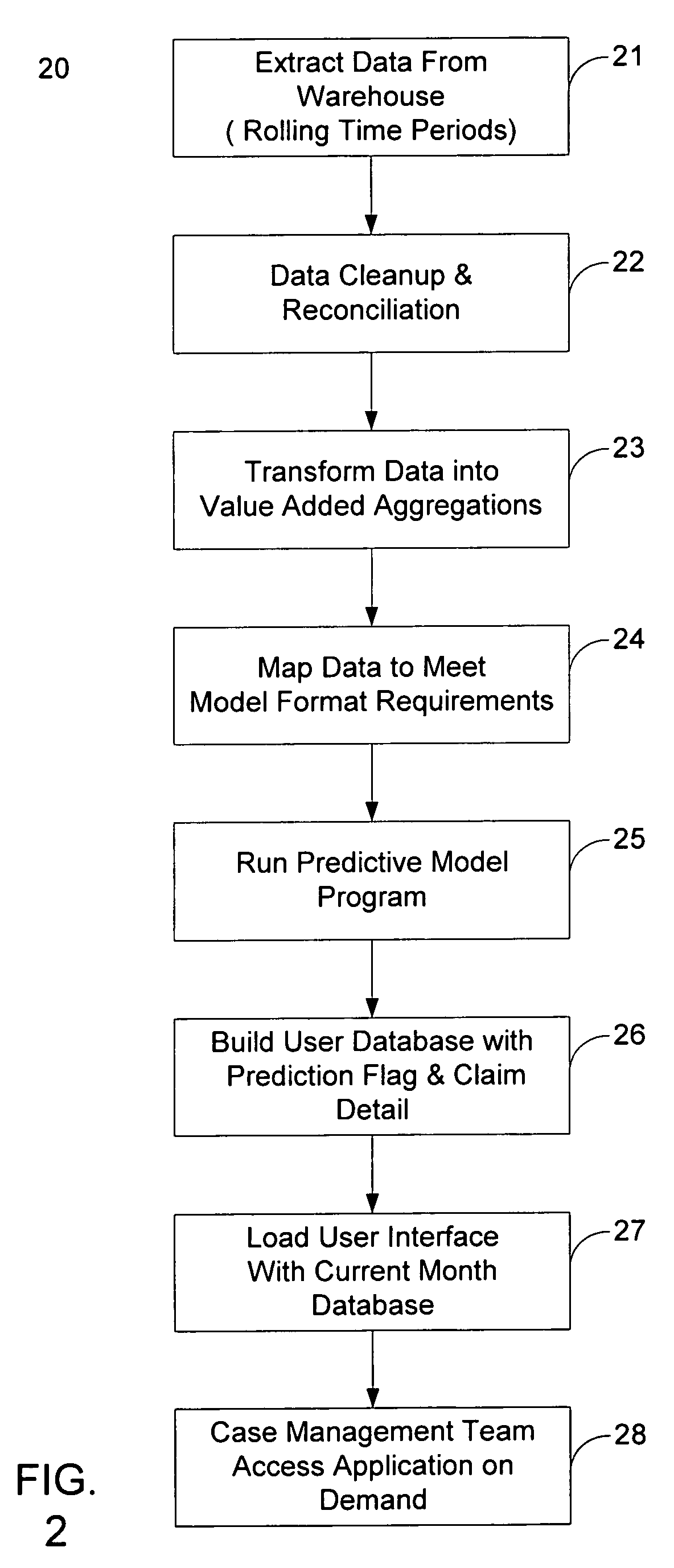
System and method for administering health care cost reduction
A system and method for the efficient administration of health care plans, particularly as to the reduction and / or elimination of avoidable medical costs for select individuals who participate in the plan, is disclosed. Existing health care data is processed to determine an indication as to the relative desirability of an intervention in a plan participant's health care regimen. The data is also processed to determine the status of one or more flags, each of which potentially indicates the relative desirability of an intervention in a plan participant's health care regimen. A predictive model is used to determine the status of a flag relating to the likelihood of an insurance plan participant making a disability claim within a certain period of time. The information relating to desirability of an intervention in a plan participant's health care regimen, as well as a plan participant's medical information and claim history, is presented to case managers and / or health care providers in a user-friendly format
Owner:AETNA
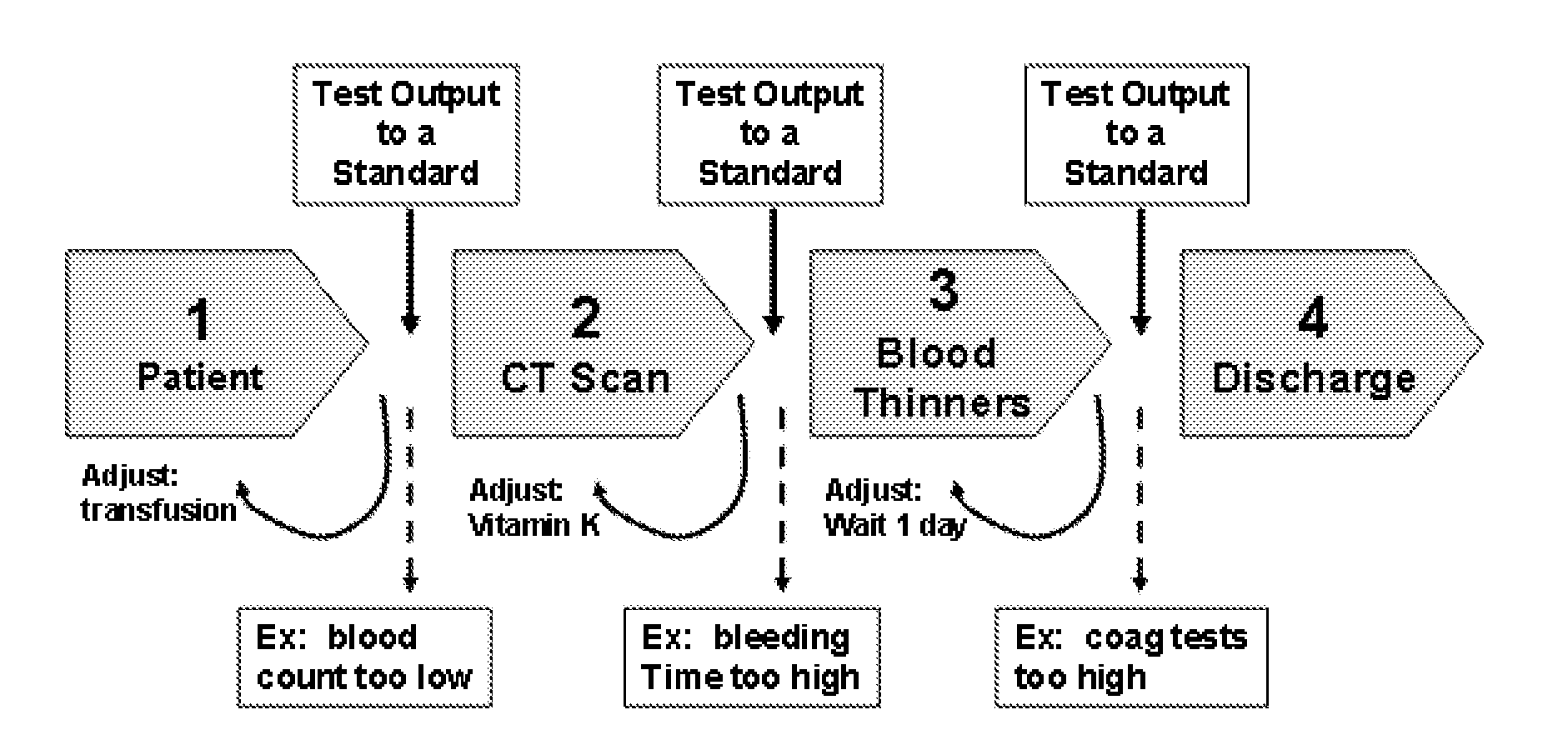
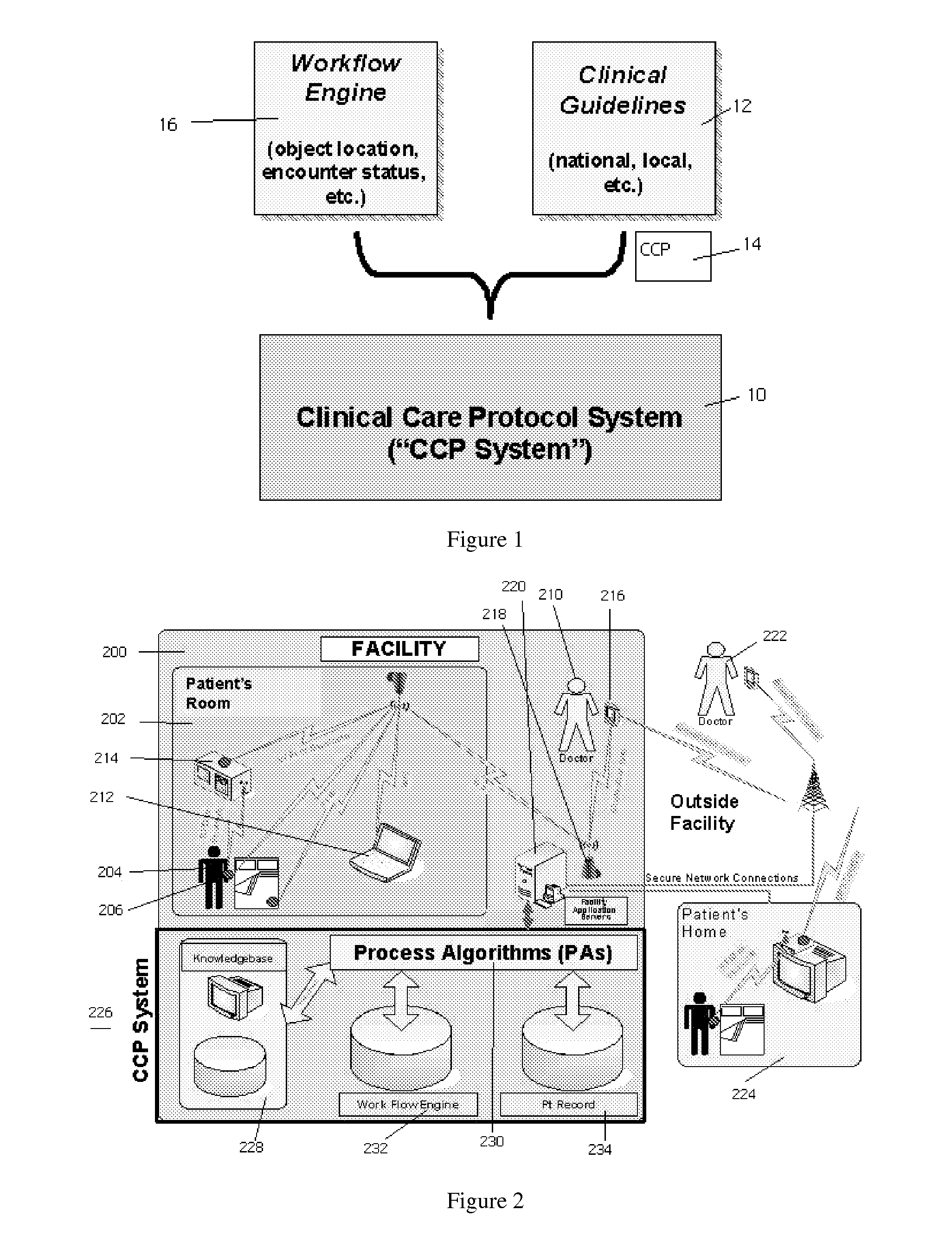
Method and system for providing clinical care
InactiveUS20070185739A1Improve implementation of and adherenceRaise the possibilityData processing applicationsMedical automated diagnosisGuidelineClinical care
The present invention describes a method that is operative within a clinical environment in which real-time locations of personnel and resources are tracked. The method begins in association with entry of a patient into the clinical environment, such as when a patient is in transit, or has been admitted, to an emergency room or to a hospital. In response, a given care guideline is identified. Using the care guideline, a set of one or more process rules, and information associated with at least one “encounter,” the system generates a patient-specific care protocol; the protocol includes a set of steps through which the patient is expected to proceed while in the clinical environment. An encounter occurs when two or more of objects (e.g., the patient, clinical personnel, and a clinical resource) are in a given physical proximity for a given time period at determined by the at least one process rule. According to the method, at least one event that occurs during at least a first step of the patient-specific care protocol is then monitored. Using information generated by the monitoring step and at least one process rule, the system then determines whether the patient moves to a next step in the patient-specific care protocol.
Owner:CLINILOGIX
Blood pressure monitoring using a multi-function wrist-worn device
ActiveUS20170340209A1Long and accurate and data setPortable and compact in designTherapiesEvaluation of blood vesselsNon invasiveBiomedical engineering
The present invention provides non-invasive devices, methods, and systems for determining a pressure of blood within a cardiovascular system of a user, the cardiovascular system including a heart and the user having a wrist covered by skin. More particularly, the present invention discloses a variety of wrist-worn devices having a variety of sensors configured to non-invasively engage the skin on the wrist of the user for sensing a variety of user signals from the cardiovascular system of the user. Generally, approaches disclosed herein may passively track blood pressure values without any interaction required on the part of the user or may allow for on demand or point measurements of blood pressure values by having a user actively interact with the sensors of the wrist-worn device. Approaches disclosed herein further allow for absolute blood pressure values to be determined directly without the requirement for any periodic calibrations or for relative blood pressure values to be tracked so as to provide relative blood pressure indices.
Owner:APPLE INC
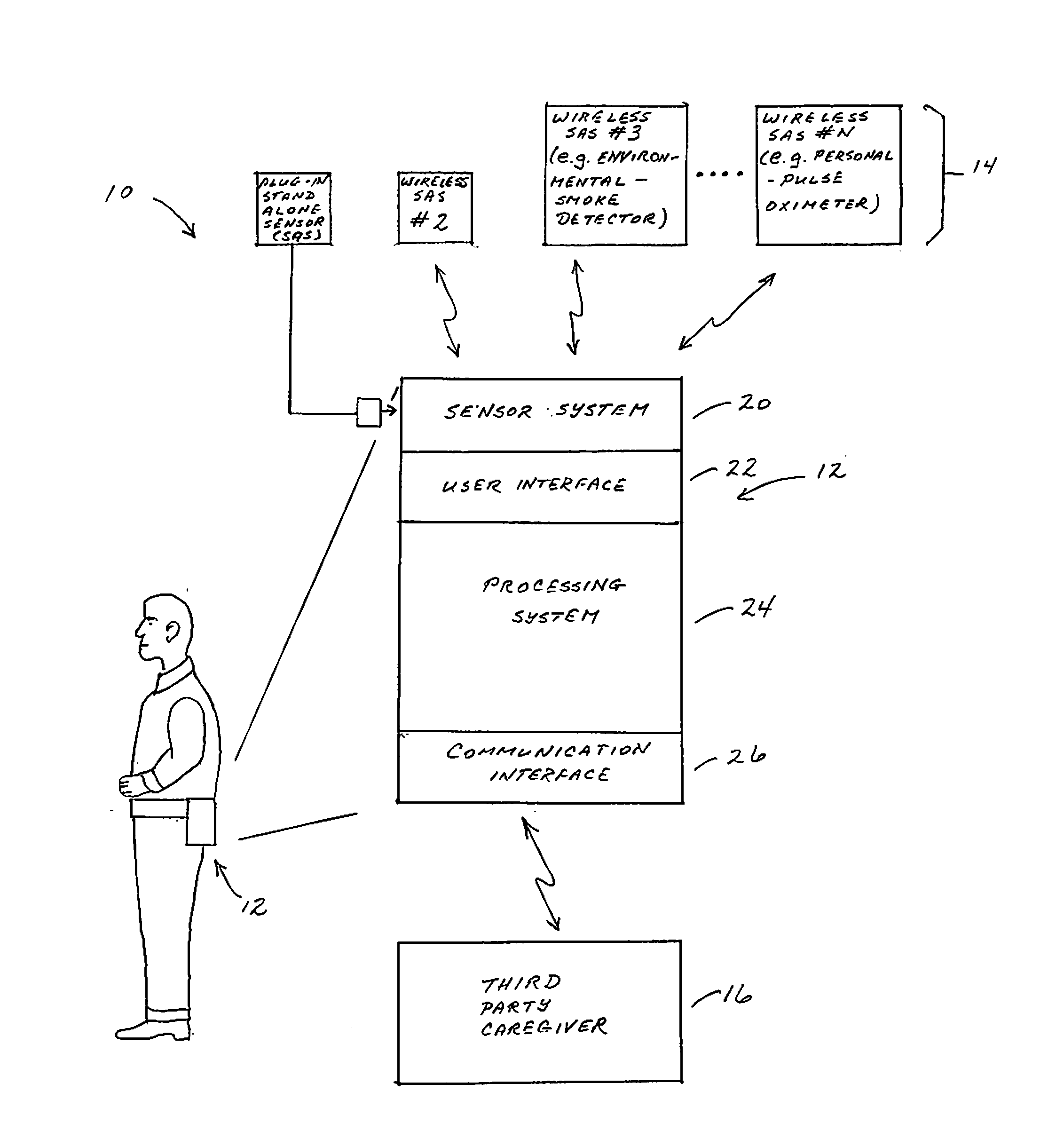
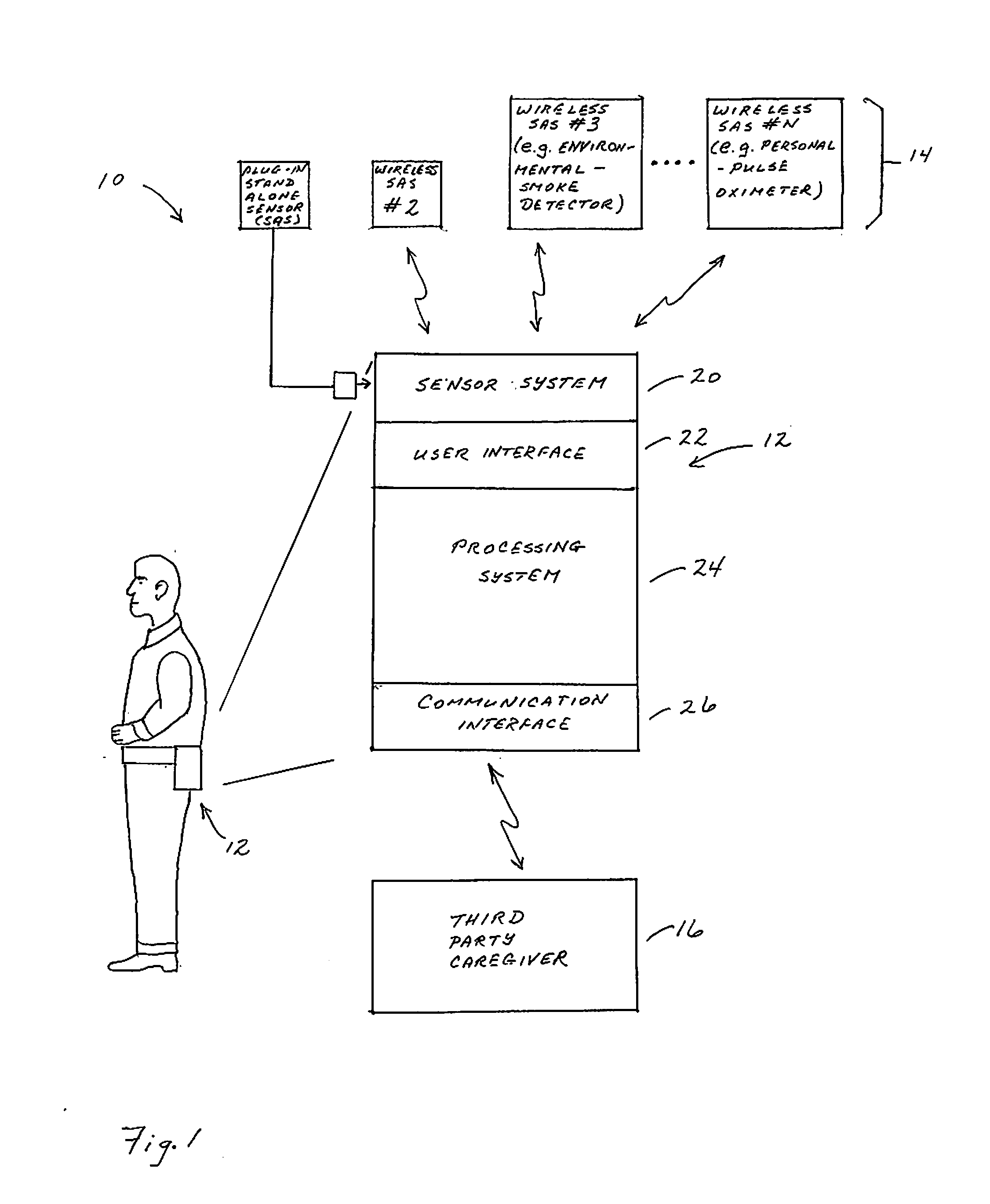
Portable Monitor for Elderly/Infirm Individuals
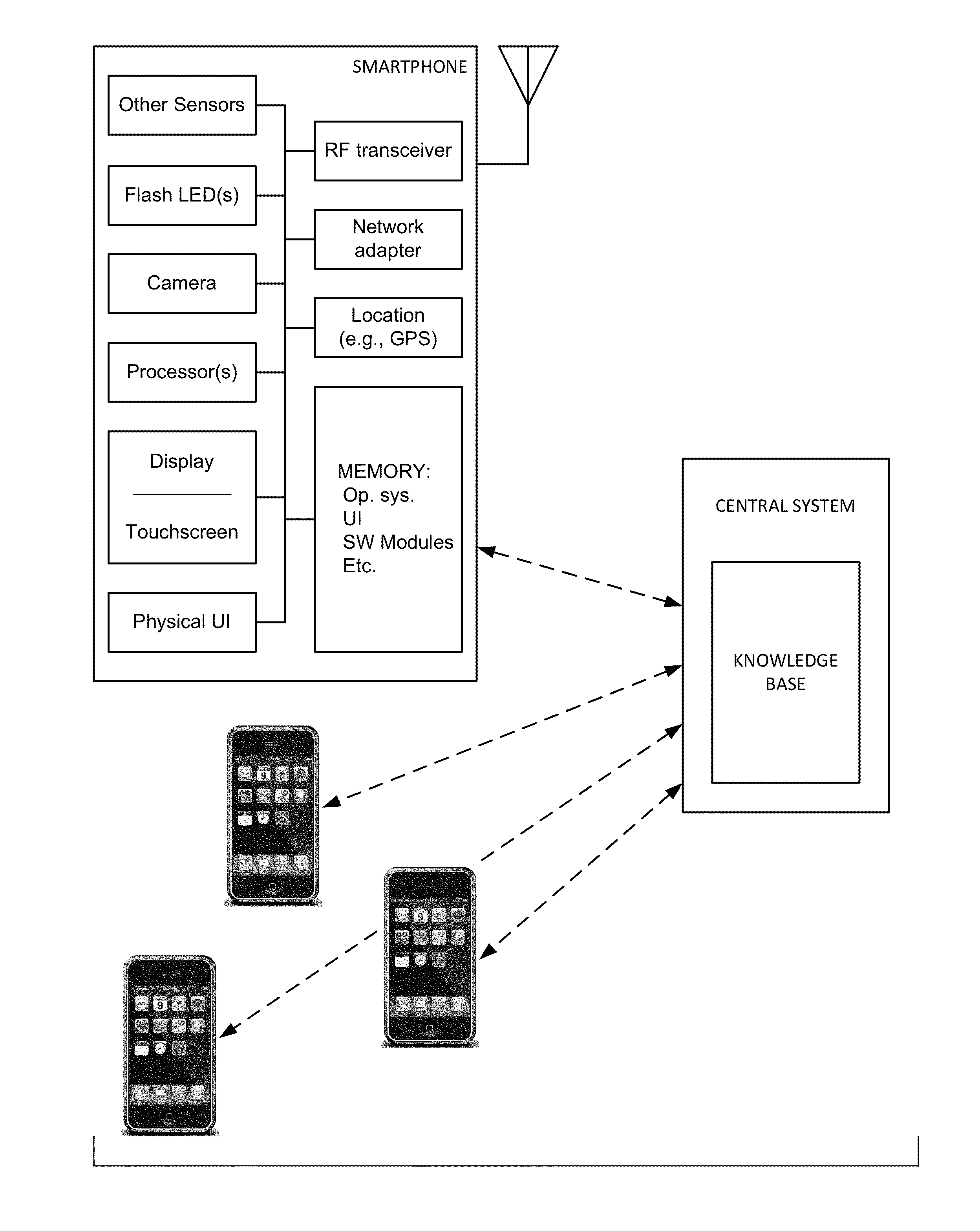
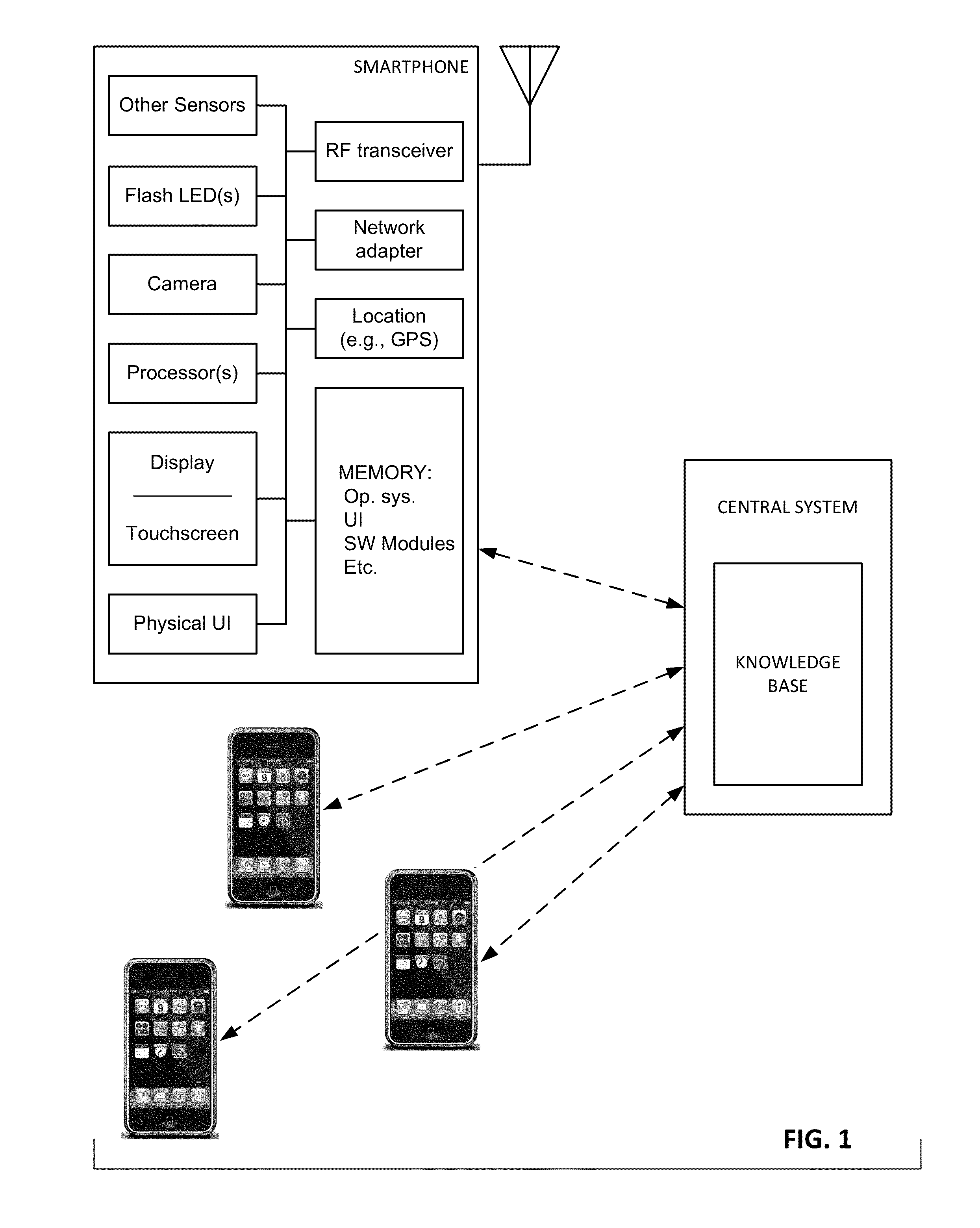
ActiveUS20110291827A1High selectivityLower cost of careAlarmsElectric signalling detailsCommunication interfaceThird party
The present invention is directed to a portable monitor for use by elderly / infirm individuals that desires a significant degree of independence but recognizes that their ability to handle many situations by themselves is or will become substantially attenuated. In one embodiment, the portable monitor includes: (a) a sensor system that receives data reflecting environmental and personal aspects associated with the monitored individual from various sensors, (b) a user interface that allows the monitored individual to interact with the monitor; (c) a communication interface that provides for the ability to communicate with a third party if the monitored individual is or is likely to enter into a situation in which assistance is likely needed; and (d) a processing system for processes data produced by sensor system and data input from the user interface to determine if the monitored individual is in a situation or likely is in a situation in which assistance is likely needed.
Owner:BALDOCCHI ALBERT S +1
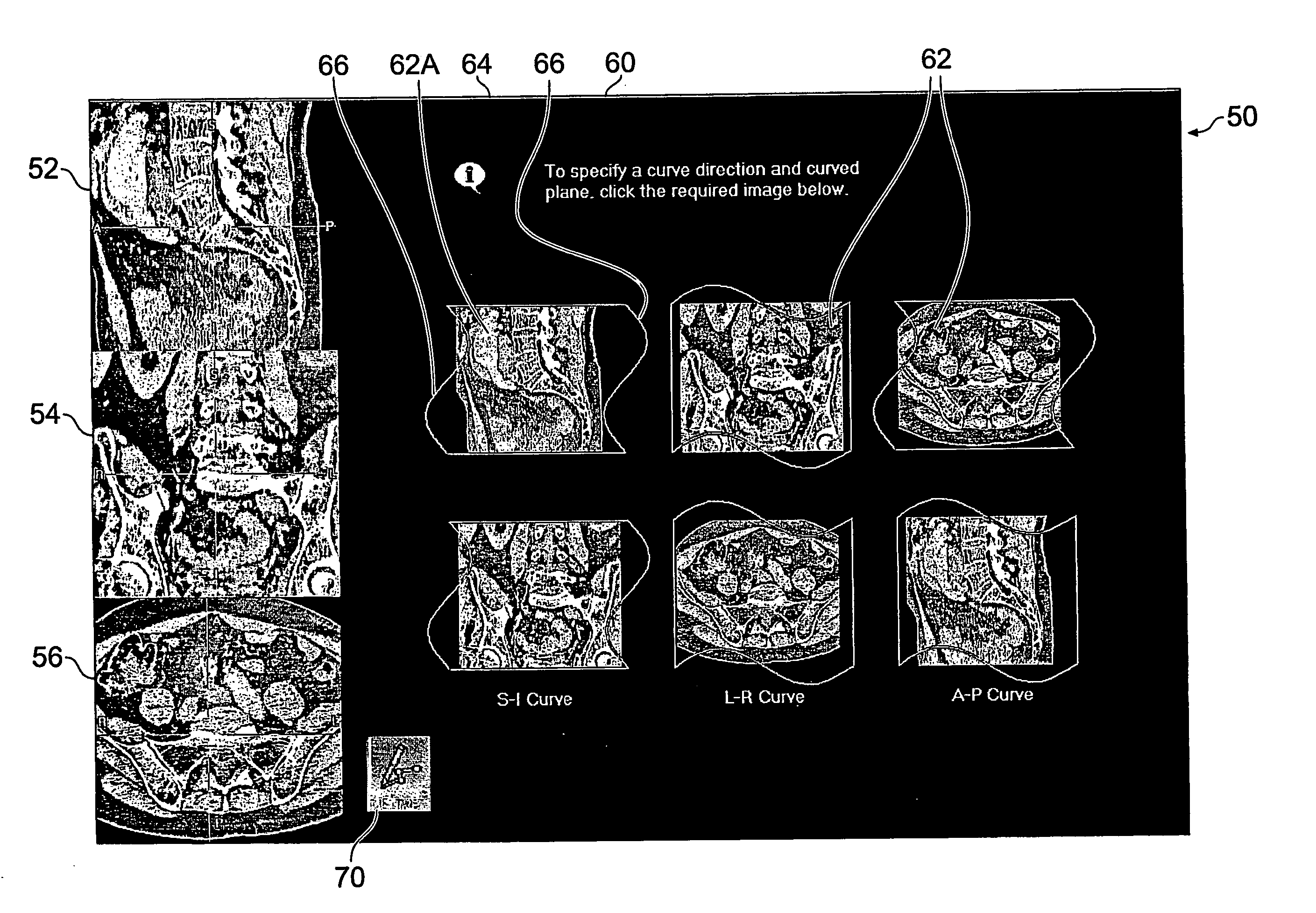
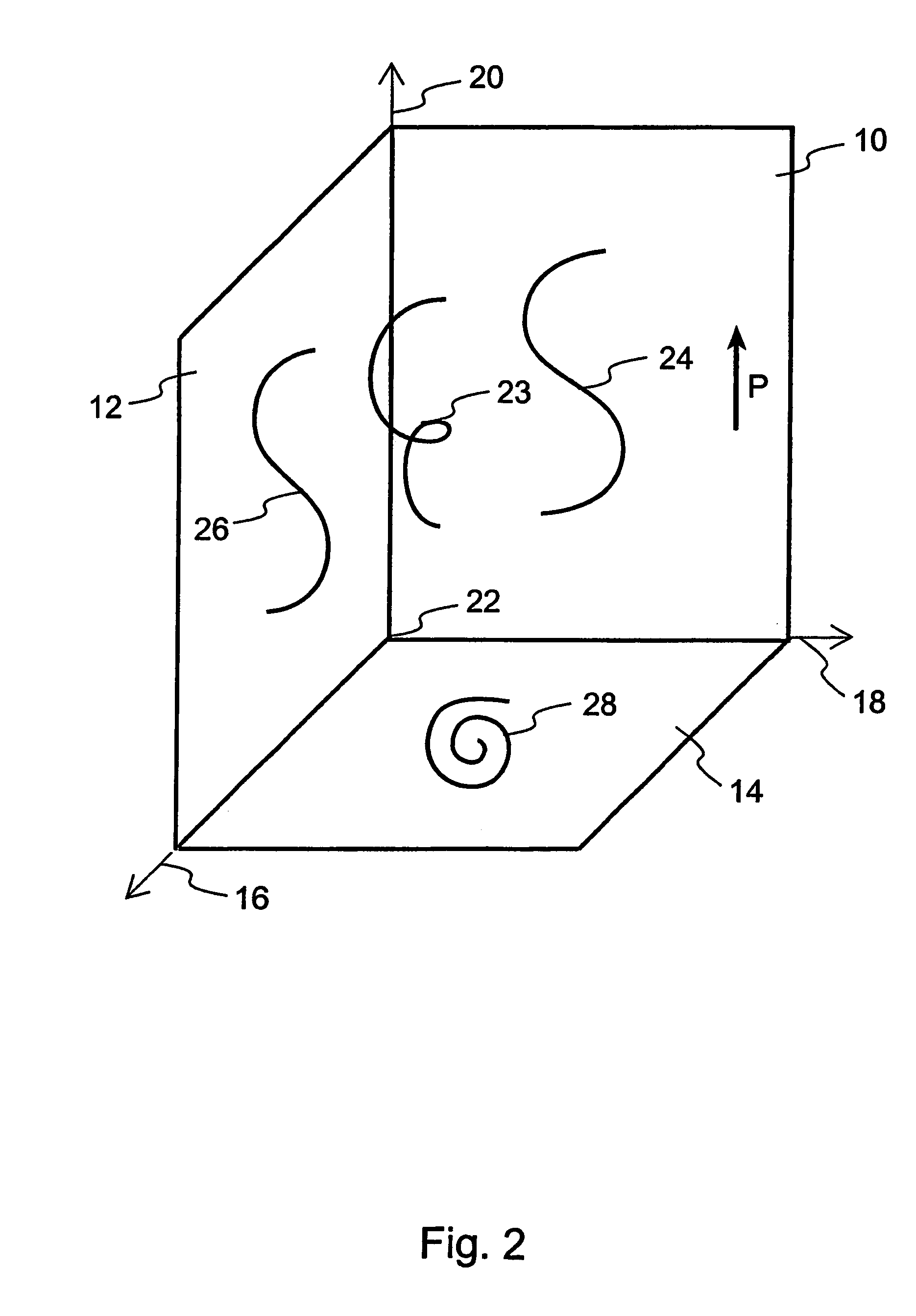
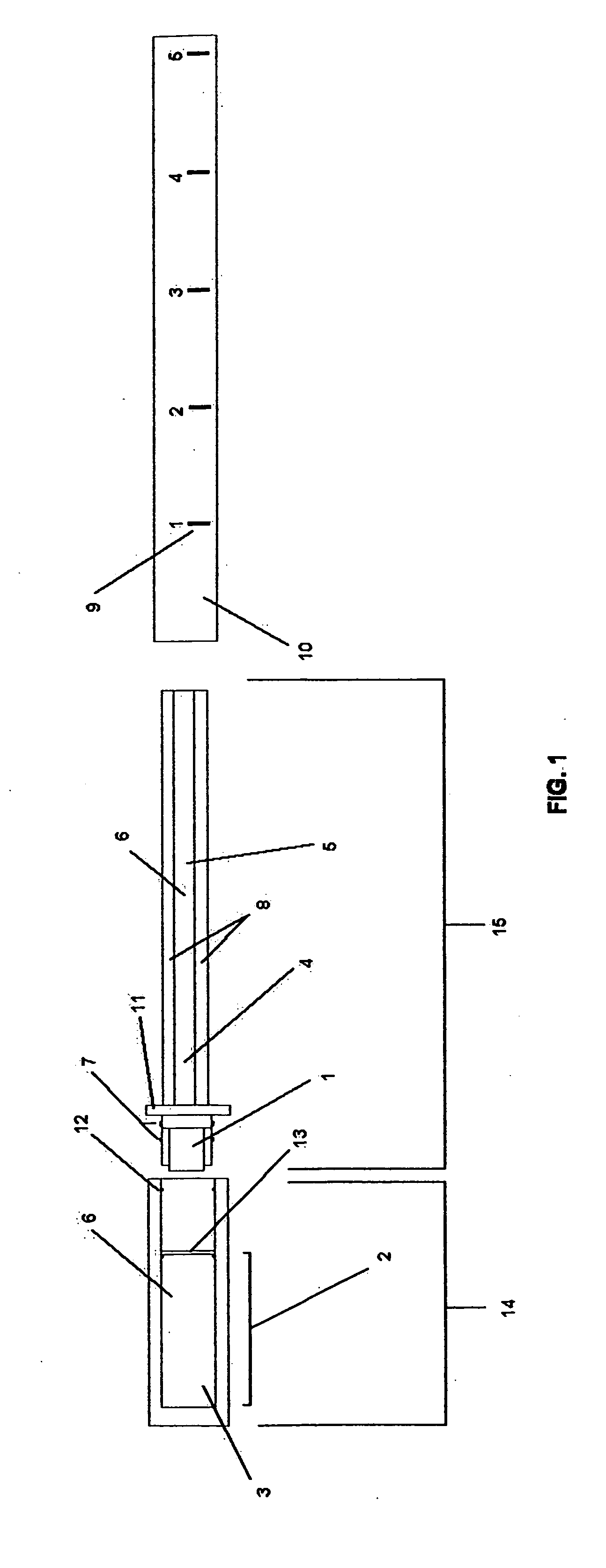
User-interface and method for curved multi-planar reformatting of three-dimensional volume data sets
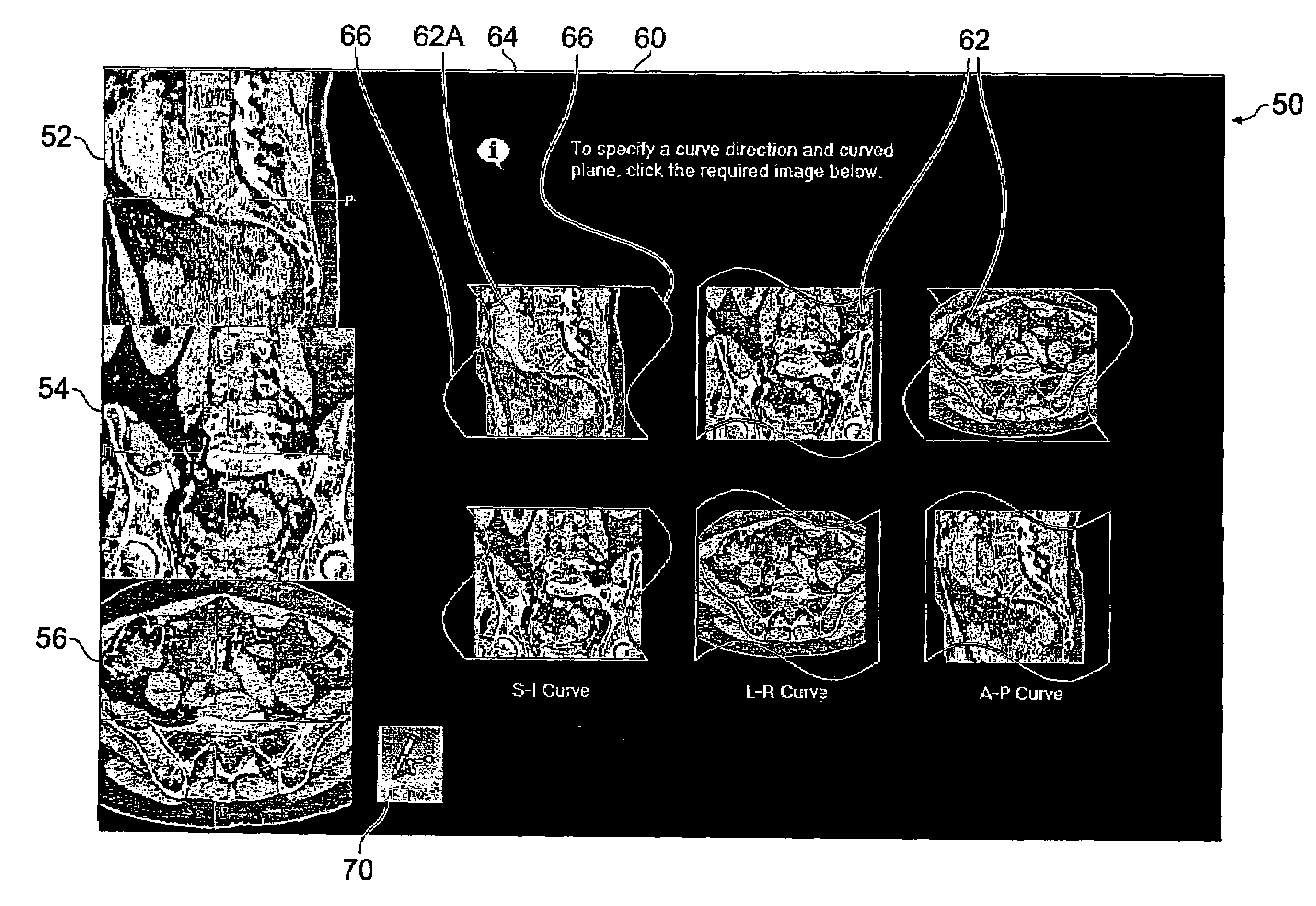
ActiveUS20050122343A1Simple accessSimplify interfaceGeometric image transformationCathode-ray tube indicatorsVolumetric dataComputer graphics (images)
Owner:TOSHIBA MEDICAL VISUALIZATION SYST EURO
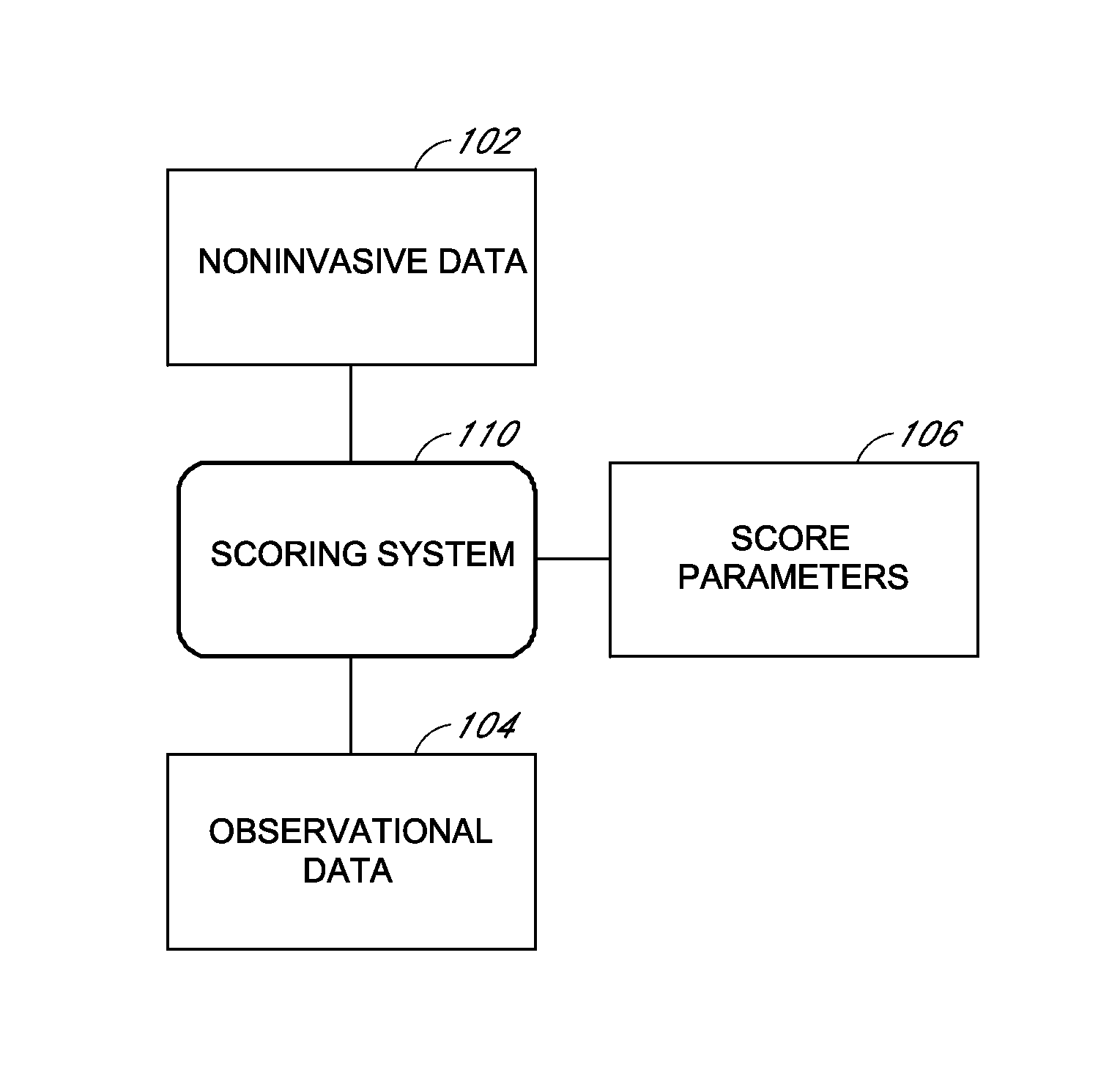
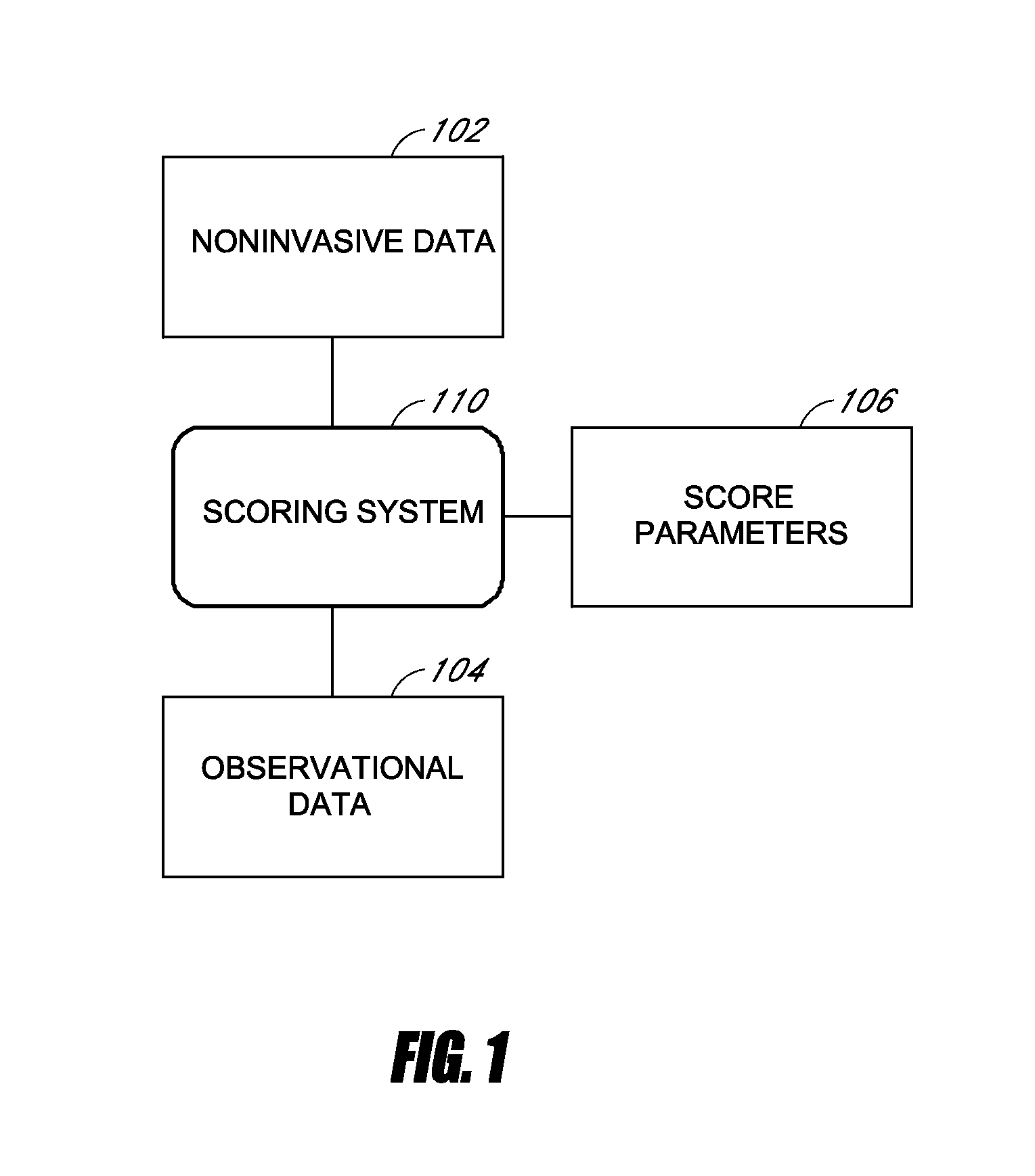
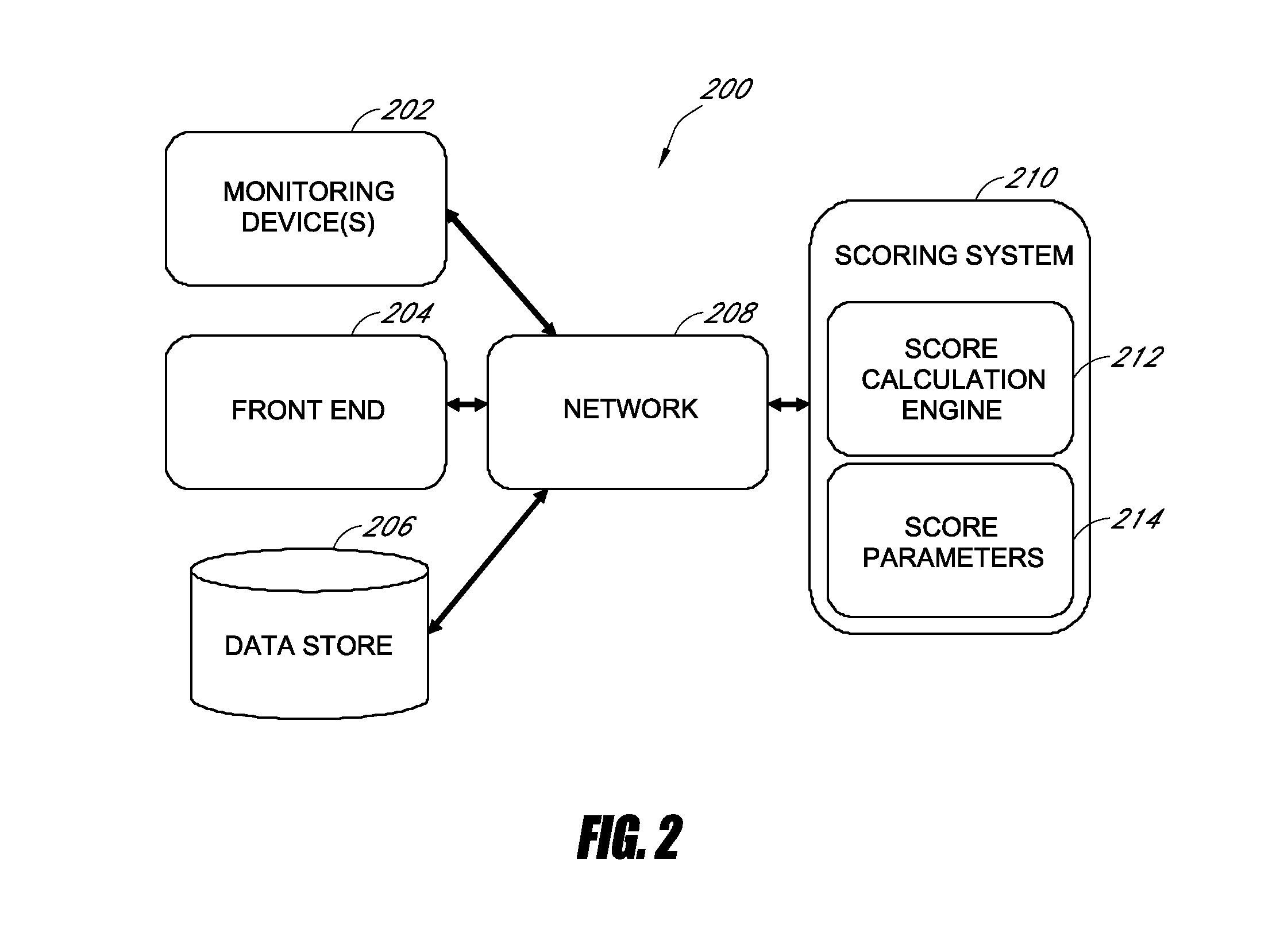
Medical scoring systems and methods
InactiveUS20130185097A1Great sensitivity and specificityImprove parental counselingData processing applicationsHealth-index calculationObservation dataICU scoring systems
Systems and methods for generating a medical score are disclosed. In some embodiments, an accurate medical score is generated within a relatively short period of time. The medical score can be derived from observational data and / or physiological time-series data collected from a subject. In some embodiments, a scoring system accesses the data, and at least a portion of the data is used in the calculation of the medical score. In certain embodiments, health care providers can use the medical score to make early predictions of complications in intensive care unit patients.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
System and method for increasing medication adherence rates
InactiveUS20120173319A1Increasing medication adherence rateDecreasing global health care costDrug and medicationsMultiple digital computer combinationsGlobal healthApplication software
Embodiments of the present disclosure generally relate to a system and method for increasing medication adherence rates. More specifically, embodiments of the present invention relate to a system and method for decreasing global health care costs by monitoring, reminding, and facilitating medication adherence utilizing mobile communication devices. In one embodiment, a method of increasing medication adherence rates comprises: enabling a user to download an application to a mobile device via a network, the application comprising a set of instructions for permitting a user to access: a medication reminder means for providing a reminder to the user to follow prescribed instructions regarding medication; and an adherence tracking means for tracking the user's compliance with the prescribed instructions; and receiving data from the mobile device at a remote server via the network, the data comprising information from the medication reminder means and the adherence tracking means.
Owner:FERRARA DANIEL
System and method for repetitive interval clinical evaluations
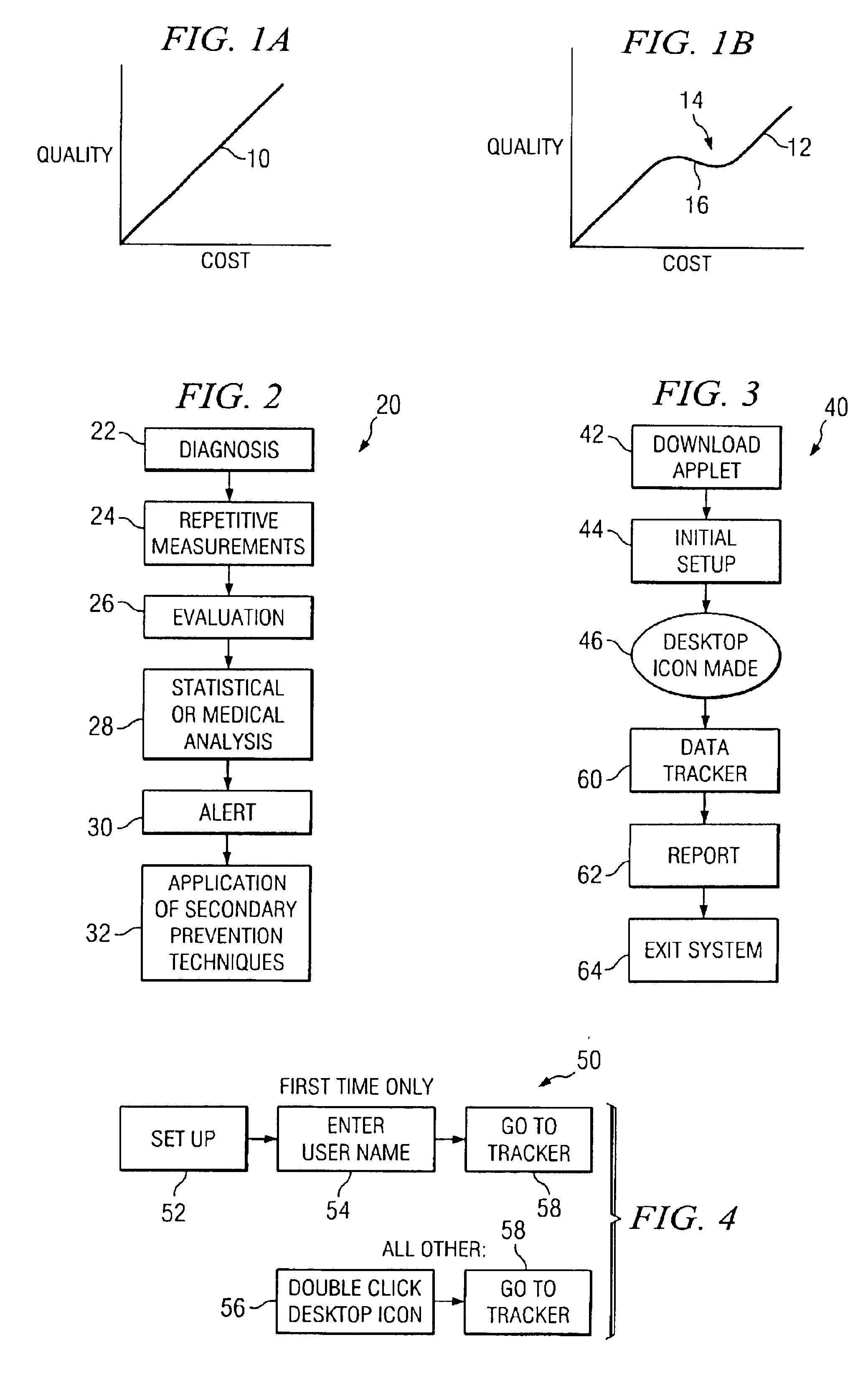
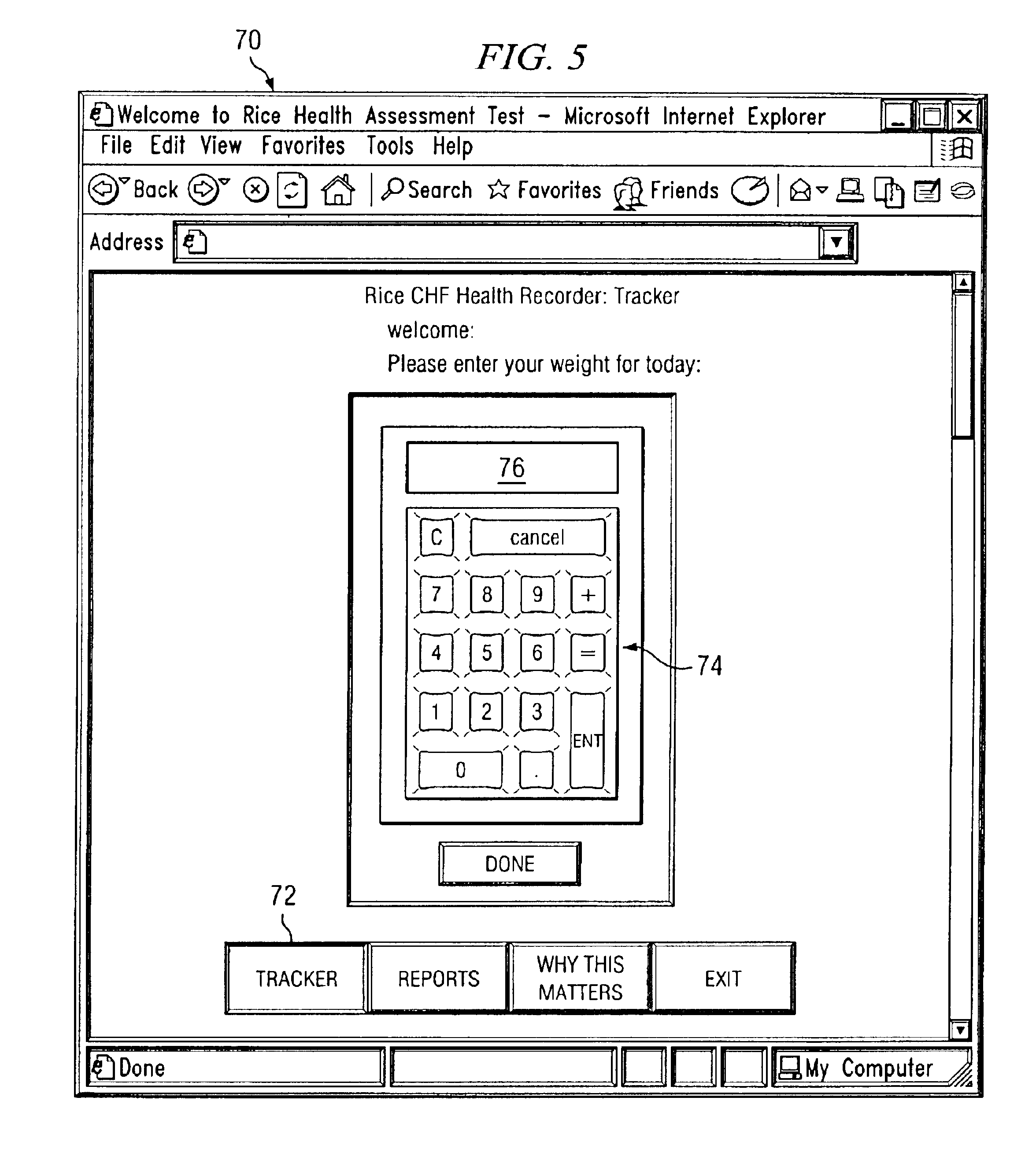
InactiveUS6955647B2Reduce associated health care costImprove the quality of lifeMedical simulationSurgeryStatistical analysisCongestive heart failure chf
A healthcare tool allows a patient to record daily parameters associated with the patient's clinical status, for example, body weight for congestive heart failure patients. A graph may be created showing the parameters on a control chart. The parameters are statistically analyzed against a control range, and when a parameter moves out of the control range, the system automatically creates a pop-up window alerting the patient that the parameter is outside the control range, and that the patient should consider informing a healthcare professional.
Owner:RICE WILLIAM H
Species-specific, genus-specific and universal DNA probes and amplification primers to rapidly detect and identify common bacterial and fungal pathogens and associated antibiotic resistance genes from clinical specimens for diagnosis in microbiology laboratories
InactiveUS20040185478A1Reduce usageDetermine rapidly the bacterial resistance to antibioticsMicrobiological testing/measurementFermentationBacteroidesNeisseria meningitidis
DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample DNA from (i) any bacterium, (ii) the species Streptococcus agalactiae, Staphylococcus saprophyticus, Enterococcus faecium, Neisseria meningitidis, Listeria monocytogenes and Candida albicans, and (iii) any species of the genera Streptococcus, Staphylococcus, Enterococcus, Neisseria and Candida are disclosed. DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample antibiotic resistance genes selected from the group consisting of blatem, blarob, blashv, blaoxa, blaZ, aadB, aacC1, aacC2, aacC3, aacA4, aac6'-lla, ermA, ermB, ermC, mecA, vanA, vanB, vanC, satA, aac(6')-aph(2''), aad(6'), vat, vga, msrA, sul and int are also disclosed. The above microbial species, genera and resistance genes are all clinically relevant and commonly encountered in a variety of clinical specimens. These DNA-based assays are rapid, accurate and can be used in clinical microbiology laboratories for routine diagnosis. These novel diagnostic tools should be useful to improve the speed and accuracy of diagnosis of microbial infections, thereby allowing more effective treatments. Diagnostic kits for (i) the universal detection and quantification of bacteria, and / or (ii) the detection, identification and quantification of the above-mentioned bacterial and fungal species and / or genera, and / or (iii) the detection, identification and quantification of the above-mentioned antibiotic resistance genes are also claimed.
Owner:GENEOHM SCI CANADA
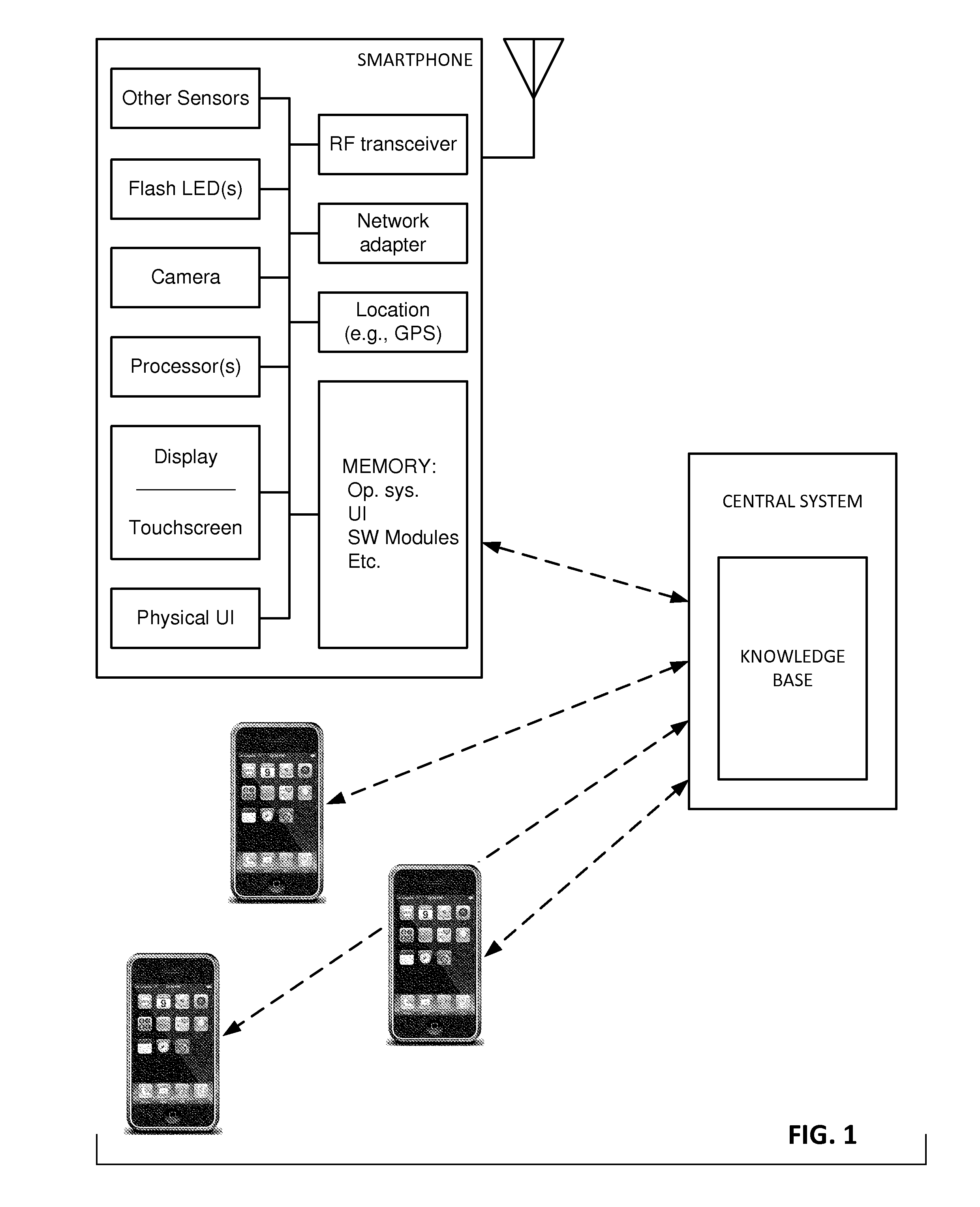
Skin imaging and applications
InactiveUS20140316235A1Lower cost of careMedical data miningMedical automated diagnosisPattern recognitionImaging analysis
The availability of high quality imagers on smartphones and other portable devices facilitates creation of a large, crowd-sourced, image reference library that depicts skin rashes and other dermatological conditions. Some of the images are uploaded with, or later annotated with, associated diagnoses or other information (e.g., “this rash went away when I stopped drinking milk”). A user uploads a new image of an unknown skin condition to the library. Image analysis techniques are employed to identify salient similarities between features of the uploaded image, and features of images in this reference library. Given the large dataset, statistically relevant correlations emerge that identify to the user certain diagnoses that may be considered, other diagnoses that may likely be ruled-out, and / or anecdotal information about similar skin conditions from other users. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
System and method for distributing healthcare products
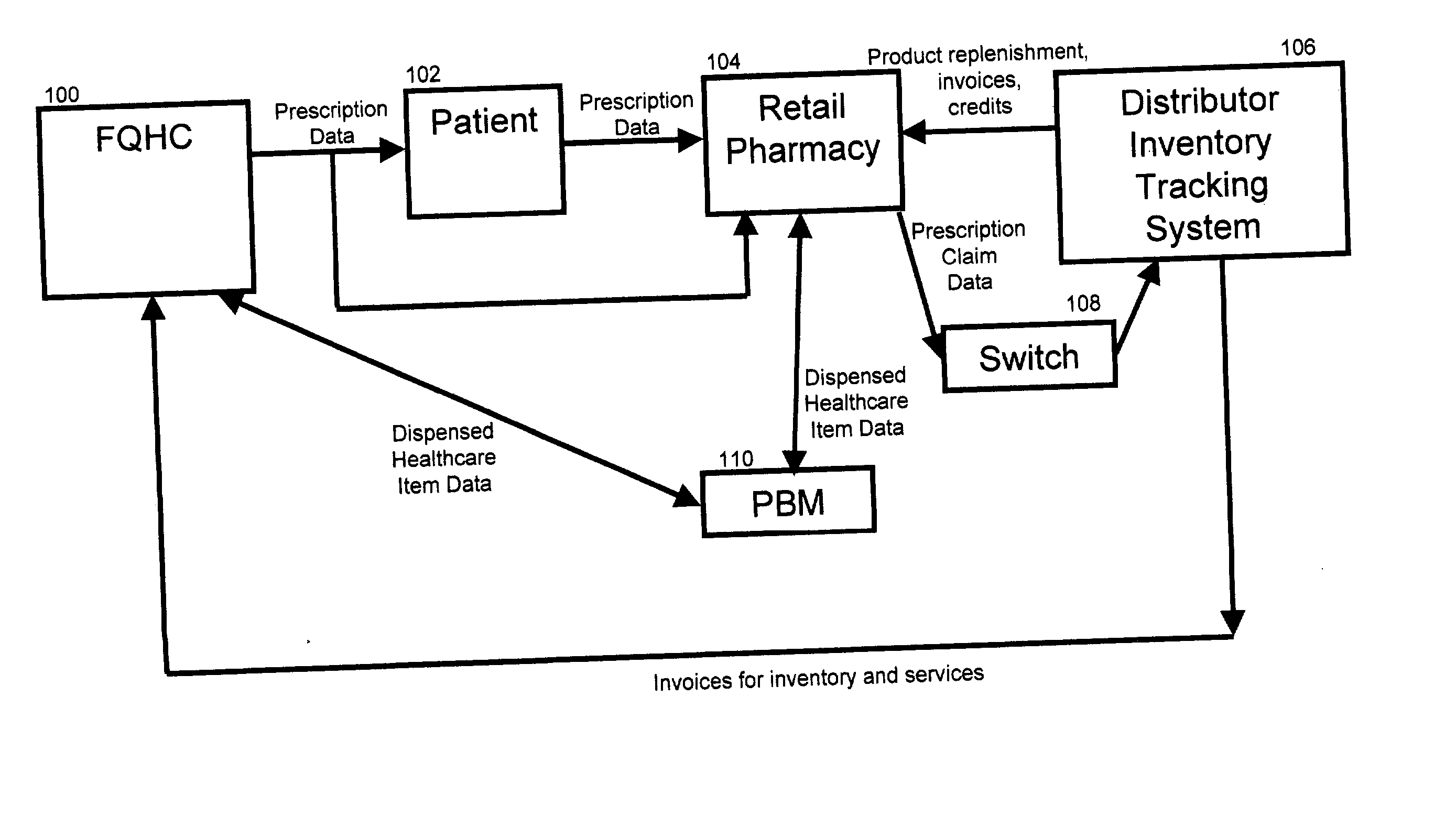
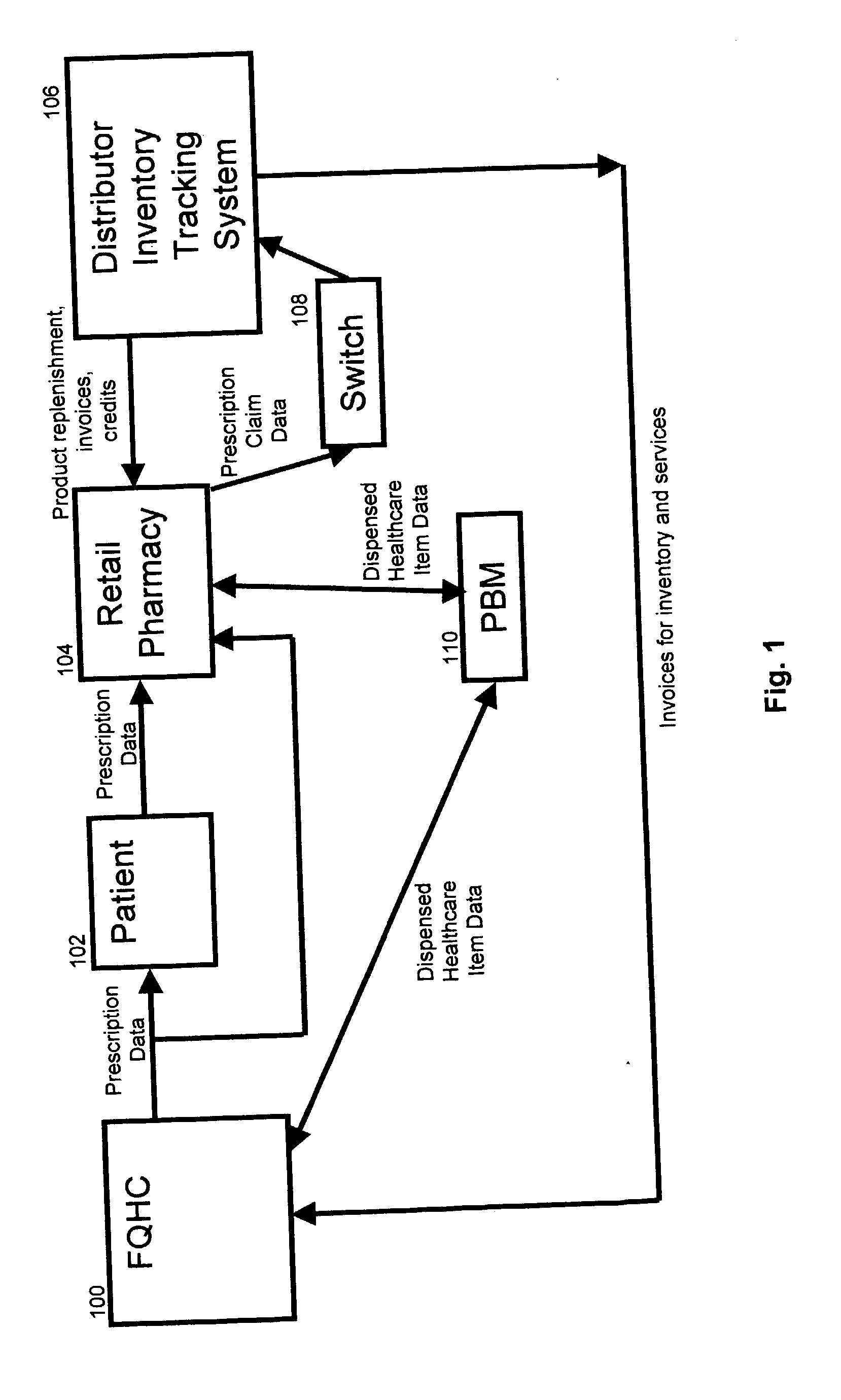
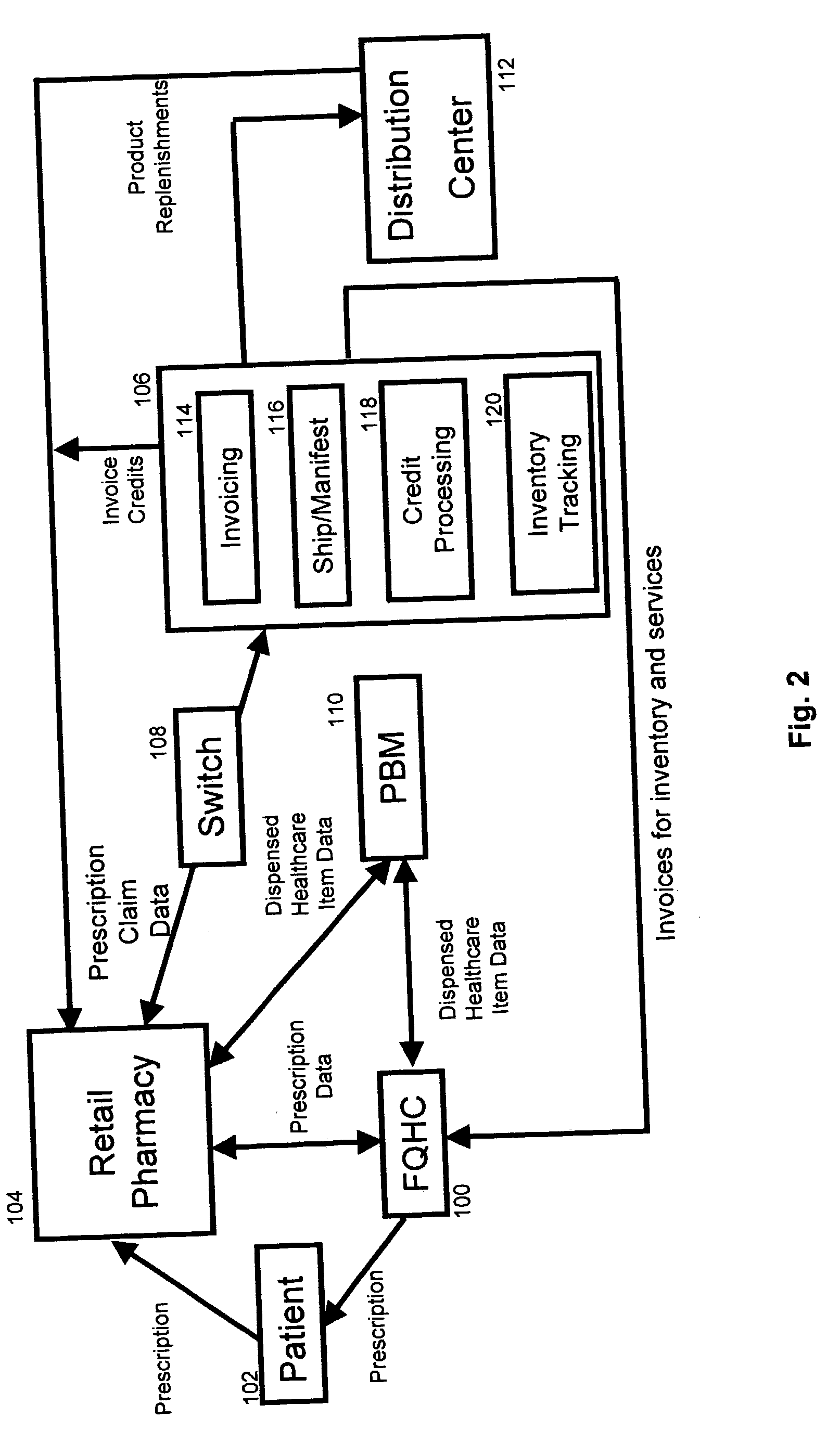
InactiveUS20040230502A1Reduce healthcare costImprove healthLogisticsSpecial data processing applicationsThird partyInvoice
A system and method for distributing healthcare products is disclosed. A patient who obtains a prescription from clinic such as a federally qualified 340B health clinic (FQHC) is entered into a retail pharmacy's computer as a third party patient. Prescription information is transmitted to a healthcare product distributor inventory tracking system where the retail pharmacy is credited at its original invoice cost for the product. The healthcare product distributor's system charges the FQHC for the product at the 340B price. The inventory system tracks each 340B product distributed. In some cases, the first time a product is dispensed, the FQHC is charged for a stock package size and subsequent prescriptions are tracked and applied against the original purchase until the amount dispensed equals or exceeds the stock package originally charged to the FQHC. Once the quantity dispensed exceeds the stock package size, the inventory accumulation process is restarted.
Owner:FIACCO JOHN +2
Hemodialysis system and method
InactiveUS20120065567A1Reducing circulating cell damageReduce turbulenceDialysis systemsMedical devicesHemodialysisHaemodialysis machine
Hemodialysis systems and methods with streamlined blood flow paths are provided. Such streamlined blood flow paths facilitate flow without undue damage to circulating cells.
Owner:ZARATE ALFREDO R
Lightweight concrete compositions containing antimicrobial agents
InactiveUS20070027224A1Decrease health care costReduce areaSolid waste managementCeramicwareCompressive strengthUltimate tensile strength
A lightweight concrete composition containing from 10 to 90 volume percent of a cement composition, from 10 to 90 volume percent of particles having an average particle diameter of from 0.2 mm to 8 mm, a bulk density of from 0.03 g / cc to 0.64 g / cc, an aspect ratio of from 1 to 3, and from 0 to 50 volume percent of aggregate, where the particles contain an antimicrobial agent; where the sum of components used does not exceed 100 volume percent, and where after the lightweight concrete composition is set it has a compressive strength of at least 1700 psi as tested according to ASTM C39 after seven days. The concrete composition can be used to make concrete masonry units, construction panels, dining tables, counter surfaces, bench tops, and / or examination tables as well as other articles.
Owner:SYNTHEON
Species-specific, genus-specific and universal DNA probes and amplification primers to rapidly detect and identify common bacterial and fungal pathogens and associated antibiotic resistance genes from clinical specimens for diagnosis in microbiology laboratories
InactiveUS20060263810A1Reduce usageDetermine rapidly the bacterial resistance to antibioticsSugar derivativesMicrobiological testing/measurementNeisseria meningitidisListeria monocytogenes
DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample DNA from (i) any bacterium, (ii) the species Streptococcus agalactiae, Staphylococcus saprophyticus, Enterococcus faecium, Neisseria meningitidis, Listeria monocytogenes and Candida albicans, and (iii) any species of the genera Streptococcus, Staphylococcus, Enterococcus, Neisseria and Candida are disclosed. DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample antibiotic resistance genes selected from the group consisting of blatem, blarob, blashv, blaoxa, blaZ, aadB, aacC1, aacC2, aacC3, aacA4, aac6′-IIa, ermA, ermB, ermC, mecA, vanA, vanB, vanC, satA, aac(6′)-aph(2″), aad(6), vat, vga, msrA, sul and int are also disclosed. The above microbial species, genera and resistance genes are all clinically relevant and commonly encountered in a variety of clinical specimens. These DNA-based assays are rapid, accurate and can be used in clinical microbiology laboratories for routine diagnosis. These novel diagnostic tools should be useful to improve the speed and accuracy of diagnosis of microbial infections, thereby allowing more effective treatments. Diagnostic kits for (i) the universal detection and quantification of bacteria, and / or (ii) the detection, identification and quantification of the above-mentioned bacterial and fungal species and / or genera, and / or (iii) the detection, identification and quantification of the above-mentioned antibiotic resistance genes are also claimed.
Owner:GENEOHM SCI CANADA
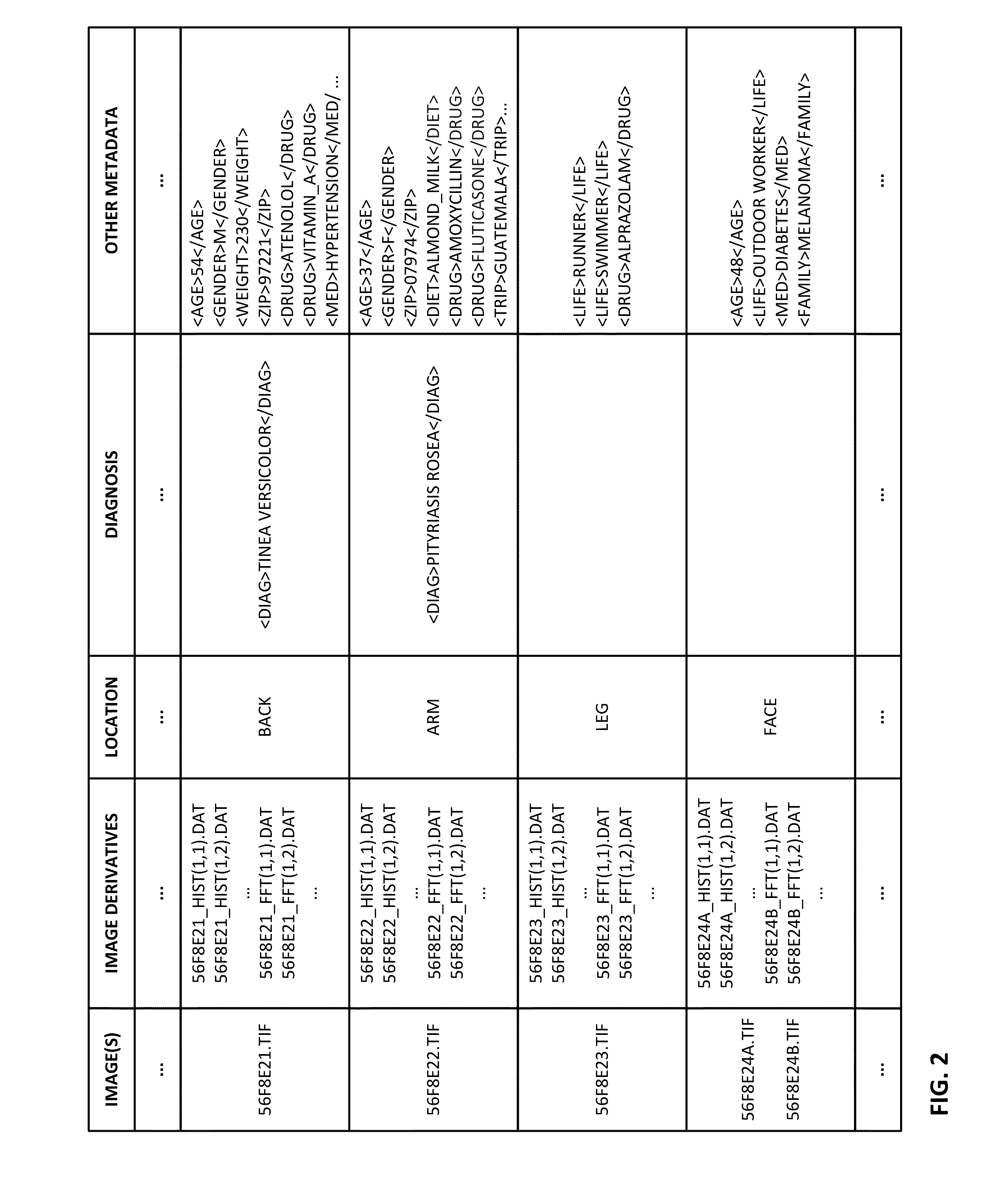
Methods and arrangements for identifying dermatological diagnoses with clinically negligible probabilties
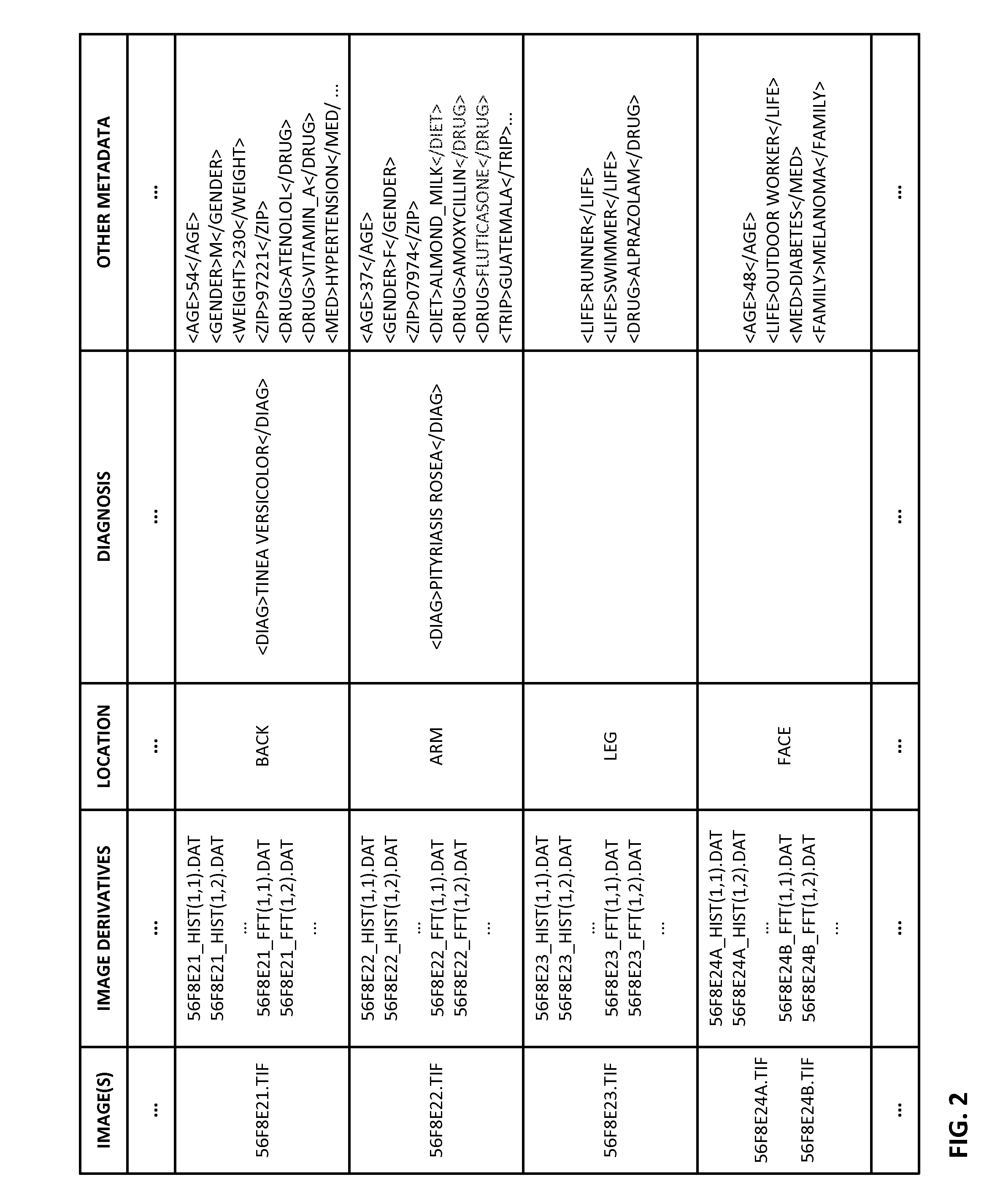
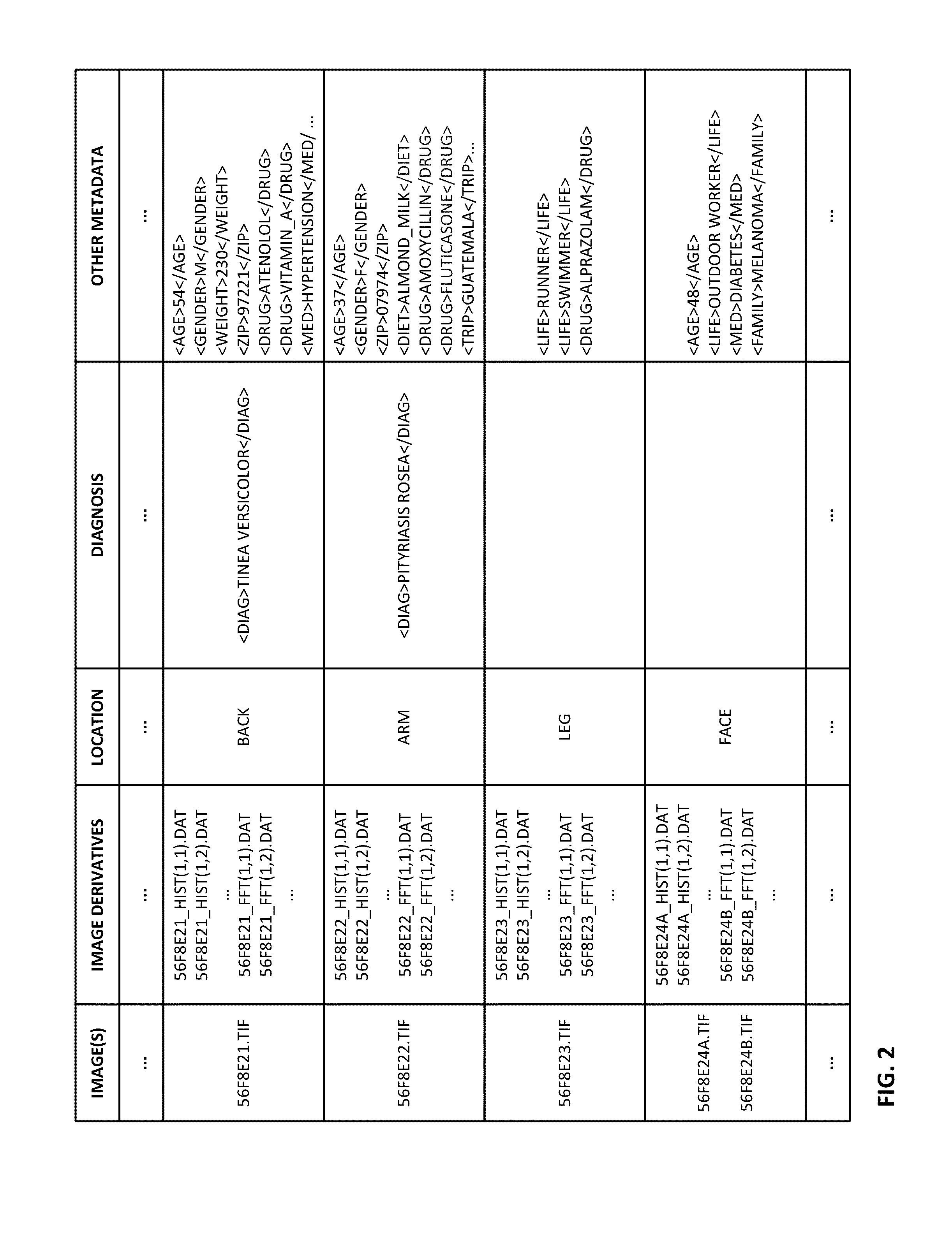
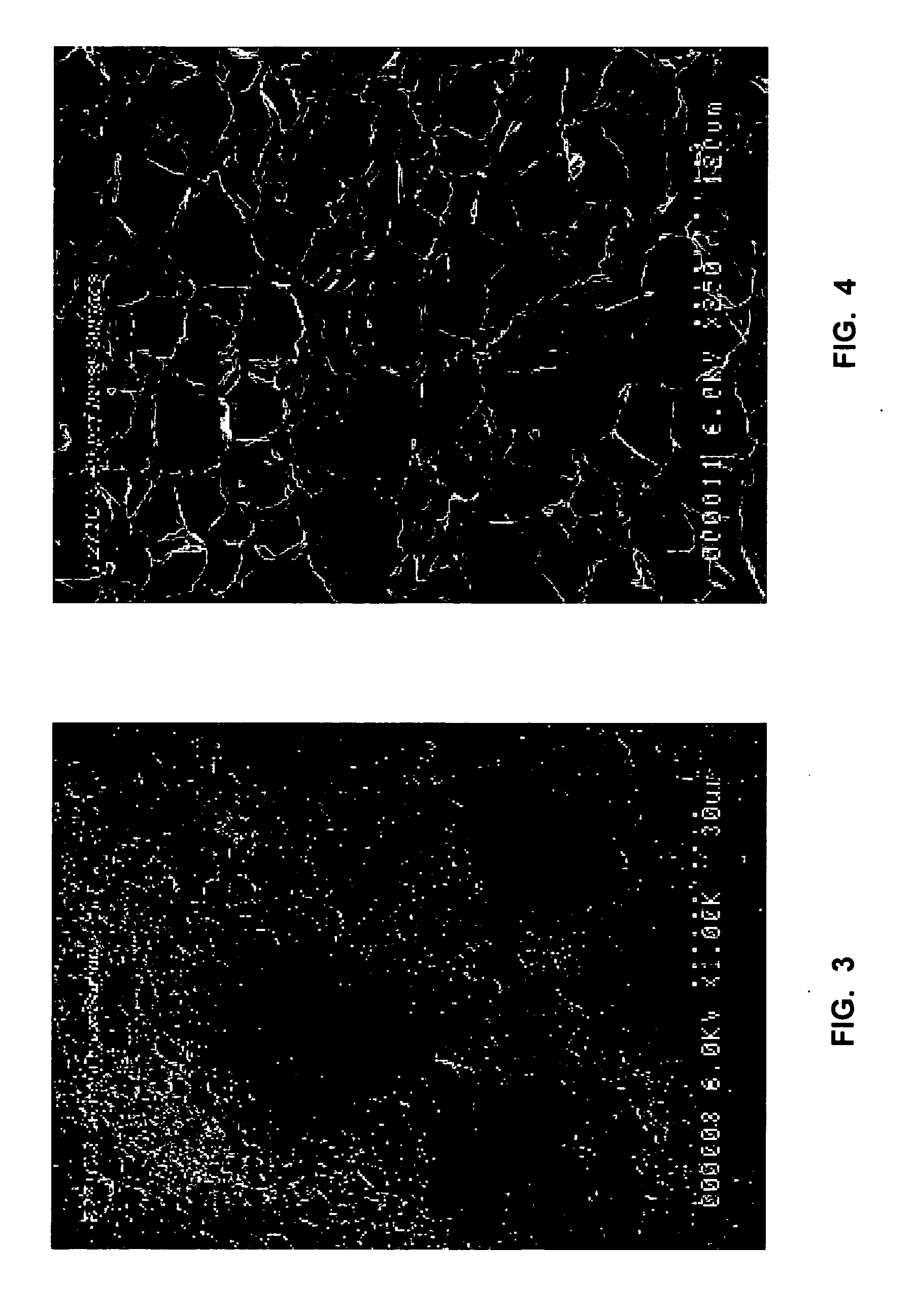
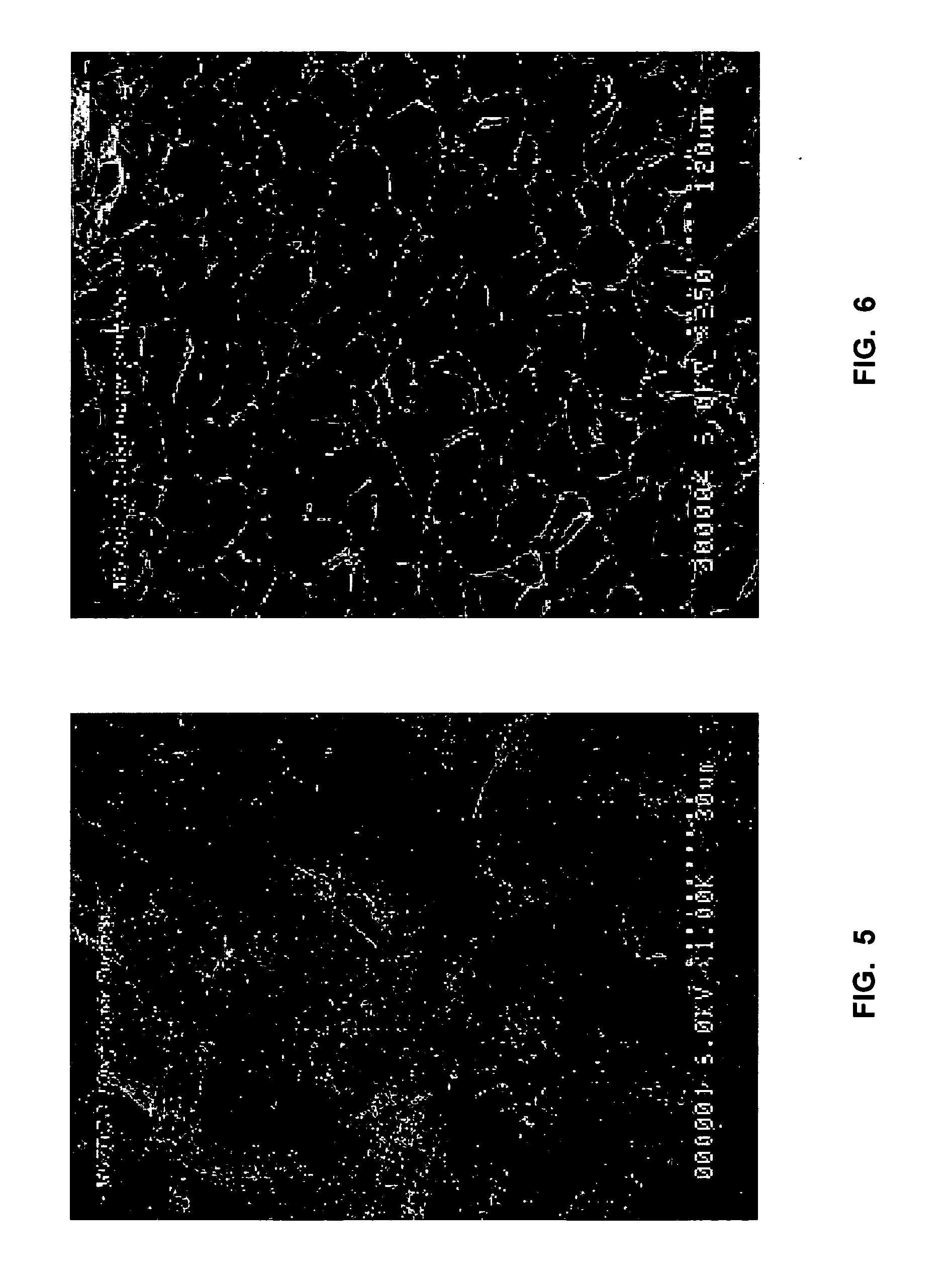
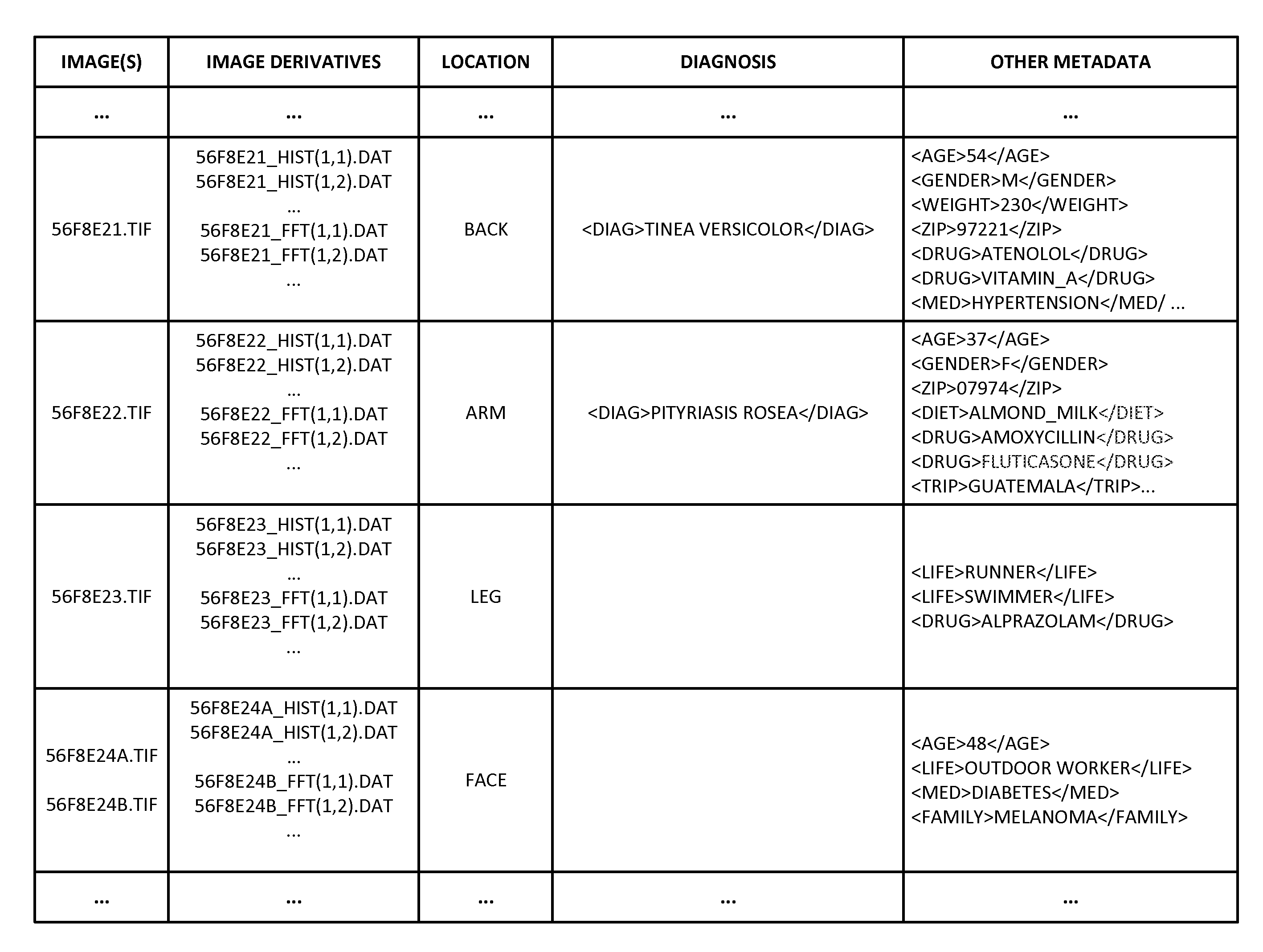
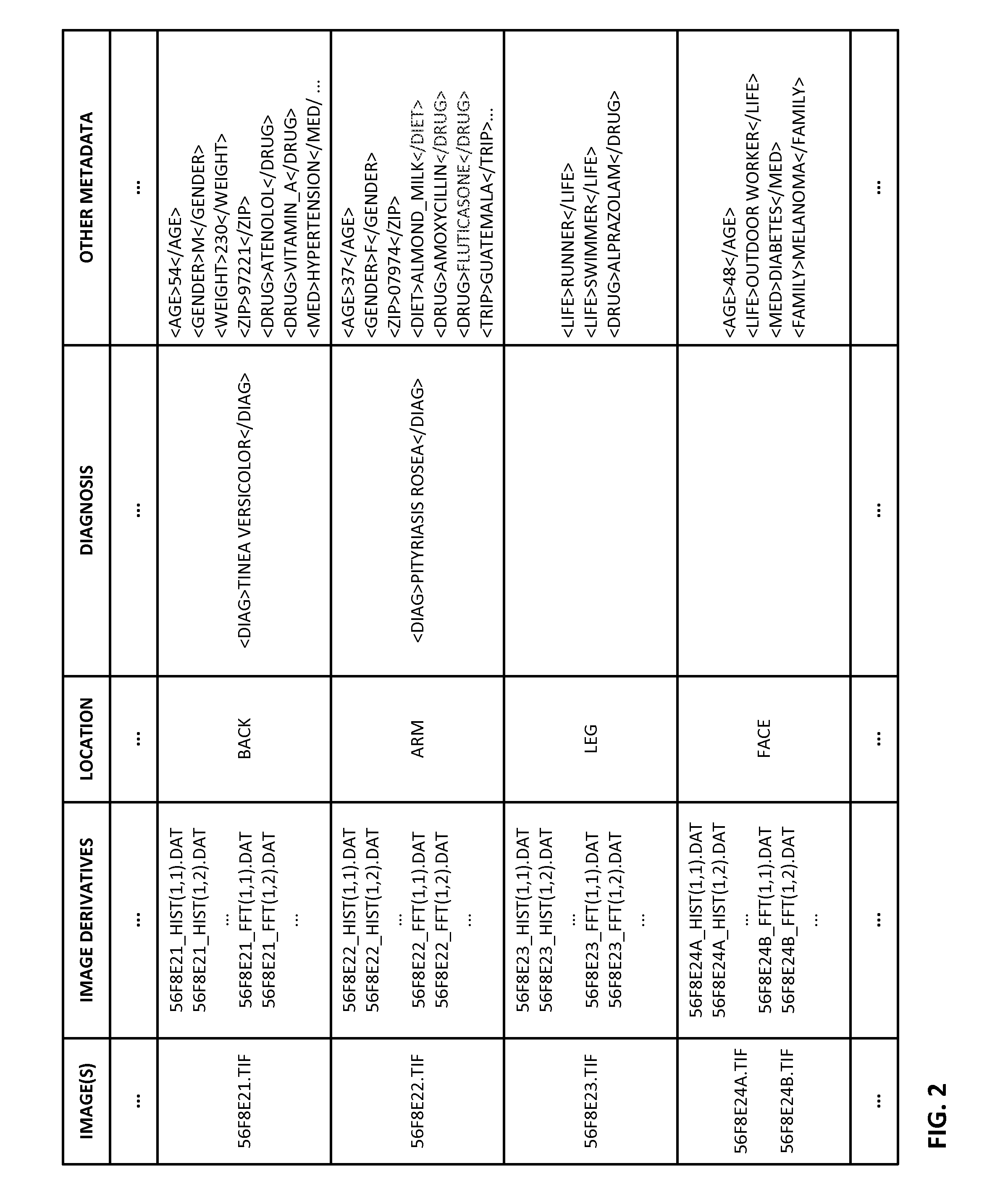
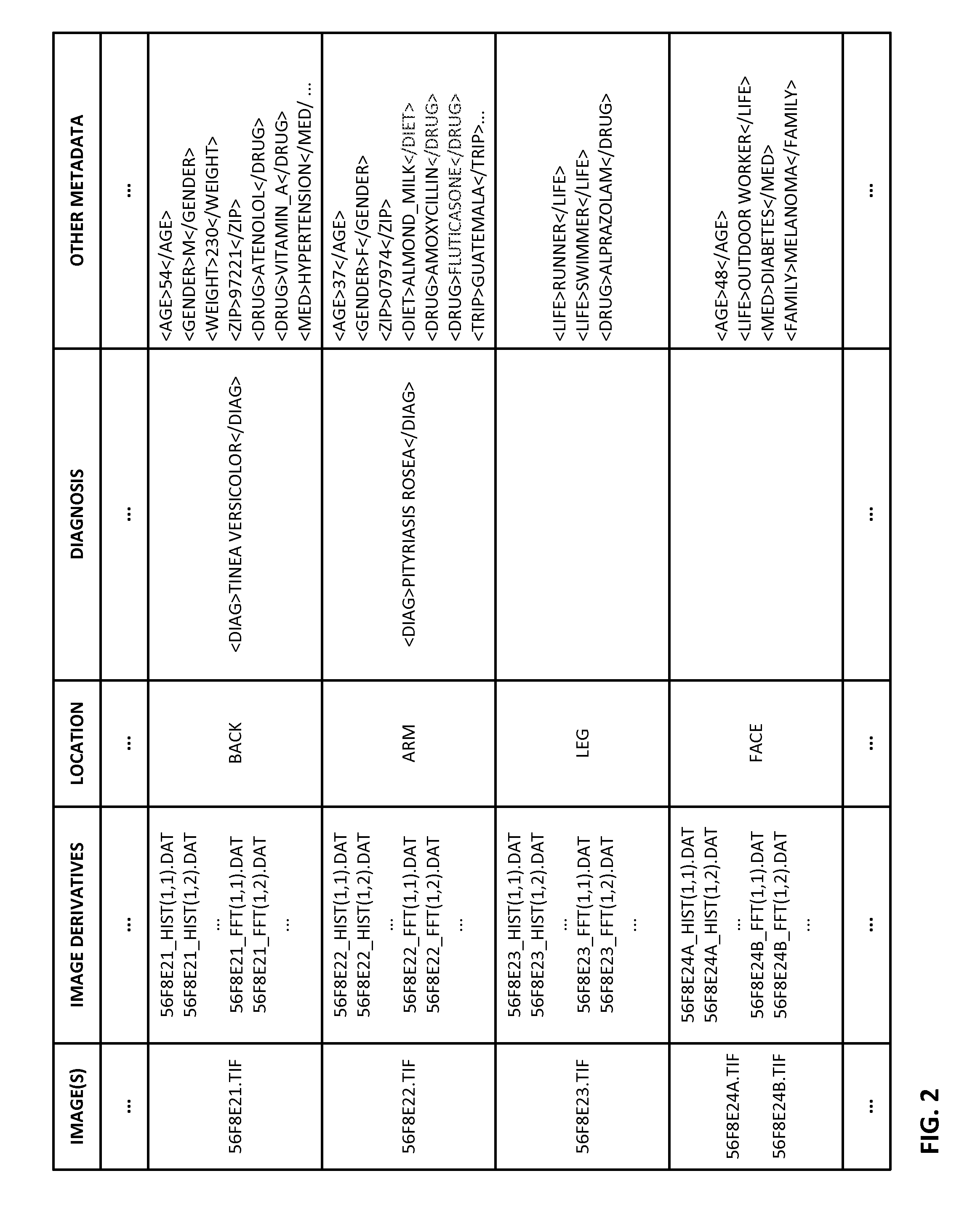
ActiveUS20150003699A1Lower cost of careImage enhancementMedical imagingPattern recognitionBiopsy procedure
Reference imagery of dermatological conditions is compiled in a crowd-sourced database (contributed by clinicians and / or the lay public), together with associated diagnosis information. A user later submits a query image to the system (e.g., captured with a smartphone). Image-based derivatives for the query image are determined (e.g., color histograms, FFT-based metrics, etc.), and are compared against similar derivatives computed from the reference imagery. This comparison identifies diseases that are not consistent with the query image, and such information is reported to the user. Depending on the size of the database, and the specificity of the data, 90% or more of candidate conditions may be effectively ruled-out, possibly sparing the user from expensive and painful biopsy procedures, and granting some peace of mind (e.g., knowledge that an emerging pattern of small lesions on a forearm is probably not caused by shingles, bedbugs, malaria or AIDS). A great number of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
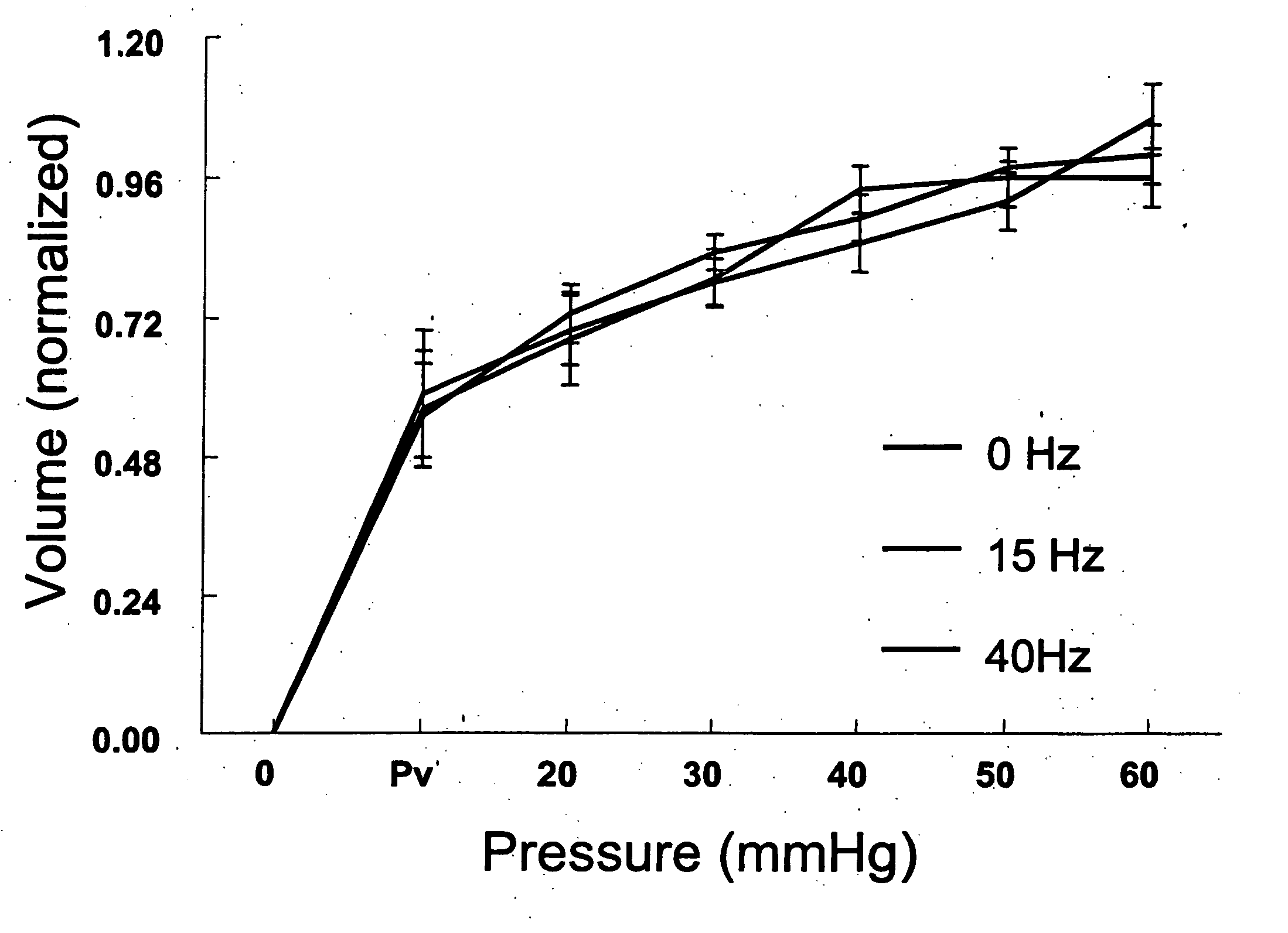
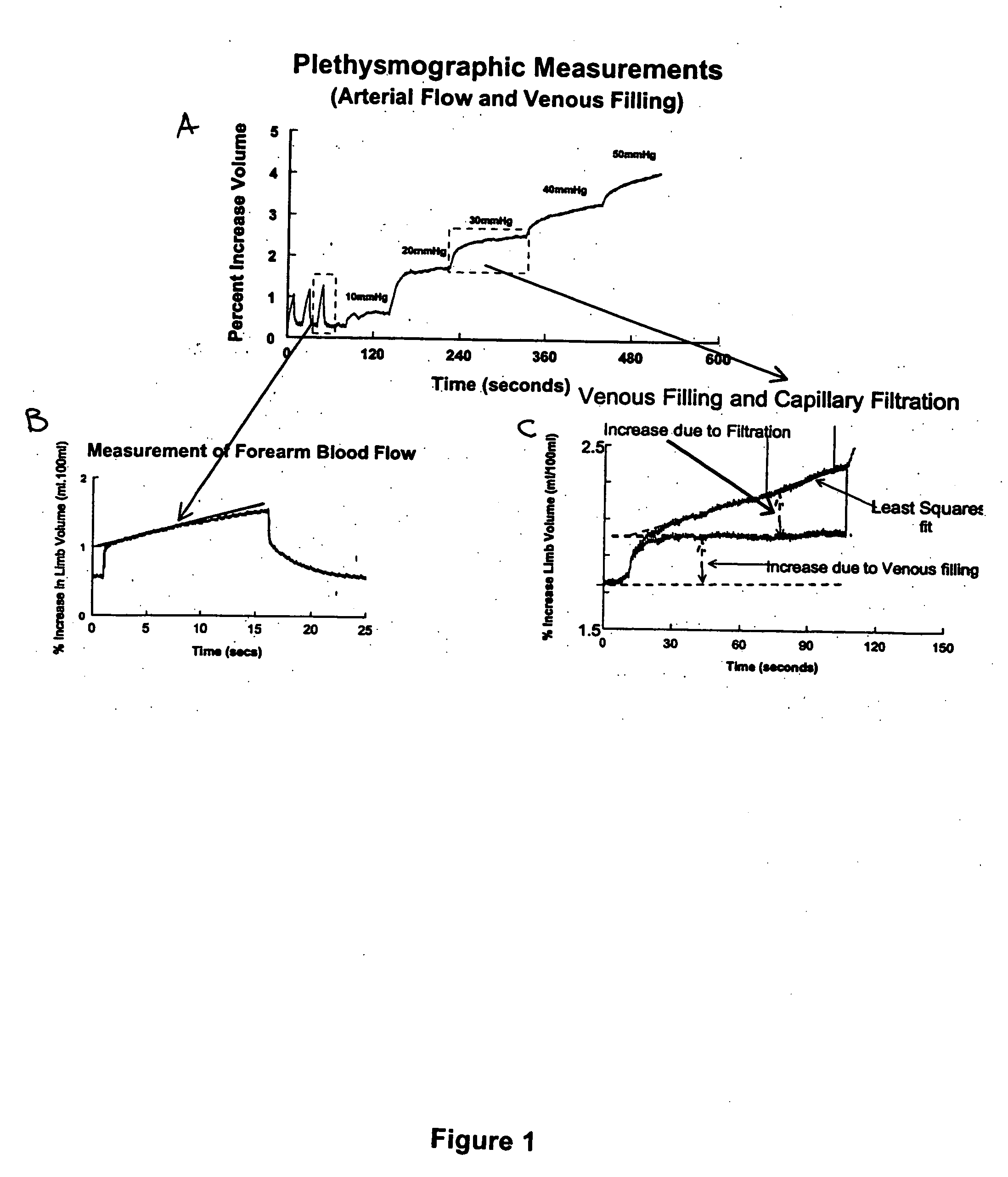
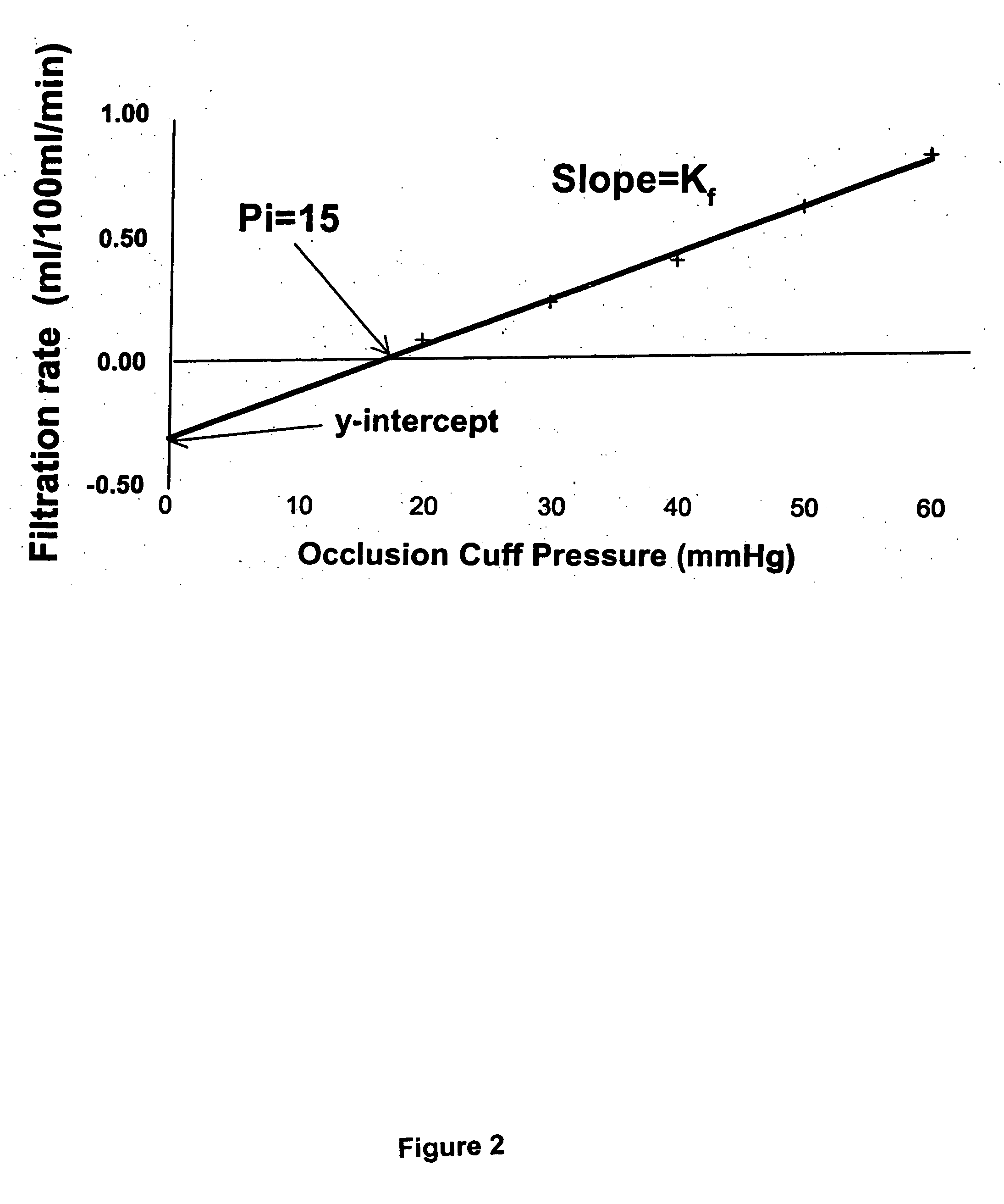
Method for enhancing blood and lymph flow in the extremities
InactiveUS20060111652A1Enhancing bloodEnhancing lymph flowBlood stagnation preventionElectrotherapyRadiologyLymph flow
Methods for enhancing blood and lymph flow in the extremities of a human subject are disclosed. In one aspect, the methods rely on a stimulus effective to displace the skin of a plantar or palmer surface of the subject, thereby enhancing blood and lymph flow in the extremity associated with the stimulated plantar or palmer surface. In another aspect, the methods rely on an electrical stimulus to directly stimulate cutaneous receptors in a plantar or palmer surface of the subject, thereby enhancing blood and lymph flow in the extremity associated with the stimulated cutaneous receptors. Apparatus for enhancing blood and lymph flow in the extremities of a human subject according to the methods of the present invention are also disclosed.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Process to Aid in Motivation of Personal Fitness, Health Monitoring and Validation of User
ActiveUS20180028896A1Enhance healthy food selectionImprove sports experienceInput/output for user-computer interactionGeometric image transformationBiometric dataImage display
A method for motivating a user including the steps of providing a device with an image capturing feature; capturing an image of the user with the image capturing feature; transmitting the captured image to an identity verification device; comparing biometric data from the image to a biometric profile provided by the user; generating a motivational image of the user with a processing device, the motivational image being produced from an acquired image of the user, the acquired image depicting at least one feature of the user; and displaying the motivational image from the processing device in substantially real time to the user; wherein the motivational image exclusively depicts a real image of the user having enhanced dimensions for the at least one feature.
Owner:ENVISIONBODY LLC
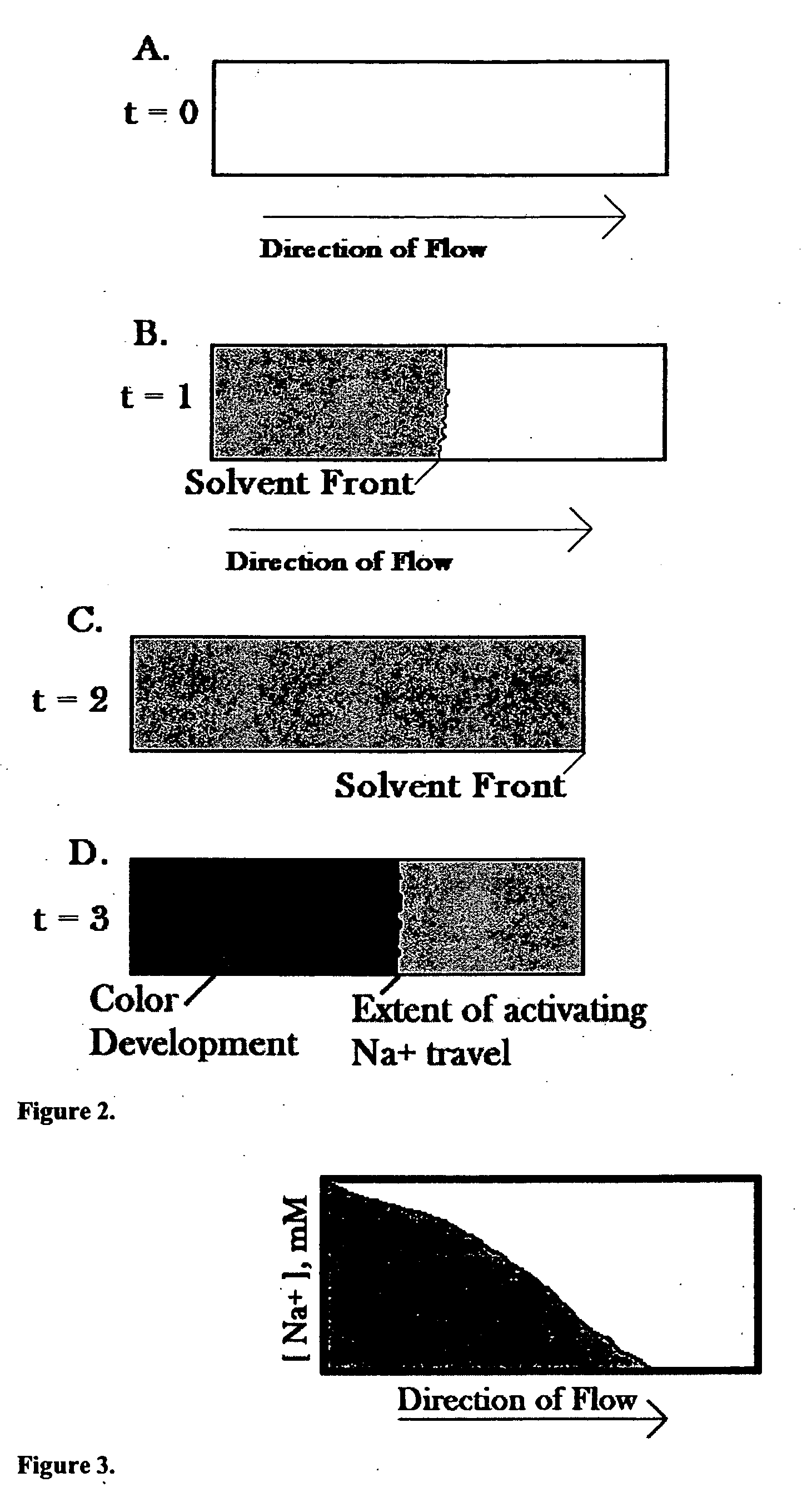
Systems and methods for measuring sodium concentration in saliva
InactiveUS20060121548A1Strict controlImproving indirect controlBioreactor/fermenter combinationsBiological substance pretreatmentsClosed loopFeedback control
Systems and methods for measuring saliva sodium concentration using a chromatographic reaction enable rapid-results, low-cost diagnosis of various medical conditions in an outpatient setting. In one embodiment, measured patient saliva sodium concentration is used by the patient or the patient's healthcare provider to guide medical decision making. In another embodiment, measured patient saliva sodium concentration is processed to mechanically adjust the concentration of sodium in an aqueous solution to be delivered to the patient for oral administration. In yet another embodiment, a closed loop system measures saliva sodium concentration and uses any of a number of different types of feedback control systems to monitor and control the fluid and / or electrolyte state of the patient.
Owner:REMOTE CLINICAL SOLUTIONS
Species-specific, genus-specific and universal DNA probes and amplification primers to rapidly detect and identify common bacterial and fungal pathogens and associated antibiotic resistance genes from clinical specimens for diagnosis in microbiology laboratories
InactiveUS20030049636A1Low costRapid positioningMicrobiological testing/measurementFermentationBacteroidesNeisseria meningitidis
DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample DNA from (i) any bacterium, (ii) the species Streptococcus agalactiae, Staphylococcus saprophyticus, Enterococcus faecium, Neisseria meningitidis, Listeria monocytogenes and Candida albicans, and (iii) any species of the genera Streptococcus, Staphylococcus, Enterococcus, Neisseria and Candida are disclosed. DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample antibiotic resistance genes selected from the group consisting of blatem, blarob, blashv, blaoxa, blaZ, aadB, aacC1, aacC2, aacC3, aacA4, aac6'-lla, ermA, ermB, ermC, mecA, vanA, vanB, vanC, satA, aac(6')-aph(2''), aad(6'), vat, vga, msrA, sul and int are also disclosed. The above microbial species, genera and resistance genes are all clinically relevant and commonly encountered in a variety of clinical specimens. These DNA-based assays are rapid, accurate and can be used in clinical microbiology laboratories for routine diagnosis. These novel diagnostic tools should be useful to improve the speed and accuracy of diagnosis of microbial infections, thereby allowing more effective treatments. Diagnostic kits for (i) the universal detection and quantification of bacteria, and / or (ii) the detection, identification and quantification of the above-mentioned bacterial and fungal species and / or genera, and / or (iii) the detection, identification and quantification of the above-mentioned antibiotic resistance genes are also claimed.
Owner:BERGERON MICHEL G +3
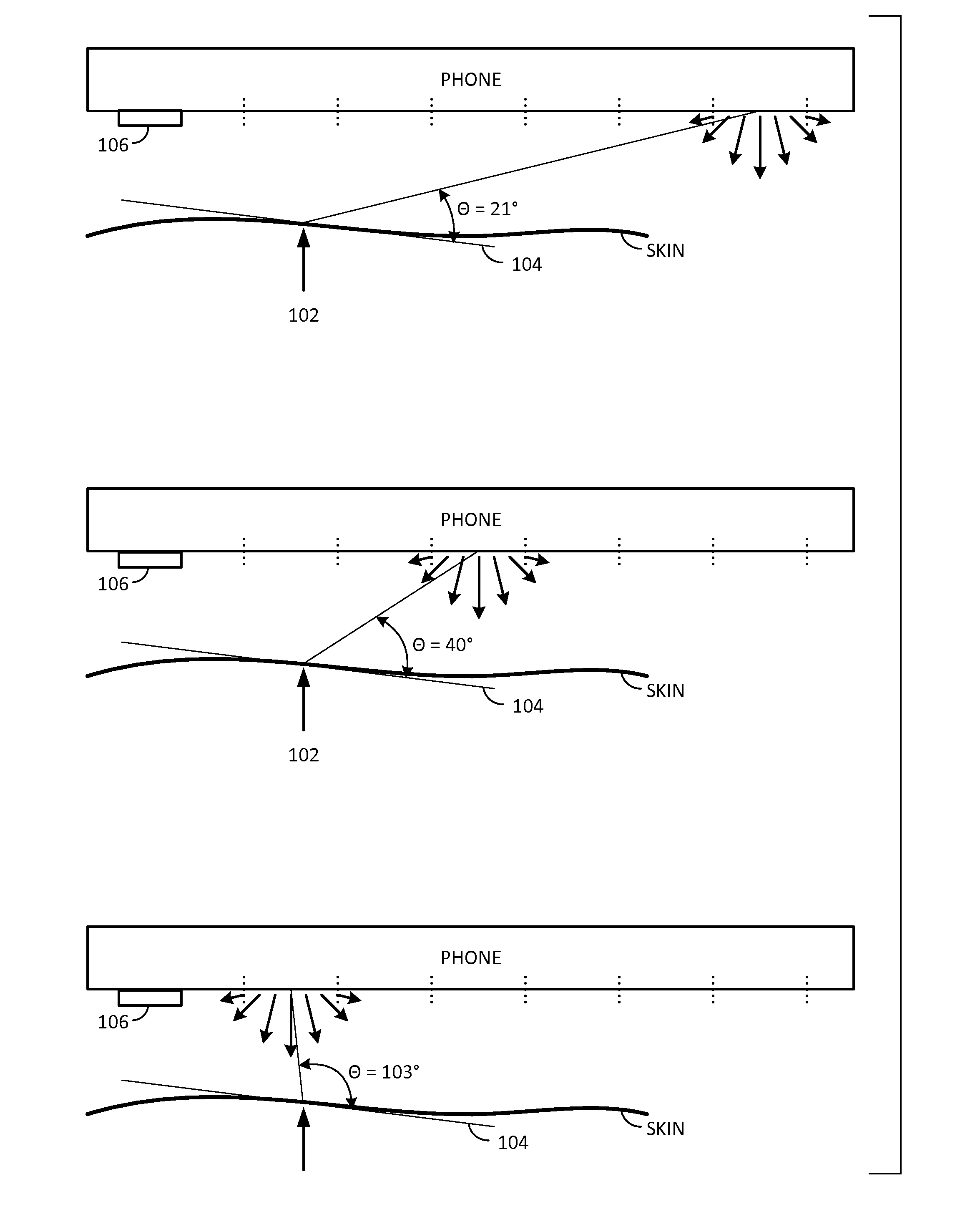
Dermoscopic data acquisition employing display illumination
ActiveUS20150005644A1Lower cost of careImage enhancementMedical imagingDisplay deviceData acquisition
In one arrangement, a smartphone camera gathers skin imagery while controlled spectral illumination is emitted from the smartphone display. In a particular embodiment, differently colored bands move across a darkened screen to illuminate the skin from different angles as plural images are being captured. The collection of imagery from different viewing angles, with different spectral illumination, and different illumination angles, can provide imagery (including composite imagery) useful in manual or automated dermoscopic analysis. A great variety of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
User-interface and method for curved multi-planar reformatting of three-dimensional volume data sets
InactiveUS7061484B2Efficient and straightforward user interfaceEfficient accessGeometric image transformationCathode-ray tube indicatorsMulti-planar ReformattingData set
A user interface for a computer-implemented Multi-Planar Reformatting (MPR) system comprises three display areas in which are displayed three orthogonal MPR images of the volume data set; a curve drawing tool to allow a user to define a curve through the volume data set; six selectable icons each representing one of six possible combinations of a plane parallel to one of the orthogonal MPR images and a direction substantially normal to a plane parallel to one of the other two MPR images, using which the user selects an approximate direction for the curve; and a fourth display area displaying a curve-related MPR image related to one or more points along the curve. The icons may be individually selectable to allow user input of adequate orientational information for a curved MPR image, or selectable in pairs for input of directional information for a cross-curve MPR image. Alternatively, three icons may be provided for a cross-curve MPR system. A tool to allow rotation of a curved MPR image about the curve can be provided, as can visual indicators of the depth of the curve within the volume, and of the orientation of the cross-curve image.
Owner:TOSHIBA MEDICAL VISUALIZATION SYST EURO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com